Tromboseprofylaxe volwassenen met maligniteit
Uitgangsvraag
In hoeverre zouden poliklinische patiënten met een maligniteit tromboseprofylaxe moeten krijgen?
Aanbeveling
Overweeg tromboseprofylaxe voor te schrijven in de vorm van een DOAC (apixaban 2,5 mg 2dd of rivaroxaban 10 mg 1dd) aan poliklinische patiënten met een solide maligniteit of maligne lymfoom en een hoog risico op veneuze trombo-embolie (Khorana score ≥2 punten) die starten met systemische kankerbehandeling (exclusief monotherapie met hormonale therapie) en geen risicofactoren hebben voor een bloeding (zie Tabel 3. Overzicht risicofactoren voor bloedingen en contra-indicaties voor het starten van tromboseprofylaxe bij patiënten met een maligniteit die starten met systemische therapie).
Overweeg tromboseprofylaxe te continueren na de eerste 6 maanden, en pas te stoppen bij het beëindigen van de systemische kankerbehandeling.
Stop tromboseprofylaxe bij het optreden van klinisch relevante bloedingen, als er een hoog risico op bloedingen ontstaat of als de terminale fase aanbreekt.
Overwegingen
Voor- en nadelen van de interventie en de kwaliteit van het bewijs
DOACs
Ten opzichte van placebo zou tromboseprofylaxe met directe orale anticoagulantia (DOACs) kunnen leiden tot een net clinical benefit die klinisch relevant is bij volwassen patiënten met een maligniteit die starten met chemotherapie en die een gemiddeld tot hoog risico op een VTE hebben en geen risicofactoren voor een bloeding. De bewijskracht voor deze cruciale uitkomst is laag. Verder zijn we onzeker over het effect van tromboseprofylaxe met DOACs op de andere cruciale uitkomst mortaliteit (zeer laag GRADE). Voor beide uitkomsten wordt de bewijskrachtbeperkt vanwege het risico op bias en imprecisie.
Ook zijn we onzeker over de effecten van tromboseprofylaxe met DOACs op de belangrijke uitkomst arteriële trombo-embolie (ATE, zeer laag GRADE). De bewijskracht voor de belangrijke uitkomstmaten VTE, majeure bloeding en klinische niet relevante majeure bloeding is laag. Ten opzichte van placebo leidt tromboseprofylaxe mogelijk tot minder veneuze trombo-embolieën (VTE). Voor de uitkomstmaten majeure bloedingen en klinisch relevante niet majeure bloedingen is er mogelijk geen verschil tussen patiënten die tromboseprofylaxe met DOAC ontvingen, vergeleken met patiënten die een placebo kregen. Daarbij moet worden opgemerkt dat patiënten met risicofactoren op een bloeding of een eerdere bloeding werden uitgesloten. Geen van de studies rapporteerde over de uitkomstmaten kwaliteit van leven, aanvulling of stoppen van oncologische behandeling en kwaliteit van sterven en dood. Er kan derhalve geen conclusie worden getrokken over de effecten van tromboseprofylaxe met DOACs op de genoemde uitkomstmaten.
LMWH
Op basis van de geïncludeerde studies zijn we onzeker over het effect van tromboseprofylaxe met laagmoleculairgewicht heparine (LMWH) op de cruciale uitkomstmaten net clinical benefit en mortaliteit, vergeleken met placebo of geen tromboseprofylaxe bij volwassen patiënten met een maligniteit die starten met chemotherapie (zeer laag GRADE). De bewijskracht van de gevonden resultaten is erg beperkt vanwege het risico op bias, inconsistentie en imprecisie. De gegevens met betrekking tot de uitkomstmaat net clinical benefit waren beperkt; er waren slechts twee studies die voldoende gegevens rapporteerden om de net clinical benefit te kunnen berekenen. Dit bemoeilijkt de interpretatie van deze gegevens.
De bewijskracht voor de belangrijke uitkomstmaat VTE is laag. Ten opzichte van placebo of standaardzorg leidt tromboseprofylaxe met LMWH mogelijk tot minder VTE. Verder zijn we onzeker over het effect van tromboseprofylaxe met LMWH op de belangrijke uitkomstmaten ATE, majeure bloeding, klinische relevante niet majeure bloeding en kwaliteit van leven. Ook hiervoor geldt dat de bewijskracht beperkt werd door het risico op bias, inconsistentie en imprecisie.
Geen van de studies rapporteerde over de uitkomstmaten aanvulling of stoppen van oncologische behandeling en kwaliteit van sterven en dood. Er kan derhalve geen conclusie worden getrokken over de effecten van tromboseprofylaxe met LMWH op de genoemde uitkomstmaten.
Fondaparinux
Er zijn geen studies gevonden die de effectiviteit van tromboseprofylaxe met fondaparinux onderzochten bij patiënten met een maligniteit die startten met systeemtherapie.
Selectie van patiënten en generaliseerbaarheid
Het risico op VTE verschilt zeer sterk tussen patiëntengroepen. Het nut (meestal uitgedrukt als Number Needed tot Treat (NNT)) van tromboseprofylaxe hangt in belangrijke mate af van dit uitgangsrisico op VTE. Het risico is met name hoger bij patiënten met lokaal gevorderde of gemetastaseerde maligniteit en bij patiënten met een hoog-risico tumor, zoals een maag- of pancreascarcinoom. De meest gebruikte risicoscore om hoog-risico patiënten te identificeren is de Khorana score. Het voordeel van de Khorana score is dat deze goed gevalideerd is en makkelijk te berekenen is o.b.v. tumortype, bloedbeeld en BMI. Het nadeel is dat de discriminerende waarde beperkt is en dat de sensitiviteit en specificiteit variëren tussen tumortypen. In het onderzoek van Alexander (2023, TARGET-TP) werden hoog-risico patiënten geïdentificeerd op basis van D-dimeer en fibrinogeenconcentraties, maar deze manier van risicostratificatie is niet gevalideerd.
In de twee DOAC-trials werden enkel patiënten geïncludeerd met een Khorana score van twee of hoger (Carrier, 2019 en Khorana, 2019). Als deze trials als uitgangspunt worden genomen, dan heeft iedere aanbeveling over DOACs dus automatisch ook betrekking op het gebruik van de Khorana score om hoog-risico patiënten te identificeren. Door gebruik van de Khorana score werd een groep patiënten geselecteerd met een uitgangsrisico van 9.3% in zes maanden (cumulatieve incidentie VTE in placebogroepen: 65/696 = 9.3%). Dit komt goed overeen met de zes-maanden incidentie van 8.9% die werd geschat in een meta-analyse (Mulder, 2019). Bij gebruik van een afkapwaarde van drie punten stijgt de zes-maanden incidentie naar 11%. In deze groep is het nut van tromboseprofylaxe groter (Bosch, 2020).
In één van de DOAC-trials (Khorana, 2019) werden patiënten geëxcludeerd bij wie een DVT van het been werd geconstateerd bij een screeningsecho vóór randomisatie; dit betrof 4.5% van de patiënten. Ook werd een screenende echo verricht na acht, 16 en 26 weken. Een asymptomatisch proximale DVT werd hierbij geconstateerd bij 4.3% in de placebogroep en 2.1% in de rivaroxaban groep. Dit beperkt de generaliseerbaarheid van de uitkomsten van deze studie, aangezien in de Nederlandse praktijk geen screenende echografie wordt toegepast. Het is onbekend of het effect van rivaroxaban groter of kleiner was geweest als screening voorafgaand aan randomisatie en tijdens het onderzoek níet was toegepast.
In de DOAC-trials werden patiënten geïncludeerd met een solide maligniteit of maligne lymfoom die gingen starten met nieuwe chemotherapie of andere systemische kankertherapie (behoudens hormoontherapie als monotherapie). Het is niet gerapporteerd welk deel van de patiënten neoadjuvante, adjuvante of palliatieve behandeling kregen. Hoewel het overgrote deel van de patiënten behandeld werd met chemotherapie, is de werkgroep van mening dat de resultaten ook toepasbaar zijn op patiënten die andere vormen van systemische kankerbehandeling krijgen, zoals immuuntherapie of tyrosine kinase remmers, aangezien deze behandelingen geassocieerd zijn met een gelijk dan wel hoger tromboserisico. Aangezien er niet tot nauwelijks patiënten met acute leukemie of multipel myeloom werden geïncludeerd, kunnen de resultaten niet naar deze groepen vertaald worden. Overigens krijgen patiënten met multipel myeloom meestal reeds tromboseprofylaxe aangeboden op basis van patiënt- en behandeling specifieke criteria conform hematologische richtlijnen (Module: Supportive care bij patiënten met (symptomatisch) multipel myeloom) .
Patiënten met een verhoogd risico op bloedingen werden uitgesloten van deelname. Er zijn geen gevalideerde scores beschikbaar die specifiek bij patiënten met kanker het bloedingsrisico schatten. Bepaalde patiëntgroepen dienen doorgaans geen tromboseprofylaxe te krijgen vanwege het verhoogde risico op bloedingen of andere contra-indicaties (zie Tabel 3, welke grotendeels gebaseerd is op de exclusiecriteria van de trials die tromboseprofylaxe hebben onderzocht in patiënten met kanker i.c.m. internationale richtlijnen (Lyman, 2021)). Er dient opgemerkt te worden dat het baseline risico op bloedingen (met name gastro-intestinale bloedingen) waarschijnlijk het hoogst is bij patiënten met een tumor van de slokdarm of maag in situ. Deze patiënten werden niet uitgesloten van de trials die tromboseprofylaxe onderzochten. Derhalve is de werkgroep van mening dat deze groep patiënten ook in aanmerking dient te komen voor tromboseprofylaxe. Een subgroepanalyse van de CASSINI-trial toonde dat ernstige bloedingen optraden bij 4 van 88 patiënten met een maagcarcinoom of gastro-oesofageaal carcinoom in de rivaroxaban groep (4.6%; waarvan 3 gastro-intestinale bloedingen) vergeleken met 1 van 85 patiënten in de placebogroep (1.2%) (HR 3.77; 95% BI 0.42-33.73; Mones, 2021). In de AVERT-trial ontwikkelde geen van de patiënten met een tumor van de bovenste tractus digestivus (n=44) of colorectaal carcinoom (n=11) een ernstige bloeding (Ladha, 2021).
|
Verhoogd bloedingsrisico |
|
Bekende bloedingsziekte |
|
Leverdysfunctie met coagulopathie (bv. verlengde PT en/of aPTT) |
|
(Verwachte) trombopenie <50 x 109/L |
|
eGFR <30 mL/min/1.73 m2 |
|
Geplande stamceltransplantatie |
|
Dubbele trombocytenaggregatieremming |
|
Chronisch gebruik NSAID |
|
Zeer laag lichaamsgewicht (<40 kg) |
|
Gelijktijdig gebruik van sterke remmers van CYP3A4 of P-glycoproteine≠ |
|
Absolute contra-indicatie voor gebruik directe orale anticoagulantia |
|
Zwangerschap |
|
Borstvoeding |
|
Relatieve contra-indicatie |
|
Levensverwachting <6 maanden |
* Hiervoor kan gebruikt gemaakt worden van de tabellen uit de EHRA-Practical Guide to NOAC use in AF uit 2021 (Steffel, 2021). ≠ Bijvoorbeeld ketoconazol, itraconazol, voriconazol, posaconazol of HIV-proteaseremmers
De meeste onderzoeken naar tromboseprofylaxe hadden een follow-up duur van zes maanden. De meeste VTE ontstaan in de eerste drie maanden na het starten van de kankerbehandeling. Het is derhalve onduidelijk of de voordelen van tromboseprofylaxe ook na zes maanden blijven bestaan. De werkgroep is echter van mening dat systemische kankerbehandeling een persisterende risicofactor is voor VTE, en dat tromboseprofylaxe dan ook gecontinueerd dient te worden tijdens de gehele behandeling, ook als deze langer dan zes maanden duurt, om het risico op een VTE na staken van tromboseprofylaxe te verlagen. De werkgroep is van mening dat bij patiënten bij wie de terminale palliatieve fase aanbreekt tromboseprofylaxe ook gestaakt dient te worden omdat het risico op bloedingen in deze fase groter lijkt dan het risico op trombose.
Net clinical benefit
Er is in deze module gekozen voor net clinical benefit als één van de cruciale uitkomstmaten. Hierdoor wordt naast een eventuele reductie in VTE ook de belangrijkste bijwerking van tromboseprofylaxe (majeure bloeding) meegewogen. Daarnaast kan tromboseprofylaxe in theorie een direct effect hebben op mortaliteit door het voorkómen van een fatale longembolie of het induceren van een fatale bloeding, en een indirect effect door het voorkómen van gevolgen van een VTE (bv. gecompliceerde ziekenhuisopname of bloeding door therapeutische antistolling). Daarbij moet aangetekend worden dat er geen bewijs is dat tromboseprofylaxe tot significante verbetering van overleving leidt (Schünemann, 2020). De beperking van de net clinical benefit is dat VTE en majeure bloeding binnen deze uitkomst een gelijke waarde hebben, terwijl dit voor de patiënt of arts niet zo hoeft te zijn. Daarnaast zijn andere relevante uitkomstmaten die beïnvloed kunnen worden door tromboseprofylaxe, zoals klinisch relevante niet-majeure bloedingen en ATE, geen onderdeel van de gebruikte definitie van net clinical benefit. Ook kan niet voor alle onderzoeken een net clinical benefit berekend worden doordat fatale bloedingen en VTE niet apart gerapporteerd worden, waardoor een risico staat op dubbele telling met mortaliteit.
Waarden en voorkeuren van patiënten (en evt. hun verzorgers)
Er is weinig onderzoek gedaan naar de waarden en voorkeuren van patiënten met betrekking tot tromboseprofylaxe. De uitkomst van de kankerbehandeling (bijvoorbeeld progressievrije overleving en mortaliteit) en kwaliteit van leven zijn voor de meeste patiënten met kanker doorgaans het belangrijkst. Aangezien VTE kan interfereren met de kankerbehandeling (bv. door een ziekenhuisopname of uitgestelde ingreep) en kan leiden tot morbiditeit (bv. pijn of dyspneu) is de werkgroep van mening dat het relevant is om het risico op VTE te bespreken en eventueel counseling over tromboseprofylaxe aan te bieden. Recent internationaal onderzoek laat echter zien dat 62% van de patiënten met kanker geen informatie had gekregen over het verhoogde risico op VTE en dat 69% geen informatie over symptomen passend bij VTE had gekregen (Potere, 2022).
Besluitvorming over tromboseprofylaxe dient expliciet gedeeld te worden met de patiënt. Er dient aangemerkt te worden dat er geen bewijs is dat tromboseprofylaxe tot verbetering van overleving leidt. Bij de counseling dient besproken te worden: het effect van tromboseprofylaxe op net clinical benefit, VTE en (majeure) bloedingen. Daarnaast dient de toedieningsvorm besproken te worden. Tromboseprofylaxe kan aangeboden worden middels LMWH (dagelijkse subcutane injecties) of DOACs (tabletten rivaroxaban 10 mg eenmaal daags of apixaban 2,5 mg tweemaal daags). Hoewel weinig onderzoek is gedaan naar het effect van subcutane injecties vs orale medicatie op de kwaliteit van leven, neemt de werkgroep aan dat patiënten (bij gelijke effectiviteit en veiligheid) een voorkeur zullen hebben voor orale medicatie, mits orale medicatie mogelijk is.
Kosten (middelenbeslag)
Er is geen kosteneffectiviteitsanalyse gedaan naar tromboseprofylaxe voor poliklinische patiënten met kanker in de Nederlandse setting. In een onderzoek uit Spanje werd geschat dat tromboseprofylaxe met apixaban leidde tot een daling van de kosten met €64,- en een klinisch niet significante toename van 0.008 quality adjusted life years (QALY’s) op de korte termijn (zes maanden, Muñoz, 2023). Tromboseprofylaxe middels rivaroxaban was geassocieerd met een stijging van de kosten met €121,- en een klinisch niet significante toename van 0.008 QALY’s. Een Canadees onderzoek schatte dat tromboseprofylaxe middels apixaban geassocieerd is met een daling van de kosten met 257 CAD en een toename van 0.001 QALY’s op de korte termijn (zes maanden) en een daling van 6.973 CAD en toename van 0.083 QALY’s op de lange termijn (Kimpton, 2021). Tot slot toonde onderzoek uit de Verenigde Staten aan dat tromboseprofylaxe met rivaroxaban of apixaban leidde tot een toename in kosten van $1.445 en toename van 0.12 QALY’s op de korte termijn (zes maanden) (Li, 2020)). Op basis van deze onderzoeken lijkt, voor de Nederlandse praktijk, tromboseprofylaxe middels apixaban en rivaroxaban niet tot onaanvaardbare zorgkosten te leiden, en waarschijnlijk een geringe toename in QALY’s. Wat niet is meegewogen in de kosteneffectiviteitsanalyses, zijn de kosten die gepaard gaan met de eventuele counseling van hoog-risico patiënten m.b.t. tromboseprofylaxe. De kosten van LMWH en DOACs in profylactische dosering zijn ten tijde van het opstellen van de richtlijn ongeveer gelijk, afhankelijk van het gekozen preparaat, waardoor er vanuit kostenperspectief geen duidelijke voorkeur is.
Aanvaardbaarheid, haalbaarheid en implementatie
Het bespreken van het risico op VTE en klachten passend bij VTE zou bij voorkeur onderdeel van het informatiegesprek met patiënten die starten met systemische kankerbehandeling moeten zijn. De praktijk leert echter dat het merendeel van de Nederlandse kankerbehandelaren het risico op VTE nooit of soms bespreken, en dat slechts een klein aantal de Khorana score kent en ook daadwerkelijk gebruikt (Kapteijn, 2022). Hieruit kan afgeleid worden dat counseling over tromboseprofylaxe momenteel zelden wordt aangeboden aan patiënten met kanker in Nederland. Mogelijke verklaringen hiervoor zijn dat (i) patiënten al veel informatie krijgen over de prognose, behandeling en andere complicaties, (ii) er niet voldoende tijd is voor het bespreken van het risico op VTE en counseling over tromboseprofylaxe, (iii) VTE niet beschouwd wordt als een relevante complicatie of (iv) dat kankerbehandelaren niet op de hoogte zijn van de literatuur over tromboseprofylaxe.
De resultaten uit de beschikbare gerandomiseerde onderzoeken suggereren dat er een mogelijk voordeel is van tromboseprofylaxe met beperkte belasting voor de patiënt (orale behandeling). Daarom is de werkgroep van mening dat tromboseprofylaxe overwogen dient te worden bij hoog-VTE-risico patiënten zonder risicofactoren voor bloedingen die starten met systemische behandeling, bijvoorbeeld patiënten met een hoog-risico tumor, zoals maag- of pancreascarcinoom, of (andere) patiënten met een Khorana score van twee punten of hoger. Daarnaast dienen patiënten geïnformeerd te worden over de klachten en symptomen die kunnen duiden op een DVT of longembolie en, indien gekozen wordt voor tromboseprofylaxe, de klachten en symptomen die kunnen duiden op een bloeding. Deze informatievoorziening kan onderdeel zijn van het algemene informatiegesprek dat reeds voorafgaand aan het starten van een nieuwe behandeling wordt gevoerd. Tromboseprofylaxe dient doorgaans gestaakt te worden bij het optreden van ernstige bloedingen en bij het staken van de kankerbehandeling. Ook in andere situaties kan besloten worden dat het voordeel van tromboseprofylaxe niet meer opweegt tegen het risico op bloedingen, bijvoorbeeld bij het ontstaan van ernstige nierinsufficiëntie of trombopenie.
