Beoordeling valrisicoverhogende medicatie
Uitgangsvraag
Wat is de plaats van het gebruik van instrumenten om valrisicoverhogende medicatie te identificeren en/of af te bouwen bij ouderen?
De uitgangsvraag omvat de volgende deelvraag:
- Welke instrumenten kun je gebruiken om valrisicoverhogende medicatie te identificeren en/of af te bouwen bij ouderen?
Aanbeveling
1. Voer altijd een gestructureerd medicatiereview met passende medicatieafbouw uit als onderdeel van een multifactoriële valrisico beoordeling.
2. Gebruik een gestructureerd medicatiereview instrument, bij voorkeur de STOPPFall, ter ondersteuning van het in kaart brengen van valrisicoverhogende en de gepersonaliseerde stappen van passende medicatieafbouw te ondersteunen, zowel bij routine medicatiereviews als bij medicatiereviews gericht op valpreventie bij ouderen.
Zie bijlage STOPPFall medicatielijst en adviezen voor medicatie afbouw)+ bijlage Overzicht van risicoverschillen voor STOPPFall medicatielijst & het interactieve STOPPFall instrument
3. Neem het valrisico mee in de overweging om valrisicoverhogende medicatie bij ouderen wel of niet voor te schrijven. Vraag hiertoe voorafgaand aan het voorschrijven of iemand in het afgelopen jaar gevallen is, moeite heeft met bewegen, lopen of balans houden en/of bezorgd is om te vallen.
Overwegingen
Associatie tussen medicatie en valrisico
Medicijnen kunnen via verschillende mechanismen een val veroorzaken, ofwel als gevolg van een bijwerking, of als gevolg van (relatieve) overdosering. Gezien de aanzienlijke stijging van medicatiegebruik en polyfarmacie bij ouderen sinds de jaren ’80, is de impact van medicatiebijwerkingen zoals vallen op deze groep zeer groot (Craftman, 2016). Juist bij ouderen spelen medicatiebijwerkingen een belangrijke rol bij het ontstaan van vallen, onder andere door het veelvuldige gebruik van medicatie, polyfarmacie, waardoor een hoger risico op interacties ontstaat (zie ook de multidisciplinaire richtlijn Polyfarmacie bij Ouderen). Daarnaast speelt bij ouderen toegenomen gevoeligheid voor bijwerkingen een rol, door onder andere veranderde lichaamssamenstelling, veranderde receptorgevoeligheid en verminderde reservecapaciteit van organen. Voorbeelden van medicatie die ten grondslag kan liggen aan een val zijn: cardiovasculaire medicatie, middelen met een sederende werking (bv psychofarmaca) en middelen met een hypoglycemische werking. Om medicatie die deze interacties en bijwerkingen veroorzaken te identificeren en eventueel af te bouwen, wordt in de richtlijn Polyfarmacie bij ouderen sterk aanbevolen een systematische werkwijze te hanteren.
Effectiviteit van medicatiereview met passende afbouw
Gezien de associatie tussen medicatie en valrisico, is het identificeren van valrisicoverhogende medicatie en het eventuele afbouwen hiervan een belangrijke factor voor de verlaging van het valrisico (Montero-Odasso, 2022). Gepubliceerde literatuur, waaronder een recente systematische review met netwerkanalyse bevestigt dat er sterk bewijs is dat een beoordeling en aanpassing van valrisicoverhogende medicatie één van de effectieve onderdelen van de multifactoriële valrisicobeoordeling is (Dautzenberg, 2021; Hopewell 2018, Montero-Odasso, 2022; Montero-Odasso, 2021). In de recent (gepubliceerde wereldrichtlijn Valpreventie (Montero-Odasso, 2022) staat op basis van GRADE beoordeling van deze literatuur de sterke aanbeveling om om een gestructureerde medicatiereview met passende medicatieafbouw standaard uit te voeren als onderdeel van de multifactoriële valrisicobeoordeling. Daarnaast is door de werkgroep van de wereldrichtlijn onderzocht in een systematisch review of een (algemeen) medicatiereview als enkelvoudige interventie ook effectief is (Seppala, 2022a). Dit bleek niet het geval. Dit benadrukt de noodzaak om het medicatiereview gericht op passende afbouw van valrisicoverhogende medicatie uit te voeren in de context van een multifactoriële valrisicobeoordeling, idealiter in de vorm van een comprehensive geriatric assessment (Montero-Odasso, 2022; Seppala, 2022b; van der Velde, 2022). Mogelijkerwijs is in de verpleeghuissetting een medicatiereview als enkelvoudige interventie wel al effectief (Seppala, 2022a). Gezien de multifactoriële aard van vallen heeft het desalniettemin toch de sterke voorkeur om altijd een multifactoriële valrisicobeoordeling uit te voeren in deze kwetsbare, hoog-risico populatie, uiteraard met een medicatiereview als vast onderdeel (Seppala, 2022; Montero-Odasso, 2022).
Om onnodig valrisico te voorkomen, heeft het daarnaast conform de wereldrichtlijn de sterke voorkeur om in algemene zin bij ouderen al voorafgaand aan het voorschrijven van valrisicoverhogende medicatie het valrisico (inclusief valhistorie) in kaart te brengen en dit mee te nemen in de besluitvorming om wel of niet valrisicoverhogende medicatie voor te schrijven (Montero-Odasso, 2022).
Medicatiereviewinstrumenten voor valrisicoverhogende medicatie
Ten aanzien van de optimale aanpak van een medicatiereview zijn de wereldrichtlijn valpreventie (Montero-Odasso, 2022) en de multidisciplinaire richtlijn Polyfarmacie bij Ouderen eenduidig. In beide richtlijnen staat de sterke aanbeveling om een medicatiereview gepersonaliseerd en gestructureerd uit te voeren met behulp van een instrument (Montero-Odasso 2022).
Algemene medicatiereview instrumenten bevatten veelal een categorie ‘valrisicoverhogende medicatie’ (STOPP/START, BEERS, etc). Deze beperken zich meestal tot de meest gebruikte, hoog-risico medicatie. De werkgroep is van mening dat het de voorkeur heeft om een instrument te gebruiken dat specifiek ontwikkeld is om valrisicoverhogende medicatie in kaart te brengen, en zowel veel gebruikte als minder frequent voorgeschreven middelen bevat. Daarnaast is de werkgroep van mening dat een instrument idealiter informatie verschaft over (1) de symptomen/bijwerkingen waarop gelet dient te worden (bv. Duizeligheid) evenals (2) welke symptomen en klachten na afbouw vervolgd dienen te worden (bv. stemmingsklachten). Dit om zowel de positieve als mogelijke negatieve effecten van de medicatieafbouw te kunnen vervolgen. Advies ten aanzien van de frequentie van follow-up is hierbij ook van belang (Seppala, 2020; van der Velde, 2023) . Een voorbeeld van een Delphi consensus instrument, waarin rekening is gehouden met bovenstaande punten is de STOPPFall (Seppala, 2021), ontwikkeld door Europese experts. In bijlage STOPPFall medicatielijst en adviezen voor medicatie afbouw) vindt u het overzicht van de consensuslijst van valrisicoverhogende medicatie conform STOPPFall. In bijlage Overzicht van risicoverschillen vindt u een overzicht van risicoverschillen tussen subklassen van valrisicoverhogende medicatie, gebaseerd op appendix III van de Delphi studie over de ontwikkeling van de STOPFall (Seppala, 2021). Voor meer gedetailleerde informatie ten aanzien van verschillende bijwerkingenprofielen van subklassen van valrisicoverhogende medicatie en individuele middelen wordt verwezen naar een serie van clinical reviews over dit onderwerp (van Poelgeest, 2023; Korkatti-Puoskari, 2023; Capiau, 2022; Ilhan, 2023; Portlock, 2023; Welsh, 2023; Virnes; 2022; van Poelgeest, 2021).
Om een weloverwogen gezamenlijke keuze te kunnen maken zijn onderstaande punten belangrijk om mee te nemen (Seppala, 2021):
- Of de patiënt(e) en behandelaar goed op de hoogte zijn van de indicatie waarvoor het medicijn gegeven wordt en of de indicatie nog wel bestaat;
- Indien er een behandelindicatie is, besproken wordt of de dosering verlaagd moet worden of het doseerinterval of -tijdstip aangepast moet worden, en of er mogelijk veiliger (niet-) medicamenteuze alternatieven er beschikbaar zijn;
- Indien geen aanpassingen in dosering en geen veiliger alternatieven mogelijk zijn: in samenspraak met de patiënt en andere behandelaars de behandeldoelen prioriteren met specifieke aandacht voor bijwerkingen die kunnen leiden tot vallen.
- Welke symptomen zouden kunnen terugkeren na afbouwen van het betreffende medicijn, en of er onttrekkingsverschijnselen zouden kunnen optreden.
Bij deze stappen is in de STOPPFall de relevante informatie beschikbaar in de beslisbomen. Een interactieve versie van het STOPPFall instrument is vrij beschikbaar. Ter ondersteuning van deze besluitvorming is daarnaast recent een serie reviewartikelen verschenen van de Europese werkgroep naar valrisicoverhogende medicatie, waarin nog diepgaander voor de meest voorgeschreven groepen van valrisicoverhogende medicatie per medicatiesubgroep de indicaties, veiligere alternatieven, en de mogelijke symptomen die kunnen leiden tot vallen als benodigde follow-up worden beschreven. Onder andere bevatten deze klinische reviews een tabel waarin per medicijn en per bijwerking semi-kwantitatief de prevalentie wordt weergegeven, zodat makkelijker een veiliger alternatief gevonden kan worden (Capiau, 2022; van Poelgeest, 2021; van Poelgeest, 2023; Virnes, 2022; Korkatti-Puoskari, 2023; Ilhan, 2023; Portlock, 2023; Welsh, 2023). Daarnaast kunnen ook geneesmiddelteksten van EPHOR app (Expertisecentrum pharmacotherapie bij ouderen) ter ondersteuning van de besluitvorming worden gebruikt, aangezien daarin standaard de voor vallen relevante bijwerkingen worden gerapporteerd (o.a. duizeligheid, valneiging, orthostatische hypotensie).
De werkgroep wil benadrukken dat de STOPPFall consensus lijst van valrisicoverhogende medicatie de meest belangrijke valrisicoverhogende medicamenten bevat, maar dat deze niet uitputtend is. De lijst van medicijnen die bijwerkingen kunnen hebben die tot valincidenten kunnen leiden is vele malen langer. Bij een medicatiebeoordeling is het zodoende van belang om niet strikt aan een instrument vast te houden, maar deze als leidraad te gebruiken en specifieke aandacht te hebben voor de mogelijke relevante bijwerkingen voor de individuele patiënt(e). In de genoemde EPHOR app worden mogelijke relevante bijwerkingen in het kader van valrisico per geneesmiddel beschreven.
Voor- en nadelen van de beschikbare instrumenten en de kwaliteit van het bewijs
Aangezien het reeds bekend is welke medicatie valrisicoverhogend is en dat een gestructureerd medicatiereview een effectief onderdeel van de multifactoriële valanalyse is uit de recente literatuursearch van de Wereldrichtlijn Valpreventie, is de bewijsvoering en GRADE beoordeling van de Wereldrichtlijn valpreventie overgenomen in de aanbevelingen van de huidige richtlijn. Maar omdat het nog onduidelijk is welk instrument het beste gebruikt kan worden voor de uitvoer van een medicatiereview in het kader van valpreventie, iseen literatuuronderzoek verricht naar de voorspellende en diagnostische waarde van bestaande instrumenten om valrisicoverhogende medicatie te identificeren bij ouderen van 65 jaar en ouder. Voor de cruciale uitkomstmaat, de voorspellende waarde, zijn negen studies geïncludeerd. Echter, de bewijskracht voor deze uitkomstmaat is zeer laag omdat het kleine observationele studies betreffen. Geen van de geïncludeerde studies rapporteert de diagnostische waarde van de instrumenten. Hier ligt een kennislacune.
Ten aanzien van diagnostische waarde is het nog onduidelijk welk instrument het beste in staat is om patiënten te identificeren die een hoog risico hebben om te vallen op basis van hun medicatiegebruik. Op basis van de gevonden literatuur kan er dan ook geen conclusie worden getrokken over welk instrument het beste kan worden gebruikt. Om toch antwoord te kunnen geven op deze vraag, worden argumenten ontleend aan aanpalende literatuur, internationale richtlijnen en expert opinion binnen de richtlijn werkgroep.
Naar aanleiding van de literatuursearch is in tabel 2 een overzicht gegeven van alle instrumenten die gebruikt kunnen worden om valrisicoverhogende medicatie in kaart te brengen als onderdeel van een medicatiereview. De meeste instrumenten zijn algemene medicatiereview instrumenten gericht op Potentially Inappropriate Medications (PIMs) en Potentially Omitted Medications (POMs) in de brede zin. Sommige hebben een specifieke sectie voor valrisicoverhogende medicatie (STOPP/START sectie K). Voor de STOPP/START lijst is ook een Nederlandse gevalideerde versie beschikbaar: STOPP/START NL 2020.
De werkgroep sluit zich aan bij de sterke aanbevelingen in de internationale richtlijn valpreventie (Montero-Odasso, 2022) om een gestructureerde medicatiebeoordeling met specifieke aandacht voor valrisicoverhogende medicatie, uit te voeren met behulp van een ondersteunend instrument. Bij voorkeur wordt hiervoor een instrument gebruikt dat (1) een lijst van alle relevante valrisicoverhogende medicatie behelst en daarnaast (2) ondersteuning biedt bij alle stappen van een gestructureerde medicatiebeoordeling. Hierdoor zijn instrumenten die zich op slechts één of enkele groepen valrisicoverhogende medicatie richten (zoals de DBI) minder geschikt voor deze toepassing. Een breder instrument zoals STEADI-Rx of STOPP-sectie K zijn vollediger, maar beperken zich tot een set veel voorgeschreven en/of zeer sterk valrisicoverhogende medicatie. Zodoende adviseert de werkgroep om het STOPPFall instrument als ondersteunend instrument in te zetten voor een gestructureerde medicatiebeoordeling in het kader van valpreventie. Dit omdat het:
1) Niet alleen een screeningslijst omvat maar ook alle stappen van medicatiebeoordeling per geneesmiddelgroep bevat en specifieke informatie geeft ten aanzien van diagnoses, klachten en symptomen die per geneesmiddelgroep in ogenschouw genomen moeten worden, inclusief een follow-up advies
2) ontwikkeld is met het specifieke doel om valrisicoverhogende medicatie te identificeren en zowel veel voorgeschreven als minder vaak voorgeschreven middelen bevat
3) gebaseerd is op de bestaande bewijsvorming in de literatuur aangevuld met input van Europese experts;
4) een uitgebreide Delphi procedure heeft doorlopen, met als gevolg een breed gedragen consensus van Europese en internationale experts.
Validatie van het instrument moet echter nog plaatsvinden, dit is een kennislacune.
Waarden en voorkeuren van patiënten (en evt. hun verzorgers)
Gezamenlijke besluitvorming is cruciaal bij medicatiebeoordeling en –optimalisatie in het kader van valpreventie. Er zijn aanwijzingen dat de kans op succes van medicatieafbouw hierdoor wordt vergroot (zie richtlijn Polyfarmacie, module minderen en stoppen van medicatie). Het betrekken van de patiënt(e) is zodoende een absolute voorwaarde voor het succes van minderen en stoppen van medicatie (richtlijn Polyfarmacie bij ouderen). Richtlijnen met bijbehorend patiënten informatiefolders over minderen en stoppen van medicatie kunnen kennis vergroten en helpen bij verwachtingenmanagement (richtlijn Polyfarmacie bij ouderen). Conform de gestructureerde stappen van medicatiebeoordeling dient bij besluitvorming ten aanzien van minderen en stoppen van medicijnen altijd meegenomen worden wat de wensen en doelen van een individuele patiënt(e) zijn (richtlijn Polyfarmacie bij ouderen, statementpaper T&F group (Van der Velde, 2023). Eerdere studies hebben laten zien dat veel ouderen met een verhoogd valrisico minder vallen en bijwerkingen verkiezen boven een lager cardiovasculair risico (Tinetti, 2008). In een algemene oudere populatie bleek ruim 75% zelfredzaamheid en behoud van functioneren te verkiezen boven overlijden, evenals boven pijn of andere symptomen (Fried, 2011). Goede individuele afstemming waar de behandeling op gericht is (levensverlengend, voorkomen van cardiovasculair event, voorkomen van vallen of verbeteren van fysiek functioneren bijvoorbeeld) is zodoende essentieel. Visuele instrumenten die patiënten kunnen helpen bij complexe medicatie-keuze momenten (wensen, doelen) kunnen hierbij helpen (richtlijn Polyfarmacie bij ouderen, Schuling, 2013; van Summeren, 2016; van Summeren, 2017).
Kosten (middelenbeslag)
In het algemeen zijn er aanwijzingen dat minderen en stoppen van medicatie, ongeacht de setting waarin het gebeurt, kan leiden tot lagere directe geneesmiddelenkosten (MDR-polyfarmacie). Er is in de literatuur slechts een klein aantal economische evaluaties beschikbaar van interventies gericht op het minderen en stoppen van medicijnen bij thuiswonende ouderen. De meesten bleken kosteneffectief volgens de afkapwaarde van de WHO (Romano, 2022). Kosteneffectiviteitsstudies naar het toepassen van een instrument voor beoordeling van valrisicoverhogende medicatie ontbreken in de literatuur. Een systematische review liet wel zien dat in algemene zin (dus zonder focus op valrisicoverhogende medicatie) de toepassing van STOPP-START geneesmiddelkosten kan verlagen (Hill-Taylor, 2016). Ten aanzien van valrisicoverhogende medicatie zijn er aanwijzingen dat medicatie-gerelateerde valincidenten substantiële verborgen (gezondheidszorg-) kosten met zich meebrengen (Tannenbaum, 2015). Een Nederlandse studie heeft laten zien dat een gestructureerde beoordeling van valrisicoverhogende medicatie/afbouw en staken van valrisicoverhogende medicatie kosteneffectief was en zelfs leidde tot een aanzienlijke besparing van de kosten (netto €1.691 per patiënt of €491 per val) op korte termijn (van der Velde, 2007).
Aanvaardbaarheid, haalbaarheid en implementatie
Zowel bij artsen als bij patiënten is er terughoudendheid ten aanzien van afbouw en stoppen van medicatie. Hierbij speelt zowel kennis ten aanzien van (prevalentie) van bijwerkingen (onderschatting) als kennis ten aanzien van beoogde effecten van de medicatie (overschatting) een rol (van der Velde, 2023). Gezondheidsvaardigheden van oudere patiënten en specifieke groepen spelen hierbij een rol. Dit behoeft extra aandacht. Een recente systematische review naar minderen en stoppen van medicijnen als enkelvoudige interventie in het kader van valpreventie liet zien dat de aanbevelingen van bijvoorbeeld een apotheker of klinische beslissingsondersteuning in beperkte mate werden opgevolgd door voorschrijvers (Seppala 2022). Ook de procesevaluaties van 2 recente grote RCTs naar het effect van medicatiereviews met STOPP/START V2 lieten zien dat implementatie niet succesvol was, onder meer als gevolg van terughoudendheid bij patiënten en behandelaars, alsmede transmurale informatieoverdracht en timing van de interventie (Blum, 2021; Dalton, 2020). Uit een recente Europese survey studie onder geriaters (in opleiding) kwam naar voren dat de belangrijkste barrières om medicatie te stoppen of te minderen waren: ontbreken bereidheid patiënten, angst voor negatieve gevolgen, gebrek aan tijd en slechte communicatie tussen voorschrijvers (Van Poelgeest, 2022). Factoren die minderen en stoppen van medicijnen juist bevorderen waren: betere informatie-uitwisseling tussen verschillende voorschrijvers, richtlijnen voor minderen en stoppen van medicijnen, en meer scholing en training (Van Poelgeest, 2022).
Implementatie strategieën voor het effectief minderen en stoppen van medicatie moeten zodoende gericht zijn op meerdere lagen van het gezondheidszorgsysteem. Eerder beschreven elementen/stappen voor implementatie van valpreventie die hierbij doorlopen kunnen worden zijn (1) creëer draagvlak, (2) breng de beginsituatie in kaart, (3) bepaal prioriteiten en doelstellingen, (4) werk acties uit, (5) evalueer en stuur bij en (6) veranker zoals beschreven in de module organisatie van zorg bij valpreventie ouderen en het handboek valkliniek (van der Velde, 2020). Daarnaast is het belangrijk dat het verwijsproces voor een medicatiebeoordeling voor ouderen met een hoog medicatie-gerelateerd valrisico eenvoudig moet zijn met zo min mogelijk tussenstappen, omdat bij iedere stap in het verwijsproces er uitval is (Bhasin, 2020; Bruce, 2021). Er is momenteel beperkte adaptatie in de implementatie van wetenschapsliteratuur naar het gebied van medicatiebeoordelingen en minderen en stoppen van medicatie (Ailabouni, 2022). Dit is een kennislacune.
Rationale van de Aanbevelingen
Aanbeveling-1
Rationale van de aanbeveling: weging van argumenten voor en tegen de interventies
Conform de voor deze richtlijn overgenomen GRADE literatuurbeoordeling van de recent gepubliceerde wereldrichtlijn valpreventie, is er sterk bewijs dat bepaalde medicatie het risico op vallen verhoogt (Montero-Odasso, 2022). In de wereldrichtlijn valpreventie wordt geconcludeerd dat een medicatiereview met passende afbouw is een bewezen effectief onderdeel van de multifactoriële valrisicobeoordeling en dient naar de mening van de werkgroep hier dan ook altijd onderdeel van uit te maken (GRADE 1B). Voor de effectiviteit van een medicatiereview als enkelvoudige interventie is geen bewijs. Daarom, en gezien de multifactoriële aard van vallen, is de mening van de werkgroep dat een medicatiereview altijd als onderdeel van een multifactoriële valrisicobeoordeling uitgevoerd moet worden. Dit geldt zowel bij thuiswonende ouderen als in de verpleeghuisetting, gezien juist de laatste groep een kwetsbare hoog-risico populatie betreft. Een Nederlandse studie heeft laten zien dat een gestructureerde beoordeling en afbouw van valrisicoverhogende medicatie kosteneffectief is en zelfs een aanzienlijke besparing van de kosten kan opleveren.
