Veno-Arteriële ECMO bij cardiogene shock
Uitgangsvraag
Wat is de plaats van veno-arteriële (VA) extracorporele membraanoxygenatie (ECMO) bij patiënten met cardiogene shock?
Aanbeveling
Overweeg behandeling met veno-arteriële extracorporele membraanoxygenatie bij patiënten met een zeer ernstige cardiogene shock (SCAI classificatie C of meer) die niet of onvoldoende reageren op conventionele therapie en met en reële kans op herstel of als brug naar een permanente LVAD-implantatie of harttransplantatie.
Overwegingen
Voor- en nadelen van de interventie en de kwaliteit van het bewijs
De werkgroep heeft een systematische literatuuranalyse uitgevoerd naar het effect van veno-arteriële extracorporele membraanoxygenatie (VA-ECMO) als behandeling voor patiënten met cardiogene shock. De huidige studies zijn voornamelijk gericht op patiënten met cardiogene shock ten gevolge van een acuut coronair probleem. Deze studies includeren patiënten met ernstige cardiogene shock (SCAI stage C tot E) of patiënten die gezien hypoperfusie, lactaat en inotropica behoefte tot diezelfde categorieën zouden behoren. Het effect van een behandeling met VA–ECMO op de cruciale uitkomstmaat mortaliteit is onduidelijk (bewijskracht ‘zeer laag’). De zeer lage bewijskracht wordt veroorzaakt door beperkingen in de studieopzet (cross-overs en vroegtijdige beëindiging van de studie) en brede betrouwbaarheidsintervallen. Er bestaat hier dan ook een forse kennislacune.
Belangrijke uitkomstmaten konden geen verdere richting geven aan de besluitvorming vanwege een zeer lage bewijskracht (gunstige neurologische uitkomsten) of geen beschikbare data (kwaliteit van leven, dagen overleeft en uit het ziekenhuis), succesvol weanen en succesvolle overbrugging naar linker ventrikel assist device (LVAD) of harttransplantatie).
Ondanks dat de onderzochte literatuur geen duidelijk bewijskracht levert naar het effect van VA-ECMO behandeling voor ernstige cardiogene shock, wordt het in gespecialiseerde centra wel voor die indicatie toegepast, gesteund door lokale expertise en voornamelijk observationele data. De bewijsvoering voor de juiste timing en effectiviteit blijft, om bovengenoemde redenen, ver achter bij de dagelijkse klinische praktijk. De werkgroep is van mening dat er sprake is van een situatie waarbij de technologische ontwikkelingen sneller gaan dan dat het klinisch bewijs geleverd wordt door klassieke gerandomiseerde, prospectieve klinische studies. Deze zijn zeer kostbaar en tijdrovend in een relatief jong, opkomend vakgebied. Nieuwe onderzoeksmethoden, zoals bijvoorbeeld prospectief, registry-based gerandomiseerde multicenter studies zijn mogelijk een oplossing.
Ondertussen wordt, bij cardiogene shock met hoge klinische nood (i.e. leven of dood) in het laatste decennium tijdelijke mechanische circulatoire ondersteuning in toenemende mate ingezet.
Waarden en voorkeuren van patiënten (en evt. hun verzorgers)
De werkgroep is van mening dat de persoonlijke wensen en levenshouding van de patiënt/familie van groot belang zijn voor de initiatie, duur en timing van de ECMO ondersteuning. Patiënten ontvangen bij voorkeur de beste behandeling met een zo hoog mogelijke overlevingskans en kwaliteit van leven. Mogelijk bepaalt de verhoogde kans op ernstige complicaties met of zonder VA-ECMO mede de survival: Banning (2023): vasculaire complicaties (21.4% vs. 0%) en bloedingscomplicaties (35.7% vs. 5.6%); Thiele (2023): bloedingen 23.4% vs. 9.6% en interventie noodzakelijke vasculaire complicaties 11.0% vs. 3.8%). Onduidelijk is in hoeverre VA-ECMO-therapie bij ernstige refractaire cardiogene shock positief bijdraagt in vergroten van de kans op overleven. Op dit moment zijn er geen enkele data beschikbaar met betrekking tot patiënt voorkeuren en -perspectief bij deze zeer gespecialiseerde, jonge behandelmodaliteit. Een behandeling met ECMO is zeer intensief en invasief, en daarom is het van groot belang continu aandacht te hebben voor optimalisatie van het comfort van de patiënt (zie ook de richtlijn Nazorg en Revalidatie van IC-Patiënten, ‘module preventie van PICS’).
Kosten (middelenbeslag)
VA-ECMO bij ernstige cardiogene shock is een intensieve behandeling die naast inzet van kostbare ECMO-apparatuur ook leidt tot meer diagnostiek en langere ligduur. Bij herstel volgt veelal een uitgebreid revalidatieproces. Een Nederlandse economische analyse laat zien dat ondanks hoge kosten VA-ECMO mogelijk wel kosteneffectief is (Oude Lansink-Hartgring, 2023).
Aanvaardbaarheid, haalbaarheid en implementatie
Doordat de ECMO-therapie bij ernstige refractaire shock alleen in gespecialiseerde centra wordt toegepast, is er potentieel onrechtvaardigheid in toegang tot deze potentieel levensreddende therapie. Een belangrijk zorgpunt hierbij is dat regionale toegang tot de mogelijk levensreddende behandelingen met VA-ECMO in beperkte centra beschikbaar is.
Het betreft waarschijnlijk een zeer geselecteerde patiëntenpopulatie. De werkgroep beveelt aan dat ieder regionale hartcentrum materiaal en expertise beschikbaar heeft voor een vorm van mechanisch circulatoire ondersteuning en actieve samenwerkingsverband heeft met een nationaal harttransplantatie-/ LVAD-centrum.
Rationale van de aanbeveling: weging van argumenten voor en tegen de interventies
Cardiogene shock is een acuut levensbedreigend ziektebeeld met een zeer hoge mortaliteit in uren tot dagen. In het laatste decennium is steeds meer tijdelijke mechanische circulatoire ondersteuning (mechanical circulatory support, MCS) beschikbaar gekomen. In deze leven of dood situatie wordt er in toenemende tijdelijke MCS, waaronder VA-ECMO, ingezet. De tijdelijke MCS kan de meeste patiënten in leven houden totdat het onderliggend ziektebeeld wordt behandeld, dan wel alternatieve oplossingen zoals bijvoorbeeld een permanent steunhart (LVAD) of spoed harttransplantatie worden ingezet. Tot op heden ontbreek echter een goede klinische bewijsvoering voor de juiste timing, patiëntenpopulatie en effectiviteit van VA-ECMO. De werkgroep beveelt aan om VA-ECMO alleen te overwegen bij geselecteerde patiënten met reële kans op overleving, dan wel patiënten met goede kans op een succesvolle LVAD- implantatie of (spoed) harttransplantatie. Bij dergelijke patiënten in centra waar geen ECMO kan worden toegepast, moet overplaatsing overwogen worden naar een ECMO centrum (zie module Organisatie van zorg).
Onderbouwing
Achtergrond
Veno-arterial (VA) extracorporeal membrane oxygenation (ECMO), a temporary mechanical circulatory and respiratory support, is worldwide increasingly used in patients with cardiogenic shock, severe (acute-on-chronic) heart failure with progressive secondary organ failure and resuscitation for cardiac arrest. However, despite using in more and more centers in the last 15 years or more, firm clinical evidence is still missing for appropriate indication, efficacy, cost-effectiveness, timely weaning and management. As a result, there is a significant variation in the use, availability, and clinical management in these patients, which is mainly dictated by the local experience and insights of the treating physicians. Furthermore, lack of patient and society perspectives hampers in depth discussion in this young, upcoming treatment modality, like long-term treatment goal, quality of life and end-of-life questions are far from crystallized.
Conclusies / Summary of Findings
|
No GRADE |
No evidence was found about the effect of VA-ECMO treatment on in hospital mortality when compared with standard treatment without VA-EMCO in patients with indication ‘cardiogenic shock’. |
|
No GRADE |
No evidence was found about the effect of VA-ECMO treatment on in ICU mortality when compared with standard treatment without VA-EMCO in patients with indication ‘cardiogenic shock’. |
|
Very low GRADE |
The evidence is very uncertain about the effect of VA-ECMO treatment on 30-day mortality when compared with standard treatment without VA-ECMO in patient with indication ‘cardiogenic shock’.
Source: Brunner, 2019; Banning, 2023; Ostadal, 2023; Thiele, 2023 |
|
Very low GRADE |
The evidence is very uncertain about the effect of VA-ECMO treatment on 1-year mortality when compared with standard treatment without VA-ECMO in patient with indication ‘cardiogenic shock’.
Source: Lackermair, 2020; Banning, 2023 |
|
Very low GRADE |
The evidence is very uncertain about the effect of VA-ECMO treatment on favourable neurological outcome at state 30 days when compared with standard treatment without VA-ECMO in patient with indication ‘cardiogenic shock’.
Source: Ostadal, 2023; Thiele, 2023 |
|
Very low GRADE |
The evidence is very uncertain about the effect of VA-ECMO treatment on favourable neurological outcome at state 12 months when compared with standard treatment without VA-ECMO in patient with indication ‘cardiogenic shock’.
Source: Lackermair, 2020 |
|
No GRADE |
No evidence was found about the effect of VA-ECMO treatment on quality of life when compared with standard treatment without VA-ECMO in patients with indication ‘cardiogenic shock’. |
|
No GRADE |
No evidence was found about the effect of VA-ECMO treatment on days alive and out of hospital when compared with standard treatment without VA-ECMO in patients with indication ‘cardiogenic shock’. |
|
No GRADE |
No evidence was found about the effect of VA-ECMO treatment on success of weaning when compared with standard treatment without VA-ECMO in patients with indication ‘cardiogenic shock’. |
|
No GRADE |
No evidence was found about the effect of VA-ECMO treatment on success of weaning when compared with standard treatment without VA-ECMO in patients with indication ‘cardiogenic shock’. |
Samenvatting literatuur
Description of studies
Brunner (2019) and Lackermair (2020) performed a monocentric, randomized, controlled, prospective, open-label trial, in which veno-arterial extracorporeal membrane oxygenation (VA-ECMO) treatment in acute myocardial infarction patients complicated by cardiogenic shock was investigated, versus standard treatment which include best medical treatment alone according to current guidelines. In total 21 patients were randomized to VA-ECMO and 21 patients to no VA-ECMO. Groups were mostly comparable at baseline although the authors found a statistically significant difference in age (62y in intervention group versus 70y in control group). In the intervention group 76% was men, compared to 95% men in the control group. Patients were followed for 12 months. Cross over of patients in the control group to VA-ECMO therapy were not allowed. Brunner (2019) describes the primary outcome left ventricular ejection fraction (LVEF) at 30 days and 30-day mortality. Lackermair (2020) investigated the secondary outcomes all-cause mortality and neurological outcomes at 12 months. Furthermore, the rate of major adverse events (MACE) was reported. First, the intervention group included significantly more patients with more diseased vessels compared to the control group. Additionally, Lackermair (2020) has missing verifiable data about the initial neurological state of patients before initiation of the study. Therefore, there was severe risk of bias.
Banning 2023 performed a prospective, randomized, open-label trial in patients with cardiogenic shock complicated with myocardial infarction. They compared treatment of 1) immediate percutaneous coronary intervention (PCI) with early peripheral VA-ECMO and standard care versus 2) immediate PCI and standard care. This EURO SHOCK trial aimed to evaluate whether early VA-ECMO could reduce mortality and morbidity. In total 17 patients were randomized to the intervention group and 18 to the control group. Groups were comparable at baseline concerning median age (in intervention group 68 versus 67 in control group) and percentage of male patients (81% in intervention group compared to 89% in control group). Cross-over took place in both directions. The primary outcome was 30-day all-cause mortality. Patients were followed for 12 months. The trial lacked from severe risk of bias because it aimed to include 428 patients but was terminated earlier due to COVID.
Ostadal (2023) performed a multicenter randomized investigator initiated academic clinical trial on national level in patients with rapidly deteriorating or severe cardiogenic shock. This study compared immediate VA-ECMO and currents standard (including mechanical or medical cardiovascular interventions) with early conservative and standard care. Hence, this study allowed downstream use of VA-ECMO in the control group in case of further worsening of the hemodynamic status in patients. In total, 58 patients were randomized to the intervention group and 59 to the control group. Baseline characteristics such as age were comparable between groups (with median age of 67y in the intervention and 65y in the control group). Furthermore, the percentage of male patients was similar between groups (74.1% in intervention group compared to 72.9% in control group). However, there was a significant difference in number of smokers with fewer smokers in the intervention group; 14 smokers (25.9%) compared to 27 smokers (47.4%) in the control group. The primary outcome was a composite outcome of death (from any cause), resuscitated circulatory arrest and implementation of another mechanical circulatory device at 30 days. Secondary outcomes included all-cause mortality (which was also part of the composite outcome) and neurological outcome (using CPC score) at 30 days. There was risk of bias in this study, due to baseline differences and lack of blinding.
Thiele (2023) performed an international investigator initiated multicentre randomized open-label trial in patients with acute myocardial infarction complicated by cardiogenic shock. They compared early unselective ECMO (where ECMO was initiated preferably before PCI) versus usual medical therapy according to current guidelines. In total 209 patients were randomized to the intervention group and 208 patients to the control group. Baseline characteristics (with median age of 62y in intervention and 63y in control group and percentage of men 81.3% in intervention group and 81.2% in control group) were similar between groups. Cross over took place and the study allowed specific other devices in the control group when certain criteria were fulfilled. The primary outcome was death from any cause at 30 days. Secondary outcomes included, among others, poor neurological outcome (defined as a post-hoc CPC score 3 or 4) at 30 days. Patients were followed for 30 days. There was some risk of bias due to lack of blinding.
