Palliatieve immuuntherapie
Uitgangsvraag
Wat is de rol van eerstelijns immunotherapie in de palliatieve fase bij een carcinoom van de maag, gastro-oesofageale overgang of oesofagus?
Aanbeveling
Aanbevelingen adenocarcinoom
Overweeg eerstelijns behandeling met pembrolizumab plus chemotherapie bij fitte patiënten met een lokaal gevorderd irresectabel of gemetastaseerd HER2-negatief adenocarcinoom van de gastro-oesofageale overgang (Siewert 1) en een CPS van 10 of hoger.
Overweeg eerstelijns behandeling met nivolumab en chemotherapie bij patiënten met een lokaal gevorderd irresectabel of gemetastaseerd HER2-negatief adenocarcinoom van maag, gastro-oesofageale overgang of oesofagus met een CPS van 5 of hoger.
Indien de patiënt aan beide indicaties voldoet, is er geen voorkeur voor pembrolizumab of nivolumab.
Aanbevelingen plaveiselcelcarcinoom
Overweeg eerstelijns behandeling met nivolumab en chemotherapie bij fitte patiënten met een irresectabel, gerecidiveerd of gemetastaseerd plaveiselcelcarcinoom van de oesofagus en een TPS van tenminste 1%.
Overweeg eerstelijns behandeling met pembrolizumab en chemotherapie bij patiënten met een irresectabel, gerecidiveerd of gemetastaseerd plaveiselcelcarcinoom van de oesofagus en een CPS van 10 of hoger.
Indien de patiënt aan beide indicaties voldoet, is er geen voorkeur voor nivolumab of pembrolizumab.
Overwegingen
Voor- en nadelen van de interventie en de kwaliteit van het bewijs
In de eerste lijn bestaat de palliatieve behandeling van een carcinoom van de maag, gastro-oesofageale overgang of oesofagus vaak uit chemotherapie. Afhankelijk van de plaats van origine, histologie, HER2 expressie en PD-L1-expressie kan immuuntherapie, meestal in combinatie met chemotherapie, een alternatief zijn.
De beschikbare gerandomiseerde trials vertonen op verschillende vlakken tekortkomingen, zoals in de literatuursamenvatting vermeld. Er is een aanzienlijk risico op bias en de GRADE-scores voor bewijskracht voor de studies zijn daarom naar beneden bijgesteld.
Echter, de studieresultaten wijzen wel op een mogelijk voordeel in totale overleving en progressievrije overleving voor verschillende immuuntherapie behandelingen op basis van pembrolizumab, ipilimumab of nivolumab. Voor specifieke PD-L1 subgroepen zijn positieve commissie BOM adviezen uitgebracht (NVMO-commissie ter Beoordeling van Oncologische Middelen (BOM), 2022), op basis van de toen geldende PASKWIL-criteria. De richtlijnwerkgroep heeft de beschikbare data beoordeeld op klinische relevantie naar aanleiding van de nieuwe (2023) PASKWIL-criteria.
De individuele onderzochte studies gebruikten verschillende anti-PD-1 antilichamen. Directe onderlinge vergelijking naar de effectiviteit van de verschillende antilichamen is er niet, en een simpele vergelijking van twee verschillende studies is onmogelijk gezien de bekende vormen van bias. Op basis van het werkingsmechanisme valt echter geen verschil in effectiviteit te verwachten.
De individuele onderzochte studies zijn bij subgroepen en in uiterst geselecteerde patiënten verricht. Het is aan te raden een zekere mate van voorzichtigheid bij de interpretatie van de resultaten te betrachten.
De chemotherapie-backbone verschilde tussen de studies, zowel voor adeno- als plaveiselcelcarcinoom. In de CheckMate 649 werd oxaliplatin gecombineerd met capecitabine dan wel 5FU. In de KEYNOTE-590 werd cisplatin gecombineerd met 5FU, maar in een voor Nederland ongebruikelijk schema. Vanuit meta-analyses is er qua effectiviteit in de gemetastaseerd setting een voorkeur voor oxaliplatin boven cisplatine, al zijn die data gebaseerd op studies die met name patiënten met een adenocarcinoom includeerden. Gerandomiseerde studies in de gemetastaseerde setting die eerstelijns chemotherapeutische behandelingen vergeleken voor enkel het plaveiselcelcarcinoom van de oesofagus zijn helaas niet beschikbaar. De EMA-registratie voor zowel nivolumab als pembrolizumab laat een combinatie met elke platinum- en fluoropyrimidinebevattende chemotherapie toe, hetgeen ruimte biedt voor keuzes passend bij de individuele patiënt.
Adenocarcinoom
Doordat patiënten met een adenocarcinoom slechts een subgroep vormden in de KEYNOTE-590 studie, en patiënten alleen konden worden geïncludeerd bij een tumor die volledig in de oesofagus was gelegen (t/m Siewert 1), is het de vraag hoe de resultaten voor de gepoolde groep van patiënten met deze twee zeer verschillende tumortypes uit KEYNOTE-590 naar de klinische praktijk vertaald kunnen worden.
De CheckMate 649 studie includeerde alleen patiënten met een adenocarcinoom, ongeacht de lokalisatie in slokdarm, gastro-oesofageale overgang of maag. De ATTRACTION-4 en de KEYNOTE-062 studies includeerden ook alleen patiënten met een adenocarcinoom, gelegen in de maag of de gastro-oesofageale overgang, waarbij onduidelijk is of ook Siewert 1 tumoren werden geïncludeerd. Hoewel het adenocarcinoom van de oesofagus op moleculair niveau sterkt lijkt op het chromosomaal instabiele subtype van het maagcarcinoom, zijn er dus strikt genomen beperkte data beschikbaar over de effectiviteit van immuuntherapie voor het adenocarcinoom van de oesofagus.
De resultaten van de CheckMate 649 in de subgroep met een CPS≥5 tumor hebben in 2022 geleid tot een positief commissie BOM advies voor deze populatie, en daarna tevens tot vergoeding in Nederland. Dit advies is gegeven op basis van de oude PASKWIL criteria: op basis van de 2023 PASKWIL criteria voldoet de HR van 0.71 net niet. Echter, aangezien de beroepsgroep heeft besloten eerder uitgebrachte positieve adviezen niet opnieuw te beoordelen en aangezien er (voorzichtig) positieve resultaten in de meeste studies in HER2 negatief adenocarcinoom worden gezien, acht de richtlijnwerkgroep het gezamenlijk bewijs voldoende om niet af te wijken van het eerdere positieve commissie BOM advies. De ATTRACTION-4 studie laat geen effect op overleving zien. De redenen daarvoor blijven speculatief maar zouden te wijten kunnen zijn aan het gebruik van een andere selectie biomarker (TPS in plaats van CPS), en de kleine populatie met PD-L1 expressie (114 patiënten) waardoor er mogelijk onvoldoende statistische power was om een effect te zien.
In de KEYNOTE-062 studie werd naast de superioriteit van de toevoeging van immuuntherapie aan chemotherapie, ook de waarde van alleen immuuntherapie vergeleken met chemotherapie in een non-inferioriteit opzet. Statistisch gezien waren de beide regimes non-inferior en werden er geen significante verschillen gezien in toxiciteit. Er zijn geen bevestigende studies voor enkel immuuntherapie. In de klinische praktijk zal slechts bij een zeer klein deel van de patiënten immuuntherapie in plaats van chemotherapie met immuuntherapie worden overwogen, bijvoorbeeld bij patiënten met een specifieke contra-indicatie voor chemotherapie.
Voor de CPS-positieve subgroep (CPS≥1) met een HER2 positief adenocarcinoom zou er mogelijk voordeel kunnen zijn voor de toevoeging van pembrolizumab aan trastuzumab en chemotherapie op totale overleving (Janjigian, 2023). Echter is de evidence beperkt, waardoor er geen aanbeveling geformuleerd is voor deze groep.
Plaveiselcelcarcinoom
Voor het plaveiselcelcarcinoom lieten zowel de CheckMate 648 als de KEYNOTE-590 een meerwaarde zien van het toevoegen van immuuntherapie aan chemotherapie in de respectievelijke biomarker-positieve populatie. In de CheckMate 648 werd ook de combinatie van ipilimumab met nivolumab vergeleken met chemotherapie alleen, maar niet met chemotherapie met immuuntherapie. Gezien het toxiciteitsprofiel van dubbele immuuntherapie en het gebrek aan bevestigende studies voor deze behandeling, lijkt er vooralsnog geen duidelijke plek voor dubbele immuuntherapie voor het oesofaguscarcinoom.
Waarden en voorkeuren van patiënten (en evt. hun verzorgers)
Het doel van het toedienen (wanneer dit geïndiceerd is) van palliatieve immunotherapie in combinatie met chemotherapie is het bewerkstelligen van levensverlenging en verbetering van de kwaliteit van leven. Er is geen onderzoek gedaan naar de waarden en voorkeuren van patiënten. Het is goed om te realiseren dat in de studies selectie heeft plaatsgevonden, bijvoorbeeld op basis van leeftijd en de aan- of afwezigheid van ernstige comorbiditeit. Met de patiënt moet duidelijk gecommuniceerd worden wat met de huidige behandelopties bereikt kan worden, en tegen welke prijs. Op basis hiervan en in combinatie met de eigen doelen van de patiënt kan een gewogen beslissing worden genomen.
Kosten (middelenbeslag)
De werkgroep heeft geen informatie gevonden over de kosteneffectiviteit. De werkgroep heeft dit aspect daarom niet meegewogen bij het formuleren van de aanbeveling. De werkgroep is zich bewust van de relevante impact op de zorgkosten.
Aanvaardbaarheid, haalbaarheid en implementatie
De werkgroep is van mening dat de aanbevelingen aanvaardbaar zijn voor zowel zorgverleners als patiënten. De werkgroep verwacht dat het uitvoeren van de aanbeveling haalbaar en implementeerbaar is. De aanbeveling sluit aan bij de huidige werkwijze in de praktijk.
Rationale van de aanbeveling adenocarcinoom: weging van de argumenten voor en tegen de interventies
Hoewel de toegevoegde waarde van immuuntherapie bovenop chemotherapie bij adenocarcinomen een beperkte winst in overleving laat zien, lijkt dit in de meeste studies het geval. De bijwerkingen als gevolg van de toevoeging van immuuntherapie zijn in de meeste gevallen hanteerbaar.
Rationale van de aanbeveling plaveiselcelcarcinoom: weging van argumenten voor en tegen de interventies
Hoewel de toegevoegde waarde van immuuntherapie bovenop chemotherapie bij plaveiselcelcarcinomen een beperkte winst in overleving laat zien, lijkt dit in alle studies het geval. De bijwerkingen als gevolg van de toevoeging van immuuntherapie zijn in de meeste gevallen hanteerbaar.
De plek van dubbele immuuntherapie blijft vooralsnog onduidelijk.
Onderbouwing
Achtergrond
Esophageal and gastric cancers have limited treatment options in the locally advanced and metastatic setting, with chemotherapy resistance limiting efficacy beyond the first- or second-line setting. With the exception of trastuzumab and ramucirumab, results of clinical trials utilizing targeted agents have been disappointing. The last years a lot of research has been focusing on the use of immunotherapy. Immunotherapy can have an anti-tumor effect by activating the innate immune systems through blocking of PD-1, PD-L1 or CTLA-4, among others, all checkpoint molecules involved in immune activation. These so-called checkpoint have been incorporated in the treatment paradigm of various solid cancer types, as monotherapy or in combination or following chemotherapy, in a biomarker-unselected population or only in patients with certain molecular features or upregulation of checkpoint proteins. PD-L1 upregulation occurs in approximately 40% of gastroesophageal cancers.
Here we discuss the role of first-line immunotherapy in the palliative setting in patients with gastric carcinoma, gastro-oesophageal junction, or oesophageal carcinoma.
Conclusies / Summary of Findings
HER2-negative adenocarcinoma
Overall survival - PD-L1 subgroups
|
Very low GRADE |
Immunotherapy (plus chemotherapy) may have a positive effect on overall survival compared to (placebo plus) chemotherapy in patients with a HER2-negative adenocarcinoma of the oesophagus, gastro-oesophageal junction or stomach with a PD-L1 CPS ≥5 or PD-L1 CPS≥10, but the evidence is very uncertain.
Source: Shitara, 2020; Janjigian, 2021; Kang, 2022; Sun, 2021 |
Progression-free survival - PD-L1 subgroups
|
Very low GRADE |
The evidence is very uncertain about the effect of immunotherapy (plus chemotherapy) on progression-free survival when compared with (placebo plus) chemotherapy in patients with a HER2-negative adenocarcinoma of the oesophagus, gastro-oesophageal junction or stomach with a PD-L1 CPS ≥5, PD-L1 CPS≥10 or PD-L1 TPS≥1%.
Source: Shitara, 2020; Janjigian, 2021; Kang, 2022; Sun, 2021 |
Adverse events (≥grade 3)
|
Very low GRADE |
The evidence is very uncertain about the effect of immunotherapy plus chemotherapy on adverse events when compared with (placebo plus) chemotherapy in patients with a HER2-negative adenocarcinoma of the oesophagus, gastro-oesophageal junction or stomach. Immunotherapy alone might result in less adverse events than chemotherapy, but this evidence is also very uncertain.
Source: Shitara, 2020; Janjigian, 2021; Kang, 2022; Sun, 2021 |
Quality of life
|
Low GRADE |
The evidence suggests that immunotherapy plus chemotherapy results in little to no difference in quality of life when compared with (placebo plus) chemotherapy in patients with a HER2-negative adenocarcinoma of the oesophagus, gastro-oesophageal junction or stomach.
Source: Janjigian, 2021; Kang, 2022 |
HER2-positive adenocarcinoma
Overall survival - PD-L1 subgroup
|
Very low GRADE |
The evidence is very uncertain about the effect of immunotherapy plus trastuzumab plus chemotherapy on overall survival when compared with placebo plus trastuzumab plus chemotherapy in patients with a HER2-positive adenocarcinoma of the gastro-oesophageal junction or stomach with a PD-L1 CPS≥1.
Source: Janjigian, 2021; Janjigian, 2023 |
Progression-free survival - PD-L1 subgroup
|
Very low GRADE |
The evidence is very uncertain about the effect of immunotherapy plus trastuzumab plus chemotherapy on progression-free survival when compared with placebo plus trastuzumab plus chemotherapy in patients with a HER2-positive adenocarcinoma of the gastro-oesophageal junction or stomach with a PD-L1 CPS≥1.
Source: Janjigian, 2021; Janjigian, 2023 |
Adverse events (≥grade 3)
|
Low GRADE |
The evidence suggests that immunotherapy plus trastuzumab plus chemotherapy results in little to no difference in adverse events when compared with placebo plus trastuzumab plus chemotherapy in patients with a HER2-positive adenocarcinoma of the gastro-oesophageal junction or stomach.
Source: Janjigian, 2021; Janjigian, 2023 |
Quality of life
|
- GRADE |
There is no evidence on the effect of immunotherapy plus trastuzumab plus chemotherapy on quality of life when compared with placebo plus trastuzumab plus chemotherapy in patients with a HER2-positive adenocarcinoma of the gastro-oesophageal junction or stomach.
Source: - |
Squamous cell carcinoma
Overall survival - PD-L1 subgroups
|
Very low GRADE |
Double immunotherapy may have a positive effect on overall survival compared to chemotherapy in patients with a squamous cell carcinoma of the oesophagus with a PD-L1 TPS≥1%, but the evidence is very uncertain.
Immunotherapy plus chemotherapy may have a positive effect on overall survival compared to (placebo plus) chemotherapy in patients with a squamous cell carcinoma of the oesophagus with a PD-L1 CPS≥10 or PD-L1 TPS≥1%, but the evidence is very uncertain.
Source: Doki, 2022; Sun, 2021 |
Progression-free survival - PD-L1 subgroups
|
Very low GRADE |
The evidence is very uncertain about the effect of double immunotherapy on progression-free survival when compared with chemotherapy in patients with with a squamous cell carcinoma of the oesophagus with a PD-L1 TPS≥1%.
Immunotherapy plus chemotherapy may have a positive effect on progression-free survival compared to (placebo plus) chemotherapy in patients with a squamous cell carcinoma of the oesophagus with a PD-L1 CPS≥10 or PD-L1 TPS≥1%, but the evidence is very uncertain.
Source: Doki, 2022; Sun, 2021 |
Adverse events (≥grade 3)
|
Low GRADE |
The evidence suggests that (double) immunotherapy (plus chemotherapy) results in little to no difference in adverse events when compared with (placebo plus) chemotherapy in patients with a squamous cell carcinoma of the oesophagus.
Source: Doki, 2022; Sun, 2021 |
Quality of life
|
Very low GRADE |
The evidence is very uncertain about the effect of (double) immunotherapy (plus chemotherapy) on quality of life when compared with chemotherapy in patients with a squamous cell carcinoma of the oesophagus.
Source: Doki, 2022 |
Samenvatting literatuur
Description of studies
Song (2023) - ASTRUM-007 describes a randomized, double-blind, phase 3 trial, which was conducted in 70 institutes in China with a median follow-up length of 15.0 months. The researchers evaluated the efficacy and safety of first-line serplulimab plus chemotherapy versus placebo plus chemotherapy in patients with previously untreated, PD-L1-positive advanced oesophageal squamous cell carcinoma. A total of 551 patients was randomized to receive serplulimab (n=368) (3 mg/kg) on day 1 once every 2 weeks for up to 2 years plus cisplatin (50 mg/m2) on day 1 for up to 8 cycles and continuous infusion of 5-fluorouracil (1,200 mg/m2) on days 1 and 2, for up to 12 cycles, both administered every 2 weeks, or placebo (n=183) plus the same chemotherapy regimen. The median age (range) was 64 (57-68) in the serplulimab group and 64 (57-68) in the placebo group. In the serplulimab group 86% of the participants was male, compared with 84% in the placebo group. The following relevant outcomes were reported: overall survival (OS), progression-free survival (PFS), and adverse events (AEs).
Xu (2023) - RATIONALE-306 describes a randomized, double-blind, phase 3 trial, which was conducted in 162 institutes across Asia, Europe, Oceania, and North America. The researchers evaluated the efficacy and safety of first-line tislelizumab plus chemotherapy versus placebo plus chemotherapy in patients with advanced or metastatic oesophageal squamous cell carcinoma. The median follow-up length was 16.4 months in the intervention group and 9.8 months in the control group. A total of 649 patients was randomized to receive tislelizumab (n=326) (200 mg) every 3 weeks on day 1 of 21-day cycles plus an investigator-chosen chemotherapy doublet, or matching placebo (n=323) plus an investigator-chosen chemotherapy doublet. The median age (range) was 64 (59-68) in the tislelizumab group and 65 (58-70) in the placebo group. In both the tislelizumab and the placebo group 87% of the participants was male. The following relevant outcomes were reported: OS, PFS, and AEs.
Doki (2022) - CHECKMATE-648 describes a randomized, open-label, three-arm phase 3 trial, which was conducted in 182 institutions in 26 countries with a minimum follow-up length of 13 months. The researchers evaluated the efficacy and safety of first-line nivolumab plus ipilimumab versus nivolumab plus chemotherapy versus chemotherapy alone in patients with previously
untreated, unresectable advanced, recurrent, or metastatic oesophageal squamous cell
carcinoma. A total of 970 patients was randomized to receive nivolumab (3 mg/kg every 2 weeks) plus ipilimumab (1 mg/kg every 6 weeks) (n=325), nivolumab (240 mg every 2 weeks) plus chemotherapy (4-week cycle of intravenous fluorouracil and cisplatin) (n=321), or chemotherapy alone (n=324). The median age (range) was 63 (28-81) in the nivolumab plus ipilimumab group, 64 (40-90) in the nivolumab plus chemotherapy group and 64 (26-81) in the chemotherapy alone group. The percentage of male participants was 83%, 79% and 85% for the three groups, respectively. The following relevant outcomes were reported: OS, pFS, AEs, and quality of life (QoL) (measured with FACT-E).
