Optimale type lijn
Uitgangsvraag
Wat is het optimale type lijn voor een centraal veneuze toegang bij volwassen patiënten?
Aanbeveling
Kies bij het plaatsen van een centraal veneuze katheter:
- bij kortdurend gebruik (tot twee weken) voor een PICC of een niet-getunnelde centraal veneuze katheter zonder cuff;
- bij middellang gebruik (twee weken tot drie maanden) bij voorkeur voor een PICC;
- bij langdurig intermitterend gebruik (langer dan drie maanden) in overleg met de patiënt voor een PICC of een poortkatheter;
- bij langdurig dagelijks gebruik (langer dan drie maanden) voor een PICC of een getunnelde centraal veneuze katheter met cuff.
Voor patiënten met chronische nierschade stadium 4 en 5 (eGFR <30 mL/min) zijn aanvullende aanbevelingen opgenomen in de richtlijn ‘Zorg bij eindstadium nierfalen’ (Module Vaatpreservatie).
Overwegingen
Voor- en nadelen van de interventie en de kwaliteit van het bewijs
Er is literatuuronderzoek uitgevoerd naar het optimale type lijn voor een centraal veneuze toegang. Mortaliteit, complicaties (waaronder infecties, diep veneuze trombose, katheter-gerelateerde bloedbaaninfecties, longembolieën) en patiënttevredenheid werden gedefinieerd als cruciale uitkomstmaten. Het aantal katheter-gerelateerde ingrepen per jaar en kwaliteit van leven werden als belangrijke uitkomstmaat gedefinieerd. De module is opgebouwd uit vier verschillende PICO’s. Perifeer geplaatste centrale veneuze katheters (PICC) werden vergeleken met poortkatheters (PAC; PICO 1), getunnelde centraal veneuze katheters met cuff ( PICO 2) en niet-getunnelde centraal veneuze katheters zonder cuff (PICO 3). Tenslotte werden getunnelde centraal veneuze katheters met niet-getunnelde centraal veneuze katheters vergeleken (PICO 4).
Er werden vijf studies geïncludeerd die een PICC vergeleken met een poortkatheter bij patiënten die gedurende drie tot twaalf maanden chemotherapie kregen. Er was een klinisch relevant verschil in het optreden van complicaties (alle complicaties samen) in het voordeel van poortkatheters. Ten aanzien van specifieke complicaties was er minder kans op een diep veneuze trombose, maar meer kans op een infectie (inclusief katheter-gerelateerde bloedbaaninfectie) en een longembolie bij een poortkatheter. Eén studie rapporteerde een hogere mortaliteit bij patiënten met een poortkatheter dan bij patiënten met een PICC. De belangrijke uitkomstmaat ‘katheter-gerelateerde ingrepen per jaar’ werd niet gerapporteerd. De bewijskracht voor de uitkomsten in het voordeel van PICCs is zeer laag, wat betekent dat de gevonden klinisch relevante verschillen voorzichtig geïnterpreteerd moeten worden. De bewijskracht voor de uitkomstmaten in het voordeel van poortkatheters is laag. De voornaamste reden voor de lage en zeer lage bewijskracht is het gebrek aan blindering van patiënten en de kleine aantallen patiënten in de geïncludeerde studies. We concluderen dat een poortkatheter mogelijk resulteert in minder complicaties en diepe veneuze trombose in vergelijking met een PICC.
Voor de vergelijking van een PICC met een getunnelde centraal veneuze katheter met cuff werd één studie geïncludeerd met patiënten die gedurende drie tot twaalf maanden chemotherapie kregen. Er was geen klinisch relevant verschil in het optreden van complicaties (alle complicaties samen) tussen de twee soorten vaattoegangen. Ten aanzien van specifieke complicaties was er meer kans op infectie (inclusief katheter-gerelateerde bloedbaaninfectie) en minder kans op diep veneuze trombose bij getunnelde centraal veneuze katheters. De bewijskracht voor deze uitkomsten varieerde echter van laag tot zeer laag. De cruciale uitkomstmaat mortaliteit en de belangrijke uitkomstmaat ‘katheter-gerelateerde ingrepen per jaar’ werden niet gerapporteerd. We concluderen dat er met het literatuuronderzoek geen belangrijke verschillen werden gevonden tussen getunnelde centraal veneuze katheters en PICCs.
Er werden vier studies geïncludeerd die een PICC vergeleken met een niet-getunnelde centraal veneuze katheter zonder cuff bij diverse patiëntengroepen die overwegend kortdurend een centraal veneuze toegang nodig hadden. De bewijskracht voor alle uitkomstmaten was zeer laag. De klinisch relevante verschillen die werden gevonden voor het optreden van complicaties (alle complicaties samen, diep veneuze trombose en katheter-gerelateerde bloedbaaninfecties) en mortaliteit moeten daarom voorzichtig worden geïnterpreteerd. De belangrijke uitkomstmaat ‘katheter-gerelateerde ingrepen per jaar’ werd niet gerapporteerd. We concluderen dat er met het literatuuronderzoek geen belangrijke verschillen werden gevonden tussen niet-getunnelde centraal veneuze katheters en PICCs.
Voor de vergelijking tussen getunnelde en niet-getunnelde centraal veneuze katheters werden vijf studies geïncludeerd. In geen van deze studies werd een getunnelde centraal veneuze katheter met cuff vergeleken met een niet-getunnelde centraal veneuze katheter zonder cuff. De studies werden in diverse situaties uitgevoerd: twee studies vonden plaats op de intensive care unit bij patiënten die een centraal veneuze katheter in v. jugularis of in v. femoralis kregen met een gemiddelde gebruiksduur van een week, twee studies werden uitgevoerd bij patiënten die een PICC in de arm kregen voor chemotherapie met een gemiddelde gebruiksduur van twee tot drie maanden, en één studie werd uitgevoerd bij patiënten die een centraal veneuze katheter in v. subclavia kregen voor chemotherapie met een gemiddelde gebruiksduur van vier maanden. In deze gerandomiseerde studies hadden patiënten met getunnelde centraal veneuze katheters mogelijk minder infecties dan patiënten met niet-getunnelde centraal veneuze katheters. De bewijskracht voor dit voordeel van getunnelde centraal veneuze katheter is echter zeer laag, omdat er weinig katheter-gerelateerde infecties optraden waardoor de effectgrootte niet precies kon worden vastgesteld en omdat er een grote klinische heterogeniteit was tussen de studies.
In meerdere studies werd de cruciale uitkomstmaat patiënttevredenheid en de belangrijke uitkomstmaat kwaliteit van leven beschreven. Slechts één studie rapporteerde deze informatie op een manier die bruikbaar was voor de systematische literatuuranalyse. De informatie over deze uitkomstmaten wordt kwalitatief geïnterpreteerd in de sectie ‘Waarden en voorkeuren van patiënten’.
Waarden en voorkeuren van patiënten (en evt. hun verzorgers)
Enkele onderzoeken die geïncludeerd zijn in de literatuuranalyse hebben de tevredenheid van patiënten met de vaattoegang gemeten (Clatot, 2020; Moss, 2021; Taxbro, 2019; Xiao 2021). Hoewel deze gegevens niet gebruikt konden worden voor een meta-analyse, geven deze onderzoeken wel inzicht in de waarden en voorkeuren van patiënten bij het maken van een keuze tussen een PICC en een poortkatheter. In één onderzoek zorgde het maken van een kort subcutaan tunneltraject niet tot meer discomfort tijdens het inbrengen van een PICC. In een ander onderzoek was zowel het inbrengen als het gebruik van een poortkatheter pijnlijker dan een PICC. In drie onderzoeken gaf een poortkatheter meer gemak bij persoonlijke hygiëne (douchen en baden) dan een PICC. In twee onderzoeken ervaarden patiënten een poortkatheter als meer comfortabel dan een PICC (bijvoorbeeld bij slapen, aankleden, intimiteit en sport). Als onderdeel van de CAVA studie werd een kwalitatief onderzoek uitgevoerd bij 42 patiënten in zes focus groepen (Ryan, 2019). Hoewel de deelnemers tevreden waren met elk van de vaattoegangen, hadden poortkatheters in de beleving van de patiënten praktische voordelen: ze zijn minder opvallend en verstorend en eenvoudiger te onderhouden, waardoor er meer vrijheid en minder indringing in persoonlijke relaties wordt ervaren. Deze voordelen hadden een belangrijke invloed op het psychologisch en emotioneel welzijn van de deelnemers. Metingen van algemene of ziekte-specifieke kwaliteit van leven in de onderzoeken van de literatuuranalyse (Clatot, 2020; Moss, 2021; Patel, 2013) lieten echter geen verschil zien tussen PICCs en poortkatheters. Waarschijnlijk werd de kwaliteit van leven in deze onderzoeken meer bepaald door de onderliggende ziekte en haar behandeling dan door het soort vaattoegang.
Kosten (middelenbeslag)
In de CAVA studie werden de kosten van een PICC, centraal ingebrachte getunnelde centraal veneuze katheter en poortkatheter met elkaar vergeleken (Moss, 2021). Een poortkatheter was gemiddeld €1915,- duurder dan een PICC en een centraal ingebrachte getunnelde centraal veneuze katheter was gemiddeld €1786,- duurder dan een PICC. Voor kortdurend gebruik bij patiënten die parenterale voeding nodig hebben lijkt een niet-getunnelde centraal veneuze katheter goedkoper te zijn dan een PICC (€15,- versus €21,- per dag; Cowl, 2010).
Aanvaardbaarheid, haalbaarheid en implementatie
In de MAGIC studie werden verschillende scenario’s waarin patiënten een intraveneuze toegang nodig hadden voorgelegd aan een panel van vijftien experts (Chopra, 2015). Wanneer medicatie moest worden toegediend waarvoor een centraal veneuze toegang nodig was, vond het panel dat een niet-getunnelde centraal veneuze katheter gepast was voor kortdurend gebruik tot twee weken. Voor een langere periode werd de voorkeur gegeven aan een getunnelde centraal veneuze katheter (vanaf twee weken) of een poortkatheter (vanaf één maand). Een PICC werd beschouwd als een gepaste vaattoegang voor iedere gebruiksduur.
Door het tunnelen van een centraal veneuze katheter komt de katheter op een andere plaats uit de huid dan de plaats waar de katheter in de vene gaat. Hierdoor kan de operateur een huidpoort kiezen die minder gevoelig is voor infectie, die eenvoudiger te verzorgen is of die comfortabeler is voor de patiënt. Zo wordt een centraal veneuze katheter in v. jugularis doorgaans getunneld tot onder het sleutelbeen en wordt een getunnelde centraal veneuze katheter in v. femoralis naar de laterale zijde van het bovenbeen geleid. Een cuff is bedoeld om de katheter te fixeren en om infectie tegen te gaan. De cuff ligt bij voorkeur twee centimeter voorbij de huidpoort, zodat deze vanuit de huidpoort kan worden losgemaakt bij het verwijderen van de centraal veneuze katheter maar toch diep genoeg ligt om niet spontaan naar buiten te komen. Het tunnelen van een PICC kan zinvol zijn bij patiënten met smalle venen in de bovenarm. Door het maken van een subcutaan tunneltraject is het soms mogelijk om een grotere vene proximaal in de bovenarm aan te prikken en de katheter toch op een comfortabele plaats halverwege de bovenarm uit de huid te laten komen. Deze techniek leidt mogelijk tot minder veneuze trombose.
Rationale van de aanbeveling: weging van argumenten voor en tegen de interventies
Voor kortdurend gebruik (minder dan twee weken; bijvoorbeeld voor postoperatieve parenterale voeding) is er keuze tussen een PICC of een niet-getunnelde centraal veneuze katheter. Een niet-getunnelde centraal veneuze katheter zonder cuff heeft in deze situatie de voorkeur boven een getunnelde centraal veneuze katheter met cuff, vanwege de lagere complexiteit van het plaatsen en verwijderen van deze katheters. Het wetenschappelijk bewijs geeft geen richting bij de keuze tussen PICC en niet-getunnelde centraal veneuze katheter omdat het bewijs van zeer lage kwaliteit is en zich vooral richt op specifieke patiënten populaties (neurologische intensive care unit en hematologische maligniteit). Omdat de duur van het gebruik niet altijd goed is in te schatten en een niet-getunnelde centraal veneuze katheter minder geschikt is voor gebruik buiten het ziekenhuis lijkt een PICC in veel gevallen de aangewezen keuze.
Voor middellang gebruik (twee weken tot drie maanden; bijvoorbeeld voor toediening van antibiotica) is er keuze tussen een PICC of een getunnelde centraal veneuze katheter met cuff. Een getunnelde centraal veneuze katheter met cuff heeft in deze situatie de voorkeur boven een niet-getunnelde centraal veneuze katheter zonder cuff, vanwege de mogelijke reductie van het aantal infecties, de verminderde kans op dislocatie van de katheter wanneer de cuff (een manchet rond de katheter) na enkele weken is ingegroeid in het omringende weefsel, en de mogelijkheid om de uittredeplaats van de katheter zo te plaatsen dat deze het meest comfortabel is voor de patiënt. De CAVA studie suggereert dat er meer infecties optreden bij getunnelde centraal veneuze katheters dan bij PICCs, terwijl er vaker mechanische problemen zijn bij PICCs. Er is geen verschil in patiënttevredenheid en kwaliteit van leven bij gebruik van een PICC of een getunnelde centraal veneuze katheter. Het is goedkoper en in veel ziekenhuizen logistiek eenvoudiger om een PICC te plaatsen dan een getunnelde centraal veneuze katheter. We adviseren daarom om in deze situatie voor een PICC te kiezen.
Voor langdurig intermitterend gebruik (langer dan drie maanden; bijvoorbeeld voor toediening van chemotherapie) is er keuze tussen een PICC of een poortkatheter. De meta-analyse suggereert dat er minder complicaties optreden bij een poortkatheter dan bij een PICC. Dit verschil betreft met name het optreden van diep veneuze trombose. Het plaatsen, verzorgen, gebruiken en verwijderen van een PICC en een poortkatheter verschilt aanzienlijk. Dit maakt dat de voorkeuren van individuele patiënten een belangrijke rol kunnen spelen bij de keuze. Kwalitatief onderzoek laat zien dat poortkatheters voor veel patiënten praktische voordelen hebben.
Voor langdurig dagelijks gebruikt is er keuze tussen een PICC of een getunnelde centraal veneuze katheter met cuff; de argumenten die bij middellang gebruik werden genoemd zijn hier ook van toepassing. Wanneer de vaattoegang mogelijk levenslang nodig is (bijvoorbeeld bij chronische parenterale voeding), is een getunnelde centraal veneuze katheter met cuff echter de aangewezen keuze. Een diep veneuze trombose als gevolg van een PICC (ook wanneer deze geen symptomen geeft) kan het aanleggen van een arterioveneuze vaattoegang in de arm veel moeilijker maken. Zo’n vaattoegang kan nodig zijn wanneer er recidiverende infecties optreden van centraal veneuze katheters.
Onderbouwing
Achtergrond
Wanneer een patiënt een centraal veneuze toegang nodig heeft, kan er een keuze worden gemaakt tussen vier verschillende soorten lijnen: een perifeer ingebrachte centrale katheter (PICC), een poortkatheter, een getunnelde centraal veneuze katheter met cuff en een niet-getunnelde centraal veneuze katheter zonder cuff. Elke soort lijn heeft voor- en nadelen bij het inbrengen, gebruiken en verwijderen van de lijn en heeft een eigen kans op complicaties (met name veneuze trombose en infectie). De verwachte gebruiksduur (kortdurend [tot twee weken], middellang [twee weken tot drie maanden], of langdurend [meer dan drie maanden]) en de gebruiksfrequentie (dagelijks of intermitterend) spelen mee bij de keuze voor een bepaald soort lijn. In deze module worden aanbevelingen gedaan over de keuze voor het soort lijn in verschillende klinische situaties.
Voor patiënten op de intensive care wordt verwezen naar de Richtlijn Centraal veneuze lijn van de NVIC. De aanleg van een vaattoegang voor hemodialyse valt buiten de afbakening van deze richtlijn en wordt beschreven in de richtlijn ‘Vaattoegang voor hemodialyse’.
Conclusies / Summary of Findings
PICO 1. Peripherally inserted central (venous) catheters (PICC) versus Port catheter (PAC)
1. Mortality (critical)
|
Low GRADE |
A peripherally inserted central (venous) catheter may reduce overall mortality when compared with a port catheter in adult patients with a central venous access.
Source: Taxbro, 2019 |
2. Complications (all combined) (critical)
|
Low GRADE |
A port catheter may reduce the number of complications (all combined) when compared with a peripherally inserted central (venous) catheter in adult patients with a central venous access.
Sources: Moss, 2021; Patel, 2013; Taxbro, 2019; Clemons, 2020 |
2a. Infections
|
Very low GRADE |
The evidence was very uncertain about the effect of a peripherally inserted central (venous) catheter on infections when compared with a port catheter in adult patients with a central venous access.
Sources: Clatot, 2020; Clemons, 2020; Moss, 2021; Patel, 2013; Taxbro, 2019 |
2b. Deep venous thrombosis
|
Low GRADE |
A port catheter may reduce the number of deep venous thromboses when compared with a peripherally inserted central (venous) catheter in adult patients with a central venous access.
Sources: Clatot, 2020; Clemons, 2020; Moss, 2021; Patel, 2013; Taxbro, 2019 |
2c. Catheter-related bloodstream infections
|
Very low GRADE |
The evidence was very uncertain about the effect of a peripherally inserted central (venous) catheter on catheter-related bloodstream infections when compared with a port catheter in adult patients with a central venous access.
Sources: Moss, 2021; Taxbro, 2019 |
2d. Catheter removal
|
Very low GRADE |
The evidence was very uncertain about the effect of a peripherally inserted central (venous) catheter on catheter removals when compared with a port catheter in adult patients with a central venous access.
Sources: Clatot, 2020; Clemons, 2020 |
2e. Pulmonary embolism
|
Very low GRADE |
The evidence was very uncertain about the effect of a peripherally inserted central (venous) catheter on pulmonary embolism when compared with a port catheter in adult patients with a central venous access.
Sources: Clemons, 2020; Moss, 2021 |
3. Patient satisfaction (critical)
|
No GRADE |
Due to a lack of relevant literature, it was not possible to draw a conclusion with regards to the outcome patient satisfaction for treatment with a peripherally inserted central (venous) catheter versus a port catheter in adult patients with a central venous access.
