Lisfranc letsel
Uitgangsvraag
Welke behandeling reduceert de meest voorkomende gevolgen (korte en lange termijn) van Lisfranc fracturen?
Aanbeveling
Behandel patiënten met een open fractuur en/of persisterende luxatiestand van het Lisfranc gewricht operatief. Behandel operatief als er een evidente instabiliteit dan wel discongruentie van het Lisfranc gewricht bestaat (>5 mm).
Bespreek bij subtiele Lisfranc letsels, waarbij er geen duidelijke dislocatie is (2-5 mm) met de patiënt of er operatief of conservatief behandeld wordt en kom door middel van samen beslissen tot een keuze.
Behandel conservatief indien aanvullend onderzoek laat zien dat het letsel stabiel is (<2 mm; zie ook het stroomschema diagnostiek traumatische complexe voetletsels)
Geef bij de operatieve behandeling van Lisfranc letsel een lichte voorkeur aan behandeling middels primaire artrodese.
Overwegingen
Voor- en nadelen van de interventie en de kwaliteit van het bewijs
Er is literatuuronderzoek gedaan naar de optimale behandelstrategie van Lisfranc fracturen. Hierbij is gezocht naar studies die een operatieve behandeling vergeleken met een conservatieve behandeling en naar studies die een primaire open reductie en interne fixatie (ORIF) vergeleken met een primaire arthrodese (PA). Deze vraag betreft met name de minder ernstig gedisluxeerde Lisfranc fracturen (< 2 mm dislocatie, in deze module: subtiel Lisfranc letsel), aangezien bij duidelijk gedisluxeerde Lisfranc fracturen (2 – 5 mm, in deze module: evident Lisfranc letsel) operatieve behandeling de voorkeur geniet. Voor de vergelijking tussen operatief ingrijpen en conservatieve behandeling werden geen RCTs gevonden, maar wel drie relevante retrospectieve observationele studies (Ren, 2019; Garriguez-Pérez, 2021; Graef, 2021). Observationeel onderzoek heeft van nature een lage bewijskracht. Daarbij was er in de studies van Ren (2019) en Garriguez-Pérez (2021) een gebrek aan correctie voor confounders, waardoor het aannemelijk is dat verschillen in de karakteristieken van de patiëntenpopulaties een effect hebben gehad op de resultaten. Bijvoorbeeld in de studie van Garriguez-Pérez (2021), werden patiënten met een dislocatie kleiner dan 2 mm conservatief behandeld, en patiënten met een dislocatie van 2 tot 5 mm operatief behandeld. In de studie van Graef (2021) is wel rekening gehouden met confounders, middels ‘propensity score matching’ in de statistische analyse. De bewijskracht voor effecten op de uitkomstmaten functionele uitkomst (AOFAS en FFI score, cruciale uitkomstmaat) en risico op artrose of infectie (belangrijke uitkomstmaten) is zeer laag. Voor de belangrijke uitkomstmaten bloeding, zenuwschade en slechte/geen genezing werd geen bewijs gevonden. Samenvattend is er op basis van dit bewijs niet te zeggen welke behandeling (operatief of conservatief) leidt tot betere uitkomsten.
Wel is in zowel de studie van Garriguez-Perez (2021) als Graef (2021) te zien dat patiënten met een minimale tot geen instabiliteit (<2 mm dislocatie) conservatief werden behandeld. Patiënten met een duidelijke dislocatie (2-5 mm) werden operatief behandeld. Zoals beschreven in de module diagnostiek (zie stroomschema diagnostiek traumatische complexe voetletsels) is de werkgroep van mening dat bij subtiele afwijkingen immobilisatie en aanvullende beeldvorming d.m.v. CT overwogen dient te worden. Er dient binnen twee weken een klinische herbeoordeling te worden gedaan, met zo nodig additionele CT of belaste voetfoto. Indien additionele diagnostiek laat zien dat het letsel stabiel is, kan er gekozen worden voor conservatieve behandeling. Mogelijk zal de RCT van Ponkilainen (2018; study protocol) meer sturing kunnen geven in de toekomst in de beste keuze van behandeling in dit soort letsels.
Voor de vergelijking tussen ORIF en PA werden vier RCTs gevonden (Sun, 2022; Stødle, 2020; Henning, 2009; Ly, 2006). Het gevonden bewijs suggereert dat er nauwelijks tot geen verschil is tussen ORIF en PA op functionele uitkomst (AOFAS/FFI-score na 12 maanden en na 24 maanden; cruciale uitkomstmaat). Ten aanzien van het risico op artrose (belangrijke uitkomstmaat) werd een absoluut risico verschil van 0.20 gevonden (95% BI 0,07 tot 0,33), in het voordeel van PA. De bewijskracht voor deze gevonden effecten is laag, mede vanwege kleine studie populaties en een laag aantal cases (n = 78 en n = 54). Voor de effecten op het risico op infectie, zenuwschade, malunion/nonunion, en chirurgisch verwijderen van bijvoorbeeld schroeven (belangrijke uitkomstmaten) werd slechts bewijs met een zeer lage bewijskracht gevonden. Zo waren de uitkomsten over het risico op verwijderen van materiaal erg tegenstrijdig. Mogelijk kan dit worden verklaard door het feit dat in Stødle (2020) alleen low-energy Lisfranc letsels waren geïncludeerd. Geen van de studies rapporteerden data over het risico op een bloeding. Redenen voor de (zeer) lage bewijskracht zijn o.a. een gebrek aan blindering en brede 95% BI’s die de grenzen van klinische besluitvorming doorkruizen. Er is geen sterk bewijs over welke behandeling de beste is, een primaire arthrodese of primaire fixatie (ORIF).
Bij een primaire fixatie tracht men het gewricht te behouden en niet op te offeren, maar de vraag is of het opweegt om 1-4 graden beweeglijkheid van het Lisfranc gewricht op te offeren met als doelmeer stabiliteit te geven door een primaire artrodese. Dit sluit aan bij de gevonden resultaten ten aanzien van functionele uitkomst, die laten zien dat de functionele uitkomst na PA en ORIF vergelijkbaar zijn. Hoewel het verschil niet klinisch relevant was, laten zowel Sun (2022) als Stødle (2020) zien dat de AOFAS-score enigszins hoger was in de patiënten die met primaire artrodese werden behandeld. Om deze beweeglijkheid postoperatief na een primaire fixatie enigszins te verwezenlijken zal een tweede operatie noodzakelijk zijn om het materiaal te verwijderen. Garriguez-Pérez (2021) adviseert om het materiaal alleen te verwijderen bij osteolyse en klachten, terwijl in de praktijk veelal de eerste keuze is om osteosynthese materiaal te verwijderen na een ORIF. Over de noodzaak tot het routinematig verwijderen van osteosynthese materiaal bestaat een kennislacune.
Een ander veel genoemd argument ten faveure van behandeling met primaire artrodese is dat het risico op artrose lager is. Dit komt mede doordat bij een primaire artrodese het gewricht wordt opgeofferd en gefixeerd. De gevonden literatuur (Sun, 2022), laat een risico verschil van 0.20 (95% 0.07 tot 0.22) zien, wat suggereert dat de kans op artrose 20% groter is in de groep patiënten die ORIF ondergaat. Het mogelijk lagere risico op artrose dient meegenomen te worden in de behandelkeuze.
Een complicatie van een artrodese die kan optreden is een nonunion. Daarentegen suggereert de gevonden literatuur over Lisfranc letsels geen verhoogd risico op nonunion bij primaire artrodese, al is de bewijskracht laag.
Het succes van de ingreep hangt sterk samen met de nabehandeling (zie ook module nabehandeling),de fysieke toestand van de patiënt,weke delen van de voet na het letsel, comorbiditeiten (o.a. diabetes mellitus; perifeer vaatlijden) en andere leefstijlfactoren (bijv. roken).
Wanneer je kiest voor primaire fixatie, is de richtlijnwerkgroep van mening dat een plaatfixatie de aangewezen keuze is. Dit wordt ook ondersteund in de retrospectieve studie van Lau (2016) waarin 62 patiënten gedurende 6 jaar behandeld zijn voor hun Lisfranc fracturen. Patiënten ondergingen fixatie met trans articulaire schroeven (14/62, 22.5%), dorsal locking plates (17/62, 27.4%) of een combinatie van schroeven en platen (29/62, 46.8%). De fracturen die werden behandeld met een combinatie van schroeven en platen hadden een 3.01 (95% CI: 1.4-8.74) keer verhoogd risico op het ontwikkelen van stadium III of IV-osteoartritis vergeleken met een behandeling van alleen platen. Er werden geen verschillen in risico op osteoartritis gevonden wanneer een behandeling van platen werd vergeleken met schroeven.
Waarden en voorkeuren van patiënten (en evt. hun verzorgers)
Wanneer er geen duidelijke instabiliteit is door een Lisfranc letsel, kan de keuze voor een conservatieve of operatieve behandeling in overleg met de patiënt worden gemaakt. Een conservatieve behandeling middels gips is minder ingrijpend voor de patiënt, maar heeft als nadeel dat je een lange periode in het gips zit wat het dagelijks functioneren beperkt.
Ook de keuze voor primaire artrodese, danwel primaire fixatie kan in overleg met de patiënt worden gemaakt. De voor- en nadelen van beide ingrepen dienen te worden besproken en de patiënt moet worden geïnformeerd over de verwachtingen t.a.v. het herstel. Voor de patiënt is primaire artrodese mogelijk minder belastend omdat er niet het risico is dat zij een tweede ingreep moeten ondergaan voor verwijdering van materiaal en de kans op artrose mogelijk hoger is bij een primaire fixatie.
Kosten-effectiviteit (middelenbeslag)
Albright (2018) is de enige studie die ons beeld geeft dat primaire artrodese een meer kosten-effectievere techniek is dan ORIF. De primaire artrodese zou $1429/QALY kosten, terwijl de primaire fixatie $3958/QALY zou kosten. Dit is met name door de afname in re-operaties bij primaire artrodese.
Aanvaardbaarheid, haalbaarheid en implementatie
Expertise over- en exposure aan dit letsel is in de meeste ziekenhuizen beperkt. Met name een beperking in de ‘index of suspicion’ zal meespelen met de problemen met detectie van instabiliteit bij een letsel in het bereik van een Lisfranc letsel. Ook bij evidente instabiliteit of complexe fracturen in de ossale structuren rondom het Lisfranc gewricht is de expertise beperkt door de lage frequentie van voorkomen. De expertise op traumatologisch gebied is beperkt, zeker waar het chirurgische interventies betreft, dit is mogelijk een belemmerende factor. Naar verwachting zijn er geen andere belemmerende factoren (bijv. aanwezigheid van apparatuur).
Acute zorg is voor eenieder toegankelijk ongeacht niveau van gezondsheidsvaardigheden, sociale klasse, opleidingsniveau, inkomen of migratie-achtergrond.
Rationale:
Aanbeveling-1
Er is geen bewijs dat operatieve behandeling bij patiënten met een subtiel Lisfranc letsel betere uitkomsten geeft dan conservatieve behandeling. In de geselecteerde retrospectieve studies werden patiënten met een minimaal tot geen verplaatst Lisfranc letsel (subtiel letsel) conservatief behandeld en patiënten met een duidelijke verplaatsing/instabiliteit operatief behandeld.
Er zijn geen prospectieve vergelijkende studies beschikbaar. De werkgroep is het, gezien deze resultaten, erover eens dat open fracturen en persisterende luxatiestand in het Lisfranc gewricht operatief behandeld dienen te worden. Aangezien intra-articulaire fracturen en ligamentair letsel van het Lisfranc gewricht kunnen leiden tot discongruentie van het gewricht of persisterende instabiliteit kunnen hebben, is de werkgroep van mening dat deze groep ook baat kan hebben bij operatieve behandeling om de mogelijke late gevolgen te minimaliseren. Onduidelijk is of intra-articulaire fracturen in het Lisfranc bereik zonder discongruentie of gewrichten die bij stresstesten een goede alignment behouden ook beter operatief behandeld kunnen worden of dat gipsimmobilisatie dan een gelijkwaardige optie is. Deze keuze zou samen met de patiënt gemaakt kunnen worden.
Aanbeveling-2
De primaire artrodese lijkt mogelijk iets beter te doen dan de primaire fixatie van een Lisfranc letsel, maar de verschillen waren niet signifcant. Mogelijk dat de verschillen kleiner worden als het osteosynthese materiaal na een primaire fixatie alleen verwijderd wordt op indicatie, aangezien we op dit moment vooral alleen osteosynthese materiaal verwijderen bij primaire fixatie. Het gevonden bewijs suggereert dat artrose mogelijk vaker voor postoperatief voorkomt in de primaire fixatie groep, vergeleken met de primaire artrodese groep. Hierdoor lijkt er een lichte voorkeur te zijn voor de primaire artrodese.
