Type graft bij behandeling VKB-letsel
Uitgangsvraag
Welke soort graft geeft het beste resultaat bij voorste kruisband-reconstructie?
Aanbeveling
Kies bij een primaire VKB-reconstructie voor een autograft, waarbij zowel een bone-patellar-tendon-bone of hamstring graft kan worden gebruikt.
Verricht een VKB-reconstructie met een single-bundel of dubbel-bundel techniek.
Gebruik geen synthetische grafts in verband met inferieure resultaten en nadelige complicerende factoren op lange termijn.
Er kan geen wetenschappelijk onderbouwde aanbeveling worden gegeven over de keuze van het type fixatie van de diverse grafts.
Er kan geen wetenschappelijk onderbouwde aanbeveling worden gegeven over het gebruik van een hedendaagse (wel of niet geaugmenteerde) VKB-hechttechniek bij operatieve behandeling van acuut VKB-letsel.
Overwegingen
Autograft of Allograft
Het gebruik van een allograft bij de VKB-reconstructie heeft als voordeel dat er geen sprake is van donorplaats morbiditeit, maar een belangrijk nadeel is een inferieure mechanische kwaliteit van de allograft ten opzichte van een autograft. Andere nadelen zijn het beperkte aanbod van allograftweefsel en de hoge kosten. Vanwege bovenstaande nadelen wordt een allograft ondanks het feit dat de resultaten van een allograft bij een primaire reconstructie vergelijkbaar zijn met een de resultaten van een autograft in Nederland zelden gebruikt bij primaire reconstructie, maar is te overwegen indien een autograft niet mogelijk/wenselijk is.
Double-bundle (DB) versus Single-bundle (SB)
Een DB-reconstructie met HT is te overwegen gezien de mogelijk superieure biomechanische clinimetrische uitkomsten (Zhu, 2013; Karikis, 2016; Ventura, 2013). Echter, studies laten tot nu toe geen superieure uitkomsten zien ten aanzien van failure (Liu, 2016; Zhang, 2014; Soumalainen, 2012) en klinische uitkomsten (Zhu, 2013; Zhang, 2014; Soumalainen, 2012; Lee, 2012).
Anatomisch versus niet-anatomisch
De laatste jaren is er bij de reconstructie van de VKB een verschuiving gaande van een niet-anatomische naar een meer anatomische plaatsing van de graft. Een onlangs gepubliceerde systematische review Chen, (2017) bestaande uit vijf RCT’s concludeerde dat de uitkomst van een single bundle anatomische reconstructie beter is dan de niet-anatomische techniek qua stabiliteit en functioneel herstel van de geopereerde knie. Omdat vier van de vijf geïncludeerde RCTs geen follow-up van tenminste twee jaar hadden, kunnen we bij de revisie van deze richtlijn de anatomische techniek (nog) niet aanbevelen, maar is gezien de resultaten van de systematische review van Chen wel te overwegen.
Fixatietechniek
Vanwege de grote heterogeniteit in studies met betrekking tot de gebruikte graft types, operatietechnieken en fixatietechnieken kon het effect van fixatietechniek niet worden geanalyseerd, en blijft de aanbeveling uit de oude richtlijn staan.
Primary repair
Hooggradig bewijs voor de effectiviteit van het hechten van de geruptureerde voorste kruisband in de acute fase ontbreekt Van Eck, (2017). Hoewel in biomechanische studies bij het hechten van de geruptureerde voorste kruisband alleen dynamische augmentatie leidde tot normalisatie van de anterieure tibiale translatie (Hoogeslag, 2018; Schliemann, 2017) zijn er resultaten van meerdere retrospectieve en prospectieve klinische series na hedendaagse arthroscopische, niet-, statisch- en dynamisch-geaugmenteerde voorste kruisband hechttechnieken beschreven (Achtning, 2016; Ateschrang, 2017; Buchler, 2016; DiFelice, 2018; Eggli, 2016; Evangelopoulos, 2017; Henle, 2015; Kohl, 2016; Kosters, 2015; Mackay, 2015; Meister, 2017; Murray, 2016; Schliemann, 2017; Van der List, 2017). Over het algemeen betreft het studies met korte of hooguit middellange follow-up. Hoewel de resultaten van patiënt gerapporteerde uitkomstmaten over het algemeen goed tot uitstekend lijken te zijn, wordt er met name een hoge frequentie van heringrepen vanwege zwelling of strekbeperking gerapporteerd (Ateschrang, 2016; Buchler, 2016; Eggli, 2016; Haberli, 2018; Henle, 2015; Kohl, 2013; Kosters, 2015; Meister, 2017). Daarnaast lijkt er een associatie te bestaan tussen de lokalisatie van het letsel en de kans op failure/re-ruptuur, waarbij proximale rupturen een betere prognose lijken te hebben in vergelijking met midsubstance of distale rupturen (DiFelice, 2018; Evangelopoulos, 2017; Henle, 2017; Krismer, 2017; Mackay, 2015; Van der List, 2017). Het toevoegen van een letsel-overbruggende collageen bioscaffold lijkt de kans op failure/re-rupturen te verkleinen, en mogelijk wordt ook het resultaat na het hechten van proximaal VKB-letsel hierdoor ook in positieve zin beïnvloed. Hierover zijn echter slechts twee studies beschreven (Evangelopoulos, 2017; Murray, 2016). Eén prospectieve cohortstudie van patiënten die een dynamisch geaugmenteerde VKB-hechting ondergingen bij midsubstance VKB-letsel, van wie bij een deel wel en bij een deel geen letsel overbruggende bioscaffold werd geplaatst. Het complicatie risico daalde van 79% zonder bioscaffold naar 9% met bioscaffold binnen twee jaar postoperatief Evangelopoulos, (2017). En één prospectieve (met VKB-reconstructie vergelijkende) cohortstudie van patiënten die een statisch geaugmenteerde VKB-hechting ondergingen bij proximaal VKB-letsel, bij wie ook een letsel overbruggende bioscaffold werd geplaatst; dit betrof een veiligheidsstudie van het gebruik van een dergelijke bioscaffold bij de mens Murray, (2016). Er werd geen verschil gevonden tussen beide groepen, binnen 3 maanden postoperatief.
Er is slechts één RCT gepubliceerd die een hedendaagse VKB-hechttechniek vergeleek met de gouden standaard, de VKB-reconstructie Schliemann, (2017). Hoewel er ook nu op basis van patiënt gerapporteerde uitkomst maten een goed tot uitstekend resultaat werd bereikt binnen 1 jaar postoperatief, zonder verschil tussen beide groepen, werden geen adverse events (zoals complicaties, operatieve heringrepen of failure/re-rupturen) gerapporteerd.
Geconcludeerd kan worden dat hedendaagse hechttechnieken van de acute VKB, met alleen korte(re) termijn resultaten op het eerste oog goed zijn. Mogelijk bieden deze een belofte van een alternatieve behandeling naast of in plaats van de huidige gouden standaard, de VKB-reconstructie. Echter, ondanks dat hedendaagse VKB-hechttechnieken op dit moment bij patiënten worden toegepast, ontbreekt het aan hooggradige bewijslast betreffende de effectiviteit hiervan en aan langere termijn follow-up. Derhalve zou toepassing binnen een METC-goedgekeurd studieverband kunnen worden overwogen, totdat er sufficiënt hooggradig en lange(re) termijn bewijs is over de effectiviteit van dergelijke hedendaagse VKB-hechttechnieken.
Synthetische grafts
Vanwege het gebrek aan nieuwe studies voor synthetische grafts blijft de aanbeveling uit de oude richtlijn staan.
Onderbouwing
Achtergrond
In the past twenty-five years, there have been many developments surrounding anterior cruciate ligament (ACL) reconstruction using different graft types. Various types of research have been performed into outcomes using different grafts. However, until now this has not yet led to the definition of one graft as the superior graft. Therefore, the working group investigated the advantages and disadvantages of various graft types in order to reach a recommendation for graft selection. Moreover, nowadays there is trend to repair acute ACL ruptures without or with statistic of dynamic augmentation.
1. Type of grafts
Autologous Bone-Patellar Tendon-Bone (BPTB) graft or Hamstrings tendon (HT) graft or Quadriceps tendon (QT) graft
The BPTB grafts and HT grafts are the most commonly used graft types in reconstructive ACL surgery. Initially, BPTB reconstructions were the first choice, but the choice has shifted more and more towards the use of HT grafts. The advantage of BPTB grafts is that they may lead to less laxity, the disadvantage is that a subset of patients suffers from pain in the knee (anterior knee pain). The risk of anterior knee pain is lower after HT reconstructions (RR 0.49) Poolman, (2007), which is thereby the greatest advantage of this technique.
The QT graft with or without bone block from the patella is used much less often, but this might be a good alternative for the BPTB or HT grafts.
Autograft or Allograft
The use of allograft in ACL reconstruction (for example BPTB, HT, QT, tibialis anterior or Achilles tendon graft) has the advantage that there is no morbidity of the donor site, such as pain or weakness of the muscle group from which the tendon is harvested. A major disadvantage is an inferior mechanical quality of the allograft when compared to an autograft due to the fact that it has undergone a sterilisation process, as well as the fact that the tissue is often from older donors. Other disadvantages are the risk of infection or rejection of the graft, the limited availability of allograft and the higher costs. Due to the aforementioned disadvantages, allografts are rarely used in the Netherlands in primary ACL reconstructions.
2. Types of surgical techniques
Double-bundle (DB) or Single-bundle (SB) Hamstrings tendon (HT) graft
Generally, the ACL is described as having two bundles, the anteromedial and the posterolateral bundle. To restore the original anatomy as much as possible, a DB hamstring reconstruction could be performed. This guideline text has chosen to use the common English nomenclature: the two grafts (semitendinosus and gracilis tendon) are placed in one of the two positions of the original bundles, usually in two femoral and one or two tibial tunnels. In a SB reconstruction, both grafts are placed together as one bundle in one femoral and one tibial tunnel.
The main advantage of the DB reconstruction might be that it is comparable to the original anatomy, which possibly results in more stability (and possibly in better long-term effects). Advantages of SB reconstruction might be that it is less complicated and a less time-consuming procedure. Strong evidence supports that in patients undergoing intra-articular ACL reconstruction the practitioner should use either single bundle or double bundle technique, because the measured outcomes are similar Shea, (2015).
3. Type of Fixation
There have been many developments around fixation of the selected graft. Mechanically, the point of fixation is the weakest link. Therefore, it is important that the selected fixation technique is sufficiently strong to enable a functional post-treatment. For both proximal and distal fixation, there is a great diversity of fixation techniques. The fixation greatly depends on the graft that is used.
4. Primary repair
The concept of primary repair of the ruptured ACL is not new. Late in the last century multiple clinical series were published, mostly on open repair of acute ruptures of the ACL. Eventually ACL suture repair was abandoned in favour of ACL reconstruction because of disappointing results.
Nevertheless, suture repair of the ruptured ACL has reawakened in recent years. Pre-clinical animal model studies led to good results. Moreover, some promising short to medium term results have been reported using non-augmented, as well as static or -mostly- dynamic augmented ACL suture repair techniques. Contrary to static augmentation, in dynamic augmentation a braid is fixed to an additional elastic link (spring-in-screw mechanism) on the tibial side, instead of fixation to the tibial and the femoral bone directly.
However, the body of evidence for clinical studies using contemporary ACL suture techniques is rather small and there is a lack of high-quality evidence. Therefore, the working group researched the evidence about the efficacy of this treatment modality in patients with an ACL rupture.
Conclusies / Summary of Findings
1. Graft types
Hamstrings tendon (HT) autograft versus Bone-patellar tendon-bone (BPTB) autograft
Failure
|
Very low GRADE |
The probability of graft failure is similar after an ACL injury reconstruction with a HT or BPTB autograft.
Sources (Li, 2012; Kautzner, 2015) |
Clinimetrics (Pivot shift test)
|
Low GRADE |
The probability of a positive pivot shift test is higher after a reconstruction with an HT autograft compared with a BPTB autograft.
Sources (Li, 2012; Mohtadi, 2015 and 2016; Razi, 2014) |
Clinimetrics (Lachman test)
|
Very low GRADE |
The probability of a positive Lachman test after an ACL injury reconstruction with a BPTB autograft seems to be similar to a reconstruction with an HT autograft.
Sources (Konrads, 2016; Kautzner, 2015; Razi, 2014) |
Clinimetrics (IKDC score)
|
Moderate GRADE |
The probability for a (nearly) Normal IKDC Physical Examination score is similar after an ACL injury reconstruction with an HT or BPTB autograft.
Sources (Li, 2012) |
Adverse events
|
Very low GRADE |
The probability of experiencing an adverse event is likely to be lower after an HT autograft compared to a BPTB autograft reconstruction.
Sources (Li, 2012; Kautzner, 2015; Razi, 2014) |
PROMs (Lysholm score)
|
Low GRADE |
There is no difference in average Lysholm score between patients with an HT autograft or BPTB autograft reconstruction.
Sources (Razi, 2015) |
Autograft versus allograft
Failure
|
Very low GRADE |
The risk of failure is lower in autograft compared to allograft ACL reconstruction.
Sources (Zeng, 2016) |
Clinimetrics (Pivot shift test)
|
Moderate GRADE |
The probability of a positive pivot shift test after an ACL reconstruction with an autograft is similar to the probability after reconstruction with an allograft.
Sources (Zeng, 2016) |
Clinimetrics (Lachman test)
|
Low GRADE |
There seems to be no difference in a positive Lachman test between patients with an allograft or autograft after a reconstruction.
Sources (Zeng, 2016) |
Clinimetrics (Instrumented laxity test)
|
Very low GRADE |
The mean score measured with an instrumented anterior laxity test is likely to be closer to normal in autograft compared to allograft ACL reconstruction.
Sources (Zeng, 2016) |
Clinimetrics (IKDC Physical Examimation score)
|
Moderate GRADE |
The probability for a (nearly) Normal (grade A and B) overall IKDC Physical Examination score is similar for patients with autograft or allograft ACL reconstruction.
Sources (Zeng, 2016) |
Adverse events
|
- GRADE |
As no data on adverse events were reported, no conclusion could be drawn on the risk of an adverse event with an allograft or autograft. |
PROMs (Lysholm score)
|
Low GRADE |
There is no difference in average Lysholm score between patients with an autograft or allograft after an ACL injury.
Sources (Zeng, 2016) |
2. Type of surgical techniques
Failure
|
Very low GRADE |
The probability of failure seems to be similar for a double-bundle and single-bundle ACL reconstructions.
Sources (Liu, 2016; Zhang, 2014; Soumalainen, 2012) |
Clinimetrics (Pivot shift test)
|
Low GRADE |
The probability of a positive pivot shift test is lower after a double-bundle ACL reconstruction compared to a single-bundle autograft.
Sources (Zhu, 2013; Liu, 2016; Karikis, 2016; Koken, 2014; Ventura, 2013; Soumalainen, 2012; Lee, 2012) |
Clinimetrics (Lachman test)
|
Low GRADE |
The probability of a positive Lachman test is lower after a double-bundle ACL reconstruction compared to a single-bundle reconstruction.
Sources (Zhu, 2013; Karikis, 2016; Zhang, 2014; Ventura, 2013; Lee, 2012) |
Clinimetrics (IKDC score)
|
Low GRADE |
There is no difference in IKDC Physical Examination score between a double-bundle or single-bundle ACL reconstruction.
Sources (Zhu, 2013; Koken, 2014; Soumalainen, 2012; Lee, 2012) |
Adverse events
|
Very low GRADE |
There seems to be no difference in the risk of adverse events between a double-bundle or single-bundle ACL reconstruction.
Sources (Zhu, 2013; Liu, 2016) |
PROMs (Lysholm score)
|
Moderate GRADE |
There is no difference in Lysholm score between a double-bundle or single-bundle ACL reconstruction.
Sources (Zhu, 2013; Zhang, 2014; Soumalainen, 2012; Lee, 2012) |
3. Type of fixation
Because of the large heterogeneity in graft types used, surgery techniques and fixation techniques of the published studies, the effect of type of fixation could not be analysed.
4. Primary repair
Failure
|
- GRADE |
As no data with a high level of evidence on the risk of failure were reported, no conclusion could be drawn on failure after contemporary primary ACL suture repair. |
Adverse events
|
- GRADE |
As no data with a high level of evidence on adverse events were reported, no conclusion could be drawn on the risk of adverse events with a contemporary primary ACL suture repair. |
Clinimetrics
Lachman test
|
Very low GRADE |
There seems to be no difference in anterior tibial translation as measured with the Lachman test between those having contemporary primary ACL suture repair compared to those having ACL reconstruction.
Sources (Schliemann, 2017) |
Pivot shift test
|
- GRADE |
As no data with a high level of evidence on the pivot shift test were reported, no conclusion could be drawn on rate of a positive pivot shift test with a contemporary primary ACL suture repair. |
PROMs
|
Very low GRADE |
There seems to be no difference in PROMs (Lysholm and IKDC score) between those having contemporary primary ACL suture repair compared to those having ACL reconstruction.
