rTMS Neglect en functiestoornissen
Uitgangsvraag
Wat is het effect van rTMS op taalvaardigheid?
Aanbeveling
Pas geen rTMS toe ter bevordering van herstel van neglect en cognitieve functiestoornissen na een herseninfarct of hersenbloeding.
Overwegingen
Voor- en nadelen van de interventie en de kwaliteit van het bewijs
Er werden zes onderzoeken (n=238) naar het effect van rTMS op herstel van neglect en andere cognitieve functiestoornissen na een herseninfarct of hersenbloeding geïdentificeerd die voldeden aan onze inclusiecriteria. Er zijn uiteenlopende resultaten gerapporteerd. Er werden klinisch relevante effecten gevonden in het voordeel van rTMS op “visuele en ruimtelijke aandacht” en “globaal cognitief functioneren” wanneer de behandeling werd toegepast binnen drie maanden na de herseninfarct/hersenbloeding. Effecten van rTMS op geheugen waren uiteenlopend. Er werden geen klinisch relevante effecten gevonden van rTMS op cognitieve functies zoals aandacht en geheugen. Alle studies waren klein met brede betrouwbaarheidsintervallen.
Waarden en voorkeuren van patiënten (en eventueel hun verzorgers)
Voor patiënten is het belangrijk dat de behandeling met rTMS veilig is en een positief resultaat oplevert. Echter op dit moment lijkt het bewijs voor de effectiviteit van de behandeling met rTMS nog zeer gering. Ook zijn er geen afzonderlijke subgroepen bekend waarbij meer effect te verwachten is. Als er toch vragen zijn van patiënten over deze behandeling dan moet duidelijk aangegeven worden dat het effect van deze behandeling op dit moment nog onduidelijk is en dat er meer onderzoek nodig is.
Rationale van de aanbeveling: weging van argumenten voor en tegen de interventies
De werkgroep is van mening dat rTMS een potentieel veelbelovende behandeling is ter verbetering van hersenfuncties na een herseninfarct of hersenbloeding. Er is vooral bewijs voor effect van vormen van ‘inhiberende’ rTMS van de gezonde hemisfeer, al dan niet in combinatie met stimulatie van hersengebieden in de aangedane hemisfeer. De bewijskracht voor alle effecten is echter laag tot zeer laag en vooral aangetoond op niveau van lichaamsfuncties en op niveau van activiteiten dan wel vaardigheden. De totale bewijskracht is zeer laag. Om aan te tonen of rTMS daadwerkelijk effectief is en welke patiënten het meest baat hebben van rTMS, in welke fase en met welke vorm van rTMS, zijn kwalitatief hoogwaardige fase III en IV-trials nodig. Er is momenteel nog geen bewijs voor of rTMS vooraf, tijdens of na oefentherapie het beste gegeven kan worden.
Onderbouwing
Conclusies / Summary of Findings
1. Conclusions rTMS ≤ 3 months after stroke onset
1.1 Visual and spatial attention (crucial)
|
Very low GRADE |
The evidence is very uncertain about the effect of low frequency rTMS on visual and spatial attention when compared with sham stimulation in patients within three months after stroke.
Sources: (Cha, 2016; Iwánski, 2020) |
1.2 Global cognitive functioning (crucial)
|
Low GRADE |
The evidence suggests that low frequency rTMS may improve global cognitive functioning when compared with sham stimulation in patients within three months after stroke.
Sources: (Lu, 2015) |
|
Low GRADE |
The evidence suggests that high frequency rTMS may improve global cognitive functioning when compared with sham stimulation in patients within three months after stroke.
Sources: Yin, 2020 |
1.3 Memory (crucial)
|
Low GRADE |
The evidence suggests that low frequency rTMS may improve short- and long-term memory when compared with sham stimulation in patients within three months after stroke.
Sources: (Lu, 2015) |
|
Low GRADE |
The evidence suggests that high frequency rTMS does not improve or worsen memory when compared with sham stimulation in patients within three months after stroke.
Sources: (Yin, 2020) |
1.4 Executive functioning (crucial)
|
Low GRADE |
The evidence suggests that high frequency rTMS does not improve or worsen executive functioning when compared with sham stimulation in patients within three months after stroke.
Sources: (Yin, 2020) |
2. Conclusions rTMS >3 months after stroke onset
2.1 Visual and spatial attention (crucial)
|
Low GRADE |
The evidence suggests that high frequency rTMS may improve visual and spatial attention when compared with sham stimulation in patients beyond three months after stroke.
Sources: (Liu, 2020) |
2.2 Global cognitive functioning (crucial)
|
Low GRADE |
The evidence suggests that low frequency rTMS over the lesion hemisphere does not improve or worsen global cognitive functioning when compared with sham stimulation in patients beyond three months after stroke.
Sources: (Tsai, 2020) |
|
Low GRADE |
The evidence suggests that high frequency rTMS may improve global cognitive functioning when compared with sham stimulation in patients beyond three months after stroke.
Sources: (Liu, 2020) |
|
Low GRADE |
The evidence suggests that iTBS over the lesion hemisphere may improve global cognitive functioning when compared with sham stimulation in patients beyond three months after stroke.
Sources: (Tsai, 2020) |
2.3 Memory (crucial)
|
- GRADE |
There were no studies describing the effect of rTMS on memory when compared with sham stimulation in patients beyond three months after stroke. |
2.4 Executive functioning (crucial)
|
- GRADE |
There were no studies describing the effect of rTMS on executive functioning when compared with sham stimulation in patients beyond three months after stroke. |
Samenvatting literatuur
Description of studies
As a starting point, we included studies from the review from Lieshout (2019). This systematic review describes the effects of noninvasive brain stimulation on poststroke cognitive function. In total, 40 studies were included, from which were 21 RCTs, nine studies with a crossover design and ten studies with other designs. To answer our clinical question and based on the selection criteria for this module, data of two RCTs were extracted from this review (Cha, 2016; Lu, 2015).
In addition, four separate RCTs were included in the analysis of the literature (Iwanski, 2020; Yin, 2020; Liu, 2020; Tsai, 2020). rTMS treatment can be performed at different time points after stroke onset. We distinguished between treatment within or at three months after stroke onset and treatment more than three months after stroke onset.
1. Start of treatment ≤ 3 months after stroke onset
From the review of Lieshout (2019), both RCTs described the effects of rTMS treatment in patients who were treated ≤ 3 months after stroke onset (Cha, 2016; Lu, 2016). Cha (2016) applied low frequency stimulation over the right posterior parietal areas (P3 and P4) to patients with ischaemic/haemorrhagic stroke (stroke side unknown) for four weeks, five times each week and 10 minutes each day. Lu (2015) applied low frequency rTMS at the right side of the dorsolateral prefrontal cortex to patients with left or right ischaemic/haemorrhagic stroke once a day, five days per week for four weeks.
Apart from the studies included in the review, two additional RCTs described the effect of rTMS treatment in patients who were treated ≤ 3 months after stroke onset (Iwanski, 2020; Yin, 2020).
Iwánski (2020) performed a randomized controlled trial, and evaluated the effects of 1Hz rTMS of the left angular gyrus combined with visuospatial therapy in post-stroke neglect. A total of 28 patients (mean age 65y; 79% male; 7% haemorrhagic stroke; 100% right side) were randomly allocated to two groups. The intervention group (n=14) received 30 minutes of rTMS (1Hz, 90% RMT, 1800 pulses) over the left angular gyrus (unaffected hemisphere). The control group (n=14) received sham stimulation over the same area. The effects were evaluated on patients’ visual and spatial attention, assessed with the behavioral inattention test, after the intervention.
Yin (2020) performed a randomized clinical trial, and evaluated the effects of rTMS on cognitive impairment and resting-state brain activity in stroke patients. A total of 34 patients with cognitive impairment after stroke (mean age 57y; 88% male; 32% haemorrhagic stroke; 38% right side) were randomly allocated to two groups. The intervention group (n=16) received 20 sessions of 10Hz rTMS over the left dorsal lateral prefrontal cortex and the control group (n=18) received no stimulation over the same area. All patients were treated for four weeks (once a day, 5 days per week) and received a 30-minute computer-assisted cognitive rehabilitation. The effects were evaluated on patients’ global cognitive functioning (assessed with the MoCA); and memory (assessed with the RBMT/VST) after two weeks and after four weeks of treatment.
2. Start of treatment beyond three months after stroke onset
From the review of Lieshout (2019), no RCTS described the effects of rTMS treatment in patient who were treated beyond three months after stroke onset. However, we found two separate RCTs (Liu, 2020; Tsai, 2020).
Liu (2020) performed a randomized controlled trial, and evaluated the effects of TMS on the performance of the activities of daily living and attention function after stroke. A total of 62 patients (mean age 58y; 45% male; 40% haemorrhagic stroke; 57% right side) were randomly allocated to two groups. The intervention group received TMS (10Hz, 700 pulses) over the left dorsolateral prefrontal cortex five times a week for four weeks. The control group received sham TMS for four weeks. All patients received an additional 30-minute comprehensive cognitive training once daily, five times a week for four weeks . Four patients did not finish the treatment, so 58 (29 per group) patients finished the four-week treatment program and end-point examinations. The effects were evaluated on patients’ visual and spatial attention assessed with the digit symbol test/digital span test and trail making test); and global cognitive functioning (assessed with the MMSE).
Tsai (2020) performed a randomized controlled trial, and evaluated the effects of rTMS and iTBS on cognitive impairment after stroke. A total of 41 patients (mean age 58y; 80% male; 51% haemorrhagic stroke; 0% right side) were randomly allocated to three groups. Each patient received 10 stimulation sessions over the affected hemisphere and were treated for 10 days in the morning from Monday to Friday for two consecutive weeks. The rTMS group received 5Hz of rTMS. The iTBS group received three pulses of 50Hz bursts repeated at 5Hz for 10 seconds. For the sham group a placebo coil was used, delivering less than 5% of the magnetic output. The effects were evaluated on patients’ global cognitive functioning (assessed with the RBANS).
Results
1. Start of treatment ≤ 3 months after stroke onset
1.1 Visual and spatial attention (crucial)
Two RCT’s described visual and spatial attention in patients who were treated within three months after stroke onset (Cha, 2016; Iwánski, 2020).
1.1.1 LF rTMS
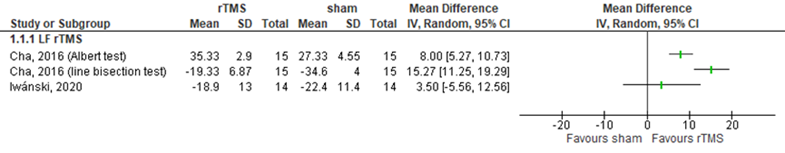
Cha (2016) assessed visual and spatial attention in patients wo received LF rTMS over the right posterior parietal areas (stroke side unknown) by the line bisection test (lower score means better outcome) and by the Albert test (higher score means better outcome) (n=30). Results on the line bisection test showed a score of 19.33 cm (SD 6.87 cm) in the intervention group, compared to 34.60 cm (SD 4.00 cm) in the control group. This difference was statistically significant and clinically relevant in favour of the intervention group. Results on the Albert test showed a score of 35.33% (SD 2.9) in the intervention group, compared to 27.33% (SD 4.55) in the control group. This difference was statistically significant and clinically relevant.
Iwánski (2020) assessed attention in patients who received LF rTMS over the unaffected hemisphere by two subtests of the behavioral inattention test (lower score means better outcome) (n=28). At the behavioral subtest (range 1 to 81), the intervention group and the control group scored 18.9 (SD 13) and 22.4 (SD 11.4) respectively. Both differences were not statistically significant nor clinically relevant. Results are shown in figure 15.
The level of evidence in the literature
The level of evidence regarding the outcome visual and spatial attention started at high because it was based on randomized controlled trials, but was downgraded by three levels due to limited number of included studies and low number of included patients (-2, imprecision) and statistical heterogeneity (-1, inconsistency). The final GRADE level of evidence of LF rTMS within three months after stroke onset regarding the outcome visual and spatial attention is very low.
Figure 15 Forest plot summarizing the effect of low frequency rTMS (LF rTMS) on visual and spatial attention, assessed by the Albert test, line bisection test and the behavioral inattention test in ischaemic/ haemorrhagic stroke patients who received treatment within three months after stroke onset
1.2 Global cognitive functioning (crucial)
Two RCTs assessed global cognitive functioning in patients who were treated within three months after stroke onset (Lu, 2015; Yin, 2020).
1.2.1 LF rTMS
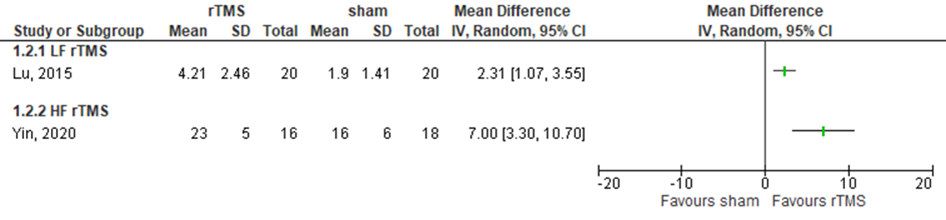
Lu (2015) assessed global cognitive functioning in patients who received LF rMS over the right dorsolateral prefrontal cortex (affected or unaffected side) by the MoCA test (range 0 to 30, higher score means better outcome) (n=40). The intervention group showed a change of 4.21 (SD 2.46) from baseline to three days post treatment, compared to a change of 1.90 (SD 1.41) in the control group. This difference was statistically significant and clinically relevant in favour of the intervention group. Results are shown in figure 16.
The level of evidence in the literature
The level of evidence regarding the outcome global cognitive functioning started at high because it was based on a randomized controlled trial, but was downgraded by two levels due to limited number of included studies and low number of included patients (-2, imprecision). The final GRADE level of evidence of HF rTMS within three months fer stroke onset regarding the outcome global cognitive functioning is low.
1.1.2 HF rTMS
Yin (2020) assessed global cognitive functioning in patients who received HF rTMS over the left dorsal lateral prefrontal cortex (affected or unaffected side) by the MoCA test (range 0 to 30, higher score means better outcome) (n=34). Results showed a score of approximately 23 (SD 5) in the intervention group, compared to 18 (SD 6) in the control group (based on estimated scores from figure 2a in the article of Yin (2020). This difference was statistically significant and clinically relevant in favour of the intervention group. Results are shown in figure 16.
The level of evidence in the literature
The level of evidence regarding the outcome global cognitive functioning started at high because it was based on a randomized controlled trial, but was downgraded by two levels due to limited number of included studies and low number of included patients (-2, imprecision). The final GRADE level of evidence of HF rTMS within three months after stroke onset regarding the outcome global cognitive functioning is low.
Figure 16 Forest plot summarizing the effect of low frequency rTMS (LF rTMS) and high frequency rTMS (HF rTMS) on global cognitive functioning in ischaemic/ haemorrhagic stroke patients who received treatment within three months after stroke onset
1.3 Memory (crucial)
Two RCTs assessed memory in patients who were treated within three months after stroke onset (Lu, 2015; Yin, 2020).
1.3.1 LF rTMS
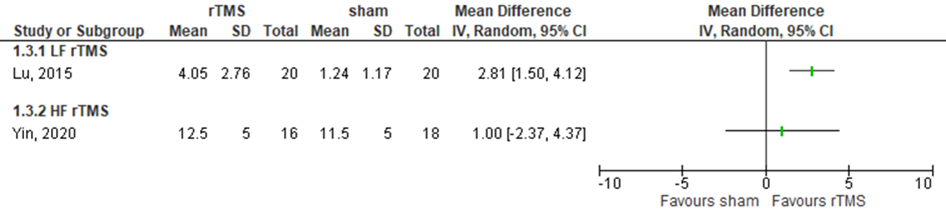
Lu (2015) assessed memory in patients who received LF rTMS over the right dorsolateral prefrontal cortex (affected or unaffected side) by the RBMT (range 0 to 24, higher score means better outcome) (n=40). The intervention group showed a change of 4.05 (SD 2.76) from baseline to three days post treatment, compared to a change of 1.24 (SD 1.17) in the control group. This difference was statistically significant and clinically relevant in favour of the intervention group. Results are shown in figure 17.