Onderbouwing
Achtergrond
Lisfranc-fracturen zijn met name verwondingen aan de tarsometatarsale gewrichten (TMT's) en het Lisfranc-ligament. Tot 20% van de subtiele ligamenteuze Lisfranc-verwondingen wordt naar verluid ongepast behandeld, hetzij als gevolg van gemiste diagnoses of een onderschatting van de ernst van het letsel. Gevolgen van Lisfranc-fracturen kunnen resulteren in aanhoudende voetpijn, forse standsafwijkingen van de voet, abnormaal lopen en functionele beperkingen, vooral bij sportactiviteiten. Er is behoefte aan consensus met betrekking tot behandelingsmogelijkheden. Momenteel is er nog veel onduidelijkheid over:
- Welke (subtiele) Lisfranc fracturen conservatief behandeld dienen te worden en welke operatief
- Of primaire artrodese leidt tot betere uitkomsten dan primaire fixatie
- Welke invloed de verschillende manieren van ingrijpen hebben op functionaliteit
Er is een grote variatie aan behandelingen mogelijk variërend van gipsimmobilisatie, open repositie en interne fixatie tot artrodese van het Lisfranc gewricht. Onduidelijkheid over de uitkomsten van de individuele behandeling maakt dat er ook een grote praktijkvariatie in behandelkeuzes aanwezig is.
Conclusies
PICO A: operative fixation compared to conservative treatment
Functional outcome (AOFAS and FFI score), (osteo)arthritis
|
Very low GRADE |
The evidence is very uncertain about the effect of operative fixation on functional outcome (AOFAS; FFI) and (osteo)arthritis when compared to conservative treatment in patients with subtle Lisfranc injury.
Source: Garriguez-Pérez, 2021; Ren, 2019; Graef 2021 |
Infection
|
Very low GRADE |
The evidence is very uncertain about the effect of operative fixation on infection when compared to conservative treatment in patients with subtle Lisfranc injury.
Source: Ren, 2019 |
Bleeding, nerve injury, malunion/non-union
|
No GRADE |
No evidence was found regarding the effect of operative fixation on bleeding, nerve injury and malunion/non-union when compared to conservative treatment in patients with subtle Lisfranc injury.
Source: - |
PICO B: open reduction and internal fixation (ORIF) compared to primary arthrodesis (PA)
Functional outcome: AOFAS-score
|
Low GRADE |
ORIF may result in little to no difference in functional outcome (AOFAS) when compared to PA in patients with Lisfranc injury.
Source: Sun, 2022; Stødle, 2020 |
Infection, nerve injury
|
Very low GRADE |
The evidence is very uncertain about the effect of ORIF on infection, and nerve injury, when compared to PA in patients with Lisfranc injury.
Source: Henning, 2009 |
Malunion/nonunion
|
Very Low GRADE |
The evidence is very uncertain about the effect of ORIF on malunion/nonunion when compared to PA in patients with Lisfranc injury.
Source: Sun, 2022; Stødle, 2020 |
Arthrosis
|
Low GRADE |
ORIF may result in an increased risk for developing (osteo)arthritis when compared to PA in patients with Lisfranc injury.
Source: Sun, 2022 |
Hardware removal
|
Very low GRADE |
The evidence is very uncertain about the effect of ORIF on hardware removal, when compared to PA in patients with Lisfranc injury.
Source: Stødle, 2020; Henning, 2009; Ly and Coetzee, 2006 |
Bleeding
|
No GRADE |
No evidence was found regarding the effect of ORIF on bleeding when compared to PA in patients with Lisfranc injury.
Source: - |
Samenvatting literatuur
Description of studies
PICO A: operative fixation compared to conservative treatment
The retrospective study of Ren (2019) analyzed 61 patients (mean age 39.4 years, 38 males; 62.3%) with undisplaced subtle ligamentous Lisfranc injuries who either had an operation (n=20 patients) or received conservative treatment (n=41 patients) during the period May 2012 to May 2017. Patients were treated in two orthopaedic centres (Beijing United Family Hospital and Tianjin Hospital, China). Patients were followed for on average 12.3 months, and the mean age of the study population was 39.4 (range, 19-64) years. The choice of treatment was done by the patients, which was based on full explanation of pros and cons of both treatments. Outcomes of interest were AOFAS and different types of complications such as infection and arthritis. There is no correction for confounding, which is considered a limitation of the study.
Garriguez-Pérez (2021) performed a retrospective study in which foot function of 42 patients with a subtle Lisfranc injury, who were treated operatively or received conservative treatment between 2009 and 2019 in a hospital (the setting was not further specified), were evaluated by means of the AOFAS Midfoot Score. Apart from AOFAS, other reported outcomes were complications such as nerve injury (however quantitative data was not presented), and (osteo)arthritis Patients with Lisfranc injuries that were displaced between 2 and 5 mm were operatively treated (n=34), whereas Lisfranc injuries that were displaced less than 2 mm received conservative treatment (n=8). The mean age of the total study population was 49 (SD 17.5) years and 35.7% was male. Mean follow-up was 4.3 (range, 1-8) years. There is no correction for confounding, which is considered a limitation of the study.
The study of Graef (2021) retrospectively analyzed treatment decisions (operative or conservative) of Lisfranc injuries treated in a single German level I trauma centre between January 2011 and December 2020. The patients who received operative treatment had subtle or evident instability, whereas patients who received conservative treatment had no instability. In total, 99 patients were included, of which 79 patients (79.8%) were operatively treated and 20 patients (20.2%) received conservative treatment. The median age of the operative group was 45.47 (IQR 15.84) years and 59.5% was male. The median age of the conservative group was 37.95 (IQR 15.13) years and 50.0% was male. This study reported the Foot Function Index (FFI) as clinical outcome. The FFI was only available for 10 patients in the conservative group and 10 patients in the operative treatment group. The mean follow-up time was only reported for the patients with available FFI score. In 10 patients of the operative group with data on FFI, the follow-up duration was 4.50 (SD 2.42) years, and this was 4.20 (SD 2.04) years in 10 patients of the conservative treatment group with data on FFI. Propensity score matching was performed for age, sex, fracture classification, injury mechanisms, total number of collateral fractures of the foot and ankle joint, and follow-up time after the initial treatment.
Table 1: Baseline characteristics of the studies included for PICO A (operative fixation versus conservative treatment)
AOFAS = American Orthopaedic Foot and Ankle Society, FFI = Foot Function index, SD = standard deviation, IQR = inter quartile range, mm = millimeters
|
Study |
Total patient population |
Operative treatment |
Conservative treatment |
|
Ren, 2019 |
· N = 61 patients with undisplaced subtle ligamentous Lisfranc injuries · Mean age 39.4 (range, 19-64) years · 62.3% male · Mean follow-up 12.3 months |
N = 20 patients
|
N = 41 patients
|
|
Garriguez-Pérez, 2021 |
· n = 42 patients with a subtle Lisfranc injury · Mean age 49 (SD 17.5) years · 35.7% male · Mean follow-up 4.3 (range, 1-8) years |
N = 34 patients displaced 2-5 mm
|
N= 8 patients displaced <2 mm
|
|
Graef, 2021 |
N = 99 patients with Lisfranc injury
|
· N = 79 patients with subtle or evident instability · Median age: 45.47 [IQR, 15.84] years · 59.5% male · Follow-up time: 4.50 (SD, 2.42) years (n = 10 patients with FFI data) |
· N =2 0 patients without instability · Median age: 37.95 [IQR, 15.13) years · 50.0% male · Follow-up time: 4.20 (SD, 2.04) years (n = 10 patients with FFI data) |
PICO B: open reduction and internal fixation (ORIF) compared to primary arthrodesis (PA)
The RCT by Sun (2022) was a multicenter trial, involving 10 foot and ankle centers of nine cities in China (setting not further specified). Sun (2022) evaluated whether ORIF or PA provided more beneficial effects in adult patients with Lisfranc injuries that involved the first tarsometatarsal (TMT) joint dislocation. A total of 88 patients were included in the trial; 44 patients were randomized to the ORIF group (intervention) and 44 patients were randomized to the PA group (control). Patients were on average 40.7 years old (range, 20-78), and 64.1% were male. Patients were followed-up at three weeks, six weeks, three months, six months, and 12 months. Outcomes were AOFAS and arthritis. ORIF and PA operation techniques were exactly similar, with the only difference being that articular cartilage was not removed from both surfaces of the first TMT joint in the ORIF group, whereas this was the case in the PA group.
The studies mentioned below were extracted from the systematic review of Van der Boom (2021). Since Van der Boom (2021) also included non-randomized studies, and only randomized controlled trials were considered relevant for answering the research question, it was decided to retrieve data from the original papers (Stødle, 2020; Henning, 2009; Ly, 2006).
Stødle (2020) compared temporary bridge plating (ORIF) with PA of the first TMT joint treatment. In total, 48 patients with low-energy unstable Lisfranc injuries were included in the trial. The trial was performed in a level 1 trauma center of the University Hospital of Oslo, Norway. Twenty-four patients randomized in the ORIF group were on average 34 (IQR, 28-40) years old, and the average age of 24 patients randomized in the PA group was 30 (IQR, 23-40) years. In both groups, 45.8% were males. The AOFAS Midfoot score was the main outcome, which was measured at 12 months and 24 months follow-up. Other outcomes were infection, nonunion, osteoarthritis, and hardware removal.
The RCT of Henning (2009) compared ORIF with PA treatment in patients with Lisfranc injuries over a five-year period (March 2000 and August 2005). The setting was not further specified. Thirty-two patients were eligible for this study; 14 patients were randomized to ORIF treatment, and 18 patients were randomized to PA treatment. Patients in the ORIF treatment group were on average 37 (range, 20-58) years old and 64.3% were male. The average age of patients in the PA treatment group was 40 (range, 25-73) years, and 66.7% was male. Outcomes that were studied included infection, nerve injury, nonunion, and hardware removal.
Ly (2006) compared the effect of ORIF with PA for the treatment of primarily ligamentous Lisfranc injuries. The setting was not further specified. Forty-one patients were considered eligible for this RCT and were followed for at least two years. Twenty patients were included in the ORIF group, and were on average 32.4 (range, 19-52) years old. Twenty-one patients constituted the PA group, and this group was on average 32.0 (range, 19-42) years old. The majority of each group was male (65% in the ORIF group and 66.7% in the PA group). AOFAS was measured at 24 months follow-up. Other outcomes were nonunion, delayed union, and hardware removal.
Results
PICO A: operative fixation compared to conservative treatment
Functional outcome
AOFAS-score
Two studies measured functional outcome with the AOFAS-score (Garriguez-Pérez, 2021; Ren, 2019). The study of Garriguez-Pérez (2021) reported a MD of 3.30 (95% CI: -13.05, 6.45) points lower in the operative group (N = 34), compared to the conservative group (N= 8), after 4.3 (range 1-8) years follow-up. The study of Ren (2019) reported a MD of 13.70 (95% CI: 9.40, 18.00) points higher in the operative group (N= 20) compared to the conservative group (N=41), after 12.3 months follow-up.
FFI score
One study measured functional outcome with the FFI score (Graef, 2021). Graef (2021) reported the FFI score after a mean follow-up time of 4.50 (SD, 2.42) years in the operative treatment and after 4.20 (SD, 2.04) years in the conservative management group. This study reported a MD of 9.06 (95% CI: -15.94, 34.06) points higher in the operative group compared to the conservative group. This was not considered statistically significant, nor clinically relevant.
Infection
Ren (2019) reported the outcome infection In this study, it was reported (in the text) that 1/20 (5.0%) patients had an infection in the surgical group, and 0/41 (0%) patients had an infection in the conservative treatment group. The risk difference (RD) was 0.05 (95% CI: -0.06, 0.16).
Bleeding
None of the included studies reported the outcome bleeding for operative fixation compared to conservative treatment.
Nerve injury
None of the included studies reported the outcome nerve injury for operative fixation compared to conservative treatment.
Malunion/non-union
None of the included studies reported the outcome malunion/non-union for operative fixation compared to conservative treatment.
(Osteo)arthritis
Two studies, Garríguez-Pérez (2021) and Ren (2019) reported the outcome (osteo)arthritis. Garriguez-Pérez (2021) reported 4/34 (11.8%) cases of osteoarthritis in the operative treatment group, compared to 3/8 (37.5%) cases in the conservative treatment group. The RR was 0.31 (95% CI: 0.09 to 1.13). In the study of Ren (2019), 0/20 (0%) cases with arthritis were reported in the operative treatment group and 2/41 (4.88%) cases were reported in the conservative treatment group. The RR was 0.40 (95% CI: 0.02 to 7.96).
Hardware removal
One study (Garríguez-Pérez, 2021) reported the outcome hardware removal. In this study, hardware removal was only done “if discomfort was referred by the patient or if the follow-up radiographs showed signs of osteolysis”. Hardware was removed because of discomfort in 10/34 (29.4%) patients of the operative group. Hardware removal because of asymptomatic osteolysis was performed in 6/34 (17.6%) patients in the operative group. Hardware removal was not performed in the patients undergoing conservative treatment, as hardware is not used when applying a conservative management strategy. As hardware removal is not needed in patients undergoing conservative treatment, a comparative analysis could not be made, and the GRADE-approach could not be applied.
