Duration of treatment
Uitgangsvraag
What is the optimal duration of treatment for patients with necrotizing otitis externa?
Aanbeveling
Treat patients with necrotizing otitis externa for 6 weeks.
Evaluate treatment response after 6 weeks and consider successful in case of:
- Resolution of symptoms (otalgia, otorrhea, fever)
- Normalisation of ear canal
- Recovery from previous facial nerve palsy (not mandatory)
If performed, in combination with
- Response on FDG-PET/CT or MRI (complete resolution not mandatory)
Consider prolonging treatment in case of:
- No / partial resolution of symptoms
- Presence of abscesses or empyema which cannot be drainaged
- Fungal cause
If performed, in combination with
- No / partial response seen on 18 FDG PET-CT/MRI
Prolong treatment until criteria of successful treatment are met and follow-up should be at least 3 months.
Overwegingen
Balance between desired and undesired effects
The systematic literature review reveals a lack of high-quality comparative studies assessing the optimal treatment duration for necrotizing otitis externa. Only one study (Pulcini, 2012) directly compared different treatment durations: a fixed duration of 6 weeks versus a variable duration (minimum 6 weeks, maximum 12 weeks), based on clinicians' assessment of clinical parameters. However, since both treatment modalities in this study showed a favorable outcome of 100% in both groups, combined with a small sample size (N=19 for the 6-week group, N=13 for the variable-duration group), no clear indication of the optimal treatment regimen can be determined. Notably, the standardized treatment regimen resulted in a shorter treatment duration, with a mean difference (MD) of 3.60 weeks (95% CI: 2.05 to 5.14), which is clinically significant.
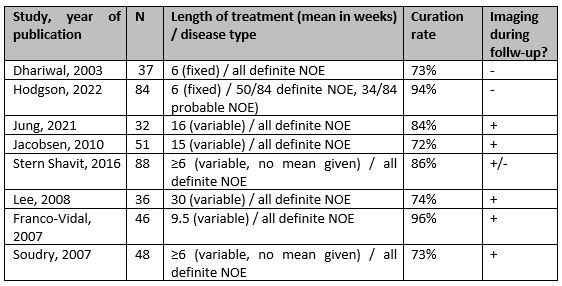
Looking at non-comparative studies in the literature, there is a significant variation in the reported treatment durations, combined with varying remission rates of the disease. To illustrate this variability, the results of non-comparative studies from the original systematic literature search are presented in Figure 1. These data are compiled from studies involving 30 or more patients. The cure rate was defined as complete remission of the disease without relapse.
These results demonstrate a significant variation in the mean weeks of treatment provided and the corresponding cure rates. This variation can possibly be attributed to substantial differences between cases, including the extent of the disease, causative agents, patient comorbidities, and differences in the definition of necrotizing otitis externa across studies. The guideline committee believes that all these factors play a role in treatment planning. Also, based on the available literature, it is impossible to exclude local practice variation.
In the available literature, and based on the experiences of the working group, the chosen treatment duration seems to roughly fall into two categories: 1) the treatment duration is predetermined and/or adjusted based on clinical findings or 2) the treatment duration is adjusted based on imaging findings during the course of treatment.
The latter, the role of imaging in assessing the effectiveness of therapy, may have had a positive effect on the outcome of NOE (Byun, 2020). Contrast enhanced and functional imaging make it possible to easily determine whether there is a reduction in the severity of the infection. In the Netherlands, this has led to imaging playing an increasingly important role in the decision-making process regarding when to stop treatment. A significant drawback, however, appears to be that clinical recovery does not always align with the normalization of imaging findings: abnormalities in contrast or isotope uptake may occur long after adequate treatment of the infection (Al-Noury 2011, Chhabria 2023, van der Meer 2023). In our view, this seems to be an important factor that could lead to overtreatment (in terms of duration) of patients. Therefore, it is relevant to consider the assessment of symptoms as the cornerstone of the judgment to discontinue treatment. Imaging can guide this judgement. Additionally, strict follow-up after stopping treatment, with the option to restart in case of deterioration, is another way to prevent overtreatment.
Influence of severity or extent?
The disease severity is reasonably embedded within the definitions, which the working group advises to use (Module 1): definite versus complex NOE, proposed by the UK consensus paper. Several staging protocols have been proposed earlier in the literature, all aimed at guiding the determination of the appropriate treatment (duration). Some studies that use disease extent are mentioned below.
Before the UK consensus paper, the staging system from the textbook Scott-Brown's Otorhinolaryngology, Head and Neck Surgery was mostly proposed, which has been modified by Lambor (2013):
- Stage I corresponds to possible NOE (only soft tissue abnormalities and "beyond," without bone erosion of the ear canal on CT).
- Stage II: the above with bone erosion.
- Stage IIIa: the above, including facial nerve involvement.
- Stage IIIb: multiple cranial nerves are involved.
- Stage IV: the above, including intracranial complications.
The staging system by Gleeson is similar but incorporates the use of a bone scan. In the article by Cho, the modified Gleeson staging system is used. The conclusion of the article states that the Gleeson staging system was valuable in assessing the extent of the disease, and all early-stage Gleeson patients had good outcomes. However, patients with higher severity staging on the Gleeson system did not necessarily require prolonged treatment (Cho, 2021).
This may reflect the observation that an advanced infection does not necessarily equate to a difficult-to-treat infection. There is also the possibility that, due to absent or inadequate previous therapy, the infection involves a large volume of tissue but still responds quickly and similarly to an infection in a smaller area.
Another proposed staging system involves stratifying the condition into severe and non-severe. This classification is based on a combination of several clinical and radiological parameters. In the article, it is retrospectively concluded that the treatment duration is, on average, longer in the group with severe NOE (Stevens, 2015). Another article reports similar findings. Here, a distinction is made between a single or a complex spreading pattern based on radiological findings, with patients exhibiting a complex spreading pattern being treated significantly longer (Van der Meer, 2022). The main limitation of these studies is, again, that no fixed endpoints for treatment have been established, making overtreatment difficult to control.
Based on the current literature, it is not possible to identify the difficult-to-treat group, since both good and poor responders are found within the unfavorable groups. On the other hand, extensive bacterial infections other than NOE generally respond well to a 6-week regimen, even including bone or central nervous infections. However, infections with presence of large abscesses or empyema where drainage (which is preferable if feasible) is anatomically not possible may sometimes need longer treatment, often guided by imaging (i.e., in the case of brain abcesses). In the absence of evidence in NOE, similar importance of the presence of undrained abcesses or empyema in determining treatment duration may be assumed.
Based on the available literature, it seems appropriate to treat patients with necrotizing otitis externa (NOE) for 6 weeks. This applies to both the definite and complex NOE groups according to the UK consensus definition. Evaluate the treatment effect at least based on clinical findings. Imaging appears warranted for therapy evaluation and as an adjunct to clinical assessment, particularly in the complex NOE group.
In our view, patients diagnosed with probable NOE fall outside the scope of this guideline. In the Dutch context, these patients are classified under the diagnosis of otitis externa, for which short-term antibiotic treatment (≤2 weeks) may be sufficient. Follow-up imaging is not necessary in this patient group (provided they are symptom-free).
Therapy response: symptoms, laboratory results, imaging results
A condition of the aforementioned treatment duration, as guided by the definition, is that the treatment should only be discontinued if the symptoms of otorrhea and otalgia have resolved. If infection parameters were elevated initially, these should also have normalized.
