BICAT
Uitgangsvraag
Bij welke patiënten met indicatie voor cataractchirurgie is ISBCS een geschikte optie en wat is de plaats van deze methode binnen de huidige behandelingsmogelijkheden?
Aanbeveling
Maak bij elke patiënt de overweging of deze geschikt is voor simultane bilaterale cataractchirurgie (ISBCS).
Bespreek de mogelijkheid voor simultane bilaterale cataractchirurgie (ISBCS) bij hiervoor geschikte patiënten (patiënten zonder oculaire comorbiditeiten en zonder verhoogd operatierisico).
Indien bilaterale simultane cataractoperaties gepland worden, dienen deze beschouwd en behandeld te worden als twee afzonderlijke procedures, conform de principal practice guideline for bilateral surgery.1
Bij het optreden van een complicatie bij de eerste operatie moet heroverwogen worden of het tweede oog aansluitend wordt geopereerd.
1 Zie voor een specificatie van deze maatregelen voor de Nederlandse situatie de paragraaf ‘Aanvaardbaarheid, haalbaarheid en implementatie’.
Overwegingen
De onderstaande overwegingen gelden alleen voor patiënten met cataract in beide ogen bij wie het doel is beide ogen te opereren. Voorts betreft het alleen ogen zonder verhoogd operatierisico en zonder oogheelkundige comorbiditeit. In het geval van cataractoperatie aan beide ogen op dezelfde dag worden de beide operaties beschouwd als twee afzonderlijke ingrepen. Hierbij moeten diverse logistieke aanpassingen worden verricht en specifieke procedures worden gevolgd, zoals beschreven in ‘general principles for excellence in immediate sequential bilateral cataract surgery 2009’ (bron: eye foundation of canada - principles of ISBCS). Zie voor een specificatie van deze maatregelen voor de Nederlandse situatie de paragraaf ‘Aanvaardbaarheid, haalbaarheid en implementatie’.
Voor- en nadelen van de interventie en de kwaliteit van het bewijs
Er is literatuuronderzoek gedaan naar de plaats van ISBCS bij cataractchirurgie. Hierbij is gezocht naar studies die hebben gekeken naar patiënten die tegelijk aan beide ogen werden geopereerd (ISBCS) en die dit hebben vergeleken met patiënten die een cataractoperatie op verschillende dagen (>10-14) hebben ondergaan. Voor deze PICO werden zes relevante studies gevonden (waarvan twee nieuwe ten opzichte van de vorige update van de richtlijn in 2021): een systematisch review en meta-analyse waarvan de RCTs al beschreven waren in de oude richtlijn (Dickman, 2022), een systematische review en meta-analyse waarin drie observationele studies relevant bleken (Aiello, 2023), een systematisch review en meta-analyse die alleen RCTs heeft geïncludeerd (Kessel, 2015), één losse RCT (Spekreijse, 2023) en twee losse observationele studies (Herrinton, 2017; Arshinoff, 2011).
Voor de cruciale uitkomstmaat ‘verschil in afwijking van de doelrefractie’ kan er met zekerheid geconcludeerd worden dat er geen verschil is tussen patiënten die tegelijk aan beide ogen worden geopereerd (ISBCS) en patiënten die op twee verschillende dagen worden geopereerd (DSBCS) (hoge bewijskracht).
Endophthalmitis komt weinig voor en daarom is voor deze cruciale uitkomstmaat alleen gebruikgemaakt van observationele studies. Bilaterale endophthalmitis kwam niet voor in de ISBCS groep, maar is wel gerapporteerd door twee studies in de DSBCS groep. Er is met redelijke zekerheid te stellen dat er geen verschil is in risico op bilaterale endophthalmitis tussen ISBCS en DSBCS. Dezelfde conclusie kan getrokken worden voor unilaterale endophthalmitis. Antibioticagebruik kan effect hebben op endophthalmitis. Daarom hebben we ook een analyse gedaan binnen de ISBCS en DSBCS-groepen naar patiënten bij wie alleen intracameraal antibiotica zijn gebruikt. Tussen die twee groepen bestond geen verschil in het optreden van endophthalmitis.
Voor wat betreft serious adverse events (exclusief endophthalmitis) en achterste kapselruptuur is onderscheid gemaakt in bewijs afkomstig uit RCTs (zeer weinig events, maar geen bias doordat patienten met oogheelkundige comorbiditeit waarschijnlijk vaker in de DSBCS groep zaten) en cohort studies (meer events). Bewijs afkomstig uit RCTs liet zien dat er weinig tot geen verschil is tussen ISBCS en DSBCS (lage bewijskracht). Voor het bewijs afkomstig uit cohort studies is het erg onzeker of er een verschil is in effect tussen ISBCS en DSBCS (zeer lage bewijskracht). Dit komt met name doordat er een grote spreiding is rondom het gevonden effect en vanwege inconsistentie tussen studies. Er was geen verschil in adverse events tussen de ISBCS groep en de DSBCS groep, de bewijskracht hiervoor is laag. Voor de belangrijke uitkomstmaat macula oedeem werd ook onderscheid gemaakt tussen bewijs afkomstig uit RCTs en uit cohort studies. Het bewijs uit RCTs liet zien dat het met lage zekerheid te stellen is dat er geen verschil in macula oedeem is tussen ISBCS en DSBCS. Voor het bewijs uit cohort studies werd geen GRADE beoordeling gedaan, omdat de DSBCS groep meer mensen bevatten met een oculaire comorbiditeit, die automatisch een hoger risico op macula oedeem hebben.
Er werd geen verschil in patiënt-gerapporteerd functioneren gevonden tussen ISBCS en DSBCS op het moment dat beide ogen zijn geopereerd, de bewijskracht hiervoor is redelijk. Echter, in de periode tussen twee operaties is met redelijke zekerheid te zeggen dat ISBCS patiënten minder klachten van hun functioneren ervaren dan DSBCS patiënten. Het bewijs dat is gevonden heeft alleen betrekking op patiënten zonder oogheelkundige comorbiditeit. Bij patiënten met oogheelkundige comorbiditeit wordt in de literatuur standaard gekozen voor DSBCS.
Samenvattend lijken er geen tot weinig verschillen in eerder genoemde uitkomsten te zijn tussen ISBCS en DSBCS.
Waarden en voorkeuren van patiënten (en evt. hun verzorgers)
Bij het merendeel van de patiënten met cataract is er een indicatie voor een cataractoperatie aan beide ogen. Hoewel een cataractoperatie aan één oog al leidt tot herstel van functionele visus, leidt de operatie van het tweede oog (met cataract) tot een verdere verbetering in kwaliteit van leven en is het ook kosteneffectief (Frampton, 2014; Shekhawat, 2017). Voordelen voor patiënten indien beide ogen op dezelfde dag worden geopereerd zijn een verwacht verminderd aantal bezoeken aan het ziekenhuis, een minder belastend druppelschema, een mogelijk korter hersteltraject, een snellere definitieve aanpassing van refractie na de operaties en (vooral in geval van anisometropie) minder functionele beperking in de periode tussen de twee operaties. Nadelen voor de patiënt kunnen zijn een langere operatietijd, geen mogelijkheid voor subtenonale anaesthesie, verlies van gevoel van ‘veiligheid/risicoreductie’ door de operaties van beide ogen niet te scheiden. Patiënten met een hogere refractieafwijking zijn mogelijk meer gebaat bij een kortere periode tussen de twee opeenvolgende operaties vanwege de functionele beperkingen bij anisometropie en problemen met refractiecorrectie in de tussenliggende periode.
Voor deze keuze-optie is een keuzekaart ontwikkeld op basis waarvan het gesprek gevoerd kan worden: keuzekaart Staar bij volwassenen. Hierin staat ook uitleg over de voor-en nadelen van afwachten, operatie aan 1 of aan 2 ogen op aparte dagen en operatie aan 2 ogen op 1 dag.
Ethische overwegingen
Vanuit technisch perspectief lijkt er weinig in de weg te staan van een bilaterale cataractoperatie in één sessie, de vraag is dan of er op andere gronden hiertegen bedenkingen zijn. We moeten ervan uitgaan dat de beslissing om überhaupt tot lensextractie van beide ogen over te gaan al goed doordacht door de maximaal geïnformeerde patiënt genomen werd zonder dat bij die beslissing andere factoren een rol speelden dan het belang van de patiënt. Het was in de Nederlandse oogheelkunde tot slechts een tiental jaren geleden een algemeen geldend principe dat er nooit aan beide ogen tegelijkertijd intracoluair of direct aansluitend intra-oculair geopereerd werd, tenzij daar een dwingende reden toe bestond. Dit was een vorm van risicobeperking die door de meeste oogartsen als zeer fundamenteel gevoeld werd. Ook aan de patiënten werd uitgelegd dat er altijd maar één oog geopereerd werd en pas het tweede wanneer duidelijk geworden was dat er geen complicaties waren. In het algemeen werd een wachtperiode van minimaal twee weken aangehouden. Deze periode werd zelfs een prestatie-indicator toen er door verzekeraars gesignaleerd werd dat men er in een aantal praktijken toe neigde de wachtperiode te verkorten of twee ogen van een patiënt op één dag wilde opereren. Ook in andere landen werd een dergelijke tendens gezien. Bij deze herziening van de richtlijn cataract werd een literatuuronderzoek verricht naar onmiddellijk consecutieve bilaterale cataractchirurgie met als resultaat dat in de aanbevelingen opgenomen werd dat chirurgie aan twee ogen in één sessie overwogen kan worden.
Dat zal echter niet betekenen dat heel oogheelkundig Nederland in het vervolg zal overstappen op deze procedure en dat alle patiënten het zullen willen ondergaan. Daarvoor zit de reserve tegen intra-oculaire ingrepen aan beide ogen in één sessie waarschijnlijk te diep en zullen er ongetwijfeld oogartsen zijn die huiverig blijven voor complicaties in geval van bilaterale consecutieve chirurgie. Het is dan ook raadzaam om enige omzichtigheid te betrachten bij het inzetten van deze procedure. Een problematisch verlopen bilaterale cataractoperatie in één sessie zou de operateur wel eens zwaarder aangerekend kunnen worden dan in geval van ingrepen met tussentijds een wachtperiode. Een ingrijpende verschuiving van inzicht over emotioneel geladen kwesties kunnen soms onverwachte en ongewenste gevolgen hebben, als bijvoorbeeld psychische of juridische.
Oogartsen en andere professioneel betrokkenen doen er verstandig aan zich kritisch op te stellen in hun handelen en het welbevinden en de autonomie van de patiënt te respecteren en het uitgangspunt van ‘primum non nocere’, ook in niet-lichamelijke betekenis, niet uit het oog te verliezen.
Kosten (middelenbeslag)
Van belang is dat de mogelijkheid om beide ogen direct na elkaar te opereren op één dag in beginsel alleen geldt voor ogen zonder bijkomende risicofactoren en voor patiënten die voor beide ogen een indicatie hebben om aan cataract geopereerd te worden. Het verschil in kosten tussen DSBCS en ISBCS zal daarom alleen gelden voor deze groep patiënten. In Nederland wordt 55% (mediaan) van de patiënten binnen 12 maanden aan beide ogen aan staar geopereerd. Naar verwachting komt 80% van de patiënten die momenteel binnen 12 maanden aan beide ogen worden geopereerd in aanmerking voor ISBCS. In 2023 is een studie gepubliceerd die de doelmatigheid van bilateraal opereren van cataract op dezelfde dag versus een periode tussen de operaties vergelijkt in de Nederlandse situatie (Spekreijse, 2023). Dit onderzoek beschrijft dat het opereren van beide ogen op dezelfde dag kosteneffectief is. Er was enerzijds sprake van een betere kwaliteit van leven (uitgedrukt in QALY’s) bij de groep patiënten die aan beide ogen op dezelfde dag was geopereerd. Anderzijds was er sprake van een kostenbesparing. In Nederland zijn de besparingen mogelijk minder hoog vergeleken met andere landen waar gekeken is naar de kosteneffectiviteit van bilateraal opereren, omdat patiënten in Nederland minder ver hoeven te reizen voor de operaties en onderzoeken. Uit het Nederlands onderzoek blijkt met name een besparing in het aantal ziekenhuisbezoeken voor patiënten die aan beide ogen op dezelfde dag worden geopereerd. In de totale operatietijd en daarmee bezetting van de operatiekamer werd geen verschil gevonden. Gezien het feit dat ook bij het opereren van twee ogen op één dag de ingreep als twee aparte ingrepen worden beschouwd wat betreft middelen is er op dit vlak alleen een kostenbesparing buiten het ziekenhuis in thuiszorgkosten en apotheekkosten na de operatie. De besparing op thuiszorg, doordat ogen tegelijkertijd gedruppeld worden, geldt alleen voor patiënten die in aanmerking komen voor thuiszorg. De patiënten die in aanmerking komen voor ISBCS zijn mogelijk niet dezelfde als voor wie thuiszorg om te druppelen nodig is, waardoor besparing op thuiszorg in praktijk ook beperkt is, zie ook: https://www.zorginstituutnederland.nl/passende-zorg/publicaties/publicatie/2023/12/18/tweezijdige-staaroperatie. De uitvoering van het opereren van twee ogen op één dag heeft implicaties voor het voorraadbeheer van de operatieafdeling en is een logistieke uitdaging om aan de gestelde vereisten van de ‘ISBCS general principles for excellence (2009)’ en de specificatie hiervan voor de Nederlandse situatie, zie hiervoor ‘Aanvaardbaarheid, haalbaarheid en implementatie‘ te voldoen. Kosten die hier mogelijk verband mee houden zijn niet meegenomen in de Nederlandse kosteneffectiviteitsstudie. De mogelijkheid, bekostiging en inrichting van het operatieve proces voor elk oog afzonderlijk zal moeten blijven bestaan. Patiënten beide mogelijkheden kunnen aanbieden speelt een belangrijke rol in het "samen beslissen" over de beste zorg op maat voor de patiënt. Het effect op kosten speelt hierbij een ondergeschikte rol.
