Preventie van PICS
Uitgangsvraag
Wat is de plaats van op de intensive care geïnitieerde farmacologische en niet-farmacologische interventies gericht op het voorkomen of reduceren van Post Intensive Care Syndroom (PICS)?
Aanbeveling
Zet geen farmacologische behandeling in specifiek ter voorkoming of reductie van Post Intensive Care Syndroom (PICS).
Bied IC-patiënten fysieke training aan, en in het bijzonder het (vroeg) mobiliseren.
Bied IC-patiënten (en de naasten; zie de module Preventie PICS-F) het gebruik van een IC-dagboek aan.
Gebruik geen oordoppen en oogmaskers om PICS-symptomen te voorkomen dan wel te verminderen.
Zet niet standaard psychologische ondersteuning in op de IC ter voorkoming van PICS. Bij preexistente psychologische factoren of (bij PICS passende) symptomen die zich tijdens de IC-opname manifesteren, kan de inzet van psychologische ondersteuning wel overwogen worden.
Overwegingen
Voor- en nadelen van de interventie en de kwaliteit van het bewijs
In deze module zijn twee zoekvragen geformuleerd om te bepalen welke medicamenteuze en of niet-medicamenteuze interventies effectief zijn om PICS-symptomen te voorkomen of te verminderen. Bij deze vragen zijn loopafstand, geheugen en angst op de lange termijn als cruciale uitkomstmaten en depressie, PTSD, terugkeer naar werk en kwaliteit van leven op de lange termijn als belangrijke uitkomstmaten gedefinieerd door de werkgroep. De uitkomstmaten omvatten dus respectievelijk het fysieke, cognitieve en het mentale domein aangevuld met terugkeer naar werk en kwaliteit van leven.
Concluderend na uitvoerige bestudering van de beschikbare internationale wetenschappelijke literatuur is vastgesteld dat bewijs voor concrete evidence based aanbevelingen ten faveure van één of meerdere interventies ontbreekt. Er zijn echter wel overwegingen te formuleren bij de verschillende interventies.
Zoekvraag 1: Medicamenteuze interventies
Slechts één studie voldeed aan de in- en exclusiecriteria van de literatuursearch. Rosuvastatin zou mogelijk geen effect hebben op geheugen (lage bewijskracht). Er zijn geen studies gevonden waarin de overige uitkomstmaten zijn bestudeerd. De overall bewijskracht komt daarmee op zeer laag. Er ligt hier op gebied van medicamenteuze interventies een duidelijke kennislacune. Opgemerkt moet worden dat de search mogelijk onvoldoende sensitief is geweest om alle mogelijke studies te vinden.
Zoekvraag 2: niet medicamenteuze interventies
Voor deze zoekvraag zijn 13 studies geïncludeerd (waarvan negen eveneens werden geïncludeerd in de systematic review van Geense, 2019). Zes studies hebben het effect van fysieke training onderzocht, drie studies het effect van dagboeken, één studie het effect van counseling, twee studies het effect van informatie en scholing en één studie heeft het effect van de optimalisatie van de IC-omgeving (oordopjes en oogmaskers) onderzocht. Hieronder worden de overwegingen gegeven per type interventie.
Fysieke training
Het is (nog) onduidelijk of fysieke training geïnitieerd op de IC ten opzichte van standaard zorg, effect heeft op loopafstand, geheugen, angst en depressie. Studies naar fysieke training laten weinig effect zien op de kwaliteit van leven van patiënten bij follow-up na 3 maanden. Er zijn geen studies gevonden die de uitkomstmaten ‘posttraumatic stress disorder’ (PTSD) en terugkeer naar werk hebben onderzocht. De overall bewijskracht is zeer laag, hier ligt een kennislacune.
Er is op dit moment (nog) onvoldoende bewijs voor preventief effect van fysieke training ten opzichte van standaard zorg tijdens de IC-opname op PICS. Hierbij moet opgemerkt worden dat binnen de standaard zorg ook vaak fysieke training werd gegeven. Dit zal het contrast tussen de studiearmen waarschijnlijk hebben verkleind. De werkgroep is daarom van mening dat de resultaten vooral laten zien dat er niet één specifieke vorm van fysieke training kan worden aangeraden, en niet dat er geen meerwaarde is van fysieke training ten opzichte van helemaal geen training. Het is ook duidelijk dat bij kritiek zieke patiënten die inactief zijn, de inactiviteit leidt tot verminderde spierkracht en conditie (Mendez-Tellez, 2012). Vanuit verschillende patiëntenpopulaties is verder bekend dat fysieke training de spierkracht en het uithoudingsvermogen verbetert. Mobiliseren, en in het bijzonder geldt dit voor vroegmobilisatie (hetgeen als belangrijk onderdeel van fysieke training wordt gezien), vormt heden ten dage een standaard onderdeel van goede kwaliteit van IC-zorg. Ondanks dat er (nog) geen gunstige effecten van fysieke training in vergelijking met standaard zorg (wat ook fysieke training kon inhouden) ter preventie of reductie van PICS zijn gevonden en omdat er (nog) geen nadelige effecten van fysieke training zijn gevonden in studies, is de werkgroep van mening dat dit de huidige werkwijze, is de werkgroep van mening dat dit de huidige werkwijze, waarin fysieke training tijdens IC-opname als standaardzorg wordt beschouwd, niet mag veranderen.
IC-dagboek
Er zijn drie studies gevonden die het effect van het IC-dagboek hebben onderzocht bij IC-patiënten. De studiekwaliteit varieerde sterk. Waarschijnlijk hebben IC-dagboeken geen klinisch relevant effect op angst, depressie en PTSD bij IC-patiënt na ontslag uit het ziekenhuis (GRADE redelijk). Het effect op kwaliteit van leven is onduidelijk (GRADE zeer laag), en het effect op loopafstand, geheugen en terugkeer naar werk is niet onderzocht. Hierbij moet opgemerkt worden dat een effect op loopafstand ook niet direct voor de hand lijkt te liggen. Belangrijk is ook om op te merken dat, al was het niet gekozen als uitkomstmaat, bij geen van de studies is de patiënttevredenheid meegenomen. Het is belangrijk dat toekomstig onderzoek ook deze uitkomstmaat meeneemt.
Ondanks bovenstaande, is de ervaring van de werkgroepleden (voornamelijk gebaseerd op patiëntverhalen bij de IC-nazorgpoli’s en reacties tijdens lotgenoten contactbijeenkomsten van voormalig IC-patiënten en hun naasten) dat de dagboeken in het algemeen positief worden gewaardeerd door zowel de voormalig IC-patiënten als hun naasten. Niet iedere patiënt of nabestaande geeft aan behoefte te hebben aan een dagboek, maar het niet aanbieden van het (bijhouden van een) dagboek kan in de praktijk achteraf als een gemiste kans worden ervaren. Het gebruik van een IC-dagboek lijkt tijdens de IC-opname bij te kunnen dragen aan het verwerken en omgaan met stress bij de naasten, en na de IC-opname ‘aan het vullen van de leegte in het geheugen van de patiënt’ dat is ontstaan tijdens de IC-periode. Daarnaast draagt het mogelijk bij aan het aangaan van het gesprek over de IC-opname met elkaar, tussen naasten onderling, maar ook met zorgverleners.
Begeleiding/ondersteuning
Er is slechts één studie gevonden. Hieruit blijkt dat counseling tijdens de IC-opname waarschijnlijk geen klinisch relevant effect heeft op angst, depressie, PTSD en kwaliteit van leven bij IC-patiënten na ontslag uit het ziekenhuis (GRADE redelijk). Voor de andere uitkomstmaten loopafstand, geheugen en terugkeer naar werk zijn geen data beschikbaar.
Deze resultaten sluiten niet uit dat er individuele casuïstiek (bijvoorbeeld bij preexistente psychologische factoren) is waarbij de inzet van een psycholoog tijdens de IC-opname is geïndiceerd.
Informatie en scholing
Er zijn twee studies gevonden, maar het effect van dit type interventie op de uitkomsten angst, depressie en PTSD blijft grotendeels onduidelijk. Er lijkt geen effect te zijn op kwaliteit van leven na ontslag uit het ziekenhuis (GRADE laag). Deze bevindingen moeten echter slechts gezien worden in het perspectief van preventie en reductie van PICS. Het betekent niet dat het geven van informatie aan patiënten tijdens, reeds voorafgaand aan een IC-opname of vóór een bepaalde (be-)handeling, niet zinvol is. Het goed informeren van de patiënt voorafgaand aan de (be-) handeling en na de behandeling is een standaard onderdeel van goed zorgverlenerschap (zie ook module Organisatie van zorg – submodule ‘Informatievoorziening aan en communicatie met de patiënt en naasten’). Uiteraard maakt een ongeplande, acute opname voorlichting voorafgaand aan de IC-opname in veel gevallen onmogelijk.
Optimaliseren van de IC-omgeving
In één studie is het gebruik van oordoppen en een oogmasker onderzocht, maar het blijft onduidelijk of het gebruik van deze materialen effect heeft op angst, depressie en PTSD (GRADE zeer laag). Voor de overige uitkomstmaten zijn geen studies gevonden. Er is hierbij sprake van een kennislacune. Het kortetermijneffect van toepassingen van oordoppen op slaap, angst en delier is hier verder niet onderzocht. Er kan aan de individuele patiënt op basis van klinische inschatting altijd oordoppen worden aangeboden. Bij gebruik van oordoppen en oogmaskers bestaat echter ook een risico dat de patiënt teveel gedepriveerd kan raken van prikkels uit de omgeving.
Waarden en voorkeuren van patiënten (en hun naasten)
Er is op dit moment nog weinig onderzoek gedaan naar de patiënttevredenheid en de patiëntvoorkeuren met betrekking tot de preventieve interventies voor PICS (Geense, 2019). Patiënten hechten eraan om na de IC-opname het ‘normale leven’ weer zo snel mogelijk op te kunnen pakken. Het voorkomen of verminderen van PICS-symptomen draagt hier waarschijnlijk aan bij, en heeft waarschijnlijk een gunstig effect op het herstel na de intensive care opname. De beschreven interventies ([vroeg] mobiliseren, bijhouden van dagboeken, informatievoorziening) zijn onderdeel van het geven van goede IC-zorg, en kennen een lage of beperkte belasting voor de patiënt. Over het algemeen zijn de meeste patiënten positief over fysieke training en is er een substantiële groep die de inzet van dagboeken nuttig vindt. Een goede informatievoorziening is daarnaast essentieel. Patiënten zullen over het algemeen het niet uitvoeren van deze interventies dan ook als nadelig ervaren.
Kosten (middelenbeslag)
Zoals hierboven beschreven zijn de onderzochte interventies (fysieke training, mobiliseren, dagboeken en informeren) al standaard onderdeel van de goede zorg en behandeling op de IC-afdeling en zullen daardoor als zodanig niet leiden tot extra kosten.
Aanvaardbaarheid, haalbaarheid en implementatie
De werkgroep stelt dat fysieke training tijdens de IC en het gebruik van een IC-dagboek zowel aanvaardbaar als haalbaar zijn omdat dit al gangbare interventies zijn en gezien wordt als standaard zorg. Deze interventies dragen bij aan betere kwaliteit van zorg.
De implementatiegraad van beide interventies is hoog in de Nederlandse IC’s, zo wordt het gebruik van IC-dagboeken in bijna 90% van de IC’s toegepast (Hendriks, 2019). Fysieke training en mobiliseren kent mogelijkerwijs een nog hogere toepassingsgraad dan van de IC-dagboeken. Meestal is bij de fysieke training en (vroeg)mobilisatie een fysiotherapeut betrokken.
Ook het geven van informatie aan patiënten en naasten over onderzoeken en behandelingen zijn standaard onderdeel van goede IC-zorg. De werkgroep is van mening dat het onthouden van fysieke training, mobiliseren, het aanbieden (en bijhouden) van een IC-dagboek en het informeren van de patiënt onaanvaardbaar is omdat het leidt tot vermindering van de kwaliteit van zorg.
Rationale van de aanbeveling: weging van argumenten voor en tegen de interventies
Medicamenteuze interventies
Er is weinig literatuur gevonden, derhalve raadt de werkgroep een farmacologische behandeling specifiek ter voorkoming of vermindering van de PICS-symptomen niet aan.
Niet-medicamenteuze interventies
Ondanks dat bewijs voor concrete evidence based aanbevelingen ten faveure van één of meerdere interventies ontbreekt, is de werkgroep van mening dat fysieke training (inclusief (vroeg) mobiliseren, zie ook Behandelprotocol Fysiotherapie op de IC (KNGF, 2021)) en het gebruik van een IC-dagboek aangeboden moet worden. Beide worden beschouwd als onderdeel van goede IC-zorg, en is op de meeste IC’s reeds geïmplementeerd.
Er is geen evidentie voor de inzet van oordoppen en oogmaskers en standaard psychologische ondersteuning ter voorkomen van PICS. De werkgroep raadt derhalve deze interventies niet standaard aan. De werkgroep hecht eraan dat de patiënten en de naasten goed geïnformeerd worden, ook als dit niet direct van invloed lijkt te zijn op het voorkomen van PICS. In de module ‘Organisatie van zorg – submodule ‘Informatievoorziening aan en communicatie met de patiënt en naasten’ worden specifieke aanbevelingen gedaan over dit onderwerp.
Onderbouwing
Achtergrond
Ernstig zieke patiënten die op de intensive care (IC) zijn behandeld kunnen het Post Intensive Care Syndroom (PICS) ontwikkelen. PICS is gedefinieerd als nieuwe of verergerende problemen in het lichamelijke, mentale en/of cognitieve domein, ontstaan na het doormaken van een kritieke, ernstige ziekte en die blijven bestaan na verblijf op een IC afdeling (Needham, 2012). PICS komt regelmatig voor bij voormalig IC-patiënten, maar het is onduidelijk welke preventieve interventies effectief zijn. Onder de preventieve interventies[1] voor PICS worden hier alle interventies bedoeld die ingezet worden tijdens de IC-opname, om langetermijn klachten te voorkomen of te verminderen.
Conclusies / Summary of Findings
Search question 1: pharmacological interventions versus no treatment or placebo
1, 3-7. Walking distance (critical), anxiety (critical), depression (important), PTSD (important), return to work (important) and quality of life (important)
|
- GRADE |
The effect of pharmacological interventions in the intensive care unit on walking distance, anxiety, depression, PTSD, return to work and quality of life is unknown. No studies were found examining these outcome measures in adult patients who were admitted to the intensive care unit. |
2. Cognitive functioning (critical)
|
Low GRADE |
The evidence suggests that treatment with rosuvastatin in the intensive care unit results in little to no difference in cognitive functioning in adult patients at six months after discharge.
Source: Needham, 2016 |
Search question 2: non-pharmacological interventions versus no treatment or usual care
1. Physical training
1. Walking distance (critical)
2-6. Cognitive functioning (critical), PTSD (important), return to work (important)
3-4. Anxiety (critical), depression (important)
7. Quality of life (important)
2. Diaries
1-2, 6. Walking distance (critical), cognitive functioning (critical), return to work (important)
|
- GRADE |
The effect of the use of diaries in the intensive care unit on walking distance, cognitive functioning and return to work is unknown. No studies were found examining these outcome measures in adult patients who were admitted to the intensive care unit. |
3. Anxiety (critical)
4. Depression (important)
5. PTSD
7. Quality of life
|
Very low GRADE |
The evidence is very uncertain about the effect of diaries in the intensive care unit on quality of life in adult patients after hospital discharge.
Source: Nielsen, 2020 |
3. Psychological counseling
1-2, 6. Walking distance (critical), cognitive functioning (critical) and return to work (important)
|
- GRADE |
The effect of psychological counseling in the intensive care unit on walking distance, cognitive functioning and return to work is unknown. No studies were found examining these outcome measures in adult patients who were admitted to the intensive care unit. |
3. Anxiety (critical)
|
Moderate GRADE |
Psychological counseling in the intensive care unit probably results in little to no difference in anxiety in adult patients after hospital discharge.
Source: Wade, 2019 |
4. Depression (important)
|
Moderate GRADE |
Psychological counseling in the intensive care unit probably results in little to no difference in depression in adult patients after hospital discharge.
Source: Wade, 2019 |
5. PTSD (important)
|
Moderate GRADE |
Psychological counseling in the intensive care unit probably results in little to no difference in PTSD in adult patients after hospital discharge.
Source: Wade, 2019 |
7. Quality of life (important)
|
Moderate GRADE |
Psychological counseling in the intensive care unit probably results in little to no difference in quality of life in adult patients after hospital discharge.
