Intrathecale baclofen
Uitgangsvraag
Wat is de plaats van intrathecale toediening van baclofen bij volwassenen met cerebrale en/of spinale spasticiteit?
Aanbeveling
Start bij systemische spasticiteit (≥ 2 betrokken extremiteiten) een proefbehandeling met ITB zo vroeg mogelijk, zodra men merkt dat behandeling met orale spasmolytica, eventueel aangevuld met focale spasmolyse, onvoldoende verlichting of te veel bezwaren geeft.
Maak voor de beoordeling van het effect van de proefbehandeling onderscheid in patiënten met aanwezige of afwezige sta- en/of loopvaardigheid:
- Patiënten met sta- en/of loopvaardigheid: deze patiënten mogen niet achteruit gaan door de behandeling; het lopen en transfers kunnen maken moeten behouden blijven. Monitor actief op deze vaardigheden, naast de ervaren effecten.
- Patiënten zonder sta- en loopvaardigheid: bij deze patiënten staat comfort en toename in kwaliteit van leven centraal. Monitor primair op ervaren effecten.
Titreer na een positieve proefbehandeling en implantatie van de definitieve pomp individueel de dosering totdat een optimale balans is bereikt.
Neem contact op met expertisecentra bij twijfels over de indicatiestelling en de (proef)behandeling met ITB.
Overwegingen
Voor- en nadelen van de interventie en de kwaliteit van het bewijs
Drie studies (Creamer, 2018; McCormick, 2015; Sammaraiee, 2020) hebben het effect van intrathecale baclofen vergeleken met standaardzorg (waaronder orale spasmolytica) bij patiënten met cerebrale of spinale spasticiteit. De literatuur biedt echter geen overtuigend bewijs om een voorkeur voor een van beide opties te rechtvaardigen; de algehele bewijskracht is zeer laag. Wel bestaat er een hoge bewijskracht voor intrathecale baclofen ten opzichte van placebo.
De enige RCT die gericht was op CVA-patiënten (Creamer, 2018) had een belangrijke beperking door een waarschijnlijke verdunning van het effect: de uitkomstmaat was gebaseerd op een gemiddelde Ashworth Score van zes spiergroepen, waarvan drie spiergroepen (heupflexoren, heupextensoren en enkeldorsaalflexoren) na CVA meestal geen relevante tonusverhoging laten zien. Als inclusiecriterium in deze studie gold een Ashworth score ≥ 3 in minimaal 2 spiergroepen van het been, waarbij de kans dus groot is dat het grootste deel van de patiënten alleen last had van spasticiteit in de enkelplantairflexoren, de knie-extensoren en/of knieflexoren (en dus niet van de heupflexoren, heupextensoren en enkeldorsaalflexoren). Gegevens op spierniveau worden door de studie echter niet gerapporteerd. De effect-verdunning kan derhalve oplopen tot 50%, wat zou betekenen dat het effect van ITB op de daadwerkelijk aangedane spiergroepen tot 2 keer zo hoog zou zijn, en daarmee klinisch relevant. Bovendien was de follow-up te kort om lange termijneffecten van ITB op functionele uitkomstmaten te beoordelen.
Opvallend is dat voor de klinisch belangrijke doelgroepen – de patiënten met dwarslaesie en MS – in de literatuur in het geheel geen RCT’s beschikbaar zijn die ITB vergelijken met gangbare zorg.
Vanwege deze beperkingen is het moeilijk om de resultaten naar de praktijk te generaliseren. De dagelijkse praktijk omvat een breed scala aan patiënten met verschillende oorzaken van spasticiteit en wisselende niveaus van functioneren – en daarmee samenhangende revalidatiedoelen. De ervaring leert dat veel patiënten baat kunnen hebben bij ITB, omdat het de spiertonus verlaagt, waardoor bijvoorbeeld het zitgemak verbetert en het risico op drukplekken vermindert. Bij rolstoelafhankelijke mensen kan het rijden in de rolstoel makkelijker worden, waardoor mobiliteit en zelfstandigheid worden vergroot. Ook kan de verzorgbaarheid verbeteren, en bestaat er op de lange duur minder kans op contracturen. Tevens kan tonusvermindering pijn verminderen en de slaapkwaliteit verhogen. Bij patiënten die nog lopen kunnen dezelfde effecten optreden, waarbij de loopvaardigheid meestal behouden kan blijven. Veel lopers ervaren de benen als ‘minder zwaar’ om te verplaatsen. Sommigen leveren wat in op de loopvaardigheid, hetgeen dan vaak kan worden opgevangen door dosisverlaging. Dit is steeds een individueel proces van ‘dose-finding’, ook na de proeffase. In de praktijk blijkt dat ITB minder centrale (bijv. sufheid) of perifere bijwerkingen (bijv. gastro-intestinaal) geeft dan orale spasmolytica. Mogelijke nadelen van ITB zijn het infectierisico, problemen met de hardware van het ITB-systeem leidend tot onderdosering dan wel overdosering, en (meer zeldzaam) onder andere het optreden van darmatonie.
In de praktijk moet, ondanks het gebrek aan hard bewijs, een afweging worden gemaakt of ITB de kwaliteit van leven van de patiënt kan verbeteren. In de studie van McCormick (2015) was het opvallend dat patiënten met een geïmplanteerde pomp die regelmatig moest worden bijgevuld door middel van transcutane injecties, geen afgenomen kwaliteit van leven rapporteerden ten opzichte van patiënten die orale baclofen gebruikten (waarbij de patiënten moesten reizen voor het vullen van de pomp; dit is anders dan in Nederland waarbij het thuis kan worden gedaan). Revalidatieartsen verwijzen steeds vaker voor ITB vanwege waargenomen subjectieve verbeteringen, zowel bij ambulante als niet-ambulante patiënten. Een positieve proef met een externe pomp (altijd bij lopers) of een bolusinjectie (soms bij niet-lopers) voorafgaand aan implantatie geeft een redelijke inschatting of een ITB-pomp geschikt is.
ITB is in principe geïndiceerd bij mensen met matige tot ernstige spasticiteit van minimaal 2 ledematen, waarbij focale behandeling met BoNT-A injecties vaak te belastend of onvoldoende effectief is (door het bereiken van de maximale dosis). De beslissing om bij deze patiënten over te gaan op ITB hangt vaak samen met te veel bijwerkingen en/of onvoldoende effect van orale spasmolytica. De doelen van ITB zijn veelal gericht op symptoomreductie (o.a. spasmen, kramp, pijn) en/of verlichting van ADL problemen (o.a. verzorging, toiletgang, slapen). Daarbij is individuele bijstelling van de dosering extra belangrijk bij mensen met residuele loopvaardigheid om deze vaardigheid in stand te houden. In de praktijk betekent dit dat met de patiënt – op basis van de doelen – de therapie besproken wordt, en dat soms niet alle ongewenste symptomen voldoende met ITB kunnen worden gereduceerd. In dat geval kan (aanvullende) focale spasmolyse nog steeds geïndiceerd zijn. De werkgroep adviseert om voor de indicatiestelling ITB gebruik te maken van de laagdrempelige ondersteuning door deskundige collegae in de daarvoor aangewezen centra (zie onder) of door Care4homecare.
Waarden en voorkeuren van patiënten (en evt. hun verzorgers)
De afweging voor de patiënt om wel of niet ITB te willen is enerzijds gebaseerd op effectiviteit en mogelijke bijwerkingen (waaronder ook gewenning), zoals hierboven genoemd. Anderzijds kunnen ook logistieke factoren een rol spelen. In het verleden moesten patiënten met een ITB-pomp elke 3 maanden naar het ziekenhuis voor navulling en revisie. Inmiddels is door zorgconcentratie gepersonaliseerde zorg mogelijk, aan huis en op de werkplek, waarbij de pomp thuis kan worden gevuld; als nodig eens per 3 maanden, of juist in enkele individuele gevallen elke maand. Deze navulling is niet hinderlijk of pijnlijk, dit is een enkele prik in de pomp door de huid.
ITB kan ook de belasting verminderen voor familie, verzorgers en/of mantelzorgers. Dit komt door het gerichtere effect van ITB op de spinale reflexen in vergelijking met orale medicatie, waardoor de ernst van de spasticiteit minder wordt, de patiënt zich comfortabeler voelt, en dus beter verzorgbaar en mobiliseerbaar is. De zorg voor de pomp wordt door verzorgenden aan huis geregeld, die deze on site kunnen instellen (een bezoek aan het ziekenhuis voor aanpassingen is dus niet meer nodig). Meer informatie over thuiszorg bij ITB is ook online te vinden (https://care4homecare.nl/itb-therapie).
Kosten (middelenbeslag)
Een ITB-pomp is kostenintensief, omdat het gaat om geïndividualiseerde zorg. In vergelijking met ‘reguliere’ zorg is ITB duurder dan orale baclofen, maar ITB is geïndiceerd zodra orale therapie faalt of te veel bijwerkingen geeft. In Nederland hebben rond de duizend patiënten een ITB-pomp, dus op maatschappelijk niveau zijn de kosten voor een pomp acceptabel, wanneer uitgezet tegen de winst die het geeft in kwaliteit van leven, en in sociale en maatschappelijke participatie van patiënten die anders niet zouden kunnen mobiliseren of werken.
In de Amerikaanse literatuur is de kosteneffectiviteit van de ITB-pomp onderzocht, en wordt geconcludeerd dat er in het eerste jaar hoge kosten aan verbonden zijn, maar dat vervolgens een afname in kosten wordt gezien. Na ongeveer 3 jaar is de behandeling kosteneffectief (Venkatraman, 2023). In de Spaanse literatuur wordt een incremental cost effectiveness ratio (ICER) van 30.000/QALY berekend, wat voor veel Westerse landen een acceptabel bedrag is (Vidal, 2017).
