tDCS Onderste extremiteit
Uitgangsvraag
Wat is het effect van tDCS op functies van de onderste extremiteit?
Aanbeveling
Pas geen tDCS toe ter bevordering van herstel van beenfunctie, loopvaardigheid of stabalans na een herseninfarct of hersenbloeding.
Overwegingen
Voor- en nadelen van de interventie en de kwaliteit van het bewijs
Enkele onderzoeken suggereren een gunstig effect van tDCS op diverse functies van de onderste extremiteit. Deze zijn echter alle klein (n=5-15 per behandelgroep) en worden gekenmerkt door methodologische zwaktes. Dit geldt voor anodale, cathodale en bihemisferale tDCS, en voor behandeling binnen drie maanden en na drie maanden na ontstaan van herseninfarct of -bloeding. De bewijskracht voor alle bevindingen is laag tot zeer laag. De werkgroep is van mening dat er onvoldoende wetenschappelijke bewijs is voor effectiviteit van tDCS in welke vorm en op welk moment dan ook ter bevordering van de loopvaardigheid, stabalans en andere functies van de onderste extremiteit.
Waarden en voorkeuren van patiënten (en eventueel hun verzorgers)
Voor patiënten is het belangrijk dat de behandeling met tDCS veilig is en een positief resultaat oplevert. Echter op dit moment lijkt het bewijs voor de effectiviteit van de behandeling met tDCS nog zeer gering. Ook zijn er geen afzonderlijke subgroepen bekend waarbij meer effect te verwachten is. Als er toch vragen zijn van patiënten over deze behandeling dan moet duidelijk aangegeven worden dat het effect van deze behandeling op dit moment nog onduidelijk is en dat er meer onderzoek nodig is.
Rationale van de aanbeveling: weging van argumenten voor en tegen de interventies
De werkgroep is van mening dat er onvoldoende wetenschappelijk bewijs is voor effect van tDCS ter bevordering van herstel van patiënten met een herseninfarct of hersenbloeding. Kwalitatief hoogwaardige fase 3 trials ontbreken.
Onderbouwing
Conclusies / Summary of Findings
1. Conclusions rTMS ≤ 3 months after stroke onset
1.1 Walking ability (crucial)
|
- GRADE |
There were no studies describing the effect of tDCS on walking ability when compared with sham stimulation in patients within three months after stroke. |
1.2 Maintaining standing balance (crucial)
|
Very low GRADE |
We are uncertain about the effect of anodal tDCS on maintaining standing balance in patients within three months after stroke.
Sources: (Andrade, 2017; Chang, 2015) |
|
Low GRADE |
Cathodal tDCS results in little to no difference in maintaining standing balance in patients within three months after stroke.
Sources: (Andrade, 2017) |
|
Low GRADE |
Bihemispheric tDCS may improve maintaining standing balance in patients within three months after stroke.
Sources: (Andrade, 2017) |
1.3 Muscle power/synergies (important)
|
Low GRADE |
Anodal tDCS results in little to no difference in patients’ muscle power/synergies within three months after stroke.
Sources: (Chang, 2015) |
1.4 Sitting balance (important)
|
- GRADE |
There were no studies describing the effect of tDCS on sitting balance when compared with sham stimulation in patients within three months after stroke. |
1.5 Transferring oneself (important)
|
Low GRADE |
Anodal tDCS may improve patients’ transferring within three months after stroke
Sources: (Andrade, 2017) |
|
Low GRADE |
Cathodal tDCS may improve patients’ transferring within three months after stroke.
Sources: (Andrade, 2017) |
|
Low GRADE |
Bihemispheric tDCS may improve patients’ transferring within three months after stroke.
Sources: (Andrade, 2017) |
1.6 Walking distance (important)
|
Low GRADE |
Anodal tDCS may improve walking distance in patients within three months after stroke.
Sources: (Andrade, 2017) |
|
Low GRADE |
Cathodal tDCS may improve walking long distances in patients within three monhts after stroke.
Sources: (Andrade, 2017) |
|
Low GRADE |
Bihemispheric tDCS may improve walking long distances in patients within three months after stroke.
Sources: (Andrade, 2017) |
1.7 Walking speed (important)
|
Low GRADE |
Anodal tDCS results in little to no difference in patients’ walking speed within three months after stroke.
Sources: (Chang, 2015) |
1.8 Falling (important)
|
Low GRADE |
Anodal tDCS results in little to no difference in falling occurrence within three months after stroke.
Sources: (Andrade, 2017) |
|
Low GRADE |
Cathodal tDCS results in little to no difference in falling occurrence within three months after stroke.
Sources: (Andrade, 2017) |
|
Low GRADE |
Bihemispheric tDCS may reduce falling incidence in patients after stroke.
Sources: (Andrade, 2017) |
Conclusions tDCS > 3 months after stroke onset
2.1 Walking ability (crucial)
|
- GRADE |
No evidence was found regarding the effect of tDCS on walking ability when compared with sham stimulation in patients beyond three months after stroke. |
2.2 Maintaining standing balance (crucial)
|
- GRADE |
There were no studies describing the effect of tDCS on maintaining standing balance when compared with sham stimulation in patients beyond three months after stroke. |
2.3 Muscle power/synergies (important)
|
- GRADE |
There were no studies describing the effect of tDCS on muscle power/synergies when compared with sham stimulation in patients beyond three months after stroke. |
2.4 Sitting balance (important)
|
- GRADE |
There were no studies decribing the effect of tDCS on sitting balance when compared with sham stimulation in patients beyond three months after stroke. |
2.5 Transferring oneself (important)
|
Low GRADE |
Anodal tDCS results in little to no difference in transferring oneself in patients beyond three months after stroke.
Sources: (Manji, 2018) |
2.6 Walking distance (important)
|
Low GRADE |
Anodal tDCS results in little to no difference in walking distance in patients beyond three months after stroke.
Sources: (Manji, 2018) |
2.7 Walking speed (important)
|
Low GRADE |
Anodal tDCS results in little to no difference in patients’ walking speed beyond three months after stroke.
Sources: (Cattagni, 2019) |
2.8 Falling (important)
|
- GRADE |
There were no studies describing the effect of tDCS on falling when compared with sham stimulation in patients beyond three months after stroke. |
Samenvatting literatuur
Description of studies
As a starting point, we included studies from the review from Tien (2020). This systematic review and meta-analysis investigates the effects of tDCS for improving ambulation ability following ischaemic or haemorrhagic stroke. In total, 12 RCTs, including those with a crossover design, comprising 248 patients, were included in the meta-analysis. To answer our clinical question, and based on the selection criteria for this module, the data from one RCT and two crossover studies were extracted from this review (Chang, 2015; Saeys, 2015; Manji, 2018).
In addition, two RCTs were included in the analysis of the literature (Andrade, 2017; Cattagni, 2019). tDCS treatment can be performed at different time points after stroke onset. We distinguished between treatment within or at three months after stroke onset and treatment beyond three months after stroke onset.
1. Start of treatment ≤ 3 months after stroke onset
From the review of Tien (2020), one RCT and one crossover study described the effects of tDCS treatment in patients who were treated ≤ 3 months after stroke onset (Chang, 2015; Saeys, 2015). Chang (2015) assessed walking ability by the lower limb subscale of the Fugl-Meyer-Assessment (FMA-LE), the lower limb Motricity Index (MI-LE) and the Functional Ambulatory Category (FAC). Standing balance was assessed by the Berg Balance Scale (BBS).
Apart from the studies included in the review, one separate RCTs described the effects of tDCS treatment in patients who were treated > 3 months after stroke onset (Andrade, 2017). This RCT describes a sham-controlled, double-blind, parallel clinical trial and evaluates the effects of anodal, bilateral and cathodal tDCS on falls and lower limb function after stroke. A total of 60 adult stroke patients were randomly allocated in four groups. The anodal tDCS group (n=15), the bilateral tDCS group (n=15), and the cathodal tDCS group (n=15) received 10 sessions of stimulation at 2mA intensity and a density equivalent to 0.05 A/m2. In the sham tDCS group (n=15), the current was ramped up over 30 seconds and then turned off. All participants received the same physical rehabilitation program, including individualized motor task training and activities of daily living training. The effects were evaluated on standing balance by the Overall Stability Index (OSI) and the Berg Balance Scale (BBS) the Four Square Step Test (FSST). Also walking speed was assessed by gait analysis. Outcomes were assessed at 10 days, one month and three months post treatment. Apart from the study in the review, one separate RCT described the effects of rTMS treatment in patients who were treated ≤3 months after stroke onset (Huang, 2018). Huang (2018) evaluates the effects 1-Hz rTMS over the contralesional leg motor area followed by physical therapy on regaining ambulation on the recovery of lower limbs after stroke. A total of 38 adult stroke patients (mean age 62y; 61% male; 55% left side) were randomly allocated into two groups. The experimental group (n=19) received 15 sessions of 15-minute 1Hz rTMS stimulation over the hot spot and 45 minutes of physical therapy. The control group (n=19) received sham rTMS for 15 minutes followed by 45 of physical therapy. The effects were evaluated on muscle power/synergies, assessed by the lower limb subscale of the Fugl-Meyer Assessment (FMA-LE) and transferring oneself, assessed by the Timed Up and Go (TUG) test. Outcomes were assessed at three months follow-up.
2. Start of treatment > 3 months after stroke onset
From the review of Tien (2020), one RCT described the effects of tDCS treatment in patients who were treated more than three months after stroke onset (Manji, 2018). The effects were evaluated on sitting/standing up by the Timed Up and Go Test (TUG) and walking distance by the 10 Minute Walking Test (10MWT) after one and after two weeks of treatment.
Apart from the studies included in the review, one separate RCT described the effects of tDCS treatment in patients who were treated > 3 months after stroke onset (Cattagni, 2019). This study was a randomized, sham-controlled, double-blind study with a crossover design and evaluates the effects of anodal tDCS during and immediately after application on leg muscle activity during gait, and on spatiotemporal and kinematic gait parameters in patients with chronic stroke. A total of 24 chronic stroke patients underwent one 30-minute session of effective anodal tDCS at 2 mA and a current density of 2MA/35 cm2. Also, patients received sham tDCS, which consisted of a 120 second stimulation at the beginning of the application to reproduce the sensation of an increase in current intensity. The effects were evaluated on patients’ walking speed and quality by assessing kinematic and spatiotemporal gait parameters.
Results
Results were extracted from the data reported in the studies as means ± standard deviations (SDs). If data were presented by medians (interquartile ranges), results were converted into means ± SD by the conversion tool described by Weir (2018). If data were presented in figures only, exact data were estimated. If only baseline data and difference scores were presented, post-intervention scores were calculated.
1. Start of treatment ≤ 3 months after stroke onset
1.1 Walking ability
One RCT described walking ability in patients who were treated within three months after stroke onset (Chang, 2015).
1.1.1 Anodal tDCS
Chang (2015) assessed walking ability by using the FAC (n=24). Data resulted in a mean difference (MD) of 0.42 (95% Confidence Interval (CI) -0.05 to 0.89), favouring tDCS. This effect was not statistically significant nor clinically relevant.
The level of evidence in the literature
The level of evidence regarding the outcome walking ability started at high because it was based on a randomized controlled trial, but was downgraded by two levels due to limited number of included patients (imprecision, -2). The final GRADE level of evidence regarding the outcome walking in stroke patients who received anodal tDCS within three months after stroke onset is low.
1.2 Maintaining standing balance
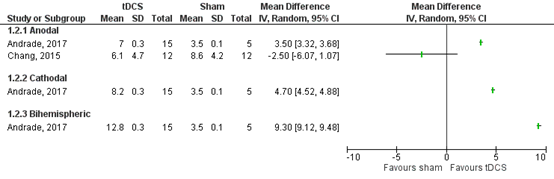
Two RCTs described maintaining standing balance in patients who were treated within three months after stroke onset (Andrade, 2017; Chang, 2015). Andrade (2017) used anodal, cathodal and bihemispheric tDCS stimulation and Chang (2015) only used anodal tDCS stimulation.
1.2.1 Anodal tDCS
Andrade (2017) and Chang (2015) assessed maintaining standing balance by the difference in BBS (0-56) before and after the intervention (n=45). Andrade (2017) reported a MD of 3.5 (95% CI 3.32 to 3.58), favouring anodal tDCS. Chang (2015) reported a MD of -2.50 (95% CI -6.07 to 1.07), favouring sham tDCS. These effects were not statistically significant nor clinically relevant. Results are shown in figure 6.
The level of evidence in the literature
The level of evidence regarding the outcome maintaining standing balance started high because it was based on randomized controlled trials, but was downgraded by three levels due to heterogenous results (inconsistency, -1) and limited number of included patients (resulting in wide confidence intervals) (imprecision, -2). The final GRADE level of evidence regarding the outcome maintaining standing balance in stroke patients who received anodal tDCS within three months after stroke onset is very low.
1.2.2 Cathodal tDCS
Andrade (2017) assessed maintaining standing balance by BBS (0-56) (n=20). Data resulted in a MD of 4.7 (95% CI 4.52 to 4.88), favouring tDCS. This effect was not statistically significant nor clinically relevant. Results are shown in figure 6.
The level of evidence in the literature
The level of evidence regarding the outcome maintaining standing balance started at high because it was based on randomized controlled trials, but was downgraded by two levels due to limited number of included patients (imprecision, -2). The final GRADE level of evidence regarding the outcome maintaining standing balance in stroke patients who received cathodal tDCS within three months after stroke onset is low.
1.2.3 Bihemispheric tDCS
Andrade (2017) assessed maintaining standing balance by the change in BBS (0-56) before and after treatment (n=20). Data resulted in a MD of 9.30 (95% CI 9.12 to 9.48), favouring tDCS. This effect was statistically significant and clinically relevant. Results are shown in figure 6.
The level of evidence in the literature
The level of evidence regarding the outcome maintaining standing balance started at high because it was based on randomized controlled trials, but was downgraded by two levels due to limited number of included patients (imprecision, -2). The final GRADE level of evidence regarding the outcome maintaining standing balance in stroke patients who received bihemispheric tDCS within three months after stroke onset is low.
Figure 6 Forest plot summarizing the effect of anodal, cathodal and bihemispheric tDCS on maintaining standing balance in ischaemic/haemorrhagic stroke patients who were treated within 3 months after stroke onset
1.3 Muscle power/synergies
One RCT described muscle power/synergies in patients who were treated within three months after stroke onset (Chang, 2015).
1.1.1 Anodal tDCS
Chang (2015) assessed muscle power/synergies by using the FMA-LE (n=24). Data resulted in a MD of 2.20 (95% CI -0.17 to 4.57), favouring tDCS. This effect was not statistically significant nor clinically relevant.
The level of evidence in the literature
The level of evidence regarding the outcome muscle synergy started at high because it was based on randomized controlled trials, but was downgraded by two levels due to limited number of included patients (imprecision, -2). The final GRADE level of evidence regarding the outcome muscle synergy in stroke patients who received anodal tDCS within three months after stroke onset is low.
