Tranexaminezuur ter preventie van HPP
Uitgangsvraag
Wat is de rol van tranexaminezuur in de preventie van HPP?
Aanbeveling
Aanbeveling-1
Geef niet routinematig preventief tranexaminezuur aan alle vrouwen die per sectio bevallen, maar beperk het gebruik tot vrouwen met een verhoogd risico op HPP op basis van meerdere risicofactoren.
Voor de identificatie van risicofactoren voor HPP en de behandeling van HPP wordt verwezen naar de Fluxus Implementatie Strategie en naar het modelprotocol behandeling van HPP.
Aanbeveling-2
Geef vrouwen die vaginaal baren zonder een verhoogd risico op haemorrhagia postpartum niet routinematig tranexaminezuur na de geboorte van het kind.
Overweeg het preventief toedienen van tranexaminezuur postpartum bij vrouwen die vaginaal baren en die een verhoogd risico op haemorrhagia postpartum hebben op basis van meerdere risicofactoren.
Voor de identificatie van risicofactoren voor HPP en de behandeling van HPP wordt verwezen naar de Fluxus Implementatie Strategie en naar het modelprotocol behandeling van HPP.
Overwegingen
Voor- en nadelen van de interventie en de kwaliteit van het bewijs
Gezien het vrij hoge en mogelijk toenemende percentage haemorrhagia postpartum, ook in de hoge inkomenslanden, is het een overweging om profylactisch tranexaminezuur te geven.
Uit de literatuur blijkt dat bij een bevalling per sectio caesarea de hoeveelheid bloedverlies en de noodzaak van toedienen van additionele uterotonica, en in mindere mate de noodzaak tot toedienen van bloedtransfusie, minder is als direct na afnavelen of voor incisie TXA toegediend is. De systematische review van Cheema et al. laat zien dat het effect van TXA bij een sectio groter is wanneer het vóór de incisie wordt toegediend, vergeleken met toediening direct na afnavelen (RR voor >1000 mL bloedverlies: 0,33 versus 0,86). Daarnaast lijkt het effect bij high risk zwangeren groter dan bij low risk zwangeren. In deze review laten twee grote, methodologisch sterke studies echter minder effect zien dan meerdere kleinere studies die tevens bredere betrouwbaarheidsintervallen hebben (Pacheco 2023, Sentilhes 2021). Daarom lijkt het zinvol om de afweging om routinematig TXA toe te dienen apart te maken voor een hoog risico geboorte met naast sectio caesarea nog andere risicofactoren.
Voor vrouwen die vaginaal bevallen lijkt er wel een positief effect van de toediening van TXA en de noodzaak tot toediening van additionele uterotonica, maar in mindere mate dan bij vrouwen die per sectio bevallen. Ook hier geldt dat het effect bij high risk zwangeren groter is dan bij low risk zwangeren. Op grond van de beschikbare literatuur is het niet mogelijk om een goed onderbouwde uitspraak te doen of en voor welke subgroep van (high risk) barenden eventuele routinematige toediening van TXA bij een vaginale baring wel van voordeel zou zijn.
De vraag is of de klinische relevantie over de totale groep groot genoeg is om TXA aan iedereen preventief toe te dienen.
Verder is het goed om te realiseren dat in de preventieve setting de controlegroepen in de studies niet overeenkomen met de huidige klinische praktijk in Nederland: in de studies wordt preventie vergeleken met geen tranexaminezuur, de huidige klinische praktijk in de meeste klinieken in Nederland is dat tranexaminezuur wordt gegeven bij bloedverlies van meer dan 500 mL.
In praktische zin zal de routinematige toediening van TXA bij sectio geen probleem zijn, daar alle vrouwen die een sectio ondergaan een iv toegang zullen hebben.
Ook high risk vrouwen die vaginaal bevallen zullen in het algemeen een IV toegang hebben.
Hoewel TXA een veilig middel is, worden ook bijwerkingen gerapporteerd die moeten worden meegenomen bij de afweging wel of niet routinematig geven. Wanneer TXA bij sectio voor incisie gegeven wordt, zijn in de praktijk weinig bijwerkingen als misselijkheid en braken waargenomen. Als het na afnavelen gegeven wordt, kunnen eventuele misselijkheid en braken ook veroorzaakt worden door andere middelen die dan toegediend worden. Wat betreft de foetus vermeldt het LAREB dat tranexaminezuur de placenta passeert. Vooral tijdens de tweede helft van de zwangerschap is ervaring met dit middel opgedaan. Deze beperkte gegevens laten tot nu toe geen verhoogde kans zien op trombose bij moeder en neonaat of op andere nadelige effecten bij de foetus.
Waarden en voorkeuren van patiënten (en evt. hun verzorgers)
Voor veel vrouwen in Nederland is baren een fysiologisch proces, waarbij zij wensen dat toediening van (preventieve) medicijnen die geen bewezen meerwaarde hebben, wordt tegengegaan. Daarom moet zorgvuldig worden afgewogen of er voldoende reden is om routinematig een intraveneus product toe te dienen tijdens alle baringen. Zoals hierboven beschreven lijkt dit niet verdedigbaar voor alle barenden. Een infuus kan zorgen voor een beperktere bewegingsvrijheid. Gebonden zijn aan bed kan leiden tot negatievere bevalervaringen. Een waaknaald kan een oplossing zijn om dit te voorkomen. Belangrijk om deze keuzes en mogelijkheden voorafgaand aan de bevalling met de zwangere te bespreken. Echter, een waaknaald zal de bewegingsvrijheid niet negatief beïnvloeden. Voor vrouwen die een sectio ondergaan lijken er echter voldoende voordelen om TXA routinematig toe te dienen. Ook bij hoog risico op fluxus bij een vaginale bevalling is TXA toediening een overweging. Deze vrouwen zullen hier in de regel geen hinder van ondervinden, omdat behoudens de toediening zelf er geen extra handelingen nodig zijn, en de bijwerkingen acceptabel zijn.
Voor vrouwen is het belangrijk dat zij goed geïnformeerd zijn over de kans op HPP en eerlijke, neutrale informatie ontvangen over mogelijke preventieve maatregelen.
Kosten (middelenbeslag)
TXA is een relatief goedkoop en goed te bewaren middel. De prijs van 2 ampullen tranexaminezuur (5 ml (100mg/ml) per ampul) is 3,08 euro.
Aanvaardbaarheid, haalbaarheid en implementatie
Zoals hierboven beschreven lijkt routinematige toediening van TXA niet verdedigbaar voor alle barenden. Voor vrouwen die een sectio ondergaan met daarnaast andere risicofactoren op bloedverlies lijken er echter voldoende voordelen om TXA profylactisch toe te dienen. Deze vrouwen zullen hier in de regel geen hinder van ondervinden, omdat behoudens de toediening zelf er geen extra handelingen nodig zijn, en de bijwerkingen acceptabel zijn.
Voor high risk vrouwen die vaginaal gaan bevallen kan een aparte afweging gemaakt worden of routinematige TXA toediening wenselijk is.
Uiteraard zullen vrouwen van tevoren net als over alle andere handelingen die verricht worden geïnformeerd worden.
Rationale van aanbeveling-1: weging van argumenten voor en tegen de interventies.
Omdat bij routinematige toediening van TXA bij vrouwen die een sectio ondergaan, met naast deze risiciofactor tevens andere risicofactoren op bloedverlies postpartum, met betrekking tot hoeveelheid bloedverlies, additionele uterotonica en bloedtransfusie, de voordelen groter lijken dan de nadelen is de aanbeveling te overwegen deze vrouwen routinematig TXA te geven, bij voorkeur voor de incisie en anders direct na de geboorte van het kind.
Rationale van aanbeveling-2: weging van argumenten voor en tegen de interventie
Omdat de werkgroep van mening is dat er onvoldoende voordelen zijn van routinematige toediening van TXA bij vaginaal barenden, met betrekking tot hoeveelheid bloedverlies en bloedtransfusie, wordt routinematige toediening afgeraden. Voor high risk vrouwen kan echter een andere afweging gemaakt worden.
Onderbouwing
Achtergrond
Tranexamic acid (TXA) is an antifibrinolytic which reduces blood loss and transfusion requirements in cardiac surgery and trauma patients by preventing breakdown of fibrinogen and fibrin. Pregnant women in the third trimester are hypercoagulable.
The question is whether TXA adds any improvement in prevention of postpartum haemorrhage in addition to usual care with the uterotonics oxytocin or carbetocin.
Furthermore, it is unclear if all women would benefit from this preventing measure and at which time and in which dosage TXA should be given.
Conclusies / Summary of Findings
Women after a cesarean section
|
Very low GRADE |
The effect of routine TXA-administration on blood loss > 500 mL is very uncertain in comparison with no routine administration of TXA in women during a cesarean section.
Source: Cheema (2023) |
|
Low GRADE |
The routine administration of TXA may reduce the risk of blood loss > 1000 mL in comparison with no routine administration of TXA in women during a cesarean section.
Source: Cheema (2023) |
|
Moderate GRADE |
The routine administration of TXA likely reduces mean blood loss in comparison with no routine administration of TXA in women during a cesarean section.
Source: Cheema (2023), Ali (2019), Singh (2014) |
|
No GRADE |
No evidence was found regarding the effect of TXA on shock when compared with no routine administration of TXA in women duringa cesarean section.
|
|
High GRADE |
The routine administration of TXA results in little to no difference in maternal death when compared with no routine administration of TXA in women during a cesarean section.
Source: Cheema (2023) |
|
Moderate GRADE |
The routine administration of TXA likely reduces the need for additional uterotonics in comparison with no routine administration of TXA in women during a cesarean section.
Source: Cheema (2023) |
|
Low GRADE |
The routine TXA may reduce the need for blood transfusion in comparison with no routine administration of TXA in women during a cesarean section.
Source: Cheema (2023) |
|
Low GRADE |
The routine administration of TXA may reduce the need for transfer for higher level of care when compared with no routine administration of TXA in women during a cesarean section.
Source: El-Gaber (2018), Ghosh (2014), Nargis and Dewan (2020), Pacheco (2023), Yehia (2014). |
Women after a vaginal delivery
|
Very low GRADE |
The evidence is very uncertain about the effect of routine administration of TXA on blood loss > 500 mL when compared with no routine administration of TXA in women after a vaginal delivery.
Source: Igboke, 2022; Sentilhes, 2018; Sujita, 2018 |
|
Very low GRADE |
The evidence is very uncertain about the effect of routine administration of TXA on blood loss > 1000 mL when compared with no routine administration of TXA in women after a vaginal delivery.
Source: Sentilhes (2018), Sujita (2018) |
|
Very low GRADE |
The evidence is very uncertain about the effect of routine administration of TXA on mean blood loss when compared with no routine administration of TXA in women after a vaginal delivery.
Source: Assis (2023), Igboke (2022), Kashanian (2022) |
|
No GRADE |
No evidence was found regarding the effect of routine TXA on shock or maternal death when compared with no routine administration of TXA in women after vaginal delivery. |
|
Low GRADE |
The routine administration of TXA may reduce the need for additional uterotonics when compared with no routine administration of TXA in women after a vaginal delivery.
Source: Assis (2023), Igboke (2022), Kashanian (2022) |
|
Very low GRADE |
The evidence is very uncertain about the effect of administration of TXA on the need for blood transfusion when compared with no routine administration of TXA in women after a vaginal delivery.
Source: Assis (2023), Igboke (2022), Kashanian (2022) |
|
Moderate GRADE |
Administration of TXA likely reduces the need for transfer for higher level of care when compared with no routine administration of TXA in women after a vaginal delivery.
Source: Sentilhes (2018) |
Women postpartum (both cesarean section and vaginal delivery)
|
Moderate GRADE
Low GRADE
Very low GRADE
|
The administration of TXA likely increases mild side effects, vomiting and diarrhea in women postpartum in comparison with no routine administration of TXA.
Administration of TXA may result in an increase in nausea when compared with no routine administration of TXA in women postpartum .
Administration of TXA may result in little to no difference in headache when compared with no routine administration of TXA in women postpartum .
Source: Ghosh (2014), Malathi (2016), Obi (2019), Sentilhes (2018), Sujita (2018), Fahmy (2021), Gungorduk (2013), Ray (2016), Igboke (2022), Kashanian (2022). |
|
No GRADE |
No evidence was found regarding the effect of TXA on women’s sense of wellbeing, acceptability and satisfaction with the intervention, breastfeeding, post-traumatic stress syndrome when compared with no routine administration of TXA in women postpartum . |
Samenvatting literatuur
Description of studies
Cheema (2023) conducted a systematic review of the literature and meta-analysis to evaluate the efficacy and safety of TXA in low- and high-risk cesarean deliveries. Databases were searched until February 2023 for RCTs that investigated the effect of prophylactic use of intravenous TXA in addition to standard uterotonic agents in comparison with placebo or standard treatment on blood loss in women who underwent a cesarean delivery. In total, 50 studies were included in the review. Yet, since some of these studies did not match the intervention as described in our PICO, these studies were excluded from our literature summary. We included twenty-two studies from the review from Cheema (2023) in our literature summary. All information from these studies, including the risk of bias assessment, is derived from Cheema (2023).