Aanvaardbaarheid, haalbaarheid en implementatie
Tegenwoordig is het gebruik van ITB steeds aanvaardbaarder. Recente studies tonen aan dat ITB – naast de doelgroepen dwarslaesie en MS – ook goed bij patiënten met niet-aangeboren hersenaandoeningen kan worden gegeven als de proefbehandeling positief is gebleken (McCormick, 2015; Creamer, 2018).
Twee zaken zitten de verdere implementatie van ITB echter in de weg: enerzijds de onbekendheid met de behandeling zelf, en anderzijds het argument van de toegenomen technologische transformatie van de zorg. Voor twijfels of onduidelijkheden rondom de indicatiestelling voor ITB zijn er expertisecentra in Nederland laagdrempelig bereikbaar: Erasmus MC, Radboud UMC, Medisch Spectrum Twente, Elisabeth-Tweesteden Ziekenhuis, Maastricht UMC+ en Amsterdam UMC. De centra in Amsterdam en Maastricht behandelen ook kinderen met ernstige spasticiteit. Het tweede punt is dat er soms bij behandelaren een angst heerst voor de techniek zelf; de onbekendheid met het ITB-systeem. Bekende vragen zijn bijvoorbeeld wat er moet gebeuren als de pomp ineens begint te piepen. Voor dit soort technische vragen zijn er specialistische teams bereikbaar, die ook familie en/of mantelzorgers ontzorgen met het gebruik van de pomp (bijv. de ambulante kliniek "Care4homecare")
Om zorg in de toekomst nog beter te kunnen leveren voor deze patiënten, is het nodig om het effect van ITB beter te evalueren, en verdere bewijslast te verzamelen. Hiervoor zijn o.a. gevalideerde vragenlijsten en/of PROMS nodig die zich vooral richten op de ervaring van patiënten en hun mantelzorgers (de Ashworth score heeft inherente tekortkomingen, deze meet alleen spierweerstand bij passieve rek en neemt geen individueel functioneren mee). Ook een kwaliteitsregistratie voor ITB/neuromodulatie (bijvoorbeeld via DICA) kan inzicht geven in de individuele vooruitgang van patiënten met ITB.
Rationale van de aanbeveling: weging van argumenten voor en tegen de interventies
Uit de literatuur komt geen hard bewijs naar voren voor de meerwaarde van ITB ten opzichte van reguliere zorg. Echter, de kwaliteit van de studies is beperkt, en ook de onderzoekspopulatie is niet goed generaliseerbaar naar de ‘echte’ patiëntenpopulatie in Nederland. In de praktijk worden veel positieve ervaringen gezien met ITB, die echter wel verschillend zijn afhankelijk van de revalidatiedoeleinden van de patiënt. Familieleden of verzorgers kunnen door een pomp worden ontzorgd door de gepersonaliseerde zorg die beschikbaar is. De kosten zijn op maatschappelijk niveau acceptabel. Onderstaande aanbevelingen gelden voor revalidatieartsen, artsen VG, specialisten ouderengeneeskunde, en geriaters.
Onderbouwing
Achtergrond
Gamma-aminobutyric acid (GABA) serves as the principal inhibitory neurotransmitter within the central nervous system. Baclofen is a potent and selective GABAB-receptor agonist that results in presynaptic suppression of hyperreflexia and is therefore used as a treatment for spasticity. When baclofen is administered orally, relatively low concentrations (12-96 μg/ml) are reached in the cerebrospinal fluid of the spinal cord, due to the blood-brain barrier. Therefore, this often renders an inadequate treatment effect for moderate to severe spasticity. In contrast to oral administration, intrathecal administration of baclofen (ITB) reaches higher concentrations in the spinal cord and can therefore be administered in lower dosage to yield improved spasticity reduction with fewer side effects. Furthermore, continuous infusion via an implanted pump system has been shown to induce significant alterations in serotonergic and dopaminergic activity, which are not observed with oral administration. While ITB produces similar side effects, they occur far less frequently due to the significantly lower dosage (over 100 times lower). The use of ITB in patients with spasticity was first described in 1984; however, its comparative effectiveness against conventional treatment in therapy-resistant spasticity remains a subject of ongoing research.
Conclusies / Summary of Findings
Effect on spasticity symptoms
|
Low GRADE |
The evidence suggests a small effect, statistically significant but not reaching clinical relevance, on muscle tone or spasticity in favour of intrathecal baclofen (ITB) versus usual care, in patients with cerebral or spinal spasticity.
Sources: (Creamer, 2018; McCormick, 2015) |
|
No GRADE |
Clonus and cramps were not reported in the included studies, and these outcomes could not be graded.
|
|
Very low GRADE |
The evidence is very uncertain about the effect on pain of intrathecal baclofen (ITB) compared to usual care, in patients with cerebral or spinal spasticity.
Sources: (Creamer, 2018; McCormick, 2015) |
Effects on daily tasks and activities
|
Very low GRADE |
The evidence is very uncertain about the potential higher chance of preserving walking ability over time for patients with cerebral or spinal spasticity with ITB treatment when compared to patients who did not receive ITB.
The evidence is very uncertain about the effect of intrathecal baclofen (ITB) on activities of daily living, compared to usual care in patients with cerebral or spinal spasticity.
Sources: (Creamer, 2018; Sammaraiee, 2020) |
|
No GRADE |
Balance and falling were not reported in the included studies, and these outcomes could not be graded. |
3. Adverse events
|
Very low GRADE |
The evidence is very uncertain about potential increased occurrences of adverse events after intrathecal baclofen (ITB), compared to usual care in patients with cerebral or spinal spasticity.
Source: (Creamer, 2018) |
Samenvatting literatuur
Description of studies
Intrathecal baclofen vs. usual care
Of the three newly included studies in the analysis, there was one RCT, one cross-sectional study and one observational cohort.
The multicenter RCT by Creamer (2018; results published in two different publications) studied the efficacy of intrathecal baclofen (ITB) as compared to conventional medical management (CMM). The study had a parallel, open label design, and they included adult patients with spasticity due to stroke, who could not reach their therapy goals with other treatment interventions. The study was conducted in rehabilitation hospitals at eleven European centers (Austria, Belgium, Germany, Italy, the Netherlands, Spain, UK, Slovenia) and seven centers in the United States. Additional inclusion criteria were a poststroke duration >6 months, spasticity in at least two extremities and an Ashworth Scale (AS) score ≥3 in a minimum of two muscle groups of the lower extremities (LE) on the affected body side. Patients were randomized to a CMM group (n = 29) and ITB group (n = 31) group, Patients in the CMM group received a combination of oral antispastic medication (at least one of oral baclofen, tizanidine, diazepam or other benzodiazepines, or dantrolene) and physiotherapy. Patient in the ITB group would first have an ITB trial to evaluate drug response before receiving the implant. Patients were evaluated at baseline, at week 6, month 3 and finally after 6 months.
The cross-sectional matched cohort survey study by McCormick (2015) compared spasticity levels and pain between individuals receiving treatment with intrathecal (n =31) versus oral baclofen (n = 31). The patient population existed of adult spasticity patients (quadriplegia or paraplegia, cerebral palsy, MS or stroke) who had been treated with intrathecal or oral baclofen for at least 1 year before recruitment, and patients in the intervention and control group were matched 1:1 for age, gender and diagnosis. Dose was dependent on physician, and dose-information was collected for baseline and at 1, 2, and 3 years before time of the survey, so that long-term baclofen dose stability could be determined by calculating the change in dose over time.
The prospective cohort by Sammaraiee (2020) evaluated the impact of ITB on walking ability in people with moderate to severe MS-related spasticity. The study included ambulatory patients with a score of seven or lower on the Expanded Disability Status Scale (EDSS) and patients who had recently stopped walking due to spasticity with an EDSS of 7.5. All subjects were submitted to a trial of intrathecal baclofen as a bolus dose via lumbar puncture. Trial doses ranged from 12.5– 50 mcg. Those who reported subjective benefit, achieved goals, and demonstrated an objective response, proceeded with pump implantation during the same admission or shortly thereafter. In those who failed to respond to the initial trial, subsequent trials were performed at a higher dose up to a maximum of 75 mcg. If initial trials failed, patients were elected not to proceed. These subjects were treated with care-as-usual (antispasmodic agents) and acted as controls in the analysis. In the end, 20 patients proceeded to pump implantation, and 10 patients acted as controls.
Results
Intrathecal baclofen versus usual care
The results below are based on newly collected studies via the current literature search, and regard the comparison of ITB with usual care, as described by the PICO.
1. Effects on spasticity symptoms
1a. Muscle tone/spasticity
One study (Creamer, 2018) reported the Ashworth score for ITB compared to usual care for the upper and lower extremity separately. The mean group difference in change from baseline to month 6 for the lower extremity and upper extremity was -0.56 (95% CI -0.97 to -0.15) and -0.49 (95% CI -0.86 to -0.13), respectively, in favor of the ITB group (both not clinically relevant).
1b. Clonus
None of the included studies reported on clonus.
1c. Cramps and pain
Pain was reported by Creamer (2018) and McCormick (2015) using the Numeric (Pain) Rating Scale (NRS) or the Brief Pain Inventory (BPI), respectively. Both the NRS and the BPI contain self-assessment scales for pain intensity for average, least and worst pain during the last 24 hours, ranging from 0 (no pain) to 10 (worst possible pain). A change of 1.7 has been found a minimal clinically important difference among patients with chronic pain. The mean group differences in change of pain scores between ITB and usual care are shown in table 2. Creamer (2018) found a clinically relevant and significant difference of -1.85 for least pain, in favor of ITB. This was not found by McCormick (2015), who instead found a clinically relevant and significant difference for worst pain, with a mean difference of -2.4, also in favor of the ITB group.
