Behandeling intermediair-hoog risico longembolie
Uitgangsvraag
Wat is de optimale behandeling van patiënten met acute intermediair-hoog risico longembolie?
Aanbeveling
Behandel patiënten met een acute intermediair-hoog risico longembolie bij initiële presentatie alleen met anticoagulantia. Start bij voorkeur met LMWH vanwege later mogelijke aanvullende reperfusietherapie bij therapiefalen.
Overweeg bij hemodynamische verslechtering aanvullende reperfusietherapie. Bepaal op individuele basis de reperfusietherapie van voorkeur (systemische trombolyse, percutane katheter geleide interventies of chirurgische embolectomie).
Overwegingen
Voor- en nadelen van de interventie en de kwaliteit van het bewijs
Voor deze module is een literatuuranalyse gedaan naar de effectiviteit en veiligheid van systemische trombolyse, percutane katheter-geleide interventies en chirurgische embolectomie in vergelijking met het initiëren van therapeutische antistolling bij patiënten met een acute intermediar-hoog risico longembolie. De bewijskracht voor de cruciale uitkomstmaten (longembolie-gerelateerde mortaliteit, all-cause mortaliteit, hemodynamische verslechtering, bloedingscomplicaties en recidief longembolie) varieerde van hoog tot zeer laag. Voor de literatuuranalyse hebben we gekozen om naast RCT’s ook cohortstudies te includeren. Bij het selecteren van de studies zijn we streng geweest door alleen studies mee te nemen die van voldoende grootte waren en correctie voor confounding hadden toegepast. Echter, de cohortstudies zijn allen registratiestudies met de nodige methodologische tekortkomingen. De bepaling van de interventie was bij de studies onzeker (gebaseerd op ICD-codes of declaraties zonder een check of dit klopte) en ook confounding by indication speelde een grote rol.
Ondanks dat een van de grootste RCT’s, de PEITHO-trial (Meyer, 2014), aantoont dat systemische thrombolyse het risico op hemodyamische decompensatie verminderd in patiënten met intermediar-hoog risico longembolie (bewijskracht hoog), adviseert de werkgroep dit niet als standaardbehandeling. Dit omdat in deze zelfde studie een sterkverhoogd risco op ernstige bloedingen gezien werd, met name cerebrale bloedingen in de oudere populatie (>65 jaar).
Ook moet de conclusie en bewijskracht over mineure bloedingen in perspectief geplaatst worden. De bewijskracht en conclusie wordt vooral gedreven door de resultaten uit deze PEITHO-trial van Meyer en collegae (Meyer, 2014). Echter, een mineure bloeding heeft geen eenduidige definitie. Daarom is het lastig een gegeneraliseerde conclusie te trekken gebaseerd op alle studies.
Er is geen geschikte relevante literatuur over chirurgische embolectomie en dit maakt dat de werkgroep geen uitspraak kan doen over de effectiviteit en veiligheid van een chirurgische embolectomie in deze patiëntengroep.
Vanwege deze onzekerheden in de literatuur adviseert de werkgroep om patiënten met een acute intermediair-hoog risico longembolie bij initiële presentatie te behandelen met alleen anticoagulantia.
Anticoagulantia
Aanvullende reperfusietherapie, te weten systemische trombolyse (volle en gereduceerde dosis), percutane katheter-geleide interventies en chirurgische embolectomie, moeten worden gereserveerd voor patiënten met een intermediair-hoog risico longembolie, die hemodynamische instabiliteit ontwikkelen. In de PEITHO-trial (Meyer, 2014) was de gemiddelde tijd tussen randomisatie en overlijden of hemodynamisch decompensatie 1,79 ± 1,60 dagen in de placebo (alleen heparine) arm. Daarom lijkt het redelijk om patiënten met een acute intermediair-hoog risico longembolie gedurende de eerste 2-3 dagen met LMWH te behandelen, en nadat een patiënt gestabiliseerd is, na deze initiële periode pas over te stappen op orale antistolling. Het is minder wenselijk om deze patiënten initieel met orale antistolling, zoals DOACs te behandelen, vanwege de verhoogde kans op escalatie van de therapie, waaronder systemische trombolyse, en daarmee potentiële bloedingscomplicaties. De werkgroep adviseert bij patiënten met een acute intermediair-hoog risico longembolie te starten met LMWH gezien de kans op hemodynamische verslechtering en mogelijke escalatie naar aanvullende reperfusietherapie.
Patiënten met een acute intermediair-hoog risico longembolie bij wie therapeutische antistolling wordt geïnitieerd, dienen gecontroleerd te worden op therapie succes dan wel therapie falen. In het geval van therapiefalen is er sprake van progressief rechterventrikelfalen, zich uitend in hemodynamische verslechtering of circulatoire collaps, of het persisteren van rechterventrikeldysfunctie met bijbehorende kliniek onder adequate therapeutische antistolling. Er zijn geen eenduidige definities beschikbaar van therapie succes dan wel falen. De werkgroep sluit zich aan bij de volgende voorgestelde definities (Pruszczyk, 2022):
1. Therapiesucces: De initiële behandeling resulteert in de verbetering van de aanvankelijk gecompromitteerde hemodynamische status: een verlaging van de hartslag en ademhalingsfrequentie, verbetering van de systemische bloeddruk, zuurstofsaturatie en verbetering van de perifere perfusie. In dit scenario is geen escalatie van de therapie vereist.
2. Therapiefalen: Er is geen verbetering van de vitale parameters na 24-48 uur adequate therapeutische antistolling dan wel hemodynamische verslechtering en of circulatoire collaps. Als de patiënt na het starten van de behandeling met anticoagulantia hemodynamische instabiliteit ontwikkelt waarvoor vasopressie, reanimatie of ECMO (Extra Corporale Membraan Oxygenatie) noodzakelijk is, is er een duidelijke indicatie voor escalatie van therapie. Verslechtering bij aanvankelijk hemodynamisch stabiele PE-patiënten kan zich ook kenmerken door een progressieve tachycardie of ademhalingsfrequentie, daling van de systemische bloeddruk of zuurstofsaturatie, of door tekenen van orgaanhypoperfusie (afname van de urineproductie, stijging van het lactaat) gedurende ten minste 15 minuten, zonder te voldoen aan de officiële criteria van shock.
In het geval van therapiefalen dient reperfusietherapie in de vorm systemische trombolyse, percutane katheter-geleide interventies dan wel chirurgische embolectomie overwogen te worden.
In het geval van absolute of relatieve contra-indicaties voor therapeutische antistolling of systemische thrombolyse kunnen katheter-geleide interventies dan wel chirurgische embolectomie overwogen worden. De therapeutische interventie van voorkeur, de te verwachten winst en risico’s, dienen per patiënt op individuele basis afgewogen te worden. Indien in het ziekenhuis een multidisciplinair EXPERT-PE-team aanwezig is, lijkt het rationeel om deze beslissing te nemen binnen dit team (Huisman, 2017).
Monitoring
Internationale richtlijnen (Konstantinides, 2020) adviseren dat patiënten met acute intermediair-hoog risico longembolieën gedurende de eerste uren tot dagen gemonitord moeten worden gezien het risico op vroege hemodynamische decompensatie. Er worden geen uitspraken gedaan over de duur van monitoring, wat deze monitoring inhoudt (welke parameters vervolgt moeten worden) en waar deze idealiter plaatsvindt. De werkgroep heeft in het kader van een SSC Subcommittee Project/Collaborative Project, genaamd Standardized risk stratification of acute pulmonary embolism, een literatuur search verricht (zie bijlage bij deze module (onder Zoekstrategie)) en geen geschikte literatuur geïdentificeerd om hierover een aanbeveling te kunnen doen. De werkgroep kan daarom geen aanbevelingen doen over de soort en duur van monitoring van patiënten met acute intermediair-hoog risico longembolieën. Onderzoek in de toekomst zal mogelijk meer inzicht geven in een betere identificatie van patiënten met een hoge kans op hemodynamische decompensatie en mortaliteit en wat dit betekent voor monitoring.
Waarden en voorkeuren van patiënten (en evt. hun verzorgers).
Er werd aandacht besteed aan het patiëntenperspectief door in de literatuur search kwaliteit van leven en PROMS mee te nemen. Er waren echter geen studies beschikbaar die deze uitkomstmaten hadden gerapporteerd.
Het patiëntenperspectief bij de behandeling van intermediair-hoog risico acute longembolie met reguliere antistolling en de keuze tussen wel of geen aanvullende reperfusietherapie indien zich een verslechtering voordoet is belangrijk. Antistolling kan voor sommige patiënten een minder invasieve en minder riskante behandelingsoptie zijn met mogelijk minder bijwerkingen. Aan de andere kant is systemische trombolyse snel en effectief bij een acuut potentieel levensgevaarlijke situatie, maar brengt het ook potentiële bloedingsrisico's met zich mee.
Het patiëntenperspectief kan variëren afhankelijk van verschillende factoren zoals de ernst van de symptomen, reeds bestaande andere gezondheidsproblemen en pre-existent functioneren, risico's en bijwerkingen van de behandeling, en persoonlijke voorkeuren. Het is belangrijk om dit met de patiënt te bespreken en samen tot een passende behandeling te komen. Dit houdt ook in om samen met de patiënt een duidelijk gewogen keuze te maken in de behandeling gaat zijn, mocht er na starten van reguliere antistolling verslechtering optreden en aanvullende reperfusietherapie noodzakelijk geacht worden. Uiteraard kan in samenspraak altijd worden afgezien van behandeling met reguliere antistolling of van escalatie met reperfusietherapie, afhankelijk van bovengenoemde factoren en voorkeuren.
Kosten (middelenbeslag)
Het gebruik van anticoagulantia, zoals beschreven in deze module, brengt geen of nauwelijks gevolgen met zich mee voor de zorgkosten. De kosten voor aanvullende reperfusietherapie zijn hoger vanwege de prijs van de trombolyse medicatie en materiaalkosten van de katheters. Perlroth (2007) heeft een analyse verricht waaruit blijkt dat trombolyse kosteneffectief kan zijn voor een selecte subgroep hemodynamisch stabiele patiënten met een intermediair hoog risico longembolie, waarbij het risico van overlijden hoog is.
Aanvaardbaarheid, haalbaarheid en implementatie
In de verschillende fasen van de richtlijnontwikkeling is rekening gehouden met de implementatie van de richtlijn (module) en de praktische uitvoerbaarheid van de aanbevelingen. De aanbevelingen zullen de huidige klinische praktijk zodanig weinig veranderen dat er geen problemen voorzien zijn in aanvaardbaarheid, haalbaarheid en er geen separaat implementatieplan is ontwikkeld. Het initiëren van aanvullende reperfusietherapie zal mogelijk meer en langdurige opnamecapaciteit van de ziekenhuizen vragen.
Rationale van de aanbeveling: weging van argumenten voor en tegen de interventies
De werkgroep is van mening dat momenteel patiënten met acute intermediair-hoog risico longembolie alleen behandeld dienen te worden met therapeutische antistolling. Huidige studies leveren onvoldoende bewijskracht om systemische thrombolyse, percutane katheter-geleide interventies en chirurgische embolectomie aan te kunnen bevelen in deze patiëntengroep. Studies tonen geen eenduidige verlaging van de longembolie gerelateerde mortaliteit, terwijl het effect op de bloedingscomplicaties onzeker is. Op basis van de huidige beschikbare literatuur kan de werkgroep geen uitspraak doen over het effect van systemische trombolyse en percutane katheter-geleide interventies op recidief longembolieën, kwaliteit van leven en het voorkomen van CTEPH/CTEPD zonder PH op de langere termijn. In de afweging van de aanbeveling zijn ook belasting van de ziekenhuiscapaciteit en kosten meegenomen.
Onderbouwing
Achtergrond
Acute intermediate-high risk pulmonary embolism compasses patients with acute PE who are hemodynamically stable but have a high early mortality risk. Early mortality of patients with acute intermediate-high risk pulmonary embolism (PE) is around 3%. Despite hemodynamic stability at the time of presentation, the risk of hemodynamic decompensation is around 4% in the first 48 hours after diagnosis (Meyer, 2014). According to the ESC-guidelines (Konstantinides, 2020), the first choice of treatment is initiation of anticoagulants and not rescue reperfusion therapy, i.e. systemic thrombolysis, percutaneous catheter-guided intervention (encompassing local thrombolysis, and/or fragmentation, and/or thrombus aspiration), or surgical embolectomy.
This patiënt group with acute intermediate-high risk PE is identified following the risk-adjusted management strategy of the ESC guidelines (Konstantinides, 2020). Risk stratification of patients with acute PE is recommended for determining the appropriate therapeutic management approach and begins upon suspicion of the disease and initiation of the diagnostic workup. This prognostic risk score combines clinical, imaging, and laboratory parameters to permit a (semi)quantitative assessment of early PE-related risk of death.
However the implications of this risk score for patient management in the acute setting in some domains is not always clear because of gaps in evidence. Also the severity of symptoms at time of clinical presentation in, the high early mortality rates, and uncertainties regarding long-term outcomes (including quality of life, post-pulmonary embolism syndrome, and chronic thromboembolic pulmonary hypertension), leads to variation in therapeutic management and sometimes the use of rescue reperfusion therapy. In daily clinical practice, this also leads to variation in how long and in what way these patients are monitored during admission in the hospital. Sometimes patients are admitted to a regular nursing ward and in other hospitals to an medium care or intensive care unit, with consequences for hospital capacity and costs. This latter subject is not the primary focus of this module.
Conclusies / Summary of Findings
PE-related mortality
|
Very low GRADE |
The evidence is very uncertain about the effect of CDT or ST on PE-related mortality when compared with AC in patients with acute intermediate-high risk PE.
Source: Kroupa, 2022; Sadeghipour, 2022; Zhang, 2018; Kucher, 2014; Fasullo, 2011 |
All-cause mortality
|
Very low GRADE |
The evidence is very uncertain about the effect of CDT or ST on all-cause mortality when compared with AC in patients with acute intermediate-high risk PE.
Source: Kroupa, 2022; Sadeghipour, 2022; Kucher, 2014; Zhang, 2018; Meyer, 2014; Sharifi, 2012; Fasullo, 2011; Konstantinides, 2002; Krishnan, 2022; Hobohm, 2021; Lin, 2021; Stein, 2020; Arora, 2017; Patel, 2015 |
Hemodynamic deterioration
|
Very low GRADE
High GRADE |
CDT + AC vs AC The evidence is very uncertain about the effect of CDT + AC on hemodynamic deterioration when compared with AC in patients with acute intermediate-high risk PE.
Source: Sadeghipour, 2022
ST + AC vs AC ST + AC reduces the risk of hemodynamic deterioration when compared with AC in patient with acute intermediate-high risk PE.
Source: Meyer, 2014 |
Bleeding complications
|
Very low GRADE
Very low GRADE
High GRADE |
Major bleeding events The evidence is very uncertain about the effect of CDT or ST on major bleedings when compared with AC in patients with acute intermediate-high risk PE.
Source: Kroupa, 2022; Sadeghipour, 2022; Lin, 2021; Kucher, 2014; Meyer, 2014; Sharifi, 2012; Fasullo, 2011; Konstantinides, 2002
Minor bleeding events CDT + AC vs AC The evidence is very uncertain about the effect of CDT + AC on minor bleeding events when compared with AC in patients with acute intermediate-high risk PE.
Source: Kroupa, 2022; Sadeghipour, 2022; Kucher, 2014
ST + AC vs AC ST + AC increases the risk of minor bleeding events when compared with AC in patient with acute intermediate-high risk PE.
Source: Zhang, 2018; Meyer, 2014; Sharifi, 2012; Fasullo, 2011 |
Recurrence of pulmonary embolism
|
Very low GRADE |
ST + AC vs AC The evidence is very uncertain about the effect of ST + AC on recurrence of pulmonary embolism when compared with AC in patients with acute intermediate-high risk PE.
Source: Zhang, 2018; Meyer, 2014; Sharifi, 2012; Konstantinides, 2002
CDT + AC vs ST + AC The evidence is very uncertain about the effect of CDT + AC on recurrence of pulmonary embolism when compared with ST + AC in patients with acute intermediate-high risk PE.
Source: Lin, 2021 |
Samenvatting literatuur
Description of studies
Planer and colleagues (Planer, 2023) undertook a systematic review into therapeutic options for patients with intermediate or high-risk PE. Anticoagulation, ST, and CDT were considered as treatment options. The authors searched multiple databases from inception to 18 October 2022. RCTs, cohort studies, and case-control studies were included if patients presented with intermediate or high-risk PE and a comparison between the stated treatment options was made.
Of the total of 44 studies included by Planer (Planer, 2023), a total of 8 RCTs and 7 cohort studies were included in our literature analysis (Table 2). The majority of studies were excluded because of a publication date before 2000 or a sample size smaller than 500 for cohort studies. An overview of the characteristics of the included studies is given in Table 2. Study populations of these studies contains at least partially acute intermediate high-risk PE patients. Although not explicitly stated in some cohort studies, it is assumed that CDT or ST is always followed by the initiation of AC.
Table 2: Overview of included studies from Planer (2023)
|
Author, year |
Na |
Intervention |
Comparator |
Follow-up |
|
RCT |
|
|
|
|
|
Kroupa, 2022 |
23 |
CDT + AC |
AC |
30 days |
|
Sadeghipour, 2022 |
94 |
CDT + AC |
AC |
3 months |
|
Zhang, 2018b |
66 |
ST (low dose) + AC |
AC |
3 months after discharge |
|
Kucher, 2014 |
59 |
CDT (USAT) + AC |
AC (UFH) |
3 months after discharge |
|
Meyer, 2014 |
1005 |
ST + AC |
AC |
30 days |
|
Sharifi, 2013b |
121 |
ST (low dose) + AC |
AC |
28 months |
|
Fasullo, 2011 |
72 |
ST + AC |
AC |
6 months |
|
Konstantinides, 2002 |
256 |
ST + AC |
AC (UFH) |
30 daysc |
|
Cohort studyd |
|
|
|
|
|
Krishnan, 2022 |
13.325 |
CDT + AC |
ST + AC or AC |
Unclear |
|
Hobohm, 2021 |
> 40000 |
CDT + AC |
ST + AC or AC |
Unclear |
|
Lin, 2021 |
1303 |
CDT + AC |
ST |
> 3 years |
|
Stein, 2020 |
6340 |
CDT + AC |
AC |
Unclear |
|
Arora, 2017 |
3384 |
CDT + AC |
ST + AC |
Unclear |
|
Patel, 2015 |
Unmatched: 1521 |
CDT + AC |
ST + AC |
Unclear |
AC, anticoagulation; CDT, catheter-directed thrombolysis; ST, systemic thrombolysis; USAT, Ultrasound-accelerated thrombolysis; UFH, unfractionated heparine
a. Total number of included patients; for cohort studies, the number of matched participant if applicable.
b. These studies used a low-dose ST. The meta-analyses showed no inconsistency potentially caused by low-dose ST. Therefore, no separate analyse on dosage was performed.
c. Not reported but deduced from figure in paper.
d. In this sudies ICD codes were used to determine use of CDT; frequently groups with ultrasound assistance and without were analyzed together.
Results
PE-related mortality
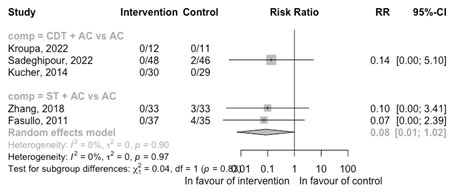
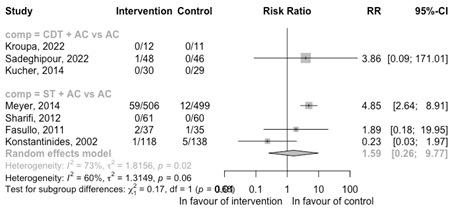
Only the RCTs reported data on PE-related mortality. The result of the meta-analysis is shown in Figure 3.
CDT + AC versus AC
Three RCTs compared CDT + AC with AC. Two RCTs reported no deaths due to PE. The one remaining RCT saw no deaths due to PE in the intervention group and two deaths in the control group. Because of the low number of deaths, no conclusion could be drawn.
ST + AC versus AC
Two RCTs compared ST + AC versus AC. The RCTs saw no deaths due to PE in the intervention group. The total of seven deaths due to PE were all in the group which received only anticoagulation. The low number of events results in effect estimate with very broad confidence intervals. It is therefore difficult to conclude anything of the interventions on the outcome PE-related mortality.
Figure 3: Meta-analysis of RCTs for the outcome PE mortality
ST, systemic thrombolysis; CDT, catheter-directed thrombolysis; AC, anticoagulation
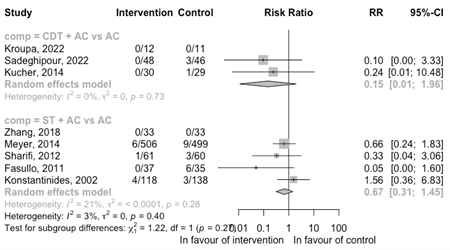
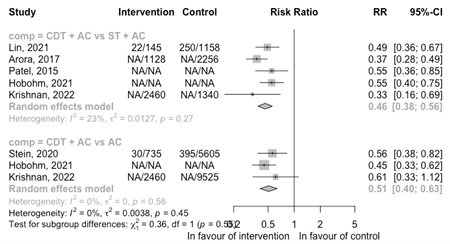
All-cause mortality
The results of the meta-analysis per comparisons for the outcome all-cause mortality is shown in Figure 4 for the RCTs and in Figure 5 for cohort studies.