Assis (2023) conducted a systematic review of the literature and meta-analysis to evaluate the efficacy of prophylactic administration of TXA on blood loss in women during cesarean or vaginal delivery. Databases were searched for RCTs until January 2020. In total, sixteen studies were included in the review. Of these studies, 5 studies were also included in the review of Cheema (2023) and eight studies did not meet our PICO. Therefore, only three studies were included in our literature summary. All information from these studies, including the risk of bias assessment, is derived from Assis (2023).
Kashanian (2022) performed a double-blind placebo controlled RCT in Iran, to evaluate the effect of TXA on the amount of blood loss after vaginal delivery. To this aim, 207 low-risk pregnant women who delivered vaginally were randomized into the intervention group (received 10 mg/kg TXA in 100 ml normal saline, n=104) or control group (received one vial of distilled water in 100 ml normal saline, n=103). Outcome measures were evaluated 6 hours postpartum and included blood loss, need for additional uterotonics and need for blood transfusion. There were some concerns regarding the risk of bias for this study, due to no clearly reported allocation concealment, loss to follow-up and no study protocol available.
Khurshid (2022) performed a RCT in Pakistan, to evaluate the use of prophylactic TXA in reducing blood loss during the third stage of labour. In this study, 116 women with a singleton pregnancy, labouring at term were randomized into the intervention group (1 gram of TXA in 20 ml 5% dextrose water, n=58) or the control group (20 ml of 5% dextrose water, n=58). Both medications were administered at the time of delivery of the shoulder. In this study, the mode of delivery was not specified. Blood loss during the third stage of labour was the main outcome measure in this study. The risk of bias for this study was considered high, due to a very brief description of the study and no description of randomization, allocation concealment and blinding.
Igboke (2022) conducted a double-blind placebo controlled RCT to study the efficacy and safety of intravenous TXA in a university hospital in Nigeria. In this study, 176 women who delivered vaginally were randomized into the intervention group (1 gr TXA, n=78) or the control group (10 ml water, n=84). In both groups, medications were administered within 2 minutes after childbirth and prophylactic oxytocin was administered after cord clamping. Outcome measures were blood loss 2 hours postpartum, blood loss>500 mL, blood transfusion and use of uterotonics. There were some concerns regarding the risk of bias for this study, due to a brief description of the randomization method, no clear description of the allocation concealment, loss to follow-up and no study protocol available.
Masood (2023) performed a RCT in Pakistan, to evaluate the effect of TXA on the reduction of blood loss. Women who underwent elective cesarean section were randomized into the intervention group (1 gr TXA 15 minutes prior to incision, n=30) or the control group (no TXA, n=30). Both groups received 10 IU oxytocin after delivery. The main outcome was estimated blood loss. The risk of bias for this study was considered high, due to a very brief description of the study and no description of randomization, allocation concealment and blinding.
Singh (2014) performed a RCT in India, in 200 women undergoing cesarean section, to study the efficacy and safety of TXA in reducing blood loss. Women were randomized into the intervention group (1 gr TXA immediately 20 minutes before incision, n=100) or the control group (no TXA, n=100). All women received 20 units of Pitocin in 500 mL ringer lactate immediately after childbirth. Outcome measures were intraoperative blood loss and 2 hours postoperative blood loss. The risk of bias for this study was considered high, due to a very brief description of the study and no description of randomization, allocation concealment and blinding.
Ali (2019) performed a RCT in Egypt in 200 pregnant women who underwent elective cesarean section, to determine the efficacy and safety of TXA in reducing blood loss after cesarean section. In total, 200 women were randomized to the intervention group (1 gr TXA in 200 ml normal saline, n=100) or the control group (no TXA, n=100). After delivery, 5 units of oxytocin in 500 mL normal saline was given to all participants. Blood loss was measured from delivery of the placenta to the end of surgery and from the end of surgery to 2 hours postpartum . Other outcome measures included blood transfusion and maternal side effects. The risk of bias for this study was considered high, due to a very brief description of the study and no description of randomization, allocation concealment and blinding.
Results
1. Blood loss
Blood loss after a cesarean section was reported in two studies as blood loss > 500 mL (Lakshmi and Abraham, 2016; Oseni, 2021), in five studies as blood loss > 1000 mL (El-Gaber, 2018; Gungorduk, 2011; Obi, 2019; Oseni, 2021; Pacheco, 2023) and in eighteen studies as mean blood loss (Ali, 2019; El-Gaber, 2018; Ghosh, 2014; Hemapriya, 2020; Jafarbegloo, 2021; Kamel, 2018; Lakshmi and Abraham, 2016; Malathi, 2016; Masood, 2023; Milani 2019; Movafegh, 2011; Nargis and Dewan, 2020; Oseni, 2021; Ray, 2016; Sentürk, 2012; Singh, 2014; Thavare and Patil, 2019; Yehia, 2014).
Blood loss after a vaginal delivery was reported in three studies as blood loss > 500 mL (Igboke, 2022; Sentilhes, 2018; Sujita 2018), in two studies as blood loss > 1000 mL (Sentilhes, 2018; Sujita, 2018) and in four studies as mean blood loss (Igboke, 2022; Kashanian, 2022; Sentilhes, 2018; Sujita, 2018).
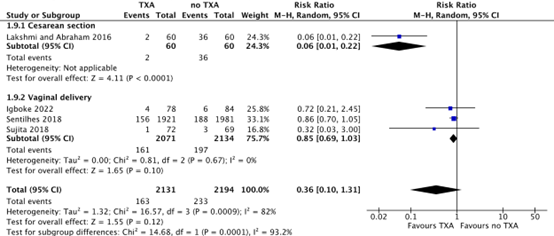
Blood loss > 500 mL
For cesarean section, blood loss > 500 mL was measured from placental delivery to end of surgery (Lakshmi and Abraham, 2016). For vaginal deliveries, blood loss > 500 mL was measured during delivery (Sentilhes 2018, Igboke 2022) and two hours postpartum (Sujita 2018).
For cesarean section, blood loss > 500 mL was only reported by Lakshmi and Abraham (2016), and occurred in 2/60 (3.3%) women after a cesarean section in the TXA-group, while 36/60 (60%) women in the no TXA-group had blood loss > 500 mL. This difference was considered clinically relevant in favour of TXA (RR 0.06, 95% CI 0.01 to 0.22) (Figure 1).
During a vaginal delivery, 161/2071 (7.8%) women in the TXA-group had blood loss > 500 mL, while 197/2134 (9.7%) women in the no-TXA group had blood loos > 500 mL. The difference in blood loss > 500 mL during a vaginal delivery was considered clinically relevant (RR 0.85, 95% CI 0.69 to 1.03) (Figure 1).
Figure 1. Blood loss >500 mL after a cesarean section and vaginal delivery.
Source: Cheema, 2023; Igboke, 2022; Sentilhes, 2018; Sujita, 2018
Abbreviations: TXA; Tranexamic Acid, CI; Confidence Interval
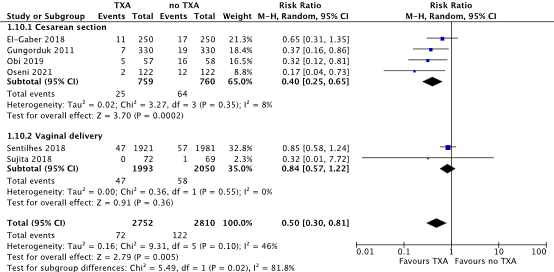
Blood loss > 1000 mL
For cesarean section, blood loss > 1000 mL was measured intra-operative (Oseni, 2021). For vaginal deliveries, blood loss > 1000 mL was measured during delivery (Sentilhes 2018) and two hours postpartum (Sujita, 2018).
For cesarean section, blood loss > 1000 mL occurred in 25/759 (3.3%) women in the TXA-group, while 64/760 (8.4%) women in the no TXA-group lost > 1000 mL blood after a cesarean section. This difference was considered clinically relevant in favour of TXA (RR 0.40, 95% CI 0.25 to 0.65) (Figure 2).
During a vaginal delivery, 47/1993 (2.4%) women in the TXA-group had blood loss > 1000 mL, while 58/2050 (2.8%) women in the no-TXA group had blood loos > 1000 mL. The difference in blood loss > 1000 mL during a vaginal delivery was considered clinically relevant (RR 0.84, 95% CI 0.57 to 1.22) (Figure 2).
Figure 2. Blood loss >1000 mL after a cesarean section and vaginal delivery.
Source: Cheema, 2023; Sentilhes, 2018; Sujita, 2018
Abbreviations: TXA; Tranexamic Acid, CI; Confidence Interval
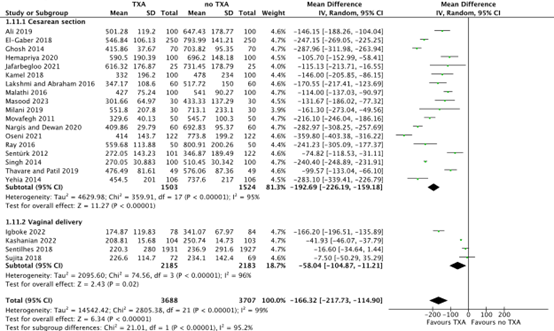
Mean blood loss
For cesarean section, mean blood loss was measured six hours postpartum (Yehia, 2014: El-Gaber, 2018; Kamel 2018), two hours postpartum (Singh, 2014; Ali, 2019; Ghosh, 2014; Ray, 2016; Thavare, 2019; Nargis, 2020; Jafarbegloo, 2021; Milani, 2019; Movafegh, 2011; Malathi, 2016; Lakshmi and Abraham, 2016; Hemapriya, 2020; Oseni, 2021; Sentürk, 2012) and from surgery to second post cesarean day (Masood, 2023). For vaginal deliveries, mean blood loss was measured six hours postpartum (Kashanian, 2022), two hours postpartum (Sujita, 2018) and during delivery (Igboke, 2022; Sentilhes, 2018).
For cesarean section, the mean difference in blood loss was 192.69 ml (95% CI -226.19 to -159.18) in favour of TXA. This difference was considered clinically relevant in favour of TXA (Figure 3).
For vaginal delivery, the mean difference in blood loss was 58.04 ml (95% CI -104.87 to -11.21) in favour of TXA. This difference was considered clinically relevant in favour of TXA (Figure 3).
Figure 3. Mean blood loss after a cesarean section and vaginal delivery.
Source: Ali, 2019; Singh, 2014; Cheema, 2023; Assis, 2023; Igboke, 2022; Kashanian, 2022
Abbreviations TXA: Tranexamic Acid, SD: Standard Deviation; CI: Confidence Interval
2. Shock
Not reported
3. Maternal death
The outcome measure maternal death was reported in one study (Pacheco, 2023) after a cesarean section.
Maternal death occurred in 0/5525 (0%) women in the TXA-group, and in 1/5470 (<0.1%) in the no TXA-group after a cesarean section. No information was provided in this study about the exact causes of this case. No relative risk can be calculated, since no events are reported in the TXA-group.
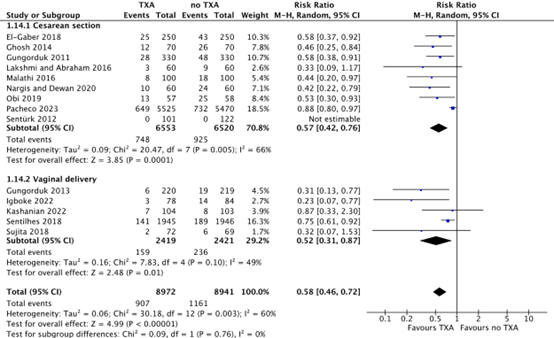
4. Use of additional uterotonics
For cesarean section, the use of additional uterotonics was reported in nine studies (El-Gaber, 2018; Ghosh, 2014; Gungorduk, 2011; Lakshmi and Abraham, 2016; Nargis and Dewan, 2020; Obi, 2019; Pacheco, 2023; Sentürk, 2012). For vaginal deliveries, use of additional uterotonics was reported in five studies (Gungorduk, 2013; Igboke, 2022; Kashanian, 2022; Sentilhes, 2018; Sujita, 2018).
After a cesarean section, additional uterotonics were used in 748/6553 (11.4%) women in the TXA-group, while additional uterotonics were used in 925/6520 (14.2%) women in the no TXA-group. This difference was considered clinically relevant in favour of TXA (RR 0.57, 95% CI 0.42 to 0.76) (Figure 4).
After a vaginal delivery, additional uterotonics were used in 159/2419 (6.6%) women in the TXA-group, while additional uterotonics were used in 236/2421 (10.9%) women in the no-TXA group. This difference was considered clinically relevant in favour of TXA (RR 0.52, 95% CI 0.31 to 0.87) (Figure 4).
Figure 4. Use of additional uterotonics after a cesarean section and vaginal delivery.