If the function of an affected cranial nerve recovers during the course of treatment, this is a very strong indication of successful therapy. Conversely, if the function has not been restored by the end of the intended treatment (according to the definition), this does not indicate anything about therapy response. Sensorineural hearing loss is by definition irreversible.
The role of imaging has already been mentioned above. In severe infections or infections with an unclear clinical course, imaging can be highly valuable. However, clinical presentation should always guide decision-making. Additionally, the treatment team should be aware that a partial response seen on imaging may lead to overtreatment. This is discussed in module Imaging to monitor treatment response.
Invasive fungal infection
A superficial otomycosis is not uncommon, which merely involves colonization of the ear canal and is treated accordingly. An invasive fungal infection generally occurs only in immunocompromised patients and is a rare life-threatening condition. For a comprehensive rationale regarding the treatment of invasive fungal infections, we refer to the specific guideline (SWAB, 2017), chapter 2.2 & 2.3 (type of therapy), 2.5 (duration), and 2.7). It is stated that duration of therapy is at least 6 to 12 weeks and, in neutropenic patients, not less than 2 weeks after resolution of neutropenia.
This corresponds to the guideline of the Infectious Diseases Society of America for treatment of Aspergillus infections recommend a minimum of 6 to 8 weeks of therapy. This range is based upon expert opinion and no systematic literature review or prospective study (Walsh, 2008). A large UK review study reports a median treatment of 13 weeks in cranial invasive aspergillosis patients (Gamaletsou, 2014).
The aforementioned guideline does mention a potential role for surgical debridement in cases of Aspergillus osteomyelitis. However, this committee also states that there is no standard role for surgery in osteomyelitis / skull base osteomyelitis caused by invasive aspergillosis.
Follow-up
Follow up should be done regularly, at least until the definition cure is met. The definition cure is set at a minimum period of 3 months after completing antibiotic therapy, without (recurrent) pain or otorrhoea (Hodgson, 2022).
Some authors disscuss the course of each patient in a monthly meeting for the first six months (Hutson, 2021). They suggest continuing follow up every eight weeks for the next six months in order to diagnose reccurences. Recurrence rate of NOE could reach 15- 20%. Recurrence is possible to occur up to 1 year after treatment. It is important to tailor follow-up to the patient's individual profile.
Quality of the evidence
The overall quality of evidence is very low. This means that the estimated effect of the critical outcomes that were found are very uncertain. There was a downgrade due to the following:
- Risk of Bias: methodological limitations
- Inconsistency: inconsistency of the results.
- Indirectness: indirectness of the evidence, due to differences in the use of surrogate outcomes.
- Imprecision: inaccuracy, due to a very small number of events in a small sample size.
Values and preferences of patients (and possibly their caregivers)
As previously stated, NOE can be an extensive infection. The duration of treatment varies but typically consists of a minimum of 6 weeks and may extend to several months. This prolonged therapy can lead to unwanted side effects (discussed in Module Additional conditions and optimizing care).
When administered intravenously for such a long duration, antibiotics significantly restrict patients' daily activities. Hospitalization further exacerbates these limitations. However, these burdens can be reduced through Outpatient Parenteral Antibiotic Therapy (OPAT).
On the other hand, untreated NOE carries a high mortality risk, justifying the extended treatment duration. A careful balance must be struck between undertreatment and minimizing the severity/side effects of therapy, ideally through shared decision-making between physician and patient.
Ultimately, all patients benefit from the shortest possible antibiotic exposure while maintaining a high likelihood of cure. This underscores the importance of appropriate treatment evaluation.
Costs (resources)
Shorter treatment durations reduce costs. However, undertreatment leading to disease recurrence may result in even higher expenses. To the guideline committee's knowledge, no formal cost-effectiveness analysis has been conducted on this subject.
Equity ((health) equity/equitable)
The guideline committee does not foresee any problems regarding equity.
Acceptability
The guideline committee does not foresee any problems regarding acceptability.
Feasibility
The intervention of long antibiotic treatment seems feasible. Feasibilty rises when OPAT is available.
Rationale of the recommendation: weighing arguments for and against the interventions
Based on the available literature, adjusting treatment duration according to disease extent does not appear to provide significant benefit. A standard 6-week course remains aligned with the core principles of osteomyelitis management. Extended therapy should be considered only in cases of inadequate clinical response, guided by imaging findings, disease severity, and the causative pathogen.
Onderbouwing
Achtergrond
The duration of treatment for necrotizing otitis externa varies significantly depending on the region or clinic. For osteomyelitis in long bones or the spine, the standard treatment duration is typically 6 weeks for acute cases and up to 12 weeks or longer for chronic or complicated cases, such as those involving prosthetic joints or hardware (Zimmerli, 2010). While the treatment duration for osteomyelitis in other body parts is broadly similar, the treatment of NOE appears more heterogenous in literature, most probably due to the fact that there is more uncertainty regarding the minimal treatment duration and whether there is a need to individualize the treatment per patient. High-quality research on this topic remains limited.Both conditions can benefit from a combination of clinical, laboratory, and imaging assessments to guide therapy, as long as necessary, but preferrably not longer than necessary.
Conclusies / Summary of Findings
|
Outcome
|
Study results and measurements |
Absolute effect estimates |
Certainty of the evidence (Quality of evidence) |
Summary |
|
|
6 weeks treatment |
Treament based on clinical parameters |
||||
|
Survival
|
-
|
- |
- |
No evidence was found regarding the effect of a standard 6-weeks treatment on improving survival in patients with necrotizing otitis externa.
(Pucini, 2012) |
|
|
Remission
|
As no events occured it was not possible to provide any indication of either the direction or magnitude of the relative treatment effect, based on 30 participants from one study.
In both study arms, no events occured, based on 30 participants from 1 study |
- |
Very low By very serious imprecision1 |
The evidence is unclear regarding the effect of a standard 6-weeks treatment to improving disease remission in patients with necrotizing otitis externa.
(Pulcini, 2012) |
|
|
Length of treatment (weeks)
|
The mean difference (MD) is 3.60 (95% CI: 2.05 to 5.14), based on data from 30 participants in one study. |
|
Very low By very serious imprecision2 |
The evidence is very uncertain about the effect of standard 6 weeks treatment to reduce the length of treatment disease control in patients with necrotizing otitis externa
(Pulcini, 2012) |
|
|
Adverse events
|
-
|
|
- |
No evidence was found regarding the effect of a standard 6-week treatment on reducing adverse events in patients with skull base osteomyelitis.
(Pulcini, 2012) |
|
1. Imprecision: very serious. Low population (<100)
2. Imprecision: very serious. Low population (<100)
Samenvatting literatuur
Description of studies
One study was included in the literature analysis. Key study characteristics and results are summarized in Table 1. The risk of bias assessment is summarized in the risk of bias tables (under the tab ‘Evidence Tables’).
Table 1. Characteristics of included studies
|
Study |
Participants (number, age, other important characteristics) |
Comparison |
Follow-up |
Outcome measures |
Comments |
Risk of bias (per outcome measure)* |
|
Individual study |
||||||
|
Pulcini, 2012 |
N at baseline Intervention: 19 Control: 13
Age Intervention: 72±13 Control: 76±8
Sex Intervention: 13 males, 5 females Control: 10 males, 3 females
|
Intervention: Patients received a standard regimen of 3 weeks i.v. ceftazidim + oral ciprofloxacin, followed by 3 weeks of oral ciprofloxacin.
