Prophylaxe met NAC en hydratie tegen PC-AKI
Uitgangsvraag
Dient profylaxe met N-acetylcysteine (NAC) te worden aanbevolen naast hydratie om de kans om PC-AKI te verkleinen bij patiënten met een normale nierfunctie of met een chronische nierziekte die intravasculair contrastmiddel (CM) krijgen toegediend?
Aanbeveling
Geef geen NAC ter preventie van PC-AKI aan patiënten met een normale of verminderde (eGFR <60 ml/min/1,73m2) nierfunctie.
Overwegingen
Our meta-analysis regarding patients with a normal renal function yielded no benefit of NAC for prevention of PC-AKI, both for patients receiving CT scan and/or for patients undergoing CAG.
The evidence regarding NAC benefit for prevention of PC-AKI in patients with an impaired renal function is weak due to the quality of the trials and the heterogeneity of the results. For example, follow-up time was only 2 to 5 days in the majority of included studies; thus meaningful conclusions could not be drawn about the consequences of NAC use for mid and long term morbidity and mortality. Furthermore, the studies were not powered to draw conclusions about morbidity and mortality, only for the short-term PC-AKI laboratory diagnosis.
A meta-analysis (Sun, 2013) concluded that the evidence on use of IV NAC to prevent PC-AKI was too inconsistent to determine the efficacy. Another meta-analysis concluded that NAC may help to prevent PC-AKI in patients undergoing coronary angiography, but does not have any impact on clinical outcomes such as dialysis or mortality (Submaramiam, 2016). Furthermore, the dose and route of administration of NAC differed between studies. In our own meta-analysis for patients with an impaired kidney function the use of NAC did not decrease the risk of PC-AKI significantly. Of note, only studies that described hydration strategies representative to those used in the Netherlands were included in this analysis. No studies were found that compared oral to intravenous N-acetylcysteine route of administration in patients undergoing intravascular contrast administration.
Intervention with NAC is without risk, cheap, and generally available, and there are theoretical arguments that NAC may provide reduction of CI-AKI. Despite the theoretically potential kidney protection arguments, we do not recommend adding NAC to hydration routinely in patients with an impaired kidney function. Reason is that the level of evidence is weak and the demonstrated benefit is small at best, and clinically not proven relevant. Moreover, the low costs of NAC itself is offset by extra handling time and a more complex AKI preventive protocol, which are unnecessary confounding and cost enhancing factors. None of the studies showed significant differences in clinical meaningful endpoints such as need of renal replacement therapy and/or mortality.
Onderbouwing
Achtergrond
The mechanism of PC-AKI is not completely understood. Direct cell damage by the iodine-containing contrast medium with subsequent oxidative stress, endothelial dysfunction and decreased nitric oxide (NO) availability is supposed to play major role. Intrarenal NO is crucial for maintaining perfusion and oxygen supply in the renal medulla. NO depletion causes vasoconstriction with hypoperfusion of the renal medulla and local hypoxia. In addition, NO depletion affects tubular fluid composition, tubule-glomerular feed-back signalling and decreases glomerular filtration rate (Liu, 2014).
However, some experts have questioned whether acute kidney injury occurring after intravascular administration of iodine-containing CM is not caused by co-existing risk factors and only coincidentally related to the CM especially if contrast media are administered by the intravenous route. In a meta-analysis of controlled studies the incidence of acute kidney injury was similar between patients receiving IV contrast and patients receiving an imaging procedure without contrast media (McDonald, 2013).
In addition, it is also difficult to distinguish the effects of contrast media from the effects of physiologic confounders that could either elevate or reduce serum creatinine in patients undergoing radiologic studies (Hofmann, 2004; Krasuski, 2003).
There is also a possibility that the effectiveness of NAC could vary by type of iodine-containing contrast medium used, LOCM vs IOCM.
A recent analysis did not demonstrate a clear benefit of NAC for patients receiving IV contrast media (Subramaniam, 2016). The same analysis found no association between the effect of NAC on the incidence of PC-AKI and mean baseline serum creatinine levels.
The argument for NAC in the decision making process has always been the low risk, the low costs and general availability of the NAC intervention. However, the low costs of NAC itself is offset by extra handling time and a more complex AKI preventive protocol, which are also confounding factors.
Thus, it is unclear whether NAC-administration should be recommended to prevent PC-AKI.
Conclusies / Summary of Findings
|
Low GRADE |
There is evidence of low quality that N-acetylcysteine does not reduce the risk of PC-AKI in patients with normal kidney function undergoing computer tomography with intravascular iodine-containing contrast administration when compared to placebo.
(Hsu, 2012) |
|
Low GRADE |
There is evidence of low quality that N-acetylcysteine does not reduce the risk of PC-AKI in patients with impaired kidney function undergoing computed tomography with intravascular iodine-containing contrast administration when compared to placebo.
(Kama, 2014; 2006; Kitzler, 2012; Poletti, 2007; Poletti, 2013; Tepel, 2000) |
|
Low GRADE |
There is evidence of low quality that N-acetylcysteine does not reduce the risk of PC-AKI in patients with normal kidney function undergoing coronary angiography with intravascular iodine-containing contrast administration when compared to placebo.
(Berwanger, 2013; Carbonell 2007; Jaffrey, 2015; Kim, 2010; Kinbara, 2010; Lawlor, 2007; Sadat, 2011; Sandhu, 2006; Tanaka, 2011; Thiele 2010) |
|
Low GRADE |
There is evidence of low quality that N-acetylcysteine does not reduce the risk of PC-AKI in patients with decreased kidney function undergoing coronary angiography with intravascular iodine-containing contrast administration when compared to placebo.
(ACT, 2011; Castini, 2010; Ferrario, 2009; Gulel, 2005; Habib, 2016, Izani Wan, 2008; Koc, 2012; Kotlyar, 2005; Sadenini, 2017; Seyon, 2007) |
|
No studies were found that compared oral to intravenous N-acetylcysteine route of administration in patients undergoing intravascular iodine-containing contrast administration. |
Samenvatting literatuur
Description of studies
CT scan, normal kidney function
One RCT (Hsu, 2012) reported on effects of NAC plus saline hydration (n=106) versus saline hydration only (n=103) in terms of incidence of PC-AKI in patients undergoing CT-scans with intravascular contrast medium. NAC was administered intravenously (600mg) prior to the CT-scan.
CT scan, decreased kidney function
A total of 5 RCTs (Kama, 2014; Kitzler, 2012; Poletti, 2007; Poletti, 2013; Tepel, 2000) with 386 patients was included. Three studies described emergency patients (Kama, 2014; Poletti, 2007; Poletti, 2013) while two studies described elective patients (Kitzler, 2012; Tepel, 2000). In two RCTs the N-acetylcysteine was administered orally (Kitzler, 2014; Tepel, 2000), with the total doses varying between 2.4g and 4.8g. In three RCTs the N-acetylcysteine was administered intravenously (Kama, 2014; Poletti, 2007; Poletti, 2013) with total doses varying between 1.05 g (150mg/kg) and 6g. The follow-up time in the studies varied between 3 days and 10 days (for laboratory parameters).
Coronary angiography and/or percutaneous intervention, normal kidney function
A total of 8 RCTs was included (Carbonell, 2007; Jaffrey, 2012; Kim, 2010; Kinbara, 2010; Lawlor, 2007; Sadat, 2011; Tanaka, 2011; Thiele, 2010) with 3093 patients was included. Four studies described emergency patients (Carbonell, 2007; Jaffrey, 2012; Tanaka, 2011; Thiele, 2010) while four studies described elective patients (Kim, 2010; Kinbara, 2010; Lawlor, 2007; Sadat, 2011). In four RCTs the N-acetylcysteine was administered orally (Kim, 2010; Kinbara, 2010; Sadat, 2011; Tanaka, 2011), with the total doses varying between 2.4g and 2.8g. In four RCTs the N-acetylcysteine was administered intravenously (Carbonell, 2007; Jaffrey, 2012; Lawlor, 2007; Thiele, 2010) with total doses varying between 1g and 6g. The follow-up time in the studies varied between 2 days and 7 days (for laboratory parameters).
Coronary angiography and/or percutaneous intervention, impaired kidney function
A total of 10 RCTs was included (ACT, 2011; Castini, 2010; Ferrario, 2009; Gulel, 2005; Habib, 2016; Izani Wan, 2008; Koc, 2012; Kotlyar, 2005; Sadineni, 2017; Seyon, 2007) with 1188 patients was included. One study described emergency patients (Seyon, 2007) while 7 studies described elective patients (ACT, 2011; Castini, 2010; Ferrario, 2009; Gulel, 2005; Izani Wan, 2008; Koc, 2012; Kotlyar, 2005). In 6 RCTs the N-acetylcysteine was administered orally (ACT, 2011; Castini, 2010; Ferrario, 2009; Gulel, 2005; Izani Wan, 2008; Seyon, 2007), with the total doses varying between 2.4g and 4.8g. In 2 RCTs the N-acetylcysteine was administered intravenously (Koc, 2012; Kotlyar, 2005) with total doses varying between 0.6g and 2.4g. The follow-up time (for laboratory parameters) in the studies varied between 2 days and 30 days.
Results
CT scans, normal kidney function
Hsu (2012) reported that 8/106 patients in the NAC group versus 15/103 patients in the control group developed PC-AKI; this difference was not significant: Relative Risk (RR): 0.12 (95% CI: 0.01 to 2.11).
CT scans, impaired kidney function
Pooling of data of 5 RCTs (Kama, 2014; 2006; Kitzler, 2012; Poletti, 2007; Poletti, 2013; Tepel, 2000) with 386 patients with 60 events showed that risk ratio of PC-AKI was not reduced significantly in the NAC group: RR: 0.64 (95% CI: 0.24 to 1.70), p=0.37, see Figure 1.
Coronary angiography, normal kidney function
Pooling of data of 8 RCTs (Carbonell, 2007; Jaffrey, 2012; Kim, 2010; Kinbara, 2010; Lawlor, 2007; Sadat, 2011; Tanaka, 2011; Thiele, 2010) with 3093 patients with 394 events showed that risk ratio of PC-AKI was not reduced in the NAC group: RR: 0.97 (0.74 to 1.28); p=0.82, see Figure 2.
Coronary angiography, impaired kidney function
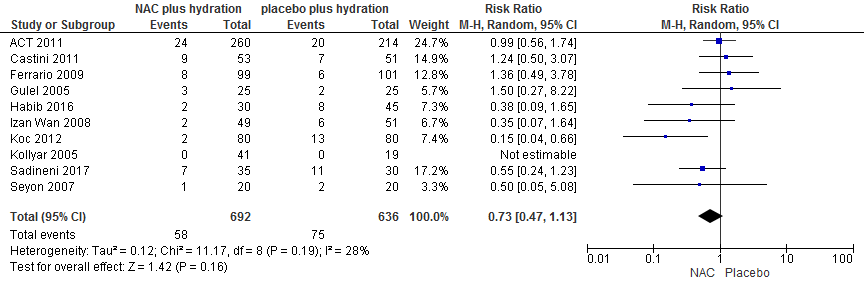
Pooling of data of 8 RCTs (ACT, 2011; Castini, 2010; Ferrario, 2009; Gulel, 2005; Habib, 2016; Izani Wan, 2008; Koc, 2012; Kotlyar, 2005; Sadineni, 2017; Seyon, 2007) with 1388 patients with 146 events showed that risk ratio of PC-AKI was not reduced in the NAC group: RR: 0.71 (0.51 to 0.98); p=0.16, see Figure 3.
Quality of evidence
The quality of evidence for the outcome PC-AKI was downgraded by two for imprecision (low number of events and overlap with 10% border of clinical significance) for all analyses.
Figure 1 Meta-analysis of NAC vs Placebo in CT with intravenous CM administration in patients with eGFR <60 ml/min/1.73m2.