Kang (2022) - ATTRACTION-4 describes a randomized, double-blind, phase 3 trial, which was conducted in 130 institutions across Japan, South Korea and Taiwan with a median follow-up of 26.6 months. The researchers evaluated the efficacy and safety of first-line nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer. A total of 724 patients was randomized to receive nivolumab (360 mg every 3 weeks) plus chemotherapy (oxaliplatin 130 mg/m2 plus either oral S-1 40 mg/m2 or oral capecitabine) (n=362), or matching placebo plus the same chemotherapy regimen (n=362). The median age (range) was 64 (25-86) in the nivolumab plus chemotherapy group and 65 (27-89) in the placebo plus chemotherapy group. In the nivolumab group 70% of the participants was male, compared with 75% in the placebo group. The following relevant outcomes were reported: OS, pFS, AEs, and QoL (measured with EQ-5D-3L and FACT-Ga).
Lu (2022) - ORIENT-15 describes a randomized, double-blind, phase 3 trial, which was conducted in 79 institutions in 5 countries (China, France, Spain, United States and Australia). The researchers evaluated the efficacy and safety of first-line sintilimab plus chemotherapy versus placebo plus chemotherapy in patients with locally advanced or metastatic oesophageal squamous cell carcinoma. The median follow-up length was 16.0 months in the intervention group and 16.9 months in the control group. A total of 658 patients was randomized to receive sintilimab (3 mg/kg in patients weighing <60 kg or 200 mg in patients weighing ≥60 kg on day 1 of each cycle) plus chemotherapy (regimen chosen by the investigator) (n=327) or matching placebo plus chemotherapy (n=332). The median age (range) was 63 (57-67) in the sintilimab plus chemotherapy group and 63 (56-67) in the placebo plus chemotherapy group. In the sintilimab group 85% of the participants was male, compared with 87% in the placebo group. The following relevant outcomes were reported: OS, pFS, AEs, and QoL (measured with QLQ-C30, QLQ-OES18 and EQ-5D-5L).
Wang (2022) - JUPITER-06 describes a randomized, double-blind, phase 3 trial, which was conducted in 72 institutions across China with a median follow-up of 7.1 months. The researchers evaluated the efficacy and safety of first-line toripalimab plus chemotherapy versus placebo plus chemotherapy in patients with treatment-naïve advanced oesophageal squamous cell carcinoma. A total of 514 patients was randomized to receive toripalimab (240 mg) plus chemotherapy (paclitaxel 175 mg/m2 and cisplatin 75 mg/m2) (n=257) or matching placebo plus the same chemotherapy regimen (n=257). The median age (range) was 63 (20-75) in the toripalimab group and 62 (40-74) in the placebo group. In the toripalimab group 84% of the participants was male, compared with 86% in the placebo group. The following relevant outcomes were reported: OS, PFS, and AEs.
Janjigian (2021) - CHECKMATE-649 describes a randomized, open-label, phase 3 trial, which was conducted in 175 institutions in 29 countries across Asia, Australia, Europe, North America and South America with a median follow-up of 13.1 months. The researchers evaluated the efficacy and safety of first-line nivolumab plus chemotherapy versus chemotherapy alone in patients with advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma. A total of 1581 patients was randomized to receive nivolumab (360 mg every 3 weeks or 240 mg every 2 weeks) plus chemotherapy (investigator’s choice) (n=789) or chemotherapy alone (n=792). The median age (range) was 62 (53-68) in the nivolumab group and 61 (53-68) in the chemotherapy group. In the nivolumab group 68% of the participants was male, compared with 71% in the chemotherapy group. The following relevant outcomes were reported: OS, pFS, AEs, and QoL (measured with FACT-Ga).
Janjigian (2021), Janjigian (2023) - KEYNOTE-811 describes a randomized, double-blind, phase 3 trial, which was conducted in 186 institutions in 20 countries with a median follow-up of 38.4 (intervention) and 38.6 (control) months. The researchers evaluated the efficacy and safety of first-line pembrolizumab plus trastuzumab (targeted therapy) plus chemotherapy versus placebo plus trastuzumab plus chemotherapy in patients with previously untreated unresectable or metastatic, HER2-positive gastric or gastro-oesophageal junction adenocarcinoma. A total of 698 patients was randomized to receive pembrolizumab (200 mg every 3 weeks) plus trastuzumab (6 mg/kg every 3 weeks) plus chemotherapy (investigator’s choice) (n=350) or matching placebo plus the same trastuzumab and chemotherapy regimen (n=348). The median age (range) was 62 (54-69) in the pembrolizumab plus trastuzumab plus chemotherapy group and 63 (55-70) in the trastuzumab plus chemotherapy group. The percentage of male participants was 81% and 80% for the two groups, respectively. The following relevant outcomes were reported: OS, PFS, and AEs.
Luo (2021) - ESCORT-1ST describes a randomized, double-blind, phase 3 trial, which was conducted in 60 institutions in China with a median follow-up of 10.8 months. The researchers evaluated the efficacy and safety of first-line camrelizumab plus chemotherapy versus placebo plus chemotherapy in patients with advanced or metastatic oesophageal
squamous cell carcinoma. A total of 596 patients was randomized to receive camrelizumab (200 mg) plus chemotherapy (up to 6 cycles of paclitaxel and cisplatin) every 3 weeks (n=298) or matching placebo plus the same chemotherapy regimen (n=298). The median age (range) was 62 (56-66) in the camrelizumab group and 62 (56-67) in the placebo group. In the camrelizumab group 87% of the participants was male, compared with 88% males in the placebo group. The following relevant outcomes were reported: OS, pFS, AEs, and QoL (measured with QLQ-C30 and QLQ-OES18).
Sun (2021) - KEYNOTE-590 describes a randomized, double-blind, phase 3 trial, which was conducted in 168 institutions in 26 countries with a median follow-up of 22.6 months. The researchers evaluated the efficacy and safety of first-line pembrolizumab plus chemotherapy versus placebo plus chemotherapy in patients with advanced oesophageal cancer and Siewert type 1 gastro-oesophageal junction cancer (both adenocarcinoma and squamous cell carcinoma). A total of 749 patients was randomized to receive pembrolizumab (200 mg) plus chemotherapy (5-fluorouracil and cisplatin) every 3 weeks (n=373) or matching placebo plus the same chemotherapy regimen (n=376). The median age (range) was 64 (28-94) in the pembrolizumab group and 62 (27-89) in the placebo group. In the pembrolizumab group 82% of the participants was male, compared with 85% males in the placebo group. The following relevant outcomes were reported: OS, PFS and AEs.
Shitara (2020) - KEYNOTE-062 describes a randomized, partially blinded, three-arm phase 3 trial, which was conducted in 200 institutions in 29 countries with a median follow-up of 29.4 months. The researchers evaluated efficacy and safety of first-line pembrolizumab versus pembrolizumab plus chemotherapy versus placebo plus chemotherapy in patients with untreated, advanced gastric or gastro-oesophageal junction cancer with programmed cell death ligand 1 (PD-L1) combined positive score (CPS) of 1 or greater. A total of 763 patients was randomized to receive pembrolizumab (200 mg) (n=256), pembrolizumab plus chemotherapy (cisplatin plus fluorouracil) (n=257) or matching placebo plus chemotherapy (n=250). The median age (range) was 61 (20-83) in the pembrolizumab group, 62 (22-83) in the pembrolizumab plus chemotherapy group and 63 (23-87) in the placebo plus chemotherapy group. The percentage of male participants was 70%, 76% and 72% for the three groups, respectively. The following relevant outcomes were reported: OS, PFS and AEs.
Results
Currently, pembrolizumab, nivolumab, and ipilimumab are the only available immunotherapy regimens in the Netherlands within the palliative setting for patients with unresectable or metastatic gastric, gastro‑oesophageal junction, or oesophageal carcinoma. Therefore, the analysis of outcomes below is restricted to these regimens.
The studies with immunotherapy regimens containing pembrolizumab, nivolumab, and/or ipilimumab are presented in table 1.
Table 1. Study characteristics of the analysed studies
|
Study (author, year) |
Study design |
Intervention |
Control |
Type |
PD-L1 subgroups |
Reported outcomes |
|
HER2-negative adenocarcinoma |
||||||
|
KEYNOTE-062 (a) |
RCT (3 arms*) |
Pembrolizumab n= 256 |
Placebo + CT n= 250
|
GC/GEJC
|
CPS 1 or more, CPS 10 or more |
OS PFS AE
|
|
KEYNOTE-062 (b) |
RCT (3 arms*) |
Pembrolizumab + CT n= 257 |
Placebo + CT n= 250
|
GC/GEJC
|
CPS 1 or more, CPS 10 or more |
OS PFS AE
|
|
CHECKMATE-649 |
RCT |
Nivolumab + CT n= 789 |
CT n= 792
|
GC/GEJC/EC
|
CPS 1 or more, CPS 5 or more |
OS PFS AE QoL (FACT-Ga)
|
|
ATTRACTION-4 |
RCT |
Nivolumab + CT n= 362 |
Placebo + CT n= 362
|
GC/GEJC
|
TPS 1% or more |
OS PFS AE QoL (FACT-Ga) |
|
KEYNOTE-590 (Sun, 2021) |
RCT |
Pembrolizumab + CT n= 373 (AC: n=99) |
Placebo + CT n= 376
(AC: n=102) |
EC/GEJCa
|
CPS 10 or more |
OS PFS AE
|
|
HER2-positive adenocarcinoma |
||||||
|
KEYNOTE-811 |
RCT |
Pembrolizumab + trastuzumab + CT n= 350 |
Placebo + trastuzumab + CT n= 348
|
GC/GEJC
|
CPS 1 or more |
OS
|
|
Squamous cell carcinoma |
||||||
|
CHECKMATE-648 (a) |
RCT |
Nivolumab + ipilimumab n= 325 |
CT n= 324 |
EC
|
TPS 1% or more |
OS PFS AE QoL (FACT-E) |
|
CHECKMATE-648 (b) |
RCT |
Nivolumab + CT n= 321 |
CT n= 324
|
EC
|
TPS 1% or more |
OS PFS AE QoL (FACT-E)
|
|
KEYNOTE-590 (Sun, 2021) |
RCT |
Pembrolizumab + CT n= 373 (SCC: n=274) |
Placebo + CT n= 376
(SCC: n=274) |
EC/GEJCa
|
CPS 10 or more |
OS PFS AE
|
|
* three-arm RCT: two comparisons AC = adenocarcinoma, AE = adverse events, CPS = combined positive score, CT = chemotherapy EC = esophageal cancer, GC = gastric cancer, GEJC = gastro‑oesophageal junction cancer, NR = not reported , OS = overall survival, PFS = progression-free survival, TPS = tumor proportion score, QoL = quality of life, SCC = squamous cell carcinoma a Siewert type 1 (GEJC not defined in the other included studies) |
||||||
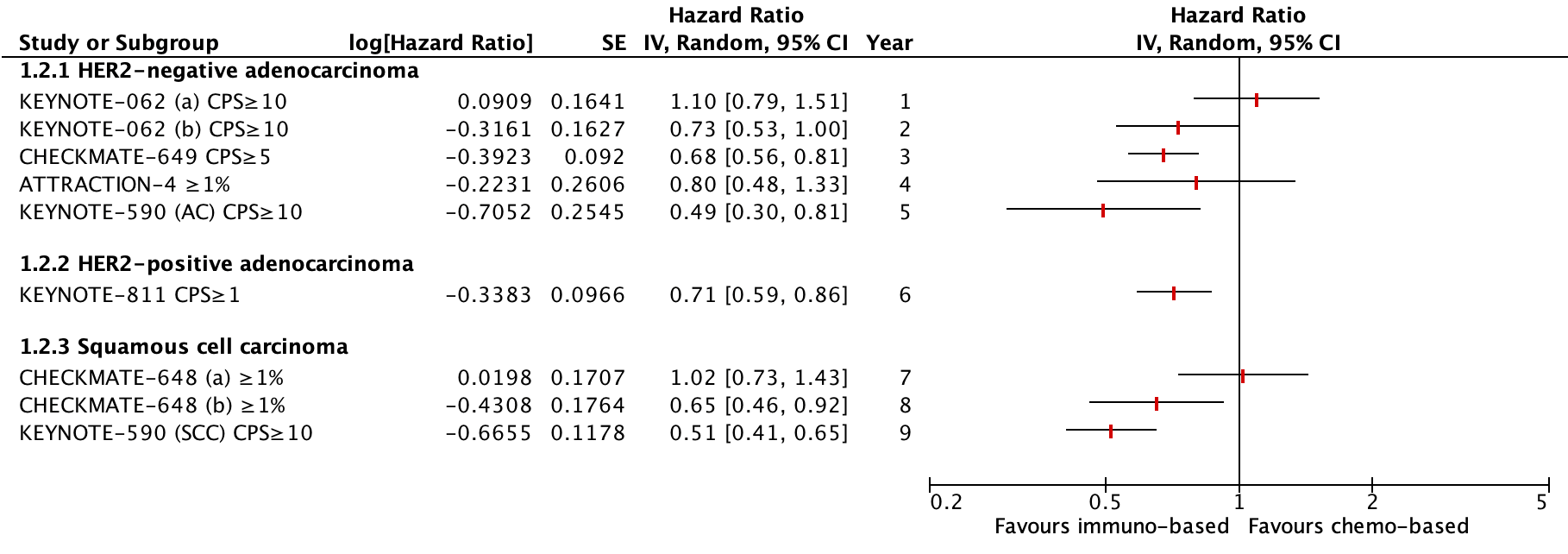
Figure 1. Outcome Overall survival with immunotherapy based regimen versus chemotherapy based regimen alone in PD-L1 subgroups
Figure 2. Outcome Progression-free survival with immunotherapy based regimen versus chemotherapy based regimen alone in PD-L1 subgroups
HER2-negative adenocarcinoma
Four studies reported outcomes for patients with a HER2-negative adenocarcinoma: KEYNOTE-062 (Shitara, 2020), CheckMate 649 (Janjigian, 2021), ATTRACTION-4 (Kang, 2022) and KEYNOTE-590 (Sun, 2021). The KEYNOTE-590 trial also included patients with a squamous cell carcinoma.
Overall survival (critical)
The median OS in the three-armed KEYNOTE-062 study was 10.6 months (95% CI: 7.7 to 13.8) in the pembrolizumab group versus 11.1 months (95% CI: 9.2 to 12.8) in the CT group (all randomized patients, PD-L1 CPS≥1). The HR was 0.91 (95% CI: 0.74 to 1.10).
The median OS was 12.5 months (95% CI: 10.8 to 13.9) in the pembrolizumab plus CT group versus 11.1 months (95% CI: 9.2 to 12.8) in the CT group (all randomized patients, PD-L1 CPS≥1). The HR was 0.85 (95% CI: 0.70 to 1.03). Nor pembrolizumab plus chemotherapy nor pembrolizumab monotherapy was superior to chemotherapy for OS in this population. Pembrolizumab was found to be non-inferior to chemotherapy for OS (all randomized patients, PD-L1 CPS≥1).
The median OS in the CheckMate 649 study was 13.8 months (95% CI: 12.6 to 14.6) in the nivolumab plus CT group versus 11.6 months (95% CI: 10.9 to 12.5) in the CT group. The HR was 0.80 (99.3% CI: 0.68 to 0.94) favoring nivolumab plus CT (Janjigian, 2021). This difference was not considered clinically relevant.
The median OS in the ATTRACTION-4 study was 17.5 months (95% CI: 15.7 to 20.8) in the nivolumab plus CT group versus 17.2 months (95% CI: 15.2 to 19.7) in the placebo plus CT group. The HR was 0.90 (95% CI: 0.75 to 1.08) favoring nivolumab plus CT (Kang, 2022). This difference was not considered clinically relevant.
The median OS in the KEYNOTE-590 study was 12.4 months (95% CI: 10.5 to 14.0) in the pembrolizumab plus CT group versus 9.8 months (95% CI: 8.8 to 10.8) in the placebo plus CT group (all randomized patients). The hazard ratio (HR) was 0.73 (95% CI: 0.62 to 0.86) favoring pembrolizumab plus CT. In patients with adenocarcinoma (n=201), the HR for OS was 0.74 (95% CI: 0.54 to 1.02) (Sun, 2021). This difference was not considered clinically relevant.
Overall survival – PD-L1 subgroups (critical)
In the three-armed KEYNOTE-062 study, an OS subgroup analysis was done for patients with a PD-L1 CPS of 10 or more. The median OS in this subgroup was 17.4 months (95% CI: 9.1 to 23.1) in the pembrolizumab group (n=92) versus 10.8 months (95% CI: 8.5 to 13.8) in the CT group (n=90). The HR for this comparison was 0.69 (95% CI: 0.49 to 0.97) favoring pembrolizumab. This difference was considered clinically relevant.
The median OS in this subgroup was 12.3 months (95% CI: 9.5 to 14.8) in the pembolizumab plus CT group (n=99) versus 10.8 months (95% CI: 8.5 to 13.8) in the CT group (n=90). The HR for this comparison was 0.85 (95% CI: 0.62 to 1.17) (Shitara, 2020) favoring pembrolizumab plus CT. This difference was not considered clinically relevant.
In the CheckMate 649 study, an OS subgroup analysis was done for patients with a PD-L1 CPS of 5 or more. The median OS in this subgroup was 14.4 months (95% CI: 13.1 to 16.2) in the nivolumab plus CT group (n=473) versus 11.1 months (95% CI: 10.0 to 12.1) in the CT group (n=482). The HR was 0.71 (98.4% CI: 0.59 to 0.86) favoring nivolumab plus CT (Janjigian, 2021). This difference was not considered clinically relevant.
In the ATTRACTION-4 study, an OS subgroup analysis was done for patients with a PD-L1 TPS of 1% or more. The median OS in this subgroup was 16.6 months (95% CI: 10.5 to 22.7) in the nivolumab plus CT group (n=58) versus 16.6 months (95% CI: 10.1 to 23.5) in the placebo plus CT group (n=56). The HR was 1.06 (95% CI: 0.67 to 1.68) favoring placebo plus CT (Kang, 2022). This difference was not considered clinically relevant
In the KEYNOTE-590 study, an OS subgroup analysis was done for patients with a PD-L1 CPS of 10 or more. The median OS in this subgroup was 13.5 months (95% CI: 11.1 to 15.6) in the pembrolizumab plus CT group (n=186) versus 9.4 months (95% CI: 8.0 to 10.7) in the placebo plus CT group (n=197). The HR was 0.62 (95% CI: 0.49 to 0.78) (Sun, 2021) favoring pembrolizumab plus CT. This difference was considered clinically relevant.
In patients in this subgroup with adenocarcinoma (n=97), the HR was 0.83 (95% CI: 0.52 to 1.34) favoring pembrolizumab plus CT. This difference was not considered clinically relevant.
Progression-free survival
The median PFS in the three-armed KEYNOTE-062 study was 2.0 months (95% CI: 1.5 to 2.8) in the pembrolizumab group versus 6.4 months (95% CI: 5.7 to 7.0) in the CT group (all randomized patients, PD-L1 CPS≥1). The HR was 1.66 (95% CI: 1.37 to 2.01).
The median PFS was 6.9 months (95% CI: 5.7 TO 7.3) in the pembrolizumab plus CT group versus 6.4 months (95% CI: 5.7 to 7.0) in the CT group (all randomized patients, PD-L1 CPS≥1). The HR was 0.84 (95% CI: 0.70 to 1.02). Pembrolizumab plus chemotherapy was not superior to chemotherapy for PFS in this population.
The median PFS in the CheckMate 649 study was 7.7 months (95% CI: 7.1 to 8.5) in the nivolumab plus CT group versus 6.9 months (95% CI: 6.6 to 7.1) in the CT group. The HR was 0.77 (95% CI: 0.68 to 0.87) favoring nivolumab plus CT (Janjigian, 2021). As the median overall survival in the control group was <12 months, the clinical relevance of PFS was not considered.
