Hydratie en complicaties
Uitgangsvraag
Welke hydratiestrategie dient te worden toegepast bij patiënten die intravasculair jodiumhoudend contrastmiddel (CM)-toediening ondergaan en een hoog PC-AKI risico hebben?
Subvragen:
- Is er een significant verschil in de incidentie van PC-AKI bij hydratie versus geen hydratie?
- Is er een significant verschil in de incidentie van PC-AKI bij intraveneuze NaCl versus NaHCO3?
- Is er een significant verschil in de incidentie van PC-AKI bij intraveneuze prehydratie (alleen) versus pre- en posthydratie (gecombineerd)?
- Is er een significant verschil in de incidentie van PC-AKI bij orale versus intraveneuze pre- en posthydratie?
- Is er een significant verschil in de incidentie van PC-AKI bij patiënten die gecontroleerde diurese ondergaan versus standaard hydratieschema’s?
Aanbeveling
Aanbeveling-1
Zorg voor een normale hydratie status voorafgaand aan toediening van jodiumhoudend contrastmiddel, ongeacht de eGFR. Corrigeer hypovolemie met intraveneuze NaCl 0.9% of Ringer’s lactaat.
Vermijd zo veel mogelijk de toediening van intravasculair jodiumhoudend contrastmiddel bij patiënten met dehydratie/hypovolemie.
Overweeg posthydratie bij twijfel over adequate hydratiestatus (1 ml/kg/uur gedurende 4 – 6 uur, of 500 ml, na contrasttoediening, bijv. NaHCO3 1.4%).
Aanbeveling-2
Voer zonder vertraging acute, niet uitstelbare diagnostiek uit bij vitaal bedreigde patiënten. Laat beeldvorming in deze situatie voorgaan boven prehydratie, of afronding van ingezette prehydratie, ter preventie van PC-AKI.
Start hydratie vóór de procedure bij vitaal bedreigde patiënten met eGFR < 30 ml/min/1.73 m2 die intra-arterieel jodiumhoudend contrastmiddel krijgen.
Als starten vóór de procedure niet mogelijk is, begin dan de hydratie bij aanvang van de procedure.
Continueer hydratie tot het eindpunt van het hydratieschema, volgens de overige aanbevelingen in deze module.
Aanbeveling-3
Beschouw het risico op PC-AKI verhoogd bij intra-arteriële contrastmiddel toediening met first-pass renale blootstelling aan jodiumhoudende contrastmiddelen.
Definieer first-pass renale blootstelling als intra-arteriële contrasttoediening binnen:
- Linkerharthelft en kransslagaders.
- aorta thoracalis.
- suprarenale aorta abdominalis.
- arteria renalis.
Beschouw overige vormen van intra-arteriële jodiumhoudende contrasttoediening als second-pass renale blootstelling, met een PC-AKI risico vergelijkbaar met intraveneuze contrasttoediening.
Aanbeveling-4
Pas bij patiënten met een eGFR ≥30 ml/min/1.73m2 geen hydratie toe ter preventie van PC-AKI bij intravasculaire toediening van jodiumhoudend contrastmiddel.
Aanbeveling-5
Kies bij patiënten met eGFR <30 ml/min/1.73m2 voor een alternatief onderzoek zonder jodiumhoudend contrastmiddel als de diagnostische waarde daarvan even goed is.
Pas bij patiënten met eGFR <30 ml/min/1.73m2 die een intravasculaire procedure ondergaan beschikbare technieken toe om de totale dosis jodiumhoudend contrastmiddel te minimaliseren.
Aanbeveling-6
Pas bij niet-dialyse-afhankelijke patiënten met eGFR < 30 ml/min/1.73m2 profylactische hydratie toe ter preventie van PC-AKI bij intraveneuze, of intra-arteriële toediening van jodiumhoudende contrastmiddelen met second-pass renale blootstelling.
Hydreer niet-dialyse-afhankelijke patiënten met eGFR < 30 ml/min/1,73 m² ter preventie van PC-AKI bij geplande intraveneuze of intra-arteriële toediening van jodiumhoudend contrastmiddel met second-pass renale blootstelling volgens één van de volgende schema’s:
- NaHCO₃ 1,4% 3 ml/kg/uur gedurende 1 uur (of 250 ml) vóór contrasttoediening; of
- NaHCO₃ 1,4% 3 ml/kg/uur gedurende 1 uur (of 250 ml) vóór contrasttoediening en NaHCO₃ 1,4% 1 ml/kg/uur gedurende 4–6 uur (of 500 ml) na contrasttoediening.
Aanbeveling-7
Overweeg bij niet-dialyse-afhankelijke patiënten met eGFR < 30 ml/min/1.73m2 rondom intra-arteriële toediening van jodiumhoudend contrastmiddel met first-pass renale blootstelling laagdrempelig naast prehydratie ook posthydratie met NaHCO3 toe te passen:
- Vóór contrasttoediening: NaHCO3 1.4% 3 ml/kg/uur gedurende 1 uur (of 250 ml)
- Na contrasttoediening: NaHCO3 1.4% 1 ml/kg/uur gedurende 4 – 6 uur (of 500 ml)
Overweeg te volstaan met prehydratie alleen bij patiënten met goede hydratiestatus, in electieve setting en bij naar verwachting een beperkte totale dosis jodiumhoudend contrastmiddel.
Aanbeveling-8
Pas bij patiënten met chronische nierschade G5 (eGFR <15 ml/min/1.73m2) het hydratieschema individueel aan in overleg met de nefroloog.
Individualiseer in overleg met de cardioloog het hydratieschema bij patiënten met hartfalen, rekening houdend met de actuele hydratietoestand en het risico op decompensatio cordis.
Aanbeveling-9
Pas bij dialyse-afhankelijkheid (hemodialyse of peritoneaal dialyse) géén hydratie toe. Wijzig het dialyseschema niet rondom contrasttoediening; voer géén extra dialyse uit na contrasttoediening, behalve bij aanwijzing voor overhydratie.
Aanbeveling-10
Pas geen hydratie met gecontroleerde diurese toe ter preventie van PC-AKI bij patiënten die (cardiale) angiografie - met of zonder interventie - ondergaan, tenzij in studieverband.
Aanbeveling-11
Pas geen orale hydratie toe als enige preventiemaatregel tegen PC-AKI.
Zie stroomschema
Overwegingen
Balance between desired and undesired effects
All studies
In risk stratification of patients, it is essential to discriminate between CA-AKI (correlative diagnosis) and CI-AKI (causative diagnosis), which requires studies with control populations in which no contrast medium is administered (Davenport, Rad 2020; McDonald RJ, Rad Clin North Am 2024). In addition, the risk might be influenced by the route of iodine-based contrast medium administration: for intravenous (CT-scan) and intra-arterial contrast medium administration with second-pass renal exposure the risk of CI-AKI is similar. For intra-arterial contrast medium administration with first-pass renal exposure, the risk has been suggested to be higher. However, the increased incidence of AKI in this population is likely partly due to comorbidity (Van Der Molen, 2018).
The number of patients with eGFR <30 ml/min/1.73m2 is absent or very low in most described studies. Only Chen 2008 studied a relatively large subgroup with an average eGFR 28±2 ml/min/1.73m2. Subanalyses for this group were not performed.
In addition to prevention of PC-AKI, optimal nephrology care is important to prevent AKI in patients with impaired renal function. Currently, end stage renal disease (ESRD) is most often caused by atherosclerotic vascular disease, hypertension and type 2 diabetes. The goal in patients with chronic kidney disease (CKD) stage 3 to 5 (non-dialysis) is to slow down deterioration of renal function and prevent or postpone cardiovascular morbidity and mortality. According to the guideline Care of the Patient with Chronic Renal Damage (Chronische nierschade (CNS); 2018) of the Dutch Federation of Nephrology (NFN), the following advice for optimal nephrology care are relevant for the present guideline: avoid nephrotoxic medications, avoid dehydration and hypovolemia, and refer patients to a nephrologist in accordance to section 5.1 of the guideline Care of the Patient with Chronic Renal Damage (Chronische nierschade (CNS); 2018).
Furthermore, independent of eGFR, all patients receiving CM should have a normal hydration status. Apart from preventive hydration, patients should receive adequate volume replacement therapy (with normal saline or Ringer’s lactate) if they have clinical signs of hypovolemia, i.e. hypotension, tachycardia, oliguria and / or loss of renal function.
Q1 Hydration versus no hydration
Three available RCTs comparing hydration with no hydration were performed in the Netherlands and one RCT with two subgroups in China.
Intravenous and intra-arterial iodine-based contrast medium with second pass renal exposure in patients with eGFR 30-60 ml/min/1.73m2
In the larger two Dutch studies (Nijssen 2017 & Timal 2020) iodine-based contrast medium was administered mainly intravenously in stable patients. In the smaller study of Kooiman 2014, contrast medium was administered intravenously in the urgent setting of CTA for suspected pulmonary embolism in patients with eGFR < 60 ml/min/1.73 m2. For prophylactic hydration, Nijssen 2017 used a short or long pre- and posthydration protocol with NaCl 0.9%, whereas Kooiman 2014 and Timal 2020 from the same study group only used short, one-hour prehydration with NaHCO3 1.4%.
In the available studies with contrast medium administration in elective settings (Nijssen 2017 & Timal 2020), the risk of PC-AKI in the control group without prehydration was relatively low with 2.6%, and 2.7%, respectively which support the notion that the risk of PC-AKI is relatively low in patients with eGFR 30-60 ml/min/1.73m2 when iodine containing contrast medium is administered intravenously or intra-arterially with second-pass renal exposure. Of note, in Nijssen 2017 approximately 50% of the patients nephrotoxic medications including mainly diuretics were temporarily interrupted.
In the setting of urgent CTA for suspected pulmonary embolism, the incidence of PC-AKI was somewhat higher with 9.2% in the control group without prophylaxis.
In the study of Nijssen prophylactic pre- and posthydration with NaCl 0.9% did not decrease the relatively low incidence of PC-AKI (2.7% vs. 2.6%). Subgroup analysis of Nijssen also could not demonstrate an advantage of prophylactic hydration in patients with diabetes, lower eGFR 30-44 ml/min/1.73m2 or intracoronary administration of contrast medium. This study also demonstrated that pre- and posthydration with NaCl 0.9% led to cardiac side effects in 5.5% of the patients (4.0% heart failure, 1.2% arrhythmia, and 0.3% hyponatremia). This risk of serious cardiac problems, combined with the absence of a clear protective effect, makes pre- and posthydration with NaCl 0.9% in these patients an undesirable procedure.
In the studies of Kooiman 2014 and Timal 2020 only prehydration with NaHCO3 1.4% resulted in a slightly, but not significantly lower incidence of PC-AKI after urgent or elective intravenous contrast medium (7.1% vs 9.5% and 1.5% vs 2.7% in the two studies respectively). In Timal 2020 only in the group without prehydration 4 out of 262 (1.5%) had a persistent mean decline of eGFR of 3 ml/min/1.73m2. Because of the small sample size, a type 2 error cannot be excluded. None of the patients receiving prehydration with NaHCO3 developed heart failure. Because of these results, it cannot be fully excluded that prophylactic prehydration for elective or urgent intravenous administration of iodine containing contrast medium has a (very) small protective effect against PC-AKI and irreversible loss of renal function without obvious side effects.
