Flow disfunctie bij vaattoegang voor hemodialyse
Uitgangsvraag
Wat is de optimale behandeling bij een stenose van de vaattoegang voor hemodialyse?
Aanbeveling
Voer bij de behandeling van een stenose van de vaattoegang voor hemodialyse bij voorkeur een conventionele percutane ballondilatatie uit.
Overweeg om aanvullend een covered stent te gebruiken bij de veneuze anastomose van een arterioveneuze graft of om in overleg met de patiënt aanvullend een paclitaxel-coated ballon te gebruiken bij een arterioveneuze fistel:
- bij patiënten met een hoog risico op een recidief stenose (bijvoorbeeld bij behandeling van een restenose die binnen 3 tot 4 maanden is teruggekomen); of
- bij patiënten voor wie een recidief stenose grote consequenties heeft (bijvoorbeeld wanneer de behandeling onder sedatie moet worden uitgevoerd, wanneer de patiënt beperkte mogelijkheden voor een nieuwe vaattoegang heeft, of wanneer er sprake is van een trombose van de vaattoegang).
Gebruik bij voorkeur geen bare metal stents bij de endovasculaire behandeling van een stenose van de vaattoegang.
Overweeg om een open chirurgische behandeling uit te voeren wanneer endovasculaire behandeling van de stenose niet succesvol is.
Overwegingen
Voor- en nadelen van de interventie en de kwaliteit van het bewijs
Er is literatuuronderzoek gedaan naar de voor- en nadelen van endovasculaire interventies om stenose van een vaattoegang voor hemodialyse te behandelen, te weten percutane ballondilatatie (PTA) met een paclitaxel gecoate ballon (drug-coated balloon, DCB), plaatsing van een covered stent en plaatsing van een bare metal stent, ten opzichte van PTA met een conventionele ballon. Functie van de volledige vaattoegang werd als cruciale uitkomstmaat gedefinieerd. Hiertoe werden primaire en secundaire patency van de volledige vaattoegang gerekend, maar ook het aantal interventies dat nodig is om de vaattoegang te onderhouden. Functie van de “target lesion” (waaronder primaire patency en aantal interventies), mortaliteit en kwaliteit van leven werden beschouwd als belangrijke uitkomstmaten voor de besluitvorming.
Er waren 10 gerandomiseerde studies die PTA met een DCB vergeleken met PTA met een conventionele ballon. Deze studies werden bijna uitsluitend bij patiënten met een arterioveneuze fistel uitgevoerd. Op basis van deze studies werd geconcludeerd dat PTA met een DCB de primaire patency van de gehele vaattoegang in de eerste 6 maanden (69% versus 50%) en 12 maanden (39% versus 28%) waarschijnlijk verbetert ten opzichte van PTA met een conventionele ballon. Op het niveau van de target lesion zien we hetzelfde patroon. Secundaire patency van de vaattoegang lijkt niet verschillend tussen PTA met een gecoate en een conventionele ballon (95% versus 95% na 12 maanden). Er lijkt geen klinisch relevant verschil te zijn in het aantal interventies aan de vaattoegang tussen deze behandelingen (0,3 interventies per jaar minder bij gebruik van een DCB ten opzichte van een conventionele ballon). Er zijn geen aanwijzingen voor een verschil in mortaliteit tussen de twee behandelingen. Kwaliteit van leven werd niet beschreven als uitkomstmaat in de gevonden studies. De totale bewijskracht, de laagste bewijskracht voor de cruciale uitkomstmaten, is laag tot redelijk.
Er waren 5 gerandomiseerde studies die PTA met covered stentplaatsing vergeleken met alleen PTA met een conventionele ballon. Deze studies werden bijna uitsluitend bij patiënten met een arterioveneuze graft uitgevoerd. De covered stent werd geplaatst over de veneuze anastomose van de graft (de typische plaats van de stenose bij arterioveneuze grafts). Omdat het niet de bedoeling is om dialysenaalden door de stent te prikken, dient een covered stent niet in het aanpriktraject van de vaattoegang te worden geplaatst. Primaire patency van de gehele vaattoegang lijkt beter te zijn in de eerste 6 maanden (41% versus 25%) en 12 maanden (24% versus 11%) na covered stentplaatsing dan na alleen PTA. Op het niveau van de target lesion zien we hetzelfde patroon. Secundaire patency van de vaattoegang lijkt niet verschillend tussen PTA met covered stent of alleen PTA (89% versus 94% na 12 maanden). Er lijkt geen klinisch relevant verschil te zijn in het aantal interventies om de vaattoegang patent te houden tussen deze interventies (0,7 interventies per jaar minder bij gebruik van een covered stent ten opzichte van alleen PTA). Mortaliteit lijkt hoger te zijn na plaatsing van een covered stent dan na PTA alleen, maar dit verschil was niet statistisch significant. Kwaliteit van leven werd in de gevonden studies niet beschreven. De totale bewijskracht, de laagste bewijskracht voor de cruciale uitkomstmaten, is laag tot redelijk. Deze gerandomiseerde studies hebben betrekking op het plaatsen van een covered stent als aanvulling op een ballondilatatie om de duurzaamheid van de behandeling te verbeteren. Een andere indicatie voor het gebruik van een covered stent is om een bloeding te behandelen wanneer een PTA wordt gecompliceerd door een ruptuur van het bloedvat.
De effecten van plaatsing van bare metal stents werden voornamelijk in oudere studies beschreven. Deze studies werden bijna uitsluitend bij patiënten met een arterioveneuze graft uitgevoerd. Door beperkingen in de onderzoeksopzet, lage patiëntaantallen, brede betrouwbaarheidsintervallen en/of inconsistentie van de resultaten werden alleen uitkomstmaten beschreven met een zeer lage bewijskracht. Het is daarom onduidelijk of primaire patency, secundaire patency en mortaliteit verschillend zijn na bare metal stentplaatsing ten opzichte van alleen PTA. De overige uitkomstmaten werden niet beschreven in de gevonden literatuur. Er kan op basis van de data geen richting gegeven worden aan de besluitvorming wat betreft plaatsing van een bare metal stent. Wel is op basis van theoretische gronden het gebruik van bare metal stents niet aan te bevelen. Door het open karakter van de stents kan intima hyperplasie namelijk door de stent heen groeien. Het gebruik van een bare metal stent geeft daardoor geen bescherming tegen intima hyperplasie.
Waarden en voorkeuren van patiënten (en eventueel hun verzorgers)
Een goede functie van de arterioveneuze fistel of graft wordt door patiënten en zorgverleners als een van belangrijkste aspecten van de vaattoegang beschouwd. Een effectieve manier of flow disfunctie van de vaattoegang te behandelen of voorkomen is daarom zeer relevant vanuit het perspectief van de patiënt.
Een percutane ballondilatatie van een stenose in de vaattoegang is een minimaal invasieve interventie, die meestal kort duurt en als poliklinische behandeling kan worden uitgevoerd. Wanneer de interventie voor een dialysebehandeling wordt gepland, hoeft de patiënt er niet speciaal voor naar het ziekenhuis te komen. Desondanks wordt de ballondilatatie door veel patiënten als pijnlijk ervaren en treden er soms complicaties op (ruptuur van het bloedvat of bloeding bij de toegangsweg). De hoeveelheid jodiumhoudend contrastmiddel die tijdens de behandeling wordt toegediend is doorgaans te weinig om effect te hebben op eventuele restfunctie van de nieren. Vanwege deze gunstige eigenschappen van een percutane ballondilatatie heeft deze behandeling de voorkeur boven een open chirurgische behandeling van een stenose in de vaattoegang door middel van een patch angioplastiek, een interponaat of een nieuwe arterioveneuze anastomose. Deze open chirurgische behandeling heeft bovendien als nadeel dat het behandelde deel van de vaattoegang pas na wondgenezing weer gecannuleerd kan worden. Hierdoor moet soms een tijdelijke centraal veneuze katheter worden ingebracht.
Kosten (middelenbeslag)
De kosten voor een percutane ballondilatatie met een conventionele ballon zijn ongeveer 1000 euro. De meerprijs van een DCB is ongeveer 500 euro en de meerprijs van een covered stent is ongeveer 2000 euro. Voor een kosteneffectieve behandeling zou gebruik van een DCB dus ongeveer 0,5 interventies in de toekomst moeten besparen en zou gebruik van een covered stent ongeveer 2 interventies moeten besparen. Op basis van de huidige data lijkt er daarom geen relevant financieel voordeel te zijn van deze behandelingen.
Aanvaardbaarheid, haalbaarheid en implementatie
De technische aspecten van de endovasculaire behandeling zijn grotendeels gelijk voor een PTA met een conventionele ballon, een drug coated ballon en een covered stent. Bij elk van deze behandelingen wordt gestart met een ballondilatatie van de stenose. Hierbij dient de ballon volledig te zijn ontplooid. Wanneer een insnoering te zien blijft op de plaats van de stenose, kan gebruik van een high-pressure ballon uitkomst bieden. De verschillende behandelingen kunnen in alle ziekenhuizen in Nederland worden uitgevoerd door interventie-radiologen en vaatchirurgen met een endovasculaire aantekening.
Het gebruik van paclitaxel gecoate ballonnen is controversieel geworden na de publicatie van een meta-analyse van gerandomiseerde onderzoeken naar het gebruik van deze ballonnen in het femoropopliteale traject bij patiënten met perifeer arterieel vaatlijden (Katsanos, 2018). In deze meta-analyse werd een verhoogde mortaliteit gevonden na 2 jaar follow-up. Een biologische verklaring voor deze bevinding lijkt onwaarschijnlijk. Op basis van de resultaten van deze eerste meta-analyse werd door de Inspectie Gezondheidszorg en Jeugd geadviseerd om alternatieve ballonnen te gebruiken bij de behandeling van patiënten met perifeer arterieel vaatlijden en om paclitaxel gecoate ballonnen alleen te gebruiken met instemming van de patiënt na het bespreken van deze bevindingen. In een tweede meta-analyse werd de vitale status nagezocht van patiënten die aanvankelijk buiten follow-up waren geraakt. Dit resulteerde in een forse reductie van de effectgrootte (Rocha-Signh, 2020). In de grote SWEDEPAD trial waarin de vitale status van vrijwel alle patiënten bekend was werd geen effect van DCB op mortaliteit waargenomen tot 4 jaar follow-up (Nordanstig, 2020). Mogelijk worden de resultaten van de eerdere studies vertekend door uitval van patiënten. In ieder geval lijkt er na 1 jaar follow-up geen verhoogde mortaliteit te zijn bij gebruik van DCB voor de behandeling van stenoses in de vaattoegang voor hemodialyse. Toch dienen paclitaxel gecoate ballonnen alleen te worden gebruikt voor de behandeling van stenoses in de vaattoegang voor hemodialyse na instemming van de patiënt nadat de bovenstaande informatie is besproken (zie publicatie van Inspectie Gezondheidszorg en Jeugd: publicatie 2020).
Rationale van de aanbeveling: weging van argumenten voor en tegen de interventies
Het gebruik van een DCB bij de behandeling van een stenose in een arterioveneuze fistel en het gebruik van een covered stent bij de behandeling van een stenose in een arterioveneuze graft leidt tot een langere tijd tot de volgende interventie (primaire patency) maar niet tot een langere levensduur (secundaire patency) van de vaattoegang. Het gebruik van deze behandelingen leidt tot een statistisch significante vermindering van het aantal interventies aan de vaattoegang, maar we beschouwen dit effect te klein om klinisch relevant te zijn. De behandelingen met DCB en covered stents lijken bovendien niet kosteneffectief te zijn. Op basis van deze argumenten dient het gebruik van een DCB bij de behandeling van een stenose in een arterioveneuze fistel en het gebruik van een covered stent bij de behandeling van een stenose in een arterioveneuze graft alleen in bijzondere omstandigheden te worden overwogen.
Inbedding in de zorg
Behalve deze geprioriteerde uitgangsvraag zijn er in de dagelijkse praktijk diverse andere aandachtspunten van belang, die kort zijn samengevat in de onderstaande tekst. De werkgroep sluit zich hiervoor aan bij de aanbevelingen van nationale en internationale richtlijnen. Voor verdere toelichting en onderbouwing wordt verwezen naar de betreffende richtlijnen.
Preventie van flow disfunctie van arterioveneuze fistels en grafts
In de KDOQI 2019 update (Lok, 2020; Guideline 14) en in de ERBP richtlijn (Gallieni, 2019; Chapter 8) wordt aandacht besteed aan het voorkomen van flow disfunctie van arterioveneuze fistels en grafts. Omdat de bewijskracht van de beschikbare literatuur beperkt is, hebben we alleen de aanbevelingen overgenomen die in beide richtlijnen worden geadviseerd.
- Overweeg om infrarood behandeling toe te passen bij patiënten met een arterioveneuze fistel (3x per week 40 minuten). Hoewel er in een meta-analyse van gerandomiseerde trials geen statistische heterogeniteit was, werd de infrarood behandeling op verschillende momenten in de ‘levensfase’ van de fistel onderzocht (tijdens maturatie, bij functioneel gebruik en na ballon angioplastiek van een stenose). Omdat de meeste interventies tijdens de maturatie plaatsvinden, zal het absolute behandeleffect in deze periode het grootst zijn:
- Overweeg om visolie supplementen te adviseren aan patiënten met een arterioveneuze graft (1x per dag 1,6 g EPA en 0,8 g DHA). Hoewel er in een meta-analyse van gerandomiseerde trials geen verschil was in het relatieve behandeleffect tussen patiënten met een arterioveneuze fistel en graft, is het absolute behandeleffect bij patiënten met een arterioveneuze graft groter (0,74 interventies per jaar minder bij gebruik van visolie). Dit voordeel moet worden afgewogen tegen een mogelijk verhoogd bloedingsrisico bij gebruik van visolie supplementen bij dialysepatiënten (RR 1,40; 95% CI: 0,78 tot 2,49).
- Schrijf geen orale anticoagulantia of een combinatie van acetylsalicylzuur en clopidogrel voor om flow disfunctie van arterioveneuze fistels of grafts te voorkomen. In gerandomiseerde trials bleek dat deze behandeling niet effectief was en meer bloedingen veroorzaakte.
- Voor andere behandelingen om flow disfunctie van arterioveneuze fistels of grafts te voorkomen was onvoldoende bewijs om een advies te geven.
Behandeling van trombose van arterioveneuze fistels en grafts
De behandeling van een getromboseerde arterioveneuze fistel of graft dient te bestaan uit twee onderdelen: het verwijderen van de stolsels en het behandelen van de onderliggende stenose. Voor het behandelen van de stenose gelden dezelfde overwegingen als hierboven beschreven bij open arterioveneuze fistels en grafts. De getromboseerde vaattoegang dient bij voorkeur voor de volgende dialysebehandeling te worden behandeld om plaatsing van een tijdelijke centraal veneuze katheter te vermijden. Na het verwijderen van de stolsels dient een angiografie te worden gemaakt om de onderliggende oorzaak van de trombose te detecteren.
De stolsels kunnen met een open chirurgische of met een endovasculaire benadering uit de vaattoegang worden verwijderd. Bij een endovasculaire benadering zijn diverse technieken (en combinaties daarvan) mogelijk: trombolyse, trombosuctie en trombus fragmentatie. In prospectieve observationele studies werden na een endovasculaire benadering bij ongeveer 40% van de patiënten longembolieën gevonden op een ventilatie-perfusie scan; bij 4% van de patiënten was er sprake van symptomatische longembolieën. In een meta-analyse van gerandomiseerde studies naar getromboseerde grafts had de chirurgische benadering aanvankelijk meer technisch succes, maar was er geen verschil in verlies van de vaattoegang na 3 maanden. De waarde van deze meta-analyse voor de huidige praktijk is echter beperkt, omdat de onderzoeken meer dan 20 jaar geleden werden uitgevoerd. In de tussentijd zijn zowel de open chirurgische als de endovasculaire technieken verder ontwikkeld. Voor de behandeling van getromboseerde arterioveneuze fistels zijn alleen observationele studies voorhanden, die geen duidelijk verschil laten zien tussen de open chirurgische en de endovasculaire benadering. Er zijn geen doorslaggevende voor- of nadelen van deze technieken die aanleiding vormen om een voorkeur uit te spreken voor een van beide behandelingen (KDOQI 2019 update, Recommendation 15.4). De ervaring van het behandelteam of specifieke omstandigheden (bijvoorbeeld de beschikbaarheid van de behandelruimte) kunnen de behandelkeuze sturen.
Wanneer het behandelteam verwacht dat de behandeling van een getromboseerde arterioveneuze fistel of graft niet zal resulteren in een duurzame vaattoegang, kan op individuele basis overwogen worden om een getromboseerde vaattoegang niet te behandelen maar om een nieuwe vaattoegang te creëren.
Behandeling van flow disfunctie van dialysekatheters
Er is sprake van flow disfunctie van dialysekatheters wanneer de voorgeschreven pompstand op het dialyseapparaat niet wordt behaald en een langere behandeling nodig is om adequate hemodialyse te bereiken (KDOQI 2019 Update, recommendation 21.1).
In een meta-analyse van 5 gerandomiseerde studies bij 479 patiënten werd geen significant verschil gevonden in het optreden van flow disfunctie wanneer preventief orale anticoagulantia werden toegediend. In 1 gerandomiseerde studie bij 180 patiënten werd onvoldoende bewijs gevonden voor een gunstig effect van acetylsalicylzuur op het voorkomen van flow disfunctie. Op basis van deze studies wordt het niet aanbevolen om systemische bloedverdunners voor te schrijven om flow disfunctie van dialysekatheters te voorkomen (KDOQI 2019 Update, recommendation 21.8).
