Liquoronderzoek bij vermoeden op de ziekte van Alzheimer
Uitgangsvraag
Wat is de waarde van liquormarkers in de diagnostiek bij de ziekte van Alzheimer en in de differentiële diagnostiek bij andere oorzaken van dementie?
Aanbeveling
Verricht niet standaard liquoronderzoek bij patiënten met dementie om de ziekte van Alzheimer aan te tonen of uit te sluiten.
Overweeg liquoronderzoek als er een sterke wens is om de ziekte van Alzheimer als oorzaak van de dementie uit te sluiten.
Overweeg bij jongere (<65 jaar) patiënten met dementie liquoronderzoek te verrichten, indien er twijfel is over de ziekte van Alzheimer als oorzaak. Liquor zonder een Alzheimer profiel kan leiden tot noodzakelijke diagnostiek naar een andere oorzaak van de dementie. Liquor met een Alzheimer profiel bij jonge patiënten wijst sterk op de ziekte van Alzheimer.
Bij twijfel over de interpretatie van de testresultaten neem contact op met een klinische expert.
Overwegingen
Voor- en nadelen van de interventie en de kwaliteit van het bewijs
Drie systematische reviews en een post-mortem case-control studie zijn gebruikt om te de accuratesse van liquormarkers om de klinische diagnose ziekte van Alzheimer vast te stellen bij patiënten met cognitieve klachten (Ritchie, 2014; Ritchie, 2017) of een vermoeden op Alzheimer dementie (Kokkinou, 2021; Struyfs, 2015) te evalueren. In totaal zijn 18 studies over amyloidβ42 (14 studies in Ritchie, 2014; 13 studies in Kokkinou, 2021; Struyfs 2015), acht studies over t-tau (zeven studies in Ritchie, 2017; Struyfs, 2015), zeven studies over p-tau (zes studies in Ritchie, 2017; Struyfs, 2015) en zes studies over p-tau/ amyloidβ ratio (vijf studies in Ritchie, 2017; Struyfs, 2015). Er zijn geen studies geïncludeerd over de diagnostische accuratesse van neurofilament light. In de geïncludeerde studies is er sprake van een hoog risico op bias, en de sensitiviteit en specificiteit vertonen een grote spreiding tussen studies. De overall bewijskracht voor de verschillende gerapporteerde liquormarkers (amyloidβ42, t-tau, p-tau en p-tau/amyloidβ ratio) komt daarmee op zeer laag. Op basis van de huidige literatuur kan er geen conclusie worden getrokken over de accuratesse van liquormarkers voor het stellen van de klinische diagnose ziekte van Alzheimer.
Een verlaagd amyloidβ 1-42, verhoogd tau en verhoogd p-tau181 in liquor wordt beschouwd als passend bij de ziekte van Alzheimer (Delaby, 2022). Bij ambigue testuitslagen kan de ratio tussen de verschillende markers, met name p-tau181/amyloidβ 1-42, worden meegewogen. De interpretatie hiervan vergt ervaring en de vooraf kans op basis van de klinische bevindingen dient hierbij altijd meegewogen te worden. Overleg bij twijfel met een collega met ervaring op het gebied van interpretatie van liquor uitslagen.
Er is onvoldoende onderbouwing voor een harde leeftijdsgrens. Daarom hanteert het cluster pragmatisch een leeftijdsgrens waarbij gesproken wordt over jonge (<65 jaar) en oude (>75 jaar) patiënten met dementie. Het cluster beschouwt de liquorwaarden boven de 75 jaar als onvoldoende sensitief en specifiek. Tussen de 65 en 75 kan de liquoruitslag in specifieke gevallen mogelijk van diagnostische meerwaarde zijn, maar met name vanwege de lagere specificiteit is voorzichtigheid geboden bij de interpretatie (Bouwman, 2009). Overleg bij twijfel met een collega met ervaring op het gebied van interpretatie van liquor uitslagen.
De huidige module gaat over patiënten bij wie dementie al is vastgesteld. Ook bij deze patiënten is de specificiteit waarschijnlijk laag, aangezien patiënten met Lewy body dementie (LBD) en vasculaire dementie ook afwijkend amyloidβ en (p-)tau in de liquor kunnen hebben. Dit komt bij 25-40% van de patiënten met LBD voor (van Harten, 2011; van Steenoven, 2016). Voor het klinisch onderscheid tussen LBD en AD heeft het geen toegevoegde waarde.
De toegevoegde waarde van liquoronderzoek is hoger bij relatief jonge patiënten (<65 jaar) met dementie (Bouwman, 2009). Liquor met normale waarden voor amyloidβ en (p-)tau maken de ziekte van Alzheimer als oorzaak onwaarschijnlijk (Spies, 2013; Spies, 2024; Vos, 2014). Dit heeft als consequentie dat bij die patiënten met dementie verder gezocht kan worden naar een andere oorzaak. De kans op een behandelbare oorzaak voor dementie, zoals een auto-immuun encefalitis, is echter klein.
Het liquor onderzoek kan helpen bij het onderscheid met fronto-temporale dementie (FTD). Een afwijkend amyloidβ past niet bij FTD, terwijl een verhoogd tau wel bij FTD kan worden gezien (Schoonenboom, 2012). Ook hier moet men rekening houden met afwijkende uitslagen bij toename van de leeftijd. De PICO voor de huidige module is niet specifiek gericht op de diagnostische waarde van liquor bij het onderscheid tussen AD en FTD of DLB. Hierover kan dan ook geen kwantitatieve uitspraak worden gedaan. Bij twijfel is het advies om met een academische geheugenpolikliniek te overleggen. Neurofilament light (NfL), in zowel liquor of bloed, is hier niet verder onderzocht omdat er geen meta-analyses gedaan zijn. Onderzoek laat zien dat NfL diagnostische waarde heeft voor het onderscheid tussen FTD en psychiatrische symptomen (Ducharme, 2020; Willemse, 2021), en de waarden zijn ook hoger in FTD dan in AD (Bridel, 2019). Het overgrote deel van het onderzoek naar liquor is gedaan bij witte mensen. Er is weinig bekend over testkarakteristieken bij patiënten met een andere etniciteit.
De huidige module gaat niet over het voorspellen van dementie bij mensen met lichte cognitieve stoornissen (MCI). Zie hiervoor de richtlijn MCI (NVN, 2018). Bij deze patiënten speelt een prognostische vraag.
Voor deze module wordt gebruikt gemaakt van klinische criteria voor dementie en voor de ziekte van Alzheimer voor toepassing in de klinische praktijk. Op dit moment is er geen aanbeveling om recentere onderzoekscriteria standaard toe te passen in de klinische praktijk waarin de definitie van de ziekte van Alzheimer verschuift van een klinische naar een biologische definitie.
Bij een verdenking van dementie diagnose kan liquordiagnostiek de diagnose van de ziekte van Alzheimer uitsluiten. Echter is het belangrijk om de leeftijd als belangrijke factor voor de interpretatie mee te nemen met name bij een afwijkend Alzheimer liquor profiel in combinatie met het klinisch beeld. Bij twijfel met betrekking tot de interpretatie van de liquoruitslagen wordt geadviseerd contact op te nemen met een collega met expertise op het gebied van dementie op jonge leeftijd (<65 jaar).
Waarden en voorkeuren van patiënten (en evt. hun verzorgers)
Bespreek duidelijk met patiënten de toegevoegde waarde van wat liquoronderzoek wel en niet kan. Bespreek expliciet wat het doel is en of dat doel bereikt kan worden met liquoronderzoek. Met name bij oudere patiënten met dementie (> 75 jaar) zal de toegevoegde waarde overwegend beperkt zijn. Zorg dat de patiënt goed begrijpt dat de uitslag van liquoronderzoek niet helpt om de prognose van de dementie te bepalen en niet nodig is om de indicatie voor eventuele behandeling met cholinesteraseremmers te starten. Dit gebeurt op basis van een klinische diagnose. Leg bij jongere patiënten met dementie (<65 jaar) uit dat een normale liquor kan betekenen dat er verder onderzoek naar een andere oorzaak van de dementie moet plaatsvinden. Liquoronderzoek wordt door patiënten soms als belastend ervaren. Patiënten willen vooral goed begrijpen waarom het onderzoek wel of niet nodig is om een diagnose te kunnen stellen. Het is een invasief onderzoek, maar met een zeer lage kans op complicaties. In de toekomst kan de niet-invasieve afname van bloed wellicht liquorafname deels vervangen.
Kosten (middelenbeslag)
Er zijn geen berekeningen naar kosteneffectiviteit van het gebruik van liquor onderzoek bij de diagnostiek bij patiënten met dementie gedaan. Omdat de toegevoegde waarde van liquor onderzoek nooit is onderzocht, kan ook niet worden geschat wat de eventuele kosteneffectiviteit is.
Aanvaardbaarheid, haalbaarheid en implementatie
Het uitvoeren van liquor onderzoek bij patiënten met dementie in de tweede en derde lijn (het domein van deze module) is in alle ziekenhuizen in Nederland mogelijk. Aangezien het slechts bij een beperkte groep patiënten in Nederland van mogelijke toegevoegde waarde is, is hiervoor geen aparte infrastructuur of extra zorgpersoneel nodig. Deze zorg is al voor iedereen in Nederland toegankelijk. De ingreep heeft een zeer laag risico op ernstige bijwerkingen.
Rationale van de aanbeveling: weging van argumenten voor en tegen de interventies
Er is geen onderzoek van voldoende methodologische kwaliteit waaruit blijkt wat de toegevoegde waarde van liquor onderzoek is bovenop klinische beoordeling met onderzoek van de cognitieve functies bij patiënten met dementie. De gerapporteerde sensitiviteit en specificiteit voor de testen in isolatie (dus los van de vooraf kans op basis van klinisch onderzoek inclusief beoordeling van de cognitieve functies) hebben een grote spreiding en zijn overwegend matig tot slecht. Met name de relatief lage specificiteit maken de test minder geschikt voor grootschalige toepassing. Vanwege het huidige gebrek aan prognostische waarde in ouderen en gebrek aan therapeutische consequenties op dit moment, is het uitvoeren van liquoronderzoek bij oudere patiënten met dementie (>75 jaar) waarschijnlijk van beperkte toegevoegde waarde. Een uitzondering hierop vormen patiënten met dementie op relatief jonge leeftijd (<65 jaar), bij wie bevestiging van de ziekte van Alzheimer belangrijk kan zijn, maar ook uitsluiten van de ziekte van Alzheimer belangrijk is omdat er dan verder onderzoek naar een andere oorzaak van dementie dient plaats te vinden.
Onderbouwing
Achtergrond
Dementia is a clinical diagnosis. Several diseases can cause demen’ia. Alzheimer's disease (AD) is the most common cause of dementia. Distinguishing AD from other forms of dementia can have consequences for the patient. Patients with mild cognitive impairment have a higher risk of developing dementia when they have abnormal AD cerebrospinal fluid (CSF)-biomarkers. The most studied CSF-biomarkers are amyloid β (1 to 42, Aβ42), total tau (Ttau), phosphorylated tau (Ptau), and neurofilament light (NfL). These CSF-biomarkers are nowadays used in clinical care in specialised centres. Aβ42 and Ptau proteins are the core proteins in the characteristic AD plaques and tangles, and the concentrations in CSF can be used to detect these AD pathologies. NfL and Ttau reflect an axonal loss and neuronal cell decay, which also occurs in other neurodegenerative diseases, and are thus less specific diagnostic markers.
Currently, it is unclear what the examination of CSF AD-biomarkers adds to the diagnostic accuracy in clinical practice in addition to non-invasive clinical assessment with cognitive examination for the diagnosis of dementia. The search question and PICO was limited to AD. In the considerations-section the role of CSF-biomarkers in differential diagnosis of different other major forms of dementia (such as lewy body dementia (LBD) and frontotemporal dementia (FTD)) is discussed in more general terms.
The golden standard for neurodegenerative diseases is a pathological anatomic (PA) diagnosis post-mortem. In absence of data on autopsy examinations and the long interval between diagnosis and post-mortem evaluation, the clinical diagnosis of AD in a multidisciplinary setting has been the gold standard. Regarding the usage of clinical guideline, it is important that the index test is not part of the clinical consensus diagnosis, to prevent circularity and an artificial increase in diagnostic accuracy.
Conclusies / Summary of Findings
1. Amyloidβ42
1.1 Specificity and sensitivity
|
Very low GRADE |
The evidence is uncertain about whether CSF amyloidβ42 is accurate in diagnosing ADD in patients suspected of having dementia.
Sources: Ritchie 2014, Kokkinou 2021, Struyfs 2015 |
1.2 Positive predictive value
|
- GRADE |
No evidence was found regarding the positive predictive value of CSF amyloidβ42 to diagnose Alzheimer disease in patients referred to a secondary care setting.
Sources: - |
1.3 Negative predictive value
|
- GRADE |
No evidence was found regarding the negative predictive value of CSF amyloidβ42 to diagnose Alzheimer disease in patients referred to a secondary care setting.
Sources: - |
2. Total-tau (CSF t-tau)
2.1 Specificity and sensitivity
|
Very low GRADE |
The evidence is uncertain about whether CSF t-tau is accurate in diagnosing ADD in patients suspected of having dementia.
Sources: Ritchie 2017, Struyfs 2015 |
2.2 Positive predictive value
|
- GRADE |
No evidence was found regarding the positive predictive value of CSF t-tau to diagnose Alzheimer disease in patients referred to a secondary care setting.
Sources: - |
2.3 Negative predictive value
|
- GRADE |
No evidence was found regarding the negative predictive value of CSF t-tau to diagnose Alzheimer disease in patients referred to a secondary care setting.
Sources: - |
3. Phosphorylated tau (CSF p-tau)
3.1 Specificity and sensitivity
|
Very low GRADE |
The evidence is uncertain about whether CSF p-tau is accurate in diagnosing ADD in patients suspected of having dementia.
Sources: Ritchie 2017, Struyfs 2015 |
3.2 Positive predictive value
|
- GRADE |
No evidence was found regarding the positive predictive value of CSF p-tau to diagnose Alzheimer disease in patients referred to a secondary care setting.
Sources: - |
3.3 Negative predictive value
|
- GRADE |
No evidence was found regarding the negative predictive value of CSF p-tau to diagnose Alzheimer disease in patients referred to a secondary care setting.
Sources: - |
4. CSF Amyloidβ /t-tau ratio
4.1 Specificity and sensitivity
|
Very low GRADE |
The evidence is uncertain about whether CSF Amyloidβ /t-tau ratio is accurate in diagnosing ADD in patients suspected of having dementia.
Sources: Struyfs 2015 |
4.2 Positive predictive value
|
- GRADE |
No evidence was found regarding the positive predictive value of CSF Amyloidβ/t-tau ratio to diagnose Alzheimer disease in patients referred to a secondary care setting.
Sources: - |
4.3 Negative predictive value
|
- GRADE |
No evidence was found regarding the negative predictive value of CSF Amyloidβ/t-tau ratio to diagnose Alzheimer disease in patients referred to a secondary care setting.
Sources: - |
5. CSF p-tau/Amyloidβ ratio
5.1 Specificity and sensitivity
|
Very low GRADE |
The evidence is uncertain about whether CSF p-tau/Amyloidβ ratio is accurate in diagnosing ADD in patients suspected of having dementia.
Sources: Ritchie 2017, Struyfs 2015 |
5.2 Positive predictive value
|
- GRADE |
No evidence was found regarding the positive predictive value of CSF p-tau/Amyloidβ ratio to diagnose Alzheimer disease in patients referred to a secondary care setting.
Sources: - |
5.3 Negative predictive value
|
- GRADE |
No evidence was found regarding the negative predictive value of CSF p-tau/Amyloidβ ratio to diagnose Alzheimer disease in patients referred to a secondary care setting.
Sources: - |
6. Neurofilament light
|
- GRADE |
No evidence was found regarding the diagnostic accuracy (sensitivity, specificity, positive predictive value or negative predictive value) of neurofilament light to diagnose Alzheimer disease in patients referred to a secondary care setting.
Sources: - |
Samenvatting literatuur
Description of studies
Kokkinou (2021) performed a systematic review and meta-analysis to determine the diagnostic accuracy of plasma and CSF amyloidß42 levels for distinguishing ADD from other dementia subtypes in people who meet the criteria for a dementia syndrome. A systematic literature search was performed up until 18 February 2020. Cross-sectional studies were included if they 1) differentiated people with ADD from other dementia subtypes, 2) had clinical assessment for dementia subtype, 3) measured CSF or plasma amyloidß42 levels, 4) had a reference standard based on diagnostic criteria for each dementia subtype such as the DSM-IV criteria, 5) sufficient data to construct two by two tables expressing amyloidß42 results by dementia subtype. Thirty-nine studies (5000 participants) were included that used CSF amyloidß42 to differentiate ADD from other dementia subtypes. Thirteen studies with 1704 participants assessed the accuracy of CSF amyloidß42 for distinguishing ADD from non-ADD. The other studies included comparison to specific dementia types. Positive cases were determined based on the amyloidß42 thresholds employed in the respective primary studies. Characteristics of the individual studies are summarized in the evidence tables.
Ritchie (2014) performed a systematic review and meta-analysis to determine the diagnostic accuracy of plasma and CSF Aß levels for detecting mild cognitive impairment (MCI) patients who would convert to Alzheimer's disease dementia (ADD) or other forms of dementia over time. A systematic literature search was performed up until 3 December 2012. Studies were included if they had 1) prospectively well-defined cohorts, 2) any accepted definition of MCI but no dementia, 3) baseline CSF or plasma Aß levels, or both, documented at or around the time the MCI diagnosis was made, 4) reference standard for Alzheimer’s dementia diagnosis based on the National Institute for National Institute of Neurological and Communicative Diseases and Stroke/ Alzheimer's Disease and Related Disorders Association (NINCDS- ADRDA) or the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria, 5) sufficient data to construct two by two tables expressing biomarker results by disease status. Studies were excluded if they included patients with possible other causes for dementia: psychiatric, neurological, metabolic, immunological, hormonal or cerebrovascular disorders, genetic cause or early onset ADD (if studies included patients below the age of 50 years, they were excluded). Although Ritchie 2014 was not limited to studies reporting amyloidß42, no studies were identified that reported on diagnostic accuracy for ADD for CSF amyloidß40 or CSF amyloidß42/amyloidß40 ratio. Fourteen studies with 1349 participants evaluated the use of CSF amyloidß42 to detect conversion to ADD. Of the 1349 participants, 436 developed Alzheimer’s dementia. Positive cases were determined based on the amyloidß42 thresholds employed in the respective primary studies. Because of variation in assay thresholds, the authors did not estimate summary sensitivity and specificity. Instead, the authors derived estimates of sensitivity at fixed values of specificity from the model fitted to produce a summary receiver operating characteristic (ROC) curve. The authors used a hierarchical summary receiver operating characteristic (HSROC) model that accounted for between study variability through the inclusion of random effects and derived sensitivities with 95% confidence intervals (CI) at median, lower and upper quartile values of the specificities from the included studies. Characteristics of the individual studies are summarized in the evidence tables.