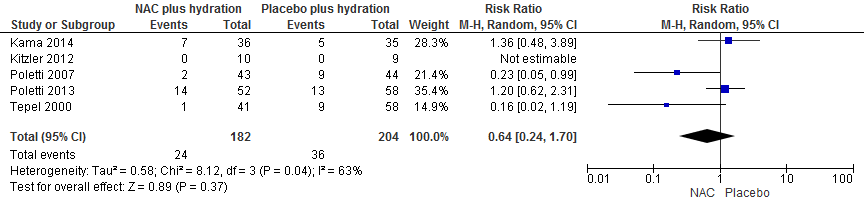
Figure 2 Meta-analysis of NAC vs Placebo in Coronary angiography with intra-arterial CM administration in patients with normal kidney function
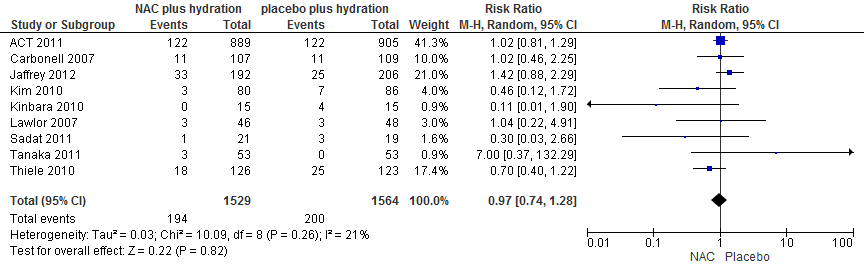
Figure 3 Meta-analysis of NAC vs Placebo in Coronary angiography with intra-arterial CM administration in patients with eGFR <60 ml/min/1.73m2.
Zoeken en selecteren
To answer our clinical question a systematic literature analysis was performed for the following research question:
Can prophylactic N-acetylcysteine in addition to hydration reduce the incidence of CI-AKI in patients receiving intravascular contrast?
Sub question:
Can prophylactic N-acetylcysteine in addition to hydration reduce the incidence of CI-AKI in patients receiving intravascular contrast in certain subgroups of patient (For example, patients with reduced kidney function)?
P (patient category) Adult patients undergoing radiological examinations receiving intravascular contrast.
I (intervention) N-acetylcysteine acid in combination with hydration, N-acetylcysteine alone.
C (comparison) Hydration alone, no preventive measures.
O (outcome) Post-contrast acute kidney injury (PC-AKI), start dialysis, decrease in residual kidney function, adverse effects of hydration (congestion, intensive care unit admittance, and mortality), cost-effectiveness.
Relevant outcome measures
The working group considered PC-AKI, mortality and start dialysis critical outcome measures for the decision making process and the intensive care admission important outcome measures for the decision-making process.
A difference of at least 10% in relative risk was defined as a clinically relevant difference; by expert opinion of the working group (no literature was available to substantiate the decision). To illustrate, if PC-AKI occurs with an incidence of 10% in the patient population, a difference of 10% of relative risk would mean a difference of 1% in absolute risk. Thus the number needed to treat would be 100, ergo: a doctor would need to treat 100 patients to prevent one case of PC-AKI. When the incidence of PC-AKI is 5%, a difference of 10% in relative risk would mean a difference of 0.5% in absolute risk, and a number needed to treat of 200.
Search and select (method)
The databases Medline (OVID), Embase and the Cochrane Library were searched from January 2005 to 23rd of July 2015 using relevant search terms for systematic reviews (SRs) and randomized controlled trials (RCTs). This search was updated on 1 May 2017.
A total of 341 studies were found. The initial literature search produced 302 hits and the update produced 39 hits. The following search criteria were applied:
- adult patients who underwent radiological examination using intravascular iodine-containing contrast media (including radiological examination during percutaneous angiography);
- patients with impaired kidney function, at least eGFR <60 ml/min1.73m2 were analysed separately from those with a normal kidney function
- hydration types: hydration with NaCl, hydration with bicarbonate, oral hydration, pre-hydration, pre- and posthydration;
- N-acetylcysteine that was administered in one of the treatment arms;
- the control arm consisted of patients that received hydration or no hydration;
- at least one of the outcome measures was described: Contrast-induced nephropathy (CIN) / contrast-induced acute kidney injury (CI-AKI), start dialysis, decrease in residual kidney function, adverse effects of hydration (overfilling, intensive care unit admittance, and mortality), and cost-effectiveness.
Based on title and abstract a total of 91 studies were selected. After examination of full texts a total of 67 studies were excluded and 24 studies definitely included in the literature summary. Reasons for exclusion are described in the exclusion table. During the search update, no more papers were included that described patients with a normal kidney function (eGFR≥60 ml/min1.73m2). The reason for this was that the working group decided to focus the recommendations on patients with an impaired eGFR (<60 ml/min1.73m2) only, because in regular clinical practice no one will consider inserting the administration of NAC in the study protocol in the population with a normal kidney function (eGFR≥60 ml/min1.73m2).
Results
24 studies were included in the literature analysis, the most important study characteristics and results were included in the evidence tables. The evidence tables and assessment of individual study quality are included under the tab Onderbouwing.
Referenties
- ACT Investigators. Acetylcysteine for prevention of renal outcomes in patients undergoing coronary and peripheral vascular angiography main results from the randomized Acetylcysteine for Contrast-Induced Nephropathy Trial (ACT). Circulation 2011;124(11):1250-1259.
- Berwanger O, Cavalcanti AB, Sousa AM, et al. Acetylcysteine for the Prevention of Renal Outcomes in Patients With Diabetes Mellitus Undergoing Coronary and Peripheral Vascular Angiography Circ Cardiovasc Interv. 2013;(2), 139-145.
- Carbonell N, Blasco M, Sanjuán R, et al. Intravenous N-acetylcysteine for preventing contrast-induced nephropathy: a randomised trial. Int J Cardiol. 2007;115(1):57-62.
- Castini D, Lucreziotti S, Bosotti L, et al. Prevention of Contrast-induced Nephropathy: A Single Center Randomized Study. Clin Cardiol. 2010;33(3):E63-8.
- Ferrario F, Barone MT, Landoni G, et al. Acetylcysteine and non-ionic isosmolar contrast-induced nephropathya randomized controlled study. Nephrol Dial Transplant. 2009;24(10):3103-7.
- Gulel O, Keles T, Eraslan H, et al. Prophylactic acetylcysteine usage for prevention of contrast nephropathy after coronary angiography. J Cardiovasc Pharm. 2005;46(4):464-7.
- Habib M, Hillis A, Hammad A. N-acetylcysteine and/or ascorbic acid versus placebo to prevent contrast-induced nephropathy in patients undergoing elective cardiac catheterization: The NAPCIN trial; A single-center, prospective, randomized trial. Saudi J Kidney Dis Transpl 2016;27:55-61
- Hoffmann U, Fischereder M, Krüger B, et al. The value of N-acetylcysteine in the prevention of radiocontrast agent-induced nephropathy seems questionable. J Am Soc Nephrol. 2004;15(2):407-10.
- Hsu TF, Huang MK, Yu SH, et al. N-acetylcysteine for the prevention of contrast-induced nephropathy in the emergency department. Intern Med. 2012;51(19):2709-14.
- Izani Wan MWM, Zainel D, Zurkurnai Y. Oral N-Acetylcysteine in prevention of contrast induced nephropathy following coronary angiogram. Interl Med Internat Med J. 2008;15(5): 353-361.
- Jaffery Z, Verma A, White CJ, et al. A randomized trial of intravenous N-acetylcysteine to prevent contrast induced nephropathy in acute coronary syndromes. Cathetern Cardiovasc Intervent. 2012;79(6):921-6.
- Kama A, Yilmaz S, Yaka E, et al. Comparison of Short-term Infusion Regimens of N-Acetylcysteine Plus Intravenous Fluids, Sodium Bicarbonate Plus Intravenous Fluids, and Intravenous Fluids Alone for Prevention of Contrast-induced Nephropathy in the Emergency Department. Acad Emerg Med. 2014;21(6):615-22.
- Kim BJ, Sung KC, Kim BS, et al. Effect of N-acetylcysteine on cystatin C-based renal function after elective coronary angiography (ENABLE Study): a prospective, randomized trial. Int J Cardiol. 2010;138(3):239-45.
- Kinbara T, Hayano T, Ohtani N, et al. Efficacy of N-acetylcysteine and aminophylline in preventing contrast-induced nephropathy. J Cardiol. 2010;55(2):174-9.
- Kitzler TM, Jaberi A, Sendlhofer G, et al. Efficacy of vitamin E and N-acetylcysteine in the prevention of contrast induced kidney injury in patients with chronic kidney disease: a double blind, randomized controlled trial. Wiener Klin Wochenschr. 2012;124(9-10):312-9.
- Koc F, Ozdemir K, Kaya MG, et al. Intravenous N-acetylcysteine plus high-dose hydration versus high-dose hydration and standard hydration for the prevention of contrast-induced nephropathy: CASISa multicenter prospective controlled trial. Int J Cardiol. 2012;155(3):418-23.
- Kotlyar E, Keogh AM, Thavapalachandran S, et al. Prehydration alone is sufficient to prevent contrast-induced nephropathy after day-only angiography proceduresa randomised controlled trial. Heart Lung Circ. 2005;14(4):245-51.
- Krasuski RA, Beard BM, Geoghagan JD, et al. Optimal timing of hydration to erase contrast-associated nephropathy: the OTHER CAN study. J Invas Cardiol. 2003;15(12):699-702.
- Lawlor DK, Moist L, DeRose G, et al.. Prevention of contrast-induced nephropathy in vascular surgery patients. Ann Vasc Surg. 2007 Sep;21(5):593-7.
- Liu ZZ, Schmerbach K, Lu Y, et al. Iodinated contrast media cause direct tubular cell damage, leading to oxidative stress, low nitric oxide, and impairment of tubuloglomerular feedbackAm J Physiol Renal Physiol. 2014;306(8):F864-72.
- McDonald JS, McDonald RJ, Carter RE, et al. Risk of intravenous contrast materialmediated acute kidney injury: a propensity scorematched study stratified by baseline-estimated glomerular filtration rate. Radiology. 2014;271(1):65-73.
- O'Sullivan S, Healy DA, Moloney MC, et al. The role of N--acetylcysteine in the prevention of contrast-induced nephropathy in patients undergoing peripheral angiography a structured review and meta-analysis. Angiology. 2013;64(8):576-82.
- Poletti PA, Platon A, De Seigneux S, et al. N-acetylcysteine does not prevent contrast nephropathy in patients with renal impairment undergoing emergency CT: a randomized study. BMC Nephrol. 2013;14:119.
- Poletti PA, Saudan P, Platon A, et al. Iv N-acetylcysteine and emergency CT: use of serum creatinine and cystatin C as markers of radiocontrast nephrotoxicity. AJR Am J Roentgenol. 2007;189(3):687-92.
- Sadat U, Walsh SR, Norden AG, et al. Does oral N-acetylcysteine reduce contrast-induced renal injury in patients with peripheral arterial disease undergoing peripheral angiography? A randomized-controlled study. Angiology. 2011;62(3):225-30.
- Sadineni R, Karthik K, Swarnalatha G, et al. N-acetyl cysteine versus allopurinol in the prevention of contrast nephropathy in patients with chronic kidney disease: A randomized controlled trial. Indian J Nephrol. 2017 Mar-Apr; 27(2): 9398.
- Sandhu C, Belli AM, Oliveira DB. The role of N-acetylcysteine in the prevention of contrast-induced nephrotoxicity. Cardiovasc Intervent Radiol. 2006;29(3), 344-347.
- Seyon RA, Jensen LA, Ferguson IA, et al. Efficacy of N-acetylcysteine and hydration versus placebo and hydration in decreasing contrast-induced renal dysfunction in patients undergoing coronary angiography with or without concomitant percutaneous coronary intervention. Heart Lung. 2007;36(3):195-204.
- Subramaniam RM, Suarez-Cuervo C, Wilson RF, et al. Effectiveness of Prevention Strategies for Contrast-Induced Nephropathy: A Systematic Review and Meta-analysis. Ann Intern Med. 2016;164(6):406-16.
- Sun Z, Fu Q, Cao L, et al. Intravenous N-acetylcysteine for prevention of contrast-induced nephropathy: a meta-analysis of randomized, controlled trials. PloS One 8.1. 2013;8(1):e55124.
- Tanaka A, Suzuki Y, Suzuki N, et al. Does N-acetylcysteine reduce the incidence of contrast-induced nephropathy and clinical events in patients undergoing primary angioplasty for acute myocardial infarction? Intern Med.. 2011;50(7):673-7.
- Tepel M, van der Giet M, Schwarzfeld C, et al. Prevention of radiographic-contrast-agentinduced reductions in renal function by acetylcysteine. N Engl J Med. 2000;343(3):180-4.