Bij de behandeling van flow disfunctie van dialysekatheters kan het volgende stappenplan worden gevolgd (KDOQI 2019 update, Guideline 22):
- Controleer de positie van de dialysekatheter met een röntgenfoto. Wanneer de tip van de dialysekatheter niet in het rechter atrium ligt of wanneer de katheter knikt in de bocht van het tunneltraject kan de positie van de katheter worden gecorrigeerd door deze te wisselen over een voerdraad.
- Wanneer de positie van de dialysekatheter correct is kan flow disfunctie veroorzaakt worden door stolsels in het lumen of aan de tip van de dialysekatheter. Deze stolsels kunnen worden behandeld met trombolytica. Plaats hiervoor een slot met alteplase (1 mg/mL) of met urokinase in beide lumina van de dialysekatheter. Na 30 tot 60 minuten kan het slot worden verwijderd en kan de dialysekatheter in 90% van de gevallen weer worden gebruikt.
- Wanneer trombolyse niet succesvol is kan flow disfunctie veroorzaakt worden door een huls van fibrine en bindweefsel die zich rondom de dialysekatheter vormt. Dit probleem kan worden behandeld door de dialysekatheter over een voerdraad te wisselen en de fibrinehuls met een ballondilatatie te doorbreken.
- Kies pas voor het verwijderen en verplaatsen van een centraal veneuze dialysekatheter naar een andere vene wanneer de bovenstaande medicamenteuze en mechanische behandelingen niet succesvol zijn geweest om thoracale centraal veneuze obstructie te voorkomen (KDOQI 2019 Update, recommendation 22.8).
Onderbouwing
Achtergrond
De belangrijkste reden voor disfunctie van de vaattoegang voor hemodialyse is een stenose in de arterioveneuze fistel of graft. Deze stenose wordt meestal veroorzaakt door intima hyperplasie. De meest toegepaste behandeling van een stenose van de vaattoegang is een percutane ballondilatatie. Helaas is de duurzaamheid van deze behandeling vaak beperkt, waardoor veel patiënten herhaaldelijk invasieve interventies moeten ondergaan om hun vaattoegang te behouden. In deze module bespreken we wanneer een aanvullende behandeling met een drug-coated ballon, covered stent of bare metal stent geïndiceerd is.
Daarnaast komen in de paragraaf ‘Inbedding in de zorg’ de volgende onderwerpen aan bod:
- Preventie van flow disfunctie van arterioveneuze fistels en grafts.
- Behandeling van trombose van arterioveneuze fistels en grafts.
- Behandeling van flow disfunctie van centraal veneuze katheters.
De indicatie voor behandeling van stenoses in arterioveneuze fistels en grafts wordt behandeld in Module ‘Monitoring en surveillance’.
Conclusies / Summary of Findings
PICO 1 Drug-coated balloons
|
Moderate GRADE |
In patients with vascular access flow dysfunction, angioplasty with a drug-coated balloon probably results in higher access circuit primary patency at 6 months follow-up compared to angioplasty with a plain balloon.
Sources: (Chen, 2020; Liao, 2019; Lookstein, 2020) |
|
Low GRADE |
In patients with vascular access flow dysfunction, angioplasty with a drug-coated balloon may not result in clinically relevant higher access circuit primary patency at 12 months follow-up compared to angioplasty with a plain balloon.
Sources: (Chen, 2020; Liao, 2019; Trerotola, 2020) |
|
Low GRADE |
In patients with vascular access flow dysfunction, angioplasty with a drug-coated balloon may not result in different access circuit secondary patency at 6 to 12 months follow-up compared to angioplasty with a plain balloon.
Sources: (Liao, 2019; Trerotola, 2020) |
|
Moderate GRADE |
In patients with vascular access flow dysfunction, angioplasty with a drug-coated balloon probably does not result in a clinically relevant decrease in interventions to keep the access circuit patent at 6 and 12 months follow-up compared to angioplasty with a plain balloon.
Sources: (Liao, 2019; Lookstein, 2020; Trerotola, 2020) |
|
- GRADE |
For the outcome quality of life, it was not possible to draw conclusions or grade the level of evidence, due to the absence data. |
|
Moderate GRADE |
In patients with vascular access flow dysfunction, angioplasty with a drug-coated balloon probably results in higher target lesion primary patency at 6 months follow-up compared to angioplasty with a plain balloon.
Sources: (Chen, 2020; Kim, 2020; Liao, 2019; Lookstein, 2020) |
|
Low GRADE |
In patients with vascular access flow dysfunction, angioplasty with a drug-coated balloon may not result in higher target lesion primary patency at 12 to 24 months follow-up compared to angioplasty with a plain balloon.
Sources: (Chen, 2020; Kim, 2020; Liao, 2019; Trerotola, 2020) |
|
Low GRADE |
In patients with vascular access flow dysfunction, angioplasty with a drug-coated balloon may not result in higher mortality compared to angioplasty with a plain balloon.
Sources: (Chen, 2020; Kim, 2020; Liao, 2019; Lookstein, 2020) |
PICO 2 Covered stent
|
Low GRADE |
In patients with a dysfunctional arteriovenous access for hemodialysis, covered stent placement may result in higher access circuit primary patency at 6 and 12 months follow-up, compared to balloon angioplasty.
Sources: (Hu, 2018) |
|
Low GRADE |
In patients with a dysfunctional arteriovenous access for hemodialysis, covered stent placement may not result in lower secondary patency at 12 and 24 months follow-up compared to balloon angioplasty.
Sources: (Kavan, 2019) |
|
Moderate GRADE |
In patients with a dysfunctional arteriovenous access for hemodialysis, covered stent placement probably does not result in a clinically relevant reduction of the number of interventions to maintain access circuit patency at 24 months follow-up compared to balloon angioplasty.
Sources: (Haskal, 2016; Kavan, 2019; Mohr, 2019) |
|
Moderate GRADE |
In patients with a dysfunctional arteriovenous access for hemodialysis, covered stent placement probably results in higher target lesion primary patency at 6 and 12 months follow-up compared to balloon angioplasty.
Sources: (Hu, 2018; Kavan, 2019; Yang, 2018) |
|
Moderate GRADE |
In patients with a dysfunctional arteriovenous access for hemodialysis, covered stent placement probably does not result in a clinically relevant reduction of the number of interventions to maintain target lesion patency at 24 months follow-up compared to balloon angioplasty.
Sources: (Mohr, 2019) |
|
Low GRADE |
In patients with a dysfunctional arteriovenous access for hemodialysis, covered stent placement may increase mortality compared to balloon angioplasty.
Sources: (Hu, 2019; Mohr, 2019) |
|
- GRADE |
It was not possible to draw conclusions or grade the level of evidence for the outcome quality of life, due to the absence of comparative studies reporting the outcome. |
PICO 3 Bare metal stent
|
- GRADE |
It was not possible to draw conclusions or grade the level of evidence for the outcome access circuit primary patency, due to the absence of comparative studies reporting the outcome. |
|
Very low GRADE |
It is unclear whether secondary patency is different after stent placement compared to balloon angioplasty in patients with a dysfunctional arteriovenous access for hemodialysis.
Sources: (Hoffer, 1997; Quin, 1995) |
|
- GRADE |
It was not possible to draw conclusions or grade the level of evidence for the outcome number of interventions to maintain access circuit patency, due to the absence of comparative studies reporting the outcome. |
|
Very low GRADE |
It is unclear whether target lesion primary patency is different after stent placement compared to balloon angioplasty in patients with a dysfunctional arteriovenous access for hemodialysis.
Sources: (Beathard, 1992; Hoffer, 1997; Kavan, 2019; Quin, 1995) |
|
- GRADE |
It was not possible to draw conclusions or grade the level of evidence for the outcome number of interventions to maintain target lesion patency, due to the absence of comparative studies reporting the outcome. |
|
Very low GRADE |
It is unclear whether mortality is different after stent placement compared to balloon angioplasty in patients with a dysfunctional arteriovenous access for hemodialysis.
Sources: (Hoffer, 1997; Beathard, 1992) |
|
- GRADE |
It was not possible to draw conclusions or grade the level of evidence for the outcome quality of life, due to the absence of comparative studies reporting the outcome. |
Samenvatting literatuur
PICO 1 Drug-coated balloons
Description of studies
Chen (2020) performed a systematic review and meta-analysis of paclitaxel-coated devices in hemodialysis (HD) access maintenance to analyze the risk of death associated with paclitaxel and the patency of HD access in patients with end-stage renal disease. The systematic review reported all-cause mortality as well as hazard ratios and odds ratios for lesion primary patency. All retrospective, prospective, and observational studies reporting on paclitaxel-coated or eluting balloons versus plain balloon angioplasty in the HD access published up to April 2019 with at least 10 patients per treatment arm were included. For the current analysis, only randomized studies with target lesions in arteriovenous fistula (AVF) or graft (AVG) were considered, which were 9 RCTs with a total of 687 patients. Study characteristics are summarized in table 5.1. The systematic review had a low risk of bias, but the individual studies had risk of bias due to lack of blinding of care providers and/or outcome assessors.
In addition to the systematic review by Chen (2020), four RCTs were included.
The RCT by Kim (2020) compared paclitaxel-coated with plain balloon angioplasty in patients with dysfunctional radiocephalic AVF or AVG. In this Korean single-center study, 20 patients with clinical signs of a failing vascular access were treated with drug-coated balloon angioplasty and 19 with plain balloon angioplasty between June 2016 and June 2018. Both de novo stenosis and restenosis were included. Target lesion primary patency was assessed with a follow-up of 3 years. There was risk of bias due to inadequate blinding of care providers and outcome assessors.
Liao (2019) compared the efficacy of DCBs and conventional balloons for angioplasty on vein-graft anastomosis of dialysis AV grafts in a RCT from Taiwan. In this single-center study, patients with clinical evidence of a hemodynamically significant stenosis from July 2015 to August 2017 were randomized to receive angioplasty with either a DCB (N=22) or a conventional balloon (N=22). Target lesion primary patency and access circuit primary patency were reported at 6 and 12 months, as well as mortality and number of interventions within 12 months. The study had a low risk of bias.
In the international multicenter RCT by Lookstein (2020), 330 patients with new or restenotic lesions in native upper-extremity AVFs were enrolled from April 2017 to December 2018. Exclusion criteria included any history of or current access-circuit thrombosis or a previous stent in the access circuit. Participants were randomly assigned to receive angioplasty either with a drug-coated balloon (N=170) or a standard balloon (N=160). The primary effectiveness end point was target-lesion primary patency at 6 months. Number of interventions performed to maintain target-lesion primary patency, access circuit primary patency and mortality were also reported. The study had a risk of bias due to inadequate blinding of care providers and outcome assessors.
Trerotola (2020) is a follow-up article to Trerotola (2018), which was reported in the meta-analysis. Target lesion and circuit primary patency, number of interventions per patient and mortality were reported at 12 and 24 months. The study had a risk of bias due to inadequate blinding of outcome accessors to treatment allocation.
The main study characteristics of all included studies are outlined in table 1.
Table 1 Study characteristics for drug-coated balloon versus plain balloon angioplasty
|
Publication |
Drug-coated balloon |
Number of patients (drug-coated/plain) |
Age (years) |
Vascular access |
Follow-up (months) |
|
Analysed by Chen, 2020 |
|
|
|
|
|
|
Björkman, 2019 |
IN.PACT Admiral |
18 / 18 |
67.0 / 67.4 |
AVF |
12 |
|
Katsanos, 2012 |
IN.PACT Admiral |
20 / 20 |
64.1 ± 14.3 |
AVF, AVG |
6 |
|
Kitrou, 2015a |
IN.PACT Admiral |
20 / 20 |
61 ± 14.6 |
AVF |
12 |
|
Kitrou, 2015b (follow-up to Katsanos, 2012) |
IN.PACT Admiral |
20 / 20 |
64 ± 14.3 |
AVG, AVG |
12 |
|
Lai, 2014 |
SeQuent Please |
10 / 10 |
67.2 ± 9.4 |
AVF |
12 |
|
Maleux, 2018 |
IN.PACT Admiral |
33 / 31 |
68.1 ± 15.9 |
AVF |
12 |
|
Roosen, 2017 |
IN.PACT Admiral |
16 / 18 |
81.6 |
AVF |
12 |
|
Swinnen, 2018 |
IN.PACT Admiral |
68 / 60 |
64.9 ± 13.7 |
AVF |
12 |
|
Trerotola, 2018 |
Lutonix |
141 / 144 |
62.0 ± 14 |
AVF |
6 |
|
Additional RCTs |
|
|
|
|
|
|
Kim, 2020 |
IN.PACT Admiral |
20 / 19 |
60.7 / 63.7 |
AVF, AVG |
36 |
|
Liao, 2019 |
IN.PACT Admiral |
22 / 22 |
70.4 / 65.9 |
AVG |
12 |
|
Lookstein, 2020 |
IN.PACT AV |
170 / 160 |
65.8 / 65.5 |
AVF |
6 |
|
Trerotola, 2020 (Follow-up to Trerotola, 2018) |
Lutonix |
141 / 144 |
62.0 ± 14 |
AVF |
24 |
Results
Access circuit function: primary patency (critical outcome)
Six studies reported access circuit primary patency at 6 and/or 12 months follow-up, as outlined in figure 1. At 6 months, 5 studies reported in total 123 events (loss of primary patency) in 395 patients (31%) treated with DCB angioplasty, versus 196 events in 394 patients (50%) treated with plain balloon angioplasty, resulting in a RR of 0.62 (95% CI 0.48 to 0.80) in favor of DCB treatment. This difference was clinically relevant.
At 12 months, 3 studies reported a total of 147 events in 242 patients (61%) in the DCB group, versus 176 events in 246 patients (72%) in the plain balloon group, resulting in a RR of 0.87 (95% CI 0.78 to 0.97) in favor of DCBs. This difference was statistically significant, but not clinically relevant.
Figure 1 Access circuit primary patency after DCB versus plain balloon angioplasty
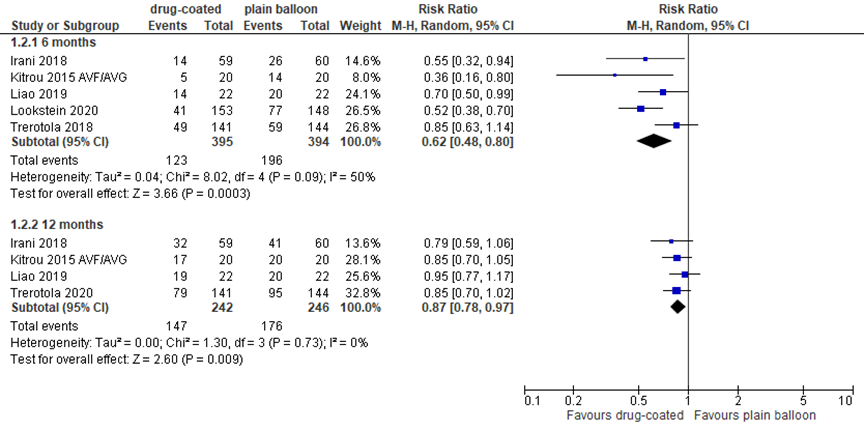
DCB: drug-coated balloon; event: loss of circuit primary patency; Random effects model; df: degrees of freedom; I2: statistical heterogeneity; CI: confidence interval; Z: p-value of pooled effect
Access circuit function: secondary patency (critical outcome)
Three studies reported access circuit or target lesion secondary patency, as presented in figure 2. Secondary patency at 6 and 12 months was high in both groups. Due to the limited number of events (loss of secondary patency), pooled results are presented as risk differences (RD). Loss of secondary patency occurred in 4/183 (2%) patients treated with DCB, versus 3/185 (2%) patients treated with plain balloon at 6 months, resulting in a RD of 0.00 (95% CI -0.03 to 0.02). At 12 months, loss of secondary patency occurred in 10/183 (5%) patients treated with DCB, versus 7/185 (4%) patients treated with plain balloon, resulting in a RD of 0.01 (95% CI -0.03 to 0.05). In addition, Trerotola (2020) reported access circuit secondary patency of 95% after treatment with DCB (N=141) versus 94% after treatment with plain balloon angioplasty (N=144) at 24 months. Kim (2020) reported (target lesion) secondary patency and found in the DCB (N=20) versus the control group (N=19) secondary patency of 90% versus 94% at 24 months, and 90% versus 94% at 36 months. Differences were not clinically relevant or statistically significant.
Figure 2 Access circuit secondary patency after DCB versus plain balloon angioplasty
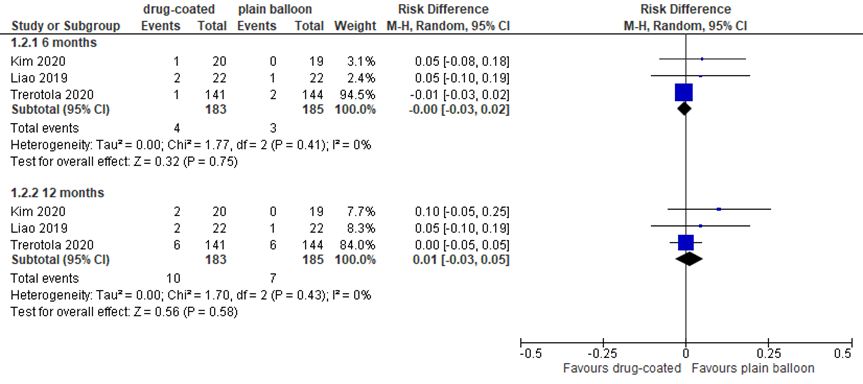
Results presented as risk differences. Event: loss of secondary patency. DCB: drug-coated balloon; random effects model; df: degrees of freedom; I2: statistical heterogeneity; CI: confidence interval; Z: p-value of pooled effect
Access circuit function: number of interventions to maintain patency (critical outcome)
Vascular access circuit function was also expressed as the number of interventions to keep the vascular access circuit patent. Liao (2019) en Lookstein (2020) reported fewer interventions at 12 months in DCBs compared to plain balloons, with mean differences of -1.70 (95% CI -4.05 to 0.65; N=44) and -0.30 (95% CI -0.47 to -0.13; N=301), respectively. Taken together, the reported differences were not clinically relevant.