Aanvaardbaarheid, haalbaarheid en implementatie
Onderzoek naar de haalbaarheid van bilateraal opereren van cataract op dezelfde dag loopt op dit moment in Nederland. Om te voldoen aan de principal practice guideline for bilateral surgery (www.ISBCS.org) zijn diverse logistieke aanpassingen nodig (Sandhu, 2023). De operatie van het tweede oog wordt als een volledig nieuwe operatie beschouwd. Extra aanpassingen omvatten onder meer het gebruik van een nieuwe instrumentenset, gesteriliseerd in een aparte sterilisatiecyclus dan die van de set die gebruikt werd tijdens de ingreep aan het eerste oog.
-
- Niet-medicamenteuze producten (zoals disposable instrumenten, visco-elasticum en BSS) die tijdens de tweede operatie gebruikt worden, dienen uit andere productie-batches afkomstig te zijn dan bij de operatie van het eerste oog, vanwege risico op TASS aangetoond bij deze producten uit dezelfde batch.
- Voor intraoculaire medicatie die in de ziekenhuisapotheek conform GMP-z (zie https://nvza.nl/voor-professionals/gmp/) worden klaargemaakt, zijn gescheiden batches geen vereiste.
- Voor alle overige intra-oculaire medicatie gebruikt op OK (zoals b.v. aprokam), dienen verschillende batches gebruikt te worden en genoteerd in het dossier. De werkwijze is vastgelegd in een lokaal protocol waarbij 1 ampul bedoeld is voor single use en dus NIET voor meerdere patiënten gebruikt mag worden (NB. dit geldt ook voor sequentiële bilaterale cataractoperaties). Zie hiervoor de nieuwe SRI richtlijn “Voor Toediening Gereed Maken (VTGM) buiten de apotheek en toediening medicatie” die in de autorisatiefase is en binnenkort wordt gepubliceerd op de richtlijnendatabase.
- Voor extra-oculaire medicatie verwijzen we naar de SRI Richtlijn “Voor Toediening Gereed Maken (VTGM) buiten de apotheek en toediening medicatie”.
Deze logistieke aanpassingen dienen lokaal geïmplementeerd te worden.
Duurzaamheid
ISBSC is duurzamer dan DSBSC omdat bij ISBCS de patiënt minimaal 2 reisbewegingen minder hoeft te maken (één operatie afspraak en (minimaal) één controle afspraak na de eerste operatie) en er tevens minder thuiszorg noodzakelijk is.
Rationale van de aanbeveling: weging van argumenten voor en tegen de interventies
Gezien het zeer geringe tot afwezige verschil in cruciale uitkomstmaten bij patiënten met cataract aan twee ogen bij wie de cataractextractie aan beide ogen ofwel gelijktijdig (ISBCS) ofwel met een periode van minimaal twee weken tussentijd (DSBCS) plaatsvindt, kunnen beide interventies worden overwogen. Het gaat hierbij om ogen zonder oculaire comorbiditeit bij wie geen verhoogd complicatierisico is en patiënten die onder topicale dan wel algehele anaesthesie geopereerd kunnen worden. Van belang is dat de operaties in geval van ISBCS beschouwd worden als twee afzonderlijke operaties en dat de principal practice guideline for bilateral surgery (www.ISBCS.org) wordt gevolgd. Zie voor een specificatie van deze maatregelen voor de Nederlandse situatie de paragraaf ‘Aanvaardbaarheid, haalbaarheid en implementatie’. Vanuit kosten van vervoer, tijd en verzorging betreffende de patiënt en duurzaamheidperspectief gaat de voorkeur uit naar ISBCS.
Onderbouwing
Achtergrond
Currently, a minimum time frame of two weeks between consecutive cataract extractions in bilateral cataracts, with a check-up in the intervening period, is the standard. Arguments for waiting at least two weeks before operating on the second eye include the risk of vision-threatening postoperative complications, particularly the risk of endophthalmitis and the occurrence of a 'refractive surprise'. In these cases, it is advised to delay or adjust the operation of the second eye. With current techniques and materials, the cataract procedure appears to be so safe and precise that it may be considered to operate on both eyes simultaneously. In the Netherlands, more than 180,000 cataract surgeries are performed annually. It is plausible that operating on both eyes simultaneously would shorten the total treatment and recovery process for the patient, resulting in a faster final outcome and functional recovery. For these reasons, simultaneous operations could also be more favorable from a cost-effectiveness perspective and additionally provide health benefits for the patient.
Conclusies / Summary of Findings
|
High GRADE |
ISBCS results in little to no difference in deviation from the target refraction when compared with DSBCS in patients with indication for immediate bilateral sequential cataract surgery.
Sources: Sarikkola, 2011; Spekreijse, 2023 |
|
Moderate GRADE |
Bilateral endophthalmitis ISBCS likely does not reduce or increase bilateral endophthalmitis when compared with DSBCS in patients with indication for bilateral cataract surgery.
Sources: Malwankar, 2022; Lacy, 2022
Unilateral endophthalmitis ISBCS likely does not reduce or increase unilateral endophthalmitis when compared with DSBCS in patients with indication for bilateral cataract surgery.
Sources: Malwankar, 2022; Lacy, 2022; Herrinton, 2017; Buchan, 2020; Arshinoff, 2011 |
|
Low GRADE |
ISBCS may result in little to no difference in SAEs (only RCTs) when compared with DSBCS in patients with indication for immediate bilateral sequential cataract surgery.
Sources: Sarikkola, 2011; Serrano-Aguilar, 2012; Spekreijse, 2023 |
|
Very low GRADE |
The evidence is very uncertain about the effect of ISBCS on SAEs (only cohort studies) when compared with DSBCS in patients with indication for bilateral cataract surgery.
Sources: Herrinton, 2017; Buchan, 2020; Malwankar, 2022 |
|
Low GRADE |
ISBCS may result in little to no difference in AEs when compared with DSBCS in patients with for immediate bilateral sequential cataract surgery.
Sources: Sarikkola, 2011; Serrano-Aguilar, 2012; Spekreijse, 2023 |
|
Moderate GRADE |
ISBCS likely results in little to no difference in PCR (only RCTs) when compared with DSBCS in patients with indication for immediate bilateral sequential cataract surgery.
Sources: Sarikkola, 2011; Serrano-Aguilar, 2012; Spekreijse, 2023 |
|
Very low GRADE |
The evidence is very uncertain about the effect of ISBCS on PCR (only cohort studies) when compared with DSBCS in patients with indication for bilateral cataract surgery.
Sources: Herrinton, 2017; Buchan, 2020 |
|
Low GRADE |
ISBCS may result in little to no difference in macular edema (only RCTs) when compared with DSBCS in patients with indication for immediate bilateral sequential cataract surgery.
Sources: Sarikkola, 2011; Serrano-Aguilar, 2012; Spekreijse, 2023 |
|
no GRADE |
It is unclear whether there is a difference in macular edema between ISBCS and DSBCS.
Sources: Herrinton, 2017; Malwankar, 2022 |
|
Moderate GRADE |
ISBCS likely results in little to no difference in patient-reported functioning after 2nd eye surgery when compared with DSBCS in patients with indication for immediate bilateral sequential cataract surgery.
Sources: Lundström, 2006; Sarikkola, 2011; Serrano-Aguilar, 2012; Spekreijse, 2023 |
|
Moderate GRADE |
ISBCS likely increases patient-reported functioning between two surgeries when compared with DSBCS in patients with indication for immediate bilateral sequential cataract surgery.
Sources: Lundström, 2006; Serrano-Aguilar, 2012 |
Samenvatting literatuur
Description of studies
Dickman (2022) is a Cochrane review which assesses the safety and visual and patient-reported outcomes of ISBCS compared to DSBCS in people with bilateral age-related cataract and summarizes current evidence for the incremental resource use, utilities, costs, and cost effectiveness associated with the use of ISBCS compared to DSBCS. RCTs that were included in this review were described in the previous update of this guideline: Sarikkola (2011) and Serrano-Aguilar (2012).
Kessel (2015) is a SR and MA, including GRADE assessment, that investigated the effectiveness of ISBCS vs. DSBCS on patient-reported functioning, visual acuity, and residual refraction, and adverse events in patients without ocular comorbidity and without significantly deviating axial length (Lundström, 2006: <21 mm or >27 mm; Sarikkola, 2011: >26 mm). Only RCTs were included: Lundström (2006), Sarikkola (2011), and Serrano-Aguilar (2012). Due to the lack of data (e.g., SDs), the data from Lundström (2006) were not included for all outcomes in the calculation of the pooled effect. Lundström (2006) included 50 and 46 patients, Sarikkola (2011) included 247 and 255 patients, and Serrano-Aguilar (2012) included 417 and 390 patients in the ISBCS and DSBCS groups, respectively. Patient-reported functioning in the study by Lundström (2006) was measured using the Catquest questionnaire, which includes questions about the difficulty of performing daily activities and satisfaction with vision, providing a total score ranging from seven to 34. No SDs were reported by this study. Sarikkola (2011) assessed patient-reported functioning using the visual function questionnaire-7 (VF-7), and Serrano-Aguilar (2012) used the visual function questionnaire-14 (VF-14) (score ranging from 0-100), which includes items such as “How much difficulty do you have reading the newspaper?” scored on a five-point scale: 1; no difficulty – 5; stopped due to vision. Visual acuity (corrected) was described, and serious adverse events (SAE) and adverse events (AE) were reported. Missing data regarding residual refraction were retrieved from the individual studies. Sarikkola (2011) used the SRK/T formula for calculating lens strength. Lundström (2006) does not provide information about the lens formula used.
Spekreijse (2023) performed a randomized controlled trial (RCT) in which safety, effectiveness and cost-effectiveness of ISBCS and DSBCS were compared among 845 patients with indication for bilateral cataract surgery. Patients unable to follow study procedures, or with increased risk of endophthalmitis or refractive surprise were excluded. Ten hospitals in the Netherlands participated in this trial. In total, 865 patients were randomly assigned to a cataract surgery: 427 to ISBCS and 438 to DSBCS. Finally, 420 patients received ISBCS and 425 received DSBCS. The outcomes deviations from target refraction, SAEs, AEs, PCR, macular oedema, and patient-reported functioning (measured with NEI-VFQ-25 [range, 0-100] and Catquest-9SF Rasch [range, -3.94-+3.52 logits]) were reported. A limitation of the study is that patients could not be blinded to the treatment allocation, which could have led to bias in patient-reported functioning.
Herrinton (2017) performed a retrospective observational study comparing 1) ISBCS (n=3561) with 2) DSBCS (n=13711) in the United States. Data from patients were included if 1) there were data available on postoperative refraction and 2) the second eye (in the case of DSBCS) was operated on within one year. Patients operated on by a specialist ophthalmologist (glaucoma, oculoplastic surgeon, or retina specialist) were not included. Previous cases of endophthalmitis were also excluded. The groups differed in 1) the year the first eye was operated on; 2) ethnicity; 3) multi- or monofocal intraocular lens implant; and 4) preoperative comorbidities such as glaucoma, diabetic retinopathy, and macular degeneration. Both groups included surgeries with intracameral antibiotics (after 2013) or topical antibiotics (before 2013). Postoperative refraction was calculated as the spherical equivalent (sphere + (cylinder/2)) measured in diopters as soon as possible, but at least 3 weeks to 1 year postoperatively. Complications such as posterior capsule rupture/vitrectomy, endophthalmitis, and central macular edema were reported. For postoperative residual refraction, potential confounding from patient-reported variables, including the time to postoperative refraction, and chosen intraocular lens formula, which changed over the years, were adjusted for. The types of intraocular lens formulas were not reported.
Arshinoff (2011) describes a multicenter cohort study in which data on the incidence of endophthalmitis were collected through 1) a survey among members of the ‘International Society of Bilateral Cataract Surgeons’ and 2) a literature review. Data on the incidence of endophthalmitis were reported for 1) ISBCS (n=95606) and 2) DSBCS (n=29582). The incidence of endophthalmitis with 1) no intracameral antibiotic prophylaxis (no intraocular antibiotics or topical antibiotics) or 2) intracameral antibiotic prophylaxis was reported for ISBCS. For DSBCS, only cases where intracameral antibiotic prophylaxis was given were reported.
