Indicatie operatieve behandeling bij distale radiusfractuur
Uitgangsvraag
Wanneer is er een indicatie voor fixatie (operatieve behandeling) van een intra- dan wel extra-articulaire distale radius fractuur?
Aanbeveling
Distale radius fracturen met acceptabele stand
Overweeg niet-operatieve behandeling bij extra- en intra-articulaire distale radius fracturen met een acceptabele stand, al dan niet na repositie.
Controleer standaard na 7 tot 10 dagen met radiologisch onderzoek of de acceptabele stand van een gereponeerde distale radius fractuur behouden blijft. Controleer bij twijfel nogmaals 2 tot 3 weken na trauma.
Informeer de patiënt over:
- De reële kans op secundaire fractuur dislocatie in gips met een uitgestelde aanvullende behandeling en mogelijke operatie tot gevolg.
- Een mogelijk sneller herstel qua functie bij een operatieve behandeling.
Distale radius fracturen met niet-acceptabele stand
Overweeg operatieve behandeling bij extra- en intra-articulaire distale radiusfracturen
die niet acceptabel gereponeerd kunnen worden of secundair disloceren.
Overweeg niet-operatieve behandeling bij distale radius fracturen die ook na repositie gedisloceerd blijven staan of secundair disloceren bijvoorbeeld bij patiënten met:
- onvermogen om de aangedane hand en pols in het dagelijks leven in te zetten door beperkingen of ernstige inactiviteit;
- gevorderde dementie;
- hoge leeftijd.
Overwegingen
Voor- en nadelen van de interventie en de kwaliteit van het bewijs
Op basis van de beschikbare literatuur is er per uitkomstmaat gekeken of er een voorkeur is voor operatieve dan wel niet-operatieve behandeling van een distale radius fractuur.
De geïncludeerde studies bevatten kleine patiënten aantallen en hadden een aantal beperkingen. De meeste studies waren niet geblindeerd en het was vaak onduidelijk of deelnemers exact geanalyseerd zijn in de groepen waarin ze gerandomiseerd waren (intention-to-treat analyse). De gehele bewijskracht varieert daardoor van laag (DASH/PRWE, secundaire behandeling, complicaties) tot zeer laag (range of motion, pijn).
Op basis van de cruciale uitkomstmaten en kijkend naar de uitkomsten na 12-maanden follow-up, kan geen uitspraak worden gedaan over de voorkeur voor operatieve of niet-operatieve behandeling. Het is onduidelijk wat het effect van operatieve en niet-operatieve behandeling is op de cruciale uitkomstmaten ROM en pijn (zeer lage bewijskracht). De functionele uitkomsten aangaande de DASH en PRWE zijn mogelijk vergelijkbaar na operatieve dan wel niet-operatieve behandeling (lage bewijskracht). Tot 6-maanden follow-up lijken de functionele uitkomsten van de DASH en PRWE in het voordeel van operatie te wijzen.
Ook op basis van de belangrijke uitkomstmaten kan geen uitspraak worden gedaan over de voorkeur voor operatieve of niet-operatieve behandeling. Er is mogelijk minder noodzaak voor een secundaire behandeling (operatief dan wel niet-operatief) na een primair operatieve behandeling in vergelijking met een primair niet-operatieve behandeling (lage bewijskracht). De kans op een infectie is mogelijk groter na een primair operatieve behandeling (lage bewijskracht). De kans op pees- en zenuwletsel is mogelijk vergelijkbaar na een primair operatieve dan wel niet-operatieve behandeling (lage bewijskracht). Na interne fixatie met volaire plaat komen er mogelijk meer peesproblemen en minder zenuwproblemen voor na operatieve behandeling in vergelijking met niet-operatieve behandeling.
Waarden en voorkeuren van patiënten (en eventueel hun verzorgers)
Het belangrijkste doel voor een patiënt met een distale radiusfractuur is het verkrijgen van een zo normaal mogelijk functionerend polsgewricht zonder pijn, in een zo kort mogelijke periode.
Een potentieel voordeel van de niet-operatieve behandeling met gips van een patiënt met een distale radiusfractuur is het voorkomen van een operatie met anesthesie met eventuele peroperatieve complicaties. De mogelijke nadelen zijn een pijnlijke repositie op de spoedeisende hulp, gipsimmobilisatie gedurende 4 tot 5 weken en bij secundaire dislocatie van de fractuur alsnog noodzaak tot een secundaire ingreep (in 25% van de gevallen) waaronder mogelijk een operatieve behandeling.
Het grote voordeel van operatieve behandeling is het voorkomen van secundaire fractuur dislocatie. Een bijkomend voordeel van operatieve behandeling met plaatosteosynthese is het direct postoperatief kunnen oefenen van de pols. Dit lijkt met name in de eerste 3 tot 6 maanden een betere functie van de pols te geven. Dit verschil lijkt te verdwijnen na 12 tot 24 maanden.
Bij jonge patiënten zal mogelijk eerder gekozen worden voor een operatie om hen zo snel mogelijk weer aan het algemeen dagelijks leven te kunnen laten deelnemen. Bij ouderen zal de voorkeur meestal uitgaan naar niet-operatieve behandeling van een distale radiusfractuur, aangezien ouderen vaak ook met minder polsfunctie hun dagelijkse activiteiten kunnen uitvoeren en perioperatieve zorg cognitieve achteruitgang kan bewerkstelligen. Ook in het geval van patiënten met dementieel syndroom en/of een hoge comorbiditeit zal eerder gekozen worden voor een niet-operatieve behandeling.
Kosten (middelenbeslag)
De grootste kosten bij de behandeling van distale radiusfracturen komen voort uit medische kosten en economische kosten die veroorzaakt worden door werkverzuim (Swart, 2017). De medische kosten bestaan enerzijds uit kosten gemaakt in het ziekenhuis (spoedeisende hulp, operaties, personeel, implantaat, poliklinische follow-up, radiologische onderzoeken) en anderzijds uit kosten gemaakt buiten het ziekenhuis (fysio- en ergotherapie).
Bij operatieve behandeling middels plaatosteosynthese is de pols vaak direct post-operatief zonder gips te bewegen hetgeen een snellere terugkeer in de maatschappij kan bevorderen en daarmee de economische kosten kan verkleinen bij patiënten die werken.
De medische kosten zullen in het algemeen hoger zijn in geval van operatieve behandeling en de kosten gepaard met werkverzuim zullen in het algemeen hoger zijn in geval van niet-operatieve behandeling.
Bij patiënten met een gedisloceerde extra-articulaire distale radiusfractuur is operatie middels volaire plaat osteosynthese kosteneffectief gebleken (Mulders, 2020).
Aanvaardbaarheid, haalbaarheid en implementatie
De werkgroep voorziet geen bezwaren in haalbaarheid en aanvaardbaarheid voor zowel de patiënt als de arts van enerzijds de niet-operatieve en anderzijds de operatieve behandeling in Nederlandse ziekenhuizen.
De aanbevelingen in deze richtlijn zullen niet veel afwijken van de huidige manier van werken. Er is voldoende ruimte in de aanbevelingen om samen met de patiënt een afgewogen beslissing te nemen en een goed behandelvoorstel op te stellen. Aanvullend zijn in ieder Nederlands ziekenhuis de benodigde middelen aanwezig en kan de behandeling uitgevoerd worden.
De literatuur laat mogelijk zien dat een niet volledig anatomische consolidatie van een distale radius fractuur niet hoeft te betekenen dat het functionele resultaat in directe relatie hiermee zal staan. Dit houdt in dat de steeds kritischer wordende patiënt goed voorgelicht moet worden over dit gegeven, met name als er gekozen wordt voor een gipsimmobilisatie bij een niet volledig anatomische repositie (conform de criteria zoals vermeld in de module 'Indicatiestelling repositie'). Een belangrijk nadeel van een gekozen niet-operatief traject na repositie van een distale radius fractuur is alsnog een operatie indicatie bij een verslechterende stand van de radius op termijn. Het percentage van converteren van een niet-operatieve behandeling naar een secundaire behandeling in de vorm van een operatieve ingreep in verband met een onacceptabel verlies aan repositie in de eerste weken, wordt in de literatuur beschreven met een aandeel van 6% tot 41 % (Bartl, 2014; Mardani Kivi, 2011; Mulders, 2019; Sirnio, 2019).
Iedereen in Nederland kan gebruik maken van dezelfde middelen in de verschillende ziekenhuizen. Voor patiënten met een distale radius fractuur waar samen met de patiënt besloten kan worden om wel of niet een operatieve correctie uit te voeren, zal een inhoudelijk gesprek hierover mogelijk moeten zijn (shared decision making en informed consent). De behandelaar moet inschatten of de patiënt deze afweging kan maken en zal anders zelf het behandelplan moeten opstellen.
Er zijn geen belemmerende factoren om deze zorg te leveren in de ziekenhuizen. In alle gevallen gaat het om verzekerde zorg. Het grootste deel van deze zorg wordt geleverd door SEH-artsen, traumachirurgen of orthopedisch chirurgen.
In alle ziekenhuizen in Nederland wordt voldaan aan de voorwaarden om deze zorg goed uit te voeren.
Er zijn geen subgroepen waarvoor aanvullende overwegingen nodig zijn. De richtlijn geeft voldoende handvatten voor implementatie.
Rationale van de aanbeveling: weging van argumenten voor en tegen de interventies
Na een jaar worden er ten aanzien van de geformuleerde uitkomst parameters geen verschillen gevonden tussen patiënten met een distale radiusfractuur die operatief zijn behandeld, of niet-operatief zijn behandeld met behoud van een goede stand van de fractuur. Er zijn aanwijzingen dat patiënten die primair operatief behandeld worden de eerste 6 maanden na het trauma hoger scoren qua functionele uitkomsten. De medische kosten vallen hoger uit bij operatief behandelde patiënten, terwijl de kosten van arbeidsverzuim hoger uitvallen bij de niet-operatief behandelde patiënten. Indien er wordt gekozen voor een niet-operatieve behandeling is het van belang de fractuur zowel klinisch als radiologisch te controleren, om bij eventuele secundaire dislocatie alsnog een operatieve correctie te kunnen verrichten en daarmee een malunion te voorkomen. Alhoewel een malunion niet altijd leidt tot functionele klachten is de werkgroep wel van mening dat een malunion zo mogelijk voorkomen moet worden. De criteria voor repositie en eventuele operatieve behandeling in geval van secundaire dislocatie worden in de module ‘Indicatiestelling repositie’ behandeld.
Onderbouwing
Achtergrond
Gedisloceerde distale radius fracturen worden in het algemeen gesloten gereponeerd, waarna immobilisatie met gips. Na repositie kan de fractuur in het gips opnieuw verplaatsen. Een distale radiusfractuur met een acceptabele stand wordt meestal met gips uitbehandeld, terwijl een distale radiusfractuur met primaire of secundaire niet-acceptabele stand bij vitale patiënten meestal wordt behandeld middels een operatie. Alhoewel er steeds meer DRF operatief behandeld worden kan men zich afvragen of dit gedragen wordt door wetenschappelijk bewijs.
Conclusies
PROMS
|
Low GRADE |
The functional outcomes of the DASH and PRWE 12 months after non-operative treatment with an acceptable reduction of a displaced distal radius fracture are possibly comparable with an operative treatment.
Sources: (Bartl, 2014; Martinez-Mendez, 2018; Sirnio, 2019; Arora, 2011; Saving, 2019; Sharma, 2014) |
|
Low GRADE |
The functional outcomes of the DASH and PRWE in the first 3 to 6 months after operative treatment are possibly better than after a non-operative treatment of distal radius fractures.
Sources: (Arora 2011, Mulders, 2019) |
Range of motion
|
Very low GRADE |
It is unclear whether non-operative treatment results in less flexion, extension, radial deviation, ulnar deviation, pronation and supination compared to operative treatment in patients with distal radius fracture after 12 months of follow-up.
Sources: (Bartl, 2014; Martinez-Mendez, Mulders, 2019; 2018; Sirnio, 2019; Arora, 2011; Saving, 2019; Sharma, 2014) |
Pain
|
Very low GRADE |
It is unclear whether non-operative treatment results in less pain compared to operative treatment in patients with distal radius fracture after 12 months of follow-up.
Sources: (Martinez-Mendez, 2018; Arora, 2011) |
Secondary treatment
|
Low GRADE |
Operative treatment possibly results in less secondary treatment compared to non-operative treatment of both intra- and extra-articular distal radius fractures in adults after 12 months of follow up.
Sources: (Bartl, 2014; Lagerström, 1999; Rodriguez-Merchan, 1997; Stein, 1990 |
Complications
|
Low GRADE |
Patients who received operative treatment possibly have higher rates of infection compared to patients who received non-operative treatment for distal radius fracture after 12 months of follow up.
Sources: (Handoll, 2007; Karantana, 2020; Arora, 2011; Bartl, 2014, McQueen, 1996; Mulders, 2019, Sharma, 2014; Sirnio, 2019) |
|
Low GRADE |
Patients who received operative treatment possibly have comparable rates of tendon and nerve pathology compared to patients who received non-operative treatment for distal radius fracture after 12 months of follow up.
Patients who received operative treatment using a volar plate possibly have higher rates of tendon pathology and lower rates of nerve pathology compared to patients who received non-operative treatment for distal radius fracture after 12 months of follow up.
Sources: (Handoll, 2007; Karantana, 2020; Arora, 2011; Bartl, 2014, McQueen, 1996; Mulders, 2019, Sharma, 2014; Sirnio, 2019) |
Samenvatting literatuur
Description of studies
A total of 2 systematic reviews (Karantana, 2020; Handoll, 2007) and 8 individual studies were included in this literature summary.
Karantana (2020) included (quasi) randomized controlled trials of percutaneous pinning (PP) for treating distal radial fractures in adults. Studies were excluded if the study: compared PP with other methods of surgical fixation; evaluated the use of supplementary PP in addition to another method of surgical fixation; evaluated surgical versus non-surgical treatment trials where the type of surgery was chosen by the surgeon or where PP was one of different surgical fixation methods used; evaluated pin site maintenance or other measures to prevent wound infection. Electronic databases up to June 2019 were searched. A total of 21 (quasi) RCTs was included in the review. Four studies in this review met the PICO (Azzopardi, 2005; Rodriguez-Merchan, 1997; Stoffelen, 1998; Wong, 2010) and were included in this analysis of the literature. Risk of bias was assessed with the Cochrane Risk of Bias Tool. One trial was adequately randomized (Azzopardi, 2005), one trial was quasi-randomized (Stoffelen, 1998) and randomization methods of the other two trials were not clearly described. For the four trials, blinding of participants and assessors was not described or not adequate. It was unclear whether these studies used intention-to-treat analyses.
Handoll (2007) included (quasi) randomized controlled clinical trials involving adults with a fracture of the distal radius, which compared external fixation (EF) with non-operative treatment. Studies were excluded if studies: evaluated treatment in a mixed population of adults and children, with proportion children > 5% and no separate data for adults; compared different methods, including techniques and devices, of external fixation; compared external fixation with other methods of surgical fixation (for example percutaneous pinning); evaluated the use of supplementary methods, such as bone grafts and substitutes, other than percutaneous pinning, to external fixation compared with non-operative treatment. A total of 15 (quasi) RCTs was included in the review. Eleven studies included in this review met the PICO (Abbaszadegan, 1990; Hegeman, 2004; Horne, 1990; Kapoor, 2000; Kreder, 2006; Lagerstrom, 1999; McQueen, 1996; Rodriguez-Merchan, 1992; Stein, 1990; Young, 2003; Zheng, 2003) and were included in this analysis. Only for one trial (Kreder, 2006) allocation concealment was adequately performed; for other trials it was unclear whether randomization was adequately concealed (Hegeman, 2004; Lagerstrom, 1999; McQueen, 1996 and Young, 2003), trials were quasi-randomized (Stein, 1990; Zheng, 2003), or randomization methods were not described (Abbaszadegan, 1990; Horne, 1990; Kapoor, 2000; Rodriguez-Merchan, 1992). No trials reported blinding of participants. Although no trial reported blinding of outcome assessors, 3 trials referred to independent assessors for some outcomes. Evidence for intention-to-treat analysis was available for 5 trials (Abbaszadegan, 1990; Hegeman, 2004; Kreder, 2006; McQueen, 1996; Young, 2003).