CDT + AC versus (ST +) AC
Three RCTs compared CDT + AC with AC (Figure 4). One RCT reported no death. The remaining RCTs saw no deaths in the intervention group and four deaths in the control group. Because of the low number of deaths, no conclusion could be drawn.
Three cohort studies, comparing CDT + AC with AC, reported a reduced risk of mortality (RR 0.51 95%CI 0.40 to 0.63) (Figure 5). A similar reduced risk was observed for the comparison with ST + AC (RR 0.46 95%CI 0.38 to 0.56). Included cohortstudies were registry-based studies with problems with the intervention assessment and confounding by indication resulting in a high risk of bias. Therefore, no conclusion could be drawn regarding the outcome all-cause mortality.
ST + AC versus AC
Four RCTs compared ST + AC versus AC (Figure 4). There is an indication that the risk of death is reduced with ST + AC versus AC (RR 0.67 95%CI 0.31 to 1.45). However, the confidence interval is broad.
Figure 4: Meta-analysis of RCTs for the outcome all-cause mortality
ST, systemic thrombolysis; CDT, catheter-directed thrombolysis; AC, anticoagulation
Figure 5: Meta-analysis of cohort studies for the outcome all-cause mortality
ST, systemic thrombolysis; CDT, catheter-directed thrombolysis; AC, anticoagulation; NA, not reported in the original article.
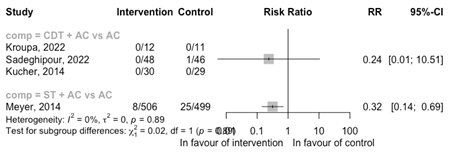
Hemodynamic deterioration
CDT + AC versus AC
Three RCTs reported on the outcome hemodynamic deterioration (Figure 6). Only one RCT reported one hemodynamic instability despite treatment with vasopressor agent (Sadeghipour, 2022). Because of the low number of events, no conclusion could be drawn.
ST + AC versus AC
One RCT reported events of hemodynamic decompensation (Meyer, 2014; Figure 6) comparing ST + AC with AC. A reduced risk of hemodynamic deterioration is observed with ST + AC versus AC (RR 0.32 95%CI 0.14 to 0.69).
Figure 6: Meta-analysis of RCTs for the outcome hemodynamic deterioration
ST, systemic thrombolysis; CDT, catheter-directed thrombolysis; AC, anticoagulation
Bleeding complications
Major bleeding events
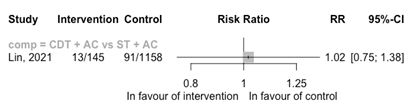
CDT + AC versus (ST +) AC
Three RCTs comparing CDT + AC with AC reported on the outcome major bleedings (Figure 7). Only one RCT reported one major bleeding event in the intervention group (Sadeghipour, 2022). Because of the low number of events, no conclusion could be drawn.
One cohort study compared CDT + AC with ST + AC (Figure 8). The risk of a major bleeding event was similar between the groups (RR 1.02 95%CI 0.75 to 1.38). The included cohort study was registry based with problems with the intervention assessment and confounding by indication resulting in a high risk of bias.
ST + AC versus AC
Four RCTs reported major bleeding events (Figure 7 & 8) comparing ST + AC with AC. The results from the RCTs are inconsistent. The largest RCT reported an increased risk; however, the other two RCTs with a low number of events (n ≤ 5) indicated an increased risk or a reduced risk both with broad confidence intervals.
Figure 7: Meta-analysis of RCTs for the outcome major bleeding events
ST, systemic thrombolysis; CDT, catheter-directed thrombolysis; AC, anticoagulation
Figure 8: Meta-analysis of cohort studies for the outcome major bleeding events
ST, systemic thrombolysis; CDT, catheter-directed thrombolysis; AC, anticoagulation
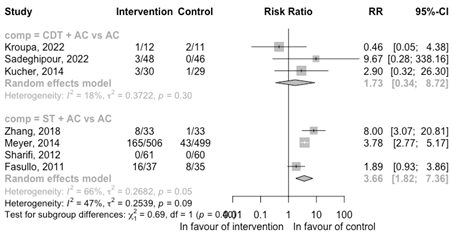
Minor bleeding events
CDT + AC versus AC
Three RCTs reported on the outcome minor bleedings (Figure 9). The RCTs suggest a potential increased risk of minor bleeding events for CDT + AC (RR 1.73 95%CI 0.34 to 8.72); however, the number of events is low (in total n ≤ 10).
ST + AC versus AC
Four RCTs reported events on minor bleeding events (Figure 9) comparing ST + AC with AC. A increased risk of minor bleeding events is observed with ST + AC versus AC (RR 3.66 95%CI 1.82 to 7.36).
Figure 9: Meta-analysis of RCTS for the outcome minor bleeding events
ST, systemic thrombolysis; CDT, catheter-directed thrombolysis; AC, anticoagulation
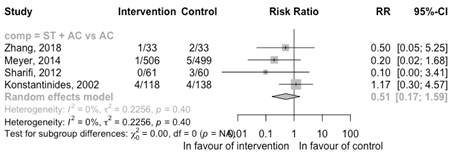
Recurrence of pulmonary embolism
CDT + AC versus ST + AC
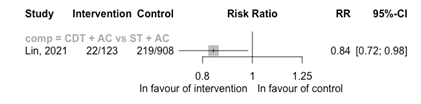
No RCTs reported the outcome of a recurrence. One cohort study reported on the comparison CDT + AC versus ST + AC (Figure 11). The risk of a recurrence is reduced with CDT + AC versus ST + AC (RR 0.84 95%CI 0.72 to 0.98). The cohort study was a registry-based study with problems with the intervention assessment and confounding by indication resulting in a high risk of bias.
ST +AC versus AC
Four RCTs comparing ST + AC with AC reported data on a recurrence of PE (Figure 10). There may be a reduced risk of a recurrence with ST + AC compared with AC (RR 0.51 95%CI 0.17 to 1.59); however, the confidence interval is broad suggesting potentially a decreased risk, no risk or increased risk.
Figure 10 Meta-analysis of RCTs for the outcome recurrence of pulmonary embolism
ST, systemic thrombolysis; CDT, catheter-directed thrombolysis; AC, anticoagulation
Figure 11: Meta-analysis of cohort studies for the outcome recurrence of pulmonary embolism
ST, systemic thrombolysis; CDT, catheter-directed thrombolysis; AC, anticoagulation
Quality of life/PROMS
None of the included studies reported any results on quality of life or PROMS.
Chronic thrombo-embolic pulmonary hypertension (CTEPH)/Chronic thrombo-embolic pulmonary disease (CTEPD) without pulmonary hypertension
None of the included studies reported any results on this outcome.
Level of evidence of the literature
PE-related mortality
The level of evidence starts at high as RCTs reported on this outcome. The level of evidence was downgraded by three levels to very low because of imprecision (very broad confidence interval).
All-cause mortality
The level of evidence is assessed separately for each study design. As the assessment of the level of evidence was the same per comparison, no distinction between the comparions was made.
- RCTs: the level of evidence starts at high and was downgraded by three levels to very low because of imprecision (very broad confidence interval).
- Cohort studies: The level of evidence starts at low for observational studies for the domain intervention. Risk of bias assessment of the cohortstudies showed issues with the intervention definition and confounding by indication. Therefore, we further downgraded from low to very low due to risk of bias.
Hemodynamic deterioration
The level of evidence starts at high as RCTs reported on this outcome.
- The level of evidence was downgraded by three levels for the comparison CDT+ AC versus AC, because of imprecision (very broad confidence interval).
- The level of evidence was not downgraded for the comparison ST + AC versus AC. Although the number of events was low, the sample size (N > 337 per arm) was sufficient to detect an effect.
Bleeding complications
Major bleeding:
- Because of the low number of events, the level of evidence for the comparison CDT + AC versus AC was not assessed.
- The level of evidence starts at low as cohort studies reported for this outcome and the comparison CDT + AC versus ST + AC. Risk of bias assessment of the cohort studies showed issues with the intervention definition and confounding by indication. Therefore, we further downgraded from low to very low due to risk of bias.
- The level of evidence starts at high as RCTs reported for this outcome and comparison. The level of evidence was downgraded by three levels for the comparison ST + AC versus AC, because of imprecision (confidence interval crosses both boundaries of minimal important difference).
Minor bleeding:
The level of evidence starts at high as RCTs reported on this outcome.
- The level of evidence was downgraded by three levels for the comparison CDT+ AC versus AC, because of imprecision (very broad confidence interval).
- The level of evidence was not downgraded for the comparison ST + AC versus AC. The sample size (N > 98 per arm) was sufficient to detect an effect.
Recurrence of pulmonary embolism
- The level of evidence starts at high as RCTs reported for this outcome and the comparison ST + AC versus AC. The level of evidence was downgraded by three levels, because of inconsistency (one level) and imprecision (confidence interval crosses both boundaries of minimal important difference).
- The level of evidence starts at low as cohort studies reported for this outcome and the comparison CDT + AC versus ST + AC. The level of evidence was downgraded by one level from low to very low, because of imprecision (confidence interval crosses one boundary of minimal important difference) and risk of bias (issues with the intervention definition and confounding by indication).
Zoeken en selecteren
A systematic review of the literature was performed to answer the following question: What are the desirable and undesirable effects of systemic thrombolysis, percutaneous catheter-guided interventions, or surgical embolectomy in comparison with initiation of anticoagulation in patients with acute intermediate-high risk pulmonary embolism?
Table 1: PICO
| Patients |
Adult patients with acute intermediate-high risk pulmonary embolism Defined by objectively demonstrated PE, right ventricular dysfunction on CTPA and/or echocardiogram, and increased troponin as defined by the ESC guidelines (Konstantinides, 2020) |
| Intervention |
Systemic thrombolysis (ST) Percutaneous catheter-guided intervention (CDI)* Surgical embolectomy (SE) |
| Control | Anticoagulation (any type) |
| Outcomes |
|
| Other selection criteria | Study design: systematic reviews, randomized controlled trials and cohort studies (include at least 500 patients and adjusted for confounding (any type of analysis)) |
*CDI includes catheter-directed local thrombolysis with or without mechanical or ultrasound-assisted fragmentation or thrombus aspiration).
Relevant outcome measures
The guideline development group considered PE-related mortality, all-cause mortality, hemodynamic deterioration, bleeding complications, and recurrence of pulmonary embolism as critical outcome measures for decision making; and quality of life/PROMS and chronic thrombo-embolic pulmonary hypertension (CTEPH) or chronic thrombo-embolic pulmonary disease (CTEPD) without pulmonary hypertension as important outcome measures for decision making.
A priori, the guideline panel did not use one definition for the patient population listed above. In the literature, different terms are used which can indicate intermediate-high risk PE patients, e.g. moderate or submassive PE. In the broadest terms, the population from the selected studies included hemodynamically stable patients with acute PE and signs of right ventricle dysfunction, irrespective of the term used to describe the population.
The working group defined a risk difference (RD) of 3%* as a minimal clinically (patient) important difference.
*Based on the differences applied in the guidelines on thromboprophylaxis in patients with COVID-19. This working group derived the minimal clinically (patient) important differences from the ACCP (2012).
Search and select (Methods)
The databases Medline (via OVID) and Embase (via Embase.com) were searched with relevant search terms until 5 July 2023. The detailed search strategy is depicted under the tab Methods. The systematic literature search resulted in 520 hits. Studies were selected based on the following criteria:
- Original study published after 2000.
- Systematic review with a detailed search strategy and risk of bias assessment of the included studies.
- Randomized controlled trials.
- Cohort studies conform the PICO with at least 500 patients and adjustment for confounding (any type of analysis).
162 studies were initially selected based on title and abstract screening. After reading the full text, 161 studies were excluded (see the table with reasons for exclusion under the tab Methods), and one study was included.
A systematic review and network meta-analysis by Planer (Planer, 2023) was included. This review included RCTs, observational cohort and case-control studies comparing catheter-directed thrombolysis (CDT) with or without mechanical or ultrasound-assisted fragmentation with other therapeutic options including anticoagulation and systemic thrombolysis (ST). Small cohort studies without adjustment for confounding were also included in this systematic review. Planer and colleagues excluded 8 observational studies based on high risk of bias. These selection criteria were different than the selection criteria for this PICO. Because the search strategy used by Planer (2023) was adequate, we decided to review the included and excluded observational studies. The final selection of the reviewed studies by Planer (2023) to answer this PICO, can be found in the table with reasons for exclusion. Of the total of 44 studies included by Planer (2023), 8 RCTs and 7 cohort studies were finally included in our analyses. No RCTs investigating surgical embolectomy were found.
Results
15 studies were included in the analysis of the literature. Important study characteristics and results are summarized in the evidence tables. The assessment of the risk of bias is summarized in the risk of bias tables.
Referenties
- Fasullo S, Scalzo S, Maringhini G, Ganci F, Cannizzaro S, Basile I, Cangemi D, Terrazzino G, Parrinello G, Sarullo FM, Baglini R, Paterna S, Di Pasquale P. Six-month echocardiographic study in patients with submassive pulmonary embolism and right ventricle dysfunction: comparison of thrombolysis with heparin. Am J Med Sci. 2011 Jan;341(1):33-9.
- Huisman MV, Montero Cabezas JM, Klok FA. Longembolie-interventieteams [Pulmonary embolism response teams: what is the added value for patients with acute pulmonary embolism?]. Ned Tijdschr Geneeskd. 2017;161:D1570. Dutch. PMID: 28831930.
- Konstantinides S, Geibel A, Heusel G, Heinrich F, Kasper W; Management Strategies and Prognosis of Pulmonary Embolism-3 Trial Investigators. Heparin plus alteplase compared with heparin alone in patients with submassive pulmonary embolism. N Engl J Med. 2002 Oct 10;347(15):1143-50.
- Konstantinides SV, Meyer G, Becattini C, Bueno H, Geersing GJ, Harjola VP, Huisman MV, Humbert M, Jennings CS, Jiménez D, Kucher N, Lang IM, Lankeit M, Lorusso R, Mazzolai L, Meneveau N, Ní Áinle F, Prandoni P, Pruszczyk P, Righini M, Torbicki A, Van Belle E, Zamorano JL; ESC Scientific Document Group. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020 Jan 21;41(4):543-603.
- Kroupa J, Buk M, Weichet J, Malikova H, Bartova L, Linkova H, Ionita O, Kozel M, Motovska Z, Kocka V. A pilot randomised trial of catheter-directed thrombolysis or standard anticoagulation for patients with intermediate-high risk acute pulmonary embolism. EuroIntervention. 2022 Oct 7;18(8):e639-e646.
- Kucher N, Boekstegers P, Müller OJ, Kupatt C, Beyer-Westendorf J, Heitzer T, Tebbe U, Horstkotte J, Müller R, Blessing E, Greif M, Lange P, Hoffmann RT, Werth S, Barmeyer A, Härtel D, Grünwald H, Empen K, Baumgartner I. Randomized, controlled trial of ultrasound-assisted catheter-directed thrombolysis for acute intermediate-risk pulmonary embolism. Circulation. 2014 Jan 28;129(4):479-86.
- Lin DS, Lin YS, Wu CK, Lin HH, Lee JK. Midterm Prognosis of Patients With Pulmonary Embolism Receiving Catheter-Directed Thrombolysis or Systemic Thrombolysis: A Nationwide Population-Based Study. J Am Heart Assoc. 2021 Apr 6;10(7):e019296.
- Meyer G, Vicaut E, Danays T, Agnelli G, Becattini C, Beyer-Westendorf J, Bluhmki E, Bouvaist H, Brenner B, Couturaud F, Dellas C, Empen K, Franca A, Galiè N, Geibel A, Goldhaber SZ, Jimenez D, Kozak M, Kupatt C, Kucher N, Lang IM, Lankeit M, Meneveau N, Pacouret G, Palazzini M, Petris A, Pruszczyk P, Rugolotto M, Salvi A, Schellong S, Sebbane M, Sobkowicz B, Stefanovic BS, Thiele H, Torbicki A, Verschuren F, Konstantinides SV; PEITHO Investigators. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med. 2014 Apr 10;370(15):1402-11.
- Perlroth DJ, Sanders GD, Gould MK. Effectiveness and cost-effectiveness of thrombolysis in submassive pulmonary embolism. Arch Intern Med. 2007 Jan 8;167(1):74-80. doi: 10.1001/archinte.167.1.74. PMID: 17210881.
- Planer D, Yanko S, Matok I, Paltiel O, Zmiro R, Rotshild V, Amir O, Elbaz-Greener G, Raccah BH. Catheter-directed thrombolysis compared with systemic thrombolysis and anticoagulation in patients with intermediate- or high-risk pulmonary embolism: systematic review and network meta-analysis. CMAJ. 2023 Jun 19;195(24):E833-E843.
- Pruszczyk P, Klok FA, Kucher N, Roik M, Meneveau N, Sharp ASP, Nielsen-Kudsk JE, Obradović S, Barco S, Giannini F, Stefanini G, Tarantini G, Konstantinides S, Dudek D. Percutaneous treatment options for acute pulmonary embolism: a clinical consensus statement by the ESC Working Group on Pulmonary Circulation and Right Ventricular Function and the European Association of Percutaneous Cardiovascular Interventions. EuroIntervention. 2022 Oct 7;18(8):e623-e638.
- Sadeghipour P, Jenab Y, Moosavi J, Hosseini K, Mohebbi B, Hosseinsabet A, Chatterjee S, Pouraliakbar H, Shirani S, Shishehbor MH, Alizadehasl A, Farrashi M, Rezvani MA, Rafiee F, Jalali A, Rashedi S, Shafe O, Giri J, Monreal M, Jimenez D, Lang I, Maleki M, Goldhaber SZ, Krumholz HM, Piazza G, Bikdeli B. Catheter-Directed Thrombolysis vs Anticoagulation in Patients With Acute Intermediate-High-risk Pulmonary Embolism: The CANARY Randomized Clinical Trial. JAMA Cardiol. 2022 Dec 1;7(12):1189-1197.Sharifi M, Awdisho A, Schroeder B, Jiménez J, Iyer P, Bay C. Retrospective comparison of ultrasound facilitated catheter-directed thrombolysis and systemically administered half-dose thrombolysis in treatment of pulmonary embolism. Vasc Med. 2019 Apr;24(2):103-109.
Evidence tabellen
Research question: What are the desirable and undesirable effects of percutaneous catheter-directed thrombolysis, systemic thrombolysis, or anticoagulation in patients with acute intermediate-high risk pulmonary embolism?
|
Study reference |
Study characteristics |
Patient characteristics |
Intervention (I) |
Comparison / control (C)
|
Follow-up |
Outcome measures and effect size |
Comments |
|
RCT |
|
|
|
|
|
|
|
|
Kroupa, 2022 |
Type of study: RCT
Setting and country: Tertiary care center, Czech Republic
Funding and conflicts of interest: Non-commercial |
Inclusion criteria:
Exclusion criteria:
N total at baseline: Intervention: 12 Control: 11
Important prognostic factors2: age ± SD: I: 60 (14) C: 63 (15)
Sex: I: 67% M C: 46% M
|
CDT + AC
|
Anticoagulation
|
Length of follow-up: 30 days
Loss-to-follow-up: None
Incomplete outcome data: None
|
No death occurred at 30 days
No instability in any patient observed
Major bleeding No major bleeding episodes at 3 months.
I: 1 (24%) C: 2 (3%) RR 0.46 (95%CI 0.05 to 4.38)
Not reported
Not reported
Not reported |
|
|
Sadeghipour, 2022 |
Type of study: RCT
Setting and country: Hospital, Iran
Funding and conflicts of interest: Non-commercial |
Inclusion criteria:
Exclusion criteria:
N total at baseline: Intervention: 48 (46 at FU) Control: 46 (39 at FU)
Important prognostic factors2 at FU: Age ± SD: I: 57 (15) C: 57 (15)
Sex: I: 72% M C: 72% M
Prior PE: I: 1 (2%) C: 1 (2%)
|
CDT + AC
A fixed dose of alteplase a rate of 0.5 mg per catheter per hour for 24 hours was administered. A fixed dose of unfractionated heparin (UFH; 500 units/hour) was administered to all the patients in the cCDT group during fibrinolytic therapy. After the termination of cCDT and removal of catheter(s), UFH was increased to therapeutic levels. Afterward, UFH was changed to twice-daily subcutaneous LMWH (enoxaparin, 1 mg/kg) in patients without procedural complication (eg, major vascular access complication or bleeding events) or unstable hemodynamics necessitating other invasive therapies. LMWH was planned to be continued for the first 48 hours after completion of fibrinolytic therapy.
|
AC (LMWH, enoxaparin)
|
Length of follow-up: 3 months
Loss-to-follow-up: Intervention: N 2 (4%) Reasons (2 did not accept on-site follow-up)
Control: N 4 (9%) Reasons (4 did not accept on-site follow-up)
Incomplete outcome data: Intervention: N 2 (4%) Reasons (2 did not accept on-site follow-up)
Control: N 7 (15%) Reasons (4 did not accept on-site follow-up; 3 died before end of FU)
|
PE-related mortality I: 0 (0%) C: 2 (4%)
All-cause mortality I: 0 (0%) C: 3 (7%)
I: 0 (0%) C: 1 (2%)
Major: I: 1 (2%) C: 0 (0%)
Minor: I: 3 (6%) C: 0 (0%)
Not reported
Not reported
Not reported |
|
|
Zhang, 2018 |
Type of study: RCT
Setting and country: Hospital, China
Funding and conflicts of interest: No conflicts. |
Inclusion criteria: Acute symptomatic PE confirmed by CTPA with an embolus located in at least 1 main or proximal lower lobe pulmonary artery and RV/LV ratio ≥0.9 obtained on echocardiographic examination
Exclusion criteria:
N total at baseline: Intervention: 33 Control: 33
Important prognostic factors2: age ± SD: I: 60 (13) C: 58 (11)
Sex: I: 55% M C: 42% M
Prior VTE I: 12% C: 9%
Concomitant DVT I: 57% C: 48%
|
rt-PA at a dose of 30 mg over 2 hours with concomitant low molecular-weight heparin (LMWH)
|
LMWH anticoagulation alone
|
Length of follow-up: 3 months after discharge
Loss-to-follow-up: None
Incomplete outcome data: None
|
No death occurred at 3 months.