Source: Wade, 2019 |
4. Information and education
1-2, 5-6. Walking distance (critical), cognitive functioning (critical), PTSD (important) and return to work (important)
|
- GRADE |
The effect of information and education interventions in the intensive care unit on walking distance, cognitive functioning, PTSD and return to work is unknown. No studies were found examining these outcome measures in adult patients who were admitted to the intensive care unit. |
3. Anxiety (critical)
|
The evidence is very uncertain about the effects of information and education interventions in the intensive care unit on anxiety in adult patients after hospital discharge.
Source: Demircelik, 2016 |
4. Depression (important)
7. Quality of life (important)
5. Optimizing the ICU environment
1-2, 6-7. Walking distance (critical), cognitive functioning (critical), return to work (important) and quality of life (important)
|
- GRADE |
The effect of the use of earplugs and eye masks in the intensive care unit on walking distance, cognitive functioning, return to work and quality of life is unknown. No studies were found examining these outcome measures in adult patients who were admitted to the intensive care unit. |
3. Anxiety (critical)
Very low GRADE |
The evidence is very uncertain about the effect of earplugs and eye masks in the intensive care unit on anxiety in adult patients after hospital discharge.
Source: Demoule, 2017 |
4. Depression (important)
|
Very low GRADE |
The evidence is very uncertain about the effect of earplugs and eye masks in the intensive care unit on depression in adult patients after hospital discharge.
Source: Demoule, 2017 |
5. PTSD (important)
|
Very low GRADE |
The evidence is very uncertain about the effect of earplugs and eye masks in the intensive care unit on PTSD in adult patients after hospital discharge.
Source: Demoule, 2017 |
Samenvatting literatuur
Search question 1: pharmacological interventions versus no treatment or placebo
One study was found that matched the search question. This study compared treatment with rosuvastatin versus placebo in patients with sepsis-associated acute respiratory distress syndrome (ARDS) in intensive care (Needham, 2016).
Description of studies
Needham (2016) performed an ancillary study within a larger trial in which 37 hospitals in the United States participated. Administration of rosuvastatin was compared with a placebo in patients with sepsis-associated acute respiratory distress syndrome. The intervention group received a 40 mg loading dose and then 20 mg daily until the earliest of death, three days after discharge from the ICU, or 28 days of study participation. At first delirium and cognition were only assessed at 12 hospitals, but as new data indicated that use of statins was associated with reduced odds of delirium, the study protocol was amended and delirium and cognition subsequently assessed in 35 hospitals. The first 75 patients were also included in another RCT comparing initial trophic with full enteral feeding. Because of ineffectiveness, the trial was stopped early. The intervention group consisted of 164 patients and the control group of 165 patients. Attention and working memory were assessed with the Digit Span age-adjusted scaled score from the Wechsler Adult Intelligence Scale (third edition). Immediate and delayed memory were assessed with the Logical Memory I and II age adjusted scaled scores. At six months follow-up, these outcomes were available for 53 patients in the rosuvastatin group and 77 patients in the placebo group. Outcomes were also assessed at 12 months, but the results were not reported in detail.
Results
1. Walking distance (critical), 3. Anxiety (critical), 4. Depression (important), 5. PTSD (important), 6. Return to work (important) and 7. Quality of life (important)
Needham (2016) did not report these outcome measures.
2. Cognitive functioning (critical)
Needham (2016) found no statistically significant differences on attention and working memory (intervention group, mean (SD): 9.2 (2.5); control group, mean (SD): 9.5 (2.6) ; β (95% CI): -0.3 (-1.2 to 0.6), p = 0.49) and immediate memory (intervention group, mean (SD): 8.6 (3.4); control group, mean (SD): 8.9 (3.3); β (95% CI): -0.4 (-1.5 to 0.7), p = 0.49). The difference in delayed memory was statistically significant (intervention group, mean (SD): 7.9 (3.3); control group, mean (SD): 8.8 (2.7); β (95% CI): -1.2 (-2.2 to -0.2), p = 0.02), with worse scores for the patients who received rosuvastatin. This difference was likely not clinically relevant.
Level of evidence of the literature
The level of evidence regarding the outcome measures walking distance, anxiety, depression, PTSD, return to work and quality of life could not be assessed; no studies were found investigating these outcome measures.
RCTs start at high GRADE. The level of evidence regarding the outcome measure cognitive functioning was downgraded by 2 levels because of the substantial attrition (risk of bias; -1); and the inclusion of only one study with a limited sample size (imprecision; -1). The level of evidence is low.
The review of Geense (2019) was used as starting point for the second search question. This systematic review described non-pharmacological interventions aimed at the prevention of PICS. The databases PubMed, CINAHL, PsycINFO, Embase and Cochrane Library were searched until 19th July 2018. The risk of bias (ROB) of the individual studies was assessed with the Cochrane Collaboration’s ROB tool.
Nine of 36 studies from this review matched the PICO and our inclusion and exclusion criteria. We excluded two non-RCTs, three RCTs with an intervention which was started before ICU admission, 13 RCTs with interventions that started after ICU discharge, one RCT with an intervention that didn’t match our PICO, one RCT in which the control group received another intervention instead of no treatment or usual care, one RCT with outcome measures that didn’t match our PICO and six studies with less than twenty patients per study arm.
Our literature search resulted in four additional studies that answer the second PICO. Thus, 13 studies were included in total. Of these studies, six RCTs investigated the effect of physical training (Denehy, 2013; Eggmann, 2018; Hodgson, 2016; Morris, 2016; Schaller, 2016 and Wright, 2018). Three studies investigated the effect of diaries (Jones, 2010; Garrouste-Orgeas, 2019 and Nielsen, 2020). One study focused on psychological counseling (Wade, 2019), two studies on information and education (Demircelik, 2016 and Fleischer, 2014) and one study on optimizing the ICU environment by using earplugs and eye masks (Demoule, 2017).
No studies were found that investigated cognitive training, communication tools or behaviour of health care providers.
1. Physical training
Description of studies
The review of Geense (2019) included five studies that examined the effect of physical training and met the inclusion and exclusion criteria (Denehy, 2013; Hodgson, 2016; Morris, 2016; Schaller, 2016 and Wright, 2018). The literature search revealed one additional study (Eggmann, 2018).
Studies included in Geense (2019)
Denehy (2013) investigated a rehabilitation program which was commenced at day five after ICU admission (<15 min/day), progressing daily on the acute hospital ward (2x 30 min) and being administered twice weekly for eight weeks in the outpatient setting (60 min). The intervention was performed by physiotherapists and consisted of marching in place, moving from sitting to standing, cardiovascular progressive resistance strength training and functional exercise. The control group received usual care, which may have included active bed exercises, sitting out of bed and marching/walking. The intervention group consisted of 74 patients and the control group of 76 patients. Walking distance was measured with the 6-minute walk test and quality of life was determined with the SF-36.
Hodgson (2016) evaluated the effects of exercise active functional activities, (e.g. walking, standing, sitting) during ICU admission. Intervention duration was 30 to 60 min per day, depending on patient level. The goal of the intervention was to maximize safe physical activity. The intervention group consisted of 29 patients and the control group of 21 patients. Quality of life was determined with the EQ-5D VAS and EQ-5D Utility, anxiety and depression with the HADS.
Morris (2016) investigated the effect of standardized rehabilitation therapy. This program consisted of three separate sessions per day. The team comprised a physical therapist, an ICU nurse, and a nursing assistant. Components of the intervention were passive range of motion (which included five repetitions for each upper and lower extremity joint), physical therapy (which included bed mobility, transfer training, and balance training), and progressive resistance exercises (which included dorsiflexion, knee flexion and extension, hip flexion, elbow flexion and extension, and shoulder flexion). The control group received usual care. Physical therapy could be ordered as part of usual care, but only Monday through Friday. Both the intervention group and the control group consisted of 150 patients. Quality of life was determined with the SF-36.
Schaller (2016) examined daily early, goal-directed mobilization in the ICU. The intervention consisted of two parts. First, a mobilization goal was defined during daily morning ward rounds and second, goal implementation across shifts was facilitated by inter-professional closed-loop communication. The control group received usual care. There were 104 patients in the intervention group and 96 in the control group. Quality of life was determined with SF-36.
Wright (2018) investigated intensive physical rehabilitation therapy which was provided by a physical therapist for 90 minutes per day on weekdays (Monday - Friday), split between at least two sessions. The physical rehabilitation therapy included functional training and individually tailored exercise programs. The control group received physical rehabilitation for 30 minutes per day. The physical rehabilitation therapy received by the standard care group was the same as that provided normally in participating ICUs. There were 150 patients in the intervention group and 158 patients in the control group. Walking distance was measured with the 6-minute walk test and quality of life was determined with the SF-36 and EQ-5D.
Studies published after Geense (2019)
Eggmann (2018) published a RCT in which early, progressive endurance and resistance training combined with mobilization was compared to standard physiotherapy with early mobilization. All patients received mechanical ventilation and both groups also received standard ICU care with protocol-guided sedation, weaning and nutrition. The intervention group consisted of 58 ICU patients, the control group consisted of 57 patients. Quality of life was determined with the SF-36.
Results for interventions with physical training
1. Walking distance (critical)
Hodgson (2016), Morris (2016), Schaller (2016) and Eggmann (2018) did not report this outcome measure.
Denehy (2013) and Wright (2018) administered the 6-minute walk test at three and six months after discharge. Data could not be pooled, since Denehy (2013) reported means with standard deviations, while Wright (2018) reported medians with interquartile ranges (IQR). Results are provided separately for short term and long term effects.
6-minute walk test short term (three months)
At three months after discharge no clinically relevant and statistically significant differences were reported by either Denehy (2013) or Wright (2018). In Denehy (2013) the intervention group had a mean (SD) walking distance of 244.2 (124.0) meters at hospital discharge and 384.5 (147.9) meters at three months after discharge. The control group had a mean (SD) walking distance of 266.7 (136.8) meters at hospital discharge and 382.1 (139.4) meters at three months after discharge. The mean difference (95% CI) was 2.40 (-60.57 to 65.37) meters (calculated using RevMan version 5.3).
In Wright (2018) the intervention group had a median (IQR) walking distance of 195 (120-260) meters at hospital discharge and 293 (124-444) meters at three months follow-up. The control group had median (IQR) walking distance of 173 (123-274) meters at hospital discharge and 255 (120-337) meters at three months follow-up. In a multiple linear regression model with stratification variables and baseline variables the adjusted difference in means (95% CI) was −6.3 (−125.8 to 107.3) meters.
6-minute walk test long term (six months)
At six months after discharge no clinically relevant and statistically significant differences were found on this outcome measure by Denehy (2013). The intervention group had a mean (SD) walking distance of 394.2 (156.2) meters and the control group 402.4 (166.6) meters. The mean difference (95% CI) was -8.20 (-79.17 to 62.77) meters (calculated using RevMan version 5.3).
Wright (2018) did not find a statistically significant difference in walking distance at six months after discharge. The intervention group had a median (IQR) walking distance of 374 (203-435) meters and the control group of 321 (197-400) meters. The adjusted difference in means (95% CI) was 61.7 (−47.2 to 157.0). The difference seems clinically relevant, but given the broad interval this should be interpreted with caution.
2. Cognitive functioning (critical), 5. PTSD (important), 6. return to work (important)
These outcome measures were not reported in the included studies about physical training.
3. Anxiety (critical)
Hodgson (2016) reported the total scores of the HADS, the summed anxiety and depression score, at six months follow-up. There was no statistically significant difference between the groups (mean (SD) intervention group 11.6 (9.1); control group: 11.3 (7.1), p=0.91). The difference is likely also not clinically relevant.
This outcome measure was not reported in the other included studies.
4. Depression (important)
See Anxiety.
7. Quality of life (important) – short-term
Various outcome measures were used to assess quality of life. The results are presented separately for the overall quality of life scales (EQ-5D and SF-36 total score), the SF-36 PCS and the SF-36 MCS.
Morris (2016) and Wright (2018) reported the SF-36 PCS at two months and Denehy (2013) at three months follow-up. The results are pooled. Physical training did not result in clinically relevant and not statistically significant between-group differences (Figure 1).
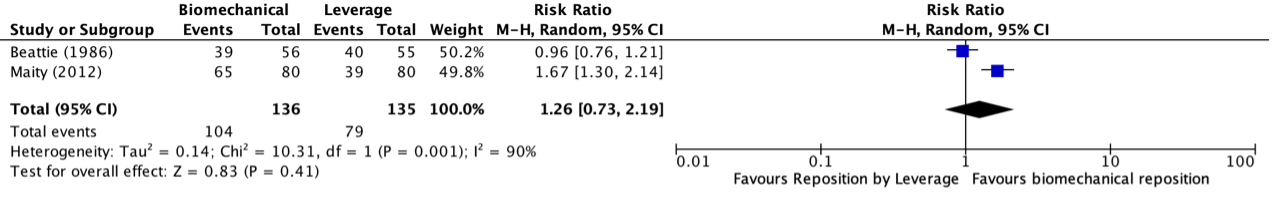
Figure 1 Short term effects of physical training on SF-36 PCS scores
Morris (2016) and Wright (2018) reported the SF-36 MCS at two months and Denehy (2013) at three months follow-up. The results are pooled. Physical training did not result in clinically relevant and not statistically significant between-group differences (Figure 3).
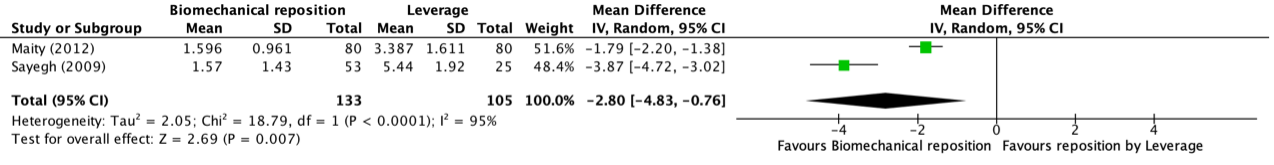
Figure 2 Short term effects of physical training on SF-36 MCS scores
Schaller (2016) found no statistical significant difference on quality of life measured with SF-36 total scores at three months follow-up (intervention group, mean (SD): 61.3 (18.4); control group, mean (SD) 63.0 (19.9); group difference mean (95% CI): -1.7 (-10.1 to 6.7), p = 0.69).
Wright (2018) reported no statistically significant difference on quality of life assessed with the EQ-5D at three months (mean difference (95% CI): 0.07 (-0.06 to 0.19)).
7. Quality of life (important) – long-term
Various outcome measures were used to assess quality of life. The results are presented separately for the overall quality of life scales (EQ-5D and SF-36 total score), the SF-36 PCS and de SF-36 MCS.
Denehy (2013), Eggmann (2018), Morris (2016) and Wright (2018) reported the SF-36 PCS at six months follow-up. The results are pooled. Physical training did not result in clinically relevant and not statistically significant between-group differences (Figure 2).
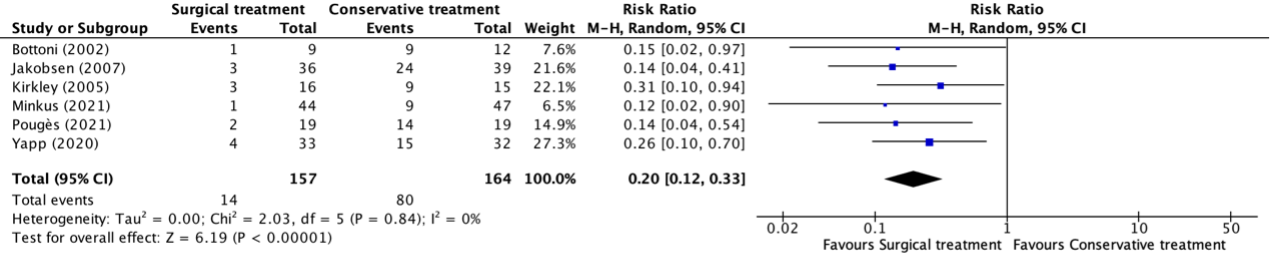
Figure 3. Long term effects of physical training on SF-36 PCS scores
Denehy (2013), Eggmann (2018), Morris (2016) and Wright (2018) reported the SF-36 MCS at six months follow-up. The results are pooled. Physical training did not result in clinically relevant and not statistically significant between-group differences (Figure 4).
