Nacontrole na resectie
Uitgangsvraag
Wat is de optimale nacontrole voor patiënten met oesofaguscarcinoom na chemo(radiatie) en resectie?
Aanbeveling
Voer geen routinematig intensieve follow-up uit bij patiënten na een slokdarmresectie
Overweeg aanvullende diagnostiek bij patiënten met verdenking op een recidief, bij wie een vroege diagnose zou kunnen leiden tot aanvullende behandeling.
Overwegingen
Voor- en nadelen van de interventie en de kwaliteit van het bewijs
De literatuursearch resulteerde in één systematic review (Chidambaram, 2022) en één RCT (Bjerring, 2019) die verschillende vormen van surveillance met elkaar vergeleken bij patiënten met oesofaguscarcinoom na een chirurgische behandeling.
De RCT van Bjerring (2019) rapporteert uitkomsten met betrekking tot overleving en her-interventies en de SR van Chidambaram (2022) rapporteert overleving. Bjerring (2019) rapporteert met betrekking tot de uitkomst algehele overleving een klinisch relevant overlevingsvoordeel voor de groep die gedurende de follow-up FDG-PET-CT scans en endoscopieën krijgen (imaging groep) in vergelijking met de groep die standaard follow-up met klinisch onderzoek krijgt (standaard groep). De RCT van Bjerring (2019) rapporteert ook meer behandelingen met chemotherapie naar aanleiding van recurrence in de imaging groep.
Drie studies uit de SR van Chidambaram (2022) rapporteren algehele overleving waarbij één studie voordeel geeft voor de ongeplande follow-up strategie (Antonowicz, 2015) en twee studies voordeel voor intensieve follow-up (Jiang, 2020; Elliot, 2020).
Wanneer er naar de belangrijke uitkomstmaten locoregionale recurrence en detectie van afstandsmetastasen wordt gekeken, rapporteren de systematic review van Chidambaram (2022) en de RCT van Bjerring (2019) alleen uitkomsten met betrekking tot locoregionale recurrence. Chidambaram (2022) rapporteert uitkomsten van zes cohortstudies waarbij er meer locoregionale recurrence wordt gedetecteerd met een gepland follow-up programma in vergelijking met een follow-up op indicatie (Abate, 2009; Antonowicz, 2015; DeSouza, 2018; Jiang, 2020; Lou, 2013; Sicic, 2017). Daarnaast rapporteert Chidambaram (2022) post-recurrence overleving, waarbij er een voordeel gevonden wordt voor de geplande follow-up. Er moet daarbij gezegd worden, dat er maar drie studies in de review van Chidambaram (2022) hebben gekeken naar post-recurrence overleving, waarvan één van de drie een voordeel aangaf voor follow-up op indicatie (Antonowicz, 2015).
Voor alle uitkomstmaten is de bewijskracht laag. Dit heeft te maken met het risico op bias, de kleine patiëntaantallen en de uitkomsten overleving en her-interventies waarbij er alleen door Bjerring (2019) uitkomsten zijn gerapporteerd. Daarnaast includeren de RCT van Bjerring (2019) en de cohortstudies van Jiang (2020) en Sicic (2017) zowel patiënten met oesofaguscarcinoom als patiënten met maagcarcinoom, wat niet overeenkomt met de patiëntpopulatie zoals gesteld in de PICO. Ook komen de interventies en controlegroepen niet geheel overeen met de gestelde PICO’s en vergelijken de studies verschillende follow-up strategieën met elkaar.
Waarden en voorkeuren van patiënten (en evt. hun verzorgers)
Aan de ene kant kunnen frequente controles rust en vertrouwen geven voor de patiënt, omdat eventuele afwijkingen tijdig ontdekt kunnen worden, wat de kans op (succesvolle) behandeling vergroot. Echter, controles kunnen ook onzeker en stressvol zijn, met een grote psychische belasting tot gevolg. CT-scans en PET-CT scans geven geen grote lichamelijke belasting voor de patiënt, maar wel stralingsbelasting. MRI-scans geven geen grote lichamelijke belasting voor de patiënt.
Kosten (middelenbeslag)
Een intensieve follow-up met terugkerende beeldvormende onderzoeken gaat gepaard met aanzienlijke kosten die variëren van een paar honderd (CT) tot ruim duizend euro (PET-CT) per onderzoek. Beoordeling van de onderzoeken en bespreking hiervan in MDO’s kosten eveneens tijd en middelen.
Aanvaardbaarheid, haalbaarheid en implementatie
Een intensieve follow-up is voor patiënten bij wie een vroege detectie zou kunnen leiden tot aanvullende behandeling en een betere kans op overleving een aanvaardbare aanvulling op de reeds zeer intensieve behandeling die zij hebben ondergaan. In alle slokdarmcentra in Nederlands is de kennis en kunde aanwezig om deze follow-up uit te voeren. Een eventuele implementatie van een intensiever follow-up schema zou daarom relatief eenvoudig kunnen zijn, mits er voldoende scancapaciteit is.
Er is onvoldoende bewijs voor het routinematig uitvoeren van intensieve follow-up bij patiënten na een slokdarmresectie. In de praktijk blijkt echter dat er klinische follow-up wordt uitgevoerd met aanvullende diagnostiek op indicatie en in overleg met de patiënt.
Rationale van de aanbeveling: weging van argumenten voor en tegen de interventies
Resultaten uit een RCT en resultaten van 7 relevante studies uit een SR van in totaal 11 studies tonen wisselende uitkomsten over het nut van intensieve follow-up. Vroege detectie van een lokaal recidief of metastase kan mogelijk leiden tot een kans op gerichte lokale behandeling en uitstel van palliatieve systeemtherapie. Identificatie van patiënten met oligometastatische ziekte kan in geselecteerde gevallen resulteren in een conversie van een palliatief naar een curatief traject en daarmee leiden tot een betere overall survival.
Er is geen bewijs over de invloed van intensieve follow-up op kwaliteit van leven.
De kwaliteit van het bewijs voor deze aanbeveling is zeer zwak.
Onderbouwing
Achtergrond
Follow-up after curative treatment with neo-adjuvant chemo(radio)therapy and esophageal resection is currently based on local preference and varies widely from outpatient visits with or without vitamin studies during several years up to routine imaging studies and follow-up of tumor markers. The optimal follow-up strategy has yet to be determined and both length and intensity of follow-up should be based on objective outcomes such as overall survival, cost-efficiency and patient burden.
Conclusies / Summary of Findings
|
Very low GRADE |
The evidence is very uncertain about the effect of follow-up with imaging on overall survival compared with follow-up without imaging in patients with esophageal carcinoma after neoadjuvant chemo(radiotherapy) and resection.
The evidence is very uncertain about the effect of follow-up shorter than five years on overall survival compared with 5-year follow-up in patients with esophageal carcinoma after neoadjuvant chemo(radiotherapy) and resection.
Sources: Bjerring, 2019; Chidambaram, 2022 |
|
Very low GRADE |
The evidence is very uncertain about the effect of follow-up with imaging on re-interventions compared with follow-up without imaging in patients with esophageal carcinoma after neoadjuvant chemo(radiotherapy) and resection.
The evidence is very uncertain about the effect of follow-up shorter than five years on re-interventions compared with 5-year follow-up in patients with esophageal carcinoma after neoadjuvant chemo(radiotherapy) and resection.
Source: Bjerring, 2019 |
|
Very low GRADE |
The evidence is very uncertain about the effect of follow-up with imaging on locoregional recurrence compared with follow-up without imaging in patients with esophageal carcinoma after neoadjuvant chemo(radiotherapy) and resection.
The evidence is very uncertain about the effect of follow-up shorter than five years on locoregional recurrence compared with 5-year follow-up in patients with esophageal carcinoma after neoadjuvant chemo(radiotherapy) and resection.
Sources: Bjerring, 2019; Chidambaram, 2022 |
|
No GRADE |
No evidence was found regarding the effect of follow-up shorter than five years on quality of life and on detection of metastases compared with 5-year follow-up in patients with esophageal carcinoma after neoadjuvant chemo(radiotherapy) and resection.