The median PFS in the ATTRACTION-4 study was 10.5 months (95% CI: 8.4 to 14.8) in the nivolumab plus CT group versus 8.3 months (95% CI: 7.0 to 9.4) in the placebo plus CT group. The HR was 0.68 (98.5% CI: 0.51 to 0.90) favoring nivolumab plus CT (Kang, 2022). This difference was not considered clinically relevant.
The median PFS in the KEYNOTE-590 study was 6.3 months (95% CI: 6.2 to 6.9) in the pembrolizumab plus CT group versus 5.8 months (95% CI: 0.55 to 0.76) in the placebo plus CT group (all randomized patients). The HR was 0.65 (95% CI: 0.55 to 0.76) favoring pembrolizumab plus CT. In patients with adenocarcinoma (n=201), the HR for PFS was 0.63 (95% CI: 0.46 to 0.87) (Sun, 2021). As the median overall survival in the control group was <12 months, the clinical relevance of PFS was not considered.
Progression-free survival – PD-L1 subgroups
In the three-armed KEYNOTE-062 study, a PFS subgroup analysis was done for patients with a PD-L1 CPS of 10 or more. The median PFS in this subgroup was 2.9 months (95% CI: 1.6 to 5.4) in the pembrolizumab group (n=92) versus 6.1 months (95% CI: 5.3 to 6.9) in the CT group (n=90). The HR for this comparison was 1.10 (95% CI: 0.79 to 1.51) favoring CT.
The HR for the comparison pembrolizumab plus CT (n=99) versus CT (n=90) was 0.73 (95% CI: 0.53 to 1.00) favoring pembrolizumab plus CT, but the median PFS in months was not given (Shitara, 2020). As the median overall survival in the control group was <12 months, the clinical relevance of PFS was not considered.
In the CheckMate 649 study, a PFS subgroup analysis was done for patients with a PD-L1 CPS of 5 or more. The median PFS in this subgroup was 7.7 months (95% CI: 7.0 to 9.2) in the nivolumab plus CT group (n=473) versus 6.0 months (95% CI: 5.6 to 6.9) in the CT group (n=482). The HR was 0.68 (98% CI: 0.56 to 0.81) favoring nivolumab plus CT (Janjigian, 2021). As the median overall survival in the control group was <12 months, the clinical relevance of PFS was not considered.
In the ATTRACTION-4 study, a PFS subgroup analysis was done for patients with a PD-L1 TPS of 1% or more. The median PFS in this subgroup was 8.3 months (95% CI: 4.3 to 12.5) in the nivolumab plus CT group (n=58) versus 4.4 months (95% CI: 3.6 to 11.1) in the placebo plus CT group (n=56). The HR was 0.80 (95% CI: 0.48 to 1.33) favoring nivolumab plus CT (Kang, 2022). This difference was not considered clinically relevant.
In the KEYNOTE-590 study, a PFS subgroup analysis was done for patients with a PD-L1 CPS of 10 or more. The median PFS in this subgroup was 7.5 months (95% CI: 6.2 to 8.2) in the pembrolizumab plus CT group (n=186) versus 5.5 months (95% CI: 4.3 to 6.0) in the placebo plus CT group (n=197). The HR was 0.51 (95% CI: 0.41 to 0.65) (Sun, 2021) favoring pembrolizumab plus CT. As the median overall survival in the control group was <12 months, the clinical relevance of PFS was not considered.
In patients in this subgroup with adenocarcinoma (n=97), the HR was 0.49 (95% CI: 0.30 to 0.81) favoring pembrolizumab plus CT.
Adverse events
In the three-armed KEYNOTE-062 study, treatment-related adverse events of grade 3 or higher occurred in 43/254 patients (17%) in the pembrolizumab group, versus 183/250 patients (73%) in the pembrolizumab plus CT group, versus 169/244 (69%) in the CT group (Shitara, 2020).
The RR for the comparison pembrolizumab versus CT was 0.24 (95% CI: 0.18 to 0.33) favoring pembrolizumab. This difference was considered clinically relevant.
The RR for the comparison pembrolizumab plus CT versus CT was 1.06 (95% CI: 0.94 to 1.18) favoring CT. This difference was not considered clinically relevant.
In the CheckMate 649 study, adverse events of grade 3 or higher occurred in 466/782 patients (60%) in the nivolumab plus CT group versus 341/767 patients (44%) in the CT group. The most common adverse events were nausea, diarrhea and peripheral neuropathy (Janjigian, 2021). The RR for this comparison was 1.34 (95% CI: 1.22 to 1.48) favoring CT. This difference was not considered clinically relevant.
In the ATTRACTION-4 study, adverse events of grade 3 or higher occurred in 71/359 patients (20%) in the nivolumab plus CT group versus 57/358 patients (16%) in the placebo plus CT group. The most common adverse events were neutrophil count decreased, platelet count decreased and decreased appetite (Kang, 2022). The RR for this comparison was 1.24 (95% CI: 0.91 to 1.70) favoring placebo plus CT. This difference was not considered clinically relevant.
In the KEYNOTE-590 study, adverse events of grade 3 or higher occurred in 370/370 patients (100%) in the pembrolizumab plus CT group versus 318/370 patients (86%) in the placebo plus CT group. The most common adverse events were decreased neutrophil count, anaemia and neutropenia (Sun, 2021). The risk ratio (RR) for this comparison was 1.03 (95% CI: 0.97 to 1.10) favoring placebo plus CT. This difference was not considered clinically relevant.
Adverse events were not reported for histology subgroups.
Quality of life
Quality of life was assessed with the Functional Assessment of Cancer Therapy-Gastric (FACT-Ga) or Functional Assessment of Cancer Therapy-Esophagus (FACT-E) questionnaires.
Quality of life was a pre-specified end point in the KEYNOTE-062 study, but no results were reported yet (Shitara, 2020).
In the CheckMate 649 study, baseline mean FACT-Ga scores were similar between the nivolumab plus CT group (126.6 [28.3]) and the CT group (126.8 [26.8]). The least squares mean difference between the two groups favored nivolumab plus CT, but the result did not exceed the minimally important difference of 15.1 points (Janjigian, 2021).
In the ATTRACTION-4 study, baseline FACT-Ga total scores were similar between the nivolumab plus CT group and the placebo plus CT group. The HR for time to symptom deterioration was 0.86 (95% CI: 0.70 to 1.06) (Kang, 2022).
Quality of life was a pre-specified end point in the KEYNOTE-590 study, but no results were reported yet (Sun, 2021).
HER2-positive adenocarcinoma
One study reported outcomes for patients with a HER2-positive adenocarcinoma: KEYNOTE-811 (Janjigian, 2021; Janjigian, 2023).
Overall survival (critical)
OS was not reported in the results of the protocol-specified first interim analysis of the KEYNOTE-811 study (Janjigian, 2021).
At the third interim analysis (Janjigian, 2023), the median OS was 20.0 months (95% CI: 17.8-22.1) in the pembrolizumab plus trastuzumab plus CT group versus 16.8 months (95% CI: 15.0-18.7) in the placebo plus trastuzumab plus CT group. The HR was 0.84 (95% CI: 0.70 to 1.01) favoring pembrolizumab plus trastuzumab plus CT (Janjigian, 2023). This difference was not considered clinically relevant.
Overall survival – PD-L1 subgroup (CPS≥1) (critical)
In the KEYNOTE-811 study, an OS subgroup analysis was done for patients with a PD-L1 CPS of 1 or more. The median OS in this subgroup was 20.0 months (95% CI: 17.9 to 22.7) in the pembrolizumab plus trastuzumab plus CT group versus 15.7 months (95% CI: 13.5 to 18.5) in the placebo plus trastuzumab plus CT group. The HR was 0.81 (95% CI: 0.67 to 0.98) favoring pembrolizumab plus trastuzumab plus CT (Janjigian, 2023). This difference was not considered clinically relevant.
Progression-free survival
PFS was not reported in the results of the protocol-specified first interim analysis of the KEYNOTE-811 study (Janjigian, 2021).
At the third interim analysis (Janjigian, 2023), the median PFS was 10.0 months (95% CI: 8.6 to 12.2) in the pembrolizumab plus trastuzumab plus CT group versus 8.1 months (95% CI: 7.1 to 8.6) in the placebo plus trastuzumab plus CT group. The HR was 0.73 (95% CI: 0.61 to 0.87) favoring pembrolizumab plus trastuzumab plus CT (Janjigian, 2023). This difference was not considered clinically relevant.
Progression-free survival – PD-L1 subgroup (CPS≥1)
In the KEYNOTE-811 study, a PFS subgroup analysis was done for patients with a PD-L1 CPS of 1 or more. The median PFS in this subgroup was 10.9 months (95% CI: 8.5 to 12.5) in the pembrolizumab plus trastuzumab plus CT group versus 7.3 months (95% CI: 6.8 to 8.5) in the placebo plus trastuzumab plus CT group. The HR was 0.71 (95% CI: 0.59 to 0.86) favoring pembrolizumab plus trastuzumab plus CT (Janjigian, 2023). This difference was not considered clinically relevant.
Adverse events
In the KEYNOTE-811 study, adverse events of grade 3 or higher occurred in 248/350 patients (71%) in the pembrolizumab plus trastuzumab plus CT group versus 225/348 patients (65%) in the placebo plus trastuzumab plus CT group. The most common adverse events were diarrhea and anaemia (Janjigian, 2023). This difference was not considered clinically relevant.
Quality of life
Quality of life was not an endpoint of the KEYNOTE-811 study (Janjigian, 2021; Janjigian, 2023).
Squamous cell carcinoma
Two studies reported outcomes for patients with a squamous cell carcinoma: CheckMate 648 (Doki, 2022) and KEYNOTE-590 (Sun, 2021). The KEYNOTE-590 trial also included patients with a HER2-negative adenocarcinoma.
Overall survival (critical)
The median OS in the three-armed CheckMate 648 study was 13.2 months (95% CI: 11.1 to 15.7) in the nivolumab plus CT group versus 10.7 months (95% CI: 9.4 to 11.9) in the CT group. The HR was 0.74 (99.1% CI: 0.58 to 0.96) favoring nivolumab plus CT (Doki, 2022). This difference was not considered clinically relevant.
The median OS was 12.7 months (95% CI: 11.3 to 15.5) in the nivolumab plus ipilimumab group versus 10.7 months (95% CI: 9.4 to 11.9) in the CT group. The HR was 0.78 (98.2% CI: 0.62 to 0.98) favoring nivolumab plus ipilimumab (Doki, 2022). This difference was not considered clinically relevant.
The median OS in the KEYNOTE-590 study was 12.4 months (95% CI: 10.5 to 14.0) in the pembrolizumab plus CT group versus 9.8 months (95% CI: 8.8 to 10.8) in the placebo plus CT group (all randomized patients). The hazard ratio (HR) was 0.73 (95% CI: 0.62 to 0.86) favoring pembrolizumab plus CT. In patients with squamous cell carcinoma (n=548), the HR for OS was 0.72 (95% CI: 0.60 to 0.88) (Sun, 2021). This difference was not considered clinically relevant.
Overall survival – PD-L1 subgroups (critical)
In the three-armed CheckMate 648 study, an OS subgroup analysis was done for patients with a PD-L1 TPS of 1% or more. The median OS in this subgroup was 15.4 months (95% CI: 11.9 to 19.5) in the nivolumab plus CT group (n=158) versus 9.1 months (95% CI: 7.7 to 10.0) in the CT group (n=157). The HR was 0.54 (99.5% CI: 0.37 to 0.80) favoring nivolumab plus CT (Doki, 2022). This difference was considered clinically relevant.
The median OS in this subgroup was 13.7 months (95% CI: 11.2 to 17.0) in the nivolumab plus ipilimumab group (n=158) versus 9.1 months (95% CI: 7.7 to 10.0) in the CT group (n=157). The HR was 0.64 (98.6% CI: 0.46 to 0.90) favoring nivolumab plus ipilimumab (Doki, 2022). This difference was considered clinically relevant.
In the KEYNOTE-590 study, a OS subgroup analysis was done for patients with a PD-L1 CPS of 10 or more. The median OS in this subgroup was 13.5 months (95% CI: 11.1 to 15.6) in the pembrolizumab plus CT group (n=186) versus 9.4 months (95% CI: 8.0 to 10.7) in the placebo plus CT group (n=197). The HR was 0.62 (95% CI: 0.49 to 0.78) (Sun, 2021) favoring pembrolizumab plus CT. This difference was considered clinically relevant.
In patients in this subgroup with squamous cell carcinoma (n=286), the HR was 0.57 (95% CI: 0.43 to 0.75) favoring pembrolizumab plus CT. This difference was considered clinically relevant.
Progression-free survival
The median PFS in the three-armed CheckMate 648 study was 5.8 months (95% CI: 5.6 to 7.0) in the nivolumab plus CT group versus 5.6 months (95% CI: 4.3 to 5.9) in the CT group. The HR was 0.81 (98.5% CI: 0.64 to 1.04) favoring nivolumab plus CT (Doki, 2022). As the median overall survival in the control group was <12 months, the clinical relevance of PFS was not considered.
The median PFS was 2.9 months (95% CI: 2.7 to 4.2) in the nivolumab plus ipilimumab group versus 5.6 months (95% CI: 4.3 to 5.9) in the CT group. The HR was 1.26 (95% CI: 1.04 to 1.52) favoring CT (Doki, 2022). As the median overall survival in the control group was <12 months, the clinical relevance of PFS was not considered.
The median PFS in the KEYNOTE-590 study was 6.3 months (95% CI: 6.2 to 6.9) in the pembrolizumab plus CT group versus 5.8 months (95% CI: 0.55 to 0.76) in the placebo plus CT group (all randomized patients). The HR was 0.65 (95% CI: 0.55 to 0.76) favoring pembrolizumab plus CT. In patients with squamous cell carcinoma (n=548), the HR for PFS was 0.65 (95% CI: 0.54 to 0.78) (Sun, 2021). As the median overall survival in the control group was <12 months, the clinical relevance of PFS was not considered.
Progression-free survival – PD-L1 subgroups
In the three-armed CheckMate 648 study, a PFS subgroup analysis was done for patients with a PD-L1 TPS of 1% or more. The median PFS in this subgroup was 6.9 months (95% CI: 5.7 to 8.3) in the nivolumab plus CT group (n=158) versus 4.4 months (95% CI: 2.9 to 5.8) in the CT group (n=157). The HR was 0.65 (98.5% CI: 0.46 to 0.92) favoring nivolumab plus CT (Doki, 2022). As the median overall survival in the control group was <12 months, the clinical relevance of PFS was not considered.
The median PFS in this subgroup was 4.0 months (95% CI: 2.4 to 4.9) in the nivolumab plus ipilimumab group (n=158) versus 4.4 months (95% CI: 2.9 to 5.8) in the CT group (n=157). The HR was 1.02 (98.5% CI: 0.73 to 1.43) favoring CT (Doki, 2022). As the median overall survival in the control group was <12 months, the clinical relevance of PFS was not considered.
In the KEYNOTE-590 study, a PFS subgroup analysis was done for patients with a PD-L1 CPS of 10 or more. The median PFS in this subgroup was 7.5 months (95% CI: 6.2 to 8.2) in the pembrolizumab plus CT group (n=186) versus 5.5 months (95% CI: 4.3 to 6.0) in the placebo plus CT group (n=197). The HR was 0.51 (95% CI: 0.41 to 0.65) (Sun, 2021) favoring pembrolizumab plus CT. As the median overall survival in the control group was <12 months, the clinical relevance of PFS was not considered.
In patients in this subgroup with squamous cell carcinoma (n=286), the HR was 0.53 (95% CI: 0.40 to 0.69) favoring pembrolizumab plus CT. This difference was considered clinically relevant.
Adverse events
In the three-armed CheckMate 648 study, adverse events of grade 3 or higher occurred in 147/310 patients (47%) in the nivolumab plus CT group versus 108/304 patients (36%) in the CT group. The most common adverse events were nausea, decreased appetite and stomatitis (Doki, 2022). The RR for this comparison was 1.33 (95% CI: 1.10 to 1.62) favoring CT. This difference was not considered clinically relevant.
Adverse events of grade 3 or higher occurred in 102/322 patients (32%) in the nivolumab plus ipilimumab group versus 108/304 patients (36%) in the CT group. The most common adverse events were nausea, decreased appetite and stomatitis (Doki, 2022). The RR for this comparison was 0.89 (95% CI: 0.72 to 1.11) favoring nivolumab plus ipilimumab. This difference was not considered clinically relevant.
In the KEYNOTE-590 study, adverse events of grade 3 or higher occurred in 370/370 patients (100%) in the pembrolizumab plus CT group versus 318/370 patients (86%) in the placebo plus CT group. The most common adverse events were decreased neutrophil count, anaemia and neutropenia (Sun, 2021). The risk ratio (RR) for this comparison was 1.03 (95% CI: 0.97 to 1.10) favoring placebo plus CT. This difference was not considered clinically relevant.
Adverse events were not reported for histology subgroups.
Quality of life
Quality of life was assessed with the Functional Assessment of Cancer Therapy-Gastric (FACT-Ga) or Functional Assessment of Cancer Therapy-Esophagus (FACT-E) questionnaires.
In the three-armed CheckMate 648 study, the least squares mean FACT-E change from baseline was 4.98 points (95% CI: 2.68 to 7.27) in the nivolumab plus CT group and 1.54 points (95% CI: -1.26 to 4.33) in the CT group. This result did not exceed the minimally important difference of 9.5 points (Doki, 2022).
The least squares mean FACT-E change from baseline was 3.45 points (95% CI: 0.96 to 5.94) in the nivolumab plus ipilimumab group and 1.54 points (95% CI: -1.26 to 4.33) in the CT group. This result did not exceed the minimally important difference of 9.5 points (Doki, 2022).
Quality of life was a pre-specified end point in the KEYNOTE-590 study, but no results were reported yet (Sun, 2021).
Level of evidence of the literature
The evidence for the outcomes overall survival (total population), progression-free survival (total population), adverse events and quality of life started at ‘high’.
The evidence for the outcomes overall survival (PD-L1 subpopulations) and progression-free survival (PD-L1 subpopulations) was derived from observational data from subgroups of an RCT. Therefore the level of evidence started at ‘low’.
HER2-negative adenocarcinoma
The level of evidence regarding the outcome measure overall survival - PD-L1 subgroups was downgraded by three levels because of study limitations (risk of bias), inconsistency (unexplained heterogeneity) and indirectness (differences in interventions). Therefore, the level of evidence was graded as very low.
The level of evidence regarding the outcome measure progression-free survival - PD-L1 subgroups was downgraded by two levels because of study limitations (risk of bias) and indirectness (differences in interventions). Therefore, the level of evidence was graded as very low.
The level of evidence regarding the outcome measure adverse events was downgraded by three levels because of study limitations (risk of bias), inconsistency (unexplained heterogeneity) and indirectness (differences in populations (histology) and interventions). Therefore, the level of evidence was graded as very low.
The level of evidence regarding the outcome measure quality of life was downgraded by two levels because of study limitations (risk of bias) and imprecision (number of included patients). Therefore, the level of evidence was graded as low.
HER2-positive adenocarcinoma
The level of evidence regarding the outcome measure overall survival – PD-L1 subgroup was downgraded by two levels because of study limitations (risk of bias) and number of included patients (imprecision). Therefore, the level of evidence was graded as very low.
The level of evidence regarding the outcome measure progression-free survival – PD-L1 subgroup was downgraded by two levels because of study limitations (risk of bias) and number of included patients (imprecision). Therefore, the level of evidence was graded as very low.
The level of evidence regarding the outcome measure adverse events was downgraded by two levels because of study limitations (risk of bias) and number of included patients (imprecision). Therefore, the level of evidence was graded as low.
The level of evidence regarding the outcome measure quality of life was not graded for the comparison pembrolizumab plus trastuzumab plus CT versus placebo plus trastuzumab plus CT, because none of the included studies reported this outcome.
Squamous cell carcinoma
The level of evidence regarding the outcome measure overall survival - PD-L1 subgroups was downgraded by two levels because of study limitations (risk of bias) and indirectness (differences in interventions). Therefore, the level of evidence was graded as very low.
