Statines en hydratie tegen PC-AKI
Uitgangsvraag
Dienen statines te worden aanbevolen naast hydratie om de kans om PC-AKI te verkleinen bij patiënten met chronische nierschade die intravasculair jodiumhoudend contrastmiddel (CM) krijgen toegediend?
Aanbeveling
Overweeg het gebruik van een kortdurende (48 uur) hoge dosering atorvastatine of rosuvastatine naast hydratie in statine-naïeve patiënten met een eGFR <60 ml/min/1,73m2 die coronair-angiografie ondergaan met of zonder coronaire interventie.
Overwegingen
Patients with reduced renal function have a higher chance to develop PC-AKI. There have been multiple randomized clinical trials performed to evaluate the efficacy of statin pretreatment with conflicting results. The results of this meta-analysis strongly support the benefit of pretreatment with high doses of atorvastatin and rosuvastatin in patients with impaired renal function undergoing coronary angiography or percutaneous coronary intervention (PCI). Since most of the included trials have excluded patients with a GFR <30ml/min/1.73m2, it remains unclear whether statins will be beneficial in patients with chronic kidney disease stage 4 or 5. Uncertainty remains about the timing and duration of pretreatment. Furthermore, the additional effect of temporarily increasing the dosage of statin for a planned procedure in chronic statin using patients is unknown. No studies are available that examined the role of pretreatment with statins for prevention of PC-AKI during administration of intravenous contrast or during percutaneous replacement of aortic valves (TAVR) or placement of a left ventricular pacemaker lead (resynchronization therapy).
In conclusion, atorvastatin and rosuvastatin, when administered at high doses and before iodine-containing contrast administration in statin-naïve patients with reduced renal function undergoing coronary angiography or percutaneous coronary intervention (PCI), have a beneficial effect on the prevention of PC-AKI.
Onderbouwing
Achtergrond
Statins are primarily used in cardiovascular medicine for their lipid lowering effects. In addition to their impact on cholesterol, statins are known to have multiple non-lipid inhibiting effects on endothelial function, inflammation responses, oxidative stress, and apoptotic pathways. The pathophysiology of PC-AKI is not completely understood, but may in part be due to high oxidative stress, inflammation and vasoconstriction. Therefore, statins may be beneficial for the prevention of PC-AKI. Clinical studies with statins to prevent PC-AKI have shown conflicting results, but there seems to be a beneficial effect in patients undergoing coronary angiography or percutaneous coronary intervention (PCI), especially in the setting of an acute coronary syndrome.
Conclusies / Summary of Findings
|
Low GRADE |
There is evidence of low quality that short-term high dose rosuvastatin or atorvastatin in addition to hydration is more effective than hydration alone in the prevention of PC-AKI in statin-naive patients with eGFR <60 ml/min/1.73m2 undergoing coronary angiography or percutaneous coronary intervention.
(Liu, 2015) |
|
The effects of statins on mortality start of dialysis and number of ICU admissions are uncertain in statin-naive patients with impaired kidney function undergoing coronary angiography or percutaneous coronary intervention. |
|
|
No studies were found evaluating the effects of statins on PC-AKI in patients receiving intravenous contrast administration. |
|
|
No studies were found evaluating the effects of short term high dose statins on PC-AKI in patients already receiving chronic low dose statin therapy. |
|
|
It is unclear whether increasing the dosage of statin prior to an iodinated CM administration in non-statin-naïve patients reduces the risk of PC-AKI. |
Samenvatting literatuur
Description of studies
Risk of PC-AKI
Table 1 presents the characteristics of the included studies. The systematic review and meta-analysis of Liu, 2015 evaluated the protective effects of statins on PC-AKI, renal replacement therapy and mortality in patients undergoing coronary angiography/percutaneous intervention. Here we encompassed only the 6 RCTs (n=1684) that were included in the subgroup analysis that focused on patients with renal dysfunction. The intervention protocol differed across studies (table). In 3 of the 6 studies both patients in the intervention as the control group were given N-acetylcysteine. The definition of PC-AKI varied (table). Where possible, the definition of PC-AKI as described in the introduction of the guideline was used to interpret the results.
As Liu, 2015 did not include specific subgroup analyses including patients with renal dysfunction for the outcomes renal replacement therapy and all-cause death; the data of the original articles were included.
Abaci, 2015 was a RCT exploring the efficacy of high-dose rosuvastatin in decreasing the incidence of PC-AKI in statin-naive patients with an eGFR between 30 and 60mL/min/1.73m2 the day before elective coronary angiography. 208 patients completed the study. Patients in the intervention group were given 40mg rosuvastatin <24h before the procedure and 20mg/day for the 2 days hereafter. Patients in the control group did not get statins. All patients received intravenous hydration. The primary outcome measure was the incidence of PC-AKI, defined as a rise of ≥25% or ≥0.5mg/dl in serum creatinine from baseline, <48 or 72 hours after contrast exposure.
In the RCTs of Shehata, 2015 and Qiao, 2015, a total of 250 diabetic patients with mild to moderate chronic kidney diseases were included. The participants in the intervention group in the study of Shehata, 2015 received oral atorvastatin (80 mg daily for 48 h) before PCI. Qiao, 2015 treated the intervention group with rosuvastatin (10 mg everyday for at least 48 hours before and 72 hours after CM administration for PCI). Shehata, 2015 provided both the intervention and control group in addition to periprocedural intravenous infusion of isotonic saline with oral N-acetylcysteine.
No studies were found where statins were compared to a control group in terms of PC-AKI, in patients undergoing computed tomography with intravenous CM administration.
Table 1 Description of the study population, definition of PC-AKI, type and dose of the statins used, type of hydration and incidence of PC-AKI
|
|
Inclusion |
Definition PC-AKI |
Type and dose of statin |
Normal saline iv hydration |
Incidence statins (%) |
Incidence Control (%) |
|
Jo, 2008 |
CrCl≤60 mL/min or SCr ≥ 1.1 mg/dl
Only patients who did not recently (<30 days before procedure) used statins and undergoing coronary angiography were included. |
A relative increase in baseline SCr of ≥25% and/or an absolute increase of ≥0.5 mg/dl within 48h after contrast administration |
Simvastatin 40mg every 12h for 2 days, in total 80 mg before procedure and 80 mg after the procedure, starting the evening of the day of the procedure. |
Half-isotonic saline, 1 L/kg/h 12h before and after the procedure. |
PC-AKI: 2.5 Mortality: 0 Start dialysis: 0 ICU admission: NR
|
PC-AKI: 3.4 Mortality: 0 Start dialysis: 1 ICU admission: NR
|
|
Toso, 2010 |
CrCL<60 mL/min
Patients without current statin treatment who underwent elective coronary angiography and/or other intervention. |
Primary: absolute serum creatinine increase of ≥0.5 mg/dl over baseline within 5 days after the admission of contrast medium. Secondary: a relative increase of ≥25% over baseline within 5 days. |
Atorvastatin 80 mg/d for 48h before and after the procedure. All patients received oral NAC 1200mg twice a day from the day before to the day after procedure. |
Isotonic saline, 1 mL/kg/h, 0.9% sodium chloride 12h before and after the procedure. |
PC-AKI: primary 10/secondary: 17 Mortality: 1 Start dialysis: 0 ICU admission: NR
|
PC-AKI: primary 11/secondary: 15 Mortality: 0 Start dialysis: 1 ICU admission: NR
|
|
Patti, 2011 |
sCr ≤3mg/dl, subgroup with pre-existing renal failure: serum creatinine level ≥1.5mg/dl or CrCl≤60.
Statin-naïve patients (patients with statin treatment <3 months were excluded) with acute coronary syndrome undergoing percutaneous coronary intervention. |
Increase in serum creatinine ≥0.5mg/dl or >25% from baseline at 24h or 48h after PCI. |
Atorvastatin 80 mg 12h before and 40 mg 2 hours before angiography. All patients received atorvastatin 40mg/day after PCI. |
For patients with preprocedural serum creatinine level ≥1.5mg/dl or CrCl≤60: saline, 1mL/kg/h for ≥12h before and ≥24h after procedure. |
PC-AKI: 14.3 Mortality: NR Start dialysis: NR ICU admission: NR
|
PC-AKI: 25.6 Mortality: NR Start dialysis: NR ICU admission: NR
|
|
Quintavalle, 2012 |
eGRF≤60mL/min/1.73m2
Naïve patients scheduled for elective coronary angiography or percutaneous coronary intervention. |
Three different definitions are used. Here, we choose to include the results associated an increase of sCr concentration ≥25% at 48 hours from baseline |
Atorvastatin 80mg within 24h before procedure. All patients received oral NAC 1200mg twice, a day before and the day of the procedure. |
Sodium bicarbonate, 3mL/kg/h for 1 hour before contrast injection, 1 mL/kg/h during and for 6 hours after the procedure. |
PC-AKI:3 Mortality: NR Start dialysis: NR ICU admission: NR
|
PC-AKI: 7 Mortality: NR Start dialysis: NR ICU admission: NR
|
|
Han, 2014 |
30≤eGRF≤90 mL/min/1.73m2. Here only the results of patients with eGRF≤60 mL/min/1.73m2 were included.