Source(s): - |
4. Catheter-related interventions per year (important)
|
No GRADE |
Due to a lack of relevant literature, it was not possible to draw a conclusion with regards to the outcome catheter-related interventions per year for treatment with a peripherally inserted central (venous) catheter versus a port catheter in adult patients with a central venous access.
Source(s): - |
5. Quality of life (important)
|
Very low GRADE |
The evidence was very uncertain about the effect of a peripherally inserted central (venous) catheter on quality of life when compared with a port catheter in adult patients with a central venous access.
Source: Moss, 2021 |
PICO 2. Peripherally inserted central (venous) catheters (PICC) versus tunneled venous catheter (CVC)
1. Mortality (critical)
|
Very low GRADE |
Due to a lack of relevant literature, it was not possible to draw a conclusion with regards to the outcome mortality for treatment with a peripherally inserted central (venous) catheter versus a tunneled venous catheter in adult patients with a central venous access.
Source(s): - |
2. Complications (all combined) (critical)
|
Very low GRADE |
The evidence was very uncertain about the effect of a peripherally inserted central (venous) catheter on complications (all combined) when compared with a tunneled venous catheter in adult patients with a central venous access.
Source: Moss, 2021 |
2a. Infections
|
Low GRADE |
A peripherally inserted central (venous) catheter may reduce infections when compared with a tunneled venous catheter in adult patients with a central venous access.
Source: Moss, 2021 |
2b. Deep venous thrombosis
|
Very low GRADE |
The evidence was very uncertain about the effect of a peripherally inserted central (venous) catheter on deep venous thrombosis when compared with a tunneled venous catheter in adult patients with a central venous access.
Source: Moss, 2021 |
2c. Catheter-related bloodstream infections
|
Low GRADE |
A peripherally central (venous) catheter may reduce catheter-related bloodstream infections when compared with a tunneled venous catheter in adult patients given central venous access.
Source: Moss, 2021 |
2d. Catheter removal
|
No GRADE |
Due to a lack of relevant literature, it was not possible to draw a conclusion with regards to the outcome catheter removal for treatment with a peripherally inserted central (venous) catheter versus a tunneled venous catheter in adult patients with a central venous access.
Source(s): - |
2e. Pulmonary embolism
|
Very low GRADE |
Due to a lack of relevant literature, it was not possible to draw a conclusion with regards to the outcome pulmonary embolism for treatment with a peripherally inserted central (venous) catheter versus a tunneled venous catheter in adult patients with a central venous access.
Source(s): - |
3. Patient satisfaction (critical)
|
No GRADE |
Due to a lack of relevant literature, it was not possible to draw a conclusion with regards to the outcome patient satisfaction for treatment with a peripherally inserted central (venous) catheter versus a tunneled venous catheter in adult patients with a central venous access.
Source(s): - |
4. Catheter-related interventions per year (important)
|
No GRADE |
Due to a lack of relevant literature, it was not possible to draw a conclusion with regards to the outcome catheter-related interventions per year for treatment with a peripherally inserted central (venous) catheter versus a tunneled venous catheter in adult patients with a central venous access.
Source(s): - |
5. Quality of life (important)
|
No GRADE |
Due to a lack of relevant literature, it was not possible to draw a conclusion with regards to the outcome quality of life for treatment with a peripherally inserted central (venous) catheter versus a tunneled venous catheter in adult patients with a central venous access.
Source(s): - |
PICO 3. Peripherally inserted central (venous) catheters (PICC) versus non-tunneled CVC
1. Mortality (critical)
|
Very low GRADE |
The evidence was very uncertain about the effect of a peripherally inserted central (venous) catheter on mortality when compared with a non-tunneled venous catheter in adult patients with a central venous access.
Sources: Brandmeir, 2020; Picardi, 2019; Chopra, 2013; Cowl, 2000 |
2. Complications (all combined) (critical)
|
Very low GRADE |
The evidence was very uncertain about the effect of a peripherally inserted central (venous) catheter on complications (all combined) when compared with a non-tunneled venous catheter in adult patients with a central venous access
Source: Brandmeir, 2020 |
2a. Infections
|
Very low GRADE |
The evidence was very uncertain about the effect of a peripherally inserted central (venous) catheter on infections when compared with a non-tunneled venous catheter in adult patients with a central venous access.
Sources: Chopra, 2013; Cowl, 2000 |
2b. Deep venous thrombosis
|
Very low GRADE |
The evidence was very uncertain about the effect of a peripherally inserted central (venous) catheter on deep venous thrombosis when compared with a non-tunneled venous catheter in adult patients with a central venous access.
Source: Fletcher, 2016 |
2c. Catheter-related bloodstream infections
|
Very low GRADE |
The evidence was very uncertain about the effect of a peripherally inserted central (venous) catheter on catheter-related bloodstream infections when compared with a non-tunneled venous catheter in adult patients with a central venous access.
Source: Picardi, 2019 |
2d. Catheter removal
|
Very low GRADE |
The evidence was very uncertain about the effect of a peripherally inserted central (venous) catheter on catheter removal when compared with a non-tunneled venous catheter in adult patients with a central venous access.
Sources: Brandmeir, 2020; Picardi, 2019 |
2e. Pulmonary embolism
|
Very low GRADE |
The evidence was very uncertain about the effect of a peripherally inserted central (venous) catheter on pulmonary embolism when compared with a non-tunneled venous catheter in adult patients with a central venous access.
Source: Fletcher, 2016 |
3. Patient satisfaction (critical)
|
No GRADE |
Due to a lack of relevant literature, it was not possible to draw a conclusion with regards to the outcome patient satisfaction for treatment with a peripherally inserted central (venous) catheter versus a non-tunneled venous catheter in adult patients with a central venous access.
Source(s): - |
4. Catheter-related interventions per year (important)
|
No GRADE |
Due to a lack of relevant literature, it was not possible to draw a conclusion with regards to the outcome catheter-related interventions per year for treatment with a peripherally inserted central (venous) catheter versus a non-tunneled venous catheter in adult patients with a central venous access.
Source(s): - |
5. Quality of life (important)
|
No GRADE |
Due to a lack of relevant literature, it was not possible to draw a conclusion with regards to the outcome quality of life for treatment with a peripherally inserted central (venous) catheter versus a non-tunneled venous catheter in adult patients with a central venous access.
Source(s): - |
PICO 4. Tunneled CVC versus non-tunneled CVC
1. Catheter-related bloodstream infection (critical)
|
Very low GRADE |
The evidence is very uncertain about the effect of tunneled catheterization on catheter-related bloodstream infections when compared with non-tunneled catheterization in adult patients given central venous access.
Sources: Andrivet, 1994; Timsit, 1996; Timsit, 1999; Xiao, 2021 |
2. Catheter-related infection (important)
|
Very low GRADE |
The evidence is very uncertain about the effect of tunneled catheterization on catheter-related infections when compared with non-tunneled catheterization in adult patients given central venous access.
Sources: Andrivet, 1994); Dai, 2020; Xiao, 2021 |
Samenvatting literatuur
PICO 1. PICC versus PAC
Systematic review(s)
-
Randomized controlled trial(s)
The randomized controlled phase II trial of Clatot (2020) aimed to compare the safety between peripherally inserted central catheters (PICC) with implanted port catheters (PAC) in patients with early breast cancer who were eligible for adjuvant chemotherapy administration. Catheters were used intermittently for nine months. In total, 256 participants were included and randomly allocated to either a PICC (n=128) or PAC (n=128). The median (IQR) age of the study population was 56.0 (30.0 to 74.0) years. The PICCs were removed on the day of the last chemotherapy administration, whereas PACs were removed four weeks after the last chemotherapy administration and before the start of radiation therapy. Outcomes were measured on the first day of each cycle of chemotherapy and at three weeks and six months after the last chemotherapy administration. None of the participants in the study were lost to follow-up. The reported outcomes in Clatot (2020) were complications (deep vein thrombosis (diagnosis based on clinical suspicion), infections, catheter removal), and quality of life.
The randomized controlled trial of Clemons (2020) aimed to compare PICCs versus PACs in patients receiving trastuzumab-based chemotherapy for early-stage breast cancer. Catheters were used intermittently for one year. In total, 56 participants were included and randomly allocated to either a PICC (n=29) or PAC (n=27). The median (IQR) age of the study population was 53.0 (32.0 to 84.0) years. The length of follow-up was not reported. No patients were lost to follow-up. The reported outcomes in Clemons (2020) were complications (deep venous thrombosis, infections, catheter removal).
The randomized controlled trial of Moss (2021) aimed to compare the safety of PICCs and PACs in patients who were expected to receive systemic anticancer treatment via a central vein. Catheters were used intermittently for more than 3 months. In total, 1061 participants were included. Of these, 346 participants were randomized to PICC (n=199) versus PAC (n=147). The mean age in each group was 61.0 years. Outcomes were measured after device removal, withdrawal or one year follow-up. All patients were included in the intention-to-treat analysis. The reported outcomes in Moss (2021) were complications (such as deep venous thrombosis, infections, pulmonary embolism, catheter-related bloodstream infections, catheter removal).
The randomized controlled trial of Patel (2013) aimed to compare the safety of peripherally inserted central catheters (PICC) with port catheters (PAC) in the delivery of chemotherapy in patients with non-hematological malignancies. In total, 70 participants were included and randomly allocated to either a PICC (n=36) or PAC (n=34). The median (IQR) age of the included participants in the PICC group was 59 (29 to 84) years compared to 60.0 (34.0 to 78.0) years in the PAC group. Outcomes were measured every three weeks until central venous catheter removal or six months, whichever was sooner. None of the participants in the study were lost to follow-up. The reported outcomes in Patel (2013) were complications (such as deep vein thrombosis (diagnosed based on clinical suspicion), infections).
The randomized controlled trial of Taxbro (2019) aimed to investigate the safety of PICCs and PACs in non-hematological cancer patients. In total, 399 participants were included and randomly allocated to either a PICC (n=201) or PAC (n=198). The median (IQR) age of the included participants in the PICC group was 66 (19 to 84) years compared to 65.0 (30.0 to 89.0) years in the PAC group. Outcomes were measured after one, three, six and twelve months. None of the participants in the study were lost to follow-up. The reported outcomes in Taxbro (2019) were complications (deep venous thrombosis (diagnosis based on clinical suspicion)).
PICO 2. PICC versus tunneled CVC
Systematic review(s)
-
Randomized controlled trial(s)
The randomized controlled trial of Moss (2021) aimed to compare the safety of PICCs and CVC in patients who were expected to receive intermittent systemic anticancer treatment via a central vein for at least three months. In total, 424 participants were randomized to PICC versus a tunneled central venous catheter. The mean age between the group ranged from 61.0 to 62.0 years. Outcomes were measured after device removal, withdrawal or one year follow-up. All patients were included in the intention-to-treat analysis. The reported outcomes in Moss (2021) were complications (such as deep venous thrombosis, infections, pulmonary embolism, catheter-related bloodstream infections, and catheter removal).
PICO 3. PICC versus non-tunneled CVC
Systematic review(s)
The systematic review of Chopra (2013) aimed to compare the safety between peripherally inserted central catheters (PICC) with non-tunneled central venous catheters in patients receiving parental nutrition. Chopra (2013) searched the electronic databases of Medline, Cinahl, Scopus, Embase, and Cochrane Central registry up to the 1st of February 2013. Studies were included if they involved participants eighteen years of age or older and when they systematically compared the frequency of central line–associated bloodstream infection between PICCs and non-tunneled CVCs. Studies that involved neonates or children, compared infection rates associated with PICCs to those associated with devices that were not CVCs, and case reports, case-control studies, editorials, reviews or studies that did not report central-line associated bloodstream infections were excluded. In total, Chopra (2013) included 23 studies. Of these, only one study matched our PICO and was therefore included in the analysis of the literature of this guideline (Cowl, 2000). All other studies were excluded because of their observational study design. Cowl (2000) included 102 hospitalized patients and compared a peripherally inserted central catheter with a non-tunneled catheter (placed in the subclavian vein) to deliver parenteral nutrition for a median of ten days. The reported outcomes in the study of Cowl (2000) were mortality, and complications (such as infections).
Randomized controlled trial(s)
The randomized controlled trial of Brandmeir (2020) aimed to compare the safety of PICCs and non-tunneled central venous catheters in patients admitted to an intensive care unit who required central venous access. In total, 152 participants were included and randomly allocated to either a PICC (n=72) or non-tunneled CVC (n=80). The mean (SD) age of the study population was 61.4 (15.9) years. The length of follow-up was not reported. Catheters were used continuously for one week and placed in the jugular or subclavian vein. None of the participants in the study were lost to follow-up. The reported outcomes in Brandmeir (2020) were deep vein thrombosis (diagnosis based on clinical suspicion), catheter-related bloodstream infections, mechanical failure, catheter removal, length of stay on the intensive care unit, and mortality.
The randomized controlled trial of Picardi (2019) aimed to compare the safety of PICCs versus non-tunneled central venous catheters in patients with acute myeloid leukemia who underwent intensive chemotherapy for hematologic remission induction. In total, 93 participants were included and randomly allocated to either a PICC (n=46) or a non-tunneled CVC (n=47). The median (IQR) age of the study population was 53.8 (18 to 80) years. Outcomes were measured after 30 days. None of the participants in the study were lost to follow-up. Catheters were used continuously for more than one month and were placed in the jugular or subclavian vein. The reported outcomes in Picardi (2019) were mortality and complications (deep venous thrombosis (diagnosis based on ultrasound screening), catheter-related bloodstream infections, catheter removal).
The randomized controlled trial of Fletcher (2016) aimed to compare the safety of PICCs and non-tunneled central venous catheters in critically ill neurologic patients. In total, 80 participants were included and randomly allocated to either a PICC (n=39 or non-tunneled CVC (n=41). The mean (SD) age of the study population was 60.0 (14.0) years. Outcomes were measured after 15 days or death. None of the participants in the study were lost to follow-up. Catheters were used continuously for two weeks and were placed in the jugular or subclavian vein. The reported outcomes in Fletcher (2016) were mortality and complications (venous thrombosis (diagnosis based on ultrasound screening of deep and superficial veins), catheter-related bloodstream infections, pulmonary embolism, catheter removal).
PICO 4. Tunneled CVC versus non-tunneled CVC
Systematic review(s)
-
Randomized controlled trial(s)
The randomized controlled trial of Andrivet (1994) investigated the efficacy of subcutaneous tunneled catheters in comparison with non-tunneled catheters in immunocompromised patients with malignancies who needed a long-term central venous access. Participants were randomly assigned to one of the treatment groups. For patients allocated in the tunneled catheters group (n=107), tunneling (for seven or eight centimeter) was performed each time with the same dilator and introducer by using the Powell-Tuck technique. In de non-tunneled catheter group (n=105), polyurethane catheters were inserted instead of silicone catheters. This type of catheter includes an integrated hub that cannot be tunneled easily. The mean (standard error) life span of the catheters used in the entire study population was 116 (6.5) days, during which 3.7 (0.2) antineoplastic sources per patient were administered. Catheters were used for 45 (2.6) days in inpatients and for 71 (5.6) days in outpatients. Fifty-one catheters were used in subjects who were inpatients only and remained in place for 40.5 (7.5) days in the tunneled group and 45.0 (6.4) days in the non-tunneled group. The central venous catheters were inserted in the subclavian vein. The tunneled catheters were non-cuffed. Five patients in the tunneled catheter group and one patient in the non-tunneled catheter group were lost the follow-up. The reported outcome was incidence of catheter-related infection.
The randomized controlled trial of Dai (2020) investigated the efficacy of tunneled and non-tunneled peripherally inserted central catheter (PICC) placement under B-mode ultrasound for oncological patients. Participants were randomly assigned to one of the treatment groups. The tunneled group (n=87) received tunneled PICC placement with B-mode ultrasound and modified Seldinger technique for which the puncture site was three centimeter above the catheter exit site. The non-tunneled group (n=87) receive routine non-tunneled PICC placement with B-mode ultrasound guidance and modified Seldinger technique for which the puncture site and catheter exit site were at the same position. The PICCs were inserted in the basilic or brachial vein. The tunneled catheters were non-cuffed. The study did not report how long the catheters remained in place. None of the patients were lost to follow-up. The reported outcome was catheter-related infection.
The randomized controlled trial of Timsit (1999) investigated the effect of tunneled or non-tunneled femoral catheters in critically ill patients on the intensive care unit. Participants were randomly assigned to one of the treatment groups. The tunneled catheter group (n=168) received polyurethane monolumen or bilumen tunneled catheters, inserted by using the Seldinger method. The non-tunneled catheter group (n=168) received the same polyurethane catheters as used in the tunneled catheter group with the same external diameter. The central venous catheters were inserted in the femoral vein. The tunneled catheters were non-cuffed. The mean (standard deviation) duration of catheter maintenance in the tunneled-catheter group was 8.2 (4.7) days, compared to 7.6 (4.5) days in the non-tunneled catheter group. None of the patients were lost to follow-up. The reported outcomes were incidence of catheter-related bloodstream infection and catheter-related infection.
The randomized controlled trial of Timsit (1996) investigated the effect of tunneled or non-tunneled catheters in critically ill patients on the intensive care unit. Participants were randomly assigned to one of the treatment groups. The tunneled catheter group (n=117) received polyurethane monolumen or bilumen tunneled catheters, inserted by using the Seldinger method. The non-tunneled catheter group (n=114) received the same polyurethane catheters used in the tunneled catheter group with the same internal and external diameter. The central venous catheters were inserted in the jugular vein. The tunneled catheters were non-cuffed. The mean (standard deviation) duration of catheter placement was about the same in both groups (8.48 (4.8) days in the tunneled group and 8.19 (4.8) days in the non-tunneled group). None of the patients were lost to follow-up. The reported outcome was incidence of catheter-related infection.
