Non-farmacologische pijnbestrijding
Uitgangsvraag
Wat is de meerwaarde van non-farmacologische pijnbestrijding bij de behandeling van kinderen met pijn?
Aanbeveling
Gebruik bij alle vormen van pijn altijd non-farmacologische pijnbestrijding. Baseer de pijnbestrijding primair op het biopsychosociaal model, waarbij de situatie op alle domeinen (biologische, psychologische en sociale factoren) zoveel mogelijk genormaliseerd wordt.
Gebruik standaard helpend taalgebruik om verwachtingen en pijn cognities van ouders en kind op een positieve manier te beinvloeden.
Geef non-farmacologische pijnbestrijding aangepast op maat, afhankelijk van de leeftijd en ontwikkelingsniveau van het kind, de wensen van ouder en kind, en wat in het verleden al is toegepast en zo nodig multidisciplinair (zie figuur 2 Stroomschema).
Overweeg in het geval van acute pijn en chronische pijn de volgende mogelijkheden (zie ook figuur 2 Stroomschema):
- Psychoeducatie;
- Afleidingstechnieken;
- Psychologische interventie;
- Stimuleren van het opbouwen van het activiteiten- en participatieniveau.
Hierbij kunnen de volgende zorgverleners betrokken worden: medisch pedagogisch zorgverlener, medisch psycholoog, (kinder)fysiotherapeut en/of psychosomatisch fysiotherapeut, revalidatiearts.
Streef ernaar om een kind met chronische pijn dat al meerdere behandelingen heeft gehad en/of naar de derde lijn wordt verwezen, multidisciplinair te behandelen of naar een pijnteam te verwijzen (zie module Organisatie van zorg).
Gebruik voor postoperatieve pijn en Sedatie, Analgesie en niet-farmacologische interventies voor begeleiding van kinderen bij medische procedures desbetreffende richtlijnen.
Gebruik voor aandoeningsspecifieke behandeling van pijn desbetreffende richtlijnen, zoals functionele buikpijn bij kinderen, somatisch onvoldoende verklaarde klachten (SOLK) bij kinderen
Overwegingen
Voor- en nadelen van de interventie en de kwaliteit van het bewijs
In deze literatuursamenvatting is naar verschillende vormen non-farmacologische pijnbestrijding gekeken voor kinderen met acute en chronische pijn. Er is onderscheid gemaakt tussen psycho-educatie, afleidingstechnieken, ,helpend taalgebruik, fysieke interventies, psychologische en andere non-farmacologische interventies.
Voor de non-farmacologische techniek psycho-educatie werden vooral studies geïncludeerd die gebruik maakten van cognitieve gedragstherapie, psychologische behandelingen en biofeedback. Er was wel overlap in de geïncludeerde studies tussen de reviews, want Abbott (2017), Abbott (2018) en Gordon (2022) includeerden veel dezelfde studies. Hierbij werd gevonden dat psycho-educatie mogelijk effectief is en pijn kan verminderen, maar geen effect heeft op functioneren en kwaliteit van leven. Echter is de bewijskracht hiervan laag, wat voornamelijk wordt veroorzaakt doordat studiedeelnemers niet geblindeerd waren (risico op vertekening).
De studies die onderzoek deden naar afleidingstechnieken maakten gebruik van luisteren naar muziek. De review van See (2023) includeerde dezelfde artikelen als de review van Ting (2022). Voor de uitkomstmaten pijn en angst lijkt de kwaliteit van het bewijs onvoldoende om conclusies te kunnen trekken over de effecten van afleidingstechnieken. De kwaliteit van bewijs is voor deze uitkomsten zeer laag, wat met name het gevolg is van het niet blinderen van studiedeelnemers (risico op vertekening) en kleine en weinig studies. Er werden geen studies gevonden die de andere uitkomstmaten hebben gemeten. Daarom kan er geen conclusie worden getrokken over de effectiviteit van afleidingstechnieken bij kinderen.
De studies die psychologische interventies onderzochten, gingen over psychologische therapie of hypnotherapie. Er was wel overlap in de geïncludeerde studies tussen de reviews, want Abbott (2017), Abbott (2018) en Gordon (2022) includeerden veel dezelfde studies. Voor psychologische behandelingen werd geen effect gevonden op angst (gemiddelde bewijskracht) en kwaliteit van leven (lage bewijskracht) bij kinderen. Psychologische interventies zouden mogelijk pijn kunnen verminderen, maar de bewijskracht hiervan is laag. Er werd onvoldoende kwaliteit van bewijs gevonden om een conclusie te kunnen trekken over het effect op functioneren. De lagere kwaliteit van bewijs werd met name veroorzaakt door risico op vertekening en tegenstrijdige resultaten. Het bewijs over de effecten van hypnotherapie op pijn en kwaliteit van leven is van zeer lage kwaliteit, waardoor hier geen conclusies over kunnen worden getrokken.
In de literatuuranalyse werden studies geïncludeerd die gebruik maakten van relaxatie en gevoelsdagboek. Hierbij werd gevonden dat relaxatie mogelijk effectief is, maar de bewijskracht hiervan is laag. Het bewijs over de effecten van gevoelsdagboek op pijn en kwaliteit van leven is van zeer lage kwaliteit, waardoor hier geen conclusies over kunnen worden getrokken. De lage tot zeer lage bewijskracht wordt voornamelijk veroorzaakt door risico op vertekening door het niet blinderen van studiedeelnemers, kleine en weinig studies en heterogeniteit in de resultaten.
In de geïncludeerde reviews werd geen onderscheid gemaakt tussen de vier vooraf gedefinieerde subgroepen. De reviews van Abbott et al. (2017), Gordon et al. (2022) en Abbott et al. (2018) includeerden allen studies in kinderen tussen de 5 en 18 jaar. De overige studies includeerden kinderen onder de 18 of 21 jaar. Er is met behulp van ASReview nog gekeken naar eventuele observationele studies en RCTs voor de subgroep neonaten en pre-verbale kinderen, maar er zijn helaas geen studies gevonden in deze subgroep die voldoen aan de PICO. Daarnaast werden er geen studies gevonden die de belangrijke uitkomstmaten (opnameduur en kosten) meenamen als uitkomstmaat.
Samenvattend kan er op basis van de resultaten met lage bewijskracht richting gegeven worden aan de besluitvorming. Psycho-educatie, relaxatie en psychologische interventies kunnen een positief effect hebben op het verminderen van acute en chronische pijn bij kinderen. Er werd onvoldoende kwaliteit van bewijs gevonden om iets te kunnen concluderen over de effectiviteit van afleidingstechnieken, hypnotherapie, gevoelsdagboek en fysieke interventies.
De werking van non-farmacologische pijnbestrijding op pijnreductie is niet altijd duidelijk. Non-farmacologische interventies kunnen relatief eenvoudig toegevoegd worden aan bestaande medicamenteuze interventies. Indien interventies een positief effect hebben op bijvoorbeeld angst en spanning, zal dit ook de ervaren pijn positief kunnen beïnvloeden. Non-farmacologische pijnbestrijding dienen te worden aangepast aan leeftijd en ontwikkelingsniveau van het kind.
In de huidige praktijk worden reeds veel verschillende non-farmacologische pijnbehandelingen toegepast. Keuzes en een plan op maat voor een kind kunnen ook effect op zelfredzaamheid en autonomie heben. Dit in het kader van gezamenlijke besluitvoering.
Waarden en voorkeuren van patiënten (en evt. hun verzorgers)
Het is van belang dat het gehele multidisciplinaire team dezelfde taal spreekt en hetzelfde verklaringsmodel/psychoeducatie uitdraagt naar het kind en zijn of haar gezin. Hierbij wordt het gebruik van helpend taalgebruik geadviseerd. Non-farmacologische technieken kunnen bijdragen aan de ontspanning en afleiding van de patiënt waardoor angst kan verminderen en comfort en patiënttevredenheid kan toenemen. Hieronder worden voor verschillende patiëntengroepen uit de PICO specifieke aandachtspunten benoemd.
Verbale kinderen en adolescenten
Goede psycho/pijneducatie voor het kind en zijn of haar gezin is van belang. Aangeraden wordt hierbij van verschillende modaliteiten gebruik te maken, denk hierbij aan mondelinge uitleg met gebruik van bijvoorbeeld metaforen, folders en beeldmateriaal.
Juist de behandeling afgestemd op de individuele patiënt, rekening houdend met kindfactoren, gezinsfactoren en omgevingsfactoren (biopsychosociaal model), gericht op het optimaliseren van de omstandigheden zodat de pijnklachten kunnen reduceren (gevolgenmodel) en het functioneringsniveau van het kind wordt vergroot, passend bij het ontwikkelingsniveau van het kind, is belangrijk.
Denk hierbij aan het doel weer volledig onderdeel zijn van de maatschappij, waar het kind net als zijn leeftijdsgenoten naar school gaat, kan spelen met vrienden en andere activiteiten kan ondernemen.
Herstel belemmerende factoren dienen hierbij in kaart te worden gebracht, zoals faseproblematiek, ontwikkelingsproblematiek (bijvoorbeeld ASS en ADHD) en problemen op het gebied van voeding, slapen, school, cognitie en stemming (bijvoorbeeld catastroferende gedachten, angst en somberheid).
Zo kan bijvoorbeeld bij het ene kind EMDR (psychologische traumabehandeling) op basis van wat hij heeft meegemaakt een onderdeel van het behandelplan zijn, terwijl voor het andere kind medicatie en activering middels kinderfysiotherapeutische begeleiding meer op de voorgrond staat.
De systemische visie (Family Integrated Care (FICare)/Samenzorg) is in het geval van kinderen van groot belang. Het kind kan niet los van zijn ouders/gezin/systeem worden gezien. Ouders spelen een zeer belangrijke rol in het ondersteunen van hun kind. Het kan echter ook voorkomen dat ouders onvoldoende ondersteuning kunnen bieden of belemmerde factoren in stand houden en dan dient naar alternatieven te worden gekeken.
Overweeg bij onvoldoende vooruitgang een revalidatietraject.
Preverbale kinderen/neonaten
Voor de allerjongste groep, preverbale kinderen, is het belangrijk om vanuit de Infant Mental Health (IMH) gedachte het kind en zijn of haar ouders te benaderen. Hierbij staat het oog hebben voor het samenspel tussen omgeving, biologische factoren en de ouder-kindrelatie centraal. Aanwezigheid van ouders is bijvoorbeeld van groot belang indien mogelijk en daarnaast is toepassen van de NIDCAP methode (Newborn Individualized Development Care and Asssessment Program)/ontwikkelingsgerichte zorg essentieel in de zorg van een kwetsbare pasgeborene.
Vanuit onderzoek naar non-farmacologische interventies bij procedurele pijn bij neonaten weten we dat onder andere huid-op-huid contact (kangaroo care of skin to skin care), begrenzing bieden, borstvoeding en het zuigen op een speen (met sucrose) pijn verlagend werken. We adviseren deze interventies tevens te overwegen in het geval van pijn niet gerelateerd aan procedures ondanks het ontbreken van overtuigend bewijs hiervoor.
Kinderen met neurobiologische ontwikkelingsstoornissen
Naast veel van bovenstaande overwegingen is het specifiek voor kinderen met neurobiologische ontwikkelingsstoornissen van belang om comfort te bieden, bijvoorbeeld door het bieden van ergonomische optimalisatie, detonisatie en/of afleiding. Ouders/verzorgers dienen expliciet betrokken te worden bij kinderen met neurobiologische ontwikkelingsstoornissen gezien hun ervaringsperspectief in hoe comfort het beste aan het individuele kind geboden kan worden.
Kosten (middelenbeslag)
Non-farmacologische pijnbestrijdingstechnieken en materialen worden al veel ingezet in ziekenhuizen, maar dienen verder geoptimaliseerd te worden. Dit kan gepaard gaan met extra kosten, zoals kosten voor scholing en materiaalkosten. Tevens kan gedacht worden aan personeelskosten wanneer bv uren medisch pedagogisch zorgverlener wordt uitgebreid. Er is weinig bekend over de kosteneffectiviteit van deze interventies. De interventies lijken over het algemeen eenvoudig uitvoerbaar en de kosten lijken beperkt. Daardoor zou het een relatief goedkope manier zijn om de patiëntbeleving positief te beïnvloeden. Er zou meer kwalitatief goed onderzoek gedaan kunnen worden, bijvoorbeeld door het vergelijken van twee non-farmacologische technieken of bij verschillende doelgroepen. Tevens zou een passende DBC structuur met verzwaring bij extra kosten hierin kunnen bijdragen.
Aanvaardbaarheid, haalbaarheid en implementatie
Naast het ruime aanbod van non-farmacologische technieken is de keuze ook afhankelijk van de voorkeur van het kind en zijn/haar ouders. Door hen te betrekken bij de keuze voor en bepaalde techniek kan een passende keus worden gemaakt waardoor de kans op een positief effect en toename van de patiënttevredenheid wordt vergroot
Belangrijk is dat de zorgverleners op de hoogte zijn van de mogelijkheden en getraind zijn in de non-farmacologische technieken die lokaal worden toegepast.
Voor het toepassen van non-farmacologische technieken en materialen is vaak scholing nodig, bijvoorbeeld bij het gebruik van helpend taalgebruik. Als een VR bril wordt gebruikt moet rekening gehouden worden met benodigde software, abonnementen, trainingen, beschikbaarheid van brillen. Dit kan mogelijk een barrière zijn voor implementatie.
Rationale van de aanbeveling: weging van argumenten voor en tegen de interventies.
In de literatuursearch van deze module is gebruik gemaakt van de vergelijking van non-farmacologische met farmacologische pijnbestrijding. Hierbij is voorbijgegaan aan het additieve effect van non-farmacologische pijnbestrijding.
Voor postoperatieve pijn en procedurele sedatie en analgesie (PSA) wordt verwezen naar desbetreffende richtlijnen. Voor aandoeningsspecifieke behandeling van pijn wordt verwezen naar desbetreffende richtlijnen, zoals functionele buikpijn bij kinderen en hoofdpijn (bij volwassenen).
Overweeg bij alle vormen van pijn bij kinderen altijd non-farmacologische en farmacologische pijnbestrijding. Hierbij is het biopsychosociaal model belangrijk. Zowel biologische, psychologische als sociale factoren spelen een rol bij pijn, pijnbeleving en pijnbestrijding. Leg aan ouder(s)/verzorger(s) en kinderen uit wat maakt dat dit werkt (naast farmacologische interventie) om te voorkomen dat zij zich niet serieus genomen voelen en weten waarom en wanneer zij dit in kunnen zetten.
Door aandacht te besteden aan alle relevante factoren ontstaat een deecsalerend effect op de pijnbeleving. Het is dan ook van belang dat de twee vormen van behandeling, zowel non-farmacologisch als farmacologisch, samen gaan voor het behandelen van pijn bij kinderen. Non-farmacologische pijnbestrijding is relatief makkelijk in te zetten en geeft weinig tot geen bijwerkingen. Een goed voorbeeld hiervan zijn afleidende technieken die naar mening van de werkgroep ondanks gebrek aan bewijs wel aanbevolen worden. Te denken valt aan zoekboeken, cognitief belastende taak, muziek, VRbril. Bij zuigelingen voeding, speen en fysiek contact met ouders.
Vanuit expert opinion wordt sterk aanbevolen multidisciplinair te werk te gaan, waarbij men vanuit de gezamenlijkheid van verschillende disciplines, zoals artsen, verpleegkundigen, medisch pedagogisch zorgverleners, fysiotherapeuten en psychologen, een behandelplan opstelt, evalueert en zo nodig bijstelt. Bepaal met elkaar welke non-farmacologische pijnbestrijdingstechieken kunnen worden toegepast, zodat keuzeopties bekend zijn, dezelfde taal gesproken wordt en voorzien kan worden in de bijbehorende materialen en scholing.
Onderbouwing
Achtergrond
Non-pharmacological pain management involves methods of pain management in children that do not involve the use of pharmacological agents, such as analgesics. Non-pharmacological pain management includes a wide range of techniques and interventions that aim to reduce or control pain without the use of medication. This can range from psychological interventions, physiotherapy, and alternative therapies to simple comfort measures such as distraction, communication and reassurance.
Communication is the exchange of thoughts, messages, or information, as by speech, signals, writing, and/or behavior.
For the non-pharmacological treatment of pain in children undergoing a procedure, we refer to the modules on non-pharmacological (psychological and physical) techniques for procedural sedation and analgesia (PSA) in children.
Conclusies / Summary of Findings
Psychoeducation
|
Low GRADE |
Psychoeducation ((self-administered) psychological treatment/ biofeedback) might be more effective on migraine related outcomes (headache days per month, number of migraine days per month, frequency of headache attacks, frequency of migraine attacks, and headache index and activity) than waitlist control in children aged <18 years.
Source: Koechlin, 2021 |
|
Low GRADE |
Psychoeducation (cognitive behaviour therapy) might reduce pain compared with control in children aged 5 to 18 years.
Source: Abbott, 2017; Abbott, 2018; Gordon, 2022 |
|
Low GRADE |
Psychoeducation (cognitive behaviour therapy) might have no effect on functioning compared with control in children with recurrent abdominal pain aged 5 to 18 years.
Source: Abbott, 2017 |
|
Low GRADE |
Psychoeducation (cognitive behaviour therapy) might have no effect on quality of life compared with control in children with recurrent abdominal pain aged 5 to 18 years.
Source: Abbott, 2017 |
Distraction techniques
|
Very low GRADE |
The evidence is very uncertain about the effect of distraction techniques (music listening) on pain compared with control in children aged ≤21 years.
Source: e, 2023; Ting, 2022 |
Physical interventions
|
Very low GRADE |
The evidence is very uncertain about the effect of physical interventions (physical therapy/ yoga) on pain compared with control in children aged ≤19 years.
Source: Abbott, 2017; Fisher, 2022; Gordon, 2022 |
|
Very low GRADE |
The evidence is very uncertain about the effect of physical interventions (physical therapy) on anxiety compared with control in children aged ≤19 years.