No evidence was found regarding the effect of follow-up with imaging on quality of life and on detection of metastases compared with follow-up without imaging in patients with esophageal carcinoma after neoadjuvant chemo(radiotherapy) and resection.
Source: - |
Samenvatting literatuur
Description of studies
Chidabaram (2022) conducted a systematic review to investigate the effect of various surveillance protocols in patients who underwent a surgical intervention for esophago-gastric cancer. Literature was searched up to July 2021. RCTs, quasi-randomized trials, cohort studies and case-control studies, investigating surveillance including any form of surveillance, studies with any type of surgery carried out with a curative intent and studies involving patients who underwent surgery alongside adjuvant or neoadjuvant chemotherapy, were included.
In total Chadabaram (2022) included 11 studies, regarding the scope of this summary, only seven cohort studies in patients with esophageal cancer, were included (Abate, 2009; Antonowicz, 2015; DeSouza, 2018; Jiang, 2020; Lou, 2013; Sicic, 2017; Elliot, 2020). The included studies compared a planned surveillance protocol with unplanned surveillance (table 1). Studies reported different follow-up period varying from 18 to 60 months. Chidabaram (2022) used the Newcastle-Ottawa tool (NOS) to assess the risk of bias.
Chidabaram (2022) reported detection of recurrence and post-recurrence survival.
Table 1. Surveillance protocols of included studies in review of Chidabaram (2022)
|
Study |
Planned surveillance protocol (intervention) |
Unplanned surveillance protocol (comparator) |
|
Abate (2009) |
History and physical examination, complete blood count, serum chemistry tests, CEA level and contrast-enhanced CT scan of chest and abdomen every 3 months for first 3 years, every 6 months for next 2 years and yearly thereafter. PET-scans were done yearly. |
Unplanned surveillance. |
|
Antonowicz (2015) |
History and physical examination at 6 weeks, 3 months, 6 months, 1 year, 18 months, 2 years than annually to 5 years. CT-scanning at 6 months, 1 year then annually to 5 years. |
Unplanned CT following urgent referral due to symptoms. |
|
DeSouza (2018) |
CT scanning every 3 months for the first year, every 6 months for the 2nd year, then annually from the 3rd year forward until the 5th year postoperatively. |
Unplanned surveillance. |
|
Jiang (2020) |
Bloodwork, imaging, or EGD’s were performed at the discretion of treating physicians. Interval of £ 4 months with respect to imaging at any time during surveillance period. |
Bloodwork, imaging, or EGD’s were performed at the discretion of treating physicians. Interval of > 4 months. |
|
Lou (2013) |
History, physical examination and chest and abdominal CT scan every 4-6 months for the first 2 years after surgery and then yearly thereafter. |
Endoscopy every 6 months for 2 years and then yearly thereafter. |
|
Sicic (2017) |
Standardized follow-up protocol every 3 months during first 2 years, every 6 months during 3rd and 4th year and after 12 months in the 5th year. Follow-up examinations included CT-scan and endoscopy. |
Individual follow-up by other physicians |
|
Elliot (2020) |
Routine annual CT/PET-CT along with clinical assessment during the first three postoperative years. |
Investigation as clinically indicated. |
Bjerring (2019) performed a randomized controlled trial comparing a structured follow-up program including FDG PET-CT and endoscopic ultrasonography (EUS) with standard follow-up visits including a clinical assessment. Patients who underwent radical resection for adenocarcinomas in the gastro-esophageal junction (GOJ), stomach and pancreas and who were fit for oncological treatment, were included in the study. Patients were randomly allocated to the intervention (imaging group) or control (standard group). Follow-up visits in both groups were scheduled at 3, 6, 9, 12, 18 and 24 months after surgery.
90 patients were included in the imaging group with a mean age of 63 years. In the imaging group, 32% of the patients had GOJ cancer, 24% stomach cancer and 43% pancreas cancer. The standard group consisted of 93 patients with a mean age of 67 years. In this group 41% had GOJ cancer, 34% stomach cancer and 25% pancreas cancer.
Bjerring (2019) reported overall survival, number of patients receiving chemotherapy for recurrence (re-interventions), disease recurrence and detection of isolated locoregional recurrence.
Results
Overall survival
The systematic review of Chidambaram (2022) and the RCT of Bjerring (2019) reported overall survival (OS).
Three studies in the review of Chidambaram (2022) reported overall survival (Antonowicz, 2015; Jiang, 2020; Elliot, 2020). Antonowicz reported OS of 15 months in the intervention group (planned follow-up) versus 20 months in the control group (unplanned follow-up). This is in favor of the unplanned follow-up. The study of Jiang (2020) reported overall survival of 36.2 months in the intervention group (surveillance) and 23.7 months in de control group (routine). Elliot (2020) reported a Hazard Ratio of 0.90 favoring intensive surveillance group.
Bjerring (2019) reported median survival of 46 months (95%CI 29-not reached) in the intervention (imaging) group and 36 months (95%CI 21-50) in the control (standard follow-up) group. This difference is clinically relevant.
Quality of life
None of the studies included in the summary of literature, reported quality of life as outcome.
Re-interventions
One study reported re-interventions (Bjerring, 2019). Bjerring (2019) reported number of patients receiving chemotherapy for recurrence. In the intervention (imaging) group, 29 patients (56%) received chemotherapy and in the control (standard follow-up) group, 19 patients (38%) received chemotherapy. This difference is clinically relevant.
Locoregional recurrence
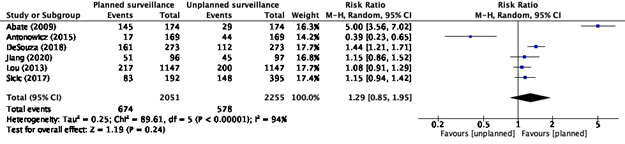
Two studies reported locoregional recurrence (Chidambaram, 2022; Bjerring, 2019). Chidambaram (2022) reported detection of recurrence (figure 1).
Figure 1. Detection of recurrence, Chidambaram (2022)
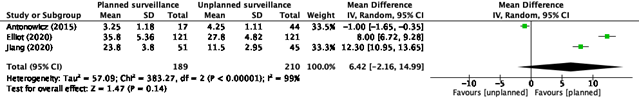
Chidambaram (2022) also reported post-recurrence survival (figure 2).
Figure 2. Post-recurrence survival, Chidambaram (2022)
The RCT of Bjerring (2019) reported disease recurrence in 48 patients (53.3%) in the intervention (imaging) group and in 42 patients (45.2%) in the control (standard follow-up) group. This difference is clinically relevant.
Bjerring (2019) also reported detection of isolated locoregional recurrence in 13 patients (14.4%) in the intervention (imaging) group and in 4 patients (4%) in the control (standard follow-up) group. This difference is clinically relevant. Detecting recurrences at an early stage can be important for patients presenting with ILR. Nine patients in the imaging group who presented with ILR were offered therapy with curative intent. In the standard group, only three patients diagnosed with ILR were offered therapy for cure.
Detection of distant metastases
None of the studies included in the summary of literature, reported detection of distant metastases as outcome.
Level of evidence of the literature
The level of evidence regarding the outcome measure overall survival was downgraded to very low GRADE because of study limitations (-1; risk of bias), conflicting results (-1; inconsistency) and applicability (-1; bias due to indirectness because of difference in study population, intervention and comparison in the study and as stated in the PICO).
The level of evidence regarding the outcome measure re-interventions was downgraded to very low GRADE because of study limitations (-1; risk of bias), applicability (-1; bias due to indirectness because of difference in study population, intervention and comparison in the study and as stated in the PICO) and number of included patients (-1; imprecision).
The level of evidence regarding the outcome measure locoregional recurrence was downgraded by to very low GRADE because of study limitations (-1; risk of bias), conflicting results (-1; inconsistency) and applicability (-1; bias due to indirectness because of difference in study population, intervention and comparison in the studies and as stated in the PICO).