PICO B: open reduction and internal fixation (ORIF) compared to primary arthrodesis (PA)
Functional outcome
AOFAS-score
Sun (2022) and Stødle (2020) reported on the outcome AOFAS Midfoot score after 12 months follow-up. The results were not pooled, as only two studies reported this outcome. The results are shown in Figure 1 below. Stødle (2020) reported a mean AOFAS-score of 79 (SD, 16) points in the ORIF group compared to 85 (SD, 10) points in the PA group. The MD was -6.00 (95% CI, -13.71, 1.71) points, which means that AOFAS was lower in the ORIF vs. PA group. This difference was not considered statistically significant, nor clinically relevant. Sun (2022) reported a mean AOFAS-score of 77.4 (SD, 5.3) points in the ORIF group vs. 84.3 (SD, 7.3) points in the PA group. The MD was -6.90 (95% CI, -9.74, -4.06) points in the ORIF compared to the PA group. This was considered statistically significant, but not clinically relevant.

Figure 1 Forest plot that shows the results of individual RCTs on the effect of ORIF vs. PA treatment on the outcome AOFAS after 12 months follow-up in adult patients with all types of Lisfranc injuries (Stødle 2020) and in patients with Lisfranc injuries that involved the first TMT joint dislocation (Sun 2022). Abbreviations: ORIF, open reduction internal fixation; PA, primary arthrodesis; SD, standard deviation; 95% CI, 95% confidence interval; IV, inverse variance (statistical method);
Two studies reported AOFAS midfoot score at 24 months follow-up (Stødle, 2020; Ly, 2006)
Stødle (2020) reported AOFAS-score at 24 months follow-up additional to the results at 12 months follow-up. The study reported a mean score of 85 (SD, 15) points in the ORIF group compared to 89 (SD, 9) points in the PA group, which resulted in a MD of -4.00 (95% CI, -11.15, 3.15) points. Ly (2006) reported a mean AOFAS of 68.6 (range, 16-100) points in the ORIF group vs. 88.0 (range, 63-100) points in the PA group. A MD could not be calculated for this study, because the SD was not reported. The GRADE-approach could not be applied.
Infection
Tabular data on infection rates were not presented in the included studies. However, two studies reported the following on infections. Henning (2009) described that “no infection […] was noted” in the ORIF group (0/14, 0%) whereas in the PA group, one patient had “presumed superficial cellulitis” also called a superficial wound infection and “no deep infection” was noted (1/18, 5.6%)”. The RD was -0.06 (95% CI, -0.21, 0.10), which was not statistically significant. Stødle (2020) reported in the text that “one patient had a superficial wound infection in the ORIF group (1/24, 4.2%)” but data on infection were not reported for the PA group. Therefore, the GRADE assessment could not be applied for the article of Stødle (2020).
Bleeding
None of the included studies reported the outcome bleeding for the for ORIF compared to PA.
Nerve injury
Information about nerve injury was reported by Henning (2009), stating in the text that “no neural injury […] was noted” in both the ORIF (0/14) and PA group (0/18). This resulted in a risk difference of 0.00 (95% CI, -0.12, 0.12).
Nonunion, delayed union
In total, three studies reported on the outcomes nonunion and delayed union (Stødle, 2020; Henning, 2009; Ly, 2006). Stødle (2020) reported that the rate of nonunion was low in both groups, with two patients in the ORIF group (2/23, 8.7%) and one patient in the PA group (1/22, 4.5%). This resulted in a RD of 0.04 (95% CI, -0.10, 0.19). Henning (2009) reported in the text that “one delayed union associated with a broken first TMT joint screw healed at the 6-month mark, and one nonunion of a first TMT joint was treated nonoperatively” in the PA group. This information was not provided for the ORIF group. Finally, Ly (2006) reported no information about nonunion/delayed union in the ORIF group, but “one patient had a delayed union” and “one patient with a nonunion […]” in the PA group. As the studies of Henning (2009) and Ly (2006) only reported data on nonunion/malunion rates for one of the treatment groups, a comparative analysis could not be made and the GRADE-approach could not be applied.
(Osteo)arthritis
Sun (2022) and Stødle (2020) reported the outcome osteo(arthritis) after ORIF or PA treatment in adult patients with all types of Lisfranc injuries (Stødle, 2020) and Lisfranc injuries that involved the first TMT joint dislocation (Sun, 2022). Sun (2022) reported 8/40 (20%) (osteo)arthritis events in the ORIF group compared to 0/38 (0%) events in the PA group. This resulted in a RD of 0.20 (95% CI 0.07, 0.33). The study of Stødle (2020) reported 11/24 (45.9%) (osteo)arthritis events in the ORIF group and did not report any data for the PA group.
Hardware removal surgery
Stødle (2020), Henning (2009), and Ly (2006) reported the outcome hardware removal surgery as potential risk of complication after ORIF or PA treatment for Lisfranc injuries. The results of the individual RCTs were pooled, see Figure 2. In the ORIF group, the pooled number of patients undergoing hardware removal was 32/58 (55.2%) compared to 14/62 (22.6%) patients in the PA group. The pooled RR was 2.38 (95% CI 0.70, 8.06).
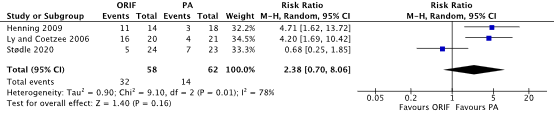
Figure 2 Forest plot that shows the results of individual RCTs on the effect of ORIF vs. PA treatment on the outcome hardware removal surgery in adult patients with all types of Lisfranc injuries Abbreviations: ORIF, open reduction internal fixation; PA, primary arthrodesis; SD, standard deviation; 95% CI, 95% confidence interval; IV, inverse variance (statistical method);
Level of evidence of the literature
PICO A: operative fixation compared to conservative treatment
The level of evidence regarding the outcome measures AOFAS and (osteo)arthritis was derived from observational studies and therefore started low. The level of evidence for each outcome measure was downgraded by two levels because of study limitations including lack of adequate correction for confounding factors (-1 risk of bias) and the 95% CI of both studies crossing the threshold for clinical decision making (-1 imprecision). For both outcome measures, the final level of evidence was graded ‘very low’.
The level of evidence regarding the outcome measure FFI was derived from observational studies and therefore started low. The level of evidence was downgraded by three levels because of study limitations including problems with the selection of Lisfranc Injuries (-1 risk of bias) and the 95% CI crossing both thresholds for clinical decision making (-2 imprecision). The final level of evidence was graded ‘very low’.
The level of evidence regarding the outcome measure infection was derived from observational studies and therefore started low. The level of evidence was downgraded by two levels because of study limitations including lack of adequate correction for confounding factors (-1 risk of bias) and small study population (-1 imprecision). The final level of evidence was graded ‘very low’.
The level of evidence regarding the outcome measures bleeding, nerve injury and malunion/nonunion could not be graded since no studies were found that reported these outcomes.
PICO B: open reduction and internal fixation (ORIF) compared to primary arthrodesis (PA)
The level of evidence regarding the outcome measure AOFAS was derived from RCTs and therefore started high. The level of evidence was downgraded by two levels because of study limitations including lack of blinding (-1 risk of bias) and the 95% CI crossing the thresholds of clinical decision making (-1 imprecision). The final level of evidence was graded ‘low’.
The level of evidence regarding the outcome measures infection and nerve injury were derived from RCTs and therefore started high. The level of evidence for each outcome measure was downgraded by three levels because of study limitations including lack of blinding and premature discontinuation of one trial (-2 risk of bias) and small study population (-1 imprecision). For both outcome measures, the final level of evidence was graded ‘very low’.
The level of evidence regarding the outcome measures bleeding could not be graded as none of the studies reported the outcome ‘bleeding’.
The level of evidence regarding the outcome measure malunion/non-union was derived from RCTs and therefore started high. The level of evidence was downgraded by three levels because of study limitations including lack of blinding and lack of external validity (-2 risk of bias); and 95% CI crossing the threshold of clinical decision making (-1 imprecision). The final level of evidence was graded ‘very low’.
The level of evidence regarding the outcome measure (osteo)arthritis was derived from RCTs and therefore started high. The level of evidence was downgraded by two levels because of study limitations including lack of blinding (-1 risk of bias); and 95% CI crossing the threshold of clinical decision making (-1 imprecision). The final level of evidence was graded ‘low’.
The level of evidence regarding the outcome measure hardware removal was derived from RCTs and therefore started high. The level of evidence was downgraded by three levels because of study limitations including lack of blinding, lack of external validity, and one trial discontinued prematurely (-1 risk of bias); conflicting results (-1 inconsistency); and 95% CI crossing the threshold of clinical decision making (-1 imprecision). The final level of evidence was graded ‘very low’.
Zoeken en selecteren
A systematic review of the literature was performed to answer the following questions:
PICO A: What is the effectiveness and safety of an operative fixation compared to conservative treatment in patients with a Lisfranc fracture?
P: Patients with Lisfranc fracture
I: Operative fixation
C: Conservative treatment
O: Functional outcomes, infection, bleeding, nerve injury, malunion, nonunion, (osteo)arthritis, hardware removal
PICO B: What is the effectiveness and safety of open reduction and internal fixation (ORIF) compared to primary arthrodesis (PA) in patients with a Lisfranc fracture?
P: Patients with Lisfranc fracture
I: Open reduction internal fixation, with e.g. trans articular screws or bridge plating
C: Primary arthrodesis
O: Functional outcome, infection, bleeding, nerve injury, malunion, nonunion, (osteo)arthritis, hardware removal
Relevant outcome measures
The guideline development group considered functional outcome and (osteo)arthritis as critical outcome measures for decision making; and infection, bleeding, nerve injury, malunion and nonunion and hardware removal as important outcome measures for decision making.
A priori, the guideline development group decided that the American Orthopaedic Foot and Ankle Society (AOFAS) score was the preferred measure for functional outcome. If a study did not include the AOFAS-score but alternative measures for functional outcome were presented (e.g. mobility or Foot Function Index; FFI-score), these alternative measures were included in the summary of the literature. For the other outcome measures listed above, the guideline development group decided to use the definitions used in the studies.
The guideline development group defined the following thresholds as a minimal clinically (patient) important difference:
- For functional outcome (measured with the AOFAS or FFI) a mean difference (MD) of 10 points was considered clinically relevant.
- For dichotomous outcomes (infection, bleeding, nerve injury, malunion and nonunion, (osteo)arthritis, and hardware removal) a Risk Ratio (RR) < 0.80 or >1.25, or a Risk Difference (RD) of 10% was considered clinically relevant.
Search and select (Methods)
The databases Medline (via OVID) and Embase (via Embase.com) were searched with relevant search terms until January 23rd, 2023. The detailed search strategy is depicted under the tab Methods. The systematic literature search resulted in 802 unique hits. Studies were selected based on the following criteria: systematic reviews and RCTs evaluating operative fixation with conservative treatment (PICO A) and comparing open reduction and internal fixation with primary arthrodesis (PICO B) of Lisfranc injuries. For PICO A (operative fixation compared with conservative treatment), also observational and/or non-randomized studies were screened and included if relevant. Twenty-nine studies were initially selected based on title and abstract screening. After reading the full text, 22 studies were excluded (see the table with reasons for exclusion under the tab Methods). In total, three observational studies (PICO A) and four RCTs (PICO B) were included.
Results
Three observational studies (PICO A) and four RCTs (PICO B) were included in the analysis of the literature. Important study characteristics and results are summarized in the evidence tables. The assessment of the risk of bias is summarized in the risk of bias tables.
Referenties
- Albright RH, Haller S, Klein E, Baker JR, Weil L Jr, Weil LS Sr, Fleischer AE. Cost-Effectiveness Analysis of Primary Arthrodesis Versus Open Reduction Internal Fixation for Primarily Ligamentous Lisfranc Injuries. J Foot Ankle Surg. 2018 Mar-Apr;57(2):325-331. doi: 10.1053/j.jfas.2017.10.016. Epub 2017 Dec 20. PMID: 29275036.
- Garríguez-Pérez D, Puerto-Vázquez M, Tomé Delgado JL, Galeote E, Marco F. Impact of the Subtle Lisfranc Injury on Foot Structure and Function. Foot Ankle Int. 2021 Oct;42(10):1303-1310. doi: 10.1177/10711007211012956. Epub 2021 Jun 10. PMID: 34109830.
- Graef J, Tsitsilonis S, Niemann M, Gehlen T, Nadler P, Graef F. Retrospective analysis of treatment decisions and clinical outcome of Lisfranc injuries: operative vs. conservative treatment. Int Orthop. 2021 Dec;45(12):3213-3219. doi: 10.1007/s00264-021-05135-w. Epub 2021 Aug 6. PMID: 34357433; PMCID: PMC8626366.