Aanbeveling-2
Rationale van de aanbeveling: weging van argumenten voor en tegen de interventie
In de wereldrichtlijn valpreventie (Montero-Odasso, 2022) en de richtlijn Polyfarmacie bij ouderen wordt aanbevolen de medicatie op een gestructureerde wijze in kaart te brengen en daarbij ondersteunende instrumenten te gebruiken De werkgroep sluit zich bij deze aanbevelingen aan. Ondanks dat er op basis van de geïncludeerde geen sterk bewijs voor is gevonden ten faveure van een van de beschikbare instrumenten, is de werkgroep toch van mening dat een ondersteunend instrument van toegevoegde waarde is. In de praktijk is er vaak terughoudendheid ten aanzien van het afbouwen en stoppen van medicatie. Een ondersteunend instrument dat zowel een lijst van relevante valrisicoverhogende medicatie als een overzicht van de te nemen stappen bij beoordeling bevat, kan helpend zijn bij het afbouwen en stoppen. De voorkeur van de werkgroep valt hierbij op het STOPPFall instrument. Dit omdat dit instrument specifiek is ontwikkeld voor de identificatie van valrisicoverhogende medicatie en gebaseerd is op bestaande bewijsvorming aangevuld met de input van Europese experts. Ook bevat het instrument naast een screeningslijst ook alle stappen van medicatiebeoordeling per geneesmiddelengroep en specifieke informatie ten aanzien van diagnoses, klachten en symptomen die in ogenschouw genomen moeten worden, inclusief een follow-up advies. Er is voor dit instrument een uitgebreide Delphi procedure doorlopen, wat heeft geleid tot een breed gedragen consensus van experts. Het ontbreken van bereidheid van de patiënt is één van de belangrijkste barrières om medicatie te stoppen. Er zijn aanwijzingen dan de kans op succes van medicatieafbouw wordt vergroot door gezamenlijke besluitvorming. De werkgroep is dan ook van mening dat een gepersonaliseerde aanpak en gezamenlijke besluitvorming noodzakelijk zijn voor een succesvol minderen en stoppen van medicatie. Om weloverwogen keuzes te maken samen met de patiënt moet gewerkt worden volgens de gestructureerde stappen van medicatiebeoordeling. Hierin worden de wensen en doelen van de individuele patiënt meegewogen en wordt ook met de patiënt samen gekeken naar de indicatie, alternatieven, dosering, bijwerkingen en symptomen die opnieuw zouden kunnen optreden bij stoppen van een middel. In de STOPPFall is bij deze stappen de relevante informatie beschikbaar in beslisbomen (zie bijlage STOPPFall medicatielijst en adviezen voor medicatie afbouw). Een interactieve digitale versie van de beslisbomen is online beschikbaar via STOPPFall instrument. Een instrument moet gezien worden als een leidraad, maar is niet uitputtend. Individuele afwegingen van mogelijke bijwerkingen van andere groepen van medicatie die mogelijk het valrisico verhogen zijn essentieel. In de geneesmiddelenteksten van de EPHOR app (Expertisecentrum pharmacotherapie bij ouderen) worden mogelijke relevante bijwerkingen (zoals duizeligheid, orthostatische hypotensie, valrisico) in het kader van valrisico per geneesmiddel beschreven(Van der Velde, 2023; van Poelgeest, 2023; Korkatti-Puoskari, 2023; Capiau, 2022; Ilhan, 2023; Portlock, 2023; Welsh, 2023; Virnes; 2022; van Poelgeest, 2021).
Aanbeveling-3
Rationale van de aanbeveling: weging van argumenten voor en tegen de interventies
Zoals onder andere beschreven in de internationale richtlijn valpreventie (Montero-Odasso, 2022), de clinical review series overzichten en de richtlijn Polyfarmacie bij ouderen, kan medicatie n via verschillende mechanismen een val veroorzaken, onder andere als gevolg van bijwerkingen. Juist bij ouderen spelen medicatiebijwerkingen een belangrijke rol, dit door het veelvuldige gebruik van medicatie, polyfarmacie, waardoor een hoger risico op interacties. Daarnaast is er bij ouderen sprake van een toegenomen gevoeligheid voor bijwerkingen, door onder andere veranderde lichaamssamenstelling, veranderde receptorgevoeligheid en verminderde reservecapaciteit. De werkgroep is daarom van mening dat voorafgaand aan het voorschrijven van valrisicoverhogende medicatie een inschatting moet worden gemaakt van het valrisico (inclusief valhistorie). Dit moet worden meegenomen in de overweging om valrisicoverhogende medicatie te starten. Eventuele veiliger alternatieven moeten overwogen worden Als starten van valrisicoverhogende medicatie toch nodig is, moet gekozen worden voor de minimale effectieve dosis en een zo kort mogelijke duur.
Onderbouwing
Achtergrond
Eén van de belangrijkste behandelbare valrisicofactoren is het gebruik van valrisicoverhogende medicatie (NL Richtlijn 2017; Hopewell, 2018; Montero-Odasso 2022;). Conform eerder gepubliceerde literatuur en richtlijnen bevestigt een recente systematische review met netwerkanalyse dat een beoordeling en aanpassing van valrisicoverhogende medicatie één van de effectieve onderdelen van de multifactoriële valrisicobeoordeling is (Dautzenberg, 2021). De recent (eind 2022) gepubliceerde wereldrichtlijn Valpreventie (Montero-Odasso, 2022) beveelt dan ook aan om standaard als onderdeel van de multifactoriële valrisicobeoordeling een gestructureerde medicatiereview met passende medicatieafbouw uit te voeren. Dit is in overeenstemming met de module Verlaging valrisico bij thuiswonende ouderen van de Nederlandse richtlijn ‘Preventie van valincidenten bij ouderen’. Er is veel onderzoek gedaan naar de associatie tussen verschillende medicatie groepen en valrisico. Psychotrope medicatie is een onafhankelijke risicofactor voor vallen. Ook anti-epileptica, anticholinergica en sommige klassen van cardiovasculaire medicatie zijn valrisico verhogend (Deandra, 2010; De Vries, 2018; Hartikainen, 2007; Leipzig, 1999a; Leipzig, 1999b; Park, 2015; Seppala, 2018a; Seppala, 2018b; Woolcott, 2009).
Mede gezien de onderliggende aandoening – die ook valrisicoverhogend kan zijn – is een gepersonaliseerde afweging ten aanzien van wel of niet afbouwen van het mogelijk valrisicoverhogende medicijn wenselijk (Montero-Odasso, 2022). Zodoende dient deze informatie meegenomen te worden in de medicatiebeoordeling, en de daaropvolgende besluitvorming om wel of niet af te bouwen. Gezien de complexiteit wordt een gestructureerde aanpak aanbevolen. In algemene zin wordt in de richtlijnmodule Medicatiebeoordeling van de richtlijn Polyfarmacie bij ouderen aanbevolen om bij een medicatiereview gestructureerde instrumenten te gebruiken. Dit is in overeenstemming met de wereldrichtlijn Valpreventie (Montero-Odasso, 2022).
In de huidige situatie wordt zowel door zorgverleners als door patiënten vallen als mogelijke medicatiebijwerking veelal niet herkend. Ook ontbreekt er een gevalideerde en gestructureerde aanpak voor uitvoering van de medicatiereview in het kader van valpreventie. Het is onduidelijk of en welk instrument hiervoor het beste gebruikt kan worden. Ook is het onduidelijk hoe het vervolg na medicatieafbouw in het kader van valpreventie er idealiter uit zou moeten zien.
Conclusies / Summary of Findings
|
Very low GRADE |
The evidence is very uncertain about the predictive value of risk for falls based on a medication review tool when compared with no tool or another tool in older adults (≥ 65 years old). Source: Blalock, 2020a; Byrne, 2019; Cardwell, 2020; Damoiseau-Volman, 2022; Lavrador, 2021; Lukazewski, 2012; Nyborg, 2017; Prudent, 2008; Stewart, 2021 |
|
no GRADE |
No evidence was found regarding the diagnostic accuracy of a medication review tool when compared with no tool or another tool in older adults (≥ 65 years old). Source: - |
Samenvatting literatuur
Description of studies
Blalock (2020a) examined the association between the drug burden index (DBI) and medication-related fall risk using a retrospective cohort study design. Adults (age 65 or older) using four or more chronic medications or at least one medication associated with an increased risk of falling were included from community pharmacies (n=1562). Pharmacy staff screened patients by asking the following key questions derived from the Stopping Elderly Accidents, Deaths, & Injuries (STEADI) Initiative:
- Have you fallen in the past year?
- Do you feel unsteady when standing or walking?
- Do you worry about falling?
In addition, patients who reported one or more falls within the past year were asked if any of the falls had resulted in injury. Patients who answered ‘yes’ to any of the key STEADI questions were classified as having screened positive for increased fall risk. These patients were eligible to receive medication review provided by a pharmacist using evidence-based algorithms developed by the study team to 1) identify medications associated with an increased risk of falling, and 2) provide therapeutic recommendations to reduce risk. In total, 1058 of the included patients were screened for fall risk using the STEADI questions. The DBI was used to assess each participant’s cumulative exposure to medications with anticholinergic or sedative properties during the 1-year follow-up time. The DBI is a validated measure used to assess a person’s total exposure to medications with anticholinergic and sedative properties. The following relevant outcome measure was reported: predictive value.
Byrne (2019) validated the drug burden index (DBI) by examining the association of the DBI score with important health outcomes in Irish community-dwelling older people. This was a cohort study using data from the Irish Longitudinal Study on Ageing (TILDA) with linked pharmacy claims data. Individuals aged ≥65 years participating in TILDA and enrolled in the General Medical Services scheme were eligible for inclusion. The drug burden index (DBI) score was determined by applying the DBI tool to participants’ medication dispensing data. In total, 1924 participants were included, and the outcomes were assessed at the time of interview (cohort 1). The DBI is a validated measure used to assess a person’s total exposure to medications with anticholinergic and sedative properties. The follow-up was 12 months. The following relevant outcome measure was reported: predictive value.
Cardwell (2020) aimed to determine whether a higher DBI was associated with poorer outcomes (hospitalization, falls, mortality, cognitive function, and functional status) over 36 months follow-up. Data from the Life Living in Advanced Age, a Cohort study in New Zealand (LiLACS NZ) was used for this study. LiLACS NZ consist of two cohorts: Māori (the Indigenous population of New Zealand) aged ≥80 years and non-Māori aged 85 years at the time of enrolment. For this guideline module, only the non-Māori population was included to increase the generalizability of the study towards the Dutch population. In total, 404 non-Māori patients were included at baseline. Medications with anticholinergic and/or sedative properties (i.e. medications with a DBI > 0) were identified using the Monthly Index of Medical Specialities (MIMS) medication formulary, New Zealand. The DBI was calculated for everyone enrolled at each time point. The DBI is a validated measure used to assess a person’s total exposure to medications with anticholinergic and sedative properties. The follow-up was 36 months. The following relevant outcome measure was reported: predictive value.
Damoiseaux-Volman (2022) investigated the effect of potentially inappropriate medications (PIMs) on inpatient falls and to identify whether PIMs as defined by STOPPFall, STOPP/START v2, or the designated section K for falls of STOPP v2 have a stronger association with inpatient falls when compared to the general tool STOPP v2. The STOPPFAll is an instrument to identify fall risk inducing drugs. STOPP/START is a general list with potentially inappropriate drugs, including a designated section K, which is a specific section to identify high risk fall risk inducing drugs. This retrospective observational study used an electronic health records dataset of patients ≥70 years admitted to an academic hospital due to a fall. In total, 16,687 patients were included in this study. The PIM exposure was calculated as the number of PIMs (sum of the unique PIMs each day) administered during hospital stay, divided by the hospital length of stay in days. The follow-up was at least 24 hours but was not further specified. The following relevant outcome measure was reported: predictive value.
Nyborg (2017) performed a cross-sectional observation study and assessed the level of inappropriate medication use in older nursing home residents in Norway according to the NORGEP-NH (≥ 65 years old). The NORGEP criteria were developed in Norway in 2008, intended for use in general practice and for a home-dwelling older population. In order to have an updated tool for assessment of medication use in nursing homes that was also suited for the Norwegian pharmaceutical market, the NORGEP-NH criteria were developed. The NORGEP-NH criteria consist of three parts: single substance criteria (criteria to avoid for regular use whenever possible), combination criteria (criteria of drug combinations that should be avoided when possible), and deprescribing criteria (criteria for continuation). The participants in the study constitute the part of the nursing home population in need of antibiotic or intravenous fluid therapy during the study period. In total, 881 patients were included. All these patients were evaluated according to the NORGEP-NH criteria. There was no control group available. The length of follow-up was not specified. The following relevant outcome measure was reported: predictive value.
Lavrador (2020) evaluated the effect size of the associations between the anticholinergic scales on cumulative anticholinergic burden instruments with peripheral or central anticholinergic adverse outcomes in older patients. This case-control study was conducted in patients over 65 years who were admitted to two internal medicine wards of a Portuguese university hospital. The Anticholinergic Drug Scale (ADS), Anticholinergic Risk scale (ARS), Anticholinergic Cognitive Burden Scale (ACBS), and Drug Burden Index (DBI) were used to calculate the patients’ anticholinergic burden. In total, 250 patients were included. There is no control group available. The association between falls in the preceding 6 months and the anticholinergic burden scales scores were reported. The length of follow-up was not specified. The following relevant outcome measure was reported: predictive value.
Lukazewski (2012) evaluated the effectiveness of a web-based program, Monitor-Rx, in identifying adults at risk for drug-related geriatric syndromes or inappropriate medicines. One of the geriatric problems that Monitor-Rx could identify is falls. This prospective pilot study compared medication-related risks generated by the Web-based program with those identified by a certified geriatric pharmacist (CGP). Eligible patients were members of Supporting Active Independent Lives (SAIL), a community-based grass roots organization intended to help older adults age in place in their homes. Monitor-Rx correlates medication effects with physical, functional, and cognitive decline in older adults by identifying medications as a cause or aggravating factor contributing to common geriatric conditions. The program also identifies medications with anticholinergic effects and medications inappropriate for use in older adults (≥ 65 years old). The study compared the incidence of falls identified by the Monitor-Rx versus identification by the GCP. In total, 29 patients were included. The participants were not followed-up over time. The following relevant outcome measure was reported: predictive value.
Prudent (2008) studied the consumption of potentially inappropriate medication (PIM) among patients aged ≥75 years, paying particular attention to psychotropic drugs and the factors influencing the use of potentially inappropriate psychotropics (PIPs). This cross-sectional analysis of a prospective multicenter cohort including 1176 hospitalized French patients aged ≥75 years. The Beers list as updated in 2003 defined which medications were considered PIPs. There was no follow-up as the analysis is cross-sectional. The following relevant outcome measure was reported: predictive value.
Stewart (2021) compared the evidence behind anticholinergic burden (ACB) measures in relation with their ability to predict risk of falling in older people. Medline (OVID), EMBASE (OVID), CINAHL (EMBSCO) and PsycINFO (OVID) were searched from 2006 until september 2020. Inclusion criteria included: participants aged 65years and older, use of one or more ACB measure(s) as a prognostic factor, cohort or case-control in design, and reporting falls as an outcome. In total, 8 studies reporting temporal associations between ACB and falls were included. The identified ACB measures were the anticholinergic cognitive burden scale (ACBS) and the anticholinergic risk score (ARS). The evidence supports an association between moderate to high ACB and risk of falling in older people, but no conclusion can be made regarding which ACB scale offers best prognostic value in older people. The following relevant outcome measure was reported: predictive value.
Sixteen studies did not comply with the PICO and were therefore not included in the literature summary and GRADE assessment. The data of these studies is not incorporated in the Evidence tables and risk of bias tables as these studies did not report the relevant outcome measures. To be able to give an overview of all available instruments to identify fall-risk increasing drugs, the studies are described shortly below. Table 2 gives an overview of all available instruments for medication review.
Ackroyd-Stolarz (2009) examined the association between potentially inappropriate prescribing of benzodiazepines, as defined by the Beers criteria, by older adults (at least 65 years of age) and the risk of having a fall during acute inpatient care.
Aizenberg (2002) retrospectively assessed the characteristics of older psychiatric inpatients (≥ 65 years old) that had sustained a fall during hospitalization. Patients that were aged ≥65 years and intact cognition were included in the study. The control group consisted of the previous and next admission of an older patient to the same ward. The anticholinergic burden score (ABS) was calculated for each patient.
Arnold (2017) conducted a retrospective chart review covering all patients aged ≥ 65 years who were admitted to Evangelisches Krankenhaus Göttingen-Weende. Potentially inappropriate psychotropic drugs were identified according to the PRISCUS list. The PRISCUS list is an expert opinion-based general list of potentially inappropriate drugs in older adults (≥ 65 years old) that is currently used as a guideline in Germany. Psychotropic drugs that were prescribed in this study were antipsychotics, antidepressants, Z-drugs and benzodiazepines. In total, 2130 patients were included.
Blalock (2020b) evaluated the effects of a community pharmacy-based fall prevention intervention (STEADI-Rx) on the risk of falling and use of medications associated with an increased risk of falling. This randomized controlled trial included adults (age ≥ 65 years) using either four or more chronic medications or one or more medications associated with an increased risk of falling and continuous insurance coverage through Medicare Part D and NC Medicaid for the entire study period. Pharmacy staff screened patients for fall risk using questions from the Stopping Elderly Accidents Deaths and Injuries (STEADI) algorithm. The Steady-Rx includes tricyclic antidepressants, antispasmodics, antihistamines, sedative hypnotics, benzodiazepines, anticonvulsants, opioid, antipsychotics, and antidepressants. Patients who screened positive were eligible to receive a pharmacist-conducted review, with recommendations sent to patient’s healthcare providers following the review. In total, 1,467 patients were included, of which 1000 were screened and 467 of the patients were not screened.
Cardwell (2015) identified tools used to quantify anticholinergic medication burden and aimed to determine the most appropriate tool for use in longitudinal research, conducted in those aged 80 years and older. An electronic search was performed in Ovid Medline, Embase, PubMed, Web of Science, PsycINFO, and international Pharmaceutical Abstracts from the inception of each database until February 2015. Inclusion criteria were published in the English literature, including populations with an average age ≥ 80 years, studies with an intention to quantify anticholinergic medication burden, and those with outcome measures that included at least one of the clinical outcome measures of interest (cognitive function, physical function, frequency of falls, hospitalization, and all-cause mortality).
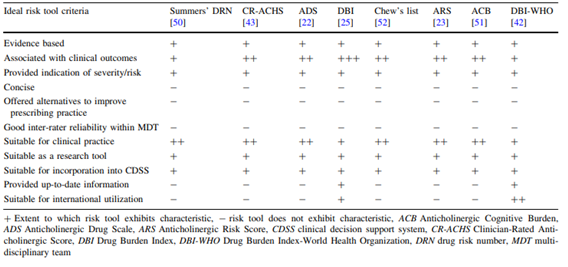
In total, 13 studies were eligible for inclusion and 8 tools were identified. The identified tools were Drug Burden Index (DBI), modified Anticholinergic Risk Scale (mARS), Drug Burden Index-World Health Organization (DBI-WHO), Clinician-Rated Anticholinergic Score (CR-ACHS), Summers’ Drug Risk Number (DRN), Anticholinergic Drug Scale (ADS), Anticholinergic Cognitive Burden (ACB) score, Anticholinergic Risk Scale (ARS), and Chew’s list. The criteria used to identify the most appropriate tool for use in longitudinal research are shown below (Table 1). The drug burden index (DBI) exhibited most of the key attributes of an ideal anticholinergic risk tool.
Table 1. Criteria used to identify the most appropriate tool for use in longitudinal research. From: Cardwell (2015).
 Di Martino (2020) applied the Beers (2015 version), STOPP/START criteria (2014 version) and Improving Prescribing in the Elderly Tool (IPET) criteria (2000 version) as key tool to improve the quality of prescribing. The Beers, STOPP/START, and IPET criteria are general lists of potentially inappropriate drugs. Adult patients (≥65 years old) admitted to the Mediterranean Institute for Transplantation and Advanced Specialists Therapies were included. These criteria were used to assess the medication use in older patients in terms of PIMs and PPOs.
Di Martino (2020) applied the Beers (2015 version), STOPP/START criteria (2014 version) and Improving Prescribing in the Elderly Tool (IPET) criteria (2000 version) as key tool to improve the quality of prescribing. The Beers, STOPP/START, and IPET criteria are general lists of potentially inappropriate drugs. Adult patients (≥65 years old) admitted to the Mediterranean Institute for Transplantation and Advanced Specialists Therapies were included. These criteria were used to assess the medication use in older patients in terms of PIMs and PPOs.
Frankenthal (2014) assessed the effect of a Screening Tool of Older Persons potentially inappropriate Prescriptions/Screening Tool to Alert doctors to Right Treatment (STOPP/START) medication intervention on clinical and economic outcomes. The STOPP/START is a general list with potentially inappropriate drugs, including section K specific for fall risk inducing drugs. This parallel group randomized trial was performed in a chronic care geriatric facility. Residents aged 65 and older prescribed with at least one medication (N=359) were randomized to receive usual pharmaceutical care or undergo medication intervention. The intervention group (N=183) received a medication review by the study pharmacist at study opening, and at 6 and 12 months. The STOPP/START criteria were applied to identify potentially inappropriate prescriptions (PIPs) and potentially prescription omissions (PPOs). The control group (N=176) received usual pharmaceutical care. The follow-up was 12 months. The following relevant outcome measure was reported: predictive value (accepted recommendations by physician).
Frankenthal (2016) assessed the effect of a Screening Tool of Older Persons potentially inappropriate Prescriptions/Screening Tool to Alert doctors to Right Treatment (STOPP/START) medication intervention on clinical and economic outcomes at 24-months of follow-up. The STOPP/START is a general list with potentially inappropriate drugs, including section K specific for fall risk inducing drugs. For study details, see Frankenthal (2014).
Jamieson (2019) evaluated the association between the Drug Burden Index (DBI) and hip fractures, after correcting for mortality and multiple potential confounding factors. Patients included home-based people aged 65 years and older. The DBI exposure was calculated for medicines with anticholinergic and sedative properties. The DBI is a validated measure used to assess a person’s total exposure to medications with anticholinergic and sedative properties. In total, 70,553 individuals from a community-dwelling older population were included in the study.