In addition, eight individuals RCTs were included (Arora, 2011; Bartl, 2014; Földhazy, 2010; Martinez-Mendez, 2018; Mulders, 2019; Saving, 2019; Sharma, 2014 and Sirnio, 2010). One of these (Földhazy, 2010) compared the operative treatment of using an external fixator with the non-operative treatment using plaster immobilization. The other seven studies compared the operative treatment of using internal fixation with a locking plate, with a non-operative treatment using closed reduction and plaster immobilization. All studies except one (Sharma, 2014), used adequate randomization methods. One study described blinding of assessors (Martinez-Mendez, 2018). Because the assignment involved a surgical procedure, neither participants nor treating physicians were blinded to the treatment allocation. Evidence for intention-to-treat analysis was available for 3 studies (Bartl, 2014; Mulders, 2019; Sirnio, 2019).
Of the 23 studies that were included in this analysis, 9 compared operative and non-operative treatment in intra-articular fractures, 4 in extra-articular fractures. Nine studies included patients with both intra- and extra-articular fractures. One study did not specify the type of fracture and was added to the group with mixed fractures.
The operative treatment, non-operative treatment, type of fracture under study, the number of participants, the age criterium and the mean age (range) of the study population of the 23 included studies are summarized in Table 1.
Other study characteristics, the details on the treatments and the results are summarized in the evidence tables.
Table 1 Study characteristics
|
Study (1st author, year) |
Operative treatment |
Non-operative treatment |
Age, mean (range) |
Intra/extra- articular |
Source data |
|
intra-articular |
|||||
|
Bartl, 2014 |
Internal fixation N = 86 |
Cast N = 88 |
≥60y; 75y ± 7 |
intra-articular |
Individual study |
|
Hegeman, 2004 |
External fixation N = 15 |
Cast N = 17 |
55-80y; 70y |
intra-articular |
Handoll 2007 |
|
Kapoor, 2000 |
External fixation N = 28 |
Cast N = 33 |
adult; 39y |
intra-articular |
Handoll 2007 |
|
Lagerstrom, 1999 |
External fixation N = 18 |
Cast N = 17 |
45-75y; 58y (45-72) |
intra-articular |
Handoll 2007 |
|
Martinez-Mendez, 2018 |
Internal fixation N = 50 |
Cast N = 47 |
>60y; 70y (60-80) |
intra-articular |
Individual study |
|
Rodriguez-Merchan, 1992 |
External fixation N = 35 |
Cast N = 35 |
≤45y; 36y (20-45) |
intra-articular |
Handoll 2007 |
|
Rodriguez-Merchan, 1997 |
percutaneous pinning N = 20 |
Cast N = 20 |
age 45-65y; 57 (46-65) |
intra-articular |
Karantana 2020 |
|
Sharma, 2014 |
Internal fixation N = 32 |
Cast N = 32 |
>25 to <55y; 50y ± 9.5 |
intra-articular |
Individual study |
|
Stein, 1990 |
External fixation N = 40 |
Cast N = 22 |
n.a.; 50y (19-79) |
intra-articular |
Handoll 2007 |
|
extra-articular |
|||||
|
Azzopardi, 2005 |
percutaneous pinning N = 30 |
Cast N = 27 |
age ≥60y; 71.5y (60-80)) |
extra-articular |
Karantana 2020 |
|
Mulders, 2019 |
Internal fixation N = 48 |
Cast N = 44 |
18-75y; median 59/60 (IQR 42-66/ 52-65) |
extra-articular |
Individual study |
|
Stoffelen, 1998 |
percutaneous pinning N = 48 |
Cast N = 50 |
Age ≤80y; 58y |
extra-articular |
Karantana 2020 |
|
Wong, 2010 |
percutaneous pinning N = 32 |
Cast N = 30 |
≥65y; 70.5y (65-76) |
extra-articular |
Karantana 2020 |
|
Mixed fractures, or not specified |
|||||
|
Abbaszadegan, 1990 |
External fixation N = 23 |
Cast N = 24 |
≤75y; 63y (22-75) |
mixed |
Handoll 2007 |
|
Arora, 2011 |
Internal fixation N = 45 |
Cast N = 45 |
≥65y; I: 75.9 (65-88), C: 77.4 (65-89) |
mixed |
Individual study |
|
Földhazy, 2010 |
External fixation N = 28 |
Cast N = 31 |
60-85y; 71 (60-85) |
mixed |
Individual study |
|
Horne, 1990 |
External fixation N = 15 |
Cast N = 16 |
>60y; 72y (61-91) |
mixed |
Handoll 2007 |
|
Kreder, 2006 |
External fixation N = 54 |
Cast N = 59 |
16-75y; 53y |
mixed |
Handoll 2007 |
|
McQueen, 1996 |
External fixation N = 30 |
Cast N = 30 |
n.a.; 63y (16-86) |
mixed |
Handoll 2007 |
|
Saving, 2019 (2 sub studies) |
Internal fixation N = 68 |
Cast N = 72 |
≥75y; 79y (70-98) |
mixed
|
Individual study |
|
Sirnio, 2019 |
Internal fixation N = 38 |
Cast N = 42 |
≥50y; 63y (50-82) |
mixed |
Individual study |
|
Young, 2003 |
External fixation N = 59 |
Cast N = 66 |
Age 16-75 57y (16-75) |
mixed |
Handoll 2007 |
|
Zheng, 2003 |
External fixation N = 12 |
Cast N = 17 |
n.a. (18-52) |
mixed |
Handoll 2007 |
Results
Overall, the results of follow-up of at least 12 months are reported. In addition, for the PROMs the 6-week, 3-month and 6-month follow-up results are also provided. The level of evidence and conclusions are based on the results after at least 12-months.
As the results of Sharma (2014) for ulnar en radial deviation and supination and pronation differ from the general scale of these ROM outcomes and no definition or method for the measurement was provided, these results were not included in the analysis.
PROMs
DASH
Eight studies reported outcomes on the Disabilities of the Arm, Shoulder, and Hand (DASH) questionnaire. The DASH questionnaire comprises 30 items and focuses on two components, disability and symptoms of the upper extremity. It is scored from zero (good status) to 100 (poor status).
Pooled analysis
Seven studies that reported the mean and SD scores were pooled in a meta-analysis (figure 3). The overall mean difference (MD) was -6.86 (95% CI -9.50 to -4.22), in favour of the operative treatment group. As the threshold for clinically meaningful differences was set at 10.8 points, this is not a clinically relevant difference.
Studies on intra-articular fractures (n=3)
The MD of the studies on intra-articular fractures was -8.47 (95% CI -12.06 to -4.89). This difference is not clinically relevant.
Operative treatment methods:
The studies included in the analysis all compared internal plate fixation with immobilization using a cast. For studies using volar plate fixation as operative treatment (n=6), the MD was -6.91 (95% CI -10.11 to -3.70). This difference is not clinically relevant.
Figure 1 DASH score after operative treatment versus non-operative treatment of distal radial fractures
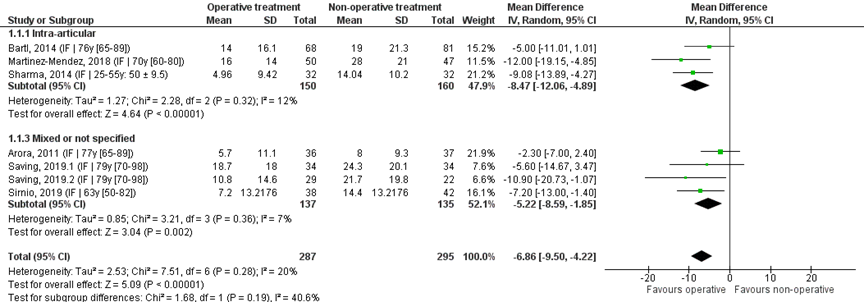
Z: p-value of overall effect; df: degrees of freedom; I2: statistical heterogeneity; CI: confidence interval; IF: internal fixation
Individual study on extra-articular fractures
One study (Mulders, 2019) reported the median (IQR) of the treatment groups. The operative treatment group had a median DASH score of 2.5 (IQR 0.0 to 12.7) and the non-operative treatment group 9.2 (IQR 1.7 to 17.7), in favour of the operative treatment group. As the threshold for clinically meaningful differences was set at 10.8 points, this difference is not clinically relevant. This result is in line with the result of the meta-analysis.
Additional follow-up periods
6 weeks
Two studies (Arora, 2011; Mulders, 2019) reported DASH scores at 6-week follow-up. Arora (2011) reported a DASH score of 18.8 (SD 17.9) for the operative treatment group and 34.4 (SD 22.5) for the non-operative treatment group. This difference is in favour of the operative treatment group and is clinically relevant. Mulders (2019) reported a median (IQR) score of 22.5 (IQR 14.2 to 35.8) operative treatment group and 48.3 (IQR 35.6 to 57.7) for the non-operative treatment group. This difference is in favour of the operative treatment group and is clinically relevant.
3 months
Five studies (Arora, 2011; Bartl, 2014; Mulders, 2019; Saving I, 2019; Saving II, 2019) reported the DASH score at 3 months or 12 weeks. Four studies reported the mean (SD) and were pooled. The MD was -7.98 (95% CI -11.88 to -4.08), in favour of the operative treatment group. This is not a clinically relevant difference. Mulders (2019) reported a median (IQR) DASH score of 6.7 (IQR 2.5 to 18.3) in the operative treatment group and 27.5 (IQR 10.0 to 38.3) in the non-operative treatment group. This difference is clinically relevant and in favour of the operative treatment group.
6 months
Two studies (Arora, 2011; Mulders, 2019) reported the DASH score at 6 months. Arora (2011) reported a mean (SD) DASH score of 12.2 (SD 14.4) for the operative treatment group and 12.4 (SD 17.0) for the non-operative treatment group. This is not a clinically relevant difference. Mulders (2019) reported a median (IQR) DASH score of 5.8 (IQR 0.0 to 17.5) in the operative treatment group and 14.2 (IQR 7.9 to 29.6) in the non-operative treatment group. This difference is in favour of the operative treatment group but is not clinically relevant.
PRWE
Five studies reported the PRWE scores. The PRWE is a 15-item questionnaire that focuses on measurement of wrist pain and disability in activities of daily living. It is scored from zero (minimum pain and disability) to 100 (maximum pain and disability).
Pooled analysis
Four studies reported the mean and SDs, and were pooled in a meta-analysis, see Figure 2. The overall mean difference was -9.67 (95% CI -14.74 to -4.59), in favour of the operative treatment group. This is not a clinically relevant difference.
Study on intra-articular fracture (n=1):
The MD of the study on intra-articular fracture was -13.00 (95% CI -21.00 to -5.00), in favour of the operative group. This is a clinically relevant difference.
Operative treatment methods:
The studies included in the analysis all compared internal plate fixation using a volar plate with immobilization using a cast.
Figure 2 PRWE score after operative treatment versus non-operative treatment of distal radial fractures
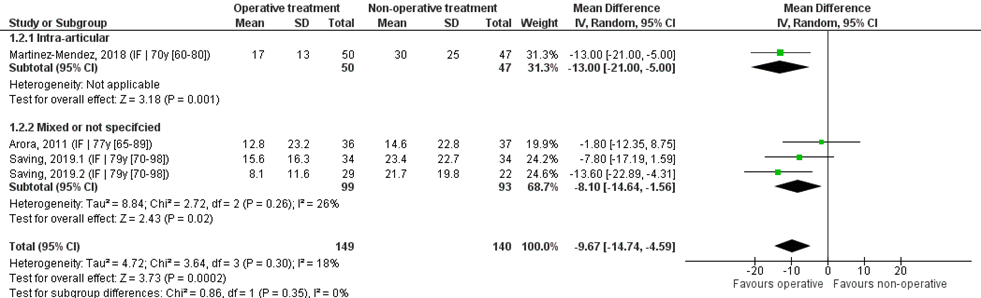
Z: p-value of overall effect; df: degrees of freedom; I2: statistical heterogeneity; CI: confidence interval; IF: internal fixation; EF: external fixation; PP: percutaneous pinning
Individual study on extra-articular fractures
One study (Mulders, 2019) reported the median and IQR. The operative treatment group had a median PRWE score of 4.0 (IQR 0.0 to 12.6) and the non-operative treatment group 10.0 (IQR 3.0 to 27.0), in favour of the operative treatment group. As the threshold for clinically meaningful differences was set at 11.5 points, this is not a clinically relevant difference. This result is in line with the result of the meta-analysis.
Additional follow-up periods
6 weeks
Two studies (Arora, 2011; Mulders, 2019) reported PRWE scores at 6-week follow-up. Arora (2011) reported a mean PRWE score of 36.4 (SD 28.7) in the operative treatment group and 64.9 (SD 29.0) in the non-operative treatment group. This difference is in favour of the operative treatment group and is clinically relevant. Mulders (2019) reported a median (IQR) PRWE score of 29.0 (IQR 18.5 to 48.5) for the operative treatment group and 55.8 (IQR 40.9 to 70.9) for the non-operative treatment group. This difference is in favour of the operative treatment group. This is a clinically relevant difference.
3 months
Four studies (Arora, 2011; Mulders, 2019; Saving I, 2019; Saving II, 2019) reported the PRWE score at 3 months or 12 weeks. Three studies reported the mean (SD) and were pooled. The MD was -15.48 (95% CI -22.18 to -8.77), in favour of the operative treatment group. This is a clinically relevant difference. Mulders (2019) reported a median (IQR) PRWE score of 11.0 (IQR 4.0 to 22.5) in the operative treatment group and 32.5 (IQR 12.0 to 50.0) in the non-operative treatment group. This difference is clinically relevant and in favour of the operative treatment group.
6 months
Two studies (Arora, 2011; Mulders, 2019) reported the PRWE score at 6 months. Arora (2011) reported a mean (SD) PRWE score of 27.7 (SD 32.0) for the operative treatment group and 31.4 (SD 33.0) for the non-operative treatment group. This is not a clinically relevant difference. Mulders (2019) reported a median (IQR) PRWE score of 7.0 (IQR 3.0 to 29.0) in the operative treatment group and 20.0 (IQR 7.5 to 45.8) in the non-operative treatment group. This difference is in favour of the operative treatment group and is clinically relevant.
Level of evidence of the literature
The level of evidence regarding function, as measured with the DASH and PRWE started as high, because studies were (quasi) RCTs. The level of evidence was downgraded by 2 levels, because of study limitations (blinding of patients and assessors, intention-to-treat analysis: risk of bias, -1) and number of included patients (imprecision, -1).
Range of motion
Flexion
Thirteen studies reported the flexion after operative and non-operative treatment.
The threshold for clinical relevance was set at 20 degrees for flexion.
Pooled analysis
Ten studies reported the mean difference and SD values of flexion in degrees and were pooled in a meta-analysis. The overall mean difference was 4.05 (95% CI -2.92 to 11.02), in favour of the operative treatment group. This is not a clinically relevant difference.
Studies on intra-articular fractures (n=3):
The MD of the study on intra-articular fractures is 5.26 (95% CI -8.26 to 18.79) in favour of the operative treatment group. This is not a clinically relevant difference.
Study on extra-articular fractures (n=1):
The MD of the studies on extra-articular fractures is 1.00 (95% CI -3.63 to 5.63) in favour of the operative group. This is not a clinically relevant difference.
Operative treatment methods
The MD of the study using percutaneous pinning as operative treatment (n=1) is 1.00 (95% CI -3.63, 5.63) in favour of the non-operative treatment group. For the studies using external fixation as operative treatment (n=2) the MD is -1.40 (95% CI -14.62 to 11.83) in favour of the non-operative group. The MD of the studies using internal fixation (n=7) is 6.09 (95% CI -0.69 to 12.84) in favour of the operative group. Of the studies using a volar plate (n=6), the MD is 5.92 (95% CI –2.31 to 14.14) in favour of the operative group.
Figure 3 Flexion score after operative treatment versus non-operative treatment of distal radial fractures
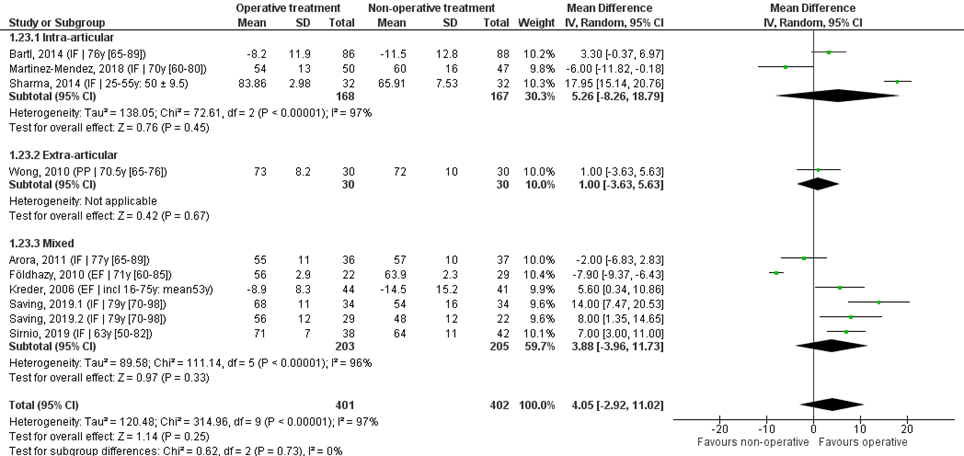
Z: p-value of overall effect; df: degrees of freedom; I2: statistical heterogeneity; CI: confidence interval; IF: internal fixation; EF: external fixation; PP: percutaneous pinning
Individual study on intra-articular fractures
One study (Hegeman, 2004) on intra-articular fractures reported the flexion relative to the uninjured side, as a percentage. The flexion of the injured wrist was 89 (SD 19) percent in the operative treatment group, and 89 (SD 17) percent in the non-operative treatment group. The MD was 0.00 (95% CI -12.56 to 12.56), favouring none of the treatment groups. No clinically relevant difference was found, which is in line with the result of the meta-analysis.
