SGLT2-remmers
Uitgangsvraag
Wat is de waarde van SGLT2-remmers bij patiënten met een verminderde nierfunctie die jodiumhoudend intravasculair contrastmiddel krijgen toegediend?
Aanbeveling
Continueer SGLT2-remmers bij alle hemodynamisch stabiele patiënten die intravasculair jodiumhoudend contrastmiddel krijgen toegediend omdat er ook bij hoog-risico groepen met gestoorde nierfunctie geen aanwijzingen zijn voor een verhoogd risico op PC-AKI en er mogelijk zelfs een beschermend effect is.
Start niet met een SGLT2-remmer binnen 1 week vóór jodiumhoudende contrasttoediening met als doel om PC-AKI te voorkomen gezien hun diuretische effect met passagère verslechtering van nierfunctie.
Overweeg de start van SGLT2-remmers kortdurend uit te stellen als duidelijk is dat een jodiumhoudend contrastonderzoek binnen 3-4 dagen noodzakelijk zal zijn.
Overweeg de behandeling met een SGLT2-remmer kortdurend te onderbreken als deze binnen één week voor de geplande jodiumhoudende contrasttoediening gestart is.
Pas bij patiënten met hoog-risico op PC-AKI ook tijdens SGLT2-remmers de gebruikelijke profylactische hydratie toe.
Overwegingen
Balans tussen gewenste en ongewenste effecten
Pros and cons of starting and/or continuing SGLT2-inhibitors (SGLT2i) during administration of iodine-containing contrast in patients with high risk of PC-AKI
Many trials have demonstrated that SGLT2i have renoprotective effects not only in patients with type 2 diabetes mellitus, but also in patients with heart failure or chronic renal failure without diabetes (Heerspink, 2020; Neal, 2017; Packer, 2020; Perkovic, 2019). However, trials have also reported an acute decline in eGFR of approximately 5 ml/min/1.73 m2 at the initiation of SGLT2i due to increased sodium delivery to the distal tubule and macula densa, leading to the activation of the tubuloglomerular feedback, which results in the vasoconstriction of the afferent glomerular arterioles and hence possibly higher risk of PC-AKI (Heerspink, 2010). It is therefore important to answer the question whether the renoprotective effects of SGLTi outweigh the theoretical increased risk of PC-AKI due to vasoconstriction of afferent glomerular arterioles when an SGLT2i is started shortly before contrast administration and/or is continued during radiologic examinations with iodine containing contrast.
Risk of PC-AKI during chronic treatment with SGLT2i in diabetic patients with high risk of PC-AKI due to renal failure
Since administration of iodine-containing contrast in radiologic investigations with high risk of PC-AKI such as CAG with PCI are in most cases not planned in advance and often performed in emergency situations in the setting of acute coronary syndrome, it is almost impossible to perform a RCT with start of a SGLTi more than 2 weeks before contrast administration. Therefore, only retrospective or prospective observational studies of cohorts of patients comparing SGLT2i-users and non-SGLT2i-users were available to answer this question and we selected the studies with subgroup analysis of patients with an eGFR <60 ml/min/1.73m2 and statistical matching between the groups for example with propensity scores.
The 3 available studies did not indicate an increased risk of PC-AKI of continuing SGLT2i during contrast in CKD patients with a relatively high level of evidence with HR well below the predefined margin of 1.09. Two out of these 3 studies even suggested a beneficial effect leading to an overall HR of 0.45 (CI 0.20-0.98) for the SGLT2i with a low level of evidence due to overlap of upper limit of CI with predefined limit of 0.91 besides other limitations of these retrospective and observational studies. In two of these studies, patients received traditional prophylactic pre- and/or posthydration with intravenous saline (NaCl 0.9%) and in the third study of Paolisso probably also. Therefore, the conclusions of the possible beneficial effects of SGLTi on risk of PC-AKI only apply to situations where adequate hydration was given around high-risk contrast administration.
Risk of PC-AKI during chronic treatment with SGLT2i in patients with heart failure without diabetes
In a single center retrospective study in Italy (Nardi, 2024) the incidence of PC-AKI according to ESUR criteria was also significantly lower during long-term (>6 months) treatment with SGLT2i because of reduced or mildly reduced LV systolic function (HFrEF or HRmrEF) with 9.3% compared to 26.7% (P=0.016) in propensity matched controls. All patients had received prophylactic intravenous pre- and posthydration with normal saline or Hartmann’s solution and more than half of the patients probably had an eGFR <60 ml/min/1.73m2 because of mean eGFR ml/min/1.73m2 of 58±26 in SGLT2i-users and 58±37 ml/min in matched controls.
Risk of accelerated decline in renal function and earlier start of RRT after contrast administration during chronic treatment with SGLT2i
One study (Chen, 2024) indicated that SGLT2i also protects against long-term risk of contrast of iodine containing contrast for the important long-term clinical outcome measures of accelerated decrease in kidney function and the earlier start of renal replacement therapy. In this study, SGLTi-users with a moderate CV-risk had significantly less MAKE (major adverse kidney events between 7-180 days after contrast) with HR 0.68 (CI 0.52-0.88), but in the smaller group with a high CV-risk this advantage failed to reach significance with a HR 0.90 (CI 0.61-1.34). None of the patients had to start RRT in both groups. This database retrospective analysis therefore suggests that the renoprotective effect of SGLTi is also maintained after contrast administration.
Beneficial or detrimental effect of starting SGLT2i shortly before contrast administration in patients with high risk of PC-AKI due to renal failure
In a prospective cohort trial in China in type 2 diabetes with only approximately 11% CKD stage 3 (Zang, 2024) PC-AKI was observed more frequently during SGLT2i (10.7% vs. 2.9% in propensity matched controls, P=0.027) despite standard saline pre- and posthydration. Subgroup analysis demonstrated that a higher incidence of PC-AKI was only observed in the subgroup who started SGLTi after admission to the hospital (1.7±1.4 days before coronary angiography) and not in patients who started SGLTi 191±223 days before admission ((20.5% for start SGLTi after admission vs. 3.4% chronic use, P=0.018). However, since PC-AKI was defined according to ESUR criteria (increase in creatinine >0.5 mg/ dL, or >25% above the baseline 48–72 h after contrast) it is possible and even likely that such an acute rise in creatinine after contrast shortly after start of SGLT2i is at least partly due to the well-known acute decline of eGFR at the initiation of SGLTi. On the other hand, it cannot be excluded that the concomitant afferent glomerular vasoconstriction after initiation of SGLTi makes patients more susceptible to toxic effects of iodine-containing contrast.
In another single centre retrospective study also in China in 655 patients with type 2 diabetes with mostly normal renal function (Yang, 2024), start of SGLTi after admission and on average 2.8 (1.8-4.0) days before CAG or PCI resulted in higher incidence of PC-AKI according to ESUR criteria than in propensity score matched controls (OR 1.51, CI 1.02-2.24) due to a higher percentage increase of creatinine >25% (11.1% vs 7.5%, P=0.03). Partial or full recovery of kidney function after 90 days however did not differ between SGLTi users and matched controls.
In absence of further evidence, it remains unclear whether it is safe to start SGLT2i shortly before contrast administration and should better be avoided when possible.
Quality of evidence
The overall quality of evidence is very low. This means that we are very uncertain about the estimated effect of the crucial outcome measures. The evidence has been downgraded because of serious:
- Imprecision: because the confidence interval exceeds the limits of clinical relevance.
Values and preferences of patients (and possibly their relatives/caregivers)
Kidney patients are prescribed SGLT2 inhibitors to prevent further deterioration of their kidney function. It is therefore an important medication for them, which they prefer not to stop taking. Temporary discontinuation can cause confusion for the patients themselves, their caregivers and healthcare providers. However, it is important for patients that SGLT2 inhibitors can be used safely, also during the administration of contrast agents. If it is nevertheless considered to temporarily discontinue the SGLT2 inhibitor in connection with contrast administration (e.g. if this was started within one week before the planned contrast administration), it is important to discuss this thoroughly with the patient. This also applies to the short-term postponement of the start of an SGLT2 inhibitor. Patients want to know what the possible risks are and want to receive a clear explanation of when they can start (again).
Cost aspects
It is unknown whether continuing SGLT2 inhibitors during contrast examination will result in cost savings because it is uncertain whether this can reduce the risk of PC-AKI, and the same applies to postponing the start of SGLT2 inhibitors shortly before contrast examination. It is therefore not possible to perform a cost-benefit analysis.
Equality ((health) equity/equitable)
The advice in this module is not expected to lead to differences in health equity because it can be applied to all types of patients.
Acceptability
Ethical acceptability
The advice seems acceptable to all patients involved.
Sustainability
There are no sustainability aspects to the proposed intervention.
Feasibility
The intervention is generally already standard care in practice and can therefore be applied without any problems.
Rationale van de aanbeveling: weging van argumenten voor en tegen de interventies
Gezien het ontbreken van gerandomiseerde studies berust de zeer lage kwaliteit van het beschikbare bewijs op retrospectieve en observationele onderzoeken met (propensity score) matched controles. Al deze onderzoeken laten echter geen aanwijzingen zien voor een nadelig effect van continueren van chronisch gebruik van SGLT2-remmers tijdens contrastonderzoek bij hemodynamisch stabiele patiënten die bovendien adequate pre- en posthydratie krijgen en deze middelen hebben mogelijk zelfs een voordelig effect zodat er geen reden is om de SGLT2-remmers te onderbreken alleen omdat er contrast toegediend zal worden. Gezien hun diuretische effect en de bijkomende afferente preglomulaire vasoconstrictie is er wel een reden om terughoudend te zijn met start van SGLT2-remmers kort voor contrasttoediening omdat de beschikbare onderzoeken onvoldoende bewijs leveren dat dit ook veilig is.
Onderbouwing
Achtergrond
At present, SGLT2-inhibitors are not indicated for prophylaxis of post contrast acute kidney injury (PC-AKI). Current indications are type 2 diabetes mellitus, chronic heart failure, and chronic kidney disease. Although many studies have clearly demonstrated a renoprotective effect of SGLT2-inhibitors in the long-term, the question arises whether these drugs are also renoprotective when used during a radiological examination with intravascular iodine-containing contrast in patients at risk for PC-AKI because of pre-existing chronic or acute renal failure.