Sources (Schliemann, 2017) |
Samenvatting literatuur
1. Graft types
Hamstrings tendon (HT) autograft versus Bone-patellar tendon-bone (BPTB) autograft
Description of included studies
Li (2012) evaluated the effectiveness of ACL reconstruction using either HT autografts or BPTB autografts. Authors systematically searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE and EMBASE up to December 2011. Only RCTs comparing HT with BTPB autografts among patients with unilateral ACL injury were included. Quasi-RCTs or RCTs with follow-up time less than 24 months were excluded. Nine RCTs in eleven reports were included. Study-specific estimates were not reported for all outcomes of interest.
Five RCTs published after the search date of Li were included, from which two RCTs contained the same population (Mohtadi, 2015; Mohtadi, 2016). Therefore, the data of four RCTs were included (Konrads, 2016; Mohtadi, 2016; Kautzner, 2015; Razi, 2014). The number of patients in these studies ranged from 62 to 230, and the follow-up ranged from two to ten years. The mean age ranged from 26 to 30 years, and most participants were males (range 55 to 83%). Failure rate was reported in only one of these studies Kautzner, (2015) and the pivot shift test was also only reported in one of these studies Razi, (2014).
Meta-analyses were performed for the outcomes failure, clinimetrics, PROMs and adverse events including the nine RCTs by Li and the four additional RCTs.
Results
1. Failure
Li (2012) reported a pooled relative risk for post-operative graft failure, without reporting the results of individual trials and/or a definition of graft failure. Therefore, the reported failure of the study by Kautzner (2015) could not be added to the meta-analysis. The pooled estimate for HT autograft in the meta-analysis by Li et al. was RR 1.37 (95%CI 0.67 to 2.81), meaning that the probability of graft failure is not statistically significantly different between an HT autograft or a BPTB autograft. The study by Kautzner also found no statistically significant difference in graft failure between HT autograft and BPTB autograft (RR 2.00; 95% CI 0.38 to 10.59).
2. Clinimetrics
The results for the pivot shift test, the Lachman test and the IKDC physical examination were used as indicators for clinimetrics.
Five studies included in the review by Li and two of the additional trials reported data on the pivot shift test. Li used a negative pivot shift test as outcome in their meta-analysis, therefore the results of the included individual studies were transformed to a positive pivot shift test outcome. The results of the final meta-analysis are depicted in figure 1. The pooled estimate for HT autograft was RR 1.73 (95%CI 1.06 to 2.82), meaning that the rate of a positive pivot shift test (indicating knee joint instability) is 73% higher after a reconstruction with an HT autograft compared with a BPTB autograft. Thus, the results on knee stability measured with the pivot shift test are better using BPTB autograft compared to HT autograft.
Five studies included in the review by Li and three of the additional trials reported data on the pivot shift test. Li (2012) reported only pooled data of the Lachman test. Therefore, the reported Lachman test of the three additional studies (Konrads, 2016; Kautzner, 2015; Razi, 2014) could not be added to the meta-analysis. The pooled RR for HT autograft in the meta-analysis of Li was 0.65 (95% CI 0.18 to 2.34), meaning that the rate of a negative Lachman test is not statistically significantly different between an HT autograft or a BPTB autograft. In the study by Konrads (2016) the RR for HT autograft of a negative Lachman test was 0.78 (95% CI 0.48 to 1.27) and in the study by Razi (2014) the RR for HT autograft was 0.52 (95% CI 0.30 to 0.90). The study by Kautzner (2015) reported the mean Lachman test. The mean Lachman test laxity after 2 years follow-up was 1.3 mm (range 0 to 10) in the HT group and 1 mm (range 0 to 12) in the BPTB group (P=0.624). Due to the limited data, no meta-analysis was performed for the Lachman test.
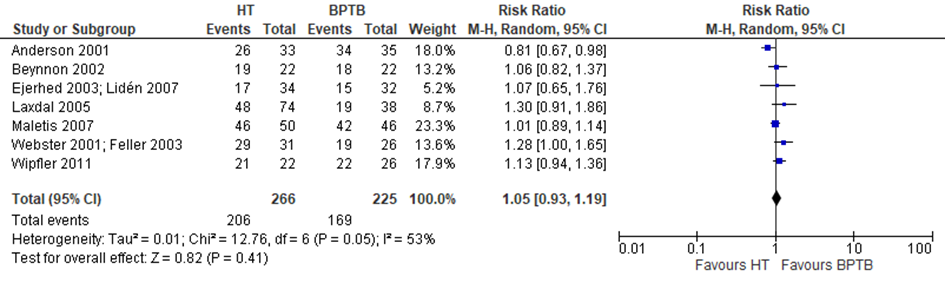
Seven trials in the review by Li (2012) reported data on the IKDC Physical Examination score. The additional trials did not report IKDC scores. A meta-analysis was performed and depicted in figure 2. The probability of a (nearly) Normal (grade A and B) overall IKDC score does not differ between an HT autograft or a BPTB autograft (RR 1.05 95% CI: 0.93-1.19).
Figure 1 Meta-analysis HT versus BPTB of the outcome positive pivot
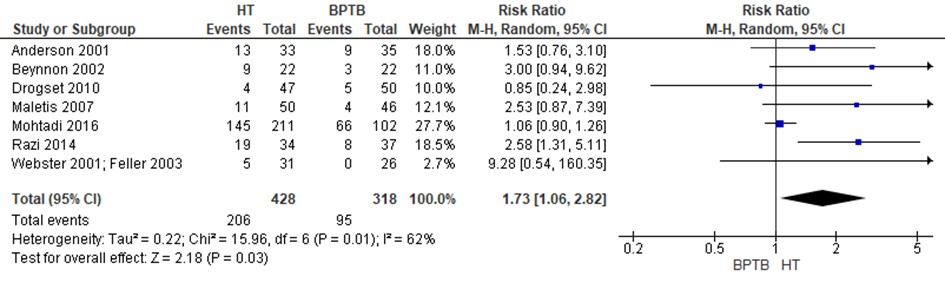
Figure 2 Meta-analysis HT versus BPTB of the outcome IKDC Physical Examination score
3. Adverse events
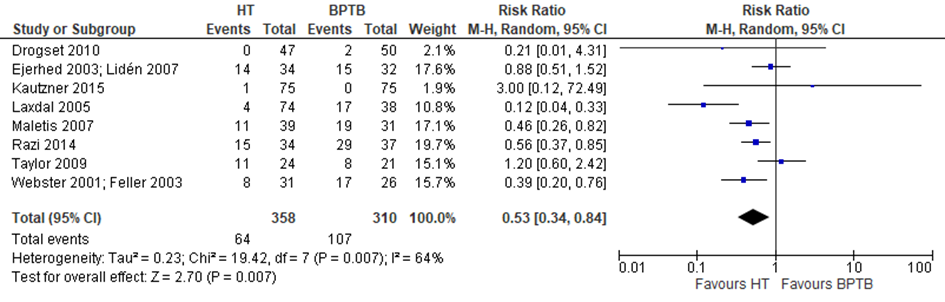
Li reported data on adverse events, defined by kneeling pain. The studies by Kautzner (2015) and Razi (2014) both reported postoperative complications (wound infection), these were considered as adverse events as well and included in the meta-analysis (figure 3). The probabily of experiencing an adverse event is 47% lower after a reconstruction with an HT autograft compared to a BPTB autograft (RR 0.53; 95% CI 0.34 to 0.84).
Figure 3 Meta-analysis HT versus BPTB of the outcome adverse events
4. PROMs
The results of the Lysholm score were considered as an indicator of PROMs.
The Lysholm score was reported in only one of the included trials Razi, (2014). The mean Lysholm score after 2 years follow-up was 90 (SD 7.6) in the HT autograft group and 88 (SD 7.5) in the BPTB autograft group, which was not a statistically significant difference (P=0.30).
Level of evidence
Failure: The level of evidence was downgraded by three levels because of the risk of bias (none of the included trials used a blinding method) and imprecision (too few patients with a failure and in total included in the meta-analysis).
Clinimetrics: The level of evidence of the outcome pivot shift test was downgraded by two levels because of the risk of bias (none of the included trials used a blinding method) and imprecision (confidence interval crossed the boundary of a clinically meaningful difference). The level of evidence of the outcome Lachman test was downgraded by three levels because of the risk of bias (none of the included trials used a blinding method), inconsistency (large heterogeneity) and imprecision (too few patients). The level of evidence of the outcome IKDC Physical Examination score was downgraded by one level because of the risk of bias (none of the included trials used a blinding method).
Adverse events: The level of evidence of the outcome adverse events was downgraded by three levels because of the risk of bias (none of the included trials used a blinding method), inconsistency (minimal or no overlap of confidence intervals), indirectness (differences in outcomes measures) and imprecision (too few patients with an event).
PROMs: The level of evidence of the outcome Lysholm score was downgraded by two levels because of the risk of bias (none of the included trials used a blinding method) and imprecision (too few patients).
Quadriceps graft
Only one RCT was found comparing quadriceps grafts with BPTB graft in 26 patients Lund, (2014). The working group interpreted this as insufficient evidence to assess the effect of quadriceps graft.
Autograft versus allograft
Description of included studies
Zeng (2016) undertook a meta-analysis of RCTs to compare autografts with allograft in ACL reconstruction. Medline, Embase and the Cochrane Library were searched from 1 January 1966 till 28 June 2014. RCTs, systematic reviews, or meta-analyses concerning primary ACL reconstruction patients were included. The reconstruction had to be arthroscopically assisted, whereby autograft was compared to allograft. The methodological quality scores of RCTs had to be 2 or greater, assessed by the Modified Oxford Scale. Inclusion criterium for follow-up was a minimum of 2 years and the minimum sample size of RCTs was 20 participants per group.
For this clinical question, only the data from RCTs will be described. Nine RCTs were included in the meta-analysis of Zeng. The meta-analysis was not updated with studies published from June 2014 till February 2017.
Results
1. Failure
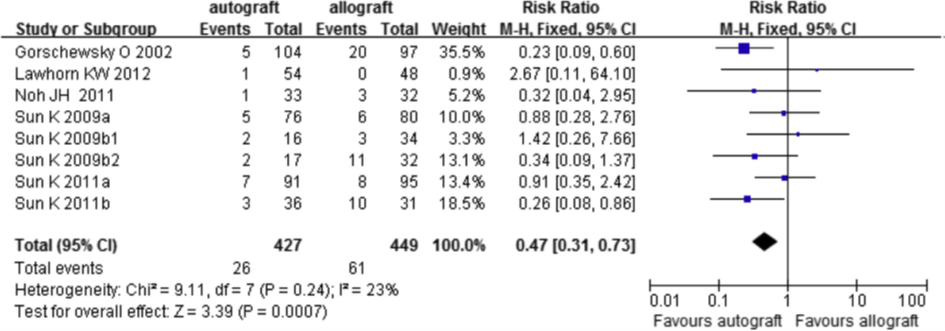
Clinical failure of the graft included revision surgery, graft rupture, +2 pivot shift or higher, and side-to-side arthrometer difference >5 mm). Seven out of nine trials reported data on failure. The individual study effect estimates and the pooled estimate are given in figure 4. The risk of failure is 53% lower with an autograft compared with an allograft (RR 0.47; 95%CI 0.31 to 0.73).
Figure 4 Meta-analysis autograft versus allograft of the outcome failure (Zeng, 2016)
2. Clinimetrics
As an indicator for clinimetrics, the results from the pivot shift test, the Lachman test and the IKDC Physical Examination score and were used. All nine included trials reported data on the pivot shift test. The results are depicted in figure 5. There was no difference in a positive pivot shift test between an autograft or an allograft (RR 1.05; 95%CI 0.99 to 1.13).
Seven out of nine trials reported results on the Lachman test (figure 6). The pooled estimate was RR 1.18 (95%CI 1.02 to 1.36) meaning that the risk of a negative Lachman test is 18% higher after a reconstruction with an autograft compared to an allograft. Because of insufficient description of the analysis method in the review of Zeng, the working group could not interpret the clinical relevance of this difference.
Five out of nine trials used an instrumented anterior laxity test (figure 7). The pooled weighted mean difference was -0.88 (95%CI -1.47 to -0.28), which was interpreted by the working group as a non-clinically relevant difference.
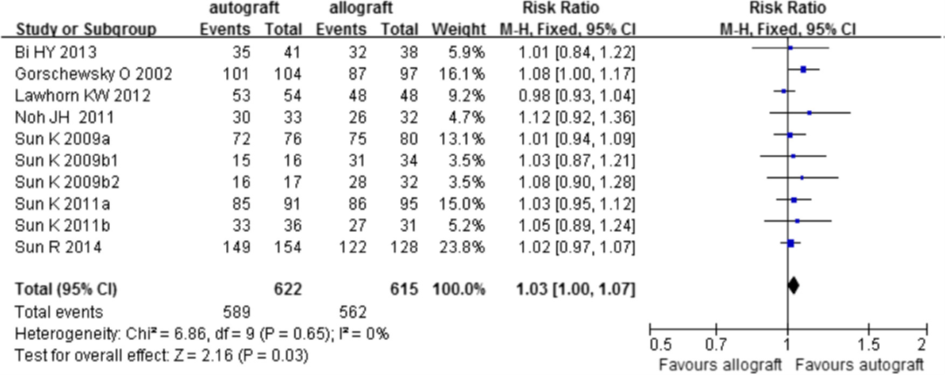
All nine trials reported data on the IKDC Physical Examination score. The meta-analysis is depicted in figure 8. The probability of a (nearly) Normal (grade A and B) overall IKDC score is not different between an allograft or an autograft (RR 1.03; 95%CI 1.00 to 1.07).
Figure 5 Meta-analysis allograft versus autograft of the outcome pivot shift test (Zeng, 2016)
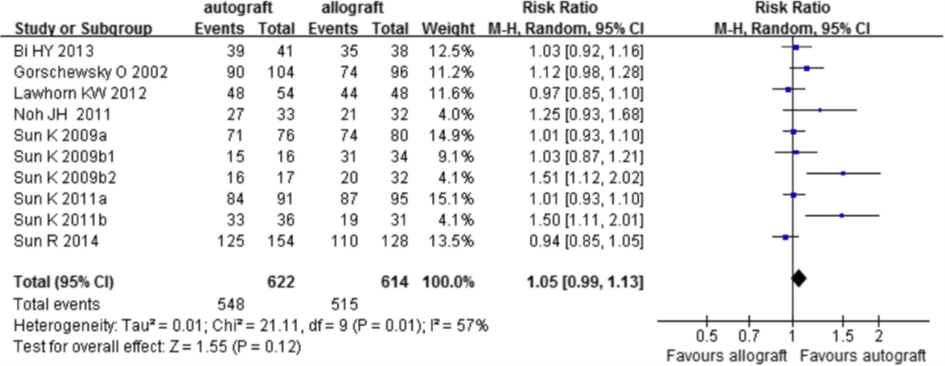
Figure 6 Meta-analysis allograft versus autograft of the outcome Lachman test (Zeng, 2016)
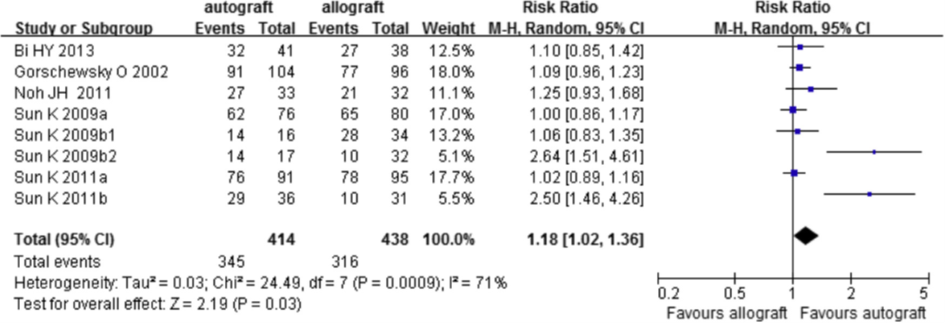
Figure 7 Meta-analysis autograft versus allograft of the outcome instrumented laxity test (Zeng, 2016)
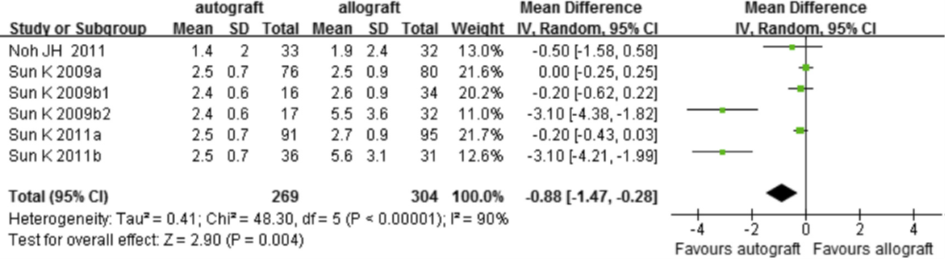
Figure 8 Meta-analysis allograft versus autograft of the outcome IKDC Physical Examination score (Zeng, 2016)
3. Adverse events
Zeng (2016) reported no data on adverse events.
