Behandeling buikvenetrombose
Uitgangsvraag
Wat is de optimale antistollingsbehandeling voor patiënten met acute buikvenetrombose?
Aanbeveling
Behandel patiënten met een buikvenetrombose (inclusief patiënten met Budd-Chiari syndroom (BCS)) in de acute fase met therapeutisch gedoseerde laagmoleculairgewicht heparine (LMWH).
Overweeg patiënten met een buikvenetrombose na de acute fase te behandelen met een vitamine K antagonist (VKA). Behandeling met een directe orale anticoagulantia (DOAC, Factor Xa-remmer) is ook een optie.
- Overweeg behandeling met een DOAC alleen als er geen sprake is van BCS, Child-Pugh C levercirrose, veneuze darmischemie of ernstige nierfunctiestoornissen.
- Indien gekozen wordt voor behandeling met een DOAC: kies voor een Factor Xa-remmer bij patiënten met buikvenetrombose zonder levercirrose.
Overwegingen
Voor- en nadelen van de interventie en de kwaliteit van het bewijs
Op basis van de geïncludeerde studies zijn we onzeker over het effect van direct orale anticoagulantia (DOACs) op de cruciale uitkomstmaten majeure bloeding, progressie van buikvenetrombose, (partiële) resolutie van buikvenetrombose en mortaliteit, vergeleken met conventionele antistollingsmedicatie (vitamine K antagonisten (VKA) en laagmoleculairgewicht heparine (LMWH)) in patiënten met een acute buikvenetrombose. Dit geldt voor de totale groep patiënten, alsook voor de patiënten met levercirrose en patiënten zonder levercirrose. De bewijskracht van de gevonden resultaten is erg beperkt, met name vanwege het risico op bias en imprecisie. Ook zijn we onzeker over het effect van DOACs op de belangrijke uitkomstmaten recidief veneuze trombo-embolie (VTE, inclusief buikvenetrombose) en noodzaak voor chirurgische of radiologische ingreep. Geen van de geïncludeerde studies rapporteerde gegevens over de uitkomstmaten leverfalen en levertransplantatie. De overall bewijskracht voor deze module is dan ook zeer laag en er is duidelijk sprake van een kennislacune.
De literatuur is niet eenduidig over de toepassing van DOACs bij de diverse types buikvenetrombose. In de geïncludeerde studies zijn voornamelijk patiënten met portatrombose en in mindere mate met v. mesenterica, v. lienalis trombose en trombose van meerdere buikvenen tegelijkertijd. Patiënten met een Budd-Chiari syndroom (BCS) ontbraken in de geïncludeerde studies. Het is daarom ook niet zeker of de gevonden effecten van DOACs op de cruciale uitkomstmaten van toepassing zijn op de diverse types buikvenetrombose, en in het bijzonder bij patiënten met BCS. Ook varieerde het gebruikte type DOAC tussen de studies, waarbij de meerderheid van de patiënten een Factor Xa-remmer (FXa-remmers) kreeg en een kleine minderheid dabigatran. Helaas zijn er ook geen RCT’s waarin behandeling met DOACs vergeleken worden met VKA/LMWH bij patiënten met een acute SVT. Resultaten uit een niet-vergelijkende cohortstudie waarin patiënten met non-cirrotische acute buikvenetrombose werden behandeld met een DOAC (rivaroxaban), suggereren dat behandeling met rivaroxaban een alternatief zou kunnen zijn voor de standaard antistollingsbehandeling (Ageno, 2022).
De standaardbehandeling van patiënten met een acute buikvenetrombose die een indicatie hebben voor behandeling met antistolling, is therapeutisch LMWH en voor de langere termijn meestal gevolgd door VKA met een streef International Normalized Ratio (INR) tussen 2,0 en 3,0. Hierbij wordt veelal gekozen voor LMWH en niet voor ongefractioneerde heparine (UFH), tenzij er sprake is van een contra-indicatie voor LMWH. Het doel van deze module was te onderzoeken of behandeling met DOACs een plaats heeft bij deze patiënten. Ondanks het feit dat we onzeker zijn over het effect van DOACs op de cruciale uitkomstmaten, suggereren de gevonden resultaten dat DOACs, waarbij met name de FXa-remmers zijn onderzocht en in mindere mate dabigatran, in ieder geval niet meer bloedingen of meer recidief trombose gaven, maar minstens vergelijkbare bloedingsrisico’s en effectiviteit hadden als LMWH of VKA. Daarnaast is het in de klinische praktijk vaak lastig of niet goed mogelijk om bij het stellen van de diagnose buikvenetrombose te weten hoe lang deze al aanwezig is, en daarmee of het om een acute of een chronische buikvenetrombose gaat. Ook is onbekend welke invloed dergelijke kennis heeft op de kansen op een bloeding of trombose en daarmee ook op de veiligheid en de effectiviteit van de behandeling.
Met de acute fase wordt het moment van diagnose bedoeld, waarin sommige patiënten klinisch worden behandeld. Het post-acute moment kenmerkt zich door belangrijk herstel van klachten en de ambulante setting waarbij orale intake geen probleem (meer) is.
Een buikvenetrombose kent meerdere mogelijke oorzaken of omstandigheden waarin deze kan ontstaan. Een bekende setting is bij een infectieuze of inflammatoire ziekte in de buikholte, zoals pancreatitis of inflammatoire darmziekten (IBD). In de studie van Naymagon (2021-B) zijn patiënten met IBD geïncludeerd. Het aantal events voor de uitkomstmaten majeure bloeding en mortaliteit was te laag om uitspraken te kunnen doen over het effect van DOACs vergeleken met conventionele antistolling. Een andere risicogroep zijn patiënten met een maligniteit, in het bijzonder patiënten met een hepatocellulair carcinoom. Zij waren wisselend vertegenwoordigd in de studies en de resultaten en aantallen zijn te beperkt om hier conclusies uit te trekken. Tenslotte is er ook een verschil in patiënten met en zonder levercirrose. Het is mogelijk dat DOACs bij patiënten met gevorderde levercirrose een ander risicoprofiel hebben. In het BAVENO-consensus document wordt aangegeven dat het daarom niet mogelijk is een aanbeveling te doen over de inzet van DOACs bij deze groep patiënten (de Franchis, 2022). In de recente ISTH-richtlijn over antistolling bij een vena porta trombose en levercirrose is de aanbeveling voor patiënten met Child-Pugh A of B levercirrose om te behandelen met een DOAC of LMWH, eventueel gevolgd door VKA. Bij patiënten met Child-Pugh C levercirrose heeft LMWH, eventueel gevolgd door VKA, de voorkeur (Carlin, 2024). Het behandelen van patiënten met een verminderde leverfunctie en een daarbij verlengde INR, kan de behandeling met VKA bemoeilijken. Dit geldt ook voor de interpretatie van de zogenaamde MELD-score, die gebruikt wordt voor de urgentiebepaling bij patiënten op de levertransplantatie-wachtlijst. LMWH blijft dan de antistolling van voorkeur, al kent dit middel voor chronisch gebruik ook beperkingen.
Er is onvoldoende bewijs om het gebruik van DOACs bij patiënten met BSC te adviseren. Er is wel ervaring vanuit de klinische praktijk om dit in individuele gevallen voor te schrijven door voorschrijvers die ervaren zijn met de behandeling van patiënten met BSC.
In het BAVENO-consensus document worden verschillende uitspraken gedaan over het gebruik van DOACs bij patiënten met een buikvenetrombose (de Franchis, 2022). Zo geven zij aan dat er bij patiënten met een verhoogd risico op bloedingen, zoals in het geval van trombocytopenie bij splenomegalie, een individuele afweging gemaakt moet worden. Een ander bloedingsrisico is het hebben van slokdarmvarices, zoals kan ontstaan door portale hypertensie ten gevolge van buikvenetrombose. Derhalve wordt in het BAVENO-consensus document aangegeven dat het raadzaam is om patiënten te screenen op slokdarmvarices en deze te behandelen, indien mogelijk voor de start van antistolling (de Franchis, 2022). De werkgroep is van mening dat het gebruik van antistolling bij patiënten met slokdarmvarices op individuele basis afgewogen moet worden. Als er een indicatie is voor antistollingsmedicatie, heeft de aanwezigheid van slokdarmvarices geen consequenties voor de keuze van het antistollingsmiddel.
De voordelen van parenterale - boven orale - behandeling van een buikvenetrombose zijn theoretisch, tenzij er sprake is van darmischemie. In dat geval is orale therapie niet mogelijk door het kritisch ziek zijn van de patiënt. Ook is de absorptie van orale medicatie in die setting redelijkerwijs verstoord. Het ontbreken van een streefwaarde maakt monitoring van het therapeutische effect van DOACs in deze setting onmogelijk. Het is onbekend wat het effect is van buikvenetrombose die niet gepaard gaat met fulminante darmischemie, maar wel met macro- of microscopisch oedeem van de darmwand, op de absorptie.
Kortom, realiserend dat gerandomiseerde studies ontbreken, de kwaliteit van het bewijs erg laag is en de geïncludeerde studies onvoldoende antwoord geven op de gestelde vragen, is het voorstelbaar dat er, na stabilisatie van het ziektebeeld, toch gekozen wordt voor het gebruik van een DOAC (FXa-remmer) als behandeling van een acute buikvenetrombose, tenzij er sprake is van BCS en cruciale comorbiditeit zoals Child-Pugh C levercirrose, darmischemie of ernstige nierfunctiestoornissen. Bij patiënten met matige levercirrose of Child-Pugh B, is voorzichtigheid geboden (de Franchis, 2022). Bij patiënten met Child-Pugh A kunnen DOACs worden voorgeschreven. De duur van de behandeling moet op individuele basis bepaald worden. Daarbij kunnen de volgende factoren meegewogen worden: locatie van de buikvenetrombose, aanwezigheid van slokdarmvarices of andere risicofactoren voor een bloeding, aanwezigheid van persisterende risicofactoren, andere relevante comorbiditeiten en de voorkeuren van de patiënt.
Waarden en voorkeuren van patiënten (en evt. hun verzorgers)
Naast een mogelijk verschillend risico op bloedingen of recidief trombose, waar de huidige studies onvoldoende uitsluitsel over geven, is voor patiënten het verschil in gebruiks(on)gemak tussen de verschillende anticoagulantia van belang. Waar DOACs een een- of tweemaal daagse inname vereisen, is bij een behandeling met VKA een eenmaal daagse dosering voldoende. Daarnaast is het meten van de INR nodig bij behandeling met VKA, meestal eens per twee tot zes weken, waar bij chronisch gebruik zelfmeten wordt gestimuleerd. In 2020 publiceerde het Zorginstituut Nederland het rapport ‘Evaluatie van de ervaringen en kosten van antistollingszorg (van Dijk, 2020). In dit rapport komt naar voren dat DOAC-gebruikers meer vrijheid ervaren door het overbodig worden van bezoeken aan de trombosedienst. Tegelijkertijd ervaren VKA-gebruikers die zelf meten en doseren ook een grote regie over hun leven.
Het grootste nadeel van DOACs (FXa-remmer) is het ontbreken of beperkte ervaring met een adequaat en direct antidotum wat het gebruik bij een hoog bloedingsrisico of in de setting van een levertransplantatie-wachtlijst onpraktischer maakt. Daarnaast is de klaring van de DOACs grotendeels afhankelijk van de nier- en leverfunctie, wat bij deze patiëntengroep relevant kan zijn.
Het grootste nadeel van de VKA-behandeling is de potentiële ontregeling van de INR door diverse factoren, zoals infecties, koorts, interacterende medicatie maar ook stress of veranderde voeding. Indien door een onderliggende leveraandoening de INR al spontaan verlengd is, kan deze de (stabiliteit van de) behandeling met VKA bemoeilijken. Ook kan het de zogenaamde MELD-score beïnvloeden, die gebruikt wordt voor patiënten op de levertransplantatie-wachtlijst.
Naast een behandeling met tabletten is behandeling met subcutane injecties (LMWH) mogelijk, die een- of tweemaal daags geprikt moeten worden. De meeste patiënten vinden injecties meer belastend dan tabletten en niet iedere patiënt kan zichzelf die injecties toedienen. Hierdoor zijn mantelzorgers of thuiszorg voor deze behandeling noodzakelijk.
Onderzoek naar voorkeuren van patiënten is schaars, maar in het algemeen wordt gevonden dat orale behandeling de voorkeur heeft boven injecties, mits deze even effectief en veilig is (Hutchinson, 2019). Het is van belang om bovenstaande afwegingen met de patiënt te bespreken, om zo samen tot een passende behandeling te komen.
Kosten (middelenbeslag)
In het rapport ‘Evaluatie van de ervaringen en kosten van antistollingszorg’ wordt ook aandacht gegeven aan de kosten. Hierbij was de conclusie dat de kosten voor antistollingszorg gestegen waren, met name door de hogere kosten van DOAC ten opzichte van VKA. LMWH is daarentegen bij de hoogste therapeutische dosering iets duurder dan een DOAC (Farmacotherapeutisch kompas). Tenslotte zijn de meeste DOACs inmiddels (2024) uit patent en zijn de kosten van deze medicatie daardoor ook lager geworden.
Aanvaardbaarheid, haalbaarheid en implementatie
Alle drie de vormen van antistolling, dus LMWH, VKA en DOAC zijn gebruikelijk en gekend in de zorg voor patiënten met trombose. De verwachting is daarom dat de aanbevelingen haalbaar zijn in de klinische praktijk.
Rationale van de aanbeveling: weging van argumenten voor en tegen de interventies
Er is geen literatuur van goede kwaliteit over de voorkeursbehandeling met antistolling bij patiënten met een acute buikvenetrombose. De behandeling met LWMH in de acute fase en vervolgens LMWH of VKA in de post-acute fase is de huidige standaard en kan nog steeds worden aanbevolen. In overleg met de patiënt kan een DOAC als alternatief worden voorgeschreven voor de post-acute fase, mits in afwezigheid van BCS en cruciale comorbiditeit zoals Child-Pugh C levercirrose, darmischemie of ernstige nierfunctiestoornissen.
Met de acute fase wordt het moment van diagnose bedoeld, waarin sommige patiënten klinisch worden behandeld. Het post-acute moment kenmerkt zich door belangrijk herstel van klachten en de ambulante setting waarbij orale intake geen probleem (meer) is. Daarnaast is het belangrijk bij de start van antistollingsbehandeling, of binnen enkele weken na start, de aanwezigheid van slokdarmvarices te beoordelen en indien aanwezig te behandelen. Dit met het risico op potentieel levensbedreigende bloedingen.
Gezien het ontbreken van voldoende bewijs over de veiligheid en effectiviteit van DOACs in vergelijking met de huidige behandeling met LMWH of VKA zijn de aanbevelingen zwak geformuleerd. Deze onzekerheid maakt dat de behandeling met LMWH of VKA ook in de post-acute fase de keuze van voorkeur blijft, waarbij het niet zeker is of een DOAC een verstandig(er) alternatief is.
Onderbouwing
Achtergrond
Patients with acute symptoms of splanchnic vein thrombosis (SVT, thrombosis of mesenteric vein, portal vein, splenic vein, and/or hepatic veins) are treated with anticoagulants. Treatment with anticoagulants seems beneficial for prevention of recurrent thrombosis, bleeding and development of portal hypertension (Candeloro, 2022; Valeriani, 2021 and Valeriani, 2021). Traditionally vitamin K antagonists (VKA) or low molecular weight heparins (LMWH) have been described for this type of venous thromboembolism (VTE). The large direct oral anticoagulants (DOAC) RCTs were performed in patients with deep vein thrombosis or pulmonary embolism and patients with SVT were not included. The question is whether DOACs can be recommended for the treatment of patients with SVT having an indication for anticoagulants. Since many patients with SVT may have comorbidities/ bleeding risk factors, including portal hypertension and oesophageal varices, that are of clinical relevance for the use of anticoagulation and particularly DOACs, the lack of (experience in) a widely available antidote, can have clinical implications and part of decision making. Here we reviewed the present literature on the use of DOACs in patients with SVT.
Conclusies / Summary of Findings
General remarks
In selection of the studies, it seemed difficult to select the studies that only included patients with an acute SVT. In part of the studies, it was not specified whether patients with acute and/or chronic SVT were included (Nagaoki, 2018 and Zhang, 2023). The working group decided to include those studies in the literature analysis, as in clinical practice it is also often not known if a SVT is recent or of older age. Besides, in most of the studies patients with PVT were included and it is therefore not sure whether results are also applicable to patients with other SVT. It is important to take this into account in interpreting the results.
Major bleeding
Total group and patients with cirrhosis (all comparisons)
|
Very low GRADE |
The evidence is very uncertain about the effect of DOACs on the outcome measure major bleeding when compared with LMWH/VKA in adult patients with SVT/in adult patients with SVT and cirrhosis.
Sources: Valeriani, 2021; Ilcewicz (2021); Naymagon (2021); Kawata (2021) and Zhang (2023) |
Patients without cirrhosis (all comparisons)
|
No GRADE |
None of the included studies reported on the outcome measure major bleeding (according to ISTH criteria) for patients without cirrhosis. Therefore no conclusion can be drawn on the effect of DOAC on the outcome measure major bleeding in patients with SVT without cirrhosis, compared to LMWH/VKA.
|
Progression of SVT
Total group and patients with cirrhosis (all comparisons)
|
Very low GRADE |
The evidence is very uncertain about the effect of DOACs on the outcome measure progression of SVT when compared with LMWH/VKA in adult patients with SVT/in adult patients with SVT and cirrhosis.
Source: Valeriani, 2021; Ilcewicz, 2021; Kawata, 2021 and Naymagon, 2021 |
Patients without cirrhosis (all comparisons)
|
No GRADE |
None of the included studies reported on the outcome measure progression of SVT for patients without cirrhosis. Therefore no conclusion can be drawn on the effect of DOAC on the outcome measure progression of SVT in patients with SVT without cirrhosis, compared to LMWH/VKA.
|
(Partial) Resolution of SVT
Total group and patients with cirrhosis and without cirrhosis (all comparisons)
|
Very low GRADE |
The evidence is very uncertain about the effect of DOACs on the outcome measure (partial) resolution of SVT when compared with LMWH/VKA in adult patients with SVT.
Sources: Valeriani, 2021; Kawata, 2021; Naymagon, 2020; Naymagon, 2021, Naymagon, 2021_B and Zhang, 2023 |
Mortality
Total group and patients with cirrhosis (all comparisons)
|
Very low GRADE |
The evidence is very uncertain about the effect of DOACs on the outcome measure mortality when compared with LMWH/VKA in adult patients with SVT/in adult patients with SVT and cirrhosis.
Source: Naymagon, 2021 |
Patients without cirrhosis (all comparisons)
|
No GRADE |
Data is too limited. Therefore no conclusion can be drawn on the effect of DOAC on the outcome measure mortality in patients with SVT without cirrhosis, compared to LMWH/VKA.
