Postoperatieve samenwerking bij chirurgie bij kwetsbare ouderen
Uitgangsvraag
Wat is de meerwaarde van het betrekken van geriatrische expertise in de postoperatieve fase bij kwetsbare ouderen na acute- en of electieve chirurgie?
Aanbeveling
Organiseer een samenwerkingsvorm waar medebehandeling door de geriatrie/ouderengeneeskunde samen met de hoofdbehandelaar laagdrempelig of standaard ingezet wordt bij kwetsbare ouderen die electieve of acute chirurgie hebben ondergaan.
Richt een multidisciplinair overlegmoment in, waarbij alle betrokken (para)medische disciplines vertegenwoordigd zijn, om de zorg van gecompliceerde en langdurig opgenomen kwetsbare ouderen te evalueren ten opzichte van diens doelen en wensen.
Inventariseer gezamenlijk en vroegtijdig de benodigde nazorg en ontslagbestemming voor kwetsbare ouderen en streef ernaar om de huisarts en/of specialist ouderengeneeskunde hierbij te betrekken.
Investeer in goede samenwerkingsverbanden met de eerste lijn, en de geriatrische revalidatiezorg in het bijzonder, om snel ontslag naar de juiste bestemming te bevorderen.
Overwegingen
Voor- en nadelen van de interventie en de kwaliteit van het bewijs
Uit een literatuuranalyse voor de richtlijn CGA en een literatuuranalyse voor de proximale femurfractuur bij ouderen, onderdeel van de oudere versie van de richtlijn Behandeling kwetsbare ouderen bij chirurgie, blijkt dat structurele medebehandeling door een geriatrisch expertise team bij patiënten ouder dan 70 jaar met een heupfractuur leidt tot minder postoperatieve complicaties tijdens de opname. Ook zijn er aanwijzingen dat de kans op overlijden afneemt, de kans op herstel tot oorspronkelijk functieniveau toeneemt en ook de kans op ontslag naar oorspronkelijke woonsituatie toeneemt.
In de huidige literatuuranalyse, die zich niet beperkt tot patiënten met een heupfractuur maar zich richt op kwetsbare ouderen die chirurgie hebben ondergaan, wordt een zeer lage tot lage bewijskracht gevonden voor de effectiviteit van (mede) behandeling van een professional(s) met geriatrische expertise (hierna: co-management) bij kwetsbare ouderen die een chirurgische ingreep hebben ondergaan. De belangrijkste reden voor de lage kwaliteit van bewijs is de inclusie van cohortstudies, en het gebrek aan RCT’s, wat kan leiden tot vertekening van de resultaten. Daarnaast zijn veel uitkomstmaten maar bij kleine aantallen patiënten onderzocht. Verder bestond de interventie vaak niet alleen uit co-management, maar werd aanvullend een preoperatief comprehensive geriatric assessment (CGA) gedaan of werd er een snellere doorstroming van opname tot ontslag gewaarborgd bij de interventiegroep, terwijl dit niet bij de controlegroep werd gedaan.
De gevonden studies laten een positief en duidelijk significant effect van co-management zien op enkele patiëntuitkomsten: postoperatieve complicaties (overall) met een relatieve risicoreductie van 26% (Hafner (2021) Yee (2022) Bub (2022) Fan (2021) Kalmet (2019) McDonald (2018)), postoperatieve urineweginfecties (Bub (2022) Fan (2021) Yee (2022) Natesan (2022)) en pneumonie (Bub (2022) Fan (2021) Giannotti (2022) Natesan (2022)), en heropname na 30 dagen (McDonald (2018) Giannotti (2022) Natesan (2022) Pernik (2021)). De bewijskracht hiervan is laag te noemen. Er werd ook een positief effect gevonden op zelfstandig functioneren (ADL) (Yee (2022)), maar de bewijskracht hiervan is zeer laag. Voor de andere gedefinieerde uitkomstmaten zoals delier, kwaliteit van leven, mortaliteit, opnameduur en ontslagbestemming, werd geen effect aangetoond. Ook van deze laatste uitkomstmaten is de bewijskracht zeer laag te noemen.
Desondanks is een deel van de factoren die de studies vertekenen ten dele wel toe te schrijven aan de interventie, zoals het doen van een CGA. Welk deel van de interventie precies tot welk gevolg leidt, is dan echter niet te onderscheiden. De werkgroep is van mening dat de gevonden positieve effecten toch representatief kunnen zijn voor het werkelijke effect van co-management bij deze patiëntengroep.
Specificering van de meest doelmatige invulling van geriatrische medebehandeling is niet te geven door de heterogeniteit van de interventies. Daarnaast is bij deze literatuurstudie gezocht met de term ‘co-management´ als synoniem voor de Nederlandse term medebehandeling. De term ‘multidomain intervention´ of ‘multidisciplinary management’ wordt echter ook regelmatig gebruikt in Engelse literatuur om medebehandeling te omschrijven.
Internationale richtlijnen
Met de aanbevelingen in deze module sluiten wij aan bij een internationale beweging om de postoperatieve zorg voor kwetsbare ouderen multidisciplinair in te richten, waarbij geriatrische expertise wordt geborgd. In internationale richtlijnen worden vergelijkbare adviezen gegeven om óf geriatrische medebehandeling óf specifieke geriatrische zorg te bieden in de postoperatieve fase om de kans op complicaties te verkleinen.
In de richtlijn Hip fracture management, laatst geüpdatet in 2023, van het National Institute of Health and Care excellence (NICE) uit de Verenigd Koninkrijk wordt bijvoorbeeld aanbevolen om ‘multidisciplinary management’ te bieden bij patiënten die orthopedische chirurgie ondergaan waarbij specifiek geadviseerd wordt om postoperatief te beoordelen op een delier en delierpreventieve maatregelen in te zetten (NICE, 2023).
In de richtlijn The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Perioperative Evaluation and Management of Frailty Among Older Adults Undergoing Colorectal Surgery (Saur et al., 2022) wordt beschreven dat kwetsbare ouderen mogelijk baat hebben van een multidisciplinaire aanpak van perioperatieve zorg met daarbij specifiek betrokkenheid van een zorgprofessional met geriatrische expertise. Hierbij refereren zij o.a. naar de studie van Shahrokni et al. uit 2022. Deze studie beschrijft dat de kans op 90 dagen mortaliteit in de groep van kwetsbare ouderen met meer dan 50% afnam nadat geriatrische medebehandeling na oncologische chirurgie werd ingevoerd. Ook werd er meer gebruik gemaakt van paramedische inzet. Een andere studie beschrijft dat er minder ´geriatrische´ complicaties voorkomen in de geriatrische medebehandeling groep (Tarazona-Santabalbina et al., 2019). The American Society of Colon and Rectal Surgeons voerde een review van 12 andere studies uit, waaruit ook blijkt dat geriatrische medebehandeling leidt tot korte opnameduur, lagere mortaliteit en kleinere kans op heropnames. Ook adviseren zij sterk dat er postoperatief beoordeeld moet worden op een delier met zonodig behandeling. Op basis van deze aanbevelingen is een programma ontwikkeld om perioperatieve zorg te optimaliseren voor de kwetsbare ouderen genaamd de Geriatric Surgery Verification Program in 2019 (American College of Surgeons, 2019). Ook hier adviseren zij om postoperatief bij hoog risicopatiënten (die als zeer kwetsbaar beschouwd worden op basis van geriatrische screeningsinstrumenten) multidisciplinaire zorg te bieden met onder andere een zorgprofessional met geriatrische expertise. Daarnaast adviseren zij ook dat kwetsbare ouderen postoperatief gescreend moeten worden op een delier en hier adequaat voor behandeld moeten worden.
Ook de richtlijn ‘Perioperative Care of People Living with Frailty van Centre of Perioperative Care’ (Centre for Perioperative Care, 2021) uit het Verenigd Koningrijk beschrijft dat elk ziekenhuis een perioperatief ´kwetsbaarheid´ team zou moeten hebben met geriatrische expertise die gedurende het gehele zorgpad aangeboden wordt. Hierbij dient specifiek aandacht te zijn voor postoperatieve complicaties, revalidatie en ontslagbestemming, proactieve zorgplanning, effectieve communicatie met patiënt en familie en er moet zorg worden gedragen voor gestructureerde samenwerking met andere zorgprofessionals van andere specialismen.
Waarden en voorkeuren van patiënten (en evt. hun verzorgers)
In het kader van postoperatieve zorg bij kwetsbare ouderen zijn de wensen en behoeftes van de patiënt en zijn omgeving erg belangrijk. Het is belangrijk dat de behoefte en niveau van benodigde ondersteuning wordt ingeschat alsook de omvang en belastbaarheid van het sociaal vangnet waarover de patiënt beschikt. Herstel- en revalidatiedoelen moeten worden besproken.
De doelen en wensen van de patiënt zijn bij voorkeur preoperatief al in kaart gebracht (zie module 3). Echter, in geval van spoed chirurgie kan dit in sommige gevallen pas postoperatief plaatsvinden. In dat geval moeten de doelen en wensen geïnventariseerd worden op het vroegst mogelijk moment in de postoperatieve zorg van de patiënt.
In het beste geval gebeurt deze inventarisatie multidisciplinair in het kader van co-management waarbij gelet kan worden op praktische (on)mogelijkheden op basis van beschikbaarheid van disciplines (of diens vertegenwoordigers), moment van de dag (avond- nacht- en weekend), aard en ernst van eventuele spoedeisendheid van de ingreep.
Kosten (middelenbeslag)
Kosteneffectiviteit van gestructureerde postoperatieve geriatrische medebehandeling is met name onderzocht voor de orthogeriatrische samenwerking. Allereerst concludeerde een systematic review en meta-analyse van Van Heghe et al. (2022) op basis van vijf eerdere studies dat orthogeriatrische samenwerking waarschijnlijk leidt tot lagere kosten. Met name de geïncludeerde studie van Miura et al. (2009) liet een significante afname van kosten zien na invoering van de samenwerking. Nieuwe studies laten ook een significante kostenreductie zien door orthogeriatrische samenwerking (Breda et al., 2023). Baji et al. (2023) identificeerde organisatie-gerelateerde factoren die geassocieerd waren met mortaliteit en kosten bij ouderen (>60 jaar) met een heupfractuur met behulp van een grote registratiedatabase van 178.757 patiënten uit 172 ziekenhuizen in het Verenigd Koninkrijk. Deze studie toonde dat betrokkenheid van een orthogeriater (geriater met orthopedische expertise) binnen 72 uur na opname geassocieerd was met een gemiddelde kostenbesparing van £529 (95% CI £148-910) per patiënt. Wanneer een orthogeriater daarnaast ook aanwezig was bij de grote visite was dit geassocieerd met een kostenreductie van £356 (95% CI £188-525) per patiënt. Naar kosteneffectiviteit van gestructureerde postoperatieve geriatrische medebehandeling bij andere vormen van (electieve of acute) chirurgie is weinig onderzoek gedaan. Een retrospectieve studie van Cizginer et al. (2023) bij oudere patiënten met colorectale chirurgie liet een kostenreductie van $10.297 per patiënt zien na invoering van structurele postoperatieve geriatrische medebehandeling. Op gebied van arbeidsintensiteit, benodigd personeel en middelen zal medebehandeling duurder zijn. Echter, het is aannemelijk dat wanneer medebehandeling van de kwetsbare oudere leidt tot een afname van complicaties en ligduur, dit ook bij andere typen operaties tot kostenreductie zal leiden. Bovendien kan intensievere begeleiding en oog voor patiënt, omgeving en hun kwaliteit van leven tot positievere ervaring van het ziekteproces leiden en daarmee ook tot goed sociaal en maatschappelijk draagvlak voor co-management.
Aanvaardbaarheid, haalbaarheid en implementatie
De literatuur voorziet niet specifiek in een advies, heldere onderbouwing of praktische invulling van postoperatieve samenwerking bij kwetsbare ouderen na chirurgie. De samenwerkingsvormen die beschreven worden in de gevonden literatuur betreffen vaak een preoperatief CGA en postoperatief structurele medebehandeling met dagelijkse, twee- of driewekelijkse beoordelingen van een zorgprofessional met geriatrische expertise samen met het chirurgische team en daarnaast ook dagelijks of wekelijks een multidisciplinair overleg.
Voor de postoperatieve zorg van kwetsbare ouderen is aan te bevelen dat er afspraken worden gemaakt over de samenwerkingsvorm met de geriatrie/ouderengeneeskunde. Bij voorkeur moet er sprake zijn van intensieve samenwerking waarbij de geriatrie/ouderengeneeskunde standaard in medebehandeling is bij kwetsbare ouderen. De vorm waarin deze medebehandeling geboden wordt, kan op verschillende manieren ingezet worden, bijvoorbeeld standaard medebehandeling de eerste 3 dagen na operatie, of aansluiten van het geriatrisch expertise team bij een reguliere (multidisciplinaire) bespreking, danwel het overdragen van het hoofdbehandelaarschap wat bij de orthogeriatrische patiënten vaak gedaan wordt. De precieze functie van de afvaardigingen van de betrokken disciplines (bijvoorbeeld medisch specialist, arts-assistent, verpleegkundig specialist, physician assistant etc.) kan ook variabel zijn. Hierbij moeten duidelijke lokale afspraken gemaakt worden over de betrokkenheid van elk van deze disciplines.
Op geleide van de gezamenlijke beoordeling van de patiënt kunnen laagdrempelig andere disciplines betrokken worden. Hierbij kan gedacht worden aan vertegenwoordiging van fysiotherapie, diëtiek, ergotherapie, maatschappelijk werk etc. De anesthesioloog is standaard betrokken in de pre- en peroperatieve fase, en postoperatief vaak in de vorm van het acute pijnteam. De intensive care kan laagdrempelig gevraagd worden om een kwetsbare oudere mee te beoordelen in de kliniek indien nodig.
Een wekelijkse bijeenkomst waarin vertegenwoordigers van alle betrokken disciplines aanwezig zijn, kan de samenwerking en kwaliteit van zorg mogelijk verder verbeteren. In de praktijk lijkt het bij kwetsbare ouderen die een gecompliceerd beloop hebben of langdurig zijn opgenomen moeilijk om de behandeling aan te passen dan wel te stoppen als hun kwaliteit van leven sterk verminderd lijkt te zijn met beperkte kans op herstel. Specifiek bij deze patiëntengroep is het evalueren van de zorg, eventueel opnieuw op basis van de vier assen van de geriatrie/ouderengeneeskunde, zeer belangrijk. Dit kan laagdrempelig worden gedaan in deze wekelijkse bijeenkomst waarbij ook de benodigde nazorg en passende ontslagbestemming besproken kan worden
Voor veelvoorkomende patiënt categorieën kunnen deze werkafspraken in een lokaal nazorgprotocol worden vastgelegd die centraal via de instelling beschikbaar zijn.
Rationale van de aanbeveling: weging van argumenten voor en tegen de interventies
In de voorgaande versie van de richtlijn ‘Behandeling kwetsbare ouderen bij chirurgie’ en de richtlijn ‘Comprehensive Geriatric Assessment’ zijn de bewijskracht en overwegingen voor geriatrische medebehandeling bij patiënten met een heupfractuur uitgebreid beschreven. Dit leidde tot de aanbeveling om structurele geriatrische medebehandeling in te zetten voor patiënten van 70 jaar en ouder met en heupfractuur (Zie richtlijn Comprehensive Geriatric Assessment, module 8.3). Voor patiënten met electieve operaties, of een niet-orthopedische acute operatie is minder bewijskracht. Dit wordt veroorzaakt door het grotendeels afwezig zijn van randomised controlled trials (RCT’s) en de heterogeniteit van de interventies. Echter, vele observationele voor-na studies laten een gunstig effect zien van geriatrische medebehandeling op het verbeteren van postoperatieve uitkomsten voor ouderen. Op basis van de meta-analyse van de literatuur voor deze richtlijn is geriatrische medebehandeling geassocieerd met een lagere kans op postoperatieve complicaties en heropnames <30 dagen, en infecties. Het is dan ook aannemelijk dat het organiseren van een samenwerkingsvorm met postoperatief geriatrische medebehandeling voor kwetsbare ouderen zal leiden tot een significante verbetering van het postoperatief beloop en herstel, patiënttevredenheid en het postoperatief functioneren.
Specificering van de meest doelmatige invulling van geriatrische medebehandeling is niet te geven door de heterogeniteit van de interventies. Het van belang dat er voor de daadwerkelijke invulling van de postoperatieve samenwerking gekeken wordt naar de lokale behoeftes en mogelijkheden. In Nederland zijn verschillende soorten samenwerkingsvormen ontstaan die als ´best practices´ gezien kunnen worden, waaronder de Geriatrische Trauma Unit. In 2024 zal er een handboek verschijnen waarin wordt beschreven hoe dit het beste te organiseren. Belangrijk is dat voor de invulling van de postoperatieve samenwerking voor kwetsbare ouderen op lokaal niveau, de snijdende specialismen en de geriatrie/interne geneeskunde-ouderengeneeskunde samen de verbinding zoeken om dit uit te voeren en te coördineren.
Een potentieel nadeel van geriatrische medebehandeling en betrokkenheid van andere (para)medische diensten is de arbeidsintensiteit en daarmee gepaard gaande kosten. Echter, de verwachting is ook dat deze investering zal leiden tot minder complicaties, kortere ligduur, en minder heropnames. Hiermee worden ook kosten bespaard. Ook is aangetoond dat orthogeriatrische samenwerkingen voor de kwetsbare ouderen met heupfracturen kosteneffectief is. Tevens zal deze multidisciplinaire inspanning ertoe leiden dat het behandelplan voor patiënten met een gecompliceerd beloop regelmatig geëvalueerd zal worden, en dat de wensen en doelen van de patiënt hierin worden meegenomen. Ook zal dit bijdragen aan vroegtijdige nazorg en ontslagplanning. In het bijzonder indien er ook geïnvesteerd wordt in een (structurele) samenwerkings- of overlegvorm met de eerstelijns- of geriatrische revalidatiezorg. Daarbij heeft het ook de voorkeur om de ontslagbestemming reeds preoperatief te bespreken met patiënt, familie, (mede)behandelaren zowel in de eerstelijns- als geriatrische revalidatiezorg. Deze inspanningen zullen leiden tot het leveren van passende zorg op de juiste plek en snellere doorstroming.
Onderbouwing
Achtergrond
Kwetsbare ouderen worden steeds vaker naar het ziekenhuis verwezen voor een operatieve behandeling. Dit kan zowel acuut als electief zijn. Op dit moment bestaat er praktijkvariatie óf, en op welke manier het specialisme geriatrie of interne geneeskunde-ouderengeneeskunde betrokken wordt in de postoperatieve fase. In de postoperatieve fase zijn er in de Nederlandse zorg verschillende samenwerkingsvormen ontstaan. Samenwerking kan variëren van een eenmalig consult tot aan een geïntegreerd team met geriatrische en chirurgische expertise dat gezamenlijk intensieve zorg biedt in het postoperatieve traject. Het bekendste voorbeeld daarvan is de Geriatrische Trauma Unit (GTU) waarbij er standaard medebehandeling is van het geriatrisch expertise team bij patiënten van 70 jaar of ouder met een heupfractuur. Op dit moment is het onduidelijk hoe de postoperatieve samenwerking het beste ingericht kan worden en wat de effectiviteit daarvan is. Deze module beschrijft de verschillende samenwerkingsvormen die er zijn vanuit de literatuur met daarbij postoperatieve uitkomsten en geeft aanbevelingen hoe dit in de dagelijkse praktijk te organiseren.
Conclusies
Functioning
Mobility
Postoperative mobilization
|
Very low GRADE
|
The evidence is very uncertain about the effect of co-management on postoperative mobilization when compared with control in frail older patients.
Source: Hafner (2021); Pernik (2021) |
Transfers
|
Very low GRADE
|
The evidence is very uncertain about the effect of co-management on the ability to make transfers when compared with control in frail older patients.