The level of evidence in the literature
The level of evidence regarding the outcome memory started at high because it was based on a randomized controlled trial, but was downgraded by two levels due to limited number of included studies and low number of included patients (-2, imprecision). The final GRADE level of evidence of LF rTMS within three months after stroke onset regarding the outcome memory is low.
1.3.2 HF rTMS
Yin (2020) assessed memory in patients who received HF rTMS over the left dorsal lateral prefrontal cortex (affected or unaffected side) by the RBMT (range 0 to 24, higher score means better outcome) (n=34). Results showed a mean score of 12.5 (SD 5) in the intervention group, compared to 11.5 (SD 5) in the control group (based on estimated scores from figure 2b in the article of Yin (2020)). This difference was not statistically significant nor clinically relevant. Results are shown in figure 17.
The level of evidence in the literature
The level of evidence regarding the outcome memory started at high because it was based on a randomized controlled trial, but was downgraded by two levels due to limited number of included studies and low number of included patients (-2, imprecision). The final GRADE level of evidence of HF rTMS within three months after stroke onset regarding the outcome memory is low.
Figure 17 Forest plot summarizing the effect of low frequency rTMS (LF rTMS) and high frequency rTMS (HF rTMS) on memory in ischaemic/ haemorrhagic stroke patients who received treatment within three months after stroke onset
1.4 Executive functioning (crucial)
1.4.1 HF rTMS
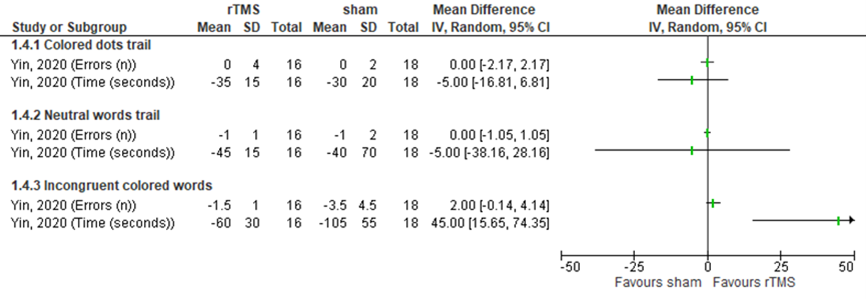
Yin (2020) assessed executive functioning in patients who received HF over the left dorsal lateral prefrontal cortex (affected or unaffected side) by the VST, consisting of three test cards: 1. colored dots trail; 2. neutral words trail; 3. incongruent colored words trail (24 words/dots each). Results were assessed by the number of errors when reading (n) and the time consumed (s) (lower scores mean better outcomes). Results are based on estimations from figure 2 D-I in the article of Yin (2020) and expressed in table 18. Differences were not statistically significant nor clinically relevant. Results are shown in figure 19.
The level of evidence in the literature
The level of evidence regarding the outcome executive functioning started at high because it was based on a randomized controlled trial, but was downgraded by two levels due to limited number of included studies and low number of included patients (-2, imprecision). The final GRADE level of evidence of HF rTMS within three months after stroke onset regarding the outcome executive functioning is low.
Table 18 Effect of HF rTMS on executive functioning, assessed by the VST
|
|
Colored dots trail |
Neutral words trail |
Incongruent colored words |
|
Time consumed (s) |
I: 35 (SD 15) C: 30 (SD 20) |
I: 45 (SD 15) C: 40 (SD 70) |
I: 60 (SD 30) C: 105 (SD 55) |
|
Error words (n) |
I: 0 (SD 4) C: 0 (SD 2) |
I: 1 (SD 1) C: 1 (SD 2) |
I: 1.5 (SD 1) C: 3.5 (SD 4.5) |
Figure 19 Forest plot summarizing the effect of high frequency rTMS (HF rTMS) on executive functioning, assessed by the colored dots trail, the neutral words trail and the incongruent words tail (number of errors and time) in ischaemic/haemorrhagic stroke patients who received treatment within three months after stroke onset
2. Start of treatment beyond three months after stroke onset
2.1 Visual and spatial attention
2.1.1 HF rTMS
Liu (2020) assessed visual and spatial attention in patients who received HF rTMS over the left dorsolateral prefrontal cortex (affected or unaffected side) by the Digit Symbol Test (range 0 to 100, higher score means better outcome) (n=58). The intervention group had a mean score of 8.59 (SD 4.18), compared to 5.31 (SD 3.09) in the control group. This difference was statistically significant and clinically relevant in favour of the intervention group. Data is shown in figure 20.
The level of evidence in the literature
The level of evidence regarding the outcome visual and spatial attention started at high because it was based on a randomized controlled trial, but was downgraded by two levels due to limited number of included studies and low number of included patients (-2, imprecision). The final GRADE level of evidence of HF rTMS beyond three months after stroke onset regarding the outcome visual and spatial attention is low.
Figure 20 Forest plot summarizing the effect high frequency rTMS (HF rTMS) on visual and spatial attention, assessed by the Digit Symbol Test in ischaemic/ haemorrhagic stroke patients who received treatment beyond three months after stroke onset

2.2 Global cognitive functioning
2.2.1 LF rTMS
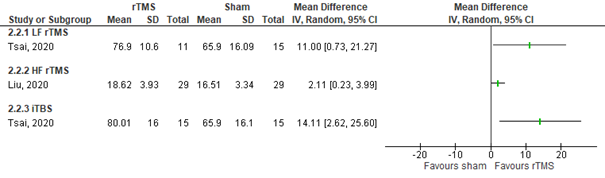
Tsai (2020) assessed global cognitive functioning in patients who received LF rTMS (5 Hz) over the affected hemisphere by the RBANS total (range 40 to 160, higher score means better outcome) (n=26). The intervention group had a mean score of 76.9 (SD 10.6) compared to 65.9 (SD 16.09) in the control group. This difference was not statistically significant nor clinically relevant. Data is shown in figure 21.
The level of evidence in the literature
The level of evidence regarding the outcome global cognitive functioning started at high because it was based on a randomized controlled trial, but was downgraded by two levels due to limited number of included studies and low number of included patients (-2, imprecision). The final GRADE level of evidence of LF rTMS beyond three months after stroke onset regarding the outcome global cognitive functioning is low.
2.2.2 HF rTMS
Liu (2020) assessed global cognitive functioning in patients who received HF rTMS over the left dorsolateral prefrontal cortex (affected or unaffected side) by the MMSE (range 0 to 30, higher score means better outcome) (n=58). The intervention group had a mean score 18.62 (SD 3.93) compared to 16.51 (SD 3.34) in the control group. This difference was statistically significant and clinically relevant in favour of the intervention group. Data is shown in figure 21.
The level of evidence in the literature
The level of evidence regarding the outcome global cognitive functioning started at high because it was based on a randomized controlled trial, but was downgraded by two levels due to limited number of included studies and low number of included patients (-2, imprecision). The final GRADE level of evidence of HF rTMS beyond three months after stroke onset regarding the outcome global cognitive functioning is low.
2.2.3 iTBS
Tsai (2020) assessed global cognitive functioning in patients who received iTBS over the affected hemisphere by the RBANS total (range 40 to 160, higher score means better outcome) (n=30). The intervention group had a mean score of 80.1 (SD 16) compared to 65.9 (SD 16.1) in the control group. This difference was statistically significant and clinically relevant in favour of the intervention group. Data is shown in figure 21.
The level of evidence in the literature
The level of evidence regarding the outcome global cognitive functioning started at high because it was based on a randomized controlled trial, but was downgraded by two levels due to limited number of included studies and low number of included patients (-2, imprecision). The final GRADE level of evidence of iTBS beyond three months after stroke onset regarding the outcome global cognitive functioning is low.
Figure 21 Forest plot summarizing the effect of low frequency rTMS (LF rTMS), high frequency rTMS (HF rTMS) and intermittent theta burst stimulation (iTBS) on global cognitive functioning, assessed by the RBANS/MMSE in ischaemic/ haemorrhagic stroke patients who received treatment beyond three months after stroke onset
2.3 Memory (crucial)
Memory was not assessed as an outcome in the included studies on rTMS treatment beyond three months after stroke onset.
2.4 Executive functioning (crucial)
Executive functioning was not assessed as an outcome in the included studies on rTMS treatment beyond three months after stroke onset.
Zoeken en selecteren
A systematic review of the literature was performed to answer the following question:
What is the effect of rTMS on visual and spatial attention, global cognitive functioning, memory and executive functioning in patients after stroke?
P: patients with ischaemic/haemorrhagic stroke with neglect and other cognitive functions;
I: non-invasive brain stimulation with repetitive transcranial magnetic stimulation (rTMS);
C: sham rTMS;
O: visual and spatial attention, global cognitive functioning, memory and executive functioning.
In het literature rTMS treatment was applied at different time points after stroke onset. On
the basis of a critical time window of spontaneous neurological recovery of maximal 3
months (Bernhardt, 2017), we decided to distinguish between treatment ≤3 months after
stroke onset and treatment >3 months after stroke onset. Within this distinguishment, the
effects were evaluated per intervention type (i.e., low frequency, high frequency, the
combination of both frequencies and intermittent theta burst stimulation), according to Pino (2014). This resulted in the following
(sub-)groups:
a. Start of treatment at or within three months after ischaemic/haemorrhagic stroke:
- low frequency rTMS (< 5Hz);
- high frequency rTMS (≥ 5 Hz);
- combining low frequency rTMS and high frequency rTMS (LF-HF rTMS);
- cerebellar intermittent theta-burst stimulation (CRB-iTBS).
b. Start of treatment beyond three months after ischaemic/haemorrhagic stroke:
- low frequency rTMS (<5Hz);
- high frequency rTMS (≥5 Hz);
- combining low frequency rTMS and high frequency rTMS (LF-HF rTMS);
- cerebellar intermittent theta-burst stimulation (CRB-iTBS).
Relevant outcome measures
The working group considered all outcome measures critical for decision-making.
Definitions
The working group classified the used outcome measures following the codes of the International Classification of Functioning, Disability and Health (ICF) in the following groups: (Steiner, 2002):
1. Visual and spatial attention: Catherine Bergego Scale (CBS), Vienna Test System, Paper-pencil assessment, attention score, Attention Matrices, Copy of Figure, visual search and cancellation task, Trail Making Test (TMT), Star Cancellation Test (SCT), Line Bisection Test (LBT), letter cancellation test, figure and shape copying test, Albert test, center of cancellation score, X-position of leftmost cancelled target, number of cancelled targets, digit span test, structured cancellation test, letter-structure cancellation test, behavioural inattention test, NIHSS scale (cognitive/neglect domains), visual search and cancellation task, figure and shape copying test.
2. Global cognitive functioning: Montreal Cognitive Assessment (MoCA), Loewenstein Occupational Therapy of Cognitive Assessment (LOTCA), Repeatable Batter for the Assessment of Neuropsychological Status (RBANS), Mini Mental State Examination (MMSE), Korean version of the MMSE, neuropsychological test battery.
3. Memory: Rivermead Behaviour Memory Test (RBMT), Memory function, Forward and Backward Digit Span Test (FDST/BDST), Forward and Backward Visual Span Test (FVST/BVST), short story test, Figural Memory Level (FML), Visual Learning Test-delayed Recall (ViLT-R), Verbal Learning Test-delayed Recall (VeLT-R).
4. Executive functioning: Victoria Stroop Test, copy of figure, logic reasoning level (LRL).
The working group defined a difference of 10% on each test scale as a clinically important difference. For standardized mean differences (SMD), results were clinically relevant if they were smaller than -0.5 or higher than 0.5.
Search and select (Methods)
The databases Medline (via OVID) and Embase (via Embase.com) were searched with relevant search terms until October 22, 2020. The detailed search strategy is depicted under the tab Methods. The systematic literature search resulted in 798 hits. Studies were selected based on the following criteria:
• Patients with ischaemic/haemorrhagic stroke.
• RCTs and SRs about non-invasive brain stimulation with rTMS.
• Subgroups with low frequency rTMS (LF-rTMS), high frequency rTMS (HF-rTMS), intermittent theta burst stimulation (iTBS), paired associative stimulation (PAS), transcranial rotating permanent magnet stimulation (TRMS), short inter-train interval (ITI) rTMS and long ITI rTMS.
• A control group receiving sham rTMS.
• More than 10 patients per treatment arm.
• For cross-over studies: a baseline measurement and one at the first cross-over point.
• A description of at least one outcome measure, as described in the PICO.
18 systematic reviews and randomized controlled trials were initially selected based on title and abstract. After reading the full-text, 13 studies were excluded (see table with reasons for exclusion under the tab Methods) and five studies were included, including one systematic review and four RCTs.
Results
Five studies were included in the analysis of the literature, including one systematic review and four RCTs. The most important study characteristics and results are included in the evidence-tables. The judgement of the individual studies (risk of bias) are included in risk-of-bias tables.
Referenties
- Di Pino G, Pellegrino G, Assenza G, Capone F, Ferreri F, Formica D, Ranieri F, Tombini M, Ziemann U, Rothwell JC, Di Lazzaro V. Modulation of brain plasticity in stroke: a novel model for neurorehabilitation. Nat Rev Neurol. 2014 Oct;10(10):597-608. doi: 10.1038/nrneurol.2014.162. Epub 2014 Sep 9. PMID: 25201238.
- Iwański S, Leśniak M, Polanowska K, Bembenek J, Czepiel W, Seniów J. Neuronavigated 1 Hz rTMS of the left angular gyrus combined with visuospatial therapy in post-stroke neglect. NeuroRehabilitation. 2020;46(1):83-93. doi: 10.3233/NRE-192951. PMID: 32039875.
- van Lieshout ECC, van Hooijdonk RF, Dijkhuizen RM, Visser-Meily JMA, Nijboer TCW. The Effect of Noninvasive Brain Stimulation on Poststroke Cognitive Function: A Systematic Review. Neurorehabil Neural Repair. 2019 May;33(5):355-374. doi: 10.1177/1545968319834900. Epub 2019 Apr 25. PMID: 31021702.
- Liu Y, Yin M, Luo J, Huang L, Zhang S, Pan C, Hu X. Effects of transcranial magnetic stimulation on the performance of the activities of daily living and attention function after stroke: a randomized controlled trial. Clin Rehabil. 2020 Dec;34(12):1465-1473. doi: 10.1177/0269215520946386. Epub 2020 Aug 4. PMID: 32748630.
- Tsai PY, Lin WS, Tsai KT, Kuo CY, Lin PH. High-frequency versus theta burst transcranial magnetic stimulation for the treatment of poststroke cognitive impairment in humans. J Psychiatry Neurosci. 2020 Jul 1;45(4):262-270. doi: 10.1503/jpn.190060. PMID: 32159313; PMCID: PMC7828923.
- Yin M, Liu Y, Zhang L, Zheng H, Peng L, Ai Y, Luo J, Hu X. Effects of rTMS Treatment on Cognitive Impairment and Resting-State Brain Activity in Stroke Patients: A Randomized Clinical Trial. Front Neural Circuits. 2020 Sep 30;14:563777. doi: 10.3389/fncir.2020.563777. PMID: 33117131; PMCID: PMC7561423.
- Yin M, Liu Y, Zhang L, Zheng H, Peng L, Ai Y, Luo J, Hu X. Effects of rTMS Treatment on Cognitive Impairment and Resting-State Brain Activity in Stroke Patients: A Randomized Clinical Trial. Front Neural Circuits. 2020 Sep 30;14:563777. doi: 10.3389/fncir.2020.563777. PMID: 33117131; PMCID: PMC7561423.