Results
Mortality
In-hospital mortality
Included studies did not report in-hospital mortality.
ICU mortality
Included studies did not report ICU mortality.
30-day mortality
Four studies reported 30-day mortality. A meta-analysis was performed (see figure 1).
140 out of 305 (46%) patients treated with VA-ECMO died and 148 out of 306 (48%) patients treated with usual care died (RR 0.95, 95%CI 0.80 to 1.12). The point estimated difference in 30-day mortality between groups was clinically relevant in favour of VA-ECMO.
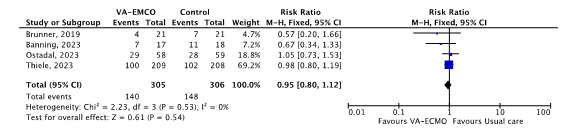
Figure 1. 30-day mortality; VA-ECMO versus usual care.
Z: p-value of overall effect; df: degrees of freedom; I2: statistical heterogeneity; CI: confidence interval; UFH: unfractionated heparin.
1-year mortality
Two studies reported 1-year mortality. One-year mortality in the study of Banning (2023) was 8/17 (47%) for the intervention group and 14/18 (78%) for control group (RR 0.61; 95%CI 0.35 to 1.06). Lackermair (2020) reported a one-year mortality of 4/21 (19%) in the intervention group versus 38% in the control group (RR 0.50; 95%CI 0.18 to 1.41). Overall, the point estimated difference in 1-year mortality was clinically relevant in favour of VA-ECMO.
Favourable neurological state
Three studies described the effect of VA-ECMO on favourable neurological state as shown in Table 1.
Favourable neurological state at 30 days
Ostadal (2023) and Thiele (2023) evaluated Cerebral Performance Score (CPC) after 30 days. Overall, the point estimated differences in favourable neurological state were not clinically relevant.
Favourable neurological state at 12 months
Lackermair (2020), used the modified Rankin Scale (mRS) score at 12 months. Lackermair (2020) reports a clinically relevant point estimated difference in favourable neurological state after 12 months in favour of VA-ECMO.
Table 1. Favourable neurological state at follow-up period of 30 days and 12 months.
|
Study |
Intervention group n/N (%) |
Control group RR n/N (%) |
RR (95%CI) |
|
30 days (CPC score) |
|
|
|
|
Thiele (2023) |
81/109 (74%) |
82/106, 77% |
0.96 (0.83 to 1.12) |
|
Ostadal (2023) |
16/58 (28%) |
17/59 (29%) |
0.96 (0.54 to 1.71) |
|
12 months (mRS score) |
|
|
|
|
Lackermair (2020) |
13/21 (62%) |
12/21 (57%) |
1.08 (0.66 to 1.79) |
Quality of life
Included studies did not report Quality of Life.
Days alive and out of hospital
Included studies did not report days alive and out of hospital.
Success of weaning
Included studies did not report success of weaning.
Successful bridge to LVAD or transplantation
Included studies did not report successful bridge to LVAD or transplantation.
Level of evidence of the literature
According to GRADE, RCTs start at a high level of evidence.
The level of evidence regarding in-hospital mortality was not reported and therefore could not be assessed with GRADE.
The level of evidence regarding ICU mortality was not reported and therefore could not be assessed with GRADE.
The level of evidence regarding the outcome measure 30-day mortality
was downgraded to very low because of cross-over (-1, risk of bias), differences in individual study results (-1, inconsistency) and wide confidence intervals crossing both borders of clinical relevance (-2, imprecision).
The level of evidence regarding the outcome measure 1 year mortality was downgraded to very low because of cross-over and early termination (-1, risk of bias), wide confidence intervals and a low number of events in a small sample size (-2, imprecision).
The level of evidence regarding the outcome measure favourable neurological outcome at state 30 days was downgraded to very low because of cross-over, lack of blinding and lack of data (-1, risk of bias) and wide confidence intervals crossing both borders of clinical relevance (-2, imprecision)
The level of evidence regarding the outcome measure favourable neurological outcome at state 12 months was downgraded to very low because of lack of blinding (-1, risk of bias) and wide confidence intervals crossing both borders of clinical relevance (-3, imprecision)
The level of evidence regarding the outcome measures quality of life, days alive and out of hospital, success of weaning and successful bridge to LVAD or transplantation were not reported and therefore could not be assessed with GRADE.
Zoeken en selecteren
A systematic review of the literature was performed to answer the following question: What is the efficacy of treatment with Veno-Arterial (VA) Extra Corporeal Membrane Oxygenation (ECMO) compared to no VA-ECMO in patients with cardiogenic shock?
| P: | Patients with cardiogenic shock |
| I: | VA-ECMO |
| C: | No VA-ECMO treatment: drug treatment, intra-aortic balloon pump (IABP), micro axial flow pump. |
| O: | Mortality, favourable neurological state, quality of life, days alive and out of hospital, success of weaning, successful bridge to left ventricular assist device (LVAD) or transplantation |
Relevant outcome measures
The guideline development group considered mortality as a critical outcome measure for decision making; and favourable neurological state, quality of life, days alive and out of hospital, success of weaning, successful bridge to LVAD or transplantation as an important outcome measure for decision making.
The working group defined the outcomes measures as follows:
- Mortality reported as events
- In-hospital
- ICU mortality
- 30-day mortality
- 1-year mortality
- Favourable neurological state:
- Cerebral Performance Category (CPC) scale, using a 5-point scale
- Modified Rankin Scale (mRS), using a 6-point scale.
For both scales, lower scores (≤2) indicate favourable neurological outcomes.
- Quality of Life score measured after one year by a validated questionnaire.
- Days alive and out of hospital measured after one year.
The working group did not define the other outcome measures listed above but used the definitions used in the studies.
The working group defined clinical relevance boundaries as follows:
- Mortality: 0.95 ≥ RR ≥ 1.05
- Favorable neurological state: 0.95 ≥ RR ≥ 1.05
- Quality of life: 20% of the of the maximum score of a validated scale
- Days alive and out of hospital: mean difference between treatments of 30 days
- Success of weaning: 0.91 ≥ RR ≥ 1.1
- Successful bridge to LVAD or transplantation: 0.91 ≥ RR ≥1.1
Search and select (Methods)
The databases Medline (via OVID) and Embase (via Embase.com) were searched with relevant search terms from 2005 until 7th of December 2022. The search was then updated from the 8th of December until the 10th of July 2023. The detailed search strategy is depicted under the tab Methods. It was a combined search for veno-arterial extracorporeal membrane oxygenation (VA-EMCO) and extracorporeal cardiopulmonary resuscitation (eCPR). The systematic literature search resulted in 472 hits. Studies were selected based on the following criteria:
- Systematic review (searched in at least two databases, and detailed search strategy, risk of bias assessment and results of individual studies available) or randomized controlled trial.
- comparing VA-ECMO with no VA-ECMO treatment (drug treatment, IABP or micro axial flow pump)
- Included patients with a cardiogenic shock.
- Patients aged ≥ 18 years.
- At least one of the following outcomes: mortality, favourable neurological state, Quality of Life, days alive and out of hospital, success of weaning and successful bridge to LVAD or transplantation.
- Follow-up periods of 30 days and 1 year for mortality (also in hospital or ICU) and favourable neurological outcome, 1 year for Quality of Life, days alive and out of hospital.
- Full-text English language publication.
48 studies were initially selected based on title and abstract screening. After reading the full text, 45 studies were excluded (see the table with reasons for exclusion under the tab Methods). Three studies were included for the literature review about VA-ECMO. The working group added two other full text to the analysis for VA-ECMO: Brunner (2019) and Thieme (2023). Brunner (2019) is a RCT which was obtained using a snowball strategy from the paper by Lackermair (2020), which was the second paper coming from the study protocol of Brunner (2019). The paper by Thiele (2023) was recently published after our second literature search. The working group decided that this paper should be added to the guideline due to its importance. These papers were conform our PICO and were therefore included as well.
Results
Five articles describing four studies were included in the analysis of the literature. Important study characteristics and results are summarized in the evidence tables. The assessment of the risk of bias is summarized in the risk of bias tables.
Referenties
- Banning AS, Sabate M, Orban M, Gracey J, López-Sobrino T, Massberg S, Kastrati A, Bogaerts K, Adriaenssens T, Berry C, Erglis A, Haine S, Myrmel T, Patel S, Buera I, Sionis A, Vilalta V, Yusuff H, Vrints C, Adlam D, Flather M, Gershlick AH. Venoarterial extracorporeal membrane oxygenation or standard care in patients with cardiogenic shock complicating acute myocardial infarction: the multicentre, randomised EURO SHOCK trial. EuroIntervention. 2023 May 19:EIJ-D-23-00204. doi: 10.4244/EIJ-D-23-00204. Epub ahead of print. PMID: 37334659.
- Brunner S, Guenther SPW, Lackermair K, Peterss S, Orban M, Boulesteix AL, Michel S, Hausleiter J, Massberg S, Hagl C. Extracorporeal Life Support in Cardiogenic Shock Complicating Acute Myocardial Infarction. J Am Coll Cardiol. 2019 May 14;73(18):2355-2357. doi: 10.1016/j.jacc.2019.02.044. PMID: 31072581.
- Lackermair K, Brunner S, Orban M, Peterss S, Orban M, Theiss HD, Huber BC, Juchem G, Born F, Boulesteix AL, Bauer A, Pichlmaier M, Hausleiter J, Massberg S, Hagl C, Guenther SPW. Outcome of patients treated with extracorporeal life support in cardiogenic shock complicating acute myocardial infarction: 1-year result from the ECLS-Shock study. Clin Res Cardiol. 2021 Sep;110(9):1412-1420. doi: 10.1007/s00392-020-01778-8. Epub 2020 Nov 12. PMID: 33180150.
- Ostadal P, Rokyta R, Karasek J, Kruger A, Vondrakova D, Janotka M, Naar J, Smalcova J, Hubatova M, Hromadka M, Volovar S, Seyfrydova M, Jarkovsky J, Svoboda M, Linhart A, Belohlavek J; ECMO-CS Investigators. Extracorporeal Membrane Oxygenation in the Therapy of Cardiogenic Shock: Results of the ECMO-CS Randomized Clinical Trial. Circulation. 2023 Feb 7;147(6):454-464. doi: 10.1161/CIRCULATIONAHA.122.062949. Epub 2022 Nov 6. PMID: 36335478.
- Oude Lansink-Hartgring A, Miranda DDR, Mandigers L, Delnoij T, Lorusso R, Maas JJ, Elzo Kraemer CV, Vlaar APJ, Raasveld SJ, Donker DW, Scholten E, Balzereit A, van den Brule J, Kuijpers M, Vermeulen KM, van den Bergh WM; Dutch ECLS Study group. Health-related quality of life, one-year costs and economic evaluation in extracorporeal membrane oxygenation in critically ill adults. J Crit Care. 2023 Feb;73:154215. doi: 10.1016/j.jcrc.2022.154215. Epub 2022 Nov 17. PMID: 36402123.
- Thiele H, Zeymer U, Akin I, Behnes M, Rassaf T, Mahabadi AA, Lehmann R, Eitel I, Graf T, Seidler T, Schuster A, Skurk C, Duerschmied D, Clemmensen P, Hennersdorf M, Fichtlscherer S, Voigt I, Seyfarth M, John S, Ewen S, Linke A, Tigges E, Nordbeck P, Bruch L, Jung C, Franz J, Lauten P, Goslar T, Feistritzer HJ, Pöss J, Kirchhof E, Ouarrak T, Schneider S, Desch S, Freund A; ECLS-SHOCK Investigators. Extracorporeal Life Support in Infarct-Related Cardiogenic Shock. N Engl J Med. 2023 Oct 5;389(14):1286-1297. doi: 10.1056/NEJMoa2307227. Epub 2023 Aug 26. PMID: 37634145.
Evidence tabellen
|
Study reference |
Study characteristics |
Patient characteristics |
Intervention (I) |
Comparison / control (C)
|
Follow-up |
Outcome measures and effect size 4 |
Comments |
|
Lackermair, 2020 |
Type of study: a monocentric, randomized, controlled, prospective, open-label trial
Setting and country: Germany, Ludwig Maximilians University of Munich
Funding and conflicts of interest: LivaNova supported the study but without any involvement in trial design or evaluation. |
Inclusion criteria: - Presented with an AMI (with or without ST segment elevation) complicated by CS (if their systolic blood pressure was less than 90 mmHg for more than 30 min or if inotropes were necessary to maintain a systolic blood pressure higher than 90 mmHg, if they had clinical signs of left heart failure with pulmonary congestion and of end-organ hypoperfusion)
-undergone cardiopulmonary resuscitation for more than 60 min or if ischemic time was more than 10 min; -had symptoms of damage to the central nervous system with fixed dilatation of pupils that was not induced by drugs; -had a mechanical cause of CS (e.g., ventricular septal defect, papillary muscle rupture or dysfunction); disease precluding ECLS implantation; greater than grade II in severity (scale ranging from I to IV, with higher grades indicating more severe regurgitation); -were older than 80 years; -suffered from shock from another cause (e.g., bradycardia, sepsis, hypovolemia);
N total at baseline: Intervention: 21 Control: 21
Important prognostic factors2: Median age (IQR) I: 62 (50-68) C: 70 (60-74)
Sex: I: 76% M C: 95% M
Number of diseased vessels: 1, n[%] 3, [n%] P value = 0.003
Groups comparable at baseline? No, age groups and number of disease vessels are significantly different between groups. For diseased vessels the following differences were reported
No of diseased vessel 3,n(%): 5/21 (24%) in ECLS versus 16/21 (76%) in control group.
