Veiligheidscultuur
Uitgangsvraag
Hoe bevorderen we een goede veiligheidscultuur op IC-afdelingen?
Aanbeveling
Iedere IC-afdeling werkt aantoonbaar aan een zo goed mogelijke veiligheidscultuur.
Overweeg hierbij het introduceren van:
- een educatie programma over patiëntveiligheid voor alle zorgverleners op de IC;
- een multidisciplinair moreel beraad;
- het trainen van teamsamenwerking, bijvoorbeeld met simulatietraining (CRM);
- een interventie voor het versterken van het geven en ontvangen van feedback;
- een interventie gericht op het verbeteren van psychologische veiligheid.
Overweeg het structureel meten van veiligheidscultuur.
Overwegingen
Voor- en nadelen van de interventie en de kwaliteit van het bewijs
Er is literatuuronderzoek verricht naar factoren die de veiligheidscultuur op IC-afdelingen verbeteren. Idealiter zouden er prognostische modellen worden gevonden met (voorspellende) factoren die een positieve invloed hebben op de veiligheidscultuur op een IC-afdeling. Vanwege afwezigheid van dit soort studies is een tweede PICO geformuleerd die de vergelijking maakt tussen een interventie die de veiligheidscultuur op IC-afdelingen bevordert versus geen interventie. Er zijn twee studies geïncludeerd die voldeden aan deze PICO. Beide studies rapporteerden een andere interventie: educational empowerment program (Amiri, 2018) en moral case deliberation (Kok, 2023b).
De cruciale uitkomstmaat, veiligheidscultuur, werd in beide studies met andere vragenlijsten gemeten. In Amiri (2018) werd de patiëntveiligheidscultuur gemeten door middel van de Hospital Survey on Patient Safety Culture (HSOPSC). De totaalscore van deze vragenlijst liet een klinisch relevant verschil zien ten faveure van de interventiegroep. De bewijskracht was echter laag. In Kok (2023) werd teamklimaat gemeten middels de Safety Attitude Questionnaire (SAQ) en organisatiecultuur met de Culture of Care Barometer (CoCB). Beide uitkomstmaten lieten geen klinisch relevant verschil zien. De bewijskracht voor beide uitkomsten was laag.
De lage bewijskracht werd veroorzaakt door het mogelijke risico op bias in beide studies, afwezige blindering en de twijfelachtige randomisatie procedure in Amiri (2018) en imprecisie (lage proefpersoonaantallen of het overschrijden van het betrouwbaarheidsinterval rondom de puntschatter). De totale bewijskracht (de laagste bewijskracht van alle cruciale uitkomstmaten samen genomen) komt uit op laag. Daarom zal de aanbeveling ook afhangen van andere factoren.
Kosten (middelenbeslag)
De twee studies (Amiri, 2018; Kok, 2023b) rapporteren geen gegevens over de kosten gerelateerd aan de interventie. De kosten van een tweedaagse training (Amiri, 2018) zullen met name personele kosten betreffen voor de zorgverleners die aanwezig zijn en verder afhangen van de mate waarin expertise buiten de ziekenhuisorganisatie wordt ingehuurd. Moreel beraad vindt plaats tijdens de dagdienst binnen de normale klinische taken (Kok, 2023b), waardoor geen extra personele kosten worden gemaakt. Aan de batenkant kan men redeneren dat het voorkomen van complicaties en uitval van personeel door mentale klachten door het bevorderen van een betere veiligheidscultuur leidt tot minder faalkosten (behandelen complicaties en inhuren invalkrachten).
Aanvaardbaarheid, haalbaarheid en implementatie
Amiri (2018) beschrijft dat de betrokkenheid van bestuurders en leidinggevenden het effect van de interventie zou kunnen vergroten. Daarnaast heeft een educatieprogramma (tijdelijk) impact op de inzetbaarheid van de deelnemende zorgverleners, die immers uitgepland worden van zorgtaken. Dit is een mogelijke barrière in tijden van schaarste van zorgpersoneel.
Kok (2023b) beschrijft dat een vrijwillige deelname aan een moreel beraad en het door zorgverleners zelf aandragen van morele problemen het draagvlak vergroten voor het organiseren en succesvol maken van het moreel beraad. Keerzijde van vrijwillige deelname is dat vaak dezelfde zorgverleners aanwezig zijn en de interventie niet alle zorgverleners bereikt. Daarnaast zijn ervaren gespreksleiders een voorwaarde voor het houden/organiseren van een moreel beraad.
Samenvatting overige literatuur
Binnen en buiten de IC zijn weinig studies uitgevoerd naar de effecten van interventies op de veiligheidscultuur (Weaver, 2013; Morello, 2013) en deze zijn van zeer lage kwaliteit.
Ondanks de zeer beperkte wetenschappelijke bewijskracht hebben verschillende cultuurinterventies echter wel een sterke face-validiteit. Daarom volgt hieronder een bespreking van de enkele cultuurinterventies:
Veiligheidsprogramma
Diverse studies hebben de effecten van een ‘Comprehensive Unit-Based Safety Program’ (CUSP) geëvalueerd op veiligheidscultuur en patiënt uitkomsten. Het programma is gericht op zowel niet-technische vaardigheden, zoals teamtraining en strategieën gericht op continu leren en verbeteren, als technische interventies, zoals het gebruik van wetenschappelijk bewezen interventies voor het verbeteren van de patiëntenzorg. Stappen binnen het programma zijn: (1) veiligheidscultuur meting; (2) educatie over veiligheid; (3) teamtraining in het identificeren, prioriteren en elimineren van onveilige situaties; (4) het betrekken van senior leidinggevenden (bijvoorbeeld lid van Raad van Bestuur) voor het draagvlak en financiële middelen; (5) implementeren en evalueren van verbeteracties en delen van resultaten; en (6) het opnieuw meten van de veiligheidscultuur. Geëvalueerde effecten van het CUSP programma zijn minder ziekenhuisinfecties en VAEs, een verbeterde veiligheidscultuur en minder personele uitval (Berenholtz, 2015; Pronovost, 2008; Sexton, 2011; Pronovost, 2005; Timmel, 2010; Vigorito, 2011). Een vervolgstudie laat zien dat continue aandacht voor onderdelen van het uitgebreide programma en monitoring van de effecten (audit and feedback) leiden tot duurzame verbetering van patiëntuitkomsten zoals lijnsepsis (Pronovost, 2010; Pronovost, 2016).
Trainen op teamsamenwerking
Ziekenhuizen zijn ‘high reliability organisations’. De veiligheidscultuur van een afdeling wordt in positieve zin beïnvloed door een open communicatie, elkaar aanspreken, een systeem van veilig incidenten melden en het ‘blamefree’ bespreken van gebeurtenissen (Valentin, 2009). Suboptimale teamsamenwerking, zoals inadequate coördinatie, leiderschap of communicatie, leiden tot veiligheidsincidenten (Schmutz, 2013). Crew Resource Management (CRM) training is geïntroduceerd vanuit de luchtvaart om teamsamenwerking en communicatie te verbeteren in zeer dynamische domeinen van de gezondheidszorg, zoals operatiekamers, IC-afdelingen en SEH’s. Simulatietraining is een veilige methode om niet-technische vaardigheden (soft skills), zoals communicatie en leiderschap, onderdelen van CRM, te versterken. Aangeleerde CRM-vaardigheden leiden tot verbeterde veiligheidskennis en attitudes van personeel en betere patiëntuitkomsten (Boet, 2014; Kemper, 2015; Haerkens, 2015).
Psychologische veiligheid
Naast introductie van teamtraining, is psychologische en sociale veiligheid binnen teams een belangrijk en actueel onderwerp (Edmondson, 2014; Pauw, 2023; Alingh, 2019; Rock, 2023; Ng, 2017). Actief uitspreken bij onveilige situaties of aanspreken bij ongewenst gedrag blijken in de praktijk weerbarstig (Pauw, 2023; Brennan, 2019; Alingh, 2019). Factoren die hierbij een rol spelen zijn onder andere: hiërarchie (Brennan, 2019; Appelbaum, 2020), samenwerking tussen artsen en verpleegkundigen (Porter 2023), gedrag van de leidinggevende (Alingh, 2019) en diversiteit (Leigh, 2019). Er is beperkt wetenschappelijk onderzoek gedaan naar deze factoren en het effect van het verbeteren van psychologische veiligheid op team functioneren en patiëntuitkomsten. Desondanks lijkt aandacht hiervoor belangrijk, zeker in het licht van de actualiteit (Rock, 2023; Pauw, 2023; Rump, 2024).
Meten van veiligheidscultuur
Er zijn diverse meetinstrumenten voor het evalueren van veiligheidscultuur. Internationaal is het meest gebruikte instrument de Hospital Survey On Patient Safety culture (HSOPS) (AHRQ, 2009), welke is gebruikt in de studie van Amiri (2018). De Nederlandstalige versie hiervan is de COMPaZ (Smits, 2008). Andere veel gebruikte instrumenten waarvan een Nederlandstalige versie beschikbaar is zijn de Culture of Care Barometer (CoCB) (Maassen 2024), welke is gebruikt in de studie van Kok (2023b), en de Safety Attitudes Questionnaire (SAQ) (Haerkens, 2016). Onderdelen van de veiligheidscultuur meetinstrumenten zijn onder andere: teamsamenwerking, leiderschap, steun vanuit het management, relaties met directe collega’s en openheid over incidenten in de zorg. Het voordeel van dergelijke meetinstrumenten is dat onder een grote groep respondenten onderzoek kan worden gedaan. De vraag is of op basis van vragenlijstonderzoek de echt belangrijke culturele aspecten die patiëntveiligheid beïnvloeden worden gemeten. Bovendien bestaat de kans op sociaal wenselijke antwoorden. Om beter inzicht te krijgen in waarom men handelt zoals men doet, wordt daarom naast meetinstrumenten vaak gebruik gemaakt van participerend-observerend onderzoek (Czarniawska-Joerges, 1992; Rock, 2023).
Rationale van de aanbeveling: weging van argumenten voor en tegen de interventies
De aanbevelingen berusten op bovengenoemde literatuur en interpretatie hiervan door de werkgroepleden.
De veiligheidscultuur van een afdeling is een belangrijke determinant van de veiligheid van de patiënten op een IC (Sexton, 2011; Braitwaite, 2017; Kok, 2023).
Onderbouwing
Achtergrond
Een goede veiligheidscultuur kenmerkt zich door risicobewustzijn, een open aanspreekcultuur, openheid voor verandering en leren en verbeteren. Een goede veiligheidscultuur is geassocieerd met betere patiëntuitkomsten, zoals minder ziekenhuissterfte, valincidenten en ziekenhuisinfecties, grotere patiënttevredenheid (Braithwaite, 2017) en met beter mentaal welzijn van IC-medewerkers (Kok, 2023a). Het is daarom aannemelijk dat het bevorderen van een goede veiligheidscultuur leidt tot zowel betere uitkomsten voor patiënten als zorgverleners.
Conclusies / Summary of Findings
Comparison 1. Educational empowerment program versus no intervention
Safety culture (critical outcome)
Total score for patient safety culture (Hospital Survey on Patient Safety Culture)
|
Low GRADE |
An educational empowerment program in intensive care units may increase patient safety culture when compared with no intervention in ICU-personnel.