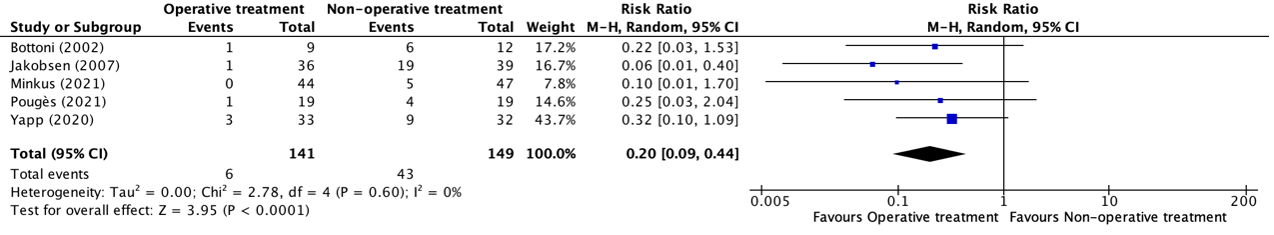
Figure 4. Long term effects of physical training on SF-36 MCS scores
Hodgson (2016) found no statistically significant differences on quality of life assessed with the EQ-5D VAS (intervention group, mean (SD): 61 (19); control group, mean (SD): 68 (19), p = 0.25) and the EQ-5D Utility (intervention group, mean (SD): 0.63 (0.27); control group, mean (SD): 0.63 (0.33), p = 0.98) at six months follow-up.
Wright (2018) report no statistical significant difference on quality of life assessed with the EQ-5D at six months follow-up (mean difference (95% CI): 0.06 (-0.08 to 0.20)).
Level of evidence of the literature
The level of evidence regarding the outcome measures cognitive functioning, PTSD and return to work could not be graded; no studies were found investigating these outcome measures.
RCTs start at high GRADE. The level of evidence regarding the outcome measure walking distance in the short-term was downgraded by 2 levels because of study limitations (risk of bias, bias due to incomplete outcome data and lack of blinding and selective outcome reporting in Wright (2018) ; -1); and the confidence intervals exceeding the minimal clinically (patient) important difference at both sides (imprecision; -2). The level of evidence is very low.
The level of evidence regarding the outcome measure walking distance in the long-term was downgraded by 3 levels because of study limitations (risk of bias, bias due to incomplete outcome data and lack of blinding and selective outcome reporting in Wright (2018); -1); and the confidence intervals exceeding the minimal clinically (patient) important difference at both sides (imprecision; -2. The level of evidence is very low.
The level of evidence regarding the outcome measures anxiety and depression in the long-term was downgraded by 3 levels because of study limitations (risk of bias, unclear ROB for blinding and incomplete outcome data, and high risk of bias for other bias, limited reporting of the findings; -2) and the inclusion of a single study with a very limited sample size (imprecision; -2). The level of evidence is very low.
The level of evidence regarding the outcome measure quality of life in the short term was downgraded by two levels because of study limitations (risk of bias; -1, see ROB table); and because the confidence interval crosses the clinical decision threshold (imprecision; -). The level of evidence is moderate.
The level of evidence regarding the outcome measure quality of life in the long-term was downgraded by 2 levels because of bias due to study limitations (risk of bias; -1); and because the confidence interval crosses the clinical decision threshold (imprecision; -1). The level of evidence is low.
2. Diaries
Description of studies
Three studies investigating the effect of diaries were included (Garrouste-Orgeas, 2019; Jones, 2010 and Nielsen, 2020). One of these studies were included in Geense (2019). All studies evaluated the effects on short-term.
Studies included in Geense (2019)
Jones (2010) examined the effect of diaries in the ICU written by healthcare staff and family members. Photos and a daily record of the ICU stay were also added. The intervention was performed by a research nurse or a doctor and took a few minutes per day. The intervention group consisted of 177 patients and the control group of 175 patients. The control group received the diary after 3 months. PTSD was measured with the Post-Traumatic Stress Syndrome (PTSS)-14 score, the PTSD Diagnostic Scale (PDS) and the number of patients with new onset PTSD. PTSD was assessed at one and three months post ICU, the other outcomes only at three months post ICU.
Studies published after Geense (2019)
Garrouste-Orgeas (2019) performed a large multicenter RCT evaluating the effects of an ICU diary filled out by clinicians and family members during ICU stay when compared to usual care (no ICU diary). Thirty-five ICUs participated and 332 patients and family members were allocated to the intervention group and 325 patients to the control group. In this summary we only focus on the data of the patients. Assessments were performed at three months follow-up. Anxiety and depression were determined with HADS. PTSD symptoms were measured by the Impact of Event Scale-Revised (IES-R) questionnaire. A large proportion of the included patients (n=255) had died before the follow-up. Furthermore, 63 patients were excluded from analyses (declined, had cognitive dysfunction, missing data, or no contact). Analyses were, therefore, performed for 164 patients in the intervention group and 175 patients in the control group.
Nielsen (2020) performed a multicenter RCT evaluating the effects of a diary authored by a close relative of a critically ill patient when compared to no dairy on psychological difficulties in patients and their relatives. In this summery we only focus on the data of the patients. The study was underpowered: it was estimated that 71 patients and relatives were required in each group, but due to slow inclusion only 36 patients were allocated to the intervention group (dairy) and 39 to the control group (no dairy). Attrition was also substantial (intervention n=10, control n=17, mainly “non-responders”). There were some differences in demographics between the groups analyzed, the percentage of female patients was higher in the control group (55 vs 42%), and the median age was lower (60 vs 68). Outcome measures were assessed three months after ICU discharge. Anxiety and depression were determined with HADS. PTSD was measured with PTSS-14 scores. Quality of life was determined with the SF-36.
Results for interventions with diaries
1. Walking distance (critical), 2. Cognitive functioning (critical), 6. Return to work (important)
These outcome measures were not reported in the included studies.
3. Anxiety (critical)
Garrouste-Orgeas (2019) found no statistically significant difference on anxiety (HADS-A, continuous scale) at three months follow-up (intervention group, median (IQR): 5 (2-8); control group, median (IQR): 6 (2-8); difference (95% CI): -0.36 (-1.22 to 0.50), p = 0.72). This difference is also considered as not clinically relevant. The number of patients with symptoms of anxiety (HADS>8) was not statistically or clinically relevant different between the groups (intervention group n=51, 31.3%; control group n=53, 30.6%, risk difference: 0.7%, 95%CI -9 to 11%, p=0.91).
Nielsen (2020) reported the number of patients scoring ≥ 11 on the HADS-A. No statistically significant difference was observed between the intervention group and control group three months after discharge from the ICU (intervention group, number: 5/25; control group, number: 3/22; relative risk (95% CI): 1.47 (0.40 to 5.44), p = 0.71).
Jones (2010) did not report this outcome measure.
4. Depression (important)
Garrouste-Orgeas (2019) found no statistically significant difference on depression at three months follow-up (intervention group, median (IQR): 4 (1-7); control group, median (IQR): 3 (2-7); difference (95% CI): -0.39 (-1.29 to 0.52), p = 0.66). This difference is also considered as not clinically relevant. The number of patients with symptoms of depression (HADS>8) was not statistically or clinically relevant different between the groups (intervention group n=31, 19%; control group n=41, 23.7%, risk difference: 5%, 95%CI -5 to 13%, p=0.35).
Nielsen (2020) reported the number of people who score ≥ 11 on the HADS-D (continuous scale). They found no statistically significant difference between the groups three months after discharge from the ICU (intervention group, number: 2/25; control group, number: 2/22; relative risk (95% CI): 0.88 (0.14 to 5.73), p = 1.0).
Jones (2010) did not report this outcome measure.
5. PTSD (important)
Garrouste-Orgeas (2019) found no statistically significant difference on IES-R scores at three months follow-up (intervention group, median (IQR): 12 (5-25); control group, median (IQR): 13 (6-27); difference (95% CI): -1.47 (-1.93 to 4.87), p = 0.38). The number of patients with an IES-R score >22 was not statistically or clinically relevant different between the groups (intervention group n=49, 29.9%; control group n=60, 34.3%, risk difference: -4%, 95%CI -15 to 6%, p=0.39).
Jones (2010) reported no statistically significant differences on PTSD determined with total PTSS-14 scores at one month (intervention group: 22.5 (14-84); control group: 25 (13-65)) and three months post ICU (intervention group, 24 (12.2); control group, 24 (11.6); unclear which measures of central tendency and statistical dispersion were reported). The number of patients that experienced the ICU as traumatically (PDS) was also not statistically significant at three months post ICU (intervention group, n (%): 70 (43.2); control group, n (%): 76 (47.5), p = 0.36). The number of patients with new onset PTSD was statistically significant different at three months post ICU: (intervention group, n (%): 8 (5); control group, n (%): 21 (13.1), p = 0.02).
Nielsen (2020) found no statistically significant differences three months after ICU discharge on total PTSS-14 scores (intervention group, median (range): 21 (14-75); control group, median (range): 28 (14-75); difference (95% CI): 11.2% (-15.7 to 46.8%), p = 0.44) or actual cases, defined as PTSS-14 scores > 31 (intervention group, number: 8/26; control group, number: 9/22; relative risk (95% CI): 0.75 (0.35 to 1.62), p = 0.55).
7. Quality of life (important)
Nielsen (2020) found no statistically significant differences on the eight subscales of the SF-36, three months after discharge from the ICU. 1. Physical function, intervention group mean: 45.8, control group mean: 38.6, p = 0.46. 2. Role physical, intervention group mean: 18.0, control group mean: 31.3, p = 0.25. 3. Bodily pain, intervention group mean: 62.6, control group mean: 52.7, p = 0.26. 4. global health, intervention group mean: 53.2, control group mean: 47.0, p = 0.36. 5. Vitality, intervention group mean: 48.5, control group mean: 55.2, p = 0.47. 6. Social function, intervention group mean: 68.8, control group mean: 64.2, p = 0.46. 7. Role emotional, intervention group mean: 49.3, control group mean: 50.8, p = 0.94. 8. Mental health, intervention group mean: 74.5, control group mean: 74.0, p = 0.76.
Garrouste-Orgeas (2019) and Jones (2010) did not report this outcome measure.
Level of evidence of the literature
The level of evidence regarding the outcome measures walking distance, cognitive functioning and return to work could not be graded; no studies were found investigating these outcome measures.
RCTs start at high GRADE. The level of evidence regarding the outcome measure anxiety was mainly based on the results of Garrouste-Orgeas (2019); continuous data) and was downgraded by 1 level because of study limitations (risk of bias; lack of blinding and loss-to follow-up -1). The level of evidence is moderate.
The level of evidence regarding the outcome measure depression was mainly based on the results of Garrouste-Orgeas (2019; continuous data) and was downgraded by 1 level because of study limitations (lack of blinding and loss-to follow-up; -1). The level of evidence is moderate.
The level of evidence regarding the outcome measure PTSD was downgraded by 2 levels because of study limitations (risk of bias, lack of blinding and loss-to follow-up; -1) and the limited number of included patients/events and the broader confidence intervals (imprecision; -1). The level of evidence is low.
The level of evidence regarding the outcome measure quality of life was downgraded by 3 levels because of study limitations (risk of bias; underpowered study, substantial attrition, differences in demographics between the groups, lack of blinding, limited reporting of the results; -2); and the inclusion of a single study with a limited number of patients (imprecision; -2). The level of evidence is very low.
3. Psychological counseling
Description of studies
One study was included that focused on psychological counseling. Wade (2019) was a cluster RCT with randomization occurring at ICU level. ICUs in the intervention group provided usual care during the baseline period (months 1-5). During the intervention period (months 7-onwards), these ICUs delivered a preventive, complex psychological intervention. This comprised 1) promotion of a therapeutic ICU environment; and for patients scoring ≥7 on the Intensive care Psychological Assessment Tool (IPAT) 1) three stress support sessions; and 2) a relaxation and recovery program. The intervention was delivered by trained ICU nurses. The ICUs in the control group provided usual care during both the baseline period and the intervention period. The intervention group consisted of 12 ICUs with 669 patients (285 patients recruited during the baseline period, 43 during the transition period and 341 during the intervention period) and the control group of 12 ICUs with 789 patients (285 patients recruited during the baseline period, 58 during the transition period and 446 during the intervention period). Anxiety and depression symptoms were determined with the HADS. PTSD symptoms were measured with the PTSD Symptom Scale - Self-Report (PSS-SR) questionnaire and quality of life with the EQ-5D-5L. Generalized linear mixed models were used and analyses were adjusted for age, sex, race/ethnicity, deprivation, preexisting anxiety/depression, planned admission following elective surgery, and ICNARC Physiology Score. A total of 63.6% of the patients in the intervention group that were assessed with the IPAT, scored ≥7. More than one-third of these patients received less than 3 stress support sessions.
Results for interventions with psychological counseling
1. Walking distance (critical), 2. Cognitive functioning (critical), Return to work (important)
Wade (2019) did not report these outcome measures.
3. Anxiety (critical)
Wade (2019) reported that there was no statistically significant difference on anxiety (HADS-A) at six months (mean (95%CI) intervention ICUs baseline period: 6.9 (6.2 to 7.6), intervention period: 6.3 (5.7 to 7.0); control ICUs baseline period: 5.9 (5.3 to 6.7), intervention period: 5.7 (5.2 to 6.2), adjusted difference in difference (95% CI): −0.24 (−1.50 to 1.01), p = 0.70. This difference is considered not clinically relevant.
4. Depression (important)
Wade (2019) reported that there was no statistically significant difference on depression (HADS-D) at six months (mean (95% CI) intervention ICUs baseline period: 6.0 (5.3 to 6.7), intervention period: 5.8 (5.1 to 6.4); control ICUs baseline period: 5.3 (4.7 to 6.0), intervention period: 5.3 (4.8 to 5.8), adjusted difference in difference (95% CI): −0.22 (−1.40 to 0.95), p = 0.71. This difference is considered not clinically relevant.
5. PTSD (important)
Wade (2019) reported that there was no statistically significant difference in PTSD symptom severity (PSS-SR score) at six months (mean (95% CI) intervention ICUs baseline period: 11.8 (10.3 to 13.3), intervention period: 11.5 (10.0 to 12.9); control ICUs baseline period 10.1 (8.7 to 11.6), intervention period 10.2 (9.1 to 11.3), treatment effect (interaction between treatment group and time period) estimate: −0.03 (95%CI: −2.58 to 2.52), p = 0.98. This difference is considered not clinically relevant.
7. Quality of life (important)
Wade (2019) reported that there were no statistically significant difference in quality of life (mean (95%CI) EQ-5D-5L; intervention ICUs baseline period: 0.66 (0.62 to 0.70), intervention period: 0.67 (0.63 to 0.71); control ICUs baseline period: 0.70 (0.66 to 0.74), intervention period: 0.69 (0.66 to 0.72), adjusted difference in difference (95% CI) of 0.01 (−0.06 to 0.08), p = 0.85).
Level of evidence of the literature
The level of evidence regarding the outcome measures walking distance, cognitive functioning and return to work could not be graded; no studies were found investigating these outcome measures.
RCTs start at high GRADE. The level of evidence regarding the outcome measure anxiety was downgraded by one levels because of the lack of blinding (risk of bias), the unclear risk of bias due to incomplete outcome data and the inclusion of only one study (imprecision; -1). The level of evidence is moderate.
The level of evidence regarding the outcome measure depression was downgraded by one level because of inclusion of the lack of blinding (risk of bias), the unclear risk of bias due to incomplete outcome data only one study (imprecision; -1). The level of evidence is moderate.
The level of evidence regarding the outcome measure PTSD was downgraded by one level because of the lack of blinding (risk of bias), the unclear risk of bias due to incomplete outcome data and the inclusion of only one study (imprecision; -1). The level of evidence is moderate.
The level of evidence regarding the outcome measure quality of life was downgraded by one level because of the lack of blinding (risk of bias), the unclear risk of bias due to incomplete outcome data and the inclusion of only one study (imprecision; -1). The level of evidence is moderate.
4. Information and education
Description of studies
The review of Geense (2019) included two studies that examined information and education interventions and met our inclusion and exclusion criteria. The literature search did not reveal any additional studies.
Demircelik (2016) investigated a multimedia informational education program for patients admitted to a coronary ICU. The intervention group received multimedia education which was carried out by ICU nurses while the control group did not receive multimedia nursing education. Technical materials were prepared for multimedia nursing education. Materials were the same for each patient. No further information was provided regarding the content of the intervention. Both the intervention group and the control group consisted of 50 patients. The control group received usual care. Anxiety and depression were assessed with the HADS.
Fleischer (2014) studied the effect of a structured information program during ICU-stay. The intervention was designed as a guided conversation with both a standardized and an individualized part. The standardized part of the intervention covered general information on nine topics: 1) people in the ICU, 2) devices and monitoring, 3) room furnishing, 4) individual safety, 5) schedule, 6) communication, 7) staff duties, 8) conveniences, and 9) helpful thoughts. In the individual part the patients were allowed to choose any number of cards from seven that depicted common fears: 1) fear of complications, 2) fear of suffocating, 3) fear of pain, 4) fear of being helpless, 5) fear of death, 6) fear of being lonely, and 7) fear of being confined. The intervention group consisted of 104 patients and the control group of 107 patients. The control group received a non-specific episodic conversation of similar length additional to standard care. Quality of life was determined three months after discharge with the SF-12 PCS, SF-12 MCS and Schedule for evaluation of Individual quality of life (SEIQoL).
