Tranexaminezuur in de behandeling van HPP
Uitgangsvraag
Wat is de rol van tranexaminezuur in de behandeling van HPP?
Aanbeveling
Geef 1g TXA zo snel mogelijk bij het optreden van aanhoudend bloedverlies postpartum vanaf 500 mL.
Herhaal deze dosering bij onverminderd bloedverlies.
Voor de identificatie van risicofactoren voor HPP en de behandeling van HPP wordt verwezen naar de Fluxus Implementatie Strategie en naar het modelprotocol behandeling van HPP.
Overwegingen
Voor- en nadelen van de interventie en de kwaliteit van het bewijs
In deze uitgangsvraag heeft de werkgroep gekeken naar het effect van tranexaminezuur op de behandeling van aanhoudend bloedverlies na een bevalling. TXA kan bloedverlies verminderen door de afbraak van fibrinogeen en fibrine tegen te gaan. In studies bij trauma patiënten en cardiothoracale chirurgie werd van TXA een positief effect op bloedverlies en mortaliteit beschreven (CRASH-2, BART).
Omdat bij aanhoudend bloedverlies bij obstetrische patiënten over het algemeen meerdere interventies tegelijkertijd plaatsvinden is de afzonderlijke bijdrage van TXA niet altijd duidelijk.
Therapeutisch gebruik van TXA bij obstetrisch bloedverlies is slechts in twee trials onderzocht: een Franse studie van Ducloy-Bouthors en de WOMAN trial die grotendeels in lage inkomenslanden uitgevoerd is, waar vaak weinig andere opties voorhanden zijn om massaal bloedverlies te voorkomen of te behandelen. Uit de RCT van Ducloy-Bouthors blijkt dat TXA een afname van bloedverlies en een kortere tijdsduur van bloedverlies gaf. Tevens was er minder anemie, transfusiebehoefte en noodzaak tot invasieve interventies. In deze studie werden vrouwen geïncludeerd met bloedverlies vanaf 800 mL na een vaginale partus. Het gemiddelde bloedverlies bij inclusie was 1000 mL in TXA groep en 950 mL in de controle groep. Om het bloedverlies te beperken was in de richtlijn uit 2019 afgesproken om TXA te geven vanaf 500 mL bloedverlies.
In de WOMAN trial werd een grote populatie vrouwen geïncludeerd met bloedverlies vanaf 500 mL na een vaginale partus en 1000 mL na een sectio caesarea. TXA heeft een positief effect op mortaliteit. In hoge inkomenslanden lijkt het effect van TXA op mortaliteit van minder belang gezien de vele andere mogelijkheden die er voorhanden zijn voor de behandeling van bloedverlies.
Vanwege weinig andere behandelingsmogelijkheden in lage inkomenslanden was bij inclusie vaak al het besluit tot hysterectomie genomen. Om die reden werd de studie uitgebreid, maar alsnog werd geen significant positief effect op het aantal hysterectomieën gevonden. Wel werden significant minder laparotomieën verricht.
Vanwege imprecisie, geen mogelijkheid tot blindering en retrospectieve aspecten zijn de conclusies afgewaardeerd en meestal van low GRADE. Ook is het gepoolde effect moeilijk te beoordelen vanwege de verschillen in hoge – en lage inkomenslanden.
Zowel in de CRASH-2 trial als de WOMAN trial lijkt het effect van TXA het grootst als het binnen 3 uur na start bloedverlies gegeven wordt.
Het gebruik van TXA leidt niet tot een toename van tromboembolische complicaties. Het is echter nog niet duidelijk of dit ook geldt voor vrouwen met een stollingsziekte waarbij een verhoogde kans op trombose is, zoals het antifosfolipidensyndroom (APS).
Er zijn geen doseringsstudies gedaan bij obstetrisch bloedverlies. De oplaaddosis in de twee geïncludeerde studies waren 1 en 4 gram. De studie waarbij 1 gram gebruikt werd, adviseert bij doorgaand bloedverlies een herhalingsdosis van 1 gram na 30 minuten. Een oplaaddosis van 4 gram TXA lijkt gepaard te gaan met een verhoogd risico op nierfunctiestoornissen.
Op basis van de literatuur kan geen aanbeveling gedaan worden ten aanzien van een minimaal tijdsinterval tussen twee giften. Een maximale dosering is niet bekend. In het farmacotherapeutisch compas wordt 4 gram per dag aangehouden.
Het tijdstip van starten lijkt het meest zinvol zo snel mogelijk na het optreden van aanhoudend bloedverlies van meer dan 500 mL. LAREB vermeldt het volgende over TXA tijdens de borstvoeding periode: Er is nog weinig bekend over het gebruik van TXA bij het geven van borstvoeding. De studies die verricht zijn laten geen nadelige effecten zien op de zuigeling. Op de eerste dag drinken zuigelingen over het algemeen kleine hoeveelheden moedermelk.
Waarden en voorkeuren van patiënten (en evt. hun verzorgers)
Hevig bloedverlies na de bevalling kan voor vrouwen een ingrijpende en verwarrende ervaring zijn, waarbij het gevoel van controle verloren kan gaan. Deze vrouwen geven aan het belangrijk te vinden dat zij voorafgaand aan de bevalling geïnformeerd worden over mogelijke complicaties zoals postpartum bloedverlies en de verschillende behandelopties die in dat geval beschikbaar zijn. Hierbij kunnen diverse medicamenten gebruikt worden waaronder tranexaminezuur. Deze informatie vooraf draagt bij aan een gevoel van voorbereiding en betrokkenheid, en kan de communicatie tijdens acute situaties ondersteunen.
Tegelijkertijd erkennen patiënten dat tijdens acute, kritieke medische situaties – zoals bij ernstig bloedverlies – vaak snel handelen vereist is en dat uitgebreide communicatie of expliciete toestemming voor elk afzonderlijk middel niet altijd mogelijk is.
Vrouwen geven aan vertrouwen te hechten aan het handelen volgens professionele standaarden in zulke situaties, mits hierover op een later moment uitleg wordt gegeven.
Daar waar de situatie het toelaat, is het wenselijk om patiënten en hun eventuele partners zo goed mogelijk te informeren over de aard van de interventie en wat zij kunnen verwachten. Transparantie en duidelijke communicatie, ook in hectische omstandigheden, worden door patiënten gewaardeerd en dragen bij aan het gevoel van veiligheid en regie.
Kosten (middelenbeslag)
Zoals uit de studie van Ducloy-Bouthors blijkt zijn door TXA gebruik minder bloedproducten en invasieve interventies nodig. In het licht daarvan kan TXA kostenbesparend zijn. Daarnaast is TXA een goedkoop product en makkelijk op voorraad te houden.
Op het moment van het schrijven van deze richtlijn is de gemiddelde prijs per ampul tranexaminezuur van 5 ml 1,54 EUR.
Aanvaardbaarheid, haalbaarheid en implementatie
TXA wordt voornamelijk toegediend als vrouwen in het ziekenhuis zijn en daar is het makkelijk te verkrijgen. In veel ziekenhuizen wordt het reeds protocollair toegepast.
Rationale van de aanbeveling: weging van argumenten voor en tegen de interventies
In hoge inkomenslanden wordt TXA gegeven om bloedverlies te voorkomen en behandelen bij zowel chirurgische als trauma patiënten. In lage inkomenslanden reduceert TXA maternale sterfte en de noodzaak tot laparotomie. Gezien deze gunstige effecten is de werkgroep van mening dat tranexaminezuur een plaats heeft bij de behandeling van aanhoudend bloedverlies postpartum (> 500 mL) zowel na vaginale baring als na sectio.
TXA is niet duur, veilig en makkelijk te bewaren. Toevoeging van TXA aan andere interventies lijkt geen toegenomen kans op tromboembolische complicaties te geven.
