Neuromodulatie
Uitgangsvraag
Wat zijn de (on)gunstige effecten van non-invasieve neuromodulatie (zoals rTMS en rDCS) bij patiënten met chronische subjectieve tinnitusklachten?
Aanbeveling
rTMS
Verricht bij patiënten met chronische tinnitusklachten geen repetitieve transcraniële magnetische stimulatie (rTMS) als onderdeel van klinische behandeling.
TDCS
Verricht bij patiënten met chronische tinnitusklachten geen transcranial direct current stimulation (tDCS) als onderdeel van klinische behandeling.
Overwegingen
Voor- en nadelen van de interventie en de kwaliteit van het bewijs
Er is een literatuuronderzoek verricht naar de effectiviteit van non-invasieve neuromodulatie ten opzichte van afwachten, placebo of sham controle bij patiënten met chronische subjectieve tinnitus. Er is één systematische review en er zijn drie RCT’s uitgewerkt die repetitieve transcraniële magnetische stimulatie (rTMS) onderzochten en er is één systematische review uitgewerkt die transcranial direct current stimulation (tDCS) onderzocht. Het gevonden bewijs voor de cruciale en belangrijke uitkomstmaten is echter van zeer lage bewijskracht, waardoor het veel onzekerheid met zich meebrengt.
Repetitieve transcraniële magnetische stimulatie (rTMS)
Voor de cruciale uitkomstmaat tinnitus last, gemeten door middel van THI, werd er, in studies met kleine aantallen proefpersonen, een klinisch relevant verschil gevonden tussen rTMS neuromodulatie en sham rTMS. Neuromodulatie (rTMS) vermindert mogelijk tinnitus last, wanneer vergeleken met sham rTMS in volwassen patiënten met chronische subjectieve tinnitus. Voor de belangrijke uitkomstmaat ernstige bijwerkingen werd geen klinisch relevant verschil gevonden tussen de rTMS en de sham rTMS groepen. De belangrijke uitkomstmaat kwaliteit van leven werd niet gerapporteerd. Meer prospectieve, grote RCT-studies zijn nodig om de effectiviteit van rTMS voor patiënten met tinnitusklachten te onderzoeken.
Transcranial direct current stimulation (tDCS)
Voor de cruciale uitkomstmaat tinnitus last, gemeten door middel van VAS annoyance, tinnitus loudness en tinnitus distress (based on VAS distress, VAS annoyance, THI, VAS discomfort, TQ, THI, mTQ, STSS and TFI), werd geen klinisch relevant verschil gevonden tussen tDCS neuromodulatie en sham tDCS. De belangrijke uitkomstmaten kwaliteit van leven en ernstige bijwerkingen werden niet gerapporteerd. De werkgroep acht het zinvol om meer onderzoek af te wachten alvorens tDCS klinisch toe te passen.
Waarden en voorkeuren van patiënten (en evt. hun verzorgers)
Tinnitus is een veelvoorkomend, complex symptoom, waarvoor op dit moment wereldwijd geen medische oplossing bestaat. Huidige behandelingen richten zich vaak op vermindering van de tinnitushinder. Ook komt tinnitus vaak voor met andere aandoeningen waar gerichte behandelingen voor zijn. Denk aan gehoorverlies (te behandelen met hoortoestellen en/of implantaten) en stemmingsklachten (depressie/angst, te behandelen in overleg met psychiater/psycholoog). De invasiviteit van een behandeling moet worden afgewogen tegen de ernst van de last die een patiënt met tinnitus ondervindt. Tinnitus patiënten die meer tinnitushinder ervaren zullen vaker open staan voor invasievere behandelingen dan patiënten die minder hinder ervaren.
De Goal Attainment Scale (Wagenaar, 2024) laat zien dat patiënten, los van individuele doelen, vijf gemeenschappelijke behandeldoelen hebben:
- Het verkrijgen van controle waardoor er beter met tinnitus kan worden omgegaan
- Het verbeteren van het welzijn en zich minder depressief of angstig voelen
- Het verbeteren van slaap
- Het verminderen van de negatieve effecten van tinnitus op het gehoor
- Het verbeteren van het begrip van tinnitus
Het is voor patiënten van belang dat een behandeling voor tinnitus invloed heeft op een of meerdere van bovenstaande behandeldoelen.
Kosten (middelenbeslag)
De kosten van tinnitus voor de maatschappij zijn door Maes (2013) onderzocht. Zij berekenden de medische kosten op € 1544 per patiënt per jaar (95%CI € 679-2649), en de maatschappelijke kosten op € 5315 per patiënt per jaar (95%CI € 1319-9001). Mede door het chronische karakter van tinnitusklachten, en door de hoge prevalentie zijn deze kosten substantieel te noemen. De kans is dan ook groot dat interventies die ernstige tinnitusklachten mogelijk verminderen uiteindelijk ook kosteneffectief zullen zijn. Dat geldt in het bijzonder bij non-invasieve interventies die geen beroep hoeven te doen op de relatief hoge kosten van operatieve medisch-specialistische zorg. Wetenschappelijke studies naar de kosteneffectiviteit zullen echter moeten uitwijzen of de bereikte effecten niet alleen klinisch relevant maar ook de investering waard zijn.
Aanvaardbaarheid, haalbaarheid en implementatie
Gezien de ernst van de klachten en de forse impact die tinnitus op het dagelijks leven kan hebben, staan een relatief groot deel van de patiënten open voor verschillende interventies om hun klachten te verminderen (Tyler, 2012). Tinnitus wordt vaak ervaren als een relatief complex probleem en het gebrek aan eenvoudige behandelingen leidt bij patiënten tot frustratie en soms zelfs tot machteloosheid (Schenk-Sandbergen, 2012). Ongeveer 30-40% van de patiënten staat zelfs open voor operatieve, invasieve interventies (Smit, 2018); ook indien ze daarvoor zelf zouden moeten betalen. Het niet invasieve karakter van non-invasieve neuromodulatie kan de acceptatie van patiënten ten gunste komen.
Non-invasieve neuromodulatie is op dit moment geen standaard aangeboden of beschikbare zorg voor patiënten met chronische subjectieve tinnitus in Nederland; in deze richtlijn wordt verdere implementatie van deze zorg niet aangeraden.
Rationale van de aanbeveling: weging van argumenten voor en tegen de interventies
De prevalentie van tinnitusklachten is hoog, met daarbij een forse impact op kwaliteit van leven voor een deel van de patiënten. De behoefte aan een eenvoudige interventie om de klachten te verminderen is dan ook hoog. Op basis van het verrichte literatuuronderzoek is het effect van rTMS neuromodulatie op tinnitusklachten onvoldoende overtuigend effectief. In studies waar wel een klein effect gevonden werd, was er sprake van een kleine onderzoekspopulatie en grote kans op vertekening van de uitkomst, waardoor het ‘level of evidence’ zeer beperkt bleef.
Onderbouwing
Achtergrond
Momenteel zijn er meerdere behandelingen of combinaties hiervan beschikbaar voor de behandeling van tinnitus. Deze module richt zich op de effectiviteit van non-invasieve neuromodulatie bij patiënten met chronische subjectieve tinnitusklachten.
Conclusies / Summary of Findings
Repetitive Transcranial Magnetic Stimulation (rTMS)
Tinnitus burden
|
Very low GRADE |
The evidence is very uncertain about the effect of rTMS neuromodulation on tinnitus burden when compared with sham rTMS in adult patients with chronic subjective tinnitus. Source: Ciminelli, 2021; Hong, 2021; Liang, 2020; Noh, 2020 |
Quality of life
|
No GRADE |
No evidence was found regarding the effect of rTMS neuromodulation on quality of life when compared with watchful waiting in adult patients with chronic subjective tinnitus. Source: - |
Serious adverse events
|
Very low GRADE |
The evidence is very uncertain about the effect of rTMS neuromodulation on serious adverse events when compared with sham rTMS in adult patients with chronic subjective tinnitus. Source: Liang, 2020 |
Transcranial Direct Current Stimulation (tDCS)
Tinnitus burden
|
Very low GRADE |
The evidence is very uncertain about the effect of tDCS neuromodulation on tinnitus burden when compared with sham tDCS in adult patients with chronic subjective tinnitus. Source: Martins, 2022 |
Quality of life
|
No GRADE |
No evidence was found regarding the effect of tDCS neuromodulation on quality of life when compared with watchful waiting in adult patients with chronic subjective tinnitus. Source: - |
Serious adverse events
|
No GRADE |
No evidence was found regarding the effect of tDCS neuromodulation on serious adverse events when compared with watchful waiting in adult patients with chronic subjective tinnitus. Source: - |
Samenvatting literatuur
Description of studies
Repetitive Transcranial Magnetic Stimulation (rTMS)
Liang (2020) performed a systematic review and meta-analysis to examine the effects of rTMS and to evaluate its clinical efficacy and safety. The databases PubMed, Embase, and Cochrane Library were searched for randomized controlled trials published from database inception to April 2020. Studies that reported the clinical efficacy and safety of rTMS in chronic tinnitus, that were RCTs, and that recruited participants without limitations to regions, age, or social status were included in the systematic review. Studies were excluded if they were non-randomized controlled studies, duplicate trials or overlapping data, animal experiments, conference abstracts, letters, and review articles. In total, 29 studies were included in the qualitative synthesis and fourteen studies were included in the quantitative synthesis and meta-analysis. The studies compared an intervention group receiving rTMS with a control group receiving sham rTMS. The following relevant outcome measures were reported: clinical efficacy and safety measured by THI scores (tinnitus burden) and adverse events.
Ciminelli (2020) performed a randomized placebo-controlled, single-blinded clinical trial to evaluate the use of bilateral, high frequency, dorsomedial prefrontal cortex (DMPFC) rTMS in treatment of chronic subjective tinnitus. Patients from both genders with age ranging from 20 to 80 years, with a diagnosis of tinnitus for at least three months and for 5 years at the most, with moderate severity (a minimum of 38 points in the THI), and normal hearing or sensorineural hearing loss compatible with age were eligible for inclusion. Exclusion criteria were conductive hearing loss (outer and middle ear disease), objective tinnitus, somatosensory tinnitus, patients taking mediation acting in the central nervous system, other current tinnitus treatment, presence of psychiatric disorders, and contraindications for rTMS. In total, 36 patients were included, but seven patients dropped out in different stages of the study. The final sample was composed of 29 patients, of whom fifteen patients received active rTMS stimulation and fourteen patients underwent sham stimulation. The rTMS protocol consisted of 10 Hz stimulation with 3000 pulses to each DMPFC. The sham group received the same protocol using a placebo figure of eight coil that produced the same sound and sensation as the active coil. There were multiple follow-up visits, at one, two, four and sixteen weeks after the end of the four weeks of treatment. The following relevant outcome measure was reported: tinnitus burden (THI and VAS loudness).
Hong (2021) performed a randomized, double-blind, sham-controlled trial to explore the safety and effectiveness of multiple daily rounds of theta burst stimulation (TBS) over five consecutive days. Patients over 18 years old with subjective tinnitus for more than two months and who had no improvement with medication were eligible for inclusion. Exclusion criteria were: Meniere’s diseases, conductive hearing loss, objective tinnitus, a history of seizure disorder, previous symptomatic stroke, surgically or traumatically implanted foreign bodies such as a pacemaker, an implanted medication pump, metal in the skull or eyes (other than dental appliances or fillings), or intracardiac lines that might pose a physical hazard during magnetic stimulation. In total, fifteen patients were included, but two patients were excluded due to technical problems during the experiment. The final sample was composed of thirteen patients, of whom ten patients received real rTMS (cTBS), and three patients received sham rTMS. One session of cTBS involved three TMS pulses of 50 Hz, repeated at a 200 ms interval. The patients who received rTMS had a mean tinnitus duration of 28.1 months and the patients who received sham rTMS had a mean tinnitus duration of 81.3 monhts. The follow-up of the study was one to three months. The following relevant outcome measures were reported: tinnitus burden (THI, VAS annoyance) and adverse events.