Source: Yee (2022) |
Activities of daily living
|
Very low GRADE
|
Co-management may increase independence in ADL when compared with control in frail older patients. The evidence is very uncertain.
Source: Yee (2022) |
Instrumental activities of daily living
|
No GRADE
|
No evidence was found regarding the effect of co-management on instrumental activities of daily living when compared with control in frail older patients. |
Cognitive disorders
|
No GRADE
|
No evidence was found regarding the effect of co-management on cognitive disorders in frail older patients. |
Quality of life
|
Very low GRADE
|
The evidence is very uncertain about the effect of co-management on quality of life when compared with control in frail older patients.
Source: Kalmet (2019) |
Post-operative Complications
|
Low GRADE
|
Co-management may reduce the incidence of overall post-operative complications when compared with control in frail older patients.
Source: Bub (2022); Fan (2021); Hafner (2021); Kalmet (2019); McDonald (2018); Yee (2022) |
Delirium
|
Very low GRADE
|
The evidence is very uncertain about the effect of co-management on post-operative delirium when compared with control in frail older patients.
Source: Fan (2021); Hafner (2021); Kalmet (2019); McDonald (2018); Natesan (2022); Pernik (2021); Yee (2022) |
Infections
Urinary tract infection
|
Low GRADE
|
Co-management may reduce the occurrence of post-operative urinary tract infections compared with control in frail older patients.
Source: Bub (2022); Fan (2021); Natesan (2022); Yee (2022) |
Pneumonia
|
Low GRADE
|
Co-management may reduce the occurrence of post-operative pneumonia compared with control in frail older patients.
Source: Bub (2022); Fan (2021); Natesan (2022); Giannotti (2022) |
Sepsis
|
Very low GRADE
|
Co-management may reduce the occurrence of post-operative sepsis compared with control in frail older patients. The evidence is very uncertain.
Source: Giannotti (2022) |
Falls
In-hospital falls
|
Very low GRADE
|
The evidence is very uncertain about the effect of co-management on in-hospital falls when compared with control in frail older patients.
Source: Hafner (2021) |
Cardiopulmonary complications
Pulmonary complications
|
Very low GRADE
|
The evidence is very uncertain about the effect of co-management on post-operative pulmonary complications when compared with control in frail older patients.
Source: Hafner (2021) |
Cardiac complications
|
Very low GRADE
|
The evidence is very uncertain about the effect of co-management on post-operative cardiac complications when compared with control in frail older patients.
Source: Hafner (2021) |
Myocardial infarction
|
Very low GRADE
|
The evidence is very uncertain about the effect of co-management on post-operative myocardial infarction when compared with control in frail older patients.
Source: Bub (2022); Natesan (2022); Fan (2021) |
Thromboembolic complications
|
Very low GRADE
|
The evidence is very uncertain about the effect of co-management on post-operative thromboembolic complications when compared with control in frail older patients.
Source: Bub (2022); Fan (2021) |
Cerebral vascular accident
|
Very low GRADE
|
The evidence is very uncertain about the effect of co-management on post-operative cerebral vascular accidents when compared with control in frail older patients.
Source: Bub (2022); Fan (2021) |
Haematological complications
|
Very low GRADE
|
Co-management may reduce haematological complications when compared with control in frail older patients. The evidence is very uncertain.
Source: Giannotti (2022) |
Length of stay
|
Very low GRADE
|
The evidence is very uncertain about the effect of co-management on length of stay when compared with control in frail older patients.
Source: Bub (2022); Fan (2021); Giannotti (2022); Hafner (2021); Kalmet (2019); McDonald (2018); Natesan (2022); Shahrokni (2020); Pernik (2021); Yee (2022) |
Discharge destination
Facility
|
Very low GRADE
|
The evidence is very uncertain about the effect of co-management on discharge destination when compared with control in frail older patients.
Source: Bub (2022); McDonald (2018) |
Returning home (with self-care)
|
Very low GRADE
|
The evidence is very uncertain about the effect of co-management on returning home when compared with control in frail older patients.
Source: Bub (2022); McDonald (2018); Yee (2022) |
Usage of health services when returning home
|
Very low GRADE
|
The evidence is very uncertain about the effect of co-management on usage of health services when returning home when compared with control in frail older patients.
Source: McDonald (2018) |
Readmission
7-days readmission
|
Very low GRADE
|
The evidence is very uncertain about the effect of co-management on 7-days readmission when compared with control in frail older patients.
Source: McDonald (2018); Pernik (2021) |
28-days readmission
|
Very Low GRADE
|
The evidence is very uncertain about the effect of co-management on 28-days readmission rates when compared with control in frail older patients.
Source: Yee (2022) |
30-days readmission
|
Low GRADE
|
Co-management may reduce 30-days readmission rates when compared with control in frail older patients.
Source: McDonald (2018); Natesan (2022); Pernik (2021); Giannotti (2022) |
90-days readmission
|
Very low GRADE
|
The evidence is very uncertain about the effect of co-management on 90-days readmission when compared with control in frail older patients.
Source: Giannotti (2022); Pernik (2021) |
1-year readmission
|
Very low GRADE
|
Co-management may reduce rates of 1-year readmissions when compared with control in frail older patients. The evidence is very uncertain.
Source: Giannotti (2022) |
Mortality
30-day mortality
|
Very low GRADE
|
The evidence is very uncertain about the effect of co-management on 30-day mortality when compared with control in frail older patients.
Source: Fan (2021); Giannotti (2022); Kalmet (2019); Yee (2022) |
90-day mortality
|
Very low GRADE
|
The evidence is very uncertain about the effect of co-management on 90-day mortality when compared with control in frail older patients.
Source: Giannotti (2022); Shahrokni (2020) |
1-year mortality
|
Very low GRADE
|
The evidence is very uncertain about the effect of co-management on 1-year mortality when compared with control in frail older patients.
Source: Giannotti (2022); Kalmet (2019); Yee (2022) |
In-hospital mortality
|
Very low GRADE
|
The evidence is very uncertain about the effect of co-management on in-hospital mortality when compared with control in frail older patients.
Source: Bub (2022); Fan (2021); Hafner (2021) |
Overgenomen zoekvraag
De zoekvraag en samenvatting van de literatuur uit de module ‘Herstelmaatregelen na proximale femurfractuur’ uit 2016 is toegevoegd, omdat voor de literatuursearch bij de nieuwe richtlijn studies geïncludeerd zijn vanaf 2016. De bewijskracht van alle eerdere studies is echter van blijvende relevantie voor met name de oudere patiënten met een proximale femurfractuur.
Uitgangsvraag
Welke maatregelen kunnen functioneel herstel bevorderen en mortaliteit voorkomen bij een kwetsbare oudere patiënt na een operatie voor een proximale femurfractuur?
Literatuurconclusies
Overgenomen van de module structurele medebehandeling van de richtlijn CGA bij consult en medebehandeling (NVKG, 2013).
|
Niveau 2 |
Het is aangetoond dat structurele medebehandeling dan wel structurele ortho-geriatrische medebehandeling door een geriatrisch expertise team bij patiënten ouder dan 70 jaar met proximale femurfractuur, leidt tot minder postoperatieve complicaties tijdens de ziekenhuisopname.
Bronnen (B: Fisher, 2006; Friedman, 2009; Lundstrum, 1998; Stenvall, 2007a; Stenvall, 2007b; Vidan, 2005) |
|
Niveau 3 |
Er zijn aanwijzingen dat structurele ortho-geriatrische medebehandeling voor patiënten ouder dan 70 jaar met proximale femurfractuur de kans op herstel tot oorspronkelijk functieniveau en de kans op behoud van de mobiliteit vergroot.
Bronnen (B: Leung, 2011; Lundstrum, 1998; Shyu, 2008; Stenvall, 2007a; Swanson, 1998; Vidan, 2005) |
|
Niveau 3 |
Er zijn aanwijzingen dat structurele ortho-geriatrische medebehandeling de kans op vallen en het valrisico verlaagt.
Bronnen (B: Stenvall, 2007b) |
|
Niveau 3 |
Er zijn aanwijzingen dat structurele ortho-geriatrische medebehandeling voor patiënten ouder dan 70 jaar met proximale femurfractuur de kans op ontslag naar oorspronkelijke woonsituatie verhoogt.
Bronnen (B: Lundstrum, 1998; Stenvall, 2007b) |
|
Niveau 3 |
Er zijn aanwijzingen dat structurele ortho-geriatrische medebehandeling voor patiënten ouder dan 70 jaar met proximale femurfractuur de kans op mortaliteit na 30 dagen en na één jaar vermindert.
Bronnen (B: Leung, 2011; Shyu, 2008) |
|
Niveau 3 |
Er zijn aanwijzingen dat structurele geriatrische medebehandeling bij patiënten ouder dan 70 jaar met proximale femurfractuur het aantal opnamedagen in het ziekenhuis verkort.
Bronnen (B: Friedman, 2009; Naglie, 2003; Stenvall, 2007a; Stenvall, 2007b; Swanson, 1998;) |
|
Niveau 2 |
Het is aannemelijk/waarschijnlijk dat standaard geïntegreerde orthopedische en geriatrische zorg voor patiënten ouder dan 70 jaar met proximale femurfractuur de kans op overlijden in het ziekenhuis vermindert.
Bronnen (A2: Fisher, 2006; Vidan, 2005) |
NOTE: Voor bovenstaande conclusies is de bewijskracht beoordeeld met behulp van de EBRO-methodiek. Dit is in tegenstelling met wat in de methodiek voor de ontwikkeling van deze richtlijn beschreven staat. Dit komt doordat bovenstaande conclusies letterlijk zijn overgenomen van de richtlijn CGA bij consult en medebehandeling (NVKG, 2013).
Samenvatting literatuur
In total, ten articles studied the effects of co-treatment of geriatricians in frail older people. All studies were cohort studies comparing a pre-intervention with post-intervention cohort. Most studies (n=5) focused on fragility hip fractures (Bub, 2022; Fan, 2022; Hafner, 2021; Kalmet, 2019; Yee, 2022), other studies focused on vascular surgery (Natesan, 2022), spine surgery (Pernik, 2021), abdominal surgery (McDonald, 2018), gastrointestinal surgery (Giannotti, 2022), surgery for various cancer types (Shahrokni, 2022).
Description of the included studies
Bub (2022) conducted a retrospective chart review study in patients ≥65 years with low-energy hip fractures at a large urban academic tertiary centre in the USA. They evaluated whether the implementation of a geriatrics-focused orthopaedic and hospitalist co-management program improved perioperative outcomes, including time to operating room, length of stay, perioperative complications, and mortality. This was compared with a historical cohort, in which patients with hip fractures were admitted to either orthopaedics or hospitalist service with the other on board as a consultant. The co-management program consisted of a hospitalist (e.g., trained geriatrics comanager) who comanaged all patients. Additionally, team members from orthopaedics, hospital medicine, nursing, and geriatrics rounded together two times a day and discussed medical and surgical management as well as psychosocial and discharge issues. The intervention group consisted of 162 patients, and the control group of 128 patients.
Fan (2021) conducted a pre-post retrospective study in patients ≥60 years with fragility fractures at a level 1 trauma centre in China. They assessed the efficacy of a multidisciplinary team co-management program (n=241) on time-to-surgery, length of stay, and postoperative complications within 30-days, compared with the historical traditional care model (n=249). The multidisciplinary team co-management program involved orthopaedic surgeons, geriatricians, anaesthesiologists, specialists of the intensive care unit, and physiotherapists. In this program, the geriatrician managed comorbidities and polypharmacy to make patients clinically stable and ready for surgery. The historical traditional care model did not include geriatric expertise.
Giannotti (2022) conducted a pre-post retrospective study in patients aged ≥ 70 years admitted for elective gastrointestinal cancer surgery or palliative treatments and required a hospital stay of at least one day, in a hospital in Italy. They studied the effects of a geriatric co-management model (n=90) to a surgeon-led model (n=117) and assessed post-operative complications. The geriatric co-management model consisted of daily targeted geriatrician-led ward rounds focusing on older patients with cancer. Prior to surgery, patients received a CGA and frailty assessment. During the inpatient postoperative period, patients were followed by the same geriatrician in a consulting role, with the surgical team in a primary role. The geriatric co-management group included a daily board round led by a geriatrician who discussed the care management during the clinical sessions. The surgeon-led model consisted of optional referral for a preoperative CGA based on clinical judgement, not on a formal frailty screening team. During hospitalization and the perioperative phase, patients were assessed daily by the surgical team and medical consultants were called in as needed.
Hafner (2021) performed a retrospective single-centre cohort study in patients ≥70 years with fragility fractures in a university hospital in Germany. They studied the effects of an ortho-geriatric model (n=224) on complications, delirium, mortality, time-to-surgery, length of stay, and other clinical outcomes, compared with usual care (n=137) before implementation of the intervention. The ortho-geriatric model involved patients who were admitted to and treated at the trauma surgery ward, with the routine consultation of a geriatrician in an interdisciplinary ward round twice a week and an interdisciplinary team conference once a week. Moreover, representatives of nursing, occupational therapy, physiotherapy, and case management took part in the treatment process from admission until discharge.
Kalmet (2019) conducted a retrospective cohort study in patients ≥65 years with a surgically treated low-energy fracture in a university hospital in the Netherlands. They compared patient-reported outcomes, such as pain and quality of life, between patients treated in the context of a multidisciplinary clinical pathway (n=182) and patients receiving usual care before implementation of the intervention (n=216). Quality of life was measured using the SF-12. The SF-12 measures various aspects of physical and mental health from which physical composite score (PCS) and mental composite score (MCS) can be calculated, ranging from a minimum of 0 to a maximum of 100 (Ware, 2002). The multidisciplinary clinical pathway involved an orthopaedic trauma surgeon, a geriatrician, an anaesthesiologist, and a physiotherapist. These disciplines are all actively involved in the decision-making process from presentation at the emergency department until discharge and other specialities could be involved when necessary.
McDonald (2018) performed a prospective cohort study in patients ≥85 years and patients ≥65 years at risk for complications who underwent elective abdominal surgery in a university hospital in the USA. They assessed clinical outcomes in patients receiving a collaborative intervention by surgery, geriatrics, and anaesthesia focused on perioperative health optimization (n=183) compared to a comparable group of patients before implementation of the intervention (n=143). The collaborative intervention consisted of a team including a geriatrician, geriatric resource nurse, social worker, program administrator, and nurse practitioner. These were all present during the preoperative visit and all participated in rounds on the in-patient geriatrics consult service.
Natesan (2022) performed a single-centre retrospective cohort study in patients ≥65 years, with one or more geriatric syndromes and one or more comorbidities, who were scheduled for vascular surgery or endovascular intervention in a tertiary hospital in Singapore. They studied the efficacy of a co-management model between geriatric medicine and vascular surgery services, on length of stay, readmission, mortality, and postoperative complications, compared to a retrospective similar cohort before implementation of the care model. The co-management model consisted of the geriatric team who visited patients once preoperative to optimize outcomes and postoperative on a daily basis to optimize comorbidity treatments and address postoperative complications early. Additionally, a practice geriatric nurse identified social support, caregiver stress, and rehabilitation potential. The intervention group included 198 patients and the control group consisted of135 patients.
Pernik (2021) conducted a retrospective cohort study in patients with an increased perioperative risk of adverse outcomes undergoing elective spine surgery in the USA. The study assessed the impact of an interdisciplinary perioperative intervention involving geriatrics, surgery, and anaesthesiology (n=147) compared to a matched historical control group treated with usual care (n=177) on delirium incidence, length of stay, and readmission rates. The interdisciplinary perioperative intervention consisted of a pre-operative assessment with a geriatrician, including a comprehensive assessment, which was communicated to the anaesthesia and surgical teams. Patients and families received specific education on delirium and delirium prevention. Approximately one or two weeks later, anaesthesia planning was performed in coordination with the geriatric and surgical teams. Postoperatively, patients were co-managed by the primary surgical and geriatric consult team daily until discharge. The geriatric team assisted with adherence to nonpharmacologic delirium prevention strategies, management of medical comorbidities, pain management, optimizing bowel and bladder function, nutritional status, avoidance of high-risk medications, administration of a daily delirium screen, and facilitating smooth transition to post-acute care.
Shahrokni (2020) conducted a retrospective cohort study in patients aged ≥ 75 years who underwent cancer related surgical treatment of various cancer types, and a hospital stay of at least 1 day. They studied the effects of a geriatric co-management model (n=1020) to a surgeon-led model (n=872), and assessed length of stay, 90-day mortality and adverse surgical outcomes within 30 days (major complications, readmission or emergency room visit). The geriatric co-management consisted of two phases: preoperative and postoperative care. Referral for pre-operative geriatric evaluation was based on the surgery teams’ clinical judgment. Preoperative evaluation consisted of a Rapid Fitness Assessment, discussion with the surgery and anaesthesiology team, and recommendations to optimize the patients’ status. Postoperatively, the geriatrics team visits all inpatients on day 1 and day 3, and further follow up if necessary. They assist with management of comorbid conditions, medication management, delirium-reducing interventions, stimulate early mobility, prevent complications and pain management. For the control group, the patients are not evaluated pre-operatively by a geriatrician and post-operatively only upon request.
Yee (2022) performed a prospective cohort study in patients ≥65 years with a low-energy hip fracture in three hospitals in Hong Kong. They studied the effect of an orthogeriatric co-management model on clinical outcomes (n=207), compared to a historical cohort (n=194) before implementation of the intervention (usual care). The orthogeriatric co-management model included a geriatrician during the postoperative phase, who co-managed the patient. Patients were co-managed by a geriatrician during combined ward rounds three times a week on the acute ward or in care of medical problems on the rehabilitation ward. The geriatrician actively reviewed all hip fracture patients to allow prompt diagnosis and management of medical complications, optimization of pain control and monitoring of comorbidities.
Results
Functional status
Postoperative mobilization
The cohort study of Hafner (2021) demonstrated that the number of patients with fragility fractures who were mobilized on the first day after surgery was statistically significantly (P<0.001) higher in the ortho-geriatric model compared with usual care before implementation of the intervention (Risk Ratio (RR) 1.52; 1.24-1.84).
In the cohort study of Pernik (2021) no difference was found in the mean number of days until walking in patients who underwent spine surgery (p=0.77; Mean Difference (MD)=0.0; 95% Confidence Interval (CI) not provided) between an interdisciplinary perioperative intervention compared with a historical usual care group.
Transfers (mobility)
In the study of Yee (2022) no statistically significant effect was found in transfer scores in patients with a fragility hip fracture between the orthogeriatric co-management model group and the historical cohort before implementation of the model (p=0.07; median (Interquartile range (IQR)) Intervention Group (IG) 12 (8) vs Control Group (CG) 9 (8); 95%CI not provided). The ability of patients to make a transfer was measured using the elderly mobility scale (EMS), which is a 20-point validated scale. A score >14 indicating an ability to perform a transfer alone and safely, a score between 10 and 14 indicating borderline in terms of safe mobility and independence in activities of daily living (ADL), and a score <10 indicating a high level of help with mobility and ADL.
Activities of Daily Living
The study of Yee (2022) demonstrated that use of an orthogeriatric co-management model increases the independence in ADL in patients with a fragility hip fracture, compared to a historical cohort before implementation of the model (p<0.001; median (IQR) IC 81 (27) vs CG 63.5 (28); 95%CI not provided). This study used the Modified Bartel ADL index (MBI), which is a 100-point validated scale. A lower score indicates more dependence and a higher score indicated more independence.
Instrumental Activities of Daily Living (i-ADL)
No study reported the outcome instrumental activities of daily living.