Control: Patients received a combination of i.v. ceftazidim followed by oral ciprofloxacin. Duration of both was not set and treatment duration was based on clinical findings (not specified). |
14 months (6–21) |
Remission: 100% remission in both groups.
Length of treatment: 5.8±0.7 weeks in the intervention group. 9.4±3.2 weeks in the control group |
Remission is reported in the article as “favourable outcome”. What constitutes a favourable outcome is not further discribed in the article. |
Remission: high Length of treatment: low |
*For further details, see risk of bias table in the appendix
Results
Survival (critical)
No studies reported survival as an outcome measure.
Remission (critical)
One study reported remission of disease (Pulcini, 2012). However, the authors only describe the outcome as a “favourable outcome”, the definition of which is not further explained. Therefore, the following interpretation is based on the assumption that “favourable outcome” is interchangeable with remission of disease. In the intervention group, 19 out of 19 patients achieved a favourable outcome. This is the same rate as in the control group, where 13 out of 13 patients achieved a favourable outcome.
Length of treatment (important)
One study reported on the length of treatment (Pulcini, 2012). With a set number of 6 weeks in the intervention group, the length of treatment was shortened in comparison to the control group. (5.8±0.7 weeks versus 9.4±3.2 weeks; mean difference is 3.60 (95% CI: 2.05 to 5.14).
Adverse events (important)
No studies reported adverse events as an outcome measure.
Zoeken en selecteren
What is the added value of a standard treatment duration compared to basing the treatment duration on clinical parameters?
| Patients | Patients with confirmed necrotizing otitis externa |
| Intervention |
Antibiotic treatment for 6 weeks |
| Control | Standard of care; treatment duration based on clinical parameters |
| Outcomes | Disease remission, survival, treatment duration, adverse events |
| Other selection criteria |
Study design: systematic reviews and randomized controlled trials, cohort studies [Minimum follow-up: 3 months] |
Relevant outcome measures
The guideline panel considered remmision of disease and survival as a critical outcome measure for decision making; and length of treatment and adverse events as an important outcome measure for decision making.
The guideline panel defined the outcome measures as follows:
- (Disease specific) survival: time to death, caused by the effects of necrotizing otitis externa.
- Remission: rate of curation of disease. Defined after prolonged disappearance of the signs and symptoms of a disease.
- Adverse events: an unexpected or harmful medical occurrence after exposure to treatment.
The guideline panel defined the following as a minimal clinically (patient) important difference.
- (Disease specific) survival: absolute difference > 5%.
- Remission: GRADE standard limits*.
- Length of treatment: GRADE standard limits*. In case of similar outcome measures for different treatment duration, the guideline panel opts for shorter treatment duration, taking into account side effects, costs, and healthcare burden.
- Adverse events: GRADE standard limits*.
* Default thresholds proposed by the international GRADE working group were used: a 25% difference in relative risk (RR) for dichotomous outcomes (RR <0.80 or RR >1.25), or 0.5 standard deviations (SD) for continuous outcomes
Search and select (Methods)
The databases [Medline (via OVID) and Embase (via Embase.com)] were searched using relevant search terms up to July 17, 2023. The detailed search strategy is provided under the tab ‘Literature Search Strategy’. The systematic literature search yielded 124 results. Studies were selected based on the following criteria: systematic reviews, randomized controlled trials (RCTs), observational studies, and other non-comparative research on the duration of treatment for necrotizing otitis externa. Eighteen studies were initially selected through title and abstract screening. After full-text review, seventeen studies were excluded (see the exclusion table under the tab ‘Evidence Tables’), and one study was included.
Referenties
- Al-Noury K, Lotfy A. Computed tomography and magnetic resonance imaging findings before and after treatment of patients with malignant external otitis. Eur Arch Otorhinolaryngol. 2011 Dec;268(12):1727-34. doi: 10.1007/s00405-011-1552-8. Epub 2011 Mar 15. PMID: 21400256.
- Byun YJ, Patel J, Nguyen SA, Lambert PR. Necrotizing Otitis Externa: A Systematic Review and Analysis of Changing Trends. Otol Neurotol. 2020 Sep;41(8):1004-1011. doi: 10.1097/MAO.0000000000002723. PMID: 32569149.
- Chhabria S, Vishnurag A. Observational Study on Clinical Features and Management of Skull Base Osteomyelitis in Hospitalised Patients at a Tertiary Care Hospital. Indian J Otolaryngol Head Neck Surg. 2023 Apr;75(Suppl 1):635-643. doi: 10.1007/s12070-023-03675-8. Epub 2023 Mar 18. PMID: 37206859; PMCID: PMC10188806.
- Cho WS, Bonduelle Q, Ghasemi A, Baskaran V, O'Connor R, Shah J, Andrewartha F, Fergie N. Prognosticating patients with necrotising otitis externa based on response to treatment. Ann R Coll Surg Engl. 2021 Apr;103(4):285-290. doi: 10.1308/rcsann.2020.7133. Epub 2021 Mar 8. PMID: 33682472; PMCID: PMC10335042.
- Dhariwal A, Manjaly JG, Patel B, Morris-Jones S, David K, Khetarpal P, Beale T, Mehta N, Logan S. Management and Clinical Outcomes of 37 Patients with Necrotizing Otitis Externa: Retrospective Review of a Standardized 6-Week Treatment Pathway. J Int Adv Otol. 2023 Jun;19(3):223-227. doi: 10.5152/iao.2023.22637. PMID: 37272640; PMCID: PMC10331632.
- Franco-Vidal V, Blanchet H, Bebear C, Dutronc H, Darrouzet V. Necrotizing external otitis: a report of 46 cases. Otol Neurotol. 2007 Sep;28(6):771-3. doi: 10.1097/MAO.0b013e31805153bd. PMID: 17721365.
- Gamaletsou MN, Rammaert B, Bueno MA, Moriyama B, Sipsas NV, Kontoyiannis DP, Roilides E, Zeller V, Prinapori R, Taj-Aldeen SJ, Brause B, Lortholary O, Walsh TJ. Aspergillus osteomyelitis: epidemiology, clinical manifestations, management, and outcome. J Infect. 2014 May;68(5):478-93. doi: 10.1016/j.jinf.2013.12.008. Epub 2013 Dec 27. PMID: 24378282; PMCID: PMC4214682.
- Gleeson, MJ, ed. Scott-Brown's Otorhinolaryngology, Head and Neck Surgery, 7th edn, vol 3. London: Hodder Arnold, 3336–9Google Scholar.
- Hodgson SH, Khan MM, Patrick-Smith M, Martinez-Devesa P, Stapleton E, Williams OM, Pretorius P, McNally M, Andersson MI. UK consensus definition for necrotising otitis externa: a Delphi study. BMJ Open. 2023 Feb 20;13(2):e061349. doi: 10.1136/bmjopen-2022-061349.
- Hodgson SH, Sinclair VJ, Arwyn-Jones J, Oh K, Nucken K, Perenyei M, Sivapathasingam V, Martinez-Devesa P, Pendlebury ST, Ramsden JD, Matthews PC, Pretorius P, Andersson MI. Characteristics, management and outcome of a large necrotising otitis externa case series: need for standardised case definition. J Laryngol Otol. 2022 Jul;136(7):604-610. doi: 10.1017/S002221512100462X. Epub 2022 Jan 19. PMID: 35042578; PMCID: PMC9257435.