Evidence tabellen
|
Study reference |
Study characteristics |
Patient characteristics |
Intervention (I) |
Comparison / control (C) |
Follow-up |
Outcome measures and effect size |
Comments |
|
Tung, 2019 |
SR and meta-analysis of RCTs Literature search up to January 2019 A: Wang, 2012
Study design: A: RCT
Setting and Country:
Source of funding and conflicts of interest:
The author(s) received no financial support for the research, authorship, and/or publication of this article. The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. |
Inclusion criteria SR: 1) the patients were diagnosed with stroke; (2) the outcomes included the effects of rTMS on 1104 Clinical Rehabilitation 33 lower limb motor function; and (3) they were randomized controlled trials. Exclusion criteria SR: We excluded articles with only protocols and non-peer-reviewed articles, such as conference papers and letters to the editor. Randomized controlled trials that investigated the effect of combined treatments, where the effects of rTMS could not be isolated, were also excluded. 3 studies included Important patient characteristics at baseline: N, mean age A: 28 patients, 63.9 yrs. Groups comparable at baseline? Yes |
Describe intervention:
A: 10 10-minute sessions of rTMS + 30-minute task-oriented training
|
Describe control:
A: 10 10-minute sessions of sham rTMS + 30-minute task-oriented training |
End-point of follow-up:
A: Posttreatment
For how many participants were no complete outcome data available? (intervention/control) A: 2/2
|
≤ 3 months Walking
Maintaining standing balance
Muscle power/synergies Effect measure: median, (IQR): HF-rTMS
Sitting balance
Transferring oneself Effect measure: RR (95% CI): Walking long distances Walking speed Falling > 3 months Walking HF-rTMS Maintaining standing balance Muscle power/synergies Effect measure: SMD (95% CI) LF-rTMS HF-rTMS Sitting balance Walking speed Effect measure: MD (95% CI) LF-rTMS HF-rTMS Falling |
Author’s conclusion In conclusion, rTMS may exhibit short-term beneficial effects on the lower limbs of patients with stroke, and it is safe for application. The long-term effects of rTMS on the lower limbs could not be discerned from this meta-analysis. Therefore, additional high-quality, large-scale randomized controlled trials are required to clarify both the long-term effects of and standard protocols for rTMS on the lower limbs of patients with stroke.
|
|
Van Lieshout, 2019 |
SR and meta-analysis of RCTs and cross-over studies.
Literature search up to February 2018
A: Cha, 2016
Study design: A: RCT
Setting and Country: Department of Radiology and Imaging Institute of Rehabilitation and Development of Brain Function, The Second Clinical Medical College of North Sichuan Medical College Nanchong Central Hospital, Nanchong, China
Source of funding and conflicts of interest: The authors declare that they have no conflicts of interest. |
Inclusion criteria SR: In adult patients (>18 years) with stroke (population), does rTMS aimed at improvement of upper limb function (intervention) as compared with sham rTMS or no rTMS (comparison) improve function or activity of the upper limb (outcome) Exclusion criteria SR: Studies were excluded if rTMS was part of a coupling/primingprotocol or if it was bilateral; if there was no upper limb outcome or stroke severity scale measurement (e.g.,NIHSS score) as outcome assessment; or if information required to perform a meta-analysis (e.g., mean scores, standard deviations) was missing.
38 studies included
Important patient characteristics at baseline:
N, mean age A: 30, 63.7 yrs Groups comparable at baseline? No |
Describe intervention:
A: 1Hz, 90% RMT, 1,200 pulses, 20 sessions Right hemisphere, P3 10/20 EEG system. |
Describe control:
A: Sham coil |
End-point of follow-up:
A: After treatment (4 wk)
For how many participants were no complete outcome data available? (intervention/control) A: n.r.
|
≤ 3 months Upper limb capacity LF-rTMS Pooled effect (random effects model): 0.45 (95% CI -0.17 to 1.07) favouring rTMS. Heterogeneity (I2): 88% HF-rTMS
Total pooled effect (random effects model): 0.68 (95% CI 0.02 to 1.35) favoring rTMS. Heterogeneity (I2): 77%
Upper limb muscle synergies LF-rTMS
Pooled effect (random effects model): 0.46 (95% CI -0.29 to 1.21) favoring rTMS
HF-rTMS Total pooled effect (random effects model): 0.39 (95% CI -0.23 to 1.02) favouring rTMS
Strength LF-rTMS
Pooled effect (random effects model): 0.39 (95% CI -0.39 to1.16) favouring rTMS
HF-rTMS
Pooled effect (random effects model): 0.74 (95% CI -0.02 to1.49) favouring rTMS
Total pooled effect (random effects model): 0.58 (95% CI -0.09 to1.07) favouring rTMS
Activities of daily living LF-rTMS
Pooled effect (random effects model model): 2.86 (95% CI -0.84 to 6.55) favouring rTMS
HF-rTMS
Total pooled effect (random effects model model): 1.79 (95% CI: 0.24 to 3.35) favouring rTMS
>3 months Upper Limb Capacity LF-rTMS
Pooled effect (random effects model model): -0.02 (95% CI: -1.91 to 1.87) favouring sham
iTBS
Total pooled effect (random effects model model): 0.22 (95% CI: -0.50 to 0.94) favouring rTMS
Upper limb muscle synergies LF-rTMS
Strength
Activities of daily living |
Facultative: rTMS treatment within the first month after stroke seems more beneficial in increasing upper limb function than after 1–3 months or in the chronic phase post-stroke (>6 months). Improvements after rTMS can most likely be detected with outcome measures assessing body functions, like the FMA score, than tests at the level of activity (e.g., JTT, ARAT). However, rTMS treatment studies in stroke patients are highly heterogeneous, with varying outcome measures and relatively small sample sizes. Another source of uncertainty is that we are unable to identify whether improved outcomes were primarily caused by rTMS per se or by rTMS in combination with an additional therapy (of a certain intensity). Further research and international cooperation should be undertaken to develop a standardized, core set of measurements for testing upper limb function. We recommend to conduct measurements at the different levels of function, activity (and participation). Future studies should incorporate these standardized tests, include a follow-up measurement at 3months after stroke onset (if the trial starts within 1-month post-stroke), and report their findings in a uniform manner (e.g., using final scores or change scores, and subtest scores). High heterogeneity and wide confidence intervals of effect sizes were found for some analyses on activity outcome measures, which could also account for the absence of rTMS effects in activity. |
|
Bucur, 2019
|
SR and meta-analysis of RCTs
Literature search up to February 2019
A: Hu, 2018 C: Seniow, 2013 D: Tsai, 2014 E: Waldowski, 2012 F: Wang, 2014
Study design: A: RCT C: RCT (pilot) D: RCT E: RCT F: RCT
Setting and Country: A: B: C: D: Taiwan E: Poland F: Taiwan
Source of funding and conflicts of interest: n.r.
|
Inclusion criteria SR: interventions designed for adults with post-stroke aphasia; rTMS or tDCS stimulation studies (alone or combined with other therapies); rTMS or tDCS were specified as the main intervention/ treatment, ; cephalic stimulation designs only (at least one electrode was positioned on the scalp, the reference electrode could be extra-cephalic, such as on a shoulder); minimum 4 weeks (1 month) of follow-up; at least four aphasic participants; peer-reviewed publications; only RCTs or crossover designs; published in English; when several articles derived from the same study, either with increased recruitment or extended follow-up evaluations, we chose the one with the higher number of participants and the most complete data reported at follow-up.
Exclusion criteria SR: interventions designed for other types of post-stroke disorders or aphasia not due to stroke; other types of brain stimulations such as transcranial random noise stimulation, electroconvulsive therapy, et cetera.; open-label studies, e.g. (55–57); studies involving less than 3 stimulation sessions over the same cortical region per patient; extra-cephalic stimulation sites, e.g.; a short follow-up period (less than one month), e.g. ; case reports and research studies with less than four participants; articles from the gray literature (i.e., literature that is not formally published in sources such as books or journal articles, e.g., unpublished Ph.D. thesis); presentations from international meetings with no specific data provided, perspective and opinion publications, case reports, series of cases, previous reviews or meta-analyses; studies not published in or translated into English; studies that did not provide adequate information to analyse treatment effects (i.e., when we could not extract useful quantitative data) and we got no reply from the authors.
16 studies included
Important patient characteristics at baseline:
N, mean age A: C: 40, D: 56, 62.5y E: 26, 61.2y F: 30, 60.9y
Sex: A: % Male C: D: 26.8% Male E: 50% Male F: 90% Male
Groups comparable at baseline? |
Describe intervention:
A: a high-frequency rTMS (HF-rTMS) group (10 Hz), a low-frequency rTMS (LF-rTMS) group (1 Hz) C: 3-week aphasia rehabilitation protocol in combination with real rTMS D: Group A (n = 33), who underwent 10 sessions of 1-Hz rTMS over the contralesional pars triangularis (PTr) E: speech and language therapy combined with real rTMS F: the TMSsyn group and underwent synchronous picture-naming training together with contralesional 1 Hz-rTMS for 10 daily sessions.
|
Describe control:
A: sham stimulation group C: 3-week aphasia rehabilitation protocol in combination with sham rTMS D: Group B (n = 23), who received sham 1-Hz stimulation E: speech and language therapy combined with sham rTMS F: the TMS sham group received concurrent naming task along with the sham 1 Hz-rTMS.
|
End-point of follow-up:
A: post treatment and 2 months post-treatment C: Immediately after therapy, and 15 weeks after completing treatment. D: on the day after the 10th session (post 1), and at 3 months after the last intervention session (post 2) E: after treatment F: immediately, and after 3 months of the intervention
For how many participants were no complete outcome data available? (intervention/control) A: B: C: D: 2/1 E: 0/0 F: 0/1
|
≤ 3 months Functional communication LF-rTMS Verbal comprehension n.r.
Expressive naming LF-rTMS >3 months Functional communication
Verbal comprehension
Defined by the picture naming test. Effect measure: mean difference (95% CI):
LF-rTMS A: n.r. Total pooled effect (random effects model model): 15.73 (95% CI: -5.06 to 26.41) favouring rTMS
|
Conclusion In conclusion, each technique has advantages and disadvantages: rTMS seems more effective but also more expensive and with a higher safety risk, while tDCS appears less effective but is user-friendly and could be applied at home with a relatively small cost. For these reasons, further evaluation of the utility of these methods for aphasia rehabilitation should combine efficacy and feasibility data, making a cost benefit analysis possible. Still, in the future the most important challenge will be to collect clear evidence of the long-term efficacy in the everyday life of these methods.
Risk of bias A: Moderate (sequence allocation, loss to follow-up, selective outcome reporting, trial ended early) C: High (all domains) D: High (all domains) E: High (all domains) F: High (all domains)
|
|
Van Lieshout, 2019 |
SR and meta-analysis of RCTs, cross-over design trials, case studies and mixed design studies.
Literature search up to January 2018
A: Cha, 2016 B: Lu, 2015
Study design: A: RCT Setting and Country: A: Korea Source of funding and conflicts of interest: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Netherlands Organization for Scientific Research (VICI 016.130.662). |
Inclusion criteria SR: 1) patients with ischaemic or haemorrhagic stroke; (2) age ≥ 18 years; (3) the use of NIBS (TMS, TBS, or tDCS); (4) objective, standardized tests or test batteries for assessment of cognitive function; and (5) baseline measurement and posttreatment measurement(s)
Exclusion criteria SR: (1) nonhuman studies and (2) studies that only tested effects on motor, language functions and perception.
2 studies included
Important patient characteristics at baseline:
N, mean age A: 30 patients, 63.7 yrs B: 40 patients, 44.9 yrs Sex: A: 53.3% Male B: 62.5% Male
Stroke B: n.r.
comparable at baseline? Yes |
Describe intervention:
A: LF rTMS + PT (5/wk x 4 weeks) B: LF rTMS + comp.-assisted cognitive training, 10 min. x 20 sessions, x5/wk x 4 weeks
|
Describe control:
A: PT, 5/wk x 4 weeks B: sham rTMS + comp.-assisted cognitive training, 10 min. x 20 sessions, x5/wk x 4 weeks
|
End-point of follow-up:
A: post-intervention B: 3 days and 2 months post treatment.
For how many participants were no complete outcome data available? (intervention/control) A: 0 B: 0
|
≤ 3 months Visual and spatial attention Defined by the line bisection test (A1) and Albert Test (A2). LF-rTMS A2: 15.27 (11.25 – 19.29) B: n.r. Global cognitive functioning Defined by the MoCA test. Effect measure: mean difference (95% CI): LF-rTMS Memory Defined by the RBMT LF-rTMS Executive functioning n.r. >3 months Visual and spatial attention
Global cognitive functioning
n.r.
Executive functioning n.r.
|
Author’s conclusion: Our review suggests that NIBS is able to alleviate neglect after stroke. However, the results are still inconclusive and preliminary for the effect of NIBS on other cognitive domains. A standardized core set of outcome measures of cognition, also at the level of daily life activities and participation, and international agreement on treatment protocols, could lead to better evaluation of the efficacy of NIBS and comparisons between studies.
Risk-of-bias: A: B: publication bias (funding)
|
|
Study reference |
Study characteristics |
Patient characteristics 2 |
Intervention (I) |
Comparison / control (C) 3
|
Follow-up |
Outcome measures and effect size 4 |
Comments |
|
Choi, 2016 |
Type of study: Cross-over study design Setting and Country: Department of Rehabilitation Medicine, Gwangju Veterans Hospital, Gwangju, Korea. Funding and conflicts of interest: |
Inclusion criteria: (1) brain lesion detectable by magnetic resonance imaging or computed tomography which were taken at the onset of the symptom; (2) the location of subcortical lesion was in middle cerebral artery (MCA) territory; (3) the time gap between enrollment in the present study and occurrence of cerebral infarct was a minimum of 6 months and a maximum of 10 years; (4) mild to moderate balance impairment (score of Berg Balance Scale (BBS) was ≥20 and ≤46; and (5) Korean version of Mini-Mental State Examination score was ≥24, indicative of cognitive ability, which was sufficient to understand the nature of study. Exclusion criteria: N total at baseline: Important prognostic factors2: Age: mean (SD) Sex (% Male): Groups comparable at baseline? |
The rTMS cycles were composed of 10 sessions each administered over two weeks, and separated by a 4-week washout period. Real rTMS was delivered at 10 Hz and 90% of RMT for 5 seconds with 25-second inter-train interval. A total of 1,000 pulses were delivered over a period of 10 minutes. |
The sham rTMS cycles were composed of 10 sessions each administered over two weeks, and separated by a 4-week washout period. For sham rTMS, the treatment cycles were same as that of real rTMS; however, sham coil (MCF-P-B70, MagVenture) which provides sound and the sensation of scalp similar to the real rTMS coil, but does not induce a magnetic field was used. |
Length of follow-up: Loss-to-follow-up (3 months) N=3
Incomplete outcome data:
|
Walking
Maintaining standing balance Effect measure: MD (95% CI)
Muscle power/synergies
Sitting balance
Walking speed
Falling
|
In conclusion, to the best of our knowledge, this is the first study to investigate the therapeutic effects of rTMS over the trunk motor cortex on balance function in stroke patients. Our results show that high frequency rTMS may be used as one of the strategies for treating chronic stroke patients with balance impairment. Further studies are required to assess not only underlying mechanisms but also detailed protocol. |
|
Forogh, 2017 |
Type of study: Setting and Country: Neuromusculoskeletal Research Center, Firoozgar Hospital, Tehran, Iran. Funding and conflicts of interest: |
Inclusion criteria: stroke patient with subacute and chronic ischaemic and hemiplegic stroke documented by Computed Tomography (CT) or MRI; at least one month has elapsed from stroke; first-ever cerebral infarction; ability to perform 3-step command (3 points); no cognitive impairment, impaired patient’s balance and gait; the ability to walk with or without support; and with Functional Ambulation Categories more than one. Exclusion criteria: Patients were not included in the study if they had: a second stroke, bilateral weakness; the cerebel-lum or brain stem involvement, proprioception impairment, hemianopsia or another visual impairment, vestibular dysfunction, neurologic comorbidity other than stroke like neuropathy, severe postural instability, orthopedic problems, significant cognitive problem, receptive aphasia, epilepsy or seizures after stroke, and pathological conditions referred as contraindica-tions of rTMS (presence of a metallic implant inside the eye or the brain, the external fixator, cardiac pacemaker). N total at baseline: Important prognostic factors2: Age: range Sex (% Male): Groups comparable at baseline? |
Treatment was carried in 5 consecutive days, with 1 Hz rTMS in contralateral brain hemisphere over the primary motor area for 20 minutes (1200 pulses), in sitting position. Low-frequency rTMS was administered by a 70-mm figure-8 coil connected to Magstim R30 stimulator (MagVenture, Denmark). The optimal site and intensity of stimulation was deter-mined based on proposed method of Kondo et al., (2013). |
for Sham stimulation, we recorded the sound of stimulator. A small speaker was installed on the stimulation coil handle. The coil was placed on the head, adjustments were done on the rTMS monitor, but speaker was activated by a switch behind the patient. A sound mimicking the real rTMS was played for the patient. |
Length of follow-up: Directly after treatment, 3 weeks and 3 months thereafter.