Kimura (2016) evaluated the prevalence of PIMs and the efficacy of hospital pharmacists’ assessment and intervention based on STOPP criteria version 2. New inpatients aged ≥65 years who were prescribed ≥1 daily medicine were included in the study. Pharmacists assessed and detected PIMs based on STOPP criteria version 2 and considered the patient’s intention to change the prescription at the time of admission of each patient. The pharmacist and doctors discussed and finally decided whether or not to change the PIMs or not. A total of 822 patients were included in this study. Patients were not followed-up over time. The STOPP v2 is a general list with potentially inappropriate drugs, including section K specific for fall risk inducing drugs.
McMahon (2014) performed a before-and-after cohort study to assess if prescribing modification occurs in older people presenting to an emergency department due to a fall over a 4-year period. In total, 1016 patients were included in the study. The STOPP screening tool and Beers prescribing criteria were applied to identify potentially inappropriate prescribing. The individual STOPP, STOPP section K, psychotropic medication, and beers criteria were compared in the 12 months pre- and post-fall. The STOPP is a general list of potentially inappropriate drugs. STOPP includes a section K specific for fall risk inducing drugs.
Onatade (2013) determined the prevalence and types of potentially inappropriate medication (PIM) in older people admitted to and discharged from an Acute UK hospital and to determine how often PIMs prescribed on discharge are accompanied by a plan for follow-up. Patients aged ≥65 years admitted to the Specialist Health and Ageing Unit were included. Data were obtained by applying STOPP criteria to electronic admission and discharge medication lists. In total, the admission and discharge medication lists were assessed for 195 patients. STOPP is a general list of potentially inappropriate drugs. STOPP includes a section K specific for fall risk inducing drugs. STOPP includes a section K specific for fall risk inducing drugs. Section K PIMS were among the most common identified PIMS on admission and discharge.
Seppala (2021) describes a comprehensive STOPPFall by Delphi consensus by a European expert group. The STOPPFall was created by two facilitators based on evidence from recent meta-analyses and national fall prevention guidelines in Europe. Twenty-four panelists chose their level of agreement on a Likert scale with the items in the STOPPFall in three Delphi panel rounds. A threshold of 70% was selected for consensus a priori. The panelists agreed on 14 medication classes to be included in the STOPPFall, including benzodiazepines, antipsychotics, benzodiazepine-related drugs, opioids, antidepressants, anticholinergics, antiepileptics, diuretics, alpha-blockers used as antihypertensives, alpha-blockers for prostate hyperplasia, centrally acting antihypertensives, antihistamines, vasodilators used in cardiac diseases, and overactive bladder and urge incontinence medications. They indicated 18 differences between pharmacological subclasses regarding fall-risk-increasing properties. In addition, practical deprescribing guidance was developed for STOPPFall medication classes.
Shaver (2020) determined whether there was an increase in fall risk increasing drug prescribing and if this is concurrent with an increase in fall-related mortality in persons 65 years and older in the United States. This serial cross-sectional analysis utilized data from both the National Vital Statistics System, as the medical expenditure panel survey for years 1999-2017. Adults aged 65 years and older were evaluated for death due to falls and for prescription fills of fall risk increasing drugs using the STEADI-Rx tool. The STEADI-Rx tool is a specific list of fall risk increasing drugs, comprising of the following FRID: tricyclic antidepressants, antispasmodics, antihistamines, sedative hypnotics, benzodiazepines, anticonvulsants, opioid, antipsychotics, and antidepressants. The analysis included 374.972 fall-related mortalities and 7.858.177.122 fills of fall risk increasing drugs.
Thevelin (2019) aimed to compare the prevalence and types of drug-related admissions identified by STOPP/START version 1 and STOPP/START version 2. The STOPP/START is a general list with potentially inappropriate drugs, including section K specific for fall risk inducing drugs. They applied the STOPP/START version 2 criteria to a subset of 100 consecutively admitted geriatric patients selected from our original cross-sectional study of 302 patients. A geriatrician and a pharmacist adjudicated whether the identified PIMs and PPOs were related to acute hospitalization.
Walsh (2019) performed a before-and-after cohort study to explore patterns of relevant potentially inappropriate prescribing in older people with fall-related hospitalizations. Adults hospitalized due to a fall, fracture, or syncope were included in the study. For participants who experiences more than one fall-related hospitalization, only the first eligible record was selected to avoid overlapping and interdependence of observations. In total, 927 individuals were identified as having a fall-related hospitalization within the study timeframe and had prescription data available. These individuals were included in the study. Fall-related prescribing was defined using the STOPP/START section K. Medication use including sedatives (benzodiazepines, Z-drugs, and neuroleptics), vasodilators, and vitamin D was compared prior and post hospitalization.
Table 2. Overview of instruments to identify fall-risk inducing drugs
|
Reference |
Tool |
Population of interest |
Brief description |
|
Aizenberg, 2002 |
ABS |
Adults aged ≥65 years |
Tool to assess the anticholinergic burden. |
|
Stewart, 2021 Lavrador, 2021 |
ACBS |
Adults aged ≥65 years |
Tool to assess the anticholinergic burden. |
|
Stewart, 2021 Lavrador, 2021 |
ARS |
Adults aged ≥65 years |
Tool to assess the anticholinergic burden. |
|
Lavrador, 2021 |
ADS |
Adults aged ≥65 years |
Tool to assess the anticholinergic burden. |
|
McMahon, 2014 Prudent, 2008 Di Martino, 2020 Ackroyd-Stolar, 2009 |
Beers list |
Adults aged > 70 years |
Screening tools to identify potentially inappropriate prescribing (PIPs). Including a section on fall-risk increasing drugs. |
|
Byrne, 2019 Cardwell, 2020 Blalock, 2020a Jamieson, 2018 Lavrador, 2021 |
DBI |
Adults aged ≥65 years |
The DBI is a risk assessment tool to quantify older individuals’ cumulative exposure to medications with clinically significant anticholinergic and/or sedative effects. |
|
Di Martino, 2020 |
IPET |
Adults aged ≥65 years |
The IPET criteria (2000 version) consist of a list of 14 PIMs identified by a panel of Canadian experts. |
|
Arnold, 2017 |
PRISCUS |
Adults aged ≥65 years |
A list of potentially inappropriate drugs in older adults (≥ 65 years old) that is currently used as a guideline in Germany. |
|
Blalock, 2020a, Blalock, 2020b Shaver, 2020 |
STEADI-Rx |
Adults aged ≥65 years |
The STEADI algorithm is adapted for use in the community pharmacy setting and provides a list of fall-risk increasing drugs. |
|
Frankenthal, 2014 Frankenthal, 2016 Walsh, 2019 Di Martino, 2020 |
STOPP-START |
Adults aged ≥65 years |
Screening tool to identify potentially inappropriate prescriptions (PIPs) and potential prescription omissions (PPOs). In section K fall-risk increasing drugs are listed. |
|
Thevelin, 2019 |
STOPP/START version 2 |
Adults aged ≥65 years |
Screening tool to identify potentially inappropriate prescriptions (PIPs) and potential prescription omissions (PPOs). In section K fall-risk increasing drugs are listed. |
|
McMahon, 2014 Onatade, 2013 |
STOPP |
Adults aged >65 years |
Screening tools to identify potentially inappropriate prescribing (PIPs). In section K fall-risk increasing drugs are listed. |
|
Kimura, 2016 Damoiseaux-Volman, 2022 |
STOPP version 2 |
Hospital patients who were prescribed ≥1 daily medicine |
Screening tool to identify potentially inappropriate medications (PIMs). In section K fall-risk increasing drugs are listed. |
|
Damoiseaux-Volman, 2022 |
Section K for falls of STOPP version 2 |
Adults aged ≥70 years |
Screening tools to identify potentially inappropriate prescribing (PIPs). This section contains fall-risk increasing drugs. |
|
Seppala, 2021 Damoiseaux-Volman, 2022 |
STOPPFall |
Older adults with high fall risk |
Comprehensive screening tool developed by Delphi consensus to identify fall risk increasing drugs and practical deprescribing guidance for STOPPFall medication classes. Consensus was achieved for 14 medication classes to create a comprehensive list of fall-risk inducing drugs. |
|
Lukozewski, 2012 |
Monitor-Rx |
Eligible patients were members of Supporting Active Independent Lives (SAIL), a community-based grass roots organization intended to help older adults age in place in their homes. |
Web-based program which associates medication effects with geriatric problems (including falls) to identify older adults at risk who would benefit from a comprehensive or targeted medication review. The program also identified medications with anticholinergic effects and medications inappropriate for use in older adults (≥ 65 years old). |
|
Nyborg, 2017 |
NORGEP-NH criteria |
The participants in the study constitute the part of the nursing home population in need of antibiotic or intravenous fluid therapy during the study period |
Screening tool to identify substances to avoid for regular use, drug combinations that should be avoided, and criteria for continuation. In section A and B, several fall-risk inducing drugs are listed. |
ABS: anticholinergic burden score, ACBS: anticholinergic cognitive burden scale, ARS: anticholinergic risk score, ADS: Anticholinergic Drug Scale, DBI: Drug Burden Index, IPET: Improving Prescribing in the Elderly Tool, STEADI: Stopping Elderly Accidents Deaths and Injuries, STEADI-Rx: Stopping Elderly Accidents, Deaths, and Injuries-Rx, STOPP: Screening Tool of Older Persons potentially inappropriate Prescriptions, STOPP-START: Screening Tool of Older Persons potentially inappropriate Prescriptions/Screening Tool to Alert doctors to Right Treatment, NORGEP-N: Norwegian General Practice Nursing Home, PIPs: potentially inappropriate prescribings, PPOs: potential prescription omissions.
Results
Predictive value of risk for falls based on a medication review tool
Nine studies reported the predictive value at risk for falls based on risk medication use according to the respectively assessed tools (Blalock, 2020a; Byrne, 2019; Cardwell, 2020; Damoiseau-Volman, 2022; Lavrador, 2021; Lukazewski, 2012; Nyborg, 2017; Prudent, 2008; Stewart, 2021). The included studies presented the predictive value as association (OR, RR or AUC) between scores on a medication review tool and outcomes (falls). Due to study heterogeneity, the results were not pooled.
Blalock (2020a) reported the predicting odds of screening positive for fall risk for a low (0 to < 0.20), moderate (0.20 to <0.50), high (0.50 to < 1.0) and very high DBI (≥1.0). The adjusted odds ratio (OR) for a low DBI was 1.55 (95%CI 1.00 to 2.39), for a moderate DBI 2.41 (95%CI 1.54 to 3.78), for a high DBI 3.08 (2.02 to 4.69), and for a very high DBI 3.27 (95%CI 2.07 to 5.16). The OR was adjusted for age, prescription fills and sex. This suggests that there was an association between the DBI and positive fall risk screening.
Byrne (2019) reported the association of the DBI score with the adverse health outcome falls (one or more self-reported falls in the previous 12 months). Patients with a low DBI score (>0 to <1) had an OR of 1.40 (95%CI 1.08 to 1.81), and patients with a high DBI score (≥1) had an OR of 1.50 (95%CI 1.03 to 2.18). This suggests that there was an association between the DBI and the adverse health outcome falls.
Cardwell (2020) reported the association between a higher DBI at baseline and an increased rate of falls at 12, 24 and 26 months of follow-up: RR 1.09 (95%CI 0.76 to 1.56), RR 1.06 (95%CI 0.75 to 1.51), and RR 1.13 (95%CI 0.80 to 1.62), respectively. This suggests that there was an association between the DBI and the adverse health outcome falls.
Damoiseaux-Volman (2022) reported the effect of exposure to potentially inappropriate medications (PIMs) on falls. The adjusted OR for STOPP was 2.24 (95%CI 2.01 to 2.49), the adjusted OR for STOPP Section K (= section Fall risk increasing drugs) was 7.89 (95%CI 6.06 to 10.26), and the adjusted OR for STOPPFall was 1.42 (95%CI 1.30 to 1.54). This suggests there was an association between the PIM exposure identified with different tools and falls.
Nyborg (2017) reported the association with potentially inappropriate medications according to the NORGEP-NH single substance and combined criteria and falls (adjusted OR 1.10 (95%CI 0.76 to 1.36). This suggests that there was an association between the NORGEP-NH criteria and falls.
Lukazewski (2012) reported the predictive value of the Monitor-Rx instrument and compared this with the judgement of a certified geriatric pharmacist (CGP). In total, all 29 patients included in the study were identified at risk for falls by both Monitor-Rx and the CGP. The predictive value for the outcome measure at risk for falls is 100%.
Lavrador (2021) reported the effect sizes (measured as areas under the curve) of the association between anticholinergic burden scales scores and the occurrence of the anticholinergic adverse outcome falls. The AUC of the ADS was 0.527 (95%CI 0.434 to 0.620), the AUC of the ARS was 0.591 (95%CI 0.496 to 0.687), the AUC of the ACB was 0.524 (95%CI 0.428 to 0.619), and the AUC of the DBI was 0.569 (0.477 to 0.662). This suggests that the anticholinergic burden scales alone are poor predictors for fall risk.
Prudent (2008) reported the association between patients taking psychotropics with at least one potentially inappropriate psychotropic (PIP) identified using the 2003 Beers list and fall risk (adjusted OR 1.1, 95%CI 0.8 to 1.6). This suggests that there was an association between the predictive value of the Beers list and falls.
The systematic review from Stewart (2021) reported the pooled analysis of adjusted HR, suggesting a modest increase in risk of falling attributed to the anticholinergic burden (HR 1.21, 95%CI 1.08 to 1.36). This suggests that there is an association between the anticholinergic burden scores and risk of falling.
Diagnostic accuracy
None of the included studies reported the outcome measure diagnostic accuracy.
Level of evidence of the literature
The level of evidence for the outcome measure predictive value comes from an RCT and observational studies and therefore starts low. The level of evidence was downgraded by 2 levels because of study limitations (risk of bias, -1) and because of a low number of included patients (imprecision, -1). The level of evidence is therefore very low.
The level of evidence for the outcome measure diagnostic accuracy could not be established as none of the included studies reported the outcome measure.
Zoeken en selecteren
A systematic review of the literature was performed to answer the following question:
What is the predictive/diagnostic value of using an instrument to identify and/or reduce fall-risk increasing drugs in older adults (≥ 65 years old)?
| P: patients | Adults (≥ 65 years old) |
| I: intervention | Instrument to identify fall-risk increasing drugs |
| C: control | No instrument or comparison between instruments |
| O: outcome measure | Predictive value of risk for falls based on a medication review tool, diagnostic value |
Relevant outcome measures
The guideline development group considered the predictive value as a critical outcome measure for decision making; and the diagnostic value as an important outcome measure for decision making.
A priori, the working group did not define the outcome measures listed above but used the definitions used in the studies.
The working group defined the following differences as a minimal clinically (patient) important differences:
- Predictive value: prevention of every single fall is clinically relevant for older adults.
- Diagnostic value: A difference of 10% in diagnostic value was considered clinically relevant.
Search and select (Methods)
The databases Medline (via OVID) and Embase (via Embase.com) were searched with relevant search terms until 07 June 2022. The detailed search strategy is depicted under the tab Methods. The systematic literature search resulted in 497 hits. Studies were selected based on the following criteria: systematic reviews or comparative studies on instruments to identify fall-risk inducing risks in adults ≥ 65 years old. In total, 59 studies were initially selected based on title and abstract screening. After reading the full text, 50 studies were excluded (see the table with reasons for exclusion under the tab Methods), and 9 studies were included.
Results
Nine studies were included in the analysis of the literature. Important study characteristics and results are summarized in the evidence tables. The assessment of the risk of bias is summarized in the risk of bias tables.
Referenties
- Ackroyd-Stolarz S, Mackinnon NJ, Sketris I, Sabo B. Potentially inappropriate prescribing of benzodiazepines for older adults and risk of falls during a hospital stay: a descriptive study. Can J Hosp Pharm. 2009 Jul;62(4):276-83. doi: 10.4212/cjhp.v62i4.808. PMID: 22478905; PMCID: PMC2826959.
- Ailabouni NJ, Reeve E, Helfrich CD, Hilmer SN, Wagenaar BH. Leveraging implementation science to increase the translation of deprescribing evidence into practice. Res Social Adm Pharm. 2022 Mar;18(3):2550-2555. doi: 10.1016/j.sapharm.2021.05.018. Epub 2021 Jun 6. PMID: 34147372.
- Aizenberg D, Sigler M, Weizman A, Barak Y. Anticholinergic burden and the risk of falls among elderly psychiatric inpatients: a 4-year case-control study. Int Psychogeriatr. 2002 Sep;14(3):307-10. doi: 10.1017/s1041610202008505. PMID: 12475091.
- Arnold I, Straube K, Himmel W, Heinemann S, Weiss V, Heyden L, Hummers-Pradier E, Nau R. High prevalence of prescription of psychotropic drugs for older patients in a general hospital. BMC Pharmacol Toxicol. 2017 Dec 4;18(1):76. doi: 10.1186/s40360-017-0183-0. PMID: 29202811; PMCID: PMC5715648.
- Bhasin S, Gill TM, Reuben DB, Latham NK, Ganz DA, Greene EJ, Dziura J, Basaria S, Gurwitz JH, Dykes PC, McMahon S, Storer TW, Gazarian P, Miller ME, Travison TG, Esserman D, Carnie MB, Goehring L, Fagan M, Greenspan SL, Alexander N, Wiggins J, Ko F, Siu AL, Volpi E, Wu AW, Rich J, Waring SC, Wallace RB, Casteel C, Resnick NM, Magaziner J, Charpentier P, Lu C, Araujo K, Rajeevan H, Meng C, Allore H, Brawley BF, Eder R, McGloin JM, Skokos EA, Duncan PW, Baker D, Boult C, Correa-de-Araujo R, Peduzzi P; STRIDE Trial Investigators. A Randomized Trial of a Multifactorial Strategy to Prevent Serious Fall Injuries. N Engl J Med. 2020 Jul 9;383(2):129-140. doi: 10.1056/NEJMoa2002183. PMID: 32640131; PMCID: PMC7421468.
- Blalock SJ, Renfro CP, Robinson JM, Farley JF, Busby-Whitehead J, Ferreri SP. Using the Drug Burden Index to identify older adults at highest risk for medication-related falls. BMC Geriatr. 2020a Jun 12;20(1):208. doi: 10.1186/s12877-020-01598-5. PMID: 32532276; PMCID: PMC7291506.
- Blalock SJ, Ferreri SP, Renfro CP, Robinson JM, Farley JF, Ray N, Busby-Whitehead J. Impact of STEADI-Rx: A Community Pharmacy-Based Fall Prevention Intervention. J Am Geriatr Soc. 2020b Aug;68(8):1778-1786. doi: 10.1111/jgs.16459. Epub 2020 Apr 21. PMID: 32315461.
- Blum MR, Sallevelt BTGM, Spinewine A, O'Mahony D, Moutzouri E, Feller M, Baumgartner C, Roumet M, Jungo KT, Schwab N, Bretagne L, Beglinger S, Aubert CE, Wilting I, Thevelin S, Murphy K, Huibers CJA, Drenth-van Maanen AC, Boland B, Crowley E, Eichenberger A, Meulendijk M, Jennings E, Adam L, Roos MJ, Gleeson L, Shen Z, Marien S, Meinders AJ, Baretella O, Netzer S, de Montmollin M, Fournier A, Mouzon A, O'Mahony C, Aujesky D, Mavridis D, Byrne S, Jansen PAF, Schwenkglenks M, Spruit M, Dalleur O, Knol W, Trelle S, Rodondi N. Optimizing Therapy to Prevent Avoidable Hospital Admissions in Multimorbid Older Adults (OPERAM): cluster randomised controlled trial. BMJ. 2021 Jul 13;374:n1585. doi: 10.1136/bmj.n1585. Erratum in: BMJ. 2022 Dec 1;379:o2859. PMID: 34257088; PMCID: PMC8276068.
- Bruce J, Hossain A, Lall R, Withers EJ, Finnegan S, Underwood M, Ji C, Bojke C, Longo R, Hulme C, Hennings S, Sheridan R, Westacott K, Ralhan S, Martin F, Davison J, Shaw F, Skelton DA, Treml J, Willett K, Lamb SE. Fall prevention interventions in primary care to reduce fractures and falls in people aged 70 years and over: the PreFIT three-arm cluster RCT. Health Technol Assess. 2021 May;25(34):1-114. doi: 10.3310/hta25340. PMID: 34075875; PMCID: PMC8200932.
- Byrne CJ, Walsh C, Cahir C, Bennett K. Impact of drug burden index on adverse health outcomes in Irish community-dwelling older people: a cohort study. BMC Geriatr. 2019 Apr 29;19(1):121. doi: 10.1186/s12877-019-1138-7. PMID: 31035946; PMCID: PMC6489229.
- Capiau A, Huys L, van Poelgeest E, van der Velde N, Petrovic M, Somers A; EuGMS Task, Finish Group on FRIDs. Therapeutic dilemmas with benzodiazepines and Z-drugs: insomnia and anxiety disorders versus increased fall risk: a clinical review. Eur Geriatr Med. 2022 Dec 28. doi: 10.1007/s41999-022-00731-4. Epub ahead of print. PMID: 36576689.
- Cardwell K, Hughes CM, Ryan C. The Association Between Anticholinergic Medication Burden and Health Related Outcomes in the 'Oldest Old': A Systematic Review of the Literature. Drugs Aging. 2015 Oct;32(10):835-48. doi: 10.1007/s40266-015-0310-9. PMID: 26442862.
- Cardwell K, Kerse N, Ryan C, Teh R, Moyes SA, Menzies O, Rolleston A, Broad J, Hughes CM. The Association Between Drug Burden Index (DBI) and Health-Related Outcomes: A Longitudinal Study of the 'Oldest Old' (LiLACS NZ). Drugs Aging. 2020 Mar;37(3):205-213. doi: 10.1007/s40266-019-00735-z. PMID: 31919805.
- Craftman ÅG, Johnell K, Fastbom J, Westerbotn M, von Strauss E. Time trends in 20 years of medication use in older adults: Findings from three elderly cohorts in Stockholm, Sweden. Arch Gerontol Geriatr. 2016 Mar-Apr;63:28-35. doi: 10.1016/j.archger.2015.11.010. Epub 2015 Nov 28. PMID: 26791168.
- Dalton K, Curtin D, O'Mahony D, Byrne S. Computer-generated STOPP/START recommendations for hospitalised older adults: evaluation of the relationship between clinical relevance and rate of implementation in the SENATOR trial. Age Ageing. 2020 Jul 1;49(4):615-621. doi: 10.1093/ageing/afaa062. PMID: 32484853.