- Thiele H, Hildebrand L, Schirdewahn C, et al. Impact of high-dose N-acetylcysteine versus placebo on contrast-induced nephropathy and myocardial reperfusion injury in unselected patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention: the LIPSIA-N-ACC (Prospective, Single-Blind, Placebo-Controlled, Randomized Leipzig Immediate PercutaneouS Coronary Intervention Acute Myocardial Infarction N-ACC) Trial. J Am Coll Cardiol. 2010;55(20):2201-9.
Evidence tabellen
Table: Exclusion after revision of full text
|
Author and year |
Reason for exclusion |
|
ACT Investigators, 2009 |
description of study design, not an original article |
|
Amini, 2009 |
Prehydration only, not comparable to Dutch clinical practice |
|
Ashworth, 2010 |
overlaps with Loomba, 2013 and is a less recent review |
|
Azmus, 2005 |
Not specifically patients with normal or abnormal kidney function (mix of impaired kidney function and diabetics) |
|
Bagshaw, 2006 |
review, not systematic |
|
Berwanger, 2012 |
Sub-analysis of ACTT studty (which is already included in literature analysis) |
|
Briguori, 2011 |
Does not compare N-acetylcysteine to placebo |
|
Briguori, 2007 |
Not specifically patients with normal or abnormal kidney function (mix of impaired kidney function and diabetics) |
|
Brown, 2009 |
overlaps with Loomba, 2013 and is a less recent review |
|
Burns, 2010 |
Not specifically patients with normal or abnormal kieny function (mix of impaired kidney function and diabetics) |
|
Busch, 2013 |
overlaps with Loomba, 2013 and is a less recent review |
|
Buyukhatipoglu, 2010 |
outcome measures as described in PICO not reported |
|
Calabro, 2011 |
observational study |
|
Carbonell, 2010 |
already included in Loomba 2013, and Sun, 2013 |
|
Carbonell, 2007 |
already included in Loomba 2013, and Sun, 2013 |
|
Chen, 2008 |
does not compare no NAC to NAC (both treatment arms recieve NAC) |
|
Coyle, 2006 |
Not specifically patients with normal or abnormal kidney function (mix of impaired kidney function and diabetics) |
|
Duong, 2005 |
overlaps with Loomba, 2013 and is a less recent review |
|
Gomes, 2005 |
Not specifically patients with normal or abnormal kidney function (mix of impaired kidney function and diabetics) |
|
Gonzales, 2007 |
overlaps with Loomba, 2013 and is a less recent review |
|
Gouveira, 2015 |
review, not systematic |
|
Gulel, 2005 |
already included in Loomba 2013 |
|
Gurm, 2011 |
Does not answer study question |
|
Hafiz, 2012 |
Acetylcysteine not compared to control |
|
Hassan, 2011 |
observational study |
|
Housseinjani, 2013 |
review, not systematic |
|
Hsu, 2012 |
already included in review Wu 2013 |
|
Hsu, 2007 |
already included in review Wu 2013 |
|
Izcovich, 2015 |
systematic review, poor quality (no clear description of included studies) |
|
Jo, 2009 |
does not compare no NAC to NAC |
|
Juergens, 2010 |
does not compare no NAC to NAC (both treatment arms recieve NAC) |
|
Khalili, 2006 |
Prehydration only, not comparable to Dutch clinical practice |
|
Kim, 2010 |
already included in Loomba 2013 |
|
Kotlyar, 2005 |
Dubbel met Kotlyar, 2005 |
|
Lee, 2011 |
does not compare no NAC to NAC (both treatment arms recieve NAC) |
|
Liu, 2006 |
overlaps with Loomba, 2013 and is a less recent review |
|
Marenzi, 2006 |
Not specifically patients with normal or abnormal kidney function (mix of impaired kidney function and diabetics) |
|
Mittal, 2014 |
review, not systematic |
|
Momeni, 2012 |
Observational study |
|
O’Sullivan 2013 |
Does not answer reseach question broadly enough, used for cross refernecing |
|
Ratcliffe, 2009 |
Not specifically patients with normal or abnormal kidney function (mix of impaired kidney function and diabetics) |
|
Ritz, 2006 |
letter to the editor, not an original article |
|
Sandhu, 2006 |
Unclear if patients were hydrated next to the NAC administration or not |
|
Sar, 2010 |
Not specifically patients with normal or abnormal kidney function (mix of impaired kidney function and diabetics) |
|
Shabbir, 2015 |
Article not found |
|
Shalansky, 2006 |
review, not systematic |
|
Solomon, 2014 |
review, not systematic |
|
Staniloae, 2009 |
subanalysis of trial, observational data |
|
Thiele, 2010 |
already included in Loomba 2013 |
|
Trivedi, 2009 |
overlaps with Loomba, 2013 and is a less recent review |
|
Zagler, 2006 |
overlaps with Loomba, 2013 and is a less recent review |
Risk of bias table for intervention studies (randomized controlled trials)
Research question:
|
Study reference
(first author, publication year) |
Describe method of randomisation1 |
Bias due to inadequate concealment of allocation?2
(unlikely/likely/unclear) |
Bias due to inadequate blinding of participants to treatment allocation?3
(unlikely/likely/unclear) |
Bias due to inadequate blinding of care providers to treatment allocation?3
(unlikely/likely/unclear) |
Bias due to inadequate blinding of outcome assessors to treatment allocation?3
(unlikely/likely/unclear) |
Bias due to selective outcome reporting on basis of the results?4
(unlikely/likely/unclear) |
Bias due to loss to follow-up?5
(unlikely/likely/unclear) |
Bias due to violation of intention to treat analysis?6
(unlikely/likely/unclear) |
|
CT scan, normal kidney function |
||||||||
|
Hsu, 2012 |
Computer-generated random numbers |
Unlikely |
Unlikely |
Unlikely |
Unlikely |
Unlikely |
Unlikely |
Unclear |
|
CT scan, decreased kidney function |
||||||||
|
Kama, 2014 |
By website randomization.com |
Unlikely |
Unlikely |
Unlikely |
Unlikely |
Unlikely |
Unlikely |
Unclear |
|
Kitzler, 2012 |
Not reported |
Unlikely |
Unlikely |
Unlikely |
Unlikely |
Unclear |
Unclear |
Unclear |
|
Poletti, 2007 |
Randomized by serial enrolment |
Unlikely |
Unlikely |
Unlikely |
Unlikely |
Unlikely |
Unclear |
Unclear |
|
Poletti, 2013 |
Computer generated randomization list |
Unlikely |
Unlikely |
Unlikely |
Unlikely |
Unlikely |
Unclear |
Unlikely |
|
Tepel, 2000 |
“Randomly assigned” |
Unlikely |
Unlikely |
Unlikely |
Unlikely |
Unlikely |
Unclear |
Unlikely |
|
CAG or PCI, normal kidney function |
||||||||
|
Carbonell, 2007 |
Computer-generated random numbers |
Unlikely |
Unlikely |
Unlikely |
Unlikely |
Unlikely |
Unlikely |
Unclear |
|
Jaffery, 2012 |
“Randomly assigned” |
Unlikely |
Unlikely |
Unlikely |
Unlikely |
Likely |
Unlikely |
Unclear |
|
Kim, 2010 |
Computer-generated random numbers |
Unlikely |
Unlikely |
Unlikely |
Unlikely |
Unlikely |
Unlikely |
Unclear |
|
Kinbara, 2010 |
“Randomly assigned” |
Unlikely |
Unlikely |
Unlikely |
Unlikely |
Unlikely |
Unlikely |
Unclear |
|
Lawlor, 2004 |
“randomization was performed by the hospital clinical trials pharmacist” |
Unlikely |
Unlikely |
Unlikely |
Unlikely |
Unlikely |
Unlikely |
Unclear |
|
Sadat, 2011 |
Computer generated randomization scheme |
Unlikely |
Unlikely |
Unlikely |
Unlikely |
Unlikely |
Unlikely |
Unclear |
|
Tanaka, 2011 |
“Randomly assigned” |
Unlikely |
Unlikely |
Unlikely |
Unlikely |
Unlikely |
Unlikely |
Unclear |
|
Thiele, 2010 |
Computer generated random numbers |
Unlikely |
Unlikely |
Unlikely |
Unlikely |
Unlikely |
Unlikely |
Unclear |
|
CAG or PCI, decreased kidney function |
||||||||
|
ACT, 2011 |
24-hour Web-based automated randomization system |
Unlikely |
Unlikely |
Unlikely |
Unlikely |
Unlikely |
Unlikely |
Unlikely |
|
Castini, 2010 |
Computer generated randomization table |
Unlikely |
Unlikely |
Unlikely |
Unlikely |
Unlikely |
Unclear |
Unclear |
|
Ferrario, 2009 |
Computer generated randomization list |
Unlikely |
Unlikely |
Unlikely |
Unlikely |
Unlikely |
Unlikely |
Unclear |
|
Gulel, 2005 |
Random allocation table |
Unlikely |
Unlikely |
Unlikely |
Unlikely |
Unlikely |
Unclear |
Unclear |
|
Habib, 2016 |
Patients were randomized into three groups |
Unlikely |
Unlikely |
Unlikely |
Unlikely |
Unlikely |
Unclear |
Unclear |
|
Izani Wan (Mohamed), 2008 |
Computer generated randomization list |
Unlikely |
Unlikely |
Unlikely |
Unlikely |
Unlikely |
Unlikely |
Unclear |
|
Koc, 2012 |
Not described |
Unlikely |
Unlikely |
Unlikely |
Unlikely |
Unlikely |
Unclear |
Unclear |
|
Kotlyar, 2005 |
Not described |
Unlikely |
Unlikely |
Unlikely |
Unlikely |
Unlikely |
Unclear |
Unclear |
|
Sadineni, 2017 |
Patients were randomly assigned |
Unlikely |
Unlikely |
Unlikely |
Unlikely |
Unlikely |
Unclear |
Unclear |
|
Seyon, 2007 |
Not described |
Unlikely |
Unlikely |
Unlikely |
Unlikely |
Unlikely |
Unclear |
Unclear |
- Randomisation: generation of allocation sequences have to be unpredictable, for example computer generated random-numbers or drawing lots or envelopes. Examples of inadequate procedures are generation of allocation sequences by alternation, according to case record number, date of birth or date of admission.
- Allocation concealment: refers to the protection (blinding) of the randomisation process. Concealment of allocation sequences is adequate if patients and enrolling investigators cannot foresee assignment, for example central randomisation (performed at a site remote from trial location) or sequentially numbered, sealed, opaque envelopes. Inadequate procedures are all procedures based on inadequate randomisation procedures or open allocation schedules..
- Blinding: neither the patient nor the care provider (attending physician) knows which patient is getting the special treatment. Blinding is sometimes impossible, for example when comparing surgical with non-surgical treatments. The outcome assessor records the study results. Blinding of those assessing outcomes prevents that the knowledge of patient assignement influences the proces of outcome assessment (detection or information bias). If a study has hard (objective) outcome measures, like death, blinding of outcome assessment is not necessary. If a study has “soft” (subjective) outcome measures, like the assessment of an X-ray, blinding of outcome assessment is necessary.
- Results of all predefined outcome measures should be reported; if the protocol is available, then outcomes in the protocol and published report can be compared; if not, then outcomes listed in the methods section of an article can be compared with those whose results are reported.
- If the percentage of patients lost to follow-up is large, or differs between treatment groups, or the reasons for loss to follow-up differ between treatment groups, bias is likely. If the number of patients lost to follow-up, or the reasons why, are not reported, the risk of bias is unclear
- Participants included in the analysis are exactly those who were randomized into the trial. If the numbers randomized into each intervention group are not clearly reported, the risk of bias is unclear; an ITT analysis implies that (a) participants are kept in the intervention groups to which they were randomized, regardless of the intervention they actually received, (b) outcome data are measured on all participants, and (c) all randomized participants are included in the analysis.
Evidence table for intervention studies (randomized controlled trials and non-randomized observational studies [cohort studies, case-control studies, case series])1
This table is also suitable for diagnostic studies (screening studies) that compare the effectiveness of two or more tests. This only applies if the test is included as part of a test-and-treat strategy – otherwise the evidence table for studies of diagnostic test accuracy should be used.