- Jacobsen LM, Antonelli PJ. Errors in the diagnosis and management of necrotizing otitis externa. Otolaryngol Head Neck Surg. 2010 Oct;143(4):506-9. doi: 10.1016/j.otohns.2010.06.924. PMID: 20869559.
- Jung DJ, Hong J, Cho HJ, Yoo MH, Lee KY. Clinical outcomes of otogenic skull base osteomyelitis. Eur Arch Otorhinolaryngol. 2021 Aug;278(8):2817-2822. doi: 10.1007/s00405-020-06366-0. Epub 2020 Sep 22. PMID: 32960351.
- Kohut RI, Lindsay JR. Necrotizing ("malignant") external otitis histopathologic processes. Ann Otol Rhinol Laryngol. 1979 Sep-Oct;88(5 Pt 1):714-20. doi: 10.1177/000348947908800520. PMID: 496204.
- Lambor DV, Das CP, Goel HC, Tiwari M, Lambor SD, Fegade MV. Necrotising otitis externa: clinical profile and management protocol. J Laryngol Otol. 2013 Nov;127(11):1071-7. doi: 10.1017/S0022215113002259. Epub 2013 Oct 29. PMID: 24169084.
- Lee S, Hooper R, Fuller A, Turlakow A, Cousins V, Nouraei R. Otogenic cranial base osteomyelitis: a proposed prognosis-based system for disease classification. Otol Neurotol. 2008 Aug;29(5):666-72. doi: 10.1097/MAO.0b013e318179972f. PMID: 18520626.
- van der Meer WL, Mitea C, Waterval JJ, Kunst HPM, Mottaghy FM, Postma AA. The Role of F18-FDG PET-MRI in Necrotising External Otitis Follow-Up: A Single Centre Experience. Ann Otolaryngol Rhinol 2023; 10(2): 1313.
- Pulcini C, Mahdyoun P, Cua E, Gahide I, Castillo L, Guevara N. Antibiotic therapy in necrotising external otitis: case series of 32 patients and review of the literature. Eur J Clin Microbiol Infect Dis. 2012 Dec;31(12):3287-94. doi: 10.1007/s10096-012-1694-7. Epub 2012 Jul 19. PMID: 22810173.
- Soudry E, Joshua BZ, Sulkes J, Nageris BI. Characteristics and prognosis of malignant external otitis with facial paralysis. Arch Otolaryngol Head Neck Surg. 2007 Oct;133(10):1002-4. doi: 10.1001/archotol.133.10.1002. PMID: 17938323.
- Stern Shavit S, Soudry E, Hamzany Y, Nageris B. Malignant external otitis: Factors predicting patient outcomes. Am J Otolaryngol. 2016 Sep-Oct;37(5):425-30. doi: 10.1016/j.amjoto.2016.04.005. Epub 2016 May 6. PMID: 27311346.
- SWAB Guidelines for the Management of Invasive Fungal Infections (2017).
- Walsh TJ, Anaissie EJ, Denning DW, Herbrecht R, Kontoyiannis DP, Marr KA, Morrison VA, Segal BH, Steinbach WJ, Stevens DA, van Burik JA, Wingard JR, Patterson TF; Infectious Diseases Society of America. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis. 2008 Feb 1;46(3):327-60. doi: 10.1086/525258. PMID: 18177225.
Evidence tabellen
Risk of Bias tables
Risk of bias table for intervention studies (observational: non-randomized clinical trials, cohort and case-control studies)
|
Study reference
(first author, year of publication) |
Bias due to a non-representative or ill-defined sample of patients?1
(unlikely/likely/unclear) |
Bias due to insufficiently long, or incomplete follow-up, or differences in follow-up between treatment groups?2
(unlikely/likely/unclear) |
Bias due to ill-defined or inadequately measured outcome ?3
(unlikely/likely/unclear) |
Bias due to inadequate adjustment for all important prognostic factors?4
(unlikely/likely/unclear) |
|
Pulcini, 2013 |
Unlikely |
Unlikely |
likely |
Unclear |
Table of excluded studies
|
Reference |
Reason for exclusion |
|
Saxena A, Paul BS, Singh G, Ahluwalia A, Paul G. Predicting Outcome in Skull Base Osteomyelitis: An Assessment of Demographic, Clinical, and Pathological Attributes. J Neurosci Rural Pract. 2021 Sep 28;12(4):751-757. doi: 10.1055/s-0041-1735324. PMID: 34737511; PMCID: PMC8559086. |
Wrong outcome |
|
van der Meer WL, Bayoumy AB, Otten JJ, Waterval JJ, Kunst HPM, Postma AA. The association between radiological spreading pattern and clinical outcomes in necrotizing external otitis. J Otol. 2022 Jul;17(3):156-163. doi: 10.1016/j.joto.2022.05.002. Epub 2022 Jun 3. PMID: 35847573; PMCID: PMC9270564. |
No comparison made |
|
Hodgson SH, Sinclair VJ, Arwyn-Jones J, Oh K, Nucken K, Perenyei M, Sivapathasingam V, Martinez-Devesa P, Pendlebury ST, Ramsden JD, Matthews PC, Pretorius P, Andersson MI. Characteristics, management and outcome of a large necrotising otitis externa case series: need for standardised case definition. J Laryngol Otol. 2022 Jul;136(7):604-610. doi: 10.1017/S002221512100462X. Epub 2022 Jan 19. PMID: 35042578; PMCID: PMC9257435. |
No comparison made |
|
Sadé J, Lang R, Goshen S, Kitzes-Cohen R. Ciprofloxacin treatment of malignant external otitis. Am J Med. 1989 Nov 30;87(5A):138S-141S. doi: 10.1016/0002-9343(89)90044-2. PMID: 2589357. |
Wrong intervention |
|
Jung DJ, Hong J, Cho HJ, Yoo MH, Lee KY. Clinical outcomes of otogenic skull base osteomyelitis. Eur Arch Otorhinolaryngol. 2021 Aug;278(8):2817-2822. doi: 10.1007/s00405-020-06366-0. Epub 2020 Sep 22. PMID: 32960351. |
No comparison made |
|
Chaabouni, M.A., Achour, I., Yousfi, G. et al. Culture-negative necrotizing otitis externa: diagnosis and management. Egypt J Otolaryngol 39, 30 (2023). https://doi.org/10.1186/s43163-022-00363-2 |
No comparison made |
|
Chawdhary G, Pankhania M, Douglas S, Bottrill I. Current management of necrotising otitis externa in the UK: survey of 221 UK otolaryngologists. Acta Otolaryngol. 2017 Aug;137(8):818-822. doi: 10.1080/00016489.2017.1295468. Epub 2017 Mar 16. PMID: 28301961. |
No comparison made |
|
Stapleton E, Watson G. Emerging themes in necrotising otitis externa: a scoping review of the literature from 2011 to 2020 and recommendations for future research. J Laryngol Otol. 2022 Jul;136(7):575-581. doi: 10.1017/S0022215121003030. Epub 2021 Oct 20. PMID: 34666847. |
No comparison made |
|