Individual study on extra-articular fractures
One study on extra-articular fractures (Mulders, 2019) reported the median and IQR scores of flexion in both groups. The median flexion score of the operative treatment group was 80 (IQR 70 to 86) and of the non-operative treatment group 70 (IQR 60 to 80), in favour of the operative treatment group. This is not a clinically relevant difference. This result is in line with the result of the meta-analysis.
Individual study on mixed fractures
One study on mixed fractures (McQueen, 1996) reported the flexion/extension relative to the uninjured side, in percentages. The flexion/extension of the injured wrist was 86.56 (SD 11.65) percent in the operative treatment group, and 83 (SD 14) percent in the non-operative treatment group. The mean difference was 3.56% (95% CI -2.49 to 9.61), in favour of the operative treatment group. This is not a clinically relevant difference. This result is in line with the result of the meta-analysis.
Level of evidence of the literature
The level of evidence regarding function, assessed with flexion of the wrist, started as high, because studies were (quasi) RCTs. The level of evidence was downgraded by 3 levels, because of study limitations (blinding of patients and assessors, intention-to-treat analysis: risk of bias, -1), conflicting results (inconsistency, -1) and number of included patients (imprecision, -1).
Extension
Twelve studies reported the extension after operative and non-operative treatment. The threshold for clinical relevance was set at 20 degrees for extension.
Pooled analysis
Ten studies reported the mean and SD values of extension in degrees and were pooled in a meta-analysis, see Figure 4. The overall mean difference was 1.71 (95% CI -3.62 to 7.04), in favour of the operative treatment group. This is not a clinically relevant difference.
Studies on intra-articular fractures (n=3):
The MD of the studies on intra-articular fractures (n=2) is 6.20 (95% CI -4.99 to 17.38) in favour of the operative treatment group. This is not a clinically relevant difference.
Study on extra-articular fractures (n=1):
The MD of the study on extra-articular fractures is 1.00 (95% CI -2.80 to 4.80) in favour of the operative treatment group. This is not a clinically relevant difference.
Operative treatment methods
The MD of the study using percutaneous pinning as operative treatment (n=1) is 1.00 (95% CI -2.80 to 4.80), in favour of the operative treatment group. For the studies using external fixation as operative treatment (n=2) the MD is -1.05 (95% CI -5.73 to 3.63) in favour of the non-operative treatment group. The MD of the studies using internal fixation (n=7) is 2.56 (95% CI -4.24 to 9.36) in favour of the operative treatment group. For studies using a volar plate, the MD was 2.81 (95% CI -5.08 to 10.71) in favour of the operative treatment group. This is not a clinically relevant difference.
Figure 4 Extension score after operative treatment versus non-operative treatment of distal radial fractures
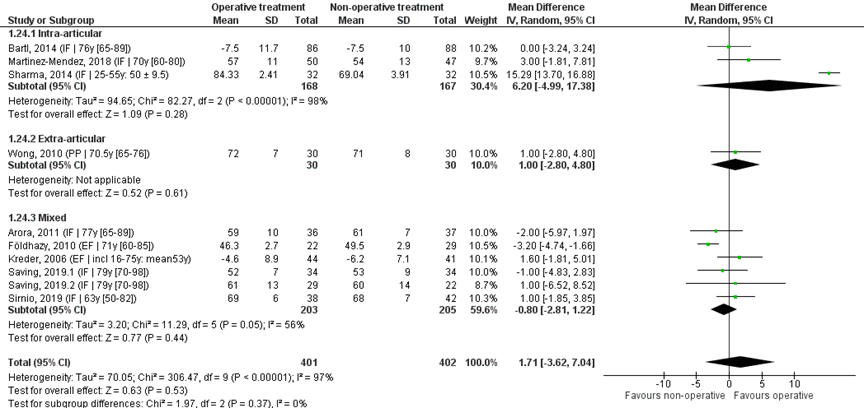
Z: p-value of overall effect; df: degrees of freedom; I2: statistical heterogeneity; CI: confidence interval; IF: internal fixation; EF: external fixation; PP: percutaneous pinning
Individual study on intra-articular fractures
One study on intra-articular fractures (Hegeman, 2004) reported the extension relative to the uninjured side, as a percentage. The extension of the injured wrist was 88 (SD 20) percent in the operative treatment group, and 72 (SD 21) percent in the non-operative treatment group. The MD was 16.00 (95% CI 1.78 to 30.22), in favour of the operative treatment group. This is not a clinically relevant difference. This result is in line with the result of the meta-analysis.
Individual study on extra-articular fractures
One study on extra-articular fractures (Mulders, 2019) reported the median and IQR scores of extension in both groups. The median extension score of the operative treatment group was 85 (IQR 80 to 90) and of the non-operative treatment group 80 (IQR 70 to 90). This is not a clinically relevant difference. This result is in line with the result of the meta-analysis.
Level of evidence of the literature
The level of evidence regarding function, assessed with extension of the wrist, started as high, because studies were (quasi) RCTs. The level of evidence was downgraded by 3 levels, because of study limitations (blinding of patients and assessors, intention-to-treat analysis: risk of bias, -1), conflicting results (inconsistency, -1) and number of included patients (imprecision, -1).
Radial deviation
Nine studies reported the radial deviation after operative and non-operative treatment. The threshold for clinical relevance was set at 5 degrees for radial deviation.
Pooled analysis
Eight studies reported the mean and SD values of radial deviation in degrees and were pooled in a meta-analysis, see Figure 5. The overall MD was 0.15 (95% CI -2.03 to 2.32), in favour of the operative treatment group. This is not a clinically relevant difference.
Study on intra-articular fractures (n=1)
The MD of the study on intra-articular fractures was -0.90 (95% CI -2.69 to 0.89) in favour of the non-operative treatment group. This is not a clinically relevant
Study on extra-articular fractures (n=1)
The MD of the study on extra-articular fractures (n=1) was -3.00 (95% CI -6.30 to 0.30) in favour of the non-operative treatment group. This is not a clinically relevant difference.
Operative treatment methods
The MD of the study using percutaneous pinning as operative treatment (n=1) is 3.00 (95% CI -6.30 to 0.30) in favour of the non-operative treatment group. For the study using external fixation as operative treatment (n=1) the MD is 3.79 (95% CI 3.08 to 4.49) in favour of the operative treatment group. The MD of the studies using internal fixation (n=5) is -0.78 (95% CI -1.82 to 0.26), in favour of the non-operative treatment group. For studies using a volar plate, the MD is (n=4) the MD was -0.96 (95% CI -2.11 to 0.19) in favour of the non-operative treatment group.
Figure 5 Radial deviation score after operative treatment versus non-operative treatment of distal radial fractures
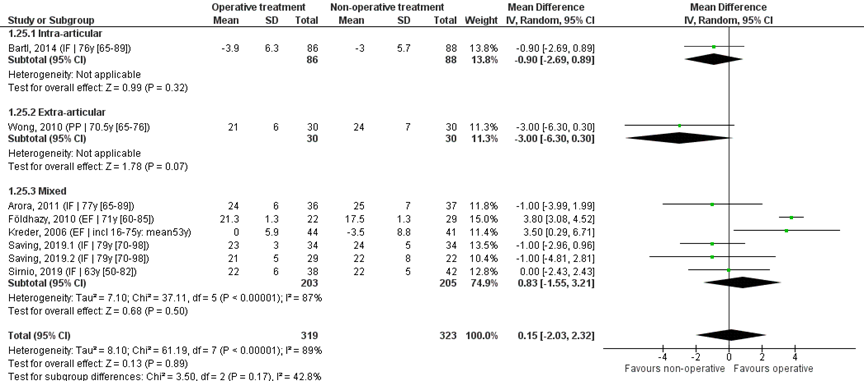
Z: p-value of overall effect; df: degrees of freedom; I2: statistical heterogeneity; CI: confidence interval; IF: internal fixation; EF: external fixation; PP: percutaneous pinning
Individual study on extra-articular fractures
One study on extra-articular fractures (Mulders, 2019) reported the median and IQR scores of radial deviation in degrees in both groups. The median radial deviation score of the operative treatment group was 15 (IQR 10 to 20) and of the non-operative treatment group 15 (IQR 10 to 15). This indicates no difference between groups. This result is in line with the result of the meta-analysis.
Level of evidence of the literature
The level of evidence regarding function, assessed with radial deviation of the wrist, started as high, because studies were (quasi) RCTs. The level of evidence was downgraded by 3 levels, because of study limitations (blinding of patients and assessors, intention-to-treat analysis: risk of bias, -1), conflicting results (inconsistency, -1) and number of included patients (imprecision, -1).
Ulnar deviation
Nine studies reported the ulnar deviation after operative and non-operative treatment. The threshold for clinical relevance was set at 7 degrees for ulnar deviation.
Pooled analysis
Eight studies reported the mean and SD values of ulnar deviation in degrees and were pooled in a meta-analysis, see Figure 6. The overall MD was 1.84 (95% CI 0.18 to 6.55), in favour of the operative treatment group. This is not a clinically relevant difference.
Study on intra-articular fractures (n=1):
The MD of the studies on intra-articular fractures is 1.50 (95% CI -0.80 to 3.80) in favour of the operative treatment group. This is not a clinically relevant difference.
Study on extra-articular fractures (n=1):
The MD of the study on extra-articular fractures is -1.00 (95% CI -4.30 to 2.30) in favour of the non-operative treatment group. This is not a clinically relevant difference.
Operative treatment methods
The MD of the study using percutaneous pinning as operative treatment (n=1) is 1.00 (95% CI -4.30 to 2.30) in favour of the non-operative treatment group. For the studies using external fixation as operative treatment (n=2) the MD is 3.84 (95% CI 2.77 to 4.92) in favour of the operative group. The MD of the studies using internal fixation (n=5) is 1.49 (95% CI -0.46 to 3.44) in favour of the operative treatment group. For studies using a volar plate, the MD is (n=4) the MD was 0.93 (95% CI -1.40 to 3.27), in favour of the operative group.
Figure 6 Ulnar deviation score after operative treatment versus non-operative treatment of distal radial fractures
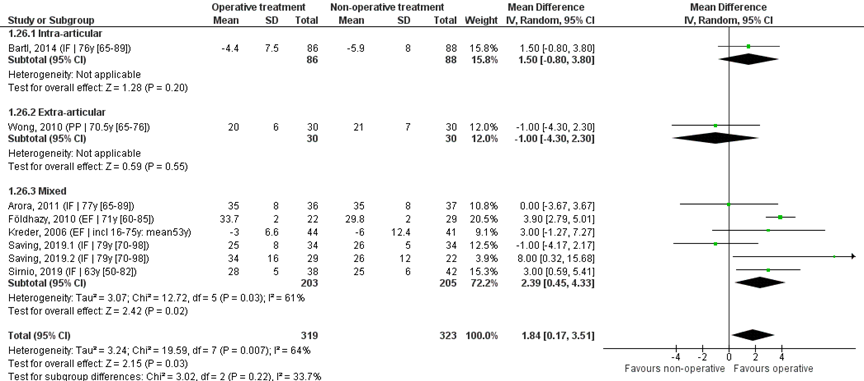
Z: p-value of overall effect; df: degrees of freedom; I2: statistical heterogeneity; CI: confidence interval; IF: internal fixation; EF: external fixation; PP: percutaneous pinning
Individual study on extra-articular fractures
One study on extra-articular fractures (Mulders, 2019) reported the median and IQR scores of ulnar deviation in both groups. The median ulnar deviation score of the operative treatment group was 25 (IQR 25 to 31) and of the non-operative treatment group 25 (IQR 20 to 30). This was a comparable score between groups.
Level of evidence of the literature
The level of evidence regarding function, assessed with ulnar deviation of the wrist, started as high, because studies were (quasi) RCTs. The level of evidence was downgraded by 3 levels, because of study limitations (blinding of patients and assessors, intention-to-treat analysis: risk of bias, -1), conflicting results (inconsistency, -1) and number of included patients (imprecision, -1).
Pronation
Ten studies reported the pronation after operative and non-operative treatment. The threshold for clinical relevance was set at 20 degrees for pronation.
Pooled analysis
Nine studies reported the mean and SD values of pronation in degrees and were pooled in a meta-analysis, see Figure 7. The overall MD was 0.90 (95% CI -1.63 to 3.43), in favour of the operative treatment group. This is not a clinically relevant difference.
Studies on intra-articular fractures (n=2):
The MD of the study on intra-articular fractures is 6.09 (95% CI -6.84 to 19.01) in favour of the operative treatment group. This is not a clinically relevant difference.
Study on extra-articular fractures (n=1):
The MD of the study on extra-articular fractures is 2.00 (95% CI -0.88 to 4.88) in favour of the operative treatment group. This is not a clinically relevant difference.
Operative treatment methods
The MD of the study using percutaneous pinning as operative treatment (n=1) was 2.00 (95% CI -0.88 to 4.88) in favour of the operative treatment group. For the study using external fixation as operative treatment (n=2) the MD is -3.87 (95% CI -5.14 to -2.60) in favour of the non-operative group. The MD of the studies using internal fixation (n=6) is 1.81 (95% CI -0.97 to 4.60), in favour of the operative treatment group. For studies using a volar plate (n=5), the MD was 2.53 (95% CI -1.22 to 6.29) in favour of the operative treatment group.
Figure 7 Pronation score after operative treatment versus non-operative treatment of distal radial fractures
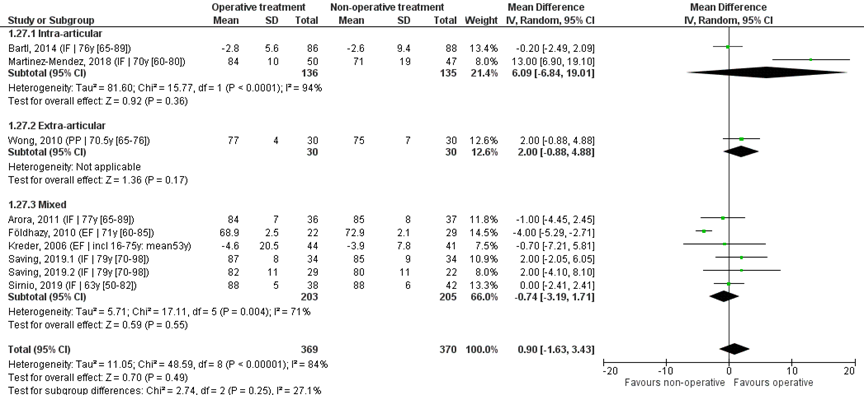
Z: p-value of overall effect; df: degrees of freedom; I2: statistical heterogeneity; CI: confidence interval; IF: internal fixation; EF: external fixation; PP: percutaneous pinning
Individual study on extra-articular fractures
One study on extra-articular fractures (Mulders, 2019) reported the median and IQR scores of pronation of both groups. The median pronation of the operative treatment group was 90 (IQR 80 to 90) degrees and of the non-operative treatment group 85 (IQR 75 to 90) degrees, in favour of the operative treatment group. This is not a clinically relevant difference. This result is in line with the meta-analysis.
Level of evidence of the literature
The level of evidence regarding function, assessed with pronation of the wrist, started as high, because studies were (quasi) RCTs. The level of evidence was downgraded by 3 levels, because of study limitations (blinding of patients and assessors, intention-to-treat analysis: risk of bias, -1), conflicting results (inconsistency, -1) and number of included patients (imprecision, -1).
Supination
Ten studies reported the supination after operative and non-operative treatment. The threshold for clinical relevance was set at 20 degrees for supination.