Rationale van de aanbeveling: weging van argumenten voor en tegen de interventies
De werkgroep is van mening dat er op basis van lage bewijskracht een voordeel zou kunnen zijn van tromboseprofylaxe met een DOAC bij poliklinische patiënten met een solide maligniteit of maligne lymfoom die starten met systemische behandeling (o.a. chemotherapie, immuuntherapie of orale kankerbehandeling zoals tyrosine kinase remmers) en een hoog risico hebben op VTE en geen risicofactoren voor een bloeding. Dit voordeel bestaat uit een klinisch relevant lager risico op het gecombineerd eindpunt van VTE, ernstige bloedingen en mortaliteit. Dit eindpunt wordt gedreven door een klinisch relevant lager risico op VTE ten koste van een gering verhoogd risico op ernstige bloedingen, zonder een effect op mortaliteit. Het risico op VTE kan ingeschat worden met de Khorana score, waarbij patiënten met twee of meer punten geclassificeerd worden als ‘hoog risico’. Patiënten met een verhoogd risico op bloedingen dienen geen tromboseprofylaxe te krijgen, zoals patiënten met (verwachte) diepe trombopenie, een eerdere klinisch relevante bloeding, moeilijk te behandelen ernstige hypertensie, hersenmetastasen of primaire hersentumor of een eGFR <30 mL/min/1.73 m2. De werkgroep geeft de voorkeur aan een DOAC (tabletten rivaroxaban 10 mg eenmaal daags of apixaban 2,5 mg tweemaal daags) boven LMWH op basis van de aanvaardbaarheid voor patiënten (orale vs. subcutane toediening) bij gelijke kosten. Indien orale toediening niet mogelijk is of er contra-indicaties zijn voor een DOAC of belangrijke interacties met andere medicatie, kan LMWH als alternatief gegeven worden, hoewel het effect van LMWH op het net clinical benefit zeer onzeker is. De werkgroep schat in dat de kosteneffectiviteit van tromboseprofylaxe met een DOAC voor de Nederlandse maatschappij acceptabel is o.b.v. extrapolatie van buitenlandse kosteneffectiviteitsanalyses. Ondanks dat de onderzoeken een follow-up duur hadden van maximaal zes maanden, is de werkgroep van mening dat overwogen moet worden de tromboseprofylaxe te continueren gedurende de duur van de systemische kankerbehandeling, aangezien het risico op VTE verhoogd is en blijft tijdens kankerbehandeling. Daarnaast is de werkgroep van mening dat tromboseprofylaxe gestaakt dient te worden als er contra-indicaties optreden (bijvoorbeeld ernstige trombopenie, ernstige nierinsufficiëntie, ernstige hypertensie of ernstige bloedingen). Het besluit omtrent tromboseprofylaxe (zowel start als stop) dient zeer nadrukkelijk genomen te worden samen met de patiënt (‘gedeelde besluitvorming’). Daarnaast dienen patiënten voorlichting te krijgen over het verhoogde risico op VTE en klachten passend bij een DVT of longembolie, zodat adequate diagnostiek tijdig ingezet kan worden, en over het risico op bloedingen als gekozen wordt voor tromboseprofylaxe.
Onderbouwing
Achtergrond
Patients with cancer have an 8.5-fold increased risk of venous thromboembolism compared to the general population (Mulder, 2021). The 1-year cumulative incidence varies widely across different tumor types, ranging from 1% in patients with breast or prostate cancer to 10-20% in patients with gastric or pancreatic cancer. Cancer-associated thrombosis can lead to morbidity, decreased quality of life, interruption or delays in cancer treatment, and mortality, and is associated with increased healthcare costs. Ambulatory patients with cancer currently do not receive routine pharmacological thromboprophylaxis, but prevention of venous thromboembolism may prevent morbidity and mortality, particularly in high-risk patients. The term ambulatory is used for patients for whom care is usually organized and provided at the outpatient clinic, excluding those admitted to the hospital because of surgery or a medical illness, for whom the risk-benefit ratio of thromboprophylaxis is different. The current guideline does also not discuss prevention of catheter-associated thrombosis in patients with a central venous catheter, but focuses on thromboprophylaxis for prevention of any form of venous thromboembolism in a broad oncology population. Finally, recommendations do not pertain to patients with multiple myeloma, since these patients are already routinely treated with either aspirin or anticoagulation during cancer treatment based on hematological guidelines.
Conclusies / Summary of Findings
All outcome measures – fondaparinux
|
No GRADE |
None of the included studies used fondaparinux as thromboprophylaxis in patients with malignancy receiving chemotherapy. Therefore, no conclusion can be drawn on the effect of thromboprophylaxis with fondaparinux on all outcome measures in adult ambulatory patients with malignancy initiating chemotherapy.
Sources: none |
Net clinical benefit
DOAC
|
Low GRADE |
In adult ambulatory patients with malignancy initiating chemotherapy and an intermediate to high risk Khorana-score and no risk factors for bleeding, thromboprophylaxis with DOACs may result in a net clinical benefit which is clinically relevant, compared to placebo.
Sources: Carrier, 2019 and Khorana, 2019 |
LMWH
|
Very low GRADE |
The evidence is very uncertain about the effect of thromboprophylaxis with LMWH on the outcome net clinical benefit when compared with placebo or standard of care in adult ambulatory patients with malignancy initiating chemotherapy.
Sources: Agnelli, 2009 and Sideras, 2006 |
Mortality
DOAC
|
Very low GRADE |
The evidence is very uncertain about the effect of thromboprophylaxis with DOACs on the outcome mortality when compared with placebo in adult ambulatory patients with malignancy initiating chemotherapy and an intermediate to high risk Khorana-score.
Sources: Carrier, 2019 and Khorana, 2019 |
LMWH
|
Very low GRADE |
The evidence is very uncertain about the effect of thromboprophylaxis with LMWHs on the outcome mortality when compared with placebo or standard of care in adult ambulatory patients with malignancy initiating chemotherapy.
Sources: Agnelli, 2009; Alexander, 2023; Altinbas, 2004; Kakkar, 2004; Macbeth, 2016; Perry, 2010 and Sideras, 2006 |
Venous thromboembolism
DOAC
|
Low GRADE |
In adult ambulatory patients with malignancy initiating chemotherapy and an intermediate to high risk Khorana-score and no risk factors for bleeding, thromboprophylaxis with DOACs may result in a reduction in number of patients with a VTE, compared to placebo.
Sources: Carrier, 2019 and Khorana, 2019 |
LMWH
|
Low GRADE |
In adult ambulatory patients with malignancy initiating chemotherapy, thromboprophylaxis with LMWHs may result in a reduction in number of patients with a VTE, compared to placebo or standard of care.
Sources: Agnelli, 2009; Alexander, 2023; Altinbas, 2004; Kakkar, 2004; Macbeth, 2016; Perry, 2010 and Sideras, 2006 |
Major bleeding
DOAC
|
Low GRADE |
In adult ambulatory patients with malignancy initiating chemotherapy and an intermediate to high risk Khorana-score and no risk factors for bleeding, thromboprophylaxis with DOACs may result in little to no difference in number of patients with a major bleeding, compared to placebo.
Sources: Carrier, 2019 and Khorana, 2019 |
LMWH
|
Very low GRADE |
The evidence is very uncertain about the effect of thromboprophylaxis with LMWHs on the outcome major bleeding when compared with placebo or standard of care in adult ambulatory patients with malignancy initiating chemotherapy.
Sources: Alexander, 2023; Agnelli, 2009; Kakkar, 2004; Macbeth, 2016; Perry, 2010 and Sideras, 2006 |
Clinically relevant non-major bleeding (CRNMB)
DOAC
|
Low GRADE |
In adult ambulatory patients with malignancy initiating chemotherapy and an intermediate to high risk Khorana-score and no risk factors for bleeding, thromboprophylaxis with DOACs may result in little to no difference in number of patients with a CRNMB, compared to placebo.
Source: Carrier, 2019 and Khorana, 2019 |
LMWH
|
Very low GRADE |
The evidence is very uncertain about the effect of thromboprophylaxis with LMWHs on the outcome CRNMB when compared with standard of care in adult ambulatory patients with malignancy initiating chemotherapy and a high risk on thrombosis.
Source: Alexander, 2023 |
Arterial thromboembolism
DOAC
|
Very low GRADE |
The evidence is very uncertain about the effect of thromboprophylaxis with DOACs on the outcome ATE when compared with placebo in adult ambulatory patients with malignancy initiating chemotherapy and an intermediate to high risk Khorana-score and no risk factors for bleeding.
Source: Carrier, 2019 and Khorana, 2019 |
LMWH
|
Very low GRADE |
The evidence is very uncertain about the effect of thromboprophylaxis with LMWHs on the outcome ATE when compared with standard of care in adult ambulatory patients with malignancy initiating chemotherapy.
Source: Agnelli, 2009; Alexander, 2023 and Macbeth, 2016 |
Quality of life
DOAC
|
No GRADE |
None of the included studies on the effect of thromboprophylaxis with DOACs in patients with malignancy initiating chemotherapy reported on the outcome measure quality of life. Therefore, no conclusion can be drawn on the effect of thromboprophylaxis with DOACs on the outcome measure quality of life in adult ambulatory patients with malignancy initiating chemotherapy.
Sources: none |
LMWH
|
Very low GRADE |
The evidence is very uncertain about the effect of thromboprophylaxis with LMWHs on the outcome measure quality of life when compared with placebo or standard of care in adult ambulatory patients with malignancy initiating chemotherapy.
Sources: Macbeth, 2016 and Sideras, 2006 |
Discontinuation of oncological treatment/complementary treatments and quality of dying and death
|
No GRADE |
None of the included studies reported on the outcome measures discontinuation of oncological treatment/complementary treatments and quality of dying and death. Therefore, no conclusion can be drawn on the effect of thromboprophylaxis with DOAC or LMWH on the outcome measures discontinuation of oncological treatment/complementary treatments and quality of dying and death in adult ambulatory patients with malignancy initiating chemotherapy.
Sources: none |
Samenvatting literatuur
Description of studies
Rutjes (2020) performed a systematic review to evaluate the effect of primary thromboprophylaxis in adult ambulatory patients with malignancy initiating chemotherapy. Several databases were searched up to August 2020, including The Cochrane Vascular Specialized Register, Cochrane Central Register of Controlled Trials, Medline, Embase, CINAHL EBSCO, and AMED Ovid. They included studies including ambulatory patients (outpatient) (children and adults) with either solid or hematological cancer at any stage. In total, 32 studies (N=15,678) were included. Of those studies, eight (≥ Phase III) studies* reported data on the effect of thromboprophylaxis with DOAC, LMWH, or fondaparinux in adult patients, compared to placebo or standard of care (no thromboprophylaxis). Quality of the studies was assessed using the Cochrane’s risk of bias tool. Table 1 lists more details on the eight studies that were included in our literature analysis. Most studies were (partly) funded by pharmaceutical companies. For the studies of Agnelli (2009) and Khorana (2019), it was reported that the sponsoring party had an active role in writing and/or editing the manuscript and/or in interpreting the data.
In general, the studies included patients who are at (higher) risk of developing thrombosis. Therefore, the included study population will not reflect the full spectrum of the target population, namely patients with a malignancy initiating systemic treatment.
*Reasons for exclusion of studies are described in the table under the tab Methods.
Alexander (2023) performed an open-label RCT (TARGET-TP) to evaluate the effect of thromboprophylaxis with enoxaparin in adult patients with lung or gastrointestinal cancer, receiving anti-cancer therapy with or without radiotherapy/immunotherapy. Based on fibrinogen and D-dimer levels, 200 patients (61%) were classified as being at high risk of venous thromboembolism. These 200 high-risk patients were randomized to receive either enoxaparin (40 mg, subcutaneously, once daily for ≤ 90 days, N=100) or no thromboprophylaxis (control group, N=100). Median age (range) was 67 (30-87) years in the enoxaparin group and 66 (31-85) in the control group. Of the patients in the enoxaparin group, 62 (62%) were male, compared to 55 (55%) in the control group. In the enoxaparin group were 52 (52%) and 5 (5%) patients with respectively metastatic disease and prior thromboembolism, compared to 44 (44%) and 7 (7%) patients in the control group. Primary follow-up was 180±30 days.
Table 1. Details of studies that are included in the literature analysis on the effect of thromboprophylaxis in ambulatory patients with cancer initiating chemotherapy, adapted from Rutjes (2020)
|
Author, year |
Participants (N) |
Disease characteristics |
Intervention |
Comparison |
Follow-up (median (IQR)) |
|
Agnelli, 2009 (PROTECHT-trial) |
I: 779, C: 387 |
Metastatic or locally advanced lung, gastrointestinal, pancreatic, breast, ovarian, or head and neck cancer |
Nadroparin
3800 IU SC, once daily for max. 120±10 days /duration of chemotherapy, median duration: NR |
Placebo |
I: 111 days (NR), C: 113 (NR) days |
|
Altinbas, 2004 |
I: 42, C: 42 |
Small-cell lung carcinoma, ECOG performance <3 and normal haematological, renal, and hepatic function tests |
Dalteparin
5000 IU SC, once daily, median (IQR) duration: 18 weeks (NR) |
No dalteparin |
10 (2-33 (range)) months |
|
Sideras, 2006* |
first part: n=50, I: 24, C:26 second part: n=88, I: 44, C: 44
|
Advanced breast cancer, failed first-line chemotherapy; advanced prostate cancer, failed primary hormonal therapy; advanced lung cancer; or advanced colorectal cancer |
Dalteparin
5000 IU SC, for 18 weeks or until disease progression, median duration: NR |
First part from dec 1998 to feb 2020 placebo, second part from feb 2020 to June 2021 standard care alone |
NR, 18 months was planned |
|
Kakkar, 2004 (FAMOUS-trial) |
I: 196, C: 189 |
Advanced stage III/IV cancer of the breast, lung, gastrointestinal tract, pancreas, liver, genitourinary tract, ovary, or uterus. |
Dalteparin
5000 IU SC, once daily for 1 year or until death, median duration: NR |
Placebo |
I: 10 (NR) months, C: 9 (NR) months |
|
Perry, 2010 (PRODIGE trial) |
I: 98, C: 88 |
Grade 3 or grade 4 Glioma |
Dalteparin
5000 IU SC, once daily, median (IQR) duration: 183 (NR) days. |
Placebo
Median (IQR) duration: 157 (NR) days |
NR, 12 months was planned |
|
Macbeth, 2016 (FRAGMATIC-trial) |
I: 102, C: 1101 |
Primary bronchial carcinoma of any stage and histology |
Dalteparin
5000 IU SC, once daily for 24 weeks (median duration: NR, reported was that 180 (18.4%) patients received full number of syringes) |
Standard care |
23.1 (3.6-31.2) months |
|
Khorana, 2019 (CASSINI-trial) |
I: 436, C: 421 |
High-risk ambulatory patients with solid cancer or lymphoma who had a Khorana score of ≥ 2, had a plan to start a new systemic regimen within 1 week before or after initiating the trial regimen and had no DVT on baseline screening ultrasonography. |
Rivaroxaban
10 mg, once daily up to 180 days, mean (range) duration: 4.3 (NR) months |
Placebo |
NR |
|
Carrier, 2019 (AVERT- trial) |
I: 291, C: 283 |
Patients with a newly diagnosed cancer site or progression of the malignant disease after complete or partial remission who were initiating a new course of chemotherapy with a minimum intent of 3 months' therapy and who had a Khorana score of ≥ 2. |
Apixaban
2.5 mg, twice daily for 6 months, median (IQR) duration:157 (78-168) days |
Placebo
Median (IQR) duration:155 (83-168) days |
183 (NR) days |
C: control, I: intervention, NR: not reported, ECOG: Eastern Cooperative Oncology Group
*The study was modified because of concerns that the low accrual rate was related to the requirements for placebo injections (first phase). The saline placebo injections were eliminated, then, unblinded LMWH was compared with standard clinical care (second phase)
Results
Net clinical benefit
Net clinical benefit was defined as a composite outcome of non-fatal VTE, non-fatal major bleeding and mortality. We used non-fatal VTE and non-fatal major bleeding in calculating net clinical benefit to prevent double counting of fatal events. In randomized controlled trials evaluating thromboprophylaxis, major bleeding events are usually assessed during the study treatment period, while VTE is assessed during the whole follow-up period. This should have prevented double counting of patients who potentially developed a non-fatal VTE and subsequently developed a non-fatal major bleeding after initiating anticoagulation. Only studies that reported on non-fatal VTE, non-fatal major bleeding, and mortality are included in the analysis on the outcome measure net clinical benefit.
We first describe the results on the outcomes VTE, major bleeding, and mortality separately and thereafter report the results on the outcome measure net clinical benefit.
Venous thromboembolism
Rutjes (2020) defined the outcome VTE as symptomatic and incidental VTE (DVT and PE).
DOACs
Two studies reported on the outcome VTE (Carrier, 2019 and Khorana, 2019). In the trial evaluating apixaban (Carrier, 2019), the outcome was symptomatic or incidentally detected VTE (DVT and PE). In the trial evaluating rivaroxaban (Khorana, 2019), the outcome was symptomatic or incidentally detected VTE (DVT and PE), also including DVT detected by serial screening ultrasonography, which was performed at weeks 8, 16, and 26.
In the apixaban group, 12/288 (4.2%) patients developed VTE compared to 28/275 (10.2%) patients in the placebo group (Carrier, 2019). This corresponds to a risk ratio (RR, 95%CI) of 0.41 (0.21 to 0.79). Risk difference (RD, 95%CI) was -0.06 (-0.10 to -0.02), which was in favour of the apixaban group and was considered clinically relevant. Corresponding NNT is -17 (-10 to -50).
In the rivaroxaban group, 25/420 (6.0%) patients developed VTE compared to 37/421 (8.8%) patients in the placebo group (Khorana, 2019). This corresponds to a RR (95%CI) of 0.68 (0.42 to 1.10). RD (95%CI) was -0.03 (-0.06 to 0.01), which was in favour of the rivaroxaban group and considered to be (borderline) clinically relevant. Corresponding NNT (95%CI) is -33 (-17 to 100).
LMWH
Three studies reported on the outcome VTE (Agnelli, 2009; Alexander, 2023 and Macbeth, 2016). Other studies reported on symptomatic VTE (Altinbas, 2004; Kakkar, 2004; Sideras, 2006 and Perry, 2010). Despite this, results of those seven studies were pooled.
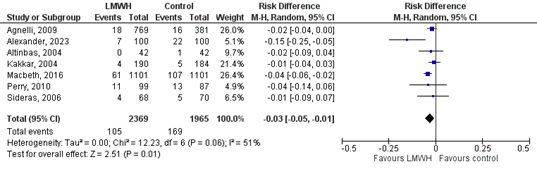
In the LMWH-group 105/2369 (4.4%) patients developed VTE, compared to 169/1965 (8.6%) patients in the control group (Figure 1). This corresponds to a RR (95%CI) of 0.57 (0.45 to 0.72). RD (95%CI) was -0.03 (-0.05 to -0.01), which was in favour of the LMWH-group and considered to be clinically relevant (borderline). Corresponding NNT (95%CI) is -33 (-20 to -100).
Figure 1. The effect of LMWH on the outcome VTE in adult patients with malignancy initiating chemotherapy
Based on Rutjes (2020) and Alexander (2023)
Fondaparinux
None of the included studies used fondaparinux as thromboprophylaxis in patients with malignancy initiating chemotherapy.
Major bleeding
Rutjes (2020) defined the outcome major bleeding as an overt bleeding associated with a decrease in hemoglobin of 2 g/dL or more or leading to a transfusion of two or more units of packed red blood cells or whole blood; bleeding that occurred at a critical site (intracranial, intraspinal, intraocular, pericardial, intra-articular, intramuscular with compartment syndrome, retroperitoneal); or bleeding contributing to death. This definition is identical to the definition of major bleeding as per the International Society on Thrombosis and Haemostasis.
DOACs
Two studies reported on the outcome major bleeding (Carrier, 2019 and Khorana, 2019). In the apixaban group 10/288 (3.5%) patients had a major bleeding while receiving study drug, compared to 5/275 (1.8%) patients in the placebo group (Carrier, 2019). This corresponds to a RR (95%CI) of 1.91 (0.66 to 5.52). RD (95%CI) was 0.02 (-0.01 to 0.04), which was in favour of the placebo group and not considered to be clinically relevant. Corresponding NNH (95%CI) is 50 (-100 to 25).