Aiello (2023) performed a systematic review (SR) and meta-analysis (MA), including risk of bias and GRADE assessment, in which the efficacy and safety profile of ISBCS was compared with DSBCS among patients with indication of bilateral cataract surgery. This SR and MA included both RCTs and non-randomized studies (NRS), however, only three big NRS were selected for the purpose of this guideline: Malwankar (2022); Lacy (2022) and Buchan (2020). Malwankar (2022) included 4014 patients (8028 eyes) and 1.940.965 patients (3.881.930 eyes), Lacy (2022) included 165.609 patients (331.218 eyes) and 3.695.440 patients (7.390.880 eyes), and Buchan (2020) included 1073 patients (2146 eyes) and 248.414 (496.682 eyes) in the ISBCS group and DSBCS group respectively. In the study of Malwankar (2022), the mean age of the cohort was 74.9 years (60% female). In the study by Lacy (2022), the mean age was not reported (58% female). The mean age in the study of Buchan (2020) was 75.5 years (60% female). The following outcomes were reported: (bilateral) endophthalmitis (Malwankar, 2022; Lacy, 2022; Buchan, 2020), serious adverse events (SAEs) (Malwankar, 2022; Buchan, 2020), posterior capsule rupture (PCR) (Buchan, 2020), and cystoïd macular edema (CME) (Malwankar, 2022). Each of the three studies had a high risk of bias, because of the study design being a multicenter retrospective cohort study.
Results
Deviation from target refraction
The outcome deviation from target refraction was reported by four studies (Herrinton, 2017; Lundström, 2006; Sarikkola, 2011; Spekreijse, 2023). Herrinton (2017) reported a mean spherical equivalent of -0.36D in the ISBCS group (n=3220) and -0.39D in the DSBCS group (n=12630), SDs were not reported. Lundström (2006) reported a mean rest spherical equivalent of -0.36D in the ISBCS group (n=50 patients) and -0.39D in the DSBCS group (n=46 patients) four months after surgery (also here, no SDs were reported). Sarikkola (2011) reported a rest spherical equivalent of 0.42±0.39D in the ISBCS group (n=244 patients) and 0.44±0.39D in the DSBCS group (n=247 patients), which resulted in a MD of -0.02 (95% CI -0.05 to 0.01). Spekreijse (2023) reported a mean ± SE deviation from target refraction of 0.32 ± 0.02D in the ISBCS group (n=420 patients) and 0.34 ± 0.02D in the DSBCS group (n=425 patients).
Complications
The outcome complications will be described for endophthalmitis, SAEs (excluding endophthalmitis), all potential AEs, and separately for PCR and macula edema.
Endophthalmitis
Bilateral endophthalmitis was reported by two studies (Malwankar, 2022; Lacy, 2022). The risk difference (RD) rather than risk ratio (RR) was reported here, because of the low incidence of endophthalmitis cases. Malwankar (2022) reported 0/4014 (0%) cases (patients) in the ISBCS group vs. 29/1 940 965 (0.0015%) cases of bilateral endophthalmitis in the DSBCS group (mean delay between surgeries 11.8 +/- 3.3 days). The RD was -0.00 (95% CI -0.00 to 0.00). Lacy (2022) reported 0/165 609 ISBCS (0%) cases vs. 7/3 695 440 DSBCS (0.00019%) cases, with a calculated RD of -0.00 (95% CI -0.00 to 0.00)(delay between surgery not specifically reported for these cases, author writes ‘they indeed had endophthalmitis in two eyes following cataract surgery in each eye’). This means no difference in risk of bilateral endophthalmitis between ISBCS and DSBCS. The studies were not pooled, because of the low number of studies.
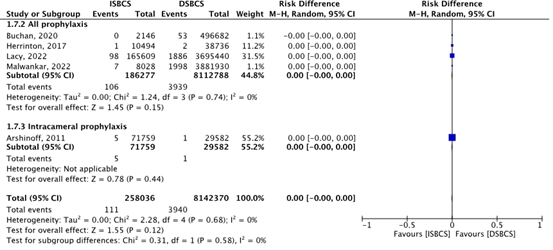
Unilateral endophthalmitis was reported by five studies (Malwankar, 2022; Lacy, 2022; Herrinton, 2017; Buchan, 2020; Arshinoff, 2011). In the group that used topical or intracameral prophylaxis, the total incidence of eyes with endophthalmitis was 106/186 277 (0.057%) in the ISBCS group and 3939/8 112 788 (0.049%) in the DSBCS group (Malwankar, 2022; Lacy, 2022; Herrinton, 2017; Buchan, 2020). In the group that used intracameral prophylaxis only, the total incidence of eyes with endophthalmitis was 5/71 759 (0.0070%) in the ISBCS group and 1/29 582 (0.0034%) in the DSBCS group (Arshinoff, 2011). This resulted in a pooled incidence of eyes with endophthalmitis of 111/258 036 (0.043%) in the ISBCS group and 3940/8 142 370 (0.048%) in the DSBCS group. The pooled RD was -0.00 (95% CI -0.00 to 0.00), which means no difference in risk of unilateral endophthalmitis between ISBCS and DSBCS (Figure 1).
Figure 1. Outcome endophthalmitis
The pooled incidence of eyes with unilateral endophthalmitis was 111/258 036 (0.043%) in the ISBCS group and 3940/8 142 370 (0.048%) in the DSBCS group
Z: p-value of the pooled effect; df: degrees of freedom; I2: statistical heterogeneity; CI: confidence interval; ISBCS: immediate sequential bilateral cataract surgery; DSBCS: delayed sequential bilateral cataract surgery. * The DSBCS cases in the study of Arshinoff (2011) were all treated with intracameral prophylaxis. In the study of Herrinton (2017), topical and intracameral prophylaxis are reported together. Totals are number of eyes
Serious adverse events (SAEs)
RCTs
In total, SAEs (which was the sum of corneal edema, macula edema, wound leak, iris prolapse, and posterior capsule rupture (PCR) incidence) were reported in three RCTs (Sarikkola, 2011; Serrano-Aguilar, 2012; Spekreijse, 2023). SAEs occurred in 34/2167 (1.57%) ISBCS eyes as compared to 34/2133 (1.59%) DSBCS eyes. The pooled RD was 0.00 (95% CI -0.01 to 0.01), which means no difference in risk of SAEs between ISBCS and DSBCS patients in RCTs (Figure 2).
Figure 2. Outcome serious adverse events (SAEs) - RCTs
The pooled number of SAEs was 34/2167 (1.57%) in the ISBCS group and 34/2133 (1.59%) in the DSBCS group
Z: p-value of the pooled effect; df: degrees of freedom; I2: statistical heterogeneity; CI: confidence interval; ISBCS: immediate sequential bilateral cataract surgery; DSBCS: delayed sequential bilateral cataract surgery. Totals are number of eyes
Cohort studies
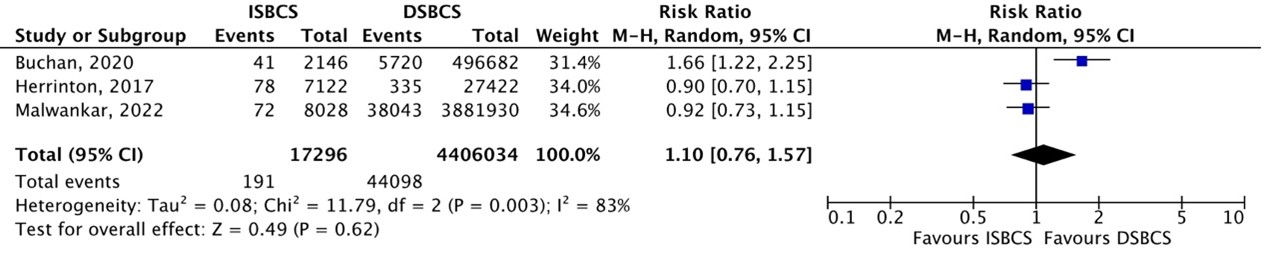
In total, SAEs (which was the sum of corneal edema, macular edema, wound leak, iris prolapse, and posterior capsule rupture (PCR) incidence) were reported in three cohort studies (Buchan, 2020; Herrinton, 2017; Malwankar, 2022;). SAEs occurred in 191/17296 (1.10%) ISBCS eyes as compared to 44098/4406034 (1.0%) DSBCS eyes. The pooled RR was 1.10 (95% CI 0.76 to 1.57), which means no difference in risk of SAEs between ISBCS and DSBCS patients in cohort studies (Figure 3).
Figure 3. Outcome serious adverse events (SAEs) – cohort studies
The pooled number of SAEs was 191/17296 (1.10%) in the ISBCS group and 44098/4406034 (1.0%) in the DSBCS group
Z: p-value of the pooled effect; df: degrees of freedom; I2: statistical heterogeneity; CI: confidence interval; ISBCS: immediate sequential bilateral cataract surgery; DSBCS: delayed sequential bilateral cataract surgery. Totals are number of eyes
Adverse events (AEs) (all types)
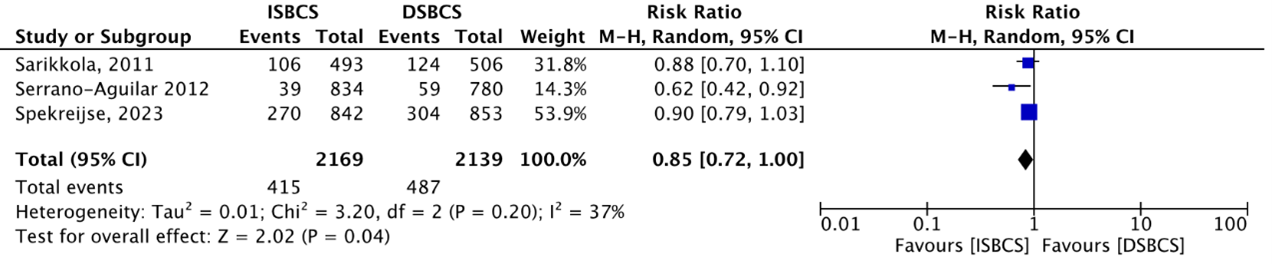
The outcome AEs (all types) was reported by three studies (Sarikkola, 2011; Serrano-Aguilar, 2012 and Spekreijse, 2023). The meta-analysis below showed that AEs occurred in a total of 415/2169 (19.1%) ISBCS eyes as compared to 487/2139 (22.8%) DSBCS eyes. This resulted in a RR of 0.85 (95% BI; 0.72 to 1.00), in favour of the ISBCS group (Figure 4), but not considered clinically relevant.
Figure 4. Outcome Adverse events (AEs)
The pooled number of AEs was 415/2169 (19.1%) in the ISBCS group and 487/2139 (22.8%) in the DSBCS group
Z: p-value of the pooled effect; df: degrees of freedom; I2: statistical heterogeneity; CI: confidence interval; ISBCS: immediate sequential bilateral cataract surgery; DSBCS: delayed sequential bilateral cataract surgery. Totals are number of eyes
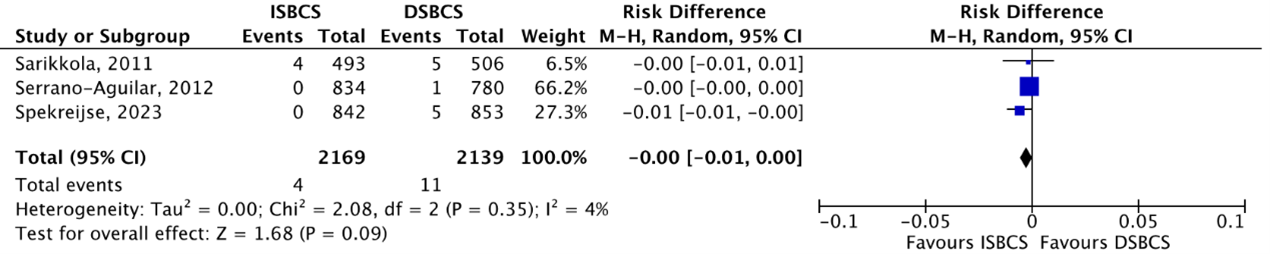
Posterior capsule rupture (PCR)
RCTs
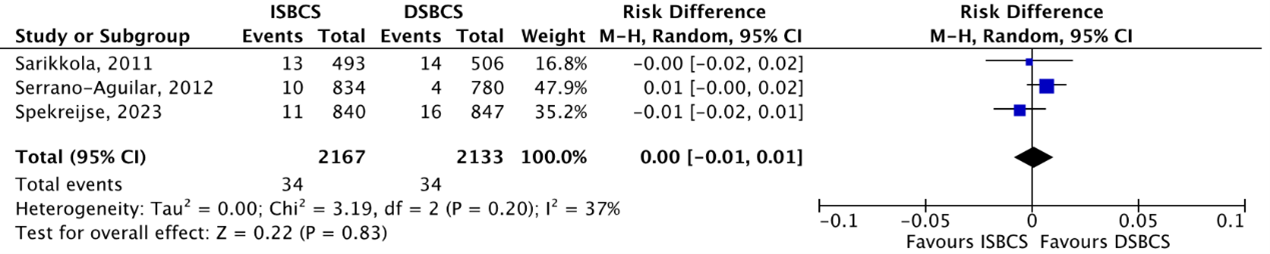
The outcome PCR was reported by three RCTs (Sarikkola, 2011; Serrano-Aguilar, 2012; Spekreijse, 2023). PCR was reported in 4/2169 (0.18%) ISBCS eyes as compared to 11/2139 (0.51%) DSBCS eyes. This resulted in a pooled RD of -0.00 (95% CI -0.01 to 0.00), which means there is no difference in effect on PCR between the two groups (Figure 5).