Noh (2020) performed a double-blind randomized clinical trial to compare the outcome of tinnitus treatment among dual-site rTMS stimulation, auditory cortex only rTMS stimulation and sham stimulation. Patients who had suffered from chronic essential tinnitus for more than six months, who gave informed consent and had all tried some of the several standard treatment modalities (such as vasodilators, antidepressants, hearing aids, noise generators, and tinnitus retraining therapy), but were unsatisfied, were eligible for inclusion. Exclusion criteria were: a history of seizure, suspected diagnosis of organic brain damage, a cardiac pacemaker, other electronic implants, including cochlear implants or intraocular ferromagnetic materials and particles, taking concomitant medications (such as antidepressants and antipsychotics), and serious heart disease or another unstable major medical condition. In total, 48 patients were included in the analysis, of whom sixteen patients received dual-site rTMS, sixteen patients received auditory only rTMS and sixteen patients received sham rTMS. The dual-site and auditory rTMS groups were treated with 12,000 pulses. There were multiple follow-up measurements, at four, eight and twelve weeks after rTMS treatment. The following relevant outcome measure was reported: tinnitus burden (THI score, and VAS awareness/loudness/annoyance/effect on daily life).
Transcranial Direct Current Stimulation (tDCS)
Martins (2022) performed a systematic review and meta-analysis to evaluate the effect of tDCS on tinnitus distress, loudness and psychiatric symptoms. The databases PubMed, Web of Science, Cochrane Library, EMBASE, PsycINFO, OVID, and CINAHL were searched for studies published until 17th of July 2021. Studies were included that enrolled: adult patients (≥18 years old) with tinnitus complaint, with no comorbidities associated with tinnitus symptoms (previous head trauma, use of ototoxic substances, Ménière’s disease, epilepsy, intracranial tumor, cochlear implants, cardiac pacemakers and pregnancy), studies including individuals with hearing loss and psychiatric symptoms were maintained since these are commonly associated with tinnitus; a group with tDCS alone, delivered by conventional or high-definition tDCS; articles measuring tinnitus-related characteristics such as loudness , tinnitus burden (THI or TFI) and/or annoyance (VAS). In total, fourteen studies were included in the qualitative analysis and six studies were included in the quantitative analysis (meta-analysis). The studies compared tDCS stimulation with a control group that received sham stimulation or waitlist control. The following relevant outcome measures were reported: tinnitus burden (THI, TFI or VAS annoyance) and tinnitus distress (based on VAS distress, VAS annoyance, THI, VAS discomfort, TQ, THI, mTQ, STSS and TFI).
Results
Repetitive Transcranial Magnetic Stimulation (rTMS)
Tinnitus burden (critical)
Tinnitus Handicap Inventory (THI)
All four included studies reported the outcome measure tinnitus burden measured by THI at different time points (Ciminelli, 2021; Hong, 2021; Liang, 2020; Noh, 2020). Due to differences in time points of measurements, the results were not pooled.
Ciminelli (2021) reported THI at 16 weeks after the start of treatment. In total, fifteen patients received rTMS and fourteen patients received sham rTMS. The mean difference between the two groups was -11.53 (95% CI -23.12 to 0.06), in favour of the patients who received rTMS. This difference is considered clinically relevant.
Hong (2021) reported THI directly after the intervention and one to three months after rTMS. In total, ten patients receiving rTMS and three patients receiving sham rTMS were taken into account directly after the intervention. The mean difference between the two groups was 4.6, in favour of the patients who received sham rTMS. This difference is not considered clinically relevant. In total, six patients receiving rTMS and one patient receiving sham rTMS were taken into account one to three months after the intervention. The mean difference between the two groups was -53.5, in favour of the patients who received rTMS. This difference is considered clinically relevant.
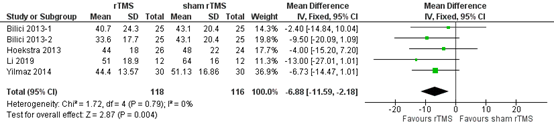
Liang (2020) reported THI at one month and at six months after rTMS. In total, 118 patients receiving rTMS and 116 patients receiving sham rTMS were taken into account one month post intervention. The pooled analysis showed a mean difference of -6.88 (95% CI -11.59 to -2.18), in favour of the patients who received rTMS (Figure 1). This difference is not considered clinically relevant.
Figure 1. THI one month after rTMS versus sham rTMS (Liang, 2020).
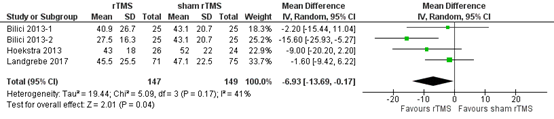
In total, 147 patients receiving rTMS and 149 patients receiving sham rTMS were taken into account six months post intervention. The pooled analysis showed a mean difference of -6.93 (95% CI -13.69 to -0.17), in favour of the patients who received rTMS (Figure 2). This difference is not considered clinically relevant.
Figure 2. THI six months after rTMS versus sham rTMS (Liang, 2020).
Noh (2020) reported difference in THI between pre and at two, four, eight, and twelve weeks post intervention. In total, 16 patients receiving dual site rTMS, 16 patients receiving auditory only rTMS and 16 patients receiving sham rTMS were considered.
The mean difference in ΔTHI at two weeks between the patients who received dual site rTMS and the patients who received sham rTMS was -2.40 (95% CI -13.70 to 8.90), in favour of the patients who received dual site rTMS. This difference is not considered clinically relevant. The mean difference in ΔTHI at two weeks between the patients who received auditory rTMS and the patients who received sham rTMS was -1.40 (95% CI -7.67 to 4.87), in favour of the patients who received auditory rTMS. This difference is not considered clinically relevant.
The mean difference in ΔTHI at four weeks between the patients who received dual site rTMS and the patients who received sham rTMS was -13.00 (95% CI 21.70 to -4.30), in favour of the patients who received dual site rTMS. This difference is considered clinically relevant. The mean difference in ΔTHI at four weeks between the patients who received auditory rTMS and the patients who received sham rTMS was -3.90 (95% CI -11.53 to 3.73), in favour of the patients who received auditory rTMS. This difference is not considered clinically relevant.
The mean difference in ΔTHI at eight weeks between the patients who received dual site rTMS and the patients who received sham rTMS was -13.00 (95% CI -26.58 to 0.58), in favour of the patients who received dual site rTMS. This difference is considered clinically relevant. The mean difference in ΔTHI at eight weeks between the patients who received auditory rTMS and the patients who received sham rTMS was -8.10 (95% CI -18.78 to 2.58), in favour of the patients who received auditory rTMS. This difference is considered clinically relevant.
The mean difference in ΔTHI at twelve weeks between the patients who received dual site rTMS and the patients who received sham rTMS was -13.10 (95% CI -26.58 to 0.38), in favour of the patients who received dual site rTMS. This difference is considered clinically relevant. The mean difference in ΔTHI at twelve weeks between the patients who received auditory rTMS and the patients who received sham rTMS was -7.40 (95% CI -19.60 to 4.80), in favour of the patients who received auditory rTMS. This difference is considered clinically relevant.
Tinnitus Questionnaire (TQ)
One study reported the outcome measure tinnitus burden measured by TQ score one month post intervention and 6 months post intervention (Liang, 2020). In total, 38 patients receiving rTMS and 34 patients receiving sham rTMS were considered one month post intervention. The pooled analysis showed a mean difference of -8.97 (95% CI -20.41 to 2.48), in favour of the patients who received rTMS. This difference is considered clinically relevant. In total, 97 patients receiving rTMS and 99 patients receiving sham rTMS were considered six months post intervention. The pooled analysis showed a mean difference of -7.02 (95% CI -18.18 to 4.13), in favour of the patients who received rTMS. This difference is not considered clinically relevant.
Visual Analogue Score (VAS) annoyance
Four studies reported the outcome measure tinnitus burden measured by VAS annoyance (Ciminelli, 2021; Hong, 2021; Liang, 2020; Noh, 2020). Due to differences in time points of measurements, the results were not pooled.
Ciminelli (2021) reported VAS annoyance at 16 weeks. In total, fifteen patients received rTMS and fourteen patients received sham rTMS. The mean difference between the two groups was 0.80 (95% CI -2.21 to 0.61), in favour of the patients who received rTMS. This difference is not considered clinically relevant.
Hong (2021) reported VAS annoyance directly after the intervention and one to three months after rTMS. In total, ten patients receiving rTMS and three patients receiving sham rTMS were considered directly after the intervention. The mean difference between the two groups was -1.2, in favour of the patients who received rTMS. This difference is not considered clinically relevant. In total, six patients receiving rTMS and one patient receiving sham rTMS were considered one to three months after the intervention. The mean difference between the two groups was -0.3, in favour of the patients who received rTMS. This difference is not considered clinically relevant.
Liang (2020) reported VAS score one month after rTMS. In total, 56 patients receiving rTMS and 54 patients receiving sham rTMS were considered one month post intervention. The pooled analysis showed a mean difference of -0.64 (95% CI -1.77 to 0.48), in favour of the patients who received rTMS. This difference is not considered clinically relevant.
Noh (2020) reported difference in VAS annoyance between pre and at two, four, eight, and twelve weeks post intervention. In total, 16 patients receiving dual site rTMS, 16 patients receiving auditory only rTMS and 16 patients receiving sham rTMS were considered.
The mean difference in ΔVAS annoyance at two weeks between the patients who received dual site rTMS and the patients who received sham rTMS was -1.20 (95% CI -2.66 to 0.26), in favour of the patients who received dual site rTMS. This difference is not considered clinically relevant. The mean difference in ΔVAS annoyance at two weeks between the patients who received auditory rTMS and the patients who received sham rTMS was -0.60 (95% CI -1.77 to 0.57), in favour of the patients who received auditory rTMS. This difference is not considered clinically relevant.
The mean difference in ΔVAS annoyance at four weeks between the patients who received dual site rTMS and the patients who received sham rTMS was -1.70 (95% CI -3.39 to -0.01), in favour of the patients who received dual site rTMS. This difference is considered clinically relevant. The mean difference in ΔVAS annoyance at four weeks between the patients who received auditory rTMS and the patients who received sham rTMS was -0.60 (95% CI -1.70 to 0.50), in favour of the patients who received auditory rTMS. This difference is not considered clinically relevant.
The mean difference in ΔVAS annoyance at eight weeks between the patients who received dual site rTMS and the patients who received sham rTMS was -2.10 (95% CI -3.65 to -0.55), in favour of the patients who received dual site rTMS. This difference is considered clinically relevant. The mean difference in ΔVAS annoyance at eight weeks between the patients who received auditory rTMS and the patients who received sham rTMS was -1.10 (95% CI -2.13 to -0.07), in favour of the patients who received auditory rTMS. This difference is not considered clinically relevant.
The mean difference in ΔVAS annoyance at twelve weeks between the patients who received dual site rTMS and the patients who received sham rTMS was -2.10 (95% CI -3.69 to -0.51), in favour of the patients who received dual site rTMS. This difference is considered clinically relevant. The mean difference in ΔVAS annoyance at twelve weeks between the patients who received auditory rTMS and the patients who received sham rTMS was -0.50 (95% CI -1.68 to 0.68), in favour of the patients who received auditory rTMS. This difference is not considered clinically relevant.
Quality of life (important)
None of the included studies reported the outcome measure quality of life.
Serious adverse events (important)
One study reported the outcome measure adverse events (Liang, 2020).
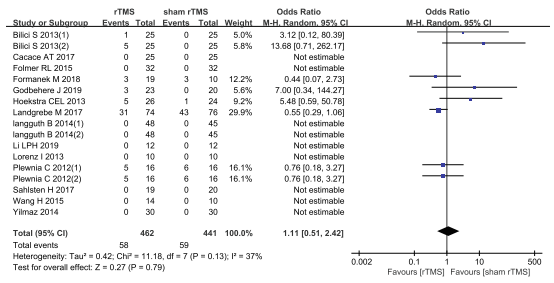
Liang (2020) reported adverse events after treatment. In total, 58 of 462 patients who received rTMS experienced adverse events, and 59 of 441 patients who received sham rTMS experienced adverse events. Among these adverse events, 21 patients reported headache, seven patients reported worsening of tinnitus, five patients reported sleep disturbances, three patients reported facial muscle discomfort, back pain, muscle hardening and ENT symptoms, two patients reported neck and shoulder stiffness, one patient reported increased sensitivity to noise, painful sensation in the affected ear, anxiety and panic attacks, and nine patients reported other events. The pooled analysis showed an odds ratio of 1.11 (95% CI 0.51 to 2.42), in favour of the patients who received sham rTMS (Figure 3). This difference is not considered clinically relevant.
Figure 3. Adverse events after rTMS versus sham rTMS. From Liang (2020).
Transcranial Direct Current Stimulation (tDCS)
Tinnitus burden (critical)
Tinnitus distress
One study reported the outcome measure tinnitus distress (Martins, 2022). In total, 70 patients receiving active tDCS and 73 patients receiving placebo were considered. The pooled analysis showed a standardized mean difference of -0.50 (95% CI -0.91 to -0.10), in favour of the patients who received active tDCS. This difference is not considered clinically relevant.