Zoeken en selecteren
A systematic review of the literature was performed to answer the following questions:
1. What is the effect of follow-up with imaging on overall survival, quality of life, re-interventions, locoregional recurrence and detection of distant metastases compared with no follow-up in patients with esophageal carcinoma after neoadjuvant chemo(radiotherapy) and resection?
| P: | Patients with esophageal carcinoma after neoadjuvant chemo(radiotherapy) and resection |
| I: | Follow-up with imaging (CT-scan, PET-CT scan, MRI) |
| C: | No follow-up |
| O: | Overall survival, quality of life, re-interventions, locoregional recurrence, detection of distant metastases |
2. What is the effect of follow-up shorter than 5-years on overall survival, quality of life, re-interventions, locoregional recurrence and detection of distant metastases compared with 5-year follow-up in patients with esophageal carcinoma after neoadjuvant chemo(radiotherapy) and resection?
| P: | Patients with esophageal carcinoma after neoadjuvant chemo(radiotherapy) and resection |
| I: | Follow-up shorter than 5-years |
| C: | 5-year follow-up |
| O: | Overall survival, quality of life, re-interventions, locoregional recurrence, detection of distant metastases |
Relevant outcome measures
The guideline development group considered overall survival, quality of life and re-interventions as critical outcome measures for decision making and locoregional recurrence and detection of distant metastases as important outcome measures for decision making.
A priori, the working group did not define the outcome measures listed above but used the definitions used in the studies.
The working group defined the following differences as a minimal clinically (patient) important difference:
- Overall survival: >5% or >3% with a HR<0.70.
- Quality of life: A difference of 10 points on the quality of life instrument EORTC QLQ-C30 or a difference of a similar magnitude on other quality of life instruments.
- Re-interventions including local treatment for isolated local recurrences and/or systemic treatment for more diffuse recurrence: >5%.
- Locoregional recurrence: >5%.
- Detection of distant metastases: >5%.
Search and select (Methods)
The databases Medline (via OVID) and Embase (via Embase.com) were searched with relevant search terms until 08-06-2023. The detailed search strategy is depicted under the tab Methods. The systematic literature search resulted in 559 hits. Studies were selected based on the following criteria:
- The study population had to meet the criteria as defined in the PICO.
- The intervention or comparison had to be as defined in the PICO.
- At least one of the outcomes as defined in the PICO had to be reported.
- Full text available.
- Articles written in English or Dutch.
- Study design: Systematic review or randomized controlled trial (RCT).
- In case of a systematic review:
- Minimum of two databases searched.
- Search strategy available with search date.
- In- and exclusion criteria reported.
- Evidence table for included studies available.
- Risk of bias assessment per study available.
Four studies were initially selected based on title and abstract screening. After reading the full text, two studies were excluded (see the table with reasons for exclusion under the tab Methods), and two studies were included.
Results
One systematic review and one RCT were included in the analysis of the literature. Important study characteristics and results are summarized in the evidence table. The assessment of the risk of bias is summarized in the risk of bias tables.
Referenties
- 1 - Abate E, DeMeester SR, Zehetner J, Oezcelik A, Ayazi S, Costales J, Banki F, Lipham JC, Hagen JA, DeMeester TR. Recurrence after esophagectomy for adenocarcinoma: defining optimal follow-up intervals and testing. J Am Coll Surg. 2010 Apr;210(4):428-35.
- 2 - Antonowicz SS, Lorenzi B, Parker M, Tang CB, Harvey M, Kadirkamanathan SS. Annual computed tomography scans do not improve outcomes following esophagectomy for cancer: a 10-year UK experience. Dis Esophagus. 2015 May-Jun;28(4):365-70.
- 3 - Bjerring OS, Fristrup CW, Pfeiffer P, Lundell L, Mortensen MB. Phase II randomized clinical trial of endosonography and PET/CT versus clinical assessment only for follow-up after surgery for upper gastrointestinal cancer (EUFURO study). Br J Surg. 2019 Dec;106(13):1761-1768.
- 4 - Chidambaram S, Sounderajah V, Maynard N, Markar SR. Evaluation of post-operative surveillance strategies for esophageal and gastric cancers: a systematic review and meta-analysis. Dis Esophagus. 2022 Dec 14;35(12):doac034.
- 5 - DeSouza ML, Drexel SE, Dewey E, Hunter JG, Mallak N, Dolan JP. Timing and pattern of recurrence follwong esophagectomy for esophageal carcinoma. Gastroenterology. 2018; 154(6): S-1257.
- 6 - Elliott JA, Markar SR, Klevebro F, Johar A, Goense L, Lagergren P, Zaninotto G, van Hillegersberg R, van Berge Henegouwen MI, Nilsson M, Hanna GB, Reynolds JV; ENSURE Study Group. An International Multicenter Study Exploring Whether Surveillance After Esophageal Cancer Surgery Impacts Oncological and Quality of Life Outcomes (ENSURE). Ann Surg. 2023 May 1;277(5):e1035-e1044.
- 7 - Jiang DM, Suzuki C, Espin-Garcia O, Lim CH, Ma LX, Sun P, Sim HW, Natori A, Chan BA, Moignard S, Chen EX, Liu G, Swallow CJ, Darling GE, Wong R, Jang RW, Elimova E. Surveillance and outcomes after curative resection for gastroesophageal adenocarcinoma. Cancer Med. 2020 May;9(9):3023-3032.
- 8 - Sisic L, Strowitzki MJ, Blank S, Nienhueser H, Dorr S, Haag GM, Jäger D, Ott K, Büchler MW, Ulrich A, Schmidt T. Postoperative follow-up programs improve survival in curatively resected gastric and junctional cancer patients: a propensity score matched analysis. Gastric Cancer. 2018 May;21(3):552-568.
Evidence tabellen
Research question:
- What is the effect of follow-up with imaging on overall survival, quality of life, re-interventions, locoregional recurrence and detection of distant metastases compared with no follow-up in patients with esophageal carcinoma after neoadjuvant chemo(radiotherapy) and resection?
- What is the effect of follow-up shorter than 5-years on overall survival, quality of life, re-interventions, locoregional recurrence and detection of distant metastases compared with 5-year follow-up in patients with esophageal carcinoma after neoadjuvant chemo(radiotherapy) and resection?
|
Study reference |
Study characteristics |
Patient characteristics |
Intervention (I) |
Comparison / control (C)
|
Follow-up |
Outcome measures and effect size |
Comments |
|
Chidambaram, 2022
PS. Study characteristics and results are extracted from the SR (unless stated otherwise) |
SR and meta-analysis of cohort studies
Literature search up to July 2021
A: Abate, 2009 B: Antonowicz, 2015 C: DeSouza, 2018 D: Jiang, 2020 E: Lou, 2013 F: Sisic, 2017 G: Elliot, 2020
Study design: A: Retrospective cohort study B: Retrospective cohort study C: Retrospective cohort study D: Retrospective cohort study E: Retrospective cohort study F: Retrospective cohort study G: Prospective cohort study
Setting and Country: Not reported
Source of funding and conflicts of interest: Not reported
|
Inclusion criteria SR: - RCT’s, quasi-randomized trials, cohort studies, case control studies; - Investigating the use of surveillance protocol in patients who had undergone surgery for gastric or esophageal cancer or compared factors between symptomatic and asymptomatic recurrence; - Surveillance included any form such as regular clinical follow-up (history and examination), blood tests, radiological investigations and endoscopy; - Studies with any type of surgery carried out with a curative intent; - Studies involving patients who underwent surgery alongside adjuvant or neoadjuvant chemotherapy
Exclusion criteria SR: - Review articles and case reports; - Studies with no comparative group; - Comparative studies with no outcome data provided for the control or intervention group - Studies with post-surgery surveillance in a cohort of patients who would have required monitoring regardless, such as patients with hereditary syndromes - Studies which included patients who did not undergo surgery but underwent only other curative therapies and if other cancer types were included
Regarding the scope of this data extraction, only 6 studies regarding patients with esophageal cancer, were included
Important patient characteristics at baseline:
N; mean age A: 174 patients; I: 56 years, C: 69 years B: I: 17 patients, C: 44 patients; I: 61 years, C: 64 years C: 273 patients; NR D: 210 patients; 64.1 years E: 1147 patients; NR F: 587, 63 years
Sex (males): A: 16% B: I: 94%, C: 86% C: 84% D: 73% E: NR F: I: 72.4%, C: 72.7%
Stage: A: NR B: T1: 8%, T2: 21%, T3: 67%, T4: 2% C: NR D: T1: 7%, T2: 17%, T3: 22%, T4: 54% E: T1: 32%, T2: 30%, T3: 25% F: T1: I: 4.2%, C: 12.4%; T2: I: 18.8%, C: 30.4%; T3: I: 64.6%, C: 46.8%; T4: I: 9.4%, C: 4.6%
Operation: A: Transthoracic en bloc, transhiata, vagal-sparing or minimally invasive B: Open: 21%, minimally invasive: 74%, transhiatal: 5% C: Esophagectomy D: Esophagectomy: 12%, Gastrectomy: 47%, Esophago-gastrectomy: 41% E: Esophagectomy F: Subtotal gastrectomy, total gastrectomy, THG, Ivor Lewos procedure or other.