Source: Naymagon (2020) |
Clinically relevant non major bleeding
Total group (DOAC vs VKA or LMWH)
|
Very low GRADE |
The evidence is very uncertain about the effect of DOACs on the outcome measure CRNMB (according to ISTH criteria) when compared with LMWH/VKA in adult patients with SVT.
Source: Kawata,2021 |
Patients with cirrhosis and patients without cirrhosis (all comparisons)
|
No GRADE |
None of the included studies reported on the outcome measure CRNMB (according to ISTH criteria) for patients with cirrhosis and patients without cirrhosis. Therefore no conclusion can be drawn on the effect of DOAC on the outcome measure CRNMB in patients with SVT with cirrhosis and patients with SVT without cirrhosis, compared to LMWH/VKA.
|
Recurrent VTE
Total group (DOAC vs VKA)
|
Very low GRADE |
The evidence is very uncertain about the effect of DOACs on the outcome measure recurrent VTE when compared with VKA in adult patients with SVT.
Source: Ilcewicz, 2021 |
Total group (DOAC vs LMWH)
|
No GRADE |
None of the included studies reported on the outcome measure recurrent VTE for patients without cirrhosis. Therefore no conclusion can be drawn on the effect of DOAC on the outcome measure recurrent VTE in patients with SVT nor on patients with SVT with cirrhosis, compared to LMWH.
|
Patients with cirrhosis and patients without cirrhosis (all comparisons)
|
No GRADE |
Data is too limited. Therefore no conclusion can be drawn on the effect of DOAC on the outcome measure mortality in patients with SVT without cirrhosis, compared to LMWH/VKA.
Sources: Naymagon, 2020 and Ilcewicz, 2021 |
Liver failure
|
No GRADE |
None of the included studies reported on the outcome measure liver failure. Therefore, no conclusion can be drawn on the effect of DOACs on the outcome measure liver failure in adults with acute SVT compared to LMWH/VKA.
|
Liver transplantation
|
No GRADE |
None of the included studies reported on the outcome measure liver transplantation. Therefore, no conclusion can be drawn on the effect of DOACs on the outcome measure liver transplantation in adults with SVT compared to LMWH/VKA.
|
Need for surgical or radiological intervention
|
No GRADE |
Data was too limited. Therefore, no conclusion can be drawn on the effect of DOACs on the outcome measure need for surgical or radiological intervention in adults with acute SVT compared to LMWH/VKA.
Sources: Naymagon, 2020 and Naymagon, 2021_B |
Samenvatting literatuur
Description of studies
Valeriani (2021) performed a systematic review to evaluate the effect of anticoagulation treatments in adults with splanchnic vein thrombosis. Several databases were searched up to December 2019, including Medline and Embase. They included 97 studies (N=7969), of which four studies reported data on the effect of treatment with direct oral anticoagulants (DOAC) (Hanafy, 2019; Nagaoki, 2018; Wille, 2019 and Sharma, 2020). However, only the study of Nagaoki (2018) was included in our literature analysis, since the publication of Hanafy (2019) was retracted, Sharma (2020) included also patients in the DOAC-group, when they switched from treatment with VKA to treatment with DOAC and the number of patients using DOAC was too limited in Wille (2018). Quality of the observational studies was assessed using the ROBINS-I tool.
Naymagon (2020) performed a retrospective cohort study to evaluate the efficacy and safety of DOACs in patients with non-cirrhotic acute portal vein thrombosis (PVT), compared to treatment with VKA, LMWH or no anticoagulation. They included 330 patients in a large urban tertiary center in the USA. In total, 108 patients received warfarin, 70 patients received enoxaparin and 93 patients received DOACs. Results on the no anticoagulation group are not considered in our literature analysis. Results on the outcome bleeding events and mortality were reported for the different anticoagulation groups, which was the case for other outcome measures.
Ilcewicz (2021) performed a retrospective cohort study to evaluate the effectiveness and safety of DOACs in patients with new PVT, compared to treatment with warfarin. They included 33 patients admitted to a large academic medical center in the USA. In total, 20 patients received DOACs and 13 patients received warfarin. Patients with cirrhosis were also included (N=10). Important outcome measures were bleeding events and recurrence of thrombo-embolic events.
Kawata (2021) performed a retrospective cohort study to evaluate the management and outcomes of patients with SVT. They included 155 patients with a newly diagnosed episode of SVT at the Thrombosis Clinic in a tertiary care hospital in Canada, of which 47 received DOAC and 98 received LWMH or VKA. SVT should have been objectively documented by imaging. Patients with cirrhosis were also included. Progression of SVT, major bleeding and clinically relevant non major bleeding (CRNMB) were important outcome measures.
Naymagon (2021) performed a retrospective cohort study to share their experiences with the anticoagulation treatment of patients with PVT and liver cirrhosis. They included 214 patients with an acute PVT and cirrhosis which were seen in a tertiary care hospital in the USA and primarily focused on the comparison between patients treated with anticoagulation (DOAC (N=18), warfarin (N=26) and enoxaparin (N=42)), and patients not treated with anticoagulation. Patients not receiving any anticoagulant were not included in our analysis. They also reported outcomes by the different anticoagulants, namely major bleeding, mortality and extension of the PVT.
Naymagon (2021-B) performed a retrospective cohort study to compare anticoagulants in the treatment of patients with acute PVT and inflammatory bowel disease (IBD). They included 63 patients which were seen in a tertiary care hospital in the USA. Patients received DOAC (N=23), warfarin (N=22) or enoxaparin (N=13). Five patients did not receive any anticoagulation, but were not included in our analysis. They reported on amongst others mortality, major bleeding and need for an additional intervention.
Zhang (2023) performed a retrospective cohort study to evaluate the safety and efficacy of anticoagulants in the treatment of PVT in patients with cirrhosis. They included 77 patients who were admitted to the liver disease center of a tertiary hospital in China. Their study focused primarily on the comparison between anticoagulation (DOAC, N=18; warfarin, N=6, heparin, N=1 and nadroparin, N=2)) and no-anticoagulation (N=50). They only compared the safety of DOAC versus warfarin. Therefore only data on the outcome measure major bleeding is included in our analysis.
Table 1 lists more details on the included studies.
|
Author, year (design) |
Participants |
Characteristics of PVT |
Liver cirrhosis and malignancy (%) |
Intervention |
Comparison |
Follow-up |
|
Nagaoki (2018), retrospective |
N=50 (I: 20, C: 30)
Age: I: 69 (53-74), C: 67 (24-83)
Sex (M): I: 35, C: 57 |
PVT, not reported whether it was acute or chronic |
Cirrhosis: 100
Malignancy: HCC I: 30; C: 63
|
Edoxaban*
mean duration: 6 months |
VKA*
mean duration: 6 months |
6 months |
|
Naymagon (2020), retrospective |
N=330, of which N=57 not using AC (I 93, C1 108, C2 70)
Age: I: 47.1 (15.2); C1: 50.4 (14.8); C2: 51.4 (16.9)
Sex (M): I: 50.5; C1: 52.8; C2: 38.6 |
PVT, acute |
Cirrhosis: 0
Malignancy: Non-HCC malignancy I: 5.4; C1: 1.9; C2: 14.3 |
Apixaban, Dabigatran, Rivaroxaban
At least for 3 months |
VKA (C1), LMWH (C2), Fondaparinux**, no AC**
At least for 3 months |
I: 28.1 ± 11.3 months C1: 55.8 ± 27.4 months C2: 33.0 ± 18.9 months |
|
Ilcewicz (2021), retrospective |
N=33 (I:13, C: 20)
Age: I: 60 ± 18; C: 51 ± 12
Sex (M) I: 69; C: 75 |
New PVT |
Cirrhosis: Previous diagnosis of cirrhosis I:38; C: 25
Malignancy: NR |
Apixaban, rivaroxaban
mean duration: 3 months |
VKA
mean duration: 3 months |
90 days |
|
Kawata (2021), retrospective |
N=136 (I: 43, C: 93)
Age: I: 59±15; C: 55±15
Sex (M): I: 64; C: 59 |
New episode of SVT |
Cirrhosis: 19.4
Malignancy: Abdominal malignancy 31 |
Apixaban, edoxaban, rivaroxaban
mean duration: 483 (359 –606) days |
VKA/LMWH/no AC**
mean duration: 483 (359 – 606) days |
6 (3-10) months for thrombotic outcomes and 9 (4-15) months for bleeding outcomes |
|
Naymagon (2021), retrospective |
N=214, of which N=86 using AC
Age:**** 60 (54–67)
Sex (M): **** 60.5 |
Acute PVT |
Cirrhosis: 100
Malignancy: Concurrent HCC: 15.1 |
Rivaroxaban, apixaban, dabigatran
median duration of AC: 18.8 (10.8–52.8) months
|
Warfarin/ enoxaparin/no AC**
median duration of AC: 18.8 (10.8–52.8) months |
NR
Median of 21 (11–44) months for patients using AC |
|
Naymagon (2021_B) retrospective |
N=63,of which N=58 using AC
Age (median (IQR)): I: 42 (29-53); C1: 43 (33-54); C2: 44 (32-53)
Sex (M): I: 73.9; C1: 46.2; C2: 63.6 |
Acute PVT and IBD |
Cirrhosis: 6
Malignancy: NR |
Rivaroxaban, apixaban, dabigatran
median duration 3.9 (2.7-6.1) months
|
Warfarin (C1), enoxaparin (C2)/no AC**
median duration of warfarin 8.5 (3.9-NA) months, for enoxaparin not reported |
I: 12 (6-35) months C1: 43 (9-80) months C2: 23 (10-58) months |
|
Zhang (2023), retrospective |
N=77 of which N=27 using AC
Age: 60.4 ± 12.3***
Sex (M): 67***
|
PVT, not reported whether it was acute or chronic |
Cirrhosis: 100
Malignancy: HCC 7 |
DOAC
Median duration of AC: 6 (2-11) months |
Warfarin
Median duration of AC: 6 (2-11) months |
NR
Median of 28.5 months for patients using AC |
*All patients were first treated with danaparoid sodium and Anthrobin P for 3 days at 1500 units/day i.v. in those patients whose antithrombin III activity decreased by less than 70%. ** Not included in our literature analysis. ***Age and sex are not reported by AC used but only for the total group patients receiving anticoagulants.
Results are reported as % (sex, cirrhosis and malignancy) or as mean ± SD/Median (IQR, age). DOAC: direct oral anticoagulants; HCC: hepatocellular carcinoma; IBD: inflammatory bowel disease; LMWH: low-molecular weight heparin; M: Male; NR: not reported; PVT: Portal vein thrombosis; VKA: vitamin K antagonist
Results
Since part of the studies compared data on treatment with DOAC versus VKA, and on DOAC versus LMWH, it was decided to present the data on those comparisons separately.
Major bleeding
Valeriani (2021), Ilcewicz (2021), Kawata (2021), Naymagon (2021) and Zhang (2023) reported on major bleeding, which was defined as a fatal and/or symptomatic bleeding in a critical area or organ, bleeding leading to a reduction of 2 g/dl or more in hemoglobin concentration or necessitating transfusion of two or more blood units (according to ISTH criteria). Valeriani (2021) included only data of Nagaoki (2018).
Besides, Naymagon (2020) and Naymagon (2021_B) reported on major bleeding, which was however defined as GRADE 3 or 4 according to WHO criteria. We decided not to include these data in the pooled data analysis, but report them separately.
Total group
DOAC vs VKA
Nagaoki (2018), Ilcewicz (2021), Naymagon (2021) and Zhang (2023) reported on major bleeding, for the comparison DOAC versus VKA. In total for 7/69 (10.1%) patients a major bleeding was reported in the DOAC group, compared to 8/82 (9.8%) patients in the VKA group (Figure 1). This corresponds to a risk ratio (RR, 95%CI) of 1.13 (0.44 to 2.86), which is in favor of the VKA group. Corresponding risk difference (RD, 95%CI) is 0.01 (-0.09 to 0.10), which is not considered to be clinically relevant.
Figure 1: Forest plot for the effect of DOACs on the outcome measure major bleeding (according to ISTH criteria) in patients with acute splanchnic vein thrombosis, compared to VKA.
Naymagon (2020) and Naymagon (2021_B) reported that in respectively 2/93 (2.2%) and 0/18 (0%) of the patients in the DOAC group major bleeding was reported, compared to 26/108 (24.1) and 3/22 (13.6%) patients in the VKA group. This corresponds to RRs (95%CI) of respectively 0.09 (0.02 to 0.37) and 0.17 (0.01 to 3.14) which are in favor of the DOAC groups. Corresponding RDs (95%CI) are -0.22 (-0.31 to -0.13) and -0.20 (-0.28 to -0.13), which is considered clinically relevant.
DOAC vs LMWH
Naymagon (2021) reported on major bleeding for the comparison DOAC versus LMWH. In the DOAC group, for 3/18 (16.7%) patients major bleeding was reported, compared to 9/42 (21.4%) patients in the LMWH group. This corresponds to a RR (95%CI) of 0.78 (0.24 to 2.54) which is in favor of the DOAC group. Corresponding RD (95%CI) is -0.05 (-0.26 to 0.16), which is considered clinically relevant.
Naymagon (2020) and Naymagon (2021_B) reported that in respectively 2/93 (2.2%) and 0/18 (0%) of the patients in the DOAC group major bleeding was reported, compared to 10/70 (14.3%) and 1/13 (7.7%) patients in the LMWH group. This corresponds to RRs (95%CI) of respectively 0.15 (0.03 to 0.67) and 0.25 (0.01 to 5.59) which are in favor of the DOAC group. Corresponding RDs (95%CI) are -0.26 (-0.37 to -0.15) and -0.08 (-0.25 to 0.10) which are considered clinically relevant.
DOAC vs LMWH/VKA
Kawata (2021) did not differentiate between patients receiving LMWH or VKA. They reported major bleeding in 3/47 (6.4%) patients in the DOAC group, compared to 6/98 (6.1%) patients in the LMWH/VKA group. This corresponds to a RR (95%CI) of 1.04 (0.27 to 3.99), which is in favor of the LMWH/VKA group. The corresponding RD (95%CI) is 0.00 (-0.08 to 0.09), which is not considered to be clinically relevant.
Patients with cirrhosis
DOAC vs VKA
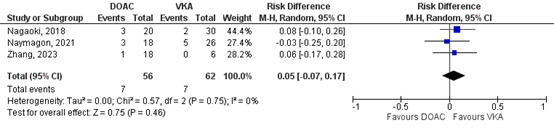
Nagaoki (2018), Naymagon (2021) and Zhang (2023) reported on major bleeding in patients with cirrhosis for the comparison between DOAC versus VKA. In total, for 7/56 (12.5%) patients, major bleeding was reported in the DOAC group, compared to 7/62 (11.3%) patients in the VKA group (Figure 2). This corresponds to a RR (95%CI) of 1.22 (0.46 to 3.24) which is in favor of the VKA group. The corresponding RD (95%CI) is 0.05 (-0.07 to 0.17), which is considered clinically relevant.
Besides, Ilcewicz (2021) reported that for none of the 10 patients with a previous diagnosis of cirrhosis a bleeding event was reported.
Figure 2: Forest plot for the effect of DOACs on the outcome measure major bleeding (according to ISTH criteria) in patients with acute splanchnic vein thrombosis and cirrhosis, compared to VKA.
DOAC vs LMWH
Naymagon (2021) reported on the outcome measure major bleeding in patients with cirrhosis for the comparison between DOAC versus LMWH – see also the results for the total group: in the DOAC group, for 3/18 (16.7%) patients a major bleeding was reported, compared to 9/42 (21.4%) patients in the LMWH group. This corresponds to a RR (95%CI) of 0.78 (0.24 to 2.54) which is in favor of the DOAC group. Corresponding RD (95%CI) is -0.05 (-0.26 to 0.16), which is considered clinically relevant.
Patients without cirrhosis
Naymagon (2020) reported on major bleeding in patients without cirrhosis, which was defined as GRADE 3 or 4 according to the WHO criteria. Data are reported here, but not used to draw conclusions on the effect of DOAC on the outcome measure major bleeding in patients without cirrhosis, compared to either VKA or LMWH.
DOAC vs VKA
Naymagon (2020) reported on major bleeding in patients without cirrhosis for the comparison DOAC versus VKA. For 2/93 (2.2%) of the patients in the DOAC group major bleeding was reported, compared to 26/108 (24.1%) patients in the VKA group. This corresponds to a RR (95%CI) of 0.09 (0.02 to 0.37), which is in favor of the DOAC group. Corresponding RD (95%CI) is -0.22 (-0.31 to -0.13), which is considered clinically relevant.
DOAC vs LMWH
Naymagon (2020) reported on major bleeding in patients without cirrhosis for the comparison DOAC versus LMWH. For 2/93 (2.2%) in the DOAC group major bleeding was reported, compared to 10/70 (14.3%) patients in the LMWH group. This corresponds to a RR (95%CI) of 0.15 (0.03 to 0.67) which is in favor of the DOAC group. Corresponding RD (95%CI) is -0.12 (-0.21 to -0.03), which is considered clinically relevant.
Progression of SVT
Valeriani (2021), Ilcewicz (2021), Kawata (2021) and Naymagon (2021) reported on progression of SVT which was respectively defined as progression of SVT at follow-up imaging (Valeriani, 2021), extension into previously uninvolved vessels or increase in the length/volume of the clot in the same vessel on imaging at 3, 6, and between 6 and 24 months, with a minimum observation period of 6 months (Kawata, 2021), PVT extension (Naymagon, 2021) or not defined (Ilcewicz, 2021). Valeriani (2021) included data of Nagaoki (2018).
Total group
DOAC vs VKA
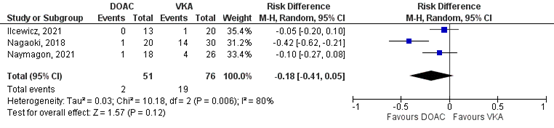
Nagaoki (2018), Ilcewicz (2021) and Naymagon (2021) reported on progression SVT for the comparison DOAC vs VKA. In total, for 2/51 (3.9%) of the patients in the DOAC group was progression of SVT reported, compared to 19/76 (25%) patients in the VKA-group (Figure 4). This corresponds to a RR (95%CI) of 0.22 (0.06 to 0.82), which is in favor of the DOAC group. Corresponding RD (95%CI) is -0.18 (-0.41 to 0.05), which is considered clinically relevant.
Figure 3: Forest plot for the effect of DOACs on the outcome measure progression of SVT in patients with acute splanchnic vein thrombosis, compared to VKA.
DOAC vs LMWH
Naymagon (2021) reported on progression of SVT for the comparison DOAC vs LMWH. For one of the patients in the DOAC group (N=18) progression of SVT was reported, compared to 8/42 (19.0%) patients in the LMWH group. The corresponding RR (95%CI) is 0.29 (0.04 to 2.16), which is in favor of the DOAC group. The corresponding RD (95%CI) is -0.13 (-0.29 to 0.02), which is considered clinically relevant.