Onderbouwing
Achtergrond
Tranexamic acid (TXA) is an antifibrinolytic which reduces blood loss and transfusion requirements in cardiac surgery and trauma patients by preventing breakdown of fibrinogen and fibrin. Pregnancy induced hypercoagulability in the third trimester is an adapted mechanism to prevent postpartum haemorrhage. The questions are whether TXA adds any improvement in obstetric ongoing blood loss compared to or in addition to routine use of uterotonic, at which moment it has to be started and in which dosage.
Conclusies / Summary of Findings
|
Low GRADE |
The use of tranexamic acid may result in little to no difference in the amount of blood loss when compared with placebo in women postpartum with ongoing blood loss >500 mL.
Source: Ducloy-Bouthors, 2011 |
|
No GRADE |
The outcome measures shock and coagulopathy were not reported in the included studies. |
|
High GRADE |
The use of tranexamic acid results in little to no difference in hysterectomy in comparison with placebo in women postpartum with ongoing blood loss >500 mL.
Source: WOMAN trial, 2017 |
|
Moderate GRADE |
The use of tranexamic acid likely results in little to no difference in organ dysfunction in comparison with placebo in women postpartum with ongoing blood loss >500 mL.
Source: Ducloy-Bouthors, 2011; WOMAN trial, 2017 |
|
Moderate GRADE |
The use of tranexamic acid likely reduces maternal death in comparison with placebo in women postpartum with ongoing blood loss >500 mL.
Source: WOMAN trial, 2017 |
|
Low GRADE |
The use of tranexamic acid may reduce blood transfusion in comparison with placebo in women postpartum with ongoing blood loss >500 mL.
Source: Ducloy-Bouthors, 2011; WOMAN trial, 2017 |
|
Moderate GRADE
Moderate GRADE
Low GRADE
High GRADE |
For the outcome measure use of additional hemostatic interventions, the following conclusions were formulated:
The use of tranexamic acid likely results in little to no difference in arterial ligation in comparison with placebo in women postpartum with ongoing blood loss >500 mL. Source: Ducloy-Bouthors, 2011; WOMAN trial, 2017
The use of tranexamic acid likely results in little to no difference in brace sutures in comparison with placebo in women postpartum with ongoing blood loss >500 mL. Source: WOMAN trial, 2017
The use of tranexamic acid may result in little to no difference in embolization in comparison with placebo in women postpartum with ongoing blood loss >500 mL. Source: Ducloy-Bouthors, 2011; WOMAN trial, 2017
The use of tranexamic acid results in little to no difference in intrauterine tamponade and manual removal of the placenta in comparison with placebo in women postpartum with ongoing blood loss >500 mL. Source: WOMAN trial, 2017 |
|
Very low GRADE |
The evidence is very uncertain about the effect of tranexamic acid on the need for transfer to higher level of care in comparison with placebo in women postpartum with ongoing blood loss >500 mL.
Source: Ducloy-Bouthors, 2011 |
|
No GRADE |
The outcome measures women’s sense of wellbeing, acceptability and satisfaction with the intervention, breastfeeding, adverse effects and PTSD were not reported in the included studies. |
Samenvatting literatuur
Description of studies
One systematic review (Shakur et al., 2018), describing three RCTs, was included in the analysis of the literature.
Shakur et al. (2018) provided a systematic review of the literature to determine the effectiveness and safety of antifibrinolytic drugs for treating primary PPH. Three trials involving 20.412 women were included in this review, but one trial (Sahhaf et al., 2014) was excluded in the current literature analysis because the studied control medication (misoprostol) did not meet our PICO. The other two trials (WOMAN trial, 2017; Ducloy-Bouthors et al., 2011), involving 20.212 women, contributed data to analyse blood loss, hysterectomy, organ dysfunction, maternal death, blood transfusion and transfer for higher level of care. The studied intervention and control treatment for the two trials are described in Table 1.
Ducloy-Bouthors et al. (2011) studied 152 women (1 woman assigned to TXA was later found not to be eligible and was excluded for analysis) with PPH (i.e. blood loss > 800 mL within 2 hours after vaginal delivery) who were recruited from eight centres (i.e. five tertiary care centres (102 patients) and three secondary care obstetric units (50 patients) in France. Women were randomized to either the intervention (n=77) or the control group (n=74) to study mortality, hysterectomy, blood transfusion, blood loss, estimated blood loss. Due to protocol violation 72 patients in both groups fully completed the protocol. There were some concerns regarding risk of bias for this trial due to a lack of blinding.
The WOMAN trial (2017) is a multi-centre RCT in which women with PPH (i.e. estimated blood loss after vaginal delivery >500 mL, or after caesarean section >1000ml) were recruited from hospital settings in high- (UK: 569 women), and low- and middle- income countries (Nigeria: 5711; Pakistan: 5282; Uganda: 2235; Kenya: 1031; Cameroon: 893; Sudan: 860; Tanzania: 538; Nepal: 533; Zambia: 496; Albania: 485; Democratic Republic of Congo: 457; Bangladesh: 325; Ethiopia: 302; Burkina Faso: 142; Jamaica: 73; Ghana: 41; Papua New Guinea: 38; Egypt: 33; Colombia: 8; Cote d’Ivoire: 8). Women were randomized to either the intervention (n=10051) or the control group (n=10009) to study mortality, serious maternal morbidity, maternal complications (such as organ failure, respiratory failure, seizure), hysterectomy, surgical and invasive interventions, blood transfusion and blood loss. The risk of bias of this trial was considered low.
|
Trial |
Intervention |
Control |
|
Ducloy-Bouthors (2011) |
Loading dose of 4 g TXA mixed with 50 mL saline, administered IV over one hour followed by a maintenance dose of 1 g/hour for six hours. |
Usual care (no TXA). |
|
WOMAN trial (2017) |
1 g IV TXA as an IV bolus over 10 minutes. If bleeding continued after 30 min, or had stopped and restarted within 24 hours of the first dose, women were given a second dose of 1 g. |
Placebo (sodium chloride 0.9% contained in ampoules and packaging with identical appearance to active treatment) with the same regimen as that used in the experimental group. |
Table 1. Description of treatments in the included trials. Abbreviations: TXA = tranexaminezuur, IV=intravenous.
Results
1: Blood loss
In the systematic review (Shakur, 2018), one study was included that reported on the outcome measure blood loss (Ducloy-Bouthors et al., 2011). In this study, women were included with blood loss >800 mL. Median blood loss at inclusion was 1000 mL in the TXA group and 950 mL in the control group. Blood loss as outcome measure was defined as mean blood loss (in mL) in the period from study inclusion (= maximal 2 hours after vaginal delivery) until 6 hours after study inclusion. In this literature summary, we included the absolute amount of blood loss (in mL).
The WOMAN trial did not measure blood loss after inclusion.
Figure 1. The effect of TXA on blood loss
Source: Shakur et al. (2018). Z: p-value of pooled effect, df: degrees of freedom, I2: statistical heterogeneity, CI: confidence interval.
Mean blood loss after inclusion in the study of Ducloy was, 280 ± 320 mL in the TXA-group and 387 ± 409 mL in the control group (MD -107.00, 95% CI -224.44 to 10. 44, Figure 1). The difference in blood loss between the TXA-group and the control group was considered not clinically important .
2: Shock
Not included in the SR
3: Coagulopathy
Not included in the SR
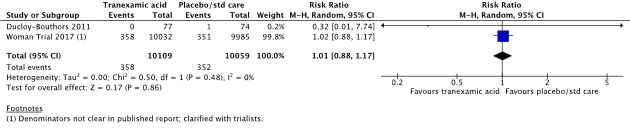
4: Hysterectomy
In the systematic review (Shakur, 2018), two studies were included that reported on the outcome measure hysterectomy (Ducloy-Bouthors et al., 2011; WOMAN trial, 2017).