Chhabria S, Vishnurag A. Observational Study on Clinical Features and Management of Skull Base Osteomyelitis in Hospitalised Patients at a Tertiary Care Hospital. Indian J Otolaryngol Head Neck Surg. 2023 Apr;75(Suppl 1):635-643. doi: 10.1007/s12070-023-03675-8. Epub 2023 Mar 18. PMID: 37206859; PMCID: PMC10188806. |
No comparison made |
|
Durojaiye OC, Slucka A, Kritsotakis EI. Retrospective analysis of outcomes of outpatient parenteral antimicrobial therapy (OPAT) for necrotising otitis externa. Eur J Clin Microbiol Infect Dis. 2022 Jun;41(6):941-949. doi: 10.1007/s10096-022-04455-y. Epub 2022 May 13. PMID: 35556187. |
No comparison made |
|
Loh S, Loh WS. Malignant otitis externa: an Asian perspective on treatment outcomes and prognostic factors. Otolaryngol Head Neck Surg. 2013 Jun;148(6):991-6. doi: 10.1177/0194599813482107. Epub 2013 Apr 4. PMID: 23558287. |
No comparison made |
|
Dhariwal A, Manjaly JG, Patel B, Morris-Jones S, David K, Khetarpal P, Beale T, Mehta N, Logan S. Management and Clinical Outcomes of 37 Patients with Necrotizing Otitis Externa: Retrospective Review of a Standardized 6-Week Treatment Pathway. J Int Adv Otol. 2023 Jun;19(3):223-227. doi: 10.5152/iao.2023.22637. PMID: 37272640; PMCID: PMC10331632. |
No comparison made |
|
Cho WS, Bonduelle Q, Ghasemi A, Baskaran V, O'Connor R, Shah J, Andrewartha F, Fergie N. Prognosticating patients with necrotising otitis externa based on response to treatment. Ann R Coll Surg Engl. 2021 Apr;103(4):285-290. doi: 10.1308/rcsann.2020.7133. Epub 2021 Mar 8. PMID: 33682472; PMCID: PMC10335042. |
No comparison made |
|
Frost J, Samson AD. Standardised treatment protocol for necrotizing otitis externa: retrospective case series and systematic literature review. J Glob Antimicrob Resist. 2021 Sep;26:266-271. doi: 10.1016/j.jgar.2021.06.015. Epub 2021 Jul 14. PMID: 34273591. |
No comparison made |
|
Lang R, Goshen S, Kitzes-Cohen R, Sadé J. Successful treatment of malignant external otitis with oral ciprofloxacin: report of experience with 23 patients. J Infect Dis. 1990 Mar;161(3):537-40. doi: 10.1093/infdis/161.3.537. PMID: 2313132. |
No comparison made No comparison made |
|
Djalilian HR, Shamloo B, Thakkar KH, Najme-Rahim M. Treatment of culture-negative skull base osteomyelitis. Otol Neurotol. 2006 Feb;27(2):250-5. doi: 10.1097/01.mao.0000181185.26410.80. PMID: 16436997. |
No comparison made |
Verantwoording
Beoordelingsdatum en geldigheid
Publicatiedatum : 29-09-2025
Beoordeeld op geldigheid : 25-09-2025
Algemene gegevens
For more details on the guideline methodology used, we refer you to the Werkwijze. Relevant information for the development of this guideline is presented below.
The revision of this guideline module was supported by the Knowledge Institute of the Federation of Medical Specialists (www.demedischspecialist.nl/kennisinstituut) and was funded by the Quality Funds for Medical Specialists (SKMS).
Samenstelling werkgroep
For the development of the guideline, a multidisciplinary guideline development group was established in 2022, consisting of representatives from all relevant specialties (see Composition of the working group) involved in the care of patients with necrotizing otitis externa.
Werkgroep
- Dr. J.J. (Jérôme) Waterval (chairman), Nederlandse Vereniging voor Keel-Neus-Oorheelkunde en Heelkunde van het Hoofd-Halsgebied, otorhinolaryngologist, Maastricht University Medical Center, Maastricht; Academic Alliance Skull Base Pathology Maaastricht University Medical Center – Radboud University Medical Center
- Dr. M.J. (Mark) van Tilburg, Nederlandse Vereniging voor Keel-Neus-Oorheelkunde en Heelkunde van het Hoofd-Halsgebied, otorhinolaryngologist, Elistabeth-TweeSteden Ziekenhuis, Tilburg
- Drs. S.A.H. (Sjoert) Pegge, Nederlandse Vereniging voor Radiologie, radiologist, Radboud University Medical Center, Nijmegen; Academic Alliance Skull Base Pathology Maaastricht University Medical Center – Radboud University Medical Center
- Prof. Dr. A.W.J.M. (Andor) Glaudemans, Nederlandse Vereniging voor Nucleaire Geneeskunde, nuclear physicist UMCG, Groningen
- Dr. M. (Moniek) Heusinkveld, Nederlandse Vereniging voor Medische Microbiologie, medical microbiologist, Gelderse Vallei Hospital, Ede
- Dr. E.J.G. (Edgar) Peters, Nederlandse Internisten Vereniging, infectious disease specialist, Amsterdam University Medical Center (tot oktober 2022)
- Dr. J.J. (Jonne) Sikkens, Nederlandse Internisten Vereniging, infectious disease specialist, Amsterdam University Medical Center (vanaf october 2022)
- Dr. I.R. (Raluca) Mihailescu, Nederlandse Internisten Vereniging, infectious disease specialist, Onze Lieve Vrouwe Gasthuis, Amsterdam (vanaf juli 2024)
- Dr. S.H. (Selwyn) Lowe, Nederlandse Internisten Vereniging, infectious disease specialist, Maastricht University Medical Center, Maastricht (vanaf juli 2024)
Klankbordgroep
- Dr. N.G.L. (Nynke) Jager, NVZA, hospital pharmacist Radboud University Medical Center, Nijmegen
- Drs. F.S. (Fleur) Sinkeler, NVZA, hospital pharmacist Radboudumc Nijmegen
Ondersteuning
- Drs. J.M.H. (Jasper) Janssen, NVKNO, otorhinolaryngologist in training, Maastricht University Medical Center, Maastricht
- Dr. A. (Anja) van der Hout, advisor Knowledge Institute of the Dutch Association of Medical Specialists
Belangenverklaringen
An overview of the conflicts of interests of the guideline development group members and the assessment of how potential conflicts of interest were addressed can be found in the table below. The signed declarations of interest are available upon request from the Secretariat of the Knowledge Institute of the Dutch Federation of Medical Specialists at secretariaat@kennisinstituut.nl.
