Andere uterotonica dan oxytocine ter preventie van HPP
Uitgangsvraag
Wat is de rol van andere uterotonica dan oxytocine ter preventie van haemorrhagia postpartum (HPP)?
Aanbeveling
Aanbeveling-1
Geef aan alle vrouwen zonder risicofactoren voor HPP die klinisch bevallen, indien gekozen wordt voor profylaxe, oxytocine ter preventie van haemorrhagia postpartum.
Voor de identificatie van risicofactoren voor HPP en de behandeling van HPP wordt verwezen naar de Fluxus Implementatie Strategie en naar het modelprotocol behandeling van HPP.
Aanbeveling-2
Geef aan vrouwen met een verhoogd risico op haemorrhagia postpartum een oxytocine bolus gevolgd door een onderhoudsdosering, of carbetocine in plaats van oxytocine.
In geselecteerde gevallen kunnen combinaties van oxytocine met methylergometrine of misoprostol worden overwogen, met inachtneming van de contra-indicaties en het bijwerkingenprofiel.
Voor de identificatie van risicofactoren voor HPP en de behandeling van HPP wordt verwezen naar de Fluxus Implementatie Strategie en naar het modelprotocol behandeling van HPP.
Overwegingen
Voor- en nadelen van de interventie en de kwaliteit van het bewijs
De werkgroep heeft een literatuurstudie verricht naar de effectiviteit van verschillende soorten uterotonica voor de preventie van bloedverlies postpartum ≥1L, het optreden van haemodynamische shock en maternale sterfte. Daarnaast is gekeken naar het optreden van verschillende belangrijke uitkomstmaten waaronder totale hoeveelheid bloedverlies, additionele interventies, bloedtransfusie en bijwerkingen van de interventies. Hierbij werden verschillende uterotonica vergeleken met onze standaardbehandeling oxytocine: 1. methylergometrine versus oxytocine, 2. misoprostol versus oxytocine, 3. carbetocine versus oxytocine, 4. methylergometrine+oxytocine versus oxytocine en 5. misoprostol+oxytocine versus oxytocine. De cruciale uitkomstmaten bloedverlies postpartum ≥1L en maternale sterfte werden voor alle vergelijkingen gerapporteerd, echter maternale shock voor geen enkele vergelijking. Maternale sterfte was voor alle vergelijkingen een zeldzame gebeurtenis waardoor hierover geen uitspraken kan worden gedaan.
De bevindingen vanuit de verschillende vergelijkingen kunnen als volgt worden samengevat:
- Methylergometrine versus oxytocine: methylergometrine leidde, vergeleken met oxytocine, niet tot een lager risico op bloedverlies postpartum ≥1L (RR 1.30 (95%CI 0.52 tot 3.27), very low grade). Voor de uitkomstmaten hoeveelheid bloedverlies, additionele interventies en bloedtransfusie werden ook geen verschillen gevonden (low to very low grade), terwijl het risico op bijwerkingen bij behandeling met methylergometrine iets verhoogd leekvergeleken met oxytocine (low grade).
- Misoprostol versus oxytocine: bij gebruik van misoprostol was het risico op bloedverlies ≥1L verhoogd vergeleken met oxytocine, met RR 1.26 ((95%CI 1.11 tot 1.43), moderate grade). Voor de uitkomstmaten totale hoeveelheid bloedverlies, additionele interventies en bloedtransfusie werden geen verschillen gevonden (moderate tot low grade), met een vergelijkbaar risico op bijwerkingen (low grade).
- Carbetocine versus oxytocine: carbetocine leidde , vergeleken met oxytocine, niet of nauwelijks tot een lager risico op bloedverlies postpartum ≥1L (RR 0.92 (95%CI 0.79 tot 1.06), low grade). Voor andere uitkomstmaten werd wel een verbetering gezien bij gebruik van carbetocine vergeleken met oxytocine: hoeveelheid bloedverlies -79 ml (95%CI -125 tot -33; low grade), additionele interventies RR 0.57 (95%CI 0.47 tot 0.69; low grade) en bloedtransfusie RR 0.62 (95%CI 0.43-0.90; moderate grade). In het optreden van bijwerkingen werden geen verschillen gezien (low grade).
- Metylergometrine+oxytocine versus oxytocine: het combinatiepreparaat syntometrine leidde, vergeleken met alleen oxytocine tot een lager risico op bloedverlies ≥1L (RR 0.74 (95%CI 0.57 tot 0.96), moderate grade). Voor de uitkomstmaten hoeveelheid bloedverlies en bloedtransfusie werden geen verschillen gevonden (low tot very low grade), en het risico op additionele interventies leek lager bij het combinatiepreparaat (RR 0.75 (95%CI 0.60-0.94; very low grade). Het combinatiepreparaat gaf een iets hoger risico op misselijkheid en braken vergeleken met alleen oxytocine (low grade).
- Misoprostol+oxytocine versus oxytocine: het combineren van misoprostol met oxytocine, vergeleken met alleen oxytocine, leidde tot een lager risico op bloedverlies ≥1L (RR 0.88 (95%CI 0.70 tot 1.10; moderate grade). Voor de andere uitkomstmaten werd een verbetering gezien in het voordeel van de combinatie misoprostol+oxytocine: -75 ml bloedverlies (95%CI -134 tot -15; moderate grade), additionele interventies RR 0.57 (0.47 tot 0.69; high grade) en bloedtransfusie RR 0.51 (0.39 tot 0.68; low grade). Bij de combinatie van misoprostol met oxytocine leken er meer bijwerkingen op te treden vergeleken met alleen oxytocine (low grade).
Uit deze bevindingen kunnen we concluderen dat oxytocine een effectief middel is ter preventie van HPP ≥1L, met een gunstig bijwerkingenprofiel, vergeleken met de overige beschikbare uterotonica. Het combineren van oxytocine met methylergometrine of met misoprostol is mogelijk nog effectiever dan alleen oxytocine toedienen, maar leidt potentieel tot meer bijwerkingen, met name misselijkheid en braken en bij misoprostol ook rillingen. Het meewegen van het risico op bijwerkingen door deze combinaties van uterotonica wordt echter bemoeilijkt door de lage bewijskracht voor deze uitkomstparameter, door risico op bias en inconsistentie. Daarnaast moet er rekening worden gehouden met verschillende contra-indicaties bij methylergometrine, zoals hypertensie/prëeclampsie, uterus myomatosus, oblitererende hartziekten en glaucoom.
Hoewel carbetocine niet of nauwelijks leidt tot een lager risico op postpartum bloedverlies ≥1L vergeleken met ‘standaard’ oxytocine, lijkt er wel een verbetering te zijn in de belangrijke uitkomstmaten hoeveelheid bloedverlies, additionele interventies en bloedtransfusie. De reductie in de hoeveelheid bloedverlies bij carbetocine was klein, (79 ml), maar de risicoreductie op de additionele interventies en bloedtransfusies ten opzichte van oxytocine lijkt wel groot. Hierbij dient opgemerkt te worden dat het in klinisch opzicht niet goed te verklaren is via welk mechanisme carbetocine tot een lager risico op additionele interventies en bloedtransfusies bij vrouwen na de bevalling zou leiden, gezien geen/klein verschil in het optreden van bloedverlies ≥1L. Daarnaast zijn zowel studies geïncludeerd waarin carbetocine vergeleken is met alleen een bolus oxytocine als studies met een bolus gevolgd door een intraveneuze onderhoudsdosering. Het kan zijn dat in studies met alleen een bolus oxytocine een groter effect van carbetocine wordt gezien dan in studies met aansluitend ook een oxytocine onderhoudsdosering gedurende de eerste uren postpartum .
Alles afwegende is de werkgroep van mening dat oxytocine routinematig aangeboden dient te worden aan postpartum vrouwen postpartum ter preventie van een HPP. Bij hoog-risico vrouwen kan gekozen worden voor carbetocine in plaats van oxytocine, of een oxytocinepomp aansluitend aan de bolus oxytocine. In geselecteerde gevallen kunnen combinaties van oxytocine met methylergometrine of misoprostol worden overwogen, met inachtneming van de contra-indicaties en het bijwerkingenprofiel. Zie voor doseringsadviezen UV2 en de flowchart.
Waarden en voorkeuren van patiënten (en evt. hun verzorgers)
Als vrouwen kiezen voor profylaxe, willen zij graag het meest effectieve middel met de minste bijwerkingen.
De vooraf gedefinieerde relevante patiëntgerelateerde uitkomsten (zoals women's sense of wellbeing, acceptability and satisfaction with the intervention, breastfeeding, PTSD) zijn in de geïncludeerde studies niet gemeten of gerapporteerd.
Het is belangrijk om het gesprek over het toedienen van profylaxe ter preventie van HPP in de antenatale periode te voeren, zodat informed consent kan worden opgenomen in het bevalplan. Dit informed consent kan in de postpartum periode kort en bondig worden herbevestigd.
Tijdens dit gesprek is het essentieel om naast het risico op HPP en de bijwerkingen van het preventieve middel ook de voor de patiënt belangrijke uitkomsten te bespreken.
Kosten (middelenbeslag)
Het gebruiken van een combinatie van uterotonica of carbetocine zal, vergeleken met alleen oxytocine, leiden tot hogere kosten van deze preventieve interventie. Echter, de kostprijzen van zowel oxytocine, methylergometrine als misoprostol zijn in de Nederlandse setting laag. Carbetocine is wel duurder dan ‘standaard’ oxytocine; in 2024 €26,70 per flacon carbetocine van 100 mcg en €1,89 per ampul oxytocine van 5 EH. De gemiddelde prijs per ampul methyergometrine (Methergin) 1 ml injectievloeistof 0.2mg/ml) is €0,55.
Indien de combinaties van uterotonica of het gebruik van carbetocine leiden tot een reductie in het optreden van HPP ≥1L of minder interventies vanwege het bloedverlies postpartum, zouden deze uterotonica juist tot lagere kosten kunnen leiden.
Aanvaardbaarheid, haalbaarheid en implementatie
Preventie van HPP door het toedienen van oxytocine na geboorte kind is reeds standaardbeleid in Nederland. Het toevoegen van methylergometrine of misoprostol hieraan zal voor de dagelijkse praktijk betekenen dat een extra handeling verricht zal moeten worden wanneer het kind geboren is, maar het betreffen middelen waar al grote bekendheid mee is, zowel op de verloskamers als op de operatiekamers. Het is te overwegen in het lokale fluxusprotocol ook de contra-indicaties voor methylergometrine te vermelden, als ook de mogelijkheid voor het eventueel toedienen van een anti-emeticum gezien de bijwerkingen van methylergometrine en misoprostol.
Carbetocine is sinds langere tijd beschikbaar in Nederland, echter de meeste klinieken hebben op dit moment nog geen ervaring met dit middel.
Rationale van aanbeveling-1: weging van argumenten voor en tegen de interventies
Oxytocine is een effectief middel voor de preventie van HPP, met weinig bijwerkingen vergeleken met andere uterotonica.
Rationale van aanbeveling-2: weging van argumenten voor en tegen de interventie
Bij vrouwen met verhoogd risico op HPP kan gekozen worden voor een oxytocine bolus gevolgd door een onderhoudsdosering, of carbetocine in plaats van oxytocine. In geselecteerde gevallen kunnen combinaties van oxytocine met methylergometrine of misoprostol worden overwogen, met inachtneming van de contra-indicaties en het bijwerkingenprofiel.
Onderbouwing
Achtergrond
The administration of oxytocin in the third stage of labour to prevent postpartum haemorrhage is routinely offered in Dutch obstetric practice, as recommended by international guidelines (NICE, WHO). Despite this preventive measure, postpartum haemorrhage is still one of the most common obstetric complications, occurring in 6-7% of women after giving birth (Perined, van Stralen 2016). Thus, evaluation of other uterotonic agents that may further reduce the risk of postpartum haemorrhage in women after giving birth remains relevant. It is uncertain whether or not other uterotonic agents (methylergometrine, misoprostol and carbetocin) should also be included in our management of third stage of labour to prevent postpartum haemorrhage and its sequelae in these women.
Conclusies / Summary of Findings
1. Methylergometrine versus oxytocin
|
Very low GRADE |
The evidence is very uncertain about the effect of methylergometrine on the risk of blood loss ≥1000mL when compared with oxytocin in postpartum women.
Source: Jago 2007, Modi 2014, Moir 1979, Orji 2008, Penaranda 2002, Zachariah 2006. |
|
No GRADE |
No evidence was found regarding the effect of methylergometrine on maternal shock when compared with oxytocin in postpartum women.
Source: None. |
|
Very low GRADE |
The evidence is very uncertain about the effect of methylergometrine on maternal death when compared with oxytocin in postpartum women.
Source: Zachariah 2006. |
|
Low GRADE |
Methylergometrine may result in little to no difference on total blood loss when compared with oxytocin in postpartum women.
Source: Dhananjaya 2014, Ezeama 2014, Jago 2007, Modi 2014, Moir 1979, Moodie 1976, Orji 2008, Penaranda 2002, Singh 2009, Zachariah 2006. |
|
Very low GRADE |
The evidence is very uncertain about the effect of methylergometrine on the use of additional uterotonics when compared with oxytocin in postpartum women.
Source: Dhananjaya 2014, Ezeama 2014, Modi 2014, Orji 2008, Singh 2009, Zachariah 2006. |
|
Very low GRADE |
The evidence is very uncertain about the effect of methylergometrine on the use of blood transfusion when compared with oxytocin in postpartum women.
Source: Dhananjaya 2014, Ezeama 2014, Modi 2014, Singh 2009, Zachariah 2006. |
|
No GRADE |
No evidence was found regarding the effect of methylergometrine on transfer to higher level of care when compared with oxytocin in postpartum women.
Source: None. |
|
No GRADE |
No evidence was found regarding the effect of methylergometrine on women's sense of wellbeing when compared with oxytocin in postpartum women.
Source: None. |
|
No GRADE |
No evidence was found regarding the effect of methylergometrine on acceptability and satisfaction with the intervention when compared with oxytocin in postpartum women.
Source: None. |
|
No GRADE |
No evidence was found regarding the effect of methylergometrine on breastfeeding when compared with oxytocin in postpartum women.
Source: None. |
|
Low GRADE |
Methylergometrine may result in more adverse effects when compared with oxytocin in postpartum women.
Source: Dhananjaya 2014, Ezeama 2014, Jago 2007, Moir 1979, Moodie 1976, Orji 2008, Penaranda 2002, Singh 2009, Zachariah 2006. |
|
No GRADE |
No evidence was found regarding the effect of methylergometrine on PTSD when compared with oxytocin in postpartum women.
Source: None. |
2. Misoprostol versus oxytocin
|
Moderate GRADE |
Oxytocin probably decreases the risk of blood loss ≥1000mL when compared with misoprostol in postpartum women.
Source: Acharya 2001, Afolabi 2010, Al-Sawaf 2013, Atukunda 2014, Baskett 2007, Bellad 2012, Benchimol 2001, Bhatti 2014, Bugalho 2001, Caliskan 2002, Caliskan 2003, Chaudhuri 2010, Chaudhuri 2012, Cook 1999, Diallo 2017, Elbohoty 2016, Gavilanes 2016, Gerstenfeld 2001, Gulmezoglu 2001, Gupta 2006, Kundodyiwa 2001, Lokugamage 2001, Lumbiganon 1999, Modi 2014, Muhammad 2019, Nasr 2009, Oboro 2003, Owonikoko 2011, Parsons 2006, Parsons 2007, Penaranda 2002, Perez-Rumbos 2017, Sadiq 2011, Snehalata 2023, Sultana 2007, Tewatia 2014, Vagge 2014, Vimala 2006, Walley 2000, Zachariah 2006. |
|
No GRADE |
No evidence was found regarding the effect of misoprostol on maternal shock when compared with oxytocin in postpartum women.
Source: None. |
|
Very low GRADE |
The evidence is very uncertain about the effect of misoprostol on maternal death when compared with oxytocin in postpartum women.
Source: Afolabi 2010, Alwani 2014, Amin 2014, Atukunda 2014, Baskett 2007, Bellad 2012, Bhatti 2014, Chaudhuri 2010, Chaudhuri 2012, Diop 2016, Elbohoty 2016, Gulmezoglu 2001, Kundodyiwa 2001, Lumbiganon 1999, Musa 2015, Nasr 2009, Oboro 2003, Parsons 2006, Parsons 2007, Perez-Rumbos 2017, Shrestha 2011, Tewatia 2014, Walley 2000, Zachariah 2006. |
|
Low GRADE |
Misoprostol may result in little to no difference in total blood loss when compared with oxytocin in postpartum women.
Source: Acharya 2001, Afolabi 2010, Al-Sawaf 2013, Amin 2014, Asmat 2017, Atukunda 2014, Bellad 2012, Benchimol 2001, Bhatti 2014, Bugalho 2001, Burman 2021, Caliskan 2003, Chaudhuri 2010, Chaudhuri 2012, Cook 1999, Diallo 2017, Dutta 2016, Eftekhari 2009, Fazel 2013, Firouzbakht 2013, Gavilanes 2016, Gulmezoglu 2001, Gupta 2006, Kundodyiwa 2001, Lokugamage 2001, Lumbiganon 1999, Mishra 2023, Modi 2014, Musa 2015, Narrey 2023, Oboro 2003, Othman 2016, Owonikoko 2011, Parsons 2006, Parsons 2007, Penaranda 2002, Perez-Rumbos 2017, Rajaei 2014, Roy 2017, Sadiq 2011, Shrestha 2011, Singh 2009, Tewatia 2014, Vagge 2014, Vimala 2006, Walley 2000, Zachariah 200. |
|
Low GRADE |
Misoprostol may result in little to no difference in the use of additional uterotonics when compared with oxytocin in postpartum women.
Source: Acharya 2001, Afolabi 2010, Al-Sawaf 2013, Alwani 2014, Atukunda 2014, Baskett 2007, Bellad 2012, Bhatti 2014, Bugalho 2001, Caliskan 2002, Caliskan 2003, Chaudhuri 2010, Chaudhuri 2012, Cook 1999, Diallo 2017, Eftekhari 2009, Elbohoty 2016, Gavilanes 2016, Gerstenfeld 2001, Gulmezoglu 2001, Gupta 2006, Karkanis 2002, Kundodyiwa 2001, Lokugamage 2001, Lumbiganon 1999, Mishra 2023, Modi 2014, Mukta 2013, Musa 2015, Nankaly 2016, Narrey 2023, Oboro 2003, Othman 2016, Owonikoko 2011, Pakniat 2015, Parsons 2006, Parsons 2007, Perez-Rumbos 2017, Rajaei 2014, Roy 2017, Sadiq 2011, Shady 2017, Singh 2009, Snehalata 2023, Sultana 2007, Tewatia 2014, Vagge 2014, Vimala 2006, Walley 2000, Zachariah 2006. |
|
Moderate GRADE |
Misoprostol probably results in little to no difference in the use of blood transfusion when compared with oxytocin in postpartum women.
Source: Acharya 2001, Afolabi 2010, Al-Sawaf 2013, Alwani 2014, Atukunda 2014, Baskett 2007, Bellad 2012, Bhatti 2014, Bugalho 2001, Caliskan 2002, Caliskan 2003, Chaudhuri 2010, Chaudhuri 2012, Cook 1999, Diallo 2017, Elbohoty 2016, Fazel 2013, Gerstenfeld 2001, Gulmezoglu 2001, Gupta 2006, Karkanis 2002, Kundodyiwa 2001, Lokugamage 2001, Lumbiganon 1999, Modi 2014, Nankaly 2016, Nasr 2009, Oboro 2003, Owonikoko 2011, Parsons 2006, Parsons 2007, Perez-Rumbos 2017, Rajaei 2014, Roy 2017, Sadiq 2011, Shady 2017, Singh 2009, Snehalata 2023, Sultana 2007, Tewatia 2014, Vagge 2014, Walley 2000, Zachariah 2006. |
|
Very low GRADE |
The evidence is very uncertain about the effect of misoprostol on transfer to higher level of care when compared with oxytocin in postpartum women.
Source: Afolabi 2010, Amin 2014, Atukunda 2014, Chaudhuri 2010, Gulmezoglu 2001, Kundodyiwa 2001, Musa 2015, Nasr 2009, Shrestha 2011, Tewatia 2014. |
|
No GRADE |
No evidence was found regarding the effect of misoprostol on women's sense of wellbeing when compared with oxytocin in postpartum women.
Source: None. |
|
Low GRADE |
Misoprostol may result in little to no difference in acceptability and satisfaction with the intervention when compared with oxytocin in postpartum women.
Source: Diop 2016. |
|
No GRADE |
No evidence was found regarding the effect of misoprostol on breastfeeding when compared with oxytocin in postpartum women.
Source: None. |
|
Low GRADE |
Misoprostol may result in little to no difference in adverse effects when compared with oxytocin in postpartum women.
Source: Acharya 2001, Adanikin 2013, Afolabi 2010, Alwani 2014, Amin 2014, Atukunda 2014, Baskett 2007, Bellad 2012, Benchimol 2001, Bhatti 2014, Bugalho 2001, Burman 2021, Caliskan 2002, Caliskan 2003, Chaudhuri 2010, Chaudhuri 2012, Cook 1999, Dasuki 2002, Diallo 2017, Diop 2016, Dutta 2016, Elbohoty 2016, Fazel 2013, Firouzbakht 2013, Gavilanes 2016, Gerstenfeld 2001, Gulmezoglu 2001, Gupta 2006, Karkanis 2002, Kundodyiwa 2001, Lokugamage 2001, Lumbiganon 1999, Mishra 2023, Mukta 2013, Musa 2015, Nankaly 2016, Narrey 2023, Nasr 2009, Oboro 2003, Othman 2016, Owonikoko 2011, Pakniat 2015, Parsons 2006, Parsons 2007, Penaranda 2002, Perez-Rumbos 2017, Rajaei 2014, Shady 2017, Shrestha 2011, Singh 2009, Snehalata 2023, Sultana 2007, Tewatia 2014, Vagge 2014, Vimala 2006, Walley 2000, Zachariah 2006. |
|
No GRADE |
No evidence was found regarding the effect of misoprostol on PTSD when compared with oxytocin in postpartum women.
Source: None. |
3. Carbetocin versus oxytocin
|
Low GRADE |
Carbetocin may result in little to no difference in the risk of blood loss ≥1000mL when compared with oxytocin in postpartum women.
Source: Al Zubaidi 2022, Attilakos 2010, Boucher 1998, El Behery 2015, Elbohoty 2016, Fenix 2012, Kabir 2015, Kang 2022, Liu 2020, Maged 2016, McDonagh 2022, Nahaer 2020, Rosseland 2013, Sudjai 2022, vd Nelson 2021, Whigham 2016, Widmer 2018, Yesmin 2022. |
|
No GRADE |
No evidence was found regarding the effect of carbetocin on maternal shock when compared with oxytocin in postpartum women.
Source: None. |
|
Very low GRADE |
The evidence is very uncertain about the effect of carbetocin on maternal death when compared with oxytocin in postpartum women.