Source: Fisher, 2022 |
|
Very low GRADE |
The evidence is very uncertain about the effect of physical interventions (physical therapy/ yoga) on functioning compared with control in children aged ≤19 years.
Source: Abbott, 2017; Fisher, 2022 |
|
Very low GRADE |
The evidence is very uncertain about the effect of physical interventions (physical therapy) on quality of life compared with control in children aged ≤19 years.
Source: Fisher, 2022 |
Psychological interventions
|
Low GRADE |
Psychological interventions (psychological therapy) might reduce pain compared with control in children aged ≤19 years.
Source: Fisher, 2022 |
|
Moderate GRADE |
Psychological interventions (psychological therapy) probably have no effect on anxiety compared with control in children aged ≤19 years.
Source: Fisher, 2022 |
|
Very low GRADE |
The evidence is very uncertain about the effect of psychological interventions (psychological therapy) on functioning compared with control in children aged ≤19 years.
Source: Fisher, 2022 |
|
Low GRADE |
Psychological interventions (psychological therapy) might have no effect on quality of life compared with control in children aged ≤19 years.
Source: Fisher, 2022 |
Other non-pharmacological interventions
|
Low GRADE |
Relaxation might be more effective on migraine related outcomes (headache days per month, number of migraine days per month, frequency of headache attacks, frequency of migraine attacks, and headache index and activity) than waitlist control in children aged <18 years.
Source: Koechlin, 2021 |
|
Very low GRADE |
The evidence is very uncertain about the effect of written self-disclosure on pain compared with control in children aged 5 to 18 years.
Source: Abbott, 2017 |
|
Very low GRADE |
The evidence is very uncertain about the effect of hypnotherapy on pain compared with control in children aged 5 to 18 years.
Source: Abbott, 2017; Abbott, 2018; Gordon, 2022 |
Samenvatting literatuur
Description of studies
Distraction techniques
See (2023) performed a systematic review and meta-analysis of RCTs, quasi-experimental studies, cohort studies, case-control studies, and pilot studies, to estimate the effect of music interventions on pain, compared with standard care in paediatric, adult, and elderly patients visiting the emergency department (ED) for any condition. We only reported the results on paediatric patients. This review reported pain and included 11 studies, of which three studies focused on the paediatric population (≤21 years). Two of the three included studies (Hartling et al., 2013 and van der Heijden et al., 2019) were also included in the review of Ting (2022). Two studies were included in the meta-analysis. The review found no significant effect on pain. The evidence was of very low quality, due to lack of blinding, inconsistency, indirectness, and imprecision. The results from this review are summarized in Table 2.
Ting (2022) performed a systematic review and meta-analysis of RCTs, quasi-experimental studies, cohort studies, case-control studies, and pilot studies to estimate the effect of listening to music, compared with music control in patients <18 years in various clinical settings. Although the comparison was described as music control, the authors described that the control group may not receive any components of music (i.e., rhythm, melody, and harmony). This review reported pain release and included 38 studies, of which 8 articles were in children with postoperative pain. We only reported these results, because chronic/procedural and prick pain does not comply with the PICO. In general, a moderately beneficial effect of music listening on pain release was reported. The quality of the included studies was low, mostly due to performance bias and detection bias. The results from this review are summarized in Table 2.
Psychoeducation and therapeutic communication
Koechlin (2021) performed a systematic review and network meta-analysis of RCTs to estimate the efficacy of nonpharmacological interventions, compared with waitlist control in patients <18 years with episodic migraine. The review reported short-term and long-term efficacy, based on a lumping and splitting approach. Outcomes were number of headache days per month, number of migraine days per month, frequency of headache attacks, frequency of migraine attacks, and headache index and activity. In general, beneficial effects were reported of self-administered treatments, biofeedback, relaxation, and psychological treatments on short-term and long-term using the lumping approach. No effects were found using the splitting approach. The evidence was of low quality, mostly due to within-study bias, imprecision, and incoherence. The results from this review are summarized in Tables 1 and 3.
Abbott (2017) performed a systematic review and meta-analysis of RCTs to estimate the effect of psychosocial interventions, compared with usual care or waitlist control in children aged 5 to 18 years with recurrent abdominal pain. The review included 18 studies of which 14 were included in the meta-analysis. Interventions consisted of cognitive behaviour therapy (CBT), hypnotherapy (including guided imagery), yoga, and written self-disclosure. Reported outcomes were pain intensity, pain duration, social or psychological functioning, quality of life, and functional impairment of daily activities. In general, beneficial effects in the short term on reducing pain with CBT and hypnotherapy were found. No effects were found on the other outcomes and on the use of yoga and written self-disclosure. The evidence was of very low to low quality due to the high risk of bias across the studies, such as unblinded participants and unblinded outcome assessment, along with some outcomes having a high level of unexplained heterogeneity (greater than 70%), wide confidence intervals, and a low number of participants included in the analyses. The results from this review are summarized in Tables 1, 3, and 4.
Gordon (2022) performed a systematic review and meta-analysis of RCTs to estimate the effect of psychosocial interventions, compared with no intervention or other interventions in children aged 4 to 18 years with functional abdominal pain disorders. We only reported the results of the intervention compared to no intervention. The review included 33 studies, of which 26 were included in the meta-analysis and 17 studies used standard care, placebo, or waitlist control as comparison. Of these 33 studies, 18 were also included in Abbott (2017) and Abbott (2018). Interventions consisted of CBT, yoga, and hypnotherapy. Reported outcomes were pain frequency and pain intensity. In general, no effects were found for CBT, with moderate quality of evidence, and the evidence for yoga and hypnotherapy was of very low quality so no conclusions could be drawn. This was mostly caused by imprecision and high risk of bias. The results from this review are summarized in Tables 1, 3, and 4.
Abbott (2018) performed a systematic review and meta-analysis of RCTs and randomized cross-over trials to estimate the effect of psychosocial intervention, compared with placebo, waiting list, no treatment, active control, or standard care in children aged 5 to 18 years with abdominal pain-related function gastrointestinal disorder. This review is a summary of three reviews, including the review of Abbott et al. (2017). Therefore, the same studies are included in this review as in the review of Abbott et al. (2017). This review updated the search and found two additional studies (Bonnert et al., 2017 and Korterink et al., 2016). Additionally, 16 studies included in Gordon et al., 2022 are also included in this review. Only the results not described in Abbott et al. (2017) are reported here. The review included 3 additional studies, next to the three reviews. Interventions consisted of CBT and hypnotherapy and reported outcomes were pain intensity postintervention, pain intensity after 3 to 6 months follow-up and pain intensity after 12 months or more follow-up. In general, beneficial effects of CBT and hypnotherapy were found on pain-intensity, with low quality of evidence due to lack of blinding. The results from this review are summarised in Tables 1 and 3.
Physical and psychological interventions
The systematic review and meta-analysis (Fisher, 2022) for the World Health Organization (WHO) guidelines on the management of chronic pain in children from 2020, was included for the psychological therapy and physical therapy interventions.
Fisher (2022) performed a systematic review and meta-analysis on efficacy and safety of pharmacological, physical, and psychological intervention for the management of chronic pain in children. In our literature analysis, only the studies researching physical therapy and psychological therapy were included. They searched CENTRAL, MEDLINE and MEDLINE in Process, and EMBASE from inception to April 2020, for pharmacological and physical therapy trials. For psychological therapy trials, they updated searches using the same three databases, and PsycINFO from previous Cochrane reviews and they searched for trials in children with cancer from inception of each database to March 2020. For pharmacological and physical therapies, RCTs and nonrandomized comparative observational trials were included. For psychological trials, only RCTs were included. Inclusion criteria were studies of children (ages 0-19 years) with chronic pain, researching any analgesic drug, bodily movement therapies, and therapies delivering recognizable psychological content. The comparison could be active or placebo comparison, treatment as usual, waitlist control, or other pharmacological or physical therapy interventions. Interventions directed at parents of children with pain were also included. Exclusion criteria were passive physical interventions such as massage or manipulation and passive delivered psychological interventions (such as reading about CBT).
Critical outcomes were (reduction in) pain intensity, health-related quality of life (HRQOL), functional disability, role functioning, emotion functioning, sleep, and adverse events. Important outcomes were activity participation, global judgement of satisfaction with treatment, patient global impression of change, and fatigue. The authors used to Cochrane Risk of Bias tool to assess risk of bias in RCTs and the ROBINS-I tool for non-randomized studies and the level of evidence was assessed using GRADE. Overall, the authors included 34 pharmacological therapy trials (29 RCTs, 5 nonrandomised studies, and 37 reports) and 5 ongoing RCTs (no results), 24 physical therapy RCTs (33 reports) and 12 further ongoing trials (11 RCTs and 1 nonrandomised trial [which reported results]), and 63 psychological therapy RCTs and 16 ongoing RCT studies. No studies with data of children with cancer diagnosis, needing palliative care, or an intellectual disability were identified. The results from this review are summarised in Tables 4 and 5.
Results
The results of the reviews are summarized in Tables 1-5.
Authors’ conclusions
The review of See (2023) concluded that music interventions can improve pain and anxiety levels in children in the emergency department, but this evidence is of low quality. The high heterogeneity observed indicates insufficient evidence.
The review of Ting (2022) concluded that listening to music reliefs pain in both psychological and physiological domains. However, most included articles had high risk of performance bias and detection bias.
The review of Koechlin (2021) concluded that the network meta-analysis revealed different findings depending on the structure of the nodes in the network. Significant short- and long-term effects were found for most nonpharmacological interventions (ie, relaxation, biofeedback, psychological treatments, and self-administered psychological treatments) as well as for 1 control group (ie, psychological placebo) when compared with the waiting list. In terms of the second aim (ie, to systematically compare the different nonpharmacological treatment options relative to each other), the included interventions did not significantly differ from one another. In a second step, they split the various published interventions into individual nodes. Here, in the short- and long-term analyses, none of the included interventions were significantly more effective than the waiting list, which was the control group that most interventions were compared with and that connected 2 otherwise unconnected networks. Also, none of the interventions differed significantly from each other in the head-to-head comparisons. However, the quality of the evidence was low.
The review of Abbott (2017) concluded that they provided low‐quality evidence that cognitive behavioural therapy (CBT) and hypnotherapy may be effective in reducing pain in the short term for children and adolescents presenting with recurrent abdominal pain (RAP). Sustained effects of both CBT and hypnotherapy on pain have also been reported, but the evidence to date is limited. There was little evidence that CBT or hypnotherapy affected school functioning, psychological well‐being, or quality of life. The review found no evidence to support the use of yoga or written self‐disclosure for the treatment of recurrent abdominal pain in children and adolescents.
The review of Gordon (2022) concluded that CBT and hypnotherapy should be considered as a treatment for functional abdominal pain disorders in childhood, with moderate quality. No conclusions could be drawn about the effect of yoga due to very low quality evidence.
The review of Abbott (2018) concluded that low-quality evidence was found to suggest that CBT and hypnotherapy may be effective in treating recurrent abdominal pain, with both reported to be effective in reducing pain in the short term. Sustained effects of CBT and hypnotherapy were also reported but the evidence is limited. We found insufficient evidence to support the use of yoga therapy or written self-disclosure.
The review of Fisher (2022) concluded that each modality of intervention separately showed some benefits of reducing pain intensity posttreatment, but these effects were not maintained at follow-up. Some physical and psychological interventions also reduced functional disability posttreatment, and effects were maintained for psychological interventions at follow-up. Physical therapies presented very little data that could be analysed for other outcomes, meaning any absence of effects and beneficial effects should be interpreted with caution. Most data were available for psychological therapies; however, we found no beneficial effects for improving HRQOL, emotional functioning, role functioning, or sleep in the meta-analyses. A beneficial effect of psychological interventions was found for global satisfaction with treatment. Adverse events were poorly reported, particularly in physical and psychological trials.
Table 1. Summary of findings table for the intervention ‘psychoeducation’
|
Outcome |
Time |
Type of intervention |
Author (year) |
N studies in meta-analysis |
Effect measure (95%CI) |
Figure for meta-analysis (see appendix) |
Certainty |
|
Efficacy |
|||||||
|
Efficacy* |
Short-term |
Self-administered psychological treatments |
Koechlin (2021) |
12 |
SMD 1.44 (95%CI -0.26 to 2.26) |
Figure 1A |
ꚛꚛ○○ low |
|
Biofeedback |
Koechlin (2021) |
12 |
SMD 1.41 (95%CI -0.64 to 2.17) |
ꚛꚛ○○ low |
|||
|
Psychological treatments |
Koechlin (2021) |
12 |
SMD 1.36 (95%CI -0.15 to 2.57) |
ꚛꚛ○○ low |
|||
|
Efficacy* |
Long term |
Self-administered psychological treatments |
Koechlin (2021) |
12 |
SMD 1.40 (95%CI -0.28 to 2.52) |
Figure 1B |
ꚛꚛ○○ low |
|
Biofeedback |
Koechlin (2021) |
12 |
SMD 1.21 (95%CI -0.47 to 1.94) |
ꚛꚛ○○ low |
|||
|
Psychological treatments |
Koechlin (2021) |
12 |
SMD 1.33 (95%CI -0.18 to 2.47) |
ꚛꚛ○○ low |
|||
|
Pain |
|||||||
|
Pain intensity |
Post intervention |
Cognitive behaviour therapy |
Abbott (2017) |
7 |
SMD -0.33 (95%CI -0.74 to 0.08) |
Figure 2 |
ꚛꚛ○○ low |
|
Cognitive behaviour therapy |
Abbott (2018) |
4 |
OR 5.67 (95%CI 1.18 to 27.32), NNTB=4 |
Figure 3 |
ꚛꚛ○○ low |
||
|
After 3-6 months follow-up |
Cognitive behaviour therapy |
Abbott (2018) |
3 |
OR 3.08 (95%CI 0.93 to 10.16), NNTB=5 |
Not provided |
|
|
|
After 3-12 months follow-up |
Cognitive behaviour therapy |
Abbott (2017) |
4 |
SMD -0.32 (95%CI -0.85 to 0.20) |
Figure 4 |
ꚛꚛ○○ low |
|
|
After ≥12 months follow-up |
Cognitive behaviour therapy |
Abbott (2017) |
3 |
SMD -0.04 (95%CI -0.39 to 0.31) |
Figure 5 |
ꚛꚛ○○ low |
|
|
Cognitive behaviour therapy |
Abbott (2018) |
2 |
OR 1.29 (95%CI 0.50 to 3.33) |
Not provided |
ꚛꚛ○○ low |
||
|
|
Cognitive behaviour therapy |
Gordon (2022) |
6 |
SMD -0.58 (95%CI -0.83 to -0.32) |
Figure 6 |
ꚛꚛꚛ○ moderate |
|
|
Pain duration |
|
Cognitive behaviour therapy |
Abbott (2017) |
1 |
No difference |
NA |
|
|
Pain frequency |
|
Cognitive behaviour therapy |
Gordon (2022) |
7 |
SMD -0.36 (95%CI -0.63 to -0.09) |
Figure 7 |
ꚛꚛꚛ○ moderate |
|
Functioning |
|||||||
|
Social or psychological functioning |
|
Cognitive behaviour therapy |
Abbott (2017) |
3 |
No difference |
Not performed |
|
|
Functional impairment of daily activities |
|
Cognitive behaviour therapy |
Abbott (2017) |
4 |
SMD -0.57 (95%CI ‐1.34 to 0.19) |
Figure 8 |
ꚛ○○○ very low |
|
Quality of life |
|||||||
|
Physical quality of life |
|
Cognitive behaviour therapy |
Abbott (2017) |
3 |
SMD 0.71 (95%CI ‐0.25 to 1.66) |
Figure 9 |
ꚛ○○○ very low |
|
Psychosocial quality of life |
|
Cognitive behaviour therapy |
Abbott (2017) |
3 |
SMD 0.43 (95%CI ‐0.21 to 1.06) |
Figure 10 |
ꚛꚛ○○ low |
* Lumping approach
Green represents in favour of the intervention. Red represents in favour of the control. Yellow represents no significant difference between intervention and control. Grey represents no conclusions could be drawn.
SMD: standardized mean difference, 95%CI: 95% confidence interval, RR: risk ratio, OR: odds ratio, HRQoL: health-related quality of life, IG: intervention group, CG: control group, NNTB: number needed to benefit
Table 2. Summary of findings table for the intervention ‘distraction techniques’
|
Outcome |
Time |
Type of intervention |
Author (year) |
N studies in meta-analysis |
Effect measure (95%CI) |
Figure for meta-analysis (see appendix) |
Certainty |
|
Pain |
|||||||
|
Pain |
|
Music listening |
See (2023) |
2 |
SMD -0.40 (95%CI -0.78 to -0.03) |
Figure 11 |
ꚛ○○○ very low |
|
Pain release |
Post-operative |
Listening to music |
Ting (2022) |
8 |
SMD -0.49 (95%CI -0.90 to -0.08) |
Not provided |
|
|
Anxiety |
|||||||
Green represents in favour of the intervention. Red represents in favour of the control. Yellow represents no significant difference between intervention and control. Grey represents no conclusions could be drawn.