|
Werkgroeplid |
Functie |
Nevenfuncties |
Gemelde belangen |
Ondernomen actie |
|
Waterval (voorzitter) |
KNO-arts MUMC |
Accreditatiecommissie Stichting Audiciensregister |
Geen |
Geen |
|
Glaudemans |
Nucleair geneeskundige UMCG
|
Voorzitter NVNG (onbetaald) |
We hebben als ziekenhuis en afdeling een samenwerking met Siemens (UMCG-Siemens PUSH collaboration/Partnership of UMCG-Siemens for building the future of Health). Hieruit vloeit uit voort dat de nieuwste camera’s bij ons komen (bv UMCG neemt nieuwe Whole-Body PET/CT-scanner in gebruik) en dat er gezamenlijk onderzoek gedaan wordt. Hierbij heb ik een aantal promovendi die door Siemens betaald worden (niet op het gebied van osteomyelitis schedelbasis) |
Geen restricties. Extern gefinancierd onderzoek valt buiten bestek richtlijn
|
|
Heusinkveld |
Arts-microbioloog in ziekenhuis Gelders Vallei |
Richtlijn otitis externa
Bestuur SKML sectie infectieserologie (onbetaald) |
Geen |
Geen |
|
Peters (tot oktober 2022) |
Internist-infectioloog-acute geneeskundige, Amsterdam UMC |
richtlijnontwikkeling: Covid-19 FMS, diabetische voet NIV, diabetische voet IWGDF, alle onbetaald
|
afdeling krijgt geld van Roche voor biomarker onderzoek bij diabetische voet osteomyelitis Diabetische voet onderzoek (extern gefinancierd)
|
Geen restricties. Extern gefinancierd onderzoek valt buiten bestek richtlijn
|
|
Pegge |
Radioloog (Neuro/Hoofdhals) Radboud UMC Nijmegen |
Geen |
Geen |
Geen |
|
Van Tilburg |
KNO-arts ETZ
|
Geen |
Geen |
Geen |
|
Sikkens |
Internist acute geneeskunde & infectioloog, Amsterdam UMC |
post-doc onderzoeker Amsterdam UMC, onbetaald
|
Ja, via ZonMw (onderzoek naar COVID bij een medewerkerscohort, onderwerp infectiepreventie en vaccin-immunologie)
|
Geen restricties. Extern gefinancierd onderzoek valt buiten bestek richtlijn
|
|
Lowe
|
Internist-infectioloog. Afdeling Medische Microbiologie, Infectieziekten en Infectiepreventie (MMI), Maastricht UMC+
|
Geen |
Geen |
Geen |
|
Mihailescu
|
Internist-infectioloog OLVG Amsterdam |
Geen |
Geen |
Geen |
|
Jasper Janssen
|
KNO-arts in opleiding bij het MUMC+ (0,8 FTE), promovendus (0,2 FTE). |
Geen |
Geen |
Geen |
|
Sinkeler
|
Ziekenhuisapotheker AmsterdamUMC
|
Geen |
Geen |
Geen |
|
Jager |
Ziekenhuisapotheker
|
Geen |
Geen |
Geen |
Inbreng patiëntenperspectief
Attention was paid to the patient perspective by inviting Stichting Hoormij and Patiëntenfederatie Nederland for the invitational conference, and close contact with Stichting Hoormij during the development of the guideline. The report of this [see related products] was discussed in the guideline development group. The input obtained was taken into account when formulating the key questions, selecting the outcome measures, and drafting the considerations. The draft guideline was also submitted for comments to Stichting Hoormij and Patiëntenfederatie Nederland, and any comments received were reviewed and processed.
Kwalitatieve raming van mogelijke financiële gevolgen in het kader van de Wkkgz
Bij de richtlijnmodule voerde de werkgroep conform de Wet kwaliteit, klachten en geschillen zorg (Wkkgz) een kwalitatieve raming uit om te beoordelen of de aanbevelingen mogelijk leiden tot substantiële financiële gevolgen. Bij het uitvoeren van deze beoordeling is de richtlijnmodule op verschillende domeinen getoetst (zie het stroomschema bij Werkwijze).
|
Module |
Uitkomst raming |
Toelichting |
|
Duration of treatment |
geen financiële gevolgen |
Uit de toetsing volgt dat de aanbeveling(en) niet breed toepasbaar zijn (<5.000 patiënten) en daarom naar verwachting geen substantiële financiële gevolgen zal hebben voor de collectieve uitgaven. |
Zoekverantwoording
Algemene informatie
|
Richtlijn: NVKNO Osteomyelitis schedelbasis – Maligne otitis externa |
|
|
Uitgangsvraag: Wat is de optimale behandelduur van therapie bij patiënten met maligne otitis externa? |
|
|
Database(s): Ovid/Medline, Embase |
Datum:17-7-2023 |
|
Periode: nvt |
Talen: nvt |
|
Literatuurspecialist: Ingeborg van Dusseldorp |
|
|
BMI zoekblokken: voor verschillende opdrachten wordt (deels) gebruik gemaakt van de zoekblokken van BMI-Online https://blocks.bmi-online.nl/ Bij gebruikmaking van een volledig zoekblok zal naar de betreffende link op de website worden verwezen. |
|
|
Toelichting: Voor deze vraag is gezocht met de volgende concepten: Maligne otitis externa EN antibiotica EN behandelduur Vanwege de kleine aantallen is geen onderscheid gemaakt in studiedesign. NB. Voor deze vraag wordt uitgegaan van de strategie van UV4 dubbeltherapie antibiotica, waarin ook naar alle antibiotica is gezocht. Als er geen evidence wordt gevonden, kan worden overwogen om een volledige update te doen van UV4 en deze opnieuw te selecteren, ook omdat een specifieke behandelduur lastig is te formuleren in een zoekstrategie. |
|
|
Te gebruiken voor richtlijnen tekst: In de databases Embase en Ovid/Medline is op 17-7-2023 met relevante zoektermen gezocht naar studies over de behandelduur van therapie bij patiënten met maligne otitis externa. De literatuurzoekactie leverde 124 unieke treffers op. |
|
Zoekopbrengst
|
|
EMBASE |
OVID/MEDLINE |
Ontdubbeld |
|
SRs |
|
|
|
|
RCTs |
|
|
|
|
Observationele studies |
|
|
|
|
Overig |
101 |
31 |
124 |
|
Totaal |
|
|
|
Zoekstrategie
Embase.com
|
No. |
Query |
Results |
|
#48 |
#38 AND #47 |
101 |
|
#47 |
'treatment duration'/exp OR '6 week*':ti,ab,kw OR 'six week*':ti,ab,kw OR '6.0':ti,ab,kw OR '6.5':ti,ab,kw OR '6-7':ti,ab,kw OR '6-8':ti,ab,kw OR ((six NEAR/3 week*):ti,ab,kw) |
1265464 |
|
#46 |
#42 OR #43 OR #44 OR #45 |
773 |
|
#45 |
#38 NOT (#42 OR #43 OR #44) |
586 |
|
#44 |
#38 AND #41 NOT (#42 OR #43) |
146 |
|
#43 |
#38 AND #40 NOT #42 |
3 |
|
#42 |
#38 AND #39 |
18 |
|
#41 |