In the rivaroxaban-group 8/405 (2%) patients had a major bleeding while receiving study drug, compared to 4/404 (1%) patients in the placebo group (Khorana, 2019). This corresponds to a RR (95%CI) of 2.00 (0.61 to 6.57). RD (95%CI) was 0.01 (-0.01 to 0.03), which was in favour of the placebo group and not considered to be clinically relevant. Corresponding NNH (95%CI) is 100 (-100 to 33).
LMWH
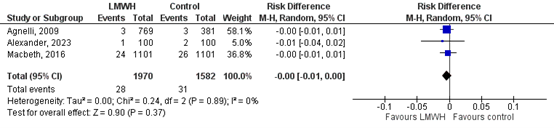
Six studies reported on the outcome major bleeding (Alexander, 2023; Agnelli, 2009; Kakkar, 2004; Macbeth, 2016; Perry, 2010 and Sideras, 2006).
In the LMWH-group 26/2327 (1.1%) patients had a major bleeding while receiving study drug, compared to 16/1923 (0.8%) patients in the control group (Figure 2). This corresponds to a RR (95%CI) of 1.35 (0.67 to 2.70). RD (95%CI) was 0.01 (0.00 to 0.01), which was in favour of the control group and not considered to be clinically relevant. Corresponding NNH (95%CI) is 100 (0 to 100).
Figure 2. The effect of LMWH on the outcome major bleeding in adult patients with malignancy initiating chemotherapy
 Based on Rutjes (2020) and Alexander (2023)
Based on Rutjes (2020) and Alexander (2023)
Fondaparinux
None of the included studies used fondaparinux as thromboprophylaxis treatment in patients with malignancy initiating chemotherapy.
Mortality
Rutjes (2020) defined the outcome mortality as one year overall mortality and therefore we derived the data on all-cause mortality from the individual studies. Macbeth (2016), Carrier (2019) and Khorana (2019) defined mortality as all-cause mortality within the study period.
DOACs
Two studies reported on the outcome mortality (Carrier, 2019 and Khorana, 2019). In the apixaban group 35/288 (12.2%) patients died, compared to 27/275 (9.8%) patients in the placebo group (Carrier, 2019). This corresponds to a RR (95%CI) of 1.24 (0.77 to 1.99). RD (95%CI) was 0.02 (-0.03 to 0.07), which was in favour of the placebo group and not considered to be clinically relevant. Corresponding NNT (95%CI) is 50 (-33.3 to 14.3).
In the rivaroxaban group 84/420 (20%) patients died, compared to 100/421 (23.8%) patients in the placebo group (Khorana, 2019). This corresponds to a RR (95%CI) of 0.84 (0.65 to 1.09). RD (95%CI) was -0.04 (-0.09 to 0.02), which was in favour of the rivaroxaban group and not considered to be clinically relevant. Corresponding NNT (95%CI) is -25 (-11 to 50).
LMWH
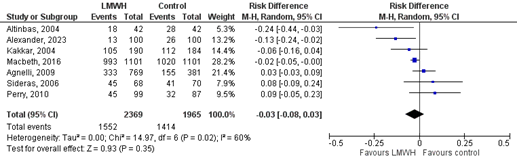
Seven studies reported on the outcome mortality (Agnelli, 2009; Alexander, 2023; Altinbas, 2004; Kakkar, 2004; Macbeth, 2016; Perry, 2010 and Sideras, 2006). In the LMWH-group 1552/2369 (65.5.%) patients died compared to 1414/1965 (72.0%) patients in the control group (Figure 3). This corresponds to a RR (95%CI) of 0.97 (0.87 to 1.08). RD (95%CI) was -0.03 (-0.08 to 0.03), which was in favour of the LMWH-group and considered to be (borderline) clinically relevant. Corresponding NNT (95%CI) was -33 (-12.5 to 33).
Figure 3. The effect of LMWH on the outcome all-cause mortality in adult patients with malignancy initiating chemotherapy
Based on Rutjes (2020) and Alexander (2023)
Fondaparinux
None of the included studies used fondaparinux as thromboprophylaxis in patients with malignancy initiating chemotherapy.
Net clinical benefit
DOACs
Carrier (2019) and Khorana (2019) reported on the outcomes non-fatal VTE, non-fatal major bleeding and all-cause mortality, from which the net clinical benefit could be calculated. Results are described in Table 2. Net clinical benefit calculated using results of Khorana (2019) was considered clinically relevant, which was not the case for net clinical benefit calculated based on Carrier (2019).
LMWH
Agnelli (2009) and Sideras (2006) reported on the outcomes non-fatal VTE, non-fatal major bleeding and one-year mortality, from which the net clinical benefit could be calculated. Results are described in Table 2. The calculated net clinical benefit was not considered to be clinically relevant.
Table 2. Net clinical benefit of thromboprophylaxis in adult patients with malignancy initiating chemotherapy (Agnelli, 2009; Sideras, 2006; Carrier, 2019 and Khorana, 2019)
|
|
Non-fatal VTE (n/N) |
Non-fatal major bleeding (n/N) |
Mortality (n/N) |
Net clinical benefit (n/N) |
|
LMWH |
||||
|
Agnelli (2009) |
I: 18/769 C: 16/381 |
I: 4/769 C: 0/381 |
I: 333/769 C: 155/381 |
RD (95%CI): 0.01 (-0.05 to 0.07) |
|
Sideras (2006) |
I: 4/68 C: 5/70 |
I: 1/68 C: 4/70 |
I: 45/68 C: 41/70 |
RD (95%CI): 0.02 (-0.13 to 0.17) |
|
DOAC |
||||
|
Carrier (2019) |
I: 12/288 C: 28/275
|
I: 10/288 C: 5/275
|
I: 35/288 C: 27/275 |
RD (95%CI): -0.02 (-0.09 to 0.05) |
|
Khorana (2019) |
I: 24/420 C: 34/421
|
I: 7/405 C: 4/404
|
I: 84/420 C: 100/421 |
RD (95%CI): -0.05 (-0.12 to 0.01) |
DOAC: direct oral anticoagulants; LMWH: low molecular weight heparin, RD: Risk Difference, VTE: venous thromboembolism, I:intervention , C:comparison, CI: confidence interval
Clinically relevant non-major bleeding
Rutjes (2020) reported on the outcome clinically relevant bleeding, which was defined as major and clinically relevant non-major bleeding. Therefore, data on CRNMB is derived from the individual studies.
DOACs
Two studies reported on the outcome CRNMB, which was defined according to ISTH criteria as bleeding not meeting the definition of major bleeding, but leading to an intervention, hospitalization, increased level of care, or face to face evaluation (Carrier, 2019 and Khorana, 2019). In the apixaban group 21/288 (7.3%) patients developed CRNMB while receiving study drug, compared to 15/275 (5.5%) patients in the placebo group (Carrier, 2019). This corresponds to a hazard ratio (HR, 95%CI) 1.28 (0.89 to 1.84). RD (95%CI) was 0.02 (-0.02 to 0.06), which was in favour of the placebo group and not considered to be clinically relevant. Corresponding NNH (95%CI) is 50 (-50 to 17).
In the rivaroxaban-group 11/405 (2.7%) patients developed CRNMB while receiving study drug, compared to 8/404 (2%) patients in the placebo group (Khorana, 2019). This corresponds to a hazard ratio (HR, 95%CI) 1.34 (0.54 to 3.32). RD (95%CI) was 0.01 (-0.01 to 0.03), which was in favour of the placebo group and not considered to be clinically relevant. Corresponding NNH (95%CI) is 100 (-100 to 33).
LMWH
One study reported on the outcome CRNMB, which was defined as bleeding not meeting criteria for major bleeding but that would be considered relevant and not trivial by a patient (Alexander, 2023). In the LMWH-group, 16/100 (16%) patients developed CRNMB while receiving study drug, compared to 9/100 (9%) patients in the control group. Adjusted HR (95%CI) was 2.63 (0.23 to 29.71). RD (95%CI) was 0.07 (-0.02 to 0.16), which was in favour of the control group and considered to be clinically relevant. Corresponding NNH (95%CI) was 14 (-50 to 6).
Fondaparinux
None of the included studies used fondaparinux as thromboprophylaxis in patients with malignancy initiating chemotherapy.
Arterial thromboembolism
Rutjes (2020) reported on the outcome ATE, which was defined as symptomatic ATE. However, for the study of Carrier (2019) data on ATE were not available in Rutjes (2020). The working group knew the systematic review and meta-analysis of Xu (2023) in which this data was reported. Therefore, for the study of Carrier (2019) data on the outcome ATE are deduced from Xu (2023). Xu (2023) did not have complementary data on the outcome ATE for the comparisons on LMWH.
DOACs
Two studies reported on the outcome ATE (Carrier, 2019 and Khorana, 2019).
In the apixaban group 1/288 (0.3%) patients developed ATE, compared to 0/275 (0%) patients in the placebo group. This corresponds to a RR (95%CI) of 2.87 (0.12 to 70.03). RD (95%CI) was 0.00 (-0.01 to 0.01), which was in favour of the placebo group and not considered to be clinically relevant. Corresponding NNT (95%CI) was 0 (-100 to 100).
In the rivaroxaban-group 4/420 (1%) patients developed ATE, compared to 7/421 (1.7%) patients in the placebo group. This corresponds to a RR (95%CI) of 0.57 (0.17 to 1.94). RD (95%CI) was -0.01 (-0.02 to 0.01), which was in favour of the rivaroxaban group and not considered to be clinically relevant. Corresponding NNT (95%CI) was -100 (-50 to 100).
LMWH
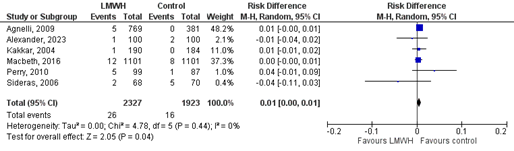
Three studies reported on the outcome ATE, which was not further specified in Rutjes (2020) (Agnelli, 2009; Alexander, 2023 and Macbeth, 2016). In the LMWH-group, 28/1970 (1.4%) patients developed ATE, compared to 31/1582 (2.0%) patients in the control group (Figure 4). This corresponds to a RR (95%CI) of 0.84 (0.51 to 1.40). RD (95%CI) was -0.00 (-0.01 to 0.00), which was in favour of the LMWH-group and not considered to be clinically relevant. Corresponding NNT (95%CI) was 0 (-100 to 0).
Figure 4. The effect of LMWH on the outcome arterial thromboembolic events in adult patients with malignancy initiating chemotherapy
Based on Rutjes (2020) and Alexander (2023)
Fondaparinux
None of the included studies used fondaparinux as thromboprophylaxis in patients with malignancy initiating chemotherapy.
Quality of life
DOACs
None of the included studies on the effect of thromboprophylaxis with DOACs in patients with malignancy initiating chemotherapy reported on the outcome QoL.
LMWH
Two studies reported on the outcome QoL (Macbeth, 2016 and Sideras, 2006). Sideras (2006) measured QoL using the single-item tool Uniscale and a 5-item series of self-assessment measures supplemented by a 13-item symptom distress scale. In the LMWH-group, 37/68 (54.4%) patients had a decrease of ≥ 10 points on the 100-point scale Uniscale, compared to 36/70 (51.4%) patients in the control group. This corresponds to a RR (95%CI) of 1.06 (0.77 to 1.45), which was in favour of the control group. This difference was not considered clinically relevant.
Macbeth (2016) used the Hospital Anxiety and Depression Score and the EuroQol 5 Dimensions (EQ-5D) to assess QoL. At six months follow-up, the mean EQ-5D score (±SD) was 69.95±26.65 in the LMWH-group (N=486), compared to 69.84±24.89 in the control group (N=454). This corresponds to a mean difference (95%CI) of 0.11 (–3.18 to 3.40), which was in favour of the LMWH-group. This difference was not considered clinically relevant.
At 12 months follow-up, the mean EQ-5D score (±SD) was 68.08±25.92 in the LMWH-group (N=221), compared to 68.42±26.95 in the control group (N=224). This corresponds to a mean difference (95%CI) of –0.34 (–5.25 to 4.57), which is in favour of the control group. This difference was not considered clinically relevant.
Fondaparinux
None of the included studies used fondaparinux as thromboprophylaxis in patients with malignancy initiating chemotherapy.
Discontinuation of oncological treatment/complementary treatments and quality of dying and death
None of the included studies reported on the outcome measures discontinuation of oncological treatment/complementary treatments and quality of dying and death.
Level of evidence of the literature
Fondaparinux
None of the included studies used fondaparinux as thromboprophylaxis in patients with malignancy initiating chemotherapy. Therefore, no conclusion can be drawn on the effect of thromboprophylaxis with fondaparinux on all outcome measures in adult ambulatory patients with malignancy, initiating chemotherapy compared with placebo or no thromboprophylaxis.
General remarks on industry sponsorship
Most studies were (partly) funded by pharmaceutical companies. For the studies of Agnelli (2009) and Khorana (2019) it was reported that the sponsoring party had an active role (e.g. interpretation of the data, writing and/or editing the manuscript). However, the level of evidence was not further downgraded because it is unlikely to have had an influence on the results because of the a priori defined outcomes, double blind design, and adjudication of outcome events.
Net clinical benefit
DOACs
Evidence regarding the outcome measure net clinical benefit comes from RCTs and therefore the level of evidence started as high. The level of evidence was downgraded to low. There was risk of bias (attrition bias, sampling bias, serial screening for DVT; downgraded one level). Furthermore, the 95%CI of the effect estimate crossed one of the thresholds for clinical relevance (imprecision, downgraded one level).
LMWH
Evidence regarding the outcome measure net clinical benefit comes from RCTs and therefore the level of evidence started as high. The level of evidence was downgraded to very low. There was risk of bias (open label trial, attrition bias, selection bias; downgraded one level). Furthermore, the 95%CI of the effect estimate crossed the thresholds for clinical relevance (imprecision, downgraded two levels).
Venous thromboembolism
DOACs
Evidence regarding the outcome VTE comes from RCTs and therefore the level of evidence started as high. The level of evidence was downgraded to low. There was risk of bias (attrition bias, sampling bias, serial screening on DVT; downgraded one level). Furthermore, the 95%CI of the effect estimate crossed one of the thresholds for clinical relevance (imprecision, downgraded one level).
LMWH
Evidence regarding the outcome VTE comes from RCTs and therefore the level of evidence started as high. The level of evidence was downgraded to low. There was risk of bias (e.g. attrition bias and open-label trials, downgraded one level). Furthermore, the 95%CI of the effect estimate crossed one of the thresholds for clinical relevance (imprecision, downgraded one level).
Major bleeding
DOACs
Evidence regarding the outcome major bleeding comes from RCTs and therefore the level of evidence started as high. The level of evidence was downgraded to low. There was risk of bias (attrition bias, sampling bias; downgraded one level). Furthermore, the 95%CI of the effect estimate crossed one of the thresholds for clinical relevance (imprecision, downgraded one level).
LMWH
Evidence regarding the outcome major bleeding comes from RCTs and therefore the level of evidence started as high. The level of evidence was downgraded to very low. There was high risk of bias (attrition bias, open label trials, downgraded one level). The results were inconsistent (inconsistency, downgraded one level) and the number of events was low (imprecision, downgraded one level).
Mortality
DOACs
Evidence regarding the outcome mortality comes from RCTs and therefore the level of evidence started as high. The level of evidence was downgraded to very low. There was risk of bias (attrition bias, sampling bias; downgraded one level). Furthermore, the 95%CI of the effect estimate crossed the thresholds for clinical relevance (imprecision, downgraded two levels).
LMWH
Evidence regarding the outcome mortality comes from RCTs and therefore the level of evidence started as high. The level of evidence was downgraded to very low. There was risk of bias (e.g. attrition bias, downgraded one level). Results were inconsistent (inconsistency, downgraded one level) and 95%CI of the effect estimate crossed the thresholds for clinical relevance (imprecision, downgraded one level). It was decided to downgrade one level for imprecision, due to the fact that part of the imprecision might be attributable to the inconsistent results.
Clinically relevant non-major bleeding
DOACs
Evidence regarding the outcome mortality comes from RCTs and therefore the level of evidence started as high. The level of evidence was downgraded to low. There was risk of bias (attrition bias, sampling bias; downgraded one level). Furthermore, the number of events was low (imprecision, downgraded one level).
LMWH
Evidence regarding the outcome CRNMB comes from a RCT and therefore the level of evidence started as high. The level of evidence was downgraded to very low. There was risk of bias (unclear follow-up and open label trial, downgraded one level). Furthermore, the 95%CI of the effect estimate crossed one of the thresholds for clinical relevance and the number of events was low (imprecision, downgraded two levels).
Arterial thromboembolism
DOACs
Evidence regarding the outcome ATE comes from RCTs and therefore the level of evidence started as high. The level of evidence was downgraded to very low. There was risk of bias (attrition bias, sampling bias; downgraded one level). Furthermore, the number of events was very low (serious imprecision, downgraded two levels).
LMWH
Evidence regarding the outcome ATE comes from RCTs and therefore the level of evidence started as high. The level of evidence was downgraded to very low. There was risk of bias (e.g. attrition bias, open label trials, downgraded one level). Furthermore, the number of events was very low (imprecision, downgraded two levels).
Quality of life
DOACs
None of the included studies on the effect of thromboprophylaxis with DOACs in patients with malignancy receiving chemotherapy reported on the outcome measure quality of life. Therefore, no conclusion can be drawn on the effect of thromboprophylaxis with DOACs on the outcome measure quality of life in adult ambulatory patients with malignancy, receiving chemotherapy.
LMWH
Evidence regarding the outcome measure QoL comes from RCTs and therefore the level of evidence started as high. The level of evidence was downgraded to very low. There was risk of bias (e.g. open label trials, downgraded two levels). Furthermore the 95%CI of the effect estimate crossed the thresholds for clinical relevance (imprecision, downgraded two levels).
Discontinuation of oncological treatment/complementary treatments and quality of dying and death in the palliative care setting
None of the included studies reported on the outcome measures discontinuation of oncological treatment/complementary treatments and quality of dying and death. Therefore, no conclusion can be drawn on the effect of thromboprophylaxis with DOAC, LMWH, or fondaparinux on the outcome measures discontinuation of oncological treatment/complementary treatments and quality of dying and death in adult ambulatory patients with malignancy, initiating chemotherapy compared to placebo or no thromboprophylaxis.
Zoeken en selecteren
A systematic review of the literature was performed to answer the following question: what are the (un)desirable effects of thromboprophylaxis with Direct Oral Anticoagulants (DOAC), Low-Molecular-Weight Heparin (LMWH) or fondaparinux in adult ambulatory patients with malignancy* in whom systemic anticancer treatment was initiated, compared to placebo or standard of care?
| P (Patients): | Adult ambulatory patients with malignancy* initiating systemic anticancer treatment |
| I (Intervention): |
Thromboprophylaxis (DOAC, LMWH, or fondaparinux) |
| C (Comparison): | Placebo, standard of care |
| O (Outcomes): | Net clinical benefit, venous thromboembolism, major bleeding, mortality, clinically relevant non-major bleeding (CRNMB), arterial thromboembolism (ATE), quality of life, discontinuation of oncological treatment/complementary treatments, quality of dying and death |
*Excluding patients with multiple myeloma, since these patients are already routinely treated with either aspirin or anticoagulation during cancer treatment based on hematological guidelines.
Relevant outcome measures
The guideline development group considered net clinical benefit and mortality as critical outcome measures for decision making; any thromboembolism and CRNMB, quality of life, discontinuation of oncological treatment/complementary treatments, and quality of dying and death as an important outcome measure for decision making.
The working group defined the outcomes as follows:
- VTE: incidental or symptomatic deep vein thrombosis (DVT) or pulmonary embolism (PE)
- Net clinical benefit: composite of non-fatal VTE, non-fatal major bleeding and mortality
- Major bleeding: fatal bleeding, and/or symptomatic bleeding in a critical area or organ, such as intracranial, intraspinal, intraocular, retroperitoneal, intra-articular or pericardial, or intramuscular with compartment syndrome, and/or bleeding causing a fall in hemoglobin levels of 1.24 mmol/L (20 g/L or greater) or more, or leading to a transfusion of 2 U or more of whole blood or red cells, as defined by International Society on Thrombosis and Haemostasis;
A priori, the working group did not define the other outcome measures listed above but used the definitions used in the studies.