Source: Al Zubaidi 2022, Attilakos 2010, Boucher 1998, El Behery 2015, Elbohoty 2016, Widmer 2018. |
|
Low GRADE |
Carbetocin may result in a small reduction on total blood loss when compared with oxytocin in postpartum women.
Source: Ahmed 2014, Attilakos 2010, Burruto 2009, Boucher 1998, Boucher 2004, Carrilo-Gaucin 2016, Cetin 2023, El Behery 2015, Fahmy 2015, Fahmy 2016, Fenix 2012, Ibrahim 2020, Jha 2023, Kabir 2015, Kang 2022, Liu 2020, Maged 2016, Mohamed 2015, Rosseland 2013, Sudjai 2022, Taheripanah 2018, Whigham 2016, Yesmin 2022. |
|
Low GRADE |
Carbetocin may decrease the use of additional uterotonics when compared with oxytocin in postpartum women.
Source: Aabha 2023, Al Zubaidi 2022, Attilakos 2010, Borruto 2009, Boucher 1998, Boucher 2004, Carrilo-Gaucin 2016, Cetin 2023, Dansereau 1999, El Behery 2015, Elbohoty 2016, Fahmy 2015, Fahmy 2016, Fenix 2012, Gh 2023, Ibrahim 2020, Jha 2023, Kabir 2015, Kang 2022, Liu 2020, Maged 2016, Mannaerts 2018, McDonagh 2022, Moertl 2011, Nahaer 2020, Ortiz-Gomez 2013, Reyes Gonzalez 2011, Reyes 2011, Rosseland 2013, Sudjai 2022, Taheripanah 2018, vd Nelson 2021, Whigham 2016, Widmer 2018. |
|
Moderate GRADE |
Carbetocin probably decreases the use of blood transfusion when compared with oxytocin in postpartum women.
Source: Aabha 2023, Al Zubaidi 2022, Attilakos 2010, Boucher 1998, Carrilo-Gaucin 2016, Cetin 2023, Dansereau 1999, El Behery 2015, Elbohoty 2016, Fahmy 2015, Fahmy 2016, Fenix 2012, Ibrahim 2020, Jha 2023, Kabir 2015, Kang 2022, Liu 2020, Maged 2016, Nahaer 2020, Reyes Gonzalez 2011, Reyes 2011, Taheripanah 2018, vd Nelson 2021, Whigham 2016, Widmer 2018. |
|
Very low GRADE |
The evidence is very uncertain about the effect of carbetocin on transfer to higher level of care when compared with oxytocin in postpartum women.
Source: Attilakos 2010, Widmer 2018. |
|
No GRADE |
No evidence was found regarding the effect of carbetocin on women's sense of wellbeing when compared with oxytocin in postpartum women.
Source: None. |
|
No GRADE |
No evidence was found regarding the effect of carbetocin on acceptability and satisfaction with the intervention when compared with oxytocin in postpartum women.
Source: None. |
|
Low GRADE |
Carbetocin may result in little to no difference in breastfeeding when compared with oxytocin in postpartum women.
Source: Reyes Gonzalez 2011, Reyes 2011. |
|
Low GRADE |
Carbetocin may result in little to no difference in adverse effects when compared with oxytocin in postpartum women.
Source: Attilakos 2010, Borruto 2009, Boucher 1998, Boucher 2004, Dansereau 1999, El Behery 2015, Elbohoty 2016, Fenix 2012, Jha 2023, Kabir 2015, Maged 2016, Mannaerts 2018, McDonagh 2022, Moertl 2011, Nahaer 2020, Ortiz-Gomez 2013, Reyes Gonzalez 2011, Reyes 2011, Rosseland 2013, Sudjai 2022, Taheripanah 2018, vd Nelson 2021, Widmer 2018. |
|
No GRADE |
No evidence was found regarding the effect of carbetocin on PTSD when compared with oxytocin in postpartum women.
Source: None. |
4. Methylergometrine plus oxytocin (=syntometrine) versus oxytocin
|
Moderate GRADE |
Syntometrine probably reduces the risk of blood loss ≥1000mL when compared with oxytocin in postpartum women.
Source: Caliskan 2002, Caliskan 2003, Choy 2002, Cook 1999, Khan 1995, Masse 2022, McDonald 1993, Mitchell 1993, Nuamsiri 2016, Rashid 2009, vd Nelson 2021, Yuen 1995. |
|
No GRADE |
No evidence was found regarding the effect of syntometrine on maternal shock when compared with oxytocin in postpartum women.
Source: None. |
|
Low GRADE |
The risk of maternal death was too low to determine whether syntometrine made a difference on maternal death.
Source: Nuamsiri 2016, Yuen 1995. |
|
Very low GRADE |
The evidence is very uncertain about the effect of syntometrine on total blood loss when compared with oxytocin in postpartum women.
Source: Balki 2008, Caliskan 2003, Choy 2002, Cook 1999, Docherty 1981, Koen 2016, Kumru 2005, Masse 2022, Mitchell 1993, Nuamsiri 2016, Rashid 2009. |
|
Very low GRADE |
The evidence is very uncertain about the effect of syntometrine on the use of additional uterotonics when compared with oxytocin in postpartum women.
Source: Balki 2008, Caliskan 2002, Caliskan 2003, Choy 2002, Cook 1999, Docherty 1981, Koen 2016, Kumru 2005, Masse 2022, Mitchell 1993, Nuamsiri 2016, Rashid 2009. |
|
Low GRADE |
Syntometrine may result in little to no difference in the use of blood transfusion when compared with oxytocin in postpartum women.
Source: Balki 2008, Caliskan 2002, Caliskan 2003, Choy 2002, Cook 1999, Khan 1995, Koen 2016, Masse 2022, McDonald 1993, Nuamsiri 2016, Rashid 2009, vd Nelson 2021, Yuen 1995. |
|
Very low GRADE |
The evidence is very uncertain about the effect of syntometrine on transfer to higher level of care when compared with oxytocin in postpartum women.
Source: Yuen 1995. |
|
No GRADE |
No evidence was found regarding the effect of carbetocin on women's sense of wellbeing when compared with oxytocin in postpartum women.
Source: None. |
|
No GRADE |
No evidence was found regarding the effect of carbetocin on acceptability and satisfaction with the intervention when compared with oxytocin in postpartum women.
Source: None. |
|
Low GRADE |
Syntometrine may result in little to no difference in breastfeeding when compared with oxytocin in postpartum women.
Source: McDonald 1993. |
|
Low GRADE |
Syntometrine may result in little to no difference in adverse effects when compared with oxytocin in postpartum women.
Source: Balki 2008, Caliskan 2002, Caliskan 2003, Choy 2002, Cook 1999, Khan 1995, Koen 2016, McDonald 1993, Nuamsiri 2016, Rashid 2009, vd Nelson 2021, Yuen 1995. |
|
No GRADE |
No evidence was found regarding the effect of syntometrine on PTSD when compared with oxytocin in postpartum women.
Source: None. |
5. Misoprostol plus oxytocin versus oxytocin
|
Moderate GRADE |
Misoprostol plus oxytocin probably reduces the risk of blood loss ≥1000mL when compared with oxytocin in postpartum women.
Source: Adanikin 2012, Badejoko 2012, Caliskan 2002, Caliskan 2003, Carbonell 2009, Chaudhuri 2015, Chaudhuri 2016, Elsedeek 2012, Fekih 2009, Hamm 2005, Hernandez-Castro 2016, Hofmeyr 2011, Lapaire 2006, Muhammad 2019, Quibel 2016, Sitaula 2017, Sood 2012, Ugwu 2014. |
|
No GRADE |
No evidence was found regarding the effect of misoprostol plus oxytocin on maternal shock when compared with oxytocin in postpartum women.
Source: None. |
|
Very low GRADE |
The evidence is very uncertain about the effect of misoprostol plus oxytocin on maternal death when compared with oxytocin in postpartum women.
Source: Badejoko 2012, Bhullar 2004, Carbonell 2009, Chaudhuri 2015, Chaudhuri 2016, El Tahan 2012, Hofmeyr 2011, Lapaire 2006, Ugwu 2014. |
|
Moderate GRADE |
Misoprostol plus oxytocin results probably in a small reduction in total blood loss when compared with oxytocin in postpartum women.
Source: Badejoko 2012, Bhullar 2004, Caliskan 2003, Carbonell 2009, Cetin 2023, Chaudhuri 2015, Chaudhuri 2016, Elsedeek 2012, El Tahan 2012, Fekih 2009, Hamm 2005, Hofmeyr 2011, Lapaire 2006, Muhammad 2019, Nayak 2017, Ottun 2022, Quibel 2016, Sitaula 2017, Sood 2012, Ugwu 2014. |
|
High GRADE |
Misoprostol plus oxytocin reduces the use of additional uterotonics when compared with oxytocin in postpartum women.
Source: Badejoko 2012, Bhullar 2004, Caliskan 2002, Caliskan 2003, Carbonell 2009, Cetin 2023, Chaudhuri 2015, Chaudhuri 2016, Elsedeek 2012, El Tahan 2012, Hamm 2005, Hernandez-Castro 2016, Hong 2007, Lapaire 2006, Muhammad 2019, Nayak 2017, Ottun 2022, Pakniat 2015, Quibel 2016, Sood 2012, Ugwu 2014. |
|
Low GRADE |
Misoprostol plus oxytocin may reduce the use of blood transfusion when compared with oxytocin in postpartum women.
Source: Badejoko 2012, Bhullar 2004, Caliskan 2002, Caliskan 2003, Carbonell 2009, Cetin 2023, Chaudhuri 2015, Chaudhuri 2016, Elsedeek 2012, El Tahan 2012, Fekih 2009, Hamm 2005, Hernandez-Castro 2016, Hong 2007, Lapaire 2006, Muhammad 2019, Nayak 2017, Ottun 2022, Quibel 2016, Sitaula 2012, Sood 2012, Ugwu 2014. |
|
Very low GRADE |
The evidence is very uncertain about the effect of misoprostol plus oxytocin on transfer to higher level of care when compared with oxytocin in postpartum women.
Source: Carbonell 2009, El Tahan 2012, Ugwu 2014. |
|
No GRADE |
No evidence was found regarding the effect of misoprostol plus oxytocin on women's sense of wellbeing when compared with oxytocin in postpartum women.
Source: None. |
|
No GRADE |
No evidence was found regarding the effect of misoprostol plus oxytocin on acceptability and satisfaction with the intervention when compared with oxytocin in postpartum women.
Source: None. |
|
No GRADE |
No evidence was found regarding the effect of misoprostol plus oxytocin on breastfeeding when compared with oxytocin in postpartum women.
Source: None. |
|
Low GRADE |
Misoprostol plus oxytocin may result in more adverse effects when compared with oxytocin in postpartum women.
Source: Adanikin 2012, Badejoko 2012, Bhullar 2004, Caliskan 2002, Caliskan 2003, Carbonell 2009, Cayan 2010, Chaudhuri 2015, Chaudhuri 2016, Elsedeek 2012, El Tahan 2012, Fekih 2009, Hofmeyr 2011, Hong 2007, Lapaire 2006, Muhammad 2019, Ottun 2022, Pakniat 2015, Quibel 2016, Sood 2012, Ugwu 2014. |
|
No GRADE |
No evidence was found regarding the effect of misoprostol plus oxytocin on PTSD when compared with oxytocin in postpartum women.
Source: None. |
Samenvatting literatuur
Description of studies
Inclusion criteria of the Cochrane review were (1) randomized controlled trials or cluster-randomized trials, (2) women in the third stage of labor following a vaginal or cesarean birth in hospital or community settings, and (3) trials comparing the effectiveness and side effects of uterotonic agents with other uterotonic agents, placebo or no treatment for preventing postpartum haemorrhage (PPH). Exclusion criteria were (1) no systemic administration, and (2) exclusive comparison of different dosages, routes or regimens of the same uterotonic agent. Uterotonic agents were allowed to be administered in any dosage, route or regimen systemically at birth. The systematic review included studies from inception up until May 24, 2018. The studies that we excluded from the Cochrane review regarded comparisons not stated in our PICO (mainly an uterotonic versus no treatment or placebo).
The 23 studies that we included besides the ones of the review, were comparing a systemically administered uterotonic agent to oxytocin. Uterotonic agents were allowed to be administered in any dosage, route or regimen systemically at birth, with or without combination with oxytocin, similar to the studies included in the review. Also, similar to the review, all studies regarded participants in the third stage of labor following a vaginal or cesarean birth in hospital or community settings.
Across all 147 studies, providing data on 111.101 participants, misoprostol was investigated in 67 studies, carbetocin in 38, misoprostol plus oxytocin in 28, syntometrine (methylergometrine + oxytocine) in 17, and methylergometrine in 10 (the total of these numbers exceeds 147 as some studies had more than two arms). Dosages, timing and route of administration ranged between studies: misoprostol was administered in dosages ranging from 200 to 800 micrograms and was provided either orally, sublingually or rectally. Carbetocin was administered in 100 micrograms either intravenous or intramuscular. Methylergometrine was provided in dosages ranging from 200 to 500 micrograms either intravenous or intramuscular. Oxytocin dosages ranged from 5 to 40 international units and was administered intravenously or intramuscularly as a single shot or as infusion, or a combination of bolus and infusion. All uterotonic agents were administered after delivery of the anterior shoulder, delivery of the baby, clamping of the umbilical cord or after delivery of the placenta. Of all studies, 81 studies researched women after vaginal birth, 63 studies women after cesarean delivery (26 elective, 7 emergency, 30 both types of cesarean), two studies did not describe the mode of delivery, and one study included women after any birth. Included participants were judged to be at a high a priori risk of PPH in 64 studies, to be at a low priori risk of PPH in 39 studies, 40 studies included both low and high risk participants and a priori risk was not specified in four studies.
Results
1. Methylergometrine versus oxytocin
1.1 Blood loss ≥1000mL
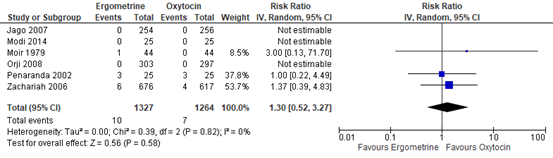
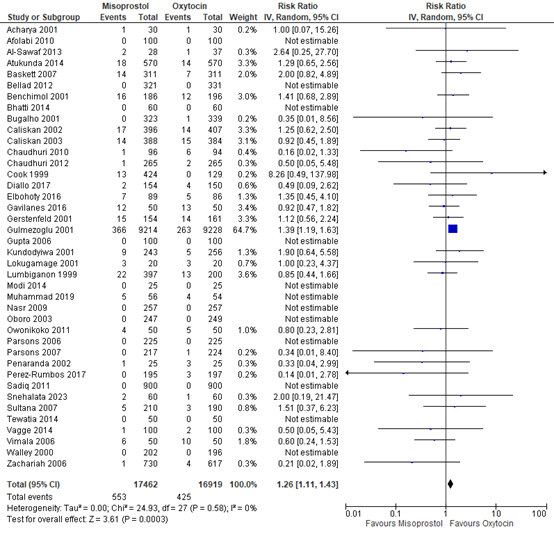
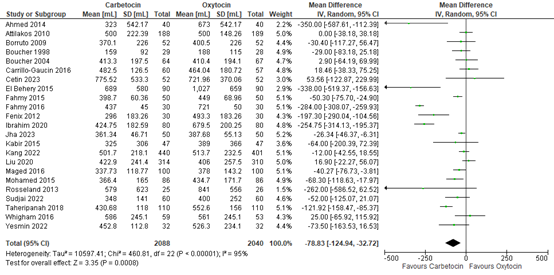
The outcome of blood loss ≥1000mL was described in six studies with data of 2.591 participants. In three studies this outcome occurred: in Moir 1979, one out of 44 participants had blood loss ≥1000mL in the methylergometrine arm versus zero of 44 participants in the oxytocin arm. In Penaranda 2002, in both arms three out of 25 participants had blood loss ≥1000mL. In Zachariah 2006, six out of 676 participants in the methylergometrine arm versus four out of 617 in the oxytocin arm had blood loss ≥1000mL. The pooled risk ratio was found to be 1.30 (95% CI 0.52 to 3.27), favouring oxytocin in the prevention of Blood loss ≥1000mL though the confidence interval was wide (figure 1).
Figure 1. Comparison of methylergometrine and oxytocin for in the prevention of blood Loss ≥1000mL.
1.2 Maternal shock
No studies reported on maternal shock.
1.3 Maternal death
One study with 1293 participants described the outcome of maternal death (Zachariah 2006). No deaths occurred.
1.4 Blood loss
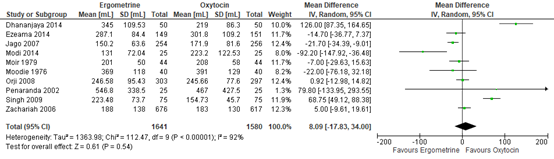
Blood loss in participants receiving methylergometrine or oxytocin was investigated in ten studies covering data of 3.221 participants. The pooled mean difference in blood loss was 8 mL (95% CI -18 to 34), favouring oxytocin (figure 2).
Figure 2. Mean Blood loss in the Comparison of methylergometrine versus oxytocin.
1.5 Use of additional uterotonics
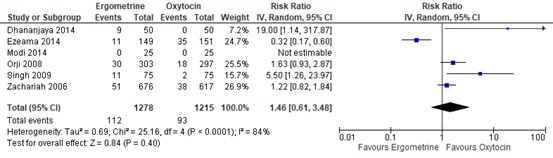
The use of additional uterotonics was assessed in six studies with data of 2.493 participants. The pooled risk ratio was 1.46 (95% CI 0.61 to 3.48), in favour of oxytocin though the confidence interval was wide (figure 3).
Figure 3. Forest plot of use of additional uterotonics in the comparison of methylergometrine versus oxytocin.
1.6 Use of blood transfusion
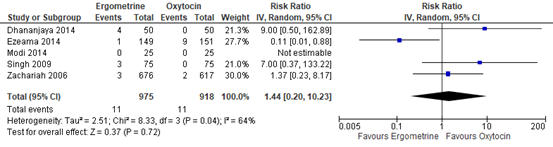
The use of blood transfusion was assessed in five studies including 1.893 participants. The pooled risk ratio was 1.44 (95% CI 0.20 to 10.23), in favour of oxytocin (figure 4).
Figure 4. Forest plot of use of blood transfusion in the comparison of methylergometrine versus oxytocin.
1.7 Transfer to higher level of care
No studies reported on the outcome of intensive care admission.
1.8 Women's sense of wellbeing
Maternal wellbeing was not assessed in any of the studies.
1.9 Acceptability and satisfaction with the intervention
No studies investigated maternal satisfaction.
1.10 Breastfeeding
No studies assessed breastfeeding.
1.11 Adverse effects
The following adverse effects were assessed: nausea, vomiting, headache, hypertension, shivering, fever and diarrhea.
- Nausea was assessed in six studies with 2.529 participants. The pooled risk ratio for nausea was 4.56 (95% CI 1.13 to 18.44), favouring oxytocin though the confidence interval was wide.
- Vomiting was assessed in five studies with 2.343 participants. The pooled risk ratio for vomiting was 3.83 (95% CI 1.10 to 13.28), favouring oxytocin though the confidence interval was wide.
- Headache was assessed in four studies with 2.293 participants. The pooled risk ratio for headache was 5.63 (95% CI 0.93 to 33.96), favouring oxytocin though the confidence interval was wide.
- Hypertension was assessed in three studies with 1.410 participants. The pooled risk ratio for hypertension was 13.39 (95% CI 2.01 to 89.44), favouring oxytocin though the confidence interval was wide.
- Shivering was assessed in three studies with 1.493 participants. The pooled risk ratio for shivering was 1.73 (95% CI 0.93 to 3.25), favouring oxytocin.
- Fever was assessed in two studies with 1.443 participants. The pooled risk ratio for fever was 2.97 (95% CI 0.97 to 9.05), favouring oxytocin though the confidence interval was wide.
- Diarrhea was assessed in two studies with 1.393 participants. It occurred in three out 726 participants in the methylergometrine arm versus zero out 667 in the oxytocin arm. The pooled risk ratio was 3.74 (95% CI 0.42 to 33.53), favouring oxytocin though the confidence interval was wide.
1.12 PTSD
No studies reported on this outcome.
2. Misoprostol versus oxytocin
2.1 Blood loss ≥1000mL
The outcome measure blood loss ≥1000mL was researched in 39 studies providing data on 34.381 participants. The pooled risk ratio for blood loss ≥1000mL among participants receiving misoprostol compared to oxytocin was 1.26 (95% CI 1.11 to 1.43), favouring oxytocin (figure 5).
Figure 5. Comparison of misoprostol versus oxytocin in the prevention of blood loss ≥1000mL.
2.2 Maternal shock
No studies reported on the outcome maternal shock.
2.3 Maternal death
Maternal death was investigated in 24 studies with 28.520 participants. In three studies death occurred: In the study (Alwani 2014), no participants out of 100 in the misoprostol arm died, while one participant out of 100 in the oxytocin arm died. In the study (Parsons 2007), no deaths occurred in the misoprostol arm (224 participants), while one participant out of 226 in the oxytocin arm died. In the study (Gulmezoglu 2001), two participants died in each arm: two out of 9,230 in the misoprostol arm, and two out of 9,225 in the oxytocin arm. Thus in total two out of 14.459 in the misoprostol arm died and four out of 14.061 in the oxytocin arm. This corresponding pooled risk difference is 0.01 (95% CI -0.02 to 0.05) and risk ratio of 0.62 (95% CI 0.14 to 2.74), favouring misoprostol.
2.4 Blood loss
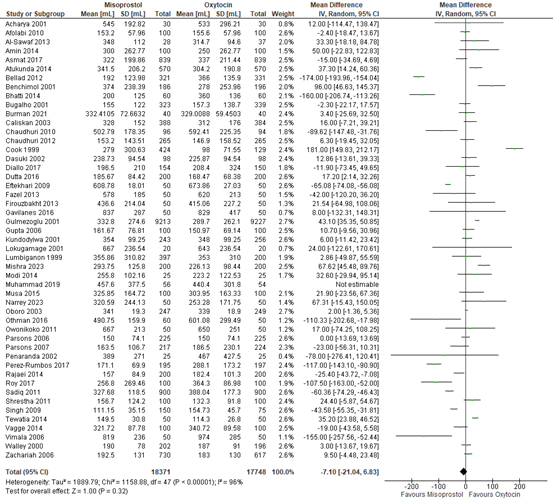
The amount of blood loss in participants receiving either misoprostol or oxytocin was assessed in 48 studies with data on 36.119 participants. The mean difference in blood loss was -7 mL (95% CI -21 to 7), in favour of misoprostol (figure 6).
Figure 6. Mean blood loss in the comparison of misoprostol versus oxytocin.