- Henning JA, Jones CB, Sietsema DL, Bohay DR, Anderson JG. Open reduction internal fixation versus primary arthrodesis for Lisfranc injuries: a prospective randomized study. Foot Ankle Int. 2009 Oct;30(10):913-22. doi: 10.3113/FAI.2009.0913. PMID: 19796583.
- Lau S, Howells N, Millar M, De Villiers D, Joseph S, Oppy A. Plates, Screws, or Combination? Radiologic Outcomes After Lisfranc Fracture Dislocation. J Foot Ankle Surg. 2016 Jul-Aug;55(4):799-802. doi: 10.1053/j.jfas.2016.03.002. Epub 2016 Apr 12. PMID: 27079306.
- Ly TV, Coetzee JC. Treatment of primarily ligamentous Lisfranc joint injuries: primary arthrodesis compared with open reduction and internal fixation. A prospective, randomized study. J Bone Joint Surg Am. 2006 Mar;88(3):514-20. doi: 10.2106/JBJS.E.00228. PMID: 16510816.
- Nunley JA, Vertullo CJ. Classification, investigation, and management of midfoot sprains: Lisfranc injuries in the athlete. Am J Sports Med. 2002 Nov-Dec;30(6):871-8. doi: 10.1177/03635465020300061901. PMID: 12435655.
- Ponkilainen VT, Mattila VM, Laine HJ, Paakkala A, Mäenpää HM, Haapasalo HH. Nonoperative, open reduction and internal fixation or primary arthrodesis in the treatment of Lisfranc injuries: a prospective, randomized, multicenter trial - study protocol. BMC Musculoskelet Disord. 2018 Aug 21;19(1):301. doi: 10.1186/s12891-018-2222-4. PMID: 30126393; PMCID: PMC6102864.
- Ren W, Li HB, Lu JK, Hu YC. Undisplaced subtle ligamentous Lisfranc injuries, conservative or surgical treatment with percutaneous position screws? Chin J Traumatol. 2019 Aug;22(4):196-201. doi: 10.1016/j.cjtee.2019.03.005. Epub 2019 May 27. PMID: 31235287; PMCID: PMC6667927.
- Richter M, Thermann H, Huefner T, Schmidt U, Kretter C. Aetiology, treatment and outcome in Lisfranc joint dislocations and fracture dislocations. J Foot Ankle Surg. 2002; 8(1): 21-32. Doi: 10.1046/j.1460-9584.2002.00294.x.
- Stødle AH, Hvaal KH, Brøgger HM, Madsen JE, Husebye EE. Temporary Bridge Plating vs Primary Arthrodesis of the First Tarsometatarsal Joint in Lisfranc Injuries: Randomized Controlled Trial. Foot Ankle Int. 2020 Aug;41(8):901-910. doi: 10.1177/1071100720925815. Epub 2020 Jun 5. PMID: 32501109; PMCID: PMC7406968.
- Sun C, Miao X, Zhang M, Yang Y, Zhao H, Tang X, Yu G. Lisfranc injuries with dislocation the first tarsometatarsal joint: primary arthrodesis or internal fixation (a randomized controlled trial). Int Orthop. 2022 Nov;46(11):2529-2537. doi: 10.1007/s00264-022-05478-y. Epub 2022 Jun 20. PMID: 35723701.
- van den Boom NAC, Stollenwerck GANL, Lodewijks L, Bransen J, Evers SMAA, Poeze M. Lisfranc injuries: fix or fuse? : a systematic review and meta-analysis of current literature presenting outcome after surgical treatment for Lisfranc injuries. Bone Jt Open. 2021 Oct;2(10):842-849. doi: 10.1302/2633-1462.210.BJO-2021-0127.R1. PMID: 34643414; PMCID: PMC8558450. Individual papers were included in the summary of literature
Evidence tabellen
Evidence table for intervention studies (randomized controlled trials and non-randomized observational studies [cohort studies, case-control studies, case series])
|
Study reference |
Study characteristics |
Patient characteristics 2 |
Intervention (I) |
Comparison / control (C) 3
|
Follow-up |
Outcome measures and effect size 4 |
Comments |
|
Ren, 2019 |
Type of study: retrospective cohort study
Setting and country: Beijing United Family Hospital and Tianjin Hospital, China
Funding and conflicts of interest:The authors declared that there are no conflicts of interest. No funding received. |
Inclusion criteria: no fractures in initial radio graphs; the radiographic Images showed that the first and second metatarsal had no diastasis (less than 2 mm in gap), but only weight bearing view showed the diastasis more than 3 mm; further images from CT showed some abnormality including ‘fleck sign’ or MRI showed plantar and interosseous branches of Lisfranc ligament rupture. ‘Fleck sign’ is a ‘small chip of bone found in the space between the first and second metatarsal bases, which indicates avulsion of the Lisfranc ligament.
Exclusion criteria: not applicable
N total at baseline: 61 patients with subtle ligamentous Lisfranc injuries Operative: n=20 (32.8%) Conservative: n=41 (67.2%)
Important prognostic factors2: Mean age total study group: 39 (range, 19-64) years
Sex: Total study group: 62.2% Male
Groups comparable at baseline? Unclear. |
Describe intervention (treatment/procedure/test):
Operative For the surgical treatment, a reduction clamp was used to hold the position of the first and second metatarsal, one or two position screw/screws (depending on whether there is a diastasis between first and second cuneiform) were inserted. A posterior plaster splint was used for two weeks after the wound was well healed, followed by a walking boot with a foot arch supporter for the followed four weeks. |
Describe control (treatment/procedure/test):
Conservative For the conservative management of the undisplaced subtle ligamentous Lisfranc injury, a posterior plaster splint was used for initial three to five days, followed by a full cast to fix the ankle in 90 with foot arch remolding without weight bearing for totally six weeks. After the cast was removed, a walking boot with foot arch supporter was used to allow patient to fully weight bear for another six weeks.
|
Length of follow-up: 12.3 months
Loss-to-follow-up: Not applicable |
Outcome measures and effect size (include 95%CI and p-value if available):
AOFAS, mean (SD) Operative: 90.0 (3.7) Conservative: 76.3 (13.0) P-value: <0.05 Interpretation: higher scores is better outcome
Infection, n (%) Operative: 1 (5.0) Conservative: 0 (0) P-value: not reported
Arthrosis, n (%) Operative: 4 (11.8) Conservative: 3 (37.5) P-value: not reported
|
Authors’ conclusions Although surgical intervention for treating ligamentous injuries to Lisfranc joint is still controversial, we can learn a lesson and inform patients to give an appropriate warning to consider conservative and surgical management for undisplaced subtle Ligamentous Lisfranc injuries.
Remarks Unclear whether groups are comparable in terms of age and sex
|
|
Garriguez-Pérez, 2021 |
Type of study: retrospective cohort study
Setting and country: hospital, country not reported
Funding and conflicts of interest: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. ICMJE forms for all authors are available online. The author(s) received no financial support for the research, authorship, and/or publication of this article.
|
Inclusion criteria: (a) traumatic widening of the first intermetatarsal or intercuneal space, (b) complete clinical and radiographic history, and (c) minimum 1-year follow-up.
Exclusion criteria:(a) complete dislocation or fracture- dislocation of the tarsometatarsal joint, (b) displaced fracture of the base of the metatarsals, (c) open or crush injuries, (d) associated injuries in the ipsilateral limb, and (e) associated comorbidities that made operative risk too high to propose operative treatment.
N total at baseline: 42 patients with subtle Lisfranc injuries Operative: n=34 (81.0%) Conservative: n=8 (19.0%)
Important prognostic factors2: Mean age total study group: 49 (SD 17.5) years
Sex: Total study group: 35.7% Male
Groups comparable at baseline? Unclear. Characteristics such as age, sex and BMI only provided for the whole study population. |
Describe intervention (treatment/procedure/test):
Operative for injuries displaced between 2 and 5 mm (stage II and III). A limited approach over the C1-M2 space was used to remove fat and scar tissue that might prevent proper reduction. The space was reduced using a clamp, and K-wires, screws, or dorsal plating were used for definitive fixation of at least 2 of the 3 possible elements (C1-M2, M1-M2, or C1-C2), according to surgeon’s preference (Figure 3). After operative treatment, an ankle splint was used for 1 week to allow healing of soft tissues. When the splint was removed, active ankle range of motion was promoted but weight bearing was not allowed for 6 weeks. Hardware removal was not done routinely, performing it only if discomfort was referred by the patient or if the follow-up radiographs showed signs of osteolysis.
|
Describe control (treatment/procedure/test):
Conservative for injuries displaced less than 2 mm (stage I). Use of posterior ankle splint for the first week until initial swelling has subsided and then replaced for a full ankle cast, which was kept for 6-8 weeks. Nonweight bearing was indicated until the 12th week after the beginning of treatment.
|
Length of follow-up: 4.3 (range, 1-8) years
Loss-to-follow-up: Not applicable
|
Outcome measures and effect size (include 95%CI and p-value if available):
AOFAS, mean (SD) Operative: 86.8 (6.6) Conservative: 90.1 (13.7) P-value: not reported Interpretation: higher scores is better outcome
Arthrosis, n (%) Operative: 4 (11.8) Conservative: 3 (37.5) P-value: not reported
|
Authors’ conclusions Foot function does not seem diminished after sustaining injuries
Remarks Groups are not comparable in types of complications
Relatively small sample size
Retrospective study and including different treatment modalities with different injury stages
No adjustment for confounding in statistical analyses |
|
Graef, 2021 |
Type of study: retrospective cohort study
Setting and country: level I trauma centre, Germany
Funding and conflicts of interest: Open Access funding enabled and organized by Projekt DEAL. Authors declare no competing interests. |
Inclusion criteria: All patients who were treated due to an injury of the Lisfranc joint in a single German level I trauma centre from January 2011 until December 2020 were included in this Study
Exclusion criteria: not reported
N total at baseline: 99 patients with Lisfranc injuries Operative: n=79 (79.8%) Conservative: n=20 (20.2%)
Important prognostic factors2: Mean age operative: 45.47 [IQR, 15.84] years Mean age conservative: 37.95 [IQR, 15.13)
Sex: Operative: 50% male Conservative: 59.5% male
Groups comparable at baseline? Baseline characteristics in terms of age, sex, trauma mechanisms etc not evenly distributed. |
Describe intervention (treatment/procedure/test):
Operative not clear / no information |
Describe control (treatment/procedure/test):
Conservative not clear / no information |
Length of follow-up: Operative: 4.50 (SD 2.42) years *using n=10 patients with FFI data Conservative: 4.20 (SD 2.04) years *using n=10 patients with FFI data
Loss-to-follow-up: Not applicable
|
Outcome measures and effect size (include 95%CI and p-value if available):
FFI, mean (SD) Operative: 30.09 (28.59) Conservative: 21.03 (28.46) P-value: 0.487 Interpretation: higher scores is worse outcome |
Authors’ conclusions the decision to treat Lisfranc injuries operatively or conservatively should always include qualitative parameters such as the grade of displacement (Buehren criteria A and C) and quantitative variables like the M1-M2 distance (Buehren criterion B) but also take into account the trauma mechanism. If conservative treatment is chosen, regular checkups are required to not miss secondary displacements.
Remarks Propensity score matching applied to evenly match groups A and B. groups were matched for age, sex, fracture classification, injury mechanism, total number of collateral fractures of the foot and ankle joint, and follow-up time after treatment.
Bonferroni correction was applied to account for multiple testing
Possibility of selection bias due to design. Operative group consisted mainly of homolateral dislocations and partial or complete divergent dislocations. Conservative group particularly consisted of isolated partial displacement. |
Evidence tables bij module Lisfranc letsel (PICO B)
Evidence table for intervention studies (randomized controlled trials and non-randomized observational studies [cohort studies, case-control studies, case series])
|
Study reference |
Study characteristics |
Patient characteristics 2 |
Intervention (I) |
Comparison / control (C) 3
|
Follow-up |
Outcome measures and effect size 4 |
Comments |
|
Sun, 2022 |
Type of study: RCT
Setting and country: hospital, China
Funding and conflicts of interest: This work was supported by the Natural Science Foundation of Beijing (No. 7212020), the Science and Technology Planning Project of Beijing Municipal Education commission (No. KM202110025013), and the Beijing Thousand Talents Project (No. 2020A43). The authors declare no competing interests |
Inclusion criteria: (1) age more than sixteen (closed osteoepiphysis), (2) purely ligamentous and fracture dislocation, and (3) with or without other TMT joint injuries.
