Meetmethoden Humeraal botverlies
Uitgangsvraag
Welke meetmethode wordt aanbevolen om botverlies aan humerus-zijde te bepalen bij patiënten met verdenking op chronische posttraumatische anterieure schouderinstabiliteit?
Aanbeveling
Meet humeraal botverlies bij patiënten met chronische posttraumatische anterieure schouderinstabiliteit op cross-sectionele beeldvorming op CT of MRI.
Kies hiervoor het liefst een lineaire meetmethode:
- bij voorkeur middels de maximale lengte op een axiale CT of MRI-coupe.
- Kies voor MRI indien lokale voorkeur uitgaat naar het bepalen van bipolair/ humeraal botverlies middels Hill Sachs Interval (meting vanaf de meest mediale grens van het defect tot aan de insertie van de infraspinatuspees).
Overwegingen
Voor- en nadelen van de interventie en de kwaliteit van het bewijs
Ondanks dat er in de laatste jaren steeds meer waarde wordt gehecht aan de mate van humeraal botverlies bij het bepalen van de indicatie voor en techniek van een eventuele operatie, bestaat er voor de diagnose hiervan geen gouden standaard. Het blijkt een uitdaging consensus te vinden over de keuze voor beeldvormende modaliteit en de optimale meetmethode (en bijbehorende afkapwaarde).
Meerdere methoden om de mate van humeraal botverlies te kwantificeren zijn beschreven, maar hierover bestaat echter nog geen universele consensus (Rossi, 2021). De systematische literatuuranalyse zoals verricht voor deze module leverde zeven studies op, echter vijf van deze alle recente studies gebruiken relatief nieuwe MRI-sequenties die in Nederland in de dagelijkse praktijk nog geen gemeengoed zijn (Lander, 2022; Stillwater, 2017; Breighner, 2018; Cui, 2023; Feuerriegel, 2023). In deze sequenties, die in experimentele/onderzoek setting verschillende namen dragen, wordt een CT-achtig beeld op MRI gecreëerd door de echotijden te verlagen tot zeer laag of bijna nul. Ondanks dat dit hypothetisch gezien een betere inschatting geeft van het ossale defect, is er nog geen onderzoek gedaan naar hoe deze experimentele sequenties zich verhouden tot de conventionele T1- en T2-gewogen (vet gesatureerde) MRI-sequenties. Twee van deze studies gebruikten zelfs een post-processing methode (van 15 minuten) wat voor de routine klinische praktijk van een ziekenhuis niet voorstelbaar en praktisch is. Alhoewel zero echo time (ZTE of UTE) sequenties veelbelovend zijn voor de toekomst heeft de werkgroep besloten de conclusies van deze vijf studies niet mee te nemen in de overwegingen en aanbevelingen.
Lineaire meetmethoden om humeraal botverlies te kwantificeren in studies op CT en MRI variëren van een eenvoudige lengte en dieptemeting tot het aantal graden betrokkenheid op een 360 graden best-fit circle om de humeruskop of de verhouding restdiameter humeruskop ten opzichte van de best fit cirkel humeruskop. Metingen op CT versus MRI lijken geen significante verschillen te laten zien. Zowel CT als MRI toonde goede tot zeer goede inter- en intrabeoordelaars betrouwbaarheid (Adriani, 2024).
Naast lineaire meetmethoden om humeraal botverlies te bepalen zijn er ook oppervlakte-gebaseerde meetmethoden. Ondanks de relatief hoge zoekopbrengst van de zoekstrategie konden er geen relevante studies worden geïncludeerd die (ook) oppervlakte-gebaseerde meetmethoden hebben onderzocht.
Een hedendaags frequent gebruikte methode om gecombineerd botverlies aan zowel glenoïdale als humerale zijde (bipolair botverlies) te duiden middels beeldvorming is het zogenaamde on-track/off-track concept (DiGiacomo, 2014). In deze methode wordt de mate van glenoïdaal botverlies ten opzichte van het glenoïd contactoppervlak (glenoid track) gerelateerd aan de mate van humeraal botverlies in de vorm van het Hill Sachs Interval. On-track laesies worden als stabiel beschouwd, waarbij de Hill-Sachs laesie binnen de glenoid track blijft. Bij off-track laesies (grotere Hill-Sachs laesies en/of significant botverlies van het glenoïd) reikt de Hill-Sachs laesie buiten de glenoid track, met een verhoogd risico op recidiverende luxaties. Deze bepaling kan derhalve bijdragen aan het te bepalen (chirurgisch) beleid. De literatuurstudie voor deze module toonde wel verschillen in CT vs MRI bij het bepalen van een on-track/off-track laesie. Sgroi (2021) verklaarde een accurater gemeten Hill Sachs Interval (HSI) op MRI door de betere afgrensbaarheid van de rotator cuff musculatuur. Deze studie spreekt door vergelijkbare lineaire metingen op CT en MRI, een accuratere HSI-meting op MRI en minder stralenbelasting voorkeur uit voor het gebruik van MRI ter bepaling humeraal botverlies.
Aangezien er geen studies gevonden zijn naar accuratesse en betrouwbaarheid in verschillende meetmethodes voor de bepaling van humeraal botverlies, kiest de werkgroep er –op basis van expert opinion– één uit. Dit om een consistente manier van meten door verschillende centra te waarborgen. Net zoals in de submodule ‘Meetmethoden Glenoïdaal botverlies’ is gekozen is voor een lineaire meetmethode, namelijk een lengtemeting van het Hill Sachs defect op een axiale coupe. Dit betreft een eenvoudige, snelle, en vermoedelijk al veelgebruikte meting. Naar lokale voorkeur kan deze meting eventueel worden aangepast naar het HSI, in het kader van de conceptuele on-track/off-track bepaling.
Aangezien er geen significante verschillen beschreven zijn tussen CT en MRI, neemt de werkgroep beide modaliteiten op in haar advies. Gebaseerd op lokale voorkeur, waarbij men rekening houdt met logistieke beschikbaarheid, MRI contra-indicaties bij bepaalde patiëntengroepen, stralingshygiëne en eventuele wens tot bijkomend in kaart willen brengen van weke delen schade. Door de bewezen accuratere meting van het Hill Sachs interval op MRI, adviseert de werkgroep om te kiezen voor MRI i.p.v. CT bij een lokale voorkeur voor het bepalen van bipolair botverlies middels de on-track/off-track methode. Belangrijk advies van de werkgroep blijft - conform submodule ‘Meetmethoden Glenoïdaal botverlies’ - om te kiezen voor slechts één vorm van beeldvorming ter bepaling van humeraal botverlies: ofwel CT of MRI.
Waarden en voorkeuren van patiënten (en evt. hun verzorgers)
CT kent een lagere belasting voor de patiënt; het onderzoek is sneller uitvoerbaar en heeft minder contra-indicaties, zoals aanwezigheid van metaal/ implantaten in het lichaam. Door de snelheid van het onderzoek is claustrofobie ook minder een probleem. CT is in de meeste Nederlandse ziekenhuizen toegankelijker, waardoor de patiënt minder lang op de wachtlijst staat. Waarschijnlijk hebben patiënten hierdoor een voorkeur voor CT. Daarentegen zal door het nadeel van stralenbelasting de patiënt mogelijk de voorkeur geven aan MRI. Het verrichten van slechts één preoperatief onderzoek, in plaats van de combinatie CT én MRI, draagt vanzelfsprekend ook bij aan verhoging van patiënten comfort.
Kosten (middelenbeslag)
Een MRI-onderzoek is duurder dan een CT-onderzoek. Buiten de bovengenoemde voor- en nadelen van beide modaliteiten, kan dit ook nog worden meegenomen in de overwegingen.
Naar verwachting kan deze richtlijn voor een vermindering in kosten zorgen, omdat nu vaak zowel CT als MRI worden gemaakt. Het doen van één onderzoek brengt minder kosten met zich mee.
Aanvaardbaarheid, haalbaarheid en implementatie
De werkgroep voorziet geen problemen met het implementeren van de aanbevelingen. In de huidige situatie wordt er gebruik gemaakt van ofwel CT, ofwel MRI (artrografie) of een combinatie van beide onderzoeken, gebaseerd op lokale voorkeur van orthopedisch/ traumachirurgen en radiologen. Metingen om ossale schade aan glenoïd en humeruskop te bepalen kunnen op beide modaliteiten op dezelfde manier worden verricht, mits 2D CT en MRI onderzoeken optimaal worden geanguleerd parallel aan het glenoïd, of er gebruik wordt gemaakt van een 3D-techniek zodat de beoordelaar zelf reconstructies kan maken. De werkgroep adviseert hierbij om een MRI-scanner met tenminste een veldsterkte van 1.5 Tesla te gebruiken, omdat hierbij een resolutie wordt gehaald die de corticale contouren van het glenoïd betrouwbaar weergeeft. Bij voorkeur worden ossale structuren beoordeeld op een T1-gewogen sequentie zonder vetonderdrukking.
Volgens de werkgroep worden hier geen problemen verwacht. In de aanbevelingen is gekozen voor de meest eenvoudige en snelle meetmethode, bovendien is dit een methode die reeds in de dagelijkse praktijk wordt toegepast.
Rationale van de aanbeveling: weging van argumenten voor en tegen de diagnostische procedure
De literatuur laat het de werkgroep niet toe een gouden standaard meetmethode te definiëren, vanwege heterogeniteit aan data en beperkte kwaliteit van de onderzoeken.
Alhoewel de meeste beschreven meetmethoden acceptabel zijn - er is wetenschappelijk niet één methode per se beter of slechter - geeft de werkgroep de voorkeur aan het meten van humeraal botverlies op een axiaal beeld middels een lineaire meetmethode, bij voorkeur een maximale lengtemeting in mm. Dit omdat het de in de literatuur meest gebruikte meetmethode is, het een eenvoudige en snelle methode is, die geen extra software behoeft. Met deze aanbeveling wil de werkgroep zorgdragen dat er voor een consistente meetmethode wordt gekozen.
Onderbouwing
Achtergrond
Bone loss on the humerus side occurs in 67% of primary shoulder dislocations and up to 100% of recurrent shoulder dislocations. The size, direction and location of the humeral bone loss contribute to the instability of the shoulder joint. As a result, the presence of humeral bone loss is becoming increasingly important in determining the risk of recurrent dislocations and in determining the indication and technique of a possible operation. There are various measurement methods available for which a universally optimal measurement method and cut-off value are lacking. Furthermore, it is unclear whether and which measurements on different imaging modalities (CT/MRI) correspond with each other.
Conclusies / Summary of Findings
|
LOW GRADE |
Use of MRI compared to CT to determine humeral bone loss may on average result in comparable measurements when using linear based measurement methods. All studies used different methods to calculate the humeral bone loss.
Sources: Lander, 2022; Sgroi, 2021; Stillwater, 2017 |
|
NO GRADE |
No studies could be included that reported on area-based measurement methods to determine humeral head bone loss.
Source: - |
|
LOW GRADE |
Individual measurements between MRI and CT for Hill-Sachs lesion length or depth may result in agreement. On average, use of MRI may result in comparable measurements compared to CT when measuring the Hill-Sachs lesion length and depth.
Sources: Cui, 2023; Feuerriegel, 2023; Sgroi, 2021 |
|
VERY LOW GRADE |
The evidence is very uncertain whether MRI results in agreement with CT when classifying on/off-track lesions.