SMD: standardized mean difference, 95%CI: 95% confidence interval, RR: risk ratio, OR: odds ratio, HRQoL: health-related quality of life, IG: intervention group, CG: control group
Table 3. Summary of findings table for the intervention ‘physical interventions’
|
Outcome |
Time |
Type of intervention |
Author (year) |
N studies in meta-analysis |
Effect measure (95%CI) |
Figure for meta-analysis (see appendix) |
Certainty |
|
Pain |
|||||||
|
Pain intensity |
Post treatment |
Physical therapy |
Fisher (2022) |
6 |
SMD -0.60 (95%CI -1.15 to -0.04) |
Figure 17 |
ꚛ○○○ very low |
|
Yoga |
Abbott (2017) |
3 |
SMD -0.31 (95%CI -0.67 to 0.05 |
Figure 18 |
ꚛꚛ○○ low |
||
|
At follow-up |
Physical therapy |
Fisher (2022) |
3 |
SMD -0.13 (95%CI -0.74 to 0.48) |
Figure 19 |
ꚛ○○○ very low |
|
|
Yoga |
Abbott (2017) |
1 |
No difference |
NA |
|
||
|
|
Yoga |
Gordon (2022) |
1 |
No conclusions could be drawn |
NA |
ꚛ○○○ very low |
|
|
Pain frequency |
|
Yoga |
Abbott (2017) |
1 |
No difference postintervention, at 6, and at 12 months follow-up |
NA |
|
|
|
Yoga |
Gordon (2022) |
1 |
No conclusions could be drawn |
NA |
ꚛ○○○ very low |
|
|
Anxiety |
|||||||
|
Anxiety post-treatment |
|
Physical therapy |
Fisher (2022) |
2 |
SMD 0.06 (95%CI -1.39 to 1.51) |
Figure 20 |
ꚛ○○○ very low |
|
Functioning |
|||||||
|
Social or psychological function |
|
Yoga |
Abbott (2017) |
3 |
No difference |
Not performed |
|
|
Functional disability |
Post intervention |
Yoga |
Abbott (2017) |
2 |
SMD -0.32 (95%CI -1.07 to 0.43) |
Figure 21 |
|
|
Physical therapy |
Fisher (2022) |
4 |
SMD -0.64 (95%CI -0.95 to -0.34) |
Figure 22 |
ꚛ○○○ very low |
||
|
At follow-up |
Physical therapy |
Fisher (2022) |
1 |
SMD -0.36 (95%CI -1.04 to 0.28) |
Figure 23 |
ꚛ○○○ very low |
|
|
Quality of life |
|||||||
|
HRQoL post-treatment |
|
Physical therapy |
Fisher (2022) |
2 |
SMD -0.64 (95%CI -1.91 to 0.63) |
|
ꚛ○○○ very low |
Green represents in favour of the intervention. Red represents in favour of the control. Yellow represents no significant difference between intervention and control. Grey represents no conclusions could be drawn.
SMD: standardized mean difference, 95%CI: 95% confidence interval, RR: risk ratio, OR: odds ratio, HRQoL: health-related quality of life, IG: intervention group, CG: control group
Table 4. Summary of findings table for the intervention ‘psychological interventions’
|
Outcome |
Time |
Type of intervention |
Author (year) |
N studies in meta-analysis |
Effect measure (95%CI) |
Figure for meta-analysis (see appendix) |
Certainty |
|
Pain |
|||||||
|
Pain intensity |
Post-treatment |
Psychological therapy |
Fisher (2022) |
38 |
SMD -0.29 (95%CI -0.43 to -0.16) |
Figure 24 |
ꚛꚛ○○ low |
|
At follow-up |
Psychological therapy |
Fisher (2022) |
21 |
SMD of -0.14 (95%CI -0.30 to 0.02) |
Figure 25 |
ꚛꚛ○○ low |
|
|
Pain intensity
|
Post intervention |
Hypnotherapy |
Abbott (2017) |
4 |
SMD -1.01 (95%CI -1.41 to -0.61) |
Figure 14 |
ꚛꚛ○○ low |
|
|
Hypnotherapy |
Abbott (2018) |
4 |
OR 6.78 (95%CI 2.41 to 19.07), NNTB = 3 |
Figure 15 |
ꚛꚛ○○ low |
|
|
|
After 5 years follow-up |
Hypnotherapy |
Abbott (2017) |
1 |
IG mean 2.9 ± SD 4.4 vs CG mean 7.7 ± SD 5.3 |
NA |
|
|
|
Hypnotherapy |
Gordon (2022) |
1 |
No conclusions could be drawn |
NA |
ꚛ○○○ very low |
|
|
30% pain reduction |
Post-treatment |
Psychological therapy |
Fisher (2022) |
1 |
RR 1.13 (95%CI 0.64 to 2.02) |
NA |
ꚛ○○○ very low |
|
At follow-up |
Psychological therapy |
Fisher (2022) |
1 |
RR 1.07 (95%CI 0.77 to 1.49) |
NA |
ꚛ○○○ very low |
|
|
50% pain reduction
|
Post-treatment |
Psychological therapy |
Fisher (2022) |
22 |
RR 2.11 (95%CI 1.61 to 2.77) |
Figure 26 |
ꚛꚛ○○ low |
|
At follow-up |
Psychological therapy |
Fisher (2022) |
9 |
RR 2.09 (95%CI 1.29 to 3.38) |
Figure 27 |
ꚛ○○○ very low |
|
|
Pain frequency |
Post-intervention |
Hypnotherapy |
Abbott (2017) |
1 |
SMD -1.28 (95%CI -1.84 to -0.72) |
Figure 16 |
ꚛꚛ○○ low |
|
|
After 5 years follow-up |
Hypnotherapy |
Gordon (2022) |
1 |
No conclusions could be drawn |
NA |
ꚛ○○○ very low |
|
|
|
Hypnotherapy |
Abbott (2017) |
1 |
IG mean 2.3 ± SD 4.0 vs CG mean 7.1 ± SD 6.0 |
NA |
|
|
Pain duration |
|
Hypnotherapy |
Abbott (2017) |
1 |
IG mean 1.20 ± SD 1.47 vs CG mean 3.50 ± SD 2.53 |
NA |
|
|
Anxiety |
|||||||
|
Anxiety
|
Post-treatment |
Psychological therapy |
Fisher (2022) |
19 |
SMD -0.08 (95%CI -0.21 to 0.04) |
Figure 28 |
ꚛꚛꚛ○ moderate |
|
At follow-up |
Psychological therapy |
Fisher (2022) |
13 |
SMD -0.07 (95%CI -0.17 to 0.03) |
Figure 29 |
ꚛꚛꚛꚛ high |
|
|
Functioning |
|||||||
|
School absence
|
Post-treatment |
Psychological therapy |
Fisher (2022) |
9 |
SMD -0.21 (95%CI -0.52 to 0.10) |
Figure 30 |
ꚛ○○○ very low |
|
At follow-up |
Psychological therapy |
Fisher (2022) |
4 |
SMD 0.14 (95%CI -0.32 to 0.60) |
Figure 31 |
ꚛ○○○ very low |
|
|
Activity participation |
At follow-up |
Psychological therapy |
Fisher (2022) |
1 |
SMD -0.99 (95%CI -1.62 to -0.36) |
NA |
ꚛ○○○ very low |
|
Functional disability |
Post-treatment |
Psychological therapy |
Fisher (2022) |
24 |
SMD -0.25 (95%CI -0.39 to -0.11) |
Figure 32 |
ꚛꚛ○○ low |
|
At follow-up |
Psychological therapy |
Fisher (2022) |
14 |
SMD -0.23 (95%CI -0.38 to -0.08) |
Figure 33 |
ꚛꚛꚛ○ moderate |
|
|
Quality of life |
|||||||
|
HRQoL |
Post-treatment |
Psychological therapy |
Fisher (2022) |
13 |
SMD -0.14 (95%CI -0.33 to 0.05) |
Figure 34 |
ꚛꚛ○○ low |
|
At follow-up |
Psychological therapy |
Fisher (2022) |
7 |
SMD -0.09 (95%CI -0.35 to 0.16) |
Figure 35 |
ꚛꚛ○○ low |
|
|
|
Hypnotherapy |
Abbott (2017) |
3 |
Inconclusive results, one study found a beneficial effect and two studies found no effect |
Not performed |
|
|
Green represents in favour of the intervention. Red represents in favour of the control. Yellow represents no significant difference between intervention and control. Grey represents no conclusions could be drawn.
SMD: standardized mean difference, 95%CI: 95% confidence interval, RR: risk ratio, OR: odds ratio, HRQoL: health-related quality of life, IG: intervention group, CG: control group
Table 5. Summary of findings table for ‘other non-farmacological interventions’
|
Outcome |
Time |
Type of intervention |
Author (year) |
N studies in meta-analysis |
Effect measure (95%CI) |
Figure for meta-analysis (see appendix) |
Certainty |
|
Efficacy |
|||||||
|
Efficacy |
Short-term |
Relaxation* |
Koechlin (2021) |
12 |
SMD 1.38 (95%CI 0.61 to 2.14) |
Figure 1A |
ꚛꚛ○○ low |
|
Relaxation stress management, relaxation education, biofeedback stress management, biofeedback relaxation education, transcendental meditation, autogenic feedback, autogenic training, progressive muscle relaxation, education, and hypnotherapy** |
Koechlin (2021) |
12 |
No differences with waiting list control |
Figure 13C |
ꚛꚛ○○ low |
||
|
Efficacy |
Long-term |
Relaxation* |
Koechlin (2021) |
12 |
SMD 1.35 (95%CI 0.60 to 2.09) |
Figure 2A |
ꚛꚛ○○ low |
|
Relaxation stress management, relaxation education, biofeedback stress management, biofeedback relaxation education, transcendental meditation, autogenic feedback, autogenic training, progressive muscle relaxation, education, and hypnotherapy** |
Koechlin (2021) |
12 |
No differences with waiting list control |
Figure 13D |
ꚛꚛ○○ low |
||
|
Pain |
|||||||
|
Pain frequency |
Post-intervention |
Written self-disclosure |
Abbott (2017) |
1 |
No difference |
NA |
|
|
After 3 months follow-up |
Written self-disclosure |
Abbott (2017) |
1 |
No difference |
NA |
|
|
|
After 6 months follow-up |
Written self-disclosure |
Abbott (2017) |
1 |
IG mean 1.35 ± SD 1.39 vs CG mean 2.32 ± SD 1.72 |
NA |
|
|
*Lumping approach
**Splitting approach
Green represents in favour of the intervention. Red represents in favour of the control. Yellow represents no significant difference between intervention and control. Grey represents no conclusions could be drawn.
SMD: standardized mean difference, 95%CI: 95% confidence interval, RR: risk ratio, OR: odds ratio, HRQoL: health-related quality of life, IG: intervention group, CG: control group
Level of evidence of the literature
The level of evidence of the literature is determined per intervention, per outcome.
Psychoeducation
The level of evidence regarding the outcome measure efficacy was downgraded by two levels because of study limitations (risk of bias) and applicability (bias due to indirectness) to LOW.
The level of evidence regarding the outcome measure pain was downgraded by two levels because of study limitations (risk of bias) and conflicting results (inconsistency) to LOW.
The level of evidence regarding the outcome measure functioning was downgraded by two levels because of study limitations (risk of bias) and number of included patients (imprecision) to LOW.
The level of evidence regarding the outcome measure quality of life was downgraded by two levels because of study limitations (risk of bias) and number of included patients (imprecision) to LOW.
Distraction techniques
The level of evidence regarding the outcome measure pain was downgraded by three levels because of study limitations (risk of bias), conflicting results (inconsistency), and number of included patients (imprecision) to VERY LOW.
Physical interventions
The level of evidence regarding the outcome measure pain was downgraded by three levels because of study limitations (risk of bias), conflicting results (inconsistency), and number of included patients (imprecision) to VERY LOW.
The level of evidence regarding the outcome measure anxiety was downgraded by three levels because of study limitations (risk of bias), conflicting results (inconsistency), and number of included patients (imprecision) to VERY LOW.
The level of evidence regarding the outcome measure functioning was downgraded by three levels because of study limitations (risk of bias), conflicting results (inconsistency), and number of included patients (imprecision) to VERY LOW.
The level of evidence regarding the outcome measure quality of life was downgraded by three levels because of study limitations (risk of bias), conflicting results (inconsistency), applicability (bias due to indirectness), and number of included patients (imprecision) to VERY LOW.
Psychological interventions
The level of evidencefor psychological therapy regarding the outcome measure pain was downgraded by two levels because of study limitations (risk of bias) and number of included patients (imprecision) to LOW.
The level of evidence for written self-disclosure regarding the outcome measure pain was downgraded by three levels because of study limitations (risk of bias), conflicting results (inconsistency), and number of included patients (imprecision) to VERY LOW.
The level of evidence for hypnotherapy regarding the outcome measure pain was downgraded by three levels because of study limitations (risk of bias), conflicting results (inconsistency), and number of included patients (imprecision) to VERY LOW.
The level of evidence for psychological therapy regarding the outcome measure anxiety was downgraded by one level because of study limitations (risk of bias) to MODERATE.
The level of evidence for psychological therapy regarding the outcome measure functioning was downgraded by three levels because of study limitations (risk of bias) and conflicting results (inconsistency) to VERY LOW.
The level of evidence for psychological therapy regarding the outcome measure quality of life was downgraded by two levels because of study limitations (risk of bias) and conflicting results (inconsistency) to LOW.
The level of evidence for hypnotherapy regarding the outcome measure quality of life was downgraded by three levels because of study limitations (risk of bias), conflicting results (inconsistency), and number of included patients (imprecision) to VERY LOW.
Other non-farmacological interventions
The level of evidence for relaxation therapy regarding the outcome measure efficacy was downgraded by two levels because of study limitations (risk of bias) and applicability (bias due to indirectness) to LOW.
The level of evidence for written self-disclosure regarding the outcome measure pain was downgraded by three levels because of study limitations (risk of bias), conflicting results (inconsistency), and number of included patients (imprecision) to VERY LOW.
Zoeken en selecteren
A systematic review of the literature was performed to answer the following question: What are the (un)favourable effects of non-pharmacological pain management compared to treatment without non-pharmacological pain management in the treatment of children with acute and chronic pain?
| P: |
1: preverbal children/neonates (<1 month) 2: verbal children 3: adolescents (<18 years) 4: children with neurobiological development disorders |
| I: |
1: psycho-education 2: distraction techniques (child care worker/pedagogical employee, clown, virtual reality, massage, sway, music therapy, physiological comfort techniques) 3: physiotherapeutic/physical interventions 4: psychological(behavioral therapy, hypnotherapy) 5: other non-farmacological interventions |
| C: | Pharmacological pain management or no pain management |
| O: | Crucial: prevention of pain, reduction of pain, anxiety, stress/distress, psychosocial functioning, (health-related) quality of life; Important: length of (hospital) stay, costs |
Relevant outcome measures
A priori, the working group did not define the outcome measures listed above but used the definitions used in the studies.
The working group defined one point difference on the VAS scale (Voepel-Lewis et al., 2011), or 10% difference on a pain scale as a minimal clinically (patient) important difference for pain prevention and reduction.
For dichotomic outcome measures, the working group used the GRADE default limits as limits for clinical decision making, which are defined as a risk ratio (RR) of >1.25 and <0.8 as clinically relevant.
For the meta-analysis, the working group defined a difference of 0.5 standard deviation (SD) as clinically relevant. When standardized mean difference (SMD) was used, 0.2 represented a small effect size, 0.5 a medium effect size and 0.8 a large effect size, based on Cohen, 1988.
Search and select (Methods)
We performed a systematic search for the PICO. The databases Medline (via OVID) and Embase (via Embase.com) were searched with relevant search terms until September 29, 2023. The detailed search strategy is depicted under the tab Methods. Studies were selected based on the following criteria: non-pharmacological treatment, pain, and children. The systematic literature search resulted in 3137 hits. Due to the high amount of hits, we decided to only screen the 697 systematic reviews.
Guideline working group members proposed an extensive World Health Organization (WHO) review and guideline about physiotherapeutic/physical (I4) and psychological (I5) interventions. We decided to use this systematic review (Fisher et al., 2022) for the literature summary of these two interventions. Therefore, we did not perform Title/Abstract selection in physiotherapeutic/physical and psychological interventions.
697 systematic reviews were screened on title and abstract for the interventions psycho-education (I1), distraction techniques (I2), and therapeutic communication (I3) by one working group member for the first selection, of which the 148 included articles were screened on title and abstract by an advisor and 48 were selected for full text reading. Parallel, another advisor screened the 697 systematic reviews using the artificial intelligence tool ASReview and included 122 articles based on title and abstract. Of these, 48 articles were not included by the manual title and abstract screening of the working group members. 15 additional articles were included based on title and abstract screening using ASReview. After reading the full text, 525 studies were excluded (see the table with reasons for exclusion under the tab Methods), and in total 7 studies, based on both the manual and ASReview screening, were included.
The working group decided to screen on title and abstract of the observational studies and RCTs on nonpharmacological treatments for preverbal children and neonates, because no systematic reviews were found for this subgroup. Screening was performed by an advisor using ASReview. One possibly suitable article provided by a working group member was used as prior knowledge. However, after screening, this article was researching pain in procedures and therefore excluded. Screening was stopped after 50 consecutive irrelevant records. No additional articles were included using ASReview screening.
Articles about procedures were excluded because these are part of the PSA guideline. A procedure was defined as: ‘an activity directed at or performed on an individual with the object of improving health, treating disease or injury, or making a diagnosis’. It is a course of action intended to achieve a result in the delivery of healthcare, which can be to diagnose, measure, monitor, or treat problems such as diseases or injuries. These are carried out by a healthcare professional. Procedures can be surgical, anaesthesia, propaedeutic, diagnostic, therapeutic, rehabilitative, screening, and cosmetic (Landau, 1986).
Results
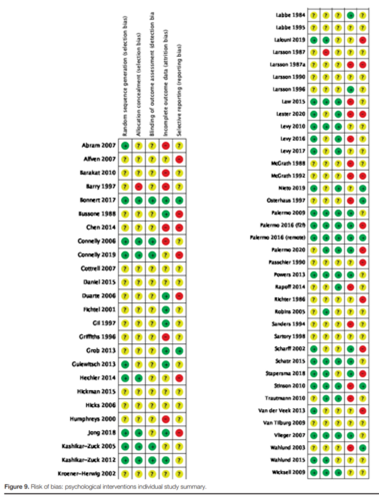
For the interventions psychoeducation, distraction techniques, and therapeutic communication, 6 studies were included in the analysis of the literature. Important study characteristics and results are summarized in the evidence tables. The assessment of the risk of bias is summarized in the risk of bias tables.
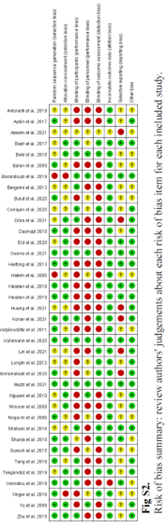
For the physical and psychological interventions, we did not include any studies in addition to the WHO review of Fisher et al., 2022. We adapted the evidence tables and risk of bias tables of the WHO review.