Loss-to-follow-up (3 months) After treatment: 0 Reasons: Study withdrawal Incomplete outcome data:
|
Walking
Maintaining standing balance Effect measure: MD (95% CI)
Muscle power/synergies Effect measure: SMD (95% CI)
Sitting balance
Walking speed
Falling
|
The present study showed that rTMS as an adjuvant therapy may improve the static postural stability, falling risk, coordination, motor recovery, and muscle strength in patients with stroke. These effects could persist up to 3 months. Further research should be conducted with larger sample size. |
|
Huang, 2018 |
Type of study: Setting and Country: From the Neuroscience Research Center and Department of Neurology, Chang Gung Memorial Hospital and Chang Gung University College of Medicine, Taoyuan, Taiwan (Y-ZH). Funding and conflicts of interest: |
Inclusion criteria: (1) first-ever supratentorial stroke in the past 10–90 days, (2) an age of 18–80 yrs, and (3) displaying substantial leg disabilities and inability to complete a Timed Up and Go (TUG) test within 2 mins independently even with orthosis. Exclusion criteria: Patients were excluded if they had contraindications to (r)TMS,21 had reported walking impairment before the stroke, or had no recordable motor evoked potentials (MEPs) from the quadriceps of M1CL in the pretreatment assessment. N total at baseline: Important prognostic factors2: Age: mean ± SD Sex (% Male): Groups comparable at baseline? |
One-Hertz rTMS was produced by a Magstim Rapid2 stimulator with a double-cone coil. A stimulus intensity at 120% of the aMTwas adopted. The aMTand stimulation location (hot spot) were determined during the TMS procedure in the pretreatment assessment. Group E received real rTMS. Interventions included 15 daily sessions of 15 mins of 1-Hz rTMS (900 pulses, either real or sham) immediately followed by 45 mins of regular PT by physical therapists who were blinded to the treatment assignment. The preceding rTMS could potentially prime functional networks for the following physical intervention and lead to superior outcomes17 probably through the mechanism of metaplasticity. The routine PT program was customized and based on the participant's performance. All the participants also received occupational therapy, and some of them had speech therapy. However, only rTMS and PTwere provided in sequence. |
One-Hertz rTMS was produced by a Magstim Rapid2 stimulator with a double-cone coil. A stimulus intensity at 120% of the aMTwas adopted. The aMTand stimulation location (hot spot) were determined during the TMS procedure in the pretreatment assessment. Group C group C received sham rTMS using a customized sham coil. included 15 daily sessions of 15 mins sham immediately followed by 45 mins of regular PT by physical therapists who were blinded to the treatment assignment. The preceding rTMS could potentially prime functional networks for the following physical intervention and lead to superior outcomes17 probably through the mechanism of metaplasticity. The routine PT program was customized and based on the participant's performance. All the participants also received occupational therapy, and some of them had speech therapy. However, only rTMS and PTwere provided in sequence. |
Length of follow-up:
Loss-to-follow-up (3 months) After treatment: 0/1 Reasons: refused to continue, loss of contact, rejection of the assessment. Incomplete outcome data:
|
Walking
Maintaining standing balance
Muscle power/synergies
Sitting balance Effect measure: RR (95% CI)
Walking speed
Falling
|
The present study found insufficient evidence that contralesional priming with 1-Hz rTMS improves ambulatory and other motor functions among patients with a severe leg dysfunction in subacute stroke. |
|
Koch, 2019 |
Type of study: Setting and Country: Santa Lucia Foundation IRCCS Funding and conflicts of interest: |
Inclusion criteria: (1) first ever chronic ischaemic stroke (i.e., at least 6 months after the stroke event); (2) hemiparesis due to left or right subcortical or cortical lesion in the territory of the middle cerebral artery; and (3) residual gait and balance impairment. Exclusion criteria: (1) history of seizures; (2) severe general impairment or concomitant diseases; (3) patients older than 80 years; and (4) treatment with benzodiazepines, baclofen, and antidepressants. N total at baseline: Important prognostic factors2: Age: mean ± SD Sex (% Male): Groups comparable at baseline?
|
Each patient performed 1 session per day of conventional PT. Physiotherapy consisted of exercises designed to promote recovery of voluntary motor and balance functions, including muscle stretching, active-assisted mobilizations, progressive neuromuscular facilitation training balance exercises, and gait training,21 lasting 90 minutes including rest periods between exercises. During gait training, the therapist (A.M.C.) was positioned behind the patient to support hip and trunk stability. Cerebellar intermittent θ-burst stimulation was carried out using a Magstim Rapid magnetic biphasic stimulator connected with a figure-8 coil with a 70-mm diameter (Magstim Company). Before each daily PT session, 2 runs of CRB-iTBS were applied over the contralesional lateral cerebellum, spaced by an interval of 5 minutes.12 For each stimulation session, in total, we delivered 1200 pulses over the lateral cerebellum, contralateral to the affected hemisphere.13,14,15,16 Cerebellar intermittent θ-burst stimulation intensity was set at 80% of the active motor threshold,22 adjusted according to the individual scalp-to-cortex distance.23 The coil was positioned tangentially to the scalp, with the handle pointing superiorly.24 A neuronavigation system (SofTaxic; EMS) coupled with a Polaris Vicra infrared camera was used to ensure that in each patient, CRB-iTBS was applied over the same spot across different sessions. |
sham iTBS |
Length of follow-up:
Loss-to-follow-up T1: 0/0 Reasons: Discontinued intervention (pneumonia / depression) Incomplete outcome data:
|
Walking
Maintaining standing balance Effect measure: MD (95% CI)
Muscle power/synergies
Sitting balance
Walking speed
Falling
|
In conclusion, we provide novel evidence that combining CRB-iTBS with traditional PT is an effective strategy to promote gait and balance recovery by engaging successful cerebello-cortical reorganization in patients with ischaemic stroke. |
|
Chiu, 2020 |
Type of study: A phase 1/2a randomized trial
Funding and conflicts of interest: This study was funded by grants from the Translational Research Initiative of the Houston Methodist Research Institute and Seraya Medical, LLC to Drs. Helekar and Chiu. We thank Dr. Susan Xu, Ph.D. of Houston Methodist Research Institute for conducting statistical analysis of the data. Dr. Helekar is listed as an inventor on U.S. patent numbers 9456784, 10398907 and 10500408 covering the device used in this study. The patent is licensed to Seraya Medical, LLC. On behalf of all other authors, the corresponding author states that they do not have a conflict of interest. |
Inclusion criteria 1) Chronic stable ischaemic stroke (>3 months from acute event); 2) Persistent unilateral weakness involving at least the upper extremity verified by clinical neurological examination; and 3) Age 18 to 80 years.
Exclusion criteria: Any active unstable medical condition; 3) Pregnancy, schizophrenia, bipolar disorder, alcoholism, or substance abuse; 4) Any condition precluding MRI; and 5) Botulinum toxin use within 2 months.
N total at baseline: Intervention: 16 Control: 15
Important prognostic factors2: There were no significant treatment group differences in baseline physical and demographic characteristics including age, gender, race, affected side, cortical versus subcortical lesion location, time since stroke, and pre-treatment fMRI profile (Supplemental Table S1). |
TRPMS microstimulators were attached (using Velcro) to a neoprene cap (Fig. 2) resembling in appearance and feel to a modified diving or swimming cap. On the contralesional side, two microstimulators were placed on primary motor cortical (PMC) sites 1 cm and 4 cm lateral to midline. Contralesional PMC was identified by locating the activation of the hand motor area in the precentral gyrus in an fMRI scan involving gripping movements of the normal hand. On the ipsilesional side, microstimulators were placed on the lateral premotor cortical (LPC) site 5 cm anterior to the international 10 20 system electroencephalographic (EEG) electrode locus C3 or C4, and the supplementary motor cortical (SMC) site 15% of the nasion-to-inion distance anterior to EEG locus Cz.21 In addition, two microstimulators were placed on ipsilesional sites surrounding the infarct lesion on the PMC and the postcentral gyrus 4 cm apart. In subjects who had a subcortical infarct and intact PMC, the microstimulator pair was placed over the MRI-localized precentral gyrus 1 cm and 4 cm lateral to midline. The stimulus protocol was programmed to a Bluetooth-enabled microcontroller operated by smartphone. Treatment consisted of 40-minute sessions of TRPMS stimulation each day 5 times per week for a total of 20 sessions over 4 weeks. The subject sat in a relaxed position during stimulation. Stimulus pulse duration was 100 msec and frequency 0.2 Hz on the contralesional side. Stimulus duration and frequency on the ipsilesional side (perilesional/PMC, LPC and SMC) were 25 ms and 5 Hz respectively. The strength of the stimuli was the maximum generated by the TRPMS device prototype and known to modulate muscle activity and cortical excitability upon stimulation of the motor representation of the muscle. |
Sham stimulation. |
Length of follow-up: After treatment.
Loss-to-follow-up: Intervention: 1 Control: 0 Reasons: Early termination.
Incomplete outcome data: n.r.
|
≤ 3 months Upper Limb Capacity
Upper limb muscle synergies
Strength
Activities of daily living
>3 months Upper limb capacity TRPMS
Upper limb muscle synergies TRPMS
Strength TRPMS
Activities of daily living |
Multifocal bilateral TRPMS was safe and showed significant fMRI changes suggestive of functional reorganization of cortical circuits in patients with chronic ischaemic stroke. A larger randomized clinical trial is warranted to verify recovery of motor function. |
|
Ren, 2019 |
Type of study: A Randomized Sham-Controlled Study
Setting and country: Department of Neurological Rehabilitation, Wuxi Tongren Rehabilitation Hospital of NanjingMedical University, Wuxi, Jiangsu Province, China
Funding and conflicts of interest: The authors declare that there are no conflicts of interest regarding the publication of this paper. The authors thank all those who participated in the trial. This work was supported by the National Natural Science Foundation of China (Grant number 1501949). Contributor Shuyuan Wu participated in the treatment of participants. |
Inclusion criteria: 1) a first-ever leftsidedmiddle cerebral artery (MCA) strokewith the lesion site verified by magnetic resonance imaging (MRI); (2) the time between 4 and 12 weeks after suffering from the stroke; (3) global aphasia defined by WAB-AQ scores; and (4) written informed consent from all subjects who participated in the study.
Exclusion criteria: (1) vision and hearing disabilities that might interfere with diagnostic and therapeutic treatment; (2) medications altering the level of cortical excitability (e.g., antiepileptics, neuroleptics or benzodiazepines); (3) a history of substance abuse, premorbid dementia or any neuropsychiatric diseases; and (4) contraindications for rTMS according to the safety guidelines.
N total at baseline: rTMS-w: 18 rTMS-b: 13 Sham: 15
Important prognostic factors2: Age: mean ± SD rTMS-w: 65.95 ± 8.53 rTMS-b: 62.46 ± 10.95 Sham: 63.60 ± 16.71
Sex: rTMS-w: 66.7% rTMS-b: 53.8% Sham: 60.0%
Groups comparable at baseline? All three groups were balanced at baseline with respect to the severity of aphasia, time since onset, participant age, gender and concomitant diseases (P>0.05) |
Those receiving real inhibiting rTMS on the right pars triangularis of the pIFG, which is the homolog of the left Broca’s area (the rTMS-b group); those receiving real inhibiting rTMS on the right pSTG, which is the homolog of the left Wernicke’s area (the rTMS-w group); and those receiving sham rTMS (the sham group), all in combination with SLT. The allocations were stored in sealed, numbered envelopes. The subjects did not know whether they were receiving real or sham rTMS. The language therapist assessed speech and language abilities and was blinded to the patients’ group assignments. All subjects, investigators (except the investigator responsible for rTMS application), clinicians, speech, and language therapists were blinded to patient assignment to real or sham rTMS. The therapeutic procedure consisted of rTMS sessions and SLT. Subjects in all three groups underwent SLT sessions for 30 minutes immediately after finishing rTMS treatment from Monday to Friday for 3 weeks. The speech and language training mainly focused on the comprehension and expression of spoken language. The rehabilitation program focused on specific training to stimulate various aspects of the language system (e.g., semantic, phonological, syntactic or motor). |
The sham group was given sham rTMS, also incombination with SLT. |
Length of follow-up: After treatment and 3 weeks of follow-up.
Loss-to-follow-up: rTMS-w: 0 Sham: 2
Reasons: Complications
Incomplete outcome data: rTMS-w: 0 Sham: 1
Reasons: Discontinued intervention (refusal).
|
≤ 3 months Functional communication Effect measure: mean difference (95% CI) in WAB-AQ:
rTMS-w 0.72 (-0.14 – 1.58)
rTMS-b 0.75 (0.21 – 1.70)
Verbal comprehension Effect measure: mean difference (95% CI) in standardized z-scores of the comprehension test:
rTMS-w 1.02 (0.13 – 1.91)
rTMS-b 0.77 (-0.18 – 1.73)
Expressive naming Effect measure: mean difference (95% CI) in WAB naming score:
LF-rTMS-w 0.01 (-0.82 – 0.84)
LF-rTMS-b 0.41 (-0.52 – 1.34) > 3 months n.r.
|
Many studies have reported that low frequency rTMS is beneficial for rehabilitating patients with aphasia, but the ideal stimulation sites for rTMS are not known. Lowfrequency rTMS applied to the right pIFG and pSTG can be assumed to be an effective treatment for global aphasia following subacute stroke. Even immediately after the 15-day treatment, LF-rTMS inhibited the right pSTG and promoted significantly increased gains in auditory comprehension and repetition, whereas LF-rTMS inhibited the right pIFG and apparently caused changes in spontaneous speech and repetition. Further investigations are necessary to explore the neural mechanisms that underlie the differences in functional recovery observed between the different stimulation sites in this study. |
|
Rubi-Fessen, 2015 |
Type of study: A Randomized Controlled Study
Setting and country: Neurologic rehabilitation hospital, Germany.
Funding and conflicts of Suppliers a. Magstim Rapid2 stimulator; The Magstim Company Ltd. b. SPSS version 20; IBM Corp. |
Inclusion criteria: 1) subacute aphasia with testability for the Aachen Aphasia Test (AAT)30; (2) poststroke period up to 16 weeks (but most were 4e6wk poststroke); (3) right handedness as measured by the Laterality Questionaire31; (4) German as the first language; and (5) age between 55 and 85 years. other neurological disease.
Exclusion criteria: 1) prior symptomatic cerebrovascular accidents; (2) neurodegenerative or psychiatric disease; (3) epilepsy; and (4) auditory or visual deficits that might impair testing.