Table 2. Mean difference for pain in 3 different domains: Average pain, Least pain, and Worst pain for different studies
|
|
Study |
|||||
|
Creamer, 2018 (NRS, 0-10) |
McCormick, 2015 (BPI, 0-10) |
|||||
|
Pain domain |
ITB group, ∆ 3 mo- baseline (n =23) |
Usual care group ∆ 3 mo- baseline (n= 24) |
Mean difference (95% CI) |
ITB group (n= 31) |
Usual care group (n = 31) |
Mean difference (95% CI) |
|
Average pain |
-1.2 |
0.0 |
-1.17 (-3.02 to 0.68) |
3.8 |
5.4 |
-1.63 (-3.01 to -0.25) |
|
Least pain |
-1.6 |
+0.2 |
-1.85 (-3.39 to -0.31) |
2.2 |
3.1 |
-0.89 (-2.09 to 0.31) |
|
Worst pain |
-1.4 |
-0.04 |
-1.31 (-3.09 to 0.47) |
4.2 |
6.6 |
-2.37 (-3.94 to -0.80) |
2. Effects on motor skills and abilities
2a. Balance and ambulation
Sammaraiee (2020) reported on preservation of ambulation (walking ability) by assessing the Expanded Disability Status Scale (EDSS) and using a cut-off of ≤ 7 (EDSS 7; unable to walk 20m even with aid, essentially restricted to wheelchair; wheels self and transfers alone; up and about in wheelchair some 12 h a day). Risk Ratios for follow up of 1 year was calculated based on number of events per group and resulted in a risk ratio of 2.0 (95% CI 0.91 to 4.41) in favor of ITB. This was a clinically relevant difference, showing that the patients who received ITB have a twice as high chance of preserving walking ability over time, when compared to patients who did not receive ITB.
2b. Activities of daily living
Creamer (2018) reported on activities in daily living via the Functional Independence measure (FIM). The FIM is often considered the golden standard for assessing basic activities in daily living (ADL). The total score ranges from 18 (totally dependent) to 126 (totally independent). The minimal clinically important difference as established by the Spinal Cord Injury Research Evidence (SCIRE) Project is a difference of 22 points. The mean group difference in change in FIM Total from baseline to month 6 between ITB and control was 5.26 (95% CI -0.59 to 11.11) in favor of ITB. This was not a clinically relevant difference.
3. Adverse events and side effects
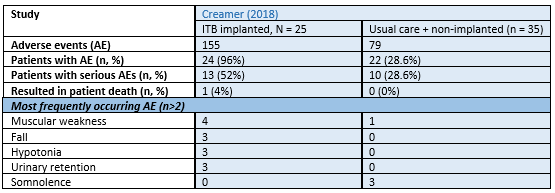
Adverse events were reported by Creamer (2018). The total number of adverse events (AEs), proportion of patients experiencing AEs, serious AEs and AEs resulting in patient death per respective arm are presented in Table 3. None of the included studies reported on falling.
Table 3. Number of adverse events as reported by Creamer (2018)
Level of evidence of the literature
1. Effects on spasticity symptoms
The level of evidence regarding the spasticity symptoms was downgraded as follows:
- 1a. Muscle tonus/spasticity: For spasticity, the level of evidence was downgraded by 2 levels because it was based on a single study with a low number of included patients (-1, imprecision) and risk of bias due to sponsoring by- and conflicts of interest related to ITB-pump fabricant (-1, publication bias).
- 1b. Clonus: No assessment of the level of evidence regarding the outcome clonus was performed.
- 1c. Cramps and pain: The level of evidence regarding pain was downgraded by 4 levels because of study limitations, including (risk of) selection bias, confounding, and information bias (-2, Risk of Bias); estimate confidence intervals crossing a border of clinical relevance (-1, imprecision) and risk of bias due to sponsoring by- and conflicts of interest related to ITB-pump fabricant (-1, publication bias).
2. Effects on daily tasks and activities
The level of evidence regarding the effects on daily tasks and activities was downgraded as follows:
- 2a. Balance and ambulation: The level of evidence for balance and ambulation was downgraded by 4 levels, because of study limitations including high risk of selection bias, residual confounding, and information bias (-2, Risk of Bias); small study population, and estimate confidence intervals crossing a border of clinical relevance (-2, imprecision).
- 2b. Activities of daily living: The level of evidence started for ADL was downgraded by 3 levels because of study limitations such as information bias (-1, risk of bias), small study population (-1, imprecision); and risk of bias due to sponsoring by- and conflicts of interest related to ITB-pump fabricant (-1, publication bias).
3. Adverse events and side effects
The level of evidence regarding the adverse events was downgraded by 4 levels, due to study limitations such as information bias and selection bias due to stringent inclusion criteria (-2, Risk of bias); number of included patients and studies (-1, imprecision); and risk of bias due to sponsoring by- and conflicts of interest related to ITB-pump fabricant (-1, publication bias).
Zoeken en selecteren
A systematic review of the literature was performed to answer the following question:
What is the effectiveness of intrathecal baclofen as compared to usual care in adults with cerebral or spinal spasticity?
| P: | Adults with cerebral or spinal spasticity |
| I: | Intrathecal antispasmodic baclofen |
| C: | Usual care, including continued oral spasmolytics |
| O: | Effects on body functions and structures (muscle tone, spasticity, clonus, pain, and muscle cramps), effects on motor skills and abilities, adverse events or side effects (allergic reactions, infection, somnolence, withdrawal symptoms) |
Relevant outcome measures
The guideline development group considered effects on body functions and structures and effects on motor skills and abilities as crucial outcome measures for decision making; and adverse events as an important outcome measure for decision making.
The working group defined the outcome measures as shown in Table 1.
Table 1. Definitions and minimally clinical important differences for assessed outcome measures
|
Outcome units |
Outcome measures |
Definition |
Minimal clinically important difference |
|
Effects on body functions and structures (ICF-impairments) |
Muscle tone/ spasticity |
measured with the (modified) Ashworth scale (AS) or (modified) Tardieu scale (TS) |
1 point on the (m)AS, PSFS or (m)TS |
|
Clonus |
measured with clonus score, frequency, clonus reflex scale or duration |
10% difference |
|
|
Cramps and pain |
measured with Visual Analog Scale, or Numeric (Pain) Rating Scale |
1.65 point difference (scale 0 to 10) or 16.55 (scale 0 to 100) (Bahreini, 2020) |
|
|
Effects on skills and abilities (ICF-activities) |
Gait pattern and gait speed (walking) |
measured with 10-meter walking test (10MWT), 6-min WT, |
0.1 m/s gait speed difference for 10-meter WT; 54 meter difference for 6MWT (Wise, 2005) |
|
Standing and balance |
Berg Balance Scale (or other) |
6 points on Berg Balance Scale |
|
|
Activities of Daily Living (ADL) |
for example using Barthel Index, including social functioning |
Barthel Index: 1.85 points (scale 0-20) (Hsieh, 2007) |
|
|
Adverse events and patient safety |
Considered of specific interest were allergic reactions, infection, somnolence, withdrawal symptoms. Also falls was an outcome of interest. However, a priori, the working group did not define adverse events but used the definitions used in the studies. |
not applicable (for falling: >1) |
|
Search and select (Methods)
The previous guideline module on this topic has been revised, in which the PICO is updated to compare intrathecal baclofen to usual care, whereas the previous module compared intrathecal baclofen to placebo. The process for the search and selection of the new literature is described below.
The databases Medline (via OVID) and Embase (via Embase.com) were searched with relevant search terms from the search date of the last revision of the module (June 2015) until the 11th of July 2023. The detailed search strategy is depicted under the tab Methods. The systematic literature search resulted in 228 hits. Studies were selected based on the following criteria:
- Randomized controlled trials, systematic review and/or meta-analysis, or other comparative study designs.
- Included adults with cerebral or spinal spasticity.
- Described intrathecal baclofen as an intervention.
- Described usual care as a comparison.
- Described at least one of the outcome measures as described in the PICO.
Eight studies were initially selected based on title and abstract screening. After reading the full text, four studies were excluded (see the table with reasons for exclusion under the heading Evidence tables) and four studies were included. As two articles regarded the same study but reported different outcomes (primary and secondary outcomes respectively), these were considered as being one study. This resulted in three newly included studies.
Results
Results from the previous module (intrathecal baclofen compared to placebo) can be found in Appendix 1. In this updated version of the module, the new results comparing intrathecal baclofen to usual care are described. Important study characteristics and results are summarized in the evidence tables. The assessment of the risk of bias is summarized in the risk of bias tables.
Referenties
- Creamer M, Cloud G, Kossmehl P, Yochelson M, Francisco GE, Ward AB, Wissel J, Zampolini M, Abouihia A, Calabrese A, Saltuari L. Effect of Intrathecal Baclofen on Pain and Quality of Life in Poststroke Spasticity. Stroke. 2018 Sep;49(9):2129-2137. doi: 10.1161/STROKEAHA.118.022255. PMID: 30354975; PMCID: PMC6116794.
- Creamer M, Cloud G, Kossmehl P, Yochelson M, Francisco GE, Ward AB, Wissel J, Zampolini M, Abouihia A, Berthuy N, Calabrese A, Loven M, Saltuari L. Intrathecal baclofen therapy versus conventional medical management for severe poststroke spasticity: results from a multicentre, randomised, controlled, open-label trial (SISTERS). J Neurol Neurosurg Psychiatry. 2018 Jun;89(6):642-650. doi: 10.1136/jnnp-2017-317021. Epub 2018 Jan 11. PMID: 29326296; PMCID: PMC6031277.
- McCormick ZL, Chu SK, Binler D, Neudorf D, Mathur SN, Lee J, Marciniak C. Intrathecal Versus Oral Baclofen: A Matched Cohort Study of Spasticity, Pain, Sleep, Fatigue, and Quality of Life. PM R. 2016 Jun;8(6):553-62. doi: 10.1016/j.pmrj.2015.10.005. Epub 2015 Oct 20. PMID: 26498518.