Referenties
- 1 - Abbott RA, Martin AE, Newlove-Delgado TV, Bethel A, Thompson-Coon J, Whear R, Logan S. Psychosocial interventions for recurrent abdominal pain in childhood. Cochrane Database Syst Rev. 2017 Jan 10;1(1):CD010971. doi: 10.1002/14651858.CD010971.pub2. PMID: 28072460; PMCID: PMC6464036.
- 2 - Abbott RA, Martin AE, Newlove-Delgado TV, Bethel A, Whear RS, Thompson Coon J, Logan S. Recurrent Abdominal Pain in Children: Summary Evidence From 3 Systematic Reviews of Treatment Effectiveness. J Pediatr Gastroenterol Nutr. 2018 Jul;67(1):23-33. doi: 10.1097/MPG.0000000000001922. PMID: 29470291.
- 3 - Fisher E, Villanueva G, Henschke N, Nevitt SJ, Zempsky W, Probyn K, Buckley B, Cooper TE, Sethna N, Eccleston C. Efficacy and safety of pharmacological, physical, and psychological interventions for the management of chronic pain in children: a WHO systematic review and meta-analysis. Pain. 2022 Jan 1;163(1):e1-e19. doi: 10.1097/j.pain.0000000000002297. Erratum in: Pain. 2023 Feb 1;164(2):e121. PMID: 33883536.
- 4 - Gordon M, Sinopoulou V, Tabbers M, Rexwinkel R, de Bruijn C, Dovey T, Gasparetto M, Vanker H, Benninga M. Psychosocial Interventions for the Treatment of Functional Abdominal Pain Disorders in Children: A Systematic Review and Meta-analysis. JAMA Pediatr. 2022 Jun 1;176(6):560-568. doi: 10.1001/jamapediatrics.2022.0313. PMID: 35404394; PMCID: PMC9002716.
- 5 - Koechlin H, Kossowsky J, Lam TL, Barthel J, Gaab J, Berde CB, Schwarzer G, Linde K, Meissner K, Locher C. Nonpharmacological Interventions for Pediatric Migraine: A Network Meta-analysis. Pediatrics. 2021 Apr;147(4):e20194107. doi: 10.1542/peds.2019-4107. Epub 2021 Mar 9. PMID: 33688031.
- 6 - Landau S. International Dictionary of Medicine and Biology. Page 2297. ISBN 0-471-01849-X.
- 7 - See C, Ng M, Ignacio J. Effectiveness of music interventions in reducing pain and anxiety of patients in pediatric and adult emergency departments: A systematic review and meta-analysis. Int Emerg Nurs. 2023 Jan;66:101231. doi: 10.1016/j.ienj.2022.101231. Epub 2022 Dec 16. PMID: 36528945.
- 8 - Ting B, Tsai CL, Hsu WT, Shen ML, Tseng PT, Chen DT, Su KP, Jingling L. Music Intervention for Pain Control in the Pediatric Population: A Systematic Review and Meta-Analysis. J Clin Med. 2022 Feb 14;11(4):991. doi: 10.3390/jcm11040991. PMID: 35207263; PMCID: PMC8877634.
Evidence tabellen
Evidence table for systematic review of RCTs and observational studies (intervention studies)
|
Study reference |
Study characteristics |
Patient characteristics |
Intervention (I) |
Comparison / control (C)
|
Follow-up |
Outcome measures and effect size |
Comments |
|
|
|||
|
See, 2023
Study characteristics and results are extracted from the SR (unless stated otherwise) |
SR and meta-analysis of RCTs, quasi-experimental studies, cohort studies, case-control studies, and pilot studies.
Literature search between 2011 and 2021.
Source of funding and conflicts of interest: No conflicts of interest. Funding not reported.
|
Inclusion criteria SR:
Exclusion criteria SR:
11 studies included, of which 9 studies and 2 reported of included studies. Three studies focused on the paediatric population (aged ≤21 years) (Hartling ea 2013; Park ea 2016; van der Heijden ea 2019)
Country and setting: 5 studies were conducted in the United States, 2 in Turkey, and one each from Australia, Canada, France, and South Africa |
Of the studies in paediatric patients, music interventions consisted of:
|
Standard care, or cartoon and standard care. |
Follow-up not reported.
|
Pain* Pooled effect (random effect model): SMD -0.40 (95%CI -0.78 to -0.03) favouring the intervention. Heterogeneity (I2): 81%. n=2
The quality of evidence was GRADE Very low, due to the lack of blinding for RCTs (risk of bias), inconsistency (high heterogeneity), indirectness (variations in population age and music interventions), and imprecision (small sample sized with wide CIs).
|
Intervention subgroup Distraction techniques
Risk of Bias Risk of Bias assessment not provided. Authors state that due to the nature of music interventions, the quality criteria of RCTs for blinding participants, providers and outcome assessors were poorly scored.
Authors conclusion This systematic review and meta-analysis present findings that contribute to the evidence supporting the effectiveness of music interventions in improving pain and anxiety levels in paediatrics and adults in the EDs.
See fiure 2 for evidence table of the included studies in the review.
*Only the results in paediatric patients are reported. |
|
|
|||
|
Fisher, 2022*
Study characteristics and results are extracted from the SR (unless stated otherwise) |
SR and meta-analysis of RCTs and nonrandomised comparative observational trials (latter only for physical therapies)
Literature search between inception and March 2020.
Source of funding and conflicts of interest: E. Fisher, G. Villanueva, N. Henschke, K Probyn, B. Buckley, and C. Eccleston report funding from the World Health Organisation during the conduct of the study. W. Zempsky is a consultant for GSK, GlycoMimetics, and the Institute for Advanced Clinical Trials for Children. He receives funding form the National Institutes of Health and the U.S. Department of Defence. The remaining authors have no conflicts of interest to declare |
Inclusion criteria SR:
Exclusion criteria SR:
24 studies in physical therapy were included, of which 33 reported of RCTs and 12 further ongoing trials (11 RCTs and 1 nonrandomised trial (of which 1 reported results)). 18 studies were included in the quantitative synthesis. 63 studies in psychological therapy were included, of which 81 reports of RCTs and 16 ongoing RCT studies.56 studies were included in the quantitative synthesis.
Country and setting: Of the 25 trials that had results, 4 studies each were conducted in the United Kingdom and the United States, 3 in Turkey, 2 each in Canada and Sweden, and one each in Australia, Brazil, Chile, Denmark, India, Iran, Israel, the Netherlands, Portugal, and Saudi Arabia. We found 34 studies conducted in North America, 11 in Sweden, 6 in the Netherlands, 6 in Germany, 2 in Australia, and one inBrazil, Spain, Italy, and China each. |
Physical trials Physical intervention
Psychological trials We found 43 arms of CBT, 15 arms of relaxation, 7 arms of behavioural therapy, 3 arms of hypnosis, 2 arms of problem-solving therapy, and one arm of acceptance commitment therapy. |
Physical trials Nonphysical intervention control, standard care, or waitlist control.
Psychological trials We found 36 active control arms, 16 standard or usual care arms, and 17 waitlist control arms (note, because some studies included multiple arms, these numbers will not add up to 63). |
Follow-up not reported.
|
Random effect models were used for all meta-analysis.
For the outcomes, see figure 4.
|
Intervention subgroup Physical and psychological interventions
Risk of Bias Risk of Bias assessment of physical intervention provided by Fisher 2022:
Risk of Bias assessment of psychological intervention provided by Fisher 2022:
Authors conclusion We conducted a systematic review and meta-analysis to determine the efficacy and safety of pharmacological, physical, and psychological therapies for the management of chronic pain in youth, used to support the World Health Organisation guidelines on the management of chronic pain in children.40 We found each modality of intervention separately showed some benefits of reducing pain intensity posttreatment, but these effects were not maintained at follow-up. Some physical and psychological interventions also reduced functional disability posttreatment, and effects were maintained for psychological interventions at follow-up. Pharmacological and physical therapies presented very little data that could be analysed for other outcomes, meaning any absence of effects and beneficial effects should be interpreted with caution. Most data were available for psychological therapies; however, we found no beneficial effects for improving HRQOL, emotional functioning, role functioning, or sleep in the meta-analyses. A beneficial effect of psychological interventions was found for global satisfaction with treatment. Adverse events were poorly reported, particularly in physical and psychological trials. See figure 3 for evidence table of the included studies in the review.
*Data on pharmacological interventions not reported. |
|
|
|||
|
Ting, 2022
Study characteristics and results are extracted from the SR (unless stated otherwise) |
SR and meta-analysis of RCTs, quasi-experimental studies, cohort studies, case-control studies, and pilot studies.
Literature search from inception to January 2022
Source of funding and conflicts of interest: The authors declare no conflict of interest.
The authors of this work were supported by the following grants: MOST108-2410-H-039-003, 109-2320-B-038-057-MY3, 109-2320-B-039-066, 110-2321-B-006-004, 110-2811-B-039-507, 110-2320-B-039-048-MY2, and 110-2320-B-039-047-MY3 from the Ministry of Science and Technology, Taiwan; ANHRF109-31, 110-13, and 110-26 from An Nan Hospital, China Medical University, Tainan, Taiwan; CMRC-CMA-2 from Higher Education Sprout Project by the Ministry of Education (MOE), Taiwan; CMU108-SR-106 from the China Medical University, Taichung, Taiwan; and CMU104-S-16-01, CMU103-BC-4-1, CRS-108-048, DMR-102-076, DMR-103-084, DMR-106-225, DMR-107-204, DMR-108-216, DMR-109-102, DMR-109-244, DMR-HHC-109-11, DMR-HHC-109-12, DMR-HHC-110-10, DMR-110-124, and CMU110-AWARD-02 from the China Medical University Hospital, Taichung, Taiwan |
Inclusion criteria SR: (1) randomized controlled trials; (2) intervention group receiving music intervention that included all three factors of music (i.e., rhythm, melody, and harmony); (3) outcome assessments included pain measures; (4) age of all participants was less than 18 years.
Exclusion criteria SR: (1) reviews, protocols, conference papers, case reports, letters, or editorials; (2) MI was administered with other types of therapy or was a part of complementary and alternative therapy; (3) the control group received any components of music, i.e., rhythm, melody, and harmony; (4) studies that did not provide information for meta-analysis.
38 studies included, which were all included in the meat-analysis.
Country and setting: Eight rticles were conducted in America, 14 in Europe, 13 in Asia, and three in Africa. 29 studies were conducted in the hospital setting and 9 in the clinic setting.
Two articles included adolescents, 12 newborns, and 24 infants and children.
8 articles included patients with postoperative pain. |
Listening to music. Among all 38 studies, the participants listened to classical music in 11 articles, to kids’ music in 8 articles, to world music in 3 articles, to pop music in 3 articles, to special composition in 5 articles, and to multiple combinations of music in 4 articles. Additionally, in one article, two groups of participants listened to either kids’ music or world music, and thus this article was separated into two datasets. As for the listening instruments, 9 articles used headphones, 4 used earphones, 19 used speakers, and 4 used live performance. In one article, participants listened to music using either headphones or speakers, and thus this study was separated into two datasets. |
Music control (not further described, but the control group does not receive any components of music) |
Follow-up not reported.
|
Pain release* Pooled effect (random effect model): SMD -0.49 (95%CI -0.90 to -0.08, p=0.018) favouring the intervention. Heterogeneity (I2): 81%. n=8
|
Intervention subgroup Distraction techniques
Risk of Bias Risk of Bias assessment provided by Ting et al., 2022:
Authors conclusion Our meta-analysis of 38 RCT articles with a total of 5601 participants provides evidence supporting the thesis that MI releases pain in both psychological and physiological domains. A consistent music style and a pure auditory experience might be important in MI for pain. MI is an appropriate, low-stress, and safe non-pharmacological treatment for clinical pain relief in the paediatric population.
No evidence table provided.
*Only the results of patients with postoperative pain are reported. |
|
|
|||
|
Koechlin, 2021 |
SR and network meta-analysis of RCTs
Literature search from inception to August 5, 2019
Source of funding and conflicts of interest: The authors declare no conflict of interest.
Dr Koechlin is sponsored by the Swiss National Science Foundation fellowship P400PS_186658. Dr Locher received funding for this project from the Swiss National Science Foundation: P400PS_180730. Dr Meissner received support from the Schweizer-Arau-Foundation and the Theophrastus Foundation, Germany. This work was supported in part by the Sara Page Mayo Endowment for Pediatric Pain Research, Education, and Treatment. |
Inclusion criteria SR: Nonpharmacological interventions for children and adolescents <18 years were included in this study. Participants had to have a diagnosis of episodic migraine (with or without aura) according to the International Headache Society (IHS) criteria, or criteria for migraine diagnosis had to be in close agreement with the IHS classification. Trials had to specifically state that they were focusing on paediatric migraine but were not required to specify the exact diagnosis of migraine to be included. Eligible trial designs included RCTs that make head-to-head comparisons of at least 2 nonpharmacological interventions as well as RCTs that compare at least 1 nonpharmacological intervention with a control group. To be included, trials had to report at least 1 migraine-related outcome. Crossover studies were only included if we were able to extract the results of the first period separately.
Exclusion criteria SR: Studies in which migraine was associated with other neurologic disorders as well as studies on menstrual migraine were excluded.
12 studies were included. 2 other studies were excluded because they did not connect with the other studies in the network.
Country and setting: Six (5%) of the included trials recruited children and adolescents from the United States, 3 (25%) from Europe, and 3 (25%) from Canada. |
Relaxation stress management, relaxation education, relaxation, biofeedback, biofeedback stress management, biofeedback relaxation education, transcendental meditation, autogenic feedback, autogenic training, psychological treatments, progressive muscle relaxation, self-administered psychological treatments, education, and hypnotherapy |
Psychological placebo, sham biofeedback, and waiting list |
Follow-up not reported.
|
Lumping approach* (interventions combined into one group) Short term efficacy Self-administered treatments (SMD: 1.44; 95% CI, 0.26 to 2.62), biofeedback (SMD: 1.41; 95% CI, 0.64 to 2.17), relaxation (SMD: 1.38; 95% CI, 0.61 to 2.14), psychological treatments (SMD: 1.36; 95% CI, 0.15 to 2.57), and psychological placebos (SMD: 1.17; 95% CI, 0.06 to 2.27) were significantly more effective than the waiting list.
Long-term efficacy Self-administered treatments (SMD: 1.40; 95% CI, 0.28 to 2.52), relaxation (SMD: 1.35; 95% CI, 0.60 to 2.09), psychological treatments (SMD: 1.33; 95% CI, 0.18 to 2.47), biofeedback (SMD: 1.21; 95% CI, 0.47 to 1.94), and psychological placebos (SMD: 1.14; 95% CI, 0.09 to 2.19) revealed significantly higher effects than the waiting list.
Splitting approach* (less heterogeneity because interventions are split into groups)
Short-term efficacy No nonpharmacological intervention was significantly more effective than the waiting list. There were no significant differences between the included nonpharmacological interventions.
Long-term efficacy No nonpharmacological intervention was significantly more effective than the waiting list. None of the included nonpharmacological interventions did differ significantly from one another.
*Outcomes were (1) number of headache days per month, (2) the number of migraine days per month, (3) frequency of headache attacks (means and SDs), (4) frequency of migraine attacks (means and SDs), or (5) headache index and activity. |
Intervention subgroup Psychoeducation, distraction techniques, therapeutic communication Quality assessment See https://view.officeapps.live.com/op/view.aspx?src=https%3A%2F%2Faap2.silverchair-cdn.com%2Faap2%2Fcontent_public%2Fjournal%2Fpediatrics%2F147%2F4%2F10.1542_peds.2019-4107%2F1%2Fpeds_20194107supplementarydata.doc%3FExpires%3D1705498669%26Signature%3DDntYW92gQkpbZfMHFqAbrI3Tbb7J~ozzJ3zZzG7y-WaxLcmNBZ4nRbDqkHuGDPhb~2GpB8zKUpGDavelrYWm59RAQxmq~djBTXQxBQUy2YsddVvvQ4Orfts1rNzexXYJ~ukZeRWs2LM9NpOFWJgTpLYpLssIfR2rIPspdnmXRGZSQY1M7DUQcog4acEk~F5aAXHAh0q8~3vc8oOR-UtsmVcSzeSOryjuNSw1A-uu3ve6UNrE2JTjPQ4xiorl6R5jFmDzhUA1~0HxaGWxqzfCDZRqq5JHWNYQ7Cqo95Ryw07MahN8FVDqoyIBJX7jWXR~HMuXlc7NY3ddyMhk7f8gqw__%26Key-Pair-Id%3DAPKAIE5G5CRDK6RD3PGA&wdOrigin=BROWSELINKhttp GRADE assessments.
Authors conclusion Interestingly, our NMA revealed different findings depending on the structure of the nodes in the network. In a first step, we lumped the interventions into broader classes. Significant short- and long-term effects were found for most nonpharmacological interventions (ie, relaxation, biofeedback, psychological treatments, and self-administered psychological treatments) as well as for 1 control group (ie, psychological placebo) when compared with the waiting list. In terms of our second aim (ie, to systematically compare the different nonpharmacological treatment options relative to each other), the included interventions did not significantly differ from one another. In a second step, we split the various published interventions into individual nodes. Here, in the short- and long-term analyses, none of the included interventions were significantly more effective than the waiting list, which was the control group that most interventions were compared with and that connected 2 otherwise unconnected networks. Also, none of the interventions differed significantly from each other in the head-to-head comparisons.
See https://publications.aap.org/view-large/8236846https://punce table of the included studies in the review.
|
|
|
|||
|
Abbott, 2017 |
SR and meta-analysis of RCTs
Literature search up to June 2016
Source of funding and conflicts of interest: The authors declare no conflict of interest.