2.5 Use of additional uterotonics
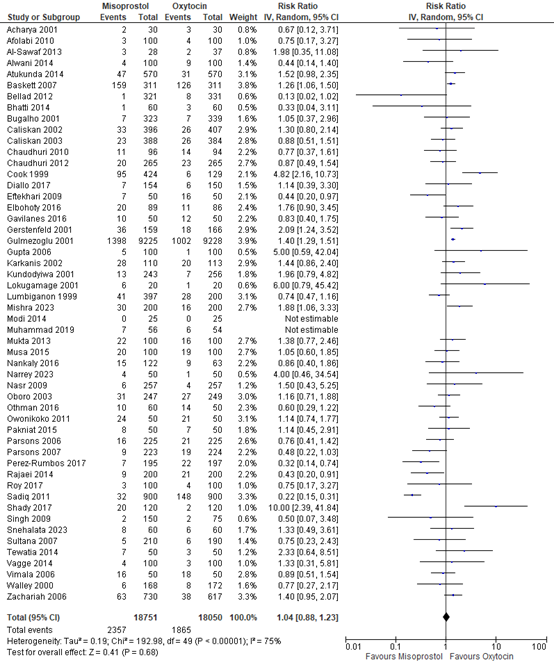
The use of additional uterotonics was described in 51 studies with data of 36.801 participants. The pooled risk ratio was 1.04 (95% CI 0.88 to 1.23), in favour of oxytocin (figure 7).
Figure 7. Forest plot of use of additional uterotonics in the comparison of misoprostol versus oxytocin.
2.6 Use of blood transfusion
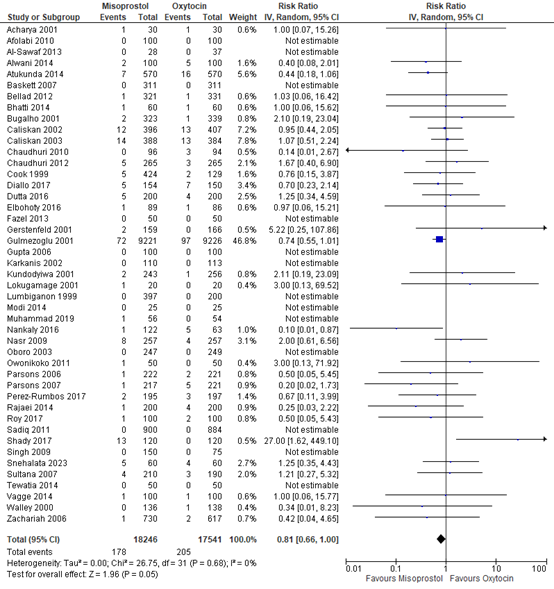
The use of blood transfusion was described in 44 studies with data of 35.787 participants. The pooled risk ratio was 0.81 (95% CI 0.66 to 1.00), in favour of misoprostol (figure 8).
Figure 8. Forest plot of use of blood transfusion in the comparison of misoprostol versus oxytocin.
2.7 Transfer to higher level of care
Ten studies with data on 21.698 participants researched intensive care admission. In two studies participants were admitted to the intensive care: in Atakunda 2014, 11 out of 570 participants in the misoprostol arm versus eight out of 570 participants in the oxytocin arm were admitted. In Gulmezoglu 2001, four out of 9224 participants in the misoprostol arm versus five out of 9231 participants in the oxytocin arm were admitted. The pooled risk ratio was 1.16 (95% CI 0.55 to 2.43), favouring oxytocin although events were rare and the confidence interval was wide.
2.8 Women's sense of wellbeing
No studies assessed the outcome maternal wellbeing.
2.9 Acceptability and satisfaction with the intervention
One study with 1.338 participants investigated maternal satisfaction (Diop 2016). For the outcome being satisfied or very satisfied with the drug, the risk ratio was 1.01 (95% CI 1.00 to 1.02), favouring oxytocin. The risk ratio for complaints about or problems with the drug was 0.36 (95% CI 0.20 to 0.64), favouring misoprostol. For the outcome ‘would take the drug again after subsequent deliveries’ the risk ratio was 1.01 (95% CI 1.00 to 1.02), and for ‘would recommend the drug to a friend’ the risk ratio was 1.01 (95% CI 1.00 to 1.02), both favouring oxytocin.
2.10 Breastfeeding
No studies assessed the outcome breastfeeding.
2.11 Adverse effects
The adverse effects of misoprostol versus oxytocin that were assessed included nausea, vomiting, headache, abdominal pain, hypertension, shivering, fever, and diarrhea.
- Nausea was assessed in 36 studies with 30.352 participants. The pooled risk ratio for nausea was 1.23 (95% CI 0.95 to 1.58), favouring oxytocin.
- Vomiting was assessed in 45 studies with 33.087 participants. The pooled risk ratio for vomiting was 1.52 (95% CI 1.22 to 1.90), favouring oxytocin.
- Headache was assessed in 13 studies with 4.699 participants. The pooled risk ratio for headache was 0.91 (95% CI 0.60 to 1.38), favouring misoprostol.
- Abdominal pain was assessed in nine studies with 3.482 participants. The pooled risk ratio for abdominal pain was 0.91 (95% CI 0.78 to 1.05), favouring misoprostol.
- Hypertension was assessed in three studies with 1.028 participants and occurred in four out 510 participants in the misoprostol arm and zero out 518 in the oxytocin arm. The pooled risk ratio was 3.64 (95% CI 0.60 to 22.27), favouring oxytocin, though the confidence interval is wide.
- Shivering was assessed in 54 studies with 35.585 participants. The pooled risk ratio for shivering was 3.82 (95% CI 3.11 to 4.69), favouring oxytocin.
- Fever was assessed in 45 studies with 33.708 participants. The pooled risk ratio for fever was 3.66 (95% CI 2.73 to 4.92), favouring oxytocin.
- Diarrhea pain was assessed in 30 studies with 31.433 participants. The pooled risk ratio for diarrhea was 2.06 (95% 1.53 to 2.78), favouring oxytocin.
2.12 PTSD
No studies assessed the outcome PTSD.
3. Carbetocin versus oxytocin
3.1 Blood loss ≥1000mL
The outcome of blood loss ≥1000mL was described in 18 studies with data of 36.615 participants. The pooled risk ratio among participants receiving carbetocin compared to oxytocin was found to be 0.92 (95% CI 0.79 to 1.06), favouring carbetocin (figure 9).
Figure 9. Comparison of carbetocin versus oxytocin in the prevention of blood loss ≥1000mL.
3.2 Maternal shock
No studies investigated maternal shock.
3.3 Maternal death
Maternal death was described in six studies with data of 30.627 participants. Death occurred in one study (Widmer 2018): four out of 14.771 participants died in the carbetocin arm versus two out of 14.768 in the oxytocin arm (risk difference 0.00014 (or 0.014%), 95% CI (-0.019%, 0.046%) and risk ratio 2.00, 95%CI 0.37 to 10.92), favouring oxytocin though the confidence interval is very wide.
3.4 Blood loss
Blood loss in participants receiving carbetocin or oxytocin was investigated in 23 studies covering data of 4.128 participants, including both vaginal and caesarean births. The pooled mean difference in blood loss was -79 mL (95% CI -125 to -33), favouring carbetocin (figure 10).
Figure 10. Mean blood loss in the comparison of carbetocin versus oxytocin.
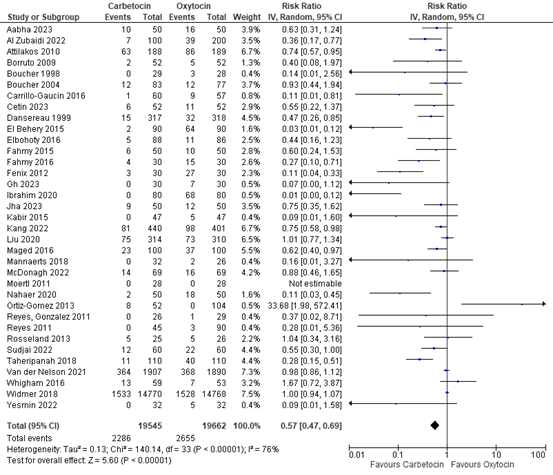
3.5 Use of additional uterotonics
The use of additional uterotonics was assessed in 35 studies with data of 39.207 participants. The pooled risk ratio was 0.57 (95% CI 0.47 to 0.69), in favour of carbetocin figure 11).
Figure 11. Forest plot of use of additional uterotonics in the comparison of carbetocin versus oxytocin.
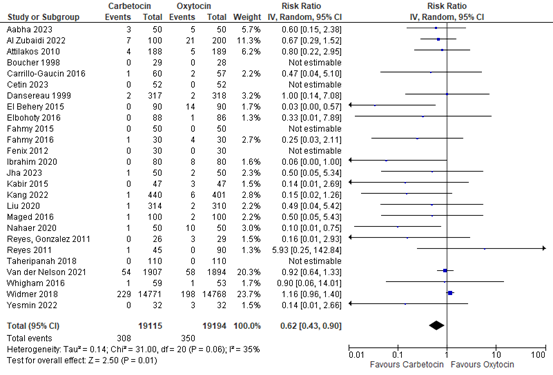
3.6 Use of blood transfusion
The use of blood transfusion was assessed in 26 studies covering data of 38.309 participants. The pooled risk ratio was 0.62 (95% CI 0.43 to 0.90), in favour of carbetocin (figure 12).
Figure 12. Forest plot of use of blood transfusion in the comparison of carbetocin versus oxytocin.
3.7 Transfer to higher level of care
Admission to intensive care was investigated in two studies with data of 29.847 participants. In one study (Attilakos 2010), one out of 188 participants was admitted in the carbetocin arm, while none of 189 participants were admitted in the oxytocin arm. In Widmer 2018, 26 out of 14.737 participants were admitted to intensive care in the carbetocin arm versus 23 out of 14.733 participants in the oxytocin arm. This resulted in a pooled risk ratio of 1.16 (95% CI 0.67 to 2.02), favouring oxytocin.
3.8 Women's sense of wellbeing
Maternal wellbeing was not assessed in any of the studies.
3.9 Acceptability and satisfaction with the intervention
No studies investigated maternal satisfaction.
3.10 Breastfeeding
Two studies with 190 participants investigated breastfeeding at discharge. In Reyes Gonzalez 2011, 22 out of 26 participants in the carbetocin arm breastfed compared to 25 out of 29 in the oxytocin arm. In Reyes 2011, in the carbetocin arm 41 out of 45 breastfed versus 88 out of 90 in the oxytocin arm. The overall pooled risk ratio was 0.94 (95% CI 0.86 to 1.03), favouring carbetocin.
3.11 Adverse effects
The following adverse effects were assessed: nausea, vomiting, headache, abdominal pain, hypertension, shivering, and fever.
- Nausea was assessed in 17 studies with 6.548 participants. The pooled risk ratio for nausea was 0.99 (95% CI 0.82 to 1.19), favouring carbetocin.
- Vomiting was assessed in 17 studies with 35.973 participants. The pooled risk ratio for vomiting was 0.89 (95% CI 0.62 to 1.27), favouring carbetocin.
- Headache was assessed in 18 studies with 6.660 participants. The pooled risk ratio for headache was 0.88 (95% CI 0.67 to 1.15), favouring carbetocin.
- Abdominal pain was assessed in 14 studies with 35.415 participants. The pooled risk ratio for abdominal pain was 1.05 (95% CI 0.84 to 1.32), favouring oxytocin.
- Hypertension was assessed in 2 studies with 3.930 participants. The pooled risk ratio for hypertension was 0.98 (95% CI 0.78 to 1.24), favouring carbetocin.
- Shivering was assessed in nine studies with 1.998 participants. The pooled risk ratio for shivering was 0.78 (95% CI 0.49 to 1.23), favouring carbetocin.
- Fever was assessed in five studies with 566 participants. The pooled risk ratio for fever was 1.01 (95% CI 0.21 to 4.83), favouring oxytocin, though the confidence interval is wide.
3.12 PTSD
No studies reported on this outcome.
4. Methylergometrine plus oxytocin (=syntometrine) versus oxytocin
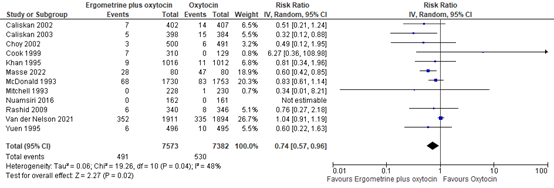
4.1 Blood loss ≥1000mL
The outcome of blood loss ≥1000mL was described in 12 studies with data of 14.955 participants. The pooled risk ratio for blood loss ≥1000mL when comparing syntometrine with oxytocin was found to be 0.74 (95% CI 0.57 to 0.96), favouring syntometrine (figure 13).
Figure 13. Comparison of syntometrine versus oxytocin in the prevention of blood loss ≥1000mL
4.2 Maternal shock
No studies investigated maternal shock.
4.3 Maternal death
Maternal death was described in two studies with data of 1.314 participants. Death did not occur in any of the participants (Nuamsiri 2016, Yuen 1995).
4.4 Blood loss
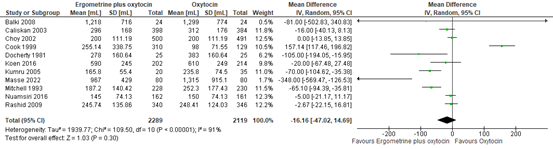
Blood loss in participants receiving syntometrine or oxytocin was investigated in 11 studies covering data of 4.408 participants. The pooled mean difference in blood loss was -16 mL (95% CI -47 to 15), favouring syntometrine (figure 14).
Figure 14. Mean blood loss in the comparison of syntometrine versus oxytocin.
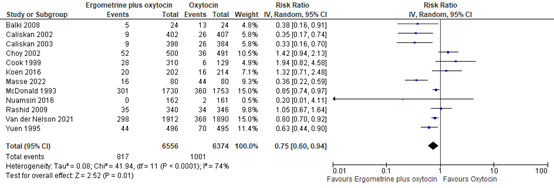
4.5 Use of additional uterotonics
The use of additional uterotonics was assessed in 12 studies with data of 12.930 participants. The pooled risk ratio was 0.75 (95% CI 0.60 to 0.94), in favour of syntometrine (figure 15).
Figure 15. Forest plot of use of additional uterotonics in the comparison of syntometrine versus oxytocin.
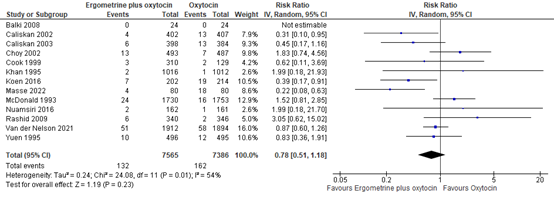
4.6 Use of blood transfusion
The use of blood transfusion was assessed in 13 studies covering data of 14.951 participants. The pooled risk ratio was 0.78 (95% CI 0.57 to 1.18), in favour of syntometrine (figure 16).
Figure 16. Forest plot of use of blood transfusion in the comparison of syntometrine versus oxytocin.
4.7 Transfer to higher level of care
One study with 991 participants investigated admission to intensive care (Yuen 1995). Here, one participant in the syntometrine arm was admitted to the intensive care.
4.8 Women's sense of wellbeing
Maternal wellbeing was not assessed in any of the studies.
4.9 Acceptability and satisfaction with the intervention
No studies investigated maternal satisfaction.
4.10 Breastfeeding
One study with 3.483 participants investigated breastfeeding at hospital discharge (McDonald 1993). In the syntometrine arm, 1478 out of 1730 participants breastfed compared to 1518 out of 1753 in the oxytocin arm. The pooled risk ratio was 0.99 (95% CI 0.96 to 1.01), favouring syntometrine.
4.11 Adverse effects
The following adverse effects were assessed: nausea, vomiting, headache, abdominal pain, hypertension, shivering, fever and diarrhea.
- Nausea was assessed in eight studies with 10.737 participants. The pooled risk ratio for nausea was 1.86 (95% CI 1.09 to 3.20), favouring oxytocin.
- Vomiting was assessed in ten studies with 14.033 participants. The pooled risk ratio for vomiting was 3.46 (95% CI 2.37 to 5.04), favouring oxytocin.
- Headache was assessed in six studies with 8.911 participants. The pooled risk ratio for headache was 1.62 (95% CI 0.94 to 2.78), favouring oxytocin.
- Abdominal pain was assessed in one study with 3.806 participants. The pooled risk ratio for abdominal pain was 1.24 (95% CI 1.00 to 1.55), favouring oxytocin.
- Hypertension was assessed in four studies with 5.159 participants. The pooled risk ratio for hypertension was 1.83 (95% CI 0.84 to 4.01), favouring oxytocin.
- Shivering was assessed in two studies with 1.591 participants. The pooled risk ratio for shivering was 0.96 (95% CI 0.60 to 1.53), favouring syntometrine.
- Fever was assessed in two studies with 1.591 participants. The pooled risk ratio for fever was 1.08 (95% CI 0.48 to 2.43), favouring oxytocin.
- Diarrhea was assessed in three studies with 2.030 participants. The pooled risk ratio for diarrhea was 1.26 (95% CI 0.72 to 2.22), favouring oxytocin.
4.12 PTSD
No studies reported on this outcome.
5. Misoprostol plus oxytocin versus oxytocin
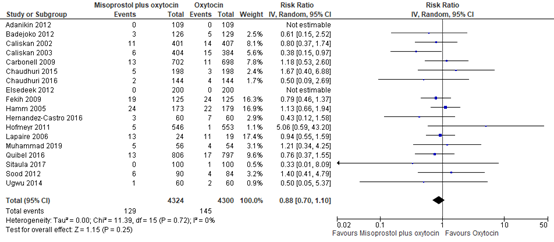
5.1 Blood loss ≥1000mL
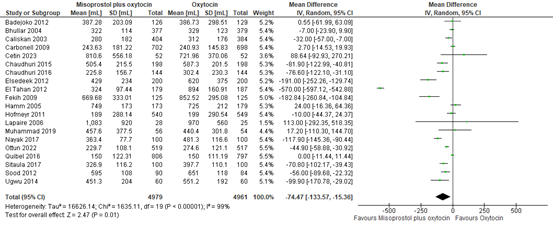
The outcome of blood loss ≥1000mL was described in 18 studies providing data on 8.624 participants. The pooled risk ratio for blood loss ≥1000mL when comparing misoprostol plus oxytocin to oxytocin was 0.88 (95% CI 0.70 to 1.10), favouring misoprostol plus oxytocin (figure 17).
Figure 17. Comparison of misoprostol plus oxytocin versus oxytocin.in the prevention of blood loss ≥1000mL.
5.2 Maternal shock
No studies investigated maternal shock.
5.3 Maternal death
Maternal death was described in nine studies and none of the 4.737 participants died.
5.4 Blood loss
Blood loss in participants receiving misoprostol and oxytocin or oxytocin only was investigated in 20 studies covering data of 9.940 participants. The pooled mean difference in blood loss was -75 mL (95% CI -134 to -15), favouring misoprostol plus oxytocin (figure 18).
Figure 18. Mean blood loss in the comparison of misoprostol plus oxytocin versus oxytocin.
5.5 Use of additional uterotonics
The use of additional uterotonics was assessed in 21 studies with data of 9.641 participants. The pooled risk ratio was 0.57 (95% CI 0.47 to 0.69), in favour of misoprostol plus oxytocin (figure 19).
Figure 19. Forest plot of use of additional uterotonics in the comparison of misoprostol plus oxytocin versus oxytocin.
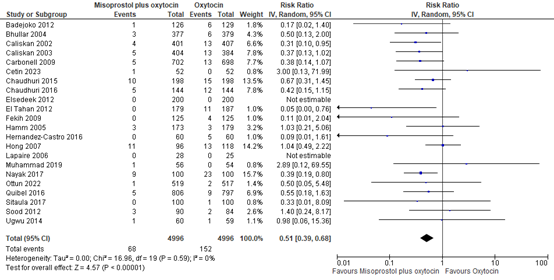
5.6 Use of blood transfusion
The use of blood transfusion was assessed in 22 studies covering data of 9.992 participants. The pooled risk ratio was 0.51 (95% CI 0.39 to 0.68), in favour of misoprostol plus oxytocin (figure 20).
Figure 20. Forest plot of use of blood transfusion in the comparison of misoprostol plus oxytocin versus oxytocin.
5.7 Transfer to higher level of care
Three studies with a total of 1.886 participants investigated admission to intensive care. In two studies no participants were admitted to intensive care (El Tahan 2012, Ugwu 2014). In Carbonell 2009, one out of 702 participants in the misoprostol plus oxytocin arm and two out of 698 participants in the oxytocin arm were admitted to the intensive care. The pooled risk ratio is 0.50 (95%CI 0.05 to 5.47) in favour of misoprostol plus oxytocin, though the confidence interval is wide.
5.8 Women's sense of wellbeing
Maternal wellbeing was not assessed in any of the studies.
5.9 Acceptability and satisfaction with the intervention
No studies investigated maternal satisfaction.
5.10 Breastfeeding
No studies reporting on breastfeeding.
5.11 Adverse effects
The following adverse effects were assessed: nausea, vomiting, headache, abdominal pain, shivering, fever and diarrhea.
- Nausea was assessed in seven studies with 3.798 participants. The pooled risk ratio for nausea was 2.21 (95% CI 1.19 to 4.10), favouring oxytocin only.
- Vomiting was assessed in 11 studies with 6.718 participants. The pooled risk ratio for vomiting was 2.24 (95% CI 1.52 to 3.31), favouring oxytocin only.
- Headache was assessed in two studies with 303 participants. Headache occurred in four out of 153 and in three out of 150 participants in the misoprostol plus oxytocin versus oxytocin arm, respectively (pooled risk ratio 1.26, 95% CI 0.26 to 6.23), favouring oxytocin only.
- Abdominal pain was assessed in one study with 366 participants. The pooled risk ratio for abdominal pain was 1.93 (95% CI 1.01 to 3.67), favouring oxytocin only.
- Shivering was assessed in 21 studies with 10.604 participants. The pooled risk ratio for shivering was 3.49 (95% CI 2.65 to 4.59), favouring oxytocin only.
- Fever was assessed in 18 studies with 9.643 participants. The pooled risk ratio for fever was 3.10 (95% CI 2.12 to 4.53), favouring oxytocin only.
- Diarrhea was assessed in eight studies with 6.685 participants. The pooled risk ratio for diarrhea was 1.98 (95% CI 1.01 to 3.87), favouring oxytocin only.
5.12 PTSD
No studies reported on this outcome.
Level of evidence of the literature
- Methylergometrine versus oxytocin
The level of evidence regarding the outcome measure Blood loss ≥1000mL was downgraded by three levels because of study limitations (risk of bias), number of events (imprecision) and a wide confidence interval (imprecision).
There was no evidence found for the outcome measure Maternal shock and thus no level of evidence was determined.
The level of evidence regarding the outcome measure Maternal death was downgraded by three levels because of study limitations (risk of bias), and low number of included participants (imprecision).
The level of evidence regarding the outcome measure Blood loss was downgraded by two levels because of study limitations (risk of bias) and conflicting results (inconsistency).