Quality of life (important)
None of the included studies reported the outcome measure quality of life.
Serious adverse events (important)
None of the included studies reported the outcome measure serious adverse events.
Level of evidence of the literature
Repetitive Transcranial Magnetic Stimulation (rTMS)
Tinnitus burden
THI
The level of evidence for tinnitus burden measured by THI was based on RCTs and therefore starts high. The level of evidence was downgraded by three levels because of study limitations (risk of bias, -1), because of the small sample size (imprecision, -1), and because of conflicting results (inconsistency, -1). The level of evidence is therefore graded as very low.
TQ
The level of evidence for tinnitus burden measured by THI was based on RCTs and therefore starts high. The level of evidence was downgraded by three levels because of study limitations (risk of bias, -1), because of a small sample size (imprecision, -1), and because of conflicting results (inconsistency, -1). The level of evidence is therefore graded as very low.
VAS annoyance
The level of evidence for tinnitus burden measured by THI was based on RCTs and therefore starts high. The level of evidence was downgraded by three levels because of study limitations (risk of bias, -1), because of a small sample size (imprecision, -1), and because of conflicting results (inconsistency, -1). The level of evidence is therefore graded as very low.
Tinnitus loudness
The level of evidence for tinnitus burden measured by THI was based on RCTs and therefore starts high. The level of evidence was downgraded by three levels because of study limitations (risk of bias, -1), and because of a very small sample size (imprecision, -2). The level of evidence is therefore graded as very low.
Quality of life
The level of evidence for quality of life could not be established as no studies reported this outcome measure.
Serious adverse events
The level of evidence was based on a systematic review that includes RCTs and therefore starts high. The level of evidence was downgraded by three levels because of study limitations (risk of bias, -1), because of a small sample size (imprecision, -1), and because of conflicting results (inconsistency, -1). The level of evidence is therefore graded as very low.
Transcranial Direct Current Stimulation (tDCS)
Tinnitus burden
The level of evidence was based on a systematic review and therefore starts high. The level of evidence was downgraded by three levels because of study limitations (risk of bias, -1), and because of a very small sample size (imprecision, -2). The level of evidence is therefore graded as very low.
Quality of life
The level of evidence for quality of life could not be established as no studies reported this outcome measure.
Serious adverse events
The level of evidence for serious adverse events could not be established as no studies reported this outcome measure.
Zoeken en selecteren
A systematic review of the literature was performed to answer the following question:
What are the (un)favorable effects of neuromodulation compared to watchful waiting, placebo/sham control, or care as usual in adult patients with chronic subjective tinnitus on tinnitus burden, quality of life, and serious adverse events?
| P: patients | adult patients with chronic subjective tinnitus (≥3 months) |
| I: intervention | neuromodulation (rTMS, rTDS) |
| C: control | watchful waiting, placebo/sham control, or care as usual |
| O: outcome measure | tinnitus burden, quality of life, serious adverse events. |
Relevant outcome measures
The guideline development group considered tinnitus burden as a critical outcome measure for decision making; and quality of life and serious adverse events as important outcome measures for decision making.
A priori, the working group defined the outcome measures as follows:
- Care as usual as defined by the authors (see description of studies);
- Tinnitus burden measured with single- (NRS) or multi-item questionnaires (e.g. THI (tinnitus Handicap Inventory, TQ (Tinnitus Questionary), TFI (Tinnitus Functional Index), VAS impact, intrusiveness); Tinnitus burden includes aspects such as impact on daily life, distress and disability (de Ridder, 2021).
- Quality of life in general;
- Serious adverse events.
The working group defined the following differences as a minimal clinically (patient) important:
- THI: 7 points (Fuller, 2020).
- TFI: 13 (Meikle, 2012)
- TQ: 8 (Zeman, 2012)
- Quality of life: 10%
- Serious adverse events: 25% (RR < 0.8 or RR > 1.25).
- VAS score: 15 points on a 100 scale, 1.5 on a 10 scale (Adamchic, 2021)
If different measurement instruments were pooled, then differences were defined as: a trivial effect (standardized mean difference (SMD) = 0 to 0.2), a small effect (SMD = 0.2 to 0.5), a moderate effect (SMD = 0.5 to 0.8), and a large effect (SMD >0.8).
Search and select (Methods)
In original search for the guideline Tinnitus – Behandeling van patiënten met tinnitus (2016), Medline (via OVID) and Embase (via Embase.com) were searched with relevant search terms from 1979 until January 2014. This resulted in 471 hits. Studies were selected based on the following criteria: systematic reviews and RCT’s that compared tinnitus treatments with watchful waiting (placebo) in adult patients with chronic subjective tinnitus. In total, 26 studies were included based on title and abstract screening. After reading the full text, two studies were included regarding neuromodulation (Meng, 2011; Song, 2012).
For this update for the guideline Tinnitus – behandeling van patiënten met tinnitus – neuromodulatie (2024), the databases Medline (via OVID) and Embase (via Embase.com) were searched with relevant search terms from 01-01-2014 until 02-05-2022 (SR’s) and 07-07-2022 (RCT’s). The detailed search strategy is depicted under the tab Methods. The systematic literature search resulted in 363 hits. Studies were selected based on the following criteria: systematic reviews and RCT’s that compared neuromodulation with watchful waiting, placebo, or sham control in adult patients with chronic subjective tinnitus. In total, 33 studies were initially selected based on title and abstract screening. After reading the full text, 28 studies were excluded (see the table with reasons for exclusion under the tab Methods). This resulted in the inclusion of two systematic reviews and three randomized controlled trials. The two studies from the original search (Meng, 2011; Song, 2012) were excluded, because they have both been included in the more recent systematic review of Martins (2022). See table with reasons for exclusion under the tab Methods.
Results
Two systematic reviews and three randomized controlled trials were included in the analysis of the literature. Important study characteristics and results are summarized in the evidence tables. The assessment of the risk of bias is summarized in the risk of bias tables.
Referenties
- Ciminelli P, Machado S, Palmeira M, Coutinho ESF, Sender D, Nardi AE. Dorsomedial Prefrontal Cortex Repetitive Transcranial Magnetic Stimulation for Tinnitus: Promising Results of a Blinded, Randomized, Sham-Controlled Study. Ear Hear. 2021 Jan/Feb;42(1):12-19. doi: 10.1097/AUD.0000000000000908. PMID: 32639254.
- Hong SM, Kim SK, Seo MY, Kang SY. Multiple Daily Rounds of Theta-Burst Stimulation for Tinnitus: Preliminary Results. Medicina (Kaunas). 2021 Jul 23;57(8):743. doi: 10.3390/medicina57080743. PMID: 34440949; PMCID: PMC8401076.
- Liang Z, Yang H, Cheng G, Huang L, Zhang T, Jia H. Repetitive transcranial magnetic stimulation on chronic tinnitus: a systematic review and meta-analysis. BMC Psychiatry. 2020 Nov 23;20(1):547. doi: 10.1186/s12888-020-02947-9. PMID: 33228598; PMCID: PMC7684956.
- Maes IH, Cima RF, Vlaeyen JW, Anteunis LJ, Joore MA. Tinnitus: a cost study. Ear Hear. 2013 Jul-Aug;34(4):508-14. doi: 10.1097/AUD.0b013e31827d113a. PMID: 23411656.
- Martins ML, Souza DDS, Cavalcante MEOB, Barboza HN, de Medeiros JF, Dos Santos Andrade SMM, Machado DGDS, da Rosa MRD. Effect of transcranial Direct Current stimulation for tinnitus treatment: A systematic review and meta-analysis. Neurophysiol Clin. 2022 Feb;52(1):1-16. doi: 10.1016/j.neucli.2021.12.005. Epub 2022 Jan 10. PMID: 35027291.
- Meikle MB, Henry JA, Griest SE, Stewart BJ, Abrams HB, McArdle R, Myers PJ, Newman CW, Sandridge S, Turk DC, Folmer RL, Frederick EJ, House JW, Jacobson GP, Kinney SE, Martin WH, Nagler SM, Reich GE, Searchfield G, Sweetow R, Vernon JA. The tinnitus functional index: development of a new clinical measure for chronic, intrusive tinnitus. Ear Hear. 2012 Mar-Apr;33(2):153-76. doi: 10.1097/AUD.0b013e31822f67c0. Erratum in: Ear Hear. 2012 May;33(3):443. PMID: 22156949.
- Noh TS, Kyong JS, Park MK, Lee JH, Oh SH, Suh MW. Dual-site rTMS is More Effective than Single-site rTMS in Tinnitus Patients: A Blinded Randomized Controlled Trial. Brain Topogr. 2020 Nov;33(6):767-775. doi: 10.1007/s10548-020-00797-y. Epub 2020 Sep 17. PMID: 32944806.
- Smit JV, Pielkenrood BJ, Arts RAGJ, Janssen ML, Temel Y, Stokroos RJ. Patient Acceptance of Invasive Treatments for Tinnitus. Am J Audiol. 2018 Jun 8;27(2):184-196. doi: 10.1044/2017_AJA-17-0015. PMID: 29507954.
- Tyler RS. Patient preferences and willingness to pay for tinnitus treatments. J Am Acad Audiol. 2012 Feb;23(2):115-25. doi: 10.3766/jaaa.23.2.6. PMID: 22353680.
- Wagenaar O, Gilles A, Van Rompaey V, Blom H. Goal Attainment Scale in tinnitus (GAS-T): treatment goal priorities by chronic tinnitus patients in a real-world setting. Eur Arch Otorhinolaryngol. 2024 Feb;281(2):693-700. doi: 10.1007/s00405-023-08134-2. Epub 2023 Jul 25. PMID: 37488402.
- Zeman F, Koller M, Schecklmann M, Langguth B, Landgrebe M; TRI database study group. Tinnitus assessment by means of standardized self-report questionnaires: psychometric properties of the Tinnitus Questionnaire (TQ), the Tinnitus Handicap Inventory (THI), and their short versions in an international and multi-lingual sample. Health Qual Life Outcomes. 2012 Oct 18;10:128. doi: 10.1186/1477-7525-10-128. PMID: 23078754; PMCID: PMC3541124.
Evidence tabellen
Evidence table for systematic review of RCTs and observational studies (intervention studies)
Research question:
|
Study reference |
Study characteristics |
Patient characteristics |
Intervention (I) |
Comparison / control (C)
|
Follow-up |
Outcome measures and effect size |
Comments |
|
Liang, 2020
Individual study characteristics deduced from [Liang, 2020] |
SR and meta-analysis of RCTs
Literature search up to April 2020
A: Bilici, 2013 (1) B: Bilici, 2013 (2) C: Cacace, 2017 D: Folmer, 2015 E: Formanek, 2018 F: Godbehere, 2019 G: Hoekstra, 2013 H: Landgrebe, 2017 I: Langguth, 2014 (1) J: Langguth, 2014 (2) K: Li, 2019 L: Lorenz, 2013 M: Plewnia, 2012 (1) N: Plewnia, 2012 (2) O: Sahlsten, 2017 P: Wang, 2015 Q: Yilmaz, 2014
Study design: A: randomized, double-blind, placebo-controlled study B: randomized, double-blind, placebo-controlled study C: Randomized, single-blinded sham-controlled crossover study D: randomized, participant and clinical or observer-blinded, placebo-controlled clinical trial E: randomized double-blind controlled trial F: Two-arm, single-blind, randomized controlled trial G: randomized, double-blind placebo-controlled clinical trial H: Sham-controlled, randomized multi-centre trial I: randomized, double-blind, parallel-group, controlled clinical trial J: randomized, double-blind, parallel-group, controlled clinical trial K: randomized controlled trial L: randomized, single-blind sham-controlled trial M: randomized controlled trial N: randomized controlled trial O: randomized, placebo-controlled study P: randomized controlled trial Q: randomized controlled trial
Source of funding and conflicts of interest: Not reported. |
Inclusion criteria SR: Studies were included if they: Reported the clinical efficacy and safety of rTMS in chronic tinnitus, were RCTs, and recruited participants without limitations to regions, ages, or social status.
Exclusion criteria SR: Studies were excluded if they fulfilled the following criteria: Non-randomized controlled studies, duplicate trials or overlapping data, animal experiments, conference abstracts, letters, and review articles.