Figure 5. Outcome posterior capsule rupture (PCR) - RCTs
The pooled number of eyes with PCR was 4/2169 (0.18%) in the ISBCS group and 11/2139 (0.51%) in the DSBCS group
Z: p-value of the pooled effect; df: degrees of freedom; I2: statistical heterogeneity; CI: confidence interval; ISBCS: immediate sequential bilateral cataract surgery; DSBCS: delayed sequential bilateral cataract surgery; PCR, posterior capsule rupture. Totals are number of eyes
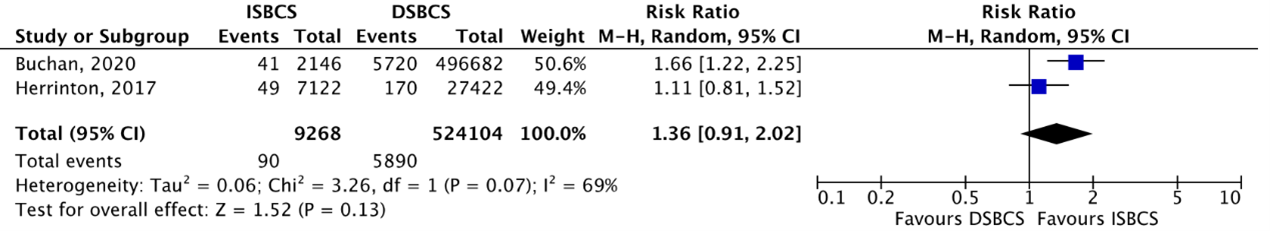
Cohort studies
The outcome PCR was reported by two cohort studies (Buchan, 2020; Herrinton, 2017). PCR was reported in 90/9268 (0.97%) ISBCS eyes as compared to 5890/524104 (1.12%) DSBCS eyes. This resulted in a pooled RR of 1.36 (95% CI 0.91 to 2.02), slightly in favour of DSBCS, but not clinically relevant (Figure 6).
Figure 6. Outcome posterior capsule rupture (PCR) – cohort studies
The pooled number of eyes with PCR was 90/9268 (0.97%) in the ISBCS group and 5890/524104 (1.12%) in the DSBCS group
Z: p-value of the pooled effect; df: degrees of freedom; I2: statistical heterogeneity; CI: confidence interval; ISBCS: immediate sequential bilateral cataract surgery; DSBCS: delayed sequential bilateral cataract surgery; PCR, posterior capsule rupture. Totals are number of eyes
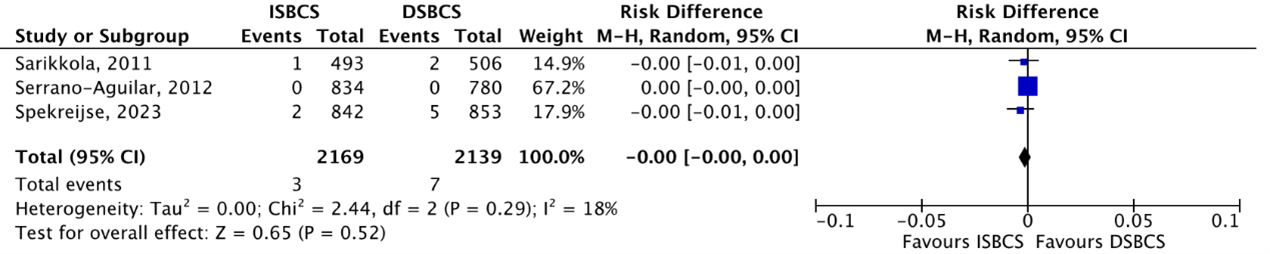
Macular edema
RCTs
The outcome macular edema was reported by three RCTs (Sarikkola, 2011; Serrano-Aguilar, 2012; Spekreijse, 2023). Macular edema occurred in 3/2169 (0.14%) ISBCS eyes compared to 7/2139 (0.33%) DSBCS eyes. This resulted in a pooled RD of -0.00 (95% CI -0.01 to 0.00), which means no difference in macular edema risk between the two groups (Figure 7).
Figure 7. Outcome macular edema – RCTs
The pooled number of eyes with macular edema was 2/2169 (0.14%) in the ISBCS group and 7/2139 (0.33%) in the DSBCS group
Z: p-value of the pooled effect; df: degrees of freedom; I2: statistical heterogeneity; CI: confidence interval; ISBCS: immediate sequential bilateral cataract surgery; DSBCS: delayed sequential bilateral cataract surgery;. Totals are number of eyes
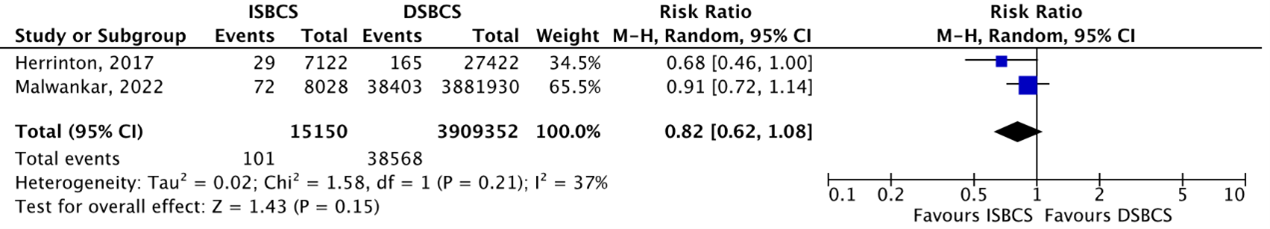
Cohort studies
The outcome macular edema was reported by two cohort studies (Herrinton, 2017; Malwankar, 2022). In the study of Herrinton (2017), it is important to mention that all patients with ocular comorbidity (and thus a higher risk of macular edema), were automatically included in the DSBCS group (source of selection bias). A GRADE assessment for this outcome could therefore not be performed. Macular edema occurred in 101/15150 (0.67%) ISBCS eyes and 38208/3909352 (0.98%) DSBCS eyes. This resulted in a pooled RR of 0.82 (95% CI 0.29 to 1.09), slightly in favour of the ISBCS group, but not considered clinically relevant (Figure 8).
Figure 8. Outcome macular edema – cohort studies
The pooled number of eyes with macular edema was 101/15150 (0.67%) in the ISBCS group and 38208/3909352 (0.98%) in the DSBCS group
Z: p-value of the pooled effect; df: degrees of freedom; I2: statistical heterogeneity; CI: confidence interval; ISBCS: immediate sequential bilateral cataract surgery; DSBCS: delayed sequential bilateral cataract surgery;. Totals are number of eyes
Patient-reported functioning
Patient-reported functioning after surgery in both eyes
Four studies reported the outcome patient-reporting functioning after surgery in both eyes (Lundström, 2006; Sarikkola, 2011; Serrano-Aguilar, 2012 and Spekreijse, 2023). The study of Lundström (2006) measured subjective functioning using the Catquest questionnaire (range, 7-34), but did not report SDs. The results could therefore not be used in the meta-analysis. The study of Sarikkola (2011) used the VF-7 score, and the VF-14 score was used by the study of Serrano-Aguilar (2012) (range, 0-100). In the study of Spekreijse (2023), vision-specific quality of life was measured by the NEI-VFQ-25 (range, 0-100) and the Catquest-9SF questionnaire (range, -3.94 logits - +3.52 logits). The NEI-VFQ-25 scores were used in the meta-analysis below. The reported Catquest-9SF Rasch Scores were -4.71 ± 1.75 (ISBCS, n=392 patients) and -4.70 ± 1.69 (DSBCS, n=381 patients). The studies were pooled, and a standardized mean difference was reported, because each study used a different questionnaire. The pooled standardized mean difference was -0.06 (95% BI -0.24, 0.11), which means there is no difference in effect on patient-reported functioning between the ISBCS and DSBCS group (Figure 9).
Figure 9. Outcome patient-reported functioning
The pooled standardized mean difference of the outcome patient-reported functioning was -0.06 (95% BI -0.24,0.11).
Z: p-value of the pooled effect; df: degrees of freedom; I2: statistical heterogeneity; CI: confidence interval; ISBCS: immediate sequential bilateral cataract surgery; DSBCS: delayed sequential bilateral cataract surgery. Totals are number of patients
Patient-reported functioning between first-eye and second-eye surgery
Lundström (2006) and Serrano-Aguilar (2012) reported about patient-reported functioning between first-eye and second-eye surgery. Using the VF-14 questionnaire, the mean ± SD score in the ISBCS group was 93.3 ± 12.8 and 81.3 ± 18.3 in the DSBCS group. This resulted in a MD of 12.0 (95% CI, 10.45 to 13.55) points, in favour of the ISBCS group (Serrano-Aguilar, 2012). In the study of Lundström (2006) who used the Catquest questionnaire, the mean score after two months was 8 (ISBCS) and 11.0 (DSBCS) points (P<0.001). SDs were not reported in this study.
Level of evidence of the literature
The level of evidence regarding the outcome measure deviation from the target refraction was retrieved from RCTs, and therefore started high. The level of evidence was not downgraded. The final level of evidence was graded ‘high’.
The level of evidence regarding the outcome measure endophthalmitis was retrieved from observational studies, and therefore started low. The level of evidence was not downgraded for risk of bias, because this has already been done by starting at a low level of evidence. There is also no downgrading for inconsistency, indirectness, imprecision, and publication bias. The evidence is upgraded with one level, because of the strong association (no difference between the groups). The final level of evidence was graded ‘moderate’.
The level of evidence regarding the outcome measure SAE was retrieved from RCTs and therefore started high. The level of evidence was downgraded by one level because of study limitations including problems with blinding of the patients and outcome assessors (-1, risk of bias) and 1 level because of a low number of cases (-1, imprecision). The final level of evidence was graded ‘low’.
The level of evidence regarding the outcome measure SAE was retrieved from observational studies, and therefore started low. The level of evidence was not downgraded for risk of bias, because this has already been done by starting at a low level of evidence. The level of evidence was downgraded by one level because of conflicting results between the three observational studies (-1, inconsistency). The final level of evidence was graded ‘very low’.
The level of evidence regarding the outcome measure AE was retrieved from RCTs and therefore started high. The level of evidence was downgraded by two levels because of study limitations including problems with blinding of the outcome assessor (-1, risk of bias) and the 95% CI around the pooled estimate crosses the left boundary of clinical relevance (-1, imprecision). The final level of evidence was graded ‘low’.
The level of evidence regarding the outcome measure PCR was retrieved from RCTs and therefore started high. The level of evidence was downgraded with one level because of study limitations including problems with blinding of the patients and outcome assessors (-1, risk of bias) and 2 levels because of a low number of cases (-2, imprecision). The final level of evidence was graded ‘low’.
The level of evidence regarding the outcome measure PCR was retrieved from observational studies, and therefore started low. The level of evidence was not downgraded for risk of bias, because this has already been done by starting at a low level of evidence. The level of evidence was downgraded by one level because of the 95% CI around the pooled estimate crosses one boundary of clinical relevance (-1, imprecision). The final level of evidence was graded ‘very low’.
The level of evidence regarding the outcome measure macular edema was retrieved from RCTs, and therefore started high. The level of evidence was downgraded by 2 levels because of a low number of cases (-2, imprecision). The final level of evidence was graded ‘low’.
A GRADE assessment could not be performed for macular edema that reported in observational studies, because selection bias was likely present in the study of Herrinton (2017). In this study, all patients with a higher risk of macular edema were automatically included in the DSBCS group.
The level of evidence regarding the outcome measure patient-reported functioning after surgery of two eyes was retrieved from RCTs and therefore started high. The level of evidence was downgraded by two levels because of study limitations including lack of blinding (-1, risk of bias). Even though the 95% CI crosses the left boundary of clinical relevance, the working group decided not to downgrade further for imprecision, because a larger study was added, that reported similar results as previous studies. The final level of evidence was graded ‘moderate’.
The level of evidence regarding the outcome measure patient-reported functioning between two surgeries was retrieved from RCTs and therefore started high. The level of evidence was downgraded by one level because of study limitations including lack of blinding (-1, risk of bias). The final level of evidence was graded ‘moderate’.
Zoeken en selecteren
A systematic review of the literature was performed to answer the following question:
What is the efficacy and safety of immediate sequential bilateral cataract surgery (ISBCS) compared with delayed sequential bilateral cataract surgery (DSBCS)?
| P: | Patients (18y) with an indication of bilateral cataract surgery |
| I: |
Immediate sequential bilateral cataract surgery (ISBCS) |
| C: | Delayed sequential bilateral cataract surgery (DSBCS) |
| O: | Deviations from the target refraction, complications (endophthalmitis, posterior capsule rupture (PCR), toxic anterior segment syndrome (TASS), corneal edema, macular edema, wound leakage, iris prolapse), patient-reported functioning |
Relevant outcome measures
The guideline development group considered deviations from target refraction and endophthalmitis as critical outcome measures for decision making. Other complications and patient-reported functioning were considered important outcome measures for decision making.
The working group defined the outcome measures as follows:
- Deviations from target refraction: proportions of eyes outside 0.50D or 1.0D
- Patient-reported functioning: standardized questionnaires (e.g. Catquest)
A priori, the working group did not define the outcome measure complications but used the definitions used in the studies.