Cognitive disorders
No study reported the outcome cognitive disorders (except for delirium, see: complications)
Quality of life
The cohort study of Kalmet (2019) assessed quality of life (QoL) using the Short Form 12 (SF-12), two years after surgery. No difference was found in overall QoL between patients with fragility fractures receiving a multidisciplinary clinical pathway and patients receiving usual care before implementation of the pathway (p=0.65; IG 47.9 ± 24.4 vs CG 45.4 ± 27.6, MD 2.5). They found no difference in physical QoL between patients with fragility fractures receiving a multidisciplinary clinical pathway and patients receiving usual care before implementation of the pathway (p=0.93; IG 36.3 ± 28.6 vs CG 35.8 ± 28.9, MD 0.5). They found no difference in mental QoL between patients with fragility fractures receiving a multidisciplinary clinical pathway and patients receiving usual care before implementation of the pathway (p=0.45; IG 59.5 ± 25.0 vs CG 54.9 ± 31.0, MD 4.6).
Complications
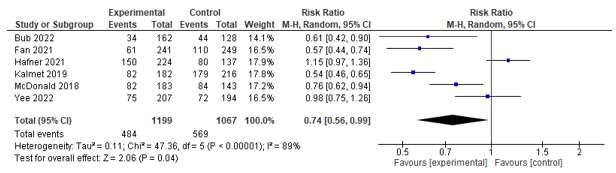
Six studies assessed the incidence of post-operative complications (<30 days). These studies were pooled, whereby a statistically significant effect between co-management and control in favour of co-management was found (RR 0.74 [0.56; 0.99]) (see Figure 1).
Figure 1. Forest plot depicting risk ratio for complications.
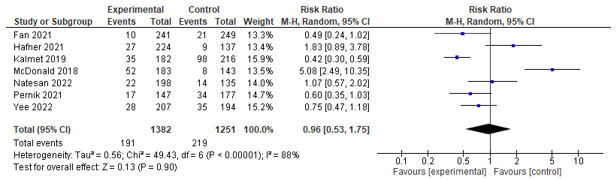
Delirium
Eight studies assessed post-operative delirium incidence, of which seven could be pooled. These studies could be pooled, but found no significant effect between co-management and control (RR 0.96; 0.53-1.75) (see Figure 2).
The pre-post study of Giannotti (2022) reported mean 4AT scores, of which a score of 5 or higher suggests delirium but is not diagnostic, and showed that 4AT scores were lower in the geriatric co-management group compared to control (p=0.03; 1.44 ± 2.62 vs. 2.19 ± 3.27).
Figure 2. Forest plot depicting risk ratio for delirium incidence.
Infections
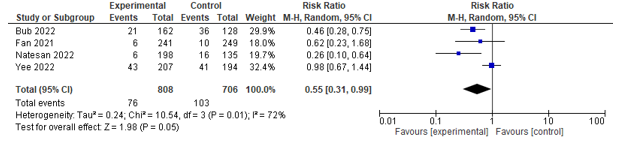
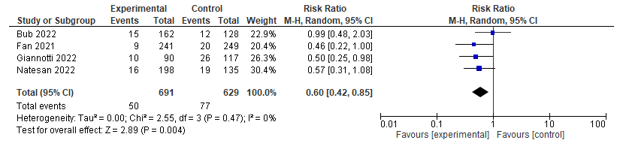
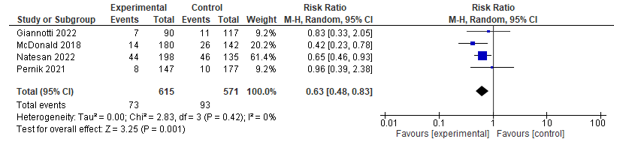
Six studies reported on the incidence of infections. Five studies assessed urinary tract infection rates and could be pooled, and a significant effect was found between co-management and control in favour of co-management (RR 0.55; 0.31-0.99) (see Figure 3). Four of these studies also assessed the incidence of pneumonia, and could be pooled, and a significant effect was found between co-management and control in favour of co-management (RR 0.60; 0.42-0.85) (see Figure 4).
Figure 3. Forest plot depicting risk ratio for urinary tract infection.
Figure 4. Forest plot depicting risk ratio for pneumonia.
The pre-post study of Giannotti (2022) reported on the incidence of bacteriemia/sepsis and reported lower rates of bacteriemia/sepsis in the geriatric co-management group (p <0.005; RR 0.33; 0.14-0.76).
Falls
One study reported on in-hospital falls, but no effect was found.
The cohort study of Hafner (2021) found no difference in the incidence of in-hospital falls between the ortho-geriatric model and usual care before implementation of the intervention in patients with fragility fractures (p=0.292; IG 1.3% vs CG 0%; 95%CI not estimable).
Cardiopulmonary complications
Four studies reported on cardiopulmonary complications, including pulmonary complications, cardiac complications and myocardial infarction. No effect was found.
Pulmonary complications
The cohort study of Hafner (2021) found no difference in the incidence pulmonary complications between the ortho-geriatric model and usual care before implementation of the intervention in patients with fragility fractures (p=0.072; IG 17.9% vs CG 12.4%; RR1.44; 0.85-2.4).
Cardiac complications
The cohort study of Hafner (2021) found no difference in the incidence of cardiac complications between the ortho-geriatric model and usual care before implementation of the intervention in patients with fragility fractures (p=0.405; IG 8.0% vs CG 8.8%; RR0.91; 0.46-1.8).
The pre-post study of Giannotti (2022) found no difference in the incidence of cardiovascular complications between groups (p=0.434; IG 17.8% CG 21.4%)
Myocardial infarction
The cohort study of Bub (2022) found no difference in the incidence of myocardial infarction between a geriatrics-focused co-management program compared with a historical care model in patients with fragility hip fractures (p=1.000; IG 0.6% vs CG 0.0%; 95%CI not estimable).
The cohort study of Natesan (2022) found no difference in the incidence of myocardial infarction between a co-management model and a retrospective cohort in patients scheduled for vascular surgery or endovascular interventions (p=n/s; IG 6% vs CG 6%; RR 0.94 [0.39;2.27]).
The cohort study of Fan (2021) found no difference in the incidence of acute coronary syndrome between a multidisciplinary team co-management program and a historical traditional care model in patients with fragility fractures (p=0.299; IG 3.3% vs CG 5.2%; RR0.64 [0.27;1.50]).
Thromboembolic complications
Three studies reported on thromboembolic complications. No effect was found.
The cohort study of Bub (2022) found no difference in deep vein thrombosis incidence between a geriatrics-focused co-management program compared with a historical care model in patients with fragility hip fractures (p=1.000; IG 0.6% vs CG 0.8%; 95%CI not provided).
The cohort study of Fan (2021) found that a multidisciplinary team co-management program in patients with fragility fractures decreases deep venous thrombosis incidence compared with a historical traditional care model (p=0.049; IG 7.9% vs CG 12.9%; RR0.56; 0.32-0.97).
The cohort study of Bub (2022) found no difference in the incidence of pulmonary embolism between a geriatrics-focused co-management program compared with a historical care model in patients with fragility hip fractures (p=1.000; IG 0.6% vs CG 0.0%; 95%CI not estimable).
Neurological complications
Two studies reported on the effects of co-management programs on the incidence of Cerebral Vascular Incidents. No effect was found. One study reported on overall neurological complications and also found no effect.
The cohort study of Bub (2022) found no difference in the incidence of cerebral vascular accident between a geriatrics-focused co-management program compared with a historical care model in patients with fragility hip fractures (p=0.194; IG 0.0% vs CG 1.6%; 95%CI not provided).
The cohort study of Fan (2021) found no difference in the incidence of cerebral vascular accident between a multidisciplinary team co-management program and a historical traditional care model in patients with fragility fractures (p=0.802; IG 2.1% vs CG 2.4%; RR0.86; 0.26-2.78).
The pre-post study of Giannotti (2022) found no difference on overall neurological complications between groups (p=0.202; IG 16.7% vs. CG 24.8%).
Haemotological complications
The pre-post study of Giannotti (2022) found fewer haematological complications in the geriatric co-management group (p=0.31; IG 7.8% vs. CG 19.7%; RR0.40; 0.18-0.88)
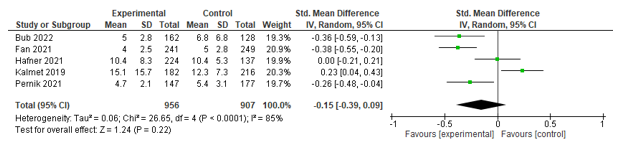
Length of stay
Ten studies assessed length of stay (LOS). Five of these could be pooled, but no significant effect between co-management and control was found (SMD -0.15; -0.39-0.09) (see Figure 5). McDonald (2018), Yee (2022), and Shahrokni (2022) could not be included in the meta-analysis because only medians and interquartile ranges were provided, and Natesan (2022) could not be included because only means were provided.
The cohort study of McDonald (2018) found that a collaborative intervention decreases median length of stay in patients with abdominal surgery compared with care before implementation of the intervention (p<0.001; median difference -2.0 days; 95%CI 1.1 to 4.2).
The cohort study of Natesan (2022) found that a co-management model decreases mean length of stay compared with a retrospective cohort in patients scheduled for vascular surgery or endovascular interventions (p=0.01; MD -9.2; 95%CI not provided).
The cohort study of Yee (2022) found that an orthogeriatric co-management model group decreases length of stay in patients with fragility hip fractures compared with the historical cohort before implementation of the intervention (p<0.001; median difference -2.0; 95%CI not provided).
The cohort study of Shahrokni (2020) found that the geriatric co-management group had a longer length of stay than the surgeon-led model (p<0.001); median difference -1.0 day; 95%CI not provided).
The pre-post study of Giannotti (2022) found no difference between groups (p=0.506; median difference 0.0 day; 95% CI not provided).
Figure 5. Forest plot depicting standardized mean difference for length of stay.
Discharge destination
Discharge to a facility
Two studies assessed the amount of patients that were discharged to a facility, but both found no effect.
The cohort study of Bub (2022) found no difference in the amount of patients with fragility hip fractures who were discharged to a skilled nursing facility between a geriatrics-focused co-management program compared with a historical care model (p=0.197; IG 39.5% vs CG 28.1%; 95%CI not provided).
The cohort study of McDonald (2018) found no difference in the amount of patients with abdominal surgery who were discharged to a facility between patients that received a collaborative intervention and patients that received care before implementation of the intervention (p=0.26; IG 14.2% vs CG 18.9%; 95%CI 0.39-1.28).
Living at home independently
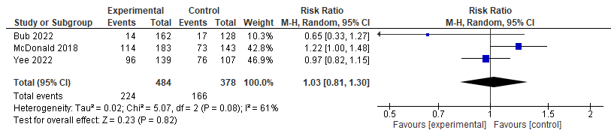
Three studies assessed the amount of patients who returned home without assistance. After pooling of the results, no significant effect between co-management and control was found (RR 1.03; 0.81- 1.30) (see Figure 6). Of these, one study also assessed the amount of patients who used health services when returning home, no effect was found.
Figure 6. Forest plot depicting risk ratio for returning home (with self-care).
Readmission
Four studies reported on readmission within different time frames. Two studies assessed 7-days readmission, of which one found no effect and the other found a positive effect in favour of co-management. One study assessed 28-days readmission, but found no difference between both groups. Three studies reported on 30-days readmission and could be pooled. A significant effect was found between co-management and control in favour of co-management (RR 0.61; 0.43-0.87) (see Figure 7). Lastly, one study assessed 90-days readmission, but found no effect.
7-days readmission
The cohort study of McDonald (2018) found that a collaborative intervention decreases 7-days readmission rates in patients with abdominal surgery compared with care before implementation of the intervention (p<0.001; IG 2.8% vs CG 9.9%; 95%CI 0.09-0.74).
The cohort study of Pernik (2021) found no difference in 7-days readmission rates in patients who underwent spine surgery between an interdisciplinary perioperative intervention compared with a historical usual care group (p>0.99; IG 2.1% vs CG 2.3%; RR0.9; 0.21-3.97).
28-days readmission
The cohort study of Yee (2022) found no difference in 28-days unplanned readmission rates in patients with fragility hip fractures between the orthogeriatric co-management model group and the historical cohort before implementation of the intervention (p=0.55; IG 12.6% vs CG 14.9%; 95%CI not provided).
30-days readmission
Four studies assessed the difference in 30-day readmission rates between groups, and a pooled effect in favour of geriatric co-management was found (RR0.63; 0.48-0.83) (see Figure 7).
Figure 7. Forest plot depicting risk ratio for 30-days readmission.
90-days readmission
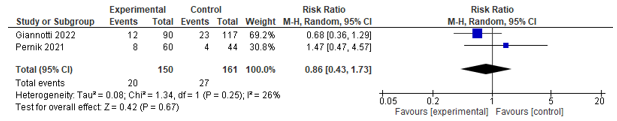
Two studies assessed the difference in 90-day readmission rates, no difference was found between groups (See Figure 8).
Figure 8. Forest plot depicting risk ratio for 90-day readmission.
1-year readmission
The pre-post study of Giannotti (2022) found fewer rehospitalizations in the geriatric co-management group compared to the surgery-led group (p=0.058; IG 23.5% vs CG 35.9%; RR0.65; 0.42-1.02).
Mortality
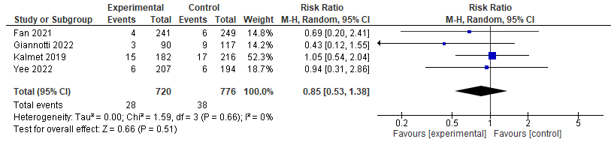
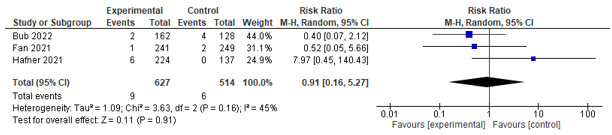
Three studies assessed mortality on different time frames; four studies assessed 30-day mortality and could be pooled. However, no significant effect was found between co-management and control (RR 0.85; 0.53-1.38) (see Figure 9). Three studies assessed in-hospital mortality, and could be pooled. However, no significant effect between co-management and control was found (RR 0.91; 0.16-5.27) (see Figure 10). Aadditionally, two studies reported on 1-year mortality, and both found no difference.
Figure 9. Forest plot depicting risk ratio for 30-day mortality.
Figure 10. Forest plot depicting risk ratio for in-hospital mortality.
90-day mortality
The cohort study of Shahrokni (2020) found that patients in the geriatric co-management group were less likely to die within 90 days after surgical treatment (p<0.001; OR 0.43 96%CI 0.28-0.67).
The pre-post study of Giannotti (2022) found no difference between groups (p=0.89; RR1.06; 0.46-2.41)
1-year mortality
The cohort study of Yee (2022) found no difference in 1-year mortality rates in patients with fragility hip fractures between the orthogeriatric co-management model group and the historical cohort before implementation of the intervention (p=0.24; difference 3.3%; 95%CI -3.1% to 9.7%).
The cohort study of Kalmet (2019) found no difference in 1-year mortality rates between a multidisciplinary clinical pathway compared with usual care before implementation of the pathway in patients with fragility fractures (p=0.50; IG 33.5% vs CG 37.0%; RR0.90 [0.69;1.18]).
The pre-post study of Giannotti (2022) found no difference in 1-year mortality rates between groups (p= 0.15 IG18.9% vs. CG12.8%; RR1.60 [0.84;3.03])
Level of evidence of the literature
The level of evidence regarding all outcomes started at Low because all included studies are observational (cohort) studies. The reasons for downgrading the evidence from ‘Low’ (cohort study) to ‘Very low’ are given below. When assessment for reasons to downgrade the evidence level led to the conclusion that no reason for downgrading was present, an assessment was made for any special strengths that would allow for an increase in the level of evidence.
Postoperative mobilization
The level of evidence regarding the outcome measure postoperative mobilization was downgraded by 1 level because of conflicting results (inconsistency) and small study population (imprecision) to Very low.
Transfers
The level of evidence regarding the outcome measure transfers was downgraded by 1 level because only one study was included in the analysis, and no effect was shown (imprecision), to Very low.
Activities of daily living
The level of evidence regarding the outcome measure activities of daily living was downgraded by 1 level because only one small study was included in the analysis (imprecision) to Very low.
Instrumental activities of daily living
No evidence was found for this outcome.
Cognitive disorders
No evidence was found for this outcome.
Quality of life
The level of evidence regarding the outcome measure quality of life was downgraded by 1 level because only one small study was included in the analysis and no effect was shown (imprecision) to Very low.
Complications
The level of evidence regarding the outcome measure complications was not downgraded and remained at Low. There were no reasons to increase the level of evidence.
Delirium
The level of evidence regarding the outcome measure delirium was downgraded by 1 level because of conflicting results (inconsistency), and no effect shown (imprecision).
Urinary tract infections
The level of evidence regarding the outcome measure urinary tract infections was not downgraded and remained at Low. There were no reasons to increase the level of evidence.
Pneumonia
The level of evidence regarding the outcome measure pneumonia was not downgraded and remained at Low. There were no reasons to increase the level of evidence.
Sepsis
The level of evidence regarding the outcome measure bacteraemia/sepsis was downgraded by 1 level because the sample size was too small (inconsistency).
In-hospital falls
The level of evidence regarding the outcome measure in-hospital falls was downgraded by 1 level to Very low because only one small study was available for the analysis and no effect was shown (imprecision).
Pulmonary complications
The level of evidence regarding the outcome measure pulmonary complications was downgraded by 1 level to Very low because only one small study was available for analysis and no effect was shown (imprecision).
Cardiac complication
The level of evidence regarding the outcome measure cardiac complications was downgraded by 1 level to Very low because only one small study was available for analysis and no effect was shown (imprecision).
Myocardial infarction
The level of evidence regarding the outcome measure myocardial infarction was downgraded by 1 level to Very low because the confidence interval includes the null effect (imprecision).
Thromboembolic complications
The level of evidence regarding the outcome measure deep venous thrombosis was downgraded by 1 level to Very low because of conflicting results (inconsistency) and small study population without effect shown (imprecision).
Cerebral vascular accident
The level of evidence regarding the outcome measure cerebral vascular accident was downgraded because of study limitations (risk of bias), conflicting results (inconsistency) and because the confidence interval includes the null effect (imprecision).
Haematological complications
The level of evidence regarding the outcome measure haematological complications was downgraded by 1 level because the sample size was too small (inconsistency).
Length of stay
The level of evidence regarding the outcome measure length of stay was downgraded by 1 level to Very low because of conflicting results (inconsistency), and because the confidence interval includes the null effect (imprecision).
Discharge destination: facility
The level of evidence regarding the outcome measure discharge destination was downgraded by 1 level to Very low because the analysis only includes one study and no effect is shown (imprecision).
Discharge destination: living at home independently
The level of evidence regarding the outcome measure living at home independently was downgraded by 1 level to Very low because of conflicting results (inconsistency), and wide 95%CI including the null effect (imprecision).
7-days readmission
The level of evidence regarding the outcome measure 7-days readmission was downgraded by 1 level to Very low because of a small population size and no effect was shown (imprecision).
28-days readmission
The level of evidence regarding the outcome measure 28-days readmission was downgraded by 1 level to Very low because only one study was available for analysis (imprecision).
30-days readmission
The level of evidence regarding the outcome measure 30-days readmission was not downgraded and remained at Low. There were no reasons to increase the level of evidence.
90-days readmission
The level of evidence regarding the outcome measure 90-days readmission was downgraded by 1 level to Very low because of conflicting results (inconsistency) and no effect was found (imprecision).
1-year readmission
The level of evidence regarding the outcome measure 1-year readmission was downgraded by 1 level because the sample size was too small (inconsistency).
In-hospital mortality
The level of evidence regarding the outcome measure in-hospital mortality was downgraded by 1 level to Very low because of conflicting results (inconsistency), and wide 95%CI including the null effect (imprecision).
30-day mortality
The level of evidence regarding the outcome measure 30-day mortality was downgraded by 1 level to Very low because of conflicting results (inconsistency), and wide 95%CI including the null effect (imprecision).