Ritchie (2017) performed a systematic review and meta-analysis to determine the diagnostic accuracy of 1) CSF t-tau, 2) CSF p-tau, 3) the CSF t-tau/Aβ ratio and 4) the CSF p-tau/Aβ ratio index tests for detecting MCI patients who would convert to ADD or other forms of dementia over time. A systematic literature search was performed in January 2013. Studies were included if they had 1) prospectively well-defined cohorts, 2) any accepted definition of MCI but no dementia, 3) CSF t-tau or p-tau and CSF tau (t-tau or p-tau)/Aβ ratio documented at or around the time the MCI diagnosis was made, 4) reference standard for Alzheimer’s dementia diagnosis based on the NINCDS- ADRDA or DSM-IV criteria, 5) sufficient data to construct two by two tables expressing biomarker results by disease status. Fifteen studies were included with 1282 participants with MCI at baseline. 1172 participants had analysable data, 430 participants developed Alzheimer’s dementia and 130 participants other forms of dementia. Follow-up ranged from less than one year to over four years, but in the majority of studies was in the range of one to three years. Diagnostic accuracy was evaluated for CSF t-tau in seven studies (291 cases and 418 non-cases), CSF p-tau in six studies (164 cases and 328 non-cases), CSF p-tau/Aβ ratio in five studies (140 cases and 293 non-cases) and CSF t-tau/Aβ ratio in only one study. Characteristics of the individual studies are summarized in the evidence tables.
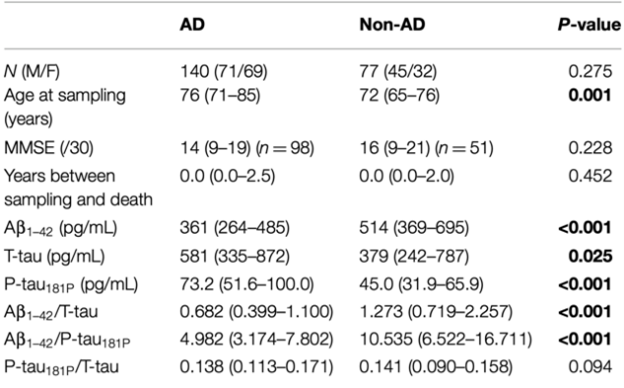
Struyfs 2015 performed a post mortem case-control study to examine the value of CSF p-tau as an ADD biomarker for a differential dementia diagnosis. 140 autopsy confirmed AD dementia and 77 autopsy confirmed non-AD dementia patients with CSF t-tau, p-tau and Aβ levels from lumbar puncture were included. All diagnoses were made according to established neuropathological criteria. For ADD, vascular dementia and dementia with Lewy bodies the Montine criteria were applied, frontotemporal dementia according to Cairns or Mackenzie and Creutzfeldt Jakob disease according to Markesbery. The ADD group also contained mixed dementia diagnoses where patients fulfilled the criteria for ADD in combination with minor pathology for other diseases such as cerebrovascular or Parkinson’s disease. Diagnostic test accuracy was assessed with ROC curve analyses to obtain the optimal cutoff values to discriminate AD. The cutoff values were determined by maximizing the Youden index: calculating the maximal sum of sensitivity and specificity. Characteristics are further summarized in the evidence tables.
Results
1. Amyloidβ42
1.1 Specificity and sensitivity
The systematic review and meta-analysis of Ritchie (2014) included fourteen studies with 1349 participants to assess the accuracy of CSF amyloidß42 to detect conversion from MCI to ADD compared to non-ADD. Of the 1349 participants, 436 were diagnosed with ADD. Individual study estimates of sensitivity were between 36% and 100% while the specificities were between 29% and 91%. Results are shown in Table 1.
The systematic review and meta-analysis of Kokkinou (2021) included thirteen studies with 1704 participants to assess the accuracy of CSF amyloidß42 for distinguishing ADD from non-ADD in people who meet the criteria for a dementia syndrome. Of the 1704 participants, 880 were diagnosed with ADD. Individual study estimates of sensitivity were between 42% and 100% while the specificities were between 25% and 82%. Results are shown in Table 1.
Struyfs 2015 assessed accuracy of CSF amyloidß42 for distinguishing ADD from non-ADD in 140 autopsy confirmed AD dementia and 77 autopsy confirmed non-AD dementia patients. The sensitivity was 79,3% and specificity 53,2% with an area under the curve (AUC) of 0,677 (95%CI 0,597-0,757) and an amyloidß42 cut-off of 500,27. Results are shown in Table 1.
1.2 Positive predictive value
Neither Ritchie (2014) or Kokkinou (2021) reported on the outcome positive predictive value.
1.3 Negative predictive value
Neither Ritchie (2014) or Kokkinou (2021) reported on the outcome negative predictive value.
Table 1. Sensitivity and specificity for diagnosing ADD based on CSF amyloidß42 (Ritchie, 2014 and Kokkinou, 2019).
|
Systematic review |
Study |
N |
TP |
FP |
FN |
TN |
Sensitivity |
Specificity |
Amyloidß42 threshold |
|
NA |
Struyfs 2015 |
217 |
- |
- |
- |
- |
0.793 |
0.532 |
500.27 ng/l |
|
Ritchie (2014) |
Bjerke 2009 |
162 |
18 |
43 |
2 |
99 |
0.90 (0.68 to 0.99) |
0.70 (0.61 to 0.77) |
512.0 ng/l |
|
Blom 2009 |
28 |
9 |
9 |
5 |
5 |
0.64 (0.35 to 0.87) |
0.36 (0.13 to 0.65) |
82.0 pM |
|
|
Chiasserini 2010 |
41 |
17 |
2 |
6 |
16 |
0.74 (0.52 to 0.90) |
0.89 (0.65 to 0.99) |
741.0 ng/l |
|
|
Galluzzi 2010 |
64 |
18 |
17 |
4 |
25 |
0.82 (0.60 to 0.95) |
0.60 (0.43 to 0.74) |
500.0 ng/l |
|
|
Hampel 2004 |
52 |
24 |
10 |
5 |
13 |
0.83 (0.64 to 0.94) |
0.57 (0.34 to 0.77) |
679.0 ng/l |
|
|
Hansson 2007 |
134 |
53 |
36 |
4 |
41 |
0.93 (0.83 to 0.98) |
0.53 (0.42 to 0.65) |
0.64 ng/ml |
|
|
Hertze 2010 |
160 |
47 |
33 |
5 |
75 |
0.90 (0.79 to 0.97) |
0.69 (0.60 to 0.78) |
209.0 pg/ml |
|
|
Kester 2011 |
100 |
32 |
16 |
10 |
42 |
0.76 (0.61 to 0.88) |
0.72 (0.59 to 0.83) |
495.0 pg/ml |
|
|
Monge-Argiles 2011 |
37 |
9 |
10 |
2 |
16 |
0.82 (0.48 to 0.98) |
0.62 (0.41 to 0.80) |
320.0 pg/ml |
|
|
Papaliagkas2009 |
53 |
5 |
11 |
0 |
37 |
1.00 (0.48 to 1.00) |
0.77 (0.63 to 0.88) |
472.0 pg/ml |
|
|
Parnetti 2006 |
55 |
4 |
4 |
7 |
40 |
0.36 (0.11 to 0.69) |
0.91 (0.78 to 0.97) |
500.0 pg/l |
|
|
Shaw 2009 |
196 |
32 |
113 |
5 |
46 |
0.86 (0.71 to 0.95) |
0.29 (0.22 to 0.37) |
192.0 pg/ml |
|
|
Vos 2013 |
214 |
62 |
41 |
29 |
82 |
0.68 (0.58 to 0.78) |
0.67 (0.58 to 0.75) |
500.0 pg/ml |
|
|
Zetterberg 2003 |
53 |
15 |
12 |
7 |
19 |
0.68 (0.45 to 0.86) |
0.61 (0.42 to 0.78) |
532.0 ng/l |
|
|
Kokkinou (2021) |
Brettschneider 2006 |
165 |
89 |
30 |
20 |
26 |
0.82 (0.73 to 0.88) |
0.46 (0.33 to 0.60) |
612.0 pg/ml |
|
Kapaki 2003 |
64 |
35 |
3 |
14 |
12 |
0.71 (0.57 to 0.83) |
0.80 (0.52 to 0.96) |
435.0 pg/ml |
|
|
Knapskog 2018 |
71 |
25 |
8 |
34 |
4 |
0.42 (0.30 to 0.56) |
0.33 (0.10 to 0.65) |
550.0 pg/ml |
|
|
Lewczuk 2004 |
33 |
19 |
2 |
3 |
9 |
0.86 (0.65 to 0.97) |
0.82 (0.48 to 0.98) |
500.0 pg/ml |
|
|
Lombardi 2018 |
35 |
28 |
1 |
4 |
2 |
0.88 (0.71 to 0.96) |
0.67 (0.09 to 0.99) |
650.0 pg/ml |
|
|
Maddalena2003 |
81 |
40 |
9 |
11 |
21 |
0.78 (0.65 to 0.89) |
0.70 (0.51 to 0.85) |
490.0 pg/ml |
|
|
Montine 2001 |
27 |
19 |
6 |
0 |
2 |
1.00 (0.82 to 1.00) |
0.25 (0.03 to 0.65) |
1125.0 pg/ml |
|
|
Perani 2016 |
75 |
40 |
15 |
7 |
13 |
0.85 (0.72 to 0.94) |
0.46 (0.28 to 0.66) |
500.0 pg/ml |
|
|
Rosler 2001 |
51 |
21 |
10 |
6 |
14 |
0.78 (0.58 to 0.91) |
0.58 (0.37 to 0.78) |
375.0 pg/ml |
|
|
Smach 2008 |
108 |
60 |
10 |
13 |
25 |
0.82 (0.71 to 0.90) |
0.71 (0.54 to 0.85) |
505.0 pg/ml |
|
|
Spies 2010 |
138 |
57 |
18 |
12 |
51 |
0.83 (0.72 to 0.91) |
0.74 (0.62 to 0.84) |
NR |
|
|
Tapiola 2000 |
107 |
55 |
11 |
25 |
16 |
0.69 (0.57 to 0.79) |
0.59 (0.39 to 0.78) |
340.0 pg/ml |
|
|
Tariciotti 2018 |
749 |
197 |
233 |
46 |
273 |
0.81 (0.76 to 0.86) |
0.54 (0.49 to 0.58) |
500.0 pg/ml |
Abbreviations: NA = Not applicable, NR = Not specified by authors, N= number of participants in study, TN = true negatives, TP = true positives, FN = false negatives, FP = false positives, CI = confidence interval.
2. Total-tau (CSF t-tau)
2.1 Specificity and sensitivity
The systematic review and meta-analysis of Ritchie (2017) included seven studies with 709 participants to assess the accuracy of CSF t-tau to detect conversion from MCI to ADD compared to non-ADD. Of the 709 participants, 291 were diagnosed with ADD. Individual study estimates of sensitivity were between 51% and 95% while the specificities were between 48% and 88%. Results are shown in Table 2.
Struyfs (2015) assessed accuracy of CSF t-tau for distinguishing ADD from non-ADD in 140 autopsy confirmed AD dementia and 77 autopsy confirmed non-AD dementia patients. The sensitivity was 62,1% and specificity 63,6% with an area under the curve (AUC) of 0,592 (95%CI 0,508-0,675) and a t-tau cut-off of 472,35. Results are shown in Table 2.
2.2 Positive predictive value
Ritchie (2017) did not report on the outcome positive predictive value.
2.3 Negative predictive value
Ritchie (2017) did not report on the outcome negative predictive value.
Table 2. Sensitivity and specificity for diagnosing ADD based on CSF t-tau (Ritchie, 2017).
|
Systematic review |
Study |
N |
TP |
FP |
FN |
TN |
Sensitivity |
Specificity |
|
NA |
Struyfs 2015 |
217 |
- |
- |
- |
- |
0.621 |
0.636 |
|
Ritchie (2017) |
Amlien 2013 |
39 |
5 |
4 |
4 |
26 |
0.56 (0.21 to 0.86) |
0.87 (0.69 to 0.96) |
|
Hampel 2004 |
52 |
26 |
12 |
3 |
11 |
0.90 (0.73 to 0.98) |
0.48 (0.27 to 0.69) |
|
|
Hansson 2006 |
134 |
29 |
9 |
28 |
68 |
0.51 (0.37 to 0.64) |
0.88 (0.79 to 0.95) |
|
|
Kester 2011 |
100 |
35 |
29 |
7 |
29 |
0.83 (0.69 to 0.93) |
0.50 (0.37 to 0.63) |
|
|
Monge-Argiles 2011 |
37 |
8 |
8 |
3 |
18 |
0.73 (0.39 to 0.94) |
0.69 (0.48 to 0.86) |
|
|
Palmqvist 2012 |
133 |
42 |
23 |
10 |
58 |
0.81 (0.67 to 0.90) |
0.72 (0.60 to 0.81) |
|
|
Vos 2013 |
214 |
65 |
28 |
26 |
95 |
0.71 (0.61 to 0.80) |
0.77 (0.69 to 0.84) |
Abbreviations: NA = Not applicable, N= number of participants in study, TN = true negatives, TP = true positives, FN = false negatives, FP = false positives, CI = confidence interval.
3. Phosphorylated tau (CSF p-tau)
3.1 Specificity and sensitivity
The systematic review and meta-analysis of Ritchie (2017) included six studies with 492 participants to assess the accuracy of CSF p-tau to detect conversion from MCI to ADD compared to non-ADD. Of the 492 participants, 164 were diagnosed with ADD. Individual study estimates of sensitivity were between 40% and 100% while the specificities were between 22% and 86%. Results are shown in Table 3.
Struyfs (2015) assessed accuracy of CSF p-tau for distinguishing ADD from non-ADD in 140 autopsy confirmed AD dementia and 77 autopsy confirmed non-AD dementia patients. The sensitivity was 77,9% and specificity 61,0% with an area under the curve (AUC) of 0,720 (95%CI 0,648-0,792) and a p-tau cut-off of 50,35. Results are shown in Table 3.
3.2 Positive predictive value
Ritchie (2017) did not report on the outcome positive predictive value.
3.3 Negative predictive value
Ritchie (2017) did not report on the outcome negative predictive value.
Table 3. Sensitivity and specificity for diagnosing ADD based on CSF p-tau (Ritchie, 2017).
|
Systematic review |
Study |
N |
TP |
FP |
FN |
TN |
Sensitivity |
Specificity |
|
NA |
Struyfs 2015 |
217 |
- |
- |
- |
- |
0.779 |
0.610 |
|
Ritchie (2017) |
Fellgiebel 2007 |
16 |
4 |
8 |
0 |
4 |
1.00 (0.40 to 1.00) |
0.33 (0.10 to 0.65) |
|
Hansson 2006 |
134 |
39 |
11 |
18 |
66 |
0.68 (0.55 to 0.80) |
0.86 (0.76 to 0.93) |
|
|
Koivunen 2008 |
14 |
2 |
7 |
3 |
2 |
0.40 (0.05 to 0.85) |
0.22 (0.03 to 0.60) |
|
|
Monge-Argiles 2011 |
37 |
9 |
11 |
2 |
15 |
0.82 (0.48 to 0.98) |
0.58 (0.37 to 0.77) |
|
|
Palmqvist 2012 |
133 |
35 |
11 |
17 |
70 |
0.67 (0.53 to 0.80) |
0.86 (0.77 to 0.93) |
|
|
Visser 2009 |
158 |
31 |
77 |
4 |
46 |
0.89 (0.73 to 0.97) |
0.37 (0.29 to 0.47) |
Abbreviations: NA = Not applicable, N= number of participants in study, TN = true negatives, TP = true positives, FN = false negatives, FP = false positives, CI = confidence interval.
4. CSF amyloidß/ t-tau ratio
4.1 Specificity and sensitivity
Struyfs (2015) assessed accuracy of CSF amyloidß42/ t-tau ratio for distinguishing ADD from non-ADD in 140 autopsy confirmed AD dementia and 77 autopsy confirmed non-AD dementia patients. The sensitivity was 75,0% and specificity 57,1% with an area under the curve (AUC) of 0,678 (95%CI 0,601-0,755) and an amyloidß42/ t-tau ratio cut-off of 1,08.
4.2 Positive predictive value
Struyfs (2015) did not report on the outcome positive predictive value.
4.3 Negative predictive value
Struyfs (2015) did not report on the outcome negative predictive value.
5. CSF p-tau/Aß ratio
5.1 Specificity and sensitivity
The systematic review and meta-analysis of Ritchie (2017) included five studies with 433 participants to assess the accuracy of CSF p-tau/Aß to detect conversion from MCI to ADD compared to non-ADD. Of the 433 participants, 140 were diagnosed with ADD. Individual study estimates of sensitivity were between 80% and 96% while the specificities were between 33% and 95%. Results are shown in Table 4.
Struyfs (2015) assessed accuracy of CSF amyloidß42/ p-tau ratio for distinguishing ADD from non-ADD in 140 autopsy confirmed AD dementia and 77 autopsy confirmed non-AD dementia patients. The sensitivity was 82,9% and specificity 59,7% with an area under the curve (AUC) of 0,770 (95%CI 0,703-0,837) and an amyloidß42/ p-tau ratio cut-off of 9,11.
5.2 Positive predictive value
Ritchie (2017) did not report on the outcome positive predictive value.
5.3 Negative predictive value
Ritchie (2017) did not report on the outcome negative predictive value.
Table 4. Sensitivity and specificity for diagnosing ADD based on CSF p-tau/Aß (Ritchie, 2017).
|
Systematic review |
Study |
N |
TP |
FP |
FN |
TN |
Sensitivity |
Specificity |
|
NA |
Struyfs 2015 |
217 |
- |
- |
- |
- |
0.829 |
0.597 |
|
Ritchie (2017) |
Hansson 2006 |
134 |
55 |
19 |
2 |
58 |
0.96 (0.88 to 1.00) |
0.75 (0.64 to 0.84) |
|
Koivunen 2008 |
14 |
4 |
6 |
1 |
3 |
0.80 (0.28 to 0.99) |
0.33 (0.07 to 0.70) |
|
|
Monge-Argiles 2011 |
37 |
9 |
9 |
2 |
17 |
0.82 (0.48 to 0.98) |
0.65 (0.44 to 0.83) |
|
|
Parnetti 2012 |
90 |
26 |
3 |
6 |
55 |
0.81 (0.64 to 0.93) |
0.95 (0.86 to 0.99) |
|
|
Visser 2009 |
158 |
28 |
49 |
7 |
74 |
0.80 (0.63 to 0.92) |
0.60 (0.51 to 0.69) |
Abbreviations: NA = Not applicable, NR = Not specified by authors, N= number of participants in study, TN = true negatives, TP = true positives, FN = false negatives, FP = false positives, CI = confidence interval.