For the outcome ‘deviations from target refraction’, the working group defined a mean difference (MD) of 5% between the groups as a minimal clinically (patient) important difference. For endophthalmitis, a risk difference (RD) >0 was defined as clinically relevant. For other complications a RR <0.80 or >1.25 was considered clinically relevant. For patient-reported functioning, a MD of 15% (based on the Catquest) was defined as clinically important.
Search and select (Methods)
The databases Medline (via OVID) and Embase (via Embase.com) were searched with -relevant search terms from may 2021 until 10-6-2024. The detailed search strategy is depicted under the tab Methods. The systematic literature search resulted in 246 hits. Studies were selected based on the following criteria: systematic reviews, randomized controlled trials and observational studies comparing ISBCS and DSBCS among patients with an indication for bilateral cataract surgery. Nine studies were initially selected based on title and abstract screening. After reading the full texts, 7 studies were excluded (see the table with reasons for exclusion under the tab Methods), 2 new studies were added to the previous selection. For the period <15-08-2019 the studies were selected from the previous guideline update. For the period 15-08-2019 to May 2021 the Cochrane review from Dickman (2022) was used, as the search and select from this review corresponds to the selection criteria of this guideline chapter. A total of 6 studies were included.
Results
Six studies were included in the analysis of the literature. Important study characteristics and results are summarized in the evidence tables. The assessment of the risk of bias is summarized in the risk of bias tables.
Referenties
- Aiello F, Gallo Afflitto G, Leviste K, Swaminathan SS, Yoo SH, Findl O, Maurino V, Nucci C. Immediate sequential vs delayed sequential bilateral cataract surgery: systematic review and meta-analysis. J Cataract Refract Surg. 2023 Nov 1;49(11):1168-1179. doi: 10.1097/j.jcrs.0000000000001230. PMID: 37276258.
- Arshinoff SA, Bastianelli PA. Incidence of postoperative endophthalmitis after immediate sequential bilateral cataract surgery. J Cataract Refract Surg. 2011 Dec;37(12):2105-14. doi: 10.1016/j.jcrs.2011.06.036. PMID: 22108106.
- Buchan JC, Donachie PHJ, Cassels-Brown A, Liu C, Pyott A, Yip JLY, Zarei-Ghanavati M, Sparrow JM. The Royal College of Ophthalmologists' National Ophthalmology Database study of cataract surgery: Report 7, immediate sequential bilateral cataract surgery in the UK: Current practice and patient selection. Eye (Lond). 2020 Oct;34(10):1866-1874. doi: 10.1038/s41433-019-0761-z. Epub 2020 Jan 7. PMID: 31911654; PMCID: PMC7608287.
- Herrinton LJ, Liu L, Alexeeff S, Carolan J, Shorstein NH. Immediate Sequential vs. Delayed Sequential Bilateral Cataract Surgery: Retrospective Comparison of Postoperative Visual Outcomes. Ophthalmology. 2017 Aug;124(8):1126-1135. doi: 10.1016/j.ophtha.2017.03.034. Epub 2017 Apr 21. PMID: 28438415; PMCID: PMC5531866.
- Lacy M, Kung TH, Owen JP, Yanagihara RT, Blazes M, Pershing S, Hyman LG, Van Gelder RN, Lee AY, Lee CS; IRIS® Registry Analytic Center Consortium. Endophthalmitis Rate in Immediately Sequential versus Delayed Sequential Bilateral Cataract Surgery within the Intelligent Research in Sight (IRIS®) Registry Data. Ophthalmology. 2022 Feb;129(2):129-138. doi: 10.1016/j.ophtha.2021.07.008. Epub 2021 Jul 13. PMID: 34265315; PMCID: PMC8755857.
- Lundström M, Albrecht S, Nilsson M, Aström B. Benefit to patients of bilateral same-day cataract extraction: Randomized clinical study. J Cataract Refract Surg. 2006 May;32(5):826-30. doi: 10.1016/j.jcrs.2006.01.075. PMID: 16765801.
- Malwankar J, Son HS, Chang DF, Dun C, Woreta F, Prescott C, Makary M, Srikumaran D. Trends, Factors, and Outcomes Associated with Immediate Sequential Bilateral Cataract Surgery among Medicare Beneficiaries. Ophthalmology. 2022 May;129(5):478-487. doi: 10.1016/j.ophtha.2021.12.015. Epub 2021 Dec 28. PMID: 34971649.
- Sandhu S, Liu D, Mathura P, Palakkamanil M, Kurji K, Rudnisky CJ, Kassiri K. Immediately sequential bilateral cataract surgery (ISBCS) adapted protocol during COVID-19. Can J Ophthalmol. 2023 Jun;58(3):171-178. doi: 10.1016/j.jcjo.2021.10.003. Epub 2021 Nov 9. PMID: 34919840; PMCID: PMC8576115.
- Sarikkola AU, Uusitalo RJ, Hellstedt T, Ess SL, Leivo T, Kivelä T. Simultaneous bilateral versus sequential bilateral cataract surgery: Helsinki Simultaneous Bilateral Cataract Surgery Study Report 1. J Cataract Refract Surg. 2011 Jun;37(6):992-1002. doi: 10.1016/j.jcrs.2011.01.019. Epub 2011 Apr 15. PMID: 21497049.
- Serrano-Aguilar P, Ramallo-Fariña Y, Cabrera-Hernández JM, Perez-Silguero D, Perez-Silguero MA, Henríquez-de la Fe F, Goás-Iglesias de Ussel J. Immediately sequential versus delayed sequential bilateral cataract surgery: safety and effectiveness. J Cataract Refract Surg. 2012 Oct;38(10):1734-42. doi: 10.1016/j.jcrs.2012.05.024. Epub 2012 Aug 9. PMID: 22884569.
- Spekreijse L, Simons R, Winkens B, van den Biggelaar F, Dirksen C, Bartels M, de Crom R, Goslings O, Joosse M, Kasanardjo J, Lansink P, Ponsioen T, Reus N, Schouten J, Nuijts R. Safety, effectiveness, and cost-effectiveness of immediate versus delayed sequential bilateral cataract surgery in the Netherlands (BICAT-NL study): a multicentre, non-inferiority, randomised controlled trial. Lancet. 2023 Jun 10;401(10392):1951-1962. doi: 10.1016/S0140-6736(23)00525-1. Epub 2023 May 15. PMID: 37201546.
Evidence tabellen
Evidence table for intervention studies (randomized controlled trials and non-randomized observational studies [cohort studies, case-control studies, case series])1
This table is also suitable for diagnostic studies (screening studies) that compare the effectiveness of two or more tests. This only applies if the test is included as part of a test-and-treat strategy – otherwise the evidence table for studies of diagnostic test accuracy should be used.
Research question:
|
Study reference |
Study characteristics |
Patient characteristics 2 |
Intervention (I) |
Comparison / control (C) 3
|
Follow-up |
Outcome measures and effect size 4 |
Comments |
|
Herrinton, 2017 |
Type of study: retrospective observational
Setting and country: Hospital, USA
Funding and conflicts of interest: Funded by the Garfield Memorial Fund, Kaiser Permanente, and the National Eye Institute (NEI R01 EY027329). These sponsors had no role in the design or conduct of this research. Kaiser Permanente potentially derives a benefit from improvements in operating room and clinic efficiency and implementation of immediate sequential bilateral cataract surgery, and this represents a potential conflict of interest. |
Inclusion criteria: health plan members who underwent their first noncomplex phacoemulsification for cataract. Information from manifest refractions for postoperative BCVA analysis. at least 1 year of enrollment before cataract surgery on the first eye.
Exclusion criteria: complex phacoemulsification cases and cases performed by glaucoma, oculoplastic, or retinal specialists, as well as procedures by any surgeon combined with corneal transplant or glaucoma surgery. Cases with previous endophthalmitis
N total at baseline: Intervention: 3561 Control: 13711
Important prognostic factors2: For example age ≤ 74y: I:54% C:51%
Sex: I: 39% M C: 38% M
Groups comparable at baseline? Different in: -year at first surgery -Race /ethnicity -Multifocal / monofocal -Glaucoma |
Describe intervention (treatment/procedure/test):
ISBCS, with surgery in the right and left eyes performed back-to-back on the same day,
|
Describe control (treatment/procedure/test):
DSBCS, with the 2 eyes operated on separate days, the second eye within 1 year of the first
|
Length of follow-up: 1 year
Incomplete outcome data: Intervention: N =341 (9,6%) Reasons (at random)
Control: N=1081 (7.9%) Reasons (at random)
|
Outcome measures and effect size (include 95%CI and p-value if available):
Refractive error (RE): RE ametropia vs emmetropia OR: 1.02 (95%CI 0.92 – 1.12), p=0.75 I: n=3220 C: n= 12630
Average postoperative RE I: -0.36D C: -0.39D
Surgical complications N=25 (0.7%) converted from ISBCS to DSBCS; due to: -Intraoperative posterior capsular rupture/ Vitrectomy (N=6) -Patient agitation or BP elevation (n=4) -Equipment problem (n=3) -Iris-related (n=3) -IOL had to be removed (n=2) Intraoperative corneal edema (n=2) -Zonular dehiscence (n=2) -Floppy capsular bag (n=1) -Ant capsular tear (n=1) -Intra-operative bleeding (n=1) à these not reported for the initial DSBCS group
Incidence Intraoperative posterior capsular rupture: I: 0.84 C: 0.67 (p=0.23)
Vitrectomy I: 0.42 C: 0.45 (p=0.82)
Post capsular rupture and/or vitrectomy: I: 0.93 (N=6 (0,2%) small post capsular rent) C: 0.88, p=0.79
Endophthalmitis (<120 days post op) I: 1/10494 eyes C: 2/38736 eyes, p=0.32
Macula edema (<120 days) (# patients), by OCT and treated with prednisolone I: 29/5247 pt = 0.55% C: 165/19368 pt = 0.85%, p=0.03 |
Postoperative RE was calculated as the spherical equivalent (sphere þ cylinder/2), measured in diopters (D); nearest the date of surgery during the interval 3 weeks to 1 year after surgery:
Emmetropia was defined as spherical error of _0.5 to 0 D, whereas eyes that were more myopic or hyperopic were defined as ametropic. |
|
Arshinoff, 2011 |
Type of study: cohort study
Setting and country: multicentre; International
Funding and conflicts of interest: Supported by the Eye Foundation of Canada (Mr. Bastianelli). |
Inclusion criteria: -Members of the iSBCS were surveyed, and the overall incidence of postoperative endophthalmitis after ISBCS was calculated from the collected data. -In addition, the literature was reviewed to determine the recent incidence of postoperative endophthalmitis in unilateral cataract surgery with and without the use of intracameral antibiotics; the data were compared with the collected results.
Exclusion criteria: All cases of postoperative endophthalmitis, irrespective of cause, were included except 2, 1 each in the vancomycin and moxifloxacin groups because in both cases the eye was noted to be clear 2 weeks postoperatively and the patients subsequently experienced trauma, causing wound rupture and then infection.