90-day mortality
The level of evidence regarding the outcome measure 30-day mortality was downgraded by 1 level to Very low because of conflicting results (inconsistency), and wide 95%CI (imprecision).
1-year mortality
The level of evidence regarding the outcome measure 1-year mortality was downgraded by 1 level to Very low because the study population was small, and no effect was found (imprecision).
Overgenomen zoekvraag
De zoekvraag en samenvatting van de literatuur uit de module ‘Herstelmaatregelen na proximale femurfractuur’ uit 2016 is toegevoegd, omdat voor de literatuursearch bij de nieuwe richtlijn studies geïncludeerd zijn vanaf 2016. De bewijskracht van alle eerdere studies is echter van blijvende relevantie voor met name de oudere patiënten met een proximale femurfractuur.
Uitgangsvraag
Welke maatregelen kunnen functioneel herstel bevorderen en mortaliteit voorkomen bij een kwetsbare oudere patiënt na een operatie voor een proximale femurfractuur?
Literatuurconclusies
Overgenomen van de module structurele medebehandeling van de richtlijn CGA bij consult en medebehandeling (NVKG, 2013).
|
Niveau 2 |
Het is aangetoond dat structurele medebehandeling dan wel structurele ortho-geriatrische medebehandeling door een geriatrisch expertise team bij patiënten ouder dan 70 jaar met proximale femurfractuur, leidt tot minder postoperatieve complicaties tijdens de ziekenhuisopname.
Bronnen (B: Fisher, 2006; Friedman, 2009; Lundstrum, 1998; Stenvall, 2007a; Stenvall, 2007b; Vidan, 2005) |
|
Niveau 3 |
Er zijn aanwijzingen dat structurele ortho-geriatrische medebehandeling voor patiënten ouder dan 70 jaar met proximale femurfractuur de kans op herstel tot oorspronkelijk functieniveau en de kans op behoud van de mobiliteit vergroot.
Bronnen (B: Leung, 2011; Lundstrum, 1998; Shyu, 2008; Stenvall, 2007a; Swanson, 1998; Vidan, 2005) |
|
Niveau 3 |
Er zijn aanwijzingen dat structurele ortho-geriatrische medebehandeling de kans op vallen en het valrisico verlaagt.
Bronnen (B: Stenvall, 2007b) |
|
Niveau 3 |
Er zijn aanwijzingen dat structurele ortho-geriatrische medebehandeling voor patiënten ouder dan 70 jaar met proximale femurfractuur de kans op ontslag naar oorspronkelijke woonsituatie verhoogt.
Bronnen (B: Lundstrum, 1998; Stenvall, 2007b) |
|
Niveau 3 |
Er zijn aanwijzingen dat structurele ortho-geriatrische medebehandeling voor patiënten ouder dan 70 jaar met proximale femurfractuur de kans op mortaliteit na 30 dagen en na één jaar vermindert.
Bronnen (B: Leung, 2011; Shyu, 2008) |
|
Niveau 3 |
Er zijn aanwijzingen dat structurele geriatrische medebehandeling bij patiënten ouder dan 70 jaar met proximale femurfractuur het aantal opnamedagen in het ziekenhuis verkort.
Bronnen (B: Friedman, 2009; Naglie, 2003; Stenvall, 2007a; Stenvall, 2007b; Swanson, 1998;) |
|
Niveau 2 |
Het is aannemelijk/waarschijnlijk dat standaard geïntegreerde orthopedische en geriatrische zorg voor patiënten ouder dan 70 jaar met proximale femurfractuur de kans op overlijden in het ziekenhuis vermindert.
Bronnen (A2: Fisher, 2006; Vidan, 2005) |
NOTE: Voor bovenstaande conclusies is de bewijskracht beoordeeld met behulp van de EBRO-methodiek. Dit is in tegenstelling met wat in de methodiek voor de ontwikkeling van deze richtlijn beschreven staat. Dit komt doordat bovenstaande conclusies letterlijk zijn overgenomen van de richtlijn CGA bij consult en medebehandeling (NVKG, 2013).
Zoeken en selecteren
A systematic review of the literature was performed to answer the following primary question: What is the effect on post-operative outcomes of co-management during post-operative care by a health care professional (HCP) with geriatric expertise and the surgical team compared to treatment during post-operative care by the surgical team alone in frail elderly patients?
P: Frail older people (≥65 years) who have undergone surgery
I: Post-operative co-management with an HCP with geriatric expertise
C: No post-operative co-management with an HCP with geriatric expertise
O: Functional status, quality of life, complications, readmissions, mortality, length of stay, costs
Functional status includes mobility, transfers, Activities of Daily Living (ADL), Instrumental Activities of Daily Living (iADL), living independently at home and cognitive impairment.
Complications include delirium, infection, falls, cardiopulmonary complications, neurological complications and thromboembolic complications.
Relevant outcome measures
The working group considered all outcome measures relevant in the patient-centred decision-making process. Whether an outcome measure is critical or important depends on the individual patient, so the working group did not distinguish critical or important outcome measures.
Search and select (methods)
A search from 2018 until September 6th, 2022 was conducted in the following databases: Embase and Ovid/Medline. The detailed search strategy is depicted in Appendix 1. The systematic search resulted in 518 records after removing duplicates. Studies were selected based on the following criteria:
Systematic review, randomized controlled trial (RCT), or cohort study;
Written in English or Dutch language;
Describing the effects of co-treatment with a geriatrician/geriatric team in frail older people.
One hundred and twenty-two articles were initially selected based on title and abstract screening. After reading the full text, 112 articles were excluded (see the table with reasons for exclusion in Appendix 2), and ten studies were included.
Results
Ten studies were included in the analysis of literature. Important study characteristics are summarized in Appendix 3 and results from the pooled outcomes are summarized in the evidence table in Appendix 4. The assessment of risk of bias is summarized in the risk of bias table (see Appendix 5) and the GRADE assessment in Appendix 6.
Overgenomen zoekvraag
De zoekvraag en samenvatting van de literatuur uit de module ‘Herstelmaatregelen na proximale femurfractuur’ uit 2016 is toegevoegd, omdat voor de literatuursearch bij de nieuwe richtlijn studies geïncludeerd zijn vanaf 2016. De bewijskracht van alle eerdere studies is echter van blijvende relevantie voor met name de oudere patiënten met een proximale femurfractuur.
Uitgangsvraag
Welke maatregelen kunnen functioneel herstel bevorderen en mortaliteit voorkomen bij een kwetsbare oudere patiënt na een operatie voor een proximale femurfractuur?
Zoeken en selecteren
Voor deze vraag is gekeken naar de systematische literatuuranalyse die werd uitgevoerd voor de module structurele medebehandeling van de richtlijn CGA bij consult en medebehandeling (NVKG, 2013). De vraagstelling voor deze literatuuranalyse is: Heeft structurele geriatrische medebehandeling een meerwaarde ten opzichte van de gebruikelijke zorg op een niet geriatrische afdeling?
Er is alleen gekeken naar de studies in deze literatuuranalyse die betrekking hebben op patiënten met een proximale femurfractuur. Deze studies kijken naar de effectiviteit van geriatrische medebehandeling, waar de meervoudige geriatrische interventie deel van uitmaakt. De meervoudige geriatrische interventie in deze studies bestaat uit een aantal enkelvoudige interventies die in aard en aantal per studie verschillen. Om deze reden is besloten om geen aanvullende systematische literatuuranalyse te verrichten naar de effectiviteit van de enkelvoudige geriatrische interventies afzonderlijk.
Referenties
- American College of Surgeons. Optimal resources for geriatric surgery; 2019 standards. 2019. https://www.facs.org/media/yldfbgwz/19_re_manual_gsv-standards_digital-linked-pdf-1.pdf
- Baji P, Patel R, Judge A, Johansen A, Griffin J, Chesser T, Griffin XL, Javaid MK, Barbosa EC, Ben-Shlomo Y, Marques EMR, Gregson CL; REDUCE Study Group. Organisational factors associated with hospital costs and patient mortality in the 365 days following hip fracture in England and Wales (REDUCE): a record-linkage cohort study. Lancet Healthy Longev. 2023 Aug;4(8):e386-e398. doi: 10.1016/S2666-7568(23)00086-7. Epub 2023 Jul 10. PMID: 37442154.
- Breda K, Keller MS, Gotanda H, Beland A, McKelvey K, Lin C, Rosen S. Geriatric fracture program centering age-friendly care associated with lower length of stay and lower direct costs. Health Serv Res. 2023 Feb;58 Suppl 1(Suppl 1):100-110. doi: 10.1111/1475-6773.14052. Epub 2022 Sep 2. PMID: 36054014; PMCID: PMC9843078.
- Bub C, Stapleton E, Iturriaga C, Garbarino L, Aziz H, Wei N, Mota F, Goldin ME, Sinvani LD, Carney MT, Goldman A. Implementation of a Geriatrics-Focused Orthopaedic and Hospitalist Fracture Program Decreases Perioperative Complications and Improves Resource Utilization. J Orthop Trauma. 2022 Apr 1;36(4):213-217. doi: 10.1097/BOT.0000000000002258. PMID: 34483320.Centre for Perioperative Care. Guideline for Perioperative Care for People Living with Frailty Undergoing Elective and Emergency Surgery. 2021. https://cpoc.org.uk/sites/cpoc/files/documents/2021-09/CPOC-BGS-Frailty-Guideline-2021.pdf
- Cizginer S, Prohl EG, Monteiro JFG, Yildiz F, Jones RN, Schechter S, Patterson R, Klipfel A, Katlic MR, Daiello LA, Mujahid N, Neupane I, Cioffi WG, Ducharme M, Vrees MD, McNicoll L. Integrated postoperative care model for older colorectal surgery patients improves outcomes and reduces healthcare costs. J Am Geriatr Soc. 2023 May;71(5):1452-1461. doi: 10.1111/jgs.18216. Epub 2023 Jan 31. PMID: 36721263.
- Fan J, Lv Y, Xu X, Zhou F, Zhang Z, Tian Y, Ji H, Guo Y, Yang Z, Hou G. The Efficacy of Multidisciplinary Team Co-Management Program for Elderly Patients With Intertrochanteric Fractures: A Retrospective Study. Front Surg. 2022 Feb 24;8:816763. doi: 10.3389/fsurg.2021.816763. PMID: 35284470; PMCID: PMC8907576.
- Giannotti C, Massobrio A, Carmisciano L, Signori A, Napolitano A, Pertile D, Soriero D, Muzyka M, Tagliafico L, Casabella A, Cea M, Caffa I, Ballestrero A, Murialdo R, Laudisio A, Incalzi RA, Scabini S, Monacelli F, Nencioni A. Effect of Geriatric Comanagement in Older Patients Undergoing Surgery for Gastrointestinal Cancer: A Retrospective, Before-and-After Study. J Am Med Dir Assoc. 2022 Nov;23(11):1868.e9-1868.e16. doi: 10.1016/j.jamda.2022.03.020. Epub 2022 May 13. PMID: 35569527.
- Hafner T, Kollmeier A, Laubach M, Knobe M, Hildebrand F, Pishnamaz M. Care of Geriatric Patients with Lumbar Spine, Pelvic, and Acetabular Fractures before and after Certification as a Geriatric Trauma Center DGU®: A Retrospective Cohort Study. Medicina (Kaunas). 2021 Jul 31;57(8):794. doi: 10.3390/medicina57080794. PMID: 34441000; PMCID: PMC8398181.
- Kalmet PHS, de Joode SGCJ, Fiddelers AAA, Ten Broeke RHM, Poeze M, Blokhuis T. Long-term Patient-reported Quality of Life and Pain After a Multidisciplinary Clinical Pathway for Elderly Patients With Hip Fracture: A Retrospective Comparative Cohort Study. Geriatr Orthop Surg Rehabil. 2019 Jun 6;10:2151459319841743. doi: 10.1177/2151459319841743. PMID: 31218092; PMCID: PMC6557012.
- McDonald SR, Heflin MT, Whitson HE, Dalton TO, Lidsky ME, Liu P, Poer CM, Sloane R, Thacker JK, White HK, Yanamadala M, Lagoo-Deenadayalan SA. Association of Integrated Care Coordination With Postsurgical Outcomes in High-Risk Older Adults: The Perioperative Optimization of Senior Health (POSH) Initiative. JAMA Surg. 2018 May 1;153(5):454-462. doi: 10.1001/jamasurg.2017.5513. PMID: 29299599; PMCID: PMC5875304.
- Miura LN, DiPiero AR, Homer LD. Effects of a geriatrician-led hip fracture program: improvements in clinical and economic outcomes. J Am Geriatr Soc. 2009 Jan;57(1):159-67. doi: 10.1111/j.1532-5415.2008.02069.x. Epub 2008 Nov 18. PMID: 19054192.
- Natesan S, Li JY, Kyaw KK, Soh Z, Yong E, Hong Q, Zhang L, Chong LRC, Tan GWL, Chandrasekar S, Lo ZJ. Effectiveness of Comanagement Model: Geriatric Medicine and Vascular Surgery. J Am Med Dir Assoc. 2022 Apr;23(4):666-670. doi: 10.1016/j.jamda.2021.10.022. Epub 2021 Nov 30. PMID: 34861223.
- National Institute for Health and Care Excellence (NICE). Hip fracture: management. Clinical guideline [CG124]. 06 January 2023. https://www.nice.org.uk/guidance/cg124/chapter/Recommendations
- Pernik MN, Deme PR, Nguyen ML, Aoun SG, Adogwa O, Hall K, Stewart NA, Dosselman LJ, El Tecle NE, McDonald SR, Bagley CA, Wingfield SA. Perioperative Optimization of Senior Health in Spine Surgery: Impact on Postoperative Delirium. J Am Geriatr Soc. 2021 May;69(5):1240-1248. doi: 10.1111/jgs.17006. Epub 2020 Dec 31. PMID: 33382460.
- Saur NM, Davis BR, Montroni I, Shahrokni A, Rostoft S, Russell MM, Mohile SG, Suwanabol PA, Lightner AL, Poylin V, Paquette IM, Feingold DL; Clinical Practice Guidelines Committee of the American Society of Colon and Rectal Surgeons. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Perioperative Evaluation and Management of Frailty Among Older Adults Undergoing Colorectal Surgery. Dis Colon Rectum. 2022 Apr 1;65(4):473-488. doi: 10.1097/DCR.0000000000002410. PMID: 35001046.
- Shahrokni, A., Tin, A., Sarraf, S., Alexander, K., Sun, S., Jung Kim, S., McMillan, S., Yulico, H., Amirnia, F., Downey, R., Vickers, A.J. & Korc-Grodzicki, B. (2020). Association of Geriatric Comanagement and 90-Day Postoperative Mortality Among Patients Aged 75 Years and Older with Cancer. JAMA network open. 8 doi:10.1001/jamanetworkopen.2020.9265
- Tarazona-Santabalbina FJ, Llabata-Broseta J, Belenguer-Varea Á, Álvarez-Martínez D, Cuesta-Peredo D, Avellana-Zaragoza JA. A daily multidisciplinary assessment of older adults undergoing elective colorectal cancer surgery is associated with reduced delirium and geriatric syndromes. J Geriatr Oncol. 2019 Mar;10(2):298-303. doi: 10.1016/j.jgo.2018.08.013. Epub 2018 Sep 11. PMID: 30217699.
- Van Heghe A, Mordant G, Dupont J, Dejaeger M, Laurent MR, Gielen E. Effects of Orthogeriatric Care Models on Outcomes of Hip Fracture Patients: A Systematic Review and Meta-Analysis. Calcif Tissue Int. 2022 Feb;110(2):162-184. doi: 10.1007/s00223-021-00913-5. Epub 2021 Sep 30. PMID: 34591127; PMCID: PMC8784368.
- Vilans. Stappenplan - Samen beslissen met kwetsbare ouderen. 2018. https://www.vilans.nl/kennis/stappenplan-samen-beslissen-met-ouderen
- Ware, J., Kosinski, M., Turner-Bowker, D., & Gandek, B. SF-12: How to score the SF-12 physical and mental health summary scales. 1995. https://www.researchgate.net/profile/John-Ware-6/publication/291994160_How_to_score_SF-12_items/links/58dfc42f92851c369548e04e/How-to-score-SF-12-items.pdf
- Yee DKH, Lau TW, Fang C, Ching K, Cheung J, Leung F. Orthogeriatric Multidisciplinary Co-Management Across Acute and Rehabilitation Care Improves Length of Stay, Functional Outcomes and Complications in Geriatric Hip Fracture Patients. Geriatr Orthop Surg Rehabil. 2022 Apr 11;13:21514593221085813. doi: 10.1177/21514593221085813. PMID: 35433103; PMCID: PMC9006372.
- <strong>Referenties Overgenomen zoekvraag
- Aloia JF. Clinical Review: The 2011 report on dietary reference intake for vitamin D: where do we go from here? J Clin Endocrinol Metab. 2011 Oct;96(10):2987-96. doi: 10.1210/jc.2011-0090. Epub 2011 Jul 27.
- Aspray TJ, Bowring C, Fraser W, et al. National osteoporosis society vitamin D guideline summary. Age Ageing. 2014;43(5):592-5.
- Avenell A, Mak JC, O'Connell D. Vitamin D and vitamin D analogues for preventing fractures in post-menopausal women and older men. Cochrane Database Syst Rev. 2014;4:CD000227.
- Bacon CJ, Gamble GD, Horne AM, et al. High-dose oral vitamin D3 supplementation in the elderly. Osteoporos Int. 2009;20(8):1407-15.
- Beaudart C, Buckinx F, Rabenda V, et al. The effects of vitamin D on skeletal muscle strength, muscle mass, and muscle power: a systematic review and meta-analysis of randomized controlled trials. J Clin Endocrinol Metab. 2014;99(11):4336-45.
- Bellelli G, Mazzola P, Morandi A, et al. Duration of postoperative delirium is an independent predictor of 6-month mortality in older adults after hip fracture. J Am Geriatr Soc. 2014;62(7):1335-40.
- Belmont PJ Jr, Garcia EJ, Romano D, et al. Risk factors for complications and in-hospital mortality following hip fractures: a study using the National Trauma Data Bank. Arch Orthop Trauma Surg. 2014;134(5):597-604.
- Berry SD, Samelson EJ, Bordes M, et al. Survival of aged nursing home residents with hip fracture. J Gerontol A Biol Sci Med Sci. 2009;64(7):771-7.
- Bischoff-Ferrari HA, Dawson-Hughes B, Staehelin HB, et al. Fall prevention with supplemental and active forms of vitamin D: a meta-analysis of randomised controlled trials. BMJ. 2009 1;339:b3692.
- Bischoff-Ferrari HA, Dawson-Hughes B, et al. Monthly High-Dose Vitamin D Treatment for the Prevention of Functional Decline: A Randomized Clinical Trial. JAMA Intern Med. 2016;176(2):175-83.
- Bischoff-Ferrari HA, Willett WC, Wong JB, et al. Prevention of nonvertebral fractures with oral vitamin D and dose dependency: a meta-analysis of randomized controlled trials. Arch Intern Med. 2009;169(6):551-61.
- Bolland MJ, Grey A, Gamble GD, et al. Vitamin D supplementation and falls: a trial sequential meta-analysis. Lancet Diabetes Endocrinol. 2014;2(7):573-80.
- Boonen S, Lips P, Bouillon R, et al. Need for additional calcium to reduce the risk of hip fracture with vitamin d supplementation: evidence from a comparative metaanalysis of randomized controlled trials. J Clin Endocrinol Metab. 2007;92(4):1415-23. Epub 2007 Jan 30.
- Brunskill SJ, Millette SL, Shokoohi A, et al. Red blood cell transfusion for people undergoing hip fracture surgery. Cochrane Database Syst Rev. 2015;4:CD009699.
- Cameron ID, Chen JS, March LM, et al. Hip fracture causes excess mortality owing to cardiovascular and infectious disease in institutionalized older people: a prospective 5-year study. J Bone Miner Res. 2010;25(4):866-72.