DOAC vs LMWH/VKA
Kawata (2021) did not differentiate between patients receiving LMWH or VKA. In total for 3/43 (7.0%) patients, progression of SVT was reported in the DOAC group, compared to 5/93 (5.4%) patients in the LMWH/VKA group. This corresponds to a RR (95%CI) of 1.16 (0.29 to 4.62), which is in favor of the LMWH/VKA group. The corresponding RD (95%CI) is 0.01 (-0.08 to 0.10), which is not considered clinically relevant.
Patients with cirrhosis
DOAC vs VKA
Nagaoki (2018), Ilcewicz (2021) and Naymagon (2021) reported on progression of SVT for the comparison DOAC versus VKA in patients with cirrhosis. As is depicted in Figure 3, RDs (95%CI) based on data of Nagaoki (2018) and Naymagon (2021) are respectively -0.42 (-0.62 to -0.21) and -0.10 (-0.27 to 0.08), which are in favor of the DOAC group and are not considered clinically relevant. Finally, Ilcewicz (2021) reported that in none of the 10 patients with previous diagnosis of cirrhosis progression of SVT was found.
DOAC vs LMWH
Naymagon (2021) reported on progression of SVT for the comparison DOAC vs LMWH in patients with cirrhosis – see also results for the total group: for one of the patients in the DOAC group (N=18) progression of SVT was reported, compared to 8/42 (19.0%) patients in the LMWH group. The corresponding RR (95%CI) is 0.29 (0.04 to 2.16), which is in favor of the DOAC group. The corresponding RD (95%CI) is -0.13 (-0.29 to 0.02), which is considered clinically relevant.
Patients without cirrhosis
None of the included studies reported on progression of SVT for patients without cirrhosis.
(partial) Resolution of SVT
Valeriani (2021), Kawata (2021), Naymagon (2020), Naymagon (2021-B), Naymagon (2021) and Zhang (2023) reported on (partial) resolution of SVT which was respectively defined as:
- any grade of recanalization (partial or complete) at follow-up imaging (Valeriani, 2021);
- either complete resolution (no evidence of thrombus in subsequent imaging) or partial resolution (objective reduction in the number of vessels involved or the length of the clot), on imaging at three, six, and between six and 24 months, with a minimum observation period of six months (Kawata, 2021);
- complete radiographic resolution of PVT established on follow-up imaging (Naymagon, 2020; Naymagon, 2021 and Naymagon, 2021_B);
- partial (> 50% reduction of the thrombus) or complete (complete disappearance of the thrombus) PVT recanalization (Zhang, 2023).
Valeriani (2021) included data of Nagaoki (2018).
Total group
DOAC vs VKA
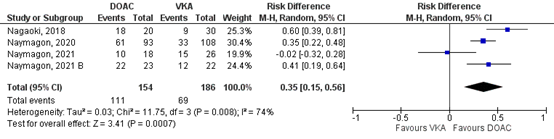
Nagaoki (2021), Naymagon (2020), Naymagon (2021), Naymagon (2021_B) and Zhang (2023) reported on (partial) resolution of SVT for the comparison DOAC vs VKA. In total, for 111/154 (72.1%) of the patients in the DOAC-group was complete resolution of SVT reported, compared to 69/186 (37.7%) patients in the VKA-group (Figure 4). This corresponds to a RR (95%CI) of 5.18 (1.49 to 18.09), which is in favor of the DOAC-group. Corresponding RD (95%CI) is 0.35 (0.15 to 0.56), which is considered clinically relevant.
Zhang (2023) reported on partial or complete SVT recanalization. However, number of events was not reported. Therefore, these data could not be included in the pooled analysis. Hazard ratio (95%CI) was 4.05 (0.5 to 37.7), which is in favor of the DOAC-group and is considered clinically relevant.
Figure 4: Forest plot for the effect of DOACs on the outcome measure (partial) resolution of SVT in patients with acute splanchnic vein thrombosis, compared to VKA.
DOAC vs LMWH
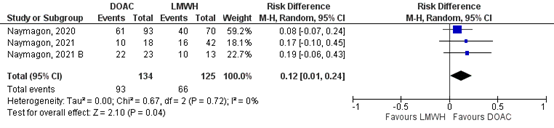
Naymagon (2020), Naymagon (2021), Naymagon (2021_B) reported on (partial) resolution of SVT for the comparison DOAC vs LMWH. In total, for 93/134 (69.4%) of the patients in the DOAC group was complete resolution of SVT reported, compared to 66/125 (52.8%) patients in the VKA-group (Figure 5). This corresponds to a RR (95%CI) of 1.21 (1.01 to 1.46), which is in favor of the DOAC-group. Corresponding RD (95%CI) is 0.12 (0.01 to 0.24), which is considered clinically relevant.
Figure 5: Forest plot for the effect of DOACs on the outcome measure (partial) resolution of SVT in patients with acute splanchnic vein thrombosis, compared to LMWH.
DOAC vs LMWH/VKA
Kawata (2021) did not differentiate between patients receiving LMWH or VKA. In total, for 25/43 (58.1%) patients (partial) resolution of SVT was reported in the DOAC-group, compared to 54/83 (65.1%) patients in the LMWH/VKA-group. This corresponds to a RR (95%CI) of 0.89 (0.66 to 1.20), which is in favor of the LMWH/VKA-group. The corresponding RD (95%CI) is -0.07 (-0.25 to 0.11), which is considered clinically relevant.
Patients with cirrhosis
DOAC vs VKA
Nagaoki (2021), Naymagon (2021) and Zhang (2023) reported on (partial) resolution of SVT for the comparison DOAC vs VKA. Zhang (2023) did not report the number of events, so results could not be pooled and are reported in Table 2. Results are inconsistent, since Nagaoki (2021) and Zhang (2023) reported results in favor of the DOAC-group while Naymagon (2021) reported a non-clinically relevant difference in favor of the VKA-group.
Table 2: Results on the effect of DOACs on the outcome measure (partial) resolution of SVT in patients with acute splanchnic vein thrombosis and cirrhosis, compared to VKA.
|
Study |
DOAC-group (n/N (%)) |
VKA-group (n/N (%)) |
Effect estimate (95%CI) |
|
Nagaoki (2021) |
18/20 (90) |
9/30 (30) |
RD: 0.60 (0.39 to 0.81) RR: 3.00 (1.70 to 5.28) |
|
Naymagon (2021) |
10/18 (55.6) |
15/26 (57.7) |
RD: -0.02 (-0.32 to 0.28) RR: 0.96 (0.57 to 1.63) |
|
Zhang (2023) |
NR |
NR |
HR: 4.045 (0.52 to 37.67) |
RD: Risk difference, RR: risk ratio, HR: Hazard ratio, DOAC: direct oral anticoagulants, NR: not reported, VKA: vitamin K antagonists
DOAC vs LMWH
Naymagon (2021) reported on (partial) resolution of SVT for the comparison DOAC vs LMWH. For 10/18 (55.6%) patients (partial) resolution of SVT was reported in the DOAC-group, compared to 16/42 (0.4%) patients in the LMWH/VKA-group. This corresponds to a RR (95%CI) of 1.46 (0.83 to 2.57), which is in favor of the DOAC-group. The corresponding RD (95%CI) is 0.17 (-0.10 to 0.45), which is considered clinically relevant.
Patients without cirrhosis
DOAC vs VKA
Naymagon (2020) and Naymagon (2021_B) reported on (partial) resolution of SVT for the comparison DOAC vs VKA. Results are reported in Table 3. Differences are in favor of the DOAC-group and are considered clinically relevant.
Table 3: Results on the effect of DOACs on the outcome measure (partial) resolution of SVT in patients with acute splanchnic vein thrombosis without cirrhosis, compared to VKA.
|
Study |
DOAC-group (n/N (%)) |
VKA-group (n/N (%)) |
Effect estimate (95%CI) |
|
Naymagon (2020) |
61/93 (65.6) |
33/108 (30.6) |
RD: 0.35 (0.22 to 0.48) RR: 2.15 (1.56 to 2.96) |
|
Naymagon (2021_B) |
22/23 (95.7) |
12/22 (54.5) |
RD: 0.41 (0.19 to 0.64) RR: 1.75 (1.19 to 2.59) |
RD: Risk difference, RR: risk ratio, DOAC: direct oral anticoagulants, VKA: vitamin K antagonist
DOAC vs LMWH
Naymagon (2020) and Naymagon (2021_B) reported on (partial) resolution of SVT for the comparison DOAC vs LMWH. Results are reported in Table 4. Differences are in favor of the DOAC-group and are considered clinically relevant.
Table 4: Results on the effect of DOACs on the outcome measure (partial) resolution of SVT in patients with acute splanchnic vein thrombosis without cirrhosis, compared to LMWH.
|
Study |
DOAC-group (n/N (%)) |
LMWH-group (n/N (%)) |
Effect estimate (95%CI) |
|
Naymagon (2020) |
61/93 (65.6) |
40/70 (57.1) |
RD: 0.08 (-0.07 to 0.24) RR: 1.15 (0.89 to 1.47) |
|
Naymagon (2021_B) |
22/23 (95.7) |
10/13 (76.9) |
RD: 0.19 (-0.06 to 0.43) RR: 1.24 (0.91 to 1.70) |
RD: Risk difference, RR: risk ratio, DOAC: direct oral anticoagulants, LMWH: low molecular weight heparin
Mortality
Naymagon (2020), Naymagon (2021) and Naymagon (2021_B) reported on mortality, which was not further defined.
Total group
DOAC vs VKA
Naymagon (2021) reported on mortality for the comparison DOAC versus VKA. In the DOAC group, 3/18 (16.7%) of the patients died, compared to 4/26 (15.4%) in the warfarin group. This corresponds to a RR (95%CI) of 1.08 (0.28 to 4.27), which is in favor of the VKA group. The corresponding RD (95%CI) is 0.01 (-0.21 to 0.23) which is not considered clinically relevant.
DOAC vs LMWH
Naymagon (2021) reported on mortality for the comparison DOAC versus LMWH. In the DOAC group, 3/18 (16.7%) of the patients died, compared to 8/42 (19.1%) in respectively the warfarin and enoxaparin group. This corresponds to a RR (95%CI) of 0.88 (0.26 to 2.92), which is in favor of the DOAC group. The corresponding RD (95%CI) is -0.02 (-0.23 to 0.19) which is not considered clinically relevant.
Naymagon (2020) and Naymagon (2021_B) reported also on mortality, but data was very limited. Naymagon (2020) reported that 12/330 (3.6%) patients died, of which three were related to PVT (enoxaparin group: N=1/70, warfarin group: N=1/108, DOAC group: N=0/93). Naymagon (2021_B) reported that 2/58 patients died during follow-up, of which one was related to PVT (warfarin group: N=1/22, enoxaparin group: N=0/13 and DOAC group: N=0/23). Since data is too limited, it cannot be used to draw conclusions on the effect of DOAC on the outcome measure mortality in patients with SVT, compared to conventional anticoagulation.
Patients with cirrhosis
DOAC vs VKA
Naymagon (2021) reported on mortality for the comparison DOAC versus VKA in patients with cirrhosis – see also results on the total group: in the DOAC group, 3/18 (16.7%) of the patients died, compared to 4/26 (15.4%) in the warfarin group. This corresponds to a RR (95%CI) of 1.08 (0.28 to 4.27), which is in favor of the VKA group. The corresponding RD (95%CI) is 0.01 (-0.21 to 0.23) which is not considered clinically relevant.
DOAC vs LMWH
Naymagon (2021) reported on mortality for the comparison DOAC vs LMWH – see also results on the total group: in the DOAC group, 3/18 (16.7%) of the patients died, compared to 8/42 (19.1%) in respectively the warfarin and enoxaparin group. This corresponds to a RR (95%CI) of 0.88 (0.26 to 2.92), which is in favor of the DOAC group. The corresponding RD (95%CI) is -0.02 (-0.23 to 0.19) which is not considered clinically relevant.
Patients without cirrhosis
Naymagon (2020) reported on mortality in patients without cirrhosis – see also results on the total group: they reported that 12/330 (3.6%) patients died, of which three were related to PVT (enoxaparin group: N=1/70, warfarin group: N=1/108, DOAC group: N=0/93). Results are too limited to draw conclusions.
Clinically relevant non-major bleeding (CRNMB)
Kawata (2021) reported on CRNMB, which was defined according to the criteria of ISTH.
Total group – DOAC versus LMWH/VKA
Kawata (2021) reported on CRNMB for the comparison DOAC versus LMWH/VKA. In the DOAC group, for 1/47 (2%) patients a CRNMB was reported, compared to 4/98 (4%) patients in the LMWH/VKA group. This corresponds to an RR (95%CI) of 0.52 (0.06 to 4.54), which is in favor of the DOAC group. Corresponding RD (95%CI) is -0.02 (-0.08 to 0.04). This difference is not considered clinically relevant.
Patients with cirrhosis
None of the included studies reported on CRNMB for patients with SVT and cirrhosis.
Patients without cirrhosis
None of the included studies reported on CRNMB for patients with SVT, without cirrhosis.
Recurrent VTE
Ilcewicz (2021) reported on recurrent thrombo-embolic events, which was defined as VTE of typical locations, including peripheral deep vein thrombosis and pulmonary embolism, new or worsened index PVT, and other atypical VTE. We considered data on worsening of PVT in the systematic analysis for the outcome measure progression of SVT.
Total group
DOAC vs VKA
Ilcewicz (2021) reported on recurrent thrombo-embolic events. In the DOAC group for none of the 13 patients a recurrent thrombo-embolic was reported, compared to 3/20 (15%) patients in the warfarin group. For all of them a new SVT was reported.
All comparisons
Naymagon (2020) reported that recurrence of SVT was not associated with type of anticoagulants and occurred in 9 of all included 330 patients (2.7%, patients without receiving anticoagulants were also included in this group, and effect measure was not reported). Data was too limited and was therefore not used to draw conclusions on the effect of DOAC on the outcome measure recurrent VTE in patients with SVT, compared to conventional anticoagulation.
Patients with cirrhosis
Ilcewicz (2021) reported that in none of the 10 patients with cirrhosis a recurrent VTE was found. Therefore, data is too limited to draw conclusions.
Patients without cirrhosis
Naymagon (2020) reported on recurrence of SVT in patients without cirrhosis – see also results for the total group. In short, they reported that recurrence of SVT was not associated with type of anticoagulants and occurred in 9 of all included 330 patients (2.7%, patients without receiving anticoagulants were also included in this group, and effect measure was not reported).
Liver failure and liver transplantation
None of the included studies reported on liver failure and liver transplantation.
Need for surgical or radiological intervention
Total group
None of the included studies reported on need for surgical or radiological intervention by the different anticoagulation groups. However, Naymagon (2021-B) reported that three of the patients who developed symptomatic portal hypertension (SPH) received a transjugular intrahepatic portosystemic shunt (enoxaparin-group: N=2/13, warfarin group: N=1/22, DOAC group: N=0/23). Besides, Naymagon (2020) reported that 24/104 (23%) of the patients developing chronic SPH received a TIPS. They concluded that this was not significantly different between the groups (including also the non-anticoagulants group). Data was too limited and was therefore not used to draw conclusions on the effect of DOAC on the outcome measure need for surgical or radiological intervention in patients with SVT, compared to conventional anticoagulation.
Patients with cirrhosis
None of the included studies reported on need for surgical or radiological intervention for patients with cirrhosis.
Patients without cirrhosis
Naymagon (2020) reported on patients without cirrhosis who developed chronic SPH and received a TIPS – see also results for the total group. In short, they reported that 24/104 (23%) of the patients developing chronic SPH received a TIPS. They concluded that this was not significantly different between the groups (including also the non-anticoagulants group).
Level of evidence of the literature
General remarks
In part of the studies, it was not specified whether patients with acute and/or chronic SVT were included (Nagaoki, 2018 and Zhang, 2023).
Major bleeding
All comparisons
The evidence regarding the outcome measure major bleeding (according to ISTH criteria) came from observational studies and therefore the level of evidence started as low. The level of evidence was downgraded to very low. There was high risk of bias in the studies (e.g. events might have been missed due to retrospective design of the study, risk on indication bias, time frame differed between the groups and risk on (residual) confounding, downgraded 2 levels). Besides, the effect estimate (95%CI) crossed the thresholds for clinical relevance (serious imprecision, downgraded 2 levels).
Patients without cirrhosis
None of the included studies reported on major bleeding (according to ISTH criteria) for patients without cirrhosis.
Progression of SVT
Total group (DOAC vs LMWH)
The evidence regarding the outcome measure progression of SVT came from observational studies and therefore the level of evidence started as low. The level of evidence was downgraded to very low. There was high risk of bias in the studies (e.g. progression might have been missed since there was no routine imaging/imaging was not standardized, risk on indication bias, time frame differed between the groups and risk on (residual) confounding, downgraded 2 levels). Besides, the effect estimate (95%CI) crossed one of the thresholds for clinical relevance (serious imprecision, downgraded 1 level).
Total group (DOAC vs VKA), patients with cirrhosis (DOAC vs VKA and DOAC vs LMWH)
The evidence regarding the outcome measure progression of SVT came from observational studies and therefore the level of evidence started as low. The level of evidence was downgraded to very low. There was high risk of bias in the studies (e.g. progression might have been missed since it was not sure whether there was routine imaging, risk on indication bias, time frame differed between the groups and risk on (residual) confounding, downgraded 2 levels). Besides, the effect estimate (95%CI) crossed the thresholds for clinical relevance (serious imprecision, downgraded 2 levels).
Patients without cirrhosis
None of the included studies reported on progression of SVT for patients without cirrhosis.
(partial) resolution of SVT
Total group (DOAC vs VKA) and patients without cirrhosis (DOAC vs VKA)
The evidence regarding the outcome measure progression of SVT came from observational studies and therefore the level of evidence started as low. The level of evidence was downgraded to very low. There was high risk of bias in the studies (e.g. (partial) resolution of SVT might have been missed since it was not sure whether there was routine imaging, risk on indication bias, time frame differed between the groups and risk on (residual) confounding, downgraded 2 levels). Besides, number of included patients was low (imprecision, downgraded 1 level).
Total group (DOAC vs LMWH)
The evidence regarding the outcome measure (partial) resolution of SVT came from observational studies and therefore the level of evidence started as low. The level of evidence was downgraded to very low. There was high risk of bias in the studies (e.g. (partial) resolution might have been missed since there was no routine imaging/imaging was not standardized, risk on indication bias, time frame differed between the groups and risk on (residual) confounding, downgraded 2 levels). Besides, the effect estimate (95%CI) crossed one of the thresholds for clinical relevance (imprecision, downgraded 1 level).