Pooled analysis
Nine studies reported the mean and SD values of supination in degrees and were pooled in ameta-analysis, see Figure 8. The overall MD was 1.58 (95% CI -0.99 to 4.15), in favour of the operative treatment group. This is not a clinically relevant difference.
Studies on intra-articular fractures (n=2):
The MD of the studies on intra-articular fractures the MD is 6.50 (95% CI -5.53 to 18.53), in favour of the operative treatment group. This is not a clinically relevant difference.
Study on extra-articular fractures (n=1):
The MD of the study on extra-articular fractures (n=1) the MD is 1.00 (95% CI -1.88 to 3.88) in favour of the operative treatment group. This is not a clinically relevant difference.
Operative treatment methods
The MD of the study using percutaneous pinning as operative treatment (n=1) is 1.00 (95% CI -1.88 to 3.88) in favour of the operative treatment group. For the studies using external fixation as operative treatment (n=2) the MD is -3.30 (95% CI -6.57 to -0.03) in favour of the non-operative group. The MD of the studies using internal fixation (n=6) is 3.63 (95% CI 0.58 to 6.68), in favour of the operative treatment group. For studies using a volar plate (n=5), the MD was 3.71 (95% CI -0.11 to 7.53), in favour of the operative treatment group.
Figure 8 Supination score after operative treatment versus non-operative treatment of distal radial fractures
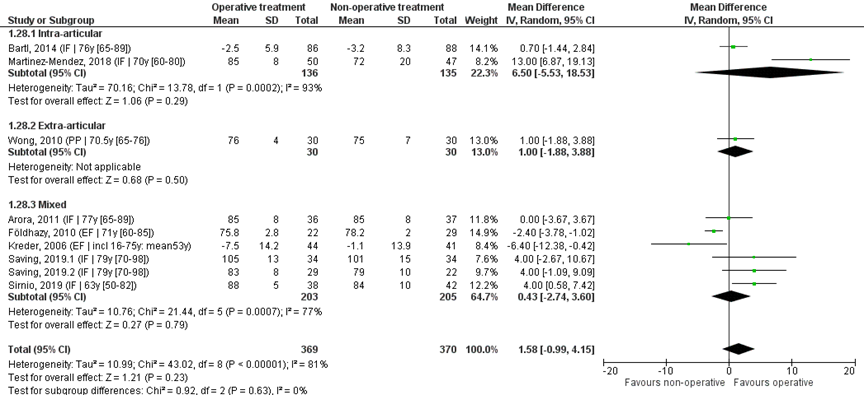
Z: p-value of overall effect; df: degrees of freedom; I2: statistical heterogeneity; CI: confidence interval; IF: internal fixation; EF: external fixation; PP: percutaneous pinning
Individual study on extra-articular fractures
One study on extra-articular fractures (Mulders, 2019) reported the median and IQR scores of supination of both groups. The median supination score of the operative treatment group was 85 (IQR 75 to 90) and of the non-operative treatment group 75 (IQR 70 to 85). This is not a clinically relevant difference.
Level of evidence of the literature
The level of evidence regarding function, assessed with supination of the wrist, started as high, because studies were (quasi) RCTs. The level of evidence was downgraded by 3 levels, because of study limitations (blinding of patients and assessors, intention-to-treat analysis: risk of bias, -1), conflicting results (inconsistency, -1) and number of included patients (imprecision, -1).
Pain
Two studies reported pain using a VAS-scale. The VAS scale used ranged from 0 to 10, with 0 indicating no pain, and 10 indicating worst pain.
The first study (Martinez-Mendez, 2018) reported general pain with a mean of 2 (SD 2) in the operative treatment group and 3 (SD 2) in the non-operative treatment group. The MD was -1.00 (95% CI -1.80 to -0.20), in favour of the operative treatment group. This is not a clinically relevant difference.
The second study (Arora, 2011) reported pain at rest and pain under stress on a VAS-scale. The score for pain at rest was 0.1 (SD 0.3) in the operative treatment group and 0.1 (SD 0.5) in the non-operative treatment group. The MD was 0.00 (95% CI -0.19, 0.19) in favour of neither of the treatment groups. This is not a clinically relevant difference.
The score for pain under stress was 0.7 (SD 1) in the operative treatment group and 0.6 (SD 1.4) in the non-operative treatment group. The MD was 0.10 (95% CI -0.46 to 0.66), in favour of the non-operative treatment group. This is not a clinically relevant difference.
Level of evidence of the literature
The level of evidence regarding pain, started as high, because studies were (quasi) RCTs. The level of evidence was downgraded by 3 levels, because of study limitations (blinding of patients and assessors; risk of bias, -1), inconsistent results (inconsistency, -1) and number of included patients (imprecision, -1).
Secondary treatment
Eleven studies reported the number of secondary treatments that were necessary within the follow-up time of the study. The NNT for infection was set at 40.
As the majority of the studies did not specify the secondary treatment that was needed, a variety of secondary treatments has been pooled. Secondary treatment included reduction loss necessitating revision, malposition of implant necessitating revision, redisplacement resulting in secondary treatment, reduction or reduction and K-wire fixation. They all indicate an additional treatment, independent of the nature (operative or non-operative) of the primary treatment.
When comparing the rates of (possibly operative) secondary treatment, it is important to note that the complete intervention group already underwent primary operative treatment, whereas it would be the first operative treatment for patients in the non-operative treatment group.
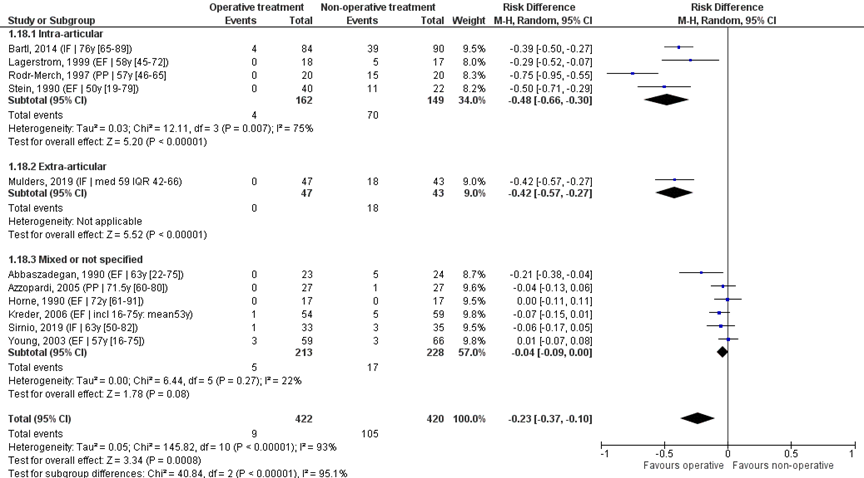
Pooled analysis
The eleven studies were pooled in a meta-analysis to calculate the risk difference (RD), see Figure 9. Overall, 9/422 (2%) of the operative and 105/420 (25%) of the non-operative treatment group needed secondary treatment. The RD was -0.23 (95% CI -0.37 to -0.10), in favour of the operative treatment group. The NNT was 5 (1 / 0.23 = 4.3), meaning that 5 patients have to be treated with primary operative treatment, to prevent 1 secondary treatment. This is a clinically relevant difference.
Studies on intra-articular fractures (n=4):
Studies on intra-articular fractures reported 4/162 (2%) patients of the operative treatment group needing secondary treatment, whereas 70/149 (47%) of the non-operative treatment group needed secondary treatment. The RD of these studies is -0.48 (95% CI -0.66 to -0.30), in favour of the operative treatment group. The NNT was 3 (1 / 0.48 = 2.1), meaning that 3 patients have to be treated with primary operative treatment, to prevent 1 secondary treatment. This is a clinically relevant difference.
Study on extra-articular fractures (n=1):
A study on extra-articular fractures reported 0/47 (0%) patients of the operative treatment group needing secondary treatment, whereas 18/43 (42%) of the non-operative treatment group needed secondary treatment. The RD of this studies is -0.42 (95% CI -0.57 to -0.27), in favour of the operative treatment group. The NNT was 3 (1 / 0.42 = 2.4), meaning that 3 patients have to be treated with primary operative treatment, to prevent 1 secondary treatment. This is a clinically relevant difference.
Operative treatment methods
Percutaneous pinning (n=2): 0/47 (0%) of the patients in the operative and 16/47 (34%) of patients in the non-operative treatment group needed secondary treatment. The RD was -0.39 (95% CI -1.20 to 0.42), in favour of the operative treatment group. The NNT was 3 (1 / 0.39 = 2.6).
External fixation (n=6): 4/211 (2%) of the patients in the operative and 29/205 (14%) of patients in the non-operative treatment group needed secondary treatment. The RD was -0.15 (95% CI -0.29 to -0.02), in favour of the operative treatment group. The NNT was 7 (1 / 0.15 = 6.7).
Internal fixation studies (n=3): 5/164 (3%) of the patients in the operative and 60/168 (36%) of patients in the non-operative treatment group needed secondary treatment. The RD was -0.28 (95% CI -0.54 to -0.03), in favour of the operative treatment group. The NNT was 4 (1 / 0.28 = 3.6).
Figure 9 (Re-)operation after operative treatment versus non-operative treatment of distal radial fractures
Z: p-value of overall effect; df: degrees of freedom; I2: statistical heterogeneity; CI: confidence interval; IF: internal fixation; EF: external fixation; PP: percutaneous pinning
Implant removal
One study (Mulders, 2019) specifically reported implant removal. In 9/47 (19%) patients in the intervention group and 1/43 (2%) of patients in the control group an additional operation took place in order to remove the volar plate. The possible necessity for implant removal primarily depends on whether an implant was used and therefore is implied by the primary treatment (operative versus. non-operative treatment). No RR has been calculated for ‘implant removal’.
Level of evidence of the literature
The level of evidence regarding secondary treatment started as high, because studies were (quasi) RCTs. The level of evidence was downgraded by 2 levels, because of study limitations (blinding of patients and assessors, intention-to-treat analysis: risk of bias, -1) and number of included patients (imprecision, -1).
Complications
Infection
Fifteen studies reported the number of infections that were administered during the study. This included pin track infections, wound infection, deep infection, joint infection and osteomyelitis. The NNT for infection was set at 20.
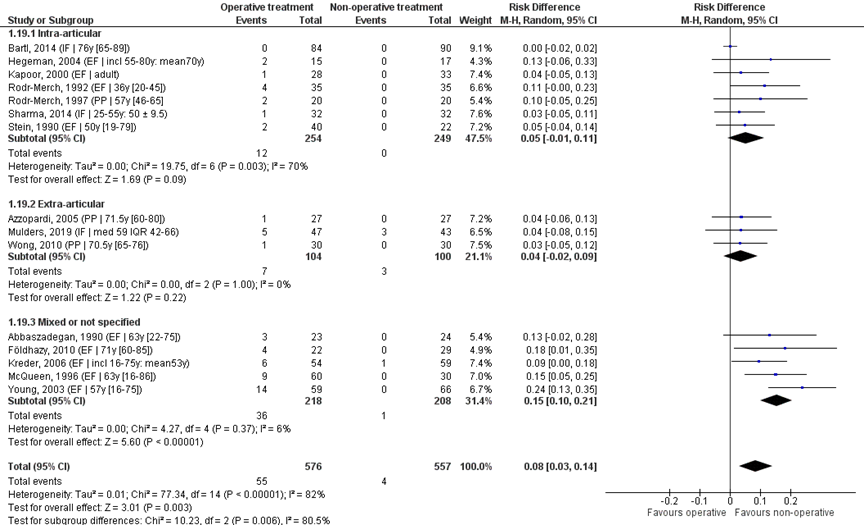
Pooled analysis
The fifteen studies were pooled in a meta-analysis, see Figure 10. Overall, infection was reported for 55/576 (10%) of patients in the operative and 4/557 (1%) of patients in the non-operative treatment group. The RD was 0.08 (95% CI 0.03 to 0.14) in favour of the non-operative treatment group. The NNT was 13 (1 / 0.08 = 12.5), meaning that 13 patients have to receive a non-operative treatment, to prevent 1 infection. This is a clinically relevant difference.
Studies on intra-articular fractures (n=7):
Studies on intra-articular fractures reported infection in 12/254 (5%) patients of the operative treatment group and in 0/249 (0%) patients of the non-operative treatment group. The RD of these studies is 0.05 (95% CI -0.02 to 0.14), in favour of the non-operative treatment group. The NNT was 20 (1 / 0.05 = 20), meaning that 20 patients have to receive a non-operative treatment, to prevent 1 infection. This is a clinically relevant difference.
Study on extra-articular fractures (n=3):
Studies on extra-articular fractures reported infection in 7/104 (7%) patients of the operative treatment group and in 3/100 (3%) of patients of the non-operative treatment group. The RD of this studies is 0.04 (95% CI -0.02 to 0.09), in favour of the non-operative treatment group. The NNT was 25 (1 / 0.04 = 25), meaning that 25 patients have to receive a non-operative treatment, to prevent 1 infection. This is not a clinically relevant difference.
Operative treatment methods
Percutaneous pinning (n=3): infection was reported for 4/77 (5%) of the patients in the operative and 0/77 (0%) of patients in the non-operative treatment group. The RD was 0.04 (95% CI -0.01 to 0.10), in favour of the non-operative treatment group. The NNT was 25 (1 / 0.04 = 25).
External fixation (n=9): infection was reported for 45/336 (13%) of the patients in the operative and 1/315 (<1%) of patients in the non-operative treatment group. The RD was 0.12 (95% CI 0.07 to 0.16), in favour of the non-operative treatment group. The NNT was 9 (1 / 0.12 = 8.3).
Internal fixation (n=3): infection was reported for 6/163 (4%) of the patients in the operative and 3/165 (2%) of patients in the non-operative treatment group needed secondary treatment. The RD was 0.01 (95% CI -0.02 to 0.04), in favour of the non-operative treatment group. The NNT was 100 (1 / 0.01 = 100). These three studies all used a volar plate.
Figure 10 Infection after operative treatment versus non-operative treatment of distal radial fractures
Z: p-value of overall effect; df: degrees of freedom; I2: statistical heterogeneity; CI: confidence interval; IF: internal fixation; EF: external fixation; PP: percutaneous pinning
Tendon pathology
Eleven studies reported pathology involving the tendon during the study. This included tendon rupture, de Quervain tenosynovitis and tendinitis. The NNT for tendon pathology was set at 40.
Pooled analysis
The eleven studies were pooled in a meta-analysis, see Figure 11. Overall, tendon pathology was reported for 18/432 (4%) of patients in the operative and 7/428 (2%) of patients in the non-operative treatment group. The RD was 0.01 (95% CI -0.02 to 0.05) in favour of the non-operative treatment group. The NNT was 100 (1 / 0.01 = 100), meaning that 100 patients have to receive a non-operative treatment, to prevent 1 tendon pathology. This is not a clinically relevant difference.
Studies on intra-articular fractures (n=4):
Studies on intra-articular fractures reported tendon pathology in 3/171 (2%) patients of the operative treatment group and in 2/177 (1%) patients of the non-operative treatment group. The RD of these studies is -0.00 (95% CI -0.03 to 0.03), indicating no difference between treatments.
Study on extra-articular fractures (n=2):
Studies on extra-articular fractures reported tendon pathology in 3/74 (4%) patients of the operative treatment group and in 3/70 (4%) of patients of the non-operative treatment group. The RD of this studies is -0.00 (95% CI -0.06 to 0.06), indicating no difference between treatments.
Operative treatment methods
Percutaneous pinning (n=2): tendon pathology was not reported for both treatment groups. The RD was 0.00 (95% CI -0.06 to 0.06), indicating no difference between treatments.
External fixation studies (n=4): tendon pathology was reported for 1/153 (13%) of the patients in the operative and 2/144 (%) of patients in the non-operative treatment group. The RD was -0.00 (95% CI -0.04 to 0.03), indicating no difference between treatments.
Internal fixation studies (n=5): tendon pathology was reported for 17/232 (7%) of the patients in the operative and 5/237 (2%) of patients in the non-operative treatment group. The RD was 0.06 (95% CI -0.03 to 0.14), in favour of the non-operative treatment group. The NNT was 17 (1 / 0.06 = 16.7). For studies using a volar plate (n=4), the RD was 0.07 (95% CI -0.05 to 0.19) with a NNT of 15 (1 / 0.07 = 14.3). This is a clinically relevant difference.
Figure 11 Tendon pathology after operative treatment versus non-operative treatment of distal radial fractures

Z: p-value of overall effect; df: degrees of freedom; I2: statistical heterogeneity; CI: confidence interval; IF: internal fixation; EF: external fixation; PP: percutaneous pinning
Nerve pathology
Sixteen studies reported pathology involving a nerve during the study. This included superficial radial nerve injury, median nerve compression, non-specified/other neuropathy. The NNT for nerve pathology was set at 20.