Results for information and education interventions
1. Walking distance (critical), 2. Cognitive functioning (critical), 5. PTSD (important), 6. Return to work (important)
Demircelik (2016) and Fleischer (2014) did not report these outcome measures.
3. Anxiety (critical)
Demircelik (2016) found a likely statistically significant and clinically relevant difference between the groups in HADS-A change scores between ICU stay and one week after discharge from the hospital, p<0.01. The intervention group had a mean (SD) score of 6.1 (0.7) during the ICU stay and 1.9 (0.2) at one week after discharge. The control group had a mean (SD) score of 5.7 (0.6) during ICU stay and 5.1 (0.6) at one week after discharge.
Fleischer (2014) did not report this outcome measure.
4. Depression (important)
Demircelik (2016) found a statistically significant and clinically relevant difference between groups in HADS-D change scores between ICU stay and 1 week after discharge from the hospital, p<0.01. The intervention group had a mean (SD) score of 5.4 (0.6) during the ICU stay and 1.9 (0.25) at one week after discharge. The control group had a mean (SD) of 5.1 (0.5) during ICU stay and 4.8 (0.5) at one week after discharge.
Fleischer (2014) did not report this outcome measure.
7. Quality of life (important)
Demircelik (2016) did not report this outcome measure.
Fleischer (2014) found no statistically significant differences in quality of life between the intervention group (mean (SD): 40.6 (9.4)) and control group (mean (SD): 40.4 (10.0)) on the SF-12 PCS (adjusted mean difference (95% CI): 0.3 (-3.4 to 3.6), p = 0.87), SF-12 MCS (intervention group, mean (SD): 46.9 (11.3); control group, mean (SD): 48.2 (11.2); adjusted mean difference (95% CI): -1.3 (-5.3 to 2.6), p = 0.50) and SEIQoL (intervention group, mean (SD): 74.9 (18.2); control group, mean (SD): 73.6 (20.1); adjusted mean difference (95% CI): 2.0 (-5.1 to 9.2), p = 0.58). These differences are considered not clinically relevant.
Level of evidence of the literature
The level of evidence regarding the outcome measures walking distance, cognitive functioning, PTSD and return to work could not be graded; no studies were found investigating these outcome measures.
RCTs start at high GRADE. The level of evidence regarding the outcome measure anxiety was downgraded by three levels because of the limited description of the methods used (risk of bias; -2) and the inclusion of only one study with a limited sample size (imprecision; -1). The level of evidence is very low.
The level of evidence regarding the outcome measure depression was downgraded by three levels because of the limited description of the methods used (risk of bias; -2) and the inclusion of only one study with a limited sample size (imprecision; -1). The level of evidence is very low.
The level of evidence regarding the outcome measure quality of life was downgraded by two level because of lack of blinding of the outcome assessment (risk of bias; -1) and the inclusion of only one study with a limited sample size (imprecision; -1). The level of evidence is low.
5. Optimizing the ICU environment
Description of studies
One study was included investigating optimizing the ICU environment. This study by Demoule (2017) was included in the review of Geense (2019). The effect of earplugs and eye masks during ICU-stay was investigated. The intervention was performed by trained nurses between 10:00 PM and 8:00 AM until discharge from the ICU. The control group received routine care during the night. Both the intervention group and control group consisted of 32 patients. Anxiety and depression were determined with the HADS and PTSD symptoms with the IES-R.
Results for interventions on optimizing the ICU environment
1. Walking distance (critical), 2. Cognitive functioning (critical), 6. Return to work (important), 7. Quality of life (important)
Demoule (2017) did not report these outcome measures.
3. Anxiety (critical)
Demoule (2017) found no statistically significant difference on HADS-A at day 90 following randomization (intervention group, median (IQR): 8 (4-11); control group, median (IQR): 6 (4-12), p = 0.69). The clinical relevance could not be determined because the data is non-parametric.
4. Depression (important)
Demoule (2017) found no statistically significant difference on HADS-D at day 90 following randomization (intervention group, median (IQR): 6 (3-12); control group, median (IQR): 6 (2-9), p = 0.63). The clinical relevance could not be determined because the data is non-parametric. However, this difference is likely not clinically relevant.
5. PTSD (important)
Demoule (2017) found no statistically significant difference in PTSD symptoms at day 90 following randomization (intervention group, median (IQR): 11 (5-18); control group, median (IQR): 16 (9-27), p = 0.15).
Level of evidence of the literature
The level of evidence regarding the outcome measures walking distance, cognitive functioning, return to work and quality of life could not be graded; no studies were found investigating these outcome measures.
RCTs start at high GRADE. The level of evidence regarding the outcome measure anxiety was downgraded by 3 levels because of the risk of bias (for instance risk of bias due to incomplete outcome data and due to selective outcome reporting ; -2), and the inclusion of only one study with a limited sample size (imprecision; -1). The level of evidence is very low.
The level of evidence regarding the outcome measure depression was downgraded by 3 levels because of risk of bias (for example risk of bias due to incomplete outcome data and due to selective outcome reporting; -2), and the inclusion of only one study with a limited sample size (imprecision; -1). The level of evidence is very low.
The level of evidence regarding the outcome measure PTSD was downgraded by 3 levels because of risk of bias (for example risk of bias due to incomplete outcome data and due to selective outcome reporting; -2), and the inclusion of only one study with a limited sample size (imprecision; -1). The level of evidence is very low.
Zoeken en selecteren
In order to answer the clinical question, a systematic literature analysis was carried out for the following search questions:
Search question 1. What are the effects of pharmacological interventions initiated in the ICU aiming at prevention of PICS in adult patients who are admitted to the intensive care unit?
P: adult patients (18 years or older) who are admitted to the intensive care unit
I: pharmacological interventions (medication) aiming at prevention of new or worsening of physical, cognitive and/or psychological complaints (symptoms of PICS) and initiated in the ICU
C: no treatment or placebo
O: walking distance, cognitive functioning, anxiety, depression, post-traumatic stress disorder (PTSD), return to work and quality of life
Search question 2.What are the effects of non-pharmacological interventions aimed at prevention of PICS in adult patients who are admitted to the intensive care unit when the intervention is initiated?
P: adult patients (18 years or older) who are admitted to the intensive care unit
I: non-pharmacological interventions (physical training, diaries, cognitive training, psychological counseling, optimizing the ICU environment, information and education, communication tools, behavior of health care providers) aiming at prevention of new or worsening of physical, cognitive and/or psychological complaints (symptoms of PICS) and initiated in the ICU
C: no treatment or usual care
O: walking distance, cognitive functioning, anxiety, depression, PTSD, return to work and quality of life
Relevant outcome measures
The guideline development group considered walking distance, cognitive functioning and anxiety as critical outcome measures for decision making and depression, PTSD, return to work and quality of life as important outcome measures for decision making.
The working group defined the outcome measures as follows:
- Cognitive functioning: as determined with a validated questionnaire or neuropsychological test to determine cognitive functioning.
- Anxiety, depression and PTSD: as determined with a validated questionnaire to identify anxiety, depression or PTSD, respectively.
- Quality of life: as determined with a validated quality of life questionnaire.
A priori, the working group did not define the outcome measures ‘walking distance’ and ‘return to work’ but applied the definitions used in the studies.
The working group defined 30 meters on the 6-minute walk test (Dajczman, 2015), 1.5 points on the Hospital Anxiety and Depression Scale (HADS) anxiety scale, 1.5 points on the HADS depression scale (Puhan, 2008), 3 points on the SF-36 PCS and 3 points on the SF-36 MCS (Frendl, 2014) as a minimal clinically (patient) important difference.
Outcome measures are included if measured after discharge from the hospital. If possible, they are subdivided into short term (after discharge from the hospital until 6 months after discharge) and long term (6 months or more after discharge from the hospital) effects.
Search and select (Methods)
For search question 1, the databases Medline (via OVID) and Embase (via Embase.com) were searched with relevant search terms until 15th of January 2020 for systematic reviews and randomized controlled trials (RCTs). The detailed search strategies are depicted under the tab Methods. This search yielded in 210 hits.
For search question 2, a recent systematic review was available (Geense, 2019). In addition, the databases Medline (via OVID) and Cinahl (via Ebsco) were searched with relevant search terms until 15th of January 2020 for systematic reviews and randomized controlled trials (RCTs) published in 2018 or later. The detailed search strategies are depicted under the tab Methods. This search yielded in 144 hits.
Studies were selected based on the following criteria: systematic reviews (searched in at least two databases, and detailed search strategy, risk of bias assessment and results of individual studies available) and RCTs answering one of the PICO’s above. In addition, at least twenty patients per study arm have had to be included.
For search question 1, 13 studies were initially selected based on title and abstract screening. After reading the full text, 12 studies were excluded (see the table with reasons for exclusion under the tab Methods) and one study was included.
For search question 2, 22 studies were initially selected based on title and abstract screening. After reading the full text, 18 studies were excluded (see the table with reasons for exclusion under the tab Methods). Four studies answered the second search question, in addition to the review of Geense (2019).
Results
One study (Needham, 2016) was included in the analysis of the literature for search question 1.
For search question 2, four studies (Eggmann, 2018; Garrouste-Orgeas, 2019; Nielsen, 2020 and Wade, 2019) were included besides the review from Geense, 2019. Given the large variation in studied interventions, we decided to classify the analysis of the literature for the second search question by intervention type: 1) physical training, 2) diaries, 3) psychological counseling, 4) information and education, and 5) optimizing the ICU environment. No literature has been found on interventions consisting of cognitive training, communication tools and behavior of health care providers. Important study characteristics and results are summarized in the evidence tables. The assessment of the risk of bias is summarized in the risk of bias tables.
Referenties
- Dajczman, E., Wardini, R., Kasymjanova, G., Préfontaine, D., Baltzan, M. A., & Wolkove, N.
(2015). Six minute walk distance is a predictor of survival in patients with chronic
obstructive pulmonary disease undergoing pulmonary rehabilitation. Canadian respiratory journal, 22(4), 225-229. - Demircelik, M. B., Cakmak, M., Nazli, Y., Şentepe, E., Yigit, D., Keklik, M., ... & Eryonucu, B.
(2016). Effects of multimedia nursing education on disease-related depression and anxiety in patients staying in a coronary intensive care unit. Applied Nursing Research, 29, 5-8. - Demoule, A., Carreira, S., Lavault, S., Pallanca, O., Morawiec, E., Mayaux, J., ... & Similowski,
T. (2017). Impact of earplugs and eye mask on sleep in critically ill patients: a prospective randomized study. Critical Care, 21(1), 284. - Denehy, L., Skinner, E. H., Edbrooke, L., Haines, K., Warrillow, S., Hawthorne, G., ... &
Berney, S. (2013). Exercise rehabilitation for patients with critical illness: a randomized controlled trial with 12 months of follow-up. Critical Care, 17(4), R156. - Eggmann, S., Verra, M. L., Luder, G., Takala, J., & Jakob, S. M. (2018). Effects of early,
combined endurance and resistance training in mechanically ventilated, critically ill patients: A randomised controlled trial. PloS one, 13(11), e0207428. - Fleischer, S., Berg, A., Behrens, J., Kuss, O., Becker, R., Horbach, A., & Neubert, T. R. (2014).
Does an additional structured information program during the intensive care unit stay reduce anxiety in ICU patients?: a multicenter randomized controlled trial. BMC anesthesiology, 14(1), 48. - Frendl, D. M., & Ware Jr, J. E. (2014). Patient-reported functional health and well-being
outcomes with drug therapy: a systematic review of randomized trials using the SF-36 health survey. Medical care, 439-445. - Garrouste-Orgeas, M., Flahault, C., Vinatier, I., Rigaud, J. P., Thieulot-Rolin, N., Mercier, E.,
... & Hamidfar, R. (2019). Effect of an ICU diary on posttraumatic stress disorder symptoms among patients receiving mechanical ventilation: a randomized clinical trial. Jama, 322(3), 229-239. - Geense, W. W., van den Boogaard, M., van der Hoeven, J. G., Vermeulen, H., Hannink, G., &
Zegers, M. (2019). Nonpharmacologic interventions to prevent or mitigate adverse
long-term outcomes among ICU survivors: a systematic review and meta-analysis. Critical care medicine, 47(11), 1607-1618. - Hendriks, M. M. C., Janssen, F.A.M., Te Pas, M.E., Kox, J. H. J. M., van de Berg, P.J.E.J.,
Bruise, M. P., de Bie, A. J. R. (2019). Post-icu care after a long intensive care
admission: A Dutch inventory study. Translated Name Netherlands Journal of Critical Care, 27(5):190-5. - Hodgson, C. L., Bailey, M., Bellomo, R., Berney, S., Buhr, H., Denehy, L., ... & Papworth, R.
(2016). A binational multicenter pilot feasibility randomized controlled trial of early goal-directed mobilization in the ICU. Critical care medicine, 44(6), 1145-1152. - Jones, C., Bäckman, C., Capuzzo, M., Egerod, I., Flaatten, H., Granja, C., ... & Griffiths, R. D.
(2010). Intensive care diaries reduce new onset post traumatic stress disorder following critical illness: a randomised, controlled trial. Critical care, 14(5), R168. - KNMG (2021) Behandelprotocol Fysiotherapie op de intensive care. Beschikbaar via kngf.nl/kennisplatform
- Mendez-Tellez, P. A., & Needham, D. M. (2012). Early physical rehabilitation in the ICU and
ventilator liberation. Respiratory care, 57(10), 1663–1669. https://doi.org/10.4187/respcare.01931 - Morris, P. E., Berry, M. J., Files, D. C., Thompson, J. C., Hauser, J., Flores, L., ... & Bakhru, R.
N. (2016). Standardized rehabilitation and hospital length of stay among patients with acute respiratory failure: a randomized clinical trial. Jama, 315(24), 2694-2702. - Needham, D. M., Davidson, J., Cohen, H., Hopkins, R. O., Weinert, C., Wunsch, H., ... &
Brady, S. L. (2012). Improving long-term outcomes after discharge from intensive
care unit: report from a stakeholders' conference. Critical care medicine, 40(2), 502-509. - Needham, D. M., Colantuoni, E., Dinglas, V. D., Hough, C. L., Wozniak, A. W., Jackson, J. C.,
... & Hopkins, R. O. (2016). Rosuvastatin versus placebo for delirium in intensive care and subsequent cognitive impairment in patients with sepsis-associated acute respiratory distress syndrome: an ancillary study to a randomised controlled trial. The lancet Respiratory medicine, 4(3), 203-212. - Nielsen, A. H., Angel, S., Egerod, I., Lund, T. H., Renberg, M., & Hansen, T. B. (2020). The
effect of family-authored diaries on posttraumatic stress disorder in intensive care unit patients and their relatives: a randomised controlled trial (DRIP-study). Australian Critical Care, 33(2), 123-129. - Puhan, M. A., Frey, M., Büchi, S., & Schünemann, H. J. (2008). The minimal important
difference of the hospital anxiety and depression scale in patients with chronic
obstructive pulmonary disease. Health and quality of life outcomes, 6(1), 46. - Schaller, S. J., Anstey, M., Blobner, M., Edrich, T., Grabitz, S. D., Gradwohl-Matis, I., ... & Lee,
J. (2016). International Early SOMS-guided Mobilization Research Initiative: Early,
goal-directed mobilisation in the surgical intensive care unit: A randomised controlled trial. Lancet, 388(10052), 1377-1388. - Wade, D. M., Mouncey, P. R., Richards-Belle, A., Wulff, J., Harrison, D. A., Sadique, M. Z., ...
& Als, N. (2019). Effect of a nurse-led preventive psychological intervention on symptoms of posttraumatic stress disorder among critically ill patients: a randomized clinical trial. Jama, 321(7), 665-675. - Wright, S. E., Thomas, K., Watson, G., Baker, C., Bryant, A., Chadwick, T. J., ... & Stafford, V.
(2018). Intensive versus standard physical rehabilitation therapy in the critically ill (EPICC): a multicentre, parallel-group, randomised controlled trial. Thorax, 73(3), 213-221.