Sources: Breighner, 2018; Sgroi, 2021 |
Samenvatting literatuur
Description of studies
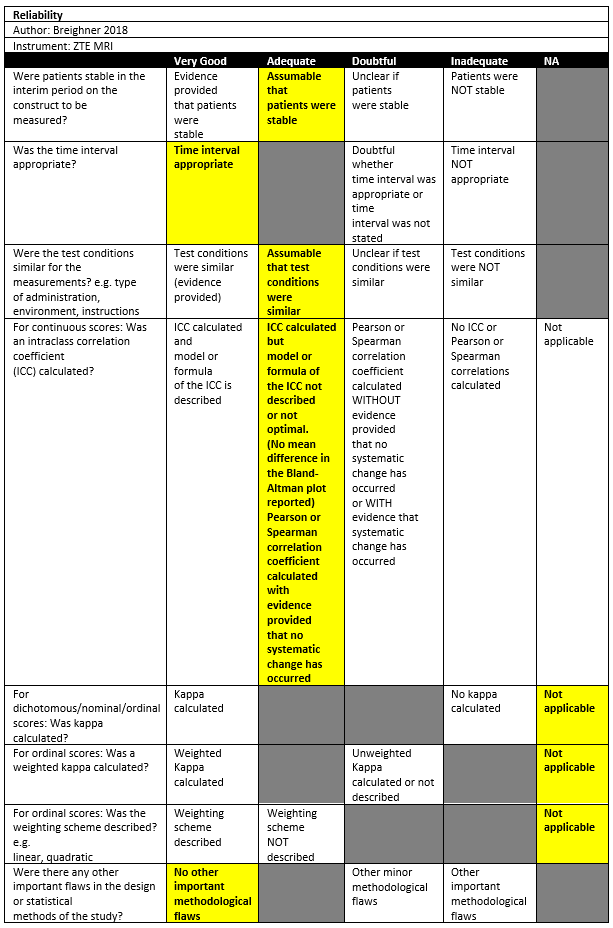
Breighner (2018) investigated a cross-sectional study in a single institution in the USA. The inclusion criteria were: Patients underwent MR imaging assessment of the shoulder, had or scheduled CT examination within 6 months of MR imaging without intervening shoulder surgery or trauma, and provided informed consent for the inclusion of this study. The exclusion criteria were excessive metal in the shoulder, as this produces artifacts in both modalities. There were 14 out of 34 patients included in the study. The mean age was 40 ±22, with 76% males. The index tests were CT and ZTE MRI. The average time between CT and MR imaging was 10 days ±32 (standard deviation); in most patients’ (29/34) CT and MR imaging examinations performed on the same day. All patients had an Hill-Sachs lesion.
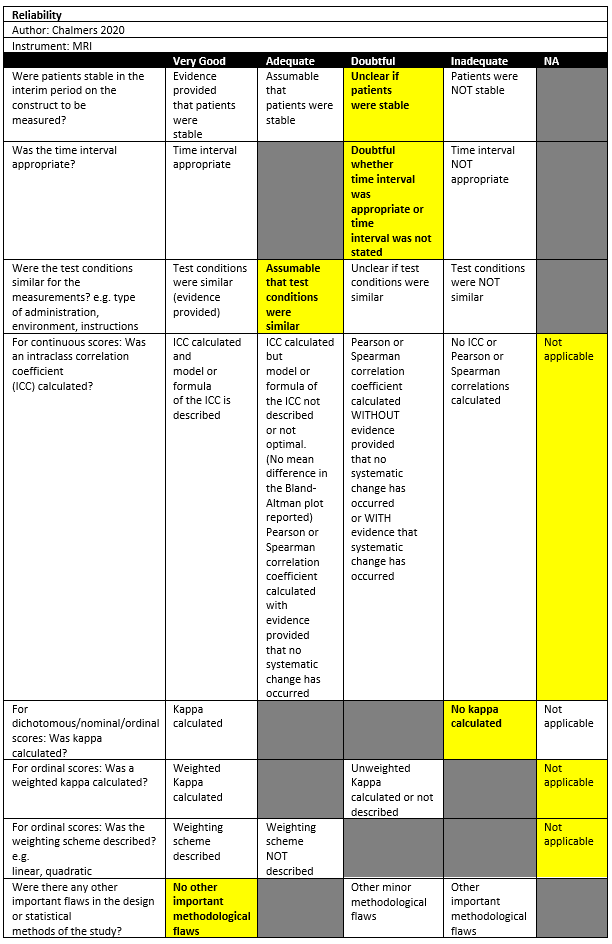
Chalmers (2020) conducted a retrospective study in a single institution in the setting of orthopedic surgical department in the USA. The inclusion criteria were: patients who underwent the surgical treatment for glenohumeral instability as coded using the Common Procedure Terminology codes 29806, 23455, 23466, 23462, 23460, and 23465 at the University of Utah, and who underwent both a CT and an MRI performed within 1 year of each other. The exclusion criteria were: Patients in whom an intervening surgical procedure was performed on the shoulder. There were 53 out of 55 patients included in the study. The mean age was 31 ± 11, with 49% males. The index tests were CT and MRI (sequences not specified). All prevalence of Hill-Sachs lesion was not reported.
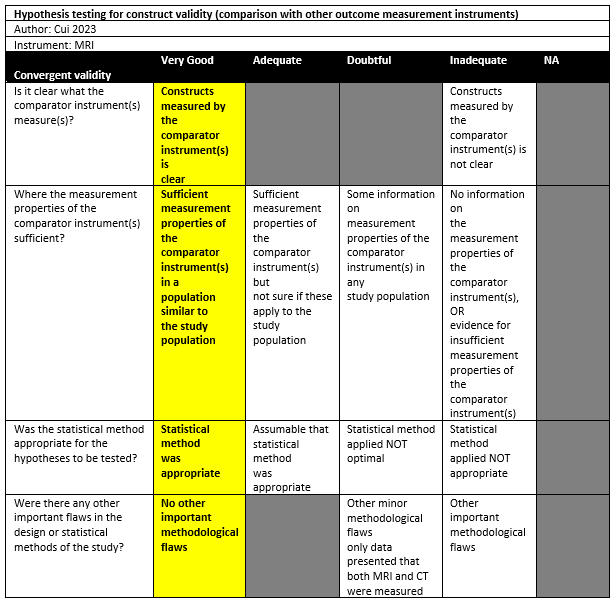
Cui (2023) investigated a cross-sectional study in a single institution in the setting of radiology and orthopedics departments in China. The inclusion criteria were: Patients with shoulder dislocation between July 2022 and June 2023, age of 18 years or older, shoulder anterior dislocation, with completion of both MRI and CT of the shoulder joint, and the interval between MRI and CT was 1 week or less. Both patients with primary dislocation and patients with recurrent dislocations were included. Exclusion criterium was a history of shoulder osseous surgery. 21 out of 56 patients were included in the study. The mean age was 27.5 ± 9.5, with 70% males. The index tests were 3D CT and 3D MRI 3D (FRACTURE). All patients had a Hill-Sachs lesion, 16 patients had a bipolar bone defect.
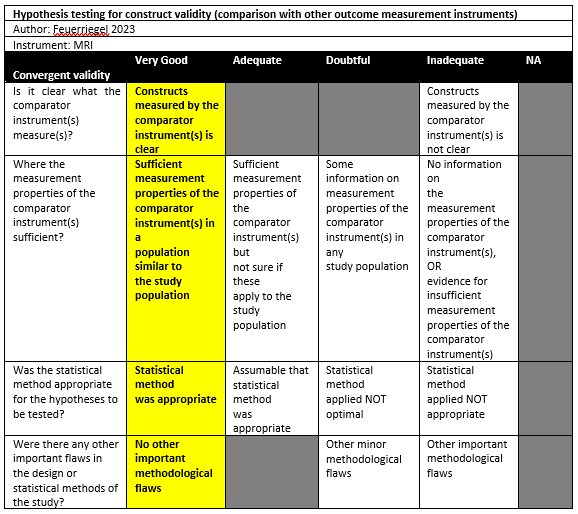
Feuerriegel (2023) conducted a cross-sectional study in a single institution in the setting of emergency department in Germany. The inclusion criteria were: Patients admitted to the emergency department with suspected traumatic dislocation of the shoulder, underwent 3-T MRI of the shoulder within 2 days after trauma, with a CT examination as part of the diagnostic workup in clinical routine. No exclusion criteria were reported. 25 Out of 46 patients were included in the study. The mean age was 40 ± 14.5, with 59% males. The index tests were CT and MRI (T1 GRE, Fracture, UTE). The interval between MRI and CT was unclear but seemed both in clinical routine. All patients had a Hill-Sachs lesion.
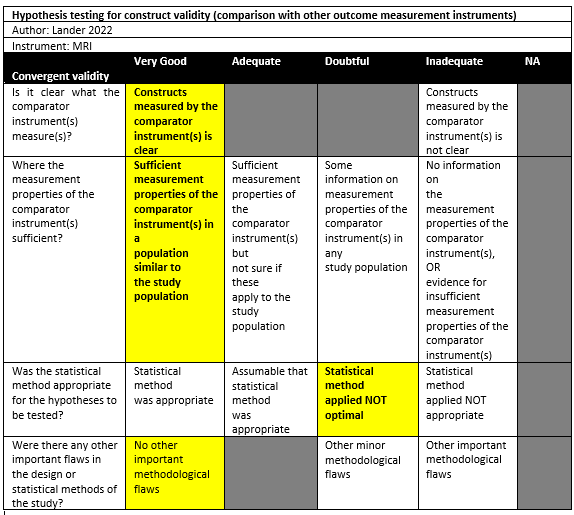
Lander (2022) performed a comparative study in a single institution in the setting of orthopedic surgical department in the USA. The inclusion criteria were: Consecutive patients with recurrent glenohumeral instability dislocations, older than 16 years, without prior surgery, and underwent both CT and MRI imaging modalities as well as 3D osseous reformats, which were ordered by their orthopedic surgeon. No exclusion criteria were reported. 18 patients were included in the study. The mean age was 34 (sd not reported), with 66% males. The index tests were CT and MRI or MRA (VIBE), which were executed in no particular order and the interval between MRI and CT was unclear. All patients had a Hill-Sachs lesion.
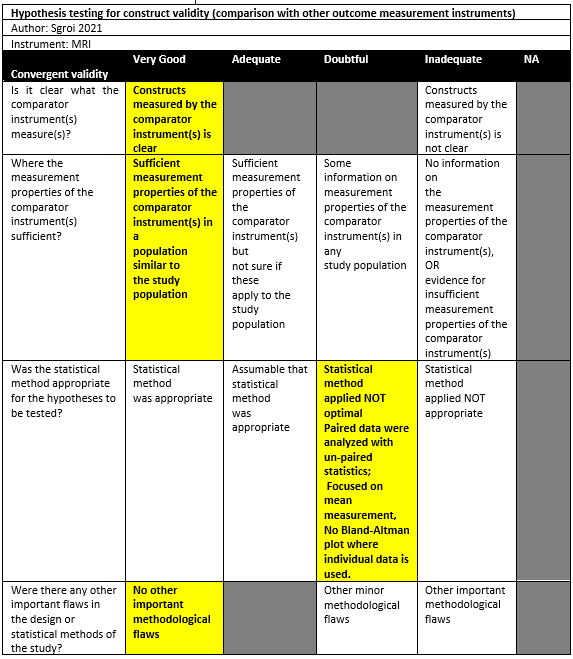
Sgroi (2021) conducted a cross-sectional study in a single institution in the setting of orthopedic surgical department in Germany. The inclusion criteria were: Consecutive patients with anterior shoulder instability scheduled for arthroscopy were retrospectively enrolled postoperatively with arthroscopic or open shoulder stabilization and available CT and MRI scans of the affected shoulder. The exclusion criteria were: concomitant rotator cuff tear, incomplete imaging diagnostics, and insufficient CT or MRI scans quality. 50 out of 80 patients were included in the study. The mean age was 26.4 ± 11.8, with 74% males. The index tests were CT and MRI (sequences not specified), which were performed as part of their preoperative diagnostic screening according to the routine clinical setup of the research setting. All patients had an Hill-Sachs lesion.