P=0.003 Other characteristics were comparable.
|
ECLS implantation using mechanical circulatory support (Stöckert Centrifugal Pump System). Also treated by anticoagulation.
As described in the trial Brunner S. Clinical Study of Extra-Corporal Life Support in Cardiogenic Shock Complicating Acute Myocardial Infarction (ECLS-SHOCK). Available via: Clinical Study of Extra-Corporal Life Support in Cardiogenic Shock Complicating Acute Myocardial Infarction - Full Text View - ClinicalTrials.gov) |
Standard treatment and no ECLS.
*All patients were expected to undergo early revascularization and to receive the best available medical treatment according to current guidelines. |
Length of follow-up: 12 months
Loss-to-follow-up: Intervention N (%) 0 Reasons (describe)
Control: N (%) 0 Reasons (describe). NA
Incomplete outcome data: Intervention: N (%) 0 Reasons (describe) NA
Control: 0 Reasons (describe): NA
|
Outcome measures and effect size (include 95%CI and p-value if available):
Mortality In hospital/ICU mortality Not reported
30 d mortality 1 year mortality (all cause)
Favourable neurological status - Survival with favourable neurological outcomes (modified Rankin Score <2) at 12 months.
Survival with severe neurological outcomes (modified Rankin score 3-5) at 12 months.
Quality of life (1 year) Not reported
Days alive and out of hospital (1 year). Success of weaning
Succesful bridge to LVAD or transplantation. |
*Comparative arm very poorly described and no further information on clinicaldata.gov.
*Second publication of the SHOCK trial. Trial focussed on LVAD. With this publication focus is on neurological outcomes after 1 year.
|
|
Brunner, 2019 |
See Lackermair, 2020 |
See Lackermair, 2020 |
See Lackermair, 2020 |
See Lackermair, 2020 |
Length of follow-up: 30 days
Loss-to-follow-up: Intervention N (%) 0 Reasons (describe)
Control: N (%) 0 Reasons (describe). NA
Incomplete outcome data: Intervention: N (%) 0 Reasons (describe) NA
Control: 0 Reasons (describe): NA
|
Outcome measures and effect size (include 95%CI and p-value if available):
Mortality In hospital mortality Not reported
ICU mortality Not reported
30 d mortality P= 0.37 1 year mortality
Favourable neurological status
Quality of life (1 year) Not reported
Days alive and out of hospital (1 year). Success of weaning
Successful bridge to LVAD or transplantation. Not reported |
This article was published before Lackermair. Lackermair was a second publication of the study protocol from Brunner, 2019. Since Lackermair was more detailed in description, more information can be found in the study description of Lackermair, 2020. |
|
Banning, 2023 |
Type of study: a prospective, randomised, open -label, study design
Setting and country: Spain, Norway, Germany, UK
Funding and conflicts of interest: Funding from European Unions Horizon 2020 research and innovation programma under grant agreement number 754946-2. Several authors reveceive royalties and payments from pharmaceutical parties. They all declare no conflict of interest. |
Inclusion: Patients presenting with CGS due to myocardial infarction and who have had attempted/successful primary PCI (PPCI) of the culprit lesion were considered if there was persistent CGS 30mins following PPCI. CGS was defined by the presence of SBP <90mmHg or maintained above 90mmHg with the addition of vasopressor or inotropic support, with evidence of hypoperfusion. All patients had a bedside echocardiogram within 30 mins post-PCI to exclude the presence of structural complication leading to CGS (ventricular septal rupture, ischaemic mitral regurgitation, left ventricular free-wall rupture). Exclusion: -Echocardiographic evidence) of mechanical cause for CGS: eg ventricular septal defect, LV-free wall rupture, ischaemic mitral regurgitation (recorded within 30 mins of end of PCI procedure). -Age <18 and>90 years. -Deemed appropriately frail (≥ 5 Canadian frailty score) -Shock from another cause (sepsis, haemorrhagic/hypovolaemic shock, anaphylaxis, myocarditis etc.). -Significant systemic illness Known dementia of any severity. -Comorbidity with life expectancy <12 months. -Severe peripheral vascular disease (precluding access making ECMO contra- indicated). -Severe allergy or intolerance to pharmacological or antithrombotic anti-platelet agents. -Out-of-hospital cardiac arrest (OHCA) under any of the following circumstances:- without return of spontaneous circulation (ongoing resuscitation effort). without pH or >7 without bystander CPR within 10 minutes of collapse. -Involved in another randomised research trial within the last 12 months. -Arterial lactate level of <2.0 mmol per litre.
N total at baseline: Intervention: 17 Control: 18
Important prognostic factors2: Median age (IQR) I: 68 (60-73) C: 67 (56-77) Sex: I: %81 M C: %89 M
Groups comparable at baseline? Yes
|
Immediate PCI plus standard care (pharmacological support) with early peripheral veno-arterial ECMO.
Referred to study protocol and design paper Banning AS, Adriaenssens T, Berry C, Bogaerts K, Erglis A, Distelmaier K, Guagliumi G, Haine S, Kastrati A, Massberg S, Orban M, Myrmel T, Vuylsteke A, Alfonso F, Van de Werf F, Verheugt F, Flather M, Sabaté M, Vrints C, Gershlick AH; Collaborators. Veno-arterial extracorporeal membrane oxygenation (ECMO) in patients with cardiogenic shock: rationale and design of the randomised, multicentre, open-label EURO SHOCK trial. EuroIntervention. |
Immediate PCI + standard care (pharmacological support titrated to attain SBP >90mmHg).
Mechanical device is not allowed. However, it is permitted to use IABP but it is considered as violation of study protocol. |
Length of follow-up: 12 months
Loss-to-follow-up: Intervention N (%) 0 Reasons (describe)
Control: N (%) 0 Reasons (describe). NA
Incomplete outcome data: Intervention: N (%) NA Reasons (describe): NA
Control: NA Reasons (describe): NA
|
Crucial
Mortality In hospital mortality Not reported
ICU mortality Not reported
30 d mortality 1 year mortality
Favourable neurological status - Not reported
Quality of life (1 year) Not reported – only for 3 months
Days alive and out of hospital (1 year). Success of weaning Not reported Succeful bridge to LVAD or transplantation
*Based on intention to treat analysis. They also used ‘as treated’. In this case, 22 were in the control arm. |
*Terminated earlier due to COVID, before completion of recruitment.
*Cross over took place for which they performed both an intention to treat as well as an ‘as treated’ analysis. Of the intervention group 5/17 (29%) did not receive ECMO treatment. Of the control group 1/18 (5.6%) received. This was however a violation of the protocol.
*In a protocol published before this study they wanted to perform a cost-effectiveness analysis. This outcome was not reported in the article
|
|
Ostadal, 2023 |
Type of study: a multicenter. randomized investigator-initiated, academic clinical trial.
Setting and country: Prague, Czech Republic.
Funding and conflicts of interest: Funding from a grant from the Czech Health Research Council. (n0. 15-27994A). 754946-2. Two authors receive speaker’s honoraria. The other authors report no conflicts. |
Inclusion: Patients were included if the had rapidly detoriating or severe cardiogenic shock. This is defined using the Society for cardiovasculair angiography and Intervention (SCAI) stage scale: - For Rapidly deteroriating cardiogenic shock (best corresponds to SCAI stage D-E). bolus administration of vasopressors to maintain mean arterial pressure >50 mm Hg + impaired left ventricle systolic function
-For severe cardiogenic shock (best corresponds to SCAI stage D); following criteria should thereby be met: 1. Hemodynamic: Cardiac index <2.2 L•min-1•m-2 + norepinephrine dose >0.1 μg•kg-1•min-1 + dobutamine dose >5 μg•kg-1•min-1 Systolic blood pressure <100 mm Hg + norepinephrine dose >0.2 μg•kg-1•min-1 + dobutamine dose >5 μg•kg-1•min-1 + (LVEF < 35% or LVEF 35%–55% + severe mitral regurgitation or aortic stenosis)
samples), with nondecreasing trend on steady doses of inotropes or vasopressors or SvO2—2 consecutive values <50% (with at least 30 min between measurements), with nonincreasing trend on steady doses of inotropes or vasopressors.
Exclusion: -high suspicion of pulmonary emboli or cardiac tamponade as a cause of shock, significant bradycardia or tachycardia that could be responsible for hemodynamic instability and was not treated pacing or cardioversion -cardiac arrest surcicors remaining comatose, -hypertophic obstructive cardiomyopathy, -peripheral arterial disease precluding arterial cannula insertion in the femoral artery, -moderate to severe aortic regurgitation, -aortic dissection, -uncontrolled bleeding or thrombolysis in Myocardial Infarction major bleeding within the last 6 months -Known encephalopathy.
N total at baseline: Intervention: 58 Control: 59
Important prognostic factors2: Median age (IQR) I: 67(60-74) C: 65 (58-71) Sex: I: %74.1 M C: %72.9 M
Groups comparable at baseline? Overall baseline characteristics are comparable. However, there is a significant difference in number of smokers in the groups with 14/58 (25.9%) smokers in VA-ECMO groups versus 27/59 in control group (47.4%).
|
Immediate VA-ECMO and current standard care (e.g. cardiovascular interventions such as percutaneous coronary, noncoronary intervention and cardiac surgery) or mechanical circulatory support. |
Early conservative and current standard care similar the intervention group.
VA-ECMO was allowed to be used downstream in case of further worsening of hemodynamic status (defined as rise of serum lactate by 3 mmol/L compared to the lowest lactate value in the last 24 hours).
|
Length of follow-up: 30 days
Loss-to-follow-up Intervention N (%): 3/61 Reasons (describe):
Control N (%): 2/61 Reasons (describe): Died after randomization (n = 122) and informed consent was not given by next of kin.
Incomplete outcome data: Unknown neurological outcome / state Intervention N (%) : 14/58 (24.1%) Reasons (describe): CPC score status at 30 days was unknown for 14 patients. The reason (e.g. absence of data, undefined score is not further explained)
Control Reasons (describe):. CPC score status at 30 days was unknown for 15 patients. The reason (e.g. absence of data, undefined score is not further explained)
|
Crucial
Mortality In hospital mortality Not reported
ICU mortality
30 d mortality
“HR – adjusted for smoking” = 0.916 (0.528 to 1.590)
“HR as – treated” = 1.254 (0.703 to 2.238) 1 year mortality
Favourable neurological status - Survival with favourable neurological outcomes (CPC <2) at 30 days. I: 16/58, 28%
Quality of life (1 year) Not reported
Days alive and out of hospital (1 year). Success of weaning Not reported Succeful bridge to LVAD or transplantation Not reported
*Based on intention to treat analysis. They also used ‘as treated’. In this case, 36 were in the control arm. |
*Cross over took place from control to intervention group, for which they performed both an intention to treat as well as an ‘as treated’ analysis
*The study did not reach precalculated sample size of 120, to detect a 50% reduction of primary endpoint.
Patients 23/59 (39%) received downstream VA-ECMO support. This was however allowed by the protocol. Furthermore, intention to treat analysis was performed |
|
Thiele, 2023 |
Type of study: an investigator-initiated, multicenter, randomized, open-label trial
Setting and country: Germany (Leipzig) and Slovenia
Funding and conflicts of interest: Authors declare no conflict of interest in the disclosure. The study was supported by Else Kröner Fresenius Foundation, the German Heart Research Foundation, and the Helios Health Institute (formerly Leipzig Heart Institute).
|
Inclusion: -Patients with acute myocardial infarction complicated by cardiogenic shock (SCAI stage C,D or E). Following definition of cardiogenic shock was used: 1. Systolic blood pressure <90 mm Hg for more than 30 minutes pressure of more than 90 mm Hg, lactate level of more than 3 mmol per liter, one of the following criteria: altered mental status, cold or clammy skin and limbs, or urine output of less than 30 ml per hour. Exclusion: -Patient that had a mechanical cause of cardiogenic shock or severe peripheral-artery disease precluding the insertion of ECLS cannulae
N total at baseline: Intervention: 209 Control: 208
Important prognostic factors2: Median age (IQR) I: 62 (56-69) C: 63 (57-71) Sex: I: %81.3 M C: %81.2 M
Groups comparable at baseline? Yes. However there are some differences in groups concerning total number of diseased vessels in groups (3). ECLS 61/203 (30%) in ECLS group compared to 84/200 (42%) in control group. Furthermore there are differences in laboratory values on admission with 1540(232-6630) ng/L in ECLS compared to 987(173-5700) in control group. |
Early unselective ECLS (where ECLS was initiated preferably before PCI in addition to usual medical therapy.
Reffered to study design and protocol paper: Thiele H, Freund A, Gimenez MR, et al.; ECLS-SHOCK Investigators. Extracorporeal life support in patients with acute myocardial infarction complicated by cardiogenic shock - Design and rationale of the ECLS-SHOCK trial. Am Heart J. 2021 Apr;234:1-11. doi: 10.1016/j.ahj.2021.01.002. Epub 2021 Jan 8. PMID: 33428901. |
Usual medical therapy according to current guidelines.
Protocol stated that crossover to ECLS was to be avoided. However, under several predefined criteria escalation to other devices such as intraaortic balloon pomp or micro axial transvalvular. |
Length of follow-up: 30 days
Loss-to-follow-up Intervention N (%): 4/209 Reasons (describe):
Control N (%): 0 Reasons (describe): NA
Incomplete outcome data: Intervention N (%) : 0 Reasons (describe): NA
Control Reasons (describe): NA
|
Crucial
Mortality In hospital mortality Not reported
ICU mortality Not reported
30 d mortality 1 year mortality
Favourable neurological status at 30 days n/N, % (RR, 95%CI)
Quality of life (1 year) Not reported
Days alive and out of hospital (1 year). Success of weaning Not reported Succeful bridge to LVAD or transplantation Not reported
*Based on intention to treat analysis. Tye also performed per protocol and as treated procedures. |
Cross over total of n=39 (9.4%). Cross over occurred. N=17 (8.1%) in intervention did not receive ECLS (of which n=4 died before receiving ECLS). N=26 (12.5%) in the control group received ECLS.