Evidence tabellen
Evidence table for intervention studies – pharmacological interventions
|
Study reference |
Study characteristics |
Patient characteristics |
Intervention (I) |
Comparison / control (C)
|
Follow-up |
Outcome measures and effect size |
Comments |
|
Needham, 2016 |
Type of study:
Setting and country: 35 hospitals in the USA
Funding and conflicts of interest: |
Inclusion criteria:
Exclusion criteria:
For the assessment of cognitive function, we also excluded patients
interview with the patient or their proxy) N total at baseline: Intervention: 164 Control: 165
Important prognostic factors2: Age ± SD: I: 52 (18) C: 53 (15)
Sex: I: 50% women C: 52% women APACHE II ± SD: I: 90 (27) C: 89 (28)
Groups comparable at baseline? Baseline patient and intensive care unit characteristics were similar in each group |
Rosuvastatin (40 mg loading dose and then 20 mg daily until the earliest of 3 days after discharge from intensive care, study day 28, or death) |
Matched placebo |
Length of follow-up
Intervention: N (%): 111 (67.7%) Reasons (describe) 12 died between discharge and initial follow-up 3 enrolled before addition of cognitive substudy 44 exclusion criteria for follow-up 7 missed follow-up at 6 months 23 ineligible for follow-up
Control: N (%): 88 (53.3%) Reasons (describe) 12 died between discharge and initial follow-up 2 enrolled before addition of cognitive substudy 31 exclusion criteria for follow-up 7 missed follow-up at 6 months 22 ineligible for follow-up
|
Cognitive functioning
Working memory and attention (mean Digit Span score, SD) I: 9.2 (2.5) p = 0.49
Immediate memory (mean Logical Memory I score, SD) I: 8.6 (3.4) C: 8.9 (3.3) p = 0.49
Delayed memory (mean Logical Memory II score, SD) I: 7.9 (3.3) C: 8.8 (2.7) Effect (95% CI): –1.2 (–2.2 to –0.2) p = 0.017
|
There were significant associations between assignment to rosuvastatin and worse delayed memory, with a worse mean continuous score and a greater percentage of patients with impaired delayed memory |
Evidencetable for systematic review of RCTs and observational studies (intervention studies) – non-pharmacological interventions
|
Study reference |
Study characteristics |
Patient characteristics |
Intervention (I) |
Comparison / control (C)
|
Follow-up |
Outcome measures and effect size |
Comments |
|
Geense, 2019
PS., study characteristics and results are extracted from the SR (unless stated otherwise) |
SR and meta-analysis of RCTs
Literature search up until July 19, 2018.
A: Denehy, 2013 B: Demircelik, 2016 C: Demoule, 2017 D: Fleischer, 2014 E: Hodgson, 2016 F: Jones, 2010 G: Morris, 2016 H: Schaller, 2016 I: Wright, 2018
Setting and country: F: UK, Denmark, Italy, Norway, Portugal, Sweden I: UK
Source of funding: Not reported for the individual studies
|
Inclusion criteria SR:
● Adult patients admitted to the ICU for at least 12 hours. ● Interventions performed before, during, or after ICU admission and aimed to prevent or mitigate long-term adverse outcomes. ● Outcomes measured after hospital discharge. Physical (e.g., pain, fatigue), mental (e.g., anxiety, depression), or cognitive (e.g., memory, attention) outcomes were included, as well as quality of life and outcomes such as social functioning and daily activities. ● The study design was a randomized controlled trial (RCT), non-RCT (NRCT), controlled before-after, or interrupted time series.
Exclusion criteria SR:
● Studies that included patients in the PICU, postanesthesia care unit, or coronary care unit ● Pharmacologic and nutritional interventions ● Outcomes related to healthcare utilization, costs, length of stay, ICU and hospital mortality, and readmissions
36 studies included, of which 9 could be included in this part of the literature summary.
We excluded two non-RCTs, three RCTs with an intervention which was started before ICU admission, 13 RCTs with interventions that started after ICU discharge, one RCT with an intervention that didn’t match our PICO, one RCT in which the control group received another intervention instead of no treatment or usual care, one RCT with outcome measures that didn’t match our PICO and six studies with less than twenty patients per study arm. |
A: Exercise rehabilitation program B: Multimedia informational education program F: Diary Duration and frequency of treatment A: Start day 5 > ICU admission (<15 min/ day). 7 d/w on ward(2x 30 min), 2x p/w for 8 weeks in outpatient setting (60 min) ICU discharge level: 30 to 60 min p/d F: Few min p/d (except for starting diary)
|
A: Usual care, may included active bed exercises, sitting out of bed/ marching/walking F: Diary (received diary after 3 mo)
|
A: Baseline; ICU discharge (ICU-D); hospital discharge (HD) to home; 3 mo; 6 mo; 12 mo F: 1 mo; 3 mo post ICU
|
Walking distance A: mean difference from usual care (95% CI) ICU discharge: −44.7 (−82.3 to −7.1) 3 mo: 15.4 (−40.1 to 71) 6 mo: −4.9 (−68.0 to 58.3) 12 mo: 4.7 (−59.7 to 69.2) B: not reported F: not reported I: adj. difference in means (95%CI) hospital discharge: −27.9 (−86.1 to 31.8) 6 mo: 61.7 (−47.2 to 157.0)
Anxiety D: not reported F: not reported
HADS-D A: not reported F: not reported G: not reported A: not reported I: 11 (5-18) E: not reported Intervention: 22.5 (14-84) Control: 25 (13-65) NS PTSS 14 at 3 months, median (range) Intervention: 24 (±12.2) Control: 24 (±11.6) Intervention: 70 (43.2) Intervention: 8 (5) Control: 21 (13.1) G: not reported 3 mo: mean (sd) 6 mo: mean (sd) 12 mo: mean (sd) B: not reported C: not reported D: not reported E: not reported F: not reported G: difference least square mean (95%CI) 6 mo: 3.4 (−0.02 to 7.0) H: not reported
3 mo: 6 mo: 12 mo: F: not reported HD: 0.3 (−2.7 to 3.3) p = 0.86 2 mo: 0.1 (−3.5 to 3.7) p = 0.96 p = 0.91 p = 0.19 3 mo: 4.2 (−1.2 to 9.5)
SF-36 A: not reported B: not reported C: not reported D: not reported E: not reported F: not reported H: 3 mo: group difference mean (95%CI) I: not reported
SF-12 B: not reported C: not reported D: SF-12 PCS, 3 mo after discharge: mean (SD) E: not reported F: not reported A: not reported B: not reported C: not reported D: 3 mo after discharge: mean (SD) E: not reported F: not reported
EQ-5D B: not reported C: not reported D: not reported E: EQ-5D VAS 6 months, mean (SD) p = 0.98 F: not reported G: not reported H: not reported 6 months, mean (SD) |
Risk of bias (ROB): Random sequence generation Low ROB: A, C, D, E, F, G, H, I, Unclear: B
Allocation concealment Low ROB: A, D, E, F, G, H Unclear: B, C, I High ROB: -
Blinding of patients and personnel High ROB: -
Blinding of outcome assessment Low ROB: A, G, I Unclear: B, C, E, F, H
Incomplete outcome data Low ROB: F High ROB: C, I
Selective reporting Unclear: B, G
Other bias Low ROB: B, D, G |
Evidence table for intervention studies – non-pharmacological interventions
|
Study reference |
Study characteristics |
Patient characteristics 2 |
Intervention (I) |
Comparison / control (C) 3
|
Follow-up |
Outcome measures and effect size 4 |
Comments |
|
Eggmann, 2018 |
Type of study: RCT
mixed ICU of an academic centre in Switzerland
|
Inclusion criteria: Adults (≥18 years) expected to stay on MV for at least 72 hours and who had been independent before the onset of critical illness. Independence was defined as living without assistance and qualitatively determined by the patient’s family or medical-health records.
previous muscle weakness, contraindications to cycling, enrolment in another intervention study, palliative care, admission diagnosis that excluded the possibility of walking at hospital discharge, and patients who did not understand German or French N total at baseline: Intervention: 58 Control: 57
Important prognostic factors2: age ± SD: I: 65±15 Sex: I: 38% female C: 28% female
Groups comparable at baseline?
|
Early, progressive endurance and resistance training programme combined with early mobilisation. Sedation was reduced if medically permitted prior to physiotherapy |
Both groups received standard ICU care with protocol-guided sedation, weaning and nutrition. Patients in the control group received European standard physiotherapy including early mobilisation, respiratory therapy and passive or active exercises. |
Length of follow-up: 6 mo after hospital discharge
Loss-to-follow-up: Intervention: N (%): 24 (41.4) Reasons (describe)
Control: 33 (57.9) N (%) Reasons (describe) |
Outcome measures and effect size (include 95%CI and p-value if available): p = 0.520 |
Except for mental health (p = 0.023), there were no significant differences in quality of life at 6-months after hospital discharge. Because of substantial missing data for the SF-36, characteristics for patients with and without the 6-month follow-up were compared. There were no significant differences between patients that completed the SF-36 versus patients that did not return the questionnaire |
|
Garrouste-Orgeas, 2019 |
Type of study: RCT
Funding and conflicts of interest: Network.
|
Inclusion criteria: Patients who were undergoing mechanical ventilation. Aged at least 18 years, receiving mechanical ventilation for at least 48 hours that was initiated within 48 hours of ICU admission, and have a family member present during the inclusion period and able to visit the patient during the ICU stay. Both the patient and family member had to have sufficient French-language skills for follow-up telephone interviews.
N total at baseline: Intervention: 332 Control: 325 Important prognostic factors2: age, median (IQR): I: 62.5 (49.5-70) C: 61 (51-70) Sex: I: 67.1% M C: 58.9% M
|
ICU diary completed by clinicians and family members of patients admitted to the ICU. Prior to patient transfer out of the ICU, a concluding note was written by an ICU clinician. The ICU diary was detailed to the patient in the ICU room a few days before discharge and was given to the patient or, if the patient was confused, to the family member who consented to the study at or as close as possible to the day of discharge. |
Usual ICU care with no ICU diary. |
Length of follow-up: Intervention: N (%): 168 (50.6) Reasons (describe) 139 died 6 Cognitive dysfunction 4 No IES-R score 3 Investigators were unable to contact Control: N (%): 150 (46.2) Reasons (describe) 116 died 15 Declined 1 No IES-R score 1 Investigators were unable to contact |
Anxiety (HADS-A) p = 0.72
The number of patients with symptoms of anxiety (HADS>8) was not statistically or clinically relevant different between the groups (intervention group n=51, 31.3%; control group n=53, 30.6%, risk difference: 0.7%, 95%CI -9 to 11%, p=0.91).
p = 0.66 The number of patients with symptoms of depression (HADS>8) was not statistically or clinically relevant different between the groups (intervention group n=31, 19%; control group n=41, 23.7%, risk difference: 5%, 95%CI -5 to 13%, p=0.35).
PTSD
The number of patients with an IES-R score >22 was not statistically or clinically relevant different between the groups (intervention group n=49, 29.9%; control group n=60, 34.3%, risk difference: -4%, 95%CI -15 to 6%, p=0.39).
|
|
|
Nielsen, 2020 |
Type of study: RCT Setting and country: Four mixed medical-surgical ICUs at two university hospitals and two regional hospitals in Western Denmark.
|
Inclusion criteria:
Exclusion criteria:
Intervention: 36 Control: 39
Important prognostic factors2: Age, median (IQR): I: 70 (27-88) C: 68 (18-89)
Sex: I: 49% M C: 59% M
Groups comparable at baseline? |
The relative was supplied with a hardcover notebook and guided by ICU nurses on how to write a diary for the ICU patient and later share the diary with the patient. |
Usual care (not diary intervention) |
Length of follow-up: Intervention: N (%): 10 (27.8) Reasons (describe) 3 excluded for LOS-ICU < 48 h or ventilator time < 24 h
Control: N (%): 17 (43.6) Reasons (describe) 1 excluded for LOS-ICU < 48 h or ventilator time < 24 h
|
Anxiety (HADS-A ≥ 11) RR 1.47 control group, number: 2/22 p = 1.0 Role physical C: 31.3 p = 0.25 Bodily pain I: 62.6 C: 52.7 p = 0.26 Global health I: 53.2 C: 47.0 p = 0.36 Vitality I: 48.5 C: 55.2 p = 0.47 Social function I: 68.8 C: 64.2 p = 0.46 Role emotional I: 49.3 C: 50.8 p = 0.94 Mental health I: 74.5 C: 74.0 p = 0.76 (difference (95% CI): 11.2% (-15.7 to 46.8%) p = 0.44
Actual cases, defined as PTSS-14 scores > 13 Relative risk (95% CI): p = 0.55 |
|
|
Wade, 2019 |
Type of study: |
Inclusion criteria: ICUs were eligible if they were active participants in the ICNARC Case Mix Programme, able to adhere to randomization and deliver the intervention, and could demonstrate potential to recruit to target.
Exclusion criteria: Stand-alone high-dependency units and specialist critical care units were excluded, along with ICUs offering formal psychological support (ICU diary use was permitted as not deemed an early intervention)
Intervention: 669 Control: 789
Important prognostic factors2: Intervention ICUs – baseline period APACHE II score, mean (SD): 17.7 (6.4)
age, mean (SD): 57.2 (16.2)
Groups comparable at baseline? |
Intervention ICUs delivered usual care during a baseline period followed by an intervention period.
|
Control ICUs delivered usual care in both baseline and intervention periods |
Length of follow-up: At follow-up, 978 patients (79.3%) who survived to 6 months completed questionnaires (range, 78.4% to 79.9% across treatment group and time period), with no difference in response rates or characteristics of responders between group or period 1 Ineligible
|
Anxiety (HADS-A) −1.50 to 1.01; p = 0.70)
|
|
Risk of bias table for intervention studies (randomized controlled trials) - pharmacological interventions
|
Study reference
|
Describe method of randomisation / (unlikely/likely/unclear) |
Bias due to inadequate concealment of allocation?
(unlikely/likely/unclear) |
Bias due to inadequate blinding of participants or care providers to treatment allocation?
(unlikely/likely/unclear) |
Bias due to inadequate blinding of outcome assessors to treatment allocation?
|
Bias due to incomplete outcome data?
(unlikely/likely/unclear) |
Bias due to selective outcome reporting on basis of the results?
(unlikely/likely/unclear) |
Bias due to violation of intention to treat analysis / other bias?
(unlikely/likely/unclear) |
|
Needham, 2016 |
Unlikely |
unlikely |
unlikely |
unlikely |
likely |
unlikely |
unlikely |
Risk of bias table for intervention studies (randomized controlled trials) - non-pharmacological interventions
|
Study reference
|
Describe method of randomisation / (unlikely/likely/unclear) |
Bias due to inadequate concealment of allocation?
(unlikely/likely/unclear) |
Bias due to inadequate blinding of participants or care providers to treatment allocation?
(unlikely/likely/unclear) |
Bias due to inadequate blinding of outcome assessors to treatment allocation?
|
Bias due to incomplete outcome data?
(unlikely/likely/unclear) |
Bias due to selective outcome reporting on basis of the results?
(unlikely/likely/unclear) |
Bias due to violation of intention to treat analysis / other bias?
(unlikely/likely/unclear) |
|
Denehy, 2013 (Geense, 2019) |
Unlikely |
unlikely |
unlikely |
unlikely |
unclear |
unlikely |
unclear |
|
Demircelik, 2016 (Geense, 2019) |
Unclear |
unclear |
unclear |
unclear |
unclear |
unclear |
unlikely |
|
Demoule, 2017 (Geense, 2019) |
Unlikely |
unclear |
unclear |
unclear |
likely |
likely |
likely |
|
Fleischer, 2014 (Geense, 2019) |
Unlikely |
unlikely |
unclear |
likely |
unclear |
likely |
unlikely |
|
Hodgson, 2016 (Geense, 2019) |
Unlikely |
unlikely |
unclear |
unclear |
unclear |
unlikely |
likely |
|
Jones, 2010 (Geense, 2019) |
Unlikely |
unlikely |
unclear |
unclear |
unlikely |
unlikely |
likely |
|
Morris, 2016 (Geense, 2019) |
Unlikely |
unlikely |
unclear |
unlikely |
unclear |
unclear |
unlikely |
|
Schaller, 2016 (Geense, 2019) |
Unlikely |
unlikely |
unlikely |
unclear |
unclear |
unlikely |
unclear |
|
Wright, 2018 (Geense, 2019) |
Unlikely |
unclear |
unclear |
unlikely |
likely |
likely |
unclear |
|
Eggmann, 2018 |
unlikely |
unlikely |
unclear |
unclear |
likely
loss-to follow-up was substantial. |
unlikely |
unlikely |
|
Garrouste-Orgeas, 2019 |
unlikely |
unlikely |
likely |
Unclear, the outcome assessor who contacted patients and family members was blind to the randomization group, however patients of course provided the answers to the questions. |
Unclear, there was some loss to follow-up, but the reasons for the loss of follow-up were only minimally described. |
unlikely |
unlikely |
|
Nielsen, 2020 |
|
Unclear, block randomisation, but not clear if multiple block sizes were used. Furthermore, unclear whether the envelops were sealed.
|
Likely (“blinding of participants and staff was not possible because of the nature of the intervention”) |
Likely (“blinding of participants and staff was not possible because of the nature of the intervention”) |
likely. Only 64% of relatives completed the questionnaire. The % females in the analysed control group was higher when compared to the percentage in the intervention group and the median age was lower. Nielsen: “As lower age and female gender are risk factors for development of PTSD, the possibility of an overestimation of positive effects of the diary should at least be considered”. Furthermore, reasons for not responding partially unknown, and there were more non-responders in the control group.
|
unlikely |
Likely, patients lost to follow-up were not included in the analysis. There was some contamination. |
|
Wade, 2019 |
unlikely |
unclear |
likely |
unclear |
unclear |
unlikely |
unlikely |
Table of quality assessment for systematic reviews of RCTs and observational studies
Based on AMSTAR checklist (Shea et al.; 2007, BMC Methodol 7: 10; doi:10.1186/1471-2288-7-10) and PRISMA checklist (Moher et al 2009, PLoS Med 6: e1000097; doi:10.1371/journal.pmed1000097)
|
Study
First author, year |
Appropriate and clearly focused question?