Research question:
|
Study reference |
Study characteristics |
Patient characteristics 2 |
Intervention (I) |
Comparison / control (C) 3
|
Follow-up |
Outcome measures and effect size 4 |
Comments |
|
CT scan, normal kidney function |
|||||||
|
Hsu, 2012 |
Type of study: Randomized controlled trial
Setting: emergency department, medical teaching center
Country: Taiwan
Source of funding: non-commercial |
Inclusion criteria: 1) all adult patients who received chest or abdominal contrast-enchanced computed tomography (CECT)
Exclusion criteria: 1) patients undergoing long-term hemodialysis or peritoneal hemodialysis 2) patients who received another dose of contrast medium within 72 hours 3) patient refused to sign concent forms 4) patients had a knon allergic reaction to N-acetlycysteine (NAC)
N total at baseline: Intervention: 106 Control: 103
Important prognostic factors2: For example age ± SD: I: 80 ± 9 C: 80 ± 11
Sex: I: 74% M C: 76% M Baseline SCr (mg/dL) ± SD I: 1.40 ± 0.58 C: 1.26 ± 0.43
Groups comparable at baseline? |
Describe intervention (treatment/procedure/test):
600mg NAC In 0.9% sodium chloride (3 mL/kg/h) for 60 minutes prior to the CECT
0.9% sodium chloride (1 mL/kg/h) for 6 hours after CECT
|
Describe control (treatment/procedure/test):
0.9% sodium chloride (3 mL/kg/h) for 60 minutes prior to the CECT
0.9% sodium chloride (1 mL/kg/h) for 6 hours after CECT
|
Length of follow-up: 72 hours
Loss-to-follow-up: Not reported
Incomplete outcome data: Not reported
|
Outcome measures and effect size (include 95%CI and p-value if available):
CIN05: (=a rise in SCr ≥0.5mg/dL within 48-72 hours after CECT imaging) I: 7.5% C: 14.6% Odds Ratio (OR): 0.31 (95% CI: 0.10 – 0.96, p=0.04)
CINor: (=a rise in SCr ≥0.5mg/dL or 25% within 48-72 hours after CECT imaging) I: 11.3% C: 19.4% OR: 0.35 (95% CI: 0.13 – 0.91, 0=0.03)
Mortality: I: 7.5% C: 12.6% OR: 0.49 (95% CI: 0.15 – 1.55, p=0.22)
Permanent renal replacement therapy: 0% in both groups |
Authors’ conclusion: A singe dose of NAC before CECT imagingcan prevent CIN in an ED setting. However it does not improve mortality rate or the need for dialysis.
Patients with congestive pulmonary edema received an adjusted hydration schedule where the rates of fluid loading were decreased by 50%. |
|
CT scan, decreased kidney function |
|||||||
|
Kama, 2014 |
Type of study: randomized controlled trial
Setting: emergency department, academic tertiary hospital
Country: Turkey
Source of funding: not reported |
Inclusion criteria: 1) adult patients (≥18 years) who presented to the emergency department 2) patients who received CECT as part of their emergency care 3) moderate or high risk for contrast induced nephropathy (CIN) according to Mehran score (>5)
Exclusion criteria: 1) CIN risk determine as Low by Mehran score 2) history of contrast-related allergies 3) hemodynamically unstable patients requiring resuscitation or surgery 4) patients receiving renal replacement therapy 5) patients did not provide infomed consent
N total at baseline: Intervention: 36 Control: 35
Important prognostic factors2: For example age (95% CI): I: 69 (65-73) C: 67 (62-72)
Sex: I:69 % M C: 65% M
eGFR <20 mL/min/1.73m2 I: 25% C: 9% eGFR 40-20 mL/min/1.73m2 I: 36% C: 46% eGFR 60-40mL/min/1.73m2 I: 11% C: 14%
Groups comparable at baseline? Yes |
Describe intervention (treatment/procedure/test):
150mg/kg NAC In 1000mL in 0.9% saline at the rate of 350ml/hour for 3 hours Before, after and during administration of contrast
|
Describe control (treatment/procedure/test):
1000mL 0.9% saline at the rate of 350ml/hour for 3 hours Before, after and during administration of contrast
|
Length of follow-up: 48-72 hours Patients who were diagnosed with CIN – 1 months
Loss-to-follow-up: Not reported
Incomplete outcome data: Not reported
|
Outcome measures and effect size (include 95%CI and p-value if available):
CIN (=25% increase or greater than 0.5mg/dL (44µmol/L) increase in the serum creatinine level, 48-72 hours after administration of the contrast agent compared with the baseline creatinine measurement) I: 7 (19%) C: 5 (14%) p>0.05
No contrast- or treatment-induced adverse events were detected during emergency department care |
Authors’conclusion: None of the short-term protocols with normal saline or NAC was superior in the emergency department pateints requiring CECT who had a moderate or high risk of CIN. |
|
Kitzler, 2012 |
Type of study: randomized controlled trial
Setting: single-center, elective patients
Country:
Source of funding: |
Inclusion criteria: -patients with chronic kidney disease stage 1-4 undergoing elective computer-assisted tomography with non-ionic radiocontrast agents when compared to 0.45% saline alone
Exclusion criteria: -
N total at baseline: Intervention: 10 Control: 10
Important prognostic factors2: For example age ± SD: mean: 75 years (not reported per group)
Sex: 38% M (not reported per group)
Groups comparable at baseline? Unc;ear |
Describe intervention (treatment/procedure/test):
N-acetylcysteine 4800mg per os
0.45% saline, 1mL/kg/h over 24 hours
|
Describe control (treatment/procedure/test):
0.45% saline, 1mL/kg/h over 24 hours
|
Length of follow-up: Not reported
Loss-to-follow-up: Not reported
Incomplete outcome data: Not reported
|
Outcome measures and effect size (include 95%CI and p-value if available):
No patients developed contrast induced acute kidney injury.
There was no significant difference in serum creatinine change between the three study arms. |
Authors’ conclusion: Following radiocontrast administration neither vitamin E nor NAC in addition to saline demonstrated an additional beneficial effect on kidney fi=unction when compared to saline alone. |
|
Poletti, 2007 |
Type of study: randomized controlled trial
Setting: emergency patients
Country: Switzerland
Source of funding: not reported |
Inclusion criteria: 1) patients admitted consecutively to the emergency department during daytime hours 2) serum creatinine >1.2md/dL
Exclusion criteria: 1) pregnancy 2) end stage renal failure with dialysis 3) suspicion of acute renal obstruction 4) asthma 5) severe cardiac failure 6) hemodynamically unstable condition contraindicating iv hydration 7) nonurgent indications for CT
N total at baseline: 87 Intervention: 44 Control: 43
Important prognostic factors2: For example age ± SD: I: 70 ± 19 C: 73 ± 17
Sex: I: 59% M C: 67% M
Groups comparable at baseline? Yes |
Describe intervention (treatment/procedure/test):
900mg NAC diluted in 5% glucose solution administered iv 1 hour before CT
0.45% saline iv at a rate of 5mL/kg body weight over the course of an hour before CT
900mg NAC mixed into the 0.45% saline perfusion administered iv after completion of CT at a rate of 1mL/kg body weight per hour for 12 hours
|
Describe control (treatment/procedure/test):
placebo in 5% glucose solution administered iv 1 hour before CT
0.45% saline iv at a rate of 5mL/kg body weight over the course of an hour before CT
placebo mixed into the 0.45% saline perfusion administered iv after completion of CT at a rate of 1mL/kg body weight per hour for 12 hours
|
Length of follow-up: 4 days
Loss-to-follow-up: 7 (8%) 3 died, 3 left hospital 1 transferred to another hospital (not reported per group)
Incomplete outcome data: As above
|
Outcome measures and effect size (include 95%CI and p-value if available): Nephrotoxicity (=≥25% increase in serum creatinine value) I: 2/44 (5%) C: 9/43 (21%) P=0.026 |
Authors’ conclusion:
On the basis of the serum creatinine concentration, iv administration of NAC appears protective against the nephrotoxicity of contrast medium. |
|
Poletti, 2013 |
Type of study: randomized controlled trial
Setting: emergency department patients
Country: Switzerland
Source of funding: not reported |
Inclusion criteria: 1) patients admitted consecutively to the emergency department 2) estimated creatinine clearance by MDRD of <60ml/min/1.73m2
Exclusion criteria: 1) asthma 2) pregnancy 3) obstructive nephropathy 4) patient’s refusal
N total at baseline: 104 Intervention: 55 Control: 59
Important prognostic factors2: For example age ± SD: I: 78 ± 12 C: 78 ± 12
Sex: I: 49% M C: 51% M
Groups comparable at baseline? Yes |
Describe intervention (treatment/procedure/test):
6000mg NAC iv diluted in 100mL saline, administered in the 60 minutes before the CT-scan
Hydration of 250mL of 0.45% saline before CT-scan
1000mL saline 0.45% after CT-scan
|
Describe control (treatment/procedure/test):
placebo diluted in 100mL saline, administered in the 60 minutes before the CT-scan
Hydration of 250mL of 0.45% saline before CT-scan
1000mL saline 0.45% after CT-scan
|
Length of follow-up: 10 days
Loss-to-follow-up: Intervention: 3 (5%) Reasons not reported
Control: 1 (2%) Reasons not reported
Incomplete outcome data: As above
|
Outcome measures and effect size (include 95%CI and p-value if available):
Nephropathy (=increase of at least 25% or 44µmol/l in serum creatinine level at day 2,4 or 10 compared to day 0) I: 8 (15%) C: 10 (17%) P=0.99
Composite event of death or acute kidney injury I: 33% C: 24% p-value not reported |
Authors’ conclusion:
An ultra-high dose of intravenous NAC is ineffective at preventing nephrotoxicity in patients with renal impairment undergoing emergency contrast CT. |
|
Tepel, 2000 |
Type of study: Randomized controlled trial
Setting: elective patients receiving CT-scan at hospital
Country: Germany
Source of funding: not reported |
Inclusion criteria: 1) patients with a serum creatinine >1.2mg/dL or creatinine clearance <50mL/min 2) known chronic renal failure and a stable serum creatinine concentration 3) patients receiving elective CT-scans
Exclusion criteria: 1) acute renal failure
N total at baseline: Intervention: 41 Control: 42
Important prognostic factors2: For example age ± SD: I: 66±11 C: 65 ± 15
Sex: I:59 % M C: 55% M
Groups comparable at baseline? Yes |
Describe intervention (treatment/procedure/test):
Acetylcycsteine orally 600mg twice daily on the day before and on the day of administration of the contrast agent
Saline (0.45%) iv. 1ml/kg/h for 12 hours before and 12 hours after contrast administration
|
Describe control (treatment/procedure/test):
Saline (0.45%) iv. 1ml/kg/h for 12 hours before and 12 hours after contrast administration
|
Length of follow-up: 48 hours, 6 days
Loss-to-follow-up: Not reported
Incomplete outcome data: Not reported
|
Outcome measures and effect size (include 95%CI and p-value if available):
Increase of at least 0.5mg/dL (44µmol/L) in serum creatinine concentration 48 hours after administration of contrast agent: I: 1/41 (2%) C: 9/42 (21%) RR: 0.1 (95% CI: 0.01 – 0.9) P=0.01
None of the patients required dialysis |
Authors’ conclusion:
Prophylactic administration of the antioxidant acetylcysteine, along with hydration, prevents the reduction in renal function induced by iopromide, a non-ionic, low-osmolality contrast agent, in patients with chronic renal insufficiency. |
|
CAG or PCI, normal kidney function |
|||||||
|
Carbonell, 2007 |
Type of study: randomized controlled trial
Setting: tertiary hospital, cardiac unit
Country: Spain
Source of funding: not reported |
Inclusion criteria: 1) patients with acute coronary syndrome and normal renal function, admitted to the cardiac unit and referred for cardiac catheterization 2) angina at rest or post-myocardial infarction Or they had received thrombolytic therapy with failed recanalization so the cardiac catheterisation was an emergency procedure
Exclusion criteria: 1) chronic renal failure or acute renal dysfunction 2) hemodynamic instability (systolic blood pressure <90mmHg) 3) known allergy to NAC or contrast agents 4) untreated gastrointestinal bleeding 5) previous treatment with theophylline, mannitol or nephrotoxic antibiotics
N total at baseline: Intervention: 107 Control: 109
Important prognostic factors2: For example age ± SD: I: 63 ± 14 C: 61 ± 12
Sex: I: 80% M C: 73% M
Creatinine clearance (ml/min) I: 86 ± 29 C: 88 ± 30
Groups comparable at baseline? |
Describe intervention (treatment/procedure/test):
NAC (600mg diluted in 50mL of 0.9% saline) iv for 30 minutes twice daily for a total of 4 times Starting at least for 6 hours before the administration of contrast media
0.9% saline iv at least 6 hours before procedure, maintained for 12 hours after contrast dosing
|
Describe control (treatment/procedure/test):
placebo (diluted in 50mL of 0.9% saline) iv for 30 minutes twice daily for a total of 4 times Starting at least for 6 hours before the administration of contrast media
0.9% saline iv at least 6 hours before procedure, maintained for 12 hours after contrast dosing
|
Length of follow-up: 48 hours
Loss-to-follow-up: Not reported
Incomplete outcome data: Not reported
|
Outcome measures and effect size (include 95%CI and p-value if available):
Contrast induced nephropathy (=an acute increase in the serum creatinine concentration ≥0.5mg/dL and/or >25% increase above baseline level at 48 hours after contrast dosing) I; 10.3% C: 10.1% P=0.50
None of the patients required dialysis. |
Patients with congestive heart failure received a reduced hydration volume.