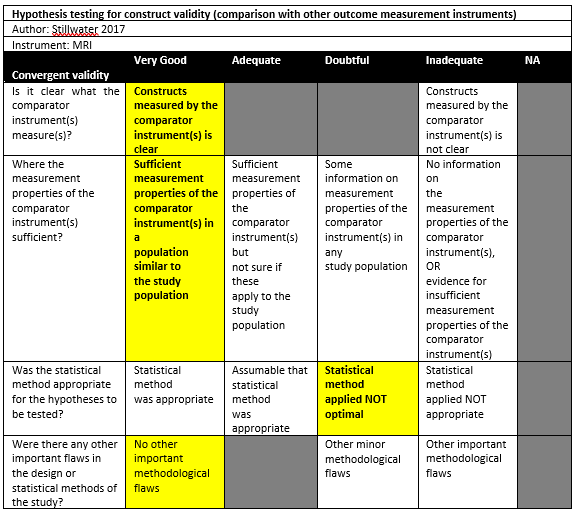
Stillwater (2017) performed a cross-sectional study in a single institution in Canada, without reporting the detailed setting of the study. The inclusion criteria were: All patients with glenohumeral instability or recurrent dislocations (either the first or recurrent), >18 years of age, imaged with both CT and MRI (VIBE) as requested by their orthopedic surgeons. One patient was excluded because of not presenting for the CT component of the study. There were 10 patients (12 shoulders) included in the study. The mean age was 29 (sd not reported), with 70% males. The index tests were CT and MRI (VIBE), which were performed within 24 h of one another without a particular order. All patients had an Hill-Sachs lesion.
Results
Results on linear based measurement methods for bone loss
Reference circle: [(circle diameter – residual humeral head width)/ circle diameter)*100]
Lander (2022) reported the percentage humeral defect for 2DCT (6.04%), 2D MRI (5.98%), 3D CT (8.29%), and 3D MRI (8.17%). There were no statistically significant differences between 3D CT and 3D MRI (p=0.769). Other comparisons do not seem to be tested and reported.
Hall method: (width of articular Hill Sachs lesion in degrees on arc / 180 degrees) *100
Sgroi (2021) reported a percentage humeral bone loss of 21.6% (SD 11.4) using CT compared to 21.0% (SD 10.2) using MRI. There were no statistically significant differences.
Flatow method using a reference circle: the quotient of the humeral head diameter without taking the Hill-Sachs lesion into account and the humeral head diameter taking the Hill-Sachs lesion into account
Using this method, Sgroi (2021) measured the humeral head diameter parallel to the articular surface of glenoid (without taking HS lesion into account) and the humeral head diameter parallel to the first diameter now taking HS lesion into account. The quotient of two diameters is calculated: for CT a mean of 17.4% (SD 8.3) was found, while for MRI 15.4% (SD 9.3) was found. There were no statistically significant differences.
Measurement without reference circle: [(humeral head height parallel to Hill-Sachs lesion – residual humeral head width)/ humeral head height parallel to Hill-Sachs lesion)*100]
Stillwater (2017) found a mean of 12.7% (SD 4.1) humeral head bone loss when measuring with CT, compared to 12.6% (SD 4.1) with MRI. There were no statistically significant differences.
Results on area based measurement methods for bone loss
No studies could be included that reported on area-based measurement methods to determine humeral head bone loss.
Hill-Sachs measurements
Length
Beighner (2018) measured the Hill-Sachs length (width on axial view) with 2 raters using CT and MRI. Rater 1 had an intraclass correlation coefficient (ICC) of 0.77 for measurements between modalities and 95% limits of agreement (LoAs) of -5.57 to 8.03. For rater 2, the ICC was 0.66 and the 95%LoAs ranged from -7.53 to 4.91.
Lander (2022) measured the maximal Hill-Sachs defect on 2D CT (18.19mm), 2D MRI (18.65mm), 3D CT (14.14mm), 3D MRI (12.39mm), and 2D MRI VIBE (19.28mm). No statistically significant differences were found for the measurements on 2D CT compared to 2D MRI and 2D MRI VIBE. Furthermore, the means of 3D CT and 3D MRI measurements also did not differ statistically significantly from each other.
Sgroi (2021) measured the length of the Hill-Sachs lesion in centimetres and found a mean of 1.4cm (SD 0.7) with CT measurements and 1.3cm (SD 0.7) with MRI measurements (not statistically significant). Sgroi (2021) also used Richards arc to determine the Hill-Sachs lesion size in degrees. A reference circle is drawn, and its center point is determined by the cross section of diameter lines. A line from the circle to its centre along the anterior edge of the articular surface is defined as 0 degrees. Size of the Hill Sachs lesion was determined by establishing its location on the 360-degree frame. It was observed that the mean arc size was 37.4 degrees (SD 34.6) on CT images and 34.9 degrees (SD 19.8) on MRI images (not statistically significant).
Depth
Beighner (2018) measured the Hill-Sachs depth on an axial view with 2 raters using CT and MRI. Rater 1 had an ICC of 0.85 for measurements between modalities and 95% limits of agreement (LoAs) of -2.09 to 2.40. For rater 2, the ICC was 0.90 and the 95%LoAs ranged from -2.27 to 1.50.
Sgroi (2021) measured the depth of the Hill-Sachs lesion in centimetres using both CT (mean: 0.7cm, SD 0.3) and MRI (mean: 0.7cm, SD 0.4). There were no statistically significant differences.
Interval
Cui (2023) drew parallel lines at both the medial edge of the lesion to the medial margin of the rotator cuff insertion on 3D humeral models. Distances between those lines in millimetres were Hill-Sachs interval measurements. A mean of 14.29mm (SD 1.93) was found for CT, compared to 14.35mm (SD 2.07) for MRI. This difference was not statistically significant. From a Bland-Altman plot, the mean difference was -0.06mm with 95%LoAs from -1.24 to 1.12.
Feuerriegel (2023) compared the Hill-Sachs interval on the axial view as measured with CT (mean: 17.4mm, SD 4.1), T1 GRE MRI (mean: 17.4, SD 4.2), FRACTURE MRI (mean: 17.3 mm, SD 4.1), UTE MRI (mean: 17.4mm, SD 4.2) and found no statistically significant differences between CT and MRI. The 95%LoAs could be approximated from a figure reported in the manuscript. For the agreement between CT and T1 GRE MRI, the 95%LoA seems to range from approximately -1.20 to 0.99. For CT versus FRACTURE MRI, this range was -1.05 to 0.8 approximately and, finally, for CT vs. UTE MRI the range was approximately -0.85 to 0.85.
Sgroi (2021) found a statistically significant difference between the measurements with CT (mean: 16.6mm, SD 0.5) and MRI (mean: 14.3mm, SD 0.5).
On/off-track classification
In all studies, the glenoid track was calculated using (0.83 * circle diameter) – diameter bone loss.
Lander (2022) reported that the imaging modalities were identical for classifying on/off track.
Sgroi (2021) observed how CT classified 33.3% of all lesions as being off-track, compared to 17.1% using MRI, although no statistically significant differences were found.
Fuerriegel (2023) found that CT and MRI all classified on-track (n=14) and off-track (n=6) in agreement, resulting in kappa=1.00.
Chalmers (2020) reported the proportion classified as on-track as measured by CT and MRI by two raters. Rater one classified 28.3% as on-track using CT and 41.5% on MRI. Rater two classified 37.7% and 39.6% on-track for CT and MRI, respectively.
Table 2. Overview of the results
|
Method |
Measurement |
Difference |
Correlation |
Agreement |
Outcome assessment* |
Risk of bias |
||
|
CT |
MRI |
|||||||
|
Linear based measurement methods |
||||||||
|
Reference circle: [(circle diameter – residual humeral head width) / circle diameter)*100] |
||||||||
|
Lander (2022) |
8.29% |
8.17%). |
There were no statistically significant differences. |
- |
- |
? |
Doubtful |
|
|
Hall method: (width of articular Hill Sachs lesion in degrees on arc / 180 degrees) *100 |
||||||||
|
Sgroi (2021) |
21.6% (SD 11.4) |
21.0% (SD 10.2) |
There were no statistically significant differences. |
- |
- |
? |
Doubtful |
|
|
Flatow method using a reference circle: the quotient of the humeral head diameter without taking the Hill-Sachs lesion into account and the humeral head diameter taking the Hill-Sachs lesion into account |
||||||||
|
Sgroi (2021) |
17.4% (SD 8.3) |
15.4% (SD 9.3) |
There were no statistically significant differences. |
- |
- |
? |
Doubtful |
|
|
Measurement without a reference circle: [(humeral head height parallel to Hill-Sachs lesion – residual humeral head width)/ humeral head height parallel to Hill-Sachs lesion)*100] |
||||||||
|
Stillwater (2017) |
12.7% (SD 4.1) |
12.6% (SD 4.1) |
There were no statistically significant differences. |
- |
- |
? |
Doubtful |
|
|
Area based measurement methods |
||||||||
|
No studies could be included that reported on area-based measurement methods to determine humeral head bone loss. |
||||||||
|
Hill-Sachs measurements |
||||||||
|
Length |
||||||||
|
Beighner (2018) |
R1: – R2: – |
R1: – R2: – |
- |
R1: ICC=0.77 R2: ICC=0.66 |
R1: 95%LoA: -5.57 to 8.03 R2: 95%LoAs -7.53 to 4.91 |
– |
Adequate |
|
|
Lander (2022) |
2D CT: 18.19mm 3D CT: 14.14mm |
2D MRI: 18.65mm 3D MRI: 12.39mm 2D MRI VIBE: 19.28mm |
No statistically significant differences were found for the measurements on 2D CT compared to 2D MRI and 2D MRI VIBE. Furthermore, the means of 3D CT and 3D MRI measurements also did not differ statistically significantly from each other. |
- |
- |
? |
Doubtful |
|
|
Sgroi (2021) |
1.4cm (SD 0.7 |
1.3cm (SD 0.7) |
There were no statistically significant differences. |
- |
- |
? |
Doubtful |
|
|
Sgroi (2021), Richards arc |
37.4 degrees (SD 34.6) |
34.9 degrees (SD 19.8) |
There were no statistically significant differences. |
- |
- |
? |
Doubtful |
|
|
Depth |
||||||||
|
Beighner (2018) |
R1: – R2: – |
R1: – R2: – |
- |
R1: ICC=0.85 R2: ICC=0.90 |
R1: 95%LoA: -2.09 to 2.40 R2: 95%LoAs -2.27 to 1.50 |
– |
Adequate |
|
|
Sgroi (2021) |
0.7cm (SD 0.3) |
0.7cm (SD 0.4) |
There were no statistically significant differences. |
- |
- |
? |
Doubtful |
|
|
Interval |
||||||||
|
Cui (2023) |
14.29mm (SD 1.93) |
14.35mm (SD 2.07) |
- |
- |
Mean difference: -0.06mm, 95%LoA: -1.24 to 1.12. |
+ |
Very good |
|
|
Feuerriegel (2023) |
17.4mm (SD 4.1) |
T1 GRE: 17.4 (SD 4.2) FRACTURE: 17.3 mm (SD 4.1) UTE: 17.4mm (SD 4.2) |
There were no statistically significant differences. |
- |
T1 GRE: approximated 95% LoA: -1.20 to 0.99 FRACTURE: approximated 95% LoA: -1.05 to 0.8 UTE: approximated 95% LoA: -0.85 to 0.85 |
+ |
Very good |
|
|
Sgroi (2021) |
16.6mm (SD 0.5) |
14.3mm (SD 0.5) |
Statistically significant difference (p=0.016) using the Wilcoxon signed-rank test. |
- |
- |
? |
Doubtful |
|
|
On/off-track classification |
||||||||
|
To calculate the glenoid track, all studies used (0.83 * circle diameter) – diameter bone loss |
||||||||
|
Lander (2022) |
- |
- |
|
- |
It was reported that the imaging modalities were identical for classifying on/off track |
? |
Doubtful |
|
|
Sgroi (2021) |
33.3% of lesions as off-track |
17.1% of lesions as off-track |
There were no statistically significant differences. |
- |
- |
? |
Doubtful |
|
|
Feuerriegel (2023) |
On-track n=14, off-track n=6 |
On-track n=14, off-track n=6 |
- |
- |
Kappa = 1.00 |
+ |
Very good |
|
|
Chalmers (2020) |
R1:28.3% on-track R2: 37.7% on-track |
R1: 41.5% on-track R2: 39.6% on-track |
- |
- |
- |
? |
Inadequate |
|
|
Abbreviations: GBL, glenoid bone loss. 95% LoA: 95% limit of agreement. ICC, Intraclass correlation coefficient. R1: rater 1, R2: rater 2 * Outcomes were rated as + (sufficient, when: correlation ≥0.70, AUC ≥0.70, Kappa ≥7.0), – (insufficient, when correlation <0.70, AUC <0.7, Kappa <7.0), or ? (indeterminate, when correlation, AUC or Kappa not reported) based on the criteria for good measurement properties (Prinsen, 2018). Bland-Altman plots showing 95% limits of agreement within the intervals of clinical relevance (±2mm [glenoid track], ±5% [proportion bone loss] from 0 [i.e. no difference]) were also rated as + (sufficient) even if a correlation coefficient is absent. |
||||||||
Level of evidence of the literature
Linear based measurement methods
The level of evidence regarding linear measurement methods was downgraded by 2 levels because of the study limitations (1 level for risk of bias: there are multiple studies of doubtful quality); the number of included patients (1 level for imprecision: total sample size <100). We did not downgrade for inconsistency (reason: all studies report similar results) and indirectness (reason: there seem to be no deviations). Publication bias was not assessed.