The level of evidence regarding the outcome measure progression-free survival - PD-L1 subgroups was downgraded by two levels because of study limitations (risk of bias) and indirectness (differences in interventions). Therefore, the level of evidence was graded as very low.
The level of evidence regarding the outcome measure adverse events was downgraded by two levels because of study limitations (risk of bias) and indirectness (differences in populations (histology) and interventions). Therefore, the level of evidence was graded as low.
The level of evidence regarding the outcome measure quality of life was downgraded by three levels because of study limitations (risk of bias), indirectness (differences in interventions) and imprecision (number of included patients). Therefore, the level of evidence was graded as very low.
Zoeken en selecteren
A systematic review of the literature was performed to answer the following question:
What is the effect of immunotherapy (with or without chemotherapy) compared to standard of care or best supportive care on overall survival, progression-free survival, adverse events and quality of life as first line therapy for patients with unresectable or metastatic gastric, gastro‑oesophageal junction, or oesophageal carcinoma?
| P: | patients with unresectable or metastatic gastric, gastro‑oesophageal junction, or oesophageal adenocarcinoma or squamous cell carcinoma |
| I: | first-line immunotherapy +/- chemotherapy |
| C: | standard of care (chemotherapy) or best supportive care |
| O: | overall survival, progression-free survival, adverse events, quality of life |
Relevant outcome measures
The guideline development group considered overall survival as a critical outcome measure for decision making, and progression-free survival, adverse events and quality of life as important outcome measures for decision making.
The working group defined the outcome measures as follows:
- Overall survival: Time to death from any cause
- Progression-free survival: Time from randomization or initiation of treatment to the occurrence of disease progression or death from any cause
- Adverse events: Grade 3 or higher adverse events of any cause
- Quality of life: Quality of life measured by a validated instrument
The working group defined the following differences as a minimal clinically (patient) important difference:
If median overall survival in control group ≤12 months:
- Overall survival: >12 weeks and hazard ratio (HR)<0.7
If median overall survival in control group >12 months:
- Overall survival: >16 weeks and hazard ratio (HR)<0.7
- Progression-free survival: >16 weeks and hazard ratio (HR)<0.7
- Adverse events: absolute difference >5% for lethal complications, or >25% for serious complications
- Quality of life: validated questionnaire, e.g. Functional Assessment of Cancer Therapy-Gastric (FACT-Ga): 15.1 points or Functional Assessment of Cancer Therapy-Esophagus (FACT-E): 9.5 points
Search and select (Methods)
The databases Medline (via OVID) and Embase (via Embase.com) were searched from 2015 until 29-05-2023. The detailed search strategy is depicted under the tab Methods. The systematic literature search resulted in 807 hits. Studies were selected based on the following criteria:
- Systematic reviews or randomized controlled trials (RCTs)
- Full-text English language publication
- Complying with the PICO criteria
We initially selected 176 studies based on title and abstract screening, from which one recent systematic review (Duan, 2023) was used as the basis for this literature summary. This systematic review included phase III RCTs with patients with unresectable locally advanced or metastatic gastric oesophageal cancer, and compared immunotherapy-based regimens with chemotherapy alone. The outcomes that were reported were OS, PFS, ORR, DCR and/or AEs. Duan searched PubMed, Embase and Cochrane Library electronic databases with relevant search terms until June 2022. Detailed search strategies were published in supplemental tables (Duan, 2023).
After reading the full text of the RCTs published after the search date from the systematic review by Duan, seventeen RCTs were excluded (see the table with reasons for exclusion under the tab Methods), and 2 additional RCTs were included (Song, 2023; Xu, 2023).
Results
The selected systematic review included nine RCTs. With the two additional RCTs, this resulted in eleven studies that were included in the analysis of the literature. One publication of the KEYNOTE-811 study (Janjigian, 2023) was added, as the first interim analysis (Janjigian, 2021) did not report the selected outcomes. Important study characteristics and results are summarized in the evidence tables. The assessment of the risk of bias is summarized in the risk of bias tables.
GRADE
GRADE was applied to assess the certainty of the evidence for the main comparisons, subgroups and critical outcome measures. The credibility of subgroup effects was assessed using the following criteria:
- Is the subgroup variable a characteristic specified at baseline or after randomization? (subgroup hypotheses should be developed a priori)
- Is the subgroup difference suggested by comparisons within rather than between studies?
- Does statistical analysis suggest that chance is an unlikely explanation for the subgroup difference?
- Did the hypothesis precede rather than follow the analysis and include a hypothesized direction that was subsequently confirmed?
- Was the subgroup hypothesis one of a smaller number tested?
- Is the subgroup difference consistent across studies and across important outcomes?
- Does external evidence (biological or sociological rationale) support the hypothesized subgroup difference?
When subgroup analyses were considered convincing and showed an interaction between the PD-L1 status and the magnitude of effect, we only graded the evidence for the subgroups and only presented conclusions for the subgroups.
Referenties
- 1 - Doki Y, Ajani JA, Kato K, Xu J, Wyrwicz L, Motoyama S, Ogata T, Kawakami H, Hsu CH, Adenis A, El Hajbi F, Di Bartolomeo M, Braghiroli MI, Holtved E, Ostoich SA, Kim HR, Ueno M, Mansoor W, Yang WC, Liu T, Bridgewater J, Makino T, Xynos I, Liu X, Lei M, Kondo K, Patel A, Gricar J, Chau I, Kitagawa Y; CheckMate 648 Trial Investigators. Nivolumab Combination Therapy in Advanced Esophageal Squamous-Cell Carcinoma. N Engl J Med. 2022 Feb 3;386(5):449-462. doi: 10.1056/NEJMoa2111380. PMID: 35108470.
- 2 - Duan X, Du H, Qi R, Yuan M, Shi J. The efficacy and safety of first line anti PD 1/PD L1 immunotherapy for gastric esophageal cancer: A systematic review and meta analysis of phase III randomized controlled trials. Exp Ther Med. 2023 Mar 28;25(5):216. doi: 10.3892/etm.2023.11915. PMID: 37123204; PMCID: PMC10133786.
- 3 - Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, Wyrwicz L, Yamaguchi K, Skoczylas T, Campos Bragagnoli A, Liu T, Schenker M, Yanez P, Tehfe M, Kowalyszyn R, Karamouzis MV, Bruges R, Zander T, Pazo-Cid R, Hitre E, Feeney K, Cleary JM, Poulart V, Cullen D, Lei M, Xiao H, Kondo K, Li M, Ajani JA. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021 Jul 3;398(10294):27-40. doi: 10.1016/S0140-6736(21)00797-2. Epub 2021 Jun 5. PMID: 34102137; PMCID: PMC8436782.
- 4 - Janjigian YY, Kawazoe A, Yañez P, Li N, Lonardi S, Kolesnik O, Barajas O, Bai Y, Shen L, Tang Y, Wyrwicz LS, Xu J, Shitara K, Qin S, Van Cutsem E, Tabernero J, Li L, Shah S, Bhagia P, Chung HC. The KEYNOTE-811 trial of dual PD-1 and HER2 blockade in HER2-positive gastric cancer. Nature. 2021 Dec;600(7890):727-730. doi: 10.1038/s41586-021-04161-3. Epub 2021 Dec 15. PMID: 34912120; PMCID: PMC8959470.
- 5 - Janjigian YY, Kawazoe A, Bai Y, Xu J, Lonardi S, Metges JP, Yanez P, Wyrwicz LS, Shen L, Ostapenko Y, Bilici M, Chung HC, Shitara K, Qin SK, Van Cutsem E, Tabernero J, Li K, Shih CS, Bhagia P, Rha SY; KEYNOTE-811 Investigators. Pembrolizumab plus trastuzumab and chemotherapy for HER2-positive gastric or gastro-oesophageal junction adenocarcinoma: interim analyses from the phase 3 KEYNOTE-811 randomised placebo-controlled trial. Lancet. 2023 Oct 19:S0140-6736(23)02033-0. doi: 10.1016/S0140-6736(23)02033-0. Epub ahead of print. PMID: 37871604.
- 6 - Kang YK, Chen LT, Ryu MH, Oh DY, Oh SC, Chung HC, Lee KW, Omori T, Shitara K, Sakuramoto S, Chung IJ, Yamaguchi K, Kato K, Sym SJ, Kadowaki S, Tsuji K, Chen JS, Bai LY, Oh SY, Choda Y, Yasui H, Takeuchi K, Hirashima Y, Hagihara S, Boku N. Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): a randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2022 Feb;23(2):234-247. doi: 10.1016/S1470-2045(21)00692-6. Epub 2022 Jan 11. PMID: 35030335.
- 7 - Lu Z, Wang J, Shu Y, Liu L, Kong L, Yang L, Wang B, Sun G, Ji Y, Cao G, Liu H, Cui T, Li N, Qiu W, Li G, Hou X, Luo H, Xue L, Zhang Y, Yue W, Liu Z, Wang X, Gao S, Pan Y, Galais MP, Zaanan A, Ma Z, Li H, Wang Y, Shen L; ORIENT-15 study group. Sintilimab versus placebo in combination with chemotherapy as first line treatment for locally advanced or metastatic oesophageal squamous cell carcinoma (ORIENT-15): multicentre, randomised, double blind, phase 3 trial. BMJ. 2022 Apr 19;377:e068714. doi: 10.1136/bmj-2021-068714. PMID: 35440464; PMCID: PMC9016493.
- 8 - Luo H, Lu J, Bai Y, Mao T, Wang J, Fan Q, Zhang Y, Zhao K, Chen Z, Gao S, Li J, Fu Z, Gu K, Liu Z, Wu L, Zhang X, Feng J, Niu Z, Ba Y, Zhang H, Liu Y, Zhang L, Min X, Huang J, Cheng Y, Wang D, Shen Y, Yang Q, Zou J, Xu RH; ESCORT-1st Investigators. Effect of Camrelizumab vs Placebo Added to Chemotherapy on Survival and Progression-Free Survival in Patients With Advanced or Metastatic Esophageal Squamous Cell Carcinoma: The ESCORT-1st Randomized Clinical Trial. JAMA. 2021 Sep 14;326(10):916-925. doi: 10.1001/jama.2021.12836. PMID: 34519801; PMCID: PMC8441593.
- 9 - NVMO-commissie ter Beoordeling van Oncologische Middelen (BOM). Nivolumab en chemotherapie als eerstelijnsbehandeling van het gemetastaseerd HER2negatief adenocarcinoom van de maag, gastrooesofageale overgang of oesofagus. Med Oncol 2022;25(4):29-34
- 10 - NVMO-commissie ter Beoordeling van Oncologische Middelen (BOM). Pembrolizumab en chemotherapie als eerstelijnsbehandeling van het lokaal irresectabel of gemetastaseerd oesofaguscarcinoom. Med Oncol 2022;25(4):37-42
- 11 - NVMO-commissie ter Beoordeling van Oncologische Middelen (BOM). Nivolumab combinatietherapie als eerstelijnsbehandeling bij het plaveiselcelcarcinoom van de oesofagus. Med Oncol 2022; 25(10):42-48
- 12 - Shitara K, Van Cutsem E, Bang YJ, Fuchs C, Wyrwicz L, Lee KW, Kudaba I, Garrido M, Chung HC, Lee J, Castro HR, Mansoor W, Braghiroli MI, Karaseva N, Caglevic C, Villanueva L, Goekkurt E, Satake H, Enzinger P, Alsina M, Benson A, Chao J, Ko AH, Wainberg ZA, Kher U, Shah S, Kang SP, Tabernero J. Efficacy and Safety of Pembrolizumab or Pembrolizumab Plus Chemotherapy vs Chemotherapy Alone for Patients With First-line, Advanced Gastric Cancer: The KEYNOTE-062 Phase 3 Randomized Clinical Trial. JAMA Oncol. 2020 Oct 1;6(10):1571-1580. doi: 10.1001/jamaoncol.2020.3370. PMID: 32880601; PMCID: PMC7489405.
- 13 - Song Y, Zhang B, Xin D, Kou X, Tan Z, Zhang S, Sun M, Zhou J, Fan M, Zhang M, Song Y, Li S, Yuan Y, Zhuang W, Zhang J, Zhang L, Jiang H, Gu K, Ye H, Ke Y, Li J, Wang Q, Zhu J, Huang J; ASTRUM-007 investigators. First-line serplulimab or placebo plus chemotherapy in PD-L1-positive esophageal squamous cell carcinoma: a randomized, double-blind phase 3 trial. Nat Med. 2023 Feb;29(2):473-482. doi: 10.1038/s41591-022-02179-2. Epub 2023 Feb 2. PMID: 36732627; PMCID: PMC9941045.
- 14 - Sun JM, Shen L, Shah MA, Enzinger P, Adenis A, Doi T, Kojima T, Metges JP, Li Z, Kim SB, Cho BC, Mansoor W, Li SH, Sunpaweravong P, Maqueda MA, Goekkurt E, Hara H, Antunes L, Fountzilas C, Tsuji A, Oliden VC, Liu Q, Shah S, Bhagia P, Kato K; KEYNOTE-590 Investigators. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet. 2021 Aug 28;398(10302):759-771. doi: 10.1016/S0140-6736(21)01234-4. Erratum in: Lancet. 2021 Nov 20;398(10314):1874. PMID: 34454674.
- 15 - Wang ZX, Cui C, Yao J, Zhang Y, Li M, Feng J, Yang S, Fan Y, Shi J, Zhang X, Shen L, Shu Y, Wang C, Dai T, Mao T, Chen L, Guo Z, Liu B, Pan H, Cang S, Jiang Y, Wang J, Ye M, Chen Z, Jiang D, Lin Q, Ren W, Wang J, Wu L, Xu Y, Miao Z, Sun M, Xie C, Liu Y, Wang Q, Zhao L, Li Q, Huang C, Jiang K, Yang K, Li D, Liu Y, Zhu Z, Chen R, Jia L, Li W, Liao W, Liu HX, Ma D, Ma J, Qin Y, Shi Z, Wei Q, Xiao K, Zhang Y, Zhang Y, Chen X, Dai G, He J, Li J, Li G, Liu Y, Liu Z, Yuan X, Zhang J, Fu Z, He Y, Ju F, Liu Z, Tang P, Wang T, Wang W, Zhang J, Luo X, Tang X, May R, Feng H, Yao S, Keegan P, Xu RH, Wang F. Toripalimab plus chemotherapy in treatment-naïve, advanced esophageal squamous cell carcinoma (JUPITER-06): A multi-center phase 3 trial. Cancer Cell. 2022 Mar 14;40(3):277-288.e3. doi: 10.1016/j.ccell.2022.02.007. Epub 2022 Mar 3. PMID: 35245446.
- 16 - Xu J, Kato K, Raymond E, Hubner RA, Shu Y, Pan Y, Park SR, Ping L, Jiang Y, Zhang J, Wu X, Yao Y, Shen L, Kojima T, Gotovkin E, Ishihara R, Wyrwicz L, Van Cutsem E, Jimenez-Fonseca P, Lin CY, Wang L, Shi J, Li L, Yoon HH. Tislelizumab plus chemotherapy versus placebo plus chemotherapy as first-line treatment for advanced or metastatic oesophageal squamous cell carcinoma (RATIONALE-306): a global, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2023 May;24(5):483-495. doi: 10.1016/S1470-2045(23)00108-0. Epub 2023 Apr 17. PMID: 37080222.
Evidence tabellen
|
Study reference |
Study characteristics |
Patient characteristics |
Intervention (I) |
Comparison / control (C)
|
Follow-up |
Outcome measures and effect size |
Comments |
|
Duan, 2023
[individual study characteristics deduced from Duan, 2023] |
SR and meta-analysis of 9 phase III RCTs
Literature search up to June 2022
A: Luo, 2021 ESCORT-1st B: Sun, 2021 KEYNOTE-590 C: Janjigian, 2021 and Janjigian, 2023 KEYNOTE-811 D: Shitara, 2020 KEYNOTE-062 E: Janjigian, 2021 CHECKMATE-649 F: Doki, 2022 CHECKMATE-648 G: Kang, 2022 ATTRACTION-4 H: Lu, 2022 ORIENT-15 I: Wang, 2022 JUPITER-06
Source of funding: A: Jiangsu Hengrui Pharmaceuticals Co, Ltd. B: Merck Sharp & Dohme D: Merck Sharp & Dohme E: Bristol Myers Squibb and Ono Pharmaceutical G: Bristol Myers Squibb and Ono Pharmaceutical I: Shanghai Junshi Biosciences |
Inclusion criteria SR: - RCTs published in English or metastatic gastric esophageal cancer patients of any race, nationality, gender and age group were treated with immunotherapy‑ based regimens (including nivolumab + ipilimumab, nivolumab + FP/SOX, toripalimab + TP, etc.), while patients in the control group were treated with chemotherapy alone (including SOX, FP, CAPOX, TP or DP) - outcome indicators included OS and PFS in the total population, PD‑L1 CPS ≥10 and PD‑L1 <10, ORR, DCR, AEs and adverse event grade ≥3.