14 studies included in the quantitative analysis
Important patient characteristics at baseline:
N (I/C): A: 75, 75 B: 75, 75 C: 30, 30 D: 32, 32 E: 20, 12 F: unclear G: 26, 24 H: 71, 75 I: 48, 45 J: 48, 45 K: 12, 12 L: 10, 10 M: 16, 16 N: 16, 16 O: 19, 20 P: 14, 10 Q: 60, 60
Gender (male/female), mean age: A: I: 33/42, 40 ± 13.2. C: 33/42, 40 ± 13.2. B: I: 33/42, 40 ± 13.2. C: 33/42, 40 ± 13.2. C: I: 30/0, 54.2 ± 14.2. C: 30/0, 42.2 ± 14.2. D: I: 25/7, 58.3 ± 9.5. C: 26/6, 62.8 ± 8.3. E: I: 13/7, 47.9 ± 14.31. C: 10/2, 51.8 ± 10.34. F: unclear G: I: 26/0, 50 ± 12. C: 15/9, 50 ± 12. H: I: 54/17, 48.1 ± 12.5. C: 51/24, 49.9 ± 13.2. I: I: 35/13, 44.9 ± 11.5. C: 31/14, 50.3 ± 12.9. J: I: 32/16, 50.4 ± 12.5. C: 31/14, 50.3 ± 12.9. K: I: 7/5, 57 ± 10.1. C: 7/5, 54 ± 7.5. L: I: 7/3, 49.8. C: 7/3, 49.8. M: I: 10/6, 46.4 ± 13.0. C: 8/8, 45.6 ± 10.3. N: I: 7/9, 55.8 ± 9.7. C: 8/8, 45.6 ± 10.3. O: I: 13/6, 48.9 ± 13.1. C: 14/6, 51.5 ± 10.7. P: I: 6/8, 62.1 ± 9.91. C: 3/7, 56.4 ± 11.8. Q: I: 27/33, 49.8 ± 8.03. C: 27/33, 49.8 ± 8.03.
Duration of tinnitus (months): A: I: >12. C: >12. B: I: >12. C: >12. C: Unclear D: I: >12. C: >12. E: I: 53.4 ± 61.89. C: 76.8 ± 76.85. F: Unclear G: I: 58 (8-240). C: 38 (12-420). H: I: 6.2 ± 5.3. C: 8.1 ± 8.4. I: I: 68.0 ± 97.0. C: 74.4 ± 74.2. J: I: 78.3 ± 64.9. C: 78.3 ± 64.9. K: I: >6. C: >6. L: I: 21.6. C: 21.6. M: I: 27 ± 14. C: 22 ± 14. N: I: 28 ± 13. C: 22 ± 14. O: I: >6. C: >6. P: I: 6-72. C: 6-72. Q: I: >6. C: >6. |
Intervention: A: rTMS (1-Hz, 900 stimuli, 110% motor threshold) B: rTMS (10-Hz, 600 stimuli, 110% motor threshold) C: rTMS (1-Hz, 1200 stimuli, 110% motor threshold) D: rTMS (1-Hz, 2000 stimuli, 110% or lower motor threshold) E: rTMS (1-Hz, 1000 stimuli, 110% motor threshold) F: rTMS (5-Hz, 1200 stimuli, 80% motor threshold) G: rTMS (1-Hz, 2000 stimuli, 110% motor threshold) H: rTMS (1-Hz, 2000 stimuli, 110% motor threshold) I: rTMS (1-Hz, 2000 stimuli, 110% motor threshold) J: rTMS (1-Hz, 2000 stimuli, 110% motor threshold) K: rTMS (1-Hz, 1800 stimuli, 110% or lower motor threshold) L: rTMS (1-Hz, 1000 stimuli, 110% motor threshold) M: rTMS (5-Hz, 2400 stimuli, 80% motor threshold) N: rTMS (5-Hz, 2400 stimuli, 80% motor threshold) O: rTMS (1-Hz, 4000 stimuli, 100% motor threshold) P: rTMS (1-Hz, 1000 stimuli, 110% motor threshold) Q: rTMS (1-Hz, 1800 stimuli, motor threshold unclear)
|
Control: A: sham rTMS B: sham rTMS C: sham rTMS D: sham rTMS E: sham rTMS F: sham rTMS G: sham rTMS H: sham rTMS I: sham rTMS J: sham rTMS K: sham rTMS L: sham rTMS M: sham rTMS N: sham rTMS O: sham rTMS P: sham rTMS Q: sham rTMS
|
End-point of follow-up: A: 6 months B: 6 months C: Unclear D: 6 months E: 6 months F: 4 weeks G: 6 months H: 6 months I: 11 weeks J: 11 weeks K: 1 month L: Unclear M: 12 weeks N: 12 weeks O: 6 months P: Unclear Q: 1 month
For how many participants were no complete outcome data available? Not reported.
|
Tinnitus burden THI 1-month post-intervention Effect measure: mean difference, [95% CI]: A: -2.40 (-14.84 to 10.04) B: -9.50 (-20.09 to 1.09) G: -4.00 (-15.20 to 7.20) K: -13.00 (-27.01 to 1.01) Q: -6.73 (-14.47 to 1.01)
Pooled effect (random effects model): -6.88 [95% CI -11.59 to -2.18] favoring rTMS. Heterogeneity (I2): 0%
THI 6-month post-intervention Effect measure: mean difference, [95% CI]: A: -2.20 (-15.44 to 11.04) B: -15.60 (-25.93 to -5.27) G: -9.00 (-20.20 to 2.20) H: -1.60 (-9.42 to 6.22)
Pooled effect (random effects model / fixed effects model): -6.51 [95% CI -11.55 to -1.48] favoring rTMS Heterogeneity (I2): 41%
TQ score 1 month post-intervention: Pooled effect (random effects model / fixed effects model): -8.97 [95% CI -20.41 to 2.48] favoring rTMS Heterogeneity (I2): 53%
TQ score 6 months post-intervention: Pooled effect (random effects model / fixed effects model): -7.02 [95% CI -18.18 to 4.13] favoring rTMS Heterogeneity (I2): 79%
VAS score 1 month post-intervention: Pooled effect (random effects model / fixed effects model): -0.64 [95% CI -1.77 to 0.48] favoring rTMS Heterogeneity (I2): 69%
Adverse events Effect measure odds ratio, [95% CI]: A: 3.12 (0.12 to 80.39) B: 13.68 (0.71 to 262.17) C: not estimable D: not estimable E: 0.44 (0.07 to 2.73) F: 7.00 (0.34 to 144.27) G: 5.48 (0.59 to 50.78) H: 0.55 (0.29 to 1.06) I: not estimable J: not estimable K: not estimable L: not estimable M: 0.76 (0.18 to 3.27) N: 0.76 (0.18 to 3.27) O: not estimable P: not estimable Q: not estimable
Pooled effect (random effects model): 1.11 [95% CI 0.51 to 2.42] favoring sham rTMS Heterogeneity (I2): 37%
|
Risk of bias (high, some concerns, or low): Tool used by authors: Cochrane Handbook Version 5.3
A: Unclear (selection) B: Unclear (selection) C: Unclear (selection, detection) D: Unclear (selection) E: Unclear (selection) F: High (attrition), unclear (selection, detection) G: Unclear (selection) H: Unclear (selection) I: Unclear (selection) J: Unclear (selection) K: Unclear (selection, detection) L: Unclear (selection, detection) M: Unclear (selection, performance, detection) N: Unclear (selection, performance, detection) O: Unclear (selection) P: Unclear (selection, performance, detection) Q: Unclear (selection, detection)
|
|
Martins, 2022
Individual study characteristics deduced from [Martins, 2022] |
SR and meta-analysis of RCTs
Literature search up to 17 July 2021
A: Abtahi, 2018 B: De Ridder, 2012 C: Faber, 2012 D: Forogh, 2016 E: Fregni, 2006 F: Garin, 2011 G: Henin, 2016 H: Hyvärinen, 2016 I: Pal, 2015 J: Shekhawat, 2018 K: Souza, 2020 L: To, 2016 M: Vanneste, 2013 N: Vanneste, 2011
Study design: A: parallel-arm B: parallel-arm C: crossover D: parallel-arm E: crossover F: crossover G: crossover H: parallel-arm I: parallel-arm J: crossover K: parallel-arm L: parallel-arm M: parallel-arm N: crossover
Setting and Country: A: Hospital, Iran B: University Hospital, Belgium C: University Hospital, Belgium D: not reported, Iran E: not reported F: not reported, Belgium G: School of Medicine, USA H: University Hospital, Helsinki I: University Hospital, Switzerland J: University Clinic, New Zealand K: Clinical School, Brazil L: not reported M: University Hospital, Belgium N: University Hospital, Belgium
Source of funding and conflicts of interest: Not reported. |
Inclusion criteria SR: Studies were included that enrolled: adult patients (≥18 years old) with tinnitus complaint, with no comorbidities associated with tinnitus symptoms (previous head trauma, use of ototoxic substances, Meniere’s disease, epilepsy, intracranial tumor, cochlear implants, cardiac pacemakers and pregnancy), studies including individiuals with hearing loss and psychiatric symptoms were maintained since these are commonly associated with tinnitus; a group with tDCS alone, delivered by conventional or high-definition tDCS; articles measuring tinnitus-related characteristics such as loudness , tinnitus severity (THI or TFI) and/or annoyance (VAS).
Exclusion criteria SR: Not reported.
14 studies included in the quantitative analysis
Important patient characteristics at baseline:
N (total): A: 51 B: 675 C: 15 D: 20 E: 7 F: 20 G: 14 H: 43 I: 42 J: 13 K: 24 L: 40 M: 50 N: 12
Gender (male/female), mean age: A: NR, 46.5 ± 14.5 B: 260/415, 48.33 ± 14.57 C: 11/4, 49.43 ± 14.89 D: NR, 48.22 ± 4.7 E: 4/3, 51.7 ± 8.4 F: 16/4, 50.9 ± 12.9 G: 10/4, 55.2 ± 6.8 H: 23/20, 51 ± 15.4 I: 24/18, 49.8 ± 11.05 J: 13/0, 53.6 K: 8/16, 47.54 ± 12.96 L: 22/18, 48.33 ± 10.74 M: 24/26, 51.37 ± 12.99 N: 9/3, 50.45
Duration of tinnitus: A: >1 B: 5.14 ± 4.24 C: 7.44 ± 5.69 D: 7.77 ± 2.55 E: 9.8 ± 6.3 F: NR G: NR H: NR I: 5.58 ± 6.96 J: NR K: 27 L: 10.83 ± 14.35 M: 2.85 ± 2.10 N: 4.75 ± 1.02 |
Intervention: A: Anodal stimulation, cathodal stimulation B: tDCS (cathodal in left DLPFC and anode in right DLPFC), tDCS based on EEG connectivity C: Anodal left/cathodal right DLPFC D: real anodal E: five types of stimulation F: anodal + cathodal G: Cas-alone, tDCS alone, CAS + HD-tDCS H: real bifrontal, real LTA I: teal tDCS J: real K: real tDCS L: tDCS M: tACS, tDCS N: tDCS
|
Control: A: control B: waiting list C: sham D: sham E: sham tDCS F: sham G: sham H: sham, control I: sham J: sham K: sham tDCS L: waiting list M: sham N: sham
|
End-point of follow-up: Not reported.
For how many participants were no complete outcome data available? Not reported. |
Tinnitus burden Tinnitus loudness Pooled effect (absolute): SMD -0.37 [95% CI -0.68 to -0.06] favoring tDCS Heterogeneity (I2): 0%
5 studies Active tDCS: 105 patients Placebo: 107 patients
Tinnitus distress (annoyance/severity) Pooled effect (absolute): SMD -0.50 [95% CI -0.91 to -0.10] favoring tDCS Heterogeneity (I2): 29%
5 studies Active tDCS: 70 patients Placebo: 73 patients
|
Risk of bias (high, some concerns, or low): Tool used by authors: A: High (attrition), unclear (selection) B: High (performance, detection, attrition), unclear (selection) C: Unclear (selection) D: Low E: Unclear (performance, detection) F: Low G: High (attrition), unclear (detection) H: Unclear (selection) I: Low J: Unclear (selection) K: Unclear (selection) L: Unclear (selection, performance, detection) M: Unclear (selection) N: Unclear (selection, performance, detection)
|
This table is also suitable for diagnostic studies (screening studies) that compare the effectiveness of two or more tests. This only applies if the test is included as part of a test-and-treat strategy – otherwise the evidence table for studies of diagnostic test accuracy should be used.
|
Study reference |
Study characteristics |
Patient characteristics 2 |
Intervention (I) |
Comparison / control (C) 3
|
Follow-up |
Outcome measures and effect size 4 |
Comments |
|
Ciminelli, 2021 |
Randomized placebo-controlled, single-blinded clinical trial
Setting and country: Rio de Janeiro, Brazil
Funding: Not reported.