In the primary analysis intention to treat was performed.
Using other devices in the control group was only allowed when certain criteria were fulfilled. A total of 28 patients (15.4%) in control group received support of medical device (non-ECLS). For n=2 patients, the criteria were not fulfilled. |
Notes: List of abbreviations.
AMI acute myocardial infarction
CS/CGS: cardiogenic shock
ELCS: extracorporeal life support
PCI: percutaneous coronary intervention
PPCI: primary percutaneous coronary intervention
VA ECMO: veno-arterial Extracorporeal Membrane Oxygenation
CPR: cardiopulmonary resuscitation
IABP: intra-aortic balloon pump
SBP: systolic blood pressure
LV: left ventricular
OHCA: out-of hospital cardiac arrest
Risk of Bias Table
|
Study reference
(first author, publication year) |
Was the allocation sequence adequately generated?
Definitely yes Probably yes Probably no Definitely no |
Was the allocation adequately concealed?
Definitely yes Probably yes Probably no Definitely no |
Blinding: Was knowledge of the allocated interventions adequately prevented?
Were patients blinded?
Were healthcare providers blinded?
Were data collectors blinded?
Were outcome assessors blinded?
Were data analysts blinded?
Definitely yes Probably yes Probably no Definitely no |
Was loss to follow-up (missing outcome data) infrequent?
Definitely yes Probably yes Probably no Definitely no |
Are reports of the study free of selective outcome reporting?
Definitely yes Probably yes Probably no Definitely no |
Was the study apparently free of other problems that could put it at a risk of bias?
Definitely yes Probably yes Probably no Definitely no |
Overall risk of bias If applicable/necessary, per outcome measure
LOW Some concerns HIGH
|
|
Lackermair, 2021 |
No information;
Reason: 1:1 randomization, further information not available |
Probably yes;
Reason: Sealed envelopes were used for randomization |
Definitely no;
|
Definitely yes;
All patients completed 12-month follow-up (100%) |
Probably no;
Reason: MACE is mentioned with rehospitalization as an example. This is not mentioned in Results section. |
Probably no:
Reason:
|
Crucial Mortality
Important Favourable neurological status - mRS at 12 months
|
|
Brunner, 2019 |
No information;
Reason: 1:1 randomization, further information not available |
Probably yes
Coming from the same protocol, Lackermair, 2021 mentioned sealed envolopes. Further information was not provided by Brunner or the study protocol. |
Definitely no;
|
Definitely yes
Reason: All patients were taken into account in the analyses. |
Probably yes;
There is no selective reporting. However safety assessments were not further defined in Methods section. |
Probably no:
Reason:
|
Crucial Mortality Some concern Randomisation method is unclear |
|
Banning, 2023 |
Probably yes: Patients were randomised to receive VA-ECMO as soon as possible and within 6 hours of randomisation or continue standard therapy in a 1:1 fashion
A web-based randomisation system stratified by outof- hospital cardiac arrest (OHCA) was used. |
Probably yes:
Randomisation will be primarily by telephone using a 24/7 interactive voice response system (IVRS), with back-up via the trial-specific web portal. |
Definitely no:
Reason: in design paper authors state that the trial is open label which could lead to systematic bias. But there was independent adjudication and blinding of endpoint evaluation. |
Definitely yes:
Reason: All patients were taken into account in the analyses. |
Probably no:
In protocol they wanted to perform a cost-effectiveness analysis. This was not mentioned within the article, but it can be published in a separate article. |
Definitely no:
-Cross over took place on both sides, however intention to treat was performed. |
Crucial 30 d mortality
|
|
Ostadal, 2023 |
Probably yes:
Patients that fulfilled the inclusion criteria were randomised in a 1:1 ratio. Furthermore, permuted blocks were used with stratification according to the type of cardiogenic centre. |
Probably yes:
An automated, web based system was used for randomization. |
Definitely no:
The protocol mentions a single masked study (outcome assessor). The published study, however, mentions that the study was unblinded. Further information was not given. |
Probably no:
Neurological outcomes. |
Probably no:
In the protocol the mentioned follow up visits (to measure all-casue mortality) at: 30 days, 6 months and 12 months. This study only reports the all-cause mortality at 30 months. |
Defintely no:
-Cross over took place from control to intervention group, for which however ITT and as treated was performed. -There are differences in baseline characteristics: number of smokers |
Crucial Mortality Some concern Cross-over took place
Important Favourable neurological status - CPC at 30 days Some concerns
|
|
Thiele, 2023 |
Probably yes:
Patients that fulfilled inclusion criteria were randomized in a 1:1 ratio. Furthermore the web – based system used randomly changing blocks and stratification. |
Probably yes:
A Web-based system was used for randomization |
Definitely no:
The authors state that blinding of the intervention was not possible and that this might have influences the physicians’ decisions. |
Probably yes:
Reason: All patients were taken into account in the analyses. |
Defintely yes:
No selective outcome reporting was performed. Protocol also mentions long term outcomes, however this study is estimated to finish only in November 2023. |
Definitely no
-Cross over took place (n=39, 9,4%) (from intervention to control and vice versa), for which however ITT was performed.
|
Crucial 30 d mortality Some concerns cross-over took place
CPC at 30 days Some concerns
|
Table of excluded studies
|
Reference |
Reason for exclusion |
|
Alfalasi R, Downing J, Cardona S, Lowie BJ, Fairchild M, Chan C, Powell E, Pourmand A, Grazioli A, Tran QK. A Comparison between Conventional and Extracorporeal Cardiopulmonary Resuscitation in Out-of-Hospital Cardiac Arrest: A Systematic Review and Meta-Analysis. Healthcare (Basel). 2022 Mar 21;10(3):591. doi: 10.3390/healthcare10030591. PMID: 35327068; PMCID: PMC8955421.
|
RCTs individual in included in current literature review
|
|
Kaso ER, Pan JA, Salerno M, Kadl A, Aldridge C, Haskal ZJ, Kennedy JLW, Mazimba S, Mihalek AD, Teman NR, Giri J, Aronow HD, Sharma AM. Venoarterial Extracorporeal Membrane Oxygenation for Acute Massive Pulmonary Embolism: a Meta-Analysis and Call to Action. J Cardiovasc Transl Res. 2022 Apr;15(2):258-267. doi: 10.1007/s12265-021-10158-0. Epub 2021 Jul 19. PMID: 34282541; PMCID: PMC8288068. |
Wrong study design: No RCTs included |
|
Scquizzato T, Bonaccorso A, Consonni M, Scandroglio AM, Swol J, Landoni G, Zangrillo A. Extracorporeal cardiopulmonary resuscitation for out-of-hospital cardiac arrest: A systematic review and meta-analysis of randomized and propensity score-matched studies. Artif Organs. 2022 May;46(5):755-762. doi: 10.1111/aor.14205. Epub 2022 Feb 23. PMID: 35199375; PMCID: PMC9307006.
|
RCTs individual in included in current literature review
|
|
Karami M, Mandigers L, Miranda DDR, Rietdijk WJR, Binnekade JM, Knijn DCM, Lagrand WK, den Uil CA, Henriques JPS, Vlaar APJ; DUTCH ECLS Study Group. Survival of patients with acute pulmonary embolism treated with venoarterial extracorporeal membrane oxygenation: A systematic review and meta-analysis. J Crit Care. 2021 Aug;64:245-254. doi: 10.1016/j.jcrc.2021.03.006. Epub 2021 Mar 24. PMID: 34049258. |
Wrong study design: No RCTs included |
|
Wyckoff MH, Singletary EM, Soar J, Olasveengen TM, et al.; COVID-19 Working Group. 2021 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations: Summary From the Basic Life Support; Advanced Life Support; Neonatal Life Support; Education, Implementation, and Teams; First Aid Task Forces; and the COVID-19 Working Group. Resuscitation. 2021 Dec;169:229-311. doi: 10.1016/j.resuscitation.2021.10.040. Epub 2021 Nov 11. PMID: 34933747; PMCID: PMC8581280. |
Wrong study design: Refers to Bierens 2021 (excluded in ti,ab selection) |
|
Mariani S, Richter J, Pappalardo F, Bělohlávek J, Lorusso R, Schmitto JD, Bauersachs J, Napp LC. Mechanical circulatory support for Takotsubo syndrome: a systematic review and meta-analysis. Int J Cardiol. 2020 Oct 1;316:31-39. doi: 10.1016/j.ijcard.2020.05.033. Epub 2020 May 28. PMID: 32473281. |
Re-analysis of primary study data |
|
Miraglia D, Miguel LA, Alonso W. The evolving role of novel treatment techniques in the management of patients with refractory VF/pVT out-of-hospital cardiac arrest. Am J Emerg Med. 2020 Mar;38(3):648-654. doi: 10.1016/j.ajem.2019.11.003. Epub 2019 Nov 18. PMID: 31836341. |
Wrong study design: No quality assesment performed |
|
Miraglia D, Miguel LA, Alonso W. Extracorporeal cardiopulmonary resuscitation for in- and out-of-hospital cardiac arrest: systematic review and meta-analysis of propensity score-matched cohort studies. J Am Coll Emerg Physicians Open. 2020 May 28;1(4):342-361. doi: 10.1002/emp2.12091. PMID: 33000057; PMCID: PMC7493557. |
Wrong study design: No RCTs included |
|
Miraglia D, Miguel LA, Alonso W. Long-term neurologically intact survival after extracorporeal cardiopulmonary resuscitation for in-hospital or out-of-hospital cardiac arrest: A systematic review and meta-analysis. Resusc Plus. 2020 Dec 11;4:100045. doi: 10.1016/j.resplu.2020.100045. PMID: 34223320; PMCID: PMC8244502. |
Wrong study design: No RCTs included |
|
Modarresi M, Amro A, Amro M, Sobeih A, Okoro U, Mansoor K, Rueda C, Elhamdani R, BenHamed N, Kocher T, Elhamdani M. Management of Cardiogenic Shock due to Thyrotoxicosis: A Systematic Literature Review. Curr Cardiol Rev. 2020;16(4):326-332. doi: 10.2174/1573403X16666200313103657. PMID: 32167428; PMCID: PMC7903499. |
Wrong study design: review of case reports |
|
Chen Z, Liu C, Huang J, Zeng P, Lin J, Zhu R, Lu J, Zhou Z, Zuo L, Liu G. Clinical Efficacy of Extracorporeal Cardiopulmonary Resuscitation for Adults with Cardiac Arrest: Meta-Analysis with Trial Sequential Analysis. Biomed Res Int. 2019 Jul 9;2019:6414673. doi: 10.1155/2019/6414673. PMID: 31360719; PMCID: PMC6652040. |
Wrong study design: No RCTs included |
|
Twohig CJ, Singer B, Grier G, Finney SJ. A systematic literature review and meta-analysis of the effectiveness of extracorporeal-CPR versus conventional-CPR for adult patients in cardiac arrest. J Intensive Care Soc. 2019 Nov;20(4):347-357. doi: 10.1177/1751143719832162. Epub 2019 Mar 4. PMID: 31695740; PMCID: PMC6820228. |
Wrong study design: No RCTs included |
|
Urban M, Siddique A, Merritt-Genore H, Um J. What are the results of venoarterial extracorporeal membrane oxygenation bridging to heart transplantation? Interact Cardiovasc Thorac Surg. 2019 Oct 1;29(4):632-634. doi: 10.1093/icvts/ivz096. PMID: 31321425. |
Wrong study design: No RCTs included |
|
Holmberg MJ, Geri G, Wiberg S, Guerguerian AM, Donnino MW, Nolan JP, Deakin CD, Andersen LW; International Liaison Committee on Resuscitation’s (ILCOR) Advanced Life Support and Pediatric Task Forces. Extracorporeal cardiopulmonary resuscitation for cardiac arrest: A systematic review. Resuscitation. 2018 Oct;131:91-100. doi: 10.1016/j.resuscitation.2018.07.029. Epub 2018 Jul 29. PMID: 30063963; PMCID: PMC6441971. |
Wrong study design: No RCTs included |
|
den Uil CA, Akin S, Jewbali LS, Dos Reis Miranda D, Brugts JJ, Constantinescu AA, Kappetein AP, Caliskan K. Short-term mechanical circulatory support as a bridge to durable left ventricular assist device implantation in refractory cardiogenic shock: a systematic review and meta-analysis. Eur J Cardiothorac Surg. 2017 Jul 1;52(1):14-25. doi: 10.1093/ejcts/ezx088. PMID: 28472406. |