The randomized controlled trial of Xiao (2021) investigated the effect of subcutaneous tunneling versus the normal technique in patients undergoing chemotherapy with peripherally inserted central catheters (PICC). Participants were randomly assigned to one of the treatment groups. The subcutaneous tunneling group (n=64) received subcutaneous tunneling technique combined with the normal PICC placement technique for which the puncture site was located five centimeter above the catheter exit site on the patient’s upper arm. Participants who were allocated to the control group (n=65) received non-tunneled PICC catheterization by using the normal PICC placement technique under B-mode ultrasound guidance combined with the modified Seldinger technique. The PICCs were inserted in the basilic or brachial vein. The tunneled catheters were non-cuffed. The median (interquartile range) duration of catheter placement in the subcutaneous tunneling group was 88 (8 to 191) days, compared to 72 (15 to 192) days in the non-tunneled group. None of the patients were lost to follow-up. The reported outcomes were incidence of catheter-related bloodstream infection and catheter-related infection.
Results
PICO 1. Peripherally inserted central (venous) catheter (PICC) versus Port catheter (PAC)
1. Mortality rates (critical)
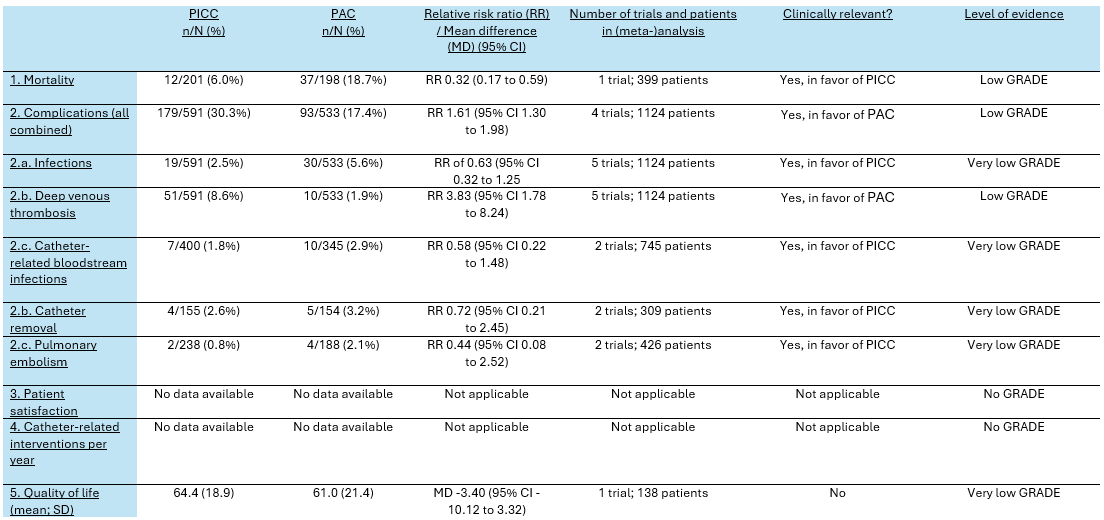
Overall mortality was reported in one trial (Taxbro, 2019). The mortality rate in the PICC group was 12/201 (6.0%), compared to 37/198 (18.7%) in the PAC group. This resulted in a RR of 0.32 (95% CI 0.17 to 0.59), in favor of the PICC group. This was considered as a clinically relevant difference.
2. Complications (all combined) (critical)
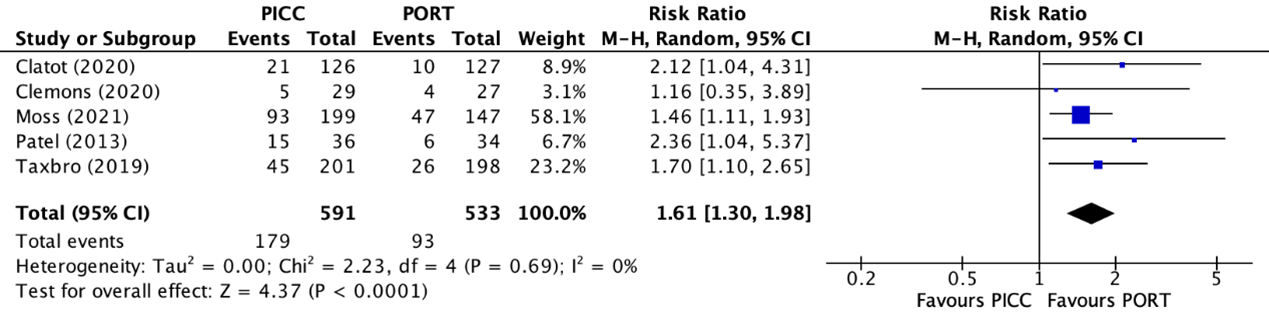
Five trials reported the combined number of complications (Clatot, 2020; Clemons, 2020; Moss, 2021; Patel, 2013; Taxbro, 2019). The total number of combined complications in the PICC group was 179/591 (30.3%), compared to 93/533 (17.4%) in the PAC group. This resulted in a relative risk ratio (RR) of 1.61 (95% CI 1.30 to 1.98), in favor of the PAC group (figure 1). This was considered as a clinically relevant difference.
Figure 1. Forest plot showing the comparison between PICC and PAC for all combined complications
Pooled relative risk ratio, random effects model. Z: p-value of overall effect; df: degrees of freedom; I2; statistical heterogeneity
2a. Infection rate
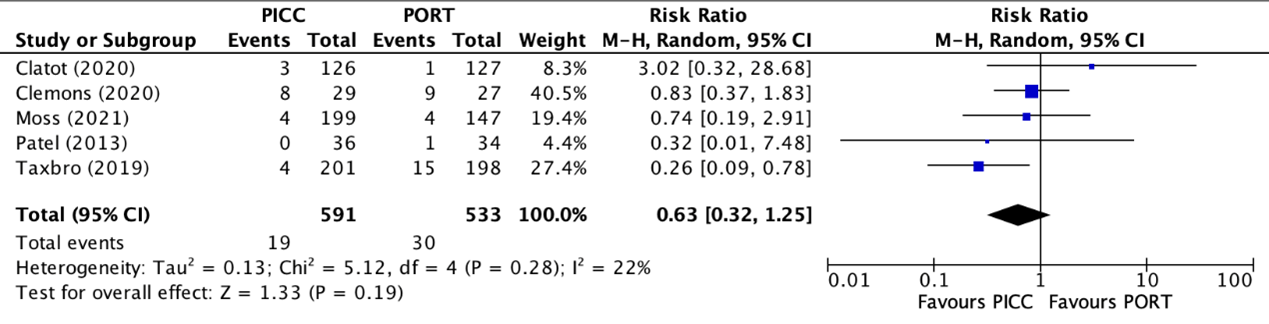
Infection rates were reported in five trials (Clatot, 2020; Clemons, 2020; Moss, 2021; Patel, 2013; Taxbro, 2019). Clatot (2020), Moss (2021), and Taxbro (2019) defined infections as pocket/exit site infections. Clemons (2020) and Patel (2013) did not specify the definition of infections in their studies. The results were pooled in a meta-analysis. The pooled infection rate in the PICC group was 19/591 (2.5%), compared to 30/533 (5.6%) in the PAC group. This resulted in a pooled RR of 0.63 (95% CI 0.32 to 1.25), in favor of the PICC group (figure 2). This was considered as a clinically relevant difference.
Figure 2. Forest plot showing the comparison between PICC and PAC for infections
Pooled relative risk ratio, random effects model. Z: p-value of overall effect; df: degrees of freedom; I2; statistical heterogeneity
2b. Deep venous thrombosis
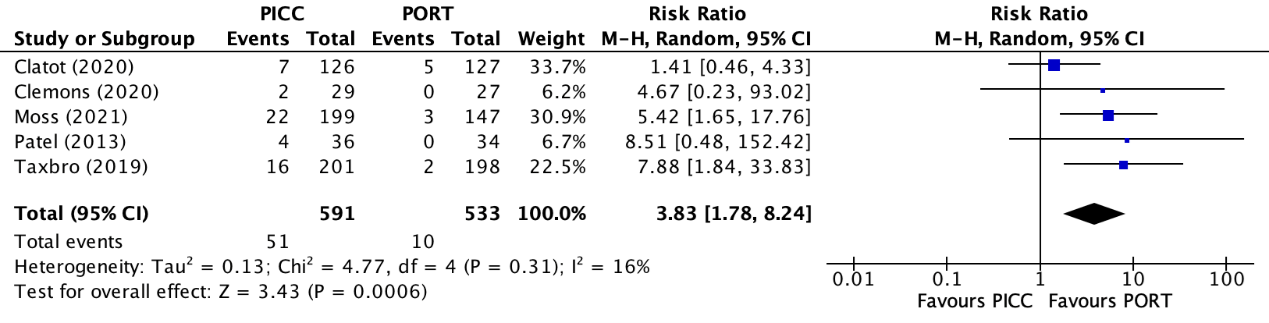
Deep venous thrombosis was reported in five trials (Clatot, 2020; Clemons, 2020; Moss, 2021; Patel, 2013; Taxbro, 2019). Clatot (2020) reported deep venous thrombosis without local infection or septicaemia. Patel (2013) reported the combined number of patients wiFCIth deep venous thrombosis and/or line occlusion, which must be considered when interpreting the results. The results were pooled in a meta-analysis. The pooled number of patients with deep venous thrombosis in the PICC group was 51/591 (8.6%), compared to 10/533 (1.9%) in the PAC group. This resulted in a pooled relative risk ratio (RR) of 3.83 (95% CI 1.78 to 8.24), in favor of the PAC group (figure 3). This was considered as a clinically relevant difference.
Figure 3. Forest plot showing the comparison between PICC and PAC for deep venous thrombosis
Pooled relative risk ratio, random effects model. Z: p-value of overall effect; df: degrees of freedom; I2; statistical heterogeneity
2c. Catheter-related bloodstream infections rates
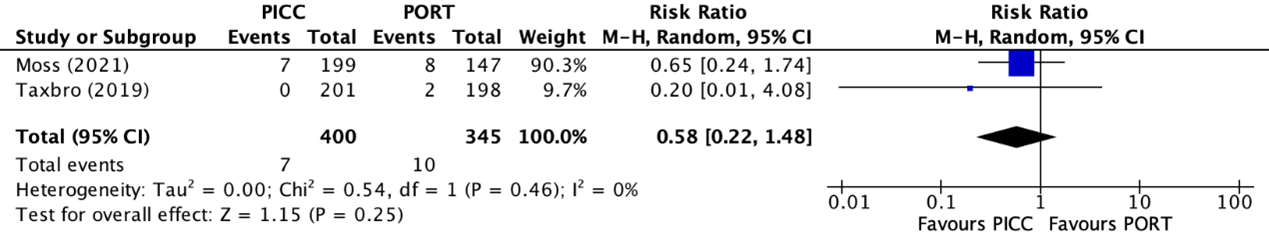
The catheter-related bloodstream infection rates were reported in two trials (Moss, 2021; Taxbro, 2019). The results were pooled in a meta-analysis. The pooled catheter-related bloodstream infection rate in the PICC group was 7/400 (1.8%), compared to 10/345 (2.9%) in the PAC group. This resulted in a pooled RR of 0.58 (95% CI 0.22 to 1.48), in favor of the PICC group (figure 4). This was considered as a clinically relevant difference.
Figure 4. Forest plot showing the comparison between PICC and PAC for catheter-related bloodstream infections
Pooled relative risk ratio, random effects model. Z: p-value of overall effect; df: degrees of freedom; I2; statistical heterogeneity
2d. Catheter removals
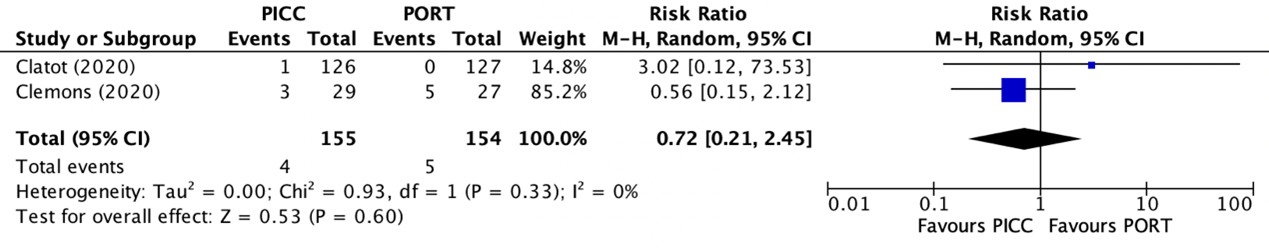
Catheter removals were reported in two trials (Clatot, 2020; Clemons, 2020). The results were pooled in a meta-analysis. The pooled number of catheter removals in the PICC group was 4/155 (2.6%), compared to 5/154 (3.2%) in the PAC group. This resulted in a pooled relative risk ratio (RR) of 0.72 (95% CI 0.21 to 2.45), in favor of the PICC group (figure 5). This was considered as a clinically relevant difference.
Figure 5. Forest plot showing the comparison between PICC and PAC for catheter removals
Pooled relative risk ratio, random effects model. Z: p-value of overall effect; df: degrees of freedom; I2; statistical heterogeneity
2e. Pulmonary embolism
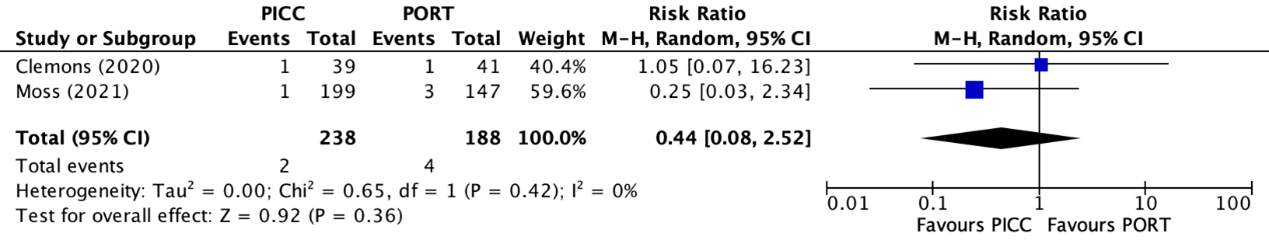
Pulmonary embolisms were reported in two trials (Clemons, 2020; Moss, 2021). The results were pooled in a meta-analysis. The pooled number of pulmonary embolisms in the PICC group was 2/238 (0.8%), compared to 4/188 (2.1%) in the PAC group. This resulted in a pooled relative risk ratio (RR) of 0.44 (95% CI 0.08 to 2.52), in favor of the PICC group (figure 6). This was considered as a clinically relevant difference.
Figure 6. Forest plot showing the comparison between PICC and PAC for pulmonary embolism
Pooled relative risk ratio, random effects model. Z: p-value of overall effect; df: degrees of freedom; I2; statistical heterogeneity
3. Patient satisfaction (critical)
None of the included studies reported information regarding patient satisfaction for treatment with a PICC or PAC.
4. Catheter-related interventions per year (important)
None of the included studies reported information regarding catheter-related interventions per year for treatment with a PICC or PAC.
5. Quality of life (important)
One trial reported quality of life (Clatot, 2020). Quality of life was measured with the QLQ-C30. The EORTC QLQ-C30 is a disease-specific measurement tool for use in patients with or cured of cancer that describes several aspects of quality of life on a zero (minimum) to 100 (maximum) numeric scale. A higher score indicates better quality of life.
The mean (SD) QLQ-C30 score in the PICC group (n=66) was 64.4 (18.9) points, compared to 61.0 (21.4) points in PAC group (n=72). This resulted in a mean difference of -3.40 (95% -10.12 CI to 3.32), in favor of the PICC group. This was not considered as a clinically relevant difference.
Level of evidence of the literature
PICO 1. PICC versus PAC
1. Mortality (critical)
The level of evidence regarding the outcome mortality was derived from a randomized controlled trial and therefore started high. The level of evidence was downgraded by two levels because the small number of events (imprecision, -2). The level of evidence was considered as low.
2. Complications (all combined) (critical)
The level of evidence regarding the outcome complications (all combined) was derived from randomized controlled trials and therefore started high. The level of evidence was downgraded by one level because of a lack of blinding in the included studies (risk of bias, -1) and the small number of events (imprecision, -1). The level of evidence was considered as low.
2a. Infections
The level of evidence regarding the outcome complications (all combined) was derived from randomized controlled trials and therefore started high. The level of evidence was downgraded by three levels because of a lack of blinding in the included studies (risk of bias, -1), the wide confidence interval crossing both boundaries of clinical relevance, and the small number of events (imprecision, -2). The level of evidence was considered as very low.
2b. Deep venous thrombosis
The level of evidence regarding the outcome deep venous thrombosis was derived from randomized controlled trials and therefore started high. The level of evidence was downgraded by two levels because of a lack of blinding in the included studies (risk of bias, -1) and the small number of events (imprecision, -1). The level of evidence was considered as low.
2c. Catheter-related bloodstream infections
The level of evidence regarding the outcome catheter-related bloodstream infections was derived from randomized controlled trials and therefore started high. The level of evidence was downgraded by three levels because of a lack of blinding in the included studies (risk of bias, -1), the wide confidence interval crossing both boundaries of clinical relevance, and the small number of events (imprecision, -2). The level of evidence was considered as very low.
2d. Catheter removal
The level of evidence regarding the outcome catheter removal was derived from randomized controlled trials and therefore started high. The level of evidence was downgraded by three levels because of a lack of blinding in the included studies (risk of bias, -1), the wide confidence interval crossing both boundaries of clinical relevance, and the small number of events (imprecision, -2). The level of evidence was considered as very low.
2e. Pulmonary embolism
The level of evidence regarding the outcome pulmonary embolism was derived from randomized controlled trials and therefore started high. The level of evidence was downgraded by three levels because of a lack of blinding in the included studies (risk of bias, -1), the wide confidence interval crossing both boundaries of clinical relevance, and the small number of events (imprecision, -2). The level of evidence was considered as very low.
3. Patient satisfaction (critical)
Because of a lack of data, it was not possible to grade the literature for the outcome patient satisfaction for treatment with PICC versus PAC.
4. Catheter-related interventions per year (important)
Because of a lack of data, it was not possible to grade the literature for the outcome catheter-related interventions per year for treatment with PICC versus PAC.
5. Quality of life (important)
The level of evidence regarding the outcome quality of life was derived from a randomized controlled trial and therefore started high. The level of evidence was downgraded by three levels because of a lack of blinding in the included study (risk of bias, -1), the wide confidence interval crossing the lower boundary of clinical relevance, and the small number of included patients (imprecision, -2). The level of evidence was considered as very low.