Authors’ conclusion: The prophylactic administration of intravenous NAC provides no additional benefit to saline in high-risk coronary patients with normal renal function. |
|
Jaffery, 2012 |
Type of study: randomized controlled trial
Setting: single-center inpatients, emergency procedure
Country: United States of America
Source of funding: not reported |
Inclusion criteria: 1) patients hospitalized with a primary diagnosis of acute coronary syndrome 2) scheduled for coronary angiography (CAG) or intervention during this hospitalization 3) age ≥18 years
Exclusion criteria: 1) end stage renal disease requiring dialysis 2) hypersensitivity to NAC 3) history of life-threatening contrast reaction
N total at baseline: Intervention: 192 Control: 206
Important prognostic factors2: For example age ± SD: I: 66 ± 13 C: 65 ± 13
Sex: I: 67 % M C: 59 % M
Baseline creatinine clearance (ml/min) I: 87 ± 41 C: 92 ± 44
Groups comparable at baseline? Yes |
Describe intervention (treatment/procedure/test):
NAC: 1200mg bolus followed by 200mg/h for 24 hours
In 500ml 5% dextrose solution of water iv
Normal saline (0.9%) iv; 1/ml/kg for 24 hours
|
Describe control (treatment/procedure/test):
Placebo in 500ml 5% dextrose solution of water iv
Normal saline (0.9%) iv; 1/ml/kg for 24 hours
|
Length of follow-up: 72 hours for lab parameters 30 days for mortality and hospital stay
Loss-to-follow-up: Not reported
Incomplete outcome data: Not reported
|
Outcome measures and effect size (include 95%CI and p-value if available):
CIN (=increase in serum creatinine concentration ≥25% above the baseline level within 72 hours of the administration of intravenous contrast) I: 16% C: P=0.40
Outcomes of mortality and length of hospital not reported. |
Patients with clinical evidence of heart failure received only NAC iv or placebo
Authors’ conclusion: In acute coronary syndrome patients undergoing CAG with or without percutaneous intervention (PCI), high-dose intravenous NAC failed to reduce the incidence of CIN. |
|
Kim, 2010 |
Type of study: randomized controlled trial
Setting: elective patients, one hospital
Country: South Korea
Source of funding: not reported |
Inclusion criteria: 1) patients scheduled for elective CAG and/or PCI with apparently normal renal function
Exclusion criteria: 1) acute coronary syndrome requiring emergency CAG/PCI 2) cardiogenic shock 3) iodinated contrast media administration within a monthor NAC within 48 hours before study entry 4) current dialysis or a serum creatinine >1.4mg/dL for men or >1.2mg/dL for women 5) thyroid diseases 6) allergy to the study medication
N total at baseline: Intervention: 80 Control: 86
Important prognostic factors2: For example age ± SD: I: 62 ± 11 C: 62 ± 10
Sex: I: 79% M C: 67% M
SCr (mg/dL) I: 1.03 ± 0.17 C: 1.03 ± 0.14
Groups comparable at baseline? Yes |
Describe intervention (treatment/procedure/test):
Oral acetylcysteine 600mg twice a day on the day before and the day of coronary angiography
0.9% saline 1/mL/kg/h for 12 hours before and 6hours after CAG
|
Describe control (treatment/procedure/test):
0.9% saline 1/mL/kg/h for 12 hours before and 6hours after CAG
|
Length of follow-up: 48 hours
Loss-to-follow-up: Not reported
Incomplete outcome data: Not reported
|
Outcome measures and effect size (include 95%CI and p-value if available):
CIN (=increase in sCR of at least 0.5mg/dL or >25% within 48 hours of contrast exposure) I: 3.8% C: 8.1% p>0.05 |
Authors’ conclusion:
Not relevant – based on cystatin-C defined CIN results and not the sCR based CIN. |
|
Kinbara, 2010 |
Type of study: randomized controlled trial
Setting: elective patients, one hospital
Country: Japan
Source of funding: not reported |
Inclusion criteria: 1) Patients with stable coronary artery disease scheduled to undergo CAG and/or PCI, with stable serum creatinine concentrations
Exclusion criteria: 1) acute myocardial infarction 2) use of vasopressors before PCI 3) cardiogenic shock 4) current peritoneal or hemodialysis 5) planned post-contrast dialysis 6) allergies to ths study medications 7) congestive heart disease 8) severe valvular disease 9) pregnancy 10) multiple myeloma 11) amyloidosis
N total at baseline: Intervention: 15 Control: 15
Important prognostic factors2: For example age ± SD: I: 70 ± 10 C: 70 ± 8
Sex: I: 80% M C: 80% M
SCr (mg/dL) I: 1.00 ± 0.36 C: 0.94 ± 0.21
Groups comparable at baseline? Yes |
Describe intervention (treatment/procedure/test):
NAC 704mg orally twice daily on the day before ond on the day of CAG and/or PCI
0.9% saline iv 1/ml/kg/hour For 30 minutes before and 10 hours after angiography
|
Describe control (treatment/procedure/test):
0.9% saline iv 1/ml/kg/hour For 30 minutes before and 10 hours after angiography
|
Length of follow-up: 48 hours
Loss-to-follow-up: Not reported
Incomplete outcome data: Not reported
|
Outcome measures and effect size (include 95%CI and p-value if available):
CIN (=SCr increase of >0.5mg/dL from baseline to 48 hours to angiography) I: 0 (0%) C: 4 (27%) 96% CI: 0.10 – 5.991, p=0.011 |
Authors’ conclusion:
These results suggest that both prophylactic NAC and aminophylline administration are effective in preventing CIN, but not with hydration alone. |
|
Lawlor, 2004 |
Type of study: randomized controlled trial
Setting: elective patients, single center
Country: United Kingdom
Source of funding: not reported |
Inclusion criteria: 1) patients with peripheral vascular disease going for elective angiography or angioplasty to participate in this trial
Exclusion criteria: -
N total at baseline: Intervention: 46 Control: 48
Important prognostic factors2: For example age ± SD: I: 72 ± 12 C: 69 ± 12
Sex: I: 59% M C: 69% M
SCr (µmol/L) I: 110 ± 42 C: 124 ± 63
Groups comparable at baseline? Yes |
Describe intervention (treatment/procedure/test):
1g of NAC in each bag of 0.9% saline
0.9% saline (500mL over 4-6 hours) 6-12 hours prior to angiography and again after angiography |
Describe control (treatment/procedure/test):
0.9% saline (500mL over 4-6 hours) 6-12 hours prior to angiography and again after angiography with placebo |
Length of follow-up: 7 days
Loss-to-follow-up: Not reported
Incomplete outcome data: Not reported
|
Outcome measures and effect size (include 95%CI and p-value if available):
CIN (=a rise of 25% or 0.5mg/dL in sCR at 48 hours after contrast administration)
Patients with normal kidney function: I: 0/29 (0%) C: 0/27 (0%) p>0.05
Patients with decreased kidney function: I: 3/17 (18%) C: 3/21 (14%) p>0.05 |
Authors’ conclusion:
NAC pre-contrast and post-contrast does not confer any benefit in preventing radiocontrast induced nephropathy in vascular patients |
|
Sadat, 2011 |
Type of study: randomized controlled trial
Setting: elective patients, single center
Country: United Kingdom
Source of funding: no funding |
Inclusion criteria: 1) patients undergoing peripheral angiography for peripheral artery disease
Exclusion criteria: 1) patients with established renal failure – on renal replacement therapy
N total at baseline: Intervention: 21 Control: 19
Important prognostic factors2: For example age ± SD: I: 75 ± 11 C: 70 ± 14
Sex: Not reported
Groups comparable at baseline? Unclear |
Describe intervention (treatment/procedure/test):
NAC 600mg twice daily orally on the ay before and on the day of CAG (2.4g in total)
Iv hydration 0.9% saline 1L over 12 hours before CAG 1L over 12 hours after CAG
|
Describe control (treatment/procedure/test):
Iv hydration 0.9% saline 1L over 12 hours before CAG 1L over 12 hours after CAG
|
Length of follow-up: 72 hours
Loss-to-follow-up: Not reported
Incomplete outcome data: Not reported
|
Outcome measures and effect size (include 95%CI and p-value if available):
CIN (=0.5mg/dL or 25% increase in sCr from baseline value within 48 hours of exposure to intravascular radiographic contrast media that is not attributable to other causes) I: 1/21 (5%) C: 3/19 (16%) P=0.33 |
Authors’ conclusion:
A clear conclusion is not formulated. |
|
Tanaka, 2011 |
Type of study: randomized controlled trial
Setting: emergency patients, single center
Country: Japan
Source of funding: not reported |
Inclusion criteria: 1) patients admitted for ST-segment elevation acute myocardial infarction treated with primary PCI
Exclusion criteria: 1) dialysis 2) known allergy to NAC 3) inability to take NAC orally
N total at baseline: Intervention: 38 Control: 38
Important prognostic factors2: For example age ± SD: I: 63 ± 13 C: 61 ± 14
Sex: I: 82% M C: 82% M
SCr (mg/dL) I: 0.95 ± 0.34 C: 0.88 ± 0.25
Groups comparable at baseline? Yes |
Describe intervention (treatment/procedure/test):
NAC 705mg orally before and 12, 24, 26 pours after intervention (2.8g in total)
Hydration with iv Ringer lactate solution at a rate of 1-2ml/kg/hour for more than 12 hours after primary CAG |
Describe control (treatment/procedure/test):
Hydration with iv Ringer lactate solution at a rate of 1-2ml/kg/hour for more than 12 hours after primary CAG |
Length of follow-up: 36 hours
Loss-to-follow-up: Not reported
Incomplete outcome data: Not reported
|
Outcome measures and effect size (include 95%CI and p-value if available):
CIN (=an increase in sCr level of 25% or more from baseline value within 72 hours after primary angioplasty) I: 2/38 (5%) C: 5/38 (13%) P=0.21
No major adverse events (death, acute renal failure requiring temporary replacement therapy, need for mechanical ventilation) occurred in either group during the in-hospital follow-up period. |
Authors’ conclusion:
While N=acetylcysteine might have the possibility to reduce the incidence of contrast-induced nephropathy in patients undergoing primary angioplasty for acute myocardial infarction, the in-hospital mortality and morbidity were not significantly different between the two groups. |
|
Thiele, 2010 |
Type of study: randomized controlled trial
Setting: emergency patients, one tertiary hospital
Country: Germany
Source of funding: not reported |
Inclusion criteria: 1) patients with acute myocardial infarction undergoing primary PCI 2) symptoms <12 hours and ST-segment elevation ≥0.1mV in ≥2 extremity leads or ≥o.2 mV in ≥2 ore-cordial leads
Exclusion criteria: 1) previous fibrinolysis <12 hours 2) known NAC allergy 3) chronic dialysis 4) pregnancy 5) contra-indications for magnetic resonance imaging
N total at baseline: Intervention: 126 Control: 125
Important prognostic factors2: For example age (interquartile range): I: 68 (57-75) C: 68 (56-76)
Sex: I: 71% M C: 66% M
SCr (µmol/L; interquartile range) I: 81 (69-97) C: 78 (67-90)
Groups comparable at baseline? Yes |
Describe intervention (treatment/procedure/test):
NAC intravenous bolus 1200mg before CAG And 1200mg twice daily for 48 hours (total dose 6g)
Hydration with intravenous 0.9% saline; infusion rate 1ml/kg/hour for 12 hours (or 0.5mg/kg/h in overt heart failure)
|
Describe control (treatment/procedure/test):
10mL of 0.9% saline at each injection
Hydration with intravenous 0.9% saline; infusion rate 1ml/kg/hour for 12 hours (or 0.5mg/kg/h in overt heart failure)
|
Length of follow-up: Laboratory parameters: 72 hours Clinical endpoints: 6 months
Loss-to-follow-up: none
Incomplete outcome data: none
|
Outcome measures and effect size (include 95%CI and p-value if available):
CIN (=increase in sCr of ≥25% from baseline within 72 hours after PCI) I: 18/126 (14%) C: 25/125 (20%) P=0.28
Mortality after 6 months I: 12/126 (14%) C: 12/125 (14%) p>0.05
New congestive heart failure I: 11/126 (9%) C: 7/125 (6%) p>0.05 |
Authors’ conclusion:
High-dose iv NAC does not provide additional clinical benefit to placebo with respect to CIN in non-selected patients undergoing angioplasty with moderate doses of contrast medium and optimal hydration. |