Yes/no/unclear |
Comprehensive and systematic literature search?
Yes/no/unclear |
Description of included and excluded studies?
Yes/no/unclear |
Description of relevant characteristics of included studies?
Yes/no/unclear |
Appropriate adjustment for potential confounders in observational studies?
Yes/no/unclear/not applicable |
Assessment of scientific quality of included studies?
Yes/no/unclear |
Enough similarities between studies to make combining them reasonable?
Yes/no/unclear |
Potential risk of publication bias taken into account?
Yes/no/unclear |
Potential conflicts of interest reported?
Yes/no/unclear |
|
Geense, 2019 |
Yes |
Yes |
Yes |
No, description of the patients is not described for the individual studies |
Not applicable |
Yes |
Unclear |
Yes |
Yes |
Exclusietabel – farmacologische interventies
Tabel Exclusie na het lezen van het volledige artikel
|
Auteur en jaartal |
Redenen van exclusie |
|
Fernandez-Gonzalo, 2018 |
Zoekstrategie en risk of bias beoordeling zijn niet gerapporteerd. |
|
Jackson, 2018 |
Patiënten verblijven niet op de IC bij initiëren van de maatregel. |
|
Marik, 2018 |
Zoekstrategie en risk of bias beoordeling zijn niet gerapporteerd. |
|
Matsuoka, 2015 |
Geen farmacologische interventie |
|
Page, 2017 |
Bevat geen uitkomstmaten uit onze PICO. |
|
Patel, 2014 |
Bevat geen uitkomstmaten uit onze PICO. |
|
Roberts, 2018 |
Review zonder kwantitatieve data (kwalitatieve review). |
|
Rozendaal, 2009 |
Bevat geen uitkomstmaten uit onze PICO. |
|
Schelling, 2004 |
Patiënten verblijven niet op de IC bij initiëren van de maatregel. |
|
Shephard, 2016 |
Review met fysiek functioneren als uitkomstmaten, maar geen loopafstand. |
|
Treggiari, 2009 |
Geen farmacologische interventie |
|
Weis, 2006 |
Patiënten verblijven niet op de IC bij initiëren van de maatregel. |
Exclusietabel – niet-farmacologische interventies
Tabel Exclusie na het lezen van het volledige artikel
|
Auteur en jaartal |
Redenen van exclusie |
|
Barreto, 2019 |
Review is minder compleet dan Geense, enkel het effect van dagboeken is onderzocht. |
|
Birk, 2019 |
Review gaat niet specifiek voor IC-patiënten. |
|
Costigan, 2019 |
Review zonder kwantitatieve data (scoping review). |
|
Doiron, 2018 |
Review is minder breed dan Geense, alleen op mobiliseren en activeren. |
|
Fuke, 2018 |
Review gaat over vroege revalidatie, niet specifiek tijdens de IC. |
|
Kalfon, 2019 |
Geen review: observationeel cohort. |
|
Kredentser, 2018 |
Minder dan 20 patiënten per studiegroep. |
|
Lewis, 2018 |
Review is minder breed dan Geense, alleen op informatie en voorlichting. |
|
McIlroy, 2018 |
Review neemt studies anders dan RCT mee (observationeel), de vermelde RCT’s staan ook in Geense. |
|
Mouncey, 2019 |
Artikel ontbreekt |
|
Nuwi, 2018 |
Review vermeld niet de resultaten van de individuele studies. |
|
Roberts, 2018 |
Review zonder kwantitatieve data (kwalitatieve review). |
|
Rosa, 2019 |
Review over interventies na ontslag van de IC (post-ICU follow-up interventions). |
|
Schmidt, 2019 |
Patiënten verblijven niet op de IC bij initiëren van de maatregel. |
|
Wollersheim, 2019 |
Minder dan 20 patiënten per studiegroep. |
|
Zang, 2019 |
Review bevat geen uitkomstmaten uit onze PICO. |
|
Zayed, 2019 |
Review bevat geen uitkomstmaten uit onze PICO. |
|
Zhang, 2019 |
Review is minder breed dan Geense, alleen op vroege mobilisatie. |
Verantwoording
Beoordelingsdatum en geldigheid
Publicatiedatum : 20-09-2022
Beoordeeld op geldigheid : 19-09-2022
Herbeoordeling module Identificatie van patiënten met risico op PICS - 2025
Herbeoordeling modules Preventie van PICS en
Preventie van PICS-F, Screeningsinstrumenten, Organisatie van zorg - 2027
Algemene gegevens
Autorisatie van de VRA onder voorbehoud van bekrachtiging tijdens de ALV.
De ontwikkeling van deze richtlijnmodule werd ondersteund door het Kennisinstituut van de Federatie Medisch Specialisten (www.demedischspecialist.nl/kennisinstituut) en werd gefinancierd uit de Stichting Kwaliteitsgelden Medisch Specialisten (SKMS). De financier heeft geen enkele invloed gehad op de inhoud van de richtlijnmodule.
Samenstelling werkgroep
Voor het ontwikkelen van de richtlijnmodule is in 2019 een multidisciplinaire werkgroep en klankbordgroep ingesteld, bestaande uit vertegenwoordigers van alle relevante specialismen (zie hiervoor de Samenstelling van de werkgroep) die betrokken zijn bij de zorg voor (voormalig) IC-patiënten.
Werkgroep
- Prof. dr. D. van Dijk, intensivist, NVIC (voorzitter)
- Dr. H.R. Holtslag, revalidatiearts, VRA (voorzitter)
- Dr. L.L.A. Bisschops, internist-intensivist, NIV
- Dr. M. van den Boogaard, senior onderzoeker, V&VN
- Drs. M.A.E.A. Brackel, voorzitter patiëntorganisatie IC Connect (tot 1 januari 2022), jeugdarts niet praktiserend
- Drs. H. van Dis, klinisch psycholoog, epidemioloog, NIP
- Dhr. R. Klerks, patiëntvertegenwoordiger, stichting FCIC
- Dr. M.M.C. van Mol, postdoc onderzoeker, psycholoog en IC Verpleegkundige, V&VN
- Dr. M. van der Schaaf, lector Revalidatie in de Acute Zorg en senior onderzoeker, KNGF
- Drs. M.S. van der Steen, internist-intensivist, NVIC
- Dr. M.Ch.E. van de Woude, anesthesioloog-intensivist, NVA
Klankbordgroep
- Drs. M.W. Brouwer, GZ-psycholoog, NIP
- Mw. N.A. Bruins, internist-intensivist, NIV
- Dr. D.S. Dettling-Ihnenfeldt, fysiotherapeut/onderzoeker, KNGF
- Drs. M.P.M. Dremmen, revalidatiearts, VRA
- Dr. H. Endeman, internist-intensivist, NIV
- Mw. S. op ’t Hoog, verpleegkundig specialist ICU en voorzitter netwerkgroep V&VN VS ICU (tot februari 2022)
- Mw. M.W.J.C. Prins-Smulders, verpleegkundig specialist ICU, V&VN ICU (vanaf februari 2022)
- Dr. K.A.J. Kuijpers, revalidatiearts, VRA
- Dr. F.A. van de Laar, huisarts
- Drs. A.J. Meinders, internist-intensivist, NIV
- Dr. A.F.C. Schut, intensivist, NIV
- Mw. M. Siebel, patiëntorganisatie IC Connect
- Dr. G.J. Versteegen, klinisch psycholoog, NIP
- Dr. L.C.M. Vloet, lector Acute Intensieve zorg HAN, voorzitter FCIC
- Prof. dr. A.R.H. van Zanten, internist-intensivist, NIV
Met ondersteuning van
- Dr. G. Peeters, senior adviseur Kennisinstituut van de Federatie Medisch Specialisten (tot november 2019)
- Dr. S. Persoon, senior adviseur, Kennisinstituut van de Federatie Medisch Specialisten (vanaf november 2019)
- Dr. V.C.M. Cox, adviseur, Kennisinstituut van de Federatie Medisch Specialisten (vanaf februari 2020 tot november 2020)
- Drs. M.E. Wessels, medisch informatiespecialist Kennisinstituut van de Federatie Medisch Specialisten
- Dr. S.N. Hofstede, senior adviseur, Kennisinstituut van de Federatie Medisch Specialisten (vanaf april 2022)
Belangenverklaringen
De Code ter voorkoming van oneigenlijke beïnvloeding door belangenverstrengeling is gevolgd. Alle werkgroepleden hebben schriftelijk verklaard of zij in de laatste drie jaar directe financiële belangen (betrekking bij een commercieel bedrijf, persoonlijke financiële belangen, onderzoeksfinanciering) of indirecte belangen (persoonlijke relaties, reputatiemanagement) hebben gehad. Gedurende de ontwikkeling of herziening van een module worden wijzigingen in belangen aan de voorzitter doorgegeven. De belangenverklaring wordt opnieuw bevestigd tijdens de commentaarfase.
Een overzicht van de belangen van werkgroepleden en het oordeel over het omgaan met eventuele belangen vindt u in onderstaande tabel. De ondertekende belangenverklaringen zijn op te vragen bij het secretariaat van het Kennisinstituut van de Federatie Medisch Specialisten.
Werkgroep
|
Werkgroeplid |
Functie |
Nevenfuncties |
Gemelde belangen |
Ondernomen actie |
|
Dijk, van |
Hoogleraar IC geneeskunde UMC Utrecht |
Geen |
Geven van voordrachten voor de stichting Family and Patient centered Intensive Care (FCIC) (onbetaald) |
Geen |
|
Holtslag |
Revalidatiearts, opleider & chef de polikliniek, Amsterdam UMC, locatie AMC |
Lid Raad van Toezicht van De Zorgcirkel (betaald) Adviseur NOC*NSF, Papendal, Arnhem, (betaald) |
IC-revalidatie is een onderwerp waar onze afdeling (Revalidatie) belang aan hecht, er wordt op onze afdeling ook onderzoek naar gedaan |
Geen |
|
Bisschops |
Internist-intensivist afdeling intensive care Radboudumc |
Geen |
Geen |
Geen |
|
Boogaard, van den |
Senior onderzoeker afdeling lntensive Care Radboudumc en projectleider MONITOR-IC
|
Onbezoldigde functies: - Bestuurslid European Delirium Association - Adviseur Network for lnvestigation of Delirium: Unifying Scientists (NIDUS) - Organisator IC-café regio Nijmegen & Omstreken - Lid werkgroep Longterm Outcome and ICU Delirium van de European Society of lntensive Care Medicine |
Projectleider MONITOR-lC (langetermijngevolgen lC-opname project; multicenter). De MONITOR-IC studie is niet door de industrie gesponsord.
Studie naar de effectiviteit van gestructureerde en gepersonaliseerde IC-nazorg. |
Geen trekker/meelezer bij de module organisatie van zorg. Geen verdere actie. |
|
Brackel-Welten |
Voorzitter IC Connect, patientenorganisatie voor (voormalig) IC-patienten en naasten, tot 1 januari 2022 (vrijwilligerswerk)
Bestuurslid Stichting Family and patient Centered Intensive Care (FCIC), tot 1 januari 2022 (vrijwilligerswerk)
Jeugdarts KNMG niet praktiserend |
Geen |
Geen |
Geen |
|
Dis, van |
Gepensioneerd Afdeling Medische Psychologie en Psychiatrie, Onze Lieve Vrouwe Gasthuis, Amsterdam en Afdeling Psychologie, Brein en Cognitie, van de Universiteit van Amsterdam
Thans Gastdocent Medische Psychologie & Farmacopsychologie, Afdeling Psychologie, Brein & Cognitie, Universiteit van Amsterdam, Roeterseiland Campus, Nieuwe Achtergracht 126 B, 1018 WT, Amsterdam
Docent RINO-Amsterdam (postacademische onderwijs GGZ), Leidseplein 5, Amsterdam |
Nederlandse Vereniging Gezondheidszorgpsychologie en haar specialismen (NVGzP): secretaris bestuur, Vz Cie Kwaliteit, Vz Cie Somatiek (onbetaald).
Landelijke Vereniging Medische Psychologie: Beroepsbelangen, Vz Werkgroep Farmacopsychologie, Werkgroep Slaap (onbetaald) |
Geen |
Geen |
|
Klerks |
Patiëntenvertegenwoordiger/ ervaringsdeskundige naaste, FCIC |
Geen |
Geen |
Geen |
|
Mol, van |
Onderzoeker Psycholoog en IC-verpleegkundige Erasmus MC (1 fte) |
Vrijwilliger Stichting FCIC Secretaris
Vacatiegeld en reiskostenvergoeding |
Geen |
Geen |
|
Schaaf, van der |
Associate professor Amsterdam UMC (afd. revalidatiegeneeskunde) en Hogeschool van Amsterdam |
Vrijwilliger Stichting FCIC (onbetaald) Bestuurslid REACH netwerk (onbetaald) |
Geen |
Geen |
|
Steen, van der |
lnternist-lntensivist Pantein Ziekenhuis Beugen
Associate professor Amsterdam UMC (afd. revalidatiegeneeskunde) en Hogeschool van Amsterdam |
CVO van vereniging voor Ostheopatie (onkostenvergoeding)
CMIO netwerk (onbezoldigd)
Vrijwilliger FCIC en coorzitter Stichting REACH netwerk (onbezoldigd) |
ZonMw Project Citizen Health; projectleider (betaald/onkostenvergoeding) DIGNIC project; Digitale gepersonaliseerde Nazorg na een opname op de Intensive Care Afdeling. |
Geen |
|
Woude, van der |
Anesthesioloog-Intensivist Zuyderland Medisch Centrum 0,6 FTE Medisch manager RVE Verpleegcentrum Zuyderland 0,3 FTE |
Geen |
Geen |
Geen |
|
Peeters (tot 11-2019) |
Senior adviseur/teamleider Kennisinstituut van de Federatie Medisch Specialisten |
Senior Atlantic Fellow for Equity in Brain Health, Global Brain Health Institute, Trinity College Dublin (onderzoeker; onbetaald).
Verzorgen van onderwijs voor de epidemiologische onderwerpen in het curriculum voor de eerstejaars fellows in het programma van de Global Brain Health Institute. |
Functie bij het Global Brain Health Institute: Er zijn soms financiële voordelen in de vorm van vergoeding van registratie en reiskosten voor congresbezoek.
|
Geen |
|
Persoon (vanaf 11-2019) |
Adviseur Kennisinstituut van de Federatie Medisch Specialisten |
Tot oktober 2018 Gastvrijheidsaanstelling afdeling Revalidatie Academisch Medisch Centrum, Amsterdam, in verband met promotietraject. Project: Physical fitness to improve fitness and combat fatigue in patients with multiple myeloma or lymphoma treated with high dose chemotherapy.
April 2018-september 2018: Docent Team Technologie, Fontys Paramedische Hogeschool. Begeleiden van studenten bij afstudeerstages. Max 1 dag in de week, betaald. |
Geen promotieonderzoek werd gefinancierd door KWF, financier had geen invloed op uitkomsten onderzoek of op huidige werkzaamheden. |
Geen |
|
Cox (vanaf 02-2020) |
Adviseur Kennisinstituut van de Federatie Medisch Specialisten |
Promotieonderzoek, getiteld: “Partners of patients with acquired brain injury – impact dyadic relationships and support”
Gefinancierd door ZonMW, projectnummer #630000002 CARE4BRAIN: personalized CAREgiver and patient support in rehabilitation FOR patients with acquired BRAIN Deficits |
Geen, financier promotieonderzoek had geen invloed op de uitkomsten onderzoek of op de huidige werkzaamheden. |
Geen |
Klankbordgroep
|
Klankbord-groeplid |
Functie |
Nevenfuncties |
Gemelde belangen |
Ondernomen actie |
|
Brouwer Mengerink |
GZ-psycholoog, St. Antoniusziekenhuis |
Geen |
Geen |
Geen |
|
Bruins
|
Internist-intensivist Medisch Centrum Leeuwarden
|
Commissie Nazorg NVIC (onbetaald).