Level of evidence of the literature
The level of evidence for the outcomes expressing access circuit function was based on randomized studies and therefore starts at high. Regarding the outcome access circuit primary patency at 6 months, the quality of evidence was downgraded by one level to moderate because of the limited number of included patients (imprecision, -1). For the outcome access circuit primary patency at more than 6 months, the quality of evidence was downgraded by two levels to low because of the limited number of included patients and because the CI crossed the threshold of clinical decision-making (both imprecision, -2). For the outcome access circuit secondary patency, the quality of evidence was downgraded by two levels to low because of the limited number of included patients and because only single studies reported a form of secondary patency (both imprecision, -2). For the outcome number of interventions to keep the access circuit patent, the quality of evidence was downgraded by one level to moderate because of the limited number of included patients (imprecision, -1).
AVF/AVG function: target lesion primary patency
Most studies reported AV access function as target lesion primary patency (figure 3). At 6 months, 10 studies with in total 1,080 patients reported loss of target lesion primary patency, as depicted in figure 1. With 127 events in 546 patients (23%) treated with DCB angioplasty versus 236 events in 534 patients (44%) treated with plain balloon angioplasty, the pooled relative risk (RR) was 0.55 in favor of DCB treatment, with a 95% confidence interval (CI) from 0.46 to 0.65. This difference was clinically relevant and statistically significant.
Figure 3 Target lesion primary patency at 6 to 24 months after DCB versus plain balloon angioplasty
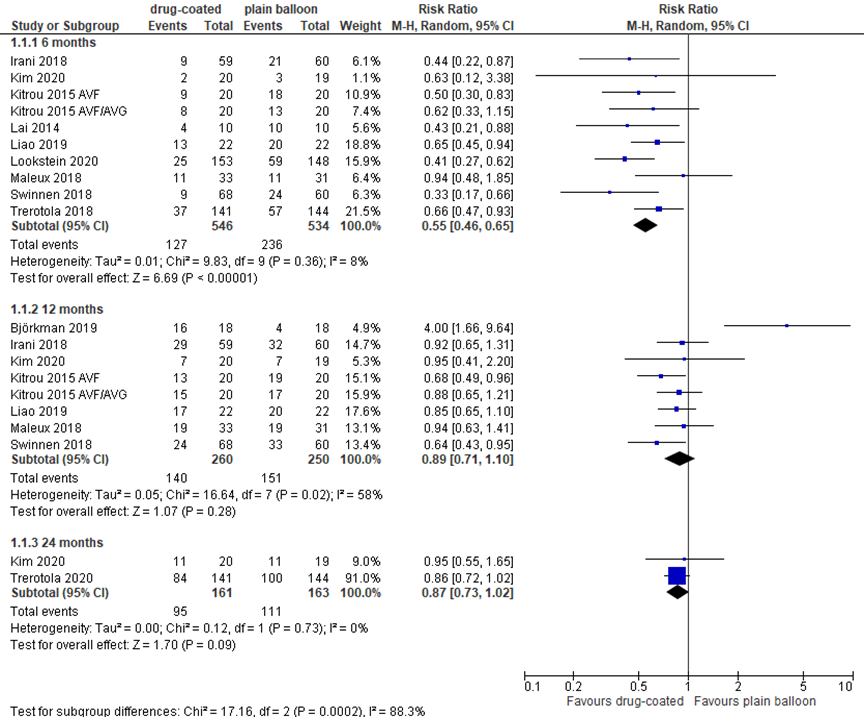
DCB: drug-coated balloon; event: loss of primary patency; random effects model; df: degrees of freedom; I2: statistical heterogeneity; CI: confidence interval; Z: p-value of pooled effect
At 12 months, 8 studies reported loss of target lesion primary patency. In 510 patients in total, 140 events were found in 260 (54%) patients treated with DCB angioplasty, versus 151 events in 250 (60%) patients treated with plain balloon angioplasty, resulting in a RR of 0.89 (95% CI 0.71 to 1.10). This difference is not clinically relevant or statistically significant.
At 24 months follow-up, Trerotola (2020) reported 84/141 (60%) events in the DCB group versus 100/144 (69%; P<0.0001) in the plain balloon group. Kim (2020) reported a target lesion primary patency of 11/20 (55%) after treatment with DCB, versus 11/19 (57%) in the plain balloon group (P=0.900). At 36 months follow-up, Kim (2020) reported a primary patency of 55% versus 48.9% (P=0.714) in DCB and control group, respectively. The differences were not clinically relevant.
AVF/AVG function: number of interventions to maintain patency
Two studies reported the number of interventions to maintain target lesion patency. Lookstein (2020) and Trerotola (2020) reported fewer interventions in DCB’s compared to plain balloons, with mean differences of -0.40 (95% CI -0.55 to -0.25) and -0.15 (95% CI -0.39 to 0.09), respectively. At 24 months follow-up, Trerotola (2020) found 1.48 ± 1.72 interventions per patient (N=141) to keep the target lesion patent in the DCB group versus 1.55 ± 1.60 per patient (N=144) in the control group, a mean difference of -0.07 (95% CI -0.46 to 0.32). Overall, the reported differences were not clinically relevant.
Quality of life
The outcome quality of life could not be determined as it was not reported in the included literature.
Level of evidence of the literature
The level of evidence for the outcomes expressing AVF/AVG function was based on randomized studies and therefore starts at high. Regarding the outcomes target lesion primary patency at 6 months, the quality of evidence was downgraded by one level to moderate because of the limited number of included patients (imprecision, -1). For the outcome target lesion primary patency at more than 6 months, the quality of evidence was downgraded by two levels to low because of the limited number of included patients and because the CI crossed the threshold of clinical decision-making (both imprecision, -2). For the outcome number of interventions to keep the target lesion patent, the quality of evidence was downgraded by one level to moderate because of the limited number of included patients (imprecision).
Mortality
Seven studies reported mortality at 6 or 12 months. Due to the very low number of events in most studies, data are presented as risk difference. With 34 events in 420 patients (8%) treated with DCB angioplasty and 27 events in 412 patients (6.6%) treated with plain balloon angioplasty, the RD was 0.02 (95% CI -0.01 to 0.04) in favor of plain balloon angioplasty (figure 4). The difference is not considered clinically relevant.
Figure 4 Mortality at 12 months after DCB versus plain balloon angioplasty
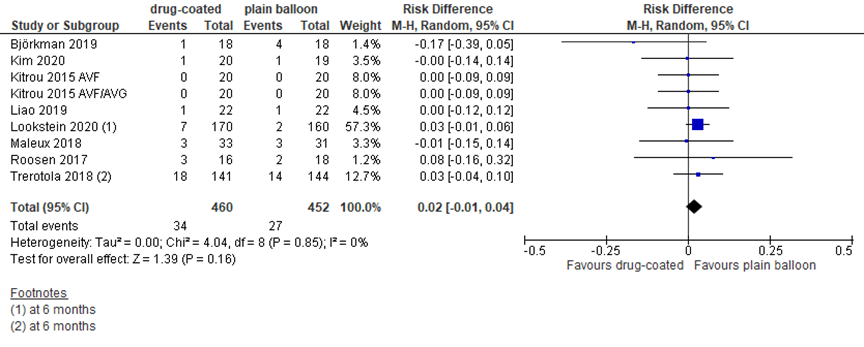
All-cause mortality (risk difference); DCB: drug-coated balloon; random effects model; df: degrees of freedom; I2: statistical heterogeneity; CI: confidence interval; Z: p-value of pooled effect
Level of evidence of the literature
The level of evidence for the outcome mortality was based on randomized studies and therefore starts at high. The quality of evidence was downgraded by 2 levels to low because of conflicting results (inconsistency) and limited number of included patients (imprecision).
PICO 2 Covered stent
Description of studies
The meta-analysis by Hu (2018), compared covered stent placement with balloon angioplasty for hemodialysis access failure. Outcomes of interest were primary patency of the treatment area and primary patency of the access circuit of hemodialysis access. The meta-analysis included four RCTs with 762 patients in total. Studies had a follow-up of 6 to 24 months. Risk of bias of the included studies was considered low, although blinding of participants and care givers was not always performed. Study characteristics are outlined in table 2.
In addition to the meta-analysis, the current analysis included three RCTs. The study by Kavan (2019) compared covered stent, stent and balloon angioplasty. It was therefore included in both the second and third research question. In this Czech study, 60 subjects were randomized in three study groups for treatment of the restenosis in the venous anastomosis or outflow vein of prosthetic AVG. Inclusion criteria were i) Age above 18 years; ii) AVG located in the upper extremity; iii) restenosis in the venous anastomosis or adjacent segment of the outflow vein up to the axilla; iv) at least 2 previous PTAs during the previous year; v) last PTA of the stenosis < 3 months and vi) referral for angiography due to malfunction of the fistula (low flow rate, elevated venous pressure during dialysis, increased intradialytic recirculation). With a follow-up of two years, the study reported primary and secondary patency of the target lesion, as well as the number of interventions to keep the access patent. The study had a high risk of bias because loss to follow-up was high (10%) and reasons were not specified. In addition, care providers were not blinded, which might affect reintervention decision.
The multicenter REVISE trial by Mohr (2019) compared covered stent placement with angioplasty in patients with dysfunctional and thrombosed hemodialysis access graft circuits and was a follow-up to the study by Vesely (2016). Patients were eligible for enrollment if they had a prosthetic AVG older than 30 days that was dysfunctional or thrombosed with a primary lesion having > 50% stenosis and was ≤ 30 mm from the venous anastomosis. In a total of 269 patients, the American study reported the number of interventions to keep the target lesion patent, the number of interventions to keep the access circuit patent, and mortality with a follow-up of 24 months. The study had a high risk of bias because care providers were not blinded, which might affect reintervention decision.
Yang (2018) evaluated the efficacy and durability of covered stent placement after balloon angioplasty in comparison to balloon angioplasty alone for the treatment of graft outflow stenosis in hemodialysis patients. In this Taiwanese RCT with a follow-up of 6 months, target lesion primary patency was reported in 98 patients with clinically significant dialysis graft outflow stenosis treated in the vascular surgery section of a tertiary medical center. Patients with end-stage renal disease undergoing regular dialysis treatment with expanded polytetrafluoroethylene (ePTFE) grafts were included in the study if they met the following criteria: age between 18 and 90 years with full functionality and the ability to comply with the follow-up protocol; ability to understand and to provide informed consent; ability to present for follow-up in an outpatient clinic three consecutive times for worsening clinical or physiologic parameters; ePTFE graft outflow stenosis > 50% on preintervention conventional angiography. The study had a high risk of bias because loss to follow-up was high (10%) and reasons were not specified. Furthermore, care providers were not blinded, which might affect reintervention decision.
Table 2 Study characteristics for covered stent placement versus balloon angioplasty
|
Publication |
Covered stent |
Number of patients (covered stent/angioplasty) |
Age (years) |
Vascular access |
Follow-up (months) |
|
Analysed by Hu, 2018 |
|||||
|
Haskal, 2010 |
Flair |
95/90 |
61.8±14.6/ 59.8±13.6 |
AVG |
6 |
|
Haskal, 2016 |
Flair |
132/138 |
63.2±13.2/ 63.1±12.3 |
AVG |
24 |
|
Rajan, 2015 |
Viabahn |
9/5 |
61 |
AVF |
12 |
|
Vesely, 2016 |
Viabahn |
145/148 |
62±13/ 61±15 |
AVG |
6 |
|
Additional RCTs |
|||||
|
Kavan, 2019 |
Fluency Plus |
20/20 |
65±13/ 61±17 |
AVG |
12 |
|
Mohr, 2019 (follow-up to Vesely, 2016) |
Viabahn |
131/138 |
61.9 ± 14.0 |
AVG |
24 |
|
Yang, 2018 |
Viabahn |
49/49 |
65.7±10.9/ 63.4±14.6 |
AVG |
6 |
Results
Access circuit function: primary patency (critical outcome)
Three studies reported primary patency of the access circuit at 6 months (figure 5). With 145 events (loss of primary patency) in 246 patients (59%) after covered stent placement versus 180 events in 239 patients (75%), the pooled RR was 0.78 (95% CI 0.66 to 0.92) in favor of covered stent placement.
Figure 5 Access circuit primary patency at 6 months after covered stent placement versus angioplasty
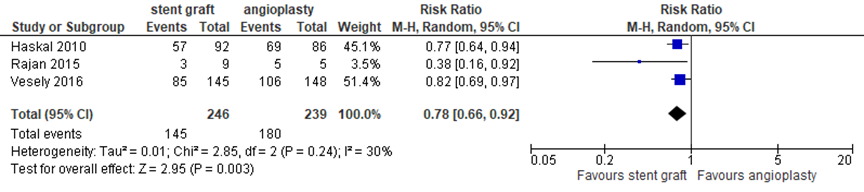
Random effects model; df: degrees of freedom; I2: statistical heterogeneity; CI: confidence interval; Z: p-value of pooled effect
In addition, 2 studies reported access circuit primary patency at 12 months. Rajan (2015) reported 22% patency in the covered stent group (N=9) versus 0% in the angioplasty group (N=5), corresponding to a RR of 0.82 (95% CI 0.53 to 1.26). Haskal (2016) reported 24% circuit primary patency after covered stent placement (N=138) versus 11% after angioplasty (N=132) at 12 months, which corresponds to a RR of 0.86 (95% CI 0.77 to 0.96). At 24 months, this study reported 9.5% access circuit primary patency in the covered stent group versus 5.5% in the control group, corresponding to a RR of 0.96 (95% CI 0.89 to 1.02).
Access circuit function: secondary patency (critical outcome)
Only one study reported vascular access secondary patency. Kavan (2019) reported 89% versus 94% vascular access secondary patency at 12 months, and 79% versus 94% at 24 months in covered stent and angioplasty groups (both N=20), respectively. The differences were not clinically relevant or statistically significant (P=0.58 in survival analysis of covered stent versus bare metal stent versus angioplasty).
Access circuit function: number of interventions to maintain access patency (critical outcome)
Few studies reported the number of interventions to maintain the target lesion or access circuit patency. Three studies reported the number of interventions to keep the access circuit patent, as outlined in figure 6. Taken together, the studies found a pooled mean difference of -1.38 (95% CI -1.56 to -1.19 in favor of covered stent placement (N=289) compared to angioplasty (N=290) at 24 months. This difference was statistically significant but not clinically relevant. In addition, Haskal (2016) reported the number of interventions to maintain access circuit patency at other time points and found 1.0 ± 1.29 versus 1.3 ± 1.24 at 6 months and 1.9 ± 2.18 versus 2.4 ± 2.31 at 12 months after covered stent (N=138) versus angioplasty (N=132), respectively. The mean difference (95% CI) at these time points was -0.30 (-0.60 to 0.00) and -0.50 (-1.04 to 0.04), these differences were not clinically relevant.
Figure 6 Number of interventions to maintain access circuit patency at 24 months after covered stent placement versus angioplasty
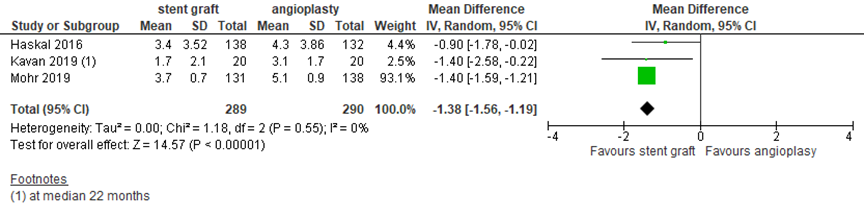
Random effects model; df: degrees of freedom; I2: statistical heterogeneity; CI: confidence interval; Z: p-value of pooled effect
Level of evidence of the literature
The level of evidence for all aspects of the outcome access circuit function was based on randomized studies and therefore starts at high. For the number of interventions to maintain access circuit patency, the level of evidence was downgraded by 1 level to moderate because of the limited number of included patients (imprecision, -1). For access circuit primary patency and secondary patency, the level of evidence was downgraded by 2 levels to low because of the limited number of included patients and because the CI crossed the clinical decision threshold (both imprecision, -2).
AVF/AVG function: target lesion primary patency
Six studies reported target lesion primary patency at different time points. At 6 months, 4 studies with in total 582 patients reported (loss of) primary patency, as depicted in figure 7. With 123 events (loss of primary patency) in 294 patients (42%) after covered stent placement versus 203 events in 288 patients (70%) after angioplasty, the pooled RR was 0.50 in favor of covered stent placement (95% CI 0.32 to 0.80). Four studies reported target lesion primary patency at 12 months. With 111 events in 216 patients (51%) with covered stents versus 169 events in 206 patients (82%) with angioplasty, the pooled RR was 0.61 (95% CI 0.49 to 0.76) in favor of treatment with a covered stent. Only two studies reported target lesion primary patency at 24 months. With 114 events in 158 patients (72%) in the covered stent groups versus 134 events in 152 patients (88%) in the control groups, the pooled RR was 0.78 (95% CI 0.62 to 0.98) in favor of covered stents. The pooled differences at the separate time points were clinically relevant and statistically significant.