Exclusion criteria: (1) previous Lisfranc injuries or foot abnormalities, (2) pathological fracture, (3) with neurovascular or head injury, and (4) with cognitive impairment
N total at baseline: 88 Intervention: 44 Control: 44
Important prognostic factors2: For example age ± SD: I: 41.9 ± 7.8 C: 39.4 ± 8.4
Sex: I: 65.0% M C: 63.2% M
Diabetes: I: 8.0% C: 10.0%
Smoker: I: 20.0% C: 16.0%
Groups comparable at baseline? More men than women in both groups. More smokers in the ORIF group, but both not statistically significant. |
Describe intervention (treatment/procedure/test):
ORIF For open reduction and internal fixation, because dislocation of the first TMT joints may potentially loss some essential anatomical landmarks which influenced precise reduction and alignment, the second ray should be reduced first. Then, the first ray was reduced regarding the second ray as an anatomical template and fixed temporarily by K-wires. The other TMT joints were reduced finally. A large point reduction clamp was used to maintain the reduction between the second metatarsal base and the medial cuneiform. The homerun screw was inserted to fix the joint using solid 2.7-mm cortical screws. The anatomic reduction and alignment of joints were confirmed visually and under fluoroscopy. Bridging plates (DePuy Synthes, Warsaw, IN, USA) between joints were used for the medial three rays. If the lateral column was unstable or dislocated, it was assessed under fluoroscopy after treatment of the medial three rays. When without satisfactory reduction, the lateral column was reduced and stabilized by temporary K-wires but not fused. The K-wires were removed at six weeks post-operatively. If the lateral column could not be closed reduced, then the second incision along the fourth metatarsal was made for reduction and fixation.
|
Describe control (treatment/procedure/test):
PA The exact same approach and principles were used as that in performing open reduction and internal fixation. The first TMT joint was fused only, and ORIF was completed for the second and third TMT joints. And the joints were also fused by the same bridging plates. The only additional step was that the articular cartilage was removed from both the surfaces of the first TMT joint. The lateral column was also reduced and stabilized by temporary K-wires but not fused. No additional bone graft was needed. The joint alignment and implant position were checked under fluoroscopy during surgery, and then, the wound was irrigated, closed, and dressed. |
Length of follow-up: 37.8 (range, 24-48) months
Loss-to-follow-up: Intervention: 0 (0%) N (%) Reasons (describe): not applicable
Control: 1 / 44 N (%) Reasons (describe): not reported
Incomplete outcome data: Intervention: 4 (10%) N (%) Reasons (describe): not reported
Control: 5 (11.4%) N (%) Reasons (describe): not reported
|
Outcome measures and effect size (include 95%CI and p-value if available):
AOFAS, mean ± SD Note, this outcome is measured at 12 months ORIF: 77.4 ± 5.3 PA: 84.3 ± 7.3 P value: <0.01
Arthrosis, n (%) ORIF: 8 (20) PA: 0 P value: <0.05
|
Authors’ conclusions: PA of the first TMT joint provided a better medium-term outcome than ORIF for Lisfranc injuries with the first TMT dislocation. PA prevented redislocation, pain, and revision as well. Therefore, PA of the first TMT joint was indicated for Lisfranc injuries with the first TMT joint dislocation.
Remarks During follow-up visits, neither patients nor the surgeons were blinded.
Small sample size
Relatively high drop-out rate
|
|
Stødle, 2020 |
Type of study: RCT
Setting and country: Level 1 trauma center, country not reported
Funding and conflicts of interest: Are H. Stødle, MD, reports grants from Sofies Mindes Ortopedi AS, Oslo, Norway, during the conduct of the study. The authors disclosed receipt of financial support from Sophies Minde Ortopedi.
|
Inclusion criteria: Lisfranc injuries with instability of the medial 3 TMT joints and no fractures in relation to the first TMT joint, in patients between 18 and 65 years old. Minor capsular avulsions in relation to the first TMT joint was accepted as a primarily ligamentous injury.
Exclusion criteria: concomitant other major lower extremity injuries / polytrauma, open injuries, previous foot pathology, diabetes mellitus, neuropathy, and peripheral vascular disease.
N total at baseline: 48 ORIF: 24 PA: 24
Important prognostic factors2: For example age ± SD: I: 34 (IQR 28-40) C: 30 (IQR 23-40)
Sex: I: 45.8% M C: 45.8% M
Groups comparable at baseline? Mostly yes, but a higher rate of ligamentous injuries in the PA group was observed |
Describe intervention (treatment/procedure/test):
ORIF A 2-incision technique was used, with one longitudinal dorsomedial incision over the first TMT joint and a second incision over the third TMT joint. A skin bridge of at least 4 cm between the incisions was preserved to reduce the risk of wound complications. The 3 medial TMT joints were exposed. In the patients randomized to dorsal bridge plating, the cartilage of the first TMT joint was left intact.
In the BP group, the first TMT joint was bridged with 2.7-mm locking plate. |
Describe control (treatment/procedure/test):
PA As all of the patients were treated with a primary arthrodesis of the second and third TMT joint, the cartilage was removed from these joints and the subchondral bone was multiperforated using a 2-mm drill bit to enhance fusion. No bone graft was used.
In the PA group, the arthrodesis of the first TMT joint was fixed with two 2.7- or 3.5-mm fully threaded screws with interfragmentary compression.
The primary arthrodesis of the second and third TMT joints was either fixed using 2.7- or 3.5-mm fully threaded screws or, in case of a severely comminuted joint, a locking plate was used.
A “homerun-screw” was then placed from the medial cuneiform to the base of the second metatarsal securing the Lisfranc mortise. After reduction and fixation of the 3 medial TMT joints, the reduction of the fourth and fifth TMT joints was assessed. If displaced, the 2 lateral TMT joints were reduced and stabilized using 1.6-mm Kirschner wires. |
Length of follow-up: 24 months
Loss-to-follow-up: Intervention: not reported N (%) Reasons (describe): not reported
Control: not reported N (%) Reasons (describe): not reported
Incomplete outcome data: Intervention: not reported N (%) Reasons (describe): not reported
Control: not reported N (%) Reasons (describe): not reported
|
Outcome measures and effect size (include 95%CI and p-value if available):
AOFAS, mean ± SD 12 months follow-up ORIF: 79 ± 16 PA: 85 ± 10 P-value: 0.12 24 months follow-up ORIF: 85 ± 15 PA: 89 ± 9 P-value: 0.32
Nonunion, n (%) ORIF: 2 (8.70) PA: 1 (4.55) P-value: >0.99
Arthrosis, n (%) ORIF: 11 (45.8) PA: 0 (0) P-value: not reported
Hardware removal, n (%) ORIF: 5 (20.8) PA: 7 (30.4) P-value: 0.45
Infection “One patient had a superficial wound infection in the BP group”(only in the text) Information not provided for PA group. |
Authors’ conclusions Both the temporary bridge plate (BP) group and the primary arthrodesis (PA) group yielded good outcome scores when treating Lisfranc injuries. We did not find superiority of the BP group compared to the PA group according to the AOFAS midfoot score. The first metatarsal was better aligned in the BP group. Despite avoiding transarticular screw damage by bridge plating the first TMT joints, there was a high rate of radiologically detected osteoarthritis in the first TMT joint. The long-term effects of post-traumatic osteoarthritis is still unknown and longer follow-up is required.
Remarks Lack of external validity, since only ‘low-energy’ injuries were included, which are known to have better results than high-energy injuries.
Possibility to have missed patients with minor symptoms for nonunion, because only patients with clinically suspected nonunion were evaluated.
Patients and examiner were not blinded for the treatment. |
|
Henning, 2009 |
Type of study: RCT
Setting and country: not reported
Funding and conflicts of interest: not reported |
Inclusion criteria: Acute Lisfranc injury of less than 3 months duration and closed physes/skeletal maturity.
Exclusion criteria Major intra-articular fracture pattern, prior foot trauma, prior foot infection, prior foot surgery, prior foot pathology, chronic injury of greater than three months duration, or associated medical comorbidities such as diabetes mellitus, peripheral vascular disease, peripheral neuropathy, or autoimmune disease.
N total at baseline: 32 ORIF: 14 PA: 18
Important prognostic factors2: For example age ± SD: ORIF: 37 (range, 20-58) years PA: 40 (range, 25-73) years
Sex: ORIF: 64.3% M PA: 66.7% M
Groups comparable at baseline? Yes, sex, age, mechanism of injury, and smoking rate was similar between groups (p >0.05) |
Describe intervention (treatment/procedure/test):
ORIF primary ORIF consisted of a 9- to 10-cm, dorsal longitudinal incision over the interval at the base of the first and second TMT joints. This approach allowed visualization and reduction of the first, second, and medial half of the third TMT joints. The first TMT was reduced with a tenaculum clamp and flexion force avoided plantar gapping or malreduction. Crossed 0.062 Kirschner wires secured the reduction. With a tenaculum clamp compressing the joint, a retrograde 0.062 Kirschner wire secured the joint. The medial aspect of the third TMT was visualized through the same incision. When necessary, the lateral 8 cm longitudinal, universal incision over the fourth metatarsal allowed access to the lateral aspect of the third and entire visualization of the fourth and fifth TMT joints. After the third TMT was reduced with a tenaculum clamp, a 0.062 Kirschner wire was percutaneously inserted retrograde to stabilize the third TMT joint. If the Kirschner wires were inserted close to the TMT, the wire would not interfere with retrograde drilling and screw insertion. The fourth and fifth TMTs were reduced with dental picks and tenaculum clamps. Retrograde percutaneous 0.062 Kirschner wires were inserted perpendicular to the TMT joint and into the subchondral bone of the cuboid. Temporary reduction was confirmed with anterior-posterior (AP), lateral (Lat), and oblique (Obl) intraoperative fluoroscopic views. Final stabilization was performed in a medial to lateral direction. A step was created on the mid anterior first MT cortical surface with a perpendicular drill through the first cortex only. A 2.5-mm drill with drill sleeve was used to cross the joint about 30 degrees from the anterior cortical surface.17 Periarticular screws (Zimmer, Warsaw, IN) with a 3.5-mm shaft and a 2.7-mm head size increased joint stability and screw longevity with lessened cortical splitting. Two crossed 3.5-mm cortical screws were inserted at the first TMT joint. The retrograde screw was inserted along the medial half of the first TMT perpendicular to the joint. The antegrade screw was inserted from the lateral half of the medial cuneiform into the base of the first MT. A single retrograde 3.5-mm periarticular screw was inserted perpendicularly across the second TMT joint on the AP view. A percutaneous incision over the mid portion of the third metatarsal was used for insertion of a single retrograde 2.7-mm or 3.5-mm periarticular cortical screw across the third TMT joint. All screws were inserted in a neutral, not lag technique. The final screw position and TMT reductions were confirmed with fluoroscopy. The fourth and fifth TMT Kirschner wires were cut below the skin. A posterior splint in neutral position was applied. When present, an associated cuboid fracture was reduced and stabilized via the lateral longitudinal incision. A 2.5-mm external fixator (Synthes, Paoli, PA) was inserted across the cuboid from the calcaneus to the fifth MT shaft. The impacted articular surface was carefully elevated with an osteotome or elevator followed by insertion of allograft bone in the void. A mini “T” plate (Synthes, Paoli, PA) stabilized the cuboid fracture and allowed for insertion of “raft” screws into the subchondral bone. Because anatomical fixation of the cuboid articular surface determined TMT reduction and restoration of the lateral column length, the cuboid fracture fixation was performed before fourth and fifth TMT reduction and stabilization with Kirschner wires. |
Describe control (treatment/procedure/test):
PA For PA, the same dorsal access was provided with the two longitudinal incisions. Since the first, second, and third TMT joints are “non-essential” or relatively immobile, the TMT joints were fused. Since the fourth and fifth TMT “essential” or more mobile, the fourth and fifth TMT joints were not fused. The reduction and fixation sequence was similar to PORIF, i.e. medial to lateral. The articular surface was removed with one-quarter inch osteotomes and small curettes. Final subchondral preparation required 2.0 mm drill perforation through the subchondral bone into cancellous bone. The temporary stabilization of the joints was similar to the PORIF group. Screws were inserted via a lag technique to compress the subchondral surfaces with the same screw configuration as PORIF. No additional bone graft or allograft was necessary. |
Length of follow-up: 24 months
Loss-to-follow-up:
3 patients dropped out postoperatively and 5 patients were lost to follow-up early in the study. At the time of the final phone survey, an additional 9 patients were unable to be contacted after an exhaustive search.
Note: drop out rate and reasons were not reported per treatment group.
Incomplete outcome data: ORIF: not reported N (%) Reasons (describe): not reported
PA: not reported N (%) Reasons (describe): not reported |
Outcome measures and effect size (include 95%CI and p-value if available):
Hardware removal, n (%) ORIF: 11 (79) PA: 3 (17) P-value: <0.05
Infection, n (%) ORIF: 0 (0) PA: 1/18 (5.56) à superficial cellulitis P-value: not reported
Neural injury No neural injury was noted in both ORIF and PA group (only in text)
Nonunion “one delayed union associated with a broken first TMT joint screw healed at the 6-month mark, and one nonunion of a first TMT joint was treated nonoperatively” in the PA group” (only in the text). This information was not provided for the ORIF group. |
Authors conclusion: PA resulted in a statistically significant decrease in the number of follow-up surgeries performed compared to ORIF if hardware removal is routinely performed.