- Sammaraiee Y, Stevenson VL, Keenan E, Buchanan K, Lee H, Padilla H, Farrell RA. Evaluation of the impact of intrathecal baclofen on the walking ability of people with Multiple Sclerosis related spasticity. Mult Scler Relat Disord. 2020 Nov;46:102503. doi: 10.1016/j.msard.2020.102503. Epub 2020 Sep 20. PMID: 33032053.
- Venkatraman V, Spears CA, Futch BG, Yang LZ, Parente BA, Lee HJ, Lad SP. Assessment of Health Care Costs and Total Baclofen Use Associated With Targeted Drug Delivery for Spasticity. Neuromodulation. 2023 Aug;26(6):1247-1255. doi: 10.1016/j.neurom.2023.01.017. Epub 2023 Mar 6. PMID: 36890089; PMCID: PMC10440289.
- Vidal J, Slof J, Serrano D, Marqués T, Kumru H, Benito-Penalva J. Cost-effectiveness of Intrathecal Baclofen Therapy in severe refractory non-focal disabling spasticity: a Spanish hospital perspective. Expert Rev Pharmacoecon Outcomes Res. 2017 Feb;17(1):67-76. doi: 10.1080/14737167.2016.1180247. Epub 2016 May 3. PMID: 27142176.
Evidence tabellen
Evidence table for intervention studies (randomized controlled trials and non-randomized observational studies [cohort studies, case-control studies, case series])
|
Study reference |
Study characteristics |
Patient characteristics 2 |
Intervention (I) |
Comparison / control (C) |
Follow-up |
Outcome measures and effect size 4 |
Comments |
|
McCormick, 2015 |
Type of study: Cross-sectional matched cohort survey study.
Setting and country: Urban academic rehabilitation outpatient clinics in the USA
Funding and conflicts of interest: Authors had nothing to disclose. Funding information not reported. |
Inclusion criteria: ³18 years treated with intrathecal or oral baclofen for at least 1 year before recruitment.
Exclusion criteria: Individuals who were not able to understand the consent process because of cognitive impairment.
N total at baseline: matched 1:1 for age, gender, and diagnosis. Intervention: 31 Control: 31
Important prognostic factors: age ± SD: I: 45 y (±11) C: 46 y (±12)
Sex: I: 64 % M C: 64 % M
Mean duration of Treatment, ± SD: I: 11 (±6) years C: 12 (±10) years
Groups comparable at baseline? YES |
Intrathecal administration of baclofen (with internal pump).
On date of survey, mean baclofen dose (SD) was 577(1429) µg/day
|
Oral administration of baclofen.
Mean baclofen dose on date of survey was 86 (50) mg/day. Dose differed per patient and depended on physician for real life management of spasticity, there was no dosing protocol.
|
N/A, cross-sectional
No information on missing outcome data is described.
Incomplete intervention data: Baclofen dosage missing at date of survey: I: 2/31 (6%) C: 3/31 (10%)
|
Penn Spasm Frequency and Severity Scale (PSFS) Mean Spasm Frequency Score, from 0-4 increasing frequency (SD): Mean Spasm Severity Score from 1-3 with increasing severity (SD): I: 1.44 (0.92) | C: 2.16 (0.83) *
Brief Pain Inventory (BPI)
I: 3.79 (2.72), C: 5.42 (2.81)
I: 4.21 (2.99), C: 6.58 (3.31)
I: 2.16 (2.43), C: 3.05 (2.39)
Mean pain severity score (SD) I: 3.3 (2.54), C: 4.96 (2.65)
* = P<0.05) |
Author’s conclusions: “Long-term treatment with intrathecal compared with oral baclofen is associated with reduced spasm frequency and severity as well as greater dose stability.”
Strengths:
Limitations:
|
|
Creamer, 2018
(published in two separate journals, same study different reported outcomes) |
Type of study: phase 4, randomized, controlled, open-label, parallel- group, multicentre study
Setting and country: rehabilitation hospitals at 11 centres across Europe (Austria, Belgium, Germany, Italy, the Netherlands, Spain, United Kingdom, and Slovenia) and 7 centres in the United States
Funding and conflicts of interest: This study was supported by Medtronic International Trading Sàrl.
several authors report personal fees from Medtronic. And one author also from, Allergan, Ipsen, Merz, and Sintetica. Two authors are employees of Medtronic. The other author reports no conflicts |
Inclusion criteria: (1) Patients with generalised spasticity aged 18–75 years; (2) poststroke duration >6 months; (3) Therapy goal not reached with other treatment interventions; (4) spasticity in at least two extremities; (5) Ashworth Scale (AS) score ≥3 in a minimum of two muscle groups of the lower extremities (LE) on the affected body side
Exclusion criteria: (1) known baclofen sensitivity; (2) uncontrolled refractory epilepsy; (3) active systemic infection; (4) presence of a cardiac pacemaker; (5) implantable cardioverter defibrillator, neurostimulator or drug delivery device; (6) use of oral vitamin K antagonists; (7) use of botulinum toxin within the 4 months prior to inclusion. Chemodenervation (including botulinum toxin) and surgery affecting limb mobility were prohibited during the study.
N total at baseline: Intervention: 31 Control:29
Important prognostic factors2: For example age ± SD: I: 56.1 (11.1) C: 55.7 (8.6)
Sex: I: 77.4 % M C: 62.1 % M
Ability to transfer: (% high-level functional patient) I: 71.0% C:82.8%
Type of stroke: % Cerebral ischaemic vs. Cerebral haemorrhagic: I: 58.1% C: 41.4%
Groups comparable at baseline? NO, comparable except for ability to transfer (high- vs. low-level functional patients) and type of stroke, but difference was not statistically tested. |
Intrathecal baclofen (ITB) therapy
Lioresal Intrathecal (ITB arm patients underwent an ITB trial between days 1 and 10 during the run-in phase to evaluate drug response. Patients could continue their oral antispastic medications during this phase. Patients fulfilling the test success criterion (1-point drop in the AS score in three muscle groups in the affected LE) were implanted between days 2 and 25 with the marketed SynchroMed II infusion system (Medtronic). After implant, patients underwent a 6-week titration period during which the ITB dose was increased until the desired clinical effect was achieved or reduced for side-effect management; oral antispastics were gradually reduced with complete discontinuation by week 6. Patients randomised to ITB who were not implanted remained on oral antispastic medications and physiotherapy until the study end.
|
oral medication (conventional medical management (CMM))
CMM arm patients received a combination of oral antispastic medication (at least one of oral baclofen, tizanidine, diazepam (or other benzodiazepines), or dantrolene) and physiotherapy throughout the study. Oral antispastic medications were prescribed by the investigator at randomisation; medications (type and dose) were then reassessed at the end of the run-in phase at the second assessment visit (day 21±2) and could be adjusted as deemed necessary by the investigator at any time during the trial, in accordance with usual clinical practice and the needs of the individual patient. |
Length of follow-up: 6 months
Only patients missing in both 3-month and 6-month follow-up were excluded from the analysis. If only 6-month follow up was missing, Last observation carried forward (LOCF) imputation was used.
Loss-to-follow-up: Intervention: 7 (23%), of whom 6 (19%) before 3-month follow up Reasons:
Control: 5 (17%), all before 3-month follow up Reasons:
Incomplete outcome data:
Primary outcomes (ITT with LOCF for month 6): Ashworth score in lower extremity & Ashworth score in higher extremity: I = 6/31 (19%) C = 4/29 (14%)
Reasons: loss-to-follow-up (see above) with LOCF from month 3 to month 6, further reasons unknown
Adverse events (modified. ITT with intervention = implanted ITB and control: control + non-implanted ITB): I = 6/31 C: 35/29 Reasons: (not) implanted
|
First article (primary outcomes)
Spastic hypertonia and muscle tone Ashworth score in the lower extremity Mean change in AS score from baseline (SD) I: -0.99 (0.75), C: - 0.43 (0.72) *
Ashworth score in the upper extremity Mean change in AS score from baseline: I: −0.66 (0.59), C: -0.17(0.70) *
Functional Independence FIM total score, mean change from baseline: I: 2.68(10.31), C: -2.58(11.00)
Adverse events (in modified ITT population, only described) All AEs (%): I: 24/25 (96.0%), C:22/35(62.9%); Serious AEs I: 13/25(/52%), C; 10/35(28.6%)
Second article Pain Mean change in NPRS (SD) Actual pain: I: -1.17(3.17), C: 0.00(3.29)*
Least pain I: −1.61(2.29), C: +0.24 (3.07)*
Worst pain I: −1.35 (2.42), C: −0.04 (3.69)
* = P<0.05) |
Author’s conclusion: “Our results suggest that intrathecal delivery of baclofen provides an improved therapeutic effect versus conventional oral medications, with a reduction of the AS score in both upper and lower limbs when used in conjunction with physiotherapy.” and secondly “…results suggest that ITB delivery provides an improved therapeutic effect when compared with CMM using oral spastic medications and physiotherapy.”
Limitations:
|
|
Summaraiee, 2020 |
Type of study: Prospective cohort
Setting and country: Center for MS-related spasticity in London, UK
Funding and conflicts of interest: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Authors have nothing to disclose. Only R. Farrell has received honoraria / consultant fees from GW Pharma, Biogen Idec, Merck, Allergan PLC
|
Inclusion criteria: (1) Ambulatory subjects based on EDSS ≤ 7 (Some subjects with EDSS 7.5 who had recently stopped walking due to spasticity were included) (2) considered suitable for ITB.