4. PROMs
The results of the Lysholm score were considered as an indicator of PROMs. Seven out of nine trials used the Lysholm questionnaire as an indicator for knee instability (Figure 9). The Lysholm score was no different between patients with an allograft or patients with an autograft (mean difference in score 0.02; 95%CI -0.71 to 0.75).
Figure 9 Meta-analysis of the outcome Lysholm score (Zeng, 2016)
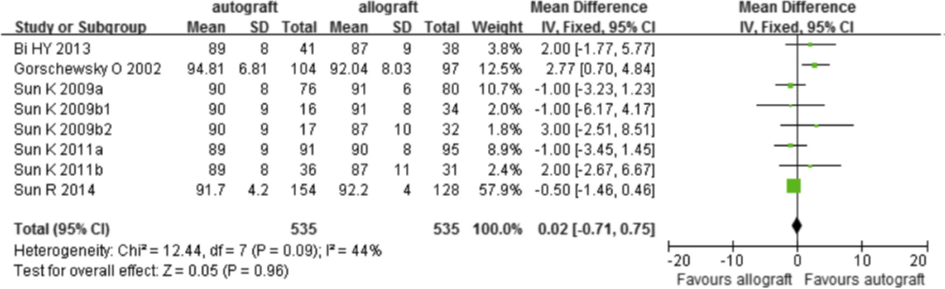
Level of evidence
Failure: The level of evidence was downgraded by three levels because of the risk of bias (none of the included trials used a blinding method), unexplained inconsistency (four trials reported a reduced risk of failure, while four studies reported either a null effect or an increased risk of failure) and imprecision (in total less than 100 failures were included in the meta-analysis).
Clinimetrics: The level of evidence of the outcome pivot shift test was downgraded by one level because of the risk of bias (none of the included trials used a blinding method). The level of evidence of the outcome Lachman test was downgraded by two levels because of the risk of bias (none of the included trials used a blinding method) and imprecision (confidence interval crossed the boundary of a clinically meaningful difference). The level of evidence of the outcome instrumented laxity test was downgraded by three levels because of the risk of bias (none of the included trials used a blinding method), inconsistency and imprecision (confidence interval crossed the boundary of a clinically meaningful difference). The level of evidence of the outcome IKDC Physical Examination score was downgraded by one level because of the risk of bias (none of the included trials used a blinding method).
Adverse events: Due to a lack of data, the level of evidence for adverse events could not be graded.
PROMs: The level of evidence of the outcome Lysholm score was downgraded by two levels because of the risk of bias (none of the included trials used a blinding method) and imprecision (too few patients).
2. Type of surgical techniques
Double-bundle (DB) versus Single-bundle (SB)
Description of included studies
Zhu (2013) performed a meta-analysis of RCTs to compare double-bundle (DB) with single-bundle (SB) ACL reconstruction. PubMed, Embase and the Cochrane Library were searched from 1 January 1966 till September 2011. Only prospective and randomised studies comparing the clinical outcomes of DB versus SB ACL reconstruction, with patients over 18 years were included. Trails with non-clinical outcomes and without a follow-up were excluded.
A total of 18 trials were included in the meta-analysis of Zhu, of which 9 trials were prospective randomised trials with a minimum mean follow-up of two years and will be described.
An additional eight studies were included in the meta-analysis published after the search date (September 2011) by Zhu (Liu, 2016; Karikis, 2016; Zhang, 2014; Koken, 2014; Ventura, 2013; Suomalainen, 2012; Nunez, 2012; Lee, 2012). The number of patients in these studies ranged from 42 to 108 and the follow-up ranged from 2 to 6.7 years. The mean age ranged from 25 to 30 years and most participants were males (range 66 to 97%). Adverse events were only reported in one of the studies Liu, (2016).
Results
1. Failure
Zhu reported no data on failure of the graft. Three of the additional trials reported data on failures. The study by Liu (2016) identified two traumatic failures at the final follow-up in the DB group and zero in the SB groups (RR 5.00; 95% CI 0.25 to 101.0). In the study by Zhang (2014), one patient in the DB and two patients in the SB group (2% and 3% respectively) had a re-rupture (RR 0.58; 95% CI 0.05 to 6.21). In the study by Soumalainen (2012), one patient in the DB group (3%) and ten in the SB group (17%) required an ACL revision surgery (RR 0.20; 95% CI 0.03 to 1.49). No meta-analysis was performed, due to the limited data.
2. Clinimetrics
The results from the pivot shift test, the Lachman test and the IKDC Physical Examination score were used as an indicator for clinimetrics. Six trials included in Zhu and six out of the nine additional trials reported data on the pivot shift test. Zhu used a negative pivot shift test as outcome in their meta-analysis, therefore the results of the included individual studies were transformed to a positive pivot shift test outcome. The results of the final meta-analysis with the twelve studies are depicted in figure 10. The pooled estimate for DB reconstruction was RR 0.52 (0.31 to 0.85), meaning that the rate of a positive pivot shift test (indicating worse knee joint stability) is 47% lower after a DB reconstruction compared with a SB reconstruction. Thus, the results on knee stability measured with the pivot shift test are better using DB compared to SB.
Two trials included in Zhu and four out of the nine additional trials reported data on the Lachman test. Zhu used a negative Lachman test as outcome in their meta-analysis, therefore the results of the included individual studies were transformed to a positive Lachman test outcome. A meta-analysis was performed with these seven studies. The results are depicted in figure 11. The pooled estimate was RR 0.56 (95% CI 0.39 to 0.81), meaning that the probability of increased anterior laxity as measured by a positive Lachman test was 44% lower after a reconstruction with a DB compared to SB graft.
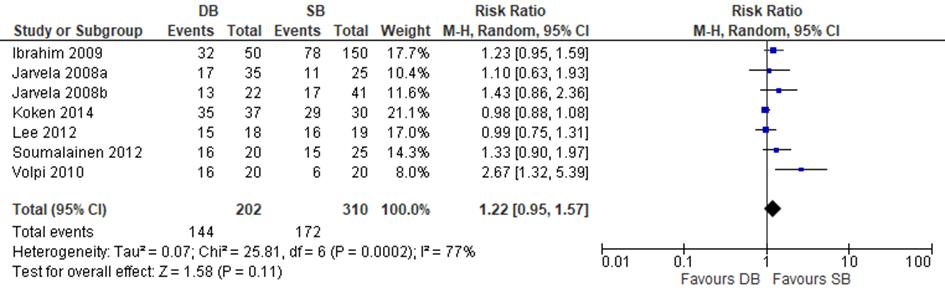
Four trials included in Zhu and three of the additional trials reported dichotomous data on the IKDC score (normal/nearly normal versus abnormal/severely) and could be included in a meta-analysis to pool the risk ratio. The results of the meta-analysis are depicted in figure 12. The pooled estimate for (nearly) normal IKDC was RR 1.22 (95% CI 0.95 to 1.57), meaning that there was no statistically significant difference in the IKDC Physical Examination score between a reconstruction with a DB graft compared to a reconstruction with a SB graft.
Figure 10 Meta-analysis DB versus SB of the outcome positive pivot shift test
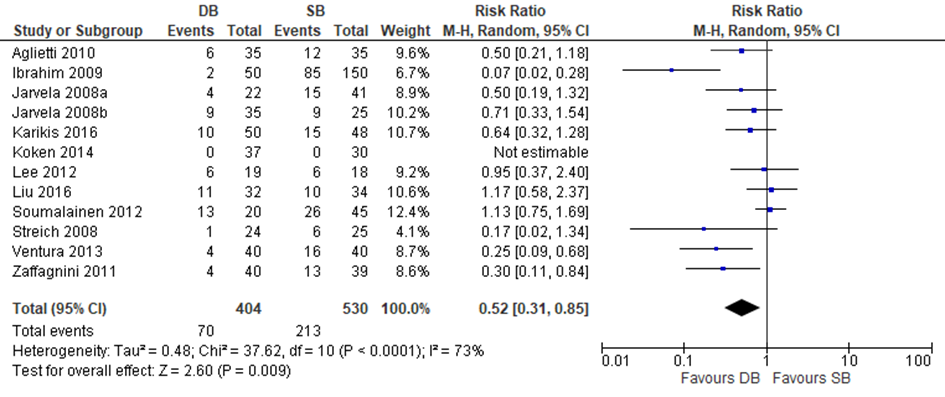
Figure 11 Meta-analysis DB versus SB of the outcome positive Lachman test
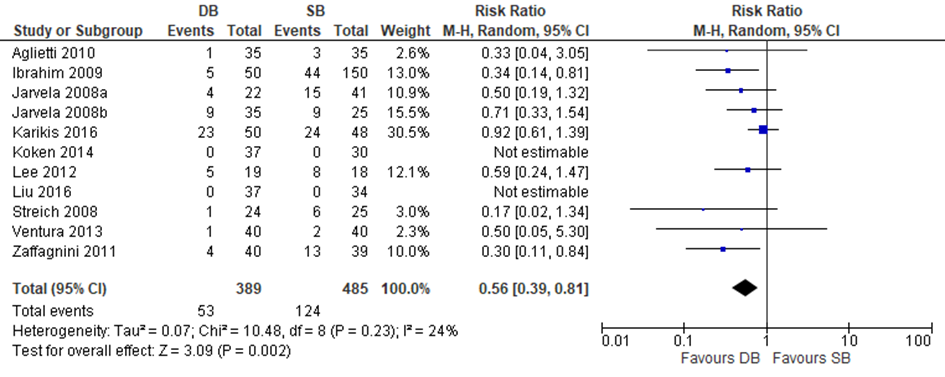
Figure 12 Meta-analysis DB versus SB of the outcome IKDC Physical Examination score (normal versus abnormal/severely)
3. Adverse events
Complications were reported in the review by Zhu and were taken as an indicator for adverse events. Zhu reported that complications are the composite of secondary meniscal
tears or unhealed meniscal fixation requiring a second arthroscopy, deep infections, graft failures, cyclops lesions, thrombosis as well as pain and swelling. Six trials included in Zhu reported data to allow a meta-analysis of the results. Liu reported that no participants developed intra-operative or post-operative complications. The results of the meta-analysis are depicted in figure 13. Pooled estimates indicated that the risk of complications is slightly lower with a DB reconstruction compared to an SB reconstruction, but the difference was not statistically significant (RR 0.73 95%CI: 0.32-1.80).
Figure 13 Meta-analysis DB versus SB of the outcome adverse events
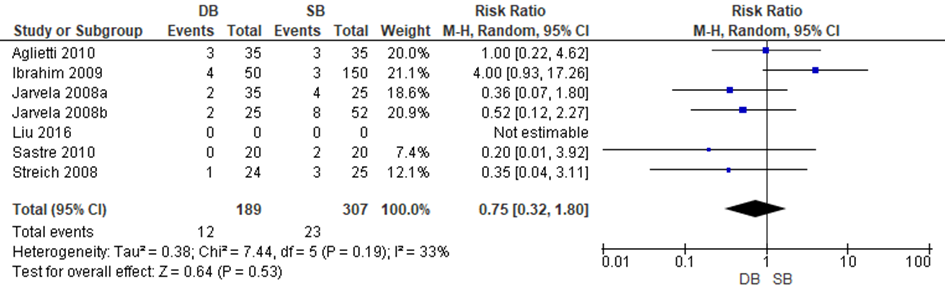
4. PROMs
The results from the Lysholm scores were used as an indicator for PROMs.
Three trials included in Zhu and three of the additional trials reported mean differences in Lysholm scores and could be included in a meta-analysis. The results of the meta-analysis are depicted in figure 14. The pooled mean difference was 0.58 (95% CI -1.55 to 2.71), meaning that there was no statistically significant difference in the Lysholm score between a reconstruction with a DB graft compared and a reconstruction with an SB graft.
Figure 14 Meta-analysis DB versus SB of the outcome Lysholm score
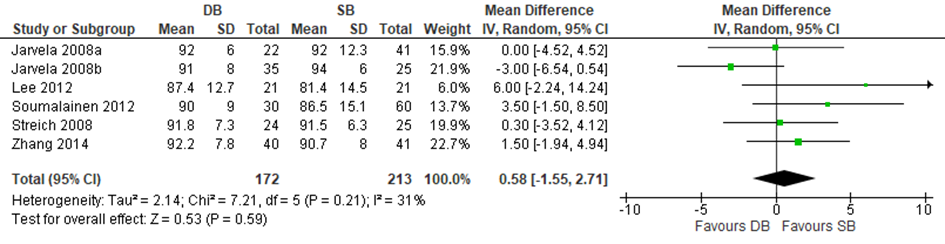
Level of evidence
Failure: The level of evidence of the outcome failure was downgraded by three levels because of the risk of bias (none of the included trials used a blinding method), indirectness (differences in outcomes measures) and imprecision (too few patients with an event).
Clinimetrics: The level of evidence of the outcome pivot shift test was downgraded by two levels because of the risk of bias (none of the included trials used a blinding method), inconsistency (large heterogeneity). The level of evidence of the outcome Lachman test was downgraded by two levels because of the risk of bias (none of the included trials used a blinding method) and imprecision (confidence interval crossed the boundary of a clinically meaningful difference). The level of evidence of the outcome IKDC Physical Examination score was downgraded by two levels because of the risk of bias (none of the included trials used a blinding method) and inconsistency (large heterogeneity).
Adverse events: The level of evidence of the outcome adverse events was downgraded by three levels because of the risk of bias (none of the included trials used a blinding method), indirectness (differences in outcomes measures) and imprecision (too few patients with an event).
PROMs: The level of evidence of the outcome Lysholm score was downgraded by one level because of the risk of bias (none of the included trials used a blinding method).
3. Type of fixation
Because of the large heterogeneity in graft types used, surgery techniques and fixation techniques of the published studies, the effect of type of fixation could not be analysed.
4. Primary repair
Description of included studies
Only one of the included studies, Schliemann (2017), reported a contemporary ACL suture repair technique) and performed a randomised controlled trial in 60 patients with acute ACL tears. Thirty patients (mean age 28.2±11.4 yr) were randomised to the intervention group and underwent a contemporary arthroscopic ACL suture repair technique (dynamic intra-ligamentary stabilisation (DIS)) and 30 patients (mean age 29.1±12.0 yr) were randomised to the control group, undergoing an anatomic semitendinosus autograft ACL reconstruction. After both procedures, the knee was placed in a knee immobiliser for four days. Afterwards, patients underwent a brace-free rehabilitation programme. Follow-up time was twelve months. Outcomes were based on the International Knee Documentation Committee (IKDC) score and Lysholm score.
Meunier (2007) described the long-term follow-up of a randomised controlled trial in 100 patients with an acute and total rupture of the ACL who were treated between 1980 and 1983. A total of 44 patients (mean age 22 yr; range 14-30) were randomised to the intervention group and underwent surgical repair (proximal ruptures non-augmented, mid-substance ruptures augmented with ITB) and 56 patients (mean age 21 yr; range 14-30) were randomised to the control group, undergoing conservative treatment. After repair, the limb was immobilised in a long leg cast and no weight bearing was allowed until the cast was removed after six weeks. An intensive rehabilitation programme was then instituted. The rehabilitation of the conservative group was not described. Follow-up time was 15 years. Outcomes were having secondary treatment, Lachman test, pivot shift test, osteoarthritis and Lysholm score. The analyses were stratified afterwards, whereby the repair group was divided into those without augmentation (Sr) and those with augmentation (Sar). The conservative group was divided into those without surgery (NSns) and those who underwent late reconstruction (NSrec). For our literature analysis, the groups were combined (Sr/Sar versus NSns/NSrec).
Results
1. Failure/ re-rupture
In the RCT of Meunier (2007), there was no statistically significant difference in having secondary treatment of meniscus injury between the repair group and the conservative group (RR 0.41; 95% CI 0.17 to 1.04; P=0.06).
Schliemann did not report the outcome failure/re-rupture rate.
2. Clinimetrics
Lachman test
In the RCT by Meunier (2007), there was no statistically significant difference in a positive Lachman test between the repair group and the conservative group after 15-yr follow-up (RR 0.87; 95% CI 0.61 to 1.24; P=0.44).
In the RCT by Schliemann (2017) it was reported that the difference in anterior tibial translation between the injured and the contralateral knee was 7.6 mm in the DIS group and 8.4 mm in the ACLR group prior to the intervention. There was no statistically significant difference between the groups after 12-month follow-up (DIS: 1.7 mm, ACLR: 1.4 mm: NS).
Pivot shift test
In the RCT by Meunier (2007), there was no statistically significant difference in a positive pivot shift test between the repair group and the conservative group after 15-yr follow-up (RR 0.69; 95% CI 0.39 to 1.22; P=0.20).
Schliemann did not report the outcome pivot shift.