Figure 7 Target lesion primary patency at 6 to 24 months after covered stent placement versus angioplasty
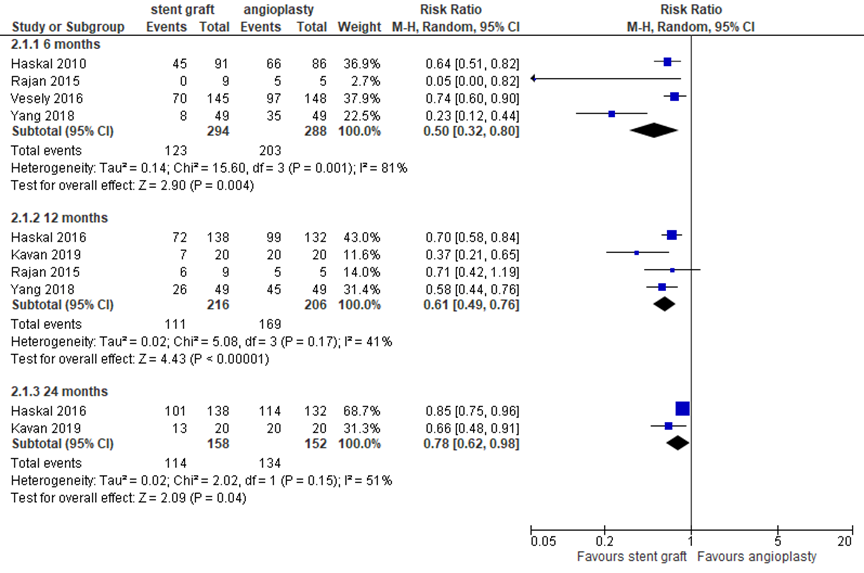
Random effects model; df: degrees of freedom; I2: statistical heterogeneity; CI: confidence interval; Z: p-value of pooled effect
AVF/AVG function: number of interventions to maintain access patency
One study reported the number of interventions to maintain target lesion patency. Mohr (2019) reported 2.7 ± 0.5 (N=131) interventions at 24 months in the covered stent group versus 3.7 ± 0.7 (N=138; P=0.007) in the angioplasty group, a mean difference of -1.00 (95% CI -1.14 to -0.86), which is statistically significant but not clinically relevant.
Level of evidence of the literature
The level of evidence for all aspects of the outcome AVF/AVG function was based on randomized studies and therefore starts at high. For target lesion primary patency at 6 and 12 months, the level of evidence was downgraded by 1 level to moderate because of the limited number of included patients (imprecision, -1).
For target lesion primary patency at 24 months, and the number of interventions to maintain target lesion patency, the level of evidence was downgraded by 2 levels to low because of the limited number of included patients and because the CI crossed the clinical decision threshold (both imprecision, -2).
Mortality
Several studies reported mortality within the study follow-up, as presented in figure 8. Two studies reported mortality at 24 months, one study at 12 months and one study at 6 months. Four studies with in total 738 patients reported 64 events in 367 patients (17%) after bare metal placement versus 60 events in 371 patients (16%), resulting in a pooled RR of 1.10 (95% CI 0.81 to 1.50) in favor of angioplasty, which is a clinically relevant difference, but statistically not significant.
Figure 8 Mortality at study endpoint after covered stent placement versus angioplasty
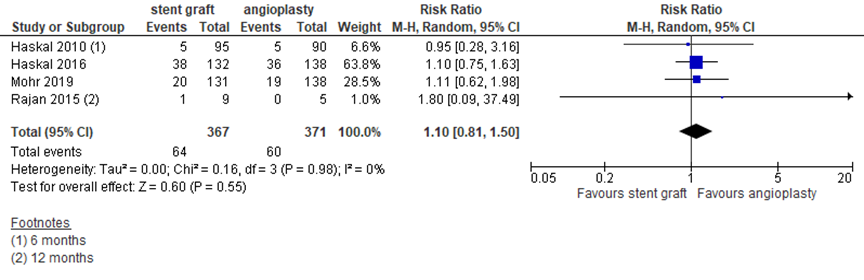
Random effects model; df: degrees of freedom; I2: statistical heterogeneity; CI: confidence interval; Z: p-value of pooled effect. Follow-up 24 months, unless indicated otherwise. If two studies described the same study population, only the most recent study was reported
Level of evidence of the literature
The level of evidence for the outcome mortality was based on randomized studies and therefore starts at high. The quality of evidence was downgraded by 2 levels to low because of the limited number of included patients and because the CI crossed the clinical decision threshold (both imprecision).
Quality of life
The outcome quality of life could not be determined as it was not reported in the included literature.
PICO 3 Bare metal stent
Description of studies
The systematic review and meta-analysis by Fu (2014), analysed clinical studies that addressed stent placement and angioplasty in dialysis access. Inclusion criteria were a measure of primary patency, secondary patency, or access dysfunction. It was decided, a priori, to exclude any of the following categories of articles: wrong topic, reviews, pediatric studies, and non-English language studies. The meta-analysis included 10 studies. To obtain the highest level of evidence, the current analysis only included the 3 randomized trials about non-covered stents reported in the review (one RCT about covered stents was already included in research question 2), i.e. Hoffer (1997), Beathard (1992) and Quin (1995). Fu (2014) reported 6-month patency rates. Due to the limited information provided in the meta-analysis, missing information was retrieved from the original publications. Main study characteristics are outlined in table 3. Fu (2014) only assessed risk of bias based on study design. The original publication had a risk of bias due to heterogeneity in the interventions in 2 out of 3 studies.
In addition to the meta-analysis, the current analysis included the study by Kavan (2019), as described for the second research question.
Table 3 Study characteristics for stent placement versus balloon angioplasty
|
Publication |
Stent |
Number of patients |
Age (stent/ angioplasty) |
Vascular access |
Follow-up (months) |
|
Analysed by Fu, 2014 |
|||||
|
Hoffer, 1997 |
Wallstent |
37 |
50.9±19.0 / 54.5±21.7 |
AVG |
12 |
|
Beathard, 1992 |
Gianturco |
58 |
Not specified |
AVG |
12 |
|
Quin, 1995 |
Gianturco, Palmaz, Wallstent |
87 |
58 (26 - 84) |
86 AVG, 1 AVF |
12 |
|
Additional RCT |
|||||
|
Kavan, 2019 |
E‑Luminexx |
39 |
68±11 / 61±17 |
AVG |
12 |
Results
Access circuit function (critical outcome)
The outcomes access circuit primary patency, secondary patency and number of interventions to keep the access circuit patent could not be determined as they were not reported in the included literature.
AVF/AVG function: target lesion primary patency
All included studies reported target lesion primary patency, as presented in figure 9. At 2 months follow-up, 28 events in 77 patients (36%) in the stent group versus 29 events in 93 patients (31%) in the angioplasty group resulted in a pooled RR of 1.24 (95% CI 0.85 to 1.82) in favor of angioplasty. At 6 months follow-up, 48 events in 77 patients (62%) after stent placement versus 57 events in 93 patients (61%) resulted in a RR of 1.09 (95% CI 0.91 to 1.32) in favor of angioplasty. At 12 months follow-up, with 84 events in 96 patients (88%) with a stent versus 100 events in 113 patients (88%) in the control group, the RR was 0.99 (95% CI 0.90 to 1.08) in favor of stent placement. The differences were not clinically relevant or statistically significant.
Figure 9 Target lesion primary patency at 2 to 12 months after stent placement versus angioplasty
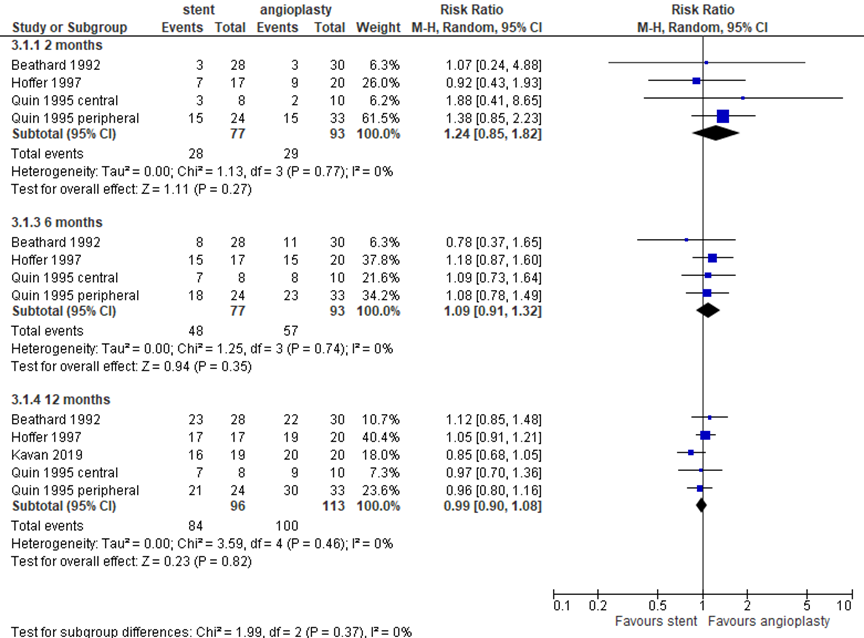
Events are loss of primary patency. Random effects model; df: degrees of freedom; I2: statistical heterogeneity; CI: confidence interval; Z: p-value of pooled effect. Quin (1995) presented data based on lesion location
AVF/AVG function: target lesion secondary patency
Two studies reported the outcome target lesion secondary patency. At 2 months follow-up, secondary patency was 81% in the stent group versus 100% in the angioplasty group in the study by Hoffer (1997). Quin (1995) reported data based on lesion location and found 73% secondary patency after stent placement versus 94% after angioplasty for peripheral lesions, and 100% in both groups for central lesions. At 6 months follow-up, Hoffer (1997) reported 69% secondary patency versus 81%, Quin (1995) reported 64% versus 80% for peripheral lesions and 89% versus 100% for central lesions, after stent placement versus angioplasty, respectively. At 12 months follow-up, for stent versus angioplasty groups Hoffer (1997) reported 60% versus 47%, and Quin (1995) reported 64% versus 71% for peripheral lesions and 78% versus 100% for central lesions, respectively.
AVF/AVG function: number of interventions to keep access patent (critical)
The outcome number of interventions to keep access patent could not be determined as it was not reported in the included literature.
Level of evidence of the literature
The level of evidence for all aspects of the outcome function was based on randomized studies and therefore starts at high. For target lesion primary patency and target lesion secondary patency, the quality of evidence was downgraded by 3 levels to very low due to limitations in study design (risk of bias, -1), because of the limited number of included patients and because the CI crossed the clinical decision threshold (both imprecision, -2).
For the number of interventions to keep the access patent, the level of evidence could not be determined due to a lack of data.
Mortality
Two studies reported all-cause mortality within the study follow-up. Hoffer (1997) found 2/28 (7%) mortality after stent placement versus 1/30 (3%) after angioplasty, whereas Beathard (1992) reported 0/17 (0%) versus 1/20 (5%), respectively.
Level of evidence of the literature
The level of evidence for the outcome mortality was based on randomized studies and therefore starts at high. The quality of evidence was downgraded by 3 levels to very low due to limitations in study design (risk of bias), because of conflicting results (inconsistency) and because of limited number of included patients (imprecision).
Quality of life
The outcome quality of life could not be determined as it was not reported in the included literature.
Zoeken en selecteren
A systematic review of the literature was performed to answer the following questions:
1. Drug-coated balloon
P: patients receiving an endovascular intervention for vascular access flow dysfunction;
I: drug-coated balloon (DCB) angioplasty;
C: plain balloon angioplasty;
O: health-related quality of life, vascular access function, mortality.
2. Covered stent
P: patients receiving an endovascular intervention for vascular access flow dysfunction;
I: covered stent placement;
C: plain balloon angioplasty;
O: health-related quality of life, vascular access function, mortality.
3. Bare metal stent
P: patients receiving an endovascular intervention for vascular access flow dysfunction;
I: bare metal stent (BMS) placement;
C: plain balloon angioplasty;
O: health-related quality of life, vascular access function, mortality.
Relevant outcomes
The guideline development group considered health-related quality of life and vascular access function (i.e. access-related intervention rates and vascular access patency) as critical outcomes for decision making; and and mortality as important outcomes for decision making.
The working group defined the outcome vascular access function a priori as access circuit primary and secondary patency, and the number of interventions to maintain access circuit patency. Target lesion primary patency and the number of interventions to maintain target lesion patency were considered to be of secondary importance from the patient’s perspective. Primary patency was defined as the interval between the index intervention and the first re-intervention for vascular access dysfunction or thrombosis, or the time of its abandonment (i.e. intervention-free vascular access survival). Secondary patency was defined as the interval between the index intervention and the abandonment of the vascular access. Mortality was defined as all-cause mortality at any time point within the study follow-up. The working group did not define the outcome health-related quality of life a priori but used the definitions used in the studies.
As minimal clinically (patient) important differences, the working group used 5% for mortality (risk ratio, RR), 25% for other RR, 0.8 interventions per patient per year for the number of interventions to maintain access circuit or target lesion patency, 10% of the maximum score for continuous outcomes (quality of life scales) and 0.5 for standardized mean differences.
Search and select (Methods)
The databases Medline (via OVID) and Embase (via Embase.com) were searched with relevant search terms up to and including September 7, 2020. All 3 PICOs were covered with one search. The detailed search strategy is depicted under the tab Methods. The systematic literature search resulted in 190 hits. Studies were selected based on the following criteria: (meta-analysis of) randomized controlled trials comparing one of the interventions with plain balloon angioplasty in patients with an arteriovenous fistula or graft for hemodialysis. Based on title and abstract screening, 40 studies were initially selected. After reading the full text, 30 studies were excluded (see the table with reasons for exclusion under the tab Methods) and 10 were included.
Results
For analysis of the literature for the first PICO (DCB), one systematic review and four RCTs were included. For the second PICO (covered stent), one systematic review and two RCTs were included and for the third PICO (BMS), one systematic review was included. In addition, one RCT was included for both the second and third PICO. Important study characteristics and results are summarized in the evidence tables. The assessment of the risk of bias is summarized in the risk of bias tables.
Referenties
- Beathard GA. Gianturco self-expanding stent in the treatment of stenosis in dialysis access grafts. Kidney Int. 1993 Apr;43(4):872-7. doi: 10.1038/ki.1993.122. PMID: 8479123.
- Chen X, Liu Y, Wang J, Zhao J, Singh N, Zhang WW. A systematic review and meta-analysis of the risk of death and patency after application of paclitaxel-coated balloons in the hemodialysis access. J Vasc Surg. 2020 Dec;72(6):2186-2196.e3. doi: 10.1016/j.jversus2020.04.525. Epub 2020 Jun 12. PMID: 32540324.
- Fu N, Joachim E, Yevzlin AS, Shin JI, Astor BC, Chan MR. A Meta-analysis of Stent Placement versus Angioplasty for Dialysis Vascular Access Stenosis. Semin Dial. 2015 May-Jun;28(3):311-7. doi: 10.1111/sdi.12314. Epub 2014 Oct 9. PMID: 25303220.
- Gallieni M, Hollenbeck M, Inston N, Kumwenda M, Powell S, Tordoir J, Al Shakarchi J, Berger P, Bolignano D, Cassidy D, Chan TY, Dhondt A, Drechsler C, Ecder T, Finocchiaro P, Haller M, Hanko J, Heye S, Ibeas J, Jemcov T, Kershaw S, Khawaja A, Labriola L, Lomonte C, Malovrh M, Marti I Monros A, Matthew S, McGrogan D, Meyer T, Mikros S, Nistor I, Planken N, Roca-Tey R, Ross R, Troxler M, van der Veer S, Vanholder R, Vermassen F, Welander G, Wilmink T, Koobasi M, Fox J, Van Biesen W, Nagler E. Clinical practice guideline on peri- and postoperative care of arteriovenous fistulas and grafts for haemodialysis in adults. Nephrol Dial Transplant. 2020 Dec 4;35(12):2203. doi: 10.1093/ndt/gfaa106. Erratum for: Nephrol Dial Transplant. 2019 Jun 1;34(Suppl 2):ii1-ii42. PMID: 32365363.
- Hoffer EK, Sultan S, Herskowitz MM, Daniels ID, Sclafani SJ. Prospective randomized trial of a metallic intravascular stent in hemodialysis graft maintenance. J Vasc Interv Radiol. 1997 Nov-Dec;8(6):965-73. doi: 10.1016/s1051-0443(97)70695-x. PMID: 9399465.
- Hu H, Wu Z, Zhao J, Wang J, Huang B, Yang Y, Xiong F. Stent graft placement versus angioplasty for hemodialysis access failure: a meta-analysis. J Surg Res. 2018 Jun;226:82-88. doi: 10.1016/j.jss.2018.01.030. Epub 2018 Feb 22. PMID: 29661293.
- Kim JW, Kim JH, Byun SS, Kang JM, Shin JH. Paclitaxel-Coated Balloon versus Plain Balloon Angioplasty for Dysfunctional Autogenous Radiocephalic Arteriovenous Fistulas: A Prospective Randomized Controlled Trial. Korean J Radiol. 2020 Nov;21(11):1239-1247. doi: 10.3348/kjr.2020.0067. Epub 2020 Jul 27. PMID: 32729275; PMCID: PMC7462765.