Source: Cheema, 2023; Assis, 2023; Igboke, 2022; Kashanian, 2022
Abbreviations TXA: Tranexamic Acid, CI: Confidence Interval
5. Blood transfusion
For cesarean section, the need for blood transfusion was reported in ten studies (Ali, 2019; El-Gaber, 2018; Ghosh, 2014; Gungorduk, 2011; Jafarbegloo, 2021; Nargis and Dewan, 2020; Obi, 2019; Pacheco, 2023; Sentürk, 2012; Yehia, 2019). For vaginal deliveries, the need for blood transfusion was reported in five studies (Gungorduk, 2013; Igboke, 2022; Kashanian, 2022; Sentilhes, 2018; Sujita, 2018).
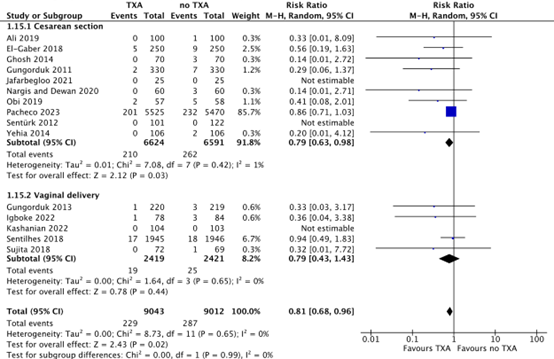
After a cesarean section, 210/6624 (3.2%) women in the TXA-group needed blood transfusion, while 262/6591 (4.0%) women in the no TXA-group needed blood transfusion. This difference was considered clinically relevant in favour of TXA (RR 0.79, 95% CI 0.63 to 0.98) (Figure 5).
After a vaginal delivery, 19/2419 (0.8%) women in the TXA-group needed blood transfusion, while 25/2421 (1.0%) women in the no-TXA group needed blood transfusion. This difference was considered clinically relevant in favour of TXA (RR 0.79, 95% CI 0.43 to 1.43) (Figure 5).
Figure 5. Need for blood transfusion after a cesarean section and vaginal delivery.
Source: Cheema, 2023; Assis, 2023; Igboke, 2022; Kashanian, 2022
Abbreviations TXA: Tranexamic Acid, CI: Confidence Interval
6. Transfer for higher level of care
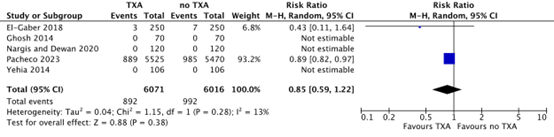
Five studies reported on the outcome measure transfer for higher level of care, defined as the need for additional surgical interventions after a cesarean section (Yehia, 2014; Ghosh, 2014; El Gaber, 2018; Nargis & Dewan, 2020; Pacheco, 2023). In the TXA-group, 892/6071 (14.7%) women needed additional surgical interventions after cesarean section, while 992/6016 (16.5%) women in the no TXA-group needed additional surgical interventions. This difference was considered clinically relevant in favour of TXA (RR 0.85, 95% CI 0.59 to 1.22) (Figure 6).
Figure 6. Additional surgical intervention after a cesarean section
Source: El-Gaber, 2018; Ghosh, 2014; Nargis and Dewan, 2020; Pacheco, 2023; Yehia, 2014.
Abbreviations TXA: Tranexamic Acid, CI: Confidence Interval
Two studies reported on the outcome measure transfer for higher level of care, defined as hysterectomy after a cesarean section (Ali, 2019; Nargis & Dewan, 2020). Yet, no events were reported in both studies. Hence, no RR can be calculated for this outcome measure.
One study reported on the outcome measure transfer for higher level of care, defined as arterial embolization or surgery for postpartum haemorrhage after vaginal delivery (Sentilhes, 2018). In the TXA-group, 3/1945 (0.2%) women needed arterial embolization or surgery for postpartum haemorrhage after a vaginal delivery, while this was the case for 5/1945 (0.3%) in the no TXA-group. This difference was considered clinically relevant in favour of TXA (RR 0.60, 95% CI 0.14 to 2.51), but the confidence interval is very wide.
7. Women’s sense of wellbeing
Not reported
8. Acceptability and satisfaction with the intervention
The outcome measure satisfaction with the intervention was reported by Sentilhes (2018) as number of women who were satisfied with the delivery (Table 1). In this study, only women who had a vaginal delivery participated.
|
Number of women that felt satisfied with the delivery |
TXA-group (n=1526) |
Placebo group (n=1540) |
|
Not at all |
1 (0.1%) |
3 (0.2%) |
|
A little |
2 (0.2%) |
10 (0.6%) |
|
Moderately |
36 (2.3%) |
40 (2.5%) |
|
Very |
766 (48.1%) |
752 (46.9%) |
|
Extremely |
785 (49.3%) |
800 (49.8%) |
Table 1. Satisfaction with delivery
Source: Sentilhes (2018). Values are reported as numbers (%).
In the group who received TXA, 766/1526 (48.1%) women were very satisfied with their delivery and 785/1526 (49.3%) were extremely satisfied with their delivery. In the group who received no TXA, 752/1540 (46.9%) women were very satisfied with their delivery and 800/1540 (49.8%) were extremely satisfied with their delivery. The difference in satisfaction was not significantly different between groups (p=0.28).
9. Breastfeeding
Not reported
10. Adverse effects
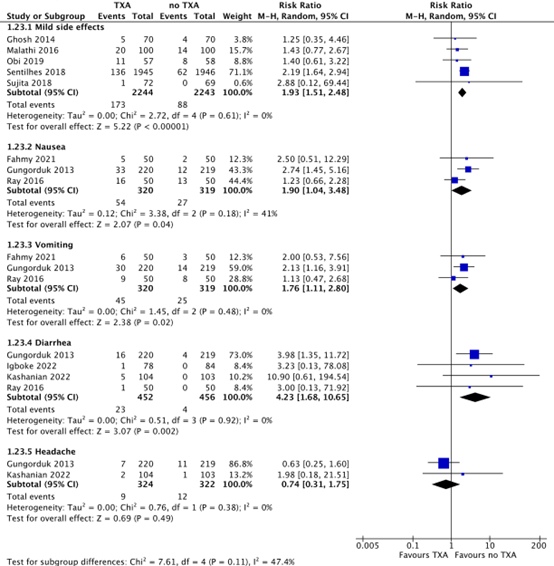
Five studies reported on the outcome measure adverse effects, defined as mild side effects (Ghosh, 2014; Malathi, 2016; Obi, 2019; Sentilhes, 2018; Sujita, 2018). In the TXA-group, 173/2244 (7.7%) women reported mild adverse effects, while this was the case for 88/2243 (3.9%) women in the no TXA-group. This difference was considered clinically relevant in favour of no TXA (RR 1.93, 95% CI 1.51 to 2.48).
Three studies reported on the outcome measure adverse effects, defined as nausea and vomiting (Fahmy, 2021; Gungorduk, 2013; Ray, 2016). In the TXA-group, 54/320 (16.9%) women felt nauseous and 45/320 (14.1%) women reported vomiting, while 27/319 (8.5%) women in the no TXA-group felt nauseous and 25/319 (7.8%) women reported vomiting. The differences in nausea (RR 1.90, 95% CI 1.04 to 3.48) and vomiting (RR 1.76, 95% CI 1.11 to 2.80) are considered clinically relevant in favour of no TXA.
Four studies reported on the outcome measure adverse effects, defined as diarrhea (Gungorduk, 2013; Igboke, 2022; Kashanian, 2022; Ray, 2016). In the TXA-group, 23/452 (5.1%) women reported diarrhea, while 4/456 (0.9%) women in the no TXA-group reported diarrhea. This difference is considered clinically relevant in favour of no TXA (RR 4.23, 95% CI 1.68 to 10.65).
Two studies reported on the outcome measure adverse effects, defined as headache (Gungorduk, 2013; Kashanian, 2022). In the TXA-group, 9/324 (2.8%) women had headache, while 12/322 (3.7%) women in the no-TXA group had headache (RR 0.75, 95% CI 0.32 to 1.74).
Figure 7. Side effects of TXA versus no TXA
Source: Ghosh, 2014; Malathi, 2016; Obi, 2019; Sentilhes, 2018; Sujita, 2018; Fahmy, 2021; Gungorduk, 2013; Ray, 2016; Igboke, 2022, Kashanian, 2022.
Abbreviations TXA: Tranexamic Acid, CI: Confidence Interval
11. Post-traumatic stress syndrome (PTSD)
Not reported
Level of evidence of the literature
Women after a cesarean section
The level of evidence regarding the outcome measure blood loss > 500 mL was downgraded by three levels to a very low GRADE, because of study limitations (-1, due to some concerns with regard to the risk of bias) and because of a very small number of included patients (-2, imprecision).
The level of evidence regarding the outcome measure blood loss > 1000 mL was downgraded by two levels to a low GRADE, because of study limitations (-1, due to some concerns with regard to the risk of bias) and because the upper limit of the 95% confidence intervals crosses the margin of clinical relevance (-1; imprecision).
The level of evidence regarding the outcome measure mean blood loss was downgraded by one level to a moderate GRADE, because of study limitations (-1; some concerns with regard to the risk of bias).
The level of evidence regarding the outcome measure shock could not be assessed with GRADE since these outcomes were not reported in the included studies.
The level of evidence regarding the outcome measure maternal death could not be assessed with GRADE since no events were reported in the included studies.
The level of evidence regarding the outcome measure use of additional uterotonics was downgraded by one level to a moderate GRADE, because of study limitations (-1; some concerns with regard to the risk of bias).
The level of evidence regarding the outcome measure blood transfusion was downgraded by two levels to a low GRADE, because of study limitations (-1, due to some concerns with regard to the risk of bias) and because the upper limit of the 95% confidence intervals crosses the margin of clinical relevance (-1; imprecision).
The level of evidence regarding the outcome measure transfer for higher level of care was downgraded by two levels to a low GRADE, because of study limitations (-1, due to some concerns with regard to the risk of bias) and because the upper limit of the 95% confidence intervals crosses the margin of clinical relevance (-1; imprecision).
Women after a vaginal delivery
The level of evidence regarding the outcome measure blood loss > 500 mL was downgraded by three levels to a very low GRADE, because of study limitations (-1, due to some concerns with regard to the risk of bias) and because the upper limit of the 95% confidence interval crosses both borders of clinical relevance (-2, imprecision).
The level of evidence regarding the outcome measure blood loss > 1000 mL was downgraded by three levels to a very low GRADE, because of study limitations (-1, due to some concerns with regard to the risk of bias) and because the lower limit of the 95% confidence interval crosses both borders of clinical relevance (-2, imprecision).
The level of evidence regarding the outcome measure mean blood loss was downgraded by three levels to a very low GRADE because of study limitations (-1, due to some concerns with regard to the risk of bias), conflicting results (-1, inconsistency) and because of the confidence intervals cross the margin of clinical relevance (-1; imprecision).
The level of evidence regarding the outcome measure shock could not be assessed with GRADE since this outcome was not reported in the included studies.
The level of evidence regarding the outcome measure maternal death was not downgraded and stayed as High GRADE.
The level of evidence regarding the outcome measure use of additional uterotonics was downgraded by two levels to a low GRADE because of study limitations (-1, due to some concerns with regard to the risk of bias) and because the upper limit of the 95% confidence interval crosses the margin of clinical relevance (-1, imprecision).
The level of evidence regarding the outcome measure blood transfusion was downgraded by three levels to a very low GRADE because of study limitations (-1, due to some concerns with regard to the risk of bias) and because the upper limit of the 95% confidence interval crosses both margins of clinical relevance (-2, imprecision).
The level of evidence regarding the outcome measure transfer for higher level of care was downgraded by one level to a moderate GRADE because the upper limit of the 95% confidence interval crosses the border of clinical relevance (-1, imprecision).
Women postpartum (both cesarean section and vaginal delivery)
The level of evidence regarding the outcome measure women’s sense of wellbeing could not be assessed with GRADE since these outcomes were not reported in the included studies.
The level of evidence regarding the outcome measure acceptability and satisfaction with the intervention could not be assessed with GRADE since no risk ratio could be calculated.
The level of evidence regarding the outcome measure breastfeeding could not be assessed with GRADE since these outcomes were not reported in the included studies.
The level of evidence regarding the outcome measure adverse effects (i.e. mild side effects, vomiting, diarrhea) was downgraded by one level to a moderate GRADE, because of study limitations (-1, due to some concerns with regard to the risk of bias).
The level of evidence regarding the outcome measure adverse effects (i.e. nausea) was downgraded by two levels to a low GRADE, because of study limitations (-1, due to some concerns with regard to the risk of bias) and because the upper limit of the 95% confidence intervals crosses the margin of clinical relevance (-1; imprecision).
The level of evidence regarding the outcome measure adverse effects (i.e. headache) was downgraded by three levels to a very low GRADE because of study limitations (-1, due to some concerns with regard to the risk of bias), conflicting results (-1; inconsistency) and because of the upper limit of the 95% confidence interval crosses the margin of clinical relevance (-1; imprecision).
The level of evidence regarding the outcome measure post-traumatic stress syndrome could not be assessed with GRADE since these outcomes were not reported in the included studies.