Other forms of treatment: A: 27% had neoadjuvant therapy B: 67.4% had neoadjuvant chemotherapy C: 75.4% had chemotherapy, 72% had radiotherapy D: Surgery + adjunctive therapy: 71% E: 63% had neoadjuvant chemotherapy F: Neoadjuvant treatment: I: 64.6%, C: 32.2%
Groups comparable at baseline: No |
Describe intervention:
A: History and physical examination, complete blood count, serum chemistry tests, CEA level and contrast-enhanced CT scan of chest and abdomen every 3 months for first 3 years, every 6 months for next 2 years and yearly thereafter. PET-scans were done yearly. B: History and physical examination at 6 weeks, 3 months, 6 months, 1 year, 18 months, 2 years than annually to 5 years. CT-scanning at 6 months, 1 year then annually to 5 years. C: CT scanning every 3 months for the first year, every 6 months for the 2nd year, then annually from the 3rd year forward until the 5th year postoperatively D: Bloodwork, imaging, or EGD’s were performed at the discretion of treating physicians. Interval of £ 4 months with respect to imaging at any time during surveillance period E: History, physical examination and chest and abdominal CT scan every 4-6 months for the first 2 years after surgery and then yearly thereafter. F: Standardized follow-up protocol every 3 months during first 2 years, every 6 months during 3rd and 4th year and after 12 months in the 5th year. Follow-up examinations included CT-scan and endoscopy. G: Routine annual CT/PET-CT along with clinical assessment during the first three postoperative years
|
Describe control:
A: Unplanned surveillance B: Unplanned CT following urgent referral due to symptoms C: Unplanned surveillance D: Bloodwork, imaging, or EGD’s were performed at the discretion of treating physicians. Interval of > 4 months E: Endoscopy every 6 months for 2 years and then yearly thereafter F: Individual follow-up by other physicians G: Investigation as clinically indicated
|
Follow-up:
A: Median 18 months B: Minimum 37 months C: 24 months D: Median 38.3 months E: Median 31 months F: Median 64.5 months G: Median 60 months
For how many participants were no complete outcome data available? Not reported
|
Outcome measure-1 Defined as detection of recurrence
Effect measure: OR [95% CI]: A: 25.00 [14.23-43.94] B: 0.32 [0.17-0.58] C: 2.07 [1.47-2.91] D: 1.31 [0.74-2.31] E: 1.27 [0.89-1.80] F: 1.27 [0.89-1.80]
Pooled effect (random effects model): 1.76 [95% CI 0.78 to 3.97] favoring the intervention (planned surveillance) Heterogeneity (I2): 96%
Outcome measure-2 Defined as post-recurrence survival
Effect measure: MD [95% CI]: A: NR B: -1.00 [-1.65- -0.35] C: NR D: 12.30 [10.95-13.65] E: NR F: 8.00 [6.72-9.28]
Pooled effect (random effects model): 6.42 [95% CI -2.16 to 14.99] favoring the intervention (planned surveillance) Heterogeneity (I2): 99%
Outcome measure-3 Defined as overall survival
B: I: 15 months, C: 20 months D: I: 36.2 months, C: 23.7 months G: HR 0.90
|
Risk of bias (NOS score): Tool used by authors:
A: 8/9 (high quality) B: 8/9 (high quality) C: 8/9 (high quality) D: 8/9 (high quality) E: 8/9 (high quality) F: NR G: 8/9 (high quality)
Brief description of author’s conclusion: Our study shows that there is still no consensus regarding monitoring patients after their operation. Our study highlights that planned surveillance had good survival benefit for patients with esophageal cancer.
|
|
Bjerring, 2019 |
Type of study: RCT
Setting and country: Single center, Odense Denmark
Funding and conflicts of interest: Authors declare no conflict of interest |
Inclusion criteria: - Patients who underwent radical resection for adenocarcinomas in the gastro-oesophageal junction (GOJ), stomach and pancreas at Odense University Hospital - Patients who were fit for oncological treatment (Eastern Cooperative Oncology Group performance status score < 3) at time of assessment 1 month after surgery - Written informed consent
Exclusion criteria: - Not reported
N total at baseline: Intervention: 90 Control: 93
Important prognostic factors2: Mean age in years: I: 63 (95%CI 61-65) C: 67 (95%CI 66-69)
Sex: I: 60% M C: 59% M
Location of cancer: GOJ: I: 32% C: 41%
Stomach: I: 24% C: 34%
Pancreas: C: 25%
Preoperative chemotherapy: C: 59%
Postoperative chemotherapy: I: 71% C: 73%
Groups comparable at baseline: No |
Describe intervention: Follow-up at 3, 6, 9, 12, 18 and 24 months after surgery with clinical assessment in combination with FDG-PET/CT and endoscopic ultrasonography (EUS) at all time points.
|
Describe control: Standard follow-up with regular visits to the outpatients clinical at 3, 6, 9, 12, 18 and 24 months after surgery. Standard follow-up included a clinical assessment. Diagnostic tests were undertaken only when there was a clinical suspicion of recurrent disease.
|
Length of follow-up: Regular follow-up: 24 months Chart review follow-up: 8 years (June 2017)
Loss-to-follow-up: No loss-to-follow-up reported
Incomplete outcome data: Not reported
|
Outcome measures and effect size (include 95%CI and p-value if available): Overall survival I: Median 46 months (95%CI 29-not reached) C: Median 36 months (95%CI 21-50)
Re-interventions (number of patients receiving chemotherapy for recurrence) I: N=29/52 (56%) C: N=19/50 (38%)
Disease recurrence I: N=48 (53.3%) C: N=42 (45.2%)
Detection of isolated locoregional recurrences I: N=13 (14.4%) C: N=4 (4%)
|
Brief description of author’s conclusion: It was hypothesized that the addition of imaging at the follow-up visits would detect recurrences earlier after surgery. Although the time from surgery to the detec- tion of recurrence was not statistically significantly differ- ent between the two groups, most patients in the imaging group were asymptomatic at the time of recurrence detec- tion (33 of 42 patients).
|
Table of quality assessment for systematic reviews of RCTs and observational studies
|
Study
First author, year |
Appropriate and clearly focused question?1
Yes/no/unclear |
Comprehensive and systematic literature search?2
Yes/no/unclear |
Description of included and excluded studies?3
Yes/no/unclear |
Description of relevant characteristics of included studies?4
Yes/no/unclear |
Appropriate adjustment for potential confounders in observational studies?5
Yes/no/unclear/not applicable |
Assessment of scientific quality of included studies?6
Yes/no/unclear |
Enough similarities between studies to make combining them reasonable?7
Yes/no/unclear |
Potential risk of publication bias taken into account?8
Yes/no/unclear |
Potential conflicts of interest reported?9
Yes/no/unclear |
|
Chidambaram, 2022 |
Yes |
Yes |
No, no description of excluded studies |
Yes |
No |
Yes, NOS was used to assess quality and Risk of Bias |
Yes |
No |
No |
Risk of bias table for intervention studies (randomized controlled trials; based on Cochrane risk of bias tool and suggestions by the CLARITY Group at McMaster University)
|
Study reference
(first author, publication year) |
Was the allocation sequence adequately generated?