N total at baseline: Intervention: 15 Control: 15
Important prognostic factors2: For example age ± SD: I: 67.9 ± 8.12 C: 69.6 ± 6.67
Sex: I: 33.3% M C: 60% M
Groups comparable at baseline? independent-sample t tests did not reveal any significant group differences with respect to age, disease duration, lesion size and aphasia severity. |
The rTMS group received 20 minutes of 1-Hz rTMS over the right triangular part of the inferior frontal gyrus (center of Brodmann area 45), Both groups were given an intensity of 90% of the individual resting motor threshold. The resting motor threshold was defined as the minimum stimulator output that elicited a visible contraction on the first dorsal interosseus muscle of the unaffected hand in more than 5 of 10 stimulation trials. Stimulation parameters were in accordance with the guidelines suggested by Wassermann.36 Before stimulation, T1-weighted, diffusion-weighted, and T2 fluid-attenuated inversion recovery MRI images were obtained to locate the optimal coil position. The respective brain areas were stimulated using a Magstim Rapid2 stimulatora with a double 70-mm coil. The stimulation point was determined using reference lines defined on the reconstruction of the respective patient’s head from the MRIs, which were then transferred to the patient’s head (For details, see Weiduschat et al37). This method has an accuracy of 10mm when compared with neuronavigated methods. |
The sham group) received the same stimulation over the vertex. Both groups were given an intensity of 90% of the individual resting motor threshold. The resting motor threshold was defined as the minimum stimulator output that elicited a visible contraction on the first dorsal interosseus muscle of the unaffected hand in more than 5 of 10 stimulation trials. Stimulation parameters were in accordance with the guidelines suggested by Wassermann.36 Before stimulation, T1-weighted, diffusion-weighted, and T2 fluid-attenuated inversion recovery MRI images were obtained to locate the optimal coil position. The respective brain areas were stimulated using a Magstim Rapid2 stimulatora with a double 70- mm coil. The stimulation point was determined using reference lines defined on the reconstruction of the respective patient’s head from the MRIs, which were then transferred to the patient’s head (For details, see Weiduschat et al37). This method has an accuracy of 10mm when compared with neuronavigated methods. |
Length of follow-up: After treatment (10 sessions) and after 2 weeks follow-up
Loss-to-follow-up: Intervention: 0 Control: 0
Incomplete outcome data: Intervention: 0 Control: 0
|
≤ 3 months Functional communication Effect measure: mean difference (95% CI) in ANELT-A scale
LF-rTMS 0.09 (-0.62 – 0.81)
Verbal comphrehension Effect measure: mean difference (95% CI) in Token Test
LF-rTMS -0.09 (-0.82 – 0.62)
Expressive naming Effect measure: mean difference (95% CI) in the accuracy of the naming screening test:
LF-rTMS -0.07 (-0.78 – 0.65)
> 3 months n.r.
|
The present study delivers further evidence that combining 1-Hz rTMS with SLT leads to significant add-on treatment effects in the subacute stage of aphasia. As indicated by the results, the outcome of behavioral therapy is enhanced not only for a variety of basic linguistic skills but also for functional communication. Longitudinal studies are required to evaluate the long-term stability of these benefits. |
|
Zumbansen, 2020 |
Type of study: three-armed sham-controlled blinded prospective proof-of-concept study
Setting and country: Jewish General Hospital, Lady Davis Institute for Medical Research, Department of Neurology & Neurosurgery, McGill University, Montreal, Quebec
Funding and conflicts of interest: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This trial was supported by research grants from the Canadian Institutes for Health Research (CIHR, MOP#125954), W.-D. Heiss Foundation, and the Lady Davis Institute at the JGH (CLIPP#2014). AZ was funded by a CIHR postdoctoral fellowship.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
|
Inclusion criteria: stroke patients presenting with speech or language problems.
Exclusion criteria: Withdrawal of consent, patients with very severe aphasia (who most likely exclusively depend on the right hemisphere) were excluded from the study.
N total at baseline: Intervention: 20 Control: 19
Important prognostic factors2: For example age ± SD: I: 66.7 ± 9.8 C: 67.4 ± 11.7
Sex: I: 50% M C: 36.8% M
Groups comparable at baseline? Yes
|
rTMS was applied over the non-affected right hemisphere (pars triangularis of the right inferior frontal gyrus) using a figure-of-eight coil at 1Hz for 900 pulses (15min) at 90% resting motor threshold (RMT). RMT was determined prior to each treatment session over the right primary motor area.13 ST sessions were given immediately following the rTMS procedure to ensure treatment within the period of maximum rTMS after-effect (about 45 min).14
|
For sham-stimulation, the coil was placed over the interhemispheric fissure at the vertex, and stimulation was applied with 10% RMT. |
Length of follow-up: After treatment and after one month follow-up.
Loss-to-follow-up (after treatment) Intervention: 2 Control: 1
Reasons: Withdrew before starting treatment and off before starting treatment.
Incomplete outcome data: n.r.
|
≤ 3 months Functional communication n.r.
Verbal comprehension Effect measure: mean difference (95% CI) in standardized Z-scores of the comphrehension test
LF-rTMS
Expressive naming
LF-rTMS
> 3 months n.r.
|
Contralesional NIBS is a safe add-on therapy for poststroke aphasia. Low frequency rTMS improved naming recovery one month after a 10-day treatment course. tDCS effect was not significantly different from sham stimulation. Our results raise the possibility that inhibitory NIBS over the right pars triangularis may have negative effects in patients where Broca’s Area is affected, supporting the view that NIBS presently should not be applied outside clinical trials. Future trials should specifically investigate individual factors for patient stratification (e.g., lesion location) and include longer-term follow-up outcome measures (>6months). |
|
Iwánski, 2020 |
Type of study: RCT
Setting and country: Inpatient inpatient or outpatient ward of the Neurorehabilitation Unit of the Institute of Psychiatry and Neurology, Poland.
Funding and conflicts of interest:
This work was supported by the National Science Center, grant number: UMO-2012/07/N/NZ7/01138.
The authors declare that there is no conflict of interest. |
Inclusion criteria: 1) MRI or CT (in case of contraindications to MRI) confirming a first-ever stroke in the right hemisphere; 2) time after onset 2–12 weeks (early subacute stroke; Bernhardt et al., 2017); 3) severe to moderate VSN recognized in a neuropsychological assessment; 4) age 18–75 years; 5) signed informed consent by the patient.
Exclusion criteria: 1) severe cognitive impairment; 2) a history of premorbid dementia, substance abuse, or any neuropsychiatric disease; 3) medications altering the level of cortical excitability (e.g., anti-epileptics, neuroleptics, or benzodiazepines); 4) contraindications for rTMS (intracranial metallic objects, implanted stimulator devices, or a history of seizures or epilepsy).
N total at baseline: Intervention: 14 Control: 14
Important prognostic factors2: age ± SD: I: 65 ± 87.5 C: 64.6 ± 7.7
Sex: I: 78.6 % M C: 78.% M
Stroke type: C: 7.1% haemorrhagic stroke
Groups comparable at baseline? Yes |
Stimulation was applied at 90% of the RMT at 1Hz frequency. A total of 1800 pulses were generated during a 30-min session. All the participants underwent comprehensive cognitive training five days a week for four weeks. During the long-lasting rTMS, the accuracy of stimulation was constantly monitored by neuronavigation, while coil position error was recorded every 10 pulses. Deviation of up to four millimetres from the target was considered acceptable. For every participant, the percentage of pulses “in target” was estimated for each session.
VSN therapy was focused mainly on visuospatial scanning with active and purposeful direction of sight to the left visual field in cognitive tasks performed in two computer programs. Additionally, paper-and-pencil tasks to improve visual scanning were used. Patients were asked to draw, copy, and analyze complex visual stimuli. The visual-scanning training was guided by verbal instruction, contralesional cues (e.g., visual stimuli), and the therapist’s feedback to orientate attention to the neglected part of space.
|
The control group received sham stimulation performed with a sham coil that looked and sounded similar to real stimulation.
VSN therapy was focused mainly on visuospatial scanning with active and purposeful direction of sight to the left visual field in cognitive tasks performed in two computer programs. Additionally, paper-and-pencil tasks to improve visual scanning were used. Patients were asked to draw, copy, and analyze complex visual stimuli. The visual-scanning training was guided by verbal instruction, contralesional cues (e.g., visual stimuli), and the therapist’s feedback to orientate attention to the neglected part of space. |
Length of follow-up: End of treatment
Loss-to-follow-up: 0 (0%)
Incomplete outcome data: 0 (0%)
|
≤ 3 months Visual and spatial attention Effect measure: mean difference (95% CI) in behavioral inattention test
LF-rTMS 3.50 (-5.56 – 12.56)
Global cognitive functioning n.r.
Memory n.r.
Executive functioning n.r.
> 3 months n.r.
|
Our study did not confirm the efficacy of focused low frequency (1 Hz) rTMS over the left angular gyrus as a therapeutic method to ameliorate VSN in early subacute stroke patients (up to three months). Future studies should explore the efficacy of more extensive rTMS targeted to neural substrates of visuospatial attention. |
|
Liu, 2020 |
Type of study: RCT
Setting and country: Inpatient rehabilitation hospital.
Funding and conflicts of interest:
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China (Grant Number: 81871847), the National Natural Science Foundation of China (Grant Number: 81672261), the National Natural Science Foundation of China (Grant Number: 81702232), the Natural Science Foundation of Guangdong Province (Grant Number: 2017A030313493) and the Medical Science and Technology Foundation of Guangdong Province (Grant Number: A2016251).
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. |
Inclusion criteria: Patient with a clear history of stroke who had imaging data showing clear lesions, sudden aggravation or ladder-like progression of attention dysfunction, or definite time between attention dysfunction and stroke or causality were included in the study. Patients with attention dysfunction screened by Mini-Mental State Examination and Cancellation Test, those with a stable condition and good motor function of the upper extremities of the habitual hand were also included. Besides, the patients had to have first onset of the disease (course of disease; 6–12 months) and aged between 40 and 75 years old.
Exclusion criteria: Patients with metal implants in the body or those with a history of seizures or family history, severe cardiopulmonary disease and multiple organ failure, malignant tumors, and severe mental illness were excluded from the study. We also excluded patients with a previous history of encephalitis, brain trauma, Alzheimer’s disease and dementia, those with severe visual, hearing or communication impairment as well as those who had received TMS treatment.
N total at baseline: Intervention: 31 Control: 31
Important prognostic factors2: age ± SD: I: 58.6 ± 6.2 C: 57.7 ± 7.3
Sex: I: 34.5 % M C: 55.2% M
Stroke type: C: 51.7% infarction
Groups comparable at baseline? Yes |
Patients in the TMS group received 10 Hz, 700 pulses of TMS for four weeks. TMS was applied using a magnetic stimulation device (CCY-IA Wuhan Yiruide Co., Ltd.), connected to a focal figure-of-eight shape coil (each loop had a diameter of 3.5 cm) cooled in liquid nitrogen. The “8”-shaped coil, was placed at the F3 point-left dorsolateral prefrontal cortex according to the 10– 20 electroencephalogram coordinate system, and the coil was at a tangent with the F3 point. We only targeted the left dorsolateral prefrontal cortex for stimulation, irrespective of the lesion hemisphere.
All the participants underwent comprehensive cognitive training five days a week for four weeks.
|
The patients in the sham group were treated with sham TMS, and the coil was held at a 90°angles to the scalp using the parameters as described for the TMS group. |
Length of follow-up: End of treatment
Loss-to-follow-up: Intervention: 2 (6.5%) Reasons: Did not receive allocated overall intervention due to discharge or withdrawal of consent.
Control: 2 (6.5%) Reasons: Did not receive allocated overall intervention due to discharge or surgery.
Incomplete outcome data: n.r.
|
≤ 3 months n.r.
> 3 months Visual and spatial attention Effect measure: mean difference (95% CI) on digit symbol test
HF-rTMS
Global cognitive functioning Effect measure: mean difference (95% CI) on the MMSE.
HF-rTMS 2.11 (0.23 – 3.99)
Memory n.r.
Executive functioning Effect measure: mean difference (95% CI) in TMT-A (errors and time in seconds)
HF-rTMS Errors: 1.73 (0.81 – 2.65) Time: 67.34 (6.90 – 167.78) |
In summary, this study suggests that TMS might be effective in improving the performance in the activities of daily living and attention function of stroke patients. The treatment parameters of TMS in our study is safe and does not cause brain tissue damage. Our findings, thus support the inclusion of the specific intervention programs in clinical practice. Further studies are necessary to evaluate the underlying mechanism of TMS for the performance of the activities of daily living and attention function after stroke. |
|
Tsai, 2020 |
Type of study: RCT
Setting and country: Taiwan.
Funding and conflicts of interest: This work was supported by the Taipei Veterans General Hospital Grant (V104C058). No conflicts of interests declared. |
Inclusion criteria: left hemispheric ischaemic or haemorrhagic stroke more than 3 months previously with cognitive impairment, defined by a Repeatable Battery for the Assessment of Neuropsychological Status (RBANS)24 score below 85; no seizure history; no intracranial occupying lesion, including arteriovenous malformation or brain tumour, according to imaging results; and no concurrent use of antidepressants or neurostimulators.
Exclusion criteria: unstable cardiac dysrhythmia, fever, infection, hyperglycemia, epilepsy or previous administration of tranquilizers, neurostimulators or other medication that significantly affected the cortical motor threshold.25 We excluded patients with metallic intracranial devices, pacemakers or other electronic devices in their bodies. The rTMS protocols used in the current study were in accordance with the safety guidelines for rTMS applications.
N total at baseline: rTMS: 14 iTBS: 15 Sham: 15
Important prognostic factors2: For example age ± SD: r: 47.45 ± 12.3
Sex: r: 81.8% Male i: 73.3% Male
Stroke type:
Groups comparable at baseline? |
Each patient received 10 days of rTMS treatment, administered in the morning from Monday to Friday for 2 consecutive weeks. The left dlPFC (F3 point) was stimulated according to the international 10/20 electroencephalography (EEG) recording system to stimulate the left prefrontal cortex.11 The intensity for the 5 Hz rTMS and iTBS groups was set at 80% of the resting motor threshold.
The iTBS treatment consisted of 3 pulses of 50 Hz bursts repeated at 5 Hz (2 s on and 8 s off) for a total of 190 seconds (600 pulses). The 5 Hz rTMS protocol was applied at an intensity of 80% of the resting motor threshold, with 2 s trains (10 pulses) at an intertrain interval of 8 seconds, repeated Every 10 seconds for a total of 10 minutes (600 pulses).
|
The sham condition involved similar procedures, except that a sham coil was used. We used a placebo coil (Magstim) for the sham stimulation, which delivered less than 5% of the magnetic output with an audible click on discharge.
|
Length of follow-up:
Loss-to-follow-up: rTMS: 3 (20%) Reasons: withdrawal because of commuting difficulties.
iTBS: 0 (0%)
Incomplete outcome data: n.r.
|
≤ 3 months n.r.
> 3 months Visual and spatial attention n.r.
Global cognitive functioning Effect measure: mean difference (95% CI) on the RBANS
LF-rTMS 11 (0.73 – 21.27)
Memory n.r.
Executive functioning n.r.
|
Our results demonstrated that both 5 Hz rTMS and iTBS were effective for poststroke cognitive impairment in terms of global cognition, attention and memory function; the domain of attention was susceptible to 5 Hz modulation. Treatment with 5 Hz rTMS may slow cognitive decline, representing both a pivotal process in poststroke cognitive impairment and an aspect of neuroplasticity that contributes to disease-modifying strategies. |
|
Yin (2020) |
Type of study: Randomized Clinical Trial.
Setting and country: China
Funding and conflicts of interest: This work was supported by the National Natural Science Foundation of China (81871847, 81672261, 1601979, 81702232, and 81972151), the Science and Technology Planning Key Project of Guangzhou (201803010119), Guangdong Basic and Applied Basic Research Foundation (2019A1515011106), and the Natural Science Foundation of Guangdong Province of China (2017A030313493).