Patients with cirrhosis (DOAC vs VKA)
The evidence regarding the outcome measure (partial) resolution of SVT came from observational studies and therefore the level of evidence started as low. The level of evidence was downgraded to very low. There was high risk of bias in the studies (e.g. (partial) resolution might have been missed since there was no routine imaging/imaging was not standardized, risk on indication bias, time frame differed between the groups and risk on (residual) confounding, downgraded 2 levels). Besides, results were inconsistent (inconsistency, downgraded 1 level) and the effect estimate (95%CI) crossed the thresholds for clinical relevance (imprecision, downgraded 1 level).
Patients with cirrhosis (DOAC vs LMWH) and patients without cirrhosis (DOAC vs LMWH)
The evidence regarding the outcome measure (partial) resolution of SVT came from observational studies and therefore the level of evidence started as low. The level of evidence was downgraded to very low. There was high risk of bias in the study (e.g. (partial) resolution might have been missed since there was no routine imaging/imaging was not standardized, risk on indication bias, time frame differed between the groups and risk on (residual) confounding, downgraded 2 levels). Besides, the effect estimate (95%CI) crossed the thresholds for clinical relevance (imprecision, downgraded 2 levels).
Mortality
Total group (DOAC vs VKA and DOAC vs LMWH) and patients with cirrhosis (DOAC vs VKA and DOAC vs LMWH)
The evidence regarding the outcome measure mortality came from observational studies and therefore the level of evidence started as low. The level of evidence was downgraded to very low. There was high risk of bias in the studies (e.g. risk on indication bias, time frame differed between the groups, patients with short follow-up were excluded and risk on (residual) confounding, downgraded 2 levels). Besides, the effect estimate (95%CI) crossed the thresholds for clinical relevance (serious imprecision, downgraded 2 levels).
Patients without cirrhosis
Data was too limited and could therefore not be used to draw conclusions on the effect of DOAC on the outcome measure recurrent mortality in patients with SVT without cirrhosis, compared to conventional anticoagulation.
Clinically relevant non major bleeding
Total group (DOAC vs VKA or LMWH)
The evidence regarding the outcome measure clinically relevant non major bleeding came from an observational study and therefore the level of evidence started as low. The level of evidence was downgraded to very low. There was high risk of bias in the studies (e.g. events might have been missed due to retrospective design of the study, risk on indication bias, time frame differed between the groups and risk on (residual) confounding, downgraded 2 levels). Besides, the effect estimate (95%CI) crossed the thresholds for clinical relevance (serious imprecision, downgraded 2 levels).
Total group (DOAC vs VKA and DOAC vs LMWH), patients with cirrhosis and patients without cirrhosis
None of the included studies reported on CRNMB for patients with or without cirrhosis, nor for the comparisons between DOAC and VKA or DOAC and LMWH for the total group.
Recurrent VTE
Total group (DOAC vs VKA)
The evidence regarding the outcome measure recurrent VTE came from an observational study and therefore the level of evidence started as low. The level of evidence was downgraded to very low. There was high risk of bias in the study (e.g. recurrent VTE’s might have been missed since there was no routine imaging, risk on indication bias, time frame differed between the groups and risk on (residual) confounding, downgraded 2 levels). Besides, the number of events (and patients) was very low (serious imprecision, downgraded 2 levels).
Total group (DOAC vs LMWH) and patients with cirrhosis
None of the included studies reported on recurrent VTE for patients with cirrhosis nor for the comparison between DOAC and LMWH.
Patients without cirrhosis
Data was too limited and could therefore not be used to draw conclusions on the effect of DOAC on the outcome measure recurrent VTE in patients with SVT without cirrhosis, compared to conventional anticoagulation.
Liver failure, liver transplantation and clinically relevant non major bleeding
None of the included studies reported on liver failure and liver transplantation. Therefore, no conclusion can be drawn on the effect of DOACs on the outcome measures liver failure and liver transplantation in adults with SVT.
Need for surgical or radiological intervention
Data was too limited and could therefore not be used to draw conclusions on the effect of DOAC on need for surgical or radiological intervention in patients with SVT, compared to conventional anticoagulation.
Zoeken en selecteren
A systematic review of the literature was performed to answer the following question: what are the (un)desirable effects of treatment with Direct Oral Anticoagulants (DOAC) in adult patients with acute SVT, compared to treatment with low-molecular weight heparin (LMWH), vitamin K antagonist (VKA) or heparin?
| P (Patients): | adult patients with acute SVT (acute portal vein thrombosis, acute Budd Chiari syndrome, acute hepatic vein thrombosis, acute splenic vein thrombosis, acute SVT, acute mesenteric vein thrombosis) |
| I (Intervention): | DOAC |
| C (Comparison): | LMWH, VKA, heparin |
| O (Outcomes): | major bleeding, clinically relevant non major bleeding (CRNMB), mortality, recurrent VTE, progression of SVT, (partial) resolution of SVT, liver failure, liver transplantation, need for surgical or radiological intervention |
Relevant outcome measures
The guideline development group considered major bleeding, progression of SVT, (partial) resolution of SVT and mortality as critical outcome measures for decision making; CRNMB, recurrent venous thromboembolic event (VTE), liver failure, liver transplantation and need for surgical or radiological intervention as important outcome measures for decision making.
The working group defined the outcome measures as follows:
- Major bleeding: fatal bleeding, and/or symptomatic bleeding in a critical area or organ, such as intracranial, intraspinal, intraocular, retroperitoneal, intra-articular or pericardial, or intramuscular with compartment syndrome, and/or bleeding causing a fall in hemoglobin levels of 1.24 mmol/L (20 g/L or greater) or more, or leading to a transfusion of 2 U or more of whole blood or red cells, as defined by International Society of Thrombosis and Haemostasis;
- Recurrent VTE: objectively confirmed VTE, including recurrent SVT;
- Need for surgical or radiological intervention: Transjugular Intrahepatic Portosystemic Shunt (TIPS), thrombosuction, bowel resection.
A priori, the working group did not define the other outcome measures listed above but used the definitions used in the studies.
The working group defined a risk difference of 3%* as a minimal clinically (patient) important difference for major bleeding, progression of SVT, (partial) resolution of SVT, mortality, clinically relevant non major bleeding and recurrent VTE. For all other outcome measures, the default thresholds proposed by the international GRADE working group were used as a threshold for clinically relevant differences: a 25% difference in relative risk (RR) for dichotomous outcomes (RR< 0.8 or RR> 1.25), and 0.5 standard deviations (SD) for continuous outcomes.
Since presence of liver cirrhosis is an important underlying cause for SVT and factor in deciding on type of anticoagulants for the treatment of patients with SVT, subgroup analysis will be performed for patients with liver cirrhosis and patients without liver cirrhosis.
*Based on the differences applied in the guidelines on thromboprophylaxis in patients with COVID-19. This working group derived the minimal clinically (patient) important differences from the ACCP (2012).
Search and select (Methods)
Two literature searches were performed. At first, we searched for systematic reviews and RCT and after this we performed an additional search to supplement the selected review(s) with observational studies that were published after the search date of the selected review(s).
Search 1: Systematic reviews (SR) and RCTs
The databases Medline (via OVID) and Embase (via Embase.com) were searched with relevant search terms until July 5th, 2023. The detailed search strategy is depicted under the tab Methods. The systematic literature search resulted in 669 hits. Studies were selected based on the following criteria: (systematic reviews of) RCTs and observational studies which evaluated the effectiveness of DOAC in adult patients with acute SVT, compared to treatment with heparin, LMWH or VKA. 37 studies were initially selected based on title and abstract screening. After reading the full text, 36 studies were excluded (see the table with reasons for exclusion under the tab Methods), and only one study was included (Valeriani, 2021).
Search 2: Observational studies
The search strategy of the systematic review (Valeriani, 2021) was completed on December 19th, 2019. Therefore, we performed an additional search on observational studies in the databases Medline (via OVID) and Embase (via Embase.com) with relevant search terms between 1st of January 2019 until the 30th of October 2023. The detailed search strategy is depicted under the tab Methods. The systematic literature search resulted in 553 hits. Studies were selected based on following criteria: observational studies which compared the effectiveness of DOAC in adult patients with acute SVT. The following studies were excluded:
- Switch over studies in which patients that switched from (long-term) conventional anticoagulants to DOACs are considered as patients in the DOAC group.
- Studies that primarily included patients with chronic SVT.
- Studies that primarily included patients with hepatocellular carcinoma and SVT, because characteristics/treatment options/prognosis of those patients differ significantly from other patients with acute SVT.
In selection of the studies, it seemed difficult to select the studies that only included patients with an acute SVT. In the study of Khan (2022) 43% of the patients had a chronic SVT. This study was excluded. However, in other studies it was not specified whether patients with acute and/or chronic SVT were included (Nagaoki, 2018 and Zhang, 2023). The working group decided to include those studies in the literature analysis, as in clinical practice it is also often not known if an SVT is acute or of older age. It is however important to take this into account in interpreting the results.
18 studies were initially selected based on title and abstract screening. After reading the full text, 12 studies were excluded (see the table with reasons for exclusion under the tab Methods), and six studies were included.
In total, 1 SR (search 1) and six observational studies (search 2) were included.
Results
Seven studies were included in the analysis of the literature. Important study characteristics and results are summarized in the evidence tables. The assessment of the risk of bias is summarized in the risk of bias tables.
Referenties
- Ageno W, Beyer Westendorf J, Contino L, Bucherini E, Sartori MT, Senzolo M, Grandone E, Santoro R, Carrier M, Delluc A, De Stefano V, Pomero F, Donadini MP, Tosetto A, Becattini C, Martinelli I, Nardo B, Bertoletti L, Di Nisio M, Lazo-Langner A, Schenone A, Riva N. Rivaroxaban for the treatment of noncirrhotic splanchnic vein thrombosis: an interventional prospective cohort study. Blood Adv. 2022 Jun 28;6(12):3569-3578. doi: 10.1182/bloodadvances.2022007397. PMID: 35439303; PMCID: PMC9631568.
- Candeloro M, Valeriani E, Monreal M, Ageno W, Riva N, Lopez-Reyes R, Peris ML, Beyer Westendorf J, Schulman S, Rosa V, López-Núñez JJ, Garcia-Pagan JC, Magaz M, Senzolo M, De Gottardi A, Di Nisio M. Anticoagulant therapy for splanchnic vein thrombosis: an individual patient data meta-analysis. Blood Adv. 2022 Aug 9;6(15):4516-4523. doi: 10.1182/bloodadvances.2022007961. PMID: 35613465; PMCID: PMC9636325.
- Carlin S, Cuker A, Gatt A, Gendron N, Hernández-Gea V, Meijer K, Siegal DM, Stanworth S, Lisman T, Roberts LN. Anticoagulation for stroke prevention in atrial fibrillation and treatment of venous thromboembolism and portal vein thrombosis in cirrhosis: guidance from the SSC of the ISTH. J Thromb Haemost. 2024 Sep;22(9):2653-2669. doi: 10.1016/j.jtha.2024.05.023. Epub 2024 May 31. PMID: 38823454.
- Van Dijk CE, Heim N and Witteveen J. Evaluatie van de ervaringen en kosten van antistollingszorg. Zorginstituut Nederland. 2020. https://www.zorginstituutnederland.nl/publicaties/rapport/2020/10/20/evaluatie-antistollingszorg
- de Franchis R, Bosch J, Garcia-Tsao G, Reiberger T, Ripoll C; Baveno VII Faculty. Baveno VII - Renewing consensus in portal hypertension. J Hepatol. 2022 Apr;76(4):959-974. doi: 10.1016/j.jhep.2021.12.022. Epub 2021 Dec 30. Erratum in: J Hepatol. 2022 Apr 14;: PMID: 35120736; PMCID: PMC11090185.
- Hutchinson A, Rees S, Young A, Maraveyas A, Date K, Johnson MJ. Oral anticoagulation is preferable to injected, but only if it is safe and effective: An interview study of patient and carer experience of oral and injected anticoagulant therapy for cancer-associated thrombosis in the select-d trial. Palliat Med. 2019 May;33(5):510-517. doi: 10.1177/0269216318815377. Epub 2018 Nov 29. PMID: 30488789; PMCID: PMC6506899.
- Ilcewicz HN, Martello JL, Piechowski K. Evaluation of the efficacy and safety of direct oral anticoagulants in the treatment of portal vein thrombosis. Eur J Gastroenterol Hepatol. 2021 Jun 1;33(6):911-916. doi: 10.1097/MEG.0000000000001958. PMID: 33079786; PMCID: PMC8371984.
- Kawata E, Siew DA, Payne JG, Louzada M, Kovacs MJ, Lazo-Langner A. Splanchnic vein thrombosis: Clinical manifestations, risk factors, management, and outcomes. Thromb Res. 2021 Jun;202:90-95. doi: 10.1016/j.thromres.2021.03.018. Epub 2021 Mar 21. PMID: 33798804.
- Nagaoki Y, Aikata H, Daijyo K, Teraoka Y, Shinohara F, Nakamura Y, Hatooka M, Morio K, Nakahara T, Kawaoka T, Tsuge M, Hiramatsu A, Imamura M, Kawakami Y, Ochi H, Chayama K. Efficacy and safety of edoxaban for treatment of portal vein thrombosis following danaparoid sodium in patients with liver cirrhosis. Hepatol Res. 2018 Jan;48(1):51-58. doi: 10.1111/hepr.12895. Epub 2017 Apr 27. PMID: 28342265.
- Naymagon L, Tremblay D, Zubizarreta N, Moshier E, Troy K, Schiano T, Mascarenhas J. The efficacy and safety of direct oral anticoagulants in noncirrhotic portal vein thrombosis. Blood Adv. 2020 Feb 25;4(4):655-666. doi: 10.1182/bloodadvances.2019001310. PMID: 32078681; PMCID: PMC7042983.
- Naymagon L, Tremblay D, Zubizarreta N, Moshier E, Mascarenhas J, Schiano T. Safety, Efficacy, and Long-Term Outcomes of Anticoagulation in Cirrhotic Portal Vein Thrombosis. Dig Dis Sci. 2021 Oct;66(10):3619-3629. doi: 10.1007/s10620-020-06695-4. Epub 2020 Nov 5. PMID: 33151401.
- Naymagon L_B, Tremblay D, Zubizarreta N, Moshier E, Naymagon S, Mascarenhas J, Schiano T. The Natural History, Treatments, and Outcomes of Portal Vein Thrombosis in Patients With Inflammatory Bowel Disease. Inflamm Bowel Dis. 2021 Jan 19;27(2):215-223. doi: 10.1093/ibd/izaa053. PMID: 32185400; PMCID: PMC8427727.
- Valeriani E, Di Nisio M, Riva N, Cohen O, Garcia-Pagan JC, Magaz M, Porreca E, Ageno W. Anticoagulant therapy for splanchnic vein thrombosis: a systematic review and meta-analysis. Blood. 2021 Mar 4;137(9):1233-1240. doi: 10.1182/blood.2020006827. PMID: 32911539.
- Valeriani E, Di Nisio M, Riva N, Cohen O, Porreca E, Senzolo M, De Gottardi A, Magaz M, Garcia-Pagan JC, Ageno W. Anticoagulant Treatment for Splanchnic Vein Thrombosis in Liver Cirrhosis: A Systematic Review and Meta-Analysis. Thromb Haemost. 2021 Jul;121(7):867-876. doi: 10.1055/s-0040-1722192. Epub 2021 Feb 1. PMID: 33525037.
- Zhang Z, Zhao Y, Li D, Guo M, Li H, Liu R, Cui X. Safety, efficacy and prognosis of anticoagulant therapy for portal vein thrombosis in cirrhosis: a retrospective cohort study. Thromb J. 2023 Jan 30;21(1):13. doi: 10.1186/s12959-023-00454-x. PMID: 36717831; PMCID: PMC9885579.
Evidence tabellen
Evidence table for systematic review of RCTs and observational studies (intervention studies)
Research question: What are the (un)desirable effects of treatment with Direct Oral Anticoagulants (DOAC) in adult patients with acute abdominal vein thrombosis, compared to treatment with low-molecular weight heparine (LMWH), vitamin K antagonist (VKA) or heparin?
|
Study reference |
Study characteristics |
Patient characteristics |
Intervention (I) |
Comparison / control (C)
|
Follow-up |
Outcome measures and effect size |
Comments |
|
Valeriani, 2021
Study characteristics and results are extracted from the SR (unless stated otherwise) |
SR and meta-analysis of RCTs/ cohort studies.
Literature search up to December, 2019
A: Hanafy (2018)* B: Nagaoki (2018) C: Sharma (2020)** D: Wille (2019)***
*Retracted article and therefore not considered in this literature review. **Part of the patients switched from VKA to DOACs and were considered in the DOAC-group. Study is excluded. ***Data on DOACs is to limited and therefore this study is not considered in this literature review.
Study design: Retrospective studies
Setting and Country: B: University hospital, Japan C: Tertiary centre, India
Source of funding and conflicts of interest: B: No funding declared, no COI C: None
Systematic review was not funded. However, publication costs of article were defrayed in part by page charge payment. |
Inclusion criteria SR: diagnosis of SVT; observational study or RCT including ≥10 patients; availability of radiological or clinical outcomes; and anticoagulant treatment with LMWH, unfractionated heparin, fondaparinux, VKAs, DOACs or no anticoagulant therapy.
Exclusion criteria SR: study design different from those specified in the inclusion criteria; inclusion of < 10 patients; and anticoagulant therapy different from those specified in the inclusion criteria.
97 studies included of which 4 studies on DOACs.
Important patient characteristics at baseline:*
N B: I: 20, C: 30) C: I: 36, C:62
Age B: I: 69 (53-74) C: 67 (24-83) C: I:29.5 (22–35) C: 28 (23–37)
Sex (male): B: I: 65%, C: 57% C: I: 53%, C: 61%
Child-Pugh score (mean or %) B: A/B/C I: 15/5/0, C: 15/10/5 C: I: 7 (6-8), C: 6 (5.7-7)
MELD score B: NR C: I: 10.8 (8.6-13), C: 11.7 (9.2-13.8)
INR B: NR C: I: 1.3 (1.2-1.4), C: 1.4 (1.2-1.6)
Etiology (%) HBV/HCV/NBNC: I: 4/6/10, C: 7/16/7
*Extracted from individual studies.
Groups comparable at baseline? |
Describe intervention:
B: Edoxaban (60 mg once daily for 6 months, dosage was halved in patients with eGFR 30-50 ml/min, patients <60 kg and patients with concurrent treatment with a strong P-glycoprotein inhibitor) C: Dabigatran (duration of therapy: 14.1 ± 6.9 months)
|
Describe control:
B: Warfarin (dosage adjusted to achieve INR 1.5-2.0) C: vitamin K antagonists (duration of therapy: 10.5 ± 6.7 months)
|
End-point of follow-up:
B: 6 months (regular clinical FU at 2 wks, 1 month, 3 months and 6 months) C: NR (once a month for the initial 3 months followed by once in 3 months)
For how many participants were no complete outcome data available? B: NR C: NR
|
Outcome major bleeding Defined as major bleeding by study authors or interpreted as major by the review authors.