Conflicts of interest: The authors have no conflicts of interest to disclose. |
Inclusion criteria: Patients from both genders with age ranging from 20 to 80 years, with a diagnosis of tinnitus for at least three months and for 5 years at the most, with moderate severity (a minimum of 38 points in the THI), and normal hearing or sensorineural hearing loss compatible with age were eligible for inclusion.
Exclusion criteria: Exclusion criteria were conductive hearing loss (outer and middle ear disease), objective tinnitus, somatosensory tinnitus, patients taking mediation acting in the central nervous system, other current tinnitus treatment, presence of psychiatric disorders, and contraindications for rTMS
N total at baseline: 36 patients randomized, 7 dropped out. Intervention: 15 Control: 14
Important prognostic factors2: Age 47-54, 55-64, 65-78: I: 5, 8, 2 C: 1, 9, 4
Gender (male): I: 8 (53.3%) C: 9 (64.3%)
Time since beginning of tinnitus (years): I: 3.2 (1.69) C: 2.99 (1.80)
Imbalance in sex and age, therefore all comparisons were controlled by these two variables. |
Intervention: 10 Hz stimulation at 120% of the resting motor threshold of the extensor hallucis longus. 3000 pulses per session to each DMPFC non simultaneously. First the coil was positioned to the left side and then to the right side.
|
Control: Same protocol using placebo figure of eight coil that produces the same sound and sensation as the active coil. |
Length of follow-up: Measurements at 1, 2, 4 and 16 weeks.
Loss-to-follow-up: Intervention: N=3 (16.7%) Reasons: discontinued intervention -pain during procedure (n=2) -abandoned (n=1)
Control: N=4 (22.2%) Reasons: discontinued intervention -fear of adverse events (n=1) -abandoned (n=3)
Incomplete outcome data: Intervention: N=0
Control: N=0 |
Outcome measures and effect size (include 95% CI and p-value if available)
Tinnitus burden (16 weeks) THI MD 11.53 (95% CI -23.12 to 0.06), favouring rTMS.
Tinnitus loudness MD 4.46 dB (95% CI -9.60 to 0.68 dB), favouring rTMS.
VAS annoyance MD 0.80 (95% CI -2.21 to 0.61), favouring rTMS.
|
|
|
Hong, 2021 |
Type of study: Randomized, double-blind, sham-controlled trial.
Setting and country: Hallym University College of Medicine, Hwaseong, Korea.
Funding: This research received no external funding.
Conflicts of interest: The authors declare no conflict of interest. |
Inclusion criteria: Patients over 18 years old with subjective tinnitus for more than 2 months and who had no improvement with medication.
Exclusion criteria: Meniere’s disease, conductive hearing loss, objective tinnitus, a history of seizure disorder, previous symptomatic stroke, surgically or traumatically implanted foreign bodies such as a pacemaker, an implanted medication pump, metal in the skull or eyes, or intracardiac lines that might pose a physical hazard during magnetic stimulation.
N total at baseline: Post-intervention Intervention: 10 Control: 3 1-3 months after Intervention: 6 Control: 1
Important prognostic factors2: Age ± SD: I: 55.1 ± 11.6 C: 62.0 ± 16.7
Women, n (%) I: 3 (30.0) C: 2 (66.7)
Duration of disease (months): I: 28.1 ± 39.6 C: 81.3 ± 67.0
Groups were comparable at baseline. |
Intervention: Theta-burst stimulation, a new method of repetitive transcranial magnetic stimulation (rTMS). One session involved 3 TMS pulses of 50 Hz repeated at a 200 ms interval for 20 s at a stimulus intensity of 70% RMT. They applied 4 sessions at a 1-s interval, after 15 minutes, another 4 sessions with a 1-s gap between sessions, 200 per day (2400 pulses/day).
|
Control: Sham repetitive transcranial magnetic stimulation (rTMS).
|
Length of follow-up: Follow-up visit at 1-3 months (7 patients participated).
Loss-to-follow-up: Total: n=2 (13.3%) Reason: technical problems during the experiment
|
Tinnitus burden VAS for annoyance after Intervention: 5.6 Control: 6.8 MD -1.2
VAS for annoyance 1-3 months Intervention: 4.7 Control: 5.0 MD -0.3
THI total subscale after Intervention: 47.0 Control: 42.4 MD 4.6
THI total subscale 1-3 months Intervention: 38.9 Control: 92.4 MD -53.5
Adverse events Intervention: No adverse events.
|
|
|
Noh, 2020 |
Type of study: Blind randomized clinical trial.
Setting and country: Seoul National University College of medicine, Seoul, Korea.
Funding: This research was supported by the Korea Health Industry Development Institute (KHDI) of Korean Ministry of Health and Welfare.
Conflicts of interest: The authors declare no competing financial interests. |
Inclusion criteria: Patients who had suffered from chronic essential tinnitus for more than six months, who gave informed consent and had all tried some of the several standard treatment modalities (such as vasodilators, antidepressants, hearing aids, noise generators, and tinnitus retraining therapy), but were unsatisfied, were eligible for inclusion.
Exclusion criteria: Exclusion criteria were: a history of seizure, suspected diagnosis of organic brain damage, a cardiac pacemaker, other electronic implants, including cochlear implants or intraocular ferromagnetic materials and particles, taking concomitant medications (such as antidepressants and antipsychotics), and serious heart disease or another unstable major medical condition.
N total at baseline: Dual site: 16 Auditory: 16 Control: 16 Total: 48 Total before loss to follow-up: 51 17-18-16
Important prognostic factors2: Age ± SD: Dual site: 54.1 ± 9.4 Auditory: 51.1 ± 12.6 C: 55.9 ± 8.8
Gender (M:F): Dual site: 13:3 Auditory: 10:6 C: 11:5
Duration of illness (months): Dual site: 76.1 ± 129.3 Auditory: 76.1 ± 98.8 C: 85.1 ± 102.4
Groups were comparable at baseline. |
Intervention I: Dual site Magnetic stimulation at AC + DLPFC site at a frequency of 1 Hz with 110% of resting motor threshold stimulation intensity. The train duration and off-time were 40 and 20 seconds respectively. The group was treated with a total of 12,000 pulses: 2000 pulses over the AC and 1000 pulses over the DLPFC daily for four consecutive days.
Intervention II: Auditory only Magnetic stimulation at AC site at a frequency of 1 Hz with 110% of resting motor threshold stimulation intensity. The train duration and off-time were 40 and 20 seconds respectively. The group was treated with a total of 12,000 pulses: 3000 pulses over the AC daily for four consecutive days.
|
Control: Sham rTMS (same scalp position as dual site group). The sham group also visited the clinic for four days.
|
Length of follow-up: There were multiple follow-up measurements, at four, eight and twelve weeks after rTMS treatment.
Loss-to-follow-up: Dual site: N=1 (2%) Reasons: -personal reasons unrelated to the treatment
Auditory: N=2 (3.9%) Reasons: -personal reasons unrelated to the treatment -headaches after rTMS
Control: N=0 (%)
|
Tinnitus burden ΔTHI 2 weeks: Dual site: -4.8 ± 21.2 Auditory: -3.8 ± 9.0 Control: -2.4 ± 9.1
ΔTHI 4 weeks: Dual site: -12.0 ± 13.0 Auditory: -2.9 ± 9.8 Control: 1.0 ± 12.1
ΔTHI 8 weeks: Dual site: -12.3 ± 21.8 Auditory: -7.4 ± 13.5 Control: 0.7 ± 17.1
ΔTHI 12 weeks: Dual site: -13.5 ± 16.9 Auditory: -7.8 ± 12.2 Control: -0.4 ± 21.7
VAS score for tinnitus symptoms (mean ± SD): Awareness 2 weeks Dual site: -1.2 ± 2.0 Auditory: -0.3 ± 0.8 Control: -0.4 ± 2.3
Awareness 4 weeks Dual site: -0.5 ± 2.4 Auditory: -0.1 ± 0.5 Control: -1.0 ± 1.8
Awareness 8 weeks Dual site: -1.0 ± 2.8 Auditory: -1.0 ± 2.0 Control: -0.4 ± 1.2
Awareness 12 weeks Dual site: -2.5 ± 2.8 Auditory: -0.8 ± 1.6 Control: -1.2 ± 1.7
Annoyance 2 weeks Dual site: -1.6 ± 2.2 Auditory: -1.0 ± 1.3 Control: -0.4 ± 2.0
Annoyance 4 weeks Dual site: -2.0 ± 2.8 Auditory: -0.9 ± 1.0 Control: -0.3 ± 2.0
Annoyance 8 weeks Dual site: -1.9 ± 2.6 Auditory: -0.9 ± 1.1 Control: 0.2 ± 1.8
Annoyance 12 weeks Dual site: -2.3 ± 2.7 Auditory: -0.7 ± 1.6 Control: -0.2 ± 1.8
Loudness 2 weeks Dual site: -1.6 ± 1.8 Auditory: -0.6 ± 1.2 Control: -0.3 ± 1.6
Loudness 4 weeks Dual site: -1.5 ± 1.8 Auditory: -0.9 ± 1.7 Control: 0.0 ± 1.7
Loudness 8 weeks Dual site: 1.6 ± 2.5 Auditory: -0.7 ± 1.9 Control: -0.3 ± 2.1
Loudness 12 weeks Dual site: -1.4 ± 2.1 Auditory: -1.0 ± 1.9 Control: -0.8 ± 1.9
Effect on daily life 2 weeks Dual site: -1.5 ± 1.6 Auditory: 0.4 ± 3.0 Control: 0.5 ± 1.4
Effect on daily life 4 weeks Dual site: -1.5 ± 2.1 Auditory: 1.1 ± 2.7 Control: 0.2 ± 1.4
Effect on daily life 8 weeks Dual site: -1.2 ± 2.2 Auditory: -0.5 ± 3.6 Control: 0.0 ± 2.1
Effect on daily life 12 weeks Dual site: -1.5 ± 2.2 Auditory: 0.2 ± 2.6 Control: 0.0 ± 2.5 |
|
Risk of bias table for intervention studies (randomized controlled trials; based on Cochrane risk of bias tool and suggestions by the CLARITY Group at McMaster University)
|
Study reference
(first author, publication year) |
Was the allocation sequence adequately generated?
Definitely yes Probably yes Probably no Definitely no |
Was the allocation adequately concealed?
Definitely yes Probably yes Probably no Definitely no |
Blinding: Was knowledge of the allocated interventions adequately prevented?
Definitely yes Probably yes Probably no Definitely no |
Was loss to follow-up (missing outcome data) infrequent?
Definitely yes Probably yes Probably no Definitely no |
Are reports of the study free of selective outcome reporting?
Definitely yes Probably yes Probably no Definitely no |
Was the study apparently free of other problems that could put it at a risk of bias?