Exclusion criteria: None
N total at baseline: Intervention (proceeded to pump): 20 Control (did not proceed to pump): 10 (of whom 1 non-responder)
Important prognostic factors2: For example Mean Age (range): I:51.3(26-59) C: 46.1 (39-64)
Sex: I: 30% M C: 50% M
Groups comparable at baseline? No, significant difference in baseline mean MRC power grade in subjects who proceeded to pump (Grade 4) compared to those who did not (Grade 3; p=0.015), but no significant difference in any other baseline measures including spasticity or 10MTW. |
Intrathecal baclofen
Subjects were admitted for a trial of intrathecal baclofen as a bolus dose via lumbar puncture. Trial doses ranged from 12.5– 50 mcg…. Those who reported subjective benefit, achieved goals, and demonstrated an objective response, proceeded with pump implantation during the same admission or shortly thereafter. In those who failed to respond to initial trial, subsequent trials were performed at a higher dose up to a maximum of 75 mcg.’
Medtronic Synchromed II pumps with 20 or 40ml reservoirs were implanted.
Subjects had regular follow up to optimise ITB dose and other medications, refill the pump, review spasticity, and monitor for any complications.
|
Case as usual (antispasmodic agents)
After the initial trials failed, subjects were elected not to proceed due to concerns over lower limb weakness impairing ambulation and independent transfers. |
Length of follow-up: minimum 12 months of follow- up. mean years of follow-up(range) I: 3.75(1-9), C: 4.1(1-8)
Loss-to-follow-up: Follow-up was different for each patient, barely reasons for loss-to-follow-up reported.
One subject died during follow up for reasons unrelated to the ITB pump at 8 years post-pump insertion.
Incomplete outcome data: Not reported in methods/results, yet stated in discussion: “This was a retrospective study that relied on routinely collected clinical data that was at times incomplete.”
10mtw (both pre-and post-trial): 15/30 (50%)
Reasons: Two subjects who were unable to complete a timed walk at baseline, completed it at peak dose, due to reduced spasms facilitating improved walking. Conversely three subjects who could complete a 10MTW pre-trial were unable to complete one post-trial due to reduced strength post-trial 10mtw. 9 of the 10 subjects that did not proceed to implant were unable to complete a 10MTW at peak dose, whereas only 3 out of the 20 that did proceed could not complete a 10MTW at peak dose. |
Outcome measures and effect size (include 95%CI and p-value if available):
4-hours post trial-dose (not implanted, n = 30) Mean Ashworth score (range) Pre-dose 1.44 (0.75-2.25); post-dose 0.98 (0.08-1.5) Mean penn spasm score (range) Pre-dose 3.0 (1.5-4.0), post-dose: 1.0 (0.0-3.5)* Mean time 10m timed walk mobility (range) Pre-dose: 76sec (20-186), post-dose: 94sec (23-401)
After implant, Number of subjects ambulatory (able to walk) t = 1 year I: 16 (80%), C: 4 (40%)* t = 2 years I: 14 (70%), C: 3 (30%)* t = 3 years I = 12 (60%), C: 30 (30%)*
Differences in the preservation of ambulation in patients with or without ITB implant (Kaplan-Meier) log rank test χ2(1) = 2.733, p =0.09.
* = P<0.05)
|
|
Risk of bias table for intervention studies (randomized controlled trials)
|
Study reference |
Was the allocation sequence adequately generated? |
Was the allocation adequately concealed? |
Blinding: Was knowledge of the allocated interventions adequately prevented? |
Was loss to follow-up (missing outcome data) infrequent? |
Are reports of the study free of selective outcome reporting? |
Was the study apparently free of other problems that could put it at a risk of bias? |
Overall risk of bias
LOW Some concerns HIGH |
|
Creamer, 2018
|
Definitely yes
Reason: Central randomization with computer generated random numbers |
Definitely no,
Reason: Due to nature of the treatments (implant vs. oral) the study was not blinded to either subject or investigator |
Probably no
Reason: Patients and health care providers were not blinded due to nature of the treatment. To avoid AS score assessment bias and inter-rater variability, all AS scores (except for those during the ITB test) were performed by the same blinded assessor. Assessment of patient-reported outcome measures could not be blinded |
Probably no
Reason: LTFU was 23% in intervention group and 19% in control group. One LTFU was a major protocol deviation: subject discontinued after switching from intervention to control group. Last Observation Carried Forward was used as an imputation method for 6-month measurement (inadequate). For each (secondary) outcome, data from some subjects is missing, which does not all seem to be related to LTFU |
Definitely yes
Reason: All relevant outcomes were reported |
Definitely no
Potential publication bias due to commercial sponsoring and conflicts of interest.
|
Some concerns
|
Risk of bias table for intervention studies (observational: non-randomized clinical trials, cohort, and case-control studies)
|
Author, year |
Selection of participants Was selection of exposed and non-exposed cohorts drawn from the same population? |
Exposure Can we be confident in the assessment of exposure? |
Outcome of interest Can we be confident that the outcome of interest was not present at start of study? |
Confounding-assessment Can we be confident in the assessment of confounding factors? |
Confounding-analysis Did the study match exposed and unexposed for all variables that are associated with the outcome of interest or did the statistical analysis adjust for these confounding variables? |
Assessment of outcome Can we be confident in the assessment of outcome? |
Follow up Was the follow up of cohorts adequate? In particular, was outcome data complete or imputed? |
Co-interventions Were co-interventions similar between groups? |
Overall Risk of bias |
|
McCormick, 2015 |
Probably yes, All participants recruited from single site practice during routine visits and matched by age (+-10 years), gender, and etiologic diagnosis of spasticity. Only MS patients included. |
Definitely no, Due to nature of exposure probably no classification bias (implant), But dose was dependent on management style of physician and differs per subject. |
Definitely no, cross-sectional study, all outcomes only measured once. |
Probably yes, Due to nature of confounding factors (age (+-10 years), gender, and etiologic diagnosis of spasticity). |
Probably no, Matched subjects, but not via propensity score (or at least not reported). Also, it is a cross-sectional study, thus baseline differences in outcomes or other related confounding factors may exist.
Other measured patient characteristics don’t differ significantly between groups. |
Probably yes, Outcomes assessed via standardized questionnaires. |
Definitely no, No follow-up because of cross- sectional design. No information on missing data provided. |
Definitely yes Reason: Additional used medication was balanced between groups (no statistically significant difference between groups). |
High
|
|
Summaraiee, 2020 |
Definitely yes,
Study includes all ambulatory subjects treated with ITB in a center for MS related spasticity |
Definitely no,
Comparator selection based on exclusion of intervention because of bad response (outcome related selection) |
Definitely no,
Difference in outcome (walking preservation) at baseline. |
Definitely no,
Selection based on first outcome. Also, statistically significant baseline differences between groups and no correction for confounding. |
Definitely no,
Selection based on first outcome |
Probably yes,
Outcomes assessed via standardized scales, but no information on how it was assessed and who did the assessment.
|
Definitely no,
Follow-up was different for each patient, barely reasons for loss-to-follow-up reported.
Study was based on routinely collected clinical data that was at times incomplete. |
Probably no,
Additional use of medication was balanced between groups at baseline, but changed during follow-up, which was not considered for the analyses. |
High
|
Table of excluded studies
|
Reference |
Reason for exclusion |
|
Marathe A, Allahabadi S, Abd-Elsayed A, Saulino M, Hagedorn JM, Orhurhu V, Karri J. Intrathecal Baclofen Monotherapy and Polyanalgesia for Treating Chronic Pain in Patients with Severe Spasticity. Curr Pain Headache Rep. 2021 Dec 11;25(12):79. doi: 10.1007/s11916-021-00994-9. Erratum in: Curr Pain Headache Rep. 2022 Mar;26(3):279. PMID: 34894303. |
Systematic review of insufficient quality, including wrong publication types (non-comparative studies) |
|
Jacobs NW, Maas EM, Brusse-Keizer M, Rietman HJS. Effectiveness and safety of cervical catheter tip placement in intrathecal baclofen treatment of spasticity: A systematic review. J Rehabil Med. 2021 Jul 9;53(7):jrm00215. doi: 10.2340/16501977-2857. PMID: 34160624; PMCID: PMC8638738. |
Systematic review including wrong publication types (case series and case reports) |
|
Lee HP, Win T, Balakrishnan S. The impact of intrathecal baclofen on the ability to walk: A systematic review. Clin Rehabil. 2023 Apr;37(4):462-477. doi: 10.1177/02692155221135827. Epub 2022 Nov 4. PMID: 36330654. |
Systematic review of insufficient quality, including wrong publication types |
|
Dietz N, Wagers S, Harkema SJ, D'Amico JM. Intrathecal and Oral Baclofen Use in Adults With Spinal Cord Injury: A Systematic Review of Efficacy in Spasticity Reduction, Functional Changes, Dosing, and Adverse Events. Arch Phys Med Rehabil. 2023 Jan;104(1):119-131. doi: 10.1016/j.apmr.2022.05.011. Epub 2022 Jun 22. PMID: 35750207. |
Systematic review with wrong study comparators (placebo instead of usual care) |
Verantwoording
Beoordelingsdatum en geldigheid
Publicatiedatum : 06-01-2026
Beoordeeld op geldigheid : 06-01-2026
De Nederlandse Vereniging van Revalidatieartsen geeft bestuurlijke goedkeuring onder voorwaarde van autorisatie door de ALV van 17 april 2026.
Algemene gegevens
De ontwikkeling/herziening van deze richtlijnmodule werd ondersteund door het Kennisinstituut van de Federatie Medisch Specialisten (www.demedischspecialist.nl/kennisinstituut) en werd gefinancierd uit de Stichting Kwaliteitsgelden Medisch Specialisten (SKMS). De financier heeft geen enkele invloed gehad op de inhoud van de richtlijnmodule.
Samenstelling werkgroep
Voor het ontwikkelen van de richtlijnmodule is in 2023 een multidisciplinaire werkgroep ingesteld, bestaande uit vertegenwoordigers van alle relevante specialismen (zie hiervoor de Samenstelling van de werkgroep) die betrokken zijn bij de zorg voor patiënten met Cerebrale en/of spinale spasticiteit.