DOAC (n/N) B: 3/20 C: 1/36
VKA (n/N) B: 2/30 C: 3/62
Outcome mortality Defined as overall mortality
DOAC (n/N) B: NR C: 1/36
VKA (n/N) B: NR C: 3/62
LMWH (n/N) B: NA C: NA
Outcome progressive SVT Defined as progression of SVT at follow-up imaging.
DOAC (n/N) B: 1/20 C: NR
VKA (n/N) B: 14/30 C: NR
LMWH (n/N) B: NR C: NA
(recurrent) VTE Defined as deep vein thrombosis of the lower or upper extremities, pulmonary embolism, or recurrent SVT
DOAC (n/N) B: NR C: 4/36
VKA (n/N) B: NR C: 4/62
LMWH (n/N) B: NA C: NA
Recanalization of SVT Defined as any grade of recanalization (partial or complete) at follow-up imaging
DOAC B: 18/20
VKA B: 9/30
|
Risk of bias (high, some concerns or low): Tool used by authors: ROBINS-I
B: high C: high
Author’s conclusion In summary, anticoagulant therapy for SVT is associated with vein recanalization and low probability of thrombosis progression. The risks of recurrent VTE and major bleeding in patients receiving anticoagulation therapy and the proportion of events in those left untreated strongly suggest the need for additional studies to optimize SVT management.
Remarks Sharma (2020): Any patient who was switched over to dabigatran from VKAs was considered in dabigatran arm.
|
Evidence table for intervention studies (randomized controlled trials and non-randomized observational studies [cohort studies, case-control studies, case series])
Research question: What are the (un)desirable effects of treatment with Direct Oral Anticoagulants (DOAC) in adult patients with acute abdominal vein thrombosis, compared to treatment with low-molecular weight heparin (LMWH), vitamin K antagonist (VKA) or heparin?
|
Study reference |
Study characteristics |
Patient characteristics |
Intervention (I) |
Comparison / control (C)
|
Follow-up |
Outcome measures and effect size |
Comments |
|
Naymagon, 2020 |
Type of study: Retrospective study
Setting and country: Urban tertiary care center, USA
Funding and conflicts of interest: Non commercial funding and no COI. |
Inclusion criteria: Patients with ICD code for non-cirrhotic (acute) PVT seen between January 2000 and February 2019.
Exclusion criteria: Splanchnic vein thrombosis without portal vein involvement, had cirrhosis or tumor thrombus, received interventional thrombolysis/thrombectomy, lacked baseline imaging of PVT at diagnosis, lacked subsequent follow-up imaging at least 3 months after diagnosis, or seemed to have chronic rather than acute PVT (eg, had known prior history of PVT or had evidence of cavernous transformation or other radiographic features to suggest chronic PVT at the time of initial diagnosis).
N total at baseline: 330 of which 57 did not receive anticoagulation Intervention: 93 Control1: 108 Control 2: 70
Important prognostic factors2: age (median (IQR)): I: 47.1 (15.2) C1: 50.4 (14.8) C2: 51.4 (16.9)
Sex (M/F (%)): I: 50.5/49.5 C1: 52.8/47.2 C2: 38.6/61.4
Etiology IBD (%) I: 6.5 C1: 5.6 C2: 4.3
IBD + surgery I: 14 C1: 12 C2: 7.1
Intraabdominal infection (%) I: 5.4 C1: 8.3 C2: 7.1
JAK2V617 mutation (%) I: 7.5 C1: 13.9 C2: 7.1
Non-HCC malignancy (%) I: 5.4 C1: 1.9 C2: 14.3
Pancreatitis (%) I: 5.4 C1: 3.7 C2: 2.9
Pregnancy (%) I: 2.2 C1: 0 C2: 2.9
Multiple (%) I: 9.7 C1: 5.6 C2: 27.1
OCP use (%) I: 6.5 C1: 1.9 C2: 5.7
Other (%) I:1.1 C1: 2.8 C2: 2.9
PV occlusion (%) Occlusive I: 57 Cl1: 63 Cl2: 51.4 Nonocclusive I: 43 Cl1: 37 Cl2: 48.6
Meld-score, Child-pugh score, INR: NR
Groups comparable at baseline? No |
DOAC
At least for 3 months |
Control 1: Warfarin Control 2: Enoxaparin
At least for 3 months |
Length of follow-up (mean number of months): Intervention: 28.1±11.3 Control1: 55.8±27.4 Control2: 33.0±18.9
Loss-to-follow-up: NR
Incomplete outcome data: NR
|
Major bleeding Defined as grade 3 or 4 bleeding, WHO. Intervention: 2/93 Control1: 26/108 Control2: 10/70 Effect measure (95%CI) I vs C1: 0.20 (0.05-0.86) P=.0307;
Mortality* Twelve patients (3.6%) died during follow-up. Three of these deaths were related to PVT (one on enoxaparin, one no AC and one warfarin).
*Study excluded patients < 3 months follow-up, and thus patients who may have died of acute complications of their initial PVT would not have been included.
Recurrence of SVT Recurrence of SVT was rare in this cohort, occurring in only 9/330 (2.7%) of patients. (not associated with type of AC).
Need for intervention 23% who developed chronic portal hypertensive symptoms received a TIPS. Frequency did not differ significantly among groups.
Complete radiographic resolution (CRR) Defined as complete radiographic resolution of PVT established on follow-up imaging
I:61/93 C1: 33/108 C2: 40/70 P=NR |
Authors conclusion This study should help establish the role of DOACs in the treatment of ncPVT. Given that more than half of the patients in this cohort had concurrent thrombosis of at least 1 other splanchnic vessel, our conclusions can likely be generalized to all ncSVT. These findings further the ongoing trend toward expanding the indications for DOACs across subtypes of venous thromboembolism, most recently exemplified by evidence favoring their use in cancer associated thrombosis, cerebral venous thrombosis, and among morbidly obese patients.Long-term outcomes among patients with ncPVT remain somewhat disappointing, and future studies should investigate the role of early thrombolysis and/or thrombectomy (in addition to AC), particularly among those patient groups most recalcitrant to AC alone (those with JAK2V617F, those with no evident predisposing factor for PVT, and those with occlusive thrombus at diagnosis).
Remarks
|
|
Ilcewicz (2021) |
Type of study: Retrospective cohort study
Setting and country: Single academic center, USA
Funding and conflicts of interest:
|
Inclusion criteria: Adults, admitted to academic medical center, from 2014 to 2018, initiated DOAC or warfarin for the treatment of a new PVT based on radiographic imaging.
Exclusion criteria: Receiving full-dose anticoagulation for any indication other than PVT, active hepatocarcinoma, and pregnancy.
N total at baseline: 33 Intervention: 13 Control: 20
Important prognostic factors2: Age (mean ± SD): I: 60 ± 18 C: 51 ± 12
Sex (M/F (%)) I: 69/31 C: 75/25
Admitting diagnosis Pancreatitis (%) I: 15 C: 20
GI-bleed (%) I: 8 C: 0
Abdominal pain (%) I: 46 C: 70
Cholecystitis (%) I: 23 C: 5
Cirrhosis (%) I: 38 C: 25
INR: I: 1.15 ± 0.6 C: 1.1 ± 0.2
Meld-score, PV occlusion and Child-pugh score: NR
Groups comparable at baseline? No |
DOAC for three months
Dosing Rivaroxaban 20 mg daily, N=5 (38%) Rivaroxaban 15 mg daily, N=1 (8%) Apixaban 2.5 mg BID, N=3 (23%) Apixaban 5 mg BID, N=4 (31%) |
Warfarin for three months |
Length of follow-up: 90 days
Loss-to-follow-up: NR
Incomplete outcome data: NR
|
Recurrent thrombo-embolic events Defined as VTE of typical locations, including peripheral deep vein thrombosis (DVT) and pulmonary embolism, new or worsened index PVT, and other atypical VTE I: 0 C: 4 (of which one worsening of index PVT, and 3 experienced new SVT)
Bleeding events Severity was determined according to ISTH I: 0 C: 1 (which fulfilled criteria of major bleeding) Effect measure (95%CI): NR P= NR
Of the 10 patients with previous diagnoses of cirrhosis, five patients received a DOAC, and the other five received warfarin. No patients with previous diagnoses of cirrhosis experienced a recurrent thrombo-embolic event or bleeding. |
Authors conclusion DOACs appear to be effective and safe in the treatment of PVT and prevention of worsening and recurrent VTE. Future studies with larger sample sizes are warranted to confirm DOACs’ efficacy and safety in the treatment of PVT, particularly in regard to optimal DOAC dosing regimens as well as in patients with Child-Pugh B and C cirrhosis.
Remarks
|
|
Kawata (2021) |
Type of study: Retrospective study
Setting and country: Single center, tertiary care, Canada
Funding and conflicts of interest: funding not reported, no COI
|
Inclusion criteria: Adults with newly diagnosed episode of SVT evaluated at the Thrombosis Clinic between 2007 and 2018. SVT was defined as any objectively documented thrombosis by imaging such as ultrasound (US), computed tomography (CT) or magnetic resonance imaging (MRI), including portal, superior/inferior mesenteric, hepatic, and/or splenic veins, or Budd Chiari syndrome
Exclusion criteria: NR
N total at baseline: 155 Intervention: NR Control: NR
Important prognostic factors2:
Sex (M/F): I 64/36: C: 59/41
Age (years±SD): I: 59±15 C: 55±15
Any local risk factors (%) I 74: C: 83
Local risk factors (%) NR for separate groups, only for total group. Malignancy: 31 Cirrhosis: 19.4 Abdominal infection: 12.9 IBD: 3.9 Abdominal surgery: 20.6 Liver transplantation: 5.2 Splenectomy: 4.5 Abdominal trauma: 1.3 Pancreatitis: 11 Cholecystitis: 5.2 Appendicitis: 2
Groups comparable at baseline? NR
|
DOAC |
LMWH or VKA |
Length of follow-up (median (IQR)): 9 (4-16) months
Loss-to-follow-up: NR
Incomplete outcome data: Total: N=22 for SVT status/progression
|
SVT progression (n/N (%)) Defined as extension into previously uninvolved vessels or increase in the length/volume of the clot in the same vessel.
I: 3/43 (7) C: 5/83 (6) Effect measure (95%CI): P= NR
Major bleeding (n/N (%)) Defined according to criteria of ISTH. I: 3/47 (6) C: 6/98 (6) Effect measure (95%CI): P>0.9999
Clinically relevant non-major bleeding (n/N (%)) Defined according to criteria of ISTH. I: 1/47 (2) C: 4/98 (4) Effect measure (95%CI):NR P>0.9999
SVT status Complete/partial resolution Defined as no evidence of thrombus in subsequent imaging) or objective reduction in the number of vessels involved or the length of the clot. I: 25/43 (58) C: 54/83 (65) P = 0.446
|
Authors conclusion In conclusion, our study results suggest that in SVT patient anticoagulation results in partial or complete thrombosis resolution in a significant proportion of patients with an acceptable bleeding risk and that outcomes are similar between patients with or without local risk factors.
Remarks Two patients received more than one DOAC. |
|
Naymagon (2021) |
Type of study: Retrospective study
Setting and country: Single center, tertiary care, USA
Funding and conflicts of interest: Non-commercial funding, no COI
|
Inclusion criteria: Patients with ICD code for PVT and cirrhosis, seen between 2000 and 2019.
Exclusion criteria: Splanchnic vein thrombosis without portal vein involvement, did not have cirrhosis, tumor thrombus, received interventional thrombolysis/ thrombectomy, lack of baseline imaging of PVT at diagnosis, lack of subsequent follow-up imaging at least 3 months following diagnosis, or seem to be chronic rather than acute PVT (e.g., had known prior history of PVT or had evidence of cavernous transformation or other radiographic features to suggest chronic PVT at the time of initial diagnosis), proceeded to orthotropic liver transplantation within 3 months of PVT diagnosis, or without interval re-imaging prior to surgery.
N total at baseline: 86 Intervention: 18 Control 1: 26 Control 2: 42
Important prognostic factors2: NR for separate groups, only for total group of 86 patients.
Sex (M/F (%)): 60.5/39.5 Age (median, IQR): 60 (54–67) Concurrent HCC (%): 15.1% MELD (median, IQR): 10 (7-13) Child-Pugh (%): A: 24.4, B: 48.8, C: 26.7 INR (median, IQR): 1.3 (1.2-1.4) Degree of PV occlusion (%): Occlusive 29.9: Non-occlusive: 70.1
Groups comparable at baseline? NR |
DOAC
Dosing Rivaroxaban 20 mg daily (following a 21 day loading period of 15 mg twice daily), Apixaban 5 mg twice daily (following a 7 day loading period of 10 mg twice daily), and Dabigatran 150 mg twice daily. |
Control 1: Warfarin Control 2: enoxaparin
Dosing Warfarin titrated to INR of 2–3 Enoxaparin 1 mg/kg twice daily |
Length of follow-up (median, IQR) 21 (11–44) – total group AC
Loss-to-follow-up: Patients were followed from time of diagnosis until the end of the study period, or until they were lost to follow up within the health system
Incomplete outcome data: NR
|
Major bleeding (n/N (%)) Defined according to criteria of ISTH. I: 3/18 (16.7) C: 5/26 (19.2) C2: 9/42 (21.4) Effect measure (95%CI):NR P=NR
PVT extension (n/N (%)) Not defined I: 1/18 (5.6) C1: 4/26 (15.4) C2: 8/42 (19.1) Effect measure (95%CI):NR P=NR
Mortality (n/N (%)) I: 3/18 (16.7) C1: 4/26 (15.4) C2: 8/42 (19.1) Effect measure (95%CI): NR P=NR
Complete radiographic resolution (CRR) Defined as CRR established at follow-up imaging
I: 10/18 (56) C1: 15/26 (58) C2: 16/42 (38) P=NR
|
Authors conclusion AC appeared safe in this large cohort of patients with cirrhotic PVT and was associated with higher rates of CRR and RCO, albeit without a significant improvement in OS. More data are needed to clarify whether AC may be associated with improvement in clinical outcomes such as decompensation of cirrhosis, eventual liver transplantation, and mortality. Prospective clinical trials will be needed to effectively evaluate such outcomes.
Remarks
|
|
Naymagon (2021_IBD) |
Type of study: Retrospective study
Setting and country: Single center, tertiary care, USA
Funding and conflicts of interest: No funding, no COI
|
Inclusion criteria: Patients with an ICD code for PVT seen between 2000 – 2019, concurrent history of IBD, medical records confirmed a history of acute PVT, with or without concurrent thrombosis in additional splanchnic vessels.
Exclusion criteria: Tumor thrombus, receipt of thrombolysis/ thrombectomy, absence of baseline imaging at PVT diagnosis, absence of subsequent follow-up imaging 3 or more months after diagnosis, and evidence of chronic as opposed to acute PVT at diagnosis (eg, known clinical history of long-standing PVT, or presence of portal cavernoma, portal collaterals, or other radiographic findings suggestive of chronic PVT at diagnosis).
N total at baseline: 58 Intervention: 23 Control1:22 Control2:13
Important prognostic factors2: Age (median (IQR)): I: 42 (29-53) C1: 43 (33-54) C2: 44 (32-53)
Sex (M/F (%)): I: 73.9/26.1 C1: 46.2/53.8 C2: 63.6/36.4
Type of IBD (%) UC I: 65.2 C1: 54.5 C2: 69.2 CD I: 34.8 C1: 45.5 C2: 30.8
Degree of PVT occlusion Occlusive I: 30.4 C1: 36.4 C2: 38.5 Nonocclusive I: 69.6 C1: 63.6 C2: 61.5
Meld-score, Child-pugh score, INR: NR
Groups comparable at baseline? No
|
DOAC |
Control1: Warfarin Control2: Enoxaparin |
Length of follow-up: I: 12 (6-35) C1: 43 (9-80) C2: 23 (10-58)
Loss-to-follow-up: NR
Incomplete outcome data: NR
|
Major bleeding Defined as WHO grade 3 or 4 I: 0/18 C1: 3/22 C2: 1/13
Mortality Two patients died during follow-up, with 1 death a direct complication of PVT (this person was treated with warfarin).
Need for intervention One of the 3 patients (N=2 on enoxaparin and N=1 on warfarin), who developed SPH went on to receive a transjugular intrahepatic portosystemic shunt with subsequent improvement in portal hypertensive symptoms
Complete radiographic resolution (CRR) Definition not reported
I: 22/23 (96) C1: 12/22 (55) C2: 10/13 (77) P=NR |
Authors conclusion This study demonstrates that the use of AC may potentially lead to excellent outcomes in IBD-associated PVT. Administered DOACs were associated with particularly favorable outcomes among such patients in this retrospective study and were in particular associated with favorable efficacy and safety profiles relative to warfarin. Rates of recurrent VTE were low, and such patients may likely discontinue AC following at least 3 months of treatment, assuming that follow-up imaging shows resolution of PVT. The incidence of clinically meaningful results in thrombophilia testing was low, and such testing need not be routinely sent among this patient population. Further studies, both prospective and retrospective, would be helpful to confirm these findings.
Remarks
|
|
Zhang (2023) |
Type of study: Retrospective study
Setting and country: Single center, liver disease center, China
Funding and conflicts of interest: No funding, no COI
|
Inclusion criteria:
liver cirrhosis was diagnosed according to the criteria of the JSGE and (3) PVT was diagnosed by abdominal Doppler ultrasound, MRI and CT.
Exclusion criteria: 1)malignant related PVT; (2) isolate splenic or mesenteric venous thrombosis; (3) those receiving non-anticoagulant treatment such TIPS, antithrombotic, thrombolysis, or thrombectomy during liver transplantation; (4) platelet count < 10 × 109/L; (5) creatinine clearance ≤30 mL/min; (6) primary thrombophilia; (7) Budd-Chiari syndrome; (8) pregnancy or breast-feeding women; (9) severe cardiopulmonary diseases; (10) cases without imaging followup information
N total at baseline: 77 of which 27 were using anticoagulants. Only 24 patients were included in the analysis (DOAC vs warfarin).
Intervention: 18 Control: 6
Important prognostic factors2: NR for separate groups, only for total group of 27 patients
Age (mean±SD): 60.4 ± 12.3
Sex (M/F (%)): 67
Meld-score (mean±SD): 5.2 ± 4.0
Etiology (%) HBV: 37 PBC: 11 Alcohol 26: NASH: 7 Drug: 4 Other: 15
Malignancies (HCC): 7
PV-occlusion (%) Occlusive: 4 Non-occlusive: 96
INR (mean±SD): 1.2 ± 0.1
Child-pugh score: NR
Groups comparable at baseline? NR
|
DOAC
Dosing rivaroxaban 20 mg qd (n = 3), rivaroxaban 10 mg qd (n = 14), edoxaban 30 mg qd (n = 1). |
Warfarin
Dosing INR target level of 1.5–2.5 |
Length of follow-up (median): 28.5 months – total group anticoagulants
Loss-to-follow-up: In case of loss to follow-up, patients were followed until the last record within our health system.