Remarks Small sample size
High drop out rate during follow-up
Non-blinding of the surgeon who directed examinations and grading of radiographs may have resulted in bias
The study was discontinued prematurely, because patients undergoing PA were doing clinically as well as the ORIF patients with significantly fewer follow-up surgical procedures and without adverse outcomes. |
|
Ly, 2006 |
Type of study: RCT
Setting and country: not reported
Funding and conflicts of interest: The authors did not receive grants or outside funding in support of their research for or preparation of this manuscript. They did not receive payments or other benefits or a commitment or agreement to provide such benefits from a commercial entity. No commercial entity paid or directed, or agreed to pay or direct, any benefits to any research fund, foundation, educational institution, or other charitable or nonprofit organization with which the authors are affiliated or associated. |
Inclusion criteria: the Lisfranc injury had to be primarily ligamentous, with no major fractures present. Lisfranc injuries with a fleck sign were considered to be primarily ligamentous.
Exclusion criteria: commuted intra-articular fracture at the base of the first or second metatarsal; any other substantial foot, ankle, or leg injury; a previous attempt at surgical management of the same injury; insulin-dependent diabetes mellitus; ipsilateral ankle fusion; peripheral vascular disease; peripheral neuropathy; and rheumatoid arthritis.
N total at baseline: 41 Intervention: 20 Control: 21
Important prognostic factors2: For example age ± SD: I: 32.4 (range, 19-52) years C: 32 (range, 19-42) years
Sex: I: 65% M C: 66.7% M
Groups comparable at baseline? Yes |
Describe intervention (treatment/procedure/test):
ORIF Two dorsal longitudinal incisions—one between the first and second metatarsals and the second centered between the fourth and fifth metatarsals—were made. Open reduction and screw fixation of the first, second, and third metatarsal-cuneiform joints was performed. Then, if necessary, Kirschner wires were placed in each of the lateral two rays, but the rays were not fused. Seven patients in the open-reduction group required Kirschner-wire fixation of the lateral rays. The Kirschner wires were removed between six and eight weeks postoperatively. The screws were not routinely removed unless they caused symptoms, and they were never removed before three months. |
Describe control (treatment/procedure/test):
PA Standard incisions were made as described for the open-reduction group. Open reduction was performed, cartilage and fibrous tissue were resected, and the joints were decorticated. Reduction and screw fixation was then performed. If the third metatarsal-cuneiform joint was seen to be displaced on the computed tomography scan or was clearly unstable on direct examination, it was fused in the same fashion. The rationale for treatment of the lateral two rays was exactly the same as the rationale in the open-reduction group. Nine patients in the arthrodesis group underwent temporary Kirschner-wire fixation of the lateral two rays. |
Length of follow-up: 42.5 months
Loss-to-follow-up: ORIF: not reported N (%) Reasons (describe): not reported
PA: not reported N (%) Reasons (describe): not reported
Incomplete outcome data: ORIF: not reported N (%) Reasons (describe): not reported
PA: not reported N (%) Reasons (describe): not reported
|
Outcome measures and effect size (include 95%CI and p-value if available):
AOFAS, mean (range) Measured at 24 months follow-up ORIF: 68.6 (16-100) PA: 88.0 (63-100) P-value: 0.005 Measured at final follow-up ORIF: 57.1 (16-100) PA: 86.9 (63-100) P-value: 0.0001
Hardware removal, n (%) ORIF: 16 (80) PA: 4 (19) P-value: not reported
Nonunion, delayed union “One patient had a delayed union” and “one patient with a nonunion […]” in the PA group (only in the text). No such information was provided for the ORIF group.
|
Authors conclusion: Because of the poor healing potential of the ligament-osseous interface and the trend toward a higher rate of correction loss, increasing deformity, and degenerative arthritic changes, we believe that primarily ligamentous injuries are a subset of Lisfranc joint injuries that are not as amenable to internal fixation. We believe that stable arthrodesis is a better primary treatment for these injuries, with superior short and medium-term outcomes than those following open reduction and internal fixation.
Remarks The allocation of treatment with an open-randomization, odd-or-even format could have introduced selection bias. Using a random-numbers table or computerized randomization would have been a better way to allocate our patients.
The attending surgeon performed the follow-up clinical examination and gathered the questionnaires, which raises concern about bias.
Hardware removal could have contributed to the poor results observed in the ORIF group. |
Risk of Bias table module Lisfranc letsel (PICO A)
|
Author, year |
Selection of participants
Was selection of exposed and non-exposed cohorts drawn from the same population?
|
Exposure
Can we be confident in the assessment of exposure?
|
Outcome of interest
Can we be confident that the outcome of interest was not present at start of study?
|
Confounding-assessment
Can we be confident in the assessment of confounding factors?
|
Confounding-analysis
Did the study match exposed and unexposed for all variables that are associated with the outcome of interest or did the statistical analysis adjust for these confounding variables?
|
Assessment of outcome
Can we be confident in the assessment of outcome?
|
Follow up
Was the follow up of cohorts adequate? In particular, was outcome data complete or imputed?
|
Co-interventions
Were co-interventions similar between groups?
|
Overall Risk of bias
|
|
Definitely yes, probably yes, probably no, definitely no |
Definitely yes, probably yes, probably no, definitely no |
Definitely yes, probably yes, probably no, definitely no |
Definitely yes, probably yes, probably no, definitely no |
Definitely yes, probably yes, probably no, definitely no |
Definitely yes, probably yes, probably no, definitely no |
Definitely yes, probably yes, probably no, definitely no |
Definitely yes, probably yes, probably no, definitely no |
Low, Some concerns, High |
|
|
Ren, 2019 |
Definitely yes
Reason: all patients had undisplaced subtle ligamentous Lisfranc injury |
Definitely yes
Reason: surgeons performed the procedures (i.e. exposures) |
Definitely yes
Reason: all outcomes were assessed after treatment |
Definitely no
Reason: confounding factors are not assessed. |
Definitely no
Reason: no adjustment for confounding in statistical analyses, because confounders were not defined a priori. |
Probably yes
Reason: not clearly described, but no reason to believe that this was not well performed. |
Definitely yes
Reason: Outcome data were complete. |
No information
|
Some concerns for all outcomes
Reason: no adjustment for confounding |
|
Garriguez-Pérez, 2021 |
Definitely no
Reason: different types of Lisfranc injuries (stage I, II and III) constituted the cohort and were differently treated. |
Definitely yes
Reason: Stage I injuries received conservative treatment, stage II and III injuries received operative treatment. The treatments were well performed. |
Definitely yes
Reason: all outcomes were assessed after treatment. |
Definitely no
Reason: confounding factors are not assessed. |
Definitely no
Reason: no adjustment for confounding in statistical analyses, because confounders were not defined a priori. |
No information |
No information
|
No information |
Some concerns for all outcomes
Reason: patients not comparable, no adjustment for confounding |
|
Graef, 2021 |
Definitely no
Reason: different types of Lisfranc fractures were differently treated. |
Definitely yes
Reason: treatments were well performed. |
Definitely yes
Reason: all outcomes were assessed after treatment. |
Definitely yes
Reason: the authors of the study thought a priori about potential confounders, such as age, sex, and trauma mechanism. |
Definitely yes
Reason: propensity score matching was applied. |
Probably yes
Reason: the FFI-F is a valid scale to assess foot function |
No information |
No information |
Some concerns for all outcomes (FFI)
Reason: concerns related to study population |
Risk of Bias table Lisfranc letsel (PICO B)
|
Study reference
(first author, publication year) |
Was the allocation sequence adequately generated?
Definitely yes Probably yes Probably no Definitely no |
Was the allocation adequately concealed?
Definitely yes Probably yes Probably no Definitely no |
Blinding: Was knowledge of the allocated interventions adequately prevented?
Were patients blinded?
Were healthcare providers blinded?
Were data collectors blinded?
Were outcome assessors blinded?
Were data analysts blinded?
Definitely yes Probably yes Probably no Definitely no |
Was loss to follow-up (missing outcome data) infrequent?
Definitely yes Probably yes Probably no Definitely no |
Are reports of the study free of selective outcome reporting?
Definitely yes Probably yes Probably no Definitely no |
Was the study apparently free of other problems that could put it at a risk of bias?
Definitely yes Probably yes Probably no Definitely no |
Overall risk of bias If applicable/necessary, per outcome measure
LOW Some concerns HIGH
|
|
Sun, 2022 |
Definitely yes
Reason: a random number generated system was used for the blinded process.
|
Probably yes
Reason: numbered envelopes were used.
|
Definitely no
Reason: Patients and healthcare providers were not blinded
Data analysts were blinded. |
Probably yes
Reason: it was stated that 1 patient was lost to follow-up and 9 patients had missing data. Missing data were not imputed |
Probably yes
Reason: the protocol is not on clinical trials.gov. However, all outcomes that have been described are presented in tables |
Definitely yes
Reason: authors have no conflicts of interest. No reason to believe that the funders of this project influenced study outcomes. |
Some concerns for all outcomes.
Reason: lack of blinding and potential problem with loss to follow-up.
|
|
Stødle, 2020 |
Definitely yes
Reason: A random allocation rule was used. |
Definitely yes
Reason: Allocations were kept in sealed, opaque envelopes and were manually shuffled. |
Definitely no
Reason: healthcare provider and patients were not blinded. |
Definitely no
Reason: there was no loss to follow-up |
Probably yes
Reason: more outcomes reported in article than in protocol |
Definitely no
Reason: potential of selection bias due to inclusion of only low-energy injuries that are known to have better results than high-energy injuries. And main reports a grant from Sofies Mindes Ortopedi, but no reason to believe that this institution influenced study results. |
Some concerns for all outcomes
Reason: lack of blinding and problem with selection bias
|
|
Henning, 2009 |
Definitely yes
Reason: A random number generating system was used. |
Probably yes
Reason: envelopes were used, but not clear whether envelopes were sealed and opaque. |
Definitely no
Reason: healthcare providers were not blinded. Not clear whether patients were blinded. |
Probably yes
Reason: three patients dropped out postoperatively and five patients were lost to follow-up early in the study. At the time of the final phone survey, an additional nine patients were unable to be contacted. |
Probably yes
Reason: all outcomes that have been described in the article are presented in tables |
Definitely no
Reason: three different surgeons performed the procedures and the trial was discontinued prematurely |
Some concerns
Reason: lack of blinding and trial was discontinued prematurely.
|
|
Ly, 2006 |
Definitely no
Reason: An odd-or-even process was used. |
No information
|
Definitely no
Reason: healthcare providers were not blinded. |
No information
|
Probably yes
Reason: all outcomes that have been described in methods are presented in tables or in text. |
Definitely yes
Reason: the authors did not receive grants or outside funding in support of their research. They did not get payments from commercial entities |
Some concerns for all outcomes
Reason: randomization process and lack of blinding.
|
Table of excluded studies
|
Reference |
Reason for exclusion |
|
Ahluwalia R, Yip G, Richter M, Maffulli N. Surgical controversies and current concepts in Lisfranc injuries. Br Med Bull. 2022 Dec 12;144(1):57-75. doi: 10.1093/bmb/ldac020. PMID: 36151742. |
Wrong design: narrative review |
|
Attia AK, Mahmoud K, Alhammoud A, d'Hooghe P, Farber D. Return to Play After Low-Energy Lisfranc Injuries in High-Demand Individuals: A Systematic Review and Meta-Analysis of Athletes and Active Military Personnel. Orthop J Sports Med. 2021 Mar 8;9(3):2325967120988158. doi: 10.1177/2325967120988158. PMID: 33763497; PMCID: PMC7944543. |
Wrong outcome: return to play / evt achtergrond, goed uitgevoerde review |
|
Cosculluela, Pedro E., Andrew M. Ebert, and Kevin E. Varner. "Dorsomedial bridge plating of Lisfranc injuries." Techniques in Foot & Ankle Surgery 8.4 (2009): 215-220. |
Wrong design: description of a technique |
|
Crates JM, Barber FA, Sanders EJ. Subtle Lisfranc subluxation: results of operative and nonoperative treatment. J Foot Ankle Surg. 2015 May-Jun;54(3):350-5. doi: 10.1053/j.jfas.2014.07.015. Epub 2015 Mar 4. PMID: 25746769. |
wrong population: all patients underwent initially non-operative management, those who failed underwent surgery |
|
Ettinger S, Altemeier A, Stukenborg-Colsman C, Yao D, Plaass C, Lerch M, Claassen L. Comparison of Isolated Screw to Plate and Screw Fixation for Tarsometatarsal Arthrodesis Including Clinical Outcome Predictors. Foot Ankle Int. 2021 Jun;42(6):734-743. doi: 10.1177/1071100720980014. Epub 2021 Feb 6. PMID: 33550860. |
Wrong comparison: comparison of two surgical techniques |
|
Gentchos, Christopher. "Lisfranc Injuries." Techniques in Foot & Ankle Surgery 20.2 (2021): 66-74. |
Narrative review |
|
Grewal US, Onubogu K, Southgate C, Dhinsa BS. Lisfranc injury: A review and simplified treatment algorithm. Foot (Edinb). 2020 Dec;45:101719. doi: 10.1016/j.foot.2020.101719. Epub 2020 Jul 6. PMID: 33038662. |
Non-systematic review |
|
van Hoeve S, Stollenwerck G, Willems P, Witlox MA, Meijer K, Poeze M. Gait analysis and functional outcome in patients after Lisfranc injury treatment. Foot Ankle Surg. 2018 Dec;24(6):535-541. doi: 10.1016/j.fas.2017.07.003. Epub 2017 Jul 18. PMID: 29409269. |
Patient characteristics of Lisfranc injuries are compared with healthy controls and only 5 patients in the conservative treatment group |
|
Kushare I, Wunderlich N, Elabd A, Attia E. Pediatric and adolescent Lisfranc injuries - Presentation, treatment and outcomes. Foot (Edinb). 2021 Mar;46:101737. doi: 10.1016/j.foot.2020.101737. Epub 2020 Sep 9. PMID: 33853714. |
Wrong population: paediatric patients |
|
Lau S, Howells N, Millar M, De Villiers D, Joseph S, Oppy A. Plates, Screws, or Combination? Radiologic Outcomes After Lisfranc Fracture Dislocation. J Foot Ankle Surg. 2016 Jul-Aug;55(4):799-802. doi: 10.1053/j.jfas.2016.03.002. Epub 2016 Apr 12. PMID: 27079306. |
Comparison of four groups (rather than two), and only two patients in the conservative treatment group. Comparison is therefore not possible.