The level of evidence regarding the outcome measure Use of additional uterotonics was downgraded by three levels because of study limitations (risk of bias), conflicting results (inconsistency) and a wide confidence interval (imprecision).
The level of evidence regarding the outcome measure Use of blood transfusion was downgraded by three levels because of study limitations (risk of bias), conflicting results (inconsistency) and a wide confidence interval (imprecision).
There was no evidence found for the outcome measure Transfer to higher level of care and thus no level of evidence was determined.
There was no evidence found for the outcome measure Women's sense of wellbeing and thus no level of evidence was determined.
There was no evidence found for the outcome measure Acceptability and satisfaction with the intervention and thus no level of evidence was determined.
There was no evidence found for the outcome measure Breastfeeding and thus no level of evidence was determined.
The level of evidence regarding the outcome measure Adverse effects was downgraded by two levels because of study limitations (risk of bias) and conflicting results (inconsistency).
There was no evidence found for the outcome measure PTSD and thus no level of evidence was determined.
- Misoprostol versus oxytocin
The level of evidence regarding the outcome measure Blood loss ≥1000mL was downgraded.
There was no evidence found for the outcome measure Maternal shock and thus no level of evidence was determined.
The level of evidence regarding the outcome measure Maternal death was downgraded by three levels because of number of events (imprecision), number of included participants (imprecision) and a wide confidence interval (imprecision).
The level of evidence regarding the outcome measure Blood loss was downgraded by two levels because of study limitations (risk of bias) and conflicting results (inconsistency).
The level of evidence regarding the outcome measure Use of additional uterotonics was downgraded by two levels because of study limitations (risk of bias) and conflicting results (inconsistency).
The level of evidence regarding the outcome measure Use of blood transfusion was downgraded by one level because of a wide confidence interval (imprecision).
The level of evidence regarding the outcome measure Transfer to higher level of care was downgraded by three levels because of conflicting results (inconsistency), number of events (imprecision) a wide confidence interval (imprecision).
There was no evidence found for the outcome measure Women's sense of wellbeing and thus no level of evidence was determined.
The level of evidence regarding the outcome measure Acceptability and satisfaction with the intervention was downgraded by two levels because of study limitations (risk of bias) and number of participants (imprecision).
There was no evidence found for the outcome measure Breastfeeding and thus no level of evidence was determined.
The level of evidence regarding the outcome measure Adverse effects was downgraded by two levels because of study limitations (risk of bias) and grouping of outcomes (inconsistency).
There was no evidence found for the outcome measure PTSD and thus no level of evidence was determined.
- Carbetocin versus oxytocin
The level of evidence regarding the outcome measure Blood loss ≥1000mL was downgraded by two levels because of study limitations (risk of bias) and suspicion of publication bias.
There was no evidence found for the outcome measure Maternal shock and thus no level of evidence was determined.
The level of evidence regarding the outcome measure Maternal death was downgraded by three levels because of number of events (imprecision) and a very wide confidence interval (imprecision, two times).
The level of evidence regarding the outcome measure Blood loss was downgraded by two levels because of study limitations (risk of bias) and conflicting results (inconsistency).
The level of evidence regarding the outcome measure Use of additional uterotonics was downgraded by two levels because of study limitations (risk of bias) and conflicting results (inconsistency).
The level of evidence regarding the outcome measure Use of blood transfusion was downgraded by one level because of a wide confidence interval (imprecision).
The level of evidence regarding the outcome measure Transfer to higher level of care was downgraded by three levels because of number of events (imprecision) and a very wide confidence interval (imprecision, two times).
There was no evidence found for the outcome measure Women's sense of wellbeing and thus no level of evidence was determined.
There was no evidence found for the outcome measure Acceptability and satisfaction with the intervention and thus no level of evidence was determined.
The level of evidence regarding the outcome measure Breastfeeding was downgraded by two levels because of study limitations (risk of bias) and number of participants (imprecision).
The level of evidence regarding the outcome measure Adverse effects was downgraded by two levels because of study limitations (risk of bias) and grouping of outcomes (inconsistency).
There was no evidence found for the outcome measure PTSD and thus no level of evidence was determined.
- Methylergometrine plus oxytocin (=syntometrine) versus oxytocin
The level of evidence regarding the outcome measure Blood loss ≥1000mL was downgraded by one level because of a wide confidence interval (imprecision).
There was no evidence found for the outcome measure Maternal shock and thus no level of evidence was determined.
The level of evidence regarding the outcome measure Maternal death was downgraded by two levels because of study limitations (risk of bias) and number of events (imprecision).
The level of evidence regarding the outcome measure Blood loss was downgraded by three levels because of study limitations (risk of bias, two times) and a wide confidence interval (imprecision).
The level of evidence regarding the outcome measure Use of additional uterotonics was downgraded by three levels because of study limitations (risk of bias), conflicting results (inconsistency) and a wide confidence interval (imprecision).
The level of evidence regarding the outcome measure Use of blood transfusion was downgraded by two levels because of study limitations (risk of bias) and a wide confidence interval (imprecision).
The level of evidence regarding the outcome measure Transfer to higher level of care was downgraded by three levels because of study limitations (risk of bias), number of events (imprecision) and a wide confidence interval (imprecision).
There was no evidence found for the outcome measure Women's sense of wellbeing and thus no level of evidence was determined.
There was no evidence found for the outcome measure Acceptability and satisfaction with the intervention and thus no level of evidence was determined.
The level of evidence regarding the outcome measure Breastfeeding was downgraded by two levels because of study limitations (risk of bias) and number of studies (imprecision).
The level of evidence regarding the outcome measure Adverse effects was downgraded by two levels because of study limitations (risk of bias) and grouping of outcomes (inconsistency).
There was no evidence found for the outcome measure PTSD and thus no level of evidence was determined.
- Misoprostol plus oxytocin versus oxytocin
The level of evidence regarding the outcome measure Blood loss ≥1000mL was downgraded by one level because of a wide confidence interval (imprecision).
There was no evidence found for the outcome measure Maternal shock and thus no level of evidence was determined.
The level of evidence regarding the outcome measure Maternal death was downgraded by three levels because of and number of events (imprecision) and non-interpretable confidence interval (imprecision, two times).
The level of evidence regarding the outcome measure Blood loss was downgraded by one level because of conflicting results (inconsistency).
The level of evidence regarding the outcome measure Use of additional uterotonics was not downgraded.
The level of evidence regarding the outcome measure Use of blood transfusion was downgraded by two levels because of study limitations (risk of bias) and a strong suspicion of publication bias.
The level of evidence regarding the outcome measure Transfer to higher level of care was downgraded by three levels because of a very wide confidence interval (imprecision).
There was no evidence found for the outcome measure Women's sense of wellbeing and thus no level of evidence was determined.
There was no evidence found for the outcome measure Acceptability and satisfaction with the intervention and thus no level of evidence was determined.
There was no evidence found for the outcome measure Breastfeeding and thus no level of evidence was determined.
The level of evidence regarding the outcome measure Adverse effects was downgraded by two levels because of study limitations (risk of bias) and grouping of outcomes (inconsistency).
There was no evidence found for the outcome measure PTSD and thus no level of evidence was determined.
Zoeken en selecteren
A systematic review of the literature was performed to answer the following question: what is the role of uterotonics other than oxytocin to prevent postpartum haemorrhage?
| P: | Postpartum women |
| I: | Administration of methylergometrine, misoprostol or carbetocin, whether or not in combination with oxytocin |
| C: | Administration of oxytocin |
| O: | Blood loss>1000mL, blood loss, shock, maternal death, use of additional uterotonics, blood transfusion, transfer to higher level of care, women's sense of wellbeing, acceptability and satisfaction with the intervention, breastfeeding, adverse effects, PTSD |
Relevant outcome measures
The guideline development group considered blood loss >1000mL, shock, transfer to higher level of care and maternal death as a critical outcome measure for decision making; and blood loss, use of additional uterotonics, blood transfusion, women's sense of wellbeing, acceptability and satisfaction with the intervention, breastfeeding, adverse effects and PTSD as an important outcome measure for decision making.
The working group defined a 1% difference as a minimal clinically (patient) important difference for maternal death (RR<0.99 or >1.01) and a 10% difference as a minimal clinically (patient) important difference for the other critical outcomes (RR<0.9 or >1.1). For the important outcomes, a 25% difference for dichotomous outcomes (RR< 0.8 or >1.25) and 0.5 SD for continuous outcomes was taken as minimal clinically (patient) important difference.
Search and select (Methods)
The databases Medline (via OVID) and Embase (via Embase.com) were searched with relevant search terms from 1990 until December 12th, 2023. The detailed search strategy is depicted under the tab Methods. The systematic literature search resulted in 771 hits. Studies were selected based on the PICO criteria. Studies were initially selected based on title and abstract screening. After reading the full text, 33 studies were excluded (see the table with reasons for exclusion under the tab Methods), and 110 studies were selected. Of these 110 studies, one was a systematic Cochrane review, 23 RCTs were not included in the review, and the others had an observational design. Due to the large body of evidence, the observational studies were excluded. The systematic review included 196 studies of which 124 fulfilled our PICO criteria. For our analyses, we included the 124 studies from the review plus the 23 RCTs that were not included in the systematic review. Thus, our analyses are based on 147 studies.
Results
147 studies were included in the analysis of the literature. Important study characteristics and results of the 23 RCTs are summarized in the evidence tables. Characteristics of the 124 studies included in the Cochrane review can be found in the review itself (from page 128 and onwards). The assessment of the risk of bias is summarized in the risk of bias tables.
Referenties
- Aabha, Verma, Hosamani. A Comparative Study was done on Oxytocin and Carbetocin in Order to Prevent Atonic Postpartum Haemorrhages after Repeated Elective Cesarean Sections. International Journal of Pharmaceutical and Clinical Research 2023; 15(1); 434-440
- Acharya G, Al-Sammarai MT, Patel N, Al-Habib A, Kiserud T. A randomized, controlled trial comparing effect of oral misoprostol and intravenous syntocinon on intra-operative blood loss during cesarean section. Acta Obstet Gynecol Scand. 2001 Mar;80(3):245-50. doi: 10.1034/j.1600-0412.2001.080003245.x. PMID: 11207490.
- Adanikin AI, Orji EO, Adanikin PO, Olaniyan O. Comparative study of rectal misoprostol to oxytocin infusion in preventing postpartum haemorrhage post-caesarean section. International Journal of Gynecology & Obstetrics 2012;119(Suppl 3):S825.
- Adanikin AI, Orji EO, Fasubaa OB, Onwudiegwu U, Ijarotimi OA, Olaniyan O. The effect of post-cesarean rectal misoprostol on intestinal motility. Int J Gynaecol Obstet. 2012 Nov;119(2):159-62. doi: 10.1016/j.ijgo.2012.05.033. Epub 2012 Aug 25. PMID: 22925817.
- Afolabi EO, Kuti O, Orji EO, Ogunniyi SO. Oral misoprostol versus intramuscular oxytocin in the active management of the third stage of labour. Singapore Med J. 2010 Mar;51(3):207-11. PMID: 20428741.
- Ahmed WA, Ibrahim ZM, Mostafa I, Kishk EA, Elbahie MA. Safety and efficacy of carbetocin in hypertensive pregnant women undergoing cesarean delivery. Journal of Maternal-Fetal & Neonatal Medicine 2014;27(Suppl 1):49.
- Al Zubaidi S, Alhaidari T. Heat stable carbetocin vs. oxytocin for the prevention of post-partum haemorrhage in emergency caesarean delivery: a randomized controlled trial. J Perinat Med. 2021 Sep 20;50(2):150-156. doi: 10.1515/jpm-2021-0206. PMID: 34535047.
- Alwani M, Singh S, Thakur R, Mishra S. A randomized study comparing rectally administered misoprostol after spinal anesthesia versus intramuscular oxytocin for prevention of postpartum haemorrhage in caesarean section. International Journal of Reproduction, Contraception, Obstetrics and Gynecology 2014;3(3):512-5.
- Amin N. Prophylactic use of misoprostol in management of third stage of labour and prevention of atonic uterus. Journal of Postgraduate Medical Institute 2014;28(2):196-200.
- Asmat R, Ashraf T, Asmat F, Asmat S, Asmat N. Effectiveness of Per Rectal Misoprostol Versus Intramuscular Oxytocin for Prevention of Primary Postpartum haemorrhage. J Coll Physicians Surg Pak. 2017 Jan;27(1):13-17. PMID: 28292361.
- Attilakos G, Psaroudakis D, Ash J, Buchanan R, Winter C, Donald F, Hunt LP, Draycott T. Carbetocin versus oxytocin for the prevention of postpartum haemorrhage following caesarean section: the results of a double-blind randomised trial. BJOG. 2010 Jul;117(8):929-36. doi: 10.1111/j.1471-0528.2010.02585.x. Epub 2010 May 19. PMID: 20482535.
- Atukunda EC, Siedner MJ, Obua C, Mugyenyi GR, Twagirumukiza M, Agaba AG. Sublingual misoprostol versus intramuscular oxytocin for prevention of postpartum haemorrhage in Uganda: a double-blind randomized non-inferiority trial. PLoS Med. 2014 Nov 4;11(11):e1001752. doi: 10.1371/journal.pmed.1001752. PMID: 25369200; PMCID: PMC4219663.
- Badejoko OO, Ijarotimi AO, Awowole IO, Loto OM, Badejoko BO, Olaiya DS, Fatusi AO, Kuti O, Orji EO, Ogunniyi SO. Adjunctive rectal misoprostol versus oxytocin infusion for prevention of postpartum haemorrhage in women at risk: a randomized controlled trial. J Obstet Gynaecol Res. 2012 Nov;38(11):1294-301. doi: 10.1111/j.1447-0756.2012.01869.x. Epub 2012 May 21. PMID: 22612662.
- Balki M, Dhumne S, Kasodekar S, Kingdom J, Windrim R, Carvalho JC. Oxytocin-ergometrine co-administration does not reduce blood loss at caesarean delivery for labour arrest. BJOG. 2008 Apr;115(5):579-84. doi: 10.1111/j.1471-0528.2007.01658.x. PMID: 18333937.
- Baskett TF, Persad VL, Clough HJ, Young DC. Misoprostol versus oxytocin for the reduction of postpartum blood loss. Int J Gynaecol Obstet. 2007 Apr;97(1):2-5. doi: 10.1016/j.ijgo.2006.12.016. Epub 2007 Feb 26. PMID: 17321529.
- Begum T, Yeasmin S, Chakma S. Sublingual misoprostol versus oxitocin infusion to reduce blood loss in caesarean section. BJOG: an international journal of obstetrics and gynaecology 2015;122(Suppl S1):258.
- Bellad MB, Tara D, Ganachari MS, Mallapur MD, Goudar SS, Kodkany BS, Sloan NL, Derman R. Prevention of postpartum haemorrhage with sublingual misoprostol or oxytocin: a double-blind randomised controlled trial. BJOG. 2012 Jul;119(8):975-82; discussion 982-6. doi: 10.1111/j.1471-0528.2012.03341.x. PMID: 22703421.
- Benchimol M, Gondry J, Mention JE, Gagneur O, Boulanger JC. Place du misoprostol dans la direction de la délivrance [Role of misoprostol in the delivery outcome]. J Gynecol Obstet Biol Reprod (Paris). 2001 Oct;30(6):576-83. French. PMID: 11883025.
- Bhatti K, Mahar T, Hafeez R, Shoaib-u-Nisa. A randomized controlled trial on prevention of postpartum haemorrhage with sublingual misoprostol or oxytocin. Medical Forum Monthly 2014;25(1):10-2.
- Bhullar A, Carlan SJ, Hamm J, Lamberty N, White L, Richichi K. Buccal misoprostol to decrease blood loss after vaginal delivery: a randomized trial. Obstet Gynecol. 2004 Dec;104(6):1282-8. doi: 10.1097/01.AOG.0000144119.94565.18. PMID: 15572491.
- Borruto F, Treisser A, Comparetto C. Utilization of carbetocin for prevention of postpartum haemorrhage after cesarean section: a randomized clinical trial. Arch Gynecol Obstet. 2009 Nov;280(5):707-12. doi: 10.1007/s00404-009-0973-8. Epub 2009 Feb 20. PMID: 19229549.
- Boucher M, Horbay GL, Griffin P, Deschamps Y, Desjardins C, Schulz M, Wassenaar W. Double-blind, randomized comparison of the effect of carbetocin and oxytocin on intraoperative blood loss and uterine tone of patients undergoing cesarean section. J Perinatol. 1998 May-Jun;18(3):202-7. PMID: 9659650.
- Boucher M, Nimrod CA, Tawagi GF, Meeker TA, Rennicks White RE, Varin J. Comparison of carbetocin and oxytocin for the prevention of postpartum haemorrhage following vaginal delivery:a double-blind randomized trial. J Obstet Gynaecol Can. 2004 May;26(5):481-8. doi: 10.1016/s1701-2163(16)30659-4. PMID: 15151735.
- Bugalho A, Daniel A, Faúndes A, Cunha M. Misoprostol for prevention of postpartum haemorrhage. Int J Gynaecol Obstet. 2001 Apr;73(1):1-6. doi: 10.1016/s0020-7292(01)00346-0. PMID: 11336714.
- Burman, Samanta, Lata, Mukherjee, Dey. Prophylactic Administration of Per Rectal Misoprostol vs Intramuscular Injection of Oxytocin in Third-stage of Labour for Prevention of Postpartum haemorrhage: A Randomised Controlled Trial. Journal of Clinical and Diagnostic Research. 2021 Sep, Vol-15(9): QC09-QC13. doi: 10.7860/JCDR/2021/50020.15387.
- Calişkan E, Meydanli MM, Dilbaz B, Aykan B, Sönmezer M, Haberal A. Is rectal misoprostol really effective in the treatment of third stage of labor? A randomized controlled trial. Am J Obstet Gynecol. 2002 Oct;187(4):1038-45. doi: 10.1067/mob.2002.126293. PMID: 12389002.
- Caliskan E, Dilbaz B, Meydanli MM, Oztürk N, Narin MA, Haberal A. Oral misoprostol for the third stage of labor: a randomized controlled trial. Obstet Gynecol. 2003 May;101(5 Pt 1):921-8. doi: 10.1097/00006250-200305000-00017. PMID: 12738151.
- Carbonell i Esteve JL, Hernandez JM, Piloto M, Setien SA, Texido CS, Tomasi G, et al. Active management of the third phase of labour plus 400 mug of sublingual misoprostol and 200 mug of rectal misoprostol versus active management only in the prevention of post-partum haemorrhage. A randomized clinical trial [Manejo activo de la tercera fase del parto mas 400 mug de misoprostol sublingual y 200 mug de misoprostol rectal frente a manejo activo solo en la prevencion de la hemorragia posparto. Ensayo clinico aleatorizado]. Progresos de Obstetricia y Ginecologia 2009;52(10):543-51.
- Carrillo-Gaucín S, Torres-Gómez LG. Carbetocina y oxitocina: prevención de hemorragia posparto en pacientes con factores de riesgo para atonía uterina [Carbetocin and oxytocin: Prevention of postpartum haemorrhage in patients with risk factors for uterine atony]. Rev Med Inst Mex Seguro Soc. 2016;54 Suppl 3:S284-S290. Spanish. PMID: 27855051.
- Cayan F, Doruk A, Sungur MA, Dilek S. Comparison of the different dosages of rectal misoprostol on intestinal motility and pain score in high risk cesarean delivery. Turkiye Klinikleri Journal of Medical Sciences 2010;30(4):1154-9.
- Çetin Ç, Dural HR, Özcan P, Tanoğlu FB, Kütük MS, Pasin Ö, Ateş S. The efficacy of three regimes of uterotonic agents for prevention of postpartum blood loss at undergoing cesarean section: a prospective randomized clinical trial. Ginekol Pol. 2023 Aug 29. doi: 10.5603/gpl.93374. Epub ahead of print. PMID: 37642248.
- Chalermpolprapa V. EPicacy of sublingual misoprostol in prevention of postpartum haemorrhage in cesarean section: A randomized double-blinded, placebo-controlled trial. Region 4-5 Medical Journal 2010;29(3):325-35.
- Chaudhuri P, Banerjee GB, Mandal A. Rectally administered misoprostol versus intravenous oxytocin infusion during cesarean delivery to reduce intraoperative and postoperative blood loss. Int J Gynaecol Obstet. 2010 Apr;109(1):25-9. doi: 10.1016/j.ijgo.2009.11.009. Epub 2010 Jan 13. PMID: 20070961.
- Chaudhuri P, Biswas J, Mandal A. Sublingual misoprostol versus intramuscular oxytocin for prevention of postpartum haemorrhage in low-risk women. Int J Gynaecol Obstet. 2012 Feb;116(2):138-42. doi: 10.1016/j.ijgo.2011.09.016. Epub 2011 Nov 17. PMID: 22100204.
- Chaudhuri P, Majumdar A. Sublingual misoprostol as an adjunct to oxytocin during cesarean delivery in women at risk of postpartum haemorrhage. Int J Gynaecol Obstet. 2015 Jan;128(1):48-52. doi: 10.1016/j.ijgo.2014.07.029. Epub 2014 Sep 16. PMID: 25277789.
- Chaudhuri P, Majumdar A. A randomized trial of sublingual misoprostol to augment routine third-stage management among women at risk of postpartum haemorrhage. Int J Gynaecol Obstet. 2016 Feb;132(2):191-5. doi: 10.1016/j.ijgo.2015.06.064. Epub 2015 Nov 6. PMID: 26613819.
- Choy CM, Lau WC, Tam WH, Yuen PM. A randomised controlled trial of intramuscular syntometrine and intravenous oxytocin in the management of the third stage of labour. BJOG. 2002 Feb;109(2):173-7. doi: 10.1111/j.1471-0528.2002.01204.x. PMID: 11905429.
- Cook CM, Spurrett B, Murray H. A randomized clinical trial comparing oral misoprostol with synthetic oxytocin or syntometrine in the third stage of labour. Aust N Z J Obstet Gynaecol. 1999 Nov;39(4):414-9. doi: 10.1111/j.1479-828x.1999.tb03124.x. PMID: 10687755.
- Dabbaghi Gale T, Elmizadeh KH, Moradi SD, Rashvand Melli E. Comparison of intravenous oxytocin and oral misoprostol in reduction of postpartum haemorrhage. Journal of Zanjan University of Medical Sciences and Health Services 2012;20(81):1-8.
- Dansereau J, Joshi AK, Helewa ME, Doran TA, Lange IR, Luther ER, Farine D, Schulz ML, Horbay GL, Griffin P, Wassenaar W. Double-blind comparison of carbetocin versus oxytocin in prevention of uterine atony after cesarean section. Am J Obstet Gynecol. 1999 Mar;180(3 Pt 1):670-6. doi: 10.1016/s0002-9378(99)70271-1. PMID: 10076146.
- Dasuki D, Emilia O, Harini S. Randomized clinical trial: the effectiveness of oral misoprostol versus oxytocin in prevention of postpartum haemorrhage [abstract]. Journal of Obstetrics and Gynaecology Research 2002;28(1):46.