Source: Amiri, 2018 |
Comparison 2: Moral case deliberation versus no intervention
Safety culture (critical outcome)
Team climate (Safety Attitude Questionnaire)
|
Low GRADE |
Moral case deliberation may result in little to no difference in team climate when compared with no intervention in ICU-personnel.
Source: Kok, 2023b |
Organizational culture (Culture of Care Barometer)
|
Low GRADE |
Moral case deliberation may result in little to no difference in organizational culture when compared with no intervention in ICU-personnel.
Source: Kok, 2023b |
Samenvatting literatuur
Description of studies
No prognostic studies predicting factors with a positive influence on the safety culture in intensive care units were found (PICO 1). Two studies were included that describe an intervention to promote safety culture in intensive care units (PICO 2).
Amiri (2018) performed a randomized controlled trial with pre-test and post-test control groups to determine the effect of an educational empowerment program on patient safety culture in adult ICUs. The study was conducted between April and September 2015 in six adult ICUs located at Namazi Hospital in Shiraz, Iran. Included ICUs were similar in terms of patient safety policies. A total of 60 nurses and 20 supervisors (nurses with at least a Bachelor’s degree and responsible for oversight nursing services in the studied ICUs) were included from a sample of 160 nurses and 20 supervisors. The intervention and control group both consisted of 30 nurses and 10 supervisors from hospital 1, 3, and 6, and hospital 2, 4, and 5, respectively. The intervention consisted of an educational empowerment program. The educational program started with a two-day workshop, followed by hanging posters and handing out educational pamphlets to the nurses and supervisors . The workshop included education on patient safety, patient safety culture, and training in speak out in a situation of a threat to patient safety, communication, leadership, mutual support, and situational monitoring skills. The control group did not receive any intervention.
Data was collected using the Persian version of Hospital Survey on Patient Safety Culture (HSOPSC) developed by the AHRQ. The HSOPSC questionnaire has 42 items in 12 dimensions. These dimensions include: teamwork within units; manager expectations and actions promoting patient safety; organizational learning and continuous improvement; Management support for patient safety; overall perception of patient safety; feedback and communication on errors; communication openness; frequency of events reported; team work across hospital units; staffing; handoffs and transitions; non-punitive response to errors. The items were answered on a five-point Likert scale, from completely disagree (1) to completely agree (5) or from never (1) to always (5). The post-test scores were measured at three months follow-up. The mean age in the intervention group was 34.87 years (SD 7.8), versus 36.05 years (SD 8.03) in the control group. The intervention group consisted of 27 females (90%), versus 26 females (SD 83.8%) in the control group. Groups were comparable at baseline.
Kok (2023b) performed a parallel cluster randomized trial to assess whether structural moral case deliberation (MCD) in ICUs reduces burn-out symptoms and moral distress and strengthens the team climate among ICU professionals. The study was conducted between Januari 1, 2020 and October 1, 2021 in six ICUs in two hospitals located in Nijmegen, the Netherlands. Five of the ICUs were part of a university medical center (two adult ICUs, an ICU specializing in weaning patients off mechanical ventilation, a step-down unit, and a PICU) and one ICU was an adult ICU, also including step-down care beds. The intervention consisted of organizing structural MCD in the ICU. “Structural” means that MCD was embedded into the regular ICU workflow and that it was organized at least once a month. Moral issues were prospectively or retrospectively discussed and related either to medical-ethical decision-making in patient cases, or to broader moral issues arising from ICU practice. Control ICUs did not receive team-based ethical dialogue, but they still retained the possibility to request MCD. A total of 435 ICU professionals were included, of whom 288 had analyzable responses for one or more of the outcome variables in the baseline survey. At baseline, 178 respondents were included in the intervention group (three ICUs), versus 110 respondents in the control group (three ICUs). Additionally, 86 and 61 respondents were enrolled at follow-up measurements in the intervention and control group, respectively. Characteristics per ICU are provided in the evidence table. This article reported on team climate using the Team Climate Inventory from the Safety Attitude Questionnaire, which contains six items with Likert scales ranging from completely disagree (0) to completely agree (4). Additionally, this study reported on organizational culture measured by the culture of care barometer which includes five dimensions of organizational culture: supportive organization, leadership, relational atmosphere, relationship with supervisor, and participation opportunities. All were measured on five-point Likert scales also ranging from completely disagree (0) to completely agree (4). Follow-up measurements took place at 6, 12 and 21 months.
Results
Comparison 1: educational empowerment program versus no intervention
Safety culture (critical outcome)
Total score of patient safety culture
In Amiri (2018) the total post scores of the outcome measure patient safety culture was measured 3 months after the workshop (intervention) using the Persian version of Hospital Survey on Patient Safety Culture (HSOPSC). In the group whom received the intervention, the total post-test mean scores of the patient safety culture was 3.46 ± 0.26, in the control group the post-test mean scores of the patient safety culture was 2.84 ± 0.37 (MD: 0.62, 95% CI 0.48 to 0.76). This difference is considered clinically relevant in favor of the intervention group.
Dimensions of the HSOPSC
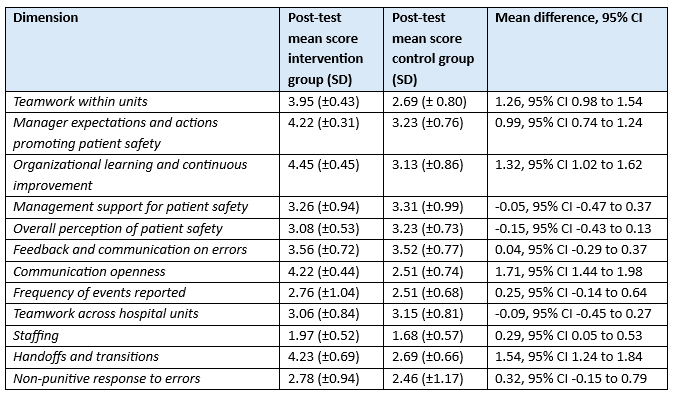
Amiri (2018) also reported on the following dimensions: teamwork within units, manager expectations and actions promoting patient safety, organizational learning and continuous improvement, management support for patient safety, overall perception of patient safety, feedback and communication on errors, communication openness, frequency of events reported, teamwork across hospital units, staffing, handoffs and transitions, and non-punitive response to errors. All dimensions were measured 3 months after the workshop (intervention). Results are reported in Table 1.
Table 1. Dimensions of patient safety culture measured with the Persian version of Hospital Survey on Patient Safety Culture, reported in Amiri (2018)
Abbreviations: SD, standard deviation; CI, confidence interval
Comparison 2: Moral case deliberation versus no intervention
Safety culture (critical outcome)
Team climate (measured with the Safety Attitude Questionnaire)
Kok (2023b) reported on Team climate using the Team Climate Inventory from the Safety Attitude Questionnaire, which contains six items with Likert scales ranging from completely disagree (0) to completely agree (4). Post intervention, team climate score did increase in favor of the intervention group (effect size: 0.03, 95%CI: −0.07 to 0.12). This difference was not considered clinically relevant.
Organizational culture (measured with the Culture of care Barometer)
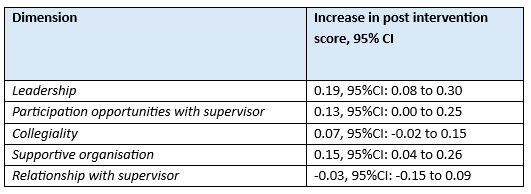
Kok (2023b) reported on multiple dimensions of organizational culture measured with the Culture of care Barometer, which measured the items on five-point Likert scales ranging from completely disagree (0) to completely agree (4). Results are reported in Table 2. All differences were not considered clinically relevant.
Table 2. Dimensions of organizational culture measured with the Culture of care Barometer, reported in Kok (2023b)
Abbreviations: CI, confidence interval
Level of evidence of the literature
Comparison 1. Educational empowerment program versus no intervention
Safety culture (critical outcome)
Total score for patient safety culture (Hospital Survey on Patient Safety Culture)
The level of evidence regarding the outcome measure patient safety culture was downgraded by two levels to low because of study limitations (high risk of bias, -1) and the OIS not being met (imprecision, -1).
Comparison 2: Moral case deliberation versus no intervention
Safety culture (critical outcome)
Team climate (Safety Attitude Questionnaire)
The level of evidence regarding the outcome measure team climate was downgraded by two levels to low because of study limitations (high risk of bias, -1) and the OIS not being met (imprecision, -1).
Organizational culture (Culture of Care Barometer)
The level of evidence regarding the outcome measure organizational culture was downgraded by two levels to low because of study limitations (high risk of bias, -1) and the confidence intervals around the point estimates crossing the upper threshold for clinical relevance (imprecision, -1).
Zoeken en selecteren
A systematic review of the literature was performed to answer the following question:
Which factors promote the safety culture in intensive care units?
For this question, we ideally wanted to find prognostic models predicting what factors have a positive influence on the safety culture in intensive care units. Because of a lack of such studies, an additional PICO was formulated.
PICO 1
| P (Patiënten): | ICU-personnel |
| I (Interventie): | Model predicting a positive influence on safety culture (for example: ethical decision climate, psychological safety, collaboration between physicians and nurses, leadership, organizational environment, work environment, CRM, team training, cooperation/teamwork job/employee satisfaction, work values, employee engagement and empowerment) |
| C (Comparison): | Other model / no model |
| O (Outcomes): | Predictive value / model performance |
| T/S (Timing): | Intensive care units |
PICO 2
| P (Patiënten): | ICU-personnel |
| I (Interventie): | Intervention to promote safety culture in intensive care units |
| C (Comparison): | No intervention |
| O (Outcomes): | Safety culture (measured with questionnaires, e.g. the Hospital Survey on Patient Safety Culture) |
Relevant outcome measures
The guideline development group considered patient safety culture as a critical outcome measure for decision making.
A priori, the working group did not define the outcome measure safety culture but used the definitions used in the studies.
The working group defined the following clinically important difference:
- safety culture (measured with questionnaires specified in included studies): 5% (5 points on a 100 points scale, 0.5 point on a 10 points scale).
Search and select (Methods)
The databases Embase.com, Ovid/Medline, Ovid/PsycInfo and Ebsco/CINAHL were searched with relevant search terms from 2000 until November 28th, 2023. The detailed search strategy is depicted under the tab Methods. The systematic literature search resulted in 462 hits. Studies were selected based on the following criteria:
- systematic review or Randomized Controlled Trial;
- describing prognostic factors or comparing interventions promoting the safety culture in intensive care units.
Nineteen studies were initially selected based on title and abstract screening. After reading the full text, seventeen studies were excluded (see the table with reasons for exclusion under the tab Methods), and two studies were included.
Results
Two studies were included in the analysis of the literature. Important study characteristics and results are summarized in the evidence tables. The assessment of the risk of bias is summarized in the risk of bias tables.
Referenties
- Amiri M, Khademian Z, Nikandish R. The effect of nurse empowerment educational program on patient safety culture: a randomized controlled trial. BMC Med Educ. 2018 Jul 3;18(1):158. doi: 10.1186/s12909-018-1255-6. PMID: 29970054; PMCID: PMC6029022.