Werkgroep
- prof. dr. A.C.H. Geurts (voorzitter), hoogleraar neurorevalidatie, Radboud UMC en Sint Maartenskliniek, namens de Nederlandse Vereniging voor Revalidatieartsen
- drs. A.M.V. Dommisse, revalidatiearts, Isala Klinieken Zwolle, namens de Nederlandse Vereniging voor Revalidatieartsen
- drs. P.J. van Dongen, patiëntvertegenwoordiger bij Hersenletsel.nl
- Dr. M. van Eijk, specialist ouderengeneeskunde, Marnix Medisch B.V., namens Verenso
- dr. J.F.M. Fleuren, revalidatiearts, Jeroen Bosch Ziekenhuis / Tolbrug, ‘s Hertogenbosch, namens de Nederlandse Vereniging voor Revalidatieartsen
- F. van Gorp-Swart, MSc, ziekenhuisapotheker, Diakonessenhuis, Utrecht/Zeist/Doorn, namens de Nederlandse Vereniging voor Ziekenhuisapothekers
- prof. dr. G. Kwakkel, hoogleraar neurorevalidatie, Amsterdam UMC, Amsterdam, namens het Koninklijk Nederlands Genootschap voor Fysiotherapie
- drs. E. Kurt, neurochirurg, Radboud UMC en Canisius Wilhelmina Ziekenhuis, Nijmegen, namens de Nederlandse Vereniging voor Neurochirurgie
- Prof. dr. C.G.M. Meskers, hoogleraar revalidatiegeneeskunde, Amsterdam UMC, Amsterdam, namens de Nederlandse Vereniging voor Revalidatieartsen
- dr. H.A. Moser, anesthesioloog, Radboud UMC, Nijmegen en Care4homecare, Bladel, namens de Nederlandse Vereniging voor Anesthesiologie
- drs. W.P. Polomski, revalidatiearts (gepensioneerd), voorheen in Spaarne Gasthuis, Hoofddorp, namens de Nederlandse Vereniging voor Revalidatieartsen
- drs. M.N. Ruissen-Eversdijk, ergotherapeut en bewegingswetenschapper, Reade, Amsterdam, namens Ergotherapie Nederland
- dr. A.V.C.M. Zeegers, orthopedisch chirurg, Medisch Spectrum Twente, Enschede, namens de Nederlandse Orthopaedische Vereniging
- dr. J.M. Zuidam, plastisch chirurg, Erasmus MC, namens de Nederlandse Vereniging voor Plastische Chirurgie
Klankbordgroep
- P.M. van Lamoen, gepensioneerd, namens Dwarslaesieorganisatie Nederland
- M. Pol, Dwarslaesie Organisatie Nederland, tot september 2024*
- Dr. A.E. Tigchelaar, Dwarslaesie Organisatie Nederland, vanaf september 2024
- Dr. W.J. Kruithof, revalidatiearts, Universitair Medisch Centrum Utrecht
- Dr. I.H. Zaal-Schuller, arts verstandelijk gehandicapten/kaderarts palliatieve zorg
*Overleden
Met ondersteuning van
- Dr. M.L. Molag, adviseur, Kennisinstituut van de Federatie Medisch Specialisten
- Dr. M.M.J. van Rooijen, adviseur, Kennisinstituut van de Federatie Medisch Specialisten
Belangenverklaringen
De Code ter voorkoming van oneigenlijke beïnvloeding door belangenverstrengeling is gevolgd. Een overzicht van de belangen van werkgroepleden en het oordeel over het omgaan met eventuele belangen vindt u in onderstaande tabel. De ondertekende belangenverklaringen zijn op te vragen bij het secretariaat van het Kennisinstituut van de Federatie Medisch Specialisten via secretariaat@kennisinstituut.nl.
|
Werkgroeplid |
Functie |
Nevenfuncties |
Gemelde belangen |
Ondernomen actie |
|
Geurts (voorzitter) |
Hoogleraar neurorevalidatie, Radboud UMC, Nijmegen en Sint Maartenskliniek |
|
Geen. |
Geen restricties. |
|
Dommisse |
Revalidatiearts, Vogellanden |
Geen |
Speakerfee bij Ipsen farmaceutica voor:
|
Geen restricties; e-learning en presentaties betreffen de volle breedte van de behandel opties |
|
van Dongen |
Patiëntvertegenwoordiger Hersenletsel.nl |
Deelname andere werkgroepen |
Geen |
Geen restricties. |
|
van Eijk |
Specialist Ouderengeneeskunde, Marnix Medisch B.V |
|
Extern gefinancierd onderzoek over heupfracturen: 1. FITHIP; onderzoek naar valangst bij patienten met heupfractuur 2. GR HIP; onderzoek naar herstel na heupfractuur 3. HIPCARE; onderzoek naar herstel en biomarkers bij heupfractuur |
Geen restricties; De webinars waren gericht op samenwerking specialisten ouderengeneeskunde en revalidatieartsen |
|
Fleuren |
Revalidatiearts, Tolbrug |
Bestuurslid VRA (onbetaald) |
Geen. |
Geen restricties. |
|
van Gorp |
Ziekenhuisapotheker |
Lid werkgroep interacties KNMP |
Geen. |
Geen restricties. |
|
Kwakkel |
Hoogleraar neurorevalidatie, Amsterdam UMC, locatie VUMC |
|
Geen |
Geen restricties. |
|
Kurt |
Neurochirurg, Radboud UMC, Nijmegen |
Geen |
Geen |
Geen restricties. |
|
Meskers |
Revalidatiearts, Amsterdam UMC locatie VUMC |
Geen |
Geen. |
Geen restricties. |
|
Moser |
Anesthesioloog, Radboud UMC, Nijmegen en Care4homecare, Bladel |
Geen |
Geen |
Geen restricties. |
|
Polomski |
Revalidatiearts Spaarne Gasthuis (gepensioneerd vanaf 1 mei 2023). |
Geen |
Lid Adviesraad Merz Benelux, raakt niet aan de modules |
Restrictie ten aanzien van besluitvorming met betrekking tot botulinetoxine |
|
Ruissen-Eversdijk |
Ergotherapeut bij Reade Revalidatie. |
Geen. |
Geen. |
Geen restricties. |
|
Zeegers |
Orthopedisch chirurg, Medisch Spectrum Twente, Enschede (tot 1-6-2025), en UMCG (vanaf 1-6-2025) |
|
Geen. |
Geen restricties. |
|
Zuidam |
Plastisch chirurg, Erasmus MC Rotterdam |
Geen. |
Geen. |
Geen restricties. |
Inbreng patiëntenperspectief
Er werd aandacht besteed aan het patiëntenperspectief door uitnodigen van Hersenletsel.nl en Dwarslaesie Organisatie Nederland (DON) bij de schriftelijke knelpuntenanalyse. DON heeft een enquête bij hun achterban uitgezet, en knelpunten werden meegenomen in het proces. Het verslag van deze enquête is besproken in de werkgroep. De verkregen input is meegenomen bij het opstellen van de uitgangsvragen, de keuze voor de uitkomstmaten en bij het opstellen van de overwegingen. In de werkgroep heeft een vertegenwoordiger van Hersenletsel.nl deelgenomen. De conceptrichtlijn is tevens voor commentaar voorgelegd aan Hersenletsel.nl, DON, MS Nederland en Spierziekten Nederland.
Kwalitatieve raming van mogelijke financiële gevolgen in het kader van de Wkkgz
Bij de richtlijnmodule is conform de Wet kwaliteit, klachten en geschillen zorg (Wkkgz) een kwalitatieve raming uitgevoerd om te beoordelen of de aanbevelingen mogelijk leiden tot substantiële financiële gevolgen. Bij het uitvoeren van deze beoordeling is de richtlijnmodule op verschillende domeinen getoetst (zie het stroomschema op de Richtlijnendatabase). Uit deze kwalitatieve raming bleek dat er geen grote financiële gevolgen te verwachten zijn.
|
Module |
Uitkomst raming |
Toelichting |
|
Intrathecale baclofen |
geen financiële gevolgen |
Uit de toetsing volgt dat de aanbevelingen niet breed toepasbaar zijn (<5.000 patiënten) en daarom naar verwachting geen substantiële financiële gevolgen zullen hebben voor de collectieve uitgaven. |
Werkwijze
Voor meer details over de gebruikte richtlijnmethodologie verwijzen wij u naar de Werkwijze. Relevante informatie voor de ontwikkeling/herziening van deze richtlijnmodule is hieronder weergegeven.