- Del Angel-Garcia G, Garcia-Contreras F, Constantino-Casas P, Nevarez-Sida A, Lopez-Gonzalez N, Garcia-Constantino M, et al. Economic evaluation of carbetocin for the prevention of uterine atony in patients with risk factors in Mexico. Value in Health 2006;9(6):A254.
- Dhananjaya BS, Charishma S. Comparative study of efficacy and safety of intramuscular oxytocin with intramuscular methylergometrine in the active management of third stage of labour. Research Journal of Pharmaceutical, Biological and Chemical Sciences 2014;5(3):734-9.
- Diallo M, Sylla T, Diouf AA, Moreira PM, Gassama O, Gueye MD, et al. Active management of third stage of labour with low doses of oral misoprostol and oxytocin on low: risk parturient in a Sub-Saharan hospital, Dakar, Sénégal. International Journal of Reproduction, Contraception, Obstetrics and Gynecology 2017;6(2):516-22.
- Diop A, Daff B, Sow M, Blum J, Diagne M, Sloan NL, Winikoff B. Oxytocin via Uniject (a prefilled single-use injection) versus oral misoprostol for prevention of postpartum haemorrhage at the community level: a cluster-randomised controlled trial. Lancet Glob Health. 2016 Jan;4(1):e37-44. doi: 10.1016/S2214-109X(15)00219-3. PMID: 26718808.
- Docherty PW, Hooper M. Choice of an oxytocic agent for routine use at delivery. Journal of Obstetrics and Gynaecology 1981;2:60.
- Dutta BK, Gupta KR. A comparative study on rectal misoprostol versus intramuscular oxytocin to prevent postpartum haemorrhage. New Indian Journal of OBGYN 2016;2(2):98-103.
- Eftekhari N, Doroodian M, Lashkarizadeh R. The effect of sublingual misoprostol versus intravenous oxytocin in reducing bleeding after caesarean section. J Obstet Gynaecol. 2009 Oct;29(7):633-6. doi: 10.1080/01443610903061744. PMID: 19757270.
- El Behery MM, El Sayed GA, El Hameed AA, Soliman BS, Abdelsalam WA, Bahaa A. Carbetocin versus oxytocin for prevention of postpartum haemorrhage in obese nulliparous women undergoing emergency cesarean delivery. J Matern Fetal Neonatal Med. 2016;29(8):1257-60. doi: 10.3109/14767058.2015.1043882. Epub 2015 May 6. PMID: 25946576.
- El Tahan MR, Warda OM, Rashad A, Yasseen AM, Ramzy EA, Ahmady MS, Diab DG, Matter MK. Effects of preoperative sublingual misoprostol on uterine tone during isoflurane anesthesia for cesarean section. Rev Bras Anestesiol. 2012 Sep-Oct;62(5):625-35. doi: 10.1016/S0034-7094(12)70162-9. PMID: 22999396.
- Elbohoty AE, Mohammed WE, Sweed M, Bahaa Eldin AM, Nabhan A, Abd-El-Maeboud KH. Randomized controlled trial comparing carbetocin, misoprostol, and oxytocin for the prevention of postpartum haemorrhage following an elective cesarean delivery. Int J Gynaecol Obstet. 2016 Sep;134(3):324-8. doi: 10.1016/j.ijgo.2016.01.025. Epub 2016 May 25. PMID: 27350226.
- Elsedeek MS. Impact of preoperative rectal misoprostol on blood loss during and after elective cesarean delivery. Int J Gynaecol Obstet. 2012 Aug;118(2):149-52. doi: 10.1016/j.ijgo.2012.03.038. Epub 2012 Jun 13. PMID: 22698700.
- Ezeama CO, Eleje GU, Ezeama NN, Igwegbe AO, Ikechebelu JI, Ugboaja JO, Ezebialu IU, Eke AC. A comparison of prophylactic intramuscular ergometrine and oxytocin for women in the third stage of labor. Int J Gynaecol Obstet. 2014 Jan;124(1):67-71. doi: 10.1016/j.ijgo.2013.07.020. Epub 2013 Oct 5. PMID: 24365208.
- Fahmy AA, Fawzy M. Oxytocin infusion after oxytocin bolus and carbetocin bolus to reduce blood loss during and after cesarean section - a randomized clinical trial. Medical Journal of Cairo University 2015;83(1):79-83.
- Fahmy NG, Yousef HM, Zaki HV. Comparative study between effect of carbetocin and oxytocin on isoflurane-induced uterine hypotonia in twin pregnancy patients undergoing cesarean section. Egyptian Journal of Anaesthesia 2016;32(1):117-21.
- Fakour F, Mirzayi M, Reza Naghipour M, Ebrahimi H, Mahdavi M. Comparison between sublingual misoprostol and intravenous oxytocin in management of third stage of labor. Iranian Journal of Obstetrics, Gynecology and Infertility 2013;15(34):7-14.
- Fazel MR, Mansoure-Samimi, Esmaeil-Fakharian. A comparison of rectal misoprostol and intravenous oxytocin on haemorrhage and homeostatic changes during cesarean section. Middle East J Anaesthesiol. 2013 Feb;22(1):41-6. PMID: 23833849.
- Fekih M, Jnifene A, Fathallah K, Ben Regaya L, Memmi A, Bouguizene S, Chaieb A, Bibi M, Khairi H. Intérêt du misoprostol dans la prévention de l'hémorragie du post-partum immédiat en cas de césarienne : essai prospectif randomisé [Benefit of misoprostol for prevention of postpartum haemorrhage in cesarean section: a randomized controlled trial]. J Gynecol Obstet Biol Reprod (Paris). 2009 Nov;38(7):588-93. French. doi: 10.1016/j.jgyn.2009.09.006. Epub 2009 Oct 14. PMID: 19833454.
- Fenix AM. Double-blind randomized controlled trial comparing the effect of carbetocin with oxytocin for the prevention of postpartum haemorrhage among high risk women following vaginal delivery. International Journal of Gynaecology & Obstetrics 2012;119(Suppl 3):S347-S348.
- Firouzbakht M, Kiapour A, Omidvar S. Prevention of post-partum haemorrhage by rectal Misoprostol: A randomized clinical trial. J Nat Sci Biol Med. 2013 Jan;4(1):134-7. doi: 10.4103/0976-9668.107277. PMID: 23633849; PMCID: PMC3633263.
- Fuks AM, Khanna P, Yusaf T, Aslian A, Kowalska D, Salafia CM. Use of prophylactic misoprostol in reduction of blood loss at vaginal delivery. Obstetrics and Gynecology 2014;123(5 Suppl):144S-5S.
- Gavilanes P, Morales MF, Velasco S, Teran E. Sublingual misoprostol is as effective as intravenous oxytocin to reduce intra-operative blood loss during cesarean delivery in women living at high altitude. J Matern Fetal Neonatal Med. 2016;29(4):559-61. doi: 10.3109/14767058.2015.1011115. Epub 2015 Feb 10. PMID: 25666740.
- Gerstenfeld TS, Wing DA. Rectal misoprostol versus intravenous oxytocin for the prevention of postpartum haemorrhage after vaginal delivery. Am J Obstet Gynecol. 2001 Oct;185(4):878-82. doi: 10.1067/mob.2001.117360. PMID: 11641670.
- Gh M, Nagbhushan T. Carbetocin vs oxytocin in caesarean section to prevent post-partum haemorrhage: A randomized controlled trial. Journal of Cardiovascular disease Research. VOL14, ISSUE 10, 2023.
- Gülmezoglu AM, Villar J, Ngoc NT, Piaggio G, Carroli G, Adetoro L, Abdel-Aleem H, Cheng L, Hofmeyr G, Lumbiganon P, Unger C, Prendiville W, Pinol A, Elbourne D, El-Refaey H, Schulz K; WHO Collaborative Group To Evaluate Misoprostol in the Management of the Third Stage of Labour. WHO multicentre randomised trial of misoprostol in the management of the third stage of labour. Lancet. 2001 Sep 1;358(9283):689-95. doi: 10.1016/s0140-6736(01)05835-4. PMID: 11551574.
- Gupta B, Jain V, Aggarwal N. Rectal misoprostol versus oxytocin in the prevention of postpartum haemorrhage - A pilot study. Int J Gynaecol Obstet. 2006 Nov;94 Suppl 2:S139-S140. doi: 10.1016/S0020-7292(06)60014-3. PMID: 29644697.
- Hamm J, Russell Z, Botha T, Carlan SJ, Richichi K. Buccal misoprostol to prevent haemorrhage at cesarean delivery: a randomized study. Am J Obstet Gynecol. 2005 May;192(5):1404-6. doi: 10.1016/j.ajog.2004.12.033. PMID: 15902121.
- Hernández-Castro F, López-Serna N, Treviño-Salinas EM, Soria-López JA, Sordia-Hernández LH, Cárdenas-Estrada E. Randomized double-blind placebo-controlled trial of buccal misoprostol to reduce the need for additional uterotonic drugs during cesarean delivery. Int J Gynaecol Obstet. 2016 Feb;132(2):184-7. doi: 10.1016/j.ijgo.2015.06.060. Epub 2015 Oct 23. PMID: 26534874.
- Hofmeyr GJ, Fawole B, Mugerwa K, Godi NP, Blignaut Q, Mangesi L, Singata M, Brady L, Blum J. Administration of 400 μg of misoprostol to augment routine active management of the third stage of labor. Int J Gynaecol Obstet. 2011 Feb;112(2):98-102. doi: 10.1016/j.ijgo.2010.08.019. Epub 2010 Dec 4. PMID: 21130990.
- Hong SC, Kim JW, Park HT, Seol HJ, Kim HJ, Kim SH, et al. Additional rectal misoprostol plus intravenous oxytocin versus intravenous oxytocin for the prevention of postpartum haemorrhage after cesarean section. American Journal of Obstetrics and Gynecology 2007;197(6 Suppl 1):S99, Abstract no: 321.
- Ibrahim ZM, Sayed Ahmed WA, Abd El-Hamid EM, Taha OT, Elbahie AM. Carbetocin versus oxytocin for prevention of postpartum haemorrhage in hypertensive women undergoing elective cesarean section. Hypertens Pregnancy. 2020 Aug;39(3):319-325. doi: 10.1080/10641955.2020.1768268. Epub 2020 May 18. PMID: 32421401.
- Jago AA, Ezechi OC, Achinge GI, Okunlola MA. Effect of oxytocics on the blood pressure of normotensive Nigerian parturients. J Matern Fetal Neonatal Med. 2007 Sep;20(9):703-5. doi: 10.1080/14767050701500406. PMID: 17701671.
- Jha A, Kaul KK, Choubey R, Keshri R, Kaushav R. Efficacy and Safety of Carbetocin versus Oxytocin for Prevention of Postpartum haemorrhage in Women Undergoing Normal Vaginal Delivery in Tertiary Care Centre of Bihar. Int. J. Pharm. Sci. Rev. Res., 80(1), May – June 2023; Article No. 14, Pages: 100-105
- Kabir N , Akter D, Daisy TA, Jesmin S, Razzak M,Tasnim S, Islam GMR. Efficacy and Safety of Carbetocin in Comparison to Oxytocin in the Active Management of Third Stage of Labour Following Vaginal Delivery: An Open Label Randomized Control Trial. Bangladesh J Obstet Gynaecol, 2015; Vol. 30(1): 3-9
- Kang S, Zhou L, Zhu L, Wang Y, Yue Y, Yan L. Carbetocin versus oxytocin for the prevention of postpartum haemorrhage after elective caesarean section in high risk women: a prospective, randomized, open-label, controlled trial in China. Clin. Exp. Obstet. Gynecol. 2022; 49(1): 023. http://doi.org/10.31083/j.ceog4901023
- Karkanis SG, Caloia D, Salenieks ME, Kingdom J, Walker M, Meffe F, Windrim R. Randomized controlled trial of rectal misoprostol versus oxytocin in third stage management. J Obstet Gynaecol Can. 2002 Feb;24(2):149-54. doi: 10.1016/s1701-2163(16)30296-1. PMID: 12196880.
- Khan GQ, John IS, Chan T, Wani S, Hughes AO, Stirrat GM. Abu Dhabi third stage trial: oxytocin versus Syntometrine in the active management of the third stage of labour. Eur J Obstet Gynecol Reprod Biol. 1995 Feb;58(2):147-51. doi: 10.1016/0028-2243(94)02004-3. PMID: 7774741.
- Koen S, Snyman LC, Pattinson RC, Makin JA. A randomised controlled trial comparing oxytocin and oxytocin + ergometrine for prevention of postpartum haemorrhage at caesarean section. S Afr Med J. 2016 Mar 7;106(4):55-6. doi: 10.7196/SAMJ.2016.v106i4.10133. PMID: 27032858.
- Kumru S, Gurates B, Parmaksiz C. Investigation of the usefulness of methyl ergonovine application in cesarean section cases [Sezaryen olgularinda metil ergonovin uygulamasinin yararliliginin arastirilmasi]. Journal of the Turkish German Gynecology Association Artemis 2005;6(1):42-5.
- Kundodyiwa TW, Majoko F, Rusakaniko S. Misoprostol versus oxytocin in the third stage of labor. Int J Gynaecol Obstet. 2001 Dec;75(3):235-41. doi: 10.1016/s0020-7292(01)00498-2. PMID: 11728483.
- Lapaire O, Schneider MC, Stotz M, Surbek DV, Holzgreve W, Hoesli IM. Oral misoprostol vs. intravenous oxytocin in reducing blood loss after emergency cesarean delivery. Int J Gynaecol Obstet. 2006 Oct;95(1):2-7. doi: 10.1016/j.ijgo.2006.05.031. Epub 2006 Aug 23. PMID: 16934269.
- Lokugamage AU, Paine M, Bassaw-Balroop K, Sullivan KR, Refaey HE, Rodeck CH. Active management of the third stage at caesarean section: a randomised controlled trial of misoprostol versus syntocinon. Aust N Z J Obstet Gynaecol. 2001 Nov;41(4):411-4. doi: 10.1111/j.1479-828x.2001.tb01319.x. PMID: 11787915.
- Liu H, Xu X-Y, Gu N, Ye X-D, Wang Z-Q, Hu Y-L, Dai Y-M. Intravenous Administration of Carbetocin Versus Oxytocin for Preventing Postpartum haemorrhage After Vaginal Delivery in High Risk Women: A Double-blind, Randomized Controlled Trial. Maternal-Fetal Medicine (2020) 2:2.
- Lumbiganon P, Hofmeyr J, Gülmezoglu AM, Pinol A, Villar J. Misoprostol dose-related shivering and pyrexia in the third stage of labour. WHO Collaborative Trial of Misoprostol in the Management of the Third Stage of Labour. Br J Obstet Gynaecol. 1999 Apr;106(4):304-8. doi: 10.1111/j.1471-0528.1999.tb08266.x. PMID: 10426235.
- Maged AM, Hassan AM, Shehata NA. Carbetocin versus oxytocin for prevention of postpartum haemorrhage after vaginal delivery in high risk women. J Matern Fetal Neonatal Med. 2016;29(4):532-6. doi: 10.3109/14767058.2015.1011121. Epub 2015 Mar 3. Update in: J Matern Fetal Neonatal Med. 2022 Dec;35(26):10707. doi: 10.1080/14767058.2022.2156874. PMID: 25731657.
- Mannaerts D, Van der Veeken L, Coppejans H, Jacquemyn Y. Adverse Effects of Carbetocin versus Oxytocin in the Prevention of Postpartum haemorrhage after Caesarean Section: A Randomized Controlled Trial. J Pregnancy. 2018 Jan 2;2018:1374150. doi: 10.1155/2018/1374150. PMID: 29484209; PMCID: PMC5816867.
- Masse N, Dexter F, Wong CA. Prophylactic Methylergonovine and Oxytocin Compared With Oxytocin Alone in Patients Undergoing Intrapartum Cesarean Birth: A Randomized Controlled Trial. Obstet Gynecol. 2022 Aug 1;140(2):181-186. doi: 10.1097/AOG.0000000000004857. Epub 2022 Jul 6. PMID: 35852267.
- McDonagh F, Carvalho JCA, Abdulla S, Cordovani D, Downey K, Ye XY, Farine D, Morais M, Balki M. Carbetocin vs. oxytocin at elective caesarean delivery: a double-blind, randomised, controlled, non-inferiority trial of low- and high-dose regimens. Anaesthesia. 2022 Aug;77(8):892-900. doi: 10.1111/anae.15714. Epub 2022 Mar 28. Erratum in: Anaesthesia. 2024 Feb;79(2):216-217. doi: 10.1111/anae.16196. PMID: 35343585.
- McDonald SJ, Prendiville WJ, Blair E. Randomised controlled trial of oxytocin alone versus oxytocin and ergometrine in active management of third stage of labour. BMJ. 1993 Nov 6;307(6913):1167-71. doi: 10.1136/bmj.307.6913.1167. Erratum in: BMJ 1993 Dec 4;307(6917):1454. PMID: 8251842; PMCID: PMC1679299.
- Mishra S, Tirkey S, Prasad A, Trivedi K. A Comparative Study of Sublingual Misoprostol Versus Intramuscular Oxytocin in the Active Management of Third Stage of Labor. Cureus. 2023 Jan 4;15(1):e33339. doi: 10.7759/cureus.33339. PMID: 36741612; PMCID: PMC9896297.
- Mitchell GG, Elbourne DR. The Salford Third Stage Trial. Oxytocin plus ergometrine versus oxytocin alone in the active management of the third stage of labor. Online J Curr Clin Trials. 1993 Aug 13;Doc No 83:[2305 words; 32 paragraphs]. PMID: 8306013.
- Modi V, Goel JK, Kashyap A, Arya SB, Kar J, Goel R. Active management of third stage of labor: A comparison of various uterotonic. Journal of South Asian Federation of Obstetrics and Gynaecology - SAFOG 2014;6(3):151-5.
- Moertl MG, Friedrich S, Kraschl J, Wadsack C, Lang U, Schlembach D. Haemodynamic effects of carbetocin and oxytocin given as intravenous bolus on women undergoing caesarean delivery: a randomised trial. BJOG. 2011 Oct;118(11):1349-56. doi: 10.1111/j.1471-0528.2011.03022.x. Epub 2011 Jun 14. Erratum in: BJOG. 2011 Nov;118(12):1549. PMID: 21668768.
- Mohamed HF, Mustafa GF, Ibrahim MA, Stefanos GE. Comparative study between intravenous bolus dose of carbetocin versus oxytocin during cesarean delivery in healthy parturients on blood loss: a randomized control trial. Medical Journal of Cairo University 2015;83(2):167-72.
- Moir DD, Amoa AB. Ergometrine or oxytocin? Blood loss and side-effects at spontaneous vertex delivery. Br J Anaesth. 1979 Feb;51(2):113-7. doi: 10.1093/bja/51.2.113. PMID: 371650.
- Moodie JE, Moir DD. Ergometrine, oxytocin and extradural analgesia. Br J Anaesth. 1976 Jun;48(6):571-4. doi: 10.1093/bja/48.6.571. PMID: 952692.
- Muhammad R, Isah A, Agida T, Akaba G. A prospective study to compare the effectiveness of adjunctive rectal misoprostol or oxytocin titration in the prevention of primary post-partum haemorrhage in at risk patients. Afr Health Sci. 2019 Mar;19(1):1517-1524. doi: 10.4314/ahs.v19i1.25. PMID: 31148979; PMCID: PMC6531961.
- Mukta M, Sahay PB. Role of Misoprostol 600 mcg Oral in Active Management of Third Stage of Labor: A Comparative Study with Oxytocin 10 IU i.m. J Obstet Gynaecol India. 2013 Oct;63(5):325-7. doi: 10.1007/s13224-012-0330-x. Epub 2013 May 3. PMID: 24431668; PMCID: PMC3798442.
- Musa AO, Ijaiya MA, Saidu R, Aboyeji AP, Jimoh AA, Adesina KT, Abdul IF. Double-blind randomized controlled trial comparing misoprostol and oxytocin for management of the third stage of labor in a Nigerian hospital. Int J Gynaecol Obstet. 2015 Jun;129(3):227-30. doi: 10.1016/j.ijgo.2015.01.008. Epub 2015 Mar 20. PMID: 25835642.
- Nahaer MK,Nurunnobi AKM,Talukder SI, Ferdousy H, Sharmin F, IslamGMR, Parvin R. Carbetocin Versus Oxytocin for Prophylaxis of PPH Used During Caesarean Section: An Open Label Randomized Control Trial. Bangladesh J Obstet Gynaecol, 2018; Vol. 33(2): 113-118
- Nankaly A, Jalilian N, Eshghiali S, Rezaei M. The effects of sublingual misoprostol and intravenous oxytocin in reducing bleeding among cesarean deliveries. Acta Medica Mediterranea 2016;32:953-7.
- Narrey N, Thakre S, Lemos CL, Tripathi S, Upadhyay R. Sublingual misoprostol versus intramuscular oxytocin for prevention of post-partum haemorrhage: a competitive study. International Journal of Toxicological and Pharmalogical Research 2023; 13(3); 73-79.
- Nasr A, Shahin AY, Elsamman AM, Zakherah MS, Shaaban OM. Rectal misoprostol versus intravenous oxytocin for prevention of postpartum haemorrhage. Int J Gynaecol Obstet. 2009 Jun;105(3):244-7. doi: 10.1016/j.ijgo.2009.01.018. Epub 2009 Feb 26. PMID: 19249048.
- Nayak L Pradhan K Mishra S. Role of 400 mcg intraoperative sublingual misoprostol for reduction of caesarean blood loss. Journal of Evidence Based Medicine and Healthcare 2017;4(10):573-7.
- Ng PS, Chan AS, Sin WK, Tang LC, Cheung KB, Yuen PM. A multicentre randomized controlled trial of oral misoprostol and i.m. syntometrine in the management of the third stage of labour. Hum Reprod. 2001 Jan;16(1):31-35. doi: 10.1093/humrep/16.1.31. PMID: 11139532.
- Nuamsiri T Kaewkiattikun K. Prevention of postpartum haemorrhage with oxytocin versus ergometrine plus oxytocin in the third stage of labor. Thai Journal of Obstetrics and Gynaecology 2016;24:97-103.
- Oboro VO, Tabowei TO. A randomised controlled trial of misoprostol versus oxytocin in the active management of the third stage of labour. J Obstet Gynaecol. 2003 Jan;23(1):13-6. doi: 10.1080/0144361021000043146. PMID: 12623474.
- Orji E, Agwu F, Loto O, Olaleye O. A randomized comparative study of prophylactic oxytocin versus ergometrine in the third stage of labor. Int J Gynaecol Obstet. 2008 May;101(2):129-32. doi: 10.1016/j.ijgo.2007.11.009. Epub 2008 Mar 4. PMID: 18164304.