Area based measurement methods
No studies could be included that reported on area-based measurement methods to determine humeral head bone loss.
Hill Sachs measurements
Length
The level of evidence regarding linear measurement methods was downgraded by 2 levels because of the study limitations (1 level for risk of bias: there is one study of adequate quality and there are multiple studies of doubtful quality); the number of included patients (1 level for imprecision: total sample size <100). We did not downgrade for inconsistency (reason: studies report similar results for mean differences; However, it is difficult to compare outcomes between studies that test for mean differences vs reporting agreement parameters) and indirectness (reason: there seem to be no deviations). Publication bias was not assessed.
Depth
The level of evidence regarding linear measurement methods was downgraded by 2 levels because of the study limitations (1 level for risk of bias: there is one study of adequate quality and one of doubtful quality); the number of included patients (1 level for imprecision: total sample size <100). We did not downgrade for indirectness (reason: there seem to be no deviations). Inconsistency could not be assessed (reason: the outcomes of both studies are difficult to compare [i.e. mean differences vs. agreement]). Publication bias was not assessed.
Interval
The level of evidence regarding linear measurement methods was downgraded by 2 levels because of the number of included patients (1 level for imprecision: total sample size <100), heterogeneity (1 level for inconsistency: the largest study contributing >50% of the total sample size found differences between mean CT and MRI measurements [>2mm], where other studies found mean CT and MRI measurements close to each other. Not enough information was provided on the MRI procedures to assess if this heterogeneity could be explained by different MRI procedures, and thus it remains unexplained. When focusing on agreement, two studies reporting agreement parameters seem to report similar 95% limits of agreement. However, if Sgroi 2021 would have reported 95%LoAs, it would probably differ from the 95%LoAs reported by Cui 2023 and Feuerriegel 2023. Conclusively, we decided to downgrade by 1 level). We did not downgrade for the study limitations (reason: there are two studies of very good quality available), indirectness (reason: there seem to be no deviations). Publication bias was not assessed. (NB: if we would ignore the study of doubtful quality and only assess both studies of very good quality, then the imprecision assessment would result in downgrading of 2 levels as the total sample size is then n<50, also resulting in ‘low GRADE’).
On/off-track classification
The level of evidence regarding linear measurement methods was downgraded by 3 levels because of the study limitations (1 level for risk of bias: there is one study of adequate quality, however it provided only 17.1% of the sample size. The other studies providing 82.9% of the sample size were assessed as being either doubtful or inadequate. Therefore, we decided to downgrade one level); heterogeneity (2 levels for inconsistency: there seem to be studies reporting perfect agreement and studies reporting potentially relevant disagreement between CT and MRI; some narratively, some proportionally, one statistically). We did not downgrade for imprecision (reason: total sample size >100) and indirectness (reason: there seem to be no deviations). Publication bias was not assessed.
Zoeken en selecteren
A systematic review of the literature was performed to answer the following question:
‘’Which measurement method is the most accurate to measure humeral bone loss in patients diagnosed with posttraumatic shoulder instability?’’
Table 1. PICO
| Patients | Patients with posttraumatic shoulder instability and humeral bone loss, confirmed by CT or MRI |
| Intervention | Different measurement methods in literature: linear based bone loss methods, area based bone loss methods, Hill-Sachs interval by MRI, on/off-track classification |
| Control | Different measurement methods in literature: linear bone loss (mm), surface-based bone loss, Hill-Sachs interval by CT, on/off-track classification |
| Outcomes | Agreement and correlation parameters on a continuous or categorical level of bone loss measurement (e.g. ICC, Bland-Altman plot, Kappa) |
Relevant outcome measures
The guideline development group considered agreement parameters as a critical outcome measure for decision making; and tests for differences and correlations as an important outcome measure for decision making.
A priori, the guideline development group did not define the outcome measures listed above but used the definitions used in the studies. Outcomes were rated as ‘sufficient’ (correlation ≥0.70, AUC ≥0.70, Kappa ≥7.0), ‘insufficient’ (correlation <0.70, AUC <0.7, Kappa <7.0), or ‘indeterminate’ (correlation, AUC or Kappa not reported) based on the criteria for good measurement properties (Prinsen, 2018).
The guideline development group defined a clinically relevant difference of more than 5% compared to CT assessment as a clinically important disagreement of humeral bone loss measurements (both linear and area based), and 2mm for measurement of length/width/interval. This is acknowledged to be an arbitrary choice since evidence regarding the clinical importance of these differences are lacking. Bland-Altman plots showing 95% limits of agreement within these intervals (±2mm, ±5% from 0 [i.e. no difference]) will be rated as ‘sufficient’, even when a correlation coefficient is absent.
Search and select (Methods)
The databases Medline (via OVID) and Embase (via Embase.com) were searched with relevant search terms (after 2000) until February 6, 2024. The detailed search strategy is depicted under the tab Methods. The systematic literature search resulted in 960 hits. Studies were selected based on the following criteria: the population were patients with post-traumatic shoulder instability, the measurement of bone loss was performed by both MRI and CT tests, the agreement of the measurements by MRI and CT was reported. Cadaveric studies were excluded as these studies were not performed in the clinical setting.
Sixty-eight studies were initially selected based on title and abstract screening. The guideline development group checked the methods of the full-text studies to determine whether different measurement methods of humeral bone loss by MRI and CT was reported. After reading the full text, 61 studies were excluded (see the table with reasons for exclusion under the tab Methods), and 7 studies were included.
Results
Seven primary studies were included in the analysis of the literature. Important study characteristics and results were extracted in the evidence tables. Results are summarized in Table 1. The assessment of the COSMIN risk of bias is summarized in the risk of bias tables.
Referenties
- Adriani M, Saccomanno MF, Bergomi A, De Filippo F, Daffara V, Milano G. Accuracy and reliability of imaging modalities for studying bipolar bone loss in anterior shoulder instability: A systematic review. Knee Surg Sports Traumatol Arthrosc. 2025 May;33(5):1844-1852. doi: 10.1002/ksa.12531. Epub 2024 Nov 4. PMID: 39497437; PMCID: PMC12022830.
- Cui DD, Long Y, Yan Y, Li C, Yang YT, Zhong JL, Yang R. Three-Dimensional Magnetic Resonance Imaging Fast Field Echo Resembling a Computed Tomography Using Restricted Echo-Spacing Sequence Is Equivalent to 3-Dimensional Computed Tomography in Quantifying Bone Loss and Measuring Shoulder Morphology in Patients With Shoulder Dislocation. Arthroscopy. 2024 Jun 1;40(6):1777-88.
- Di Giacomo G, Itoi E, Burkhart SS. Evolving concept of bipolar bone loss and the Hill-Sachs lesion: from "engaging/non-engaging" lesion to "on-track/off-track" lesion. Arthroscopy. 2014 Jan;30(1):90-8. doi: 10.1016/j.arthro.2013.10.004. PMID: 24384275.
- Feuerriegel GC, Kronthaler S, Weiss K, Haller B, Leonhardt Y, Neumann J, Pfeiffer D, Hesse N, Erber B, Schwaiger BJ, Makowski MR. Assessment of glenoid bone loss and other osseous shoulder pathologies comparing MR-based CT-like images with conventional CT. European Radiology. 2023 Dec;33(12):8617-26.
- Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. Journal of chiropractic medicine. 2016 Jun 1;15(2):155-63.
- Lander ST, Liles JL, Kim BI, Taylor DC, Lau BC. Comparison of computed tomography and 3D magnetic resonance imaging in evaluating glenohumeral instability bone loss. Journal of Shoulder and Elbow Surgery. 2022 Nov 1;31(11):2217-24.
- Mokkink LB, Prinsen C, Patrick DL, Alonso J, Bouter L, De Vet HC, Terwee CB, Mokkink L. COSMIN methodology for systematic reviews of patient-reported outcome measures (PROMs). User manual. 2018 Feb;78(1):6-3.
- Rossi LA, Frank RM, Wilke D, Provencher CMT, Millet PJ, Romeo A, Walch G, Lo I, Yamamoto N, Bokor D, Di Giacomo G, Tokish J, Lech O, Itoi E, Garrigues G, Scheibel M, Boileau P, Calvo E, Arce G, Toro F, Sugaya H, Ranalletta M, Parada S, Savoie F, Verma NN, Chahla J. Evaluation and Management of Glenohumeral Instability With Associated Bone Loss: An Expert Consensus Statement Using the Modified Delphi Technique. Arthroscopy. 2021 Jun;37(6):1719-1728. doi: 10.1016/j.arthro.2020.12.237. Epub 2021 Jan 13. Erratum in: Arthroscopy. 2025 Feb;41(2):532. doi: 10.1016/j.arthro.2024.07.008. PMID: 33453347.
- Sgroi M, Huzurudin H, Ludwig M, Dornacher D, Reichel H, Kappe T. With the exception of the Hill–Sachs interval, CT and MRI show no significant differences in the diagnostic value of the HSL measurement regardless of the measurement technique. Knee Surgery, Sports Traumatology, Arthroscopy. 2021 Dec;29(12):3981-8.
- Stillwater L, Koenig J, Maycher B, Davidson M. 3D-MR vs. 3D-CT of the shoulder in patients with glenohumeral instability. Skeletal radiology. 2017 Mar;46:325-31.
- Terwee CB, Bot SD, de Boer MR, Van der Windt DA, Knol DL, Dekker J, Bouter LM, de Vet HC. Quality criteria were proposed for measurement properties of health status questionnaires. Journal of clinical epidemiology. 2007 Jan 1;60(1):34-42.
Evidence tabellen
Summary of included studies
|
Study reference |
Study characteristics |
Patient characteristics
|
Index test (test of interest) |
Measurement method
|
Follow-up |
Outcome measures and effect size |
|||||||||||||||||||||||||
|
Stillwater 2017 |
Type of study: Cross-sectional study
Setting and country: Setting not reported, Canada
Funding and conflicts of interest: None declared. |
Inclusion criteria: All patients with glenohumeral instability or recurrent dislocations were identified. Those patients >18 years of age, who were to be imaged with both CT and MRI, as requested by their orthopedic surgeons, were included in the study. This included patients who either had their first dislocation or had recurrent dislocations. Patients underwent CT and MRI examinations in no particular order; however, the CT and MRI examination were performed within 24 h of one another.