Exclusion criteria SR: - Duplicate literature, case reports, editorials or review literature, etc - literature with missing primary data articles
9 studies included
Population – histology A: EC - SCC B: EC/GEJC - AC/SCC C: GC/GEJC - AC D: GC/GEJC - AC E: GC/GEJC/EC - AC F: EC - SCC G: GC/GEJC - AC H: EC - SCC I: EC – SCC
Ethnicity A: Asian B: Asian/non-Asian C: Asian/non-Asian D: Asian/non-Asian E: Asian/non-Asian F: Asian/non-Asian G: Asian H: Asian/non-Asian I: Asian
Important patient characteristics at baseline:
N, median age A: 596 patients, 62 yrs B: 749 patients, 62 yrs C: 698 patients, 62 yrs D: 763 patients, 62 yrs E:1581 patients, 62 yrs F: 970 patients, 64 yrs G: 724 patients, 65 yrs H: 659 patients, 63 yrs I: 514 patients, 63 yrs
Sex (% Male): A: 87.8% B: 83.4% C: 81% D: 72.6% E: 69.6% F: 82.2% G: 72.2% H: 86.0% I: 85.0%
PD-L1 expression A: subgroups in article B: 51% CPS ≥10, 46% CPS<10, 3% unknown C: 85% CPS ≥1, 15% CPS<1 D: 37% CPS ≥10 E: 16% TPS≥1%, 84% <1% F: 49% TPS≥1%, 51% <1% or indeterminate G: 16% TPS≥1%, 84% <1% H: subgroups in article I: subgroups in article |
Describe intervention:
A: Camrelizumab + CT B: Pembrolizumab + CT C: Pembrolizumab + Trastuzumab + CT D(1): Pembrolizumab E: Nivolumab + CT F(1): Nivolumab + Ipilimumab G: Nivolumab + CT H: Sintilimab + CT I: Toripalimab + CT
Chemotherapy: A: 6 cycles of paclitaxel (175mg/m2) and cisplatin (75mg/m2) B: 5-fluorouracil 800 mg/m² on days 1–5 plus cisplatin 80 mg/m² on day 1 once every 3 weeks for up to 35 cycles C: 5-flurouacil (800 mg per m2 of body-surface area administered intravenously on days 1–5 of each 3-week cycle) and cisplatin (80 mg per m2 administered intravenously once every 3 weeks) or capecitabine (1,000 mg per m2 administered orally twice daily on days 1–14 of each 3-week cycle) and oxaliplatin (130 mg per m2 administered intravenously once every 3 weeks) for up to 35 cycles D: cisplatin 80mg/m2/d on day 1 plus fluorouracil 800mg/m2/d on days 1 to 5 or capecitabine 1000mg/m2 twice daily), E: XELOX [capecitabine 1000 mg/m² twice a day, days 1–14 and oxaliplatin 130 mg/m², day 1, every 3 weeks] or FOLFOX [leucovorin 400 mg/m², day 1, fluorouracil 400 mg/m², day 1 and 1200 mg/m², days 1–2, and oxaliplatin 85 mg/m², day 1, every 2 weeks] F: 4-week cycle of intravenous fluorouracil at a dose of 800 mg per square meter of body-surface area on days 1 through 5 and intravenous cisplatin at a dose of 80 mg per square meter on day 1 G: intravenous oxaliplatin 130 mg/m2 on day 1 plus either oral S-1 40 mg/m2 [SOX] or oral capecitabine 1000 mg/m2 [CAPOX], twice daily on days 1-14 every three weeks or cisplatin plus 5-fluorouracil (800 mg/m2 continuous infusion on days 1-5) I: paclitaxel (175 mg/m2) and cisplatin (75 mg/m2) on Day 1 of each 3-week cycle |
Describe control:
A: Placebo + CT B: Placebo + CT C: Placebo + D: Placebo + CT
E: CT F: CT
G: Placebo + CT H: Placebo + CT I: Placebo + CT
|
Follow-up (median):
A: 10.8 months B: 22.6 months C: 38.4 months (I) and 38.6 months (C) D: 29.4 months E: 13.1 months F: min. 13 months G: 26.6 months H: 16.0 months (I) and 16.9 months (C) I: 7.1 months
Discontinuation treatment (intervention/control) A: I: 220 (74%), C: 270 (91%) B: I: 328 (89%), C: 359 (97%) C: I: 286 (82%), C: 304 (88%) D: I(1): 233 (91%), I(2): 226 (88%), C: 237 (95%) E: I: 698 (88%), C: 728 (92%) F: I(1): 285 (89%), I(2): 301 (93%), C: 300 (93%) G: I: 314 (87%), C: 334 (92%) H: I: 254 (78%), C: 296 (89%) I: I: 166 (65%), C: 188 (73%)
|
I vs C
Overall survival
Effect measure: HR [95% CI]: A: 0.70 [0.56, 0.88] B: 0.73 [0.62, 0.86] C: 0.84 [0.70-1.01] D(1): 0.91 [0.74, 1.10] E: 0.80 [0.68, 0.94] F(1): 0.78 [0.62, 0.98] G: 0.90 [0.75, 1.08] H: 0.63 [0.51, 0.78] I: 0.58 [0.43, 0.78]
Pooled effect (fixed effects model): 0.74* [95% CI 0.69 to 0.80] favoring the intervention. Heterogeneity (I2): 26%, P<0.01
Overall survival – subgroup PD-L1 – CPS ≥1* D(1): 0.91 [0.69-1.18] (99.2% CI) E: 0.77 [0.64, 0.92] (99.3% CI)
Overall survival – subgroup PD-L1 – CPS ≥5* E: 0.71 [0.59, 0.86] (98.4% CI)
Overall survival – subgroup PD-L1 – CPS ≥10*
Overall survival – subgroup PD-L1 CPS≥1%*
Progression-free survival Effect measure: HR [95% CI]: A: 0.56 [0.45, 0.68] B: 0.65 [0.55, 0.76] C: 0.73 [0.61-0.87] D(1): 1.66 [1.37, 2.01] E: 0.77 [0.68, 0.87] F(1): 1.26 [1.04, 1.52] G: 0.68 [0.51, 0.90] H: 0.56 [0.46, 0.68] I: 0.58 [0.46, 0.74]
Pooled effect (fixed effects model): 0.71* [95% CI 0.59 to 0.86] favoring the intervention. Heterogeneity (I2): 87%, P<0.01
Progression-free survival – subgroup PD-L1 – CPS ≥1* D(1): 1.66 [1.37, 2.01] E: 0.74 [0.65, 0.85]
Progression-free survival – subgroup PD-L1 – CPS ≥5*
Progression-free survival – subgroup PD-L1 – CPS ≥10*
Progression-free – subgroup PD-L1 CPS≥1%*
Adverse events (≥Grade 3)
Effect measure: RR [95% CI]: A: 0.94 [0.83, 1.05] B: 1.03 [0.97, 1.10] C: 1.00 [0.85, 1.17] D(1): 0.24 [0.18, 0.33] D(2): 1.06 [0.94, 1.18] E: 1.34 [1.22, 1.48] F(1): 0.89 [0.72, 1.11] F(2): 1.33 [1.10, 1.62] G: 1.24 [0.91, 1.70] H: 1.00 [0.85, 1.17] I: 1.04 [0.94, 1.16]
Pooled effect (random effects model): 0.97 [95% CI 0.84 to 1.12] slightly favoring the intervention. Heterogeneity (I2): 93%, P=0.69
Quality of life* E: 6.9 [3.9, 9.9] versus 2.5 [-1.6, 6.6] G: 0.86 [0.70, 1.06]
FACT-E F(2): 4.98 [2.68, 7.27] versus 1.54 [-1.26, 4.33] |
Risk of bias: Tool used by authors:
A: Some concerns B: Low C: Low D: Some concerns E: Some concerns F: Low G: Low H: Some concerns I: Low
Author’s conclusion: “Immunotherapy‑based regimens are superior to standard chemotherapy in the first‑line treatment of advanced gastric oesophageal cancer, with significantly improved OS, PFS, DCR, and ORR. Furthermore, patients in the PDL1 CPS ≥10 subgroup appeared to benefit more significantly than the total population. The incidence of adverse reactions in the immunotherapy‑based group was not higher than that in the chemotherapy‑based group.” |
|
*deduced from original full-text study article: this was only done for studies with immunotherapy regimens that were available in the Netherlands when this module was written (nivolumab/pembrolizumab/ipilimumab). AC = adenocarcinoma; CPS = combined positive score; CT = chemotherapy; EC = esophageal cancer; FACT-E = Functional Assessment of Cancer Therapy-Esophagus; FACT-Ga = Functional Assessment of Cancer Therapy-Gastric; GC = gastric cancer; GEJC = gastro‑oesophageal junction cancer; HR = hazard ratio; NR = not reported; RR = risk ratio; SCC = squamous cell carcinoma |
|||||||
Evidence table for intervention studies
|
Study reference |
Study characteristics |
Patient characteristics 2 |
Intervention (I) |
Comparison / control (C) 3
|
Follow-up |
Outcome measures and effect size 4 |
Comments |
|
Song, 2023
ASTRUM-007 |
Type of study: Randomized, double-blind phase 3 trial
Setting and country: Multicenter (70 hospitals), China
Funding and conflicts of interest: Shanghai Henlius Biotech, Inc. supported in study execution, study design, data acquisition, statistical analyses and manuscript revisions.
|
Inclusion criteria: - aged 18–75 years lesion based on central imaging in accordance with RECIST v1.1 (Full eligibility criteria in the article appendix)
Exclusion criteria: - previously received PD-1 or PD-L1 inhibitors metastases (Full eligibility criteria in the article appendix)
N total at baseline: Intervention: 368 Control: 183
Population – histology:
Ethnicity: Asian
Important prognostic factors2:
Median age (IQR): I: 64 (57-68) C: 64 (57-68)
Sex: I: 86% M C: 84% M
PD-L1 expression: I: 56% 1≤CPS<10, 44% CPS≥ 10 C: 57% 1≤CPS<10, 43% CPS≥ 10
Groups comparable at baseline? Yes |
Serplulimab + CF Serplulimab (3 mg kg−1) on day 1 once every 2 weeks for up to 2 years Cisplatin (50 mg m−2) on day 1 for up to 8 cycles and 5-FU (1,200 mg m−2) continuous administration daily on days 1 and 2 of each cycle for up to 12 cycles, both administered intravenously every 2 weeks.
Treatment was continued until disease progression, intolerable toxicities, investigator decision, patient withdrawal of consent, completion of 2 years of therapy or death, whichever occurred first.
|
Placebo + CF Placebo (3 mg kg−1) on day 1 once every 2 weeks for up to 2 years Cisplatin (50 mg m−2) on day 1 for up to 8 cycles and 5-FU (1,200 mg m−2) continuous administration daily on days 1 and 2 of each cycle for up to 12 cycles, both administered intravenously every 2 weeks.
Treatment was continued until disease progression, intolerable toxicities, investigator decision, patient withdrawal of consent, completion of 2 years of therapy or death, whichever occurred first. |
Length of follow-up, median (IQR): I: 14.9 (8.8-19.7) months C: 15.0 (9.4-19.9) months
Loss-to-follow-up: Intervention: 278 (76%) discontinued treatment: 184 disease progression 31 patient decision 20 adverse event 18 died 6 physician decision 19 other reasons
Control: 165 (90%) discontinued treatment: 118 disease progression 17 patient decision 10 adverse event 10 died 4 physician decision 6 other reasons
|
Overall survival I: 15.3 months (95% CI: 14.0 to 18.6) C: 11.8 months (95% CI: 9.7 to 14.0 months) HR = 0.68 (95% CI: 0.53 to 0.87), P<0.01
Progression-free survival I: 5.8 months (95% CI: 5.7 to 6.9) C: 5.3 months (95% CI: 4.3 to 5.6) HR = 0.60 (95% CI: 0.48 to 0.75), P<0.01
Adverse events I: 201 patients (53%) C: 81 patients (48%)
Quality of life No results reported.
|
Author’s conclusion: “In conclusion, first-line serplulimab in combination with chemotherapy significantly improved PFS and OS in patients with previously untreated, PD-L1-positive, locally advanced or metastatic ESCC, compared with chemotherapy alone, with a manageable safety profile.”
N.B.: fifteen patients in the placebo plus chemotherapy group received serplulimab owing to an error in drug distribution. |
|
Xu, 2023
RATIONALE-306 |
Type of study: Randomized, double-blind phase 3 trial
Setting and country: 162 medical centres across Asia, Europe, Oceania, and North America
Funding and conflicts of interest: BeiGene had a role in study design, data collection, data analysis, data interpretation, and writing of the clinical study report. Medical writing support was provided by the funder. Detailed declarations of interests are provided in the article. |
Inclusion criteria: - aged 18 years or older - ECOG performance status of 0 or 1 (Full eligibility criteria in the article appendix)
Exclusion criteria: - brain or leptomeningeal metastases that were symptomatic or required treatment (Full eligibility criteria in the article appendix)
N total at baseline: Intervention: 326 Control: 323
Population – histology:
Ethnicity: I: 75% Asian, 24% non-Asian C: 75% Asian, 24% non-Asian
Important prognostic factors2:
Median age (IQR): I: 64.0 (59.0-68.0) C: 65.0 (58.0-70.0)
Sex: I: 87% C: 87%
PD-L1 expression: I: 36% TAP score ≥10%, 46% TAP score <10%, 18% unknown C: 33% TAP score ≥10%, 52% TAP score <10%, 15% unknown
Groups comparable at baseline? Yes |
Tislelizumab+chemotherapy Tislelizumab 200 mg intravenously every 3 weeks on day 1 of 21-day cycles, together with an investigator-chosen chemotherapy doublet.
Chemotherapy options:
Treatment was continued until investigator-assessed disease progression per RECIST version 1.1, unacceptable toxicity, death, or withdrawal of consent. |
Placebo+chemotherapy Matching placebo intravenously every 3 weeks on day 1 of 21-day cycles, together with an investigator-chosen chemotherapy doublet.
Chemotherapy options:
Treatment was continued until investigator-assessed disease progression per RECIST version 1.1, unacceptable toxicity, death, or withdrawal of consent. |
Length of follow-up, median (IQR): I: 16.4 (8.6-21.8) C: 9.8 (5.8-19.0)
Loss-to-follow-up: Intervention: 286 (88%) discontinued treatment: 177 progressive disease 41 withdrew 39 adverse event 5 treatment interruption 4 doctor’s decision 20 other (eg, death)
Control: 306 (95%) discontinued treatment: 223 progressive disease 35 withdrew 20 adverse event 1 treatment interruption 7 doctor’s decision 1 non-compliance with study drug 19 other (eg, death) |
Overall survival I: 17.2 months (95% CI: 15.8-20.1) C: 10.6 months (95% CI: 9.3-12.1) HR = 0.66 (95% CI: 0.54 to 0.80), P<0.01
Progression-free survival I: 7.3 months (95% CI: 6.9-8.3) C: 5.6 months (95% CI: 4.9-6.0) HR = 0.62 (95% CI: 0.52 to 0.75), P<0.01
Adverse events I: 216 patients (67%) C: 207 patients (64%)
Quality of life No results reported, will be published in a separate publication. |
Author’s conclusion: “First-line treatment of advanced or metastatic oesophageal squamous cell carcinoma with tislelizumab plus chemotherapy resulted in significant and clinically meaningfully overall survival benefit versus placebo plus chemotherapy. The safety profile of tislelizumab in combination with chemotherapy was manageable, with no new safety signals identified.” |
|
AC = adenocarcinoma; CF = cisplatin and 5-fluorouracil; EC = esophageal cancer; ECOG = Eastern Cooperative Oncology Group; GC = gastric cancer; GEJC = gastro‑oesophageal junction cancer; NR = not reported; SCC = squamous cell carcinoma |
|||||||
Risk of bias table for intervention studies (randomized controlled trials; based on Cochrane risk of bias tool and suggestions by the CLARITY Group at McMaster University)
|
Study reference
(first author, publication year) |
Was the allocation sequence adequately generated?
|
Was the allocation adequately concealed?
|
Blinding: Was knowledge of the allocated interventions adequately prevented?
Were patients blinded?
Were healthcare providers blinded?
Were data collectors blinded?
Were outcome assessors blinded?
Were data analysts blinded?
|
Was loss to follow-up (missing outcome data) infrequent?
|
Are reports of the study free of selective outcome reporting?
|
Was the study apparently free of other problems that could put it at a risk of bias?
|
Overall risk of bias
|
|
Luo, 2021 ESCORT-1st |
Definitely yes;
Reason: Randomization with a centralized interactive web-response system |
Probably yes;
Reason: The randomization was centralized. |
Definitely yes;
Reason: Double-blind trial. Patients, investigators, and the sponsor’s study team were masked to treatment assignment. |
Probably no;
Reason: Discontinuation treatment percentages were higher in the control group than in the intervention group. |
Definitely yes;
Reason: All relevant outcomes from the trial registry were reported. |
Definitely no;
Reason: The funder was involved in data collection, analysis, interpretation, and guaranteed the accuracy, completeness of the data, writing of the report, and the decision to submit the manuscript for publication. |
Some concerns |
|
Sun, 2021 KEYNOTE-590 |
Definitely yes;
Reason: An interactive voice response system (IVRS) or integrated web response system was used for randomization. |
Probably yes;
Reason: The randomized allocation schedule was generated by the sponsor and implemented in the IVRS. |
Definitely yes;
Reason: Double-blind trial. Patients, investigators, and site staff were masked to group assignment. |
Probably no;
Reason: Discontinuation treatment percentages were higher in the control group than in the intervention group. |
Probably yes;
Reason: All relevant outcomes from the trial registry were reported, except for quality of life (secondary outcome). |
Definitely no;
Reason: The funder participated in study design, data interpretation, and the writing of the report, and maintained the study database. Many authors have interests linked to the funding company. |
Some concerns |
|
Janjigian, 2021; Janjigian, 2023 KEYNOTE-811 |
Definitely yes;
Reason: Randomization by means of an integrated interactive voice-response and Web-response system. |
Probably yes;
Reason: Randomization was central (protocol). |
Probably yes;
Reason: Double-blind study. Outcomes were assessed by blinded, independent central review. |
Probably no;
Reason: Discontinuation treatment percentages were higher in the control group than in the intervention group. |
Probably yes;
Reason: The dual primary endpoints were reported. However, quality of life (exploratory endpoint) was not reported in the articles. |
Definitely no;
Reason: The study was designed by academic advisors and employees of the study sponsor. Many authors have interests linked to the funding company. The funder participated in the study design, data collection, data analysis, data interpretation, report writing and study database maintenance. |
Some concerns |
|
Shitara, 2020 KEYNOTE-062 |
Definitely yes;
Reason: The sponsor generated the allocation schedule using a computerized random list generator (IVRS/IWRS). |
No information. |
Probably no;
Reason: Partially blinded. Patients and site and sponsor personnel were blinded to pembrolizumab or placebo in the combination and chemotherapy groups. Investigators and patients were unblinded to pembrolizumab monotherapy. Imaging data were centrally reviewed and the central reviewer was blinded to subject treatment assignment; and allocation schedule was blinded in the database preventing aggregate analysis. |
Probably no;
Reason: Discontinuation treatment percentages were higher in the control group than in the intervention group. |
Probably yes;
Reason: All relevant outcomes from the trial registry were reported, except for quality of life (secondary outcome). |
Definitely no;
Reason: The funder was involved in the design and conduct of the study, in collection, management, analysis, and interpretation of the data. Several authors were employees of the sponsor and were involved in the review, or approval of the manuscript, and decision to submit the manuscript for publication. |
HIGH
|
|
Janjigian, 2021 CHECKMATE-649 |
Definitely yes;
Reason: Randomization with interactive web response technology. |
Probably yes;
Reason: A treatment allocation list was generated by the sponsor. The web registration system was implemented by a third party, which ensured that the assignment sequence was concealed until the treatment allocation was completed. |
Definitely no;
Reason: Open label study. Outcomes were assessed by blinded independent central review. |
Probably yes;
Reason: Discontinuation treatment percentages were similar in the control group and intervention group. |
Definitely yes;
Reason: All relevant outcomes from the trial registry were reported. |
Definitely no;
Reason: The funder of the study was involved in study design, data collection, data analysis, data interpretation, and writing of the clinical study report. Many authors have interests linked to the funding company. |
HIGH |
|
Doki, 2022 CHECKMATE-648 |
Definitely yes;
Reason: Randomization was performed using web-based interactive response technology. |
Probably yes;
Reason: A treatment allocation list was generated by the sponsor. The web registration system was implemented by a third party, which ensured that the assignment sequence was concealed until the treatment allocation was completed. |
Definitely no;
Reason: Open label study. Outcomes were assessed by blinded independent central review. |
Probably yes;