1.4 Sitting balance
No studies described sitting balance in patients who were treated within three months after stroke onset.
1.5 Transferring oneself
One RCT described transferring oneself in patients who were treated within three months of stroke onset (Andrade, 2017). Results were based on estimations from figure 3D at timepoint post 1 (posttreatment assessment) in the RCT from Andrade (2017).
1.5.1 Anodal tDCS
Andrade (2017) assessed transferring oneself by the change in STS (%) (n=20). Data resulted in a MD of 13.5% (95% CI 12.2 to 14.8), favouring tDCS. This effect was statistically significant and clinically relevant.
The level of evidence in the literature
The level of evidence regarding the outcome transferring oneself started at high because it was based on a randomized controlled trial, but was downgraded by two levels due to limited number of included patients (imprecision, -2). The final GRADE level of evidence of efficacy of anodal tDCS within three months after stroke onset regarding the outcome transferring oneself is low.
1.5.2 Cathodal tDCS
Andrade (2017) assessed transferring oneself by the change in STS (%) (n=20). Data resulted in a MD of 9.5% (95% CI 8.3 to 10.7), favouring tDCS. This effect was statistically significant, but not clinically relevant.
The level of evidence in the literature
The level of evidence regarding the outcome transferring oneself started at high because it was based on a randomized controlled trial, but was downgraded by two levels due to limited number of included patients (imprecision, -2). The final GRADE level of evidence of efficacy of cathodal tDCS within three months after stroke onset regarding the outcome transferring oneself is low.
1.5.3 Bihemispheric tDCS
Andrade (2017) assessed transferring oneself by the change in STS (%) (n=20). Data resulted in a MD of 24.5% (95% CI: 23.0 to 26.0), favouring tDCS. This effect was statistically significant and clinically relevant.
The level of evidence in the literature
The level of evidence regarding the outcome transferring oneself started at high because it was based on a randomized controlled trial, but was downgraded by two levels due to limited number of included patients (imprecision, -2). The final GRADE level of evidence of efficacy of bihemispheric tDCS within three months after stroke onset regarding the outcome transferring oneself is low.
1.6 Walking distance
One RCT described walking distance in patient who were treated within three months after stroke onset (Andrade, 2017). Results were estimated from figure 3C at timepoint post 1 (posttreatment assessment) in the RCT from Andrade (2017).
1.6.1 Anodal tDCS
Andrade (2017) assessed walking distance by the change in 6MWT (%) (n=20). Data resulted in a MD of 28.3% (95% CI 27.3 to 29.2), favouring tDCS. This effect was statistically significant and clinically relevant.
The level of evidence in the literature
The level of evidence regarding the outcome walking distance started at high because it was based on a randomized controlled trial, but was downgraded by two levels due to limited number of included patients (imprecision, -2). The final GRADE level of evidence of efficacy of anodal tDCS within three months after stroke onset regarding the outcome walking long distances is low.
1.6.2 Cathodal tDCS
Andrade (2017) assessed walking long distances by the change in 6MWT (%) (n=20). Data resulted in a MD of 27.0% (95% CI 26.12to 27.9), favouring tDCS. This effect was statistically significant and clinically relevant.
The level of evidence in the literature
The level of evidence regarding the outcome walking long distances started at high because it was based on a randomized controlled trial, but was downgraded by two levels due to limited number of included patients (imprecision, -2). The final GRADE level of evidence of efficacy of cathodal tDCS within three months after stroke onset regarding the outcome walking long distances is low.
1.6.3 Bihemispheric
Andrade (2017) assessed walking long distances by the change in 6MWT (%) (n=20). Data resulted in a MD of 33.5% (95% CI 32.5 to 34.5), favouring tDCS. This effect was statistically significant and clinically relevant.
The level of evidence in the literature
The level of evidence regarding the outcome walking long distances started at high because it was based on a randomized controlled trial, but was downgraded by two levels due to limited number of included patients (imprecision, -2). The final GRADE level of evidence of efficacy of bihemispheric tDCS within three months after stroke onset regarding the outcome walking long distances is low.
1.7 Walking speed
One RCT described walking speed in patients who were treated within three months after stroke onset (Chang, 2015).
1.7.1 Anodal tDCS
Chang (2015) assessed walking speed by measuring the speed in the gait analysis in meters per second (m/s). Data resulted in a MD of 0.05 m/s (95% CI -0.15 to 0.25). This effect was not statistically significant nor clinically relevant.
The level of evidence in the literature
The level of evidence regarding the outcome walking speed started at high because it was based on a randomized controlled trial, but was downgraded by two levels due to limited number of included patients (imprecision, -2). The final GRADE level of evidence of efficacy of anodal tDCS within three months after stroke onset regarding the outcome walking speed is low.
1.8 Falling
One RCT described falling in patients who were treated within three months after stroke onset (Andrade, 2017).
1.8.1 Anodal tDCS
Andrade (2017) assessed falling by the change in the FES-I (%) (n=20). Data resulted in a MD of 8.0% (95% CI 7.3 to 8.7), favouring tDCS. This effect was statistically significant, but not clinically relevant.
The level of evidence in the literature
The level of evidence regarding the outcome falling started at high because it was based on a randomized controlled trial, but was downgraded by two levels due to limited number of included patients (imprecision, -2). The final GRADE level of evidence of efficacy of anodal tDCS within three months after stroke onset regarding the outcome falling is low.
1.8.2 Cathodal tDCS
Andrade (2017) assessed falling by the change in FES-I (%) (n=20). Data resulted in a MD of 6.3% (95% CI 5.7 to 7.0), favouring tDCS. This effect was statistically significant, but not clinically relevant.
The level of evidence in the literature
The level of evidence regarding the outcome falling started at high because it was based on a randomized controlled trial, but was downgraded by two levels due to limited number of included patients (imprecision, -2). The final GRADE level of evidence of efficacy of cathodal tDCS within three months after stroke onset regarding the outcome falling is low.
1.8.3 Bihemispheric tDCS
Andrade (2017) assessed falling by the change in FES-I (%) (n=20). Data resulted in a MD of 20.7% (95% CI 19.8 to 21.5), favouring tDCS. This effect was statistically significant and clinically relevant.
The level of evidence in the literature
The level of evidence regarding the outcome falling started at high because it was based on a randomized controlled trial, but was downgraded by two levels due to limited number of included patients (imprecision, -2). The final GRADE level of evidence of efficacy of bihemispheric tDCS within three months after stroke onset regarding the outcome falling is low.
2. Start of treatment > 3 months after stroke onset
2.1 Walking
No studies described walking in patients who were treated beyond three months of stroke onset.
2.2 Maintaining standing balance
No studies described standing balance in patients who were treated beyond three months of stroke onset.
2.3 Muscle power/synergies
No studies described muscle power/synergies in patients who were treated beyond three months of stroke onset.
2.4 Sitting balance
No studies described sitting balance in patients who were treated beyond three months after stroke onset.
2.5 Transferring oneself
One RCT described transferring oneself in patients who were treated beyond three months after stroke onset (Manji, 2018).
2.5.1 Anodal tDCS
Manji (2018) assessed sitting and standing balance up by the TUG time (s) (n=30). Data resulted in a MD of -0.90 seconds (95% CI -6.34 to 4.54), favouring tDCS, after one week of treatment. After another week of treatment, data resulted in a MD of -3.10 seconds (95% CI -8.44 to 2.24), favouring tDCS. Both effects were not statistically significant nor clinically relevant.
The level of evidence in the literature
The level of evidence regarding the outcome sitting/standing up started at high because it was based on a randomized controlled trial, but was downgraded by two levels due to limited number of included patients (imprecision, -2). The final GRADE level of evidence regarding the outcome sitting and standing balance in stroke patients who received anodal tDCS beyond three months after stroke onset is low.
2.6 Walking long distances
One RCT described walking long distances up in patients who were treated beyond three months after stroke onset (Manji, 2018).
2.6.1 Anodal tDCS
Manji (2018) assessed walking long distances by the 10MWT (n=30). Data resulted in a MD of -2.30 seconds (95% CI -6.17 to 1.57), favouring tDCS, after one week of treatment. After another week of treatment, data resulted in a MD of -1.20 seconds (95% CI -4.64 to 2.24), favouring tDCS. Both effects were not statistically significant nor clinically relevant.
The level of evidence in the literature
The level of evidence regarding the outcome walking long distances started at high because it was based on a randomized controlled trial, but was downgraded by two levels due to limited number of included patients (imprecision, -2). The final GRADE level of evidence regarding the outcome walking long distances in stroke patients who received anodal tDCS beyond three months after stroke onset is low.
2.7 Walking speed
One RCT described walking speed in patients who were treated beyond three months after stroke onset (Cattagni, 2019).
2.7.1 Anodal tDCS
Cattagni (2019) assessed walking speed by the gait speed (n=24). Data resulted in a MD of 0.20 cm/second (95% CI -20.88 to 21.28), favouring tDCS. This effect was not statistically significant nor clinically relevant.
The level of evidence in the literature
The level of evidence regarding the outcome walking speed started at high because it was based on a randomized controlled trial, but was downgraded by two levels due to limited number of included patients (imprecision, -2). The final GRADE level of evidence regarding the outcome walking speed in stroke patients who received anodal tDCS beyond three months after stroke onset is low.
2.8 Falling
No studies described falling in patients who were treated beyond three months after stroke onset.
Zoeken en selecteren
A systematic review of the literature was performed to answer the following question:
What is the effect of rTMS on lower limb capacity in patients after stroke?
P: patients with ischaemic/haemorrhagic stroke with persisting lower limb dysfunction;
I: non-invasive brain stimulation with transcranial direct current stimulation (tDCS);
C: sham tDCS;
O: walking ability, maintaining standing balance, muscle power/synergies, sitting balance, transferring oneself, walking distance, walking speed and falling.
In the literature, tDCS treatment was applied at different time points after stroke onset. On the basis of a critical time window of spontaneous neurological recovery of maximal 3 months (Bernhardt, 2017), we decided to distinguish between treatment ≤ 3 months after stroke onset and treatment >3 months after stroke onset. Within this distinguishment, the effects were evaluated per intervention type. This resulted in the following (sub-)groups:
a. Start of treatment at or within three months after ischaemic/haemorrhagic stroke:
- Anodal tDCS;
- Cathodal tDCS;
- Bihemispheric tDCS.
b. Start of treatment beyond three months after ischaemic/haemorrhagic stroke:
- Anodal tDCS;
- Cathodal tDCS;
- Bihemispheric tDCS.
Relevant outcome measures
The working group considered ‘walking ability’ and ‘maintaining standing balance; as critical outcome measure for decision making. ‘Muscle power/synergies’, ‘sitting balance’, ‘transferring oneself’, ‘walking distance’, ‘walking speed’ and ‘falling’ were considered as important outcome measures for decision making. .
Definitions
The working group classified the used outcome measures following the codes of the International Classification of Functioning, Disability and Health (ICF) in the following groups: (Steiner, 2002):
1. Walking ability (d450): Functional Ambulatory Category (FAC), Rivermead Mobility Index (RMI).
2. Maintaining standing balance (d154) : Berg Balance Scale (BBS), Four Square Step Test (FSST), Overall Stability Index (OSI).
3. Muscle Power (b730)/synergies: Fugl-Meyer Assessment of the Lower Extremity (FMA-LE), Lower Limb Motricity Index (MI-LE).
4. Sitting balance (d4153): Trunk Impairment Scale (TIS), Trunk Control Test (TCT).
5. Transferring onseself (d420): Tinetti Test, Timed Up and Go Test (TUG), Sit to Stand Test (STS).
6. Walking distance (d4501): 6-Minute Walk Test (6MWT),
7. Walking speed (d4500): Gait speed, gait velocity /Walking short distances 10-Metre Walk Test (10MWT).
8. Falling (d4403): Incidence of falls.
The working group defined a difference of 10% on each test scale as a clinically important difference. For standardized mean differences (SMD), results were clinically relevant if they were smaller than -0.5 or higher than 0.5.
Search and select (Methods)
The databases Medline (via OVID) and Embase (via Embase.com) were searched with relevant search terms until October 22, 2020. The detailed search strategy is depicted under the tab Methods. The systematic literature search resulted in 798 hits. Studies were selected based on the following criteria:
- Patients with ischaemic/haemorrhagic stroke.
- RCTs and SRs about non-invasive brain stimulation with rTMS.
- Subgroups with anodal, cathodal or bihemispheric tDCS.
- A control group receiving sham tDCS.
- More than 10 patients per treatment arm.
- For cross-over studies: a baseline measurement and at the first cross-over point.
- A description of at least one outcome measure, as described in the PICO.
11 studies were initially selected based on title and abstract. After reading the full-text, eight studies were excluded (see table with reasons for exclusion under the tab Methods) and three studies were included.
Results
Three studies were included in the analysis of the literature, including one systematic review and two RCTs. The most important study characteristics and results are included in the evidence tables. The judgement of the individual studies (risk of bias) is included in risk-of-bias tables.
Referenties
- Andrade SM, Ferreira JJA, Rufino TS, Medeiros G, Brito JD, da Silva MA, Moreira RN. Effects of different montages of transcranial direct current stimulation on the risk of falls and lower limb function after stroke. Neurol Res. 2017 Dec;39(12):1037-1043. doi: 10.1080/01616412.2017.1371473. Epub 2017 Sep 8. PMID: 28885111.
- Cattagni T, Geiger M, Supiot A, de Mazancourt P, Pradon D, Zory R, Roche N. A single session of anodal transcranial direct current stimulation applied over the affected primary motor cortex does not alter gait parameters in chronic stroke survivors. Neurophysiol Clin. 2019 Sep;49(4):283-293. doi: 10.1016/j.neucli.2019.07.012. Epub 2019 Jul 30. PMID: 31375380.
- Chang MC, Kim DY, Park DH. Enhancement of Cortical Excitability and Lower Limb Motor Function in Patients With Stroke by Transcranial Direct Current Stimulation. Brain Stimul. 2015 May-Jun;8(3):561-6. doi: 10.1016/j.brs.2015.01.411. Epub 2015 Jan 31. PMID: 25736569.
- Manji A, Amimoto K, Matsuda T, Wada Y, Inaba A, Ko S. Effects of transcranial direct current stimulation over the supplementary motor area body weight-supported treadmill gait training in hemiparetic patients after stroke. Neurosci Lett. 2018 Jan 1;662:302-305. doi: 10.1016/j.neulet.2017.10.049. Epub 2017 Oct 28. PMID: 29107706.
- Tien HH, Liu WY, Chen YL, Wu YC, Lien HY. Transcranial direct current stimulation for improving ambulation after stroke: a systematic review and meta-analysis. Int J Rehabil Res. 2020 Dec;43(4):299-309. doi: 10.1097/MRR.0000000000000427. PMID: 32675686; PMCID: PMC7643800.