Hysterectomy was reported in 358/10109 (3.5%) women in the TXA-group and in 352/10059 (3.5%) women in the control group (RR 1.01, 95% CI 0.88 to 1.17) (Figure 2). There was no clinically relevant difference in hysterectomy between the TXA-group and the control group.
Figure 2. The effect of TXA on hysterectomy
Source: Shakur et al. (2018). Z: p-value of pooled effect, df: degrees of freedom, I2: statistical heterogeneity, CI: confidence interval.
5) Organ dysfunction
Defined in the SR as multiple organ failure, renal failure, hepatic failure, cardiac arrest, seizure and respiratory failure.
In the systematic review (Shakur, 2018), two studies were included that reported on the outcome measure organ dysfunction (Ducloy-Bouthors et al., 2011; WOMAN trial, 2017).
Organ dysfunction was reported in 99/10109 (1.0%) women in the TXA-group and in 105/10059 (1.0%) women in the control group (RR 0.94, 95% CI 0.71 to 1.23) (Figure 3). There was no clinically relevant difference in organ dysfunction between the TXA-group and the control group.
Figure 3. The effect of TXA on organ dysfunction
Source: Shakur et al. (2018). Z: p-value of pooled effect, df: degrees of freedom, I2: statistical heterogeneity, CI: confidence interval.
In the systematic review (Shakur, 2018), two studies were included that reported on the outcome measure renal failure (Ducloy-Bouthors et al., 2011; WOMAN trial, 2017). Renal failure was reported in 129/10110 (1.3%) women in the TXA-group and in 118/10059 (1.2%) women in the control group (RR 1.09, 95% CI 0.85 to 1.39) (Figure 4). There was no clinically relevant difference in organ dysfunction between the TXA-group and the control group.
Figure 4. The effect of TXA on renal failure
Source: Shakur et al. (2018). Z: p-value of pooled effect, df: degrees of freedom, I2: statistical heterogeneity, CI: confidence interval.
In the systematic review (Shakur, 2018), one study was included that reported on the outcome measure hepatic failure (WOMAN trial, 2017). Hepatic failure was reported in 29/10033 (0.3%) women in the TXA-group and in 30/9985 (0.3%) women in the control group (RR 0.96, 95% CI 0.58 to 1.60) (Figure 5). There was no clinically relevant difference in organ dysfunction between the TXA-group and the control group.
Figure 5. The effect of TXA on hepatic failure
Source: Shakur et al. (2018). Z: p-value of pooled effect, df: degrees of freedom, I2: statistical heterogeneity, CI: confidence interval.
In the systematic review (Shakur, 2018), one study was included that reported on the outcome measure cardiac arrest (WOMAN trial, 2017). Cardiac arrest was reported in 110/10033 (1.1%) women in the TXA-group and in 115/9985 (1.2%) women in the control group (RR 0.95, 95% CI 0.73 to 1.23) (Figure 6). There was no clinically relevant difference in organ dysfunction between the TXA-group and the control group.
Figure 6. The effect of TXA on cardiac arrest
Source: Shakur et al. (2018). Z: p-value of pooled effect, df: degrees of freedom, I2: statistical heterogeneity, CI: confidence interval.
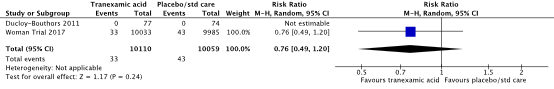
In the systematic review (Shakur, 2018), two studies were included that reported on the outcome measure seizure (Ducloy-Bouthors et al., 2011; WOMAN trial, 2017). Seizure was reported in 33/10110 (0.3%) women in the TXA-group and in 43/10059 (0.4%) women in the control group (RR 0.76, 95% CI 0.49 to 1.20) (Figure 7). The difference seizure between the TXA-group and the control group was clinically important in favour of the TXA group.
Figure 7. The effect of TXA on seizure
Source: Shakur et al. (2018). Z: p-value of pooled effect, df: degrees of freedom, I2: statistical heterogeneity, CI: confidence interval.
In the systematic review (Shakur, 2018), one study was included that reported on the outcome measure maternal respiratory failure (WOMAN trial, 2017). Respiratory failure was reported in 108/10033 (1.1%) women in the TXA-group and in 115/9985 (1.2%) women in the control group (RR 0.87, 95% CI 0.67 to 1.12) (Figure 8). There was clinically relevant difference in maternal respiratory failure between the TXA-group and the control group in favour of TXA.
Figure 8. The effect of TXA on Maternal respiratory failure
Source: Shakur et al. (2018). Z: p-value of pooled effect, df: degrees of freedom, I2: statistical heterogeneity, CI: confidence interval.
6) Maternal death
In the systematic review (Shakur, 2018), two studies were included that reported on the outcome measure maternal death (Ducloy-Bouthors et al., 2011; WOMAN trial, 2017), defined as maternal mortality due to bleeding.
Maternal mortality was reported in 155/10113 (1.5%) women in the TXA-group and in 191/10059 (1.9%) women in the control group (RR 0.81, 95% CI 0.65 to 1.00) (Figure 9). There was a clinically relevant difference in maternal death in favour of the TXA-group.
Figure 9. The effect of TXA on maternal death
Source: Shakur et al. (2018). Z: p-value of pooled effect, df: degrees of freedom, I2: statistical heterogeneity, CI: confidence interval.
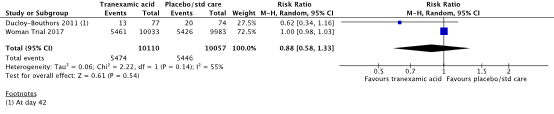
7) blood transfusion
In the systematic review (Shakur, 2018), two studies were included that reported on the outcome measure blood transfusion (Ducloy-Bouthors et al., 2011; WOMAN trial, 2017).
Blood transfusion was required in 5474/10110 (54.1%) women in the TXA-group and in 5446/10057 (54.1%) women in the control group (RR 0.88, 95% CI 0.58 to 1.33) (Figure 10). There was clinically relevant difference in the need for blood transfusion between the TXA-group and the control group, in favour of the TXA-group.
Figure 10. The effect of TXA on the need for blood transfusion
Source: Shakur et al. (2018). Z: p-value of pooled effect, df: degrees of freedom, I2: statistical heterogeneity, CI: confidence interval.
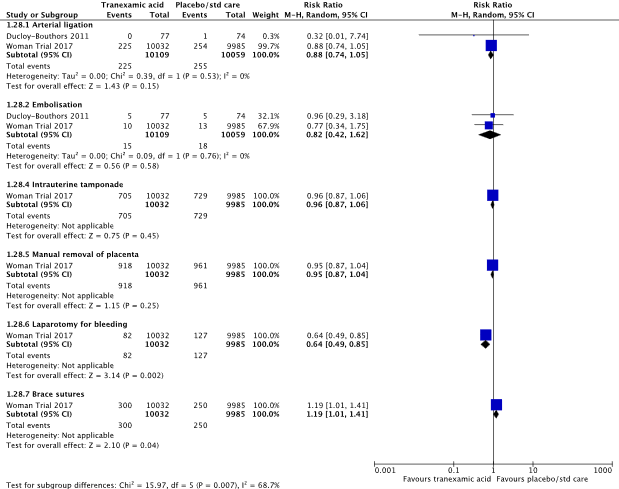
8) use of additional surgical hemostatic intervention
In the systematic review (Shakur, 2018), both included studies (Ducloy-Bouthors et al., 2011; WOMAN trial, 2017) reported on the outcome measure arterial ligation. Arterial ligation was performed in 225/10109 (2.3%) women in the TXA-group and in 255/10059 (2.5%) women in the control group (RR 0.88, 95% CI 0.74 to 1.05) (Figure 11). There was no clinically relevant difference in the need for arterial ligation between the TXA-group and the control group.