Medisch manager ZE Acuut. 0,2 FTE |
Ondersteunt lopend onderzoek naar het herstel van IC patiënten, niet als hoofdonderzoeker. |
Geen |
|
Dettling-Ihnenfeldt
|
Fysiotherapeut/onderzoeker AmsterdamUMC, locatie AMC
|
Secretaris Stichting REACH netwerk (onbetaald) |
Geen |
Geen |
|
Dremmen
|
Revalidatiearts Zuyderland Medisch Centrum Heerlen - Sittard-Geleen. Voorheen via Adelante Zorggroep |
Geen |
Geen |
Geen |
|
Endeman
|
Internist-intensivist, Erasmus MC, Rotterdam |
Geen |
Geen |
Geen |
|
Op ’t Hoog (tot februari 2022) |
Verpleegkundig specialist ICU Elisabeth Tweesteden-ziekenhuis
|
Voorzitter V&VNVS ICU netwerk (onbetaald).
Lid Verpleegkundige Advies Raad Elisabeth Tweesteden-ziekenhuis – vanuit loondienst.
Lid Dagelijks bestuur Vakgroep Verpleegkundig Specialisten Elisabeth Tweesteden-ziekenhuis (onbetaald). |
Geen |
Geen |
|
Kuijpers |
Revalidatiearts, Medisch spectrum Twente |
Geen |
Geen |
Geen |
|
Laar, van de |
Huisarts (0,6 fte), onderzoeker/docent Academisch Gezondheidscentrum Thermion (0,4 fte), Radboud UMC, afdeling eerstelijns geneeskunde |
Geen |
Werkt samen in onderzoek naar post-IC patiënten (en familie) met onderzoekers IC RaboudUMC ( MiCare studie) |
Geen |
|
Meinders |
Internist-intensivist St Antonius ziekenhuis, Utrecht |
Geen |
Geen |
Geen |
|
Prins-Smulders (vanaf februari 2022) |
Verpleegkundig specialist ICU Elisabeth Tweesteden-ziekenhuis
|
Vrijwilliger IC-connect FCIC (onbetaald) |
Geen |
Geen |
|
Schut |
Intensivist Ikazia ziekenhuis Rotterdam |
Geen |
Geen |
Geen |
|
Siebel |
Ervaringsdeskundige patiëntenorganisatie ICConnect |
Geen |
Geen |
Geen |
|
Versteegen |
Klinisch psycholoog, UMCG |
Plaatsvervangend Hoofdopleider Psychotherapie, PPO, Groningen (0,1 fte) (mede)verantwoordelijk voor de opleiding tot Psychotherapeut |
Geen |
Geen |
|
Vloet
|
Voorzitter FCIC, vrijwilligersfunctie |
Bestuurslid Venticare (onbetaald) |
Vanuit lectoraat diverse gesubsidieerde trajecten op IC gebied. Momenteel geen op gebied van nazorg. |
Geen |
|
Zanten, van |
lnternist-intensivist, Medisch Hoofd & Opleider, afdeling lntensive Care, Ziekenhuis Gelderse Vallei, Ede (0.8 FTE)
Buitengewoon Hoogleraar Wageningen Univeristy & Research, Wageningen (0.2 FTE) |
Lid GIC, Lid platform Kwaliteit NlV, Lid ESPEN richtlijn commissie Voeding volwassen lC patienten, Lid MEN sectie ESICM, working group gastrointestinal failure Lid SKMS/ZonMw richtlijn Voeding en IC, Lid klankbordgroep SKMS/ZonMw richtlijn Nazorg en Revalidatie IC, Lid diverse congrescommissies (Nationale Voedingscongres, lnternational Sepsis Symposium Netherlands, Mythen Missers en Maatwerk lnfectieuze bedreigingen,Mythen Missers en Maatwerk Circulatie, Mythen Missers en Maatwerk Beademing, Begrenzingen Intensive Care, Masterclass lC Schiermonnikoog, Masterclass Voeding en IC)
Betaald: Medisch Lid METC-Oost, kamer 4 WUR. Expertiserapportages: Medirisk, Hofman Letselschade, Centramed, Rechtbank Midden-Nederland spreker/onkostenvergoedingen bovengenoemde congressen in NL. Spreker/onkostenvergoedingen internationale congressen: ISICEM, ESICM, ESPEN, ASPEN, CSPEN, IrSPEN, PENSA, BAPEN, BRASPEN, CSCCM, Middle-East Nutrition Summit. |
Lezingen, consultancy, reiskostenvergoedingen voor bedrijven in afgelopen 5 jaar: Abbott, AOP Pharma (Amomed), BBraun, Baxter, Cablon Medical, Cardinal Health, Danone-Nutricia, DIM3, Fresenius-Kabi, Lyric, Mermaid, Nestle-Novartis. Rousselot.
Partner die Congresbureau Interactie voert.
Scholingen in acute zorg en IC geneeskunde.
Participatie als onderzoeker in lC sepsis trials (b.v. AKPA-ART 123 trombomoduline, adrenomedulline trial, Revival trial), VitaCCa trial (OHCA, vitamine C), opbrengsten vloeien naar Stichting lC research en worden niet uitgekeerd aan onderzoekers. Zelf geinitieerd onderzoek: VIRS-studie, (investigator initiated registry argipressine bij septische shock, grant AOP Pharma), Protein supplement RCT post ICU (grant Rousselot), nutrition intake post ICU (Valifood study, Prospect I&II study, grant Nutricia).
Gemelde belangen zijn niet relevant voor deze richtlijnmodule. |
Geen |
Inbreng patiëntenperspectief
Er werd aandacht besteed aan het patiëntenperspectief door participatie van een afgevaardigde in de werkgroep vanuit Stichting FCIC en een afgevaardigde vanuit patiëntorganisatie IC Connect. De conceptmodule is tevens voor commentaar voorgelegd aan Stichting FCIC, patiëntorganisatie IC Connect en de Patiëntenfederatie Nederland.
Methode ontwikkeling
Evidence based
Implementatie
|
Aanbeveling |
Tijdspad voor implementatie: 1-3 jaar of >3 jaar |
Verwacht effect op kosten |
Randvoorwaarden voor implementatie (binnen aangegeven tijdspad) |
Mogelijke barrières voor implementatie1 |
Te ondernemen acties voor implementatie2 |
Verantwoordelijken voor acties3 |
Overige opmerkingen |
|
Alle aanbevelingen uit deze module |
<1 jaar, de meeste aanbevelingen zijn al op veel intensive cares geïmplementeerd. |
Nauwelijks, de meeste aanbevelingen zijn al op veel intensive cares geïmplementeerd. |
- |
- |
- |
Zorgverleners op de IC. |
- |
Werkwijze
AGREE
Deze richtlijnmodule is opgesteld conform de eisen vermeld in het rapport Medisch Specialistische Richtlijnen 2.0 van de adviescommissie Richtlijnen van de Raad Kwaliteit. Dit rapport is gebaseerd op het AGREE II instrument (Appraisal of Guidelines for Research & Evaluation II; Brouwers, 2010).
Kwalitatieve raming van mogelijke financiële gevolgen in het kader van de Wkkgz
Bij de richtlijn is conform de Wet kwaliteit, klachten en geschillen zorg (Wkkgz) een kwalitatieve raming uitgevoerd of de aanbevelingen mogelijk leiden tot substantiële financiële gevolgen. Bij het uitvoeren van deze beoordeling zijn richtlijnmodules op verschillende domeinen getoetst (zie het stroomschema op de Richtlijnendatabase).
Uit de kwalitatieve raming blijkt dat er waarschijnlijk geen substantiële financiële gevolgen zijn, zie onderstaande tabel.
|
Module |
Uitkomst kwalitatieve raming |
Toelichting |
|
Module Preventie van PICS |
Waarschijnlijk geen substantiële financiële gevolgen |
Hoewel uit de toetsing volgt dat de aanbeveling(en) breed toepasbaar zijn (>40.000 patiënten), volgt ook uit de toetsing dat het geen nieuwe manier van zorgverlening of andere organisatie van zorgverlening betreft en het overgrote deel (±90%) van de zorgaanbieders en zorgverleners al aan de norm voldoet. Er worden daarom geen substantiële financiële gevolgen verwacht. |
|
Module Preventie PICS-F |
Waarschijnlijk geen substantiële financiële gevolgen |
Hoewel uit de toetsing volgt dat de aanbeveling(en) breed toepasbaar zijn (>40.000 patiënten), volgt ook uit de toetsing dat het geen nieuwe manier van zorgverlening of andere organisatie van zorgverlening betreft die substantiële financiële investering vraagt. |
|
Module Identificatie van patiënten met risico op PICS |
Waarschijnlijk geen substantiële financiële gevolgen |
Hoewel uit de toetsing volgt dat de aanbeveling(en) breed toepasbaar zijn (>40.000 patiënten), volgt ook uit de toetsing dat het geen nieuwe manier van zorgverlening of andere organisatie van zorgverlening betreft die substantiële financiële investering vraagt. |
|
Module Screeningsinstrumenten voor PICS |
Waarschijnlijk geen substantiële financiële gevolgen |
Hoewel uit de toetsing volgt dat de aanbeveling(en) breed toepasbaar zijn (>40.000 patiënten), volgt ook uit de toetsing dat het geen nieuwe manier van zorgverlening of andere organisatie van zorgverlening betreft die substantiële financiële investering vraagt. |
|
Module Coördinatie en taken met betrekking tot de organisatie van IC-nazorg |
Waarschijnlijk geen substantiële financiële gevolgen |
Hoewel uit de toetsing volgt dat de aanbeveling(en) breed toepasbaar zijn (>40.000 patiënten), volgt ook het geen nieuwe manier van zorgverlening of andere organisatie van zorgverlening betreft die substantiële financiële gevolgen met zich meebrengt, dat er geen toename van >5% is in het aantal in te zetten voltijdsequivalenten aan zorgverleners en het geen wijziging in het opleidingsniveau van zorgpersoneel betreft. Er worden daarom geen substantiële financiële gevolgen verwacht. |
|
Module Overdracht klinische informatie in de keten |
Waarschijnlijk geen substantiële financiële gevolgen |
Hoewel uit de toetsing volgt dat de aanbeveling(en) breed toepasbaar zijn (>40.000 patiënten), volgt ook uit de toetsing dat het geen nieuwe manier van zorgverlening of andere organisatie van zorgverlening betreft die substantiële financiële investering vraagt. Er worden daarom geen substantiële financiële gevolgen verwacht. |
|
Module Informatievoorziening aan en communicatie met de patiënt en naasten |
Waarschijnlijk geen substantiële financiële gevolgen |
Hoewel uit de toetsing volgt dat de aanbeveling(en) breed toepasbaar zijn (>40.000 patiënten), volgt ook uit de toetsing dat het geen nieuwe manier van zorgverlening of andere organisatie van zorgverlening betreft die substantiële financiële investering vraagt. Er worden daarom geen substantiële financiële gevolgen verwacht. |
Knelpuntenanalyse en uitgangsvragen
Tijdens de voorbereidende fase inventariseerde de werkgroep de knelpunten met betrekking tot de preventie, herkenning en behandeling van PICS. Tevens zijn er knelpunten aangedragen door Inspectie Gezondheidszorg en Jeugd (IGJ), Koninklijk Nederlands Genootschap voor Fysiotherapie (KNGF), Nederlandse Internisten Vereniging (NIV), NVIC, VRA, Nederlandse Vereniging voor Klinische Geriatrie (NVKG), Nederlandse Vereniging voor Heelkunde (NVvH), Nederlandse Vereniging voor Psychiatrie (NVvP), Stichting FCIC, IC Connect en Verpleegkundigen en Verzorgenden Nederland - Intensive Care (V&VN-IC) tijdens een Invitational conference. Een verslag hiervan is opgenomen in de bijlagen.
Op basis van de uitkomsten van de knelpuntenanalyse zijn door de werkgroep concept-uitgangsvragen opgesteld en definitief vastgesteld.
Uitkomstmaten
Na het opstellen van de zoekvraag behorende bij de uitgangsvraag inventariseerde de werkgroep welke uitkomstmaten voor de patiënt relevant zijn, waarbij zowel naar gewenste als ongewenste effecten werd gekeken. Hierbij werd een maximum van acht uitkomstmaten gehanteerd. De werkgroep waardeerde deze uitkomstmaten volgens hun relatieve belang bij de besluitvorming rondom aanbevelingen, als cruciaal (kritiek voor de besluitvorming), belangrijk (maar niet cruciaal) en onbelangrijk. Tevens definieerde de werkgroep tenminste voor de cruciale uitkomstmaten welke verschillen zij klinisch (patiënt) relevant vonden.
Methode literatuursamenvatting
Een uitgebreide beschrijving van de strategie voor zoeken en selecteren van literatuur is te vinden onder ‘Zoeken en selecteren’ onder Onderbouwing. De beoordeling van de kracht van het wetenschappelijke bewijs wordt hieronder toegelicht.
Beoordelen van de kracht van het wetenschappelijke bewijs
De kracht van het wetenschappelijke bewijs werd bepaald volgens de GRADE-methode. GRADE staat voor ‘Grading Recommendations Assessment, Development and Evaluation’ (zie http://www.gradeworkinggroup.org). De basisprincipes van de GRADE-methodiek zijn: het benoemen en prioriteren van de klinisch (patiënt) relevante uitkomstmaten, een systematische review per uitkomstmaat en een beoordeling van de bewijskracht per uitkomstmaat op basis van de acht GRADE-domeinen (domeinen voor downgraden: risk of bias, inconsistentie, indirectheid, imprecisie en publicatiebias; domeinen voor upgraden: dosis-effect relatie, groot effect en residuele plausibele confounding).
GRADE onderscheidt vier gradaties voor de kwaliteit van het wetenschappelijk bewijs: hoog, redelijk, laag en zeer laag. Deze gradaties verwijzen naar de mate van zekerheid die er bestaat over de literatuurconclusie, in het bijzonder de mate van zekerheid dat de literatuurconclusie de aanbeveling adequaat ondersteunt (Schünemann, 2013; Hultcrantz, 2017).
|
Definitie |
|
|
Hoog |
|
|
Redelijk |
|
|
Laag |
|
|
Zeer laag |
|
Bij het beoordelen (graderen) van de kracht van het wetenschappelijk bewijs in richtlijnen volgens de GRADE-methodiek spelen grenzen voor klinische besluitvorming een belangrijke rol (Hultcrantz, 2017). Dit zijn de grenzen die bij overschrijding aanleiding zouden geven tot een aanpassing van de aanbeveling. Om de grenzen voor klinische besluitvorming te bepalen moeten alle relevante uitkomstmaten en overwegingen worden meegewogen. De grenzen voor klinische besluitvorming zijn daarmee niet één op één vergelijkbaar met het minimaal klinisch relevant verschil (Minimal Clinically Important Difference, MCID). Met name in situaties waarin een interventie geen belangrijke nadelen heeft en de kosten relatief laag zijn, kan de grens voor klinische besluitvorming met betrekking tot de effectiviteit van de interventie bij een lagere waarde (dichter bij het nul-effect) liggen dan de MCID (Hultcrantz, 2017).
Overwegingen (van bewijs naar aanbeveling)
Om te komen tot een aanbeveling zijn naast (de kwaliteit van) het wetenschappelijke bewijs ook andere aspecten belangrijk en worden meegewogen, denk bijvoorbeeld aan aanvullende argumenten uit bijvoorbeeld de biomechanica of fysiologie, waarden en voorkeuren van patiënten, kosten (middelenbeslag), aanvaardbaarheid, haalbaarheid en implementatie. Deze aspecten zijn systematisch vermeld en beoordeeld (gewogen) onder het kopje ‘Overwegingen’ en kunnen (mede) gebaseerd zijn op expert opinion. Hierbij is gebruik gemaakt van een gestructureerd format gebaseerd op het evidence-to-decision framework van de internationale GRADE Working Group (Alonso-Coello, 2016a; Alonso-Coello 2016b). Dit evidence-to-decision framework is een integraal onderdeel van de GRADE methodiek.
Formuleren van aanbevelingen
De aanbevelingen geven antwoord op de uitgangsvraag en zijn gebaseerd op het beschikbare wetenschappelijke bewijs en de belangrijkste overwegingen en een weging van de gunstige en ongunstige effecten van de relevante interventies. De kracht van het wetenschappelijk bewijs en het gewicht dat door de werkgroep wordt toegekend aan de overwegingen, bepalen samen de sterkte van de aanbeveling. Conform de GRADE-methodiek sluit een lage bewijskracht van conclusies in de systematische literatuuranalyse een sterke aanbeveling niet a priori uit en zijn bij een hoge bewijskracht ook zwakke aanbevelingen mogelijk (Agoritsas, 2017; Neumann, 2016). De sterkte van de aanbeveling wordt altijd bepaald door weging van alle relevante argumenten tezamen. De werkgroep heeft bij elke aanbeveling opgenomen hoe zij tot de richting en sterkte van de aanbeveling zijn gekomen.