Only type 2 DM patients who did not received any statin treatment for at least 14 days who were undergoing coronary/peripheral arterial diagnostic angiography, left ventriculography or percutaneous coronary intervention were included. |
Increase in sCr concentration ≥0.5 mg/dl or ≥25% above baseline at 72h after exposure. |
Rosuvastin 10 mg/day from 2 days before to 3 days after procedure. |
Isotonic saline, 0.9% sodium chloride, 1mL/kg/h started 12h before and continued for 24h after the procedure. |
PC-AKI: 3.6 Mortality: NR Start dialysis: NR ICU admission: NR
|
PC-AKI: 4.4 Mortality: NR Start dialysis: NR ICU admission: NR
|
|
Leoncini, 2014 |
sCr ≤3mg/dL or without acute renal failure or renal replacement therapy. Here the results of a subgroup with eCrCL<60mL/min are presented.
Statin-naïve patients with acute coronary syndrome undergoing early invasive strategy. |
Primary: increase in sCR concentration ≥0.5 mg/dL or ≥25% above baseline at 72h after exposure.
|
Rosuvastatin 40mg and 20mg/d. At discharge patients continued treatment (20mg/d), while patients in the control group received 40 mg/day atorvastatin. All patients received oral NAC 1200 mg twice a day from the day before through the day after procedure |
0.9% Sodium chloride, 1 mL/kg/h for 12h before and after procedure. |
PC-AKI: 8.6 Mortality: NR Start dialysis: NR ICU admission: NR
|
PC-AKI: 21.0 Mortality: NR Start dialysis: NR ICU admission: NR
|
|
Abaci, 2015 |
30≤eGRF≤60 mL/min/1.73m2.
Patients were naïve to statins and scheduled for elective coronary angiography |
Increase in serum creatinine of ≥0.5 mg/dl or ≥25% from baseline <48 or 72 hours after angiography. |
Rosuvastin 40mg <24h before procedure and then 20mg/day for 2 days. |
Isotonic saline, 1ml/kg/h, 0.9% sodium chloride for 12h before and 24h after procedure. |
PC-AKI: 5.8 Mortality: NR Start dialysis: NR ICU admission: NR
|
PC-AKI: 8.6 Mortality: NR Start dialysis: NR ICU admission: NR
|
|
Shehata, 2015 |
Diabetic patients, carrying the diagnosis of chronic stable angina and suffering from mild or moderate CKD. (eGFR 30– <90 mL/min/1.73 m
|
Increase in serum creatinine by >0.5 mg/dl (44.2 µmol/L) or >25% of baseline value |
Oral atorvastatin (80 mg daily) for 48 h before PCI, in addition to periprocedural intravenous infusion of isotonic saline and oral N-acetylcysteine. Standard parenteral hydration protocol in both groups. |
Intravenous infusion of isotonic saline and oral N-acetylcysteine, in addition to placebo formula. |
PC-AKI: 7.7 Mortality: NR Start dialysis: 0 ICU admission: NR |
PC-AKI: 20 Mortality: NR Start dialysis: 0 ICU admission: NR |
|
Qiao, 2015 |
1. Diabetic patients; 2. Mild to moderate CKD, which was defined as estimated glomerular filtration rate (eGFR) 30 to 89 ml/min per 1.73 m2; 3. Total CM administrated dose of volume ≥ 100 ml. |
Relative increase in baseline SCr of ≥ 25% and/or an absolute increase of ≥ 0.5 mg/dl (44.2 μmol/L) within 72 hours after contrast administration |
The rosuvastatin group received 10 mg everyday for at least 48 hours before and 72 hours after CM administration. |
Received no statins during the trial. All patients received intravenous hydration with isotonic saline (0.9% sodium chloride 1-1.5 ml/kg/hour for 3-12 hours before and 6-24 hours after the procedure). |
PC-AKI: 3 Mortality: NR Start dialysis: 0 ICU admission: 0 |
PC-AKI: 3 Mortality: NR Start dialysis: 0 ICU admission: 0 |
Results
Risk on PC-AKI
Pooled results of Liu (2015) showed that statin pretreatment significantly decreased the risk of PC-AKI compared to placebo treatment: risk ratio 0.51 (95% CI: 0.37 to 0.70), fixed effects model. However, this meta-analysis might have overestimated the effects of statins, as the results of one study (Quintavalle, 2012) in which PC-AKI was primarily defined as an increase CysC concentration of 10% above the baseline value at 24h after administration of contrast were included.
Abaci (2015) reported that 6 of the 103 patients in de rosuvastatin group and 9 of the 105 patients in the control group developed PC-AKI after the procedure.
Meta-analysis
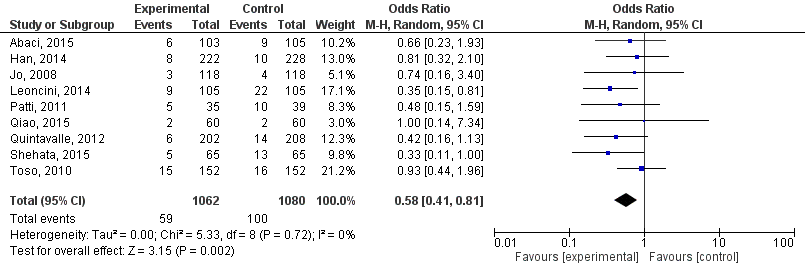
The six studies from the subgroup analysis of Liu, 2015 (adapted results for Quintavalle, 2012) and the studies of Abaci, 2015, Shehata, 2015 and Qiao, 2015 were pooled (Figure 1).
Statins significantly decreased the risk of PC-AKI: risk ratio 0.58 (95% CI: 0.41; 0.81, p=0.002, random effects model) in patients undergoing coronary angiography/percutaneous interventions.
Figure 1 Meta-analysis of studies in patients undergoing coronary angiography/percutaneous interventions
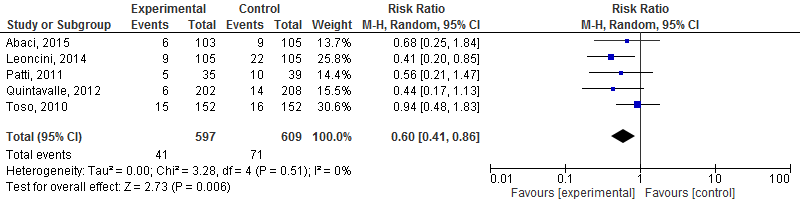
A separate meta-analysis (Figure 2) was performed to determine the effects of high dose rosuvastatin or atorvastatin on the risk of PC-AKI.
High dose rosuvastatin or atorvastatin significantly decreased the risk of PC-AKI: risk ratio 0.60 (95% CI: 0.41; 0.86, p=0.006, random effects model) in patients undergoing coronary angiography/percutaneous interventions.
Figure 2 Meta-analysis of studies that evaluated the effects of high dose rosuvastatin or atorvastatin on risk of PC-AKI in patients undergoing coronary angiography/percutaneous interventions
Start dialysis
In the study of Jo (2008) one patient in the placebo group needed haemodialysis for renal failure 3 days after coronary angiography. Toso (2010) reported one case of temporally hemofiltration in the placebo group. In five studies (Abaci, 2015; Han, 2014; Leoncini, 2014; Patti, 2011; Quintavalle, 2012) there were no patients with a need of dialysis, the studies did not report on this outcome, did not provide the results for this specific subgroup of patients (impaired kidney function) or did not report the results for the control and intervention group separately. Thus, in the studies that examined start of dialysis, 0/270 patients in the statin group versus 2/270 in the control group developed need of dialysis after CAG. None of the included studies were powered to detect differences in the outcome start of dialysis and the incidence of this outcome was very low. Because this very low number of cases, no conclusions can be drawn for this outcome.