Incomplete outcome data: NR
|
Major bleeding According to the criteria of the ISTH I: 1/18 C: 0/6 Effect measure (95%CI): NR P= 1.000
PVT recanalization Defined as both complete and partial recanalization. Complete recanalization referred to the complete disappearance of the thrombus and partial recanalization to more than 50% reduction of the thrombus
I: NR C: NR HR (95%CI): 4.045 (0.52-37.67)
|
Authors conclusion In conclusion, anticoagulant therapy could increase the rate of PVT recanalization without increasing the rate of bleeding in patients with liver cirrhosis and could reduce the rate of variceal bleeding. Compared with the non-anticoagulant group, anticoagulant therapy may be beneficial to the liver function of patients with cirrhotic PVT. There was no significant difference in the safety and efficacy of different anticoagulants in the treatment of cirrhotic PVT. Further studies are needed to optimize the use of anticoagulants in patients with cirrhotic PVT.
Remarks
|
Risk of bias table for interventions studies (cohort studies based on risk of bias tool by the CLARITY Group at McMaster University)
|
Author, year |
Selection of participants
Was selection of exposed and non-exposed cohorts drawn from the same population?
|
Exposure
Can we be confident in the assessment of exposure?
|
Outcome of interest
Can we be confident that the outcome of interest was not present at start of study? |
Confounding-assessment
Can we be confident in the assessment of confounding factors? |
Confounding-analysis
Did the study match exposed and unexposed for all variables that are associated with the outcome of interest or did the statistical analysis adjust for these confounding variables? |
Assessment of outcome
Can we be confident in the assessment of outcome?
|
Follow up
Was the follow up of cohorts adequate? In particular, was outcome data complete or imputed? |
Co-interventions
Were co-interventions similar between groups? |
Overall Risk of bias
|
|
|
|
|
|
|
|
|
|
|
|
|
Naymagon (2020) |
Probably no
Reason: Time frame was different between the groups and there might have been indication bias. Patients with short follow-up were excluded, thus patients who may have died of acute complication of PVT were not included. |
Probably yes
Reason: No information (initial long-term AC used in each instance was recorded), but it is most likely that exposure is determined correctly. |
Definitely yes
Reason: mortality was logically not present at the start of the study, for the outcome measure major bleeding it is also not likely.
|
Probably yes
Reason: radiological reports were used; for other confounders it is not sure how they were assessed.
|
Definitely no
Reason: analysis was adjusted for confounding, but high risk on residual confounding
|
Probably no
Reason: due to the retrospective nature of the study, it is possible that bleeding events were missed.
|
Probably no
Reason: Follow-up period differed significantly between the groups. Loss to follow-up is not reported.
|
No information
|
High (all outcomes)
|
|
Ilcewicz (2021) |
Probably no
Reason: Time frame might be different between the groups, there might have been indication bias. |
Probably yes
Reason: No information, but it is most likely that exposure is determined correctly. |
Definitely yes
Reason: recurrent thrombo-embolic event was logically not present at the start of the study, for the outcome measure major bleeding it is also not likely.
|
No information
|
Definitely no
Reason: no adjustment for confounding
|
Probably no
Reason: due to retrospective nature of the study, it is possible that bleeding events were missed. Besides, there was no routine imaging and therefore worsening or recurrence of PVT might have been underestimated. |
Probably no
Reason: follow-up time might be short, especially for the outcome measure PVT recurrence. Loss to follow-up is not reported. |
Probably no
Reason: Groups differed significantly with regard to concomitant medications (e.g. antiplatelet, beta-blocker, PPI and NSAID), which were, but NSAIDS, more frequently used in DOAC group. |
High (all outcomes) |
|
Kawata (2021) |
Probably no
Reason: Time frame might be different between the groups, there might have been indication bias. |
Probably yes
Reason: No information, but it is most likely that exposure is determined correctly (i.e. initial anticoagulant therapy). |
Definitely yes
Reason: progression of SVT was logically not present at the start of the study, for the outcome measure bleeding it is also not likely.
|
Probably yes
Reason: imaging reports were used; for other confounders it is not sure how they were assessed.
|
Definitely no
Reason: no adjustment for confounding
|
Probably no
Reason: Due to retrospective nature of the study, it is possible that bleeding events were missed. |
No information
Reason: Follow-up period was not reported by anticoagulant group. Loss to follow-up is not reported. Imaging was performed at 3, 6, and between 6 and 24 months, with a minimum observation period of 6 months. Result at the last assessment was reported. |
No information
|
High (all outcomes |
|
Naymagon (2021) |
Probably no
Reason: Time frame might be different between the groups, there might have been indication bias. Patients with short follow-up (imaging < 3 months) were excluded, thus patients who may have died of acute complication of PVT were not included. |
Probably yes
Reason: No information (initial long-term AC used in each instance was recorded), but it is most likely that exposure is determined correctly. |
Definitely yes
Reason: PVT extension and mortality was logically not present at the start of the study, for the outcome measure major bleeding it is also not likely.
|
Probably yes
Reason: radiological reports were used; for other confounders it is not sure how they were assessed.
|
Definitely no
Reason: no adjustment for confounding
|
Probably no
Reason: due to retrospective nature of the study, it is possible that bleeding events were missed. |
No information
Reason: Follow-up period was not reported by anticoagulatn group. Loss to follow-up is not reported. Follow-up intervals (imaging) were not standardized. |
No information
|
High (all outcomes) |
|
Naymagon (2021 -–B) |
Probably no
Reason: Time frame might be different between the groups, there might have been indication bias. Patients with short follow-up (imaging < 3 months) were excluded, thus patients who may have died of acute complication of PVT were not included. |
Probably yes
Reason: No information (initial long-term AC used in each instance was recorded), but it is most likely that exposure is determined correctly. |
Definitely yes
Reason: It is not likely that the outcome measure major bleeding was present at the start of the study.
|
Probably yes
Reason: radiological reports were used; for other confounders it is not sure how they were assessed.
|
Definitely no
Reason: no adjustment for confounding
|
Probably no
Reason: due to retrospective nature of the study, it is possible that bleeding events were missed. |
Probably no
Reason: length of follow-up differed between the groups. Loss to follow-up is not reported. Follow-up intervals (imaging) were not standardized. |
No information
|
High (all outcomes) |
|
Zhang (2023) |
Probably no
Reason: Time frame might be different between the groups, there might have been indication bias. |
Probably yes
Reason: Information was collected from medical records, and it is most likely that exposure is determined correctly. |
Definitely yes
Reason: It is not likely that the outcome measure major bleeding was present at the start of the study.
|
Probably yes
Reason: Information was collected from medical records, and confounders might be determined correctly |
Definitely no
Reason: No adjustment for confounding
|
Probably no
Reason: Due to retrospective nature of the study, it is possible that bleeding events are missed. |
No information
Reason: Follow-up period was not reported by anticoagulatn group. Loss to follow-up is not reported. |
No information |
High (all outcomes) |
Table of excluded studies
Search I – Systematic reviews and RCTs
|
Reference |
Reason for exclusion |
|
Ding H, Zhang Y, Zhao L, Wu S, Liu J, Wang C, Pei T, Su Y. What intervention regimen is most effective prevention for Portal venous system thrombosis after splenectomy in cirrhotics patients with Portal hypertension? Systematic review and network meta-analysis. Pharmacol Res. 2020 Jul;157:104825. doi: 10.1016/j.phrs.2020.104825. Epub 2020 Apr 21. PMID: 32330553. |
Wrong comparison |
|
Yao W, Feng Y, Liu T, Li W, Zhang M, Yao Y, Wu S. Rivaroxaban versus low-molecular weight heparin plus warfarin prevents portal vein system thrombosis after splenectomy and pericardial devascularization: A randomized clinical trial. EXCLI J. 2021 Mar 4;20:537-549. doi: 10.17179/excli2020-3120. PMID: 33883982; PMCID: PMC8056059. |
Wrong population, wrong outcome |
|
Yao C, Zhao M, Ibrahim B, Saab S. Anticoagulation for the Treatment of Portal Vein Thrombosis in Cirrhosis: A Systematic Review and Meta-Analysis of Comparative Studies. J Clin Exp Hepatol. 2023 May-Jun;13(3):404-413. doi: 10.1016/j.jceh.2022.12.016. Epub 2023 Jan 3. PMID: 37250883; PMCID: PMC10213860. |
Wrong comparison (anticoagulation vs no treatment) |
|
Li Z, Xu W, Wang L, Chai L, Ageno W, Romeiro FG, Li H, Qi X. Risk of Bleeding in Liver Cirrhosis Receiving Direct Oral Anticoagulants: A Systematic Review and Meta-analysis. Thromb Haemost. 2023 Jun 19. doi: 10.1055/s-0043-1770100. Epub ahead of print. PMID: 37336474. |
Wrong population |
|
Guerrero A, Campo LD, Piscaglia F, Scheiner B, Han G, Violi F, Ferreira CN, Téllez L, Reiberger T, Basili S, Zamora J, Albillos A; Baveno Cooperation: an EASL consortium. Anticoagulation improves survival in patients with cirrhosis and portal vein thrombosis: The IMPORTAL competing-risk meta-analysis. J Hepatol. 2023 Jul;79(1):69-78. doi: 10.1016/j.jhep.2023.02.023. Epub 2023 Feb 28. PMID: 36858157. |
Wrong comparison (anticoagulation vs no treatment) |
|
Candeloro M, Valeriani E, Monreal M, Ageno W, Riva N, Schulman S, Bang SM, Mellado M, Díaz-Peromingo JA, Moisés J, Díaz-Brasero AM, Garcia-Pagan JC, Perez-Campuzano V, Senzolo M, De Gottardi A, Di Nisio M. Clinical course and treatment of incidentally detected splanchnic vein thrombosis: an individual patient data meta-analysis. J Thromb Haemost. 2023 Jun;21(6):1592-1600. doi: 10.1016/j.jtha.2023.03.002. Epub 2023 Mar 11. PMID: 36907381. |
Wrong comparison |
|
Zhang Z, Zhao Y, Han B, Zhu Z, Sun L, Cui X. The Efficacy and Safety of Anticoagulants in the Treatment of Cirrhotic Portal Vein Thrombosis: A Systematic Review and Meta-Analysis. Clin Appl Thromb Hemost. 2022 Jan-Dec;28:10760296221104797. doi: 10.1177/10760296221104797. PMID: 35656719; PMCID: PMC9168872. |
Incomplete search strategy/search strategy is very specific |
|
Koh JH, Liew ZH, Ng GK, Liu HT, Tam YC, De Gottardi A, Wong YJ. Efficacy and safety of direct oral anticoagulants versus vitamin K antagonist for portal vein thrombosis in cirrhosis: A systematic review and meta-analysis. Dig Liver Dis. 2022 Jan;54(1):56-62. doi: 10.1016/j.dld.2021.07.039. Epub 2021 Aug 13. PMID: 34393072. |
Wrong population |
|
Candeloro M, Valeriani E, Monreal M, Ageno W, Riva N, Lopez-Reyes R, Peris ML, Beyer Westendorf J, Schulman S, Rosa V, López-Núñez JJ, Garcia-Pagan JC, Magaz M, Senzolo M, De Gottardi A, Di Nisio M. Anticoagulant therapy for splanchnic vein thrombosis: an individual patient data meta-analysis. Blood Adv. 2022 Aug 9;6(15):4516-4523. doi: 10.1182/bloodadvances.2022007961. PMID: 35613465; PMCID: PMC9636325. |
Missing information on included studies (subgroup analysis on DOAC) |
|
Valeriani E, Di Nisio M, Riva N, Cohen O, Porreca E, Senzolo M, De Gottardi A, Magaz M, Garcia-Pagan JC, Ageno W. Anticoagulant Treatment for Splanchnic Vein Thrombosis in Liver Cirrhosis: A Systematic Review and Meta-Analysis. Thromb Haemost. 2021 Jul;121(7):867-876. doi: 10.1055/s-0040-1722192. Epub 2021 Feb 1. PMID: 33525037. |
Included case series |
|
Ng CH, Tan DJH, Nistala KRY, Syn N, Xiao J, Tan EXX, Woo FZ, Chew NWS, Huang DQ, Dan YY, Sanyal AJ, Muthiah MD. A network meta-analysis of direct oral anticoagulants for portal vein thrombosis in cirrhosis. Hepatol Int. 2021 Oct;15(5):1196-1206. doi: 10.1007/s12072-021-10247-x. Epub 2021 Aug 21. PMID: 34417718. |
Included retracted RCT of Hanafy, no update possible |
|
Ghazaleh S, Beran A, Aburayyan K, Nehme C, Patel D, Khader Y, Sharma S, Aziz M, Abdel-Aziz Y, Hammad T, Nawras A. Efficacy and safety of anticoagulation in non-malignant portal vein thrombosis in patients with liver cirrhosis: a systematic review and meta-analysis. Ann Gastroenterol. 2021;34(1):104-110. doi: 10.20524/aog.2020.0544. Epub 2020 Oct 2. PMID: 33414629; PMCID: PMC7774659. |
Wrong comparison |
|
Qi X, De Stefano V, Li H, Dai J, Guo X, Fan D. Anticoagulation for the treatment of portal vein thrombosis in liver cirrhosis: a systematic review and meta-analysis of observational studies. Eur J Intern Med. 2015 Jan;26(1):23-9. doi: 10.1016/j.ejim.2014.12.002. Epub 2015 Jan 5. PMID: 25566699. |
Wrong comparison |
|
Semmler G, Lindorfer A, Schäfer B, Bartl S, Hametner-Schreil S, Gensluckner S, Balcar L, Pomej K, Lampichler K, Trauner M, Aigner E, Datz C, Zoller H, Hofer H, Schöfl R, Mandorfer M, Reiberger T, Scheiner B. Outcome of Budd-Chiari Syndrome Patients Treated With Direct Oral Anticoagulants: An Austrian Multicenter Study. Clin Gastroenterol Hepatol. 2023 Apr;21(4):978-987.e2. doi: 10.1016/j.cgh.2022.04.024. Epub 2022 May 6. PMID: 35533994. |
Retrospective study, insufficient information on the control group |
|
Sharma S, Kumar R, Rout G, Gamanagatti SR, Shalimar. Dabigatran as an oral anticoagulant in patients with Budd-Chiari syndrome post-percutaneous endovascular intervention. J Gastroenterol Hepatol. 2020 Apr;35(4):654-662. doi: 10.1111/jgh.14843. Epub 2019 Nov 25. PMID: 31476024. |
Included in Valeriani, 2021 |
|
Salim S, Ekberg O, Elf J, Zarrouk M, Gottsäter A, Acosta S. Evaluation of direct oral anticoagulants and vitamin K antagonists in mesenteric venous thrombosis. Phlebology. 2019 Apr;34(3):171-178. doi: 10.1177/0268355518779517. Epub 2018 May 31. PMID: 29848218. |
Switch over study |
|
Hanafy AS, Abd-Elsalam S, Dawoud MM. Randomized controlled trial of rivaroxaban versus warfarin in the management of acute non-neoplastic portal vein thrombosis. Vascul Pharmacol. 2019 Feb;113:86-91. doi: 10.1016/j.vph.2018.05.002. Epub 2018 Jun 7. Retraction in: Vascul Pharmacol. 2023 Jan 12;:107142. PMID: 29886103. |
Retracted article |
|
Janczak DT, Mimier MK, McBane RD, Kamath PS, Simmons BS, Bott-Kitslaar DM, Lenz CJ, Vargas ER, Hodge DO, Wysokinski WE. Rivaroxaban and Apixaban for Initial Treatment of Acute Venous Thromboembolism of Atypical Location. Mayo Clin Proc. 2018 Jan;93(1):40-47. doi: 10.1016/j.mayocp.2017.10.007. Epub 2017 Dec 6. PMID: 29217335. |
Wrong population |
|
Yin Y, Wang L, Gao F, Liu L, Qi X. Anticoagulation Therapy for Splanchnic Vein Thrombosis Associated With Acute Pancreatitis: A Systematic Review and Meta-Analysis. Clin Appl Thromb Hemost. 2023 Jan-Dec;29:10760296231188718. doi: 10.1177/10760296231188718. PMID: 37461391; PMCID: PMC10357047. |
Wrong comparison |
|
Sissingh NJ, Groen JV, Koole D, Klok FA, Boekestijn B, Bollen TL, van Santvoort HC, Verdonk RC, Bonsing BA, van Eijck CHJ, van Hooft JE, Mieog JSD; Dutch Pancreatitis Study Group. Therapeutic anticoagulation for splanchnic vein thrombosis in acute pancreatitis: A systematic review and meta-analysis. Pancreatology. 2022 Mar;22(2):235-243. doi: 10.1016/j.pan.2021.12.008. Epub 2021 Dec 22. PMID: 35012902. |
Wrong comparison |
|
Ghazaleh S, Chuang J, Sayeh W, Iqbal A, Beran A, Khader Y, Burmeister C, Aziz M, Assaly R, Nawras A. Comparative Efficacy of Anticoagulant Medications in Nonmalignant Portal Vein Thrombosis in Liver Cirrhosis-A Systematic Review and Network Meta-analysis. Am J Ther. 2022 Jul 8. doi: 10.1097/MJT.0000000000001538. Epub ahead of print. PMID: 36927678. |
Wrong publication type |
|
Anis FS, Adiamah A, Lobo DN, Sanyal S. Incidence and treatment of splanchnic vein thrombosis in patients with acute pancreatitis: A systematic review and meta-analysis. J Gastroenterol Hepatol. 2022 Mar;37(3):446-454. doi: 10.1111/jgh.15711. Epub 2021 Nov 3. PMID: 34657310. |
Wrong comparison |
|
Wang L, Guo X, Xu X, De Stefano V, Plessier A, Noronha Ferreira C, Qi X. Anticoagulation Favors Thrombus Recanalization and Survival in Patients With Liver Cirrhosis and Portal Vein Thrombosis: Results of a Meta-Analysis. Adv Ther. 2021 Jan;38(1):495-520. doi: 10.1007/s12325-020-01550-4. Epub 2020 Nov 5. PMID: 33155180; PMCID: PMC7854392. |
Missing studies compared to other systematic reviews |
|
Gupta S, Hidalgo J, Singh B, Iyer A, Yang Y, Short A, Singh S, Bhatt H, Gupta S. Usage of Direct Acting Oral Anticoagulants in Cirrhotic and Non-Cirrhotic Portal Vein Thrombosis: A Systematic Review. Cureus. 2021 Aug 5;13(8):e16922. doi: 10.7759/cureus.16922. PMID: 34367844; PMCID: PMC8342267. |