- Damoiseaux-Volman BA, Raven K, Sent D, Medlock S, Romijn JA, Abu-Hanna A, van der Velde N. Potentially inappropriate medications and their effect on falls during hospital admission. Age Ageing. 2022 Jan 6;51(1):afab205. doi: 10.1093/ageing/afab205. PMID: 34673915; PMCID: PMC8753037.
- Deandrea S, Lucenteforte E, Bravi F, Foschi R, La Vecchia C, Negri E. Risk factors for falls in community-dwelling older people: a systematic review and meta-analysis. Epidemiology. 2010 Sep;21(5):658-68. doi: 10.1097/EDE.0b013e3181e89905. PMID: 20585256.
- de Vries M, Seppala LJ, Daams JG, van de Glind EMM, Masud T, van der Velde N; EUGMS Task and Finish Group on Fall-Risk-Increasing Drugs. Fall-Risk-Increasing Drugs: A Systematic Review and Meta-Analysis: I. Cardiovascular Drugs. J Am Med Dir Assoc. 2018 Apr;19(4):371.e1-371.e9. doi: 10.1016/j.jamda.2017.12.013. Epub 2018 Feb 12. PMID: 29396189.
- Di Martino E, Provenzani A, Polidori P. Evidence-based application of explicit criteria to assess the appropriateness of geriatric prescriptions at admission and hospital stay. PLoS One. 2020 Aug 25;15(8):e0238064. doi: 10.1371/journal.pone.0238064. PMID: 32841285; PMCID: PMC7446960.
- Eckstrom E, Parker EM, Lambert GH, Winkler G, Dowler D, Casey CM. Implementing STEADI in Academic Primary Care to Address Older Adult Fall Risk. Innov Aging. 2017 Sep;1(2):igx028. doi: 10.1093/geroni/igx028. PMID: 29955671; PMCID: PMC6016394.
- Frankenthal D, Israeli A, Caraco Y, Lerman Y, Kalendaryev E, Zandman-Goddard G, Lerman Y. Long-Term Outcomes of Medication Intervention Using the Screening Tool of Older Persons Potentially Inappropriate Prescriptions Screening Tool to Alert Doctors to Right Treatment Criteria. J Am Geriatr Soc. 2017 Feb;65(2):e33-e38. doi: 10.1111/jgs.14570. Epub 2016 Dec 9. PMID: 27943247.
- Fried TR, Tinetti ME, Iannone L, O'Leary JR, Towle V, Van Ness PH. Health outcome prioritization as a tool for decision making among older persons with multiple chronic conditions. Arch Intern Med. 2011 Nov 14;171(20):1854-6. doi: 10.1001/archinternmed.2011.424. Epub 2011 Sep 26. PMID: 21949032; PMCID: PMC4036681.
- Hartikainen S, Lönnroos E, Louhivuori K. Medication as a risk factor for falls: critical systematic review. J Gerontol A Biol Sci Med Sci. 2007 Oct;62(10):1172-81. doi: 10.1093/gerona/62.10.1172. PMID: 17921433.
- Hill-Taylor B, Walsh KA, Stewart S, Hayden J, Byrne S, Sketris IS. Effectiveness of the STOPP/START (Screening Tool of Older Persons' potentially inappropriate Prescriptions/Screening Tool to Alert doctors to the Right Treatment) criteria: systematic review and meta-analysis of randomized controlled studies. J Clin Pharm Ther. 2016 Apr;41(2):158-69. doi: 10.1111/jcpt.12372. Epub 2016 Mar 17. PMID: 26990017.
- Hopewell S, Adedire O, Copsey BJ, Boniface GJ, Sherrington C, Clemson L, Close JC, Lamb SE. Multifactorial and multiple component interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2018 Jul 23;7(7):CD012221. doi: 10.1002/14651858.CD012221.pub2. PMID: 30035305; PMCID: PMC6513234.
- Ilhan, B., Erdo?an, T., Topinková, E., Bahat, G., & EuGMS Task and Finish Group on FRIDs (2023). Management of use of urinary antimuscarinics and alpha blockers for benign prostatic hyperplasia in older adults at risk of falls: a clinical review. European geriatric medicine, 14(4), 733-746. https://doi.org/10.1007/s41999-023-00798-7
- Jamieson HA, Nishtala PS, Scrase R, Deely JM, Abey-Nesbit R, Hilmer SN, Abernethy DR, Berry SD, Mor V, Lacey CJ, Schluter PJ. Drug Burden Index and Its Association With Hip Fracture Among Older Adults: A National Population-Based Study. J Gerontol A Biol Sci Med Sci. 2019 Jun 18;74(7):1127-1133. doi: 10.1093/gerona/gly176. PMID: 30084928.
- Kimura T, Ogura F, Yamamoto K, Uda A, Nishioka T, Kume M, Makimoto H, Yano I, Hirai M. Potentially inappropriate medications in elderly Japanese patients: effects of pharmacists' assessment and intervention based on Screening Tool of Older Persons' Potentially Inappropriate Prescriptions criteria ver.2. J Clin Pharm Ther. 2017 Apr;42(2):209-214. doi: 10.1111/jcpt.12496. Epub 2016 Dec 31. PMID: 28039932.
- Korkatti-Puoskari, N., Tiihonen, M., Caballero-Mora, M. A., Topinkova, E., Szczerbińska, K., Hartikainen, S., & on the Behalf of the EuGMS Task & Finish group on FRIDs (2023). Therapeutic dilemma's: antipsychotics use for neuropsychiatric symptoms of dementia, delirium and insomnia and risk of falling in older adults, a clinical review. European geriatric medicine, 14(4), 709-720. https://doi.org/10.1007/s41999-023-00837-3
- Lavrador M, Cabral AC, Figueiredo IV, Veríssimo MT, Castel-Branco MM, Fernandez-Llimos F. Size of the associations between anticholinergic burden tool scores and adverse outcomes in older patients. Int J Clin Pharm. 2021 Feb;43(1):128-136. doi: 10.1007/s11096-020-01117-x. Epub 2020 Aug 29. PMID: 32860598.
- Leipzig RM, Cumming RG, Tinetti ME. Drugs and falls in older people: a systematic review and meta-analysis: I. Psychotropic drugs. J Am Geriatr Soc. 1999a Jan;47(1):30-9. doi: 10.1111/j.1532-5415.1999.tb01898.x. PMID: 9920227.
- Leipzig RM, Cumming RG, Tinetti ME. Drugs and falls in older people: a systematic review and meta-analysis: II. Cardiac and analgesic drugs. J Am Geriatr Soc. 1999b Jan;47(1):40-50. doi: 10.1111/j.1532-5415.1999.tb01899.x. PMID: 9920228.
- Lukazewski A, Mikula B, Servi A, Martin B. Evaluation of a web-based tool in screening for medication-related problems in community-dwelling older adults. Consult Pharm. 2012 Feb;27(2):106-13. doi: 10.4140/TCP.n.2012.106. PMID: 22330951.
- McMahon CG, Cahir CA, Kenny RA, Bennett K. Inappropriate prescribing in older fallers presenting to an Irish emergency department. Age Ageing. 2014 Jan;43(1):44-50. doi: 10.1093/ageing/aft114. Epub 2013 Aug 8. PMID: 23927888.
- Montero-Odasso MM, Kamkar N, Pieruccini-Faria F, Osman A, Sarquis-Adamson Y, Close J, Hogan DB, Hunter SW, Kenny RA, Lipsitz LA, Lord SR, Madden KM, Petrovic M, Ryg J, Speechley M, Sultana M, Tan MP, van der Velde N, Verghese J, Masud T; Task Force on Global Guidelines for Falls in Older Adults. Evaluation of Clinical Practice Guidelines on Fall Prevention and Management for Older Adults: A Systematic Review. JAMA Netw Open. 2021 Dec 1;4(12):e2138911. doi: 10.1001/jamanetworkopen.2021.38911. PMID: 34910151; PMCID: PMC8674747.
- Montero-Odasso M, van der Velde N, Martin FC, Petrovic M, Tan MP, Ryg J, Aguilar-Navarro S, Alexander NB, Becker C, Blain H, Bourke R, Cameron ID, Camicioli R, Clemson L, Close J, Delbaere K, Duan L, Duque G, Dyer SM, Freiberger E, Ganz DA, Gómez F, Hausdorff JM, Hogan DB, Hunter SMW, Jauregui JR, Kamkar N, Kenny RA, Lamb SE, Latham NK, Lipsitz LA, Liu-Ambrose T, Logan P, Lord SR, Mallet L, Marsh D, Milisen K, Moctezuma-Gallegos R, Morris ME, Nieuwboer A, Perracini MR, Pieruccini-Faria F, Pighills A, Said C, Sejdic E, Sherrington C, Skelton DA, Dsouza S, Speechley M, Stark S, Todd C, Troen BR, van der Cammen T, Verghese J, Vlaeyen E, Watt JA, Masud T; Task Force on Global Guidelines for Falls in Older Adults. World guidelines for falls prevention and management for older adults: a global initiative. Age Ageing. 2022 Sep 2;51(9):afac205. doi: 10.1093/ageing/afac205. PMID: 36178003; PMCID: PMC9523684.
- Nyborg G, Brekke M, Straand J, Gjelstad S, Romøren M. Potentially inappropriate medication use in nursing homes: an observational study using the NORGEP-NH criteria. BMC Geriatr. 2017 Sep 19;17(1):220. doi: 10.1186/s12877-017-0608-z. PMID: 28927372; PMCID: PMC5606129.
- Onatade R, Auyeung V, Scutt G, Fernando J. Potentially inappropriate prescribing in patients on admission and discharge from an older peoples' unit of an acute UK hospital. Drugs Aging. 2013 Sep;30(9):729-37. doi: 10.1007/s40266-013-0097-5. PMID: 23780641.
- Park H, Satoh H, Miki A, Urushihara H, Sawada Y. Medications associated with falls in older people: systematic review of publications from a recent 5-year period. Eur J Clin Pharmacol. 2015 Dec;71(12):1429-40. doi: 10.1007/s00228-015-1955-3. Epub 2015 Sep 26. PMID: 26407688.
- Portlock, G. E., Smith, M. D., van Poelgeest, E. P., Welsh, T. J., & EuGMS Task and Finish Group on FRIDs(Fall-Risk-Increasing Drugs) (2023). Therapeutic dilemmas: cognitive enhancers and risk of falling in older adults-a clinical review. European geriatric medicine, 14(4), 721-732. https://doi.org/10.1007/s41999-023-00821-x
- Prudent M, Dramé M, Jolly D, Trenque T, Parjoie R, Mahmoudi R, Lang PO, Somme D, Boyer F, Lanièce I, Gauvain JB, Blanchard F, Novella JL. Potentially inappropriate use of psychotropic medications in hospitalized elderly patients in France: cross-sectional analysis of the prospective, multicentre SAFEs cohort. Drugs Aging. 2008;25(11):933-46. doi: 10.2165/0002512-200825110-00004. PMID: 18947261.
- Romano S, Figueira D, Teixeira I, Perelman J. Deprescribing Interventions among Community-Dwelling Older Adults: A Systematic Review of Economic Evaluations. Pharmacoeconomics. 2022 Mar;40(3):269-295. doi: 10.1007/s40273-021-01120-8. Epub 2021 Dec 16. PMID: 34913143.
- Schuling J, Sytema R, Berendsen AJ. Aanpassen medicatie: voorkeur oudere patiënt telt mee [Adjusting medication: elderly patient's preference counts]. Ned Tijdschr Geneeskd. 2013;157(47):A6491. Dutch. PMID: 24252406.
- Seppala LJ, Wermelink AMAT, de Vries M, Ploegmakers KJ, van de Glind EMM, Daams JG, van der Velde N; EUGMS task and Finish group on fall-risk-increasing drugs. Fall-Risk-Increasing Drugs: A Systematic Review and Meta-Analysis: II. Psychotropics. J Am Med Dir Assoc. 2018a Apr;19(4):371.e11-371.e17. doi: 10.1016/j.jamda.2017.12.098. PMID: 29402652.
- Seppala LJ, van de Glind EMM, Daams JG, Ploegmakers KJ, de Vries M, Wermelink AMAT, van der Velde N; EUGMS Task and Finish Group on Fall-Risk-Increasing Drugs. Fall-Risk-Increasing Drugs: A Systematic Review and Meta-analysis: III. Others. J Am Med Dir Assoc. 2018b Apr;19(4):372.e1-372.e8. doi: 10.1016/j.jamda.2017.12.099. Epub 2018 Mar 2. PMID: 29402646.
- Seppala LJ, Petrovic M, Ryg J, Bahat G, Topinkova E, Szczerbi?ska K, van der Cammen TJM, Hartikainen S, Ilhan B, Landi F, Morrissey Y, Mair A, Gutiérrez-Valencia M, Emmelot-Vonk MH, Mora MÁC, Denkinger M, Crome P, Jackson SHD, Correa-Pérez A, Knol W, Soulis G, Gudmundsson A, Ziere G, Wehling M, O'Mahony D, Cherubini A, van der Velde N. STOPPFall (Screening Tool of Older Persons Prescriptions in older adults with high fall risk): a Delphi study by the EuGMS Task and Finish Group on Fall-Risk-Increasing Drugs. Age Ageing. 2021 Jun 28;50(4):1189-1199. doi: 10.1093/ageing/afaa249. PMID: 33349863; PMCID: PMC8244563.
- Seppala LJ, Kamkar N, van Poelgeest EP, Thomsen K, Daams JG, Ryg J, Masud T, Montero- Odasso M, Hartikainen S, Petrovic M, van der Velde N; Task Force on Global Guidelines for Falls in Older Adults. Medication reviews and deprescribing as a single intervention in falls prevention: a systematic review and meta-analysis. Age Ageing. 2022a Sep 2;51(9):afac191. doi: 10.1093/ageing/afac191. PMID: 36153749; PMCID: PMC9509688.
- Seppala LJ, van der Velde N. How to tackle the global challenge of falls? Age Ageing. 2022b Nov 2;51(11):afac264. doi: 10.1093/ageing/afac264. PMID: 36414218; PMCID: PMC9681598.
- Shaver AL, Clark CM, Hejna M, Feuerstein S, Wahler RG Jr, Jacobs DM. Trends in fall-related mortality and fall risk increasing drugs among older individuals in the United States,1999-2017. Pharmacoepidemiol Drug Saf. 2021 Aug;30(8):1049-1056. doi: 10.1002/pds.5201. Epub 2021 Feb 16. PMID: 33534172; PMCID: PMC8254780.
- Stewart C, Taylor-Rowan M, Soiza RL, Quinn TJ, Loke YK, Myint PK. Anticholinergic burden measures and older people's falls risk: a systematic prognostic review. Ther Adv Drug Saf. 2021 May 31;12:20420986211016645. doi: 10.1177/20420986211016645. PMID: 34104401; PMCID: PMC8170331.
- Tannenbaum C, Diaby V, Singh D, Perreault S, Luc M, Vasiliadis HM. Sedative-hypnotic medicines and falls in community-dwelling older adults: a cost-effectiveness (decision-tree) analysis from a US Medicare perspective. Drugs Aging. 2015 Apr;32(4):305-14. doi: 10.1007/s40266-015-0251-3. PMID: 25825121.
- Thevelin S, Mounaouar LE, Marien S, Boland B, Henrard S, Dalleur O. Potentially Inappropriate Prescribing and Related Hospital Admissions in Geriatric Patients: A Comparative Analysis between the STOPP and START Criteria Versions 1 and 2. Drugs Aging. 2019 May;36(5):453-459. doi: 10.1007/s40266-018-00635-8. PMID: 30694444.
- Tinetti ME, McAvay GJ, Fried TR, Allore HG, Salmon JC, Foody JM, Bianco L, Ginter S, Fraenkel L. Health outcome priorities among competing cardiovascular, fall injury, and medication-related symptom outcomes. J Am Geriatr Soc. 2008 Aug;56(8):1409-16. doi: 10.1111/j.1532-5415.2008.01815.x. Epub 2008 Jul 24. PMID: 18662210; PMCID: PMC3494099.
- van der Velde N, Meerding WJ, Looman CW, Pols HA, van der Cammen TJ. Cost effectiveness of withdrawal of fall-risk-increasing drugs in geriatric outpatients. Drugs Aging. 2008;25(6):521-9. doi: 10.2165/00002512-200825060-00005. PMID: 18540690.
- van der Velde N, Seppala L, Petrovic M, Ryg J, Tan MP, Montero-Odasso M, Martin FC, Masud T. Sustainable fall prevention across Europe: challenges and opportunities. Aging Clin Exp Res. 2022 Oct;34(10):2553-2556. doi: 10.1007/s40520-022-02178-w. Epub 2022 Jul 13. PMID: 35829992; PMCID: PMC9637581.
- van der Velde N, Emmelot-Vonk M, Daal J, van Deudekom F, Hegge HHM, Hoekstra W, Lugtenberg M, de Ruiter-Bout N. Handboek valkliniek. Een praktische handleiding voor de implementatie van een valkliniek en regionale transmurale samenwerking. 2020 (2e versie).van der Velde, N., Seppala, L. J., Hartikainen, S., Kamkar, N., Mallet, L., Masud, T., Montero-Odasso, M., van Poelgeest, E. P., Thomsen, K., Ryg, J., Petrovic, M., & EuGMS Task, Finish Group on Fall-risk-increasing drugs (2023). European position paper on polypharmacy and fall-risk-increasing drugs recommendations in the World Guidelines for Falls Prevention and Management: implications and implementation. European geriatric medicine, 14(4), 649-658.
- van Poelgeest EP, Pronk AC, Rhebergen D, van der Velde N. Depression, antidepressants and fall risk: therapeutic dilemmas-a clinical review. Eur Geriatr Med. 2021 Jun;12(3):585-596. doi: 10.1007/s41999-021-00475-7. Epub 2021 Mar 15. PMID: 33721264; PMCID: PMC8149338.
- van Poelgeest EP, Seppala LJ, Lee JM, Bahat G, Ilhan B, Lavan AH, Mair A, van Marum RJ, Onder G, Ryg J, Fernandes MA, Garfinkel D, Guðmundsson A, Hartikainen S, Kotsani M, Montero-Errasquín B, Neumann-Podczaska A, Pazan F, Petrovic M, Soulis G, Vankova H, Wehling M, Wieczorowska-Tobis K, van der Velde N; EuGMS SIG Pharmacology. Deprescribing practices, habits and attitudes of geriatricians and geriatricians-in-training across Europe: a large web-based survey. Eur Geriatr Med. 2022 Dec;13(6):1455-1466. doi: 10.1007/s41999-022-00702-9. Epub 2022 Nov 2. PMID: 36319837; PMCID: PMC9722796.
- van Poelgeest EP, Handoko ML, Muller M, van der Velde N; EUGMS Task & Finish group on Fall-risk-increasing drugs. Diuretics, SGLT2 inhibitors and falls in older heart failure patients: to prescribe or to deprescribe? A clinical review. Eur Geriatr Med. 2023 Feb 3. doi: 10.1007/s41999-023-00752-7. Epub ahead of print. PMID: 36732414.
- van Summeren JJ, Haaijer-Ruskamp FM, Schuling J. Eliciting Preferences of Multimorbid Elderly Adults in Family Practice Using an Outcome Prioritization Tool. J Am Geriatr Soc. 2016 Nov;64(11):e143-e148. doi: 10.1111/jgs.14415. Epub 2016 Sep 9. PMID: 27612181.
- van Summeren JJ, Schuling J, Haaijer-Ruskamp FM, Denig P. Outcome prioritisation tool for medication review in older patients with multimorbidity: a pilot study in general practice. Br J Gen Pract. 2017 Jul;67(660):e501-e506. doi: 10.3399/bjgp17X690485. Epub 2017 Mar 27. PMID: 28347987; PMCID: PMC5565860.
- Virnes RE, Tiihonen M, Karttunen N, van Poelgeest EP, van der Velde N, Hartikainen S. Opioids and Falls Risk in Older Adults: A Narrative Review. Drugs Aging. 2022 Mar;39(3):199-207. doi: 10.1007/s40266-022-00929-y. Epub 2022 Mar 15. PMID: 35288864; PMCID: PMC8934763.
- Walsh ME, Boland F, Moriarty F, Fahey T. Modification of Potentially Inappropriate Prescribing Following Fall-Related Hospitalizations in Older Adults. Drugs Aging. 2019 May;36(5):461-470. doi: 10.1007/s40266-019-00646-z. PMID: 30834489.
- Welsh, T. J., & Mitchell, A. (2023). Centrally acting antihypertensives and alpha-blockers in people at risk of falls: therapeutic dilemmas-a clinical review. European geriatric medicine, 14(4), 675-682. https://doi.org/10.1007/s41999-023-00813-x
- Woolcott JC, Richardson KJ, Wiens MO, Patel B, Marin J, Khan KM, Marra CA. Meta-analysis of the impact of 9 medication classes on falls in elderly persons. Arch Intern Med. 2009 Nov 23;169(21):1952-60. doi: 10.1001/archinternmed.2009.357. Erratum in: Arch Intern Med. 2010 Mar 8;170(5):477. PMID: 19933955.
Evidence tabellen
Evidence table for intervention studies (randomized controlled trials and non-randomized observational studies [cohort studies, case-control studies, case series])
Research question: What is the predictive/diagnostic value of using an instrument to identify and/or reduce fall-risk increasing drugs in older adults (≥ 65 years old)?
|
Study reference |
Study characteristics |
Patient characteristics 2 |
Intervention (I) |
Comparison / control (C) 3
|
Follow-up |
Outcome measures and effect size 4 |
Comments |
|
Blalock, 2020a |
Type of study: Retrospective cohort study
Setting and country: data were derived from a RCT, USA
Funding and conflicts of interest: This work was supported by Cooperative Agreement Number 1 U01 CE002769–01 from the Centers for Disease Control and Prevention and Grant Number 1C1CMS331338 from the Department of Health and Human Services, Centers for Medicare & Medicaid Services. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the U.S. Department of Health and Human Services or any of its agencies. The research presented here was conducted by the awardee. Findings might or might not be consistent with or confirmed by the findings of the independent evaluation contractor.
The authors declared no conflicts of interest with respect to the authorship or publication of this article. |
Inclusion criteria: For this paper, we limited the sample to older adults who received their medications from one of the pharmacies assigned to the intervention group (n = 4719) and used data only from the 1-year period prior to intervention implementation. In addition, because medication use was assessed using Medicare Part D and North Carolina (NC) Medicaid claim records, we limited the sample to intervention group participants who had continuous coverage for prescription medications through either Medicare Part D or NC Medicaid for the entire 1-year period of observation (n = 1562).