6. Neurofilament light
None of the included studies (Ritchie 2014, Ritchie 2017, Kokkinou 2019) reported on the diagnostic accuracy (specificity, sensitivity, positive predictive value or negative predictive value) of neurofilament light.
Level of evidence of the literature
1. Amyloidβ42
The evidence was derived from two systematic reviews (Ritchie 2014, Kokkinou 2019) and one case-control study (Struyfs 2015). The level of evidence for all reported outcome measures started at ‘high quality’.
1.1 Specificity and sensitivity
The level of evidence regarding the outcome measure specificity was downgraded by 3 levels because of study limitations including issues with patient selection, index test and flow/timing (-2 risk of bias) and conflicting results (-1 inconsistency).
The level of evidence regarding the outcome measure sensitivity was downgraded by 3 levels because of study limitations including issues with patient selection, index test and flow/timing (-2 risk of bias) and conflicting results (-1 inconsistency).
1.2 Positive predictive value
The level of evidence was not assessed since none of the included studies reported on positive predictive value.
1.3 Negative predictive value
The level of evidence was not assessed since none of the included studies reported on negative predictive value.
2. Total-tau (CSF t-tau)
The evidence was derived from one systematic review (Ritchie 2017) and one case-control study (Struyfs 2015). The level of evidence for all reported outcome measures started at ‘high quality’.
2.1 Specificity and sensitivity
The level of evidence regarding the outcome measure specificity was downgraded by 3 levels because of study limitations including issues with patient selection, index test and flow/timing (-2 risk of bias) and conflicting results (-1 inconsistency).
The level of evidence regarding the outcome measure sensitivity was downgraded by 3 levels because of study limitations including issues with patient selection, index test and flow/timing (-2 risk of bias) and conflicting results (-1 inconsistency).
2.2 Positive predictive value
The level of evidence was not assessed since none of the included studies reported on positive predictive value.
2.3 Negative predictive value
The level of evidence was not assessed since none of the included studies reported on negative predictive value.
3. Phosphorylated tau (CSF p-tau)
The evidence was derived from one systematic review (Ritchie 2017) and one case-control study (Struyfs 2015). The level of evidence for all reported outcome measures started at ‘high quality’.
3.1 Specificity and sensitivity
The level of evidence regarding the outcome measure specificity was downgraded by 3 levels because of study limitations including issues with patient selection, index test and flow/timing (-2 risk of bias) and conflicting results (-1 inconsistency).
The level of evidence regarding the outcome measure sensitivity was downgraded by 3 levels because of study limitations including issues with patient selection, index test and flow/timing (-2 risk of bias) and conflicting results (-1 inconsistency).
3.2 Positive predictive value
The level of evidence was not assessed since none of the included studies reported on positive predictive value.
3.3 Negative predictive value
The level of evidence was not assessed since none of the included studies reported on negative predictive value.
4. CSF Amyloidβ /t-tau ratio
The level of evidence regarding the diagnostic accuracy of CSF t-tau/Amyloidβ ratio was not assessed since none of the included studies reported on CSF t-tau/Amyloidβ ratio.
The evidence was derived from one case-control study (Struyfs 2015). The level of evidence for all reported outcome measures started at ‘high quality’.
4.1 Specificity and sensitivity
The level of evidence regarding the outcome measure specificity was downgraded by 3 levels because of study limitations including issues or unclear reporting about patient selection, index test and flow/timing (-1 risk of bias) and only one study reporting a point estimate without a confidence interval (-2 imprecision).
The level of evidence regarding the outcome measure sensitivity was downgraded by 3 levels because of study limitations including issues or unclear reporting about patient selection, index test and flow/timing (-1 risk of bias) and only one study reporting a point estimate without a confidence interval (-2 imprecision).
4.2 Positive predictive value
The level of evidence was not assessed since none of the included studies reported on positive predictive value.
4.3 Negative predictive value
The level of evidence was not assessed since none of the included studies reported on negative predictive value.
5. CSF p-tau/Amyloidβ ratio
The evidence was derived from one systematic review (Ritchie 2017) and one case-control study (Struyfs 2015). The level of evidence for all reported outcome measures started at ‘high quality’.
5.1 Specificity and sensitivity
The level of evidence regarding the outcome measure specificity was downgraded by 3 levels because of study limitations including issues with patient selection, index test and flow/timing (-2 risk of bias) and number of included patients (-1 imprecision).
The level of evidence regarding the outcome measure sensitivity was downgraded by 3 levels because of study limitations including issues with patient selection, index test and flow/timing (-2 risk of bias) and conflicting results (-1 inconsistency).
5.2 Positive predictive value
The level of evidence was not assessed since none of the included studies reported on positive predictive value.
5.3 Negative predictive value
The level of evidence was not assessed since none of the included studies reported on negative predictive value.
6. Neurofilament light
The level of evidence regarding the diagnostic accuracy of neurofilament light was not assessed since none of the included studies reported on neurofilament light.
Zoeken en selecteren
A systematic review of the literature was performed to answer the following question: What is the diagnostic value of CSF markers (amyloidβ (1 to 42), ratio of amyloidβ (1 to 42)/ amyloidβ (1 to 40), total-tau, phosphorylated tau181, ratio ptau181/ amyloidβ(1-42), neurofilament light) in patients with cognitive impairment and suspected Alzheimer dementia?
P (Patients) = patients with cognitive impairment and suspected Alzheimer dementia
I (Intervention) = biomarkers in CSF (p-tau181/amyloidβ1-42, totaal-tau, gefosforyleerd tau, ratio ptau/aβ, neurofilament light)
C (Comparison) = no CSF markers (diagnosis based on clinical judgement)
R (Reference) = pathological anatomical (PA) diagnosis or the clinical consensus diagnosis.
O (Outcome measures) = diagnostic value (sensitivity, specificity, positive predictive value, negative predictive value)
T/S (Timing/setting) = Additional investigations to diagnose Alzheimer's disease after completing non-invasive clinical judgment with a suspicion of Alzheimer dementia.
Relevant outcome measures
The guideline development group considered specificity, sensitivity and positive and negative predictive value as crucial outcome measures for decision making. From a clinical perspective high specificity is more important than high sensitivity. In other words, avoiding false positive diagnoses is more important than avoiding false negative diagnoses, due to the absence of available disease-modifying treatments.
A priori, the working group did not define the outcome measures listed above but used the definitions used in the studies. The working group deliberately did not define minimal clinically important differences.
Search and select (Methods)
On 19 June 2023, a systematic search was performed in the databases Embase.com and Ovid/Medline for systematic reviews and randomized controlled trials (RCTs) on the diagnostic value of cerebrospinal fluid markers for Alzheimer's disease. The detailed search strategy is depicted under the tab Methods. The systematic literature search resulted in 574 unique hits. Studies were selected based on the following criteria:
- Systematic reviews (searched in at least two databases, detailed search strategy with search date, in- and exclusion criteria, exclusion table, risk of bias assessment and results of individual studies available) or RCTs;
- Full-text English language publication; and
- Studies according to the PICROTS.
Initially, 16 studies were selected based on title and abstract screening. After reading the full text, 12 studies were excluded (see the table with reasons for exclusion under the tab Methods), and four studies were included.
Results
Three systematic reviews and one observational study were included in the analysis of the literature. The three systematic reviews included 14 studies (Ritchie, 2014), 15 studies (Ritchie, 2017) and 39 studies (Kokkinou 2021). Important study characteristics and results are summarized in the evidence tables. The assessment of the risk of bias is summarized in the risk of bias tables.
Referenties
- Bouwman FH, Schoonenboom NS, Verwey NA, van Elk EJ, Kok A, Blankenstein MA, Scheltens P, van der Flier WM. CSF biomarker levels in early and late onset Alzheimer's disease. Neurobiol Aging. 2009 Dec;30(12):1895-901. doi: 10.1016/j.neurobiolaging.2008.02.007. Epub 2008 Apr 9. PMID: 18403055.
- Bridel C, van Wieringen WN, Zetterberg H, Tijms BM, Teunissen CE; and the NFL Group; Alvarez-Cermeño JC, Andreasson U, Axelsson M, Bäckström DC, Bartos A, Bjerke M, Blennow K, Boxer A, Brundin L, Burman J, Christensen T, Fialová L, Forsgren L, Frederiksen JL, Gisslén M, Gray E, Gunnarsson M, Hall S, Hansson O, Herbert MK, Jakobsson J, Jessen-Krut J, Janelidze S, Johannsson G, Jonsson M, Kappos L, Khademi M, Khalil M, Kuhle J, Landén M, Leinonen V, Logroscino G, Lu CH, Lycke J, Magdalinou NK, Malaspina A, Mattsson N, Meeter LH, Mehta SR, Modvig S, Olsson T, Paterson RW, Pérez-Santiago J, Piehl F, Pijnenburg YAL, Pyykkö OT, Ragnarsson O, Rojas JC, Romme Christensen J, Sandberg L, Scherling CS, Schott JM, Sellebjerg FT, Simone IL, Skillbäck T, Stilund M, Sundström P, Svenningsson A, Tortelli R, Tortorella C, Trentini A, Troiano M, Turner MR, van Swieten JC, Vågberg M, Verbeek MM, Villar LM, Visser PJ, Wallin A, Weiss A, Wikkelsø C, Wild EJ. Diagnostic Value of Cerebrospinal Fluid Neurofilament Light Protein in Neurology: A Systematic Review and Meta-analysis. JAMA Neurol. 2019 Sep 1;76(9):1035-1048. doi: 10.1001/jamaneurol.2019.1534. PMID: 31206160; PMCID: PMC6580449.
- Delaby C, Teunissen CE, Blennow K, Alcolea D, Arisi I, Amar EB, Beaume A, Bedel A, Bellomo G, Bigot-Corbel E, Bjerke M, Blanc-Quintin MC, Boada M, Bousiges O, Chapman MD, DeMarco ML, D'Onofrio M, Dumurgier J, Dufour-Rainfray D, Engelborghs S, Esselmann H, Fogli A, Gabelle A, Galloni E, Gondolf C, Grandhomme F, Grau-Rivera O, Hart M, Ikeuchi T, Jeromin A, Kasuga K, Keshavan A, Khalil M, Körtvelyessy P, Kulczynska-Przybik A, Laplanche JL, Lewczuk P, Li QX, Lleó A, Malaplate C, Marquié M, Masters CL, Mroczko B, Nogueira L, Orellana A, Otto M, Oudart JB, Paquet C, Paoletti FP, Parnetti L, Perret-Liaudet A, Peoc'h K, Poesen K, Puig-Pijoan A, Quadrio I, Quillard-Muraine M, Rucheton B, Schraen S, Schott JM, Shaw LM, Suárez-Calvet M, Tsolaki M, Tumani H, Udeh-Momoh CT, Vaudran L, Verbeek MM, Verde F, Vermunt L, Vogelgsang J, Wiltfang J, Zetterberg H, Lehmann S. Clinical reporting following the quantification of cerebrospinal fluid biomarkers in Alzheimer's disease: An international overview. Alzheimers Dement. 2022 Oct;18(10):1868-1879. doi: 10.1002/alz.12545. Epub 2021 Dec 22. PMID: 34936194; PMCID: PMC9787404.
- Ducharme S, Dols A, Laforce R, Devenney E, Kumfor F, van den Stock J, Dallaire-Théroux C, Seelaar H, Gossink F, Vijverberg E, Huey E, Vandenbulcke M, Masellis M, Trieu C, Onyike C, Caramelli P, de Souza LC, Santillo A, Waldö ML, Landin-Romero R, Piguet O, Kelso W, Eratne D, Velakoulis D, Ikeda M, Perry D, Pressman P, Boeve B, Vandenberghe R, Mendez M, Azuar C, Levy R, Le Ber I, Baez S, Lerner A, Ellajosyula R, Pasquier F, Galimberti D, Scarpini E, van Swieten J, Hornberger M, Rosen H, Hodges J, Diehl-Schmid J, Pijnenburg Y. Recommendations to distinguish behavioural variant frontotemporal dementia from psychiatric disorders. Brain. 2020 Jun 1;143(6):1632-1650. doi: 10.1093/brain/awaa018. Erratum in: Brain. 2020 Jul 1;143(7):e62. PMID: 32129844; PMCID: PMC7849953.
- Kokkinou M, Beishon LC, Smailagic N, Noel-Storr AH, Hyde C, Ukoumunne O, Worrall RE, Hayen A, Desai M, Ashok AH, Paul EJ, Georgopoulou A, Casoli T, Quinn TJ, Ritchie CW. Plasma and cerebrospinal fluid ABeta42 for the differential diagnosis of Alzheimer's disease dementia in participants diagnosed with any dementia subtype in a specialist care setting. Cochrane Database Syst Rev. 2021 Feb 10;2(2):CD010945. doi: 10.1002/14651858.CD010945.pub2. PMID: 33566374; PMCID: PMC8078224.
- van Harten AC, Kester MI, Visser PJ, Blankenstein MA, Pijnenburg YA, van der Flier WM, Scheltens P. Tau and p-tau as CSF biomarkers in dementia: a meta-analysis. Clin Chem Lab Med. 2011 Mar;49(3):353-66. doi: 10.1515/CCLM.2011.086. Epub 2011 Feb 23. PMID: 21342021.
- Nichols E, Merrick R, Hay SI, Himali D, Himali JJ, Hunter S, Keage HAD, Latimer CS, Scott MR, Steinmetz JD, Walker JM, Wharton SB, Wiedner CD, Crane PK, Keene CD, Launer LJ, Matthews FE, Schneider J, Seshadri S, White L, Brayne C, Vos T. The prevalence, correlation, and co-occurrence of neuropathology in old age: harmonisation of 12 measures across six community-based autopsy studies of dementia. Lancet Healthy Longev. 2023 Mar;4(3):e115-e125. doi: 10.1016/S2666-7568(23)00019-3. PMID: 36870337; PMCID: PMC9977689.
- NVN, 2018. Richtlijn Mild Cognitive Impairment (MCI). Beoordeeld: 04-09-2018. Link: https://richtlijnendatabase.nl/richtlijn/mild_cognitive_impairment_mci/startpagina_-_mild_cognitive_impairment_mci.html
- Ritchie C, Smailagic N, Noel-Storr AH, Takwoingi Y, Flicker L, Mason SE, McShane R. Plasma and cerebrospinal fluid amyloid beta for the diagnosis of Alzheimer's disease dementia and other dementias in people with mild cognitive impairment (MCI). Cochrane Database Syst Rev. 2014 Jun 10;2014(6):CD008782. doi: 10.1002/14651858.CD008782.pub4. PMID: 24913723; PMCID: PMC6465069.
- Ritchie C, Smailagic N, Noel-Storr AH, Ukoumunne O, Ladds EC, Martin S. CSF tau and the CSF tau/ABeta ratio for the diagnosis of Alzheimer's disease dementia and other dementias in people with mild cognitive impairment (MCI). Cochrane Database Syst Rev. 2017 Mar 22;3(3):CD010803. doi: 10.1002/14651858.CD010803.pub2. PMID: 28328043; PMCID: PMC6464349.
- Schoonenboom NS, Reesink FE, Verwey NA, Kester MI, Teunissen CE, van de Ven PM, Pijnenburg YA, Blankenstein MA, Rozemuller AJ, Scheltens P, van der Flier WM. Cerebrospinal fluid markers for differential dementia diagnosis in a large memory clinic cohort. Neurology. 2012 Jan 3;78(1):47-54. doi: 10.1212/WNL.0b013e31823ed0f0. Epub 2011 Dec 14. PMID: 22170879.
- van Steenoven I, Aarsland D, Weintraub D, Londos E, Blanc F, van der Flier WM, Teunissen CE, Mollenhauer B, Fladby T, Kramberger MG, Bonanni L, Lemstra AW; European DLB consortium. Cerebrospinal Fluid Alzheimer's Disease Biomarkers Across the Spectrum of Lewy Body Diseases: Results from a Large Multicenter Cohort. J Alzheimers Dis. 2016 Aug 18;54(1):287-95. doi: 10.3233/JAD-160322. PMID: 27567832; PMCID: PMC5535729.
- Struyfs H, Niemantsverdriet E, Goossens J, Fransen E, Martin JJ, De Deyn PP, Engelborghs S. Cerebrospinal Fluid P-Tau181P: Biomarker for Improved Differential Dementia Diagnosis. Front Neurol. 2015 Jun 17;6:138. doi: 10.3389/fneur.2015.00138. PMID: 26136723; PMCID: PMC4470274.
- Spies PE, Claassen JA, Peer PG, Blankenstein MA, Teunissen CE, Scheltens P, van der Flier WM, Olde Rikkert MG, Verbeek MM. A prediction model to calculate probability of Alzheimer's disease using cerebrospinal fluid biomarkers. Alzheimers Dement. 2013 May;9(3):262-8. doi: 10.1016/j.jalz.2012.01.010. Epub 2012 Nov 2. PMID: 23123231.
- Spies PE, Verbeek MM, Sjogren MJ, de Leeuw FE, Claassen JA. Alzheimer biomarkers and clinical Alzheimer disease were not associated with increased cerebrovascular disease in a memory clinic population. Curr Alzheimer Res. 2014 Jan;11(1):40-6. doi: 10.2174/1567205010666131120101352. PMID: 24251395.
- Vos SJ, Visser PJ, Verhey F, Aalten P, Knol D, Ramakers I, Scheltens P, Rikkert MG, Verbeek MM, Teunissen CE. Variability of CSF Alzheimer's disease biomarkers: implications for clinical practice. PLoS One. 2014 Jun 24;9(6):e100784. doi: 10.1371/journal.pone.0100784. PMID: 24959687; PMCID: PMC4069102.
- Vos SJ, Xiong C, Visser PJ, Jasielec MS, Hassenstab J, Grant EA, Cairns NJ, Morris JC, Holtzman DM, Fagan AM. Preclinical Alzheimer's disease and its outcome: a longitudinal cohort study. Lancet Neurol. 2013 Oct;12(10):957-65. doi: 10.1016/S1474-4422(13)70194-7. Epub 2013 Sep 4. PMID: 24012374; PMCID: PMC3904678.
- Willemse EAJ, Scheltens P, Teunissen CE, Vijverberg EGB. A neurologist's perspective on serum neurofilament light in the memory clinic: a prospective implementation study. Alzheimers Res Ther. 2021 May 18;13(1):101. doi: 10.1186/s13195-021-00841-4. PMID: 34006321; PMCID: PMC8132439.