Definitely yes Probably yes Probably no Definitely no |
Overall risk of bias If applicable/necessary, per outcome measure
LOW Some concerns HIGH
|
|
Ciminelli, 2021 |
Definitely yes
Reason: The patients were randomized using a computer-generated randomization list in Microsoft Excel. |
No information
Reason: No information about concealment of allocation. |
Probably yes
Reason: Patients were blinded. |
Definitely no
Reason: After randomization, there was 16.7% loss-to-follow-up in the intervention group and 22.2% loss-to-follow-up in the control group. |
Probably yes
Reason: Outcome measures in the abstract were reported.
|
Definitely no
Reason: Small number of patients, relatively short follow-up period.
|
HIGH (all outcomes)
Reason: No information about allocation concealment, frequent loss-to-follow-up, small sample size, relatively short follow-up period. |
|
Hong, 2021 |
Definitely yes
Reason: They used computer-generated random numbers for the randomization. |
Probably yes
Reason: After enrolment, each patient was determined to the type of intervention with a random number. |
Probably yes
Reason: Both patients and investigators (outcome assessors) were blinded to treatment conditions. |
Definitely no
Reason: Loss-to-follow-up was 13.3%.
|
Probably yes
Reason: Outcome measures in the abstract were reported.
|
Definitely no
Reason: small sample size, effect of comorbidities, broad follow-up period.
|
HIGH (all outcomes)
Reason: Loss-to-follow-up, small sample size, effect of comorbidities, broad follow-up period.
|
|
Noh, 2020 |
Probably yes
Reason: An unrestricted randomization technique was used. |
Definitely yes
Reason: Sealed opaque envelopes containing the group information were used. |
Probably yes
Reason: Except for the individual who performed the rTMS, all other researchers and patients were blinded to the treatment method. |
Definitely yes
Reason: Loss-to-follow-up was 2-4%.
|
Probably yes
Reason: Outcome measures in the abstract were reported.
|
Definitely no
Reason: small sample size, - difference between dual site and auditory only was not determined, variability among subjects. |
Some concerns (all outcomes)
Reason: small sample size, difference between dual site and auditory only was not determined, variability among subjects. |
Table of excluded studies
|
Reference |
Reason for exclusion |
|
Cacace AT, Hu J, Romero S, Xuan Y, Burkard RF, Tyler RS. Glutamate is down-regulated and tinnitus loudness-levels decreased following rTMS over auditory cortex of the left hemisphere: A prospective randomized single-blinded sham-controlled cross-over study. Hear Res. 2018 Feb;358:59-73. doi: 10.1016/j.heares.2017.10.017. Epub 2017 Nov 15. PMID: 29150051. |
Included in the search period from the SR from Liang, 2020. |
|
Chen JJ, Zeng BS, Wu CN, Stubbs B, Carvalho AF, Brunoni AR, Su KP, Tu YK, Wu YC, Chen TY, Lin PY, Liang CS, Hsu CW, Hsu SP, Kuo HC, Chen YW, Tseng PT, Li CT. Association of Central Noninvasive Brain Stimulation Interventions With Efficacy and Safety in Tinnitus Management: A Meta-analysis. JAMA Otolaryngol Head Neck Surg. 2020 Sep 1;146(9):801-809. doi: 10.1001/jamaoto.2020.1497. PMID: 32644131; PMCID: PMC7349076. |
Network meta-analysis, is hard to update with recent literature. |
|
Deklerck AN, Marechal C, Pérez Fernández AM, Keppler H, Van Roost D, Dhooge IJM. Invasive Neuromodulation as a Treatment for Tinnitus: A Systematic Review. Neuromodulation. 2020 Jun;23(4):451-462. doi: 10.1111/ner.13042. Epub 2019 Sep 16. PMID: 31524324. |
The review from Stegeman, 2021 is more complete. |
|
Dong C, Chen C, Wang T, Gao C, Wang Y, Guan X, Dong X. Low-Frequency Repetitive Transcranial Magnetic Stimulation for the Treatment of Chronic Tinnitus: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Biomed Res Int. 2020 May 2;2020:3141278. doi: 10.1155/2020/3141278. PMID: 32461976; PMCID: PMC7218966. |
The review from Liang, 2020 is more recent. |
|
Godbehere J, Sandhu J, Evans A, Twigg V, Scivill I, Ray J, Barker A. Treatment of Tinnitus Using Theta Burst Based Repetitive Transcranial Magnetic Stimulation-A Single Blinded Randomized Control Trial. Otol Neurotol. 2019 Jun;40(5S Suppl 1):S38-S42. doi: 10.1097/MAO.0000000000002207. PMID: 31225821. |
Included in the search period from the SR from Liang, 2020. |
|
Folmer RL, Theodoroff SM, Casiana L, Shi Y, Griest S, Vachhani J. Repetitive Transcranial Magnetic Stimulation Treatment for Chronic Tinnitus: A Randomized Clinical Trial. JAMA Otolaryngol Head Neck Surg. 2015 Aug;141(8):716-22. doi: 10.1001/jamaoto.2015.1219. PMID: 26181507. |
Included in the search period from the SR from Liang, 2020. |
|
Formánek M, Migaľová P, Krulová P, Bar M, Jančatová D, Zakopčanová-Srovnalová H, Tomášková H, Zeleník K, Komínek P. Combined transcranial magnetic stimulation in the treatment of chronic tinnitus. Ann Clin Transl Neurol. 2018 Jun 8;5(7):857-864. doi: 10.1002/acn3.587. PMID: 30009203; PMCID: PMC6043768. |
Included in the search period from the SR from Liang, 2020. |
|
Forogh B, Mirshaki Z, Raissi GR, Shirazi A, Mansoori K, Ahadi T. Repeated sessions of transcranial direct current stimulation for treatment of chronic subjective tinnitus: a pilot randomized controlled trial. Neurol Sci. 2016 Feb;37(2):253-9. doi: 10.1007/s10072-015-2393-9. Epub 2015 Oct 24. PMID: 26498289. |
Included in the SR from Martins, 2022 |
|
Hyvärinen P, Mäkitie A, Aarnisalo AA. Self-Administered Domiciliary tDCS Treatment for Tinnitus: A Double-Blind Sham-Controlled Study. PLoS One. 2016 Apr 28;11(4):e0154286. doi: 10.1371/journal.pone.0154286. PMID: 27124116; PMCID: PMC4849783. |
Wrong study design, no RCT. |
|
Labree B, Hoare DJ, Gascoyne LE, Scutt P, Del Giovane C, Sereda M. Determining the Effects of Transcranial Direct Current Stimulation on Tinnitus, Depression, and Anxiety: A Systematic Review. Brain Sci. 2022 Apr 8;12(4):484. doi: 10.3390/brainsci12040484. PMID: 35448015; PMCID: PMC9029345. |
Does not comply with PICO: not only tinnitus patients were included. |
|
Landgrebe M, Hajak G, Wolf S, Padberg F, Klupp P, Fallgatter AJ, Polak T, Höppner J, Haker R, Cordes J, Klenzner T, Schönfeldt-Lecuona C, Kammer T, Graf E, Koller M, Kleinjung T, Lehner A, Schecklmann M, Pöppl TB, Kreuzer P, Frank E, Langguth B. 1-Hz rTMS in the treatment of tinnitus: A sham-controlled, randomized multicenter trial. Brain Stimul. 2017 Nov-Dec;10(6):1112-1120. doi: 10.1016/j.brs.2017.08.001. Epub 2017 Aug 5. PMID: 28807845. |
Included in the search period from the SR from Liang, 2020. |
|
Lefebvre-Demers M, Doyon N, Fecteau S. Non-invasive neuromodulation for tinnitus: A meta-analysis and modeling studies. Brain Stimul. 2021 Jan-Feb;14(1):113-128. doi: 10.1016/j.brs.2020.11.014. Epub 2020 Dec 1. PMID: 33276156. |
The included SR’s are more complete compared to this study. |
|
Noh TS, Kyong JS, Park MK, Lee JH, Oh SH, Chung CK, Kim JS, Suh MW. Treatment Outcome of Auditory and Frontal Dual-Site rTMS in Tinnitus Patients and Changes in Magnetoencephalographic Functional Connectivity after rTMS: Double-Blind Randomized Controlled Trial. Audiol Neurootol. 2019;24(6):293-298. doi: 10.1159/000503134. Epub 2019 Dec 12. PMID: 31830753. |
Included in the search period from the SR from Liang, 2020. |
|
Pal N, Maire R, Stephan MA, Herrmann FR, Benninger DH. Transcranial Direct Current Stimulation for the Treatment of Chronic Tinnitus: A Randomized Controlled Study. Brain Stimul. 2015 Nov-Dec;8(6):1101-7. doi: 10.1016/j.brs.2015.06.014. Epub 2015 Jun 27. PMID: 26198363. |
Included in the SR from Martins, 2022 |
|
Sahlsten H, Virtanen J, Joutsa J, Niinivirta-Joutsa K, Löyttyniemi E, Johansson R, Paavola J, Taiminen T, Sjösten N, Salonen J, Holm A, Rauhala E, Jääskeläinen SK. Electric field-navigated transcranial magnetic stimulation for chronic tinnitus: a randomized, placebo-controlled study. Int J Audiol. 2017 Sep;56(9):692-700. doi: 10.1080/14992027.2017.1313461. Epub 2017 Apr 18. PMID: 28415897. |
Included in the search period from the SR from Liang, 2020. |
|
Santos ADHM, Santos APS, Santos HS, Silva ACD. The use of tDCS as a therapeutic option for tinnitus: a systematic review. Braz J Otorhinolaryngol. 2018 Sep-Oct;84(5):653-659. doi: 10.1016/j.bjorl.2018.02.003. Epub 2018 Mar 9. PMID: 29573997; PMCID: PMC9452266. |
The SR from Martins, 2022 is more recent. |
|
Schecklmann M, Giani A, Tupak S, Langguth B, Raab V, Polak T, Várallyay C, Großmann W, Herrmann MJ, Fallgatter AJ. Neuronavigated left temporal continuous theta burst stimulation in chronic tinnitus. Restor Neurol Neurosci. 2016;34(2):165-75. doi: 10.3233/RNN-150518. PMID: 26890094. |
Included in the search period from the SR from Liang, 2020. |
|
Stegeman I, Velde HM, Robe PAJT, Stokroos RJ, Smit AL. Tinnitus treatment by vagus nerve stimulation: A systematic review. PLoS One. 2021 Mar 11;16(3):e0247221. doi: 10.1371/journal.pone.0247221. PMID: 33705401; PMCID: PMC7951891. |
Not conform PICO: wrong comparison |
|
Souza DDS, Almeida AA, Andrade SMDS, Machado DGDS, Leitão M, Sanchez TG, Rosa MRDD. Transcranial direct current stimulation improves tinnitus perception and modulates cortical electrical activity in patients with tinnitus: A randomized clinical trial. Neurophysiol Clin. 2020 Sep;50(4):289-300. doi: 10.1016/j.neucli.2020.07.002. Epub 2020 Aug 28. PMID: 32863109. |
Included in the SR from Martins, 2022 |
|
Teismann H, Wollbrink A, Okamoto H, Schlaug G, Rudack C, Pantev C. Combining transcranial direct current stimulation and tailor-made notched music training to decrease tinnitus-related distress--a pilot study. PLoS One. 2014 Feb 25;9(2):e89904. doi: 10.1371/journal.pone.0089904. PMID: 24587113; PMCID: PMC3934956. |
Different intervention: combined intervention of tDCS and TMNMT. |
|
To WT, Ost J, Hart J Jr, De Ridder D, Vanneste S. The added value of auditory cortex transcranial random noise stimulation (tRNS) after bifrontal transcranial direct current stimulation (tDCS) for tinnitus. J Neural Transm (Vienna). 2017 Jan;124(1):79-88. doi: 10.1007/s00702-016-1634-2. Epub 2016 Oct 19. PMID: 27761741. |
Included in the SR from Martins, 2022 |
|
Tyler R, Cacace A, Stocking C, Tarver B, Engineer N, Martin J, Deshpande A, Stecker N, Pereira M, Kilgard M, Burress C, Pierce D, Rennaker R, Vanneste S. Vagus Nerve Stimulation Paired with Tones for the Treatment of Tinnitus: A Prospective Randomized Double-blind Controlled Pilot Study in Humans. Sci Rep. 2017 Sep 20;7(1):11960. doi: 10.1038/s41598-017-12178-w. PMID: 28931943; PMCID: PMC5607328. |
Different comparison: VNS with paired tones versus VNS without paired tones. |
|
Wang TC, Tyler RS, Chang TY, Chen JC, Lin CD, Chung HK, Tsou YA. Effect of Transcranial Direct Current Stimulation in Patients With Tinnitus: A Meta-Analysis and Systematic Review. Ann Otol Rhinol Laryngol. 2018 Feb;127(2):79-88. doi: 10.1177/0003489417744317. Epub 2017 Dec 1. PMID: 29192507. |
The review from Martins, 2022 is more recent. |
|
Yadollahpour A, Mayo M, Saki N, Rashidi S, Bayat A. A chronic protocol of bilateral transcranial direct current stimulation over auditory cortex for tinnitus treatment: Dataset from a double-blinded randomized controlled trial. F1000Res. 2018 Jun 12;7:733. doi: 10.12688/f1000research.14971.1. PMID: 30356442; PMCID: PMC6178906. |
Included in the search period from Martins, 2022. |
|
Yadollahpour A, Bayat A, Rashidi S, Saki N, Karimi M. Dataset of acute repeated sessions of bifrontal transcranial direct current stimulation for treatment of intractable tinnitus: A randomized controlled trial. Data Brief. 2017 Sep 13;15:40-46. doi: 10.1016/j.dib.2017.09.006. PMID: 28971121; PMCID: PMC5609868. |
Included in the search period from Martins, 2022. |
|
Yang T, Zhang J, Wang B, Zhang W, Xu M, Yang S, Liu H. Electrical stimulation to treat tinnitus: a meta-analysis and systemic review of randomized controlled trials. Ther Adv Chronic Dis. 2021 Sep 13;12:20406223211041069. doi: 10.1177/20406223211041069. PMID: 34729140; PMCID: PMC8442493. |
The SR from Martins, 2022 and Liang, 2020 are more complete. |
|
Yilmaz M, Yener MH, Turgut NF, Aydin F, Altug T. Effectiveness of transcranial magnetic stimulation application in treatment of tinnitus. J Craniofac Surg. 2014 Jul;25(4):1315-8. doi: 10.1097/SCS.0000000000000782. PMID: 25006914. |
Included in the search period from the SR from Liang, 2020. |
|
Yin L, Chen X, Lu X, An Y, Zhang T, Yan J. An updated meta-analysis: repetitive transcranial magnetic stimulation for treating tinnitus. J Int Med Res. 2021 Mar;49(3):300060521999549. doi: 10.1177/0300060521999549. PMID: 33729855; PMCID: PMC7975580. |
The SR from Liang, 2020 is more complete. |
Verantwoording
Beoordelingsdatum en geldigheid
Laatst beoordeeld : 19-11-2024
Algemene gegevens
De ontwikkeling van deze richtlijnmodule werd ondersteund door het Kennisinstituut van de Federatie Medisch Specialisten (www.demedischspecialist.nl/kennisinstituut) en werd gefinancierd uit de Kwaliteitsgelden Medisch Specialisten (SKMS). De financier heeft geen enkele invloed gehad op de inhoud van de richtlijnmodule.