Table 1. Summary of findings PICO 1
PICO 2. PICC versus tunneled CVC
1. Mortality (critical)
None of the included studies reported information regarding mortality for treatment with a PICC or tunneled CVC.
2. Complications (critical)
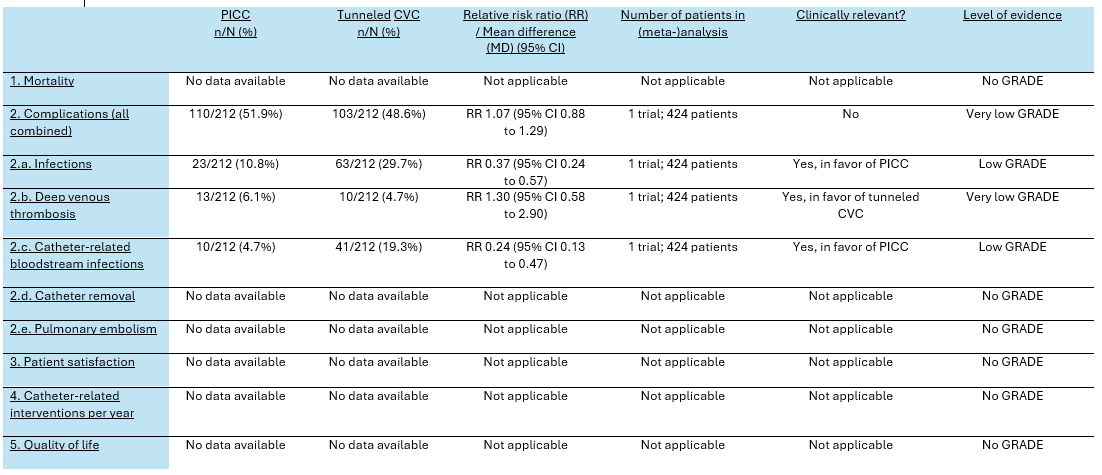
One trial reported the combined number of complications (Moss, 2021). The number of combined complications in the PICC group was 110/212 (51.9%), compared to 103/212 (48.6%) in the CVC group. This resulted in a RR of 1.07 (95% CI 0.88 to 1.29), in favor of the tunneled CVC group. This was not considered as a clinically relevant difference.
2a. Infections
Infections were reported in one trial (Moss, 2021). The number of infections in the PICC group was 23/212 (10.8%), compared to 63/212 (29.7%) in the tunneled CVC group. This resulted in a pooled relative risk ratio (RR) of 0.37 (95% CI 0.24 to 0.57), in favor of the PICC group. This was considered as a clinically relevant difference.
2b. Deep venous thrombosis
Deep venous thrombosis was reported in one trial (Moss, 2021). The number of patients with deep venous thrombosis in the PICC group was 13/212 (6.1%), compared to 10/212 (4.7%) in the tunneled CVC group. This resulted in a RR of 1.30 (95% CI 0.58 to 2.90), in favor of the tunneled CVC group. This was considered as a clinically relevant difference.
2c. Catheter-related bloodstream infections
Catheter-related bloodstream infections were reported in one trial (Moss, 2021). The number of catheter-related bloodstream infections in the PICC group was 10/212 (4.7%), compared to 41/212 (19.3%) in the tunneled CVC group. This resulted in a RR of 0.24 (95% CI 0.13 to 0.47), in favor of the PICC group. This was considered as a clinically relevant difference.
2d. Catheter removal
None of the included studies reported information regarding catheter removal for treatment with a PICC or tunneled CVC.
2e. Pulmonary embolism
None of the included studies reported information regarding pulmonary embolism for treatment with a PICC or tunneled CVC.
3. Patient satisfaction (critical)
None of the included studies reported information regarding patient satisfaction for treatment with a PICC or tunneled CVC.
4. Catheter-related interventions per year (important)
None of the included studies reported information regarding catheter-related interventions per year for treatment with a PICC or tunneled CVC.
5. Quality of life (important)
None of the included studies reported information regarding quality of life for treatment with a PICC or tunneled CVC.
Level of evidence of the literature
PICO 2. PICC versus tunneled CVC
1. Mortality (critical)
Because of a lack of data, it was not possible to grade the literature for the outcome mortality for treatment with PICC versus tunneled CVC.
2. Complications (all combined) (critical)
The level of evidence regarding the outcome complications (all combined) was derived from a randomized controlled trial and therefore started high. The level of evidence was downgraded by three levels because of a lack of blinding in the included studies (risk of bias, -1), the wide confidence interval crossing the upper boundary of clinical relevance, and the small number of events (imprecision, -2). The level of evidence was considered as very low.
2a. Infections
The level of evidence regarding the outcome infections was derived from a randomized controlled trial and therefore started high. The level of evidence was downgraded by two levels because of a lack of blinding in the included studies (risk of bias, -1) and the small number of events (imprecision, -1). The level of evidence was considered as low.
2b. Deep venous thrombosis
The level of evidence regarding the outcome deep venous thrombosis was derived from randomized controlled trials and therefore started high. The level of evidence was downgraded by three levels because of a lack of blinding in the included studies (risk of bias, -1), the wide confidence interval crossing both boundaries of clinical relevance, and the small number of events (imprecision, -2). The level of evidence was considered as very low.
2c. Catheter-related bloodstream infections
The level of evidence regarding the outcome catheter-related bloodstream infections was derived from randomized controlled trials and therefore started high. The level of evidence was downgraded by two levels because of a lack of blinding in the included studies (risk of bias, -1) and the small number of events (imprecision, -1). The level of evidence was considered as low.
2d. Catheter removal
Because of a lack of data, it was not possible to grade the literature for the outcome catheter removal for treatment with PICC versus tunneled CVC.
2e. Pulmonary embolism
Because of a lack of data, it was not possible to grade the literature for the outcome pulmonary embolism for treatment with PICC versus tunneled tunneled CVC.
3. Patient satisfaction (critical)
Because of a lack of data, it was not possible to grade the literature for the outcome patient satisfaction for treatment with PICC versus tunneled tunneled CVC.
4. Catheter-related interventions per year (important)
Because of a lack of data, it was not possible to grade the literature for the outcome catheter-related interventions per year for treatment with PICC versus tunneled CVC.
5. Quality of life (important)
Because of a lack of data, it was not possible to grade the literature for the outcome quality of life for treatment with PICC versus tunneled CVC.
Table 2. Summary of findings PICO 2
PICO 3. Peripherally inserted central (venous) catheter (PICC) versus non-tunneled central venous catheter
1. Mortality (critical)
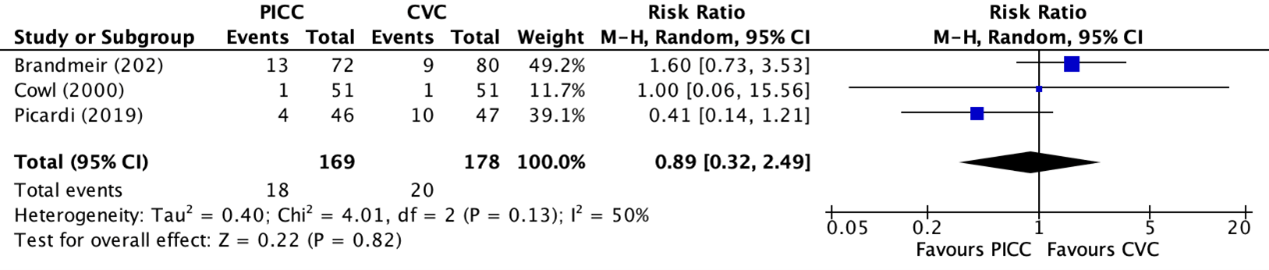
Mortality was reported in one trial, retrieved from the systematic review of Chopra (2013) (Cowl, 2000) and in two individual trials (Brandmeir, 2020; Picardi, 2019). Picardi (2019) reported the 30-day mortality. Brandmeir (2020) and Cowl (2000) did not specify the length of follow-up for mortality. The results were pooled in a meta-analysis. The pooled number of mortalities in the PICC group was 18/169 (10.7%), compared to 20/178 (11.2%) in the non-tunneled CVC group. This resulted in a RR of 0.89 (95% CI 0.32 to 2.49), in favor of the non-tunneled CVC group. This was not considered as a clinically relevant difference.
Figure 7. Forest plot showing the comparison between PICC and non-tunneled CVC for mortality
Pooled relative risk ratio, random effects model. Z: p-value of overall effect; df: degrees of freedom; I2; statistical heterogeneity
2. Complications (critical)
One trial reported the combined number of complications (Brandmeir, 2020). The number of combined complications in the PICC group was 14/72 (19.4%), compared to 10/80 (12.5%) in the non-tunneled CVC group. This resulted in a RR of 1.56 (95% CI 0.74 to 3.28), in favor of the non-tunneled CVC group. This was considered as a clinically relevant difference.
2a. Infection rates
One trial, retrieved from the systematic review of Chopra (2013) reported the combined number of infections (Cowl, 2000). The number of infections in the PICC group was 2/51 (3.9%), compared to 3/51 (5.9%) in the non-tunneled CVC group. This resulted in a RR of 0.67 (95% CI 0.12 to 3.82), in favor of the PICC group. This was considered as a clinically relevant difference.
2b. Deep venous thrombosis
Deep venous thrombosis was reported in one trial (Fletcher, 2016). The number of patients with deep venous thrombosis in the PICC group was 7/39 (17.9%), compared to 1/41 (2.4%) in the non-tunneled CVC group. This resulted in a RR of 7.36 (95% CI 0.95 to 57.10), in favor of the non-tunneled CVC group. This was considered as a clinically relevant difference.
2c. Catheter-related bloodstream infections
Catheter-related bloodstream infections were reported in one trial (Picardi, 2019). The number of catheter-related bloodstream infections in the PICC group was 2/46 (4.3%), compared to 11/47 (23.4%) in the non-tunneled CVC group. This resulted in a RR of 0.19 (95% CI 0.04 to 0.79), in favor of the PICC group. This was considered as a clinically relevant difference.
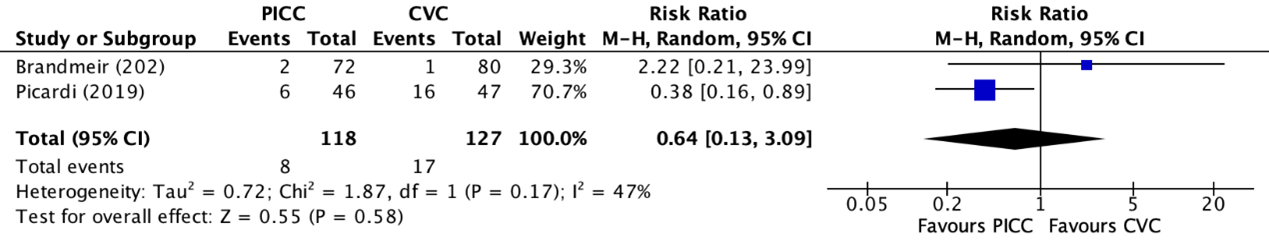
2d. Catheter removal
Catheter removal was reported in two trials (Brandmeir, 2020; Picardi, 2019). The results were pooled in a meta-analysis. The number of catheter removals in the PICC group was 8/118 (6.8%), compared to 17/127 (13.4%) in the non-tunneled CVC group. This resulted in a RR of 0.64 (95% CI 0.13 to 3.09), in favor of the PICC group (figure 10). This was considered as a clinically relevant difference.
Figure 8. Forest plot showing the comparison between PICC and non-tunneled CVC for catheter removal
Pooled relative risk ratio, random effects model. Z: p-value of overall effect; df: degrees of freedom; I2; statistical heterogeneity
2e. Pulmonary embolism
Pulmonary embolisms were reported in one trial (Fletcher, 2016). The number of pulmonary embolisms in the PICC group was 1/39 (2.6%), compared to 1/41 (2.4%) in the non-tunneled CVC group. This resulted in a RR of 1.05 (95% CI 0.07 to 16.23), in favor of the CVL group. This was not considered as a clinically relevant difference.
3. Patient satisfaction (critical)
None of the included studies reported information regarding patient satisfaction for treatment with a PICC or non-tunneled CVC.
4. Catheter-related interventions per year (important)
None of the included studies reported information regarding catheter-related interventions per year for treatment with a PICC or non-tunneled CVC.
5. Quality of life (important)
None of the included studies reported information regarding quality of life for treatment with a PICC or non-tunneled CVC.
Level of evidence of the literature
PICO 3: PICC versus non-tunneled CVC
1. Mortality (critical)
The level of evidence regarding the outcome mortality was derived from randomized controlled trials and therefore started high. The level of evidence was downgraded by three levels because of the wide confidence interval crossing the upper boundary of clinical relevance, the small number of events (imprecision, -2), and heterogeneity in the study results (inconsistency, -1). The level of evidence was considered as very low.
2. Complications (all combined) (critical)
The level of evidence regarding the outcome complications (all combined) was derived from a randomized controlled trial and therefore started high. The level of evidence was downgraded by three levels because of a lack of blinding in the included studies (risk of bias, -1), the wide confidence interval crossing the upper boundary of clinical relevance, and the small number of events (imprecision, -2). The level of evidence was considered as very low.
2a. Infections
The level of evidence regarding the outcome infections was derived from a randomized controlled trial and therefore started high. The level of evidence was downgraded by three levels because of the lack of blinding in the included study (risk of bias, -1), the wide confidence interval crossing the lower and upper boundaries of clinical relevance, the small number of events (imprecision, -2). The level of evidence was considered as very low.
2b. Deep venous thrombosis
The level of evidence regarding the outcome deep venous thrombosis was derived from a randomized controlled trial and therefore started high. The level of evidence was downgraded by three levels because of a lack of blinding in the included studies (risk of bias, -1), the wide confidence interval crossing the upper boundary of clinical relevance, and the small number of events (imprecision, -2). The level of evidence was considered as very low.
2c. Catheter-related bloodstream infections
The level of evidence regarding the outcome catheter-related bloodstream infections was derived from randomized controlled trials and therefore started high. The level of evidence was downgraded by two levels because of a lack of blinding in the included studies (risk of bias, -1), the wide confidence interval crossing the lower boundary of clinical relevance, and the small number of events (imprecision, -2). The level of evidence was considered as very low.
2d. Catheter removal
The level of evidence regarding the outcome catheter removal was derived from a randomized controlled trial and therefore started high. The level of evidence was downgraded by three levels because of a lack of blinding in the included studies (risk of bias, -1), the wide confidence interval crossing both boundaries of clinical relevance, and the small number of events (imprecision, -2). The level of evidence was considered as very low.
2e. Pulmonary embolism
The level of evidence regarding the outcome pulmonary embolism was derived from a randomized controlled trial and therefore started high. The level of evidence was downgraded by three levels because of a lack of blinding in the included studies (risk of bias, -1), the wide confidence interval crossing the upper boundary of clinical relevance, and the small number of events (imprecision, -2). The level of evidence was considered as very low.
3. Patient satisfaction (critical)
Because of a lack of data, it was not possible to grade the literature for the outcome patient satisfaction for treatment with PICC versus non-tunneled CVC.
4. Catheter-related interventions per year (important)
Because of a lack of data, it was not possible to grade the literature for the outcome catheter-related interventions per year for treatment with PICC versus non-tunneled CVC.
5. Quality of life (important)
Because of a lack of data, it was not possible to grade the literature for the outcome quality of life for treatment with PICC versus non-tunneled CVC.
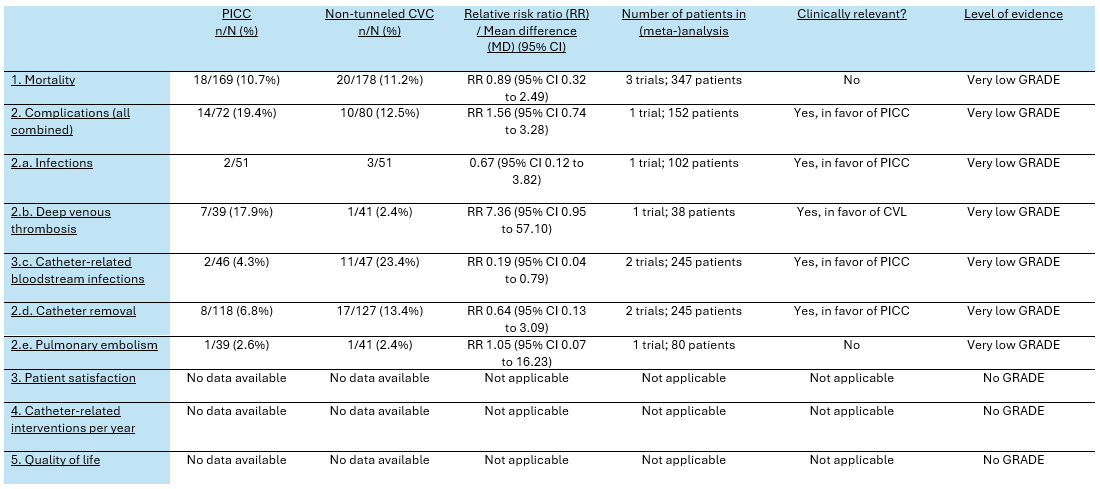
Table 3. Summary of findings PICO 3
PICO 4. Tunneled CVC versus non-tunneled CVC
1. Catheter-related bloodstream infection
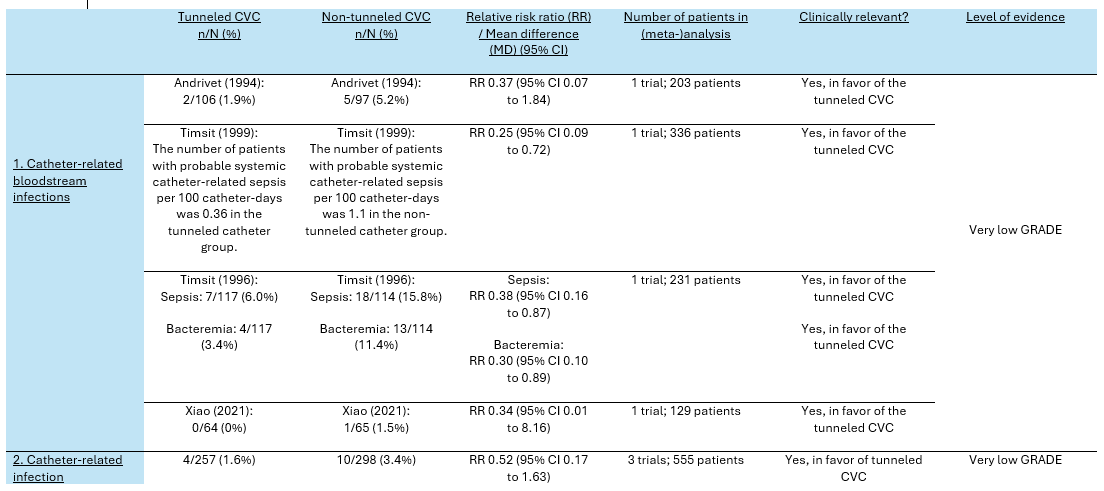
Catheter-related bloodstream infection was reported in four trials (Andrivet, 1994; Timsit; 1996; Timsit, 1999; Xiao, 2021). Catheter-related sepsis and catheter-related bacteremia were also categorized under catheter-related bloodstream infections. Andrivet reported the number of catheter-related bacteremia. Timsit (1999) reported the incidence of catheter-related bloodstream infections and the incidence of catheter-related sepsis per 100 catheter-days. Xiao (2021) reported the absolute number of catheter-related bloodstream infections between tunneled- and non-tunneled catheters.