Zoeken en selecteren
A systematic review of the literature was performed to answer the following question: What is the role of tranexamic acid (TXA) in the prevention of postpartum haemorrhage (PPH)?
| P: patients | Women postpartum |
| I: intervention | Administration of TXA |
| C: control | Placebo or standard care (no TXA) |
| O: outcome measure | Blood loss (>500, >1000, mean), shock, maternal death, use of additional uterotonics, blood transfusion, transfer for higher level of care, women’s sense of wellbeing, acceptability and satisfaction with the intervention, breastfeeding, adverse effects, post-traumatic stress syndrome (PTSD) |
Relevant outcome measures
The working group considered blood loss more than 1000 mL (dichotomous), shock, transfer for higher level of care, maternal death and blood transfusion as critical outcome measures for decision making; and blood loss (continuous outcome measure), use of additional hemostatic intervention, women's sense of wellbeing, acceptability, satisfaction with the intervention, breastfeeding, adverse effects and PTSD as important outcome measures for decision making.
The working group defined the outcome measures based on the definitions from Meher (2018)
The working group defined a 1% difference for maternal death (RR < 0.99 or > 1.01) and 10% (RR < 0.90 or > 1.10) for other critical outcome measures as a minimal clinically (patient) important difference. For the other outcomes, a 25% difference for dichotomous outcomes (RR < 0.8 or > 1.25) and 0.5 SD for continuous outcomes was taken as minimal clinically (patient) important difference.
Search and select (Methods)
The databases Medline (via OVID) and Embase (via Embase.com) were searched with relevant search terms until August 31th, 2023. The detailed search strategy is depicted under the tab Methods. The systematic literature search resulted in 430 hits. Studies were selected based on the following criteria:
- Randomized controlled trials (RCTs) or systematic reviews in which a minimum of 2 databases were searched and in which a detailed search strategy, in- and exclusion criteria, exclusion table, evidence table and risk of bias assessment are included
- Studying women postpartum (within 2 hours) with blood loss > 500 mL
- Comparing TXA with standard treatment, no treatment or placebo
- Assessing one or more of the predefined outcomes
Seventy-two studies were initially selected based on title and abstract screening. After reading the full text, sixty-six studies were excluded (see the table with reasons for exclusion under the tab Methods) and six studies were included. Furthermore, two studies were identified through snowballing.
Results
Eight studies (two systematic reviews (Cheema, 2023; Assis, 2023) and six RCTs (Kashanian, 2022; Khurshid, 2022; Igboke, 2022; Masood, 2023; Singh, 2014; Ali, 2019)) were included in the analysis of the literature. Important study characteristics and results are summarized in the evidence tables. The assessment of the risk of bias is summarized in the risk of bias tables.
Referenties
- Ali SAA, Oof TF, Elmolla MF. Efficacy of intravenous tranexamic acid in reducing blood loss after elective cesarean section. The Egyptian Journal of Hospital Medicine. 2019;74(6):1353-1358.
- Assis IC, Govêia CS, Miranda DB, Ferreira RS, Riccio LGC. Analysis of the efficacy of prophylactic tranexamic acid in preventing postpartum bleeding: systematic review with meta-analysis of randomized clinical trials. Braz J Anesthesiol. 2023 Jul-Aug;73(4):467-476.
- Cheema HA, Ahmad AB, Ehsan M, Shahid A, Ayyan M, Azeem S, Hussain A, Shahid A, Nashwan AJ, Mikuš M, Laganà AS. Tranexamic acid for the prevention of blood loss after cesarean section: an updated systematic review and meta-analysis of randomized controlled trials. Am J Obstet Gynecol MFM. 2023 Aug;5(8):101049.
- Igboke FN, Obi VO, Dimejesi BI, Lawani LO. Tranexamic acid for reducing blood loss following vaginal delivery: a double-blind randomized controlled trial. BMC Pregnancy Childbirth. 2022 Mar 3;22(1):178.
- Kashanian M, Dadkhah F, Tabatabaei N, Sheikhansari N. Effects of tranexamic acid on the amount of bleeding following vaginal delivery and its adverse effects: a double-blind placebo controlled randomized clinical trial. J Matern Fetal Neonatal Med. 2022 Dec;35(25):5611-5615.
- Khurshid HN, Noor S, Tasheen H, Khokar S, Saleem S. Mean Blood Loss in Third Stage of Labour Treated with and without Prophylactic Tranexamic Acid. Pakistan Journal of Medical & Health Sciences. 2022;16(5):45-45.
- Masood J, Hayat Z, Ahmed N, Jabeen R, Shifa N, Masud F. Comparison of Estimated Blood Loss between Tranexamic Acid and Control in Women Undergoing Elective Cesarean Section. Pakistan Journal of Medical & Health Sciences. 2023;17(04):288-288.
- Singh T, Burute SB, Deshpande HG, Jethani S, Ratwani K. Efficacy of tranexamic acid in decreasing blood loss during and after caesarean section: a randomized case control prospective study. Journal of Evolution of Medical and Dental Sciences. 2014;3(11):2780-2789.
Evidence tabellen
Evidence table for SRs
|
Study reference |
Study characteristics |
Patient characteristics |
Intervention (I) |
Comparison / control (C)
|
Follow-up |
Outcome measures and effect size |
Comments |
|
Cheema, 2023
PS., study characteristics and results are extracted from the SR (unless stated otherwise) |
SR and meta-analysis of RCTs
Literature search up to February 2023
A: Gungorduk, 2011 B: Movafegh, 2011 C: Abdel-Aleem, 2013 D: Sentürk, 2013 E: Ghosh, 2014 F: Yehia, 2014 G: Lakshmi, 2016 H: Malathi, 2016 I: Ray, 2016 J: El-Gaber, 2018 K: Kamel, 2018 L: Milani, 2019 M: Obi, 2019 N: Thavare, 2019 O: Hemapriya, 2020 P: Nargis and Dewan, 2020 Q: Shalabi, 2020 R: Fahmy, 2021 T: Naeiji, 2021 U: Oseni, 2021 V: Pacheco, 2023 All studies are RCTs
Setting and Country: A: Turkey B: Iran C: Egypt D: Turkey E: India F: Egypt G: India H: India I: India J: Egypt K: Egypt L:Iran M: Nigeria N: India O: India P: Bangladesh Q: Egypt R: Egypt S: Iran T: Iran U: Nigeria V: United States
Source of funding and conflicts of interest: Not reported in the SR
|
Inclusion criteria SR: - Study design: RCTs - Population: women undergoing cesarean delivery who received TXA irrespective of age or ethnicity - Intervention: prophylactic intravenous TXA at cesarean delivery irrespective of type or dosage or timing of administration - Comparator: placebo, no treatment, standard treatment, or prostaglandin analogs - Outcome: reporting at least 1 outcome of interest.
Studies that combined TXA with another agent provided that the same agent was also administered to the control arm were included in the review. We sought to include all RCTs regardless of their publication status.
Exclusion criteria SR: - Study designs other than RCTs - Studies that administered TXA after a diagnosis of PPH was made instead of prophylactically. - Studies conducted on animals - Studies evaluating outcomes in women undergoing vaginal delivery.
50 studies were included in the SR, but 22 were included in our literature analysis.
Important patient characteristics at baseline:
Number of patients (intervention vs. control) A: 660 (330 vs. 330) B: 100 (50 vs 50) C: 740 (373 vs 376) D: 223 (101 vs 122) E: 140 (70 vs 70) F: 212 (106 vs 106) G: 120 (60 vs 60) H: 200 (100 vs 100) I: 100 (50 vs 50) J: 500 (250 vs 250)
Groups comparable at baseline? Yes |
Describe intervention:
A: 1 g TXA 10 min before incision B: 10 mg/kg TXA 20 min before anesthesia C: 1 g TXA 10 min before incision D: 1 g TXA 10 min before incision E: 1 g TXA before skin incision F: 1 g TXA with anesthesia G: 1 g TXA 20 min before incision H: 10 mg/kg TXA 15−20 min before incision I: 1 g TXA 20 min before anesthesia J: 1 g TXA after birth after delivery anesthesia Following umbilical cord clamping
|
Describe control:
A: 5% glucose B: 200 ml normal saline C: Standard treatment D: 5% dextrose solution E: 10 ml sterile water F: Placebo G: Standard treatment H: Standard treatment I: 5% dextrose solution J: Normal saline |
End-point of follow-up:
A: 6 wks after surgery B: 24h after surgery C: 24h after surgery D: 8h after surgery E: 24h postoperatively F: 24h postoperatively G: 24h after surgery H: 24h after surgery I: 24h after surgery J: 24h after surgery L: Within 12-24h after surgery N: 2h postpartum O: 24h postoperative P: 24h postoperatively Q: 24h postpartum R: 24h postoperatively T: 6h after surgery
For how many participants were no complete outcome data available? (intervention/control) Not reported in the SR
|
Outcome measure-1: Blood loss
Blood loss defined as blood loss > 1000 ml
Effect measure: RR, RD, mean difference [95% CI]: A: 0.37 [0.16, 0.86] C: 0.98 [0.15, 6.95] J: 0.65 [0.31, 1.35] U: 0.17 [0.04, 0.73] V: 0.91 [0.79, 1.05]
Pooled effect (random effects model / fixed effects model): Not reported in the SR since more studies are included in the SR.
Blood loss defined as mean blood loss.
Effect measure: RR, RD, mean difference [95% CI]: B: -216.10 [-246.04, -186.16] E :-287.96 [-311.98, -263.94] F: -283.10 [-339.41, -226.79] G: -170.55 [-217.41, -123.69] H: -114.00 [-137.03, -90.97] I: -241.23 [-305.09, -177.37] J: -247.15 [-269.05, -225.25] K: -146.00 [-205.85, -86.15] L: -161.30 [-273.04, -49.56] P: -282.97 [-308.25, -257.69]
Pooled effect (random effects model / fixed effects model): Not reported in the SR since more studies are included in the SR.
Outcome measure-2: shock Not reported
Outcome measure-3: maternal death Not reported
Outcome measure-4: use of additional uterotonics
Effect measure: RR, RD, mean difference [95% CI]: A: 0.58 [0.38, 0.91] D: Not estimable E: 0.46 [0.25, 0.84] G: 0.33 [0.09, 1.17] H: 0.44 [0.20, 0.97] J: 0.58 [0.37, 0.92] P: 0.42 [0.22, 0.79] M: 0.53 [0.30, 0.93] V: 0.88 [0.80, 0.97]
Pooled effect (random effects model): 0.57 [95% CI 0.42 to 0.76] favoring intervention (TXA) Heterogeneity (I2): 66%
Outcome measure-5: blood transfusion
Effect measure: RR, RD, mean difference [95% CI]: A: 0.29 [0.06, 1.37] D: Not estimable E: 0.14 [0.01, 2.72] F: 0.20 [0.01, 4.12] J: 0.56 [0.19, 1.63] M: 0.41 [0.08, 2.01] P: 0.14 [0.01, 2.71] S: Not estimable V: 0.86 [0.71, 1.03]
Pooled effect (random effects model): 0.79 [95% CI 0.63 to 0.98] favoring intervention (TXA) Heterogeneity (I2): 1%
Outcome measure-6: transfer for higher level of care
Outcome measure-7: women’s sense of wellbeing Not reported
Outcome measure-8: Acceptability and satisfaction with the intervention
Outcome measure-9: breastfeeding Not reported
Outcome measure-10: adverse effects
Outcome measure-11: post-traumatic stress syndrome Not reported
|
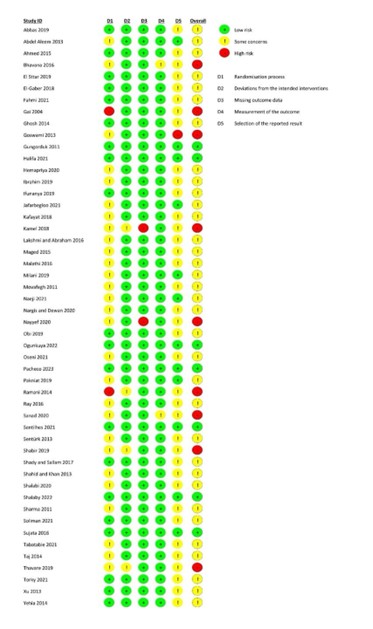
Risk of bias (high, some concerns or low): Tool used by authors:
Facultative:
Brief description of author’s conclusion
“Tranexamic acid may reduce the risk for blood loss in caesarean deliveries with a higher benefit observed in high-risk patients, but the lack of high-quality evidence precludes any strong conclusions. The administration of tranexamic acid before skin incision, but not after cord clamping, was associated with a large benefit. Additional studies, especially in the high-risk population and focused on evaluating the timing of tranexamic acid dministration, are needed to confirm or refute these findings.”
|
|
Assis, 2023 |
SR and meta-analysis of blinded RCTs
Literature search up to January 2020
A: Gungorduk, 2013 B: Sentilhes, 2018 C: Sujita, 2018
Setting and Country: A: Turkey B: France C: Thailand
Source of funding and conflicts of interest: Not reported in the SR
|
Inclusion criteria SR: - women undergoing vaginal or cesarean delivery - English written
Exclusion criteria SR: - non-English publications
Number of patients (intervention vs. control) A: 220/219 B: 1931/1927 C: 72/69 |
Describe intervention:
A: 1g TXA, for 5 min after extracting the anterior shoulder B: 1g TXA, 2 min after delivery C: 1g TXA + 20 mL saline (30 mL total)
|
Describe control:
A: 30 mL glucose 5% B: Saline IV C: 30 ml saline
|
End-point of follow-up: Not reported in the SR
For how many participants were no complete outcome data available? (intervention/control) Not reported in the SR
|
Outcome measure-1: Blood loss
Effect measure: RR, RD, mean difference [95% CI]: A: -88,480 (-120,115 to 56,845) B: -16,6 (-34,640 to 1,440) C: -7,5 (-50,094 to 35,094)
Pooled effect (random effects model / fixed effects model): Not reported in the SR (since more studies are included in the SR)
Outcome measure-2: shock Not reported
Outcome measure-3: maternal death Not reported
Outcome measure-4: use of additional uterotonics Not reported
Outcome measure-5: blood transfusion Not reported
Outcome measure-6: transfer for higher level of care Not reported
Outcome measure-7: women’s sense of wellbeing Not reported
Outcome measure-8: Acceptability and satisfaction with the intervention Not reported
Outcome measure-9: breastfeeding Not reported
Outcome measure-10: adverse effects Not reported
Outcome measure-11: post-traumatic stress syndrome Not reported
|
Facultative:
Brief description of author’s conclusion
“The prophylactic use of tranexamic acid is effective in reducing the bleeding post-partum volume.” |
Evidence table for RCTs
|
Study reference |
Study characteristics |
Patient characteristics 2 |
Intervention (I) |
Comparison / control (C) 3
|
Follow-up |
Outcome measures and effect size 4 |
Comments |
|
Kashanian, 2022
|
Type of study: RCT
Setting and country: Akbarabadi Teaching Hospital in Tehran, Iran.