3. Adverse events
Adverse events were not reported as outcome in the included studies.
4. PROMs
Lysholm score
In the RCT by Meunier (2007), there was no difference between the repair group and the conservative group in Lysholm score (good/excellent versus fair) after 15-yr follow-up (RR 0.72; 95% CI 0.29 to 1.80; P=0.27).
In the RCT by Schliemann (2017), the mean Lysholm score after 1-yr follow-up was 89.8 (SD 11.0) in the repair group and 89.9 (SD 15.5) in the reconstruction group, which was not a statistically significant difference (MD 0.1; 95% CI -6.85 to 7.05; P=0.98).
IKDC score
In the RCT by Schliemann (2017), the mean IKDC score after 1-yr follow-up was 85.7 (SD 12.4) in the repair group and 84.8 (SD 19.4) in the reconstruction group, which was not a statistically significant difference (MD 0.9; 95% CI -9.32 to 7.51; P=0.83).
Meunier (2007) did not report the outcome IKDC score.
Level of evidence
Failure/re-rupture: Because of limited data, the level of evidence for failure/re-rupture could not be graded.
Clinimetrics: The level of evidence was downgraded by three levels because of the risk of bias (inadequate allocation concealment, unclear if a blinding method was used and bias due to violation of intention to treat analysis), imprecision (too few patients) and indirectness (conservative treatment instead of reconstruction as comparison).
Adverse events: Because of limited data, the level of evidence for adverse events could not be graded.
PROMs: The level of evidence for the outcome PROMs was downgraded by three levels because of the risk of bias (unclear or inadequate blinding and bias due to violation of intention to treat analysis) and indirectness (difference in outcome measures).
Zoeken en selecteren
A systematic review of the literature was performed to answer the following questions:
- What is the effectiveness of different autografts (BPTB, QT) compared to HT autograft in ACL reconstruction?
- What is the effectiveness of autograft compared to allograft in ACL reconstruction?
- What is the effectiveness of SB hamstring compared to DB hamstring in ACL reconstruction?
- What is the effectiveness of different fixation methods in ACL reconstruction (bio-absorbable versus metallic, suspensory versus aperture, cortical button versus transfemoral suspensory, inter-tunnel versus extra-tunnel)?
- What is the effectiveness of a repair compared to reconstruction in patients with ACL injury?
P: patients with ACL reconstruction;
I: 1. Graft type (BPTB, HT, QT);
2. Use of autograft;
3. SB HT reconstruction;
4. Fixation technique (screw (metal, bio-absorbable), button, et cetera);
5. Non-augmented or augmented primary suture repair.
C: 1. One (or more) other graft types;
2. Use of allograft;
3. DB HT reconstruction;
4. One (or more) of other fixation techniques;
5. Reconstruction;
O: Failure, clinimetrics, adverse event and PROMs.
Relevant outcome measures
The working group considered failure, clinimetrics, adverse event and PROMs as critical outcome measures for decision-making.
The working group did not define the outcome measures a priori, but applied the definitions used in the studies.
The working group did not define clinical (patient) relevant differences a priori.
Search and select (Method)
The databases Medline (via OVID) and Embase (via Embase.com) were searched with relevant search terms. For the update of the literature, both databases were searched from the previous search date (December 2009) till February 2017. A separate search was performed for the question of repair. Therefore, both databases were searched with relevant search terms from 2000 till September 2017. The detailed strategy of both searches is depicted under the tab Methods. The updated systematic literature search resulted in 469 hits, and the literature search for repair resulted in 308 hits. Studies were selected based on the following criteria:
- Systematic review with evidence tables, risk of bias evaluation and a detailed search strategy.
- Prospective randomised controlled trials of patients with ACL injury evaluating the effect of different grafts (BPTB, HT (DB – SB), QT, allograft, artificial), different graft techniques (tunnel variation, double bundle – single bundle) different fixation techniques and non-augmented or augmented primary suture repair on PROMs or failure after a minimum follow-up of 2 years.
Initially, 149 studies were selected from the updated reconstruction search based on title and abstract by one or both reviewers. After reading the full text, 132 studies were excluded (see the table with reasons for exclusion under the tab Methods) and 17 studies were included.
Seventeen studies were included in the literature analysis, three systematic reviews and fourteen additional RCTs.
Nine studies were selected by one or both reviewers from the repair search, based on title and abstract. After reading the full text, seven studies were excluded (see the table with reasons for exclusion under the tab Methods) and two studies were included in the literature analysis, both RCTs (Schliemann, 2017; Meunier, 2007). Important study characteristics and results are depicted in the evidence tables. The assessment of the risk of bias is depicted in the risk of bias tables.
Referenties
- Achtnich A, Herbst E, Forkel P, et al. Acute Proximal Anterior Cruciate Ligament Tears: Outcomes After Arthroscopic Suture Anchor Repair Versus Anatomic Single-Bundle Reconstruction. Arthroscopy. 2016;32(12):2562-2569.
- Ahlden M, Sernert N, Karlsson J, et al. A prospective randomized study comparing double- and single-bundle techniques for anterior cruciate ligament reconstruction. Am J Sports Med. 2013;41(11):2484-91.
- Ateschrang A, Ahmad SS, Stockle U, et al. Recovery of ACL function after dynamic intraligamentary stabilization is resultant to restoration of ACL integrity and scar tissue formation. Knee Surg Sports Traumatol Arthrosc. 2017.
- Buchler L, Regli D, Evangelopoulos DS, et al. Functional recovery following primary ACL repair with dynamic intraligamentary stabilization. Knee. 2016;23(3):549-553.
- Chen H, Tie K, Qi Y,et al. Anteromedial versus transtibial technique in single-bundle autologous hamstring ACL reconstruction: a meta-analysis of prospective randomized controlled trials. J Orthop Surg Res. 2017;12(1):167. doi: 10.1186/s13018-017-0671-3. Review. PubMed PMID: 29115973; PubMed Central PMCID: PMC5678560.
- DiFelice GS, van der List JP. Clinical Outcomes of Arthroscopic Primary Repair of Proximal Anterior Cruciate Ligament Tears Are Maintained at Midterm Follow-up. Arthroscopy. 2018. 29373290.
- Eggli S, Roder C, Perler G,et al. Five year results of the first ten ACL patients treated with dynamic intraligamentary stabilisation. BMC Musculoskelet Disord. 2016;17:105.
- Evangelopoulos DS, Kohl S, Schwienbacher S, et al. Collagen application reduces complication rates of mid-substance ACL tears treated with dynamic intraligamentary stabilization. Knee Surg Sports Traumatol Arthrosc. 2017;25(8):2414-2419.
- Henle P, Bieri KS, Brand M, et al. Patient and surgical characteristics that affect revision risk in dynamic intraligamentary stabilization of the anterior cruciate ligament. Knee Surg Sports Traumatol Arthrosc. 2017.
- Henle P, Roder C, Perler G, et al. Dynamic Intraligamentary Stabilization (DIS) for treatment of acute anterior cruciate ligament ruptures: case series experience of the first three years. BMC Musculoskelet Disord. 2015;16:27.
- Hoogeslag RAG, Brouwer RW, Huis In 't Veld R,et al. Dynamic augmentation restores anterior tibial translation in ACL suture repair: a biomechanical comparison of non-, static and dynamic augmentation techniques. Knee Surg Sports Traumatol Arthrosc. 2018.
- Karikis I, Desai N, Sernert N, et al. Comparison of Anatomic Double- and Single-Bundle Techniques for Anterior Cruciate Ligament Reconstruction Using Hamstring Tendon Autografts: A Prospective Randomized Study With 5-Year Clinical and Radiographic Follow-up. Am J Sports Med. 2016;44(5):1225-36.
- Kautzner J, Kos P, Hanus M, et al. A comparison of ACL reconstruction using patellar tendon versus hamstring autograft in female patients: a prospective randomised study. Int Orthop. 2015;39(1):125-30.
- Kohl S, Evangelopoulos DS, Kohlhof H, et al. Anterior crucial ligament rupture: self-healing through dynamic intraligamentary stabilization technique. Knee Surg Sports Traumatol Arthrosc. 2013;21(3):599-605.
- Kohl S, Evangelopoulos DS, Schar MO, et al. Dynamic intraligamentary stabilisation: initial experience with treatment of acute ACL ruptures. Bone Joint J. 2016;98-B(6):793-798.
- Koken M, Akan B, Kaya A, et al. Comparing the anatomic single-bundle versus the anatomic double-bundle for anterior cruciate ligament reconstruction: A prospective, randomized, single blind, clinical study. European Orthopaedics and Traumatology. 2014;5(3):247-52.
- Konrads C, Reppenhagen S, Plumhoff P, et al. No significant difference in clinical outcome and knee stability between patellar tendon and semitendinosus tendon in anterior cruciate ligament reconstruction. Arch Orthop Trauma Surg. 2016;136(4):521-5.
- Kosters C, Herbort M, Schliemann B, et al. Dynamic intraligamentary stabilization of the anterior cruciate ligament. Operative technique and short-term clinical results. Unfallchirurg. 2015;118(4):364-371.
- Krismer AM, Gousopoulos L, Kohl S,et al. Factors influencing the success of anterior cruciate ligament repair with dynamic intraligamentary stabilisation. Knee Surg Sports Traumatol Arthrosc. 2017;25(12):3923-3928.
- Lee S, Kim H, Jang J, et.al. Comparison of anterior and rotatory laxity using navigation between single- and double-bundle ACL reconstruction: prospective randomized trial. Knee Surg Sports Traumatol Arthrosc. 2012;20(4):752-61.
- Li S, Chen Y, Lin Z, et al. A systematic review of randomized controlled clinical trials comparing hamstring autografts versus bone-patellar tendon-bone autografts for the reconstruction of the anterior cruciate ligament. Arch Orthop Trauma Surg. 2012;132(9):1287-97.
- Liu Y, Cui G, Yan H, et al. Comparison Between Single- and Double-Bundle Anterior Cruciate Ligament Reconstruction With 6- to 8-Stranded Hamstring Autograft: A Prospective, Randomized Clinical Trial. Am J Sports Med. 2016;44(9):2314-22.
- Mackay GM, Blyth MJ, Anthony I, et al. A review of ligament augmentation with the InternalBrace: the surgical principle is described for the lateral ankle ligament and ACL repair in particular, and a comprehensive review of other surgical applications and techniques is presented. Surg Technol Int. 2015;26:239-255.
- Meister M, Koch J, Amsler F, et al. ACL suturing using dynamic intraligamentary stabilisation showing good clinical outcome but a high reoperation rate: a retrospective independent study. Knee Surg Sports Traumatol Arthrosc. 2017.
- Mohtadi N, Chan D, Barber R,et al. Reruptures, Reinjuries, and Revisions at a Minimum 2-Year Follow-up: A Randomized Clinical Trial Comparing 3 Graft Types for ACL Reconstruction. Clin J Sport Med. 2016;26(2):96-107.
- Mohtadi N, Chan D, Barber R, et al. A Randomized Clinical Trial Comparing Patellar Tendon, Hamstring Tendon, and Double-Bundle ACL Reconstructions: Patient-Reported and Clinical Outcomes at a Minimal 2-Year Follow-up. Clin J Sport Med. 2015;25(4):321-31.
- Murray MM, Flutie BM, Kalish LA, et al. The Bridge-Enhanced Anterior Cruciate Ligament Repair (BEAR) Procedure: An Early Feasibility Cohort Study. Orthop J Sports Med. 2016;4(11):2325967116672176.
- Nunez M, Sastre S, Nunez E, et al. Health-related quality of life and direct costs in patients with anterior cruciate ligament injury: single-bundle versus double-bundle reconstruction in a low-demand cohort--a randomized trial with 2 years of follow-up. Arthroscopy. 2012;28(7):929-35.
- Razi M, Sarzaeem MM, Kazemian GH, et al. Reconstruction of the anterior cruciate ligament: a comparison between bone-patellar tendon-bone grafts and fourstrand hamstring grafts. Med J Islam Repub Iran. 2014;28:134.
- Shea KG, Carey JL, Richmond J, et al. The American Academy of Orthopaedic Surgeons Evidence-Based Guideline on Management of Anterior Cruciate Ligament Injuries. JBJS. 2015;97(8):672674
- Schliemann B, Glasbrenner J, Rosenbaum D, et al. Changes in gait pattern and early functional results after ACL repair are comparable to those of ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2017.
- Schliemann B, Lenschow S, Domnick C, et al. Knee joint kinematics after dynamic intraligamentary stabilization: cadaveric study on a novel anterior cruciate ligament repair technique. Knee Surg Sports Traumatol Arthrosc. 2017;25(4):1184-1190.
- Suomalainen P, Jarvela T, Paakkala A, et al. Double-bundle versus single-bundle anterior cruciate ligament reconstruction: a prospective randomized study with 5-year results. Am J Sports Med. 2012;40(7):1511-8.
- Van der List JP, DiFelice GS. Range of motion and complications following primary repair versus reconstruction of the anterior cruciate ligament. Knee. 2017;24(4):798-807.
- Van der List JP, DiFelice GS. Role of tear location on outcomes of open primary repair of the anterior cruciate ligament: A systematic review of historical studies. Knee. 2017;24(5):898-908. 28803759.
- van Eck CF, Limpisvasti O, ElAttrache NS. Is There a Role for Internal Bracing and Repair of the Anterior Cruciate Ligament? A Systematic Literature Review. Am J Sports Med. 2017:363546517717956.
- Ventura A, Iori S, Legnani C, et al. Single-bundle versus double-bundle anterior cruciate ligament reconstruction: assessment with vertical jump test. Arthroscopy. 2013;29(7):1201-10.
- Zeng C, Gao SG, Li H, et al. Autograft Versus Allograft in Anterior Cruciate Ligament Reconstruction: A Meta-analysis of Randomized Controlled Trials and Systematic Review of Overlapping Systematic Reviews. Arthroscopy. 2016;32(1):153-63.e18.
- Zhang Z, Gu B, Zhu W, et al. Double-bundle versus single-bundle anterior cruciate ligament reconstructions: a prospective, randomized study with 2-year follow-up. Eur. 2014;24(4):559-65.
- Zhu Y, Tang RK, Zhao P,et al. Double-bundle reconstruction results in superior clinical outcome than single-bundle reconstruction. Knee Surg Sports Traumatol Arthrosc. 2013;21(5):1085-96.
Evidence tabellen
|
Study reference |
Study characteristics |
Patient characteristics |
Intervention (I) |
Comparison / control (C) |
Follow-up |
Outcome measures and effect size |
Comments |
|
HS autograft versus BPTB autograft |
|||||||
|
Li, 2012 |
SR and meta-analysis of RCTs
Literature search up to December 2011
A: Anderson, 2001 B: Beynnon, 2002 C: Ejerhed, 2003/ Lidén, 2007 D: Webster 2001/ Feller, 2003 E: Laxdal, 2005 F: Maletis, 2007 G: Taylor, 2009 H: Drogset, 2010 I: Wipfler, 2011
Study design: RCT
Setting and Country: not reported
Source of funding: Not reported
|
Inclusion criteria SR:
Exclusion criteria SR:
9 studies included
N, mean age A: 70 patients, 14-44 yr B: 56 patients, 18-52 yr C: 71 patients, 14-59 yr D: 65 patients, 18-40 yr E: 134 patients, 12-52 yr F: 99 patients, 14-48 yr G: 64 patients, 17-45 yr H: 115 patients, 18-45 yr I: 62 patients, 25-64 yr |
HS autograft
|
BPTB autograft
|
A: 35 months B: 36 months C: 86 months D: 36 months E: 24 months F: 24 months G: 36 months H: 24 months I: 105 months
Incomplete outcome data, N (intervention/control) A: 2/0 B: 6/6 C: 3/2 D: 3/5 E: 12/4 F: 3/0 G: 5/3 H: 10/8 I: 9/5
|
1. Failure No definition reported
Individual trials results were not reported. Total n/N HT: 17/340 BPTB: 11/293
Pooled RR: 1.37 (95% CI 0.67 to 2.81) favouring BPTB Heterogeneity (I2): 23%
2. Clinimetrics Pivot shift test, Lachman test
Pivot shift test (negative) Effect measure: RR (95%CI) A: 0.82 (0.58 till1.14) B: 0.68 (0.47 till 1.01) D: 0.84 (0.71 till 1.00) F: 0.85 (0.72 till 1.01) H: 1.02 (0.90 till 1.15)
Pooled RR: 0.87 (95% CI 0.79 to 0.96) favouring HT Heterogeneity (I2): 47%
Lachman test (negative) Individual trials results were not reported. Total n/N HT: 40/85 BPTB: 44/78
Pooled RR: 0.65 (95% CI 0.18 to 2.34) favouring HT Heterogeneity (I2): ? (P<0.0001)
3. Adverse events Anterior knee pain
Anterior knee pain Individual trials results were not reported. Total n/N HT: 35/161 BPTB: 41/118
Pooled RR: 0.66 (95% CI 0.45 to 0.96) favouring HT Heterogeneity (I2): ? (P=0.88)
Kneeling pain Effect measure: RR (95%CI) C: 0.88 (0.51 till 1.52) D: 0.39 (0.20 till 0.76) E: 0.12 (0.04 till 0.33) F: 0.46 (0.26 till 0.82) G: 1.20 (0.60 till 2.42) H: 0.21 (0.01-4.31)
Pooled RR: 0.49 (95% CI 0.27 to 0.91) favouring HT Heterogeneity (I2): 73%
4. PROMS IKDC score
IKDC (normal/nearly normal versus abnormal/severely) Effect measure: RR (95%CI) A: 0.81 (0.67 till 0.98) B: 1.06 (0.82 till 1.37) C: 1.07 (0.65 till 1.76) D: 1.28 (1.00 till 1.65) E: 1.30 (0.91 till 1.86) F: 1.01 (0.89 till 1.14) I: 1.13 (0.94 till 1.36)
Pooled RR: 1.05 (95% CI 0.93 to 1.19) favouring BPTB Heterogeneity (I2): 53% |
Brief description of author’s conclusion: There was a statistically significant lower rate of negative Pivot test, anterior knee pain and kneeling pain in the HT group than in the BPTB group.