Samenstelling werkgroep
Voor het ontwikkelen van de richtlijnmodules is in 2020 een multidisciplinaire cluster ingesteld. Dit cluster bestaat uit vertegenwoordigers van alle relevante organisaties die betrekking hebben op de zorg voor patiënten met gehoorproblemen.
Het cluster Otologie bestaat uit meerdere richtlijnen, zie hier voor de actuele clusterindeling. De stuurgroep bewaakt het proces van modulair onderhoud binnen het cluster. De expertisegroepsleden worden indien nodig gevraagd om hun expertise in te zetten voor een specifieke richtlijnmodule. Het cluster Otologie bestaat uit de volgende personen:
Clusterstuurgroep
- Dr. H. (Hilke) van Det-Bartels, voorzitter cluster otologie, KNO-arts, Isala, Zwolle, NVKNO (tot en met oktober 2023)
- Drs. M. (Monique) Campman-Verhoeff, voorzitter cluster otologie, KNO-arts, Treant Zorggroep, Emmen (vanaf november 2023)
- Dr. E. (Erik) van Spronsen, voorzitter cluster otologie, KNO-arts, Amsterdam UMC locatie AMC, Amsterdam
- Drs. R.M. (Robert) van Haastert, KNO-arts, Dijklander Ziekenhuis, Purmerend
- Dr. A.L. (Diane) Smit, KNO-arts, UMC Utrecht, Utrecht
- Drs. M.J. (Thijs) Vaessen, Oogarts, Deventer Ziekenhuis, Deventer
- Dr. Y.J.W. (Yvonne) Simis, Klinisch fysicus, audioloog, Amsterdam UMC locatie VUmc, Amsterdam (tot augustus 2023)
- Dr. ir. F.L. (Femke) Theelen, Audioloog, Amsterdam UMC, Amsterdam (vanaf augustus 2023)
- Drs. S.A.H. (Sjoert) Pegge, Radioloog, Radboudumc, Nijmegen
Betrokken clusterexpertisegroep
- Dr. A.L. (Diane) Smit, KNO-arts, UMC Utrecht, Utrecht
- Dr. R. (Rutger) Hofman, KNO-arts, UMCG, Groningen
- Dr. C.A. (Katja) Hellingman, KNO-arts, Maastricht UMC, Maastricht
- Dr. E.L.J. (Erwin) George, Klinisch fysicus-audioloog, Maastricht UMC, Maastricht
- M. (Michael) Huinink, patiëntvertegenwoordiger, Stichting Hoormij·NVVS (tot en met mei 2023)
- Drs. O.V.G. (Olav) Wagenaar, klinisch neuropsycholoog, Rijndam revalidatie (vanaf januari 2024)
- Ir. C.W.M. (Chris) van den Dries, patiëntvertegenwoordiger, Stichting Hoormij·NVVS (vanaf februari 2024)
Met ondersteuning van
- Dr. R. (Romy) Zwarts-van de Putte, adviseur, Kennisinstituut van Medisch Specialisten
- Drs. E.R.L. (Evie) Verweg, junior adviseur, Kennisinstituut van Medisch Specialisten
Belangenverklaringen
De Code ter voorkoming van oneigenlijke beïnvloeding door belangenverstrengeling is gevolgd. Alle clusterstuurgroepleden en actief betrokken expertisegroepleden (fungerend als schrijver en/of meelezer bij tenminste één van de geprioriteerde richtlijnmodules) hebben schriftelijk verklaard of zij in de laatste drie jaar directe financiële belangen (betrekking bij een commercieel bedrijf, persoonlijke financiële belangen, onderzoeksfinanciering) of indirecte belangen (persoonlijke relaties, reputatiemanagement) hebben gehad. Gedurende de ontwikkeling of herziening van een richtlijnmodule worden wijzigingen in belangen aan de projectleider doorgegeven. De belangenverklaring wordt opnieuw bevestigd tijdens de commentaarfase. Een overzicht van de belangen van de clusterleden en betrokken expertisegroepleden en het oordeel over het omgaan met eventuele belangen vindt u in onderstaande tabel. De ondertekende belangenverklaringen zijn op te vragen bij het secretariaat van het Kennisinstituut van de Federatie Medisch Specialisten.
Clusterstuurgroep
Tabel 1. Gemelde (neven)functies en belangen stuurgroep
|
Clusterlid |
Functie |
Nevenfuncties |
Gemelde belangen |
Ondernomen actie |
|
Van Det |
KNO arts Isala Zwolle |
Voorzitter koploperproject, lid kerngroep otologie KNO vereniging, lid klankbordgroep vestibulologie, lid KNO vereniging |
Geen |
Geen restrictie |
|
Spronsen |
KNO-arts, Amsterdam UMC |
Raad van Advies START |
Geen |
Geen restrictie |
|
Vaessen |
Oogarts Deventer Ziekenhuis, Oogarts Streekziekenhuis Koningin Beatrix te Winterswijk |
Geen |
Samen met Sallandse Specialisten Coorporatie aandeelhouder in een drietal |
Geen restrictie |
|
Simis (tot augustus 2023) |
Klinisch Fysicus – Audioloog |
Geen |
Geen |
Geen restrictie |
|
Van Haastert |
KNO-arts Dijklanderziekenhuis Hoorn/Purmerend |
Geen |
Geen |
Geen restrictie |
|
Smit |
KNO arts UMC Utrecht, afdeling KNO |
Medisch adviseur Stichting Hoormij·NVVS, onbetaald |
-Medeaanvrager en onderzoeker van MinT-studie over toepassing van mindfulness bij tinnitus, gefinancierd door HandicapNL |
Geen restrictie, de studie die betrekking heeft op tinnitus is niet gefinancierd door een commerciële partij. |
|
Campman-Verhoeff |
KNO-arts Treant Zorggroep |
Bestuurslid stichting nascholing KNO – onbetaald |
Geen |
Geen restrictie |
|
Pegge |
Radioloog Radboud UMC |
Geen |
Geen |
Geen restrictie |
|
Theelen (vanaf augustus 2023) |
Klinisch Fysicus – Audioloog |
Geen |
Geen |
Geen restrictie |
Clusterexpertisegroep
Tabel 2. Gemelde (neven)functies en belangen expertisegroep
|
Clusterlid |
Functie |
Nevenfuncties |
Gemelde belangen |
Ondernomen actie |
|
Hofman |
KNO-arts UMCG, Groningen |
Geen |
Copromotor van een promovendus die onderzoek doet naar beengeleidingsimplantaten (BCD), onderzoek is gesponsord door Oticon medical. Een BCD helpt niet tegen tinnitus. |
Geen restrictie, geen connectie tussen extern gefinancierd onderzoek en herziene modules. |
|
Hellingman |
KNO-arts/otoloog MUMC+, Maastricht |
Geen |
Geen |
Geen restrictie |
|
George |
Klinisch fysicus-audioloog, Maastricht UMC+ |
- bestuurslid Stichting Opleiding Klinische Fysica (onbetaald) |
ELEPHANT-studie (2018-2022) en RHINO-studie (vanaf 2022), wetenschappelijke research; promovendus grotendeels gefinancieerd door Advanced Bionics, op gebied van optimalisatie van outcomes van cochleaire implantatie. Geen vergoeding voor mijzelf. -Reguliere samenwerking met patientenorganisatie Stichting Hoormij·NVVS, geen boegbeeldfunctie. |
Geen restrictie. Deze studies hebben geen betrekking op de modules die in deze cyclus zijn herzien. |
|
Huinink (tot mei 2023) |
Directeur Grootzakelijk Rabobank Vrijwilliger Stichting Hoormij·NVVS |
Bestuurslid (toezichthouder) Stichting NMCX |
Geen |
Geen restrictie |
|
Wagenaar (vanaf januari 2024) |
Klinisch neuropsycholoog/opleider bij Revalidatiecentrum Rijndam |
'Tinnitus (onbetaald) Columnist Earline (betaald) |
Royalty’s boek EHBOorsuizen Royalty’s online zelf-CGT eHBOorsuizen Financieel belang in TinniVRee Neuropsychologisch verklaringsmodel tinnitus & hyperacusis. EHBOorsuizen FB peersupportgroup |
Geen restrictie, de mogelijke belangen hebben geen betrekking op de modules die in deze cyclus zijn herzien. |
|
Van den Dries (vanaf februari 2024) |
Vrijwilliger bij Stichting Hoormij·NVVS Lid van Commissie Tinnitus en Hyperacusis als belangenbehartiger en voorlichter |
Moderator van de Facebookgroep Eerste Hulp bij Oorsuizen/Tinnitus |
Geen |
Geen restrictie |
Inbreng patiëntenperspectief
Er werd aandacht besteed aan het patiëntenperspectief door deelname van relevante patiëntenorganisaties aan de need-for-update en/of prioritering. De verkregen input is meegenomen bij het opstellen van de uitgangsvragen, de keuze voor de uitkomstmaten en bij het opstellen van de overwegingen. De conceptrichtlijnmodule is tevens ter commentaar voorgelegd aan alle relevante patiëntenorganisaties in de stuur- en expertisegroep (zie ‘Samenstelling cluster’ onder ‘Verantwoording’) en aan alle patiëntenorganisaties die niet deelnemen aan de stuur- en expertisegroep, maar wel hebben deelgenomen aan de need-for-update (zie ‘Need-for-update’ onder ‘Verantwoording’). De eventueel aangeleverde commentaren zijn bekeken en verwerkt.
Wkkgz & Kwalitatieve raming van mogelijke substantiële financiële gevolgen
Bij de richtlijnmodule is conform de Wet kwaliteit, klachten en geschillen zorg (Wkkgz) een kwalitatieve raming uitgevoerd om te beoordelen of de aanbevelingen mogelijk leiden tot substantiële financiële gevolgen. Bij het uitvoeren van deze beoordeling is de richtlijnmodule op verschillende domeinen getoetst (zie het stroomschema op de Richtlijnendatabase).
Module |
Uitkomst raming |
Toelichting |
|
Module [Behandeling van patiënten met tinnitus – neuromodulatie] |
Geen substantiële financiële gevolgen |
Hoewel uit de toetsing volgt dat de aanbeveling(en) breed toepasbaar zijn (>40.000 patiënten), volgt ook uit de toetsing dat het overgrote deel (±90%) van de zorgaanbieders en zorgverleners al aan de norm voldoet. Er worden daarom geen substantiële financiële gevolgen verwacht. |
Werkwijze
AGREE
Deze richtlijnmodule is opgesteld conform de eisen vermeld in het rapport Medisch Specialistische Richtlijnen 3.0 van de adviescommissie Richtlijnen van de Raad Kwaliteit. Dit rapport is gebaseerd op het AGREE II instrument (Appraisal of Guidelines for Research & Evaluation II; Brouwers, 2010).