Defined by the need for cardiopulmonary resuscitation, a drop in SBP by ≥40 mm Hg for ≥15 min or SBP <90 mm Hg for ≥15 min in accompanied with hypoperfusion of end-organ, or the need for vasopressors.
I: 0 (0) C: 3 (9%)
Major bleeding No major bleeding episodes at 3 months.
Minor bleeding I: 8 (24%) C: 1 (3%) RR 8.00 (95%CI 1.06 to 60.43)
I: 1 (3%) C: 2 (6%) RR 0.50 (95%CI 0.05 to 5.25)
Not reported
Not reported |
|
|
Kucher, 2014 |
Type of study: RCT
Setting and country: Hospital, Germany & Switzerland
Funding and conflicts of interest: Commercial funding |
Inclusion criteria: Symptomatic PE confirmed by contrast-enhanced computed tomography (CT) with embolus located in at least 1 main or proximal lower lobe pulmonary artery and RV to left ventricular dimension (RV/LV) ratio ≥1 obtained from the echocardiographic apical 4-chamber view.
Exclusion criteria: age <18 or >80 years; index PE symptom duration >14 days; insufficient echocardiographic image quality in the apical 4-chamber view that prohibited the measurement of the RV/LV ratio; known significant bleeding risk; administration of thrombolytic agents within the previous 4 days; active bleeding; known bleeding diathesis; known coagulation disorder; platelet count <100000/mm3; previous use of vitamin K antagonists with international normalized ratio >2.5 on admission; history of any intracranial or intraspinal surgery or trauma or intracranial/intraspinal bleeding; intracranial neoplasm, arteriovenous malformation, or aneurysm; gastrointestinal bleeding <3 months; internal eye surgery or hemorrhagic retinopathy <3 months; major surgery, cataract surgery, trauma, obstetric delivery, cardiopulmonary resuscitation, or other invasive procedure <10 days; allergy, hypersensitivity, or thrombocytopenia from heparin or rtPA; severe contrast allergy to iodinated contrast; known right-to-left cardiac shunt (eg, from a large patent foramen ovale or atrial septal defect); large (>10 mm) right atrial or RV thrombus; hemodynamic decompensation, defined as the need for cardiopulmonary resuscitation, or systolic blood pressure <90 mmHg for at least 15 minutes, or drop of systolic blood pressure by at least 40 mmHg for at least 15 minutes with signs of end-organ hypoperfusion (cold extremities or low urinary output <30 mL/h or mental confusion), or need for catecholamine administration to maintain adequate organ perfusion and a systolic blood pressure of >90 mmHg; severe hypertension on repeated readings (systolic >180 mmHg or diastolic >105 mmHg); pregnancy, lactation, or parturition <30 days; participation in any other investigational drug or device study; life expectancy <90 days; and inability to comply with study assessments.
N total at baseline: Intervention: 30 Control: 29
Important prognostic factors2: age ± SD: I: 64 (15) C: 62 (13)
Sex: I: 63% F C: 41% F
Prior PE I: 13% C: 7%
|
Unfractionated heparin (UFH) and an ultrasound-assisted catheter-directed thrombolysis (USAT) regimen of 10 mg recombinant tissue plasminogen activator (rtPA) over 15 hours per treated lung via the EkoSonic Endovascular System.
|
UFH alone |
Length of follow-up: 90 days
Loss-to-follow-up: None
Incomplete outcome data: None
|
PE-related mortality No deaths after 3 months
All-cause mortality I: 0 (0%) C: 1 (3%)
No hemodynamic decompensation after 3 months
Major bleeding No major bleeding episodes at 3 months.
I: 3 (10%) C: 1 (3%) RR 2.90 (95%CI 0.32 to 26.30)
No recurrences after 3 months
Not reported
Not reported |
|
|
Meyer, 2014 |
Type of study: RCT
Setting and country: Hospital, Frnace, Germany, Poland, and Italy.
Funding and conflicts of interest: Mixed (non-commercial and commercial) |
Inclusion criteria: Age of 18 years or older, objectively confirmed acute pulmonary embolism with an onset of symptoms 15 days or less before randomization, right ventricular dysfunction confirmed by echocardiography or spiral computed tomography (CT) of the chest, and myocardial injury confirmed by a positive test for troponin I or troponin T.
Exclusion criteria: See supplementary table of paper
N total at baseline: Intervention: 506 Control: 499
Important prognostic factors2: Age ± SD: I: 66 (15) C: 65 (16)
Sex: I: 48% M C: 46% M
|
a single weight-based intravenous bolus (given over a period of 5 to 10 seconds) of the fibrinolytic agent tenecteplase.
the administration of unfractionated heparin was started as an intravenous bolus immediately after randomization in both groups
|
Patients assigned to placebo were given a single intravenous bolus of the same volume and appearance as the bolus of Tenecteplase
the administration of unfractionated heparin was started as an intravenous bolus immediately after randomization in both groups
|
Length of follow-up: 30 days
Loss-to-follow-up: None
Incomplete outcome data: None
|
PE-related mortality Not reported
All-cause mortality I: 6 (1%) C: 9 (2%) OR 0.65 (95%CI 0.23 to 1.85)
I: 8 (2%) C: 25 (5%) OR 0.30 (95%CI 0.14 to 0.68)
Major bleedings I: 59 (12%) C: 12 (2.4%) RR 4.77 (95%CI 2.59 to 8.77)
Minor bleedings I: 165 (33) C: 43 (9) RR 3.78 (95%CI 2.77 tot 5.17)
I: 1 (0%) C: 5 (1%) OR 0.20 (95%CI 0.02 to 1.68)
Not reported
Not reported |
|
|
Sharifi, 2012 |
Type of study: RCT
Setting and country: USA
Funding and conflicts of interest: Not reported |
Inclusion criteria: Adult patients presenting with signs and symptoms suggestive of PE plus imaging documentation on computed tomographic angiography or ventilation/perfusion scanning were potentially eligible for the study. “Moderate” PE was defined as the presence of signs and symptoms of PE plus computed tomographic pulmonary angiographic involvement of >70% involvement of thrombus in ≥2 lobar or left or right main pulmonary arteries (Figure 1) or by a high probability ventilation/perfusion scan showing ventilation/perfusion mismatch in ≥2 lobes
Exclusion criteria: Onset of symptoms >10 days; >8 hours since the start of parenteral anticoagulation; systemic arterial systolic blood pressure <95 or ≥200/100 mm Hg; eligibility for full-dose thrombolysis; a contraindication to unfractionated or low-molecular-weight heparin; severe thrombocytopenia (platelet count <50,000/mm3); major bleeding within <2 months requiring transfusion; surgery or major trauma within <2 weeks; brain mass; neurologic surgery, intracerebral hemorrhage, or subdural hematoma within <1 year; end-stage illness with no plan for PE treatment; and an inability to perform echocardiography.
N total at baseline: Intervention: 61 Control: 60
Important prognostic factors2: Age ± SD: I: 58 (9) C: 59 (10)
Sex: I: 46% M C: 45% M
Prior VTE I: 13 (21%) C: 12 (20%)
Concomitant DVT I: 35 (57%) C: 33 (55%)
|
ST (with tPA) + AC
The dose of tPA was ≤50% of the standard dose (100 mg) commonly used for the treatment of PE, which we termed “safe dose” thrombolysis.
Warfarin was started at admission in all patients.
All patients received either unfractionated heparin or subcutaneous enoxaparin, with initial preference given to the latter drug.
|
AC
Warfarin was started at admission in all patients.
All patients received either unfractionated heparin or subcutaneous enoxaparin, with initial preference given to the latter drug.
|
Length of follow-up: Mean 28 ± 5 months
Loss-to-follow-up: Intervention: N 3 (5%) Reasons (Not stated)
Control: N 4 (7%) Reasons (Not stated)
Incomplete outcome data: Intervention: N 3 (5%) Reasons (Not stated)
Control: N 4 (7%) Reasons (Not stated)
|
PE-related mortality Not reported
All-cause mortality I: 1 (2%) C: 3 (5%) RR 0.33 (95%CI 0.04 to 3.06)
Not reported
No bleeding event
I: 0 (0%) C: 3 (5%)
Not reported
Not reported
|
|
|
Fasullo, 2011 |
Type of study: RCT
Setting and country: Emergency department, Italy
Funding and conflicts of interest: Not stated |
Inclusion criteria: (1) symptoms onset since no more than 6 hours, for first episode of acute SPE; (2) normal blood pressure [systolic blood pressure (SBP) 100 mm Hg]; (3) RVD at echocardiogram; (4) positive lung spiral computed tomography (CT) and (5) dyspnea, chest pain, tachypnea, hypoxemia PO2 75 mm Hg, PCO2 40 mm Hg, oxygen saturation 90% in room air, D-dimer elevation, electrocardiography (ECG) with S1-Q3-T3 pattern, inversion of T waves in V1 to V4, a right bundle-branch block or right axis deviation.
Exclusion criteria: active internal bleeding, recent intracranial bleeding, intracranial tumor or seizure history, ischemic stroke until 2 months, neurosurgery during last month, recent surgery within 10 days, puncture of uncompressible vessel within 10 days, trauma within 15 days, uncontrolled hypertension (SBP ≥180 mm Hg and diastolic BP ≥110 mm Hg), hemorrhagic disorder of thrombocytopenia (100,000), severe impaired hepatic or renal function, gastrointestinal bleeding within 10 days, pregnancy, age older than 75 years. Patients were also excluded if they had arterial aneurysm or arterial/venous malformation and cancer at increased risk for bleeding. In addition, patients with chronic pulmonary hypertension, severe chronic obstructive pulmonary disease and who had received therapeutic doses of heparin (unfractionated or low-molecular-weight heparin) for more than 72 hours before randomization, thrombolytic treatment within the previous 4 days, or glycoprotein IIb/IIIa antagonists within the preceding 7 days were also excluded, and so were excluded the ones who were under oral anticoagulation
N total at baseline: Intervention: 37 Control:3 35
Important prognostic factors2: For example age ± SD: I: 55 (17) C: 57 (16)
Sex: I: 57% M C: 57% M
|
ST (100 mg of alteplase (Actilyse as a 10-mg bolus, followed by a 90-mg intravenous infusion over a period of 2 hours)
In addition to alteplase, both groups continued to receive unfractionated heparin treatment (1000 U/hr and/or accordingly activated partial thromboplastin time [aPTT]), in combination with warfarin (started on day 1 after randomization), until the international normalized ratio was within the therapeutic range for 2 consecutive days; after this point, heparin was stopped, and only warfarin was kept after discharge and during follow-up
|
AC Matching placebo to alteplase.
In addition to placebo, both groups continued to receive unfractionated heparin treatment (1000 U/hr and/or accordingly activated partial thromboplastin time [aPTT]), in combination with warfarin (started on day 1 after randomization), until the international normalized ratio was within the therapeutic range for 2 consecutive days; after this point, heparin was stopped, and only warfarin was kept after discharge and during follow-up
|
Length of follow-up: 6 months
Loss-to-follow-up: None
Incomplete outcome data: None
|
PE-related mortality I: 0 C: 4 (11%)
All-cause mortality I: 0 C: 6 (17%)
Not reported
Major bleeding I: 2 (5%) C: 1 (3%) RR 1.89 (95%CI 0.18 to 19.95)
Minor bleeding I: 16 (43%) C: 8 (22%) RR 1.89 (95%CI 0.93 to 3.86)
I: 0 C: 5
Not reported
Not reported |
|
|
Konstantinides, 2002 |
Type of study: RCT
Setting and country: Germany
Funding and conflicts of interest: Commercial |
Inclusion criteria: echocardiographically detected right ventricular dysfunction, defined as right ventricular enlargement combined with loss of inspiratory collapse of the inferior vena cava, without left ventricular or mitral-valve disease12; echocardiographically detected pulmonary-artery hypertension,13 defined as a tricuspid regurgitant jet velocity greater than 2.8 m per second, followed by confirmation of pulmonary embolism (by ventilation–perfusion lung scanning, spiral computed tomography [CT], or pulmonary angiography); a diagnosis of precapillary pulmonary hypertension based on catheterization of the right side of the heart, defined as a mean pulmonary-artery pressure above 20 mm Hg and a pulmonary-capillary wedge pressure below 18 mm Hg, followed by confirmation of pulmonary embolism; or new electrocardiographic signs of right ventricular strain (defined as complete or incomplete right bundlebranch block, S waves in lead I combined with Q waves in lead III, or inverted T waves in precordial leads V1, V2, and V3), followed by confirmation of pulmonary embolism.
Exclusion criteria: age over 80 years; hemodynamic instability, defined as persistent arterial hypotension (i.e., systolic pressure below 90 mm Hg), with or without signs of cardiogenic shock; onset of symptoms more than 96 hours before diagnosis; thrombolytic treatment, major surgery, or biopsy within the preceding 7 days; major trauma within the preceding 10 days; stroke, transient ischemic attack, craniocerebral trauma, or neurologic surgery within the preceding 6 months; gastrointestinal bleeding within the preceding 3 months; uncontrolled hypertension; a known bleeding disorder; known inability to tolerate alteplase; known diabetic retinopathy; current therapy with an oral anticoagulant; current pregnancy or lactation; a life expectancy of less than 6 months because of underlying disease; or planned use of thrombolytic agents for extensive deep-vein thrombosis.
N total at baseline: Intervention: 118 Control: 138
Important prognostic factors2: age ± SD: I: C:
Sex: I: 46% M C: 49% M
|
Heparin plus alteplase
intravenous bolus of 5000 U of unfractionated heparin before undergoing further diagnostic workup.
Patients who met the inclusion criteria and were enrolled in the study were then randomly assigned to receive 100 mg of alteplase as a 10-mg bolus, followed by a 90-mg intravenous infusion over a period of two hours, or matching placebo.
In addition to alteplase or placebo, patients in both groups received an intravenous infusion of unfractionated heparin. |
Heparin plus placebo
|
Length of follow-up: Based on figure 1, 30 days
Loss-to-follow-up: Not reported
Incomplete outcome data: Not reported
|
PE-related mortality Not reported
All-cause mortality I: 4 (3%) C: 3 (2%) RR 1.56 (95%CI 0.36 to 6.83)
Not reported
Major bleeding I: 1 (5%) C: 5 (3%) RR 0.23 (95%CI 0.03 to 1.97)
Minor bleeding Not reported
I: 4 C: 4 RR 1.17 (95%CI 0.30 to 4.57)
Not reported
Not reported |
|
|
Cohort studies |
|
|
|
|
|
|
|
|
Krishnan, 2022 |
Type of study: Cohort study
Setting and country: Nationwide Inpatient Sample from 2017, USA
Funding and conflicts of interest: None |
Inclusion criteria: Patients above the age of 18 years admitted with the principal diagnosis of Acute PE with cor pulmonale in 2017
Exclusion criteria: None
N total at baseline: Intervention1: 2460 Intervention2: 1340 Control: 9525
Important prognostic factors2: Mean age: I1: 61 I2: 61 C: 65
Sex: I1: 51% M I2: 59% M C: 48% M
|
Intervention1: CDT Intervention2: ST |
AC |
Length of follow-up: Not reported
Loss-to-follow-up: Not reported
Incomplete outcome data: Not reported
|
PE-related mortality Not reported
All-cause mortality CDT vs AC OR 0.61 (95%CI 0.33 to 1.11) CDT vs ST OR 0.33 (95%CI 0.14 to 0.61)
Not reported
Not reported
Not reported
Not reported
Not reported
|
Analyses adjusted for age, liver disease, obesity, OSA, hypertension, race, annual income, Charlson Comorbidity Score, and hospital bed size. |
|
Hobohm, 2021 |
Type of study: Cohort study
Setting and country: Database of the federal Office of Statistics, Germany
Funding and conflicts of interest: Non-commercial |
Inclusion criteria: Hospitalized patients diagnosed with PE between the years 2005 and 2016
Exclusion criteria: patients who underwent (i) surgical embolectomy or (ii) percutaneous treatment (thrombus fragmentation; or rotational thrombectomy) without thrombolytic drugs at any dosage were excluded from all analyses. Patients who received both systemic thrombolysis and CDT were also excluded from analysis.
N total at baseline: Intervention: 1175 Control: 40728
Important prognostic factors2: Age median (IQR): I: 68 (53-76) C: 69 (57-77)
Sex: I: 49% M C: 48% M
|
CDT |
ST Or no ST (AC alone) |
Length of follow-up: Not reported
Loss-to-follow-up: Not reported
Incomplete outcome data: Not reported
|
PE-related mortality Not reported
All-cause mortality CDT vs AC OR 0.45 (95%CI 0.33 to 0.62) CDT vs ST (among hemodynamically stable PE patients) OR 0.55 (95%CI 0.40 to 0.75)
Not reported
Not reported
Not reported
Not reported
Not reported
|
We fitted multivariate logistic regression models including the following covariates chosen based on clinical relevance and no obvious collinearity: age, sex, cancer (ICD codes C00-C97), coronary artery disease (ICD code I25), heart failure (ICD code I50), chronic obstructive pulmonary disease (COPD, ICD code J44), essential arterial hypertension (ICD code I10), diabetes mellitus (ICD codes E10–E14), chronic renal insufficiency (chronic renal insufficiency stages 3–5 with glomerular filtration rate <60mL/min/1.73 m2: ICD codes N18.3, N18.83, N18.84, N18.4, N18.5), surgery during inhospital stay (OPS code 5), tachycardia (ICD codes I47 and R000), syncope (ICD code R55), and hypoxia (ICD code J96). The multivariate analyses were extended by adding the Charlson index. |
|
Lin, 2021 |
Type of study: Cohort study
Setting and country: Health insurance database, Taiwan
Funding and conflicts of interest: Non-commercial |
Inclusion criteria: Patients who were first admitted for PE (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM], code 415.1) between January 1, 2001, and December 31, 2013, were identified.
Exclusion criteria: (1) had missing demographical data (<0.1%), (2) were aged <20 years, or (3) were not treated by thrombolysis during the index PE admission.
N total at baseline: Intervention: 145 Control: 1158
Important prognostic factors2: Age ± SD: I: 61 (16) C: 62 (16)
Sex: I: 39% M C: 46% M
History of PE I: 2 (1%) C: 13 (1%)
|
CDT (thrombolytic agent received through multi-side-hole catheters)
Thrombolytic agent consisted of:
|
ST (thrombolytic agent NOT received through multi-side-hole catheters) |
Length of follow-up: Mean follow-up of 3.8 yrs and 3.4 yrs for CDT and ST, respectively
Loss-to-follow-up: Not reported
Incomplete outcome data: Not reported
|
Only results of the IPTW analyses are reported. Adjusted for sex, hyperlipidemia, hyperthyroidism, previous stroke, Charlson Comorbidity Index score, statin use, anticoagulant use, and intubation.
PE-related mortality Not reported
All-cause mortality I: 22 (15%) C: 250 (22%) OR 0.49 (95%CI 0.36 to 0.67)
Not reported
Major bleeding I: 13 (9%) C: 91 (8%) OR 1.02 (95%CI 0.75 to 1.37)
Minor bleeding Not reported
Among the group who survived initial hospitalization I: 22 (18% out of 123) C: 219 (24% out of 908) HR 0.84 (95%CI 0.72 to 0.98)
Not reported
Not reported
|
|
|
Stein, 2020 |
Type of study: Cohort study
Setting and country: Nationwide Inpatient sample, USA
Funding and conflicts of interest: None |
Inclusion criteria: Stable patients with acute PE and acute cor pulmonale. Stable patients were defined as not in shock and not on ventilator support.
Exclusion criteria: Patients who underwent pulmonary embolectomy or received intravenous thrombolytic therapy.
N total at baseline: Intervention: 735 Control: 5605
Important prognostic factors2: Age ± SD: I: 60 (13) C: 60 (11)
Sex: I: 47% M C: 45% M
|
CDT plus AC
|
AC
|
Length of follow-up: Not reported
Loss-to-follow-up: Not reported
Incomplete outcome data: Not reported
|
PE-related mortality Not reported
All-cause mortality I: 30 (4%) C: 395 (7%) OR 0.56 (95%CI 0.38 to 0.82)
Not reported
Not reported
Not reported
Not reported
Not reported
|
Patients with PE an acute pulmonale were assumed to treated with anticoagulants if they did not receive intravenous thrombolytic therapy, catheter-directed thrombolytic therapy, or pulmonary embolectomy. Authors also assumed that patients treated with catheter-directed thrombolysis also received anticoagulants.
Patients were matched on age, gender, and co-morbid conditions. |
|
Arora, 2017 |
Type of study: Cohort study
Setting and country: National Readmission Database, USA
Funding and conflicts of interest: None |
Inclusion criteria: Patient admitted for PE and received trombolysis
Exclusion criteria: we excluded patients with secondary diagnostic codes for acute ST elevation myocardial infarction, ischemic stroke, and hospice care. We excluded patients with age <18 years, with missing data for age, gender, or mortality. We also excluded procedures performed in the month of December, as we did not have follow-up data for the same.