- Katsanos K, Spiliopoulos S, Kitrou P, Krokidis M, Karnabatidis D. Risk of Death Following Application of Paclitaxel-Coated Balloons and Stents in the Femoropopliteal Artery of the Leg: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J Am Heart Assoc. 2018 Dec 18;7(24):e011245. doi: 10.1161/JAHA.118.011245. PMID: 30561254; PMCID: PMC6405619.
- Kavan J, Kudlicka J, Malik J, Chytilova E, Lambert L, Slavikova M, Matras P, Burgetova A. Treatment of failing arterio-venous dialysis graft by angioplasty, stent, and stent graft: Two-years analysis of patency rates and cost-effectiveness. Exp Ther Med. 2019 Nov;18(5):4144-4150. doi: 10.3892/etm.2019.8050. Epub 2019 Sep 25. PMID: 31641387; PMCID: PMC6796405.
- Liao MT, Lee CP, Lin TT, Jong CB, Chen TY, Lin L, Hsieh MY, Lin MS, Chie WC, Wu CC. A randomized controlled trial of drug-coated balloon angioplasty in venous anastomotic stenosis of dialysis arteriovenous grafts. J Vasc Surg. 2020 Jun;71(6):1994-2003. doi: 10.1016/j.jversus2019.07.090. Epub 2019 Oct 11. PMID: 31611105.
- Lok CE, Huber TS, Lee T, et al; KDOQI Vascular Access Guideline Work Group. KDOQI clinical practice guideline for vascular access: 2019 update. Am J Kidney Dis 2020;75:S1-S164.
- Lookstein RA, Haruguchi H, Ouriel K, Weinberg I, Lei L, Cihlar S, Holden A; IN.PACT AV Access Investigators. Drug-Coated Balloons for Dysfunctional Dialysis Arteriovenous Fistulas. N Engl J Med. 2020 Aug 20;383(8):733-742. doi: 10.1056/NEJMoa1914617. PMID: 32813949.
- Mohr BA, Sheen AL, Roy-Chaudhury P, Schultz SR, Aruny JE; REVISE Investigators. Clinical and Economic Benefits of Stent Grafts in Dysfunctional and Thrombosed Hemodialysis Access Graft Circuits in the REVISE Randomized Trial. J Vasc Interv Radiol. 2019 Feb;30(2):203-211.e4. doi: 10.1016/j.jvir.2018.12.006. PMID: 30717951.
- Nordanstig J, James S, Andersson M, Andersson M, Danielsson P, Gillgren P, Delle M, Engström J, Fransson T, Hamoud M, Hilbertson A, Johansson P, Karlsson L, Kragsterman B, Lindgren H, Ludwigs K, Mellander S, Nyman N, Renlund H, Sigvant B, Skoog P, Starck J, Tegler G, Toivola A, Truedson M, Wahlgren CM, Wallinder J, Öjersjö A, Falkenberg M. Mortality with Paclitaxel-Coated Devices in Peripheral Artery Disease. N Engl J Med. 2020 Dec 24;383(26):2538-2546. doi: 10.1056/NEJMoa2005206. Epub 2020 Dec 9. PMID: 33296560.
- Rocha-Singh KJ, Duval S, Jaff MR, Schneider PA, Ansel GM, Lyden SP, Mullin CM, Ioannidis JPA, Misra S, Tzafriri AR, Edelman ER, Granada JF, White CJ, Beckman JA; VIVA Physicians, Inc. Mortality and Paclitaxel-Coated Devices: An Individual Patient Data Meta-Analysis. Circulation. 2020 Jun 9;141(23):1859-1869. doi: 10.1161/CIRCULATIONAHA.119.044697. Epub 2020 May 6. PMID: 32370548; PMCID: PMC8029645.
- Quinn SF, Schuman ES, Demlow TA, Standage BA, Ragsdale JW, Green GS, Sheley RC. Percutaneous transluminal angioplasty versus endovascular stent placement in the treatment of venous stenoses in patients undergoing hemodialysis: intermediate results. J Vasc Interv Radiol. 1995 Nov-Dec;6(6):851-5. doi: 10.1016/s1051-0443(95)71200-3. PMID: 8850659.
- Schmidli J, Widmer M. Vascular Access: 2018 clinical practical guidelines of the European Society for Vascular Surgery. Eur J Vasc Endovasc Surg 2018;55:757-818.
- Trerotola SO, Saad TF, Roy-Chaudhury P; Lutonix AV Clinical Trial Investigators. The Lutonix AV Randomized Trial of Paclitaxel-Coated Balloons in Arteriovenous Fistula Stenosis: 2-Year Results and Subgroup Analysis. J Vasc Interv Radiol. 2020 Jan;31(1):1-14.e5. doi: 10.1016/j.jvir.2019.08.035. Epub 2019 Nov 6. PMID: 31706886.
- Yang HT, Yu SY, Su TW, Kao TC, Hsieh HC, Ko PJ. A prospective randomized study of stent graft placement after balloon angioplasty versus balloon angioplasty alone for the treatment of hemodialysis patients with prosthetic graft outflow stenosis. J Vasc Surg. 2018 Aug;68(2):546-553. doi: 10.1016/j.jversus2017.12.062. Epub 2018 Apr 2. PMID: 29622355.
Evidence tabellen
Evidence table for systematic review of RCTs and observational studies (intervention studies)
Research question 1: What are the (un)favorable effects of drug-coated balloon angioplasty compared to plain balloon angioplasty in patients with a dysfunctional vascular access for hemodialysis?
|
Study reference |
Study characteristics |
Patient characteristics |
Intervention (I) |
Comparison / control (C)
|
Follow-up |
Outcome measures and effect size |
Comments |
|
Chen, 2020 |
SR and meta-analysis of RCTs and cohort studies.
Literature search up to April 2019
A: Bjorkman, 2019 B: Trerotola, 2018 C: Swinnen, 2018 D: Maleux, 2018 E: Roosen, 2017 F: Kitrou, 2015 A G: Kitrou, 2015 B H: Lai, 2014 I: Irani, 2018 J: Katsanos, 2012 (Teo, 2013 was not included because it was only an abstract)
Study design: SR included RCTs and cohort studies in AVF, AVG and CVS. In current analysis, only RCTs in AVF and AVG were included, conform the PICO.
Setting and Country: West China Hospital of Sichuan University, Chengdu, Sichuana; and University of Washington and Puget Sound VA Health Care System, Seattle.
Source of funding and conflicts of interest: No funding. Authors declare no conflict of interest.
|
Inclusion criteria SR: All retrospective, prospective, and observational studies investigating PCB or PES versus plain balloon angioplasty (PBA) in the HD access (arteriovenous fistula (AVF), arteriovenous graft (AVG), or CVS); population of patients with ESRD needing long-term HD; clinical follow-up of at least 6 months available; and studies having sufficient data to pool the effect estimate.
Exclusion criteria SR: Review articles, case reports, and editorial comments; studies with duplicate data; comparative studies that compared target lesion interventions with previous interventions on the same target lesion as a control; studies with a sample size of fewer than 10 patients.
16 studies included (10 used for current analysis)
Important patient characteristics at baseline: N, mean age A: 36, 67y B: 285, 62±14y C: 128, 64.9±13.7y D: 64, 68.1±15.9y E: 34, 81.6y F: 40, 61±14.6 G: 40, 64±14.3 H: 20, 67.2±9.4 I: 119, 59.2±10.3 J: 40, 64.1±14.3
Target lesion: A: AVF B: AVF C: AVF D: AVF E: AVF F: AVF G: AVF, AVG H: AVF I: AVF, AVG J: AVF, AVG
Lesion type: A: Primary/ recurrent B: Primary/ recurrent C: Recurrent D: Primary/ recurrent E: Recurrent F: Not specified G: Primary/ recurrent H: Primary/ recurrent I: Primary/ recurrent J: Primary/ recurrent |
Paclitaxel-coated balloon angioplasty A: IN.PACT Admiral (Medtronic), 3 mg/mm2 paclitaxel B: Lutonix (Bard), 2 mg/mm2 paclitaxel C: IN.PACT Admiral D: IN.PACT Admiral E: IN.PACT Admiral F: IN.PACT Admiral G: IN.PACT Admiral H: SeQuent Please (B. Braun), paclitaxel dose not specified I: IN.PACT Admiral J: IN.PACT Admiral
|
Conventional balloon angioplasty Not specified |
End-point of follow-up (months): A: 12 B: 6 C: 12 D: 12 E: 12 F: 12 G: 12 H: 12 I: 12 J: 6
For how many participants were no complete outcome data available? Not specified
|
Function (critical): Target lesion primary patency (Event: loss of primary patency) 6 months A: - B: I 37/141; C 57/144 C: I 9/68; C 24/60 D: I 11/33; C 11/31 E: - F: I 9/20; C 18/20 G: I 8/20; C 13/20 H: I 4/10; C 10/10 I: I 9/59: C 21/60 J: same study population as Kitrou 2015 Pooled effect (random effects model): RR 0.56 (95% CI 0.45 to 0.70) favoring drug-coated balloons. Heterogeneity (I2): 11%
12 months A: I 16/18; C 4/18 B: - C: I 24/68; C 33/60 D: I 19/33; C 19/31 E: - F: I 13/20; C 19/20 G: I 15/20; C 17/20 H: - I: I 29/59; C 32/60 J: - Pooled effect (random effects model): RR 0.91 (95% CI 0.68 to 1.23) favoring drug-coated balloons. Heterogeneity (I2): 70%
Access circuit primary patency HR (95% CI) at 6/12 months A: - B: 0.70 (0.44 to 1.11) at 6m C: - D: - E: - F: 0.48 (0.23 to 0.97) at 12m G: - H: - I: 0.699 (0.442 to 1.107) at 12m J: 0.32 (0.14 to 0.75) at 6m
Events at 6 months A: - B: events I 49/141 (35%); C 59/144 (41%) C: - D: - E: - F: I 63%; C 32% G: - H: - I: I 14/59; C 26/60 J: same study population as Kitrou 2015 Pooled effect (random effects model): RR 0.61 (95% CI 0.38 to 0.98) favoring drug-coated balloons. Heterogeneity (I2): 60%
Events at 12 months A: - B: - C: - D: - E: - F: I 15%; C 0%; 12 months G: - H: - I: I 32/59; C 41/60 at 12m J: - Pooled effect (random effects model): RR 0.83 (95% CI 0.71 to 0.98) favoring drug-coated balloons. Heterogeneity (I2): 0%
Number of interventions to keep access patent Not reported in review, data retrieved from original papers A: - B: I: 0.31; C: 0.44 per patient at 6 months; P=0.03 C: - D: - E: - F: - G: - H: - I: J: -
Secondary lesion/circuit patency Not reported in review, nor in original papers
Mortality At 12 months, unless stated otherwise A: I 1/18; C 4/18 B: I 18/141; C 14/144 (6 months) C: - D: I 3/33; C 3/31 E: I 3/16; C 2/18 F: - G: - H: - I: I 1/59: C 1/60 J: - Pooled effect (random effects model): RR 1.19 (95% CI 0.69 to 2.04) favoring conventional balloons. Heterogeneity (I2): 0%
Quality of Life Not reported |
Authors’ conclusions This meta-analysis showed a significant improvement of short-term and midterm primary patency of HD access after treatment with PCB angioplasty. There was a trend suggesting that PCB angioplasty might increase the risk of 24- month mortality compared with PBA.
Risk of bias was considered low. Blinding of care providers was not performed or feasible in most studies, but risk of bias was considered unlikely. |
Evidence table for intervention studies (randomized controlled trials and non-randomized observational studies (cohort studies, case-control studies, case series))1
This table is also suitable for diagnostic studies (screening studies) that compare the effectiveness of two or more tests. This only applies if the test is included as part of a test-and-treat strategy - otherwise the evidence table for studies of diagnostic test accuracy should be used.
Research question 1: What are the (un)favorable effects of drug-coated balloon angioplasty compared to plain balloon angioplasty in patients with a dysfunctional vascular access for hemodialysis?
Notes:
- Prognostic balance between treatment groups is usually guaranteed in randomized studies, but non-randomized (observational) studies require matching of patients between treatment groups (case-control studies) or multivariate adjustment for prognostic factors (confounders) (cohort studies); the evidence table should contain sufficient details on these procedures.
- Provide data per treatment group on the most important prognostic factors ((potential) confounders).
- For case-control studies, provide sufficient detail on the procedure used to match cases and controls.
- For cohort studies, provide sufficient detail on the (multivariate) analyses used to adjust for (potential) confounders.
Table of quality assessment for systematic reviews of RCTs and observational studies
Based on AMSTAR checklist (Shea, 2007; BMC Methodol 7: 10; doi:10.1186/1471-2288-7-10) and PRISMA checklist (Moher, 2009; PLoS Med 6: e1000097; doi:10.1371/journal.pmed1000097)
|
Study
First author, year |
Appropriate and clearly focused question?1
Yes/no/unclear |
Comprehensive and systematic literature search?2
Yes/no/unclear |
Description of included and excluded studies?3
Yes/no/unclear |
Description of relevant characteristics of included studies?4
Yes/no/unclear |
Appropriate adjustment for potential confounders in observational studies?5
Yes/no/unclear/notapplicable |
Assessment of scientific quality of included studies?6
Yes/no/unclear |
Enough similarities between studies to make combining them reasonable?7
Yes/no/unclear |
Potential risk of publication bias taken into account?8
Yes/no/unclear |
Potential conflicts of interest reported?9
Yes/no/unclear |
|
Chen, 2020 |
Yes |
Yes |
No Reasons for exclusion are indicated, but not with references |
Yes |
NA |
Yes |
Yes |
Yes, publication bias not suspected. |
Unclear, conflict of interest stated for SR, but not for individual studies. |
- Research question (PICO) and inclusion criteria should be appropriate and predefined.
- Search period and strategy should be described; at least Medline searched; for pharmacological questions at least Medline + EMBASE searched.
- Potentially relevant studies that are excluded at final selection (after reading the full text) should be referenced with reasons.
- Characteristics of individual studies relevant to research question (PICO), including potential confounders, should be reported.
- Results should be adequately controlled for potential confounders by multivariate analysis (not applicable for RCTs).
- Quality of individual studies should be assessed using a quality scoring tool or checklist (Jadad score, Newcastle-Ottawa scale, risk of bias table et cetera).
- Clinical and statistical heterogeneity should be assessed; clinical: enough similarities in patient characteristics, intervention and definition of outcome measure to allow pooling? For pooled data: assessment of statistical heterogeneity using appropriate statistical tests (for example Chi-square, I2)?
- An assessment of publication bias should include a combination of graphical aids (for example funnel plot, other available tests) and/or statistical tests (for example Egger regression test, Hedges-Olken). Note: If no test values or funnel plot included score “no”. Score “yes” if mentions that publication bias could not be assessed because there were fewer than 10 included studies.
- Sources of support (including commercial co-authorship) should be reported in both the systematic review and the included studies. Note: To get a “yes,” source of funding or support must be indicated for the systematic review AND for each of the included studies.
Risk of bias table for intervention studies (randomized controlled trials)
Research question 1: What are the (un)favorable effects of drug-coated balloon angioplasty compared to plain balloon angioplasty in patients with a dysfunctional vascular access for hemodialysis?
- Randomisation: generation of allocation sequences have to be unpredictable, for example computer generated random-numbers or drawing lots or envelopes. Examples of inadequate procedures are generation of allocation sequences by alternation, according to case record number, date of birth or date of admission.
- Allocation concealment: refers to the protection (blinding) of the randomisation process. Concealment of allocation sequences is adequate if patients and enrolling investigators cannot foresee assignment, for example central randomisation (performed at a site remote from trial location) or sequentially numbered, sealed, opaque envelopes. Inadequate procedures are all procedures based on inadequate randomisation procedures or open allocation schedules.
- Blinding: neither the patient nor the care provider (attending physician) knows which patient is getting the special treatment. Blinding is sometimes impossible, for example when comparing surgical with non-surgical treatments. The outcome assessor records the study results. Blinding of those assessing outcomes prevents that the knowledge of patient assignement influences the proces of outcome assessment (detection or information bias). If a study has hard (objective) outcome measures, like death, blinding of outcome assessment is not necessary. If a study has “soft” (subjective) outcome measures, like the assessment of an X-ray, blinding of outcome assessment is necessary.
- Results of all predefined outcome measures should be reported; if the protocol is available, then outcomes in the protocol and published report can be compared; if not, then outcomes listed in the methods section of an article can be compared with those whose results are reported.
- If the percentage of patients lost to follow-up is large, or differs between treatment groups, or the reasons for loss to follow-up differ between treatment groups, bias is likely. If the number of patients lost to follow-up, or the reasons why, are not reported, the risk of bias is unclear.
- Participants included in the analysis are exactly those who were randomized into the trial. If the numbers randomized into each intervention group are not clearly reported, the risk of bias is unclear; an ITT analysis implies that (a) participants are kept in the intervention groups to which they were randomized, regardless of the intervention they actually received, (b) outcome data are measured on all participants, and (c) all randomized participants are included in the analysis.
Evidence table for systematic review of RCTs and observational studies (intervention studies)
Research question 2: What are the (un)favorable effects of stent graft placement compared to balloon angioplasty in patients with a dysfunctional vascular access for hemodialysis?
Evidence table for intervention studies (randomized controlled trials and non-randomized observational studies (cohort studies, case-control studies, case series))1
This table is also suitable for diagnostic studies (screening studies) that compare the effectiveness of two or more tests. This only applies if the test is included as part of a test-and-treat strategy - otherwise the evidence table for studies of diagnostic test accuracy should be used.
Research question 2: What are the (un)favorable effects of covered stent placement compared to balloon angioplasty in patients with a dysfunctional vascular access for hemodialysis?