Exclusion criteria: One patient was excluded because of not presenting for the CT component of the study.
N=10 patients (12 shoulders)
Prevalence: Hill-Sachs lesions: 100%
Mean age ± SD: 29
Sex: 70% M/ 30% F |
Describe index test: MRI: a 3-T Siemens scanner (MAGNETOM Verio, Siemens Heatlhcare, Erlangen, Germany). Additionally, for the purposes of this study, a 3D isotropic volumetric interpolated breath-hold examination (VIBE) with a water excitation sequence was added on to the standard protocol.
Comparator test: CT: a 64- multidetector row CT (VCT, GE Medical Systems, Milwaukee, WI), with a protocol consisting of: helical imaging, 20% adaptive statistical iterative reconstruction dose reduction, detector coverage 20 mm, pitch 0.531:1, table speed 10.62, slice thickness 0.625 mm, FOV 18 cm, kVp 120, rotation time 0.8 s, auto mA and noise index 22.
A radiology resident with 3 years of experi- ence performed the CT and MRI measurements. CT and MRI measurements were performed separately, approximately 2 days apart, with the measurer blinded to measurements from the other modality. |
A line parallel to the orientation of the Hill-Sachs lesion was drawn to determine the maximal humeral head height (A). A line perpendicular to the humeral head height line was then drawn to determine the residual humeral head width (B). Using these measurements, the width of the humeral head defect could be determined (A-B), as could the percent humeral head bone loss [(A-B/A)*100]
The definition of size of Hill-Sachs lesions was not clearly described, assumed to be similar with Hill-Sachs depths.
|
Time between the index test and reference test: Not applicable as no reference test were included.
For how many participants were no complete outcome data available? 1 (9%)
Reasons for incomplete outcome data described? A total of 11 patients who met the criteria were consecutively enrolled over a 6-month period, from November 2014 to April 2015. A total of 13 shoulders were imaged. One patient was excluded because of not presenting for the CT component of the study. A total of 12 shoulders were therefore included in the study.
|
Mean measurements
TOST (Two one-sided t-tests for equivalence) were used with an equivalence margin of 1 mm for measurements and 1% for percent bone loss. A significant result indicates significantly equivalence within the priori defined margin.
|
|||||||||||||||||||||||||
|
Breighner 2018 |
Type of study: Cross-sectional study
Setting and country: a single institution, Department of radiology and imging and department of orthopedic surgery and sports medicine, the USA
Funding and conflicts of interest: This study was performed with financial support and technical assistance from GE Healthcare.
|
Inclusion criteria: Thirty-four patients undergoing MR imaging assessment of the shoulder were identified for prior or scheduled CT studies within 6 months of MR imaging without intervening shoulder surgery or trauma and were enrolled in the study after providing informed consent/assent.
Exclusion criteria: excessive metal in the shoulder, as this produces artifacts in both modalities. Suture an- chors were not cause for exclusion.
N=14/34
Prevalence: HSL: 100% in the population with HSL
Mean age ± SD: 40 ± 22 for the whole 34 patients
Sex: 76% M / 24% F fort he whole 34 patients |
Describe index test: ZTE MRI: a 1.5-Tesla MRI scanner (Siemens Symphony, Germany)
Comparator test: CT: a GE Discovery CT750 HD scanner (n = 30) or a GE LightSpeed VCT scanner (n = 4) (GE Healthcare, Waukesha, Wis).
The average time between CT and MR imaging was 10 days ±32 (standard deviation), with most patients’ (29 of 34) CT and MR imaging examinations performed on the same day.
|
Hill-Sachs lesion size was measured in the radial and tangential directions depth and width, respectively
|
Time between the index test en reference test: Not applicable as no reference test were included.
For how many participants were no complete outcome data available? 0
Reasons for incomplete outcome data described? Not applicable.
|
Intermodal Agreement between CT and ZTE MRI
Note.LOA = Bland-Altman 95% limits of agreement. * Statistically significant (P = 0.05). If significant, agreement was substantial or better (ICC >=0.61). |
|||||||||||||||||||||||||
|
Chalmers 2020 |
Type of study: Retrospective study
Setting and country: a single institution, Department for Orthopaedic Surgery, the USA
Funding and conflicts of interest: The authors report potential conflicts of interest or sources of funding, details please see the article.
|
Inclusion criteria: (1) patients who underwent the surgical treatment for glenohumeral instability as coded using the Common Procedure Terminology codes 29806, 23455, 23466, 23462, 23460, and 23465 at the University of Utah, and (2) who underwent both a CT and an MRI performed within 1 year of each other.
Exclusion criteria: Patients in whom an intervening surgical pro- cedure was performed on the shoulder.
N=53/55
Prevalence: HSL: unclear
Mean age ± SD: 31 ± 11
Sex: 49% M / 51% F |
Describe index test: MRI
Comparator test: CT Both the CT and MRI images were down- loaded in DICOM format (Digital Imaging and Communications in Medicine) and uploaded into a free- available viewing software (OsiriX; Pixmeo Sarl, Berne, Switzerland). The MRI and CT scans were obtained within 1 year of each other. The mean (sd) was 45±83 days between scans.
|
on the HillSachs view, the distance from the posterior aspect of the articular surface (the anteromedial most aspect of the Hill-Sachs defect) to the rotator cuff attachment (the posterolateral most aspect of the HillSachs defect) was measured. “on- track” or “off-track.”: the width of the glenoid was multiplied by 0.83 and the width of the glenoid defect was subtracted from this number.15 If the result was larger than the width of the HillSachs, the shoulder was considered to be “on-track” and if the result was smaller than the width of the HillSachs the shoulder was considered to be “off-track.” These calculations were made for each observer and each imaging modality. |
Time between the index test en reference test: Not applicable as no reference test were included.
For how many participants were no complete outcome data available? 53 (96.3%)
Reasons for incomplete outcome data described? Two patients with scans with more than 1 year between scans were excluded.
|
On- /Off-track |
|||||||||||||||||||||||||
|
Sgroi 2021 |
Type of study: Cross-sectional study
Setting and country: a single institution, Department for Orthopaedic Surgery, Germany
Funding and conflicts of interest: none declared. |
Inclusion criteria: Eighty consecutive patients with anterior shoulder instability scheduled from 2013 to 2017 in our department for arthroscopy were retrospectively enrolled postoperatively in this study: (1) arthroscopic or open shoulder stabilisation and (2) available CT and MRI scans of the afected shoulder.
Exclusion criteria: (1) concomitant rotator cuf tear, (2) incomplete imaging diagnostics, and (3) insuicient CT or MRI scans quality.
N=50/80
Prevalence: HSL: 100%
Mean age ± SD: 26.4 ± 11.8
Sex: 74% M / 26% F |
Describe index test: MRI: a 1.5-Tesla MRI scanner (Siemens Symphony, Germany)
Comparator test: For all patients CT: Siemens Somatom Emotion, ST: 1.0 mm, pitch: 0.8, 130 kV.
For all patients CT and MRI scans of the shoulders were performed as part of their preoperative diagnostic screening according to our routine clinical setup. Study-related radiological analysis of all patients was conducted postoperatively at 34.7 ± 11.4 months (range: 24.1–52.0 months). Two orthopaedic trainees re-analysed and re-evaluated preoperative CT and MRI scans.
|
The width of the HSL was measured by drawing a line between both of its edges. The depth of the HSL was obtained by placing a virtual circle on the humeral head. The longest perpendicular line from the ground of the lesion to the surface of the circle was deined as the depth of the HSL. The glenoid track method: First, the diameter (D) of the lower glenoid and the extent of glenoid bone loss (GBL) were measured using the best-it-circle method. Second, the glenoid track was extrapolated using the following formula: GT = (0.83 * D)-GBL. Finally, the Hill–Sachs interval (HSI) was deined as the sum of the width of the HSL and the extent of intact bone between the rotator cuf insertion and the lateral rim of the HSL. The HSL was deined as of-track if the HSI was greater than the glenoid track (HSI > GT); otherwise, it was defined as on-track. |
Time between the index test en reference test: Not applicable as no reference test were included.
For how many participants were no complete outcome data available? 30 (37.5%)
Reasons for incomplete outcome data described? Thirty patients were excluded according to the exclusion criteria: 25 due to incomplete CT scans and 5 due to the insuicient CT scan quality.
|
Measuring methods (mean)
Measurements of the glenoid track (mean)
n. s. not signiicant; none of the measurement results was normally distributed. The Wilcoxon signed-rank test was used to compare the interval-scaled measurements; Yates’s Chi- square test was used for nominal-scaled variables. Signiicant correlations are marked in bold Signiicance level = < 0.05 |
|||||||||||||||||||||||||
|
Lander 2022 |
Type of study: Unclear
Setting and country: a single institution, Department of Orthopaedic Surgery, the USA Funding and conflicts of interest: Several authors receive financial or material support from Arthrex, Inc., Breg, DJOrtho, Mitek, and Smith & Nephew; intellectual property loy- alties from DePuy, a Johnson & Johnson Company; and receives research support from Smith & Nephew, Arthrex, Inc., and Wright Medical Technology, Inc. Details please see the article. |
Inclusion criteria: Consecutive patients with recurrent glenohumeral instability dislocations were identified. Patients older than 16 years, without prior surgery, were identified and underwent both CT and MRI imaging modalities, as well as 3D osseous reformats, which were ordered by their orthopedic surgeon. Patients received CT and MRI evaluations in no particular order but whichever could be obtained first per patient and radiology schedule. interval between MRI and CT was unclear.
Exclusion criteria: Not reported.
N=18 (18 shoulders)
Prevalence: recurrent glenohumeral instability: 100%
Mean age ± SD: 34 Sex: 66% M / 34% F |
Describe index test: MRI (n=12) and MRA (n=6) were performed on a Siemens Skyra (3-tesla magnets) MRI scanner. In order to produce 3D MRI osseous reformats, a 3D isotropic volumetric interpolated breath-hold examination (VIBE) with water excitation sequence was performed in addition to the standard protocol. Comparator test: CT: a standard 64- multidetector-row CT and helical imaging, including 20% adap- tive statistical iterative reconstruction dose reduction, detector coverage 20 mm, pitch 0.531:1, table speed 10.62, slice thickness 0.625 mm, field of view 18 cm, tube voltage 120 kVp, rotation time 0.8 seconds, automatic tube current modulation, and noise index 22. A 3D osseous reconstruction was performed with a protocol to isolate the pixel values by Hounsfield units to manually segment the anatomy.
The interval between MRI and CT was unclear. |
A line (blue) was placed to determine maximal humeral head height (A) and then a perpendicular line (green) was drawn to measure the residual humeral head (B) and thereby the bone defect (A - B) (orange). Percentage bone loss was determined through the formula [(A - B)/A) * 100].
Two-dimensional humeral head defect measurements were made on 2D CT and T1 MRI axial views at the point of greatest defect, measured in millimeters. (Similar to Hill-Sachs width in other studies)
|
Time between the index test en reference test: Not applicable as no reference test were included.
For how many participants were no complete outcome data available? 0
Reasons for incomplete outcome data described? Not reported as only patients with complete outcome data were included. |
Mean measurements
Paired t tests were used. No significant differences were found between 3D CT and 3D MRI using paired t-test.
|
|||||||||||||||||||||||||
|
Cui 2023 |
Type of study: Cross-sectional study Setting and country: a single institution, Department of Radiology and department of Orthopaedics, China
Funding and conflicts of interest: none declared. |
Inclusion criteria: Patients with shoulder disloca- tion between July 2022 and June 2023 were identified retrospectively. The inclusion criteria were (1) age of 18 years or older, (2) shoulder anterior dislocation, (3) completion of both MRI and CT of the shoulder joint, and (4) interval between MRI and CT was 1 week or less. Both patients with primary dislocation and patients with recurrent dislocation were included.