Evidence tabellen
|
Study reference |
Study characteristics |
Patient characteristics |
Intervention (I) |
Comparison / control (C) |
Follow-up |
Outcome measures and effect size |
Comments |
|
Bai, 2019 |
SR and meta-analysis of RCTs and cross-over studies.
Literature search up to January 2019
A: Mazzoleni, 2017 B: Figlewski, 2017 C: Straudi, 2016 D: Hesse, 2011 E: Allman, 2016 F: Hamoudi, 2018 I: Saeys, 2015
Study design: A: RCT B: RCT C: RCT D: RCT E: RCT G: RCT Setting and Country: Department of Radiology and Imaging Institute of Rehabilitation and Development of Brain Function, The Second Clinical Medical College of North Sichuan Medical College Nanchong Central Hospital, Nanchong, China
Source of funding and conflicts of interest: The authors declare that they have no conflicts of interest. |
Inclusion criteria SR: (1) all patients were adults (≥18 years) and were diagnosed with a first stroke; (2) the articles were focused on the effect of tDCS on the recovery of motor function in stroke patients; (3) the stimulation sites were located in the primary motor cortex (M1); (4) all experiments were randomized control trials including crossover and parallel design; (5) ≥5 patients were enrolled, and all control groups were sham tDCS; (6) all included articles were peer reviewed and published in English; and (7) the results were measured with scales.
Exclusion criteria SR: (1) patients who had other diseases that could cause motor dysfunction; (2) articles that had been published but did not provide raw data, such as reviews, meta-analysis, or case reports; (3) animal experiments; and (4) results that were not expressed as mean ± standard deviation or mean ± standard error, but as median or interquartile range.
29 studies included
Important patient characteristics at baseline:
N, mean age A: 24 patients, 72.6 yrs B: 44 patients, 60.5 yrs C: 23 patients, 58.2 yrs D: 95 patients, 65.5 yrs E: 24 patients, 63.5 yrs. F: 22 patients, 63.4 yrs. G: 36 patients, 61.8 yrs yrs.
Groups comparable at baseline? |
Describe intervention:
A: wrist robot assisted therapy + tDCS (0.057 mA/cm2) B: training + anodal tDCS (0.043 mA/cm2) C: robot assisted training + tDCS (0.029 mA/cm2) D: arm robot therapy + tDCS (0.057 mA/cm2) Anodal & Cathodal E: tDCS (anodal, 0.029 mA/cm2) + motor training G: tDCS (0.04 mA/cm2) I: tDCS (0.043 mA/cm2) |
Describe control:
A: wrist robot assisted therapy + tDCS B: training + sham tDCS (1.5mA) C: Robot Assisted training + Sham tDCS D: Arm robot therapy + sham tDCS E: Sham tDCS + motor training G: Sham tDCS I: Sham tDCS |
End-point of follow-up:
A: after treatment (30 sessions) B: after treatment (9 sessions) C: one week after treatment (10 sessions) D: after treatment and 3 months after treatment (30 sessions) E: Day 10 / 1 Week / 1 Month / 3 Month (9 sessions) G: n.r. I: 4 weeks / 8 weeks after treatment (5 sessions)
For how many participants were no complete outcome data available? (intervention/control) A: n.r. B: 3/1 C: 0 D: 4/3/4 E: 1/1 G: n.r. I: n.r.
|
Treatment ≤ 3 months Upper Limb motor function Effect measure: standardized mean difference (95% CI):
Anodal A: 0.17 (-0.63 – 0.97) B: n.r. C: n.r. D: n.r.
Upper limb synergy Effect measure: standardized mean difference (95% CI): Anodal A: -0.39 (-0.53 – 0.31)
Pooled effect (random effects model): -0.11 (95% CI: -0.53 to 0.31) favoring sham Heterogeneity (I2): 0%
Cathodal A: n.r.
Strength A: n.r. B: n.r. C: n.r. D: n.r.
Activities of daily living Effect measure: mean difference (95% CI):
Anodal A: n.r. B: n.r. C: n.r. D: -2.7 (-11.62 – 6.22)
Cathodal A: n.r. B: n.r. C: n.r. D: 2.90 (-5.63 – 11.43)
Treatment > 3 months Upper Limb motor function Effect measure: standardized mean difference (95% CI): Anodal A: n.r. B: n.r. C: n.r. D: n.r.
Cathodal A: n.r. B: 0.07 (-0.52 – 0.66) C: n.r. D: n.r.
Bihemispheric A: n.r. B: n.r. C: 0.27 (-0.55 – 1.09) D: n.r.
Upper limb synergy Effect measure: standardized mean difference (95% CI):
Anodal A: n.r. B: n.r. C: n.r. D: n.r.
Bihemispheric A: n.r. B: n.r. C: n.r. D: n.r.
Strength Effect measure: mean difference (95% CI):
Cathodal A: n.r. B: 1.40 (-4.56 – 7.36 C: n.r. D: n.r.
Activities of daily living
A: n.r. B: n.r. C: n.r. D: n.r. |
Facultative:
tDCS is effective for the recovery of stroke patients with limb dysfunction after the first unilateral stroke, but the optimal parameters of tDCS for the upper and lower limbs are different. tDCS has a great impact on the recovery of upper limb function in chronic stroke patients. In addition, stroke patients with upper limb hemiplegia recover better by using anode or cathode tDCS with above 0.029mA/cm2 current density and ≤10 sessions of treatment. But for the recovery of lower limb function, subacute stroke patients benefit more from bilateral tDCS.
|
|
Tien, 2020 |
SR and meta-analysis of RCTs and cross-over studies.
Literature search up to January 2019 A: Chang, 2015
Study design: A: RCT
Setting and Country:
Source of funding and conflicts of interest: There are no conflicts of interest. |
Inclusion criteria SR: (1) application of tDCS in patients with stroke who were over 18 years of age; (2) outcome assessments including gait parameters, walking speed and endurance, functional mobility test or questionnaire for walking ability and balance; (3) pre-post and randomized controlled clinical study design; (4) active tDCS versus sham tDCS and could combine other rehabilitation treatments in two groups; (5) published in English or Chinese language.
Exclusion criteria SR: (1) patients had other types of neurological or musculoskeletal diseases or subjects were non-human subjects; (2) treatment combined other types of stimulation; (3) the articles were non-clinical trials including review, case report, editorial comment, and meta-analysis.
14 studies included
Important patient characteristics at baseline:
N, mean age A: 24 patients, 62,8 yrs.
Stroke type A: 24/0
Groups comparable at baseline? Yes |
Describe intervention:
A: 10 sessions of anodal tDCS and conventional physical therapy.
|
Describe control:
A: Sham group, in which patients received 10 sessions of sham stimulation and conventional physical therapy. |
End-point of follow-up:
A: after treatment
For how many participants were no complete outcome data available? (intervention/control) A: 0
|
Treatment ≤ 3 months Effect measure: mean difference (95% CI): Anodal Standing balance Effect measure: mean difference (95% CI): Anodal Strength and Muscle Synergy Effect measure: mean difference (95% CI): Anodal Siting balance Sitting and standing balance Walking distance Walking speed Effect measure: mean difference (95% CI): Anodal Falling Treatment > 3 months Standing balance Strength and Muscle Synergy Sitting Balance Sitting and standing balance Anodal Walking distance Effect measure: mean difference (95% CI): Anodal Walking speed
|
Facultative: In conclusion, this meta-analysis suggests that tDCS improves walking ability with an exception of walking speed and endurance in patients with stroke. Both anodal and dual- hemispheric tDCS exert positive effects on promoting walking-related performances after stroke. However, difficulty in improving balance performance by tDCS may limit the effects of tDCS on walking speed and/or walking distance.
Level of evidence: PEDro scale (per study)
|
|
Elsner, 2019 |
SR and meta-analysis of RCTs
Literature search up to July 2018
A: Fridriksson, 2018 B: Meinzer, 2016 C: Spielmann, 2018
Study design: A: RCT
Setting and Country: A: USA
Source of funding and conflicts of interest:
A: None
(commercial / non-commercial / industrial co-authorship)
|
Inclusion criteria SR: - RCTs, cross-over trials
Exclusion criteria SR: - Quasi-randomised controlled trials - Aged 18 years or above - Stroke patients (WHO definition) - tDCS alone or with SLTH
21 studies included
Important patient characteristics at N, mean age A: 74, 59.8y
Sex: A: 69.7% Male
Groups comparable at baseline? |
Describe intervention:
A: A-tDCS (1 mA) for 20 minutes over the left scalp over the individually most active cortex region identified by naming tasks fMRI during 45 minutes of computerised naming treatment B: A-tDCS over the leftM1 (1 mA for 20 minutes) at the beginning of computer-assisted naming treatment session with the ’vanishing cues’ approach (2 times for 90 minutes a day, 4 days per week for 2 weeks) C: A-tDCS for 1 mA for the first 20 minutes and word-finding therapy for 45minutes per day on 5 consecutive sessions; 225 minutes per week.
|
Describe control:
A: S-tDCS for 20minutes over the left scalp over the individually most active cortex region identified by naming tasks fMRI during 45 minutes of computerised naming treatment B: S-tDCS over the leftM1 (1 mA for 30 seconds) at the beginning of computer-assisted naming treatment session with the ’vanishing cues’ approach (2 times for 90 minutes a day, 4 days per week for 2 weeks) C: S-tDCS for the first 20 minutes and word-finding therapy for 45minutes per day on 5 consecutive sessions; 225 minutes per week.
|
End-point of follow-up:
A: 1 week after treatment B: After treatment and after 6 months follow-up C: After treatment and after 6 months follow-up
For how many participants were no complete outcome data available? (intervention/control) A: n.r. B: n.r. C: n.r.
|
Treatment ≤ 3 months
Functional communication Measured with ANELT (0-50). Effect measure: MD (95% CI)
Anodal: A: n.r.
Verbal comprehension n.r.
Expressive naming Measured with the BNT (60 items) Effect measure: SMD (95% CI)
Anodal:
Treatment >3 months
Functional communication Measured with the CETI Effect measure: MD (95% CI):
Anodal: A: n.r. B: 9.40 (-6.18 to 24.98) in favour of tDCS. C: n.r.
Verbal comprehension n.r.
Expressive naming Measured with naming ability of trained and untrained items or the picture naming test. Effect measure SMD (95% CI):
Anodal: A: 0.41 (-0.06 to 0.87) in favour of tDCS. B: 0.69 (-0.10 to 1.49) in favour of tDCS. C: n.r.
Pooled effect (random effects model: Heterogeneity (I2): 0% |
Risk of Bias: B: Unclear (allocation concealment) C: High (selective reporting) D: Unclear (allocation concealment, blinding, elective outcome reporting), High (incomplete outcome data)
Author’s conclusion There is no evidence of the effectiveness of tDCS (A-tDCS, CtDCS, Dual-tDCS) versus control (S-tDCS) for improving functional communication in people with aphasia (low quality of evidence), accuracy in naming verbs (very low quality of evidence), and cognition in stroke patients with aphasia at the present time. However, tDCS improves the accuracy in naming nouns (moderate quality of evidence), measured at the end of the intervention period and possibly also at follow-up (moderate quality of evidence). Current evidence does not support the routine use of tDCS for aphasia after stroke. |
|
Van Lieshout, 2019 |
SR and meta-analysis of RCTs, cross-over design trials, case studies and mixed design studies.
Literature search up to January 2018
A: Ládavas, 2015 B: Yun, 2015
Study design: A: RCT
Setting and Country: A: Italy
Source of funding and conflicts of interest: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Netherlands Organization for Scientific Research (VICI 016.130.662). |
Inclusion criteria SR: 1) patients with ischaemic or haemorrhagic stroke; (2) age ≥ 18 years; (3) the use of NIBS (TMS, TBS, or tDCS); (4) objective, standardized tests or test batteries for assessment of cognitive function; and (5) baseline measurement and posttreatment measurement(s)
Exclusion criteria SR: (1) nonhuman studies and (2) studies that only tested effects on motor, language functions and perception.
40 studies included
Important patient characteristics at baseline:
N, mean age A: 30 patients, 68.9 yrs B: 30 patients, 62.1 yrs C: 45 patients, 62.8 yrs Sex: A: 53.3% Male B: 70% Male C: 44.4% Male
Stroke B: n.r. C: n.r.
comparable at baseline? Yes |
Describe intervention:
A: a-tDCS + prism adaptation, c-tDCS + prism adaptation, 20 minutes x 10 sessions, x5/wk, 2 weeks. B: a-tDCS + cognitive rehabilitation, 30 minutes x 15 sessions, x5/wk x 3 wks
|
Describe control:
A: Sham a/c tDCS + prism adaptation, 20 minutes x 10 sessions, x5/wk, 2x weeks. B: sham tDCS + cognitive rehabilitation, 30 minutes, x 15 sessions, x 5/wk, x3 weeks.
|
End-point of follow-up:
A: first week after PA treatment. B: Post-treatment
For how many participants were no complete outcome data available? (intervention/control) A: n.r. B: n.r.
|
Treatment ≤ 3 months Visual and spatial attention n.r.
Global cognitive functioning Defined by the Korean version of the MMSE test (B1=left side; B2=right side)
Effect measure: Mean difference (95% Confidence Interval): A: n.r. B2: 1.40 (-1.87 – 4.67 in favour of tDCS)
Memory Defined by the ViLT-R/VeLT-R (B1=left side; B2=right side). Effect measure: Mean difference (95% Confidence Interval): A: n.r. B2: 1.80 (-11.91 – 15.51) favouring tDCS / -2.70 (-14.31 – 8.91) favouring sham.
Executive functioning n.r.
Treatment ≤ 3 months Visual and spatial attention Defined by the behavioral inattention test.
Effect measure: Mean Difference (95% Confidence Interval). Anodal
Global cognitive functioning n.r.
Memory n.r.
Executive functioning n.r.
|
Author’s conclusion: Our review suggests that NIBS is able to alleviate neglect after stroke. However, the results are still inconclusive and preliminary for the effect of NIBS on other cognitive domains. A standardized core set of outcome measures of cognition, also at the level of daily life activities and participation, and international agreement on treatment protocols, could lead to better evaluation of the efficacy of NIBS and comparisons between studies.
Risk-of-bias: A: n.r. B: n.r.
|
|
Study characteristics |
Patient characteristics 2 |
Intervention (I) |
Comparison / control (C) 3
|
Follow-up |
Outcome measures and effect size 4 |
Comments |
|
||||
|
Sik, 2015 |
Type of study: Double-Blind Sham-Controlled Study
Setting and country: Physical Medicine and Rehabilitation (PMR) Department of Baskent University Faculty of Medicine, Turkey
Funding and conflicts of interest: This study was approved by Baskent University Institutional Review Board and Ethics Committee (Project no: KA15/271) and supported by Baskent University Research Fund. The authors have no financial conflicts of interest. |
Inclusion criteria: Patients with a history of subacute or chronic stroke (disease duration of at least three months) and hand-wrist dorsiflexion of at least 10 degrees (90 degrees wrist palmar flexion posture) due to involvement of the middle cerebral artery were included in our study.