Wrong study design: No quality assesment |
|
Wang GN, Chen XF, Qiao L, Mei Y, Lv JR, Huang XH, Shen B, Zhang JS. Comparison of extracorporeal and conventional cardiopulmonary resuscitation: A meta-analysis of 2 260 patients with cardiac arrest. World J Emerg Med. 2017;8(1):5-11. doi: 10.5847/wjem.j.1920-8642.2017.01.001. PMID: 28123613; PMCID: PMC5263037. |
Wrong study design: No RCTs included |
|
Kim SJ, Kim HJ, Lee HY, Ahn HS, Lee SW. Comparing extracorporeal cardiopulmonary resuscitation with conventional cardiopulmonary resuscitation: A meta-analysis. Resuscitation. 2016 Jun;103:106-116. doi: 10.1016/j.resuscitation.2016.01.019. Epub 2016 Feb 2. PMID: 26851058. |
Wrong study design: No RCTs included |
|
Ortega-Deballon I, Hornby L, Shemie SD, Bhanji F, Guadagno E. Extracorporeal resuscitation for refractory out-of-hospital cardiac arrest in adults: A systematic review of international practices and outcomes. Resuscitation. 2016 Apr;101:12-20. doi: 10.1016/j.resuscitation.2016.01.018. Epub 2016 Feb 1. PMID: 26836946. |
Wrong study design: No RCTs included |
|
Ouweneel DM, Schotborgh JV, Limpens J, Sjauw KD, Engström AE, Lagrand WK, Cherpanath TGV, Driessen AHG, de Mol BAJM, Henriques JPS. Extracorporeal life support during cardiac arrest and cardiogenic shock: a systematic review and meta-analysis. Intensive Care Med. 2016 Dec;42(12):1922-1934. doi: 10.1007/s00134-016-4536-8. Epub 2016 Sep 19. PMID: 27647331; PMCID: PMC5106498. |
Wrong study design: No RCTs included for eCPR |
|
Tramm R, Ilic D, Davies AR, Pellegrino VA, Romero L, Hodgson C. Extracorporeal membrane oxygenation for critically ill adults. Cochrane Database Syst Rev. 2015 Jan 22;1(1):CD010381. doi: 10.1002/14651858.CD010381.pub2. PMID: 25608845; PMCID: PMC6353247. |
Wrogn study design: no relevant studies after 2005 included |
|
Affas ZR, Touza GG, Affas S. A Meta-Analysis Comparing Venoarterial (VA) Extracorporeal Membrane Oxygenation (ECMO) to Impella for Acute Right Ventricle Failure. Cureus. 2021 Nov 16;13(11):e19622. doi: 10.7759/cureus.19622. PMID: 34956754; PMCID: PMC8674946. |
Wrong study design en intervention: RCTs no quality assessment and VA-ECMO does not directly compare with Impella (ECMO + control versus Impella + control), also animal studies |
|
Ahn C, Kim W, Cho Y, Choi KS, Jang BH, Lim TH. Efficacy of extracorporeal cardiopulmonary resuscitation compared to conventional cardiopulmonary resuscitation for adult cardiac arrest patients: a systematic review and meta-analysis. Sci Rep. 2016 Sep 23;6:34208. doi: 10.1038/srep34208. PMID: 27659306; PMCID: PMC5034223. |
Wrong study design: No RCTs included |
|
Al-Fares AA, Randhawa VK, Englesakis M, McDonald MA, Nagpal AD, Estep JD, Soltesz EG, Fan E. Optimal Strategy and Timing of Left Ventricular Venting During Veno-Arterial Extracorporeal Life Support for Adults in Cardiogenic Shock: A Systematic Review and Meta-Analysis. Circ Heart Fail. 2019 Nov;12(11):e006486. doi: 10.1161/CIRCHEARTFAILURE.119.006486. Epub 2019 Nov 13. PMID: 31718322. |
Wrong outcome measurement: data was analysed on basis of LV venting |
|
Baldetti L, Gramegna M, Beneduce A, Melillo F, Moroni F, Calvo F, Melisurgo G, Ajello S, Fominskiy E, Pappalardo F, Scandroglio AM. Strategies of left ventricular unloading during VA-ECMO support: a network meta-analysis. Int J Cardiol. 2020 Aug 1;312:16-21. doi: 10.1016/j.ijcard.2020.02.004. Epub 2020 Feb 4. PMID: 32057479. |
Wrong outcome measurement: data was analysed on basis of LV venting |
|
Batchelor RJ, Wheelahan A, Zheng WC, Stub D, Yang Y, Chan W. Impella versus Venoarterial Extracorporeal Membrane Oxygenation for Acute Myocardial Infarction Cardiogenic Shock: A Systematic Review and Meta-Analysis. J Clin Med. 2022 Jul 7;11(14):3955. doi: 10.3390/jcm11143955. PMID: 35887718; PMCID: PMC9317942. |
Wrong study design: no RCT |
|
Gravesteijn BY, Schluep M, Disli M, Garkhail P, Dos Reis Miranda D, Stolker RJ, Endeman H, Hoeks SE. Neurological outcome after extracorporeal cardiopulmonary resuscitation for in-hospital cardiac arrest: a systematic review and meta-analysis. Crit Care. 2020 Aug 17;24(1):505. doi: 10.1186/s13054-020-03201-0. PMID: 32807207; PMCID: PMC7430015. |
Wrong study design/comparison: No comparison group, articles only about follow up of neurological outcomes after eCPR for in hospital cardiac arrest. |
|
Mandigers L, Boersma E, den Uil CA, Gommers D, Bělohlávek J, Belliato M, Lorusso R, Dos Reis Miranda D. Systematic review and meta-analysis comparing low-flow duration of extracorporeal and conventional cardiopulmonary resuscitation. Interact Cardiovasc Thorac Surg. 2022 Sep 9;35(4):ivac219. doi: 10.1093/icvts/ivac219. PMID: 36000900; PMCID: PMC9491846. |
Wrong outcome measurement : compare the relation between short term survival curves and low-flow duration of eCPR versus CCPR |
|
Zhang Q, Han Y, Sun S, Zhang C, Liu H, Wang B, Wei S. Mortality in cardiogenic shock patients receiving mechanical circulatory support: a network meta-analysis. BMC Cardiovasc Disord. 2022 Feb 13;22(1):48. doi: 10.1186/s12872-022-02493-0. PMID: 35152887; PMCID: PMC8842943. |
Wrong study design: no RCT |
|
Abrams D, MacLaren G, Lorusso R, Price S, Yannopoulos D, Vercaemst L, Bělohlávek J, Taccone FS, Aissaoui N, Shekar K, Garan AR, Uriel N, Tonna JE, Jung JS, Takeda K, Chen YS, Slutsky AS, Combes A, Brodie D. Extracorporeal cardiopulmonary resuscitation in adults: evidence and implications. Intensive Care Med. 2022 Jan;48(1):1-15. doi: 10.1007/s00134-021-06514-y. Epub 2021 Sep 10. PMID: 34505911; PMCID: PMC8429884. |
Wrong study design: Not a systematic review, not a RCT |
|
Grunau B, Bashir J, Cheung A, Boone R, McDonald K, Scheuermeyer F, Singer J, Jenneson S, Straight R, Twaites B, Harris L, Haig S, Harris D, Vandegriend R, Kanji H, Christenson J. A pragmatic parallel group implementation study of a prehospital-activated ECPR protocol for refractory out-of-hospital cardiac arrest. Resuscitation. 2021 Oct;167:22-28. doi: 10.1016/j.resuscitation.2021.08.004. Epub 2021 Aug 9. Erratum in: Resuscitation. 2021 Dec;169:312-313. PMID: 34384821. |
Wrong study design: No RCT |
|
Addison D, Cheng E, Forrest P, Livingstone A, Morton RL, Dennis M. Cost-effectiveness of extracorporeal cardiopulmonary resuscitation for adult out-of-hospital cardiac arrest: A systematic review. Resuscitation. 2022 Sep;178:19-25. doi: 10.1016/j.resuscitation.2022.07.010. Epub 2022 Jul 11. PMID: 35835249. |
Wrong outcome: cost effectiveness. Used for 'Overwegingen/Background' |
|
Ahn C, Kim W, Cho Y, Choi KS, Jang BH, Lim TH. Efficacy of extracorporeal cardiopulmonary resuscitation compared to conventional cardiopulmonary resuscitation for adult cardiac arrest patients: a systematic review and meta-analysis. Sci Rep. 2016 Sep 23;6:34208. doi: 10.1038/srep34208. PMID: 27659306; PMCID: PMC5034223. |
Wrong study design: No RCT included |
|
Beyea MM, Tillmann BW, Iansavichene AE, Randhawa VK, Van Aarsen K, Nagpal AD. Neurologic outcomes after extracorporeal membrane oxygenation assisted CPR for resuscitation of out-of-hospital cardiac arrest patients: A systematic review. Resuscitation. 2018 Sep;130:146-158. doi: 10.1016/j.resuscitation.2018.07.012. Epub 2018 Jul 11. PMID: 30017957. |
Wrong study design: No RCT included |
|
Grant C Jr, Richards JB, Frakes M, Cohen J, Wilcox SR. ECMO and Right Ventricular Failure: Review of the Literature. J Intensive Care Med. 2021 Mar;36(3):352-360. doi: 10.1177/0885066619900503. Epub 2020 Jan 22. PMID: 31964208. |
No systematic review |
|
Grunau B, Hornby L, Singal RK, Christenson J, Ortega-Deballon I, Shemie SD, Bashir J, Brooks SC, Callaway CW, Guadagno E, Nagpal D. Extracorporeal Cardiopulmonary Resuscitation for Refractory Out-of-Hospital Cardiac Arrest: The State of the Evidence and Framework for Application. Can J Cardiol. 2018 Feb;34(2):146-155. doi: 10.1016/j.cjca.2017.08.015. Epub 2017 Sep 9. PMID: 29249614. |
No systematic review: narrative review. |
|
Miraglia D, Almanzar C, Rivera E, Alonso W. Extracorporeal cardiopulmonary resuscitation for refractory cardiac arrest: a scoping review. J Am Coll Emerg Physicians Open. 2021 Feb 12;2(1):e12380. doi: 10.1002/emp2.12380. PMID: 33615309; PMCID: PMC7880165. |
No systematic review: scoping review. (The primary limitation of the scoping review is that risk of bias and methodological quality are generally not appraised. However eCPR versus no eCPR studies with neurological outcomes. Also forrest plot, BUT compared in adult patients versus out of hospital patients) |
|
Prondzinsky R, Werdan K. Extracorporeal life support during cardiac arrest and cardiogenic shock-how good is the evidence really? Ann Transl Med. 2017 Feb;5(3):58. doi: 10.21037/atm.2017.01.30. PMID: 28251137; PMCID: PMC5326650. |
No systematic review: narrative review. |
|
Chen CY, Tsai J, Hsu TY, Lai WY, Chen WK, Muo CH, Kao CH. ECMO Used in a Refractory Ventricular Tachycardia and Ventricular Fibrillation Patient: A National Case-Control Study. Medicine (Baltimore). 2016 Mar;95(13):e3204. doi: 10.1097/MD.0000000000003204. PMID: 27043684; PMCID: PMC4998545. |
Retrospective database study (propensity score matching). |
|
Havranek S, Fingrova Z, Rob D, Smalcova J, Kavalkova P, Franek O, Smid O, Huptych M, Dusik M, Linhart A, Belohlavek J. Initial rhythm and survival in refractory out-of-hospital cardiac arrest. Post-hoc analysis of the Prague OHCA randomized trial. Resuscitation. 2022 Dec;181:289-296. doi: 10.1016/j.resuscitation.2022.10.006. Epub 2022 Oct 13. PMID: 36243225. |
Wrong study objective: compares shocable versus non shockable |
|
Karatolios K, Chatzis G, Markus B, Luesebrink U, Ahrens H, Divchev D, Syntila S, Jerrentrup A, Schieffer B. Comparison of mechanical circulatory support with venoarterial extracorporeal membrane oxygenation or Impella for patients with cardiogenic shock: a propensity-matched analysis. Clin Res Cardiol. 2021 Sep;110(9):1404-1411. doi: 10.1007/s00392-020-01777-9. Epub 2020 Nov 13. PMID: 33185749; PMCID: PMC8405518. |
Wrong study design: no RCT |
|
Klee TE, Kern KB. A review of ECMO for cardiac arrest. Resusc Plus. 2021 Feb 6;5:100083. doi: 10.1016/j.resplu.2021.100083. PMID: 34223349; PMCID: PMC8244483. |
No RCT or systematic review: Narrative review. Is not specifically about comparing eCPR / ECMO with non eCPR |
|
Patel JK, Meng H, Qadeer A, Parikh PB. Impact of Extracorporeal Membrane Oxygenation on Mortality in Adults With Cardiac Arrest. Am J Cardiol. 2019 Dec 15;124(12):1857-1861. doi: 10.1016/j.amjcard.2019.09.013. Epub 2019 Sep 25. PMID: 31679644. |
Wrong study design: no RCT |
Verantwoording
Beoordelingsdatum en geldigheid
Publicatiedatum : 25-11-2024
Beoordeeld op geldigheid : 07-11-2024
De richtlijn zal modulair onderhouden worden in het cluster “Intensive Care” en frequent worden beoordeeld op de geldigheid van de aanbeveling vanaf 2026. Meer informatie over werken in clusters en modulair onderhoud vindt u hier.
Algemene gegevens
De ontwikkeling/herziening van deze richtlijnmodule werd ondersteund door het Kennisinstituut van de Federatie Medisch Specialisten (www.demedischspecialist.nl/kennisinstituut) en werd gefinancierd uit de Kwaliteitsgelden Medisch Specialisten (SKMS). De financier heeft geen enkele invloed gehad op de inhoud van de richtlijnmodule.
Samenstelling werkgroep
Voor het ontwikkelen van de richtlijnmodule is in 2022 een multidisciplinaire werkgroep ingesteld, bestaande uit vertegenwoordigers van alle relevante specialismen (zie hiervoor de Samenstelling van de werkgroep) die betrokken zijn bij de zorg voor patiënten die extracorporele membraanoxygenatie (ECMO) ondergaan.