Exclusion criteria: Patients with a history of shoulder osseous surgery.
N=21/56
Prevalence: 21/56 HSL 16 bipolar bone defect (both HSL and glenoid defect) 35/56 without bone defect
Mean age ± SD: 27.5 ±9.5
Sex: 70% M / 30% F |
Describe index test: MRI: a Philips 3-T MRI scanner (Amsterdam, The Netherlands) and an 8- channel phased-array coil.
Comparator test: CT: a Siemens Dual Source CT scanner (Erlangen, Germany).
The interval between MRI and CT was 1 week or less.
|
Two parallel lines were drawn at the medial edge of the Hill-Sachs lesion and the medial margin of the posterior rotator cuff attachment, along the orientation parallel to the Hill-Sachs lesion. The width between these 2 parallel lines was measured as the HSI (Fig 2). the GT was calculated using the glenoid diameter (D) and gle- noid defect (d) measured on the en face view (GT= 0.83D-d). If the HSI was greater than the GT, the lesion was determined to be off-track; if the HSI was less than the GT, the lesion was determined to be on-track.
|
Time between the index test en reference test: Not applicable as no reference test were included.
For how many participants were no complete outcome data available? 0 (%)
Reasons for incomplete outcome data described? Not reported as only patients with complete outcome data were included. |
Mean measurement
*paired t test was used.
Bland-Altman plots
|
|||||||||||||||||||||||||
|
Feuerriegel 2023 |
Type of study: Cross-sectional study
Setting and country: Single institution, emergency department, Germany
Funding and conflicts of interest: K.W. is employed by Philips GmbH Market DACH but was not involved in data acquisition or analysis.
|
Inclusion criteria: Patients admitted to the emergency department with suspected traumatic dislocation of the shoulder. All participants underwent 3-T MRI of the shoulder within 2 days after trauma, and in patients with fractures a CT examination was commenced as part of the diagnostic workup in clinical routine.
Exclusion criteria: Not reported.
N=46
Prevalence: 25/46 with osseous pathologies Prevalence of bony Bankart lesions and HSL were 100%.
Mean age ± SD: 40±14.5
Sex: 59% M / 41% F |
Describe index test: MRI: a 3-T MR scanner (Ingenia Elition X; Philips Healthcare) with a dedicated 16-channel shoulder coil (dStream shoulder 16ch coil, Philips Healthcare). UTE images were acquired in the sag- ittal plane. Due to the isotropic acquisition voxel size, the T1 GRE and FRACTURE sequences were acquired in axial orientation und reformatted in the sagittal and coronal plane as well as inverted to resemble a bright CT-like bone contrast.
Comparator test: CT: either an IQon Spectral CT scanner (Philips Healthcare) or a Siemens Somatom go.Top scanner (Sie- mens Healthineers).
The interval between MRI and CT unclear, but seemed both in clinial routine.
|
The humerus was assessed for Hill-Sachs lesions and in patients with a bipolar lesion. The definition and measurement of Hill-Sachs interval was not clear.
|
Time between the index test en reference test: Not applicable as no reference test were included.
For how many participants were no complete outcome data available? 0
Reasons for incomplete outcome data described? Not reported. |
Mean measurement
Bland-Altman plots
Bland-Altman plots (estimated based on the figure): CT vs T1 GRE mean difference not clear, 95% limits of agreement: -1.20,0.99; CT vs Fracture mean difference not clear, 95% limits of agreement: -1.05,0.75; CT vs T1 GRE mean difference not clear, 95% limits of agreement: -0.80,0.80;
Correlation regarding percentage of glenoid bone loss
|
|||||||||||||||||||||||||
COSMIN risk of bias assessment of included studies
List of excluded studies
|
Reference |
Reason for exclusion |
|
Lee 2013 |
Wrong outcome |
|
E Souza 2014 |
Wrong intervention |
|
Markenstein 2014 |
Wrong outcome |
|
Gyftopoulos 2015 |
Wrong intervention |
|
Acid 2012 |
Wrong population |
|
Aliprandi 2006 |
Wrong population |
|
Aygün 2017 |
Wrong population |
|
Bencardino 2013 |
Wrong study design |
|
Bishop 2013 |
Wrong population |
|
Bitzer 2004 |
Article in German |
|
Cagle 2019 |
Wrong outcome |
|
Crossan 2023 |
Wrong study design |
|
Cusmano 2000 |
Article in italian |
|
Dickens 2019 |
No intervention |
|
Dobson 2009 |
No intervention |
|
Elkharbotly 2016 |
Wrong population |
|
Foster 2023 |
Wrong intervention |
|
Galvin 2016 |
Wrong intervention |
|
Gómez Bermúdez 2022 |
Article in spain |
|
Gyftopoulos 2012 |
Wrong population |
|
Gyftopoulos 2013 |
Wrong population |
|
Gyftopoulos 2014 |
Wrong intervention |
|
Huijsmans 2007 |
Wrong population |
|
Jezycki 2024 |
Article in german |
|
Khan 2023 |
Wrong population |
|
Khedr 2013 |
Wrong population |
|
Koh 2018 |
No intervention |
|
Vopat 2020 |
Wrong outcome |
|
Madhuchandra 2022 |
Wrong outcome |
|
Mahmoud 2013 |
Wrong population |
|
Moroder 2013 |
Wrong population |
|
Oh 2010 |
Wrong population |
|
Owens 2014 |
Wrong population |
|
Parmar 2002 |
Wrong population |
|
Rossi 2021 |
Wrong study design |
|
Rutgers 2022 |
Wrong population |
|
Thacher 2023 |
Wrong study design |
|
Vopat 2021 |
Wrong intervention |
|
Weel 2016 |
Wrong study design |
|
Weil 2022 |
Wrong study design |
|
Wu 2022 |
Wrong population |
|
Yanke 2017 |
Wrong population |
|
DGMSR 2023 |
Wrong study design |
|
Stecco 2013 |
Wrong population (Glenoid) |
|
Ma 2018 |
Wrong population (Glenoid) |
|
de Mello 2020 |
Wrong population (Glenoid) |
|
Vopat 2018 |
Wrong population (Glenoid) |
|
Lansdown 2019 |
Wrong population (Glenoid) |
|
Friedman 2014 |
Wrong population (Glenoid) |
|
Verweij 2020 |
Wrong population (Glenoid) |
|
Weber 2021 |
Wrong population (Glenoid) |
|
Sgroi 2022 |
Wrong population (Glenoid) |
|
Rerko 2013 |
Wrong population (Glenoid) |
|
Kumar 2023 |
Wrong population (Glenoid) |
|
Makovicka 2023 |
Wrong population (Glenoid) |
|
Min 2023 |
Wrong population (Glenoid) |
|
Zappia 2023 |
Wrong population (Glenoid) |
|
Tian 2012 |
Wrong population (Glenoid) |
|
Saliken 2015 |
Wrong intervention (Review) |
|
Miao 2019 |
Wrong intervention (Review) |
|
Walter 2019 |
Wrong intervention (Review) |
Verantwoording
Beoordelingsdatum en geldigheid
Publicatiedatum : 17-02-2026
Beoordeeld op geldigheid : 17-02-2026
Algemene gegevens
De ontwikkeling/herziening van deze richtlijnmodule werd ondersteund door het Kennisinstituut van de Federatie Medisch Specialisten (www.demedischspecialist.nl/kennisinstituut) en werd gefinancierd door de Stichting Kwaliteitsgelden Medisch Specialisten (SKMS). De financier heeft geen enkele invloed gehad op de inhoud van de richtlijnmodule.
Samenstelling werkgroep
Voor het ontwikkelen van de richtlijnmodules is in 2022 een multidisciplinaire werkgroep ingesteld, bestaande uit vertegenwoordigers van alle relevante specialismen (zie hiervoor de Samenstelling van de werkgroep) die betrokken zijn bij de zorg voor patiënten met schouderinstabiliteit.
Werkgroep richtlijn schouderinstabiliteit
- Prof. Dr. M.P.J (Michel) Bekerom, orthopedisch chirurg OLVG en MC Jan van Gooijen, NOV – voorzitter richtlijnwerkgroep
- Dr. J.J.A.M. (Jos) van Raaij, orthopedisch chirurg, niet praktiserend, NOV
- Dr. O.A.J. (Olivier) van der Meijden, orthopedisch chirurg Albert Schweitzer Ziekenhuis, NOV
- Dr. A.R. (Alex) Poublon, orthopedisch chirurg Ziekenhuis Gelderse Vallei, NOV
- Dr. T.D.W. (Tjarco) Alta, orthopedisch chirurg Spaarne Gasthuis, NOV
- Dr. R.J. (Robert Jan) Derksen, traumachirurg, Zaans Medisch Centrum, NVvH
- MSc. F. (Femke) Boon, fysio-, manueel therapeut, extended scope specialist Schoudercentrum IBC, KNGF
- MSc. K.M.C. (Karin) Hekman, fysio-, manueel therapeut, extended scope specialist, Schoudercentrum IBC, KNGF
- Dr. I.D. (Iris) Kilsdonk, radioloog Deventer Ziekenhuis, NVvR
- Dr. H.J. (Henk-Jan) van der Woude, radioloog OLVG, NVvR
- Drs. H.K. (Rik) van der Kolk, sportarts OLVG, VSG
- Mevr. drs. G. (Gerardine) Willemsen-de Mey, patiëntvertegenwoordiger Nationale Vereniging ReumaZorg Nederland
Met ondersteuning van
- Dr. J. (Jacqueline) Jennen, adviseur Kennisinstituut van de Federatie Medisch Specialisten (tot december 2023)
- Dr. F. (Floor) Willeboordse, senior adviseur Kennisinstituut van de Federatie Medisch Speciaisten (tot januari 2025)
- Dr. M.S. (Matthijs) Ruiter, senior adviseur, Kennisinstituut van de Federatie Medisch Specialisten (vanaf januari 2025)
- MSc. D.G. (Dian) Ossendrijver, adviseur kennisinstituut van de Federatie Medisch Specialisten (vanaf december 2023)
- Dr. M. (Michiel) Oerbekke, adviseur Kennisinstituut van de Federatie Medisch Specialisten
- Dr. J. (Jing) de Haan-Du, adviseur Kennisinstituut van de Federatie Medisch Specialisten
- E. (Esther) van Bijl, medisch informatiespecialist, kennisinstituut van de Federatie Medisch Specialisten
Belangenverklaringen
Een overzicht van de belangen van werkgroepleden en het oordeel over het omgaan met eventuele belangen vindt u in onderstaande tabel. De ondertekende belangenverklaringen zijn op te vragen bij het secretariaat van het Kennisinstituut van de Federatie Medisch Specialisten via secretariaat@kennisinstituut.nl.