Need-for-update, prioritering en uitgangsvragen
Tijdens de need-for-update fase (voorjaar, 2021) inventariseerde het cluster de geldigheid van de richtlijnmodules binnen het cluster. Naast de partijen die deelnemen aan de stuur- en expertisegroep zijn hier ook andere stakeholders voor benaderd. Per richtlijnmodule is aangegeven of deze geldig is, herzien moet worden, kan vervallen of moet worden samengevoegd. Ook was er de mogelijkheid om nieuwe onderwerpen aan te dragen die aansluiten bij één (of meerdere) richtlijn(en) behorend tot het cluster. De richtlijnmodules waarbij door één of meerdere partijen werd aangegeven herzien te worden, werden doorgezet naar de prioriteringsronde. Ook suggesties voor nieuwe richtlijnmodules werden doorgezet naar de prioriteringsronde. Afgevaardigden vanuit de partijen in de stuur- en expertisegroep werden gevraagd om te prioriteren (zie ‘Samenstelling cluster’ onder ‘Verantwoording’). Hiervoor werd de RE-weighted Priority-Setting (REPS) – tool gebruikt. De uitkomsten (ranglijst) werd gebruikt als uitgangspunt voor de discussie. Voor de geprioriteerde richtlijnmodules zijn door de het cluster concept-uitgangsvragen herzien of opgesteld en definitief vastgesteld.
Uitkomstmaten
Na het opstellen van de zoekvraag behorende bij de uitgangsvraag inventariseerde het cluster welke uitkomstmaten voor de patiënt relevant zijn, waarbij zowel naar gewenste als ongewenste effecten werd gekeken. Hierbij werd een maximum van acht uitkomstmaten gehanteerd. Het cluster waardeerde deze uitkomstmaten volgens hun relatieve belang bij de besluitvorming rondom aanbevelingen, als cruciaal (kritiek voor de besluitvorming), belangrijk (maar niet cruciaal) en onbelangrijk. Tevens definieerde het cluster tenminste voor de cruciale uitkomstmaten welke verschillen zij klinisch (patiënt) relevant vonden.
Methode literatuursamenvatting
Een uitgebreide beschrijving van de strategie voor zoeken en selecteren van literatuur is te vinden onder ‘Zoeken en selecteren’. Indien mogelijk werd de data uit verschillende studies gepoold in een random-effects model. Review Manager 5.4 werd indien mogelijk gebruikt voor de statistische analyses. De beoordeling van de kracht van het wetenschappelijke bewijs wordt hieronder toegelicht.
Beoordelen van de kracht van het wetenschappelijke bewijs
De kracht van het wetenschappelijke bewijs werd bepaald volgens de GRADE-methode. GRADE staat voor ‘Grading Recommendations Assessment, Development and Evaluation’ (zie http://www.gradeworkinggroup.org). De basisprincipes van de GRADE-methodiek zijn: het benoemen en prioriteren van de klinisch (patiënt) relevante uitkomstmaten, een systematische review per uitkomstmaat, en een beoordeling van de bewijskracht per uitkomstmaat op basis van de acht GRADE-domeinen (domeinen voor downgraden: risk of bias, inconsistentie, indirectheid, imprecisie, en publicatiebias; domeinen voor upgraden: dosis-effect relatie, groot effect, en residuele plausibele confounding). GRADE onderscheidt vier gradaties voor de kwaliteit van het wetenschappelijk bewijs: hoog, redelijk, laag en zeer laag. Deze gradaties verwijzen naar de mate van zekerheid die er bestaat over de literatuurconclusie, in het bijzonder de mate van zekerheid dat de literatuurconclusie de aanbeveling adequaat ondersteunt (Schünemann, 2013; Hultcrantz, 2017).
Tabel 3. Gradaties voor de kwaliteit van wetenschappelijk bewijs
|
GRADE |
Definitie |
|
Hoog |
|
|
Redelijk |
|
|
Laag |
|
|
Zeer laag |
|
Bij het beoordelen (graderen) van de kracht van het wetenschappelijk bewijs in een richtlijnmodule volgens de GRADE-methodiek spelen grenzen voor klinische besluitvorming een belangrijke rol (Hultcrantz, 2017). Dit zijn de grenzen die bij overschrijding aanleiding zouden geven tot een aanpassing van de aanbeveling. Om de grenzen voor klinische besluitvorming te bepalen moeten alle relevante uitkomstmaten en overwegingen worden meegewogen. De grenzen voor klinische besluitvorming zijn daarmee niet één op één vergelijkbaar met het minimaal klinisch relevant verschil (Minimal Clinically Important Difference, MCID). Met name in situaties waarin een interventie geen belangrijke nadelen heeft en de kosten relatief laag zijn, kan de grens voor klinische besluitvorming met betrekking tot de effectiviteit van de interventie bij een lagere waarde (dichter bij het nuleffect) liggen dan de MCID (Hultcrantz, 2017).
Overwegingen (van bewijs naar aanbeveling)
Om te komen tot een aanbeveling zijn naast (de kwaliteit van) het wetenschappelijke bewijs ook andere aspecten belangrijk en worden meegewogen, zoals aanvullende argumenten uit bijvoorbeeld de biomechanica of fysiologie, waarden en voorkeuren van patiënten, kosten (middelenbeslag), aanvaardbaarheid, haalbaarheid en implementatie. Deze aspecten zijn systematisch vermeld en beoordeeld (gewogen) onder het kopje ‘Overwegingen’ en kunnen (mede) gebaseerd zijn op expert opinion. Hierbij is gebruik gemaakt van een gestructureerd format gebaseerd op het evidence-to-decision framework van de internationale GRADE Working Group (Alonso-Coello, 2016a; Alonso-Coello 2016b). Dit evidence-to-decision framework is een integraal onderdeel van de GRADE methodiek.
Formuleren van aanbevelingen
De aanbevelingen geven antwoord op de uitgangsvraag en zijn gebaseerd op het beschikbare wetenschappelijke bewijs en de belangrijkste overwegingen, en een weging van de gunstige en ongunstige effecten van de relevante interventies. De kracht van het wetenschappelijk bewijs en het gewicht dat door het cluster wordt toegekend aan de overwegingen, bepalen samen de sterkte van de aanbeveling. Conform de GRADE-methodiek sluit een lage bewijskracht van conclusies in de systematische literatuuranalyse een sterke aanbeveling niet a priori uit, en zijn bij een hoge bewijskracht ook zwakke aanbevelingen mogelijk (Agoritsas, 2017; Neumann, 2016). De sterkte van de aanbeveling wordt altijd bepaald door weging van alle relevante argumenten tezamen. Het cluster heeft bij elke aanbeveling opgenomen hoe zij tot de richting en sterkte van de aanbeveling zijn gekomen.
In de GRADE-methodiek wordt onderscheid gemaakt tussen sterke en zwakke (of conditionele) aanbevelingen. De sterkte van een aanbeveling verwijst naar de mate van zekerheid dat de voordelen van de interventie opwegen tegen de nadelen (of vice versa), gezien over het hele spectrum van patiënten waarvoor de aanbeveling is bedoeld. De sterkte van een aanbeveling heeft duidelijke implicaties voor patiënten, behandelaars en beleidsmakers (zie onderstaande tabel). Een aanbeveling is geen dictaat, zelfs een sterke aanbeveling gebaseerd op bewijs van hoge kwaliteit (GRADE gradering HOOG) zal niet altijd van toepassing zijn, onder alle mogelijke omstandigheden en voor elke individuele patiënt.
Tabel 4. Sterkte van de aanbevelingen
|
Implicaties van sterke en zwakke aanbevelingen voor verschillende richtlijngebruikers |
||
|
|
Sterke aanbeveling |
Zwakke (conditionele) aanbeveling |
|
Voor patiënten |
De meeste patiënten zouden de aanbevolen interventie of aanpak kiezen en slechts een klein aantal niet. |
Een aanzienlijk deel van de patiënten zouden de aanbevolen interventie of aanpak kiezen, maar veel patiënten ook niet. |
|
Voor behandelaars |
De meeste patiënten zouden de aanbevolen interventie of aanpak moeten ontvangen. |
Er zijn meerdere geschikte interventies of aanpakken. De patiënt moet worden ondersteund bij de keuze voor de interventie of aanpak die het beste aansluit bij zijn of haar waarden en voorkeuren. |
|
Voor beleidsmakers |
De aanbevolen interventie of aanpak kan worden gezien als standaardbeleid. |
Beleidsbepaling vereist uitvoerige discussie met betrokkenheid van veel stakeholders. Er is een grotere kans op lokale beleidsverschillen. |
Organisatie van zorg
Bij de ontwikkeling van de richtlijnmodule is expliciet aandacht geweest voor de organisatie van zorg: alle aspecten die randvoorwaardelijk zijn voor het verlenen van zorg (zoals coördinatie, communicatie, (financiële) middelen, mankracht en infrastructuur). Randvoorwaarden die relevant zijn voor het beantwoorden van deze specifieke uitgangsvraag zijn genoemd bij de overwegingen. Meer algemene, overkoepelende, of bijkomende aspecten van de organisatie van zorg worden behandeld in de richtlijnmodule Organisatie van zorg.
Commentaar- en autorisatiefase
De conceptrichtlijnmodule werd voorgelegd aan alle partijen die benaderd zijn voor de need-for-update fase. De commentaren werden verzameld en besproken met het cluster. Naar aanleiding van de commentaren werd de conceptrichtlijnmodule aangepast en definitief vastgesteld door het cluster. De definitieve richtlijnmodule werd ter autorisatie of goedkeuring voorgelegd aan de partijen die beschreven staan bij ‘Initiatief en autorisatie’ onder ‘Verantwoording’.
Literatuur
Agoritsas T, Merglen A, Heen AF, Kristiansen A, Neumann I, Brito JP, Brignardello-Petersen R, Alexander PE, Rind DM, Vandvik PO, Guyatt GH. UpToDate adherence to GRADE criteria for strong recommendations: an analytical survey. BMJ Open. 2017 Nov 16;7(11):e018593. doi: 10.1136/bmjopen-2017-018593. PubMed PMID: 29150475; PubMed Central PMCID: PMC5701989.
Alonso-Coello P, Schünemann HJ, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Rada G, Rosenbaum S, Morelli A, Guyatt GH, Oxman AD; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ. 2016 Jun 28;353:i2016. doi: 10.1136/bmj.i2016. PubMed PMID: 27353417.
Alonso-Coello P, Oxman AD, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Vandvik PO, Meerpohl J, Guyatt GH, Schünemann HJ; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 2: Clinical practice guidelines. BMJ. 2016 Jun 30;353:i2089. doi: 10.1136/bmj.i2089. PubMed PMID: 27365494.
Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, Fervers B, Graham ID, Grimshaw J, Hanna SE, Littlejohns P, Makarski J, Zitzelsberger L; AGREE Next Steps Consortium. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010 Dec 14;182(18):E839-42. doi: 10.1503/cmaj.090449. Epub 2010 Jul 5. Review. PubMed PMID: 20603348; PubMed Central PMCID: PMC3001530.
Hultcrantz M, Rind D, Akl EA, Treweek S, Mustafa RA, Iorio A, Alper BS, Meerpohl JJ, Murad MH, Ansari MT, Katikireddi SV, Östlund P, Tranæus S, Christensen R, Gartlehner G, Brozek J, Izcovich A, Schünemann H, Guyatt G. The GRADE Working Group clarifies the construct of certainty of evidence. J Clin Epidemiol. 2017 Jul;87:4-13. doi: 10.1016/j.jclinepi.2017.05.006. Epub 2017 May 18. PubMed PMID: 28529184; PubMed Central PMCID: PMC6542664.
Medisch Specialistische Richtlijnen 2.0 (2012). Adviescommissie Richtlijnen van de Raad Kwalitieit. http://richtlijnendatabase.nl/over_deze_site/over_richtlijnontwikkeling.html
Neumann I, Santesso N, Akl EA, Rind DM, Vandvik PO, Alonso-Coello P, Agoritsas T, Mustafa RA, Alexander PE, Schünemann H, Guyatt GH. A guide for health professionals to interpret and use recommendations in guidelines developed with the GRADE approach. J Clin Epidemiol. 2016 Apr;72:45-55. doi: 10.1016/j.jclinepi.2015.11.017. Epub 2016 Jan 6. Review. PubMed PMID: 26772609.
Schünemann H, Brożek J, Guyatt G, et al. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group, 2013. Available from http://gdt.guidelinedevelopment.org/central_prod/_design/client/handbook/handbook.html.
Wagenaar O, Gilles A, Van Rompaey V, Blom H. Goal Attainment Scale in tinnitus (GAS-T): treatment goal priorities by chronic tinnitus patients in a real-world setting. Eur Arch Otorhinolaryngol. 2024 Feb;281(2):693-700. doi: 10.1007/s00405-023-08134-2. Epub 2023 Jul 25. PMID: 37488402.
Zoekverantwoording
Zoekacties zijn opvraagbaar. Neem hiervoor contact op met de Richtlijnendatabase.