Werkgroep
Dhr. dr. L.C. (Luuk) Otterspoor (voorzitter), cardioloog-intensivist, Catharina Ziekenhuis, Eindhoven; NVIC
Mw. dr. J.M.D. (Judith) van den Brule, cardioloog-intensivist, Radboudumc, Nijmegen; NVIC
Dhr. drs. C.V. (Carlos) Elzo Kraemer, internist-intensivist, Leids Universitair Medisch Centrum, Leiden; NVIC
Mw. dr. A. (Annemieke) Oude Lansink-Hartgring, internist-intensivist, Universitair Medisch Centrum Groningen, Groningen; NVIC
Dhr. drs. J.E. (Jorge) Lopez Matta, longarts-intensivist, Leids Universitair Medisch Centrum, Leiden; NVALT
Dhr. dr. M. (Meindert) Palmen, cardiothoracaal chirurg, Leids Universitair Medisch Centrum, Leiden; NVT
Dhr. dr. K. (Kadir) Çaliskan, cardioloog, Erasmus Medisch Centrum, Rotterdam; NVVC
Dhr. dr. (Krischan) Sjauw (vanaf januari 2023), interventiecardioloog, St. Antonius Ziekenhuis, Nieuwegein; NVVC
Dhr. M.A. (Michel) de Jong, klinisch perfusionist, Universitair Medisch Centrum Utrecht, Utrecht; NeSECC
Dhr. drs. E. (Erik) Scholten, anesthesioloog-intensivist, St. Antonius Ziekenhuis, Nieuwegein; NVA
Mw. K.S.M. (Kimberley) Amatdasim (vanaf februari 2023), intensive care verpleegkundige, Maastricht UMC+, Maastricht; V&VN
Mw. J. (Juul) van de Steeg (tot 30 oktober 2023), verpleegkundig specialist intensive- en medium care, St. Antonius Ziekenhuis, Nieuwegein; V&VN
Mw. J. (José) Joustra, patiëntvertegenwoordiger; FCIC/IC Connect
Klankbordgroep
Dhr. dr. F.J. (Erik) Slim, revalidatiearts, Ziekenhuis Rivierenland, Tiel; VRA
Mw. drs. M.P. (Marijn) Mulder, technisch geneeskundige en docent-onderzoeker, Universiteit Twente, Twente; NVvTG
Dhr. R. (Robert) van der Stoep, fysiotherapeut, Erasmus Medisch Centrum, Rotterdam; KNGF, NVZF
Met ondersteuning van
Mw. drs. I. (Ingeborg) van Dusseldorp, senior medisch informatiespecialist, Kennisinstituut van de Federatie van Medisch Specialisten
Mw. drs. F.M. (Femke) Janssen, junior adviseur, Kennisinstituut van de Federatie van Medisch Specialisten
Mw. dr. J.C. (José) Maas, adviseur, Kennisinstituut van de Federatie van Medisch Specialisten
Mw. drs. L.H.M. (Linda) Niesink-Boerboom, medisch informatiespecialist, Kennisinstituut van de Federatie van Medisch Specialisten
Belangenverklaringen
De Code ter voorkoming van oneigenlijke beïnvloeding door belangenverstrengeling is gevolgd. Alle werkgroepleden hebben schriftelijk verklaard of zij in de laatste drie jaar directe financiële belangen (betrekking bij een commercieel bedrijf, persoonlijke financiële belangen, onderzoeksfinanciering) of indirecte belangen (persoonlijke relaties, reputatiemanagement) hebben gehad. Gedurende de ontwikkeling of herziening van een module worden wijzigingen in belangen aan de voorzitter doorgegeven. De belangenverklaring wordt opnieuw bevestigd tijdens de commentaarfase.
Een overzicht van de belangen van werkgroepleden en het oordeel over het omgaan met eventuele belangen vindt u in onderstaande tabel. De ondertekende belangenverklaringen zijn op te vragen bij het secretariaat van het Kennisinstituut van de Federatie Medisch Specialisten.
|
Werkgroeplid |
Functie |
Nevenfuncties |
Gemelde belangen |
Ondernomen actie |
|
Annemieke Oude Lansink - Hartgring |
Intensivist bij Universitair Medisch Centrum Groningen |
Bestuurslid bij Stichting Venticare - vrijwilligersvergoeding |
Geen |
Geen restricties |
|
Carlos Elzo Kraemer |
Internist-Intensivist volwassen IC, LUMC |
Geen |
Toegevoegd 24-1-24 |
Geen restricties |
|
Erik Scholten |
Anesthesioloog-intensivist st Antonius ziekenhuis |
Geen |
Deelgenomen aan de INCEPTION trial (rol: lokale onderzoeker, PI Antonius ziekenhuis; geen overkoepelend projectleider) |
Geen restricties |
|
Jorge E. Lopez Matta |
Longarts-Intensivist. Werkzaam als full time intensivist in het Leids Universitair Medisch Centrum. |
Longarts-intensivist in het LUMC: Betaald. Full time. Behalve regulier IC, ook actief betrokken bij het opstellen van protocollen en onderwijs rondom ECMO en point of care ultrasound. |
Geen |
Geen restricties |
|
Jose Joustra |
Ervaringsdeskundige |
Communicatiemedewerker gemeente Purmerend |
Geen |
Geen restricties |
|
Judith van den Brule |
Cardioloog-intensivist Radboudumc |
- |
Geen |
Geen restricties |
|
Juul van de Steeg, vertrokken 30-10-2023 |
Verpleegkundig Specialist Intensive- en Medium Care |
Literatuur selecteren |
Geen |
Geen restricties |
|
Kadir Çaliskan |
Cardioloog |
Werkgroep MCS |
Geen |
Geen restricties |
|
Kimberley Amatdasim |
Intensive Care Verpleegkundige, Maastricht UMC+ |
Geen |
Geen |
Geen restricties |
|
Krischan Sjauw |
Interventiecardioloog Medisch Centrum Leeuwarden. |
Afgevaardigde NVVC - inhoudelijk voorzitter ZiN/Programma Uitkomstgerichte zorg, project "Samen beslissen in Acuut coronaire syndromen"; vacatie/uren vergoeding |
- 1. Industry initiated international trial Philips/Volcano: DEFINE-GPS trial (ClinicalTrials.gov Identifier: NCT04451044); Multi-center, prospective, randomized controlled study comparing PCI guided by angiography versus iFR Co-Registration using commercially available Philips pressure guidewires and the SyncVision co-registration system, employing an adaptive design study for interim sample size re-estimation; rol Nationale PI en local PI MCL; betaling trial patient fees aan Research afdeling Cardiologie/HAVA.
|
Geen restricties. Onderwerp van industrie gesponsorde onderzoek valt buiten bestek van de richtlijn. |
|
Luuk Otterspoor (voorzitter) |
Intensivist (50%) Cardioloog (50%), Catharina Ziekenhuis |
Geen |
1EURO-ICE Toegev (restricted grant, geen PI) INCEPTION. Deze werd deels gefinancierd door Getinge. ON SCENE trial, initiatiefnemer is Erasmus MC. |
Geen restricties |
|
Meindert Palmen |
Cardiothoracaal chirurg LUMC. Uit hoofde van deze functie leid ik het LVAD programma in het LUMC |
Geen. |
Cytosorbents voor inclusie patiënten cytosorb tijdens hartfalen studie. Zoals hierboven aangegeven komen alle financiële vergoedingen ten bate van het afdelingsfonds van de afdeling cardiothoracale chirurgie van het LUMC
|
Geen restricties. Onderwerp van industrie gesponsorde onderzoek valt buiten bestek van de richtlijn. |
|
Michel de Jong |
Klinisch perfusionist UMCU, betaald ( niet in loondienst maar vanuit de maatschap heartbeat)
|
LVAD specialist, betaald
|
Geen |
Geen restricties |
|
Erik Slim |
Revalidatiearts |
n.v.t. |
Geen |
Geen restricties
|
|
Marijn Mulder |
Docent/onderzoeker - Universiteit Twente (1.0 FTE) |
n.v.t. |
Betrokken bij research consultancy voor Maquet Critical Care, AB, maar krijg daarvoor geen persoonlijke vergoeding, deze wordt uitbetaald aan de universiteit.
De scope van dit onderzoeksproject valt buiten de scope van de richtlijn.
|
Geen restricties
|
|
Robert van der Stoep |
Fysiotherapeut Erasmus Medisch Centrum (36 uur per week, betaald) |
Gastdocent voor het Nederlands Paramedisch Instituut bij verschillende cursussen over fysiotherapie op de IC. (2 à 3 keer per jaar, betaald). |
Visual incentivised and monitored rehabilitation for early mobilisation in the Intensive Care Unit - ICMOVE
|
Geen restricties
|
|
Valentijn Schweitzer
NVMM heeft zich teruggetrokken uit klankbordgroep, omdat voor NVMM geen relevante modules zijn opgenomen in het definitieve raamwerk |
AIOS MMB |
Geen |
Geen |
Geen restricties
|
|
Nelianne Verkaik, Vertrokken 22-11-2023
NVMM heeft zich teruggetrokken uit klankbordgroep, omdat voor NVMM geen relevante modules zijn opgenomen in het definitieve raamwerk |
Arts-microbioloog Erasmus MC |
Deelnemersraad Stichting Werkgroep Antibiotica Beleid onbetaald |
Intellectueel gewin in zin van meer kennis opdoen/expertise opbouwen omtrent antibiotica bij ECLS, zonder mogelijkheden voor vermarkting
|
Geen restricties
|
|
Femke Janssen |
Junior adviseur kennisinstituut van de Federatie van Medisch Specialisten |
Geen |
Geen |
Geen restricties
|
|
José Maas |
Adviseur kennisinstituut van de Federatie van Medisch Specialisten |
Geen |
Geen |
Geen restricties
|
Inbreng patiëntenperspectief
Er werd aandacht besteed aan het patiëntenperspectief door het uitnodigen van Family and Patient Centered Intensive Care/IC Connect (FCIC/IC Connect), Harteraad en het Longfonds voor deelname aan de invitational conference. Een afgevaardigde van Family and Patient Centered Intensive Care/IC Connect (FCIC/IC Connect) nam deel aan de werkgroep. De verkregen input is meegenomen bij het opstellen van de uitgangsvragen, de keuze voor de uitkomstmaten en bij het opstellen van de overwegingen. De conceptrichtlijn is tevens voor commentaar voorgelegd aan FCIC/IC Connect, Harteraad, het Longfonds en de Patiëntenfederatie Nederland en de eventueel aangeleverde commentaren zijn bekeken en verwerkt.
Kwalitatieve raming van mogelijke financiële gevolgen in het kader van de Wkkgz
Bij de richtlijnmodule is conform de Wet kwaliteit, klachten en geschillen zorg (Wkkgz) een kwalitatieve raming uitgevoerd om te beoordelen of de aanbevelingen mogelijk leiden tot substantiële financiële gevolgen. Bij het uitvoeren van deze beoordeling is de richtlijnmodule op verschillende domeinen getoetst (zie het stroomschema op de Richtlijnendatabase).
Module |
Uitkomst raming |
Toelichting |
|
Module Veno-Arteriële ECMO bij cardiogene shock |
geen financiële gevolgen |
Uit de toetsing volgt dat de aanbeveling(en) niet breed toepasbaar zijn (<5.000 patiënten) en zal daarom naar verwachting geen substantiële financiële gevolgen hebben voor de collectieve uitgaven. |
Werkwijze
AGREE
Deze richtlijnmodule is opgesteld conform de eisen vermeld in het rapport Medisch Specialistische Richtlijnen 2.0 van de adviescommissie Richtlijnen van de Raad Kwaliteit. Dit rapport is gebaseerd op het AGREE II instrument (Appraisal of Guidelines for Research & Evaluation II; Brouwers, 2010).
Knelpuntenanalyse en uitgangsvragen
Tijdens de voorbereidende fase inventariseerde de werkgroep de knelpunten in de zorg voor patiënten die extracorporele membraanoxygenatie (ECMO) ondergaan. Tevens zijn er knelpunten aangedragen door verschillende partijen/vertegenwoordigers via een invitational conference. Een verslag hiervan is opgenomen onder aanverwante producten.
Op basis van de uitkomsten van de knelpuntenanalyse zijn door de werkgroep concept-uitgangsvragen opgesteld en definitief vastgesteld.
Uitkomstmaten
Na het opstellen van de zoekvraag behorende bij de uitgangsvraag inventariseerde de werkgroep welke uitkomstmaten voor de patiënt relevant zijn, waarbij zowel naar gewenste als ongewenste effecten werd gekeken. Hierbij werd een maximum van acht uitkomstmaten gehanteerd. De werkgroep waardeerde deze uitkomstmaten volgens hun relatieve belang bij de besluitvorming rondom aanbevelingen, als cruciaal (kritiek voor de besluitvorming), belangrijk (maar niet cruciaal) en onbelangrijk. Tevens definieerde de werkgroep tenminste voor de cruciale uitkomstmaten welke verschillen zij klinisch (patiënt) relevant vonden.
Methode literatuursamenvatting
Een uitgebreide beschrijving van de strategie voor zoeken en selecteren van literatuur is te vinden onder ‘Zoeken en selecteren’ onder Onderbouwing. Indien mogelijk werd de data uit verschillende studies gepoold in een random-effects model. Review Manager 5.4 werd gebruikt voor de statistische analyses. De beoordeling van de kracht van het wetenschappelijke bewijs wordt hieronder toegelicht.
Beoordelen van de kracht van het wetenschappelijke bewijs
De kracht van het wetenschappelijke bewijs werd bepaald volgens de GRADE-methode. GRADE staat voor ‘Grading Recommendations Assessment, Development and Evaluation’ (zie http://www.gradeworkinggroup.org/). De basisprincipes van de GRADE-methodiek zijn: het benoemen en prioriteren van de klinisch (patiënt) relevante uitkomstmaten, een systematische review per uitkomstmaat, en een beoordeling van de bewijskracht per uitkomstmaat op basis van de acht GRADE-domeinen (domeinen voor downgraden: risk of bias, inconsistentie, indirectheid, imprecisie, en publicatiebias; domeinen voor upgraden: dosis-effect relatie, groot effect, en residuele plausibele confounding).