Exclusion criteria: Patients with severe cognitive deficits, history of epileptic convulsion, severe depression, neglect syndrome, aphasia, severe spasticity, static deformity in the upper extremity, non-ambulated, cerebellar or anterior cerebral artery involvement, brain stem involvement, basal ganglia involvement, intracranial metallic implant, cardiac pacemaker, significant visual loss, significant hearing loss, complex regional pain syndrome in plegic upper extremity, uncontrolled systemic problems, and application of botulinum A toxin to the plegic upper extremity in the past 6 months were excluded from the study.
N total at baseline: Anodal tDCS: 12 Bihemispheric tDCS: 12 Sham: 12
Important prognostic factors2: Age: mean (25-75 percentile) A: 62,0 (54,8–70,0)
Sex: A: 60% B: 40% S: 72.7%
Groups comparable at baseline? Yes |
In addition to a conventional physiotherapy and occupational therapy program, anodal tDCS application was used in one group (A) for 20 minutes and 15 sessions in 3 weeks, bihemispheric anodal/cathodal tDCS application in another group (B) for 40 minutes and 15 sessions in 3 weeks. The anodal tDCS application involved the placement of the active electrode to the C3-C4 area of the affected hemisphere and the reference electrode to the opposite supraorbital region. The application of bihemispheric anodal-cathodal tDCS was performed by the placement of the active electrode to the C3-C4 area of the unaffected hemisphere in addition to its anodal application, and the placement of the reference electrode to the opposite supraorbital region with the reversal of the current against the anodal tDCS |
In addition to a conventional physiotherapy and occupational therapy program, sham tDCS application in the third group (S). In the sham tDCS group, electrodes were placed as in the anodal group, with the first tingling sensation (1 min) achieved by turning on the device followed by interruption of the current, performed carefully so that the patient did not notice.
|
Length of follow-up: 6 months.
Loss-to-follow-up: A: 2 (16.7%)
Reasons: Did not continue study because of social reasons.
Incomplete outcome data: A: 0
|
Treatment ≤ 3 months
Upper limb motor function n.r.
Upper limb synergy n.r.
Strength n.r.
Activities of daily living n.r.
Treatment > 3 months Upper limb motor function Effect measure: standardized mean difference (95% Confidence Interval)
0.47 (-0.56 – 0.97)
Upper limb synergy n.r.
Strength n.r.
Activities of daily living n.r.
|
In conclusion, tDCS is an important non-invasive brain stimulation technique that can provide additional benefits to plegic upper extremity motor function when coadministered with a rehabilitation program. |
|
|||
|
Alisar, 2020 |
Type of study: prospective, randomized, sham-controlled study Setting and country: Physical Medicine and Rehabilitation, Department of Physical Medicine and Rehabilitation Haydarpaşa Numune Educatıon and Research Hospital İstanbul, Turkey
Funding and conflicts of interest: n.r. |
Inclusion criteria: (1) age 18-75 years (2) first time stroke sufferer (3) stroke of vascular aetiology as determined by computerised tomography or magnetic resonance imaging (4) at least 3 months since stroke onset (5) mini mental state examination score 23 6) stable medical condition.
Exclusion criteria: (1) presence of sensory aphasia/neglect/significant hearing or visual loss/significant spasticity (modified Ashworth scale grade 3-4) (2) history of epilepsy/brain tumour/cranial surgery (3) presence of a pacemaker/intracranial metallic implant.
N total at baseline: Intervention: 16 Control: 16
Important prognostic factors2: For example age ± SD: I: 63.56 ± 10.19 C: 63.50 ± 12.60
Sex: I: 38% M C: 44% M
Groups comparable at baseline? Yes |
tDCS was applied using a double channelled direct current stimulator (ZMI Electronics Ltd. Taiwan 2012). The consistency of the direct current was constantly monitored and determined by the direct current stimulator. The current was applied using 5.5 cm £ 4 cm (22 cm2) rectangular electrodes.
|
The electrodes were placed using the same method in the sham group and the stimulator switched on and current increased until the patient felt the typical ''tingling'' sensation on their scalp for a duration of 30 seconds. It is well known that tDCS does not elicit auditory or somatosensory perceptions beyond the initial minute of its application,12 therefore, the stimulation was gradually reduced and switched off after approximately a minute. The electrodes remained in situ for 30 minutes during the OT session.
|
Length of follow-up: After treatment (15th)
Loss-to-follow-up: Intervention: 0 Control: 0
Incomplete outcome data: Intervention: 2 (11.1%) Control: 4 (20%)
Reasons: Did not receive allocated intervention.
|
Treatment ≤ 3 months
Upper limb motor function n.r.
Upper limb synergy n.r.
Strength n.r.
Activities of daily living n.r.
Treatment > 3 months Upper limb motor function n.r.
Upper limb synergy Effect measure: standardized mean difference (95% Confidence Interval)
0.30 (-0.39 – 1.00)
Strength n.r.
Activities of daily living Effect measure: Mean difference (95% Confidence Interval):
29.82 (11.83 – 47.81)
|
In this double-blind randomized controlled study, bihemispheric tDCS therapy combined with OT and conventional rehabilitation methods were found to be effective in improving upper extremity motor functions and ADL in stroke patients. Functional improvement was greater in chronic stroke sufferers who received tDCS. In order for tDCS to routinely enter stroke rehabilitation practice; optimum duration and intensity of tDCS, ideal time since stroke onset, and lesion location should be identified. With this in mind, randomized controlled clinical trials in larger patient populations with longer follow-up periods should be conducted. |
|
|||
|
Nicolo, 2018 |
Type of study: Double-blinded, randomized, placebo-controlled study.
Funding and conflicts of interest: n.r. |
Inclusion criteria: (1) ischaemic or haemorrhagic stroke, (2) <10 weeks after stroke, (3) unilateral lesion in the territory of the middle cerebral artery, and (4) first-ever appearance of upper extremity motor impairment based on the Fugl- Meyer upper extremity scale (score<50).
Exclusion criteria: Participants were excluded if they met any of the following criteria: epileptic seizures, presence of metallic objects in the brain, skull breach after craniectomy, presence of implants or neural stimulators, pregnancy, sleep deprivation, recent traumatic brain injury, delirium or disturbed vigilance, inability to participate in 1-hour treatment sessions, severe language comprehension deficits, new stroke lesions during rehabilitation, or medical complications.
N total at baseline: Intervention: 14 Control: 13
Important prognostic factors2: For example age ± SD: I: 68.5 ± 10.8 C: 64.3 ± 17.1
Sex: I: 57.1% M C: 61.5% M
Groups comparable at baseline? Yes |
tDCS was applied for 25 minutes at an intensity of 1mA38 using a constant-current electrical stimulator. Two 35-cm2 electrodes with sponge surfaces were placed over the ipsilesional supraorbital region (anodal electrode) and the contralesional M1 (cathodal electrode) using the positions of C3 or C4 electrodes of the international 10-20 electroencephalography system.
|
For sham stimulation, the current was ramped up for 30 seconds and then slowly tapered down to zero. This modus operandi has been used to prevent participants from differentiating between real and sham stimulation. Physical therapy was started after approximately minutes of tDCS. |
Length of follow-up: After intervention.
Loss-to-follow-up: Intervention: 0 Control: 0
Incomplete outcome data: Intervention: 0 Control: 0
|
Treatment ≤ 3 months
Upper limb motor function Effect measure: Standardized mean difference (95% Confidence Interval):
0.55 (-0.22 – 1.32)
Upper limb synergy Effect measure: Standardized mean difference (95% Confidence Interval):
-0.19 (-0.95 – 0.57)
Strength Effect measure: Mean Difference (95% Confidence Interval):
0.00 (-4.99 -4.99)
Activities of daily living n.r.
Treatment > 3 months Upper limb motor function n.r.
Upper limb synergy n.r.
Strength n.r.
Activities of daily living n.r.
|
This study demonstrates that tDCS and rTMS can target different aspects of stroke plasticity. An inhibition of the contralesional M1or a reduction of interhemispheric interactions did not lead to improved motor recovery in our sample. Conversely, exploratory subgroup analyses suggest that motor recovery might be enhanced by early interventions that seek to increase FC of ipsilesional motor nodes. This hypothesis will need to be confirmed in future trials applying tDCS within the first 4 weeks after stroke. |
|
|||
|
Shahweiwola, 2018 |
Type of study: Randomized Controlled Study.
Funding and conflicts of interest: This work was supported by the National Natural Science Foundation of China (No. 51475292, No. 61761166006), and the Shanghai Municipal Commission of Health and Family lanning (No. 2017ZZ01006). The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. |
Inclusion criteria: (1) age between 35 and 70 years; (2) cerebral haemorrhage or cerebral infraction for the first time; (3) confirmed by head CT or MRI; (4) at least 6 months since stroke onset and an ipsilateral arm Brunnstrom recovery at stages 0–3; (5) conscious and able to communicate; and (6) able to sign informed consent himself/herself or with the help of his/her immediate family member.
Exclusion criteria: (1) sequelae after lacunar cerebral infraction; (2) peripheral neuropathy in upper limbs; (3) unconsciousness, sensory aphasia or mental disorders, that may lead to failures in coordinating examination and treatment; (4) history of seizure. (5) serious illnesses, such as heart, liver or kidney diseases, or serious coagulation disorders; (6) history of cognitive disorder, neuropsychiatric disorder, drug or alcohol abuse; (7) organ failure, carcinoma or terminal stroke that seriously affect quality of life beyond hand dysfunction; (8) inability to complete basic course, to persist treatment, or difficult to follow-up; (9) with metal implants or skull defect; (10) existence of skin rash, allergy or wounds at the locations where stimulation electrodes would be placed.
N total at baseline: Intervention: 15 Control: 15
Important prognostic factors2: For example age ± SD: I: 49.3 ± 9.4 C: 51.9 ± 11.0
Sex: I: 93.3% M C: 86.7% M
Groups comparable at baseline? Yes |
The active tDCS protocol (intensity: 2.0 mA, time of ramp-up: 10 s, time of ramp-down: 10 s). We placed the anode electrode (5 cm×5 cm) of tDCS over the hot spot on the lesioned hemisphere and the cathode electrode (5 cm×5 cm) on the contralateral symmetrical area of non-lesioned hemisphere. If MEPs could not be detected on the paralyzed arm, the hot spot for APB on the non-lesioned hemisphere was first determined, and then the symmetrical area on the contralateral hemisphere was regarded as the APB hot spot corresponding to the paralyzed arm. tDCS intervention was delivered for 20 min via a pair of sponge electrodes moistened with 0.9% NaCl solution. To deliver the FES therapy, we used the Bio-feedback Neuromuscular Stimulator (MyoNet-BOW-III, NCC Medical Co., LTD, China), a parameter-adjustable transcutaneous stimulator that used selfadhesive surface electrodes. The amplitude of the electric current and the tasks were selected based on patients’ needs and adjusted weekly. |
the sham protocol was programmed by a dedicated computer software package and saved on the device ahead of the usage. Regular parameters of tDCS were chosen based on pilot study prior to the experiment. To deliver the FES therapy, we used the Bio-feedback Neuromuscular Stimulator (MyoNet-BOW-III, NCC Medical Co., LTD, China), a parameter-adjustable transcutaneous stimulator that used selfadhesive surface electrodes. The amplitude of the electric current and the tasks were selected based on patients’ needs and adjusted weekly. |
Length of follow-up: After intervention (4 weeks)
Loss-to-follow-up: Intervention: 0 Control: 0
Incomplete outcome data: Intervention: 0 Control: 0
|
Treatment ≤ 3 months
Upper limb motor function n.r.
Upper limb synergy n.r.
Strength n.r.
Activities of daily living n.r.
Treatment > 3 months Upper limb motor function Effect measure: Standardized mean difference (95% Confidence Interval):
0.49 (-0.24 – 1.22)
Upper limb synergy Effect measure: Standardized mean difference (95% Confidence Interval): 0.28 (-1.25 – 0.79)
Strength n.r.
Activities of daily living n.r.
|
Our results suggest new trials with combined intervention in both the central nervous system and the peripheral nervous system. In terms of limitations, since only stroke patients with unilateral arm paralysis were recruited and tested, more types and cases of stroke are needed. In addition, due to the demographics of the patients that were available for the study, both groups were skewed toward males. To further investigate in the interaction between the tDCS treatment and the FES therapy in the protocol, more clinical trials with various intervention conditions and more diverse demographics are also needed. |
|
|||
|
Salazar, 2020 |
Type of study: A double-blind randomized controlled trial
Funding and conflicts of interest: This study received financial support from Conselho Nacional de Pesquisa (CNPq, Brazil) - grant universal 461254/2014-0) and from the Coordenac¸a˜o de Aperfeic¸oamento de Pessoal de Nı´vel Superior (CAPES, Brazil) - finance code 001). The authors declare that they have no competing interest. |
Inclusion criteria individuals with ischaemic or haemorrhagic chronic stroke confirmed by head CT or MRI at least 6 months before recruitment, aged between 18 and 80 years, with moderate (32-47/66) or severe hemiparesis (9-31/66) according to the Fugl–Meyer score (17). Participants had to have minimal cognitive ability on the Mini Mental State Examination (> 20/30 points (illiterate) or > 24/ 30 points (literate) (18) and no history of seizures. Furthermore, participants had to be able to reach forward with both ULs.
Exclusion criteria Individuals who presented shoulder pain, adhesive capsulitis or glenohumeral luxation and any contraindications for electrical stimulation were excluded.
N total at baseline: Intervention: 15 Control: 15
Important prognostic factors2: For example age ± SD: I: 49.3 ± 9.4 C: 51.9 ± 11.0
Sex: I: 93.3% M C: 86.7% M
Groups comparable at baseline? Yes |
This group underwent 10 sessions of concurrent tDCS and FES during 30 min, 5 times a week for 2 weeks (excluding weekends). Before each stimulation session, participants underwentscapular, shoulder, elbow, wrist and finger passive mobilization for approximately 10 min. |
This group underwent 10 sessions of placebo tDCS (sham tDCS) and FES during 30 min, 5 times a week for 2 weeks (excluding weekends). Before each stimulation session, participants underwentscapular, shoulder, elbow, wrist and finger passive mobilization for approximately 10 min. |
Length of follow-up: After intervention (2 weeks)
Loss-to-follow-up: Intervention: 0 Control: 0
Incomplete outcome data: Intervention: 0 Control: 0
|
Treatment ≤ 3 months
Upper limb motor function n.r.
Upper limb synergy n.r.
Strength n.r.
Activities of daily living n.r.