Intra-arterial contrast medium with first pass renal exposure in patients with eGFR <30 ml/min/1.73m2
Iodine-based contrast medium was given intra-arterially with risk of first pass of contrast medium through the kidneys in approximately 50% of the patients in Nijssen 2017 for predominantly elective CAG or PCI and in Chen 2008 in all patients for elective PCI. For prophylactic hydration, Chen 2008 used a long pre- and posthydration protocol with hypotonic NaCl 0.45%. Nijssen 2017 used a short or long pre- and posthydration protocol with NaCl 0.9%. In the subgroup with low eGFR in Chen 2008, pre-and posthydration with NaCl 0.45% had a significant protective effect against PC-AKI (21.3% vs. 34.04% without hydration) but could not prevent it in the majority of patients (6.67% vs. 6.97%).
In one meta-analysis (Wang, 2019) including 3 RCTs with only a small percentage (13-31%) of patients with renal impairment, hydration with NaCl 0.9% pre- and/or during and after the PCI significantly reduced the risk of PC-AKI in these not always hemodynamically stable STEMI patients (RR 0.64; 95% CI 0.50-0.82). Hydration also tended to reduce the need for renal replacement therapy (RR 0.30; 95% CI 0.08-1.08) and mortality (RR 0.58; 95% CI 0.31-1.08). In all these studies, the infusion rate of hydration was reduced in patients with reduced left ventricular function. In this meta-analysis only the effect of hydration with NaCl 0.9% of the study of Maioli (Maioli, 2011: see below) was included.
Further relevant details of the studies in the meta-analysis are described below (Wang, 2019):
In the RCT of Maioli 2011 with three groups of 150 patients each with 23-31% eGFR <60 ml/min/1.73m2, one hour pre- and 12 hour posthydration with isotonic NaHCO3 resulted in a significantly lower incidence of PC-AKI (12%) than 12 hours posthydration with NaCl 0.9% (22.5%) and the control group without hydration (27.3%, P for trend =0.001). There was no significant difference between posthydration with saline and no hydration. The risk of PC-AKI was especially lower with hydration volumes >960 ml.
The RCT of Luo 2014 (Luo, 2014) with two groups of 108 patients each, 12 hours posthydration with NaCl 0.9% significantly reduced PC-AKI only in the high and very high Mehran risk groups.
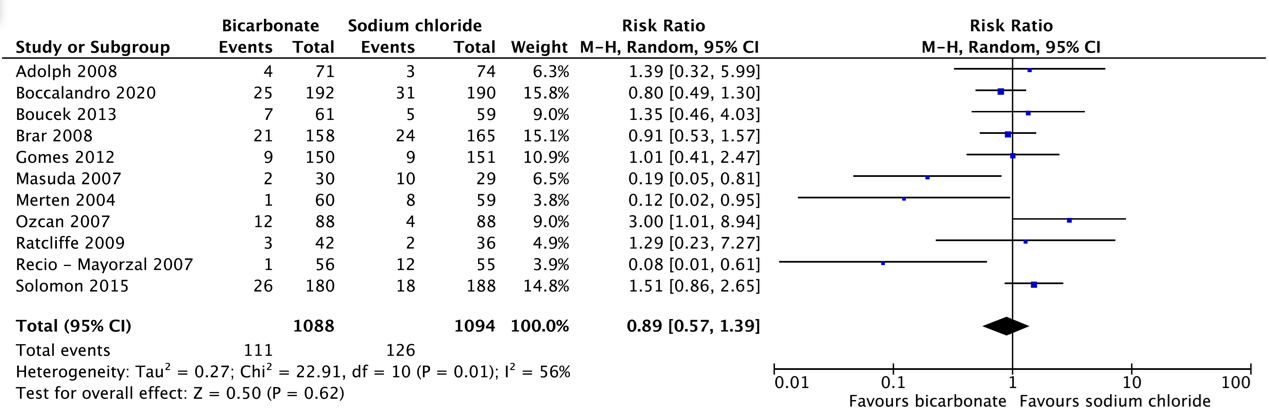
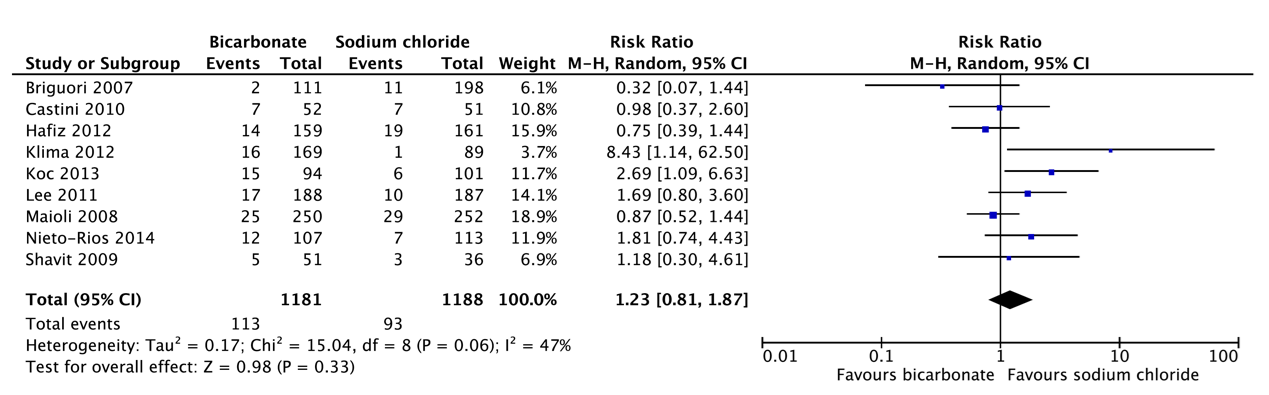
Q2 sodium chloride versus bicarbonate
Pooled analysis of all older studies and the new study of Boccolandro 2020 in patients with moderate to severe renal failure indicated that short pre- and post-hydration with isotonic NaHCO3 1.4% or NaCl 0.9% resulted in a comparable incidence of PC-AKI after CAG/PCI (10.1% vs. 11.4%, respectively).
Pooled analysis of older studies in patients with moderate to severe renal failure indicated that short pre- and post-hydration with NaHCO3 1.4% resulted in a slightly, but not significantly higher incidence of PC-AKI after CAG/PCI (9.4%) than long pre- and posthydration with NaCl 0.9% (6.8%).
Additionally, the very large recent RCT of Weisbord 2018 compared the incidence of PC-AKI after CAG between pre- and posthydration with NaHCO3 1.4% or NaCl 0.9% with the same specified minimum required volume and variable, but identical short to long infusion durations in both arms depending on the local policies in the different participating centers. In this RCT the incidence of PC-AKI was comparable after bicarbonate (9.5%) and saline (8.3%). Also, in the sub study of Garcia 2018 of patients who underwent PCI, the incidence of PC-AKI did not differ between bicarbonate (11.3%) and saline (12.0%).
Although the quality of evidence for the effectiveness of short sodium bicarbonate pre- and post-hydration versus short or long sodium chloride pre- and post-hydration for preventing PC-AKI after CAG/PCI and need for renal replacement therapy is very low to low, for practical reasons the short pre- and post-hydration with NaHCO3 seems more attractive. Moreover, despite the similar incidence of heart failure or pulmonary edema in the studies included in this analysis (see table 5) the RCT of Nijssen 2017 (AMACING) indicated that short and long duration of hydration with NaCl 0.9% in relatively low risk procedures in patients with CKD stage 3 resulted in 5.5% major cardiologic adverse events. Outside the research setting, it is likely that especially long duration of NaCl 0.9% hydration will have an inherent risk of fluid overloading in daily practice. Therefore, also in view of the probable risks of hydration with larger volumes of NaCl 0.9%, a short pre- and posthydration with NaHCO3 is to be preferred.
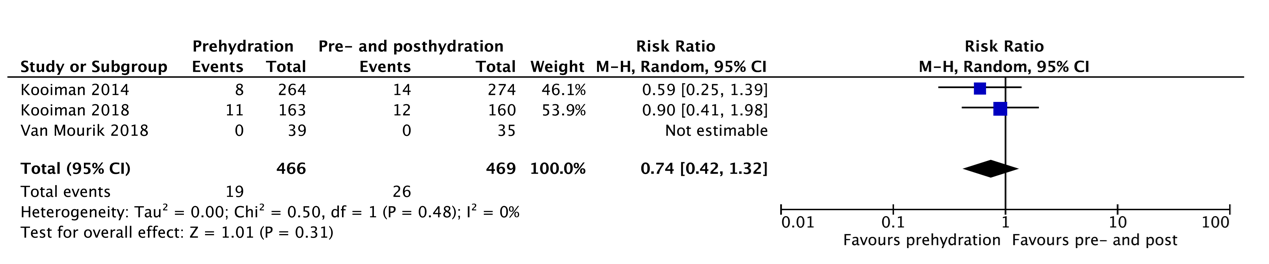
Q3 Pre-hydration only versus pre- and posthydration
Studies comparing prehydration to pre- and posthydration are limited and only focus on prehydration with bicarbonate versus pre- and posthydration with NaCl 0,9% in relatively low-risk populations (>90% eGFR 30-60ml/min/1,73m2) (Kooiman 2014, 2018, van Mourik 2018). Kooiman 2014 and van Mourik 2018 included patients undergoing an elective CT scan, whereas Kooiman 2018 focused on procedures with intra-arterial contrast medium administration, which in ~20% of the patients entailed CAG or PCI with first-pass renal exposure. There was no difference in the incidence of PC-AKI between the prehydration only and the pre- and posthydration group in these studies. A subgroup analysis for first pass versus second-pass renal exposure was not performed. Of note, van Mourik 2018 reported 0% incidence of PC-AKI in both groups. In total, only 78 patients with an eGFR <30ml/min/1,73m2 were included in these studies, and there are no studies focusing specifically on patients with an eGFR <30ml/min/1,73m2. The evidence for prehydration only in high-risk populations is therefore limited. This has, however, been applied broadly in the Netherlands since 2017 without reports of adverse effects, but these results have not been confirmed by other studies.
In contrast, in international guidelines pre-and posthydration is often recommended based on the expert opinion that the amount of urine output is the most important factor regardless how this is achieved. This may especially be valid for intra-arterial CM administration with first pass renal exposure such as coronary angiography or PCI (Solomon, Intervent Cardiol Clin 2023). However, studies investigating PC-AKI in patients undergoing emergent CAG or PCI (such as in STEMI) are inherently prone to bias, since the myocardial infarction is an important independent risk factor for AKI.
Given the fact that Nijssen 2017 showed an elevated risk of cardiac complications following NaCl 0.9% pre- and posthydration, lack of evidence for NaCl 0.9% prehydration alone, reduced patient and healthcare burden of 1 hour prehydration with bicarbonate 1.4% compared to pre- and posthydration with NaCl 0,9%, the Working Group has an expert-based preference for bicarbonate prehydration.