Because of their renoprotective effect these drugs might be an interesting option to prevent PC-AKI when they are started (also) for this indication shortly before administration of iodine-containing contrast and if so, it would be important to know how far in advance they should be started. On the other hand, another important clinical dilemma is whether long term use of SGLT2-inhibitors increase the risk of PC-AKI due to their diuretic effect with increased sodium delivery to the distal renal tubule and consequently vasoconstriction of afferent glomerular arterioles and should therefore be interrupted during contrast examination.
Conclusies / Summary of Findings
Table 3. Summary of Findings
Population: Patients with reduced kidney function (eGFR <60 ml/min/1.73m2) undergoing radiological examinations or interventions with intravascular iodine-containing contrast media.
Intervention: SGLT2-inhibitors (such as Canagliflozin, dapagliflozin, empagliflozin, ertugliflozin)
Comparator: No SLGT2-inhibitors
Click here to see this table in a document
|
Outcome Timeframe |
Study results and measurements |
Absolute effect estimates |
Certainty of the evidence (Quality of evidence) |
Conclusions |
|
|
No SLGT2-inhibitors |
SGLT2-inhibitors (such as Canagliflozin, dapagliflozin, empagliflozin, ertugliflozin) |
||||
|
CI-AKI (critical)
|
Relative risk: 0.45 (CI 95% 0.20 – 0.98) Based on data from 508 participants in 3 studies
|
246 per 1000 |
111 per 1000 |
Very low Due to serious imprecision1 |
The evidence is very uncertain about the effect of SGLT2-inhibitors on CI-AKI when compared with no SGLT2-inhibitors in patients with reduced kidney function (eGFR <60 ml/min/1.73m2) undergoing radiological examinations or interventions with intravascular iodine-containing contrast media. |
|
Difference: 135 fewer per 1000 (CI 95% 197 fewer – 5 fewer) |
|||||
|
Start renal replacement therapy (important) |
Based on data from 1 study |
None of the patients had to start renal replacement therapy in the first 6 months after contrast administration in both SGLT2-users and non-SGLT2-users. |
Very low Due to very serious imprecision2 |
The evidence is very uncertain about the effect of SGLT2-inhibitors on start of renal replacement therapy when compared with no SGLT2-inhibitors in patients with reduced kidney function (eGFR <60 ml/min/1.73m2) undergoing radiological examinations or interventions with intravascular iodine-containing contrast media. |
|
|
Accelerated decrease in kidney function (important)
|
Based on data from 1 study |
HR for risk of MAKEs for group:
|
Very low Due to serious imprecision3 |
The evidence is very uncertain about the effect of SGLT2-inhibitors on accelerated decrease in kidney function when compared with no SGLT2-inhibitors in patients with reduced kidney function (eGFR <60 ml/min/1.73m2) undergoing radiological examinations or interventions with intravascular iodine-containing contrast media. |
|
1. Imprecision: serious. Due to overlap of the lower limit of the 95% confidence interval with the minimal clinically important difference
2. Imprecision: very serious. Due to no events occurred and the optimal information size was not achieved
3. Imprecision: serious. Due to the optimal information size which was not achieved
Samenvatting literatuur
Description of studies
A total of four studies were included in the analysis of the literature. Important study characteristics and results are summarized in table 2. The assessment of the risk of bias is summarized in the risk of bias tables (under the tab ‘Evidence tabellen’).
Since SGLT2-inhibitors were contra-indicated in patients with eGFR <20-30 ml/min/1.73m2, most studies have excluded patients with an eGFR <30 ml/min/1.73m2 and only Chen included a very small number of patients (20 out of 920) on SGLT2-inhibitors with an eGFR <30 ml/min/1.73m2. For the same reasons, patients with diabetes mellitus type 1 were excluded.
Çabuk (2024) performed a cross-sectional, prospective observational single-center study to determine the effect of SGLT2-inhibitors on the occurrence of contrast-associated acute kidney injury (CA-AKI) in patients with type II diabetes who underwent elective coronary angiography (CAG) with or without percutaneous coronary intervention (PCI) and patients with acute coronary syndrome who underwent emergency CAG with or without PCI within 24 hours after admission. In both groups, approximately 80% of the patients were treated with PCI. All patients received intravenous isotonic saline starting at the beginning of angiography and continued for 12 hours after CAG as prophylaxis for PC-AKI. Baseline characteristics in the overall study population were similar between the groups with and without SGLT2-inhibitors. In total, a subgroup of 113 patients had an eGFR between 30-60 mL/min/1.73m2 of which 36 patients received SGLT2-inhibitors (empagliflozin or dapagliflozin) for at least 6 months and 77 patients did not receive SGLT2-inhibitors. The outcome of interest was the occurrence of CA-AKI defined according to European Society of Urogenital Radiology (ESUR) criteria as an increase in serum creatinine of ≥0.5 mg/dL (44 µmol/L) or an absolute increase of ≥25% from baseline 72 hours after contrast media exposure.
Chen (2024) aimed to determine whether SGLT2-inhibitors affect the risk of contrast-induced-acute kidney injury (CI-AKI) by retrospectively analyzing data from the Taipei Medical University Clinical Research Database. Patients with type 2 diabetes who had contrast exposure while undergoing computer tomography or coronary angiography between 2016 and 2020 were included. In total, 250 SGLT-users and 4016 non-SGLT2-users had an eGFR <60 ml/min/1.73m2 (of these 20 and 512 eGFR <30 ml/min/1.73m2, respectively). Besides, 475 SGLT2-users and 6469 non-users had a moderate risk of cardiovascular events due to chronic kidney disease (CKD) defined by an eGFR ≥ 60 and uACR ≥ 300, eGFR = 45–59 and uACR = 30–300, or eGFR = 30–44 and uACR < 30, and respectively 75 and 1484 patients a high risk defined by eGFR = 45–59 and uACR ≥ 300, eGFR = 30–44 and uACR ≥ 30, or eGFR < 30. Overlap weighting for differences in propensity score was used to adjust for the effects of potential confounders consisting mainly of a higher prevalence of comorbidities in SGLT2-users. The outcome of interest is the risk of major adverse kidney events (MAKE), defined as the composite of Acute Kidney Disease (AKD) 7-90 days after contrast, CKD progression 91-180 days after contrast, and start of Renal Replacement Therapy (RRT). Both AKD and CKD progression were defined as an increase in the serum level of creatinine by ≥0.3 mg/dl or by 1.5 times the baseline value (KDIGO AKI criteria), new onset of microalbuminuria, or a ≥40% decline in the eGFR.
Liu (2023) performed a retrospective single center matched cohort analysis to determine the effect of dapagliflozin in the prevention of post-contrast acute kidney injury (PC-AKI) in patients with type 2 diabetes and CKD. Inclusion criteria were an age between 18 and 75 years, an eGFR between 45 and 89 ml/min/1.73m2 and receiving elective coronary angiography or percutaneous coronary intervention procedures. All patients received standard hydration with an intravenous infusion of 0.9% saline at a rate of 1 mL/kg/h for 6 h before and 12 h after the procedure. In total, 348 patients had a CKD stage of G3a (eGFR between 45 and 59) of which 74 patients received dapagliflozin for at least 2 weeks before contrast and 274 patients did not receive dapagliflozin. The outcome of interest was the occurrence of PC-AKI according to ESUR criteria as a serum creatinine level ≥1.25 times of the baseline level and/or increase of 0.5 mg/dL (44.2 μmol/L) within 72 h after exposure to the contrast medium. Propensity score-matched analysis was conducted to explore the association between dapagliflozin usage and the occurrence of PC-AKI leading to a comparison of 74 dapagliflozin users with 72 matched control patients.
Paolisso (2023) performed a multicenter, international, prospective observational cohort study to determine the association between chronic SGLT2-inhibitor treatment for at least 3 months and the development of CI-AKI. Diabetic patients with acute myocardial infarction (both ST- and non-ST segment elevation myocardial infarction) and with or without CKD treated with PCI were included. Patients were treated according to the 2020 ESC guidelines for the management of acute coronary syndrome and therefore should have received prophylactic hydration around the PCI. In total, 249 patients had CKD according to KDIGO criteria of which 28 patients received SGLT2-inhibitors and 221 patients were non-SGLT2-inhibitor users. Of note, the Mehran risk score for PC-AKI did not differ between the 2 groups in this latter CKD group. The outcome of interest was the rate of CI-AKI which was defined according to ESUR criteria as an absolute (≥0.5 mg/dl = 44 µmol/L) or relative increase (≥25%) in creatinine at 48-72 h after PCI compared to baseline values.