Notes:
- Prognostic balance between treatment groups is usually guaranteed in randomized studies, but non-randomized (observational) studies require matching of patients between treatment groups (case-control studies) or multivariate adjustment for prognostic factors (confounders) (cohort studies); the evidence table should contain sufficient details on these procedures.
- Provide data per treatment group on the most important prognostic factors ((potential) confounders).
- For case-control studies, provide sufficient detail on the procedure used to match cases and controls.
- For cohort studies, provide sufficient detail on the (multivariate) analyses used to adjust for (potential) confounders.
Table of quality assessment for systematic reviews of RCTs and observational studies
Based on AMSTAR checklist (Shea, 2007; BMC Methodol 7: 10; doi:10.1186/1471-2288-7-10) and PRISMA checklist (Moher, 2009; PLoS Med 6: e1000097; doi:10.1371/journal.pmed1000097)
|
Study
First author, year |
Appropriate and clearly focused question?1
Yes/no/unclear |
Comprehensive and systematic literature search?2
Yes/no/unclear |
Description of included and excluded studies?3
Yes/no/unclear |
Description of relevant characteristics of included studies?4
Yes/no/unclear |
Appropriate adjustment for potential confounders in observational studies?5
Yes/no/unclear/notapplicable |
Assessment of scientific quality of included studies?6
Yes/no/unclear |
Enough similarities between studies to make combining them reasonable?7
Yes/no/unclear |
Potential risk of publication bias taken into account?8
Yes/no/unclear |
Potential conflicts of interest reported?9
Yes/no/unclear |
|
Hu, 2018 |
Yes |
Yes |
Yes Reasons for exclusion are indicated, but not with references |
Yes |
N/A |
Yes |
Yes |
Yes |
Unclear Not specified for included studies |
- Research question (PICO) and inclusion criteria should be appropriate and predefined.
- Search period and strategy should be described; at least Medline searched; for pharmacological questions at least Medline + EMBASE searched.
- Potentially relevant studies that are excluded at final selection (after reading the full text) should be referenced with reasons.
- Characteristics of individual studies relevant to research question (PICO), including potential confounders, should be reported.
- Results should be adequately controlled for potential confounders by multivariate analysis (not applicable for RCTs).
- Quality of individual studies should be assessed using a quality scoring tool or checklist (Jadad score, Newcastle-Ottawa scale, risk of bias table et cetera).
- Clinical and statistical heterogeneity should be assessed; clinical: enough similarities in patient characteristics, intervention and definition of outcome measure to allow pooling? For pooled data: assessment of statistical heterogeneity using appropriate statistical tests (for example Chi-square, I2)?
- An assessment of publication bias should include a combination of graphical aids (for example funnel plot, other available tests) and/or statistical tests (for example Egger regression test, Hedges-Olken). Note: If no test values or funnel plot included score “no”. Score “yes” if mentions that publication bias could not be assessed because there were fewer than 10 included studies.
- Sources of support (including commercial co-authorship) should be reported in both the systematic review and the included studies. Note: To get a “yes,” source of funding or support must be indicated for the systematic review AND for each of the included studies.
Risk of bias table for intervention studies (randomized controlled trials)
Research question 2: What are the (un)favorable effects of covered stents compared to balloon angioplasty in patients with a dysfunctional vascular access for hemodialysis?
|
Study reference
(first author, publication year) |
Describe method of randomisation1 |
Bias due to inadequate concealment of allocation?2
(unlikely/likely/unclear) |
Bias due to inadequate blinding of participants to treatment allocation?3
(unlikely/likely/unclear) |
Bias due to inadequate blinding of care providers to treatment allocation?3
(unlikely/likely/unclear) |
Bias due to inadequate blinding of outcome accessors to treatment allocation?3
(unlikely/likely/unclear) |
Bias due to selective outcome reporting on basis of the results?4
(unlikely/likely/unclear) |
Bias due to loss to follow-up?5
(unlikely/likely/unclear) |
Bias due to violation of intention to treat analysis?6
(unlikely/likely/unclear) |
|
Kavan, 2019 |
Not reported. |
Likely. Concealment of allocation was not reported. |
Unlikely. The study was not blinded, but this is not likely to affect the outcomes of interest. |
Likely. The study was not blinded. Knowledge of treatment allocation might affect reintervention decision. |
Unlikely. The study was not blinded, but this is not likely to affect outcome assessment. |
Unlikely. |
Likely. Reasons for censoring in survival analysis not specified, resulting in risk of bias. |
Likely. No intention-to-treat analysis. |
|
Mohr, 2019 |
1:1 block randomization, not further specified. |
Likely. Concealment of allocation was not reported. |
Unlikely. The study was not blinded, but this is not likely to affect the outcomes of interest. |
Likely. The study was not blinded. Knowledge of treatment allocation might affect reintervention decision. |
Unlikely. The study was not blinded, but this is not likely to affect outcome assessment. |
Unlikely. |
Unlikely. |
Likely. No intention-to-treat analysis. |
|
Yang, 2018 |
The randomization was conducted by a third party by drawing an envelope from a container of evenly mixed envelopes. |
Likely. Concealment of allocation was not reported. |
Unlikely. The study was not blinded, but this is not likely to affect the outcomes of interest. |
Likely. The study was not blinded. Knowledge of treatment allocation might affect reintervention decision. |
Unlikely. The study was not blinded, but this is not likely to affect outcome assessment. |
Unlikely. |
Likely. Loss to follow-up is high (10%) and reasons are not specified. |
Likely. No intention-to-treat analysis. |
- Randomisation: generation of allocation sequences have to be unpredictable, for example computer generated random-numbers or drawing lots or envelopes. Examples of inadequate procedures are generation of allocation sequences by alternation, according to case record number, date of birth or date of admission.
- Allocation concealment: refers to the protection (blinding) of the randomisation process. Concealment of allocation sequences is adequate if patients and enrolling investigators cannot foresee assignment, for example central randomisation (performed at a site remote from trial location) or sequentially numbered, sealed, opaque envelopes. Inadequate procedures are all procedures based on inadequate randomisation procedures or open allocation schedules.
- Blinding: neither the patient nor the care provider (attending physician) knows which patient is getting the special treatment. Blinding is sometimes impossible, for example when comparing surgical with non-surgical treatments. The outcome assessor records the study results. Blinding of those assessing outcomes prevents that the knowledge of patient assignement influences the proces of outcome assessment (detection or information bias). If a study has hard (objective) outcome measures, like death, blinding of outcome assessment is not necessary. If a study has “soft” (subjective) outcome measures, like the assessment of an X-ray, blinding of outcome assessment is necessary.
- Results of all predefined outcome measures should be reported; if the protocol is available, then outcomes in the protocol and published report can be compared; if not, then outcomes listed in the methods section of an article can be compared with those whose results are reported.
- If the percentage of patients lost to follow-up is large, or differs between treatment groups, or the reasons for loss to follow-up differ between treatment groups, bias is likely. If the number of patients lost to follow-up, or the reasons why, are not reported, the risk of bias is unclear
- Participants included in the analysis are exactly those who were randomized into the trial. If the numbers randomized into each intervention group are not clearly reported, the risk of bias is unclear; an ITT analysis implies that (a) participants are kept in the intervention groups to which they were randomized, regardless of the intervention they actually received, (b) outcome data are measured on all participants, and (c) all randomized participants are included in the analysis.
Evidence table for systematic review of RCTs and observational studies (intervention studies)
Research question: Research question: What are the (un)favorable effects of stent placement compared to balloon angioplasty in patients with a dysfunctional vascular access for hemodialysis?
|
Study reference |
Study characteristics |
Patient characteristics |
Intervention (I) |
Comparison / control (C) |
Follow-up |
Outcome measures and effect size |
Comments |
|
Fu, 2014 |
SR and meta-analysis
Literature search up to December 2013
A: Hoffer, 1997 B: Beathard, 1992 C: Quin, 1995
Study design: Experimental and observational clinical studies
Setting and Country: Single Center, USA
Source of funding and conflicts of interest: No funding reported. No conflict of interest reported by authors. |
Inclusion criteria SR: Inclusion criteria were a measure of primary patency, secondary patency, or access dysfunction.
Exclusion criteria SR: wrong topic, reviews, pediatric studies, and non-English language studies.
10 studies included in SR
3 RCTs included in current analysis
Important patient characteristics at baseline:
N, mean age (I / C) A: 37 patients, age 50.9±19.0 / 54.5±21.7 B: 58 patients, age not specified C: 87 patients, age 58 (range 26 - 84)
Sex: A: 29% / 15% male B: not specified C: 47% male
Vascular access A: AVG B: AVG C: 86 AVG, 1 AVF
Groups were comparable at baseline. |
Stent placement after balloon angioplasty
A: Wallstent (BMS) N=17 B: Gianturco (BMS) N=28 - in the first 11 cases a regular stent design with a rigid strut and no barbs was used; - in the remaining 17 cases, the rigid strut was replaced with one that was hinged in the middle so that the two elements could articulate and provide flexibility to the unit. C: Gianturco, Palmaz, Wallstent (BMS) N=40
|
Balloon angioplasty alone.
A: N=20 B: N=30 C: N=47
|
End-point of follow-up:
A: 12 months B: 12 months C: 12 months
For how many participants were no complete outcome data available? (intervention/control) A: not specified B: not specified C: not specified
|
Function (critical) Target lesion primary patency* 2 months A: I 56% (N=17); C 53% (N=20) B: I 91% (N=28); C 89% (N=30) C: peripheral lesions I 36% (N=24); C 55% (N=33) Central lesions I 67%(N=8); C 81% (N=10)^^
Pooled effect (random effects model): 1.24 (0.85, 1.82) favoring angioplasty. Heterogeneity (I2): 0%
3 months A: - B: I 85%; C 79% C: -
6 months A: I 12%; C 23% B: I 72%; C 64% C: peripheral lesions I 27%; C 31% Central lesions I 11%; C 23%
Pooled effect (random effects model): 1.09 (0.91, 1.32) favoring angioplasty. Heterogeneity (I2): 0%
12 months A: I 0%; C 7% B: I 17%; C 28% (differences not significant at any time interval, P>0.07) C: peripheral lesions I 11%; C 10%; P=0.653 (survival analysis) Central lesions I 11%; C 12% P=0.460 (survival analysis)
Pooled effect (random effects model): 1.02 (0.93, 1.13) favoring angioplasty. Heterogeneity (I2): 0%
Target lesion secondary patency 2 months A: I 81%; C 100% B: - C: peripheral lesions I 73%; C 94% Central lesions I 100%; C 100%
6 months A: I 69%; C 81% B: - C: peripheral lesions I 64%; C 80% Central lesions I 89%; C 100%
12 months A: I 60%; C 47% B: - C: peripheral lesions I 64%; C 71% P=0.168 (survival analysis); Central lesions I 78%; C 100%
Access circuit primary patency Not reported in SR nor in original papers
Access circuit secondary patency Not reported in SR nor in original papers
Number of interventions to keep access patent Not reported in SR nor in original papers
Mortality A: I 2/28; C 1/30 B: I 0/17; C 1/20 C: -
Quality of life Not reported in SR nor in original papers
* data retrieved from original papers
^^Quin reported data based on lesion location. 36 stents in 24 patients for peripheral and 14 stents in 8 patients for central lesions |
Author’s conclusion: In conclusion, our meta-analysis suggests significantly higher primary patency rates in stenotic AV access receiving stent placement versus angioplasty.
The SR had a risk of bias because quality of individual studies was not assessed using a quality scoring tool or checklist, and publication bias was not taken into account. The individual studies had a risk of bias due to heterogeneity of the interventions within the studies. |
Evidence table for systematic review of RCTs and observational studies (intervention studies)
Research question 3: What are the (un)favorable effects of stents compared to balloon angioplasty in patients with a dysfunctional vascular access for hemodialysis?
See study by Kavan (2019) under research question 2.
Table of quality assessment for systematic reviews of RCTs and observational studies
Based on AMSTAR checklist (Shea, 2007; BMC Methodol 7: 10; doi:10.1186/1471-2288-7-10) and PRISMA checklist (Moher, 2009; PLoS Med 6: e1000097; doi:10.1371/journal.pmed1000097)
|
Study
First author, year |
Appropriate and clearly focused question?1
Yes/no/unclear |
Comprehensive and systematic literature search?2
Yes/no/unclear |
Description of included and excluded studies?3
Yes/no/unclear |
Description of relevant characteristics of included studies?4
Yes/no/unclear |
Appropriate adjustment for potential confounders in observational studies?5
Yes/no/unclear/notapplicable |
Assessment of scientific quality of included studies?6
Yes/no/unclear |
Enough similarities between studies to make combining them reasonable?7
Yes/no/unclear |
Potential risk of publication bias taken into account?8
Yes/no/unclear |
Potential conflicts of interest reported?9
Yes/no/unclear |
|
Fu, 2014 |
Yes |
Yes |
Yes Reasons for exclusion are indicated, but not with references. |
Yes |
N/A |
No Only study design was taken into account. |
Yes |
No |
Unclear Not specified for included studies. |
- Research question (PICO) and inclusion criteria should be appropriate and predefined.
- Search period and strategy should be described; at least Medline searched; for pharmacological questions at least Medline + EMBASE searched.
- Potentially relevant studies that are excluded at final selection (after reading the full text) should be referenced with reasons.
- Characteristics of individual studies relevant to research question (PICO), including potential confounders, should be reported.
- Results should be adequately controlled for potential confounders by multivariate analysis (not applicable for RCTs).
- Quality of individual studies should be assessed using a quality scoring tool or checklist (Jadad score, Newcastle-Ottawa scale, risk of bias table et cetera).
- Clinical and statistical heterogeneity should be assessed; clinical: enough similarities in patient characteristics, intervention and definition of outcome measure to allow pooling? For pooled data: assessment of statistical heterogeneity using appropriate statistical tests (for example Chi-square, I2)?
- An assessment of publication bias should include a combination of graphical aids (for example funnel plot, other available tests) and/or statistical tests (for example Egger regression test, Hedges-Olken). Note: If no test values or funnel plot included score “no”. Score “yes” if mentions that publication bias could not be assessed because there were fewer than 10 included studies.
- Sources of support (including commercial co-authorship) should be reported in both the systematic review and the included studies. Note: To get a “yes,” source of funding or support must be indicated for the systematic review AND for each of the included studies.
Risk of bias table for intervention studies (randomized controlled trials)
Research question 3: What are the (un)favorable effects of stents compared to balloon angioplasty in patients with a dysfunctional vascular access for hemodialysis?
See study by Kavan (2019) under research question 2.
Table of excluded studies for all PICOs
|
Author and year |
Reason for exclusion |
|
Björkman, 2019 |
Analysed in systematic review |
|
D’Cruz, 2019 |
No comparative data |
|
Dinh, 2019 |
Overlap with used systematic review |
|
Georgiadis, 2010 |
No original data |
|
Haskal, 2010 |
Analysed in systematic review |
|
Haskal, 2016 |
Analysed in systematic review |
|
Irani, 2018 |
Analysed in systematic review |
|
Katsanos, 2012 |
Analysed in systematic review |
|
Kavan, 2016 |
Follow-up study 2019 analysed |
|
Khawaya, 2016 |
More recent systematic review used |
|
Kitrou, 2015 a |
Analysed in systematic review |
|
Kitrou, 2015 b |
Analysed in systematic review |
|
Kitrou, 2017 |
Central venous stenosis, not AVG/AVF |
|
Kennedy, 2019 |
Overlap with used systematic review |
|
Kouvelos, 2018 |
Overlap with used systematic review |
|
Lai, 2014 |
Analysed in systematic review |
|
Liao, 2020 |
Overlap with used systematic review |
|
Maleux, 2018 |
Analysed in systematic review |
|
McLennan, 2016 |
Narrative review |
|
Moreno-Sánchez, 2020 |
Unclear presentation of data, and outcomes reported per lesion, not per patiënt. |
|
Nikopoulos, 2019 |
Overlap with used systematic review |
|
Rokoszak, 2020 |
Overlap with used systematic review |
|
Roy-Chaudhury, 2005 |
No clinical outcomes |
|
Salim, 2020 |
Overlap with used systematic review |
|
Shemesh, 2008 |
Comparison different from PICOs |
|
Swinnen, 2019 |
Analysed in systematic review |
|
Trerotola, 2018 |
Analysed in systematic review |
|
Vasanthamohan, 2015 |
More recent systematic review used |
|
Yan, 2019 |
Overlap with used systematic review |
|
Yuan, 2020 |
Overlap with used systematic review |
Verantwoording
Beoordelingsdatum en geldigheid
Publicatiedatum : 30-03-2022
Beoordeeld op geldigheid : 24-02-2022
Algemene gegevens
De ontwikkeling van deze richtlijnmodule werd ondersteund door het Kennisinstituut van de Federatie Medisch Specialisten en werd gefinancierd uit de Stichting Kwaliteitsgelden Medisch Specialisten (SKMS). De financier heeft geen enkele invloed gehad op de inhoud van de richtlijnmodule.
Samenstelling werkgroep
Voor het ontwikkelen van de richtlijnmodule is in 2019 een multidisciplinaire werkgroep ingesteld, bestaande uit vertegenwoordigers van alle relevante specialismen die betrokken zijn bij de zorg voor patiënten die een vaattoegang voor hemodialyse hebben of krijgen.