Q4 Oral versus intravenous hydration
The quality of evidence for the effectivity of oral hydration for the prevention of PC-AKI is low. Furthermore, the oral intake of patients could not be quantified and could therefore lead to PC-AKI due to lack of adherence to oral hydration instructions. Therefore, it is the recommendation of the working group that oral hydration should not be used in the prevention of PC-AKI. However, the encouragement of patients using oral fluids unrestrictedly on the day of CM exposure, besides other preventive measures, is advisable.
Q5 Hydration with controlled diuresis
The ratio behind this technique is to increase renal blood flow and urinary output in a controlled environment, based on patient’s parameters, such as central venous pressure, left ventricular end diastolic pressure or urinary output. The amount of additional intravenous fluids and, if necessary, a low dose diuretic, is individualized by the abovementioned parameters. These techniques can only be applied in an in-patient setting as intravenous or intra-arterial catheters are necessary, combined with a urinary catheter for monitoring urinary production. This makes these techniques applicable for a subgroup of patients. The Working Group thinks that controlled diuresis is a promising new invasive strategy to prevent PC-AKI in hospitalized patients undergoing (cardiac) angiography with or without intervention. Which technique is optimal is unknown. Outside of the scope of the current modular revision, a recent meta-analysis of Cossette 2025 demonstrated that tailored hydration based on left-ventricular end-diastolic pressure significantly reduced the incidence of PC-AKI after CAG/PCI as compared to standard hydration (RR 0.61; 95% CI 0.41-0.90). Showing comparable efficacy to aforementioned urinary flow rate solutions, with the advantage of a possible reduced barrier to implementation in clinical practice. However, more information and research are needed before reliable conclusions can be drawn regarding the effectiveness and preferred type of controlled diuresis, or its application in an outpatient setting. Therefore, the Working Group recommends that, for now, this technique should be reserved for research setting only.
Quality of evidence
Q1 Hydration versus no hydration
The overall quality of evidence is very low. This means that we are very uncertain about the estimated effect found for the crucial outcome measures.
Downgraded due to (very) serious:
- Risk of bias: methodological limitations, due to loss to follow-up
- Inconsistency: inconsistency of results
- Imprecision: inaccuracy, because the confidence interval exceeds both limits of clinical relevance and because of a very small number of events with a small sample size
Q2 Sodium chloride versus bicarbonate
The overall quality of evidence is very low. This means that we are very uncertain about the estimated effect found for the crucial outcome measures.
Downgraded due to (very) serious:
- Inconsistency: inconsistency of results
- Imprecision: inaccuracy because the confidence interval exceeds the limit/both limits of clinical relevance
Q3 Pre-hydration only versus pre- and posthydration
The overall quality of evidence is very low. This means that we are very uncertain about the estimated effect found for the crucial outcome measures.
Downgraded due to (very) serious:
- Inconsistency: inconsistency of results
- Indirectness: Indirectness of evidence, due to differences in the type of hydration used for pre- and post-hydration
- Imprecision: inaccuracy, due to failure to achieve the optimal sample size or a very small number of events with a small sample size
Q4 Oral versus intravenous hydration
For the comparison of oral versus intravenous hydration in all patients the level of evidence was graded as low due to imprecision and heterogeneity of included studies.
Q5 Hydration with controlled diuresis
For the comparison of controlled diuresis versus IV hydration in all patients the level of evidence was graded as low due to imprecision and heterogeneity of included studies.
Values and preferences of patients (and possibly their relatives/caregivers)
For patients, it is very important that the use of iodine-based contrast agents does not cause PC-AKI or worsen kidney function. To reduce this risk, patients are willing to stay longer in the hospital for hydration. Current medical knowledge suggests that hydration lowers the chance of kidney problems and, as recommended, does not lead to serious side effects. Patients do not want unnecessarily long hydration schedules. It is important for patients to receive a good explanation of the reasons for applying hydration during diagnostics and procedures.
For critically ill patients, receiving treatment quickly and without delays in imaging is a priority. At the same time, preventing kidney problems remains important. These patients therefore want hydration (if it is effective) to be started as soon as possible when iodine-based contrast agents are used.
Cost aspects
The hydration interventions increase the costs of the imaging study slightly. However, these costs are low in comparison to the cost of treatment of clinically relevant CI-AKI or even CA-AKI. Per equal infusion quantity, NaCl 0.9% comes at reduced cost in comparison to NaHCO3 1.4% according to the Zorginstituut Nederland provided data on www.medicijnkosten.nl. A cost-impact analysis has not been part of the current modular revision, and as such differences in cost-effectiveness between NaCl 0.9% versus NaHCO3 1.4% cannot be provided.
Equality ((health) equity/equitable)
The intervention has no expected impact on health equity.
Acceptability
Ethical acceptability
The Working Group expects no ethical concerns with regard to the proposed hydration strategies. Comparable hydration strategies are part of current routine clinical practice, with a low burden as well as a low risk to the patient, whilst providing possible protection against iodine contrast-medium associated risk of kidney injury.
Sustainability
Although any form of hydration strategy will have an environmental impact, environmental sustainability has not been part of the considerations for an optimal hydration strategy.
Feasibility
The feasibility of the provided recommendations is considered high. Hydration strategies consisting of either NaCl 0.9% or NaHCO 1.4% are part of current routine clinical care. Supply chain availability of either products is generally well. And application of hydration strategies is generally well accepted on a national level.
Rationale van aanbeveling-1: weging van argumenten voor en tegen de interventies
De preventie van PC-AKI kent als randvoorwaarde een adequate hydratie status ongeacht eGFR (zie ook: 2.3 Evaluatie van eGFR). Aanbevelingen met oogpunt op PC-AKI preventie worden gedaan in veronderstelling van een normale hydratie status.
Eindoordeel:
Sterke aanbeveling voor (Doen).
Rationale van aanbeveling-2: weging van argumenten voor en tegen de interventies
In geval van 1) acute, niet uitstelbare diagnostiek, of 2) behandeling, van een vitaal bedreigde patiënt, prevaleert het belang van beeldvorming en eventuele behandeling boven hydratie. Hydratie in het kader van PC-AKI preventie dient derhalve geen reden te zijn voor vertraging in het diagnostisch traject of aanvang van eventuele interventies bij vitaal bedreigde patiënten.
Eindoordeel:
Sterke aanbeveling voor (Doen).
Rationale van aanbeveling-3: weging van argumenten voor en tegen de interventies
Geraadpleegde literatuur toont een verhoogde incidentie van PC-AKI binnen patiënten populaties met intra-arteriële toediening van jodiumhoudend contrastmiddel met first-pass renale blootstelling.
Eindoordeel:
Sterke aanbeveling voor (Doen).
Rationale van aanbeveling-4: weging van argumenten voor en tegen de interventies
Er bestaat onvoldoende evidentie om bij eGFR ≥30 ml/min/1.73m2 hydratie aan te bevelen voor PC-AKI profylaxe na intravasculaire toediening van jodiumhoudend contrast. Daarnaast zijn er aanwijzingen dat gecombineerde pre- en posthydratieschema’s met NaCl 0.9% het risico op hyponatriëmie, aritmie en hartfalen verhogen, waardoor deze schema’s gecontra-indiceerd zijn. Bij gebrek aan bewijs vanuit zowel de literatuur als de praktijk, is het niet doelmatig om mensen en middelen in te zetten om deze extra hydratie behandeling uit te voeren in deze groep patiënten.
Eindoordeel:
Sterke aanbeveling tegen (Niet doen).
Rationale van aanbeveling-5: weging van argumenten voor en tegen de interventies
Bij patiënten met een eGFR <30 ml/min/1.73 m2 toont geraadpleegde literatuur een verhoogde incidentie aan PC-AKI met mogelijke relatie tot de toegediende contrastdosis. Vervang jodiumhoudend contrastmiddel of verminder contrastdosis waar mogelijk. Maak tijdens intravasculaire procedures gebruik van aanwezige beschikbare technieken om jodiumhoudend contrastdosis te beperken.
Eindoordeel:
Sterke aanbeveling voor (Doen).
Rationale van aanbeveling-6: weging van argumenten voor en tegen de interventies
De geraadpleegde literatuur toont een zeer beperkte evaluatie van het mogelijk gunstige effect van hydratie bij patiënten met een eGFR <30 ml/min/1.73m2. Voor intra-arteriële toediening van jodiumhoudend contrast met first-pass van de nieren toont de beperkt beschikbare literatuur een mogelijke meerwaarde voor hydratie en in deze setting is aangetoond dat de ernst van de nierfunctiestoornis een belangrijke voorspeller is van het risico op PC-AKI. De beschikbare literatuur maakt aannemelijk dat hydratieschema’s met pre- en korte posthydratie met NaHCO3 1.4% even effectief zijn als schema’s met pre- en korte of lange posthydratie met NaCl 0.9% zodat er een voorkeur is voor hydratieschema’s met NaHCO3 gezien het eerdergenoemde verhoogde risico op hartfalen bij hydratie met NaCl 0.9%. Het effect van alleen prehydratie met NaHCO3 1.4% is helaas niet goed onderzocht in patiënten met eGFR <30 ml/min/1.73m2.
Voor intraveneuze contrasttoediening en intra-arteriële contrasttoediening met second-pass van de nieren bestaat er op dit moment onvoldoende bewijsvoering van hoge kwaliteit binnen de geraadpleegde literatuur om meerwaarde van hydratie te bevestigen noch te ontkrachten.
Gezien deze bovenstaande overwegingen is het cluster van mening om bij alle patiënten met een eGFR <30 ml/min/1.73m2 enige vorm van hydratie ter PC-AKI preventie toe te passen.
Ondanks zeer beperkt wetenschappelijk bewijs wordt door een groot deel van de Nederlandse centra een voorkeur gegeven aan een hydratieschema met alleen prehydratie met NaHCO3 1.4%, doch zonder prospectieve evaluatie op de incidentie van PC-AKI en eventuele vervroeging van indicatie tot start van nierfunctie-vervangende therapie.
Eindoordeel:
Sterke aanbeveling voor (Doen).
Zwakke aanbeveling voor (Doen).
Rationale van aanbeveling-7: weging van argumenten voor en tegen de interventies
Binnen de geraadpleegde literatuur is er géén bewijs dat alleen prehydratie met NaHCO3 1.4% even effectief als NaCl 0.9% hydratie voor preventie van PC-AKI bij intra-arteriële onderzoeken met first-pass van jodiumhoudend contrast. Deze wetenschappelijke basis ontbreekt ook volledig voor prehydratie alleen met NaCl 0.9%. Pre- en korte posthydratie met NaHCO3 1.4% wordt op basis van de literatuur bij deze patiënten als equivalent beschouwd aan korte en lange hydratieschema’s met NaCl 0.9%.
Toevoegen van korte 4-6 uur posthydratie met NaHCO3 1.4% heeft (of naar verwachting) minimale logistieke of financiële implicaties na interventies met arteriële contrasttoediening met first-pass effect. Gezien deze overwegingen is het cluster van mening dat posthydratie bij deze patiëntengroep vooralsnog laagdrempelig toegepast dient te worden.
Gelet op logistieke overwegingen, kostenoverwegingen en verhoogde risico op hartfalen bij NaCl 0.9% schema’s wordt bij voorkeur een hydratieschema met prehydratie en korte posthydratie met NaHCO3 1.4% aanbevolen.