Table 2. Characteristics of included studies
|
Study |
Participants |
Comparison |
Follow-up |
Outcome measures |
Comments |
Risk of bias (per outcome measure)* |
|
Individual studies |
||||||
|
Çabuk, 2024 |
N at baseline Intervention: 36 Control: 77
Characteristics not mentioned for subgroup with CKD
|
Intervention: SGLT2-inhibitor users (dapagliflozin or empagliflozin) Control: no SGLT2-inhibitor users |
Used SGLT2-inhibitors for at least 6 months and CA-AKI was assessed up to 72 hours |
Occurrence of CA-AKI |
The authors declared that this study has received no financial support. The authors have no conflicts of interest to declare. |
Low |
|
Chen, 2024 |
N at baseline Intervention: 75 Control: 1484
Characteristics not mentioned for subgroup with CKD
|
Intervention: SGLT2-inhibitor users Control: no SGLT2-inhibitor users |
6 months after contrast |
Risk of major adverse kidney events (MAKE), start of Renal Replacement Therapy |
Funded by The Taiwan Ministry of Science and Technology (MOST 111-2314- B-038-103, 107-2314-B-038-019-MY3, NSTC 111-2218-E-008-009); Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan (DP2-111-21121-01-O-13); Taipei Medical University Hospital (112TMU-TMUH-14, 109TMU-TMUH-22). All authors declare no conflict of interest |
Low |
|
Liu, 2023 |
N at baseline Intervention: 74 Control: 274
Characteristics not mentioned for subgroup with CKD
|
Intervention: dapagliflozin users Control: no dapagliflozin users |
PC-AKI was assessed 72 hours after contrast medium |
PC-AKI occurrence |
Funded by the Safety Capability Building for Civil Aviation (Grant No.: DFS20180601). The authors have no conflicts of interest to declare |
Low |
|
Paolisso, 2023 |
N at baseline Intervention: 28 Control: 221
Age (median, IQR) Intervention: 71 (66 to 80) years Control: 78 (71 to 84) years
Sex (male) Intervention: 21 (75%) Control: 155 (70.1%)
|
Intervention: chronic SGLT2-inhibitor therapy Control: other oral anti-diabetic agents |
At least 3 months |
Rate of CI-AKI |
Funded by research grant from the CardioPaTh PhD Program. The authors declare that they have no competing interests |
Low |
* For further details, see risk of bias table in the appendix
Results
1. Post-contrast AKI (PC-AKI)
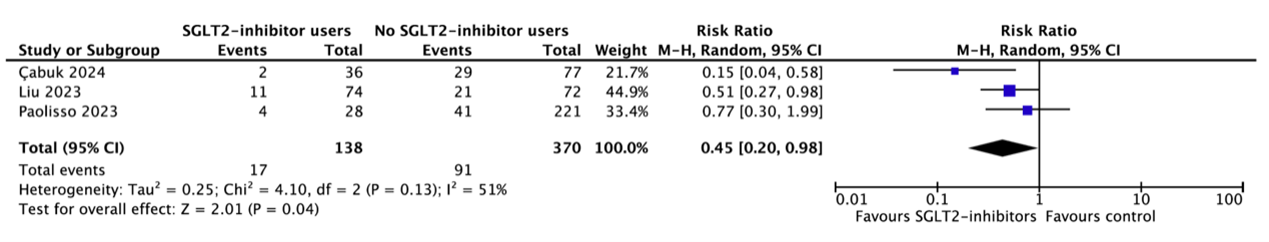
Three studies reported the occurrence of PC-AKI in subgroups of patients with eGFR 30-60 ml/min/1.73m2 (Çabuk, 2024; Liu, 2023; Paolisso, 2023) (figure 1). In total, 17 of the 138 patients (12.3%) who received SGLT2-inhibitors had PC-AKI as compared to 91 of the 370 patients (24.6%) who did not receive SGLT2-inhibitors (RR=0.45, 95%CI 0.20 to 0.98). This difference is clinically relevant favoring SGLT2-inhibitors.
Figure 1. Occurrence of PC-AKI
2. Start renal replacement therapy (RRT)
Chen (2024) reported that none of the patients with an eGFR <60 ml/min/1.73m2 had to start RRT in the first 6 months after contrast administration in both SGLT2-users and non-SGLT2-users.
3. Accelerated decrease in kidney function (according to KDIGO criteria)
Chen (2024) reported the risk of a major adverse kidney event (MAKE) which consists of acute kidney disease (AKD) 7-90 days after contrast, chronic kidney disease (CKD) progression 91-180 days after contrast, and the need for renal replacement therapy. AKD and CKD progression were defined as an increase in the serum level of creatinine by ≥0.3 mg/dl or by 1.5 times the baseline value (KDIGO AKI criteria), new onset of microalbuminuria or a ≥40% decline in eGFR. Subgroup analysis demonstrated that SGLT2-users had a significantly lower risk of MAKE compared to non-SGLT2-users if they had a moderate risk of CV events due to CKD (hazard ratio of 0.68 (95%CI 0.52-0.88)), but the difference in risk failed to reach significance in the smaller group with a high risk of CV events due to CKD (hazard ratio of 0.90 (95%CI 0.61 to 1.34)).
Zoeken en selecteren
A systematic review of the literature was performed to answer the following question(s):
What are the possible beneficial or detrimental effects of using SGLT2-inhibitors in patients with renal impairment who are receiving iodine-containing contrast?
Table 1. PICO
| Patients | Patients with reduced kidney function (eGFR <60 ml/min/1.73m2) undergoing radiological examinations or interventions with intravascular iodine-containing contrast media |
|
Intervention |
SGLT2-inhibitors (such as canagliflozin, dapagliflozin, empagliflozin, ertugliflozin) |
|
Control |
No SGLT2-inhibitors |
| Outcomes | Post-Contrast AKI (PC-AKI), start renal replacement therapy, or accelerated decrease in kidney function according to KDIGO criteria |
| Other selection criteria | Study design: systematic reviews, randomized controlled trials or observational studies |
Relevant outcome measures
The guideline panel considered incidence of Post-Contrast AKI (PC-AKI) as a critical outcome measure for decision making to start or interrupt SGLT2-inhibitors before contrast administration in patients with renal failure, and earlier start of renal replacement therapy and accelerated decrease in kidney function as important long-term outcome measures.
The working group defined PC-AKI as described in the chapter 2.1 PC-AKI: Definities, terminologie en klinisch verloop in the Dutch guideline “Veilig gebruik van contrastmiddelen (NVVR, 2017)”.
The guideline panel defined the following as a minimal clinically (patient) important difference:
- Post-contrast acute kidney injury (PC-AKI): relative risk <0.91 or >1.10.
- Complications of PC-AKI (accelerated decrease in kidney function, earlier start of dialysis): relative risk <0.91 or >1.10.
A difference of at least 10% in relative risk was defined as a minimal clinically (patient) important difference; by expert opinion of the working group (no literature was available to substantiate the decision). To illustrate, if PC-AKI occurs with an incidence of 10% in the patient population, a difference of 10% of relative risk would mean a difference of 1% in absolute risk. Thus, the number needed to treat would be 100, ergo: a doctor would need to treat 100 patients to prevent one case of PC-AKI. When the incidence of PC-AKI is 5%, a difference of 10% in relative risk would mean a difference of 0.5% in absolute risk, and a number needed to treat of 200.
Search and select (Methods)
The databases Medline (via OVID) and Embase (via Embase.com) were searched with relevant search terms from 2015 until 8-8-2024. The detailed search strategy is listed under the tab ‘Literature search strategy’. The systematic literature search resulted in 196 hits. Studies were selected based on the following criteria:
- The study population had to meet the criteria as defined in the PICO.
- The intervention had to meet the criteria as defined in the PICO.
- Research type: systematic review, randomized-controlled trials or observational studies comparing SGT2-inhibitors with no SGLT2-inhibitors.
- Articles written in English or Dutch.
Initially, 45 studies were selected based on title and abstract screening. After reading 21 studies in full text, seventeen studies were excluded (see the exclusion table under the tab ‘Evidence tabellen’), and four studies were included (Çabuk, 2024; Chen, 2024; Liu, 2023; Paolisso, 2023).
Referenties
- Çabuk G, Hazır KE. Do Sodium-Glucose Cotransporter 2 Inhibitors Decrease the Risk of Contrast-Associated Acute Kidney Injury in Patients with Type II Diabetes Mellitus? Anatol J Cardiol. 2024 Mar 20;28(5):222–8. doi: 10.14744/AnatolJCardiol.2024.3980. Epub ahead of print. PMID: 38506315; PMCID: PMC11059220.
- Cai D, Chen Q, Mao L, Xiao T, Wang Y, Gu Q, Wang Q, Ji Y, Sun L. Association of SGLT2 inhibitor dapagliflozin with risks of acute kidney injury and all-cause mortality in acute myocardial infarction patients. Eur J Clin Pharmacol. 2024 Apr;80(4):613-620. doi: 10.1007/s00228-024-03623-7. Epub 2024 Feb 6. PMID: 38319348; PMCID: PMC10937750.
- Chen CW, Su FY, Wang PP, Chuang MT, Lin YC, Kao CC, Huang CY. Renal outcomes after contrast exposure in patients with diabetes who use sodium-glucose cotransporter 2 inhibitors. Postgrad Med J. 2024 Feb 15;100(1181):142-150. doi: 10.1093/postmj/qgad118. PMID: 38055906.
- Heerspink HJL, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, Mann JFE, McMurray JJV, Lindberg M, Rossing P, Sjöström CD, Toto RD, Langkilde AM, Wheeler DC; DAPA-CKD Trial Committees and Investigators. Dapagliflozin in Patients with Chronic Kidney Disease. N Engl J Med. 2020 Oct 8;383(15):1436-1446. doi: 10.1056/NEJMoa2024816. Epub 2020 Sep 24. PMID: 32970396.
- James S, Erlinge D, Storey RF, McGuire DK, de Belder M, Eriksson N, Andersen K, Austin D, Arefalk G, Carrick D, Hofmann R, Hoole SP, Jones DA, Lee K, Tygesen H, Johansson PA, Langkilde AM, Ridderstråle W, Parvaresh Rizi E, Deanfield J, Oldgren J. Dapagliflozin in Myocardial Infarction without Diabetes or Heart Failure. NEJM Evid. 2024 Feb;3(2):EVIDoa2300286. doi: 10.1056/EVIDoa2300286. Epub 2023 Nov 11. PMID: 38320489.
- Karakasis P, Fragakis N, Kouskouras K, Karamitsos T, Patoulias D, Rizzo M. Sodium-Glucose Cotransporter-2 Inhibitors in Patients With Acute Coronary Syndrome: A Modern Cinderella? Clin Ther. 2024 Nov;46(11):841-850. doi: 10.1016/j.clinthera.2024.06.010. Epub 2024 Jul 10. PMID: 38991865.
- Liu T, Jian X, Li L, Chu S, Fan Z. The Association between Dapagliflozin Use and the Risk of Post-Contrast Acute Kidney Injury in Patients with Type 2 Diabetes and Chronic Kidney Disease: A Propensity-Matched Analysis. Kidney Blood Press Res. 2023;48(1):752-760. doi: 10.1159/000535208. Epub 2023 Nov 17. PMID: 37980899; PMCID: PMC10711763.