Zoekverantwoording
Algemene informatie
|
Cluster/richtlijn: VRA Behandeling van cerebrale en/of spinale spasticiteit bij volwassenen |
|
|
Uitgangsvraag/modules: Wat is de plaats van intrathecale toediening van baclofen bij volwassenen met cerebrale en/of spinale spasticiteit? |
|
|
Database(s): Embase.com, Ovid/Medline |
Datum: 11 juli 2023 |
|
Periode: vanaf juni 2015 |
Talen: geen restrictie |
|
Literatuurspecialist: Alies van der Wal |
Rayyan review: https://rayyan.ai/reviews/721371 |
|
BMI-zoekblokken: voor verschillende opdrachten wordt (deels) gebruik gemaakt van de zoekblokken van BMI-Online https://blocks.bmi-online.nl/ Bij gebruikmaking van een volledig zoekblok zal naar de betreffende link op de website worden verwezen. |
|
|
Toelichting: Voor deze vraag is gezocht op de elementen: cerebrale en/of spinale spasticiteit intrathecale baclofen De sleutelartikelen worden gevonden met deze search |
|
|
Te gebruiken voor richtlijnen tekst: On the 11th of July 2023, a systematic search was performed in the databases Embase.com and Ovid/Medline for systematic reviews, RCTs and observational studies about intrathecal baclofen in cerebral and/or spinal spasticity. The search resulted in 228 unique hits. |
|
Zoekopbrengst
|
|
EMBASE |
OVID/MEDLINE |
Ontdubbeld |
|
SR |
37 |
24 |
39 |
|
RCT |
49 |
20 |
56 |
|
Observationele studies |
112 |
85 |
133 |
|
Totaal |
198 |
129 |
228* |
*in Rayyan
Zoekstrategie
Embase.com
|
No. |
Query |
Results |
|
#13 |
#10 OR #11 OR #12 |
198 |
|
#12 |
#5 AND (#8 OR #9) NOT (#10 OR #11) = observationeel |
112 |
|
#11 |
#5 AND #7 NOT #10 = RCT |
49 |
|
#10 |
#5 AND #6 = SR |
37 |
|
#9 |
'case control study'/de OR 'comparative study'/exp OR 'control group'/de OR 'controlled study'/de OR 'controlled clinical trial'/de OR 'crossover procedure'/de OR 'double blind procedure'/de OR 'phase 2 clinical trial'/de OR 'phase 3 clinical trial'/de OR 'phase 4 clinical trial'/de OR 'pretest posttest design'/de OR 'pretest posttest control group design'/de OR 'quasi experimental study'/de OR 'single blind procedure'/de OR 'triple blind procedure'/de OR (((control OR controlled) NEAR/6 trial):ti,ab,kw) OR (((control OR controlled) NEAR/6 (study OR studies)):ti,ab,kw) OR (((control OR controlled) NEAR/1 active):ti,ab,kw) OR 'open label*':ti,ab,kw OR (((double OR two OR three OR multi OR trial) NEAR/1 (arm OR arms)):ti,ab,kw) OR ((allocat* NEAR/10 (arm OR arms)):ti,ab,kw) OR placebo*:ti,ab,kw OR 'sham-control*':ti,ab,kw OR (((single OR double OR triple OR assessor) NEAR/1 (blind* OR masked)):ti,ab,kw) OR nonrandom*:ti,ab,kw OR 'non-random*':ti,ab,kw OR 'quasi-experiment*':ti,ab,kw OR crossover:ti,ab,kw OR 'cross over':ti,ab,kw OR 'parallel group*':ti,ab,kw OR 'factorial trial':ti,ab,kw OR ((phase NEAR/5 (study OR trial)):ti,ab,kw) OR ((case* NEAR/6 (matched OR control*)):ti,ab,kw) OR ((match* NEAR/6 (pair OR pairs OR cohort* OR control* OR group* OR healthy OR age OR sex OR gender OR patient* OR subject* OR participant*)):ti,ab,kw) OR ((propensity NEAR/6 (scor* OR match*)):ti,ab,kw) OR versus:ti OR vs:ti OR compar*:ti OR ((compar* NEAR/1 study):ti,ab,kw) OR (('major clinical study'/de OR 'clinical study'/de OR 'cohort analysis'/de OR 'observational study'/de OR 'cross-sectional study'/de OR 'multicenter study'/de OR 'correlational study'/de OR 'follow up'/de OR cohort*:ti,ab,kw OR 'follow up':ti,ab,kw OR followup:ti,ab,kw OR longitudinal*:ti,ab,kw OR prospective*:ti,ab,kw OR retrospective*:ti,ab,kw OR observational*:ti,ab,kw OR 'cross sectional*':ti,ab,kw OR cross?ectional*:ti,ab,kw OR multicent*:ti,ab,kw OR 'multi-cent*':ti,ab,kw OR consecutive*:ti,ab,kw) AND (group:ti,ab,kw OR groups:ti,ab,kw OR subgroup*:ti,ab,kw OR versus:ti,ab,kw OR vs:ti,ab,kw OR compar*:ti,ab,kw OR 'odds ratio*':ab OR 'relative odds':ab OR 'risk ratio*':ab OR 'relative risk*':ab OR 'rate ratio':ab OR aor:ab OR arr:ab OR rrr:ab OR ((('or' OR 'rr') NEAR/6 ci):ab))) |
14236747 |
|
#8 |
'major clinical study'/de OR 'clinical study'/de OR 'case control study'/de OR 'family study'/de OR 'longitudinal study'/de OR 'retrospective study'/de OR 'prospective study'/de OR 'comparative study'/de OR 'cohort analysis'/de OR ((cohort NEAR/1 (study OR studies)):ab,ti) OR (('case control' NEAR/1 (study OR studies)):ab,ti) OR (('follow up' NEAR/1 (study OR studies)):ab,ti) OR (observational NEAR/1 (study OR studies)) OR ((epidemiologic NEAR/1 (study OR studies)):ab,ti) OR (('cross sectional' NEAR/1 (study OR studies)):ab,ti) |
7728902 |
|
#7 |
'clinical trial'/exp OR 'randomization'/exp OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp OR 'placebo'/exp OR 'prospective study'/exp OR rct:ab,ti OR random*:ab,ti OR 'single blind':ab,ti OR 'randomised controlled trial':ab,ti OR 'randomized controlled trial'/exp OR placebo*:ab,ti |
3827532 |
|
#6 |
'meta analysis'/exp OR 'meta analysis (topic)'/exp OR metaanaly*:ti,ab OR 'meta analy*':ti,ab OR metanaly*:ti,ab OR 'systematic review'/de OR 'cochrane database of systematic reviews'/jt OR prisma:ti,ab OR prospero:ti,ab OR (((systemati* OR scoping OR umbrella OR 'structured literature') NEAR/3 (review* OR overview*)):ti,ab) OR ((systemic* NEAR/1 review*):ti,ab) OR (((systemati* OR literature OR database* OR 'data base*') NEAR/10 search*):ti,ab) OR (((structured OR comprehensive* OR systemic*) NEAR/3 search*):ti,ab) OR (((literature NEAR/3 review*):ti,ab) AND (search*:ti,ab OR database*:ti,ab OR 'data base*':ti,ab)) OR (('data extraction':ti,ab OR 'data source*':ti,ab) AND 'study selection':ti,ab) OR ('search strategy':ti,ab AND 'selection criteria':ti,ab) OR ('data source*':ti,ab AND 'data synthesis':ti,ab) OR medline:ab OR pubmed:ab OR embase:ab OR cochrane:ab OR (((critical OR rapid) NEAR/2 (review* OR overview* OR synthes*)):ti) OR ((((critical* OR rapid*) NEAR/3 (review* OR overview* OR synthes*)):ab) AND (search*:ab OR database*:ab OR 'data base*':ab)) OR metasynthes*:ti,ab OR 'meta synthes*':ti,ab |
942968 |
|
#5 |
#4 AND [01-06-2015]/sd |
383 |
|
#4 |
#3 NOT ('conference abstract'/it OR 'editorial'/it OR 'letter'/it OR 'note'/it) NOT (('animal'/exp OR 'animal experiment'/exp OR 'animal model'/exp OR 'nonhuman'/exp) NOT 'human'/exp) NOT (('adolescent'/exp OR 'child'/exp OR adolescent*:ti,ab,kw OR child*:ti,ab,kw OR schoolchild*:ti,ab,kw OR infant*:ti,ab,kw OR girl*:ti,ab,kw OR boy*:ti,ab,kw OR teen:ti,ab,kw OR teens:ti,ab,kw OR teenager*:ti,ab,kw OR youth*:ti,ab,kw OR pediatr*:ti,ab,kw OR paediatr*:ti,ab,kw OR puber*:ti,ab,kw) NOT ('adult'/exp OR 'aged'/exp OR 'middle aged'/exp OR adult*:ti,ab,kw OR man:ti,ab,kw OR men:ti,ab,kw OR woman:ti,ab,kw OR women:ti,ab,kw)) |
1311 |
|
#3 |
#1 AND #2 |
2706 |
|
#2 |
'intrathecal baclofen pump'/exp OR baclofenpump*:ti,ab,kw OR ((baclofen NEAR/3 pump*):ti,ab,kw) OR itb:ti,ab,kw OR (('baclofen'/de OR 'baclofen*':ti,ab,kw OR 'baclophen':ti,ab,kw OR 'baklofen':ti,ab,kw OR 'lebic':ti,ab,kw OR lioresal:ti,ab,kw) AND ('intrathecal drug administration'/exp OR 'intrathecal pump'/exp OR 'intrathecal catheter'/exp OR intrathecal*:ti,ab,kw OR 'intra thecal*':ti,ab,kw OR subarachnoid*:ti,ab,kw OR 'codman 3000':ti,ab,kw OR 'isomed':ti,ab,kw OR 'synchromed':ti,ab,kw OR 'ascenda':ti,ab,kw OR 'spinocath':ti,ab,kw OR ((spinal NEAR/3 catheter*):ti,ab,kw))) |
4230 |
|
#1 |