The work of the evidence synthesis team is funded by the National Institute for Health Research Collaboration for Leadership in Applied Health Research and Care South West Peninsula (PenCLAHRC). However, the funder had no role in the review itself. |
Inclusion criteria SR:
Exclusion criteria SR: Not reported
18 studies were included (from 26 reports). 2 studies (from 2 reports) awaiting classification and 14 studies were included in the meta-analysis.
Country and setting: The majoity of studies recruited children through paediatric gastroenterology or paediatric pain clinics. Eight studies were conducted in the USA, three in the Netherlands, two in Germany, two in Australia, one in Canada, one in Brazil, and one recruiting from both the USA and Canada. |
Cognitive behaviour therapy (CBT), hypnotherapy (including guided imagery), yoga, written self-disclosure. |
The comparator was usual medical care in six studies, a wait‐list control in eight studies, and an education or breathing control, or both, in four studies. However, meta-analysis are compared with usual care or wait-list control. |
Follow-up not reported.
|
CBT Pain intensity: postintervention Pooled effect (random effect model): SMD -0.33 (95%CI -0.74 to 0.08, p=0.12) favouring the intervention. Heterogeneity (I2): 70%. n=7. GRADE low.
Pain intensity: between 3 to 12 months follow-up Pooled effect (random effect model): SMD -0.32 (95%CI -0.85 to 0.20, p=0.23) favouring the intervention. Heterogeneity (I2): 76%. n=4. GRADE low.
Pain intensity: 12 months or more follow-up Pooled effect (random effect model): SMD -0.04 (95%CI -0.39 to 0.31, p=0.82) favouring the intervention. Heterogeneity (I2): 52%. n=3. GRADE low.
Pain duration There was no evidence of effect of intervention compared with active control on pain duration at any time point (postintervention mean: 8.67 intervention, 6.84 control, P = 0.96; 6 months' mean: 5.34 intervention, 8.58 control, P = 0.25; 12 months' mean: 6.11 intervention, 6.89 control, P = 0.80 (no SDs reported)). n=1
Social or psychological functioning Three studies reported outcomes related to social or psychological functioning, all of which found no effect of therapy when compared to control.
Quality of life (physical subscale) Pooled effect (random effect model): SMD 0.71 (95%CI ‐0.25 to 1.66, p=0.15) favouring the intervention. Heterogeneity (I2): 79%. n=3. GRADE very low.
Quality of life (psychosocial subscale) Pooled effect (random effect model): SMD 0.43 (95%CI ‐0.21 to 1.06, p=0.19) favouring the intervention. Heterogeneity (I2): 58%. n=3. GRADE low.
Functional impairment of daily activities Pooled effect (random effect model): SMD -0.57 (95%CI ‐1.34 to 0.19, p=0.14) favouring the intervention. Heterogeneity (I2): 80%. n=4. GRADE very low.
Hypnotherapy Pain intensity: postintervention Pooled effect (random effect model): SMD -1.01 (95%CI -1.41 to -0.61, p<0.00001) favouring the intervention. Heterogeneity (I2): 21%. n=4. GRADE low.
Pain intensity: 5 year follow-up n=1 Pain intensity remained significantly lower at five years (P < 0.001) in the group that had received three months of hypnotherapy (mean 2.9 (SD 4.4)) compared to the group that had received usual care (mean 7.7 (SD 5.3)).
Pain frequency: postintervention Pooled effect (random effect model): SMD -1.28 (95%CI -1.84 to -0.72 p<0.00001) favouring the intervention. Heterogeneity (I2): 55%. n=4. GRADE low.
Pain frequency: 5 year follow-up n=1 Pain frequency remained significantly lower (P = 0.001) in the group that had received hypnotherapy (mean 2.3 (SD 4.0)) compared to the group that had received usual care (mean 7.1 (SD 6.0)).
Pain duration n=1 They reported pain duration as significantly lower for the 20 children who had received hypnotherapy compared to those in the wait‐list control group, with mean scores of 1.20 (SD 1.47) compared to 3.50 (SD 2.53), P = 0.014, respectively.
Quality of life One study found no difference in self-repoted health‐related quality of life for those receiving hypnotherapy compared to wait‐list control.
One study found that children who had received guided imagery therapy reported an improved overall quality of life (mean 28.2) compared to those in the wait‐list control group (mean 9.3) at postintervention (P = 0.49, no SDs reported).
One study reported that there were no differences in quality of life at five‐year follow‐up between those who had received hypnotherapy compared to usual care control.
Yoga Pain intensity: postintervention Pooled effect (random effect model): SMD -0.31 (95%CI -0.67 to 0.05, p=0.09) favouring the intervention. Heterogeneity (I2): 0%. n=3. GRADE low.
Pain intensity: 12-month follow-up One study found no significant effect over time for the yoga intervention compared to usual care (P = 0.09).
Pain frequency: postintervention, 6 month, 12-month follow-up One study reported no significant effect of yoga compared to usual care on pain frequency across the three follow‐ups (postintervention, 6 months, and 12 months; P = 0.20).
Social or psychological functioning None of the three studies reported significant effects of yoga intervention on social or psychological functioning.
Functional disability Pooled effect (random effect model): SMD -0.32 (95%CI -1.07 to 0.43, p=0.40) favouring the intervention. Heterogeneity (I2): 44%. n=2. GRADE low.
Written self-disclosure Pain frequency No effect of treatment on the frequency of debilitating pain episodes (using a scale of 0 to 5, where 0 = none and 5 = every day) was found postintervention or at three months' follow‐up. However, at six months' follow‐up the frequency of such episodes was lower (P < 0.05, exact P value not in report) in those who had undergone written self‐disclosure (mean 1.35 (SD 1.39)) compared to usual care (mean 2.32 (SD 1.72)).
|
Intervention subgroup Psychoeducation, therapeutic communication
Risk of Bias Risk of Bias assessment provided by Abbott et al., 2017:
Authors conclusion Overall, this review provides low‐quality evidence that cognitive behavioural therapy (CBT) and hypnotherapy may be effective in reducing pain in the short term for children and adolescents presenting with recurrent abdominal pain (RAP). Sustained effects of both CBT and hypnotherapy on pain have also been reported, but the evidence to date is limited. There was little evidence that CBT or hypnotherapy affected school functioning, psychological well‐being, or quality of life.
This review found no evidence to support the use of yoga or written self‐disclosure for the treatment of recurrent abdominal pain in children and adolescents.
See https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6464036/#CD010971-sec-0155title for evidence tables of the included studies in the review.
|
|||||
|
Gordon, 2022
Study characteristics and results are extracted from the SR (unless stated otherwise) |
SR and meta-analysis of RCTs
Literature search from inception to August 2021
Source of funding and conflicts of interest: The authors declare no conflict of interest.
Funding is not reported |
Inclusion criteria SR: Included in this systematic review and meta-analysis were all published, unpublished, and ongoing RCTs that compared psychosocial interventions to any intervention or control and studies that targeted at children aged 4 to 18 years with functional abdominal pain disorders as defined by the Rome or similar criteria. There were no date or language restrictions.
Exclusion criteria SR: Not reported.
33 studies included (67 citations), of which 26 were included in the mete-analysis.
Country and setting: Not reported. |
Twelve studies compared CBT with no intervention, 5 studies compared CBT with educational support, 3 studies compared yoga with no intervention, 2 studies compared hypnotherapy with no intervention, 2 studies compared gut-directed hypnotherapy with hypnotherapy, and 2 studies compared guided imagery with relaxation. |
See previous column |
Length of follow-up lasted from 5 days to 4 months.
|
CBT* Pain frequency Pooled effect (random effect model): SMD -0.36 (95%CI -0.63 to -0.09) favouring the intervention. Heterogeneity (I2): 46%. n=7. GRADE moderate.**
Pain intensity Pooled effect (random effect model): SMD -0.58 (95%CI -0.83 to -0.32) favouring the intervention. Heterogeneity (I2): 19%. n=6. GRADE moderate.**
Yoga* Pain frequency and intensity One study reported pain frequency and intensity and another change in pain frequency. However, the results of the meta-analyses were of very low certainty in evidence owing to high imprecision and risk of bias, and no conclusions could be drawn.
Hypnotherapy* Pain frequency, pain intensity, and composite pain score One study reported this, but the results of the meta-analyses were of very low certainty owing to high imprecision and risk of bias, and no conclusions could be drawn.
|
Intervention subgroup Psychoeducation, therapeutic communication
Risk of Bias Risk of Bias assessment provided by Gordon et al., 2022:
Authors conclusion Results of this systematic review and meta-analysis suggest that CBT and hypnotherapy should be considered as a treatment for functional abdominal pain disorders in childhood, and these new findings should be considered by guideline committees internationally. Future RCTs should address sample size and precision issues in integral areas to enhance overall certainty; in addition, studies should also consider the role of targeted interventions for susceptible patients. CBT combined with a pharmacological or microbiome-based therapy may be considered.
See https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9002716/bin/jamapediatr-e220313-s001.pdfhttps://w) for evidence tables of the included studies in the review.
*Only results of intervention versus no intervention reported here.
** Study reports SMD in figure and RR in text. Due to the outcome measure, we assumed that SMD is right. |
|||||
|
Abbott, 2018 |
SR and meta-analysis of RCTs and randomised cross-over trials
Literature search up to November 21, 2017
Source of funding and conflicts of interest: Conflict of interest not reported.
This research was funded by the National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care South West Peninsula. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health. |
Inclusion criteria SR: Children aged 5 to 18 years old with RAP or an abdominal pain-related functional gastrointestinal disorder, as defined by the Rome III criteria (13), were included. Any dietary, pharmacological, or psychosocial intervention compared to placebo, waiting list, no treatment, active control (psychosocial interventions only), or standard care were included. Included studies were restricted to randomised controlled trials (RCTs) and randomised cross-over studies.
Exclusion criteria SR: Not reported
52 studies included in 3 Cochrane reviews. 3 additional studies were included on update search in Nov 2017.
Country and setting: The studies were conducted in 15 countries, recruiting children from secondary/tertiary paediatric gastroenterology or pain clinics (n = 37), primary care (n = 1), the community (n = 1), or from a combination of these (n = 10), or not described (n = 3). |
Psychosocial interventions* |
Placebo, waiting list, no treatment, active control, or standard care |
Follow-up not reported.
|
BT** Pain intensity: postintervention Pooled effect (random effect model): OR 5.67 (95%CI 1.18 to 27.32, p=0.03) favouring the intervention. Heterogeneity (I2): 71%. NNTB = 4. n=4.
Pain intensity: between 3 to 6 months follow-up Pooled effect (random effect model): OR 3.08 (95%CI 0.93 to 10.16, p=0.06) favouring the intervention. NNTB = 5. n=3
Pain intensity: 12 months or more follow-up Pooled effect (random effect model): OR 1.29 (95%CI 0.50 to 3.33, p=0.60) favouring the intervention. n=2.
Hypnotherapy Pain intensity: postintervention Pooled effect (random effect model): OR 6.78 (95%CI 2.41 to 19.07, p<0.0003) favouring the intervention. Heterogeneity (I2): 23%. NNTB = 3. n=4.
|
Intervention subgroup Psychoeducation Risk of Bias Not provided.
Authors conclusion The review also found low-quality evidence to suggest that CBT and hypnotherapy may be effective in treating RAP, with both reported to be effective in reducing pain in the short term. Sustained effects of CBT and hypnotherapy were also reported but the evidence is limited. We found insufficient evidence to support the use of yoga therapy or written self-disclosure.
*Data on dietary and pharmacological interventions not reported here.
**This article is a summary and update of three reviews, including the review of Abbott 2017. This review is described above, only the different results of CBT and hypnotherapy are described (yoga and written self-disclosure is therefore not described here).
See figure 5 for the characteristics of the included studies. |
|||||
Figure 1.Evidence table of the included studies in See et al., 2023. Black outlined are the studies in children

Figure 2. Evidence table of the included studies in Fisher et al., 2022
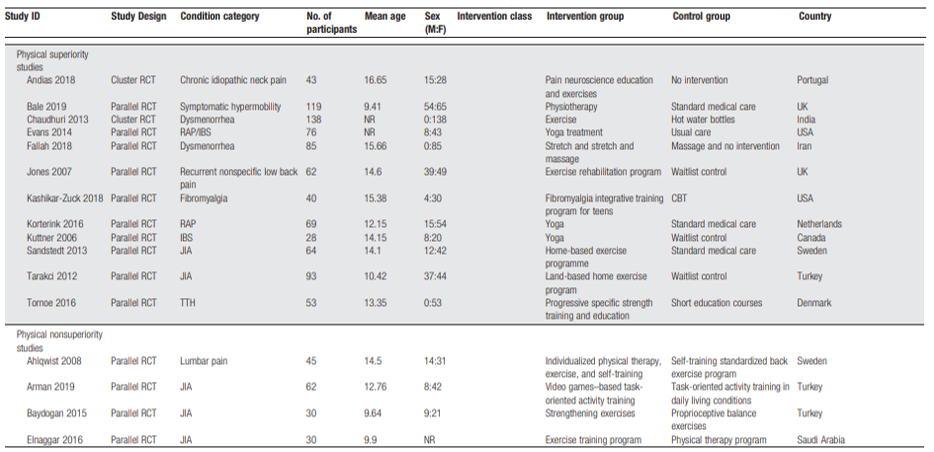
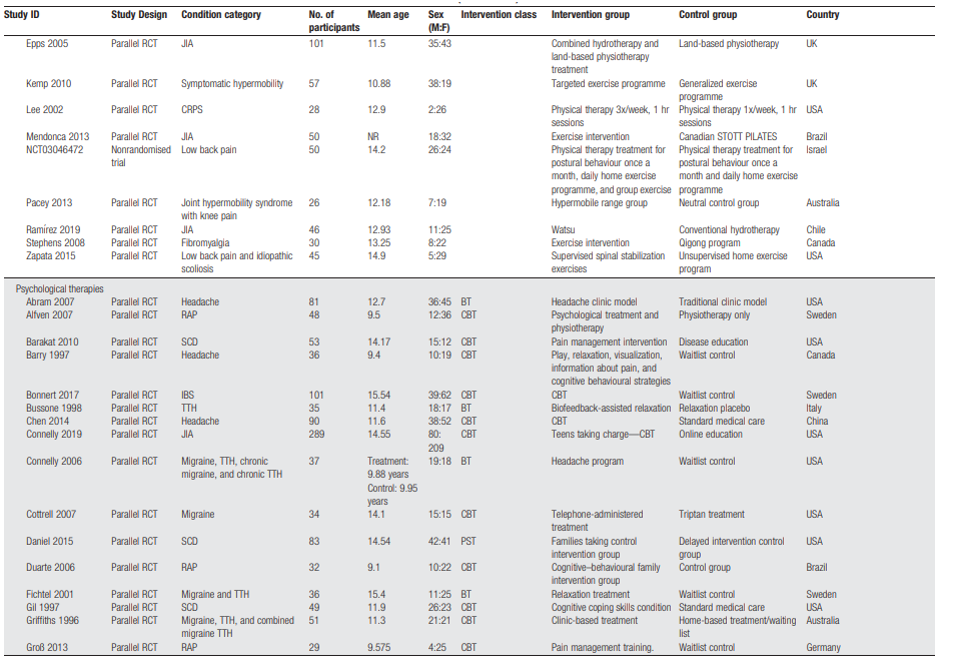
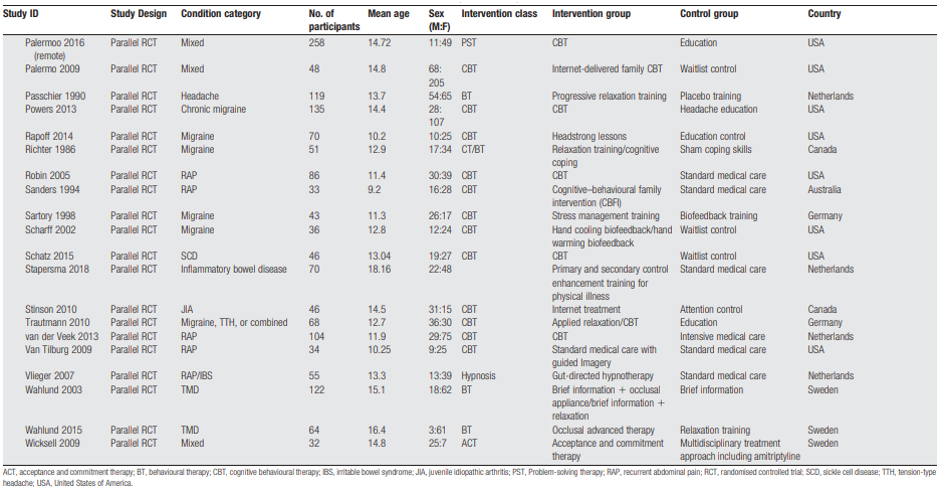
Figure 3. Meta-analysis findings of Fisher et al., 2022
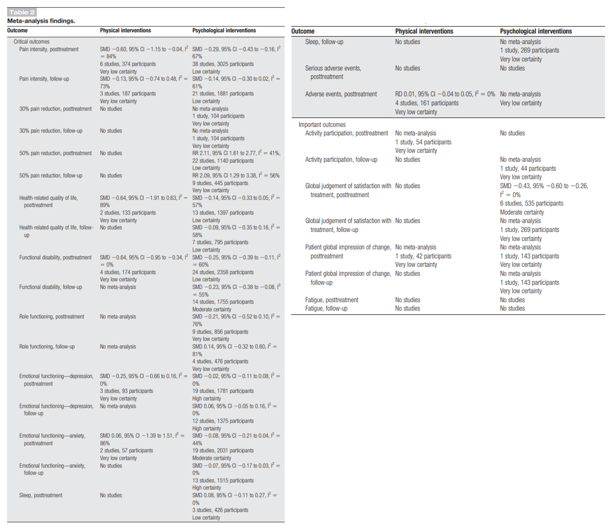
Figure 4. Characteristics of the included studies in Abbott et al. (2018)
Quality assessment of the included studies
|
Study
First author, year |
Appropriate and clearly focused question?1
Yes/no/unclear |
Comprehensive and systematic literature search?2
Yes/no/unclear |
Description of included and excluded studies?3
Yes/no/unclear |
Description of relevant characteristics of included studies?4
Yes/no/unclear |
Appropriate adjustment for potential confounders in observational studies?5
Yes/no/unclear/not applicable |
Assessment of scientific quality of included studies?6
Yes/no/unclear |
Enough similarities between studies to make combining them reasonable?7
Yes/no/unclear |
Potential risk of publication bias taken into account?8
Yes/no/unclear |
Potential conflicts of interest reported?9
Yes/no/unclear |
Risk of bias |
|
See, 2023 |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Unclear |
Yes |
Low |
|
Fisher, 2022 |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Low |
|
Ting, 2022 |
Yes |
Yes |
Yes |
No |
Not applicable |
Yes |
Yes |
Yes |
No |
High |
|
Koechlin, 2021 |
Yes |
Yes |
Yes |
Yes |
Not applicable |
Yes |
Yes |
Yes |
No |
Low |
|
Abbott, 2017 |
Yes |
Yes |
Yes |
Yes |
Not applicable |
Yes |
Yes |
Yes |
Yes |
Low |
|
Gordon, 2022 |
Yes |
Yes |
Yes |
Yes |
Not applicable |
Yes |
Yes |
No |
No |
Some concerns |
|
Abbott, 2018 |
Yes |
Yes |
Yes |
Yes |
No |
Yes |
Yes |
Yes |
No |
Low |
Table of excluded studies
|
Title |
Author |
Year |
Reason |
|
Effect modifiers of virtual reality in pain management: a systematic review and meta-regression analysis |
Lier |
2023 |
Children not reported separately |
|
Using Virtual Reality Exposure Therapy in Pain Management: A Systematic Review and Meta-Analysis of Randomized Controlled Trials |
Huang |
2022 |
Study includes children and adults and procedural and non procedural pain. No results for children with non procedural pain are reported separately. |