Andrivet (1994) reported the incidence of catheter-related bacteremia, which was categorized under catheter-related bloodstream infections for the purpose of this guideline.
The number of patients with catheter-related bloodstream infections in the tunneled catheter group was 2/106 (1.9%), compared to 5/97 (5.2%) in the non-tunneled catheter group. This resulted in a RR of 0.37 (95% CI 0.07 to 1.84), in favor of the tunneled catheter group. This was considered as a clinically relevant difference.
Timsit (1999) reported the incidence of probable catheter-related sepsis and catheter-related bloodstream infections. For this guideline, both outcomes were categorized under catheter-related bloodstream infections. Due to the absence of a standard deviation for both outcomes, it was impossible to pool both outcome measures into a pooled incidence rate. The results were therefore separately reported.
The number of patients with probable systemic catheter-related sepsis per 100 catheter-days in Timsit (1999) in the tunneled catheter group (n=168) was 0.36, compared to 1.1 in the non-tunneled catheter group (n=168). This resulted in a RR of 0.25 (95% CI 0.09 to 0.72), in favor of the tunneled catheter group. This was considered as a clinically relevant difference.
The number of patients with catheter-related bloodstream infections per 100 catheter-days in Timsit (1999) in the tunneled catheter group was 0.073, compared to 0.23 in the non-tunneled catheter group. This resulted in a RR of 0.28 (95% CI 0.03 to 1.92), in favor of the tunneled catheter group. This was considered as a clinically relevant difference.
Timsit (1996) reported the incidence of catheter-related sepsis and catheter-related bacteremia.
The number of patients with catheter-related sepsis in Timsit (1996) in the tunneled catheter group was 7/117 (6.0%), compared to 18/114 (15.8%) in the non-tunneled catheter group. This resulted in a RR of 0.38 (95% CI 0.16 to 0.87), in favor of the tunneled catheter group.
The number of patients with catheter-related bacteremia in Timsit (1996) in the tunneled catheter group was 4/117 (3.4%), compared to 13/114 (11.4%) in the non-tunneled catheter group. This resulted in a RR of 0.30 (95% CI 0.10 to 0.89), in favor of the tunneled catheter group.
Both results showed that there was a clinically relevant difference in catheter-related bloodstream infections between the tunneled- and non-tunneled cathether group.
Xiao (2021) reported the absolute incidence of catheter-related bloodstream infections. The number of patients with catheter-related bloodstream infections in Xiao (2021) in the tunneled catheter group was 0/64 (0%), compared to 1/65 (1.5%) in the non-tunneled catheter group. This resulted in a RR of 0.34 (95% CI 0.01 to 8.16), in favor of the tunneled catheter group. This was considered as a clinically relevant difference.
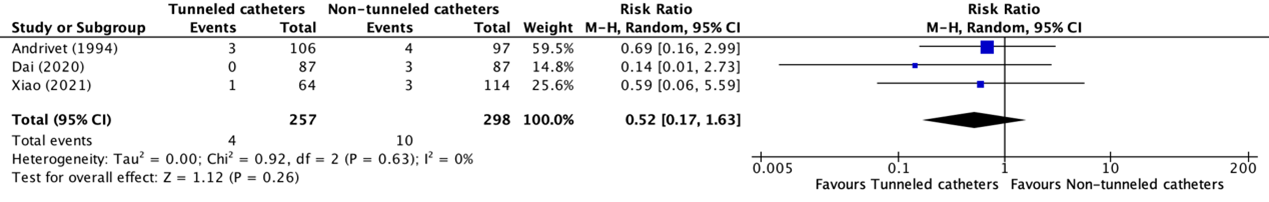
2. (Catheter-related) infection
(Catheter-related) infection was reported in three trials (Andrivet, 1994; Dai, 2020; Xiao, 2021). Andrivet (1994) reported non-bacteremic catheter-related infection, while Dai (2020), and Xiao (2021) reported regular infections. The results were pooled in a meta-analysis. The pooled number of patients with (catheter-related) infections in the tunneled catheter group was 4/257 (1.6%), compared to 10/298 (3.4%) in the non-tunneled catheter group. This resulted in a pooled relative risk ratio (RR) of 0.52 (95% CI 0.17 to 1.63), in favor of the tunneled catheter group. This was considered as a clinically relevant difference.
Figure 9. Forest plot showing the comparison between tunneled catheters and non-tunneled catheters for (catheter-related) infection
Pooled relative risk ratio, random effects model. Z: p-value of overall effect; df: degrees of freedom; I2; statistical heterogeneity; CI: confidence interval
Level of evidence of the literature
1. Catheter-related bloodstream infection
The level of evidence regarding the outcome catheter-related bloodstream infection was derived from randomized controlled trials and therefore started high. The level of evidence was downgraded by three levels because of the wide confidence interval crossing both boundaries of clinical relevance (both imprecision, -2) and because of clinical heterogeneity in the included studies (heterogeneity, -1). The level of evidence was considered as very low.
2. Catheter-related infection
The level of evidence regarding the outcome catheter-related infection was derived from randomized controlled trials and therefore started high. The level of evidence was downgraded by three levels because of a lack of blinding in the included studies (risk of bias, -1) and the wide confidence interval crossing both boundaries of clinical relevance (both imprecision, -2). The level of evidence was considered as very low.
Table 4. Summary of findings PICO 4
Zoeken en selecteren
A systematic review of the literature was performed to answer the following questions:
- What are the (un)beneficial effects of a peripherally inserted central catheter (PICC) compared to a port catheter and a tunneled central venous catheter
- What is the additional value for reducing the risk of infectious complications of tunneled vs non-tunneled central venous catheters placement?
PICO 1: Peripherally inserted central (venous) catheters (PICC) versus Port catheter (PAC)
| P: |
Adult patients given central venous access |
| I: |
Peripherally inserted central (venous) catheter |
| C: |
Port catheter |
| O: |
Quality of life, patient satisfaction, complications (such as infections, deep venous thrombosis, catheter-related bloodstream infections, catheter removal, pulmonary embolism), catheter-related intervention rate, and mortality |
PICO 2: Peripherally inserted central (venous) catheters (PICC) versus tunneled venous catheter (CVC)
| P: |
Adult patients given central venous access |
| I: |
Peripherally inserted central (venous) catheters |
| C: |
Tunneled central venous catheter |
| O: |
Quality of life, patient satisfaction, complications (such as infections, deep venous thrombosis, catheter-related bloodstream infections, catheter removal, pulmonary embolism), catheter-related intervention rate, and mortality |
PICO 3: Peripherally inserted central (venous) catheters (PICC) versus non-tunneled central venous line (CVL)
| P: |
Adult patients given central venous access |
| I: |
Peripherally inserted central (venous) catheters |
| C: |
Non-tunneled central venous catheter |
| O: |
Quality of life, patient satisfaction, complications (such as infections, deep venous thrombosis, catheter-related bloodstream infections, catheter removal, pulmonary embolism), catheter-related intervention rate, and mortality |
PICO 4: Tunneled central venous catheters versus non-tunneled central venous catheters
| P: |
Adult patients given central venous access |
| I: |
Tunneled (or cuffed) central venous access |
| C: |
Non-tunneled (or non-cuffed) central venous access |
| O: |
Catheter-related bloodstream infection (i.e., catheter-related sepsis, catheter-related bacteremia) and catheter-related infection |
Relevant outcome measures
The guideline development group considered mortality, complications (such as infections, deep venous thrombosis, catheter-related bloodstream infections, catheter removal, pulmonary embolism), and patient satisfaction as critical outcome measures for decision making and catheter-related intervention rate, catheter-related infection, and quality of life as important outcome measures for decision making.
As minimal clinically (patient) important differences, the working group used 5% for mortality (risk ratio; RR), 25% for other RR, 10% of the maximum score for continuous outcomes (quality of life scales) and 0.5 for standardized mean differences.
Search and select (Methods)
Two separate literature searches were conducted. For the first search, the databases Medline (via OVID) and Embase (via Embase.com) were searched with relevant search terms until the 23rd of August 2022 to investigate the (un)beneficial effects of a peripherally inserted central catheter (PICC) compared to a port catheter and a tunneled central venous catheter (PICO 1, PICO 2 and PICO 3 of this guideline). The systematic literature search resulted in 536 hits. Studies were selected based on the following criteria: systematic reviews and randomized controlled trials for the optimal type of line for central venous access. Twenty-nine studies were initially selected based on title and abstract screening. After reading the full text, twenty studies were excluded (see the table with reasons for exclusion under the tab Methods), and nine studies were included.
Secondly, the databases [Medline (via OVID) and Embase (via Embase.com)] were searched with relevant search terms until the 10th of January 2023 to investigate the additional value of tunneled versus non-tunneled central venous catheter placement (PICO 4 of this guideline). The systematic literature search resulted in 149 hits. Studies were selected based on the following criteria: systematic reviews and randomized controlled trials (RCTs) about the place of tunneled (versus non-tunneled) CVL to reduce infection risk. Eight studies were initially selected based on title and abstract screening. After reading the full text, three studies were excluded (see the table with reasons for exclusion under the tab Methods), and five studies were included.
The detailed search strategies are depicted under the tab Methods.
Results
Fourteen studies were included in the analysis of the literature. Important study characteristics and results are summarized in the evidence tables. The assessment of the risk of bias is summarized in the risk of bias tables.
Referenties
- Andrivet P, Bacquer A, Ngoc CV, Ferme C, Letinier JY, Gautier H, Gallet CB, Brun-Buisson C. Lack of clinical benefit from subcutaneous tunnel insertion of central venous catheters in immunocompromised patients. Clin Infect Dis. 1994 Feb;18(2):199-206. doi: 10.1093/clinids/18.2.199. PMID: 8161627.
- Benezra D, Kiehn TE, Gold JW, Brown AE, Turnbull AD, Armstrong D. Prospective study of infections in indwelling central venous catheters using quantitative blood cultures. Am J Med. 1988 Oct;85(4):495-8. doi: 10.1016/s0002-9343(88)80084-6. PMID: 3177396.
- Bjornson HS, Colley R, Bower RH, Duty VP, Schwartz-Fulton JT, Fischer JE. Association between microorganism growth at the catheter insertion site and colonization of the catheter in patients receiving total parenteral nutrition. Surgery. 1982 Oct;92(4):720-7. PMID: 6812229.
- Bozzetti F, Terno G, Camerini E, Baticci F, Scarpa D, Pupa A. Pathogenesis and predictability of central venous catheter sepsis. Surgery. 1982 Apr;91(4):383-9. PMID: 6801797.
- Brandmeir NJ, Davanzo JR, Payne R, Sieg EP, Hamirani A, Tsay A, Watkins J, Hazard SW, Zacko JC. A Randomized Trial of Complications of Peripherally and Centrally Inserted Central Lines in the Neuro-Intensive Care Unit: Results of the NSPVC Trial. Neurocrit Care. 2020 Apr;32(2):400-406. doi: 10.1007/s12028-019-00843-z. PMID: 31556001.
- Chopra V, O'Horo JC, Rogers MA, Maki DG, Safdar N. The risk of bloodstream infection associated with peripherally inserted central catheters compared with central venous catheters in adults: a systematic review and meta-analysis. Infect Control Hosp Epidemiol. 2013 Sep;34(9):908-18. doi: 10.1086/671737. Epub 2013 Jul 26. PMID: 23917904.
- Chopra V, Flanders SA, Saint S, Woller SC, O'Grady NP, Safdar N, Trerotola SO, Saran R, Moureau N, Wiseman S, Pittiruti M, Akl EA, Lee AY, Courey A, Swaminathan L, LeDonne J, Becker C, Krein SL, Bernstein SJ; Michigan Appropriateness Guide for Intravenouse Catheters (MAGIC) Panel. The Michigan Appropriateness Guide for Intravenous Catheters (MAGIC): Results From a Multispecialty Panel Using the RAND/UCLA Appropriateness Method. Ann Intern Med. 2015 Sep 15;163(6 Suppl):S1-40. doi: 10.7326/M15-0744. PMID: 26369828.
- Clatot F, Fontanilles M, Lefebvre L, Lequesne J, Veyret C, Alexandru C, Leheurteur M, Guillemet C, Gouérant S, Petrau C, Théry JC, Rigal O, Moldovan C, Tennevet I, Rastelli O, Poullain A, Savary L, Bubenheim M, Georgescu D, Gouérant J, Gilles-Baray M, Di Fiore F. Randomised phase II trial evaluating the safety of peripherally inserted catheters versus implanted port catheters during adjuvant chemotherapy in patients with early breast cancer. Eur J Cancer. 2020 Feb;126:116-124. doi: 10.1016/j.ejca.2019.11.022. Epub 2020 Jan 10. PMID: 31931269.
- Clemons M, Stober C, Kehoe A, Bedard D, MacDonald F, Brunet MC, Saunders D, Vandermeer L, Mazzarello S, Awan A, Basulaiman B, Robinson A, Mallick R, Hutton B, Fergusson D. A randomized trial comparing vascular access strategies for patients receiving chemotherapy with trastuzumab for early-stage breast cancer. Support Care Cancer. 2020 Oct;28(10):4891-4899. doi: 10.1007/s00520-020-05326-y. Epub 2020 Jan 30. PMID: 32002617.Fletcher JJ, Wilson TJ, Rajajee V, Stetler WR Jr, Jacobs TL, Sheehan KM, Brown DL. A Randomized Trial of Central Venous Catheter Type and Thrombosis in Critically Ill Neurologic Patients. Neurocrit Care. 2016 Aug;25(1):20-8. doi: 10.1007/s12028-016-0247-9. PMID: 26842716.
- Cowl CT, Weinstock JV, Al-Jurf A, Ephgrave K, Murray JA, Dillon K. Complications and cost associated with parenteral nutrition delivered to hospitalized patients through either subclavian or peripherally-inserted central catheters. Clin Nutr. 2000 Aug;19(4):237-43. doi: 10.1054/clnu.2000.0103. PMID: 10952794.
- Dai C, Li J, Li QM, Guo X, Fan YY, Qin HY. Effect of tunneled and nontunneled peripherally inserted central catheter placement: A randomized controlled trial. J Vasc Access. 2020 Jul;21(4):511-519. doi: 10.1177/1129729819888120. Epub 2019 Nov 11. PMID: 31709895.
- Ge X, Cavallazzi R, Li C, Pan SM, Wang YW, Wang FL. Central venous access sites for the prevention of venous thrombosis, stenosis and infection. Cochrane Database Syst Rev. 2012 Mar 14;2012(3):CD004084. doi: 10.1002/14651858.CD004084.pub3. PMID: 22419292; PMCID: PMC6516884.
- Hamilton HC, Foxcroft DR. Central venous access sites for the prevention of venous thrombosis, stenosis and infection in patients requiring long-term intravenous therapy. Cochrane Database Syst Rev. 2007 Jul 18;(3):CD004084. doi: 10.1002/14651858.CD004084.pub2. Update in: Cochrane Database Syst Rev. 2012;3:CD004084. PMID: 17636746.
- Moss JG, Wu O, Bodenham AR, Agarwal R, Menne TF, Jones BL, Heggie R, Hill S, Dixon-Hughes J, Soulis E, Germeni E, Dillon S, McCartney E; CAVA trial group. Central venous access devices for the delivery of systemic anticancer therapy (CAVA): a randomised controlled trial. Lancet. 2021 Jul 31;398(10298):403-415. doi: 10.1016/S0140-6736(21)00766-2. Epub 2021 Jul 21. PMID: 34297997.
- Patel GS, Jain K, Kumar R, Strickland AH, Pellegrini L, Slavotinek J, Eaton M, McLeay W, Price T, Ly M, Ullah S, Koczwara B, Kichenadasse G, Karapetis CS. Comparison of peripherally inserted central venous catheters (PICC) versus subcutaneously implanted port-chamber catheters by complication and cost for patients receiving chemotherapy for non-haematological malignancies. Support Care Cancer. 2014 Jan;22(1):121-8. doi: 10.1007/s00520-013-1941-1. Epub 2013 Sep 5. PMID: 24005884.
- Picardi M, Della Pepa R, Cerchione C, Pugliese N, Mortaruolo C, Trastulli F, Giordano C, Grimaldi F, Zacheo I, Raimondo M, Chiurazzi F, Pane F. A Frontline Approach With Peripherally Inserted Versus Centrally Inserted Central Venous Catheters for Remission Induction Chemotherapy Phase of Acute Myeloid Leukemia: A Randomized Comparison. Clin Lymphoma Myeloma Leuk. 2019 Apr;19(4):e184-e194. doi: 10.1016/j.clml.2018.12.008. Epub 2018 Dec 20. PMID: 30704933.
- Ryan C, Hesselgreaves H, Wu O, Moss J, Paul J, Dixon-Hughes J, Germeni E. Patient acceptability of three different central venous access devices for the delivery of systemic anticancer therapy: a qualitative study. BMJ Open. 2019 Jul 9;9(7):e026077. doi: 10.1136/bmjopen-2018-026077. PMID: 31292176; PMCID: PMC6624052.
- Sitges-Serra A, Hernández R, Maestro S, Pi-Suñer T, Garcés JM, Segura M. Prevention of catheter sepsis: the hub. Nutrition. 1997 Apr;13(4 Suppl):30S-35S. doi: 10.1016/s0899-9007(97)00220-7. PMID: 9178308.