The working group defined the following as a minimal clinically (patient) important difference:
- Net clinical benefit, VTE, major bleeding, mortality, CRNMB, ATE: risk difference of 3%*
- For all other outcome measures, the default thresholds proposed by the international GRADE working group were used as a threshold for clinically relevant differences: a 25% difference in relative risk (RR) for dichotomous outcomes (RR <0.8 or RR >1.25), and 0.5 standard deviations (SD) for continuous outcomes.
*Based on the differences applied in the guidelines on thromboprophylaxis in patients with COVID-19. This working group derived the minimal clinically (patient) important differences from the ACCP (2012).
Search and select (Methods)
First search
The databases Medline (via OVID) and Embase (via Embase.com) were searched with relevant search terms until July 4th, 2023. The systematic literature search resulted in 104 hits. Studies were selected based on the following criteria: (systematic reviews of) ≥ Phase III RCTs which compared the efficacy of thromboprophylaxis (DOAC, LMWH, fondaparinux) with placebo or no thromboprophylaxis in adult ambulatory patients with malignancy, initiating systemic anticancer treatment. 22 studies were initially selected based on title and abstract screening. After reading the full text, 21 studies were excluded (see the table with reasons for exclusion under the tab Methods), and one study was included (Cochrane Review of Rutjes, 2020).
Second search
An additional search was performed to complement the evidence in the Cochrane Review of Rutjes (2020). It was decided to omit the term ‘ambulatory’, in order to be sure that all relevant studies were found. The databases Medline (via OVID) and Embase (via Embase.com) were searched with relevant search terms until October 12th, 2023. The systematic literature search resulted in 819 hits. Studies were selected based on the following criteria: (systematic reviews of) ≥ Phase III RCTs which compared the efficacy of thromboprophylaxis (DOAC, LMWH, fondaparinux) with placebo or no thromboprophylaxis in adult patients with malignancy, initiating systemic anticancer treatment. One study was initially selected based on title and abstract screening. After reading the full text, this study was included (Alexander, 2023).
The detailed search strategies are shown under the tab Methods.
Results
Two studies were included in the analysis of the literature (Rutjes, 2020 and Alexander, 2023). Important study characteristics and results are summarized in the evidence tables. The assessment of the risk of bias is summarized in the risk of bias tables.
Referenties
- Agnelli G, Gussoni G, Bianchini C, Verso M, Mandalà M, Cavanna L, Barni S, Labianca R, Buzzi F, Scambia G, Passalacqua R, Ricci S, Gasparini G, Lorusso V, Bonizzoni E, Tonato M; PROTECHT Investigators. Nadroparin for the prevention of thromboembolic events in ambulatory patients with metastatic or locally advanced solid cancer receiving chemotherapy: a randomised, placebo-controlled, double-blind study. Lancet Oncol. 2009 Oct;10(10):943-9. doi: 10.1016/S1470-2045(09)70232-3. Epub 2009 Aug 31. PMID: 19726226.
- Altinbas M, Coskun HS, Er O, Ozkan M, Eser B, Unal A, Cetin M, Soyuer S. A randomized clinical trial of combination chemotherapy with and without low-molecular-weight heparin in small cell lung cancer. J Thromb Haemost. 2004 Aug;2(8):1266-71. doi: 10.1111/j.1538-7836.2004.00871.x. PMID: 15304029.
- Bosch FTM, Mulder FI, Kamphuisen PW, Middeldorp S, Bossuyt PM, Büller HR, van Es N. Primary thromboprophylaxis in ambulatory cancer patients with a high Khorana score: a systematic review and meta-analysis. Blood Adv. 2020 Oct 27;4(20):5215-5225. Doi: 10.1182/bloodadvances.2020003115. PMID: 33104795; PMCID: PMC7594395.
- Carrier M, Abou-Nassar K, Mallick R, Tagalakis V, Shivakumar S, Schattner A, Kuruvilla P, Hill D, Spadafora S, Marquis K, Trinkaus M, Tomiak A, Lee AYY, Gross PL, Lazo-Langner A, El-Maraghi R, Goss G, Le Gal G, Stewart D, Ramsay T, Rodger M, Witham D, Wells PS; AVERT Investigators. Apixaban to Prevent Venous Thromboembolism in Patients with Cancer. N Engl J Med. 2019 Feb 21;380(8):711-719. doi: 10.1056/NEJMoa1814468. Epub 2018 Dec 4. PMID: 30511879.
- Kakkar AK, Levine MN, Kadziola Z, Lemoine NR, Low V, Patel HK, Rustin G, Thomas M, Quigley M, Williamson RC. Low molecular weight heparin, therapy with dalteparin, and survival in advanced cancer: the fragmin advanced malignancy outcome study (FAMOUS). J Clin Oncol. 2004 May 15;22(10):1944-8. doi: 10.1200/JCO.2004.10.002. PMID: 15143088.
- Kaptein FHJ, Guman NAM, van Es N, Kamphuisen PW, Klok FA, Mairuhu ATA, Huisman MV. Treatment and prevention of cancer-associated thrombosis in the Netherlands: A national survey. Res Pract Thromb Haemost. 2023 Jan 24;7(1):100057. Doi: 10.1016/j.rpth.2023.100057. PMID: 36846646; PMCID: PMC9943872.
- Khorana AA, Soff GA, Kakkar AK, Vadhan-Raj S, Riess H, Wun T, Streiff MB, Garcia DA, Liebman HA, Belani CP, O'Reilly EM, Patel JN, Yimer HA, Wildgoose P, Burton P, Vijapurkar U, Kaul S, Eikelboom J, McBane R, Bauer KA, Kuderer NM, Lyman GH; CASSINI Investigators. Rivaroxaban for Thromboprophylaxis in High-Risk Ambulatory Patients with Cancer. N Engl J Med. 2019 Feb 21;380(8):720-728. doi: 10.1056/NEJMoa1814630. PMID: 30786186.
- Kimpton M, Kumar S, Wells PS, Coyle D, Carrier M, Thavorn K. Cost-utility analysis of apixaban compared with usual care for primary thromboprophylaxis in ambulatory patients with cancer. CMAJ. 2021 Oct 12;193(40):E1551-E1560. Doi: 10.1503/cmaj.210523. PMID: 35040802; PMCID: PMC8568073.
- Ladha D, Mallick R, Wang TF, Caiano L, Wells PS, Carrier M. Efficacy and safety of apixaban for primary prevention in gastrointestinal cancers: A post-hoc analysis of the AVERT trial. Thromb Res. 2021 Jun;202:151-154. doi: 10.1016/j.thromres.2021.03.013. Epub 2021 Mar 21. PMID: 33857789.
- Li A, Carlson JJ, Kuderer NM, Schaefer JK, Li S, Garcia DA, Khorana AA, Carrier M, Lyman GH. Cost-effectiveness analysis of low-dose direct oral anticoagulant (DOAC) for the prevention of cancer-associated thrombosis in the United States. Cancer. 2020 Apr 15;126(8):1736-1748. Doi: 10.1002/cncr.32724. Epub 2020 Jan 30. PMID: 31999844.
- Lyman GH, Carrier M, Ay C, Di Nisio M, Hicks LK, Khorana AA, Leavitt AD, Lee AYY, Macbeth F, Morgan RL, Noble S, Sexton EA, Stenehjem D, Wiercioch W, Kahale LA, Alonso-Coello P. American Society of Hematology 2021 guidelines for management of venous thromboembolism: prevention and treatment in patients with cancer. Blood Adv. 2021 Feb 23;5(4):927-974. Doi: 10.1182/bloodadvances.2020003442. Erratum in: Blood Adv. 2021 Apr 13;5(7):1953. PMID: 33570602; PMCID: PMC7903232.
- Macbeth F, Noble S, Evans J, Ahmed S, Cohen D, Hood K, Knoyle D, Linnane S, Longo M, Moore B, Woll PJ, Appel W, Dickson J, Ferry D, Brammer C, Griffiths G. Randomized Phase III Trial of Standard Therapy Plus Low Molecular Weight Heparin in Patients With Lung Cancer: FRAGMATIC Trial. J Clin Oncol. 2016 Feb 10;34(5):488-94. doi: 10.1200/JCO.2015.64.0268. Epub 2015 Dec 23. PMID: 26700124.
- Mones JV, Streiff MB, Khorana AA, Bendheim GA, Damaraju CV, Wildgoose P, Burton P, Riess H, Soff GA. Rivaroxaban thromboprophylaxis for gastric/gastroesophageal junction tumors versus other tumors: A post hoc analysis of the randomized CASSINI trial. Res Pract Thromb Haemost. 2021 Jul 20;5(5):e12549. doi: 10.1002/rth2.12549. PMID: 34308096; PMCID: PMC8292144.
- Mulder FI, Horváth-Puhó E, van Es N, van Laarhoven HWM, Pedersen L, Moik F, Ay C, Büller HR, Sørensen HT. Venous thromboembolism in cancer patients: a population-based cohort study. Blood. 2021 Apr 8;137(14):1959-1969. doi: 10.1182/blood.2020007338. PMID: 33171494.
- Mulder FI, Candeloro M, Kamphuisen PW, Di Nisio M, Bossuyt PM, Guman N, Smit K, Büller HR, van Es N; CAT-prediction collaborators. The Khorana score for prediction of venous thromboembolism in cancer patients: a systematic review and meta-analysis. Haematologica. 2019 Jun;104(6):1277-1287. doi: 10.3324/haematol.2018.209114. Epub 2019 Jan 3. PMID: 30606788; PMCID: PMC6545838.
- Muñoz AJ, Ortega L, Gutiérrez A, Gallardo E, Rubio-Rodríguez D, Rubio-Terrés C, Morón B, García-Alfonso P, Soria JM. Cost-effectiveness of apixaban and rivaroxaban in thromboprophylaxis of cancer patients treated with chemotherapy in Spain. J Med Econ. 2023 Jan-Dec;26(1):1145-1154. doi: 10.1080/13696998.2023.2248839. Epub 2023 Aug 21. PMID: 37602646.
- Perry JR, Julian JA, Laperriere NJ, Geerts W, Agnelli G, Rogers LR, Malkin MG, Sawaya R, Baker R, Falanga A, Parpia S, Finch T, Levine MN. PRODIGE: a randomized placebo-controlled trial of dalteparin low-molecular-weight heparin thromboprophylaxis in patients with newly diagnosed malignant glioma. J Thromb Haemost. 2010 Sep;8(9):1959-65. doi: 10.1111/j.1538-7836.2010.03973.x. PMID: 20598077.
- Potere N, Barco S, Mahé I, Cesarman-Maus G, Angchaisuksiri P, Leader A, Okoye HC, Olayemi E, Ay C, Carrier M, Connors JM, Farmakis IT, Fumagalli RM, Jing ZC, Lee LH, McLintock C, Ní Ainle F, Giannakoulas G, Goto S, Guillermo Esposito MC, Jara-Palomares L, Szlaszynska M, Tan CW, Van Es N, Wang TF, Hunt BJ, Di Nisio M. Awareness of venous thromboembolism among patients with cancer: Preliminary findings from a global initiative for World Thrombosis Day. J Thromb Haemost. 2022 Dec;20(12):2964-2971. doi: 10.1111/jth.15902. Epub 2022 Oct 21. PMID: 36201366; PMCID: PMC9828201.
- Rutjes AW, Porreca E, Candeloro M, Valeriani E, Di Nisio M. Primary prophylaxis for venous thromboembolism in ambulatory cancer patients receiving chemotherapy. Cochrane Database Syst Rev. 2020 Dec 18;12(12):CD008500. doi: 10.1002/14651858.CD008500.pub5. PMID: 33337539; PMCID: PMC8829903.
- Schünemann HJ, Ventresca M, Crowther M, Briel M, Zhou Q, Noble S, Macbeth F, Griffiths G, Garcia D, Lyman GH, Di Nisio M, Iorio A, Mbuagbaw L, Neumann I, van Es N, Brouwers M, Guyatt G, Streiff MB, Marcucci M, Baldeh T, Florez ID, Alma OG, Solh Z, Bossuyt PM, Kahale LA, Ageno W, Bozas G, Büller HR, Lebeau B, Lecumberri R, Loprinzi C, McBane R, Sideras K, Maraveyas A, Pelzer U, Perry J, Klerk C, Agnelli G, Akl EA. Evaluating prophylactic heparin in ambulatory patients with solid tumours: a systematic review and individual participant data meta-analysis. Lancet Haematol. 2020 Oct;7(10):e746-e755. doi: 10.1016/S2352-3026(20)30293-3. PMID: 32976752.
- Sideras K, Schaefer PL, Okuno SH, Sloan JA, Kutteh L, Fitch TR, Dakhil SR, Levitt R, Alberts SR, Morton RF, Rowland KM, Novotny PJ, Loprinzi CL. Low-molecular-weight heparin in patients with advanced cancer: a phase 3 clinical trial. Mayo Clin Proc. 2006 Jun;81(6):758-67. doi: 10.4065/81.6.758. PMID: 16770976.
- Steffel J, Collins R, Antz M, Cornu P, Desteghe L, Haeusler KG, Oldgren J, Reinecke H, Roldan-Schilling V, Rowell N, Sinnaeve P, Vanassche T, Potpara T, Camm AJ, Heidbüchel H; External reviewers. 2021 European Heart Rhythm Association Practical Guide on the Use of Non-Vitamin K Antagonist Oral Anticoagulants in Patients with Atrial Fibrillation. Europace. 2021 Oct 9;23(10):1612-1676. doi: 10.1093/europace/euab065. Erratum in: Europace. 2021 Oct 9;23(10):1676. doi: 10.1093/europace/euab157. PMID: 33895845.
Evidence tabellen
Evidence table for systematic review of RCTs and observational studies (intervention studies)
Research question: What are the (un)desirable effects of thromboprophylaxis with Direct Oral Anticoagulants (DOAC), Low-Molecular-Weight Heparin (LMWH) or fondaparinux in adult ambulatory patients with malignancy in which systemic anticancer treatment was initiated, compared to placebo or standard of care?
|
Study reference |
Study characteristics |
Patient characteristics |
Intervention (I) |
Comparison / control (C)
|
Follow-up |
Outcome measures and effect size |
Comments |
|
Rutjes, 2020
Study characteristics and results are extracted from the SR, for details see study characteristics in Rutjes (2020). |
SR and meta-analysis of RCTs
Literature search up to August 2020
A: Agnelli, 2009 D: Altinbas, 2004 E: Vadhan-Raj, 2013 F: Sideras, 2006 G: Kakkar, 2004 H: Perry, 2010 I: Macbeth, 2016 (TOPIC-2) K: Khorana 2019 L: Carrier, 2019
Study design: Parallel RCT’s, all included studies I: superiority trial
Setting and Country: A: multicenter, EU D: NR E: NR, USA F: multicenter, USA G: multicenter, USA H: multicenter, western countries I: multicenter, UK K: multicenter, USA L: multicenter, Canada
Source of funding and conflicts of interest: A: Commercial funding by pharmaceutical company, scientific director of pharmaceutical company was involved as author D: Funding source not reported, no disclosure of potential COI E: NR (commercial partner listed at clinicaltrials.gov) F: Non-commercial funding, COI NR G: Commercial funding by pharmaceutical company, several COI reported. H: Commercial funding by pharmaceutical companies, lead author reported COI I: Partial commercial funding by pharmaceutical company, some authors reported COI K: Partial commercial funding by pharmaceutical companies, all authors reported COI. L: Partial commercial funding by pharmaceutical companies, all authors reported COI
|
Inclusion criteria SR: RCT’s and quasi-RCT’s on participants who were ambulatory patients receiving chemotherapy at the time of randomisation or study entry. Intervention: Any type of oral or parenteral coagulation.
Exclusion criteria SR: Studies of participants receiving anticoagulation for a previous VTE or an indication other than VTE. Studies evaluating prophylaxis for catheter-related thrombosis.
Total of 32 studies included in qualitive analysis, 19 studies included in meta-analysis. Since part of these studies did not fulfil our inclusion criteria, we described 12 studies in this evidence table.
Important patient characteristics at baseline:
N A: I: 779, C: 387 D: 83 E: I: 38, C: 37 F: first part: n=52, second part: n=86 G: I: 196, C: 189 H: I: 98, C: 88 I: I: 102, C: 1101 K: I: 436, C: 421 L: I: 291, C: 283
Age (mean years±SD) A: I 62.1 (10.3) C: 63.7 (9.2) D: median 58 (IQR 34-75) E: I: 59 (range 36-75), C: 64 (38-77) F: First part: I: 64.5 (NR), C: 63.5 (NR) Second part: I: 68.5 (NR), C: 70.5 (NR) G: I: 62 (IQR 54-68), C: 60.9 (IQR 52-69)) H: I: 57 (range 30-81), C: 55 (range 26-77) I: median I: 65 (IQR 59-71), C: 64 (IQR 58-71) K: I: median 63(range 23-88), C: 62 (range 28-88) L: I: 61.2 (12.4), C: 61.7 (11.3)
Sex (% male): A: 48 D: 82 E: I: 52.6, C: 56.8 F: First part: I: 50, C: 42 Second part: I: 64, C: 70 G: I: 40.5, C: 45.7 H: I: 62, C: 57 I: I: 60, C: 59.6 K: I: 52.9, C: 48.9 L: I: 41.6, C: 42
Metastatic disease (%): A: NR D: I: n=19, C: n=17 E: NR F: NR (all incurable cancer) G: I: 85, C: 87.5 H: NR I: I: 60.9, C: 60.5 K: NR (54.5) in those with solid tumour L: I: 73 (25.1), C: 67 (23.7)
Previous VTE (n (%)): A: I: 12 (1.6), C: 6 (1.6) D: 0 (NA) E: NR F: First part: I: 4, C: 4 Second part: I: 5, C: 0 G: 0 (NA) H: NR I: NR K: I: 13 (3.1), C: 2 (0.5) L: 9 (3.1), 8 (2.8)
Disease characteristics A: Ambulatory patients receiving chemotherapy for metastatic or locally advanced lung, gastrointestinal, pancreatic, breast, ovarian, or head and neck cancer D: small-cell lung carcinoma patients with ECOG performance status of <3 and normal haematological, renal, and hepatic function tests E: Patients with advanced stage adenocarcinoma of the pancreas planning to initiate systemic chemotherapy within 2 weeks, ECOG performance status 0–2, adequate renal function. F: Patients with advanced breast cancer who had failed first-line chemotherapy; advanced prostate cancer who had failed primary hormonal therapy; advanced lung cancer; or advanced colorectal cancer. G: patients with advanced stage III or IV malignant disease of the breast, lung, gastrointestinal tract, pancreas, liver, genitourinary tract, ovary, or uterus. H: Patients with grade 3 or grade 4 Glioma I: Patients with primary bronchial carcinoma of any stage and histology K: High-risk ambulatory patients with solid cancer or lymphoma who had a Khorana score of ≥ 2, had a plan to start a new systemic regimen within 1 week before or after initiating the trial regimen and had no DVT on screening ultrasonography. L: Patients with a newly diagnosed cancer site or progression of the malignant disease after complete or partial remission who were initiating a new course of chemotherapy with a minimum intent of 3 months' therapy and who had a Khorana score of ≥ 2.