Mortality
Only Toso (2010) reported one death; one patient in the atorvastatin group died from acute heart failure aggravated by major bleeding. Six studies (Abaci, 2015; Han, 2014; Leoncini, 2014; Patti, 2011; Quintavalle, 2012) did not report on this outcome, reported zero mortality, did not provide the results for this specific subgroup of patients (impaired kidney function) or did not report the results for the control and intervention group separately. None of the included studies were powered to detect differences in the outcome start of dialysis and the incidence of this outcome was very low. Because the very low number of cases, no conclusions can be drawn for this outcome.
Intensive care admission
The included studies did not report on this outcome measure.
Quality of evidence
The level of quality of evidence for the outcome PC-AKI was decreased from level high to level low due to heterogeneity in statin types and protocol and imprecision (total number of events <300 per group).
For the outcomes start dialysis and mortality, the level of evidence was decreased from high to very low, 1 point for heterogeneity and 2 points for gross imprecision.
Zoeken en selecteren
To answer our clinical question a systematic literature analysis was performed for the following research question:
Can statins when compared to no statins reduce the incidence of PC-AKI in patients with pre-existent reduced kidney function receiving intravascular contrast?
P (patient category) Patients undergoing radiological examinations with reduced kidney function receiving intravascular contrast.
I (intervention) statins in combination with hydration.
C (comparison) Hydration alone or no preventive measures.
O (outcome) PC-AKI, start dialysis, mortality, intensive care admission.
Relevant outcome measures
The working group considered PC-AKI, mortality and start dialysis critical outcome measures for the decision making process and the intensive care admission important outcome measures for the decision-making process.
A difference of at least 10% in relative risk was defined as a clinically relevant difference; by expert opinion of the working group (no literature was available to substantiate the decision). To illustrate, if PC-AKI occurs with an incidence of 10% in the patient population, a difference of 10% of relative risk would mean a difference of 1% in absolute risk. Thus the number needed to treat would be 100, ergo: a doctor would need to treat 100 patients to prevent one case of PC-AKI. When the incidence of PC-AKI is 5%, a difference of 10% in relative risk would mean a difference of 0.5% in absolute risk, and a number needed to treat of 200.
Search and select (method)
The data bases Medline (OVID) and Embase were searched from January 1995 to 12 Augustus 2015 using relevant search terms for systematic reviews (SRs) and randomized controlled trials (RCTs). This search was updated on 1 May 2017.
A total of 174 studies were found. The initial literature search produced 131 hits and the update produced 43 hits. The following inclusion criteria were applied:
- randomized controlled trial or meta-analysis;
- adult patients who underwent radiological examination using intravascular contrast media;
- patients with impaired kidney function, at least eGFR < 60ml/min/1.73m2;
- hydration types: hydration with i.v. NaCl or bicarbonate, oral hydration;
- the intervention arm consisted of patients that received statins and hydration. All types of statins and statin protocols included;
- the control arm consisted of patients that received hydration only or no preventive measures;
- studies that provided N-acetylcysteine (NAC) were included, when both groups received the same doses;
- at least one of the outcome measures was described: PC-AKI, start dialysis, mortality, and intensive care admission.
Based on title and abstract 74 studies were selected. After examination of full text, 71 studies were excluded and one study was added after cross-referencing, leaving 4 studies to be included in the literature summary. Reasons for exclusion are described in the exclusion table.
Results
Four studies were included in the literature analysis, one meta-analysis and three randomized controlled studies. The most important study characteristics and results are included in the evidence tables.
Referenties
- Abaci O, Arat Ozkan A, Kocas C, et al. Impact of Rosuvastatin on contrast-induced acute kidney injury in patients at high risk for nephropathy undergoing elective angiography. Am J Cardiol. 2015;115(7):867-71.
- Han Y, Zhu G, Han L, et al. Short-term rosuvastatin therapy for prevention of contrast-induced acute kidney injury in patients with diabetes and chronic kidney disease. J Am Coll Cardiol. 2014 Jan 7-14;63(1):62-70.
- Jo SH, Koo BK, Park JS, Et al. Prevention of radiocontrast medium-induced nephropathy using short-term high-dose simvastatin in patients with renal insufficiency undergoing coronary angiography (PROMISS) trial--arandomized controlled study. Am Heart J. 2008 Mar;155(3):499.e1-8.
- Leoncini M, Toso A, Maioli M, et al. . Early high-dose rosuvastatin and cardioprotection in the protective effect of rosuvastatin and antiplatelet therapy on contrast-induced acute kidney injury and myocardial damage in patients with acute coronary syndrome (PRATO-ACS) study. Am Heart J. 2014 Nov;168(5):792-7.
- Liu YH, Liu Y, Duan CY, et al. Statins for the Prevention of Contrast-Induced Nephropathy After Coronary Angiography/Percutaneous Interventions: A Meta-analysis of Randomized Controlled Trials. J Cardiovasc Pharmacol Ther. 2015;20(2):181-92.
- Patti G, Ricottini E, Nusca A, et al.. Short-term, high-dose Atorvastatin pretreatment to prevent contrast-induced nephropathy in patients with acute coronary syndromes undergoing percutaneous coronary intervention (from the ARMYDA-CIN [atorvastatin for reduction of myocardial damage during angioplasty--contrast-induced nephropathy] trial. Am J Cardiol. 2011 Jul 1;108(1):1-7.
- Qiao B, Deng J, Li Y, Wang X, Han Y. Rosuvastatin attenuated contrast-induced nephropathy in diabetes patients with renal dysfunction. Int J Clin Exp Med. 2015 Feb 15;8(2):2342-9. eCollection 2015.
- Quintavalle C, Fiore D, De Micco F, et al. Impact of a high loading dose of atorvastatin on contrast-induced acute kidney injury. Circulation. 2012 Dec 18;126(25):3008-16.
- Shehata M, Hamza M. Impact of high loading dose of atorvastatin in diabetic patients with renal dysfunction undergoing elective percutaneous coronary intervention: a randomized controlled trial. Cardiovasc Ther. 2015 Apr;33(2):35-41.