|
Effectiveness of psychosocial interventions on health outcomes of children with cancer: A systematic review of randomised controlled trials |
Melesse |
2022 |
No results on pain were reported |
|
Effect of a game-based intervention on preoperative pain and anxiety in children: A systematic review and meta-analysis |
Suleiman-Martos |
2022 |
Wrong C: children in the control group in some studies also receive other distraction methods (multimedia player, VR video + parents watching the same video, bedside entertainment and relaxation theatre) |
|
The Benefits of Integrative Medicine for Pain Management in Oncology: A Narrative Review of the Current Evidence |
Carvalho |
2023 |
Children not reported separately, included studies about children also not suitable for the PICO |
|
Integrative Medicine for Pain Management in Oncology: Society for Integrative Oncology-ASCO Guideline |
Mao |
2022 |
Children not reported separately, evidence extraction cannot be found in the guideline |
|
The physical and psychological outcomes of art therapy in pediatric palliative care: A systematic review |
Motlagh |
2023 |
5 of 17 studies were before-after studies. 4 studies reported on pain, of which 2 were non-RCTs |
|
A systematic review of psychosocial therapies for children with rheumatic diseases |
Cohen |
2017 |
One study compares cognitive behaviour therapy with wait-list control, see cell A69. |
|
American Society of Hematology 2020 guidelines for sickle cell disease: Management of acute and chronic pain |
Brandow |
2020 |
Two pre-post studies could be suitable, see cell A69 and A70. Other studies were not in children. |
|
Pain management in COVID-19 pediatric patients - An evidence- based review |
Mishra |
2021 |
Wrong I: other nonpharmacological treatments than in our PICO (drink water/warm bath/etc) |
|
Nonpharmacological interventions addressing pain, sleep, and quality of life in children and adolescents with primary headache: A systematic review |
Klausen |
2019 |
Wrong C: waiting list control, telephone contact, or webbased CD rom |
|
Effect of music on patients with cardiovascular diseases and during cardiovascular interventions: A systematic review |
Ho |
2021 |
Review includes no studies in children measuring pain |
|
The effectiveness of play therapy in children with leukemia: A systematic review |
Ramdaniati |
2023 |
Wrong O: doen not report pain quantitatively |
|
A Systematic Review of Occupational Therapy-Related Interventions for Pediatric Chronic Pain |
Suder |
2023 |
Control not described |
|
Adjunctive Nonpharmacologic Interventions for the Management of Burn Pain: A Systematic Review |
Gasteratos |
2022 |
Children not reported separately |
|
Complementary and alternative medicine for children with sickle cell disease: A systematic review |
Alsabri |
2023 |
Wrong aim: cross sectional studies info the use of CAM |
|
Control effect of virtual reality technology on procedural pain in children's wound: A meta-analysis |
Li |
2022 |
Wrong I: VR for procedural pain |
|
Does virtual reality reduce pain in pediatric patients? A systematic review |
Iannicelli |
2019 |
Wrong I: VR for procedural pain |
|
Effectiveness of complementary therapies for the management of symptom clusters in palliative care in pediatric oncology: a systematic review |
Lopes-Júnior |
2021 |
wrong I: complementary therapy |
|
Effectiveness of Hospital Clowning on Pediatric Anxiety and Pain: Network Meta-Analysis |
Caci |
2023 |
Medical procedure |
|
Effectiveness of hospital clowns for symptom management in paediatrics: Systematic review of randomised and non-randomised controlled trials |
Lopes-Júnior |
2020 |
Outcome pain only reported during procedures, anxiety also reported before the procedure and during the induction of anaethesia --> prodecures |
|
Effectiveness of virtual reality interventions for adolescent patients in hospital settings: Systematic review |
Ridout |
2021 |
One study reported that measures pain during chemotherapy using VR compared to standard care. This study can be extracted, see cell A71. |
|
Effects of Infant Massage: A Systematic Review |
Mrljak |
2022 |
One study included was performed in children with non procedural pain. These study data can be extracted, see cell A72. |
|
Effects of non-pharmacological interventions on pain intensity of children with burns: A systematic review and meta-analysis |
Farzan |
2023 |
Some of the included studies compared with standard care or pharmacotherapy and some with other distraction methods (television, stories, toys) + procedures (burn wound care, physical therapy, dressing application, dressing removal) |
|
Efficacy and safety of non-pharmacological interventions for endotracheal suctioning pain in preterm infants: A systematic review |
Cai |
2023 |
Procedure (endotracheal suctioning) |
|
Efficacy and safety of non-pharmacological interventions for neonatal pain: an overview of systematic reviews |
Shen |
2022 |
Procedural pain |
|
Efficacy of non-pharmacological interventions to reduce pain in children with sickle cell disease: A systematic review |
van Veelen |
2023 |
Wrong C: education and also includes articles without control. However, three articles could be suitable, see A74. |
|
Examining the effects of music-based interventions on pain and anxiety in hospitalized children: An integrative review |
Johnson |
2021 |
Procedures |
|
Extended Reality Use in Paediatric Intensive Care: A Scoping Review |
Goldsworthy |
2023 |
Wrong design: description of studies but no effects described + no control groups described |
|
Holistic Comfort Interventions for Pediatric Nursing Procedures: A Systematic Review |
Bice |
2017 |
Wrong I: procedural comfort interventions |
|
Immersive and Non-Immersive Virtual Reality for Pain and Anxiety Management in Pediatric Patients with Hematological or Solid Cancer: A Systematic Review |
Comparcini |
2023 |
One study could be suitable, see A78 |
|
Immersive Virtual Reality as Analgesia during Dressing Changes of Hospitalized Children and Adolescents with Burns: A Systematic Review with Meta-Analysis |
Lauwens |
2020 |
Procedure |
|
Impact of Virtual Reality Technology on Pain and Anxiety in Pediatric Burn Patients: A Systematic Review and Meta-Analysis |
Smith |
2022 |
Procedure |
|
Improving vaccine-related pain, distress or fear in healthy children and adolescents–a systematic search of patient-focused interventions |
Lee |
2018 |
Procedure? Results are reported separately so can be extracted per included study |
|
Infants born preterm, stress, and neurodevelopment in the neonatal intensive care unit: might music have an impact? |
Anderson |
2018 |
Study selected the 10 most methodologically stringent and relevant articles |
|
Interventions for postoperative pain in children: An overview of systematic reviews |
Boric |
2017 |
Twee studies kunnen wel geschikt zijn, zie A80 en A81 |
|
Management of routine postoperative pain for children undergoing cardiac surgery: A Paediatric Acute Care Cardiology Collaborative Clinical Practice Guideline |
Gal |
2022 |
Gebruiken voor overwegingen |
|
Maternal voice reduces procedural pain in neonates: A meta-Analysis of randomized controlled trials |
Jin |
2023 |
Procedure |
|
Medical clowning in hospitalized children: a meta-analysis |
Kasem Ali Sliman |
2023 |
Procedure |
|
MUSIC INTERVENTIONS IN PEDIATRIC ONCOLOGY: Systematic review and meta-analysis |
da Silva Santa |
2021 |
Some interventions are combined music interventions and some controls were active controls |
|
Music-based interventions in paediatric and adolescents oncology patients: A systematic review |
González-Martín-Moreno |
2021 |
One study could be suitable, see A83 |
|
Nonpharmacologic Intervention on the Prevention of Pain and Anxiety During Pediatric Dental Care: A Systematic Review |
Goettems |
2017 |
Dental treatment no hospital care |
|
Non-pharmacological and non-surgical treatment of pain in children and adolescents with cerebral palsy: A scoping review |
Flyckt |
2022 |
Included studies with interventions within PICO are not suitable (interviews and surveys) |
|
Nonpharmacological Applications during the Retinopathy of Prematurity Examination and Their Effects on Pain Control: A Systematic Review and Meta-analysis |
Erçelik |
2022 |
Wrong I: sucrose, glucose, and breast milk |
|
Non-Pharmacological Management for Vaccine-Related Pain in Children in the Healthcare Setting: A Scoping Review |
Wu |
2022 |
Not all included studies use standard care as control. Results are reported per type of intervention. + scoping review |
|
Non-pharmacological options for managing chronic musculoskeletal pain in children with pediatric rheumatic disease: a systematic review |
Nijhof |
2018 |
Wrong C: active comparator. However, some studies could be suitable, see A85, A86, A87 |
|
Non-pharmacological pain interventions for sickle cell crisis in pediatrics: A scoping review |
Ibitoye |
2023 |
Wrong aim: overview of which interventions are used in patients with sickle cell crisis |
|
Non-pharmacological pain relief during orthodontic treatment |
Aljubaibi |
2018 |
Wrong design: summary review. Original article does not perform a subgroup analysis in children and adults, and is therefore not suitable. |
|
Pediatric Complex Regional Pain Syndrome and Occupational Therapy Intervention: A Scoping Review |
Tay |
2023 |
Wrong studies included: qualitative studies and reviews that are not suitable |
|
Pediatric Distraction Tools for Prehospital Care of Pain and Distress: A Systematic Review |
Robinson |
2023 |
Procedures |
|
The Effect of Music Therapy Applied to Neonatales on their Pain: Systematic Review |
Terzi |
2023 |
No control group reported and procedures included |
|
The impact of music, play, and pet therapies in managing pain and anxiety in paediatric patients in hospital: a rapid systematic review |
Goren |
2023 |
4 of 24 studies had no standard care as comparator. |
|
Virtual reality distraction for acute pain in children |
Lambert |
2020 |
Procedure |
|
Virtual Reality Technology for Pain and Anxiety Management among Patients with Cancer: A Systematic Review |
Ahmad |
2020 |
No included studies in children without procedures performed |
|
Virtual Reality Therapy to Control Burn Pain: Systematic Review of Randomized Controlled Trials |
De Jesus Catalã |
2022 |
Results not described properly |
|
The effectiveness of web-based mobile health interventions in paediatric outpatient surgery: A systematic review and meta-analysis of randomized controlled trials. |
Rantala |
2020 |
Procedure |
Verantwoording
Beoordelingsdatum en geldigheid
Publicatiedatum : 17-03-2025
Beoordeeld op geldigheid : 21-02-2025
Algemene gegevens
De ontwikkeling/herziening van deze richtlijnmodule werd ondersteund door het Kennisinstituut van de Federatie Medisch Specialisten (www.demedischspecialist.nl/kennisinstituut) en werd gefinancierd uit de Kwaliteitsgelden Medisch Specialisten (SKMS).
De financier heeft geen enkele invloed gehad op de inhoud van de richtlijnmodule.
Samenstelling werkgroep
Voor het ontwikkelen van de richtlijnmodule is in 2021 een multidisciplinaire werkgroep ingesteld, bestaande uit vertegenwoordigers van alle relevante specialismen (zie hiervoor de Samenstelling van de werkgroep) die betrokken zijn bij de zorg voor kinderen met pijn.
Kernwerkgroep
- Drs. M.A. (Maarten) Mensink, kinderanesthesioloog en pijnarts, werkzaam in het Prinses Máxima Centrum voor Kinderoncologie te Utrecht, NVA, voorzitter
- Drs. J.F. (Joanne) Goorhuis, algemeen kinderarts, werkzaam in het Medisch Spectrum Twente, NVK
- Dr. T. (Tessa) Sieswerda, kinderarts, werkzaam in het Amsterdam UMC te Amsterdam, NVK
- Drs. M.S. (Sukru) Genco, algemeen kinderarts, werkzaam in het OLVG te Amsterdam, NVK
- Dr. S.H. (Steven) Renes, anesthesioloog-pijnspecialist, werkzaam in het Radboud UMC te Nijmegen, NVA
- Dr. P. (Petra) Honig-Mazer, psychotherapeut, werkzaam in het Erasmus MC Sophia te Rotterdam, PAZ/LVMP
- Drs. M. (Marjorie) de Neef, kinder-IC verpleegkundige, werkzaam in het Amsterdam UMC, V&VN
- E.C. (Esen) Doganer, junior projectmanager en beleidsmedewerker, Stichting Kind en Ziekenhuis
Werkgroep
- Drs. L.A.M. (Lonneke) Aarts, algemeen kinderarts, werkzaam in het RadboudUMC Amalia kinderziekenhuis te Nijmegen, NVK
- Prof. dr. W.J.E. (Wim) Tissing, kinderoncoloog, werkzaam in het UMCG te Groningen en Prinses Máxima Centrum te Utrecht, NVK
- Drs. P. (Petra) Hissink-Muller, kinderreumatoloog, werkzaam in het Erasmus MC Sophia te Rotterdam
- Dr. A.M. (Arine) Vlieger, algemeen kinderarts, werkzaam in het St. Antonius Ziekenhuis te Utrecht, NVK
- Dr. G.E. (Gerbrich) van den Bosch, kinderarts-neonatoloog, werkzaam in het Erasmus MC Sophia te Rotterdam, NVK
- Drs. K. (Karina) Elangovan, kinderanesthesioloog, werkzaam in het Erasmus MC Sophia te Rotterdam, NVA
- Dr. C.M.G. (Claudia) Keyzer – Dekker, kinderchirurg, werkzaam in het Erasmus MC Sophia te Rotterdam, NVvH
- A.P. (Annette) van der Kaa, kinderfysiotherapeut, werkzaam in het Erasumc MC Sophia te Rotterdam, NVFK en KNGF
- Drs. A. H. (Agnes) Dommerholt, klinisch psycholoog, werkzaam in het OLVG te Amsterdam, NIP/LVMP (vanaf 1-1-2023)
Klankbordgroep
- Drs. J. (Judig) Blaauw, kinderrevalidatiearts, VRA
- Dr. H. (Hanneke) Bruijnzeel, AIOS, werkzaam in het UMC Utrecht te Utrecht, NVKNO
- Dr. A.M.J.W. (Anne-Marie) Scheepers, ziekenhuisapotheker, werkzaam in het MUMC te Maastricht, NVZA
- Dr. S.A. (Sylvia) Obermann-Borst, ervaringsdeskundige ouder & huisarts-epidemioloog, Care4Neo (voorheen Vereniging van Ouders van Couveusekinderen - VOC)
- Dr. I.H. (Ilse) Zaal-Schuller, arts voor verstandelijk gehandicapten/kaderarts palliatieve zorg i.o., werkzaam bij Prinsenstichting Purmerend/ AmsterdamUMC locatie AMC, NVAVG
- Dr. E. (Eva) Schaffrath, anesthesioloog, werkzaam in het Maastricht UMC te Maastricht, PROSA Kenniscentrum
- M. (Mirjam) Jansen op de Haar, HME-MO Vereniging Nederland
Met ondersteuning van
- Dr. J. (Janneke) Hoogervorst – Schilp, senior adviseur, Kennisinstituut van de Federatie Medisch Specialisten
- Dr. C. (Cécile) Overman, adviseur, Kennisinstituut van de Federatie Medisch Specialisten
- Dr. T. (Tim) Christen, adviseur, Kennisinstituut van de Federatie Medisch Specialisten
- Drs. D.A.M. (Danique) Middelhuis, junior adviseur, Kennisinstituut van de Federatie Medisch Specialisten
- Drs. M. (Mark) van Eck, junior adviseur, Kennisinstituut van de Federatie Medisch Specialisten
- Drs. L. (Liza) van Mun, junior adviseur, Kennisinstituut van de Federatie Medisch Specialisten
Belangenverklaringen
De Code ter voorkoming van oneigenlijke beïnvloeding door belangenverstrengeling is gevolgd. Alle werkgroepleden hebben schriftelijk verklaard of zij in de laatste drie jaar directe financiële belangen (betrekking bij een commercieel bedrijf, persoonlijke financiële belangen, onderzoeksfinanciering) of indirecte belangen (persoonlijke relaties, reputatiemanagement) hebben gehad. Gedurende de ontwikkeling of herziening van een module worden wijzigingen in belangen aan de voorzitter doorgegeven. De belangenverklaring wordt opnieuw bevestigd tijdens de commentaarfase.