Evidence tabellen
Evidence table for systematic reviews of diagnostic test accuracy studies
|
Study reference |
Study characteristics |
Patient characteristics |
Index test (test of interest) |
Reference test
|
Follow-up |
Outcome measures and effect size |
Comments |
|
Ritchie, 2014
Study characteristics and results for individual studies are extracted from the SR Ritchie, 2014 (unless stated otherwise) |
SR
Literature search up to December 2012
A: Bjerke 2009 B: Blom 2009 C: Chiasserini 2010 D: Galluzzi 2010 E: Hampel 2004 F: Hansson 2007 G: Hertze 2010 H: Kester 2011 I: Papaliagkas 2009 J: Monge-Argiles 2011 K: Parnetti 2006 L: Shaw 2009 M: Vos 2013 N: Zetterberg 2003
Study design: prospective cohort
Setting and Country: Participants were recruited from i) secondary care – outpatient clinic (n = 12); ii) secondary care – inpatients (n = 2); iii) community care (n = 2) and mixed setting (n = 1).
Source of funding and conflicts of interest: Not reported
|
Inclusion criteria SR: 1) prospectively well-defined cohorts 2) any accepted definition of MCI but no dementia 3) baseline CSF or plasma Aß levels, or both, documented at or around the time the MCI diagnosis was made 4) reference standard for Alzheimer’s dementia diagnosis based on the National Institute for National Institute of Neurological and Communicative Diseases and Stroke/ Alzheimer's Disease and Related Disorders Association (NINCDS- ADRDA) or the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria 5) sufficient data to construct two by two tables expressing biomarker results by disease status.
Exclusion criteria SR: Studies were excluded if they included patients with possible other causes for dementia: psychiatric, neurological, metabolic, immunological, hormonal or cerebrovascular disorders, genetic cause or early onset ADD (if studies included patients below the age of 50 years, they were excluded).
14 studies included
Important patient characteristics: Number of patients; characteristics important to the research question; for example, age, sex, bmi, ...
N, mean age A: 162 patients, 63.8 yrs B: 28 patients, 63.7 yrs C: 41 patients, 66.0 yrs D: 64 patients, 71.6 yrs E: 52 patients, 72.5 yrs F: 134 patients, 70.8 G: 159 patients, 72 years H: 100 patients, 67.8 yrs I: 53 patients, 68.7 yrs J: 37 patients, 73.4 yrs K: 55 patients, age NR L: 196 patients, 75.2 yrs M: 214 patients, 70.7 years N: 53 patients; mean age NR
Sex: A: 68/162 (42.0% male) B: 28/58 (46.4% male) C: 20/41 (48.9% male) D: 37/90 (41.0% male) E: 24/52 (46.2% male) F: 58/131 (32.8% male) G: 65/159 (40.8% male) H: 59/100 (59.0% male) I: 22/53 (41.5% male) J: 13/37 (35.1% male) K: gender NR L: 131/196 (66.8% male) M: 270/625 (43.2% male) N: gender NR |
Describe index and comparator tests* and cut-off point(s):
A: CSF Abeta42; 512 ng/l B: CSF Abeta42; 82 pM C: CSF Abeta42; 741 pg/ml D: CSF Abeta42; 500 pg/ml E: CSF Abeta 1-42; 679 ng/l F-1: CSF Abeta42; 0.64 ng/ml F-2: Abeta42/Abeta40 ratio; 0.95 ng/l G: CSF Abeta42; 209 pg/ml H: CSF Abeta42; 495 pg/ml I: CSF Abeta42; 472 pg/ml J: CSF Abeta42; 320 pg/ml K: CSF Abeta42; 500 pg/l L: CSF Abeta42; 192 pg/ml M: CSF Abeta1-42; 500 pg/ml N: CSF Abeta42; 532 ng/l ..... [use the format ‘character+number’ if 2 or more index tests are being compared, e.g. A-1, A-2, etc] |
Describe reference test and cut-off point(s):
NB: Cut-off NA for all reference tests
A: NINDS-ADRDA and ICD-10 criteria B: NINCDS-ADRDA C: NINCDS-ADRDA D: NINCDS-ADRDA E: NINCDS-ADRDA, DSM-IV criteria F: NINCDS-ADRDA, DSM-III-R G: NINCDS-ADRDA, DSM-II-R criteria H: NINCDS-ADRDA I: the criteria used to define progression to ADD were not clearly defined J: NINCDS-ADRDA K: NINCDS-ADRDA L: NINCDS-ADRDA M: NINCDS-ADRDA, DSM-IV criteria N: DSM-IV
Prevalence (%) [based on reference test at specified cut-off point] A: 20/174 (11.5%) B: 14/28 (50%) C: 23/41 (56.1%) D: 39/90 (43.3%) E: 29/52 (55.8%) F: 57/134 (42.5%) G: 55/166 (33.1%) H: 42/107 (39.3%) I: 5/53 (9.43%) J: 11/37 (29.7%) K: 11/55 (20%) L: 37/196 (18.9%) M: 91/214 (42.5%) N: 22/53 (41.5%)
For how many participants were no complete outcome data available? N (%) A: 12/162 (7.41%) B: 30/58 (51.7%) C: 0 (0%) D: 44/64 (68.8%) E: 0 (0%) F: 3/134 (2.24%) G: 7/160 (4.38%) H: 53/100 (53.0%) I: 0 (0%) J: 0 (0%) K: 0 (0%) L: 4/196 (2.04%) M: 17/214 (7.94%) N: 0 (0%)
Reasons for incomplete outcome data described? A: 8 patients had improved their condition at follow-up, 3 patients converted to a dementia disorder where no etiology could be clinically established, 1 patient converted to primary progressive aphasia B: reasons NR C: NA, because everyone had outcome data D: 16 refused follow-up assessment, 2 did not follow-up due to logistic problems, 24 refused LP, 2 failure to reach the arachnoid space due to osteoarthrosis E: NA, because everyone had outcome data F: 3 participants died before completion of 4 years follow-up and were excluded from the analyses because their cognitive ability was uncertain G: xMAP analysis resulted in technical errors H: 7 MCI participants who converted to other dementias were excluded from the analysis; 46 participants did not have follow-up data; no further information I: NA, because everyone had outcome data J: NA, because everyone had outcome data K: NA, because everyone had outcome data L: reasons NR M: some participants refused to participate or were untraceable or died before follow-up N: NA, because everyone had outcome data
|
Endpoint of follow-up: Outcomes were assessed
A: max 4 years B: mean 3.4 years; max 8.0 years C: max 4 years D: mean 2 years E: mean 0.7 years F: mean 5.2 years; max 6.8 years G: mean 4.7 years; max 7.2 years) H: mean 1.5 years; max 2.0 years I: mean 0.9 years; max 1.8 years J: 0.5 years K: max 1.0 years L: max 1.0 years M: mean 2.5 years (max 5 years) N: mean 1.67 years |
Outcome measures and effect size (include 95%CI and p-value if available):
Outcome measure-1/2 for Abeta42 e.g. sensitivity / specificity (%)], mean (95% CI) A: 90 [68, 99]% / 70 [61, 77]% B: 64 [35, 87]% / 36 [13, 65]% C: 74 [52, 90]% / 89 [65, 99]% D: 82 [60, 95]% / 60 [43, 74]% E: 83 [64, 94]% / 57 [34, 77]% F: 93 [83, 98]% / 53 [42, 65]% G: 90 [79, 97]% / 69 [60, 78]% H: 76 [61, 88]% / 72 [59, 83]% I: 100 [48, 100]% / 77 [63, 88%] J: 82[48, 98]% / 62 [41, 80]% K: 36 [11, 69]% / 91 [78, 97]% L: 86 [71, 95]% / 29 [22, 37]% M: 68 [58, 78]% / 67 [58, 75]% N: 68 [45, 86]% / 61 [42, 78]%
Pooled characteristics: Because of the variation in assay thresholds, the authors did not estimate summary sensitivity and specificity. Instead they derived estimates of sensitivity at fixed values of specificity from the hierarchical summary receiver operating characteristic (HSROC) model used to produce the ROC curve. At the median specificity of 64%, the sensitivity was 81% (95% CI 72 to 87). This equated to a positive likelihood ratio (LR+) of 2.22 (95% CI 2.00 to 2.47) and a negative likelihood ratio (LR–) of 0.31 (95% CI 0.21 to 0.48).
|
Study quality (ROB): method used and results per individual study
Methods used: QUADAS-2 tool Patient selection domain Studies C, F, G, I, and M scored unclear risk of bias, mainly because of poor reporting.
Studies E, H, L scored high risk of bias, mainly because the participants were not consecutively or randomly enrolled.
Index test domain Studies A, B, C, E, F, I, J, and N scored high risk of bias, mainly because the threshold used was not pre-specified and the optimal cut-off level was determined from ROC analyses; therefore, the accuracy of the plasma and CSF Aß biomarkers reported in these studies appeared to be overestimated.
Reference standard domain Studies A, B, C, D, E, F, H, J, I, K, and L scored unclear risk of bias, mainly because of poor reporting
Flow and timing domain Studies B, D, E, H, J, I, K, L, and N scored high risk of bias, because not all patients were accounted for in the analysis or the time interval between the index test and reference standard was not appropriate (duration of follow-up was shorter than one year).
Place of the index test in the clinical pathway: add-on
Choice of cut-off point: The studies defined their own cut-off points. Thus no overall choice was made and this could be a source for heterogeneity.
Author’s conclusion. We conclude that when applied to a population of patients with MCI, CSF Aß levels cannot be recommended as an accurate test for Alzheimer's disease.
Sensitivity analyses: ×Exclusion of one study (Kester 2011) – main results were robust ×Exclusion of 8 studies at high or unclear risk of bias for patient selection domain – main results were robust ×Exclusion of 8 studies at high risk of bias for Index test domain – main results were robust
|
|
Ritchie, 2017
Study characteristics and results for individual studies are extracted from the SR Ritchie, 2017 (unless stated otherwise) |
SR
Literature search up to January 2013
A: Amlien 2013 B: Hampel 2004 C: Hansson 2006 D: Kester 2011 E: Monge-Argiles 2011 F: Palmqvist 2012 G: Vos 2013 H: Fellgiebel 2007 I: Koivunen 2008 J: Visser 2009 K: Parnetti 2012
Study design: prospective cohort
Setting and Country: Participants were recruited from i) secondary care – outpatient clinic (n = 12); ii) secondary care – inpatients (n = 2); iii) community care (n = 2) and mixed setting (n = 1).
Source of funding and conflicts of interest: Not reported
|
Inclusion criteria SR: 1) prospectively well-defined cohorts, 2) any accepted definition of MCI but no dementia, 3) CSF t-tau or p-tau and CSF tau (t-tau or p-tau)/Aβ ratio documented at or around the time the MCI diagnosis was made, 4) reference standard for Alzheimer’s dementia diagnosis based on the NINCDS- ADRDA or DSM-IV criteria, 5) sufficient data to construct two by two tables expressing biomarker results by disease status.
Exclusion criteria SR: We excluded those studies that included people with MCI possibly caused by: i) a current or history of alcohol/drug abuse; ii) central nervous system (CNS) trauma (e.g. subdural haematoma), tumour, or infection; iii) other neurological conditions, e.g. Parkinson’s or Huntington’s diseases.
11 studies included
Important patient characteristics: Number of patients; characteristics important to the research question; for example, age, sex, bmi, ...
N, mean age A: 49 patients, MCI with abnormal CSF t-tau level: 64 (range 45 to 76); MC with normal CSF t-tau level: 58.5 (45 to 77) yrs B: 52 patients, mean 72.6 years C: 137 patients MCI-MCI (stable): 67 (50 to 86) yrs; MCI-AD 75 (59 to 85) yrs; MCI-other dementia 76 (54 to 82) yrs D: 153 patients, mean 67 ± 9 yrs 9 ± 7 yrs MCI-AD E: 37 patients, mean 73.43 ± 6.63 years F: 133 patients, MCI-MCI: mean 69.8 (range 55 to 85) yrs; MCI-AD: 75.3 (range 55 to 87) yrs; MCI-other dementias: 71.2 (59 to 83) yrs G: 231 patients, 70.7 ± 7.6 yrs naMCI; 70.7 yrs ± 7.8 aMC H: 16 patients, total sample: mean age 68.6 ± 7.9 yrs; MCI-MCI: 68.8 ± 10.0 yrs; MCI-progressive: 68.5 ± 5.9 yrs (4/8 MCI-AD: 69.5 ± 7.9 yrs) I: 15 patients, mean age 71.1 ± 7.2 yrs J: 168 patients, 70.0 ± 7.7 yrs naMCI; 70.0 ± 7.7 yrs aMCI; 66.0 ± 7.9 yrs SCI K: 90 patients, MCI-MCI: mean 66.35 ± 8.22 yrs; MCI-AD: mean 67.23 ± 9.04 yrs
Sex: A: 20/39 (51.3% male) B: 24/52 (46.2% male) C: 60/133 (45.1% male) D: 59/100 (59% male) E: 13/37 (35.1% male) F: MCI-AD: 16/52 (30.8% male) G: 270/605 (44.6% male) H: 9/16 (56.3% male) I: 9/15 (60% male) J: 88/168 (52.4% male) K: 66/100 (66% male) |
Describe index and comparator tests* and cut-off point(s):
A: CSF t-tau; ≥ 300 ng/L for age younger than 50 years; ≥ 450 ng/L for age 50 to 69 years; ≥ 500 ng/ L for age older than 70 years (Sjogren 2001) B: CSF t-tau; ≥ 479 ng/L C-1: CSF t-tau; > 350 ng/L C-2: CSF p-tau; ≥ 60 ng/L C-3: CSF p-tau/ABeta ratio; ˂ 6.5 ng/L D: CSF t-tau; > 356 pg/mL abnormal level; E-1: CSF t-tau; ≥77.5 pg/mL E-2: CSF p-tau; ≥ 54.5 pg/mL E-3: CSF t-tau/ABeta ratio; 0.18 E-4: CSF p-tau/ABeta ratio; 0.18 F-1: CSF t-tau; > 87 pg/mL F-2: CSF p-tau; >39 pg/mL F-3: CSF t-tau/ABeta ratio; cut-off NR G-1: CSF t-tau; > 450 pg/mL for age less than 70 years; > 500 pg/mL for age older than 70 years G-2: CSF t-tau/ABeta ratio; ABeta1–42/(240 1 [1.183 t-tau]) ˂ 1.0 H: CSF p-tau; ≥ 50 pg/mL I-1: CSF p-tau; ≥ 70 pg/mL I-2: CSF p-tau / ABeta ratio; <6.5 pg/mL J-1: CSF p-tau, used in clinical practice; ≥51 pg/mL J-2: CSF p-tau >90 percentile of controls after correction of age; ≥85pg/mL J-3: CSF p-tau/ABeta ratio; <9.92 (<10th percentile of reference group after correction for age) K: CSF p-tau / ABeta ratio; 1074.0 |
Describe reference test and cut-off point(s):
NB: cut-off points NR
A: The Global Deterioration Scale (Reisberg 1982) in combination with the research criteria for the diagnosis of Alzheimer's disease, International Working group (Dubois 2007) B: NINCDS-ADRDA criteria; DSM-IV criteria C: NINCDS-ADRDA and DSM-III-R for Alzheimer's disease dementia D: NINCDS-ADRDA criteria for Alzheimer's disease dementia E: NINCDS-ADRDA criteria F: NINCDS-ADRDA for AD; G: NINCDS-ADRDA criteria; DSM-IV criteria H: progression to Alzheimer's disease dementia was assumed if CDR reached 1 I: NINCS-ADRDA criteria; DSM-IV criteria J: NINCS-ADRDA criteria; DSM-IV criteria K: not specified. MCI participants were clinically evaluated at least once a year during 4-year follow-up period. However, it was reported that the NINCDS-ADRDA criteria were used at baseline to identify AD diagnostic group.
Prevalence (%) [based on reference test at specified cut-off point] A: 9/39 (23%) B: 29/52 (56%) C: 57/134 (42%) D: 42/100 (42%) E: 11/37 (28%) F: 52/133 (39%) G: 91/214 (42%) H: 4/16 (25%) I: 5/14 (36%) J: 35/158 (22%) K: 32/90 (35%)
For how many participants were no complete outcome data available? N (%) A: 17/39 (43.6)% B: 0 (0%) C: 43/180 (23.9%) D: 7/107 (6.54%) E: 0 (0%) F: 0 (0%) G: 17/231 (7.36%) H: 1/16 (..) I:1/15 (6.67%) J: 10/168 (5.95%) K: 0 (0%)
Reasons for incomplete outcome data described? A: 4 participants with MCI objected to re-examination, 1 died of unrelated causes, and 5 were excluded because of definite other diagnoses. 7 control subjects objected to re-examination. B: NA, because everyone had outcome data C: initially identified 180 consecutive participants with MCI; 43/180 not included in the study: 32 refused lumbar puncture and 11 non-usable CSF samples; 3 participants died before 4 years of follow-up (not included in the analysis) D: 7 MCI participants who converted to other forms of dementia were excluded from the analysis. E: NA, because everyone had outcome data F: NA, because everyone had outcome data G: some refused to participate or were untraceable or died before follow-up H: that participant was replaced by an additional, consecutively recruited patient from the memory clinic I:reasons for loss-to-follow-up NR J: CSF follow-up data were not available; the reason not given K: NA, because everyone had outcome data
|
Endpoint of follow-up: A: mean 2.6 ± 0.5 years (range 1.6 to 4 years) B: mean 0.7 ± 0.43 years (range 0.17 to 2 years) C: median 5.2 years (range 4.0 to 6.8 years) D: median 1.5 years (IQR 1.08 – 2 years) E: 0.5 years F: mean 5.9 years (range 3.2 to 8.8 years) G: mean 2.5 ± 1.0 years H: mean 1.63 ± 0.75 years I: 2 years J: range 1 to 3 years for MCI K: maximum: 4 years mean 3.40 ± 1.01 years |
Outcome measures and effect size (include 95%CI and p-value if available):
Outcome measure-1/2 [e.g. sensitivity / specificity (%)] A: CSF t-tau: 56 [21, 86]% / 87 [69, 96]% B: CSF t-tau: 90 [73, 98]% / 48 [27, 69]% C-1: CSF t-tau: 51 [37, 64]% / 88 [79, 95]% C-2: CSF p-tau: 68 [55, 80]% / 86 [76, 93]% C-3: CSF p-tau/ABeta ratio: 96 [88, 100]% / 75 [64, 84]% D: CSF t-tau: 83 [69, 93]% / 50 [37, 63]% E-1: CSF t-tau: 73 [39, 94]% / 69 [48, 86]% E-2: CSF p-tau: 82 [48, 98]% / 58 [37, 77]% E-3: CSF p-tau/ABeta ratio: 82 [48, 98]% / 65 [44, 83]% F-1: CSF t-tau: 81 [67, 90]% / 72 [60, 81]% F-2: CSF p-tau: 67 [53, 80]% / 86 [77, 93]% G: CSF t-tau: 71 [61, 80]% / 77 [69, 84]% H: CSF p-tau: 100 [40, 100]% / 33 [10, 65]% I-1: CSF p-tau: 40 [5, 85]% / 22 [3, 60]% I-2: CSF p-tau/ABeta ratio: 80 [28, 99]% / 33 [7, 70]% J-1: CSF p-tau: 89 [73, 97]% / 37 [29, 47]% J-2: CSF p-tau/ABeta ratio: 80 [63, 92]% / 60 [51, 69]% K: CSF p-tau/ABeta ratio: 81 [64, 93]% / 95 [86, 99]%
Pooled characteristics: The authors did not meta-analyse pairs of sensitivity and specificity using the bivariate model, because the results were not clinically interpretable due to the mixed thresholds used by studies. Instead, the authors fitted an HSROC meta-analysis models to estimate summary ROC curves and derived estimates of sensitivity and likelihood ratios at a fixed value of specificity (chosen a priori as the median specificity for the studies that were analysed when fitting the model).