- Toso A, Leoncini M, Maioli M, et al. Pharmacologic prophylaxis for contrast-induced acute kidney injury. Intervent Cardiol Clin. 2010(3): 405419
Evidence tabellen
Table: Exclusion after revision of full text
|
Author and year |
Reason for exclusion |
|
Aggarwal, 2014 |
Article not found |
|
Atallah, 2004 |
Published before the SR of Liu, 2015 |
|
Ball, 2014 |
Review, not systematic |
|
Barbieri, 2014 |
Did not include subgroup analyses with patients with renal dysfunction |
|
Bidram, 2015 |
Patients with eGFR<60 excluded |
|
Bouzas-Mosquera, 2009 |
Published before the search date of SR of Liu, 2015 |
|
Cheungpasitporn, 2015 |
Did not include subgroup analyses with patients with renal dysfunction |
|
Gandhi, 2014 |
Overlapping with the systematic review of Liu, 2015, that was already included in the literature analysis |
|
Giacoppo, 2014 |
Overlapping with the systematic review of Liu, 2015, that was already included in the literature analysis |
|
Han, 2014 |
Included in the review of Liu, 2015 |
|
Hoshi, 2014 |
Renal function not compromised, observational study |
|
Jo, 2015 |
Article not available |
|
Jo, 2008 |
Included in the review of Liu, 2015 |
|
Kandula, 2010 |
Published before the SR of Liu, 2015 |
|
Kaya, 2013 |
Published before the SR of Liu, 2015 |
|
Kenaan, 2014 |
Renal function not compromised, observation study |
|
Lee, 2014 |
Overlapping with the systematic review of Liu, 2015, that was already included in the literature analysis |
|
Leoncini, 2014 |
Outcomes were the cardioprotective effects |
|
Leoncini, 2014 |
Included in the review of Liu, 2015 |
|
Li, 2012 |
Published before the SR of Liu, 2015 |
|
Liu, 2014 |
Patients with eGFR of 30-90 mL/min/1.73m2 included, compared rosuvastatin with atorvastatin |
|
Mao, 2014 |
Did not include subgroup analyses with patients with renal dysfunction |
|
Marenzi, 2015 |
Did not include subgroup analyses with patients with renal dysfunction |
|
Munoz, 2011 |
Published before the SR of Liu, 2015 |
|
Ozhan, 2010 |
Published before the SR of Liu, 2015 |
|
Pappy, 2011 |
More recent SR available |
|
Patti, 2014 |
Letter to the editor, substantial subgroup of patients has no renal dysfunction |
|
Patti, 2008 |
Published before the SR of Liu, 2015 |
|
Patti, 2011 |
Included in the review of Liu, 2015 |
|
Peruzzi, 2014 |
No separate analysis for patients with renal dysfunction |
|
Qiao, 2015 |
Patients with eGFR of 30-89 mL/min/1.73m2 included |
|
Quintavalle, 2012 |
Included in the review of Liu, 2015 |
|
Sanadgol, 2012 |
Published before the SR of Liu, 2015 |
|
Sanei, 2014 |
Patients with normal renal function included |
|
Shehata, 2015 |
Patients with eGFR of 30-90 mL/min/1.73m2 included |
|
Singh, 2014 |
Overlapping with the systematic review of Liu, 2015, that was already included in the literature analysis |
|
Takagi, 2011 |
More recent SR available |
|
Toso, 2014 |
Used the data of Leoncini, 2013 |
|
Toso, 2010 |
Included in the review of Liu, 2015 |
|
Ukaigwe, 2014 |
Overlapping with the systematic review of Liu, 2015, that was already included in the literature analysis |
|
Wu, 2015 |
Article not found |
|
Xie, 2014 |
Overlapping with the systematic review of Liu, 2015, that was already included in the literature analysis |
|
Xinwei, 2009 |
Published before the SR of Liu, 2015 |
|
Yoshida, 2009 |
Published before the SR of Liu, 2015 |
|
Yun, 2014 |
Observational study |
|
Zhang, 2011 |
More recent SR available |
|
Zhao, 2008 |
Published before the SR of Liu, 2015 |
|
Zhou, 2011 |
More recent SR available |
Table: Exclusion after revision of full text (update 2017)
|
Author and year |
Reason for exclusion |
|
Ali-Hassan-Sayegh, 2016 |
Does not meet selection criteria, references were checked |
|
Chalikias, 2016 |
Does not meet selection criteria, references were checked |
|
Fan, 2016 |
No studies included after original search |
|
Gadapa, 2016 |
Full text not available |
|
Giacoppo, 2015 |
Full text not available |
|
Jo, 2015 |
Does not meet selection criteria |
|
Li, 2016 |
Does not meet selection criteria |
|
Navarese, 2017 |
Does not meet selection criteria |
|
Rabbat, 2015 |
Abstract |
|
Subramaniam, 2016 |
Does not meet selection criteria, references were checked |
|
Thompson, 2016 |
No studies included after original search |
|
Vanmassenhove, 2016 |
No studies included after original search |
|
Wang, 2016 |
No studies included after original search |
|
Zografos, 2016 |
Full text not available |
|
Zografos, 2016 |
No studies included after original search |
|
Zografos, 2016 |
No studies included after original search |
|
Fu, 2015 |
Full text not available |
|
Gaskina, 2016 |
Abstract |
|
Gaskina, 2016 |
Abstract |
|
Maskon, 2016 |
Abstract |
|
Park, 2016 |
Full text not available |
|
Kohsravi, 2016 |
Does not meet selection criteria |
|
Li, 2016 |
Does not meet selection criteria |
Table of quality assessment for systematic reviews of RCTs and observational studies
Based on AMSTAR checklist (Shea et al.; 2007, BMC Methodol 7: 10; doi:10.1186/1471-2288-7-10) and PRISMA checklist (Moher et al 2009, PLoS Med 6: e1000097; doi:10.1371/journal.pmed1000097)
|
Study
First author, year |
Appropriate and clearly focused question?1
Yes/no/unclear |
Comprehensive and systematic literature search?2
Yes/no/unclear |
Description of included and excluded studies?3
Yes/no/unclear |
Description of relevant characteristics of included studies?4
Yes/no/unclear |
Appropriate adjustment for potential confounders in observational studies?5
Yes/no/unclear/notapplicable |
Assessment of scientific quality of included studies?6
Yes/no/unclear |
Enough similarities between studies to make combining them reasonable?7
Yes/no/unclear |
Potential risk of publication bias taken into account?8
Yes/no/unclear |
Potential conflicts of interest reported?9
Yes/no/unclear |
|
Liu, 2015 |
yes |
Yes |
No (excluded studies not referenced) |
yes |
NA |
Yes |
Unclear (different definitions of PC-AKI used among included studies) |
Unclear (funnel plot not provided for subanalysis, <10 studies included) |
Yes (none of the studies were sponsored by industry) |
- Research question (PICO) and inclusion criteria should be appropriate and predefined
- Search period and strategy should be described; at least Medline searched; for pharmacological questions at least Medline + EMBASE searched
- Potentially relevant studies that are excluded at final selection (after reading the full text) should be referenced with reasons
- Characteristics of individual studies relevant to research question (PICO), including potential confounders, should be reported
- Results should be adequately controlled for potential confounders by multivariate analysis (not applicable for RCTs)
- Quality of individual studies should be assessed using a quality scoring tool or checklist (Jadad score, Newcastle-Ottawa scale, risk of bias table etc.)
- Clinical and statistical heterogeneity should be assessed; clinical: enough similarities in patient characteristics, intervention and definition of outcome measure to allow pooling? For pooled data: assessment of statistical heterogeneity using appropriate statistical tests (e.g. Chi-square, I2)?
- An assessment of publication bias should include a combination of graphical aids (e.g., funnel plot, other available tests) and/or statistical tests (e.g., Egger regression test, Hedges-Olken). Note: If no test values or funnel plot included, score “no”. Score “yes” if mentions that publication bias could not be assessed because there were fewer than 10 included studies.
- Sources of support (including commercial co-authorship) should be reported in both the systematic review and the included studies. Note: To get a “yes,” source of funding or support must be indicated for the systematic review AND for each of the included studies.
Risk of bias table for intervention studies (randomized controlled trials)
Research question:
|
Study reference
(first author, publication year) |
Describe method of randomisation1 |
Bias due to inadequate concealment of allocation?2
(unlikely/likely/unclear) |
Bias due to inadequate blinding of participants to treatment allocation?3
(unlikely/likely/unclear) |
Bias due to inadequate blinding of care providers to treatment allocation?3
(unlikely/likely/unclear) |
Bias due to inadequate blinding of outcome assessors to treatment allocation?3
(unlikely/likely/unclear) |
Bias due to selective outcome reporting on basis of the results?4
(unlikely/likely/unclear) |
Bias due to loss to follow-up?5
(unlikely/likely/unclear) |
Bias due to violation of intention to treat analysis?6
(unlikely/likely/unclear) |
|
Shehata, 2015 |
Not described |
unclear |
Unlikely |
Unlikely |
Unlikely |
Unlikely |
Unlikely |
Unclear |
|
Qiao, 2015 |
Not described |
unclear |
Unlikely |
Unlikely |
Unlikely |
Unlikely |
Unlikely |
Unclear |
|
Abaci, 2015 |
Not described |
unclear |
Unlikely |
Unlikely |
Unlikely |
unlikely |
Unclear |
unclear |
- Randomisation: generation of allocation sequences have to be unpredictable, for example computer generated random-numbers or drawing lots or envelopes. Examples of inadequate procedures are generation of allocation sequences by alternation, according to case record number, date of birth or date of admission.
- Allocation concealment: refers to the protection (blinding) of the randomisation process. Concealment of allocation sequences is adequate if patients and enrolling investigators cannot foresee assignment, for example central randomisation (performed at a site remote from trial location) or sequentially numbered, sealed, opaque envelopes. Inadequate procedures are all procedures based on inadequate randomisation procedures or open allocation schedules..
- Blinding: neither the patient nor the care provider (attending physician) knows which patient is getting the special treatment. Blinding is sometimes impossible, for example when comparing surgical with non-surgical treatments. The outcome assessor records the study results. Blinding of those assessing outcomes prevents that the knowledge of patient assignement influences the proces of outcome assessment (detection or information bias). If a study has hard (objective) outcome measures, like death, blinding of outcome assessment is not necessary. If a study has “soft” (subjective) outcome measures, like the assessment of an X-ray, blinding of outcome assessment is necessary.
- Results of all predefined outcome measures should be reported; if the protocol is available, then outcomes in the protocol and published report can be compared; if not, then outcomes listed in the methods section of an article can be compared with those whose results are reported.