Eindoordeel:
Zwakke aanbeveling voor (Doen).
Rationale van aanbeveling-8: weging van argumenten voor en tegen de interventies
Patiënten met chronische nierschade G5 (eGFR < 15 ml/min/1.73m2) en patiënten met hartfalen kennen een verhoogd risico op zowel PC-AKI en hydratie gerelateerde complicaties. Individualiseer hydratie waar nodig. Consulteer laagdrempelig relevante specialismen, denk hierbij aan respectievelijk nefrologie en cardiologie. Bij reeds dialyse-afhankelijkheid toont beschikbare literatuur geen meerwaarde voor hydratie of aanpassing van het dialyseschema.
Eindoordeel:
Sterke aanbeveling voor (Doen).
Rationale van aanbeveling-9: weging van argumenten voor en tegen de interventies
Bij reeds dialyse-afhankelijkheid toont beschikbare literatuur geen meerwaarde voor hydratie of aanpassing van het dialyseschema.
Eindoordeel:
Sterke aanbeveling tegen (Niet Doen).
Rationale van aanbeveling-10: weging van argumenten voor en tegen de interventies
Eindoordeel:
Sterke aanbeveling tegen (Niet doen).
Rationale van aanbeveling-11: weging van argumenten voor en tegen de interventies
Er bestaat onvoldoende evidentie om alleen orale hydratie aan te bevelen bij patiënten met een verhoogd risico op PC-AKI. Bij gebrek aan bewijs vanuit zowel de literatuur als de praktijk, is het niet doelmatig om mensen en middelen in te zetten om deze extra handeling uit te voeren.
Eindoordeel:
Sterke aanbeveling tegen (Niet doen).
Onderbouwing
Achtergrond
Current protocols using pre/post hydration with Bicarbonate 1.4% IV or NaCl 0.9% IV are functioning well in practice. However, it is necessary to compare the recommended hydration protocols for patients with impaired renal function in the guideline to newly published approaches and evaluate if the recommendations have to be updated. In addition, an investigation of the possibility to adapt protocols for specific situations in interventional radiology or cardiology, and tailor prehydration dependent on the route of CM administration (intravenous or intra-arterial with second pass renal exposure vs. intra-arterial with first pass renal exposure) is required.
In the current module, the following possible strategies are compared:
- Hydration versus no hydration
- Sodium chloride (NaCl) versus sodium bicarbonate (NaHCO3) hydration
- Pre-hydration only versus pre- and posthydration
- Oral versus intravenous hydration
- Hydration with controlled diuresis
Conclusies / Summary of Findings
Table 3. Summary of Findings
Population: Patients with impaired renal function (chronic kidney disease, chronic renal failure, CKD stage 3 or higher, eGFR <60 ml/min/1.73m2) undergoing radiological or cardiological examinations with iodine-containing contrast media.
Intervention: Hydration
Comparator: No hydration
Click here to see this table in a document
|
Outcome Timeframe |
Study results and measurements |
Absolute effect estimates |
Certainty of the evidence (Quality of evidence) |
Conclusions |
|
|
No hydration |
Hydration |
||||
|
Post-contrast acute kidney injury (critical) |
Based on data from 4 studies |
Kooiman (2014) reported a risk difference of -0.02 (95%CI -0.11 to 0.07). Nijssen (2017) reported a risk difference of 0.00 (95%CI -0.02 to 0.03). Timal (2020) reported a risk difference of -0.01 (95%CI -0.04 to 0.01). Chen (2008) reported a risk difference of -0.20 (95%CI -0.33 to -0.08) for patients with moderate to severely impaired renal function and -0.00 (95%CI -0.04 to 0.04) for normal or mildly impaired renal function. |
Very low Due to serious inconsistency, due to very serious imprecision1 |
The evidence is very uncertain about the effect of hydration on post-contrast acute kidney injury when compared with no hydration in patients with impaired renal function undergoing radiological or cardiological examinations with iodine-containing contrast media. |
|
|
Start renal replacement therapy (RRT) |
|||||
|
Start renal replacement therapy (critical) |
Based on data from 3 studies |
Kooiman (2014), Nijssen (2017) and Timal (2020) reported that none of the patients who either received hydration or no hydration had to start RRT. |
Very low Due to serious inconsistency, due to very serious imprecision2 |
The evidence is very uncertain about the effect of hydration on the start of renal replacement therapy when compared with no hydration in patients with impaired renal function undergoing radiological or cardiological examinations with iodine-containing contrast media. |
|
|
Start renal replacement therapy at 1 year follow-up (critical) |
Based on data from 1 study |
Nijssen (2018) reported a risk difference of 0.00 (95%CI -0.01 to 0.01). |
Very low GRADE Due to serious risk of bias, due to very serious imprecision3 |
The evidence is very uncertain about the effect of hydration on the start of renal replacement therapy at 1 year follow-up when compared with no hydration in patients with impaired renal function undergoing radiological or cardiological examinations with iodine-containing contrast media. |
|
|
Acute renal failure (important) |
- |
- |
No GRADE (no evidence was found) |
No evidence was found regarding the effect of hydration on acute renal failure when compared with no hydration in patients with impaired renal function (chronic kidney disease, chronic renal failure, CKD stage 3 or higher, eGFR <60 ml/min/1.73m2) undergoing radiological or cardiological examinations with iodine-containing contrast media. |
|
|
Irreversible loss of kidney function |
|||||
|
Irreversible loss in kidney function – persisting decline in renal function at 2 months follow-up (important) |
Based on data from 1 study |
Timal (2020) reported a risk difference of -0.43 (95%CI -0.86 to 0.00). |
Very low GRADE Due to serious risk of bias, due to very serious imprecision4 |
The evidence is very uncertain about the effect of hydration on irreversible loss in kidney function (defined as persisting decline in renal function at 2 months follow-up) when compared with no hydration in patients with impaired renal function undergoing radiological or cardiological examinations with iodine-containing contrast media.
|
|
|
Irreversible loss in kidney function – >10 eGFR decline (important) |
Based on data from 1 study |
Nijssen (2017) reported a risk difference of -0.02 (95%CI -0.05 to 0.02). |
Very low GRADE Due to serious risk of bias, due to very serious imprecision4 |
The evidence is very uncertain about the effect of hydration on irreversible loss in kidney function (defined as >10 eGFR decline) as compared with no hydration in patients with impaired renal function undergoing radiological or cardiological examinations with iodine-containing contrast media. |
|
|
Irreversible loss in kidney function – >10 eGFR decline at 1 year follow-up (important) |
Based on data from 1 study |
Nijssen (2018) reported a risk difference of -0.00 (95%CI -0.05 to 0.05). |
Very low GRADE Due to serious risk of bias, due to very serious imprecision4 |
The evidence is very uncertain about the effect of hydration on irreversible loss in kidney function (defined as >10 eGFR decline) at 1 year follow-up when compared with no hydration in patients with impaired renal function undergoing radiological or cardiological examinations with iodine-containing contrast media. |
|
|
Irreversible loss in kidney function – decline to <30 mL per min/1.73m2 (important) |
Based on data from 1 study |
Nijssen (2017) reported a risk difference of 0.00 (95%CI -0.02 to 0.03). |
Very low GRADE Due to serious risk of bias, due to very serious imprecision4 |
The evidence is very uncertain about the effect of hydration on irreversible loss in kidney function (defined as decline to <30 mL per min/1.73m2) when compared to no hydration in patients with impaired renal function undergoing radiological or cardiological examinations with iodine-containing contrast media. |
|
|
Irreversible loss in kidney function – decline to <30 mL per min/1.73m2 at 1 year follow-up (important) |
Based on data from 1 study |
Nijssen (2018) reported a risk difference of 0.00 (95%CI -0.02 to 0.02). |
Very low GRADE Due to serious risk of bias, due to very serious imprecision4 |
The evidence is very uncertain about the effect of hydration on irreversible loss in kidney function (defined as decline to <30 mL per min/1.73m2) at 1 year follow-up when compared with no hydration in patients with impaired renal function undergoing radiological or cardiological examinations with iodine-containing contrast media. |
|
|
Adverse events (important) |
Based on data from 2 studies |
Nijssen (2018) reported a risk difference of 0.04 (95%CI 0.02 to 0.06). Timal (2020) reported that none of the patients who either received hydration or no hydration had adverse events. |
Very low GRADE Due to serious risk of bias, due to serious inconsistency, due to serious imprecision5 |
The evidence is very uncertain about the effect of hydration on adverse events when compared with no hydration in patients with impaired renal function undergoing radiological or cardiological examinations with iodine-containing contrast media. |
|
1. Inconsistency: serious. Due to conflicting results and differences in the type of hydration.
Imprecision: very serious. Due to overlap of the lower and upper limit of the 95% confidence interval with the minimal clinically important difference.
2. Inconsistency: serious. Due to differences in the type of hydration.
Imprecision: very serious. Due to no events occurred and the optimal information size was not achieved.
3. Risk of bias: serious. Due to lack of blinding.
Imprecision: very serious. Due to overlap of the lower and upper limit of the 95% confidence interval with the minimal clinically important difference.
4. Risk of bias: serious. Due to loss to follow-up
Imprecision: very serious. Due to overlap of the lower and upper limit of the 95% confidence interval with the minimal clinically important difference.
5. Risk of bias: serious. Due to lack of blinding.
Inconsistency: serious. Due to conflicting results and differences in the type of hydration.
Imprecision: serious. Due to the low number of events.
Table 6. Summary of Findings
Population: Patients with impaired renal function (chronic kidney disease, chronic renal failure, CKD stage 3 or higher, eGFR <60 ml/min/1.73m2) undergoing radiological or cardiological examinations with iodine-containing contrast media.