Definitely yes Probably yes Probably no Definitely no |
Was the allocation adequately concealed?
Definitely yes Probably yes Probably no Definitely no |
Blinding: Was knowledge of the allocated interventions adequately prevented?
Were patients blinded?
Were healthcare providers blinded?
Were data collectors blinded?
Were outcome assessors blinded?
Were data analysts blinded?
Definitely yes Probably yes Probably no Definitely no |
Was loss to follow-up (missing outcome data) infrequent?
Definitely yes Probably yes Probably no Definitely no |
Are reports of the study free of selective outcome reporting?
Definitely yes Probably yes Probably no Definitely no |
Was the study apparently free of other problems that could put it at a risk of bias?
Definitely yes Probably yes Probably no Definitely no |
Overall risk of bias If applicable/necessary, per outcome measure
LOW Some concerns HIGH
|
|
Bjerring, 2019 |
Definitely yes;
Reason: Central randomization with computer generated random numbers |
Definitely yes;
Reason: Group allocation labels were placed in non-transparent envelopes. Randomization sequence was unknown to doctors responsible for patient enrolment |
Definitely no;
Reason: Patients and health care providers were not blinded. PET-CT radiologists were not offered any clinical information about the patients or EUS data |
Probably yes;
Reason: No loss to follow-up reported |
Definitely yes;
Reason: All relevant outcomes were reported |
Probably no;
Reason: Groups were not comparable at baseline regarding age and location of cancer |
Some concerns |
Table of excluded studies
|
Reference |
Reason for exclusion |
|
Bišof V, Juretić A, Stančić-Rokotov D, Rustemović N, Miletić D, Boban M, Omrčen T, Razumović JJ, Pavlović I, Fröbe A, Čonkaš M, Rakušić Z, Gugić D. [CLINICAL RECOMMENDATIONS FOR DIAGNOSIS, TREATMENT AND MONITORING OF PATIENTS WITH ESOPHAGEAL AND ESOPHAGOGASTRIC JUNCTION CANCERS]. Lijec Vjesn. 2016 Sep-Oct;138(9-10):233-9. Croatian. PMID: 30148543. |
Wrong language |
|
Bjerring OS, Hess S, Petersen H, Fristrup CW, Lundell L, Mortensen MB. Value of regular endosonography and [18F]fluorodeoxyglucose PET-CT after surgery for gastro-oesophageal junction, stomach or pancreatic cancer. BJS Open. 2021 Mar 5;5(2):zraa028. doi: 10.1093/bjsopen/zraa028. PMID: 33688946; PMCID: PMC7944502. |
Wrong design: Narrative article regarding to EUFORO study Bjerring (2019) |
Verantwoording
Beoordelingsdatum en geldigheid
Publicatiedatum : 01-12-2025
Beoordeeld op geldigheid : 01-12-2025
Samenstelling werkgroep
Voor het ontwikkelen van de richtlijnmodule is in 2022 een multidisciplinaire cluster ingesteld. Het cluster Maag-Oesofaguscarcinoom bestaat uit meerdere richtlijnen, zie hier voor de actuele clusterindeling. De stuurgroep bewaakt het proces van modulair onderhoud binnen het cluster. De expertisegroepsleden geven hun expertise in, indien nodig. De volgende personen uit het cluster zijn betrokken geweest bij de herziening van deze module:
Clusterstuurgroep
- Dhr. Prof. Dr. P.D. (Peter) Siersema (voorzitter), maag-darm-leverarts, Erasmus MC, Rotterdam; NVMDL
- Mevr. Dr. R.E. (Roos) Pouw, maag-darm-leverarts, UMC Utrecht; NVMDL
- Mevr. Prof. Dr. H.W.M. (Hanneke) van Laarhoven, internist, Amsterdam UMC, NIV
- Dhr. Dr. E. (Erik) Vegt, nucleair geneeskundige, Erasmus MC, Rotterdam, NVNG
- Dhr. Prof. Dr. M.I. (Mark) van Berge Henegouwen, chirurg, Amsterdam UMC, NVvH
- Dhr. Prof. Dr. R. (Richard) van Hillegersberg, chirurg, UMC Utrecht, NVvH
- Mevr. Dr. A. (Annemarieke) Bartels-Rutten, radioloog, AVL Amsterdam, NVvR
Clusterexpertisegroep
- Dhr. Dr. M.J. (Marc) van Det, chirurg, ZGT Almelo, NvVH
- Mevr. M.E. (Manon) Dik, verpleegkundig specialist, ZGT, Almelo, V&VN
- Dhr. C.C.G. (Carlo) Schippers, verpleegkundig specialist, UMC Utrecht, V&VN
- Dhr. R. (Remco) Huiszoon MBA, ervaringsdeskundige Stichting voor Patiënten met kanker aan het Spijsverteringskanaal; SPKS
Met ondersteuning van
- Mevr. Dr. J. (Jana) Tuijtelaars, adviseur, Kennisinstituut van de Federatie voor Medisch Specialisten
- Mevr. S.N. (Sarah) van Duijn MSc, adviseur, Kennisinstituut van de Federatie voor Medisch Specialisten
- Mevr. Dr. M.H.D. (Majke) van Bommel, adviseur, Kennisinstituut van de Federatie Medisch Specialisen
Belangenverklaringen
Een overzicht van de belangen van de clusterleden en het oordeel over het omgaan met eventuele belangen vindt u in onderstaande tabel. De ondertekende belangenverklaringen zijn op te vragen bij het secretariaat van het Kennisinstituut van de Federatie Medisch Specialisten via secretariaat@kennisinstituut.nl.
Clusterstuurgroepleden
|
Naam |
Hoofdfunctie |
Nevenwerkzaamheden |
Persoonlijke financiële belangen |
Persoonlijke relaties |
Extern gefinancierd onderzoek |
Intellectuele belangen en reputatie |
Overige belangen |
Datum |
Restrictie |
|
Peter Siersema (Voorzitter cluster) |
Maag-Darm-Leverarts en Hoogleraar Gastro-intestinale Endoscopie met focus op Innovatie en Duurzaamheid, Erasmus MC Universitair Medisch Centrum
|
Editor-in-Chief, Endoscopy - betaald
|
Geen |
Geen |
* Pentax, FujiFilm, Norgine & Magentiq Eye - Endoscopisch onderzoek - Projectleider * Astra Zeneca - Onderzoek behandeling Eosinofiele oesofagitis - Geen projectleider
|
Geen |
Geen |
24-07-2025 |
Geen restricties |
|
Hanneke van Laarhoven
|
Internist-oncoloog. Hoofd afdeling medische oncologie, Amsterdam UMC
|
Lid Raad van Toezicht IKLNL - betaald
|
Research funding e/o medicatie materialen voorziening: AMGEN, AstraZeneca, AURISTONE, BMS, Incyte, Merck, ORCA, and Servier - betaald/verstrekt aan Amsterdam UMC Consultant/adviesrol: Amphera, Astellas, Beigene, Daiichy, Myeloid - betaald aan Amsterdam UMC Spreker rol: Astellas, AstraZeneca, BMS, Benecke, Daiichi-Sankyo, JAAP, Medtalks, Novartis, Servier, and Travel Congress Management - betaald aan Amsterdam UMC
|
Geen |
Zie persoonlijke financiele belangen. Daarnaast onderzoek gefinancier door KWF Kankerbestrijding en Health Holland op het gebied van slokdarm/maagkanker (Projectleider)
|
Geen |
Geen |
28-07-2025 |
a) Werkgroeplid werkt niet als enige inhoudsdeskundige aan de module; b) Werkgroeplid werkt tenminste samen met een ander werkgroeplid met vergelijkbare expertise in alle fasen (studieselectie, data-extractie, evidence synthese, evidence-to-decision, aanbevelingen formuleren) van het ontwikkelproces; c) In alle fasen van het ontwikkelproces is een onafhankelijk methodoloog betrokken; d) Overwegingen en aanbevelingen worden besproken en vastgesteld tijdens een werkgroepvergadering onder leiding van een onafhankelijk voorzitter (zonder gemelde belangen)
|
|
Annemarieke Bartels-Rutten
|
Radioloog, Antoni van Leeuwenhoek
|
Geen |
In dienst bij ziekenhuis dat zich volledig richt op oncologische zorg.