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication. |
Inclusion criteria: (1) stroke patients in accordance with the diagnostic criteria established by the fourth National Cerebrovascular Disease Academic Conference in 1995 confirmed by a brain CT or MRI; (2) first-ever stroke, course of stroke between 1 and 6 months; (3) right-handed; (4) aged 30– 75 years; (5) the presence of cognitive impairments (Montreal Cognitive Assessment, MoCA < 26); (6) no severe aphasia and able of accomplishing cognitive tests; (7) stable vital signs, no progression of neurological symptoms; (8) normal cognitive functions before stroke; (9) capable of tolerating MRI scan; (10) voluntary participation and signed the informed consent.
Exclusion criteria: The exclusion criteria were as follows: (1) non-first stroke; (2) complete left prefrontal cortex injury confirmed by CT/MRI; (3) transcranial surgery or skull defect; (4) metal or cardiac pacemaker implants; (5) history of brain tumor, brain trauma, seizures, and risks of seizures; (6) cognitive function recession before stroke; (7) any neuropsychiatric comorbidity and affective disorder that could influence the test outcomes; (8) any other factors that could affect cognitive assessments and treatments.
N total at baseline: Intervention: 16 Control: 18
Important prognostic factors2: age ± SD: Intervention: 56.69 ± 12.92 Sex: Intervention: 87.5% male Control: 88.9% male
Stroke type: Control: 66.7% ischaemic
Groups comparable at baseline? Yes |
For rTMS group, the stimulating coil was placed tangentially to the surface of the skull. All patients received treatments once a day, 5 days per week for 4 weeks. After rTMS treatments, patients received a 30-min computer assisted cognitive rehabilitation referring to attention, executive function, memory, calculation, language and visuospatial skills, et cetera. Therapists were blinded to assignments. Besides, during hospitalization, patients received conventional drug treatments recommended by the 2016 American Heart Association/American Stroke Association recommendation (Winstein et al., 2016). |
For the no-stim control group, the coil was placed perpendicularly to the surface of the skull inducing no magnetic field. All patients received treatments once a day, 5 days per week for 4 weeks. After rTMS treatments, patients received a 30-min computer assisted cognitive rehabilitation referring to attention, executive function, memory, calculation, language and visuospatial skills, et cetera. Therapists were blinded to assignments. Besides, during hospitalization, patients received conventional drug treatments recommended by the 2016 American Heart Association/American Stroke Association recommendation (Winstein et al., 2016). |
Length of follow-up: After treatment (4 weeks)
Loss-to-follow-up: rTMS: 2 (11%) Reasons: Discontinued rTMS intervention (complained of scalp pain)
control: 0 (0%) Incomplete outcome data: n.r.
|
≤ 3 months Visual and spatial attention n.r.
Global cognitive functioning Effect measure: mean difference (95% CI) n the MoCA test
HF-rTMS 7.00 (3.30 – 10.70)
Memory Effect measure: mean difference (95% CI) on the RBMT
HF-rTMS 1.00 (-2.37 – 4.37)
Executive functioning Effect measure: mean difference (95% CI) on the coloured dots trail/neutral words trail/incongruent coloured words in time (seconds) and errors words (n)
HF-rTMS Errors: 0.00 (-2.17 – 2.17)/ 0.00 (-1.05 – 1.05)/ 2.00 (-0.14 – 4.14)
Time: -5.00 (-16.81 – 6.81)/ -5 (-38.16 – 28.16)//45 (15.85 – 74.35)
> 3 months n.r. |
In summary, our results suggest that high frequency rTMS applied on the left DLPFC could improve cognitive function for stroke patients with cognitive impairment, with accompanying changes in the left medial prefrontal cortex. |
Notes:
- Prognostic balance between treatment groups is usually guaranteed in randomized studies, but non-randomized (observational) studies require matching of patients between treatment groups (case-control studies) or multivariate adjustment for prognostic factors (confounders) (cohort studies); the evidence table should contain sufficient details on these procedures.
- Provide data per treatment group on the most important prognostic factors ((potential) confounders).
- For case-control studies, provide sufficient detail on the procedure used to match cases and controls.
- For cohort studies, provide sufficient detail on the (multivariate) analyses used to adjust for (potential) confounders.
Table of quality assessment for systematic reviews of RCTs and observational studies
Based on AMSTAR checklist (Shea, 2007; BMC Methodol 7: 10; doi:10.1186/1471-2288-7-10) and PRISMA checklist (Moher, 2009; PLoS Med 6: e1000097; doi:10.1371/journal.pmed1000097)
|
Study
First author, year |
Appropriate and clearly focused question?1
Yes/no/unclear |
Comprehensive and systematic literature search?2
Yes/no/unclear |
Description of included and excluded studies?3
Yes/no/unclear |
Description of relevant characteristics of included studies?4
Yes/no/unclear |
Appropriate adjustment for potential confounders in observational studies?5
Yes/no/unclear/notapplicable |
Assessment of scientific quality of included studies?6
Yes/no/unclear |
Enough similarities between studies to make combining them reasonable?7
Yes/no/unclear |
Potential risk of publication bias taken into account?8
Yes/no/unclear |
Potential conflicts of interest reported?9
Yes/no/unclear |
|
Tung, 2019 |
Yes |
Yes |
No |
Yes |
Not applicable |
Yes |
Yes |
Yes |
Yes |
|
Van Lieshout, 2019 |
Yes |
Yes |
Yes |
Yes |
Not applicable |
Yes |
No |
Yes |
Yes |
|
Bucur, 2019 |
Yes |
Yes |
Yes |
Yes |
Not applicable |
Yes |
Yes |
Yes |
Unclear |
|
Van Lieshout, 2019 |
Yes |
Yes |
Yes |
Yes |
Not applicable |
Yes |
Yes |
Yes |
Yes |
- Research question (PICO) and inclusion criteria should be appropriate and predefined.
- Search period and strategy should be described; at least Medline searched; for pharmacological questions at least Medline + EMBASE searched.
- Potentially relevant studies that are excluded at final selection (after reading the full text) should be referenced with reasons.
- Characteristics of individual studies relevant to research question (PICO), including potential confounders, should be reported.
- Results should be adequately controlled for potential confounders by multivariate analysis (not applicable for RCTs).
- Quality of individual studies should be assessed using a quality scoring tool or checklist (Jadad score, Newcastle-Ottawa scale, risk of bias table et cetera)
- Clinical and statistical heterogeneity should be assessed; clinical: enough similarities in patient characteristics, intervention and definition of outcome measure to allow pooling? For pooled data: assessment of statistical heterogeneity using appropriate statistical tests (for example Chi-square, I2)?
- An assessment of publication bias should include a combination of graphical aids (for example funnel plot, other available tests) and/or statistical tests (for example Egger regression test, Hedges-Olken). Note: If no test values or funnel plot included, score “no”. Score “yes” if mentions that publication bias could not be assessed because there were fewer than 10 included studies.
- Sources of support (including commercial co-authorship) should be reported in both the systematic review and the included studies. Note: To get a “yes,” source of funding or support must be indicated for the systematic review AND for each of the included studies.
|
Study reference
(first author, publication year) |
Was the allocation sequence adequately generated? a
Definitely yes Probably yes Probably no Definitely no |
Was the allocation adequately concealed?b
Definitely yes Probably yes Probably no Definitely no |
Blinding: Was knowledge of the allocated interventions adequately prevented?c
Were patients blinded?
Were healthcare providers blinded?
Were data collectors blinded?
Were outcome assessors blinded?
Were data analysts blinded?
Definitely yes Probably yes Probably no Definitely no |
Was loss to follow-up (missing outcome data) infrequent?d
Definitely yes Probably yes Probably no Definitely no |
Are reports of the study free of selective outcome reporting?e
Definitely yes Probably yes Probably no Definitely no |
Was the study apparently free of other problems that could put it at a risk of bias?f
Definitely yes Probably yes Probably no Definitely no |
Overall risk of bias If applicable/necessary, per outcome measureg
LOW Some concerns HIGH |
|
Choi, 2016 |
Probably yes;
Reason: Patients were randomly divided into two groups considering their treatment order. |
Definitely yes;
Reason: The subjects were randomized by other healthcare professional who did not participate in this study. |
Probably no;
Reason: For sham rTMS, the treatment cycles were same as that of real rTMS; however, sham coil (MCF-P-B70, MagVenture) which provides sound and the sensation of scalp similar to the real rTMS coil, but does not induce a magnetic field was used. In addition, the patients were not allowed to recognize whether it is the sham rTMS or not. |
No information;
Reason: Loss to follow-up was not reported. |
Definitely no;
Reason: All relevant outcomes were reported. |
Definitely yes;
Reason: No other problems reported. |
LOW |
|
Forogh, 2017 |
No information;
Reason: No information was provided. |
No information;
Reason: No information was provided. |
Probably yes;
Reason: A sound mimicking the real rTMS was played for the patient, but no information was provided about the blinding of outcome assessors and data analysts. |
Probably no;
Reason: Loss to follow-up was frequent, but balanced (5 and 6 from the 22 patients). |
Definitely yes;
Reason: All relevant outcomes were reported. |
Definitely yes;
Reason: No other problems reported. |
Some concerns (lack of information about the randomization and blinding) |
|
Huang, 2018 |
Definitely yes;
Reason: With stratification regarding the stroke duration (10–30 and 31–90 day), patients were randomly allocated at a 1:1 ratio (with a block of 2) into experimental group (E) or control group (C). |
No information;
Reason: No information was provided. |
Definitely yes;
Reason: Patients and the outcome measurement assessors were blinded to the treatment assignment. |
Probably yes: |
Definitely yes;
Reason: All relevant outcomes were reported. |
Definitely yes;
Reason: No other problems reported. |
LOW |
|
Koch, 2019 |
Definitely yes;
Reason: Patients were randomly assigned to 2 age-matched groups treated either with CRB-iTBS treatment or sham iTBS. The randomization algorithm used the minimal sufficient balancing method to prevent imbalances in baseline age and stroke severity |
No information;
Reason: No information was provided. |
Definitely yes;
Reason: Each evaluation was performed by a clinician or by a neurophysiologist who was blinded to the experimental condition of the patient. |
Probably yes;
Reason: loss to follow-up was infrequent (2 patients discontinued treatment from the 36 in total). |
Definitely yes;
Reason: All relevant outcomes were reported |
Probably no;
Reason: The sample included patients with stroke in both hemispheres, which could have influenced literality. |
|
|
Li, 2016 |
Definitely yes;
Reason: Patients were equally and randomly divided into three groups according to the consecutive order of admission using a random number table. |
Probably yes;
Reason: Patients were equally and randomly divided into three groups according to the consecutive order of admission using a random number table. |
Probably yes;
Reason: The paper was double-blinded. |
Definitely yes;
Reason: The remaining 127 patients completed the trial and were included in statistical analyses |
Definitely yes;
Reason: All relevant outcomes were reported |
Probably yes;
Reason: No other problems noted. |
LOW |
|
Volz, 2016 |
Probably yes;
Reasons: All subsequent patients were matched to the most similar patient regarding the randomization factors (1–3) and accordingly assigned to the other treatment group. |
Definitely yes;
matching of patients was performed by an experimenter (C.G.) not involved in the behavioural, electrophysiological, or neuroimaging assessment of patients to exclude any selection bias. |
Definitely no;
Reasons: We used a sham-controlled, pseudo-randomized, single-blinded between-subject design. |
Definitely yes;
Reasons: All patients completed the intervention protocol without adverse events. |
Definitely yes;
Reason: All relevant outcomes were reported |
Probably yes;
Reason: No other problems noted.
|
Some concerns (grip strength) |
|
Long, 2018 |
Probably yes:
Reasons: The participants were randomized into three groups. |
Probably yes:
Reasons: The participants were randomized into three groups. |
Definitely yes;
Patients, healthcare providers and outcome assessors were blinded to the study. |
Definitely yes;
All patients included underwent the intervention. |
Definitely yes;
Reason: All relevant outcomes were reported |
Probably yes;
Reason: No other problems noted.
|
LOW |
|
Ke, 2020 |
Definitely yes;
Reasons: The study participants were randomly assigned to three HF-rTMS groups using a randomization distribution table, generated by a computer. |
Definitely yes;
Reasons: The study participants were randomly assigned to three HF-rTMS groups using a randomization distribution table, generated by a computer. |
Definitely yes;
Reasons: These clinical outcome assessments were performed by a neurologist with experience in these scales who was blinded to the study assignment at baseline, after rTMS, and at follow-up. |
Probably yes;
Reasons: Four patients were lost during follow-up: two patients in the sham group and one patient in each of the short and long ITI rTMS groups. |
Definitely yes;
Reasons: All relevant outcomes were reported |
Probably yes;
Reasons: No other problems noted.
|
Some concerns |
|
Kim, 2020 |
Probably yes;
Reasons: This study was an outcome-assessor-blinded, single-center, randomized controlled pilot clinical trial with a 1:1:1:1 allocation ratio. |
Probably yes;
Reasons: This study was an outcome-assessor-blinded, single-center, randomized controlled pilot clinical trial with a 1:1:1:1 allocation ratio. |
Probably no;
Reasons: We had no choice but to adopt a single-outcome-assessor blinding approach because sham treatment was impossible due to the nature of SA, which included scalp penetration. During the study, the assessor was blinded to group assignments, and data analysts without conflicts of interest were involved in this study. |
Probably yes;
Reasons: Three did not complete treatment in the control group. One exited the study due to orthopaedic surgery, four did not complete treatment, and two were lost to follow-up in the rTMS group. |
Definitely yes;
Reasons: All relevant outcomes were reported |
Probably yes;
Reasons: we did not explore the long-term additional effects on cognitive function, dysphagia, and walking and could not exclude any biasing effect of pain. |
Some concerns |
|
Harvey, 2018 |
Probably yes;
Reasons: This was a multicentre randomized, blinded, sham-controlled pivotal trial comparing NBT comprising 1 Hz rTMS to noninjured hemisphere versus sham stimulation, delivered before upper limb rehabilitation therapy. Random assignment of participants occurred at 12 sites in the United States in a 2:1 ratio of experimental treatment to sham. An imbalanced randomization provided adequate subject exposure to therapeutic NBT for assessment of safety. |
Probably yes;
Reasons: This was a multicentre randomized, blinded, sham-controlled pivotal trial comparing NBT comprising 1 Hz rTMS to noninjured hemisphere versus sham stimulation, delivered before upper limb rehabilitation therapy. Random assignment of participants occurred at 12 sites in the United States in a 2:1 ratio of experimental treatment to sham. An imbalanced randomization provided adequate subject exposure to therapeutic NBT for assessment of safety. |
Probably no;
Blinding to group assignment included all participants and study staff except for the NBT device operator. |
Probably yes;
Reasons: Loss- to follow-up was equal in both groups. |
Definitely yes;
Reasons: All relevant outcomes were reported. |
Probably no;
Reasons: Although the target sample size was achieved, follow-up was not completed after the Data Safety Monitoring Board reported on February 26, 2016, that the second interim analysis met statistical futility for the intervention. |
LOW |
|
Chen, 2019 |
Definitely yes;
Reasons: The flow diagram of the randomization procedure and the study design are illustrated in Figs. 1 and 2, respectively. |
Definitely yes;
Reasons: The flow diagram of the randomization procedure and the study design are illustrated in Figs. 1 and 2, respectively. |
Probably yes;
Reasons: The raters (occupational therapists who were only in contact with the patients during assessment), blinded to group assignment, were trained to administer outcome measures by senior therapists prior to the experiment, and passed a written competency and reliability test. |
Definitely yes;
Reasons: One patient was lost to follow-up in the intervention group due to unavailability for intervention. |
Definitely yes;
Reasons: All relevant outcomes were reported. |
Probably yes;
Reason: No other problems noted |
LOW |
|
Chiu, 2020 |
Probably yes;
Reasons: Randomization of 30 subjects 1:1 to active and sham groups was planned. |
Probably yes;
Reasons: Randomization of 30 subjects 1:1 to active and sham groups was planned. |
Definitely yes;
Reasons: Certified physical therapist, were blinded to treatment assignment. Blinding of subjects was successfully maintained. |
Definitely yes;
Reasons: One patient was lost to follow-up in the intervention group due to early termination. |
Definitely yes;
Reasons: All relevant outcomes were reported. |
Probably yes;
Reason: No other problems noted |
LOW |
|
Ren, 2019 |
Probably yes;
Reason: The patients were randomly assigned to three groups. |
Definitely yes;
Reason: The allocations were stored in sealed, numbered envelopes. The subjects did not know whether they were receiving real or sham rTMS. |
Definitely yes;
Reason: The language therapist assessed speech and language abilities and was blinded to the patients’ group assignments. All subjects, investigators (except the investigator responsible for rTMS application), clinicians, speech, and language therapists were blinded to patient assignment to real or sham rTMS. |
Probably yes;
Reason: Loss to follow-up was 9.3%. |
Definitely yes;
Reason: All relevant outcomes were reported |
Probably yes;
Reason: No other problems noted. |
LOW |
|
Rubi-Fessen, 2015 |
Definitely yes;
Reasons: Allocation to either real or sham stimulation was performed by a computer-generated allocation sequence |
Definitely yes;
Reasons: The allocation sequence was concealed by means of consecutively numbered sealed envelopes. |
Definitely yes;
Reasons: All therapy plans were developed by the same experienced speech and language therapist, who was blinded to group allocation and not involved in conducting the treatment. |
Definitely yes;
Reasons: Ten persons discontinued the study during initial magnetic resonance imaging (MRI) or withdrew after enrolment |
Definitely yes;
Reason: All relevant outcomes were reported |
Probably yes;
Reason: No other problems noted.