Intervention: NaCl
Comparator: NaHCO3
Click here to see this table in a document
|
Outcome Timeframe |
Study results and measurements |
Absolute effect estimates |
Certainty of the evidence (Quality of evidence) |
Conclusions |
|
|
NaCl |
NaHCO3 |
||||
|
Post-contrast acute kidney injury |
|||||
|
PC-AKI: Short schedule NaHCO3 vs. short schedule NaCl |
Relative risk: 0.89 (CI 95% 0.57 – 1.39) Based on data from 2192 participants in 11 studies |
115 per 1000 |
102 per 1000 |
Very low GRADE Due to serious inconsistency, due to very serious imprecision1 |
The evidence is very uncertain about the effect of NaCl on post-contrast acute kidney injury when compared with NaHCO3 in patients with impaired renal function undergoing radiological or cardiological examinations with iodine-containing contrast media. |
|
Difference: 13 fewer per 1000 (CI 95% 49 fewer – 45 more) |
|||||
|
PC-AKI: Short schedule NaHCO3 vs. long schedule NaCl |
Relative risk: 1.23 (CI 95% 0.81 – 1.87) Based on data from 2369 participants in 9 studies |
78 per 1000 |
96 per 1000 |
Very low GRADE Due to serious inconsistency, due to very serious imprecision1 |
|
|
Difference: 18 more per 1000 (CI 95% 15 fewer – 68 more) |
|||||
|
PC-AKI: All other hydration schedules comparing bicarbonate plus sodium chloride or bicarbonate only to either sodium chloride or bicarbonate only |
Based on data from 6 studies |
Studies identified before 2017 varied in their estimates (see results above). Weisbord (2018) reported a risk difference of -0.01 (95%CI -0.03 to 0.00). |
Very low GRADE Due to serious inconsistency, due to very serious imprecision1 |
||
|
PC-AKI: NaHCO3 (ultrashort schedule) precontrast versus pre- and post-CM hydration with NaCl
|
Based on data from 1 study |
Kooiman (2014) reported a risk difference of -0.02 (95%CI -0.05 to 0.01) |
Very low GRADE Due to serious indirectness, due to very serious imprecision2 |
||
|
Start renal replacement therapy (RRT) |
|||||
|
Start RRT: Short schedule NaHCO3 vs. short schedule NaCl |
Based on data from 9 studies |
Studies identified before 2017 varied in their estimates (see table 5) Boccalandro (2020) reported a risk difference of -0.01 (95%CI -0.05 to 0.03)
|
Very low GRADE Due to serious inconsistency, due to very serious imprecision3 |
The evidence is very uncertain about the effect of NaCl on the start of renal replacement therapy when compared with NaHCO3 in patients with impaired renal function undergoing radiological or cardiological examinations with iodine-containing contrast media. |
|
|
Start RRT: Short schedule NaHCO3 vs. long schedule NaCl |
Based on data from 7 studies |
Studies identified before 2017 varied in their estimates (see table 5) |
Very low GRADE Due to serious inconsistency, due to very serious imprecision3 |
||
|
Start RRT: All other hydration schedules comparing bicarbonate plus sodium chloride or bicarbonate only to either sodium chloride or bicarbonate only |
Based on data from 5 studies |
Studies identified before 2017 varied in their estimates (see table 5) Weisbord (2018) reported a risk difference of -0.00 |
Very low GRADE Due to serious inconsistency, due to very serious imprecision3 |
||
|
Start RRT: NaHCO3 (ultrashort schedule) precontrast versus pre- and post-CM hydration with NaCl |
Based on data from 1 study |
Kooiman (2014) reported that none of the patients who either received prehydration or pre-and posthydration had to start renal replacement therapy. |
Very low GRADE Due to serious indirectness, due to very serious imprecision2 |
||
|
Start renal replacement therapy – 1 year (important) |
Based on data from 1 study |
Boccalandro (2020) reported a risk difference of -0.02 (95%CI -0.04 to 0.01) |
Very low GRADE Due to very serious imprecision4 |
The evidence is very uncertain about the effect of NaCl on the start of renal replacement therapy at 1 year follow-up when compared with NaHCO3 in patients with impaired renal function undergoing radiological or cardiological examinations with iodine-containing contrast media. |
|
|
Start renal replacement therapy – 5 year (important) |
Based on data from 1 study |
Boccalandro (2020) reported a risk difference of 0.02 (-0.05 to 0.08) |
Very low GRADE Due to very serious imprecision4 |
The evidence is very uncertain about the effect of NaCl on the start of renal replacement therapy at 5 year follow-up when compared with NaHCO3 in patients with impaired renal function undergoing radiological or cardiological examinations with iodine-containing contrast media. |
|
|
Acute renal failure (important) |
- |
- |
No GRADE (no evidence was found) |
No evidence was found regarding the effect of NaCl on acute renal failure when compared with NaHCO3 in patients with impaired renal function undergoing radiological or cardiological examinations with iodine-containing contrast media. |
|
|
Irreversible loss of kidney function (important) |
Based on data from 1 study |
Weisbord (2018) reported a risk difference of 0.00 (95%CI -0.00 to 0.01) |
Very low GRADE Due to very serious imprecision4 |
The evidence is very uncertain about the effect of NaCl on irreversible loss of kidney function when compared with NaHCO3 in patients with impaired renal function undergoing radiological or cardiological examinations with iodine-containing contrast media. |
|
|
Adverse events |
|||||
|
Adverse events: Short schedule NaHCO3 vs. short schedule NaCl |
Based on data from 5 studies |
Studies identified before 2017 varied in their estimates (see table 5). Boccalandro (2020) reported a risk difference of 0.01 (95%CI -0.06 to 0.08). |
Very low GRADE Due to serious inconsistency, due to very serious imprecision5 |
The evidence is very uncertain about the effect of NaCl on adverse events when compared with NaHCO3 in patients with impaired renal function undergoing radiological or cardiological examinations with iodine-containing contrast media |
|
|
Adverse events: Short schedule NaHCO3 vs. long schedule NaCl |
Based on data from 4 studies |
Studies identified before 2017 varied in their estimates (see table 5). |
Very low GRADE Due to serious inconsistency, due to very serious imprecision5 |
||
|
Adverse events: All other hydration schedules comparing bicarbonate plus sodium chloride or bicarbonate only to either sodium chloride or bicarbonate only |
Based on data from 4 studies |
Studies identified before 2017 varied in their estimates (see table 5). Weisbord (2018) reported a risk difference of -0.01 |
Very low GRADE Due to serious inconsistency, due to very serious imprecision5 |
||
|
Adverse events: NaHCO3 (ultrashort schedule) precontrast versus pre- and post-CM hydration with NaCl |
Based on data from 1 study |
Kooiman (2014) reported a risk difference of -0.03 (95%CI -0.10 to 0.05). |
Very low GRADE Due to serious inconsistency, due to very serious imprecision6 |
||
1. Inconsistency: serious. Due to conflicting results and heterogeneity
Imprecision: very serious. Due to overlap of the upper and lower limit of the 95% confidence interval with the minimal clinically important difference
2. Indirectness: serious. Due to differences in the hydration type (pre- versus pre- and posthydration)
Imprecision: very serious. Due to overlap of the upper and lower limit of the 95% confidence interval with the minimal clinically important difference
3. Inconsistency: serious. Due to conflicting results and heterogeneity
Imprecision: very serious. Due to the optimal information size which was not achieved and the low number of events
4. Imprecision: very serious. Due to overlap of the upper and lower limit of the 95% confidence interval with the minimal clinically important difference and the optimal information size was not achieved.
5. Inconsistency: serious. Due to heterogeneity between the studies
Imprecision: very serious. Due to the optimal information size which was not achieved and the low number of events
6. Inconsistency: serious. Due to differences in the hydration type (pre- versus pre- and posthydration)
Imprecision: very serious. Due to the optimal information size which was not achieved and the low number of events
Table 8. Summary of Findings
Population: Patients with impaired renal function (chronic kidney disease, chronic renal failure, CKD stage 3 or higher, eGFR <60 ml/min/1.73m2) undergoing radiological or cardiological examinations with iodine-containing contrast media.
Intervention: Prehydration
Comparator: Pre- and posthydration
Click here to see this table in a document
|
Outcome Timeframe |
Study results and measurements |
Absolute effect estimates |
Certainty of the evidence (Quality of evidence) |
Conclusions |
|
|
Pre- and posthydration |
Prehydration |
||||
|
Post-contrast acute kidney injury (critical) |
Relative risk: 0.74 (CI 95% 0.42 – 1.32) Based on data from 935 participants in 3 studies |
75 per 1000 |
56 per 1000 |
Very low GRADE Due to serious indirectness, due to very serious imprecision1 |
The evidence is very uncertain about the effect of prehydration on post-contrast acute kidney injury when compared with pre- and posthydration in patients with impaired renal function undergoing radiological or cardiological examinations with iodine-containing contrast media. |
|
Difference: 19 fewer per 1000 (CI 95% 43 fewer – 24 more) |
|||||
|
Start renal replacement therapy (critical) |
Based on data from 3 studies |
Kooiman (2014), Kooiman (2018) and Van Mourik (2018) reported that none of the patients who either received prehydration or pre-and posthydration had to start renal replacement therapy. |
Very low GRADE Due to serious indirectness, due to very serious imprecision2 |
The evidence is very uncertain about the effect of prehydration on the start of dialysis when compared with pre- and posthydration in patients with impaired renal function undergoing radiological or cardiological examinations with iodine-containing contrast media. |
|
|
Acute renal failure (important) |
- |
- |
No GRADE (no evidence was found) |
No evidence was found regarding the effect of prehydration on acute renal failure when compared with pre- and posthydration in patients with impaired renal function undergoing radiological or cardiological examinations with iodine-containing contrast media. |
|
|
Irreversible loss of kidney function (important) |
- |
- |
No GRADE (no evidence was found) |
No evidence was found regarding the effect of prehydration on irreversible loss of kidney function when compared with pre- and posthydration in patients with impaired renal function undergoing radiological or cardiological examinations with iodine-containing contrast media. |
|
|
Adverse events (important) |
Based on data from 2 studies |
Kooiman (2014) reported a risk difference of -0.02 (95%CI -0.04 to -0.00). Van Mourik (2018) reported a risk difference of -0.03 (95%CI -0.10 to 0.05). |
Very low GRADE Due to serious indirectness, due to very serious imprecision1 |
The evidence is very uncertain about the effect of prehydration on adverse events when compared with pre- and posthydration in patients with impaired renal function undergoing radiological or cardiological examinations with iodine-containing contrast media. |
|
1. Indirectness: serious. Due to differences in the hydration type used for pre- and posthydration
Imprecision: very serious. Due to overlap of the upper and lower limit of the 95% confidence interval with the minimal clinically important difference
2. Indirectness: serious. Due to differences in the hydration type used for pre- and posthydration
Imprecision: very serious. Due to no events occurred and the optimal information size which was not achieved
Samenvatting literatuur
Question 1-3 update 2025.
Question 1: hydration versus no hydration
Description of studies
A total of four studies were included in the analysis of the literature. Three RCTs were found in the search of 2017 and two studies were added in 2025. Important study characteristics and results are summarized in table 2. The assessment of the risk of bias is summarized in the risk of bias tables (under the tab ‘Evidence tabellen’).
Chen (2008) performed a prospective, randomized, parallel-group, open label study in three centers in China to study the effect of pre- and posthydration with 0.45% saline on major clinical endpoints after 6 months and the incidence of PC-AKI after elective PCI. They studied all consecutive patients with myocardial ischemia scheduled for elective PCI, divided into a group of 660 patients (mean age 60±8 years) with normal or mildly impaired renal function (creatinine <133, mean 114±26 umol/l and eGFR 53±16 ml/min/1.73m2) and a group of 276 patients (mean age 63±11 years) with moderate to severely impaired renal function (creatinine >133; mean creatinine 220±9 umol/l and eGFR 28±2 ml/min/1.73m2). Patients were excluded if (1) the coronary anatomy not suitable for PCI; (2) emergency coronary artery bypass grafting (CABG) being required; (3) patients in chronic peritoneal or hemodialytic treatment; (4) acute myocardial infarction (AMI) at admission. In both groups, hydration was given with hypotonic NaCl 0.45% at a rate of 1 ml/kg/min for 12 hours before and 6 hours after PCI. In patients with reduced left ventricular function (ejection fraction <40%), infusion rate was reduced to 0.8 ml/kg/min. Only in the group with moderate to severely impaired renal function, all patients received a loading dose of 1200 mg N-Acetyl Cysteine 12 hours before PCI. Primary endpoint consisted of clinical driven revascularization (either PCI or CABG), death from all causes and requiring emergency renal-replacement therapy during the 6 months follow-up. They further studied adverse events such as acute pulmonary edema during 6 months follow-up and the incidence of PC-AKI defined as an absolute increase of serum creatinine >44 umol/L at 48 hours after PCI in the two groups.