N total at baseline: Intervention: 95606 Control: 29582
Important prognostic factors2: For example age ± SD: I:NR C:NR
Sex: I: NR C: NR
Groups comparable at baseline? NR |
Describe intervention (treatment/procedure/test):
immediately sequential bilateral cataract surgery (ISBCS)
|
Describe control (treatment/procedure/test):
delayed sequential bilateral cataract surgery [DSBCS)
|
Length of follow-up:
Loss-to-follow-up: NR
Incomplete outcome data: NR
|
Outcome measures and effect size (include 95%CI and p-value if available):
Endophthalmitis incidence rate in ISBCS
NO IC agent (No IC agent (none C infusion)) :12/23847 (1/1987); 0.05%
IC profylaxis: 5/71759 (1/14352); 0.007%
All: 17/95606 (1/5759); 0.017%
In DSBCS 1/29582
|
|
|
Spekreijse, 2023 |
Type of study: RCT
Setting and country: 10 hospitals in the Netherlands
Funding and conflicts of interest: Research grant from The Netherlands Organization for Health Research and Development (ZonMw) and Dutch Ophthalmological Society. No conflicts of interest. |
Inclusion criteria: - Bilateral cataracts - Indication for cataract extraction - Surgery is expected to be uncomplicated
Exclusion criteria: - Age <18y - Insufficient understanding of the Dutch language to comply with study procedures and/or complete patient questionnaires - Inability to complete follow-up or comply with study procedures - non-routine cataract surgery - Cognitive or behavioural conditions that might interfere with surgery - Cataract surgery with premium IOL implantation - Conditions that increase the risk of endophthalmitis - Factors that increase the risk of refractive surprise - Conditions that increase the risk of corneal edema - Factors that increase the risk of complicated surgery - Sight-threatening comorbidity - Glaucoma or intraocular pressure of >24 mmHg - Uveitis - Diabetes mellitus with diabetic retinopathy and macular edema
N total at baseline: ISBCS: 421 pt DSBCS: 428 pt
Important prognostic factors2: age ± SD: ISBCS: 73 ± 6.9 yrs DSBCS: 73 ± 7.2
Sex: ISBCS: 48% M DSBCS: 43% M
Groups comparable at baseline? Yes |
Describe intervention: ISBCS
|
Describe control: DSBCS, time gap between first and second eye surgery of 2 weeks
|
Length of follow-up: 1 week, 4 weeks, and 3 months
Loss-to-follow-up: ISBCS: n=2 (0.48%) Reasons: unknown (n=1) and death (n=1)
DSBCS: n=4 (0.94%) Reasons: unknown (n=3) and death (n=1)
Incomplete outcome data: ISBCS: 1 (0.24%) Reasons: not reported
DSBCS: n=4 (0.94%) Reasons: not reported
|
Outcome measure-1 Deviation from target refraction
Mean ± SE ISBCS: 0.32 ± 0.02D DSBCS: 0.34 ± 0.02D
Outcome measure-2 SAEs Include cornea edema, CME, wound leakage, and PCR
Events/total (eyes) ISBCS: 11/842 (1.31%) DSBCS: 21/853 (2.46%) RR (95% CI): 0.53 (0.26 to 1.09)
Outcome measure-3 AEs
Events/total (eyes) ISBCS: 270/842 (32.1%) DSBCS: 304/853 (35.6%) RR (95% CI): 0.90 (0.79 to 1.03)
Outcome measure-4 PCR
Events/total (eyes) ISBCS: 0/842 (0%) DSBCS: 5/853 (0.59%) RR (95% CI): 0.09 (0.01 to 1.66)
Outcome measure-5 CME
Events/total (eyes) ISBCS: 2/842 (0.24%) DSBCS: 5/853 (0.59%) RR (95% CI): 0.41 (0.08 to 2.08)
Outcome measure-6 Patient-reported functioning Health related quality of life 3 months postoperatively
Mean ± SD ISBCS: 91.78 ± 9.54 DSBCS: 91.54 ± 9.11 SMD (95% CI): 0.03 (-0.11 to 0.17) |
Author’s conclusion: “Our results showed that ISBCS was non-inferior to DSBCS regarding postoperative refractive outcomes within 1·0 D and 0·5 D of target refraction and visual acuity outcomes. Additionally, we found no significant differences in complication rates and patient-reported outcome measures.” |
Notes:
- Prognostic balance between treatment groups is usually guaranteed in randomized studies, but non-randomized (observational) studies require matching of patients between treatment groups (case-control studies) or multivariate adjustment for prognostic factors (confounders) (cohort studies); the evidence table should contain sufficient details on these procedures
- Provide data per treatment group on the most important prognostic factors [(potential) confounders]
- For case-control studies, provide sufficient detail on the procedure used to match cases and controls
- For cohort studies, provide sufficient detail on the (multivariate) analyses used to adjust for (potential) confounders
Evidence table for systematic review of RCTs and observational studies (intervention studies)
Research question:
Risk of bias table for intervention studies (randomized controlled trials; based on Cochrane risk of bias tool and suggestions by the CLARITY Group at McMaster University)
Research question:
|
Study reference
(first author, publication year) |
Was the allocation sequence adequately generated?
Definitely yes Probably yes Probably no Definitely no |
Was the allocation adequately concealed?
Definitely yes Probably yes Probably no Definitely no |
Blinding: Was knowledge of the allocated interventions adequately prevented?
Were patients blinded?
Were healthcare providers blinded?
Were data collectors blinded?
Were outcome assessors blinded?
Were data analysts blinded?
Definitely yes Probably yes Probably no Definitely no |
Was loss to follow-up (missing outcome data) infrequent?
Definitely yes Probably yes Probably no Definitely no |
Are reports of the study free of selective outcome reporting?
Definitely yes Probably yes Probably no Definitely no |
Was the study apparently free of other problems that could put it at a risk of bias?
Definitely yes Probably yes Probably no Definitely no |
Overall risk of bias If applicable/necessary, per outcome measure
LOW Some concerns HIGH
|
|
Spekreijse, 2023 |
Definitely yes
Reason: randomization was done by using a certified electronic data capture tool (Castor EDC) |
No information |
Definitely no
Reason: surgeons, patients, and outcome assessors (medical doctors, optometrists, and ophthalmic technical assistants) were not masked to treatment allocation. |
Definitely yes
Reason: loss-to-fup was balanced across the groups |
Definitely yes
Reason: All relevant outcomes were reported |
Definitely yes
Reason: No other problems noted |
Some concerns (all outcomes)
Reason: problems with blinding
|
Randomization: generation of allocation sequences have to be unpredictable, for example computer generated random-numbers or drawing lots or envelopes. Examples of inadequate procedures are generation of allocation sequences by alternation, according to case record number, date of birth or date of admission.
Allocation concealment: refers to the protection (blinding) of the randomization process. Concealment of allocation sequences is adequate if patients and enrolling investigators cannot foresee assignment, for example central randomization (performed at a site remote from trial location). Inadequate procedures are all procedures based on inadequate randomization procedures or open allocation schedules..
Blinding: neither the patient nor the care provider (attending physician) knows which patient is getting the special treatment. Blinding is sometimes impossible, for example when comparing surgical with non-surgical treatments, but this should not affect the risk of bias judgement. Blinding of those assessing and collecting outcomes prevents that the knowledge of patient assignment influences the process of outcome assessment or data collection (detection or information bias). If a study has hard (objective) outcome measures, like death, blinding of outcome assessment is usually not necessary. If a study has “soft” (subjective) outcome measures, like the assessment of an X-ray, blinding of outcome assessment is necessary. Finally, data analysts should be blinded to patient assignment to prevents that knowledge of patient assignment influences data analysis.
Lost to follow-up: If the percentage of patients lost to follow-up or the percentage of missing outcome data is large, or differs between treatment groups, or the reasons for loss to follow-up or missing outcome data differ between treatment groups, bias is likely unless the proportion of missing outcomes compared with observed event risk is not enough to have an important impact on the intervention effect estimate or appropriate imputation methods have been used.
Selective outcome reporting: Results of all predefined outcome measures should be reported; if the protocol is available (in publication or trial registry), then outcomes in the protocol and published report can be compared; if not, outcomes listed in the methods section of an article can be compared with those whose results are reported.
Other biases: Problems may include: a potential source of bias related to the specific study design used (e.g. lead-time bias or survivor bias); trial stopped early due to some data-dependent process (including formal stopping rules); relevant baseline imbalance between intervention groups; claims of fraudulent behavior; deviations from intention-to-treat (ITT) analysis; (the role of the) funding body (see also downgrading due to industry funding https://kennisinstituut.viadesk.com/do/document?id=1607796-646f63756d656e74). Note: The principles of an ITT analysis implies that (a) participants are kept in the intervention groups to which they were randomized, regardless of the intervention they actually received, (b) outcome data are measured on all participants, and (c) all randomized participants are included in the analysis.
Overall judgement of risk of bias per study and per outcome measure, including predicted direction of bias (e.g. favors experimental, or favors comparator). Note: the decision to downgrade the certainty of the evidence for a particular outcome measure is taken based on the body of evidence, i.e. considering potential bias and its impact on the certainty of the evidence in all included studies reporting on the outcome.
Risk of bias table for interventions studies (cohort studies based on risk of bias tool by the CLARITY Group at McMaster University)
|
Author, year |
Selection of participants
Was selection of exposed and non-exposed cohorts drawn from the same population?
|
Exposure
Can we be confident in the assessment of exposure?
|
Outcome of interest
Can we be confident that the outcome of interest was not present at start of study?
|
Confounding-assessment
Can we be confident in the assessment of confounding factors?
|
Confounding-analysis
Did the study match exposed and unexposed for all variables that are associated with the outcome of interest or did the statistical analysis adjust for these confounding variables?
|
Assessment of outcome
Can we be confident in the assessment of outcome?
|
Follow up
Was the follow up of cohorts adequate? In particular, was outcome data complete or imputed?
|
Co-interventions
Were co-interventions similar between groups?
|
Overall Risk of bias
|
|
Definitely yes, probably yes, probably no, definitely no |
Definitely yes, probably yes, probably no, definitely no |
Definitely yes, probably yes, probably no, definitely no |
Definitely yes, probably yes, probably no, definitely no |
Definitely yes, probably yes, probably no, definitely no |
Definitely yes, probably yes, probably no, definitely no |
Definitely yes, probably yes, probably no, definitely no |
Definitely yes, probably yes, probably no, definitely no |
Low, Some concerns, High |
|
|
Herrinton, 2017 |
Definitely no
Reason: selection bias is likely due to the fact that patients with ocular comorbidities were automatically in the DSBCS group
|
Definitely yes
Reason: ISBCS cases were identified from a procedure code used by the health plan and from a laterality variable recorded into structured operative data
|
Probably yes
Reason: data on outcomes obtained from electronic medical records
|
Probably yes
Reason: data obtained from electronic medical records
|
Definitely yes
Reason: analyses are adjusted for patient-level variables, including stratified analyses on surgeon and various subgroup analyses
|
Probably yes
Reason: Outcomes were assessed by outcome assessors ( |
Definitely yes
Reason: missing outcome data were not imputed and after comparison with complete data, were assumed to be missing at random
|
Definitely yes
Reason: Most baseline characteristics were balanced across the groups |
Low (endophthalmitis)
Low (SAEs)
Low (PCR)
Low (CME)
|
|
Arshinoff, 2011 |
Definitely yes
Reason: |
Probably yes
Reason: Members of the ISBCS were surveyed + data of Sweden came from a national registry |
Probably yes
Reason: members of the ISBCS were asked about endophthalmitis cases and were certain about this |
No information
|
No information |
Probably yes
Reason: data about endophthalmitis cases were obtained by asking members of the ISBCS to retrospectively look back + literature review |
No information |
No information |
Low
(endophthalmitis)
|
|
Buchan, 2020 |
Definitely yes
Reason: both groups were selected from the Royal College of Ophthalmologists’ National Ophthalmology Database Audit (NOD) |
Probably yes
Reason: data could potentially be misclassified, but no reason to make this assumption |
Probably yes
Reason: data on outcomes obtained from NOD, no reason to think outcomes were present at the start of the study |
Probably yes
Reason: data could potentially be misclassified, but no reason to make this assumption |
Definitely no
Reason: there were a lot of baseline differences in confounders between ISBCS and DSBCS groups, for which there was no adjustment in the analysis |
Probably yes
Reason: data could potentially be misclassified, but no reason to make this assumption |
No information |
No information |
Some concerns (PCR, endophthalmitis)
Reason: lack of adjustment for confounders in the PCR analysis could have resulted in an overestimated OR.
|
|
Lacy, 2022 |
Probably no
Reason: selection bias is likely, because unilateral cases (which could be patients with more underlying conditions) were included in DSBCS group. |
Probably yes
Reason: clear definition of ISBCS and DSBCS |
Probably yes
Reason: no reason to think outcomes were present at the start of the study |
Probably yes
Reason: history of comorbid ophthalmic diseases were based on ICD10 and 9 codes, other confounders retracted from IRIS registry. Data could have been misclassified. |
Definitely yes
Reason: analyses with outcome endophthalmitis were adjusted for decade of life, race, insurance type, history of AMD, DR or glaucoma |
Probably yes
Reason: use of ICD10 and ICD9 codes within 4 weeks of surgery and a diagnosis code with supporting clinical findings at 2 and 6 weeks after cataract surgery. Data could have been misclassified, which could have resulted in higher endophthalmitis rate. + no access to data about complication, and patients with complication likely had higher rate of endophthalmitis |
No information |
No information |
Some concerns (endophthalmitis)
Reason: possibility of selection bias |
|
Malwankar, 2022 |
Definitely yes
Reason: both groups were selected from the Medicare fee-for-service carrier claims data. DSBCS patients could theoretically be candidates for ISBCS, because of the definition being <14 days between first and 2nd eye surgery in the DSBCS group |
Probably yes
Reason: Cataract surgery was identified by using Current Procedural Terminology (CPT) codes |
Probably yes
Reason: endophthalmitis occurring within 42 days after 2nd eye surgery and CME within 180 days after 2nd eye surgery |
Definitely yes
Reason: Patient demographic information was obtained from the Medicare Master Beneficiary Summary File. Social Security Administration Data are used to assign race and ethnicity and categories are validated. Patient zip codes were mapped to Federal Information Processing Standard codes |
Definitely no
Reason: no reason to assume that proper adjustment for confounders was done in statistical analyses |
Probably yes
Reason: ICD10 codes used to determine endophthalmitis laterality for patients undergoing bilateral cataract surgery after October 2015 & use of treatment codes CPT-4, which could have been performed to treat other medical conditions. |
Probably no
Reason: In 20% of endophthalmitis cases, laterality could not be ascertained. |
No information |
Some concerns (endophthalmitis, CME)
Reason: no proper adjustment for confounders in statistical analysis which could have led to overestimation of endophthalmitis + CME rates
|
Footnotes
Selection of participants Example of low risk of bias: Exposed and unexposed drawn for same administrative database of patients presenting at same points of care over the same time frame
Exposure Examples of low risk of bias: Secure record (e.g. surgical records, pharmacy records); Repeated interview or other ascertainment asking about current use/exposure
Confounding Examples of low risk of bias regarding assessment: Interview of all participants; Self-completed survey from all participants; Review of charts with reproducibility demonstrated; From database with documentation of accuracy of abstraction of prognostic data.
Example of low risk of bias regarding analysis: Comprehensive matching (e.g. with propensity score) or adjustment for all plausible confounding variables
NB: Preferably, the working group determined the minimal set of confounding variables which should be adjusted for. This should be done before the literature search and selection.