Een overzicht van de belangen van (kern)werkgroepleden en klankbordgroepleden en het oordeel over het omgaan met eventuele belangen vindt u in onderstaande tabel. De ondertekende belangenverklaringen zijn op te vragen bij het secretariaat van het Kennisinstituut van de Federatie Medisch Specialisten.
|
Betrokkenen |
Functie |
Nevenfuncties |
Gemelde belangen |
Ondernomen actie |
|
Werkgroep |
||||
|
Aarts |
Algemeen kinderarts in het Amalia kinderziekenhuissinds november 2017 |
Interne functies onbetaald: 1. voorzitter Pijn werkgroep Amalia kinderziekenhuis. 2. Verbonden aan werkgroep procedurele sedatie bij kinderen. 3. Implementatie VR in Amalia. |
Onderzoek naar effect comfort talk technieken; maar eenmalige subsidie gekregen voor uitvoer. Geen extern belang qua uitkomst. |
Geen actie |
|
Bosch van den |
Kinderarts-neonatoloog |
NALS (Newborn Advanced Life Support) instructeur SSHK te Riel (reanimatietraining, alleen onkostenvergoeding). Wetenschapelijk onderzoeker Erasmus MC-Sophia (onbetaald, betaald voor klinische werkzaamheden als neonatoloog). O.a. begeleider 3 PhD studenten m.b.t. neonatale pin en stress |
Vriendenloterij en De Stichting Vrienden van het Sophia - HIPPO studie - Landelijke studie naar de hoeveelheid en impact van neonatale en ouderlijke stress op de NICU, projectleider. De Stichting Vrienden van het Sophia – Pijn bij NEC project (lange termijn follow up van patienten met NEC o.a. testen van pijngevoeligheid, projectleider. Horizon 2020 - ALBINO study (RCT naar Allopurinol bij neonaten met asfyxie, Europese multicenter studie waarbij Rotterdam meedoet als 1 van deze centra) – Nee geen projectleider, alleen lokale pi van Rotterdam (maar niet inhoudelijk betrokken bij de opzet en dergelijke van de studie) |
Geen actie
|
|
Dommerholt |
Klinisch psycholoog KJ, vakgroep kindergeneeskunde OLVG |
Praktijk Dommerholt, praktijk voor kinder- en jeugdpsychotherapie, supervisie en doceren. (momenteel inactief). Lid vakgroep en medische staf, uitvoeren van psychologische diagnostiek en behandeling van kinderen en hun systeem, opleider. |
Geen |
Geen actie |
|
Elangovan |
Universitair medisch specialist Anesthesioloog-pijnspecialist; ErasmusMC |
Geen |
Geen |
Geen actie |
|
Genco |
Kinderarts, OLVG, Amsterdam |
- Eigenaar Genco Med. Beheer B.V. |
Directe belangen bij eigen B.V. maar geen relatie met de bezigheden van de werkgroep. Bijvangst van het project kan zijn: nieuwe kennis en ervaring om binnen onze organisatie te delen. |
Geen actie |
|
Goorhuis |
Algemeen kinderarts - acute kindergeneeskunde |
Geen |
Geen |
Geen actie |
|
Hissink-Muller |
Kinderreumatoloog, Erasmus MC Sophia |
Geen |
Ja, CHAMP studie en UCAN CAN DU. 1. NWO, Goed Geneesmiddelen Gebruik. UCAN CAN DU, CIHR, ZONMW, ReumaNederland. 2. CHAMP studie, mono of polytherapie bij non systemic JIA patienten. Personalised medicine bij JIA. |
Geen actie |
|
Kaa, van der |
Kinderfysiotherapeut |
-Docent Master Kinderfysiotherapie bij Breederode Hogeschool - Universitair docent -Kinderfysiotherapeut 1e lijn (Fysio van der Linden) |
Geen |
Geen actie |
|
Keyzer-Dekker |
Kinderchirurg Sophia Kinderziekenhuis ErasmusMC te Rotterdam |
APLS instructeur SSHK Riel, dagvergoeding |
Geen |
Geen actie |
|
Mensink* |
kinderanesthesioloog - pijnarts - Prinses Máxima Centrum voor kinderoncologie |
Bestuurslid sectie Pijn&palliatieve geneeskunde NVA - onbetaalde functie |
Geen |
Geen actie |
|
Neef, de |
Verpleegkundig onderzoeker, Kinder IC, Amsterdam UMC |
Geen |
Geen |
Geen actie |
|
Honig-Mazer |
Erasmus MC - Sophia Kinderziekenhuis Afdeling Kinder- en Jeugdpsychiatrie/psychologie Unit Psychosociale Zorg Psychotherapeut BIG |
Kleine eigen praktijk: Praktijk voor Psychotherapie Honig-Mazer, betaald |
Geen |
Geen actie |
|
Renes |
Anesthesioloog-pijnspecialist Radboudumc |
Kwaliteitsvisitaties Nederlandse Vereniging Anesthesiologie, vacatiegeld |
Geen |
Geen actie |
|
Sieswerda |
Kinderarts sociale pediatrie Amsterdam UMC locatie AMC |
Werkgroeplid NVK commissie richtlijnen |
2023 - heden. Initiatiefnemeer gekoelde vs niet-gekoelde sondes, met funding vanuit Janivo stichting. 2021 – heden. Initiatiefnemer studie ‘Focus on comfort - the effect of language on pain perception in pediatric patients.’ met funding vanuit ESPR. Publicatie verwacht Q4 2023 2021 – heden. Initiatiefnemer studie ‘The ARCADE study, Anxiety Reduction in Children Analyzing Data from EEG.’ met funding vanuit WAR. Publicatie verwacht Q2 2024 |
Geen actie |
|
Simons |
Kinderarts - neonatoloog - klinisch farmacoloog (Universitair Medische Specialist) |
Lid geneesmiddelencommissie Erasmus MC (onbetaald) |
Geen |
Geen actie |
|
Tissing |
Kinderoncoloog, Hoogleraar supportive care in de kinderoncologie. 0.6 fte Prinses Maxima Centrum, 0,4 fte UMCG |
PI van onderzoek naar app over invloed van laagdrempelig contact op pijn bij patiënten met kanker. |
Geen |
Geen actie |
|
Uitzinger |
Junior Project manager en beleidsmedewerker Stichting kind en ziekenhuis |
Geen |
Geen |
Geen actie |
|
Vlieger |
Kinderarts St Antonius ziekenhuis Nieuwegein |
1. Betaald les geven via Cure en Care op het gebied van hypnose bij kinderen. 2. Mede-eigenaar van Skills4Comfort, een onderwijsbedrijf, dat tegen betaling trainingen verzorgt in ziekenhuizen op het gebied van non-farmacologische technieken om het comfort van patienten te verbeteren, mn tijdens pijnlijke procedures. 3. Voorzitter Stichting Hypnose bij Kinderen. Onbetaald. 4. Tot 2021: bestuurslid en mede-oprichter van de stichting Procedureel comfort bij Kinderen (Prosa). |
Enig financieel belang door mede-eigenaarschap van Skills4comfort dat onderwijs verzorgt op hte gebied van non-farmacologische pijn bestrijding. |
Uitsluiten van besluitvorming voor modules over non-farmacologische pijnbestrijding. Mag wel meelezen. |
*Voorzitter
|
Klankbordgroep |
||||
|
Blaauw |
Kinderrevalidatiearts |
Geen |
Geen |
Geen actie |
|
Bruijnzeel |
Arts-assistent Keel-, Neus- en Oorheelkunde, UMC Utrecht |
Kerngroep Pediatrie (KNO vereniging) - onbetaald |
Geen |
Geen actie |
|
Haar, van der |
Freelance consultant Moonshot |
Bestuurslid HME-MO Vereniging Nederland |
Geen |
Geen actie |
|
Scheepers |
ziekenhuisapotheker, Maastricht UMC+ |
Geen |
Geen |
Geen actie |
|
Obermann-Borst |
Coördinator Wetenschap bij Care4Neo 10 u per week |
Coördinator Wetenschap bij Care4Neo 75% betaald/25% vrijwillig verzorgen van bijdrage vanuit patientenperspectief aan wetenschap, richtlijnen en kwaliteit van zorg namens de patientenvereniging voor ouders van en voor kinderen die te vroeg, te klein en/of ziek geboren zijn. |
Geen |
Geen actie |
|
Zaal Schuller |
Arts voor verstandelijk gehandicapten |
Arts voor verstandelijk gehandicapten, betaald. |
Geen |
Geen actie |
|
Schaffrath |
Kinderanesthesioloog MUMC |
Faculty member PROSA (tegen dagvergoeding) |
Geen |
Geen actie |
Inbreng patiëntenperspectief
Er werd aandacht besteed aan het patiëntenperspectief door afvaardiging van Stichting Kind en Ziekenhuis in de kernwerkgroep en Care4Neo en HME-MO Vereniging Nederland in de klankbordgroep. Op verschillende momenten is input gevraagd tijdens een invitational conference en bij het opstellen van het raamwerk. Het verslag van de invitational conference [zie aanverwante producten] is besproken in de werkgroep. De verkregen input is meegenomen bij het opstellen van de uitgangsvragen, de keuze voor de uitkomstmaten en bij het opstellen van de overwegingen. De conceptrichtlijn is tevens voor commentaar voorgelegd aan de patiëntenorganisaties en de eventueel aangeleverde commentaren zijn bekeken en verwerkt.
Kwalitatieve raming van mogelijke financiële gevolgen in het kader van de Wkkgz
Bij de richtlijnmodule is conform de Wet kwaliteit, klachten en geschillen zorg (Wkkgz) een kwalitatieve raming uitgevoerd om te beoordelen of de aanbevelingen mogelijk leiden tot substantiële financiële gevolgen. Bij het uitvoeren van deze beoordeling is de richtlijnmodule op verschillende domeinen getoetst (zie het stroomschema op de Richtlijnendatabase).
Module |
Uitkomst raming |
Toelichting |
|
Module Non-farmacologische pijnbestrijding
|
Geen financiële gevolgen |
Hoewel uit de toetsing volgt dat de aanbeveling(en) breed toepasbaar zijn (>40.000 patiënten), volgt uit de toetsing dat het geen nieuwe manier van zorgverlening of andere organisatie van zorgverlening betreft, het geen toename in het aantal in te zetten voltijdsequivalenten aan zorgverleners betreft en het geen wijziging in het opleidingsniveau van zorgpersoneel betreft. Er worden daarom geen financiële gevolgen verwacht. |
Werkwijze
AGREE
Deze richtlijnmodule is opgesteld conform de eisen vermeld in het rapport Medisch Specialistische Richtlijnen 2.0 van de adviescommissie Richtlijnen van de Raad Kwaliteit. Dit rapport is gebaseerd op het AGREE II instrument (Appraisal of Guidelines for Research & Evaluation II; Brouwers, 2010).
Knelpuntenanalyse en uitgangsvragen
Voorafgaand aan de voorbereidende fase is een invitational conference georganiseerd over herkenning en behandeling van pijn binnen de kindzorg. Een verslag hiervan is opgenomen onder aanverwante producten. Daarnaast werd tijdens de voorbereidende fase van de richtlijn een schriftelijke knelpunteninventarisatie gehouden. Op basis van de uitkomsten van de knelpuntenanalyse zijn door de werkgroep concept-uitgangsvragen opgesteld en definitief vastgesteld.
Uitkomstmaten
Na het opstellen van de zoekvraag behorende bij de uitgangsvraag inventariseerde de werkgroep welke uitkomstmaten voor de patiënt relevant zijn, waarbij zowel naar gewenste als ongewenste effecten werd gekeken. Hierbij werd een maximum van acht uitkomstmaten gehanteerd. De werkgroep waardeerde deze uitkomstmaten volgens hun relatieve belang bij de besluitvorming rondom aanbevelingen, als cruciaal (kritiek voor de besluitvorming), belangrijk (maar niet cruciaal) en onbelangrijk. Tevens definieerde de werkgroep tenminste voor de cruciale uitkomstmaten welke verschillen zij klinisch (patiënt) relevant vonden.
Methode literatuursamenvatting
Een uitgebreide beschrijving van de strategie voor zoeken en selecteren van literatuur en de beoordeling van de risk-of-bias van de individuele studies is te vinden onder ‘Zoeken en selecteren’ onder Onderbouwing. De beoordeling van de kracht van het wetenschappelijke bewijs wordt hieronder toegelicht.
Beoordelen van de kracht van het wetenschappelijke bewijs
De kracht van het wetenschappelijke bewijs werd bepaald volgens de GRADE-methode. GRADE staat voor ‘Grading Recommendations Assessment, Development and Evaluation’ (zie https://www.gradeworkinggroup.org/). De basisprincipes van de GRADE-methodiek zijn: het benoemen en prioriteren van de klinisch (patiënt) relevante uitkomstmaten, een systematische review per uitkomstmaat, en een beoordeling van de bewijskracht per uitkomstmaat op basis van de acht GRADE-domeinen (domeinen voor downgraden: risk of bias, inconsistentie, indirectheid, imprecisie, en publicatiebias; domeinen voor upgraden: dosis-effect relatie, groot effect, en residuele plausibele confounding).
GRADE onderscheidt vier gradaties voor de kwaliteit van het wetenschappelijk bewijs: hoog, redelijk, laag en zeer laag. Deze gradaties verwijzen naar de mate van zekerheid die er bestaat over de literatuurconclusie, in het bijzonder de mate van zekerheid dat de literatuurconclusie de aanbeveling adequaat ondersteunt (Schünemann, 2013; Hultcrantz, 2017).
|
GRADE |
Definitie |
|
Hoog |
|
|
Redelijk |
|
|
Laag |
|
|
Zeer laag |
|
Bij het beoordelen (graderen) van de kracht van het wetenschappelijk bewijs in richtlijnen volgens de GRADE-methodiek spelen grenzen voor klinische besluitvorming een belangrijke rol (Hultcrantz, 2017). Dit zijn de grenzen die bij overschrijding aanleiding zouden geven tot een aanpassing van de aanbeveling. Om de grenzen voor klinische besluitvorming te bepalen moeten alle relevante uitkomstmaten en overwegingen worden meegewogen. De grenzen voor klinische besluitvorming zijn daarmee niet één op één vergelijkbaar met het minimaal klinisch relevant verschil (Minimal Clinically Important Difference, MCID). Met name in situaties waarin een interventie geen belangrijke nadelen heeft en de kosten relatief laag zijn, kan de grens voor klinische besluitvorming met betrekking tot de effectiviteit van de interventie bij een lagere waarde (dichter bij het nuleffect) liggen dan de MCID (Hultcrantz, 2017).
Overwegingen (van bewijs naar aanbeveling)
Om te komen tot een aanbeveling zijn naast (de kwaliteit van) het wetenschappelijke bewijs ook andere aspecten belangrijk en worden meegewogen, zoals aanvullende argumenten uit bijvoorbeeld de biomechanica of fysiologie, waarden en voorkeuren van patiënten, kosten (middelenbeslag), aanvaardbaarheid, haalbaarheid en implementatie. Deze aspecten zijn systematisch vermeld en beoordeeld (gewogen) onder het kopje ‘Overwegingen’ en kunnen (mede) gebaseerd zijn op expert opinion. Hierbij is gebruik gemaakt van een gestructureerd format gebaseerd op het evidence-to-decision framework van de internationale GRADE Working Group (Alonso-Coello, 2016a; Alonso-Coello 2016b). Dit evidence-to-decision framework is een integraal onderdeel van de GRADE-methodiek.
Formuleren van aanbevelingen
De aanbevelingen geven antwoord op de uitgangsvraag en zijn gebaseerd op het beschikbare wetenschappelijke bewijs en de belangrijkste overwegingen, en een weging van de gunstige en ongunstige effecten van de relevante interventies. De kracht van het wetenschappelijk bewijs en het gewicht dat door de werkgroep wordt toegekend aan de overwegingen, bepalen samen de sterkte van de aanbeveling. Conform de GRADE-methodiek sluit een lage bewijskracht van conclusies in de systematische literatuuranalyse een sterke aanbeveling niet a priori uit, en zijn bij een hoge bewijskracht ook zwakke aanbevelingen mogelijk (Agoritsas, 2017; Neumann, 2016). De sterkte van de aanbeveling wordt altijd bepaald door weging van alle relevante argumenten tezamen. De werkgroep heeft bij elke aanbeveling opgenomen hoe zij tot de richting en sterkte van de aanbeveling zijn gekomen.
In de GRADE-methodiek wordt onderscheid gemaakt tussen sterke en zwakke (of conditionele) aanbevelingen. De sterkte van een aanbeveling verwijst naar de mate van zekerheid dat de voordelen van de interventie opwegen tegen de nadelen (of vice versa), gezien over het hele spectrum van patiënten waarvoor de aanbeveling is bedoeld. De sterkte van een aanbeveling heeft duidelijke implicaties voor patiënten, behandelaars en beleidsmakers (zie onderstaande tabel). Een aanbeveling is geen dictaat, zelfs een sterke aanbeveling gebaseerd op bewijs van hoge kwaliteit (GRADE gradering HOOG) zal niet altijd van toepassing zijn, onder alle mogelijke omstandigheden en voor elke individuele patiënt.
|
Implicaties van sterke en zwakke aanbevelingen voor verschillende richtlijngebruikers |
||
|
|
Sterke aanbeveling |
Zwakke (conditionele) aanbeveling |
|
Voor patiënten |
De meeste patiënten zouden de aanbevolen interventie of aanpak kiezen en slechts een klein aantal niet. |
Een aanzienlijk deel van de patiënten zouden de aanbevolen interventie of aanpak kiezen, maar veel patiënten ook niet. |
|
Voor behandelaars |
De meeste patiënten zouden de aanbevolen interventie of aanpak moeten ontvangen. |
Er zijn meerdere geschikte interventies of aanpakken. De patiënt moet worden ondersteund bij de keuze voor de interventie of aanpak die het beste aansluit bij zijn of haar waarden en voorkeuren. |
|
Voor beleidsmakers |
De aanbevolen interventie of aanpak kan worden gezien als standaardbeleid. |
Beleidsbepaling vereist uitvoerige discussie met betrokkenheid van veel stakeholders. Er is een grotere kans op lokale beleidsverschillen. |
Organisatie van zorg
In de knelpuntenanalyse en bij de ontwikkeling van de richtlijnmodule is expliciet aandacht geweest voor de organisatie van zorg: alle aspecten die randvoorwaardelijk zijn voor het verlenen van zorg (zoals coördinatie, communicatie, (financiële) middelen, mankracht en infrastructuur). Randvoorwaarden die relevant zijn voor het beantwoorden van deze specifieke uitgangsvraag zijn genoemd bij de overwegingen. Meer algemene, overkoepelende, of bijkomende aspecten van de organisatie van zorg worden behandeld in de module Organisatie van zorg.