- If the percentage of patients lost to follow-up is large, or differs between treatment groups, or the reasons for loss to follow-up differ between treatment groups, bias is likely. If the number of patients lost to follow-up, or the reasons why, are not reported, the risk of bias is unclear
- Participants included in the analysis are exactly those who were randomized into the trial. If the numbers randomized into each intervention group are not clearly reported, the risk of bias is unclear; an ITT analysis implies that (a) participants are kept in the intervention groups to which they were randomized, regardless of the intervention they actually received, (b) outcome data are measured on all participants, and (c) all randomized participants are included in the analysis.
Evidence table for systematic review of RCTs and observational studies (intervention studies)
Research question:
|
Study reference |
Study characteristics |
Patient characteristics |
Intervention (I) |
Comparison / control (C) |
Follow-up |
Outcome measures and effect size |
Comments |
|
Liu, 2015
[individual study characteristics deduced from [1st author, year of publication ]]
PS., study characteristics and results are extracted from the SR (unless stated otherwise) |
SR and meta-analysis of RCTs
Literature search up to Feb 2014
A: Jo, 2008 B: Toso, 2010 C: Patti, 2011 D: Quintavalle, 2012 E: Han, 2013 F: Leoncini, 2013
Study design: RCT [parallel]
Setting and Country: Not reported
Source of funding: None was sponsored by industry
|
Inclusion criteria SR: RCTs investigating the efficacy of statins in preventing CIN compared with placebo, the treatment groups received statins before the contrast exposure at any dose, for any length of time. Studies were only included if none of the arms or both received N-acetylcysteine.
Exclusion criteria SR: Trials comparing 2 different doses of statins. Only studies that included patients with renal dysfunction (defined as eGFR≤60 mL/min/1.73m2 or creatine clearance ≤60 mL/min/1.73m2) were included here.
6 studies included
Important patient characteristics at baseline:
N A: 236 B: 304 C: 74 D: 410 E: 450 F: 210
Groups comparable at baseline? Unclear |
Describe intervention:
A: Simvastin 40mg, 12 hours for 2 days, 80mg before procedure, 80mg after the procedure B: Atorvastatin 80mg/d for 48 hours before and after the procedure versus placebo, oral NAC 1200mg 2 times day before to the day after procedure C: Atorvastatin 80 mg 12 hours before and further 40mg 2 hours before angiography D: 80mg within 24h before exposure, oral NAC 1200mg2 times/day before and the day of procedure E: Rosuvastatin 10mg from 2 days before to 3 days after procedure F: Rosuvastin 40mg followed by 20mg/d, oral NAC 1200 mg 2 times/d before and day after procedure
|
Describe control:
A: Placebo
B: Oral NAC 1200mg 2 times day before to the day after procedure
C: Placebo
D: Placebo, oral NAC 1200mg2 times/day before and the day of procedure
E: placebo
F: oral NAC 1200 mg 2 times/d before and day after procedure
|
End-point of follow-up (PC-AKI): A: within 48h after contrast administration B: within 5 days C: 48h after PCI D: 48h after from baseline value E: within 72h after contrast administration F: within 72h after contrast administration
For how many participants were no complete outcome data available? Not reported
|
Outcome measure-1: PC-AKI, defined as an increase of ≥25%SCr or SCr ≥0.5mg/dL within 48-120h.
Effect measure: RR (95% CI: A: 0.75 (0.17;3.28) B: 0.94 (0.48;1.83) C: 0.56 (0.21;1.47) D: 0.44 (0.17;1.13) E: 0.82 (0.33;2.04) F: 0.41 (0.20;0.85)
Pooled effect (fixed effects model): 0.51 (0.37;0.70) favouring intervention. I2=44%
Outcome measure-2: Mortality (cases) A: intervention=0, placebo=0 B: intervention=1, placebo=0 C: NR D: NR E: NR F: NR
Outcome measure-3: Start dialysis A: intervention=0, placebo=1 B: intervention=0, placebo=1 C: NR D: NR E: NR F: NR Outcome measure-4: ICU (not reported in any of the included studies) |
Facultative:
The result presented here involves a subgroup analyses of patients with impaired kidney function.
The results of the study of Quintavalle, 2012 are adapted (secondary outcome measure is the correct PC-AKI definition)
Liu, 2015 include a fixed analyses, the use of random analyses might be preferred given the heterogeneity found (I2=44%)
For the outcome measures mortality, start of dialysis and ICU admission, data extraction took place using the original articles of the studies included in Liu, 2015.
|
Evidence table for intervention studies (randomized controlled trials and non-randomized observational studies [cohort studies, case-control studies, case series])1
This table is also suitable for diagnostic studies (screening studies) that compare the effectiveness of two or more tests. This only applies if the test is included as part of a test-and-treat strategy – otherwise the evidence table for studies of diagnostic test accuracy should be used.
Research question:
|
Study reference |
Study characteristics |
Patient characteristics 2 |
Intervention (I) |
Comparison / control (C) 3
|
Follow-up |
Outcome measures and effect size 4 |
Comments |
|
Shehata, 2015 |
Type of study: RCT
Setting: Catheterization laboratory
Country: Egypt
Source of funding: not reported, no conflicts of interest |
Inclusion criteria: Diabetic patients, carrying the diagnosis of chronic stable angina and suffering from mild or moderate CKD. (eGFR 30– <90 mL/min/1.73 m2
Exclusion criteria: Severe CKD (e GFR <30 mL/min/1.73 m) [9], end-stage renal disease (or patients on hemodialysis), intake of potentially nephrotoxic drugs, acute myocardial infarction requiring emergency coronary intervention, cardiogenic shock. See article for a complete overview of exclusion criteria.
N total at baseline: Intervention: 65 Control: 65
Important prognostic factors2: For example age ± SD: I: 55 (6) C:57 (5)
Sex: I: 53% M C: 56% M
Contrast (mL) (mean± SD) I: 274 (8) C: 278 (11)
Contrast nephropathy risk score (mean± SD) I: NR C: NR
Groups comparable at baseline? yes, no statistical significant differences |
Describe intervention (treatment/procedure/test):
Oral atorvastatin (80 mg daily) for 48 h before PCI, in addition to periprocedural intravenous infusion of isotonic saline and oral N-acetylcysteine. Standard parenteral hydration protocol in both groups. |
Describe control (treatment/procedure/test):
Intravenous infusion of isotonic saline and oral N-acetylcysteine, in addition to placebo formula. |
follow-up: 10 days
Loss-to-follow-up: Intervention: 0
Control: 0
Incomplete outcome data: No
|
Outcome measures and effect size (include 95%CI and p-value if available):
Incidence of PC-AKI (increase in serum creatinine of ≥0.5 mg/dL or an absolute increase of ≥25% from baseline <48 or72h after contrast exposure)
Intervention group: 5/65 events, control group 13/65 events, p<0.05
Mortality, initiation of dialysis and ICU-admission not reported
|
The current study results identify a high-risk population showing a pronounced benefit upon adopting the high dose atorvastatin pretreatment approach before contrast exposure. |
|
Qiao, 2015 |
Type of study: RCT
Setting: Hospital
Country: China
Source of funding: not reported, no conflicts of interest |
Inclusion criteria: 1. Diabetic patients; 2. Mild to moderate CKD, which was defined as estimated glomerular filtration rate (eGFR) 30 to 89 ml/min per 1.73 m2; 3. Total CM administrated dose of volume ≥ 100 ml.
Exclusion criteria: Pregnancy, lactation, Ketoacidosis, Lactic acidosis, prior CM administration within 7 days of study entry. Importantly, all patients who were recent statin users (with 14 days before the procedure) were excluded. See article for a complete overview of exclusion criteria.
N total at baseline: Intervention: 60 Control: 60
Important prognostic factors2: For example age ± SD: I: 62 (8) C:62 (8)
Sex: I: 68% M C: 73% M
Contrast (mL) (mean± SD) I: 204 (75) C: 212 (85)
Contrast nephropathy risk score (mean± SD) I: NR C: NR
Groups comparable at baseline? Yes, average eGFR 60 ml/min/1.73 m2 |
Describe intervention (treatment/procedure/test):
The rosuvastatin group received 10 mg everyday for at least 48 hours before and 72 hours after CM administration. |
Describe control (treatment/procedure/test):
Received no statins during the trial. All patients received intravenous hydration with isotonic saline (0.9% sodium chloride 1-1.5 ml/kg/hour for 3-12 hours before and 6-24 hours after the procedure). |
follow-up: Between 48-72h after procedure, up to 30 days.