Assessment of outcome Examples of low risk of bias: Independent blind assessment; Record linkage; For some outcomes (e.g. fractured hip), reference to the medical record is sufficient to satisfy the requirement for confirmation of the fracture
Follow up Examples of low risk of bias: No missing outcome data; Reasons for missing outcome data unlikely to be related to true outcome (for
survival data, censoring is unlikely to introduce bias); Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; Missing data have been imputed using appropriated methods
Co-interventions Example of low risk of bias: Most or all relevant co-interventions that might influence the outcome of interest are documented to be similar in the exposed and unexposed
Table of excluded studies
|
Reference |
Reason for exclusion |
|
Alio JL, Gessa-Sorroche M, Nowrouzi A, Maldonado MJ. Immediate bilateral sequential cataract surgery. Arch Soc Esp Oftalmol (Engl Ed). 2022 Jul;97(7):402-408. doi: 10.1016/j.oftale.2022.02.010. Epub 2022 Apr 19. PMID: 35459602. |
More recent review is available |
|
Dickman MM, Spekreijse LS, Winkens B, Schouten JS, Simons RW, Dirksen CD, Nuijts RM. Immediate sequential bilateral surgery versus delayed sequential bilateral surgery for cataracts. Cochrane Database Syst Rev. 2022 Apr 25;4(4):CD013270. doi: 10.1002/14651858.CD013270.pub2. PMID: 35467755; PMCID: PMC9037598. |
A more recent review is available (Aiello, 2023), which also evaluated additional outcomes
|
|
Frampton G, Harris P, Cooper K, Lotery A, Shepherd J. The clinical effectiveness and cost-effectiveness of second-eye cataract surgery: a systematic review and economic evaluation. Health Technol Assess. 2014 Nov;18(68):1-205, v-vi. doi: 10.3310/hta18680. PMID: 25405576; PMCID: PMC4781176. |
Wrong comparison (investigates effectiveness of 2nd eye surgery) |
|
Hujanen P, Vaajanen A, Felin T, Lehtonen E, Syvänen U, Huhtala H, Helminen M, Sintonen H, Tuulonen A, Uusitalo-Järvinen H. Immediate sequential bilateral cataract surgery: a 13-year real-life report of 56 700 cataract operations. Br J Ophthalmol. 2023 Nov 22;107(12):1782-1786. doi: 10.1136/bjo-2021-320588. PMID: 36229178. |
No comparative study |
|
Lacy M, Kung TH, Owen JP, Yanagihara RT, Blazes M, Pershing S, Hyman LG, Van Gelder RN, Lee AY, Lee CS; IRIS® Registry Analytic Center Consortium. Endophthalmitis Rate in Immediately Sequential versus Delayed Sequential Bilateral Cataract Surgery within the Intelligent Research in Sight (IRIS®) Registry Data. Ophthalmology. 2022 Feb;129(2):129-138. doi: 10.1016/j.ophtha.2021.07.008. Epub 2021 Jul 13. PMID: 34265315; PMCID: PMC8755857. |
This study is included in the review of Aiello (2023) |
|
Malwankar J, Son HS, Chang DF, Dun C, Woreta F, Prescott C, Makary M, Srikumaran D. Trends, Factors, and Outcomes Associated with Immediate Sequential Bilateral Cataract Surgery among Medicare Beneficiaries. Ophthalmology. 2022 May;129(5):478-487. doi: 10.1016/j.ophtha.2021.12.015. Epub 2021 Dec 28. PMID: 34971649. |
This study is included in the review of Aiello (2023) |
|
Spekreijse LS, Nuijts RMMA. An update on immediate sequential bilateral cataract surgery. Curr Opin Ophthalmol. 2023 Jan 1;34(1):21-26. doi: 10.1097/ICU.0000000000000907. Epub 2022 Oct 20. PMID: 36206058; PMCID: PMC9835660. |
Narrative review, no comparative study |
Verantwoording
Beoordelingsdatum en geldigheid
Publicatiedatum : 09-05-2025
Beoordeeld op geldigheid : 07-04-2025
Algemene gegevens
Voor meer details over de gebruikte richtlijnmethodologie verwijzen wij u naar de Werkwijze. Relevante informatie voor de ontwikkeling/herziening van deze richtlijnmodule is hieronder weergegeven.
Samenstelling werkgroep
Voor het ontwikkelen van de richtlijnmodule is in 2022 een multidisciplinaire cluster ingesteld. Het cluster Oog bestaat uit meerdere richtlijnen, zie hier voor de actuele clusterindeling. De stuurgroep bewaakt het proces van modulair onderhoud binnen het cluster. De expertisegroepsleden geven hun expertise in, indien nodig. De volgende personen uit het cluster zijn betrokken geweest bij de herziening van deze module:
Clusterstuurgroep
- Mevr. dr. M.C. (Marjolijn) Bartels, Oogarts, NOG, voorzitter Oog cluster t/m maart 2025
- Mevr. dr. N.C. (Nicole) Naus, Oogarts, NOG, voorzitter cluster Oog vanaf april 2025
- Mevr. dr. A.J. (Arlette) van Sorge, Oogarts, NOG
- Dhr. drs. L.J. (Leo) Noordzij, Oogarts, NOG
- Mevr. dr. Ir M. (Marleen) van Aartrijk, klinisch fysicus, NVKF
- Mevr. C. (Caroline) Osterholt-Bel, patiëntvertegenwoordiger, de Oogvereniging
Clusterexpertisegroep
- Dhr. drs. R.H.J. (Robert Jan) Wijdh
- Mevr. dr. N. (Nienke) Visser
Met ondersteuning van
- Mevr. drs. D. (Dian) Ossendrijver, adviseur Kennisinstituut van de Federatie Medisch Specialisten
- Mevr. dr. A.C.J. (Astrid) Balemans, senior adviseur Kennisinstituut van de Federatie Medisch Specialisten
- Mevr. E (Esther) van der Bijl, informatiespecialist Kennisinstituut van de Federatie Medisch Specialisten
Belangenverklaringen
Een overzicht van de belangen van de clusterleden en het oordeel over het omgaan met eventuele belangen vindt u in onderstaande tabel. De ondertekende belangenverklaringen zijn op te vragen bij het secretariaat van het Kennisinstituut van de Federatie Medisch Specialisten via secretariaat@kennisinstituut.nl.
Clusterstuurgroep
|
Naam |
Hoofdfunctie |
Nevenwerkzaamheden |
Persoonlijke financiële belangen |
Persoonlijke relaties |
Extern gefinancierd onderzoek |
Intellectuele belangen en reputatie |
Overige belangen |
Datum |
Restrictie |
|
Marjolijn Bartels (Voorzitter cluster tm maart 2025) |
oogarts Deventer ziekenhuis |
Lid van Concilium, bestuurslid kwaliteit NOG en lid van cornea werkgroep (NOG). |
nee |
nee |
ja, 4. Deelname ZonMW- optimise. Lokale PI (vanaf jan 2025) |
meer persoonlijk belang van goed op de hoogte zijn van alles, hetgeen gebruikt wordt in de dagelijkse praktijk en als opleider, lid van Concilium, lid van commissie kwaliteit en voorzitter van cornea werkgroep. |
nee |
18-4-2025 |
Geen trekker of 1ste meelezer vanwege intellectueel belang bij BICAT |
|
Nicole Naus - Postema |
oogarts Erasmus MC Rotterdam |
Afdelingshoofd oogheelkunde Erasmus MC Voorzitter richtlijnencommissie NOG, Lid richtlijn PCC NVDV |
Geen |
Geen |
nee |
Geen |
Geen |
23-04-2025 |
Geen restricties |
|
Verpoorten - van Sorge |
Oogarts Koninklijke Visio |
Voorzitter VRS (vereniging voor revalidatie bij slechtziendheid, onbetaald). Stuurgroeplid bij cluster OOG (NOG). Visitator visitatiecommissie NOG. Bestuurslid Oogcontact (onbetaald). |
Nee |
nee |
nee |
nee |
nee |
06-05-2025 |
Geen restricties |
|
Leo Noordzij |
Oogarts bij Coöperatie Oogheelkunde op Zuid voor 3.5 dag in de week. |
Lid richtlijn werkgroep FMS/ NOG betreffende leeftijdsgebonden macula degeneratie (tm juli 2023). |
Geen. |
Geen. |
Geen. |
Geen. |
Geen. |
20-04-2025 |
Geen restricties ('advieswerk' neergelegd per juni/augustus 2022)
|
|
Marleen van Aartrijk |
Klinisch Fysicus bij Koninklijke Visio (Revalidatie & Advies): betaald,
|
nvt |
Geen persoonlijk financieel belang. Indien een richtlijn verwijzing naar revalidatiezorg stimuleert kan dat impact hebben op mijn werkgever (en dan indirect op mijzelf) |
Nvt |
Binnen de revalidatiezorg zijn er fondsen (oa Novum, ZonMW) die exptertiseprojecten ondersteunen. Aan een aantal van deze projecten draag ik bij, maar dat gaat om een zeer beperkt deel van mijn tijd (ben geen hoofdonderzoeker oid) |
Verduidelijking van rol revalidatiezorg in de zorgketen |
nvt |
22-04-2025 |
Geen restricties |
|
Caroline Osterholt- Bel |
Adviseur Oogzorg Oogvereniging |
TOA Ikazia |
nee |
nee |
nee |
nee |
nee |
29-4-2025 |
nee |
Clusterexpertisegroep
|
Naam |
Hoofdfunctie |
Nevenwerkzaamheden |
Persoonlijke financiële belangen |
Persoonlijke relaties |
Extern gefinancierd onderzoek |
Intellectuele belangen en reputatie |
Overige belangen |
Datum |
Restrictie |
|
Robert Jan Wijdh |
Oogarts, UMCG |
Geen |
Geen |
Geen |
Geen |
Geen |
Geen |
18-04-2025 |
Geen restricties |
|
Nienke Visser |
Oogarts MUMC Maastricht |
Lid cornea werkgroep ESCRS |
Geen |
Geen |
Hoofdonderzoeker Europese klinische studie naar dropless cataract surgery (Effectiveness of periocular drug injection in cataract surgery). Deze studie is gefinancierd door de ESCRS en zal begin 2025 zijn afgerond. |
Geen |
Geen |
23-10-2024 |
Geen restricties |
Inbreng patiëntenperspectief
Kwalitatieve raming van mogelijke financiële gevolgen in het kader van de Wkkgz
Bij de richtlijnmodule voerden de clusterleden conform de Wet kwaliteit, klachten en geschillen zorg (Wkkgz) een kwalitatieve raming uit om te beoordelen of de aanbevelingen mogelijk leiden tot substantiële financiële gevolgen. Bij het uitvoeren van deze beoordeling is de richtlijnmodule op verschillende domeinen getoetst (zie het stroomschema bij Werkwijze).
|
Module |
Uitkomst raming |
Toelichting |
|
Module BICAT |
Geen financiële gevolgen |
Voldoet niet aan criterium 1,2 en 3 |
Methode ontwikkeling
Evidence based
Zoekverantwoording
Algemene informatie
|
Cluster/richtlijn: Oog - Herziening tijdsbestek cataract |
|
|
Uitgangsvraag/modules: Welk tijdsbestek dient er minimaal tussen de cataractoperatie van het eerste en het tweede oog te zitten? |
|
|
Database(s): Embase.com, Ovid/Medline |
Datum: 10 juni 2024 |
|
Periode: vanaf mei 2021 |
Talen: geen restrictie |
|
Literatuurspecialist: Esther van der Bijl |
Rayyan review: https://rayyan.ai/reviews/1059514 |
|
BMI-zoekblokken: voor verschillende opdrachten wordt (deels) gebruik gemaakt van de zoekblokken van BMI-Online https://blocks.bmi-online.nl/ Deduplication: voor het ontdubbelen is gebruik gemaakt van http://dedupendnote.nl/ |
|
|
Toelichting: Voor deze vraag is gezocht op de elementen cataractoperatie EN tijdsbestek EN endophthalmitis (voor observationele studies).