'clinical trial'/exp OR 'randomization'/exp OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp OR 'placebo'/exp OR 'prospective study'/exp OR rct:ab,ti OR random*:ab,ti OR 'single blind':ab,ti OR 'randomised controlled trial':ab,ti OR 'randomized controlled trial'/exp OR placebo*:ab,ti OR 'comparative study'/exp OR 'control group'/de OR 'controlled study'/de OR 'controlled clinical trial'/de OR 'crossover procedure'/de OR 'double blind procedure'/de OR 'phase 2 clinical trial'/de OR 'phase 3 clinical trial'/de OR 'phase 4 clinical trial'/de OR 'pretest posttest design'/de OR 'pretest posttest control group design'/de OR 'quasi experimental study'/de OR 'single blind procedure'/de OR 'triple blind procedure'/de OR (((control OR controlled) NEAR/6 trial):ti,ab,kw) OR (((control OR controlled) NEAR/6 (study OR studies)):ti,ab,kw) OR (((control OR controlled) NEAR/1 active):ti,ab,kw) OR 'open label*':ti,ab,kw OR (((double OR two OR three OR multi OR trial) NEAR/1 (arm OR arms)):ti,ab,kw) OR ((allocat* NEAR/10 (arm OR arms)):ti,ab,kw) OR placebo*:ti,ab,kw OR 'sham-control*':ti,ab,kw OR (((single OR double OR triple OR assessor) NEAR/1 (blind* OR masked)):ti,ab,kw) OR nonrandom*:ti,ab,kw OR 'non-random*':ti,ab,kw OR 'quasi-experiment*':ti,ab,kw OR crossover:ti,ab,kw OR 'cross over':ti,ab,kw OR 'parallel group*':ti,ab,kw OR 'factorial trial':ti,ab,kw OR ((phase NEAR/5 (study OR trial)):ti,ab,kw) OR ((case* NEAR/6 (matched OR control*)):ti,ab,kw) OR ((match* NEAR/6 (pair OR pairs OR cohort* OR control* OR group* OR healthy OR age OR sex OR gender OR patient* OR subject* OR participant*)):ti,ab,kw) OR ((propensity NEAR/6 (scor* OR match*)):ti,ab,kw) OR versus:ti OR vs:ti OR compar*:ti OR ((compar* NEAR/1 study):ti,ab,kw) OR (('major clinical study'/de OR 'clinical study'/de OR 'cohort analysis'/de OR 'observational study'/de OR 'cross-sectional study'/de OR 'multicenter study'/de OR 'correlational study'/de OR 'follow up'/de OR cohort*:ti,ab,kw OR 'follow up':ti,ab,kw OR followup:ti,ab,kw OR longitudinal*:ti,ab,kw OR prospective*:ti,ab,kw OR retrospective*:ti,ab,kw OR observational*:ti,ab,kw OR 'cross sectional*':ti,ab,kw OR cross?ectional*:ti,ab,kw OR multicent*:ti,ab,kw OR 'multi-cent*':ti,ab,kw OR consecutive*:ti,ab,kw) AND (group:ti,ab,kw OR groups:ti,ab,kw OR subgroup*:ti,ab,kw OR versus:ti,ab,kw OR vs:ti,ab,kw OR compar*:ti,ab,kw OR 'odds ratio*':ab OR 'relative odds':ab OR 'risk ratio*':ab OR 'relative risk*':ab OR 'rate ratio':ab OR aor:ab OR arr:ab OR rrr:ab OR ((('or' OR 'rr') NEAR/6 ci):ab))) OR 'major clinical study'/de OR 'clinical study'/de OR 'case control study'/de OR 'family study'/de OR 'longitudinal study'/de OR 'retrospective study'/de OR 'prospective study'/de OR 'comparative study'/de OR 'cohort analysis'/de OR ((cohort NEAR/1 (study OR studies)):ab,ti) OR (('case control' NEAR/1 (study OR studies)):ab,ti) OR (('follow up' NEAR/1 (study OR studies)):ab,ti) OR (observational NEAR/1 (study OR studies)) OR ((epidemiologic NEAR/1 (study OR studies)):ab,ti) OR (('cross sectional' NEAR/1 (study OR studies)):ab,ti) |
16360334 |
|
#40 |
'randomized controlled trial'/exp OR random*:ti,ab OR (((pragmatic OR practical) NEAR/1 'clinical trial*'):ti,ab) OR ((('non inferiority' OR noninferiority OR superiority OR equivalence) NEAR/3 trial*):ti,ab) OR rct:ti,ab,kw |
1839814 |
|
#39 |
'meta analysis'/exp OR 'meta analysis (topic)'/exp OR metaanaly*:ti,ab OR 'meta analy*':ti,ab OR metanaly*:ti,ab OR 'systematic review'/de OR 'cochrane database of systematic reviews'/jt OR prisma:ti,ab OR prospero:ti,ab OR (((systemati* OR scoping OR umbrella OR 'structured literature') NEAR/3 (review* OR overview*)):ti,ab) OR ((systemic* NEAR/1 review*):ti,ab) OR (((systemati* OR literature OR database* OR 'data base*') NEAR/10 search*):ti,ab) OR (((structured OR comprehensive* OR systemic*) NEAR/3 search*):ti,ab) OR (((literature NEAR/3 review*):ti,ab) AND (search*:ti,ab OR database*:ti,ab OR 'data base*':ti,ab)) OR (('data extraction':ti,ab OR 'data source*':ti,ab) AND 'study selection':ti,ab) OR ('search strategy':ti,ab AND 'selection criteria':ti,ab) OR ('data source*':ti,ab AND 'data synthesis':ti,ab) OR medline:ab OR pubmed:ab OR embase:ab OR cochrane:ab OR (((critical OR rapid) NEAR/2 (review* OR overview* OR synthes*)):ti) OR ((((critical* OR rapid*) NEAR/3 (review* OR overview* OR synthes*)):ab) AND (search*:ab OR database*:ab OR 'data base*':ab)) OR metasynthes*:ti,ab OR 'meta synthes*':ti,ab |
733409 |
|
#38 |
#36 AND #37 NOT ('conference abstract'/it OR 'editorial'/it OR 'letter'/it OR 'note'/it) NOT (('animal experiment'/exp OR 'animal model'/exp OR 'nonhuman'/exp) NOT 'human'/exp) |
773 |
|
#37 |
'antibiotic therapy'/exp/mj OR 'antibiotic agent'/exp/mj OR 'antifungal agent'/exp/mj OR 'antifungal therapy'/exp/mj OR antibiotic*:ti,kw OR antifung*:ti,ab,kw OR 'anti-biotic*':ti,ab,kw OR 'anti-fung*':ti,ab,kw OR 'cephalosporin'/exp OR 'cefalosporin*':ti,ab,kw OR 'cephalosporin*':ti,ab,kw OR 'penicillin derivative'/exp OR 'penicillin*':ti,ab,kw OR 'carbapenem'/exp OR 'carbapenem':ti,ab,kw OR 'quinoline derived antiinfective agent'/exp OR 'quinolone derivative'/exp OR fluoroquinolon*:ti,ab,kw OR quinolon*:ti,ab,kw OR 'aminoglycoside antibiotic agent'/exp OR 'aminoglycoside'/exp OR aminoglycoside*:ti,ab,kw OR 'aminoglucoside*':ti,ab,kw OR 'meropenem'/exp OR 'meropenem':ti,ab,kw OR merrem:ti,ab,kw OR 'ceftazidime'/exp OR 'ceftazidime':ti,ab,kw OR fortum:ti,ab,kw OR 'tobramycin'/exp OR 'tobramycin*':ti,ab,kw OR 'piperacillin'/exp OR 'piperacillin*':ti,ab,kw OR pipracil:ti,ab,kw OR 'tazobactam'/exp OR 'tazobactam':ti,ab,kw OR 'combination drug therapy'/exp OR (((dual OR mono OR combination* OR combined OR double OR multimodality) NEAR/3 (therap* OR treat*)):ti,ab,kw) |
1885018 |
|
#36 |
'malignant otitis externa'/exp/mj OR (((maligna* OR necroti* OR necrosis) NEAR/3 ('otitis externa' OR 'external otitis')):ti,kw) OR ('otitis externa'/mj AND (maligna*:ti,kw OR necroti*:ti,kw OR necrosis:ti,kw)) OR (('osteomyelitis'/exp/mj OR 'osteomyelitis':ti,kw) AND ('skull'/exp/mj OR 'skull disease'/exp/mj OR skull*:ti,ab,kw OR cranial:ti,ab,kw OR cranium:ti,ab,kw)) |