Groups comparable at baseline? Yes |
A: Nadroparin, 3800 IU SC, once daily for max. 120 days or for whole duration of chemotherapy D: Dalteparin, 5000 IU SC, once daily for whole duration of therapy (was stopped with disease progression or at end of 18wks chemo) E: Dalteparin, 5000 IU SC once daily for 16 weeks + standard care F: Dalteparin, 5000 IU SC for 18 weeks or until disease progression G: Dalteparin, 5000 IU SC, once daily for 1 year or until death H: Dalteparin, 5000 IU SC, once daily, median duration 183 days. I: Dalteparin, 5000 IU SC once daily for 24 weeks plus standard care K: Rivaroxaban,10 mg once daily up to 180 days, mean treatment period was 4.3 months L: Apixaban, 2.5 mg twice daily for 6 months |
A: Placebo D: no dalteparin E: Standard care F: First part placebo, second part standard care alone G: Placebo H: Placebo, median duration 157 days I: Standard care K: Placebo L: Placebo
|
End-point of follow-up (median): A: I 111 days, C: 113 days D: 10 (2-33) months E: NR F: NR, 18 months was planned G: I: 10 months, C: 9 months H: NR, 12 months was planned I: 23.1 (IQR 3.6-31.2) months K: NR L: 183 days
For how many participants were no complete outcome data available? A: NR D: NR E: NR F: NR G: NR H: NR I: NR K: NR L: NR
|
For all analyses: pooled effect is NA (since not all of the studies in Rutjes (2020) will be included in our analysis.
Symptomatic VTE Defined as objectively verified by means of Doppler (compression) ultrasonography or venography for DVT, and spiral computed tomography, ventilation/perfusion lung scan, or pulmonary angiography for PE.
DOAC (RR (95%CI)) K: 0.79 [0.41 , 1.54] L: 0.39 [0.18 , 0.83]
LMWH (RR (95%CI)) F: 0.41 [0.08 , 2.05] G: 2.91 [0.12 , 70.87] H: 4.39 [0.52 , 36.89] I: 1.50 [0.62 , 3.66]
Fondaparinux NA
Any VTE Defined as symptomatic and incidental VTE
DOAC (RR (95%CI)) K: 0.68 [0.42 , 1.10] L: 0.41 [0.21 , 0.79]
LMWH (RR (95%CI)) A: 0.56 [0.29 , 1.08] E: 0.22 [0.05 , 0.94] I: 0.57 [0.42 , 0.77]
Fondaparinux NA
Major bleed Defined as overt bleeding associated with a decrease in haemoglobin of 2 g/dL or more, or leading to a transfusion of two or more units of packed red blood cells or whole blood; bleeding that occurred at a critical site (intracranial, intraspinal, intraocular, pericardial, intra-articular,intramuscular with compartment syndrome, retroperitoneal); or bleeding contributing to death.
DOAC (RR (95%CI)) K: 2.00 [0.61 , 6.57] L: 1.91 [0.66 , 5.52]
LMWH (RR (95%CI)) F: 0.41 [0.08 , 2.05] G: 2.91 [0.12 , 70.87] H: 4.39 [0.52 , 36.89] I: 1.50 [0.62 , 3.66]
Fondaparinux NA
Clinically relevant bleeding Defined as major and clinically relevant nonmajor bleeding); typically defined as overt bleeding that does not meet the criteria for major bleeding, but is associated with the need for medical intervention, contact with a physician, or interruption of the study drug or with discomfort or impairment of activities of daily life
DOAC (RR (95%CI)) K: 1.58 [0.78 , 3.21]
LMWH (RR (95%CI)) I: 4.43 [2.49 , 7.86]
Fondaparinux NA
Mortality Defined as 1 year overall mortality DOAC (RR (95%CI)) NA
LMWH (RR (95%CI)) D: 0.64 [0.43 , 0.97] F: 1.13 [0.87 , 1.47] G: 0.91 [0.76 , 1.08] H: 1.24 [0.87 , 1.75]
Fondaparinux NA
Quality of life Defined as DOAC (RR (95%CI)) NA
LMWH (RR (95%CI)) F: 1.06 [0.77 to 1.45]
LMWH (MD (95%CI)) I (6 months): 0.11 (-3.18 to 3.40) I (12 months): –0.34 (–5.25 to 4.57)
Fondaparinux NA
Arterial thromboembolic events. Defined as DOAC (RR (95%CI)) K: 0.57 [0.17 , 1.94]
LMWH (RR (95%CI)) A: 0.50 [0.10 , 2.44] I: 0.92 [0.53 , 1.60]
Fondaparinux NA
|
Author’s conclusion In ambulatory cancer patients, primary thromboprophylaxis with direct factor Xa inhibitors may reduce the incidence of symptomatic venous thromboembolism (VTE) (low-certainty evidence) and probably increases the risk major bleeding (moderate-certainty evidence) when compared with placebo. Low-molecular-weight heparin (LMWH) reduces symptomatic VTE with 37 participants requiring prophylaxis to prevent one event (high-certainty evidence). This benefit comes at the cost of a higher incidence of major bleeding, where for each 144 participants treated, one event is expected to occur when compared against placebo or no thromboprophylaxis (moderate-certainty evidence). When deciding whether to use primary antithrombotic prophylaxis in ambulatory cancer patients receiving chemotherapy, clinicians need to determine the patient's baseline risk of VTE with the help of risk-stratification models and weigh the magnitude of benefit with antithrombotic prophylaxis, especially on major clinical endpoints, against the risk of major bleeding complications. Evidence for the use of thromboprophylaxis with anticoagulants other than direct factor Xa inhibitors and LMWH is limited.
Remarks on individual studies F: After 52 accrued participants, the study was modified because of concerns that the low accrual rate was related to the requirements for placebo injections. The saline placebo injections were eliminated, then, unblinded LMWH was compared with standard clinical care. I: The trial did not reach its intended number of events for the primary analysis. Use of prophylactic anticoagulant outside of trial (short-term use, e.g. inpatient thromboprophylaxis, and therapeutic anticoagulation were allowed if clinically indicated according to local guidelines), n (%): 106 (9.7%) in LMWH group; 88 (8.0%) in control group. |
Evidence table for systematic review of RCTs and observational studies (intervention studies)
Research question: What are the (un)desirable effects of thromboprophylaxis with Direct Oral Anticoagulants (DOAC), Low-Molecular-Weight Heparin (LMWH) or fondaparinux in adult ambulatory patients with malignancy in which systemic anticancer treatment was initiated, compared to placebo or standard of care?
|
Study reference |
Study characteristics |
Patient characteristics |
Intervention (I) |
Comparison / control (C)
|
Follow-up |
Outcome measures and effect size 4 |
Comments |
|
Alexander, 2023 |
Type of study: Open-label RCT
Setting and country: Metropolitan (N=1) and regional (N=4) hospitals, Australia
Funding and conflicts of interest: Several authors reported COI. |
Inclusion criteria: Adults with clinician-estimated life expectancy of ≤ 6 months, prior to commencement of systemic anticancer therapies with or without radiotherapy for gastrointestinal or lung cancer (newly diagnosed or relapsed or progressive disease, curative or palliative intent) at 5 hospitals in Australia.
Exclusion criteria: Patients with contraindication to enoxaparin, including conditions placing them at high risk of bleeding or requiring therapeutic anticoagulation.
N total at baseline: Intervention: 100 Control: 100
Important prognostic factors2: Age (median years (range)) I: 67 (30-87) C: 66 (31-85)
Sex: I: 62% M C: 55% M
Metastatic disease (n (%)): I: 52 (52%) C: 44 (44%)
Previous thromboembolism (n (%)) I: 5 (5%) C: 7 (7%)
Groups comparable at baseline? Yes
|
Enoxaparin
40 mg, subcutaneously daily for a minimum of 90 days (extended up to 180 days according to ongoing risk)
|
No thromboprophylaxis
|
Length of follow-up: 180 days ± 30 days
Loss-to-follow-up: Intervention: NR However it was described that 41 (41%) patients discontinued treatment: patient choice (N=8, no reason (N=7), new contraindication (N=6), end of life care (N=6), CRNM bleeding (N=6), thromboembolism (N=3), major bleeding (N=1), other (N=4)) and 2 (2%) patients did not receive intervention.
Control: 0 (0%) Reasons: NA
Incomplete outcome data: Intervention: NR
Control: NR
|
All VTE DVT must be confirmed by ultrasonography, venography or magnetic resonance angiography (cerebral events). PE must be confirmed by spiral CT, CT pulmonary angiography (CTPA) or lung ventilation/ perfusion scan. I: 7/100 (7%) C: 22/100 (22%) aHR (95%CI): 0.28 (0.12-0.65) P = NR
ATE ATE must be confirmed by relevant radiologic imaging, or specifically for myocardial infarct (MI) must meet criteria outlined in the universal definition of MI considering biomarker changes, ischemic symptoms, ECG changes, cardiac imaging abnormalities, and cardiac death. I: 1/100 (1%) C: 2/100 (2%) aHR (95%CI): 0.78 (0.05-13.20) P = NR
All thromboembolism Defined as DVT, PE and ATE. I: 8/100 (8%) * C: 23/100 (23%) HR (95%CI): 0.30 (0.13-0.68) P = 0.005 * 6/8 thromboembolic events occurred without active enoxaparin therapy.
Major bleeding Defined as clinically overt bleeding meeting at least one of the following criteria: fatal bleeding; symptomatic bleeding in a critical area or organ, such as intracranial, intraspinal, intraocular, retroperitoneal, intraarticular or pericardial, or intramuscular with compartment syndrome; bleeding causing a fall in haemoglobin level of 20 g/L or more, or leading to transfusion of two or more units of whole blood or red cells. Clinically overt was defined as new onset visible bleeding or signs and symptoms suggestive of bleeding which in the absence of visible bleeding were confirmed by relevant imaging techniques. I: 1/100 (1%) C: 2/100 (2%) aHR (95%CI): 2.63 (0.23-29.71) P = NR
CRNMB Defined as bleeding not meeting criteria for major bleeding but that would be considered relevant and not trivial by a patient and physician. I: 16/100 (16%) C: 9/100 (9%) aHR (95%CI): 0.68 (0.30-1.55) P = NR *one patient in the control group had both major bleeding and CRNMB.
Mortality Defined as 6 month all cause mortality I: 13/100 (13%) C: 26/100 (26%) aHR (95%CI): 0.45 (0.23-0.87)) P = NR
Quality of life Not reported in this article yet.
Treatment adherence NR
*Adjusted for cancer diagnosis, cancer treatment, stage and hospital site. |
ITT analysis was performed
Fibrinogen and d-dimer levels were used to identify patients with high risk on thromboembolism. In total 200 high-risk patients were randomized to receive either enoxaparin or no thromboprophylaxis. Other patients (low risk, N=128) were assigned to the observational group. These data were not taken into account in our literature analysis.
Of the 100 patients in the intervention group, N=41 discontinued treatment |
Risk of bias table for intervention studies (randomized controlled trials; based on Cochrane risk of bias tool and suggestions by the CLARITY Group at McMaster University)
Research question: What are the (un)desirable effects of thromboprophylaxis with Direct Oral Anticoagulants (DOAC), Low-Molecular-Weight Heparin (LMWH) or fondaparinux in adult ambulatory patients with malignancy in which systemic anticancer treatment was initiated, compared to placebo or standard of care?
|
Study reference
(first author, publication year) |
Was the allocation sequence adequately generated?
Definitely yes Probably yes Probably no Definitely no |
Was the allocation adequately concealed?
Definitely yes Probably yes Probably no Definitely no |
Blinding: Was knowledge of the allocated interventions adequately prevented?
Were patients blinded?
Were healthcare providers blinded?
Were data collectors blinded?
Were outcome assessors blinded?
Were data analysts blinded?
Definitely yes Probably yes Probably no Definitely no |
Was loss to follow-up (missing outcome data) infrequent?
Definitely yes Probably yes Probably no Definitely no |
Are reports of the study free of selective outcome reporting?
Definitely yes Probably yes Probably no Definitely no |
Was the study apparently free of other problems that could put it at a risk of bias?
Definitely yes Probably yes Probably no Definitely no |
Overall risk of bias If applicable/necessary, per outcome measure
LOW Some concerns HIGH
|
|
Alexander (2023) |
Definitely yes;
Reason: Randomization was performed using the randomization module created by an independent statistician.
|
Definitely yes;
Reason: Randomization was performed blinded and in advance of patient recruitment.
|
Definitely no;
Reason: Open-label trial (after randomization the study team, treating clinician and patient were unblinded to treatment allocation). TE and bleeding events were adjudicated by a committee unaware of randomisation. |
No information
|
Probably yes;
Reason: Not all relevant outcomes are reported yet, but might be reported in other (new) articles. |
Definitely yes;
Reason: No other problems noted: |
Some concerns (major bleeding, VTE, mortality, CRNMB, any thromboembolism)
HIGH (quality of life, discontinuation/ supplementation of oncological treatment, quality of dying and death) |
Table of excluded studies
Excluded studies part I
|
Reference |
Reason for exclusion |
|
Carrier M, Abou-Nassar K, Mallick R, Tagalakis V, Shivakumar S, Schattner A, Kuruvilla P, Hill D, Spadafora S, Marquis K, Trinkaus M, Tomiak A, Lee AYY, Gross PL, Lazo-Langner A, El-Maraghi R, Goss G, Le Gal G, Stewart D, Ramsay T, Rodger M, Witham D, Wells PS; AVERT Investigators. Apixaban to Prevent Venous Thromboembolism in Patients with Cancer. N Engl J Med. 2019 Feb 21;380(8):711-719. doi: 10.1056/NEJMoa1814468. Epub 2018 Dec 4. PMID: 30511879. |
Included in Rutjes (2020) |
|
Khorana AA, Soff GA, Kakkar AK, Vadhan-Raj S, Riess H, Wun T, Streiff MB, Garcia DA, Liebman HA, Belani CP, O'Reilly EM, Patel JN, Yimer HA, Wildgoose P, Burton P, Vijapurkar U, Kaul S, Eikelboom J, McBane R, Bauer KA, Kuderer NM, Lyman GH; CASSINI Investigators. Rivaroxaban for Thromboprophylaxis in High-Risk Ambulatory Patients with Cancer. N Engl J Med. 2019 Feb 21;380(8):720-728. doi: 10.1056/NEJMoa1814630. PMID: 30786186 |
Included in Rutjes (2020) |
|
Akl EA, Kahale LA, Ballout RA, Barba M, Yosuico VE, van Doormaal FF, Middeldorp S, Bryant A, Schünemann H. Parenteral anticoagulation in ambulatory patients with cancer. Cochrane Database Syst Rev. 2014 Dec 10;(12):CD006652. doi: 10.1002/14651858.CD006652.pub4. Update in: Cochrane Database Syst Rev. 2017 Sep 11;9:CD006652. PMID: 25491949. |
Recent review available. |
|
Bosch FTM, Mulder FI, Kamphuisen PW, Middeldorp S, Bossuyt PM, Büller HR, van Es N. Primary thromboprophylaxis in ambulatory cancer patients with a high Khorana score: a systematic review and meta-analysis. Blood Adv. 2020 Oct 27;4(20):5215-5225. doi: 10.1182/bloodadvances.2020003115. PMID: 33104795; PMCID: PMC7594395. |
Included only studies in high-risk patients. |
|
Agnelli G, Gussoni G, Bianchini C, Verso M, Mandalà M, Cavanna L, Barni S, Labianca R, Buzzi F, Scambia G, Passalacqua R, Ricci S, Gasparini G, Lorusso V, Bonizzoni E, Tonato M; PROTECHT Investigators. Nadroparin for the prevention of thromboembolic events in ambulatory patients with metastatic or locally advanced solid cancer receiving chemotherapy: a randomised, placebo-controlled, double-blind study. Lancet Oncol. 2009 Oct;10(10):943-9. doi: 10.1016/S1470-2045(09)70232-3. Epub 2009 Aug 31. PMID: 19726226. |
Included in Rutjes (2020) |
|
Carrier M, Abou-Nassar K, Mallick R, Tagalakis V, Shivakumar S, Schattner A, Kuruvilla P, Hill D, Spadafora S, Marquis K, Trinkaus M, Tomiak A, Lee AYY, Gross PL, Lazo-Langner A, El-Maraghi R, Goss G, Le Gal G, Stewart D, Ramsay T, Rodger M, Witham D, Wells PS; AVERT Investigators. Apixaban to Prevent Venous Thromboembolism in Patients with Cancer. N Engl J Med. 2019 Feb 21;380(8):711-719. doi: 10.1056/NEJMoa1814468. Epub 2018 Dec 4. PMID: 30511879. |
Included in Rutjes (2020) |
|
Khorana AA, Soff GA, Kakkar AK, Vadhan-Raj S, Riess H, Wun T, Streiff MB, Garcia DA, Liebman HA, Belani CP, O'Reilly EM, Patel JN, Yimer HA, Wildgoose P, Burton P, Vijapurkar U, Kaul S, Eikelboom J, McBane R, Bauer KA, Kuderer NM, Lyman GH; CASSINI Investigators. Rivaroxaban for Thromboprophylaxis in High-Risk Ambulatory Patients with Cancer. N Engl J Med. 2019 Feb 21;380(8):720-728. doi: 10.1056/NEJMoa1814630. PMID: 30786186 |
Included in Rutjes (2020) |
|
Akl EA, Kahale LA, Ballout RA, Barba M, Yosuico VE, van Doormaal FF, Middeldorp S, Bryant A, Schünemann H. Parenteral anticoagulation in ambulatory patients with cancer. Cochrane Database Syst Rev. 2014 Dec 10;(12):CD006652. doi: 10.1002/14651858.CD006652.pub4. Update in: Cochrane Database Syst Rev. 2017 Sep 11;9:CD006652. PMID: 25491949. |
Recent review available. |
|
Bosch FTM, Mulder FI, Kamphuisen PW, Middeldorp S, Bossuyt PM, Büller HR, van Es N. Primary thromboprophylaxis in ambulatory cancer patients with a high Khorana score: a systematic review and meta-analysis. Blood Adv. 2020 Oct 27;4(20):5215-5225. doi: 10.1182/bloodadvances.2020003115. PMID: 33104795; PMCID: PMC7594395. |
Included only studies in high-risk patients. |
Studies excluded in Rutjes (2020)
|
Reference |
Reason for exclusion |
|
Campos-Cabrera G, Mendez-Garcia E, Campos-CabreraS, Campos-VillagomezJL, Campos-CabreraV. Rivaroxaban or aspirin as thromboprophylaxis in multiple myeloma. Blood 2018;132(Suppl 1):5068. [DOI: 10.1182/blood-2018-99-111579] |
wrong comparison |
|
Chahinian AP, Propert KJ, Ware JH, Zimmer B, Perry MC, Hirsh V, Skarin A, Kopel S, Holland JF, Comis RL, et al. A randomized trial of anticoagulation with warfarin and of alternating chemotherapy in extensive small-cell lung cancer by the Cancer and Leukemia Group B. J Clin Oncol. 1989 Aug;7(8):993-1002. doi: 10.1200/JCO.1989.7.8.993. PMID: 2547030. |
wrong intervention |
|
van Doormaal FF, Di Nisio M, Otten HM, Richel DJ, Prins M, Buller HR. Randomized trial of the effect of the low molecular weight heparin nadroparin on survival in patients with cancer. J Clin Oncol. 2011 May 20;29(15):2071-6. doi: 10.1200/JCO.2010.31.9293. Epub 2011 Apr 18. PMID: 21502549.