- Taxbro K, Hammarskjöld F, Thelin B, Lewin F, Hagman H, Hanberger H, Berg S. Clinical impact of peripherally inserted central catheters vs implanted port catheters in patients with cancer: an open-label, randomised, two-centre trial. Br J Anaesth. 2019 Jun;122(6):734-741. doi: 10.1016/j.bja.2019.01.038. Epub 2019 Apr 17. PMID: 31005243.
- Timsit JF, Bruneel F, Cheval C, Mamzer MF, Garrouste-Orgeas M, Wolff M, Misset B, Chevret S, Regnier B, Carlet J. Use of tunneled femoral catheters to prevent catheter-related infection. A randomized, controlled trial. Ann Intern Med. 1999 May 4;130(9):729-35. doi: 10.7326/0003-4819-130-9-199905040-00004. PMID: 10357691.
- Timsit JF, Sebille V, Farkas JC, Misset B, Martin JB, Chevret S, Carlet J. Effect of subcutaneous tunneling on internal jugular catheter-related sepsis in critically ill patients: a prospective randomized multicenter study. JAMA. 1996 Nov 6;276(17):1416-20. PMID: 8892717.
- Xiao MF, Xiao CQ, Li J, Dai C, Fan YY, Cao HJ, Qin HY. Subcutaneous tunneling technique to improve outcomes for patients undergoing chemotherapy with peripherally inserted central catheters: a randomized controlled trial. J Int Med Res. 2021 Apr;49(4):3000605211004517. doi: 10.1177/03000605211004517. PMID: 33840246; PMCID: PMC8044577.
Evidence tabellen
|
Study reference |
Study characteristics |
Patient characteristics 2 |
Intervention (I) |
Comparison / control (C) 3
|
Follow-up |
Outcome measures and effect size 4 |
Comments |
|
Andrivet (1994)
|
Type of study: Randomized controlled trial.
Setting and country: Centre Médico-Chirurgical de Bligny (ICU unit).
Funding and conflicts of interest: No information. |
Inclusion criteria:
Exclusion criteria: No information.
N total at baseline: Intervention: N = 106 Control: N = 97
Important prognostic factors2: age ± SD: I: 55.7 (1.3) C: 53.7 (1.4)
Sex: I: 71/106 (66%) M C: 67/97 (65%) M
Groups comparable at baseline? Yes.
|
Describe intervention (treatment/procedure/test): Tunneled catheterization.
|
Describe control (treatment/procedure/test): Non-tunneled catheterization.
|
Length of follow-up: No information.
Loss-to-follow-up: I: N = 5 C: N = 1
|
Catheter-related bacteremia I: 2/106 (1.9%) C: 5/97 (5.2%)
Non-bacteremic catheter-related infections I: 3/106 (2.8%) C: 4/97 (4.1%)
|
Author’s conclusion: In conclusion, our findings suggest that routine subcutaneous tunneling of central venous catheters is unnecessary in immunocompromised patients, a finding that allows for easier and quicker insertion of catheters in such patients, who often are disable and algid. Because subcutaneous tunneling in the present study was not associated with an increased rate of complications, we cannot suggest that this insertion technique should be abandoned. Since the completion of this study, we do not perform subcutaneous tunneling in our patients who require prolonged central venous access via the subclavian route. This therapeutic choice may not apply to other sites of venous access such as the internal jugular veins or to other materials such as cuffed or multilumen catheters. |
|
Brandmeir (2020) |
Type of study: Prospective, randomized controlled trial.
Setting and country: NSICU.
Funding and conflicts of interest: Database software was funded by NIH/NCRR Grant Number UL1RR033184, the remainder of the study was departmentally/institutionally funded.
The authors declare that they have no conflict of interest to disclose.
|
Inclusion criteria:
Exclusion criteria:
N total at baseline: Intervention: N = 72 Control: N = 80
Important prognostic factors2: age ± SD: I: 59.7 (18.0) C: 63.3 (13.6)
Sex: I: 35/72 (48.6%)M C: 45/80 (56.3%) M
Groups comparable at baseline? Yes. |
Describe intervention (treatment/procedure/test):
PICC
|
Describe control (treatment/procedure/test):
Centrally inserted central venous catheters (internal jugular placement or subclavian placement). |
Length of follow-up:
Loss-to-follow-up: None.
|
Complications (all combined) I: 14/72 (19.4%) C: 10/80 (12.5%)
Failure to insert I: 8/72 (11.1%) C: 5/80 (6.3%)
Tip malposition I: 0/72 (0%) C: 1/80 (1.3%)
Early removal I: 2/72 (2.8%) C: 1/80 (1.3%)
Mortality I: 13/72 (18.1%) C: 9/80 (11.3%) |
Author’s conclusion: This study provides evidence that PICCs and CVCs have similar risks of complications in the NSICU when compared in a randomized controlled clinical trial.
|
|
Clatot (2020) |
Type of study: Phase II randomised study.
Setting and country: Not reported. Funding and conflicts of interest: This work was supported by La Ligue Contre le Cancer de Haute-Normandie and Centre Henri Bec- querel. This funding source had no role in the design of the study, data acquisition, interpretation, manuscript writing or decision to submit results.
The authors have no conflict of interest to declare.
|
Inclusion criteria:
Exclusion criteria:
N total at baseline: Intervention: N = 127 Control: N = 126
Important prognostic factors2: age ± SD: I: 57.5 (30 to 74) C: 56 (30 to 74)
Sex: Not reported.
Groups comparable at baseline? Yes. |
Describe intervention (treatment/procedure/test):
PICC
|
Describe control (treatment/procedure/test):
PORT |
Length of follow-up: 35 weeks.
Loss-to-follow-up: None.
|
Complications (all combined) I: 21/126 (16.6%) C: 10/127 (7.8%)
DVT without local infection or septicaemia I: 7/126 (5.6%) C: 5/127 (3.9%)
DVT with septicaemia I: 2/126 (1.6%) C: 2/127 (1.2%)
DVT with local infection only I: 1/126 (0.8%) C: 0/127 (0%)
Pocket infection/exit-site infection without septicaemia I: 3/126 (2.4%) C: 1/127 (0.8%)
Pocket infection/exit-site infection with septicaemia I: 2/126 (1.6%) C: 0/127 (0%)
Implantation failure I: 2/126(1.6%) C: 2/127 (1.2%)
Device withdrawal I: 1/126 (1.6%) C: 0/127 (0%)
|
Author’s conclusion: In conclusion, this prospective randomized study shows that CR-SAEs in patients with EBC are frequent (12.2%) but rarely impact the ACT process (4/253 ACT interruptions and 3/253 ACT delays > 1 week). PICCs are associated with a significantly higher risk of CR- SAEs than PORTs, which confirms the results from retrospective studies or prospective studies performed in various cancer situations. Moreover, patients reported more discomfort with PICCs than with PORTs. Taken together, these results support the preferential use of PORTs instead of PICCs in the case of EBC ACT. |
|
Clemons (2020) |
Type of study: Multi-centre and unblinded trial.
Setting and country: Ottawa Hospital Cancer Centre, the Irving Greenberg Family Cancer Centre, Ottawa; or the Cancer Centre of Southeastern Ontario, Kingston, Ontario, Canada.
Funding and conflicts of interest: Funding of this study was through the Rethinking Clinical Trials (REaCT program).
Dr. Awan reports participating in the Novartis Canada Advisory Board on the use of Ribociclib. Dr. Hutton reports personal fees from Cornerstone Research, outside the submitted work. The remaining authors declare that they have no conflicts of interest
|
Inclusion criteria:
Exclusion criteria:
N total at baseline: Intervention: N = 29 Control: N = 27
Important prognostic factors2: age ± SD: I: 52 (32 to 84) C: 54 (34 to 82)
Sex: Unclear.
Groups comparable at baseline? Yes. |
Describe intervention (treatment/procedure/test):
PICC
|
Describe control (treatment/procedure/test):
PORT
|
Length of follow-up: Unclear.
Loss-to-follow-up: None.
|
All complications PICC: 5/29 PORT: 4/27
Deep venous thrombosis PICC: 2/29 (6.9%) PORT: 0/27 (0%)
Pulmonary embolism PICC: 2/29 (6.9%) PORT: 0/27 (0%)
Infections PICC: 8/29 (27.6%) PORT: 9/27 (33.3%)
Device removal PICC: 3/29 (10.3%) PORT: 5/27 (18.5%)
|
Author’s conclusion: In conclusion, while reliable central vascular access may improve the patient experience by reducing the number of extra peripheral IV attempts, reducing the risk of extravasation, and reducing long term damage to the intima of the vein, these benefits have not been shown in appropriately designed prospective trials. This is particularly true as we increasingly move away from anthracycline-containing chemotherapy regimens. This is important as lines are associated with higher initial costs, delayed beginning of systemic therapy and a broad range of complications. Optimizing the type of IV access may not only reduce variability in patient care and potentially offer cost savings but also improve patient comfort and acceptability. In the current study, we have failed to demonstrate the feasibility of our novel trials methodology. In addition, the incidence of toxicities reported in our study also means that for a future study to definitively determine optimal IV access however given the generally low level of physician engagement, performing such a trial may be challenging. More trials are clearly needed. |
|
Dai (2020)
|
Type of study: Randomized controlled trial.
Setting and country: Single center at Sun Yat-sen University Cancer Center in Guangzhou, China.
Funding and conflicts of interest: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Financial support received from the Medical Scientific Research Foundation of Guangdong Province of China (A2019007).
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
|
Inclusion criteria:
Exclusion criteria:
N total at baseline: Intervention: N = 87 Control: N = 87
Important prognostic factors2: age ± SD: I: 45.70 (11.32) C: 45.66 (11.45)
Sex: I: 51/87 (58.6%) M C: 55/87 (63.2%) M
Groups comparable at baseline? Yes. |
Describe intervention (treatment/procedure/test): Tunneled PICC
|
Describe control (treatment/procedure/test): Non-tunneled PICC
|
Length of follow-up: No information.
Loss-to-follow-up: None.
|
Infection I: 0/87 (0%) C: 3/87 (3.4%) |
Author’s conclusion: Our study compared the effect of the tunneled and non- tunneled PICC techniques, and these results confirm that tunneled PICC is safe, feasible, and effective. Although tunneled PICC adds 17.87 Yuan, less than 3 min, and 0.2mL of bleeding volume to the procedure, it has a lower incidence of complications during the placement. Moreover, it can also reduce the cost of PICC maintenance and the incidence of complications after the placement, especially in wound oozing, MARSI, venous thrombosis, and catheter dislodgement. Altogether, the tunneled technique applied to PICC placement may hence be recommended.
|
|
Fletcher (2016) |
Type of study: Pragmatic, prospective, randomized, open-label, independently adjudicated outcome trial.
Setting and country: Intensive care unit, USA.
Funding and conflicts of interest: This research was funded by the Michigan Institute for Clinical & Health Research grant support (CTSA: UL1RR024986).
Dr. Brown received a Clinical and Translational Science Award from the Michigan Institute for Clinical and Health Research (see below). All other authors declare that they have no conflicts of interest.
|
Inclusion criteria:
Exclusion criteria:
N total at baseline: Intervention: N = 39 Control: N = 41
Important prognostic factors2: age ± SD: I: 61 (12) C: 59 (15)
Sex: I: 24/39 (61.5%) M C: 25/41 (61%) M
Groups comparable at baseline? Yes. |
Describe intervention (treatment/procedure/test):
PICC
|
Describe control (treatment/procedure/test):
Centrally inserted central venous catheter.
|
Length of follow-up: Unclear.
Loss-to-follow-up: None.
|
Death PICC: 5/39 (12.8%) CICVC: 5/41 (12.2%)
Pulmonary embolism (possible catheter-related) PICC: 1/39 (2.6%) CICVC: 1/41 (2.4%)
Thrombosis PICC: 7/39 (17.9%) CICVC: 1/41 (2.4%)
|
Author’s conclusion: Our trial demonstrates that critically ill neurologic patients who require a CVC have significantly lower odds of CRLVT with placement of a CICVC as compared to a PICC. Additional study seems warranted comparing safety and efficacy of CVCs in critically ill patient populations.
|
|
Moss (2021) |
Type of study: Pragmatic, open-label, multicentre, mixed methods, randomised, controlled trial.
Setting and country: 18 UK oncology units, United Kingdom.
Funding and conflicts of interest: The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
All other authors declare no competing interests.
|
Inclusion criteria:
Exclusion criteria:
N total at baseline: See original study.
Important prognostic factors2: See original study.
Sex: See original study.
Groups comparable at baseline? Yes.
|
Describe intervention (treatment/procedure/test):
I: PICC
II: PORT
III: PORT
|
Describe control (treatment/procedure/test):
I: CVC (Hickman)
II: CVC (Hickman)
III: PICC |
Length of follow-up: 12 months.
Loss-to-follow-up: None.
|
PICC vs CVC (Hickman)
Number of patients with 0 complications (all combined) PICC: 102/212 (48.1%) CVC: 110/212 (51.9%)
Number of patients with 1 or more complications (all combined) PICC: 110/212 (51.9%) CVC: 103/212 (48.1%)
Number of patients with DVT PICC: 13/212 (6.1%) CVC: 10/212 (4.7%)
Number of patients with pulmonary embolism PICC: 6/212 (2.8%) CVC: 4/212 (1.9%)
Number of patients with an infection PICC: 23/212 (10.8%) CVC: 63/212 (29.7%)
Number of patients with laboratory confirmed bloodstream infection PICC: 10/212 (4.7%) CVC: 41/212 (19.3%)
Number of patients with suspected catheter-related bloodstream infection PICC: 10/212 (4.7%) CVC: 18/212 (8.5%)
Number of patients with exit site infections PICC: 4/212 (1.9%) CVC: 19/212 (9.0%)
Number of patients with mechanical failures PICC: 31/212 (14.6%) CVC: 7/212 (3.3%)
Other complications PICC: 23/212 (10.8%) CVC: 16/212 (7.5%)
PORT vs PICC
Number of patients with 0 complications (all combined) PORT: 100/147 (68.0%) PICC: 106/199 (53.3%)
Number of patients with 1 or more complications (all combined) PORT: 47/147 (32.0%) PICC: 93/199 (46.7%)
Number of patients with DVT PORT: 3/147 (2.0%) PICC: 22/199 (11.1%)
Number of patients with pulmonary embolism PORT: 3/147 (2.0%) PICC: 1/199 (0.5%)
Number of patients with an infection PORT: 18/147 (12.2%) PICC: 16/199 (8.0%)
Number of patients with laboratory confirmed bloodstream infection PORT: 8/147 (55.1%) PICC:7/199 (3.5%)
Number of patients with suspected catheter-related bloodstream infection PORT: 8/147 (5.4%) PICC: 5/199 (2.5%)
Number of patients with exit site infections PORT: 4/147 (2.7%) PICC: 4/199 (2.0%)
Number of patients with mechanical failures PORT: 4/147 (2.7%) PICC: 21/199 (10.6%)
Other complications PORT: 16/147 (10.9%) PICC:19/199 (9.5%) |
Author’s conclusion: CAVA has expanded the knowledge base on these CVADs and the case for a PORT-dominant strategy has been strengthened. These findings should prove useful for updating national and international guidelines to recommend the adoption of PORT-delivered services for relevant patient groups.
|
|
Patel (2013) |
Type of study: Randomized controlled trial.
Setting and country: Three Australian centres, Victoria, Australia.
Funding and conflicts of interest: The authors have no conflicts of interest to declare. The authors have full control of all primary data and agree to allow the journal to review the data if requested.
|
Inclusion criteria:
Exclusion criteria:
N total at baseline: Intervention: N = 36 Control: N = 34
Important prognostic factors2: age ± SD: I: 59 (29 to 84) C: 60 (34 to 78)
Sex: I: 17/36 (47%) M C: 19/34 (56%) M
Groups comparable at baseline? Yes.
|
Describe intervention (treatment/procedure/test):
PICC
|
Describe control (treatment/procedure/test):
PORT |
Length of follow-up: 6 months or until CVC removal.
Loss-to-follow-up: None.
|
Complications (all combined) I: 15/36 (41.7%) C: 6/34 (17.6%)
DVT/line occlusion I: 4/36 (11.1%) C: 0/34 (0%)
Infection I: 0/36 (0%) C: 1/34 (2.9%)
Line disruption I: 2/36 (5.6%) C: 1/34 (2.9%)
Patient choice I: 1/36 (2.7%) C: 0/34 (0%)
Line occlusion not requiring CVC I: 3/36 (8.3%) C: 0/34 (0%)
Pain I: 1/36 (2.7%) C: 4/34 (11.8%)
Pruritus I: 1/36 (2.7%) C: 0/34 (0%)
Wound complication I: 1/36 (2.7%) C: 0/34 (0%) |
Author’s conclusion: In summary, port devices were associated with a lower complication rate in particular significantly fewer thromboses, hence supporting port use over PICCs within our patient group. Specifically, placement of a port device rather than a PICC line should be considered for patients who are at high risk of thrombosis. On consideration of complications within the first 6 months, cost analyses alone did not support the use of one CVC over the other. However, further work is required to validate quality of life questionnaires and to ascertain cost and complication rates in order to demonstrate CVC cost- effectiveness.
|
|
Picardi (2019) |
Type of study: Randomized controlled trial.
Setting and country: Unclear.
Funding and conflicts of interest: Unclear. |
Inclusion criteria:
Exclusion criteria:
N total at baseline: Intervention: N = 46 Control: N = 47
Important prognostic factors2: age ± SD: I: 54.5 (24 to 80) years C: 53 (18 to 74) years
Sex: I: 25/46 (54.3%) M C: 22/47 (46.8%) M
Groups comparable at baseline? Yes. |
Describe intervention (treatment/procedure/test):
PICC (open-ended, nonvalved pressure injectable polyurethane PICC with a flexible tip).
|
Describe control (treatment/procedure/test):
Centrally inserted central venous catheters (CICC) (external nontunneled heparin coated Vialon CVC). |
Length of follow-up: Unclear.