Commentaar- en autorisatiefase
De conceptrichtlijnmodule werd aan de betrokken (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd ter commentaar. De commentaren werden verzameld en besproken met de werkgroep. Naar aanleiding van de commentaren werd de conceptrichtlijnmodule aangepast en definitief vastgesteld door de werkgroep. De definitieve richtlijnmodule werd aan de deelnemende (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd voor autorisatie en door hen geautoriseerd dan wel geaccordeerd.
Zoekverantwoording
Embase
|
No. |
Query |
Results |
|
#19 |
#15 AND #18 sleutelartikelen gevonden |
2 |
|
#18 |
#16 OR #17 |
2 |
|
#17 |
'efficacy of non-pharmacological methods of pain management in children undergoing venipuncture in a pediatric outpatient clinic' |
1 |
|
#16 |
'non-pharmacological management of infant and young child procedural pain' AND 2023 |
1 |
|
#15 |
#12 OR #13 OR #14 |
2937 |
|
#14 |
#7 AND (#10 OR #11) NOT #12 NOT #13 OBS |
1107 |
|
#13 |
#7 AND #9 NOT #12 Clinical trials |
1178 |
|
#12 |
#7 AND #8 SR |
652 |
|
#11 |
'case control study'/de OR 'comparative study'/exp OR 'control group'/de OR 'controlled study'/de OR 'controlled clinical trial'/de OR 'crossover procedure'/de OR 'double blind procedure'/de OR 'phase 2 clinical trial'/de OR 'phase 3 clinical trial'/de OR 'phase 4 clinical trial'/de OR 'pretest posttest design'/de OR 'pretest posttest control group design'/de OR 'quasi experimental study'/de OR 'single blind procedure'/de OR 'triple blind procedure'/de OR (((control OR controlled) NEAR/6 trial):ti,ab,kw) OR (((control OR controlled) NEAR/6 (study OR studies)):ti,ab,kw) OR (((control OR controlled) NEAR/1 active):ti,ab,kw) OR 'open label*':ti,ab,kw OR (((double OR two OR three OR multi OR trial) NEAR/1 (arm OR arms)):ti,ab,kw) OR ((allocat* NEAR/10 (arm OR arms)):ti,ab,kw) OR placebo*:ti,ab,kw OR 'sham-control*':ti,ab,kw OR (((single OR double OR triple OR assessor) NEAR/1 (blind* OR masked)):ti,ab,kw) OR nonrandom*:ti,ab,kw OR 'non-random*':ti,ab,kw OR 'quasi-experiment*':ti,ab,kw OR crossover:ti,ab,kw OR 'cross over':ti,ab,kw OR 'parallel group*':ti,ab,kw OR 'factorial trial':ti,ab,kw OR ((phase NEAR/5 (study OR trial)):ti,ab,kw) OR ((case* NEAR/6 (matched OR control*)):ti,ab,kw) OR ((match* NEAR/6 (pair OR pairs OR cohort* OR control* OR group* OR healthy OR age OR sex OR gender OR patient* OR subject* OR participant*)):ti,ab,kw) OR ((propensity NEAR/6 (scor* OR match*)):ti,ab,kw) OR versus:ti OR vs:ti OR compar*:ti OR ((compar* NEAR/1 study):ti,ab,kw) OR (('major clinical study'/de OR 'clinical study'/de OR 'cohort analysis'/de OR 'observational study'/de OR 'cross-sectional study'/de OR 'multicenter study'/de OR 'correlational study'/de OR 'follow up'/de OR cohort*:ti,ab,kw OR 'follow up':ti,ab,kw OR followup:ti,ab,kw OR longitudinal*:ti,ab,kw OR prospective*:ti,ab,kw OR retrospective*:ti,ab,kw OR observational*:ti,ab,kw OR 'cross sectional*':ti,ab,kw OR cross?ectional*:ti,ab,kw OR multicent*:ti,ab,kw OR 'multi-cent*':ti,ab,kw OR consecutive*:ti,ab,kw) AND (group:ti,ab,kw OR groups:ti,ab,kw OR subgroup*:ti,ab,kw OR versus:ti,ab,kw OR vs:ti,ab,kw OR compar*:ti,ab,kw OR 'odds ratio*':ab OR 'relative odds':ab OR 'risk ratio*':ab OR 'relative risk*':ab OR 'rate ratio':ab OR aor:ab OR arr:ab OR rrr:ab OR ((('or' OR 'rr') NEAR/6 ci):ab))) |
14454665 |
|
#10 |
'major clinical study'/de OR 'clinical study'/de OR 'case control study'/de OR 'family study'/de OR 'longitudinal study'/de OR 'retrospective study'/de OR 'prospective study'/de OR 'comparative study'/de OR 'cohort analysis'/de OR ((cohort NEAR/1 (study OR studies)):ab,ti) OR (('case control' NEAR/1 (study OR studies)):ab,ti) OR (('follow up' NEAR/1 (study OR studies)):ab,ti) OR (observational NEAR/1 (study OR studies)) OR ((epidemiologic NEAR/1 (study OR studies)):ab,ti) OR (('cross sectional' NEAR/1 (study OR studies)):ab,ti) |
6767914 |
|
#9 |
'clinical trial'/exp OR 'randomization'/exp OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp OR 'placebo'/exp OR 'prospective study'/exp OR rct:ab,ti OR random*:ab,ti OR 'single blind':ab,ti OR 'randomised controlled trial':ab,ti OR 'randomized controlled trial'/exp OR placebo*:ab,ti |
3882790 |
|
#8 |
'meta analysis'/exp OR 'meta analysis (topic)'/exp OR metaanaly*:ti,ab OR 'meta analy*':ti,ab OR metanaly*:ti,ab OR 'systematic review'/de OR 'cochrane database of systematic reviews'/jt OR prisma:ti,ab OR prospero:ti,ab OR (((systemati* OR scoping OR umbrella OR 'structured literature') NEAR/3 (review* OR overview*)):ti,ab) OR ((systemic* NEAR/1 review*):ti,ab) OR (((systemati* OR literature OR database* OR 'data base*') NEAR/10 search*):ti,ab) OR (((structured OR comprehensive* OR systemic*) NEAR/3 search*):ti,ab) OR (((literature NEAR/3 review*):ti,ab) AND (search*:ti,ab OR database*:ti,ab OR 'data base*':ti,ab)) OR (('data extraction':ti,ab OR 'data source*':ti,ab) AND 'study selection':ti,ab) OR ('search strategy':ti,ab AND 'selection criteria':ti,ab) OR ('data source*':ti,ab AND 'data synthesis':ti,ab) OR medline:ab OR pubmed:ab OR embase:ab OR cochrane:ab OR (((critical OR rapid) NEAR/2 (review* OR overview* OR synthes*)):ti) OR ((((critical* OR rapid*) NEAR/3 (review* OR overview* OR synthes*)):ab) AND (search*:ab OR database*:ab OR 'data base*':ab)) OR metasynthes*:ti,ab OR 'meta synthes*':ti,ab |
965194 |
|
#7 |
#5 NOT #6 |
4228 |
|
#6 |
'labor pain'/exp OR 'childbirth'/exp |
79676 |
|
#5 |
#4 AND [2017-2023]/py NOT ('conference abstract'/it OR 'editorial'/it OR 'letter'/it OR 'note'/it) NOT (('animal'/exp OR 'animal experiment'/exp OR 'animal model'/exp OR 'nonhuman'/exp) NOT 'human'/exp) |
4399 |
|
#4 |
#3 AND ('adolescent'/exp OR 'baby'/exp OR 'boy'/exp OR 'child'/exp OR 'minors'/exp/mj OR 'pediatric patient'/exp OR 'pediatrics'/exp OR 'schoolchild'/exp OR 'childhood disease'/exp OR infan*:ti,ab OR newborn*:ti,ab OR 'new born*':ti,ab OR perinat*:ti,ab OR neonat*:ti,ab OR baby*:ti,ab OR babies:ti,ab OR toddler*:ti,ab OR minors*:ti,ab OR boy:ti,ab OR boys:ti,ab OR boyfriend:ti,ab OR boyhood:ti,ab OR girl*:ti,ab OR kid:ti,ab OR kids:ti,ab OR child*:ti,ab OR children*:ti,ab OR schoolchild*:ti,ab OR adolescen*:ti,ab OR juvenil*:ti,ab OR youth*:ti,ab OR teen*:ti,ab OR pubescen*:ti,ab OR pediatric*:ti,ab OR paediatric*:ti,ab OR peadiatric*:ti,ab OR school:ti,ab OR school*:ti,ab OR prematur*:ti,ab OR preterm*:ti,ab) |
12699 |
|
#3 |
#1 AND #2 |
46872 |
|
#2 |
'neurolinguistic programming'/exp OR 'comfort'/exp OR 'hypnosis'/exp OR 'virtual reality'/exp OR 'psychoeducation'/exp OR 'medical clowning'/exp OR 'massage'/exp OR 'music therapy'/exp OR 'music'/exp OR 'music':ti,ab,kw OR massage*:ti,ab,kw OR rock*:ti,ab,kw OR sway*:ti,ab,kw OR lull:ti,ab,kw OR (((medical OR hospital OR clinic OR therap*) NEAR/2 clown*):ti,ab,kw) OR 'childcare worker*':ti,ab,kw OR 'child care worker*':ti,ab,kw OR 'psycho education':ti,ab,kw OR psychoeducation:ti,ab,kw OR distraction*:ti,kw OR nonpharmacolog*:ti,kw OR 'non pharmacolog*':ti,kw OR (('age appropriate' NEAR/4 (communication OR approach* OR method* OR language OR strateg* OR information OR way)):ti,ab,kw) OR nlp:ti,kw OR 'neurolinguistic program*':ti,ab,kw OR comfort:ti,kw OR hypnosis:ti,kw OR 'virtual reality':ti,kw OR 'pedagogical employee*':ti,ab,kw OR 'guided attention':ti,ab,kw OR 'comfort talk*':ti,ab,kw |
242203 |
|
#1 |
'pain'/exp/mj OR 'fear'/exp OR 'pain assessment'/exp/mj OR 'anxiety assessment'/exp OR anxious*:ti,ab,kw OR anxiet*:ti,ab,kw OR fear*:ti,ab,kw OR panic*:ti,ab,kw OR dread*:ti,ab,kw OR worry*:ti,ab,kw OR agitation:ti,ab,kw OR agitated:ti,ab,kw OR apprehensi*:ti,ab,kw OR nervous*:ti,ab,kw OR distress*:ti,ab,kw OR catastrophi*:ti,ab,kw OR discomfort*:ti,ab,kw OR stress*:ti,ab,kw OR pain*:ti,ab,kw OR ache*:ti,ab,kw OR suffering:ti,ab,kw OR anguish*:ti,ab,kw OR cry*:ti,ab,kw |
4792986 |
Ovid/Medline
|
# |
Searches |
Results |
|
14 |
11 or 12 or 13 |
1028 |
|
13 |
(6 and (9 or 10)) not 11 not 12 OBS |
181 |
|
12 |
(6 and 8) not 11 Clinical trials |
565 |
|
11 |
6 and 7 SR |
282 |
|
10 |
Case-control Studies/ or clinical trial, phase ii/ or clinical trial, phase iii/ or clinical trial, phase iv/ or comparative study/ or control groups/ or controlled before-after studies/ or controlled clinical trial/ or double-blind method/ or historically controlled study/ or matched-pair analysis/ or single-blind method/ or (((control or controlled) adj6 (study or studies or trial)) or (compar* adj (study or studies)) or ((control or controlled) adj1 active) or "open label*" or ((double or two or three or multi or trial) adj (arm or arms)) or (allocat* adj10 (arm or arms)) or placebo* or "sham-control*" or ((single or double or triple or assessor) adj1 (blind* or masked)) or nonrandom* or "non-random*" or "quasi-experiment*" or "parallel group*" or "factorial trial" or "pretest posttest" or (phase adj5 (study or trial)) or (case* adj6 (matched or control*)) or (match* adj6 (pair or pairs or cohort* or control* or group* or healthy or age or sex or gender or patient* or subject* or participant*)) or (propensity adj6 (scor* or match*))).ti,ab,kf. or (confounding adj6 adjust*).ti,ab. or (versus or vs or compar*).ti. or ((exp cohort studies/ or epidemiologic studies/ or multicenter study/ or observational study/ or seroepidemiologic studies/ or (cohort* or 'follow up' or followup or longitudinal* or prospective* or retrospective* or observational* or multicent* or 'multi-cent*' or consecutive*).ti,ab,kf.) and ((group or groups or subgroup* or versus or vs or compar*).ti,ab,kf. or ('odds ratio*' or 'relative odds' or 'risk ratio*' or 'relative risk*' or aor or arr or rrr).ab. or (("OR" or "RR") adj6 CI).ab.)) |
5521050 |
|
9 |
Epidemiologic studies/ or case control studies/ or exp cohort studies/ or Controlled Before-After Studies/ or Case control.tw. or cohort.tw. or Cohort analy$.tw. or (Follow up adj (study or studies)).tw. or (observational adj (study or studies)).tw. or Longitudinal.tw. or Retrospective*.tw. or prospective*.tw. or consecutive*.tw. or Cross sectional.tw. or Cross-sectional studies/ or historically controlled study/ or interrupted time series analysis/ [Onder exp cohort studies vallen ook longitudinale, prospectieve en retrospectieve studies] |
4542669 |
|
8 |
exp clinical trial/ or randomized controlled trial/ or exp clinical trials as topic/ or randomized controlled trials as topic/ or Random Allocation/ or Double-Blind Method/ or Single-Blind Method/ or (clinical trial, phase i or clinical trial, phase ii or clinical trial, phase iii or clinical trial, phase iv or controlled clinical trial or randomized controlled trial or multicenter study or clinical trial).pt. or random*.ti,ab. or (clinic* adj trial*).tw. or ((singl* or doubl* or treb* or tripl*) adj (blind$3 or mask$3)).tw. or Placebos/ or placebo*.tw. |
2638198 |
|
7 |
meta-analysis/ or meta-analysis as topic/ or (metaanaly* or meta-analy* or metanaly*).ti,ab,kf. or systematic review/ or cochrane.jw. or (prisma or prospero).ti,ab,kf. or ((systemati* or scoping or umbrella or "structured literature") adj3 (review* or overview*)).ti,ab,kf. or (systemic* adj1 review*).ti,ab,kf. or ((systemati* or literature or database* or data-base*) adj10 search*).ti,ab,kf. or ((structured or comprehensive* or systemic*) adj3 search*).ti,ab,kf. or ((literature adj3 review*) and (search* or database* or data-base*)).ti,ab,kf. or (("data extraction" or "data source*") and "study selection").ti,ab,kf. or ("search strategy" and "selection criteria").ti,ab,kf. or ("data source*" and "data synthesis").ti,ab,kf. or (medline or pubmed or embase or cochrane).ab. or ((critical or rapid) adj2 (review* or overview* or synthes*)).ti. or (((critical* or rapid*) adj3 (review* or overview* or synthes*)) and (search* or database* or data-base*)).ab. or (metasynthes* or meta-synthes*).ti,ab,kf. |
696439 |
|
6 |
5 not ((exp animals/ or exp models, animal/) not humans/) not (letter/ or comment/ or editorial/) |
1439 |
|
5 |
limit 4 to yr="2017 -Current" |
1484 |
|
4 |
1 and 2 and 3 |
2637 |
|
3 |
(child* or schoolchild* or infan* or adolescen* or pediatri* or paediatr* or neonat* or boy or boys or boyhood or girl or girls or girlhood or youth or youths or baby or babies or toddler* or childhood or teen or teens or teenager* or newborn* or postneonat* or postnat* or puberty or preschool* or suckling* or picu or nicu or juvenile?).tw. |
2942471 |
|
2 |
exp Neurolinguistic Programming/ or Patient Comfort/ or exp Hypnosis/ or exp Virtual Reality/ or exp Massage/ or exp Music/ or exp Music Therapy/ or music.ti,ab,kf. or massage*.ti,ab,kf. or rock*.ti,ab,kf. or sway*.ti,ab,kf. or lull.ti,ab,kf. or ((medical or hospital or clinic or therap*) adj2 clown*).ti,ab,kf. or childcare worker*.ti,ab,kf. or child care worker*.ti,ab,kf. or psycho education.ti,ab,kf. or psychoeducation.ti,ab,kf. or distraction*.ti,kf. or nonpharmacolog*.ti,kf. or non pharmacolog*.ti,kf. or (age appropriate adj4 (communication or approach* or method* or language or strateg* or information or way)).ti,ab,kf. or nlp.ti,kf. or neurolinguistic program*.ti,ab,kf. or comfort.ti,kf. or hypnosis.ti,kf. or virtual reality.ti,kf. or pedagogical employee*.ti,ab,kf. or guided attention.ti,ab,kf. or comfort talk*.ti,ab,kf. |
156165 |
|
1 |
exp Pain/ or exp Fear/ or Anxiety/ or Pain Measurement/ or anxious*.ti,kf. or anxiet*.ti,kf. or fear*.ti,kf. or panic*.ti,kf. or dread*.ti,kf. or worry*.ti,kf. or agitation.ti,kf. or agitated.ti,kf. or apprehensi*.ti,kf. or nervous*.ti,kf. or distress*.ti,kf. or catastrophiz*.ti,kf. or discomfort*.ti,kf. or stress*.ti,kf. or pain*.ti,kf. or ache*.ti,kf. or suffering.ti,kf. or anguish*.ti,kf. |
1317585 |