N total at baseline: Intervention: 1128 Control: 2256
Important prognostic factors2: Sex: I: 53% M C: 52% M
|
CDT
|
ST
|
Length of follow-up: Not reported
Loss-to-follow-up: Not reported
Incomplete outcome data: Not reported
|
PE-related mortality Not reported
All-cause mortality (absolute numbers not reported) I: 6% C: 15% OR 0.37 (95%CI 0.28 to 0.49)
Not reported
Not reported
Not reported
Not reported
Not reported |
A propensity score, which was assigned to each principal hospitalization, was based on multivariable logistic regression model that examined the impact of 12 variables (patient demographics, co-morbidities, and hospital characteristics) on the likelihood of treatment assignment |
|
Patel, 2015 |
Type of study: Cohort study
Setting and country: Nationwide Inpatient Sample, USA
Funding and conflicts of interest: Not reported |
Inclusion criteria: Patients admitted with principal diagnosis of PE who received thrombolysis.
Exclusion criteria: We excluded all observations with <18 years of age. We further excluded patients with secondary diagnostic codes for deep vein thrombosis, acute ST elevation myocardial infarction, and ischemic stroke.
N total at baseline: Intervention: 352 (unmatched) Control: 1169 (unmatched)
Important prognostic factors2: Age ± SD: I: 59 (15) C: 58 (16)
Sex: I: 48% M C: 42% M
|
CDT
|
ST
|
Length of follow-up: Not reported
Loss-to-follow-up: Not reported
Incomplete outcome data: Not reported
|
PE-related mortality
All-cause mortality I: 13% C: 22% OR 0.55 (95%CI 0.36 to 0.85)
Not reported
Not reported
Not reported
Not reported
Not reported |
To adjust the possible confounding variables and to ameliorate the effect of selection and indication bias, propensity score matching was done after generating the propensity scores from demographic covariates including the Deyo-modification of Charlson score, cardiopulmonary arrest, saddle PE, and shock. |
Risk of bias assessment RCT
Research question: What are the desirable and undesirable effects of percutaneous catheter-directed thrombolysis, systemic thrombolysis, or anticoagulation in patients with acute intermediate-high risk pulmonary embolism?
|
Study reference
|
Was the allocation sequence adequately generated?
|
Was the allocation adequately concealed?
|
Blinding: Was knowledge of the allocated interventions adequately prevented?
Were patients blinded?
Were healthcare providers blinded?
Were data collectors blinded?
Were outcome assessors blinded?
Were data analysts blinded? |
Was loss to follow-up (missing outcome data) infrequent?
|
Are reports of the study free of selective outcome reporting?
|
Was the study apparently free of other problems that could put it at a risk of bias?
|
Overall risk of bias If applicable/necessary, per outcome measure
|
|
Kroupa, 2022 |
Probably no
Reason: Authors stated: “randomized using a simple evelope method [..] in a 1:1 ratio.” |
Probably no
Reason: Authors stated: “randomized using a simple evelope method [..] in a 1:1 ratio.” |
Probably no
Reason: Nothing stated on blinding. |
Definitely yes.
Reason: No participants were lost during the study. |
Probably yes
Reason: Outcomes mentioned in Methods section were reported in the results section. |
Definitely yes
Reason: No other problems |
High risk of bias
Reason: No information on the randomization, allocation concealment or blinding of participants. |
|
Sadeghipour, 2022 |
Probably yes
Reason: Authors stated: “Randomization was carried out in a 1:1 ratio to cCDT plus anticoagulation vs anticoagulation monotherapy via an electronic web-based system with permuted blocks of 4 and concealed allocation sequences.” |
Probably yes
Reason: Authors stated: “Randomization was carried out in a 1:1 ratio to cCDT plus anticoagulation vs anticoagulation monotherapy via an electronic web-based system with permuted blocks of 4 and concealed allocation sequences.” |
Probably no
Reason: Data collectors and outcome assessors were blinded. Rest of team were not blinded as no placebo or sham was used. |
Probably yes
Reason: Although more patients did not accept follow-up in the control group than intervention group, numbers are low. |
Definitely yes
Reason: Trial was registered and stated outcomes were mentioned in the results section. |
Definitely yes
Reason: No other problems |
Low risk of bias
Reason: For the outcome of interest and reported, the not blinding of patients or participants will most likely not affect the estimate. |
|
Zhang, 2018 |
Probably no
Reason: Only stated the following: “[..] randomly assigned by envelopes to receive [..]”. |
Probably no
Reason: Only stated the following: “[..] randomly assigned by envelopes to receive [..]”. |
Probably no
Reason: Nothing stated on blinding. |
Definitely yes.
Reason: No participants were lost during the study. |
Probably yes
Reason: Outcomes mentioned in Methods section were reported in the results section. However, clinical trial registry for this trial could be found. |
Definitely yes
Reason: No other problems |
High risk of bias
Reason: No information on the randomization, allocation concealment or blinding of participants. |
|
Kucher, 2013 |
Probably no
Reason: Authors stated: “Randomization was performed in blocks of 4 without stratification.” |
Probably no
Reason: Authors stated: “Randomization was performed in blocks of 4 without stratification.” |
Probably no
Reason: Nothing stated on blinding. |
Definitely yes.
Reason: No participants were lost during the study. |
Definitely yes
Reason: Trial was registered before first results and outcomes were reported in the paper. |
Definitely yes
Reason: First author was a consultant for the sponsor (device company) and the results are in favor of the sponsor. |
High risk of bias
Reason: No information on the randomization, allocation concealment or blinding of participants. Trial was sponsored by a device company for which the first author is a consultant. |
|
Meyer, 2014 |
Probably yes
Reason: Authors stated: “Eligible patients underwent central randomization with the use of a computerized Internetbased system. Randomization was stratified by center and, within centers, was performed in blocks to ensure balanced distribution of the treatment groups. |
Probably yes
Reason: Placebo similar to intervention was used. |
Probably yes
Reason: Placebo similar to intervention was used. Data was concealed from the investigators. |
Definitely yes.
Reason: No participants were lost during the study. |
Probably yes
Reason: Outcomes mentioned in Methods section were reported in the results section. However, clinical trial registry for this trial could not be found. |
Probably yes
Reason: Trial received mixed sponsor ship. However, authors stated: “None of the trial funders had any role in the design or conduct of the trial, the analysis of the data, or the preparation of the manuscript.” |
Low risk of bias
Reason: No issues |
|
Sharifi, 2012 |
Probably yes
Reason: Authors only stated: “After evaluation of the patient, the study investigator placed a telephone call to the study center, and, by opening of sealed envelopes, randomization to the TG or CG was made.” |
Probably yes
Reason: Authors only stated: “After evaluation of the patient, the study investigator placed a telephone call to the study center, and, by opening of sealed envelopes, randomization to the TG or CG was made.” |
Probably no
Reason: Nothing stated on blinding. |
Probably yes
Reason: Number of participants lost was low, even though no reasons were provided. |
Probably yes
Reason: Outcomes mentioned in Methods section were reported in the results section. However, clinical trial registry for this trial could not be found. |
Probably yes
Reason: Funding of the trial is unclear. |
Low risk of bias
Reason: No issues |
|
Fasullo, 2011 |
Probably yes
Reason: Authors stated: “Randomization was performed by using a preliminary computer algorithm, [..].” |
Probably yes
Reason: Authors stated: “[..], and the assignment of all patients was decided at admission, before echocardiogram and before lung spiral lung CT by an external team of physicians (at least 2) who were blinded about study protocol.” |
Probably yes
Reason: Authors only stated: “Two blinded physicians evaluated the clinical status and if recurrence of PE was present and side effects warfarin treatment (bleedings) were also recorded.” Participants did receive a placebo to alteplase. |
Definitely yes.
Reason: No participants were lost during the study. |
Probably yes
Reason: Outcomes mentioned in Methods section were reported in the results section. |
Probably yes
Reason: Funding of the trial is unclear. |
Low risk of bias
Reason: No issues |
|
Konstantinides, 2002
|
Probably yes
Reason: Authors stated: “Randomization was performed on a 1:1 basis with a fixed block size of six patients at each center, according to a standard randomization program. |
Probably yes
Reason: Nothing stated on allocation concealment |
Probably yes
Reason: Placebo was used, and data was analyzed by an independent organization. |
Probably yes
Reason: Not specifically stated, but most likely no participants were lost. |
Probably yes
Reason: Outcomes mentioned in Methods section were reported in the results section. |
Probably no
Reason: Author was employed by sponsor and effect estimate is in favor of the sponsor. |
Some concerns
Reason: Influence of sponsor cannot be ruled out. |
Risk of bias assessment Cohort studies
Research question: What are the desirable and undesirable effects of percutaneous catheter-directed thrombolysis, systemic thrombolysis, or anticoagulation in patients with acute intermediate-high risk pulmonary embolism?
|
Author, year |
Selection of participants
Was selection of exposed and non-exposed cohorts drawn from the same population?
|
Exposure
Can we be confident in the assessment of exposure?
|
Outcome of interest
Can we be confident that the outcome of interest was not present at start of study?
|
Confounding-assessment
Can we be confident in the assessment of confounding factors?
|
Confounding-analysis
Did the study match exposed and unexposed for all variables that are associated with the outcome of interest or did the statistical analysis adjust for these confounding variables?
|
Assessment of outcome
Can we be confident in the assessment of outcome?
|
Follow up
Was the follow up of cohorts adequate? In particular, was outcome data complete or imputed?
|
Co-interventions
Were co-interventions similar between groups?
|
Overall Risk of bias
|
|
Krishnan, 2022 |
Definitely yes
Reason: All participants came from the same database.
|
Probably no
Reason: ICD-10 codes were used. However, no checks were performed if the ICD code matched the intervention. |
Unclear
Reason: It is unclear how the outcome was determined |
Definitely no
Reason: Selection of confounding factors was based on univariate analyses. |
Definitely no
Reason: Although standard multivariate analyse was used, confounding by indication is still an issue. |
Unclear
Reason: It is unclear how the outcome was determined |
Unclear
Reason: It is unclear what the duration of follow-up was |
Unclear
Reason: no information provided
|
High (All outcomes)
Confounding by indication is an issue |
|
Hobohm, 2021 |
Definitely yes
Reason: All participants came from the same database. |
Probably no
Reason: ICD-10-GM codes were used. However, no checks were performed if the ICD code matched the intervention. |
Unclear
Reason: It is unclear how the outcome was determined |
Definitely yes
Reason: Apprioprate factors used in adjustment |
Definitely no
Reason: Although standard multivariate analyse was used, confounding by indication is still an issue. |
Definitely yes
Reason: ICD-10-GM codes were used. |
Unclear
Reason: It is unclear what the duration of follow-up was |
Unclear
Reason: no information provided
|
High (all outcomes)
Confounding by indication is an issue |
|
Lin, 2021 |
Definitely yes
Reason: All participants came from a health insurance database.
|
Probably no
Reason: Claims data was used for the exposure. However, no checks were performed if the claim matched the intervention
|
Probably yes
Reason: Mortality was defined as removal from the database. Major bleeding and recurrent PE were not clearly defined.
|
Definitely yes
Reason: Apprioprate factors used in adjustment
|
Probably no
Reason: IPTW analyses used. Confounding by indication may still be an issue
|
Probably yes
Reason: Mortality was defined as removal from the database. Major bleeding and recurrent PE were not clearly defined. |
Definitely yes
Reason: Average follow-up was at least 3 years.
|
Probably yes
Reason: Medication use was adjusted for.
|
High (All outcomes)
Confounding by indication is an issue |
|
Stein, 2020 |
Definitely yes
Reason: All participants came from Nationwide Inpatient sample.
|
Unclear
Reason: It is unclear how exposure was determined.
|
Unclear
Reason: It is unclear how the outcome was determined |
Probably yes
Reason: Patients were matched. However, only a limited number of factors were used. |
Definitely no
Reason: Although a matched analysis was used, confounding by indication is still an issue. |
Unclear
Reason: It is unclear how the outcome was determined |
Unclear
Reason: It is unclear what the duration of follow-up was |
Unclear
Reason: no information provided
|
High (all outcomes)
Too many items were unclear and confounding by indication is an issue |
|
Arora, 2017 |
Definitely yes
Reason: All participants came from the National Readmission Database
|
Probably no
Reason: ICD-9-CM codes were used. However, the interventions researched do not have unique coding. |
Unclear
Reason: It is unclear how the outcome was determined |
Definitely yes
Reason: Apprioprate factors used in adjustment
|
Probably no
Reason: Propensity score matched analyses used. Confounding by indication may still be an issue |
Unclear
Reason: It is unclear how the outcome was determined |
Unclear
Reason: It is unclear what the duration of follow-up was |
Probably yes
Reason: Cohort characteristics were balanced after matching.
|
High (All outcomes)
Information on the outcome and follow-up would be helpful. Confounding by indication is an issue |
|
Patel, 2015 |
Definitely yes
Reason: All participants came from Nationwide Inpatient sample.
|
Probably no
Reason: ICD-9-CM codes were used. However, the interventions researched do not have unique coding. |
Unclear
Reason: It is unclear how the outcome was determined |
Definitely yes
Reason: Apprioprate factors used in adjustment
|
Probably no
Reason: Propensity score matched analyses used. Confounding by indication may still be an issue
|
Unclear
Reason: It is unclear how the outcome was determined |
Unclear
Reason: It is unclear what the duration of follow-up was |
Unclear
Reason: no information provided
|
High (All outcomes)
Information on the outcome and follow-up would be helpful. Confounding by indication is an issue |
Table of excluded studies from Planer (2023)
|
Reference |
Reason for exclusion |
|
Jerjes-Sanchez C, Ramírez-Rivera A, de Lourdes García M, et al. Streptokinase and heparin versus heparin alone in massive pulmonary embolism: a randomized controlled trial. J Thromb Thrombolysis 1995;2:227-9. |
Published before 2000 |
|
Goldhaber SZ, Come PC, Lee RT, et al. Alteplase versus heparin in acute pulmonary embolism: randomised trial assessing right-ventricular function and pulmonary perfusion. Lancet 1993;341:507-11. |
Published before 2000 |
|
Dalla-Volta S, Palla A, Santolicandro A, et al. PAIMS 2: Alteplase combined with heparin versus heparin in the treatment of acute pulmonary embolism. Plasminogen activator Italian multicenter study 2. J Am Coll Cardiol 1992;20:520-6 |
Published before 2000 |
|
Tissue plasminogen activator for the treatment of acute pulmonary embolism: a collaborative study by the PIOPED Investigators. Chest 1990;97:528-33. |
Published before 2000 |
|
Dotter CT, Seaman AJ, Rösch J, et al. Streptokinase and heparin in the treatment of pulmonary embolism: a randomized comparison. Vasc Endovascular Surg 1979;13:42-52 |
Published before 2000 |
|
Ly B, Arnesen H, Eie H, et al. A controlled clinical trial of streptokinase and heparin in the treatment of major pulmonary embolism. Acta Med Scand 1978;203:465-70. |
Published before 2000 |
|
Tibbutt DA, Lee GDJ, Sharp AA, et al. Comparison by controlled clinical trial of streptokinase and heparin in treatment of life-threatening pulmonary embolism. BMJ 1974;1:343-7. |
Published before 2000 |
|
Miller GAH, Sutton GC, Kerr IH, et al. Comparison of streptokinase and heparin in treatment of isolated acute massive pulmonary embolism. BMJ 1971;2:681-4. |
Published before 2000 |
|
Urokinase Pulmonary Embolism Trial Study Group. Urokinase Pulmonary Embolism trial: Phase 1 results. JAMA 1970;214:2163-72. |
Published before 2000 |
|
Geller BJ, Adusumalli S, Pugliese SC, et al. Outcomes of catheter-directed versus systemic thrombolysis for the treatment of pulmonary embolism: a realworld analysis of national administrative claims. Vasc Med 2020;25:334-40. |
Despite using an advanced method for confounding, no effect estimates were reported. |
|
Macovei L, Presura RM, Arsenescu Georgescu C. Systemic or local thrombolysis in high-risk pulmonary embolism. Cardiol J. 2015;22(4):467-74. doi: 10.5603/CJ.a2014.0103. Epub 2015 Jan 7. PMID: 25563712. |
Wrong comparison |
|
Beyer SE, Shanafelt C, Pinto DS, et al. Utilization and outcomes of thrombolytic therapy for acute pulmonary embolism: a nationwide cohort study. Chest 2020;157:645-53. |
Only the analysis of CDT-US vs CDT was adjusted for confounding. |
|
Liang NL, Avgerinos ED, Singh MJ, et al. Systemic thrombolysis increases hemorrhagic stroke risk without survival benefit compared with catheter-directed intervention for the treatment of acute pulmonary embolism. J Vasc Surg Venous Lymphat Disord 2017;5:171-176.e1 |
Overlap in sample from the same database used by other included studies. |
|
Ahmed MA, Abdelsalam SI, Elmorsy RA. Value of thrombolytic therapy for submassive pulmonary embolism patients. Egypt J Chest Dis Tuberc 2018;67:413-8 |
Cohort study; included less than 500 patients |
|
Bradley M, Bull T, Hountras P, et al. Pragmatic use of catheter-directed thrombolysis in venous thromboembolism and a comparative evaluation with traditional therapies in submassive pulmonary embolism. J Pharm Pract 2022;35:738-46. |
Cohort study; included less than 500 patients |
|
Gorgis S, Mawri S, Dabbagh MF, et al. Ultrasound-assisted catheter-directed thrombolysis versus anticoagulation alone for management of submassive pulmonary embolism. J Cardiol 2022;80:441-8. |
Cohort study; included less than 500 patients |
|
Zimmermann L, Laufs U, Petros S, et al. Outcome after thrombolysis in patients with intermediate high-risk pulmonary embolism: a propensity score analysis. J Emerg Med 2022;62:378-89. |
Cohort study; included less than 500 patients |
|
Harrison E, Kim JS, Lakhter V, et al. Safety and efficacy of catheter directed thrombolysis (CDT) in elderly with pulmonary embolism (PE). BMJ Open Respir Res 2021;8:6-10 |
Cohort study; included less than 500 patients |
|
Kline TM, Rodino AM, Dorszynski A, et al. Ultrasound-assisted catheterdirected thrombolysis versus systemic anticoagulation alone for submassive pulmonary embolism. J Thromb Thrombolysis 2021;52:130-7 |
Cohort study; included less than 500 patients |
|