- Nardi G, Marchi E, Allinovi M, Lugli G, Biagiotti L, Di Muro FM, Valenti R, Muraca I, Tomberli B, Ciardetti N, Alterini B, Meucci F, Di Mario C, Mattesini A. Contrast-Induced Acute Kidney Injury in Patients with Heart Failure on Sodium-Glucose Cotransporter-2 Inhibitors Undergoing Radiocontrast Agent Invasive Procedures: A Propensity-Matched Analysis. J Clin Med. 2024 Apr 1;13(7):2041. doi: 10.3390/jcm13072041. PMID: 38610806; PMCID: PMC11012317.
- Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR; CANVAS Program Collaborative Group. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med. 2017 Aug 17;377(7):644-657. doi: 10.1056/NEJMoa1611925. Epub 2017 Jun 12. PMID: 28605608.
- Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, Jamal W, Kimura K, Schnee J, Zeller C, Cotton D, Bocchi E, Böhm M, Choi DJ, Chopra V, Chuquiure E, Giannetti N, Janssens S, Zhang J, Gonzalez Juanatey JR, Kaul S, Brunner-La Rocca HP, Merkely B, Nicholls SJ, Perrone S, Pina I, Ponikowski P, Sattar N, Senni M, Seronde MF, Spinar J, Squire I, Taddei S, Wanner C, Zannad F; EMPEROR-Reduced Trial Investigators. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N Engl J Med. 2020 Oct 8;383(15):1413-1424. doi: 10.1056/NEJMoa2022190. Epub 2020 Aug 28. PMID: 32865377.
- Paolisso P, Bergamaschi L, Cesaro A, Gallinoro E, Gragnano F, Sardu C, Mileva N, Foà A, Armillotta M, Sansonetti A, Amicone S, Impellizzeri A, Belmonte M, Esposito G, Morici N, Andrea Oreglia J, Casella G, Mauro C, Vassilev D, Galie N, Santulli G, Calabrò P, Barbato E, Marfella R, Pizzi C. Impact of SGLT2-inhibitors on contrast-induced acute kidney injury in diabetic patients with acute myocardial infarction with and without chronic kidney disease: Insight from SGLT2-I AMI PROTECT registry. Diabetes Res Clin Pract. 2023 Aug;202:110766. doi: 10.1016/j.diabres.2023.110766. Epub 2023 Jun 3. PMID: 37276980.
- Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, Cannon CP, Capuano G, Chu PL, de Zeeuw D, Greene T, Levin A, Pollock C, Wheeler DC, Yavin Y, Zhang H, Zinman B, Meininger G, Brenner BM, Mahaffey KW; CREDENCE Trial Investigators. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N Engl J Med. 2019 Jun 13;380(24):2295-2306. doi: 10.1056/NEJMoa1811744. Epub 2019 Apr 14. PMID: 30990260.
- von Lewinski D, Kolesnik E, Aziz F, Benedikt M, Tripolt NJ, Wallner M, Pferschy PN, von Lewinski F, Schwegel N, Holman RR, Oulhaj A, Moertl D, Siller-Matula J, Sourij H. Timing of SGLT2i initiation after acute myocardial infarction. Cardiovasc Diabetol. 2023 Sep 30;22(1):269. doi: 10.1186/s12933-023-02000-5. PMID: 37777743; PMCID: PMC10544140.
- Yang H, Yang L, Jardine MJ, Arnott C, Neuen BL, Xu K, Zhao X, Qian D, Cui B, Qiu Y, Huang Y, Yu J, Wang J, Yu S, Tan H, Huang L, Li JW, Jin J. The association between sodium-glucose cotransporter 2 inhibitors and contrast-associated acute kidney injury in patients with type 2 diabetes undergoing angiography: a propensity-matched study. Eur J Med Res. 2024 Dec 24;29(1):621. doi: 10.1186/s40001-024-02214-7. PMID: 39719658; PMCID: PMC11667978.
- Zang J, Liang J, Zhang X, Sang D, Duan X, Wang Z, Wei W, Wu G. Short term sodium glucose transport protein 2 inhibitors are associated with post contrast acute kidney injury in patients with diabetes. Sci Rep. 2024 Oct 2;14(1):22937. doi: 10.1038/s41598-024-74233-7. PMID: 39358407; PMCID: PMC11447200.
Evidence tabellen
Risk of bias table for interventions studies (cohort studies based on risk of bias tool by the CLARITY Group at McMaster University)
|
Author, year |
Selection of participants
Was selection of exposed and non-exposed cohorts drawn from the same population?
|
Exposure
Can we be confident in the assessment of exposure?
|
Outcome of interest
Can we be confident that the outcome of interest was not present at start of study?
|
Confounding-assessment
Can we be confident in the assessment of confounding factors? |
Confounding-analysis
Did the study match exposed and unexposed for all variables that are associated with the outcome of interest or did the statistical analysis adjust for these confounding variables? |
Assessment of outcome
Can we be confident in the assessment of outcome? |
Follow up
Was the follow up of cohorts adequate? In particular, was outcome data complete or imputed?
|
Co-interventions
Were co-interventions similar between groups?
|
Overall Risk of bias
|
|
Definitely yes, probably yes, probably no, definitely no |
Definitely yes, probably yes, probably no, definitely no |
Definitely yes, probably yes, probably no, definitely no |
Definitely yes, probably yes, probably no, definitely no |
Definitely yes, probably yes, probably no, definitely no |
Definitely yes, probably yes, probably no, definitely no |
Definitely yes, probably yes, probably no, definitely no |
Definitely yes, probably yes, probably no, definitely no |
Low, Some concerns, High |
|
|
Çabuk, 2024 |
Definitely yes
Reason: Participants were selected from same hospital. |
Probably yes
Reason: Derived from electronic medical records.
|
Probably yes
Reason: Outcome related to intervention.
|
Probably yes
Reason: Derived from medical records. |
Probably yes
Reason: Adjustment for confounding variables was performed. |
Probably yes
Reason: The outcome of interest was predefined. |
Probably yes
Reason: Follow-up was adequate and loss to follow-up was silimar in both groups. No missing data.
|
No information
No information presented for the subgroup of patients with eGFR <60 mL/min/1.73m2 |
Low |
|
Chen, 2024 |
Definitely yes
Reason: Participants were selected from a database.
|
Probably yes
Reason: Derived from database.
|
Probably yes
Reason: Outcome related to intervention.
|
Probably yes
Reason: Confounding factors were retrieved from database. |
Probably yes
Reason: Overlap weighting was performed to adjust for the effect of confounders.
|
Probably yes
Reason: The outcome of interest was predefined. |
Probably yes
Reason: Follow-up was adequate |
No information
No information presented for the subgroup of patients with high CKD risk |
Low |
|
Liu, 2023 |
Definitely yes
Reason: Participants were selected from same hospital.
|
Probably yes
Reason: Derived electronic medical records.
|
Probably yes
Reason: Outcome related to intervention.
|
Probably yes
Reason: Confounding factors were retrieved from electronic medical records. |
Probably yes
Reason: Propensity score-matching analysis was conducted. |
Probably yes
Reason: The outcome of interest was predefined. |
Probably yes
Reason: Follow-up was adequate |
No information
No information presented for the subgroup of patients with CKD stage G3a |
Low |
|
Paolisso, 2023 |
Definitely yes
Reason: Participants were selected from a registry.
|
Probably yes
Reason: Derived from registry.
|
Probably yes
Reason: Outcome related to intervention.
|
Probably yes
Reason: Participants characteristics were derived from registry.
|
Probably yes
Reason: Multivariate analysis adjusted for confounding factors.
|
Probably yes
Reason: The highest serum creatinine level within 72 hours after PCI was used to diagnose CI-AKI.
|
Probably yes
Reason: Follow-up was adequate.
|
Probably no
Reason: Glucose-lowering agents at admission and in-hospital glucose-lowering strategy were different between groups.