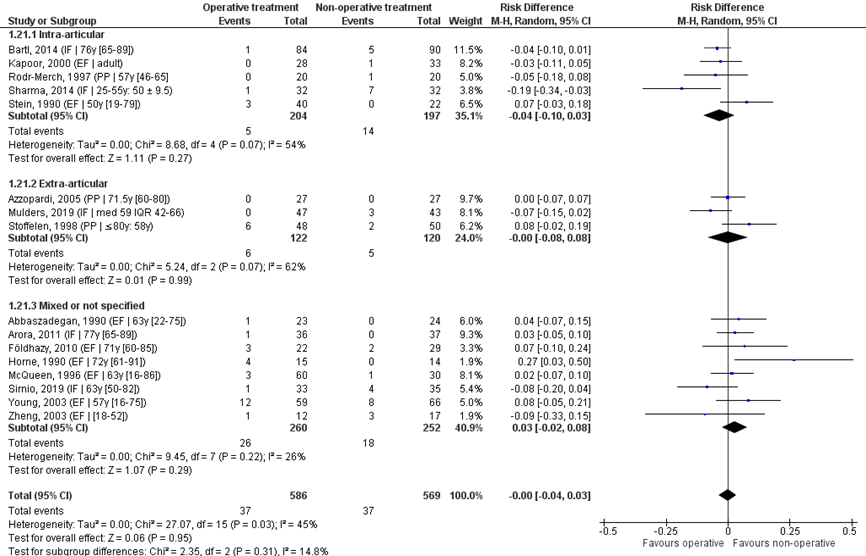
Pooled analysis
The sixteen studies were pooled in a meta-analysis, see Figure 12. Overall, nerve pathology was reported for 37/586 (%) of patients in the operative and 37/569 (%) of patients in the non-operative treatment group. The RD was -0.00 (95% CI -0.04 to 0.07), indicating no difference between treatments.
Studies on intra-articular fractures (n=4)
Studies on intra-articular fractures reported nerve pathology in 5/204 (3%) patients of the operative treatment group and in 14/197 (7%) patients of the non-operative treatment group. The RD of these studies is -0.04 (95% CI -0.10 to 0.03), in favour of the operative treatment group. The NNT was 25 (1 / 0.04 = 25), meaning that 25 patients have to receive an operative treatment, to prevent 1 nerve pathology. This is not a clinically relevant difference.
Study on extra-articular fractures (n=3)
Studies on extra-articular fractures reported nerve pathology in 6/122 (5%) patients of the operative treatment group and in 5/120 (4%) of patients of the non-operative treatment group. The RD of this studies is -0.00 (95% CI -0.08 to 0.08), indicating no difference between treatments.
Operative treatment methods
Percutaneous pinning (n=3): nerve pathology was reported for 6/95 (6%) of the patients in the operative and 3/97 (3%) of patients in the non-operative treatment group. The RD was 0.01 (95% CI -0.06 to 0.09), in favour of the non-operative treatment group. The NNT was 100 (1 / 0.01 = 100).
External fixation (n=8): nerve pathology was reported for 27/259 (10%) of the patients in the operative and 15/235 (6%) of patients in the non-operative treatment group. The RD was 0.04 (95% CI -0.01 to 0.09), in favour of the non-operative treatment group. The NNT was 25 (1 / 0.04 = 25).
Internal fixation (n=5): nerve pathology was reported for 4/232 (2%) of the patients in the operative and 19/237 (8%) of patients in the non-operative treatment group. The RD was -0.05 (95% CI -0.11 to 0.01), in favour of the operative treatment group. The NNT was 20 (1 / 0.05 = 20). For studies using a volar plate (n=4), the RD was -0.05 (95% CI -0.12 to 0.02) with a NNT of 20 (1 / 0.05 = 20). This is a clinically relevant difference.
Figure 12 Nerve pathology after operative treatment versus non-operative treatment of distal radial fractures
Z: p-value of overall effect; df: degrees of freedom; I2: statistical heterogeneity; CI: confidence interval; IF: internal fixation; EF: external fixation; PP: percutaneous pinning
Level of evidence of the literature
The level of evidence regarding complications (infection, tendon- and nerve injuries) started as high, because studies were (quasi) RCTs. The level of evidence was downgraded by 2 levels, because of study limitations (blinding of patients and assessors, intention-to-treat analysis: risk of bias, -1) and number of included patients (imprecision, -1).
Zoeken en selecteren
A systematic review of the literature was performed to answer the following question:
What is the (non)-effectiveness of operative versus non-operative treatment of patients with an intra- or extra-articular radius fracture on PROMs, range of motion, pain, re(operations) and complications after 12-month follow-up?
P: patients with an intra- or extra-articular radius fracture;
I: operative treatment;
C: non-operative treatment;
O: PROMs, range of motion, pain, (re-)operations and complications (infection, nerve pathology, tendon pathology).
Relevant outcome measures
The guideline development group considered PROMs and pain as a critical outcome measures for decision making; and secondary treatment and complications as an important outcome measure for decision making.
The working group defined PROMs (Patient Reported Outcome Measures) as follows: Michigan Hand Outcomes Questionnaire (MHOQ), Patient Rated Wrist Evaluation (PRWE) and (Quick) Disability of the Arm, Shoulder and Hand (DASH) score. Other outcome measures were not defined a priori, but definitions as described in the studies were used.
Regarding the PROMS, the working group defined 10.8 points for the DASH and 11.5 for the PRWE score (Walenkamp, 2015) as a clinically meaningful difference. Regarding the range of motion (ROM), the threshold for clinical relevance was set at 20 degrees for extension and flexion, 7 degrees for ulnar deviation, 5 degrees for radial deviation and 20 degrees for supination and pronation. For VAS scales, the threshold for clinically meaningful differences was set at 20mm or 2 points. For dichotomous outcomes a number needed to treat (NNT) was defined: secondary treatment: NNT ≤40; complications including infection: NNT ≤ 20, tendon pathology: NNT ≤ 40 and nerve pathology, NNT ≤ 20.
Search and select (Methods)
The databases (Medline (via OVID) and Embase (via Embase.com)) were searched with relevant search terms from 2000 until Feb 5, 2020. The detailed search strategy is depicted under the tab Methods. The systematic literature search resulted in 250 hits. Studies were selected based on the following criteria: RCTs with at least 1 year follow up, comparing operative treatment with non-operative treatment in patients with intra- and extra-articular radius fractures. Studies were initially selected based on title and abstract screening. Full texts of 62 papers were evaluated for inclusion. After reading the full text, 9 articles were included and 53 were excluded (see the table with reasons for exclusion under the tab Methods). Of 1 review a more recent review was found during the full-text screening, which was included in this analysis. In total 10 articles were included.
Results
One systematic review including 4 studies that met the inclusion criteria, one systematic review describing 11 studies that met the inclusion criteria, and 8 individual studies were included in the analysis of the literature. Important study characteristics and results are summarized in the evidence tables. The assessment of the risk of bias is summarized in the risk of bias tables.
Referenties
- Arora, R., Lutz, M., Deml, C., Krappinger, D., Haug, L., & Gabl, M. (2011). A prospective randomized trial comparing nonoperative treatment with volar locking plate fixation for displaced and unstable distal radial fractures in patients sixty-five years of age and older. The Journal of bone and joint surgery. American volume, 93(23), 2146–2153. https://doi.org/10.2106/JBJS.J.01597.
- Bartl, C., Stengel, D., Bruckner, T., Gebhard, F., & ORCHID Study Group (2014). The treatment of displaced intra-articular distal radius fractures in elderly patients. Deutsches Arzteblatt international, 111(46), 779–787. https://doi.org/10.3238/arztebl.2014.0779.
- Espinosa Gutiérrez, A., & Moreno Velázquez, A. (2010). Costo-beneficio de diferentes tratamientos en pacientes con fractura distal de radio (Cost-benefit of various treatments for patients with distal radius fracture). Acta ortopedica mexicana, 24(2), 61–65.
- Földhazy, Z., & Ahrengart, L. (2010). External fixation versus closed treatment of displaced distal radial fractures in elderly patients: a randomized controlled trial. Current Orthopaedic Practice, 21(3), 288–295. https://doi.org/10.1097/bco.0b013e3181cd6513.
- Handoll H.H.G., Huntley J.S., Madhok R. External fixation versus conservative treatment for distal radial fractures in adults. Cochrane Database of Systematic Reviews 2007, Issue 3. Art. No.: CD006194. DOI: 10.1002/14651858.CD006194.pub2.
- Karantana A., Handoll HHG., Sabouni A. (2020). Percutaneous pinning for treating distal radial fractures in adults. Cochrane Database of Systematic Reviews 2020, Issue 2. Art. No.: CD006080. DOI: 10.1002/14651858.CD006080.pub3. Accessed 02 December 2020.
- Mardani Kivi, M.,Asadi, K., Hashemi Motlagh, K., Shakibi, M. (2011). Distal radius fracture, a comparison between closed reduction and long arm cast versus. closed reduction and percutaneous pinning and short arm cast. Siraz E-medical Journal, 12(3), 155-161.
- Martinez-Mendez, D., Lizaur-Utrilla, A., & de-Juan-Herrero, J. (2017). Intra-articular distal radius fractures in elderly patients: a randomized prospective study of casting versus volar plating. Journal of Hand Surgery (European Volume), 43(2), 142–147. https://doi.org/10.1177/1753193417727139.
- Mulders, M. A. M., Walenkamp, M. M. J., van Dieren, S., Goslings, J. C., & Schep, N. W. L. (2019). Volar Plate Fixation Versus Plaster Immobilization in Acceptably Reduced Extra-Articular Distal Radial Fractures. The Journal of Bone and Joint Surgery, 101(9), 787–796. https://doi.org/10.2106/jbjs.18.00693.
- Mulders, M. A. M., Walenkamp, M. M. J., van Dieren, S., Goslings, J. C., & Schep, N. W. L. VIPER Trial Collaborators (2020). Volar Plate Fixation in Adults with a Displaced Extra-Articular Distal Radial Fracture Is Cost-Effective. J Bone Joint Surg Am. Apr 1;102(7):609-616. doi: 10.2106/JBJS.19.00597. PMID: 32079885.
- Saving, J., Severin Wahlgren, S., Olsson, K., Enocson, A., Ponzer, S., Sköldenberg, O., Mellstrand Navarro, C. (2019). Nonoperative Treatment Compared with Volar Locking Plate Fixation for Dorsally Displaced Distal Radial Fractures in the Elderly. The Journal of Bone and Joint Surgery, 101(11), 961–969. https://doi.org/10.2106/jbjs.18.00768.
- Sharma, H., Khare, G. N., Singh, S., Ramaswamy, A. G., Kumaraswamy, V., & Singh, A. K. (2014). Outcomes and complications of fractures of distal radius (AO type B and C): volar plating versus nonoperative treatment. Journal of orthopaedic science: official journal of the Japanese Orthopaedic Association, 19(4), 537–544. https://doi.org/10.1007/s00776-014-0560-0.
- Shauver, M. J., Clapham, P. J., & Chung, K. C. (2011). An economic analysis of outcomes and complications of treating distal radius fractures in the elderly. The Journal of hand surgery, 36(12), 1912–8.e83. https://doi.org/10.1016/j.jhsa.2011.09.039.
- Sirniö, K., Leppilahti, J., Ohtonen, P., & Flinkkilä, T. (2019). Early palmar plate fixation of distal radius fractures may benefit patients aged 50 years or older: a randomized trial comparing 2 different treatment protocols. Acta Orthopaedica, 90(2), 123–128. https://doi.org/10.1080/17453674.2018.1561614.
- Swart, E., Tulipan, J., & Rosenwasser, M. P. (2017). How Should the Treatment Costs of Distal Radius Fractures Be Measured?. American journal of orthopedics (Belle Mead, N.J.), 46(1), E54–E59.
- Walenkamp MM, de Muinck Keizer RJ, Goslings JC, Vos LM, Rosenwasser MP, Schep NW. (2015). The Minimum Clinically Important Difference of the Patient-rated Wrist Evaluation Score for Patients With Distal Radius Fractures. Clin Orthop Relat Res, Oct;473(10):3235-41. doi: 10.1007/s11999-015-4376-9. Epub 2015 Jun 4. Erratum in: Clin Orthop Relat Res. 2015 Sep;473(9):3063. PubMed PMID: 26040969; PubMed Central PMCID: PMC4562929.
Evidence tabellen
Evidence table for systematic review of RCTs and observational studies (intervention studies)
|
Study reference |
Study characteristics |
Patient characteristics |
Intervention (I) |
Comparison / control (C) |
Follow-up |
Outcome measures and effect size |
Comments |
|
Karantana, 2020
(individual study characteristics deduced from Karantana, 2020)
PS., study characteristics and results are extracted from the SR (unless stated with *) |
SR and meta-analysis of (quasi)-RCTs
Literature search up to June 2019
A: Azzopardi, 2005 B: Rodriguez-Merchan, 1997 C: Stoffelen, 1998 D: Wong, 2010
Study design: RCT
Setting and Country: School of Medicine /University setting, UK
Source of funding and conflicts of interest: Internal sources: Teesside University, University of Nottingham & University of Manchester, UK. External sources National Institute for Health Research (NIHR) via Cochrane Infrastructure funding to the Cochrane Bone, Joint and Muscle Trauma Group, UK. |
Inclusion criteria SR: (quasi) randomized controlled trials of percutaneous pinning (PP) for treating distal radial fractures in adults (completed skeletal growth)
Exclusion criteria SR:
A total of 21 (quasi) RCTs was included in the review. 4 studies on PP versus. immobilization that were a (quasi-) RCT and had a full publication (no abstract only) were used in the current analysis
Important patient characteristics at baseline:
N, age (mean (range)) A: N=57 (I:30, C:27) 71.5y (60-80) B: N=40 (I:20, C:20) 57 (46-65) C: N=98 (I:48, C:50) 58y D: N=62 (I:32, C:30) 70.5y (65-76)
Sex, n/N (%) male: A: 6/54 (11%) B: 11/40 (27%) C: 57/98 (58%) D: 11/60 (18%)
Groups comparable at baseline?
|
Surgical: percutaneous pinning (PP)
A: PP: using 2 crossed, smooth K-wires, 1.6 mm diameter, inserted through small stab incisions under fluoroscopic guidance. 1 wire through the styloid process, the other through Lister's tubercle or the dorso-ulnar border of distal fragment. Both wires engaging opposite cortex. Blunt dissection to bone. Pins left protruding percutaneously. Wrist immobilised in "well-moulded" short-arm cast. Wires/plaster removed after 5 weeks B: Closed reduction under general anaesthesia or brachial block. PP with fluoroscopic assistance using 3 K-wires. Two 0.45 mm K-wires inserted from radial styloid proximally towards the ulna and one inserted from the ulnar side proximally towards the radius. Forearm cast applied. Pins and cast removed after 7 weeks C: Percutaneous triple intrafocal Kapandji pinning (distal fragment was not transfixed; pins acted as a buttress to articular surface) followed by 1 week of plaster immobilisation until pain subsided. (By deduction: pins in place for 6 weeks) D: K-wire insertion was probably percutaneous. Pins inserted under general or regional anaesthesia. Reduction was done during the operation. Operation done under Bier's block; with the distal radius in reduced position, then insertion of 3 K-wires (percutaneous) under fluoroscopic guidance through three small stab incisions ("tripod" construct described in report); wires were not buried; no plaster of Paris was applied to augment the fracture stability but the occupational therapist made a removable palmar splint for resting purpose. Immediate gentle mobilisation under instructions of a physiotherapist was allowed. Wires were removed after fracture consolidation. |
Conservative: immobilization
A: Three-point fixation obtained in a "well-moulded" short-arm cast for 5 weeks. B: Closed manipulation under local anaesthesia, split below-elbow cast (20 degrees palmar flexion, 10 degrees ulnar deviation) (remanipulation at 1 week if dorsal angulation > 10 degrees, radial shortening > 3 mm. New cast applied). Patients given instructions to mobilise their fingers. Cast removed after 7 weeks. C: Closed reduction, followed by an above-elbow plaster cast for 3 weeks, then below-elbow plaster cast for 3 weeks D: Cast group: closed reduction under haematoma block, Jones's methods used for reduction, below- elbow plaster of Paris was applied.