In the systematic review (Shakur, 2018), two studies were included that reported on the outcome measure embolization (Ducloy-Bouthors et al., 2011; WOMAN trial, 2017). Embolization was performed in 15/10109 (0.1%) women in the TXA-group and in 18/10059 (0.2%) women in the control group (RR 0.82, 95% CI 0.42 to 1.62) (Figure 11). There was no clinically relevant difference in the need for embolization between the TXA-group and the control group.
In the systematic review (Shakur, 2018), one study was included that reported on the outcome measure intrauterine tamponade (WOMAN trial, 2017). Intrauterine tamponade was performed in 705/10032 (7.0%) women in the TXA-group and in 729/9985 (7.3%) women in the control group (RR 0.96, 95% CI 0.87 to 1.06) (Figure 11). There was no clinically relevant difference in the need for intrauterine tamponade between the TXA-group and the control group.
In the systematic review (Shakur, 2018), one study was included that reported on the outcome measure manual removal of the placenta (WOMAN trial, 2017). Manual removal of the placenta was performed in 918/10032 (9.2%) women in the TXA-group and in 961/9985 (9.6%) women in the control group (RR 0.95, 95% CI 0.87 to 1.04) (Figure 11). There was no clinically relevant difference in the need for manual removal of the placenta between the TXA-group and the control group.
In the systematic review (Shakur, 2018), one study was included that reported on the outcome measure brace sutures (WOMAN trial, 2017). Brace sutures was performed in 300/10032 (3.0%) women in the TXA-group and in 250/9985 (2.5%) women in the control group (RR 1.19, 95% CI 1.01 to 1.41) (Figure 11). There was no clinically relevant difference in the need for brace sutures between the TXA-group and the control group.
Figure 11. The effect of TXA on the use of surgical additional hemostatic interventions
Source: Shakur et al. (2018). Z: p-value of pooled effect, df: degrees of freedom, I2: statistical heterogeneity, CI: confidence interval.
9) transfer for higher level of care
In the systematic review (Shakur, 2018), one study was included that reported on the outcome measure transfer for higher level of care, which was defined in the review as maternal intensive care admission. (Ducloy-Bouthors et al., 2011).
In the TXA-group, 3/77 (3.9%) women were transferred to the intensive care, while this was the case for 5/74 (6.8%) women in the control group (RR 0.58, 95% CI 0.14 to 2.33) (Table 2). There was clinically relevant difference in the need for admission to the intensive care between the TXA-group and the control group in favour of TXA.
Table 2. The effect of TXA on transfer for higher level of care
|
|
TXA-group |
Control group |
RR [95% CI] |
||
|
Events |
Total |
Events |
Total |
||
|
Maternal intensive care admission |
3 |
77 |
5 |
74 |
0.58 [0.14, 2.33] |
Source: Ducloy-Bouthors et al. (2011). RR: Risk Ratio, CI: Confidence Interval
10) women's sense of wellbeing
Not reported in the SR.
11) acceptability and satisfaction with the intervention
Not reported in the SR.
12) breastfeeding
Not reported in the SR.
13) adverse effects
Not reported in the SR.
14) PTSD
Not reported in the SR.
Level of evidence of the literature
The level of evidence regarding the outcome measure blood loss was downgraded by two levels to a low GRADE, because of study limitations (-1; risk of bias due to a lack of blinding and retrospectively registered trial protocol) and confidence intervals that crossed the margin of clinical relevance (-1; imprecision).
The level of evidence regarding the outcome measure hysterectomy was not downgraded and was graded with a high GRADE.
The level of evidence regarding the outcome measure organ dysfunction (multiple organ failure, renal failure, hepatic failure, cardiac arrest, seizure and respiratory failure) was downgraded by 1 level to a moderate GRADE because confidence intervals crossed the margin of clinical relevance (-1; imprecision).
The level of evidence regarding the outcome measure maternal death was downgraded by one level to a moderate GRADE because of confidence intervals that crossed the margin of clinical relevance (-1; imprecision).
The level of evidence regarding the outcome measure blood transfusion was downgraded by two levels to a low GRADE because confidence intervals crossed both margins of clinical relevance (-2; imprecision).
Regarding the outcome measure use of additional hemostatic interventions, the level of evidence for arterial ligation and brace sutures was downgraded by one level to a moderate GRADE because of confidence intervals that crossed the margin of clinical relevance (-1; imprecision), the level of evidence for embolization was downgraded by two levels to a low GRADE because confidence intervals crossed both margins of clinical relevance (-2; imprecision), and the level of evidence for intrauterine tamponade and manual removal of the placenta was not downgraded and evaluated with a high GRADE.
The level of evidence regarding the outcome measure transfer to higher levels of care was downgraded by three levels to a very low GRADE, because of study limitations (-1; risk of bias due to a lack of blinding and retrospectively registered trial protocol) and confidence intervals that crossed both margins of clinical relevance (-2; imprecision).
Zoeken en selecteren
A systematic review of the literature was performed to answer the following question: What is the role of tranexamic acid in the treatment of HPP?
| P: patients | Women postpartum with blood loss > 500 mL |
| I: intervention | Tranexamic acid (TXA) |
| C: control | No TXA or placebo |
| O: outcome measure | Additional blood loss, shock, coagulopathy, hysterectomy, organ dysfunction, maternal death, blood transfusion, use of additional hemostatic intervention, transfer for higher level of care, women's sense of wellbeing, acceptability and satisfaction with the intervention, breastfeeding, and adverse effects, PTSD |
Relevant outcome measures
The guideline development group considered blood loss more than 1000 mL (dichotomous) , hysterectomy, shock, organ dysfunction, transfer for higher level of care, maternal death and blood transfusion as crucial outcome measures for decision making; and blood loss (continuous), coagulopathy, use of additional surgical hemostatic intervention, women's sense of wellbeing, acceptability and satisfaction with the intervention, breastfeeding, adverse effects and PTSD as important outcome measures for decision making.
The working group defined the outcome measures based on the definitions from Meher (2018).
The working group defined a 1% difference for maternal death (RR < 0.99 or > 1.01) and 10% (RR < 0.90 or > 1.10) for other critical outcome measures as a minimal clinically (patient) important difference. For the other outcomes, a 25% difference for dichotomous outcomes (RR < 0.8 or > 1.25) and 0.5 SD for continuous outcomes was taken as minimal clinically (patient) important difference.
Search and select (Methods)
The databases Medline (via OVID) and Embase (via Embase.com) were searched with relevant search terms until August 31th, 2023. The detailed search strategy is depicted under the tab Methods. The systematic literature search resulted in 430 hits. Studies were selected based on the following criteria:
- Randomized controlled trials (RCTs) or systematic reviews in which a minimum of 2 databases were searched and in which a detailed search strategy, in- and exclusion criteria, exclusion table, evidence table and risk of bias assessment are included
- Studying women postpartum with blood loss > 500 mL
- Comparing TXA with standard treatment, no treatment or placebo
- Assessing one or more of the predefined outcomes
Seventeen studies were initially selected based on title and abstract screening. After reading the full text, sixteen studies were excluded (see the table with reasons for exclusion under the tab Methods) and one study was included.
Results
One systematic review (Shakur et al., 2018) was included in the analysis of the literature. Important study characteristics and results are summarized in the evidence tables. The assessment of the risk of bias is summarized in the risk of bias tables.
Referenties
- Shakur H, Beaumont D, Pavord S, Gayet-Ageron A, Ker K, Mousa HA. Antifibrinolytic drugs for treating primary postpartum haemorrhage. Cochrane Database Syst Rev. 2018 Feb 20;2(2):CD012964. doi: 10.1002/14651858.CD012964. PMID: 29462500; PMCID: PMC6491317.