Treatment > 3 months Upper limb motor function n.r.
Upper limb synergy Effect measure: Standardized mean difference (95% Confidence Interval): -1.34 (-2.14 - -0.54)
Strength n.r.
Activities of daily living n.r.
|
Concurrent bi-cephalic tDCS and FES slightly improved reaching motor performance and handgrip force of chronic post-stroke individuals with moderate and severe UL impairment. |
|
|||
|
Yao, 2020 |
Type of study: a randomized controlled trial.
Funding and conflicts of interest: This work was supported by Shanghai Jiao Tong University School of Medicine - Institute of Neuroscience, Chinese Academy of Sciences, Leading Startup Project of Brain Diseases Clinical Research Center (2017NKX002). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors declare that they have no competing interests. |
Inclusion criteria stroke (silent infarct is allowed) as diagnosed by computed tomography or magnetic resonance imaging image scans; had their first ischaemic stroke between 2 weeks to 12 months; can induce motor evoked potential (MEP) of contralesional first dorsal interossei muscle (FDI) using Transcranial magnetic stimulation.
Exclusion criteria orbital metallic implants, pacemakers or artificial cochlea; previous seizure history; previous history of brain neurosurgery or cerebral trauma; aphasia, unilateral neglect or cognitive deficits (Mini-Mental State Examination score < 20); refused to sign informed consent.
N total at baseline: Intervention: 22 Control: 20
Important prognostic factors2: For example age ± SD: I: 63 ± 7.5 C: 66.2 ± 6.2
Sex: I: 70% M C: 85% M
Groups comparable at baseline? Yes |
The electrical stimulation device was a transcranial direct current stimulation model IS300 manufactured by Sichuan Intelligent Company of China. Its two conductive rubber electrodes were placed in a saline-soaked sponge (5 × 7 cm 2) when used. The cathodal electrode was placed over the patients’ scalp which corresponded to the primary motor cortex (M1) of the unaffected hemisphere, and the region was determined by the induction of stable MEP response in the FDI using transcranial magnetic stimulation. The reference electrode was placed above the contralateral supraorbital region. The current of the experimental group was constant 2 mA for 20 min. he duration of the VR therapy was 20 min. |
For the control group, the current was rapidly increased to 2 mA in the beginning and then slowly tapered down to 0. At the end of the experiment, the current again rapidly ramp up to 2 mA and then slowly ramp-down to 0. It created a scalp sensation to blind the subject. he duration of the VR therapy was 20 min. |
Length of follow-up: After treatment (2 weeks)
Loss-to-follow-up: Intervention: 0 Control: 0
Incomplete outcome data: Intervention: 2 Control: 0 Reasons: Discontinued intervention.
|
Treatment ≤ 3 months
Upper limb motor function Effect measure: Standardized mean difference (95% Confidence Interval):
0.33 (-0.07 – 0.90)
Upper limb synergy n.r.
Strength n.r.
Activities of daily living n.r.
Treatment > 3 months Upper limb motor function n.r.
Upper limb synergy n.r.
Strength n.r.
Activities of daily living n.r.
|
Our proof-of-concept single-centre phase II study showed that c-tDCS combined with VR can reduce motor impairment, improve function, increase ADL in the affected upper limb in patients with subacute or chronic ischaemic stroke than VR alone. This study provides critical preliminary data to plan a future multicentre clinical trial to systematically investigate the efficacy of combined intervention. |
|
|||
|
Edwards, 2019 |
Type of study: randomized controlled trial
Funding and conflicts of interest: This study was supported by NICHD of the NIH, under award number R01HD069776. Dr. A. Pascual- Leone was partly supported by the Sidney R. Baer Jr. Foundation, the Football Players Health Study at Harvard University, and Harvard Catalyst | The Harvard Clinical and Translational Science Centre (NCRR and the NCATS NIH, UL1 RR025758). Dr. L. Gerberwas partly supported by the Clinical Translational Science Center, grant number UL1-TR000457-06. Dr. B.T. Volpe and J. Chang were supported by the Feinstein Medical Research Foundation. |
Inclusion criteria a) a first unilateral ischaemic lesion; b) cognitive function sufficient to understand the experiments and follow instructions; and c) Motor Power score 1-4/5 (neither hemiplegic nor fully recovered motor function in the muscles of the shoulder and/or elbow and/or wrist).
Exclusion criteria n.r.
N total at baseline: Intervention: 41 Control: 41
Important prognostic factors2: For example age (mean):
Sex: 61% M
Groups comparable at baseline? No, not in age and cortical versus subcortical stroke.
|
For participants in the RobottDCS group, a 2mA current was delivered by a battery driven, constant current stimulator (1×1 tDCS device, Soterix Medical, New York) using surface rubber-carbon electrodes (35cm2) with surrounding saline soaked sponges (0.9% NaCl). Participants received stimulation for 20 minutes while seated (immediately prior to robot-assisted motor training), with the anode centered 5 cm lateral to the vertex (Fig. 3), and the cathode on the contralateral supraorbital area (Allman et al., 2016).
|
Participants in the RobotSham group had a comparable set-up to RobottDCS, with the stimulation comprising a 30 sec current ramp to 2 mA, then 30 sec ramp down to 0 mA, repeated after 20 mins. |
Length of follow-up: After treatment
Loss-to-follow-up: Intervention: 3 Control: 5 Reasons: unrelated illness, death to spouse, transportation issue.
Incomplete outcome data: Intervention: 4 Control: 1 Reasons: unrelated illness, botox treatment, illness of relative.
|
Treatment ≤ 3 months
Upper limb motor function n.r.
Upper limb synergy n.r.
Strength n.r.
Activities of daily living n.r.
Treatment > 3 months Upper limb motor function Effect measure: Standardized mean difference (95% Confidence Interval):
0.03 (-0.44 – 0.51)
Upper limb synergy n.r.
Strength n.r.
Activities of daily living n.r.
|
We conclude that; 1. Robot-assisted arm training is an effective form of rehabilitation for chronic post-stroke hemiparesis, 2. Supplementary ipsilesional-anode tDCS as performed in our study sample although well tolerated, did not augment training effects, and 3. Residual corticospinal integrity (motor evoked potential presence) in the chronic stroke hemiparetic arm, can be a predictor of clinically important response to intensive arm rehabilitation. |
|
|||
|
Andrade, 2017 |
Type of study: sham-controlled, double-blinded, parallel clinical trial. Setting and Country: Cognitive Neuroscience and Behavior Program, Department of Psychology, Federal University of Paraíba, João Pessoa, Brazil; Nursing. Department, State University of Paraíba, Campina Grande, Brazil. Funding and conflicts of interest: No potential conflict of interest was reported by the authors. |
Inclusion criteria: (a) between 18 and 75 years of age; (b) diagnosis of unilateral, non-recurring, acute ischaemic stroke, as defined by the International Classification of Diseases (ICD-10) through Computed Tomography or Magnetic Resonance conducted by neurologists; (c) able to walk 10 m independently (with or without a mobility aid); (d) high risk of falling (fall during hospital admission, leg score on the Step Test lower than 7, or Berg Balance Scale (BBS) score of less than 49). Exclusion criteria: (a) score between 25 and 32 points on the National Institute of Health Stroke Scale (11) and degree 5 according to the Rankin Scale (12); (b) Mini Mental State Examination score lower than 20. N total at baseline: Important prognostic factors2: Age: mean (SD) C: 70.40 ± 2.32 Sex (% Male): Groups comparable at baseline? Yes |
Group A days for two weeks) of anodal tDCS stimulation at 2 mA intensity and current density equivalent to 0.05 A/m2. the active electrode was placed on the affected hemisphere. Group B Group C All participants received the same physical rehabilitation program (1 h daily, three times per week). Therapy included individualized motor task training and activities of daily living training, emphasizing active participation of the affected and unaffected hemispheres.
|
For sham stimulation, the current was ramped up over 30 seconds and then turned off (15). The current was delivered through two saline-soaked sponge surface electrodes (5 × 7 cm) using a battery-driven constant current stimulator (Trans Cranial Technologies®, Hong Kong, China). |
Length of follow-up: 1 day after treatment, one-month and three-month follow-up.
Loss-to-follow-up (3 months) N=3
Incomplete outcome data:
|
Walking ability Standing balance Effect measure: mean difference (95% CI): Anodal Cathodal Bihemispheric Pooled effect (random effects model): 5.83 (95% CI 2.37 to 9.30) favoring tDCS. Heterogeneity (I2): 100% Strength and Muscle Synergy Sitting balance Sitting and standing balance Effect measure: mean difference (95% CI): Anodal: Cathodal Bihemispheric Walking distance Effect measure: mean difference (95% CI): Anodal Cathodal Bihemispheric Pooled effect (random effects model): 29.6 (95% CI 27.7 0 – 33.46) favoring tDCS. Heterogeneity (I2): 98% Falling Effect measure: mean difference (95% CI): Anodal Cathodal Bihemispheric Pooled effect (random effects model): 11.7 (95% CI 3.50 – 18.80) favoring tDCS. Heterogeneity (I2): 100% |
This is the first trial to report the effects of different electrode’s setups in reducing the risk of falls and lower limb function in acute stroke. tDCS resulted in significant motor recovery sustained at least three months beyond the intervention. A consensus on what is the best montage is yet to come (26,29) although studies that investigate the long-term effects can contribute to the experimental point of view, proposing systematic study protocols, as well as provide clinical applicability evidence of tDCS in following these patients. |
||||
|
Cattagni, 2019 |
Type of study: sham-controlled, double-blinded, parallel clinical trial. Setting and Country: Cognitive Neuroscience and Behavior Program, Department of Psychology, Federal University of Paraíba, João Pessoa, Brazil; Nursing. Department, State University of Paraíba, Campina Grande, Brazil. Funding and conflicts of interest: No potential conflict of interest was reported by the authors. |
Inclusion criteria: male or female aged 18 years or older, hemiparesis following uni-lateral hemispheric cerebral lesions of vascular origin more than 6 months previously, able to walk for 10 minutes non-stop without gait aids and able to provide informed consent. Exclusion criteria: presence of cardiac pacemaker, aphasia or cognitive difficulties that could interfere with comprehension of instructions, neuro-orthopedic surgery to the lower limbs less than 6 months previously, concomitant progressive disease, one or more epileptic seizures within the year prior to the date of inclusion, an intracerebral metal clip, non-affiliation to the social security regime or being under guardianship. N total at baseline: Important prognostic factors2: Age: mean (SD) Sex (% Male): Groups comparable at baseline? Yes |
Anodal tDCS was administered using a constant current electrical stimulator (Eldith DC-stimulator, Ilmenau, Ger-many). Rectangular electrodes (35 cm2; 7 × 5 cm) covered by a saline-soaked sponge were used for the anode and cathode. The anode was placed over the leg area of the motor cortex on the affected side with the medial border of electrode placed laterally to Cz on the international electroencephalogram 10—20 system (30) (Fig. 2A). The cathode was placed above the contralateral orbit. The stimulation intensity was set at 2 mA for 30 minutes. This intensity was reached progressively over a period of 8 seconds at the beginning of tDCS and was reduced to 0 mA over the last 8seconds. A current density of 0.06 mA cm − 2 (2 mA/35 cm2)was used in order to remain below the threshold that can lead to tissue damage. |
The electrodes were placed in the same position as for anodal tDCS and the same stimulation procedure as for the effective anodal tDCS was respected (Fig. 2A). However, a current was only delivered for 120 seconds at the begin-ning of the application to reproduce the sensation of an increase in current intensity. This stimulation duration was chosen because it is below the 180 seconds that Nitsche andPaulus (46) showed to be required to induce anodal tDCS post-effects. This sham tDCS administration has been shown to be indistinguishable from effective tDCS (18). To ensure that the session was blinded, an independent physician set-up the tDCS equipment in either anodal or placebo mode; this person was uninvolved in data recording, col-letting or processing. |
Length of follow-up: Directly after treatment.
Loss-to-follow-up (3 months) n.r.
Incomplete outcome data:
|
Walking ability
Standing balance
Strength and Muscle Synergy
Sitting Balance
Sitting and standing balance
Walking distance
Walking speed Effect measure: mean difference (95% CI) 0.20 (-20.88 – 21.28)
Falling
|
Although a single session of anodal tDCS has been shown pre-viously to modify the excitability of neurons in the leg motor area, in the present study it was not found to alter muscle activity patterns during gait in patients with chronic stroke, regardless of their BDNF genotype (Val66Met versus Val66Val).There is currently no evidence for the use of a single session of anodal tDCS on the leg motor cortex of patients with chronic stroke to alter leg muscle activities during gait and improve the gait pattern. Nevertheless, because the current study only focused on the acute effects of anodal tDCS on gait parameters in individuals with chronic stroke, any potential chronic effects of tDCS (i.e., effects following repeated sessions of anodal tDCS) cannot be ruled out. |
||||
|
Zumbansen, 2020 |
Type of study: three-armed sham-controlled blinded prospective proof-of-concept study
Setting and country: Jewish General Hospital, Lady Davis Institute for Medical Research, Department of Neurology & Neurosurgery, McGill University, Montreal, Quebec
Funding and conflicts of interest: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This trial was supported by research grants from the Canadian Institutes for Health Research (CIHR, MOP#125954), W.-D. Heiss Foundation, and the Lady Davis Institute at the JGH (CLIPP#2014). AZ was funded by a CIHR postdoctoral fellowship.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. |
Inclusion criteria: stroke patients presenting with speech or language problems.
Exclusion criteria: Withdrawal of consent, patients with very severe aphasia (who most likely exclusively depend on the right hemisphere) were excluded from the study.
N total at baseline: Intervention: 24 Control: 19
Important prognostic factors2: For example age ± SD: I: 65.3 ± 13.2 C: 67.4 ± 11.7
Sex: I: 58.3% M C: 36.8% M
Groups comparable at baseline? Yes
|
ctDCS was applied with 5-cm2 sponge electrodes placed over the target area (cathode) and the forehead over the right eye (anode). Stimulation with a 2-mA current started immediately before and lasted throughout the ST session
|
For sham stimulation, the current was turned on for 30 s to elicit a typical skin sensation and then turned off. |
Length of follow-up: After treatment and after one month follow-up.
Loss-to-follow-up (after treatment) Intervention: 1 Control: 1
Reasons: Withdrew before starting treatment.
Incomplete outcome data: n.r.
|
Treatment ≤ 3 months Functional communication n.r.
Verbal comprehension Measured with the standardized Z-scores of the token test post-intervention. Effect measure: MD (95% CI):
Cathodal: -0.07 (-1.12 to 0.98) in favour of sham tDCS.