GRADE onderscheidt vier gradaties voor de kwaliteit van het wetenschappelijk bewijs: hoog, redelijk, laag en zeer laag. Deze gradaties verwijzen naar de mate van zekerheid die er bestaat over de literatuurconclusie, in het bijzonder de mate van zekerheid dat de literatuurconclusie de aanbeveling adequaat ondersteunt (Schünemann, 2013; Hultcrantz, 2017).
|
GRADE |
Definitie |
|
Hoog |
|
|
Redelijk |
|
|
Laag |
|
|
Zeer laag |
|
Bij het beoordelen (graderen) van de kracht van het wetenschappelijk bewijs in richtlijnen volgens de GRADE-methodiek spelen grenzen voor klinische besluitvorming een belangrijke rol (Hultcrantz, 2017). Dit zijn de grenzen die bij overschrijding aanleiding zouden geven tot een aanpassing van de aanbeveling. Om de grenzen voor klinische besluitvorming te bepalen moeten alle relevante uitkomstmaten en overwegingen worden meegewogen. De grenzen voor klinische besluitvorming zijn daarmee niet één op één vergelijkbaar met het minimaal klinisch relevant verschil (Minimal Clinically Important Difference, MCID). Met name in situaties waarin een interventie geen belangrijke nadelen heeft en de kosten relatief laag zijn, kan de grens voor klinische besluitvorming met betrekking tot de effectiviteit van de interventie bij een lagere waarde (dichter bij het nuleffect) liggen dan de MCID (Hultcrantz, 2017).
Overwegingen (van bewijs naar aanbeveling)
Om te komen tot een aanbeveling zijn naast (de kwaliteit van) het wetenschappelijke bewijs ook andere aspecten belangrijk en worden meegewogen, zoals aanvullende argumenten uit bijvoorbeeld de biomechanica of fysiologie, waarden en voorkeuren van patiënten, kosten (middelenbeslag), aanvaardbaarheid, haalbaarheid en implementatie. Deze aspecten zijn systematisch vermeld en beoordeeld (gewogen) onder het kopje ‘Overwegingen’ en kunnen (mede) gebaseerd zijn op expert opinion. Hierbij is gebruik gemaakt van een gestructureerd format gebaseerd op het evidence-to-decision framework van de internationale GRADE Working Group (Alonso-Coello, 2016a; Alonso-Coello 2016b). Dit evidence-to-decision framework is een integraal onderdeel van de GRADE methodiek.
Formuleren van aanbevelingen
De aanbevelingen geven antwoord op de uitgangsvraag en zijn gebaseerd op het beschikbare wetenschappelijke bewijs en de belangrijkste overwegingen, en een weging van de gunstige en ongunstige effecten van de relevante interventies. De kracht van het wetenschappelijk bewijs en het gewicht dat door de werkgroep wordt toegekend aan de overwegingen, bepalen samen de sterkte van de aanbeveling. Conform de GRADE-methodiek sluit een lage bewijskracht van conclusies in de systematische literatuuranalyse een sterke aanbeveling niet a priori uit, en zijn bij een hoge bewijskracht ook zwakke aanbevelingen mogelijk (Agoritsas, 2017; Neumann, 2016). De sterkte van de aanbeveling wordt altijd bepaald door weging van alle relevante argumenten tezamen. De werkgroep heeft bij elke aanbeveling opgenomen hoe zij tot de richting en sterkte van de aanbeveling zijn gekomen.
In de GRADE-methodiek wordt onderscheid gemaakt tussen sterke en zwakke (of conditionele) aanbevelingen. De sterkte van een aanbeveling verwijst naar de mate van zekerheid dat de voordelen van de interventie opwegen tegen de nadelen (of vice versa), gezien over het hele spectrum van patiënten waarvoor de aanbeveling is bedoeld. De sterkte van een aanbeveling heeft duidelijke implicaties voor patiënten, behandelaars en beleidsmakers (zie onderstaande tabel). Een aanbeveling is geen dictaat, zelfs een sterke aanbeveling gebaseerd op bewijs van hoge kwaliteit (GRADE gradering HOOG) zal niet altijd van toepassing zijn, onder alle mogelijke omstandigheden en voor elke individuele patiënt.
|
Implicaties van sterke en zwakke aanbevelingen voor verschillende richtlijngebruikers |
||
|
|
||
|
|
Sterke aanbeveling |
Zwakke (conditionele) aanbeveling |
|
Voor patiënten |
De meeste patiënten zouden de aanbevolen interventie of aanpak kiezen en slechts een klein aantal niet. |
Een aanzienlijk deel van de patiënten zou de aanbevolen interventie of aanpak kiezen, maar veel patiënten ook niet. |
|
Voor behandelaars |
De meeste patiënten zouden de aanbevolen interventie of aanpak moeten ontvangen. |
Er zijn meerdere geschikte interventies of aanpakken. De patiënt moet worden ondersteund bij de keuze voor de interventie of aanpak die het beste aansluit bij zijn of haar waarden en voorkeuren. |
|
Voor beleidsmakers |
De aanbevolen interventie of aanpak kan worden gezien als standaardbeleid. |
Beleidsbepaling vereist uitvoerige discussie met betrokkenheid van veel stakeholders. Er is een grotere kans op lokale beleidsverschillen. |
Organisatie van zorg
In de knelpuntenanalyse en bij de ontwikkeling van de richtlijnmodule is expliciet aandacht geweest voor de organisatie van zorg: alle aspecten die randvoorwaardelijk zijn voor het verlenen van zorg (zoals coördinatie, communicatie, (financiële) middelen, mankracht en infrastructuur). Randvoorwaarden die relevant zijn voor het beantwoorden van deze specifieke uitgangsvraag zijn genoemd bij de overwegingen. Meer algemene, overkoepelende, of bijkomende aspecten van de organisatie van zorg worden behandeld in de module Organisatie van zorg.
Commentaar- en autorisatiefase
De conceptrichtlijnmodule werd aan de betrokken (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd ter commentaar. De commentaren werden verzameld en besproken met de werkgroep. Naar aanleiding van de commentaren werd de conceptrichtlijnmodule aangepast en definitief vastgesteld door de werkgroep. De definitieve richtlijnmodule werd aan de deelnemende (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd voor autorisatie en door hen geautoriseerd dan wel geaccordeerd.
Literatuur
Agoritsas T, Merglen A, Heen AF, Kristiansen A, Neumann I, Brito JP, Brignardello-Petersen R, Alexander PE, Rind DM, Vandvik PO, Guyatt GH. UpToDate adherence to GRADE criteria for strong recommendations: an analytical survey. BMJ Open. 2017 Nov 16;7(11):e018593. doi: 10.1136/bmjopen-2017-018593. PubMed PMID: 29150475; PubMed Central PMCID: PMC5701989.
Alonso-Coello P, Schünemann HJ, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Rada G, Rosenbaum S, Morelli A, Guyatt GH, Oxman AD; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ. 2016 Jun 28;353:i2016. doi: 10.1136/bmj.i2016. PubMed PMID: 27353417.
Alonso-Coello P, Oxman AD, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Vandvik PO, Meerpohl J, Guyatt GH, Schünemann HJ; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 2: Clinical practice guidelines. BMJ. 2016 Jun 30;353:i2089. doi: 10.1136/bmj.i2089. PubMed PMID: 27365494.
Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, Fervers B, Graham ID, Grimshaw J, Hanna SE, Littlejohns P, Makarski J, Zitzelsberger L; AGREE Next Steps Consortium. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010 Dec 14;182(18):E839-42. doi: 10.1503/cmaj.090449. Epub 2010 Jul 5. Review. PubMed PMID: 20603348; PubMed Central PMCID: PMC3001530.
Hultcrantz M, Rind D, Akl EA, Treweek S, Mustafa RA, Iorio A, Alper BS, Meerpohl JJ, Murad MH, Ansari MT, Katikireddi SV, Östlund P, Tranæus S, Christensen R, Gartlehner G, Brozek J, Izcovich A, Schünemann H, Guyatt G. The GRADE Working Group clarifies the construct of certainty of evidence. J Clin Epidemiol. 2017 Jul;87:4-13. doi: 10.1016/j.jclinepi.2017.05.006. Epub 2017 May 18. PubMed PMID: 28529184; PubMed Central PMCID: PMC6542664.
Medisch Specialistische Richtlijnen 2.0 (2012). Adviescommissie Richtlijnen van de Raad Kwalitieit. http://richtlijnendatabase.nl/over_deze_site/over_richtlijnontwikkeling.html
Neumann I, Santesso N, Akl EA, Rind DM, Vandvik PO, Alonso-Coello P, Agoritsas T, Mustafa RA, Alexander PE, Schünemann H, Guyatt GH. A guide for health professionals to interpret and use recommendations in guidelines developed with the GRADE approach. J Clin Epidemiol. 2016 Apr;72:45-55. doi: 10.1016/j.jclinepi.2015.11.017. Epub 2016 Jan 6. Review. PubMed PMID: 26772609.
Schünemann H, Brożek J, Guyatt G, et al. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group, 2013. Available from http://gdt.guidelinedevelopment.org/central_prod/_design/client/handbook/handbook.html.
Zoekverantwoording
Algemene informatie
|
Database(s): Ovid/Medline, Embase |
Datum:8-12-2022, 10-7-2023 |
|
Periode: 2005- |
Talen: nvt |
Zoekopbrengst
|
|
EMBASE |
OVID/MEDLINE |
Ontdubbeld |
|
SRs |
267 |
173 |
46 |
|
RCTs |
162 |
129 |
29 |
|
Observationele studies |
|
|
|
|
Overig |
|
|
|
|
Totaal |
|
|
472 |
|
|
EMBASE |
OVID/MEDLINE |
Ontdubbeld |
|
SRs |
217 |
151 |
234 |
|
RCTs |
141 |
112 |
163 |
|
Observationele studies |
1380* |
1000* |
|
|
Overig |
|
|
|
|
Totaal |
|
|
397 |
*niet in Rayyan
Zoekstrategie
Embase
|
No. |
Query |
Results |
|
#23 |
#5 AND #22 sleutelartikelen gevonden |
4 |
|
#22 |
#17 OR #18 OR #19 |
1732 |
|
#21 |
#19 NOT #18 NOT #17 |
1380 |
|
#20 |
#18 NOT #17 RCT |
141 |
|
#19 |
(#14 OR #15) AND #16 |
1603 |
|
#18 |
#13 AND #16 |
185 |
|
#17 |
#12 AND #16 SR |
217 |
|
#16 |
#11 NOT (('adolescent'/exp OR 'child'/exp OR adolescent*:ti,ab,kw OR child*:ti,ab,kw OR schoolchild*:ti,ab,kw OR infant*:ti,ab,kw OR girl*:ti,ab,kw OR boy*:ti,ab,kw OR teen:ti,ab,kw OR teens:ti,ab,kw OR teenager*:ti,ab,kw OR youth*:ti,ab,kw OR pediatr*:ti,ab,kw OR paediatr*:ti,ab,kw OR puber*:ti,ab,kw) NOT ('adult'/exp OR 'aged'/exp OR 'middle aged'/exp OR adult*:ti,ab,kw OR man:ti,ab,kw OR men:ti,ab,kw OR woman:ti,ab,kw OR women:ti,ab,kw)) |
3311 |
|
#15 |
'case control study'/de OR 'comparative study'/exp OR 'control group'/de OR 'controlled study'/de OR 'controlled clinical trial'/de OR 'crossover procedure'/de OR 'double blind procedure'/de OR 'phase 2 clinical trial'/de OR 'phase 3 clinical trial'/de OR 'phase 4 clinical trial'/de OR 'pretest posttest design'/de OR 'pretest posttest control group design'/de OR 'quasi experimental study'/de OR 'single blind procedure'/de OR 'triple blind procedure'/de OR (((control OR controlled) NEAR/6 trial):ti,ab,kw) OR (((control OR controlled) NEAR/6 (study OR studies)):ti,ab,kw) OR (((control OR controlled) NEAR/1 active):ti,ab,kw) OR 'open label*':ti,ab,kw OR (((double OR two OR three OR multi OR trial) NEAR/1 (arm OR arms)):ti,ab,kw) OR ((allocat* NEAR/10 (arm OR arms)):ti,ab,kw) OR placebo*:ti,ab,kw OR 'sham-control*':ti,ab,kw OR (((single OR double OR triple OR assessor) NEAR/1 (blind* OR masked)):ti,ab,kw) OR nonrandom*:ti,ab,kw OR 'non-random*':ti,ab,kw OR 'quasi-experiment*':ti,ab,kw OR crossover:ti,ab,kw OR 'cross over':ti,ab,kw OR 'parallel group*':ti,ab,kw OR 'factorial trial':ti,ab,kw OR ((phase NEAR/5 (study OR trial)):ti,ab,kw) OR ((case* NEAR/6 (matched OR control*)):ti,ab,kw) OR ((match* NEAR/6 (pair OR pairs OR cohort* OR control* OR group* OR healthy OR age OR sex OR gender OR patient* OR subject* OR participant*)):ti,ab,kw) OR ((propensity NEAR/6 (scor* OR match*)):ti,ab,kw) OR versus:ti OR vs:ti OR compar*:ti OR ((compar* NEAR/1 study):ti,ab,kw) OR (('major clinical study'/de OR 'clinical study'/de OR 'cohort analysis'/de OR 'observational study'/de OR 'cross-sectional study'/de OR 'multicenter study'/de OR 'correlational study'/de OR 'follow up'/de OR cohort*:ti,ab,kw OR 'follow up':ti,ab,kw OR followup:ti,ab,kw OR longitudinal*:ti,ab,kw OR prospective*:ti,ab,kw OR retrospective*:ti,ab,kw OR observational*:ti,ab,kw OR 'cross sectional*':ti,ab,kw OR cross?ectional*:ti,ab,kw OR multicent*:ti,ab,kw OR 'multi-cent*':ti,ab,kw OR consecutive*:ti,ab,kw) AND (group:ti,ab,kw OR groups:ti,ab,kw OR subgroup*:ti,ab,kw OR versus:ti,ab,kw OR vs:ti,ab,kw OR compar*:ti,ab,kw OR 'odds ratio*':ab OR 'relative odds':ab OR 'risk ratio*':ab OR 'relative risk*':ab OR 'rate ratio':ab OR aor:ab OR arr:ab OR rrr:ab OR ((('or' OR 'rr') NEAR/6 ci):ab))) |