- Meher S, Cuthbert A, Kirkham JJ, Williamson P, Abalos E, Aflaifel N, Bhutta ZA, Bishop A, Blum J, Collins P, Devane D, Ducloy-Bouthors AS, Fawole B, Gülmezoglu AM, Gutteridge K, Gyte G, Homer C, Mallaiah S, Smith JM, Weeks AD, Alfirevic Z. Core outcome sets for prevention and treatment of postpartum haemorrhage: an international Delphi consensus study. BJOG. 2019 Jan;126(1):83-93. doi: 10.1111/1471-0528.15335. Epub 2018 Jul 29. PMID: 29920912.
- Fergusson DA, Hébert PC, Mazer CD, Fremes S, MacAdams c, Murkin JM, Teoh K, Duke PC, Arellano R, Blajchman MA, Bussières JS, Côté D, Karski J, Martineau R, Robblee JA, Rodger M, Wells G, Clinch J, Pretorius R.A Comparison of Aprotinin and Lysine Analogues in High-Risk Cardiac Surgery. NEJM 2008 vol. 358 no. 22
- I Roberts, H Shakur, T Coats, B Hunt, E Balogun, L Barnetson, L Cook, T Kawahara, P Perel, D Prieto-Merino, M Ramos, J Cairns and C Guerriero. The CRASH-2 trial: a randomised controlled trial and economic evaluation of the effects of tranexamic acid on death, vascular occlusive events and transfusion requirement in bleeding trauma patients. Health Technology Assessment VOLUME 17 ISSUE 10 March 2013
Evidence tabellen
P: Vrouwen postpartum vanaf 500 ml bloedverlies
I: Tranexaminezuur
C: Placebo of geen tranexaminezuur
O: 1) blood loss; 2) shock; 3) coagulopathy; 4) hysterectomy; 5) organ dysfunction; 6) maternal death; 7) blood transfusion; 8) use of additional hemostatic intervention; 9) transfer for higher level of care; 10) women's sense of wellbeing; 11) acceptability and satisfaction with the intervention; 12) breastfeeding; 13) adverse effects; 14) PTSD
|
Study reference |
Study characteristics |
Patient characteristics |
Intervention (I): TXA treatment |
Comparison / control (C): no TXA
|
Follow-up |
Outcome measures and effect size |
Comments |
|
Shakur et al., 2018
Study characteristics and results are extracted from the SR |
SR and meta-analysis of RCTs
Literature search up to May 28th, 2017.
A: Woman trial 2017 B: Ducloy-Bouthors 2011 C: Sahaf, 2014
Study design: A: RCT B: RCT C: RCT
Setting and Country: A: women were recruited from hospital settings in high- (UK: 569 women), and low- and middle- income countries (Nigeria: 5711; Pakistan: 5282; Uganda: 2235; Kenya: 1031; Cameroon: 893; Sudan: 860; Tanzania: 538; Nepal: 533; Zambia: 496; Albania: 485; Democratic Republic of Congo: 457; Bangladesh: 325; Ethiopia: 302; Burkina Faso: 142; Jamaica: 73; Ghana: 41; Papua New Guinea: 38; Egypt: 33; Colombia: 8; Cote d’Ivoire: 8). Women were recruited to the WOMAN trial between 2010 and 2016. B: conducted in France in eight centres (five tertiary care centres and three secondary care obstetric centres: 152 women). This study recruited women between 2005 to 2008. C: Iran
Source of funding and conflicts of interest: A: Funded by the London School of Hygiene & Tropical Medicine, Pfizer, UK Department of Health, Wellcome Trust, and Bill & Melinda Gates Foundation B: Funded by the French Ministry of Health
|
Inclusion criteria SR: - Women after birth (vaginal or caesarean) following a pregnancy > 24 wks - Diagnosis of primary PPH. - PPH may be defined in different ways: 1) Women with > 500 ml blood loss 2) Women with primary PPH requiring blood transfusion and/or blood products 3) women with a clinical diagnosis of primary PPH (as defined by trialists).
Exclusion criteria SR: -Women with PPH with gestational age < 24 wks.
3 studies included
Important patient characteristics at baseline:
N, mean age A: 20060 patients, XX yrs B: 152 patients C: ….
Sex: A: % Male B: C: ….
Groups comparable at baseline? A: yes B: C: No
|
A: IV TXA 1 g as an IV bolus over 10 minutes; if after 30 minutes bleeding continued, or had stopped and restarted within 24 hours of the first dose, women were given a second dose (n = 10,051).
B: A loading dose of 4 g TXA mixed with 50 mL saline, administered intravenously (IV) over one hour followed by a maintenance dose of 1 g/hour for six hours (n = 78).
C: 1 g IV TXA repeated after 30 minutes (n=100). Prostatglandin F2ɑ was administered in case of treatment failure.
|
A: placebo (sodium chloride 0.9% contained in ampoules and packaging with identical appearance to active treatment) with the same regimen as that used in the experimental group (n = 10,009).
B: Usual care (no TXA) (n = 74).
C: 1000 micrograms (mcg) rectal misoprostol (five 200 mcg tablets) (n=100). Prostatglandin F2ɑ was administered in case of treatment failure.
|
End-point of follow-up:
A: 42 days postpartum (all outcomes are measured at discharge or on day 42 if still in hospital) B: 6 hours postpartum C: unclear
For how many participants were no complete outcome data available? (intervention/control) A: 4/3 participants withdrew their consent after randomization. 11/21 participants did not have primary outcome data. Reasons for incompleteness were not described in the trial report, but for these small numbers of missing data it is unlikely to impact the results.
B: 1 woman assigned to TXA was later found not to be eligible and was excluded for analysis
C: unclear
|
1) blood loss Defined in the SR as blood loss > 500ml
Effect measure: RR [95% CI]: A: 0.50 [0.27, 0.93] B: NA
Pooled effect (fixed effects model): 0.50 [95% CI 0.27 to 0.93] favoring TXA. Heterogeneity (I2): NA
Defined in the SR as blood loss > 100ml
Effect measure: RR [95% CI]: A: 0.48 [0.15, 1.53] B: NA
Pooled effect (fixed effects model): 0.48 [95% CI 0.15 to 1.53] favoring TXA. Heterogeneity (I2): NA.
Defined in the SR as mean blood loss (in ml)
Effect measure: mean difference [95% CI]: A: -107 [-224.44, 10.44] B: NA
Pooled effect (fixed effects model): -107 [95% CI -224.44 to 10.44] favoring TXA. Heterogeneity (I2): NA.
2) shock Not included in the SR
3) coagulopathy Not included in the SR
4) hysterectomy
Effect measure: RR [95% CI]: A: 1.02 [0.88, 1.17] B: 0.32 [0.01, 7.74]
Pooled effect (fixed effects model): 1.01 [95% CI 0.88 to 1.17] favoring control. Heterogeneity (I2): 0%
5) organ dysfunction Defined in the SR as multiple organ failure.
Effect measure: RR [95% CI]: A: 0.94 [0.71, 1.23] B: Not estimable
Pooled effect (fixed effects model): 0.94 [95% CI 0.71 to 1.23] favoring TXA. Heterogeneity (I2): NA
6) maternal death
Effect measure: RR [95% CI]: A: 0.81[0.65,1] B: Not estimable
Pooled effect (fixed effects model): 0.81 [95% CI 0.65 to 1] favoring TXA. Heterogeneity (I2): NA
7) blood transfusion
Effect measure: RR [95% CI]: A: 1 [0.98, 1.03] B: 0.62 [0.98, 1.16]
Pooled effect (fixed effects model): 1 [95% CI 0.97 to 1.03] Heterogeneity (I2): 54.89%
8) use of additional hemostatic intervention Not included in the SR.
9) transfer for higher level of care Defined in the SR as maternal intensive care admission.
Effect measure: RR, RD, mean difference [95% CI]: A: NA B: 0.58 [0.14, 2.33]
Pooled effect (fixed effects model): 0.58 [95% CI 0.14 to 2.33] favoring TXA. Heterogeneity (I2): NA
10) women's sense of wellbeing Not included in the SR.
11) acceptability and satisfaction with the intervention Not included in the SR.