Kooiman (2014) described 138 patients with eGFR <60 ml/min/1.73m2 undergoing chest CT for suspected pulmonary embolism. Sixty-seven patients received no hydration, and the remaining 71 patients received 250ml NaHCO3 1.4% within one hour prior to CT.
Nijssen (2017) performed a prospective, randomised, phase 3, single centre, parallel-group, open-label, non-inferiority trial (AMACING) in the Netherlands to determine whether giving no prophylaxis is non-inferior to standard care prophylactic hydration. Patients aged 18 years and older (mean age 72±9 years) who received an elective procedure requiring intravascular iodinated contrast material with known eGFR lower than 60 mL per min/1.73m2 were eligible. Inclusion criteria were patients with an eGFR between 45 and 59 mL per min/1.73 m² combined with either diabetes, or at least two predefined risk factors (age >75 years; anaemia defined as haematocrit values <0.39 L/L for men, and <0.36 L/L for women; cardiovascular disease; non-steroidal anti-inflammatory drug or diuretic nephrotoxic medication); or eGFR between 30 and 45 mL per min/1.73 m²; or multiple myeloma or lymphoplasmacytic lymphoma with small chain proteinuria. In total, 328 patients received hydration with 0.9% NaCl (52% short protocol and 48% long protocol of respectively 4 hours and 12 hours before and after contrast) and 332 patients received no hydration. Groups were comparable at baseline. Mean eGFR was 47±8 ml/min/1.73m2 and 35% had an eGFR 30-45 ml/min/1.73m2. 48% of the patients received intra-arterial contrast (of these 60% coronary catheterisations, 30% percutaneous coronary interventions and 10% other). Serum creatinine was measured immediately before, at 2-6 days, and at 26-35 days after contrast administration. PC-AKI was defined as an increase of serum creatinine >25% or >44 µmol/L 2-6 days after contrast (instead of the usual 48-72 hours after contrast). Outcomes of interest were incidence of contrast-induced nephropathy, renal failure, dialysis and renal function decline.
Nijssen (2018) presented the 1-year follow-up results of the study of Nijssen (2017). For inclusion criteria see above. Outcomes of interest were renal function decline and dialysis.
Timal (2020) performed a multicentre, noninferiority, randomized clinical trial (KOMPAS) in the Netherlands to determine the safety of omitting prophylactic prehydration prior to iodine-based contrast media administration in the setting of elective contrast-enhanced CT. Inclusion criteria were patients with an estimated glomerular filtration rate (eGFR) of 30 to 44 mL/min/1.73 m2 (CKD stage 3B) or with an eGFR of 45 to 59 mL/min/1.73 m2 (CKD stage 3A) in the presence of diabetes or at least 2 of the following risk factors: peripheral artery disease, congestive heart failure, age older than 75 years, anemia, contrast medium volume greater than 150 mL, or use of nephrotoxic medication (e.g., diuretics, nonsteroidal anti-inflammatory drugs, cyclosporin, tacrolimus, antiviral medication, amphotericin B, aminoglycosides, cisplatin, vancomycin). In total, 261 patients received prehydration with 250 mL of 1.4% sodium bicarbonate administered in a 1-hour infusion and 262 patients received no prehydration. Groups were comparable at baseline with amongst others 40% diabetes and approximately 15% heart failure in both groups. About half of the patients had an eGFR 30-45 ml/min/1.73m2. Approximately 50% of the patients were using diuretics, which could be interrupted before contrast administration. Serum creatinine was measured before hydration and contrast, at 2-5 days, and at 7-14 days after contrast administration in all patients and was repeated after 2 months in the patients who had developed PC-AKI. PC-AKI was defined as an increase of serum creatinine >25% or >44 µmol/L 2-5 days after contrast. Outcomes of interest were incidence of postcontrast acute kidney injury (PC-AKI), irreversible loss of kidney function and dialysis.
Table 2. Characteristics of included studies
|
Study |
Participants |
Comparison |
Follow-up |
Outcome measures |
Comments |
Risk of bias (per outcome measure)* |
|
Individual studies |
||||||
|
Chen, 2008 |
N at baseline:
Normal serum creatinine group Intervention: 330 Control: 330
Abnormal serum creatinine group Intervention: 188 Control: 188
Baseline characteristics not reported |
Intervention: Hydration (treated by 0.45% saline given intravenously at a rate of 1 ml/kg/h starting from 12 h before scheduled time for coronary angiogram) Control: nonhydration
All patients in abnormal group took twice orally loading dose of 1200 mg NAC at 12 h before scheduled time for coronary angiogram and immediately after procedure.
|
6 months |
CIN |
Source of funding and conflicts of interest not reported. |
High (all outcomes) |
|
Kooiman, 2014 |
N at baseline Intervention: 71 Control: 67
Age (mean, SD) Intervention: 71 years (SD=13) Control: 70 years (SD=12)
Sex (male) Intervention: 34 (48%) Control: 35 (52%)
|
Intervention: 250 mL iv 1.4% sodium bicarbonate 1 hour before CTPA Control: Withholding hydration prior to CTPA |
96 hours |
CI-AKI, need for dialysis |
Non-commercial source of funding. |
LOW (CI-AKI, dialysis)** |
|
Nijssen, 2017 |
N at baseline Intervention: 328 Control: 332
Age (mean, SD) Intervention: 71.9 years (SD=9.3) Control: 72.6 years (SD=9.3)
Sex (male) Intervention: 194 (59%) Control: 213 (64%)
|
Intervention: intravenous 0.9% NaCl Control: no prophylaxis |
Up to 35 days |
Incidence of contrast-induced nephropathy, renal failure, renal function decline, dialysis, adverse events |
The funder was not involved in trial design, patient recruitment, data collection, analysis, interpretation or presentation, writing or editing. The authors declare no competing interests. |
LOW (dialysis, adverse events)
Some concerns (incidence of contrast-induced nephropathy, renal failure, renal function decline) |
|
Nijssen, 2018 (follow-up of Nijssen, 2017) |
N at baseline Intervention: 328 Control: 332
Age (mean, SD) Intervention: 71.9 years (SD=9.3) Control: 72.6 years (SD=9.3)
Sex (male) Intervention: 194 (59%) Control: 213 (64%)
|
Intervention: intravenous 0.9% NaCl Control: no prophylaxis |
1 year |
Renal function decline, dialysis |
Funding by Stichting de Weijerhorst. The authors declare no competing interests.
|
Some concerns (end-stage renal failure, decrease in residual kidney function)
Low (dialysis) |
|
Timal, 2020 |
N at baseline Intervention: 261 Control: 262
Age (median, IQR) Intervention: 73 years (67-78) Control: 74 years (67-80)
Sex (male) Intervention: 166 (63.6%) Control: 213 (64.9%)
|
Intervention: prehydration with 250 mL of 1.4% sodium bicarbonate administered in a 1-hour infusion Control: no prehydration
|
Up to 1 year |
Incidence of PC-AKI, decrease in residual kidney function, and dialysis |
Not reported |
Low (incidence of PC-AKI, end-stage renal failure, dialysis, adverse events)
Some concerns (decrease in residual kidney function) |
*For further details, see risk of bias table in the appendix
** The risk of bias was based on the risk of bias assessment performed in the guideline of 2017
Results
1. Post-contrast acute kidney injury (PC-AKI) (critical)
Four studies reported the incidence of PC-AKI for the comparison between hydration and no hydration (Chen, 2008; Kooiman, 2014; Nijssen, 2017; Timal, 2020). Because of heterogeneity between these studies in terms of type of hydration, the results were not pooled.
Chen (2008) reported that 22 of the 330 patients (6.67%) in the group with normal or mildly impaired renal function (eGFR 53±16 ml/min/1.73m2) who received hydration with 0.45% NaCl had PC-AKI as compared to 23 of the 330 (6.97%) who received no hydration (RR=0.89, 95%CI 0.58 to 1.35). In the group with moderate to severely impaired renal function (eGFR 28±2 ml/min/1.73m2), 40 of the 188 patients (21.28%) who received hydration had PC-AKI as compared to 64 of the 188 patients (34.04%) who received no hydration (RR=0.63, 95%CI 0.46 to 0.84).
Kooiman (2014) reported that 5 of the 70 patients (7.1%) who received 1-hour pre-hydration with 250ml NaHCO3 had PC-AKI as compared to 6 of the 65 patients (9.2%) in the group withholding hydration (RR=0.77, 95%CI 0.25 to 2.41).
Nijssen (2017) reported that 8 of the 296 patients (2.7%) who received hydration had contrast-induced nephropathy as compared to 8 of the 307 patients (2.6%) who did not receive hydration (RR=1.04, 95%CI 0.39 to 2.73).
Timal (2020) reported that 4 of the 261 patients (1.5%) who received hydration had PC-AKI 2 to 5 days after contrast enhanced CT as compared to 7 of the 262 patients (2.7%) who did not receive hydration (RR=0.57, 95%CI 0.17 to 1.94).
2. Start renal replacement therapy (RRT) (critical)
Three studies reported the start of renal replacement therapy for the comparison between hydration and no hydration (Nijssen, 2014; Nijssen, 2017; Timal, 2020). Because of heterogeneity between these studies in terms of type of hydration and since no events occurred, the results were not pooled.
Nijssen (2014) reported that none of the PC-AKI patients who either received hydration or no hydration developed need for dialysis.
Nijssen (2017) reported that no patients who received either hydration or did not receive hydration required dialysis within 35 days post-contrast.
Timal (2020) reported that during the at least one-year follow-up, no patients who received either hydration or did not receive hydration required dialysis.
At 1-year follow-up
Nijssen (2018), the 1-year follow-up study of Nijssen (2017), reported that within 365 days, 2 of the 328 patients (0.6%) who received hydration required dialysis as compared to 2 of the 332 patients (0.6%) who did not receive contrast (RR=1.01, 95%CI 0.14 to 7.14).
3. Acute renal failure (important)
Not reported.
4. Irreversible loss of kidney function (important)
Timal (2020) reported that after 2 months of follow-up, 3 of the 7 patients (43%) with PC-AKI who did not receive hydration had a persisting decline of renal function (mean decrease in eGFR, 3 mL/min/1.73 m2) while this did not occur in the 4 patients who developed PC-AKI despite hydration (RR=0.23, 95%CI 0.01 to 3.56).
4.1. >10 ml/min/1.73m2 eGFR decline
Nijssen (2017) reported that 7 of the 260 patients (2.7%) who received hydration had >10 ml/min/1.73m2 renal function decline from baseline as compared to 11 of the 260 patients (4.2%) who did not receive hydration (RR=0.64, 95%CI 0.25 to 1.62). This difference is clinically relevant favoring hydration.
1-year follow-up
Nijssen (2018), the 1-year follow-up study of Nijssen (2017), reported that 28 of the 297 patients (9.4%) who received hydration had >10 ml/min/1.73m2 renal function decline from baseline as compared to 28 of the 292 patients (9.6%) who did not receive hydration (RR=0.98, 95%CI 0.60 to 1.62). This difference is not clinically relevant.