Expressive naming Measured with the standardized Z-scores of the BNT post intervention. Effect measure: MD (95% CI)
Cathodal: 0.29 (95% CI -0.34 to 0.92)
Treatment > 3 months n.r.
|
Contralesional NIBS is a safe add-on therapy for poststroke aphasia. Low frequency rTMS improved naming recovery one month after a 10-day treatment course. ctDCS effect was not significantly different from sham stimulation. Our results raise the possibility that inhibitory NIBS over the right pars triangularis may have negative effects in patients where Broca’s Area is affected, supporting the view that NIBS presently should not be applied outside clinical trials. Future trials should specifically investigate individual factors for patient stratification (e.g., lesion location) and include longer-term follow-up outcome measures (>6months).
|
|
|||
|
Wang, 2019 |
Type of study: A Randomized Sham-Controlled Study
Setting and country: Department of Rehabilitation, Wangjing Hospital of China Academy of Chinese Medical Science, Beijing, China
Funding and conflicts of interest: The authors have declared that no competing interests existed at the time of publication.
|
Inclusion criteria of a single left hemispheric stroke; (b) absence of a previous brain injury; (c) a lesion that affected the left frontal lobe; (d) slow speech, laborious pronunciation; and (e) inability or difficulty in word repetition (monosyllabic and disyllabic word repetition score < 5/10 (test scores/total scores) to avoid ceiling effect after treatment).
Exclusion criteria: (a) severely impaired auditory verbal comprehension (auditory word–picture identification < 6/30), (b) a history of seizures within 12 months until present hospitalization, and (c) psychiatric disease or dementia.
N total at baseline A-tDCS-M1: 18 S-tDCS: 16
Important prognostic factors2: Mean age A-tDCS-M1: 52.6 yrs A-tDCS-Broca: 56.1 yrs
Sex: A-tDCS-M1: 77.8% Male A-tDCS-Broca: 61.1% Male
Groups comparable at baseline? Yes
|
A-tDCS-M1 the location of the lip region of M1 for the tDCS treatment was selected according to transcranial magnetic stimulation. The transcranial magnetic stimulation was administered using a rapid magnetic stimulator (Magstim Company, P/N: 3013-00) with a figure-of-eight coil (diameter, 7 cm), a peak intensity of stimulation at 2 T, and a pulse duration of 250 s. The coil was moved over the scalp in order to determine the optimal site from which maximal amplitude motor evoked potentials were elicited in the unaffected orbicularis oris muscle. Then, the left homologous area was determined as the A-tDCS site.
A-tDCS-Broca region as the crossing point between T3-Fz and F7-Cz according to the international 10–20 system (Friederici, Hahne, & von Cramon, 1998). This crossing point was known as left Broca’s point (LBP). The cathodal electrode was placed over the right shoulder. |
For sham stimulation, the stimulator was turned off after 30 s. For both active and sham tDCS treatments, the intensity of the current was gradually increased and decreased. The anodal electrode of the S-tDCS group was placed similarly to that of the A-tDCS-M1 group. This procedure blinded the subjects to the stimulation conditions. tDCS was delivered immediately before the 30-min speech training. |
Length of follow-up: After treatment.
Loss-to-follow-up (after treatment) A-tDCS-M1: 0 A-tDCS-Broca: 0 S-tDCS: 0
Reasons: Withdrew before starting treatment.
Incomplete outcome data: n.r. |
Treatment ≤ 3 months n.r.
Treatment > 3 months Functional communication n.r.
Verbal comprehension n.r.
Expressive naming Measured with the picture naming test. Effect measure: SMD (95% CI)
Anodal: Broca
M1 0.08 (-0.76 to 0.91) in favour of tDCS.
|
In summary, our study provides the first evidence for the application of tDCS over the left lip region of M1, improving the articulation performance in patients with poststroke aphasia and AoS. tDCS, with its potential to enhance cortical activation and network connectivity, offers an exciting novel strategy for recovery from AoS. |
|
|||
|
Hosseinzadeh, 2018 |
Type of study: RCT
Setting and country: Iran
Funding and conflicts of interest:
This work was supported by Kerman Neuroscience Research Center, Kerman, Iran and Kavoush Research Center for Behavioral, Cognitive and Addiction, Guilan University of Medical Sciences, Rasht, Iran. Also, we thanks the Prof. Vahid Sheibani, Dr. Mohammad Shabani for those kindness, assistance and considerations (Ethics code number: IR.KMU.REC.1395.23).
The authors declare no conflict of interest. |
Inclusion criteria: All subjects were patients with chronic ischaemic stroke who were admitted at the Tolou Clinic, Rasht, Iran. Magnetic resonance imaging (MRI) was performed to confirm both lesion locations.
Exclusion criteria: Patients with other types of stroke were not included to reduce the heterogeneity of the study population.
N total at baseline: Anodal: 25 Cathodal: 25 Sham: 25
Important prognostic factors2: age ± SD: Anodal 58±8 Cahodal: 64±7 Sham: 59±7
Sex: Anodal: 52% M Cathodal: 48% M Sham: 48%M
Stroke type:
Groups comparable at baseline? Yes
|
tDCS was applied by a battery-powered, constant current electrical stimulator (at 2-mA intensity using a pair of surface saline-soaked 35-cm2sponge electrodes (5 7 cm), for 3 times a week for 1 month, for 30 min per session and at an electric current of 2 mA. We used two different electrode montages, including an anode and a cathode.
In anodal tDCS, 2842 5Department of Psychiatry, Guilan University of Medical Sciences, Shafa Hospital, Rasht, Iran 6Department of Neuroscience, Neuroscience Research Center, Guilan University of Medical Sciences, Rasht, Iran 7Razi Herbal Medicines Research Center, Lorestan University of Medical Sciences, Khorramabad, Iran University of Medical sciences, Rasht, Iran Biomedical Research and Therapy, (11):2841-2849 the anode electrode was mounted on the left superior temporal gyrus, while the cathode was placed over the contralateral superior region (cp5). The current was run through the brain and other tissues of the head, from the anode to the cathodal electrode.
In cathodal tDCS, the cathode was used for placement symmetrical to the left gyrus (cp6), while the anode was placed at the contralateral supraorbital region.
All patients received the same protocol, 3 times a week for 30 min per session for 1 month. Then, all of these evaluation tests were applied for the patients at 3 months after ending the tDCS sessions |
tDCS was applied by a battery-powered, constant current electrical stimulator (at 2-mA intensity using a pair of surface saline-soaked 35-cm2sponge electrodes (5 7 cm), for 3 times a week for 1 month, for 30 min per session and at an electric current of 2 mA. We used two different electrode montages, including an anode and a cathode.
In sham tDCS, the anode was placed over the left superior temporal gyrus and cathode was placed on the contralateral supraorbital region, but no current was applied.
All patients received the same protocol, 3 times a week for 30 min per session for 1 month. Then, all of these evaluation tests were applied for the patients at 3 months after ending the tDCS sessions |
Length of follow-up: 3 months after the intervention.
Loss-to-follow-up: n.r.
Incomplete outcome data: n.r.
|
Outcome measures and effect size (include 95%CI and p-value if available):
|
Our results demonstrate that anodic tDCS is beneficial and safe method for increasing the rehabilitation of movement and life–related functions in chronic stroke patients, while cathodic tDCS can enhance executive memory. The ability of anodic tDCS to modulate cortical excitability becomes useful, and exploring the preference indication of the anodic and cathodic tDCS abilities requires further investigation. One of the limitations of this study was the number of patients needed to conduct the research trial, which took a lot of research time. Among other constraints/ factors were the predictable high cost, and the provision of help and assistance from various agencies, such as the Kerman Neuroscience Research Center, Kerman, Iran and Kavoush Research Center for Behavioral, Cognitive and Addiction, Guilan University of Medical Sciences, Rasht, Iran. Moreover, while the introduction of the tDCS method for patients and neurologists has garnered greater attention, more research is necessary to fully explore the advantages of this method. |
|
|||
|
Shaker, 2018 |
Type of study: RCT
Setting and country: the outpatient clinic of Faculty of Physical Therapy, Cairo University, and neurology outpatient clinic of Cairo University Hospitals.
Funding and conflicts of interest:
The authors declare that they have no competing interests (financial and non-financial). We declare that the research was conducted in absence of any commercial relationships that could be constructed as a potential conflict of interest. |
Inclusion criteria: Included in this study were patients with ischaemic cerebrovascular stroke in the domain of carotid system diagnosed clinically and confirmed by CT and/or MRI of the brain, their age ranged from 40 to 60 years old to minimize the effect of age on cognition, duration of illness at least 6 months from stroke onset, educated patients at least 10 years of formal education, degree of spasticity was 1 and 1+ according to Modified Ashworth Scale, grade of muscle power was 3 according to manual muscle test, mini mental state examination score ranged from 19 to 24 (mild cognitive impairment), functional independence measure score ranged from 84 to 99, Beck depression inventory score from 0–9, and normal hearing and vision.
Exclusion criteria: Excluded from this study were patients with recurrent cerebrovascular stroke or concomitant neurological disorders that may affect cognition, psychiatric disorders, drug or alcohol abuse, and seizure disorders; patients receiving drugs that may affect cognitive functions (anti- depressants, anti-epileptics, sedatives, muscle relaxants); and patients with medical illness that may affect cognitive functions (thyroid, renal or hepatic disorder) and a presence of wounds in the skin of the skull.
N total at baseline: Intervention: 20 Control: 20
Important prognostic factors2: age ± SD: I: 54.45 ± 4.68 C: 53.05 ± 6.32
Sex: 100% Male
Stroke type:
Groups comparable at baseline? Yes |
in group A, patients received treatment for 1 month, three sessions per week (every other day). The duration of each session was 60 min, divided into 30 min of tDCS (2 mA) application followed by 30 min of RehaCom training.
All patients received RehaCom for cognitive training: RehaCom is a computerized program instituted in the International Neurological Restoration Center for rehabilitation of brain injury. |
In group B, patients received sham tDCS and the same cognitive training program as group A. In sham tDCS, real stimulation was applied for 5 s; then, stimulation was turned off.
All patients received RehaCom for cognitive training: RehaCom is a computerized program instituted in the International Neurological Restoration Center for rehabilitation of brain injury. |
Length of follow-up: End of treatment
Loss-to-follow-up: n.r.
Incomplete outcome data: n.r.
|
Outcome measures and effect size (include 95%CI and p-value if available):
|
In view of the results of this study, it could be concluded that tDCS is a safe and effective neurorehabilitation modality that improves cognitive functions in several domains including attention and concentration, figural memory, logical reasoning, and reaction behavior. Moreover, tDCS has a positive impact on performance of daily activities. So, it is recommended to use it in the rehabilitation of post stroke cognitive dysfunctions. |
|
|||
Notes:
- Prognostic balance between treatment groups is usually guaranteed in randomized studies, but non-randomized (observational) studies require matching of patients between treatment groups (case-control studies) or multivariate adjustment for prognostic factors (confounders) (cohort studies); the evidence table should contain sufficient details on these procedures.
- Provide data per treatment group on the most important prognostic factors(((potential) confounders).
- For case-control studies, provide sufficient detail on the procedure used to match cases and controls.
- For cohort studies, provide sufficient detail on the (multivariate) analyses used to adjust for (potential) confounders.
Table of quality assessment for systematic reviews of RCTs and observational studies
Based on AMSTAR checklist (Shea, 2007; BMC Methodol 7: 10; doi:10.1186/1471-2288-7-10) and PRISMA checklist (Moher, 2009; PLoS Med 6: e1000097; doi:10.1371/journal.pmed1000097)
|
Study
First author, year |
Appropriate and clearly focused question?1
Yes/no/unclear |
Comprehensive and systematic literature search?2
Yes/no/unclear |
Description of included and excluded studies?3
Yes/no/unclear |
Description of relevant characteristics of included studies?4
Yes/no/unclear |
Appropriate adjustment for potential confounders in observational studies?5
Yes/no/unclear/notapplicable |
Assessment of scientific quality of included studies?6
Yes/no/unclear |
Enough similarities between studies to make combining them reasonable?7
Yes/no/unclear |
Potential risk of publication bias taken into account?8
Yes/no/unclear |
Potential conflicts of interest reported?9
Yes/no/unclear |
|
Bai, 2019 |
Yes |
Yes |
No |
Yes |
Not applicable |
Yes |
Yes |
Yes |
Yes |
|
Tien, 2020 |
Yes |
Yes |
No |
Yes |
Not applicable |
Yes |
Yes |
Yes |
Yes |
|
Elsner, 2019 |
Yes |
Yes |
Yes |
Yes |
Not applicable |
Yes |
Unclear |
Yes |
Yes |
|
Van Lieshout, 2019 |
Yes |
Yes |
Yes |
Yes |
Not applicable |
Yes |
Yes |
Yes |
Yes |
- Research question (PICO) and inclusion criteria should be appropriate and predefined.
- Search period and strategy should be described; at least Medline searched; for pharmacological questions at least Medline + EMBASE searched.
- Potentially relevant studies that are excluded at final selection (after reading the full text) should be referenced with reasons.
- Characteristics of individual studies relevant to research question (PICO), including potential confounders, should be reported.
- Results should be adequately controlled for potential confounders by multivariate analysis (not applicable for RCTs).
- Quality of individual studies should be assessed using a quality scoring tool or checklist (Jadad score, Newcastle-Ottawa scale, risk of bias table et cetera).
- Clinical and statistical heterogeneity should be assessed; clinical: enough similarities in patient characteristics, intervention and definition of outcome measure to allow pooling? For pooled data: assessment of statistical heterogeneity using appropriate statistical tests (for example Chi-square, I2)?
- An assessment of publication bias should include a combination of graphical aids (for example funnel plot, other available tests) and/or statistical tests (for example Egger regression test, Hedges-Olken). Note: If no test values or funnel plot included, score “no”. Score “yes” if mentions that publication bias could not be assessed because there were fewer than 10 included studies.
- Sources of support (including commercial co-authorship) should be reported in both the systematic review and the included studies. Note: To get a “yes,” source of funding or support must be indicated for the systematic review AND for each of the included studies.
Risk of bias tables of the RCTs
|
Study reference
(first author, publication year) |
Was the allocation sequence adequately generated? a
Definitely yes Probably yes Probably no Definitely no |
Was the allocation adequately concealed?b
Definitely yes Probably yes Probably no Definitely no |
Blinding: Was knowledge of the allocated interventions adequately prevented?c
Were patients blinded?
Were healthcare providers blinded?
Were data collectors blinded?
Were outcome assessors blinded?
Were data analysts blinded?