- Boet S, Bould MD, Fung L, et al. Transfer of learning and patient outcome in simulated crisis resource management: a systematic review. Can J Anaesth. 2014;61:571-82.
- Braithwaite J, Herkes J, Ludlow K, Testa L, Lamprell G. Association between organisational and workplace cultures, and patient outcomes: systematic review. BMJ Open. 2017 Nov 8;7(11):e017708. doi: 10.1136/bmjopen-2017-017708. PMID: 29122796; PMCID: PMC5695304.
- Czarniawska-Joerges, B., Exploring Complex Organizations: a Cultural Perspective. Newbury Park (CA): Sage Publications, 1992.
- Haerkens MH, Kox M, Lemson J, Houterman S, van der Hoeven JG, Pickkers P. Crew Resource Management in the Intensive Care Unit: a prospective 3-year cohort study. Acta Anaesthesiol Scand. 2015 Nov;59(10):1319-29. doi: 10.1111/aas.12573. Epub 2015 Jun 16. PMID: 26079640; PMCID: PMC5033035.
- Haerkens MH, van Leeuwen W, Sexton JB, Pickkers P, van der Hoeven JG. Validation of the Dutch language version of the Safety Attitudes Questionnaire (SAQ-NL). BMC Health Serv Res. 2016 Aug 15;16(a):385. doi: 10.1186/s12913-016-1648-3. PMID: 27528393; PMCID: PMC4986249.
- Kemper PF, de Bruijne M, van Dyck C, So RL, Tangkau P, Wagner C. Crew resource management training in the intensive care unit. A multisite controlled before-after study. BMJ Qual Saf. 2016 Aug;25(8):577-87. doi: 10.1136/bmjqs-2015-003994. Epub 2016 Feb 3. PMID: 26843412.
- Kok N, Van Gurp J, van der Hoeven JG, Fuchs M, Hoedemaekers C, Zegers M. Complex interplay between moral distress and other risk factors of burnout in ICU professionals: findings from a cross-sectional survey study. BMJ Qual Saf. 2023a Apr;32(4):225-234. doi: 10.1136/bmjqs-2020-012239. Epub 2021 Jun 29. PMID: 34187883; PMCID: PMC10086276.
- Kok N, Zegers M, Teerenstra S, Fuchs M, van der Hoeven JG, van Gurp JLP, Hoedemaekers CWE. Effect of Structural Moral Case Deliberation on Burnout Symptoms, Moral Distress, and Team Climate in ICU Professionals: A Parallel Cluster Randomized Trial. Crit Care Med. 2023b Oct 1;51(10):1294-1305. doi: 10.1097/CCM.0000000000005940. Epub 2023 Jun 5. PMID: 37272981.
- Maassen S, van Oostveen C, Weggelaar AM, Rafferty AM, Zegers M, Vermeulen H. Measuring the work environment among healthcare professionals: Validation of the Dutch version of the Culture of Care Barometer. PLoS One. 2024 Feb 29;19(2):e0298391. doi: 10.1371/journal.pone.0298391. PMID: 38421985; PMCID: PMC10903908.
- Mannion R, Davies H. Understanding organisational culture for healthcare quality improvement. BMJ. 2018 Nov 28;363:k4907. doi: 10.1136/bmj.k4907. PMID: 30487286; PMCID: PMC6260242.
- Morello RT, Lowthian JA, Barker AL, McGinnes R, Dunt D, Brand C. Strategies for improving patient safety culture in hospitals: a systematic review. BMJ Qual Saf. 2013 Jan;22(1):11-8. doi: 10.1136/bmjqs-2011-000582. Epub 2012 Jul 31. PMID: 22849965.
- Rock LK, Morse KJ, Eppich W, Rudolph JW. Transforming Team Culture: A Case Study From Critical Care. Chest. 2023 Jun;163(6):1448-1457. doi: 10.1016/j.chest.2022.12.046. Epub 2023 Jan 12. PMID: 36642367.
- Scott T, Mannion R, Davies HT, et al. Implementing culture change in health care: theory and practice. Int J Qual Health Care 2003;15:111–8.
- Schmutz J, Manser T. Do team processes really have an effect on clinical performance? A systematic literature review. Br J Anaesth. 2013 Apr;110(4):529-44. doi: 10.1093/bja/aes513. Epub 2013 Mar 1. PMID: 23454826.
- Smits M, Christiaans-Dingelhoff I, Wagner C, Wal Gv, Groenewegen PP. The psychometric properties of the 'Hospital Survey on Patient Safety Culture' in Dutch hospitals. BMC Health Serv Res. 2008 Nov 7;8:230. doi: 10.1186/1472-6963-8-230. PMID: 18990256; PMCID: PMC2588576.
- Valentin A, Capuzzo M, Guidet B, Moreno R, Metnitz B, Bauer P, Metnitz P; Research Group on Quality Improvement of the European Society of Intensive Care Medicine (ESICM); Sentinel Events Evaluation (SEE) Study Investigators. Errors in administration of parenteral drugs in intensive care units: multinational prospective study. BMJ. 2009 Mar 12;338:b814. doi: 10.1136/bmj.b814. PMID: 19282436; PMCID: PMC2659290.
- Weaver SJ, Lubomksi LH, Wilson RF, Pfoh ER, Martinez KA, Dy SM. Promoting a culture of safety as a patient safety strategy: a systematic review. Ann Intern Med. 2013 Mar 5;158(5 Pt 2):369-74. doi: 10.7326/0003-4819-158-5-201303051-00002. PMID: 23460092; PMCID: PMC4710092.
- Literatuur CUSP
- Pronovost P, Needham D, Berenholtz S, Sinopoli D, Chu H, Cosgrove S, Sexton B, Hyzy R, Welsh R, Roth G, Bander J, Kepros J, Goeschel C. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med. 2006 Dec 28;355(26):2725-32. doi: 10.1056/NEJMoa061115. Erratum in: N Engl J Med. 2007 Jun 21;356(25):2660. PMID: 17192537.
- Pronovost PJ, Berenholtz SM, Goeschel C, Thom I, Watson SR, Holzmueller CG, Lyon JS, Lubomski LH, Thompson DA, Needham D, Hyzy R, Welsh R, Roth G, Bander J, Morlock L, Sexton JB. Improving patient safety in intensive care units in Michigan. J Crit Care. 2008 Jun;23(2):207-21. doi: 10.1016/j.jcrc.2007.09.002. PMID: 18538214.
- Timmel J, Kent PS, Holzmueller CG, Paine L, Schulick RD, Pronovost PJ. Impact of the Comprehensive Unit-based Safety Program (CUSP) on safety culture in a surgical inpatient unit. Jt Comm J Qual Patient Saf. 2010 Jun;36(6):252-60. doi: 10.1016/s1553-7250(10)36040-5. PMID: 20564886.
- Miller K, Briody C, Casey D, Kane JK, Mitchell D, Patel B, Ritter C, Seckel M, Wakai S, Drees M. Using the Comprehensive Unit-based Safety Program model for sustained reduction in hospital infections. Am J Infect Control. 2016 Sep 1;44(9):969-76. doi: 10.1016/j.ajic.2016.02.038. Epub 2016 May 13. PMID: 27184208.
- Pronovost P J, Goeschel C A, Colantuoni E, Watson S, Lubomski L H, Berenholtz S M et al. Sustaining reductions in catheter related bloodstream infections in Michigan intensive care units: observational study BMJ 2010; 340 :c309 doi:10.1136/bmj.c309.
- Pronovost PJ, Watson SR, Goeschel CA, Hyzy RC, Berenholtz SM. Sustaining Reductions in Central Line-Associated Bloodstream Infections in Michigan Intensive Care Units: A 10-Year Analysis. Am J Med Qual. 2016 May;31(3):197-202. doi: 10.1177/1062860614568647. Epub 2015 Jan 21. PMID: 25609646.
- Berenholtz SM, Lubomski LH, Weeks K, Goeschel CA, Marsteller JA, Pham JC, Sawyer MD, Thompson DA, Winters BD, Cosgrove SE, Yang T, Louis TA, Meyer Lucas B, George CT, Watson SR, Albert-Lesher MI, St Andre JR, Combes JR, Bohr D, Hines SC, Battles JB, Pronovost PJ; On the CUSP: Stop BSI program. Eliminating central line-associated bloodstream infections: a national patient safety imperative. Infect Control Hosp Epidemiol. 2014 Jan;35(1):56-62. doi: 10.1086/674384. Epub 2013 Nov 26. PMID: 24334799.
- Vigorito MC, McNicoll L, Adams L, Sexton B. Improving safety culture results in Rhode Island ICUs: lessons learned from the development of action-oriented plans. Jt Comm J Qual Patient Saf. 2011;37:509-14. [PMID: 22132663].
- Sexton JB, Berenholtz SM, Goeschel CA, Watson SR, Holzmueller CG, Thompson DA, et al. Assessing and improving safety climate in a large cohort of intensive care units. Crit Care Med. 2011;39:934-9. [PMID: 21297460].
- Literatuur psychologische veiligheid
- Alingh CW, van Wijngaarden JDH, van de Voorde K, Paauwe J, Huijsman R. Speaking up about patient safety concerns: the influence of safety management approaches and climate on nurses' willingness to speak up. BMJ Qual Saf. 2019 Jan;28(1):39-48. doi: 10.1136/bmjqs-2017-007163. Epub 2018 Jun 28. PMID: 29954948.
- Appelbaum NP, Lockeman KS, Orr S, Huff TA, Hogan CJ, Queen BA, Dow AW. Perceived influence of power distance, psychological safety, and team cohesion on team effectiveness. J Interprof Care. 2020 Jan-Feb;34(1):20-26. doi: 10.1080/13561820.2019.1633290. Epub 2019 Aug 5. PMID: 31381458.
- Brennan PA, Davidson M. Improving patient safety: we need to reduce hierarchy and empower junior doctors to speak up. BMJ. 2019 Jul 2;366:l4461. doi: 10.1136/bmj.l4461. PMID: 31266748.
- Edmondson, A. C, & Zhike, L. (2014). Psychological Safety: The History, Renaissance, and Future of an Interpersonal Construct. Annual Review of Organizational Psychology and Organizational Behavior, 1(1), 23-43. https://doi.org/10.1146/annurev-orgpsych-031413-091305.
- Leigh JP, Grood C, Ahmed SB, Ulrich AC, Fiest KM, Straus SE, Stelfox HT. Toward Gender Equity in Critical Care Medicine: A Qualitative Study of Perceived Drivers, Implications, and Strategies. Crit Care Med. 2019 Apr;47(4):e286-e291. doi: 10.1097/CCM.0000000000003625. PMID: 30855331.
- Ng GWY, Pun JKH, So EHK, et al. Speak-up culture in an intensive care unit in Hong Kong: a crosssectional survey exploring the communication openness perceptions of Chinese doctors and nurses. BMJ Open 2017;7:e015721. doi:10.1136/ bmjopen-2016-015721.
- Rock LK, Morse KJ, Eppich W, Rudolph JW. Transforming Team Culture: A Case Study From Critical Care. Chest. 2023 Jun;163(6):1448-1457. doi: 10.1016/j.chest.2022.12.046. Epub 2023 Jan 12. PMID: 36642367.