- Ortiz-Gómez JR, Morillas-Ramírez F, Fornet-Ruiz I, Palacio-Abizanda FJ, Bermejo-Albares L. Estudio clínico y farmacoeconómico de la eficacia de la carbetocina en cesáreas electivas respecto a la oxitocina a dosis bajas y a dosis habituales [Clinical and pharmacological study of the efficacy of carbetocin in elective caesareans compared to low and usual doses of oxytocin]. Rev Esp Anestesiol Reanim. 2013 Jan;60(1):7-15. Spanish. doi: 10.1016/j.redar.2012.06.013. Epub 2012 Nov 1. PMID: 23122840.
- Othman ER, Fayez MF, El Aal DE, El-Dine Mohamed HS, Abbas AM, Ali MK. Sublingual misoprostol versus intravenous oxytocin in reducing bleeding during and after cesarean delivery: A randomized clinical trial. Taiwan J Obstet Gynecol. 2016 Dec;55(6):791-795. doi: 10.1016/j.tjog.2016.02.019. PMID: 28040121.
- Owonikoko KM, Arowojolu AO, Okunlola MA. Effect of sublingual misoprostol versus intravenous oxytocin on reducing blood loss at cesarean section in Nigeria: a randomized controlled trial. J Obstet Gynaecol Res. 2011 Jul;37(7):715-21. doi: 10.1111/j.1447-0756.2010.01399.x. Epub 2011 Mar 6. PMID: 21375669.
- Pakniat H, Khezri MB. The Effect of Combined Oxytocin-Misoprostol Versus Oxytocin and Misoprostol Alone in Reducing Blood Loss at Cesarean Delivery: A Prospective Randomized Double-Blind Study. J Obstet Gynaecol India. 2015 Dec;65(6):376-81. doi: 10.1007/s13224-014-0607-3. Epub 2015 Jan 24. PMID: 26663995; PMCID: PMC4666219.
- Parsons SM, Walley RL, Crane JMG, Matthews K, Hutchens D. Oral misoprostol versus oxytocin in the management of the third stage of labour. J Obstet Gynaecol Can. 2006 Jan;28(1):20-26. doi: 10.1016/S1701-2163(16)32029-1. PMID: 16533451.
- Parsons SM, Walley RL, Crane JM, Matthews K, Hutchens D. Rectal misoprostol versus oxytocin in the management of the third stage of labour. J Obstet Gynaecol Can. 2007 Sep;29(9):711-8. doi: 10.1016/s1701-2163(16)32594-4. PMID: 17825135.
- Penaranda WA, Arrieta OB, Yances BR. Active management of childbirth with sublingual misoprostol: a controlled clinical trial in the Hospital de Maternidad Rafael Calvo [Manejo active del alumbramiento con misoprostol sublingual: un studio clinico controlado en al hospital de maternidad rafael calvo de cartagena]. Revista Colombiana de Obstetricia y Ginecologia 2002;53(1):87-92.
- Perez-Rumbos A, Reyna-Villasmil E Rondon-Tapia M, Reyna-Villasmil N. Rectal misoprostol or intramuscular oxytocin in the management of the third phase of labour. Perinatología y Reproducción Humana 2017;31(2):78-84.
- Priya GP, Veena P, Chaturvedula L, Subitha L. A randomized controlled trial of sublingual misoprostol and intramuscular oxytocin for prevention of postpartum haemorrhage. Arch Gynecol Obstet. 2015 Dec;292(6):1231-7. doi: 10.1007/s00404-015-3763-5. Epub 2015 May 20. PMID: 25990482.
- Quibel T, Ghout I, Goffinet F, Salomon LJ, Fort J, Javoise S, Bussieres L, Aegerter P, Rozenberg P; Groupe de Recherche en Obstétrique et Gynécologie (GROG). Active Management of the Third Stage of Labor With a Combination of Oxytocin and Misoprostol to Prevent Postpartum haemorrhage: A Randomized Controlled Trial. Obstet Gynecol. 2016 Oct;128(4):805-11. doi: 10.1097/AOG.0000000000001626. PMID: 27607864.
- Rajaei M, Karimi S, Shahboodaghi Z, Mahboobi H, Khorgoei T, Rajaei F. Safety and efficacy of misoprostol versus oxytocin for the prevention of postpartum haemorrhage. J Pregnancy. 2014;2014:713879. doi: 10.1155/2014/713879. Epub 2014 Mar 5. PMID: 24734184; PMCID: PMC3964754.
- Rashid M, Clark A, Rashid MH. A randomised controlled trial comparing the efficacy of intramuscular syntometrine and intravenous syntocinon, in preventing postpartum haemorrhage. J Obstet Gynaecol. 2009 Jul;29(5):396-401. doi: 10.1080/01443610902946929. PMID: 19603316.
- Reyes OA. Carbetocin vs oxytocin for the prevention of postpartum haemorrhage in grand multiparous patients: A randomized controlled trial. [Spanish]. Clinica e investigacion en ginecologia y obstetricia 2011; Vol. 38, issue 1:2-7.
- Reyes OA, Gonzalez GM. Carbetocin versus oxytocin for prevention of postpartum haemorrhage in patients with severe preeclampsia: a double-blind randomized controlled trial. J Obstet Gynaecol Can. 2011 Nov;33(11):1099-1104. doi: 10.1016/S1701-2163(16)35077-0. PMID: 22082783.
- Rosseland LA, Hauge TH, Grindheim G, Stubhaug A, Langesæter E. Changes in blood pressure and cardiac output during cesarean delivery: the effects of oxytocin and carbetocin compared with placebo. Anesthesiology. 2013 Sep;119(3):541-51. doi: 10.1097/ALN.0b013e31829416dd. PMID: 23598289.
- Roy R, Vernekar M. Comparative Study on Effect of Misoprostol and Oxytocin in the Active Management of Third Stage of Labor in A Tertiary Hospital in Manipur, India. Indian Journal of Public Health Research & Development. October-December 2017, Vol. 8, No. 4. Doi: 10.5958/0976-5506.2017.00373.4
- Sadiq UG, Kwanashie O, Mairiga G, Gamaniel S, Isa H, Abdu A, et al. A randomised clinical trial comparing the efficacy of oxytocin injection and oral misoprostol tablet in the prevention of postpartum haemorrhage in Maiduguri Nigeria. International Research Journal of Pharmacy 2011;2(8):76-81.
- Shady NW, Sallam HF, Elsayed AH, Abdelkader AM, Ali SS, Alanwar A, Abbas AM. The effect of prophylactic oral tranexamic acid plus buccal misoprostol on blood loss after vaginal delivery: a randomized controlled trial. J Matern Fetal Neonatal Med. 2019 Jun;32(11):1806-1812. doi: 10.1080/14767058.2017.1418316. Epub 2017 Dec 27. PMID: 29241383.
- Shrestha A, Dongol A, Chawla CD, Adhikari RK. Rectal misoprostol versus intramuscular oxytocin for prevention of postpartum haemorrhage. Kathmandu Univ Med J (KUMJ). 2011 Jan-Mar;9(33):8-12. doi: 10.3126/kumj.v9i1.6254. PMID: 22610801.
- Sood AK, Singh S. Sublingual misoprostol to reduce blood loss at cesarean delivery. J Obstet Gynaecol India. 2012 Apr;62(2):162-7. doi: 10.1007/s13224-012-0168-2. Epub 2012 Jun 1. PMID: 23543254; PMCID: PMC3425684.
- Sudjai D, Thambumroong S. Prophylaxis post-cesarean haemorrhage using carbetocin versus oxytocin in high-risk women: a double-blind randomized controlled trial. J Med Assoc Thai 2022; 105(6):498-504.
- Sultana N, Khatun M. Misoprostol versus oxytocin in the active management of the third stage of labour. Journal of Bangladesh College of Physicians and Surgeons 2007;25(2):73-6.
- Taheripanah R, Shoman A, Karimzadeh MA, Zamaniyan M, Malih N. Efficacy of oxytocin versus carbetocin in prevention of postpartum haemorrhage after cesarean section under general anesthesia: a prospective randomized clinical trial. J Matern Fetal Neonatal Med. 2018 Nov;31(21):2807-2812. doi: 10.1080/14767058.2017.1355907. Epub 2017 Jul 25. PMID: 28707488.
- Tewatia R, Rani S, Srivastav U, Makhija B. Sublingual misoprostol versus intravenous oxytocin in prevention of post-partum haemorrhage. Arch Gynecol Obstet. 2014 Apr;289(4):739-42. doi: 10.1007/s00404-013-3026-2. Epub 2013 Sep 18. PMID: 24045979.
- Ugwu IA, Enabor OO, Adeyemi AB, Lawal OO, Oladokun A, Olayemi O. Sublingual misoprostol to decrease blood loss after caesarean delivery: a randomised controlled trial. J Obstet Gynaecol. 2014 Jul;34(5):407-11. doi: 10.3109/01443615.2014.899329. Epub 2014 Apr 11. PMID: 24724983.
- Un Nisa S, Usmani SY. Role of intravenous syntocinon in prevention of primary postpartum Haemorrhage. Pakistan Journal of Medical and Health Sciences 2012;6(4):1020-4.
- Vagge DS, Mamatha KR, Shivamurthy G, Rohatgi V. A comparative study to assess the efficacy and tolerability of per rectal misoprostol and intravenous oxytocin in prevention of primary postpartum haemorrhage in a tertiary care hospital. Journal of Chemical and Pharmaceutical Research 2014;6(3):1134-40.
- van der Nelson H, O'Brien S, Burnard S, Mayer M, Alvarez M, Knowlden J, Winter C, Dailami N, Marques E, Burden C, Siassakos D, Draycott T. Intramuscular oxytocin versus Syntometrine® versus carbetocin for prevention of primary postpartum haemorrhage after vaginal birth: a randomised double-blinded clinical trial of effectiveness, side effects and quality of life. BJOG. 2021 Jun;128(7):1236-1246. doi: 10.1111/1471-0528.16622. Epub 2021 Jan 12. PMID: 33300296.
- Vimala N, Mittal S, Kumar S. Sublingual misoprostol versus oxytocin infusion to reduce blood loss at cesarean section. Int J Gynaecol Obstet. 2006 Feb;92(2):106-10. doi: 10.1016/j.ijgo.2005.10.008. Epub 2005 Dec 15. PMID: 16343498.
- Walley RL, Wilson JB, Crane JM, Matthews K, Sawyer E, Hutchens D. A double-blind placebo controlled randomised trial of misoprostol and oxytocin in the management of the third stage of labour. BJOG. 2000 Sep;107(9):1111-5. doi: 10.1111/j.1471-0528.2000.tb11109.x. PMID: 11002954.
- Whigham CA, Gorelik A, Loughnan TE, Trivedi A. Carbetocin versus oxytocin to reduce additional uterotonic use at non-elective caesarean section: a double-blind, randomised trial (.). J Matern Fetal Neonatal Med. 2016 Dec;29(23):3866-9. doi: 10.3109/14767058.2016.1149564. Epub 2016 Mar 3. PMID: 26940081.
- Widmer M, Piaggio G, Nguyen TMH, Osoti A, Owa OO, Misra S, Coomarasamy A, Abdel-Aleem H, Mallapur AA, Qureshi Z, Lumbiganon P, Patel AB, Carroli G, Fawole B, Goudar SS, Pujar YV, Neilson J, Hofmeyr GJ, Su LL, Ferreira de Carvalho J, Pandey U, Mugerwa K, Shiragur SS, Byamugisha J, Giordano D, Gülmezoglu AM; WHO CHAMPION Trial Group. Heat-Stable Carbetocin versus Oxytocin to Prevent Haemorrhage after Vaginal Birth. N Engl J Med. 2018 Aug 23;379(8):743-752. doi: 10.1056/NEJMoa1805489. Epub 2018 Jun 27. PMID: 29949473.
- Yesmin S, Begum F, Bain S, Tareq AHMI, Parvin S, Jahan FI, Farzana H. Carbetocin versus Oxytocin in the Prevention of Postpartum haemorrhage after Caesarean Section. Bangladesh J Obstet Gynaecol, 2020; Vol. 35(2): 63-67.
- Yuen PM, Chan NS, Yim SF, Chang AM. A randomised double blind comparison of Syntometrine and Syntocinon in the management of the third stage of labour. Br J Obstet Gynaecol. 1995 May;102(5):377-80. doi: 10.1111/j.1471-0528.1995.tb11288.x. PMID: 7612530.
- Zachariah ES, Naidu M, Seshadri L. Oral misoprostol in the third stage of labor. Int J Gynaecol Obstet. 2006 Jan;92(1):23-6. doi: 10.1016/j.ijgo.2005.08.026. Epub 2005 Nov 4. PMID: 16271721.
Evidence tabellen
|
Study reference |
Study characteristics |
Patient characteristics |
Intervention (I) |
Comparison / control (C) |
Follow-up |
Outcome measures and effect size |
Comments |
|
Aabha 2023 |
Type of study: RCT.
Setting and country: hospital in India.
Funding and conflicts of interest: None. |
N total at baseline: 100 Intervention: 50 Control: 50
Delivery mode: elective cesarean A priori risk for HPP: low |
Carbetocin 100ug iv along with delivery of the anterior shoulder. |
Oxytocin 5IU iv + 20-40IU infusion along with delivery of the anterior shoulder. |
Follow-up: 48 hours
Loss-to-follow-up and incomplete outcome data: 0 |
Use of blood transfusion: Intervention: 6%, Control: 10% (p=0.461)
Use of additional uterotonics: Intervention: 20%, Control: 32% (p=0.221) |
|
|
Cetin 2023 |
Type of study: RCT.
Setting and country: university hospital in Turkey.
Funding and conflicts of interest: None |
N total at baseline: 156 Intervention: 52/52 Control: 52
Delivery mode: cesarean A priori risk for HPP: low |
I: Carbetocin 100ug iv. II: Misoprostol 400mg intra-uterine + oxytocin 20IU in 4h. |
Oxytocin iv 20IU in 4h. |
Follow-up: Not reported
Loss-to-follow-up and incomplete outcome data: 0 |
Intraoperative blood loss (mL): Carbetocin: 756, Misoprostol + oxytocin: 811, Control: 722 (p=0.840)
Use of blood transfusion: Carbetocin: 0%, Misoprostol + oxytocin: 2%, Control: 0% (p=1)
Use of additional uterotonics: Carbetocin: 11.5%, Misoprostol + oxytocin: 28.8%, Control: 21.2% (p=0.090) |
PPH defined as >1000mL blood loss within 24 hours after delivery
3-armed study |
|
Gh 2023 |
Type of study: RCT.
Setting and country: hospital in India.
Funding and conflicts of interest: None |
N total at baseline: 60 Intervention: 30 Control: 30
Delivery mode: cesarean A priori risk for HPP: low |
Carbetocin 100ug im after delivery of baby. |
Oxytocin iv 40IU in 7h after delivery of baby. |
Follow-up: Not reported
Loss-to-follow-up and incomplete outcome data: 0 |
Use of additional uterotonics: Intervention: 0%, Control: 23.5% (p<0.01) |
|
|
Jha 2023 |
Type of study: RCT.
Setting and country: tertiary hospital in Turkey.
Funding and conflicts of interest: None |
N total at baseline: 100 Intervention: 50 Control: 50
Delivery mode: vaginal A priori risk for HPP: low
|
Carbetocin 100ug im after delivery of baby. |
Oxytocin im 10IU after delivery of baby. |
Follow-up: 24 hours
Loss-to-follow-up and incomplete outcome data: 0 |
Blood loss (mL): Intervention: 361, Control: 388 (p=0.011)
Use of additional uterotonics: Intervention: 18%, Control: 24% (p=0.624)
Use of blood transfusion: Intervention: 2%, Control: 4% (p>0.99)
Adverse events: Intervention: 10%, control 10% (p>0.99) |
Open-label trial
PPH defined as >500mL blood loss |
|
Mishra 2023 |
Type of study: RCT.
Setting and country: hospital in India.
Funding and conflicts of interest: None |
N total at baseline: 400 Intervention: 200 Control: 200
Delivery mode: vaginal A priori risk for HPP: low |
Misoprostol 600ug sublingually after delivery of baby.
|
Oxytocin im 10IU after delivery of baby. |
Follow-up: 48 hours
Loss-to-follow-up and incomplete outcome data: 0 |
Blood loss (mL): Intervention: 294, Control: 226 (p<0.001)
Use of additional uterotonics: Intervention: 15%, Control: 8% (p=0.028)
|
Open-label trial |
|
Narrey 2023 |
Type of study: RCT.
Setting and country: hospital in India.
Funding and conflicts of interest: None |
N total at baseline: 100 Intervention: 50 Control: 50
Delivery mode: vaginal A priori risk for HPP: low |
Misoprostol 600ug sublingually after delivery of baby |
Oxytocin im 10IU after delivery of baby |
Follow-up: 24 hours
Loss-to-follow-up and incomplete outcome data: 0 |
Blood loss (mL): Intervention: 321, Control: 253 (p=0.11)
Use of additional uterotonics: Intervention: 8%, Control: 2% (p=0.36)
Adverse events
|
PPH defined as >500mL blood loss |
|
Snehalata 2023 |
Type of study: RCT.
Setting and country: hospital in India.
Funding and conflicts of interest: None |
N total at baseline: 120 Intervention: 60 Control: 60
Delivery mode: vaginal A priori risk for HPP: low |
Misoprostol 800ug rectally after delivery of baby |
Oxytocin im 10IU after delivery of bab |
Follow-up: 2 hours
Loss-to-follow-up and incomplete outcome data: 0 |
Blood loss (mL): Intervention: 231, Control: 220 (p=0.138)
Blood loss ≥1000mL: Intervention: 3.3%, Control: 1.7% (not significant)
Use of additional uterotonics: Intervention: 13%, Control: 10%
Use of blood transfusion: Intervention: 8.3%, Control: 6.7%
Adverse events |
PPH defined as >500mL blood loss |
|
Al Zubaidi 2022 |
Type of study: RCT.
Setting and country: hospital in Iraq.
Funding and conflicts of interest: None |
N total at baseline: 300 Intervention: 100 Control: 200
Delivery mode: emergency cesarean A priori risk for HPP: low |
Carbetocin 100ug iv after delivery of baby
|
Oxytocin 10IU iv after delivery of baby |
Follow-up: 24 hours
Loss-to-follow-up and incomplete outcome data: 0 |
Blood loss ≥1000mL: Intervention: 13%, Control: 10.5% (p=0.52)
Use of additional uterotonics: Intervention: 7%, Control: 19.5% (p=0.005)
Use of blood transfusion: Intervention: 7%, Control: 10.5% (p=0.33)
|
Non-inferiority trial |
|
Kang 2022 |
Type of study: RCT.
Setting and country: hospital in China.
Funding and conflicts of interest: None |
N total at baseline: 841 Intervention: 440 Control: 441
Delivery mode: elective cesarean A priori risk for HPP: high |
Carbetocin 100ug iv after delivery of baby
|
Oxytocin 10IU in the body of the uterus after delivery of baby + 20IU infusion in 1h |
Follow-up: 24 hours
Loss-to-follow-up and incomplete outcome data: 0 |
Blood loss (mL): Intervention: 370, Control: 387 (p=0.2)
Blood loss ≥1000mL: Intervention: 3.5%, Control: 5.2% (p=0.1)
Use of additional uterotonics: Intervention: 18.4%, Control: 24.4% (p=0.03)
Use of blood transfusion: Intervention: 0.2%, Control: 1.5% (p=0.1) |
|
|
Masse 2022 |
Type of study: RCT.
Setting and country: hospital in the USA.
Funding and conflicts of interest: None |
N total at baseline: 160 Intervention: 80 Control: 80
Delivery mode: cesarean A priori risk for HPP: low |
Methylergonovine 0,2mg 1mL im + oxytocin iv 30IU in 2h after delivery of baby |
Oxytocin iv 30IU in 2h after delivery of baby |
Follow-up: 24 hours
Loss-to-follow-up and incomplete outcome data: 0 |
Blood loss (mL): Intervention: 967, Control: 1315 (p<0.05)
Use of additional uterotonics: Intervention: 20%, Control: 55% (not significant)
Use of blood transfusion: Intervention: 5%, Control: 23% (p<0.05) |
|
|
McDonagh 2022 |
Type of study: RCT.
Setting and country: two hospitals in Canada.
Funding and conflicts of interest: None |
N total at baseline: 138 Intervention: 69 Control: 69
Delivery mode: elective cesarean A priori risk for HPP: low |
Carbetocin 100ug iv after clamping umbilical cord |
Oxytocin 5IU bolus + 20IU infusion after clamping umbilical cord
|
Follow-up: 24 hours
Loss-to-follow-up and incomplete outcome data: 0 |
Blood loss (mL): Intervention: 887, Control: 829 (p=0.09)
Blood loss ≥1000mL: Intervention: 37%, Control: 39% (p=0.86)
Use of additional uterotonics: Intervention: 3%, Control: 7% (p=0.37)
Adverse effects |
Data analysis based on per-protocol principle.
Non-inferiority trial.
4-armed study; we included arm II and IV. |
|
Ottun 2022 |
Type of study: RCT.
Setting and country: hospital in Nigeria.
Funding and conflicts of interest: None |
N total at baseline: 1038 Intervention: 519 Control: 517
Delivery mode: vaginal A priori risk for HPP: low |
Misoprostol 400ug sublingually + oxytocin 10IU im after delivery of baby |
Oxytocin 10IU im after delivery of baby
|
Follow-up: 24 hours
Loss-to-follow-up and incomplete outcome data: 0 |
Blood loss (mL): Intervention: 230, Control: 275 (p<0.001)
Use of additional uterotonics: Intervention: 10.6%, Control: 20.7% (p<0.001)
Use of blood transfusion: Intervention: 0.2%, Control: 0.4%
Adverse effects |
|
|
Sudjai 2022 |
Type of study: RCT.
Setting and country: hospital in Thailand.
Funding and conflicts of interest: None |
N total at baseline: 120 Intervention: 60 Control: 60
Delivery mode: cesarean A priori risk for HPP: high |
Carbetocin 100ug iv |
Oxytocin 20IU iv in 8h
|
Follow-up: Not reported
Loss-to-follow-up and incomplete outcome data: 0 |
Blood loss (mL): Intervention: 348, Control: 400 (p=0.159)
Use of additional uterotonics: Intervention: 20%, Control: 36.7% (p=0.040)
Adverse effects |
|
|
Yesmin 2022 |
Type of study: RCT.
Setting and country: hospital in Bangladesh.
Funding and conflicts of interest: not reported |
N total at baseline: 64 Intervention: 32 Control: 32
Delivery mode: cesarean A priori risk for HPP: low |
Carbetocin 100ug iv after delivery of baby. |
Oxytocin 10IU iv after delivery of baby. |
Follow-up: 24 hours
Loss-to-follow-up and incomplete outcome data: 0 |
Blood loss (mL): Intervention: 453, Control: 526 (p=0.121)
Blood loss ≥1000mL: Intervention: 0%, Control: 9.4% (p=0.119)
Use of additional uterotonics: Intervention: 0%, Control: 16.7% (p<0.05)
Use of blood transfusion: Intervention: 0%, Control: 10% (not significant)
|
PPH defined as >1000mL blood loss. |
|
Burman 2021 |
Type of study: RCT.