12) breastfeeding Not included in the SR.
13) adverse effects Not included in the SR.
14) PTSD Not included in the SR.
|
Risk of bias (high, some concerns or low): Tool used by authors: Cochrane tool
A: Low risk of bias B: Some concerns C: High risk of bias
Facultative:
Brief description of author’s conclusion: “The use of the antifibrinolytic drug tranexamic acid (TXA) appears to reduce the risk of death due to bleeding without increasing the risk of vascular occlusive events. There is evidence from this review and from another major trial that the effect on death due to bleeding depends on the time interval between giving birth and the commencement of treatment, and this would suggest that TXA should be given as early as possible and within three hours of giving birth.”
|
Risk of Bias
|
Study reference
(first author, publication year) |
Was the allocation sequence adequately generated?
Definitely yes Probably yes Probably no Definitely no |
Was the allocation adequately concealed?
Definitely yes Probably yes Probably no Definitely no |
Blinding: Was knowledge of the allocated interventions adequately prevented?
Were patients blinded?
Were healthcare providers blinded?
Were data collectors blinded?
Were outcome assessors blinded?
Were data analysts blinded?
Definitely yes Probably yes Probably no Definitely no |
Was loss to follow-up (missing outcome data) infrequent?
Definitely yes Probably yes Probably no Definitely no |
Are reports of the study free of selective outcome reporting?
Definitely yes Probably yes Probably no Definitely no |
Was the study apparently free of other problems that could put it at a risk of bias?
Definitely yes Probably yes Probably no Definitely no |
Overall risk of bias If applicable/necessary, per outcome measure
LOW Some concerns HIGH
|
||||||||
|
Woman Trial 2017
Information based on Cochrane Review (Shakur et al., 2018) |
Definitely yes; Reason: Randomisation was generated and secured by an independent statistical consultant from Sealed Envelope Ltd (UK). The codes were then made available to Brecon Pharmaceuticals Limited (UK) explicitly for the treatment packs to be created in accordance with the randomisation list.
|
Definitely yes; Reason: After eligibility was confirmed and consent procedures completed, baseline information was collected on the entry form. Women were then randomly al- located to TXA or placebo group by selection of the lowest numbered treatment pack form from a box containing eight numbered packs that were identical apart from the pack number. (An emergency un-blinding service was avail- able from the pharmaceutical company.) |
Definitely yes; Reason: Participants, caregivers and study staff (site investigators and trial coordinating centre staff) were blinded to treatment allocation.
|
Definitely yes; Reason: little missing data. Reasons for incompleteness were not described in the trial report, but there were only small numbers missing so unlikely to impact on results.
|
Definitely yes; Reason: Most outcomes pre-specified in the protocol were reported in final publication except length of hospital stay and receipt of mechanical ventilation. |
Definitely yes; Reason: No other risk of bias identified.
|
LOW risk
|
|
|||||||
|
Ducloy-Bouthors et al., 2011
Information based on Cochrane Review (Shakur et al., 2018) |
Definitely yes; Reason: “The randomisation sequence was generated by a centralized computer, and randomisation was balanced by centre.” |
Probably no;
Reason: not described. |
Definitely no;
Reason: open-label trial. |
Definitely yes; Reason: 1 woman assigned to TXA was later found not to be eligible and was excluded from ITT analysis.
|
Probably no; Reason: Retrospectively registered. Unable to compare reported results to pre-speci- fied outcomes.
|
Definitely yes;
Reason: other bias not apparent. |
Some concerns
Due to a lack of blinding and retrospectively registered trial protocol. |
|
|||||||
Table of excluded studies
|
Ageron FX, Gayet-Ageron A, Ker K, Coats TJ, Shakur-Still H, Roberts I; Antifibrinolytics Trials Collaboration. Effect of tranexamic acid by baseline risk of death in acute bleeding patients: a meta-analysis of individual patient-level data from 28 333 patients. Br J Anaesth. 2020 Jun;124(6):676-683. |
Wrong P -> also studies included that studied trauma patients |
|
Bouet PE, Ruiz V, Legendre G, Gillard P, Descamps P, Sentilhes L. Policy of high-dose tranexamic acid for treating postpartum hemorrhage after vaginal delivery. J Matern Fetal Neonatal Med. 2016;29(10):1617-22. |
Wrong design: before-and-after study |
|
Brenner A, Shakur-Still H, Chaudhri R, Fawole B, Arulkumaran S, Roberts I; WOMAN Trial Collaborators. The impact of early outcome events on the effect of tranexamic acid in post-partum haemorrhage: an exploratory subgroup analysis of the WOMAN trial. BMC Pregnancy Childbirth. 2018 Jun 7;18(1):215. |
Exploratory subgroup analyse of the WOMAN trial with the same outcome measures reported as in the complete trial. |
|
Della Corte L, Saccone G, Locci M, Carbone L, Raffone A, Giampaolino P, Ciardulli A, Berghella V, Zullo F. Tranexamic acid for treatment of primary postpartum hemorrhage after vaginal delivery: a systematic review and meta-analysis of randomized controlled trials. J Matern Fetal Neonatal Med. 2020 Mar;33(5):869-874 |
Same studies included as in the Cochrane review of 2020 |
|
Ducloy-Bouthors AS, Jude B, Duhamel A, Broisin F, Huissoud C, Keita-Meyer H, Mandelbrot L, Tillouche N, Fontaine S, Le Goueff F, Depret-Mosser S, Vallet B; EXADELI Study Group; Susen S. High-dose tranexamic acid reduces blood loss in postpartum haemorrhage. Crit Care. 2011;15(2):R117. |
Included in Cochrane review |
|
Fahrenholtz CG, Bonanno LS, Martin JB. Tranexamic acid as adjuvant treatment for postpartum hemorrhage: a systematic review protocol. JBI Database System Rev Implement Rep. 2019 Aug;17(8):1565-1572. |
Wrong design: protocol artiekel |
|
Faraoni D, Carlier C, Samama CM, Levy JH, Ducloy-Bouthors AS. Efficacité et sécurité de l'acide tranexamique en prévention et/ou en traitement de l'hémorragie du post-partum : une revue systématique de la littérature avec méta-analyse [Efficacy and safety of tranexamic acid administration for the prevention and/or the treatment of post-partum haemorrhage: a systematic review with meta-analysis]. Ann Fr Anesth Reanim. 2014 Nov;33(11):563-71. French. |
French language |
|
Ferrari FA, Garzon S, Raffaelli R, Cromi A, Casarin J, Ghezzi F, Uccella S, Franchi M. Tranexamic acid for the prevention and the treatment of primary postpartum haemorrhage: a systematic review. J Obstet Gynaecol. 2022 Jul;42(5):734-746. |
Only two studies about the treatment of PPH. These two studies are also included in the Cochrane review of 2020 |
|
Ferrer P, Roberts I, Sydenham E, Blackhall K, Shakur H. Anti-fibrinolytic agents in post partum haemorrhage: a systematic review. BMC Pregnancy Childbirth. 2009 Jul 15;9:29. |
Included studies do not match our PICO |
|
Heesen M, Böhmer J, Klöhr S, Rossaint R, van de Velde M, Dudenhausen JW, Straube S. Prophylactic tranexamic acid in parturients at low risk for post-partum haemorrhage: systematic review and meta-analysis. Acta Anaesthesiol Scand. 2014 Oct;58(9):1075-85. |
Wrong P -> prevention of PPH |
|
Kumari S, Kumari P. Assessment of Uterotonics and Tranexamic Acid for the Treatment of Postpartum Hemorrhage: A Comparative Study. International Journal of Pharmaceutical and Clinical Research. 2022; 14(5):609-614. |
Wrong design -> observational study |
|
Ogunkua OT, Duryea EL, Nelson DB, Eddins MM, Klucsarits SE, McIntire DD, Leveno KJ. Tranexamic acid for prevention of hemorrhage in elective repeat cesarean delivery-a randomized study. Am J Obstet Gynecol MFM. 2022 Mar;4(2):100573. |
Wrong P |
|
Sentilhes L, Lasocki S, Ducloy-Bouthors AS, Deruelle P, Dreyfus M, Perrotin F, Goffinet F, Deneux-Tharaux C. Tranexamic acid for the prevention and treatment of postpartum haemorrhage. Br J Anaesth. 2015 Apr;114(4):576-87. |
Wrong design: narrative review |
|
Usharani N. Manjula D, Purushottam K. Comparative evaluation of anti-hemorrhagic effect of uterotonics and Tranexamic acid (TXA) for postpartum hemorrhage: An experimental study. Journal of Cardiovascular Disease Research. 2023;14(3):755-758. |
Wrong design -> observational study |
|
WOMAN Trial Collaborators. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. Lancet. 2017 May 27;389(10084):2105-2116. |
Included in Cochrane review |
|
Zeng X, Huang D, Luo X, Gong H, Wang X. Comparison of Clinical Effects of Intravenous Tranexamic Acid and Carbetocin in the Treatment of Postpartum Hemorrhage. Indian Journal of Pharmaceutical Sciences. 2022; 84( 0): 158-162. |
Wrong outcome: blood loss in ml |
Verantwoording
Beoordelingsdatum en geldigheid
Publicatiedatum : 12-12-2025
Beoordeeld op geldigheid : 12-12-2025
Algemene gegevens
De ontwikkeling/herziening van deze richtlijnmodule werd ondersteund door het Kennisinstituut van de Federatie Medisch Specialisten (www.demedischspecialist.nl/kennisinstituut) en werd gefinancierd door de Stichting Kwaliteitsgelden Medisch Specialisten (SKMS). De financier heeft geen enkele invloed gehad op de inhoud van de richtlijnmodule.