|
CAG or PCI, decreased kidney function |
|||||||
|
ACT, 2011 |
Type of study: randomized controlled trial
Setting: inpatients, elective, multi-centre
Country: Brazil
Source of funding: non-commercial |
Inclusion criteria: 1) patients undergoing CAG or peripheral arterial angiography 2) at least one risk factor for CI-AKI: -age >70 years -chronic renal failure -diabetes mellitus -clinical evidence of congestive heart failure -left ventricular ejection fraction <0.45 -hypotension
Exclusion criteria: -patients on dialysis -patients with ST-segment elevation myocardial infarction -pregnancy or breastfeeding -women <45 years who did not use contraceptive methods
N total at baseline: Intervention: 1172 Control: 1136
With eGFR<30 ml/min I: 68 C: 63
With eGFR 30 to 60 ml/min I: 515 C: 492
Important prognostic factors2: For example age ± SD: I: 68 ± 10 C: 68 ± 10
Sex: I: 62% M C:61 % M
Groups comparable at baseline? Yes
|
Describe intervention (treatment/procedure/test):
NAC 2x600mg orally every 12 hours for 2 days (2 doses before and 2 doses after contrast administration, total dose 4800mg)
Hydration with 0.9% saline 1mg/kg/hour from 6-12 hours before to 6-12 hours after angiography
|
Describe control (treatment/procedure/test):
placebo orally every 12 hours for 2 days (2 doses before and 2 doses after contrast administration)
Hydration with 0.9% saline 1mg/kg/hour from 6-12 hours before to 6-12 hours after angiography
|
Length of follow-up: 48-96 hours for laboratory parameters 30 days for clinical events
Loss-to-follow-up: Intervention: 56 (5%) 12 did not receive study drug before angiography 15 were not submitted to angiography 19 were lost to 48-96 hour serum creatinine follow-up 4 died before 48-96 hours 15 did not return to collect serum creatinine 1 was lost to 30-day follow-up
Control: 54 (5%) 7 did not receive study drug before angiography 12 were not submitted to angiography 17 were lost to 48-96 hour serum creatinine follow-up 3 died before 48-96 hours 14 did not return to collect serum creatinine 1 was lost to 30-day follow-up
Incomplete outcome data: Intervention: 1153 (98%) had data included in laboratory parameters analysis 1171 (99.9%) had data included in secondary outcome analysis Reasons not reported
Control: 1119 (98%) had data included in laboratory parameters analysis 1135 (99.9%) had data included in secondary outcome analysis Reasons not reported |
Outcome measures and effect size (include 95%CI and p-value if available):
CI-AKI (=a 25% elevation of sCr above baseline 48-986 hours after angioplasty)
All participants I: 147/1153 (12.7%) C: 142/119 (12.7%) RR: 1.00 (95% CI: 0.81 – 1.25, p=0.97)
Patients with serum creatinine >1.5mg/dL: I: 12/188 (6%) C: 10/179 (6%) P=0.75
Patients with eGFR 30 – 60 mL/min I: 30/425 (7%) C: 27/398 (7%) RR: 1.04 (0.63 – 1.72) P=0.73
Patients with eGFR<30ml/min I: 6/56 (11%) C: 3/48 (6%) RR: 1.71 (0.45 – 6.49) P=0.92
Composite outcome of death or need for dialysis: I: 2,2% C: 2.3% Hazard ratio (HR): 0.97 (95% CI: 0.56 – 1.69, p=0.92)
Cardiovascular deaths: HR: 0.99 (95% CI: 0.51 – 1.99, p=0.97)
There was also no difference in the risk of these outcomes defined post hoc. |
Authors’ conclusion
In this large randomized trial we found that acetylcysteine does not reduce the risk of contrast-induced acute kidney injury or other clinically relevant outcomes in at-risk patients undergoing coronary or peripheral vascular angiography. |
|
Castini, 2008 |
Type of study: randomized controlled trial
Setting: elective patients, single centre
Country: Italy
Source of funding: not reported |
Inclusion criteria: 1) patients undergoing CAG and/or PCI 2) age ≥18 years 3) stable sCr ≥1.2mg/dL
Exclusion criteria: 1) sCr >4mg/dL 2) a history of dialysis, multiple myeloma, pulmonary edema, cardiogenic shock, acute myocardial infarction 3) emergency catheterization 4) recent exposure to radiographic contrast media within 7 days of the study 5) allergy to iodinate contrast media or NAC 6) previous enrolment in the same or other protocols 7) administration of mannitol, theophylline, dopamine, dobutamine, nonsteroidal anti-inflammatory drugs or fenoldopam
N total at baseline: Intervention: 52 Control: 51
Important prognostic factors2: For example age ± SD: I: 71 ± 7 C:73 ± 8
Sex: I: 94% M C: 84% M
sCr (mg/dL) I: 1.57 ± 0.38 C: 1.49 ± 0.30
Groups comparable at baseline? Yes |
Describe intervention (treatment/procedure/test):
NAC 600mg orally every 12 hours for 2 days (2 doses before and 2 doses after contrast administration, total dose 2400mg)
0.9% saline iv 1ml/kg/hour for 12 hours before and 12 hours after contrast administration
|
Describe control (treatment/procedure/test):
0.9% saline iv 1ml/kg/hour for 12 hours before and 12 hours after contrast administration
|
Length of follow-up: 5 days
Loss-to-follow-up: none
Incomplete outcome data: Not reported
|
Outcome measures and effect size (include 95%CI and p-value if available):
CIN1 (=increase in sCr ≥25% over the baseline value in any of the time points: 24, 48 and 120 hours after contrast administration) I: 7 (14%) C: 9 (17%) p>0.05
CIN2 (=increase in sCr ≥0.5mg/dL over the baseline value in any of the time points: 24, 48 and 120 hours after contrast administration) I: 4 (8%) p>0.05
No acute renal failure necessitating renal replacement therapy occurred. |
Authors’ conclusion
Our findings suggest that the addition of NAC does not add further benefit in CIN prevention, compared to standard hydration with isotonic saline infusion.
|
|
Ferrario, 2009 |
Type of study: randomized controlled trial
Setting: elective patients, university hospital
Country: Italy
Source of funding: not reported |
Inclusion criteria: 1) patients scheduled for elective or diagnostic CAG and/or PCI 2) age ≥18 years 3) creatinine clearance <55ml/min and a stable renal function
Exclusion criteria: 1) ongoing acute myocardial infarction or acute coronary syndrome 2) renal replacement therapy 3) allergy to NAC 4) need for administration of mannitol, theophylline, dopamine, dobutamine, fenoldopam or nephrotoxic drugs within 1 week of procedure 5) clinical signs of dehydration and systemic hypotension
N total at baseline: Intervention: 99 Control: 101
Important prognostic factors2: For example age ± SD: I: 75 ± 8 C: 75 ± 7
Sex: I: 68% M C: 62% M
Creatinine clearance (mL/min) I: 37 ± 11.5 C: 40 ± 9.3
Groups comparable at baseline? Yes |
Describe intervention (treatment/procedure/test):
NAC 600mg orally every 12 hours for 2 days (2 doses on the day before and 2 doses on the day of contrast administration, total dose 2400mg)
0.9% saline 1ml/kg/h in 12-24 hours before the procedure and 24 hours after
|
Describe control (treatment/procedure/test):
Placebo (glucose tablets) orally every 12 hours for 2 days (2 doses on the day before and 2 doses on the day of contrast administration)
0.9% saline 1ml/kg/h in 12-24 hours before the procedure and 24 hours after
|
Length of follow-up: 3 days
Loss-to-follow-up: Intervention: 4 (4%) Reasons not reported
Control: 4 (3%) Reasons not reported
Incomplete outcome data: Not reported
|
Outcome measures and effect size (include 95%CI and p-value if available):
CIN (=increase in sCr ≥0.5mg/dL or >25% within 3 days after the procedure) I: 8/99 (8%) C: 6/101 (6%) P=0.60 |
Authors’ conclusion
In our experience, NAC did not prevent CIN in patients receiving iso-osmolar (iodixanol) contrast media and adequate hydration. |
|
Gulel, 2005 |
Type of study: randomized controlled trial
Setting: elective, single centre
Country: Turkey
Source of funding: not reported |
Inclusion criteria: 1) patients scheduled for elective diagnostic CAG 2) chronic renal impairement: sCr >1.3mg/dL 3) stable renal function
Exclusion criteria: 1) acute renal failure 2) end-stage renal failure on regular dialysis 3) clinically evident heart failure 4) allergy against contrast agents 5) serious hepatic dysfunction 6) planned PCI
N total at baseline: Intervention: 25 Control: 25
Important prognostic factors2: For example age ± SD: I: 61 ± 12 C: 62 ± 12
Sex: I: 80% M C: 72% M
Creatinine clearance (mL/min) I: 46.5 ± 4.2 C: 43.2 ± 3.9
Groups comparable at baseline? Yes |
Describe intervention (treatment/procedure/test):
NAC 600mg orally every 12 hours for 2 days (2 doses on the day before and 2 doses on the day of contrast administration, total dose 2400mg)
0.9% saline 1ml/kg/h in 12 hours before the procedure and 12 hours after
|
Describe control (treatment/procedure/test):
0.9% saline 1ml/kg/h in 12 hours before the procedure and 12 hours after
|
Length of follow-up: 48 hours
Loss-to-follow-up: Not reported
Incomplete outcome data: Not reported
|
Outcome measures and effect size (include 95%CI and p-value if available):
Contrast nephropathy (= an increase more than 0.5 mg/dL 48 hours after the procedure compared with baseline values-) I: 3/25 (12%) C: 2/25 (8%) p>0.05 |
Authors’ conclusion:
Our results show that oral acetylcysteine does not reduce the risk of contrast nephropathy when used before elective diagnostic CAG in patients with renal dysfunction. |
|
Habib, 2016 |
Type of study: randomized controlled trial
Setting: European Gaza Hospital, Gaza, Palestine (Israel)
Source of funding: not reported |
Inclusion criteria: Patients had at least one risk factor for CIN (age >70 years, baseline creatinine level >1.5 mg/dL, heart failure, diabetes mellitus or contrast media volume >300 mL)
Exclusion criteria: Not stated
N total at baseline: Group A: 40 Group C: 40
Important prognostic factors2: For example age ± SD: Group A: 63 ± 8 Group C: 63 ± 8
Sex: Group A: 67% M Group C: 76% M
Groups comparable at baseline? Yes |
Describe intervention (treatment/procedure/test):
Group A (n = 30), NAC 1200 mg orally before angiography and 1200 mg orally twice daily for three doses along with good hydration
|
Describe control (treatment/procedure/test):
Group C (n = 45), hydration with 0.9% saline started just before contrast media injection and continued for 12 h at a rate 1.0 mL/kg/min after angiography or 0.5 mL/kg/h in cases with overt heart failure for 12 h |
Length of follow-up: 48 hours
Loss-to-follow-up: Not reported
Incomplete outcome data: Not reported
|
Outcome measures and effect size (include 95%CI and p-value if available):
Contrast nephropathy (= an increase more than 0.5 mg/dL 48 hours after the procedure compared with baseline values-) I: 2/30 C: 8/45 P=0.001 |
Authors’ conclusion:
Our study indicates that high doses of NAC plus hydration provide better protection against CIN than combination therapy of NAC and ascorbic acid plus hydration, or hydration alone. |
|
Izani Wan, 2008 (Mohamed) |
Type of study: randomized controlled trial
Setting: elective patients, single centre
Country: Malaysia
Source of funding: not reported |
Inclusion criteria: 1) patients electively admitted for CAG 2) calculated creatinine clearance 40-90ml/min 3) age ≥18 years
Exclusion criteria: 1) severe renal failure 2) presence of acute or reversible component of renal failure 3) severe peptic ulcer disease 4) history of allergy to NAC 5) severe asthma 6) pregnancy or breastfeeding
N total at baseline: Intervention: 49 Control: 51
Important prognostic factors2: For example age ± SD: I: 58 ± 8 C: 56 ± 7
Sex: I: 86% M C: 82% M
SCr (µmol/L) I: 124 ± 17 C: 124 ± 22
Groups comparable at baseline? Yes |
Describe intervention (treatment/procedure/test):
NAC 600mg orally every 12 hours for 2 days (2 doses on the day before and 2 doses on the day of contrast administration, total dose 2400mg)
0.45% saline 1ml/kg/h in 12 hours before the procedure and 12 hours after