Loss-to-follow-up: Intervention: 0
Control: 0
Incomplete outcome data: No
|
Outcome measures and effect size (include 95%CI and p-value if available):
Incidence of PC-AKI (increase in serum creatinine of ≥0.5 mg/dL or an absolute increase of ≥25% from baseline <48 or72h after contrast exposure)
Intervention group: 2/60 events, control group 2/60 events, p<0.05
Mortality, initiation of dialysis and ICU-admission not specifically reported, but no post procedural adverse events occurred.
|
|
|
Abaci, 2015 |
Type of study: RCT
Setting: University cardiology institute, inpatients
Country: Turkey
Source of funding: not reported, no conflicts of interest |
Inclusion criteria: Patients naïve to statins and scheduled for coronary angiography with EGFR between 30 and 60 mL/min/1.73m2.
Exclusion criteria: Emergency coronary angiography, acute renal failure or end-stage renal failure requiring dialysis. See article for a complete overview of exclusion criteria.
N total at baseline: Intervention: 110 Control:110
Important prognostic factors2: For example age ± SD: I: 67.5 (8.9) C:67.7 (8.9)
Sex: I: 64% M C: 73.4% M
Contrast (mL) (mean± SD) I: 139.2 (77.4) C: 117.7 (56.8)
Contrast nephropathy risk score (mean± SD) I: 9.3 (3.9) C: 7.7 (3.4)
Groups comparable at baseline? Not completely, see contrast volume and contrast nephropathy risk (above) |
Describe intervention (treatment/procedure/test):
Patients were given 40mg rosuvastatin <24 h before coronary angiography and hereafter 20mg/day for 2 days.
|
Describe control (treatment/procedure/test):
No statin treatment |
follow-up: Between 48-72h after angiography, 6 months and 1 year.
Loss-to-follow-up: Intervention: 7 (6%) Reasons unknown
Control: 5 (5%) Reasons unknown
Incomplete outcome data: See loss to follow-up
|
Outcome measures and effect size (include 95%CI and p-value if available):
Incidence of PC-AKI (increase in serum creatinine of ≥0.5 mg/dL or an absolute increase of ≥25% from baseline <48 or72h after contrast exposure.
Intervention group: 6/103 events, control group 9/105 events. Relative risk (95%CI)= 0.71 (0.25;-2.0)
Mortality, initiation of dialysis and ICU-admission not reported
|
All patients received intravenous hydration with isotonic saline (14mL/kg/h, 0.9% sodium chloride) for 12h before and 24h after contrast exposure.
Statistical analyses not clear. Secondary outcomes (death and decrease in eGFR of ≥25% or renal failure requiring dialysis at 12 months) were reported as a composite outcome and exact data was not shown. |
Notes:
- Prognostic balance between treatment groups is usually guaranteed in randomized studies, but non-randomized (observational) studies require matching of patients between treatment groups (case-control studies) or multivariate adjustment for prognostic factors (confounders) (cohort studies); the evidence table should contain sufficient details on these procedures
- Provide data per treatment group on the most important prognostic factors [(potential) confounders]
- For case-control studies, provide sufficient detail on the procedure used to match cases and controls
- For cohort studies, provide sufficient detail on the (multivariate) analyses used to adjust for (potential) confounders
Verantwoording
Beoordelingsdatum en geldigheid
Publicatiedatum : 08-01-2018
Beoordeeld op geldigheid : 01-11-2017
Validity
The board of the Radiological Society of the Netherlands will determine at the latest in 2023 if this guideline (per module) is still valid and applicable. If necessary, a new working group will be formed to revise the guideline. The validity of a guideline can be shorter than 5 years, if new scientific or healthcare structure developments arise, that could be seen as a reason to commence revisions. The Radiological Society of the Netherlands is considered the keeper of this guideline and thus primarily responsible for the actuality of the guideline. The other scientific societies that have participated in the guideline development share the responsibility to inform the primarily responsible scientific society about relevant developments in their field.
Initiative
Radiological Society of the Netherlands
Authorization
The guideline is submitted for authorization to:
- Association of Surgeons of the Netherlands
- Dutch Association of Urology
- Dutch Federation of Nephrology
- Dutch Society Medical Imaging and Radiotherapy
- Dutch Society of Intensive Care
- Netherlands Association of Internal Medici
- Netherlands Society for Clinical Chemistry and Laboratory Medicine
- Netherlands Society of Cardiology
- Netherlands Society of Emergency Physicians
- Radiological Society of the Netherlands
Algemene gegevens
General Information
The guideline development was assisted by the Knowledge Institute of Medical Specialists (https://www.kennisinstituut.nl) and was financed by the Quality Funds for Medical Specialists (Kwaliteitsgelden Medisch Specialisten: SKMS).
Doel en doelgroep
Goal of the current guideline
The aim of the Part 1 of Safe Use of Iodine-containing Contrast Media guidelines is to critically review the present recent evidence with the above trend in mind, and try to formulate new practical guidelines for all hospital physicians to provide the safe use of contrast media in diagnostic and interventional studies. The ultimate goal of this guideline is to increase the quality of care, by providing efficient and expedient healthcare to the specific patient populations that may benefit from this healthcare and simultaneously guard patients from ineffective care. Furthermore, such a guideline should ideally be able to save money and reduce day-hospital waiting lists.
Users of this guideline
This guideline is intended for all hospital physicians that request or perform diagnostic or interventional radiologic or cardiologic studies for their patients in which CM are involved.
Samenstelling werkgroep
Working group members
A multidisciplinary working group was formed for the development of the guideline in 2014. The working group consisted of representatives from all relevant medical specialization fields that are involved with intravascular contrast administration.
All working group members have been officially delegated for participation in the working group by their scientific societies. The working group has developed a guideline in the period from October 2014 until July 2017.
The working group is responsible for the complete text of this guideline.
Working group
Cobbaert C., clinical chemist, Leiden University Medical Centre (member of advisory board from September 2015)
Danse P., interventional cardiologist, Rijnstate Hospital, Arnhem
Dekker H.M., radiologist, Radboud University Medical Centre, Nijmegen
Geenen R.W.F., radiologist, Noordwest Ziekenhuisgroep (NWZ), Alkmaar/Den Helder
Hoogeveen E.K., nephrologist, Jeroen Bosch Hospital, ‘s-Hertogenbosch
Kooiman J., research physician, Leiden University Medical Centre, Leiden
Oudemans - van Straaten H.M., internist-intensive care specialist, Free University Medical Centre, Amsterdam
Pels Rijcken T.H., interventional radiologist, Tergooi, Hilversum
Sijpkens Y.W.J., nephrologist, Haaglanden Medical Centre, The Hague
Vainas T., vascular surgeon, University Medical Centre Groningen (until September 2015)
van den Meiracker A.H., internist-vascular medicine, Erasmus Medical Centre, Rotterdam
van der Molen A.J., radiologist, Leiden University Medical Centre, Leiden (chairman)
Wikkeling O.R.M., vascular surgeon, Heelkunde Friesland Groep, location: Nij Smellinghe Hospital, Drachten (from September 2015)
Advisory board
Demir A.Y., clinical chemist, Meander Medical Center, Amersfoort, (member of working group until September 2015)
Hubbers R., patient representative, Dutch Kidney Patient Association
Mazel J., urologist, Spaarne Gasthuis, Haarlem
Moos S., resident in Radiology, HAGA Hospital, The Hague
Prantl K., Coordinator Quality & Research, Dutch Kidney Patient Association
van den Wijngaard J., resident in Clinical Chemistry, Leiden University Medical Center
Methodological support
Boschman J., advisor, Knowledge Institute of Medical Specialists (from May 2017)
Burger K., senior advisor, Knowledge Institute of Medical Specialists (until March 2015)
Harmsen W., advisor, Knowledge Institute of Medical Specialists (from May 2017)
Mostovaya I.M., advisor, Knowledge Institute of Medical Specialists
Persoon S., advisor, Knowledge Institute of Medical Specialists (March 2016 – September 2016)
van Enst A., senior advisor, Knowledge Institute of Medical Specialists (from January 2017)
Belangenverklaringen
Conflicts of interest
The working group members have provided written statements about (financially supported) relations with commercial companies, organisations or institutions that are related to the subject matter of the guideline. Furthermore, inquiries have been made regarding personal financial interests, interests due to personal relationships, interests related to reputation management, interest related to externally financed research and interests related to knowledge valorisation. The statements on conflict of interest can be requested at the administrative office of the Knowledge Institute of Medical Specialists and are summarised below.