Loss-to-follow-up: None.
|
Catheter-related bloodstream infections PICC: 2/46 (4.3%) CICC: 11/47 (23.4%)
Catheter-related deep venous thrombosis PICC: 4/46 (8.7%) CICC: 12/47 (25.5%)
Catheter-positioning related complications PICC: 2/26 (4.3%) CICC: 13/4727.7%)
Catheter malfunctions PICC: 4/46 (8.7%) CICC: 5/47 (10.6%)
Catheter removals PICC: 6/46 (13.0%) CICC: 16/47 (34.0%)
30-day mortality PICC: 4/46 (8.7%) CICC: 10/47 (21.3%)
|
Author’s conclusion: The presented data have shown that the PICC is an easy-to-use device that enables safe and effective central intravascular access for patients receiving intensive chemotherapy for hematologic remission induction of AML. In contrast, BSI and septic thrombophlebitis emerged as life-threatening complications for neutropenic patients with external nontunneled CICCs in situ. Our findings highlight the importance of a team experienced in PICC positioning and care, with a well-written protocol to optimize the catheter insertion procedures and subsequent management. With optimal conditions and experienced physicians, we propose the use of PICC as a new frontline option for CVC in patients with acute leukemia undergoing intensive chemotherapy.
|
|
Taxbro (2019) |
Type of study: Open-label, randomised, two-centre trial.
Setting and country: Two oncology centres in Sweden.
Funding and conflicts of interest: Futurum (Academy for Healthcare, Jo€nko€ping County Council, Sweden; grant number 767451); FORSS (Research Council in South East Sweden; grant number 295881).
The authors declare that they have no conflicts of interest.
|
Inclusion criteria:
Exclusion criteria:
N total at baseline: Intervention: N = 201 Control: N = 198
Important prognostic factors2: age ± SD: I: 66 (19 to 84) C: 65 (30 to 89)
Sex: I: 91/201 (45.3%) M C: 83/198 (41.9%) M
Groups comparable at baseline? Yes. |
Describe intervention (treatment/procedure/test):
PICC
|
Describe control (treatment/procedure/test):
PORT |
Length of follow-up: 12 months maximum.
Loss-to-follow-up: None.
|
All adverse eventsPICC: 45/201 (22.4%) PORT: 26/198 (13.1%)
Overall mortality PICC: 12/201 (6.0%) PORT: 37/198 (18.7%)
Catheter-related deep venous thrombosis PICC: 16/201 (8.0%) PORT: 2/198 (1.0%)
Catheter infection PICC: 4/201 (2.0%) PORT: 16/198 (8.1%)
Exit, local or pocket infection PICC: 4/201 (2.0%) PORT: 15/198 (7.8%)
Catheter-related bloodstream infections PICC: 0/201 (0%) PORT: 2/198 (1.0%)
Catheter occlusion PICC: 16/201 (8.0%) PORT: 1/198 (0.5%)
Mechanical failure PICC: 9/201 (4.5%) PORT: 7/198 (3.5%) |
Author’s conclusion: In conclusion, we have demonstrated that the risk for CR- DVT and overall adverse events is higher in cancer patients with a PICC than those with a PORT. These findings are of clinical importance and should be considered by anaesthetists, oncologists, and vascular access clinicians when advising patients eligible for a CVC before chemotherapy.
|
|
Timsit (1999)
|
Type of study: Randomized controlled trial.
Setting and country: Three intensive care units at academic hospitals in Paris, France.
Funding and conflicts of interest: The funding agencies had no input into the de- sign or conduct of this study or in the decision to submit the manuscript for publication.
|
Inclusion criteria:
Exclusion criteria:
N total at baseline: Intervention: N = 168 Control: N = 168
Important prognostic factors2: age ± SD: I: 61.4 (16.7) C: 61.1 (17.0)
Sex: I: 105/168 (62.5%) M C: 104/168 (61.9%) M
Groups comparable at baseline? Yes. |
Describe intervention (treatment/procedure/test): Tunneled catheters.
|
Describe control (treatment/procedure/test): Non-tunneled catheters.
|
Length of follow-up: Discharge or death.
Loss-to-follow-up: None. |
Systemic catheter-related sepsis per 100 catheter-days I: 0.36 C: 1.1 RR 0.25 (95% CI 0.09 to 0.72)
Catheter-related bloodstream infection per 100 catheter-days I: 0.073 C: 0.23 RR 0.28 (95% CI 0.03 to 1.92)
|
Author’s conclusion: We conclude that in critically ill patients in whom femoral access is mandatory, tunneled catheterization is associated with a lower rate of infectious complications than nontunneled catheterization.
|
|
Timsit (1996)
|
Type of study: Randomized controlled trial.
Setting and country: Three ICUs in Paris, France.
Funding and conflicts of interest: No information. |
Inclusion criteria:
Exclusion criteria:
N total at baseline: Intervention: N = 117 Control: N = 114
Important prognostic factors2: age ± SD: I: 63.4 (16.1) C: 66.9 (13.7)
Sex: I: 82/117 (70%) M C: 84/114 (73.7%) M
Groups comparable at baseline? Yes. |
Describe intervention (treatment/procedure/test): Tunneled catheters.
|
Describe control (treatment/procedure/test): Non-tunneled catheters.
|
Length of follow-up: Until discharge of death.
Loss-to-follow-up: None.
|
Systemic catheter-related sepsis I: 7/117 (6.0%) C: 18/114 (15.8%)
Bacteremic catheter-related sepsis I: 4/117 (3.4%) C: 13/114 (11.4%)
|
Author’s conclusion: We conclude that in critically ill pa¬ tients receiving mechanical ventilation for whom internal jugular access is chosen, tunnelization is more suitable as it is associated with a lower rate of infectious complications than nontunneled access.
|
|
Xiao (2021)
|
Type of study: Randomized controlled trial. Setting and country: Sun Yat-Sen university cancer center in Guangzhou, China.
Funding and conflicts of interest: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by the Medical Scientific Research Foundation of Guangdong Province of China (A2019007).
The authors declare that there is no conflict of interest. |
Inclusion criteria:
Exclusion criteria:
N total at baseline: Intervention: N = 64 Control: N = 65
Important prognostic factors2: age ± SD: I: 45.64 (11.59) C: 47.95 (11.96)
Sex: I: 35/64 (54.7%) M C: 39/65 (60%) M
Groups comparable at baseline? Yes. |
Describe intervention (treatment/procedure/test): Subcutaneous tunnelling technique (PICC).
|
Describe control (treatment/procedure/test): Normal technique (PICC)
|
Length of follow-up: Unclear.
Loss-to-follow-up: None.
|
Infection I: 1/64 (1.6%) C: 3/65 (4.6%)
Cathether-related bloodstream infection I: 0/64 (0%) C: 1/65 (1.5%)
|
Author’s conclusion: In this study, we evaluated the effect of the subcutaneous tunneling technique on improving outcomes in patients with PICCs. We demonstrated that the subcutaneous tunneling technique is a safe, feasible, and efficient method to expand the use of multilumen PICCs by allowing insertion of a larger PICC without increasing pain during placement. Moreover, this technique can reduce the cost of PICC maintenance and reduce complications after placement, especially with respect to catheter dislodgement, venous thrombosis, wound oozing, and unscheduled PICC removal. Therefore, the subcutaneous tunneling technique should be recommended to improve patient outcomes of PICC insertion. |
Risk of bias table
|
Study reference
(first author, publication year) |
Was the allocation sequence adequately generated?
Definitely yes Probably yes Probably no Definitely no |
Was the allocation adequately concealed?
Definitely yes Probably yes Probably no Definitely no |
Blinding: Was knowledge of the allocated interventions adequately prevented?
Were patients blinded?
Were healthcare providers blinded?
Were data collectors blinded?
Were outcome assessors blinded?
Were data analysts blinded?
Definitely yes Probably yes Probably no Definitely no |
Was loss to follow-up (missing outcome data) infrequent?
Definitely yes Probably yes Probably no Definitely no |
Are reports of the study free of selective outcome reporting?
Definitely yes Probably yes Probably no Definitely no |
Was the study apparently free of other problems that could put it at a risk of bias?
Definitely yes Probably yes Probably no Definitely no |
Overall risk of bias If applicable/necessary, per outcome measure
LOW Some concerns HIGH
|
|
Andrivet (1994)
|
Definitely yes.
Reason: each patient was randomly assigned to either the TC or the NTC group on an odd/even basis according to the order in which they presented for catheterization. |
No information.
Reason: - |
No information.
Reason: - |
Probably no.
Reason: More lost to follow-up in intervention group compared with control group. |
Probably yes.
Reason: All predefined outcomes were reported. |
Probably yes.
Reason: No other biases reported. |
Some concerns. |
|
Brandmeir (2020) |
Definitely yes
Reason: Randomization was carried out by means of a computer-generated randomized sequence with equal allocation to each arm and no blocking scheme.
|
Definitely yes
Reason: Allocation was concealed to the patients and researchers prior to enrollment.
|
Definitely no
Reason: The major limitation of this study is that outcomes were not blinded.
|
Definitely yes
Reason: no lost to follow-up reported. |
Probably yes
Reason: all predefined outcomes were reported. |
Probably yes
Reason: No other bias reported. |
Some concerns; no blinding. |
|
Clatot (2020) |
Definitely yes
Reason: Randomisation was per- formed at a 1:1 allocation ratio using a block size of 8, without a stratification factor
|
No information.
Reason: - |
No information.
Reason: - |
Definitely yes
Reason: no lost to follow-up reported.
|
Probably yes
Reason: all predefined outcomes were reported. |
Probably no
Reason: a third limitation is a potential patient selection, particularly due to the high study refusal rate (54%) that we did not expect
|
Some concerns; no information regarding allocation concealment, blinding, and other bias. |
|
Dai (2020)
|
Definitely yes.
Reason: A total of 174 participants were randomized to the experimental group (tunneled peripherally inserted central catheter) or the control group
|
Definitely yes.
Reason: At baseline, the participants were allocated to either the intervention or the control group through a computer-generated permuted- block randomization scheme using the envelope method.
|
Definitely no.
Reason: Non-blinded study. |
Definitely yes.
Reason: No lost to follow-up reported. |
Probably yes.
Reason: All predefined outcomes were reported. |
Probably yes.
Reason: No other biases reported. |
Some concerns. |
|
Fletcher (2016) |
Definitely yes
Reason: Patients were randomized to receive either a PICC or CICVC
|
Definitely yes
Reason: Allocation concealment was achieved using sequentially numbered, opaque, sealed envelopes stored in a central location.
|
Definitely no
Reason: Open-label study. |
Definitely yes
Reason: no lost to follow-up reported.
|
Probably yes
Reason: all predefined outcomes were reported.
|
Probably no.
Reason: - |
Some concerns: no blinding in the study. |
|
Moss (2021) |
Definitely yes
Reason: Randomisation was done using a minimisation algorithm stratifying by centre, body-mass index, type of cancer, device history, and treatment mode.
|
No information.
Reason: - |
Definitely no
Reason: The study was necessarily open-label with all parties aware of treatment allocation.
|
Probably yes
Reason: all patients were included in the intention-to-treat analysis. |
Probably yes
Reason: all predefined outcomes were reported. |
Probably yes
Reason: Further limitations of the trial included a reduction in power of two of the comparisons after 18 months. All comparisons were initially designed with 90% power; however, a protocol-mandated review of recruitment at this time allowed adjustments to be made on the basis of actual recruitment to each comparison and the results of the pilot study.
|
Some concerns; informating regarding allocation concealment and blinding. |
|
Patel (2013) |
Definitely yes
Reason: Patients were randomised 1:1
|
No information
Reason: - |
No information
Reason: - |
Definitely yes
Reason: no lost to follow-up reported.
|
Probably yes
Reason: all predefined outcomes were reported. |
Probably no
Reason: Finally, the general- izability of our findings may be compromised due to loco- regional factors such as availability of skills and resources, patient selection bias and selected accrual.
|
Some concerns; no information regarding allocation concealmen, blinding and probably existence of other bias in the study. |
|
Picardi (2019) |
Definitely yes
Reason: The patients were randomized 1:1
|
Definitely yes
Reason: The random allocation sequence was performed using a computerized system generated by the statistician’s study
|
Definitely no
Reason: Open-label study. |
Definitely yes
Reason: no lost to follow-up reported.
|
Probably yes
Reason: all predefined outcomes were reported.
|
Probably no.
Reason: - |
Some concerns: no blinding in the study. |
|
Taxbro (2019) |
Definitely yes
Reason: Patients were randomised in a 1:1 allocation ratio
|
No information
Reason: - |
Definitely no
Reason: It was not feasible to blind the patient, clinician, or trial assessors to the allocated arm, because of the particular properties of the catheters.
|
Definitely yes
Reason: no lost to follow-up reported.
|
Probably yes
Reason: all predefined outcomes were reported. |
Probably no.
Reason: - |
Some concerns: no blinding in the study. |
|
Timsit (1999)
|
Definitely yes.
Reason: Patients were randomly assigned to one of the treatment groups. |
Definitely yes.
Reason: we randomly assigned patients to treatment groups immediately before catheter placement by using a computer- assisted system and a computer-generated allocation schedule.
|
Probably yes.
Reason: Because clinicians were not blinded, a blinded five-physician steering committee deter- mined the presence of each study end point using all reported data (and, if necessary, the patient’s full medical record).
|
Definitely yes.
Reason: No lost to follow-up reported. |
Probably yes.
Reason: All predefined outcomes were reported. |
Probably yes.
Reason: No other biases reported. |
Low. |
|
Timsit (1996)
|
Definitely yes.
Reason: Patients were randomly assigned to one of the treatment groups. |
No information.
Reason: - |
Definitely no.
Reason: Non-blinded study. |
Definitely yes.
Reason: No lost to follow-up reported. |
Probably yes.
Reason: All predefined outcomes were reported. |
Probably yes.
Reason: No other biases reported. |
Some concerns. |
|
Xiao (2021)
|
Definitely yes.
Reason: One hundred thirty patients were randomly divided into an experimental group (subcutaneous tunneling technique) and control group (normal technique)
|
No information.
Reason: - |
Definitely no.
Reason: The first is that double blinding was not possible in our study because the wounds and surgical procedures were different between the groups, which might have influenced the degree of comfort in the two groups.
|
Definitely yes.
Reason: No lost to follow-up reported. |
Probably yes.
Reason: All predefined outcomes were reported. |
Probably yes.
Reason: No other biases reported. |
Some concerns. |
Exclusie tabel
|
Author and year |
Reason for exclusion |
|
Fukuda (2015) |
Wrong comparison. |
|
Golsorkhi (2022) |
Wrong study design. |
|
Guo (2021) |
Wrong outcomes. |
|
He (2021) |
Wrong outcomes. |
|
Hon (2019) |
Wrong study design. |
|
Li (2021) |
Wrong study design. |
|
Lv (2018) |
Studies in SR already included. |
|
Maria (2019) |
Wrong comparison. |
|
Mateo-Lobo (2019) |
Includes observational studies only; excluded because of wrong study design. |
|
Mavrovounis (2020) |
Studies in SR already included. |
|
Mitchell (2013) |
Wrong comparison. |
|
Nielsen (2021) |
Wrong comparison. |
|
Parienti (2019) |
Wrong comparison. |
|
Pikwer (2012) |
Wrong study design. |
|
Puri (2022) |
Wrong study design. |
|
Ricard (2013) |
Wrong comparison. |
|
Saber (2011) |
Wrong study design. |
|
Tran (2010) |
Wrong study design. |
|
Trerotola (2010) |
Wrong comparison. |
|
Ugas (2012) |
Wrong study design. |
|
Yeow (2022) |
Wrong study design. |
|
Zhong (2021) |
Wrong study design. |
Verantwoording
Beoordelingsdatum en geldigheid
Laatst beoordeeld : 25-06-2025
Nog in afwachting autorisatie van de volgende partijen:
- Vereniging voor Hygiëne en Infectiepreventie in de Gezondheidszorg
- Patiëntenfederatie Nederland
Algemene gegevens
De ontwikkeling/herziening van deze richtlijnmodule werd ondersteund door het Kennisinstituut van de Federatie Medisch Specialisten (www.demedischspecialist.nl/kennisinstituut) en werd gefinancierd uit de Stichting Kwaliteitsgelden Medisch Specialisten (SKMS). De financier heeft geen enkele invloed gehad op de inhoud van de richtlijnmodule.
Samenstelling werkgroep
Voor het ontwikkelen van de richtlijnmodule is in 2022 een multidisciplinaire werkgroep ingesteld, bestaande uit vertegenwoordigers van alle relevante specialismen (zie hiervoor de Samenstelling van de werkgroep) die betrokken zijn bij de zorg rondom vaattoegangswegchirurgie.
Werkgroep
- dr. C.G. (Niels) Vos (voorzitter), Nederlandse Vereniging voor Heelkunde
- dr. M.E. (Marianne) Sitsen (vice-voorzitter), Nederlandse Vereniging voor Anesthesiologie
- dr. M.J. (Marijke) Molegraaf, Nederlandse Vereniging voor Heelkunde
- dr. M.G.J. (Maarten) Snoeijs, Nederlandse Vereniging voor Heelkunde
- dr. M. (Mahir) Uslu, Nederlandse Vereniging voor Anesthesiologie
- dr. M. (Michelle) Gompelman, Nederlandse Internisten Vereniging
- dr. E.R. (Eric) van der Vorm, Nederlandse Vereniging voor Medische Microbiologie / Samenwerkingsverband Richtlijnen Infectiepreventie
- drs. Ir. P.A.A. (Pum) Le Haen, Nederlandse Vereniging voor Radiologie
Klankbordgroep
- dr. H. (Hanneke) Buter, Nederlandse Vereniging voor Intensive Care
- Werkgroep SRI-richtlijn Veneuze en arteriële katheters
Met ondersteuning van
- dr. M.S. (Matthijs) Ruiter, senior adviseur, Kennisinstituut van de Federatie Medisch Specialisten
- M. (Mitchel) Griekspoor, MSc, adviseur, Kennisinstituut van de Federatie Medisch Specialisten
Belangenverklaringen
De Code ter voorkoming van oneigenlijke beïnvloeding door belangenverstrengeling is gevolgd. Alle werkgroepleden hebben schriftelijk verklaard of zij in de laatste drie jaar directe financiële belangen (betrekking bij een commercieel bedrijf, persoonlijke financiële belangen, onderzoeksfinanciering) of indirecte belangen (persoonlijke relaties, reputatiemanagement) hebben gehad. Gedurende de ontwikkeling of herziening van een module worden wijzigingen in belangen aan de voorzitter doorgegeven. De belangenverklaring wordt opnieuw bevestigd tijdens de commentaarfase.