|
End-point of follow-up:
A: 12M B: 12M C: 12M D: 19.5M (13-24)
For how many participants were no complete outcome data available? (intervention/control) A: 9 (unclear) B: 0 C: 0 D: 2 (2/0)
|
Function
Defined as Mayo Wrist score (0-100; reflects pain, grip strength, range of motion, and return to employment; higher is better); mean±sd A: NR B: NR C: NR D: I: 82.2±6.2 C: 80.5±7.5
Defined as ROM (°); mean±SD A: NR B: NR C: NR D: Flexion I: 73.0±8.2 C: 72.0±10.0 Extension I: 72.0±7.0 C:71.0±8.0 Radial deviation I: 21.0±6.0 C:24.0±7.0 Ulnar deviation I: 20.0±6.0 C:21.0±7.0 Supination I: 76.0±4.0 C:75.0±7.0 Pronation I: 77.0±4.0 C:75.0±7.0
Pain Defined as ‘yes’ occasional pain A: NR B: I: 2/20, C: 4/20 C: NR D: NR
Complications Secondary treatment A: I: 0/27, C: 1/27 B: I: 0/20, C: 15/20 C: NR D: NR Infection A: I: 1/27, C: 0/27 B: I: 2/20, C: 0/20 C: NR D: I: 1/30, C: 0/30 Tendon injury/rupture A: I: 0/27, C: 0/27 B: I: 0/20, C: 0/20 C: NR D: NR Nerve injury (also CTS) A: I: 0/27, C: 0/27 B: I: 0/20, C: 1/20 C: I: 6/48, C: 2/50 D: NR Complex regional pain syndrome A: NR B: I: 1/20, C: 1/20 C: I: 12/48, C: 15/50 D: I: 0/30, C: 1/30
|
Cochrane review; good quality publication
Authors conclusion: Overall, there is insufficient RCT evidence to inform on the role of percutaneous pinning versus cast immobilisation alone
Risk of Bias: A: randomized by tossing coin, concealment not described; participants and personnel not blinded; assessors not blind; intention-to-treat (ITT) analysis claimed but 6/9 lost to follow up not identified; protocol available, small differences; participants balance but 3 deaths (pinning group) not included B: randomized using blinded consecutively numbered envelopes, sequence generation and allocation concealment unclear; participants and personnel not blinded; ITT: likely; loss to follow-up: none, probably; selective reporting unclear, no protocol available and results are incomplete and inadequate C: quasi-randomized: patients were alternately treated: possible selection bias; participants and personnel not blinded; no participants flow available, imbalance in groups (50 versus. 47); no protocol available, incomplete data (no SDs), imbalance gender + number of participants with high velocity injuries. D: randomization method not described, but patients randomly allocated by opening sequentially numbered opaque sealed envelopes; participants and personnel providing intervention not blinded, assessors blinded; personnel assessing complications probably not blinded; ITT analysis and no loss to follow-up, 2 death from heart disease unrelated to intervention; no protocol available, but outcomes reported in methods are adequately reported; participants balanced at baseline |
|
Handoll, 2007
(individual study characteristics deduced from (Handoll, 2020)
PS., study characteristics and results are extracted from the SR (unless stated with *) |
SR and meta-analysis of (quasi-) RCTs
Literature search up to September 2006
A: Abbaszadegan, 1990 B: Hegeman, 2004 C: Horne, 1990 D: Kapoor, 2000 E: Kreder, 2006 F: Lagerstrom, 1999 G: McQueen, 1996 H: Rodriguez- Merchan, 1992 I: Stein, 1990 J: Young, 2003 K: Zheng, 2003
Study design: (quasi-)RCT
Setting and Country: Centre for Rehabilitation Sciences, University of Teesside; Royal Infirmary of Edinburgh; Cochrane Bone, Joint and Muscle Trauma Group, University of Manchester. UK
Source of funding and conflicts of interest: Internal sources: University of Teesside, UK. External sources: • No sources of support supplied |
Inclusion criteria SR: (quasi) randomised controlled clinical trials involving adults with a fracture of the distal radius, which compared external fixation with conservative treatment.
Exclusion criteria SR: Trials with a mixed population of adults and children, with proportion children >5% and no separate data for adults. We excluded trials comparing different methods, including techniques and devices, of external fixation; or trials comparing external fixation with other methods of surgical fixation, such as percutaneous pinning, or trials evaluating the use of supplementary methods, such as bone grafts and substitutes, other than percutaneous pinning, to external fixation compared with conservative treatment.
A total of 15 (quasi) RCTs was included in the review. 11 studies on PP versus. immobilization that were a (quasi-) RCT and had a full publication (no abstract only) were used in the current analysis
Important patient characteristics at baseline:
N, mean age A: N=47 (I:23, C:24) 63y (22-75) B: N=32 (I:15, C:17) 70y (55-80) C: N=37 (at least I:15, C:16 or I:14, C:13) Of analyzed n=29: 72y (61-91) D: N=61 (I:28, C:33) 39y (of 3 study arms) E: N=113 (I:54, C:59) 53y F: N=35 (I:18, C:17) 58y (45-72) G: N=90 (I: 60 (two study arms), C: 30) 63y (16-86) (of N=120) H: N=70 (I:35, C:35) 36y (20-45) I: N=62 (I: 40, C: 22) 50y (19-79) J: N=125 (I:59, C:66) 57y (16-75) K: N=29 (I:12, C:17) - (18-52)
Sex, n/N (%) male: A: 11/47 (23%) B: 3/32 (9%) C: not stated D: 46/61 (75%) E: 74/113 (35%) F: 5/35 (14%) G: 9/90 (10%) H: 58/70 (83%) I: not stated J: 28/125 (22%) K: 15/29 (52%) |
Surgical: external fixation (EF)
A: closed reduction under local anaesthesia, temporary dorsal plaster cast. External Hoffman fixator applied at 1-3 days under regional anaesthesia: 2 pins inserted through 1 cm skin incision through middle of second metacarpal and 2 pins in radius. Fixator removed at 4w (mean 31d) B: reduction then application of Hoffmann II compact external fixator: 2 pins inserted into the second metacarpal and 2 pins in radial shaft. Fixator removed after 6 weeks C: closed reduction under ischaemic arm block then modified AO tubular external fixator for 5 weeks: 2 pins placed at right angles in 2nd metacarpal, 2 pins placed at right angles into dorsoradial aspect of distal radius. “Stab incisions” of pins. D: Roger and Anderson external frame fixator: 2 pins into 2nd and 3rd metacarpals, 2 into radius shaft. Patients encouraged to use limb (eating etc) and rotate forearm. Fixator removed 6-7 weeks. Splint for 2 days after removal of fixator, then mobilisation. E: closed reduction under regional anaesthesia. Application of small spanning AO fixator: 2.5 mm pins into 2nd metacarpal and 4 mm pins into radius via 1 cm skin incision. Additional (in 19 cases) smooth K-wires inserted from the radial styloid or dorsum of the radius across fracture fragments at surgeon’s discretion. Optional wires removed 4-6 weeks. Fixator removed at 6-8 weeks. F: light (in weight) non-cylindrical AO external fixator. Immobilised for 6 weeks. G: (1) closed reduction and Pennig external fixator. Two pins inserted into 2nd metacarpal and 2 into radial shaft using an open technique. Ball joint locked. Fixator removed after 6 weeks. (2) as above (1) but release of ball joint of fixator at 3 weeks to allow wrist movement. H: reduction under general anaesthesia or brachial block. Clyburn dynamic external fixator: 2 pins driven into radial diaphysis and 2 into diaphysis of 2nd metacarpal. Overnight hospital admission. Posterior splint applied for 3 weeks if joint disrupted; transverse pin inserted for 3 weeks if joint unstable. Device removed after 7 weeks. Pin sites dressed by medical staff at weekly intervals I: closed reduction then the “small” AO external tubular fixator, usually for 6 weeks: 2 pins placed in 2nd metacarpal, 2 pins placed into radial shaft. J: manipulation and application of bridging Pennig dynamic fixator under general anaesthesia. Pins inserted percutaneously into 2nd metacarpal and under direct vision into the radial shaft. Distal ball joint unlocked at 3 weeks, fixator removed at 6 weeks K: closed (5 participants) or open (7 participants) reduction under brachial plexus block. Then application of small size Zhongjia SGD-type unilateral multifunctional external fixator: 2 pins inserted through skin incisions (0.7 cm) into the second metacarpal and 2 pins through skin incisions in radial shaft. Kirschner wire added if fracture was still unstable. Wrist fixed in medial position, slight extension and ulnar deviation or volar flexion (opposite to the direction of the injury). Fixator made dynamic and K-wire removed from week 4. Fixator removed after 6 weeks. Immediate functional training finger mobilisation; then, finger, elbow and shoulder from week 2; wrist joint mobilisation and strengthening activities from week 4.
|
Conservative: immobilization
A: Closed manipulation under local anaesthesia, then below elbow plaster cast for 4 weeks (mean 31 days) B: closed manipulation then below elbow plaster cast for 6 weeks. Physiotherapy started after 6 weeks. C: closed reduction under ischaemic arm block then below-elbow backslab, 10- 15 degrees palmar flexion and ulnar deviation, for 5 weeks D: closed reduction and plaster cast. Remanipulated once if necessary. Immobilisation for 6 to 7 weeks E: closed reduction under haematoma block (and fluoroscopy), then long arm splint with wrist in neutral and elbow at 90 degrees - reduction repeated if necessary. Splint converted to long arm cast within 14 days, reduced to short arm cast at 3-4 weeks, removed at 6-8 weeks. F: cylindrical below elbow plaster cast for 6 weeks. G: closed manipulation, then forearm cast for 6 weeks H: closed manipulation under local anaesthesia, then forearm plaster. Remanipulation at 1 week if position unacceptable. Total 7 weeks, unless problems when kept for 1 more week. I: closed reduction then above-elbow plaster cast with the forearm in pronation, usually for 6 weeks J: manipulation under regional or general anaesthesia and application of below elbow plaster backslab; then completed to full below-elbow plaster cast at 1 week and removed at 6 weeks K: manual reduction under haematoma block with X-ray monitoring. Plaster of Paris short-arm (forearm) cast applied, position changed after 2 weeks to “medial”. Cast removed after 6 weeks. Functional training was done before and after removing the cast.
|
End-point of follow-up:
A: 12M B: 12M C: 4-15M D: 48M E: 24M F: 12M, 24M G: 12M H: 12M I: 6M-48M (mean 36M) J: 12M, 84M K: 12M
For how many participants were no complete outcome data available? (n/N interv, n/N contr) A: 1/23, 0/24 B: 0/15, 0/17 C: not reported D: 18/28, 23/33 E: 10/54, 18/59 F: 2/18, 2/17 G: 6/60, 2/30 H: 0/35, 0/35 I: 0/40, 0/22 J: 48/59, 60/66 at 12M 23/59, 16/66 at 84M K: 0/12, 0/17
|
Function
Defined as ROM A: NR B: at 1 year, % compared to uninjured side; mean (SD) Flexion: I: 89 (19), C: 89 (17) Extension: I: 88 (20), C: 72 (21) C: NR D: NR E: at 2 years; difference injured – normal side; degrees mean (SD) Flexion: I: -8.9(8.3), C: -14.5(15.2) Extensio: I: -4.6 (8.9), C: -6.2 (7.1) Radial dev: I: 0 (5.9), C: 3.5 (8.8) Ulnar dev: I: -3 (6.6), C: -6 (12.4) Supinat: I: -7.5 (14.2), C: -1.1 (13.9) Pronation: I: -4.6(20.5), C: -3.9 (7.8) F: NR G: at 1 year, % of normal side, mean (SD) Flexion/extension: I: 86.56 (11.65), C: 83 (14) Overall ROM: I: 89 (13), C: 93 (11) H: NR I: NR J: at 1 year, degrees, mean (SD) Flexion: I: 63(5.3), C: 61(5.8) Extensio: I: 60(5.7), C: 60 (7.1) Radial dev: I: 21(3.1), C: 22(4.3) Ulnar dev: I: 36(4), C: 33(5) Supinat: I: 87(5), C: 86(4) Pronation: I: 90(0.6), C: 88(0.2) K: NR
Pain Defined as ‘yes’ persistent pain A: NR B: at 1 year Radiocarpal pain: I: 2/15, C: 3/17 Ulnocarpal pain: I: 2/15, C: 3/17 Radioulnar pain: I: 2/15, C: 3/17 C: NR D: NR E: NR F: NR G: NR H: NR I: NR J: at 1 year: I: 5/48, C: 4/60 at 7 years: I: 6/36, C: 11/50 K: NR
Secondary treatment A: Redisplacement resulting in secondary treatment: I: 0/23, C: 5/24 B: NR C: Redisplacement resulting in secondary treatment: I: 0/17, C: 0/17 D: NR E: Redisplacement resulting in secondary treatment: I: 1/54, C: 5/59 F: Redisplacement resulting in secondary treatment: I: 0/18, C: 5/17 G: NR H: NR I: Redisplacement resulting in secondary treatment: I: 0/40, C: 11/22 J: Redisplacement resulting in secondary treatment: I: 3/59, C: 3/66 K: NR
Complications Defined as infection (here: pin track infection) A: (+osteomyelitis 0/0) I: 3/23, C: 0/24 B: I: 2/15, C: 0/17 C: NR D: I: 1/28, C: 0/33 E: I: 6/54, C: 1/59 F: NR G:(+wound infection 0/0) I: 9/60, C: 0/30 H: (+osteomyelitis 0/0) I: 4/35, C: 0/35 I: (+osteomyelitis 0/0) I: 2/40, C: 0/22 J: I: 14/59, C: 0/66 K: NR
Defined as Tendon injury/rupture A: NR B: NR C: NR D: NR E: NR F: NR G: I: 0/60, C: 0/30 H: I: 0/35, C: 0/35 I: NR J: at 7 years, I: 0/36, C: 2/50 K: NR
Defined as Nerve injury A: Superficial radial nerve paraesthesia or injury I: 1/23, C: 0/24 B: NR C: Radial nerve neuritis or neuropathy I: 4/15, C: 0/14 D: Median nerve compression /CTS I: 0/28, C: 1/33 E: NR F: NR G: Median nerve compression /CTS I: 3/60, C: 1/30 H: NR I: Superficial radial nerve paraesthesia or injury I: 3/40, C: 0/22 J: Median nerve compression /CTS + Radial nerve neuritis or neuropathy I: 12/59, C: 8/66 K: Median nerve compression /CTS + Superficial radial nerve paraesthesia or injury I: 1/12, C: 3/17
Defined as Complex regional pain syndrome (also: reflex symp dystrophy) A: NR B: I: 4/15, C: 1/17 C: I: 0/15, C: 0/14 D: I: 1/28, C: 0/33 E: I: 1/54, C: 2/59 F: NR G: I: 7/60, C: 1/30 H: I: 0/35, C: 2/35 I: I: 1/40, C: 0/22 J: I: 7/59, C: 7/66 K: NR |
Cochrane review; good quality publication
Authors conclusion: There is some evidence to support the use of EF for dorsally displaced fractures of the distal radius in adults. Though there is insufficient evidence to confirm a better functional outcome, EF reduces redisplacement, gives improved anatomical results and most of the excess surgically-related complications are minor.
Risk of Bias, based on allocation concealment: A: unclear risk B: unclear risk C: unclear risk D: unclear risk E: low risk F: unclear risk G: unclear risk H: unclear risk I: high risk J: unclear risk K: high risk |
Table of quality assessment for systematic reviews of RCTs and observational studies
Based on AMSTAR checklist (Shea, 2007; BMC Methodol 7: 10; doi:10.1186/1471-2288-7-10) and PRISMA checklist (Moher, 2009; PLoS Med 6: e1000097; doi:10.1371/journal.pmed1000097)
|
Study
First author, year |
Appropriate and clearly focused question?
Yes/no/unclear |
Comprehensive and systematic literature search?
Yes/no/unclear |
Description of included and excluded studies?
Yes/no/unclear |
Description of relevant characteristics of included studies?
Yes/no/unclear |
Appropriate adjustment for potential confounders in observational studies?
Yes/no/unclear/not applicable |
Assessment of scientific quality of included studies?
Yes/no/unclear |
Enough similarities between studies to make combining them reasonable?
Yes/no/unclear |
Potential risk of publication bias taken into account?
Yes/no/unclear |
Potential conflicts of interest reported?
Yes/no/unclear |
|
Karantana, 2020 |
Yes |
Yes |
Yes |
Yes |
n.a. |
Yes |
Yes |
unclear |
Yes, reported: no declarations of interest |
|
Handoll, 2007 |
Yes |
Yes |
Yes |
Yes |
n.a. |
Yes |
Yes |
No, “There were insufficient data to assess publication bias; for example, by preparing a funnel plot.” |
Yes, reported: no declarations of interest |
Evidence table for intervention studies
Risk of bias table for intervention studies (randomized controlled trials)
|
Study reference
(first author, publication year) |
Describe method of randomisation |
Bias due to inadequate concealment of allocation?