De sleutelartikelen van Dickman et al. (2022) en Spekreijse et al. (2023) worden gevonden met deze search. |
|
|
Te gebruiken voor richtlijntekst: In de databases Embase.com en Ovid/Medline is op 10 juni 2024 systematisch gezocht naar systematische reviews, RCTs en observationele studies over het tijdsbestek tussen de cataractoperatie van het eerste en het tweede oog. De literatuurzoekactie leverde 246 unieke treffers op. |
|
Zoekopbrengst
10 juni 2024
|
|
EMBASE |
OVID/MEDLINE |
Ontdubbeld |
|
SR |
11 |
18 |
19 |
|
RCT |
105 |
142 |
193 |
|
Observationele studies |
16 |
27 |
34 |
|
Totaal |
132 |
187 |
246* |
*in Rayyan
Zoekstrategie
Embase.com 10 juni 2024
|
No. |
Query |
Results |
|
#1 |
'cataract'/exp OR 'cataract extraction'/exp OR ((lens* NEAR/2 opacit*):ti,ab,kw) OR cataract*:ti,ab,kw OR pseudoaphakia*:ti,ab,kw OR capsulorhexis:ti,ab,kw OR capsulorrhexis:ti,ab,kw OR phacoemulsification*:ti,ab,kw |
127488 |
|
#2 |
(('first eye' NEAR/1 cataract*):ti,ab,kw) OR (('second eye' NEAR/1 cataract*):ti,ab,kw) OR (('fellow eye' NEAR/1 cataract*):ti,ab,kw) OR ((simultaneous NEAR/2 (phaco* OR phako* OR cataract*)):ti,ab,kw) OR ((bilateral NEAR/2 (phaco* OR phako* OR endophthalmitis)):ti,ab,kw) OR (((bilateral OR sequentia OR interval) NEAR/2 ('cataract* surg*' OR 'cataract* extract*' OR 'cataract* remov*')):ti,ab,kw) OR (('same day' NEAR/2 (phaco* OR phako* OR cataract* OR surg*)):ti,ab,kw) OR (((bilateral OR sequential OR interval) NEAR/2 ('cataract* surg*' OR 'cataract* extract*' OR 'cataract* remov*')):ti,ab,kw) |
3394 |
|
#3 |
#1 AND #2 |
1695 |
|
#4 |
#3 AND [01-05-2021]/sd NOT ('conference abstract'/it OR 'editorial'/it OR 'letter'/it OR 'note'/it) NOT (('animal'/exp OR 'animal experiment'/exp OR 'animal model'/exp OR 'nonhuman'/exp) NOT 'human'/exp) |
325 |
|
#5 |
'meta analysis'/exp OR 'meta analysis (topic)'/exp OR metaanaly*:ti,ab OR 'meta analy*':ti,ab OR metanaly*:ti,ab OR 'systematic review'/de OR 'cochrane database of systematic reviews'/jt OR prisma:ti,ab OR prospero:ti,ab OR (((systemati* OR scoping OR umbrella OR 'structured literature') NEAR/3 (review* OR overview*)):ti,ab) OR ((systemic* NEAR/1 review*):ti,ab) OR (((systemati* OR literature OR database* OR 'data base*') NEAR/10 search*):ti,ab) OR (((structured OR comprehensive* OR systemic*) NEAR/3 search*):ti,ab) OR (((literature NEAR/3 review*):ti,ab) AND (search*:ti,ab OR database*:ti,ab OR 'data base*':ti,ab)) OR (('data extraction':ti,ab OR 'data source*':ti,ab) AND 'study selection':ti,ab) OR ('search strategy':ti,ab AND 'selection criteria':ti,ab) OR ('data source*':ti,ab AND 'data synthesis':ti,ab) OR medline:ab OR pubmed:ab OR embase:ab OR cochrane:ab OR (((critical OR rapid) NEAR/2 (review* OR overview* OR synthes*)):ti) OR ((((critical* OR rapid*) NEAR/3 (review* OR overview* OR synthes*)):ab) AND (search*:ab OR database*:ab OR 'data base*':ab)) OR metasynthes*:ti,ab OR 'meta synthes*':ti,ab |
1034825 |
|
#6 |
'clinical trial'/exp OR 'randomization'/exp OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp OR 'placebo'/exp OR 'prospective study'/exp OR rct:ab,ti OR random*:ab,ti OR 'single blind':ab,ti OR 'randomised controlled trial':ab,ti OR 'randomized controlled trial'/exp OR placebo*:ab,ti |
4046230 |
|
#7 |
'major clinical study'/de OR 'clinical study'/de OR 'case control study'/de OR 'family study'/de OR 'longitudinal study'/de OR 'retrospective study'/de OR 'prospective study'/de OR 'comparative study'/de OR 'cohort analysis'/de OR ((cohort NEAR/1 (study OR studies)):ab,ti) OR (('case control' NEAR/1 (study OR studies)):ab,ti) OR (('follow up' NEAR/1 (study OR studies)):ab,ti) OR (observational NEAR/1 (study OR studies)) OR ((epidemiologic NEAR/1 (study OR studies)):ab,ti) OR (('cross sectional' NEAR/1 (study OR studies)):ab,ti) |
8261718 |
|
#8 |
'case control study'/de OR 'comparative study'/exp OR 'control group'/de OR 'controlled study'/de OR 'controlled clinical trial'/de OR 'crossover procedure'/de OR 'double blind procedure'/de OR 'phase 2 clinical trial'/de OR 'phase 3 clinical trial'/de OR 'phase 4 clinical trial'/de OR 'pretest posttest design'/de OR 'pretest posttest control group design'/de OR 'quasi experimental study'/de OR 'single blind procedure'/de OR 'triple blind procedure'/de OR (((control OR controlled) NEAR/6 trial):ti,ab,kw) OR (((control OR controlled) NEAR/6 (study OR studies)):ti,ab,kw) OR (((control OR controlled) NEAR/1 active):ti,ab,kw) OR 'open label*':ti,ab,kw OR (((double OR two OR three OR multi OR trial) NEAR/1 (arm OR arms)):ti,ab,kw) OR ((allocat* NEAR/10 (arm OR arms)):ti,ab,kw) OR placebo*:ti,ab,kw OR 'sham-control*':ti,ab,kw OR (((single OR double OR triple OR assessor) NEAR/1 (blind* OR masked)):ti,ab,kw) OR nonrandom*:ti,ab,kw OR 'non-random*':ti,ab,kw OR 'quasi-experiment*':ti,ab,kw OR crossover:ti,ab,kw OR 'cross over':ti,ab,kw OR 'parallel group*':ti,ab,kw OR 'factorial trial':ti,ab,kw OR ((phase NEAR/5 (study OR trial)):ti,ab,kw) OR ((case* NEAR/6 (matched OR control*)):ti,ab,kw) OR ((match* NEAR/6 (pair OR pairs OR cohort* OR control* OR group* OR healthy OR age OR sex OR gender OR patient* OR subject* OR participant*)):ti,ab,kw) OR ((propensity NEAR/6 (scor* OR match*)):ti,ab,kw) OR versus:ti OR vs:ti OR compar*:ti OR ((compar* NEAR/1 study):ti,ab,kw) OR (('major clinical study'/de OR 'clinical study'/de OR 'cohort analysis'/de OR 'observational study'/de OR 'cross-sectional study'/de OR 'multicenter study'/de OR 'correlational study'/de OR 'follow up'/de OR cohort*:ti,ab,kw OR 'follow up':ti,ab,kw OR followup:ti,ab,kw OR longitudinal*:ti,ab,kw OR prospective*:ti,ab,kw OR retrospective*:ti,ab,kw OR observational*:ti,ab,kw OR 'cross sectional*':ti,ab,kw OR cross?ectional*:ti,ab,kw OR multicent*:ti,ab,kw OR 'multi-cent*':ti,ab,kw OR consecutive*:ti,ab,kw) AND (group:ti,ab,kw OR groups:ti,ab,kw OR subgroup*:ti,ab,kw OR versus:ti,ab,kw OR vs:ti,ab,kw OR compar*:ti,ab,kw OR 'odds ratio*':ab OR 'relative odds':ab OR 'risk ratio*':ab OR 'relative risk*':ab OR 'rate ratio':ab OR aor:ab OR arr:ab OR rrr:ab OR ((('or' OR 'rr') NEAR/6 ci):ab))) |
15144432 |
|
#9 |
#4 AND #5 – SR’s |
11 |
|
#10 |
#4 AND #6 NOT #9 – RCT’s |
105 |
|
#11 |
#4 AND ('endophthalmitis'/exp OR endophthalmitis:ti,ab,kw) AND (#7 OR #8) NOT (#9 OR #10) – Observationele studies |
16 |
|
#12 |
#9 OR #10 OR #11 |
132 |
Ovid/Medline 10 juni 2024
|
# |
Searches |
Results |
|
1 |
exp Cataract/ or exp Cataract Extraction/ or (lens* adj2 opacit*).ti,ab,kf. or cataract*.ti,ab,kf. or pseudoaphakia*.ti,ab,kf. or capsulorhexis.ti,ab,kf. or capsulorrhexis.ti,ab,kf. or phacoemulsification*.ti,ab,kf. |
85897 |
|
2 |
(('first eye' adj1 cataract*) or ('second eye' adj1 cataract*) or ('fellow eye' adj1 cataract*) or (simultaneous adj2 (phaco* or phako* or cataract*)) or (bilateral adj2 (phaco* or phako* or endophthalmitis)) or ((bilateral or sequentia or interval) adj2 ('cataract* surg*' or 'cataract* extract*' or 'cataract* remov*')) or ('same day' adj2 (phaco* or phako* or cataract* or surg*)) or ((bilateral or sequential or interval) adj2 ('cataract* surg*' or 'cataract* extract*' or 'cataract* remov*'))).ti,ab,kf. |
2460 |
|
3 |
1 and 2 |
1394 |
|
4 |
limit 3 to rd=20210501-20240610 |
679 |
|
5 |
4 not (comment/ or editorial/ or letter/) not ((exp animals/ or exp models, animal/) not humans/) |
644 |
|
6 |
meta-analysis/ or meta-analysis as topic/ or (metaanaly* or meta-analy* or metanaly*).ti,ab,kf. or systematic review/ or cochrane.jw. or (prisma or prospero).ti,ab,kf. or ((systemati* or scoping or umbrella or "structured literature") adj3 (review* or overview*)).ti,ab,kf. or (systemic* adj1 review*).ti,ab,kf. or ((systemati* or literature or database* or data-base*) adj10 search*).ti,ab,kf. or ((structured or comprehensive* or systemic*) adj3 search*).ti,ab,kf. or ((literature adj3 review*) and (search* or database* or data-base*)).ti,ab,kf. or (("data extraction" or "data source*") and "study selection").ti,ab,kf. or ("search strategy" and "selection criteria").ti,ab,kf. or ("data source*" and "data synthesis").ti,ab,kf. or (medline or pubmed or embase or cochrane).ab. or ((critical or rapid) adj2 (review* or overview* or synthes*)).ti. or (((critical* or rapid*) adj3 (review* or overview* or synthes*)) and (search* or database* or data-base*)).ab. or (metasynthes* or meta-synthes*).ti,ab,kf. |
751976 |
|
7 |
exp clinical trial/ or randomized controlled trial/ or exp clinical trials as topic/ or randomized controlled trials as topic/ or Random Allocation/ or Double-Blind Method/ or Single-Blind Method/ or (clinical trial, phase i or clinical trial, phase ii or clinical trial, phase iii or clinical trial, phase iv or controlled clinical trial or randomized controlled trial or multicenter study or clinical trial).pt. or random*.ti,ab. or (clinic* adj trial*).tw. or ((singl* or doubl* or treb* or tripl*) adj (blind$3 or mask$3)).tw. or Placebos/ or placebo*.tw. |
2736261 |
|
8 |
Epidemiologic studies/ or case control studies/ or exp cohort studies/ or Controlled Before-After Studies/ or Case control.tw. or cohort.tw. or Cohort analy$.tw. or (Follow up adj (study or studies)).tw. or (observational adj (study or studies)).tw. or Longitudinal.tw. or Retrospective*.tw. or prospective*.tw. or consecutive*.tw. or Cross sectional.tw. or Cross-sectional studies/ or historically controlled study/ or interrupted time series analysis/ [Onder exp cohort studies vallen ook longitudinale, prospectieve en retrospectieve studies] |
4746911 |
|
9 |
Case-control Studies/ or clinical trial, phase ii/ or clinical trial, phase iii/ or clinical trial, phase iv/ or comparative study/ or control groups/ or controlled before-after studies/ or controlled clinical trial/ or double-blind method/ or historically controlled study/ or matched-pair analysis/ or single-blind method/ or (((control or controlled) adj6 (study or studies or trial)) or (compar* adj (study or studies)) or ((control or controlled) adj1 active) or "open label*" or ((double or two or three or multi or trial) adj (arm or arms)) or (allocat* adj10 (arm or arms)) or placebo* or "sham-control*" or ((single or double or triple or assessor) adj1 (blind* or masked)) or nonrandom* or "non-random*" or "quasi-experiment*" or "parallel group*" or "factorial trial" or "pretest posttest" or (phase adj5 (study or trial)) or (case* adj6 (matched or control*)) or (match* adj6 (pair or pairs or cohort* or control* or group* or healthy or age or sex or gender or patient* or subject* or participant*)) or (propensity adj6 (scor* or match*))).ti,ab,kf. or (confounding adj6 adjust*).ti,ab. or (versus or vs or compar*).ti. or ((exp cohort studies/ or epidemiologic studies/ or multicenter study/ or observational study/ or seroepidemiologic studies/ or (cohort* or 'follow up' or followup or longitudinal* or prospective* or retrospective* or observational* or multicent* or 'multi-cent*' or consecutive*).ti,ab,kf.) and ((group or groups or subgroup* or versus or vs or compar*).ti,ab,kf. or ('odds ratio*' or 'relative odds' or 'risk ratio*' or 'relative risk*' or aor or arr or rrr).ab. or (("OR" or "RR") adj6 CI).ab.)) |
5709542 |
|
10 |
5 and 6 – SR’s |
18 |
|
11 |
(5 and 7) not 10 – RCT’s |
142 |
|
12 |
(5 and (exp Endophthalmitis/ or endophthalmitis.ti,ab,kf.) and (8 or 9)) not (10 or 11) – Observationele studies |
27 |
|
13 |
10 or 11 or 12 |
187 |