Weng C, Wang X, Huang L, et al. Low-dose urokinase thrombolytic therapy for patients with acute intermediate-high-risk pulmonary embolism: a retrospective cohort study. PLoS One 2021;16:e0248603. |
Cohort study; included less than 500 patients |
|
Yilmaz ES, Uzun O. Low-dose thrombolysis for submassive pulmonary embolism. J Investig Med 2021;69:1439-46. |
Cohort study; included less than 500 patients |
|
D’Auria S, Sezer A, Thoma F, et al. Outcomes of catheter-directed thrombolysis vs. standard medical therapy in patients with acute submassive pulmonary embolism. Pulm Circ 2020;10:2045894019898368. |
Cohort study; included less than 500 patients |
|
Lee JK, Chen WH, Lin YS, Chang CH, Chen TH. Comparison of Effectiveness between Anticoagulation and Thrombolysis Therapy for Pulmonary Embolism in Patients Complicated with Shock: A Nationwide Population-Based Study. Thromb Haemost. 2020 Aug;120(8):1208-1216. |
Cohort study which included only patients with shock, no intermediate-high risk patients were included |
|
Rehman NU, Dar MI, Bansal M, et al. Clinical outcomes of submassive pulmonary embolism thrombolysis — an Indian experience. Egypt Heart J 2020;72:87 |
Cohort study; included less than 500 patients |
|
Sharifi M, Awdisho A, Schroeder B, et al. Retrospective comparison of ultrasound facilitated catheter-directed thrombolysis and systemically administered half-dose thrombolysis in treatment of pulmonary embolism. Vasc Med 2019;24:103-9. |
Cohort study; included less than 500 patients |
|
Avgerinos ED, Abou Ali AN, Liang NL, et al. Catheter-directed interventions compared with systemic thrombolysis achieve improved ventricular function recovery at a potentially lower complication rate for acute pulmonary embolism. J Vasc Surg Venous Lymphat Disord 2018;6:425-32. |
Cohort study; included less than 500 patients |
|
Schissler AJ, Gylnn RJ, Sobieszczyk PS, et al. Ultrasound-assisted catheterdirected thrombolysis compared with anticoagulation alone for treatment of intermediate-risk pulmonary embolism. Pulm Circ 2018;8:2045894018800265. |
Cohort study; included less than 500 patients |
|
Sista AK, Friedman OA, Dou E, et al. A pulmonary embolism response team’s initial 20-month experience treating 87 patients with submassive and massive pulmonary embolism. Vasc Med 2018;23:65-71. |
Cohort study; included less than 500 patients |
|
Klevanets J, Starodubtsev V, Ignatenko P, et al. Systemic thrombolytic therapy and catheter-directed fragmentation with local thrombolytic therapy in patients with pulmonary embolism. Ann Vasc Surg 2017;45:98-105. |
Cohort study; included less than 500 patients |
|
Avgerinos ED, Liang NL, El-Shazly OM, et al. Improved early right ventricular function recovery but increased complications with catheter-directed interventions compared with anticoagulation alone for submassive pulmonary embolism Presented in the Plenary Session at the 2015 Vascular Annual Meeting. J Vasc Surg Venous Lymphat Disord 2016;4:268-75. |
Cohort study; included less than 500 patients |
|
Yoo JW, Choi HC, Lee SJ, et al. American Journal of Emergency Medicine Comparison between systemic and catheter thrombolysis in patients with pulmonary embolism. Am J Emerg Med 2016;34:985-8. |
Cohort study; included less than 500 patients |
|
Hamel E, Pacouret G, Vincentelli D, et al. Thrombolysis or heparin therapy in massive pulmonary embolism with right ventricular dilation: results from a 128-patient monocenter registry. Chest 2001;120:120-5. |
Cohort study; included less than 500 patients |
|
Excluded by Planer (2023) because of high risk of bias |
|
|
Iskandar JP, Hariri E, Kanaan C, Kassis N, Kamran H, Sese D, et al. The safety and efficacy of systemic versus catheter-based therapies: application of a prognostic model by a pulmonary embolism response team. J Thromb Thrombolysis. 2022;53(3):616–25. |
Cohort study; included less than 500 patients |
|
Nicholas A Barrett, Anthony Byrne ADMH and NR. Management of massive pulmonary embolism: a retrospective single-centre cohort study. Crit Care Study Guid Text Rev Second Ed. 2010;(December):305–19. |
Cohort study; included less than 500 patients |
|
Konstantinides S, Tiede N, Geibel A, Olschewski M, Just H, Kasper W. Comparison of alteplase versus heparin for resolution of major pulmonary embolism. Am J Cardiol. 1998;82(8):966–70. |
Published before 2000 |
|
Nakamura M, Nakanishi N, Yamada N, Sakuma M, Miyahara Y, Okada O, et al. Effectiveness and safety of the thrombolytic therapy for acute pulmonary thromboembolism: Results of a multicenter registry in the Japanese Society of Pulmonary Embolism Research. Int J Cardiol. 2005;99(1):83–9. |
Cohort study; included less than 500 patients |
|
Riera-Mestre A, Jiménez D, Muriel A, Lobo JL, Moores L, Yusen RD, et al. Thrombolytic therapy and outcome of patients with an acute symptomatic pulmonary embolism. J Thromb Haemost. 2012;10(5):751–9. |
Cohort study; included less than 500 patients |
|
Sekulic I, Dzudovic B, Matijasevic J, Batranovic U, Rusovic S, Mihajlovic M, et al. Ultrasound assisted thrombolysis in intermediate-risk patients with pulmonary thromboembolism. Acta Cardiol. 2020;75(7):623–30. |
Cohort study; included less than 500 patients |
|
Zulty M, Saleh N, Hernandez J, Kalaria A, Camire L, Weisman DS. Catheter-Directed Therapy: Outcomes Versus Standard of Care and Evaluation of Current Practice. Am J Med. 2021;134(3):400–4. |
Cohort study; included less than 500 patients |
|
Omaygenc DO, Omaygenc MO. thrombolysis and anticoagulation embolism : A retrospective analysis. 2021; |
Cohort study; included less than 500 patients |
Table of excluded studies from the search strategy
|
Reference |
Reason for exclusion |
|
Agnelli, G. and Becattini, C. and Kirschstein, T. Thrombolysis vs heparin in the treatment of pulmonary embolism: A clinical outcome-based meta-analysis. Archives of Internal Medicine. 2002; 162 (22) :2537-2541 |
a more recent meta-analysis was included |
|
Wan, S. and Quinlan, D. J. and Agnelli, G. and Eikelboom, J. W. Thrombolysis compared with heparin for the initial treatment of pulmonary embolism: A meta-analysis of the randomized controlled trials. Circulation. 2004; 110 (6) :744-749 |
a more recent meta-analysis was included |
|
Cao, Y. and Zhao, H. and Gao, W. and Wang, Y. and Cao, J. Systematic review and meta-analysis for thrombolysis treatment in patients with acute submassive pulmonary embolism. Patient Preference and Adherence. 2014; 8 :275-282 |
a more recent meta-analysis was included |
|
Chatterjee, S. and Chakraborty, A. and Weinberg, I. and Kadakia, M. and Wilensky, R. L. and Sardar, P. and Kumbhani, D. J. and Mukherjee, D. and Jaff, M. R. and Giri, J. Thrombolysis for pulmonary embolism and risk of all-cause mortality, major bleeding, and intracranial hemorrhage: A meta-analysis. JAMA. 2014; 311 (23) :2414-2421 |
a more recent meta-analysis was included |
|
Riera-Mestre, Antoni and Becattini, Cecilia and Giustozzi, Michela and Agnelli, Giancarlo Thrombolysis in hemodynamically stable patients with acute pulmonary embolism: a meta-analysis. Thrombosis research. 2014; 134 (6) :1265-71 |
a more recent meta-analysis was included |
|
Janz, T. G. Using thrombolytic therapy for life-threatening pulmonary embolism. Journal of Critical Illness. 2003; 18 (3) :102-109 |
narrative review |
|
Büller, H. R. and Agnelli, G. and Hull, R. D. and Hyers, T. M. and Prins, M. H. and Raskob, G. E. Antithrombotic therapy for venous thromboembolic disease: The Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004; 126 (3) :401S-428S |
conference proceedings |
|
Goldhaber, S. Z. Pulmonary embolism. Lancet. 2004; 363 (9417) :1295-1305 |
narrative review |
|
Meyer, G. and Sanchez, O. Indications and modalities of fibrinolytic therapy for pulmonary embolism. Sang Thrombose Vaisseaux. 2009; 21 (4) :181-185 |
narrative review |
|
Meyer, G. Thrombolytic therapy for pulmonary embolism. Journal des Maladies Vasculaires. 2011; 36 :S33-S36 |
narrative review |
|
Chen, H. and Ren, C. and Chen, H. Thrombolysis versus anticoagulation for the initial treatment of moderate pulmonary embolism: A meta-analysis of randomized controlled trials. Respiratory Care. 2014; 59 (12) :1880-1887 |
a more recent meta-analysis was included |
|
Liu, Yunfeng and Lu, Youjin and Song, Jian and Li, Dan and Liu, Hongyan and Yang, Jin and Zhao, Hui Recombinant tissue plasminogen activator for hemodynamically stable patients experiencing an acute pulmonary embolism: a meta-analysis. Thrombosis research. 2014; 134 (1) :50-6 |
a more recent meta-analysis was included |
|
Nakamura, S. and Takano, H. and Kubota, Y. and Asai, K. and Shimizu, W. Impact of the efficacy of thrombolytic therapy on the mortality of patients with acute submassive pulmonary embolism: a meta-analysis. Journal of thrombosis and haemostasis : JTH. 2014; 12 (7) :1086-95 |
a more recent meta-analysis was included |
|
Konstantinides, S. and Geibel, A. and Heusel, G. and Heinrich, F. and Kasper, W. Heparin plus alteplase compared with heparin alone in patients with submassive pulmonary embolism. New England Journal of Medicine. 2002; 347 (15) :1143-1150 |
a more recent meta-analysis was included |
|
Kelly, J. and Hunt, B. J. Do anticoagulants improve survival in patients presenting with venous thromboembolism?. Journal of Internal Medicine. 2003; 254 (6) :527-539 |
narrative review |
|
Aleksic, I. and Kamler, M. and Herold, U. and Massoudy, P. and Jakob, H. G. Surgical treatment for massive pulmonary embolism. Herz. 2005; 30 (4) :269-273 |
narrative review |
|
Gao, Guang-yuan and Yang, Ping and Liu, Miao and Ding, Mei and Liu, Guo-hui and Tong, Ya-liang and Yang, Chun-yan and Meng, Fan-bo Thrombolysis for acute intermediate-risk pulmonary embolism: A meta-analysis. Thrombosis research. 2015; 136 (5) :932-7 |
a more recent meta-analysis was included |
|
Marti, C. and John, G. and Konstantinides, S. and Combescur, C. and Sanchez, O. and Lankeit, M. and Meyer, G. and Perrier, A. Systemic thrombolytic therapy for acute pulmonary embolism: A systematic review and meta-analysis. European Heart Journal. 2015; 36 (10) :605-614 |
a more recent meta-analysis was included |
|
Xu, Q. and Huang, K. and Zhai, Z. and Yang, Y. and Wang, J. and Wang, C. Initial thrombolysis treatment compared with anticoagulation for acute intermediate-risk pulmonary embolism: A meta-analysis. Journal of Thoracic Disease. 2015; 7 (5) :810-821 |
a more recent meta-analysis was included |
|
Vedantham, S. Interventional approaches to acute venous thromboembolism. Seminars in Respiratory and Critical Care Medicine. 2008; 29 (1) :56-65 |
narrative review |
|
Alcedo, P. E. and García-Perdomo, H. A. and Rojas-Hernandez, C. M. The net benefit of thrombolysis in the management of intermediate risk pulmonary embolism: Systematic review and meta-analysis. eJHaem. 2020; 1 (2) :457-466 |
a more recent meta-analysis was included |
|
Sharifi, M. and Bay, C. and Skrocki, L. and Rahimi, F. and Mehdipour, M. Moderate pulmonary embolism treated with thrombolysis (from the "mOPETT" Trial). American Journal of Cardiology. 2013; 111 (2) :273-277 |
Study already included in Planer (2023) |
|
Kucher, N. and Boekstegers, P. and Müller, O. J. and Kupatt, C. and Beyer-Westendorf, J. and Heitzer, T. and Tebbe, U. and Horstkotte, J. and Müller, R. and Blessing, E. and Greif, M. and Lange, P. and Hoffmann, R. T. and Werth, S. and Barmeyer, A. and Härtel, D. and Grünwald, H. and Empen, K. and Baumgartner, I. Randomized, controlled trial of ultrasound-assisted catheter-directed thrombolysis for acute intermediate-risk pulmonary embolism. Circulation. 2014; 129 (4) :479-486 |
Study already included in Planer (2023) |
|
Meyer, G. and Vicaut, E. and Danays, T. and Agnelli, G. and Becattini, C. and Beyer-Westendorf, J. and Bluhmki, E. and Bouvaist, H. and Brenner, B. and Couturaud, F. and Dellas, C. and Empen, K. and Franca, A. and Galiè, N. and Geibel, A. and Goldhaber, S. Z. and Jimenez, D. and Kozak, M. and Kupatt, C. and Kucher, N. and Lang, I. M. and Lankeit, M. and Meneveau, N. and Pacouret, G. and Palazzini, M. and Petris, A. and Pruszczyk, P. and Rugolotto, M. and Salvi, A. and Schellong, S. and Sebbane, M. and Sobkowicz, B. and Stefanovic, B. S. and Thiele, H. and Torbicki, A. and Verschuren, F. and Konstantinides, S. V. Fibrinolysis for patients with intermediate-risk pulmonary embolism. New England Journal of Medicine. 2014; 370 (15) :1402-1411 |
Study already included in Planer (2023) |
|
Konstantinides, Stavros V. and Vicaut, Eric and Danays, Thierry and Becattini, Cecilia and Bertoletti, Laurent and Beyer-Westendorf, Jan and Bouvaist, Helene and Couturaud, Francis and Dellas, Claudia and Duerschmied, Daniel and Empen, Klaus and Ferrari, Emile and Galie, Nazzareno and Jimenez, David and Kostrubiec, Maciej and Kozak, Matija and Kupatt, Christian and Lang, Irene M. and Lankeit, Mareike and Meneveau, Nicolas and Palazzini, Massimiliano and Pruszczyk, Piotr and Rugolotto, Matteo and Salvi, Aldo and Sanchez, Olivier and Schellong, Sebastian and Sobkowicz, Bozena and Meyer, Guy Impact of Thrombolytic Therapy on the Long-Term Outcome of Intermediate-Risk Pulmonary Embolism. Journal of the American College of Cardiology. 2017; 69 (12) :1536-1544 |
Study already included in Planer (2023) |
|
D'Auria S, Sezer A, Thoma F, Sharbaugh M, McKibben J, Maholic R, Avgerinos ED, Rivera-Lebron BN, Toma C. Outcomes of catheter-directed thrombolysis vs. standard medical therapy in patients with acute submassive pulmonary embolism. Pulm Circ. 2020 Apr 8;10(1):2045894019898368. doi: 10.1177/2045894019898368. PMID: 32292583; PMCID: PMC7144676. |
Study already included in Planer (2023) |
|
Ishisaka Y, Watanabe A, Fujisaki T, Iwagami M, So M, Steiger D, Aoi S, Secemsky EA, Wiley J, Kuno T. Comparison of interventions for intermediate to high-risk pulmonary embolism: A network meta-analysis. Catheter Cardiovasc Interv. 2023 Aug;102(2):249-265. doi: 10.1002/ccd.30745. Epub 2023 Jun 3. PMID: 37269229. |
No detailed search strategy |
|
Mathew, D. and Kim, J. and Kosuru, B. P. and Devagudi, D. and Sherif, A. and Shrestha, U. and Bedi, P. Mortality and bleeding associated with the management of sub-massive pulmonary embolism: a systematic review and Bayesian network meta-analysis. Scientific reports. 2023; 13 (1) :7169 |
search strategy not clear |
|
Kroupa, J. and Buk, M. and Weichet, J. and Malikova, H. and Bartova, L. and Linkova, H. and Ionita, O. and Kozel, M. and Motovska, Z. and Kocka, V. A pilot randomised trial of catheter-directed thrombolysis or standard anticoagulation for patients with intermediate-high risk acute pulmonary embolism. EuroIntervention. 2022; 18 (8) :E639-E646 |
Study already included in Planer (2023) |
|
Qin, Zhi-qiang and Wang, Chen [Comparison of thrombolysis and anticoagulation in pulmonary thromboembolism: a meta-analysis]. Zhonghua jie he he hu xi za zhi = Zhonghua jiehe he huxi zazhi = Chinese journal of tuberculosis and respiratory diseases. 2003; 26 (12) :772-5 |
article in chinese |
|
Goldhaber, S. Z. Thrombolytic therapy for patients with pulmonary embolism who are hemodynamically stable but have right ventricular dysfunction: Pro. Archives of Internal Medicine. 2005; 165 (19) :2197-2205 |
opinion article/ commentary |
|
Harris, T. and Meek, S. When should we thrombolyse patients with pulmonary embolism? A systematic review of the literature. Emergency Medicine Journal. 2005; 22 (11) :766-771 |
narrative review |
|
Konstantinides, S. Pulmonary embolism: Impact of right ventricular dysfunction. Current Opinion in Cardiology. 2005; 20 (6) :496-501 |
narrative review |
|
Meyer, G. and Sanchez, O. Thrombolysis in pulmonary embolism. Reanimation. 2005; 14 (3) :196-202 |
narrative review |
|
Wan, S. and Quinlan, D. J. and Agnelli, G. and Eikelboom, J. W. and Elliott, G. and Stevens, S. Review: Thrombolytic treatment does not reduce the risk of recurrent pulmonary embolism and death more than heparin. Evidence-Based Medicine. 2005; 10 (2) :41 |
narrative review |
|
Wang L. Thrombolytic Therapy for Pulmonary Embolism: Lessons from Recent Clinical Trials. Australasian Journal of Paramedicine. 2005;3:1-6. doi:10.33151/ajp.3.4.341 |
narrative review |
|
Emmerich, J. and Meyer, G. and Decousus, H. and Agnelli, G. Role of fibrinolysis and interventional therapy for acute venous thromboembolism. Thrombosis and Haemostasis. 2006; 96 (3) :251-257 |
narrative review |
|
Ramakrishnan, Naresh Thrombolysis is not warranted in submassive pulmonary embolism: a systematic review and meta-analysis. Critical care and resuscitation : journal of the Australasian Academy of Critical Care Medicine. 2007; 9 (4) :357-63 |
narrative review |
|
Skaf, E. and Beemath, A. and Siddiqui, T. and Janjua, M. and Patel, N. R. and Stein, P. D. Catheter-Tip Embolectomy in the Management of Acute Massive Pulmonary Embolism. American Journal of Cardiology. 2007; 99 (3) :415-420 |
does not fit the PICO |
|
Worster, Andrew and Smith, Camala and Silver, Shawna and Brown, Michael D. Evidence-based emergency medicine/critically appraised topic. Thrombolytic therapy for submassive pulmonary embolism?. Annals of emergency medicine. 2007; 50 (1) :78-84 |
does not fit the PICO |
|
Kuo, W. T. and Gould, M. K. and Louie, J. D. and Rosenberg, J. K. and Sze, D. Y. and Hofmann, L. V. Catheter-directed Therapy for the Treatment of Massive Pulmonary Embolism: Systematic Review and Meta-analysis of Modern Techniques. Journal of Vascular and Interventional Radiology. 2009; 20 (11) :1431-1440 |
does not fit the PICO |
|
Tardy, B. and Venet, C. and Zeni, F. and Coudrot, M. and Guyomarc'h, S. and Mismetti, P. Short term effect of recombinant tissue plasminogen activator in patients with hemodynamically stable acute pulmonary embolism: Results of a meta-analysis involving 464 patients. Thrombosis Research. 2009; 124 (6) :672-677 |
wrong I, does not fit the PICO |
|
Lankeit, M. and Konstantinides, S. Thrombolysis for pulmonary embolism: Past, present and future. Thrombosis and Haemostasis. 2010; 103 (5) :877-883 |
narrative review |
|
Kuo, W. T. Endovascular therapy for acute pulmonary embolism. Journal of Vascular and Interventional Radiology. 2012; 23 (2) :167-179 |
narrative review |
|
Lankeit, Mareike and Konstantinides, Stavros Thrombolytic therapy for submassive pulmonary embolism. Best practice & research. Clinical haematology. 2012; 25 (3) :379-89 |
review/ study protocol |
|
Tapson, V. F. Thrombolytic therapy in acute pulmonary embolism. Current Opinion in Cardiology. 2012; 27 (6) :585-591 |
narrative review |
|
Bunwaree, S. and Roffi, M. and Bonvini, J. M. and Noble, S. and Righini, M. and Bonvini, R. F. AngioJet® rheolytic thrombectomy: A new treatment option in cases of massive pulmonary embolism. Interventional Cardiology (London). 2013; 5 (1) :71-87 |
wrong C |
|
He, C. and Von Segesser, L. K. and Kappetein, P. A. and Mestres, C. A. and Smith, J. A. and Choong, C. K. C. Acute pulmonary embolectomy. European Journal of Cardio-thoracic Surgery. 2013; 43 (6) :1087-1095 |
narrative review |
|
Heberlein, Wolf E. and Meek, Mollie E. and Saleh, Omar and Meek, James C. and Lensing, Shelly Y. and Culp, William C. New generation aspiration catheter: Feasibility in the treatment of pulmonary embolism. World journal of radiology. 2013; 5 (11) :430-5 |
case series |
|
Chen, H. and Ren, C. and Chen, H. Thrombolysis versus anticoagulation for the initial treatment of moderate pulmonary embolism: A meta-analysis of randomized controlled trials. Respiratory Care. 2014; 59 (12) :1880-1887 |