|
Describe control (treatment/procedure/test):
0.45% saline 1ml/kg/h in 12 hours before the procedure and 12 hours after
|
Length of follow-up: 48 hours
Loss-to-follow-up: Intervention: 4 (8%) 1 early discharge 2 procedure cancellation 1 procedure complication
Control: 4 (7%) 2 early discharge 2 procedure cancellation
Incomplete outcome data: As above
|
Outcome measures and effect size (include 95%CI and p-value if available):
CIN (= increase of >25% in the sCr level 48 hours after the procedure) I: 2/49 (4%) C: 6/51 (12%) P=0.27
None of the patients who developed CIN required dialysis. |
Authors’ conclusion:
Addition of NAC to standard hydration therapy is not associated with reduction in incidence of CIN in patients with mild to moderate renal impairment undergoing elective CAG. |
|
Koc, 2012 |
Type of study: randomized controlled trial
Setting: elective patients, single centre
Country: Turkey
Source of funding: not reported |
Inclusion criteria: 1) patients about to undergo CAG and/or PCI 2) calculated creatinine clearance <60ml/min or sCr≥1.1mg/dL 3) age ≥18 years
Exclusion criteria: 1) contrast-agent hypersensitivity 2) pregnancy or lactation 3) decompensated heart failure 4) pulmonary edema 5) emergency catheterisation 6) acute or end-stage renal failure
N total at baseline: Intervention: 80 Control: 80
Important prognostic factors2: For example age ± SD: I: 62 ± 10 C: 65 ± 11
Sex: I: 76% M C: 79% M
Creatinine clearance (mL/min) I: 59 ± 16 C: 58 ± 16
Groups comparable at baseline? Yes |
Describe intervention (treatment/procedure/test):
NAC 600mg intravenously every 12 hours for 2 days (2 doses on the day before and 2 doses on the day of contrast administration, total dose 2400mg)
0.9% saline iv 1ml/kg/h in on the day before, on the day of, and on the day after the procedure
|
Describe control (treatment/procedure/test):
0.9% saline iv 1ml/kg/h in on the day before, on the day of, and on the day after the procedure
|
Length of follow-up: 48 hours
Loss-to-follow-up: Not reported
Incomplete outcome data: Not reported
|
Outcome measures and effect size (include 95%CI and p-value if available):
CIN (=baseline sCr ≥25% and/or an absolute increase in sCr of ≥0.5 mg/dL 48 hours after the procedure) I: 2 (3%) C: 13 (16%) P=0.006
No patients needed hemodialysis. |
Authors’conclusion:
The results of this study suggest that NAC plus high-dose hydration was superior to high-dose hydration alone as well as standard hydration for the protection of renal function in patients with mild to moderate renal dysfunction who are undergoing CAG and/or PCI. |
|
Kotlyar, 2005 |
Type of study: randomised controlled trial
Setting: elective patients admitted for 1 day
Country: Australia
Source of funding: commercial (pharmaceutical company) |
Inclusion criteria: 1) day-stay elective patients scheduled for CAG and/or PCI
Exclusion criteria: 1) allergy to the study medication 2) unstable renal function 3) undergoing chronic dialysis 4) uncontrolled asthma 5) pregnancy or breastfeeding
N total at baseline: I1: 20 I2: 21 C: 19
Important prognostic factors2: For example age ± SD: I1: 66 ± 14 I2: 67 ± 12 C: 69 ± 9
Sex: I1: 75% M I2: 86% M C: 89% M
SCR (mmol/L) I1: 0.16 ± 0.03 I2: 0.16 ± 0.03 C: 0.15 ± 0.02
Groups comparable at baseline? Yes |
Describe intervention (treatment/procedure/test):
I1: NAC 300mg intravenously, once 1-2 hours before procedure and once 2-4 hours after procedure (total dose 600mg)
Hydration iv: 0.9% saline 100ml/hour 2 hours before procedure and 5hours after procedure
I1: NAC6300mg intravenously, once 1-2 hours before procedure and once 2-4 hours after procedure (total dose 1200mg)
Hydration iv: 0.9% saline 100ml/hour 2 hours before procedure and 5hours after procedure
|
Describe control (treatment/procedure/test):
Hydration iv: 0.9% saline 100ml/hour 2 hours before procedure and 5hours after procedure
|
Length of follow-up: 2-4 days and 30 days
Loss-to-follow-up: Not reported
Incomplete outcome data: Not reported
|
Outcome measures and effect size (include 95%CI and p-value if available):
None of the patients developed CIN (=
None of the patients developed a need for dialysis. |
Authors’ conclusion:
For day-saty patients with mild to moderate renal impairement undergoing CAG and/or PCI, prehydration alone is less complicated and more cost-effective than a combination of IV NAC (at doses used) and hydration. |
|
Sadineni, 2017 |
Type of study: randomized controlled trial
Setting: Department of Nephrology, Nizam's Institute of Medical Sciences, Hyderabad, Telangana, India
Source of funding: not reported |
Inclusion criteria: Age more than 30 years + Patients should have their serum creatinine ≥1.2 mg/dl on their most recent sample drawn within 3 months of planned procedure
Exclusion criteria: Patients with acute renal failure, endstage renal disease requiring dialysis, intravascular administration of contrast material within previous 6 days, pregnancy, lactation, emergent coronary angiography, history of hypersensitivity reaction to contrast media, cardiogenic shock, pulmonary edema, mechanical ventilator, parenteral use of diuretics, recent use of NAC, recent use of ascorbic acid, and use of metformin or NSAIDS within 48 h of procedure were excluded from the study.
N total at baseline: NAC: 35 Placebo: 30
Important prognostic factors2: For example age ± SD: NAC: 61 ± 11 Placebo: 63 ± 12
Sex: Group A: 77% M Group C: 87% M
Groups comparable at baseline? Yes |
Describe intervention (treatment/procedure/test):
NAC + NS: Group of patients who received NS and NAC |
Describe control (treatment/procedure/test):
Placebo + NS: Group of patients who received NS only |
Length of follow-up: 48 hours
Loss-to-follow-up: Not reported
Incomplete outcome data: Not reported
|
Outcome measures and effect size (include 95%CI and p-value if available):
CIN, defined as either a relative increase in serum creatinine from baseline of ≥25% or an absolute increase of ≥0.3 mg/dl (44.2 µmol/L) during days 1 and 2 NAC: 7/35 Placebo: 11/30 P > 0.05 |
Authors’ conclusion:
The major finding of this study was there was no significant difference between NAC and placebo in the prevention of contrast nephropathy. |
|
Seyon, 2007 |
Type of study: randomized controlled trial
Setting: emergency patients, one centre
Country: Canada
Source of funding: not reported |
Inclusion criteria: 1) patients admitted with a diagnosis of acute coronary syndrome 2) scheduled for CAG and/or PCI 3) impaired renal function defined as: -calculated creatinine clearance <50ml/min or -sCr≥1.4mg/dL for males or sCr≥1.3mg/dL for females 4) age ≥18 years
Exclusion criteria: 1) hemodynamic instability requiring inotropic support 2) pregnancy 3) acute gastrointestinal disorder 4) Killip class III or IV or NYHA III or IV, or patients deemed by cardiologist unsuitable for iv hydration 5) known sensitivity to NAC 6) current treatment with theophylline or mannitol 7) dialysis therapy 8) participation in another study or use of experimental drugs
N total at baseline: Intervention: 20 Control: 20
Important prognostic factors2: For example age ± SD: I: 76 ± 6 C: 75 ± 10
Sex: I: 60% M C: 70% M
Groups comparable at baseline? Yes |
Describe intervention (treatment/procedure/test):
600mg NAC orally four doses in total (1 before procedure and 3 after every 12 hours)
Iv hydration 0.45% saline1ml/kg/hour 4-6 hours before and 12 hours after procedure
|
Describe control (treatment/procedure/test):
Iv hydration 0.45% saline1ml/kg/hour 4-6 hours before and 12 hours after procedure
|
Length of follow-up: 48 hours
Loss-to-follow-up: Not reported
Incomplete outcome data: Not reported
|
Outcome measures and effect size (include 95%CI and p-value if available):
CIN (=increase in sCr >44µmol/L (0.5mg/dL) and/or 25% above baseline within 48 hours) I: 1/20 (5%) C: 2/20 (10%) p<0.05
No patients required dialysis therapy.
|
Authors’ conclusion
These results suggest that this cohort gained no added protection to renal function with the use of NAC |
Notes:
- Prognostic balance between treatment groups is usually guaranteed in randomized studies, but non-randomized (observational) studies require matching of patients between treatment groups (case-control studies) or multivariate adjustment for prognostic factors (confounders) (cohort studies); the evidence table should contain sufficient details on these procedures
- Provide data per treatment group on the most important prognostic factors [(potential) confounders]
- For case-control studies, provide sufficient detail on the procedure used to match cases and controls
- For cohort studies, provide sufficient detail on the (multivariate) analyses used to adjust for (potential) confounders
CAG: coronary angiography; CECT: contrast-enhanced computed tomography; CI: confidence interval; CI-AKI: contrast-induced acute kidney injury; CIN: contrast induced nephropathy; iv: intravenous; NAC: N-acetylcysteine; NYHA: New York Heart Association; OR: odds ratio; PCI: percutaneous coronary intervention; SCr: serum creatinine
Verantwoording
Beoordelingsdatum en geldigheid
Laatst beoordeeld : 01-11-2017
Validity
The board of the Radiological Society of the Netherlands will determine at the latest in 2023 if this guideline (per module) is still valid and applicable. If necessary, a new working group will be formed to revise the guideline. The validity of a guideline can be shorter than 5 years, if new scientific or healthcare structure developments arise, that could be seen as a reason to commence revisions. The Radiological Society of the Netherlands is considered the keeper of this guideline and thus primarily responsible for the actuality of the guideline. The other scientific societies that have participated in the guideline development share the responsibility to inform the primarily responsible scientific society about relevant developments in their field.
Initiative
Radiological Society of the Netherlands
Authorization
The guideline is submitted for authorization to:
- Association of Surgeons of the Netherlands
- Dutch Association of Urology
- Dutch Federation of Nephrology
- Dutch Society Medical Imaging and Radiotherapy
- Dutch Society of Intensive Care
- Netherlands Association of Internal Medici
- Netherlands Society for Clinical Chemistry and Laboratory Medicine
- Netherlands Society of Cardiology
- Netherlands Society of Emergency Physicians
- Radiological Society of the Netherlands
Algemene gegevens
General Information
The guideline development was assisted by the Knowledge Institute of Medical Specialists (https://www.kennisinstituut.nl) and was financed by the Quality Funds for Medical Specialists (Kwaliteitsgelden Medisch Specialisten: SKMS).
Doel en doelgroep
Goal of the current guideline
The aim of the Part 1 of Safe Use of Iodine-containing Contrast Media guidelines is to critically review the present recent evidence with the above trend in mind, and try to formulate new practical guidelines for all hospital physicians to provide the safe use of contrast media in diagnostic and interventional studies. The ultimate goal of this guideline is to increase the quality of care, by providing efficient and expedient healthcare to the specific patient populations that may benefit from this healthcare and simultaneously guard patients from ineffective care. Furthermore, such a guideline should ideally be able to save money and reduce day-hospital waiting lists.
Users of this guideline
This guideline is intended for all hospital physicians that request or perform diagnostic or interventional radiologic or cardiologic studies for their patients in which CM are involved.