|
|
Levy CJ, Yatsonsky D 2nd, Moral MZ, Liu J, Ebraheim NA. Arthrodesis or Open Reduction Internal Fixation for Lisfranc Injuries: A Meta-analysis. Foot Ankle Spec. 2022 Apr;15(2):179-184. doi: 10.1177/1938640020971419. Epub 2020 Dec 3. PMID: 33269645. |
Higher quality SR available (van den Boom, 2021) |
|
McHale KJ, Rozell JC, Milby AH, Carey JL, Sennett BJ. Outcomes of Lisfranc Injuries in the National Football League. Am J Sports Med. 2016 Jul;44(7):1810-7. doi: 10.1177/0363546516645082. Epub 2016 May 10. PMID: 27166291. |
Unclear if all patients have a dislocation at start of the study |
|
Nunley JA, Vertullo CJ. Classification, investigation, and management of midfoot sprains: Lisfranc injuries in the athlete. Am J Sports Med. 2002 Nov-Dec;30(6):871-8. doi: 10.1177/03635465020300061901. PMID: 12435655. |
Comparison of different types of Lisfranc fractures (stage I, II and III) and wrong population (n = 15 athletes) |
|
Prakash, Mukara, et al. "A Prospective Study on the Surgical Management of Closed Lisfranc Injury by Various Modalities." European Journal of Molecular & Clinical Medicine (EJMCM) 8.04 (2021): 2021 |
wrong comparison: comparison of different fixation techniques |
|
Richter M, Thermann H, Huefner T, Schmidt U, Krettek C. Aetiology, treatment and outcome in Lisfranc joint dislocations and fracture dislocations. Foot and Ankle Surgery. 2002;8:21-32. https://doi.org/10.1046/j.1460-9584.2002.00294.x |
Wrong population; patients who were treated operatively may have different types of Lisfranc injuries than patients who were treated non-operatively. |
|
Robertson GAJ, Ang KK, Maffulli N, Keenan G, Wood AM. Return to sport following Lisfranc injuries: A systematic review and meta-analysis. Foot Ankle Surg. 2019 Oct;25(5):654-664. doi: 10.1016/j.fas.2018.07.008. Epub 2018 Aug 8. PMID: 30321929. |
Non-comparative studies included, only studies reporting return to sports included |
|
Shakked RJ. Lisfranc Injury in the Athlete. JBJS Rev. 2017 Sep;5(9):e4. doi: 10.2106/JBJS.RVW.17.00025. PMID: 28902660. |
Non-systematic review |
|
So E, Lee J, Pershing ML, Chu AK, Wilson M, Halaharvi C, Mandas V, Hyer CF. A Comparison of Complications and Reoperations Between Open Reduction and Internal Fixation Versus Primary Arthrodesis Following Lisfranc Injury. Foot Ankle Spec. 2021 Nov 28:19386400211058264. doi: 10.1177/19386400211058264. Epub ahead of print. PMID: 34841938. |
Wrong design; retrospective cohort. |
|
Stavrakakis IM, Magarakis GE, Christoforakis Z. Percutaneous fixation of Lisfranc joint injuries: A systematic review of the literature. Acta Orthop Traumatol Turc. 2019 Nov;53(6):457-462. doi: 10.1016/j.aott.2019.08.005. Epub 2019 Sep 28. PMID: 31575479; PMCID: PMC6939019. |
Wrong intervention: study on screw percutaneous fixation |
|
Ter Laak Bolk CS, Dahmen J, Lambers KTA, Blankevoort L, Kerkhoffs GMMJ. Adequate return to sports and sports activities after treatment of Lisfranc injury: a meta-analysis. J ISAKOS. 2021 Jul;6(4):212-219. doi: 10.1136/jisakos-2020-000477. Epub 2020 Dec 10. PMID: 34272297. |
Wrong outcome: return to play |
|
Yu, Xiao, et al. "Cannulated compressive screw compared with cortical screw for fixation of simple first tarsometatarsal joint fracture-dislocation: a finite element analysis." INTERNATIONAL JOURNAL OF CLINICAL AND EXPERIMENTAL MEDICINE 10.4 (2017): 6475-6481. |
Wrong comparison: comparison of two fixation techniques |
Verantwoording
Autorisatiedatum en geldigheid
Laatst beoordeeld : 10-09-2024
Laatst geautoriseerd : 10-09-2024
Geplande herbeoordeling : 10-09-2029
Algemene gegevens
De ontwikkeling van deze richtlijn werd ondersteund door het Kennisinstituut van de Federatie Medisch Specialisten (www.demedischspecialist.nl/kennisinstituut) en werd gefinancierd uit de Kwaliteitsgelden Medisch Specialisten (SKMS). De financier heeft geen enkele invloed gehad op de inhoud van de richtlijnmodule.
Samenstelling werkgroep
Voor het ontwikkelen van de richtlijnmodule is in februari 2022 een multidisciplinaire werkgroep ingesteld, bestaande uit vertegenwoordigers van alle relevante specialismen (zie hiervoor de Samenstelling van de werkgroep) die betrokken zijn bij de zorg voor patiënten met traumatisch complexe voetletsels.
Werkgroep
- Dhr. dr. T. (Tim) Schepers (voorzitter werkgroep); traumachirurg, Amsterdam UMC, Nederlandse Vereniging voor Heelkunde (NVvH)
- Dhr. prof. dr. M. (Martijn) Poeze; traumachirurg, Maastricht UMC, Nederlandse Vereniging voor Heelkunde (NVvH)
- Dhr. dr. S.D. (Stijn) Nelen; traumachirurg, Radboud UMC Nijmegen, Nederlandse Vereniging voor Heelkunde (NVvH)
- Dhr. drs. B.E. (Bastiaan) Steunenberg; radioloog, Elisabeth-TweeSteden Ziekenhuis Tilburg, Nederlandse Vereniging voor Radiologie (NVvR)
- Dhr. drs. H.H. (Erik) Dol; SEH-arts, Jeroen Bosch Ziekenhuis, Nederlandse Vereniging van Spoedeisende Hulp Artsen (NVSHA)
- Dhr. drs. M.W. (Menno) Bloembergen; orthopedisch-chirurg, Reinier Haga Orthopedisch Centrum en Hagaziekenhuis, Nederlandse Orthopaedische Vereniging (NOV)
- Dhr. drs. J.P.S. (Joris) Hermus; orthopedisch-chirurg, Maastricht UMC, Nederlandse Orthopaedische Vereniging (NOV)
- Dhr. E. (Erik) Wink; registerpodoloog/podotherapeut/fysiotherapeut, podologic/stichting Reyery, Koninklijk Nederlands Genootschap Fysiotherapie (KNGF) en stichting LOOP (Landelijk Overkoepelend Orgaan Podologie)
- Dhr. drs. P.W.A. (Peter) Muitjens; revalidatiearts, Adelante Zorggroep, Nederlandse Vereniging van Revalidatieartsen (VRA)
Met ondersteuning van
- Mw. dr. A.C.J. (Astrid) Balemans, senior adviseur, Kennisinstituut van Medisch Specialisten.
- Mw. MSc. D.G. (Dian) Ossendrijver, junior adviseur, Kennisinstituut van Medisch Specialisten.
Belangenverklaringen
De Code ter voorkoming van oneigenlijke beïnvloeding door belangenverstrengeling is gevolgd. Alle werkgroepleden hebben schriftelijk verklaard of zij in de laatste drie jaar directe financiële belangen (betrekking bij een commercieel bedrijf, persoonlijke financiële belangen, onderzoeksfinanciering) of indirecte belangen (persoonlijke relaties, reputatiemanagement) hebben gehad. Gedurende de ontwikkeling of herziening van een module worden wijzigingen in belangen aan de voorzitter doorgegeven. De belangenverklaring wordt opnieuw bevestigd tijdens de commentaarfase.
Een overzicht van de belangen van werkgroepleden en het oordeel over het omgaan met eventuele belangen vindt u in onderstaande tabel. De ondertekende belangenverklaringen zijn op te vragen bij het secretariaat van het Kennisinstituut van de Federatie Medisch Specialisten.
Belangentabel richtlijnwerkgroep complexe voetletsels
|
Werkgroeplid |
Functie |
Nevenwerkzaamheden |
Gemelde Persoonlijke Financiële Belangen |
Gemelde Persoonlijke Relaties |
Extern gefinancierd onderzoek |
Gemelde Intell. belangen en reputatie |
Gemelde Overige belangen |
Ondernomen actie |
|
Tim Schepers (voorzitter richtlijn complexe voetletsels) |
Traumachirurg voltijd Amsterdam UMC
|
Voet-enkel expert groep van de Arbeitsgemeinschaft für Osteosynthesefragen (AO)
|
Geen |
Geen |
Ja: wifi2 studie naar wondinfecties bij voet+enkel operaties (verwijderen schroeven/plaat): https://www.amc.nl/web/research-75/trials-collaborations/wifi-2.htm. De studie richt zich op de effectiviteit van antibiotica op het voorkomen van infecties. Rol als projectleider. Gefinancierd roor ZonMW, direct aan Amsterdam Medical Research |
Geschat +/- 200 publicaties over voet- enkelletsel
|
Geen |
Geen restricties; geen van de modules gaat over het onderwerp van de wifi2 studie |
|
Stijn Nelen |
Traumachirurg Radboud UMC Nijmegen |
ATLS-instructeur |
Geen |
Geen |
Geen |
Geen |
Geen |
Geen restricties |
|
Martijn Poeze |
Traumachirurg Maastricht UMC |
Geen |
Geen |
Geen |
Geen |
Geen |
Geen |
Geen restricties |
|
Bastiaan Steunenberg |
Radioloog Isala Zwolle |
Geen |
Geen |
Geen |
Geen |
Geen |
Geen |
Geen restricties |
|
Erik Dol |
SEH-arts KNMG |
ATLS-instructeur |
Geen |
Geen |
Geen |
Geen |
Geen |
Geen restricties |
|
Menno Bloembergen |
Orthopedisch chirurg |
Geen |
Geen |
Geen |
Geen |
Geen |
Geen |
Geen restricties |
|
Joris PS Hermus |
Orthopedisch chirurg-traumatoloog |
Voet-enkel expert groep van de Arbeitsgemeinschaft für Osteosynthesefragen (AO)
Council member & honorary secretary European Foot and Ankle Society |
Geen |
Geen |
Geen |
Geen |
Geen |
Geen restricties |
|
Erik Wink |
Registerpodoloog podotherapeut bij podologic |
Fysiotherapeut bij reyerey, langebaan schaatsploeg |
Geen |
Geen |
Geen |
Geen |
Geen |
Geen restricties |
|
Peter Muitjens |
Revalidatiearts 0,8 fte, betaald |
Geen |
Geen |
Geen |
Geen |
Geen |
Geen |
Geen restricties |
Inbreng patiëntenperspectief
Er werd aandacht besteed aan het patiëntenperspectief door het uitnodigen van Patiëntenfederatie Nederland (PFN) voor de schriftelijke knelpuntenanalyse. De verkregen input is meegenomen bij het opstellen van de uitgangsvragen, de keuze voor de uitkomstmaten en bij het opstellen van de overwegingen. De conceptrichtlijn is tevens voor commentaar voorgelegd aan Patiëntenfederatie Nederland en de eventueel aangeleverde commentaren zijn bekeken en verwerkt.