Index test-1 CSF t-tau for diagnosing ADD 77 [95% CI 67 to 85] (sens, with fixed value of spec of 72)
Index test-2 CSF p-tau for diagnosing ADD 81 [64 to 91.5] (sens, with fixed value of spec of 47.5)
Index test-3 CSF p-tau/Abeta ratio for diagnosing ADD no meta-analysis because the studies were few and small.
|
Study quality (ROB): method used and results per individual study
Methods used: QUADAS-2 tool
Patient selection domain Studies B, D, and I scored high risk of bias because the participants were not consecutively or randomly enrolled or both the sampling procedure and exclusion criteria were not described. We stated that all included studies avoided a case-control design because we only considered data on performance of the index test to discriminate between people with MCI who converted to dementia and those who remained stable.
Studies H, E, F, K, J, and G scored unclear risk of bias, due to poor reporting on sampling procedure or exclusion criteria.
Index test domain Studies H, C, E, F, and K scored high risk of bias, due to poor reporting because the threshold used was not prespecified and the optimal cutoff level was determined from ROC analyses; therefore, the accuracy of the CSF biomarkers reported in these studies appeared to be overestimated.
Study A scored unclear risk of bias, due to poor reporting.
Reference standard domain Studies A, H, B, D, I, E, and K scored unclear risk of bias, mainly because it was not reported whether clinicians conducting follow-up were aware of initial CSF biomarker analysis results. Some studies did not clearly report the reference standards used for diagnosing Alzheimer’s disease dementia. We were not able to obtain the information about how the reference standard was obtained and by whom, due to poor reporting.
Flow and timing domain Studies A, H, I, E, K, J, and G scored unclear risk of bias, because not all participants were included in the analysis and/or the follow-up period was shorter than one year and/or reporting was poor.
Studies C and D scored high risk of bias because a large number of participants with non-Alzheimer's disease dementia were excluded from the analysis.
Place of the index test in the clinical pathway: add-on
Choice of cut-off point: The studies defined their own cut-off points. Thus no overall choice was made and this could be a source for heterogeneity.
Author’s conclusion. Given the insufficient evidence to evaluate the diagnostic value in MCI of CSF t-tau, CSF p-tau, CSF t-tau/ABeta ratio and CSF p-tau/ABeta ratio for Alzheimer's disease dementia and other forms of dementias examined in this review, particular attention should be paid to the risk of misdiagnosis and overdiagnosis of dementia (and therefore overtreatment) in clinical practice.
Sensitivity analyses: not performed, due to the limited number of studies.
Heterogeneity: Due to insufficient studies, meta-regression was not performed. So, sources of heterogeneity could not be examined.
|
|
Kokkinou, 2021
Study characteristics and results for individual studies are extracted from the SR Kokkinou, 2021 (unless stated otherwise) |
SR
Literature search up to February 2020
A: Brettschneider 2006 B: Kapaki 2003 C: Knapskog 2018 D: Lewczuk 2004 E: Lombardi 2018 F: Maddalena 2003 G: Montine 2001 H: Perani 2016 I: Smach 2008 J: Rosler 2001 K: Spies 2010 L: Tapiola 2000 M: Tariciotti 2018
Study design: cross-sectional case-control (ADD vs non-ADD dementia)
Setting and Country: Participants were recruited from i) secondary care – outpatient clinic (n = 12); ii) secondary care – inpatients (n = 2); iii) community care (n = 2) and mixed setting (n = 1).
Source of funding and conflicts of interest: No known conflicts of interest. The National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Dementia and Cognitive Improvement group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service or the Department of Health
|
Inclusion criteria SR: Cross-sectional studies that differentiated people with ADD from other dementia subtypes. Eligible studies required measurement of participant plasma or CSF ABeta42 levels and clinical assessment for dementia subtype. Delayed verification studies
Exclusion criteria SR: NR
13 studies included
Important patient characteristics: Number of patients; characteristics important to the research question; for example, age, sex, bmi, ...
N, mean age A: 109 AD patients, 71 (43-88) years for AD B: 49 AD patients, 67.6 ± 9.3 years for AD C: 205 patients, 84.8 ± 8.8 years for the total sample D: 22 AD patients, 68 (62–77) years for AD E: 32 AD patients, 66.5 ± 9.9 years for ADD; F: 81 patients (n=51 AD), 70.1±8.7 years for AD G: 19 AD patients, 65.3±8.7 years for AD H: 47 AD patients, 66±6.8 years for AD I: 73 AD patients, 73 (48–85) years for AD J: 27 probable AD patients, mean age NR K: 69 AD patients, 69±8 years for AD L: 80 probable AD, 71±8 years for probable AD M: 264 AD patients, 67.7 ± 10.4 years for ADD ….
Sex: A: 39/109 AD (35.8% male) B: 31/49 AD (63.3% male) C: 53.7% male (total sample) D: 6/22 AD (27.3% male) E: 19/32 AD (59.4% male) F: 54/100 (54% male) G: NR H: 26/47 AD (55.3% male) I: 49/73 (67.1% male) J: 9/27 probable AD (33.3% male) K: 34/69 AD (49.3% male) L: 34/80 probable AD (42.5% male) M: 106/264 AD (40.2% male) |
Describe index and comparator tests* and cut-off point(s):
A: ABeta42; 612 pg/ml B: ABeta42; 435 pg/ml C: ABeta42; 550 pg/ml D: ABeta42; 500 pg/ml E: ABeta42; 650 pg/ml F: ABeta42; 490 pg/ml G: ABeta42; 1125 pg/ml H: ABeta42; 500 pg/ml I: ABeta42; 505 pg/ml J: ABeta42; 375 pg/ml K: NR L: ABeta42; 340 pg/ml M: ABeta42; 500 pg/ml
[use the format ‘character+number’ if 2 or more index tests are being compared, e.g. A-1, A-2, etc] |
Describe reference test and cut-off point(s):
A: NINCDS-ADRDA criteria B: NINCDS-ADRDA criteria C: no diagnostic criteria specified; by consensus between two experienced physicians. D: NINCDS-ADRDA criteria E: NIA-AA criteria F: NINCDS-ADRDA G: NINCDS-ADRDA H: NINCDS-ADRDA I: NINCDS-ADRDA J: NINCDS-ADRDA K: NINCDS-ADRDA L: NINCDS-ADRDA M: NINCDS-ADRDA
Prevalence (%) [based on reference test at specified cut-off point] A: 109/165 (66.1%) B: 49/70 (70%) C: 138/205 (67.3%) D: 22/33 (66.7%) E: 32/45 (71.1%) F: 51/81 (63%) G: 19/27 (70.3%) H: 47/75 (62.7%) I: 73/108 (67.6%) J: 27/51 (52.9%) K: 69/138 (50%) L: 80/107 (74.8%) M: 264/1137 (23.2%)
For how many participants were no complete outcome data available? N (%) A: NR B: NR C: there is missing data, but for how many patients is NR D: NR E: 0 (0%) F: NR G: NR H: NR I: 20/73 (27.4%) J: NR K: NR L: NR M: 121 (10.7%).
Reasons for incomplete outcome data described? A: NR B: NR C: reasons of missing data NR D: NR E: NA, because everyone had outcome data F: NR G: NR H: NR I: no adequate CSF samples obtained J: NR K: NR L: NR
|
Endpoint of follow-up:
This is NA for all individual studies, because only cross-sectional studies were included in this SR.
|
Outcome measures and effect size (include 95%CI and p-value if available):
Outcome measure-1/2 [e.g. sensitivity / specificity (%)] A: 82 [73, 88]% / 46 [33, 60]% B: 71 [57, 83]% / 80 [52, 96]% C: 42 [30, 56]% / 33 [10, 65)% D: 86 [65, 97]% / 82 [48, 98]% E: 88 [71, 96]% / 67 [9, 99]% F: 78 [65, 89]% / 70 [51, 85]% G: 100 [82, 100]% / 25 [3, 65]% H: 85 [72, 94]% / 46 [28, 66]% I: 82 [71, 90]% / 71 [54, 85]% J: 78 [58, 91]% / 58 [37, 78]% K: 83 [72, 91]% / 74 [62, 84]% L 69 [57, 79]% / 59 [39, 78]% M 81 [76, 86]% / 54 [49, 58]%
Pooled characteristic Bivariate analysis, CSF ABeta42 [pooled cut-off at all thresholds]: Pooled sensitivity 79 [73, 85]%
Bivariate analysis, CSF ABeta42 [pooled cut-off at all thresholds]: Pooled specificity 60 [52, 67]%
|
Study quality (ROB): method used and results per individual study
Methods used: QUADAS-2 tool
Patient selection domain Studies A, B, D, E, F, G, I, K, L, M scored high risk of bias because of selective inclusion or enriching the population with a certain dementia type.
Studies C and H scored unclear risk of bias, because of poor reporting.
Index test domain Studies A, B, D, F, I, K, and L scored high risk of bias, because the ABeta42 threshold used was not pre-specified.
Studies C, E, G, and M scored unclear risk of bias, because they did not report whether investigators interpreted the ABeta42 data without knowledge of the dementia classification.
Reference Standard domain Study E scored high risk of bias, because investigators made the dementia assessment with the knowledge of the ABeta42 result.
Study M scored unclear risk of bias, because they did not report whether the investigator, who interpreted the results of reference standard, conducted the assessment without the knowledge of the ABeta42 data.
Flow and timing domain Studies I and M scored high risk of bias because the final clinical diagnosis was established (reassessed) 12 months or longer after CSF sampling.
Studies C, E, H, and K scored unclear risk of bias because not all patients were included in the analysis and/or studies did not report the interval between index test and reference standard.
Place of the index test in the clinical pathway: add-on
Choice of cut-off point: The studies defined their own cut-off points. Thus no overall choice was made and this could be a source for heterogeneity.
Sensitivity analyses: ·Effect of age: removal of studies C, G and J did not substantially alter pooled estimates of sensitivity or specificity. ·Effect of studies without a pre-specified test threshold: removal of eight studies did not alter pooled estimates of sensitivity or specificity.
Heterogeneity: Sources of heterogeneity were: 1) population and dementia subtype enrolled; 2) test threshold used, and 3) the diagnostic criteria and definition of ADD and dementia subtypes.
|
*comparator test equals the C of the PICO; two or more index/ comparator tests may be compared; note that a comparator test is not the same as a reference test (golden standard)
Evidence table for diagnostic test accuracy studies
|
Study reference |
Study characteristics |
Patient characteristics
|
Index test (test of interest) |
Reference test
|
Follow-up |
Outcome measures and effect size |
Comments |
|
Struyfs 2015 |
Type of study[1]: postmortem case-control in biobank samples.
Setting and country: Biobank, België
Funding and conflicts of interest: Several sources of funding reported, all Belgium government and European research funding grants. The authors declare no conflicts of interest. |
Inclusion criteria: Pathologically confirmed diagnoses of AD and non-AD, CSF samples available in biobank.
Exclusion criteria: None described
N=140 AD, 77 non-AD. Below characteristics as reported by the authors in table 1. All data are median values with 25th and 75th quartiles between brackets, except for N. To compare gender distribution between the groups, a Chi-square test was performed, while Mann–Whitney U tests were used to compare clinical and biomarker data between the groups. AD, Alzheimer’s disease; non-AD, dementia not due to Alzheimer’s disease; MMSE, mini-mental state examination; Aβ1–42, amyloid-β peptide of 42 amino acids; T-tau, total tau-protein; P-tau181P, tau phosphorylated at threonine 181. Bold values show statistically significant P-values (P < 0.05).
|
Describe index test: Lumbar puncture (LP) to collect CSF between the intervertebral space L3/L4 or L4/L5. Concentrations of Aβ1–42, T-tau, and P-tau181P were determined with commercially available single analyte ELISA- kits (respectively, INNOTEST® β-AMYLOID(1–42), INNOTEST® hTAU-Ag, and INNOTEST® PHOSPHO-TAU(181P); Fujirebio, Ghent, Belgium).
Cut-off point(s): No predefined cut-offs were specified. By maximizing the Youden index from the ROC curve, an optimal cut-off point was derived. These were: Aβ1–42= 500,27 T-tau = 472,35 P-tau181P = 50,35 Aβ1–42/ T-tau = 1,08 Aβ1–42/ P-tau181P = 9,11 |
Describe reference test[2]:
Pathological diagnoses according to standard neuropathological criteria.
Cut-off point(s): For ADD, vascular dementia and dementia with Lewy bodies the Montine criteria were applied, frontotemporal dementia according to Cairns or Mackenzie and Creutzfeld Jacob disease according to Markesbery. The ADD group also contained mixed dementia diagnoses where patients fulfilled the criteria for ADD in combination with minor pathology for other diseases such as cerebrovascular or Parkinson’s disease. |
Time between the index test en reference test: Unclear, years between sampling and death is reported but CSF test were performed later. Years between sampling and death were: AD = median 0 year (IQR 0-2,5 years) Non-AD = median 0 year (IQR 0-2 years).
For how many participants were no complete outcome data available? There was complete data for all included patients. |
Outcome measures and effect size (include 95%CI and p-value if available)4:
Amyloidß42: sensitivity 79,3% and specificity 53,2% with an area under the curve (AUC) of 0,677 (95%CI 0,597-0,757) and an amyloidß42 cut-off of 500,27. T-tau: sensitivity 62,1% and specificity 63,6% with an area under the curve (AUC) of 0,592 (95%CI 0,508-0,675) and a t-tau cut-off of 472,35. P-tau: sensitivity 77,9% and specificity 61,0% with an area under the curve (AUC) of 0,720 (95%CI 0,648-0,792) and a p-tau cut-off of 50,35. Amyloidß42/ t-tau: sensitivity 75,0% and specificity 57,1% with an area under the curve (AUC) of 0,678 (95%CI 0,601-0,755) and a amyloidß42/ t-tau ratio cut-off of 1,08. Amyloidß42/ t-tau: sensitivity 82,9% and specificity 59,7% with an area under the curve (AUC) of 0,770 (95%CI 0,703-0,837) and a amyloidß42/ p-tau ratio cut-off of 9,11. |
None |
[1] In geval van een case-control design moeten de patiëntkarakteristieken per groep (cases en controls) worden uitgewerkt. NB; case control studies zullen de accuratesse overschatten (Lijmer et al., 1999)
[2] De referentiestandaard is de test waarmee definitief wordt aangetoond of iemand al dan niet ziek is. Idealiter is de referentiestandaard de Gouden standaard (100% sensitief en 100% specifiek). Let op! dit is niet de “comparison test/index 2”.
4 Beschrijf de statistische parameters voor de vergelijking van de indextest(en) met de referentietest, en voor de vergelijking tussen de indextesten onderling (als er twee of meer indextesten worden vergeleken).
Table of quality assessment for systematic reviews of diagnostic studies
|
Study
|
Appropriate and clearly focused question?1
Yes/no/unclear |
Comprehensive and systematic literature search?2
Yes/no/unclear |
Description of included and excluded studies?3
Yes/no/unclear |
Description of relevant characteristics of included studies?4
Yes/no/unclear |
Assessment of scientific quality of included studies?5
Yes/no/unclear |
Enough similarities between studies to make combining them reasonable?6 Yes/no/unclear |
Potential risk of publication bias taken into account?7
Yes/no/unclear |
Potential conflicts of interest reported?8
Yes/no/unclear |
|
Ritchie, 2014 |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
No |
No |
|
Ritchie, 2017 |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
No |
No |
|
Kokkinou, 2021 |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
No |
No, only reported for systematic review. |
- Research question (PICO) and inclusion criteria should be appropriate (in relation to the research question to be answered in the clinical guideline) and predefined
- Search period and strategy should be described; at least Medline searched
- Potentially relevant studies that are excluded at final selection (after reading the full text) should be referenced with reasons
- Characteristics of individual studies relevant to the research question (PICO) should be reported
- Quality of individual studies should be assessed using a quality scoring tool or checklist (preferably QUADAS-2; COSMIN checklist for measuring instruments) and taken into account in the evidence synthesis
- Clinical and statistical heterogeneity should be assessed; clinical: enough similarities in patient characteristics, diagnostic tests (strategy) to allow pooling? For pooled data: at least 5 studies available for pooling; assessment of statistical heterogeneity and, more importantly (see Note), assessment of the reasons for heterogeneity (if present)? Note: sensitivity and specificity depend on the situation in which the test is being used and the thresholds that have been set, and sensitivity and specificity are correlated; therefore, the use of heterogeneity statistics (p-values; I2) is problematic, and rather than testing whether heterogeneity is present, heterogeneity should be assessed by eye-balling (degree of overlap of confidence intervals in Forest plot), and the reasons for heterogeneity should be examined.
- There is no clear evidence for publication bias in diagnostic studies, and an ongoing discussion on which statistical method should be used. Tests to identify publication bias are likely to give false-positive results, among available tests, Deeks’ test is most valid. Irrespective of the use of statistical methods, you may score “Yes” if the authors discuss the potential risk of publication bias.
- Sources of support (including commercial co-authorship) should be reported in both the systematic review and the included studies. Note: To get a “yes,” source of funding or support must be indicated for the systematic review AND for each of the included studies.
Risk of bias assessment diagnostic accuracy studies (QUADAS II, 2011)
|
Study reference |
Patient selection |
Index test |
Reference standard |
Flow and timing |
Comments with respect to applicability |
|
Struyfs 2015 |
Was a consecutive or random sample of patients enrolled? Unclear.
Was a case-control design avoided? No
Did the study avoid inappropriate exclusions? Yes
|
Were the index test results interpreted without knowledge of the results of the reference standard? Unclear
If a threshold was used, was it pre-specified? No
|
Is the reference standard likely to correctly classify the target condition? Yes
Were the reference standard results interpreted without knowledge of the results of the index test? Yes, although the pathologist had access to clinical files and neuroimaging data.
|
Was there an appropriate interval between index test(s) and reference standard? Unclear, not reported. Although years between sampling and death was 0 years in both the AD and non-AD group, the interquartile ranges were 0-2, 5 years for AD and 0-2 years for non-AD.