|
wrong intervention |
|
Ek L, Gezelius E, Bergman B, Bendahl PO, Anderson H, Sundberg J, Wallberg M, Falkmer U, Verma S, Belting M; Swedish Lung Cancer Study Group (SLUSG). Randomized phase III trial of low-molecular-weight heparin enoxaparin in addition to standard treatment in small-cell lung cancer: the RASTEN trial. Ann Oncol. 2018 Feb 1;29(2):398-404. doi: 10.1093/annonc/mdx716. PMID: 29106448; PMCID: PMC5834130. |
wrong intervention |
|
Elit LM, Lee AY, Parpia S, Swystun LL, Liaw PC, Hoskins P, Julian DH, Julian JA, Levine MN. Dalteparin low molecular weight heparin (LMWH) in ovarian cancer: a phase II randomized study. Thromb Res. 2012 Dec;130(6):894-900. doi: 10.1016/j.thromres.2012.09.010. Epub 2012 Oct 7. PMID: 23046525. |
wrong comparison |
|
Greiner J, Schrappe M, Claviez A, Zimmermann M, Niemeyer C, Kolb R, Eberl W, Berthold F, Bergsträsser E, Gnekow A, Lassay E, Vorwerk P, Lauten M, Sauerbrey A, Rischewski J, Beilken A, Henze G, Korte W, Möricke A; THROMBOTECT Study Investigators. THROMBOTECT - a randomized study comparing low molecular weight heparin, antithrombin and unfractionated heparin for thromboprophylaxis during induction therapy of acute lymphoblastic leukemia in children and adolescents. Haematologica. 2019 Apr;104(4):756-765. doi: 10.3324/haematol.2018.194175. Epub 2018 Sep 27. PMID: 30262570; PMCID: PMC6442986. |
wrong population |
|
Haas SK, Freund M, Heigener D, HeilmannL, Kemkes- Matthes B, von TempelhoK GF, TOPIC Investigators. Lowmolecular- weight heparin versus placebo for the prevention of venous thromboembolism in metastatic breast cancer or stage III/IV lung cancer. Clinical and Applied Thrombosis Hemostasis 2012;18(2):159-65. |
wrong intervention (TOPIC 1 and TOPIC 2), study terminated |
|
Khorana AA, Francis CW, Kuderer NM, Carrier M, Ortel TL, Wun T, Rubens D, Hobbs S, Iyer R, Peterson D, Baran A, Kaproth-Joslin K, Lyman GH. Dalteparin thromboprophylaxis in cancer patients at high risk for venous thromboembolism: A randomized trial. Thromb Res. 2017 Mar;151:89-95. doi: 10.1016/j.thromres.2017.01.009. Epub 2017 Jan 26. PMID: 28139259. |
study terminated |
|
Klerk CP, Smorenburg SM, Otten HM, Lensing AW, Prins MH, Piovella F, Prandoni P, Bos MM, Richel DJ, van Tienhoven G, Büller HR. The effect of low molecular weight heparin on survival in patients with advanced malignancy. J Clin Oncol. 2005 Apr 1;23(10):2130-5. doi: 10.1200/JCO.2005.03.134. Epub 2005 Feb 7. PMID: 15699479. |
wrong intervention |
|
Larocca A, Cavallo F, Bringhen S, Di Raimondo F, Falanga A, Evangelista A, Cavalli M, Stanevsky A, Corradini P, Pezzatti S, Patriarca F, Cavo M, Peccatori J, Catalano L, Carella AM, Cafro AM, Siniscalchi A, Crippa C, Petrucci MT, Yehuda DB, Beggiato E, Di Toritto TC, Boccadoro M, Nagler A, Palumbo A. Aspirin or enoxaparin thromboprophylaxis for patients with newly diagnosed multiple myeloma treated with lenalidomide. Blood. 2012 Jan 26;119(4):933-9; quiz 1093. doi: 10.1182/blood-2011-03-344333. Epub 2011 Aug 11. PMID: 21835953. |
wrong comparison |
|
Lebeau B, Chastang C, Brechot JM, Capron F, Dautzenberg B, Delaisements C, Mornet M, Brun J, Hurdebourcq JP, Lemarie E. Subcutaneous heparin treatment increases survival in small cell lung cancer. "Petites Cellules" Group. Cancer. 1994 Jul 1;74(1):38-45. doi: 10.1002/1097-0142(19940701)74:1<38::aid-cncr2820740108>3.0.co;2-e. PMID: 8004580. |
wrong intervention |
|
Lecumberri R, López Vivanco G, Font A, González Billalabeitia E, Gúrpide A, Gómez Codina J, Isla D, Galán A, Bover I, Domine M, Vicente V, Rosell R, Rocha E. Adjuvant therapy with bemiparin in patients with limited-stage small cell lung cancer: results from the ABEL study. Thromb Res. 2013;132(6):666-70. doi: 10.1016/j.thromres.2013.09.026. Epub 2013 Sep 27. PMID: 24491267. |
wrong intervention |
|
Levine M, Hirsh J, Gent M, Arnold A, Warr D, Falanga A, Samosh M, Bramwell V, Pritchard KI, Stewart D, et al. Double-blind randomised trial of a very-low-dose warfarin for prevention of thromboembolism in stage IV breast cancer. Lancet. 1994 Apr 9;343(8902):886-9. doi: 10.1016/s0140-6736(94)90008-6. PMID: 7908358. |
wrong intervention |
|
Levine MN, Gu C, Liebman HA, Escalante CP, Solymoss S, Deitchman D, Ramirez L, Julian J. A randomized phase II trial of apixaban for the prevention of thromboembolism in patients with metastatic cancer. J Thromb Haemost. 2012 May;10(5):807-14. doi: 10.1111/j.1538-7836.2012.04693.x. PMID: 22409262. |
wrong comparison, Phase II trial |
|
Maraveyas A, Waters J, Roy R, Fyfe D, Propper D, Lofts F, Sgouros J, Gardiner E, Wedgwood K, Ettelaie C, Bozas G. Gemcitabine versus gemcitabine plus dalteparin thromboprophylaxis in pancreatic cancer. Eur J Cancer. 2012 Jun;48(9):1283-92. doi: 10.1016/j.ejca.2011.10.017. Epub 2011 Nov 17. PMID: 22100906. |
phase II trial, wrong intervention |
|
Maurer LH, Herndon JE 2nd, Hollis DR, Aisner J, Carey RW, Skarin AT, Perry MC, Eaton WL, Zacharski LL, Hammond S, Green MR. Randomized trial of chemotherapy and radiation therapy with or without warfarin for limited-stage small-cell lung cancer: a Cancer and Leukemia Group B study. J Clin Oncol. 1997 Nov;15(11):3378-87. doi: 10.1200/JCO.1997.15.11.3378. PMID: 9363869. |
wrong intervention |
|
Meyer G, Besse B, Doubre H, Charles-Nelson A, Aquilanti S, Izadifar A, Azarian R, Monnet I, Lamour C, Descourt R, Oliviero G, Taillade L, Chouaid C, Giraud F, Falcoz PE, Revel MP, Westeel V, Dixmier A, Tredaniel J, Dehette S, Decroisette C, Prevost A, Pichon E, Fabre E, Soria JC, Friard S, Stern JB, Jabot L, Dennewald G, Pavy G, Petitpretz P, Tourani JM, Alifano M, Chatellier G, Girard P. Anti-tumour effect of low molecular weight heparin in localised lung cancer: a phase III clinical trial. Eur Respir J. 2018 Oct 4;52(4):1801220. doi: 10.1183/13993003.01220-2018. PMID: 30262574. |
wrong intervention |
|
Mitchell L, Andrew M, Hanna K, Abshire T, Halton J, Wu J, Anderson R, Cherrick I, Desai S, Mahoney D, McCusker P, Chait P, Abdolell M, de Veber G, Mikulis D. Trend to efficacy and safety using antithrombin concentrate in prevention of thrombosis in children receiving l-asparaginase for acute lymphoblastic leukemia. Results of the PAARKA study. Thromb Haemost. 2003 Aug;90(2):235-44. doi: 10.1160/TH02-11-0283. PMID: 12888870. |
wrong intervention, wrong population, Phase II trial |
|
Palumbo A, Cavo M, Bringhen S, Zamagni E, Romano A, Patriarca F, Rossi D, Gentilini F, Crippa C, Galli M, Nozzoli C, Ria R, Marasca R, Montefusco V, Baldini L, Elice F, Callea V, Pulini S, Carella AM, Zambello R, Benevolo G, Magarotto V, Tacchetti P, Pescosta N, Cellini C, Polloni C, Evangelista A, Caravita T, Morabito F, Offidani M, Tosi P, Boccadoro M. Aspirin, warfarin, or enoxaparin thromboprophylaxis in patients with multiple myeloma treated with thalidomide: a phase III, open-label, randomized trial. J Clin Oncol. 2011 Mar 10;29(8):986-93. doi: 10.1200/JCO.2010.31.6844. Epub 2011 Jan 31. PMID: 21282540. |
wrong comparison |
|
Pelzer U, Opitz B, Deutschinoff G, Stauch M, Reitzig PC, Hahnfeld S, Müller L, Grunewald M, Stieler JM, Sinn M, Denecke T, Bischoff S, Oettle H, Dörken B, Riess H. Efficacy of Prophylactic Low-Molecular Weight Heparin for Ambulatory Patients With Advanced Pancreatic Cancer: Outcomes From the CONKO-004 Trial. J Clin Oncol. 2015 Jun 20;33(18):2028-34. doi: 10.1200/JCO.2014.55.1481. Epub 2015 May 18. PMID: 25987694. |
wrong intervention |
|
Zacharski LR, Henderson WG, Rickles FR, Forman WB, Cornell CJ Jr, Forcier RJ, Edwards R, Headley E, Kim SH, O'Donnell JR, O'Dell R, Tornyos K, Kwaan HC. Effect of warfarin on survival in small cell carcinoma of the lung. Veterans Administration Study No. 75. JAMA. 1981 Feb 27;245(8):831-5. PMID: 6257941. |
wrong intervention |
|
Zwicker JI, Liebman HA, Bauer KA, Caughey T, Campigotto F, Rosovsky R, Mantha S, Kessler CM, Eneman J, Raghavan V, Lenz HJ, Bullock A, Buchbinder E, Neuberg D, Furie B. Prediction and prevention of thromboembolic events with enoxaparin in cancer patients with elevated tissue factor-bearing microparticles: a randomized-controlled phase II trial (the Microtec study). Br J Haematol. 2013 Feb;160(4):530-7. doi: 10.1111/bjh.12163. Epub 2012 Dec 13. PMID: 23240761; PMCID: PMC3609903. |
phase II trial, wrong intervention |
|
Vadhan-Raj S, Zhou_X, Varadhachary_GR, Milind_J, Fogelman_D, ShroK_R, et al. Randomized controlled trial of dalteparin for primary thromboprophylaxis for venous thromboembolism (VTE) in patients with advanced pancreatic cancer (APC): risk factors predictive of VTE. 55th Annual Meeting of the American Society of Hematology; 2013 Dec 7-10; New Orleans, LA. |
abstract |
Verantwoording
Beoordelingsdatum en geldigheid
Publicatiedatum : 22-10-2025
Beoordeeld op geldigheid : 23-09-2025
Algemene gegevens
Voor meer details over de gebruikte richtlijnmethodologie verwijzen wij u naar de Werkwijze. Relevante informatie voor de ontwikkeling/herziening van deze richtlijnmodule is hieronder weergegeven.
De ontwikkeling/herziening van deze richtlijnmodule werd ondersteund door het Kennisinstituut van de Federatie Medisch Specialisten (www.demedischspecialist.nl/kennisinstituut) en werd gefinancierd uit de Stichting Kwaliteitsgelden Medisch Specialisten (SKMS). Patiëntenparticipatie bij deze richtlijn werd medegefinancierd uit de Kwaliteitsgelden Patiënten Consumenten (SKPC) binnen het programma KIDZ. De financier heeft geen enkele invloed gehad op de inhoud van de richtlijnmodule.
Samenstelling werkgroep
Voor het ontwikkelen van de richtlijnmodule is in 2021 een multidisciplinaire werkgroep ingesteld, bestaande uit vertegenwoordigers van alle relevante specialismen (zie hiervoor de Samenstelling van de werkgroep) die betrokken zijn bij de zorg voor patiënten die antitrombotische therapie dan wel tromboseprofylaxe gebruiken.
Kerngroep
- Prof. dr. M.V. (Menno) Huisman, internist-vasculaire geneeskunde, LUMC, NIV (voorzitter)
- Dr. M.J.H.A. (Marieke) Kruip, internist-hematoloog, Erasmus MC, NIV, NVVH (Nederlandse Vereniging voor Hematologie)
- Prof. Dr. F.A. (Erik) Klok, internist-vasculaire geneeskunde, LUMC, NIV
- Dr. J. (Jenneke) Leentjens, internist-vasculaire geneeskunde, RadboudUMC, NIV (vanaf 2023)
- Dr. N. (Nick) van Es, internist-vasculaire geneeskunde, Amsterdam UMC, NIV (vanaf 2023)
- Dr. M.A. (Marc) Brouwer, cardioloog, RadboudUMC, NVVC
- Dr. H.B. (Harmen) Ettema, orthopedisch chirurg, Isala, NOV
- Dr. B. (Banne) Nemeth, aios orthopedie, LUMC, NOV
- Dr. A.M. (Arno) Wiersema, vaatchirurg, Dijklander Ziekenhuis, NVVH (tot 2023)
- Dr. M.C. (Michiel) Warlé, vaatchirurg, RadboudUMC, NVVH (vanaf 2024)
- Dr. M.E. (Maarten) Tushuizen, maag-darm-leverarts, LUMC, NVMDL
- Dr. J.M. (Jonathan) Coutinho, neuroloog, Amsterdam UMC, NVN
- Drs. M.H. (Monique) Suijker, kinderarts-hematoloog, UMC Utrecht, NVK
- Drs. P (Paul) Smits, huisarts/ Kaderhuisarts HVZ, NHG
Klankbordgroep
- Dr. J.J.C.M. (Sjef) van de Leur, arts klinische chemie, Isala, NVKC
- Dr. M.G. (Mariëlle) van Pampus, gynaecoloog, OLVG, NVOG
- Drs. R.J. (Rutger) Lely, radioloog, Amsterdam UMC, NVVR
- Dr. C. (Bibi) van Montfrans, dermatoloog, Erasmus MC, NVDV
- Dr. R.A. (Richard) Faaij, klinisch geriater, Diakonessenhuis, NVKG
- Dr. B. (Baucke) van Minnen, kaakchirurg, UMCG, NVMKA
- Drs. N. (Noa) Rosenberg, beleidsadviseur, Harteraad (vanaf mei 2024)
- I.G.J. (Ilse) Verstraaten MSc, beleidsadviseur, Harteraad (tot 2024)
- Dr. N. (Nakisa) Khorsand, ziekenhuisapotheker, OLVG, NVZA
- Dr. M.F. (Margreet) van Herwaarden, openbaar apotheker, KNMP
- Dr. E.T.T.L. (Eric) Tjwa, MDL-arts, RadboudUMC, NVMDL
- Dr. L.M. (Linda) de Heer, cardio-thoracaal chirurg, UMC Utrecht, NVT
- Prof. dr. S. (Saskia) Middeldorp, internist-vasculaire geneeskunde, Radboudumc, NIV
- Dr. J.M.M.B. (Hans-Martin) Otten, internist-oncoloog, Meander MC, NIV
- Dr. E.J. (Esther) Nossent, longarts, Amsterdam UMC, NVALT
- Dr. C.H. (Heleen) van Ommen, kinderarts-hematoloog, Erasmus MC, NVK
- Dr. K.M.J. (Katja) Heitink, kinderarts-oncoloog, Prinses Maxima Centrum, NVK
- Prof. dr. N.P. (Nicole) Juffermans, intensivist, Amsterdam UMC, NVIC
- Dr. M.C.A. (Marcella) Muller, intensivist, Amsterdam UMC, NVIC
Met ondersteuning van
- H. (Hanneke) Olthuis, adviseur, Kennisinstituut van de Federatie Medisch Specialisten
- H.J. (Harm-Jan) van der Hart, adviseur, Kennisinstituut van de Federatie Medisch Specialisten
Belangenverklaringen
Een overzicht van de belangen van werkgroepleden en het oordeel over het omgaan met eventuele belangen vindt u in onderstaande tabel. De ondertekende belangenverklaringen zijn op te vragen bij het secretariaat van het Kennisinstituut van de Federatie Medisch Specialisten via secretariaat@kennisinstituut.nl.
|
Werkgroeplid |
Functie |
Nevenfuncties |
Gemelde belangen |
Ondernomen actie |
|
Huisman (voorzitter) |
Internist vasculaire geneeskunde |
|
à op geen van deze projecten projectleider, betreft unrestricted grants en veelal financiering promotietrajecten. |
Geen restricties |
|
Banne Nemeth |
Orthopedisch chirurg in opleiding |
Postdoc klinische epidemiologie en orthopedie, LUMC |
Trombosestichting - “VTE following total hip and knee arthroplasty: prediction is the future“ |
Geen restricties |
|
Harmen Ettema |
Orthopedisch chirurg |
Geen |
Gemeld bij herbevestiging in 2025: ZonMW, Distinct trial, tromboseprofylaxe bij orthopedische ingrepen, geen projectleider |
Geen restricties |
|
Jonathan Coutinho |
neuroloog Amsterdam UMC |
Geen |
Gemeld bij herbevestiging in 2025:
|
Restricties t.a.v. besluitvorming modules over andexanet alfa bij bloedingen geen restricties ten gevolge van rol pacific stroke study en dabigatran etixilate/warfarin bij CVT, aangezien deze patientengroepen geen onderwerp zijn van deze richtlijnherziening. |
|
Klok |
Internist vasculaire geneeskunde LUMC Leiden |
Gemeld bij herbevestiging in 2024:
|
|
Geen restricties |
|
Kruip |
Hematoloog Gemeld bij herbevestiging in 2024: Directeur Kwaliteit & Patientenzorg, Erasmus MC, betaald |
Gemeld bij herbevestiging in 2024:
|
|
Geen restricties |
|
Maarten Tushuizen |
MDL-arts LUMC |
Geen |
Maag-Lever-Darmstichting (MLDS) |
Geen restricties |
|
Marc Brouwer |
Cardioloog Radboudumc |
Geen |
Nee |
Geen restricties |
|
Paul Smits |
huisarts, zelfstandig |
Coördinator onderwijscommissie harvaatHAG |
geen |
Geen restricties |
|
Monique Suijker |
Kinderarts-hematoloog werkzaam bij Van Creveldkliniek, UMCU |
Geen |
Geen lopende studies Bayer en Janssen - Einstein Jr studie - gebruik Rivaroxaban bij kinderen – afgesloten |
Geen restricties |
|
Arno Wiersema (teruggetrokken, tot 2024) |
Vaatchirurg, Dijklander ziekenhuis |
Geen |
ZonMw, Amsterdam UMC, Dijklander zh en Medtronic - www.action-1.nl - betreft onderzoek naar rol van heparine bij een open buikslagader operatie, rol als projectleider |
Restricties ten aanzien van besluitvorming over heparine. |
|
Michiel Warlé (vanaf 2024) |
Vaatchirurg Radboudumc |
Werkgroep Landelijk Kennisplatform Antistolling |
ZEGG/ZonMw- GENPAD studie (Cyp2c19 genotypering bij Clopidogrel en perifeer arterieel vaatlijden – hoofdonderzoeker Gemeld bij herbevestiging in 2025: NWO-OTP, Wireless clot retriever, projectleider |
Geen restricties |
|
Nick van Es (vanaf 2023) |
Internist-vasculaire geneeskunde, Amsterdam UMC, locatie AMC |
Geen |
Gemeld bij herbevestiging in 2024:
|
Geen restricties |
|
Leentjens (vanaf 2023) |
Internist-vasculair geneeskundige, Radboudumc |
Geen |
|
Geen restricties |
|
Actieve klankbordgroepleden |
||||
|
Esther Nossent |
Longarts Amsterdam UMC |
Geen |
Gemeld bij herbevestiging in 2025:
|
Geen restricties |
|
Heleen van Ommen |
Hoofd afd. Kinderhematologie & kinderoncologie Erasmus MC Sophia Kinderziekenhuis |
Geen |
|
Geen restricties |
|
Eric Tjwa |
MDL arts, Radboudumc |
Geen |
Geen Gemeld bij herbevestiging in 2025: Geen betrokkenheid bij onderzoeken die direct/indirect verband houden met de inhoud van de richtlijn |
Geen restricties |
|
Hans-Martin Otten (vanaf 2024) |
Internist Meander MC |
Lid METC UMCU, betaald |
|
Geen restricties |
|
Noa Rosenberg (vanaf 2024) |
Beleidsadviseur |
|
Geen |
Geen restricties |
|
Katja Heitink – Pollé |
Kinderoncoloog Prinses Máxima Centrum |
Landelijke werkgroep trombose bij kinderen |
geen |
Geen restricties |
Inbreng patiëntenperspectief
De werkgroep besteedde aandacht aan het patiëntenperspectief door uitnodigen van Stichting Harteraad voor de schriftelijke knelpuntenanalyse en door een patiëntvertegenwoordiger van Stichting Harteraad toe te voegen aan de klankbordgroep. De verkregen input is meegenomen bij het opstellen van de uitgangsvragen, de keuze voor de uitkomstmaten en bij het opstellen van de overwegingen (zie alinea waarden en voorkeuren van patiënten). De conceptrichtlijn is tevens voor commentaar voorgelegd aan Stichting Harteraad en Stichting Kind en Ziekenhuis en de eventueel aangeleverde commentaren zijn bekeken en verwerkt.