Exclusion criteria: Not reported.
N total at baseline: 1562 patients.
Important prognostic factors2: Sex and age is not reported.
Groups comparable at baseline? NA
|
Describe intervention (treatment/procedure/test):
Pharmacy staff screened patients by asking the following key questions derived from the STEADI Initiative: (1) Have you fallen in the past year, (2) Do you feel unsteady when standing or walking, and (3) Do you worry about falling. In addition, patients who reported one or more falls within the past year were asked if any of the falls had resulted in injury. Patients who answered “Yes” to any of the key STEADI questions were classified as having screened positive for increased fall risk. These patients were eligible to receive a medication review provided by a pharmacist associated with the pharmacy where they obtained their medications. As part of the medication review, the pharmacist evaluated the patient’s medication regimen using evidence-based algorithms developed by the study team to: (1) identify medications associated with an increased risk of falling and (2) provide therapeutic recommendations to reduce risk. Pharmacists conducting the medication reviews were aware of participants’ responses to the key STEADI questions. After conducting a medication review, the pharmacist communicated recommendations to the patient’s prescriber using forms developed for this purpose. The study was approved by the Institutional Review Board at the University of North Carolina at Chapel Hill.
|
Describe control (treatment/procedure/test):
NA. |
Length of follow-up: 12 months
Loss-to-follow-up: Not reported.
Incomplete outcome data: N=1, reason is not reported.
|
Outcome measures and effect size (include 95%CI and p-value if available):
Predictive value The adjusted odds ratio for a low DBI is 1.55 (95%CI 1.00 to 2.39), for a moderate DBI 2.41 (95%CI 1.54 to 3.78), for a high DBI 3.08 (2.02 to 4.69), and for a very high DBI 3.27 (95%CI 2.07 to 5.16).
Diagnostic value Not reported. |
Author’s conclusion Our findings suggest that the DBI is a useful tool that could be used to improve future research and practice by focusing limited resources on those individuals at greatest risk for medication-related falls. |
|
Byrne, 2019 |
Type of study: cohort
Setting and country: the study used data from the TILDA study, Ireland.
Funding and conflicts of interest: This work was supported by the Health Research Board of Ireland (grant code RL-2015-1579). TILDA is supported by the Irish Government Department of Health and Children, The Atlantic Philanthropies and Irish Life. The funding bodies had no role in the design of the study, in the collection, analysis, and interpretation of data, and in the writing of the manuscript.
The authors declare that they have no competing interests. |
Inclusion criteria: In the present study, participants were included if they were aged ≥65 years at their TILDA Wave 1 interview, were enrolled in the General Medical Services (GMS) scheme, and presented a GMS identifier which could be linked to their pharmacy claims data.
Exclusion criteria: Not reported.
N total at baseline: 1924
Important prognostic factors2: Age (mean , SD) 75.0 (6.1)
Sex: 54.7% F.
Groups comparable at baseline? NA
|
Describe intervention (treatment/procedure/test):
The DBI tool was applied to participants’ medication dispensing data to determine DBI exposure [4]. DBI medications (with dose information) were identified using relevant ATC codes.
|
Describe control (treatment/procedure/test):
NA |
Length of follow-up: 12 months.
Loss-to-follow-up: Not reported.
Incomplete outcome data: N=198, reason is not reported.
|
Outcome measures and effect size (include 95%CI and p-value if available):
Predictive value (OR, 95% CI) DBI none: 1.00 (ref) DBI low: 1.40 (1.08 to 1.81) DBI high: 1.50 (1.03 to 2.18)
Diagnostic value Not reported. |
Author’s conclusion: This study validates the DBI tool against a spectrum of important adverse health outcomes in Irish community-dwelling older people. Using the DBI tool, increasing DBI score was independently associated with a greater risk of functional impairment, self-reported falls, frailty, and reduced QoL |
|
Cardwell, 2020 |
Type of study: cohort study
Setting and country: data from the LiLACS NZ were used, New Zealand
Funding and conflicts of interest: Funding was provided by Department for Employment and Learning, Northern Ireland.
KC, NK, CR, RT, SM, OM, AR, JB and CH declare that they have no conflict of interest. This current analysis was supported by the Department for Employment and Learning (DEL), Northern Ireland, through a postgraduate studentship to KC. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study. |
Inclusion criteria: non-Māori if aged 85 years in 2010.
Exclusion criteria: Not reported.
N total at baseline: N=404
Important prognostic factors2: age ± SD: 84.56 (0.53)
Sex: 53% F
Groups comparable at baseline? NA
|
Describe intervention (treatment/procedure/test):
Medications with anticholinergic and/or sedative properties (i.e. medications with a DBI>0) were identifed using the Monthly Index of Medical Specialities (MIMS) medication formulary, New Zealand. DBI was calculated for everyone enrolled at each time point.
|
Describe control (treatment/procedure/test):
NA |
Length of follow-up: 36 months.
Loss-to-follow-up: Not reported.
Incomplete outcome data: N=123, reasons not specified.
|
Outcome measures and effect size (include 95%CI and p-value if available):
Predictive value 12 months OR 1.09 (0.76 to 1.56) 24 months OR 1.06 (0.75 to 1.51) 36 months 1.13 (0.80 to 1.62)
Diagnostic value Not reported. |
Author’s conclusion: Using data from LiLACS NZ, a higher DBI was signifcantly associated with a greater risk of mortality (in Māori and non-Māori) and impaired cognitive function (in non-Māori). This highlights the importance of employing strate_gies to manage the prescribing of medications with a DBI>0 in older adults. |
|
Damoiseaux-Volman, 2022 |
Type of study: retrospective observational matching study.
Setting and country: Academic hospital, The Netherlands
Funding and conflicts of interest:
The innovation funds of Amsterdam UMC, location AMC, supported this work. The sponsor had no role in the design, methods, data collec_tions and analysis and preparation of this article.
The authors do not declare any conflicts of interest. |
Inclusion criteria: admissions of patients aged ≥70 years with a minimum length of stay of 24 h in a time period of 4 years (November 2015–November 2019).
Exclusion criteria: patients admitted to non-clinical departments.
N total at baseline: 16,687
Important prognostic factors2: Age: 77.2 years (5.8)
Sex: 52.4% M
Groups comparable at baseline? NA, one cohort.
|
Describe intervention (treatment/procedure/test):
STOPP criteria version 2, included 68 STOPP criteria.
4 belonged to the designated section K for falls of Stopp v2.
STOPPFALL contains 14 medication groups.
PIM exposure was calculated as the number of PIMs (sum of the unique PIMs each day) administered during hospital stay, divided by hospital length of stay.
|
Describe control (treatment/procedure/test):
Control group was not available. |
Length of follow-up: Minimum length of stay of 24 hours.
Loss-to-follow-up: NA (retrospective study)
Incomplete outcome data: NA (incomplete data was not included in the analyses).
|
Outcome measures and effect size (include 95%CI and p-value if available):
Predictive value Effect of exposure to PIM on inpatient falls showing the results of outcome ‘fall <24h of PIM’ (multinominal logistic regression).
aOR (95%CI): STOPP: 2.24 (2.01 to 2.49) STOPP section K: 7.89 (6.06 to 10.26) STOPPFall: 1.42 (1.30 to 1.54). |
Author’s conclusion We identified an independent association of PIMs on inpatient falls for all applied (de)prescribing tools. The strongest effect was identified for STOPP section K, which is restricted to high-risk medication for falls. Our results suggest that decreasing PIM exposure during hospital stay might benefit fall prevention, but intervention studies are warranted. |
|
Lavrador, 2020 |
Type of study: Case-control study
Setting and country: internal medicine ward of a Tertiary University Hospital.
Funding and conflicts of interest: Marta Lavrador acknowledges the FCT— Fundação para a Ciência e a Tecnologia for funding her with a Doctoral Grant.
Marta Lavrador obtained a complete Doctoral Grant from the FCT—Fundação para a Ciência e a Tecno_logia (SFRH/BD/123678/2016). No other external funding was received for this study.
|
Inclusion criteria: Patients over 65 years admitted to two internal medicine wards of a Portuguese university hospital. Patients taking at least one medicine at the time of admission were included.
Exclusion criteria: Patients who were unable to adequately answer to the questionnaires due to physical or mental disability, patients with diagnosed dementia, and patients who were taking acetylcholinesterase inhibitors (e.g., donepezil, galantamine, rivastigmine)
N total at baseline: 250
Important prognostic factors2: Age: 81.67 (SD = 7.8)
Sex: 50% M
Groups comparable at baseline? NA the groups are not compared within the study.
|
Describe intervention (treatment/procedure/test):
Exposure to anticholinergic drugs was calculated by the summation of the drug scores for each of the three anticholinergic scales:
The Anticholinergic Risk Scale (ARS), the Anticholinergic Drug Scale (ADS), and the Anticholinergic Cognitive Burden Scale (ACB).
Also, one equation (the anticholinergic component of the DBI was used.
|
Describe control (treatment/procedure/test):
Control group was not included. |
Length of follow-up: NA (single time point).
Loss-to-follow-up: NA
Incomplete outcome data: NA
|
Outcome measures and effect size (include 95%CI and p-value if available): Predictive value Effect sizes (measured as area under the curve) of the association between anticholinergic burden scales scores and the occurrence of anticholinergic adverse outcomes
AUC for falls ADS: 0.527 (0.434 to 0.620) ARS: 0.591 (0.496 to 0.687) ACB: 0.524 (0.428 to 0.619) DBI: 0.569 (0.477 to 0.662)
Diagnostic value Not reported.
|
Author’s conclusion Although significant differences in the scores of anticholinergic burden instruments and adverse outcomes may exist, the effect sizes of these associations ranged from ‘fail’ to ‘fair’, which limits their utility in preventing anticholinergic adverse outcomes with medication review interventions. |
|
Lukazewski, 2012 |
Type of study: prospective pilot study.
Setting and country: Wisconsin-based community pharmacy serving primarily older adults.
Funding and conflicts of interest: No funding was received for the development of this manuscript. The authors disclosed no potential conflicts of interest. |
Inclusion criteria: Eligible participants were members of Supporting Active Independent Lives (SAIL), a community-based grass roots organization intended to help older adults age in place in their homes.
Exclusion criteria: Not reported.
N total at baseline: Intervention: 29 Control: 29
Important prognostic factors2: Mean age: 81.6 (range 71-93).
Sex: 27.6% M
Groups comparable at baseline? NA as only one group is studied.
|
Describe intervention (treatment/procedure/test):
Monitor-Rx is a Web-based clinical tool, affiliated with the American Society of Consultant Pharmacists Foundation, which associates medication effects with geriatric problems to identify older adults at risk who would benefit from a comprehensive or targeted medication review.
Specifically, Monitor-Rx correlates medication effects with physical, functional, and cognitive decline in older adults by identifying medications as a cause or aggravating factor contributing to common geriatric conditions. The program also identifies medications with anticholinergic effects and medications inappropriate for use in older adults (≥ 65 years old). Anticholinergic burden is a strong predictor of cognitive impairment and reduces physical function and disability in older adults
|
Describe control (treatment/procedure/test):
Evaluation by a certified geriatric pharmacist. |
Length of follow-up: No follow-up in time.
Loss-to-follow-up: NA.
Incomplete outcome data: NA.
|
Outcome measures and effect size (include 95%CI and p-value if available):
Predictive value I: 29 (100%) C: 29 (100%)
Diagnostic value Not reported.
|
Author’s conclusion: In the older adults who were screened, Monitor-Rx was successful at identifying those with a high risk of drugrelated geriatric syndromes. |
|
Nyborg (2017) |
Type of study: Cross-sectional observational pharmaco- epidemiological study
Setting and country: nursing homes, Norway.
Funding and conflicts of interest: The project was funded by General Practice Research Fund (AMFF) hosted by the Norwegian medical Association. The AMFF had no role in the design, conduct, analyses or reporting of this study.
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper |
Inclusion criteria: The participants in our study constitute the part of the nursing home population in need of antibiotic or intravenous fluid therapy during the study period. For the purpose of this study, we included data from only the first treatment episode (990 individual residents).
Exclusion criteria: Patients hospitalized with septicemia or hospitalized for additional diagnostics or treatment were excluded in the intervention trial. Further, we excluded patients <70 years, as the NORGEP-NH criteria were developed for nursing home residents ≥70 years. For 33 subjects (3.6% of those eligible) medication lists were not available. These were also excluded from our study.
N total at baseline: Intervention: 881
Important prognostic factors2: Mean age: 85.9 (range 70–102).
Sex: I: 68.6% F
Groups comparable at baseline? Not applicable. |
Describe intervention (treatment/procedure/test):
Employment of the NORGEP-NH criteria to study the extent of potentially inappropriate medication use among nursing home residents.
|
Describe control (treatment/procedure/test):
Control group was not available. |
Length of follow-up: Not described.
Loss-to-follow-up: NA.
Incomplete outcome data: NA.
|
Outcome measures and effect size (include 95%CI and p-value if available):
Predictive value OR: 1.09 (0.77 to 1.54) aOR: 1.10 (0.76 to 1.36)
Not reported.
|
Author’s conclusion: This study analysed potentially inappropriate medication use in nursing homes according to the NORGEP-NH criteria. We found a high prevalence of PIMs, and among these, the use of psychotropic drugs was especially prevalent. Females were at higher risk of receiving both PIMs and multiple psychotropic drugs concurrently. Residents in long_term wards, and residents with a better-preserved ADL, had a higher risk of receiving multiple psychotropic drugs. The use of multiple psychotropic drugs increased the risk of falls in the course of an infection or dehydration episode. A prevalence of PIMs of this magnitude reveals a need for targeted measures. |
|
Prudent, 2008 |
Type of study: cross-sectional analysis of a prospective multicentre cohort study
Setting and country: France
Funding and conflicts of interest: The study was funded by the French Ministry of Health through the clinical Research Hospital Projects, the National Health Insurance Agency for Wage Earners, France and the Institute for Longevity and ageing. The financial sponsors played no role in the design, execution, analysis and interpretation of the data or writing of the study.
The authors have no conflicts of interest that are directly relevant to the content of this study. |
Inclusion criteria: Patients had to be aged ≥ 75 years and hospitalized in any medical ward of the same hospital.
Exclusion criteria: Patients hospitalized in the ACE unit, intensive care or surgery unit, or who were not hospitalized after emergency department admission.
N total at baseline: 1176
Important prognostic factors2: Age: 48.6% is aged ≥ 85 years.
Sex: 64.9% were female.
83.3% was living at their homes before hospitalization.
Groups comparable at baseline? NA
|
Describe intervention (treatment/procedure/test):
The Beers criteria in the 2003 update were used to identify prescription of PIMs and PIPs.
|
Describe control (treatment/procedure/test):
Not applicable. |
Length of follow-up: NA, cross-sectional analysis
Loss-to-follow-up: NA
Incomplete outcome data: Na
|
Outcome measures and effect size (include 95%CI and p-value if available):
Predictive value OR: 1.2 (0.9 to 1.5)
Diagnostic value Not reported.
|
Author’s conclusion PIM use is common among hospitalized older adults in France. The most important determinant of risk of receiving a psychotropic medication or a PIP was the number of drugs being taken. The older adults (≥ 65 years old) who have multiple comorbidities, complex chronic conditions and are usually receiving polypharmacy, are at increased risk for adverse drug events. These adverse events are often linked to problems that could be preventable such as delirium, depression and falls. Regular review of prescriptions would help optimize prescription of psycho_tropics in older adults (≥ 65 years old). The Beers list is a good tool for evaluating PIMs but is too restrictive with respect to psychotropics; in the latter respect, the list could usefully be widened |
Notes:
- Prognostic balance between treatment groups is usually guaranteed in randomized studies, but non-randomized (observational) studies require matching of patients between treatment groups (case-control studies) or multivariate adjustment for prognostic factors (confounders) (cohort studies); the evidence table should contain sufficient details on these procedures
- Provide data per treatment group on the most important prognostic factors [(potential) confounders]
- For case-control studies, provide sufficient detail on the procedure used to match cases and controls
- For cohort studies, provide sufficient detail on the (multivariate) analyses used to adjust for (potential) confounders
Risk of bias table for interventions studies
|
Author, year |
Selection of participants
Was selection of exposed and non-exposed cohorts drawn from the same population?
|
Exposure
Can we be confident in the assessment of exposure?
|
Outcome of interest
Can we be confident that the outcome of interest was not present at start of study?
|
Confounding-assessment
Can we be confident in the assessment of confounding factors?
|
Confounding-analysis
Did the study match exposed and unexposed for all variables that are associated with the outcome of interest or did the statistical analysis adjust for these confounding variables? |
Assessment of outcome
Can we be confident in the assessment of outcome?
|
Follow up
Was the follow up of cohorts adequate? In particular, was outcome data complete or imputed?
|
Co-interventions
Were co-interventions similar between groups?
|
Overall Risk of bias
|
|
Definitely yes, probably yes, probably no, definitely no |
Definitely yes, probably yes, probably no, definitely no |
Definitely yes, probably yes, probably no, definitely no |
Definitely yes, probably yes, probably no, definitely no |
Definitely yes, probably yes, probably no, definitely no |
Definitely yes, probably yes, probably no, definitely no |
Definitely yes, probably yes, probably no, definitely no |
Definitely yes, probably yes, probably no, definitely no |
Low, Some concerns, High |
|
|
Blalock, 2020a |
Definitely yes
Reason: The study used a retrospective cohort design. Participants were identified from 31 community pharmacies. |
Definitely yes
Reason: Because medication use was assessed using Medicare Part D and North Carolina (NC) Medicaid claim records, we limited the sample to intervention group participants who had continuous coverage for prescription medications through either Medicare Part D or NC Medicaid for the entire 1-year period of observation (n = 1562). |
Probably yes
Reason: The individuals were already at risk for falls, but are now identified using the tool. They were not identified before. |
Probably no
Reason: Prescription data from community pharmacies are used.
However the presence of other comorbidities are not included in the analyses. |
Probably no
Reason: The analyses are adjusted for confounders. However, not all relevant confounders are taken into account. |
Probably no
Reason: Patients are screened at risk for fall, however it is unknown whether they actually fall. |
Probably yes
Reason: Follow-up was 12 months. |
Unknown
Reason: unknown
|
Some concernes (all outcomes) |
|
Byrne, 2019 |
Definitely yes
Reason: This was a cohort study using data from The Irish Longitudinal Study on Ageing (TILDA) with linked pharmacy claims data. |
Definitely yes
Reason: pharmacy claims data. |
Probably no
Reason: They were already at risk for falls. |
Probably yes
Several confounding factors are listed, based on pharmacy claims data or the registered in the cohort study. |
Definitely yes
Reason: The analyses are adjusted for confounders. |
Probably yes
Reason: self-reported falls in the last 12 months. May be hard to remember for older adults (≥ 65 years old). (recall bias). |
Probably yes
Reason: Follow-up was 12 months. |
Unknown
Reason: unknown
|
Some concernes (all outcomes) |
|
Cardwell, 2020 |
Definitely yes
Reason: the study used data from the LiLACS NZ non-Maori cohort aged 85 years at the time of enrolment. |
Definitely yes
Reason: Review of GP medical records. Medication data were collected during the interview by review of medicine containers, including drug name, daily dose prescribed and frequency. Medications with anticholinergic and/or sedative properties (i.e. medications with a DBI>0) were identifed using the Monthly Index of Medical Specialities (MIMS) medication formulary, New Zealand. |
Probably no
Reason: They were already at risk for falls. |
Probably yes
Several confounding factors are listed, based on a standardized questionnaire (interview), a health assessment and a review of GP medical records. |
Definitely yes
Reason: Regression models were adjusted for age, gender, general practitioner (GP) visits, socioeconomic deprivation, number of medicines prescribed and one of the following: prior hospitalisation, history of falls, baseline cognitive function [Modifed Mini-Mental State Examination (3MS)] or baseline functional status [Nottingham Extended Activities of Daily Living (NEADL)].
|
Probably yes
Reason: outcomes (all-cause hospitalisations, falls, mortality, cognitive function and functional status) were evaluated at 12 months, 24 months and 36 months follow-up during the face-to-face interview and health assessment. Falls data were recorded at each interview by self-report (yes/no response). May be hard to remember for older adults (≥ 65 years old). (recall bias). |
Probably yes
Reason: Follow-up was 36 months. |
Unknown
Reason: unknown
|
Some concernes (all outcomes) |
|
Damoiseaux-Volman (2022) |
Definitely yes
Reason: all patients are included from an academic hospital. |
Definitely yes
Reason: medication administations during hospital stay. |
Probably yes
Reason: they are included when admitted to the hospital. |
Probabl yes
Reason: several confounding factors are taken into account, all based on admission data in the hospital. |
Definitely yes
Reason: matching was performed. |
Definitely yes
Reason: In-house falls are identified using the medical data. |
Probably no
Reason: admission for at least 24 hours, falls could possibly also happen later or when at home. |
Unknown
Reason: unknown
|
Some concernes (all outcomes) |
|
Nyborg, 2017 |
Definitely yes
Reason: cross-sectional observational study from residents in nursing homes in the county of Vestfold, Norway. |
Definitely yes
Reason: data obtained from medication lists. Patients with no medication list available were excluded. |
Probably no
Reason: They were already at risk for falls. |
Unclear
Reason: unknown which confounders were taken into account. |
Probably yes
Reason: multivariable models are presented. However, unknown for which confounders they adjusted. |
Probably yes
Reason: falls with fracture or injury of fall/tendency of falls. Tendency of falls may be hard to establish. |
Unclear
Reason: the length of follow-up is not specified. |
Unknown
Reason: unknown
|
Some concernes (all outcomes) |
|
Lavrador, 2020 |
Definitely yes
Reason: in patients over 65 years admitted to two internal medicine wards of a Portuguese university hospital. |
Definitely yes
Reason: medication data is obtained from electronic health records from the preceding 6 months. |
Probably yes
Reason: they are included when admitted to the hospital. |
Definitely no
Reason: confounders are not taken into account. |
Definitely no
Reason: multivariable analyses were not performed. |
Probably yes
Reason: self-reported falls in the last 12 months. May be hard to remember for older adults (≥ 65 years old). (recall bias). |
Unclear
Reason: the length of follow-up is not specified. |
Unknown
Reason: unknown
|
Some concernes (all outcomes) |
|
Lukazewski, 2012 |
Definitely yes
Reason: The participants are their own controls.
|
Probably yes
Reason: comprehensive medication phone interview using the interview protocol.
|
Probably yes
The individuals were already at risk for falls, but are now identified using the tool. They were not identified before. |
Definitely no
Reason: confounding factors are not taken into account.
|
Definitely no
Reason: multivariable analyses were not performed.
|
Definitely yes
Reason: certified geriatric pharmacist assessed the outcome as well.
|
NA
Reason: NA, cross-sectional analysis.
|
Probably no
Reason: unknown
|
Some concerns (all outcomes) |
|
Prudent, 2008 |
Definitely yes
Reason: All patients are included from the same cohort study (SAFE study) |
Definitely yes
Reason: the names and number of drugs used were collected by investigators in the same area of the patient’s general practitioners at the time of inclusion in the study. Patietns for whom the data was not available were excluded. |
Probably yes
Reason: they are included when admitted to the hospital. |
Definitely no
Reason: confounding factors are not taken into account.
|
Definitely no
Reason: multivariable analyses were not performed specific for falls in patients taking at least one psychotropic.
|
Definitely yes
Reason: fall risk were assessed by the one-leg standing test as part of a standardized geriatric evaluation. |
NA
Reason: NA, cross-sectional analysis.
|
Probably no
Reason: unknown
|
Some concerns (all outcomes) |
Footnotes
Selection of participants Example of low risk of bias: Exposed and unexposed drawn for same administrative database of patients presenting at same points of care over the same time frame
Exposure Examples of low risk of bias: Secure record (e.g. surgical records, pharmacy records); Repeated interview or other ascertainment asking about current use/exposure
Confounding Examples of low risk of bias regarding assessment: Interview of all participants; Self-completed survey from all participants; Review of charts with reproducibility demonstrated; From database with documentation of accuracy of abstraction of prognostic data.