Did all patients receive a reference standard? Yes
Did patients receive the same reference standard? Yes
Were all patients included in the analysis? Yes
|
Are there concerns that the included patients do not match the review question? No
Are there concerns that the index test, its conduct, or interpretation differ from the review question? No
Are there concerns that the target condition as defined by the reference standard does not match the review question? No
|
|
CONCLUSION: Could the selection of patients have introduced bias?
RISK: UNCLEAR |
CONCLUSION: Could the conduct or interpretation of the index test have introduced bias?
RISK: HIGH
|
CONCLUSION: Could the reference standard, its conduct, or its interpretation have introduced bias?
RISK: LOW |
CONCLUSION Could the patient flow have introduced bias?
RISK: UNCLEAR |
|
Table of excluded studies
|
Reference |
Reason for exclusion |
|
Hazan J, Wing M, Liu KY, Reeves S, Howard R. Clinical utility of cerebrospinal fluid biomarkers in the evaluation of cognitive impairment: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2023 Feb;94(2):113-120. doi: 10.1136/jnnp-2022-329530. Epub 2022 Sep 12. PMID: 36096664. |
Excluded diagnostic accuracy: unclear reference, wrong population (not only suspected AD, but mixed with depression/parkinson and other diagnosis) and wrong outcome (change in diagnosis) |
|
Ma Y, Brettschneider J, Collingwood JF. A Systematic Review and Meta-Analysis of Cerebrospinal Fluid Amyloid and Tau Levels Identifies Mild Cognitive Impairment Patients Progressing to Alzheimer's Disease. Biomedicines. 2022 Jul 15;10(7):1713. doi: 10.3390/biomedicines10071713. PMID: 35885018; PMCID: PMC9313367. |
wrong outcome (standardized mean differences in metabolite levels) |
|
Fink HA, Linskens EJ, Silverman PC, McCarten JR, Hemmy LS, Ouellette JM, Greer NL, Wilt TJ, Butler M. Accuracy of Biomarker Testing for Neuropathologically Defined Alzheimer Disease in Older Adults With Dementia. Ann Intern Med. 2020 May 19;172(10):669-677. doi: 10.7326/M19-3888. Epub 2020 Apr 28. PMID: 32340038. |
wrong outcome (reported only median values for all studies, not per study results); wrong design (does not report study characteristics in enough detail to determine if studies are conform PICO ); |
|
Rivero-Santana A, Ferreira D, Perestelo-Pérez L, Westman E, Wahlund LO, Sarría A, Serrano-Aguilar P. Cerebrospinal Fluid Biomarkers for the Differential Diagnosis between Alzheimer's Disease and Frontotemporal Lobar Degeneration: Systematic Review, HSROC Analysis, and Confounding Factors. J Alzheimers Dis. 2017;55(2):625-644. doi: 10.3233/JAD-160366. PMID: 27716663. |
Wrong design (set up to discriminate AD from FTD and reports diagnostic accuracy for this comparison; unable to extract details for individuel studies, only reported point estimates for sensitivity and specificity) |
|
Olsson B, Lautner R, Andreasson U, Öhrfelt A, Portelius E, Bjerke M, Hölttä M, Rosén C, Olsson C, Strobel G, Wu E, Dakin K, Petzold M, Blennow K, Zetterberg H. CSF and blood biomarkers for the diagnosis of Alzheimer's disease: a systematic review and meta-analysis. Lancet Neurol. 2016 Jun;15(7):673-684. doi: 10.1016/S1474-4422(16)00070-3. Epub 2016 Apr 8. PMID: 27068280. |
Wrong outcome (reported ratio between Alzheimer’s disease and control cohorts or mild cognitive impairment due to Alzheimer’s disease and stable mild cognitive impairment cohorts with 95% CIs, not diagnostic accuracy measures) |
|
Mo JA, Lim JH, Sul AR, Lee M, Youn YC, Kim HJ. Cerebrospinal fluid β-amyloid1-42 levels in the differential diagnosis of Alzheimer's disease--systematic review and meta-analysis. PLoS One. 2015 Feb 24;10(2):e0116802. doi: 10.1371/journal.pone.0116802. PMID: 25710596; PMCID: PMC4339391. |
Unclear reporting (unable to extract details for bias evaluation and assessment diagnostic accuracy) |
|
Dumurgier J, Schraen S, Gabelle A, Vercruysse O, Bombois S, Laplanche JL, Peoc'h K, Sablonnière B, Kastanenka KV, Delaby C, Pasquier F, Touchon J, Hugon J, Paquet C, Lehmann S. Cerebrospinal fluid amyloid-β 42/40 ratio in clinical setting of memory centers: a multicentric study. Alzheimers Res Ther. 2015 Jun 1;7(1):30. doi: 10.1186/s13195-015-0114-5. PMID: 26034513; PMCID: PMC4450486. |
Wrong setting (longitudinal studies for conversion MCI to AD or other forms, not differential diagnosis) |
|
Ewers M, Mattsson N, Minthon L, Molinuevo JL, Antonell A, Popp J, Jessen F, Herukka SK, Soininen H, Maetzler W, Leyhe T, Bürger K, Taniguchi M, Urakami K, Lista S, Dubois B, Blennow K, Hampel H. CSF biomarkers for the differential diagnosis of Alzheimer's disease: A large-scale international multicenter study. Alzheimers Dement. 2015 Nov;11(11):1306-15. doi: 10.1016/j.jalz.2014.12.006. Epub 2015 Mar 21. PMID: 25804998. |
Wrong design (set up to distinguish AD from other dementia types, not about diagnostic accuracy for diagnosing AD) |
|
Rosa MI, Perucchi J, Medeiros LR, Fernandes B, Fernandes Dos Reis ME, Silva BR. Accuracy of cerebrospinal fluid Aβ(1-42) for Alzheimer's disease diagnosis: a systematic review and meta-analysis. J Alzheimers Dis. 2014;40(2):443-54. doi: 10.3233/JAD-132264. PMID: 24448789. |
wrong design (AD vs control) |
|
Tang W, Huang Q, Wang Y, Wang ZY, Yao YY. Assessment of CSF Aβ42 as an aid to discriminating Alzheimer's disease from other dementias and mild cognitive impairment: a meta-analysis of 50 studies. J Neurol Sci. 2014 Oct 15;345(1-2):26-36. doi: 10.1016/j.jns.2014.07.015. Epub 2014 Jul 15. PMID: 25086857. |
wrong outcome (standardized mean differences in metabolite levels) |
|
Tang W, Huang Q, Yao YY, Wang Y, Wu YL, Wang ZY. Does CSF p-tau181 help to discriminate Alzheimer's disease from other dementias and mild cognitive impairment? A meta-analysis of the literature. J Neural Transm (Vienna). 2014 Dec;121(12):1541-53. doi: 10.1007/s00702-014-1226-y. Epub 2014 May 10. PMID: 24817210. |
wrong outcome (standardized mean differences in metabolite levels) |
|
Mattsson N, Rosén E, Hansson O, Andreasen N, Parnetti L, Jonsson M, Herukka SK, van der Flier WM, Blankenstein MA, Ewers M, Rich K, Kaiser E, Verbeek MM, Olde Rikkert M, Tsolaki M, Mulugeta E, Aarsland D, Visser PJ, Schröder J, Marcusson J, de Leon M, Hampel H, Scheltens P, Wallin A, Eriksdotter-Jönhagen M, Minthon L, Winblad B, Blennow K, Zetterberg H. Age and diagnostic performance of Alzheimer disease CSF biomarkers. Neurology. 2012 Feb 14;78(7):468-76. doi: 10.1212/WNL.0b013e3182477eed. Epub 2012 Feb 1. PMID: 22302554; PMCID: PMC3280049. |
Wrong design (set up to compare age effects on diagnostic test accuracy) |
[1] In geval van een case-control design moeten de patiëntkarakteristieken per groep (cases en controls) worden uitgewerkt. NB; case control studies zullen de accuratesse overschatten (Lijmer et al., 1999)
[2] De referentiestandaard is de test waarmee definitief wordt aangetoond of iemand al dan niet ziek is. Idealiter is de referentiestandaard de Gouden standaard (100% sensitief en 100% specifiek). Let op! dit is niet de “comparison test/index 2”.
4 Beschrijf de statistische parameters voor de vergelijking van de indextest(en) met de referentietest, en voor de vergelijking tussen de indextesten onderling (als er twee of meer indextesten worden vergeleken).
Verantwoording
Beoordelingsdatum en geldigheid
Laatst beoordeeld : 25-11-2024
Algemene gegevens
De ontwikkeling van deze richtlijnmodule werd ondersteund door het Kennisinstituut van de Federatie Medisch Specialisten (www.demedischspecialist.nl/kennisinstituut) en werd gefinancierd uit de Kwaliteitsgelden Medisch Specialisten (SKMS). De financier heeft geen enkele invloed gehad op de inhoud van de richtlijnmodule.
Samenstelling werkgroep
Voor het ontwikkelen van de richtlijnmodules is in 2021 een multidisciplinaire cluster ingesteld. Dit cluster bestaat uit vertegenwoordigers van alle relevante organisaties die betrekking hebben op de zorg voor patiënten met dementie en delier.
Het cluster Cognitieve stoornissen en Dementie bestaat uit drie richtlijnen, zie hier voor de actuele clusterindeling. De stuurgroep bewaakt het proces van modulair onderhoud binnen het cluster. De expertisegroepsleden worden indien nodig gevraagd om hun expertise in te zetten voor een specifieke richtlijnmodule. Het cluster Cognitieve stoornissen en Dementie bestaat uit de volgende personen.
Clusterstuurgroep
- Dhr. Prof. Dr. M.G.M. (Marcel) Olde Rikkert (voorzitter), klinisch geriater, Radboudumc, Nijmegen; NVKG
- Dhr. Prof. Dr. A.R. (Tony) Absalom, anesthesioloog, Universitair Medisch Centrum Groningen, Groningen; NVA
- Dhr. Dr. J.H.J.M. (Jeroen) de Bresser, radioloog, Leids Universitair Medisch Centrum, Leiden; NVvR
- Mevr. J.L.M. (Josephine) Lambregts, patiëntvertegenwoordiger; Alzheimer Nederland
- Mevr. Dr. I.K. (Indrag) Lampe, psychiater, OLVG, Amsterdam; NVvP
- Mevr. Prof. Dr. B.C. (Barbara) van Munster, internist, Universitair Medisch Centrum Groningen, Groningen; NIV
- Dhr. Prof. Dr. E. (Edo) Richard, neuroloog, Radboudumc, Nijmegen; NVN
- Mevr. Prof. Dr. Ir. C. (Charlotte) Teunissen, klinisch chemicus, AmsterdamUMC, Amsterdam; NVKC
- Dhr. Dr. R.A.W. (Ronald) Verhagen, orthopedisch chirurg, Tergooi MC, Hilversum; NOV
Clusterexpertisegroep
- Dhr. Dr. A.P.A. (Auke) Appelman, radioloog, Universitair Medisch Centrum Groningen, Groningen; NVvR
- Dhr. Prof. Dr. B.N.M. (Bart) van Berckel, nucleair geneeskundige, AmsterdamUMC, Amsterdam; NVNG
- Mevr. Dr. R.L. (Rozemarijn) van Bruchem- Visser, internist ouderengeneeskunde, Erasmus Medisch Centrum, Rotterdam; NIV
- Dhr. B.P.H. (Bas) ter Brugge, specialist ouderengeneeskunde, Vilente, Doorwerth; Verenso
- Dhr. J.P.C.M. (Jos) van Campen, klinisch geriater, OLVG, Amsterdam; NVKG
- Dhr. Dr. J.A.H.R. (Jurgen) Claassen, klinisch geriater, Radboudumc, Nijmegen; NVKG
- Dhr. Dr. P.L.J. (Paul) Dautzenberg, klinisch geriater, Jeroen Bosch Ziekenhuis, ‘s-Hertogenbosch; NVKG
- Mevr. Dr. A.M. (Agnies) van Eeghen, Arts voor Verstandelijk Gehandicapten, AmsterdamUMC, Amsterdam; NVAVG
- Mevr. Dr. M.E.A. (Marlise) van Eersel, internist, Universitair Medisch Centrum Groningen, Groningen; NIV
- Mevr. Dr. S. (Sanne) Franzen, neuropsycholoog, Erasmus Medisch Centrum, Rotterdam; NIP
- Mevr. C.M. (Christa) de Geus, neurogeneticus, AmsterdamUMC, Amsterdam; NVKG
- Mevr. M. (Marjolein) Groeneveld, MSc, verpleegkundig Consulent Geriatrie, klinisch epidemioloog, Catharina Ziekenhuis, Eindhoven; V&VN
- Dhr. Dr. R.B. (Rients) Huitema, klinisch neuropsycholoog, Universitair Medisch Centrum Groningen, Groningen; NIP
- Dhr. Drs. A. (Ali) Lahdidioui, internist, HagaZiekenhuis, Den Haag; NIV
- Dhr. Dr. J. (Jules) Lavalaye, nucleair geneeskundige, St.Antonius Ziekenhuis, Nieuwegein/Utrecht; NVNG
- Mevr. Drs. L. (Lieke) Mitrov, ziekenhuisapotheker, Maasstad Ziekenhuis, Rotterdam; NVZA – tijdelijk vervangen door Shirley Sparla.
- Mevr. Dr. M. (Marieke) Perry, huisarts/onderzoeker, Radboudumc, Nijmegen; NHG
- Dhr. Dr. G. (Gerwin) Roks, neuroloog, Elisabeth-TweeSteden Ziekenhuis, Tilburg; NVN
- Mevr. Dr. T.R. (Rikje) Ruiter, internist, Maasstad Ziekenhuis, Rotterdam; NIV
- Mevr. A.J.B.P. (Astrid) Schoonbrood, ergotherapeut, Ergotherapiepraktijk Zuid-Limburg; Ergotherapie Nederland
- Mevr. Dr. N. (Niki) Schoonenboom, neuroloog, Spaarne Gasthuis, Haarlem/Hoofdorp, NVN
- Dhr. Dr. H. (Harro) Seelaar, neuroloog, Erasmus Medisch Centrum, Rotterdam; NVN
- Dhr. Dr. K.S. (Koen) Simons, intensivist-internist, Jeroen Bosch Ziekenhuis, ‘s-Hertogenbosch; NVIC
- Mevr. Drs. M.M.E. (Marlies) Sleegers-Kerkenaar, klinisch geriater, Sint Jans Gasthuis, Weert; NVKG
- Mevr. Drs. S.C.A. (Shirley) Sparla, ziekenhuisapotheker, St. Antonius Ziekenhuis; NVZA – tijdelijke vervanging van Lieke Mitrov.
- Mevr. Dr. P.E. (Petra) Spies, klinisch geriater, Gelre ziekenhuizen, Apeldoorn/Zutphen; NVKG
- Mevr. Dr. Ir. J. (Jenny) T. van der Steen, universitair hoofddocent, LUMC, Leiden en senior onderzoeker, Radboudumc, Nijmegen; persoonlijke titel
- Drs. VCJ (Vera) van Stek-Smits, neuropsycholoog-gezondheidszorgpsycholoog, Basalt Revalidatie HMC Westeinde, Den Haag, NIP
- Mevr. Prof. Dr. M. (Meike) Vernooij, radioloog, Erasmus Medisch Centrum, Rotterdam; NVvR
- Dhr. Dr. E.G.B. (Jort) Vijverberg, neuroloog, AmsterdamUMC, Amsterdam, NVN
- Mevr. Dr. M.A. (Marjolein) Wijngaarden, internist, Leids Universitair Medisch Centrum, Leiden, Leiden; NIV
Met ondersteuning van:
- Mevr. Dr. C.T.J. (Charlotte) Michels, adviseur, Kennisinstituut van de Federatie Medisch Specialisten
- Mevr. Dr. L.C. (Lotte) Houtepen, adviseur, Kennisinstituut van de Federatie Medisch Specialisten
- Mevr. Drs. L.C. (Laura) van Wijngaarden, junior adviseur, Kennisinstituut van de Federatie Medisch Specialisten
Belangenverklaringen
De Code ter voorkoming van oneigenlijke beïnvloeding door belangenverstrengeling is gevolgd. Alle clusterstuurgroepleden en actief betrokken expertisegroepsleden (fungerend als schrijver en/of meelezer bij tenminste één van de geprioriteerde richtlijnmodules) hebben schriftelijk verklaard of zij in de laatste drie jaar directe financiële belangen (betrekking bij een commercieel bedrijf, persoonlijke financiële belangen, onderzoeksfinanciering) of indirecte belangen (persoonlijke relaties, reputatiemanagement) hebben gehad. Gedurende de ontwikkeling of herziening van een richtlijnmodule worden wijzigingen in belangen aan de projectleider doorgegeven. De belangenverklaring wordt opnieuw bevestigd tijdens de commentaarfase. Een overzicht van de belangen van de clusterleden en betrokken expertisegroepsleden en het oordeel over het omgaan met eventuele belangen vindt u in onderstaande tabel. De ondertekende belangenverklaringen zijn op te vragen bij het secretariaat van het Kennisinstituut van de Federatie Medisch Specialisten.