Funding and conflicts of interest: Funding not reported, no conflict of interest. |
Inclusion criteria: Multiparous, singleton pregnancy, gestational age >37 and <42 wks, normal blood pressure.
Exclusion criteria: History of coagulopathy, pre-eclampsia, placental abruption, hypersensitivity to TXA, history of cardiac, hepatic, renal and neurologic disorders, BMI>30, episiotomy.
N total at baseline: Intervention: 104 Control: 103
Groups comparable at baseline? Yes
|
Describe intervention (treatment/procedure/test):
10 mg/kg intravenous TXA in 100ml normal saline.
In the third stage of labour, all participants received 20 units of IM oxytocin. |
Describe control (treatment/procedure/test):
One vial of distilled water (as placebo) in 100 ml normal saline.
In the third stage of labour, all participants received 20 units of IM oxytocin. |
Length of follow-up: 3 weeks post delivery
Loss-to-follow-up: Intervention: 0 Control: 0
Incomplete outcome data: Intervention: 0 Control: 0
|
Outcome measures and effect size (include 95%CI and p-value if available):
Blood loss (in ml) Intervention: 208.81±15.68 ml Control: 250.74±14.73 ml p-value: 0.033
Need for uterotonic agents:
Intervention: 97 (93.3%) Control: 95 (92.2%) p-value: 0.187
Intervention: 0 Control: 3 (2.9%) p-value: 0.000
Intervention: 7 (6.7%) Control: 5 (4.9%) p-value: 0.685 |
|
|
Khurshid, 2022 |
Type of study: RCT
Setting and country: Department of obstretics and Gynecology Unit-I, Sir Ganga Ram Hospital Lahore, New Delhi, Inida
Funding: Not reported
Conflicts of interest: no |
Inclusion criteria: Women aged 18-35 years old with a singleton pregnancy, 37-42 wks gestation, in second stage of labour in the hospital.
Exclusion criteria: Not reported
N total at baseline: Intervention: 58 Control: 58
Groups comparable at baseline? Yes
|
Describe intervention (treatment/procedure/test):
1 gm of transamine was given in 5 minutes diluted in 20 ml 5% dextrose water at the time of delivery of the shoulders.
|
Describe control (treatment/procedure/test):
20 ml of 5% dextrose water was given at the time of delivery of the shoulder. |
Length of follow-up: Not reported
Loss-to-follow-up and incomplete outcome data: Not reported
|
Outcome measures and effect size (include 95%CI and p-value if available):
Blood loss (in ml) Intervention: 244.83±21.47 ml Control: 254.09±22.36 ml p-value: <0.001
|
|
|
Igboke, 2022 |
Type of study: RCT
Setting and country: Department of Obstetrics and Gynaecology, Alex-Ekwueme Federal University Teaching Hospital, Abakaliki, Nigeria
Funding: No funding was received
Conflicts of interest: no competing interest |
Inclusion criteria: Women in spontaneous labour, planned vaginal delivery, term pregnancy, singleton pregnancy, cephalic presentation, no contra-indications for TXA.
Exclusion criteria: Prior history of thromboembolism, auto-immune disease, sickle cell disease, bleeding disorder, renal disease, liver pathology, known cardiovascular disease, multiple pregnancy, intrauterine fetal death, previous uterine surgeries, chronic hypertension, preeclampsia, eclampsia, HELLP syndrome, antepartum haemorrhage, ruptured uterus, varicose veins at increased risk of deep vein thrombosis, history of epilepsy/seizure, episiotomy.
N total at baseline: Intervention: 88 Control: 88
Groups comparable at baseline? Yes
|
Describe intervention (treatment/procedure/test):
1 g TXA (Exacyl, Sanofi, Avenis Paris France) slowly (over 30-60 seconds) intravenously, within 2 minutes after childbirth and prophylactic oxytocin administration once the cord has been clamped.
|
Describe control (treatment/procedure/test):
10 mls of water for injection (Biofem; Juhel Anambra Nigeria) slowly (over 30-60 seconds) intravenously, within 2 minutes after childbirth and prophylactic oxytocin administration once the cord has been clamped. |
Length of follow-up:
Loss-to-follow-up: Intervention: 10 (11.4%) Control: 4 (2.5%) Reasons (describe): not reported
Incomplete outcome data: No
|
Outcome measures and effect size (include 95%CI and p-value if available):
Blood loss >500 ml Intervention: 4/78 (5.13%) Control: 6/84 (7.14%) RR (95% CI): 0.71 (0.38 to 1.79)
Blood transfusion: Intervention: 1/78 (1.28%) Control: 3/84 (3.57%) RR (95% CI): 0.25 (0.09 to 2.82)
Uterotonics: Intervention: 3/78 (3.85%) Control: 14/84 (16.67%) RR (95% CI): 0.24 (0.12 to 0.96)
Side effects (diarrhoea): Intervention: 1/78 (1.15%) Control: 0/84 (0%) RR: not estimable
|
|
|
Masood, 2023 |
Type of study: RCT
Setting and country: Obstetric and Gynaecology department, Foundation University, Fauji Foundation Hospital, Rawalpindi, Pakistan.
Funding and conflicts of interest: not reported |
Inclusion criteria: Women undergoing elective caesarean section, 18-40 years old, gestational age >37 wks. Exclusion criteria: history of DVT, epilepsy/seizures, known chronic diseases (like cardiovascular, renal or liver), autoimmunie diseases, coagulopathy or bleeding disorders, multiple pregnancies, placenta previa, morbidity adherence placenta, abruptio placenta, eclampsia, HELLP syndrome, those who have been administered low molecular weight heparin or anti platelets a week before delivery.
N total at baseline: Intervention: 30 Control: 30
Groups comparable at baseline? Yes
|
Describe intervention (treatment/procedure/test):
TXA (1 gram intravenously) 15 minutes before incision.
10 IU oxytocin was administered postdelivery.
40 IU of oxytocin mixed in Ringer Lactate’s Solution (1000 ml) at a rate of 125 ml/hour.
|
Describe control (treatment/procedure/test):
No TXA.
10 IU oxytocin was administered postdelivery.
40 IU of oxytocin mixed in Ringer Lactate’s Solution (1000 ml) at a rate of 125 ml/hour.
|
Length of follow-up: Not reported
Loss-to-follow-up: Not reported
Incomplete outcome data: Not reported
|
Outcome measures and effect size (include 95%CI and p-value if available):
Estimated blood loss (in ml) Intervention: 301.66±64.97 Control: 433.33±137.29 p-value: <0.001
|
|
|
Singh, 2014 |
Type of study: RCT
Setting and country: Department of Obstetrics and Gynaecology, Dr. D. Y. Patil medical college, Pune, India
Funding and conflicts of interest: not reported |
Inclusion criteria: All primipara and multipara delivered by caesarean section without any risk factors
Exclusion criteria: patients with hemorrhagic disorders, placenta previa, polyhydramnios, patients who can lead to increased blood loss i.e. twin pregnancies, anemia, PIH.
N total at baseline: Intervention: 100 Control: 100
Groups comparable at baseline? Not reported.
|
Describe intervention (treatment/procedure/test):
TXA 1 gram, IV, slowly over 5 minutes, 20 minutes before incision.
After delivery: 20 units of Pitocin in 500 ml if ringer lactate.
|
Describe control (treatment/procedure/test):
No TXA. |
Length of follow-up:
Loss-to-follow-up: Not described
Incomplete outcome data: Not described
|
Outcome measures and effect size (include 95%CI and p-value if available):
Mean blood loss (in ml): Intervention: 270.05 ± 30.883 Control: 510.45 ± 30.342 p-value: <0.001
|
|
|
Ali, 2019 |
Type of study: RCT
Setting and country: Labor Ward in Al-Azhar University Maternity Hospital, Egypt
Funding and conflicts of interest: not reported |
Inclusion criteria: Pregnant women undergoing cesarean delivery for many elective indications. Full term primiparas / multiparas. Singleton pregnancy being delivered by CS.
Exclusion criteria: Medical problems involving the heart, liver, kidney and brain diseases. Blood disorders. Allergy to tranexamic acid. History of thromboembolic disorders, abnormal placentation, severe pre-eclampsia, uterine anomalies and pathology. Multiple pregnancy, macrosomia. Polyhydramnios. Patients requiring blood transfusion due to anemia
|
Describe intervention:
20 minutes before taking the skin incision 1 g tranexamic acid (Kapron®, Amoun, Egypt) was given in 200 mL normal saline. After delivery of the neonate, 5 units of oxytocin (Syntocynon®, NOVARTIS, Egypt) in 500 ml normal saline was given by intravenous drip over 30 minutes. Tranexamic acid injections were prepared by diluting 1g (10 mL) tranexamic acid with 200 ml of normal saline.
|
Describe control:
Tranexamic acid was not given in the control group patients.
After delivery of the neonate, oxytocin was given as in the study group patents. |
Length of follow-up: 2 hours postpartum
Loss-to-follow-up: Not described
Incomplete outcome data: Not described
|
Blood loss in 1st period (ml) (from placental delivery till end of operation) Intervention: 386,55 ± 104,98 Control: 507,76 ± 152,14 P<0.001
Blood loss in de second period (from end of surgery to 2 hours postoperative) Intervention: 114,73 ± 53,82 Control: 139,67 ± 73,35 P=0.019
Blood transfusion Control: 1 P=0,316
Hysterectomy: Intervention: 0 Control: 0 P = NA |
|
Risk of bias table for intervention studies (randomized controlled trials; based on Cochrane risk of bias tool and suggestions by the CLARITY Group at McMaster University)
Research question: What is the role of tranexamic acid (TXA) in the prevention of postpartum hemorrhage (PPH)?
|
Study reference
(first author, publication year) |
Was the allocation sequence adequately generated?
Definitely yes Probably yes Probably no Definitely no |
Was the allocation adequately concealed?
Definitely yes Probably yes Probably no Definitely no |
Blinding: Was knowledge of the allocated interventions adequately prevented?
Were patients blinded?
Were healthcare providers blinded?
Were data collectors blinded?
Were outcome assessors blinded?
Were data analysts blinded? Definitely yes Probably yes Probably no Definitely no |
Was loss to follow-up (missing outcome data) infrequent?
Definitely yes Probably yes Probably no Definitely no |
Are reports of the study free of selective outcome reporting?
Definitely yes Probably yes Probably no Definitely no |
Was the study apparently free of other problems that could put it at a risk of bias?