Example of low risk of bias regarding analysis: Comprehensive matching (e.g. with propensity score) or adjustment for all plausible confounding variables
Assessment of outcome Examples of low risk of bias: Independent blind assessment; Record linkage; For some outcomes (e.g. fractured hip), reference to the medical record is sufficient to satisfy the requirement for confirmation of the fracture
Follow up Examples of low risk of bias: No missing outcome data; Reasons for missing outcome data unlikely to be related to true outcome (for
survival data, censoring is unlikely to introduce bias); Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; Missing data have been imputed using appropriated methods
Co-interventions Example of low risk of bias: Most or all relevant co-interventions that might influence the outcome of interest are documented to be similar in the exposed and unexposed
Evidence table for systematic review of RCTs and observational studies
|
Study reference |
Study characteristics |
Patient characteristics |
Intervention (I) |
Comparison / control (C)
|
Follow-up |
Outcome measures and effect size |
Comments |
|
Stewart, 2021
PS., study characteristics and results are extracted from the SR (unless stated otherwise) |
SR and meta-analysis of cohort and case-control studies.
Literature search up to September 2020
A: Green, 2019 B: Hwan, 2019 C: Landi, 2014 D: Richardson, 2015 E: Squires, 2020 F: Suehs, 2019 G: Tan, 2020 H: Zia, 2016
Study design: A: retrospective cohort B: retrospective cohort C: prospective cohort D: prospective cohort E: retrospective cohort F: retrospective cohort G: prospective cohort H: case-control
Setting and Country: A: USA B: Korea C: Nursing home, Italy D: Community, Italy E: RCT participants, USA F: USA G: primary care, UK H: community, Malysia
Source of funding and conflicts of interest: Not reported. |
Inclusion criteria SR: report was an observational study (longitudinal cohort or case-control); involved exclusively adults aged ⩾65years (or have a mean age ⩾65years or present data for a subset of cohort aged ⩾65years); assessed ACB exposure using any ACB measure (to include anticholinergic domain of the Drug Burden Index); any length of follow-up period; report any measure for falls as an outcome.
Exclusion criteria SR: systematic review, randomised control trial, cross-sectional study, qualitative study, editorial or opinion article; studies restricted to classes of anticholinergic medications or specific anticholinergic medications (eg psychotropics); measure of medication use not specifically directed at anticholinergic drugs (e.g. Beers Criteria, Drug Burden Index).
8 studies included
Important patient characteristics at baseline:
N A: 10698 B: 118750 C: 1490 D: 2696 E: 1635 F: 113311 G: 25639 H: 428
mean age A: 79.1 (7.99) B: 75.4 (6.6) C: 83.6 D: 72.2 E: 78.7 F: 74.8 (6.2) G: 58.0 (9.0) H: 75.3 /72.13
Sex (female): A: 58% B: 56.4% C: 71.5% D: 52.3% E: 66.9% F: 49.0% G: 55.0% H: 68.2% / 66.7%
Groups comparable at baseline? Yes |
Describe intervention:
A: ACBS B: ARS C: ARS D: ACBS E: ACBS F: ACBS G: ACBS H: ACBS
|
Describe control:
NA
|
End-point of follow-up: A: 12 months B: 3 months C: 12 months D: 24 months E: 30 months F: 38.5 months G: 24 months H: 12 months
For how many participants were no complete outcome data available? Not reported.
|
Outcome measure-1 Defined as HR of falls and ACB
Effect measure: RR, RD, mean difference [95% CI]: A: 1.080 (0.971 to 1.201) B: 1.310 (1.071 to 1.602) C: - D: - E: 1.230 (0.708 to 2.135) F: 1.280 (1.236 to 1.326) G: - H: -
Pooled effect (random effects model / fixed effects model): 1.214 [95% CI 1.062 to 1.361]
|
Facultative:
Brief description of author’s conclusion
The evidence supports an association between moderate to high ACB and risk of falling in older people, but no conclusion can be made regarding which ACB scale offers best prognostic value in older people.
|
Table of quality assessment for systematic reviews of RCTs and observational studies
Based on AMSTAR checklist (Shea et al.; 2007, BMC Methodol 7: 10; doi:10.1186/1471-2288-7-10) and PRISMA checklist (Moher et al 2009, PLoS Med 6: e1000097; doi:10.1371/journal.pmed1000097)
|
Study
First author, year |
Appropriate and clearly focused question?1
Yes/no/unclear |
Comprehensive and systematic literature search?2
Yes/no/unclear |
Description of included and excluded studies?3
Yes/no/unclear |
Description of relevant characteristics of included studies?4
Yes/no/unclear |
Appropriate adjustment for potential confounders in observational studies?5
Yes/no/unclear/notapplicable |
Assessment of scientific quality of included studies?6
Yes/no/unclear |
Enough similarities between studies to make combining them reasonable?7
Yes/no/unclear |
Potential risk of publication bias taken into account?8
Yes/no/unclear |
Potential conflicts of interest reported?9
Yes/no/unclear |
|
Stewart, 2021 |
Yes
This PROSPERO-registered systematic review (CRD42019115918) compared the evidence behind ACB measures in relation to their ability to predict risk of falling in older people. |
Yes
Medline (OVID), EMBASE (OVID), CINAHL (EMBSCO) and PsycINFO (OVID) were searched using comprehensive search terms and a validated search filter for prognostic studies.
A date restriction was applied (1 January 2006–16 November 2018) and the review was updated on 30 September 2020. |
Yes
The reasons for exclusion are listed. However, the potentially relevant studies that are excluded at final selection are not referenced with reasons. |
Yes
Table 1 |
Yes
Some of the studies adjusted for potential confounders in the observational studies, however not all included studies. |
No |
Yes
|
No |
Yes
However, not for the individual studies. |
- Research question (PICO) and inclusion criteria should be appropriate and predefined
- Search period and strategy should be described; at least Medline searched; for pharmacological questions at least Medline + EMBASE searched
- Potentially relevant studies that are excluded at final selection (after reading the full text) should be referenced with reasons
- Characteristics of individual studies relevant to research question (PICO), including potential confounders, should be reported
- Results should be adequately controlled for potential confounders by multivariate analysis (not applicable for RCTs)
- Quality of individual studies should be assessed using a quality scoring tool or checklist (Jadad score, Newcastle-Ottawa scale, risk of bias table etc.)
- Clinical and statistical heterogeneity should be assessed; clinical: enough similarities in patient characteristics, intervention and definition of outcome measure to allow pooling? For pooled data: assessment of statistical heterogeneity using appropriate statistical tests (e.g. Chi-square, I2)?
- An assessment of publication bias should include a combination of graphical aids (e.g., funnel plot, other available tests) and/or statistical tests (e.g., Egger regression test, Hedges-Olken). Note: If no test values or funnel plot included, score “no”. Score “yes” if mentions that publication bias could not be assessed because there were fewer than 10 included studies.
- Sources of support (including commercial co-authorship) should be reported in both the systematic review and the included studies. Note: To get a “yes,” source of funding or support must be indicated for the systematic review AND for each of the included studies.
Table of excluded studies
|
Reference |
Reason for exclusion |
|
Ackroyd-Stolarz S, Mackinnon NJ, Sketris I, Sabo B. Potentially inappropriate prescribing of benzodiazepines for older adults and risk of falls during a hospital stay: a descriptive study. Can J Hosp Pharm. 2009 Jul;62(4):276-83. doi: 10.4212/cjhp.v62i4.808. PMID: 22478905; PMCID: PMC2826959. |
The study did not report the relevant outcome measures. |
|
Aizenberg D, Sigler M, Weizman A, Barak Y. Anticholinergic burden and the risk of falls among elderly psychiatric inpatients: a 4-year case-control study. Int Psychogeriatr. 2002 Sep;14(3):307-10. doi: 10.1017/s1041610202008505. PMID: 12475091. |
The study did not report the relevant outcome measures. |
|
Arnold I, Straube K, Himmel W, Heinemann S, Weiss V, Heyden L, Hummers-Pradier E, Nau R. High prevalence of prescription of psychotropic drugs for older patients in a general hospital. BMC Pharmacol Toxicol. 2017 Dec 4;18(1):76. doi: 10.1186/s40360-017-0183-0. PMID: 29202811; PMCID: PMC5715648. |
The study did not report the relevant outcome measures. |
|
Blalock SJ, Ferreri SP, Renfro CP, Robinson JM, Farley JF, Ray N, Busby-Whitehead J. Impact of STEADI-Rx: A Community Pharmacy-Based Fall Prevention Intervention. J Am Geriatr Soc. 2020b Aug;68(8):1778-1786. doi: 10.1111/jgs.16459. Epub 2020 Apr 21. PMID: 32315461. |
The study did not report the relevant outcome measures. |
|
Cardwell K, Hughes CM, Ryan C. The Association Between Anticholinergic Medication Burden and Health Related Outcomes in the 'Oldest Old': A Systematic Review of the Literature. Drugs Aging. 2015 Oct;32(10):835-48. doi: 10.1007/s40266-015-0310-9. PMID: 26442862. |
The study did not report the relevant outcome measures. |
|
Di Martino E, Provenzani A, Polidori P. Evidence-based application of explicit criteria to assess the appropriateness of geriatric prescriptions at admission and hospital stay. PLoS One. 2020 Aug 25;15(8):e0238064. doi: 10.1371/journal.pone.0238064. PMID: 32841285; PMCID: PMC7446960. |
The study did not report the relevant outcome measures. |
|
Eckstrom E, Parker EM, Lambert GH, Winkler G, Dowler D, Casey CM. Implementing STEADI in Academic Primary Care to Address Older Adult Fall Risk. Innov Aging. 2017 Sep;1(2):igx028. doi: 10.1093/geroni/igx028. PMID: 29955671; PMCID: PMC6016394. |
The study did not report the relevant outcome measures. |
|
Frankenthal D, Lerman Y, Kalendaryev E, Lerman Y. Potentially inappropriate prescribing among older residents in a geriatric hospital in Israel. Int J Clin Pharm. 2013 Oct;35(5):677-82. doi: 10.1007/s11096-013-9790-z. Epub 2013 May 10. PMID: 23661173. |
The study contains the same population as Frankenthal (2014) and does not report the relevant outcome measures. |
|
Frankenthal D, Lerman Y, Kalendaryev E, Lerman Y. Intervention with the screening tool of older persons potentially inappropriate prescriptions/screening tool to alert doctors to right treatment criteria in elderly residents of a chronic geriatric facility: a randomized clinical trial. J Am Geriatr Soc. 2014 Sep;62(9):1658-65. doi: 10.1111/jgs.12993. PMID: 25243680. |
The study did not report the relevant outcome measures. |
|
Frankenthal D, Israeli A, Caraco Y, Lerman Y, Kalendaryev E, Zandman-Goddard G, Lerman Y. Long-Term Outcomes of Medication Intervention Using the Screening Tool of Older Persons Potentially Inappropriate Prescriptions Screening Tool to Alert Doctors to Right Treatment Criteria. J Am Geriatr Soc. 2017 Feb;65(2):e33-e38. doi: 10.1111/jgs.14570. Epub 2016 Dec 9. PMID: 27943247. |
The study did not report the relevant outcome measures. |
|
Jamieson HA, Nishtala PS, Scrase R, Deely JM, Abey-Nesbit R, Hilmer SN, Abernethy DR, Berry SD, Mor V, Lacey CJ, Schluter PJ. Drug Burden Index and Its Association With Hip Fracture Among Older Adults: A National Population-Based Study. J Gerontol A Biol Sci Med Sci. 2019 Jun 18;74(7):1127-1133. doi: 10.1093/gerona/gly176. PMID: 30084928. |
The study did not report the relevant outcome measures. |
|
Kiesel EK, Hopf YM, Drey M. An anticholinergic burden score for German prescribers: score development. BMC Geriatr. 2018 Oct 11;18(1):239. doi: 10.1186/s12877-018-0929-6. PMID: 30305048; PMCID: PMC6180424. |
The study does not investigate an instrument on medication review. |
|
Kimura T, Ogura F, Yamamoto K, Uda A, Nishioka T, Kume M, Makimoto H, Yano I, Hirai M. Potentially inappropriate medications in elderly Japanese patients: effects of pharmacists' assessment and intervention based on Screening Tool of Older Persons' Potentially Inappropriate Prescriptions criteria ver.2. J Clin Pharm Ther. 2017 Apr;42(2):209-214. doi: 10.1111/jcpt.12496. Epub 2016 Dec 31. PMID: 28039932. |
The study did not report the relevant outcome measures. |
|
Mayer T, Meid AD, Saum KU, Brenner H, Schöttker B, Seidling HM, Haefeli WE. Comparison of Nine Instruments to Calculate Anticholinergic Load in a Large Cohort of Older Outpatients: Association with Cognitive and Functional Decline, Falls, and Use of Laxatives. Am J Geriatr Psychiatry. 2017 May;25(5):531-540. doi: 10.1016/j.jagp.2017.01.009. Epub 2017 Jan 23. PMID: 28233606. |
The study did not report the relevant outcome measures. |
|
McMahon CG, Cahir CA, Kenny RA, Bennett K. Inappropriate prescribing in older fallers presenting to an Irish emergency department. Age Ageing. 2014 Jan;43(1):44-50. doi: 10.1093/ageing/aft114. Epub 2013 Aug 8. PMID: 23927888. |
The study did not report the relevant outcome measures. |
|
Neal SR, Wood AD, Ablett AD, Gregory JS, Guillot J, Macdonald HM, Reid DM, Myint PK. Anticholinergic burden in middle-aged women and recurrent falls in later life: findings from the Aberdeen prospective osteoporosis screening study (APOSS). Ther Adv Drug Saf. 2020 May 27;11:2042098620929852. doi: 10.1177/2042098620929852. PMID: 32547728; PMCID: PMC7273562. |
Different population: younger than 65 years. |
|
Onatade R, Auyeung V, Scutt G, Fernando J. Potentially inappropriate prescribing in patients on admission and discharge from an older peoples' unit of an acute UK hospital. Drugs Aging. 2013 Sep;30(9):729-37. doi: 10.1007/s40266-013-0097-5. PMID: 23780641. |
The study did not report the relevant outcome measures. |
|
Rafiq M, McGovern A, Jones S, Harris K, Tomson C, Gallagher H, de Lusignan S. Falls in the elderly were predicted opportunistically using a decision tree and systematically using a database-driven screening tool. J Clin Epidemiol. 2014 Aug;67(8):877-86. doi: 10.1016/j.jclinepi.2014.03.008. Epub 2014 Apr 29. PMID: 24786593. |
The instrument does not contain a part on medication review. |
|
Seppala LJ, Petrovic M, Ryg J, Bahat G, Topinkova E, Szczerbińska K, van der Cammen TJM, Hartikainen S, Ilhan B, Landi F, Morrissey Y, Mair A, Gutiérrez-Valencia M, Emmelot-Vonk MH, Mora MÁC, Denkinger M, Crome P, Jackson SHD, Correa-Pérez A, Knol W, Soulis G, Gudmundsson A, Ziere G, Wehling M, O'Mahony D, Cherubini A, van der Velde N. STOPPFall (Screening Tool of Older Persons Prescriptions in older adults with high fall risk): a Delphi study by the EuGMS Task and Finish Group on Fall-Risk-Increasing Drugs. Age Ageing. 2021 Jun 28;50(4):1189-1199. doi: 10.1093/ageing/afaa249. PMID: 33349863; PMCID: PMC8244563. |
The study did not report the relevant outcome measures. |
|
Shaver AL, Clark CM, Hejna M, Feuerstein S, Wahler RG Jr, Jacobs DM. Trends in fall-related mortality and fall risk increasing drugs among older individuals in the United States,1999-2017. Pharmacoepidemiol Drug Saf. 2021 Aug;30(8):1049-1056. doi: 10.1002/pds.5201. Epub 2021 Feb 16. PMID: 33534172; PMCID: PMC8254780. |
|
|
Thevelin S, Mounaouar LE, Marien S, Boland B, Henrard S, Dalleur O. Potentially Inappropriate Prescribing and Related Hospital Admissions in Geriatric Patients: A Comparative Analysis between the STOPP and START Criteria Versions 1 and 2. Drugs Aging. 2019 May;36(5):453-459. doi: 10.1007/s40266-018-00635-8. PMID: 30694444. |
The study did not report the relevant outcome measures. |
|
Walsh ME, Boland F, Moriarty F, Fahey T. Modification of Potentially Inappropriate Prescribing Following Fall-Related Hospitalizations in Older Adults. Drugs Aging. 2019 May;36(5):461-470. doi: 10.1007/s40266-019-00646-z. PMID: 30834489. |
The study did not report the relevant outcome measures. |
Verantwoording
Beoordelingsdatum en geldigheid
Publicatiedatum : 20-02-2024
Beoordeeld op geldigheid : 06-02-2024
Algemene gegevens
De ontwikkeling/herziening van deze richtlijnmodule werd ondersteund door het Kennisinstituut van de Federatie Medisch Specialisten (www.demedischspecialist.nl/kennisinstituut) en werd gefinancierd uit de Kwaliteitsgelden Medisch Specialisten (SKMS). De financier heeft geen enkele invloed gehad op de inhoud van de richtlijnmodule.
Samenstelling werkgroep
Voor het ontwikkelen van de richtlijnmodule is in 2020 een multidisciplinair cluster ingesteld, bestaande uit vertegenwoordigers van alle relevante specialismen (zie hiervoor de Samenstelling van het cluster) die betrokken zijn bij de zorg voor patiënten met duizeligheid en vallen.
Cluster stuurgroep duizeligheid en vallen
- Prof. dr. T.D. (Tjasse) Bruintjes, voorzitter cluster duizeligheid en vallen, KNO-arts Gelre Ziekenhuizen; NVKNO
- Dr. S. (Simon) Geerse, KNO-arts, Treant locatie Emmen, NVKNO
- Dr. W.P.A. (Willem) Kelders, KNO-arts, Franciscus Gasthuis & Vlietland; NVKNO
- Prof. dr. N. (Nathalie) van der Velde, klinisch geriater, Amsterdam UMC; NVKG
- Prof. dr. M.H. (Mariëlle) Emmelot-Vonk, klinisch geriater, UMC Utrecht; NVKG
- Dr. R.B. (Roeland) van Leeuwen, neuroloog, Gelre Ziekenhuizen; NVN
- Drs. E.M. (Egbert) Koomen, cardioloog, Gelre Ziekenhuizen; NVvC
- J.H.W. (Joost) Rutten, internist, Radboud UMC; NIV
- Dr. J. (Jan) Festen, patiëntvertegenwoordiger, Kaderlid KBO-PCOB
Betrokken cluster expertiseleden duizeligheid en vallen (cyclus 1)
- M.J.H. (Marjo) van Gils-Simons BSc, ergotherapeut; EN
- Dr. O.R. (Otto) Maarsingh, huisarts, Amsterdam UMC; NHG
- Dr. V.A. (Vincent) van Vugt, huisarts, Amsterdam UMC; NHG
- Dr. E.P. (Eveline) van Poelgeest, internist-ouderengeneeskunde, Amsterdam UMC; NIV
- Drs. C.M. (Margreet) Aalten, klinisch geriater, St. Jansdal Ziekenhuis; NVKG
- Drs. J. (Janneke) Bakker, AIOS-geriatrie, Vincent van Gogh; NVKG
- Dr. E.C.A. (Evert) Kaal, neuroloog, Maasstad Ziekhuis; NVN
- Drs. B.S. (Birgit) Jacobs, ziekenhuisapotheker, Catharina Ziekenhuis; NVZA
Met ondersteuning van
- Dr. R. (Romy) Zwarts - van de Putte, adviseur, Kennisinstituut van Medisch Specialisten
- MSc, D.G. (Dian) Ossendrijver, junior adviseur, Kennisinstituut van Medisch Specialisten
- Dr. A.C.J. (Astrid) Balemans, adviseur, Kennisinstituut van Medisch Specialisten (vanaf juli 2023 – tot november 2023).
Belangenverklaringen
De Code ter voorkoming van oneigenlijke beïnvloeding door belangenverstrengeling is gevolgd. Alle clusterleden hebben schriftelijk verklaard of zij in de laatste drie jaar directe financiële belangen (betrekking bij een commercieel bedrijf, persoonlijke financiële belangen, onderzoeksfinanciering) of indirecte belangen (persoonlijke relaties, reputatiemanagement) hebben gehad. Gedurende de ontwikkeling of herziening van een module worden wijzigingen in belangen aan de voorzitter doorgegeven. De belangenverklaring wordt opnieuw bevestigd tijdens de commentaarfase.
Een overzicht van de belangen van de clusterleden en het oordeel over het omgaan met eventuele belangen vindt u in onderstaande tabel. De ondertekende belangenverklaringen zijn op te vragen bij het secretariaat van het Kennisinstituut van de Federatie Medisch Specialisten.