- Rump B, Hartman L (2024)
Emotionele arbeid is een onderschatte stressfactor | medischcontact.
Evidence tabellen
Evidence table for intervention studies
|
Study reference |
Study characteristics |
Patient characteristics 2 |
Intervention (I) |
Comparison / control (C) 3
|
Follow-up |
Outcome measures and effect size 4 |
Comments |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Amiri, 2018 |
Type of study: RCT
Setting and country: 6 adult ICUs at Namazi Hospital, Shiraz, Iran.
Funding and conflicts of interest: The present study was financially supported by the Vice Chancellor of Research of Shiraz University of Medical Sciences, Shiraz, Iran (Grant No. 5793). The funding body did not play any roles in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript. |
Inclusion criteria: Having at least 6 months experience in an adult ICU and at least a Bachelor’s degree in nursing.
Exclusion criteria: The unwillingness to participate, failure to complete the pre-test, and lack of participation in training sessions.
N total at baseline: Intervention: 30 nurses and 10 supervisors Control: 30 nurses and 10 supervisors
Important prognostic factors Age, mean (SD) I: 34.87 (SD 7.8) C: 36.06 (SD 8.03)
Sex female, n (%) I: 27 (90%) C: 26 (83.8%)
Education, n (%) Bachelor’s degree I: 26 (86.7%) C: 30 (96.8%) Master’s degree I: 4 (13.3%) C: 1 (3.2%)
Position Nurse I: 21 (70%) C: 27 (87.1%) Supervisor I: 9 (30%) C: 4 (12.9%)
Groups comparable at baseline? Yes
|
Describe intervention (treatment/procedure/test):
Educational empowerment program
This program started with a two-day workshop (8 h), followed by hanging posters and handing out educational pamphlets to the nurses and supervisors of the experimental group at their workplace. The educational contents of the workshop, posters, and pamphlets were matched. The workshop included education on patient safety, patient safety culture, speak out in a situation of a threat to patient safety, and the skills of Team Strategies and Tools to Enhance Performance and Patient Safety (TeamSTEPPS). TeamSTEPPS was developed by the Agency for Healthcare Research and Quality (AHRQ) to improve patient outcomes. It included communication, leadership, mutual support, and situational monitoring skills. The workshop consisted of a lecture, group discussion, and presenting scenarios. In addition, some textual and graphical posters (related to TeamSTEPPS skills, speak up, and patient safety culture) were placed on the walls of patient’s unit in the ICUs of the experimental group for a period of 6 weeks. During the following 6 weeks, every week one pamphlet was handed out to the nurses in the experimental groups. Pamphlets contents included communication, mutual support, situation monitoring, leadership, speak up, and patient safety culture.
|
Describe control (treatment/procedure/test):
No intervention |
Length of follow-up: 3 months
Loss-to-follow-up: Intervention: 9 nurses and 1 supervisor were lost to follow-up
Reasons: 8 nurses and 1 supervisor did not receive the intervention, and 1 nurse did not complete the post-test
Control: 3 nurses and 6 supervisors were lost to follow-up
Reasons: 3 nurses and 4 supervisors did not complete the pre-rest, and 2 supervisors did not complete the post-test
Incomplete outcome data: Data from participants who completed the pre- and post-test were analysed.
|
Persian version of Hospital Survey on Patient Safety Culture (HSOPSC). The pre-test was completed individually before the workshop. Three months after the workshop, the post-test was conducted individually in both groups.
Dimensions (mean (SD)) Teamwork within units I: Pre-test: 2.91(±0.74) Post-test: 3.95(±0.43) C: Pre-test: 2.51 (± 0.82) Post-test: 2.69(±0.80)
Manager expectations and actions promoting patient safety I: Pre-test: 3.48 (±0.83) Post-test: 4.22 (±0.31) C: Pre-test: 3.22 (±0.68) Post-test: 3.23 (±0.76)
Organizational learning and continuous improvement I: Pre-test: 3.83 (±0.65) Post-test: 4.45 (±0.45) C: Pre-test: 3.49 (±0.82) Post-test: 3.13 (±0.86)
Management support for patient safety I: Pre-test: 3.15 (±1.05) Post-test: 3.26 (±0.94) C: Pre-test: 2.97 (±1.04) Post-test: 3.31 (±0.99)
Overall perception of patient safety I: Pre-test: 2.92 (±0.62) Post-test: 3.08 (±0.53) C: Pre-test: 3.29 (±0.63) Post-test: 3.23 (±0.73)
Feedback and communication on errors I: Pre-test: 3.25 (±0.85) Post-test: 3.56 (±0.72) C: Pre-test: 3.53 (±0.78) Post-test: 3.52 (±0.77)
Communication openness I: Pre-test: 2.72 (±0.67) Post-test: 4.22 (±0.44) C: Pre-test: 2.80 (±0.79) Post-test: 2.51 (±0.74)
Frequency of events reported I: Pre-test: 2.91 (±0.56) Post-test: 2.76 (±1.04) C: Pre-test: 2.66 (±0.66) Post-test: 2.51 (±0.68)
Teamwork across hospital units I: Pre-test: 2.94 (±0.93) Post-test: 3.06 (±0.84) C: Pre-test: 3.17 (±0.76) Post-test: 3.15 (±0.81)
Staffing I: Pre-test: 1.84 (±0.62) Post-test: 1.97 (±0.52) C: Pre-test: 1.69 (±0.46) Post-test: 1.68 (±0.57
Handoffs and transitions I: Pre-test: 2.75 (±0.91) Post-test: 4.23 (±0.69) C: Pre-test: 2.42 (±0.80) Post-test: 2.69 (±0.66)
Non-punitive response to errors I: Pre-test: 2.25 (±0.93) Post-test: 2.78 (±0.94) C: Pre-test: 2.45 (±1.15) Post-test: 2.46 (±1.17)
Total scores of the patient safety culture I: Pre-test: 2.91 (±0.4) Post-test: 3.46 (±0.26) C: Pre-test: 2.86 (±0.37) Post-test: 2.84 (±0.37)
Safety score I: Pre-test: 2.63 (±0.7) Post-test: 3.37 (±0.5) C: Pre-test: 2.88 (±0.4) Post-test: 2.90 (±0.5) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Kok, 2023 |
Type of study: Parallel cluster RCT
Setting and country: Six ICUs in two hospitals located in Nijmegen, the Netherland Funding and conflicts of interest: This research was supported by ZonMw, the Netherlands Organisation for Health Research and Development (grant 516012513). Dr. Kok’s institution received funding from ZonMw. Dr. van Gurp’s institution received funding from The Netherlands Organisation for Health Research and Development; he received support for article research from The Netherlands Organisation for Health Research and Development. The remaining authors have disclosed that they do not have any potential conflicts of interest. |
Inclusion criteria: ICU professionals with responsibilities in the provision of intensive care, that is, ICU nurses, intensivists, fellows, and residents, were eligible for inclusion.
Exclusion criteria: N.R.
N total at baseline:
Intervention and control group characteristics
Groups comparable at baseline? Yes |
Describe intervention (treatment/procedure/test):
The intervention consisted of organizing structural MCD in the ICU. “Structural” means that MCD was embedded into the regular ICU workflow and that it was organized at least once a month. Moreover, in each ICU, appointed ICU physicians and ICU nurses kept track of moral issues affecting the professionals, and organized the MCDs. All professionals in the unit were invited, but participation was not mandatory. Moral issues were prospectively or retrospectively discussed and related either to medical-ethical decision-making in patient cases, or to broader moral issues arising from ICU practice, for example, prejudices about unvaccinated COVID-19 patients or ICU nurse’s proper role in end-of-life care and death rituals on the PICU. A fixed pool of five facilitators (four ethicists and one spiritual counselor, all experienced in facilitating MCD) presided over the meetings according to availability. Facilitators were independent and not involved in conducting the study. They were informed about the research project. Generally, a single MCD lasted an hour.
MCD was implemented at the randomly assigned timepoint.
|
Describe control (treatment/procedure/test):
No team-based ethical dialogue was offered on the control ICUs. Control ICUs, however, retained the possibility to request MCD. |
Length of follow-up: 6, 12 and 21 months
Loss-to-follow-up and incomplete outcome data: Per-protocol analyses: 346 participants.
The study cohort was open. ICU professionals could therefore leave or be added to the cohort over time. ICU professionals with full data on the outcomes, but not at all timepoints, were also included in the analysis.
|
Team climate Effect: 0.03 95%CI: −0.07 to 0.12
Supportive organization Effect: 0.15 95%CI: 0.04 to 0.26
Leadership Effect: 0.19 95%CI: 0.08 to 0.30
Collegiality Effect: 0.07 95%CI: −0.02 to 0.15
Relationship with supervisor Effect: −0.03 95%CI: −0.15 to 0.09
Participation opportunities Effect: 0.13 95%CI: 0.00 to 0.25
|
ITT analyses were performed |
Risk of bias table for intervention studies
|
Study reference
(first author, publication year) |
Was the allocation sequence adequately generated?
Definitely yes Probably yes Probably no Definitely no |
Was the allocation adequately concealed?
Definitely yes Probably yes Probably no Definitely no |
Blinding: Was knowledge of the allocated interventions adequately prevented?
Were patients blinded?
Were healthcare providers blinded?
Were data collectors blinded?
Were outcome assessors blinded?
Were data analysts blinded?
Definitely yes Probably yes Probably no Definitely no |
Was loss to follow-up (missing outcome data) infrequent?
Definitely yes Probably yes Probably no Definitely no |
Are reports of the study free of selective outcome reporting?
Definitely yes Probably yes Probably no Definitely no |
Was the study apparently free of other problems that could put it at a risk of bias?
Definitely yes Probably yes Probably no Definitely no |
Overall risk of bias If applicable/necessary, per outcome measure
LOW Some concerns HIGH
|
|
Amiri, 2018 |
Definitely no
Reason: Permuted block randomization based on hospital, but not clear how
Citation: was proportional to the total number of its nurses. To randomly allocate nurses, a number was assigned to each ICU and categorized into the control and experimental groups, based on permuted block randomization. In total, 30 nurses from ICUs number 1, 3, and 6 (surgical, neurosurgical, and general ICU) were assigned to the experimental group. In addition, 30 nurses from ICUs number 2, 4, and 5 (medical, neurosurgical, and general ICU) were assigned to the control group. |
No information |
Probably no
Reason: The educational empowerment program was carried out by one of the researchers. |
Probably no
Reason: The loss to follow-up after allocation and after follow-up relatively big.
Loss-to-follow-up: Intervention: 9 nurses and 1 supervisor were lost to follow-up Reasons: 8 nurses and 1 supervisor did not receive the intervention, and 1 nurse did not complete the post-test
Control: 3 nurses and 6 supervisors were lost to follow-up Reasons: 3 nurses and 4 supervisors did not complete the pre-rest, and 2 supervisors did not complete the post-test
Only the data from participants who completed the pre- and post-test were analysed.
|
Definitely yes
Reason: All relevant outcomes were reported |
Definitely yes
Reason: No other problems noted |
HIGH (all outcomes)
Reason: No high confidence in randomisation procedure and blinding |
|
Kok, 2023 |
Probably yes
Reason: Envelopes contained the names of the participating ICU’s. |
Definitely yes
Reason: ICUs were randomized by N.K. and M.Z. using sealed envelopes containing the names of the participating ICUs apart from ICU 1, which was already in the intervention condition at the start of the study. An independent researcher was present during randomization. |
Probably no
Reason: Blinding was not possible as ICU professionals would be aware of MCD structurally taking place in their ICU. |
Probably no
Reason: Loss to follow-up and incomplete data was high. Per-protocol analyses: 346 participants.