(unlikely/likely/unclear) |
Bias due to inadequate blinding of participants to treatment allocation?
(unlikely/likely/unclear) |
Bias due to inadequate blinding of care providers to treatment allocation?
(unlikely/likely/unclear) |
Bias due to inadequate blinding of outcome assessors to treatment allocation?
(unlikely/likely/unclear) |
Bias due to selective outcome reporting on basis of the results?
(unlikely/likely/unclear) |
Bias due to loss to follow-up?
(unlikely/likely/unclear) |
Bias due to violation of intention to treat analysis?
(unlikely/likely/unclear) |
|
Arora, 2011 |
Sequentially numbered, sealed enveloped
“After the study nurse had obtained patient consent, the patients were randomized to either ORIF or nonoperative treatment with use of sequentially numbered, sealed envelopes.” |
Unlikely
Adequate procedure |
Likely
Not described |
Unclear
Not described |
Likely
Clinical assessor not blinded
“At each visit, a clinical examination was performed by an independent examiner who was not blinded to the method of treatment because the palmar scars in the operative treatment group could be easily observed.” |
Unclear
No protocol available. Outcome variables that are mentioned in methods are included in the results section. |
Unlikely
Reasons described and comparable between groups
“Four patients in the operative treatment group and two patients in the nonoperative treatment group died of unrelated conditions before the latest follow-up examination and were excluded. Five patients from the operative treatment group and six patients from the nonoperative treatment group were lost to follow-up and were excluded.” |
Unclear
Individuals lost to follow up and death were excluded from analysis |
|
Bartl, 2014 |
Computerized
“Random, center-stratified block assignment on a 1:1 basis was achieved by means of the online resource www.randomizer.at.” |
Unlikely
Adequate procedure |
Likely
Not described |
Unclear
Not described |
Likely
Not described |
Unclear
Protocol available, not all expected outcome measures described (e.g. need for pain medication)
“The study was registered at current controlledtrials.com (ISRCTN 76120052).” |
Unclear
More study drop-outs in interventions group (18/84 versus. 7/88) |
Unlikely
Inconsistent numbers in flow chart, but probably ITT analysis was performed.
“The primary analysis was oriented on the intention-to-treat (ITT) principle; all patients were analysed according to their random treatment assignment.” |
|
Földhazy, 2010 |
Closed envelopes
“The randomization procedure was conducted at the emergency department using closed envelopes.” |
Unlikely
Adequate procedure |
Likely
Not described |
Unclear
Not described |
Likely
Not described |
Unclear
No protocol available. Outcome variables that are mentioned in methods are included in the results section |
Unclear
Reasons described, unclear whether difference between groups might induce bias.
|
Unclear
Individuals lost to follow up were excluded from analysis |
|
Martinez-Mendez, 2018 |
Opaque envelopes
“Randomization was performed by the method of random number generation provided in opaque envelope.” |
Unlikely
Adequate procedure |
Likely
|
Unclear
Not described |
Unlikely
Assessors blinded at 12 and 24 months
“The clinical and radiological assessments could not be blind because of the presence of incision and hardware in the surgical patients. However, assessments at annual follow-up were performed by an independent observer not involved in the treatments.” |
Unclear
No protocol available |
Unlikely
No lost to follow up
“Final follow-up data were obtained at least 24 post-injury months for all patients with a mean follow-up of 29 months”
|
Unclear
Loss to follow up not described |
|
Mulders, 2019 |
Computerized
“Randomization was performed by means of a secured online computerized randomization procedure, using mixed block sizes of 2, 4, 6, and 8. Randomization was stratified according to age into 3 strata (18 to 30, 31 to 60, and 61 to 75 years old).” |
Unlikely
Adequate procedure |
Likely
“Because the assignment involved a surgical procedure, neither participants nor treating physicians were blinded to the treatment allocation.” |
Unclear
“Because the assignment involved a surgical procedure, neither participants nor treating physicians were blinded to the treatment allocation.” |
Likely
“Clinical assessment was performed by an independent examiner who was not blinded to the allocated treatment because the scars on the palmar side of the wrist in the operative group could easily be observed.” |
Unlikely
Protocol available “The trial protocol has previously been published.”
Primary outcome from protocol included, not all secondary outcomes (VAS pain, patient satisfaction).
|
Unlikely
|
Unclear
Individuals lost to follow up were excluded from analysis
“All analyses were performed according to the intention-to-treat principle.” |
|
Saving, 2019 |
Concealed envelopes
“Randomization was performed in a 1:1 ratio using concealed opaque envelopes without stratification.” |
Unlikely
Adequate procedure |
Likely
“Patients were not blinded to the allocation, nor were investigators.” |
Unclear
“Patients were not blinded to the allocation, nor were investigators.” |
Likely
“Patients were not blinded to the allocation, nor were investigators.” |
Unlikely
Studies in clinical trial register. Relevant outcome measures included in publication. |
Unlikely
Reasons drop out described. |
Unclear
Individuals lost to follow up were excluded from analysis
“All results were analyzed according to the intention-to-treat principle.” |
|
Sharma, 2014 |
Even / odd numbers, not further specified
“by alternate randomization: 32 patients with odd numbers went into the nonoperative group and the other 32 with even numbers went into the volar plating group.” |
Likely
Inadequate procedure |
Likely
Not described |
Unclear
Not described |
Likely
Not described |
Unclear
No protocol available |
Unclear
Intervention group: 18 study drop outs; control group: 7 study drop outs. Reasons not provided. |
Unclear
Not described |
|
Sirnio, 2019 |
Sealed opaque envelopes
“Patients were randomly allocated into study groups based on a computer-generated list. Randomization was performed in blocks, with block sizes randomly varying between 4, 6, 8, and 12. Separate lists were created for age groups of < 65 and ≥ 65 years, and for type A and C fractures. Randomization lists were sealed into numbered opaque envelopes.” |
Unlikely
Adequate procedure |
Likely
Not described |
Unclear
Not described |
Likely
Not described |
Unclear
No protocol available. |
Unlikely
Reasons drop out described, percentage of drop-out per group similar
|
Unlikely
All available data included in analysis according to allocation; missing data was imputed.
|
Table of excluded studies
|
Author and year |
Reason for exclusion |
|
Mellstrand Navarro, 2019 |
Studies included from Cochrane review 2007 or as individual studies |
|
Bruyere, 2018 |
More recent review included |
|
Wang, 2017 |
More recent review included |
|
Yu, 2016 |
More recent review included |
|
Song, 2015 |
More recent review included |
|
Ju, 2015 |
More recent review included |
|
Kvernmo, 2013 |
More recent review included |
|
Handoll, 2013 |
Content updated in more recent Cochrane reviews |
|
Diaz-Garcia, 2011 |
More recent review included |
|
Handoll, 2007 (percutaneous pinning) |
More recent review included |
|
Paksima, 2004 |
More recent review included |
|
Handoll, 2003 |
Content updated in more recent Cochrane reviews |
|
Achten, 2019 |
Protocol |
|
Drobetz, 2016 |
No data on specific PICO (no sub group to extract non-operative treatment data) |
|
Kumaravel, 2015 |
No RCT |
|
Mardani Kivi, 2011 |
Follow up period <1y |
|
Wong, 2010 |
Described in included review |
|
Kreder, 2006 |
Described in included review |
|
Azzopardi, 2005 |
Described in included review |
|
Azzopardi, 2005 |
Duplicate |
|
Moroni, 2004 |
Follow up period <1y |
|
Hegeman, 2004 |
Described in included review |
|
Young, 2003 |
Described in included review |
|
Stoffelen, 1999 |
Described in included review |
|
Gupta, 1999 |
Described in included review |
|
Stoffelen, 1998 |
Described in included review |
|
Rodriguez-Merchan, 1997 |
Described in included review |
|
Shankar, 1992 |
Described in included review |
|
Merchan, 1992 |
Described in included review |
|
Axelrod, 1991 |
Commentary |
|
Horne, 1990 |
Described in included review |
|
Abbaszadegan, 1990 |
Described in included review |
|
Bartolotta, 2019 |
Handbook |
|
Reynolds, 2014 |
More recent review included |
|
Venkatesh, 2016 |
Described in included review |
|
Kumar, 2014 |
Follow up period <1y |
|
Ismatullah, 2012 |
Follow up period <1y |
|
McQueen, 1996 |
Described in included review |
|
Chung, 2020 |
Wrong study design |
|
Bartolotta, 2019 |
Handbook |
|
Chung, 2019 |
Duplicate |
|
Vannabouathong, 2018 |
More recent review included |
|
Swedish Council on Health Technology, Assessment, 2017 |
Mellstrand is scientific publication of this work |
|
Lee, 2016 |
other patient group |
|
Zhao, 2015 |
More recent review included |
|
Reynolds, 2014 |
Duplicate |
|
Trevisan, 2013 |
Short communication |
|
ur Rahman, 2012 |
Follow up period <1y |
|
Cai, 2002 |
Foreign language |
|
Kapoor, 2000 |
Described in included review |
|
Lagerstrom, 1999 |
Described in included review |
|
Roumen, 1991 |
Described in included review |
Verantwoording
Autorisatiedatum en geldigheid
Laatst beoordeeld : 02-08-2021
Laatst geautoriseerd : 02-08-2021
Geplande herbeoordeling : 01-01-2027
Algemene gegevens
De ontwikkeling/herziening van deze richtlijnmodule werd ondersteund door het Kennisinstituut van de Federatie Medisch Specialisten en werd gefinancierd uit de Stichting Kwaliteitsgelden Medisch Specialisten (SKMS).
De financier heeft geen enkele invloed gehad op de inhoud van de richtlijnmodule.
De richtlijn is ontwikkeld in samenwerking met:
- Nederlandse Orthopaedische Vereniging
- Nederlandse Vereniging voor Plastische Chirurgie
- Nederlandse Vereniging voor Radiologie
- Nederlandse Vereniging van Spoedeisende Hulp Artsen
- Nederlandse Vereniging van Revalidatieartsen
- Koninklijk Nederlands Genootschap voor Fysiotherapie
- Nederlandse Vereniging voor Handtherapie
- Osteoporose Vereniging
Doel en doelgroep
Doel
Deze multidisciplinaire richtlijn bevat aanbevelingen ter ondersteuning van de dagelijkse praktijk voor hulpverleners die zich bezighouden met diagnostiek en behandeling van patiënten met een distale radius fractuur. De aanbevelingen zijn opgesteld op basis van de huidige wetenschappelijke inzichten. De knelpunten die behandelaars ervaren in de dagelijkse zorgpraktijk bij patiënten met een distale radius fractuur dienen als uitgangspunt bij de ontwikkeling van deze richtlijn.
Ter bevordering van de implementatie wordt geadviseerd om deze richtlijn aanknopingspunt te laten zijn voor lokale behandelprotocollen voor patiënten met een distale radius fractuur. Daarnaast kan de richtlijn gebruikt worden bij het geven van voorlichting aan patiënten met een distale radius fractuur.
Doelgroep
Deze richtlijn is bedoeld voor alle zorgverleners die betrokken zijn bij de zorg voor volwassen patiënten met een intra- of extra-articulaire distale radiusfractuur.
Samenstelling werkgroep
Voor het ontwikkelen van de richtlijnmodule is in 2019 een multidisciplinaire werkgroep ingesteld, bestaande uit vertegenwoordigers van alle relevante specialismen die betrokken zijn bij de zorg voor patiënten met een distale radius fractuur.
Werkgroep
- Dr. P.F.W. Hannemann, chirurg, werkzaam in het Maastricht UMC te Maastricht, (voorzitter), NVvH
- Dr. N.W.L. Schep, chirurg, werkzaam in het Maasstad Ziekenhuis te Rotterdam, NVvH
- Dr. D.I. Vos, chirurg, werkzaam in het Amphia ziekenhuis te Breda, NVvH
- Dr. R.L.M. Deijkers, orthopaedisch chirurg, werkzaam in het HagaZiekenhuis te Den Haag, NOV
- Dr. J.W. Colaris, orthopaedisch chirurg, werkzaam in het Erasmus UMC te Rotterdam, NOV
- Drs. J. van Loon, plastisch chirurg, werkzaam bij Blooming plastisch chirurgie te Haarlem, NVPC
- Drs. S. Bollen, radioloog, werkzaam in Het Groene Hart Ziekenhuis te Gouda,NVvR
- Drs. G.J.P. Smits, SEH-arts, werkzaam in het Catharina Ziekenhuis te Eindhoven, NVSHA
- Drs. K.S. van Wonderen, AIOS SEH, werkzaam in het St. Antonius ziekenhuis te Nieuwegein, NVSHA
- Dr. G. Zemack, revalidatiearts, werkzaam bij Libra revalidatie audiologie te Eindhoven, NVR
- Dr. F.J.B. Lötters, fysiotherapeut en docent fysiotherapie, werkzaam bij het Hand & Pols Centrum te Dordrecht en Hogeschool Leiden, KNGF, NVvHandtherapie
- H.J.G. van den Broek, voorzitter Osteoporose Vereniging te Haarlem.
Met ondersteuning van
- Drs. T. Geltink, adviseur, Kennisinstituut van de Federatie Medisch Specialisten
- Dr. S.N. Hofstede, senior adviseur, Kennisinstituut van de Federatie Medisch Specialisten
Belangenverklaringen
De code ter voorkoming van oneigenlijke beïnvloeding door belangenverstrengeling is gevolgd. Alle werkgroepleden hebben schriftelijk verklaard of zij in de laatste drie jaar directe financiële belangen (betrekking bij een commercieel bedrijf, persoonlijke financiële belangen, onderzoeksfinanciering) of indirecte belangen (persoonlijke relaties, reputatiemanagement) hebben gehad. Gedurende de ontwikkeling of herziening van een module worden wijzigingen in belangen aan de voorzitter doorgegeven. De belangenverklaring wordt opnieuw bevestigd tijdens de commentaarfase.
Een overzicht van de belangen van werkgroepleden en het oordeel over het omgaan met eventuele belangen vindt u in onderstaande tabel. De ondertekende belangenverklaringen zijn op te vragen bij het secretariaat van het Kennisinstituut van de Federatie Medisch Specialisten.