In de GRADE-methodiek wordt onderscheid gemaakt tussen sterke en zwakke (of conditionele) aanbevelingen. De sterkte van een aanbeveling verwijst naar de mate van zekerheid dat de voordelen van de interventie opwegen tegen de nadelen (of vice versa), gezien over het hele spectrum van patiënten waarvoor de aanbeveling is bedoeld. De sterkte van een aanbeveling heeft duidelijke implicaties voor patiënten, behandelaars en beleidsmakers (zie onderstaande tabel). Een aanbeveling is geen dictaat, zelfs een sterke aanbeveling gebaseerd op bewijs van hoge kwaliteit (GRADE-gradering HOOG) zal niet altijd van toepassing zijn, onder alle mogelijke omstandigheden en voor elke individuele patiënt.
|
Implicaties van sterke en zwakke aanbevelingen voor verschillende richtlijngebruikers |
||
|
|
Sterke aanbeveling |
Zwakke (conditionele) aanbeveling |
|
Voor patiënten |
De meeste patiënten zouden de aanbevolen interventie of aanpak kiezen en slechts een klein aantal niet. |
Een aanzienlijk deel van de patiënten zouden de aanbevolen interventie of aanpak kiezen, maar veel patiënten ook niet. |
|
Voor behandelaars |
De meeste patiënten zouden de aanbevolen interventie of aanpak moeten ontvangen. |
Er zijn meerdere geschikte interventies of aanpakken. De patiënt moet worden ondersteund bij de keuze voor de interventie of aanpak die het beste aansluit bij zijn of haar waarden en voorkeuren. |
|
Voor beleidsmakers |
De aanbevolen interventie of aanpak kan worden gezien als standaardbeleid. |
Beleidsbepaling vereist uitvoerige discussie met betrokkenheid van veel stakeholders. Er is een grotere kans op lokale beleidsverschillen. |
Organisatie van zorg
In de knelpuntenanalyse en bij de ontwikkeling van de richtlijnmodule is expliciet aandacht geweest voor de organisatie van zorg: alle aspecten die randvoorwaardelijk zijn voor het verlenen van zorg (zoals coördinatie, communicatie, (financiële) middelen, mankracht en infrastructuur). Randvoorwaarden die relevant zijn voor het beantwoorden van deze specifieke uitgangsvraag zijn genoemd bij de overwegingen. Meer algemene, overkoepelende, of bijkomende aspecten van de organisatie van zorg worden behandeld in de module Organisatie van zorg.
Commentaar- en autorisatiefase
De conceptrichtlijnmodule werd aan de betrokken (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd ter commentaar. De commentaren werden verzameld en besproken met de werkgroep. Naar aanleiding van de commentaren werd de conceptrichtlijnmodule aangepast en definitief vastgesteld door de werkgroep. De definitieve richtlijnmodule werd aan de deelnemende (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd voor autorisatie en door hen geautoriseerd dan wel geaccordeerd.
Literatuur
Agoritsas T, Merglen A, Heen AF, Kristiansen A, Neumann I, Brito JP, Brignardello-Petersen R, Alexander PE, Rind DM, Vandvik PO, Guyatt GH. UpToDate adherence to GRADE criteria for strong recommendations: an analytical survey. BMJ Open. 2017 Nov 16;7(11):e018593. doi: 10.1136/bmjopen-2017-018593. PubMed PMID: 29150475; PubMed Central PMCID: PMC5701989.
Alonso-Coello P, Schünemann HJ, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Rada G, Rosenbaum S, Morelli A, Guyatt GH, Oxman AD; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ. 2016 Jun 28;353:i2016. doi: 10.1136/bmj.i2016. PubMed PMID: 27353417.
Alonso-Coello P, Oxman AD, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Vandvik PO, Meerpohl J, Guyatt GH, Schünemann HJ; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 2: Clinical practice guidelines. BMJ. 2016 Jun 30;353:i2089. doi: 10.1136/bmj.i2089. PubMed PMID: 27365494.
Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, Fervers B, Graham ID, Grimshaw J, Hanna SE, Littlejohns P, Makarski J, Zitzelsberger L; AGREE Next Steps Consortium. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010 Dec 14;182(18):E839-42. doi: 10.1503/cmaj.090449. Epub 2010 Jul 5. Review. PubMed PMID: 20603348; PubMed Central PMCID: PMC3001530.
Hultcrantz M, Rind D, Akl EA, Treweek S, Mustafa RA, Iorio A, Alper BS, Meerpohl JJ, Murad MH, Ansari MT, Katikireddi SV, Östlund P, Tranæus S, Christensen R, Gartlehner G, Brozek J, Izcovich A, Schünemann H, Guyatt G. The GRADE Working Group clarifies the construct of certainty of evidence. J Clin Epidemiol. 2017 Jul;87:4-13. doi: 10.1016/j.jclinepi.2017.05.006. Epub 2017 May 18. PubMed PMID: 28529184; PubMed Central PMCID: PMC6542664.
Medisch Specialistische Richtlijnen 2.0 (2012). Adviescommissie Richtlijnen van de Raad Kwalitieit. http://richtlijnendatabase.nl/over_deze_site/over_richtlijnontwikkeling.html
Neumann I, Santesso N, Akl EA, Rind DM, Vandvik PO, Alonso-Coello P, Agoritsas T, Mustafa RA, Alexander PE, Schünemann H, Guyatt GH. A guide for health professionals to interpret and use recommendations in guidelines developed with the GRADE approach. J Clin Epidemiol. 2016 Apr;72:45-55. doi: 10.1016/j.jclinepi.2015.11.017. Epub 2016 Jan 6. Review. PubMed PMID: 26772609.
Schünemann H, Brożek J, Guyatt G, et al. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group, 2013. Available from http://gdt.guidelinedevelopment.org/central_prod/_design/client/handbook/handbook.html.
Schünemann HJ, Oxman AD, Brozek J, Glasziou P, Jaeschke R, Vist GE, Williams JW Jr, Kunz R, Craig J, Montori VM, Bossuyt P, Guyatt GH; GRADE Working Group. Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. BMJ. 2008 May 17;336(7653):1106-10. doi: 10.1136/bmj.39500.677199.AE. Erratum in: BMJ. 2008 May 24;336(7654). doi: 10.1136/bmj.a139.
Schünemann, A Holger J [corrected to Schünemann, Holger J]. PubMed PMID: 18483053; PubMed Central PMCID: PMC2386626.
Wessels M, Hielkema L, van der Weijden T. How to identify existing literature on patients' knowledge, views, and values: the development of a validated search filter. J Med Libr Assoc. 2016 Oct;104(4):320-324. PubMed PMID: 27822157; PubMed Central PMCID: PMC5079497.
Zoekverantwoording
|
Uitgangsvraag: Welke maatregelen (farmacologisch/niet-farmacologisch) gericht op de drie domeinen van PICS kunnen worden geïnitieerd op de IC om PICS te voorkomen of reduceren? |
|
|
Database(s): Medline, Cinahl (niet farma), Medline, Embase (farma) |
Datum: 15-01-2020 |
|
Periode: niet-farma: 2018-heden Farma: geen beperking |
Talen: E |
|
Database |
Zoektermen |
Totaal |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Engels
Niet-farma: 2018-dec. 2020
Engels Farma
Dec. 2020 |
1 exp Critical Care/ or Critical Care Nursing/ or critical care.ti,ab,kf. or Critical Illness/ or "critical* ill*".ti,ab,kf. or intensive care units/ or (intensive adj2 care).ti,ab,kf. or (ICU? or SICU? or MICU? or HDU? or ITU? or CCU?).ti,ab,kf. (240374) 7 Stress Disorders, Post-Traumatic/ or Survivors/ or (((post traumatic or posttraumatic) adj "stress disorder*") or PTSD* or PICS or survivor*).ti,ab,kf. or ((after adj5 discharge) or (post adj3 (ICU or "intensive care"))).ti,ab,kf. (174865) 8 1 and 7 (11722) 9 ("post intensive care syndrome*" or "postintensive care syndrome" or "post icu syndrome*" or "post ic syndrome" or "ICU-acquired weakness*" or ICUAW).ti,ab,kf. (435) 10 8 or 9 (11902) 11 limit 10 to english language (11112) Niet-farma 16 exp Physical Therapy Modalities/ or Physical Therapy Specialty/ or exp Psychotherapy/ or exp Complementary Therapies/ or exp Nutrition Therapy/ or Health Education/ or Occupational Therapy/ or exp Nutrition Therapy/ or Counseling/ or Social Work/ or Primary Health Care/ or (counseling or Counselling or "early mobili?ation" or Psychoeducation or psycho-education).ti,ab,kf. or (physiotherap* or (physical adj (therap* or activit*)) or exercis* or training* or (occupational adj therap*) or psychotherap* or (behavio?r* adj therap*) or (cogniti* adj therap*) or diet or diets or dietary or nutrition* or ((health or patient*) adj (educati* or litera* or informati*)) or "graded activit*").ti,ab,kf. or diary as topic/ or (diary or diaries).ti,ab,kf. or Intensive Care Units/og or exp Vitamins/ or exp trace elements/ or Magnesium/ or Sodium/ or Potassium/ or (Vitamin* or "trace element*" or Magnesium or Sodium or Potassium).ti,ab,kf. or Light/ or Noise/ or Smell/ or (light* or noise* or smell*).ti,ab,kf. or prevent*.ti,ab,kf. or pc.fs. or exp preventive health services/ or "early intervention (education)"/ or exp health education/ or primary prevention/ (6128320) 17 11 and 16 (2570) 18 limit 17 to yr="2018 -Current" (521) 19 (meta-analysis/ or meta-analysis as topic/ or (metaanaly* or metanaly* or meta-analy*).ti,ab,kf. or ((systematic* or scoping or evidence based) adj3 (review* or overview*)).ti,kf. or ((evidence or research) adj3 synthesis).ti,kf. or systematic review.pt. or (prisma or (("structured literature" or comprehensive or "quantitative literature" or evidence-based) adj2 (search or review)) or "systematic search" or ((systemic or systematized) adj3 review) or "systematic research synthesis" or (review* adj3 independent*)).ti,ab,kf. or (((quantitative or rapid or short or critical* or structured or comparative or comparitive or evidence or comprehensive) adj3 (review* or overview*)).ti. and search.ab.) or "Review Literature as Topic"/ or cochrane.jw. or (cochrane or embase or medline or cinahl or cinhal or cancerlit).ab. or (("selection criteria" or "data extraction").ab. and "review"/)) not (Comment/ or Editorial/ or Letter/ or (animals/ not humans/)) (350428) 20 18 and 19 (43) > 42 uniek 21 (exp randomized controlled trial/ or randomized controlled trials as topic/ or random*.ti,ab. or rct?.ti,ab. or ((pragmatic or practical) adj "clinical trial*").ti,ab,kf. or ((non-inferiority or noninferiority or superiority or equivalence) adj3 trial*).ti,ab,kf.) not (animals/ not humans/) (1164420) 22 18 and 21 (95) 23 22 not 20 (74) > 73 uniek Farma 24 exp Drug Therapy/ or ((drug adj3 therap*) or medicin*).ti,ab,kf. or (farma* or pharma*).ti,ab,kf,jw. or (dt or tu or ad or ae).fs. (6269084) 25 (prevent* or prophyla*).ti,ab,kf. or pc.fs. or exp preventive health services/ or "early intervention (education)"/ or exp health education/ or primary prevention/ (2701350) 26 24 and 25 (1011612) 27 11 and 26 (507) 28 (meta-analysis/ or meta-analysis as topic/ or (metaanaly* or metanaly* or meta-analy*).ti,ab,kf. or ((systematic* or scoping or evidence based) adj3 (review* or overview*)).ti,kf. or ((evidence or research) adj3 synthesis).ti,kf. or systematic review.pt. or (prisma or (("structured literature" or comprehensive or "quantitative literature" or evidence-based) adj2 (search or review)) or "systematic search" or ((systemic or systematized) adj3 review) or "systematic research synthesis" or (review* adj3 independent*)).ti,ab,kf. or (((quantitative or rapid or short or critical* or structured or comparative or comparitive or evidence or comprehensive) adj3 (review* or overview*)).ti. and search.ab.) or "Review Literature as Topic"/ or cochrane.jw. or (cochrane or embase or medline or cinahl or cinhal or cancerlit).ab. or (("selection criteria" or "data extraction").ab. and "review"/)) not (Comment/ or Editorial/ or Letter/ or (animals/ not humans/)) (350428) 29 27 and 28 (29) 30 (exp randomized controlled trial/ or randomized controlled trials as topic/ or random*.ti,ab. or rct?.ti,ab. or ((pragmatic or practical) adj "clinical trial*").ti,ab,kf. or ((non-inferiority or noninferiority or superiority or equivalence) adj3 trial*).ti,ab,kf.) not (animals/ not humans/) (1164420) 31 27 and 30 (143) 32 31 not 29 (121) |
Farma: 210
Niet Farma: 144
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Cinahl |
Niet-farma
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Embase |
Farma ((((((('intensive care'/exp/mj OR 'intensive care unit'/exp/mj OR 'critical illness'/exp/mj OR critical) AND care:ti,ab OR 'critical* ill*':ti,ab OR ((intensive NEAR/2 care):ti,ab) OR icu*:ti,ab OR sicu*:ti,ab OR micu*:ti,ab OR hdu*:ti,ab OR itu*:ti,ab OR ccu*:ti,ab) AND (('posttraumatic stress disorder'/exp OR 'survivor'/exp) OR (((('post traumatic' OR posttraumatic) NEAR/1 'stress disorder*'):ti,ab) OR ptsd*:ti,ab OR pics:ti,ab OR survivor*:ti,ab OR ((after NEAR/5 discharge):ti,ab) OR ((post NEAR/3 (icu OR 'intensive care')):ti,ab)))) OR ('post intensive care syndrome*':ti,ab OR 'postintensive care syndrome':ti,ab OR 'post icu syndrome*':ti,ab OR 'post ic syndrome':ti,ab OR 'icu-acquired weakness*':ti,ab OR icuaw:ti,ab)) NOT 'conference abstract':it AND [english]/lim AND ([embase]/lim OR [pubmed-not-medline]/lim)) AND (('drug therapy'/exp/mj OR ((drug NEAR/3 therap*):ti,ab) OR medicin*:ti,ab OR farma*:ti,ab,jt OR pharma*:ti,ab,jt OR drug:lnk) AND ('prevention'/exp OR prevent*:ti,ab OR prophyla*:ti,ab))) AND (('meta analysis'/exp OR 'meta analysis (topic)'/exp OR 'systematic review (topic)'/exp OR metaanaly*:ti,ab OR 'meta analy*':ti,ab OR metanaly*:ti,ab OR 'systematic review'/de OR 'cochrane database of systematic reviews'/jt OR prisma:ti,ab OR prospero:ti,ab OR (((systemati* OR scoping OR umbrella OR 'structured literature') NEAR/3 (review* OR overview*)):ti,ab) OR ((systemic* NEAR/1 review*):ti,ab) OR (((systemati* OR literature OR database* OR 'data base*') NEAR/10 search*):ti,ab) OR (((structured OR comprehensive* OR systemic*) NEAR/3 search*):ti,ab) OR (((literature NEAR/3 review):ti) AND (search*:ab OR database*:ab OR 'data base*':ab)) OR (('data extraction':ti,ab OR 'data source*':ti,ab) AND 'study selection':ti,ab) OR ('search strategy':ti,ab AND 'selection criteria':ti,ab) OR ('data source*':ti,ab AND 'data synthesis':ti,ab) OR medline:ab OR pubmed:ab OR embase:ab OR cochrane:ab OR (((critical OR rapid) NEAR/2 (review* OR overview* OR synthes*)):ti) OR ((((critical* OR rapid*) NEAR/3 (review* OR overview* OR synthes*)):ab) AND (search*:ab OR database*:ab OR 'data base*':ab)) OR metasynthes*:ti,ab OR 'meta synthes*':ti,ab) NOT (('animal'/de OR 'animal experiment'/exp OR 'animal model'/exp OR 'nonhuman'/exp) NOT 'human'/exp) NOT ('conference abstract':it OR 'conference review'/it OR 'editorial'/it OR 'letter'/it (42) AND (('randomized controlled trial'/exp OR 'randomized controlled trial (topic)'/exp OR random*:ti,ab OR rct*:ti,ab OR (((pragmatic OR practical) NEAR/1 'clinical trial*'):ti,ab) OR ((('non inferiority' OR noninferiority OR superiority OR equivalence) NEAR/3 trial*):ti,ab)) NOT (('animal experiment'/exp OR 'animal model'/exp OR 'nonhuman'/exp) NOT 'human'/exp) NOT ('conference abstract':it OR 'conference review'/it OR 'editorial'/it OR 'letter'/it OR 'note'/it)))) (78) |
|