No meta-analysis |
|
Coons EM, Staubes BA, Casey AL, Elagizi-Youssef SA, Mohammed AE, Sharma N, Kline ER. Direct Oral Anticoagulants Versus Warfarin for Treatment of Thrombosis or Atrial Fibrillation in Patients With Cirrhosis: A Retrospective Cohort Study. Ann Pharmacother. 2022 May;56(5):533-540. doi: 10.1177/10600280211025050. Epub 2021 Sep 1. PMID: 34470525. |
No meta-analysis |
|
Gao Y, Liu H, Tang F, Zhang X, Li F, Ye Q, Yuan H, Lv H, Han T. Efficacy and safety of anticoagulants in liver cirrhosis patients with portal vein thrombosis: A meta-analysis. Clin Res Hepatol Gastroenterol. 2021 Mar;45(2):101649. doi: 10.1016/j.clinre.2021.101649. Epub 2021 Feb 16. PMID: 33601064. |
Wrong comparison |
|
Dong S, Qi H, Li Y, Men P, Alifu M, Zhang Y, Li Y, Zhao R. A systematic review and meta-analysis of anticoagulation therapy for portal vein thrombosis in patients with cirrhosis: to treat or not to treat? Hepatol Int. 2021 Dec;15(6):1356-1375. doi: 10.1007/s12072-021-10233-3. Epub 2021 Sep 6. PMID: 34487316. |
Wrong comparison |
|
Chen H, Lei J, Liang S, Luo G, Deng M, Lü M. Safety and Efficacy of Anticoagulation in Patients with Cirrhosis: A Meta-Analysis. Can J Gastroenterol Hepatol. 2021 Apr 21;2021:8859602. doi: 10.1155/2021/8859602. PMID: 34007837; PMCID: PMC8102101. |
No complete information on search strategy |
|
Mohan BP, Aravamudan VM, Khan SR, Ponnada S, Asokkumar R, Adler DG. Treatment response and bleeding events associated with anticoagulant therapy of portal vein thrombosis in cirrhotic patients: Systematic review and meta-analysis. Ann Gastroenterol. 2020 Sep-Oct;33(5):521-527. doi: 10.20524/aog.2020.0503. Epub 2020 May 30. PMID: 32879600; PMCID: PMC7406805. |
Included studies which were also included in Zhang (2022), additional study was of Scheiner (no-comparative study) and did not include Ilzewics. |
|
Di Nisio M, Valeriani E, Riva N, Schulman S, Beyer-Westendorf J, Ageno W. Anticoagulant therapy for splanchnic vein thrombosis: ISTH SSC Subcommittee Control of Anticoagulation. J Thromb Haemost. 2020 Jul;18(7):1562-1568. doi: 10.1111/jth.14836. PMID: 32619346. |
Guidance document |
|
Priyanka P, Kupec JT, Krafft M, Shah NA, Reynolds GJ. Newer Oral Anticoagulants in the Treatment of Acute Portal Vein Thrombosis in Patients with and without Cirrhosis. Int J Hepatol. 2018 Jun 5;2018:8432781. doi: 10.1155/2018/8432781. PMID: 29973997; PMCID: PMC6008786. |
Narrative review |
|
Hoolwerf EW, Kraaijpoel N, Büller HR, van Es N. Direct oral anticoagulants in patients with liver cirrhosis: A systematic review. Thromb Res. 2018 Oct;170:102-108. doi: 10.1016/j.thromres.2018.08.011. Epub 2018 Aug 17. PMID: 30153564. |
Wrong population |
|
Zhang W, Zhou DM, Li Y. [Clinical effect of low-molecular-weight heparin in prevention and treatment of liver cirrhosis and portal vein thrombosis after splenectomy: a systematic review and meta-analysis]. Zhonghua Gan Zang Bing Za Zhi. 2016 Oct 20;24(10):732-737. Chinese. doi: 10.3760/cma.j.issn.1007-3418.2016.10.004. PMID: 27938557. |
Wrong language |
|
Lv Y, Bai W, Li K, Wang Z, Guo W, Luo B, Wang J, Wang Q, Wang E, Xia D, Li X, Yuan J, Han N, Niu J, Yin Z, Fan D, Han G. Anticoagulation and Transjugular Intrahepatic Portosystemic Shunt for the Management of Portal Vein Thrombosis in Cirrhosis: A Prospective Observational Study. Am J Gastroenterol. 2021 Jul 1;116(7):1447-1464. doi: 10.14309/ajg.0000000000001194. Erratum in: Am J Gastroenterol. 2022 Jan 1;117(1):200. PMID: 33630766. |
Wrong intervention/comparison |
|
Li A, Zhang MC, Li P, Eshaghpour A, Li K, Carrier M, Wells P, Crowther MA. Direct oral anticoagulants for the treatment of splanchnic vein thrombosis - A systematic review and meta-analysis. Thromb Res. 2023 Sep;229:209-218. doi: 10.1016/j.thromres.2023.06.003. Epub 2023 Jun 20. PMID: 37544136. |
Incomplete search strategy |
|
Tang K, Weinberg EM. Direct oral anticoagulants in the treatment of portal vein thrombosis in patients with portal hypertension. Clin Liver Dis (Hoboken). 2023 Jul 10;22(2):37-41. doi: 10.1097/CLD.0000000000000063. PMID: 37663556; PMCID: PMC10473309. |
No systematic review with meta-analysis, wrong publication type |
Search II – Observational studies
|
Reference |
Reason for exclusion |
|
Bergère M, Erard-Poinsot D, Boillot O, Valette PJ, Guillaud O, Chambon-Augoyard C, Dumortier J. Portal vein thrombosis and liver cirrhosis: Long-term anticoagulation is effective and safe. Clin Res Hepatol Gastroenterol. 2019 Aug;43(4):395-402. doi: 10.1016/j.clinre.2018.11.011. Epub 2018 Dec 18. PMID: 30578107. |
wrong comparison |
|
Serrao A, Merli M, Lucani B, Aprile F, Fiori L, Gioia S, Breccia M, Riggio O, Chistolini A. Outcomes of long-term anticoagulant treatment for the secondary prophylaxis of splanchnic venous thrombosis. Eur J Clin Invest. 2021 Jan;51(1):e13356. doi: 10.1111/eci.13356. Epub 2020 Aug 11. PMID: 33180323. |
wrong study population, wrong comparison |
|
Pettinari I, Vukotic R, Stefanescu H, Pecorelli A, Morelli M, Grigoras C, Sparchez Z, Andreone P, Piscaglia F; BO-LIVES (BOlogna LIVEr vascular Studies). Clinical Impact and Safety of Anticoagulants for Portal Vein Thrombosis in Cirrhosis. Am J Gastroenterol. 2019 Feb;114(2):258-266. doi: 10.1038/s41395-018-0421-0. PMID: 30538290. |
wrong comparison |
|
K T, Chan SJ, Varghese C, Lim WB, Cheemungtoo GM, Akter N, Nayar M, Pandanaboyana S. A selective anticoagulation policy for splanchnic vein thrombosis in acute pancreatitis is associated with favourable outcomes: experience from a UK tertiary referral centre. HPB (Oxford). 2022 Nov;24(11):1937-1943. doi: 10.1016/j.hpb.2022.06.003. Epub 2022 Jun 16. PMID: 35786365. |
wrong comparison |
|
Saleh S, Dalal S, Desai A, Thomas C, Chitsaz E. Outcomes of Anticoagulation in Patients With Splanchnic Vein Thrombosis From Acute Pancreatitis: A Population-Based Nationwide Retrospective Cohort Study. Pancreas. 2022 Sep 1;51(8):e105-e106. doi: 10.1097/MPA.0000000000002122. PMID: 36607956. |
wrong publication type |
|
Acuna-Villaorduna A, Tran V, Gonzalez-Lugo JD, Azimi-Nekoo E, Billett HH. Natural history and clinical outcomes in patients with portal vein thrombosis by etiology: A retrospective cohort study. Thromb Res. 2019 Feb;174:137-140. doi: 10.1016/j.thromres.2018.12.019. Epub 2018 Dec 27. PMID: 30597344. |
wrong comparison |
|
Barbui T, De Stefano V, Carobbio A, Iurlo A, Alvarez-Larran A, Cuevas B, Ferrer Marín F, Vannucchi AM, Palandri F, Harrison C, Sibai H, Griesshammer M, Bonifacio M, Elli EM, Trotti C, Koschmieder S, Carli G, Benevolo G, Ianotto JC, Goel S, Falanga A, Betti S, Cattaneo D, Arellano-Rodrigo E, Mannelli L, Vianelli N, Doyle A, Gupta V, Wille K, Tremblay D, Mascarenhas J. Direct oral anticoagulants for myeloproliferative neoplasms: results from an international study on 442 patients. Leukemia. 2021 Oct;35(10):2989-2993. doi: 10.1038/s41375-021-01279-1. Epub 2021 May 19. PMID: 34012132; PMCID: PMC8132485. |
wrong publication type |
|
Naymagon L, Tremblay D, Mascarenhas J, Schiano T. Characteristics, anticoagulation, and outcomes of portal vein thrombosis after intra-abdominal surgery. Surgery. 2021 May;169(5):1175-1181. doi: 10.1016/j.surg.2020.11.016. Epub 2020 Dec 24. PMID: 33358635. |
wrong outcomes |
|
Hajibandeh S, Hajibandeh S, Agrawal S, Irwin C, Obeidallah R, Subar D. Anticoagulation Versus No Anticoagulation for Splanchnic Venous Thrombosis Secondary to Acute Pancreatitis: Do We Really Need to Treat the Incidental Findings? Pancreas. 2020 Oct;49(9):e84-e85. doi: 10.1097/MPA.0000000000001644. PMID: 33003093. |
wrong publication type |
|
Noronha Ferreira C, Cortez-Pinto H, Serejo F, Velosa J, Marinho RT. Anticoagulation in patients with cirrhosis and portal vein thrombosis: Safety and beneficial effect on OLT-free survival. Liver Int. 2019 Oct;39(10):2002. doi: 10.1111/liv.14231. Epub 2019 Sep 18. PMID: 31461802. |
wrong publication type |
|
Andraska E, Haga L, Reitz K, Li X, Ramos R, Avgerinos E, Singh M, Eslami M, Makaroun M, Chaer R. Acute superior mesenteric venous thrombosis results in high rates of readmission and morbidity. J Vasc Surg Venous Lymphat Disord. 2020 Sep;8(5):748-755. doi: 10.1016/j.jvsv.2020.01.007. Epub 2020 Mar 3. PMID: 32139329; PMCID: PMC7434641. |
wrong comparison |
Verantwoording
Beoordelingsdatum en geldigheid
Publicatiedatum : 22-10-2025
Beoordeeld op geldigheid : 23-09-2025
Algemene gegevens
Voor meer details over de gebruikte richtlijnmethodologie verwijzen wij u naar de Werkwijze. Relevante informatie voor de ontwikkeling/herziening van deze richtlijnmodule is hieronder weergegeven.
De ontwikkeling/herziening van deze richtlijnmodule werd ondersteund door het Kennisinstituut van de Federatie Medisch Specialisten (www.demedischspecialist.nl/kennisinstituut) en werd gefinancierd uit de Stichting Kwaliteitsgelden Medisch Specialisten (SKMS). Patiëntenparticipatie bij deze richtlijn werd medegefinancierd uit de Kwaliteitsgelden Patiënten Consumenten (SKPC) binnen het programma KIDZ. De financier heeft geen enkele invloed gehad op de inhoud van de richtlijnmodule.
Samenstelling werkgroep
Voor het ontwikkelen van de richtlijnmodule is in 2021 een multidisciplinaire werkgroep ingesteld, bestaande uit vertegenwoordigers van alle relevante specialismen (zie hiervoor de Samenstelling van de werkgroep) die betrokken zijn bij de zorg voor patiënten die antitrombotische therapie dan wel tromboseprofylaxe gebruiken.
Kerngroep
- Prof. dr. M.V. (Menno) Huisman, internist-vasculaire geneeskunde, LUMC, NIV (voorzitter)
- Dr. M.J.H.A. (Marieke) Kruip, internist-hematoloog, Erasmus MC, NIV, NVVH (Nederlandse Vereniging voor Hematologie)
- Prof. Dr. F.A. (Erik) Klok, internist-vasculaire geneeskunde, LUMC, NIV
- Dr. J. (Jenneke) Leentjens, internist-vasculaire geneeskunde, RadboudUMC, NIV (vanaf 2023)
- Dr. N. (Nick) van Es, internist-vasculaire geneeskunde, Amsterdam UMC, NIV (vanaf 2023)
- Dr. M.A. (Marc) Brouwer, cardioloog, RadboudUMC, NVVC
- Dr. H.B. (Harmen) Ettema, orthopedisch chirurg, Isala, NOV
- Dr. B. (Banne) Nemeth, aios orthopedie, LUMC, NOV
- Dr. A.M. (Arno) Wiersema, vaatchirurg, Dijklander Ziekenhuis, NVVH (tot 2023)
- Dr. M.C. (Michiel) Warlé, vaatchirurg, RadboudUMC, NVVH (vanaf 2024)
- Dr. M.E. (Maarten) Tushuizen, maag-darm-leverarts, LUMC, NVMDL
- Dr. J.M. (Jonathan) Coutinho, neuroloog, Amsterdam UMC, NVN
- Drs. M.H. (Monique) Suijker, kinderarts-hematoloog, UMC Utrecht, NVK
- Drs. P (Paul) Smits, huisarts/ Kaderhuisarts HVZ, NHG
Klankbordgroep
- Dr. J.J.C.M. (Sjef) van de Leur, arts klinische chemie, Isala, NVKC
- Dr. M.G. (Mariëlle) van Pampus, gynaecoloog, OLVG, NVOG
- Drs. R.J. (Rutger) Lely, radioloog, Amsterdam UMC, NVVR
- Dr. C. (Bibi) van Montfrans, dermatoloog, Erasmus MC, NVDV
- Dr. R.A. (Richard) Faaij, klinisch geriater, Diakonessenhuis, NVKG
- Dr. B. (Baucke) van Minnen, kaakchirurg, UMCG, NVMKA
- Drs. N. (Noa) Rosenberg, beleidsadviseur, Harteraad (vanaf mei 2024)
- I.G.J. (Ilse) Verstraaten MSc, beleidsadviseur, Harteraad (tot 2024)
- Dr. N. (Nakisa) Khorsand, ziekenhuisapotheker, OLVG, NVZA
- Dr. M.F. (Margreet) van Herwaarden, openbaar apotheker, KNMP
- Dr. E.T.T.L. (Eric) Tjwa, MDL-arts, RadboudUMC, NVMDL
- Dr. L.M. (Linda) de Heer, cardio-thoracaal chirurg, UMC Utrecht, NVT
- Prof. dr. S. (Saskia) Middeldorp, internist-vasculaire geneeskunde, Radboudumc, NIV
- Dr. J.M.M.B. (Hans-Martin) Otten, internist-oncoloog, Meander MC, NIV
- Dr. E.J. (Esther) Nossent, longarts, Amsterdam UMC, NVALT
- Dr. C.H. (Heleen) van Ommen, kinderarts-hematoloog, Erasmus MC, NVK
- Dr. K.M.J. (Katja) Heitink, kinderarts-oncoloog, Prinses Maxima Centrum, NVK
- Prof. dr. N.P. (Nicole) Juffermans, intensivist, Amsterdam UMC, NVIC
- Dr. M.C.A. (Marcella) Muller, intensivist, Amsterdam UMC, NVIC
-
Dr. M.N. (Mandy) Lauw, internist-hematoloog, Erasmus MC, NIV, NVVH (Nederlandse Vereniging voor Hematologie, meelezer vanuit cluster Antitrombotisch beleid)
Met ondersteuning van
- H. (Hanneke) Olthuis, adviseur, Kennisinstituut van de Federatie Medisch Specialisten
- H.J. (Harm-Jan) van der Hart, adviseur, Kennisinstituut van de Federatie Medisch Specialisten
Belangenverklaringen
Een overzicht van de belangen van werkgroepleden en het oordeel over het omgaan met eventuele belangen vindt u in onderstaande tabel. De ondertekende belangenverklaringen zijn op te vragen bij het secretariaat van het Kennisinstituut van de Federatie Medisch Specialisten via secretariaat@kennisinstituut.nl.
|
Werkgroeplid |
Functie |
Nevenfuncties |
Gemelde belangen |
Ondernomen actie |
|
Huisman (voorzitter) |
Internist vasculaire geneeskunde |
|
à op geen van deze projecten projectleider, betreft unrestricted grants en veelal financiering promotietrajecten. |
Geen restricties |
|
Banne Nemeth |
Orthopedisch chirurg in opleiding |
Postdoc klinische epidemiologie en orthopedie, LUMC |
Trombosestichting - “VTE following total hip and knee arthroplasty: prediction is the future“ |
Geen restricties |
|
Harmen Ettema |
Orthopedisch chirurg |
Geen |
Gemeld bij herbevestiging in 2025: ZonMW, Distinct trial, tromboseprofylaxe bij orthopedische ingrepen, geen projectleider |
Geen restricties |
|
Jonathan Coutinho |
neuroloog Amsterdam UMC |
Geen |
Gemeld bij herbevestiging in 2025:
|
Restricties t.a.v. besluitvorming modules over andexanet alfa bij bloedingen geen restricties ten gevolge van rol pacific stroke study en dabigatran etixilate/warfarin bij CVT, aangezien deze patientengroepen geen onderwerp zijn van deze richtlijnherziening. |
|
Klok |
Internist vasculaire geneeskunde LUMC Leiden |
Gemeld bij herbevestiging in 2024:
|
|
Geen restricties |
|
Kruip |
Hematoloog Gemeld bij herbevestiging in 2024: Directeur Kwaliteit & Patientenzorg, Erasmus MC, betaald |
Gemeld bij herbevestiging in 2024:
|
|
Geen restricties |
|
Maarten Tushuizen |
MDL-arts LUMC |
Geen |
Maag-Lever-Darmstichting (MLDS) |
Geen restricties |
|
Marc Brouwer |
Cardioloog Radboudumc |
Geen |
Nee |
Geen restricties |
|
Paul Smits |
huisarts, zelfstandig |
Coördinator onderwijscommissie harvaatHAG |
geen |
Geen restricties |
|
Monique Suijker |
Kinderarts-hematoloog werkzaam bij Van Creveldkliniek, UMCU |
Geen |
Geen lopende studies Bayer en Janssen - Einstein Jr studie - gebruik Rivaroxaban bij kinderen – afgesloten |
Geen restricties |
|
Arno Wiersema (teruggetrokken, tot 2024) |
Vaatchirurg, Dijklander ziekenhuis |
Geen |
ZonMw, Amsterdam UMC, Dijklander zh en Medtronic - www.action-1.nl - betreft onderzoek naar rol van heparine bij een open buikslagader operatie, rol als projectleider |
Restricties ten aanzien van besluitvorming over heparine. |
|
Michiel Warlé (vanaf 2024) |
Vaatchirurg Radboudumc |
Werkgroep Landelijk Kennisplatform Antistolling |
ZEGG/ZonMw- GENPAD studie (Cyp2c19 genotypering bij Clopidogrel en perifeer arterieel vaatlijden – hoofdonderzoeker Gemeld bij herbevestiging in 2025: NWO-OTP, Wireless clot retriever, projectleider |
Geen restricties |
|
Nick van Es (vanaf 2023) |
Internist-vasculaire geneeskunde, Amsterdam UMC, locatie AMC |
Geen |
Gemeld bij herbevestiging in 2024:
|
Geen restricties |
|
Leentjens (vanaf 2023) |
Internist-vasculair geneeskundige, Radboudumc |
Geen |
|
Geen restricties |
|
Actieve klankbordgroepleden |
||||
|
Esther Nossent |
Longarts Amsterdam UMC |
Geen |
Gemeld bij herbevestiging in 2025:
|
Geen restricties |
|
Heleen van Ommen |
Hoofd afd. Kinderhematologie & kinderoncologie Erasmus MC Sophia Kinderziekenhuis |
Geen |
|
Geen restricties |
|
Eric Tjwa |
MDL arts, Radboudumc |
Geen |
Geen Gemeld bij herbevestiging in 2025: Geen betrokkenheid bij onderzoeken die direct/indirect verband houden met de inhoud van de richtlijn |
Geen restricties |
|
Hans-Martin Otten (vanaf 2024) |
Internist Meander MC |
Lid METC UMCU, betaald |
|
Geen restricties |
|
Noa Rosenberg (vanaf 2024) |
Beleidsadviseur |
|
Geen |
Geen restricties |
|
Katja Heitink – Pollé |
Kinderoncoloog Prinses Máxima Centrum |
Landelijke werkgroep trombose bij kinderen |
geen |
Geen restricties |
| Mandy Lauw
|
Internist-hematoloog, Erasmus MC Rotterdam, afdeling hematologie |
Geen |
1. ZONMW- Prevention of venous |
Restrictie t.a.v. besluitvorming over tromboseprofylaxe bij patienten met ALL en behandeling van VTE bij patienten met hematologische maligniteiten. |
Inbreng patiëntenperspectief
De werkgroep besteedde aandacht aan het patiëntenperspectief door uitnodigen van Stichting Harteraad voor de schriftelijke knelpuntenanalyse en door een patiëntvertegenwoordiger van Stichting Harteraad toe te voegen aan de klankbordgroep. De verkregen input is meegenomen bij het opstellen van de uitgangsvragen, de keuze voor de uitkomstmaten en bij het opstellen van de overwegingen (zie alinea waarden en voorkeuren van patiënten). De conceptrichtlijn is tevens voor commentaar voorgelegd aan Stichting Harteraad en Stichting Kind en Ziekenhuis en de eventueel aangeleverde commentaren zijn bekeken en verwerkt.