|
Werkgroeplid |
Functie |
Nevenfuncties |
Gemelde belangen |
Ondernomen actie |
|
Hannemann |
Traumachirurg/hand- en polschirurg (FESSH) Maastricht Universitair Medisch Centrum Maastricht |
Plaatsvervangend opleider heelkunde regio VIII (onbetaald); Course director CASH 3 cursus hand- en polschirurgie (onbetaald); Faculty lid AO (betaald); Faculty lid IBRA (betaald); Course director IBRA hand and wrist courses (betaald); Fellowship director IBRA upper limb training center MUMC Maastricht (betaald); Ontwikkeling course modules upper extremity IBRA (betaald); Faculty lid Esser Master class (onbetaald); ATLS instructor (betaald) Opleider HAIO’s (onbetaald) |
Begunstigde ZonMW subsidie aangaande onderzoek naar correctie osteotomie van de distale radius
Begunstigde Inscite subsidie (extern gefinancierd) voor onderzoek naar artrose van het polsgewricht |
Geen actie. De onderwerpen van de gesubsidieerde studies vallen buiten de afbakening van de richtlijn. |
|
Bollen |
Radioloog Groene Hart Ziekenhuis Gouda |
Geen |
Geen |
Geen actie. |
|
Deijkers |
Orthopedisch Chirurg HagaZiekenhuis Den Haag. |
Voorzitter Hand en Pols Werkgroep van de NOV (onbetaald); Organisator A&A Hand and Wrist Course Utrecht (onbetaald); Instructeur Dutch Wrist Arthroscopy Course (DWAC) (onbetaald); Voorzitter Commissie Certificering Subspecialisatie Handchirurgie van de Nederlandse Vereniging voor Handchirurgie (onbetaald) |
Geen |
Geen actie. |
|
van de Broek |
Voorzitter Osteoporose Vereniging (onbezoldigd)
|
Bestuurslid Energiek Heusden (energie coöperatie,(onbetaald); DGA eigen holding BV (pensioenrechten, betaald) |
Geen |
Geen actie. |
|
Van Loon |
Plastisch chirurg RKZ Beverwijk |
CFO Beverwijk Skin and Scar Company, Haarlem (betaald) |
Geen |
Geen actie. |
|
Colaris |
Orthopedisch chirurg - traumatoloog /hand- en polschirurg (FESSH) |
NOV bestuurslid (onkostenvergoeding); Bestuurslid Orthopedische Traumatologie Portefeuillehouder CCOC (onbetaald); Bestuurslid werkgroep hand-pols NOV (onbetaald); Bestuurslid werkgroep AI NOV (onbetaald); Bestuurslid Zuid West Overleg Traumatologie (onbetaald); Bestuurslid en co-founder BIG hand event (onbetaald); Faculty AO (betaald); Faculty OTC (betaald); Faculty Dutch wrist course (onbetaald); Faculty Esser Master Class (onbetaald) |
ZonMw subsidie voor uitwerking CAST-studie waarbij gereponeerde distale radiusfracturen ofwel in circulair gips ofwel in een gipsspalk worden geïmmobiliseerd. |
Geen actie. |
|
Zemack |
Revalidatiearts, Libra Revalidatie & Audiologie, Eindhoven, Tilburg en Weert. |
Voorzitter van de geaccrediteerde Werkgroep Trauma Revalidatie (WTR) van de VRA (Nederlandse Vereniging van Revalidatieartsen) (onbetaald) |
Geen |
Geen actie. |
|
Smits |
SEH-arts KNMG, Catharinaziekenhuis Eindhoven. |
PhD candidate, TU/e (onbetaald); Docent spoedzorg, Schola Medica Utrecht (betaald) |
Geen |
Geen actie. |
|
Lötters |
Hand-/fysiotherapeut, Bewegingswetenschapper bij Hand en Pols Revalidatie Nederland, locaties Den Haag en Dordrecht |
Docent bij de opleiding fysiotherapie bij de Hogeschool Leiden. |
Geen |
Geen actie. |
|
Schep |
Traumachirurg/hand- en polschirurg (FESSH), Maasstad Ziekenhuis Rotterdam, tevens werkzaam in het Spijkenisse Medisch Centrum |
Bestuurslid Ned. Ver. Handchirurgie (onbetaald); Bestuurslid Big Hand Event (onbetaald); Instructeur Dutch Wrist Ascopie course (onbetaald); Consultant Synthes, Arthrex: betrokken bij cursussen (betaald); Chairman diverse AO cursussen (onkosten vergoeding); Instructeur IBRA course (onkosten vergoeding); Instructeur CASH cursus handfracturen (onkosten vergoeding); Editor boek Leidraad chirurgie co- assistent revenuen; Consultant KLS Martin (betaald) |
Echtgenote heeft een medisch congres bureau. Geen relatie met deze richtlijn; Begunstigde diverse Zonmw subsidies voor onderzoek naar handfracturen, geen relatie met deze richtlijn; Meerdere publicaties aangaande de distale radius die ook in deze richtlijn aan bod komen.
|
Geen actie. |
|
Vos |
Traumachirurg Amphia Ziekenhuis Breda |
Lid klachten-commissie, Jeroen Bosch ziekenhuis den Bosch, (betaald); Lid kwaliteitsvisitatie commissie NVvH (onkostenvergoeding); ATLS instructor (betaald); AO faculty (betaald); consultant Operace Johnson & Johnson (betaald) |
Geen |
Geen trekker of meelezer bij uitgangsvragen over platen. |
|
Van Wonderen |
AIOS SEH bij st. Antoniusziekenhuis te Nieuwegein/Utrecht |
geen |
Geen |
Geen actie. |
Inbreng patiëntenperspectief
Voor de totstandkoming van deze richtlijn is aandacht besteed aan het patiëntenperspectief door uitnodigen van Patiëntenfederatie Nederland en patiëntenvereniging Osteoporose Vereniging voor de invitational conference en door middel van het aanstellen van een afgevaardigde van patiëntenvereniging Osteoporose Vereniging als lid van de werkgroep. Aanvullend zijn er door de Patiëntenfederatie Nederland patiëntervaringen verzameld door een vragenlijst uit te sturen onder het Zorgpanel van de Patiëntenfederatie. Het verslag hiervan (zie de bijlagen) is besproken in de werkgroep. De verkregen input is meegenomen bij het opstellen van de uitgangsvragen, de keuze voor de uitkomstmaten en bij het opstellen van de overwegingen. De conceptrichtlijn is tevens voor commentaar voorgelegd aan de Osteoporose Vereniging en de Patiëntenfederatie en de daarbij aangeleverde commentaren zijn bekeken en verwerkt.
Methode ontwikkeling
Evidence based
Werkwijze
AGREE
Deze richtlijnmodule is opgesteld conform de eisen vermeld in het rapport Medisch Specialistische Richtlijnen 2.0 van de adviescommissie Richtlijnen van de Raad Kwaliteit. Dit rapport is gebaseerd op het AGREE II instrument (Appraisal of Guidelines for Research & Evaluation II; Brouwers, 2010).
Knelpuntenanalyse en uitgangsvragen
Tijdens de voorbereidende fase inventariseerde de werkgroep de knelpunten in de zorg voor patiënten met een distale radius fractuur. De werkgroep beoordeelde de aanbeveling(en) uit de eerdere richtlijnmodules (Nederlandse Vereniging voor Heelkunde, 2010) op noodzaak tot revisie. Tevens zijn er knelpunten aangedragen door de Beroepsvereniging Verzorgenden Verpleegkundigen (V&VN), de Nederlandse Vereniging van Revalidatieartsen (VRA), het Koninklijk Nederlands Genootschap voor Fysiotherapie (KNGF), de Nederlandse Vereniging voor Handtherapie (NVHT) en de Nederlandse Vereniging voor Heelkunde (NVvH) via een schriftelijke invitational conference. Daarnaast is er een enquête uitgezet onder het Zorgpanel van de Patiëntenfederatie. Het rapport hiervan is opgenomen in de bijlagen.
Op basis van de uitkomsten van de knelpuntenanalyse zijn door de werkgroep concept-uitgangsvragen opgesteld en definitief vastgesteld.
Uitkomstmaten
Na het opstellen van de zoekvragen behorende bij de uitgangsvragen inventariseerde de werkgroep welke uitkomstmaten voor de patiënt relevant zijn, waarbij zowel naar gewenste als ongewenste effecten werd gekeken. Hierbij werd een maximum van acht uitkomstmaten gehanteerd. De werkgroep waardeerde deze uitkomstmaten volgens hun relatieve belang bij de besluitvorming rondom aanbevelingen als cruciaal (kritiek voor de besluitvorming), belangrijk (maar niet cruciaal) en onbelangrijk. Tevens definieerde de werkgroep tenminste voor de cruciale uitkomstmaten welke verschillen zij klinisch (patiënt gerelateerd) relevant vonden.
Methode literatuursamenvatting
Een uitgebreide beschrijving van de strategie voor zoeken en selecteren van literatuur en de beoordeling van de risk-of-bias van de individuele studies is te vinden onder ‘Zoeken en selecteren’ onder ‘Onderbouwing’. De beoordeling van de kracht van het wetenschappelijke bewijs wordt hieronder toegelicht.
Beoordelen van de kracht van het wetenschappelijke bewijs
De kracht van het wetenschappelijke bewijs werd bepaald volgens de GRADE-methode. GRADE staat voor ‘Grading Recommendations Assessment, Development and Evaluation’ (zie http://www.gradeworkinggroup.org/). De basisprincipes van de GRADE-methodiek zijn: het benoemen en prioriteren van de klinisch (patiënt) relevante uitkomstmaten, een systematische review per uitkomstmaat en een beoordeling van de bewijskracht per uitkomstmaat op basis van de acht GRADE-domeinen (domeinen voor downgraden: risk of bias, inconsistentie, indirectheid, imprecisie en publicatiebias; domeinen voor upgraden: dosis-effect relatie, groot effect en residuele plausibele confounding).
GRADE onderscheidt vier gradaties voor de kwaliteit van het wetenschappelijk bewijs: hoog, redelijk, laag en zeer laag. Deze gradaties verwijzen naar de mate van zekerheid die er bestaat over de literatuurconclusie, in het bijzonder de mate van zekerheid dat de literatuurconclusie de aanbeveling adequaat ondersteunt (Schünemann, 2013; Hultcrantz, 2017).
|
GRADE |
Definitie |
|
Hoog |
|
|
Redelijk |
|
|
Laag |
|
|
Zeer laag |
|
Bij het beoordelen (graderen) van de kracht van het wetenschappelijk bewijs in richtlijnen volgens de GRADE-methodiek spelen grenzen voor klinische besluitvorming een belangrijke rol (Hultcrantz, 2017). Dit zijn de grenzen die bij overschrijding aanleiding zouden geven tot een aanpassing van de aanbeveling. Om de grenzen voor klinische besluitvorming te bepalen moeten alle relevante uitkomstmaten en overwegingen worden meegewogen. De grenzen voor klinische besluitvorming zijn daarmee niet één op één vergelijkbaar met het minimaal klinisch relevant verschil (Minimal Clinically Important Difference, MCID). Met name in situaties waarin een interventie geen belangrijke nadelen heeft en de kosten relatief laag zijn, kan de grens voor klinische besluitvorming met betrekking tot de effectiviteit van de interventie bij een lagere waarde (dichter bij het nuleffect) liggen dan de MCID (Hultcrantz, 2017).
Overwegingen (van bewijs naar aanbeveling)
Om te komen tot een aanbeveling zijn naast (de kwaliteit van) het wetenschappelijke bewijs ook andere aspecten belangrijk en worden meegewogen, zoals aanvullende argumenten uit bijvoorbeeld de biomechanica of fysiologie, waarden en voorkeuren van patiënten, kosten (middelenbeslag), aanvaardbaarheid, haalbaarheid en implementatie. Deze aspecten zijn systematisch vermeld en beoordeeld (gewogen) onder het kopje ‘Overwegingen’ en kunnen (mede) gebaseerd zijn op expert opinion. Hierbij is gebruik gemaakt van een gestructureerd format gebaseerd op het evidence-to-decision framework van de internationale GRADE Working Group (Alonso-Coello, 2016a; Alonso-Coello, 2016b). Dit evidence-to-decision framework is een integraal onderdeel van de GRADE-methodiek.
Formuleren van aanbevelingen
De aanbevelingen geven antwoord op de uitgangsvraag en zijn gebaseerd op het beschikbare wetenschappelijke bewijs en de belangrijkste overwegingen en een weging van de gunstige en ongunstige effecten van de relevante interventies. De kracht van het wetenschappelijk bewijs en het gewicht dat door de werkgroep wordt toegekend aan de overwegingen, bepalen samen de sterkte van de aanbeveling. Conform de GRADE-methodiek sluit een lage bewijskracht van conclusies in de systematische literatuuranalyse een sterke aanbeveling niet a priori uit en zijn bij een hoge bewijskracht ook zwakke aanbevelingen mogelijk (Agoritsas, 2017; Neumann, 2016). De sterkte van de aanbeveling wordt altijd bepaald door weging van alle relevante argumenten tezamen. De werkgroep heeft bij elke aanbeveling opgenomen hoe zij tot de richting en sterkte van de aanbeveling zijn gekomen.
In de GRADE-methodiek wordt onderscheid gemaakt tussen sterke en zwakke (of conditionele) aanbevelingen. De sterkte van een aanbeveling verwijst naar de mate van zekerheid dat de voordelen van de interventie opwegen tegen de nadelen (of vice versa), gezien over het hele spectrum van patiënten waarvoor de aanbeveling is bedoeld. De sterkte van een aanbeveling heeft duidelijke implicaties voor patiënten, behandelaars en beleidsmakers (zie onderstaande tabel). Een aanbeveling is geen dictaat, zelfs een sterke aanbeveling gebaseerd op bewijs van hoge kwaliteit (GRADE-gradering HOOG) zal niet altijd van toepassing zijn, onder alle mogelijke omstandigheden en voor elke individuele patiënt.
|
Implicaties van sterke en zwakke aanbevelingen voor verschillende richtlijngebruikers |
||
|
|
Sterke aanbeveling |
Zwakke (conditionele) aanbeveling |
|
Voor patiënten |
De meeste patiënten zouden de aanbevolen interventie of aanpak kiezen en slechts een klein aantal niet. |
Een aanzienlijk deel van de patiënten zouden de aanbevolen interventie of aanpak kiezen, maar veel patiënten ook niet. |
|
Voor behandelaars |
De meeste patiënten zouden de aanbevolen interventie of aanpak moeten ontvangen. |
Er zijn meerdere geschikte interventies of aanpakken. De patiënt moet worden ondersteund bij de keuze voor de interventie of aanpak die het beste aansluit bij zijn of haar waarden en voorkeuren. |
|
Voor beleidsmakers |
De aanbevolen interventie of aanpak kan worden gezien als standaardbeleid. |
Beleidsbepaling vereist uitvoerige discussie met betrokkenheid van veel stakeholders. Er is een grotere kans op lokale beleidsverschillen. |
Organisatie van zorg
In de knelpuntenanalyse en bij de ontwikkeling van de richtlijnmodule is expliciet aandacht geweest voor de organisatie van zorg: alle aspecten die randvoorwaardelijk zijn voor het verlenen van zorg (zoals coördinatie, communicatie, (financiële) middelen, mankracht en infrastructuur). Randvoorwaarden die relevant zijn voor het beantwoorden van deze specifieke uitgangsvraag zijn genoemd bij de overwegingen. Meer algemene, overkoepelende, of bijkomende aspecten van de organisatie van zorg worden behandeld in de module 'Organisatie van zorg'.
Commentaar- en autorisatiefase
De conceptrichtlijnmodule werd aan de betrokken (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd ter commentaar. De commentaren werden verzameld en besproken met de werkgroep. Naar aanleiding van de commentaren werd de conceptrichtlijnmodule aangepast en definitief vastgesteld door de werkgroep. De definitieve richtlijnmodule werd aan de deelnemende (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd voor autorisatie en door hen geautoriseerd dan wel geaccordeerd.
Literatuur
Agoritsas T, Merglen A, Heen AF, Kristiansen A, Neumann I, Brito JP, Brignardello-Petersen R, Alexander PE, Rind DM, Vandvik PO, Guyatt GH. UpToDate adherence to GRADE criteria for strong recommendations: an analytical survey. BMJ Open. 2017 Nov 16;7(11):e018593. doi: 10.1136/bmjopen-2017-018593. PubMed PMID: 29150475; PubMed Central PMCID: PMC5701989.
Alonso-Coello P, Schünemann HJ, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Rada G, Rosenbaum S, Morelli A, Guyatt GH, Oxman AD; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ. 2016 Jun 28;353:i2016. doi: 10.1136/bmj.i2016. PubMed PMID: 27353417.
Alonso-Coello P, Oxman AD, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Vandvik PO, Meerpohl J, Guyatt GH, Schünemann HJ; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 2: Clinical practice guidelines. BMJ. 2016 Jun 30;353:i2089. doi: 10.1136/bmj.i2089. PubMed PMID: 27365494.
Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, Fervers B, Graham ID, Grimshaw J, Hanna SE, Littlejohns P, Makarski J, Zitzelsberger L; AGREE Next Steps Consortium. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010 Dec 14;182(18):E839-42. doi: 10.1503/cmaj.090449. Epub 2010 Jul 5. Review. PubMed PMID: 20603348; PubMed Central PMCID: PMC3001530.
Hultcrantz M, Rind D, Akl EA, Treweek S, Mustafa RA, Iorio A, Alper BS, Meerpohl JJ, Murad MH, Ansari MT, Katikireddi SV, Östlund P, Tranæus S, Christensen R, Gartlehner G, Brozek J, Izcovich A, Schünemann H, Guyatt G. The GRADE Working Group clarifies the construct of certainty of evidence. J Clin Epidemiol. 2017 Jul;87:4-13. doi: 10.1016/j.jclinepi.2017.05.006. Epub 2017 May 18. PubMed PMID: 28529184; PubMed Central PMCID: PMC6542664.
Medisch Specialistische Richtlijnen 2.0 (2012). Adviescommissie Richtlijnen van de Raad Kwalitieit. https://richtlijnendatabase.nl/over_deze_site/richtlijnontwikkeling.html
Neumann I, Santesso N, Akl EA, Rind DM, Vandvik PO, Alonso-Coello P, Agoritsas T, Mustafa RA, Alexander PE, Schünemann H, Guyatt GH. A guide for health professionals to interpret and use recommendations in guidelines developed with the GRADE approach. J Clin Epidemiol. 2016 Apr;72:45-55. doi: 10.1016/j.jclinepi.2015.11.017. Epub 2016 Jan 6. Review. PubMed PMID: 26772609.
Schünemann H, Brożek J, Guyatt G, et al. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group, 2013. Available from http://gdt.guidelinedevelopment.org/central_prod/_design/client/handbook/handbook.html.
Zoekverantwoording
Zoekacties zijn opvraagbaar. Neem hiervoor contact op met de Richtlijnendatabase.