|
Low
|
Table of excluded studies
|
Reference |
Reason for exclusion |
|
Basutkar RS, Cutinha RM, Sathish V, Shahil A, Saneen Ck N. Impact of SGLT2 Inhibitors on Renal Function in Type 2 Diabetic Patients with Coronary Artery Disease Undergoing Percutaneous Intervention: A Systematic Review and Meta-Analysis. Curr Diabetes Rev. 2024 Jul 3. doi: 10.2174/0115733998301228240625065230. Epub ahead of print. PMID: 38963097. |
Wrong population: with or without chronic kidney disease. |
|
Cai D, Chen Q, Mao L, Xiao T, Wang Y, Gu Q, Wang Q, Ji Y, Sun L. Association of SGLT2 inhibitor dapagliflozin with risks of acute kidney injury and all-cause mortality in acute myocardial infarction patients. Eur J Clin Pharmacol. 2024 Apr;80(4):613-620. doi: 10.1007/s00228-024-03623-7. Epub 2024 Feb 6. PMID: 38319348; PMCID: PMC10937750. |
Wrong population: no subgroup based on eGFR<60 ml/min/1.73m2 |
|
Chang TY, Lu CT, Huang HL, Chou RH, Chang CC, Liu CT, Huang PH, Lin SJ. Association of Sodium-Glucose Cotransporter 2 (SGLT2) Inhibitor Use With Cardiovascular and Renal Outcomes in Type 2 Diabetes Mellitus Patients With Stabilized Acute Myocardial Infarction: A Propensity Score Matching Study. Front Cardiovasc Med. 2022 Apr 29;9:882181. doi: 10.3389/fcvm.2022.882181. PMID: 35571176; PMCID: PMC9098830. |
Wrong population: no subgroup based on eGFR<60 ml/min/1.73m2 |
|
Feitosa MPM, Lima EG, Abizaid AAC, Mehran R, Lopes NHM, de Assis Fischer Ramos T, Hideo-Kajita A, Filho RK, Junior CVS. The safety of SGLT-2 inhibitors in diabetic patients submitted to elective percutaneous coronary intervention regarding kidney function: SAFE-PCI pilot study. Diabetol Metab Syndr. 2023 Jun 26;15(1):138. doi: 10.1186/s13098-023-01107-9. PMID: 37365618; PMCID: PMC10291785. |
Wrong population: no subgroup based on eGFR<60 ml/min/1.73m2 |
|
Hitchen SA, Lan NSR, Rankin JM, Larbalestier R, Yeap BB, Fegan PG. Real-world barriers and safety of initiating sodium-glucose co-transporter 2 inhibitor treatment immediately following an acute cardiac event in people with diabetes. J Diabetes Complications. 2021 Dec;35(12):108057. doi: 10.1016/j.jdiacomp.2021.108057. Epub 2021 Sep 29. PMID: 34610888. |
No comparison: barriers to initiating SGLT2 inhibitors |
|
Hua R, Ding N, Guo H, Wu Y, Yuan Z, Li T. Contrast-Induced Acute Kidney Injury in Patients on SGLT2 Inhibitors Undergoing Percutaneous Coronary Interventions: A Propensity-Matched Analysis. Front Cardiovasc Med. 2022 Jun 20;9:918167. doi: 10.3389/fcvm.2022.918167. PMID: 35795364; PMCID: PMC9251334. |
Wrong population: no subgroup based on eGFR<60 ml/min/1.73m2 |
|
Jang J, Park S, Kim S, Kim SH, Oh YS, Sa YK, Hwang Y, Jang SW, Ihm SH, Choi Y. Clinical outcomes with the use of sodium-glucose cotransporter-2 inhibitors in patients with atrial fibrillation and type 2 diabetes mellitus: a multi-centre, real-world cohort study. Eur J Prev Cardiol. 2024 Feb 15;31(3):320-329. doi: 10.1093/eurjpc/zwad322. PMID: 37798123. |
Unclear how chronic kidney disease was defined (no data about eGFR) |
|
Jhund PS, Solomon SD, Docherty KF, Heerspink HJL, Anand IS, Böhm M, Chopra V, De Boer RA, Desai AS, Ge J, Kitakaze M, Merkley B, O'Meara E, Shou M, Tereshchenko S, Verma S, Vinh PN, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Bengtsson O, Langkilde AM, Sjöstrand M, McMurray JJV. Efficacy of Dapagliflozin on Renal Function and Outcomes in Patients with Heart Failure with Reduced Ejection Fraction: Results of DAPA-HF. Circulation. 2021; 143 (4) :298-309 |
Wrong population: did not receive contrast media |
|
Karakasis P, Fragakis N, Kouskouras K, Karamitsos T, Patoulias D, Rizzo M. Sodium-Glucose Cotransporter-2 Inhibitors in Patients With Acute Coronary Syndrome: A Modern Cinderella? Clin Ther. 2024 Jul 10:S0149-2918(24)00149-8. doi: 10.1016/j.clinthera.2024.06.010. Epub ahead of print. PMID: 38991865. |
No systematic review |
|
Kültürsay B, Yılmaz C, Güven B, Mutlu D, Karagöz A. Potential renoprotective effect of SGLT2 inhibitors against contrast-induced AKI in diabetic STEMI patients undergoing primary PCI. Kardiol Pol. 2024;82(1):29-36. doi: 10.33963/v.kp.98260. Epub 2024 Jan 17. PMID: 38230461. |
Wrong population: no subgroup based on eGFR<60 ml/min/1.73m2 |
|
Liu X, Wang W, Xing X. Effect of SGLT2 Inhibitors on Post-PCI Outcomes after Acute Myocardial Infarction in Diabetic Patients: A Systematic Review and Meta-Analysis. Heart Surgery Forum. 2024; 27 (4) :E414-E423 |
Review of poor quality |
|
McMurray JJV, Wheeler DC, Stefánsson BV, Jongs N, Postmus D, Correa-Rotter R, Chertow GM, Greene T, Held C, Hou FF, Mann JFE, Rossing P, Sjöström CD, Toto RD, Langkilde AM, Heerspink HJL. Effect of Dapagliflozin on Clinical Outcomes in Patients With Chronic Kidney Disease, With and Without Cardiovascular Disease. Circulation. 2021; 143 (5) :438-448 |
Wrong population: eGFR between 25 and 75 mL·min–1·1.73 m–2, and |
|
Meregildo-Rodriguez ED, Asmat-Rubio MG, Vásquez-Tirado GA. SGLT-2 inhibitors and prevention of contrast-induced nephropathy in patients with diabetes undergoing coronary angiography and percutaneous coronary interventions: systematic review and meta-analysis. Front Endocrinol (Lausanne). 2023 Dec 20;14:1307715. doi: 10.3389/fendo.2023.1307715. PMID: 38179307; PMCID: PMC10765513. |
Unclear about reduced kidney function |
|
Nardi G, Marchi E, Allinovi M, Lugli G, Biagiotti L, Di Muro FM, Valenti R, Muraca I, Tomberli B, Ciardetti N, Alterini B, Meucci F, Di Mario C, Mattesini A. Contrast-Induced Acute Kidney Injury in Patients with Heart Failure on Sodium-Glucose Cotransporter-2 Inhibitors Undergoing Radiocontrast Agent Invasive Procedures: A Propensity-Matched Analysis. J Clin Med. 2024 Apr 1;13(7):2041. doi: 10.3390/jcm13072041. PMID: 38610806; PMCID: PMC11012317. |
Wrong population: no subgroup based on eGFR<60 ml/min/1.73m2 |
|
Paolisso P, Bergamaschi L, Gragnano F, Gallinoro E, Cesaro A, Sardu C, Mileva N, Foà A, Armillotta M, Sansonetti A, Amicone S, Impellizzeri A, Esposito G, Morici N, Andrea OJ, Casella G, Mauro C, Vassilev D, Galie N, Santulli G, Marfella R, Calabrò P, Pizzi C, Barbato E. Outcomes in diabetic patients treated with SGLT2-Inhibitors with acute myocardial infarction undergoing PCI: The SGLT2-I AMI PROTECT Registry. Pharmacol Res. 2023 Jan;187:106597. doi: 10.1016/j.phrs.2022.106597. Epub 2022 Dec 5. PMID: 36470546; PMCID: PMC9946774. |
Wrong population: no subgroup based on eGFR<60 ml/min/1.73m2 |
|
Schechter M, Melzer-Cohen C, Rozenberg A, Yanuv I, Chodick G, Karasik A, Kosiborod M, Mosenzon O. Cardiorenal outcomes with sodium/glucose cotransporter-2 inhibitors in patients with type 2 diabetes and low kidney risk: real world evidence. Cardiovasc Diabetol. 2021 Aug 18;20(1):169. doi: 10.1186/s12933-021-01362-y. PMID: 34407822; PMCID: PMC8375057. |
Wrong comparison: other glucose lowering agent |
|
Tsukamoto S, Kobayashi K, Toyoda M, Tone A, Kawanami D, Suzuki D, Tsuriya D, Machimura H, Shimura H, Wakui H, Takeda H, Yokomizo H, Takeshita K, Chin K, Kanasaki K, Miyauchi M, Saburi M, Morita M, Yomota M, Kimura M, Hatori N, Nakajima S, Ito S, Murata T, Matsushita T, Furuki T, Hashimoto T, Umezono T, Muta Y, Takashi Y, Tamura K. Effect of preceding drug therapy on the renal and cardiovascular outcomes of combined sodium-glucose cotransporter-2 inhibitor and glucagon-like peptide-1 receptor agonist treatment in patients with type 2 diabetes and chronic kidney disease. Diabetes, Obesity and Metabolism. 2024; 26 (8) :3248-3260 |
Wrong study aim: to examine whether the composite renal outcome differed between those who received SGLT2 inhibitor treatment first and those who received a GLP-1RA first |
Verantwoording
Beoordelingsdatum en geldigheid
Publicatiedatum : 09-12-2025
Beoordeeld op geldigheid : 09-12-2025
Samenstelling werkgroep
Voor het ontwikkelen van de richtlijnmodule is in 2023 een multidisciplinair cluster ingesteld. Het cluster Beeldvormende diagnostiek bestaat uit meerdere richtlijnen, zie hier voor de actuele clusterindeling. De stuurgroep bewaakt het proces van modulair onderhoud binnen het cluster. De expertisegroepsleden geven hun expertise in, indien nodig. De volgende personen uit het cluster zijn betrokken geweest bij de herziening van deze module:
Clusterstuurgroep
- Drs. N. (Nanko) de Graaf, voorzitter, radioloog, NVvR (tot 17-02-2025)
- Dr. S. (Stef) Levolger, (interventie)radioloog, NVvR (voorzitter vanaf 10-03-2025)
- Dr. P.M. (Marc) van der Zee, cardioloog, NVvC
- Dr. J.W. (Jan Willem) Hinnen, vaatchirurg, NVvH
- Dr. Ir. M. (Marcel) van Straten, klinisch fysicus, NVKF
Clusterexpertisegroep
- Dr. H.W. (Henk) van Hamersvelt, internist-nefroloog, NIV
- Dr. N. (Neeltje) Coolen, patiëntvertegenwoordiger, Nierpatiënten Vereniging Nederland
Met ondersteuning van
- Drs. D.A.M. (Danique) Middelhuis, adviseur, Kennisinstituut van de Federatie Medisch Specialisten
- Drs. A.L.J. (Andrea) Kortlever - van der Spek, adviseur, Kennisinstituut van de Federatie Medisch Specialisten (tot 01-07-2024)
- Dr. L. (Lotte) Houtepen, adviseur, Kennisinstituut van de Federatie Medisch Specialisten (vanaf 01-07-2024)
Belangenverklaringen
Een overzicht van de belangen van de clusterleden en het oordeel over het omgaan met eventuele belangen vindt u in onderstaande tabel. De ondertekende belangenverklaringen zijn op te vragen bij het secretariaat van het Kennisinstituut van de Federatie Medisch Specialisten via secretariaat@kennisinstituut.nl.