|
Geen |
Geen |
Geen |
Geen |
23-07-2025 |
Geen restricties |
|
Mark van Berge Henegouwen
|
chirurg slokdarm en maagchirurgie Amsterdam UMC hoogleraar slokdarm en maagchirurgie Universiteit van Amsterdam
|
Consultant: Intuitive Surgery, Johnson and Johnson, Medtronic, Stryker Alle fees voor deze functies betaald aan Amsterdam UMC.
|
Geen |
Geen |
Olympus financiering studie (researcher initiated grant) Stryker financiering studie (researcher initiated grant) uitkomsten richtlijn geen invloed op deze bedrijven of studies
|
Geen |
consultancy voor meerdere bedrijven (uitbetaling aan Amsterdam UMC), niet gerelateerd aan richtlijn. bedrijven: Alesi Surgical, Mylan, Jonson and Johnson. bij Medtronic niet meer actief. Toevoeging: bestuur DUCA, DICA en voorzitter werkgroep Upper GI (allen onbetaald). Bij consultancy uitbetaald aan A'dam UMC mag nog bij: BBraun en Viatris
|
26-08-2025 |
a) Werkgroeplid werkt niet als enige inhoudsdeskundige aan de module; b) Werkgroeplid werkt tenminste samen met een ander werkgroeplid met vergelijkbare expertise in alle fasen (studieselectie, data-extractie, evidence synthese, evidence-to-decision, aanbevelingen formuleren) van het ontwikkelproces; c) In alle fasen van het ontwikkelproces is een onafhankelijk methodoloog betrokken; d) Overwegingen en aanbevelingen worden besproken en vastgesteld tijdens een werkgroepvergadering onder leiding van een onafhankelijk voorzitter (zonder gemelde belangen)
|
|
Richard van Hillegersberg
|
Chirurg, UMC Utrecht
|
Proctor Intuitive Surgical Consultant Medtronic Consultant Olympus, betaald aan UMC Utrecht
|
Proctor Intuitive Surgical Consultant Medtronic
|
Geen
|
1. Intuitive - Telementoring trial (Projectleider JA) 2. Intuitive- UGIRA benigne registry (Projectleider JA)
|
Bestuur DUCA, DICA
|
Geen |
4-08-2025 |
a) Werkgroeplid werkt niet als enige inhoudsdeskundige aan de module; b) Werkgroeplid werkt tenminste samen met een ander werkgroeplid met vergelijkbare expertise in alle fasen (studieselectie, data-extractie, evidence synthese, evidence-to-decision, aanbevelingen formuleren) van het ontwikkelproces; c) In alle fasen van het ontwikkelproces is een onafhankelijk methodoloog betrokken; d) Overwegingen en aanbevelingen worden besproken en vastgesteld tijdens een werkgroepvergadering onder leiding van een onafhankelijk voorzitter (zonder gemelde belangen)
|
|
Erik Vegt
|
Nucleair geneeskundige, Afdeling Nucleaire Geneeskunde, Erasmus MC, Rotterdam. Werkzaam op de Afdeling Radiologie Erasmus MC
|
Voorzitter Concilium Radiologicum NVvR / NVNG, onbetaald.
|
Geen |
Geen |
ZonMW-subsidie voor de PLASTIC-studie, programma doelmatigheid van zorg, naar de kosten-effectiviteit van FDG-PET/CT en laparoscopie bij maagcarcinoom. KWF: PLASTIC-3, maagkanker FAPI-PET
|
Geen |
Geen |
23-07-2025 |
Geen restricties |
|
Roos Pouw |
Maag-Darm-Leverarts UMC Utrecht
|
Secretaris Dutch Upper Cancer Group (DUCG), onbetaald - Secretary General van de United European Gastroenterology (UEG), onbetaald - Consultancy voor Medtronic BV, betaling voor verrichte werkzaamheden gaat naar het ziekenhuis - Consultancy voor MicroTech Europe, betaling voor verrichte werkzaamheden gaat naar het ziekenhuis - Consultancy voor Cook BV, betaling voor verrichte werkzaamheden gaat naar het ziekenhuis - Consultancy voor Fujifilm BV, betaling voor verrichte werkzaamheden gaat naar het ziekenhuis - Consultancy voor Boston Scientific, betaling voor verrichte werkzaamheden gaat naar het ziekenhuis.
|
Geen |
Geen |
Projectleider MLDS: Personaliseren van follow-up na endoscopische eradicatie therapie van Barrett slokdarm met neoplasie. Projectleider KWF PREFER studie: onderzoek naar endoscopische follow-up na endoscopische resectie van T1b slokdarmcarcinoom. Projectleider MOELLER Medical: Studie naar endoscopische vacuumtherapie voor profylaxe en therapie van naadlekkage na slokdarm resectie. Projectleider NVGE: Studie die T-cell infiltratie bij vroegcarcinomen onderzoekt en relatie met metastasering
|
Geen |
Geen |
29-08-2025 |
a) Werkgroeplid werkt niet als enige inhoudsdeskundige aan de module; b) Werkgroeplid werkt tenminste samen met een ander werkgroeplid met vergelijkbare expertise in alle fasen (studieselectie, data-extractie, evidence synthese, evidence-to-decision, aanbevelingen formuleren) van het ontwikkelproces; c) In alle fasen van het ontwikkelproces is een onafhankelijk methodoloog betrokken; d) Overwegingen en aanbevelingen worden besproken en vastgesteld tijdens een werkgroepvergadering onder leiding van een onafhankelijk voorzitter (zonder gemelde belangen)
|
Betrokken clusterexpertisegroepleden
|
Naam |
Hoofdfunctie |
Nevenwerkzaamheden |
Persoonlijke financiële belangen |
Persoonlijke relaties |
Extern gefinancierd onderzoek |
Intellectuele belangen en reputatie |
Overige belangen |
Datum |
Restrictie |
|
Marc van Det
|
Gastro-intestinaal chirurg Ziekenhuis groep Twente (ZGT) full-time aanstelling
|
Proctor/Instructor voor Intuitive Surgical betreffende Robot-Assisted operaties in de upper-GI zoals: - Slokdarm resecties - Maagresecties - Hernia diafragmatica. Trainingen worden enkele keren per jaar gegeven en hiervoor ontvangt de trainee geen acreditatiepunten. - deelnemer MEC-U.
|
Proctor/Instructor voor Intuitive Surgical betreffende Robot-Assisted operaties in de upper-GI zoals: - Slokdarm resecties - Maagresecties - Hernia diafragmatica.
|
Geen |
Geen |
Geen |
Geen |
23-07-2025 |
Geen restricties |
|
Manon Dik
|
Verpleegkundig specialist AGZ bij Ziekenhuisgroep Twente
|
Geen |
Geen |
Geen |
Geen |
Geen |
Geen |
4-08-2025 |
Geen restricties |
|
Carlo Schippers
|
Verpleegkundig specialist UMCUtrecht
|
Eigenaar Patiënten begrijpen, betaald
|
Geen |
Geen |
Geen |
Geen |
Geen |
28-07-2025 |
Geen restricties |
|
Remco Huiszoon |
ING Nederland NV, teammanager
|
Bestuurslid SPKS - Stichting voor Patiënten met Kanker aan het Spijsverteringskanaal
|
Geen |
Geen |
Geen |
Geen |
Geen |
14-01-2025 |
Geen restricties |
Werkwijze
Voor meer details over de gebruikte richtlijnmethodologie verwijzen wij u naar de Werkwijze. Relevante informatie voor de ontwikkeling/herziening van deze richtlijnmodule is hieronder weergegeven.