Clusterstuurgroep: Gemelde (neven)functies en belangen stuurgroep
|
Clusterlid |
Functie |
Nevenfuncties |
Gemelde belangen |
Ondernomen actie |
|
Olde Rikkert* |
Hoogleraar Geriatrie, Radboudumc, Nijmegen |
Hoofdredacteur Nederlands Tijdschrift voor Geneeskunde |
Geen; uitsluitend ZonMw gefinancierd onderzoek dat overheidsbelang centraal stelt. Sinds 2017 geen farma-onderzoek meer. |
Geen restrictie |
|
Absalom |
Hoogleraar Anesthesiologie, UMCG, Groningen |
Consultancy werkzaamheden (betaald, alle betalingen aan UMCG) 6. Consultancy werk voor Becton Dickinson (Eysins, Switzerland) en Terumo (Tokyo, Japan) – technische advies over spuitpompen. Niet gerelateerd aan dementie/ MCI/ delier. |
Extern gefinancierd onderzoeken, maar financier heeft geen belangen bij de richtlijn. * Rigel Pharmaceuticals (San Francisco, USA) (PAST) * The Medicines Company (Parsippany, NJ, USA) (PAST) |
Geen restricties, omdat adviseurswerk niet gerelateerd is aan de afbakening van het cluster |
|
De Bresser |
- Neuroradioloog 1.0fte, LUMC, Leiden |
Geen |
Mijn onderzoek wordt mede gesponsord door Alzheimer Nederland. Deze financier heeft geen belang bij bepaalde uitkomsten van de richtlijn. |
Geen restrictie |
|
Lambregts |
Medewerker belangenbehartiging bij Alzheimer Nederland |
Geen |
Geen |
Geen restrictie |
|
Lampe |
Psychiater, OLVG Ziekenhuis, Amsterdam |
Geen |
Geen |
Geen restrictie |
|
Richard |
Neuroloog (1.0fte) Radboudumc, Nijmegen |
- Neuroloog-onderzoeker AmsterdamUMC, locatie AMC, gastvrijheidsaanstelling. |
Geen, uitsluitend onderzoek financiering van non-profit instellingen (e.g. ZonMw, Europese Commissie). |
Geen restrictie |
|
Teunissen |
Hoofd Neurochemisch laboratorium, Afdeling Klinische Chemie, AmsterdamUMC, locatie VUmc, Amsterdam |
*Adviseur voor educatief blad: Mednet Neurologie (betaald). * Alle betalingen zijn aan het AmsterdamUMC. |
Onderzoeksubsidies zijn ontvangen van European Commission (Marie Curie International Training Network, JPND), Health Holland, ZonMW, Alzheimer Drug Discovery Foundation, The Selfridges Group Foundation, Alzheimer Netherlands, Alzheimer Association, Innovative Medicines Initiatives 3TR, EPND, JPND, National MS Society, ABOARD, TAP-dementia. |
Geen restrictie |
|
Van Munster |
* Hoogleraar Interne Geneeskunde, Ouderengeneeskunde/Geriatrie, UMCG, Groningen. *Plaatsvervangend opleider Geriatrie, UMCG, Groningen. |
* 2020 – heden Voorzitter Alzheimer Centrum Groningen (alle functies zijn onbetaald) |
*2022 ZONMw: Young Onset Dementia- INCLUDED: Advance care planning. 2022 ZEGG/ZONMw: "The impact of a comprehensive geriatric assessment including advance care planning in acutely hospitalized frail patients with cognitive disorders: the GOA” study" |
Geen restrictie |
|
Verhagen |
Orthopedisch chirurg/opleider in Tergooi MC |
Geen |
Geen |
Geen restrictie |
Clusterexpertisegroep: Gemelde (neven)functies en belangen expertisegroep
Richtlijn Dementie: Module ‘Liquoronderzoek bij vermoeden op de ziekte van Alzheimer’
|
Clusterlid |
Functie |
Nevenfuncties |
Gemelde belangen |
Ondernomen actie |
|
Richard |
Neuroloog. 0.8fte Afd. Neurologie, Radboudumc, Nijmegen. 0.2fte Afd. Public and Occupational Heallth, Amsterdam UMC, Amsterdam |
- Neuroloog-onderzoeker AmsterdamUMC, locatie AMC, gastvrijheidsaanstelling. |
Geen, uitsluitend onderzoek financiering van non-profit instellingen (e.g. ZonMw, Europese Commissie). |
Geen restrictie |
|
Seelaar |
Neuroloog, Erasmus MC, Rotterdam Universitair docent, Erasmus MC, Rotterdam |
Lid van European Reference Network of rare neurological diseases (ERN-RND), specifiek van de werkgroep frontotemporale dementie. Projectleider van een fase 2 studie (INFRONT-2) NCT03987295 en een fase 3 studie (INFRONT-3). |
Extern gefinancieerd onderzoek * A phase 2 study to evaluate safety of long-term AL001 Dosing in FTD patients (INFRONTS-2). Company: Alector, Clinicaltrails.gov, NCT03987295 * ZonMW: meerdere studies naar frontotemporale dementie met daarin een rol als projectleider. * Alzheimer Nederland: meerdere studies naar frontotemporale dementie met daarin een rol als projectleider. * Gieskes Strijbis Fonds: onderzoek naar oorsprong frontotemporale dementie (rol als projectleider) Bluefield Foundation: onderzoek naar erfelijke frontotemporale dementie (rol als projectleider) De onderzoeken hebben geen inhoudelijke link met de richtlijn. |
Geen restrictie |
|
Teunissen |
Hoofd Neurochemisch laboratorium, Afdeling Klinische Chemie, AmsterdamUMC, locatie VUmc, Amsterdam |
*Adviseur voor educatief blad: Mednet Neurologie (betaald). * Alle betalingen zijn aan het AmsterdamUMC. |
Onderzoeksubsidies zijn ontvangen van European Commission (Marie Curie International Training Network, JPND), Health Holland, ZonMW, Alzheimer Drug Discovery Foundation, The Selfridges Group Foundation, Alzheimer Netherlands, Alzheimer Association, Innovative Medicines Initiatives 3TR, EPND, JPND, National MS Society, ABOARD, TAP-dementia. |
Geen restrictie |
Inbreng patiëntenperspectief
Er werd aandacht besteed aan het patiëntenperspectief door deelname van relevante patiëntenorganisaties aan de need-for-update en/of prioritering. De verkregen input is meegenomen bij het opstellen van de uitgangsvragen, de keuze voor de uitkomstmaten en bij het opstellen van de overwegingen. De conceptrichtlijnmodule is tevens ter commentaar voorgelegd aan alle relevante patiëntenorganisaties in de stuur- en expertisegroep (zie ‘Samenstelling cluster’ onder ‘Verantwoording’) en aan alle patiëntenorganisaties die niet deelnemen aan de stuur- en expertisegroep, maar wel hebben deelgenomen aan de need-for-update (zie ‘Need-for-update’ onder ‘Verantwoording’). De eventueel aangeleverde commentaren zijn bekeken en verwerkt.
Kwalitatieve raming van mogelijke financiële gevolgen in het kader van de Wkkgz
Bij de richtlijnmodule is conform de Wkkgz een kwalitatieve raming uitgevoerd of de aanbevelingen mogelijk leiden tot substantiële financiële gevolgen. Bij het uitvoeren van deze beoordeling is de richtlijnmodule op verschillende domeinen getoetst (zie het stroomschema).
Uit de kwalitatieve raming blijkt dat er waarschijnlijk geen substantiële financiële gevolgen zijn, zie onderstaande tabel.
Module |
Uitkomst raming |
Toelichting |
|
Module ‘Liquoronderzoek bij vermoeden op de ziekte van Alzheimer’ |
Geen substantiële financiële gevolgen |
Hoewel uit de toetsing volgt dat de aanbeveling(en) breed toepasbaar zijn (>40.000 patiënten), volgt uit de toetsing dat het overgrote deel van de zorgverleners al aan de norm voldoet, het geen nieuwe manier van zorgverlening betreft, het geen toename in het aantal voltijdsequivalenten of wijziging in het opleidingsniveau van zorgverleners betreft. Er worden daarom geen financiële gevolgen verwacht. |
Werkwijze
AGREE
Deze richtlijnmodule is opgesteld conform de eisen vermeld in het rapport Medisch Specialistische Richtlijnen 3.0 van de adviescommissie Richtlijnen van de Raad Kwaliteit. Dit rapport is gebaseerd op het AGREE II instrument (Appraisal of Guidelines for Research & Evaluation II; Brouwers, 2010).
Need-for-update, prioritering en uitgangsvragen
Tijdens de need-for-update fase en prioriteringsfase (februari, 2023) inventariseerde het cluster de geldigheid van de richtlijnmodules binnen het cluster. Naast de partijen die deelnemen aan de stuur- en expertisegroep zijn hier ook andere stakeholders voor benaderd. Per richtlijnmodule is aangegeven of deze geldig is, herzien moet worden, kan vervallen of moet worden samengevoegd. Ook was er de mogelijkheid om nieuwe onderwerpen aan te dragen die aansluiten bij één (of meerdere) richtlijn(en) behorend tot het cluster. De richtlijnmodules waarbij door één of meerdere partijen werd aangegeven herzien te worden, werden doorgezet naar de prioriteringsronde. Ook suggesties voor nieuwe richtlijnmodules werden doorgezet naar de prioriteringsronde. Afgevaardigden vanuit de partijen in de stuur- en expertisegroep werden gevraagd om te prioriteren (zie ‘Samenstelling cluster’ onder ‘Verantwoording’). Hiervoor werd de RE-weighted Priority-Setting (REPS) – tool gebruikt. De uitkomsten (ranklijst) werd gebruikt als uitgangspunt voor de discussie. Voor de geprioriteerde richtlijnmodules zijn door de het cluster concept-uitgangsvragen herzien of opgesteld en definitief vastgesteld.
Uitkomstmaten
Na het opstellen van de zoekvraag behorende bij de uitgangsvraag inventariseerde het cluster welke uitkomstmaten voor de patiënt relevant zijn, waarbij zowel naar gewenste als ongewenste effecten werd gekeken. Hierbij werd een maximum van acht uitkomstmaten gehanteerd. Het cluster waardeerde deze uitkomstmaten volgens hun relatieve belang bij de besluitvorming rondom aanbevelingen, als cruciaal (kritiek voor de besluitvorming), belangrijk (maar niet cruciaal) en onbelangrijk. Tevens definieerde het cluster tenminste voor de cruciale uitkomstmaten welke verschillen zij klinisch (patiënt) relevant vonden.
Methode literatuursamenvatting
Een uitgebreide beschrijving van de strategie voor zoeken en selecteren van literatuur is te vinden onder ‘Zoeken en selecteren’. Indien mogelijk werd de data uit verschillende studies gepoold in een random-effects model. Review Manager 5.4 werd indien mogelijk gebruikt voor de statistische analyses. De beoordeling van de kracht van het wetenschappelijke bewijs wordt hieronder toegelicht.
Beoordelen van de kracht van het wetenschappelijke bewijs
De kracht van het wetenschappelijke bewijs werd bepaald volgens de GRADE-methode. GRADE staat voor ‘Grading Recommendations Assessment, Development and Evaluation’ (zie http://www.gradeworkinggroup.org/). De basisprincipes van de GRADE-methodiek zijn: het benoemen en prioriteren van de klinisch (patiënt) relevante uitkomstmaten, een systematische review per uitkomstmaat, en een beoordeling van de bewijskracht per uitkomstmaat op basis van de acht GRADE-domeinen (domeinen voor downgraden: risk of bias, inconsistentie, indirectheid, imprecisie, en publicatiebias; domeinen voor upgraden: dosis-effect relatie, groot effect, en residuele plausibele confounding). GRADE onderscheidt vier gradaties voor de kwaliteit van het wetenschappelijk bewijs: hoog, redelijk, laag en zeer laag. Deze gradaties verwijzen naar de mate van zekerheid die er bestaat over de literatuurconclusie, in het bijzonder de mate van zekerheid dat de literatuurconclusie de aanbeveling adequaat ondersteunt (Schünemann, 2013; Hultcrantz, 2017).
Tabel 6. Gradaties voor de kwaliteit van wetenschappelijk bewijs
|
GRADE |
Definitie |
|
Hoog |
|
|
Redelijk |
|
|
Laag |
|
|
Zeer laag |
|
Bij het beoordelen (graderen) van de kracht van het wetenschappelijk bewijs in een richtlijnmodule volgens de GRADE-methodiek spelen grenzen voor klinische besluitvorming een belangrijke rol (Hultcrantz, 2017). Dit zijn de grenzen die bij overschrijding aanleiding zouden geven tot een aanpassing van de aanbeveling. Om de grenzen voor klinische besluitvorming te bepalen moeten alle relevante uitkomstmaten en overwegingen worden meegewogen. De grenzen voor klinische besluitvorming zijn daarmee niet één op één vergelijkbaar met het minimaal klinisch relevant verschil (Minimal Clinically Important Difference, MCID). Met name in situaties waarin een interventie geen belangrijke nadelen heeft en de kosten relatief laag zijn, kan de grens voor klinische besluitvorming met betrekking tot de effectiviteit van de interventie bij een lagere waarde (dichter bij het nuleffect) liggen dan de MCID (Hultcrantz, 2017).
Overwegingen (van bewijs naar aanbeveling)
Om te komen tot een aanbeveling zijn naast (de kwaliteit van) het wetenschappelijke bewijs ook andere aspecten belangrijk en worden meegewogen, zoals aanvullende argumenten uit bijvoorbeeld de biomechanica of fysiologie, waarden en voorkeuren van patiënten, kosten (middelenbeslag), aanvaardbaarheid, haalbaarheid en implementatie. Deze aspecten zijn systematisch vermeld en beoordeeld (gewogen) onder het kopje ‘Overwegingen’ en kunnen (mede) gebaseerd zijn op expert opinion. Hierbij is gebruik gemaakt van een gestructureerd format gebaseerd op het evidence-to-decision framework van de internationale GRADE Working Group (Alonso-Coello, 2016a; Alonso-Coello 2016b). Dit evidence-to-decision framework is een integraal onderdeel van de GRADE methodiek.
Formuleren van aanbevelingen
De aanbevelingen geven antwoord op de uitgangsvraag en zijn gebaseerd op het beschikbare wetenschappelijke bewijs en de belangrijkste overwegingen, en een weging van de gunstige en ongunstige effecten van de relevante interventies. De kracht van het wetenschappelijk bewijs en het gewicht dat door het cluster wordt toegekend aan de overwegingen, bepalen samen de sterkte van de aanbeveling. Conform de GRADE-methodiek sluit een lage bewijskracht van conclusies in de systematische literatuuranalyse een sterke aanbeveling niet a priori uit, en zijn bij een hoge bewijskracht ook zwakke aanbevelingen mogelijk (Agoritsas, 2017; Neumann, 2016). De sterkte van de aanbeveling wordt altijd bepaald door weging van alle relevante argumenten tezamen. Het cluster heeft bij elke aanbeveling opgenomen hoe zij tot de richting en sterkte van de aanbeveling zijn gekomen.
In de GRADE-methodiek wordt onderscheid gemaakt tussen sterke en zwakke (of conditionele) aanbevelingen. De sterkte van een aanbeveling verwijst naar de mate van zekerheid dat de voordelen van de interventie opwegen tegen de nadelen (of vice versa), gezien over het hele spectrum van patiënten waarvoor de aanbeveling is bedoeld. De sterkte van een aanbeveling heeft duidelijke implicaties voor patiënten, behandelaars en beleidsmakers (zie onderstaande tabel). Een aanbeveling is geen dictaat, zelfs een sterke aanbeveling gebaseerd op bewijs van hoge kwaliteit (GRADE gradering HOOG) zal niet altijd van toepassing zijn, onder alle mogelijke omstandigheden en voor elke individuele patiënt.
Tabel 7. Sterkte van de aanbevelingen
|
Implicaties van sterke en zwakke aanbevelingen voor verschillende richtlijngebruikers |
||
|
|
Sterke aanbeveling |
Zwakke (conditionele) aanbeveling |
|
Voor patiënten |
De meeste patiënten zouden de aanbevolen interventie of aanpak kiezen en slechts een klein aantal niet. |
Een aanzienlijk deel van de patiënten zouden de aanbevolen interventie of aanpak kiezen, maar veel patiënten ook niet. |
|
Voor behandelaars |
De meeste patiënten zouden de aanbevolen interventie of aanpak moeten ontvangen. |
Er zijn meerdere geschikte interventies of aanpakken. De patiënt moet worden ondersteund bij de keuze voor de interventie of aanpak die het beste aansluit bij zijn of haar waarden en voorkeuren. |
|
Voor beleidsmakers |
De aanbevolen interventie of aanpak kan worden gezien als standaardbeleid. |
Beleidsbepaling vereist uitvoerige discussie met betrokkenheid van veel stakeholders. Er is een grotere kans op lokale beleidsverschillen. |
Organisatie van zorg
Bij de ontwikkeling van de richtlijnmodule is expliciet aandacht geweest voor de organisatie van zorg: alle aspecten die randvoorwaardelijk zijn voor het verlenen van zorg (zoals coördinatie, communicatie, (financiële) middelen, mankracht en infrastructuur). Randvoorwaarden die relevant zijn voor het beantwoorden van deze specifieke uitgangsvraag zijn genoemd bij de overwegingen. Meer algemene, overkoepelende, of bijkomende aspecten van de organisatie van zorg worden behandeld in de richtlijnmodule Organisatie van zorg.
Commentaar- en autorisatiefase
De conceptrichtlijnmodule werd voorgelegd aan alle partijen die benaderd zijn voor de need-for-update fase. De commentaren werden verzameld en besproken met het cluster. Naar aanleiding van de commentaren werd de conceptrichtlijnmodule aangepast en definitief vastgesteld door het cluster. De definitieve richtlijnmodule werd ter autorisatie of goedkeuring voorgelegd aan de partijen die beschreven staan bij ‘Initiatief en autorisatie’ onder ‘Verantwoording’.
Literatuur
Agoritsas T, Merglen A, Heen AF, Kristiansen A, Neumann I, Brito JP, Brignardello-Petersen R, Alexander PE, Rind DM, Vandvik PO, Guyatt GH. UpToDate adherence to GRADE criteria for strong recommendations: an analytical survey. BMJ Open. 2017 Nov 16;7(11):e018593. doi: 10.1136/bmjopen-2017-018593. PubMed PMID: 29150475; PubMed Central PMCID: PMC5701989.
Alonso-Coello P, Schünemann HJ, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Rada G, Rosenbaum S, Morelli A, Guyatt GH, Oxman AD; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ. 2016 Jun 28;353:i2016. doi: 10.1136/bmj.i2016. PubMed PMID: 27353417.
Alonso-Coello P, Oxman AD, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Vandvik PO, Meerpohl J, Guyatt GH, Schünemann HJ; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 2: Clinical practice guidelines. BMJ. 2016 Jun 30;353:i2089. doi: 10.1136/bmj.i2089. PubMed PMID: 27365494.
Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, Fervers B, Graham ID, Grimshaw J, Hanna SE, Littlejohns P, Makarski J, Zitzelsberger L; AGREE Next Steps Consortium. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010 Dec 14;182(18):E839-42. doi: 10.1503/cmaj.090449. Epub 2010 Jul 5. Review. PubMed PMID: 20603348; PubMed Central PMCID: PMC3001530.
Hultcrantz M, Rind D, Akl EA, Treweek S, Mustafa RA, Iorio A, Alper BS, Meerpohl JJ, Murad MH, Ansari MT, Katikireddi SV, Östlund P, Tranæus S, Christensen R, Gartlehner G, Brozek J, Izcovich A, Schünemann H, Guyatt G. The GRADE Working Group clarifies the construct of certainty of evidence. J Clin Epidemiol. 2017 Jul;87:4-13. doi: 10.1016/j.jclinepi.2017.05.006. Epub 2017 May 18. PubMed PMID: 28529184; PubMed Central PMCID: PMC6542664.
Medisch Specialistische Richtlijnen 2.0 (2012). Adviescommissie Richtlijnen van de Raad Kwalitieit. http://richtlijnendatabase.nl/over_deze_site/over_richtlijnontwikkeling.html
Neumann I, Santesso N, Akl EA, Rind DM, Vandvik PO, Alonso-Coello P, Agoritsas T, Mustafa RA, Alexander PE, Schünemann H, Guyatt GH. A guide for health professionals to interpret and use recommendations in guidelines developed with the GRADE approach. J Clin Epidemiol. 2016 Apr;72:45-55. doi: 10.1016/j.jclinepi.2015.11.017. Epub 2016 Jan 6. Review. PubMed PMID: 26772609.