Citation from study: It is likely that the questionnaires were mostly returned by ICU professionals without burnout symptoms, leading to a selection bias known as the “healthy worker effect,” which may increase the likelihood of false-negative findings. This bias works in two ways. First, professionals with burnout at the start of the study who improved over time may not have participated in the baseline measurement but might have returned follow-up questionnaires: the so-called healthy worker selection effect. Second, it is likely that those ICU professionals who over the course of the study developed burnout symptoms dropped out and did not complete follow-up questionnaires: the so-called healthy worker survivor effect. |
Definitely yes
Reason: All relevant outcomes were reported |
Definitely yes
Reason: No other problems noted |
HIGH (all outcome measures)
Reason: high loss to follow-up with possible healthy-worker effect and no blinding |
Table of excluded studies
|
Reference |
Reason for exclusion |
|
Etemadifar, Shahram and Sedighi, Zeynab and Sedehi, Morteza and Masoudi, Reza The effect of situation, background, assessment, recommendation-based safety program on patient safety culture in intensive care unit nurses. Journal of education and health promotion. 2021; 10 :422 |
Wrong study design |
|
Kemper, Peter F. and de Bruijne, Martine and van Dyck, Cathy and So, Ralph L. and Tangkau, Peter and Wagner, Cordula Crew resource management training in the intensive care unit. A multisite controlled before-after study. BMJ quality & safety. 2016; 25 (8) :577-587 |
Wrong study design (no randomization took place) |
|
Sexton, J. B. and Berenholtz, S. M. and Goeschel, C. A. and Watson, S. R. and Holzmueller, C. G. and Thompson, D. A. and Hyzy, R. C. and Marsteller, J. A. and Schumacher, K. and Pronovost, P. J. Assessing and improving safety climate in a large cohort of intensive care units. Critical Care Medicine. 2011; 39 (5) :934-939 |
Wrong study design |
|
Ling, Lowell and Gomersall, Charles David and Samy, Winnie and Joynt, Gavin Matthew and Leung, Czarina C. H. and Wai-Tat, Wong and Lee, Anna and Leung, Czarina Ch and Wong, Wai-Tat The Effect of a Freely Available Flipped Classroom Course on Health Care Worker Patient Safety Culture: A Prospective Controlled Study. Journal of Medical Internet Research. 2016; 18 (7) :26 |
Wrong study design |
|
Ling, Lowell and Gomersall, Charles David and Samy, Winnie and Joynt, Gavin Matthew and Leung, Czarina Ch and Wong, Wai-Tat and Lee, Anna The Effect of a Freely Available Flipped Classroom Course on Health Care Worker Patient Safety Culture: A Prospective Controlled Study. Journal of medical Internet research. 2016; 18 (7) :e180 |
Wrong study design |
|
Ling, L. and Joynt, G. and Lee, A. and Samy, W. and Fung, H. and Gomersall, C. D. Prospective controlled study to compare the effects of a basic patient safety course on healthcare worker patient safety culture. Critical Care. 2015; 19 :S180 |
Wrong study design |
|
Al Ma'mari, Qasim and Sharour, Loai Abu and Al Omari, Omar Fatigue, burnout, work environment, workload and perceived patient safety culture among critical care nurses. British Journal of Nursing. 2020; 29 (1) :28-34 |
among critical care nurses; including neonatal intensive care units (NICUs), paediatric ICUs, adult ICUs, coronary care units and post-cardiac surgery units, |
|
Alrabae, Yaseen Mohammed A. and Aboshaiqah, Ahmad E. and Tumala, Regie B. The association between self‐reported workload and perceptions of patient safety culture: A study of intensive care unit nurses. Journal of Clinical Nursing (John Wiley & Sons, Inc.). 2021; 30 (7) :1003-1017 |
Wrong study design |
|
Armellino, Donna and Griffin, Mary T. Quinn and Fitzpatrick, Joyce J. Structural empowerment and patient safety culture among registered nurses working in adult critical care units. Journal of Nursing Management. 2010; 18 (7) :796-803 |
Wrong study design |
|
Collier, Susan L. and Fitzpatrick, Joyce J. and Siedlecki, Sandra L. and Dolansky, Mary A. Employee Engagement and a Culture of Safety in the Intensive Care Unit. JONA: The Journal of Nursing Administration. 2016; 46 (1) :49-54 |
Wrong study design |
|
de Lima Silva Nunes, Ranielle and de Camargo Silva, Ana Elisa Bauer and de Lima, Juliana Carvalho and Carvalho, Dayse Edwiges and Bernardes, Cristina Alves and Sousa, Tanielly Paula and Gimenes, Fernanda Raphael Escobar and Pires, Ana Claudia Andrade Cordeiro Factors influencing the patient safety climate in intensive care units: cross-sectional study. BMC nursing. 2021; 20 (1) :125 |
Wrong study design |
|
Dodek, Peter M. and Wong, Hubert and Jaswal, Danny and Heyland, Daren K. and Cook, Deborah J. and Rocker, Graeme M. and Kutsogiannis, Demetrios J. and Dale, Craig and Fowler, Robert and Ayas, Najib T. Organizational and safety culture in Canadian intensive care units: Relationship to size of intensive care unit and physician management model. Journal of Critical Care. 2012; 27 (1) :11-17 |
Wrong study design |
|
Meurling, Lisbet and Hedman, Leif and Sandahl, Christer and Fellander-Tsai, Li and Wallin, Carl-Johan Systematic simulation-based team training in a Swedish intensive care unit: a diverse response among critical care professions. BMJ quality & safety. 2013; 22 (6) :485-494 |
Wrong study design |
|
Ng, George Wing Yiu and Pun, Jack Kwok Hung and So, Eric Hang Kwong and Chiu, Wendy Wai Hang and Leung, Avis Siu Ha and Stone, Yuk Han and Lam, Chung Ling and Lai, Sarah Pui Wa and Leung, Rowlina Pui Wah and Luk, Hing Wah and Leung, Anne Kit Hung and Au Yeung, Kin Wah and Lai, Kang Yiu and Slade, Diana and Chan, Engle Angela Speak-up culture in an intensive care unit in Hong Kong: a cross-sectional survey exploring the communication openness perceptions of Chinese doctors and nurses. BMJ open. 2017; 7 (8) :e015721 |
Wrong study design |
|
Pronovost, P. and Weast, B. and Rosenstein, B. and Sexton, J. B. and Holzmueller, C. G. and Paine, L. and Davis, R. and Rubin, H. R. Implementing and validating a comprehensive unit-based safety program. Journal of Patient Safety. 2005; 1 (1) :33-40 |
Wrong outcome and study design |
|
Tlili, M. A. and Aouicha, W. and Sahli, J. and Mellouli, A. and Hiab, M. B. and Chelbi, S. and Rejeb, M. B. and Mallouli, M. Assessing anesthesiologists' patient safety culture and its associated factors in Tunisian intensive care units. Anesthesia and Analgesia. 2021; 133 (3) :1644 |
Wrong study design |
|
Vifladt, Anne and Simonsen, Bjoerg O. and Lydersen, Stian and Farup, Per G. Changes in patient safety culture after restructuring of intensive care units: Two cross-sectional studies. Intensive & Critical Care Nursing. 2016; 32 :58-65 |
Wrong study design |
Verantwoording
Beoordelingsdatum en geldigheid
Publicatiedatum : 16-10-2025
Beoordeeld op geldigheid : 16-10-2025
Algemene gegevens
Voor meer details over de gebruikte richtlijnmethodologie verwijzen wij u naar de Werkwijze. Relevante informatie voor de ontwikkeling/herziening van deze leidraad is hieronder weergegeven.
De ontwikkeling/herziening van deze module werd ondersteund door het Kennisinstituut van de Federatie Medisch Specialisten (www.demedischspecialist.nl/kennisinstituut) en werd gefinancierd uit de Stichting Kwaliteitsgelden Medisch Specialisten (SKMS). De financier heeft geen enkele invloed gehad op de inhoud van de module.
Samenstelling werkgroep
Voor het ontwikkelen van de module is in 2023 een multidisciplinaire werkgroep ingesteld, bestaande uit vertegenwoordigers van alle relevante specialismen (zie hiervoor de Samenstelling van de werkgroep) die betrokken zijn bij de zorg voor patiënten die zijn opgenomen op de Intensive Care.
Werkgroep
Dr. I.A. (Iwan) Meynaar (voorzitter), internist-intensivist, HagaZiekenhuis, NVIC
Drs. B. (Ben) de Jong, internist-intensivist, Saxenburgh Medisch Centrum, NVIC
Dr. M. (Marieke) Zegers, Associate Professor, Radboudumc, NVIC
Drs. T.C. (Corien) Veenstra, longarts-intensivist, UMCG, NVIC
Dr. J. (Jasper) van Bommel, anesthesioloog-intensivist, Erasmus MC, NVIC
Dr. P. (Peter) van Vliet, neuroloog-intensivist, Haaglanden Medisch Centrum, NVN/NVIC
Dr. G.J. (Jan) Zijlstra, longarts-intensivist, Amsterdam UMC, NVALT
Dr. M.A.M. (Miriam) Moviat, internist-intensivist, Jeroen Bosch Ziekenhuis, NIV
Dr. M.V. (Mark) Koning, anesthesioloog-intensivist, Rijnstate Ziekenhuis, NVA
Drs. R.W.L. (Rens) van de Weyer, cardioloog-intensivist, Elkerliek Ziekenhuis, NVVC
Drs. J.M.R. (Joost) Meijer, chirurg-intensivist, Noordwest Ziekenhuisgroep, NVvH
Drs. L. (Lea) van Duijvenbode-den Dekker, IC verpleegkundige, Amphia Ziekenhuis, V&VN-IC
Dr. W. (Willemke) Stilma (vanaf maart 2024), Hoofddocent en postdoc onderzoeker, Hogeschool van Amsterdam, V&VN-IC
Dr. P.J.T. (Paul) Rood (tot maart 2024), bestuurder V&VN-IC, senior onderzoeker HAN University of applied sciences & Ziekenhuis Rijnstate
Dr. M.M.C. (Margo) van Mol, Assistant Professor, Erasmus MC, FCIC/IC-Connect
Klankbordgroep
Mevr. J.E. (Janine) de Kleijn, MSc, Physician Assistant, Catharina Ziekenhuis, NAPA
Dr. J.M. (Joep) Droogh, intensivist, UMCG, NVIC (namens de transportcommissie)
Dr. D.J. (David) van Westerloo, intensivist, LUMC, NVIC (namens de LHIC)
Drs. C.J.G.M. (Crétien) Jacobs, anesthesioloog-intensivist, Elkerliek Ziekenhuis, NVIC (namens de werkgroep beroepsprofiel intensivisten)
Drs. C. (Coby) Heij, anesthesioloog-intensivist, Spaarne Gasthuis, NVIC (namens de commissie beroepsbelangen intensivisten)
Drs. J. (Jacco) Rozendaal, Verpleegkundig Specialist IC/MC, St. Antonius Ziekenhuis, V&VN-VS
Met dank aan
Dr J. J. Spijkstra, intensivist, AmsterdamUMC (namens de taakgroep formatie)
Drs. A. (Arianne) Doorduin-Schmeets, Unithoofd Intensive Care, Jeroen Bosch Ziekenhuis, ’s-Hertogenbosch (namens het LHIC)
Dhr. F. (Frank) van der Zee, IC verpleegkundige/Avond-nacht-weekend Hoofd, Frisius MC locatie Leeuwarden, Leeuwarden (namens LNICV)
Mw. I. (Iepie) Plagge van der Vliet, manager intensive care en medium care, Martini ziekenhuis, Groningen (namens LHIC)
Dhr. D.R. (Dick) Streefkerk, hoofd IC, Alrijne ziekenhuis, Leiderdorp, namens LHIC
Drs. T. (Toine) Klarenbeek, Intensive Care Verpleegkundige/Klinisch epidemioloog, Maxima medisch centrum, Veldhoven (namens LNICV)
Mevr, L. (Lisette) Epping - Tijdhof, Adviseur Kwaliteit en Veiligheid / niet-praktiserende IC verpleegkundige, Medisch Spectrum Twente, Enschede (namens V&VN IC expertise kwaliteit en veiligheid)
Dhr. R. (Renze) Jongstra, IC-verpleegkundige volwassenen en kinderen, Circulation Practitioner (namens bestuur V&VN-IC)
Met ondersteuning van
Drs. F.M. (Femke) Janssen, adviseur, Kennisinstituut van de Federatie Medisch Specialisten
Dr. S.N. (Stefanie) Hofstede, senior adviseur, Kennisinstituut van de Federatie Medisch Specialisten
Belangenverklaringen
Een overzicht van de belangen van werkgroepleden en het oordeel over het omgaan met eventuele belangen vindt u in onderstaande tabel. De ondertekende belangenverklaringen zijn op te vragen bij het secretariaat van het Kennisinstituut van de Federatie Medisch Specialisten via secretariaat@kennisinstituut.nl.