Kwalitatieve raming van mogelijke financiële gevolgen in het kader van de Wkkgz
Bij de richtlijnmodule voerde de werkgroep conform de Wet kwaliteit, klachten en geschillen zorg (Wkkgz) een kwalitatieve raming uitom te beoordelen of de aanbevelingen mogelijk leiden tot substantiële financiële gevolgen. Bij het uitvoeren van deze beoordeling is de richtlijnmodule op verschillende domeinen getoetst (zie het stroomschema bij Werkwijze).
Module |
Uitkomst raming |
Toelichting |
|
Tromboseprofylaxe volwassenen met maligniteit |
geen financiële gevolgen |
Hoewel uit de toetsing volgt dat de aanbevelingen breed toepasbaar zijn (>40.000 patiënten), volgt ook uit de toetsing dat het geen nieuwe manier van zorgverlening of andere organisatie van zorgverlening betreft, het geen toename in het aantal in te zetten voltijdsequivalenten aan zorgverleners betreft en het geen wijziging in het opleidingsniveau van zorgpersoneel betreft. Er worden daarom geen substantiële financiële gevolgen verwacht. |
Zoekverantwoording
Algemene informatie
|
Richtlijn: Cluster antitrombotische beleid |
|
|
Uitgangsvraag: UV3 Moeten poliklinische patiënten met kanker tijdens systemische kankerbehandeling farmacologische tromboseprofylaxe krijgen als primaire preventie voor veneuze tromboembolie?
|
|
|
Database(s): Ovid/Medline, Embase |
Datum: 4-7-2023 en 14-9-2023 |
|
Periode: nvt |
Talen: nvt |
|
Literatuurspecialist: Ingeborg van Dusseldorp en Esther van der Bijl |
|
|
BMI zoekblokken: voor verschillende opdrachten wordt (deels) gebruik gemaakt van de zoekblokken van BMI-Online https://blocks.bmi-online.nl/ Bij gebruikmaking van een volledig zoekblok zal naar de betreffende link op de website worden verwezen. |
|
|
Toelichting: 14-9-2023 Na de selectie is geconstateerd dat niet alle relevante literatuur wordt gevonden. Naar aanleiding van gevonden evidence in 2020 wordt gevraagd om een update van de zoekstrategie, waarin de setting ambulante patiënten achterwege wordt gelaten en gezocht wordt vanaf 2020. Het resultaat wordt ontdubbeld t.o.v. het eerder gevonden resultaat op 4 juli.
4-7-2023 Voor deze vraag is gezocht met de volgende concepten: Systeemtherapie EN farmacologische tromboseprofylaxe EN ambulante patiënten Er is geen onderscheid gemaakt tussen volwassenen en kinderen. |
|
Zoekopbrengst
|
Zoekopbrengst 12-10-2023 |
EMBASE vanaf 2023 |
OVID/MEDLINE vanaf 2020 |
Ontdubbeld t.o.v. Rayyan 14-9-2023 |
|
SRs |
81 |
24 |
7 |
|
RCTs |
61 |
37 |
1 |
|
Observationele studies |
|
|
|
|
Totaal |
|
|
819 |
|
Zoekopbrengst 14-9-2023 |
EMBASE |
OVID/MEDLINE |
Ontdubbeld t.o.v. Rayyan 4-7-2023 |
|
SRs |
513 |
24 |
485 |
|
RCTs |
338 |
35 |
326 |
|
Observationele studies |
|
|
|
|
Totaal |
851 |
59 |
811 |
4-7-2023
|
|
EMBASE |
OVID/MEDLINE |
Ontdubbeld |
|
SRs |
67 |
21 |
62 |
|
RCTs |
38 |
22 |
42 |
|
Observationele studies |
|
|
|
|
Totaal |
105 |
43 |
104 |
Zoekstrategie
Embase 14-9-2023
|
#16 |
#12 AND [2020-2023]py RCT |
338 |
|
#15 |
#11 AND [2020-2023]py SR |
513 |
|
#14 |
#4 AND #13 sleutelartikelen gevonden |
3 |
|
#13 |
#11 OR #12 |
2215 |
|
#12 |
#8 AND #10 NOT #11 |
988 |
|
#11 |
#8 AND #9 |
1227 |
|
#10 |
'randomized controlled trial'/exp OR random*:ti,ab OR (((pragmatic OR practical) NEAR/1 'clinical trial*'):ti,ab) OR ((('non inferiority' OR noninferiority OR superiority OR equivalence) NEAR/3 trial*):ti,ab) OR rct:ti,ab,kw |
2099619 |
|
#9 |
'meta analysis'/exp OR 'meta analysis (topic)'/exp OR metaanaly*:ti,ab OR 'meta analy*':ti,ab OR metanaly*:ti,ab OR 'systematic review'/de OR 'cochrane database of systematic reviews'/jt OR prisma:ti,ab OR prospero:ti,ab OR (((systemati* OR scoping OR umbrella OR 'structured literature') NEAR/3 (review* OR overview*)):ti,ab) OR ((systemic* NEAR/1 review*):ti,ab) OR (((systemati* OR literature OR database* OR 'data base*') NEAR/10 search*):ti,ab) OR (((structured OR comprehensive* OR systemic*) NEAR/3 search*):ti,ab) OR (((literature NEAR/3 review*):ti,ab) AND (search*:ti,ab OR database*:ti,ab OR 'data base*':ti,ab)) OR (('data extraction':ti,ab OR 'data source*':ti,ab) AND 'study selection':ti,ab) OR ('search strategy':ti,ab AND 'selection criteria':ti,ab) OR ('data source*':ti,ab AND 'data synthesis':ti,ab) OR medline:ab OR pubmed:ab OR embase:ab OR cochrane:ab OR (((critical OR rapid) NEAR/2 (review* OR overview* OR synthes*)):ti) OR ((((critical* OR rapid*) NEAR/3 (review* OR overview* OR synthes*)):ab) AND (search*:ab OR database*:ab OR 'data base*':ab)) OR metasynthes*:ti,ab OR 'meta synthes*':ti,ab |
961476 |
|
#8 |
#7 NOT ('conference abstract'/it OR 'editorial'/it OR 'letter'/it OR 'note'/it) NOT (('animal'/exp OR 'animal experiment'/exp OR 'animal model'/exp OR 'nonhuman'/exp) NOT 'human'/exp) |
15307 |
|
#7 |
#5 AND #6 |
20071 |
|
#6 |
'low molecular weight heparin'/exp OR 'dalteparin'/exp OR 'enoxaparin'/exp OR 'nadroparin'/exp OR 'tinzaparin'/exp OR 'fondaparinux'/exp OR 'apixaban'/exp OR 'dabigatran'/exp OR 'rivaroxaban'/exp OR 'bm 2123':ti,ab,kw OR 'bm2123':ti,ab,kw OR 'choay':ti,ab,kw OR 'depolymerized heparin':ti,ab,kw OR 'ebpm 1':ti,ab,kw OR 'ebpm 2':ti,ab,kw OR 'ebpm 3':ti,ab,kw OR 'ebpm1':ti,ab,kw OR 'ebpm2':ti,ab,kw OR 'ebpm3':ti,ab,kw OR 'ff 1034':ti,ab,kw OR 'ff1034':ti,ab,kw OR 'fr 860':ti,ab,kw OR 'low molecular heparin':ti,ab,kw OR 'low molecular weight heparin':ti,ab,kw OR 'nm heparin':ti,ab,kw OR 'pk 007':ti,ab,kw OR 'sandoz 5100':ti,ab,kw OR 'sandoz 6700':ti,ab,kw OR 'traxyparine':ti,ab,kw OR lmwh:ti,ab,kw OR 'assubex':ti,ab,kw OR 'kriva':ti,ab,kw OR 'naxat':ti,ab,kw OR 'rivaro':ti,ab,kw OR 'rivarolto':ti,ab,kw OR 'rivaroxaban':ti,ab,kw OR 'rivaxa':ti,ab,kw OR 'throsaben':ti,ab,kw OR 'xanirva':ti,ab,kw OR 'xarelto':ti,ab,kw OR 'xerdoxo':ti,ab,kw OR 'xindus':ti,ab,kw OR 'bibr 953':ti,ab,kw OR 'bibr953':ti,ab,kw OR 'dabigatran':ti,ab,kw OR 'aboxoma':ti,ab,kw OR 'apixaban':ti,ab,kw OR 'apixaben':ti,ab,kw OR 'bms 562247':ti,ab,kw OR 'bms562247':ti,ab,kw OR 'bms562247 01':ti,ab,kw OR 'eliques':ti,ab,kw OR 'eliquis':ti,ab,kw OR 'lunast':ti,ab,kw OR 'pf 0465257':ti,ab,kw OR 'pf0465257':ti,ab,kw OR 'tah 3311':ti,ab,kw OR 'tah3311':ti,ab,kw OR 'arixtra':ti,ab,kw OR 'fondaparin*':ti,ab,kw OR 'gsk 576428':ti,ab,kw OR 'gsk576428':ti,ab,kw OR 'ic 851589':ti,ab,kw OR 'ic851589':ti,ab,kw OR 'org 31540':ti,ab,kw OR 'org31540':ti,ab,kw OR 'quixidar':ti,ab,kw OR 'sr 90107':ti,ab,kw OR 'sr 90107a':ti,ab,kw OR 'sr90107':ti,ab,kw OR 'sr90107a':ti,ab,kw OR 'xantidar':ti,ab,kw OR 'innohep':ti,ab,kw OR 'lhn1':ti,ab,kw OR 'logiparin':ti,ab,kw OR 'tinzaparin':ti,ab,kw OR 'dalteparin':ti,ab,kw OR 'fragmin':ti,ab,kw OR 'fragmine':ti,ab,kw OR 'k 2165':ti,ab,kw OR 'k2165':ti,ab,kw OR 'kabi 2165':ti,ab,kw OR 'low liquemin':ti,ab,kw OR 'arovi':ti,ab,kw OR 'clexan':ti,ab,kw OR 'clexane':ti,ab,kw OR 'colevance':ti,ab,kw OR 'crusia':ti,ab,kw OR 'decipar':ti,ab,kw OR 'enoxaparin':ti,ab,kw OR 'ghemaxan':ti,ab,kw OR 'hepaxane':ti,ab,kw OR 'inhixa':ti,ab,kw OR 'klexane':ti,ab,kw OR 'ledraxen':ti,ab,kw OR 'losima':ti,ab,kw OR 'lovenox':ti,ab,kw OR 'neoparin':ti,ab,kw OR 'percolozin':ti,ab,kw OR 'pk 10169':ti,ab,kw OR 'pk10169':ti,ab,kw OR 'qualiop klinik':ti,ab,kw OR 'rovinadil':ti,ab,kw OR 'rp 54563':ti,ab,kw OR 'rp54563':ti,ab,kw OR 'thorinane':ti,ab,kw OR 'cy 216':ti,ab,kw OR 'cy 216d':ti,ab,kw OR 'cy216':ti,ab,kw OR 'cy216d':ti,ab,kw OR 'fraxiparin':ti,ab,kw OR 'fraxodi':ti,ab,kw OR 'nadroparin*':ti,ab,kw OR 'seledie':ti,ab,kw OR 'seleparina':ti,ab,kw OR 'seleparine':ti,ab,kw OR 'tedegliparin':ti,ab,kw |
118112 |
|
#5 |
'systemic therapy'/exp AND ('neoplasm'/exp OR neoplas*:ti,ab,kw OR carcinom*:ti,ab,kw OR cancer*:ti,ab,kw OR malignan*:ti,ab,kw OR tumor*:ti,ab,kw OR tumour*:ti,ab,kw OR metasta*) OR 'cancer immunotherapy'/exp OR 'cancer hormone therapy'/exp OR 'molecularly targeted therapy'/exp OR 'multimodality cancer therapy'/exp OR 'chemotherapy'/exp OR 'antineoplastic agent'/exp OR 'checkpoint inhibitor therapy'/exp OR 'protein kinase inhibitor'/exp OR ((((hormon* OR endocrin* OR 'combined modalit*' OR multimodal* OR 'multiple modalit*' OR target* OR immun* OR systemic* OR checkpoint OR 'check point') NEAR/3 (therap* OR treat*)):ti,ab,kw) AND ('neoplasm'/exp OR neoplas*:ti,ab,kw OR carcinom*:ti,ab,kw OR cancer*:ti,ab,kw OR malignan*:ti,ab,kw OR tumor*:ti,ab,kw OR tumour*:ti,ab,kw OR metasta*:ti,ab,kw)) OR 'biologic response modifier therapy':ti,ab,kw OR 'biological response modifier therapy':ti,ab,kw OR 'chemoradiotherapy'/exp OR 'chemoradi*':ti,ab,kw OR 'radiochemo*':ti,ab,kw OR chemotherap*:ti,ab,kw OR ((antineoplastic NEAR/3 (drug* OR agent*)):ti,ab,kw) OR 'protein kinase inhibitor*':ti,ab,kw |
3982517 |
|
#4 |
#1 OR #2 OR #3 sleutelartikelen |
3 |
|
#3 |
'primary prophylaxis for venous thromboembolism in ambulatory cancer patients receiving chemotherapy' AND [2020]/py AND rutjes |
1 |
|
#2 |
'rivaroxaban for thromboprophylaxis in high-risk ambulatory patients with cancer' AND khorana |
1 |
|
#1 |
'apixaban to prevent venous thromboembolism in patients with cancer' |
1 |
Embase 4-7-2023
|
#15 |
#4 AND #14 sleutelartikelen gevonden |
3 |
|
#14 |
#12 OR #13 |
105 |
|
#13 |
#9 AND #11 NOT #12 RCT |
38 |
|
#12 |
#9 AND #10 SR |
67 |
|
#11 |
'randomized controlled trial'/exp OR random*:ti,ab OR (((pragmatic OR practical) NEAR/1 'clinical trial*'):ti,ab) OR ((('non inferiority' OR noninferiority OR superiority OR equivalence) NEAR/3 trial*):ti,ab) OR rct:ti,ab,kw |
2070573 |
|
#10 |
'meta analysis'/exp OR 'meta analysis (topic)'/exp OR metaanaly*:ti,ab OR 'meta analy*':ti,ab OR metanaly*:ti,ab OR 'systematic review'/de OR 'cochrane database of systematic reviews'/jt OR prisma:ti,ab OR prospero:ti,ab OR (((systemati* OR scoping OR umbrella OR 'structured literature') NEAR/3 (review* OR overview*)):ti,ab) OR ((systemic* NEAR/1 review*):ti,ab) OR (((systemati* OR literature OR database* OR 'data base*') NEAR/10 search*):ti,ab) OR (((structured OR comprehensive* OR systemic*) NEAR/3 search*):ti,ab) OR (((literature NEAR/3 review*):ti,ab) AND (search*:ti,ab OR database*:ti,ab OR 'data base*':ti,ab)) OR (('data extraction':ti,ab OR 'data source*':ti,ab) AND 'study selection':ti,ab) OR ('search strategy':ti,ab AND 'selection criteria':ti,ab) OR ('data source*':ti,ab AND 'data synthesis':ti,ab) OR medline:ab OR pubmed:ab OR embase:ab OR cochrane:ab OR (((critical OR rapid) NEAR/2 (review* OR overview* OR synthes*)):ti) OR ((((critical* OR rapid*) NEAR/3 (review* OR overview* OR synthes*)):ab) AND (search*:ab OR database*:ab OR 'data base*':ab)) OR metasynthes*:ti,ab OR 'meta synthes*':ti,ab |
940686 |
|
#9 |
#8 NOT ('conference abstract'/it OR 'editorial'/it OR 'letter'/it OR 'note'/it) NOT (('animal'/exp OR 'animal experiment'/exp OR 'animal model'/exp OR 'nonhuman'/exp) NOT 'human'/exp) |
217 |
|
#8 |
#5 AND #6 AND #7 |
437 |
|
#7 |
'outpatient department'/exp OR 'ambulatory care'/exp OR 'outpatient'/exp OR 'outpatient care'/exp OR ambulator*:ti,ab,kw OR dispensar*:ti,ab,kw OR extramural:ti,ab,kw OR oupatient*:ti,ab,kw OR 'out patient':ti,ab,kw OR 'outward patient*':ti,ab,kw OR policlinic*:ti,ab,kw OR polyclinic*:ti,ab,kw |
462561 |
|
#6 |
'low molecular weight heparin'/exp OR 'dalteparin'/exp OR 'enoxaparin'/exp OR 'nadroparin'/exp OR 'tinzaparin'/exp OR 'fondaparinux'/exp OR 'apixaban'/exp OR 'dabigatran'/exp OR 'rivaroxaban'/exp OR 'bm 2123':ti,ab,kw OR 'bm2123':ti,ab,kw OR 'choay':ti,ab,kw OR 'depolymerized heparin':ti,ab,kw OR 'ebpm 1':ti,ab,kw OR 'ebpm 2':ti,ab,kw OR 'ebpm 3':ti,ab,kw OR 'ebpm1':ti,ab,kw OR 'ebpm2':ti,ab,kw OR 'ebpm3':ti,ab,kw OR 'ff 1034':ti,ab,kw OR 'ff1034':ti,ab,kw OR 'fr 860':ti,ab,kw OR 'low molecular heparin':ti,ab,kw OR 'low molecular weight heparin':ti,ab,kw OR 'nm heparin':ti,ab,kw OR 'pk 007':ti,ab,kw OR 'sandoz 5100':ti,ab,kw OR 'sandoz 6700':ti,ab,kw OR 'traxyparine':ti,ab,kw OR lmwh:ti,ab,kw OR 'assubex':ti,ab,kw OR 'kriva':ti,ab,kw OR 'naxat':ti,ab,kw OR 'rivaro':ti,ab,kw OR 'rivarolto':ti,ab,kw OR 'rivaroxaban':ti,ab,kw OR 'rivaxa':ti,ab,kw OR 'throsaben':ti,ab,kw OR 'xanirva':ti,ab,kw OR 'xarelto':ti,ab,kw OR 'xerdoxo':ti,ab,kw OR 'xindus':ti,ab,kw OR 'bibr 953':ti,ab,kw OR 'bibr953':ti,ab,kw OR 'dabigatran':ti,ab,kw OR 'aboxoma':ti,ab,kw OR 'apixaban':ti,ab,kw OR 'apixaben':ti,ab,kw OR 'bms 562247':ti,ab,kw OR 'bms562247':ti,ab,kw OR 'bms562247 01':ti,ab,kw OR 'eliques':ti,ab,kw OR 'eliquis':ti,ab,kw OR 'lunast':ti,ab,kw OR 'pf 0465257':ti,ab,kw OR 'pf0465257':ti,ab,kw OR 'tah 3311':ti,ab,kw OR 'tah3311':ti,ab,kw OR 'arixtra':ti,ab,kw OR 'fondaparin*':ti,ab,kw OR 'gsk 576428':ti,ab,kw OR 'gsk576428':ti,ab,kw OR 'ic 851589':ti,ab,kw OR 'ic851589':ti,ab,kw OR 'org 31540':ti,ab,kw OR 'org31540':ti,ab,kw OR 'quixidar':ti,ab,kw OR 'sr 90107':ti,ab,kw OR 'sr 90107a':ti,ab,kw OR 'sr90107':ti,ab,kw OR 'sr90107a':ti,ab,kw OR 'xantidar':ti,ab,kw OR 'innohep':ti,ab,kw OR 'lhn1':ti,ab,kw OR 'logiparin':ti,ab,kw OR 'tinzaparin':ti,ab,kw OR 'dalteparin':ti,ab,kw OR 'fragmin':ti,ab,kw OR 'fragmine':ti,ab,kw OR 'k 2165':ti,ab,kw OR 'k2165':ti,ab,kw OR 'kabi 2165':ti,ab,kw OR 'low liquemin':ti,ab,kw OR 'arovi':ti,ab,kw OR 'clexan':ti,ab,kw OR 'clexane':ti,ab,kw OR 'colevance':ti,ab,kw OR 'crusia':ti,ab,kw OR 'decipar':ti,ab,kw OR 'enoxaparin':ti,ab,kw OR 'ghemaxan':ti,ab,kw OR 'hepaxane':ti,ab,kw OR 'inhixa':ti,ab,kw OR 'klexane':ti,ab,kw OR 'ledraxen':ti,ab,kw OR 'losima':ti,ab,kw OR 'lovenox':ti,ab,kw OR 'neoparin':ti,ab,kw OR 'percolozin':ti,ab,kw OR 'pk 10169':ti,ab,kw OR 'pk10169':ti,ab,kw OR 'qualiop klinik':ti,ab,kw OR 'rovinadil':ti,ab,kw OR 'rp 54563':ti,ab,kw OR 'rp54563':ti,ab,kw OR 'thorinane':ti,ab,kw OR 'cy 216':ti,ab,kw OR 'cy 216d':ti,ab,kw OR 'cy216':ti,ab,kw OR 'cy216d':ti,ab,kw OR 'fraxiparin':ti,ab,kw OR 'fraxodi':ti,ab,kw OR 'nadroparin*':ti,ab,kw OR 'seledie':ti,ab,kw OR 'seleparina':ti,ab,kw OR 'seleparine':ti,ab,kw OR 'tedegliparin':ti,ab,kw |