Gemelde (neven)functies en belangen werkgroep
|
Naam |
Hoofdfunctie |
Neven werkzaamheden |
Persoonlijke financiële belangen |
Persoonlijke relaties |
Extern gefinancierd onderzoek |
Intellectuele belangen en reputatie |
Overige belangen |
Datum |
Restrictie |
|
Michel van den Bekerom (voorzitter) |
Orthopedisch chirurg, OLVG, Amsterdam |
Betaald onderwijs geven bij/voor: |
Geen |
Geen |
Onderzoek gesteund door: SECEC (European Society for surgery of the shoulder an the elbow), ZonMW, SNN (Smith and Nephew). Het OLVG ontvangt financiële support voor een shoulder and elbow clinical and research fellowship van van Smith en Nephew. Dit fellowship wordt mede (financieel) ondersteund door een firma die materiaal maakt dat gebruikt wordt voor schouderstabilisaties. Het betreft een overeenkomst tussen het OLVG en de firma vanwege educatieve doeleinden. |
Geen |
Het OLVG is voornemens om een consultancy contract met zimmer/biomet op te stellen zodat ik op vraag elders kan opereren. |
19-10-2022 |
Geen restricties
|
|
Jos van Raaij |
Orthopedisch chirurg, Martini ziekenhuis Groningen |
Geen |
Geen |
Geen |
Geen |
Geen |
Geen |
11-07-2022 |
Geen restricties |
|
Olivier van der Meijden |
Orthopedisch chirurg |
Geen |
Geen |
Geen |
Geen |
Geen |
Geen |
28-09-2023 |
Geen restricties |
|
Alexander Poublon |
Orthopedisch chirurg met aandachtsgebied schouder |
Geen |
Geen |
Geen |
Geen |
Geen |
Geen |
06-10-2023 |
Geen restricties |
|
Tjarco Alta |
Orthopedisch Chirurg |
Geen |
Geen |
Geen |
Geen |
Geen |
Geen |
28-09-2023 |
Geen restricties |
|
Karin Hekman |
Fysiotherapeut bij Schoudercentrum IBC |
Voorzitter Schoudernetwerk Nederland; onkostenvergoeding |
Ik behandel patiënten die schouderinstabiliteit hebben binnen het schoudercentrum en binnen MC Jan van Goyen, dit is de reden voor het plaatsnemen in deze expertise groep. |
Geen |
Geen |
Het enige voordeel dat deelname aan deze richtlijn oplevert is nog meer persoonlijke expertise wat mogelijk leidt tot een verdieping van de fysiotherapeutische toepassingen bij schouderinstabiliteit. Dit is voor het SchouderNetwerk Nederland van primair belang en kan leiden tot uitdragen van kennis naar de regionale netwerken. Als voorzitter van deze stichting voel ik mij hier verantwoordelijk voor. |
Geen |
09-10-2023 |
Geen restricties |
|
Femke Boon |
Fysio- manueel therapeut en Extended Scope specialist bij Schoudercentrum IBC locatie Amstelland |
- 2x/ maand elleboog-schouder orthopedie poli bij Medisch Centrum Jan van Goyen (betaald, gedetacheerd vanuit IBC) |
Werkzaam bij Schoudercentrum IBC Amstelland |
Geen |
Richtlijn ontwikkeling FMS primaire anterieure schouderluxaties |
Geen |
Geen |
4-10-2023 |
Geen restricties |
|
Iris Kilsdonk |
Deventer Ziekenhuis |
Bestuurslid NVvR, sectie muskuloskeletale radiologie |
Geen |
Geen |
Geen |
Geen |
Geen |
2-10-2023 |
Geen restricties |
|
Robert Jan Derksen |
Traumachirurg Zaans Medisch Centrum |
Bestuurslid NVT |
Geen |
Geen |
Geen |
Geen |
Geen |
28-08-2022 |
Geen restricties |
|
Rik van der Kolk |
Sportarts, OLVG |
Geen |
Geen |
Geen |
Geen |
Geen |
Geen |
10-08-2022 |
Geen restricties |
|
Henk-Jan van der Woude |
Radioloog Onze Lieve Vrouwe Gasthuis Amsterdam |
Consulent Commissie voor Beentumoren, onbezoldigd |
Geen |
Geen |
Geen |
Geen |
Geen |
07-02-2023 |
Geen restricties |
Inbreng patiëntenperspectief
De werkgroep besteedde aandacht aan het patiëntenperspectief door het uitnodigen van de Patiëntenfederatie Nederland en de Nationale Vereniging ReumaZorg Nederland voor de knelpunteninventarisatie. Daarnaast nam een patiëntvertegenwoordiger van ReumaZorg Nederland deel aan de werkgroep. De verkregen input is meegenomen bij het opstellen van de uitgangsvragen, de keuze voor de uitkomstmaten en bij het opstellen van de overwegingen. De conceptrichtlijn is tevens voor commentaar voorgelegd aan Patiëntenfederatie Nederland en ReumaZorg Nederland en de eventueel aangeleverde commentaren zijn bekeken en verwerkt
Kwalitatieve raming van mogelijke financiële gevolgen in het kader van de Wkkgz
Bij de richtlijnmodule voerde de werkgroep conform de Wet kwaliteit, klachten en geschillen zorg (Wkkgz) een kwalitatieve raming uit om te beoordelen of de aanbevelingen mogelijk leiden tot substantiële financiële gevolgen. Bij het uitvoeren van deze beoordeling is de richtlijnmodule op verschillende domeinen getoetst (zie het stroomschema bij Werkwijze).
|
Module |
Uitkomst raming |
Toelichting |
|
Meetmethoden Humeraal botverlies |
Geen financiële gevolgen |
Uit de toetsing volgt dat de aanbeveling(en) niet breed toepasbaar zijn (<5.000 patiënten) en zal daarom naar verwachting geen substantiële financiële gevolgen hebben voor de collectieve uitgaven.
|
Werkwijze
Voor meer details over de gebruikte richtlijnmethodologie verwijzen wij u naar de Werkwijze. Relevante informatie voor de ontwikkeling van deze richtlijn is hieronder weergegeven.
Zoekverantwoording
Algemene informatie
|
Cluster/richtlijn: Schouderinstabiliteit - Module 2 Posttraumatische schouderinstabiliteit |
|
|
Uitgangsvraag/modules: Welk (aanvullend?) beeldvormend onderzoek moet worden verricht bij post traumatische schouderinstabiliteit? |
|
|
Database(s): Embase.com, Ovid/Medline |
Datum: 6-2-2024 |
|
Periode: vanaf 2000 |
Talen: geen restrictie |
|
Literatuurspecialist: Esther van der Bijl |
Rayyan review: https://rayyan.ai/reviews/922035 |
|
BMI-zoekblokken: voor verschillende opdrachten wordt (deels) gebruik gemaakt van de zoekblokken van BMI-Online https://blocks.bmi-online.nl/ Bij gebruikmaking van een volledig zoekblok zal naar de betreffende link op de website worden verwezen. |
|
|
Toelichting: Voor deze vraag is gezocht op de elementen Schouderinstabiliteit en MRI.
→ De sleutelartikelen PMID 22996361, PMID 18061117 en PMID 35452020 worden gevonden met deze search.
Zoals besproken is er gezocht met de P, I en het diagnostisch filter. In overleg zijn bij de P ook de zoektermen ‘glenoid bone loss’ en ‘glenoid defect’ meegenomen, omdat anders relevante artikelen gemist worden. |
|
|
Te gebruiken voor richtlijntekst: In de databases Embase.com en Ovid/Medline is op 6 februari 2024 systematisch gezocht naar systematische reviews, RCTs en observationele studies over de diagnostische accuratesse van MRI bij patiënten met verdenking op posttraumatische schouderinstabiliteit (ossale component). De literatuurzoekactie leverde 960 unieke treffers op. |
|
Zoekopbrengst - 6 februari 2024
|
|
EMBASE |
OVID/MEDLINE |
Ontdubbeld |
|
SR |
80 |
11 |
83 |
|
RCT |
199 |
30 |
210 |
|
Observationeel |
633 |
206 |
667 |
|
Totaal |
912 |
247 |
960* |
*in Rayyan
Zoekstrategie - 6 februari 2024
Embase.com
|
No. |
Query |
Results |
|
#1 |
'shoulder dislocation'/exp OR 'recurrent shoulder dislocation'/exp OR 'bankart lesion'/exp OR ((('shoulder*' OR 'gleno-humer*' OR 'glenoid*' OR 'humer*' OR 'scapulohumer*' OR 'glenohumer*') NEAR/3 ('dislocat*' OR 'diastasis' OR 'instabil*' OR 'luxat*' OR 'subluxat*' OR 'defect*')):ti,ab,kw) OR (('shoulder*':ti,ab,kw OR glenoid*:ti,ab,kw OR 'gleno-humer*':ti,ab,kw OR 'humer*':ti,ab,kw OR 'scapulohumer*':ti,ab,kw OR 'glenohumer*':ti,ab,kw) AND (('bon*' NEAR/3 ('loss*' OR 'erosion*')):ti,ab,kw)) OR ((('bankart' OR 'hill-sachs') NEAR/3 ('fracture*' OR 'lesion*' OR 'tear*')):ti,ab,kw) OR ((('on track' OR 'off track') NEAR/3 ('hill sachs' OR 'bone loss*' OR 'shoulder*' OR 'lesion*')):ti,ab,kw) |
17298 |
|
#2 |
'nuclear magnetic resonance imaging'/exp OR 'mri scanner'/exp OR ('magnetic resonance':ab,ti AND (image:ab,ti OR images:ab,ti OR imaging:ab,ti)) OR mri:ab,ti OR mris:ab,ti OR nmr:ab,ti OR mra:ab,ti OR mras:ab,ti OR zeugmatograph*:ab,ti OR 'mr tomography':ab,ti OR 'mr tomographies':ab,ti OR 'mr tomographic':ab,ti OR 'mr imag*':ti,ab,kw OR 'proton spin':ab,ti OR ((magneti*:ab,ti OR 'chemical shift':ab,ti) AND imaging:ab,ti) OR fmri:ab,ti OR fmris:ab,ti OR rsfmri:ti,ab,kw |
1604297 |
|
#3 |
'diagnostic procedure'/exp OR 'sensitivity and specificity'/de OR sensitivity:ab,ti OR specificity:ab,ti OR predict*:ab,ti OR 'roc curve':ab,ti OR 'receiver operator':ab,ti OR 'receiver operators':ab,ti OR likelihood:ab,ti OR 'diagnostic error'/exp OR 'diagnostic accuracy'/exp OR 'diagnostic test accuracy study'/exp OR 'inter observer':ab,ti OR 'intra observer':ab,ti OR interobserver:ab,ti OR intraobserver:ab,ti OR validity:ab,ti OR kappa:ab,ti OR reliability:ab,ti OR reproducibility:ab,ti OR ((test NEAR/2 're-test'):ab,ti) OR ((test NEAR/2 'retest'):ab,ti) OR 'reproducibility'/exp OR accuracy:ab,ti OR 'differential diagnosis'/exp OR 'validation study'/de OR 'measurement precision'/exp OR 'diagnostic value'/exp OR 'reliability'/exp OR 'predictive value'/exp OR ppv:ti,ab,kw OR npv:ti,ab,kw OR (((false OR true) NEAR/3 (negative OR positive)):ti,ab) OR diagnos*:ti,ab |
24669709 |
|
#4 |
#1 AND #2 AND #3 |
2429 |
|
#5 |
#4 AND [2000-2024]/py NOT ('conference abstract'/it OR 'editorial'/it OR 'letter'/it OR 'note'/it) NOT (('animal'/exp OR 'animal experiment'/exp OR 'animal model'/exp OR 'nonhuman'/exp) NOT 'human'/exp) |
1863 |
|
#6 |
'meta analysis'/exp OR 'meta analysis (topic)'/exp OR metaanaly*:ti,ab OR 'meta analy*':ti,ab OR metanaly*:ti,ab OR 'systematic review'/de OR 'cochrane database of systematic reviews'/jt OR prisma:ti,ab OR prospero:ti,ab OR (((systemati* OR scoping OR umbrella OR 'structured literature') NEAR/3 (review* OR overview*)):ti,ab) OR ((systemic* NEAR/1 review*):ti,ab) OR (((systemati* OR literature OR database* OR 'data base*') NEAR/10 search*):ti,ab) OR (((structured OR comprehensive* OR systemic*) NEAR/3 search*):ti,ab) OR (((literature NEAR/3 review*):ti,ab) AND (search*:ti,ab OR database*:ti,ab OR 'data base*':ti,ab)) OR (('data extraction':ti,ab OR 'data source*':ti,ab) AND 'study selection':ti,ab) OR ('search strategy':ti,ab AND 'selection criteria':ti,ab) OR ('data source*':ti,ab AND 'data synthesis':ti,ab) OR medline:ab OR pubmed:ab OR embase:ab OR cochrane:ab OR (((critical OR rapid) NEAR/2 (review* OR overview* OR synthes*)):ti) OR ((((critical* OR rapid*) NEAR/3 (review* OR overview* OR synthes*)):ab) AND (search*:ab OR database*:ab OR 'data base*':ab)) OR metasynthes*:ti,ab OR 'meta synthes*':ti,ab |