a more recent meta-analysis was included |
|
Engelberger, R. P. and Kucher, N. Ultrasound-assisted thrombolysis for acute pulmonary embolism: A systematic review. European Heart Journal. 2014; 35 (12) :758-764 |
narrative review |
|
Liu, Yunfeng and Lu, Youjin and Song, Jian and Li, Dan and Liu, Hongyan and Yang, Jin and Zhao, Hui Recombinant tissue plasminogen activator for hemodynamically stable patients experiencing an acute pulmonary embolism: a meta-analysis. Thrombosis research. 2014; 134 (1) :50-6 |
a more recent meta-analysis was included |
|
Nakamura, S. and Takano, H. and Kubota, Y. and Asai, K. and Shimizu, W. Impact of the efficacy of thrombolytic therapy on the mortality of patients with acute submassive pulmonary embolism: a meta-analysis. Journal of thrombosis and haemostasis : JTH. 2014; 12 (7) :1086-95 |
wrong I |
|
Konstantinides, S. and Geibel, A. and Kasper, W. Thrombolytic treatment of pulmonary embolism: Life-saving option or unacceptable risk?. Intensivmedizin und Notfallmedizin. 2000; 37 (1) :139-145 |
narrative review |
|
Arcasoy, Selim M. and Vachani, Anil Local and systemic thrombolytic therapy for acute venous thromboembolism. Clinics in chest medicine. 2003; 24 (1) :73-91 |
narrative review |
|
Pérez de Llano, L. A. and Baloira Villar, A. and Veres Racamonde, A. and Veiga, F. and Golpe Gómez, R. and Pajuelo Fernández, F. Multicenter, prospective study comparing enoxaparin with unfractionated heparin in the treatment of submassive pulmonary thromboembolism. Archivos de Bronconeumologia. 2003; 39 (8) :341-345 |
does not fit the PICO |
|
Konstantinides, S. Should thrombolytic therapy be used in patients with pulmonary embolism?. American Journal of Cardiovascular Drugs. 2004; 4 (2) :69-74 |
narrative review |
|
Madden, B. P. and Sheth, A. and Ho, T. B. L. Thrombolytic therapy for acute proximal pulmonary embolism without significant haemodynamic compromise. Respiratory Medicine Extra. 2006; 2 (1) :34-38 |
case series |
|
Zhang, Zhu and Zhai, Zhen-guo and Liang, Li-rong and Liu, Fang-fang and Yang, Yuan-hua and Wang, Chen Lower dosage of recombinant tissue-type plasminogen activator (rt-PA) in the treatment of acute pulmonary embolism: a systematic review and meta-analysis. Thrombosis research. 2014; 133 (3) :357-63 |
wrong I, does not fit the PICO |
|
Brandt, K. and McGinn, K. and Quedado, J. Low-Dose Systemic Alteplase (tPA) for the Treatment of Pulmonary Embolism. Annals of Pharmacotherapy. 2015; 49 (7) :818-824 |
narrative review |
|
Gao, Guang-yuan and Yang, Ping and Liu, Miao and Ding, Mei and Liu, Guo-hui and Tong, Ya-liang and Yang, Chun-yan and Meng, Fan-bo Thrombolysis for acute intermediate-risk pulmonary embolism: A meta-analysis. Thrombosis research. 2015; 136 (5) :932-7 |
a more recent meta-analysis was included |
|
Meyer G, Planquette B, Sanchez O. Fibrinolysis for Acute Care of Pulmonary Embolism in the Intermediate Risk Patient. Curr Atheroscler Rep. 2015 Dec;17(12):68. doi: 10.1007/s11883-015-0546-1. PMID: 26486512. |
does not fit the PICO |
|
Meyer, G. and Sanchez, O. and Planquette, B. Intermediate-risk pulmonary embolism. Thrombolysis, yes or no?. Reanimation. 2015; 24 (2) :98-103 |
narrative review |
|
Wärntges, S. and Konstantinides, S. V. Progress in the management of acute pulmonary embolism. Current Opinion in Pulmonary Medicine. 2015; 21 (5) :417-424 |
narrative review |
|
Xu, Q. and Huang, K. and Zhai, Z. and Yang, Y. and Wang, J. and Wang, C. Initial thrombolysis treatment compared with anticoagulation for acute intermediate-risk pulmonary embolism: A meta-analysis. Journal of Thoracic Disease. 2015; 7 (5) :810-821 |
a more recent meta-analysis was included |
|
Bajaj, N. S. and Kalra, R. and Arora, P. and Ather, S. and Guichard, J. L. and Lancaster, W. J. and Patel, N. and Raman, F. and Arora, G. and Al Solaiman, F. and Clark, D. T. and Dell'Italia, L. J. and Leesar, M. A. and Davies, J. E. and McGiffin, D. C. and Ahmed, M. I. Catheter-directed treatment for acute pulmonary embolism: Systematic review and single-arm meta-analyses. International Journal of Cardiology. 2016; 225 :128-139 |
does not fit the PICO |
|
Keeling, W. B. and Leshnower, B. G. and Lasajanak, Y. and Binongo, J. and Guyton, R. A. and Halkos, M. E. and Thourani, V. H. and Lattouf, O. M. Midterm benefits of surgical pulmonary embolectomy for acute pulmonary embolus on right ventricular function. Journal of Thoracic and Cardiovascular Surgery. 2016; 152 (3) :872-878 |
does not fit the PICO |
|
Teleb, M. and Porres-Aguilar, M. and Anaya-Ayala, J. E. and Rodriguez-Castro, C. and Porres-Muñoz, M. and Mukherjee, D. Potential role of systemic thrombolysis in acute submassive intermediate risk pulmonary embolism: Review and future perspectives. Therapeutic Advances in Cardiovascular Disease. 2016; 10 (2) :103-110 |
narrative review |
|
Bloomer, T. L. and El-Hayek, G. E. and McDaniel, M. C. and Sandvall, B. C. and Liberman, H. A. and Devireddy, C. M. and Kumar, G. and Fong, P. P. and Jaber, W. A. Safety of catheter-directed thrombolysis for massive and submassive pulmonary embolism: Results of a multicenter registry and meta-analysis. Catheterization and Cardiovascular Interventions. 2017; 89 (4) :754-760 |
does not fit the PICO |
|
Kalra, R. and Bajaj, N. S. and Arora, P. and Arora, G. and Crosland, W. A. and McGiffin, D. C. and Ahmed, M. I. Surgical Embolectomy for Acute Pulmonary Embolism: Systematic Review and Comprehensive Meta-Analyses. Annals of Thoracic Surgery. 2017; 103 (3) :982-990 |
does not fit the PICO |
|
Kesselman, A. and Kuo, W. T. Catheter-Directed Therapy for Acute Submassive Pulmonary Embolism: Summary of Current Evidence and Protocols. Techniques in Vascular and Interventional Radiology. 2017; 20 (3) :193-196 |
narrative review |
|
Konstantinides, S. V. and Barco, S. Systemic Thrombolytic Therapy for Acute Pulmonary Embolism: Who Is a Candidate?. Seminars in Respiratory and Critical Care Medicine. 2017; 38 (1) :056-065 |
narrative review |
|
Li, X. F. and Wan, C. Q. and He, X. G. and Qiu, J. Y. and Li, D. Y. and Sun, Y. X. and Mao, Y. M. Catheter-directed therapy as a treatment for submassive pulmonary embolism: A meta-analysis. Life Sciences. 2017; 188 :17-25 |
a more recent meta-analysis was included |
|
Lou, B. H. and Wang, L. H. and Chen, Y. A meta-analysis of efficacy and safety of catheter-directed interventions in submassive pulmonary embolism. European review for medical and pharmacological sciences. 2017; 21 (1) :184-198 |
a more recent meta-analysis was included |
|
Sista, Akhilesh K. and Miller, Larry E. and Kahn, Susan R. and Kline, Jeffrey A. Persistent right ventricular dysfunction, functional capacity limitation, exercise intolerance, and quality of life impairment following pulmonary embolism: Systematic review with meta-analysis. Vascular medicine (London, England). 2017; 22 (1) :37-43 |
a more recent meta-analysis was included |
|
Perlroth, D. J. and Sanders, G. D. and Gould, M. K. Effectiveness and cost-effectiveness of thrombolysis in submassive pulmonary embolism. Archives of Internal Medicine. 2007; 167 (1) :74-80 |
does not fit the PICO |
|
Yoo, H. H. B. and Rodrigues, H. and Queluz, T. T. Treatment of pulmonary thromboembolism in patients with systemic blood pressure stability and right ventricular dysfunction. Current Respiratory Medicine Reviews. 2008; 4 (1) :52-56 |
narrative review |
|
Zamanian, R. T. and Gould, M. K. Effectiveness and cost effectiveness of thrombolysis in patients with acute pulmonary embolism. Current Opinion in Pulmonary Medicine. 2008; 14 (5) :422-426 |
narrative review |
|
Todd, J. L. and Tapson, V. F. Thrombolytic therapy for acute pulmonary embolism: A critical appraisal. Chest. 2009; 135 (5) :1321-1329 |
narrative review |
|
Gao, H. and Huang, G. Y. and Ma, L. L. and Wang, L. X. Combined catheter thrombus fragmentation and fibrinolysis for acute pulmonary embolism. Internal Medicine Journal. 2011; 41 (9) :687-691 |
Case series |
|
Jin, J. and Ding, W. B. and Yuan, R. F. Thrombolytic treatment of acute pulmonary thromboembolism: Comparison between catheter-directed thrombolysis and venous thrombolysis. Journal of Interventional Radiology (China). 2012; 21 (8) :667-671 |
does not fit the PICO |
|
Steering Committee. Single-bolus tenecteplase plus heparin compared with heparin alone for normotensive patients with acute pulmonary embolism who have evidence of right ventricular dysfunction and myocardial injury: rationale and design of the Pulmonary Embolism Thrombolysis (PEITHO) trial. Am Heart J. 2012 Jan;163(1):33-38.e1. doi: 10.1016/j.ahj.2011.10.003. PMID: 22172434. |
Trial protocol |
|
Tafur, A. J. and Shamoun, F. E. and Patel, S. I. and Tafur, D. and Donna, F. and Murad, M. H. Catheter-Directed Treatment of Pulmonary Embolism: A Systematic Review and Meta-Analysis of Modern Literature. Clinical and Applied Thrombosis/Hemostasis. 2017; 23 (7) :821-829 |
More recent review included |
|
Tapson, V. F. and Friedman, O. Systemic Thrombolysis for Pulmonary Embolism: Who and How. Techniques in Vascular and Interventional Radiology. 2017; 20 (3) :162-174 |
narrative review |
|
Tromeur, C. and Van Der Pol, L. M. and Couturaud, F. and Klok, F. A. and Huisman, M. V. Therapeutic management of acute pulmonary embolism. Expert Review of Respiratory Medicine. 2017; 11 (8) :641-648 |
narrative review |
|
Avgerinos, E. D. and Saadeddin, Z. and Abou Ali, A. N. and Fish, L. and Toma, C. and Chaer, M. and Rivera-Lebron, B. N. and Chaer, R. A. A meta-analysis of outcomes of catheter-directed thrombolysis for high- and intermediate-risk pulmonary embolism. Journal of Vascular Surgery: Venous and Lymphatic Disorders. 2018; 6 (4) :530-540 |
More recent review included |
|
Eberle, H. and Lyn, R. and Knight, T. and Hodge, E. and Daley, M. Clinical update on thrombolytic use in pulmonary embolism: A focus on intermediate-risk patients. American Journal of Health-System Pharmacy. 2018; 75 (17) :1275-1285 |
narrative review |
|
Harvey JJ, Huang S, Uberoi R. Catheter-directed therapies for the treatment of high risk (massive) and intermediate risk (submassive) acute pulmonary embolism. Cochrane Database Syst Rev. 2022 Aug 8;8(8):CD013083. doi: 10.1002/14651858.CD013083.pub2. PMID: 35938605; PMCID: PMC9358724. |
More recent review included |
|
Kaymaz, C. and Akbal, Ö Y. and Tanboğa, I. H. and Hakgör, A. and Yilmaz, F. and Öztürk, S. and Poçi, N. and Türkday, S. and Özdemir, N. and Konstantinides, S. Ultrasound-assisted catheter-directed thrombolysis in high-risk and intermediate-high-risk pulmonary embolism: A meta-analysis. Current Vascular Pharmacology. 2018; 16 (2) :179-189 |
a more recent NMA was included |
|
Loyalka, P. and Ansari, M. Z. and Cheema, F. H. and Miller, C. C. and Rajagopal, S. and Rajagopal, K. Surgical pulmonary embolectomy and catheter-based therapies for acute pulmonary embolism: A contemporary systematic review. Journal of Thoracic and Cardiovascular Surgery. 2018; 156 (6) :2155-2167 |
included massive PE, wrong P |
|
Riva, Nicoletta and Puljak, Livia and Moja, Lorenzo and Ageno, Walter and Schunemann, Holger and Magrini, Nicola and Squizzato, Alessandro Multiple overlapping systematic reviews facilitate the origin of disputes: the case of thrombolytic therapy for pulmonary embolism. Journal of clinical epidemiology. 2018; 97 :1-13 |
Does not fit the PICO |
|
Schultz, J. and Andersen, A. and Kabrhel, C. and Nielsen-Kudsk, J. E. Catheter-based therapies in acute pulmonary embolism. EuroIntervention. 2018; 13 (14) :1721-1727 |
narrative review |
|
Pei, D. T. and Liu, J. and Yaqoob, M. and Ahmad, W. and Bandeali, S. S. and Hamzeh, I. R. and Virani, S. S. and Hira, R. S. and Lakkis, N. M. and Alam, M. Meta-Analysis of Catheter Directed Ultrasound-Assisted Thrombolysis in Pulmonary Embolism. American Journal of Cardiology. 2019; 124 (9) :1470-1477 |
More recent review included |
|
Pillus, D. and Bruno, E. and Farcy, D. and Vilke, G. M. and Childers, R. Systematic Review: The Role of Thrombolysis in Intermediate-Risk Pulmonary Embolism. Journal of Emergency Medicine. 2019; 57 (4) :517-522 |
narrative review |
|
Alcedo, P. E. and García-Perdomo, H. A. and Rojas-Hernandez, C. M. The net benefit of thrombolysis in the management of intermediate risk pulmonary embolism: Systematic review and meta-analysis. eJHaem. 2020; 1 (2) :457-466 |
More recent review included |
|
Choi, J. H. and O'Malley, T. J. and Maynes, E. J. and Weber, M. P. and D'Antonio, N. D. and Mellado, M. and West, F. M. and Galanis, T. and Gonsalves, C. F. and Marhefka, G. D. and Awsare, B. K. and Merli, G. J. and Tchantchaleishvili, V. Surgical Pulmonary Embolectomy Outcomes for Acute Pulmonary Embolism. Annals of Thoracic Surgery. 2020; 110 (3) :1072-1080 |
past niet bij de pico |
|
Duffett L, Castellucci LA, Forgie MA. Pulmonary embolism: update on management and controversies. BMJ. 2020 Aug 5;370:m2177. doi: 10.1136/bmj.m2177. PMID: 32759284. |
narrative review |
|
Igneri, L. A. and Hammer, J. M. Systemic Thrombolytic Therapy for Massive and Submassive Pulmonary Embolism. Journal of Pharmacy Practice. 2020; 33 (1) :74-89 |
More recent review included |
|
Izcovich, A. and Criniti, J. M. and Popoff, F. and Lu, L. and Wu, J. and Ageno, W. and Witt, D. M. and Jaff, M. R. and Schulman, S. and Manja, V. and Verhamme, P. and Rada, G. and Zhang, Y. and Nieuwlaat, R. and Wiercioch, W. and Schünemann, H. J. and Neumann, I. Thrombolytics for venous thromboembolic events: A systematic review with meta-analysis. Blood Advances. 2020; 4 (7) :1539-1553 |
More recent review included |
|
Kline, J. A. and Hernandez, J. and Hogg, M. M. and Jones, A. E. and Courtney, D. M. and Kabrhel, C. and Nordenholz, K. E. and Diercks, D. B. and Rondina, M. T. and Klinger, J. R. Rationale and methodology for a multicentre randomised trial of fibrinolysis for pulmonary embolism that includes quality of life outcomes. EMA - Emergency Medicine Australasia. 2013; 25 (6) :515-526 |
Trial protocol |
|
Tapson, V. F. Thrombolytic therapy for acute pulmonary embolism. Seminars in Thrombosis and Hemostasis. 2013; 39 (4) :452-458 |
Narrative review |
|
Patra, S. and Agrawal, N. and Manjunath, C. N. and Nagesh, C. M. and Srinivas, B. C. and Ravindranath, K. S. and Reddy, B. Thrombolytic therapy in the treatment of acute sub-massive pulmonary embolism: A prospective observational study. Blood Coagulation and Fibrinolysis. 2014; 25 (2) :167-171 |
Cohot study < 500 patients |
|
Sanchez, O. and Planquette, B. and Meyer, G. Management of massive and submassive pulmonary embolism: Focus on recent randomized trials. Current Opinion in Pulmonary Medicine. 2014; 20 (5) :393-399 |
Narrative review |
|
Shukla, A. N. and Thakkar, B. and Jayaram, A. A. and Madan, T. H. and Gandhi, G. D. Efficacy and safety of tenecteplase in pulmonary embolism. Journal of Thrombosis and Thrombolysis. 2014; 38 (1) :24-29 |
Case series |
|
Stein, Paul D. and Dalen, James E. Thrombolytic therapy for acute pulmonary embolism: when do the benefits exceed the risks?. The American journal of medicine. 2014; 127 (11) :1031-1032 |
narrative review |
|
Avgerinos, E. D. and Chaer, R. A. Catheter-directed interventions for acute pulmonary embolism. Journal of Vascular Surgery. 2015; 61 (2) :559-565 |
Study included by Paner (2023) |
|
Stewart, L. K. and Peitz, G. W. and Nordenholz, K. E. and Courtney, D. M. and Kabrhel, C. and Jones, A. E. and Rondina, M. T. and Diercks, D. B. and Klinger, J. R. and Kline, J. A. Contribution of fibrinolysis to the physical component summary of the SF-36 after acute submassive pulmonary embolism. Journal of Thrombosis and Thrombolysis. 2015; 40 (2) :161-166 |
Does not fit the PICO |
|
Ucar, Elif Yilmazel and Akgun, Metin and Araz, Omer and Tas, Hakan and Kerget, Bugra and Meral, Mehmet and Kaynar, Hasan and Saglam, Leyla Comparison of LMWH versus UFH for hemorrhage and hospital mortality in the treatment of acute massive pulmonary thromboembolism after thrombolytic treatment : randomized controlled parallel group study. Lung. 2015; 193 (1) :121-7 |
Does not fit the PICO |
|
Vedantham, S. Interventional therapy for venous thromboembolism. Journal of Thrombosis and Haemostasis. 2015; 13 :S245-S251 |
narrative review |
|
Bradford, M. A. and Lindenauer, P. K. and Walkey, A. J. Practice patterns and complication rates of thrombolysis for pulmonary embolism. Journal of Thrombosis and Thrombolysis. 2016; 42 (3) :313-321 |
Analysis was stratified by vasopressor use |
|
Sista, Akhilesh K. and Goldhaber, Samuel Z. and Vedantham, Suresh and Kline, Jeffrey A. and Kuo, William T. and Kahn, Susan R. and Kabrhel, Christopher and McLaughlin, Vallerie V. and White, Sarah B. and Kim, Nick H. and Gray, Michael and Simon, Marc A. and Benenati, James F. and Misra, Sanjay and Sterling, Keith M. and Kee, Stephen T. and Konstantinides, Stavros V. and Jaff, Michael R. and Kearon, Clive Research Priorities in Submassive Pulmonary Embolism: Proceedings from a Multidisciplinary Research Consensus Panel. Journal of vascular and interventional radiology : JVIR. 2016; 27 (6) :787-94 |
Not original data included |
|
Rodriguez, D. and Jerjes-Sanchez, C. and Fonseca, S. and Garcia-Toto, R. and Martinez-Alvarado, J. and Panneflek, J. and Ortiz-Ledesma, C. and Nevarez, F. Thrombolysis in massive and submassive pulmonary embolism during pregnancy and the puerperium: a systematic review. Journal of Thrombosis and Thrombolysis. 2020; 50 (4) :929-941 |
More recent review included |
|
Tice C, Seigerman M, Fiorilli P, Pugliese SC, Khandhar S, Giri J, Kobayashi T. Management of Acute Pulmonary Embolism. Curr Cardiovasc Risk Rep. 2020;14(12):24. doi: 10.1007/s12170-020-00659-z. Epub 2020 Oct 6. PMID: 33042325; PMCID: PMC7538277. |
narrative review |
|
Wu, J. and Chen, H. and Yu, Y. and Peng, L. and Li, J. and Liang, H. and Ba, M. and Ruan, H. and Hong, C. Feasibility of ultrasound-assisted catheter-directed thrombolysis for submassive pulmonary embolism: A meta-analysis of case series. Clinical Respiratory Journal. 2020; 14 (5) :430-439 |
Meta-analysis of case series |
|
Amini, S. and Bakhshandeh, H. and Mosaed, R. and Abtahi, H. and Sadeghi, K. and Mojtahedzadeh, M. Efficacy and Safety of Different Dosage of Recombinant Tissue-type Plasminogen Activator (rt-PA) in the Treatment of Acute Pulmonary Embolism: A Systematic Review and Meta-analysis. Iranian Journal of Pharmaceutical Research. 2021; 20 (2) :441-454 |
Does not fit the PICO |
|
Bishay, V. L. and Adenikinju, O. and Todd, R. FlowTriever Retrieval System for the treatment of pulmonary embolism: overview of its safety and efficacy. Expert Review of Medical Devices. 2021; 18 (11) :1039-1048 |
Does not fit the PICO |
|
Castillo-Perez, M. and Jerjes-Sánchez, C. and Rodríguez, D. and Paredes-Vazquez, J. G. and Panneflek, J. and Vazquez-Guajardo, M. Clinical outcomes of very elderly patients treated with ultrasound-assisted catheter-directed thrombolysis for pulmonary embolism: a systematic review. Journal of Thrombosis and Thrombolysis. 2021; 52 (1) :260-271 |
Does not fit the PICO |
|
Madathil, R., Anagnostakos, J., Pereira, G. et al. Current Management of Acute Pulmonary Embolism. Curr Surg Rep 9, 16 (2021). https://doi.org/10.1007/s40137-021-00293-7 |
narrative review |
|
Nguyen PC, Stevens H, Peter K, McFadyen JD. Submassive Pulmonary Embolism: Current Perspectives and Future Directions. J Clin Med. 2021 Jul 30;10(15):3383. doi: 10.3390/jcm10153383. PMID: 34362166; PMCID: PMC8347177. |