116390 |
|
#5 |
'systemic therapy'/exp AND ('neoplasm'/exp OR neoplas*:ti,ab,kw OR carcinom*:ti,ab,kw OR cancer*:ti,ab,kw OR malignan*:ti,ab,kw OR tumor*:ti,ab,kw OR tumour*:ti,ab,kw OR metasta*) OR 'cancer immunotherapy'/exp OR 'cancer hormone therapy'/exp OR 'molecularly targeted therapy'/exp OR 'multimodality cancer therapy'/exp OR 'chemotherapy'/exp OR 'antineoplastic agent'/exp OR 'checkpoint inhibitor therapy'/exp OR 'protein kinase inhibitor'/exp OR ((((hormon* OR endocrin* OR 'combined modalit*' OR multimodal* OR 'multiple modalit*' OR target* OR immun* OR systemic* OR checkpoint OR 'check point') NEAR/3 (therap* OR treat*)):ti,ab,kw) AND ('neoplasm'/exp OR neoplas*:ti,ab,kw OR carcinom*:ti,ab,kw OR cancer*:ti,ab,kw OR malignan*:ti,ab,kw OR tumor*:ti,ab,kw OR tumour*:ti,ab,kw OR metasta*:ti,ab,kw)) OR 'biologic response modifier therapy':ti,ab,kw OR 'biological response modifier therapy':ti,ab,kw OR 'chemoradiotherapy'/exp OR 'chemoradi*':ti,ab,kw OR 'radiochemo*':ti,ab,kw OR chemotherap*:ti,ab,kw OR ((antineoplastic NEAR/3 (drug* OR agent*)):ti,ab,kw) OR 'protein kinase inhibitor*':ti,ab,kw |
3912136 |
|
#4 |
#1 OR #2 OR #3 sleutelartikelen |
3 |
|
#3 |
'primary prophylaxis for venous thromboembolism in ambulatory cancer patients receiving chemotherapy' AND [2020]/py AND rutjes |
1 |
|
#2 |
'rivaroxaban for thromboprophylaxis in high-risk ambulatory patients with cancer' AND khorana |
1 |
|
#1 |
'apixaban to prevent venous thromboembolism in patients with cancer' |
1 |
Ovid/Medline 14-9-2023
|
# |
Searches |
Results |
|
11 |
limit 8 to yr="2020 - 2024" RCT |
35 |
|
10 |
limit 7 to yr="2020 - 2024" SR |
24 |
|
9 |
7 or 8 |
264 |
|
8 |
4 and 6 (not 7) |
180 |
|
7 |
4 and 5 |
84 |
|
6 |
exp randomized controlled trial/ or randomized controlled trials as topic/ or random*.ti,ab. or rct?.ti,ab. or ((pragmatic or practical) adj "clinical trial*").ti,ab,kf. or ((non-inferiority or noninferiority or superiority or equivalence) adj3 trial*).ti,ab,kf. |
1644900 |
|
5 |
meta-analysis/ or meta-analysis as topic/ or (metaanaly* or meta-analy* or metanaly*).ti,ab,kf. or systematic review/ or cochrane.jw. or (prisma or prospero).ti,ab,kf. or ((systemati* or scoping or umbrella or "structured literature") adj3 (review* or overview*)).ti,ab,kf. or (systemic* adj1 review*).ti,ab,kf. or ((systemati* or literature or database* or data-base*) adj10 search*).ti,ab,kf. or ((structured or comprehensive* or systemic*) adj3 search*).ti,ab,kf. or ((literature adj3 review*) and (search* or database* or data-base*)).ti,ab,kf. or (("data extraction" or "data source*") and "study selection").ti,ab,kf. or ("search strategy" and "selection criteria").ti,ab,kf. or ("data source*" and "data synthesis").ti,ab,kf. or (medline or pubmed or embase or cochrane).ab. or ((critical or rapid) adj2 (review* or overview* or synthes*)).ti. or (((critical* or rapid*) adj3 (review* or overview* or synthes*)) and (search* or database* or data-base*)).ab. or (metasynthes* or meta-synthes*).ti,ab,kf. |
693090 |
|
4 |
3 not ((exp animals/ or exp models, animal/) not humans/) not (letter/ or comment/ or editorial/) |
1165 |
|
3 |
1 and 2 |
1335 |
|
2 |
exp Heparin, Low-Molecular-Weight/ or Dalteparin/ or Enoxaparin/ or Nadroparin/ or Tinzaparin/ or Dabigatran/ or Rivaroxaban/ or bm 2123.ti,ab,kf. or bm2123.ti,ab,kf. or choay.ti,ab,kf. or depolymerized heparin.ti,ab,kf. or ebpm 1.ti,ab,kf. or ebpm 2.ti,ab,kf. or ebpm 3.ti,ab,kf. or ebpm1.ti,ab,kf. or ebpm2.ti,ab,kf. or ebpm3.ti,ab,kf. or ff 1034.ti,ab,kf. or ff1034.ti,ab,kf. or fr 860.ti,ab,kf. or low molecular heparin.ti,ab,kf. or low molecular weight heparin.ti,ab,kf. or nm heparin.ti,ab,kf. or "pk 007".ti,ab,kf. or sandoz 5100.ti,ab,kf. or sandoz 6700.ti,ab,kf. or traxyparine.ti,ab,kf. or lmwh.ti,ab,kf. or assubex.ti,ab,kf. or kriva.ti,ab,kf. or naxat.ti,ab,kf. or rivaro.ti,ab,kf. or rivarolto.ti,ab,kf. or rivaroxaban.ti,ab,kf. or rivaxa.ti,ab,kf. or throsaben.ti,ab,kf. or xanirva.ti,ab,kf. or xarelto.ti,ab,kf. or xerdoxo.ti,ab,kf. or xindus.ti,ab,kf. or bibr 953.ti,ab,kf. or bibr953.ti,ab,kf. or dabigatran.ti,ab,kf. or aboxoma.ti,ab,kf. or apixaban.ti,ab,kf. or apixaben.ti,ab,kf. or bms 562247.ti,ab,kf. or bms562247.ti,ab,kf. or "bms562247 01".ti,ab,kf. or eliques.ti,ab,kf. or eliquis.ti,ab,kf. or lunast.ti,ab,kf. or "pf 0465257".ti,ab,kf. or pf0465257.ti,ab,kf. or tah 3311.ti,ab,kf. or tah3311.ti,ab,kf. or arixtra.ti,ab,kf. or fondaparin*.ti,ab,kf. or gsk 576428.ti,ab,kf. or gsk576428.ti,ab,kf. or ic 851589.ti,ab,kf. or ic851589.ti,ab,kf. or org 31540.ti,ab,kf. or org31540.ti,ab,kf. or quixidar.ti,ab,kf. or sr 90107.ti,ab,kf. or sr 90107a.ti,ab,kf. or sr90107.ti,ab,kf. or sr90107a.ti,ab,kf. or xantidar.ti,ab,kf. or innohep.ti,ab,kf. or lhn1.ti,ab,kf. or logiparin.ti,ab,kf. or tinzaparin.ti,ab,kf. or dalteparin.ti,ab,kf. or fragmin.ti,ab,kf. or fragmine.ti,ab,kf. or k 2165.ti,ab,kf. or k2165.ti,ab,kf. or kabi 2165.ti,ab,kf. or low liquemin.ti,ab,kf. or arovi.ti,ab,kf. or clexan.ti,ab,kf. or clexane.ti,ab,kf. or colevance.ti,ab,kf. or crusia.ti,ab,kf. or decipar.ti,ab,kf. or enoxaparin.ti,ab,kf. or ghemaxan.ti,ab,kf. or hepaxane.ti,ab,kf. or inhixa.ti,ab,kf. or klexane.ti,ab,kf. or ledraxen.ti,ab,kf. or losima.ti,ab,kf. or lovenox.ti,ab,kf. or neoparin.ti,ab,kf. or percolozin.ti,ab,kf. or pk 10169.ti,ab,kf. or pk10169.ti,ab,kf. or qualiop klinik.ti,ab,kf. or rovinadil.ti,ab,kf. or rp 54563.ti,ab,kf. or rp54563.ti,ab,kf. or thorinane.ti,ab,kf. or cy 216.ti,ab,kf. or cy 216d.ti,ab,kf. or cy216.ti,ab,kf. or cy216d.ti,ab,kf. or fraxiparin.ti,ab,kf. or fraxodi.ti,ab,kf. or nadroparin*.ti,ab,kf. or seledie.ti,ab,kf. or seleparina.ti,ab,kf. or seleparine.ti,ab,kf. or tedegliparin.ti,ab,kf. |
34414 |
|
1 |
Chemoradiotherapy/ or Chemotherapy, Adjuvant/ or Consolidation Chemotherapy/ or Electrochemotherapy/ or Induction Chemotherapy/ or Maintenance Chemotherapy/ or Molecular Targeted Therapy/ or Orthomolecular Therapy/ or Photochemotherapy/ or (Immunotherapy/ and (exp Neoplasms/ or (neoplas* or carcinom* or cancer* or malignan* or tumor* or tumour* or metasta*).ti,ab,kf.)) or exp Antineoplastic Agents/ or Immune Checkpoint Inhibitors/ or Protein Kinase Inhibitors/ or (((hormon* or endocrin* or combined modalit* or multimodal* or multiple modalit* or target* or immun* or systemic* or checkpoint or "check point") adj3 (therap* or treat*)).ti,ab,kf. and (exp Neoplasms/ or neoplas*.ti,ab,kf. or carcinom*.ti,ab,kf. or cancer*.ti,ab,kf. or malignan*.ti,ab,kf. or tumor*.ti,ab,kf. or tumour*.ti,ab,kf. or metasta*.ti,ab,kf.)) or biologic response modifier therapy.ti,ab,kf. or biological response modifier therapy.ti,ab,kf. or chemoradi*.ti,ab,kf. or radiochemo*.ti,ab,kf. or chemotherap*.ti,ab,kf. or (antineoplastic adj3 (drug* or agent*)).ti,ab,kf. or protein kinase inhibitor*.ti,ab,kf. |
1871870 |
Ovid/Medline 144-7-2023
|
# |
Searches |
Results |
|
11 |
9 or 10 |
43 |
|
10 |
6 and 8 not 9 RCT |
22 |
|
9 |
6 and 7 SR |
21 |
|
8 |
exp randomized controlled trial/ or randomized controlled trials as topic/ or random*.ti,ab. or rct?.ti,ab. or ((pragmatic or practical) adj "clinical trial*").ti,ab,kf. or ((non-inferiority or noninferiority or superiority or equivalence) adj3 trial*).ti,ab,kf. |
1625962 |
|
7 |
meta-analysis/ or meta-analysis as topic/ or (metaanaly* or meta-analy* or metanaly*).ti,ab,kf. or systematic review/ or cochrane.jw. or (prisma or prospero).ti,ab,kf. or ((systemati* or scoping or umbrella or "structured literature") adj3 (review* or overview*)).ti,ab,kf. or (systemic* adj1 review*).ti,ab,kf. or ((systemati* or literature or database* or data-base*) adj10 search*).ti,ab,kf. or ((structured or comprehensive* or systemic*) adj3 search*).ti,ab,kf. or ((literature adj3 review*) and (search* or database* or data-base*)).ti,ab,kf. or (("data extraction" or "data source*") and "study selection").ti,ab,kf. or ("search strategy" and "selection criteria").ti,ab,kf. or ("data source*" and "data synthesis").ti,ab,kf. or (medline or pubmed or embase or cochrane).ab. or ((critical or rapid) adj2 (review* or overview* or synthes*)).ti. or (((critical* or rapid*) adj3 (review* or overview* or synthes*)) and (search* or database* or data-base*)).ab. or (metasynthes* or meta-synthes*).ti,ab,kf. |
678494 |
|
6 |
5 not ((exp animals/ or exp models, animal/) not humans/) not (letter/ or comment/ or editorial/) |
75 |
|
5 |
3 and 4 |
81 |
|
4 |
Ambulatory Care/ or exp Outpatient Clinics, Hospital/ or Ambulatory Care Facilities/ or ambulator*.ti,ab,kf. or dispensar*.ti,ab,kf. or extramural.ti,ab,kf. or oupatient*.ti,ab,kf. or out patient.ti,ab,kf. or outward patient*.ti,ab,kf. or policlinic*.ti,ab,kf. or polyclinic*.ti,ab,kf. |
179576 |
|
3 |
1 and 2 |
1327 |
|
2 |
exp Heparin, Low-Molecular-Weight/ or Dalteparin/ or Enoxaparin/ or Nadroparin/ or Tinzaparin/ or Dabigatran/ or Rivaroxaban/ or bm 2123.ti,ab,kf. or bm2123.ti,ab,kf. or choay.ti,ab,kf. or depolymerized heparin.ti,ab,kf. or ebpm 1.ti,ab,kf. or ebpm 2.ti,ab,kf. or ebpm 3.ti,ab,kf. or ebpm1.ti,ab,kf. or ebpm2.ti,ab,kf. or ebpm3.ti,ab,kf. or ff 1034.ti,ab,kf. or ff1034.ti,ab,kf. or fr 860.ti,ab,kf. or low molecular heparin.ti,ab,kf. or low molecular weight heparin.ti,ab,kf. or nm heparin.ti,ab,kf. or "pk 007".ti,ab,kf. or sandoz 5100.ti,ab,kf. or sandoz 6700.ti,ab,kf. or traxyparine.ti,ab,kf. or lmwh.ti,ab,kf. or assubex.ti,ab,kf. or kriva.ti,ab,kf. or naxat.ti,ab,kf. or rivaro.ti,ab,kf. or rivarolto.ti,ab,kf. or rivaroxaban.ti,ab,kf. or rivaxa.ti,ab,kf. or throsaben.ti,ab,kf. or xanirva.ti,ab,kf. or xarelto.ti,ab,kf. or xerdoxo.ti,ab,kf. or xindus.ti,ab,kf. or bibr 953.ti,ab,kf. or bibr953.ti,ab,kf. or dabigatran.ti,ab,kf. or aboxoma.ti,ab,kf. or apixaban.ti,ab,kf. or apixaben.ti,ab,kf. or bms 562247.ti,ab,kf. or bms562247.ti,ab,kf. or "bms562247 01".ti,ab,kf. or eliques.ti,ab,kf. or eliquis.ti,ab,kf. or lunast.ti,ab,kf. or "pf 0465257".ti,ab,kf. or pf0465257.ti,ab,kf. or tah 3311.ti,ab,kf. or tah3311.ti,ab,kf. or arixtra.ti,ab,kf. or fondaparin*.ti,ab,kf. or gsk 576428.ti,ab,kf. or gsk576428.ti,ab,kf. or ic 851589.ti,ab,kf. or ic851589.ti,ab,kf. or org 31540.ti,ab,kf. or org31540.ti,ab,kf. or quixidar.ti,ab,kf. or sr 90107.ti,ab,kf. or sr 90107a.ti,ab,kf. or sr90107.ti,ab,kf. or sr90107a.ti,ab,kf. or xantidar.ti,ab,kf. or innohep.ti,ab,kf. or lhn1.ti,ab,kf. or logiparin.ti,ab,kf. or tinzaparin.ti,ab,kf. or dalteparin.ti,ab,kf. or fragmin.ti,ab,kf. or fragmine.ti,ab,kf. or k 2165.ti,ab,kf. or k2165.ti,ab,kf. or kabi 2165.ti,ab,kf. or low liquemin.ti,ab,kf. or arovi.ti,ab,kf. or clexan.ti,ab,kf. or clexane.ti,ab,kf. or colevance.ti,ab,kf. or crusia.ti,ab,kf. or decipar.ti,ab,kf. or enoxaparin.ti,ab,kf. or ghemaxan.ti,ab,kf. or hepaxane.ti,ab,kf. or inhixa.ti,ab,kf. or klexane.ti,ab,kf. or ledraxen.ti,ab,kf. or losima.ti,ab,kf. or lovenox.ti,ab,kf. or neoparin.ti,ab,kf. or percolozin.ti,ab,kf. or pk 10169.ti,ab,kf. or pk10169.ti,ab,kf. or qualiop klinik.ti,ab,kf. or rovinadil.ti,ab,kf. or rp 54563.ti,ab,kf. or rp54563.ti,ab,kf. or thorinane.ti,ab,kf. or cy 216.ti,ab,kf. or cy 216d.ti,ab,kf. or cy216.ti,ab,kf. or cy216d.ti,ab,kf. or fraxiparin.ti,ab,kf. or fraxodi.ti,ab,kf. or nadroparin*.ti,ab,kf. or seledie.ti,ab,kf. or seleparina.ti,ab,kf. or seleparine.ti,ab,kf. or tedegliparin.ti,ab,kf. |
34104 |
|
1 |
Chemoradiotherapy/ or Chemotherapy, Adjuvant/ or Consolidation Chemotherapy/ or Electrochemotherapy/ or Induction Chemotherapy/ or Maintenance Chemotherapy/ or Molecular Targeted Therapy/ or Orthomolecular Therapy/ or Photochemotherapy/ or (Immunotherapy/ and (exp Neoplasms/ or (neoplas* or carcinom* or cancer* or malignan* or tumor* or tumour* or metasta*).ti,ab,kf.)) or exp Antineoplastic Agents/ or Immune Checkpoint Inhibitors/ or Protein Kinase Inhibitors/ or (((hormon* or endocrin* or combined modalit* or multimodal* or multiple modalit* or target* or immun* or systemic* or checkpoint or "check point") adj3 (therap* or treat*)).ti,ab,kf. and (exp Neoplasms/ or neoplas*.ti,ab,kf. or carcinom*.ti,ab,kf. or cancer*.ti,ab,kf. or malignan*.ti,ab,kf. or tumor*.ti,ab,kf. or tumour*.ti,ab,kf. or metasta*.ti,ab,kf.)) or biologic response modifier therapy.ti,ab,kf. or biological response modifier therapy.ti,ab,kf. or chemoradi*.ti,ab,kf. or radiochemo*.ti,ab,kf. or chemotherap*.ti,ab,kf. or (antineoplastic adj3 (drug* or agent*)).ti,ab,kf. or protein kinase inhibitor*.ti,ab,kf. |
1856533 |