Setting and country: hospital in India.
Funding and conflicts of interest: None |
N total at baseline: 80 Intervention: 40 Control: 40
Delivery mode: vaginal A priori risk for HPP: low |
Misoprostol 600ug rectal after delivery of baby. |
Oxytocin 10IU im after delivery of baby. |
Follow-up: 24 hours (8 for blood loss)
Loss-to-follow-up and incomplete outcome data: 0 |
Blood loss (mL): Intervention: 427, Control: 425 (p=0.9165)
Adverse effects |
PPH defined as >500mL blood loss. |
|
Van der Nelson 2021 |
Type of study: RCT.
Setting and country: 6 hospitals in the UK
Funding and conflicts of interest: Yes, pharmacy sponsored. |
N total at baseline: 5717 Intervention: 1914 / 1909 Control: 1894
Delivery mode: vaginal A priori risk for HPP: low |
I: syntometrine 500ug/5IU im. II: carbotocin 100ug im . |
Oxytocin 10IU im after clamping of the umbilical cord. |
Follow-up: 2 hours
Loss-to-follow-up and incomplete outcome data: equal over arms. |
Blood loss ≥1000mL: Carbetocin: 17.3%, Syntometrine: 18.4%, Control: 18.7%
Use of additional uterotonics: Carbetocin: 19.1%, Syntometrine: 15.6%, Control: 19.5%
Use of blood transfusion: Carbetocin: 2.8%, Syntometrine: 2.7%, Control: 3.1%
Adverse effects |
3-armed study
PPH defined as >500mL blood loss
Pharmacy sponsored trial |
|
Ibrahim 2020 |
Type of study: RCT.
Setting and country: hospital in Egypt.
Funding and conflicts of interest: None |
N total at baseline: 160 Intervention: 80 Control: 80
Delivery mode: elective cesarean A priori risk for HPP: low |
Carbetocin 100ug iv after delivery of baby. |
Oxytocin 10IU iv in 8h after delivery of baby.
|
Follow-up: 24 hours
Loss-to-follow-up and incomplete outcome data: 0 |
Blood loss (mL): Intervention: 425, Control: 680 (p<0.001)
Use of additional uterotonics: Intervention: 0%, Control: 85% (p<0.001)
Use of blood transfusion: Intervention: 0%, Control: 10% ((p=0.1) |
Participants with hypertension only
PPH defined as blood loss >1000mL
|
|
Liu 2020 |
Type of study: RCT.
Setting and country: hospital in China.
Funding and conflicts of interest: None |
N total at baseline: 624 Intervention: 314 Control: 310
Delivery mode: vaginal A priori risk for HPP: high |
Carbetocin 100ug iv after delivery of anterior shoulder.
|
Oxytocin 10IU iv after delivery of anterior shoulder.
|
Follow-up: 24 hours
Loss-to-follow-up and incomplete outcome data: 0 |
Blood loss (mL): Intervention: 423, Control: 406 (p=0.40)
Blood loss ≥1000mL: Intervention: 3.2%, Control: 3.5% (p=0.83)
Use of additional uterotonics: Intervention: 23.9%, Control: 23.5% (p=0.93)
Use of blood transfusion: Intervention: 0.3%, Control: 0.6% (p=0.62) |
|
|
Nahaer 2020 |
Type of study: RCT.
Setting and country: hospital in Bangladesh.
Funding and conflicts of interest: Yes, by pharmacy. |
N total at baseline: 100 Intervention: 50 Control: 50
Delivery mode: cesarean A priori risk for HPP: low |
Carbetocin 100ug iv after cesarean. |
Oxytocin 10IU after cesarean.
|
Follow-up: 24 hours
Loss-to-follow-up and incomplete outcome data: 0 |
Blood loss, use of additional uterotonics, use of blood transfusion, adverse effects Blood loss ≥1000mL: Intervention: 0%, Control: 8% (p=0.001)
Use of additional uterotonics: Intervention: 4%, Control: 36% (p=0.001)
Use of blood transfusion: Intervention: 2%, Control: 20% (p=0.001)
Adverse effects |
PPH defined as blood loss >1000mL
Type of cesarean not stated. |
|
Muhammad 2019 |
Type of study: RCT.
Setting and country: hospital in Nigeria.
Funding and conflicts of interest: None |
N total at baseline: 110 Intervention: 54 Control: 56
Delivery mode: not reported A priori risk for HPP: high |
Misoprostol 600ug rectal after delivery of placenta + oxytocin 20IU iv in 4h. |
Oxytocin 10IU im after delivery of baby + 20IU iv in 4h. |
Follow-up: 24 hours
Loss-to-follow-up and incomplete outcome data: 0 |
Blood loss (mL): Intervention: 458, Control: 440 (p=0.712)
Blood loss ≥1000mL: Intervention: 8.9%, Control: 7.4% (p=0.789)
Use of additional uterotonics: Intervention: 12.5%, Control: 11.1% (p=0.841)
Use of blood transfusion: Intervention: 1.7%, Control: 0%
Adverse effects |
|
|
Roy 2017 |
Type of study: RCT.
Setting and country: hospital in India.
Funding and conflicts of interest: None |
N total at baseline: 200 Intervention: 100 Control: 100
Delivery mode: vaginal A priori risk for HPP: low |
Misoprostol 800ug oral after delivery of baby. |
Oxytocin 10IU im after delivery of baby.
|
Follow-up: 24 hours
Loss-to-follow-up and incomplete outcome data: 0 |
Use of additional uterotonics: Intervention: 3%, Control: 4% (p=0.700)
Use of blood transfusion: Intervention: 1%, Control: 2% (p=0.561)
|
PPH defined as blood loss >500mL
Nothing stated about blinding.
|
|
Priya 2015 |
Type of study: RCT.
Setting and country: hospital in India.
Funding and conflicts of interest: None |
N total at baseline: 500 Intervention: 250 Control: 250
Delivery mode: vaginal A priori risk for HPP: low |
Misoprostol 400ug sublingually at delivery of anterior shoulder. |
Oxytocin 10IU im at delivery of anterior shoulder. |
Follow-up: 24 hours
Loss-to-follow-up and incomplete outcome data: 0 |
No extractable data. |
Blood loss measured after delivery of placenta. |
|
Firouzbakht 2013 |
Type of study: RCT.
Setting and country: hospital in Iran.
Funding and conflicts of interest: None |
N total at baseline: 100 Intervention: 50 Control: 50
Delivery mode: vaginal A priori risk for HPP: low |
Misoprostol 400ug rectal. |
Oxytocin 20IU iv.
|
Follow-up: 12 hours
Loss-to-follow-up and incomplete outcome data: 0 |
Blood loss (mL): Intervention: 437, Control: 415 (p=0.069)
Use of additional uterotonics: Intervention: 6%, Control: 8% (p=0.523)
Adverse effects |
|
Risk of Bias tables
Risk of bias table for systematic reviews
|
Study reference |
Appropriate and clearly focused question?1 |
Comprehensive and systematic literature search?2 |
Description of included and excluded studies?3 |
Description of relevant characteristics of included studies?4 |
Assessment of scientific quality of included studies?5 |
Enough similarities between studies to make combining them reasonable?6 |
Potential risk of publication bias taken into account?7 |
Potential conflicts of interest reported?8
|
|
Gallos, 2018 |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Risk of bias table for intervention studies (studies not included in the systematic review of Gallos, 2018)
|
Study reference (first author, year) |
Was the allocation sequence adequately generated?
|
Was the allocation adequately concealed?
|
Blinding:
|
Was loss to follow-up (missing outcome data) infrequent?
|
Are reports of the study free of selective outcome reporting?
|
Was the study apparently free of other problems that could put it at a risk of bias? |
Overall risk of bias If applicable/necessary, per outcome measure
|
|
Aabha 2023 |
Definitely yes
Reason: Authors used computerized randomization. |
Probably no
Reason: No information is provided. |
Probably no
Reason: No information is provided. |
Probably no
Reason: No information is provided, but follow-up was only 48 hours. |
Unclear
Reason: outcome measures are not reported in the methods section. |
Probably no
Reason: No power calculation was performed, relatively small sample size. |
Use of blood transfusion, use of additional uterotonics Some concerns Reason: Probably no blinding.
|
|
Cetin 2023 |
Definitely yes
Reason: Authors used computerized randomization. |
Probably no
Reason: No information is provided. |
Probably no
Reason: No information is provided. |
Definitely no
Reason: no loss to follow-up. |
Probably yes
Reason: All outcome measures mentioned in the methods section are reported. |
Probably yes
Reason: No conflicts of interests or other concerning findings. |
Blood loss, use of blood transfusion, use of additional uterotonics Some concerns Reason: Probably no blinding. |
|
Gh 2023 |
Definitely no
Reason: selection of participants at subsequent order in the ratio of 1:1. |
Probably no
Reason: No information is provided. |
Probably no
Reason: No information is provided. |
Probably no
Reason: No information is provided on losses and on duration of follow-up. |
Unclear
Reason: outcome measures are not reported in the methods section. |
Probably no
Reason: Overfitting of the data (many outcomes and comparisons for only 30 participants per group). |
Blood loss, use of additional uterotonics High risk of bias Reason: Incorrect randomization and probably no blinding. |
|
Jha 2023 |
Definitely yes
Reason: Authors used web generated randomization. |
Probably no
Reason: No information is provided. |
Definitely no
Reason: Open-label trial. |
Definitely no
Reason: All randomized participants were included in the results section. |
Probably yes
Reason: All outcome measures mentioned in the methods section are reported. |
Probably no
Reason: No power calculation was performed, relatively small sample size. |
Blood loss, use of additional uterotonics, adverse events Some concerns Reason: No blinding. |
|
Mishra 2023 |
Definitely yes
Reason: Authors used computerized randomization. |
Probably no
Reason: No information is provided. |
Definitely no
Reason: Open-label trial. |
Definitely no
Reason: no loss to follow-up. |
Probably yes
Reason: All outcome measures mentioned in the methods section are reported. |
Probably yes
Reason: No conflicts of interests or other concerning findings. |
Blood loss, use of additional uterotonics Some concerns Reason: No blinding.
|
|
Narrey 2023 |
Probably yes
Reason: group determination by drawing envelopes. |
Probably no
Reason: No information is provided. |
Probably no
Reason: No information is provided. |
Definitely no
Reason: All participants were included in the results section. |
Probably yes
Reason: All outcome measures mentioned in the methods section are reported. |
Probably yes
Reason: No conflicts of interests or other concerning findings. |
Blood loss, use of additional uterotonics, adverse events Some concerns Reason: No blinding. |
|
Snehalata 2023 |
Unclear
Reason: method of randomization not provided. |
Probably no
Reason: No information is provided. |
Definitely yes
Reason: double blinding. |
Definitely no
Reason: All participants were included in the results section. |
Unclear
Reason: outcome measures are not reported in the methods section. |
Probably no
Reason: No power calculation was performed. |
Blood loss High risk of bias Reason: Randomization method unclear, method of measuring blood loss not provided
Use of blood transfusion, use of additional uterotonics, adverse events Some concerns Reason: Randomization method unclear. |
|
Al Zubaidi 2022 |
Probably no
Reason: ‘systematic random sampling’ in ratio of 2:1. |
Probably no
Reason: ‘systematic random sampling’ in ratio of 2:1. |
Definitely yes
Reason: double blinding (anesthesiologist who administered drug was not blinded). |
Definitely no
Reason: no loss to follow-up. |
Probably yes
Reason: All outcome measures mentioned in the methods section are reported. |
Probably no
Reason: Sample size was governed by the availability of the test drug. |
Blood loss, use of additional uterotonics, use of blood transfusion Some concerns Reason: Randomization method unclear. |
|
Kang 2022 |
Definitely yes
Reason: Authors used computerized randomization. |
Probably no
Reason: No information is provided. |
Definitely no
Reason: Open-label trial. |
Definitely no
Reason: All participants were included in the results section. |
Probably yes
Reason: All outcome measures mentioned in the methods section are reported. |
Probably no
Reason: No power calculation was performed. |
Blood loss, use of additional uterotonics, use of blood transfusion Some concerns Reason: No blinding. |
|
Masse 2022 |
Definitely yes
Reason: Authors used computerized randomization: 1-to-1 allocation with mixed block sizes (4 and 6). |
Definitely yes
Reason: Opaque, sealed envelopes with allocation sequence were prepared. |
Definitely yes
Reason: double blinding (anesthesiologist who administered drug was not blinded). |
Definitely no
Reason: All participants were included in the results section. |
Probably yes
Reason: All outcome measures mentioned in the methods section are reported. |
Probably yes
Reason: No conflicts of interests or other concerning findings. |
Blood loss, use of additional uterotonics, use of blood transfusion Low risk of bias Reason: no concerns. |
|
McDonagh 2022 |
Definitely yes
Reason: Authors used computer-generated block randomization with a block size of 8. |
Definitely yes
Reason: Opaque, sealed envelopes with group allocation by care provider who was not involved in the care of the patient. |
Definitely yes
Reason: double blinding (blinding of patient, anesthetist and obstetrician). |
Definitely no
Reason: no loss to follow-up. |
Probably yes
Reason: All outcome measures mentioned in the methods section are reported. |
Probably no
Reason: non-inferiority trial with data analysis based on the per-protocol principle. Blood loss calculated by a formula with hematocrit value and patients’ weight. |
Blood loss, use of additional uterotonics, adverse effects Some concerns Reason: no intention-to-treat analysis, blood loss estimation may not be accurate. |
|
Ottun 2022 |
Probably yes
Reason: Randomization based on treatment pack. |
Definitely yes
Reason: Opaque, sealed treatment packs that could only be taken consecutively prepared by pharmacist not further involved in the study. |
Unclear
Reason: Title and abstract mention double-blinding, but no information on blinding provided in the methods section. |
Definitely no
Reason: All participants that received the intervention were included in the results section. |
Probably yes
Reason: All outcome measures mentioned in the methods section are reported. |
Probably yes
Reason: No conflicts of interests, power calculation performed, intention-to-treat analysis. |
Blood loss, use of additional uterotonics, use of blood transfusion, adverse effects Some concerns Reason: Blinding unclear. |
|
Sudjai 2022 |
Definitely yes
Reason: Authors used computerized randomization with 1:1 ratio. |
Probably yes
Reason: identical drug packages labelled with study numbers. |
Unclear
Reason: Title and abstract mention double-blinding, but no information on blinding provided in the methods section. |
Definitely no
Reason: no loss to follow-up. |
Probably yes
Reason: All outcome measures mentioned in the methods section are reported. |
Probably yes
Reason: Relatively small sample size, though power calculation has been performed; may be too small to investigate adverse effects. |
Blood loss, use of additional uterotonics, adverse effects Some concerns Reason: Blinding unclear. |
|
Yesmin 2022 |
Probably yes
Reason: ‘lottery method using different colored cards in sealed envelopes’. |
Probably no
Reason: ‘lottery method using different colored cards in sealed envelopes’. |
Probably no
Reason: No information is provided. |
Definitely no
Reason: no loss to follow-up. |
Probably yes
Reason: All outcome measures mentioned in the methods section are reported. |
Probably no
Reason: Small sample size. |
Blood loss, use of additional uterotonics, use of blood transfusion Some concerns Reason: Probably no blinding, limited information about randomization process. |
|
Burman 2021 |
Definitely yes
Reason: Authors used computerized randomization. |
Probably no
Reason: No information is provided. |
Definitely yes
Reason: double blinding (blinding of participants and investigators). |
Definitely no
Reason: no loss to follow-up. |
Probably yes
Reason: All outcome measures mentioned in the methods section are reported. |
Probably yes
Reason: Relatively small sample size, though power calculation has been performed; may be too small to investigate adverse effects. |
Blood loss, adverse effects Some concerns Reason: No information about blinding of care providers. |
|
Van der Nelson 2021 |
Definitely yes
Reason: Authors used computerized randomization with a ratio of 1:1:1 and block size of nine, stratified by site. |
Definitely yes.
Reason: Blinded ampoules by snapper tops and opaque labels, with boxes labelled according to the allocation sequence. |
Definitely yes
Reason: double blinding (blinding of participants, clinical staff and researchers). |
Probably no.
Reason: Missing data seems equal over the arms and is limited. |
Probably yes
Reason: All outcome measures mentioned in the methods section are reported. |
Probably no
Reason: Pharmacy sponsored trial, modified intention-to-treat and per-protocol analysis. |
Blood loss, use of additional uterotonics, use of blood transfusion, adverse effects Some concerns Reason: Pharmacy sponsored trial. |
|
Ibrahim 2020 |
Definitely yes
Reason: Authors used computerized randomization with 1:1 ratio. |
Probably no
Reason: No information is provided. |
Definitely yes
Reason: single blinding (blinding of participants). |
Definitely no
Reason: no loss to follow-up. |
Probably yes
Reason: All outcome measures mentioned in the methods section are reported. |
Probably no
Reason: Small sample size, no power-calculation. |
Blood loss, use of additional uterotonics, use of blood transfusion Some concerns Reason: No blinding of care providers. |
|
Liu 2020 |
Definitely yes
Reason: Authors used computerized randomization in a 1:1 ratio. |
Probably no
Reason: No information is provided. |
Definitely yes
Reason: double blinding (blinding of participants, care providers and investigators). |
Definitely no
Reason: no loss to follow-up. |
Probably yes
Reason: All outcome measures mentioned in the methods section are reported. |
Probably no
Reason: Per-protocol analysis (12 patients excluded). |
Blood loss, use of additional uterotonics, use of blood transfusion, adverse effects Some concerns Reason: Per-protocol analysis. |
|
Nahaer 2020 |
Definitely yes
Reason: Authors used computerized randomization. |
Probably no
Reason: No information is provided. |
Definitely no
Reason: Open-label trial. |
Definitely no
Reason: no loss to follow-up. |
Probably yes
Reason: All outcome measures mentioned in the methods section are reported. |
Probably no
Reason: Pharmacy that prepared study drug is also co-author, relatively small sample size. |
Blood loss, use of additional uterotonics, use of blood transfusion, adverse effects Some concerns Reason: No blinding.