|
LOW |
|
Zumbansen, 2020 |
Probably yes:
Reasons: Computer-generated, non-restricted randomization by site was performed through an online system located at the Department of Clinical Epidemiology at the JGH (Montreal). |
Probably yes:
Reasons: Data and eligibility were entered by each study site coordinator into a web-based data capturing system. Only the technician performing the stimulation had access to the randomization information when logging into the study platform, on the first day of treatment. |
Definitely yes;
Reasons: Patients, therapist, principal investigators and research personnel assessing clinical outcomes were blinded to the treatment assignment. Therapists did not attend rTMS sessions and had no access to the tDCS device settings. |
Probably yes;
Reasons: 4 patients withdrew from the study before starting treatment, 7 patients discontinued intervention and 4 patients were lost at post evaluation. |
Definitely yes;
Reason: All relevant outcomes were reported |
Probably no;
Reason: Imprecision and multiplicity of analyses, violation of intention-to-treat analysis.
|
LOW |
|
Liu, 2020 |
Definitely yes;
Reasons: A computer-generated randomization table with a 1:1 ratio, created by a statistician not involved in the study, was used to randomly allocate patients into the TMS group or the sham group. |
Definitely yes;
Reasons: |
Definitely yes;
Reasons: |
Definitely yes;
Reasons: |
Definitely yes;
Reasons: All relevant outcomes were reported |
Probably yes;
Reasons: No other problems noted.
|
LOW |
|
Tsai, 2020 |
Definitely yes;
Reasons: Randomization order was computer generated and concealed in sequentially numbered opaque envelopes by an independent statistician. |
Probably yes;
Reasons: Therapists were blinded to group allocation. |
Probably yes;
Reasons: The study was double-blinded. |
Probably no;
Reasons: Three patients in the 5 Hz rTMS group withdrew from the intervention sessions because of commuting difficulties |
Definitely yes;
Reasons: All relevant outcomes were reported |
Probably yes;
Reasons: No other problems noted.
|
LOW |
|
Yin, 2020 |
Definitely yes;
Reasons: total of 34 PSCI patients were recruited for the present study between August 2017 and August 2019, which were subdivided for rTMS (n = 16) and no-stim control (n = 18) treatment groups using a computer-generated list of random numbers. |
Probably yes;
Reasons: No information was provided about the blinding of the randomization process. |
Probably yes:
Reasons: Therapists were blinded to assignments, but no information was provided about the blinding of patients, data collectors and outcome assessors. |
Definitely yes;
Reasons: Loss to follow-up was infrequent. |
Definitely yes;
Reasons: All relevant outcomes were reported. |
Probably no;
Reasons: Only two female patients were included in each group. It is undeniable that the findings were more suitable explaining for male patients.
|
LOW |
|
Iwanski, 2020 |
Definitely yes;
Reasons: Patients were randomly assigned (via a bespoke computer program) to either an experimental (rTMS), or control group (sham). |
Probably yes;
Reasons: Group assignments were known to the researchers who administered rTMS (ML and KP), while the neuropsychologist responsible for the cognitive assessment and leading therapy (SI) was blind to all patient treatment allocation. |
Definitely yes;
Reasons: Group assignments were known to the researchers who administered rTMS (ML and KP), while the neuropsychologist responsible for the cognitive assessment and leading therapy (SI) was blind to all patient treatment allocation. All participants had never experienced rTMS before, and were unaware whether they were receiving real or sham stimulation. |
Definitely yes;
Reasons: Loss to follow-up was infrequent. |
Definitely yes;
Reasons: All relevant outcomes were reported. |
Probably yes;
Reasons: No other problems noted.
|
LOW |
- Randomization: generation of allocation sequences have to be unpredictable, for example computer generated random-numbers or drawing lots or envelopes. Examples of inadequate procedures are generation of allocation sequences by alternation, according to case record number, date of birth or date of admission.
- Allocation concealment: refers to the protection (blinding) of the randomization process. Concealment of allocation sequences is adequate if patients and enrolling investigators cannot foresee assignment, for example central randomization (performed at a site remote from trial location). Inadequate procedures are all procedures based on inadequate randomization procedures or open allocation schedules.
- Blinding: neither the patient nor the care provider (attending physician) knows which patient is getting the special treatment. Blinding is sometimes impossible, for example when comparing surgical with non-surgical treatments, but this should not affect the risk of bias judgement. Blinding of those assessing and collecting outcomes prevents that the knowledge of patient assignment influences the process of outcome assessment or data collection (detection or information bias). If a study has hard (objective) outcome measures, like death, blinding of outcome assessment is usually not necessary. If a study has “soft” (subjective) outcome measures, like the assessment of an X-ray, blinding of outcome assessment is necessary. Finally, data analysts should be blinded to patient assignment to prevents that knowledge of patient assignment influences data analysis.
- If the percentage of patients lost to follow-up or the percentage of missing outcome data is large, or differs between treatment groups, or the reasons for loss to follow-up or missing outcome data differ between treatment groups, bias is likely unless the proportion of missing outcomes compared with observed event risk is not enough to have an important impact on the intervention effect estimate or appropriate imputation methods have been used.
- Results of all predefined outcome measures should be reported; if the protocol is available (in publication or trial registry), then outcomes in the protocol and published report can be compared; if not, outcomes listed in the methods section of an article can be compared with those whose results are reported.
- Problems may include: a potential source of bias related to the specific study design used (e.g. lead-time bias or survivor bias); trial stopped early due to some data-dependent process (including formal stopping rules); relevant baseline imbalance between intervention groups; claims of fraudulent behavior; deviations from intention-to-treat (ITT) analysis; (the role of the) funding body. Note: The principles of an ITT analysis implies that (a) participants are kept in the intervention groups to which they were randomized, regardless of the intervention they actually received, (b) outcome data are measured on all participants, and (c) all randomized participants are included in the analysis.
- Overall judgement of risk of bias per study and per outcome measure, including predicted direction of bias (e.g. favors experimental, or favors comparator). Note: the decision to downgrade the certainty of the evidence for a particular outcome measure is taken based on the body of evidence, i.e. considering potential bias and its impact on the certainty of the evidence in all included studies reporting on the outcome.
Tabel Exclusie na het lezen van het volledige artikel voor deelvraag 4 (neglect en andere cognitieve functiestoornissen)
|
Auteur en jaartal |
Redenen van exclusie |
|
Cha, 2015 |
Studie bevatte ≤ 10 patiënten per arm. |
|
Cotoi, 2019 |
Includeert maar één vorm van rTMS. |
|
Fan, 2018 |
Zoekdatum niet gespecificeerd. |
|
Fu, 2015 |
Studie bevatte ≤ 10 patiënten per arm. |
|
Cha, 2016 |
Al geïncludeerd in de review van van Lieshout (2019) |
|
Yang, 2015 |
Studie bevatte ≤ 10 patiënten per arm. |
|
Yang, 2017 |
De controlegroep kreeg ‘conventional rehabilitation’ in plaats van een sham. |
|
Kim, 2013 |
Studie bevatte ≤ 10 patiënten per arm. |
|
Kim, 2018 |
Studie bevatte ≤ 10 patiënten per arm. |
|
Lu, 2015 |
Al geïncludeerd in de review van van Lieshout (2019) |
|
Nyffeler, 2019 |
Studie bevatte ≤ 10 patiënten per arm. |
|
Salazar, 2018 |
Minder recent gezocht dan van Lieshout (2019) |
|
Vatanparasti, 2019 |
Studie bevatte ≤ 10 patiënten per arm. |
Verantwoording
Beoordelingsdatum en geldigheid
Publicatiedatum : 09-01-2023
Beoordeeld op geldigheid : 12-09-2024
Algemene gegevens
De ontwikkeling/herziening van deze richtlijnmodule werd ondersteund door het Kennisinstituut van de Federatie Medisch Specialisten (www.demedischspecialist.nl/kennisinstituut) en werd gefinancierd uit de Stichting Kwaliteitsgelden Medisch Specialisten (SKMS). De financier heeft geen enkele invloed gehad op de inhoud van de richtlijnmodule.
Samenstelling werkgroep
Voor het ontwikkelen van de richtlijnmodule is in 2021 een doorstart gemaakt met de multidisciplinaire werkgroep, bestaande uit vertegenwoordigers van alle relevante specialismen (zie hiervoor de Samenstelling van de werkgroep) die betrokken zijn bij de zorg voor patiënten met een herseninfarct of hersenbloeding.
Kerngroep
- Dr. B. (Bob) Roozenbeek (voorzitter), neuroloog, Erasmus MC Rotterdam, namens de Nederlandse Vereniging voor Neurologie (NVN)
- Prof. dr. R.M. (Renske) van den Berg-Vos, neuroloog, OLVG West Amsterdam, namens de NVN
- Prof. dr. J. (Jeannette) Hofmeijer, neuroloog, Rijnstate ziekenhuis Arnhem, namens de NVN
- Prof. dr. J.M.A. Visser-Meilij, revalidatiearts, UMC Utrecht, namens de VRA
- A.F.E. (Arianne) Verburg, huisarts, namens het Nederlands Huisartsen Genootschap (NHG)
- Prof. dr. H.B. (Bart) van der Worp, neuroloog, UMC Utrecht, namens de NVN
- Dr. S.M. (Yvonne) Zuurbier, neuroloog, Amsterdam UMC, namens de NVN
- Prof. dr. W. (Wim) van Zwam, radioloog, Maastricht UMC, namens de Nederlandse Vereniging voor Radiologie (NVvR)
- Prof. dr. G. (Gert) Kwakkel, hoogleraar neurorevalidatie, Amsterdam UMC, namens de Koninklijk Nederlands Genootschap foor Fysiotherapie (KNGF)
Met ondersteuning van
- Dr. M.L. Molag, adviseur, Kennisinstituut van de Federatie Medisch Specialisten
- Drs. F. Ham, junior adviseur, Kennisinstituut van de Federatie Medisch Specialisten
Belangenverklaringen
De Code ter voorkoming van oneigenlijke beïnvloeding door belangenverstrengeling is gevolgd. Alle werkgroepleden hebben schriftelijk verklaard of zij in de laatste drie jaar directe financiële belangen (betrekking bij een commercieel bedrijf, persoonlijke financiële belangen, onderzoeksfinanciering) of indirecte belangen (persoonlijke relaties, reputatiemanagement) hebben gehad. Gedurende de ontwikkeling of herziening van een module worden wijzigingen in belangen aan de voorzitter doorgegeven. De belangenverklaring wordt opnieuw bevestigd tijdens de commentaarfase.
Een overzicht van de belangen van werkgroepleden en het oordeel over het omgaan met eventuele belangen vindt u in onderstaande tabel. De ondertekende belangenverklaringen zijn op te vragen bij het secretariaat van het Kennisinstituut van de Federatie Medisch Specialisten.
|
Werkgroeplid |
Functie |
Nevenfuncties |
Gemelde belangen |
Ondernomen actie |
|
Van den Berg-Vos |
Neuroloog |
Geen |
Voorzitter werkgroep CVA van het Transmuraal Platform Amsterdam (betaald d.m.v. vacatiegelden) Lid focusgroep CVA ROAZ Noord-Holland (onbetaald) ‘Medical lead' Experiment Uitkomstindicatoren VWS (namens Santeon) aandoening CVA, via Santeon betaald voor 4 uur per week Projectleider “Patient-Reported Outcomes Measurement Information System (PROMIS®) voor waardegedreven zorg" (SKMS-project met subsidie FMS, betaald d.m.v. vacatiegelden) Projectleider "Regionale auditing voor kwaliteit van zorg bij patiënten met een herseninfarct” (SKMS-project met subsidie FMS , betaald dmv vacatiegelden) Voorzitterschap (namens de Nederlandse Vereniging voor Neurologie) van werkgroep CVA van het programma Uitkomstgerichte Zorg ‘Meer inzicht in uitkomsten’ (betaald d.m.v. vacatiegelden) |
Geen |
|
Hofmeijer |
Neuroloog (0,6 fte) |
Hoogleraar universiteit Twente (0,4 fte) |
Geen |
Geen |
|
Kwakkel |
Hoogleraar Neurorevalidatie |
Europees Editor NeuroRehabilitation and Neural Repair
|
Handling editor Stroke (AHA) Coordinator Stroke Unit Cursus NPI Cursusleider mCIMT bij NPI Cursusleider Neurorebvalidatie-CVA bij NPI
|
Geen |
|
Roozenbeek |
Neuroloog |
|
Lid van CONTRAST, coördineert onderzoeksprojecten op gebied van acute beroertezorg gefinancierd door Stichting BeterKeten, Stichting THEIA, Erasmus Universiteit en Erasmus MC |
Geen |
|
Verburg |
Huisarts |
Senior wetenschappelijk medewerker NHG |
Geen |
Geen |
|
Visser-Meily |
Revalidatiearts, hoogleraar en afdelingshoofd |
Geen |
Geen |
Geen |
|
Van der Worp |
Neuroloog |
|
Adviezen aan/consultancy voor Boehringer Ingelheim, producent van onder anderen alteplase en dabigatron. Adviseur van Bayer en LivaNova; subsidie van Stryker voor stroke trial (gelden via het CONTRAST consortium); mede-onderzoeker B-STARS, een inmiddels afgeronde RCT van rTMS bij patiënten met een beroerte. |
Uitsluiting besluitvorming alteplase en dabigatran |
|
Zuurbier |
Neuroloog |
Geen |
Geen |
Geen |
|
Van Zwam |
Neuro-interventieradioloog |
Geen |
Consultancy activiteiten voor stryker en Cerenovus, lid CONTRAST, MRCLEAN: ATE |
Geen invloed op richtlijnonderwerpen |
Inbreng patiëntenperspectief
Er werd aandacht besteed aan het patiëntenperspectief door gebruik te maken van kwaliteitscriteria vanuit patiëntenperspectief voor CVA, ontwikkeld door Harteraad. Verder informeren Harteraad, Hartstichting en Hersenletsel door middel van notulen vergaderingen kerngroep en worden ze betrokken bij relevante onderwerpen. De conceptmodules zijn tevens voor commentaar aan bovengenoemde verenigingen voorgelegd.