Definitely yes Probably yes Probably no Definitely no |
Overall risk of bias If applicable/necessary, per outcome measure
LOW Some concerns HIGH
|
||
|
Cheema, 2023 |
|||||||||
|
Assis, 2023 |
|||||||||
|
Kashanian, 2022 |
Probably yes
Block randomization method |
Probably yes
Vials of TXA and placebo were coded by a midwife who was not aware of the sampling process |
Probably yes
|
Probably no
Not reported |
Probably no
Not reported/no study protocol available |
Probably yes |
Some concerns
Due to no clearly reported allocation concealment, loss to follow-up and no study protocol available. |
||
|
Khurshid, 2022 |
Definitely no
Not described
|
Definitely no
Not described
|
Definitely no
Not described
|
Definitely no
Not described
|
Definitely no
Not described/no protocol available
|
Probably no
Very brief description of the study |
HIGH
|
||
|
Igboke, 2022 |
Probably yes
Participants were randomized using a computer-generated random number with a special software -> 88 numbers were generated and these were allocated to group A, while others were automatically allocated to group B. |
Probably yes
Sealed envelopes |
Probably no
Researchers were blinded. For others: not described. |
Probably no
Not reported |
Probably no
Not described/ no protocol available |
Probably yes |
Some concerns
Due to randomization method, no clear allocation concealment and limited blinding. |
||
|
Masood, 2023 |
Definitely no
Not described
|
Definitely no
Not described
|
Definitely no
Not described
|
Definitely no
Not described
|
Definitely no
Not described/no protocol available
|
Probably no
Very brief description of the study |
HIGH
|
||
|
Singh, 2014 |
Definitely no
Not described
|
Definitely no
Not described
|
Definitely no
Not described
|
Definitely no
Not described
|
Definitely no
Not described/no protocol available
|
Probably no
Very brief description of the study |
HIGH
|
||
|
Ali, 2019 |
Probably yes
Computer generated randomization list. No further information. |
Probably no
Sealed envelopes |
Probably no
The solution was prepared by an anaesthetist who was not involved in the study. No further information. |
Definitely no
Not described
|
Definitely no
Not described/no protocol available
|
Probably no
Very brief description of the study |
HIGH |
||
Table of excluded studies
|
Abdel-Aleem H, Alhusaini TK, Abdel-Aleem MA, Menoufy M, Gülmezoglu AM. Effectiveness of tranexamic acid on blood loss in patients undergoing elective cesarean section: randomized clinical trial. J Matern Fetal Neonatal Med. 2013 Nov;26(17):1705-9. |
Included in review Cheema 2023 |
|
Abu-Zaid A, Baradwan S, Alshahrani MS, Bakhsh H, Badghish E, Khadawardi K, AlRasheed MA, Turkistani A, AlNaim NF, AlNaim LF, Fodaneel M, AbuAlsaud FS, Jamjoom MZ, Tulbah M, Almugbel M, Alomar O, Al-Jundi H, Allam HS, Alabdrabalamir S, Salem H, Al-Badawi IA. Prophylactic tranexamic acid among women undergoing vaginal delivery to reduce postpartum blood loss and related morbidities: A systematic review and meta-analysis of 17 randomized controlled trials. J Gynecol Obstet Hum Reprod. 2022 Jun;51(6):102378. |
More recent review is included. Seven studies from this review are judged seperately to be included in this literature summary. |
|
Ahmed MR, Sayed Ahmed WA, Madny EH, Arafa AM, Said MM. Efficacy of tranexamic acid in decreasing blood loss in elective caesarean delivery. J Matern Fetal Neonatal Med. 2015 Jun;28(9):1014-8. |
Included in review Cheema 2023 |
|
Alam A, Choi S. Prophylactic Use of Tranexamic Acid for Postpartum Bleeding Outcomes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Transfus Med Rev. 2015 Oct;29(4):231-41. |
More recent review is included. |
|
Ali MM, El-Bromboly WH, Elnagar WM, Hashem MFA. Efficacy of tranexemic acid in prevention of hemorrhage after vaginal delivery postpartum. European Journal of Molecular and Clinical Medicine. 2021;8(4):503-512. |
Blood loss was calculated in a different way than in other studies. |
|
Bellos I, Pergialiotis V. Tranexamic acid for the prevention of postpartum hemorrhage in women undergoing cesarean delivery: an updated meta-analysis. Am J Obstet Gynecol. 2022 Apr;226(4):510-523.e22. |
More recent review is included. |
|
Binyamin Y, Frenkel A, Gruzman I, Lerman S, Bichovsky Y, Zlotnik A, Stav MY, Erez O, Orbach-Zinger S. Prophylactic Administration of Tranexamic Acid Reduces Blood Products' Transfusion and Intensive Care Admission in Women Undergoing High-Risk Cesarean Sections. J Clin Med. 2023 Aug 12;12(16):5253. |
Wrong population: high risk women |
|
Eyeberu A, Getachew T, Amare G, Yadeta E, Lemi M, Bekele H, Negash A, Degefa M, Balcha T, Balis B, Eshetu B, Habte S, Abdurke M, Alemu A, Mohammed A, Ahmed F, Musa I, Getachew A, Amin A, Tefera T, Debella A. Use of tranexamic acid in decreasing blood loss during and after delivery among women in Africa: a systematic review and meta-analysis. Arch Gynecol Obstet. 2023 Sep;308(3):709-725. |
More recent review is included. |
|
Farhadifar F, Shahgheibi S, Zare S, Rezaie M, Seyedolshohadae F, Sharami SRY, Tahmasebi F, Koohestani M, Aghamiri V, Sohrabi M, Nouri B, Mousavi M. Investigation of prophylactic effect of tranexamic acid in preventing postpartum hemorrhage in besat hospital in sanandaj. Pakistan Journal of Medical and Health Sciences. 2021;15(3):966-969. |
Concerns about quality of the study |
|
Ferrari FA, Garzon S, Raffaelli R, Cromi A, Casarin J, Ghezzi F, Uccella S, Franchi M. Tranexamic acid for the prevention and the treatment of primary postpartum haemorrhage: a systematic review. J Obstet Gynaecol. 2022 Jul;42(5):734-746. |
More recent review is included. |
|
Franchini M, Mengoli C, Cruciani M, Bergamini V, Presti F, Marano G, Pupella S, Vaglio S, Masiello F, Veropalumbo E, Piccinini V, Pati I, Liumbruno GM. Safety and efficacy of tranexamic acid for prevention of obstetric haemorrhage: an updated systematic review and meta-analysis. Blood Transfus. 2018 Jul;16(4):329-337. |
More recent review is included. Two studies from this review are judged seperately to be included in this literature summary. |
|
Gulzar J, Zahra S, Aftab I, Amir N, Liaqat RMN, Iqbal A. Determining Efficacy of Tranexamic Acid in Reducing Post Partum Haemorrhage in Elective Cesarean Section Patients Evaluating in Referance to Fall in Haemoglobin. Pakistan Journal of Medical and Health Sciences. 2022;16(10):456-458. |
Blood loss was not reported (even though it is mentioned as study aim) |
|
Gungorduk K, Asıcıoğlu O, Yıldırım G, Ark C, Tekirdağ Aİ, Besımoglu B. Can intravenous injection of tranexamic acid be used in routine practice with active management of the third stage of labor in vaginal delivery? A randomized controlled study. Am J Perinatol. 2013 May;30(5):407-13. |
Included in review Assis 2023 |
|
Gungorduk K, Yıldırım G, Asıcıoğlu O, Gungorduk OC, Sudolmus S, Ark C. Efficacy of intravenous tranexamic acid in reducing blood loss after elective cesarean section: a prospective, randomized, double-blind, placebo-controlled study. Am J Perinatol. 2011 Mar;28(3):233-40. |
Included in review Cheema 2023 |
|
Halder S, Samanta B, Sardar R, Chattopadhyay S. Tranexamic acid used before caesarean section reduces blood loss based on pre- and postoperative haemoglobin level: a case-control study. J Indian Med Assoc. 2013 Mar;111(3):184-6. |
Preliminary report |
|
Halder S, Samanta B, Sardar R, Chattopadhyay S. Tranexamic acid used before caesarean section reduces blood loss based on pre- and postoperative haemoglobin level: a case-control study. J Indian Med Assoc. 2013 Mar;111(3):184-6. |
Design: case control studie |
|
Heesen M, Böhmer J, Klöhr S, Rossaint R, van de Velde M, Dudenhausen JW, Straube S. Prophylactic tranexamic acid in parturients at low risk for post-partum haemorrhage: systematic review and meta-analysis. Acta Anaesthesiol Scand. 2014 Oct;58(9):1075-85. |
More recent review is included. |
|
Henry J, McFarland A. The effectiveness of tranexamic acid at reducing postoperative blood loss following cesarean section: a systematic review of quantitative evidence protocol. JBI Database System Rev Implement Rep. 2015 Jul 17;13(6):72-81. |
wrong study design: Protocol article |
|
Iqbal MJ, Mazhar A, Shabir A. Intravenous Tranexamic acid versus placebo during Caesarian section: A comparative study. Pak J Med Sci. 2022 May-Jun;38(5):1183-1187. |
Wrong population: high risk women |
|
Jafarbegloo E, Faridnyia F, Ahangari R, Mohammadbeigi A. Prophylactic use of tranexamic acid on blood loss in cesarean delivery: A randomized controlled-clinical trial. Jundishapur Journal of Natural Pharmaceutical Products. 2021;26(1):19-24. |
Included in review Cheema 2023 |
|
Kafayat H, Janjua M, Naheed I, Iqbal T. To assess the prophylactic role of tranexamic acid in reducing blood loss during and after two hours of caesarean section. Pakistan Journal of Medical and Health Sciences. 2018 jan;12(4):1662-1665. |
Included in review Cheema 2023 |
|
Ker K, Shakur H, Roberts I. Does tranexamic acid prevent postpartum haemorrhage? A systematic review of randomised controlled trials. BJOG. 2016 Oct;123(11):1745-52. |
More recent review is included. One study from this review is judged seperately to be included in this literature summary. |
|
Lakshmi SD, Abraham R. Role of Prophylactic Tranexamic Acid in Reducing Blood Loss during Elective Caesarean Section: A Randomized Controlled Study. J Clin Diagn Res. 2016 Dec;10(12):QC17-QC21. |
Included in review Cheema 2023 |
|
Lee A, Wang MY, Roy D, Wang J, Gokhale A, Miranda-Cacdac L, Kuntz M, Grover B, Gray K, Curley KL. Prophylactic Tranexamic Acid Prevents Postpartum Hemorrhage and Transfusions in Cesarean Deliveries: A Systematic Review and Meta-analysis. Am J Perinatol. 2023 Jul 21. |
More recent review is included. |
|
Lee SH, Kwek ME, Tagore S, Wright A, Ku CW, Teong ACA, Tan AWM, Lim SWC, Yen DYT, Ang CYX, Sultana R, Lim CHF, Mathur D, Mathur M. Tranexamic acid, as an adjunct to oxytocin prophylaxis, in the prevention of postpartum haemorrhage in women undergoing elective caesarean section: A single-centre double-blind randomised controlled trial. BJOG. 2023 Aug;130(9):1007-1015. |
More recent review is included. One study from this review is judged seperately to be included in this literature summary. |
|
Li C, Gong Y, Dong L, Xie B, Dai Z. Is prophylactic tranexamic acid administration effective and safe for postpartum hemorrhage prevention?: A systematic review and meta-analysis. Medicine (Baltimore). 2017 Jan;96(1):e5653. |
More recent review is included. Three studies from this review are judged seperately to be included in this literature summary. |
|
Maged AM, Helal OM, Elsherbini MM, Eid MM, Elkomy RO, Dahab S, Elsissy MH. A randomized placebo-controlled trial of preoperative tranexamic acid among women undergoing elective cesarean delivery. Int J Gynaecol Obstet. 2015 Dec;131(3):265-8. |
Included in review Cheema 2023 |
|
Milani F, Haryalchi K, Sharami SH, Atrkarroshan Z, Farzadi S. Prophylactic effect of tranexamic acid on hemorrhage during and after the cesarean section. International Journal of Women's Health and Reproduction Sciences. 2019;7(1)74-78. |
Included in review Cheema 2023 |
|
Mirghafourvand M, Mohammad-Alizadeh S, Abbasalizadeh F, Shirdel M. The effect of prophylactic intravenous tranexamic acid on blood loss after vaginal delivery in women at low risk of postpartum haemorrhage: a double-blind randomised controlled trial. Aust N Z J Obstet Gynaecol. 2015 Feb;55(1):53-8. |
Included in review Assis 2023 |
|