999431 |
|
#7 |
'clinical trial'/exp OR 'randomization'/exp OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp OR 'placebo'/exp OR 'prospective study'/exp OR rct:ab,ti OR random*:ab,ti OR 'single blind':ab,ti OR 'randomised controlled trial':ab,ti OR 'randomized controlled trial'/exp OR placebo*:ab,ti |
3963976 |
|
#8 |
'major clinical study'/de OR 'clinical study'/de OR 'case control study'/de OR 'family study'/de OR 'longitudinal study'/de OR 'retrospective study'/de OR 'prospective study'/de OR 'comparative study'/de OR 'cohort analysis'/de OR ((cohort NEAR/1 (study OR studies)):ab,ti) OR (('case control' NEAR/1 (study OR studies)):ab,ti) OR (('follow up' NEAR/1 (study OR studies)):ab,ti) OR (observational NEAR/1 (study OR studies)) OR ((epidemiologic NEAR/1 (study OR studies)):ab,ti) OR (('cross sectional' NEAR/1 (study OR studies)):ab,ti) |
8055848 |
|
#9 |
'case control study'/de OR 'comparative study'/exp OR 'control group'/de OR 'controlled study'/de OR 'controlled clinical trial'/de OR 'crossover procedure'/de OR 'double blind procedure'/de OR 'phase 2 clinical trial'/de OR 'phase 3 clinical trial'/de OR 'phase 4 clinical trial'/de OR 'pretest posttest design'/de OR 'pretest posttest control group design'/de OR 'quasi experimental study'/de OR 'single blind procedure'/de OR 'triple blind procedure'/de OR (((control OR controlled) NEAR/6 trial):ti,ab,kw) OR (((control OR controlled) NEAR/6 (study OR studies)):ti,ab,kw) OR (((control OR controlled) NEAR/1 active):ti,ab,kw) OR 'open label*':ti,ab,kw OR (((double OR two OR three OR multi OR trial) NEAR/1 (arm OR arms)):ti,ab,kw) OR ((allocat* NEAR/10 (arm OR arms)):ti,ab,kw) OR placebo*:ti,ab,kw OR 'sham-control*':ti,ab,kw OR (((single OR double OR triple OR assessor) NEAR/1 (blind* OR masked)):ti,ab,kw) OR nonrandom*:ti,ab,kw OR 'non-random*':ti,ab,kw OR 'quasi-experiment*':ti,ab,kw OR crossover:ti,ab,kw OR 'cross over':ti,ab,kw OR 'parallel group*':ti,ab,kw OR 'factorial trial':ti,ab,kw OR ((phase NEAR/5 (study OR trial)):ti,ab,kw) OR ((case* NEAR/6 (matched OR control*)):ti,ab,kw) OR ((match* NEAR/6 (pair OR pairs OR cohort* OR control* OR group* OR healthy OR age OR sex OR gender OR patient* OR subject* OR participant*)):ti,ab,kw) OR ((propensity NEAR/6 (scor* OR match*)):ti,ab,kw) OR versus:ti OR vs:ti OR compar*:ti OR ((compar* NEAR/1 study):ti,ab,kw) OR (('major clinical study'/de OR 'clinical study'/de OR 'cohort analysis'/de OR 'observational study'/de OR 'cross-sectional study'/de OR 'multicenter study'/de OR 'correlational study'/de OR 'follow up'/de OR cohort*:ti,ab,kw OR 'follow up':ti,ab,kw OR followup:ti,ab,kw OR longitudinal*:ti,ab,kw OR prospective*:ti,ab,kw OR retrospective*:ti,ab,kw OR observational*:ti,ab,kw OR 'cross sectional*':ti,ab,kw OR cross?ectional*:ti,ab,kw OR multicent*:ti,ab,kw OR 'multi-cent*':ti,ab,kw OR consecutive*:ti,ab,kw) AND (group:ti,ab,kw OR groups:ti,ab,kw OR subgroup*:ti,ab,kw OR versus:ti,ab,kw OR vs:ti,ab,kw OR compar*:ti,ab,kw OR 'odds ratio*':ab OR 'relative odds':ab OR 'risk ratio*':ab OR 'relative risk*':ab OR 'rate ratio':ab OR aor:ab OR arr:ab OR rrr:ab OR ((('or' OR 'rr') NEAR/6 ci):ab))) |
14796927 |
|
#10 |
#5 AND #6 – SR’s |
80 |
|
#11 |
#5 AND #7 NOT #10 – RCT’s |
199 |
|
#12 |
#5 AND (#8 OR #9) NOT (#10 OR #11) – Observationele studies |
633 |
|
#13 |
#10 OR #11 OR #12 |
912 |
Ovid/Medline
|
# |
Searches |
Results |
|
1 |
exp Shoulder Dislocation/ or exp Bankart Lesions/ or ((shoulder* or gleno-humer* or glenoid* or humer* or scapulohumer* or glenohumer*) adj3 (dislocat* or diastasis or instabil* or luxat* or subluxat* or defect*)).ti,ab,kf. or ((shoulder* or glenoid* or gleno-humer* or humer* or scapulohumer* or glenohumer*) and (bon* adj3 (loss* or erosion*))).ti,ab,kf. or ((bankart or hill-sachs) adj3 (fracture* or lesion* or tear*)).ti,ab,kf. or ((on track or off track) adj3 (hill sachs or bone loss* or shoulder* or lesion*)).ti,ab,kf. |
13706 |
|
2 |
exp magnetic resonance imaging/ or ("magnetic resonance" and (image or images or imaging)).ti,ab,kf. or mri.ti,ab,kf. or mris.ti,ab,kf. or nmr.ti,ab,kf. or mra.ti,ab,kf. or mras.ti,ab,kf. or zeugmatograph*.ti,ab,kf. or "mr tomography".ti,ab,kf. or "mr tomographies".ti,ab,kf. or "mr tomographic".ti,ab,kf. or 'mr imag*'.ti,ab,kf. or "proton spin".ti,ab,kf. or ((magneti* or "chemical shift") and imaging).ti,ab,kf. or fmri.ti,ab,kf. or fmris.ti,ab,kf. or rsfmri.ti,ab,kf. |
981588 |
|
3 |
exp "Sensitivity and Specificity"/ or (sensitivity or specificity).ti,ab. or (predict* or ROC-curve or receiver-operator*).ti,ab. or (likelihood or LR*).ti,ab. or exp Diagnostic Errors/ or (inter-observer or intra-observer or interobserver or intraobserver or validity or kappa or reliability).ti,ab. or reproducibility.ti,ab. or (test adj2 (re-test or retest)).ti,ab. or "Reproducibility of Results"/ or accuracy.ti,ab. or Diagnosis, Differential/ or Validation Study/ or ((false or true) adj3 (negative or positive)).ti,ab. |
5006646 |
|
4 |
1 and 2 and 3 |
408 |
|
5 |
limit 4 to yr="2000 -Current" |
349 |
|
6 |
5 not (comment/ or editorial/ or letter/) not ((exp animals/ or exp models, animal/) not humans/) |
343 |
|
7 |
meta-analysis/ or meta-analysis as topic/ or (metaanaly* or meta-analy* or metanaly*).ti,ab,kf. or systematic review/ or cochrane.jw. or (prisma or prospero).ti,ab,kf. or ((systemati* or scoping or umbrella or "structured literature") adj3 (review* or overview*)).ti,ab,kf. or (systemic* adj1 review*).ti,ab,kf. or ((systemati* or literature or database* or data-base*) adj10 search*).ti,ab,kf. or ((structured or comprehensive* or systemic*) adj3 search*).ti,ab,kf. or ((literature adj3 review*) and (search* or database* or data-base*)).ti,ab,kf. or (("data extraction" or "data source*") and "study selection").ti,ab,kf. or ("search strategy" and "selection criteria").ti,ab,kf. or ("data source*" and "data synthesis").ti,ab,kf. or (medline or pubmed or embase or cochrane).ab. or ((critical or rapid) adj2 (review* or overview* or synthes*)).ti. or (((critical* or rapid*) adj3 (review* or overview* or synthes*)) and (search* or database* or data-base*)).ab. or (metasynthes* or meta-synthes*).ti,ab,kf. |
724616 |
|
8 |
exp clinical trial/ or randomized controlled trial/ or exp clinical trials as topic/ or randomized controlled trials as topic/ or Random Allocation/ or Double-Blind Method/ or Single-Blind Method/ or (clinical trial, phase i or clinical trial, phase ii or clinical trial, phase iii or clinical trial, phase iv or controlled clinical trial or randomized controlled trial or multicenter study or clinical trial).pt. or random*.ti,ab. or (clinic* adj trial*).tw. or ((singl* or doubl* or treb* or tripl*) adj (blind$3 or mask$3)).tw. or Placebos/ or placebo*.tw. |
2687808 |
|
9 |
Epidemiologic studies/ or case control studies/ or exp cohort studies/ or Controlled Before-After Studies/ or Case control.tw. or cohort.tw. or Cohort analy$.tw. or (Follow up adj (study or studies)).tw. or (observational adj (study or studies)).tw. or Longitudinal.tw. or Retrospective*.tw. or prospective*.tw. or consecutive*.tw. or Cross sectional.tw. or Cross-sectional studies/ or historically controlled study/ or interrupted time series analysis/ [Onder exp cohort studies vallen ook longitudinale, prospectieve en retrospectieve studies] |
4645843 |
|
10 |
Case-control Studies/ or clinical trial, phase ii/ or clinical trial, phase iii/ or clinical trial, phase iv/ or comparative study/ or control groups/ or controlled before-after studies/ or controlled clinical trial/ or double-blind method/ or historically controlled study/ or matched-pair analysis/ or single-blind method/ or (((control or controlled) adj6 (study or studies or trial)) or (compar* adj (study or studies)) or ((control or controlled) adj1 active) or "open label*" or ((double or two or three or multi or trial) adj (arm or arms)) or (allocat* adj10 (arm or arms)) or placebo* or "sham-control*" or ((single or double or triple or assessor) adj1 (blind* or masked)) or nonrandom* or "non-random*" or "quasi-experiment*" or "parallel group*" or "factorial trial" or "pretest posttest" or (phase adj5 (study or trial)) or (case* adj6 (matched or control*)) or (match* adj6 (pair or pairs or cohort* or control* or group* or healthy or age or sex or gender or patient* or subject* or participant*)) or (propensity adj6 (scor* or match*))).ti,ab,kf. or (confounding adj6 adjust*).ti,ab. or (versus or vs or compar*).ti. or ((exp cohort studies/ or epidemiologic studies/ or multicenter study/ or observational study/ or seroepidemiologic studies/ or (cohort* or 'follow up' or followup or longitudinal* or prospective* or retrospective* or observational* or multicent* or 'multi-cent*' or consecutive*).ti,ab,kf.) and ((group or groups or subgroup* or versus or vs or compar*).ti,ab,kf. or ('odds ratio*' or 'relative odds' or 'risk ratio*' or 'relative risk*' or aor or arr or rrr).ab. or (("OR" or "RR") adj6 CI).ab.)) |
5616453 |
|
11 |
6 and 7 – SR’s |
11 |
|
12 |
(6 and 8) not 11 – RCT’s |
30 |
|
13 |
(6 and (9 or 10)) not (11 or 12) – Observationele studies |
206 |
|
14 |
11 or 12 or 13 |
247 |