|
Member |
Function |
Other offices |
Personal financial interests |
Personal relationships |
Reputation management |
Externally financed research |
Knowledge-valorisation |
Other potential conflicts of interest |
Signed |
|
Workgroup |
|||||||||
|
Burger |
Advisor, Knowledge Institute of Medical Specialists |
None |
None |
None |
None |
None |
None |
None |
Yes |
|
Cobbaert |
Member, physician clinical chemistry |
Head of clinical chemistry department in Leiden LUMC. Tutor for post-academic training of clinical chemists, coordinator/host for the Leiden region Member of several working groups within the Dutch Society for Clinical Chemistry and member of several international working groups for clinical chemistry |
None |
None |
Member of several working groups within the Dutch Society for Clinical Chemistry and member of several international working groups for clinical chemistry |
None |
None |
None |
Yes |
|
Danse |
Member, cardiologist |
Board member committee of Quality, Dutch society for Cardiology (unpaid) Board member Conference committee DRES (unpaid) |
None |
None |
None |
None |
None |
None |
Yes |
|
Dekker |
Member, radiologist |
None |
None |
None |
None |
None |
None |
None |
Yes |
|
Geenen |
Member, radiologist |
Member Contrast Media Safety Committee of the European Society of Urogenital Radiology (unpaid, meetings are partially funded by CM industry))) |
None |
None |
None |
None |
None |
Has been a public speaker during symposia organised by GE Healthcare about contrast agents (most recently in June 2014) |
Yes |
|
Hoogeveen |
Member, nephrologist |
Member of Guideline Committee of Dutch Federation of Nephrology |
None |
None |
Member of Guideline Committee of Dutch Society for Nephrology |
Grant from the Dutch Kidney Foundation to study effect of fish oil on kidney function in post-MI patients |
None |
None |
Yes |
|
Kooiman |
Member, research physician |
Resident in department of gynaecology & obstetrics |
None |
None |
None |
None |
None |
None |
Yes |
|
Mostovaya |
Advisor, Knowledge Institute of Medical Specialists |
None |
None |
None |
None |
None |
None |
None |
Yes |
|
Oudemans – van Straaten |
Member, intensive care medical specialist Professor Intensive Care |
none |
None |
None |
None |
None |
None |
None |
Yes |
|
Pels Rijcken |
Member, interventional radiologist |
None |
None |
None |
None |
None |
None |
None |
Yes |
|
Sijpkens |
Member, nephrologist |
None |
None |
None |
None |
None |
None |
None |
Yes |
|
Vainas |
Member, vascular surgeon |
None |
None |
None |
None |
None |
None |
None |
Yes |
|
Van den Meiracker |
Member, internist vascular medicine |
None |
None |
None |
None |
None |
None |
None |
Yes |
|
Van der Molen |
Chairman, radiologist |
Member Contrast Media Safety Committee of the European Society of Urogenital Radiology (unpaid,CMSC meetings are partially funded by CM industry)) |
None |
None |
Secretary section of Abdominal Radiology; Radiological Society of the Netherlands (until spring of 2015) |
None |
None |
Receives Royalties for books: Contrast Media Safety, ESUR guidelines, 3rd ed. Springer, 2015 Received speaker fees for lectures on CM safety by GE Healthcare, Guerbet, Bayer Healthcare and Bracco Imaging (2015-2016) |
Yes |
|
Wikkeling |
Member, vascular surgeon |
None |
None |
None |
None |
None |
None |
None |
Yes |
|
Advisory Board |
|||||||||
|
Demir |
Member, physician clinical chemistry |
None |
None |
None |
None |
None |
None |
None |
Yes |
|
Hubbers |
Member, patient’s representative, Dutch Society of Kidney Patients |
None |
None |
None |
None |
None |
None |
None |
Yes |
|
Mazel |
Member, urologist |
None |
None |
None |
None |
None |
None |
None |
Yes |
|
Prantl |
Member, policy maker, Dutch Society of Kidney Patients |
None |
None |
None |
None |
None |
None |
None |
Yes |
|
Van den Wijngaard |
Member, resident clinical chemistry |
Reviewer for several journals (such as American Journal of Physiology) |
None |
None |
None |
None |
None |
None |
Yes |
Inbreng patiëntenperspectief
Patients’ perspective was represented, firstly by membership and involvement in the advisory board of a policy maker and a patients’ representative from the Dutch Kidney Patient Association. Furthermore, an online survey was organized by the Dutch Kidney Patient Association about the subject matter of the guideline. A summary of the results of this survey has been discussed during a working group meeting at the beginning of the guideline development process. Subjects that were deemed relevant by patients were included in the outline of the guideline. The concept guideline has also been submitted for feedback during the comment process to the Dutch Patient and Consumer Federation, who have reported their feedback through the Dutch Kidney Patient Association.
Methode ontwikkeling
Evidence based
Implementatie
In the different phases of guideline development, the implementation of the guideline and the practical enforceability of the guideline were taken into account. The factors that could facilitate or hinder the introduction of the guideline in clinical practice have been explicitly considered. The implementation plan can be found with the Related Products. Furthermore, quality indicators were developed to enhance the implementation of the guideline. The indicators can also be found with the Related Products.
Werkwijze
AGREE
This guideline has been developed conforming to the requirements of the report of Guidelines for Medical Specialists 2.0; the advisory committee of the Quality Counsel. This report is based on the AGREE II instrument (Appraisal of Guidelines for Research & Evaluation II) (www.agreetrust.org), a broadly accepted instrument in the international community and on the national quality standards for guidelines: “Guidelines for guidelines” (www.zorginstituutnederland.nl).
Identification of subject matter
During the initial phase of the guideline development, the chairman, working group and the advisor inventory the relevant subject matter for the guideline. Furthermore, an Invitational Conference was organized, where additional relevant subjects were suggested by the Dutch Kidney Patient Association, Dutch Society for Emergency Physicians, and Dutch Society for Urology. A report of this meeting can be found in Related Products.
Clinical questions and outcomes
During the initial phase of guideline development, the chairman, working group and advisor identified relevant subject matter for the guideline. Furthermore, input was acquired for the outline of the guideline during an Invitational Conference. The working group then formulated definitive clinical questions and defined relevant outcome measures (both beneficial land harmful effects). The working group rated the outcome measures as critical, important and not important. Furthermore, where applicable, the working group defined relevant clinical differences.
Strategy for search and selection of literature
For the separate clinical questions, specific search terms were formulated and published scientific articles were sought after in (several) electronic databases. Furthermore, studies were looked for by cross-referencing other included studies. The studies with potentially the highest quality of research were looked for first. The working group members selected literature in pairs (independently of each other) based on title and abstract. A second selection was performed based on full text. The databases search terms and selection criteria are described in the modules containing the clinical questions.
Quality assessment of individual studies
Individual studies were systematically assessed, based on methodological quality criteria that were determined prior to the search, so that risk of bias could be estimated. This is described in the “risk of bias” tables.
Summary of literature
The relevant research findings of all selected articles are shown in evidence tables. The most important findings in literature are described in literature summaries. When there were enough similarities between studies, the study data were pooled.
Grading the strength of scientific evidence
A) For intervention questions
The strength of the conclusions of the scientific publications was determined using the GRADE-method. GRADE stands for Grading Recommendations Assessment, Development and Evaluation (see http://www.gradeworkinggroup.org/) (Atkins, 2004).
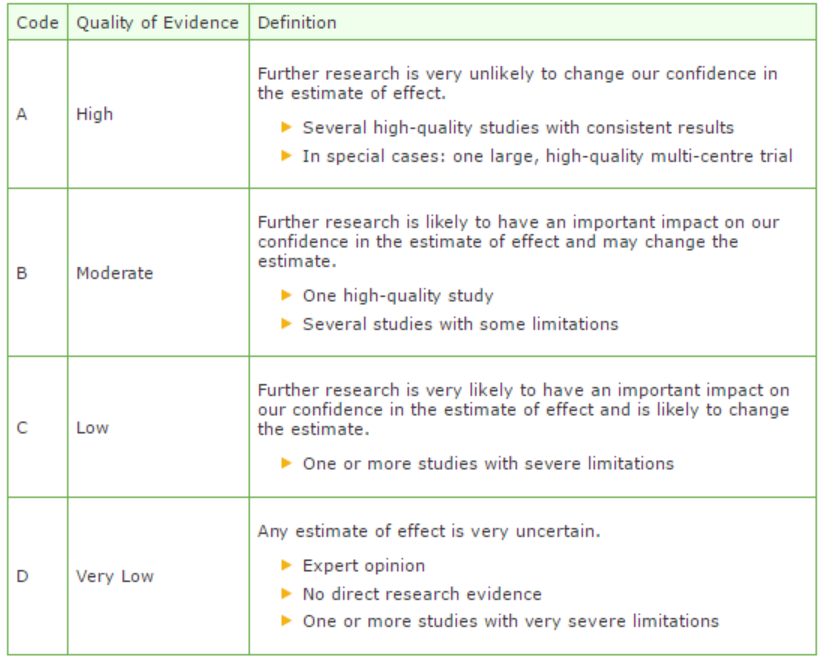
GRADE defines four gradations for the quality of scientific evidence: high, moderate, low or very low. These gradations provide information about the amount of certainty about the literature conclusions. (http://www.guidelinedevelopment.org/handbook/).