Movafegh A, Eslamian L, Dorabadi A. Effect of intravenous tranexamic acid administration on blood loss during and after cesarean delivery. Int J Gynaecol Obstet. 2011 Dec;115(3):224-6. |
Included in review Cheema 2023 |
|
Naeiji Z, Delshadiyan N, Saleh S, Moridi A, Rahmati N, Fathi M. Prophylactic use of tranexamic acid for decreasing the blood loss in elective cesarean section: A placebo-controlled randomized clinical trial. J Gynecol Obstet Hum Reprod. 2021 Jan;50(1):101973. |
Included in review Cheema 2023 |
|
Nandal I, Kochar SPS, Dahiya A, Kaur R. Role of Intravenous Tranexamic Acid in Reducing Blood Loss during Caesarean Delivery. International Medical Journal. 2022;29(1):23-25. |
Concerns about quality of the study |
|
Nargis N, Dewan F. Prophylactic use of tranexamic acid during caesarean section in preventing postpartum haemorrhage-a prospective randomised double blind placebo controlled study. Bangladesh Journal of Obstetrics and Gynecology. 2020;33(2):125-130. |
Included in review Cheema 2023 |
|
Novikova N, Hofmeyr GJ, Cluver C. Tranexamic acid for preventing postpartum haemorrhage. Cochrane Database Syst Rev. 2015 Jun 16;(6):CD007872. |
More recent review is included |
|
Ogunkua OT, Duryea EL, Nelson DB, Eddins MM, Klucsarits SE, McIntire DD, Leveno KJ. Tranexamic acid for prevention of hemorrhage in elective repeat cesarean delivery-a randomized study. Am J Obstet Gynecol MFM. 2022 Mar;4(2):100573. |
Included in review Cheema 2023 |
|
Oseni RO, Zakari M, Adamou N, Umar UA. Effectiveness of preoperative tranexamic acid in reducing blood loss during caesarean section at Aminu Kano Teaching Hospital, Kano: a randomized controlled trial. Pan Afr Med J. 2021 May 12;39:34. |
Included in review Cheema 2023 |
|
Pacheco LD, Clifton RG, Saade GR, Weiner SJ, Parry S, Thorp JM Jr, Longo M, Salazar A, Dalton W, Tita ATN, Gyamfi-Bannerman C, Chauhan SP, Metz TD, Rood K, Rouse DJ, Bailit JL, Grobman WA, Simhan HN, Macones GA; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal–Fetal Medicine Units Network. Tranexamic Acid to Prevent Obstetrical Hemorrhage after Cesarean Delivery. N Engl J Med. 2023 Apr 13;388(15):1365-1375. |
Included in review Cheema 2023 |
|
Peitsidis P, Kadir RA. Antifibrinolytic therapy with tranexamic acid in pregnancy and postpartum. Expert Opin Pharmacother. 2011 Mar;12(4):503-16. |
Wrong study design; expert opinion |
|
Priyanka, Bajpayee M, Sinha AR. Tranexamic acid to Reduce Blood Loss in Women at High Risk for Postpartum Hemorrhage undergoing Cesarean Section: A Randomized Controlled Trial. International Journal of Pharmaceutical and Clinical Research. 2023;15(3):1447-1451. |
Wrong population: high risk women |
|
Ramesh AC, Rajni S, Deka N. Efficacy of tranexamic acid in decreasing blood loss during and after cesarean section: A randamized case controlled prospective study. Indian Journal of Public Health Research and Development. 2015;6(2):12-15. |
Concerns about quality of the study |
|
Ray I, Bhattacharya R, Chakraborty S, Bagchi C, Mukhopadhyay S. Role of Intravenous Tranexamic Acid on Caesarean Blood Loss: A Prospective Randomised Study. J Obstet Gynaecol India. 2016 Oct;66(Suppl 1):347-52. doi: 10.1007/s13224-016-0915-x. Epub 2016 Jun 25. PMID: 27651628; PMCID: PMC5016480. |
Included in review Cheema 2023 |
|
Roy P, Sujatha MS, Bhandiwad A, Biswas B. Role of Tranexamic Acid in Reducing Blood Loss in Vaginal Delivery. J Obstet Gynaecol India. 2016 Oct;66(Suppl 1):246-50. |
Concerns about quality of the study |
|
Saccone G, Della Corte L, D'Alessandro P, Ardino B, Carbone L, Raffone A, Guida M, Locci M, Zullo F, Berghella V. Prophylactic use of tranexamic acid after vaginal delivery reduces the risk of primary postpartum hemorrhage. J Matern Fetal Neonatal Med. 2020 Oct;33(19):3368-3376. |
Double in search |
|
Saccone G, Della Corte L, D'Alessandro P, Ardino B, Carbone L, Raffone A, Guida M, Locci M, Zullo F, Berghella V. Prophylactic use of tranexamic acid after vaginal delivery reduces the risk of primary postpartum hemorrhage. J Matern Fetal Neonatal Med. 2020 Oct;33(19):3368-3376. |
More recent review is included. |
|
Sekhavat L, Tabatabaii A, Dalili M, Farajkhoda T, Tafti AD. Efficacy of tranexamic acid in reducing blood loss after cesarean section. J Matern Fetal Neonatal Med. 2009 Jan;22(1):72-5. |
Quasi-randomized (based on Cochrane review) |
|
Sentilhes L, Lasocki S, Ducloy-Bouthors AS, Deruelle P, Dreyfus M, Perrotin F, Goffinet F, Deneux-Tharaux C. Tranexamic acid for the prevention and treatment of postpartum haemorrhage. Br J Anaesth. 2015 Apr;114(4):576-87. |
wrong study design: narrative review |
|
Sentilhes L, Madar H, Le Lous M, Sénat MV, Winer N, Rozenberg P, Kayem G, Verspyck E, Fuchs F, Azria E, Gallot D, Korb D, Desbrière R, Le Ray C, Chauleur C, de Marcillac F, Perrotin F, Parant O, Salomon LJ, Gauchotte E, Bretelle F, Sananès N, Bohec C, Mottet N, Legendre G, Letouzey V, Haddad B, Vardon D, Mattuizzi A, Froeliger A, Bouchghoul H, Daniel V, Regueme S, Roussillon C, Georget A, Darsonval A, Benard A, Deneux-Tharaux C; Groupe de Recherche en Obstétrique et Gynécologie. Tranexamic acid for the prevention of blood loss after cesarean among women with twins: a secondary analysis of the TRAnexamic Acid for Preventing Postpartum Hemorrhage Following a Cesarean Delivery randomized clinical trial. Am J Obstet Gynecol. 2022 Dec;227(6):889.e1-889.e17. |
Wrong population: women with twins |
|
Sentilhes L, Sénat MV, Le Lous M, Winer N, Rozenberg P, Kayem G, Verspyck E, Fuchs F, Azria E, Gallot D, Korb D, Desbrière R, Le Ray C, Chauleur C, de Marcillac F, Perrotin F, Parant O, Salomon LJ, Gauchotte E, Bretelle F, Sananès N, Bohec C, Mottet N, Legendre G, Letouzey V, Haddad B, Vardon D, Madar H, Mattuizzi A, Daniel V, Regueme S, Roussillon C, Benard A, Georget A, Darsonval A, Deneux-Tharaux C; Groupe de Recherche en Obstétrique et Gynécologie. Tranexamic Acid for the Prevention of Blood Loss after Cesarean Delivery. N Engl J Med. 2021 Apr 29;384(17):1623-1634. |
Included in review Cheema 2023 |
|
Sentilhes L, Winer N, Azria E, Sénat MV, Le Ray C, Vardon D, Perrotin F, Desbrière R, Fuchs F, Kayem G, Ducarme G, Doret-Dion M, Huissoud C, Bohec C, Deruelle P, Darsonval A, Chrétien JM, Seco A, Daniel V, Deneux-Tharaux C; Groupe de Recherche en Obstétrique et Gynécologie. Tranexamic Acid for the Prevention of Blood Loss after Vaginal Delivery. N Engl J Med. 2018 Aug 23;379(8):731-742. |
Included in review Assis 2023 |
|
Sentürk MB, Cakmak Y, Yildiz G, Yildiz P. Tranexamic acid for cesarean section: a double-blind, placebo-controlled, randomized clinical trial. Arch Gynecol Obstet. 2013 Apr;287(4):641-5. |
Included in review Cheema 2023 |
|
Shahid A, Khan A. Tranexamic acid in decreasing blood loss during and after caesarean section. J Coll Physicians Surg Pak. 2013 Jul;23(7):459-62. |
Included in review Cheema 2023 |
|
Shalaby MA, Maged AM, Al-Asmar A, El Mahy M, Al-Mohamady M, Rund NMA. Safety and efficacy of preoperative tranexamic acid in reducing intraoperative and postoperative blood loss in high-risk women undergoing cesarean delivery: a randomized controlled trial. BMC Pregnancy Childbirth. 2022 Mar 14;22(1):201. doi: 10.1186/s12884-022-04530-4. Erratum in: BMC Pregnancy Childbirth. 2022 Nov 7;22(1):823. |
Included in review Cheema 2023 |
|
Simonazzi G, Bisulli M, Saccone G, Moro E, Marshall A, Berghella V. Tranexamic acid for preventing postpartum blood loss after cesarean delivery: a systematic review and meta-analysis of randomized controlled trials. Acta Obstet Gynecol Scand. 2016 Jan;95(1):28-37. |
More recent review is included. |
|
Singh KP, Singh HA, Singh KM, Laishram N. Tranexamic Acid and Blood Loss During and After Caesarean Section. A Randomized Case Control Prospective Study. European Journal of Molecular and Clinical Medicine. 2022;9(7):7870-7874. |
No fulltext available |
|
Stortroen NE, Tubog TD, Shaffer SK. Prophylactic Tranexamic Acid in High-Risk Patients Undergoing Cesarean Delivery: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. AANA J. 2020 Aug;88(4):273-281. |
Wrong Patient population (high risk pregnancy) |
|
Sujata N, Tobin R, Kaur R, Aneja A, Khanna M, Hanjoora VM. Randomized controlled trial of tranexamic acid among parturients at increased risk for postpartum hemorrhage undergoing cesarean delivery. Int J Gynaecol Obstet. 2016 Jun;133(3):312-5. |
Included in review Cheema 2023 |
|
Sujita A, Songthamwat S, Songthamwat M. Effectiveness of tranexamic acid for reducing postpartum blood loss in the first two hours after vaginal delivery: A randomised controlled trial. Journal of Clinical and Diagnostic Research. 2018 jan;12(3). |
Included in review Assis 2023 |
|
Taj N, Firdous A, Akhtar N, Chaudhary MH, Bajwa SZ. Efficacy of Tranexamic acid in reducing blood loss during and after Cesarean section Ullah, E. Rawal Medical Journal. 2014 jan; 39(3):311-313. |
Included in review Cheema 2023 |
|
Tayyba A, Anjum S, Farooq M, Nadeem N, Farooq M, Sohail H, Sohail M, Sulehri AA. Role of tranexamic acid in reducing blood loss during and after caesarean section. Pakistan Journal of Medical and Health Sciences. 2020;14(3):558-561. |
No fulltext available |
|
Urooj H, Shah M, Bukhari B, Rahim R. Comparison of therapeutic effectiveness of oxytocin versus oxytocin plus tranexamic acid in the prevention of post-partum hemorrhage. Journal of Postgraduate Medical Institute. 2021;35(2):80-84. |
Concerns about quality of the study |
|
Wang HY, Hong SK, Duan Y, Yin HM. Tranexamic acid and blood loss during and after cesarean section: a meta-analysis. J Perinatol. 2015 Oct;35(10):818-25. |
More recent review is included. |
|
Wang Y, Liu S, He L. Prophylactic use of tranexamic acid reduces blood loss and transfusion requirements in patients undergoing cesarean section: A meta-analysis. J Obstet Gynaecol Res. 2019 Aug;45(8):1562-1575. |
More recent review is included. |
|
Xia Y, Griffiths BB, Xue Q. Tranexamic acid for postpartum hemorrhage prevention in vaginal delivery: A meta-analysis. Medicine (Baltimore). 2020 Jan;99(3):e18792. |
More recent review is included. |
|
Xu J, Gao W, Ju Y. Tranexamic acid for the prevention of postpartum hemorrhage after cesarean section: a double-blind randomization trial. Arch Gynecol Obstet. 2013 Mar;287(3):463-8. |
Included in review Cheema 2023 |
|
Yang F, Wang H, Shen M. Effect of preoperative prophylactic intravenous tranexamic acid on perioperative blood loss control in patients undergoing cesarean delivery: a systematic review and meta-analysis. BMC Pregnancy Childbirth. 2023 Jun 6;23(1):420. |
More recent review is included. |
|
Yang H, Zheng S, Shi C. [Clinical study on the efficacy of tranexamic acid in reducing postpartum blood lose: a randomized, comparative, multicenter trial]. Zhonghua Fu Chan Ke Za Zhi. 2001 Oct;36(10):590-2. Chinese. |
Language: Chinese |
Verantwoording
Beoordelingsdatum en geldigheid
Publicatiedatum : 12-12-2025
Beoordeeld op geldigheid : 12-12-2025
Algemene gegevens
De ontwikkeling/herziening van deze richtlijnmodule werd ondersteund door het Kennisinstituut van de Federatie Medisch Specialisten (www.demedischspecialist.nl/kennisinstituut) en werd gefinancierd door de Stichting Kwaliteitsgelden Medisch Specialisten (SKMS). De financier heeft geen enkele invloed gehad op de inhoud van de richtlijnmodule.
Samenstelling werkgroep
Voor het ontwikkelen van de richtlijnmodule is in 2022 een multidisciplinaire werkgroep ingesteld, bestaande uit vertegenwoordigers van alle relevante specialismen (zie hiervoor de Samenstelling van de werkgroep) die betrokken zijn bij de zorg voor patiënten met haemorrhagia postpartum.