Werkgroep
- Dr. M.G.J. Snoeijs, vaatchirurg in Maastricht UMC+, NVvH (voorzitter)
- Dr. R.D. Toorop, vaatchirurg in UMC Utrecht, NVvH
- Dr. F. van Hoek, vaatchirurg in Radboudumc, NVvH
- Dr. M.J. Molegraaf, vaatchirurg in Isala ziekenhuis, NVvH
- Dr. M. Schouten, internist-nefroloog in Tergooi, NIV
- Dr. J.I. Rotmans, internist-nefroloog in LUMC, NIV
- Drs. R.J.B. Brans, radioloog in Maastricht UMC+, NVvR
- M. ter Meer, praktijkbegeleider dialyse en dialyseverpleegkundige OLVG, V&VN
- Drs. K. Prantl, Beleidsmedewerker kwaliteit & onderzoek, NVN
- H.Th. Hubbers, ervaringsdeskundige, NVN
Met ondersteuning van
- Dr. K.N.J. Burger, senior adviseur, Kennisinstituut van de Federatie Medisch Specialisten (tot mei 2021)
- Dr. M.S. Ruiter, adviseur, Kennisinstituut van de Federatie Medisch Specialisten
Belangenverklaringen
De Code ter voorkoming van oneigenlijke beïnvloeding door belangenverstrengeling is gevolgd. Alle werkgroepleden hebben schriftelijk verklaard of zij in de laatste drie jaar directe financiële belangen (betrekking bij een commercieel bedrijf, persoonlijke financiële belangen, onderzoeksfinanciering) of indirecte belangen (persoonlijke relaties, reputatiemanagement) hebben gehad. Gedurende de ontwikkeling of herziening van een module worden wijzigingen in belangen aan de voorzitter doorgegeven. De belangenverklaring wordt opnieuw bevestigd tijdens de commentaarfase.
Een overzicht van de belangen van werkgroepleden en het oordeel over het omgaan met eventuele belangen vindt u in onderstaande tabel. De ondertekende belangenverklaringen zijn op te vragen bij het secretariaat van het Kennisinstituut van de Federatie Medisch Specialisten.
|
Werkgroeplid |
Functie |
Nevenfuncties |
Gemelde belangen |
Ondernomen actie |
|
Snoeijs |
Vaatchirurg Maastricht UMC+ |
Geen |
Leading the Change, OASIS Zorgevaluatie (Wat is de beste vaattoegang voor oudere dialysepatienten?), rol als projectleider.
ZonMW, FLOW Zorgevaluatie (Is het detecteren en preventief behandelen van stenoses in de vaattoegang zinvol?), rol als projectleider.
Nierstichting, Shunt Simulatie Studie (Helpen computer simulaties van de flow bij het aanleggen van arterioveneuze fistels?), geen rol als projectleider.
Australasian Kidney Trials Network, VALID (Validatie van interventies aan de vaattoegang als care outcome measure), geen rol als projectleider.
Ik ben verantwoordelijk voor het expertisecentrum met topreferente zorgfunctie voor vaattoegangschirurgie in Maastricht UMC+. |
Geen actie |
|
Toorop |
Vaat-en transplantatiechirurg UMC Utrecht |
geen |
geen |
Geen actie |
|
Van Hoek |
Vaat- en transplantatiechirurg, Radboudumc |
geen |
geen |
Geen actie |
|
Molegraaf |
vaatchirurg MSB Isala Ziekenhuis |
geen |
geen |
Geen actie |
|
Rotmans |
Internist-nefroloog LUMC |
geen |
Uitvinder op meerdere patenten op het gebied van vaattoegang voor dialyse maar geen eigenaar (patenten zijn van LUMC). VIDI-grant ZonMW onderzoekssubsidie. Unrestricted research grant van Enceladus Pharmaceuticals (inmiddels afgerond) betrof een grant voor een investigator-initiated placebo-gecontroleerde klinische studie naar de effectiviteit van liposomaal prednisolon voor het verbeteren van shuntrijping (maturatie). Deze studie is afgerond maar nog niet gepubliceerd (is under review). De shunt maturatie bleek in beide groepen vergelijkbaar (dus geen gunstig effect van liposomaal prednison). |
Geen moduleschrijver voor uitgangsvragen met betrekking tot maturatie |
|
Schouten |
internist-nefroloog Tergooi |
Richtlijncommissie Nederlandse Federatie voor Nefrologie (betaald) |
geen |
Geen actie |
|
Brans |
Interventieradioloog Maastricht UMC+ |
Secretaris commissie accreditatie NVvR (onbetaald) |
geen |
Geen actie |
|
Ter Meer |
Praktijk begeleider dialyse OLVG te Amsterdam en |
Gast docent Amstel Academie voor opleiding dialyseverpleegundige en dialyse assistent betaald (verzorgen van lessen over vaattoegang) Gast docent albeda college opleiding vaattoegang tot 2021 betaald (verzorgen van de les VSA) Presentatie voor firma Baxter 4 x betaald, presentatie over nieuwe ontwikkelingen op gebied van vaattoegangszorg voor verpleegkundigen. |
geen |
Geen actie |
|
Prantl |
beleidsmedewerker kwaliteit & onderzoek NVN |
geen |
Namens de NVN heb ik meegedacht met de ontwikkeling van de Vascoscope, een nieuw echoapparaat dat het aanprikken waarschijnlijk kan vereenvoudigen. Inmiddels geen betrokkenheid meer bij dit onderzoek. |
Geen actie |
|
Hubbers |
Ervaringsdeskundige |
Geen |
Namens de NVN heb ik als vrijwilliger twee keer deelgenomen in de werkgroep Vascoscoop in het UMC Utrecht. Doel hiervan was te komen tot een handzaam echoapparaatje dat zowel een verpleegkundige als patiënt makkelijk zelf kan bedienen/gebruiken om misprikken bij vaattoegang voor hemodialyse te voorkomen. Nadien heb ik het doorontwikkelde apparaat twee keer gezien waarvan 1x getest in de praktijk. |
Geen actie |
|
Burger |
Senior adviseur bij Kennisinstituut van de Federatie Medisch Specialisten |
Geen |
Geen |
Geen actie |
|
Ruiter |
Adviseur bij Kennisinstituut van de Federatie Medisch Specialisten |
Geen |
Geen |
Geen actie |
Inbreng patiëntenperspectief
Er werd aandacht besteed aan het patiëntperspectief door Patiëntenfederatie Nederland en andere relevante patiëntenorganisaties uit te nodigen voor een schriftelijke knelpuntenanalyse. Het verslag hiervan (zie aanverwante producten) is besproken in de werkgroep. De verkregen input is meegenomen bij het opstellen van de uitgangsvragen. Bovendien werd het patiëntperspectief vertegenwoordigd door afvaardiging van patiëntenorganisatie Nierpatiënten Vereniging Nederland in de werkgroep. Tot slot werden de modules voor commentaar voorgelegd aan Patiëntenfederatie Nederland en andere relevante patiëntenorganisaties en de eventueel aangeleverde commentaren zijn bekeken en verwerkt.
Kwalitatieve raming van mogelijke financiële gevolgen in het kader van de Wkkgz
Bij de richtlijn is conform de Wet kwaliteit, klachten en geschillen zorg (Wkkgz) een kwalitatieve raming uitgevoerd of de aanbevelingen mogelijk leiden tot substantiële financiële gevolgen. Bij het uitvoeren van deze beoordeling zijn richtlijnmodules op verschillende domeinen getoetst. Uit de kwalitatieve raming blijkt dat er geen substantiële financiële gevolgen zijn, zie onderstaande tabel.
|
Module |
Uitkomst kwalitatieve raming |
Toelichting |
|
Module 1 Operatieve besluitvorming |
geen substantiële financiële gevolgen |
Uit de toetsing volgt dat de aanbevelingen niet breed toepasbaar zijn (<5.000 patiënten) en zullen daarom naar verwachting geen substantiële financiële gevolgen hebben voor de collectieve uitgaven. |
|
Module 2 Perioperatieve zorg |
geen substantiële financiële gevolgen |
Uit de toetsing volgt dat de aanbevelingen niet breed toepasbaar zijn (<5.000 patiënten) en zullen daarom naar verwachting geen substantiële financiële gevolgen hebben voor de collectieve uitgaven. |
|
Module 3 Gebruik en verzorging |
geen substantiële financiële gevolgen |
Hoewel uit de toetsing volgt dat de aanbevelingen breed toepasbaar zijn (5.000-40.000 patiënten), volgt ook uit de toetsing dat het geen nieuwe manier van zorgverlening of andere organisatie van zorgverlening betreft. Er worden daarom geen substantiële financiële gevolgen verwacht. |
|
Module 4 Monitoring en surveillance |
geen substantiële financiële gevolgen |
Hoewel uit de toetsing volgt dat de aanbevelingen breed toepasbaar zijn (5.000-40.000 patiënten), volgt ook uit de toetsing dat het geen nieuwe manier van zorgverlening of andere organisatie van zorgverlening betreft. Er worden daarom geen substantiële financiële gevolgen verwacht. |
|
Module 5 Flow disfunctie |
geen substantiële financiële gevolgen |
Uit de toetsing volgt dat de aanbevelingen niet breed toepasbaar zijn (<5.000 patiënten) en zullen daarom naar verwachting geen substantiële financiële gevolgen hebben voor de collectieve uitgaven. |
|
Module 6 Infectie van de vaattoegang |
geen substantiële financiële gevolgen |
Uit de toetsing volgt dat de aanbevelingen niet breed toepasbaar zijn (<5.000 patiënten) en zullen daarom naar verwachting geen substantiële financiële gevolgen hebben voor de collectieve uitgaven. |
|
Module 7 Ischemie |
geen substantiële financiële gevolgen |
Uit de toetsing volgt dat de aanbevelingen niet breed toepasbaar zijn (<5.000 patiënten) en zullen daarom naar verwachting geen substantiële financiële gevolgen hebben voor de collectieve uitgaven. |
|
Module 8 High-flow vaattoegang |
geen substantiële financiële gevolgen |
Uit de toetsing volgt dat de aanbevelingen niet breed toepasbaar zijn (<5.000 patiënten) en zullen daarom naar verwachting geen substantiële financiële gevolgen hebben voor de collectieve uitgaven. |
|
Module 9 Ongebruikte vaattoegang |
geen substantiële financiële gevolgen |
Hoewel uit de toetsing volgt dat de aanbevelingen breed toepasbaar zijn (5.000-40.000 patiënten), volgt ook uit de toetsing dat het geen nieuwe manier van zorgverlening of andere organisatie van zorgverlening betreft. Er worden daarom geen substantiële financiële gevolgen verwacht. |
|
Module 10 Randvoorwaarden (Organisatie van zorg) |
geen substantiële financiële gevolgen |
Hoewel uit de toetsing volgt dat de aanbevelingen breed toepasbaar zijn (5.000-40.000 patiënten), volgt ook uit de toetsing dat het geen nieuwe manier van zorgverlening of andere organisatie van zorgverlening betreft. Er worden daarom geen substantiële financiële gevolgen verwacht. |
Methode ontwikkeling
Evidence based
Werkwijze
AGREE
Deze richtlijnmodule is opgesteld conform de eisen vermeld in het rapport Medisch Specialistische Richtlijnen 2.0 van de adviescommissie Richtlijnen van de Raad Kwaliteit. Dit rapport is gebaseerd op het AGREE II instrument (Appraisal of Guidelines for Research & Evaluation II; Brouwers, 2010).
Knelpuntenanalyse en uitgangsvragen
Tijdens de voorbereidende fase inventariseerde de werkgroep de knelpunten in de zorg voor de vaattoegang voor hemodialyse. Tevens zijn er knelpunten aangedragen door stakeholders door middel van een schriftelijke knelpuntenanalyse. Een verslag hiervan is opgenomen onder aanverwante producten. Op basis van de uitkomsten van de knelpuntenanalyse zijn door de werkgroep concept-uitgangsvragen opgesteld en definitief vastgesteld.
Uitkomstmaten
Na het opstellen van de zoekvraag behorende bij de uitgangsvraag inventariseerde de werkgroep welke uitkomstmaten voor de patiënt relevant zijn, waarbij zowel naar gewenste als ongewenste effecten werd gekeken. Hierbij werd een maximum van acht uitkomstmaten gehanteerd. De werkgroep waardeerde deze uitkomstmaten volgens hun relatieve belang bij de besluitvorming rondom aanbevelingen, als cruciaal (kritiek voor de besluitvorming), belangrijk (maar niet cruciaal) en onbelangrijk. Tevens definieerde de werkgroep tenminste voor de cruciale uitkomstmaten welke verschillen zij klinisch (patiënt) relevant vonden.
Methode literatuursamenvatting
Een uitgebreide beschrijving van de strategie voor zoeken en selecteren van literatuur en de beoordeling van de risk-of-bias van de individuele studies is te vinden onder ‘Zoeken en selecteren’ onder Onderbouwing. De beoordeling van de kracht van het wetenschappelijke bewijs wordt hieronder toegelicht.
Beoordelen van de kracht van het wetenschappelijke bewijs
De kracht van het wetenschappelijke bewijs werd bepaald volgens de GRADE-methode. GRADE staat voor ‘Grading Recommendations Assessment, Development and Evaluation’ (zie https://www.gradeworkinggroup.org/). De basisprincipes van de GRADE-methodiek zijn: het benoemen en prioriteren van de klinisch (patiënt) relevante uitkomstmaten, een systematische review per uitkomstmaat, en een beoordeling van de bewijskracht per uitkomstmaat op basis van de acht GRADE-domeinen (domeinen voor downgraden: risk of bias, inconsistentie, indirectheid, imprecisie, en publicatiebias; domeinen voor upgraden: dosis-effect relatie, groot effect, en residuele plausibele confounding).
GRADE onderscheidt vier gradaties voor de kwaliteit van het wetenschappelijk bewijs: hoog, redelijk, laag en zeer laag. Deze gradaties verwijzen naar de mate van zekerheid die er bestaat over de literatuurconclusie, in het bijzonder de mate van zekerheid dat de literatuurconclusie de aanbeveling adequaat ondersteunt (Schünemann, 2013; Hultcrantz, 2017).
|
GRADE |
Definitie |
|
Hoog |
|
|
Redelijk |
|
|
Laag |
|
|
Zeer laag |
|
Bij het beoordelen (graderen) van de kracht van het wetenschappelijk bewijs in richtlijnen volgens de GRADE-methodiek spelen grenzen voor klinische besluitvorming een belangrijke rol (Hultcrantz, 2017). Dit zijn de grenzen die bij overschrijding aanleiding zouden geven tot een aanpassing van de aanbeveling. Om de grenzen voor klinische besluitvorming te bepalen moeten alle relevante uitkomstmaten en overwegingen worden meegewogen. De grenzen voor klinische besluitvorming zijn daarmee niet één op één vergelijkbaar met het minimaal klinisch relevant verschil (Minimal Clinically Important Difference, MCID). Met name in situaties waarin een interventie geen belangrijke nadelen heeft en de kosten relatief laag zijn, kan de grens voor klinische besluitvorming met betrekking tot de effectiviteit van de interventie bij een lagere waarde (dichter bij het nuleffect) liggen dan de MCID (Hultcrantz, 2017).
Overwegingen (van bewijs naar aanbeveling)
Om te komen tot een aanbeveling zijn naast (de kwaliteit van) het wetenschappelijke bewijs ook andere aspecten belangrijk en worden meegewogen, zoals aanvullende argumenten uit bijvoorbeeld de biomechanica of fysiologie, waarden en voorkeuren van patiënten, kosten (middelenbeslag), aanvaardbaarheid, haalbaarheid en implementatie. Deze aspecten zijn systematisch vermeld en beoordeeld (gewogen) onder het kopje ‘Overwegingen’ en kunnen (mede) gebaseerd zijn op expert opinion. Hierbij is gebruik gemaakt van een gestructureerd format gebaseerd op het evidence-to-decision framework van de internationale GRADE Working Group (Alonso-Coello, 2016a; Alonso-Coello, 2016b). Dit evidence-to-decision framework is een integraal onderdeel van de GRADE-methodiek. Daarnaast vond de werkgroep het wenselijk om aan de overwegingen een sectie toe te voegen die de uitgangsvraag in een bredere context plaatst. In dit onderdeel “inbedding in de zorg” zijn op basis van bestaande internationale richtlijnen aandachtpunten behandeld die van belang zijn in de dagelijkse praktijk. Voor verdere toelichting en onderbouwing wordt verwezen naar de betreffende richtlijnen.
Formuleren van aanbevelingen
De aanbevelingen geven antwoord op de uitgangsvraag en zijn gebaseerd op het beschikbare wetenschappelijke bewijs en de belangrijkste overwegingen, en een weging van de gunstige en ongunstige effecten van de relevante interventies. De kracht van het wetenschappelijk bewijs en het gewicht dat door de werkgroep wordt toegekend aan de overwegingen, bepalen samen de sterkte van de aanbeveling. Conform de GRADE-methodiek sluit een lage bewijskracht van conclusies in de systematische literatuuranalyse een sterke aanbeveling niet a priori uit, en zijn bij een hoge bewijskracht ook zwakke aanbevelingen mogelijk (Agoritsas, 2017; Neumann, 2016). De sterkte van de aanbeveling wordt altijd bepaald door weging van alle relevante argumenten tezamen. De werkgroep heeft bij elke aanbeveling opgenomen hoe zij tot de richting en sterkte van de aanbeveling zijn gekomen.