Zoekverantwoording
Zoekopbrengst
|
Database |
Aantallen treffers |
Aantallen treffers na ontdubbelen |
|
Medline 8 jun 2023 |
4595 |
4588 |
|
Embase 8 jun 2023 |
5826 |
2082 |
|
Totaal |
10421 |
6670 |
- Resectie: aantal SRs: 218; aantal RCT’s: 248, aantal observationele studies: 2769
- Recurrence: aantal SR’s: 271, aantal RCT’s: 288, aantal observationele studies: 2876
Zoekstrategie
Ovid/Medline 8 juni 2023
Ovid MEDLINE(R) ALL <1946 to June 07, 2023>
|
1 |
exp Esophageal Neoplasms/ or ((carcinoma* or neoplas* or adenoma* or adenocarcinoma* or tumor* or tumour* or cancer* or oncolog* or malignan* or carcinogen* or oncogen* or anticarcinogen* or squamous*) adj3 (oesophag* or esophag* or gastroesophag* or gastrooesophag* or oesogastr* or esogastr*)).ti,ab,kf. |
79157 |
|
2 |
Surgery.sh. or exp Surgical Procedures, Operative/ or (surger* or surgical* or operation* or operative* or resecti* or esophagectom* or oesophagectom*).ti,ab,kf. |
5056230 |
|
3 |
Survival Rate/ or exp Survival Analysis/ or Survivors/ or Reoperation/ or ((surviv* adj3 (rate or mean or analys* or disease-free or progression-free or event-free or overall)) or survivor* or (surg* adj3 (revis* or repeat*)) or reoperati* or re-operati* or resurg* or re-surg*).ti,ab,kf. |
993539 |
|
4 |
exp Neoplasm Metastasis/ or (metasta* or seeding* or micrometasta*).ti,ab,kf. |
717185 |
|
5 |
exp "Quality of Life"/ or (quality-of-life or qoli or qol or qolis or hrqol* or hr-qol* or life-qual*).ti,ab,kf. |
435782 |
|
6 |
3 or 4 or 5 |
1932631 |
|
7 |
Endoscopy/ or exp Laryngoscopy/ or exp Endoscopy, Gastrointestinal/ or (endoscop* or gastroscop* or laryngoscop* or esophagoscop* or oesophagoscop*).ti,ab,kf. |
336072 |
|
8 |
exp Tomography, X-Ray Computed/ or (computed-tomograph* or ct or cts or cat-scan* or computer-assisted-tomograph* or computerized-tomograph* or computerised-tomograph* or computed-x-ray-tomograph* or computed-xray-tomograph*).ti,ab,kf. |
841768 |
|
9 |
exp Tomography, Emission-Computed/ or (spect or petscan* or pet-scan* or pet or (emission adj3 tomogra*) or radionuclid*).ti,ab,kf. |
240653 |
|
10 |
exp Magnetic Resonance Imaging/ or ((magnetic-resonance adj3 imag*) or mri or mris or nmr or mra or mras or zeugmatograph* or mr-tomograph* or proton-spin or ((magneti* or chemical-shift) adj3 imag*) or fmri or fmris).ti,ab,kf. |
933350 |
|
11 |
7 or 8 or 9 or 10 |
2035704 |
|
12 |
1 and 2 and 6 and 11 |
4806 |
|
13 |
12 not ((Adolescent/ or Child/ or Infant/) not Adult/) |
4802 |
|
14 |
13 not ((exp animals/ or exp models, animal/) not humans/) |
4793 |
|
15 |
14 not (comment/ or editorial/ or letter/ or Case Reports/) |
3816 |
|
16 |
limit 15 to yr="2008 -Current" |
2381 |
|
17 |
(systematic-review.pt. or (meta-analysis/ or meta-analysis as topic/ or (metaanaly* or meta-analy* or metanaly*).ti,ab,kf. or systematic review/ or cochrane.jw. or (prisma or prospero).ti,ab,kf. or ((systemati* or umbrella or "structured literature") adj3 (review* or overview*)).ti,ab,kf. or (systemic* adj1 review*).ti,ab,kf. or ((systemati* or literature or database* or data-base*) adj10 search*).ti,ab,kf. or ((structured or comprehensive* or systemic*) adj3 search*).ti,ab,kf. or ((literature adj3 review*) and (search* or database* or data-base*)).ti,ab,kf. or (("data extraction" or "data source*") and "study selection").ti,ab,kf. or ("search strategy" and "selection criteria").ti,ab,kf. or ("data source*" and "data synthesis").ti,ab,kf. or (medline or pubmed or embase or cochrane).ab. or ((critical or rapid) adj2 (review* or overview* or synthes*)).ti. or (((critical* or rapid*) adj3 (review* or overview* or synthes*)) and (search* or database* or data-base*)).ab. or (metasynthes* or meta-synthes*).ti,ab,kf.)) not (comment/ or editorial/ or letter/ or ((exp animals/ or exp models, animal/) not humans/)) |
637672 |
|
18 |
16 and 17 |
114 |
|
19 |
exp randomized controlled trial/ or random*.ti,ab,kf. or ((pragmatic or practical) adj clinical trial*).ti,ab,kf. or ((non-inferiority or noninferiority or superiority or equivalence) adj3 trial*).ti,ab,kf. |
1557192 |
|
20 |
(16 and 19) not 18 |
149 |
|
21 |
exp Epidemiologic Studies/ or (cohort or (case adj5 (control or controll* or comparison or referent)) or risk or causation or causal or odds-ratio or etiol* or aetiol* or natural-history or predict* or prognos* or outcome or course or retrospect* or followup or follow-up).ti,ab,kf. |
8483595 |
|
22 |
(16 and 21) not (18 or 20) |
1839 |
|
23 |
exp Neoplasm Recurrence, Local/ or Recurrence/ or (recurren* or recrudescen* or relaps*).ti,ab,kf. |
958409 |
|
24 |
1 and 2 and 23 |
6342 |
|
25 |
24 not ((Adolescent/ or Child/ or Infant/) not Adult/) |
6326 |
|
26 |
25 not ((exp animals/ or exp models, animal/) not humans/) |
6316 |
|
27 |
26 not (comment/ or editorial/ or letter/ or Case Reports/) |
5188 |
|
28 |
limit 27 to yr="2008 -Current" |
3524 |
|
29 |
(17 and 28) not (22 or 20 or 18) |
153 |
|
30 |
(19 and 28) not (22 or 20 or 18 or 29) |
194 |
|
31 |
(21 and 28) not (22 or 20 or 18 or 29 or 30) |
2146 |
Embase.com - 8 juni 2023
|
No. |
Query |
Results |
|
#31 |
#21 AND #28 NOT (#18 OR #20 OR #22 OR #29 OR #30) |
2467 |
|
#30 |
#19 AND #28 NOT (#18 OR #20 OR #22 OR #29) |
247 |
|
#29 |
#17 AND #28 NOT (#18 OR #20 OR #22) |
260 |
|
#28 |
#26 NOT ('conference abstract'/it OR 'conference review'/it OR 'editorial'/it OR 'letter'/it OR 'note'/it OR 'case report'/exp) AND [2008-2023]/py |
4576 |
|
#27 |
#26 NOT ('conference abstract'/it OR 'conference review'/it OR 'editorial'/it OR 'letter'/it OR 'note'/it OR 'case report'/exp) |
6389 |
|
#26 |
#25 NOT (('animal'/exp OR 'animal experiment'/exp OR 'animal model'/exp OR 'nonhuman'/exp) NOT 'human'/exp) |