Reason: Discontinuation treatment percentages were similar in the control group and two intervention groups. |
Definitely yes;
Reason: All relevant outcomes from the trial registry were reported. |
Definitely no;
Reason: the sponsor funded the trial, provided the trial drugs, and collaborated with the academic authors on the trial design and on the collection, analysis, and interpretation of the data. |
HIGH |
|
Kang, 2022 ATTRACTION-4 |
Definitely yes;
Reason: Randomization was performed using an interactive web response system. |
Probably yes;
Reason: The allocation sequence was generated by the interactive web response system and the sponsor was masked to the allocation sequence. |
Definitely yes;
Reason: Double-blind trial. Patients, investigators, and the study sponsor were masked to the study treatment. Masking was ensured by keeping nivolumab and placebo identical in appearance, which were checked by a person responsible for investigational product allocation. |
Probably yes;
Reason: Discontinuation treatment percentages were similar in the control group and intervention group. |
Definitely yes;
Reason: All relevant outcomes from the trial registry were reported (in the article or in the appendix). |
Definitely no;
Reason: The funders of the study had a role in study design, data collection, data analysis, data interpretation, and writing of the report. Many authors have interests linked to the funding company. |
Some concerns |
|
Lu, 2022 ORIENT-15 |
Definitely yes;
Reason: Randomization was performed using an interactive web response system. |
No information. |
Probably yes;
Reason: Double-blind trial. Patients, investigators, and the sponsor’s study team were blinded to the allocation of treatment. The results of the interim analysis were reviewed by an unblinded external statistics team. |
Probably no;
Reason: Discontinuation treatment percentages were higher in the control group than in the intervention group. |
Definitely yes;
Reason: All relevant outcomes from the trial registry were reported. |
Definitely no;
Reason: The funder was involved in data analysis and interpretation, verification of data accuracy and completeness, writing of the clinical report, and the decision to submit the manuscript for publication. Several authors have interests linked to the funding company. |
Some concerns |
|
Wang, 2022 JUPITER-06 |
Definitely yes;
Reason: The patient randomization and the dispense of investigational drugs were implemented via an Interactive Web Response System (IWRS). |
Probably yes;
Reason: The random allocation sequences for patients and investigational drugs were generated and maintained by an independent unblinded statistician from a third-party vendor. |
Definitely yes;
Reason: Double-blind study. the study personnel, the investigator, study center staff, patient, sponsor, clinical contract research organization and the independent review committee were blinded. |
Probably yes;
Reason: Discontinuation treatment percentages were slightly higher in the control group than in the intervention group. |
Probably yes;
Reason: All relevant outcomes from the trial registry were reported, except for time to response and quality of life (secondary outcomes). |
Probably no;
Reason: Role of the funder is not clear. Some authors were employees of the funding company. |
LOW |
|
Song, 2023 ASTRUM-007 |
Definitely yes;
Reason: Randomization was performed using an integrated web response system. |
No information. |
Probably yes;
Reason: Double-blind study. Patients, investigators and the sponsor’s study team were masked to group assignment. The results of the final analyses were conducted by an unblinded external statistician. |
Probably no;
Reason: Discontinuation treatment percentages were higher in the control group than in the intervention group. |
Definitely yes;
Reason: All relevant outcomes from the trial registry were reported. |
Definitely no;
Reason: The funder supported in study execution, study design, data acquisition, statistical analyses and manuscript revisions. Some authors were employees of the funding company. |
Some concerns |
|
Xu, 2023 RATIONALE-306 |
Definitely yes;
Reason: Randomization was performed using an interactive response technology system. |
No information. |
Probably no;
Reason: Double-blind trial. Investigators, patients, and sponsor staff or its designees were masked to group assignment. After achieving the primary objective, the study was to be unblinded. |
Probably yes;
Reason: Discontinuation treatment percentages were slightly higher in the control group than in the intervention group. |
Definitely yes;
Reason: All relevant outcomes from the trial registry were reported (in the article or in the appendix). Quality of life will be reported in a separate publication. |
Definitely no;
Reason: The funder had a role in study design, data collection, data analysis, data interpretation, and writing of the clinical study report. |
Some concerns |
Table of excluded studies
|
Reference |
Reason for exclusion |
|
Kato K, Doki Y, Ogata T, Motoyama S, Kawakami H, Ueno M, Kojima T, Shirakawa Y, Okada M, Ishihara R, Kubota Y, Amaya-Chanaga C, Chen T, Matsumura Y, Kitagawa Y. First-line nivolumab plus ipilimumab or chemotherapy versus chemotherapy alone in advanced esophageal squamous cell carcinoma: a Japanese subgroup analysis of open-label, phase 3 trial (CheckMate 648/ONO-4538-50). Esophagus. 2023 Apr;20(2):291-301. doi: 10.1007/s10388-022-00970-1. Epub 2022 Nov 19. PMID: 36401133; PMCID: PMC10024660. |
CheckMate 648 is included in the selected systematic review (Duan, 2023)
|
|
Liu T, Bai Y, Lin X, Li W, Wang J, Zhang X, Pan H, Bai C, Bai L, Cheng Y, Zhang J, Zhong H, Ba Y, Hu W, Xu R, Guo W, Qin S, Yang N, Lu J, Shitara K, Lei M, Li M, Bao N, Chen T, Shen L. First-line nivolumab plus chemotherapy vs chemotherapy in patients with advanced gastric, gastroesophageal junction and esophageal adenocarcinoma: CheckMate 649 Chinese subgroup analysis. Int J Cancer. 2023 Feb 15;152(4):749-760. doi: 10.1002/ijc.34296. Epub 2022 Oct 31. PMID: 36121651; PMCID: PMC10092493. |
CheckMate 649 is included in the selected systematic review (Duan, 2023)
|
|
Satake H, Lee KW, Chung HC, Lee J, Yamaguchi K, Chen JS, Yoshikawa T, Amagai K, Yeh KH, Goto M, Chao Y, Lam KO, Han SR, Shiratori S, Shah S, Shitara K. Pembrolizumab or pembrolizumab plus chemotherapy versus standard of care chemotherapy in patients with advanced gastric or gastroesophageal junction adenocarcinoma: Asian subgroup analysis of KEYNOTE-062. Jpn J Clin Oncol. 2023 Mar 7;53(3):221-229. doi: 10.1093/jjco/hyac188. PMID: 36533429; PMCID: PMC9991501. |
KEYNOTE-062 is included in the selected systematic review (Duan, 2023)
|
|
Cao Y, Qin S, Luo S, Li Z, Cheng Y, Fan Y, Sun Y, Yin X, Yuan X, Li W, Liu T, Hsu CH, Lin X, Kim SB, Kojima T, Zhang J, Lee SH, Bai Y, Muro K, Doi T, Bai C, Gu K, Pan HM, Bai L, Yang JW, Cui Y, Lu W, Chen J. Pembrolizumab versus chemotherapy for patients with esophageal squamous cell carcinoma enrolled in the randomized KEYNOTE-181 trial in Asia. ESMO Open. 2022 Feb;7(1):100341. doi: 10.1016/j.esmoop.2021.100341. Epub 2021 Dec 29. PMID: 34973513; PMCID: PMC8764510. |
Second-line treatment (KEYNOTE-181)
|
|
Chung HC, Kang YK, Chen Z, Bai Y, Wan Ishak WZ, Shim BY, Park YL, Koo DH, Lu J, Xu J, Chon HJ, Bai LY, Zeng S, Yuan Y, Chen YY, Gu K, Zhong WY, Kuang S, Shih CS, Qin SK. Pembrolizumab versus paclitaxel for previously treated advanced gastric or gastroesophageal junction cancer (KEYNOTE-063): A randomized, open-label, phase 3 trial in Asian patients. Cancer. 2022 Mar 1;128(5):995-1003. doi: 10.1002/cncr.34019. Epub 2021 Dec 8. PMID: 34878659; PMCID: PMC9299889. |
Second-line treatment (KEYNOTE-063)
|
|
Doki Y, Ajani JA, Kato K, Xu J, Wyrwicz L, Motoyama S, Ogata T, Kawakami H, Hsu CH, Adenis A, El Hajbi F, Di Bartolomeo M, Braghiroli MI, Holtved E, Ostoich SA, Kim HR, Ueno M, Mansoor W, Yang WC, Liu T, Bridgewater J, Makino T, Xynos I, Liu X, Lei M, Kondo K, Patel A, Gricar J, Chau I, Kitagawa Y; CheckMate 648 Trial Investigators. Nivolumab Combination Therapy in Advanced Esophageal Squamous-Cell Carcinoma. N Engl J Med. 2022 Feb 3;386(5):449-462. doi: 10.1056/NEJMoa2111380. PMID: 35108470. |
CheckMate 648 is included in the selected systematic review (Duan, 2023)
|
|
Fuchs CS, Özgüroğlu M, Bang YJ, Di Bartolomeo M, Mandala M, Ryu MH, Fornaro L, Olesinski T, Caglevic C, Chung HC, Muro K, Van Cutsem E, Elme A, Thuss-Patience P, Chau I, Ohtsu A, Bhagia P, Wang A, Shih CS, Shitara K. Pembrolizumab versus paclitaxel for previously treated PD-L1-positive advanced gastric or gastroesophageal junction cancer: 2-year update of the randomized phase 3 KEYNOTE-061 trial. Gastric Cancer. 2022 Jan;25(1):197-206. doi: 10.1007/s10120-021-01227-z. Epub 2021 Sep 1. PMID: 34468869; PMCID: PMC8732941. |
Second-line treatment (KEYNOTE-061)
|
|
Janjigian YY, Van Cutsem E, Muro K, Wainberg Z, Al-Batran SE, Hyung WJ, Molena D, Marcovitz M, Ruscica D, Robbins SH, Negro A, Tabernero J. MATTERHORN: phase III study of durvalumab plus FLOT chemotherapy in resectable gastric/gastroesophageal junction cancer. Future Oncol. 2022 Jun;18(20):2465-2473. doi: 10.2217/fon-2022-0093. Epub 2022 May 10. PMID: 35535555. |
Study protocol (MATTERHORN)
|
|
Kang YK, Chen LT, Ryu MH, Oh DY, Oh SC, Chung HC, Lee KW, Omori T, Shitara K, Sakuramoto S, Chung IJ, Yamaguchi K, Kato K, Sym SJ, Kadowaki S, Tsuji K, Chen JS, Bai LY, Oh SY, Choda Y, Yasui H, Takeuchi K, Hirashima Y, Hagihara S, Boku N. Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): a randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2022 Feb;23(2):234-247. doi: 10.1016/S1470-2045(21)00692-6. Epub 2022 Jan 11. PMID: 35030335. |
ATTRACTION-4 is included in the selected systematic review (Duan, 2023)
|
|
Kojima T, Hara H, Tsuji A, Yasui H, Muro K, Satoh T, Ogata T, Ishihara R, Goto M, Baba H, Nishina T, Han S, Sakata T, Yatsuzuka N, Doi T, Kato K. First-line pembrolizumab + chemotherapy in Japanese patients with advanced/metastatic esophageal cancer from KEYNOTE-590. Esophagus. 2022 Oct;19(4):683-692. doi: 10.1007/s10388-022-00920-x. Epub 2022 Jun 7. PMID: 35668304; PMCID: PMC9436840. |
KEYNOTE-590 is included in the selected systematic review (Duan, 2023)
|
|
Lu Z, Wang J, Shu Y, Liu L, Kong L, Yang L, Wang B, Sun G, Ji Y, Cao G, Liu H, Cui T, Li N, Qiu W, Li G, Hou X, Luo H, Xue L, Zhang Y, Yue W, Liu Z, Wang X, Gao S, Pan Y, Galais MP, Zaanan A, Ma Z, Li H, Wang Y, Shen L; ORIENT-15 study group. Sintilimab versus placebo in combination with chemotherapy as first line treatment for locally advanced or metastatic oesophageal squamous cell carcinoma (ORIENT-15): multicentre, randomised, double blind, phase 3 trial. BMJ. 2022 Apr 19;377:e068714. doi: 10.1136/bmj-2021-068714. PMID: 35440464; PMCID: PMC9016493. |
ORIENT-15 is included in the selected systematic review (Duan, 2023)
|
|
Okada M, Kato K, Cho BC, Takahashi M, Lin CY, Chin K, Kadowaki S, Ahn MJ, Hamamoto Y, Doki Y, Yen CC, Kubota Y, Kim SB, Hsu CH, Holtved E, Xynos I, Matsumura Y, Takazawa A, Kitagawa Y. Three-Year Follow-Up and Response-Survival Relationship of Nivolumab in Previously Treated Patients with Advanced Esophageal Squamous Cell Carcinoma (ATTRACTION-3). Clin Cancer Res. 2022 Aug 2;28(15):3277-3286. doi: 10.1158/1078-0432.CCR-21-0985. PMID: 35294546; PMCID: PMC9662935. |
Second-line treatment (ATTRACTION-3)
|
|
Shen L, Kato K, Kim SB, Ajani JA, Zhao K, He Z, Yu X, Shu Y, Luo Q, Wang J, Chen Z, Niu Z, Zhang L, Yi T, Sun JM, Chen J, Yu G, Lin CY, Hara H, Bi Q, Satoh T, Pazo-Cid R, Arkenau HT, Borg C, Lordick F, Li L, Ding N, Tao A, Shi J, Van Cutsem E; RATIONALE-302 Investigators. Tislelizumab Versus Chemotherapy as Second-Line Treatment for Advanced or Metastatic Esophageal Squamous Cell Carcinoma (RATIONALE-302): A Randomized Phase III Study. J Clin Oncol. 2022 Sep 10;40(26):3065-3076. doi: 10.1200/JCO.21.01926. Epub 2022 Apr 20. PMID: 35442766; PMCID: PMC9462531. |
Second-line treatment (RATIONALE-302)
|
|
Stein A, Paschold L, Tintelnot J, Goekkurt E, Henkes SS, Simnica D, Schultheiss C, Willscher E, Bauer M, Wickenhauser C, Thuss-Patience P, Lorenzen S, Ettrich T, Riera-Knorrenschild J, Jacobasch L, Kretzschmar A, Kubicka S, Al-Batran SE, Reinacher-Schick A, Pink D, Sinn M, Lindig U, Hiegl W, Hinke A, Hegewisch-Becker S, Binder M. Efficacy of Ipilimumab vs FOLFOX in Combination With Nivolumab and Trastuzumab in Patients With Previously Untreated ERBB2-Positive Esophagogastric Adenocarcinoma: The AIO INTEGA Randomized Clinical Trial. JAMA Oncol. 2022 Aug 1;8(8):1150-1158. doi: 10.1001/jamaoncol.2022.2228. PMID: 35737383; PMCID: PMC9227706. |
Wrong comparison (Ipilimumab + Nivolumab + Trastuzumab versus FOLFOX + Nivolumab + Trastuzumab) (INTEGA) |
|
Tabernero J, Shen L, Elimova E, Ku G, Liu T, Shitara K, Lin X, Boyken L, Li H, Grim J, Ajani J. HERIZON-GEA-01: Zanidatamab + chemo ± tislelizumab for 1L treatment of HER2-positive gastroesophageal adenocarcinoma. Future Oncol. 2022 Sep;18(29):3255-3266. doi: 10.2217/fon-2022-0595. Epub 2022 Aug 24. PMID: 36000541. |
Design paper (HERIZON-GEA-01) |
|
Wang ZX, Cui C, Yao J, Zhang Y, Li M, Feng J, Yang S, Fan Y, Shi J, Zhang X, Shen L, Shu Y, Wang C, Dai T, Mao T, Chen L, Guo Z, Liu B, Pan H, Cang S, Jiang Y, Wang J, Ye M, Chen Z, Jiang D, Lin Q, Ren W, Wang J, Wu L, Xu Y, Miao Z, Sun M, Xie C, Liu Y, Wang Q, Zhao L, Li Q, Huang C, Jiang K, Yang K, Li D, Liu Y, Zhu Z, Chen R, Jia L, Li W, Liao W, Liu HX, Ma D, Ma J, Qin Y, Shi Z, Wei Q, Xiao K, Zhang Y, Zhang Y, Chen X, Dai G, He J, Li J, Li G, Liu Y, Liu Z, Yuan X, Zhang J, Fu Z, He Y, Ju F, Liu Z, Tang P, Wang T, Wang W, Zhang J, Luo X, Tang X, May R, Feng H, Yao S, Keegan P, Xu RH, Wang F. Toripalimab plus chemotherapy in treatment-naïve, advanced esophageal squamous cell carcinoma (JUPITER-06): A multi-center phase 3 trial. Cancer Cell. 2022 Mar 14;40(3):277-288.e3. doi: 10.1016/j.ccell.2022.02.007. Epub 2022 Mar 3. PMID: 35245446. |
JUPITER-06 is included in the selected systematic review (Duan, 2023)
|
|
Xu J, Li Y, Fan Q, Shu Y, Yang L, Cui T, Gu K, Tao M, Wang X, Cui C, Xu N, Xiao J, Gao Q, Liu Y, Zhang T, Bai Y, Li W, Zhang Y, Dai G, Ma D, Zhang J, Bai C, Huang Y, Liao W, Wu L, Chen X, Yang Y, Wang J, Ji S, Zhou H, Wang Y, Ma Z, Wang Y, Peng B, Sun J, Mancao C. Clinical and biomarker analyses of sintilimab versus chemotherapy as second-line therapy for advanced or metastatic esophageal squamous cell carcinoma: a randomized, open-label phase 2 study (ORIENT-2). Nat Commun. 2022 Feb 14;13(1):857. doi: 10.1038/s41467-022-28408-3. PMID: 35165274; PMCID: PMC8844279. |
Second-line treatment, phase II trial (ORIENT-2)
|
Verantwoording
Beoordelingsdatum en geldigheid
Publicatiedatum : 18-12-2024
Beoordeeld op geldigheid : 01-09-2024
Algemene gegevens
De ontwikkeling/herziening van deze richtlijnmodule werd ondersteund door het Kennisinstituut van de Federatie Medisch Specialisten (www.demedischspecialist.nl/kennisinstituut) en werd gefinancierd uit de Kwaliteitsgelden Medisch Specialisten (SKMS). De financier heeft geen enkele invloed gehad op de inhoud van de richtlijnmodule.
Samenstelling werkgroep
Voor het ontwikkelen van de richtlijnmodule is in 2021 een multidisciplinair cluster ingesteld, bestaande uit vertegenwoordigers van alle relevante specialismen (zie hiervoor de Samenstelling van het cluster) die betrokken zijn bij de zorg voor patiënten met oesofagus- en maagcarcinoom.
Het cluster oesofagus- en maagcarcinoom bestaat uit meerdere richtlijnen, zie hier voor de actuele clusterindeling. De stuurgroep bewaakt het proces van modulair onderhoud binnen het cluster. De expertisegroepsleden worden indien nodig gevraagd om hun expertise in te zetten voor een specifieke richtlijnmodule. Het cluster oesofagus- en maagcarcinoom bestaat uit de volgende personen:
Clusterstuurgroep
- Dhr. Prof. Dr. P.D. (Peter) Siersema (voorzitter), maag-darm-leverarts, Erasmus MC, Rotterdam; NVMDL
- Mevr. Dr. R.E. (Roos) Pouw, maag-darm-leverarts, Amsterdam UMC, Amsterdam; NVMDL
- Mevr. Dr. A. (Annemarieke) Bartels – Rutten, radioloog, NKI-AVL, Amsterdam; NVvR
- Dhr. Prof. Dr. M.I. (Mark) van Berge Henegouwen, chirurg, Amsterdam UMC, Amsterdam; NVvH
- Dhr. Prof. Dr. R. (Richard) van Hillegersberg, chirurg, UMC Utrecht, Utrecht; NVvH
- Dhr. M.C.C.M. (Maarten) Hulshof MD PhD, Radiotherapeut, Amsterdam UMC, Amsterdam; NVRO (neemt geen deel meer)
- Mevr. Dr. H.W.M. (Hanneke) van Laarhoven, internist, Amsterdam UMC, Amsterdam; NIV
- Mevr. Dr. E.M. (Liesbeth) Timmermans, bestuurslid Stichting voor Patiënten met Kanker aan het Spijsverteringskanaal; SPKS (tot 1 december 2023)
- Dhr. Dr. E. (Erik) Vegt, nucleair geneeskundige, Erasmus MC, Rotterdam; NVNG
Clusterexpertisegroep
- Dhr. Drs. W.W. (Weibel) Braunius, keel-neus-oorarts, UMC Utrecht, Utrecht; NVKNO
- Mevr. Dr. M.J. (Marc) van Det, chirurg, Ziekenhuisgroep Twente; NVvH
- Mevr. Dr. S.S. (Suzanne) Gisbertz, chirurg, Amsterdam UMC, Amsterdam; NVvH
- Mevr. Dr. N.C.T. (Nicole) van Grieken, patholoog, Amsterdam UMC, Amsterdam; NVVP
- Dhr. R. (Ronald) Hoekstra, internist, Ziekenhuisgroep Twente; NIV
- Dhr. R. (Remco) Huiszoon MBA, ervaringsdeskundige en bestuurslid Stichting voor Patiënten met kanker aan het Spijsverteringskanaal excl. darmkanker; SPKS
- Dhr. P.M. (Paul) Jeene MD, radiotherapeut, Radiotherapiegroep; NVRO
- Dhr. Dr. S.M. (Sjoerd) Lagarde, chirurg, Erasmus MC, Rotterdam; NVvH
- Dhr. Dr. R.W.F. (Roelof) van Leeuwen, ziekenhuisapotheker, Erasmus MC, Rotterdam; NVZA
- Dhr. Dr. S.L. (Sybren) Meijer, patholoog, Amsterdam UMC, Amsterdam; NVVP
- Mevr. B. (Bianca) Mostert, internist, Erasmus MC, Rotterdam; NIV
- Mevr. C.T. (Kristel) Muijs MD PhD, radiotherapeut, UMCG, Groningen; NVRO
- Mevr. L. (Luidmila) Peppelenbosch – Kodach, patholoog, NKI-AVL, Amsterdam; NVVP
- Mevr. Drs. H. (Heidi) Rütten, radiotherapeut, Radboud UMC, Nijmegen; NVRO
- Mevr. M. (Marije) Slingerland, internist, LUMC, Leiden; NIV
- Mevr. Prof. Dr. V.M.C.W. (Manon) Spaander, maag-darm-leverarts, Erasmus MC, Rotterdam; NVMDL
- Mevr. M.E. (Manon) Dik, verpleegkundig specialist, Ziekenhuisgroep Twente; V&VN
- Dhr. C.C.G. (Carlo) Schippers, verpleegkundig specialist, UMC Utrecht, Utrecht; V&VN
Met ondersteuning van:
- Mevr. S.N. (Sarah) van Duijn MSc, adviseur, Kennisinstituut van de Federatie Medisch Specialisten
- Mevr. M. (Miriam) te Lintel Hekkert MSc, adviseur, Kennisinstituut van de Federatie Medisch Specialisten
- Mevr. Dr. C.M.W. (Charlotte) Gaasterland, adviseur, Kennisinstituut van de Federatie Medisch Specialisten
Belangenverklaringen
De Code ter voorkoming van oneigenlijke beïnvloeding door belangenverstrengeling is gevolgd. Alle clusterleden hebben schriftelijk verklaard of zij in de laatste drie jaar directe financiële belangen (betrekking bij een commercieel bedrijf, persoonlijke financiële belangen, onderzoeksfinanciering) of indirecte belangen (persoonlijke relaties, reputatiemanagement) hebben gehad. Gedurende de ontwikkeling of herziening van een module worden wijzigingen in belangen aan de voorzitter doorgegeven. De belangenverklaring wordt opnieuw bevestigd tijdens de commentaarfase.