In de GRADE-methodiek wordt onderscheid gemaakt tussen sterke en zwakke (of conditionele) aanbevelingen. De sterkte van een aanbeveling verwijst naar de mate van zekerheid dat de voordelen van de interventie opwegen tegen de nadelen (of vice versa), gezien over het hele spectrum van patiënten waarvoor de aanbeveling is bedoeld. De sterkte van een aanbeveling heeft duidelijke implicaties voor patiënten, behandelaars en beleidsmakers (zie onderstaande tabel). Een aanbeveling is geen dictaat, zelfs een sterke aanbeveling gebaseerd op bewijs van hoge kwaliteit (GRADE-gradering HOOG) zal niet altijd van toepassing zijn, onder alle mogelijke omstandigheden en voor elke individuele patiënt.
|
Implicaties van sterke en zwakke aanbevelingen voor verschillende richtlijngebruikers |
||
|
|
Sterke aanbeveling |
Zwakke (conditionele) aanbeveling |
|
Voor patiënten |
De meeste patiënten zouden de aanbevolen interventie of aanpak kiezen en slechts een klein aantal niet. |
Een aanzienlijk deel van de patiënten zouden de aanbevolen interventie of aanpak kiezen, maar veel patiënten ook niet. |
|
Voor behandelaars |
De meeste patiënten zouden de aanbevolen interventie of aanpak moeten ontvangen. |
Er zijn meerdere geschikte interventies of aanpakken. De patiënt moet worden ondersteund bij de keuze voor de interventie of aanpak die het beste aansluit bij zijn of haar waarden en voorkeuren. |
|
Voor beleidsmakers |
De aanbevolen interventie of aanpak kan worden gezien als standaardbeleid. |
Beleidsbepaling vereist uitvoerige discussie met betrokkenheid van veel stakeholders. Er is een grotere kans op lokale beleidsverschillen. |
Organisatie van zorg
In de knelpuntenanalyse en bij de ontwikkeling van de richtlijnmodule is expliciet aandacht geweest voor de organisatie van zorg: alle aspecten die randvoorwaardelijk zijn voor het verlenen van zorg (zoals coördinatie, communicatie, (financiële) middelen, mankracht en infrastructuur). Randvoorwaarden die relevant zijn voor het beantwoorden van deze specifieke uitgangsvraag zijn genoemd bij de overwegingen. Meer algemene, overkoepelende, of bijkomende aspecten van de organisatie van zorg worden behandeld in de module Organisatie van zorg.
Commentaar- en autorisatiefase
De conceptrichtlijnmodule werd aan de betrokken (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd ter commentaar. De commentaren werden verzameld en besproken met de werkgroep. Naar aanleiding van de commentaren werd de conceptrichtlijnmodule aangepast en definitief vastgesteld door de werkgroep. De definitieve richtlijnmodule werd aan de deelnemende (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd voor autorisatie en door hen geautoriseerd dan wel geaccordeerd.
Literatuur
Agoritsas T, Merglen A, Heen AF, Kristiansen A, Neumann I, Brito JP, Brignardello-Petersen R, Alexander PE, Rind DM, Vandvik PO, Guyatt GH. UpToDate adherence to GRADE criteria for strong recommendations: an analytical survey. BMJ Open. 2017 Nov 16;7(11):e018593. doi: 10.1136/bmjopen-2017-018593. PubMed PMID: 29150475; PubMed Central PMCID: PMC5701989.
Alonso-Coello P, Schünemann HJ, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Rada G, Rosenbaum S, Morelli A, Guyatt GH, Oxman AD; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ. 2016 Jun 28;353:i2016. doi: 10.1136/bmj.i2016. PubMed PMID: 27353417.
Alonso-Coello P, Oxman AD, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Vandvik PO, Meerpohl J, Guyatt GH, Schünemann HJ; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 2: Clinical practice guidelines. BMJ. 2016 Jun 30;353:i2089. doi: 10.1136/bmj.i2089. PubMed PMID: 27365494.
Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, Fervers B, Graham ID, Grimshaw J, Hanna SE, Littlejohns P, Makarski J, Zitzelsberger L; AGREE Next Steps Consortium. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010 Dec 14;182(18):E839-42. doi: 10.1503/cmaj.090449. Epub 2010 Jul 5. Review. PubMed PMID: 20603348; PubMed Central PMCID: PMC3001530.
Hultcrantz M, Rind D, Akl EA, Treweek S, Mustafa RA, Iorio A, Alper BS, Meerpohl JJ, Murad MH, Ansari MT, Katikireddi SV, Östlund P, Tranæus S, Christensen R, Gartlehner G, Brozek J, Izcovich A, Schünemann H, Guyatt G. The GRADE Working Group clarifies the construct of certainty of evidence. J Clin Epidemiol. 2017 Jul;87:4-13. doi: 10.1016/j.jclinepi.2017.05.006. Epub 2017 May 18. PubMed PMID: 28529184; PubMed Central PMCID: PMC6542664.
Medisch Specialistische Richtlijnen 2.0 (2012). Adviescommissie Richtlijnen van de Raad Kwalitieit. https://richtlijnendatabase.nl/over_deze_site/richtlijnontwikkeling.html.
Neumann I, Santesso N, Akl EA, Rind DM, Vandvik PO, Alonso-Coello P, Agoritsas T, Mustafa RA, Alexander PE, Schünemann H, Guyatt GH. A guide for health professionals to interpret and use recommendations in guidelines developed with the GRADE approach. J Clin Epidemiol. 2016 Apr;72:45-55. doi: 10.1016/j.jclinepi.2015.11.017. Epub 2016 Jan 6. Review. PubMed PMID: 26772609.
Schünemann H, Brożek J, Guyatt G, et al. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group, 2013. Available from http://gdt.guidelinedevelopment.org/central_prod/_design/client/handbook/handbook.html.
Zoekverantwoording
|
Richtlijn: Vaattoegang voor hemodialyse |
|
|
Uitgangsvraag: UV8 optimale percutane behandeling bij stenosis van de vaattoegang |
|
|
Database(s): Ovid/Medline, Embase |
Datum: 7-9-2020 |
|
Periode: 2000-2020 |
Talen: nvt |
Zoekverantwoording
Ovid/Medline
1 exp Drug-Eluting Stents/ or exp Self Expandable Metallic Stents/ or passeo-18 lux.ti,ab,kf. or advanta v12.ti,ab,kf. or begraft.ti,ab,kf. or covera.ti,ab,kf. or covered cheatham-platinum stent*.ti,ab,kf. or cragg stent*.ti,ab,kf. or cronus.ti,ab,kf. or e-ventus bx.ti,ab,kf. or passager.ti,ab,kf. or stentor.ti,ab,kf. or symbiot.ti,ab,kf. or talent lps.ti,ab,kf. or stent graft*.ti,ab,kf. or stentgraft*.ti,ab,kf. or axios stent.ti,ab,kf. or besmooth peripheral.ti,ab,kf. or hot axios.ti,ab,kf. or strecker.ti,ab,kf. or metal stent*.ti,ab,kf. or metallic stent*.ti,ab,kf. or placlitaxel.ti,ab,kf. or ((eluting or coat* or cover*) adj2 (stent* or balloon*)).ti,ab,kf. (38901)
2 exp Arteriovenous Fistula/ or exp Arteriovenous Anastomosis/ or exp Arteriovenous Shunt, Surgical/ or av fistula*.ti,ab,kf. or anastomosis arteriovenosa.ti,ab,kf. or arterial venous anastomos*.ti,ab,kf. or arterio venous anastomos*.ti,ab,kf. or arterio venous aneurysm*.ti,ab,kf. or arterio-venous fistula*.ti,ab,kf. or arteriovenous anastomos*.ti,ab,kf. or arteriovenous aneurysm*.ti,ab,kf. or arteriovenous crossing.ti,ab,kf. or arteriovenous fistula*.ti,ab,kf. or artery vein fistula*.ti,ab,kf. or av anastomos*.ti,ab,kf. or av aneurysm*.ti,ab,kf. or artery access.ti,ab,kf. or vein access.ti,ab,kf. or vascular access.ti,ab,kf. or a-v shunt*.ti,ab,kf. or arterio venous connection.ti,ab,kf. or arterio venous shunt*.ti,ab,kf. or arteriovenous bypass.ti,ab,kf. or arteriovenous external shunt*.ti,ab,kf. or arteriovenous shunt*.ti,ab,kf. or av shunt*.ti,ab,kf. or brescia cimino shunt*.ti,ab,kf. or veno arterial shunt*.ti,ab,kf. or venoarterial bypass*.ti,ab,kf. or aorta caval fistula*.ti,ab,kf. or carotid cavernous fistula*.ti,ab,kf. or av graft*.ti,ab,kf. or flixene.ti,ab,kf. or arteriovenous graft*.ti,ab,kf. (44656)
3 1 and 2 (887)
4 limit 3 to yr="2000 -Current" (827)
5 4 not ((exp animals/ or exp models, animal/) not humans/) not (letter/ or comment/ or editorial/) (787)
6 (meta-analysis/ or meta-analysis as topic/ or (meta adj analy$).tw. or (systematic*or literature adj2 review$1).tw. or (systematic adj overview$1).tw. or exp "Review Literature as Topic"/ or cochrane.ab. or cochrane.jw. or embase.ab. or medline.ab. or (psychlit or psyclit).ab. or (cinahl or cinhal).ab. or cancerlit.ab. or ((selection criteria or data extraction).ab. and "review"/)) not (Comment/ or Editorial/ or Letter/ or (animals/ not humans/)) (301031)
7 (exp clinical trial/ or randomized controlled trial/ or exp clinical trials as topic/ or randomized controlled trials as topic/ or Random Allocation/ or Double-Blind Method/ or Single-Blind Method/ or (clinical trial, phase i or clinical trial, phase ii or clinical trial, phase iii or clinical trial, phase iv or controlled clinical trial or randomized controlled trial or multicenter study or clinical trial).pt. or random*.ti,ab. or (clinic* adj trial*).tw. or ((singl* or doubl* or treb* or tripl*) adj (blind$3 or mask$3)).tw. or Placebos/ or placebo*.tw.) not (animals/ not humans/) (2022725)
8 Epidemiologic studies/ or case control studies/ or exp cohort studies/ or Controlled Before-After Studies/ or Case control.tw. or (cohort adj (study or studies)).tw. or Cohort analy$.tw. or (Follow up adj (study or studies)).tw. or (observational adj (study or studies)).tw. or Longitudinal.tw. or Retrospective*.tw. or prospective*.tw. or consecutive*.tw. or Cross sectional.tw. or Cross-sectional studies/ or historically controlled study/ or interrupted time series analysis/ (Onder exp cohort studies vallen ook longitudinale, prospectieve en retrospectieve studies) (3513561)
9 5 and 6 (23) SR
10 5 and 7 (100)
11 5 and 8 (303)
12 10 not 9 (84) RCT
13 11 not 10 not 9 (232) observationeel
Embase
|
No. |
Query |
Results |
|
#22 |
#20 NOT #19 NOT #18, observationeel |
229 |
|
#21 |
#19 NOT #18, RCT |
124 |
|
#20 |
#13 AND #17 |
334 |
|
#19 |
#13 AND #16 |
142 |
|
#18 |
#13 AND #15 SR |
36 |
|
#17 |
'major clinical study'/de OR 'clinical study'/de OR 'case control study'/de OR 'family study'/de OR 'longitudinal study'/de OR 'retrospective study'/de OR 'prospective study'/de OR 'cohort analysis'/de OR ((cohort NEAR/1 (study OR studies)):ab,ti) OR (('case control' NEAR/1 (study OR studies)):ab,ti) OR (('follow up' NEAR/1 (study OR studies)):ab,ti) OR (observational NEAR/1 (study OR studies)) OR ((epidemiologic NEAR/1 (study OR studies)):ab,ti) OR (('cross sectional' NEAR/1 (study OR studies)):ab,ti) |
5401257 |
|
#16 |
('clinical trial'/exp OR 'randomization'/exp OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp OR 'placebo'/exp OR 'prospective study'/exp OR rct:ab,ti OR random*:ab,ti OR 'single blind':ab,ti OR 'randomised controlled trial':ab,ti OR 'randomized controlled trial'/exp OR placebo*:ab,ti) NOT 'conference abstract':it |
2340458 |
|
#15 |
('meta-analysis'/de OR cochrane:ab OR embase:ab OR psycinfo:ab OR cinahl:ab OR medline:ab OR ((systematic NEAR/1 (review OR overview)):ab,ti) OR ((meta NEAR/1 analy*):ab,ti) OR metaanalys*:ab,ti OR 'data extraction':ab OR cochrane:jt OR 'systematic review'/de) NOT (('animal experiment'/exp OR 'animal model'/exp OR 'nonhuman'/exp) NOT 'human'/exp) |
473856 |
|
#14 |
#8 AND #13 |
4 |
|
#13 |
#12 AND (1-1-2000)/sd NOT ('conference abstract'/it OR 'editorial'/it OR 'letter'/it OR 'note'/it) NOT (('animal experiment'/exp OR 'animal model'/exp OR 'nonhuman'/exp) NOT 'human'/exp) |
1190 |
|
#12 |
#6 AND #11 |
1681 |
|
#11 |
'arteriovenous fistula'/exp OR 'vascular access'/exp OR 'arteriovenous shunt'/exp OR 'arteriovenous graft'/exp OR 'av fistula*':ti,ab OR 'anastomosis arteriovenosa':ti,ab OR 'arterial venous anastomos*':ti,ab OR 'arterio venous anastomos*':ti,ab OR 'arterio venous aneurysm':ti,ab OR 'arterio-venous fistula*':ti,ab OR 'arteriovenous anastomos*':ti,ab,kw OR 'arteriovenous aneurysm*':ti,ab,kw OR 'arteriovenous crossing':ti,ab,kw OR 'arteriovenous fistula*':ti,ab,kw OR 'artery vein fistula*':ti,ab OR 'av anastomos*':ti,ab OR 'av aneurysm*':ti,ab OR 'artery access':ti,ab,kw OR 'vein access':ti,ab,kw OR 'vascular access':ti,ab,kw OR 'a-v shunt*':ti,ab,kw OR 'arterio venous connection':ti,ab,kw OR 'arterio venous shunt*':ti,ab,kw OR 'arteriovenous bypass':ti,ab,kw OR 'arteriovenous external shunt*':ti,ab,kw OR 'arteriovenous shunt*':ti,ab,kw OR 'av shunt*':ti,ab,kw OR 'brescia cimino shunt*':ti,ab,kw OR 'veno arterial shunt*':ti,ab,kw OR 'venoarterial bypass*':ti,ab,kw OR 'aorta caval fistula*':ti,ab,kw OR 'carotid cavernous fistula*':ti,ab,kw OR 'av graft*':ti,ab,kw OR 'flixene':ti,ab,kw OR 'arteriovenous graft*':ti,ab,kw |
75603 |
|
#10 |
#7 AND (1-1-2000)/sd NOT ('conference abstract'/it OR 'editorial'/it OR 'letter'/it OR 'note'/it) NOT (('animal experiment'/exp OR 'animal model'/exp OR 'nonhuman'/exp) NOT 'human'/exp) |
1797 |
|
#9 |
#7 AND #8 |
4 |
|
#8 |
#1 OR #2 OR #4 |
4 |
|
#7 |
#5 AND #6 |
2630 |
|
#6 |
'drug-coated balloon'/exp OR 'metal stent'/exp OR 'stent graft'/exp OR 'passeo-18 lux':ti,ab,kw OR 'drug-coated balloon':ti,ab,kw OR 'drug-eluting balloon':ti,ab,kw OR 'advanta v12':ti,ab,kw OR 'advanta v12 rx':ti,ab,kw OR 'begraft':ti,ab,kw OR 'begraft peripheral':ti,ab,kw OR 'covera (stent graft)':ti,ab,kw OR 'covera plus (stent graft)':ti,ab,kw OR 'covered cheatham-platinum stent':ti,ab,kw OR 'cragg stent':ti,ab,kw OR 'cronus (device)':ti,ab,kw OR 'e-ventus bx':ti,ab,kw OR 'passager (device)':ti,ab,kw OR 'stentor (device)':ti,ab,kw OR 'stentor system':ti,ab,kw OR 'symbiot (stent graft)':ti,ab,kw OR 'talent lps':ti,ab,kw OR 'covered stent':ti,ab,kw OR 'fabric covered stent':ti,ab,kw OR 'membrane covered stent':ti,ab,kw OR 'stent graft':ti,ab,kw OR 'axios stent':ti,ab,kw OR 'besmooth peripheral':ti,ab,kw OR 'hot axios':ti,ab,kw OR 'strecker':ti,ab,kw OR 'metal stent':ti,ab,kw OR 'metallic stent':ti,ab,kw OR placlitaxel:ti,ab,kw |
34399 |
|
#5 |
'percutaneous transluminal angioplasty'/exp OR 'balloon angioplasty':ti,ab,kw OR 'dotter artery dilatation':ti,ab,kw OR 'percutaneous angioplasty':ti,ab,kw OR 'percutaneous transluminal angioplasty':ti,ab,kw OR 'percutaneous transluminal artery dilatation':ti,ab,kw OR 'transluminal angioplasty':ti,ab,kw OR 'transluminal artery dilatation':ti,ab,kw |
36076 |
|
#4 |
stent AND graft AND versus AND balloon AND angioplasty AND for AND failing AND 'dialysis access' AND grafts AND 2010 AND haskal |
2 |
|
#3 |
stentgraft AND placement AND versus AND angioplasty AND for AND hemodialysis AND access AND failure AND huanrui |
0 |
|
#2 |
'drug coated' AND balloon AND angioplasty AND in AND failing AND av AND fistulas AND 2018 |
1 |
|
#1 |
a AND systematic AND review AND 'meta-analysis' AND of AND 'drug coated' AND versus AND conventional AND balloon AND angioplasty AND for AND dialysis AND access AND stenosis |
1 |