- Chel V, Wijnhoven HA, Smit JH, et al. Efficacy of different doses and time intervals of oral vitamin D supplementation with or without calcium in elderly nursing home residents. Osteoporos Int. 2008;19(5):663-71.
- Dawson-Hughes B, Mithal A, Bonjour JP, et al. IOF position statement: vitamin D recommendations for older adults. Osteoporos Int. 2010;21(7):1151-4.
- DIPART(Vitamin D Individual Patient Analysis of Randomized Trials) Group. Patient level pooled analysis of 68 500 patients from seven major vitamin D fracture trials in US and Europe. BMJ. 2010;340:b5463.
- Dolan MM, Hawkes WG, Zimmerman SI, et al. Delirium on hospital admission in aged hip fracture patients: prediction of mortality and 2-year functional outcomes. J Gerontol A Biol Sci Med Sci. 2000;55(9):M527-34.
- Dovjak P, Iglseder B, Mikosch P, Get al. Treatment and prevention of postoperative complications in hip fracture patients: infections and delirium. Wien Med Wochenschr. 2013;163(19-20):448-54.
- Fisher AA, Davis MW, Rubenach SE, et al. Outcomes for Older patients with hip fractures: the Impact of orthopedic and geriatric medicine cocare. J. Orthop. Trauma. 2006;20:172-180.
- Friedman SM, Mendelson DA, Bingham KW, et al. Impact of a comanaged geriatric fracture center on short-term hip fracture outcomes. Arch Intern Med. 2009;169(18):1712-1717.
- Friedman SM, Mendelson DA, Kates SL, et al. Geriatric co-management of proximal femur fractures: total quality management and protocol-driven care result in better outcomes for a frail patient population. J Am Geriatr Soc. 2008;56(7):1349-56.
- Gezondheidsraad. Evaluatie van de voedingsnormen voor vitamine D. Den Haag: Gezondheidsraad, 2012; publicatienr. 2012/15.
- Gould CV, Umscheid CA, Agarwal RK, et al. Guideline for prevention of catheter-associated urinary tract infections 2009. Infect Control Hosp Epidemiol. 2010;31(4):319-26.
- Gregersen M, Borris LC, Damsgaard EM. Postoperative blood transfusion strategy in frail, anemic elderly patients with hip fracture: the TRIFE randomized controlled trial. Acta Orthop. 2015;86(3):363-72.
- Grigoryan KV, Javedan H, Rudolph JL. Orthogeriatric care models and outcomes in hip fracture patients: a systematic review and meta-analysis. J Orthop Trauma. 2014;28(3):e49-55.
- Gruber-Baldini AL, Marcantonio E, Orwig D, et al. Delirium outcomes in a randomized trial of blood transfusion thresholds in hospitalized older adults with hip fracture. J Am Geriatr Soc. 2013;61(8):1286-95.
- Gustafson Y, Berggren D, Brännström B, et al. Acute confusional states in elderly patients treated for femoral neck fracture. J Am Geriatr Soc. 1988;36(6):525-30.
- Halm EA, Wang JJ, Boockvar K, et al. The effect of perioperative anemia on clinical and functional outcomes in patients with hip fracture. J Orthop Trauma. 2004;18(6):369-74.
- Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911-30. doi: 10.1210/jc.2011-0385.
- Ish-Shalom S, Segal E, Salganik T, et al. Comparison of daily, weekly, and monthly vitamin D3 in ethanol dosing protocols for two months in elderly hip fracture patients. J Clin Endocrinol Metab. 2008;93(9):3430-5.
- Johansson I, Athlin E, Frykholm L, et al. Intermittent versus indwelling catheters for older patients with hip fractures. J Clin Nurs. 2002;11(5):651-6.
- Jones KS, Assar S, Harnpanich D, et al. 25(OH)D2 half-life is shorter than 25(OH)D3 half-life and is influenced by DBP concentration and genotype. J Clin Endocrinol Metab. 2014;99(9):3373-81.
- Lawrence VA, Hilsenbeck SG, Noveck H, et al. Medical complications and outcomes after hip fracture repair. Arch Intern Med. 2002;162(18):2053-7.
- Lawrence VA, Silverstein JH, Cornell JE, et al. Higher Hb level is associated with better early functional recovery after hip fracture repair. Transfusion. 2003;43(12):1717-22.
- Lee HB, Mears SC, Rosenberg PB, et al. Predisposing factors for postoperative delirium after hip fracture repair in individuals with and without dementia. J Am Geriatr Soc. 2011;59(12):2306-13.
- Leung AH, Lam T, Cheung W, et al. An orthogeriatric Collaborative Intervention Program for Fragility Fractures: A Retrospective Cohort Study. The journal of Trauma Injury, Infection and Critical care. 2011;71:5.
- Love AL, Cornwell PL, Whitehouse SL. Oropharyngeal dysphagia in an elderly post-operative hip fracture population: a prospective cohort study. Age Ageing. 2013;42(6):782-5.
- Lundstrum M, Edlund A, Lundstrom G, et al. Reorganisation of nursing and medical care to reduce the incidence of postoperative delirium and improve rehabilitation outcome in elderly patients treated for femoral neck fractures. Scand J Caring Sci. 1998;13:193-200.
- Maier S, Sidelnikov E, Dawson-Hughes B, Eet al. Before and after hip fracture, vitamin D deficiency may not be treated sufficiently. Osteoporos Int. 2013;24(11):2765-73.
- Marcantonio ER, Flacker JM, Michaels M, et al. Delirium is independently associated with poor functional recovery after hip fracture. J Am Geriatr Soc. 2000;48(6):618-24.
- Morrison RS, Magaziner J, Gilbert M, et al. Relationship between pain and opioid analgesics on the development of delirium following hip fracture. J Gerontol A Biol Sci Med Sci. 2003;58(1):76-81.
- Naglie G, Tansey C, Kirkland JL, et al. Interdisciplinary care for elderly people with hip fracture: a randomized controlled trial. CMAJ. 2002;167(1):25-32.
- National Clinical Guideline Centre (NICE). Hip fracture. The management of hip fracture in adults. London (UK): National Institute for Health and Clinical Excellence (NICE); 2011 Jun.
- Nederland Huisartsen Genootschap (NHG). Multidisciplinaire richtlijn polyfarmacie bij ouderen, 2012. Utrecht: NHG; 2012
- Nederlandse Orthopaedische Vereniging (NOV). Richtlijn proximale femurfractuur. In ontwikkeling.
- Nederlandse Vereniging voor Anesthesiologie (NVA). Richtlijn postoperatieve pijn. Utrecht: NVA; 2012.
- Nederlandse Vereniging voor Anesthesiologie (NVA). Richtlijn preventie van perioperatieve pulmonale complicaties bij niet-pulmonale chirurgie bij patiënten met een verhoogd risico op pulmonale complicaties. Utrecht: NVA; 2012.
- Nederlandse Vereniging voor Klinische Geriatrie (NVKG). Richtlijn Comprehensive Geriatric Assessment (CGA) bij consult en medebehandeling. Addendum behorende bij de richtlijn CGA (NVKG). Utrecht: NVKG; 2013.
- Nederlandse Vereniging voor Klinische Geriatrie (NVKG). Richtlijn Delier Volwassenen. Utrecht: NVKG; 2013.
- Nederlandse Vereniging voor Reumatologie (NVvR). Richtlijn Osteoporose en Fractuurpreventie. Utrecht: NVvR; 2011.
- Papaioannou A, Kennedy CC, Giangregorio L, et al. A randomized controlled trial of vitamin D dosing strategies after acute hip fracture: no advantage of loading doses over daily supplementation. BMC Musculoskelet Disord. 2011;12:135.
- Premaor MO, Scalco R, da Silva MJ, et al. The effect of a single dose versus a daily dose of cholecalciferol on the serum 25-hydroxycholecalciferol and parathyroid hormone levels in the elderly with secondary hyperparathyroidism living in a low-income housing unit. J Bone Miner Metab. 2008;26(6):603-8.
- Prestmo A, Hagen G, Sletvold O, et al. Comprehensive geriatric care for patients with hip fractures: a prospective, randomised, controlled trial. Lancet. 2015;385(9978):1623-33.
- Ramason R, Selvaganapathi N, Ismail NH, et al. Prevalence of vitamin d deficiency in patients with hip fracture seen in an orthogeriatric service in sunny singapore. Geriatr Orthop Surg Rehabil. 2014;5(2):82-6.
- Reid IR, Bolland MJ, Grey A. Effects of vitamin D supplements on bone mineral density: a systematic review and meta-analysis. Lancet. 2014;383(9912):146-55.
- Rizzoli R, Boonen S, Brandi ML, et al. Vitamin D supplementation in elderly or postmenopausal women: a 2013 update of the 2008 recommendations from the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO). Curr Med Res Opin. 2013;29(4):305-13.
- Roche JJ, Wenn RT, Sahota O, et al. Effect of comorbidities and postoperative complications on mortality after hip fracture in elderly people: prospective observational cohort study. BMJ. 2005;331(7529):1374.
- Salonoja M, Salminen M, Vahlberg T, et al. Withdrawal of psychotropic drugs decreases the risk of falls requiring treatment. Arch Gerontol Geriatr. 2012;54(1):160-7.
- Sanders KM, Stuart AL, Williamson EJ, et al. Annual high-dose oral vitamin D and falls and fractures in older women: a randomized controlled trial. JAMA. 2010;303(18):1815-22.
- Sanquin, Landelijke Gebruikersraad Bloedvoorziening. Richtlijn bloedtransfusie. Utrecht: CBO; 2011.
- Shyu YI, Liang J, Wu CC, et al. A pilot investigation of the short-term effects of an interdisciplinary intervention program on elderly patients with hip fracture in Taiwan. J Am Geriatr Soc. 2005;53(5):811-8.
- Sjöberg C, Wallerstedt SM. Effects of medication reviews performed by a physician on treatment with fracture-preventing and fall-risk-increasing drugs in older adults with hip fracture-a randomized controlled study. J Am Geriatr Soc. 2013;61(9):1464-72.
- Sletvold O, Helbostad JL, Thingstad P, et al. Effect of in-hospital comprehensive geriatric assessment (CGA) in older people with hip fracture. The protocol of the Trondheim Hip Fracture trial. BMC Geriatr. 2011;11:18.
- Stenvall M, Olofsson B, Lundstrom M, et al. A multidisciplinary, multifactorial intervention program reduces postoperative falls and injuries after femoral neck fracture. Osteoporosis, Int. 2007a;18:167-175.
- Stenvall M, Olofsson B, Nyberg L, et al. Improved performance in activiteits of daily living and mobility after a multidisciplinary postoperative rehabilitation in older people withfemoral neck fracture: randomized controlled trial with 1-year follow-up. J. Rehabil.Med. 2007b;39:232-238.
- Swanson C, Day GA, Yelland CE, et al. The management of elderly patients with femoral fractures. A randomised controlled trial of early intervention versus standard care. MJA 1998;169:515-518.
- Tang BM, Eslick GD, Nowson C, et al. Use of calcium or calcium in combination with vitamin D supplementation to prevent fractures and bone loss in people aged 50 years and older: a meta-analysis. Lancet. 2007;370(9588):657-66.
- Thorell K, Ranstad K, Midlöv P, et al. Is use of fall risk-increasing drugs in an elderly population associated with an increased risk of hip fracture, after adjustment for multimorbidity level: a cohort study. BMC Geriatr. 2014;14:131.
- Vidan M, Serra JA, Riquilme G, et al. Efficacy of Comprehensive Geriatric Intervention in Older Patients Hospitalized for Hip Fracture: A Randomized Controlled Trial. JAGS. 2005;53:1476-1482.
- Watne LO, Torbergsen AC, Conroy S, et al. The effect of a pre- and postoperative orthogeriatric service on cognitive function in patients with hip fracture: randomized controlled trial (Oslo Orthogeriatric Trial). BMC Med. 2014;12:63.
- Werkgroep Klinische Gerontofarmacologie (WKGF) van Nederlandse Vereniging voor Klinische Geriatrie. Standpunt Vitamine D. Utrecht: NVKG; 2013 Okt.
- Wyller TB, Watne LO, Torbergsen A, et al. The effect of a pre- and post-operative orthogeriatric service on cognitive function in patients with hip fracture. The protocol of the Oslo Orthogeriatrics Trial. BMC Geriatr. 2012;12:36.
- Zheng YT, Cui QQ, Hong YM, et al. A meta-analysis of high dose, intermittent vitamin D supplementation among older adults. PLoS One. 2015 Jan 20;10(1):e0115850.
- Zuckerman JD, Sakales SR, Fabian DR, et al. Hip fractures in geriatric patients. Results of an interdisciplinary hospital care program. Clin Orthop Relat Res. 1992;(274):213-25.
- Zwart SR, Parsons H, Kimlin M, et al. A 250 µg/week dose of vitamin D was as effective as a 50 µg/d dose in healthy adults, but a regimen of four weekly followed by monthly doses of 1250 µg raised the risk of hypercalciuria. Br J Nutr. 2013;110(10):1866-72.
Evidence tabellen
|
Study reference |
Study characteristics |
Patient characteristics 2 |
Intervention (I) |
Comparison/ control (C) 3
|
Follow-up |
Outcome measures and effect size 4 |
Comments |
|
Bub, 2022 |
Type of study: Retrospective chart review study
Setting and country: Large urban, academic tertiary centre, located in the greater New York metropolitan area
Funding and conflicts of interest: Reported |
Inclusion criteria: Patients were enrolled when they were 65 years of age and older and admitted to the hospital with a low-energy (fragility) hip-fracture
Exclusion criteria: Patients younger than 65 years old, pathologic hip fractures, periprosthetic fractures, and chronic opioid users were excluded.
N total at baseline: Intervention: 162 Control: 128
Important prognostic factors2: For example age ± SD: I: 84.11 ± 8.4 C: 83.5 ± 9.0
Sex: I: 34.6% M C: 22.7% M
Groups comparable at baseline? No, the two groups were comparable for CCI, surgery type, and anaesthesia, although there were significantly more intertrochanteric fractures and fewer women in the intervention group. |
Describe intervention (treatment/procedure/test):
The intervention consisted of the AGS CoCore: Ortho program. A dedicated hospitalist (eg trained geriatrics comanager), with geriatrics oversight, comanaged all patients throughout the perioperative period. Team members from orthopaedics, hospital medicine, nursing, and geriatrics rounded together 2 times a day, in the morning and again on nightly rounds. The teams discussed medical and surgical management as well as psychosocial and discharge issues.
Additionally, evidence based standardized pre- and postoperative protocols were adapted and implemented in the HER, preoperative risk stratification, and postoperative delirium prevention and discharge planning was implemented in the intervention group.
|
Describe control (treatment/procedure/test):
Before the implementation of comanagement, patients with hip fracture were admitted to either orthopaedics or hospitalist service with the other on board as a consultant. Patients with an American Society of Anesthesiologists classification I and II were admitted to orthopaedics, whereas those over III were admitted to the hospitalist service. American Society of Anesthesiologists class III classification was admitted to either orthopaedics or hospitalists depending on the surgeon’s comfort level. There was no formal agreement between orthopaedics and hospital medicine and no standard process for preoperative assessment or perioperative care (eg, pain management). |
Length of follow-up: No follow up.
Loss-to-follow-up: NA
Incomplete outcome data: NA
|
Outcome measures and effect size (include 95%CI and p-value if available):
Surgical metrics Time to OR significant lower in I group (I 30.3 hours ± 23.4 vs C 36.2 ± 23.7, p=0.026*).
No difference in operative time (I 60.6 ± 26.2 vs C 61.5 ± 23.6, p=0.716).
Lower length of stay (I 5.0 days ± 2.8 vs C 6.8 ± 6.8, p=0.005*).
Opioid use lower in I group (I 11.1 MME/d ± 14.6 vs C 16.0 ± 17.3, p=0.011*).
Perioperative testing No difference in preoperative transthoracic echocardiogram (I 21 (13.0%) vs C 28 (21.9%), p=0.58).
Discharge disposition No difference in discharge destination (p=0.197, in-hospital mortality I 2 (1.2%) vs C 4 (3.1%); skilled nursing facility I 64 (39.5%) vs 36 (28.1%); subacute rehabilitation I 79 (48.8%) vs C 67 (52.3%); home I 14 (8.6%) vs C 17 (13.3%); hospice/specialized care I 3 (1.9%) vs C 4 (3.1%).
Perioperative complications No difference in preoperative and postoperative transfusion. No difference in failed trial of voiding and discharged with Foley.
I group had decreased use of indwelling bladder catheters preoperative (I 46.9% vs C 68.0%, p<0.001*) and postoperative (I 58.0% vs C 83.6, p<0.001*).
I group had decreased incidence of urinary tract infections (I 21 (13.0%) vs C 36 (28.1%), p=0.002*).
No difference in deep vein thrombosis (I 1 (0.6%) vs C 1 (0.8%), p=1.000).
No difference in aspiration pneumonia (I 15 (9.3%) vs C 12 (9.4%), p=0.703).
No difference in pulmonary embolism (I 1 (0.6%) vs C 0, p=1.000).
No difference in cerebrovascular accident (I 0 vs C 2 (1.6%), p=0.194).
No difference in myocardial infarction (I 1 (0.6%) vs C 0, p=1.000).
No difference in return to operating room (I 1 (0.6%) vs 0, p=1.000).
No difference in 6-month mortality rate (I 10 (6.2%) vs C 8 (6.3%), p=1.000).
Less complications in the I group (I 34 (21.0%) vs C 44 (34.4%), p=0.012*). |
No comments |
|
Fan, 2021 |
Type of study: Pre-post retrospective study
Setting and country: single trauma centre in China
Funding and conflicts of interest: Reported |
Inclusion criteria: (1) acute intertrochanteric fractures (time from injury to admission was limited to 3 weeks); (2) age ≥60 years old; and (3) low-energy injury. A low-energy injury was defined as an injury which patients would sustain while falling over slippery ground in a walking or sitting position.
Exclusion criteria: Pathological fracture; high-energy injury, such as car crash; peri-prosthetic fracture; multiply traumatized patients or terminal malignancies.
N total at baseline: Intervention: 241 Control: 249
Important prognostic factors2: For example age ± SD: I: 79.9 ± 8.1 C: 78.8 ± 7.2
Sex: I: 30.52% M C: 31.95% M
Groups comparable at baseline? Yes |
Describe intervention (treatment/procedure/test):
The MDT model involved orthopaedic surgeons, geriatricians, anaesthesiologists, specialists of the intensive care unit (ICU), and physiotherapists. This model consisted of a pathway of care spanning Emergency Department (ED) presentation to discharge from hospital. The traumatologist
Additionally to the intervention, suitability of surgical treatment, preoperative anaesthesia evaluation, post-operative evaluation in ICU, early geriatric rehabilitation, and early surgery and early discharge were also implemented in the intervention group. |
Describe control (treatment/procedure/test):
Traditional Orthopaedic Care model (TOC): When a patient suspected of having a fragility hip fracture was seen in the ED, an initial X-ray of the injured hip was obtained. Once the intertrochanteric fracture diagnosis was confirmed, the patient was admitted to the orthopaedic ward under the care of an orthopaedic surgical team. Treatment was generally managed by trauma surgeons and their team, who had no specific geriatric expertise. The geriatric assessment was not performed during inpatient treatment. Specialist consultants were called on according to their perception of patients’ clinical conditions. The preoperative anaesthesia assessment was performed on the day before surgery. Surgical treatment was performed by trauma surgeons. The patient would be transferred to the department of rehabilitation medicine after discharge. |
Length of follow-up: One months after discharge
Loss-to-follow-up: Total: 23 patients were excluded, but reasons were not reported.
|
Outcome measures and effect size (include 95%CI and p-value if available):
Time to surgery Significantly lower in I group: I 1.7 ± 1.3 days vs C 2.4 ± 1.5 days, p< 0.001*.