Clusterstuurgroepleden
|
Naam |
Hoofdfunctie |
Nevenwerkzaamheden |
Persoonlijke financiële belangen |
Persoonlijke relaties |
Extern gefinancierd onderzoek |
Intellectuele belangen en reputatie |
Overige belangen |
Datum |
Restrictie |
|
Nanko de Graaf* |
Academisch Medisch Specialist / Radioloog (betaald) Erasmus MC Rotterdam |
Bestuurslid sectie Techniek, NVvR (onbetaald) Bestuurslid NCS (Ned. Comm. Stralingsdosimetrie (onbetaald) Voorzitter richtlijn Veilig gebruik van intraveneuze contrastmiddelen bij kinderen (vacatiegelden) Adviescommissie Pocus (point of care ultrasound van de NVvR bij de NVK (onbetaald) |
Geen |
Geen |
Geen |
Geen |
Geen |
23-1-2023 |
Geen restrictie |
|
Stef Levolger* |
(Interventie)radioloog. Vrij gevestigd medisch specialist. Lid vakgroep Radiologie Maasstad Ziekenhuis/ MSB Maasstad Ziekenhuis. |
Arts-commissielid MEC-U (lokale METC). Vacatiegelden. |
Geen persoonlijke financiële belangen. |
Geen financieel baat bij de partner. Geen financieel baat in verdere directe omgeving voor zover bekend. |
- Medeaanvrager Zorginstuut Nederland (ZiN) Veelbelovende zorg subsidieregeling: ‘ThrOmbectomy in high-Risk Pulmonary Embolism – Device versus thrOmbolysis NetherLands’ (TORPEDO-NL) - Lokaal PI Short MRI Surveillance (SMS) studie. KWF gefinancierd. - ZonMw DoelmatigheidsOnderzoek Voorbereidende studies: ‘What are the direct and indirect costs for PRG and PEG in The Netherlands’ * ZiN/ZonMw - Trombectomie versus IV trombolyse. Hoog risico longembolie patiënten - Geen projectleider * ZonMw - Pilotstudie. Microcosting. PRG versus PEG gastrostomie - Geen projectleider * KWF - Leverkankerscreening. Echografie versus MRI. Surveillance - Geen projectleider |
Geen |
Geen |
27-8-2024 |
Geen restrictie |
|
Marc van der Zee |
Cardioloog St Jansdal |
Coördinator onderzoek binnen St Jansdal ziekenhuis incl. aansturing onderzoeksverpleegkundigen |
Geen |
Geen |
Geen |
Geen |
Geen |
22-11-2022 |
Geen restrictie |
|
Jan-willem Hinnen |
Vaatchirurg Jeroen Bosch Ziekenhuis, ‘s Hertogenbosch |
Bestuur Nederlandse Vereniging voor Vaatchirurige Betrokken bij onderzoeken welke niet gerelateerd zijn aan het cluster |
Geen |
Geen |
Geen |
Geen |
Geen |
19-02-2023 |
Geen restrictie |
|
Marcel van Straten |
Klinisch Fysicus Erasmus MC |
Lid Commissie Straling NVKF |
n.v.t. |
n.v.t. |
Marcel is principal investigator van de lijn Physics in CT Technology. In samenwerking met Siemens wordt de nieuwe acquisitie- (het maken van scans) en postprocessingtechnieken (het verwerken van informatie uit scans) op met name de photon-counting scanner geëvalueerd. Verschillende onderwepen uit het cluster zijn gerelateerd aan het onderzoek. Echter, worden er binnen het cluster geen aanbevelingen opgesteld die herleidbaar zijn tot fabrikanten, zoals Siemans. Tevens begeleid hij PhD-studenten die een thesis schrijven over dit onderwerp. |
n.v.t. |
n.v.t. |
9-1-2024 |
Geen restrictie |
Betrokken clusterexpertisegroepleden
|
Naam |
Hoofdfunctie |
Nevenwerkzaamheden |
Persoonlijke financiële belangen |
Persoonlijke relaties |
Extern gefinancierd onderzoek |
Intellectuele belangen en reputatie |
Overige belangen |
Datum |
Restrictie |
|
Henk van Hamersvelt |
Gepensioneerd internist-nefroloog met nul-uren aanstelling bij afdeling Nierziekten van Radboudumc te Nijmegen |
Onbezoldigd voorzitter richtlijnencommissie van de Nederlandse Federatie voor Nefrologie |
Geen |
Geen |
Geen |
Geen |
Geen |
22-11-2022 |
Geen restrictie |
|
Neeltje Coolen |
Coördinator Patiëntenparticipatie Kwaliteit van Zorg, Nierpatiënten Vereniging Nederland |
Geen |
Geen |
Geen |
AstraZeneca: Psychosocial impact of COVID-19 on immunocompromised individuals - a cross sectional survey in the Netherlands
Characteristics of immunocompromised individuals admitted to the ICU due to the Omicron corona variant - a descriptive study in the Netherlands |
Geen |
Geen |
30-11-2023 |
Geen restrictie |
Inbreng patiëntenperspectief
Kwalitatieve raming van mogelijke financiële gevolgen in het kader van de Wkkgz
Bij de richtlijnmodule voerden de clusterleden conform de Wet kwaliteit, klachten en geschillen zorg (Wkkgz) een kwalitatieve raming uit om te beoordelen of de aanbevelingen mogelijk leiden tot substantiële financiële gevolgen. Bij het uitvoeren van deze beoordeling is de richtlijnmodule op verschillende domeinen getoetst (zie het stroomschema bij Werkwijze).
|
Module |
Uitkomst raming |
Toelichting |
|
SGLT-2 remmers |
Geen financiële gevolgen |
Hoewel uit de toetsing volgt dat de aanbeveling(en) breed toepasbaar zijn (5.000-40.000 patiënten), volgt ook uit de toetsing dat het overgrote deel (±90%) van de zorgaanbieders en zorgverleners al aan de norm voldoet of het geen nieuwe manier van zorgverlening of andere organisatie van zorgverlening betreft. Er worden daarom geen financiële gevolgen verwacht. |
Werkwijze
Voor meer details over de gebruikte richtlijnmethodologie verwijzen wij u naar de Werkwijze. Relevante informatie voor de ontwikkeling/herziening van deze richtlijnmodule is hieronder weergegeven.
Zoekverantwoording
Algemene informatie
|
Cluster/richtlijn: Beeldvormende diagnostiek - Veilig gebruik van contrastmiddelen - Module SGLT2 remmers |
|
|
Uitgangsvraag/modules: Wat is de waarde van SGLT-2 remmers bij patiënten met een verminderde nierfunctie die intravasculair contrastmiddel (CM) krijgen toegediend? |
|
|
Database(s): Embase.com, Ovid/Medline |
Datum: 8 augustus 2024 |
|
Periode: vanaf 2015 |
Talen: geen restrictie |
|
Literatuurspecialist: Esther van der Bijl |
Rayyan review: https://rayyan.ai/reviews/1117417 |
|
BMI-zoekblokken: voor verschillende opdrachten wordt (deels) gebruik gemaakt van de zoekblokken van BMI-Online https://blocks.bmi-online.nl/ Deduplication: voor het ontdubbelen is gebruik gemaakt van http://dedupendnote.nl/ |
|
|
Toelichting: Voor deze vraag is gezocht op de elementen ((kidney failure AND iodinated contrast medium) OR contrast induced nephropathy) AND SGLT2.