Level of evidence: 1. Failure: VERY LOW Due to risk of bias (inadequate allocation concealment) and imprecision (too few patients with a failure and in total included in the meta-analysis)
2. Clinimetrics: Pivot shift test: MODERATE Due to risk of bias (inadequate allocation concealment) Lachman test: VERY LOW Due to risk of bias (inadequate allocation concealment), inconsistency (based on a p-value of <0.0001) and imprecision (too few patients with an event)
3. Adverse event: Anterior knee pain: LOW Due to risk of bias (inadequate allocation concealment) and imprecision (too few patients with an event) Kneeling pain: VERY LOW Due to risk of bias (inadequate allocation concealment), inconsistency (two studies reported no effect and three studies a decreased effect) and imprecision (too few patients with an event)
4. PROMS: MODERATE Due to risk of bias (inadequate allocation concealment)
|
|
Autograft versus allograft |
|||||||
|
Zeng, 2016 |
SR and meta-analysis of RCTs and SRs
Literature search up to June 2014
A: Lawhorn, 2012 B: Sun, 2011a C: Sun, 2011b D: Noh, 2011 E: Sun 2009a F: Sun 2009b G: Gorschewsky, 2002 H: Bi, 2013 I: Sun, 2014
Study design: RCT
Setting and Country: not reported
Source of funding: Non-commercial
|
Inclusion criteria SR:
Exclusion criteria SR:
9 RCT’s included
N, mean age, sex (auto/allograft) A: 102 patients, 30.3/33.3 yr, 59/79% male B: 186 patients, 29.6/31.2 yr, 78/82% male C: 67 patients, 30.9/30.3 yr, 78/77% male D: 64 patients, 32/22 yr, 81/81% male E: 156 patients, 31.7/32.8 yr, 80/79% male F: 67 patients, 29.7/31.8 yr, 73/65% male G: 301 patients, 31.6/34.4 yr, 76/73% male H: 79 patients, 33/34 yr, sex NR I: 282 patients, 27.5/27.1 yr, 69/73% male
|
Autograft A: hamstring tendon (HT) B: HT C: HT D: HT E: bone-patellar-tendon-bone (BPTB) F: BPTB G: BPTB H: HT I: HT
|
Allograft A: Anterior tibialis, freshfrozen, nonirradiated, nonechemically treated B: HT, fresh-frozen, nonirradiated C: HT, fresh-frozen, irradiated, dose of 2.5 Mrad, thawed in sterile physiological fluid at room temperature D: Free-tendon Achilles, fresh-frozen E: BPTB, fresh-frozen, nonirradiated F: BPTB, fresh-frozen, nonirradiated, and BPTB, fresh-frozen, irradiated, dose of 2.5 Mrad, thawed in sterile physiological fluid at room temperature G: BPTB, irradiated, gamma ray, 1.5 Mrad and 15 kGy, osmotic treatment, oxidation, solvent drying H: Soft-tissue graft, Co-60 ray, -80°C preservation, unfreezing at room temperature, washed by gentamicin saline solution, soaking in povidone-iodine (0.2 g/L) for 10 min I: Deep-frozen tibialis anterior tendon nonirradiated
|
End of follow-up A: >24 months B: range 72-120 months C: range 30-56 months D: auto 28.1/ allo 31.6 months E: range 48 till 96 months F: range 13 till 24 months G: range 10 till 32 months H: range 36 till 57 months I: >36 months
|
1. Failure Including revision surgery, graft rupture, +2 pivot shift or higher, and side-to-side arthrometer difference >5 mm)
Effect measure: RR (95%CI) A: 2.67 (0.11-64.10) B: 0.91 (0.35 till 2.42) C: 0.26 (0.08 till 0.86) D: 0.32 (0.04 till 2.95) E: 0.88 (0.28 till 2.76) F: 1.42 (0.26 till 7.66 & 0.34 (0.09 till 1.37) G: 0.23 (0.09-0.60)
Pooled effect (random effects model): RR: 0.47 (95% CI 0.31 to 0.73) favouring autograft Heterogeneity (I2): 23% (p=0.24)
2. Clinimetrics Pivot shift test, Lachman test; instrumented laxity test)
Pivot-shift test Effect measure: RR (95%CI) A: 0.97 (0.85 till 1.10) B: 1.01 (0.93 till 1.10) C: 1.50 (1.11 till 2.01) D: 1.25 (0.93 till 1.68) E: 1.01 (0.93 till 1.10) F: 1.03 (0.87 till 1.21) & 1.51 (1.12 till 2.02) G: 1.12 (0.98 till 1.28) H: 1.03 (0.92 till 1.16) I: 0.94 (0.85till 1.05)
Pooled RR: 1.05 (95% CI 0.99 to 1.13) favouring autograft Heterogeneity (I2): 57%
Lachman test Effect measure: RR (95%CI) B: 1.02 (0.89 till 1.16) C: 2.50 (1.46 till 4.26) D: 1.25 (0.93 till 1.68) E: 1.00 (0.86 till1.17) F: 1.06 (0.83till 1.35) & 2.64 (1.51 till 4.61) G: 1.09 (0.96 till 1.23) H: 1.10 (0.85 till 1.42)
Pooled RR: 1.18 (95% CI 1.02 to 1.36) favouring autograft Heterogeneity (I2): 71%
Instrumented laxity test Effect measure: MD (95%CI) B: -0.20 (-0.43 to 0.03) C: -3.10 (-4.21 to -1.99) D: -0.50 (-1.58 to 0.58) E: 0.00 (-0.25 to 0.25) F: -0.20 (-0.62 to 0.22 & -3.10 (-4.38 to -1.82)
Pooled MD: -0.88 (-1.47 to -0.28) favouring autograft Heterogeneity (I2): 90%
3. Adverse events Not reported
4. PROMS Overall IKDC score, Lysholm score
Overall IKDC Effect measure: RR (95%CI) A: 0.98 (0.93 to 1.04) B: 1.03 (0.92 to 1.12) C: 1.05 (0.89 to 1.24) D: 1.12 (0.92 to 1.36) E: 1.01 (0.94 to 1.09) F: 1.03 (0.87 to 1.21) G: 1.08 (0.90 to 1.28) H: 1.01 (0.84 to 1.22) I: 1.02 (0.97 to 1.07)
Pooled RR: 1.03 (95% CI 1.00 to 1.07) favouring autograft Heterogeneity (I2): 0%
Lysholm score Effect measure: MD (95%CI) B: -1.00 (-3.45 to 1.45) C: 2.00 (-2.67 to 6.67) E: -1.00 (-3.23 to 1.23) F: -1.00 (-6.17 to 4.17) & 3.00 (-2.51 to 8.51) G: 2.77 (0.70-4.84) H: 2.00 (-1.77 to 5.77) I: -0.50 (-1.46 to 0.46)
Pooled mean difference: 0.02 (95% CI -0.71 to 0.75) Heterogeneity (I2): 44% |
Subgroup analyses for (non)irradiated allograft reported.
Brief description of author’s conclusion: statistically significant differences in favour of autograft observed for clinical failure, Lachman test, instrumented laxity test and Tegner score. In subgroup analyses outcomes only significant for autograft versus irradiated allograft.
Level of evidence: 1. Failure: VERY LOW Due to risk of bias (none used a blinding method), heterogeneity (four studies reduced risk, four studies no effect or potential increased risk), imprecision (low number of events)
2. Clinimetrics: Pivot-shift test: MODERATE Due to risk of bias (none used a blinding method) Lachman test: LOW Due to risk of bias (none used a blinding method) and imprecision (CI crosses clinical meaningful difference boundary) Instrumented laxity test: VERY LOW Due to risk of bias (none used a blinding method), inconsistency and imprecision (CI crosses clinical meaningful difference boundary)
4. PROMS: Overall IKDC score: MODERATE Due to risk of bias (none used a blinding method) Lysholm score: LOW Due to risk of bias (none used a blinding method) and imprecision (too few patients)
|
|
Double bundle versus single bundle |
|||||||
|
Zhu, 2013 |
SR and meta-analysis of RCTs
Literature search up to September 2011
A: Adachi, 2004 B: Jarvela, 2008 C: Streich, 2008 D: Jarvela, 2008 E: Ibrahim, 2009 F: Volpi, 2010 G: Aglietti, 2010 H: Sastre, 2010 I: Zaffagnini, 2011
Study design: RCT’s
Setting and Country: Not stated
Source of funding: The following was reported: “There are no further sources of funding for the study, authors, or preparation of the manuscript.” It is unclear to which other sources of funding are eluded.
|
Inclusion criteria SR:
Exclusion criteria SR:
18 trials included of which 9 trials were prospective randomized trials with a mean follow-up≥ 2 yr, which will be described.
Important patient characteristics at baseline:
N, mean age DB/SB, % male DB/SB A: 108 patients 29/29 years; 62/58% B: 60 patiënt 35/30 years; 69/76% C: 49 patiënt 30/29 years; 100/100% D: 77 patients 33.0 years; NA E: 200 patients 28 years; 100% F: 40 patients 30/27 years; 75/85% G: 70 patients 28/28 years; 80/71% H: 40 patients 31/29 years; 70/60% I: 79 patients 27/26 years; 55/51% |
Double bundle
|
Single bundle
|
End-point of follow-up (mean, mo (range)):
A: 32 (24 to 36) B: 27 (24 to 36) C: 24 (23 to 25) D: 24 (24 to 35) E: 29 (25 to 38) F: 30 (21 to 37) G: 24 (NA) H: 24 (NA) I: 103 (96 to 120)
For how many participants were no complete outcome data available? (intervention/control) Not reported
|
1. Failure Not reported
2. Clinimetrics Pivot shift test, Lachman test, instrumental laxity test (KT-1000 arthrometer)
Pivot shift test Effect measure: RR (95% CI): B: 1.29 (0.95 to 1.75) C: 1.26 (1.00 to 1.60) D: 1.16 (0.82 to 1.65) E: 2.22 (1.83 to 2.68) G: 1.26 (0.95 to 1.67) I: 1.35 (1.06 to 1.72)
Pooled effect (fixed effects model) RR 1.50 (95% CI 1.35 to 1.66) favoring SB Heterogeneity (I2): 79% (0.000)
Lachman test Effect measure: RR (95% CI): E: 1.27 (1.11 to 1.46) G: 1.06 (0.95 to 1.19)
Pooled effect (fixed effects model): RR 1.14 (95% CI 1.05 to 1.24) favoring SB Heterogeneity (I2): 75% (p=0.046)
Instrumental laxity test Effect measure: MD (95% CI): B: -0.90 (-2.06 to 0.26) C: 0.20 (-0.75 to 1.15) D: -0.40 (-1.57 to 0.77) G: -1.00 (-1.63 to -0.37)
Pooled effect (fixed effects model): MD -0.64 (95% CI- 1.08 to -0.20) favoring DB Heterogeneity (I2):350% (p=0.20)
3. Adverse events Complications
Effect measure: RR (95% CI): B: 0.48 (0.09 to 2.44) C: 0.32 (0.03 to 3.30) D: 0.32 (0.05 to 1.89) E: 4.26 (0.92 to 19.74) G: 1.00 (0.19 to 5.33) H: 0.18 (0.01 to 4.01)
Pooled effect (fixed effects model): RR 0.80 (95% CI 0.37 to 1.70) favoring DB Heterogeneity (I2): 34% (p=0.19)
4. PROMS IKDC score, Lysholm score
IKDC score Effect measure: RR (95% CI): D: 1.10 (0.63 to 1.93) E: 1.23 (1.03 to 1.83) F: 2.67 (1.32 to 5.39) I: 1.31 (1.02 to 1.69)
Pooled effect (fixed effects model):
Lysholm score Effect measure: mean difference (95% CI): B: 0.00 (-4.52 to 4.52) C: 0.30 (-3.52 to 4.12) D: -3.00 (-6.54 to 0.54)
Pooled effect (fixed effects model): MD -1.11 (95% CI –3.36 to -1.14) favoring SB Heterogeneity (I2): 0% (p=0.397) |
Level of evidence: As this review could be updated with 7 trails published after 2011, the level of evidence was not graded for the outcomes reported in this review. |
Evidence table for intervention studies (randomized controlled trials and non-randomized observational studies (cohort studies, case-control studies, case series))
This table is also suitable for diagnostic studies (screening studies) that compare the effectiveness of two or more tests. This only applies if the test is included as part of a test-and-treat strategy – otherwise the evidence table for studies of diagnostic test accuracy should be used.
|
Study reference |
Study characteristics |
Patient characteristics 2 |
Intervention (I) |
Comparison / control (C) 3 |
Follow-up |
Outcome measures and effect size 4 |
Comments |
|
BPTB versus HS autograft |
|||||||
|
Mohtadi, 2016 & Mohtadi, 2015 |
Type of study: RCT
Setting: patients presenting with isolated ACL deficiency
Country: Canada
Source of funding: Commercial |
Inclusion criteria:
Exclusion criteria:
N total at baseline: Intervention: 110 Control:220
Important prognostic factors2: Age ± SD: I: 28 (9.7) C: 28 (9.8)
Sex: I: 57% M C: 55% M
|
Patellor Tendon
|
Quadrupled hamstring tendon and double-bundle hamstring tendon
|
Length of follow-up: 2 years
Loss-to-follow-up: Intervention: N = 4 (4%) Reasons (Lost to follow-up (n=1); withdrawals (n=3))
Control: N = 4 (%) Reasons (Lost to follow-up (n=3); moved, unable to return for follow-up (n=1))
Incomplete outcome data: Intervention: N = 4 (4%) Reasons (Lost to follow-up (n=1); withdrawals (n=3))
Control: N = 4 (%) Reasons (Lost to follow-up (n=3); moved, unable to return for follow-up (n=1))
|
Not reported.
Pivot shift test (negative) at two years follow-up, n/N
I: 36/102 C: 66/211 RR (HS versus BPTB) 0.89 (95%CI: 0.64-1.23)
Lachman test Not reported
Not reported
Not reported |
|
|
Konrads, 2016 |
Type of study: RCT
Setting: Consecutive patients received ACL reconstruction
Country: Germany
Source of funding: Unclear |
Inclusion criteria:
Exclusion criteria:
N total at baseline: Intervention: 31 Control: 31
Important prognostic factors2: Were not reported per group Age (min, max): 29 years (18, 44)
Sex: 73% M
Groups comparable at baseline? Unclear
|
BPTB - the middle third of the ipsilateral patellar tendon in bone–patellar tendon–bone technique (BPTB group) with interference screw fixation at femoral and tibial
|
ST - ipsilateral semitendinosus tendon (ST group), with endobutton fixation at the femur and suture-disc fixation at the tibia. The semitendinosus tendon was prepared as a quadruple or triple graft according to its length.
|
Length of follow-up: 10 years
Loss-to-follow-up: Intervention: N = 7 (23%)
Control: N = 8 (26%) Reasons (traumatic transplant tear (n=1))
Overall, five could not be located and nine refused to attend the follow-up because of business reasons or unwillingness.
Incomplete outcome data: Intervention: N = 7 (23%)
Control: N = 8 (26%) Reasons (traumatic transplant tear (n=1))
Overall, five could not be located and nine refused to attend the follow-up because of business reasons or unwillingness. |
Not reported.
Pivot shift test, not reported
Lachman test (negative) at ten years follow-up, n/N I: 16/24 C: 12/23 RR (HS versus BPTB) 0.78 (95%CI 0.48-1.27)
Not reported
Lysholm score, mean (min, max) I: 92.0 (63, 98) C: 91.8 (62, 98) P=0.66 |
The authors declare that they have no conflict of interest. However, it is unclear whether this trial received funding.