The proportion of patients receiving surgery within 24 h (I 148 (61.4%) vs. C 87 (34.9%), p <0.001*) and 48 h (I 195 (80.9%) vs. C 158 (63.5%), p < 0.001*) after admission to the ward was significantly higher in the I group compared with those in the C group.
Total length of stay Lower in I group than C group (I 4.0 ± 2.5 days vs C 5.0 ± 2.8 days, p< 0.001*).
Anaemia No difference in proportion of patients receiving red blood cell transfusion (I 80 (33.2%) vs C 75 (30.1%), p=0.464).
The proportion of patients with a haemoglobin >11 g/dl was higher in the I group (I 58 (24.1%) vs C 37 (14.9%), p=0.010*).
Postoperative complications Patients in the I group had significantly lower proportion of postoperative complications (I 61 (25.3%) vs. C 110 (44.2%), p < 0.001*)
Lower rates of deep venous thrombosis (I 18 (7.9%) vs. C 32 (12.9%), p = 0.049*) Lower rates of pneumonia (I 9 (3.8%) vs. C 20 (8.0%), p = 0.045*)
Lower rates of delirium (I 10 (4.1%) vs. C 21 (9.2%), p = 0.025*).
No statistically significant differences were observed in wound infection (I 3 (1.2%) vs C 4 (1.6%), p=0.736), urinary tract infection (I 6 (3.5%) vs C 10 (4.0%), p=0.342), cerebral vascular accident (I 5 (2.1%) vs C 6 (2.4%), p=0.802), acute coronary syndrome (I 8 (3.3%) vs C 13 (5.2%), p=0.299), and gastrointestinal bleeding (I 2 (0.8%) vs C 4 (1.6%), p=0.435).
30-day Harris Score did not differ (I 80.8 ± 7.5 vs C 80.3 ± 7.0, p=0.440).
Mortality No difference in in-hospital mortality (I 1 (0.4%) vs C 2 (0.8%), p=0.582).
No difference in 30-day mortality (I 4 (1.7%) vs C 6 (2.4%), p=0.557). |
No comments |
|
Giannotti, 2022 |
Type of study: pre-post retrospective study
Setting and country: single-centre study based at the oncological surgery of Policlinico San Martino of Genoa, Italy
Fundings and conflict of interest: not reported |
Inclusion criteria: Patients aged 70 or older admitted for elective GI cancer surgery or palliative treatments and required a hospital stay of at least 1 day.
Exclusion criteria: if patients had any instability needing acute surgery, or if they were admitted for secondary surgeries or because of a postoperative complication.
N total at baseline: Intervention: 90 Control: 117
Important prognostic factors age median (IQR): I: 79.0 (76.0-83.0) C: 82.0 (78.0-85.0) Sex: I: 53.3% M C: 64.1%
Groups comparable at baseline? Patients in control group are slightly older. Comparable in terms of cancer diagnosis and metastatic disease.
|
Describe the Intervention:
Model of geriatric care consisted of initiation of daily targeted geriatrician-led ward rounds focusing on older patients with cancer. During the inpatient postoperative period, patients were followed by the same geriatrician in a consulting role, with the surgical team in a primary role. The geriatric comanagement group included a daily board round led by a geriatrician who discussed the care management during the clinical sessions.
Patients received a pre-operative CGA and a frailty assessment.
Perioperative phase followed the major principles of the Enhanced Recovery After Surgery (ERAS) |
Describe the control:
Older patients with cancer underwent a preoperative comprehensive geriatric assessment (CGA) and frailty assessment to stratify patients’ frailty and performance status within 2 weeks before admission to the surgical department by a geriatrician working at the geriatric clinic of our hospital. This CGA was aimed at identifying high risk patients and the assessment was followed by recommendations based on the identified health issues. Referral to the geriatrics service was based on the surgical team preference and clinical judgment, but not based on a formal frailty screening tool. During hospitalization and perioperative phase, patients from the control group were assessed daily by the surgical team and medical consultants were called in as needed.
Perioperative phase followed the major principles of the Enhanced Recovery After Surgery (ERAS). |
Length of follow up One year
Loss to follow up No missing data reported |
Outcome measures and effect size (include 95%CI and p-value if available):
Post-operative complications Fewer rates of bacteriemia/sepsis in the I group (I 6.7% vs. C 20.5%; p 0.011) Fewer rates of haematological complications in the I group (I 7.8% vs. 19.7%; p 0.031). Similar rates of acute kidney failure, pneumonia, cardiovascular complications and neurological complications.
Patients in the geriatric comanagement group lower risk of grade I-V postoperative complications OR 0.43; 95% CI 0.25-0.73; p < 0.002)
Delirium Lower 4AT scores in the I group (mean (SD)): I 1.44 (2.62) C 2.19 (3.27); p 0.03
Rehospitalization Lower rates of rehospitalization within 1 year in the I group (I 23.5% vs C 35.9%; p 0.006).HR 0.49; 95% CI 0.30-0.81; p < 0.006)
Length of stay No difference in median length of stay or overall survival between groups. |
|
|
Hafner, 2021 |
Type of study: Retrospective cohort study
Setting and country: Single setting study based on data collected from the electronic patient records at the university hospital of Aachen, Germany.
Funding and conflicts of interest: Reported |
Inclusion criteria: All geriatric patients aged > 70 years who were admitted with lumbar spine, pelvic, or acetabular fractures in the period from January 2012 to September 2019 were included.
Exclusion criteria: Polytrauma patients (Injury Severity Score ≥ 16), tumour-associated fractures, and patients treated only in the ICU were excluded.
N total at baseline: Intervention: 224 Control: 137
Important prognostic factors2: For example age ± SD: I: 82.2 ± 6.2 C: 81.5 ± 6.2
Sex: I: 30.4% M C: 25.5% M
Groups comparable at baseline? No difference in age and sex. Differences in ASA classification a larger number of patients in the OGC cohort were assigned to ASA group III/IV (UC: 68.8% vs. OGC: 75.4%; p = 0.002). Moreover, the two cohorts differed significantly regarding the fracture entities. While pelvic fractures were the most common fracture |
Describe intervention (treatment/procedure/test):
The ortho-geriatric model implemented in this study is based on the ward round model in conformity with the certification guidelines of the DGU® [12,19]. Patients were admitted to and treated at the trauma surgery ward with the routine consultation of a geriatrician in an interdisciplinary ward round twice a week and an interdisciplinary team conference once a week. Moreover, representatives of nursing, occupational therapy, physiotherapy, and case management took part in the treatment process from admission until discharge. All participants were advised to provide care following the standard operating procedures (SOPs) provided by the DGU® for geriatric trauma patients.
Geriatric assessment was also performed. |
Describe control (treatment/procedure/test):
Usual care, before implementation of the ortho-geriatric model. |
Length of follow-up: No follow-up.
|
Outcome measures and effect size (include 95%CI and p-value if available):
Clinical complications Higher rate of urological complications in the I group (I 84 (37.5%) vs C 35 (25.5%), p=0.021*).
No difference in total complications (I 150 (67.0%) vs C 80 (58.4%), p=0.100).
Significant fewer numbers of revision surgery in the I group (I 7 (3.1%) vs C 3 (2.2%), p=0.012*).
No difference in dehydration, electrolyte disorder, anaemia, transfusion, pulmonary complications, delirium, cardiac complications, gastrointestinal complications, surgical complications, neurological complications, acute kidney injury, sepsis/SIRS/shock, complications during anaesthesia, in-hospital fall, and in-hospital mortality (all p>0.05).
Secondary outcome measures No difference in total length hospital stay (I 10.4 ± 8.3 vs C 10.4 ± 5.3 days, p=0.111).
Increase in time to surgery in I group (I 112.2 ± 75.8 vs C 72.4 ± 54.5 hours, p<0.001*).
Higher amount of patients with suspected dementia (I 13 (5.8%) vs C1 (0.7%), p=0.022*). Increased osteoporosis therapy at discharge (p<0.001*).
More patients were mobilized on the first day in the I group (I 101 (86.3% in 117 patients) vs 48 (57.1% in 84 patients), p<0.001*).
No difference in change in walking ability before and after the fracture (p=0.252).
No difference in therapy regimen (surgery or conservative) (p=0.255). No difference in time at ICU (I 11.2 ± 36.0 vs C 14.3 ± 47.4 hours, p=0.660).
No difference in osteoporosis therapy at discharge (I 19 (9.4% in 203 patients) vs C 16 (11.7% in 137 patients). |
No comments. |
|
Kalmet, 2019 |
Type of study: Retrospective cohort study
Setting and country: University hospital in Maastricht, the Netherlands
Funding and conflicts of interest: Reported |
Inclusion criteria: Patients aged 65 years or older with a surgically treated low-energy fracture
Exclusion criteria: Patients with a high-energy hip fracture, patients with several or complicated fractures, and patients not living in the hospital area were excluded.
N total at baseline: Intervention: 182 Control: 216
Important prognostic factors2: For example age ± SD: I: 83.4 ± 7.4 C: 82.2 ± 7.5
Sex: I: 29.1% M C: 29.2% M
Groups comparable at baseline? Patients in the C group had more comorbidities and were more likely to have a femoral neck fracture. No differences in gender, age, or ASA classification |
Describe intervention (treatment/procedure/test):
The intervention consisted of a multidisciplinary team (MCP). This MCP consists of an orthopaedic trauma surgeon, a geriatrician, an anaesthesiologist, and a physiotherapist. These
Additionally, patients in the intervention group received early surgery and early discharge. |
Describe control (treatment/procedure/test):
The comparison consisted of usual care. Usual care protocol includes standard traditional treatment by an orthopaedic trauma surgeon at the trauma unit with a follow-up at the out-patient clinic. Physiotherapy is prescribed when the patient is discharged home. |
Length of follow-up: At least two years
Loss-to-follow-up: 49.9% of patients were still alive after at least 2 years follow-up. 14 of the remaining 159 patients could not fulfil the questionnaire due to cognitive impairment. The response rate of the questionnaire was 65.6% (95 of 145 participants).
|
Outcome measures and effect size (include 95%CI and p-value if available): In-hospital outcomes Mean time to surgery was shorter in the I group (I 18.2 ± 9.3 hours vs C 25.3 ± 13.9 hours, p<0.01*).
Number of patients who had to wait more than 24 hours for surgery was lower in the I group (I 17.6% vs C 44.9%, p<0.01*).
Longer mean length of hospitalization in I group (I 15.1 ± 15.7 vs C 12.3 ± 7.3, p=0.02*).
Patient reported outcomes No difference in mean QoL (I 47.9 ± 24.4 vs C 45.4 ± 27.6, p=0.65), physical QoL (I 36.3 ± 28.6 vs C 35.8 ± 28.9, p=0.93), or mental QoL (I 59.5 ± 25.0 vs CG 54.9 ± 31.0, p=0.45).
No difference in pain 30-day after hip fracture (I 4.2 ± 2.8 vs C 3.9 ± 2.7, p=0.56), 1-year after fracture (I 2.7 ± 2.8 vs 1.9 ± 2.7, p=0.17), and at follow-up questionnaire (I 2.8 ± 4.0 vs 1.8 ± 2.7, p=0.19).
Complications and mortality outcome measures Lower incidence of postoperative complications in I group (I 82 (45.1%) vs C 179 (82.9%), p<0.01*).
Lower incidence of postoperative delirium (I 35 (19.2%) vs C 98 (45.4%), p<0.01*).
No difference in 30-day mortality (I 15 (8.2%) vs C 17 (7.9%), p=0.90) and 1-year mortality (I 61 (33.5%) vs C 80 (37.0%), p=0.50). |
No comments. |
|
McDonald, 2018 |
Type of study: Prospective cohort study
Setting and country: Duke university hospital USA
Funding and conflicts of interest: Reported |
Inclusion criteria: All surgical candidates 85 years and older undergoing elective colorectal, general, and hepatopancreaticobiliary surgical procedures (these were at higher risk). Patients between age 65 and 84 years were considered eligible when any 1 of the following conditions was present: prior diagnosis of a cognitive disorder, weight loss of more than 4.54 kg in the last year, multimorbidity (presence of 2 or more chronic medical conditions), polypharmacy (more than 5 prescription medications), any visual or hearing impairment, or if the surgeon perceived an increased estimation of risk.
Exclusion criteria: This analysis excluded participants who underwent outpatient surgical procedures.
N total at baseline: Intervention: 183 Control: 143
Important prognostic factors2: For example age ± SD: I: 75.6 ± 6.8 C: 71.9 ± 6.4
Sex: I: 46.6% M C: 51.0% M
Groups comparable at baseline? Patients in the I group were older, more often white, more often an active smoker, had higher haemoglobin levels, and more comorbid conditions
|
Describe intervention (treatment/procedure/test):
The POSH preoperative assessment team included a geriatrician, geriatric resource nurse, social worker, program administrator, and nurse practitioner
Additionally, patients in the intervention group received preoperative risk assessment and modification. |
Describe control (treatment/procedure/test):
Control cohort of adults older than 65 years who underwent similar surgical procedures as determined by Current Procedural Terminology code, performed by the same surgical group, between January 2010 and May 2011. |
Length of follow-up: 30-days
Loss-to-follow-up: Intervention: N (%): 3 Reasons (describe): NR
Control: N (%): 1 Reasons (describe): NR
Incomplete outcome data: No incomplete outcome data
|
Outcome measures and effect size (include 95%CI and p-value if available):
Health care service use Shorter median LOS in I group (I median 4.0 d range 1-75 vs C median 6.0 d range 1-60, p<0.001*, 95%CI 1.1;4.2).
Lower 7-day all-cause readmission rates (I 5 of 180 (2.8%) vs C 14 of 142 (9.9%), p<0.001*, 95%CI 0.09;0.74).
Lower 30-day all-cause readmission rates (I 14 of 180 (7.8%) vs C 26 of 142 (18.3%), p>0.001*, 95%CI 0.19; 0.75).
Patients in the I group returned home with self-care more frequently (instead of discharge with skilled services) (I 114 of 183 (62.3%) vs C 73 of 143 (51.1%), p=0.04*, 95%CI 1.02; 2.47).
No difference in usage of health service when returning to home (I 32 of 183 [17.5%] vs C 34 of 143 [23.8%]; P = 0.16; 95% CI, 0.39-1.17).
No difference in discharge to facility (I 26 of 183 [14.2%] vs C 27 of 143 [18.9%]; P = 0.26; 95% CI, 0.39-1.28).
Postoperative complications Lower rate of complications in the I group (I 82 of 183 (44.8%) vs C 84 of 143 (58.7%), p=0.01*, 95%CI 0.37; 0.89).
Lower incidence of postoperative cardiogenic or hypovolemic shock in I group (I 4 of 183 [2.2%] vs C 12 of 143 [8.4%];P = <.001*; 95% CI, 0.08-0.77), bleeding during and after surgery (I 11 of 183 [6.1%] vs C 22 of 143 [15.4%]; P = <.001*; 95% CI, 0.16-0.75), and postoperative ileus (I 9 of 183 [4.9%] vs C 29 of 143 [20.3%]; P < .001*; 95% CI, 0.09-0.45).
Higher rates in I group of nausea/vomiting (I 25 of 183 [13.7%] vs C 5 of 143 [3.5%];P = <.001*; 95% CI, 1.62-11.71) and higher rates of documented delirium (I 52 of 183 [28.4%] vs C 8 of 143 [5.6%]; P = <.001*; 95% CI, 3.06-14.65). |
No comments |
|
Natesan, 2022 |
Type of study: Retrospective cohort study
Setting and country: tertiary hospital in Singapore
Funding and conflicts of interest: Not reported |
Inclusion criteria: Eligible patients included those who were acutely admitted to vascular surgery, aged 65 years, had 1 or more geriatric syndromes (dementia, a previous history of delirium, falls, postural hypotension) and 1 or more comorbidities (diabetes mellitus, ischemic heart disease, old stroke disease, chronic kidney disease), and were scheduled for vascular surgery or endovascular interventions. The types of vascular surgical procedure included major amputation, minor amputation, lower limb angioplasty, lower limb angioplasty with minor amputation, peripheral arterial bypass, and abdominal aortic surgery (open and closed).
Exclusion criteria: NR
N total at baseline: Intervention: 198 Control: 135
Important prognostic factors2: For example age (range): I: 77 (63-94) C: 76 (65-93)
Sex: I: 53% M C: 54% M
Groups comparable at baseline? Yes for age, gender, and operative procedure. However, they were not comparable for preoperative comorbidities, operative procedures.
|
Describe intervention (treatment/procedure/test):
Patients who fulfilled the criteria were referred to a geriatric surgical service. The geriatric team assessed the patients by performing a comprehensive geriatric assessment, identifying major contributors of frailty (cognitive impairment, low mood, malnutrition), optimizing the risk factors for delirium, and helping the patient and family with shared decision making for surgical intervention and postoperative discharge planning. In the preoperative period, each patients was seen 2-3 days before surgery by the geriatric team, to optimize delirium, depression and anxiety, and malnutrition. In the postoperative period, the geriatric team observed the patients postoperatively on a daily basis (5 days a week, Monday-Friday) to optimize comorbidity treatments and address postoperative complications early. The geriatric team helped the surgical team manage medications on a daily basis. The geriatric team also managed postoperative pain relief and discontinued unnecessary intravenous lines and urinary catheters. The team also encouraged the patient to sit out of bed and made early referrals to physiotherapy. An advanced practice geriatric nurse on the geriatric surgical team spoke with family members to identify social support and caregiver stress for discharge planning. She also identified the rehabilitation potential of each patient through consultations with therapists and coordinated early transfers to community hospitals. Suitable cases
Additionally, patients in the IG received a comprehensive geriatric assessment, delirium prevention, shared decision making support, depression and anxiety prevention, and malnutrition prevention |
Describe control (treatment/procedure/test):
Retrospective cohort of 135 vascular patients without geriatric surgical service comanagement from January 2014 to July 2015 who would have fulfilled the criteria for geriatric consultation. |
Length of follow-up: 30-days
Loss-to-follow-up: NR
Incomplete outcome data: NR
|
Outcome measures and effect size (include 95%CI and p-value if available):
Length of stay Lower length of stay in the I group (I 11.6 days vs C 20.8 days, p=0.001*).
Complications No difference in postoperative delirium (I 22 (11%) vs C 14 (10%), p=n/s).
No difference in fluid overload (I 6 (3%) vs C 10 (7%), p=0.073).
No difference in acute myocardial infarction ((I 11 (6%) vs C 8 (6%), p=n/s).
No difference in nosocomial pneumonia (I 16 (8%) vs C 19 (14%), p=0.101).
Less nosocomial urinary infections (I 6 (3%) vs C 16 (12%), p=0.003).
Readmission Lower 30-day readmission (I 44 (22%) vs C 46 (34%), p=0.011). |
Article also describes results for an ‘early’ period, directly after implementation of the intervention, but these results are not reported here. This because other studies also have a washout period. |
|
Pernik, 2021 |
Type of study: Retrospective cohort study
Setting and country: USA
Funding and conflicts of interest: Reported |
Inclusion criteria: Patients with an increased perioperative risk of adverse outcomes were included.
Exclusion criteria: Patients who underwent non-spinal procedures, emergency, outpatient, or observational (<24 hour stay) surgery were excluded. We also excluded procedures involving only lumbar laminectomy without fusion, anterior cervical discectomy without myelopathy, spinal cord stimulator placement or replacement, arteriovenous fistula resection, and spinal cord or column tumours, as these cases are not referred to UTSW POSH.