4.2. Decline to <30 mL per min/1.73m2
Nijssen (2017) reported that 7 of the 260 patients (2.7%) who received hydration had a renal function decline to eGFR < 30 mL per min/1.73m2 as compared to 6 of the 260 patients (2.3%) who did not receive hydration (RR=1.17, 95%CI 0.40 to 3.42).
1-year follow-up
Nijssen (2018), the 1-year follow-up study of Nijssen (2017), reported that 9 of the 297 patients (3.0%) who received hydration had a renal function decline to eGFR
< 30 mL per min/1.73m2 as compared to 8 of the 292 patients (2.7%) who did not receive hydration (RR=1.11, 95%CI 0.43 to 2.83).
5. Adverse events (important)
Nijssen (2017) reported that 13 of the 328 patients (4.0%) who received short or long hydration with NaCl 0.9% had symptomatic heart failure, while this did not occur in patients who did not receive hydration (RR=27.33, 95%CI 1.63 to 457.82).
Timal (2020) reported that no patients who received either hydration (n=261) or did not receive hydration (n=262) developed heart failure.
Q2: NaCl versus NaHCO3
Description of studies
A total of 27 studies were included in the analysis of the literature. Twenty-four RCTs were found and described in the search of 2017. This was updated in 2025 with the description of three additional studies. Important study characteristics and results are summarized in table 4. The assessment of the risk of bias is summarized in the risk of bias tables (under the tab ‘Evidence tabellen’).
Description of three studies from update 2025
Boccalandro (2020) performed a prospective, single-centre, randomized, double-blinded
study in the USA to compare the effect of a short schedule of sodium bicarbonate with NaCl on long-term outcomes of clinically stable patients with chronic kidney disease (CKD) undergoing non-emergent coronary angiography and when indicated percutaneous coronary intervention. It included elective cases and urgent cases defined as requiring angiography within 24-hours from hospital admission with CKD Stage III-IV (eGFR 15-59 ml/min/1.73 m2). In total, 190 patients received NaCl (154mEq/l NaCl with 5% dextrose solution for 1 h before and during the angiographic procedure at a rate of 3ml/kg/h followed by 1 ml kg/h for 6 h post-procedure ) and 192 patients received sodium bicarbonate (150 mEq/l SB with 5% dextrose solution at 3 ml/kg/h for 1 h before and during the angiographic procedure, followed by 1 ml/kg/h for 6 h post-procedure). Diuretics and non-steroidal anti-inflammatory drugs were discontinued 24-hours prior to the procedure and at least for 72-hours after the procedure. Groups were comparable at baseline. Gender was equally distributed with 53% and 51% male in the sodium bicarbonate group and sodium chloride group, respectively. Within the sodium bicarbonate group, 60% were of Caucasian descent, 30% of Hispanic descent and 10% of African American descent. These figures were comparable within the sodium chloride group, being 62%, 29% and 8%, respectively. Baseline eGFR was 40±18 ml/min/1.73m2 and 63% had diabetes mellitus. Outcomes of interest were iodine containing contrast associated acute kidney injury (CA-AKI) defined by an absolute increase in creatinine >0.5 mg/dl (>44 µmol/l) or relative increase >25% within 48 hours and need to start renal replacement therapy after 1 and 5 years based on telephone interview of randomized patients.
Weisbord (2018) performed a double-blind, placebo- and comparator-controlled, multicentre, international randomized clinical trial with a 2 by 2 factorial design comparing intravenous sodium bicarbonate with intravenous sodium chloride and oral acetylcysteine with oral placebo for the prevention of major adverse outcomes and acute kidney injury in a large population of high-risk patients undergoing coronary or noncoronary angiography. Patients were enrolled at 53 medical centres in the United States of America (35 Veteran Affairs sites), 13 sites in Australia, 3 sites in Malaysia, and 2 sites in New Zealand. For the purpose of this guideline, only the comparison between sodium chloride and sodium bicarbonate were analysed. Patients (93.6% male; 80.9% with diabetes) undergoing non-emergency coronary or non-coronary angiography who had a baseline eGFR of 15 to 44.9 ml/min/1.73 m2 or both an eGFR of 45 to 59.9 ml/min/1.73 m2 and diabetes were included. In total, 2482 patients received isotonic 0.9% sodium chloride (154 mmol/l) and 2511 patients received isotonic 1.26% sodium bicarbonate (150 mmol/l). The infusion protocol allowed different volume and duration of infusion in the protocols of the participating centers with a specified minimum required volume and duration of administration of ≥3 ml/kg over 1-12 hours (1-3 ml/kg/hour) before angiographic procedure, 1–1.5 ml/kg/hour during angiography, and ≥6 ml/kg over 4-12 hours (1–1.5 ml/kg/hour) after the procedure. Blood samples were collected before, 3-5 days and 90-104 days after angiography and serum creatinine levels in these samples were measured simultaneously for each patient in the trial laboratory. Groups were comparable at baseline. The outcomes of interest were CA-AKI (increase of serum creatinine ≥25% and/or ≥0.5 mg/dl (44 µmol/l) at 96 hours) and need for dialysis or persistent decline in kidney function (increase of creatinine >50% from baseline) at 90 days. The trial was stopped prematurely because of futility after the preplanned interim analysis after inclusion of 4993 patients.
Garcia (2018) performed a subgroup analysis of the study of Weisbord (2018) to assess the effect of intravenous sodium bicarbonate and intravenous sodium chloride in patients who underwent percutaneous coronary intervention (PCI) during the angiographic examination. Patients were excluded in the case of emergency angiography (e.g., those with ST-segment elevation myocardial infarction); unstable baseline levels of serum creatinine (≥ 25% variation within 3 days prior to angiograph); decompensated heart failure requiring IV inotropic support, ultrafiltration, or intra-aortic balloon pump; recent exposure to contrast media. In total, 593 patients received sodium chloride, and 568 patients received sodium bicarbonate. Groups were comparable at baseline. Baseline median eGFR was 50.7 ml/min/1.73m2 (IQR 41.7 – 60.1). Patients undergoing PCI received a median contrast volume of 160 ml (IQR 115 – 220) versus non-PCI median 75 ml (IQR 50 – 105) in the overall cohort of Weisbord 2018. The outcome of interest was CA-AKI.
Description of all current and previous studies
Depending on the design, the RCTs comparing sodium to bicarbonate hydration were categorized into several groups:
- Short schedule NaHCO3 vs. short schedule NaCl in patients with impaired kidney function undergoing coronary angiography (CAG) and/or PCI. A total of 11 RCTs (Adolph, 2008; Boccalandro, 2020; Boucek, 2013; Brar, 2008; Gomes, 2012; Masuda, 2007; Merten, 2004; Ozcan, 2007; Ratcliffe, 2009; Recio-Mayoral, 2007; Solomon 2015) with 2,192 patients were identified, that compared bicarbonate and sodium chloride hydration in a similar hydration scheme for coronary angiography. All the studies were performed in patients with impaired kidney function;
- Short schedule NaHCO3 vs. long schedule NaCl (1ml/kg/h for 12h pre- and 12h post-CM administration) in patients with impaired kidney function undergoing CAG and/or PCI. A total of 9 RCTs (Briguori, 2007; Castini, 2010; Hafiz, 2012; Klima, 2012; Koc, 2013; Lee, 2011; Maioli, 2008; Nieto-Rios, 2014; Shavit, 2009) with 3,026 patients were identified that compared bicarbonate hydration to sodium chloride pre- and posthydration (1ml/kg, 12hour pre- and post) for coronary angiography;
- All other hydration schedules comparing bicarbonate plus sodium chloride or bicarbonate only to either sodium chloride or bicarbonate only. Four RCTs (Chong, 2015; Motohiro, 2011; Tamuro, 2009; Ueda, 2011) with 358 patients compared bicarbonate to sodium chloride hydration with divergent hydration schemes for coronary angiography, like adding a bolus NaHCO3 to sodium chloride hydration or exchanging sodium chloride by NaHCO3 hydration for multiple hours. One study (Weisbord, 2018) with 4,993 patients compared bicarbonate to sodium chloride hydration with an infusion protocol allowing different volume and duration of infusion in the protocols of the participating centers with a specified minimum required volume and duration of administration (see above) for coronary angiography and Garcia (2018) performed a subgroup analysis for 1,161 patients receiving PCI;
- One RCT compared in a non-inferiority trial, a 1-hour schedule of 250ml NaHCO3 1.4% versus 1000 ml NaCl 0,9% in 4-12h pre- and 4-12h post-CM administration in 548 CT patients. (Kooiman, 2014).
Table 4. Characteristics of included studies
|
Study |
Participants |
Comparison |
Follow-up |
Outcome measures |
Comments |
Risk of bias (per outcome measure)* |
|
Individual studies |
||||||
|
||||||
|
Adolph, 2008 |
N at baseline Intervention: 71 Control: 74
Age (mean, SD) Intervention: 70 years (SD=8) Control: 73 years (SD=7)
Sex (male) Intervention: 53 (75%) Control: 60 (81%) |
Intervention: Sodium bicarbonate 154mEq/L in 5% dextrose solution 2ml/kg body weight/hour for 2 hours before And 1ml/kg body weight/hour during and for 6 hours after contrast administration Control: Sodium chloride 154 mEq/L in 5% dextrose solution 2ml/kg body weight/hour for 2 hours before And 1ml/kg body weight/hour during and for 6 hours after contrast administration |
2 days |
CIN, dialysis |
Source of funding not reported |
Unclear** |
|
Boccalandro, 2020 |
N at baseline Intervention: 190 Control: 192
Age (mean, SD) Intervention: 68 years (SD=10) Control: 67 years (SD=13)
Sex (male) Intervention: 96 (51%) Control: 102 (53%)
|
Intervention: sodium chloride (154mEq/l NaCl with 5% dextrose solution) Control: sodium bicarbonate (150 mEq/l of sodium bicarbonate with 5% dextrose solution) Both administered at 3 ml/kg/h for 1 h before and during the angiographic procedure, followed by 1 ml/kg/h for 6 h post-procedure) |
Up to 60 months |
CA-AKI, renal replacement therapy, adverse events
|
No relation with industry and no financial support
In multivariate analysis significantly higher incidence of CA-AKI with diabetes, higher baseline creatinine, advanced age, and history of heart failure |
Low (all outcomes) |
|
Boucek, 2013 |
N at baseline Intervention: 61 Control: 59
Age (mean, SD) Intervention: 63 years (SD=11) Control: 67 years (SD=10)
Mean eGFR 44±18.