Pivot shift test was mentioned in the methods section, but not among the results. |
|
Kautzner, 2015 |
Type of study: RCT
Setting: patients presenting with ACL deficiency
Country: Czech Republic
Source of funding: Non-commercial |
Inclusion criteria:
N total at baseline: Intervention: 75 Control: 75
Important prognostic factors2: Were not reported per group Mean age (min, max): 26 years (17, 47)
Sex: not reported
|
BTB (bone-patellar tendon-bone) Authors used a combination of crosspin femoral and interference-screw tibial fixation |
HT (hamstring)
Authors used a suspension femoral fixation and interference-screw tibial fixation |
Length of follow-up: Two years with a minimum of one year
Loss-to-follow-up: Intervention: N = 1 (2%) Reasons (not reported)
Control: N = 2 (3%) Reasons (not reported)
Incomplete outcome data: Intervention: N = 1 (2%) Reasons (not reported)
Control: N = 2 (3%) Reasons (not reported)
|
Defined as graft rupture or failure determined during arthroscopy
I: 2 (2.7%) C: 4 (5.3%) RR (HS versus BPTB): 2.00 (95%CI: 0.38-10.59)
Pivot shift test, not reported
Lachman test (positive) at two years follow-up, mean (min, max) I: 1 mm (0-12) C: 1.3 mm (0-10)
Defined as postoperative complication (DVT, wound infection)
DVT I: 4 (6%) C: 4 (6%) RR: 1.00 (95%CI: 0.26-3.85)
Wound infection I: 0 (0%) C: 1 (1%) RR (HS versus BPTB): 3.00 (95%CI: 0.12-72.5)
Lysholm score at two years follow-up, mean (SD) I: 88 (7.5) C: 90 (7.6) P=0.30 MD (HS versus BPTB) -2.0 (95%CI: -4.44 to 0.44) |
Surgery was performed by three surgeons experienced with ACL reconstructions. All patients were clinically evaluated by a single surgeon according to a standardised protocol. |
|
Razi, 2014 |
Type of study: RCT
Setting: patients presenting with ACL tearing
Country: Iran
Source of funding: Not reported |
Exclusion criteria:
N total at baseline: Intervention: 46 Control: 41
Important prognostic factors2: Age (SD): I: 30 (4.5) C: 28 (3.7)
Sex: I: 83% M C: 88% M
|
BPTB (Bone patellar bone autograft) |
ST (semitendinosus-gracilis) |
Length of follow-up: Three years
Loss-to-follow-up: Intervention: N = 9 (20%) Reasons (not reported)
Control: N = 7 (17%) Reasons (not reported)
Incomplete outcome data: Intervention: N = 9 (20%) Reasons (not reported)
Control: N = 7 (17%) Reasons (not reported)
|
Not reported
Pivot shift test (negative) at three years follow-up, n/N
I: 29/37 C: 15/34 RR (HS versus BPTB) 0.56 (95%CI: 0.37-0.85)
Lachman test (negative) at three years follow-up, n/N I: 23/37 C: 11/34 RR (HS versus BPTB) 0.52 (95%CI: 0.30-0.90)
Defined as postoperative complication (DVT, wound infection)
Deep infection I: 1 (3%) C: 2 (6%)
Wound infection I: 3 (8%) C: 2 (6%) RR (HS versus BPTB) 0.75 (95%CI: 0.13-4.26)
Patellar fracture I: 1 (3%) C: 0 (0%)
Not reported |
IKDC score is reported, unclear whether objective or subjective |
|
Double bundle versus single bundle |
|||||||
|
Liu, 2016 |
Type of study: RCT
Setting: Consecutive patients with complete, isolated chronic ACL lesions
Country: China
Source of funding: Not stated
|
Inclusion criteria:
Exclusion criteria: Examination under anesthesia or intraoperative findings did not meet inclusion criteria
N total at baseline: Intervention: 40 Control: 40
Important prognostic factors2: Age (range): I: 25 (16 to 45) C: 29 (17 to 47)
Sex: I: 80% M C: 85% M
|
Double bundle |
Single bundle |
Length of follow-up: Average of 80 months (range: 74-86 months)
Loss-to-follow-up: Intervention: N = 6 (15%) Reasons (not reported)
Control: N = 8 (20%) Reasons (n=1 revision ACL surgery; n=1 unable to receive revision surgery; n=6 not reported)
Incomplete outcome data: Intervention: N = 6 (15%) Reasons (not reported)
Control: N = 8 (20%) Reasons (n=1 revision ACL surgery; n=1 unable to receive revision surgery; n=6 not reported)
|
The following was reported in the article:
“We identified 2 traumatic failures for instability at the final follow-up in the double bundle group.”
Based on this information: I: 2 (5%) C: 0 (0%) RR: 5.00 (95%CI: 0.25-101.0)
Pivot shift test at end of follow-up, n/N
I: 11/32 C: 10/34 RR 1.17 (95%CI: 0.58-2.37)
Lachman test (positive) at end of follow-up, n/N I: 0/37 C: 0/34
Defined as intra- and postoperative complication
I: 0 (0%) C: 0 (0%) RR: 1.00 (0.02 to 49.2)
IKDC and Lysholm
IKDC score at the end of follow-up, mean (range) I: 92.2 (71.6-100) C: 93.4 (59.8-100)
Lysholm score at the end of follow-up, mean (range) I: 95.4 (76 to 100) C: 95.7 (66 to 100) P=0.438 |
|
|
Karikis, 2016
Two-year follow-up data from Ahlden, 2013 |
Type of study: RCT
Setting: unselected group of patients without regard to age (if >18 years), sex, or activity level.
Country: Sweden
Source of funding: None commercial
|
Inclusion criteria:
Exclusion criteria:
N total at baseline: Intervention: 53 Control: 52
Important prognostic factors2: Age (SD): I: 30 (9) C: 28 (9)
Sex: I: 66% M C: 70% M |
Double bundle |
Single bundle |
Length of follow-up: Five years
Loss-to-follow-up: Intervention: N = 7 (13%) Reasons (n=6 lost to follow-up; n=1 sustained contralateral femur fracture)
Control: N = 11 (21%) Reasons (n=2 did not receive allocated intervention; n=9 lost to follow-up)
Incomplete outcome data: Intervention: N = 7 (13%) Reasons (n=6 lost to follow-up; n=1 sustained contralateral femur fracture)
Control: N = 11 (21%) Reasons (n=2 did not receive allocated intervention; n=9 lost to follow-up)
|
Not reported
Pivot shift test (positive) at end of follow-up, n/N
Two year follow-up I: 10/50 C: 15/48 RR 0.64 (95%CI: 0.32-1.28)
Five year follow-up I: 7/45 C: 4/36 RR 1.40 (95%CI: 0.44-4.41)
Lachman test (positive) at end of follow-up, n/N
Two year follow-up I: 23/50 C: 24/48 RR 0.92 (95%CI: 0.61-1.39)
Five year follow-up I: 25/45 C: 18/36 RR 1.11 (95%CI: 0.73-1.69)
Not reported
Defined as Lysholm score at the end of follow-up
Two year follow-up, median (range) I: 89 (39 to 100) C:89 (49 to 100) P=0.77
Five year follow-up, mean (SD) I: 90.1 (9.1) 46 C: 84.3 (21.2) 41 MD 5.80 (95%CI: -1.02 to 12.63) |
Only the results from the two follow-up will be used in the meta-analysis as the majority of the trials followed patients up for about two years. |
|
Zhang, 2014 |
Type of study: RCT
Setting: patients with a symptomatic rupture of the ACL
Country: China
Source of funding: None (“No funds were received in support of this study)” |
Inclusion criteria:
Exclusion criteria:
N total at baseline: Intervention: 50 Control: 58
Important prognostic factors2: Age (range): 31 (22-51)
Sex: 60% M
|
Double bundle |
Single bundle |
Length of follow-up: Two years
Loss-to-follow-up: Intervention: N = 10 (20%) Reasons (n=5 not available for clinical examination at 24 months; n=1 rerupture; n=1 ACL injury in the opposite knee; n=2 subsequent knee surgery and n=1 meniscus surgery)
Control: N = 17 (29%) Reasons (n=9 not available for clinical examination at 24 months; n=2 rerupture; n=1 ACL injury in the opposite knee; n=3 subsequent knee surgery and n=2 meniscus surgery)
Additionally, three patients could not be traced. It is unclear whether they used a single bundle or double reconstruction on these patients.
Incomplete outcome data: Intervention: N = 10 (20%) Reasons (n=5 not available for clinical examination at 24 months; n=1 rerupture; n=1 ACL injury in the opposite knee; n=2 subsequent knee surgery and n=1 meniscus surgery)
Control: N = 17 (29%) Reasons (n=9 not available for clinical examination at 24 months; n=2 rerupture; n=1 ACL injury in the opposite knee; n=3 subsequent knee surgery and n=2 meniscus surgery) |
Reported as rerupture
I: 1 (2%) C: 2 (3%) RR 0.58 (95%CI: 0.05-6.21)
Pivot shift test Not reported
Lachman test (positive) at end of follow-up, n/N I: 7/40 C: 8/41 RR 0.89 (95%CI: 0.36-2.24)
Not reported
Defined as Lysholm score at the end of follow-up, mean (SD)
I: 92.2 (7.8) 40 C:90.7 (8.0) 41 MD 1.5 (95%CI: -2.00 to 5.00)
|
Pivot shift test is mentioned in the methods section; however, no results were reported in the results section. |
|
Koken, 2014 |
Type of study: RCT
Setting: patients with ACL deficiency
Country: Turkey
Source of funding: Not stated |
Inclusion criteria:
Exclusion criteria:
N total at baseline: Intervention: 37 Control: 30
Important prognostic factors2: Age (range): 27.6 (16-56) yr
Sex: 97% M |
Single bundle |
Double bundle |
Mean length of follow-up: 25.8 months (range 18 to 72)
Loss-to-follow-up: Not stated
Incomplete outcome data: Not stated
|
Not reported
Pivot shift test (positive) I: 0/37 C: 0/30
Lachman test (positive) at end of follow-up, n/N I: 0/37 C: 0/30
Not reported
Defined as KOOS score at 6 months and 1 yr follow-up, mean (SD)
6 months follow-up, mean (range) I: 79.4 (60.5 to 93.6) C: 81.7 (50.9 to 95.5)
1 year follow-up, mean (range) I: 80.3 (60.7 to 95.0) C: 83.5 (50.6 to 95.0) P<0.05
IKDC score (normal/nearly normal versus abnormal/severely) at end of follow-up, n/N I: 35/37 C: 29/30 RR 0.98 (95%CI: 0.88-1.08) |
|
|
Ventura, 2013 |
Type of study: RCT
Setting: patients with an isolated ACL lesion
Country: Italy
Source of funding: Not stated |
Inclusion criteria:
Exclusion criteria:
N total at baseline: Intervention: 40 Control: 40
Important prognostic factors2: Age (SD): I: 28 (9) C: 28 (6)
Sex: I: 63% M C: 65% M |
Double bundle |
Single bundle |
Length of follow-up: Two years
Loss-to-follow-up: Not stated
Incomplete outcome data: Not stated
|
Not reported
Pivot shift test (positive) I: 4/40 C: 16/40 RR 0.25 (95%CI: 0.09-0.68)
Lachman test (positive) at end of follow-up, n/N I: 1/40 C: 2/40 RR 0.50 (95%CI: 0.05-5.30)
The following was reported: “No major complications were observed in all patients considered.”
Defined as IKDC-2000 score at the end of follow-up, mean (SD)
I: 80.8 (9.9) 40 C: 78.4 (10.3) 40 MD 2.3 (95%CI: -2.10 to 6.90) |
|
|
Soumalainen, 2012 |
Type of study: RCT
Setting: Not stated
Country: Finland
Source of funding: Not stated |
Inclusion criteria:
N total at baseline: Intervention: 30 Control: 60
Important prognostic factors2: Age (SD): I: 28 (9) C: 28 (6)
Sex: I: 63% M C: 65% M
|
Double bundle |
Single bundle with bio absorbable screw fixation and single bundle metallic screw fixation (groups were taken together) |
Length of follow-up: Five years
Loss-to-follow-up: Intervention: N=10 (33%) Reasons (n=1 revision surgery; n=9 lost to follow-up)
Control: N=15 (25%) Reasons (n=10 revision surgery; n=5 lost to follow-up)
Incomplete outcome data: Intervention: N=10 (33%) Reasons (n=1 revision surgery; n=9 lost to follow-up)
Control: N=15 (25%) Reasons (n=10 revision surgery; n=5 lost to follow-up)
|
Defined as requiring ACL revision surgery, n/N
I: 1/30 C: 10/60 RR 0.20 (95%CI: 0.03-1.49)
Pivot shift test (positive) I: 13/20 C: 26/45 RR 1.13 (95%CI: 0.75-1.69)
KT-1000, mean, mm I: 1.6 (3.0) C: 2.3 (3.3) MD -0.70 (95%CI: -2.43 to 1.03)
Not reported
Lysholm score at the end of follow-up, mean (SD)
I: 90 (9) C:86.5 (15.1) MD 3.5 (95%CI: -3.78 to 10.78)
IKDC score (normal/nearly normal versus abnormal/severely) at end of follow-up, n/N I: 16/20 C: 15/25 RR 1.33 (95%CI: 0.90-1.97) |
|
|
Núñez, 2012 |
Type of study: RCT
Setting: Urban tertiary center
Country: Spain
Source of funding: Not stated |
Inclusion criteria:
Exclusion criteria:
N total at baseline: Intervention: 30 Control: 25
Important prognostic factors2: Age (SD): I: 30 (8) C: 30 (8)
Sex: I: 90% M C: 78% M
|
Double bundle |
Single bundle |
Length of follow-up: Two years
Loss-to-follow-up: Intervention: N=1 (3%) Reasons (n=1 lost to follow-up)
Control: N=2 (8%) Reasons (n=2 lost to follow-up)
Incomplete outcome data: Intervention: N=1 (3%) Reasons (n=1 lost to follow-up)
Control: N=2 (8%) Reasons (n=2 lost to follow-up)
|
The following was reported:
“No complications due to early graft failure or superficial or deep infections were recorded.”
Not reported
The following was reported:
“No complications due to early graft failure or superficial or deep infections were recorded.”
Defined as subjective IKDC score at the end of follow-up, mean (SD)
I: 70.0 (10.5) C: 69.7 (10.6) MD 0.3 (95%CI: -5.6 to 6.21)
Defined as IKDC score at the end of follow-up, mean (SD)
I: 70.0 (10.5) 29 C: 69.7 (10.6) 23 MD -0.3 (95%CI: -6.21 to 5.61)
|
|
|
Lee, 2012 |
Type of study: RCT
Setting: Paitents with ACL-deficient knees
Country: South Korea
Source of funding: None-commercial |
Inclusion criteria:
Exclusion criteria:
N total at baseline: Intervention: 21 Control: 21
Important prognostic factors2: Age (SD): I: 31 (18-58) C: 29 (17-56)
Sex: I: 90% M C: 86% M
|
Double bundle |
Single bundle |
Length of follow-up: Two years
Loss-to-follow-up: Intervention: N=2 (10%) Reasons (n=2 lost to follow-up)
Control: N=3 (14%) Reasons (n=3 lost to follow-up)
Incomplete outcome data: Intervention: N=2 (10%) Reasons (n=2 lost to follow-up)
Control: N=3 (14%) Reasons (n=3 lost to follow-up)
|
Not stated
Pivot shift test (positive) I: 6/19 C: 6/18 RR 0.95 (95%CI: 0.37-2.40)
Lachman test (positive) I: 5/19 C: 8/18 RR 0.59 (95%CI: 0.24-1.47)
KT-1000, mean, mm I: 2.62 (1.27) C: 2.74 (1.65) MD -0.12 (95%CI: -1.10 to 0.86)
Not reported
Defined as Lysholm score at the end of follow-up, mean (SD)
I: 87.4 (12.7) C:81.4 (14.5) MD 6.0 (95%CI: -3.08 to 15.08)
IKDC score Subjective IKDC score at the end of follow-up, mean (SD)
I: 79.6 (12.9) C: 70.9 (11.1) MD 8.7 (95%CI: 0.65-16.75)
Objective IKDC score (normal/nearly normal versus abnormal/severely) at the end of follow-up, n/N I: 15/18 C: 16/19 RR 0.99 (95%CI: 0.75-1.31) |
|
|
Primary repair versus reconstruction |
|||||||
|
Meunier, 2007 |
Type of study: RCT
Setting: patients with acute and total rupture of the ACL
Country: Sweden
Source of funding: not reported |
Inclusion criteria:
Exclusion criteria: Not reported
N total at baseline: Intervention: 44 Control: 56
Age (range): I: 22 (14 to 30) yr C: 21 (14 to 30) yr
Sex: I: 75% M C: 63% M
Groups comparable at baseline? Not tested
|
Repair using multiple sutures that were fixed in both stumps of the ACL and passed through drill holes in the tibia and the femur, respectively, and tied over staples fixed to the external cortices of the tibial and femoral metaphyses.
|
Conservative treatment regimen regarding the ACL rupture |
Length of follow-up: 15 yr
Loss-to-follow-up: 6 Group unknown
Incomplete outcome data: 12 Group unknown
|
1. Failure/rerupture Defined as 2nd treatment of meniscus injury, n/N I: 5/42 C: 18/52 RR (S versus NS) 0.41 (95%CI: 0.17-1.04) P=0.06
2. Clinimetrics. Lachman positive, n/N I: 29/42 C: 46/52 RR (Sar/Sr versus NSns/NSrec) 0.87 (95%CI: 0.61-1.24) P=0.44
Pivot shift positive, n/N I: 13/42 C: 27/52 RR (Sar/Sr versus NSns/NSrec) 0.69 (95%CI: 0.39-1.22) P=0.20
3. Adverse events Not reported
4. PROMS KOOS QoL, mean (95% CI) Sar: 69 (61 to 78) Sr: 58 (44 to 73) NSns: 64 (55 to 74) NSrec: 59 (49 to 70) P=NS
Lysholm score at 15-yr follow-up (good or excellent versus fair), n/N I: 36/42 C: 41/52 RR (Sar/Sr versus NSns/NSrec) 0.72 (95% CI: 0.29 to 1.80) P=0.27
Lysholm score at 15-yr follow-up (good or excellent versus fair), mean (range) Sar: 95 (92 to 96) NSns: 90 (83-92) P=0.05 |
Analyses not by intention to treat principle. The following groups were made: Sr (repair without augmentation), Sar (augmented primary repair), NSns (conservative treatment, no surgery), NSrec (conservative treatment who underwent late reconstruction). For our analysis, results of Sr and Sar were taken together, and results of NSns and NSrec.