|
|
Muhammad 2019 |
Definitely yes
Reason: Authors used computerized randomization. |
Probably yes
Reason: ‘Sequentially numbered, opaque, sealed envelopes indicating allocation’. |
Definitely no
Reason: No blinding. |
Definitely no
Reason: no loss to follow-up. |
Probably yes
Reason: All outcome measures mentioned in the methods section are reported. |
Probably no
Reason: Per-protocol analysis (12 patients excluded). |
Blood loss, use of additional uterotonics, use of blood transfusion, adverse effects High risk of bias Reason: No blinding, per-protocol analysis. |
|
Roy 2017 |
Unclear
Reason: No information is provided. |
Probably no
Reason: No information is provided. |
Probably no
Reason: No information is provided. |
Definitely no
Reason: no loss to follow-up. |
Unclear
Reason: outcome measures are not reported in the methods section. |
Probably no
Reason: Very little information provided. |
Blood loss, use of additional uterotonics, use of blood transfusion, adverse effects High risk of bias Reason: Unclear randomization, no blinding. |
|
Priya 2015 |
Unclear
Reason: No information is provided. |
Probably no
Reason: No information is provided. |
Probably no
Reason: No information is provided. |
Definitely no
Reason: no loss to follow-up. |
Probably yes
Reason: All outcome measures mentioned in the methods section are reported. |
Probably no
Reason: Very little information provided. |
Blood loss, use of additional uterotonics, use of blood transfusion, adverse effects High risk of bias Reason: Unclear randomization, no blinding. |
|
Firouzbakht 2013 |
Unclear
Reason: No information is provided. |
Probably no
Reason: No information is provided. |
Probably no
Reason: No information is provided. |
Definitely no
Reason: no loss to follow-up. |
Probably yes
Reason: All outcome measures mentioned in the methods section are reported. |
Probably no
Reason: Very little information provided. |
Blood loss, use of additional uterotonics, use of blood transfusion, adverse effects High risk of bias Reason: Unclear randomization, no blinding. |
Table of excluded studies
|
Reference |
Reason for exclusion |
|
Gh, M. Nagbhushan, T. Carbetocin vs oxytocin in caesarean section to prevent post-partum haemorrhage: A randomized controlled trial. Journal of Cardiovascular Disease Research. 2023 |
No extractable data |
|
Cetin C, Tanoglu FB, Hanligil E, Gokce A, Pasin O, Ozcan P. Carbetocin versus Oxytocin with or without Tranexamic Acid for Prophylactic Prevention of Postpartum Hemorrhage after a Vaginal Delivery: A Randomized Clinical Trial. Gynecol Obstet Invest. 2023;88(6):366-374. doi: 10.1159/000534375. Epub 2023 Sep 30. PMID: 37778349. |
Wrong intervention/ comparison |
|
Xu R, Guo Y, Zhang Q, Zeng X. Comparison of Clinical Efficacy and Safety between Misoprostol and Oxytocin in the Prevention of Postpartum Hemorrhage: A Meta-Analysis. J Healthc Eng. 2022 Apr 11;2022:3254586. doi: 10.1155/2022/3254586. Retraction in: J Healthc Eng. 2023 Oct 4;2023:9895282. doi: 10.1155/2023/9895282. PMID: 35449871; PMCID: PMC9017444. |
Article has been retracted |
|
Huang X, Xue W, Zhou J, Zhou C, Yang F. Effect of Carbetocin on Postpartum Hemorrhage after Vaginal Delivery: A Meta-Analysis. Comput Math Methods Med. 2022 Jun 20;2022:6420738. doi: 10.1155/2022/6420738. PMID: 35770122; PMCID: PMC9236811. |
Review not the most comprehensive |
|
Kalafat E, Gokce A, O'Brien P, Benlioglu C, Koc A, Karaaslan O, Khalil A. Efficacy of carbetocin in the prevention of postpartum hemorrhage: a systematic review and Bayesian meta-analysis of randomized trials. J Matern Fetal Neonatal Med. 2021 Jul;34(14):2303-2316. doi: 10.1080/14767058.2019.1664463. Epub 2019 Sep 19. PMID: 31537134. |
Review not the most comprehensive |
|
Mirteimouri, M. Pourali, L. Akhlaghi, F. Bajgiran, R. J. Effect of sublingual Misoprostol in combination with oxytocin in reducing blood loss during and after cesarean delivery: A randomized clinical trial. Tehran University Medical Journal. 2020 |
Full text in Arabian |
|
Onwochei DN, Owolabi A, Singh PM, Monks DT. Carbetocin compared with oxytocin in non-elective Cesarean delivery: a systematic review, meta-analysis, and trial sequential analysis of randomized-controlled trials. Can J Anaesth. 2020 Nov;67(11):1524-1534. English. doi: 10.1007/s12630-020-01779-1. Epub 2020 Aug 3. PMID: 32748189. |
Review not the most comprehensive |
|
Gallos I, Williams H, Price M, Pickering K, Merriel A, Tobias A, Lissauer D, Gee H, Tunçalp Ö, Gyte G, Moorthy V, Roberts T, Deeks J, Hofmeyr J, Gülmezoglu M, Coomarasamy A. Uterotonic drugs to prevent postpartum haemorrhage: a network meta-analysis. Health Technol Assess. 2019 Feb;23(9):1-356. doi: 10.3310/hta23090. PMID: 30821683; PMCID: PMC6421507. |
Review not the most comprehensive |
|
Jin XH, Li D, Li X. Carbetocin vs oxytocin for prevention of postpartum hemorrhage after vaginal delivery: A meta-analysis. Medicine (Baltimore). 2019 Nov;98(47):e17911. doi: 10.1097/MD.0000000000017911. PMID: 31764790; PMCID: PMC6882650. |
Review not the most comprehensive |
|
Onwochei DN, Van Ross J, Singh PM, Salter A, Monks DT. Carbetocin reduces the need for additional uterotonics in elective caesarean delivery: a systematic review, meta-analysis and trial sequential analysis of randomised controlled trials. Int J Obstet Anesth. 2019 Nov;40:14-23. doi: 10.1016/j.ijoa.2019.06.007. Epub 2019 Jun 24. PMID: 31353178. |
Review not the most comprehensive |
|
Tan, Y. Liu, S. Cao, S. Wang, T. Chen, L. The efficacy and safety of carbetocin versus oxytocin on the prevention of postpartum hemorrhage for women undergoing vaginal delivery: A meta-analysis. Chinese Journal of Evidence-Based Medicine. 2018 |
Full text not available |
|
Voon HY, Suharjono HN, Shafie AA, Bujang MA. Carbetocin versus oxytocin for the prevention of postpartum hemorrhage: A meta-analysis of randomized controlled trials in cesarean deliveries. Taiwan J Obstet Gynecol. 2018 Jun;57(3):332-339. doi: 10.1016/j.tjog.2018.04.002. PMID: 29880160. |
Review not the most recent/ comprehensive |
|
Parez-Rumbos, A. Reyna-Villasmil, E. Reyna-Villasmil, N. Rondan-Tapa-a, M. Rectal misoprostol or intramuscular oxytocin in the management of the third phase of labour. Perinatologia y Reproduccion Humana. 2017 |
Full text in Spanish |
|
Jin B, Du Y, Zhang F, Zhang K, Wang L, Cui L. Carbetocin for the prevention of postpartum hemorrhage: a systematic review and meta-analysis of randomized controlled trials. J Matern Fetal Neonatal Med. 2016;29(3):400-7. doi: 10.3109/14767058.2014.1002394. Epub 2015 Sep 4. PMID: 25579116. |
Review not the most recent |
|
Maged AM, Hassan AM, Shehata NA. Carbetocin versus oxytocin in the management of atonic post partum haemorrhage (PPH) after vaginal delivery: a randomised controlled trial. Arch Gynecol Obstet. 2016 May;293(5):993-9. doi: 10.1007/s00404-015-3911-y. Epub 2015 Oct 28. PMID: 26511939. |
Wrong population |
|
Pizzagalli F, Agasse J, Marpeau L. Comparaison entre carbétocine et oxytocine en cours de césarienne dans la prévention des hémorragies du post-partum [Carbetocin versus Oxytocin during caesarean section for preventing postpartum haemorrhage]. Gynecol Obstet Fertil. 2015 May;43(5):356-60. French. doi: 10.1016/j.gyobfe.2015.03.004. Epub 2015 Apr 16. PMID: 25892107. |
Full text in French |
|
Soleimani, Z. Naini, A. A. The effectiveness of sublingual misoprostol in prevention of bleeding during cesarean delivery. Iranian Journal of Obstetrics, Gynecology and Infertility. 2014 |
Full text in Persian |
|
Chaudhuri P, Mandi S, Mazumdar A. Rectally administrated misoprostol as an alternative to intravenous oxytocin infusion for preventing post-partum hemorrhage after cesarean delivery. J Obstet Gynaecol Res. 2014 Sep;40(9):2023-30. doi: 10.1111/jog.12464. Erratum in: J Obstet Gynaecol Res. 2015 Jun;41(6):995. doi: 10.1111/jog.12761. PMID: 25181622. |
Intervention given after the third stage of labor |
|
Elgafor el Sharkwy IA. Carbetocin versus sublingual misoprostol plus oxytocin infusion for prevention of postpartum hemorrhage at cesarean section in patients with risk factors: a randomized, open trail study. Arch Gynecol Obstet. 2013 Dec;288(6):1231-6. doi: 10.1007/s00404-013-2896-7. Epub 2013 May 21. PMID: 23689739. |
Wrong comparison |
|
Hua J, Chen G, Xing F, Scott M, Li Q. Effect of misoprostol versus oxytocin during caesarean section: a systematic review and meta-analysis. BJOG. 2013 Apr;120(5):531-40. doi: 10.1111/1471-0528.12134. Epub 2013 Jan 18. PMID: 23331998. |
Review, not the most recent |
|
Al-Sawaf A, El-Mazny A, Shohayeb A. A randomised controlled trial of sublingual misoprostol and intramuscular oxytocin for prevention of postpartum haemorrhage. J Obstet Gynaecol. 2013 Apr;33(3):277-9. doi: 10.3109/01443615.2012.755503. PMID: 23550857. |
Wrong comparison |
|
Sood AK, Singh S. Sublingual misoprostol to reduce blood loss at cesarean delivery. J Obstet Gynaecol India. 2012 Apr;62(2):162-7. doi: 10.1007/s13224-012-0168-2. Epub 2012 Jun 1. PMID: 23543254; PMCID: PMC3425684. |
Wrong comparison |
|
Dabbaghi Gale, T. Elmizadeh, K. H. Moradi, S. D. Rashvand Melli, E. Comparison of intravenous oxytocin and oral misoprostol in reduction of postpartum hemorrhage. Journal of Zanjan University of Medical Sciences and Health Services. 2012 |
Full text in Persian |
|
Fawole AO, Sotiloye OS, Hunyinbo KI, Umezulike AC, Okunlola MA, Adekanle DA, Osamor J, Adeyanju O, Olowookere OO, Adekunle AO, Singata M, Mangesi L, Hofmeyr GJ. A double-blind, randomized, placebo-controlled trial of misoprostol and routine uterotonics for the prevention of postpartum hemorrhage. Int J Gynaecol Obstet. 2011 Feb;112(2):107-11. doi: 10.1016/j.ijgo.2010.08.023. Epub 2010 Dec 4. PMID: 21130446. |
Wrong comparison |
|
Triopon G, Goron A, Agenor J, Aya GA, Chaillou AL, Begler-Fonnier J, Bousquet PJ, Mares P. Utilisation de la carbétocine lors de la delivrance dirigée au cours des césariennes. Comparaison avec l'ocytocine [Use of carbetocin in prevention of uterine atony during cesarean section. Comparison with oxytocin]. Gynecol Obstet Fertil. 2010 Dec;38(12):729-34. French. doi: 10.1016/j.gyobfe.2010.10.003. PMID: 21111653. |
Full text in French |
|
Widmer M, Blum J, Hofmeyr GJ, Carroli G, Abdel-Aleem H, Lumbiganon P, Nguyen TN, Wojdyla D, Thinkhamrop J, Singata M, Mignini LE, Abdel-Aleem MA, Tran ST, Winikoff B. Misoprostol as an adjunct to standard uterotonics for treatment of post-partum haemorrhage: a multicentre, double-blind randomised trial. Lancet. 2010 May 22;375(9728):1808-13. doi: 10.1016/S0140-6736(10)60348-0. PMID: 20494730. |
Wrong population |
|
Haque N, Bilkis L, Haque N, Bari MS, Haque S. Comparative study between rectally administered misoprostol as a prophylaxis versus conventional intramuscular oxytocin in post partum hemorrhage. Mymensingh Med J. 2009 Jan;18(1 Suppl):S40-44. PMID: 19377430. |
Full text unavailable |
|
Fekih M, Jnifene A, Fathallah K, Ben Regaya L, Memmi A, Bouguizene S, Chaieb A, Bibi M, Khairi H. Intérêt du misoprostol dans la prévention de l'hémorragie du post-partum immédiat en cas de césarienne : essai prospectif randomisé [Benefit of misoprostol for prevention of postpartum hemorrhage in cesarean section: a randomized controlled trial]. J Gynecol Obstet Biol Reprod (Paris). 2009 Nov;38(7):588-93. French. doi: 10.1016/j.jgyn.2009.09.006. Epub 2009 Oct 14. PMID: 19833454. |
Full text in French |
|
Beigi, A. and Tabarestani, H. and Moini, A. and Zarrinkoub, F. and Kazempour, M. and Hadian Amree, A. Sublingual misoprostol versus intravenous oxytocin in the management of postpartum hemorrhage. Tehran University Medical Journal. 2009 |
Full text in Persian |
|
Fujimoto M, Takeuchi K, Sugimoto M, Maruo T. Prevention of postpartum hemorrhage by uterotonic agents: comparison of oxytocin and methylergometrine in the management of the third stage of labor. Acta Obstet Gynecol Scand. 2006;85(11):1310-4. doi: 10.1080/00016340600756912. PMID: 17091409. |
Wrong comparison |
|
Quiroga Díaz R, Esparza Arechiga M, Batiza Reséndiz V, Coronado López O, Hernández Ayup S, Martínez Cuervo J. Misoprostol vaginal para la prevención de la hemorragia posparto [Vaginal misoprostol in the prevention of postpartum hemorrhage]. Ginecol Obstet Mex. 2002 Nov;70:572-5. Spanish. PMID: 12561708. |
Full text in Spanish |
|
Gul, A. Zeteroglu, S. Karayel, M. Sahin, G. Kocar, M. Surucu, R. The comparison of misoprostol and oxytocin efficacy in the prevention of postpartum hemorrhage related to uterine atony. Jinekoloji ve Obstetrik Dergisi. 2000 |
Full text unavailable but probably in Turkish |
|
El-Refaey H, Nooh R, O'Brien P, Abdalla M, Geary M, Walder J, Rodeck C. The misoprostol third stage of labour study: a randomised controlled comparison between orally administered misoprostol and standard management. BJOG. 2000 Sep;107(9):1104-10. doi: 10.1111/j.1471-0528.2000.tb11108.x. PMID: 11002953. |
Wrong comparison |
Verantwoording
Beoordelingsdatum en geldigheid
Publicatiedatum : 12-12-2025
Beoordeeld op geldigheid : 12-12-2025
Algemene gegevens
De ontwikkeling/herziening van deze richtlijnmodule werd ondersteund door het Kennisinstituut van de Federatie Medisch Specialisten (www.demedischspecialist.nl/kennisinstituut) en werd gefinancierd door de Stichting Kwaliteitsgelden Medisch Specialisten (SKMS). De financier heeft geen enkele invloed gehad op de inhoud van de richtlijnmodule.
Samenstelling werkgroep
Voor het ontwikkelen van de richtlijnmodule is in 2022 een multidisciplinaire werkgroep ingesteld, bestaande uit vertegenwoordigers van alle relevante specialismen (zie hiervoor de Samenstelling van de werkgroep) die betrokken zijn bij de zorg voor patiënten met haemorrhagia postpartum.
Werkgroep
- Dr. M.(Martina) Porath (voorzitter) (NVOG)
- Dr. H.C.J. (Liesbeth) Scheepers (NVOG)
- Dr. D.D.C.A. (Dacia) Henriquez (NVOG)
- Dr. J.M. (Annemieke) Middeldorp(NVOG)
- Prof. dr. T.H. (Thomas) van den Akker (NVOG)
- Dr. P. (Paul) Ramler (NVOG) (tot September 2023)
- Dr. K.P.M. (Karin) van Galen(NIV)
- Drs. H.W. (Hannah) de Klerk (KNOV)
- Drs. L. (Lianne) Zondag (KNOV)
- Prof. dr. ir. Y.M.C. (Yvonne) Henskens (NVKC)
- Dr. I.C.M. (Ingrid) Beenakkers (NVA)
- Dr. S. (Simone) Willems (NVA)
- Mw. I. (Ilse) van Ee (PFN)
Klankboardgroep
- Drs. K. (Klaartje) Caminada (AZN)
- Mw. B. (Britt) Ketelaars (PFN)
Met ondersteuning van
- Dr. M. (Mohammadreza) Abdollahi, adviseur, Kennisinstituut van de Federatie van Medisch Specialisten
- Dr. I.M. (Irina) Mostovaya, senior adviseur, Kennisinstituut van de Federatie van Medisch Specialisten
- Dr. J. (Jana) Tuijtelaars, adviseur, Kennisinstituut van de Federatie van Medisch Specialisten
- Drs. D.A.M. (Danique) Middelhuis, adviseur, Kennisinstituut van de Federatie van Medisch Specialisten
- Dr. M. (Majke) van Bommel, adviseur, Kennisinstituut van de Federatie van Medisch Specialisten
- Dr. L. (Leanne) Küpers, adviseur, Kennisinstituut van de Federatie van Medisch Specialisten
- Drs. D.A.M. (Fieke) Pepping, junior adviseur, Kennisinstituut van de Federatie van Medisch Specialisten
- Linda Niesink, medisch informatie specialist, Kennisinstituut van de Federatie Medisch Specialisten
- Laura Boerboom, medisch informatie specialist, Kennisinstituut van de Federatie Medisch Specialisten
- Alies Oost, medisch informatie specialist, Kennisinstituut van de Federatie Medisch Specialisten
Belangenverklaringen
Een overzicht van de belangen van werkgroepleden en het oordeel over het omgaan met eventuele belangen vindt u in onderstaande tabel. De ondertekende belangenverklaringen zijn op te vragen bij het secretariaat van het Kennisinstituut van de Federatie Medisch Specialisten via secretariaat@kennisinstituut.nl.
|
Naam |
Hoofdfunctie |
Nevenwerkzaamheden |
Persoonlijke financiële belangen |
Persoonlijke relaties |
Extern gefinancierd onderzoek |
Intellectuele belangen en reputatie |
Overige belangen |
Datum |
Restrictie |
|
Martina Porath |
gynaecoloog-perinatoloog Maxima Medisch Centrum |
Voorzitter focusgroep Acute Verloskunde Noord-Brabant Lid NVOG werkgroep Otterlo |
geen |
geen |
geen |
geen |
geen |
04/28/2025 |
|
|
Liesbeth Scheepers |
perinatoloog MUMC |
Richtlijnen commissie Otterloo groep Voorzitter Regioconsortium Geboortezorg Limburg Vice voorzitter Perinatale audit Limburg |
Geen |
Nee |
ZONMW onderzoek, niet op dit onderwerp |
Nee |
Nee |
04/25/2025 |
|
|
Annemieke Middeldorp |
Gynaecoloog Perinatoloog Leids Universitair Medisch Centrum, gestopt met werken vanaf 2024 |
geen |
geen |
geen |
geen |
geen |
geen |
04/25/2025 |
|
|
Dacia Henriquez |
Gynaecoloog, Perinatoloog, Amphia Ziekenhuis |
Geen |
Geen |
Geen |
Geen |
Geen |
Geen |
04/28/2025 |
|
|
Thomas van den Akker |
Gynaecoloog perinatoloog, LUMC, hoogleraar Verloskunde |
Hoogleraar VU *Global Maternal Health, Athena Instituut. *Perined bestuurslid *RvT Wemos. *FMG Pijlervoorzitter NVOG *Vz working party for international safe motherhood and reproductive health. *Perinatale audit regiovoorzitter. Alles onbetaald. |
Geen |
Geen |
Geen |
Door werk van de commissie komt het werk van mijn voormalig promovendi Ada Gillissen en Paul Ramler meer in de aandacht staan, omdat sommige aanbevelingen hierop gebaseerd zijn (met name van laatstgenoemde ten aanzien van ballon versus embolisatie).
Het feit dat ik als bijdrager op de richtlijn sta kan gezien worden als ten goede komend aan de boegbeeldfunctie die ik bij de NVOG heb, maar het effect hiervan lijkt beperkt in het licht van mijn andere activiteiten. |
Geen |
04/25/2025 |
|
|
Paul Ramler (tot September 2023) |
AIOS Gynaecologie, cluster Leiden |
Geen |
Geen |
Geen |
Geen |
Geen |
Geen |
29/01/2023 |
|
|
Ingrid Beenakkers |
Anesthesioloog, UMCU/WKZ |
geen |
geen |
geen |
geen |
geen |
geen |
04/28/2025 |
|
|
Simone Anna Alexandra Sijm-Willems |
Anesthesioloog Radboudumc |
- |
-zs |
- |
- |
- |
- |
04/25/2025 |
|
|
Karin van Galen |
internist-hematoloog UMC Utrecht |
geen |
geen |
n.v.t. |
Octapharma: Pregnancy and inherited bleeding disorders - unresticted research grant, projectleider. |
Voorzitter Nederlands Zorgnetwerk Vrouwen met een stollingsstoornis. Tot Febr 2025 voorzitter Women and Girls with beleeding Disorders Group European Assosiation for Heamophilia and Alied Disorders - momenteel nog actief lid van deze werkgroep. |
n.v.t. |
04/25/2025 |
|
|
Yvonne Henskens |
Klinisch chemicus, waarnemend hoofd Centraal Diagnostisch Laboratorium Hoogleraar Klinische Chemie ihb Hemostase |
Voorzitter Vereniging Hematologisch Laboratoria 2020-heden Lid Vici beoordelingscie 2022 Lid raad van Advies NVKC 2023-heden Adviseur Promicol 2020-heden |
geen |
geen |
Voor alle studies in het kader van mijn leerstoel worden reagentia of apparatuur gratis of met korting verkregen, hierbij wordt geen enkele IVD methode of bedrijf uitgesloten mits het past in het doel van mijn leerstoel. Noyons stipendium NVKC (prijs) Stago / Validatie van apparatuur en/of reagentia / Ja Siemens / Validatie van apparatuur en/of reagentia / Ja Werfen / Validatie van apparatuur en/of reagentia / N Nodia / Validatie van apparatuur en/of reagentia / Nee Promicol / Validatie van apparatuur en/of reagentia / Nee |
nee |
nee |
04/25/2025 |
|
|
Hannah de Klerk |
Zelfstandig eerstelijns verloskundige (ZZP), betaald PhD aanstelling Amsterdam UMC, locatie VUMc, onbetaald |
Gastdocent HU, betaald Projectleider KNOV, betaald |
geen |
nee |
nee |
nee |
nee |
7/10/2022 |
|
|
Lianne Zondag |
Eerstelijns verloskundige - verloskundige praktijk de Toekomst - Geldermalsen KNOV - senior richtlijnonwikkelaar |
Bestuurslid Netwerk Geboortezorg Rivierenland PhD candidate Maastricht University |
Geen |
Geen |
Geen |
Geen |
Geen |
2/6/2023 |
|
|
Ilse van Ee |
Adviseur Patientenbelang - full time inbreng patientenperspectief |
Ervaringsdeskundige patientvertegenwoordiger - Eupati - fellow Psoriasispatienten Nederland - onbetaald Coordinator Patientenpraticipatie, lid centrale redactie Psoriasispatienten Nederland - onbetaald Eupati mentor - Eupati Nl - onbetaald |
nee |
nvt |
Janssen / Freedom of disease Psoriasis / ja |
nee |
nvt |
04/25/2025 |
|
|
Klaartje Caminada |
Medisch Manager Ambulancezorg, lid protocollencommissie Ambulancezorg Nederland |
geen |
geen |
geen |
geen |
geen |
geen |
04/29/2025 |
|
|
Brit Ketelaars |
Adviseur patiëntbelang bij Patiëntenfederatie Nederland (full time, betaald) |
geen |
geen |
geen |
geen |
geen |
geen |
04/25/2025 |
|
Inbreng patiëntenperspectief
De werkgroep besteedde aandacht aan het patiëntenperspectief door uitnodigen van Patiëntenfederatie Nederland, Geboortebeweging en Het Buikencollectiefvoor de invitational conference. Het verslag hiervan is besproken in de werkgroep. De verkregen input is meegenomen bij het opstellen van de uitgangsvragen, de keuze voor de uitkomstmaten en bij het opstellen van de overwegingen. De conceptrichtlijn is tevens voor commentaar voorgelegd aan Patiëntenfederatie Nederland en de eventueel aangeleverde commentaren zijn bekeken en verwerkt.
Kwalitatieve raming van mogelijke financiële gevolgen in het kader van de Wkkgz
Bij de richtlijnmodule voerde de werkgroep conform de Wet kwaliteit, klachten en geschillen zorg (Wkkgz) een kwalitatieve raming uit om te beoordelen of de aanbevelingen mogelijk leiden tot substantiële financiële gevolgen. Bij het uitvoeren van deze beoordeling is de richtlijnmodule op verschillende domeinen getoetst (zie het stroomschema bij Werkwijze).
Uit de kwalitatieve raming blijkt dat er waarschijnlijk mogelijk substantiële financiële gevolgen zijn, zie onderstaande tabel.
In Nederland bevallen per jaar ca. 160.000 vrouwen. De incidentie van haemorrhagia postpartum is 7 tot 10%. Dit zijn 11.200 tot 16.000 vrouwen per jaar. Het aantal vrouwen met risicofactoren voor HPP is niet bekend.
| Module |
Uitkomst raming |
Toelichting |
|
Andere uterotonica dan oxytocine ter preventie van HPP |
mogelijk financiële gevolgen |
Het gebruik van carbetocine is toegevoegd als alternatief voor oxytocine. Carbetocine is duurder dan oxytocine (EUR 27,13 vs 4.95) maar vergt minder materialen dan oxytocine (eenmalige toediening zonder onderhoudsdosering) en lijkt efficiënter in het voorkomen van HPP waardoor mogelijk minder vaak dure vervolgbehandelingen nodig zijn. |
Werkwijze
Voor meer details over de gebruikte richtlijnmethodologie verwijzen wij u naar de Werkwijze. Relevante informatie voor de ontwikkeling/herziening van deze richtlijnmodule is hieronder weergegeven.
Zoekverantwoording
Zoekstrategie
Embase & Ovid/Medline
|
Database |
Zoektermen |
|||||||||||||||||||||||||||||||||
|
Embase
|
|
|||||||||||||||||||||||||||||||||
|
Medline (OVID)
|
1 exp Postpartum Hemorrhage/ or 'fluxus postpartum'.ti,ab,kf. or 'postpartum hemorrhage'.ti,ab,kf. or 'post partum haemorrhage'.ti,ab,kf. or 'post partum hemorrhage'.ti,ab,kf. or 'postpartal haemorrhage'.ti,ab,kf. or 'postpartal hemorrhage'.ti,ab,kf. or 'postpartum bleeding'.ti,ab,kf. or 'postpartum haemorrhage'.ti,ab,kf. or 'puerperal haemorrhage'.ti,ab,kf. or 'puerperal hemorrhage'.ti,ab,kf. or ('blood loss' adj6 (postpartum or 'post partum' or delivery or cesarean or caesarean)).ti,ab,kf. or 'obstetric* bleeding'.ti,ab,kf. or 'obstetric* hemorrhage'.ti,ab,kf. or 'obstetric* haemorrhage'.ti,ab,kf. (14390) |