Een overzicht van de belangen van werkgroepleden en het oordeel over het omgaan met eventuele belangen vindt u in onderstaande tabel. De ondertekende belangenverklaringen zijn op te vragen bij het secretariaat van het Kennisinstituut van de Federatie Medisch Specialisten.
|
Werkgroeplid |
Hoofdfunctie |
Project Tekstveld |
Nevenwerkzaamheden |
Persoonlijke Financiële belangen |
Persoonlijke relaties |
Extern gefinancierd onderzoek |
Intell. Belangen en reputatie |
Overige belangen |
Ondernomen actie |
|
Niels Vos (vz.) |
Vaatchirurg |
Richtlijn Centraal Veneuze Toegang |
Voorzitter huidige richtlijncommissie. |
Geen. |
Geen. |
1. Studie naar functioneren van Artivion (Jotec) EVAR prothese. Gefinancierd door Artivion. Multicenter, ik ben lokale (martini ziekenhuis) hoofdonderzoeker. Het ziekenhuis krijgt een onkosten vergoeding van een paar 100 euro. Persoonlijk krijg ik er niets voor. Het is een reguliere verkrijgbare stent waar de leverancier wilde kijken hoe deze presteert (technisch/klinisch en QoL).
2.Door ZonMW gefinancierde multicenter trial naar antistolling na pta (CLEARPATH trial, RCT, clopidogrel / placebo vs clopidogrel / ascal na dotter). Hier is een onkostenvergoeding per patiënt voor die naar het ziekenhuis (wetenschappelijk instituut) gaat. Ik ben ook hier lokale hoofdonderzoeker.
|
Geen. |
Geen. |
Geen restricties. Extern gefinancierd onderzoek valt buiten bestek van de richtlijn. |
|
Eric van der Vorm |
Arts-microbioloog |
Richtlijn Centraal Veneuze Toegang |
Geen. |
Geen. |
Geen. |
Geen. |
Geen. |
Geen. |
Geen restricties. |
|
Maarten Snoeijs |
Vaatchirurg |
Centraal Veneuze Toegang & Cluster expertisegroep Arteriële en Veneuze Pathologie. |
Geen. |
Geen. |
Geen. |
1. OASIS Zorgevaluatie (Zorgevaluatie Nederland) 2. FLOW Zorgevaluatie (ZonMw) 3. Personalised hemodynamic modeling of arteriovenous grafts for prediction of vascular access stenosis and thrombosis (Vascular Access Society research grant) 4. ShuntSimulationStudy (Nierstichting).
|
Leider expertisecentrum met topreferente zorgfunctie voor vaattoegangchirurgie in MUMC+ |
Geen. |
Geen restricties. Extern gefinancierd onderzoek valt buiten bestek van de richtlijn. |
|
Mahir Uslu |
Anesthesioloog |
Richtlijn Centraal Veneuze Toegang
|
Geen. |
Geen. |
Geen. |
Geen. |
Geen. |
Geen. |
Geen restricties. |
|
Marianne Elisabeth Sitsen |
Anesthesioloog en medisch manager OK centrum |
Richtlijn Centraal Veneuze Toegang |
Geen. |
Geen. |
Geen. |
Geen. |
Geen. |
Geen. |
Geen restricties. |
|
Marijke Molegraaf |
Vaatchirurg |
Richtlijn Centraal Veneuze Toegang
|
Geen. |
Geen. |
Geen. |
Geen. |
Geen. |
Geen. |
Geen restricties. |
|
Michelle Gompelman |
Internist-infectioloog, Arts-onderzoeker |
Richtlijn Centraal Veneuze Toegang |
Hoofdredactieraad lid Tijdschrift voor Infectieziekten – onbetaald. |
Geen. |
Geen. |
CARRIER-trial (ZonMW), Staphylococcus aureus decolonization in patients on home parenteral nutrition, geen PI. |
Geen. |
Geen. |
Geen restricties. Extern gefinancierd onderzoek valt buiten bestek van de richtlijn.
|
|
Pum le Haen |
Interventieradioloog |
Richtlijn Centraal Veneuze Toegang |
Geen. |
Geen. |
Geen. |
Lokale P.I. voor onderzoek naar de verbeterde/snellere toegang tot de AFS bij antegrade punctie van de AFC met de Speedwire. Multi center internationale studie onder P.I-schap van D. van den Heuvel, interventieradioloog St Antonius Ziekenhuis in Nieuwegein. Voor de inclusie van een patiënt in het bovengenoemde onderzoek wordt een onkostenvergoeding van 100 euro betaald. Deze vergoeding loopt via het ziekenhuis en de specialisten coöperatie en wordt betaald door Speedwire. Gaat om in totaal 20-25 patiënten in de looptijd van het onderzoek. |
Geen. |
Geen. |
Geen restricties. Extern gefinancierd onderzoek valt buiten bestek van de richtlijn. |
Inbreng patiëntenperspectief
Er werd aandacht besteed aan het patiëntenperspectief door uitnodigen van de Patiëntenfederatie Nederland voor de invitational conference. Het verslag hiervan is besproken in de werkgroep. De verkregen input is meegenomen bij het opstellen van de uitgangsvragen, de keuze voor de uitkomstmaten en bij het opstellen van de overwegingen. De conceptrichtlijn is tevens voor commentaar voorgelegd aan de deelnemers van de invitational conference en de eventueel aangeleverde commentaren zijn bekeken en verwerkt.
Kwalitatieve raming van mogelijke financiële gevolgen in het kader van de Wkkgz (Volgt na de commentaarfase)
Bij de richtlijnmodule is conform de Wet kwaliteit, klachten en geschillen zorg (Wkkgz) een kwalitatieve raming uitgevoerd om te beoordelen of de aanbevelingen mogelijk leiden tot substantiële financiële gevolgen. Bij het uitvoeren van deze beoordeling is de richtlijnmodule op verschillende domeinen getoetst (zie het stroomschema op de Richtlijnendatabase).
Module |
Uitkomst raming |
Toelichting |
|
Optimale type lijn |
Geen substantiële financiële gevolgen |
Uit de toetsing volgt dat de aanbevelingen mogelijk een kostenbesparend effect hebben door voor kortdurend en middellang gebruik de voorkeur te geven aan een PICC-lijn en bij langdurig gebruik (langer dan drie maanden) te kiezen voor een PICC-lijn of een getunnelde centraal veneuze katheter met cuff. |
Werkwijze
AGREE
Deze richtlijnmodule is opgesteld conform de eisen vermeld in het rapport Medisch Specialistische Richtlijnen 2.0 van de adviescommissie Richtlijnen van de Raad Kwaliteit. Dit rapport is gebaseerd op het AGREE II instrument (Appraisal of Guidelines for Research & Evaluation II; Brouwers, 2010).
Knelpuntenanalyse en uitgangsvragen
Tijdens de voorbereidende fase inventariseerde de werkgroep de knelpunten in de zorg voor patiënten met die een centraal veneuze lijn krijgen. Tevens zijn er knelpunten aangedragen via een invitational conference. Een verslag hiervan is opgenomen onder aanverwante producten. Op basis van de uitkomsten van de knelpuntenanalyse zijn door de werkgroep concept-uitgangsvragen opgesteld en definitief vastgesteld.
Uitkomstmaten
Na het opstellen van de zoekvraag behorende bij de uitgangsvraag inventariseerde de werkgroep welke uitkomstmaten voor de patiënt relevant zijn, waarbij zowel naar gewenste als ongewenste effecten werd gekeken. Hierbij werd een maximum van acht uitkomstmaten gehanteerd. De werkgroep waardeerde deze uitkomstmaten volgens hun relatieve belang bij de besluitvorming rondom aanbevelingen, als cruciaal (kritiek voor de besluitvorming), belangrijk (maar niet cruciaal) en onbelangrijk. Tevens definieerde de werkgroep tenminste voor de cruciale uitkomstmaten welke verschillen zij klinisch (patiënt) relevant vonden.
Methode literatuursamenvatting
Een uitgebreide beschrijving van de strategie voor zoeken en selecteren van literatuur is te vinden onder ‘Zoeken en selecteren’ onder Onderbouwing. Indien mogelijk werd de data uit verschillende studies gepoold in een random-effects model. [Review Manager 5.4] werd gebruikt voor de statistische analyses. De beoordeling van de kracht van het wetenschappelijke bewijs wordt hieronder toegelicht.
Beoordelen van de kracht van het wetenschappelijke bewijs
De kracht van het wetenschappelijke bewijs werd bepaald volgens de GRADE-methode. GRADE staat voor ‘Grading Recommendations Assessment, Development and Evaluation’ (zie http://www.gradeworkinggroup.org/). De basisprincipes van de GRADE-methodiek zijn: het benoemen en prioriteren van de klinisch (patiënt) relevante uitkomstmaten, een systematische review per uitkomstmaat, en een beoordeling van de bewijskracht per uitkomstmaat op basis van de acht GRADE-domeinen (domeinen voor downgraden: risk of bias, inconsistentie, indirectheid, imprecisie, en publicatiebias; domeinen voor upgraden: dosis-effect relatie, groot effect, en residuele plausibele confounding).
GRADE onderscheidt vier gradaties voor de kwaliteit van het wetenschappelijk bewijs: hoog, redelijk, laag en zeer laag. Deze gradaties verwijzen naar de mate van zekerheid die er bestaat over de literatuurconclusie, in het bijzonder de mate van zekerheid dat de literatuurconclusie de aanbeveling adequaat ondersteunt (Schünemann, 2013; Hultcrantz, 2017).
|
GRADE |
Definitie |
|
Hoog |
|
|
Redelijk |
|
|
Laag |
|
|
Zeer laag |
|
Bij het beoordelen (graderen) van de kracht van het wetenschappelijk bewijs in richtlijnen volgens de GRADE-methodiek spelen grenzen voor klinische besluitvorming een belangrijke rol (Hultcrantz, 2017). Dit zijn de grenzen die bij overschrijding aanleiding zouden geven tot een aanpassing van de aanbeveling. Om de grenzen voor klinische besluitvorming te bepalen moeten alle relevante uitkomstmaten en overwegingen worden meegewogen. De grenzen voor klinische besluitvorming zijn daarmee niet één op één vergelijkbaar met het minimaal klinisch relevant verschil (Minimal Clinically Important Difference, MCID). Met name in situaties waarin een interventie geen belangrijke nadelen heeft en de kosten relatief laag zijn, kan de grens voor klinische besluitvorming met betrekking tot de effectiviteit van de interventie bij een lagere waarde (dichter bij het nuleffect) liggen dan de MCID (Hultcrantz, 2017).
Overwegingen (van bewijs naar aanbeveling)
Om te komen tot een aanbeveling zijn naast (de kwaliteit van) het wetenschappelijke bewijs ook andere aspecten belangrijk en worden meegewogen, zoals aanvullende argumenten uit bijvoorbeeld de biomechanica of fysiologie, waarden en voorkeuren van patiënten, kosten (middelenbeslag), aanvaardbaarheid, haalbaarheid en implementatie. Deze aspecten zijn systematisch vermeld en beoordeeld (gewogen) onder het kopje ‘Overwegingen’ en kunnen (mede) gebaseerd zijn op expert opinion. Hierbij is gebruik gemaakt van een gestructureerd format gebaseerd op het evidence-to-decision framework van de internationale GRADE Working Group (Alonso-Coello, 2016a; Alonso-Coello 2016b). Dit evidence-to-decision framework is een integraal onderdeel van de GRADE methodiek.
Formuleren van aanbevelingen
De aanbevelingen geven antwoord op de uitgangsvraag en zijn gebaseerd op het beschikbare wetenschappelijke bewijs en de belangrijkste overwegingen, en een weging van de gunstige en ongunstige effecten van de relevante interventies. De kracht van het wetenschappelijk bewijs en het gewicht dat door de werkgroep wordt toegekend aan de overwegingen, bepalen samen de sterkte van de aanbeveling. Conform de GRADE-methodiek sluit een lage bewijskracht van conclusies in de systematische literatuuranalyse een sterke aanbeveling niet a priori uit, en zijn bij een hoge bewijskracht ook zwakke aanbevelingen mogelijk (Agoritsas, 2017; Neumann, 2016). De sterkte van de aanbeveling wordt altijd bepaald door weging van alle relevante argumenten tezamen. De werkgroep heeft bij elke aanbeveling opgenomen hoe zij tot de richting en sterkte van de aanbeveling zijn gekomen.
In de GRADE-methodiek wordt onderscheid gemaakt tussen sterke en zwakke (of conditionele) aanbevelingen. De sterkte van een aanbeveling verwijst naar de mate van zekerheid dat de voordelen van de interventie opwegen tegen de nadelen (of vice versa), gezien over het hele spectrum van patiënten waarvoor de aanbeveling is bedoeld. De sterkte van een aanbeveling heeft duidelijke implicaties voor patiënten, behandelaars en beleidsmakers (zie onderstaande tabel). Een aanbeveling is geen dictaat, zelfs een sterke aanbeveling gebaseerd op bewijs van hoge kwaliteit (GRADE gradering HOOG) zal niet altijd van toepassing zijn, onder alle mogelijke omstandigheden en voor elke individuele patiënt.
|
Implicaties van sterke en zwakke aanbevelingen voor verschillende richtlijngebruikers |
||
|
|
||
|
|
Sterke aanbeveling |
Zwakke (conditionele) aanbeveling |
|
Voor patiënten |
De meeste patiënten zouden de aanbevolen interventie of aanpak kiezen en slechts een klein aantal niet. |
Een aanzienlijk deel van de patiënten zouden de aanbevolen interventie of aanpak kiezen, maar veel patiënten ook niet. |
|
Voor behandelaars |
De meeste patiënten zouden de aanbevolen interventie of aanpak moeten ontvangen. |
Er zijn meerdere geschikte interventies of aanpakken. De patiënt moet worden ondersteund bij de keuze voor de interventie of aanpak die het beste aansluit bij zijn of haar waarden en voorkeuren. |
|
Voor beleidsmakers |
De aanbevolen interventie of aanpak kan worden gezien als standaardbeleid. |
Beleidsbepaling vereist uitvoerige discussie met betrokkenheid van veel stakeholders. Er is een grotere kans op lokale beleidsverschillen. |
Organisatie van zorg
In de knelpuntenanalyse en bij de ontwikkeling van de richtlijnmodule is expliciet aandacht geweest voor de organisatie van zorg: alle aspecten die randvoorwaardelijk zijn voor het verlenen van zorg (zoals coördinatie, communicatie, (financiële) middelen, mankracht en infrastructuur). Randvoorwaarden die relevant zijn voor het beantwoorden van deze specifieke uitgangsvraag zijn genoemd bij de overwegingen. Meer algemene, overkoepelende, of bijkomende aspecten van de organisatie van zorg worden behandeld in de module Organisatie van zorg.
Commentaar- en autorisatiefase
De conceptrichtlijnmodule werd aan de betrokken (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd ter commentaar. De commentaren werden verzameld en besproken met de werkgroep. Naar aanleiding van de commentaren werd de conceptrichtlijnmodule aangepast en definitief vastgesteld door de werkgroep. De definitieve richtlijnmodule werd aan de deelnemende (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd voor autorisatie en door hen geautoriseerd dan wel geaccordeerd.
Literatuur
Agoritsas T, Merglen A, Heen AF, Kristiansen A, Neumann I, Brito JP, Brignardello-Petersen R, Alexander PE, Rind DM, Vandvik PO, Guyatt GH. UpToDate adherence to GRADE criteria for strong recommendations: an analytical survey. BMJ Open. 2017 Nov 16;7(11):e018593. doi: 10.1136/bmjopen-2017-018593. PubMed PMID: 29150475; PubMed Central PMCID: PMC5701989.
Alonso-Coello P, Schünemann HJ, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Rada G, Rosenbaum S, Morelli A, Guyatt GH, Oxman AD; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ. 2016 Jun 28;353:i2016. doi: 10.1136/bmj.i2016. PubMed PMID: 27353417.
Alonso-Coello P, Oxman AD, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Vandvik PO, Meerpohl J, Guyatt GH, Schünemann HJ; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 2: Clinical practice guidelines. BMJ. 2016 Jun 30;353:i2089. doi: 10.1136/bmj.i2089. PubMed PMID: 27365494.
Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, Fervers B, Graham ID, Grimshaw J, Hanna SE, Littlejohns P, Makarski J, Zitzelsberger L; AGREE Next Steps Consortium. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010 Dec 14;182(18):E839-42. doi: 10.1503/cmaj.090449. Epub 2010 Jul 5. Review. PubMed PMID: 20603348; PubMed Central PMCID: PMC3001530.
Hultcrantz M, Rind D, Akl EA, Treweek S, Mustafa RA, Iorio A, Alper BS, Meerpohl JJ, Murad MH, Ansari MT, Katikireddi SV, Östlund P, Tranæus S, Christensen R, Gartlehner G, Brozek J, Izcovich A, Schünemann H, Guyatt G. The GRADE Working Group clarifies the construct of certainty of evidence. J Clin Epidemiol. 2017 Jul;87:4-13. doi: 10.1016/j.jclinepi.2017.05.006. Epub 2017 May 18. PubMed PMID: 28529184; PubMed Central PMCID: PMC6542664.
Medisch Specialistische Richtlijnen 2.0 (2012). Adviescommissie Richtlijnen van de Raad Kwalitieit. http://richtlijnendatabase.nl/over_deze_site/over_richtlijnontwikkeling.html
Neumann I, Santesso N, Akl EA, Rind DM, Vandvik PO, Alonso-Coello P, Agoritsas T, Mustafa RA, Alexander PE, Schünemann H, Guyatt GH. A guide for health professionals to interpret and use recommendations in guidelines developed with the GRADE approach. J Clin Epidemiol. 2016 Apr;72:45-55. doi: 10.1016/j.jclinepi.2015.11.017. Epub 2016 Jan 6. Review. PubMed PMID: 26772609.
Schünemann H, Brożek J, Guyatt G, et al. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group, 2013. Available from http://gdt.guidelinedevelopment.org/central_prod/_design/client/handbook/handbook.html.
Zoekverantwoording
Zoekacties zijn opvraagbaar. Neem hiervoor contact op met de Richtlijnendatabase.