Definitely yes Probably yes Probably no Definitely no |
Was loss to follow-up (missing outcome data) infrequent?d
Definitely yes Probably yes Probably no Definitely no |
Are reports of the study free of selective outcome reporting?e
Definitely yes Probably yes Probably no Definitely no |
Was the study apparently free of other problems that could put it at a risk of bias?f
Definitely yes Probably yes Probably no Definitely no |
Overall risk of bias If applicable/necessary, per outcome measureg
LOW Some concerns HIGH
|
|
Alisar, 2020 |
Definitely yes;
Reasons: Patients were allocated a number and randomised into 1 of 2 treatment groups using the Random Allocation Software programme. |
Definitely yes;
Reasons: The patients and PMR trainee who performed all patient evaluations were blind to the treatment received |
Definitely no;
Reasons: The occupational therapist and PMR specialist involved in the study were not blind to the treatment received. |
Definitely yes;
Reasons: There were no dropouts. |
Definitely yes;
Reasons: All relevant outcomes were reported |
Probably no;
Reasons: Limitations of the study include the heterogeneity in lesion location and the short post treatment follow-up.
|
Some concerns |
|
Sik, 2015 |
No information;
Reasons: No information was provided about the allocation sequence. |
No information;
Reasons: No information was provided abaut the concealment of the allocation sequence. |
Probably yes;
Reasons: The evaluation of WMFT and KFET was conducted by an experienced physiotherapist who was blinded to the therapy, but no information was provided about the blinding of patients and data collectors. |
Definitely yes;
Reasons: Missing outcome data was infrequent. |
Definitely yes;
Reasons: All relevant outcomes were reported |
Probably yes;
Reasons: No other problems noted.
|
LOW |
|
Nicolo, 2018 |
Definitely yes;
Reasons: Randomization was stratified for initial motor impairment and stroke lateralization, with an allocation sequence based on a block size of 3, generated with a computer random number generator |
Definitely yes;
Reasons: The allocation sequence was generated by a researcher not involved in recruitment. |
Probably no;
Reasons: Subjects were blinded with respect to the true or sham stimulation conditions; Motor function was assessed by a trained therapist who was blinded to treatment allocation; The researcher administering NIBS was unblinded. |
Definitely yes;
Reasons: Missing outcome data was infrequent. |
Definitely yes;
Reasons: All relevant outcomes were reported |
Probably yes;
Reasons: No other problems noted.
|
LOW |
|
Shaheiwola, 2018 |
No information;
Reasons: No information was provided about the allocation sequence. |
No information;
Reasons: No information was provided about the allocation concealment. |
Probably yes;
Reasons: To ensure the reliability of results, there was a baseline observation period of 4 weeks before the intervention period; No information was provided about the blinding of the patients, data collectors and data analysts. |
Definitely yes;
Reasons: No patients were excluded from the analysis or lost to follow-up. |
Definitely yes;
Reasons: All relevant outcomes were reported |
Probably no;
Reasons: Only stroke patients with unilateral arm paralysis were recruited and tested. |
Some concerns |
|
Salazar, 2020 |
Definitely yes;
Reasons: Randomization was done by using a computer-generated random number of sequences (http://www. random.com). Concealed randomization was performed in blocks of 4 to 6 individuals. |
Probably yes;
Reasons: An investigator who was not involved in the assessment, treatment or statistical analysis conducted the randomization. |
Definitely yes;
Reasons: A trained physiotherapist delivered the treatment to both groups. The same blinded examiners performed all assessments; To assess the effective blinding of participants, they were asked to answer whether they were aware of receiving real or sham tDCS. Participants were allowed to answer only YES or NO. This assessment was performed at the end of the last evaluation session; A blinded assessor asked all participants if they noticed some improvement, no change or deterioration after the treatment. |
Definitely yes;
Reasons: Missing outcome data was infrequent. |
Definitely yes;
Reasons: All relevant outcomes were reported |
Probably yes;
Reasons: No other problems noted.
|
LOW |
|
Yao, 2020 |
Definitely yes;
Reasons: The study used a computer to generate a random number table. The physician designated the random numbers in the random number table as the experimental group or the control group according to odd or even numbers. |
Definitely yes;
Reasons: The generated random distribution sequence was put into the sequentially coded, sealed and opaque envelope.
|
Definitely no;
Reasons: Only the patients were blinded to the stimulation condition by creating a scalp sensation to the subjects receiving sham stimulation. |
Definitely yes;
Reasons: No patients were excluded from the analysis. |
Definitely yes;
Reasons: All relevant outcomes were reported |
Definitely no;
Reasons: As the study was conducted in the rehabilitation inpatient facility in real-world practice, and all of the patients likely received additional rehabilitation therapy outside of the clinical-trial setting. We could not quantify and control these therapies which may pose biases to the study. |
Some concerns |
|
Edwards, 2019 |
Definitely yes;
Reasons: The randomization was block stratified to ensure our two comparison groups were balanced with respect to this potential prognostic factor. |
Definitely yes;
Reasons: The allocation was concealed by a predetermined sealed envelope method. Both the patient and tDCS administrator were blind to the randomization. |
Probably yes;
Reasons: Patients, evaluating therapists, and analysts were blind to the tDCS randomization. |
Probably no;
Reasons: 8/77 patients were lost to follow-up due to unrelated illness, botox treatment or illness of relative. |
Definitely yes;
Reasons: All relevant outcomes were reported |
Probably yes;
Reasons: No other problems noted.
|
LOW |
|
Andrade, 2017 |
Definitely yes;
Reasons: Randomization was conducted with randomly permuted blocks, through an online program (www. random.org). After completion of baseline measures. |
Definitely yes;
Reasons: Blind allocation in the ratio of 1:1:1:1 was carried out with sequentially numbered and sealed opaque envelopes. |
Definitely yes;
Reasons: Blinding was extended from the person responsible for randomization, allocation, outcome assessing, and trialists to those who analyzed the data. |
Probably yes;
Reasons: 2/60 patients were lost to follow-up, which was infrequent and balanced. |
Definitely yes;
Reasons: All relevant outcomes were reported |
Probably yes;
Reasons: No other problems noted.
|
LOW |
|
Cattagni, 2019 |
Probably yes;
Reasons: The order of sessions was randomized in order to ensure that equal numbers (12) of participants began with each type of session (effective anodal tDCS or sham tDCS). |
No information;
Reasons: Reasons: No information was provided about the concealment of the allocation sequence.
|
Definitely yes;
Reasons: To ensure that the session was blinded, an independent physician set-up the tDCS equipment in either anodal or placebo mode; this person was uninvolved in data recording, collecting or processing. |
No information;
Reasons: No information was provided about lost to follow-up. |
Definitely yes;
Reasons: All relevant outcomes were reported |
Probably yes;
Reasons: No other problems noted.
|
Some concerns |
|
Zumbansen, 2020 |
Definitely yes;
Reasons: Computer-generated, non-restricted randomization by site was performed through an online system located at the Department of Clinical Epidemiology at the JGH (Montreal). |
Definitely yes;
Reasons: Only the technician performing the stimulation had access to the randomization information when logging into the study platform, on the first day of treatment. |
Definitely yes;
Reasons: Patients, therapist, principal investigators and research personnel assessing clinical outcomes were blinded to the treatment assignment. Therapists did not attend rTMS sessions and had no access to the tDCS device settings. |
Probably yes;
Reasons: 1/63 patients were lost to follow-up at the first day after the intervention, which was infrequent. |
Definitely yes;
|
Definitely no;
Reasons: Patient characteristics were not balanced (in terms of language variability), the recruitment goal was not reached, assessment tools lack validation. |
LOW |
|
Wang, 2019 |
Definitely yes;
computer-generated randomization list by a single investigator. |
Definitely yes;
computer-generated randomization list by a single investigator. The assigned random number was input into the stimulator device by the same investigator who did not participate in other sections of the study. |
Definitely yes;
Reasons: All subjects and speech-language therapists were blinded to the design. |
Definitely yes;
Reasons: No patients were lost to follow-up. |
Definitely yes;
|
Probably no;
Reasons: the inclusion criteria for brain lesion involved in the left frontal lobe, but most participants’ lesion was far beyond the site. |
LOW |
|
Hosseinzadeh, 2018 |
Definitely yes;
Reasons: The research groups (of patients) were based on the random selection of envelopes. |
Definitely yes;
Reasons: In order to observe the double blindness of the research, the patients chose closed envelopes for the rhombus, in which specific codes were inserted. The patients and tDCS-conducting technicians did not know the content of the envelopes, thus providing a satisfactory classification of the four groups. |
Definitely yes;
Reasons: In order to observe the double blindness of the research, the patients chose closed envelopes for the rhombus, in which specific codes were inserted. |
Probably yes;
Reasons: Loss to follow-up was not reported. |
Definitely yes;
Reasons: All relevant outcomes were reported. |
Probably yes;
Reasons: No other problems noted.
|
LOW |
|
Shaker, 2018 |
No information;
Reasons: No information was provided about the generation of the allocation sequence. |
No information;
Reasons: No information was provided about allocation concealment.
|
Definitely no;
Reasons: The study was single-blinded but did not specify who was blinded. |
Probably yes;
Reasons: Loss to follow-up was not reported. |
Definitely yes;
Reasons: All relevant outcomes were reported. |
Probably yes;
Reasons: No other problems noted.
|
Some concerns |
1. Randomization: generation of allocation sequences have to be unpredictable, for example computer generated random-numbers or drawing lots or envelopes. Examples of inadequate procedures are generation of allocation sequences by alternation, according to case record number, date of birth or date of admission.
2. Allocation concealment: refers to the protection (blinding) of the randomization process. Concealment of allocation sequences is adequate if patients and enrolling investigators cannot foresee assignment, for example central randomization (performed at a site remote from trial location). Inadequate procedures are all procedures based on inadequate randomization procedures or open allocation schedules.
3. Blinding: neither the patient nor the care provider (attending physician) knows which patient is getting the special treatment. Blinding is sometimes impossible, for example when comparing surgical with non-surgical treatments, but this should not affect the risk of bias judgement. Blinding of those assessing and collecting outcomes prevents that the knowledge of patient assignment influences the process of outcome assessment or data collection (detection or information bias). If a study has hard (objective) outcome measures, like death, blinding of outcome assessment is usually not necessary. If a study has “soft” (subjective) outcome measures, like the assessment of an X-ray, blinding of outcome assessment is necessary. Finally, data analysts should be blinded to patient assignment to prevents that knowledge of patient assignment influences data analysis.
4. If the percentage of patients lost to follow-up or the percentage of missing outcome data is large, or differs between treatment groups, or the reasons for loss to follow-up or missing outcome data differ between treatment groups, bias is likely unless the proportion of missing outcomes compared with observed event risk is not enough to have an important impact on the intervention effect estimate or appropriate imputation methods have been used.
5. Results of all predefined outcome measures should be reported; if the protocol is available (in publication or trial registry), then outcomes in the protocol and published report can be compared; if not, outcomes listed in the methods section of an article can be compared with those whose results are reported.
6. Problems may include: a potential source of bias related to the specific study design used (e.g., lead-time bias or survivor bias); trial stopped early due to some data-dependent process (including formal stopping rules); relevant baseline imbalance between intervention groups; claims of fraudulent behavior; deviations from intention-to-treat (ITT) analysis; (the role of the) funding body. Note: The principles of an ITT analysis implies that (a) participants are kept in the intervention groups to which they were randomized, regardless of the intervention they actually received, (b) outcome data are measured on all participants, and (c) all randomized participants are included in the analysis.
7. Overall judgement of risk of bias per study and per outcome measure, including predicted direction of bias (e.g. favors experimental, or favors comparator). Note: the decision to downgrade the certainty of the evidence for a particular outcome measure is taken based on the body of evidence, i.e. considering potential bias and its impact on the certainty of the evidence in all included studies reporting on the outcome.
Tabel Exclusie na het lezen van het volledige artikel voor deelvraag 2 (onderste extremiteit)
|
Auteur en jaartal |
Redenen van exclusie |
|
Montenegro, 2016 |
Te weinig patiënten geïncludeerd. |
|
Seo, 2017 |
Te weinig patiënten geïncludeerd. |
|
Geiger, 2019 |
Te weinig patiënten geïncludeerd. |
|
Lian, 2020 |
Te weinig patiënten geïncludeerd. |
|
Ojardias, 2020 |
Te weinig patiënten geïncludeerd. |
|
Klomjai, 2018 |
Te weinig patiënten geïncludeerd. |
|
Saeys, 2015 |
Verkeerd studiedesign. |
Verantwoording
Beoordelingsdatum en geldigheid
Laatst beoordeeld : 12-09-2024
Algemene gegevens
De ontwikkeling/herziening van deze richtlijnmodule werd ondersteund door het Kennisinstituut van de Federatie Medisch Specialisten (www.demedischspecialist.nl/kennisinstituut) en werd gefinancierd uit de Stichting Kwaliteitsgelden Medisch Specialisten (SKMS). De financier heeft geen enkele invloed gehad op de inhoud van de richtlijnmodule.
Samenstelling werkgroep
Voor het ontwikkelen van de richtlijnmodule is in 2021 een doorstart gemaakt met de multidisciplinaire werkgroep, bestaande uit vertegenwoordigers van alle relevante specialismen (zie hiervoor de Samenstelling van de werkgroep) die betrokken zijn bij de zorg voor patiënten met een herseninfarct of hersenbloeding.
Kerngroep
- Dr. B. (Bob) Roozenbeek (voorzitter), neuroloog, Erasmus MC Rotterdam, namens de Nederlandse Vereniging voor Neurologie (NVN)
- Prof. dr. R.M. (Renske) van den Berg-Vos, neuroloog, OLVG West Amsterdam, namens de NVN
- Prof. dr. J. (Jeannette) Hofmeijer, neuroloog, Rijnstate ziekenhuis Arnhem, namens de NVN
- Prof. dr. J.M.A. Visser-Meilij, revalidatiearts, UMC Utrecht, namens de VRA
- A.F.E. (Arianne) Verburg, huisarts, namens het Nederlands Huisartsen Genootschap (NHG)
- Prof. dr. H.B. (Bart) van der Worp, neuroloog, UMC Utrecht, namens de NVN
- Dr. S.M. (Yvonne) Zuurbier, neuroloog, Amsterdam UMC, namens de NVN
- Prof. dr. W. (Wim) van Zwam, radioloog, Maastricht UMC, namens de Nederlandse Vereniging voor Radiologie (NVvR)
- Prof. dr. G. (Gert) Kwakkel, hoogleraar neurorevalidatie, Amsterdam UMC, namens de Koninklijk Nederlands Genootschap foor Fysiotherapie (KNGF)
Met ondersteuning van
- Dr. M.L. Molag, adviseur, Kennisinstituut van de Federatie Medisch Specialisten
- Drs. F. Ham, junior adviseur, Kennisinstituut van de Federatie Medisch Specialisten
Belangenverklaringen
De Code ter voorkoming van oneigenlijke beïnvloeding door belangenverstrengeling is gevolgd. Alle werkgroepleden hebben schriftelijk verklaard of zij in de laatste drie jaar directe financiële belangen (betrekking bij een commercieel bedrijf, persoonlijke financiële belangen, onderzoeksfinanciering) of indirecte belangen (persoonlijke relaties, reputatiemanagement) hebben gehad. Gedurende de ontwikkeling of herziening van een module worden wijzigingen in belangen aan de voorzitter doorgegeven. De belangenverklaring wordt opnieuw bevestigd tijdens de commentaarfase.