N total at baseline: Intervention: 147 Control: 177
Important prognostic factors2: For example age ± SD: I: 75.6 ± 5.2 C: 71.5 ± 5.4
Sex: I: 18% M C: 12% M
Groups comparable at baseline? Patients in the I group were older, had more comorbidities and more frequent preoperative alcohol use, less often anxiety, less often hearing aid use, greater proportion of pelvic fixation, and a shorter mean aesthetic duration
|
Describe intervention (treatment/procedure/test):
Once referred to the program patients underwent a pre-operative assessment with a geriatrician within 30 days of their procedure. The geriatricians performed a comprehensive assessment including discussion of goals and expectations for surgery, functional assessment, assessment of nutritional status, evaluation of medical comorbidities, social support, cognitive assessment, evaluation for depression, thorough medication review, and discontinuation of high risk medications when possible. Patients and families also received specific education on delirium and delirium prevention. Results and recommendations from this evaluation
Additionally, patients in the IG received preoperative geriatric assessment, intraoperative specialized anaesthesia protocol and minimize time under anaesthesia, and postoperative delirium prevention |
Describe control (treatment/procedure/test):
Standard care |
Length of follow-up: 90 days post-operative
Loss-to-follow-up: NR
Incomplete outcome data: NR
|
Outcome measures and effect size (include 95%CI and p-value if available):
Delirium incidence and recognition No difference in delirium incidence (I 17 (11.6%) vs C 34 (19.2%); RR = 0.60; CI = 0.35–1.03; NNT = 14; P = .065).
Recognition of patients with delirium significantly increased in the I group (I 13 (76.5%) vs C 8 (23.5%); RR = 3.07; CI = 1.57–6.00; NNT = 3; P = .001*).
Patient outcomes Lower hospital LOS in the I group (I 4.7 d ± 2.1 vs C 5.4 ± 3.1, p=0.016*).
No difference in ICU LOS (I 0.7 ± 1.0 vs C 0.8 ± 1.3, p=0.40). No difference in postoperative day walking (I 1.7 ± 1.7 vs C 1.7 ± 1.2, p=0.77).
No difference in 7-day readmission (I 3 (2.1%) vs C 4 (2.3%), p>0.99), 30-day readmission (I 8 (5.4%) vs C 10 (5.7%), p=0.94), and 90-day readmission (I 12 (9.5%) vs C 13 (7.3%), p=0.48). |
No comments |
|
Shahrokni, 2020 |
Type of study: Retrospective cohort study
Setting and country: Tertiary care (single-centre) Memorial Sloan Kettering Cancer Centre, New York, United States of America
Funding and conflicts of interests: Reported |
Inclusion criteria: Patients aged 75 years and older who underwent cancer-related surgical treatment of various cancer types
Exclusion criteria: Procedures with length of stay of 0 days Missing mortality data (n = 78)
N total at baseline: Intervention: 1020 Control: 872
Important prognostic factors age ± SD: I: 81 ± 4 C: 80 ± 4
Sex I: 47.8% M C: 51.6% M
Groups comparable at baseline?
Intervention group is slightly older. ASA score was similar. Groups were not similar in procedure type (ie. colorectal, gynaecological, urology, thoracic etc.)
|
Describe intervention:
Geriatic comanagement includes 2 phases: preoperative and postoperative care. Referral for preoperative geriatric evaluation is based on the surgery team’s preference and clinical judgment, not using a formal frailty screening tool.
Preoperative evaluation consists of the electronic Rapid Fitness Assessment. After discussion with the surgery and anesthesiology teams, the geriatrics team recommends interventions aimed at optimizing the patient’s status (ie. Consultation with a cardiologists, exercise recommendations, etc.).
Postoperative phase: geriatrics service visits inpatients on a consultative basis on postoperative day 1 and 3, and further follow up if necessary. Assist with management of comorbid conditions and reintroduction of medications. Implement delirium-reducing interventions, assisting with early mobility, prevention of complications, pain management. Plan for follow up with community clinicians is recommended.
Outpatient care is provided by the surgical team only. |
Describe control:
Patients whose care is managed by the surgical service, without geriatric comanagement, may undergo preoperative evaluation by a nongeriatrician (ie. Cardiologist or internist). A geriatric consultation may be requested during the postoperative inpatient period. |
Length of follow-up 90 days
Incomplete outcome data Mortality data missing of 78 patients, integrated in exclusion criteria |
Outcome measures and effect size (include 95%CI and p-value if available):
Length of stay: Intervention group had a longer length of stay (median (IQR)): I 5 (3-8) days vs. C 4 (2-7) days; [p < 0.001)
90-day mortality Patients in the I group were less likely to die within 90 days after surgical treatment (OR 0.43 95% CI 0.28-0.67; p<0.001).
Adverse surgical outcomes within 30 days (major complication, readmission or emergency room visit) No difference between groups: OR 0.93 95%CI 0.73-1.18); p = 0.54 |
|
|
Yee, 2022 |
Type of study: Prospective cohort study
Setting and country: One acute hospital (Queen Mary Hospital) and two rehabilitation hospitals (Fung Yiu King Hospital and Maclehose Medical Rehabilitation Hospital) in Hong Kong.
Funding and conflicts of interest: Reported |
Inclusion criteria: Inclusion criteria were age more than or equal to 65; diagnosis of acute (time of injury within 14 days) isolated hip fracture patients from low energy trauma.
Exclusion criteria: Exclusion criteria included high-energy trauma, pathological fractures, multiple trauma, or old fractures that occurred more than 2 weeks ago.
N total at baseline: Intervention: 207 Control: 194
Important prognostic factors2: For example age ± SD: I: 83.6 ± 8.2 C: 84.8 ± 7.6
Sex: I: 28.5% M C: 26.8% M
Groups comparable at baseline? No, patients in the I group more often lived at home and had less mean postoperative blood transfusions.
|
Describe intervention (treatment/procedure/test):
The interventional model differed in the addition of a geriatrician during the postoperative phase, who co-managed the patient in both the acute and rehabilitation hospital. An orthogeriatric speciality ward was set up and implemented in Nov 2018 to concentrate resources and manpower. The new system championed collaboration between the orthopaedic surgeons and the geriatricians. In the acute ward, cases were co-managed during combined ward rounds which occurred 3 times per week. The geriatrician actively reviewed all hip fracture patients to allow prompt diagnosis and management of medical complications, optimization of pain control and monitoring of comorbidities.
Additionally, patients in the IG received expedited and streamlined transferral of patients from acute hospital to rehabilitation hospital |
Describe control (treatment/procedure/test):
In the conventional model, patients were admitted to an acute orthopaedic ward where an orthopaedic surgeon was responsible for managing care and treatment of all medical problems, including both orthopaedic and non-orthopaedic–related complications. Medical and geriatric input was only available upon ad hoc request and these interdisciplinary referrals occurred after the complications had already arisen. For semi-urgent referrals, the patients would be reviewed by the physician after 2 days, leading to delays in management and prolonged length of stay. |
Length of follow-up: One year after the index fracture
Loss-to-follow-up: NR
Incomplete outcome data: NR
|
Outcome measures and effect size (include 95%CI and p-value if available):
Length of stay Less patients with extended LOS in the I group (I 39.1% vs C 64.4%, difference 25.3% (95%CI 15.838% ; 34.767%), p<0.001*).
Lower median acute hospital LOS (I median 7.0 IQR 3-11 days vs C median 8.0 IQR 4-12 days; log-rank P=.001*; unadjusted HR for hospital discharge, 1.284 [95% CI, 1.053–1.566]; P = .01*; adjusted HR, 1.309 [95% CI,1.070–1.602]; P=.009*).
Lower median rehabilitation hospital LOS (I median 16.0 IQR 9-23 vs C median 18 IQR 9-27 days; og-rank P=.001*; unadjusted HR for hospital discharge, 1.391 [95% CI, 1.124–1.723]; P=.002*; adjusted HR, 1.357 [95% CI, 1.095–1.682]; P=.005*).
No difference in preoperative LOS (both median 1 IQR 0-2 dats, log-rank P=0.38).
Lower median postoperative LOS in the I group (I median 4.0 IQR 1-7 vs C median 6.0 IQR 2-10, log-rank p<0.001*).
Mortality No difference in 30-day mortality (I 1.9% vs C 0%; difference, 1.9% [95% CI, .0%–3.8%]; P=.99), 3-month mortality (I 2.9% vs C 3.1%; difference, 0.2% [95% CI,-3.2% to 3.6%]; P=.56), 6-month mortality (I 4.8% vs C 7.2%; difference, 2.4% [95% CI,-2.4%–7.3%]; P=.24), and 12-month mortality (I 10.6% vs C 13.9%; difference, 3.3% [95% CI,-3.1%–9.7%]; P=.24) after adjusting for covariates.
Functional recovery Higher median MBI before discharge in the I group (I median 81 IQR 27 vs C median 63.5 IQR 28, p<0.001*).
Higher EMS before discharge from the rehabilitation hospital in the I group (I median 12 IQR 8 vs C 9 IQR 8, p=0.07*).
Complications Less chest infections in the I group (I 11 (5.3%) vs C 21 (10.8%), difference 5.5% [95%CI 0.2%–10.9%], P=0.04*).
No statistically significant difference in other complications or presence of any medical complications; surgical complications, postoperative blood transfusion, wound complications, urinary tract infection, acute retention of urine, delirium, gastrointestinal bleeding, renal failure, and any medical complications.
Osteoporosis More prescription of bisphosphonate (I 138 (66.7%) vs C 25 (12.9%); difference, 53.8% [95% CI, 45.8%–61.7%], P<.001*).
There was no difference in the number of subsequent fractures within 1 year of index fracture between the orthogeriatric group and conventional group (3 (1.4%) vs 6 (3.1%), difference, 1.6% [95% CI,-1.3% to 4.6%], P=.27).
Discharge destination from the rehabilitation hospital No difference in the amount of patients being able to go back to their home (I 96 (69.1%) vs 76 (71.0%); difference, 2.0% [95% CI,-9.6 to 13.5]; P=.74).
Readmission rates No difference in 28-days hospital unplanned readmission rate (I 12.6% vs C 14.9%, p=0.55).
No difference in readmission due to medical reasons (I 8.2% vs C 11.3%, p=0.55).
No difference in readmission due to orthopaedic reasons (I 4.3% vs C 3.6%, p=0.55). |
No comments |
NM: not measured; IG: intervention group; CG: control group, SD: standard deviation; NR: not reported; NA: not applicable
Evidence table for the pooled outcomes
|
Outcome |
Result |
Heterogeneity |
References |
|
Living at home independently |
RR 1.03 [0.81; 1.30] |
61% |
Bub (2022); McDonald (2018); Yee (2022) |
|
Complications |
RR 0.74 [0.56; 0.99] |
89% |
Bub (2022); Fan (2021); Hafner (2021); Kalmet (2019); McDonald (2018); Yee (2022) |
|
Delirium |
RR 0.96 [0.53; 1.75] |
88% |
Fan (2021); Hafner (2021); Kalmet (2019); McDonald (2018); Natesan (2022); Pernik (2021); Yee (2022) |
|
Urinary tract infections |
RR 0.55 [0.31; 0.99] |
72% |
Bub (2022); Fan (2021); Natesan (2022); Yee (2022) |
|
Pneumonia |
RR 0.60 [0.42; 0.85] |
0% |
Bub (2022); Fan (2021); Giannotti (2022) Natesan (2022) |
|
90-day readmission |
RR 0.86 [0.43; 1.73] |
26% |
Giannotti (2022); Pernik (2021) |
|
30-days readmission |
RR 0.63 [0.48; 0.83] |
0% |
Giannotti (2022); McDonald (2018); Natesan (2022); Pernik (2021) |
|
30-day mortality |
RR 0.85 [0.53; 1.38] |
0% |
Fan (2022); Giannotti (2022); Kalmet (2019); Yee (2022) |
|
In-hospital mortality |
RR 0.91 [0.16; 5.27] |
45% |
Bub (2022); Fan (2021); Hafner (2021) |
|
Length of stay |
SMD -0.15 [-0.39; 0.09] |
85% |
Bub (2022); Fan (2021); Hafner (2021); Kalmet (2019); Pernik (2021) |
Green = in favour of intervention (comanagement); light-grey = not significant/no effect; red = in favour of control; values between [ ] represent 95% confidence intervals; RR = risk ratio; SDM = standardized mean difference.
Table with exclusion reasons
|
Author (first year) |
Exclusion reason |
|
Adogwa (2018) |
Wrong population |
|
Aletto (2020) |
Wrong population |
|
Anighoro (2020) |
Wrong intervention |
|
Baroni (2019) |
Wrong population |
|
Baxter (2021) |
Full text not available |
|
Blauth (2021) |
Wrong population |
|
Blood (2019) |
Wrong population |
|
Branas (2018) |
Full text not available |
|
Breda (2022) |
Wrong population and wrong intervention |
|
Burton (2020) |
Wrong intervention |
|
Bryant (2019) |
Wrong intervention |
|
Castellví Valls (2018) |
Wrong intervention |
|
Chacón-Valenzuela (2022) |
Article in Spanish |
|
Cheng (2020) |
Wrong population |
|
Christiano (2021) |
Wrong intervention |
|
Civinini (2019) |
Wrong population |
|
Cooper (2020) |
Background article |
|
De Sire (2021) |
Wrong population |
|
De Vincentis (2021) |
Wrong population and wrong publication type |
|
Deeken (2022) |
Wrong population |
|
Deschodt (2020) |
Wrong population and wrong intervention |
|
Dottorini (2020) |
Background article |
|
Eamer (2018) |
Wrong intervention |
|
Festen (2021) |
Wrong intervention |
|
Figveld (2019) |
Background article |
|
Filippova (2019) |
Wrong population |
|
Flikweert (2021) |
Wrong population |
|
Fu (2022) |
Wrong intervention |
|
Fu (2022) |
Wrong intervention |
|
González-Quevedo (2020) |
Wrong intervention |
|
González-Quevedo (2022) |
Wrong intervention |
|
González-Senac (2021) |
Wrong intervention |
|
Greenstein (2019) |
Wrong population |
|
Gupta (2021) |
Wrong intervention |
|
Handoll (2021) |
Wrong population |
|
Igwe (2020) |
Review; reference checking performed but no relevant articles |
|
Janssen (2019) |
Review; reference checking performed but no relevant articles |
|
Jiménez (2019) |
Wrong comparison |
|
Jones (2020) |
Background article |
|
Ke (2019) |
Background article |
|
Khan (2020) |
Wrong population |
|
Kotsani (2021) |
Wrong publication type |
|
Kusen (2019) |
Wrong population |
|
Kusen (2021) |
Wrong population |
|
Kusen (2021) |
Wrong population |
|
Laubach (2021) |
Wrong population |
|
Lee (2021) |
Wrong population |
|
Leung (2018) |
Wrong population |
|
Li (2022) |
Wrong population |
|
Lin (2021) |
Wrong intervention |
|
Liu (2022) |
Wrong population |
|
Liu (2022) |
Wrong population |
|
Losh (2019) |
Wrong population |
|
Mason (2018) |
Wrong comparison |
|
McMillan (2022) |
Full text not available |
|
Min (2021) |
Background article |
|
Mudge (2020) |
Wrong population |
|
Mukherjee (2020) |
Wrong publication type and wrong population |
|
Murphy (2019) |
Wrong population |
|
Oberai (2018) |
Wrong population |
|
Ogawa (2022) |
Wrong population |
|
Olotu (2019) |
Review; reference checking performed but no relevant articles |
|
O'Mara-Gardner (2020) |
Wrong intervention |
|
Park (2022) |
Wrong population |
|
Parks (2022) |
Wrong publication type |
|
Partridge (2018) |
Wrong publication type |
|
Patel (2020) |
Wrong population |
|
Peng (2020) |
Wrong population |
|
Pfeufer (2020) |
Wrong comparison |
|
Piolo (2018) |
Background article |
|
Portelli Tremont (2022) |
Wrong comparison |
|
Quaranta (2021) |
Wrong outcomes |
|
Reguant (2019) |
Wrong population |
|
Rincón Gómez (2020) |
Article in Spanish |
|
Roberts (2021) |
Full text not available |
|
Roberts (2022) |
Wrong population |
|
Roll (2019) |
Wrong intervention |
|
Rostoft (2020) |
Wrong comparison |
|
Rubin (2018) |
Wrong intervention |
|
Saber (2022) |
Wrong population |
|
Sacks (2020) |
Background article |
|
Sanli (2019) |
Wrong comparison |
|
Schoeneberg (2021) |
Wrong population |
|
Schulz (2021) |
Wrong population |
|
Sedlock (2020) |
Wrong population |
|
Shen (2022) |
Wrong study design |
|
Shigemoto (2019) |
Wrong intervention |
|
Shigemoto (2021) |
Wrong intervention |
|
Shipway (2018) |
Wrong population |
|
Steffensmeier (2022) |
Wrong population |
|
Stephens (2019) |
Wrong population |
|
Styan (2018) |
Wrong population |
|
Su (2022) |
Wrong population |
|
Subramaniam (2020) |
Review; reference checking performed but no relevant articles |
|
Sura-Amonrattana (2021) |
Wrong population |
|
Tarazona-Santabalbina (2019) |
Wrong population |
|
Tarazona-Santabalbina (2021) |
Wrong population |
|
Thillainadesan (2021) |
Background article |
|
Thillainadesan (2022) |
Review; reference checking performed but no relevant articles |
|
Thillainadesan (2022) |
Wrong population: not all patients underwent surgery |
|
Van Dartel (2021) |
Wrong population |
|
Van der Vlies (2020) |
Wrong intervention |
|
Van Grootven (2021) |
Wrong population |
|
Van Heghe (2022) |
Wrong population |
|
Vilches-Moraga (2018) |
Wrong publication type and wrong population |
|
Wallace (2019) |
Wrong population |
|
Werner (2020) |
Wrong population |
|
Wiedl (2022) |
Wrong population: not all patients underwent surgery |
|
Wu (2019) |
Wrong population |
|
Zhang (2022) |
Wrong population |
|
Zhao (2022) |
Wrong population |
Verantwoording
Autorisatiedatum en geldigheid
Laatst beoordeeld : 09-04-2024
Laatst geautoriseerd : 09-04-2024
Geplande herbeoordeling : 09-04-2028
Algemene gegevens
In samenwerking met : bovenstaande partijen, Verenso, Verpleegkundigen en Verzorgenden Nederland – Verpleegkundig Specialisten en Genero
De ontwikkeling van deze richtlijn werd ondersteund door het Kennisinstituut van de Federatie Medisch Specialisten (www.demedischspecialist.nl/kennisinstituut) en werd gefinancierd uit de Kwaliteitsgelden Medisch Specialisten (SKMS).
De financier heeft geen enkele invloed gehad op de inhoud van de richtlijn.
Samenstelling werkgroep
Voor het ontwikkelen van de richtlijn is in 2021 een multidisciplinaire werkgroep ingesteld, bestaande uit vertegenwoordigers van alle relevante specialismen (zie hiervoor de Samenstelling van de werkgroep) die betrokken zijn bij chirurgie bij kwetsbare ouderen.
Werkgroep
Dr. D.E. (Didy) Jacobsen (voorzitter), Nederlandse Vereniging voor Klinische Geriatrie
Dr. H.A. (Harmke) Polinder-Bos, Nederlandse Vereniging voor Klinische Geriatrie
Dr. S. (Suzanne) Festen, Nederlandse Internisten Vereniging
N.S. (Niamh) Landa-Hoogerbrugge, MSc Verpleegkundigen en Verzorgenden Nederland en Verpleegkundigen en Verzorgenden Nederland – Verpleegkundig Specialisten
Dr. H.P.A. (Eric) van Dongen, Nederlandse Vereniging voor Anesthesiologie
Dr. J. (Juul) Tegels, Nederlandse Vereniging voor Heelkunde
Drs. P.E. (Petra) Flikweert, Nederlandse Orthopaedische Vereniging
Drs. H.P.P.R. (Heike) de Wever, Verenso
Patiëntvertegenwoordiger
M.R. (Marike) Abel- van Nieuwamerongen, Genero
Met ondersteuning van
Drs. E.A. (Emma) Gans, adviseur Kennisinstituut van de Federatie Medisch Specialisten
Drs. L.A.M. (Liza) van Mun, junior adviseur Kennisinstituut van de Federatie Medisch Specialisten
Dr. T. (Tim) Christen, adviseur Kennisinstituut van de Federatie Medisch Specialisten
Dr. J.F. (Janke) de Groot, senior adviseur Kennisinstituut van de Federatie Medisch Specialisten
Y. (Yvonne) van Kempen, projectsecretaresse, Kennisinstituut van de Federatie Medisch Specialisten
Belangenverklaringen
De Code ter voorkoming van oneigenlijke beïnvloeding door belangenverstrengeling is gevolgd. Alle werkgroepleden hebben schriftelijk verklaard of zij in de laatste drie jaar directe financiële belangen (betrekking bij een commercieel bedrijf, persoonlijke financiële belangen, onderzoeksfinanciering) of indirecte belangen (persoonlijke relaties, reputatiemanagement) hebben gehad. Gedurende de ontwikkeling of herziening van een module worden wijzigingen in belangen aan de voorzitter doorgegeven. De belangenverklaring wordt opnieuw bevestigd tijdens de commentaarfase.