De sleutelartikelen worden gevonden met deze search. |
|
|
Te gebruiken voor richtlijntekst: In de databases Embase.com en Ovid/Medline is op 8 augustus 2024 systematisch gezocht naar systematische reviews, RCTs en observationele studies over de waarde van SGLT-2 remmers bij patiënten met een verminderde nierfunctie die intravasculair contrastmiddel krijgen toegediend. De literatuurzoekactie leverde 196 unieke treffers op. |
|
Zoekopbrengst
8-8-2024
|
|
EMBASE |
OVID/MEDLINE |
Ontdubbeld |
|
SR |
28 |
3 |
28 |
|
RCT |
82 |
11 |
87 |
|
Observationele studies |
81 |
11 |
81 |
|
Totaal |
191 |
25 |
196* |
*in Rayyan
Zoekstrategie
Embase.com - 8 augustus 2024
|
No. |
Query |
Results |
|
#1 |
'kidney failure'/exp OR 'chronic kidney failure'/exp OR 'estimated glomerular filtration rate'/exp OR 'kidney disease'/de OR 'kidney function'/de OR (((kidney* OR renal*) NEAR/3 (disease* OR disorder* OR failure* OR insufficienc* OR injur*)):ti,ab,kw) OR 'nephropath*':ti,ab,kw OR egfr:ti,ab,kw OR gfr:ti,ab,kw OR 'estimated gfr':ti,ab,kw OR 'glomerular filtration rat*':ti,ab,kw OR 'glomerulofiltration rat*':ti,ab,kw OR 'glomerulus filtration rat*':ti,ab,kw OR ckd:ti,ab,kw OR 'kidney function':ti,ab,kw OR nephrotoxicit*:ti,ab,kw OR 'aki':ti,ab,kw |
1179169 |
|
#2 |
'contrast medium'/exp OR 'iodinated contrast medium'/exp OR 'percutaneous coronary intervention'/exp OR 'arteriography'/exp OR 'ioversol'/exp OR 'iobitridol'/exp OR (((contrast OR radiocontrast) NEAR/3 (agent* OR material* OR media OR medium OR iodinated OR iodine OR iodized OR administrat* OR dose OR doses OR dosage OR enhanced OR exposure)):ti,ab,kw) OR 'percutaneous coronary intervention':ti,ab,kw OR 'contrast-induc*':ti,ab,kw OR 'radiocontrast-induc*':ti,ab,kw OR 'arter* angiograph*':ti,ab,kw OR 'arteriogram*':ti,ab,kw OR 'arteriograph*':ti,ab,kw OR hexabrix:ti,ab,kw OR iomeron:ti,ab,kw OR iopamiro:ti,ab,kw OR omnipaque:ti,ab,kw OR optiray:ti,ab,kw OR ultravist:ti,ab,kw OR xenetix:ti,ab,kw OR iodixanol:ti,ab,kw OR ioxaglate:ti,ab,kw OR iomeprol:ti,ab,kw OR iopamidol:ti,ab,kw OR iosimenol:ti,ab,kw OR iohexol:ti,ab,kw OR ioversol:ti,ab,kw OR iopromide:ti,ab,kw OR iobitridol:ti,ab,kw |
567884 |
|
#3 |
#1 AND #2 |
46000 |
|
#4 |
'contrast induced nephropathy'/exp OR ((('rc-induc*' OR 'contrast induc*' OR 'radiocontrast induc*') NEAR/4 (nephropath* OR nephrotoxicit* OR renal OR kidney*)):ti,ab,kw) OR 'ci-aki':ti,ab,kw |
8636 |
|
#5 |
#3 OR #4 |
46731 |
|
#6 |
'sodium glucose cotransporter 2 inhibitor'/exp OR sglt2:ti,ab,kw OR 'sglt 2':ti,ab,kw OR 'gliflozin*':ti,ab,kw OR 'sodium dependent glucose cotransporter 2':ti,ab,kw OR 'sodium glucose co-transporter 2':ti,ab,kw OR 'sodium glucose cotransporter 2':ti,ab,kw OR 'sodium-glucose transporter 2':ti,ab,kw OR 'sodium dependent glucose cotransporter2':ti,ab,kw OR 'sodium glucose co-transporter2':ti,ab,kw OR 'sodium glucose cotransporter2':ti,ab,kw OR 'sodium-glucose transporter2':ti,ab,kw OR 'canagliflozin'/exp OR 'canagliflocin':ti,ab,kw OR 'canagliflozin':ti,ab,kw OR 'canaglu':ti,ab,kw OR 'invokana':ti,ab,kw OR 'jnj 28431754':ti,ab,kw OR 'jnj28431754':ti,ab,kw OR 'sulisent':ti,ab,kw OR 'ta 7284':ti,ab,kw OR 'ta7284':ti,ab,kw OR 'dapagliflozin'/exp OR 'andatang':ti,ab,kw OR 'bms 512148':ti,ab,kw OR 'bms512148':ti,ab,kw OR 'ckd 380':ti,ab,kw OR 'ckd380':ti,ab,kw OR 'dapagliflozin':ti,ab,kw OR 'dwp 16001':ti,ab,kw OR 'dwp16001':ti,ab,kw OR 'edistride':ti,ab,kw OR 'farxiga':ti,ab,kw OR 'forxiga':ti,ab,kw OR 'hgp 1602':ti,ab,kw OR 'hgp 1812':ti,ab,kw OR 'hgp1602':ti,ab,kw OR 'hgp1812':ti,ab,kw OR 'lyn 045':ti,ab,kw OR 'lyn045':ti,ab,kw OR 'oxra':ti,ab,kw OR 'empagliflozin'/exp OR 'bi 10773':ti,ab,kw OR 'bi10773':ti,ab,kw OR 'ckd 398':ti,ab,kw OR 'ckd398':ti,ab,kw OR 'empagliflozin':ti,ab,kw OR 'gibtulio':ti,ab,kw OR 'jardiance':ti,ab,kw OR 'oboravo':ti,ab,kw OR 'ertugliflozin'/exp OR 'ertugliflozin':ti,ab,kw OR 'mk 8835':ti,ab,kw OR 'mk8835':ti,ab,kw OR 'pf 04971729':ti,ab,kw OR 'pf 04971729 00':ti,ab,kw OR 'pf 04971729-00':ti,ab,kw OR 'pf 4971729':ti,ab,kw OR 'pf 4971729 00':ti,ab,kw OR 'pf 4971729-00':ti,ab,kw OR 'pf04971729':ti,ab,kw OR 'pf04971729 00':ti,ab,kw OR 'pf04971729-00':ti,ab,kw OR 'pf4971729':ti,ab,kw OR 'pf4971729 00':ti,ab,kw OR 'pf4971729-00':ti,ab,kw OR 'steglatro':ti,ab,kw |
34147 |
|
#7 |
#5 AND #6 |
375 |
|
#8 |
#7 AND [2015-2024]/py NOT ('conference abstract'/it OR 'editorial'/it OR 'letter'/it OR 'note'/it) NOT (('animal'/exp OR 'animal experiment'/exp OR 'animal model'/exp OR 'nonhuman'/exp) NOT 'human'/exp) |
292 |
|
#9 |
'meta analysis'/exp OR 'meta analysis (topic)'/exp OR metaanaly*:ti,ab OR 'meta analy*':ti,ab OR metanaly*:ti,ab OR 'systematic review'/de OR 'cochrane database of systematic reviews'/jt OR prisma:ti,ab OR prospero:ti,ab OR (((systemati* OR scoping OR umbrella OR 'structured literature') NEAR/3 (review* OR overview*)):ti,ab) OR ((systemic* NEAR/1 review*):ti,ab) OR (((systemati* OR literature OR database* OR 'data base*') NEAR/10 search*):ti,ab) OR (((structured OR comprehensive* OR systemic*) NEAR/3 search*):ti,ab) OR (((literature NEAR/3 review*):ti,ab) AND (search*:ti,ab OR database*:ti,ab OR 'data base*':ti,ab)) OR (('data extraction':ti,ab OR 'data source*':ti,ab) AND 'study selection':ti,ab) OR ('search strategy':ti,ab AND 'selection criteria':ti,ab) OR ('data source*':ti,ab AND 'data synthesis':ti,ab) OR medline:ab OR pubmed:ab OR embase:ab OR cochrane:ab OR (((critical OR rapid) NEAR/2 (review* OR overview* OR synthes*)):ti) OR ((((critical* OR rapid*) NEAR/3 (review* OR overview* OR synthes*)):ab) AND (search*:ab OR database*:ab OR 'data base*':ab)) OR metasynthes*:ti,ab OR 'meta synthes*':ti,ab |
1051754 |
|
#10 |
'clinical trial'/exp OR 'randomization'/exp OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp OR 'placebo'/exp OR 'prospective study'/exp OR rct:ab,ti OR random*:ab,ti OR 'single blind':ab,ti OR 'randomised controlled trial':ab,ti OR 'randomized controlled trial'/exp OR placebo*:ab,ti |
4083147 |
|
#11 |
'major clinical study'/de OR 'clinical study'/de OR 'case control study'/de OR 'family study'/de OR 'longitudinal study'/de OR 'retrospective study'/de OR 'prospective study'/de OR 'comparative study'/de OR 'cohort analysis'/de OR ((cohort NEAR/1 (study OR studies)):ab,ti) OR (('case control' NEAR/1 (study OR studies)):ab,ti) OR (('follow up' NEAR/1 (study OR studies)):ab,ti) OR (observational NEAR/1 (study OR studies)) OR ((epidemiologic NEAR/1 (study OR studies)):ab,ti) OR (('cross sectional' NEAR/1 (study OR studies)):ab,ti) |
8351280 |
|
#12 |
'case control study'/de OR 'comparative study'/exp OR 'control group'/de OR 'controlled study'/de OR 'controlled clinical trial'/de OR 'crossover procedure'/de OR 'double blind procedure'/de OR 'phase 2 clinical trial'/de OR 'phase 3 clinical trial'/de OR 'phase 4 clinical trial'/de OR 'pretest posttest design'/de OR 'pretest posttest control group design'/de OR 'quasi experimental study'/de OR 'single blind procedure'/de OR 'triple blind procedure'/de OR (((control OR controlled) NEAR/6 trial):ti,ab,kw) OR (((control OR controlled) NEAR/6 (study OR studies)):ti,ab,kw) OR (((control OR controlled) NEAR/1 active):ti,ab,kw) OR 'open label*':ti,ab,kw OR (((double OR two OR three OR multi OR trial) NEAR/1 (arm OR arms)):ti,ab,kw) OR ((allocat* NEAR/10 (arm OR arms)):ti,ab,kw) OR placebo*:ti,ab,kw OR 'sham-control*':ti,ab,kw OR (((single OR double OR triple OR assessor) NEAR/1 (blind* OR masked)):ti,ab,kw) OR nonrandom*:ti,ab,kw OR 'non-random*':ti,ab,kw OR 'quasi-experiment*':ti,ab,kw OR crossover:ti,ab,kw OR 'cross over':ti,ab,kw OR 'parallel group*':ti,ab,kw OR 'factorial trial':ti,ab,kw OR ((phase NEAR/5 (study OR trial)):ti,ab,kw) OR ((case* NEAR/6 (matched OR control*)):ti,ab,kw) OR ((match* NEAR/6 (pair OR pairs OR cohort* OR control* OR group* OR healthy OR age OR sex OR gender OR patient* OR subject* OR participant*)):ti,ab,kw) OR ((propensity NEAR/6 (scor* OR match*)):ti,ab,kw) OR versus:ti OR vs:ti OR compar*:ti OR ((compar* NEAR/1 study):ti,ab,kw) OR (('major clinical study'/de OR 'clinical study'/de OR 'cohort analysis'/de OR 'observational study'/de OR 'cross-sectional study'/de OR 'multicenter study'/de OR 'correlational study'/de OR 'follow up'/de OR cohort*:ti,ab,kw OR 'follow up':ti,ab,kw OR followup:ti,ab,kw OR longitudinal*:ti,ab,kw OR prospective*:ti,ab,kw OR retrospective*:ti,ab,kw OR observational*:ti,ab,kw OR 'cross sectional*':ti,ab,kw OR cross?ectional*:ti,ab,kw OR multicent*:ti,ab,kw OR 'multi-cent*':ti,ab,kw OR consecutive*:ti,ab,kw) AND (group:ti,ab,kw OR groups:ti,ab,kw OR subgroup*:ti,ab,kw OR versus:ti,ab,kw OR vs:ti,ab,kw OR compar*:ti,ab,kw OR 'odds ratio*':ab OR 'relative odds':ab OR 'risk ratio*':ab OR 'relative risk*':ab OR 'rate ratio':ab OR aor:ab OR arr:ab OR rrr:ab OR ((('or' OR 'rr') NEAR/6 ci):ab))) |