'spasticity'/exp OR 'muscle hypertonia'/de OR 'muscle rigidity'/de OR 'spastic paraplegia'/exp OR 'spastic paresis'/exp OR 'muscle spasm'/de OR 'muscle tone'/de OR 'dystonia'/exp OR spastic*:ti,ab,kw OR spasm*:ti,ab,kw OR 'high tone':ti,ab,kw OR hyperton*:ti,ab,kw OR hypertonus:ti,ab,kw OR hypertonicity:ti,ab,kw OR hypermyoton*:ti,ab,kw OR dyston*:ti,ab,kw OR myodyston*:ti,ab,kw OR palsy:ti,ab,kw OR paresis:ti,ab,kw OR pareses:ti,ab,kw OR parapares*:ti,ab,kw OR paratonia:ti,ab,kw OR (((muscle* OR muscular) NEAR/3 (rigid* OR tone OR tonus OR overactiv* OR 'over activ*' OR paretic)):ti,ab,kw) OR 'spinal cord injury'/exp OR 'spine injury'/exp OR 'multiple sclerosis'/exp OR 'brain injury'/exp OR 'cerebrovascular disease'/exp OR 'brain hemorrhage'/exp OR 'brain infarction'/exp OR 'intracranial aneurysm'/exp OR 'stroke patient'/exp OR 'stroke unit'/exp OR 'cerebral palsy'/exp OR (((spinal OR spine OR head) NEAR/2 (injur* OR trauma*)):ti,ab,kw) OR tbi:ti,ab,kw OR cva:ti,ab,kw OR cvas:ti,ab,kw OR stroke:ti,ab,kw OR poststroke:ti,ab,kw OR (((cerebrovascular OR 'cerebro vascular' OR cerebral OR intracerebral OR cerebellum OR brain OR 'corpus callosum' OR intracranial OR intraventricular OR periventricular OR subarachnoid) NEAR/3 (accident* OR attack* OR infarct* OR insult* OR event* OR diseas* OR injur* OR trauma OR arrest OR failure OR bleed* OR microbleed* OR aneurysm* OR hemorrhage* OR haemorrhage* OR microhemorrhage* OR microhaemorrhage* OR ischemi* OR ischaemi* OR haematoma* OR hematoma* OR thrombo* OR emboli* OR occlus*)):ti,ab,kw) OR apoplex*:ti,ab,kw OR encephalorrhagia:ti,ab,kw OR (((multiple OR disseminated OR insular OR multiplex) NEAR/2 scleros*):ti,ab,kw) |
1875389 |
Ovid/Medline
|
# |
Searches |
Results |
|
13 |
10 or 11 or 12 |
129 |
|
12 |
(5 and (8 or 9)) not (10 or 11) = observationeel |
85 |
|
11 |
(5 and 7) not 10 = RCT |
20 |
|
10 |
5 and 6 = SR |
24 |
|
9 |
Case-control Studies/ or clinical trial, phase ii/ or clinical trial, phase iii/ or clinical trial, phase iv/ or comparative study/ or control groups/ or controlled before-after studies/ or controlled clinical trial/ or double-blind method/ or historically controlled study/ or matched-pair analysis/ or single-blind method/ or (((control or controlled) adj6 (study or studies or trial)) or (compar* adj (study or studies)) or ((control or controlled) adj1 active) or "open label*" or ((double or two or three or multi or trial) adj (arm or arms)) or (allocat* adj10 (arm or arms)) or placebo* or "sham-control*" or ((single or double or triple or assessor) adj1 (blind* or masked)) or nonrandom* or "non-random*" or "quasi-experiment*" or "parallel group*" or "factorial trial" or "pretest posttest" or (phase adj5 (study or trial)) or (case* adj6 (matched or control*)) or (match* adj6 (pair or pairs or cohort* or control* or group* or healthy or age or sex or gender or patient* or subject* or participant*)) or (propensity adj6 (scor* or match*))).ti,ab,kf. or (confounding adj6 adjust*).ti,ab. or (versus or vs or compar*).ti. or ((exp cohort studies/ or epidemiologic studies/ or multicenter study/ or observational study/ or seroepidemiologic studies/ or (cohort* or 'follow up' or followup or longitudinal* or prospective* or retrospective* or observational* or multicent* or 'multi-cent*' or consecutive*).ti,ab,kf.) and ((group or groups or subgroup* or versus or vs or compar*).ti,ab,kf. or ('odds ratio*' or 'relative odds' or 'risk ratio*' or 'relative risk*' or aor or arr or rrr).ab. or (("OR" or "RR") adj6 CI).ab.)) |
5464865 |
|
8 |
Epidemiologic studies/ or case control studies/ or exp cohort studies/ or Controlled Before-After Studies/ or Case control.tw. or cohort.tw. or Cohort analy$.tw. or (Follow up adj (study or studies)).tw. or (observational adj (study or studies)).tw. or Longitudinal.tw. or Retrospective*.tw. or prospective*.tw. or consecutive*.tw. or Cross sectional.tw. or Cross-sectional studies/ or historically controlled study/ or interrupted time series analysis/ [Onder exp cohort studies vallen ook longitudinale, prospectieve en retrospectieve studies] |
4482444 |
|
7 |
exp clinical trial/ or randomized controlled trial/ or exp clinical trials as topic/ or randomized controlled trials as topic/ or Random Allocation/ or Double-Blind Method/ or Single-Blind Method/ or (clinical trial, phase i or clinical trial, phase ii or clinical trial, phase iii or clinical trial, phase iv or controlled clinical trial or randomized controlled trial or multicenter study or clinical trial).pt. or random*.ti,ab. or (clinic* adj trial*).tw. or ((singl* or doubl* or treb* or tripl*) adj (blind$3 or mask$3)).tw. or Placebos/ or placebo*.tw. |
2609251 |
|
6 |
meta-analysis/ or meta-analysis as topic/ or (metaanaly* or meta-analy* or metanaly*).ti,ab,kf. or systematic review/ or cochrane.jw. or (prisma or prospero).ti,ab,kf. or ((systemati* or scoping or umbrella or "structured literature") adj3 (review* or overview*)).ti,ab,kf. or (systemic* adj1 review*).ti,ab,kf. or ((systemati* or literature or database* or data-base*) adj10 search*).ti,ab,kf. or ((structured or comprehensive* or systemic*) adj3 search*).ti,ab,kf. or ((literature adj3 review*) and (search* or database* or data-base*)).ti,ab,kf. or (("data extraction" or "data source*") and "study selection").ti,ab,kf. or ("search strategy" and "selection criteria").ti,ab,kf. or ("data source*" and "data synthesis").ti,ab,kf. or (medline or pubmed or embase or cochrane).ab. or ((critical or rapid) adj2 (review* or overview* or synthes*)).ti. or (((critical* or rapid*) adj3 (review* or overview* or synthes*)) and (search* or database* or data-base*)).ab. or (metasynthes* or meta-synthes*).ti,ab,kf. |
679922 |
|
5 |
4 and 20150601:20230711.(dt). |
276 |
|
4 |
3 not (comment/ or editorial/ or letter/) not ((exp animals/ or exp models, animal/) not humans/) not ((Adolescent/ or Child/ or Infant/ or adolescen*.ti,ab,kf. or child*.ti,ab,kf. or schoolchild*.ti,ab,kf. or infant*.ti,ab,kf. or girl*.ti,ab,kf. or boy*.ti,ab,kf. or teen.ti,ab,kf. or teens.ti,ab,kf. or teenager*.ti,ab,kf. or youth*.ti,ab,kf. or pediatr*.ti,ab,kf. or paediatr*.ti,ab,kf. or puber*.ti,ab,kf.) not (Adult/ or adult*.ti,ab,kf. or man.ti,ab,kf. or men.ti,ab,kf. or woman.ti,ab,kf. or women.ti,ab,kf.)) |
967 |
|
3 |
1 and 2 |
1412 |
|
2 |
(baclofenpump* or (baclofen adj3 pump*) or itb).ti,ab,kf. or ((exp Baclofen/ or 'baclofen*'.ti,ab,kf. or 'baclophen'.ti,ab,kf. or 'baklofen'.ti,ab,kf. or 'lebic'.ti,ab,kf. or lioresal.ti,ab,kf.) and (Infusion Pumps/ or exp Injections, Spinal/ or Infusion Pumps, Implantable/ or intrathecal*.ti,ab,kf. or 'intra thecal*'.ti,ab,kf. or subarachnoid*.ti,ab,kf. or 'codman 3000'.ti,ab,kf. or 'isomed'.ti,ab,kf. or 'synchromed'.ti,ab,kf. or 'ascenda'.ti,ab,kf. or 'spinocath'.ti,ab,kf. or (spinal adj3 catheter*).ti,ab,kf.)) |
2345 |
|
1 |
exp Muscle Spasticity/ or exp Spasm/ or exp Muscle Hypertonia/ or exp Paraplegia/ or exp Paraparesis, Spastic/ or exp Muscle Tonus/ or exp Dystonia/ or spastic*.ti,ab,kf. or spasm*.ti,ab,kf. or 'high tone'.ti,ab,kf. or hyperton*.ti,ab,kf. or hypertonus.ti,ab,kf. or hypertonicity.ti,ab,kf. or hypermyoton*.ti,ab,kf. or dyston*.ti,ab,kf. or myodyston*.ti,ab,kf. or palsy.ti,ab,kf. or paresis.ti,ab,kf. or pareses.ti,ab,kf. or parapares*.ti,ab,kf. or paratonia.ti,ab,kf. or ((muscle* or muscular) adj3 (rigid* or tone or tonus or overactiv* or 'over activ*' or paretic)).ti,ab,kf. or exp Spinal Cord Injuries/ or exp Spinal Injuries/ or exp Multiple Sclerosis/ or exp Brain Injuries/ or Head Injuries, Closed/ or exp Cerebrovascular Disorders/ or exp Stroke/ or exp Intracranial Hemorrhages/ or exp Brain Infarction/ or exp Cerebral Palsy/ or ((spinal or spine or head) adj2 (injur* or trauma*)).ti,ab,kf. or tbi.ti,ab,kf. or cva.ti,ab,kf. or cvas.ti,ab,kf. or stroke.ti,ab,kf. or poststroke.ti,ab,kf. or ((cerebrovascular or 'cerebro vascular' or cerebral or intracerebral or cerebellum or brain or 'corpus callosum' or intracranial or intraventricular or periventricular or subarachnoid) adj3 (accident* or attack* or infarct* or insult* or event* or diseas* or injur* or trauma or arrest or failure or bleed* or microbleed* or aneurysm* or hemorrhage* or haemorrhage* or microhemorrhage* or microhaemorrhage* or ischemi* or ischaemi* or haematoma* or hematoma* or thrombo* or emboli* or occlus*)).ti,ab,kf. or apoplex*.ti,ab,kf. or encephalorrhagia.ti,ab,kf. or ((multiple or disseminated or insular or multiplex) adj2 scleros*).ti,ab,kf. |
1152656 |