Kwalitatieve raming van mogelijke financiële gevolgen in het kader van de Wkkgz
Bij de richtlijnmodule is conform de Wet kwaliteit, klachten en geschillen zorg (Wkkgz) een kwalitatieve raming uitgevoerd om te beoordelen of de aanbevelingen mogelijk leiden tot substantiële financiële gevolgen. Bij het uitvoeren van deze beoordeling is de richtlijnmodule op verschillende domeinen getoetst (zie het stroomschema op de Richtlijnendatabase).
Module |
Uitkomst raming |
Toelichting |
|
Module Lisfranc letsel |
Geen financiële gevolgen |
Uit de toetsing volgt dat de aanbeveling(en) niet breed toepasbaar zijn (<5.000 patiënten) en daarom naar verwachting geen substantiële financiële gevolgen zullen hebben voor de collectieve uitgaven. |
Werkwijze
AGREE
Deze richtlijnmodule is opgesteld conform de eisen vermeld in het rapport Medisch Specialistische Richtlijnen 2.0 van de adviescommissie Richtlijnen van de Raad Kwaliteit. Dit rapport is gebaseerd op het AGREE II instrument (Appraisal of Guidelines for Research & Evaluation II; Brouwers, 2010).
Knelpuntenanalyse en uitgangsvragen
Tijdens de voorbereidende fase inventariseerde de werkgroep de knelpunten in de zorg voor patiënten met traumatisch complex voetletsel. Tevens zijn er knelpunten aangedragen door middel van een schriftelijke knelpuntenanalyse. Een verslag hiervan is opgenomen onder aanverwante producten.
Op basis van de uitkomsten van de knelpuntenanalyse zijn door de werkgroep concept-uitgangsvragen opgesteld en definitief vastgesteld.
Uitkomstmaten
Na het opstellen van de zoekvraag behorende bij de uitgangsvraag inventariseerde de werkgroep welke uitkomstmaten voor de patiënt relevant zijn, waarbij zowel naar gewenste als ongewenste effecten werd gekeken. Hierbij werd een maximum van acht uitkomstmaten gehanteerd. De werkgroep waardeerde deze uitkomstmaten volgens hun relatieve belang bij de besluitvorming rondom aanbevelingen, als cruciaal (kritiek voor de besluitvorming), belangrijk (maar niet cruciaal) en onbelangrijk. Tevens definieerde de werkgroep tenminste voor de cruciale uitkomstmaten welke verschillen zij klinisch (patiënt) relevant vonden.
Methode literatuursamenvatting
Een uitgebreide beschrijving van de strategie voor zoeken en selecteren van literatuur is te vinden onder ‘Zoeken en selecteren’ onder Onderbouwing. Indien mogelijk werd de data uit verschillende studies gepoold in een random-effects model (Review Manager 5.4) werd gebruikt voor de statistische analyses. De beoordeling van de kracht van het wetenschappelijke bewijs wordt hieronder toegelicht.
Beoordelen van de kracht van het wetenschappelijke bewijs
De kracht van het wetenschappelijke bewijs werd bepaald volgens de GRADE-methode. GRADE staat voor ‘Grading Recommendations Assessment, Development and Evaluation’ (zie http://www.gradeworkinggroup.org/). De basisprincipes van de GRADE-methodiek zijn: het benoemen en prioriteren van de klinisch (patiënt) relevante uitkomstmaten, een systematische review per uitkomstmaat, en een beoordeling van de bewijskracht per uitkomstmaat op basis van de acht GRADE-domeinen (domeinen voor downgraden: risk of bias, inconsistentie, indirectheid, imprecisie, en publicatiebias; domeinen voor upgraden: dosis-effect relatie, groot effect, en residuele plausibele confounding).
GRADE onderscheidt vier gradaties voor de kwaliteit van het wetenschappelijk bewijs: hoog, redelijk, laag en zeer laag. Deze gradaties verwijzen naar de mate van zekerheid die er bestaat over de literatuurconclusie, in het bijzonder de mate van zekerheid dat de literatuurconclusie de aanbeveling adequaat ondersteunt (Schünemann, 2013; Hultcrantz, 2017).
|
GRADE |
Definitie |
|
Hoog |
|
|
Redelijk |
|
|
Laag |
|
|
Zeer laag |
|
Bij het beoordelen (graderen) van de kracht van het wetenschappelijk bewijs in richtlijnen volgens de GRADE-methodiek spelen grenzen voor klinische besluitvorming een belangrijke rol (Hultcrantz, 2017). Dit zijn de grenzen die bij overschrijding aanleiding zouden geven tot een aanpassing van de aanbeveling. Om de grenzen voor klinische besluitvorming te bepalen moeten alle relevante uitkomstmaten en overwegingen worden meegewogen. De grenzen voor klinische besluitvorming zijn daarmee niet één op één vergelijkbaar met het minimaal klinisch relevant verschil (Minimal Clinically Important Difference, MCID). Met name in situaties waarin een interventie geen belangrijke nadelen heeft en de kosten relatief laag zijn, kan de grens voor klinische besluitvorming met betrekking tot de effectiviteit van de interventie bij een lagere waarde (dichter bij het nuleffect) liggen dan de MCID (Hultcrantz, 2017).
Overwegingen (van bewijs naar aanbeveling)
Om te komen tot een aanbeveling zijn naast (de kwaliteit van) het wetenschappelijke bewijs ook andere aspecten belangrijk en worden meegewogen, zoals aanvullende argumenten uit bijvoorbeeld de biomechanica of fysiologie, waarden en voorkeuren van patiënten, kosten (middelenbeslag), aanvaardbaarheid, haalbaarheid en implementatie. Deze aspecten zijn systematisch vermeld en beoordeeld (gewogen) onder het kopje ‘Overwegingen’ en kunnen (mede) gebaseerd zijn op expert opinion. Hierbij is gebruik gemaakt van een gestructureerd format gebaseerd op het evidence-to-decision framework van de internationale GRADE Working Group (Alonso-Coello, 2016a; Alonso-Coello 2016b). Dit evidence-to-decision framework is een integraal onderdeel van de GRADE methodiek.
Formuleren van aanbevelingen
De aanbevelingen geven antwoord op de uitgangsvraag en zijn gebaseerd op het beschikbare wetenschappelijke bewijs en de belangrijkste overwegingen, en een weging van de gunstige en ongunstige effecten van de relevante interventies. De kracht van het wetenschappelijk bewijs en het gewicht dat door de werkgroep wordt toegekend aan de overwegingen, bepalen samen de sterkte van de aanbeveling. Conform de GRADE-methodiek sluit een lage bewijskracht van conclusies in de systematische literatuuranalyse een sterke aanbeveling niet a priori uit, en zijn bij een hoge bewijskracht ook zwakke aanbevelingen mogelijk (Agoritsas, 2017; Neumann, 2016). De sterkte van de aanbeveling wordt altijd bepaald door weging van alle relevante argumenten tezamen. De werkgroep heeft bij elke aanbeveling opgenomen hoe zij tot de richting en sterkte van de aanbeveling zijn gekomen.
In de GRADE-methodiek wordt onderscheid gemaakt tussen sterke en zwakke (of conditionele) aanbevelingen. De sterkte van een aanbeveling verwijst naar de mate van zekerheid dat de voordelen van de interventie opwegen tegen de nadelen (of vice versa), gezien over het hele spectrum van patiënten waarvoor de aanbeveling is bedoeld. De sterkte van een aanbeveling heeft duidelijke implicaties voor patiënten, behandelaars en beleidsmakers (zie onderstaande tabel). Een aanbeveling is geen dictaat, zelfs een sterke aanbeveling gebaseerd op bewijs van hoge kwaliteit (GRADE gradering HOOG) zal niet altijd van toepassing zijn, onder alle mogelijke omstandigheden en voor elke individuele patiënt.
|
Implicaties van sterke en zwakke aanbevelingen voor verschillende richtlijngebruikers |
||
|
|
||
|
|
Sterke aanbeveling |
Zwakke (conditionele) aanbeveling |
|
Voor patiënten |
De meeste patiënten zouden de aanbevolen interventie of aanpak kiezen en slechts een klein aantal niet. |
Een aanzienlijk deel van de patiënten zouden de aanbevolen interventie of aanpak kiezen, maar veel patiënten ook niet. |
|
Voor behandelaars |
De meeste patiënten zouden de aanbevolen interventie of aanpak moeten ontvangen. |
Er zijn meerdere geschikte interventies of aanpakken. De patiënt moet worden ondersteund bij de keuze voor de interventie of aanpak die het beste aansluit bij zijn of haar waarden en voorkeuren. |
|
Voor beleidsmakers |
De aanbevolen interventie of aanpak kan worden gezien als standaardbeleid. |
Beleidsbepaling vereist uitvoerige discussie met betrokkenheid van veel stakeholders. Er is een grotere kans op lokale beleidsverschillen. |
Organisatie van zorg
In de knelpuntenanalyse en bij de ontwikkeling van de richtlijnmodule is expliciet aandacht geweest voor de organisatie van zorg: alle aspecten die randvoorwaardelijk zijn voor het verlenen van zorg (zoals coördinatie, communicatie, (financiële) middelen, mankracht en infrastructuur). Randvoorwaarden die relevant zijn voor het beantwoorden van deze specifieke uitgangsvraag zijn genoemd bij de overwegingen. Meer algemene, overkoepelende, of bijkomende aspecten van de organisatie van zorg worden behandeld in de module Organisatie van zorg.
Commentaar- en autorisatiefase
De conceptrichtlijnmodule werd aan de betrokken (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd ter commentaar. De commentaren werden verzameld en besproken met de werkgroep. Naar aanleiding van de commentaren werd de conceptrichtlijnmodule aangepast en definitief vastgesteld door de werkgroep. De definitieve richtlijnmodule werd aan de deelnemende (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd voor autorisatie en door hen geautoriseerd dan wel geaccordeerd.
Literatuur
Agoritsas T, Merglen A, Heen AF, Kristiansen A, Neumann I, Brito JP, Brignardello-Petersen R, Alexander PE, Rind DM, Vandvik PO, Guyatt GH. UpToDate adherence to GRADE criteria for strong recommendations: an analytical survey. BMJ Open. 2017 Nov 16;7(11):e018593. doi: 10.1136/bmjopen-2017-018593. PubMed PMID: 29150475; PubMed Central PMCID: PMC5701989.
Alonso-Coello P, Schünemann HJ, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Rada G, Rosenbaum S, Morelli A, Guyatt GH, Oxman AD; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ. 2016 Jun 28;353:i2016. doi: 10.1136/bmj.i2016. PubMed PMID: 27353417.
Alonso-Coello P, Oxman AD, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Vandvik PO, Meerpohl J, Guyatt GH, Schünemann HJ; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 2: Clinical practice guidelines. BMJ. 2016 Jun 30;353:i2089. doi: 10.1136/bmj.i2089. PubMed PMID: 27365494.
Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, Fervers B, Graham ID, Grimshaw J, Hanna SE, Littlejohns P, Makarski J, Zitzelsberger L; AGREE Next Steps Consortium. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010 Dec 14;182(18):E839-42. doi: 10.1503/cmaj.090449. Epub 2010 Jul 5. Review. PubMed PMID: 20603348; PubMed Central PMCID: PMC3001530.
Hultcrantz M, Rind D, Akl EA, Treweek S, Mustafa RA, Iorio A, Alper BS, Meerpohl JJ, Murad MH, Ansari MT, Katikireddi SV, Östlund P, Tranæus S, Christensen R, Gartlehner G, Brozek J, Izcovich A, Schünemann H, Guyatt G. The GRADE Working Group clarifies the construct of certainty of evidence. J Clin Epidemiol. 2017 Jul;87:4-13. doi: 10.1016/j.jclinepi.2017.05.006. Epub 2017 May 18. PubMed PMID: 28529184; PubMed Central PMCID: PMC6542664.
Medisch Specialistische Richtlijnen 2.0 (2012). Adviescommissie Richtlijnen van de Raad Kwalitieit. http://richtlijnendatabase.nl/over_deze_site/over_richtlijnontwikkeling.html
Neumann I, Santesso N, Akl EA, Rind DM, Vandvik PO, Alonso-Coello P, Agoritsas T, Mustafa RA, Alexander PE, Schünemann H, Guyatt GH. A guide for health professionals to interpret and use recommendations in guidelines developed with the GRADE approach. J Clin Epidemiol. 2016 Apr;72:45-55. doi: 10.1016/j.jclinepi.2015.11.017. Epub 2016 Jan 6. Review. PubMed PMID: 26772609.
Schünemann H, Brożek J, Guyatt G, et al. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group, 2013. Available from http://gdt.guidelinedevelopment.org/central_prod/_design/client/handbook/handbook.html.
Zoekverantwoording
Zoekacties zijn opvraagbaar. Neem hiervoor contact op met de Richtlijnendatabase.