15298363 |
|
#13 |
#8 AND #9 – SR’s |
28 |
|
#14 |
#8 AND #10 NOT #13 – RCT’s |
82 |
|
#15 |
#8 AND (#11 OR #12) NOT (#13 OR #14) – Observationele studies |
81 |
|
#16 |
#13 OR #14 OR #15 |
191 |
Ovid/Medline - 8 augustus 2024
|
# |
Searches |
Results |
|
1 |
exp Renal Insufficiency/ or exp Kidney Failure, Chronic/ or exp Glomerular Filtration Rate/ or *Kidney Diseases/ or ((kidney* or renal*) adj3 (disease* or disorder* or failure* or insufficienc* or injur*)).ti,ab,kf. or nephropath*.ti,ab,kf. or egfr.ti,ab,kf. or gfr.ti,ab,kf. or estimated gfr.ti,ab,kf. or glomerular filtration rat*.ti,ab,kf. or glomerulofiltration rat*.ti,ab,kf. or glomerulus filtration rat*.ti,ab,kf. or ckd.ti,ab,kf. or kidney function.ti,ab,kf. or nephrotoxicit*.ti,ab,kf. or aki.ti,ab,kf. |
626742 |
|
2 |
exp Contrast Media/ or exp Percutaneous Coronary Intervention/ or exp Angiography/ or ((contrast or radiocontrast) adj3 (agent* or material* or media or medium or iodinated or iodine or iodized or administrat* or dose or doses or dosage or enhanced or exposure)).ti,ab,kf. or percutaneous coronary intervention.ti,ab,kf. or contrast-induc*.ti,ab,kf. or radiocontrast-induc*.ti,ab,kf. or arter* angiograph*.ti,ab,kf. or arteriogram*.ti,ab,kf. or arteriograph*.ti,ab,kf. or hexabrix.ti,ab,kf. or iomeron.ti,ab,kf. or iopamiro.ti,ab,kf. or omnipaque.ti,ab,kf. or optiray.ti,ab,kf. or ultravist.ti,ab,kf. or xenetix.ti,ab,kf. or iodixanol.ti,ab,kf. or ioxaglate.ti,ab,kf. or iomeprol.ti,ab,kf. or iopamidol.ti,ab,kf. or iosimenol.ti,ab,kf. or iohexol.ti,ab,kf. or ioversol.ti,ab,kf. or iopromide.ti,ab,kf. or iobitridol.ti,ab,kf. |
535473 |
|
3 |
1 and 2 |
21770 |
|
4 |
(((rc-induc* or contrast induc* or radiocontrast induc*) adj4 (nephropath* or nephrotoxicit* or renal or kidney*)) or ci-aki).ti,ab,kf. |
3838 |
|
5 |
3 or 4 |
21795 |
|
6 |
exp Sodium-Glucose Transporter 2 Inhibitors/ or exp Canagliflozin/ or sglt2.ti,ab,kf. or sglt 2.ti,ab,kf. or gliflozin*.ti,ab,kf. or sodium dependent glucose cotransporter 2.ti,ab,kf. or sodium glucose co-transporter 2.ti,ab,kf. or sodium glucose cotransporter 2.ti,ab,kf. or sodium-glucose transporter 2.ti,ab,kf. or sodium dependent glucose cotransporter2.ti,ab,kf. or sodium glucose co-transporter2.ti,ab,kf. or sodium glucose cotransporter2.ti,ab,kf. or sodium-glucose transporter2.ti,ab,kf. or canagliflocin.ti,ab,kf. or canagliflozin.ti,ab,kf. or canaglu.ti,ab,kf. or invokana.ti,ab,kf. or jnj 28431754.ti,ab,kf. or jnj28431754.ti,ab,kf. or sulisent.ti,ab,kf. or ta 7284.ti,ab,kf. or ta7284.ti,ab,kf. or andatang.ti,ab,kf. or bms 512148.ti,ab,kf. or bms512148.ti,ab,kf. or ckd 380.ti,ab,kf. or ckd380.ti,ab,kf. or dapagliflozin.ti,ab,kf. or dwp 16001.ti,ab,kf. or dwp16001.ti,ab,kf. or edistride.ti,ab,kf. or farxiga.ti,ab,kf. or forxiga.ti,ab,kf. or hgp 1602.ti,ab,kf. or hgp 1812.ti,ab,kf. or hgp1602.ti,ab,kf. or hgp1812.ti,ab,kf. or "lyn 045".ti,ab,kf. or lyn045.ti,ab,kf. or oxra.ti,ab,kf. or bi 10773.ti,ab,kf. or bi10773.ti,ab,kf. or ckd 398.ti,ab,kf. or ckd398.ti,ab,kf. or empagliflozin.ti,ab,kf. or gibtulio.ti,ab,kf. or jardiance.ti,ab,kf. or oboravo.ti,ab,kf. or ertugliflozin.ti,ab,kf. or mk 8835.ti,ab,kf. or mk8835.ti,ab,kf. or "pf 04971729".ti,ab,kf. or "pf 04971729 00".ti,ab,kf. or pf 04971729-00.ti,ab,kf. or pf 4971729.ti,ab,kf. or "pf 4971729 00".ti,ab,kf. or pf 4971729-00.ti,ab,kf. or pf04971729.ti,ab,kf. or "pf04971729 00".ti,ab,kf. or pf04971729-00.ti,ab,kf. or pf4971729.ti,ab,kf. or "pf4971729 00".ti,ab,kf. or pf4971729-00.ti,ab,kf. or steglatro.ti,ab,kf. |
14908 |
|
7 |
5 and 6 |
46 |
|
8 |
limit 7 to yr="2015 -Current" |
46 |
|
9 |
8 not (comment/ or editorial/ or letter/) not ((exp animals/ or exp models, animal/) not humans/) |
42 |
|
10 |
meta-analysis/ or meta-analysis as topic/ or (metaanaly* or meta-analy* or metanaly*).ti,ab,kf. or systematic review/ or cochrane.jw. or (prisma or prospero).ti,ab,kf. or ((systemati* or scoping or umbrella or "structured literature") adj3 (review* or overview*)).ti,ab,kf. or (systemic* adj1 review*).ti,ab,kf. or ((systemati* or literature or database* or data-base*) adj10 search*).ti,ab,kf. or ((structured or comprehensive* or systemic*) adj3 search*).ti,ab,kf. or ((literature adj3 review*) and (search* or database* or data-base*)).ti,ab,kf. or (("data extraction" or "data source*") and "study selection").ti,ab,kf. or ("search strategy" and "selection criteria").ti,ab,kf. or ("data source*" and "data synthesis").ti,ab,kf. or (medline or pubmed or embase or cochrane).ab. or ((critical or rapid) adj2 (review* or overview* or synthes*)).ti. or (((critical* or rapid*) adj3 (review* or overview* or synthes*)) and (search* or database* or data-base*)).ab. or (metasynthes* or meta-synthes*).ti,ab,kf. |
766213 |
|
11 |
exp clinical trial/ or randomized controlled trial/ or exp clinical trials as topic/ or randomized controlled trials as topic/ or Random Allocation/ or Double-Blind Method/ or Single-Blind Method/ or (clinical trial, phase i or clinical trial, phase ii or clinical trial, phase iii or clinical trial, phase iv or controlled clinical trial or randomized controlled trial or multicenter study or clinical trial).pt. or random*.ti,ab. or (clinic* adj trial*).tw. or ((singl* or doubl* or treb* or tripl*) adj (blind$3 or mask$3)).tw. or Placebos/ or placebo*.tw. |
2761650 |
|
12 |
Epidemiologic studies/ or case control studies/ or exp cohort studies/ or Controlled Before-After Studies/ or Case control.tw. or cohort.tw. or Cohort analy$.tw. or (Follow up adj (study or studies)).tw. or (observational adj (study or studies)).tw. or Longitudinal.tw. or Retrospective*.tw. or prospective*.tw. or consecutive*.tw. or Cross sectional.tw. or Cross-sectional studies/ or historically controlled study/ or interrupted time series analysis/ [Onder exp cohort studies vallen ook longitudinale, prospectieve en retrospectieve studies] |
4796824 |
|
13 |
Case-control Studies/ or clinical trial, phase ii/ or clinical trial, phase iii/ or clinical trial, phase iv/ or comparative study/ or control groups/ or controlled before-after studies/ or controlled clinical trial/ or double-blind method/ or historically controlled study/ or matched-pair analysis/ or single-blind method/ or (((control or controlled) adj6 (study or studies or trial)) or (compar* adj (study or studies)) or ((control or controlled) adj1 active) or "open label*" or ((double or two or three or multi or trial) adj (arm or arms)) or (allocat* adj10 (arm or arms)) or placebo* or "sham-control*" or ((single or double or triple or assessor) adj1 (blind* or masked)) or nonrandom* or "non-random*" or "quasi-experiment*" or "parallel group*" or "factorial trial" or "pretest posttest" or (phase adj5 (study or trial)) or (case* adj6 (matched or control*)) or (match* adj6 (pair or pairs or cohort* or control* or group* or healthy or age or sex or gender or patient* or subject* or participant*)) or (propensity adj6 (scor* or match*))).ti,ab,kf. or (confounding adj6 adjust*).ti,ab. or (versus or vs or compar*).ti. or ((exp cohort studies/ or epidemiologic studies/ or multicenter study/ or observational study/ or seroepidemiologic studies/ or (cohort* or 'follow up' or followup or longitudinal* or prospective* or retrospective* or observational* or multicent* or 'multi-cent*' or consecutive*).ti,ab,kf.) and ((group or groups or subgroup* or versus or vs or compar*).ti,ab,kf. or ('odds ratio*' or 'relative odds' or 'risk ratio*' or 'relative risk*' or aor or arr or rrr).ab. or (("OR" or "RR") adj6 CI).ab.)) |
5756036 |
|
14 |
9 and 10 – SR’s |
3 |
|
15 |
(9 and 11) not 14 – RCT’s |
11 |
|
16 |
(9 and (12 or 13)) not (14 or 15) – Observationele studies |
11 |
|
17 |
14 or 15 or 16 |
25 |