3086 |
Ovid/Medline
|
# |
Searches |
Results |
|
14 |
4 and 13 |
31 |
|
13 |
"Duration of Therapy"/ or "6.0".ti,ab,kf. or "6.5".ti,ab,kf. or "6-7".ti,ab,kf. or "6-8".ti,ab,kf. or (six adj3 week*).ti,ab,kf. |
1572259 |
|
12 |
4 not 11 |
374 |
|
11 |
8 or 9 or 10 |
126 |
|
10 |
(4 and 7) not (8 or 9) |
113 |
|
9 |
(4 and 6) not 8 |
6 |
|
8 |
4 and 5 |
7 |
|
7 |
exp clinical trial/ or randomized controlled trial/ or exp clinical trials as topic/ or randomized controlled trials as topic/ or Random Allocation/ or Double-Blind Method/ or Single-Blind Method/ or (clinical trial, phase i or clinical trial, phase ii or clinical trial, phase iii or clinical trial, phase iv or controlled clinical trial or randomized controlled trial or multicenter study or clinical trial).pt. or random*.ti,ab. or (clinic* adj trial*).tw. or ((singl* or doubl* or treb* or tripl*) adj (blind$3 or mask$3)).tw. or Placebos/ or placebo*.tw. or Epidemiologic studies/ or case control studies/ or exp cohort studies/ or Controlled Before-After Studies/ or Case control.tw. or cohort.tw. or Cohort analy$.tw. or (Follow up adj (study or studies)).tw. or (observational adj (study or studies)).tw. or Longitudinal.tw. or Retrospective*.tw. or prospective*.tw. or consecutive*.tw. or Cross sectional.tw. or Cross-sectional studies/ or historically controlled study/ or interrupted time series analysis/ or Case-control Studies/ or clinical trial, phase ii/ or clinical trial, phase iii/ or clinical trial, phase iv/ or comparative study/ or control groups/ or controlled before-after studies/ or controlled clinical trial/ or double-blind method/ or historically controlled study/ or matched-pair analysis/ or single-blind method/ or (((control or controlled) adj6 (study or studies or trial)) or (compar* adj (study or studies)) or ((control or controlled) adj1 active) or "open label*" or ((double or two or three or multi or trial) adj (arm or arms)) or (allocat* adj10 (arm or arms)) or placebo* or "sham-control*" or ((single or double or triple or assessor) adj1 (blind* or masked)) or nonrandom* or "non-random*" or "quasi-experiment*" or "parallel group*" or "factorial trial" or "pretest posttest" or (phase adj5 (study or trial)) or (case* adj6 (matched or control*)) or (match* adj6 (pair or pairs or cohort* or control* or group* or healthy or age or sex or gender or patient* or subject* or participant*)) or (propensity adj6 (scor* or match*))).ti,ab,kf. or (confounding adj6 adjust*).ti,ab. or (versus or vs or compar*).ti. or ((exp cohort studies/ or epidemiologic studies/ or multicenter study/ or observational study/ or seroepidemiologic studies/ or (cohort* or 'follow up' or followup or longitudinal* or prospective* or retrospective* or observational* or multicent* or 'multi-cent*' or consecutive*).ti,ab,kf.) and ((group or groups or subgroup* or versus or vs or compar*).ti,ab,kf. or ('odds ratio*' or 'relative odds' or 'risk ratio*' or 'relative risk*' or aor or arr or rrr).ab. or (("OR" or "RR") adj6 CI).ab.)) |
8305718 |
|
6 |
exp randomized controlled trial/ or randomized controlled trials as topic/ or random*.ti,ab. or rct?.ti,ab. or ((pragmatic or practical) adj "clinical trial*").ti,ab,kf. or ((non-inferiority or noninferiority or superiority or equivalence) adj3 trial*).ti,ab,kf. |
1628800 |
|
5 |
meta-analysis/ or meta-analysis as topic/ or (metaanaly* or meta-analy* or metanaly*).ti,ab,kf. or systematic review/ or cochrane.jw. or (prisma or prospero).ti,ab,kf. or ((systemati* or scoping or umbrella or "structured literature") adj3 (review* or overview*)).ti,ab,kf. or (systemic* adj1 review*).ti,ab,kf. or ((systemati* or literature or database* or data-base*) adj10 search*).ti,ab,kf. or ((structured or comprehensive* or systemic*) adj3 search*).ti,ab,kf. or ((literature adj3 review*) and (search* or database* or data-base*)).ti,ab,kf. or (("data extraction" or "data source*") and "study selection").ti,ab,kf. or ("search strategy" and "selection criteria").ti,ab,kf. or ("data source*" and "data synthesis").ti,ab,kf. or (medline or pubmed or embase or cochrane).ab. or ((critical or rapid) adj2 (review* or overview* or synthes*)).ti. or (((critical* or rapid*) adj3 (review* or overview* or synthes*)) and (search* or database* or data-base*)).ab. or (metasynthes* or meta-synthes*).ti,ab,kf. |
680557 |
|
4 |
3 not (comment/ or editorial/ or letter/ or ((exp animals/ or exp models, animal/) not humans/)) |
500 |
|
3 |
1 and 2 |
525 |
|
2 |
exp *Anti-Bacterial Agents/ or exp Antifungal Agents/ or antibiotic*.ti,kf. or antifung*.ti,ab,kf. or 'anti-biotic*'.ti,ab,kf. or 'anti-fung*'.ti,ab,kf. or exp Cephalosporins/ or exp Penicillins/ or exp Carbapenems/ or exp Fluoroquinolones/ or exp Aminoglycosides/ or exp Meropenem/ or exp Ceftazidime/ or exp Tobramycin/ or exp Piperacillin/ or exp Tazobactam/ or 'cefalosporin*'.ti,ab,kf. or 'cephalosporin*'.ti,ab,kf. or 'penicillin*'.ti,ab,kf. or 'carbapenem'.ti,ab,kf. or fluoroquinolon*.ti,ab,kf. or quinolon*.ti,ab,kf. or aminoglycoside*.ti,ab,kf. or 'aminoglucoside*'.ti,ab,kf. or 'meropenem'.ti,ab,kf. or merrem.ti,ab,kf. or 'ceftazidime'.ti,ab,kf. or fortum.ti,ab,kf. or 'tobramycin*'.ti,ab,kf. or 'piperacillin*'.ti,ab,kf. or Pipracil.ti,ab,kf. or 'tazobactam'.ti,ab,kf. or exp Drug Therapy, Combination/ or ((dual or mono or combination* or combined or double or multimodality) adj3 (therap* or treat*)).ti,ab,kf. |
1391087 |
|
1 |
((maligna* or necroti* or necrosis) adj3 ('otitis externa' or 'external otitis')).ti,kf. or (exp *Otitis Externa/ and (maligna* or necroti* or necrosis).ti,kf.) or ((exp *Osteomyelitis/ or 'osteomyelitis'.ti,kf.) and (exp Skull/ or skull*.ti,ab,kf. or cranial.ti,ab,kf. or cranium.ti,ab,kf.)) |
2667 |