|
Werkgroeplid |
Functie |
Nevenfuncties |
Gemelde belangen |
Ondernomen actie |
|
Meynaar, voorzitter |
Intensivist, HagaZiekenhuis |
Bestuurslid Nederlandse Vereniging voor Intensive Care, onbetaald behoudens een onkostenvergoeding, onderzoeker |
Leren van Juiste Diagnoses, door ZonMw gesubsidieerd onderzoek 160.000 euro (inmiddels afgerond 2021-2023)
|
Geen restricties |
|
De Jong |
Internist-intensivist |
- Cliëntenraad Prinses Maxima Centrum (onkostenvergoeding) |
Eenmalige deelname binnen adviesraad voor Paion t.a.v. positionering van giapreza binnen intensive care geneeskunde
|
Geen restricties |
|
Veenstra |
Intensivist
|
Instructeur NVIC bronchoscopie cursus (onbetaald) Lid NVIC commissie pulmonale diagnostiek en interventies (onbetaald) Lid NVIC commissie simulatie (onbetaald) FCCS instructeur (vergoeding wordt overgemaakt naar het UMCG) Medisch visiteur externe kwaliteitsvisitaties NVIC (vergoeding wordt overgemaakt naar het UMCG) Lid Zinnige Zorg traject Zorginstituut VTE (NVIC afgevaardigde, onbetaald) Secretaris sectie IC, NVALT (onbetaald) Lid sectie pulmonale interventies, NVALT (onbetaald) |
Geen |
Geen restricties |
|
Zijlstra |
Longarts-Intensivist, Dijklander Ziekenhuis, betaald |
Geen |
Geen |
Geen restricties |
|
Van Bommel |
Intensivist, Erasmus Medisch Centrum Rotterdam. Werkzaam als staflid op de Intensive Care Volwassenen (betaald). |
Geen |
Geen |
Geen restricties |
|
Meijer |
Chirurg-intensivist bij de Noordwest Ziekenhuisgroep (1,0 FTE) |
Lid toelatingscommissie binnen Noordwestziekenhuisgroep (onbetaald) |
Geen |
Geen restricties |
|
Van Duijvenbode – den Dekker |
IC verpleegkundige, Amphia Ziekenhuis |
Docent Erasmus MC Academie |
Geen |
Geen restricties |
|
Van Mol |
Assistant Professor |
Bestuurslid Stichting FCIC (onbezoldigd) |
1. ZonMw - hoofdonderzoeker van de ICNaVen-studie, ontwikkelen digitale ondersteuning in IC-nazorg voor naasten van een IC-patiënt. Dit is een multicenter studie (nationale en internationale samenwerking) waarbij eerst de behoeften en prioritering wordt verkend en vervolgens een daarop aangepaste interventie wordt ontwikkeld. (Projectleider) 2. ZonMw - hoofdonderzoeker van de DIPIC-studie, een implementatiestudie voor een digitaal dagboek op de IC, als opmaat naar persoonsgerichte zorg. Dit is een multicenter studie in een multi-methods benadering, om het gebruik van een digitaal dagboek op de IC te stimuleren. (Projectleider) 3. ZonMw - Ik ben mede-onderzoeker bij het ontwikkelen van een PGO-IC(na)zorg. Hierbij wordt in co-creatie met verschillende stakholders en Quli een digitale omgeving specifiek ingericht op de voormalig IC-patiënt. Stichting FCIC is penvoerder. (Projectleider) |
Geen restricties |
|
Zegers |
Associate Professor Radboudumc |
Geen |
1. Zorginstituut - Evaluatie van IC Nazorg (Projectleider) 2. ZonMw/NWO- Evaluatie van de kosten-effectivieti van IC-zorg (Projectleider) 3. NFU-Zire (Projectleider) 4. ZonMw - Safety 2 (Projectleider) |
Geen restricties |
|
Koning |
Anesthesioloog-intensivist, Rijnstate Ziekenhuis, Arnhem |
Geen
|
Geen |
Geen restricties |
|
Moviat |
Intensivist Jeroen Bosch ziekenhuis
|
FCCS instructeur
|
Geen |
Geen restricties |
|
Rood (tot 11-03-2024) |
Senior onderzoeker - Projectleider, HAN University of applied sciences |
Vicevoorzitter, V&VN-IC, beroepsvereniging van IC verpleegkundigen |
Ja, NWO Raak SIA |
Geen restricties |
|
Stilma (vanaf 11-03-2024) |
Hogeschool hoofddocent bij cluster verpleegkunde, Hogeschool van Amsterdam, Amsterdam (0,8 FTE) |
Bestuurslid V&VN-IC |
1. NWO - NWO docentenbeurs - promotietraject (Projectleider) 2. KIEM-MV - Circulaire kansen beademingszorg (Projectleider) |
Geen restricties |
|
Van Vliet |
Intensivist / Haaglanden Medisch Centrum |
Bestuursvoorzitter MuzIC (onbetaald) ATLS instructeur (onbetaald) Docent bij de Hogeschool Utrecht bij de PA-opleiding (betaald) |
Geen |
Geen restricties |
|
Weyer |
Cardioloog-intensivist Elkerliek ziekenhuis Helmond |
FCCS instructeur |
Geen |
Geen restricties |
|
De Kleijn |
Physician assistant Intensive care Catharina ziekenhuis Eindhoven |
Commissielid NVIC richtlijnontwikkeling lid NAPA vakgroep intensive care |
Geen |
Geen restricties |
|
Droogh |
Intensivist, UMCG |
Voorzitter commissie transport NVIC, onbetaald Hoofd MICU UMCG |
Geen |
Geen restricties |
|
Van Westeloo |
Intensivist LUMC |
MICU Zuidwest Nederland Eurocross |
Circadiaan onderzoek Philips |
Geen restricties |
|
Jacobs |
Intensivist |
Geen |
Geen |
Geen restricties |
|
Heij |
Intensivist, Spaarne Gasthuis |
Bestuurslid NVIC, onkostenvergoeding Voorzitter cie Beroepsbelangen NVIC Lid ledenraad LAD, onkostenvergoeding |
Geen |
Geen restricties |
|
Rozendaal |
Verpleegkundig Specialist IC/MC |
Docent respiratie en beademing, St. Antoniusacademie (parttime) |
Geen |
Geen restricties |
|
Janssen |
Adviseur Kennisinstituut FMS |
Promovendus UMCU |
Geen |
Geen restricties |
|
Hofstede |
Senior adviseur Kennisinstituut FMS |
Geen |
Geen |
Geen restricties |
Inbreng patiëntenperspectief
Er werd aandacht besteed aan het patiëntenperspectief door een afgevaardigde patiëntenvereniging in de werkgroep (FCIC/IC-Connect). De verkregen input is meegenomen bij het opstellen van de uitgangsvragen, de keuze voor de uitkomstmaten en bij het opstellen van de overwegingen. De conceptleidraad is tevens voor commentaar voorgelegd aan de FCIC/IC-Connect en de eventueel aangeleverde commentaren zijn bekeken en verwerkt.
Kwalitatieve raming van mogelijke financiële gevolgen in het kader van de Wkkgz
Bij de leidraad voerde de werkgroep conform de Wet kwaliteit, klachten en geschillen zorg (Wkkgz) een kwalitatieve raming uit om te beoordelen of de aanbevelingen mogelijk leiden tot substantiële financiële gevolgen. Bij het uitvoeren van deze beoordeling is de richtlijnmodule op verschillende domeinen getoetst (zie het stroomschema bij Werkwijze).
| Module |
Uitkomst raming |
Toelichting |
|
Veiligheidscultuur |
geen financiële gevolgen |
Deze aanbevelingen zijn onveranderd t.o.v. de huidige aanbeveling/huidige zorg. |
Werkwijze
Achtergrond voor de herziening
In 2006 is de eerste kwaliteitsstandaard over de organisatie van de intensive care gepubliceerd en in werking getreden (NVA, 2006). In 2016 werd een herziene kwaliteitsstandaard gepubliceerd door het Zorginstituut Nederland (2016). Deze kwaliteitsstandaard werd vanuit de NVIC aangevuld met de zogenaamde blauwdruk (NVIC, 2021). Daaruit werd een visitatie normenkader ontwikkeld, wat deel uit maakt van de feitelijke handhaving en controle op de kwaliteit door de NVIC (NVIC, 2022).
De kwaliteitsstandaard uit 2016 had een looptijd van vijf jaar en moest na vijf jaar worden geëvalueerd en herzien. Door de COVID-19 pandemie kon de evaluatie pas in 2022 plaatsvinden. De NVIC benoemde een werkgroep die de evaluatie uitvoerde door middel van een enquête die werd gevolgd door interviews (NVIC, 2023). Het Kennisinstituut van de Federatie Medisch Specialisten ondersteunde deze evaluatie. In 2023 is gestart met de herziening van de kwaliteitsstandaard. Gezien de organisatorische aard van de uitgangsvragen, wordt de herziene versie een leidraad genoemd. Dit sluit aan bij de beschreven definities in het rapport Medisch Specialistische Richtlijnen 3.0.