Indirect evidence: repair versus conservative treatment instead of repair versus reconstruction. |
|
Schliemann, 2017 |
Type of study: RCT
Setting: patients with acute ACL tear
Country: Germany
Source of funding: non-commercial |
Inclusion criteria:
Exclusion criteria:
N total at baseline: Intervention: 30 Control: 30
age ± SD: I: 28.2±11.4 C: 29.1±12.0
Sex: I: 50% M C: 73% M
Groups comparable at baseline? Not tested |
Dynamic intraligamentary stabilization (new technique to repair), according to the technique described by Kösters et al.
|
Anatomic single-bundle ACL reconstruction using a four-stranded semitendinosus autograft.
|
Length of follow-up: 1 year
Loss-to-follow-up: 0
Incomplete outcome data: 0
|
1. Failure/rerupture Not reported
2. Clinimetrics. Not reported
3. Adverse events Not reported
4. PROMS Lysholm score at 1-yr follow-up, mean (SD) I: 89.8 (11.0) C: 89.9 (15.5) MD (repair versus reconstruction) 0.1 (95%CI: -6.85 to 7.05) P=0.98
IKDC score at 1-yr follow-up, mean (SD) I: 85.7 (12.4) C: 84.8 (19.4) MD (repair versus reconstruction) -0.9 (95% CI: -9.32 to 7.51) P=0.83
|
|
Notes:
- Prognostic balance between treatment groups is usually guaranteed in randomized studies, but non-randomized (observational) studies require matching of patients between treatment groups (case-control studies) or multivariate adjustment for prognostic factors (confounders) (cohort studies); the evidence table should contain sufficient details on these procedures.
- Provide data per treatment group on the most important prognostic factors ((potential) confounders).
- For case-control studies, provide sufficient detail on the procedure used to match cases and controls.
- For cohort studies, provide sufficient detail on the (multivariate) analyses used to adjust for (potential) confounders.
Risk of bias
Table of quality assessment for systematic reviews of RCTs and observational studies
|
Study
First author, year |
Appropriate and clearly focused question?1
Yes/no/unclear |
Comprehensive and systematic literature search?2
Yes/no/unclear |
Description of included and excluded studies?3
Yes/no/unclear |
Description of relevant characteristics of included studies?4
Yes/no/unclear |
Appropriate adjustment for potential confounders in observational studies?5
Yes/no/unclear/notapplicable |
Assessment of scientific quality of included studies?6
Yes/no/unclear |
Enough similarities between studies to make combining them reasonable?7
Yes/no/unclear |
Potential risk of publication bias taken into account?8
Yes/no/unclear |
Potential conflicts of interest reported?9
Yes/no/unclear |
|
Zeng, 2016 |
Yes |
Yes |
No |
Yes |
NA |
Yes |
Yes |
Yes |
No |
|
Li, 2012 |
Yes |
Yes |
No |
Yes |
NA |
Yes |
Yes |
Yes |
No |
|
Zhu, 2013 |
Yes |
Yes |
No |
Yes |
NA |
Yes |
Yes |
Yes |
No |
Based on AMSTAR checklist (Shea, 2007; BMC Methodol 7: 10; doi:10.1186/1471-2288-7-10) and PRISMA checklist (Moher, 2009; PLoS Med 6: e1000097; doi:10.1371/journal.pmed1000097)
- Research question (PICO) and inclusion criteria should be appropriate and predefined.
- Search period and strategy should be described; at least Medline searched; for pharmacological questions at least Medline + EMBASE searched.
- Potentially relevant studies that are excluded at final selection (after reading the full text) should be referenced with reasons.
- Characteristics of individual studies relevant to research question (PICO), including potential confounders, should be reported.
- Results should be adequately controlled for potential confounders by multivariate analysis (not applicable for RCTs).
- Quality of individual studies should be assessed using a quality scoring tool or checklist (Jadad score, Newcastle-Ottawa scale, risk of bias table et cetera.)
- Clinical and statistical heterogeneity should be assessed; clinical: enough similarities in patient characteristics, intervention and definition of outcome measure to allow pooling? For pooled data: assessment of statistical heterogeneity using appropriate statistical tests (for example Chi-square, I2)?
- An assessment of publication bias should include a combination of graphical aids (for example funnel plot, other available tests) and/or statistical tests (for example Egger regression test, Hedges-Olken). Note: If no test values or funnel plot included, score “no”. Score “yes” if mentions that publication bias could not be assessed because there were fewer than 10 included studies.
- Sources of support (including commercial co-authorship) should be reported in both the systematic review and the included studies. Note: To get a “yes,” source of funding or support must be indicated for the systematic review AND for each of the included studies.
Risk of bias table for intervention studies (randomized controlled trials)
|
Study reference
(first author, publication year) |
Describe method of randomisation1 |
Bias due to inadequate concealment of allocation?2
(unlikely/likely/unclear) |
Bias due to inadequate blinding of participants to treatment allocation?3
(unlikely/likely/unclear) |
Bias due to inadequate blinding of care providers to treatment allocation?3
(unlikely/likely/unclear) |
Bias due to inadequate blinding of outcome assessors to treatment allocation?3
(unlikely/likely/unclear) |
Bias due to selective outcome reporting on basis of the results?4
(unlikely/likely/unclear) |
Bias due to loss to follow-up?5
(unlikely/likely/unclear) |
Bias due to violation of intention to treat analysis?6
(unlikely/likely/unclear) |
|
|
Double versus single bundle |
|||||||||
|
Liu, 2016 |
“The randomization was performed via a computer generated list of random numbers, while the patients were recruited according to the general waiting list by the hospital secretary, who was not aware of the study.” |
Unclear. Not stated |
Unlikely. “(…) they were blinded to the type of procedure performed.” |
Likely. “All ACL reconstructions were performed by a single experienced arthroscopic surgeon.” |
Unlikely. “All patients were evaluated before surgery and at final follow-up by an independent, blinded observer.” |
Unclear. No mention of a trial register. However, outcomes stated in the methods section were reported in the results section. |
Unclear. Only stated that patients were lost to follow-up, but no reason was given. |
Unclear. Not stated. |
|
|
Karikis, 2016 |
“Randomization was by closed envelopes administered by the study coordinator, who drew them from a box with equal amounts of envelopes for both study groups. Moreover, all the envelopes were sealed and opened just before the operation when the patients were anaesthetized.” |
Unclear. “(..) closed envelopes administered by the study coordinator, who drew them from a box with equal amounts of envelopes for both study groups. Moreover, all the envelopes were sealed and opened just before the operation when the patients were anaesthetized.” |
Unlikely. “(..), all the envelopes were (…) opened just before the operation when the patients were anaesthetized.” |
Unlikely. “The physical therapist was blinded to the surgical technique to which the patient had been randomized (…).” |
Unclear. Not stated. |
Unclear. No mention of a trial register. However, outcomes stated in the methods section were reported in the results section. |
Unclear. Only stated that patients were lost to follow-up, but no reason was given. |
Unclear. Not stated |
|
|
Zhang, 2014 |
“The patients were randomized preoperatively with the aid of sealed envelopes, to one of the two surgical procedures.” |
Unclear. Not stated. |
Unclear. Not stated |
Unclear. Not stated |
Unclear. Not stated |
Likely. No mention of a trial register.In the methods section is was stated that the pivot shift test was also performed. However, no results were given. |
Unclear. The main difference between groups is unavailability for follow-up. |
Unclear. Not stated. |
|
|
Koken, 2014 |
“Randomization was performed according to the patients’ date of birth. Patients whose date of birth ended with an odd number comprised group 1 and patients whose date of birth ended with an even number comprised group 2.” |
Likely |
Unclear. Not stated |
Unclear. Not stated |
Unclear. Not stated |
Unclear. No mention of a trial register. However, outcomes stated in the methods section were reported in the results section. |
Unlikely. No patients had lost to follow-up |
Unclear. Not stated. |
|
|
Ventura, 2013 |
“The study population was preoperatively randomized into either the SB group or the DB group by closed envelopes.” |
Unclear. Only stated by closed envelopes. |
Unclear. Not stated |
Unclear. Not stated |
Unlikely. “The examined knee of the patient was covered with a stockinette to prevent information being obtained about the incision site. In this way the examiner who supervised the assessment was blinded to the technique adopted. Patients’ evaluations were performed by the same team.” |
Unclear. No mention of a trial register. However, outcomes stated in the methods section were reported in the results section. |
Unclear. Not stated that the patients were all follow-up until the end of the study. |
Unclear. Not stated. |
|
|
Soumalainen, 2012 |
“Ninety patients were randomized with closed envelopes into 3 groups of ACL reconstruction (…).” |
Unclear. Only stated by closed envelopes. |
Unclear. Not stated |
Unclear. Not stated |
Unlikely. “All clinical assessments were made by a single blinded and independent examiner.” |
Unclear. No mention of a trial register. However, outcomes stated in the methods section were reported in the results section. |
Likely. In both group, at least 25% of the patients were lost to follow-up at the end of the trial. |
Unclear. Not stated. |
|
|
Núñez, 2012 |
“Patients were randomized in a 2-step process. One investigator drew up a random list of numbers using an ad hoc randomization generator specifically designed for this study. Another investigator, who was blinded to the randomization, allocated 1 of the numbers generated to each patient, who was then assigned to the group indicated.” |
Unclear |
Unclear. Not stated |
Unclear. Not stated |
Unlikely. “Assessments were carried out by an independent investigator blinded to the treatment procedures. |
Unclear. No mention of a trial register. However, outcomes stated in the methods section were reported in the results section. |
Unlikely. Only 1 and 2 patients had lost to follow-up data in each group. |
Unclear. Not stated. |
|
|
Primary repair versus reconstruction |
|||||||||
|
Meunier, 2007 |
Randomisation based on birth year (odd or even), in a consecutive order |
Likely |
Unclear, not stated |
Unclear, not stated |
Unlikely |
Unlikely, results reported of outcomes described in methods |
Unlikely |
Likely, results only stratified to surgery during follow-up |
|
|
Schliemann, 2017 |
Block randomization, using a sealed envelope |
Unlikely |
Unclear, envelope opened in theatre just before surgery but not stated if participant was aware |
Likely |
Unclear |
Unlikely, results reported of outcomes described in methods |
Unlikely, no loss to follow-up |
Unlikely |
|
- Randomisation: generation of allocation sequences have to be unpredictable, for example computer generated random-numbers or drawing lots or envelopes. Examples of inadequate procedures are generation of allocation sequences by alternation, according to case record number, date of birth or date of admission.
- Allocation concealment: refers to the protection (blinding) of the randomisation process. Concealment of allocation sequences is adequate if patients and enrolling investigators cannot foresee assignment, for example central randomisation (performed at a site remote from trial location) or sequentially numbered, sealed, opaque envelopes. Inadequate procedures are all procedures based on inadequate randomisation procedures or open allocation schedules.
- Blinding: neither the patient nor the care provider (attending physician) knows which patient is getting the special treatment. Blinding is sometimes impossible, for example when comparing surgical with non-surgical treatments. The outcome assessor records the study results. Blinding of those assessing outcomes prevents that the knowledge of patient assignement influences the proces of outcome assessment (detection or information bias). If a study has hard (objective) outcome measures, like death, blinding of outcome assessment is not necessary. If a study has “soft” (subjective) outcome measures, like the assessment of an X-ray, blinding of outcome assessment is necessary.
- Results of all predefined outcome measures should be reported; if the protocol is available, then outcomes in the protocol and published report can be compared; if not, then outcomes listed in the methods section of an article can be compared with those whose results are reported.
- If the percentage of patients lost to follow-up is large, or differs between treatment groups, or the reasons for loss to follow-up differ between treatment groups, bias is likely. If the number of patients lost to follow-up, or the reasons why, are not reported, the risk of bias is unclear.
- Participants included in the analysis are exactly those who were randomized into the trial. If the numbers randomized into each intervention group are not clearly reported, the risk of bias is unclear; an ITT analysis implies that (a) participants are kept in the intervention groups to which they were randomized, regardless of the intervention they actually received, (b) outcome data are measured on all participants, and (c) all randomized participants are included in the analysis.
Verantwoording
Beoordelingsdatum en geldigheid
Laatst beoordeeld : 26-10-2018
Voor het beoordelen van de actualiteit van deze richtlijn is de werkgroep niet in stand gehouden. Uiterlijk in 2023 bepaalt het bestuur van de Nederlandse Orthopaedische Vereniging of de modules van deze richtlijn nog actueel zijn. Op modulair niveau is een onderhoudsplan beschreven. Bij het opstellen van de richtlijn heeft de werkgroep per module een inschatting gemaakt over de maximale termijn waarop herbeoordeling moet plaatsvinden en eventuele aandachtspunten geformuleerd die van belang zijn bij een toekomstige herziening (update). De geldigheid van de richtlijn komt eerder te vervallen indien nieuwe ontwikkelingen aanleiding zijn een herzieningstraject te starten.
De Nederlandse Orthopaedische Vereniging is regiehouder van deze richtlijn en eerstverantwoordelijke op het gebied van de actualiteitsbeoordeling van de richtlijn. De andere aan deze richtlijn deelnemende wetenschappelijke verenigingen of gebruikers van de richtlijn delen de verantwoordelijkheid en informeren de regiehouder over relevante ontwikkelingen binnen hun vakgebied.
Algemene gegevens
De richtlijnontwikkeling werd ondersteund door het Kennisinstituut van de Federatie Medisch Specialisten (www.kennisinstituut.nl) en werd gefinancierd uit de Stichting Kwaliteitsgelden Medisch Specialisten (SKMS).
Doel en doelgroep
Doel
Deze richtlijn beoogt uniform beleid ten aanzien van de diagnostiek, indicatiestelling, behandeling en nazorg van patiënten met voorste kruisbandletsel.
Doelgroep
Deze richtlijn is geschreven voor alle leden van de beroepsgroepen die betrokken zijn bij de zorg voor patiënten met VKB-letsel, zoals, maar niet beperkend tot, orthopedisch chirurgen, fysiotherapeuten en andere professionals, zoals revalidatieartsen, chirurgen en sportartsen. Deze andere beroepsgroepen worden betrokken in de knelpuntenanalyse en indien van toepassing toegevoegd aan de richtlijnwerkgroep. Aangezien richtlijnen de klinische besluitvorming ondersteunen is de richtlijn ook bedoeld voor patiënten met VKB-letsel.
Samenstelling werkgroep
Voor het ontwikkelen van de richtlijn is in 2016 een multidisciplinaire werkgroep ingesteld, bestaande uit vertegenwoordigers van alle relevante specialismen die betrokken zijn bij de zorg voor patiënten met voorste kruisbandletsel te maken hebben.
De werkgroepleden zijn door hun beroepsverenigingen gemandateerd voor deelname.
De werkgroep is verantwoordelijk voor de integrale tekst van deze richtlijn.