11355 |
|
#25 |
#24 NOT (('adolescent'/exp OR 'child'/exp) NOT ('adult'/exp OR 'aged'/exp OR 'middle aged'/exp)) |
11400 |
|
#24 |
#1 AND #2 AND #23 |
11448 |
|
#23 |
'tumor recurrence'/exp OR 'cancer recurrence'/de OR 'recurrent disease'/exp OR recurren*:ti,ab,kw OR recrudescen*:ti,ab,kw OR relaps*:ti,ab,kw |
1467322 |
|
#22 |
#16 AND #21 NOT (#18 OR #20) |
2416 |
|
#21 |
'epidemiology'/de OR 'prospective study'/exp OR 'cohort analysis'/exp OR cohort:ti,ab,kw OR ((case NEAR/5 (control OR controll* OR comparison OR referent)):ti,ab,kw) OR risk:ti,ab,kw OR causation:ti,ab,kw OR causal:ti,ab,kw OR 'odds ratio':ti,ab,kw OR etiol*:ti,ab,kw OR aetiol*:ti,ab,kw OR 'natural history':ti,ab,kw OR predict*:ti,ab,kw OR prognos*:ti,ab,kw OR outcome:ti,ab,kw OR course:ti,ab,kw OR retrospect*:ti,ab,kw OR 'case control':ti,ab,kw OR 'multivariate':ti,ab,kw OR followup:ti,ab,kw OR 'follow up':ti,ab,kw |
11181411 |
|
#20 |
#16 AND #19 NOT #18 |
218 |
|
#19 |
'randomized controlled trial'/exp OR random*:ti,ab,kw OR (((pragmatic OR practical) NEAR/1 'clinical trial*'):ti,ab,kw) OR ((('non inferiority' OR noninferiority OR superiority OR equivalence) NEAR/3 trial*):ti,ab,kw) |
2056252 |
|
#18 |
#16 AND #17 |
218 |
|
#17 |
('meta analysis'/exp OR 'meta analysis (topic)'/exp OR metaanaly*:ti,ab OR 'meta analy*':ti,ab OR metanaly*:ti,ab OR 'systematic review'/de OR 'cochrane database of systematic reviews'/jt OR prisma:ti,ab OR prospero:ti,ab OR (((systemati* OR umbrella OR 'structured literature') NEAR/3 (review* OR overview*)):ti,ab) OR ((systemic* NEAR/1 review*):ti,ab) OR (((systemati* OR literature OR database* OR 'data base*') NEAR/10 search*):ti,ab) OR (((structured OR comprehensive* OR systemic*) NEAR/3 search*):ti,ab) OR (((literature NEAR/3 review*):ti,ab) AND (search*:ti,ab OR database*:ti,ab OR 'data base*':ti,ab)) OR (('data extraction':ti,ab OR 'data source*':ti,ab) AND 'study selection':ti,ab) OR ('search strategy':ti,ab AND 'selection criteria':ti,ab) OR ('data source*':ti,ab AND 'data synthesis':ti,ab) OR medline:ab OR pubmed:ab OR embase:ab OR cochrane:ab OR (((critical OR rapid) NEAR/2 (review* OR overview* OR synthes*)):ti) OR ((((critical* OR rapid*) NEAR/3 (review* OR overview* OR synthes*)):ab) AND (search*:ab OR database*:ab OR 'data base*':ab)) OR metasynthes*:ti,ab OR 'meta synthes*':ti,ab) NOT (('animal'/exp OR 'animal experiment'/exp OR 'animal model'/exp OR 'nonhuman'/exp) NOT 'human'/exp) NOT ('conference abstract'/it OR 'conference review'/it OR 'editorial'/it OR 'letter'/it OR 'note'/it) |
712296 |
|
#16 |
#14 NOT ('conference abstract'/it OR 'conference review'/it OR 'editorial'/it OR 'letter'/it OR 'note'/it OR 'case report'/exp) AND [2008-2023]/py |
3369 |
|
#15 |
#14 NOT ('conference abstract'/it OR 'conference review'/it OR 'editorial'/it OR 'letter'/it OR 'note'/it OR 'case report'/exp) |
4706 |
|
#14 |
#13 NOT (('animal'/exp OR 'animal experiment'/exp OR 'animal model'/exp OR 'nonhuman'/exp) NOT 'human'/exp) |
8852 |
|
#13 |
#12 NOT (('adolescent'/exp OR 'child'/exp) NOT ('adult'/exp OR 'aged'/exp OR 'middle aged'/exp)) |
8899 |
|
#12 |
#1 AND #2 AND #6 AND #11 |
8922 |
|
#11 |
#7 OR #8 OR #9 OR #10 |
2952430 |
|
#10 |
'nuclear magnetic resonance imaging'/exp OR (('magnetic resonance' NEAR/3 imag*):ti,ab,kw) OR mri:ti,ab,kw OR mris:ti,ab,kw OR nmr:ti,ab,kw OR mra:ti,ab,kw OR mras:ti,ab,kw OR zeugmatograph*:ti,ab,kw OR 'mr tomograph*':ti,ab,kw OR 'proton spin':ti,ab,kw OR (((magneti* OR 'chemical shift') NEAR/3 imag*):ti,ab,kw) OR fmri:ti,ab,kw OR fmris:ti,ab,kw |
1528271 |
|
#9 |
'computer assisted emission tomography'/exp OR 'gated single photon emission computed tomography'/exp OR 'single photon emission computer tomography'/exp OR spect:ti,ab,kw OR petscan*:ti,ab,kw OR 'pet scan*':ti,ab,kw OR ((emission NEAR/3 tomograph*):ti,ab,kw) OR radionuclid*:ti,ab,kw |
375357 |
|
#8 |
'x-ray tomography'/exp OR 'computed tomograph*':ti,ab,kw OR ct:ti,ab,kw OR cts:ti,ab,kw OR 'cat scan*':ti,ab,kw OR 'computer assisted tomograph*':ti,ab,kw OR 'computerized tomograph*':ti,ab,kw OR 'computerised tomograph*':ti,ab,kw OR 'computed x ray tomograph*':ti,ab,kw OR 'computed xray tomograph*':ti,ab,kw |
1033079 |
|
#7 |
'endoscopy'/de OR 'digestive tract endoscopy'/de OR 'esophagogastroduodenoscopy'/de OR 'gastrointestinal endoscopy'/de OR 'pharyngoscopy'/de OR 'laryngoscopy'/exp OR endoscop*:ti,ab,kw OR gastroscop*:ti,ab,kw OR laryngoscop*:ti,ab,kw OR esophagoscop*:ti,ab,kw OR oesophagoscop*:ti,ab,kw |
508653 |
|
#6 |
#3 OR #4 OR #5 |
2740438 |
|
#5 |
'quality of life'/exp OR 'quality of life':ti,ab,kw OR qoli:ti,ab,kw OR qol:ti,ab,kw OR qolis:ti,ab,kw OR hrqol*:ti,ab,kw OR 'hr qol*':ti,ab,kw OR 'life qual*':ti,ab,kw |
783387 |
|
#4 |
'metastasis'/exp OR metasta*:ti,ab,kw OR seeding*:ti,ab,kw OR micrometasta*:ti,ab,kw |
1138969 |
|
#3 |
'survival rate'/exp OR 'survival analysis'/exp OR 'survivor'/de OR 'cancer survivor'/de OR 'reoperation'/de OR ((surviv* NEAR/3 (rate OR mean OR analys* OR 'disease free' OR 'progression free' OR 'event free' OR overall)):ti,ab,kw) OR survivor*:ti,ab,kw OR (surg*:ti,ab,kw AND adj3:ti,ab,kw AND (revis*:ti,ab,kw OR repeat*:ti,ab,kw)) |
1155212 |
|
#2 |
'surgery'/exp OR surger*:ti,ab,kw OR surgical*:ti,ab,kw OR operation*:ti,ab,kw OR operative*:ti,ab,kw OR resecti*:ti,ab,kw OR esophagectom*:ti,ab,kw OR oesophagectom*:ti,ab,kw |
7267570 |
|
#1 |
'esophagus tumor'/exp OR (((oesophag* OR esophag* OR gastroesophag* OR gastrooesophag* OR oesogastr* OR esogastr*) NEAR/3 (carcinoma* OR neoplas* OR tumour* OR adenoma* OR adenocarcinoma* OR tumor* OR cancer* OR oncolog* OR malignan* OR carcinogen* OR oncogen* OR anticarcinogen* OR squamous*)):ti,ab,kw) |
126151 |