Cluster stuurgroep
|
Clusterlid |
Functie |
Nevenfuncties |
Gemelde belangen |
Ondernomen actie |
|
Bruintjes (voorzitter cluster duizeligheid en vallen) |
- KNO-arts (vrijgevestigd) Gelre ziekenhuizen Apeldoorn (0,8 fte) |
- lid beroepsgenoot Regionaal Tuchtcollege voor de Gezondheidszorg Zwolle (onkostenvergoeding) |
Geen |
Geen restrictie |
|
Van der Velde |
Staflid en hoogleraar onderafdeling Geriatrie Amsterdam UMC, lokatie AMC |
Onbetaald: |
nee; alleen subsidies van onafhankelijke subsidieverstrekkers (NWO, ZonMW, Amsterdams Universiteitsfonds). De onderzoekslijn betreft valpreventie (hart- en vaatziekte gerelateerd aan vallen, medicatie gerelateerd aan vallen en de doorontwikkeling van de valanalyse van veiligheidNL).
Co-auteur van de STOPPFall tool die ontwikkeld is samen met Europese leden van de Task & Finish group on fall-risk inducing drugs (voorzitter van de werkgroep). |
Geen restrictie |
|
Van Leeuwen |
Neuroloog, Gelre ziekenhuizen Apeldoorn |
Lid beroepsgenoot Regionaal Tuchtcollege voor de Gezondheidszorg. Betaald |
Geen |
Geen restrictie |
|
Emmelot-Vonk |
Hoogleraar en medisch afdelingshoofd klinische geriatrie, UMC Utrecht |
Voorzitter wetenschapscommissie NVKG - onbetaald |
Geen |
Geen restrictie |
|
Koomen |
Cardioloog, Gelre Apeldoorn |
Plaatsvervangend lid beroepsgenoot Centraal Tucht College gezondheidszorg |
Geen |
Geen restrictie |
|
Rutten |
Internist-Vasculair Geneeskunde 0.9 FTE/Radboudumc, Nijmegen |
Consultant op projectbasis voor Volw International - project ontwikkeling screeningstool ter opsporing patiënten met lipodystrofie |
Principle Investigator Radboudumc CALM DIEM en CALM START studies, sponsor Vascular Dynamics (studies naar effect van specifiek type carotisstent ter verlaging van de bloeddruk)
|
Geen restrictie. Deze studies hebben geen betrekking op de modules die in deze cyclus zijn herzien.
|
|
Geerse |
KNO-arts (loondienst) Amsterdam UMC, locatie AMC (1.0 fte) Per 1-3-2022 KNO-arts, Treant locatie Emmen |
per 1-3-2013: lid kerngroep vestibulogie KNO-vereniging (vacatievergoeding)
|
Geen |
Geen restrictie |
|
Kelders |
- KNO-arts (vrijgevestigd) Franciscus Gasthuis en Vlietland Rotterdam (0,8 fte) |
- lid kerngroep vestibulogie KNO-vereniging (vacatievergoeding) (tot 1-3-2023) |
Geen |
Geen restrictie |
|
Festen |
Kaderlid KBO-PCOB |
lid van de commissie Langdurige Zorg en Ondersteuning, |
Geen |
Geen restrictie |
Betrokken cluster expertiseleden
|
Clusterlid |
Functie |
Nevenfuncties |
Gemelde belangen |
Ondernomen actie |
|
Maarsingh |
Huisarts, Amsterdam UMC, locatie Vumc, Universitaire Huisartsenpraktijk Senior-onderzoeker, Amsterdam UMC, locatie Vumc, afdeling Huisartsgeneeskunde |
Geen |
Ons onderzoek op het gebied van duizeligheid werd in het verleden gefinancierd door ZonMw. Het is onze inzet omook in de toekomst subsidies te werven, zodat dit onderzoek kan blijven plaatsvinden |
Geen restrictie |
|
Kaal |
Neuroloog Maasstad Ziekenhuis 0,8 FTE |
Voorzitter Werkgroep voor Syncope en Autonome Aandoeningen, onbetaald |
Geen
|
Geen restrictie |
|
Jacobs |
Ziekenhuisapotheker-klinisch farmacoloog bij Catharina Ziekenhuis, Eindhoven |
Geen |
Geen |
Geen restrictie |
|
Bakker |
Arts assistent in opleiding tot specialist |
Lid van werkgroep kwaliteitsbeleid 3.0: herziening kwaliteitsbeleid beroepsvereniging |
Geen |
Geen restrictie |
|
Van Poelgeest |
Internist ouderengeneeskunde AUMC, betaald |
Expertisecentrum farmacotherapie bij ouderen (Ephor), betaald |
Geen |
Geen restrictie |
|
Van Vugt |
-Huisarts (0.6 FTE), ZZP. |
2x dag per jaar KNGF-geaccrediteerde nascholing geven bij SOMT, University of Physiotherapy in Amersfoort. Betaald. |
Betrokken geweest bij de ontwikkeling van een medisch hulpmiddel, Vertigo Training. Het intellectueel eigendom van deze online vorm van de oefenbehandeling vestibulaire revalidatie ligt bij de afdeling huisartsgeneeskunde van Amsterdam UMC. Ik heb geen commercieel belang bij Vertigo Training. Het is wel mogelijk dat deze behandeling meer bekendheid zal krijgen door het werk van de commissie. |
Geen restrictie. Deze studies hebben geen betrekking op de modules die in deze cyclus zijn herzien.
|
|
van Gils-Simons |
1e lijns ergotherapeut in dienst bij de Wever, ouderenorganisatie in Tilburg |
Geen |
Geen |
Geen restrictie |
|
Aalten |
Klinisch geriater Ziekenhuis St. Jansdal Harderwijk 0,6 FTE |
Lid accreditatie beoordelingscommissie NVKG onbezoldigd. |
Geen |
Geen restrictie |
Inbreng patiëntenperspectief
Er werd aandacht besteed aan het patiëntenperspectief door de afvaardiging van KBO-PCOB in de cluster stuurgroep. De verkregen input is meegenomen bij het opstellen van de uitgangsvragen, de keuze voor de uitkomstmaten en bij het opstellen van de overwegingen. De conceptmodule is tevens voor commentaar voorgelegd aan de KPO-PCOB en stichting Hoormij. De eventueel aangeleverde commentaren zijn bekeken en verwerkt.
Kwalitatieve raming van mogelijke financiële gevolgen in het kader van de Wkkgz
Bij de richtlijn is conform de Wet kwaliteit, klachten en geschillen zorg (Wkkgz) een
kwalitatieve raming uitgevoerd of de aanbevelingen mogelijk leiden tot substantiële
financiële gevolgen. Bij het uitvoeren van deze beoordeling zijn richtlijnmodules op
verschillende domeinen getoetst (zie het stroomschema op de Richtlijnendatabase).
Uit de kwalitatieve raming blijkt dat er waarschijnlijk geen substantiële financiële gevolgen
zijn, zie onderstaande tabel.
|
Module |
Uitkomst raming |
Toelichting |
|
Module effect medicijnen valrisico |
Geen substantiële financiële gevolgen |
Hoewel uit de toetsing volgt dat de aanbeveling(en) breed toepasbaar zijn (>40.000 patiënten), volgt ook uit de toetsing dat het geen nieuwe manier van zorgverlening of andere organisatie van zorgverlening betreft. Er worden daarom geen substantiële financiële gevolgen verwacht. |
Werkwijze
AGREE
Deze richtlijnmodule is opgesteld conform de eisen vermeld in het rapport Medisch Specialistische Richtlijnen 2.0 van de adviescommissie Richtlijnen van de Raad Kwaliteit. Dit rapport is gebaseerd op het AGREE II instrument (Appraisal of Guidelines for Research & Evaluation II; Brouwers, 2010).
Need-for-update, prioritering en uitgangsvragen
Tijdens de need-for-update fase inventariseerde het cluster de geldigheid van de modules binnen het cluster. Naast de betrokken wetenschappelijke verenigingen en patiëntenorganisaties zijn hier ook andere stakeholders voor benaderd in het voorjaar van 2021, waaronder IGJ, NHG, NVZ, V&VN, NAPA, ZiNL, ZKN, ZN, en het KNGF.
Per module is aangegeven of deze geldig is, kan worden samengevoegd met een andere module, obsoleet is en kan vervallen of niet meer geldig is en moet worden herzien. Ook was er de mogelijkheid om nieuwe onderwerpen voor modules aan te dragen die aansluiten bij één (of meerdere) richtlijn(en) behorend tot het cluster. De modules die door één of meerdere partijen werden aangekaart als ‘niet geldig’ zijn meegegaan in de prioriteringsfase. Deze modules zijn geprioriteerd door het cluster.
Voor de geprioriteerde modules zijn door de het cluster concept-uitgangsvragen herzien of opgesteld en definitief vastgesteld.
Uitkomstmaten
Na het opstellen van de zoekvraag behorende bij de uitgangsvraag inventariseerde het cluster welke uitkomstmaten voor de patiënt relevant zijn, waarbij zowel naar gewenste als ongewenste effecten werd gekeken. Hierbij werd een maximum van acht uitkomstmaten gehanteerd. Het cluster waardeerde deze uitkomstmaten volgens hun relatieve belang bij de besluitvorming rondom aanbevelingen, als cruciaal (kritiek voor de besluitvorming), belangrijk (maar niet cruciaal) en onbelangrijk. Tevens definieerde het cluster tenminste voor de cruciale uitkomstmaten welke verschillen zij klinisch (patiënt) relevant vonden.
Methode literatuursamenvatting
Een uitgebreide beschrijving van de strategie voor zoeken en selecteren van literatuur is te vinden onder ‘Zoeken en selecteren’ onder Onderbouwing. Indien mogelijk werd de data uit verschillende studies gepoold in een random-effects model. Review Manager 5.4 werd gebruikt voor de statistische analyses. De beoordeling van de kracht van het wetenschappelijke bewijs wordt hieronder toegelicht.
Beoordelen van de kracht van het wetenschappelijke bewijs
De kracht van het wetenschappelijke bewijs werd bepaald volgens de GRADE-methode. GRADE staat voor ‘Grading Recommendations Assessment, Development and Evaluation’ (zie http://www.gradeworkinggroup.org/). De basisprincipes van de GRADE-methodiek zijn: het benoemen en prioriteren van de klinisch (patiënt) relevante uitkomstmaten, een systematische review per uitkomstmaat, en een beoordeling van de bewijskracht per uitkomstmaat op basis van de acht GRADE-domeinen (domeinen voor downgraden: risk of bias, inconsistentie, indirectheid, imprecisie, en publicatiebias; domeinen voor upgraden: dosis-effect relatie, groot effect, en residuele plausibele confounding).
GRADE onderscheidt vier gradaties voor de kwaliteit van het wetenschappelijk bewijs: hoog, redelijk, laag en zeer laag. Deze gradaties verwijzen naar de mate van zekerheid die er bestaat over de literatuurconclusie, in het bijzonder de mate van zekerheid dat de literatuurconclusie de aanbeveling adequaat ondersteunt (Schünemann, 2013; Hultcrantz, 2017).
|
GRADE |
Definitie |
|
Hoog |
|
|
Redelijk |
|
|
Laag |
|
|
Zeer laag |
|
Bij het beoordelen (graderen) van de kracht van het wetenschappelijk bewijs in richtlijnen volgens de GRADE-methodiek spelen grenzen voor klinische besluitvorming een belangrijke rol (Hultcrantz, 2017). Dit zijn de grenzen die bij overschrijding aanleiding zouden geven tot een aanpassing van de aanbeveling. Om de grenzen voor klinische besluitvorming te bepalen moeten alle relevante uitkomstmaten en overwegingen worden meegewogen. De grenzen voor klinische besluitvorming zijn daarmee niet één op één vergelijkbaar met het minimaal klinisch relevant verschil (Minimal Clinically Important Difference, MCID). Met name in situaties waarin een interventie geen belangrijke nadelen heeft en de kosten relatief laag zijn, kan de grens voor klinische besluitvorming met betrekking tot de effectiviteit van de interventie bij een lagere waarde (dichter bij het nuleffect) liggen dan de MCID (Hultcrantz, 2017).
Overwegingen (van bewijs naar aanbeveling)
Om te komen tot een aanbeveling zijn naast (de kwaliteit van) het wetenschappelijke bewijs ook andere aspecten belangrijk en worden meegewogen, zoals aanvullende argumenten uit bijvoorbeeld de biomechanica of fysiologie, waarden en voorkeuren van patiënten, kosten (middelenbeslag), aanvaardbaarheid, haalbaarheid en implementatie. Deze aspecten zijn systematisch vermeld en beoordeeld (gewogen) onder het kopje ‘Overwegingen’ en kunnen (mede) gebaseerd zijn op expert opinion. Hierbij is gebruik gemaakt van een gestructureerd format gebaseerd op het evidence-to-decision framework van de internationale GRADE Working Group (Alonso-Coello, 2016a; Alonso-Coello 2016b). Dit evidence-to-decision framework is een integraal onderdeel van de GRADE methodiek.
Formuleren van aanbevelingen
De aanbevelingen geven antwoord op de uitgangsvraag en zijn gebaseerd op het beschikbare wetenschappelijke bewijs en de belangrijkste overwegingen, en een weging van de gunstige en ongunstige effecten van de relevante interventies. De kracht van het wetenschappelijk bewijs en het gewicht dat door het cluster wordt toegekend aan de overwegingen, bepalen samen de sterkte van de aanbeveling. Conform de GRADE-methodiek sluit een lage bewijskracht van conclusies in de systematische literatuuranalyse een sterke aanbeveling niet a priori uit, en zijn bij een hoge bewijskracht ook zwakke aanbevelingen mogelijk (Agoritsas, 2017; Neumann, 2016). De sterkte van de aanbeveling wordt altijd bepaald door weging van alle relevante argumenten tezamen. Het cluster heeft bij elke aanbeveling opgenomen hoe zij tot de richting en sterkte van de aanbeveling zijn gekomen.
In de GRADE-methodiek wordt onderscheid gemaakt tussen sterke en zwakke (of conditionele) aanbevelingen. De sterkte van een aanbeveling verwijst naar de mate van zekerheid dat de voordelen van de interventie opwegen tegen de nadelen (of vice versa), gezien over het hele spectrum van patiënten waarvoor de aanbeveling is bedoeld. De sterkte van een aanbeveling heeft duidelijke implicaties voor patiënten, behandelaars en beleidsmakers (zie onderstaande tabel). Een aanbeveling is geen dictaat, zelfs een sterke aanbeveling gebaseerd op bewijs van hoge kwaliteit (GRADE gradering HOOG) zal niet altijd van toepassing zijn, onder alle mogelijke omstandigheden en voor elke individuele patiënt.
|
Implicaties van sterke en zwakke aanbevelingen voor verschillende richtlijngebruikers |
||
|
|
||
|
|
Sterke aanbeveling |
Zwakke (conditionele) aanbeveling |
|
Voor patiënten |
De meeste patiënten zouden de aanbevolen interventie of aanpak kiezen en slechts een klein aantal niet. |
Een aanzienlijk deel van de patiënten zouden de aanbevolen interventie of aanpak kiezen, maar veel patiënten ook niet. |
|
Voor behandelaars |
De meeste patiënten zouden de aanbevolen interventie of aanpak moeten ontvangen. |
Er zijn meerdere geschikte interventies of aanpakken. De patiënt moet worden ondersteund bij de keuze voor de interventie of aanpak die het beste aansluit bij zijn of haar waarden en voorkeuren. |
|
Voor beleidsmakers |
De aanbevolen interventie of aanpak kan worden gezien als standaardbeleid. |
Beleidsbepaling vereist uitvoerige discussie met betrokkenheid van veel stakeholders. Er is een grotere kans op lokale beleidsverschillen. |
Organisatie van zorg
Bij de ontwikkeling van de richtlijnmodule is expliciet aandacht geweest voor de organisatie van zorg: alle aspecten die randvoorwaardelijk zijn voor het verlenen van zorg (zoals coördinatie, communicatie, (financiële) middelen, mankracht en infrastructuur). Randvoorwaarden die relevant zijn voor het beantwoorden van deze specifieke uitgangsvraag zijn genoemd bij de overwegingen. Meer algemene, overkoepelende, of bijkomende aspecten van de organisatie van zorg worden behandeld in de module Organisatie van zorg.
Commentaar- en autorisatiefase
De conceptrichtlijnmodule werd aan de betrokken (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd ter commentaar. De commentaren werden verzameld en besproken met het cluster. Naar aanleiding van de commentaren werd de conceptrichtlijnmodule aangepast en definitief vastgesteld door het cluster. De definitieve richtlijnmodule werd aan de deelnemende (wetenschappelijke) verenigingen en (patiënt)organisaties voorgelegd voor autorisatie en door hen geautoriseerd dan wel geaccordeerd.
Literatuur
Agoritsas T, Merglen A, Heen AF, Kristiansen A, Neumann I, Brito JP, Brignardello-Petersen R, Alexander PE, Rind DM, Vandvik PO, Guyatt GH. UpToDate adherence to GRADE criteria for strong recommendations: an analytical survey. BMJ Open. 2017 Nov 16;7(11):e018593. doi: 10.1136/bmjopen-2017-018593. PubMed PMID: 29150475; PubMed Central PMCID: PMC5701989.
Alonso-Coello P, Schünemann HJ, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Rada G, Rosenbaum S, Morelli A, Guyatt GH, Oxman AD; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ. 2016 Jun 28;353:i2016. doi: 10.1136/bmj.i2016. PubMed PMID: 27353417.
Alonso-Coello P, Oxman AD, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Vandvik PO, Meerpohl J, Guyatt GH, Schünemann HJ; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 2: Clinical practice guidelines. BMJ. 2016 Jun 30;353:i2089. doi: 10.1136/bmj.i2089. PubMed PMID: 27365494.
Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, Fervers B, Graham ID, Grimshaw J, Hanna SE, Littlejohns P, Makarski J, Zitzelsberger L; AGREE Next Steps Consortium. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010 Dec 14;182(18):E839-42. doi: 10.1503/cmaj.090449. Epub 2010 Jul 5. Review. PubMed PMID: 20603348; PubMed Central PMCID: PMC3001530.
Hultcrantz M, Rind D, Akl EA, Treweek S, Mustafa RA, Iorio A, Alper BS, Meerpohl JJ, Murad MH, Ansari MT, Katikireddi SV, Östlund P, Tranæus S, Christensen R, Gartlehner G, Brozek J, Izcovich A, Schünemann H, Guyatt G. The GRADE Working Group clarifies the construct of certainty of evidence. J Clin Epidemiol. 2017 Jul;87:4-13. doi: 10.1016/j.jclinepi.2017.05.006. Epub 2017 May 18. PubMed PMID: 28529184; PubMed Central PMCID: PMC6542664.
Medisch Specialistische Richtlijnen 2.0 (2012). Adviescommissie Richtlijnen van de Raad Kwalitieit. http://richtlijnendatabase.nl/over_deze_site/over_richtlijnontwikkeling.html
Neumann I, Santesso N, Akl EA, Rind DM, Vandvik PO, Alonso-Coello P, Agoritsas T, Mustafa RA, Alexander PE, Schünemann H, Guyatt GH. A guide for health professionals to interpret and use recommendations in guidelines developed with the GRADE approach. J Clin Epidemiol. 2016 Apr;72:45-55. doi: 10.1016/j.jclinepi.2015.11.017. Epub 2016 Jan 6. Review. PubMed PMID: 26772609.
Schünemann H, Brożek J, Guyatt G, et al. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group, 2013. Available from http://gdt.guidelinedevelopment.org/central_prod/_design/client/handbook/handbook.html.