B) For diagnostic, etiological, prognostic or adverse effect questions, the GRADE-methodology cannot (yet) be applied. The quality of evidence of the conclusion is determined by the EBRO method (van Everdingen, 2004)
Formulating conclusion
For diagnostic, etiological, prognostic or adverse effect questions, the evidence was summarized in one or more conclusions, and the level of the most relevant evidence was reported. For intervention questions, the conclusion was drawn based on the body of evidence (not one or several articles). The working groups weighed the beneficial and harmful effects of the intervention.
Considerations
Aspects such as expertise of working group members, patient preferences, costs, availability of facilities, and organization of healthcare aspects are important to consider when formulating a recommendation. These aspects were discussed in the paragraph Considerations.
Formulating recommendations
The recommendations answer the clinical question and were based on the available scientific evidence and the most relevant considerations.
Constraints (organization of healthcare)
During the development of the outline of the guideline and the rest of the guideline development process, the organization of healthcare was explicitly taken into account. Constraints that were relevant for certain clinical questions were discussed in the Consideration paragraphs of those clinical questions. The comprehensive and additional aspects of the organization of healthcare were discussed in a separate chapter.
Development of quality indicators
Internal (meant for use by scientific society or its members) quality indicators are developed simultaneously with the guideline. Furthermore, existing indicators on this subject were critically appraised; and the working group produces an advice about such indicators. Additional information on the development of quality indicators is available by contacting the Knowledge Institute for Medical Specialists. (secretariaat@kennisinstituut.nl).
Knowledge Gaps
During the development of the guideline, a systematic literature search was performed the results of which help to answer the clinical questions. For each clinical question the working group determined if additional scientific research on this subject was desirable. An overview of recommendations for further research is available in the appendix Knowledge Gaps.
Comment- and authorisation phase
The concept guideline was subjected to commentaries by the involved scientific societies. The commentaries were collected and discussed with the working group. The feedback was used to improve the guideline; afterwards the working group made the guideline definitive. The final version of the guideline was offered for authorization to the involved scientific societies, and was authorized.
References
Atkins D, Eccles M, Flottorp S, et al. GRADE Working Group. Systems for grading the quality of evidence and the strength of recommendations I: critical appraisal of existing approaches The GRADE Working Group. BMC Health Serv Res. 2004 Dec 22;4(1):38.
Van Everdingen JJE, Burgers JS, Assendelft WJJ, et al. Evidence-based richtlijnontwikkeling. Bohn Stafleu van Loghum. Houten, 2004
Zoekverantwoording
|
Database |
Search terms |
Total |
|
Medline (OVID) 1995-aug. 2015
Engels, Nederlands |
1 exp Contrast Media/ or ((contrast adj3 iodine) or (contrast adj3 medi*)).ti,ab. (112282) 2 exp Kidney Diseases/ or (((kidney or renal) adj2 (disease* or injur* or failure*)) or nephropath* or (renal adj2 (insufficienc* or function* or disease* or failure*))).ti,ab. (536907) 3 1 and 2 (8955) 4 (((contrast* or ci) adj2 (nephropath* or 'kidney injury' or aki or nephrotoxicity)) or ciaki).ti,ab. (1969) 5 3 or 4 (9449) 6 limit 5 to (yr="1995-Current" and (dutch or english)) (5521) 7 exp hydroxymethylglutaryl-coa reductase inhibitors/ or (statin* or lovastatin* or meglutol* or pravastatin* or simvastatin* or rosuvastatin* or atorvastatin*).).ti,ab,kw. or (hydroxymethylglutaryl* adj4 inhibitor*).ti,ab,kw. (45277) 8 6 and 7 (131) 9 (meta-analysis/ or meta-analysis as topic/ or (meta adj analy$).tw. or ((systematic* or literature) adj2 review$1).tw. or (systematic adj overview$1).tw. or exp "Review Literature as Topic"/ or cochrane.ab. or cochrane.jw. or embase.ab. or medline.ab. or (psychlit or psyclit).ab. or (cinahl or cinhal).ab. or cancerlit.ab. or ((selection criteria or data extraction).ab. and "review"/)) not (Comment/ or Editorial/ or Letter/ or (animals/ not humans/)) (248141) 10 8 and 9 (32) – 31 uniek 11 (exp clinical trial/ or randomized controlled trial/ or exp clinical trials as topic/ or randomized controlled trials as topic/ or Random Allocation/ or Double-Blind Method/ or Single-Blind Method/ or (clinical trial, phase i or clinical trial, phase ii or clinical trial, phase iii or clinical trial, phase iv or controlled clinical trial or randomized controlled trial or multicenter study or clinical trial).pt. or random*.ti,ab. or (clinic* adj trial*).tw. or ((singl* or doubl* or treb* or tripl*) adj (blind$3 or mask$3)).tw. or Placebos/ or placebo*.tw.) not (animals/ not humans/) (1508278) 12 8 and 11 (71) 13 Epidemiologic studies/ or case control studies/ or exp cohort studies/ or Controlled Before-After Studies/ or Case control.tw. or (cohort adj (study or studies)).tw. or Cohort analy$.tw. or (Follow up adj (study or studies)).tw. or (observational adj (study or studies)).tw. or Longitudinal.tw. or Retrospective.tw. or prospective.tw. or Cross sectional.tw. or Cross-sectional studies/ or historically controlled study/ or interrupted time series analysis/ [Onder exp cohort studies vallen ook longitudinale, prospectieve en retrospectieve studies] (2209511) 14 8 and 13 (38) 15 12 not 10 (45) 22 (12 or 14) not 10 (58) – 56 uniek |
131 |
|
Embase (Elsevier) |
'contrast induced nephropathy'/exp/dm_pc OR ((contrast* OR ci) NEAR/2 (nephropath* OR 'kidney injury' OR aki OR nephrotoxicity)):ab,ti OR ciaki:ab,ti OR ('contrast medium'/exp OR (contrast NEAR/3 iodine):ab,ti OR (contrast NEAR/3 medi*):ab,ti AND ('kidney disease'/exp OR 'kidney function'/exp OR (kidney NEAR/2 (disease* OR injur* OR failure*)):ab,ti OR nephropath*:ab,ti OR (renal NEAR/2 (insufficienc* OR function* OR disease* OR failure*)):ab,ti))
AND ('hydroxymethylglutaryl coenzyme a reductase inhibitor'/exp/mj OR statin*:ab,ti OR lovastatin*:ab,ti OR meglutol*:ab,ti OR pravastatin*:ab,ti OR simvastatin*:ab,ti OR rosuvastatin*:ab,ti OR atorvastatin*:ab,ti OR (hydroxymethylglutaryl* NEAR/4 inhibitor*):ab,ti)
AND ([dutch]/lim OR [english]/lim) AND [embase]/lim AND [1995-2015]/py
'meta analysis'/de OR cochrane:ab OR embase:ab OR psychlit:ab OR cinahl:ab OR medline:ab OR (systematic NEAR/1 (review OR overview)):ab,ti OR (meta NEAR/1 analy*):ab,ti OR metaanalys*:ab,ti OR 'data extraction':ab OR cochrane:jt OR 'systematic review'/de NOT ('animal experiment'/exp OR 'animal model'/exp OR 'nonhuman'/exp NOT 'human'/exp)) (34) – 6 uniek
AND ('clinical trial'/exp OR 'randomization'/exp OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp OR 'placebo'/exp OR 'prospective study'/exp OR rct:ab,ti OR random*:ab,ti OR 'single blind':ab,ti OR 'randomised controlled trial':ab,ti OR 'randomized controlled trial'/exp OR placebo*:ab,ti) NOT 'conference abstract':it OR 'clinical study'/exp (87) – 38 uniek |