Wkkgz & Kwalitatieve raming van mogelijke substantiële financiële gevolgen
Kwalitatieve raming van mogelijke financiële gevolgen in het kader van de Wkkgz
Bij de richtlijn is conform de Wet kwaliteit, klachten en geschillen zorg (Wkkgz) een kwalitatieve raming uitgevoerd of de aanbevelingen mogelijk leiden tot substantiële financiële gevolgen. Bij het uitvoeren van deze beoordeling zijn richtlijnmodules op verschillende domeinen getoetst (zie het stroomschema op de Richtlijnendatabase).
Uit de kwalitatieve raming blijkt dat er waarschijnlijk geen substantiële financiële gevolgen zijn, zie onderstaande tabel.
Submodule |
Uitkomst raming |
Toelichting |
|
Non-invasieve hersenstimulatie met rTMS |
Geen financiële gevolgen |
Hoewel uit de toetsing volgt dat de aanbeveling(en) breed toepasbaar zijn (>40.000 patiënten), volgt ook uit de toetsing dat [het overgrote deel (±90%) van de zorgaanbieders en zorgverleners al aan de norm voldoet OF het geen nieuwe manier van zorgverlening of andere organisatie van zorgverlening betreft, het geen toename in het aantal in te zetten voltijdsequivalenten aan zorgverleners betreft en het geen wijziging in het opleidingsniveau van zorgpersoneel betreft]. Er worden daarom geen substantiële financiële gevolgen verwacht. |
|
Non-invasieve hersenstimulatie met tDCS |
Geen financiële gevolgen |
Hoewel uit de toetsing volgt dat de aanbeveling(en) breed toepasbaar zijn (>40.000 patiënten), volgt ook uit de toetsing dat [het overgrote deel (±90%) van de zorgaanbieders en zorgverleners al aan de norm voldoet OF het geen nieuwe manier van zorgverlening of andere organisatie van zorgverlening betreft, het geen toename in het aantal in te zetten voltijdsequivalenten aan zorgverleners betreft en het geen wijziging in het opleidingsniveau van zorgpersoneel betreft]. Er worden daarom geen substantiële financiële gevolgen verwacht. |
De kwalitatieve raming volgt na de commentaarfase.
Werkwijze
AGREE
Deze richtlijnmodule is opgesteld conform de eisen vermeld in het rapport Medisch Specialistische Richtlijnen 2.0 van de adviescommissie Richtlijnen van de Raad Kwaliteit. Dit rapport is gebaseerd op het AGREE II instrument (Appraisal of Guidelines for Research & Evaluation II; Brouwers, 2010).
Knelpuntenanalyse en uitgangsvragen
Tijdens de voorbereidende fase inventariseerden de werkgroep de knelpunten in de zorg voor patiënten na een herseninfarct of hersenbloeding. Op basis van de uitkomsten van de knelpuntenanalyse zijn door de werkgroep concept-uitgangsvragen opgesteld en definitief vastgesteld.
Uitkomstmaten
Na het opstellen van de zoekvraag behorende bij de uitgangsvraag inventariseerde de werkgroep welke uitkomstmaten voor de patiënt relevant zijn, waarbij zowel naar gewenste als ongewenste effecten werd gekeken. Hierbij werd een maximum van acht uitkomstmaten gehanteerd. De werkgroep waardeerde deze uitkomstmaten volgens hun relatieve belang bij de besluitvorming rondom aanbevelingen, als cruciaal (kritiek voor de besluitvorming), belangrijk (maar niet cruciaal) en onbelangrijk. Tevens definieerde de werkgroep tenminste voor de cruciale uitkomstmaten welke verschillen zij klinisch (patiënt) relevant vonden.
Methode literatuursamenvatting
Een uitgebreide beschrijving van de strategie voor zoeken en selecteren van literatuur en de beoordeling van de risk-of-bias van de individuele studies is te vinden onder ‘Zoeken en selecteren’ onder Onderbouwing. De beoordeling van de kracht van het wetenschappelijke bewijs wordt hieronder toegelicht.
Beoordelen van de kracht van het wetenschappelijke bewijs
De kracht van het wetenschappelijke bewijs werd bepaald volgens de GRADE-methode. GRADE staat voor ‘Grading Recommendations Assessment, Development and Evaluation’ (zie http://www.gradeworkinggroup.org/). De basisprincipes van de GRADE-methodiek zijn: het benoemen en prioriteren van de klinisch (patiënt) relevante uitkomstmaten, een systematische review per uitkomstmaat, en een beoordeling van de bewijskracht per uitkomstmaat op basis van de acht GRADE-domeinen (domeinen voor downgraden: risk of bias, inconsistentie, indirectheid, imprecisie, en publicatiebias; domeinen voor upgraden: dosis-effect relatie, groot effect, en residuele plausibele confounding).
GRADE onderscheidt vier gradaties voor de kwaliteit van het wetenschappelijk bewijs: hoog, redelijk, laag en zeer laag. Deze gradaties verwijzen naar de mate van zekerheid die er bestaat over de literatuurconclusie, in het bijzonder de mate van zekerheid dat de literatuurconclusie de aanbeveling adequaat ondersteunt (Schünemann, 2013; Hultcrantz, 2017).
|
GRADE |
Definitie |
|
Hoog |
|
|
Redelijk |
|
|
Laag |
|
|
Zeer laag |
|
Bij het beoordelen (graderen) van de kracht van het wetenschappelijk bewijs in richtlijnen volgens de GRADE-methodiek spelen grenzen voor klinische besluitvorming een belangrijke rol (Hultcrantz, 2017). Dit zijn de grenzen die bij overschrijding aanleiding zouden geven tot een aanpassing van de aanbeveling. Om de grenzen voor klinische besluitvorming te bepalen moeten alle relevante uitkomstmaten en overwegingen worden meegewogen. De grenzen voor klinische besluitvorming zijn daarmee niet één op één vergelijkbaar met het minimaal klinisch relevant verschil (Minimal Clinically Important Difference, MCID). Met name in situaties waarin een interventie geen belangrijke nadelen heeft en de kosten relatief laag zijn, kan de grens voor klinische besluitvorming met betrekking tot de effectiviteit van de interventie bij een lagere waarde (dichter bij het nuleffect) liggen dan de MCID (Hultcrantz, 2017).
Overwegingen (van bewijs naar aanbeveling)
Om te komen tot een aanbeveling zijn naast (de kwaliteit van) het wetenschappelijke bewijs ook andere aspecten belangrijk en worden meegewogen, zoals aanvullende argumenten uit bijvoorbeeld de biomechanica of fysiologie, waarden en voorkeuren van patiënten, kosten (middelenbeslag), aanvaardbaarheid, haalbaarheid en implementatie. Deze aspecten zijn systematisch vermeld en beoordeeld (gewogen) onder het kopje ‘Overwegingen’ en kunnen (mede) gebaseerd zijn op expert opinion. Hierbij is gebruik gemaakt van een gestructureerd format gebaseerd op het evidence-to-decision framework van de internationale GRADE Working Group (Alonso-Coello, 2016a; Alonso-Coello, 2016b). Dit evidence-to-decision framework is een integraal onderdeel van de GRADE-methodiek.
Formuleren van aanbevelingen
De aanbevelingen geven antwoord op de uitgangsvraag en zijn gebaseerd op het beschikbare wetenschappelijke bewijs en de belangrijkste overwegingen, en een weging van de gunstige en ongunstige effecten van de relevante interventies. De kracht van het wetenschappelijk bewijs en het gewicht dat door de werkgroep wordt toegekend aan de overwegingen, bepalen samen de sterkte van de aanbeveling. Conform de GRADE-methodiek sluit een lage bewijskracht van conclusies in de systematische literatuuranalyse een sterke aanbeveling niet a priori uit, en zijn bij een hoge bewijskracht ook zwakke aanbevelingen mogelijk (Agoritsas, 2017; Neumann, 2016). De sterkte van de aanbeveling wordt altijd bepaald door weging van alle relevante argumenten tezamen. De werkgroep heeft bij elke aanbeveling opgenomen hoe zij tot de richting en sterkte van de aanbeveling zijn gekomen.
In de GRADE-methodiek wordt onderscheid gemaakt tussen sterke en zwakke (of conditionele) aanbevelingen. De sterkte van een aanbeveling verwijst naar de mate van zekerheid dat de voordelen van de interventie opwegen tegen de nadelen (of vice versa), gezien over het hele spectrum van patiënten waarvoor de aanbeveling is bedoeld. De sterkte van een aanbeveling heeft duidelijke implicaties voor patiënten, behandelaars en beleidsmakers (zie onderstaande tabel). Een aanbeveling is geen dictaat, zelfs een sterke aanbeveling gebaseerd op bewijs van hoge kwaliteit (GRADE gradering HOOG) zal niet altijd van toepassing zijn, onder alle mogelijke omstandigheden en voor elke individuele patiënt.
|
Implicaties van sterke en zwakke aanbevelingen voor verschillende richtlijngebruikers |
||
|
|
Sterke aanbeveling |
Zwakke (conditionele) aanbeveling |
|
Voor patiënten |
De meeste patiënten zouden de aanbevolen interventie of aanpak kiezen en slechts een klein aantal niet. |
Een aanzienlijk deel van de patiënten zouden de aanbevolen interventie of aanpak kiezen, maar veel patiënten ook niet. |
|
Voor behandelaars |
De meeste patiënten zouden de aanbevolen interventie of aanpak moeten ontvangen. |
Er zijn meerdere geschikte interventies of aanpakken. De patiënt moet worden ondersteund bij de keuze voor de interventie of aanpak die het beste aansluit bij zijn of haar waarden en voorkeuren. |
|
Voor beleidsmakers |
De aanbevolen interventie of aanpak kan worden gezien als standaardbeleid. |
Beleidsbepaling vereist uitvoerige discussie met betrokkenheid van veel stakeholders. Er is een grotere kans op lokale beleidsverschillen. |
Organisatie van zorg
In de knelpuntenanalyse en bij de ontwikkeling van de richtlijnmodule is expliciet aandacht geweest voor de organisatie van zorg: alle aspecten die randvoorwaardelijk zijn voor het verlenen van zorg (zoals coördinatie, communicatie, (financiële) middelen, mankracht en infrastructuur). Randvoorwaarden die relevant zijn voor het beantwoorden van deze specifieke uitgangsvraag zijn genoemd bij de overwegingen. Meer algemene, overkoepelende, of bijkomende aspecten van de organisatie van zorg worden behandeld in de module Organisatie van zorg.
Commentaar- en autorisatiefase
De conceptmodule werd aan de betrokken (wetenschappelijke) verenigingen, instanties en (patiënt) organisaties voorgelegd ter commentaar. De commentaren werden verzameld en besproken met de werkgroep. Naar aanleiding van de commentaren werd de conceptmodule aangepast en definitief vastgesteld door de werkgroep. De definitieve module werd aan de deelnemende (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd voor autorisatie en door hen geautoriseerd dan wel geaccordeerd. De commentaartabel is op te vragen bij het Kennisinstituut via secretariaat@kennisinstituut.nl.
Zoekverantwoording
Algemene informatie
|
Richtlijn: Herseninfarct en hersenbloeding |
|
|
Uitgangsvraag: Welke patiënten hebben baat bij non-invasieve hersenstimulatie TMS? |
|
|
Database(s): Embase, Medline |
Datum: 22-10-2020 |
|
Periode: Geen restrictie |
Talen: Engels |
|
Literatuurspecialist: Linda Niesink |
|
|
Toelichting en opmerkingen:
→ Voor deze vraag is gezocht op de elementen hersenbloeding/herseninfarct (in het blauw) en Transcranial Magnetic Stimulation (TMS) (in het groen).
→ De opgegeven sleutelartikelen van Elsner (2016) (2017) (2020) en van Lieshout (2019) worden gevonden met de zoekopdracht.
à Voor de RCT’s is gezocht vanaf 2015. |
|
Zoekopbrengst
|
|
Embase |
OVID/MEDLINE |
Ontdubbeld |
|
SRs |
286 |
179 |
286 |
|
RCT |
437 |
479 |
512 |
|
Totaal |
723 |
564 |
798 |
Zoekverantwoording
|
Database |
Zoektermen |
|||||||||||||||||||||||||||
|
Embase
|
|
|||||||||||||||||||||||||||
|
Medline (OVID)
|
1 exp brain ischemia/ (narrower terms zijn: exp brain infarction/ or brain stem infarctions/ or lateral medullary syndrome/ or cerebral infarction/ or dementia, multi-infarct/ or infarction, anterior cerebral artery/ or infarction, middle cerebral artery/ or infarction, posterior cerebral artery/ or hypoxia-ischemia, brain/ or ischaemic attack, transient/ or vertebrobasilar insufficiency/ or subclavian steal syndrome/) (109955) 2 exp "intracranial embolism and thrombosis"/ or (((infarct* or isch?emi* or accident* or hemorrhage* or haemorrhage*) adj3 (brain or cerebral or cerebro or cerebrovascular or intracerebral or intracranial)) or ('transient ischaemic attack*' or tia or cva or stroke or poststroke)).ti,ab,kw. (343978) 3 1 or 2 (376270) 4 limit 3 to english (333779) 5 Transcranial Magnetic Stimulation/ or Transcranial Direct Current Stimulation/ or ((transcranial or 'trans cranial' or noninvasive or 'non-invasive') adj3 ('magnetic stimulat*' or 'electric stimulat*' or 'brain stimulat*')).ti,ab,kw. or ('theta burst stimulat*' or tms or nibs or tes tdcs or tbs or neurostimulat* or 'neuro stimulat*' or neuromodulat* or 'neuro modulat*').ti,ab,kw. (47279) 6 4 and 5 (2390) 7 (meta-analysis/ or meta-analysis as topic/ or (metaanaly* or meta-analy* or metanaly*).ti,ab,kf. or systematic review/ or cochrane.jw. or (prisma or prospero).ti,ab,kf. or ((systemati* or scoping or umbrella or "structured literature") adj3 (review* or overview*)).ti,ab,kf. or (systemic* adj1 review*).ti,ab,kf. or ((systemati* or literature or database* or data-base*) adj10 search*).ti,ab,kf. or ((structured or comprehensive* or systemic*) adj3 search*).ti,ab,kf. or ((literature adj3 review*) and (search* or database* or data-base*)).ti,ab,kf. or (("data extraction" or "data source*") and "study selection").ti,ab,kf. or ("search strategy" and "selection criteria").ti,ab,kf. or ("data source*" and "data synthesis").ti,ab,kf. or (medline or pubmed or embase or cochrane).ab. or ((critical or rapid) adj2 (review* or overview* or synthes*)).ti. or (((critical* or rapid*) adj3 (review* or overview* or synthes*)) and (search* or database* or data-base*)).ab. or (metasynthes* or meta-synthes*).ti,ab,kf.) not (comment/ or editorial/ or letter/ or ((exp animals/ or exp models, animal/) not humans/))(457758) 8 (exp clinical trial/ or randomized controlled trial/ or exp clinical trials as topic/ or randomized controlled trials as topic/ or Random Allocation/ or Double-Blind Method/ or Single-Blind Method/ or (clinical trial, phase i or clinical trial, phase ii or clinical trial, phase iii or clinical trial, phase iv or controlled clinical trial or randomized controlled trial or multicenter study or clinical trial).pt. or random*.ti,ab. or (clinic* adj trial*).tw. or ((singl* or doubl* or treb* or tripl*) adj (blind$3 or mask$3)).tw. or Placebos/ or placebo*.tw.) not (animals/ not humans/) (2037813) 9 6 and 7 (179) - SRs 10 6 and 8 (840) 11 limit 10 to yr="2015 -Current" (479) - RCTs |