narrative review |
|
Zuo Z, Yue J, Dong BR, Wu T, Liu GJ, Hao Q. Thrombolytic therapy for pulmonary embolism. Cochrane Database Syst Rev. 2021 Apr 15;4(4):CD004437. doi: 10.1002/14651858.CD004437.pub6. PMID: 33857326; PMCID: PMC8092433. |
More recent review included |
|
Chandra, V. M. and Khaja, M. S. and Kryger, M. C. and Sista, A. K. and Wilkins, L. R. and Angle, J. F. and Sharma, A. M. Mechanical aspiration thrombectomy for the treatment of pulmonary embolism: A systematic review and meta-analysis. Vascular Medicine (United Kingdom). 2022; 27 (6) :574-584 |
More recent review included |
|
Chopard, R. and Meneveau, N. and Ecarnot, F. Catheter-based therapy for acute pulmonary embolism: An overview of current evidence. Archives of Cardiovascular Diseases. 2022; 115 (6) :397-405 |
narrative review |
|
Freund, Y. and Cohen-Aubart, F. and Bloom, B. Acute Pulmonary Embolism: A Review. JAMA. 2022; 328 (13) :1336-1345 |
narrative review |
|
Harvey JJ, Huang S, Uberoi R. Catheter-directed therapies for the treatment of high risk (massive) and intermediate risk (submassive) acute pulmonary embolism. Cochrane Database Syst Rev. 2022 Aug 8;8(8):CD013083. doi: 10.1002/14651858.CD013083.pub2. PMID: 35938605; PMCID: PMC9358724. |
narrative review |
|
Ismayl, Mahmoud and Machanahalli Balakrishna, Akshay and Aboeata, Ahmed and Gupta, Tanush and Young, Michael N. and Altin, S. Elissa and Aronow, Herbert D. and Goldsweig, Andrew M. Meta-Analysis Comparing Catheter-Directed Thrombolysis Versus Systemic Anticoagulation Alone for Submassive Pulmonary Embolism. The American journal of cardiology. 2022; 178 :154-162 |
wrong C |
|
Olanipekun, Titilope and Abe, Temidayo and Effoe, Valery and Chris-Olaiya, Abimbola and Biney, Isaac and Guru, Pramod and Ritchie, Charles and Sanghavi, Devang Utilization trends and outcomes of catheter-directed thrombolysis for pulmonary embolism in the US by race/ethnicity. Journal of thrombosis and thrombolysis. 2022; 54 (4) :675-685 |
does not fit the PICO |
|
Pan, Q. and Gao, H. and Wang, Y. and Chen, Q. Comparison of Efficacy and Safety between Thrombolysis Plus Anticoagulation vs. Anticoagulation Alone for the Treatment of Acute Submassive Pulmonary Embolism: A Systematic Review and Meta-analysis. Current Vascular Pharmacology. 2022; 20 (6) :491-500 |
More recent review included |
|
Pasha, A. K. and Siddiqui, M. U. and Siddiqui, M. D. and Ahmed, A. and Abdullah, A. and Riaz, I. and Murad, M. H. and Bjarnason, H. and Wysokinski, W. E. and McBane, R. D. Catheter directed compared to systemically delivered thrombolysis for pulmonary embolism: a systematic review and meta-analysis. Journal of Thrombosis and Thrombolysis. 2022; 53 (2) :454-466 |
More recent review included |
|
Kabrhel, C. and Ali, A. and Choi, J. G. and Hur, C. Systemic Thrombolysis, Catheter-Directed Thrombolysis, and Anticoagulation for Intermediate-risk Pulmonary Embolism: A Simulation Modeling Analysis. Academic Emergency Medicine. 2017; 24 (10) :1235-1243 |
No original study/data |
|
Kosova EC, Desai KR, Schimmel DR. Endovascular Management of Massive and Submassive Acute Pulmonary Embolism: Current Trends in Risk Stratification and Catheter-Directed Therapies. Curr Cardiol Rep. 2017 Jun;19(6):54. doi: 10.1007/s11886-017-0864-8. PMID: 28466280. |
narrative review |
|
Mangi, Muhammad A. and Rehman, Hiba and Bansal, Vikas and Zuberi, Omer Ultrasound Assisted Catheter-Directed Thrombolysis of Acute Pulmonary Embolism: A Review of Current Literature. Cureus. 2017; 9 (7) :e1492 |
narrative review |
|
Teleb, M. and Porres-Aguilar, M. and Rivera-Lebron, B. and Ngamdu, K. S. and Botrus, G. and Anaya-Ayala, J. E. and Mukherjee, D. Ultrasound-Assisted Catheter-Directed Thrombolysis: A Novel and Promising Endovascular Therapeutic Modality for Intermediate-Risk Pulmonary Embolism. Angiology. 2017; 68 (6) :494-501 |
narrative review |
|
Avgerinos, E. D. and Mohapatra, A. and Rivera-Lebron, B. and Toma, C. and Kabrhel, C. and Fish, L. and Lacomis, J. and Ocak, I. and Chaer, R. A. Design and rationale of a randomized trial comparing standard versus ultrasound-assisted thrombolysis for submassive pulmonary embolism. Journal of Vascular Surgery: Venous and Lymphatic Disorders. 2018; 6 (1) :126-132 |
Trial protocol |
|
Furfaro, D. and Stephens, R. S. and Streiff, M. B. and Brower, R. Catheter-directed thrombolysis for intermediate-risk pulmonary embolism. Annals of the American Thoracic Society. 2018; 15 (2) :134-144 |
More recent review included |
|
Lozier, J. N. and Elinoff, J. M. and Suffredini, A. F. and Rosing, D. R. and Sidenko, S. and Sherry, R. M. and Metwalli, A. R. and Sachdev, V. and Danner, R. L. and Chang, R. Low-dose, short course alteplase treatment of submassive pulmonary embolism: A case series from the National Institutes of Health Clinical Center. Blood Coagulation and Fibrinolysis. 2018; 29 (8) :701-707 |
case series |
|
Tapson, V. F. and Sterling, K. and Jones, N. and Elder, M. and Tripathy, U. and Brower, J. and Maholic, R. L. and Ross, C. B. and Natarajan, K. and Fong, P. and Greenspon, L. and Tamaddon, H. and Piracha, A. R. and Engelhardt, T. and Katopodis, J. and Marques, V. and Sharp, A. S. P. and Piazza, G. and Goldhaber, S. Z. A Randomized Trial of the Optimum Duration of Acoustic Pulse Thrombolysis Procedure in Acute Intermediate-Risk Pulmonary Embolism: The OPTALYSE PE Trial. JACC: Cardiovascular Interventions. 2018; 11 (14) :1401-1410 |
Dose comparison trial |
|
Zhang, L. Y. and Gao, B. A. and Jin, Z. and Xiang, G. M. and Gong, Z. and Zhang, T. T. and Lu, H. F. and Wang, Y. Q. and Gong, Y. and Lu, C. and Huang, W. L. Clinical efficacy of low dose recombinant tissue-type plasminogen activator for the treatment of acute intermediate-risk pulmonary embolism. Saudi Medical Journal. 2018; 39 (11) :1090-1095 |
Included in Planer (2023) |
|
Zhao, T. and Ni, J. and Hu, X. and Wang, Y. and Du, X. The Efficacy and Safety of Intermittent Low-Dose Urokinase Thrombolysis for the Treatment of Senile Acute Intermediate-High-Risk Pulmonary Embolism: A Pilot Trial. Clinical and Applied Thrombosis/Hemostasis. 2018; 24 (7) :1067-1072 |
Comparison between two agents for thrombolysis |
|
Barco, Stefano and Russo, Mariaconcetta and Vicaut, Eric and Becattini, Cecilia and Bertoletti, Laurent and Beyer-Westendorf, Jan and Bouvaist, Helene and Couturaud, Francis and Danays, Thierry and Dellas, Claudia and Duerschmied, Daniel and Empen, Klaus and Ferrari, Emile and Galie, Nazzareno and Jimenez, David and Klok, Frederikus A. and Kostrubiec, Maciej and Kozak, Matija and Kupatt, Christian and Lang, Irene M. and Lankeit, Mareike and Meneveau, Nicolas and Palazzini, Massimiliano and Pruszczyk, Piotr and Rugolotto, Matteo and Salvi, Aldo and Sanchez, Olivier and Schellong, Sebastian and Sobkowicz, Bozena and Meyer, Guy and Konstantinides, Stavros V. Incomplete echocardiographic recovery at 6 months predicts long-term sequelae after intermediate-risk pulmonary embolism. A post-hoc analysis of the Pulmonary Embolism Thrombolysis (PEITHO) trial. Clinical research in cardiology : official journal of the German Cardiac Society. 2019; 108 (7) :772-778 |
No comparison |
|
Bin, H. and Binxia, S. and Xufeng, C. and Lin, L. and Jinsong, Z. Effects of the combination of alteplase or urokinase with low molecular weight heparin anticoagulant therapy in the treatment of elderly patients with acute submassive pulmonary embolism. Acta Medica Mediterranea. 2019; 35 (3) :1287-1292 |
does not fit the PICO |
|
Taslakian B, Li C, Goldhaber SZ, Mikkelsen KZ, Horowitz JM, Kabrhel C, Barnes GD, Sista AK. How the Results of a Randomized Trial of Catheter-Directed Thrombolysis Versus Anticoagulation alone for Submassive Pulmonary Embolism Would Affect Patient and Physician Decision Making: Report of an Online Survey. J Clin Med. 2019 Feb 7;8(2):215. doi: 10.3390/jcm8020215. PMID: 30736480; PMCID: PMC6406864. |
does not fit the PICO |
|
Belsky J, Warren P, Stanek J, Kumar R. Catheter-directed thrombolysis for submassive pulmonary embolism in children: A case series. Pediatr Blood Cancer. 2020 Apr;67(4):e28144. doi: 10.1002/pbc.28144. Epub 2019 Dec 25. PMID: 31876109. |
Case series in children |
|
D'Auria S, Sezer A, Thoma F, Sharbaugh M, McKibben J, Maholic R, Avgerinos ED, Rivera-Lebron BN, Toma C. Outcomes of catheter-directed thrombolysis vs. standard medical therapy in patients with acute submassive pulmonary embolism. Pulm Circ. 2020 Apr 8;10(1):2045894019898368. doi: 10.1177/2045894019898368. PMID: 32292583; PMCID: PMC7144676. |
Included in Planer (2023) |
|
Geller, B. J. and Adusumalli, S. and Pugliese, S. C. and Khatana, S. A. M. and Nathan, A. and Weinberg, I. and Jaff, M. R. and Kobayashi, T. and Mazurek, J. A. and Khandhar, S. and Yang, L. and Groeneveld, P. W. and Giri, J. S. Outcomes of catheter-directed versus systemic thrombolysis for the treatment of pulmonary embolism: A real-world analysis of national administrative claims. Vascular Medicine (United Kingdom). 2020; 25 (4) :334-340 |
More recent review included |
|
Layman, S. N. and Guidry, T. J. and Gillion, A. R. Low-Dose Alteplase for the Treatment of Submassive Pulmonary Embolism: A Case Series. Journal of Pharmacy Practice. 2020; 33 (5) :708-711 |
case series |
|
Pietrasik A, Gąsecka A, Szarpak Ł, Pruc M, Kopiec T, Darocha S, Banaszkiewicz M, Niewada M, Grabowski M, Kurzyna M. Catheter-Based Therapies Decrease Mortality in Patients With Intermediate and High-Risk Pulmonary Embolism: Evidence From Meta-Analysis of 65,589 Patients. Front Cardiovasc Med. 2022 Jun 16;9:861307. doi: 10.3389/fcvm.2022.861307. PMID: 35783825; PMCID: PMC9243366. |
More recent review included |
|
Siordia JA, Kaur A. Catheter-directed Thrombolysis versus Systemic Anticoagulation for Submassive Pulmonary Embolism: A Meta-Analysis. Curr Cardiol Rev. 2022;18(1):112-117. doi: 10.2174/1573403X17666210603114116. PMID: 34082686; PMCID: PMC9241122. |
narrative review |
|
Alsamman, M. and Choudhry, A. M. and AlSaadi, A. M. and Prashad, R. Ultrasound-Accelerated Catheter-Directed Thrombolysis. Cardiology Research. 2023; 14 (3) :161-166 |
narrative review |
|
Farmakis, I. T. and Keller, K. and Barco, S. and Konstantinides, S. V. and Hobohm, L. From acute pulmonary embolism to post-pulmonary embolism sequelae: Functional and hemodynamic implications. Vasa - European Journal of Vascular Medicine. 2023; 52 (1) :29-37 |
narrative review |
|
Iannaccone, M. and Franchin, L. and Russo, F. and Botti, G. and Castellano, D. and Montorfano, M. and Boccuzzi, G. and Mamas, M. A. and Chieffo, A. Mortality across treatment strategies in intermediate-to-high risk pulmonary embolism in the modern era: A meta-analysis of observational studies and RCTs. International journal of cardiology. 2023; :131127 |
unclear/incomplete search strategy (interventional (CDT) versus medical therapy), majority observational studies |
|
Milioglou, I. and Farmakis, I. and Wazirali, M. and Ajluni, S. and Khawaja, T. and Chatuverdi, A. and Giannakoulas, G. and Shishehbor, M. and Li, J. Percutaneous thrombectomy in patients with intermediate- and high-risk pulmonary embolism and contraindications to thrombolytics: a systematic review and meta-analysis. Journal of Thrombosis and Thrombolysis. 2023; 55 (2) :228-242 |
Does not fit the PICO |
|
Murguia AR, Mukherjee D, Ojha C, Rajachandran M, Siddiqui TS, Nickel NP. Reduced-Dose Thrombolysis in Acute Pulmonary Embolism A Systematic Review. Angiology. 2024 Mar;75(3):208-218. doi: 10.1177/00033197231167062. Epub 2023 Apr 15. PMID: 37060258. |
narrative review |
|
Pietrasik, A. and Gasecka, A. and Kotulecki, A. and Karolak, P. and Araszkiewicz, A. and Darocha, S. and Grabowski, M. and Kurzyna, M. Catheter-directed therapy to treat intermediate-and high-risk pulmonary embolism: Personal experience and review of the literature. Cardiology Journal. 2023; 30 (3) :462-472 |
narrative review |
|
Planer, D. and Yanko, S. and Matok, I. and Paltiel, O. and Zmiro, R. and Rotshild, V. and Amir, O. and Elbaz-Greener, G. and Raccah, B. H. Catheter-directed thrombolysis compared with systemic thrombolysis and anticoagulation in patients with intermediate- or high-risk pulmonary embolism: systematic review and network meta-analysis. CMAJ. Canadian Medical Association Journal. 2023; 195 (24) :E833-E843 |
does not fit the PICO |
|
Meng, Y. and Zhang, J. and Ma, Q. and Qin, H. and Zhang, B. and Pang, H. and Yin, Q. and Tian, H. Pulmonary Interventional Therapy for Acute Massive and Submassive Pulmonary Embolism in Cases Where Thrombolysis Is Contraindicated. Annals of Vascular Surgery. 2020; 64 :169-174 |
Massive PE |
|
Piazza, G. and Sterling, K. M. and Tapson, V. F. and Ouriel, K. and Sharp, A. S. P. and Liu, P. Y. and Goldhaber, S. Z. One-Year Echocardiographic, Functional, and Quality of Life Outcomes After Ultrasound-Facilitated Catheter-Based Fibrinolysis for Pulmonary Embolism. Circulation: Cardiovascular Interventions. 2020; 13 (8) :E009012 |
Wrong C |
|
Avgerinos, E. D. and Jaber, W. and Lacomis, J. and Markel, K. and McDaniel, M. and Rivera-Lebron, B. N. and Ross, C. B. and Sechrist, J. and Toma, C. and Chaer, R. and Gladwin, M. and Lamberty, P. and Kabrhel, C. and Klein, A. J. and Makaroun, M. S. and Miller, C. E. and Mohapatra, A. and Phelos, H. and Sachdeva, R. and Al-Khoury, G. and Madigan, M. and Liang, N. and Fish, L. and Phelos, H. and Sheline, J. and Brimmeier, J. Randomized Trial Comparing Standard Versus Ultrasound-Assisted Thrombolysis for Submassive Pulmonary Embolism: The SUNSET sPE Trial. JACC: Cardiovascular Interventions. 2021; 14 (12) :1364-1373 |
wrong C |
|
Herzig, Matthew and Khandhar, Sameer and Palevsky, Harold and Fritz, Jason and Mehta, Mili and Matthai, William Intermediate-Term Outcomes for Patients with Submassive Pulmonary Embolism Treated With Catheter-Directed Thrombolysis. The Journal of invasive cardiology. 2021; 33 (12) :E949-E953 |
Case series |
|
Lin DS, Lin YS, Wu CK, Lin HH, Lee JK. Midterm Prognosis of Patients With Pulmonary Embolism Receiving Catheter-Directed Thrombolysis or Systemic Thrombolysis: A Nationwide Population-Based Study. J Am Heart Assoc. 2021 Apr 6;10(7):e019296. doi: 10.1161/JAHA.120.019296. Epub 2021 Mar 31. PMID: 33787288; PMCID: PMC8174309. |
Case series |
|
Maturana MA, Seitz MP, Pour-Ghaz I, Ibebuogu UN, Khouzam RN. Invasive Strategies for the Treatment of Pulmonary Embolism. Where Are We in 2020? Curr Probl Cardiol. 2021 Mar;46(3):100650. doi: 10.1016/j.cpcardiol.2020.100650. Epub 2020 Jul 22. PMID: 32839040. |
narrative review |
|
Michaud, E. and Pan, M. and Aggarwal, V. Catheter-based therapies in acute and chronic pulmonary embolism. Current Opinion in Cardiology. 2021; 36 (6) :704-710 |
narrative review |
|
Sulimov, D. S. and Freund, A. and Thiele, H. Catheter-directed therapy in pulmonary embolism. Herz. 2021; 46 (5) :399-405 |
narrative review |
|
Yilmaz, Emine Serap and Uzun, Oguz Low-dose thrombolysis for submassive pulmonary embolism. Journal of investigative medicine : the official publication of the American Federation for Clinical Research. 2021; 69 (8) :1439-1446 |
Case series |
|
Al-Khadra Y, Missula V, Al-Bast B, Singanallur P, Al Tamimi R, Albast N, Abdu M, Deshpande R, Salih M, White P, Shishehbor MH, Hafiz AM. Outcomes of Mechanical Thrombectomy Compared With Systemic Thrombolysis in Pulmonary Embolism: A Comprehensive Evaluation From the National Inpatient Sample Database. J Endovasc Ther. 2022 Dec 2:15266028221138020. doi: 10.1177/15266028221138020. Epub ahead of print. PMID: 36461672. |
Does not fit the PICO |
|
Klok, F. A. and Piazza, G. and Sharp, A. S. P. and Ní Ainle, F. and Jaff, M. R. and Chauhan, N. and Patel, B. and Barco, S. and Goldhaber, S. Z. and Kucher, N. and Lang, I. M. and Schmidtmann, I. and Sterling, K. M. and Becker, D. and Martin, N. and Rosenfield, K. and Konstantinides, S. V. Ultrasound-facilitated, catheter-directed thrombolysis vs anticoagulation alone for acute intermediate-high-risk pulmonary embolism: Rationale and design of the HI-PEITHO study. American Heart Journal. 2022; 251 :43-53 |
study design description trial protocol description |
|
Krishnan, A. M. and Gadela, N. V. and Ramanathan, R. and Jha, A. and Perkins, M. E. and Metersky, M. L. A Comparative Analysis of Catheter Directed Thrombolysis with Anticoagulation Alone or Systemic tPA in Acute Pulmonary Embolism with Cor Pulmonale. Journal of Intensive Care Medicine. 2022; 37 (10) :1336-1343 |
Included in Planer (2023) |
|
Kroupa, J. and Buk, M. and Weichet, J. and Malikova, H. and Bartova, L. and Linkova, H. and Ionita, O. and Kozel, M. and Motovska, Z. and Kocka, V. A pilot randomised trial of catheter-directed thrombolysis or standard anticoagulation for patients with intermediate-high risk acute pulmonary embolism. EuroIntervention. 2022; 18 (8) :E639-E646 |
Included in Planer (2023) |
|
Sadeghipour, Parham and Jenab, Yaser and Moosavi, Jamal and Hosseini, Kaveh and Mohebbi, Bahram and Hosseinsabet, Ali and Chatterjee, Saurav and Pouraliakbar, Hamidreza and Shirani, Shapour and Shishehbor, Mehdi H. and Alizadehasl, Azin and Farrashi, Melody and Rezvani, Mohammad Ali and Rafiee, Farnaz and Jalali, Arash and Rashedi, Sina and Shafe, Omid and Giri, Jay and Monreal, Manuel and Jimenez, David and Lang, Irene and Maleki, Majid and Goldhaber, Samuel Z. and Krumholz, Harlan M. and Piazza, Gregory and Bikdeli, Behnood Catheter-Directed Thrombolysis vs Anticoagulation in Patients With Acute Intermediate-High-risk Pulmonary Embolism: The CANARY Randomized Clinical Trial. JAMA cardiology. 2022; 7 (12) :1189-1197 |
Included by Planer (2023) |
|
Sanchez, O. and Charles-Nelson, A. and Ageno, W. and Barco, S. and Binder, H. and Chatellier, G. and Duerschmied, D. and Empen, K. and Ferreira, M. and Girard, P. and Huisman, M. V. and Jiménez, D. and Katsahian, S. and Kozak, M. and Lankeit, M. and Meneveau, N. and Pruszczyk, P. and Petris, A. and Righini, M. and Rosenkranz, S. and Schellong, S. and Stefanovic, B. and Verhamme, P. and De Wit, K. and Vicaut, E. and Zirlik, A. and Konstantinides, S. V. and Meyer, G. Reduced-Dose Intravenous Thrombolysis for Acute Intermediate-High-risk Pulmonary Embolism: Rationale and Design of the Pulmonary Embolism International THrOmbolysis (PEITHO)-3 trial. Thrombosis and Haemostasis. 2022; 122 (5) :857-866 |
description of study design |
|
Elmoghrabi, Adel and Shafi, Irfan and Abdelrahman, Ahmed and Osman, Heba and Manasrah, Nouraldeen and Zghouzi, Mohamed and Halboni, Adnan and Patino, Skarlet and Patel, Neel N. and Hakim, Zaher and Gardi, Delair and Lakkis, Nasser and Alraies, M. Chadi Outcomes of Catheter-Based Pulmonary Artery Embolectomy in Patients With Sub-Massive to Massive Pulmonary Embolism. Cureus. 2023; 15 (2) :e34877 |
retrospective case series |
|
Götzinger F, Lauder L, Sharp ASP, Lang IM, Rosenkranz S, Konstantinides S, Edelman ER, Böhm M, Jaber W, Mahfoud F. Interventional therapies for pulmonary embolism. Nat Rev Cardiol. 2023 Oct;20(10):670-684. doi: 10.1038/s41569-023-00876-0. Epub 2023 May 12. PMID: 37173409; PMCID: PMC10180624. |
narrative review |
Verantwoording
Beoordelingsdatum en geldigheid
Publicatiedatum : 22-10-2025
Beoordeeld op geldigheid : 23-09-2025
Algemene gegevens
Voor meer details over de gebruikte richtlijnmethodologie verwijzen wij u naar de Werkwijze. Relevante informatie voor de ontwikkeling/herziening van deze richtlijnmodule is hieronder weergegeven.