Kwalitatieve raming van mogelijke financiële gevolgen in het kader van de Wkkgz
Bij de richtlijnmodule voerde de werkgroep conform de Wet kwaliteit, klachten en geschillen zorg (Wkkgz) een kwalitatieve raming uitom te beoordelen of de aanbevelingen mogelijk leiden tot substantiële financiële gevolgen. Bij het uitvoeren van deze beoordeling is de richtlijnmodule op verschillende domeinen getoetst (zie het stroomschema bij Werkwijze).
Module |
Uitkomst raming |
Toelichting |
|
Behandeling buikvene trombose |
geen financiële gevolgen |
Uit de toetsing volgt dat de aanbevelingen niet breed toepasbaar zijn (<5.000 patiënten) en daarom naar verwachting geen substantiële financiële gevolgen zullen hebben voor de collectieve uitgaven. |
Zoekverantwoording
Zoekstrategie
Embase
|
No. |
Query |
Results |
|
#19 |
#17 AND #18 sleutelartikelen gevonden |
3 |
|
#18 |
#9 OR #10 |
624 |
|
#17 |
#15 AND #16 |
3 |
|
#16 |
#9 OR #10 OR #11 |
1707 |
|
#15 |
#12 OR #13 OR #14 sleutelartikelen |
3 |
|
#14 |
'anticoagulant therapy for splanchnic vein thrombosis: a systematic review and meta-analysis' AND 2021 NOT [2020]/py |
1 |
|
#13 |
'rivaroxaban for the treatment of noncirrhotic splanchnic vein thrombosis: an interventional prospective cohort study' |
1 |
|
#12 |
'randomized controlled trial of rivaroxaban versus warfarin in the management of acute non-neoplastic portal vein thrombosis' |
1 |
|
#11 |
#4 AND (#7 OR #8) NOT #9 NOT #10 Observationele studies |
1083 |
|
#10 |
#4 AND #6 NOT #9 Clinical trials, RCTs |
377 |
|
#9 |
#4 AND #5 SR |
247 |
|
#8 |
'case control study'/de OR 'comparative study'/exp OR 'control group'/de OR 'controlled study'/de OR 'controlled clinical trial'/de OR 'crossover procedure'/de OR 'double blind procedure'/de OR 'phase 2 clinical trial'/de OR 'phase 3 clinical trial'/de OR 'phase 4 clinical trial'/de OR 'pretest posttest design'/de OR 'pretest posttest control group design'/de OR 'quasi experimental study'/de OR 'single blind procedure'/de OR 'triple blind procedure'/de OR (((control OR controlled) NEAR/6 trial):ti,ab,kw) OR (((control OR controlled) NEAR/6 (study OR studies)):ti,ab,kw) OR (((control OR controlled) NEAR/1 active):ti,ab,kw) OR 'open label*':ti,ab,kw OR (((double OR two OR three OR multi OR trial) NEAR/1 (arm OR arms)):ti,ab,kw) OR ((allocat* NEAR/10 (arm OR arms)):ti,ab,kw) OR placebo*:ti,ab,kw OR 'sham-control*':ti,ab,kw OR (((single OR double OR triple OR assessor) NEAR/1 (blind* OR masked)):ti,ab,kw) OR nonrandom*:ti,ab,kw OR 'non-random*':ti,ab,kw OR 'quasi-experiment*':ti,ab,kw OR crossover:ti,ab,kw OR 'cross over':ti,ab,kw OR 'parallel group*':ti,ab,kw OR 'factorial trial':ti,ab,kw OR ((phase NEAR/5 (study OR trial)):ti,ab,kw) OR ((case* NEAR/6 (matched OR control*)):ti,ab,kw) OR ((match* NEAR/6 (pair OR pairs OR cohort* OR control* OR group* OR healthy OR age OR sex OR gender OR patient* OR subject* OR participant*)):ti,ab,kw) OR ((propensity NEAR/6 (scor* OR match*)):ti,ab,kw) OR versus:ti OR vs:ti OR compar*:ti OR ((compar* NEAR/1 study):ti,ab,kw) OR (('major clinical study'/de OR 'clinical study'/de OR 'cohort analysis'/de OR 'observational study'/de OR 'cross-sectional study'/de OR 'multicenter study'/de OR 'correlational study'/de OR 'follow up'/de OR cohort*:ti,ab,kw OR 'follow up':ti,ab,kw OR followup:ti,ab,kw OR longitudinal*:ti,ab,kw OR prospective*:ti,ab,kw OR retrospective*:ti,ab,kw OR observational*:ti,ab,kw OR 'cross sectional*':ti,ab,kw OR cross?ectional*:ti,ab,kw OR multicent*:ti,ab,kw OR 'multi-cent*':ti,ab,kw OR consecutive*:ti,ab,kw) AND (group:ti,ab,kw OR groups:ti,ab,kw OR subgroup*:ti,ab,kw OR versus:ti,ab,kw OR vs:ti,ab,kw OR compar*:ti,ab,kw OR 'odds ratio*':ab OR 'relative odds':ab OR 'risk ratio*':ab OR 'relative risk*':ab OR 'rate ratio':ab OR aor:ab OR arr:ab OR rrr:ab OR ((('or' OR 'rr') NEAR/6 ci):ab))) |
14286706 |
|
#7 |
'major clinical study'/de OR 'clinical study'/de OR 'case control study'/de OR 'family study'/de OR 'longitudinal study'/de OR 'retrospective study'/de OR 'prospective study'/de OR 'comparative study'/de OR 'cohort analysis'/de OR ((cohort NEAR/1 (study OR studies)):ab,ti) OR (('case control' NEAR/1 (study OR studies)):ab,ti) OR (('follow up' NEAR/1 (study OR studies)):ab,ti) OR (observational NEAR/1 (study OR studies)) OR ((epidemiologic NEAR/1 (study OR studies)):ab,ti) OR (('cross sectional' NEAR/1 (study OR studies)):ab,ti) |
6767914 |
|
#6 |
'clinical trial'/exp OR 'randomization'/exp OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp OR 'placebo'/exp OR 'prospective study'/exp OR rct:ab,ti OR random*:ab,ti OR 'single blind':ab,ti OR 'randomised controlled trial':ab,ti OR 'randomized controlled trial'/exp OR placebo*:ab,ti |
3302394 |
|
#5 |
'meta analysis'/exp OR 'meta analysis (topic)'/exp OR metaanaly*:ti,ab OR 'meta analy*':ti,ab OR metanaly*:ti,ab OR 'systematic review'/de OR 'cochrane database of systematic reviews'/jt OR prisma:ti,ab OR prospero:ti,ab OR (((systemati* OR scoping OR umbrella OR 'structured literature') NEAR/3 (review* OR overview*)):ti,ab) OR ((systemic* NEAR/1 review*):ti,ab) OR (((systemati* OR literature OR database* OR 'data base*') NEAR/10 search*):ti,ab) OR (((structured OR comprehensive* OR systemic*) NEAR/3 search*):ti,ab) OR (((literature NEAR/3 review*):ti,ab) AND (search*:ti,ab OR database*:ti,ab OR 'data base*':ti,ab)) OR (('data extraction':ti,ab OR 'data source*':ti,ab) AND 'study selection':ti,ab) OR ('search strategy':ti,ab AND 'selection criteria':ti,ab) OR ('data source*':ti,ab AND 'data synthesis':ti,ab) OR medline:ab OR pubmed:ab OR embase:ab OR cochrane:ab OR (((critical OR rapid) NEAR/2 (review* OR overview* OR synthes*)):ti) OR ((((critical* OR rapid*) NEAR/3 (review* OR overview* OR synthes*)):ab) AND (search*:ab OR database*:ab OR 'data base*':ab)) OR metasynthes*:ti,ab OR 'meta synthes*':ti,ab |
733409 |
|
#4 |
#3 AND [2008-2023]/py NOT ('conference abstract'/it OR 'editorial'/it OR 'letter'/it OR 'note'/it) NOT (('animal'/exp OR 'animal experiment'/exp OR 'animal model'/exp OR 'nonhuman'/exp) NOT 'human'/exp) |
3567 |
|
#3 |
#1 AND #2 |
4816 |
|
#2 |
'direct oral anticoagulant'/exp OR 'direct oral anticoagulant agent'/exp OR 'anticoagulant agent'/exp OR 'apixaban'/exp OR 'non vitamin k antagonist oral anticoagulant'/exp OR 'thrombin inhibitor'/exp OR 'edoxaban'/exp OR 'rivaroxaban'/exp OR 'dabigatran'/exp OR 'thrombin inhibitor':ti,ab,kw OR 'aboxoma':ti,ab,kw OR 'apixaban':ti,ab,kw OR 'apixaben':ti,ab,kw OR doac*:ti,ab,kw OR 'bms 562247':ti,ab,kw OR 'bms562247':ti,ab,kw OR 'eliques':ti,ab,kw OR 'eliquis':ti,ab,kw OR 'lunast':ti,ab,kw OR 'pf 0465257':ti,ab,kw OR 'pf0465257':ti,ab,kw OR 'tah 3311':ti,ab,kw OR 'tah3311':ti,ab,kw OR 'bibr 953':ti,ab,kw OR 'bibr953':ti,ab,kw OR 'dabigatran':ti,ab,kw OR 'du 176':ti,ab,kw OR 'du 176b':ti,ab,kw OR 'du176':ti,ab,kw OR 'du176b':ti,ab,kw OR 'edoxaban':ti,ab,kw OR 'endoxaban':ti,ab,kw OR 'lixiana':ti,ab,kw OR 'roteas':ti,ab,kw OR 'savaysa':ti,ab,kw OR 'assubex':ti,ab,kw OR 'ast 8294':ti,ab,kw OR 'ast8294':ti,ab,kw OR 'bay 59 7939':ti,ab,kw OR 'bay 597939':ti,ab,kw OR 'bay59 7939':ti,ab,kw OR 'bay597939':ti,ab,kw OR 'bs 112':ti,ab,kw OR 'bs112':ti,ab,kw OR 'dst 8294':ti,ab,kw OR 'dst8294':ti,ab,kw OR 'jnj 39039039':ti,ab,kw OR 'jnj39039039':ti,ab,kw OR 'kriva':ti,ab,kw OR 'naxat':ti,ab,kw OR 'rivaro':ti,ab,kw OR 'rivarolto':ti,ab,kw OR 'rivaroxaban':ti,ab,kw OR 'rivaxa':ti,ab,kw OR 'throsaben':ti,ab,kw OR 'xanirva':ti,ab,kw OR 'xarelto':ti,ab,kw OR 'xerdoxo':ti,ab,kw OR 'xindus':ti,ab,kw |
812381 |
|
#1 |
'budd chiari syndrome'/exp OR 'mesenteric thrombosis'/exp OR 'portal vein thrombosis'/exp OR 'splanchnic vein thrombosis'/exp OR (('hepatic portal vein'/exp OR 'mesenteric vein'/exp OR 'splenic vein'/exp) AND 'thrombosis'/exp) OR (((splanchnic OR splenic OR 'vena lienalis' OR mesenter* OR abdom* OR portal OR porto OR hepatic) NEAR/3 (thromb* OR occlusi*)):ti,ab,kw) OR 'pylethromb*':ti,ab,kw OR 'budd-chiari':ti,ab,kw OR ((chiari NEAR/2 (deform* OR disease* OR syndrom*)):ti,ab,kw) OR 'endophlebitis obliterans hepatica':ti,ab,kw OR 'splenic vein thrombosis'/exp |
44815 |
Ovid/Medline
|
# |
Searches |
Results |
|
9 |
(5 and 7) not 8 Clinical trials, RCTs |
103 |
|
8 |
5 and 6 SR |
84 |
|
7 |
exp clinical trial/ or randomized controlled trial/ or exp clinical trials as topic/ or randomized controlled trials as topic/ or Random Allocation/ or Double-Blind Method/ or Single-Blind Method/ or (clinical trial, phase i or clinical trial, phase ii or clinical trial, phase iii or clinical trial, phase iv or controlled clinical trial or randomized controlled trial or multicenter study or clinical trial).pt. or random*.ti,ab. or (clinic* adj trial*).tw. or ((singl* or doubl* or treb* or tripl*) adj (blind$3 or mask$3)).tw. or Placebos/ or placebo*.tw. |
2616982 |
|
6 |
meta-analysis/ or meta-analysis as topic/ or (metaanaly* or meta-analy* or metanaly*).ti,ab,kf. or systematic review/ or cochrane.jw. or (prisma or prospero).ti,ab,kf. or ((systemati* or scoping or umbrella or "structured literature") adj3 (review* or overview*)).ti,ab,kf. or (systemic* adj1 review*).ti,ab,kf. or ((systemati* or literature or database* or data-base*) adj10 search*).ti,ab,kf. or ((structured or comprehensive* or systemic*) adj3 search*).ti,ab,kf. or ((literature adj3 review*) and (search* or database* or data-base*)).ti,ab,kf. or (("data extraction" or "data source*") and "study selection").ti,ab,kf. or ("search strategy" and "selection criteria").ti,ab,kf. or ("data source*" and "data synthesis").ti,ab,kf. or (medline or pubmed or embase or cochrane).ab. or ((critical or rapid) adj2 (review* or overview* or synthes*)).ti. or (((critical* or rapid*) adj3 (review* or overview* or synthes*)) and (search* or database* or data-base*)).ab. or (metasynthes* or meta-synthes*).ti,ab,kf. |
684473 |
|
5 |
4 not ((exp animals/ or exp models, animal/) not humans/) not (letter/ or comment/ or editorial/) |
1007 |
|
4 |
limit 3 to yr="2008 - 2023" |
1132 |
|
3 |
1 and 2 |
1885 |
|
2 |
exp Anticoagulants/ or Rivaroxaban/ or exp Dabigatran/ or thrombin inhibitor.ti,ab,kf. or aboxoma.ti,ab,kf. or apixaban.ti,ab,kf. or apixaben.ti,ab,kf. or doac*.ti,ab,kf. or bms 562247.ti,ab,kf. or bms562247.ti,ab,kf. or eliques.ti,ab,kf. or eliquis.ti,ab,kf. or lunast.ti,ab,kf. or "pf 0465257".ti,ab,kf. or pf0465257.ti,ab,kf. or tah 3311.ti,ab,kf. or tah3311.ti,ab,kf. or bibr 953.ti,ab,kf. or bibr953.ti,ab,kf. or dabigatran.ti,ab,kf. or du 176.ti,ab,kf. or du 176b.ti,ab,kf. or du176.ti,ab,kf. or du176b.ti,ab,kf. or edoxaban.ti,ab,kf. or endoxaban.ti,ab,kf. or lixiana.ti,ab,kf. or roteas.ti,ab,kf. or savaysa.ti,ab,kf. or assubex.ti,ab,kf. or ast 8294.ti,ab,kf. or ast8294.ti,ab,kf. or bay 59 7939.ti,ab,kf. or bay 597939.ti,ab,kf. or bay59 7939.ti,ab,kf. or bay597939.ti,ab,kf. or bs 112.ti,ab,kf. or bs112.ti,ab,kf. or dst 8294.ti,ab,kf. or dst8294.ti,ab,kf. or jnj 39039039.ti,ab,kf. or jnj39039039.ti,ab,kf. or kriva.ti,ab,kf. or naxat.ti,ab,kf. or rivaro.ti,ab,kf. or rivarolto.ti,ab,kf. or rivaroxaban.ti,ab,kf. or rivaxa.ti,ab,kf. or throsaben.ti,ab,kf. or xanirva.ti,ab,kf. or xarelto.ti,ab,kf. or xerdoxo.ti,ab,kf. or xindus.ti,ab,kf. |
247867 |
|
1 |
Budd-Chiari Syndrome/ or exp Mesenteric Vascular Occlusion/ or ((Portal System/ or Mesenteric Veins/ or Portal Vein/ or Splenic Vein/) and exp Thrombosis/) or ((splanchnic or splenic or vena lienalis or mesenter* or abdom* or portal or porto or hepatic) adj3 (thromb* or occlusi*)).ti,ab,kf. or pylethromb*.ti,ab,kf. or budd-chiari.ti,ab,kf. or (chiari adj2 (deform* or disease* or syndrom*)).ti,ab,kf. or endophlebitis obliterans hepatica.ti,ab,kf. |
27836 |