Tijdens de voorbereidende fase voor deze herziening inventariseerde de werkgroep middels de evaluatie van kwaliteitsstandaard en een invitational conference de knelpunten met betrekking tot de organisatie van intensive care afdelingen. Op basis van de uitkomsten van de knelpuntenanalyse zijn door de werkgroep concept-uitgangsvragen opgesteld en definitief vastgesteld.
Aan de start van het proces is met de werkgroep besproken hoe de uitgangsvragen onderbouwd kunnen worden. De werkgroep heeft gekozen voor een combinatie van uitgangsvragen met en zonder literatuursearch. Dit vanwege het organisatorische karakter van de leidraad en specifieke situaties die alleen in Nederland van toepassing zijn. Een uitgebreide beschrijving van de strategie voor zoeken en selecteren van literatuur is te vinden onder ‘Zoeken en selecteren’ onder Onderbouwing. De beoordeling van de kracht van het wetenschappelijke bewijs wordt hieronder toegelicht. Daar waar de literatuur geen antwoord leverde, werd gebruik gemaakt van expert opinie.
Relevante conceptmodules zijn vóór de commentaar- en autorisatiefase eerst nog langs partijen uit de klankbordgroep gestuurd voor input. Binnen de NVIC en de V&VN betrof het de beroepsbelangen commissie, de visitatiecommissie NKIC, de richtlijncommissie, de werkgroep beroepsprofiel, het landelijk netwerk van ICs, de transportcommissie van de NVIC, de V&VN-IC, de V&VN-VS en de besturen van NVIC en V&VN. Buiten de NVIC betrof het, VPned (de vereniging voor practitioners) en NAPA (Nederlandse Associatie Physician Assistants), de NICE (Nationale Intensive Care Evaluatie), en de LHIC (landelijke IC hoofden overleg).
De conceptleidraadmodule werd aan de betrokken (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd ter commentaar. De commentaren werden verzameld en besproken met de werkgroep. Tijdens de commentaarfase heeft tevens een Webinar plaatsgevonden (d.d. 07-01-2025). Naar aanleiding van de commentaren werd de conceptleidraadmodule aangepast en definitief vastgesteld door de werkgroep. De definitieve leidraadmodule werd aan de deelnemende (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd voor autorisatie en door hen geautoriseerd dan wel geaccordeerd.
Voor meer details over de gebruikte richtlijnmethodologie verwijzen wij u naar de Werkwijze.
Zoekverantwoording
Algemene informatie
|
Cluster/richtlijn: NVIC Leidraad Organisatie van Intensive Care |
|
|
Uitgangsvraag/modules: Hoe bevorderen we een positieve veiligheidscultuur op IC-afdelingen? |
|
|
Database(s): Embase.com, Ovid/Medline |
Datum: 28 november 2023 |
|
Periode: vanaf 2000 |
Talen: geen restrictie |
|
Literatuurspecialist: Alies van der Wal |
Rayyan review: https://rayyan.ai/reviews/858695 |
|
BMI-zoekblokken: voor verschillende opdrachten wordt (deels) gebruik gemaakt van de zoekblokken van BMI-Online https://blocks.bmi-online.nl/ Deduplication: voor het ontdubbelen is gebruik gemaakt van http://dedupendnote.nl/ |
|
|
Toelichting: Voor deze vraag is gezocht op de elementen:
→De sleutelartikelen worden niet gevonden met deze search doordat ze niet specifiek over IC gaan. Er is voor gekozen om de search in eerste instantie wel op IC te richten. Eventueel kan in een later stadium breder worden gezocht, mocht deze strategie onvoldoende opleveren. |
|
|
Te gebruiken voor richtlijntekst: In de databases Embase.com, Ovid/Medline, Ovid/PsycInfo en Ebsco/CINAHL is op 28 november 2023 systematisch gezocht naar systematische reviews, RCTs en observationele studies over veiligheidscultuur op IC-afdelingen. De literatuurzoekactie leverde 462 unieke treffers op. |
|
Zoekopbrengst
|
|
EMBASE |
OVID/MEDLINE |
OVID/PSYCINFO |
EBSCO CINAHL |
Ontdubbeld |
|
SR |
9 |
12 |
11 |
15 |
41 |
|
RCT |
53 |
67 |
6 |
111 |
190 |
|
Observationele studies |
121 |
141 |
45 |
97 |
231 |
|
Totaal |
183 |
220 |
62 |
223 |
462* |
*in Rayyan
Zoekstrategie
Embase.com
|
No. |
Query |
Results |
|
#1 |
'intensive care unit'/exp OR 'intensive care'/exp OR 'intensive care':ti,ab,kw OR icu:ti,ab,kw OR 'critical care':ti,ab,kw |
1205986 |
|
#2 |
'organizational culture'/exp/mj OR (((work OR workplace OR organisation* OR organization* OR institution* OR safety OR team) NEAR/3 (cultur* OR climate)):ti,kw) |
7547 |
|
#3 |
#1 AND #2 |
366 |
|
#4 |
#3 NOT ('conference abstract'/it OR 'editorial'/it OR 'letter'/it OR 'note'/it) NOT (('animal'/exp OR 'animal experiment'/exp OR 'animal model'/exp OR 'nonhuman'/exp) NOT 'human'/exp) |
305 |
|
#5 |
#3 AND [2000-2024]/py |
362 |
|
#6 |
'meta analysis'/exp OR 'meta analysis (topic)'/exp OR metaanaly*:ti,ab OR 'meta analy*':ti,ab OR metanaly*:ti,ab OR 'systematic review'/de OR 'cochrane database of systematic reviews'/jt OR prisma:ti,ab OR prospero:ti,ab OR (((systemati* OR scoping OR umbrella OR 'structured literature') NEAR/3 (review* OR overview*)):ti,ab) OR ((systemic* NEAR/1 review*):ti,ab) OR (((systemati* OR literature OR database* OR 'data base*') NEAR/10 search*):ti,ab) OR (((structured OR comprehensive* OR systemic*) NEAR/3 search*):ti,ab) OR (((literature NEAR/3 review*):ti,ab) AND (search*:ti,ab OR database*:ti,ab OR 'data base*':ti,ab)) OR (('data extraction':ti,ab OR 'data source*':ti,ab) AND 'study selection':ti,ab) OR ('search strategy':ti,ab AND 'selection criteria':ti,ab) OR ('data source*':ti,ab AND 'data synthesis':ti,ab) OR medline:ab OR pubmed:ab OR embase:ab OR cochrane:ab OR (((critical OR rapid) NEAR/2 (review* OR overview* OR synthes*)):ti) OR ((((critical* OR rapid*) NEAR/3 (review* OR overview* OR synthes*)):ab) AND (search*:ab OR database*:ab OR 'data base*':ab)) OR metasynthes*:ti,ab OR 'meta synthes*':ti,ab |
981669 |
|
#7 |
'clinical trial'/exp OR 'randomization'/exp OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp OR 'placebo'/exp OR 'prospective study'/exp OR rct:ab,ti OR random*:ab,ti OR 'single blind':ab,ti OR 'randomised controlled trial':ab,ti OR 'randomized controlled trial'/exp OR placebo*:ab,ti |
3925045 |
|
#8 |
'major clinical study'/de OR 'clinical study'/de OR 'case control study'/de OR 'family study'/de OR 'longitudinal study'/de OR 'retrospective study'/de OR 'prospective study'/de OR 'comparative study'/de OR 'cohort analysis'/de OR ((cohort NEAR/1 (study OR studies)):ab,ti) OR (('case control' NEAR/1 (study OR studies)):ab,ti) OR (('follow up' NEAR/1 (study OR studies)):ab,ti) OR (observational NEAR/1 (study OR studies)) OR ((epidemiologic NEAR/1 (study OR studies)):ab,ti) OR (('cross sectional' NEAR/1 (study OR studies)):ab,ti) |
7953101 |
|
#9 |
'case control study'/de OR 'comparative study'/exp OR 'control group'/de OR 'controlled study'/de OR 'controlled clinical trial'/de OR 'crossover procedure'/de OR 'double blind procedure'/de OR 'phase 2 clinical trial'/de OR 'phase 3 clinical trial'/de OR 'phase 4 clinical trial'/de OR 'pretest posttest design'/de OR 'pretest posttest control group design'/de OR 'quasi experimental study'/de OR 'single blind procedure'/de OR 'triple blind procedure'/de OR (((control OR controlled) NEAR/6 trial):ti,ab,kw) OR (((control OR controlled) NEAR/6 (study OR studies)):ti,ab,kw) OR (((control OR controlled) NEAR/1 active):ti,ab,kw) OR 'open label*':ti,ab,kw OR (((double OR two OR three OR multi OR trial) NEAR/1 (arm OR arms)):ti,ab,kw) OR ((allocat* NEAR/10 (arm OR arms)):ti,ab,kw) OR placebo*:ti,ab,kw OR 'sham-control*':ti,ab,kw OR (((single OR double OR triple OR assessor) NEAR/1 (blind* OR masked)):ti,ab,kw) OR nonrandom*:ti,ab,kw OR 'non-random*':ti,ab,kw OR 'quasi-experiment*':ti,ab,kw OR crossover:ti,ab,kw OR 'cross over':ti,ab,kw OR 'parallel group*':ti,ab,kw OR 'factorial trial':ti,ab,kw OR ((phase NEAR/5 (study OR trial)):ti,ab,kw) OR ((case* NEAR/6 (matched OR control*)):ti,ab,kw) OR ((match* NEAR/6 (pair OR pairs OR cohort* OR control* OR group* OR healthy OR age OR sex OR gender OR patient* OR subject* OR participant*)):ti,ab,kw) OR ((propensity NEAR/6 (scor* OR match*)):ti,ab,kw) OR versus:ti OR vs:ti OR compar*:ti OR ((compar* NEAR/1 study):ti,ab,kw) OR (('major clinical study'/de OR 'clinical study'/de OR 'cohort analysis'/de OR 'observational study'/de OR 'cross-sectional study'/de OR 'multicenter study'/de OR 'correlational study'/de OR 'follow up'/de OR cohort*:ti,ab,kw OR 'follow up':ti,ab,kw OR followup:ti,ab,kw OR longitudinal*:ti,ab,kw OR prospective*:ti,ab,kw OR retrospective*:ti,ab,kw OR observational*:ti,ab,kw OR 'cross sectional*':ti,ab,kw OR cross?ectional*:ti,ab,kw OR multicent*:ti,ab,kw OR 'multi-cent*':ti,ab,kw OR consecutive*:ti,ab,kw) AND (group:ti,ab,kw OR groups:ti,ab,kw OR subgroup*:ti,ab,kw OR versus:ti,ab,kw OR vs:ti,ab,kw OR compar*:ti,ab,kw OR 'odds ratio*':ab OR 'relative odds':ab OR 'risk ratio*':ab OR 'relative risk*':ab OR 'rate ratio':ab OR aor:ab OR arr:ab OR rrr:ab OR ((('or' OR 'rr') NEAR/6 ci):ab))) |
14617226 |
|
#10 |
#5 AND #6 - SR |
9 |
|
#11 |
#5 AND #7 NOT #10 - RCT |
53 |
|
#12 |
#5 AND (#8 OR #9) NOT (#10 OR #11) - observationeel |
121 |
|
#13 |
#10 OR #11 OR #12 |
183 |
Ovid/Medline
|
# |
Searches |
Results |
|
1 |
exp Critical Care/ or exp Intensive Care Units/ or 'intensive care'.ti,ab,kf. or icu.ti,ab,kf. or 'critical care'.ti,ab,kf. |
307646 |
|
2 |
exp Organizational Culture/ or ((work or workplace or organisation* or organization* or institution* or safety or team) adj3 (cultur* or climate)).ti,kf. |
22491 |
|
3 |