Een overzicht van de belangen van de clusterleden en het oordeel over het omgaan met eventuele belangen vindt u in onderstaande tabel. De ondertekende belangenverklaringen zijn op te vragen bij het secretariaat van het Kennisinstituut van de Federatie Medisch Specialisten.
Clusterstuurgroep
|
Clusterlid |
Functie |
Nevenfuncties |
Gemelde belangen |
Ondernomen actie |
|
Siersema (Voorzitter) |
MDL-arts en Hoofd Endoscopisch Centrum, Radboud University Medical Center
|
Editor in Chief, Endoscopy |
Research funding/advisory board zonder invloed op deze richtlijn |
Geen restrictie |
|
Pouw (tijdelijke voorzitter) |
MDL-arts Amsterdam UMC |
Bestuurslid DUCG - onbetaald. Bestuurslid young ISDE - onbetaald. Bestuurslid Barrett Expertise Centra - onbetaald. Lid beoordelingscommissie ontwikkeling en implementatie KWF - onbetaald. Nationaal afgevaardigde NVMDL voor UEG - onbetaald. Projectleider KWF (PREFER studie). Studie protocol (inclusief inclusie criteria) PREFER studie staat vast. Publicatie resultaten worden pas over vijf jaar verwacht, ruim na datum publicatie richtlijnmodule endoscopische behandeling vroegcarcinoom maag. |
Betaalde deelname aan onderwijscursus georganiseerd door Medtronic Betaald adviseurschap voor Medtronic BV. (scholing en webminar endoscopische behandeling vroege afwijking in slokdarm, geen belang bij gebruik producten) Betaald adviseurschap voor MicroTech Europe (webminar, symposium, m.n. behandeling van lekkages na slokdarmoperaties) |
a) Werkgroeplid werkt niet als enige inhoudsdeskundige aan de module;
|
|
Timmermans |
- Bestuurslid SPKS (Stichting voor Patiënten met kanker aan het Spijsverteringskanaal) 5 uur per week - Gedragswetenschappelijk docent huisartsenopleiding Eerstelijnsgeneeskunde Radboudumc |
Onbetaald vrijwilligerswerk Bestuurslid SPKS (15 uur per week) |
Geen |
Neemt geen deel meer aan cluster - Geen restrictie
|
|
Van Laarhoven |
Hoofd afdeling medische oncologie, Amsterdam UMC |
- Wetenschappelijke raad KWF (onbetaald) - Voorzitter ESMO upper GI faculty (onbetaald) - Lid ESMO Leadership Generation programme (onbetaald) - Lid EORTC upper GI strategy commiittee (onbetaald) |
- Consultant or advisory role: Amphera, AstraZeneca, Beigene, BMS, Daiichy-Sankyo, Dragonfly, Eli Lilly, MSD, Nordic Pharma, Servier - Research funding and/or medication supply: Bayer, BMS, Celgene, Janssen, Incyte, Eli Lilly, MSD, Nordic Pharma, Philips, Roche, Servier - Speaker role: Astellas, Benecke, Daiichy-Sankyo, JAAP, Medtalks, Novartis, Travel Congress Management B.V Employment and leadership: Amsterdam UMC, the Netherlands (head of the department of medical oncology) Honorary: ESMO (chair upper GI faculty) |
Geen restrictie |
|
Bartels |
Radioloog, Antoni van Leeuwenhoek |
Geen |
Geen |
Geen restrictie |
|
Van Berge Henegouwen |
Chirurg slokdarm en maagchirurgie Amsterdam UMC Hoogleraar slokdarm en maagchirurgie Universiteit van Amsterdam |
- bestuur DUCA, DICA en voorzitter werkgroep Upper GI (allen onbetaald). |
- Olympus financiering studie (researcher initiated grant) Stryker financiering studie (researcher initiated grant) uitkomsten richtlijn geen invloed op deze bedrijven of studies - Consultancy voor meerdere bedrijven (B. Braun en Viatris) (uitbetaling aan Amsterdam UMC), niet gerelateerd aan richtlijn. |
Geen restrictie |
|
Hulshof |
Radiotherapeut oncoloog Amsterdam UMC |
Geen |
Geen |
Neemt geen deel meer aan cluster - Geen restrictie |
|
Van Hillegersberg |
Chirurg, UMC Utrecht |
Proctor Intuitive Surgical Consultant Medtronic |
- Bestuur DUCA, DICA |
Geen restrictie |
|
Vegt |
Nucleair geneeskundige, Afdeling Radiologie en Nucleaire Geneeskunde, Erasmus MC, Rotterdam |
Geen |
- ZonMW-subsidie voor de PLASTIC-studie, programma doelmatigheid van zorg, naar de kosten-effectiviteit van FDG-PET/CT en laparoscopie bij maagcarcinoom. |
Geen restrictie |
|
Van Rossum |
[volgt] |
[volgt] |
[volgt] |
[volgt] |
Clusterexpertisegroep
|
Clusterlid |
Functie |
Nevenfuncties |
Gemelde belangen |
Ondernomen actie |
|
Huiszoon |
ING Bank N.V. Agile coach expert, full-time |
Buddy voor slokdarmkanker patiënten bij het SPKS, onbetaald. Vanuit persoonlijke ervaring 'klankbord' zijn voor patiënten die nu dezelfde ziekte hebben als ik in 2017 heb gehad |
Neemt deel namens SPKS en hoopt vanuit dat perspectief als ervaringsdeskundige bij te kunnen dragen. Geen boegbeeldfunctie of ander belang |
Geen restrictie |
|
Braunius |
Oncologisch Hoofd-Halschirurg UMC Utrecht Cancer Center |
Geen |
Geen |
Geen restrictie |
|
Van Leeuwen |
Ziekenhuisapotheker - Erasmus MC Afdelingen Apotheek (80%) en Interne Oncologie (20%) |
SIG Oncologie NVZA - onbetaald Werkgroep Geneesmiddel Interacties NVZA/KNMP (betaald "onkosten") Onderwijs PAO Farmacie (betaald "onkosten") |
Industrie: Geneesmiddelen onderzoek i.s.m. Roche, Astellas, BMS, Servier, Boehringer (unrestricted research grants) Fondsen: Stichting Coolsingel, Stichting de Merel, Stichting Mitialto Geen conflict of interest |
a) Werkgroeplid werkt niet als enige inhoudsdeskundige aan de module; b) Werkgroeplid werkt tenminste samen met een ander werkgroeplid met vergelijkbare expertise in alle fasen (studieselectie, data-extractie, evidence synthese, evidence-to-decision, aanbevelingen formuleren) van het ontwikkelproces; c) In alle fasen van het ontwikkelproces is een onafhankelijk methodoloog betrokken; d) Overwegingen en aanbevelingen worden besproken en vastgesteld tijdens een werkgroepvergadering onder leiding van een onafhankelijk voorzitter (zonder gemelde belangen) |
|
Jeene |
Radiotherapeut - Oncoloog bij Radiotherapiegroep PhD candidate - AmsterdamUMC ( 0 uren aanstelling) |
Bestuurslid DUCG - onbetaald |
Studie coördinator en eerste auteur POLDER trail (effectiviteit kortdurende uitwendige radiotherapie, geen externe financiering). PICO is anders dan studie |
Geen restrictie |
|
Gisbertz |
Slokdarmkanker en maagkanker chirurg - Amsterdam UMC |
Bestuur ESDE, NVGIC, ISDE, research commitee EAES, de Groene OK: allen onbetaald |
Extern gefinancierd onderzoek: KWF: SQA n observational studies (projectleider) CCA: USPIO enhanced MRI in esophageal cancer (projectleider) |
Geen restrictie |
|
Lagarde |
Chirug, Erasmus MC, Rotterdam |
- lid wetenschappelijke commissie DKCA - bestuurslid werkgroep Upper Gi beiden onbetaald |
Geen |
Geen restrictie |
|
Hoekstra |
Internist-oncoloog, Ziekenhuisgroep Twente (ZGT) |
Lid Concillium Medicinae Internae (onbetaald) |
- Als internist-oncoloog betrokken bij inclusie van patiënten in klinische studies bij oesofagus- en maagcarcinoom. Op dit moment Critics-2 studie en Lyrics studie |
Geen restrictie |
|
Van Det |
Gastro-intestinaal chirurg Ziekenhuis groep Twente (ZGT) |
- Proctor/Instructor voor Intuitive Surgical betreffende Robot-Assisted operaties in de upper-GI zoals: - Slokdarm resecties - Maagresecties - Hernia diafragmatica. |
Geen |
Geen restrictie |
|
Rütten |
Radiotherapeut, Radboud UMC |
Geen |
Geen |
Geen restrictie |
|
Van Grieken |
Patholoog, Amsterdam UMC (locatie Vumc), Amsterdam |
Detachering Expertisepanel poliepen BVO-DK, Screeningsorganisatie BVO darmkanker (3 uur/week) |
- KWF - Identificatie van markers voor response op immunotherapie - projectleider - KWF - CRITICS-II klinische trial voor resectabel maagcarcinoom - ZonMW - Effect van chemotherapie bij patienten met microsatelliet instabiel resectabel maagcarcinoom. – projectleider - advisory boards van BMS, MSD |
a) Werkgroeplid werkt niet als enige inhoudsdeskundige aan de module; |
|
Mostert |
Internist-oncoloog, Erasmus MC |
Consultancy voor: BMS, Lilly, Servier |
- BMS: fase 2 studie: nivolumab tijdens actieve surveillance slokdarmcarcinoom Sanofi: cabazitaxel bij AR-v7 positieve prostaatcarcinoom patiënten Pfizer: DLA bij mammacarcinoompatiënten behandeld met CDK4/6 De f1/2 studie betreft research support`; investigator initiated studie waarvoor BMS de medicatie “schenkt” Astra zeneca betreft advisory board. |
a) Werkgroeplid werkt niet als enige inhoudsdeskundige aan de module; |
|
Slingerland |
Internist-oncoloog LUMC |
Geen |
- Advisory board Lilly, Astra Zeneca en BMS |
a) Werkgroeplid werkt niet als enige inhoudsdeskundige aan de module; |
|
Spaander |
MDL-arts 1.0 Fte in Erasmus Universiteit MC (betaald) Voor 6 uur per week gedetacheerd aan de screeningorganisatie voor het BVO darmkanker (betaald) |
Voorzitter NVMDL en NVGE oncologie commissie (onbetaald) |
ZonMW: Gender differencees in Barrett Surveillance, projectleidersrol. Capsulomics: Biomarkers in Barrett slokdarm, projectleidersrol. Lucid: Non-ionvasive tool for Barrett surveillance. Microtech: New Esophageal stent. CELTIC: Blood test bij FIT +patiënten
|
Geen restrictie |
|
Meijer |
Patholoog, Amsterdam UMC |
Geen |
Geen |
Geen restrictie |
|
Peppelenbosch - Kodach |
Patholoog, NKI/AVL |
Geen |
Deelname studie inter-observer variabiliteit voor PD-L1 CPS in maagcarcinomen, gefinancierd door BMS, fee naar de werkgever AVL/NKI |
Geen restrictie |
|
Muijs |
Radiotherapeut-Oncoloog Universitaire Medisch Centrum Groningen |
Lid wetenschapscommissie DUCA (Gemandateerde NVRO) Lid werkgroep indicatie protocol protonen radiotherapie (NVRO) |
Project Leider Models Project (KWf funded): ontwikkelen en valideren predictiemodellen voor complicaties na CRT en resectie Principle investigator CLARIFY studie (KWF funded): Observationeel onderzoek naar pulmonale hupertensie als complicatie na thoracale RT Participatie in PROTECT studie (EU funded): RCT fase 3: fotonen vs protonen bij nCRT voor oesofaguscarcinoom Voortrekker protonen RT bij het oesofaguscarcinoom |
Geen restrictie |
|
Dik |
[volgt] |
[volgt] |
[volgt] |
[volgt] |
|
Schippers |
[volgt] |
[volgt] |
[volgt] |
[volgt] |
Inbreng patiëntenperspectief
Er werd aandacht besteed aan het patiëntenperspectief door de afvaardiging van de Stichting voor Patiënten met kanker aan het Spijsverteringskanaal (SPKS). De verkregen input is meegenomen bij het opstellen van de uitgangsvragen, de keuze voor de uitkomstmaten en bij het opstellen van de overwegingen. De conceptmodule is tevens voor commentaar voorgelegd aan SPKS en de eventueel aangeleverde commentaren zijn bekeken en verwerkt.
Kwalitatieve raming van mogelijke financiële gevolgen in het kader van de Wkkgz
Bij de richtlijn is conform de Wet kwaliteit, klachten en geschillen zorg (Wkkgz) een kwalitatieve raming uitgevoerd of de aanbevelingen mogelijk leiden tot substantiële financiële gevolgen. Bij het uitvoeren van deze beoordeling zijn richtlijnmodules op verschillende domeinen getoetst (zie het stroomschema op de Richtlijnendatabase).
Uit de kwalitatieve raming blijkt dat er waarschijnlijk geen substantiële financiële gevolgen zijn, zie onderstaande tabel.
Module |
Uitkomst raming |
Toelichting |
|
‘Palliatieve immuuntherapie’ (oesofagus- en maagcarcinoom) |
Geen financiële gevolgen |
Hoewel uit de toetsing volgt dat de aanbeveling(en) breed toepasbaar zijn (5.000-40.000 patiënten), volgt ook uit de toetsing dat [het overgrote deel (±90%) van de zorgaanbieders en zorgverleners al aan de norm voldoet OF het geen nieuwe manier van zorgverlening of andere organisatie van zorgverlening betreft]. Er worden daarom geen financiële gevolgen verwacht. |
Werkwijze
AGREE
Deze richtlijnmodule is opgesteld conform de eisen vermeld in het rapport Medisch Specialistische Richtlijnen 2.0 van de adviescommissie Richtlijnen van de Raad Kwaliteit. Dit rapport is gebaseerd op het AGREE II instrument (Appraisal of Guidelines for Research & Evaluation II; Brouwers, 2010).
Need-for-update, prioritering en uitgangsvragen
Tijdens de need-for-update fase inventariseerde het cluster de geldigheid van de modules binnen het cluster. Naast de betrokken wetenschappelijke verenigingen en patiëntenorganisaties zijn hier ook andere stakeholders voor benaderd in juni 2021.
Per module is aangegeven of deze geldig is, kan worden samengevoegd met een andere module, obsoleet is en kan vervallen of niet meer geldig is en moet worden herzien. Ook was er de mogelijkheid om nieuwe onderwerpen voor modules aan te dragen die aansluiten bij één (of meerdere) richtlijn(en) behorend tot het cluster. De modules die door één of meerdere partijen werden aangekaart als ‘niet geldig’ zijn meegegaan in de prioriteringsfase. Deze module is geprioriteerd door het cluster.
Voor de geprioriteerde modules zijn door het cluster concept-uitgangsvragen herzien of opgesteld en definitief vastgesteld.
Uitkomstmaten
Na het opstellen van de zoekvraag behorende bij de uitgangsvraag inventariseerde het cluster welke uitkomstmaten voor de patiënt relevant zijn, waarbij zowel naar gewenste als ongewenste effecten werd gekeken. Hierbij werd een maximum van acht uitkomstmaten gehanteerd. Het cluster waardeerde deze uitkomstmaten volgens hun relatieve belang bij de besluitvorming rondom aanbevelingen als cruciaal (kritiek voor de besluitvorming), belangrijk (maar niet cruciaal) en onbelangrijk. Het cluster definieerde klinisch (patiënt) relevante verschillen, tenminste voor de cruciale uitkomstmaten.
Methode literatuursamenvatting
Een uitgebreide beschrijving van de strategie voor zoeken en selecteren van literatuur is te vinden onder ‘Zoeken en selecteren’ onder Onderbouwing. Indien mogelijk werd de data uit verschillende studies gepoold in een random-effects model. Review Manager 5.4 werd gebruikt voor de statistische analyses. De beoordeling van de kracht van het wetenschappelijke bewijs wordt hieronder toegelicht.
Beoordelen van de kracht van het wetenschappelijke bewijs
De kracht van het wetenschappelijke bewijs werd bepaald volgens de GRADE-methode. GRADE staat voor ‘Grading Recommendations Assessment, Development and Evaluation’ (zie http://www.gradeworkinggroup.org/). De basisprincipes van de GRADE-methodiek zijn: het benoemen en prioriteren van de klinisch (patiënt) relevante uitkomstmaten, een systematische review per uitkomstmaat, en een beoordeling van de bewijskracht per uitkomstmaat op basis van de acht GRADE-domeinen (domeinen voor downgraden: risk of bias, inconsistentie, indirectheid, imprecisie, en publicatiebias; domeinen voor upgraden: dosis-effect relatie, groot effect, en residuele plausibele confounding).
GRADE onderscheidt vier gradaties voor de kwaliteit van het wetenschappelijk bewijs: hoog, redelijk, laag en zeer laag. Deze gradaties verwijzen naar de mate van zekerheid die er bestaat over de literatuurconclusie, in het bijzonder de mate van zekerheid dat de literatuurconclusie de aanbeveling adequaat ondersteunt (Schünemann, 2013; Hultcrantz, 2017).
|
Definitie |
|
|
Hoog |
|
|
Redelijk |
|
|
Laag |
|
|
Zeer laag |
|
Bij het beoordelen (graderen) van de kracht van het wetenschappelijk bewijs in richtlijnen volgens de GRADE-methodiek spelen grenzen voor klinische besluitvorming een belangrijke rol (Hultcrantz, 2017). Dit zijn de grenzen die bij overschrijding aanleiding zouden geven tot een aanpassing van de aanbeveling. Om de grenzen voor klinische besluitvorming te bepalen moeten alle relevante uitkomstmaten en overwegingen worden meegewogen.
De grenzen voor klinische besluitvorming zijn daarmee niet één op één vergelijkbaar met het minimaal klinisch relevant verschil (Minimal Clinically Important Difference, MCID).
Met name in situaties waarin een interventie geen belangrijke nadelen heeft en de kosten relatief laag zijn, kan de grens voor klinische besluitvorming met betrekking tot de effectiviteit van de interventie bij een lagere waarde (dichter bij het nuleffect) liggen dan de MCID (Hultcrantz, 2017).