Samenstelling werkgroep
Voor het ontwikkelen van de richtlijnmodule is in 2022 een multidisciplinaire werkgroep ingesteld, bestaande uit vertegenwoordigers van alle relevante specialismen (zie hiervoor de Samenstelling van de werkgroep) die betrokken zijn bij de zorg voor patiënten met haemorrhagia postpartum.
Werkgroep
- Dr. M.(Martina) Porath (voorzitter) (NVOG)
- Dr. H.C.J. (Liesbeth) Scheepers (NVOG)
- Dr. D.D.C.A. (Dacia) Henriquez (NVOG)
- Dr. J.M. (Annemieke) Middeldorp(NVOG)
- Prof. dr. T.H. (Thomas) van den Akker (NVOG)
- Dr. P. (Paul) Ramler (NVOG) (tot September 2023)
- Dr. K.P.M. (Karin) van Galen(NIV)
- Drs. H.W. (Hannah) de Klerk (KNOV)
- Drs. L. (Lianne) Zondag (KNOV)
- Prof. dr. ir. Y.M.C. (Yvonne) Henskens (NVKC)
- Dr. I.C.M. (Ingrid) Beenakkers (NVA)
- Dr. S. (Simone) Willems (NVA)
- Mw. I. (Ilse) van Ee (PFN)
Klankboardgroep
- Drs. K. (Klaartje) Caminada (AZN)
- Mw. B. (Britt) Ketelaars (PFN)
Met ondersteuning van
- Dr. M. (Mohammadreza) Abdollahi, adviseur, Kennisinstituut van de Federatie van Medisch Specialisten
- Dr. I.M. (Irina) Mostovaya, senior adviseur, Kennisinstituut van de Federatie van Medisch Specialisten
- Dr. J. (Jana) Tuijtelaars, adviseur, Kennisinstituut van de Federatie van Medisch Specialisten
- Drs. D.A.M. (Danique) Middelhuis, adviseur, Kennisinstituut van de Federatie van Medisch Specialisten
- Dr. M. (Majke) van Bommel, adviseur, Kennisinstituut van de Federatie van Medisch Specialisten
- Dr. L. (Leanne) Küpers, adviseur, Kennisinstituut van de Federatie van Medisch Specialisten
- Drs. D.A.M. (Fieke) Pepping, junior adviseur, Kennisinstituut van de Federatie van Medisch Specialisten
- Linda Niesink, medisch informatie specialist, Kennisinstituut van de Federatie Medisch Specialisten
- Laura Boerboom, medisch informatie specialist, Kennisinstituut van de Federatie Medisch Specialisten
- Alies Oost, medisch informatie specialist, Kennisinstituut van de Federatie Medisch Specialisten
Belangenverklaringen
Een overzicht van de belangen van werkgroepleden en het oordeel over het omgaan met eventuele belangen vindt u in onderstaande tabel. De ondertekende belangenverklaringen zijn op te vragen bij het secretariaat van het Kennisinstituut van de Federatie Medisch Specialisten via secretariaat@kennisinstituut.nl.
|
Naam |
Hoofdfunctie |
Nevenwerkzaamheden |
Persoonlijke financiële belangen |
Persoonlijke relaties |
Extern gefinancierd onderzoek |
Intellectuele belangen en reputatie |
Overige belangen |
Datum |
Restrictie |
|
Martina Porath |
gynaecoloog-perinatoloog Maxima Medisch Centrum |
Voorzitter focusgroep Acute Verloskunde Noord-Brabant Lid NVOG werkgroep Otterlo |
geen |
geen |
geen |
geen |
geen |
04/28/2025 |
|
|
Liesbeth Scheepers |
perinatoloog MUMC |
Richtlijnen commissie Otterloo groep Voorzitter Regioconsortium Geboortezorg Limburg Vice voorzitter Perinatale audit Limburg |
Geen |
Nee |
ZONMW onderzoek, niet op dit onderwerp |
Nee |
Nee |
04/25/2025 |
|
|
Annemieke Middeldorp |
Gynaecoloog Perinatoloog Leids Universitair Medisch Centrum, gestopt met werken vanaf 2024 |
geen |
geen |
geen |
geen |
geen |
geen |
04/25/2025 |
|
|
Dacia Henriquez |
Gynaecoloog, Perinatoloog, Amphia Ziekenhuis |
Geen |
Geen |
Geen |
Geen |
Geen |
Geen |
04/28/2025 |
|
|
Thomas van den Akker |
Gynaecoloog perinatoloog, LUMC, hoogleraar Verloskunde |
Hoogleraar VU *Global Maternal Health, Athena Instituut. *Perined bestuurslid *RvT Wemos. *FMG Pijlervoorzitter NVOG *Vz working party for international safe motherhood and reproductive health. *Perinatale audit regiovoorzitter. Alles onbetaald. |
Geen |
Geen |
Geen |
Door werk van de commissie komt het werk van mijn voormalig promovendi Ada Gillissen en Paul Ramler meer in de aandacht staan, omdat sommige aanbevelingen hierop gebaseerd zijn (met name van laatstgenoemde ten aanzien van ballon versus embolisatie).