1 and 2 |
684 |
|
4 |
3 not (comment/ or editorial/ or letter/) not ((exp animals/ or exp models, animal/) not humans/) |
652 |
|
5 |
limit 4 to yr="2000 -Current" |
620 |
|
6 |
meta-analysis/ or meta-analysis as topic/ or (metaanaly* or meta-analy* or metanaly*).ti,ab,kf. or systematic review/ or cochrane.jw. or (prisma or prospero).ti,ab,kf. or ((systemati* or scoping or umbrella or "structured literature") adj3 (review* or overview*)).ti,ab,kf. or (systemic* adj1 review*).ti,ab,kf. or ((systemati* or literature or database* or data-base*) adj10 search*).ti,ab,kf. or ((structured or comprehensive* or systemic*) adj3 search*).ti,ab,kf. or ((literature adj3 review*) and (search* or database* or data-base*)).ti,ab,kf. or (("data extraction" or "data source*") and "study selection").ti,ab,kf. or ("search strategy" and "selection criteria").ti,ab,kf. or ("data source*" and "data synthesis").ti,ab,kf. or (medline or pubmed or embase or cochrane).ab. or ((critical or rapid) adj2 (review* or overview* or synthes*)).ti. or (((critical* or rapid*) adj3 (review* or overview* or synthes*)) and (search* or database* or data-base*)).ab. or (metasynthes* or meta-synthes*).ti,ab,kf. |
710647 |
|
7 |
exp clinical trial/ or randomized controlled trial/ or exp clinical trials as topic/ or randomized controlled trials as topic/ or Random Allocation/ or Double-Blind Method/ or Single-Blind Method/ or (clinical trial, phase i or clinical trial, phase ii or clinical trial, phase iii or clinical trial, phase iv or controlled clinical trial or randomized controlled trial or multicenter study or clinical trial).pt. or random*.ti,ab. or (clinic* adj trial*).tw. or ((singl* or doubl* or treb* or tripl*) adj (blind$3 or mask$3)).tw. or Placebos/ or placebo*.tw. |
2663255 |
|
8 |
Epidemiologic studies/ or case control studies/ or exp cohort studies/ or Controlled Before-After Studies/ or Case control.tw. or cohort.tw. or Cohort analy$.tw. or (Follow up adj (study or studies)).tw. or (observational adj (study or studies)).tw. or Longitudinal.tw. or Retrospective*.tw. or prospective*.tw. or consecutive*.tw. or Cross sectional.tw. or Cross-sectional studies/ or historically controlled study/ or interrupted time series analysis/ [Onder exp cohort studies vallen ook longitudinale, prospectieve en retrospectieve studies] |
4594775 |
|
9 |
Case-control Studies/ or clinical trial, phase ii/ or clinical trial, phase iii/ or clinical trial, phase iv/ or comparative study/ or control groups/ or controlled before-after studies/ or controlled clinical trial/ or double-blind method/ or historically controlled study/ or matched-pair analysis/ or single-blind method/ or (((control or controlled) adj6 (study or studies or trial)) or (compar* adj (study or studies)) or ((control or controlled) adj1 active) or "open label*" or ((double or two or three or multi or trial) adj (arm or arms)) or (allocat* adj10 (arm or arms)) or placebo* or "sham-control*" or ((single or double or triple or assessor) adj1 (blind* or masked)) or nonrandom* or "non-random*" or "quasi-experiment*" or "parallel group*" or "factorial trial" or "pretest posttest" or (phase adj5 (study or trial)) or (case* adj6 (matched or control*)) or (match* adj6 (pair or pairs or cohort* or control* or group* or healthy or age or sex or gender or patient* or subject* or participant*)) or (propensity adj6 (scor* or match*))).ti,ab,kf. or (confounding adj6 adjust*).ti,ab. or (versus or vs or compar*).ti. or ((exp cohort studies/ or epidemiologic studies/ or multicenter study/ or observational study/ or seroepidemiologic studies/ or (cohort* or 'follow up' or followup or longitudinal* or prospective* or retrospective* or observational* or multicent* or 'multi-cent*' or consecutive*).ti,ab,kf.) and ((group or groups or subgroup* or versus or vs or compar*).ti,ab,kf. or ('odds ratio*' or 'relative odds' or 'risk ratio*' or 'relative risk*' or aor or arr or rrr).ab. or (("OR" or "RR") adj6 CI).ab.)) |
5569029 |
|
10 |
5 and 6 - SR |
12 |
|
11 |
(5 and 7) not 10 - RCT |
67 |
|
12 |
(5 and (8 or 9)) not (10 or 11) - observationeel |
141 |
|
13 |
10 or 11 or 12 |
220 |
Ovid/PsycInfo
|
# |
Searches |
Results |
|
1 |
exp Intensive Care/ or (intensive care or icu or critical care).ti,ab,id. |
13663 |
|
2 |
exp Organizational Culture/ or ((work or workplace or organisation* or organization* or institution* or safety or team) adj3 (cultur* or climate)).ti,ab,id. |
33670 |
|
3 |
1 and 2 |
153 |
|
4 |
limit 3 to yr="2000 -Current" |
147 |
|
5 |
((literature review or systematic review or meta analysis).md. or "literature review"/ or meta analysis/ or (((meta adj2 analy*) or metaanaly* or (synthes* adj2 (literature* or research* or studies or data)) or (pooled and analys*) or ((data adj1 pool*) and studies) or medline or medlars or embase or cinahl or scisearch or psychlit or psyclit or cinhal or cancerlit or cochrane or bids or pubmed or ovid or ((hand or manual or database* or computer*) adj1 search*) or (electronic adj1 (database* or data base or data bases))).ti,ab,id. or (review* or overview).ti. or (bibliograph* or relevant journals or ((review* or overview*) adj9 (systematic* or methodologic* or quantitativ* or research* or literature* or studies or trial* or effective*))).ab.)) not (((retrospective* or record* or case* or patient*) adj1 review*) or ((patient* or review*) adj1 chart*)).ti,ab,id. |
482880 |
|
6 |
exp clinical trial/ or randomized controlled trial/ or randomized controlled trials as topic/ or Random Allocation/ or Double-Blind Method/ or Single-Blind Method/ or (clinical trial, phase i or clinical trial, phase ii or clinical trial, phase iii or clinical trial, phase iv or controlled clinical trial or randomized controlled trial or multicenter study or clinical trial).pt. or random*.ti,ab. or (clinic* adj trial*).tw. or ((singl* or doubl* or treb* or tripl*) adj (blind$3 or mask$3)).tw. or Placebos/ or placebo*.tw. |
293465 |
|
7 |
Epidemiologic studies/ or case control studies/ or exp Cohort Analysis/ or Controlled Before-After Studies/ or Case control.tw. or cohort*.tw. or Cohort analy$.tw. or (Follow up adj (study or studies)).tw. or (observational adj (study or studies)).tw. or Longitudinal.tw. or Retrospective*.tw. or prospective*.tw. or consecutive*.tw. or Cross sectional.tw. or Cross-sectional studies/ or historically controlled study/ or interrupted time series analysis/ |
466124 |
|
8 |
4 and 5 - SR |
11 |
|
9 |
(4 and 6) not 8 - RCT |
6 |
|
10 |
(4 and 7) not (8 or 9) - observationeel |
45 |
|
11 |
8 or 9 or 10 |
62 |
Ebsco/CINAHL
|
# |
Searches |
Results |
|
S1 |
(MH "Intensive Care Units+") OR (MH "Critical Care Nursing+") OR (MH "Critical Care+") OR TI ("critical care" OR "intensive care” OR icu) OR AB ("critical care" OR "intensive care” OR icu) |
163,47 |
|
S2 |
(MM "Organizational Culture") OR TI (((work OR workplace OR organisation* OR organization* OR institution* OR safety OR team) N3 (cultur* OR climate))) |
11,599 |
|
S3 |
S1 AND S2 |
390 |
|
S4 |
(MH "Meta Analysis") or TX (meta-analy* or metanaly* or metaanaly* or meta analy*) or TX (systematic* N5 review*) or (evidence* N5 review*) or (methodol* N5 review*) or (quantitativ* N5 review*) or TX (systematic* N5 overview*) or (evidence* N5 overview*) or (methodol* N5 overview*) or (quantitativ* N5 overview*) or TX (systematic* N5 survey*) or (evidence* N5 survey*) or (methodol* N5 survey*) or (quantitativ* N5 survey*) or TX (systematic* N5 overview*) or (evidence* N5 overview*) or (methodol* N5 overview*) or (quantitativ* N5 overview*) or TX (pool* N2 data) or (combined N2 data) or (combining N2 data) or (pool* N2 trials) or (combined N2 trials) or (combining N2 trials) or (pool* N2 studies) or (combined N2 studies) or (combining N2 studies) or (pool* N2 results) or (combined N2 results) or (combining N2 results) |
320,934 |
|
S5 |
(MH "Clinical Trials+") OR (PT (Clinical trial)) OR (MH "Random Assignment") OR (MH "Quantitative Studies") OR (TX ((clini* N1 trial*) OR (singl* N1 blind*) OR (singl* N1 mask*) OR (doubl* N1 blind*) OR (doubl* N1 mask*) OR (tripl* N1 blind*) OR (tripl* N1 mask*) OR (random* N1 allocat*) OR placebo* OR ((waitlist* OR (wait* and list*)) and (control* OR group)) OR "treatment as usual" OR tau OR (control* N3 (trial* OR study OR studies OR group*)) OR randomized OR randomised)) |
1,987,559 |
|
S6 |
(MH "Case Control Studies+") OR (MH "Case Studies") OR (MH "Cross Sectional Studies") OR (MH "Prospective Studies+") OR (MH "Retrospective Panel Studies") OR (MH "Correlational Studies") OR TI "case control" OR TI “case referent” OR AB “case referent*” OR TI “case stud*” OR AB “case stud*” OR TI “case series” OR AB “case series” OR TI cohort* OR AB cohort* OR TI “cross sectional” OR AB “cross sectional” OR TI “follow up” OR AB “follow up” OR TI longitudinal OR AB longitudinal OR TI retrospective* OR AB retrospective* OR TI prospective* OR AB prospective* OR TI observational OR AB observational OR TI “Controlled before and after” OR AB “Controlled before and after” OR TI “Interrupted time series” OR AB “Interrupted time series” OR TI Correlational OR AB Correlational |
1,575,205 |
|
S7 |
S3 AND S4 - SR |
15 |
|
S8 |
S3 AND S5 NOT S7 - RCT |
111 |
|
S9 |
S3 AND S6 NOT (S7 OR S8) - observationeel |
97 |
|
S10 |
S7 OR S8 OR S9 |
223 |