Overwegingen (van bewijs naar aanbeveling)
Om te komen tot een aanbeveling zijn naast (de kwaliteit van) het wetenschappelijke bewijs ook andere aspecten belangrijk en deze worden meegewogen, zoals aanvullende argumenten uit bijvoorbeeld de biomechanica of fysiologie, waarden en voorkeuren van patiënten, kosten (middelenbeslag), aanvaardbaarheid, haalbaarheid en implementatie. Deze aspecten zijn systematisch vermeld en beoordeeld (gewogen) onder het kopje ‘Overwegingen’ en kunnen (mede) gebaseerd zijn op expert opinion. Hierbij is gebruik gemaakt van een gestructureerd format gebaseerd op het evidence-to-decision framework van de internationale GRADE Working Group (Alonso-Coello, 2016a; Alonso-Coello 2016b). Dit evidence-to-decision framework is een integraal onderdeel van de GRADE methodiek.
Formuleren van aanbevelingen
De aanbevelingen geven antwoord op de uitgangsvraag en zijn gebaseerd op het beschikbare wetenschappelijke bewijs en de belangrijkste overwegingen, en een weging van de gunstige en ongunstige effecten van de relevante interventies. De kracht van het wetenschappelijk bewijs en het gewicht dat door het cluster wordt toegekend aan de overwegingen, bepalen samen de sterkte van de aanbeveling. Conform de GRADE-methodiek sluit een lage bewijskracht van conclusies in de systematische literatuuranalyse een sterke aanbeveling niet a priori uit, en zijn bij een hoge bewijskracht ook zwakke aanbevelingen mogelijk (Agoritsas, 2017; Neumann, 2016). De sterkte van de aanbeveling wordt altijd bepaald door weging van alle relevante argumenten tezamen. Het cluster heeft bij elke aanbeveling opgenomen hoe zij tot de richting en sterkte van de aanbeveling zijn gekomen.
In de GRADE-methodiek wordt onderscheid gemaakt tussen sterke en zwakke (of conditionele) aanbevelingen. De sterkte van een aanbeveling verwijst naar de mate van zekerheid dat de voordelen van de interventie opwegen tegen de nadelen (of vice versa), gezien over het hele spectrum van patiënten waarvoor de aanbeveling is bedoeld. De sterkte van een aanbeveling heeft duidelijke implicaties voor patiënten, behandelaars en beleidsmakers (zie onderstaande tabel). Een aanbeveling is geen dictaat, zelfs een sterke aanbeveling gebaseerd op bewijs van hoge kwaliteit (GRADE gradering HOOG) zal niet altijd van toepassing zijn, onder alle mogelijke omstandigheden en voor elke individuele patiënt.
|
Implicaties van sterke en zwakke aanbevelingen voor verschillende richtlijngebruikers |
||
|
|
||
|
|
Sterke aanbeveling |
Zwakke (conditionele) aanbeveling |
|
Voor patiënten |
De meeste patiënten zouden de aanbevolen interventie of aanpak kiezen en slechts een klein aantal niet. |
Een aanzienlijk deel van de patiënten zouden de aanbevolen interventie of aanpak kiezen, maar veel patiënten ook niet. |
|
Voor behandelaars |
De meeste patiënten zouden de aanbevolen interventie of aanpak moeten ontvangen. |
Er zijn meerdere geschikte interventies of aanpakken. De patiënt moet worden ondersteund bij de keuze voor de interventie of aanpak die het beste aansluit bij zijn of haar waarden en voorkeuren. |
|
Voor beleidsmakers |
De aanbevolen interventie of aanpak kan worden gezien als standaardbeleid. |
Beleidsbepaling vereist uitvoerige discussie met betrokkenheid van veel stakeholders. Er is een grotere kans op lokale beleidsverschillen. |
Organisatie van zorg
Bij de ontwikkeling van de richtlijnmodule is expliciet aandacht geweest voor de organisatie van zorg: alle aspecten die randvoorwaardelijk zijn voor het verlenen van zorg (zoals coördinatie, communicatie, (financiële) middelen, mankracht en infrastructuur). Randvoorwaarden die relevant zijn voor het beantwoorden van deze specifieke uitgangsvraag zijn genoemd bij de overwegingen. Meer algemene, overkoepelende, of bijkomende aspecten van de organisatie van zorg worden behandeld in de module Organisatie van zorg.
Commentaar- en autorisatiefase
De conceptrichtlijnmodule werd aan de betrokken (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd ter commentaar. De commentaren werden verzameld en besproken met het cluster. Naar aanleiding van de commentaren werd de conceptrichtlijnmodule aangepast en definitief vastgesteld door het cluster. De definitieve richtlijnmodule werd aan de deelnemende (wetenschappelijke) verenigingen en (patiënt)organisaties voorgelegd voor autorisatie en door hen geautoriseerd dan wel geaccordeerd.
Literatuur
Agoritsas T, Merglen A, Heen AF, Kristiansen A, Neumann I, Brito JP, Brignardello-Petersen R, Alexander PE, Rind DM, Vandvik PO, Guyatt GH. UpToDate adherence to GRADE criteria for strong recommendations: an analytical survey. BMJ Open. 2017 Nov 16;7(11):e018593. doi: 10.1136/bmjopen-2017-018593. PubMed PMID: 29150475; PubMed Central PMCID: PMC5701989.
Alonso-Coello P, Schünemann HJ, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Rada G, Rosenbaum S, Morelli A, Guyatt GH, Oxman AD; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ. 2016 Jun 28;353:i2016. doi: 10.1136/bmj.i2016. PubMed PMID: 27353417.
Alonso-Coello P, Oxman AD, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Vandvik PO, Meerpohl J, Guyatt GH, Schünemann HJ; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 2: Clinical practice guidelines. BMJ. 2016 Jun 30;353:i2089. doi: 10.1136/bmj.i2089. PubMed PMID: 27365494.
Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, Fervers B, Graham ID, Grimshaw J, Hanna SE, Littlejohns P, Makarski J, Zitzelsberger L; AGREE Next Steps Consortium. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010 Dec 14;182(18):E839-42. doi: 10.1503/cmaj.090449. Epub 2010 Jul 5. Review. PubMed PMID: 20603348; PubMed Central PMCID: PMC3001530.
Hultcrantz M, Rind D, Akl EA, Treweek S, Mustafa RA, Iorio A, Alper BS, Meerpohl JJ, Murad MH, Ansari MT, Katikireddi SV, Östlund P, Tranæus S, Christensen R, Gartlehner G, Brozek J, Izcovich A, Schünemann H, Guyatt G. The GRADE Working Group clarifies the construct of certainty of evidence. J Clin Epidemiol. 2017 Jul;87:4-13. doi: 10.1016/j.jclinepi.2017.05.006. Epub 2017 May 18. PubMed PMID: 28529184; PubMed Central PMCID: PMC6542664.
Medisch Specialistische Richtlijnen 2.0 (2012). Adviescommissie Richtlijnen van de Raad Kwalitieit. http://richtlijnendatabase.nl/over_deze_site/over_richtlijnontwikkeling.html
Neumann I, Santesso N, Akl EA, Rind DM, Vandvik PO, Alonso-Coello P, Agoritsas T, Mustafa RA, Alexander PE, Schünemann H, Guyatt GH. A guide for health professionals to interpret and use recommendations in guidelines developed with the GRADE approach. J Clin Epidemiol. 2016 Apr;72:45-55. doi: 10.1016/j.jclinepi.2015.11.017. Epub 2016 Jan 6. Review. PubMed PMID: 26772609.
Schünemann H, Brożek J, Guyatt G, et al. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group, 2013. Available from http://gdt.guidelinedevelopment.org/central_prod/_design/client/handbook/handbook.html.
Zoekverantwoording
Literature search strategy
|
Uitgangsvraag 6+7: Palliatieve immunotherapie: Rol in palliatieve (gemetastaseerd/niet-resectabel) fase van adenocarcinoom + Palliatieve immunotherapie: Rol in palliatieve (gemetastaseerd/niet-resectabel) fase van plaveiselcelcarcinoom |
|
|
Database(s): Medline, Embase |
Datum: 29-5-2023 |
|
Periode: >2015 |
|
Zoekverantwoording
|
Database |
Aantallen treffers |
Aantallen treffers na ontdubbelen |
|
Medline 29 mei 2023 |
337 |
335 |
|
Embase 29 mei 2023 |
545 |
472 |
|
Totaal |
882 |
807 |
- Aantal SRs: 427; aantal RCT’s: 380.
OVID/Medline 29 mei 2023
Ovid MEDLINE(R) ALL <1946 to May 25, 2023>
|
1 |
Stomach Neoplasms/ or exp Esophageal Neoplasms/ or linitis-plastica.ti,ab,kf. or ((carcinoma* or neoplas* or adenoma* or adenocarcinoma* or tumor* or tumour* or cancer* or oncolog* or malignan* or carcinogen* or oncogen* or anticarcinogen* or squamous*) adj5 (esophag* or oesophag* or stomach or gastric* or cardia or gastroesophag* or gastrooesophag* or oesogast* or esogast*)).ti,ab,kf. |
221200 |
|
2 |
exp Neoplasm Metastasis/ or exp Palliative Medicine/ or exp Palliative Care/ or Terminal Care/ or (metasta* or seeding* or micrometasta* or unresectab* or advanced or palliati* or terminal* or inoperab* or irresectab* or stage-4 or stage-four or stage-iv or end-of-life).ti,ab,kf. |
1809593 |
|
3 |
exp Immunotherapy/ or Antineoplastic Agents, Immunological/ or T-Lymphocytes, Cytotoxic/ or Nivolumab/ or exp Immune Checkpoint Proteins/ or Ipilimumab/ or Avelumab/ or Adjuvants, Immunologic/ or Immunotherapy, Adoptive/ or Nivolumab/ or (immunotherap* or radioimmunotherap* or immunopotentiat* or immunostimul* or immunonutrition or immunoactivator* or immunoadjuvant* or donor-lymphocyte-infusion* or chimeric-antigen-receptor-therap* or antigen-B7-H1 or antigen-B7H1 or antigen-CD274 or B7-H1-antigen or B7-H1-protein or B7-homolog-1-protein or B7H1-antigen or B7H1-protein or CD274-antigen* or PDCD1-ligand-1 or PDCD1LG1-protein or programmed-cell-death-1-ligand-1 or programmed-death-1-ligand-1-protein or programmed-death-ligand-1 or program-death-1 or protein-B7-H1 or protein-B7H1 or protein-PDCD1LG1 or checkpoint-inhibitor* or checkpoint-blockade* or anti-PD1 or anti-PD-1 or anti-PDL1 or anti-PD-L1 or anti-CTLA4 or cytotoxic-T-lymphocyte-associated-protein-4 or nivolumab or opdivo or pembrolizumab or keytruda or durvalumab or imfinzi or cemiplimab or libtayo or ipilimumab or yervoy or avelumab or bavencio or ((immun* or BRM or response-modifier or car or cytol*) adj3 (therap* or intervention* or lymphocyte* or cell* or adjuvant*))).ti,ab,kf. |
848787 |
|
4 |
1 and 2 and 3 |
3967 |
|
5 |
4 not ((Adolescent/ or Child/ or Infant/) not Adult/) |
3962 |
|
6 |
5 not ((exp animals/ or exp models, animal/) not humans/) |
3915 |
|
7 |
6 not (comment/ or editorial/ or letter/ or Case Reports/) |
3377 |
|
8 |
limit 7 to yr="2015 -Current" |
2393 |
|
9 |
(systematic-review.pt. or (meta-analysis/ or meta-analysis as topic/ or (metaanaly* or meta-analy* or metanaly*).ti,ab,kf. or systematic review/ or cochrane.jw. or (prisma or prospero).ti,ab,kf. or ((systemati* or umbrella or "structured literature") adj3 (review* or overview*)).ti,ab,kf. or (systemic* adj1 review*).ti,ab,kf. or ((systemati* or literature or database* or data-base*) adj10 search*).ti,ab,kf. or ((structured or comprehensive* or systemic*) adj3 search*).ti,ab,kf. or ((literature adj3 review*) and (search* or database* or data-base*)).ti,ab,kf. or (("data extraction" or "data source*") and "study selection").ti,ab,kf. or ("search strategy" and "selection criteria").ti,ab,kf. or ("data source*" and "data synthesis").ti,ab,kf. or (medline or pubmed or embase or cochrane).ab. or ((critical or rapid) adj2 (review* or overview* or synthes*)).ti. or (((critical* or rapid*) adj3 (review* or overview* or synthes*)) and (search* or database* or data-base*)).ab. or (metasynthes* or meta-synthes*).ti,ab,kf.)) not (comment/ or editorial/ or letter/ or ((exp animals/ or exp models, animal/) not humans/)) |
635721 |
|
10 |
8 and 9 |
156 |
|
11 |
exp randomized controlled trial/ or random*.ti,ab,kf. or ((pragmatic or practical) adj clinical trial*).ti,ab,kf. or ((non-inferiority or noninferiority or superiority or equivalence) adj3 trial*).ti,ab,kf. |
1554523 |
|
12 |
8 and 11 |
262 |
|
14 |
12 not 10 |
181 |
Embase.com 29 mei 2023
|
No. |
Query |
Results |
|
#12 |
#8 AND #11 NOT #10 |
361 |
|
#11 |
'randomized controlled trial'/exp OR random*:ti,ab,kw OR (((pragmatic OR practical) NEAR/1 'clinical trial*'):ti,ab,kw) OR ((('non inferiority' OR noninferiority OR superiority OR equivalence) NEAR/3 trial*):ti,ab,kw) |
2051905 |
|
#10 |
#8 AND #9 |
422 |
|
#9 |
('meta analysis'/exp OR 'meta analysis (topic)'/exp OR metaanaly*:ti,ab OR 'meta analy*':ti,ab OR metanaly*:ti,ab OR 'systematic review'/de OR 'cochrane database of systematic reviews'/jt OR prisma:ti,ab OR prospero:ti,ab OR (((systemati* OR umbrella OR 'structured literature') NEAR/3 (review* OR overview*)):ti,ab) OR ((systemic* NEAR/1 review*):ti,ab) OR (((systemati* OR literature OR database* OR 'data base*') NEAR/10 search*):ti,ab) OR (((structured OR comprehensive* OR systemic*) NEAR/3 search*):ti,ab) OR (((literature NEAR/3 review*):ti,ab) AND (search*:ti,ab OR database*:ti,ab OR 'data base*':ti,ab)) OR (('data extraction':ti,ab OR 'data source*':ti,ab) AND 'study selection':ti,ab) OR ('search strategy':ti,ab AND 'selection criteria':ti,ab) OR ('data source*':ti,ab AND 'data synthesis':ti,ab) OR medline:ab OR pubmed:ab OR embase:ab OR cochrane:ab OR (((critical OR rapid) NEAR/2 (review* OR overview* OR synthes*)):ti) OR ((((critical* OR rapid*) NEAR/3 (review* OR overview* OR synthes*)):ab) AND (search*:ab OR database*:ab OR 'data base*':ab)) OR metasynthes*:ti,ab OR 'meta synthes*':ti,ab) NOT (('animal'/exp OR 'animal experiment'/exp OR 'animal model'/exp OR 'nonhuman'/exp) NOT 'human'/exp) NOT ('conference abstract'/it OR 'conference review'/it OR 'editorial'/it OR 'letter'/it OR 'note'/it) |
709516 |
|
#8 |
#6 NOT ('conference abstract'/it OR 'conference review'/it OR 'editorial'/it OR 'letter'/it OR 'note'/it OR 'case report'/exp) AND [2015-2023]/py |
4925 |
|
#7 |
#6 NOT ('conference abstract'/it OR 'conference review'/it OR 'editorial'/it OR 'letter'/it OR 'note'/it OR 'case report'/exp) |
6688 |
|
#6 |
#5 NOT (('animal'/exp OR 'animal experiment'/exp OR 'animal model'/exp OR 'nonhuman'/exp) NOT 'human'/exp) |
10966 |
|
#5 |
#4 NOT (('adolescent'/exp OR 'child'/exp) NOT ('adult'/exp OR 'aged'/exp OR 'middle aged'/exp)) |
11233 |
|
#4 |
#1 AND #2 AND #3 |
11255 |
|
#3 |
'immunotherapy'/exp OR 'antineoplastic monoclonal antibody'/exp/mj OR 'programmed death 1 ligand 1'/de OR 'cytotoxic t lymphocyte'/de OR 'nivolumab'/de OR 'pembrolizumab'/de OR 'durvalumab'/de OR 'cemiplimab'/de OR 'ipilimumab'/de OR 'avelumab'/de OR 'immune checkpoint protein'/mj OR 'immunological adjuvant'/mj OR 'adoptive immunotherapy'/mj OR immunotherap*:ti,ab,kw OR radioimmunotherap*:ti,ab,kw OR immunopotentiat*:ti,ab,kw OR immunostimul*:ti,ab,kw OR immunoactivator*:ti,ab,kw OR immunoadjuvant*:ti,ab,kw OR immunonutrition:ti,ab,kw OR 'donor lymphocyte infusion*':ti,ab,kw OR 'chimeric antigen receptor therap*':ti,ab,kw OR 'antigen b7 h1':ti,ab,kw OR 'antigen b7h1':ti,ab,kw OR 'antigen cd274':ti,ab,kw OR 'b7 h1 antigen':ti,ab,kw OR 'b7 h1 protein':ti,ab,kw OR 'b7 homolog 1 protein':ti,ab,kw OR 'b7h1 antigen':ti,ab,kw OR 'b7h1 protein':ti,ab,kw OR 'cd274 antigen*':ti,ab,kw OR 'pdcd1 ligand 1':ti,ab,kw OR 'pdcd1lg1 protein':ti,ab,kw OR 'programmed cell death 1 ligand 1':ti,ab,kw OR 'programmed death 1 ligand 1 protein':ti,ab,kw OR 'programmed death ligand 1':ti,ab,kw OR 'program death 1':ti,ab,kw OR 'protein b7 h1':ti,ab,kw OR 'protein b7h1':ti,ab,kw OR 'protein pdcd1lg1':ti,ab,kw OR 'checkpoint inhibitor*':ti,ab,kw OR 'checkpoint blockade*':ti,ab,kw OR 'anti pd1':ti,ab,kw OR 'anti pd 1':ti,ab,kw OR 'anti pdl1':ti,ab,kw OR 'anti pd l1':ti,ab,kw OR 'anti ctla4':ti,ab,kw OR 'cytotoxic t lymphocyte associated protein 4':ti,ab,kw OR nivolumab:ti,ab,kw OR opdivo:ti,ab,kw OR pembrolizumab:ti,ab,kw OR keytruda:ti,ab,kw OR durvalumab:ti,ab,kw OR imfinzi:ti,ab,kw OR cemiplimab:ti,ab,kw OR libtayo:ti,ab,kw OR ipilimumab:ti,ab,kw OR yervoy:ti,ab,kw OR avelumab:ti,ab,kw OR bavencio:ti,ab,kw OR (((immun* OR brm OR 'response modifier' OR car OR cytol*) NEAR/3 (therap* OR intervention* OR lymphocyte* OR cell* OR adjuvant*)):ti,ab,kw) |
1036011 |
|
#2 |
'metastasis'/exp OR 'palliative therapy'/exp OR 'terminal care'/de OR 'inoperable cancer'/de OR 'advanced cancer'/de OR metasta*:ti,ab,kw OR seeding*:ti,ab,kw OR micrometasta*:ti,ab,kw OR unresectab*:ti,ab,kw OR advanced:ti,ab,kw OR palliati*:ti,ab,kw OR terminal*:ti,ab,kw OR inoperab*:ti,ab,kw OR irresectab*:ti,ab,kw OR 'stage 4':ti,ab,kw OR 'stage four':ti,ab,kw OR 'stage iv':ti,ab,kw OR 'end of life':ti,ab,kw |
2588179 |
|
#1 |
'stomach tumor'/exp OR 'esophagus tumor'/exp OR 'linitis plastica':ti,ab,kw OR (((carcinoma* OR neoplas* OR adenoma* OR adenocarcinoma* OR tumor* OR tumour* OR cancer* OR oncolog* OR malignan* OR carcinogen* OR oncogen* OR anticarcinogen* OR squamous*) NEAR/5 (esophag* OR oesophag* OR stomach OR gastric* OR cardia OR gastroesophag* OR gastrooesophag* OR oesogast* OR esogast*)):ti,ab,kw) |
329734 |