Het feit dat ik als bijdrager op de richtlijn sta kan gezien worden als ten goede komend aan de boegbeeldfunctie die ik bij de NVOG heb, maar het effect hiervan lijkt beperkt in het licht van mijn andere activiteiten. |
Geen |
04/25/2025 |
|
|
Paul Ramler (tot September 2023) |
AIOS Gynaecologie, cluster Leiden |
Geen |
Geen |
Geen |
Geen |
Geen |
Geen |
29/01/2023 |
|
|
Ingrid Beenakkers |
Anesthesioloog, UMCU/WKZ |
geen |
geen |
geen |
geen |
geen |
geen |
04/28/2025 |
|
|
Simone Anna Alexandra Sijm-Willems |
Anesthesioloog Radboudumc |
- |
-zs |
- |
- |
- |
- |
04/25/2025 |
|
|
Karin van Galen |
internist-hematoloog UMC Utrecht |
geen |
geen |
n.v.t. |
Octapharma: Pregnancy and inherited bleeding disorders - unresticted research grant, projectleider. |
Voorzitter Nederlands Zorgnetwerk Vrouwen met een stollingsstoornis. Tot Febr 2025 voorzitter Women and Girls with beleeding Disorders Group European Assosiation for Heamophilia and Alied Disorders - momenteel nog actief lid van deze werkgroep. |
n.v.t. |
04/25/2025 |
|
|
Yvonne Henskens |
Klinisch chemicus, waarnemend hoofd Centraal Diagnostisch Laboratorium Hoogleraar Klinische Chemie ihb Hemostase |
Voorzitter Vereniging Hematologisch Laboratoria 2020-heden Lid Vici beoordelingscie 2022 Lid raad van Advies NVKC 2023-heden Adviseur Promicol 2020-heden |
geen |
geen |
Voor alle studies in het kader van mijn leerstoel worden reagentia of apparatuur gratis of met korting verkregen, hierbij wordt geen enkele IVD methode of bedrijf uitgesloten mits het past in het doel van mijn leerstoel. Noyons stipendium NVKC (prijs) Stago / Validatie van apparatuur en/of reagentia / Ja Siemens / Validatie van apparatuur en/of reagentia / Ja Werfen / Validatie van apparatuur en/of reagentia / N Nodia / Validatie van apparatuur en/of reagentia / Nee Promicol / Validatie van apparatuur en/of reagentia / Nee |
nee |
nee |
04/25/2025 |
|
|
Hannah de Klerk |
Zelfstandig eerstelijns verloskundige (ZZP), betaald PhD aanstelling Amsterdam UMC, locatie VUMc, onbetaald |
Gastdocent HU, betaald Projectleider KNOV, betaald |
geen |
nee |
nee |
nee |
nee |
7/10/2022 |
|
|
Lianne Zondag |
Eerstelijns verloskundige - verloskundige praktijk de Toekomst - Geldermalsen KNOV - senior richtlijnonwikkelaar |
Bestuurslid Netwerk Geboortezorg Rivierenland PhD candidate Maastricht University |
Geen |
Geen |
Geen |
Geen |
Geen |
2/6/2023 |
|
|
Ilse van Ee |
Adviseur Patientenbelang - full time inbreng patientenperspectief |
Ervaringsdeskundige patientvertegenwoordiger - Eupati - fellow Psoriasispatienten Nederland - onbetaald Coordinator Patientenpraticipatie, lid centrale redactie Psoriasispatienten Nederland - onbetaald Eupati mentor - Eupati Nl - onbetaald |
nee |
nvt |
Janssen / Freedom of disease Psoriasis / ja |
nee |
nvt |
04/25/2025 |
|
|
Klaartje Caminada |
Medisch Manager Ambulancezorg, lid protocollencommissie Ambulancezorg Nederland |
geen |
geen |
geen |
geen |
geen |
geen |
04/29/2025 |
|
|
Brit Ketelaars |
Adviseur patiëntbelang bij Patiëntenfederatie Nederland (full time, betaald) |
geen |
geen |
geen |
geen |
geen |
geen |
04/25/2025 |
|
Inbreng patiëntenperspectief
De werkgroep besteedde aandacht aan het patiëntenperspectief door uitnodigen van Patiëntenfederatie Nederland, Geboortebeweging en Het Buikencollectiefvoor de invitational conference. Het verslag hiervan is besproken in de werkgroep. De verkregen input is meegenomen bij het opstellen van de uitgangsvragen, de keuze voor de uitkomstmaten en bij het opstellen van de overwegingen. De conceptrichtlijn is tevens voor commentaar voorgelegd aan Patiëntenfederatie Nederland en de eventueel aangeleverde commentaren zijn bekeken en verwerkt.
Kwalitatieve raming van mogelijke financiële gevolgen in het kader van de Wkkgz
Bij de richtlijnmodule voerde de werkgroep conform de Wet kwaliteit, klachten en geschillen zorg (Wkkgz) een kwalitatieve raming uit om te beoordelen of de aanbevelingen mogelijk leiden tot substantiële financiële gevolgen. Bij het uitvoeren van deze beoordeling is de richtlijnmodule op verschillende domeinen getoetst (zie het stroomschema bij Werkwijze).
Uit de kwalitatieve raming blijkt dat er waarschijnlijk mogelijk substantiële financiële gevolgen zijn, zie onderstaande tabel.
In Nederland bevallen per jaar ca. 160.000 vrouwen. De incidentie van haemorrhagia postpartum is 7 tot 10%. Dit zijn 11.200 tot 16.000 vrouwen per jaar. Het aantal vrouwen met risicofactoren voor HPP is niet bekend.
| Module |
Uitkomst raming |
Toelichting |
|
Tranexaminezuur in de behandeling van HPP |
geen financiële gevolgen |
De aanbeveling is ongewijzigd |
Werkwijze
Voor meer details over de gebruikte richtlijnmethodologie verwijzen wij u naar de Werkwijze. Relevante informatie voor de ontwikkeling/herziening van deze richtlijnmodule is hieronder weergegeven.
Zoekverantwoording
Literature search strategy
Algemene informatie
|
Richtlijn: Richtlijn Modulaire actualisatie richtlijn Hemorrhagia Postpartum (HPP) |
|
|
Uitgangsvraag: Wat is de rol van tranexaminezuur in de preventie van HPP? |
|
|
Database(s): Medline (OVID), Embase |
Datum: 31-08-2023 |
|
Periode: > 2000 |
Talen: geen beperking |
|
Literatuurspecialist: Laura Boerboom |
|
|
BMI zoekblokken: voor verschillende opdrachten wordt (deels) gebruik gemaakt van de zoekblokken van BMI-Online https://blocks.bmi-online.nl/ Bij gebruikmaking van een volledig zoekblok zal naar de betreffende link op de website worden verwezen. |
|
|
Toelichting en opmerkingen:
→ Voor deze vraag is gezocht op de elementen HPP (in het blauw) en tranexaminezuur (in het groen).
→ Alle 7 sleutelpublicaties zitten in de resultaten.
→ Resultaten staan in Rayyan.
|
|
|
Te gebruiken voor richtlijnen tekst: In de databases Embase (via embase.com) en Medline (via OVID) is op 31-08-2023 met relevante zoektermen gezocht naar systematic reviews, RCT’s en observationele studies over wat de rol is van tranexaminezuur in de preventie van HPP. De literatuurzoekactie leverde 430 unieke treffers op. |
|
Zoekopbrengst
|
|
EMBASE |
OVID/MEDLINE |
Ontdubbeld |
|
SR’s |
110 |
44 |
109 |
|
RCT’s |
190 |
81 |
202 |
|
Observationele studies |
117 |
18 |
119 |
Zoekstrategie
|
Database |
Zoektermen |
||||||||||||||||||||||||||||||||||||
|
Embase
|
|
||||||||||||||||||||||||||||||||||||
|
Medline (OVID)
|
1 exp Postpartum Hemorrhage/ or 'fluxus postpartum'.ti,ab,kf. or 'postpartum hemorrhage'.ti,ab,kf. or 'post partum haemorrhage'.ti,ab,kf. or 'post partum hemorrhage'.ti,ab,kf. or 'postpartal haemorrhage'.ti,ab,kf. or 'postpartal hemorrhage'.ti,ab,kf. or 'postpartum bleeding'.ti,ab,kf. or 'postpartum haemorrhage'.ti,ab,kf. or 'puerperal haemorrhage'.ti,ab,kf. or 'puerperal hemorrhage'.ti,ab,kf. or ('blood loss' adj6 (postpartum or 'post partum' or delivery or cesarean)).ti,ab,kf. or 'obstetric* bleeding'.ti,ab,kf. or 'obstetric* hemorrhage'.ti,ab,kf. or 'obstetric* haemorrhage'.ti,ab,kf. (14067) |