Werkgroep
- Dhr. dr. D.E. (Duncan) Meuffels, orthopaedisch chirurg, werkzaam in het Erasmus MC te Rotterdam, (NOV) (voorzitter)
- Dhr. prof. dr. R.L. (Ron) Diercks, orthopaedisch chirurg, werkzaam in het UMCG te Groningen, (NOV)
- Dhr. dr. R. (Roy) Hoogeslag, orthopaedisch chirurg, werkzaam in de OCON Sportmedische kliniek te Hengelo, (NOV)
- Dhr. dr. R.W. (Reinoud) Brouwer, orthopaedisch chirurg, werkzaam in het Martini Ziekenhuis te Groningen, (NOV)
- Dhr. dr. R.P.A. (Rob) Janssen, orthopaedisch chirurg-traumatoloog, werkzaam in het Maxima Medisch Centrum te Eindhoven/Veldhoven, (NVA)
- Dhr. drs. P.A. (Peter) Leenhouts, traumachirurg, werkzaam in het AMC te Amsterdam, (NvVH)
- Dhr. drs. E.A. (Edwin) Goedhart, manager sportgeneeskunde, werkzaam bij de KNVB, (VSG)
- Dhr. dr. A.F. (Ton) Lenssen, coördinator onderzoek, werkzaam in het Maastricht UMC te Maastricht, (KNGF)
Meelezers:
- Patiëntenfederatie Nederland te Utrecht (Patiëntenfederatie Nederland)
Met ondersteuning van:
- Mw. dr. J. (Janneke) Hoogervorst-Schilp, adviseur, Kennisinstituut van de Federatie Medisch Specialisten
- Mw. dr. B.H. (Bernardine) Stegeman, adviseur, Kennisinstituut van de Federatie Medisch Specialisten
Belangenverklaringen
De KNMG-Code ter voorkoming van oneigenlijke beïnvloeding door belangenverstrengeling” is gevolgd. Alle werkgroepleden hebben schriftelijk verklaard of ze in de laatste drie jaar directe financiële belangen (betrekking bij een commercieel bedrijf, persoonlijke financiële belangen, onderzoeksfinanciering) of indirecte belangen (persoonlijke relaties, reputatie management, kennisvalorisatie) hebben gehad. Een overzicht van de belangen van werkgroepleden en het oordeel over het omgaan met eventueel belangen vindt u in onderstaande tabel. De ondertekende belangenverklaringen zijn op te vragen bij het secretariaat van het Kennisinstituut van de Federatie Medisch Specialisten.
|
Werkgroeplid |
Functie |
Nevenfuncties |
Gemelde belangen |
Ondernomen actie |
|
Meuffels |
Orthopaedisch chirurg en staflid afdeling orthopaedie, Erasmus MC, Universitair Medisch Centrum Rotterdam |
Buitengewoon staflid revalidatie centrum Rijndam (onbetaald) |
Voorzitter congrescommissie en bestuurslid Nederlandse Arthoscopie Vereniging,
ZonMW: |
Geen actie (onderzoek niet gesponsord door de industrie) |
|
Diercks |
Orthopaedisch chirurg, Hoogleraar klinische sportgeneeskunde UMCG |
Redacteur, leerboek sportgeneeskunde; royalties auteur, leerboek voor orthopedie: royalties board of editors, American Jornal of Sports medicine: onbetaald reviewer voor wetenschappelijke tijdschriften, onbetaald. Bovengenoemd onderzoeksproject zou op lange termijn kunnen uitmonden in een productontwikkeling. Hiervan is op dit moment en in de nabije toekomst geen sprake |
Gezamenlijk onderzoek UT naar verbetering richtapparaat kruisbandreconstructie, geen externe relaties, geen externe financiering. |
Geen actie (valt buiten de afbakening van de richtlijn) |
|
Goedhart |
Medisch manager SportMedisch Centrum KNVB/Bondsarts |
Diverse docentschappen gericht op sportgeneeskunde |
Vicevoorzitter Vereniging voor Sportgeneeskunde. Bestuurslid College Clubartsen en Consulenten. |
Geen actie |
|
Hoogeslag |
Orthopaedisch chirurg 90% |
Hoofd medische staf BVO FC TWENTE 10%. |
Return to sports criteria bij vermoeidheid; Rct naar reconstructie versus repair (hechten) van de vkb ruptuur, repair uitgevoerd met Ligamys van de firma Mathys, reconstructie uitgevoerd met all-inside techniek van de firma Arthrex, niet betaald door de industrie; inclusie beeindigd; biomechanische studie naar vkb hechting met niet-, statisch- en dynamisch-gewrichtsoverbruggende stabilisatie, niet betaald door de industrie; Rct naar type graft te gebruiken voor VKB-reconstructie (quadriceps, patellapees, hamstringspees), niet betaald door de industrie: includerend; Prospectief cohort naar hechting van gescheurde vkb, niet betaald door de industrie: METC pending |
Geen actie |
|
Leenhouts |
Traumachirurg Academisch Medisch Centrum in Amsterdam |
Geen |
Geen |
Geen actie |
|
Brouwer |
Orthopaedisch chirurg Martin Ziekenhuis Groningen |
Voorzitter werkgroep knie (=DKS Dutch Knee Society onbetaald) |
Geen |
Geen actie |
|
Lenssen |
Wetenschappelijk onderzoeker afdeling fysiotherapie MUMC+ (0,8 Fte) |
Senior docent faculteit gezondheidszorg Zuyd Hogeschool tutor SOMT Kerpen (D) |
Geen |
Geen actie |
|
Janssen |
Opleider, orthopaedisch chirurg |
Onbetaalde nevenfuncties: |
Geen |
Geen actie |
Inbreng patiëntenperspectief
Er werd aandacht besteed aan het patiëntenperspectief door de Patiëntenfederatie Nederland als meelezer te betrekken in het richtlijnproces. Tijdens de oriënterende zoekactie werd gezocht op literatuur naar patiëntenperspectief (zie Strategie voor zoeken en selecteren van literatuur). De conceptrichtlijn is tevens voor commentaar voorgelegd aan de Patiëntenfederatie Nederland.
Methode ontwikkeling
Evidence based
Implementatie
In de verschillende fasen van de richtlijnontwikkeling is rekening gehouden met de implementatie van de richtlijn (module) en de praktische uitvoerbaarheid van de aanbevelingen. Daarbij is uitdrukkelijk gelet op factoren die de invoering van de richtlijn in de praktijk kunnen bevorderen of belemmeren. Het implementatieplan is te vinden bij de aanverwante producten.
Werkwijze
AGREE
Deze richtlijn is opgesteld conform de eisen vermeld in het rapport Medisch Specialistische Richtlijnen 2.0 van de adviescommissie Richtlijnen van de Raad Kwaliteit. Dit rapport is gebaseerd op het AGREE II instrument (Appraisal of Guidelines for Research & Evaluation II; Brouwers, 2010), dat een internationaal breed geaccepteerd instrument is. Voor een stap-voor-stap beschrijving hoe een evidence-based richtlijn tot stand komt taewordt verwezen naar het stappenplan Ontwikkeling van Medisch Specialistische Richtlijnen van het Kennisinstituut van Medisch Specialisten.
Knelpuntenanalyse
Tijdens de voorbereidende fase inventariseerden de voorzitter van de werkgroep en de adviseur de knelpunten. De werkgroep beoordeelde de aanbevelingen uit de eerdere richtlijn (NOV, 2011) op noodzaak tot revisie. Tevens is er een knelpuntenanalyse gehouden om te inventariseren welke knelpunten er in de praktijk bestaan rondom de zorg voor patiënten met VKB-letsel. De knelpuntenanalyse vond tijdens een Invitational conference plaats, gecombineerd met de knelpuntenanalyse voor de revisie van de richtlijn artroscopie. Hiervoor werden alle belanghebbende partijen (stakeholders) uitgenodigd. Knelpunten konden zowel medisch inhoudelijk zijn, als betrekking hebben op andere aspecten zoals organisatie van zorg, informatieoverdracht of implementatie.
De volgende partijen waren aanwezig bij de Invitational conference en hebben knelpunten aangedragen: Nefemed, Zorginstituut Nederland, ZimmerBiomet Nederland, Nederlandse Vereniging van Arbeids- en Bedrijfsgeneekunde (NVAB), Nederlands Huisartsen Genootschap (NHG), Nederlandse Vereniging voor Radiologie (NVvR), Nederlandse Orthopaedische Vereniging (NOV). Een verslag van de Invitational conference met daarin een overzicht van partijen die uitgenodigd waren, is opgenomen in de Knelpuntenanalyse.
Uitgangsvragen en uitkomstmaten
Op basis van de uitkomsten van de knelpuntenanalyse zijn door de voorzitter en de adviseur concept-uitgangsvragen opgesteld. Deze zijn met de werkgroep besproken waarna de werkgroep de definitieve uitgangsvragen heeft vastgesteld. Vervolgens inventariseerde de werkgroep per uitgangsvraag welke uitkomstmaten voor de patiënt relevant zijn, waarbij zowel naar gewenste als ongewenste effecten werd gekeken. De werkgroep waardeerde deze uitkomstmaten volgens hun relatieve belang bij de besluitvorming rondom aanbevelingen, als kritiek, belangrijk (maar niet kritiek) en onbelangrijk. Tevens definieerde de werkgroep tenminste voor de kritieke uitkomstmaten welke verschillen zij klinisch (patiënt) relevant vonden.
Strategie voor zoeken en selecteren van literatuur
Er werd eerst oriënterend gezocht naar bestaande buitenlandse richtlijnen en naar systematische reviews in Medline (OVID) en Cochrane Library, en literatuur over patiëntenvoorkeuren en patiëntrelevante uitkomstmaten (patiëntenperspectief). Vervolgens werd voor de afzonderlijke uitgangsvragen aan de hand van specifieke zoektermen gezocht naar gepubliceerde wetenschappelijke studies in (verschillende) elektronische databases. Tevens werd aanvullend gezocht naar studies aan de hand van de literatuurlijsten van de geselecteerde artikelen. In eerste instantie werd gezocht naar studies met de hoogste mate van bewijs. De werkgroepleden selecteerden de via de zoekactie gevonden artikelen op basis van vooraf opgestelde selectiecriteria. De geselecteerde artikelen werden gebruikt om de uitgangsvraag te beantwoorden. De databases waarin is gezocht, de zoekstrategie en de gehanteerde selectiecriteria zijn te vinden in de module met desbetreffende uitgangsvraag. De zoekstrategie voor de oriënterende zoekactie en patiëntenperspectief zijn opgenomen onder aanverwante producten.
Kwaliteitsbeoordeling individuele studies
Individuele studies werden systematisch beoordeeld, op basis van op voorhand opgestelde methodologische kwaliteitscriteria, om zo het risico op vertekende studieresultaten (risk of bias) te kunnen inschatten. Deze beoordelingen kunt u vinden in de Risk of Bias (RoB) tabellen. De gebruikte RoB instrumenten zijn gevalideerde instrumenten die worden aanbevolen door de Cochrane Collaboration: AMSTAR – voor systematische reviews; Cochrane – voor gerandomiseerd gecontroleerd onderzoek.
Samenvatten van de literatuur
De relevante onderzoeksgegevens van alle geselecteerde artikelen werden overzichtelijk weergegeven in evidence-tabellen. De belangrijkste bevindingen uit de literatuur werden beschreven in de samenvatting van de literatuur. Bij een voldoende aantal studies en overeenkomstigheid (homogeniteit) tussen de studies werden de gegevens ook kwantitatief samengevat (meta-analyse) met behulp van Review Manager 5.
Beoordelen van de kracht van het wetenschappelijke bewijs
A) Voor interventievragen (vragen over therapie of screening)
De kracht van het wetenschappelijke bewijs werd bepaald volgens de GRADE-methode. GRADE staat voor Grading Recommendations Assessment, Development and Evaluation (zie http://www.gradeworkinggroup.org/).
GRADE onderscheidt vier gradaties voor de kwaliteit van het wetenschappelijk bewijs: hoog, redelijk, laag en zeer laag. Deze gradaties verwijzen naar de mate van zekerheid die er bestaat over de literatuurconclusie Schünemann, (2013).
|
GRADE |
Definitie |
|
Hoog |
|
|
Redelijk |
|
|
Laag |
|
|
Zeer laag |
|
B) Voor vragen over diagnostische tests, schade of bijwerkingen, etiologie en prognose
De kracht van het wetenschappelijke bewijs werd eveneens bepaald volgens de GRADE-methode: GRADE-diagnostiek voor diagnostische vragen (Schünemann, 2008), en een generieke GRADE-methode voor vragen over schade of bijwerkingen, etiologie en prognose. In de gehanteerde generieke GRADE-methode werden de basisprincipes van de GRADE-methodiek toegepast: het benoemen en prioriteren van de klinisch (patiënt) relevante uitkomstmaten, een systematische review per uitkomstmaat, en een beoordeling van bewijskracht op basis van de vijf GRADE-criteria (startpunt hoog; downgraden voor risk of bias, inconsistentie, indirectheid, imprecisie, en publicatiebias).
Formuleren van de conclusies
Voor elke relevante uitkomstmaat werd het wetenschappelijk bewijs samengevat in een of meerdere literatuurconclusies waarbij het niveau van bewijs werd bepaald volgens de GRADE-methodiek. De werkgroepleden maakten de balans op van elke interventie (overall conclusie). Bij het opmaken van de balans werden de gunstige en ongunstige effecten voor de patiënt afgewogen. De overall bewijskracht wordt bepaald door de laagste bewijskracht gevonden bij een van de kritieke uitkomstmaten. Bij complexe besluitvorming waarin naast de conclusies uit de systematische literatuuranalyse vele aanvullende argumenten (overwegingen) een rol spelen, werd afgezien van een overall conclusie. In dat geval werden de gunstige en ongunstige effecten van de interventies samen met alle aanvullende argumenten gewogen onder het kopje Overwegingen.
Overwegingen (van bewijs naar aanbeveling)
Om te komen tot een aanbeveling zijn naast (de kwaliteit van) het wetenschappelijke bewijs ook andere aspecten belangrijk en worden meegewogen, zoals de expertise van de werkgroepleden, de waarden en voorkeuren van de patiënt (patient values and preferences), kosten, beschikbaarheid van voorzieningen en organisatorische zaken. Deze aspecten worden, voor zover geen onderdeel van de literatuursamenvatting, vermeld en beoordeeld (gewogen) onder het kopje Overwegingen.
Formuleren van aanbevelingen
De aanbevelingen geven antwoord op de uitgangsvraag en zijn gebaseerd op het beschikbare wetenschappelijke bewijs en de belangrijkste overwegingen, en een weging van de gunstige en ongunstige effecten van de relevante interventies. De kracht van het wetenschappelijk bewijs en het gewicht dat door de werkgroep wordt toegekend aan de overwegingen, bepalen samen de sterkte van de aanbeveling. Conform de GRADE-methodiek sluit een lage bewijskracht van conclusies in de systematische literatuuranalyse een sterke aanbeveling niet a priori uit, en zijn bij een hoge bewijskracht ook zwakke aanbevelingen mogelijk. De sterkte van de aanbeveling wordt altijd bepaald door weging van alle relevante argumenten tezamen.
Randvoorwaarden (Organisatie van zorg)
In de knelpuntenanalyse en bij de ontwikkeling van de richtlijn is expliciet rekening gehouden met de organisatie van zorg: alle aspecten die randvoorwaardelijk zijn voor het verlenen van zorg (zoals coördinatie, communicatie, (financiële) middelen, menskracht en infrastructuur). Randvoorwaarden die relevant zijn voor het beantwoorden van een specifieke uitgangsvraag maken onderdeel uit van de overwegingen bij de bewuste uitgangsvraag.
Kennislacunes
Tijdens de ontwikkeling van deze richtlijn is systematisch gezocht naar onderzoek waarvan de resultaten bijdragen aan een antwoord op de uitgangsvragen. Bij elke uitgangsvraag is door de werkgroep nagegaan of er (aanvullend) wetenschappelijk onderzoek gewenst is om de uitgangsvraag te kunnen beantwoorden. Een overzicht van de onderwerpen waarvoor (aanvullend) wetenschappelijk van belang wordt geacht, is als aanbeveling in de Kennislacunes beschreven (onder aanverwante producten).
Literatuur
Brouwers MC, Kho ME, Browman GP, et al. AGREE Next Steps Consortium. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010;182(18):E839-42. doi: 10.1503/cmaj.090449. Epub 2010 Jul 5. Review. PubMed PMID: 20603348.
Medisch Specialistische Richtlijnen 2.0 (2012). Adviescommissie Richtlijnen van de Raad Kwalitieit. https://richtlijnendatabase.nl/over_deze_site.html
Schünemann H, Brożek J, Guyatt G, et al. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group, 2013. Available from http://gdt.guidelinedevelopment.org/central_prod/_design/client/handbook/handbook.html.
Schünemann HJ, Oxman AD, Brozek J, et al. Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. BMJ. 2008;336(7653):1106-10. doi: 10.1136/bmj.39500.677199.AE. Erratum in: BMJ. 2008;336(7654). doi: 10.1136/bmj.a139. PubMed PMID: 18483053.
Ontwikkeling van Medisch Specialistische Richtlijnen: stappenplan. Kennisinstituut van Medisch Specialisten.
Zoekverantwoording
Zoekacties zijn opvraagbaar. Neem hiervoor contact op met de Richtlijnendatabase.