Schünemann H, Brożek J, Guyatt G, et al. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group, 2013. Available from http://gdt.guidelinedevelopment.org/central_prod/_design/client/handbook/handbook.html.
Zoekverantwoording
Literature search strategy
Algemene informatie
|
Cluster/richtlijn: Cluster cognitieve stoornissen en dementie (koploper 1, cyclus 2) |
|
|
Uitgangsvraag/modules: Wat is de diagnostische waarde van liquormarkers (amyloidβ (1 tot 42), amyloidβ (1 tot 40), totaal-tau, gefosforyleerd tau, ratio ptau/abeta, neurofilament light) bij patiënten met cognitieve stoornissen bij een vermoeden op Alzheimer dementie? |
|
|
Database(s): Embase.com, Ovid/Medline |
Datum: 19 juni 2023 |
|
Periode: Vanaf 2012 |
Talen: geen restrictie |
|
Literatuurspecialist: Alies van der Wal |
Rayyan review: https://rayyan.ai/reviews/701996 |
|
BMI-zoekblokken: voor verschillende opdrachten wordt (deels) gebruik gemaakt van de zoekblokken van BMI-Online https://blocks.bmi-online.nl/ Bij gebruikmaking van een volledig zoekblok zal naar de betreffende link op de website worden verwezen. |
|
Zoekopbrengst
|
|
EMBASE |
OVID/MEDLINE |
Ontdubbeld |
|
SR |
140 |
91 |
159 |
|
RCT |
253 |
424 |
415 |
|
Observationele studies |
1629 |
1110 |
1903 |
|
Totaal |
393 |
515 |
574* |
*in Rayyan
Zoekstrategie
Embase.com
|
No. |
Query |
Results |
|
#14 |
#7 AND (#10 OR #11) NOT (#12 OR #13) = observationeel |
1629 |
|
#13 |
#7 AND #9 NOT #12 = RCT |
253 |
|
#12 |
#7 AND #8 = SR |
140 |
|
#11 |
'case control study'/de OR 'comparative study'/exp OR 'control group'/de OR 'controlled study'/de OR 'controlled clinical trial'/de OR 'crossover procedure'/de OR 'double blind procedure'/de OR 'phase 2 clinical trial'/de OR 'phase 3 clinical trial'/de OR 'phase 4 clinical trial'/de OR 'pretest posttest design'/de OR 'pretest posttest control group design'/de OR 'quasi experimental study'/de OR 'single blind procedure'/de OR 'triple blind procedure'/de OR (((control OR controlled) NEAR/6 trial):ti,ab,kw) OR (((control OR controlled) NEAR/6 (study OR studies)):ti,ab,kw) OR (((control OR controlled) NEAR/1 active):ti,ab,kw) OR 'open label*':ti,ab,kw OR (((double OR two OR three OR multi OR trial) NEAR/1 (arm OR arms)):ti,ab,kw) OR ((allocat* NEAR/10 (arm OR arms)):ti,ab,kw) OR placebo*:ti,ab,kw OR 'sham-control*':ti,ab,kw OR (((single OR double OR triple OR assessor) NEAR/1 (blind* OR masked)):ti,ab,kw) OR nonrandom*:ti,ab,kw OR 'non-random*':ti,ab,kw OR 'quasi-experiment*':ti,ab,kw OR crossover:ti,ab,kw OR 'cross over':ti,ab,kw OR 'parallel group*':ti,ab,kw OR 'factorial trial':ti,ab,kw OR ((phase NEAR/5 (study OR trial)):ti,ab,kw) OR ((case* NEAR/6 (matched OR control*)):ti,ab,kw) OR ((match* NEAR/6 (pair OR pairs OR cohort* OR control* OR group* OR healthy OR age OR sex OR gender OR patient* OR subject* OR participant*)):ti,ab,kw) OR ((propensity NEAR/6 (scor* OR match*)):ti,ab,kw) OR versus:ti OR vs:ti OR compar*:ti OR ((compar* NEAR/1 study):ti,ab,kw) OR (('major clinical study'/de OR 'clinical study'/de OR 'cohort analysis'/de OR 'observational study'/de OR 'cross-sectional study'/de OR 'multicenter study'/de OR 'correlational study'/de OR 'follow up'/de OR cohort*:ti,ab,kw OR 'follow up':ti,ab,kw OR followup:ti,ab,kw OR longitudinal*:ti,ab,kw OR prospective*:ti,ab,kw OR retrospective*:ti,ab,kw OR observational*:ti,ab,kw OR 'cross sectional*':ti,ab,kw OR cross?ectional*:ti,ab,kw OR multicent*:ti,ab,kw OR 'multi-cent*':ti,ab,kw OR consecutive*:ti,ab,kw) AND (group:ti,ab,kw OR groups:ti,ab,kw OR subgroup*:ti,ab,kw OR versus:ti,ab,kw OR vs:ti,ab,kw OR compar*:ti,ab,kw OR 'odds ratio*':ab OR 'relative odds':ab OR 'risk ratio*':ab OR 'relative risk*':ab OR 'rate ratio':ab OR aor:ab OR arr:ab OR rrr:ab OR ((('or' OR 'rr') NEAR/6 ci):ab))) |
14177310 |
|
#10 |
'major clinical study'/de OR 'clinical study'/de OR 'case control study'/de OR 'family study'/de OR 'longitudinal study'/de OR 'retrospective study'/de OR 'prospective study'/de OR 'comparative study'/de OR 'cohort analysis'/de OR ((cohort NEAR/1 (study OR studies)):ab,ti) OR (('case control' NEAR/1 (study OR studies)):ab,ti) OR (('follow up' NEAR/1 (study OR studies)):ab,ti) OR (observational NEAR/1 (study OR studies)) OR ((epidemiologic NEAR/1 (study OR studies)):ab,ti) OR (('cross sectional' NEAR/1 (study OR studies)):ab,ti) |
7692378 |
|
#9 |
'clinical trial'/exp OR 'randomization'/exp OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp OR 'placebo'/exp OR 'prospective study'/exp OR rct:ab,ti OR random*:ab,ti OR 'single blind':ab,ti OR 'randomised controlled trial':ab,ti OR 'randomized controlled trial'/exp OR placebo*:ab,ti |
3811345 |
|
#8 |
'meta analysis'/exp OR 'meta analysis (topic)'/exp OR metaanaly*:ti,ab OR 'meta analy*':ti,ab OR metanaly*:ti,ab OR 'systematic review'/de OR 'cochrane database of systematic reviews'/jt OR prisma:ti,ab OR prospero:ti,ab OR (((systemati* OR scoping OR umbrella OR 'structured literature') NEAR/3 (review* OR overview*)):ti,ab) OR ((systemic* NEAR/1 review*):ti,ab) OR (((systemati* OR literature OR database* OR 'data base*') NEAR/10 search*):ti,ab) OR (((structured OR comprehensive* OR systemic*) NEAR/3 search*):ti,ab) OR (((literature NEAR/3 review*):ti,ab) AND (search*:ti,ab OR database*:ti,ab OR 'data base*':ti,ab)) OR (('data extraction':ti,ab OR 'data source*':ti,ab) AND 'study selection':ti,ab) OR ('search strategy':ti,ab AND 'selection criteria':ti,ab) OR ('data source*':ti,ab AND 'data synthesis':ti,ab) OR medline:ab OR pubmed:ab OR embase:ab OR cochrane:ab OR (((critical OR rapid) NEAR/2 (review* OR overview* OR synthes*)):ti) OR ((((critical* OR rapid*) NEAR/3 (review* OR overview* OR synthes*)):ab) AND (search*:ab OR database*:ab OR 'data base*':ab)) OR metasynthes*:ti,ab OR 'meta synthes*':ti,ab |
936329 |
|
#7 |
#6 AND [2012-2023]/py |
2603 |
|
#6 |
#5 NOT ('conference abstract'/it OR 'editorial'/it OR 'letter'/it OR 'note'/it) NOT (('animal'/exp OR 'animal experiment'/exp OR 'animal model'/exp OR 'nonhuman'/exp) NOT 'human'/exp) NOT (('adolescent'/exp OR 'child'/exp OR adolescent*:ti,ab,kw OR child*:ti,ab,kw OR schoolchild*:ti,ab,kw OR infant*:ti,ab,kw OR girl*:ti,ab,kw OR boy*:ti,ab,kw OR teen:ti,ab,kw OR teens:ti,ab,kw OR teenager*:ti,ab,kw OR youth*:ti,ab,kw OR pediatr*:ti,ab,kw OR paediatr*:ti,ab,kw OR puber*:ti,ab,kw) NOT ('adult'/exp OR 'aged'/exp OR 'middle aged'/exp OR adult*:ti,ab,kw OR man:ti,ab,kw OR men:ti,ab,kw OR woman:ti,ab,kw OR women:ti,ab,kw)) |
3419 |
|
#5 |
#1 AND #2 AND #3 AND #4 |
5509 |
|
#4 |
'sensitivity and specificity'/de OR sensitivity:ab,ti OR sensitive:ab,ti OR specificity:ab,ti OR predict*:ab,ti OR 'roc curve':ab,ti OR 'receiver operator':ab,ti OR 'receiver operators':ab,ti OR likelihood:ab,ti OR 'diagnostic error'/exp OR 'diagnostic accuracy'/exp OR 'diagnostic test accuracy study'/exp OR 'inter observer':ab,ti OR 'intra observer':ab,ti OR interobserver:ab,ti OR intraobserver:ab,ti OR validity:ab,ti OR kappa:ab,ti OR reliability:ab,ti OR reproducibility:ab,ti OR ((test NEAR/2 're-test'):ab,ti) OR ((test NEAR/2 'retest'):ab,ti) OR 'reproducibility'/exp OR accuracy:ab,ti OR 'differential diagnosis'/exp OR 'validation study'/de OR 'measurement precision'/exp OR 'diagnostic value'/exp OR 'reliability'/exp OR 'predictive value'/exp OR ppv:ti,ab,kw OR npv:ti,ab,kw OR (((false OR true) NEAR/3 (negative OR positive)):ti,ab) |
6690614 |
|
#3 |
'amyloid beta protein'/exp OR 'amyloid beta protein[1-42]'/exp OR 'amyloid beta protein[1-40]'/exp OR (((amyloid OR a) NEAR/3 (beta* OR β*)):ti,ab,kw) OR 'abeta*':ti,ab,kw OR aβ*:ti,ab,kw OR amyloidβ*:ti,ab,kw OR amyloidbeta:ti,ab,kw OR ab42:ti,ab,kw OR ab40:ti,ab,kw OR abp:ti,ab,kw OR 'tau protein'/exp OR 'p tau protein'/exp OR 't tau protein'/exp OR 'total tau protein'/exp OR 'phosphorylated tau protein'/exp OR 'phosphorylated tau 181'/exp OR 'phosphorylated tau 181 protein'/exp OR 'phosphorylated tau'/exp OR tau:ti,ab,kw OR ttau:ti,ab,kw OR ptau*:ti,ab,kw OR tau181:ti,ab,kw OR tau231:ti,ab,kw OR 'neurofilament l'/exp OR 'neurofilament light chain'/exp OR 'neurofilament light chain protein'/exp OR 'neurofilament light protein'/exp OR 'neurofilament light'/exp OR 'neurofilament l*':ti,ab,kw OR nfl:ti,ab,kw |
338121 |
|
#2 |
'cerebrospinal fluid'/exp OR 'cerebrospinal fluid level'/exp OR 'cerebrospinal fluid examination'/exp OR csf:ti,ab,kw OR liquor*:ti,ab,kw OR (((cerebrospinal* OR spinal*) NEAR/3 (fluid* OR biomarker*)):ti,ab,kw) |
322845 |
|
#1 |
'alzheimer disease'/exp OR alzheimer*:ti,ab,kw OR 'senile dementia*':ti,ab,kw |
301144 |
Ovid/Medline
|
# |
Searches |
Results |
|
14 |
(7 and (10 or 11)) not (12 or 13) = observationeel |
1110 |
|
13 |
(7 and 9) not 12 = RCT |
424 |
|
12 |
7 and 8 = SR |
91 |
|
11 |
Case-control Studies/ or clinical trial, phase ii/ or clinical trial, phase iii/ or clinical trial, phase iv/ or comparative study/ or control groups/ or controlled before-after studies/ or controlled clinical trial/ or double-blind method/ or historically controlled study/ or matched-pair analysis/ or single-blind method/ or (((control or controlled) adj6 (study or studies or trial)) or (compar* adj (study or studies)) or ((control or controlled) adj1 active) or "open label*" or ((double or two or three or multi or trial) adj (arm or arms)) or (allocat* adj10 (arm or arms)) or placebo* or "sham-control*" or ((single or double or triple or assessor) adj1 (blind* or masked)) or nonrandom* or "non-random*" or "quasi-experiment*" or "parallel group*" or "factorial trial" or "pretest posttest" or (phase adj5 (study or trial)) or (case* adj6 (matched or control*)) or (match* adj6 (pair or pairs or cohort* or control* or group* or healthy or age or sex or gender or patient* or subject* or participant*)) or (propensity adj6 (scor* or match*))).ti,ab,kf. or (confounding adj6 adjust*).ti,ab. or (versus or vs or compar*).ti. or ((exp cohort studies/ or epidemiologic studies/ or multicenter study/ or observational study/ or seroepidemiologic studies/ or (cohort* or 'follow up' or followup or longitudinal* or prospective* or retrospective* or observational* or multicent* or 'multi-cent*' or consecutive*).ti,ab,kf.) and ((group or groups or subgroup* or versus or vs or compar*).ti,ab,kf. or ('odds ratio*' or 'relative odds' or 'risk ratio*' or 'relative risk*' or aor or arr or rrr).ab. or (("OR" or "RR") adj6 CI).ab.)) |
5448124 |
|
10 |
Epidemiologic studies/ or case control studies/ or exp cohort studies/ or Controlled Before-After Studies/ or Case control.tw. or cohort.tw. or Cohort analy$.tw. or (Follow up adj (study or studies)).tw. or (observational adj (study or studies)).tw. or Longitudinal.tw. or Retrospective*.tw. or prospective*.tw. or consecutive*.tw. or Cross sectional.tw. or Cross-sectional studies/ or historically controlled study/ or interrupted time series analysis/ [Onder exp cohort studies vallen ook longitudinale, prospectieve en retrospectieve studies] |
4464295 |
|
9 |
exp clinical trial/ or randomized controlled trial/ or exp clinical trials as topic/ or randomized controlled trials as topic/ or Random Allocation/ or Double-Blind Method/ or Single-Blind Method/ or (clinical trial, phase i or clinical trial, phase ii or clinical trial, phase iii or clinical trial, phase iv or controlled clinical trial or randomized controlled trial or multicenter study or clinical trial).pt. or random*.ti,ab. or (clinic* adj trial*).tw. or ((singl* or doubl* or treb* or tripl*) adj (blind$3 or mask$3)).tw. or Placebos/ or placebo*.tw. |
2600532 |
|
8 |
meta-analysis/ or meta-analysis as topic/ or (metaanaly* or meta-analy* or metanaly*).ti,ab,kf. or systematic review/ or cochrane.jw. or (prisma or prospero).ti,ab,kf. or ((systemati* or scoping or umbrella or "structured literature") adj3 (review* or overview*)).ti,ab,kf. or (systemic* adj1 review*).ti,ab,kf. or ((systemati* or literature or database* or data-base*) adj10 search*).ti,ab,kf. or ((structured or comprehensive* or systemic*) adj3 search*).ti,ab,kf. or ((literature adj3 review*) and (search* or database* or data-base*)).ti,ab,kf. or (("data extraction" or "data source*") and "study selection").ti,ab,kf. or ("search strategy" and "selection criteria").ti,ab,kf. or ("data source*" and "data synthesis").ti,ab,kf. or (medline or pubmed or embase or cochrane).ab. or ((critical or rapid) adj2 (review* or overview* or synthes*)).ti. or (((critical* or rapid*) adj3 (review* or overview* or synthes*)) and (search* or database* or data-base*)).ab. or (metasynthes* or meta-synthes*).ti,ab,kf. |
674975 |
|
7 |
limit 6 to yr="2012 -Current" |
2564 |
|
6 |
5 not (comment/ or editorial/ or letter/) not ((exp animals/ or exp models, animal/) not humans/) not ((Adolescent/ or Child/ or Infant/ or adolescen*.ti,ab,kf. or child*.ti,ab,kf. or schoolchild*.ti,ab,kf. or infant*.ti,ab,kf. or girl*.ti,ab,kf. or boy*.ti,ab,kf. or teen.ti,ab,kf. or teens.ti,ab,kf. or teenager*.ti,ab,kf. or youth*.ti,ab,kf. or pediatr*.ti,ab,kf. or paediatr*.ti,ab,kf. or puber*.ti,ab,kf.) not (Adult/ or adult*.ti,ab,kf. or man.ti,ab,kf. or men.ti,ab,kf. or woman.ti,ab,kf. or women.ti,ab,kf.)) |
3335 |
|
5 |
1 and 2 and 3 and 4 |
3447 |
|
4 |
exp "Sensitivity and Specificity"/ or (Sensitiv* or Specific*).ti,ab. or (predict* or ROC-curve or receiver-operator*).ti,ab. or (likelihood or LR*).ti,ab. or exp Diagnostic Errors/ or (inter-observer or intra-observer or interobserver or intraobserver or validity or kappa or reliability).ti,ab. or reproducibility.ti,ab. or (test adj2 (re-test or retest)).ti,ab. or "Reproducibility of Results"/ or accuracy.ti,ab. or Diagnosis, Differential/ or Validation Study/ |
7865042 |
|
3 |
exp Amyloid beta-Peptides/ or ((amyloid or a) adj3 beta*).ti,ab,kf. or 'abeta*'.ti,ab,kf. or amyloidbeta.ti,ab,kf. or ab42.ti,ab,kf. or ab40.ti,ab,kf. or abp.ti,ab,kf. or exp tau Proteins/ or tau.ti,ab,kf. or ttau.ti,ab,kf. or ptau*.ti,ab,kf. or tau181.ti,ab,kf. or tau231.ti,ab,kf. or exp Neurofilament Proteins/ or 'neurofilament l*'.ti,ab,kf. or nfl.ti,ab,kf. |
282336 |
|
2 |
exp Cerebrospinal Fluid/ or csf.ti,ab,kf. or liquor*.ti,ab,kf. or ((cerebrospinal* or spinal*) adj3 (fluid* or biomarker*)).ti,ab,kf. |
189010 |
|
1 |
exp Alzheimer Disease/ or alzheimer*.ti,ab,kf. or 'senile dementia*'.ti,ab,kf. |
201504 |