13672680 |
|
#14 |
'major clinical study'/de OR 'clinical study'/de OR 'case control study'/de OR 'family study'/de OR 'longitudinal study'/de OR 'retrospective study'/de OR 'prospective study'/de OR 'comparative study'/de OR 'cohort analysis'/de OR ((cohort NEAR/1 (study OR studies)):ab,ti) OR (('case control' NEAR/1 (study OR studies)):ab,ti) OR (('follow up' NEAR/1 (study OR studies)):ab,ti) OR (observational NEAR/1 (study OR studies)) OR ((epidemiologic NEAR/1 (study OR studies)):ab,ti) OR (('cross sectional' NEAR/1 (study OR studies)):ab,ti) |
6767914 |
|
#13 |
'randomized controlled trial'/exp OR random*:ti,ab OR (((pragmatic OR practical) NEAR/1 'clinical trial*'):ti,ab) OR ((('non inferiority' OR noninferiority OR superiority OR equivalence) NEAR/3 trial*):ti,ab) OR rct:ti,ab,kw |
1839814 |
|
#12 |
'meta analysis'/exp OR 'meta analysis (topic)'/exp OR metaanaly*:ti,ab OR 'meta analy*':ti,ab OR metanaly*:ti,ab OR 'systematic review'/de OR 'cochrane database of systematic reviews'/jt OR prisma:ti,ab OR prospero:ti,ab OR (((systemati* OR scoping OR umbrella OR 'structured literature') NEAR/3 (review* OR overview*)):ti,ab) OR ((systemic* NEAR/1 review*):ti,ab) OR (((systemati* OR literature OR database* OR 'data base*') NEAR/10 search*):ti,ab) OR (((structured OR comprehensive* OR systemic*) NEAR/3 search*):ti,ab) OR (((literature NEAR/3 review*):ti,ab) AND (search*:ti,ab OR database*:ti,ab OR 'data base*':ti,ab)) OR (('data extraction':ti,ab OR 'data source*':ti,ab) AND 'study selection':ti,ab) OR ('search strategy':ti,ab AND 'selection criteria':ti,ab) OR ('data source*':ti,ab AND 'data synthesis':ti,ab) OR medline:ab OR pubmed:ab OR embase:ab OR cochrane:ab OR (((critical OR rapid) NEAR/2 (review* OR overview* OR synthes*)):ti) OR ((((critical* OR rapid*) NEAR/3 (review* OR overview* OR synthes*)):ab) AND (search*:ab OR database*:ab OR 'data base*':ab)) OR metasynthes*:ti,ab OR 'meta synthes*':ti,ab |
733409 |
|
#11 |
#9 AND #10 |
3882 |
|
#10 |
'cardiogenic shock'/exp OR 'myocarditis'/exp OR 'heart arrest'/exp OR 'postpericardiotomy syndrome'/exp OR 'lung embolism'/exp OR (((lung OR pulmonary) NEAR/3 (embol* OR microembol* OR thromboembol*)):ti,ab,kw) OR 'post cardiotomy syndrome':ti,ab,kw OR 'post pericardiotomy syndrome':ti,ab,kw OR 'post-commissurotomy syndrome':ti,ab,kw OR 'postcardiotomy syndrome':ti,ab,kw OR 'postcommissurotomy syndrome':ti,ab,kw OR 'postpericardectomy syndrome':ti,ab,kw OR 'postpericardiotomy syndrome':ti,ab,kw OR 'asystol*':ti,ab,kw OR (((cardiac OR circulat* OR heart OR cardio*) NEAR/3 (arrest OR standstill OR shock OR collapse)):ti,ab,kw) OR 'cardiomyocyte inflammation':ti,ab,kw OR 'cardiomyopathic inflammatory process':ti,ab,kw OR 'inflammatory cardiomyopathy':ti,ab,kw OR 'inflammatory myocardiopathy':ti,ab,kw OR 'myocard inflammation':ti,ab,kw OR 'myocardial inflammation':ti,ab,kw OR 'myocarditis':ti,ab,kw OR 'acute on chronic heart failure'/exp |
353662 |
|
#9 |
#8 AND [1-1-2005]/sd NOT ('conference abstract'/it OR 'editorial'/it OR 'letter'/it OR 'note'/it) NOT (('animal'/exp OR 'animal experiment'/exp OR 'animal model'/exp OR 'nonhuman'/exp) NOT 'human'/exp) |
6137 |
|
#8 |
#6 OR #7 |
11885 |
|
#7 |
'veno-arterial ecmo'/exp OR (('veno arterial' NEAR/3 (extracorporeal OR ecmo OR ecls)):ti,ab,kw) OR 'va ecmo':ti,ab,kw OR 'va ecls':ti,ab,kw |
6917 |
|
#6 |
'resuscitation'/exp AND 'extracorporeal oxygenation'/exp OR (resuscitat*:ti AND extracorporeal:ti) OR (resuscitat*:ab AND extracorporeal:ab) OR (resuscitat*:kw AND extracorporeal:kw) OR ecpr:ti,ab,kw |
6638 |
|
#5 |
#1 OR #2 OR #3 OR #4 sleutelartikelen |
4 |
|
#4 |
'advanced reperfusion strategies for patients with out-of-hospital cardiac arrest and refractory ventricular fibrillation' |
1 |
|
#3 |
'effect of intra-arrest transport, extracorporeal cardiopulmonary resuscitation, and immediate invasive assessment and treatment on functional neurologic outcome in refractory out-of-hospital cardiac arrest' |
1 |
|
#2 |
'extracorporeal membrane oxygenation in the therapy of cardiogenic shock' AND ostadal AND 2022 |
1 |
|
#1 |
'short-term mechanical circulatory support as a bridge to durable left ventricular assist device implantation in refractory cardiogenic shock' |
1 |
Ovid/Medline
|
# |
Searches |
Results |
|
17 |
15 not 14 not 13 |
1000 |
|
16 |
14 not 13 RCT |
112 |
|
15 |
(10 or 11) and 12 |
1181 |
|
14 |
9 and 12 |
157 |
|
13 |
8 and 12 SR |
151 |
|
12 |
7 not ((Adolescent/ or Child/ or Infant/ or adolescen*.ti,ab,kf. or child*.ti,ab,kf. or schoolchild*.ti,ab,kf. or infant*.ti,ab,kf. or girl*.ti,ab,kf. or boy*.ti,ab,kf. or teen.ti,ab,kf. or teens.ti,ab,kf. or teenager*.ti,ab,kf. or youth*.ti,ab,kf. or pediatr*.ti,ab,kf. or paediatr*.ti,ab,kf. or puber*.ti,ab,kf.) not (Adult/ or adult*.ti,ab,kf. or man.ti,ab,kf. or men.ti,ab,kf. or woman.ti,ab,kf. or women.ti,ab,kf.)) |
2260 |
|
11 |
Case-control Studies/ or clinical trial, phase ii/ or clinical trial, phase iii/ or clinical trial, phase iv/ or comparative study/ or control groups/ or controlled before-after studies/ or controlled clinical trial/ or double-blind method/ or historically controlled study/ or matched-pair analysis/ or single-blind method/ or (((control or controlled) adj6 (study or studies or trial)) or (compar* adj (study or studies)) or ((control or controlled) adj1 active) or "open label*" or ((double or two or three or multi or trial) adj (arm or arms)) or (allocat* adj10 (arm or arms)) or placebo* or "sham-control*" or ((single or double or triple or assessor) adj1 (blind* or masked)) or nonrandom* or "non-random*" or "quasi-experiment*" or "parallel group*" or "factorial trial" or "pretest posttest" or (phase adj5 (study or trial)) or (case* adj6 (matched or control*)) or (match* adj6 (pair or pairs or cohort* or control* or group* or healthy or age or sex or gender or patient* or subject* or participant*)) or (propensity adj6 (scor* or match*))).ti,ab,kf. or (confounding adj6 adjust*).ti,ab. or (versus or vs or compar*).ti. or ((exp cohort studies/ or epidemiologic studies/ or multicenter study/ or observational study/ or seroepidemiologic studies/ or (cohort* or 'follow up' or followup or longitudinal* or prospective* or retrospective* or observational* or multicent* or 'multi-cent*' or consecutive*).ti,ab,kf.) and ((group or groups or subgroup* or versus or vs or compar*).ti,ab,kf. or ('odds ratio*' or 'relative odds' or 'risk ratio*' or 'relative risk*' or aor or arr or rrr).ab. or (("OR" or "RR") adj6 CI).ab.)) |
5303494 |
|
10 |
Epidemiologic studies/ or case control studies/ or exp cohort studies/ or Controlled Before-After Studies/ or Case control.tw. or cohort.tw. or Cohort analy$.tw. or (Follow up adj (study or studies)).tw. or (observational adj (study or studies)).tw. or Longitudinal.tw. or Retrospective*.tw. or prospective*.tw. or consecutive*.tw. or Cross sectional.tw. or Cross-sectional studies/ or historically controlled study/ or interrupted time series analysis/ [Onder exp cohort studies vallen ook longitudinale, prospectieve en retrospectieve studies] |
4307864 |
|
9 |
exp randomized controlled trial/ or randomized controlled trials as topic/ or random*.ti,ab. or rct?.ti,ab. or ((pragmatic or practical) adj "clinical trial*").ti,ab,kf. or ((non-inferiority or noninferiority or superiority or equivalence) adj3 trial*).ti,ab,kf. |
1567084 |
|
8 |
meta-analysis/ or meta-analysis as topic/ or (metaanaly* or meta-analy* or metanaly*).ti,ab,kf. or systematic review/ or cochrane.jw. or (prisma or prospero).ti,ab,kf. or ((systemati* or scoping or umbrella or "structured literature") adj3 (review* or overview*)).ti,ab,kf. or (systemic* adj1 review*).ti,ab,kf. or ((systemati* or literature or database* or data-base*) adj10 search*).ti,ab,kf. or ((structured or comprehensive* or systemic*) adj3 search*).ti,ab,kf. or ((literature adj3 review*) and (search* or database* or data-base*)).ti,ab,kf. or (("data extraction" or "data source*") and "study selection").ti,ab,kf. or ("search strategy" and "selection criteria").ti,ab,kf. or ("data source*" and "data synthesis").ti,ab,kf. or (medline or pubmed or embase or cochrane).ab. or ((critical or rapid) adj2 (review* or overview* or synthes*)).ti. or (((critical* or rapid*) adj3 (review* or overview* or synthes*)) and (search* or database* or data-base*)).ab. or (metasynthes* or meta-synthes*).ti,ab,kf. |
633995 |
|
7 |
6 not ((exp animals/ or exp models, animal/) not humans/) not (letter/ or comment/ or editorial/) |
2552 |
|
6 |
limit 5 to yr="2005 -Current" |
2834 |
|
5 |
3 and 4 |
2990 |
|
4 |
1 or 2 |
5439 |
|
3 |
exp Myocardial Infarction/ or Myocarditis/ or Postpericardiotomy Syndrome/ or exp Pulmonary Embolism/ or ((lung or pulmonary) adj3 (embol* or microembol* or thromboembol*)).ti,ab,kf. or post cardiotomy syndrome.ti,ab,kf. or post pericardiotomy syndrome.ti,ab,kf. or post-commissurotomy syndrome.ti,ab,kf. or postcardiotomy syndrome.ti,ab,kf. or postcommissurotomy syndrome.ti,ab,kf. or postpericardectomy syndrome.ti,ab,kf. or postpericardiotomy syndrome.ti,ab,kf. or asystol*.ti,ab,kf. or ((cardiac or circulat* or heart or cardio*) adj3 (arrest or standstill or shock or collapse)).ti,ab,kf. or cardiomyocyte inflammation.ti,ab,kf. or cardiomyopathic inflammatory process.ti,ab,kf. or inflammatory cardiomyopathy.ti,ab,kf. or inflammatory myocardiopathy.ti,ab,kf. or myocard inflammation.ti,ab,kf. or myocardial inflammation.ti,ab,kf. or myocarditis.ti,ab,kf. |
345293 |
|
2 |
((Resuscitation/ or Cardiopulmonary Resuscitation/ or Heart Massage/) and Extracorporeal Membrane Oxygenation/) or (resuscitat* and extracorporeal).ti. or (resuscitat* and extracorporeal).ab. or (resuscitat* and extracorporeal).kf. or ecpr.ti,ab,kf. |
2677 |
|
1 |
(Extracorporeal Membrane Oxygenation/ and ("veno arter*" or va or venoarter*).ti,ab,kf.) or ('venoarterial' adj3 (extracorporeal or ecmo or ecls)).ti,ab,kf. or 'vaecmo'.ti,ab,kf. or 'vaecls'.ti,ab,kf. |
3188 |