De ontwikkeling/herziening van deze richtlijnmodule werd ondersteund door het Kennisinstituut van de Federatie Medisch Specialisten (www.demedischspecialist.nl/kennisinstituut) en werd gefinancierd uit de Stichting Kwaliteitsgelden Medisch Specialisten (SKMS). Patiëntenparticipatie bij deze richtlijn werd medegefinancierd uit de Kwaliteitsgelden Patiënten Consumenten (SKPC) binnen het programma KIDZ. De financier heeft geen enkele invloed gehad op de inhoud van de richtlijnmodule.
Samenstelling werkgroep
Voor het ontwikkelen van de richtlijnmodule is in 2021 een multidisciplinaire werkgroep ingesteld, bestaande uit vertegenwoordigers van alle relevante specialismen (zie hiervoor de Samenstelling van de werkgroep) die betrokken zijn bij de zorg voor patiënten die antitrombotische therapie dan wel tromboseprofylaxe gebruiken.
Kerngroep
- Prof. dr. M.V. (Menno) Huisman, internist-vasculaire geneeskunde, LUMC, NIV (voorzitter)
- Dr. M.J.H.A. (Marieke) Kruip, internist-hematoloog, Erasmus MC, NIV, NVVH (Nederlandse Vereniging voor Hematologie)
- Prof. Dr. F.A. (Erik) Klok, internist-vasculaire geneeskunde, LUMC, NIV
- Dr. J. (Jenneke) Leentjens, internist-vasculaire geneeskunde, RadboudUMC, NIV (vanaf 2023)
- Dr. N. (Nick) van Es, internist-vasculaire geneeskunde, Amsterdam UMC, NIV (vanaf 2023)
- Dr. M.A. (Marc) Brouwer, cardioloog, RadboudUMC, NVVC
- Dr. H.B. (Harmen) Ettema, orthopedisch chirurg, Isala, NOV
- Dr. B. (Banne) Nemeth, aios orthopedie, LUMC, NOV
- Dr. A.M. (Arno) Wiersema, vaatchirurg, Dijklander Ziekenhuis, NVVH (tot 2023)
- Dr. M.C. (Michiel) Warlé, vaatchirurg, RadboudUMC, NVVH (vanaf 2024)
- Dr. M.E. (Maarten) Tushuizen, maag-darm-leverarts, LUMC, NVMDL
- Dr. J.M. (Jonathan) Coutinho, neuroloog, Amsterdam UMC, NVN
- Drs. M.H. (Monique) Suijker, kinderarts-hematoloog, UMC Utrecht, NVK
- Drs. P (Paul) Smits, huisarts/ Kaderhuisarts HVZ, NHG
Klankbordgroep
- Dr. J.J.C.M. (Sjef) van de Leur, arts klinische chemie, Isala, NVKC
- Dr. M.G. (Mariëlle) van Pampus, gynaecoloog, OLVG, NVOG
- Drs. R.J. (Rutger) Lely, radioloog, Amsterdam UMC, NVVR
- Dr. C. (Bibi) van Montfrans, dermatoloog, Erasmus MC, NVDV
- Dr. R.A. (Richard) Faaij, klinisch geriater, Diakonessenhuis, NVKG
- Dr. B. (Baucke) van Minnen, kaakchirurg, UMCG, NVMKA
- Drs. N. (Noa) Rosenberg, beleidsadviseur, Harteraad (vanaf mei 2024)
- I.G.J. (Ilse) Verstraaten MSc, beleidsadviseur, Harteraad (tot 2024)
- Dr. N. (Nakisa) Khorsand, ziekenhuisapotheker, OLVG, NVZA
- Dr. M.F. (Margreet) van Herwaarden, openbaar apotheker, KNMP
- Dr. E.T.T.L. (Eric) Tjwa, MDL-arts, RadboudUMC, NVMDL
- Dr. L.M. (Linda) de Heer, cardio-thoracaal chirurg, UMC Utrecht, NVT
- Prof. dr. S. (Saskia) Middeldorp, internist-vasculaire geneeskunde, Radboudumc, NIV
- Dr. J.M.M.B. (Hans-Martin) Otten, internist-oncoloog, Meander MC, NIV
- Dr. E.J. (Esther) Nossent, longarts, Amsterdam UMC, NVALT
- Dr. C.H. (Heleen) van Ommen, kinderarts-hematoloog, Erasmus MC, NVK
- Dr. K.M.J. (Katja) Heitink, kinderarts-oncoloog, Prinses Maxima Centrum, NVK
- Prof. dr. N.P. (Nicole) Juffermans, intensivist, Amsterdam UMC, NVIC
- Dr. M.C.A. (Marcella) Muller, intensivist, Amsterdam UMC, NVIC
Met ondersteuning van
- H. (Hanneke) Olthuis, adviseur, Kennisinstituut van de Federatie Medisch Specialisten
- H.J. (Harm-Jan) van der Hart, adviseur, Kennisinstituut van de Federatie Medisch Specialisten
Belangenverklaringen
Een overzicht van de belangen van werkgroepleden en het oordeel over het omgaan met eventuele belangen vindt u in onderstaande tabel. De ondertekende belangenverklaringen zijn op te vragen bij het secretariaat van het Kennisinstituut van de Federatie Medisch Specialisten via secretariaat@kennisinstituut.nl.
|
Werkgroeplid |
Functie |
Nevenfuncties |
Gemelde belangen |
Ondernomen actie |
|
Huisman (voorzitter) |
Internist vasculaire geneeskunde |
|
à op geen van deze projecten projectleider, betreft unrestricted grants en veelal financiering promotietrajecten. |
Geen restricties |
|
Banne Nemeth |
Orthopedisch chirurg in opleiding |
Postdoc klinische epidemiologie en orthopedie, LUMC |
Trombosestichting - “VTE following total hip and knee arthroplasty: prediction is the future“ |
Geen restricties |
|
Harmen Ettema |
Orthopedisch chirurg |
Geen |
Gemeld bij herbevestiging in 2025: ZonMW, Distinct trial, tromboseprofylaxe bij orthopedische ingrepen, geen projectleider |
Geen restricties |
|
Jonathan Coutinho |
neuroloog Amsterdam UMC |
Geen |
Gemeld bij herbevestiging in 2025:
|
Restricties t.a.v. besluitvorming modules over andexanet alfa bij bloedingen geen restricties ten gevolge van rol pacific stroke study en dabigatran etixilate/warfarin bij CVT, aangezien deze patientengroepen geen onderwerp zijn van deze richtlijnherziening. |
|
Klok |
Internist vasculaire geneeskunde LUMC Leiden |
Gemeld bij herbevestiging in 2024:
|
|
Geen restricties |
|
Kruip |
Hematoloog Gemeld bij herbevestiging in 2024: Directeur Kwaliteit & Patientenzorg, Erasmus MC, betaald |
Gemeld bij herbevestiging in 2024:
|
|
Geen restricties |
|
Maarten Tushuizen |
MDL-arts LUMC |
Geen |
Maag-Lever-Darmstichting (MLDS) |
Geen restricties |
|
Marc Brouwer |
Cardioloog Radboudumc |
Geen |
Nee |
Geen restricties |
|
Paul Smits |
huisarts, zelfstandig |
Coördinator onderwijscommissie harvaatHAG |
geen |
Geen restricties |
|
Monique Suijker |
Kinderarts-hematoloog werkzaam bij Van Creveldkliniek, UMCU |
Geen |
Geen lopende studies Bayer en Janssen - Einstein Jr studie - gebruik Rivaroxaban bij kinderen – afgesloten |
Geen restricties |
|
Arno Wiersema (teruggetrokken, tot 2024) |
Vaatchirurg, Dijklander ziekenhuis |
Geen |
ZonMw, Amsterdam UMC, Dijklander zh en Medtronic - www.action-1.nl - betreft onderzoek naar rol van heparine bij een open buikslagader operatie, rol als projectleider |
Restricties ten aanzien van besluitvorming over heparine. |
|
Michiel Warlé (vanaf 2024) |
Vaatchirurg Radboudumc |
Werkgroep Landelijk Kennisplatform Antistolling |
ZEGG/ZonMw- GENPAD studie (Cyp2c19 genotypering bij Clopidogrel en perifeer arterieel vaatlijden – hoofdonderzoeker Gemeld bij herbevestiging in 2025: NWO-OTP, Wireless clot retriever, projectleider |
Geen restricties |
|
Nick van Es (vanaf 2023) |
Internist-vasculaire geneeskunde, Amsterdam UMC, locatie AMC |
Geen |
Gemeld bij herbevestiging in 2024:
|
Geen restricties |
|
Leentjens (vanaf 2023) |
Internist-vasculair geneeskundige, Radboudumc |
Geen |
|
Geen restricties |
|
Actieve klankbordgroepleden |
||||
|
Esther Nossent |
Longarts Amsterdam UMC |
Geen |
Gemeld bij herbevestiging in 2025:
|
Geen restricties |
|
Heleen van Ommen |
Hoofd afd. Kinderhematologie & kinderoncologie Erasmus MC Sophia Kinderziekenhuis |
Geen |
|
Geen restricties |
|
Eric Tjwa |
MDL arts, Radboudumc |
Geen |
Geen Gemeld bij herbevestiging in 2025: Geen betrokkenheid bij onderzoeken die direct/indirect verband houden met de inhoud van de richtlijn |
Geen restricties |
|
Hans-Martin Otten (vanaf 2024) |
Internist Meander MC |
Lid METC UMCU, betaald |
|
Geen restricties |
|
Noa Rosenberg (vanaf 2024) |
Beleidsadviseur |
|
Geen |
Geen restricties |
|
Katja Heitink – Pollé |
Kinderoncoloog Prinses Máxima Centrum |
Landelijke werkgroep trombose bij kinderen |
geen |
Geen restricties |
Inbreng patiëntenperspectief
De werkgroep besteedde aandacht aan het patiëntenperspectief door uitnodigen van Stichting Harteraad voor de schriftelijke knelpuntenanalyse en door een patiëntvertegenwoordiger van Stichting Harteraad toe te voegen aan de klankbordgroep. De verkregen input is meegenomen bij het opstellen van de uitgangsvragen, de keuze voor de uitkomstmaten en bij het opstellen van de overwegingen (zie alinea waarden en voorkeuren van patiënten). De conceptrichtlijn is tevens voor commentaar voorgelegd aan Stichting Harteraad en Stichting Kind en Ziekenhuis en de eventueel aangeleverde commentaren zijn bekeken en verwerkt.
Kwalitatieve raming van mogelijke financiële gevolgen in het kader van de Wkkgz
Bij de richtlijnmodule voerde de werkgroep conform de Wet kwaliteit, klachten en geschillen zorg (Wkkgz) een kwalitatieve raming uitom te beoordelen of de aanbevelingen mogelijk leiden tot substantiële financiële gevolgen. Bij het uitvoeren van deze beoordeling is de richtlijnmodule op verschillende domeinen getoetst (zie het stroomschema bij Werkwijze).
Module |
Uitkomst raming |
Toelichting |
|
Behandeling intermediair-hoog risico longembolie |
geen financiële gevolgen |
Uit de toetsing volgt dat de aanbevelingen niet breed toepasbaar zijn (<5.000 patiënten) en daarom naar verwachting geen substantiële financiële gevolgen zullen hebben voor de collectieve uitgaven. |
Zoekverantwoording
Literature search strategy
|
Richtlijn: Cluster antitrombotisch beleid |
|
|
Uitgangsvraag: Wat is de optimale behandeling van patiënten met acute intermediate-high risk longembolieën? |
|
|
Database(s): Ovid/Medline, Embase |
Datum:5-7-2023 |
|
Periode: 2000- |
Talen: nvt |
|
Literatuurspecialist: Ingeborg van Dusseldorp |
|
|
BMI zoekblokken: voor verschillende opdrachten wordt (deels) gebruik gemaakt van de zoekblokken van BMI-Online https://blocks.bmi-online.nl/ Bij gebruikmaking van een volledig zoekblok zal naar de betreffende link op de website worden verwezen. |
|
|
Toelichting: Voor deze vraag is gezocht met de volgende concepten: Pulmonary embolism AND intermediate high-risk patients AND hemodynamic monitoring, reperfusion, thrombolysis, embolectomy, fibrinolysis or thrombectomy Alle sleutelartikelen worden gevonden. |
|
|
Te gebruiken voor richtlijnen tekst: In de databases Embase.com en Ovid/Medline is op 5-7-2023 met relevante zoektermen gezocht naar systematische reviews en RCT’s over de optimale behandeling van patiënten met intermediate-high risk longembolieën. De literatuurzoekactie leverde 520 unieke treffers op. |
|
Zoekopbrengst
|
|
EMBASE |
OVID/MEDLINE |
Ontdubbeld |
|
SRs |
253 |
123 |
277 |
|
RCTs |
205 |
144 |
243 |
|
Observationele studies |
|
|
|
|
Totaal |
458 |
267 |
520 |
Zoekstrategie
Embase
|
No. |
Query |
Results |
|
#21 |
#16 AND #20 sleutelartikelen gevonden |
3 |
|
#20 |
#17 OR #18 OR #19 sleutelartikelen |
3 |
|
#19 |
konstantinides AND 2002 AND pulmonary AND embolism AND submassive |
1 |
|
#18 |
'randomized, controlled trial of ultrasound-assisted catheter-directed thrombolysis for acute intermediate-risk pulmonary embolism' AND kucher AND [2014]/py |
1 |
|
#17 |
'fibrinolysis for patients with intermediate-risk pulmonary embolism' AND [2014]/py AND meyer |
1 |
|
#16 |
#14 OR #15 |
458 |
|
#15 |
#8 AND #10 NOT #14 RCT |
205 |
|
#14 |
#8 AND #9 SR |
253 |
|
#10 |
'randomized controlled trial'/exp OR random*:ti,ab OR (((pragmatic OR practical) NEAR/1 'clinical trial*'):ti,ab) OR ((('non inferiority' OR noninferiority OR superiority OR equivalence) NEAR/3 trial*):ti,ab) OR rct:ti,ab,kw |
1839814 |
|
#9 |
'meta analysis'/exp OR 'meta analysis (topic)'/exp OR metaanaly*:ti,ab OR 'meta analy*':ti,ab OR metanaly*:ti,ab OR 'systematic review'/de OR 'cochrane database of systematic reviews'/jt OR prisma:ti,ab OR prospero:ti,ab OR (((systemati* OR scoping OR umbrella OR 'structured literature') NEAR/3 (review* OR overview*)):ti,ab) OR ((systemic* NEAR/1 review*):ti,ab) OR (((systemati* OR literature OR database* OR 'data base*') NEAR/10 search*):ti,ab) OR (((structured OR comprehensive* OR systemic*) NEAR/3 search*):ti,ab) OR (((literature NEAR/3 review*):ti,ab) AND (search*:ti,ab OR database*:ti,ab OR 'data base*':ti,ab)) OR (('data extraction':ti,ab OR 'data source*':ti,ab) AND 'study selection':ti,ab) OR ('search strategy':ti,ab AND 'selection criteria':ti,ab) OR ('data source*':ti,ab AND 'data synthesis':ti,ab) OR medline:ab OR pubmed:ab OR embase:ab OR cochrane:ab OR (((critical OR rapid) NEAR/2 (review* OR overview* OR synthes*)):ti) OR ((((critical* OR rapid*) NEAR/3 (review* OR overview* OR synthes*)):ab) AND (search*:ab OR database*:ab OR 'data base*':ab)) OR metasynthes*:ti,ab OR 'meta synthes*':ti,ab |
733409 |
|
#8 |
#7 AND [2000-2023]/py NOT ('conference abstract'/it OR 'editorial'/it OR 'letter'/it OR 'note'/it) NOT (('animal'/exp OR 'animal experiment'/exp OR 'animal model'/exp OR 'nonhuman'/exp) NOT 'human'/exp) |
4429 |
|
#7 |
#5 AND #6 |
8326 |
|
#6 |
'high risk patient'/exp OR 'high risk population'/exp OR 'medium risk population'/exp OR 'medium risk patient'/exp OR 'intermediate risk patient'/exp OR 'moderate risk patient'/exp OR 'moderate risk population'/exp OR intermediate:ti,ab,kw OR 'high* risk*':ti,ab,kw OR submassive:ti,ab,kw OR moderate:ti,ab,kw OR severe:ti,ab,kw OR acute:ti,ab,kw OR 'bad risk':ti,ab,kw |
5085027 |
|
#5 |
#1 AND #4 |
15846 |
|
#4 |
#2 OR #3 |
460353 |
|
#3 |
'hemodynamic monitoring'/exp OR 'lidco':ti,ab,kw OR 'haemodynamic monitoring':ti,ab,kw OR 'hemodynamic monitoring':ti,ab,kw |
80925 |
|
#2 |
'systemic thrombolysis'/exp OR 'reperfusion'/exp OR 'blood clot lysis'/exp OR 'percutaneous catheter'/exp OR 'embolectomy'/exp OR 'fibrinolysis'/exp OR 'thrombectomy'/exp OR 'thrombectom*':ti,ab,kw OR 'fibrin degradation':ti,ab,kw OR 'fibrin splitting':ti,ab,kw OR fibrinolytic*:ti,ab,kw OR fibrinolys*:ti,ab,kw OR 'pulmonary embolectomy'/exp OR (((lung OR pulmonar*) NEAR/3 embolectom*):ti,ab,kw) OR 'percutaneous catheter':ti,ab,kw OR reperfusion:ti,ab,kw OR 'clot lysis':ti,ab,kw OR 'systemic thromboly*':ti,ab,kw |
383840 |
|
#1 |
'lung embolism'/exp/mj OR (((lung OR pulmonar*) NEAR/3 (microembolism* OR embolism* OR thromboembolism* OR 'micro embolism*' OR 'thrombo embolism*')):ti,ab,kw) |
86045 |
Ovid/Medline
|
# |
Searches |
Results |
|
12 |
(8 and 10) not 11 RCT |
144 |
|
11 |
8 and 9 SR |
123 |
|
10 |
exp randomized controlled trial/ or randomized controlled trials as topic/ or random*.ti,ab. or rct?.ti,ab. or ((pragmatic or practical) adj "clinical trial*").ti,ab,kf. or ((non-inferiority or noninferiority or superiority or equivalence) adj3 trial*).ti,ab,kf. |
1625962 |
|
9 |
meta-analysis/ or meta-analysis as topic/ or (metaanaly* or meta-analy* or metanaly*).ti,ab,kf. or systematic review/ or cochrane.jw. or (prisma or prospero).ti,ab,kf. or ((systemati* or scoping or umbrella or "structured literature") adj3 (review* or overview*)).ti,ab,kf. or (systemic* adj1 review*).ti,ab,kf. or ((systemati* or literature or database* or data-base*) adj10 search*).ti,ab,kf. or ((structured or comprehensive* or systemic*) adj3 search*).ti,ab,kf. or ((literature adj3 review*) and (search* or database* or data-base*)).ti,ab,kf. or (("data extraction" or "data source*") and "study selection").ti,ab,kf. or ("search strategy" and "selection criteria").ti,ab,kf. or ("data source*" and "data synthesis").ti,ab,kf. or (medline or pubmed or embase or cochrane).ab. or ((critical or rapid) adj2 (review* or overview* or synthes*)).ti. or (((critical* or rapid*) adj3 (review* or overview* or synthes*)) and (search* or database* or data-base*)).ab. or (metasynthes* or meta-synthes*).ti,ab,kf. |
678494 |
|
8 |
7 not ((exp animals/ or exp models, animal/) not humans/) not (letter/ or comment/ or editorial/) |
2011 |
|
7 |
limit 6 to yr="2000 -Current" |
2156 |
|
6 |
4 and 5 |
2597 |
|
5 |
(intermediate or high* risk* or submassive or moderate or severe or acute or bad risk).ti,ab,kf. |
3506921 |
|
4 |
1 and (2 or 3) |
5103 |
|
3 |
exp Hemodynamic Monitoring/ or lidco.ti,ab,kf. or haemodynamic monitoring.ti,ab,kf. or hemodynamic monitoring.ti,ab,kf. |
4542 |
|
2 |
exp Thrombolytic Therapy/ or exp Reperfusion/ or exp fibrinolysis/ or exp Thrombectomy/ or exp Embolectomy/ or ((lung or pulmonar*) adj3 embolectom*).ti,ab,kf. or percutaneous catheter.ti,ab,kf. or reperfusion.ti,ab,kf. or clot lysis.ti,ab,kf. or systemic thromboly*.ti,ab,kf. |
158402 |
|
1 |
exp Pulmonary Embolism/ or ((lung or pulmonar*) adj3 (microembolism* or embolism* or thromboembolism* or micro embolism* or thrombo embolism*)).ti,ab,kf. |
64335 |
Zoekstrategie Monitoring
|
Onderwerp |
Monitoring van patiënten met een acute intermediair-hoog risico longembolie |
|
Zoekstrategie |
("Pulmonary Embolism" [MeSH Terms] OR "pulmonary embolism" [title/abstract] OR "pulmonary embolism" [tw] OR "Venous Thromboembolism"[Mesh] OR "deep vein thrombosis" [tiab] OR "deep vein thrombosis" [tw] OR "Venous Thromboembolism"[Mesh] OR "venous thromboembolism" [tiab] OR "venous thromboembolism" [tw] OR "blood clot" [tiab:~3]) AND ("vital sign monitoring" [tiab] OR "vital sign monitoring" [tw] OR "non-invasive monitoring" [tiab] OR "non-invasive monitoring" [tw] OR "Critical Care"[Mesh] OR step-down [tw] OR "intermediate care" [tw] OR "high dependency" [tw] OR "coronary care" [tw] OR "discharge" [tw] AND clinical study [Filter]) |
|
Opbrengst |
Geen |