Werkgroep
- Dr. M.(Martina) Porath (voorzitter) (NVOG)
- Dr. H.C.J. (Liesbeth) Scheepers (NVOG)
- Dr. D.D.C.A. (Dacia) Henriquez (NVOG)
- Dr. J.M. (Annemieke) Middeldorp(NVOG)
- Prof. dr. T.H. (Thomas) van den Akker (NVOG)
- Dr. P. (Paul) Ramler (NVOG) (tot September 2023)
- Dr. K.P.M. (Karin) van Galen(NIV)
- Drs. H.W. (Hannah) de Klerk (KNOV)
- Drs. L. (Lianne) Zondag (KNOV)
- Prof. dr. ir. Y.M.C. (Yvonne) Henskens (NVKC)
- Dr. I.C.M. (Ingrid) Beenakkers (NVA)
- Dr. S. (Simone) Willems (NVA)
- Mw. I. (Ilse) van Ee (PFN)
Klankboardgroep
- Drs. K. (Klaartje) Caminada (AZN)
- Mw. B. (Britt) Ketelaars (PFN)
Met ondersteuning van
- Dr. M. (Mohammadreza) Abdollahi, adviseur, Kennisinstituut van de Federatie van Medisch Specialisten
- Dr. I.M. (Irina) Mostovaya, senior adviseur, Kennisinstituut van de Federatie van Medisch Specialisten
- Dr. J. (Jana) Tuijtelaars, adviseur, Kennisinstituut van de Federatie van Medisch Specialisten
- Drs. D.A.M. (Danique) Middelhuis, adviseur, Kennisinstituut van de Federatie van Medisch Specialisten
- Dr. M. (Majke) van Bommel, adviseur, Kennisinstituut van de Federatie van Medisch Specialisten
- Dr. L. (Leanne) Küpers, adviseur, Kennisinstituut van de Federatie van Medisch Specialisten
- Drs. D.A.M. (Fieke) Pepping, junior adviseur, Kennisinstituut van de Federatie van Medisch Specialisten
- Linda Niesink, medisch informatie specialist, Kennisinstituut van de Federatie Medisch Specialisten
- Laura Boerboom, medisch informatie specialist, Kennisinstituut van de Federatie Medisch Specialisten
- Alies Oost, medisch informatie specialist, Kennisinstituut van de Federatie Medisch Specialisten
Belangenverklaringen
Een overzicht van de belangen van werkgroepleden en het oordeel over het omgaan met eventuele belangen vindt u in onderstaande tabel. De ondertekende belangenverklaringen zijn op te vragen bij het secretariaat van het Kennisinstituut van de Federatie Medisch Specialisten via secretariaat@kennisinstituut.nl.
|
Naam |
Hoofdfunctie |
Nevenwerkzaamheden |
Persoonlijke financiële belangen |
Persoonlijke relaties |
Extern gefinancierd onderzoek |
Intellectuele belangen en reputatie |
Overige belangen |
Datum |
Restrictie |
|
Martina Porath |
gynaecoloog-perinatoloog Maxima Medisch Centrum |
Voorzitter focusgroep Acute Verloskunde Noord-Brabant Lid NVOG werkgroep Otterlo |
geen |
geen |
geen |
geen |
geen |
04/28/2025 |
|
|
Liesbeth Scheepers |
perinatoloog MUMC |
Richtlijnen commissie Otterloo groep Voorzitter Regioconsortium Geboortezorg Limburg Vice voorzitter Perinatale audit Limburg |
Geen |
Nee |
ZONMW onderzoek, niet op dit onderwerp |
Nee |
Nee |
04/25/2025 |
|
|
Annemieke Middeldorp |
Gynaecoloog Perinatoloog Leids Universitair Medisch Centrum, gestopt met werken vanaf 2024 |
geen |
geen |
geen |
geen |
geen |
geen |
04/25/2025 |
|
|
Dacia Henriquez |
Gynaecoloog, Perinatoloog, Amphia Ziekenhuis |
Geen |
Geen |
Geen |
Geen |
Geen |
Geen |
04/28/2025 |
|
|
Thomas van den Akker |
Gynaecoloog perinatoloog, LUMC, hoogleraar Verloskunde |
Hoogleraar VU *Global Maternal Health, Athena Instituut. *Perined bestuurslid *RvT Wemos. *FMG Pijlervoorzitter NVOG *Vz working party for international safe motherhood and reproductive health. *Perinatale audit regiovoorzitter. Alles onbetaald. |
Geen |
Geen |
Geen |
Door werk van de commissie komt het werk van mijn voormalig promovendi Ada Gillissen en Paul Ramler meer in de aandacht staan, omdat sommige aanbevelingen hierop gebaseerd zijn (met name van laatstgenoemde ten aanzien van ballon versus embolisatie).
Het feit dat ik als bijdrager op de richtlijn sta kan gezien worden als ten goede komend aan de boegbeeldfunctie die ik bij de NVOG heb, maar het effect hiervan lijkt beperkt in het licht van mijn andere activiteiten. |
Geen |
04/25/2025 |
|
|
Paul Ramler (tot September 2023) |
AIOS Gynaecologie, cluster Leiden |
Geen |
Geen |
Geen |
Geen |
Geen |
Geen |
29/01/2023 |
|
|
Ingrid Beenakkers |
Anesthesioloog, UMCU/WKZ |
geen |
geen |
geen |
geen |
geen |
geen |
04/28/2025 |
|
|
Simone Anna Alexandra Sijm-Willems |
Anesthesioloog Radboudumc |
- |
-zs |
- |
- |
- |
- |
04/25/2025 |
|
|
Karin van Galen |
internist-hematoloog UMC Utrecht |
geen |
geen |
n.v.t. |
Octapharma: Pregnancy and inherited bleeding disorders - unresticted research grant, projectleider. |
Voorzitter Nederlands Zorgnetwerk Vrouwen met een stollingsstoornis. Tot Febr 2025 voorzitter Women and Girls with beleeding Disorders Group European Assosiation for Heamophilia and Alied Disorders - momenteel nog actief lid van deze werkgroep. |
n.v.t. |
04/25/2025 |
|
|
Yvonne Henskens |
Klinisch chemicus, waarnemend hoofd Centraal Diagnostisch Laboratorium Hoogleraar Klinische Chemie ihb Hemostase |
Voorzitter Vereniging Hematologisch Laboratoria 2020-heden Lid Vici beoordelingscie 2022 Lid raad van Advies NVKC 2023-heden Adviseur Promicol 2020-heden |
geen |
geen |
Voor alle studies in het kader van mijn leerstoel worden reagentia of apparatuur gratis of met korting verkregen, hierbij wordt geen enkele IVD methode of bedrijf uitgesloten mits het past in het doel van mijn leerstoel. Noyons stipendium NVKC (prijs) Stago / Validatie van apparatuur en/of reagentia / Ja Siemens / Validatie van apparatuur en/of reagentia / Ja Werfen / Validatie van apparatuur en/of reagentia / N Nodia / Validatie van apparatuur en/of reagentia / Nee Promicol / Validatie van apparatuur en/of reagentia / Nee |
nee |
nee |
04/25/2025 |
|
|
Hannah de Klerk |
Zelfstandig eerstelijns verloskundige (ZZP), betaald PhD aanstelling Amsterdam UMC, locatie VUMc, onbetaald |
Gastdocent HU, betaald Projectleider KNOV, betaald |
geen |
nee |
nee |
nee |
nee |
7/10/2022 |
|
|
Lianne Zondag |
Eerstelijns verloskundige - verloskundige praktijk de Toekomst - Geldermalsen KNOV - senior richtlijnonwikkelaar |
Bestuurslid Netwerk Geboortezorg Rivierenland PhD candidate Maastricht University |
Geen |
Geen |
Geen |
Geen |
Geen |
2/6/2023 |
|
|
Ilse van Ee |
Adviseur Patientenbelang - full time inbreng patientenperspectief |
Ervaringsdeskundige patientvertegenwoordiger - Eupati - fellow Psoriasispatienten Nederland - onbetaald Coordinator Patientenpraticipatie, lid centrale redactie Psoriasispatienten Nederland - onbetaald Eupati mentor - Eupati Nl - onbetaald |
nee |
nvt |
Janssen / Freedom of disease Psoriasis / ja |
nee |
nvt |
04/25/2025 |
|
|
Klaartje Caminada |
Medisch Manager Ambulancezorg, lid protocollencommissie Ambulancezorg Nederland |
geen |
geen |
geen |
geen |
geen |
geen |
04/29/2025 |
|
|
Brit Ketelaars |
Adviseur patiëntbelang bij Patiëntenfederatie Nederland (full time, betaald) |
geen |
geen |
geen |
geen |
geen |
geen |
04/25/2025 |
|
Inbreng patiëntenperspectief
De werkgroep besteedde aandacht aan het patiëntenperspectief door uitnodigen van Patiëntenfederatie Nederland, Geboortebeweging en Het Buikencollectiefvoor de invitational conference. Het verslag hiervan is besproken in de werkgroep. De verkregen input is meegenomen bij het opstellen van de uitgangsvragen, de keuze voor de uitkomstmaten en bij het opstellen van de overwegingen. De conceptrichtlijn is tevens voor commentaar voorgelegd aan Patiëntenfederatie Nederland en de eventueel aangeleverde commentaren zijn bekeken en verwerkt.
Kwalitatieve raming van mogelijke financiële gevolgen in het kader van de Wkkgz
Bij de richtlijnmodule voerde de werkgroep conform de Wet kwaliteit, klachten en geschillen zorg (Wkkgz) een kwalitatieve raming uit om te beoordelen of de aanbevelingen mogelijk leiden tot substantiële financiële gevolgen. Bij het uitvoeren van deze beoordeling is de richtlijnmodule op verschillende domeinen getoetst (zie het stroomschema bij Werkwijze).
Uit de kwalitatieve raming blijkt dat er waarschijnlijk mogelijk substantiële financiële gevolgen zijn, zie onderstaande tabel.
In Nederland bevallen per jaar ca. 160.000 vrouwen. De incidentie van haemorrhagia postpartum is 7 tot 10%. Dit zijn 11.200 tot 16.000 vrouwen per jaar. Het aantal vrouwen met risicofactoren voor HPP is niet bekend.
| Module |
Uitkomst raming |
Toelichting |
|
Tranexaminezuur ter preventie van HPP |
geen financiële gevolgen |
De aanbeveling betreft alleen vrouwen met meerdere risicofactoren voor HPP. Het aantal is niet bekend. Tranexaminezuur is goedkoop. |
Werkwijze
Voor meer details over de gebruikte richtlijnmethodologie verwijzen wij u naar de Werkwijze. Relevante informatie voor de ontwikkeling/herziening van deze richtlijnmodule is hieronder weergegeven.
Zoekverantwoording
Literature search strategy
Algemene informatie
|
Richtlijn: Richtlijn Modulaire actualisatie richtlijn Hemorrhagia Postpartum (HPP) |
|
|
Uitgangsvraag: Wat is de rol van tranexaminezuur in de preventie van HPP? |
|
|
Database(s): Medline (OVID), Embase |
Datum: 31-08-2023 |
|
Periode: > 2000 |
Talen: geen beperking |
|
Literatuurspecialist: Laura Boerboom |
|
|
BMI zoekblokken: voor verschillende opdrachten wordt (deels) gebruik gemaakt van de zoekblokken van BMI-Online https://blocks.bmi-online.nl/ Bij gebruikmaking van een volledig zoekblok zal naar de betreffende link op de website worden verwezen. |
|
|
Toelichting en opmerkingen:
→ Voor deze vraag is gezocht op de elementen HPP (in het blauw) en tranexaminezuur (in het groen).
→ Alle 7 sleutelpublicaties zitten in de resultaten.
→ Resultaten staan in Rayyan.
|
|
|
Te gebruiken voor richtlijnen tekst: In de databases Embase (via embase.com) en Medline (via OVID) is op 31-08-2023 met relevante zoektermen gezocht naar systematic reviews, RCT’s en observationele studies over wat de rol is van tranexaminezuur in de preventie van HPP. De literatuurzoekactie leverde 430 unieke treffers op. |
|
Zoekopbrengst
|
|
EMBASE |
OVID/MEDLINE |
Ontdubbeld |
|
SR’s |
110 |
44 |
109 |
|
RCT’s |
190 |
81 |
202 |
|
Observationele studies |
117 |
18 |
119 |
Zoekstrategie
|
Database |
Zoektermen |
||||||||||||||||||||||||||||||||||||
|
Embase
|
|
||||||||||||||||||||||||||||||||||||
|
Medline (OVID)
|
1 exp Postpartum Hemorrhage/ or 'fluxus postpartum'.ti,ab,kf. or 'postpartum hemorrhage'.ti,ab,kf. or 'post partum haemorrhage'.ti,ab,kf. or 'post partum hemorrhage'.ti,ab,kf. or 'postpartal haemorrhage'.ti,ab,kf. or 'postpartal hemorrhage'.ti,ab,kf. or 'postpartum bleeding'.ti,ab,kf. or 'postpartum haemorrhage'.ti,ab,kf. or 'puerperal haemorrhage'.ti,ab,kf. or 'puerperal hemorrhage'.ti,ab,kf. or ('blood loss' adj6 (postpartum or 'post partum' or delivery or cesarean)).ti,ab,kf. or 'obstetric* bleeding'.ti,ab,kf. or 'obstetric* hemorrhage'.ti,ab,kf. or 'obstetric* haemorrhage'.ti,ab,kf. (14067) |