Samenstelling werkgroep
Working group
Cobbaert C., clinical chemist, Leiden University Medical Centre (member of advisory board from September 2015)
Danse P., interventional cardiologist, Rijnstate Hospital, Arnhem
Dekker H.M., radiologist, Radboud University Medical Centre, Nijmegen
Geenen R.W.F., radiologist, Noordwest Ziekenhuisgroep (NWZ), Alkmaar/Den Helder
Hoogeveen E.K., nephrologist, Jeroen Bosch Hospital, ‘s-Hertogenbosch
Kooiman J., research physician, Leiden University Medical Centre, Leiden
Oudemans - van Straaten H.M., internist-intensive care specialist, Free University Medical Centre, Amsterdam
Pels Rijcken T.H., interventional radiologist, Tergooi, Hilversum
Sijpkens Y.W.J., nephrologist, Haaglanden Medical Centre, The Hague
Vainas T., vascular surgeon, University Medical Centre Groningen (until September 2015)
van den Meiracker A.H., internist-vascular medicine, Erasmus Medical Centre, Rotterdam
van der Molen A.J., radiologist, Leiden University Medical Centre, Leiden (chairman)
Wikkeling O.R.M., vascular surgeon, Heelkunde Friesland Groep, location: Nij Smellinghe Hospital, Drachten (from September 2015)
Advisory board
Demir A.Y., clinical chemist, Meander Medical Center, Amersfoort, (member of working group until September 2015)
Hubbers R., patient representative, Dutch Kidney Patient Association
Mazel J., urologist, Spaarne Gasthuis, Haarlem
Moos S., resident in Radiology, HAGA Hospital, The Hague
Prantl K., Coordinator Quality & Research, Dutch Kidney Patient Association
van den Wijngaard J., resident in Clinical Chemistry, Leiden University Medical Center
Methodological support
Boschman J., advisor, Knowledge Institute of Medical Specialists (from May 2017)
Burger K., senior advisor, Knowledge Institute of Medical Specialists (until March 2015)
Harmsen W., advisor, Knowledge Institute of Medical Specialists (from May 2017)
Mostovaya I.M., advisor, Knowledge Institute of Medical Specialists
Persoon S., advisor, Knowledge Institute of Medical Specialists (March 2016 – September 2016)
van Enst A., senior advisor, Knowledge Institute of Medical Specialists (from January 2017)
Belangenverklaringen
Conflicts of interest
The working group members have provided written statements about (financially supported) relations with commercial companies, organisations or institutions that are related to the subject matter of the guideline. Furthermore, inquiries have been made regarding personal financial interests, interests due to personal relationships, interests related to reputation management, interest related to externally financed research and interests related to knowledge valorisation. The statements on conflict of interest can be requested at the administrative office of the Knowledge Institute of Medical Specialists and are summarised below.
|
Member |
Function |
Other offices |
Personal financial interests |
Personal relationships |
Reputation management |
Externally financed research |
Knowledge-valorisation |
Other potential conflicts of interest |
Signed |
|
Workgroup |
|||||||||
|
Burger |
Advisor, Knowledge Institute of Medical Specialists |
None |
None |
None |
None |
None |
None |
None |
Yes |
|
Cobbaert |
Member, physician clinical chemistry |
Head of clinical chemistry department in Leiden LUMC. Tutor for post-academic training of clinical chemists, coordinator/host for the Leiden region Member of several working groups within the Dutch Society for Clinical Chemistry and member of several international working groups for clinical chemistry |
None |
None |
Member of several working groups within the Dutch Society for Clinical Chemistry and member of several international working groups for clinical chemistry |
None |
None |
None |
Yes |
|
Danse |
Member, cardiologist |
Board member committee of Quality, Dutch society for Cardiology (unpaid) Board member Conference committee DRES (unpaid) |
None |
None |
None |
None |
None |
None |
Yes |
|
Dekker |
Member, radiologist |
None |
None |
None |
None |
None |
None |
None |
Yes |
|
Geenen |
Member, radiologist |
Member Contrast Media Safety Committee of the European Society of Urogenital Radiology (unpaid, meetings are partially funded by CM industry))) |
None |
None |
None |
None |
None |
Has been a public speaker during symposia organised by GE Healthcare about contrast agents (most recently in June 2014) |
Yes |
|
Hoogeveen |
Member, nephrologist |
Member of Guideline Committee of Dutch Federation of Nephrology |
None |
None |
Member of Guideline Committee of Dutch Society for Nephrology |
Grant from the Dutch Kidney Foundation to study effect of fish oil on kidney function in post-MI patients |
None |
None |
Yes |
|
Kooiman |
Member, research physician |
Resident in department of gynaecology & obstetrics |
None |
None |
None |
None |
None |
None |
Yes |
|
Mostovaya |
Advisor, Knowledge Institute of Medical Specialists |
None |
None |
None |
None |
None |
None |
None |
Yes |
|
Oudemans – van Straaten |
Member, intensive care medical specialist Professor Intensive Care |
none |
None |
None |
None |
None |
None |
None |
Yes |
|
Pels Rijcken |
Member, interventional radiologist |
None |
None |
None |
None |
None |
None |
None |
Yes |
|
Sijpkens |
Member, nephrologist |
None |
None |
None |
None |
None |
None |
None |
Yes |
|
Vainas |
Member, vascular surgeon |
None |
None |
None |
None |
None |
None |
None |
Yes |
|
Van den Meiracker |
Member, internist vascular medicine |
None |
None |
None |
None |
None |
None |
None |
Yes |
|
Van der Molen |
Chairman, radiologist |
Member Contrast Media Safety Committee of the European Society of Urogenital Radiology (unpaid,CMSC meetings are partially funded by CM industry)) |
None |
None |
Secretary section of Abdominal Radiology; Radiological Society of the Netherlands (until spring of 2015) |
None |
None |
Receives Royalties for books: Contrast Media Safety, ESUR guidelines, 3rd ed. Springer, 2015 Received speaker fees for lectures on CM safety by GE Healthcare, Guerbet, Bayer Healthcare and Bracco Imaging (2015-2016) |
Yes |
|
Wikkeling |
Member, vascular surgeon |
None |
None |
None |
None |
None |
None |
None |
Yes |
|
Advisory Board |
|||||||||
|
Demir |
Member, physician clinical chemistry |
None |
None |
None |
None |
None |
None |
None |
Yes |
|
Hubbers |
Member, patient’s representative, Dutch Society of Kidney Patients |
None |
None |
None |
None |
None |
None |
None |
Yes |
|
Mazel |
Member, urologist |
None |
None |
None |
None |
None |
None |
None |
Yes |
|
Prantl |
Member, policy maker, Dutch Society of Kidney Patients |
None |
None |
None |
None |
None |
None |
None |
Yes |
|
Van den Wijngaard |
Member, resident clinical chemistry |
Reviewer for several journals (such as American Journal of Physiology) |
None |
None |
None |
None |
None |
None |
Yes |
Inbreng patiëntenperspectief
Patients’ perspective was represented, firstly by membership and involvement in the advisory board of a policy maker and a patients’ representative from the Dutch Kidney Patient Association. Furthermore, an online survey was organized by the Dutch Kidney Patient Association about the subject matter of the guideline. A summary of the results of this survey has been discussed during a working group meeting at the beginning of the guideline development process. Subjects that were deemed relevant by patients were included in the outline of the guideline. The concept guideline has also been submitted for feedback during the comment process to the Dutch Patient and Consumer Federation, who have reported their feedback through the Dutch Kidney Patient Association.
Methode ontwikkeling
Evidence based
Implementatie
In the different phases of guideline development, the implementation of the guideline and the practical enforceability of the guideline were taken into account. The factors that could facilitate or hinder the introduction of the guideline in clinical practice have been explicitly considered. The implementation plan can be found with the Related Products. Furthermore, quality indicators were developed to enhance the implementation of the guideline. The indicators can also be found with the Related Products.
Werkwijze
AGREE
This guideline has been developed conforming to the requirements of the report of Guidelines for Medical Specialists 2.0; the advisory committee of the Quality Counsel. This report is based on the AGREE II instrument (Appraisal of Guidelines for Research & Evaluation II) (www.agreetrust.org), a broadly accepted instrument in the international community and on the national quality standards for guidelines: “Guidelines for guidelines” (www.zorginstituutnederland.nl).
Identification of subject matter
During the initial phase of the guideline development, the chairman, working group and the advisor inventory the relevant subject matter for the guideline. Furthermore, an Invitational Conference was organized, where additional relevant subjects were suggested by the Dutch Kidney Patient Association, Dutch Society for Emergency Physicians, and Dutch Society for Urology. A report of this meeting can be found in Related Products.
Clinical questions and outcomes
During the initial phase of guideline development, the chairman, working group and advisor identified relevant subject matter for the guideline. Furthermore, input was acquired for the outline of the guideline during an Invitational Conference. The working group then formulated definitive clinical questions and defined relevant outcome measures (both beneficial land harmful effects). The working group rated the outcome measures as critical, important and not important. Furthermore, where applicable, the working group defined relevant clinical differences.
Strategy for search and selection of literature
For the separate clinical questions, specific search terms were formulated and published scientific articles were sought after in (several) electronic databases. Furthermore, studies were looked for by cross-referencing other included studies. The studies with potentially the highest quality of research were looked for first. The working group members selected literature in pairs (independently of each other) based on title and abstract. A second selection was performed based on full text. The databases search terms and selection criteria are described in the modules containing the clinical questions.
Quality assessment of individual studies
Individual studies were systematically assessed, based on methodological quality criteria that were determined prior to the search, so that risk of bias could be estimated. This is described in the “risk of bias” tables.
Summary of literature
The relevant research findings of all selected articles are shown in evidence tables. The most important findings in literature are described in literature summaries. When there were enough similarities between studies, the study data were pooled.
Grading the strength of scientific evidence
A) For intervention questions
The strength of the conclusions of the scientific publications was determined using the GRADE-method. GRADE stands for Grading Recommendations Assessment, Development and Evaluation (see http://www.gradeworkinggroup.org/) (Atkins, 2004).
GRADE defines four gradations for the quality of scientific evidence: high, moderate, low or very low. These gradations provide information about the amount of certainty about the literature conclusions. (http://www.guidelinedevelopment.org/handbook/).
B) For diagnostic, etiological, prognostic or adverse effect questions, the GRADE-methodology cannot (yet) be applied. The quality of evidence of the conclusion is determined by the EBRO method (van Everdingen, 2004)
Formulating conclusion
For diagnostic, etiological, prognostic or adverse effect questions, the evidence was summarized in one or more conclusions, and the level of the most relevant evidence was reported. For intervention questions, the conclusion was drawn based on the body of evidence (not one or several articles). The working groups weighed the beneficial and harmful effects of the intervention.
Considerations
Aspects such as expertise of working group members, patient preferences, costs, availability of facilities, and organization of healthcare aspects are important to consider when formulating a recommendation. These aspects were discussed in the paragraph Considerations.
Formulating recommendations
The recommendations answer the clinical question and were based on the available scientific evidence and the most relevant considerations.
Constraints (organization of healthcare)
During the development of the outline of the guideline and the rest of the guideline development process, the organization of healthcare was explicitly taken into account. Constraints that were relevant for certain clinical questions were discussed in the Consideration paragraphs of those clinical questions. The comprehensive and additional aspects of the organization of healthcare were discussed in a separate chapter.
Development of quality indicators
Internal (meant for use by scientific society or its members) quality indicators are developed simultaneously with the guideline. Furthermore, existing indicators on this subject were critically appraised; and the working group produces an advice about such indicators. Additional information on the development of quality indicators is available by contacting the Knowledge Institute for Medical Specialists. (secretariaat@kennisinstituut.nl).
Knowledge Gaps
During the development of the guideline, a systematic literature search was performed the results of which help to answer the clinical questions. For each clinical question the working group determined if additional scientific research on this subject was desirable. An overview of recommendations for further research is available in the appendix Knowledge Gaps.
Comment- and authorisation phase
The concept guideline was subjected to commentaries by the involved scientific societies. The commentaries were collected and discussed with the working group. The feedback was used to improve the guideline; afterwards the working group made the guideline definitive. The final version of the guideline was offered for authorization to the involved scientific societies, and was authorized.
References
Atkins D, Eccles M, Flottorp S, et al. GRADE Working Group. Systems for grading the quality of evidence and the strength of recommendations I: critical appraisal of existing approaches The GRADE Working Group. BMC Health Serv Res. 2004 Dec 22;4(1):38.
Van Everdingen JJE, Burgers JS, Assendelft WJJ, et al. Evidence-based richtlijnontwikkeling. Bohn Stafleu van Loghum. Houten, 2004
Zoekverantwoording
Zoekacties zijn opvraagbaar. Neem hiervoor contact op met de Richtlijnendatabase.