Zoekverantwoording
Literature search strategy module 1: effect medicijnen op valrisico
Algemene informatie
|
Cluster: Duizeligheid en vallen |
|
|
Richtlijn: Preventie van valincidenten bij ouderen |
|
|
Uitgangsvraag/modules: Effect medicijnen op valrisico ouderen |
|
|
Database(s): Ovid/Medline, Embase.com |
Datum: 07-06-2022 |
|
Periode: geen restrictie |
Talen: Engels, Nederlands |
|
Literatuurspecialist: Miriam van der Maten |
|
|
BMI-zoekblokken: voor verschillende opdrachten wordt (deels) gebruik gemaakt van de zoekblokken van BMI-Online https://blocks.bmi-online.nl/ Bij gebruikmaking van een volledig zoekblok zal naar de betreffende link op de website worden verwezen. |
|
|
Toelichting: Voor deze vraag is gezocht op de elementen:
|
|
|
Te gebruiken voor richtlijnen tekst: In de databases Embase.com en Ovid/Medline is op 7 juni 2022 met relevante zoektermen gezocht naar systematische reviews, RCT en observationele studies over welke instrumenten je kunt gebruiken om valrisicoverhogende medicatie te identificeren en/of af te bouwen bij ouderen. De literatuurzoekactie leverde 497 unieke treffers op. |
|
Zoekopbrengst
|
|
EMBASE |
OVID/MEDLINE |
Ontdubbeld |
|
SRs |
62 |
45 |
74 |
|
RCT |
53 |
55 |
86 |
|
Observationele studies |
236 |
179 |
337 |
|
Totaal |
351 |
279 |
497 |
Zoekstrategie
Embase.com
|
No. |
Query |
Results |
|
#16 |
#13 OR #14 OR #15 |
351 |
|
#15 |
#9 AND #12 NOT (#13 OR #14) = observationeel |
236 |
|
#14 |
#9 AND #11 NOT #13 = RCT |
53 |
|
#13 |
#9 AND #10 = SR |
62 |
|
#12 |
'case control study'/de OR 'comparative study'/exp OR 'control group'/de OR 'controlled study'/de OR 'controlled clinical trial'/de OR 'crossover procedure'/de OR 'double blind procedure'/de OR 'phase 2 clinical trial'/de OR 'phase 3 clinical trial'/de OR 'phase 4 clinical trial'/de OR 'pretest posttest design'/de OR 'pretest posttest control group design'/de OR 'quasi experimental study'/de OR 'single blind procedure'/de OR 'triple blind procedure'/de OR (((control OR controlled) NEAR/6 trial):ti,ab,kw) OR (((control OR controlled) NEAR/6 (study OR studies)):ti,ab,kw) OR (((control OR controlled) NEAR/1 active):ti,ab,kw) OR 'open label*':ti,ab,kw OR (((double OR two OR three OR multi OR trial) NEAR/1 (arm OR arms)):ti,ab,kw) OR ((allocat* NEAR/10 (arm OR arms)):ti,ab,kw) OR placebo*:ti,ab,kw OR 'sham-control*':ti,ab,kw OR (((single OR double OR triple OR assessor) NEAR/1 (blind* OR masked)):ti,ab,kw) OR nonrandom*:ti,ab,kw OR 'non-random*':ti,ab,kw OR 'quasi-experiment*':ti,ab,kw OR crossover:ti,ab,kw OR 'cross over':ti,ab,kw OR 'parallel group*':ti,ab,kw OR 'factorial trial':ti,ab,kw OR ((phase NEAR/5 (study OR trial)):ti,ab,kw) OR ((case* NEAR/6 (matched OR control*)):ti,ab,kw) OR ((match* NEAR/6 (pair OR pairs OR cohort* OR control* OR group* OR healthy OR age OR sex OR gender OR patient* OR subject* OR participant*)):ti,ab,kw) OR ((propensity NEAR/6 (scor* OR match*)):ti,ab,kw) OR versus:ti OR vs:ti OR compar*:ti OR ((compar* NEAR/1 study):ti,ab,kw) OR (('major clinical study'/de OR 'clinical study'/de OR 'cohort analysis'/de OR 'observational study'/de OR 'cross-sectional study'/de OR 'multicenter study'/de OR 'correlational study'/de OR 'follow up'/de OR cohort*:ti,ab,kw OR 'follow up':ti,ab,kw OR followup:ti,ab,kw OR longitudinal*:ti,ab,kw OR prospective*:ti,ab,kw OR retrospective*:ti,ab,kw OR observational*:ti,ab,kw OR 'cross sectional*':ti,ab,kw OR cross?ectional*:ti,ab,kw OR multicent*:ti,ab,kw OR 'multi-cent*':ti,ab,kw OR consecutive*:ti,ab,kw) AND (group:ti,ab,kw OR groups:ti,ab,kw OR subgroup*:ti,ab,kw OR versus:ti,ab,kw OR vs:ti,ab,kw OR compar*:ti,ab,kw OR 'odds ratio*':ab OR 'relative odds':ab OR 'risk ratio*':ab OR 'relative risk*':ab OR 'rate ratio':ab OR aor:ab OR arr:ab OR rrr:ab OR ((('or' OR 'rr') NEAR/6 ci):ab))) |
13168353 |
|
#11 |
'randomized controlled trial'/exp OR random*:ti,ab OR (((pragmatic OR practical) NEAR/1 'clinical trial*'):ti,ab) OR ((('non inferiority' OR noninferiority OR superiority OR equivalence) NEAR/3 trial*):ti,ab) OR rct:ti,ab,kw |
1839814 |
|
#10 |
'meta analysis'/exp OR 'meta analysis (topic)'/exp OR metaanaly*:ti,ab OR 'meta analy*':ti,ab OR metanaly*:ti,ab OR 'systematic review'/de OR 'cochrane database of systematic reviews'/jt OR prisma:ti,ab OR prospero:ti,ab OR (((systemati* OR scoping OR umbrella OR 'structured literature') NEAR/3 (review* OR overview*)):ti,ab) OR ((systemic* NEAR/1 review*):ti,ab) OR (((systemati* OR literature OR database* OR 'data base*') NEAR/10 search*):ti,ab) OR (((structured OR comprehensive* OR systemic*) NEAR/3 search*):ti,ab) OR (((literature NEAR/3 review*):ti,ab) AND (search*:ti,ab OR database*:ti,ab OR 'data base*':ti,ab)) OR (('data extraction':ti,ab OR 'data source*':ti,ab) AND 'study selection':ti,ab) OR ('search strategy':ti,ab AND 'selection criteria':ti,ab) OR ('data source*':ti,ab AND 'data synthesis':ti,ab) OR medline:ab OR pubmed:ab OR embase:ab OR cochrane:ab OR (((critical OR rapid) NEAR/2 (review* OR overview* OR synthes*)):ti) OR ((((critical* OR rapid*) NEAR/3 (review* OR overview* OR synthes*)):ab) AND (search*:ab OR database*:ab OR 'data base*':ab)) OR metasynthes*:ti,ab OR 'meta synthes*':ti,ab |
733409 |
|
#9 |
#2 AND #3 AND #4 AND (#5 OR #6 OR #7 OR #8) AND ([english]/lim OR [dutch]/lim) NOT ('conference abstract'/it OR 'editorial'/it OR 'letter'/it OR 'note'/it) NOT (('animal experiment'/exp OR 'animal model'/exp OR 'nonhuman'/exp) NOT 'human'/exp) |
504 |
|
#8 |
'antihistaminic agent'/exp OR 'anti histamin*':ti,ab,kw OR antihistamin*:ti,ab,kw OR ((histamine NEAR/2 (antagonist* OR block*)):ti,ab,kw) OR 'cholinergic receptor blocking agent'/exp OR ((('acetylcholine receptor' OR achr OR cholinergic) NEAR/2 (block* OR inhibit* OR antagonist*)):ti,ab,kw) OR parasympathicolytic*:ti,ab,kw OR parasympatholytic*:ti,ab,kw OR anticholinergic*:ti,ab,kw OR 'adrenergic receptor stimulating agent'/exp OR adrenergic*:ti,ab,kw OR adrenogenic*:ti,ab,kw OR 'spasmolytic agent'/exp OR antispasmodic*:ti,ab,kw OR antispasmic*:ti,ab,kw OR 'smooth muscle relaxant':ti,ab,kw OR 'smooth muscle relaxing':ti,ab,kw OR spasmolytic*:ti,ab,kw OR 'insulin'/exp OR insulin*:ti,ab,kw OR 'oral antidiabetic agent'/exp OR 'acarbose'/exp OR 'pioglitazone'/exp OR 'repaglinide'/exp OR 'histamine h2 receptor antagonist'/exp OR (((h2 OR 'h 2' OR 'histamine 2') NEAR/3 (block* OR antagonist* OR antihistamine*)):ti,ab,kw) OR 'proton pump inhibitor'/exp OR ((('proton pump' OR 'hydrogen potassium adenosine triphosphatase' OR 'hydrogen potassium atpase') NEAR/1 inhibit*):ti,ab,kw) |
2070088 |
|
#7 |
'opiate'/exp OR opiate*:ti,ab,kw OR opioid*:ti,ab,kw OR 'nonsteroid antiinflammatory agent'/exp OR 'nonsteroid antiinflammatory agent*':ti,ab,kw OR nsaid*:ti,ab,kw OR 'muscle relaxant agent'/exp OR 'muscle* relax*':ti,ab,kw |
1498301 |
|
#6 |
'diuretic agent'/exp OR diuretic*:ti,ab,kw OR thiazide:ti,ab,kw OR 'antiarrhythmic agent'/exp OR 'anti arrhythm*':ti,ab,kw OR antiarrhythm*:ti,ab,kw OR 'glycoside'/exp OR glycoside:ti,ab,kw OR 'calcium antagonist'/exp OR 'calcium antagonist*':ti,ab,kw OR 'diltiazem'/exp OR 'verapamil'/exp OR 'vasodilator agent'/exp OR vasodilat*:ti,ab,kw OR vasodilatat*:ti,ab,kw OR 'alpha adrenergic receptor blocking agent'/exp OR ((('alpha adrenergic' OR 'alpha adrenoceptor' OR alpha) NEAR/3 (antagonist* OR block*)):ti,ab,kw) OR 'beta adrenergic receptor blocking agent'/exp OR ((('beta adrenergic' OR 'beta adrenoceptor' OR beta) NEAR/3 (antagonist* OR block*)):ti,ab,kw) OR 'dipeptidyl carboxypeptidase inhibitor'/exp OR (((ace OR 'angiotensin converting enzyme' OR 'dipeptidyl carboxypeptidase') NEAR/1 inhibit*):ti,ab,kw) OR 'angiotensin receptor antagonist'/exp OR ((angiotensin NEAR/2 receptor NEAR/2 (antagonist* OR block*)):ti,ab,kw) OR 'hydroxymethylglutaryl coenzyme a reductase inhibitor'/exp OR statins:ti,ab,kw OR 'nitric acid derivative'/exp OR nitrate*:ti,ab,kw |
2097267 |
|
#5 |
'fall-risk increasing drug*':ti,ab,kw OR frid*:ti,ab,kw OR (((drug* OR agent* OR substance* OR medication*) NEAR/5 fall*):ti,ab,kw) OR 'hypnotic sedative agent'/exp OR hypnosedative*:ti,ab,kw OR ((hypnotic* NEAR/2 sedative*):ti,ab,kw) OR 'anxiolytic agent'/exp OR 'anti-anxiety':ti,ab,kw OR anxiolytic*:ti,ab,kw OR 'neuroleptic agent'/exp OR antipsychotic*:ti,ab,kw OR neuroleptic*:ti,ab,kw OR 'antidepressant agent'/exp OR 'anti-depress*':ti,ab,kw OR antidepress*:ti,ab,kw OR 'serotonin uptake inhibitor*':ti,ab,kw OR ssri*:ti,ab,kw OR 'serotonin noradrenalin reuptake inhibitor*':ti,ab,kw OR snri*:ti,ab,kw OR 'cholinesterase inhibitor'/exp OR 'acetylcholinesterase inhibitor*':ti,ab,kw OR 'anticholesterinase':ti,ab,kw OR 'anticholinesterase':ti,ab,kw OR 'cholinesterase block*':ti,ab,kw OR 'cholinesterase inhibit*':ti,ab,kw OR 'antivertigo agent'/exp OR antivertigo:ti,ab,kw OR 'vertigo inhibit*':ti,ab,kw OR 'benzodiazepine derivative'/exp OR benzodiazepine*:ti,ab,kw OR '4 aminobutyric acid receptor stimulating agent'/exp OR (((gaba* OR 'aminobutyric acid receptor') NEAR/3 agonist*):ti,ab,kw) OR 'anticonvulsive agent'/exp OR anticonvuls*:ti,ab,kw OR 'anti convuls*':ti,ab,kw OR antiepilepti*:ti,ab,kw OR 'anti epilepti*':ti,ab,kw OR 'antiparkinson agent'/exp OR antiparkinson:ti,ab,kw OR 'anti dyskinesia':ti,ab,kw OR antidyskinesia:ti,ab,kw |
1872036 |
|
#4 |
'screening'/exp/mj OR 'questionnaire'/exp OR 'practice guideline'/exp OR 'deprescription'/exp OR tool*:ti,ab,kw OR instrument*:ti,ab,kw OR deprescri*:ti,ab,kw OR stoppfall:ti,ab,kw OR 'stopp-fall':ti,ab,kw OR 'stop-start':ti,ab,kw OR 'prescription'/exp/mj OR screen*:ti,ab,kw |
3989777 |
|
#3 |
'fall risk'/exp/mj OR 'fall risk assessment'/exp/mj OR 'falling'/exp/mj OR ((fall* NEAR/3 risk*):ti,ab,kw) |
29319 |
|
#2 |
'aged'/exp OR 'geriatrics'/exp OR 'elderly care'/exp OR elder*:ti,ab,kw OR eldest:ti,ab,kw OR frail*:ti,ab,kw OR geriatri*:ti,ab,kw OR ((old NEAR/1 age*):ti,ab,kw) OR ((oldest NEAR/1 old*):ti,ab,kw) OR senior*:ti,ab,kw OR senium:ti,ab,kw OR ((very NEAR/1 old*):ti,ab,kw) OR septuagenarian*:ti,ab,kw OR octagenarian*:ti,ab,kw OR octogenarian*:ti,ab,kw OR nonagenarian*:ti,ab,kw OR centarian*:ti,ab,kw OR centenarian*:ti,ab,kw OR supercentenarian*:ti,ab,kw OR 'older people':ti,ab,kw OR ((older NEAR/1 subject*):ti,ab,kw) OR ((older NEAR/1 patient*):ti,ab,kw) OR ((older NEAR/1 age*):ti,ab,kw) OR ((older NEAR/1 adult*):ti,ab,kw) OR 'older man':ti,ab,kw OR 'older men':ti,ab,kw OR 'older male*':ti,ab,kw OR 'older woman':ti,ab,kw OR 'older women':ti,ab,kw OR 'older female*':ti,ab,kw OR ((older NEAR/1 population*):ti,ab,kw) OR ((older NEAR/1 person*):ti,ab,kw) |
3904758 |
Ovid/Medline
|
# |
Searches |
Results |
|
16 |
13 or 14 or 15 |
279 |
|
15 |
(9 and 12) not (13 or 14) = observationeel |
179 |
|
14 |
(9 and 11) not 13 = RCT |
55 |
|
13 |
9 and 10 = SR |
45 |
|
12 |
Case-control Studies/ or clinical trial, phase ii/ or clinical trial, phase iii/ or clinical trial, phase iv/ or comparative study/ or control groups/ or controlled before-after studies/ or controlled clinical trial/ or double-blind method/ or historically controlled study/ or matched-pair analysis/ or single-blind method/ or (((control or controlled) adj6 (study or studies or trial)) or (compar* adj (study or studies)) or ((control or controlled) adj1 active) or "open label*" or ((double or two or three or multi or trial) adj (arm or arms)) or (allocat* adj10 (arm or arms)) or placebo* or "sham-control*" or ((single or double or triple or assessor) adj1 (blind* or masked)) or nonrandom* or "non-random*" or "quasi-experiment*" or "parallel group*" or "factorial trial" or "pretest posttest" or (phase adj5 (study or trial)) or (case* adj6 (matched or control*)) or (match* adj6 (pair or pairs or cohort* or control* or group* or healthy or age or sex or gender or patient* or subject* or participant*)) or (propensity adj6 (scor* or match*))).ti,ab,kf. or (confounding adj6 adjust*).ti,ab. or (versus or vs or compar*).ti. or ((exp cohort studies/ or epidemiologic studies/ or multicenter study/ or observational study/ or seroepidemiologic studies/ or (cohort* or 'follow up' or followup or longitudinal* or prospective* or retrospective* or observational* or multicent* or 'multi-cent*' or consecutive*).ti,ab,kf.) and ((group or groups or subgroup* or versus or vs or compar*).ti,ab,kf. or ('odds ratio*' or 'relative odds' or 'risk ratio*' or 'relative risk*' or aor or arr or rrr).ab. or (("OR" or "RR") adj6 CI).ab.)) |
5171138 |
|
11 |
(exp randomized controlled trial/ or randomized controlled trials as topic/ or random*.ti,ab. or rct?.ti,ab. or ((pragmatic or practical) adj "clinical trial*").ti,ab,kf. or ((non-inferiority or noninferiority or superiority or equivalence) adj3 trial*).ti,ab,kf.) not (animals/ not humans/) |
1381317 |
|
10 |
(meta-analysis/ or meta-analysis as topic/ or (metaanaly* or meta-analy* or metanaly*).ti,ab,kf. or systematic review/ or cochrane.jw. or (prisma or prospero).ti,ab,kf. or ((systemati* or scoping or umbrella or "structured literature") adj3 (review* or overview*)).ti,ab,kf. or (systemic* adj1 review*).ti,ab,kf. or ((systemati* or literature or database* or data-base*) adj10 search*).ti,ab,kf. or ((structured or comprehensive* or systemic*) adj3 search*).ti,ab,kf. or ((literature adj3 review*) and (search* or database* or data-base*)).ti,ab,kf. or (("data extraction" or "data source*") and "study selection").ti,ab,kf. or ("search strategy" and "selection criteria").ti,ab,kf. or ("data source*" and "data synthesis").ti,ab,kf. or (medline or pubmed or embase or cochrane).ab. or ((critical or rapid) adj2 (review* or overview* or synthes*)).ti. or (((critical* or rapid*) adj3 (review* or overview* or synthes*)) and (search* or database* or data-base*)).ab. or (metasynthes* or meta-synthes*).ti,ab,kf.) not (comment/ or editorial/ or letter/ or ((exp animals/ or exp models, animal/) not humans/)) |
569361 |
|
9 |
2 and 3 and 4 and (5 or 6 or 7 or 8) |
479 |
|
8 |
exp Histamine H1 Antagonists/ or exp Histamine Antagonists/ or 'anti histamin*'.ti,ab,kf. or antihistamin*.ti,ab,kf. or (histamine adj2 (antagonist* or block*)).ti,ab,kf. or exp Cholinergic Antagonists/ or (('acetylcholine receptor' or achr or cholinergic) adj2 (block* or inhibit* or antagonist*)).ti,ab,kf. or parasympathicolytic*.ti,ab,kf. or parasympatholytic*.ti,ab,kf. or anticholinergic*.ti,ab,kf. or exp Adrenergic Agents/ or adrenergic*.ti,ab,kf. or adrenogenic*.ti,ab,kf. or exp Parasympatholytics/ or antispasmodic*.ti,ab,kf. or antispasmic*.ti,ab,kf. or 'smooth muscle relaxant'.ti,ab,kf. or 'smooth muscle relaxing'.ti,ab,kf. or spasmolytic*.ti,ab,kf. or exp Insulin/ or insulin*.ti,ab,kf. or (exp Hypoglycemic Agents/ and oral.ti,ab,kf.) or exp Acarbose/ or exp Pioglitazone/ or exp Histamine H2 Antagonists/ or ((h2 or 'h 2' or 'histamine 2') adj3 (block* or antagonist* or antihistamine*)).ti,ab,kf. or exp Proton Pump Inhibitors/ or (('proton pump' or 'hydrogen potassium adenosine triphosphatase' or 'hydrogen potassium atpase') adj1 inhibit*).ti,ab,kf. |
1015953 |
|
7 |
exp Opiate Alkaloids/ or opiate*.ti,ab,kf. or opioid*.ti,ab,kf. or exp Anti-Inflammatory Agents, Non-Steroidal/ or 'nonsteroid antiinflammatory agent*'.ti,ab,kf. or nsaid*.ti,ab,kf. or exp Muscle Relaxants, Central/ or 'muscle* relax*'.ti,ab,kf. |
444359 |
|
6 |
exp Diuretics/ or diuretic*.ti,ab,kf. or thiazide.ti,ab,kf. or exp Anti-Arrhythmia Agents/ or 'anti arrhythm*'.ti,ab,kf. or antiarrhythm*.ti,ab,kf. or exp Glycosides/ or glycoside.ti,ab,kf. or exp Calcium Channel Blockers/ or 'calcium antagonist*'.ti,ab,kf. or exp Diltiazem/ or exp Verapamil/ or exp Vasodilator Agents/ or vasodilat*.ti,ab,kf. or vasodilatat*.ti,ab,kf. or exp Adrenergic alpha-Antagonists/ or (('alpha adrenergic' or 'alpha adrenoceptor' or alpha) adj3 (antagonist* or block*)).ti,ab,kf. or exp Adrenergic beta-Antagonists/ or (('beta adrenergic' or 'beta adrenoceptor' or beta) adj3 (antagonist* or block*)).ti,ab,kf. or exp Angiotensin-Converting Enzyme Inhibitors/ or ((ace or 'angiotensin converting enzyme' or 'dipeptidyl carboxypeptidase') adj1 inhibit*).ti,ab,kf. or exp Angiotensin Receptor Antagonists/ or (angiotensin adj2 receptor adj2 (antagonist* or block*)).ti,ab,kf. or exp Hydroxymethylglutaryl-CoA Reductase Inhibitors/ or statins.ti,ab,kf. or exp Nitrates/ or nitrate*.ti,ab,kf. |
1281816 |
|
5 |
('fall-risk increasing drug*' or frid* or ((drug* or agent* or substance* or medication*) adj5 fall*)).ti,ab,kf. or exp "Hypnotics and Sedatives"/ or hypnosedative*.ti,ab,kf. or (hypnotic* adj2 sedative*).ti,ab,kf. or exp Anti-Anxiety Agents/ or 'anti-anxiety'.ti,ab,kf. or anxiolytic*.ti,ab,kf. or exp Antipsychotic Agents/ or antipsychotic*.ti,ab,kf. or neuroleptic*.ti,ab,kf. or exp Antidepressive Agents/ or 'anti-depress*'.ti,ab,kf. or antidepress*.ti,ab,kf. or 'serotonin uptake inhibitor*'.ti,ab,kf. or ssri*.ti,ab,kf. or 'serotonin noradrenalin reuptake inhibitor*'.ti,ab,kf. or snri*.ti,ab,kf. or exp Cholinesterase Inhibitors/ or 'acetylcholinesterase inhibitor*'.ti,ab,kf. or 'anticholesterinase'.ti,ab,kf. or 'anticholinesterase'.ti,ab,kf. or 'cholinesterase block*'.ti,ab,kf. or 'cholinesterase inhibit*'.ti,ab,kf. or antivertigo.ti,ab,kf. or 'vertigo inhibit*'.ti,ab,kf. or exp Benzodiazepines/ or benzodiazepine*.ti,ab,kf. or ((gaba* or 'aminobutyric acid receptor') adj3 agonist*).ti,ab,kf. or exp Anticonvulsants/ or anticonvuls*.ti,ab,kf. or 'anti convuls*'.ti,ab,kf. or antiepilepti*.ti,ab,kf. or 'anti epilepti*'.ti,ab,kf. or exp Antiparkinson Agents/ or antiparkinson.ti,ab,kf. or 'anti dyskinesia'.ti,ab,kf. or antidyskinesia.ti,ab,kf. |
670369 |
|
4 |
exp Mass Screening/ or exp "Surveys and Questionnaires"/ or exp Guideline/ or exp Practice Guideline/ or exp Deprescriptions/ or exp *Prescriptions/ or tool*.ti,ab,kf. or instrument*.ti,ab,kf. or deprescri*.ti,ab,kf. or stoppfall.ti,ab,kf. or 'stopp-fall'.ti,ab,kf. or 'stop-start'.ti,ab,kf. or screen*.ti,ab,kf. |
2962833 |
|
3 |
exp Accidental Falls/ or (fall* adj5 risk*).ti,ab,kf. |
34813 |
|
2 |
exp "Aged"/ or exp "Aged, 80 and over"/ or exp "Frail Elderly"/ or exp "Geriatrics"/ or exp "Geriatric Psychiatry"/ or exp "Geriatric Nursing"/ or exp "Geriatric Dentistry"/ or exp "Dental Care for Aged"/ or exp "Health Services for the Aged"/ or (elder* or eldest or frail* or geriatri* or old age* or oldest old* or senior* or senium or very old* or septuagenarian* or octagenarian* or octogenarian* or nonagenarian* or centarian* or centenarian* or supercentenarian* or older people or older subject* or older patient* or older age* or older adult* or older man or older men or older male* or older woman or older women or older female* or older population* or older person*).ti,ab,kf. |
3642934 |