Een overzicht van de belangen van werkgroepleden en het oordeel over het omgaan met eventuele belangen vindt u in onderstaande tabel. De ondertekende belangenverklaringen zijn op te vragen bij het secretariaat van het Kennisinstituut van de Federatie Medisch Specialisten.
|
Werkgroeplid |
Functie |
Nevenfuncties |
Gemelde belangen |
Ondernomen actie |
|
Van den Berg-Vos |
Neuroloog |
Geen |
Voorzitter werkgroep CVA van het Transmuraal Platform Amsterdam (betaald d.m.v. vacatiegelden) Lid focusgroep CVA ROAZ Noord-Holland (onbetaald) ‘Medical lead' Experiment Uitkomstindicatoren VWS (namens Santeon) aandoening CVA, via Santeon betaald voor 4 uur per week Projectleider “Patient-Reported Outcomes Measurement Information System (PROMIS®) voor waardegedreven zorg" (SKMS-project met subsidie FMS, betaald d.m.v. vacatiegelden) Projectleider "Regionale auditing voor kwaliteit van zorg bij patiënten met een herseninfarct” (SKMS-project met subsidie FMS , betaald dmv vacatiegelden) Voorzitterschap (namens de Nederlandse Vereniging voor Neurologie) van werkgroep CVA van het programma Uitkomstgerichte Zorg ‘Meer inzicht in uitkomsten’ (betaald d.m.v. vacatiegelden) |
Geen |
|
Hofmeijer |
Neuroloog (0,6 fte) |
Hoogleraar universiteit Twente (0,4 fte) |
Geen |
Geen |
|
Kwakkel |
Hoogleraar Neurorevalidatie |
Europees Editor NeuroRehabilitation and Neural Repair
|
Handling editor Stroke (AHA) Coordinator Stroke Unit Cursus NPI Cursusleider mCIMT bij NPI Cursusleider Neurorebvalidatie-CVA bij NPI
|
Geen |
|
Roozenbeek |
Neuroloog |
|
Lid van CONTRAST, coördineert onderzoeksprojecten op gebied van acute beroertezorg gefinancierd door Stichting BeterKeten, Stichting THEIA, Erasmus Universiteit en Erasmus MC |
Geen |
|
Verburg |
Huisarts |
Senior wetenschappelijk medewerker NHG |
Geen |
Geen |
|
Visser-Meily |
Revalidatiearts, hoogleraar en afdelingshoofd |
Geen |
Geen |
Geen |
|
Van der Worp |
Neuroloog |
|
Adviezen aan/consultancy voor Boehringer Ingelheim, producent van onder anderen alteplase en dabigatron. Adviseur van Bayer en LivaNova; subsidie van Stryker voor stroke trial (gelden via het CONTRAST consortium); mede-onderzoeker B-STARS, een inmiddels afgeronde RCT van rTMS bij patiënten met een beroerte. |
Uitsluiting besluitvorming alteplase en dabigatran |
|
Zuurbier |
Neuroloog |
Geen |
Geen |
Geen |
|
Van Zwam |
Neuro-interventieradioloog |
Geen |
Consultancy activiteiten voor stryker en Cerenovus, lid CONTRAST, MRCLEAN: ATE |
Geen invloed op richtlijnonderwerpen |
Inbreng patiëntenperspectief
Er werd aandacht besteed aan het patiëntenperspectief door gebruik te maken van kwaliteitscriteria vanuit patiëntenperspectief voor CVA, ontwikkeld door Harteraad. Verder informeren Harteraad, Hartstichting en Hersenletsel door middel van notulen vergaderingen kerngroep en worden ze betrokken bij relevante onderwerpen. De conceptmodules zijn tevens voor commentaar aan bovengenoemde verenigingen voorgelegd.
Wkkgz & Kwalitatieve raming van mogelijke substantiële financiële gevolgen
Kwalitatieve raming van mogelijke financiële gevolgen in het kader van de Wkkgz
Bij de richtlijn is conform de Wet kwaliteit, klachten en geschillen zorg (Wkkgz) een kwalitatieve raming uitgevoerd of de aanbevelingen mogelijk leiden tot substantiële financiële gevolgen. Bij het uitvoeren van deze beoordeling zijn richtlijnmodules op verschillende domeinen getoetst (zie het stroomschema op de Richtlijnendatabase).
Uit de kwalitatieve raming blijkt dat er waarschijnlijk geen substantiële financiële gevolgen zijn, zie onderstaande tabel.
Submodule |
Uitkomst raming |
Toelichting |
|
Non-invasieve hersenstimulatie met rTMS |
Geen financiële gevolgen |
Hoewel uit de toetsing volgt dat de aanbeveling(en) breed toepasbaar zijn (>40.000 patiënten), volgt ook uit de toetsing dat [het overgrote deel (±90%) van de zorgaanbieders en zorgverleners al aan de norm voldoet OF het geen nieuwe manier van zorgverlening of andere organisatie van zorgverlening betreft, het geen toename in het aantal in te zetten voltijdsequivalenten aan zorgverleners betreft en het geen wijziging in het opleidingsniveau van zorgpersoneel betreft]. Er worden daarom geen substantiële financiële gevolgen verwacht. |
|
Non-invasieve hersenstimulatie met tDCS |
Geen financiële gevolgen |
Hoewel uit de toetsing volgt dat de aanbeveling(en) breed toepasbaar zijn (>40.000 patiënten), volgt ook uit de toetsing dat [het overgrote deel (±90%) van de zorgaanbieders en zorgverleners al aan de norm voldoet OF het geen nieuwe manier van zorgverlening of andere organisatie van zorgverlening betreft, het geen toename in het aantal in te zetten voltijdsequivalenten aan zorgverleners betreft en het geen wijziging in het opleidingsniveau van zorgpersoneel betreft]. Er worden daarom geen substantiële financiële gevolgen verwacht. |
De kwalitatieve raming volgt na de commentaarfase.
Werkwijze
AGREE
Deze richtlijnmodule is opgesteld conform de eisen vermeld in het rapport Medisch Specialistische Richtlijnen 2.0 van de adviescommissie Richtlijnen van de Raad Kwaliteit. Dit rapport is gebaseerd op het AGREE II instrument (Appraisal of Guidelines for Research & Evaluation II; Brouwers, 2010).
Knelpuntenanalyse en uitgangsvragen
Tijdens de voorbereidende fase inventariseerden de werkgroep de knelpunten in de zorg voor patiënten na een herseninfarct of hersenbloeding. Op basis van de uitkomsten van de knelpuntenanalyse zijn door de werkgroep concept-uitgangsvragen opgesteld en definitief vastgesteld.
Uitkomstmaten
Na het opstellen van de zoekvraag behorende bij de uitgangsvraag inventariseerde de werkgroep welke uitkomstmaten voor de patiënt relevant zijn, waarbij zowel naar gewenste als ongewenste effecten werd gekeken. Hierbij werd een maximum van acht uitkomstmaten gehanteerd. De werkgroep waardeerde deze uitkomstmaten volgens hun relatieve belang bij de besluitvorming rondom aanbevelingen, als cruciaal (kritiek voor de besluitvorming), belangrijk (maar niet cruciaal) en onbelangrijk. Tevens definieerde de werkgroep tenminste voor de cruciale uitkomstmaten welke verschillen zij klinisch (patiënt) relevant vonden.
Methode literatuursamenvatting
Een uitgebreide beschrijving van de strategie voor zoeken en selecteren van literatuur en de beoordeling van de risk-of-bias van de individuele studies is te vinden onder ‘Zoeken en selecteren’ onder Onderbouwing. De beoordeling van de kracht van het wetenschappelijke bewijs wordt hieronder toegelicht.
Beoordelen van de kracht van het wetenschappelijke bewijs
De kracht van het wetenschappelijke bewijs werd bepaald volgens de GRADE-methode. GRADE staat voor ‘Grading Recommendations Assessment, Development and Evaluation’ (zie http://www.gradeworkinggroup.org/). De basisprincipes van de GRADE-methodiek zijn: het benoemen en prioriteren van de klinisch (patiënt) relevante uitkomstmaten, een systematische review per uitkomstmaat, en een beoordeling van de bewijskracht per uitkomstmaat op basis van de acht GRADE-domeinen (domeinen voor downgraden: risk of bias, inconsistentie, indirectheid, imprecisie, en publicatiebias; domeinen voor upgraden: dosis-effect relatie, groot effect, en residuele plausibele confounding).
GRADE onderscheidt vier gradaties voor de kwaliteit van het wetenschappelijk bewijs: hoog, redelijk, laag en zeer laag. Deze gradaties verwijzen naar de mate van zekerheid die er bestaat over de literatuurconclusie, in het bijzonder de mate van zekerheid dat de literatuurconclusie de aanbeveling adequaat ondersteunt (Schünemann, 2013; Hultcrantz, 2017).
|
GRADE |
Definitie |
|
Hoog |
|
|
Redelijk |
|
|
Laag |
|
|
Zeer laag |
|
Bij het beoordelen (graderen) van de kracht van het wetenschappelijk bewijs in richtlijnen volgens de GRADE-methodiek spelen grenzen voor klinische besluitvorming een belangrijke rol (Hultcrantz, 2017). Dit zijn de grenzen die bij overschrijding aanleiding zouden geven tot een aanpassing van de aanbeveling. Om de grenzen voor klinische besluitvorming te bepalen moeten alle relevante uitkomstmaten en overwegingen worden meegewogen. De grenzen voor klinische besluitvorming zijn daarmee niet één op één vergelijkbaar met het minimaal klinisch relevant verschil (Minimal Clinically Important Difference, MCID). Met name in situaties waarin een interventie geen belangrijke nadelen heeft en de kosten relatief laag zijn, kan de grens voor klinische besluitvorming met betrekking tot de effectiviteit van de interventie bij een lagere waarde (dichter bij het nuleffect) liggen dan de MCID (Hultcrantz, 2017).
Overwegingen (van bewijs naar aanbeveling)
Om te komen tot een aanbeveling zijn naast (de kwaliteit van) het wetenschappelijke bewijs ook andere aspecten belangrijk en worden meegewogen, zoals aanvullende argumenten uit bijvoorbeeld de biomechanica of fysiologie, waarden en voorkeuren van patiënten, kosten (middelenbeslag), aanvaardbaarheid, haalbaarheid en implementatie. Deze aspecten zijn systematisch vermeld en beoordeeld (gewogen) onder het kopje ‘Overwegingen’ en kunnen (mede) gebaseerd zijn op expert opinion. Hierbij is gebruik gemaakt van een gestructureerd format gebaseerd op het evidence-to-decision framework van de internationale GRADE Working Group (Alonso-Coello, 2016a; Alonso-Coello, 2016b). Dit evidence-to-decision framework is een integraal onderdeel van de GRADE-methodiek.
Formuleren van aanbevelingen
De aanbevelingen geven antwoord op de uitgangsvraag en zijn gebaseerd op het beschikbare wetenschappelijke bewijs en de belangrijkste overwegingen, en een weging van de gunstige en ongunstige effecten van de relevante interventies. De kracht van het wetenschappelijk bewijs en het gewicht dat door de werkgroep wordt toegekend aan de overwegingen, bepalen samen de sterkte van de aanbeveling. Conform de GRADE-methodiek sluit een lage bewijskracht van conclusies in de systematische literatuuranalyse een sterke aanbeveling niet a priori uit, en zijn bij een hoge bewijskracht ook zwakke aanbevelingen mogelijk (Agoritsas, 2017; Neumann, 2016). De sterkte van de aanbeveling wordt altijd bepaald door weging van alle relevante argumenten tezamen. De werkgroep heeft bij elke aanbeveling opgenomen hoe zij tot de richting en sterkte van de aanbeveling zijn gekomen.
In de GRADE-methodiek wordt onderscheid gemaakt tussen sterke en zwakke (of conditionele) aanbevelingen. De sterkte van een aanbeveling verwijst naar de mate van zekerheid dat de voordelen van de interventie opwegen tegen de nadelen (of vice versa), gezien over het hele spectrum van patiënten waarvoor de aanbeveling is bedoeld. De sterkte van een aanbeveling heeft duidelijke implicaties voor patiënten, behandelaars en beleidsmakers (zie onderstaande tabel). Een aanbeveling is geen dictaat, zelfs een sterke aanbeveling gebaseerd op bewijs van hoge kwaliteit (GRADE gradering HOOG) zal niet altijd van toepassing zijn, onder alle mogelijke omstandigheden en voor elke individuele patiënt.
|
Implicaties van sterke en zwakke aanbevelingen voor verschillende richtlijngebruikers |
||
|
|
Sterke aanbeveling |
Zwakke (conditionele) aanbeveling |
|
Voor patiënten |
De meeste patiënten zouden de aanbevolen interventie of aanpak kiezen en slechts een klein aantal niet. |
Een aanzienlijk deel van de patiënten zouden de aanbevolen interventie of aanpak kiezen, maar veel patiënten ook niet. |
|
Voor behandelaars |
De meeste patiënten zouden de aanbevolen interventie of aanpak moeten ontvangen. |
Er zijn meerdere geschikte interventies of aanpakken. De patiënt moet worden ondersteund bij de keuze voor de interventie of aanpak die het beste aansluit bij zijn of haar waarden en voorkeuren. |
|
Voor beleidsmakers |
De aanbevolen interventie of aanpak kan worden gezien als standaardbeleid. |
Beleidsbepaling vereist uitvoerige discussie met betrokkenheid van veel stakeholders. Er is een grotere kans op lokale beleidsverschillen. |
Organisatie van zorg
In de knelpuntenanalyse en bij de ontwikkeling van de richtlijnmodule is expliciet aandacht geweest voor de organisatie van zorg: alle aspecten die randvoorwaardelijk zijn voor het verlenen van zorg (zoals coördinatie, communicatie, (financiële) middelen, mankracht en infrastructuur). Randvoorwaarden die relevant zijn voor het beantwoorden van deze specifieke uitgangsvraag zijn genoemd bij de overwegingen. Meer algemene, overkoepelende, of bijkomende aspecten van de organisatie van zorg worden behandeld in de module Organisatie van zorg.
Commentaar- en autorisatiefase
De conceptmodule werd aan de betrokken (wetenschappelijke) verenigingen, instanties en (patiënt) organisaties voorgelegd ter commentaar. De commentaren werden verzameld en besproken met de werkgroep. Naar aanleiding van de commentaren werd de conceptmodule aangepast en definitief vastgesteld door de werkgroep. De definitieve module werd aan de deelnemende (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd voor autorisatie en door hen geautoriseerd dan wel geaccordeerd. De commentaartabel is op te vragen bij het Kennisinstituut via secretariaat@kennisinstituut.nl.
Zoekverantwoording
Zoekacties zijn opvraagbaar. Neem hiervoor contact op met de Richtlijnendatabase.