Een overzicht van de belangen van werkgroepleden en het oordeel over het omgaan met eventuele belangen vindt u in onderstaande tabel. De ondertekende belangenverklaringen zijn op te vragen bij het secretariaat van het Kennisinstituut van de Federatie Medisch Specialisten.
|
Naam |
Hoofdfunctie |
Nevenwerkzaamheden |
Persoonlijke Financiële Belangen |
Persoonlijke Relaties |
Extern gefinancierd onderzoek |
Intellectuele belangen en reputatie |
Overige belangen |
Datum |
Actie |
|
Didy Jacobsen (voorzitter) |
Internist-ouderengeneeskunde, academisch medisch specialist, Radboudumc afdeling geriatrie |
Geen |
Geen |
Geen |
- |
Niet aanwezig. |
Nieuwe onderzoeksvoorstel op het gebied van zorgpadoptimalisatie/zorgpadontwikkeling voor ouderen met hoogrisico plaveiselcelcarcinoom. Vanuit dermatologie (Radboudumc) wordt dit opgezet. Ik denk mee voor geriatrisch perspectief. |
30-09-2021 |
Geen restricties |
|
Heike de Wever |
Specialist ouderengeneeskunde, kaderarts geriatrische revalidatie bij de stichting TanteLouise |
Lid van kerngroep kaderartsen geriatrische revalidatie van Verenso (onbetaald) |
Geen |
Neen |
Neen |
Neen |
Neen |
1-12-2021 |
Geen restricties |
|
Suzanne Festen |
Internist ouderengeneeskunde |
Nvt |
Geen belangenverstrengeling |
Geen belangenverstrengeling |
Betrokken bij ZIN subsidie en KWF subsidie |
Behoudens dat de inhoud raakt aan mijn expertise in klinisch werk en onderzoek geen belangen. |
Nvt |
11-11-2021 |
Geen restricties |
|
Eric van Dongen |
Anesthesioloog maatschap anesthesiologie, ic en pijnbestrijding |
Bestuur E infuse, vacatiegelden |
Geen |
Geen |
Geen |
Co-founder AGE MDO, ketenzorg perioperatief proces kwetsbare oudere |
Geen |
11-10-2021 |
Geen restricties |
|
Harmke Polinder- Bos |
Klinisch Geriater, Erasmus MC, Rotterdam |
Niet van toepassing |
Niet van toepassing |
Niet van toepassing |
2021: COOP-studie |
Behoudens dat de inhoud raakt aan mijn expertise in klinisch werk en onderzoek geen belangen. |
Geen |
4-10-2021 |
Geen restricties |
|
Niamh Landa - Hoogerbruggen |
Verpleegkundig specialist GE-chirurgie/klinische geriatrie Maasstad ziekenhuis |
Bestuurslid V&VN geriatrie en gerontologie |
Nee |
Nee |
Nee |
Neveneffect kan zijn meer expertise ontwikkelen op dit gebied en zodoende integreren in huidig zorgpad dieontwikkeld is |
Nee |
29-09-2021 |
Geen restricties |
|
Juul Tegels |
Lid richtlijnwerkgroep |
Traumachirurg, fellow |
Geen |
Geen |
Geen |
|
Geen |
18-3-2022 |
Geen restricties |
|
Petra Flikweert |
Orthopedisch chirurg, Reinier haga orthopedisch centrum, zoetermeer. Vanuit de NOV gemandateerde voor de werkgroep. |
Commissie kwaliteit - Haga ziekenhuis - onbetaald Commissie kwaliteit - NOV - onbetaald Onderwijscommissie NOV - onbetaald |
Geen |
Geen |
Geen |
Geen |
Geen |
3-9-2023 |
Geen restricties |
|
Marike Abel- van Nieuwamerongen |
Lid ouderen- en mantelzorgforum; Genero (onbezoldigd> onkostenvergoeding) |
Lid RvT landelijke medezeggenschapsorganisatie cliënten Lid Cliëntenraad ziekenhuis in Tilburg Lid Cliëntenraad 1e lijnsorganisatie in Etten-Leur (onbetaalde functies, wel onkostenvergoeding) |
Geen |
Geen |
Geen |
Geen |
Geen |
6-9-2023 |
Geen restricties |
Inbreng patiëntenperspectief
Er werd aandacht besteed aan het patiëntenperspectief door het uitnodigen van de Patiëntenfederatie Nederland en KBO-PCOB voor de schriftelijke knelpuntenanalyse. De verkregen input is meegenomen bij het opstellen van de uitgangsvragen, de keuze voor de uitkomstmaten en bij het opstellen van de overwegingen. De conceptrichtlijn is tevens voor commentaar voorgelegd aan Genero, KBO-PCOB en Patiëntenfederatie Nederland en de eventueel aangeleverde commentaren zijn bekeken en verwerkt.
Kwalitatieve raming van mogelijke financiële gevolgen in het kader van de Wkkgz
Bij de richtlijn is conform de Wet kwaliteit, klachten en geschillen zorg (Wkkgz) een kwalitatieve raming uitgevoerd of de aanbevelingen mogelijk leiden tot substantiële financiële gevolgen. Bij het uitvoeren van deze beoordeling zijn richtlijnmodules op verschillende domeinen getoetst (zie het stroomschema op de Richtlijnendatabase).
Uit de kwalitatieve raming blijkt dat er [waarschijnlijk geen/ mogelijk] substantiële financiële gevolgen zijn, zie onderstaande tabel.
|
Module |
Uitkomst kwalitatieve raming |
Toelichting |
|
Module Perioperatieve samenwerking bij chirurgie bij kwetsbare ouderen |
geen substantiële financiële gevolgen |
Hoewel uit de toetsing volgt dat de aanbeveling(en) breed toepasbaar zijn (>40.000 patiënten), volgt ook uit de toetsing dat het geen nieuwe manier van zorgverlening of andere organisatie van zorgverlening betreft, het geen toename in het aantal in te zetten voltijdsequivalenten aan zorgverleners betreft en het geen wijziging in het opleidingsniveau van zorgpersoneel betreft. Er worden daarom geen substantiële financiële gevolgen verwacht. |
Werkwijze
AGREE
Deze richtlijnmodule is opgesteld conform de eisen vermeld in het rapport Medisch Specialistische Richtlijnen 2.0 van de adviescommissie Richtlijnen van de Raad Kwaliteit. Dit rapport is gebaseerd op het AGREE II instrument (Appraisal of Guidelines for Research & Evaluation II; Brouwers, 2010).
Knelpuntenanalyse en uitgangsvragen
Tijdens de voorbereidende fase inventariseerden de werkgroep de knelpunten in de chirurgische zorg voor kwetsbare ouderen. De werkgroep beoordeelde de aanbevelingen uit de eerdere richtlijnmodule (NVKG, 2016) op noodzaak tot revisie. Tevens zijn er knelpunten aangedragen door betrokken partijen via een schriftelijke knelpuntenanalyse.
Op basis van de uitkomsten van de knelpuntenanalyse zijn door de werkgroep concept-uitgangsvragen opgesteld en definitief vastgesteld.
Uitkomstmaten
Na het opstellen van de zoekvraag behorende bij de uitgangsvraag inventariseerde de werkgroep welke uitkomstmaten voor de patiënt relevant zijn, waarbij zowel naar gewenste als ongewenste effecten werd gekeken. De werkgroep waardeerde deze uitkomstmaten volgens hun relatieve belang bij de besluitvorming rondom aanbevelingen, als cruciaal (kritiek voor de besluitvorming), belangrijk (maar niet cruciaal) en onbelangrijk. Tevens definieerde de werkgroep tenminste voor de cruciale uitkomstmaten welke verschillen zij klinisch (patiënt) relevant vonden.
Methode literatuursamenvatting
Een uitgebreide beschrijving van de strategie voor zoeken en selecteren van literatuur en de beoordeling van de risk-of-bias van de individuele studies is te vinden onder ‘Zoeken en selecteren’ onder Onderbouwing. De beoordeling van de kracht van het wetenschappelijke bewijs wordt hieronder toegelicht.
Beoordelen van de kracht van het wetenschappelijke bewijs
De kracht van het wetenschappelijke bewijs werd bepaald volgens de GRADE-methode. GRADE staat voor ‘Grading Recommendations Assessment, Development and Evaluation’ (zie http://www.gradeworkinggroup.org). De basisprincipes van de GRADE-methodiek zijn: het benoemen en prioriteren van de klinisch (patiënt) relevante uitkomstmaten, een systematische review per uitkomstmaat, en een beoordeling van de bewijskracht per uitkomstmaat op basis van de acht GRADE-domeinen (domeinen voor downgraden: risk of bias, inconsistentie, indirectheid, imprecisie, en publicatiebias; domeinen voor upgraden: dosis-effect relatie, groot effect, en residuele plausibele confounding).
GRADE onderscheidt vier gradaties voor de kwaliteit van het wetenschappelijk bewijs: hoog, redelijk, laag en zeer laag. Deze gradaties verwijzen naar de mate van zekerheid die er bestaat over de literatuurconclusie, in het bijzonder de mate van zekerheid dat de literatuurconclusie de aanbeveling adequaat ondersteunt (Schünemann, 2013; Hultcrantz, 2017).
|
GRADE |
Definitie |
|
Hoog |
|
|
Redelijk |
|
|
Laag |
|
|
Zeer laag |
|
Bij het beoordelen (graderen) van de kracht van het wetenschappelijk bewijs in richtlijnen volgens de GRADE-methodiek spelen grenzen voor klinische besluitvorming een belangrijke rol (Hultcrantz, 2017). Dit zijn de grenzen die bij overschrijding aanleiding zouden geven tot een aanpassing van de aanbeveling. Om de grenzen voor klinische besluitvorming te bepalen moeten alle relevante uitkomstmaten en overwegingen worden meegewogen. De grenzen voor klinische besluitvorming zijn daarmee niet één op één vergelijkbaar met het minimaal klinisch relevant verschil (Minimal Clinically Important Difference, MCID). Met name in situaties waarin een interventie geen belangrijke nadelen heeft en de kosten relatief laag zijn, kan de grens voor klinische besluitvorming met betrekking tot de effectiviteit van de interventie bij een lagere waarde (dichter bij het nuleffect) liggen dan de MCID (Hultcrantz, 2017).
Overwegingen (van bewijs naar aanbeveling)
Om te komen tot een aanbeveling zijn naast (de kwaliteit van) het wetenschappelijke bewijs ook andere aspecten belangrijk en worden meegewogen, zoals aanvullende argumenten uit bijvoorbeeld de biomechanica of fysiologie, waarden en voorkeuren van patiënten, kosten (middelenbeslag), aanvaardbaarheid, haalbaarheid en implementatie. Deze aspecten zijn systematisch vermeld en beoordeeld (gewogen) onder het kopje ‘Overwegingen’ en kunnen (mede) gebaseerd zijn op expert opinion. Hierbij is gebruik gemaakt van een gestructureerd format gebaseerd op het evidence-to-decision framework van de internationale GRADE Working Group (Alonso-Coello, 2016a; Alonso-Coello 2016b). Dit evidence-to-decision framework is een integraal onderdeel van de GRADE-methodiek.
Formuleren van aanbevelingen
De aanbevelingen geven antwoord op de uitgangsvraag en zijn gebaseerd op het beschikbare wetenschappelijke bewijs en de belangrijkste overwegingen, en een weging van de gunstige en ongunstige effecten van de relevante interventies. De kracht van het wetenschappelijk bewijs en het gewicht dat door de werkgroep wordt toegekend aan de overwegingen, bepalen samen de sterkte van de aanbeveling. Conform de GRADE-methodiek sluit een lage bewijskracht van conclusies in de systematische literatuuranalyse een sterke aanbeveling niet a priori uit, en zijn bij een hoge bewijskracht ook zwakke aanbevelingen mogelijk (Agoritsas, 2017; Neumann, 2016). De sterkte van de aanbeveling wordt altijd bepaald door weging van alle relevante argumenten tezamen. De werkgroep heeft bij elke aanbeveling opgenomen hoe zij tot de richting en sterkte van de aanbeveling zijn gekomen.
In de GRADE-methodiek wordt onderscheid gemaakt tussen sterke en zwakke (of conditionele) aanbevelingen. De sterkte van een aanbeveling verwijst naar de mate van zekerheid dat de voordelen van de interventie opwegen tegen de nadelen (of vice versa), gezien over het hele spectrum van patiënten waarvoor de aanbeveling is bedoeld. De sterkte van een aanbeveling heeft duidelijke implicaties voor patiënten, behandelaars en beleidsmakers (zie onderstaande tabel). Een aanbeveling is geen dictaat, zelfs een sterke aanbeveling gebaseerd op bewijs van hoge kwaliteit (GRADE gradering HOOG) zal niet altijd van toepassing zijn, onder alle mogelijke omstandigheden en voor elke individuele patiënt.
|
Implicaties van sterke en zwakke aanbevelingen voor verschillende richtlijngebruikers |
||
|
|
Sterke aanbeveling |
Zwakke (conditionele) aanbeveling |
|
Voor patiënten |
De meeste patiënten zouden de aanbevolen interventie of aanpak kiezen en slechts een klein aantal niet. |
Een aanzienlijk deel van de patiënten zou de aanbevolen interventie of aanpak kiezen, maar veel patiënten ook niet. |
|
Voor behandelaars |
De meeste patiënten zouden de aanbevolen interventie of aanpak moeten ontvangen. |
Er zijn meerdere geschikte interventies of aanpakken. De patiënt moet worden ondersteund bij de keuze voor de interventie of aanpak die het beste aansluit bij zijn of haar waarden en voorkeuren. |
|
Voor beleidsmakers |
De aanbevolen interventie of aanpak kan worden gezien als standaardbeleid. |
Beleidsbepaling vereist uitvoerige discussie met betrokkenheid van veel stakeholders. Er is een grotere kans op lokale beleidsverschillen. |
Organisatie van zorg
In de knelpuntenanalyse en bij de ontwikkeling van de richtlijnmodule is expliciet aandacht geweest voor de organisatie van zorg: alle aspecten die randvoorwaardelijk zijn voor het verlenen van zorg (zoals coördinatie, communicatie, (financiële) middelen, mankracht en infrastructuur). Randvoorwaarden die relevant zijn voor het beantwoorden van deze specifieke uitgangsvraag zijn genoemd bij de overwegingen. Meer algemene, overkoepelende, of bijkomende aspecten van de organisatie van zorg worden behandeld in de module Organisatie van zorg.
Commentaar- en autorisatiefase
De conceptrichtlijnmodule werd aan de betrokken (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd ter commentaar. De commentaren werden verzameld en besproken met de werkgroep. Naar aanleiding van de commentaren werd de conceptrichtlijnmodule aangepast en definitief vastgesteld door de werkgroep. De definitieve richtlijnmodule werd aan de deelnemende (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd voor autorisatie en door hen geautoriseerd dan wel geaccordeerd.
Referenties
Agoritsas T, Merglen A, Heen AF, Kristiansen A, Neumann I, Brito JP, Brignardello-Petersen R, Alexander PE, Rind DM, Vandvik PO, Guyatt GH. UpToDate adherence to GRADE criteria for strong recommendations: an analytical survey. BMJ Open. 2017 Nov 16;7(11):e018593. doi: 10.1136/bmjopen-2017-018593. PubMed PMID: 29150475; PubMed Central PMCID: PMC5701989.
Alonso-Coello P, Schünemann HJ, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Rada G, Rosenbaum S, Morelli A, Guyatt GH, Oxman AD; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ. 2016 Jun 28;353:i2016. doi: 10.1136/bmj.i2016. PubMed PMID: 27353417.
Alonso-Coello P, Oxman AD, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Vandvik PO, Meerpohl J, Guyatt GH, Schünemann HJ; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 2: Clinical practice guidelines. BMJ. 2016 Jun 30;353:i2089. doi: 10.1136/bmj.i2089. PubMed PMID: 27365494.
Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, Fervers B, Graham ID, Grimshaw J, Hanna SE, Littlejohns P, Makarski J, Zitzelsberger L; AGREE Next Steps Consortium. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010 Dec 14;182(18):E839-42. doi: 10.1503/cmaj.090449. Epub 2010 Jul 5. Review. PubMed PMID: 20603348; PubMed Central PMCID: PMC3001530.
Hultcrantz M, Rind D, Akl EA, Treweek S, Mustafa RA, Iorio A, Alper BS, Meerpohl JJ, Murad MH, Ansari MT, Katikireddi SV, Östlund P, Tranæus S, Christensen R, Gartlehner G, Brozek J, Izcovich A, Schünemann H, Guyatt G. The GRADE Working Group clarifies the construct of certainty of evidence. J Clin Epidemiol. 2017 Jul;87:4-13. doi: 10.1016/j.jclinepi.2017.05.006. Epub 2017 May 18. PubMed PMID: 28529184; PubMed Central PMCID: PMC6542664.
Medisch Specialistische Richtlijnen 2.0 (2012). Adviescommissie Richtlijnen van de Raad Kwalitieit. http://richtlijnendatabase.nl/over_deze_site/over_richtlijnontwikkeling.html
Neumann I, Santesso N, Akl EA, Rind DM, Vandvik PO, Alonso-Coello P, Agoritsas T, Mustafa RA, Alexander PE, Schünemann H, Guyatt GH. A guide for health professionals to interpret and use recommendations in guidelines developed with the GRADE approach. J Clin Epidemiol. 2016 Apr;72:45-55. doi: 10.1016/j.jclinepi.2015.11.017. Epub 2016 Jan 6. Review. PubMed PMID: 26772609.
NVKG. Richtlijn Behandeling kwetsbare ouderen bij chirurgie. 2016. https://richtlijnendatabase.nl/richtlijn/behandeling_kwetsbare_ouderen_bij_chirurgie/generieke_zorgpad.html
Schünemann H, Brożek J, Guyatt G, et al. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group, 2013. Available from http://gdt.guidelinedevelopment.org/central_prod/_design/client/handbook/handbook.html.
Schünemann HJ, Oxman AD, Brozek J, Glasziou P, Jaeschke R, Vist GE, Williams JW Jr, Kunz R, Craig J, Montori VM, Bossuyt P, Guyatt GH; GRADE Working Group. Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. BMJ. 2008 May 17;336(7653):1106-10. doi: 10.1136/bmj.39500.677199.AE. Erratum in: BMJ. 2008 May 24;336(7654). doi: 10.1136/bmj.a139.
Schünemann, A Holger J [corrected to Schünemann, Holger J]. PubMed PMID: 18483053; PubMed Central PMCID: PMC2386626.
Wessels M, Hielkema L, van der Weijden T. How to identify existing literature on patients' knowledge, views, and values: the development of a validated search filter. J Med Libr Assoc. 2016 Oct;104(4):320-324. PubMed PMID: 27822157; PubMed Central PMCID: PMC5079497.
Zoekverantwoording
Zoekacties zijn opvraagbaar. Neem hiervoor contact op met de Richtlijnendatabase.