Planned procedure of CAG/PCI (35%) or lower limb angiography/angioplasty (60%)
Sex (male) Intervention: 46 (75%) Control: 44 (75%) |
Intervention: 1.4% sodium bicarbonate in 5% glucose 3ml/kg/hour 1 hour before contrast administration (limited to a maximum of 330mL) 1mL/kg/hour 6 hours after contrast administration (limited to a maximum of 660mL) Control: 0.9% sodium chloride in 5% glucose 3ml/kg/hour 1 hour before contrast administration (limited to a maximum of 330mL) 1mL/kg/hour 6 hours after contrast administration (limited to a maximum of 660mL)
|
2 days – laboratory parameters 1 month – clinical parameters
|
CIN defined by >25% increase of creatinine or >44 umol/l within 2 days after contrast, dialysis |
Commercial source of funding |
Low** |
|
Brar, 2008 |
N at baseline Intervention: 175 Control: 178
Age (IQR, range) Intervention: 71 (65 to 75) years Control: 71 (65 to 76) years All CAG, 32% with PCI and 46% after ACS. Emergency CAG excluded
Mean GFR 48±9 Small group GFR <30 (11 NaCl and 10 NaHCO3)
Sex (male) Intervention: 113 (65%) Control: 110 (62%) |
Intervention: 1.4% sodium bicarbonate iv infusion. Infusion was begun 1 hour prior to the start of contrast administration at 3mL/kg for 1 hour, decreased to 1.5 mL/kg per hour during the procedure and for 4 hours following completion of the procedure. For patients weighing more than 100 kg, the bolus and infusion rate were limited to those used for patients weighing 100kg Control: 0.9% sodium chloride iv infusion. Infusion was begun 1 hour prior to the start of contrast administration at 3 mL/kg for 1 hour, decreased to 1.5 mL/kg per hour during the procedure and for 4 hours following completion of the procedure. For patients weighing more than 100 kg, the bolus and infusion rate were limited to those used for patients weighing 100kg |
2-3 days for laboratory parameters 6 months for clinical effects
|
PC-AKI defined by >25% decrease in GFR, persistent decline or GFR >25% in group with PC-AKI, dialysis
|
Commercial source of funding
In subgroup with GFR <30 much higher incidence of PC-AKI (4/11=36% in NaCl and 2/10=20% in NaHCO3)
Persistent decline in GFR after 8 wks in NaCl 3% and NaHCO3 2.5% |
Low** |
|
Gomes, 2012 |
N at baseline Intervention: 150 Control: 151
Age (mean, SD) Intervention: 64 years (SD=12) Control: 65 years (SD=12)
All CAG with or without PCI. No data on elective or emergency (full text not available) GFR <50
Sex (male) Intervention: 103 (69%) Control: 113 (75%) |
Intervention: 154 mEq/l of sodium bicarbonate in 5% dextrose and H2O 3 mL/ kg/ h for 1 hour immediately before contrast injection same fluid at a rate of 1 mL/kg/h during contrast exposure and for 6 hours after the procedure Control: 0.9% sodium chloride infusion 3 mL/ kg/ h for 1 hour immediately before contrast injection same fluid at a rate of 1 mL/kg/h during contrast exposure and for 6 hours after the procedure |
48 hours
|
CIN defined by increase creatinine >44 umol/l, dialysis |
No source of funding reported |
Low** |
|
Masuda, 2007 |
N at baseline Intervention: 30 Control: 29
Age (mean, SD) Intervention: 75 years (SD=8) Control: 76 years (SD=11)
Emergency diagnostic or interventional CAG for suspected ACS. Diuretics and other medication could be started at discretion of attending physician.
Sex (male) Intervention: 19 (63%) Control: 17 (59%)
|
Intervention: 154-mEq/L infusion of sodium bicarbonate as a bolus of 3 ml/kg/hour for 1 hour before the administration of contrast, followed by an infusion of 1 ml/kg/hour for 6 hours during and after the procedure Control: 154-mEq/L infusion of sodium chloride as a bolus of 3 ml/kg/hour for 1 hour before the administration of contrast, followed by an infusion of 1 ml/kg/hour for 6 hours during and after the procedure |
2 days |
CIN defined by creatinine increase >25% or >44 umol/l Incidence in-hospital dialysis for acute renal failure
|
Source of funding not reported.
Lower GFR in patients developing CIN (29.6±15.8 vs 42.0±14.2 without CIN) – chance of absolute increase of 44 umol/l higher with lower GFR (=higher baseline creatinine).
Incidence of heart failure similar NaCl 38% and NaHCO3 37% |
Unclear** |
|
Merten, 2004 |
N at baseline Intervention: 60 Control: 59
Age (mean, SD) Intervention: 66.7 years (SD=12) Control: 69.2 years (SD=12) Non- emergency radiologic procedures (approx. 80% CAG). Diuretics continued. Mean GFR 43 (13-84)
Sex (male) Intervention: 44 (73%) Control: 45 (76%) |
Intervention: 154 mEq/L of sodium bicarbonate, as a bolus of 3 mL/kg per hour for 1 hour before iopamidol contrast, followed by an infusion of 1 mL/kg per hour for 6 hours after the procedure. Control: 154 mEq/L of either sodium chloride as a bolus of 3 mL/kg per hour for 1 hour before iopamidol contrast, followed by an infusion of 1 mL/kg per hour for 6 hours after the procedure. |
2 days |
CIN, dialysis, adverse events |
Non-commercial source of funding
In the results, strange mean increase in GFR only in bicarbonate group 8.5±22% and large changes in GFR ranging from -40% to +50% |
Unclear** |
|
Ozcan, 2007 |
N at baseline Intervention: 88 Control: 88
Non-emergency CAG (71%) or PCI (29%). Diuretics discontinued on day of procedure.
Median creatinine clearance 50 (range 21-105) ml/min
Age median (minimum – maximum) Intervention: 68 (43-86) Control: 70 (40-84)
Sex (male) Intervention: 64 (73%) Control: 66 (75%)
Creatinine clearance (mL/min) I: 53 (21 – 81) C: 50 (22-101) |
Intervention: 1.4% sodium bicarbonate Iv fluid (1 mL/kg/h, upper limit 100 mL/h) for 6 hours before and 6 hours after the procedure Control: 0.9% sodium chloride Iv fluid (1 mL/kg/h, upper limit 100 mL/h) for 6 hours before and 6 hours after the procedure
|
48 hours |
CIN defined by creatinine increase >25% or >44 umol/l, dialysis, adverse events
|
Source of funding not reported
None of patients in both groups developed heart failure |
Unclear** |
|
Ratcliffe, 2009 |
N at baseline Intervention: 19 Control: 15
Age (mean, SD) Intervention: 67 years (SD=11) Control: 64 years (SD=10)
Sex (male) Intervention: 11 (58%) Control: 9 (60%) |
Intervention: Iv 0.9% NaHCO3 hydration at an infusion rate of 3 mL/kg/h for 1 h before contrast, and continued at 1 mL/kg/h during the procedure and for 6 h following contrast exposure Control: Iv 0.9% sodium chloride hydration at an infusion rate of 3 mL/kg/h for 1 h before contrast, and continued at 1 mL/kg/h during the procedure and for 6 h following contrast exposure
|
72 hours |
CIN, dialysis, adverse events |
Source of funding not reported |
Some concerns** |
|
Recio-Mayoral, 2007 |
N at baseline Intervention: 56 Control: 55
Age (mean, SD) Intervention: 65 years (SD=10) Control: 64 years (SD=9)
Sex (male) Intervention: 38 (68%) Control: 39 (71%)
Glomerular filtration rate (mL/min) I: 75 ± 21 C: 74 ± 20
|
Intervention: Active prophylactic treatment of PCI: Intravenous bolus of 5 ml/kg/h of alkaline sodium chloride solution with 154 mEq/l of sodium bicarbonate in 5% glucose and H2O (adding 77 ml of 1,000 mEq/l sodium bicarbonate to 433 ml of 5% glucose in H2O) plus 2,400 mg of N-AC in the same solution over 1 hour the bolus was administered in the 60 min preceding contrast injection. Afterward, patients received fluid therapy, without N-AC, at 1.5 ml/kg/h perfusion rate in the 12 h after the procedure plus 2 doses of 600 mg N-AC orally the next day. Control: Standard treatment: perfusion of isotonic sodium chloride (0.9%) at rate of 1 ml/kg/h for 12 h after PCI plus 2 doses of 600 mg N-AC orally the next day |
3 days |
CIN, dialysis, adverse events |
Source of funding not reported |
Some concerns** |
|
Solomon, 2015
|
N at baseline Intervention: 195 Control: 196
Age (mean, SD) Intervention: 72 years (SD=10)) Control: 72 years (SD=9)
Sex (male) Intervention: 111 (57%) Control: 114 (58%) |
Intervention: 1.3% sodium bicarbonate (154 mEq/L). 5 ml/kg of sodium bicarbonate over 60 minutes before angiography and 1.5 ml/kg per h during and for 4 hours after angiography Control: 0.9% sodium chloride (154 mEq/L) |
6 months |
Incidence CI-AKI, dialysis |
Source of funding not reported |
Unclear** |
|
||||||
|
Briguori, 2007 |
N at baseline Intervention: 111 Control: 108
Age (mean, SD) Intervention: 70 years (SD=9) Control: 71 years (SD=9)
Sex (male) Intervention: 98 (88%) Control: 87 (81%)
|
Intervention: 154 mEq/L sodium bicarbonate in dextrose and H2O. The initial intravenous bolus was 3 mL/kg/h for 1 hour immediately before contrast injection. After this, patients received the same fluid at a rate of 1 mL/kg/h during contrast exposure and for 6 hours after the procedure. NAC orally at a dose of 1200 mg twice daily on the day before and the day of administration of the contrast agent (total of 2 days). Control: Isotonic sodium chloride (0.90%) was given intravenously at a rate of 1 mL/kg body weight per hour (0.5 mL/kg for patients with left ventricular ejection fraction _40%) for 12 hours before and 12 hours after administration of the contrast agent.NAC orally at a dose of 1200 mg twice daily on the day before and the day of administration of the contrast agent (total of 2 days). |
48 hours for laboratory parameters 5 days for clinical events
|
CIN, dialysis |
Source of funding not reported |
Low** |
|
Castini, 2008 |
N at baseline Intervention: 52 Control: 51
Age (mean, SD) Intervention: 70 years (SD=8) Control: 73 years (SD=8)
Sex (male) Intervention: 44 (85%) Control: 43 (84%)
|
Intervention: 154 mL of 1000 mEq/L SB added to 846 mL of 5% dextrose in H2O. The initial intravenous bolus was 3 mL/kg for 1 hour immediately before contrast injection. Thereafter, patients received the same fluid at a rate of 1 mL/kg per hour during contrast exposure and for 6 hours after the procedure. Control: sodium chloride (0.9%) given intravenously at a rate of 1 mL/kg body weight per hour for 12 hours before and 12 hours after administration of the contrast agent. |
5 days |
CIN, dialysis |
Source of funding not reported |
Unclear** |
|
Hafiz, 2012 |
N at baseline Intervention: 159 Control: 161
Age (median, IQR) Intervention: 74 (65 to 80) years Control: 73 (63 to 80) years
Sex (male) Intervention: 89 (56%) Control: 92 (57%)
eGFR C: 41 (33-50) |
Intervention: dextrose 5% in water containing 154 mEq/L of NaHCO3 with or without NAC. NAC was used in 50% of patients in both study arms in a similarly randomized fashion as above; 1,200 mg was administered orally 2–12 hr before the procedure followed by another 1,200 mg oral dose 6–12 hr after the procedure. Control: intravenous 0.9% normal sodium chloride with or without NAC. NAC was used in 50% of patients in both study arms in a similarly randomized fashion as above; 1,200 mg was administered orally 2–12 hr before the procedure followed by another 1,200 mg oral dose 6–12 hr after the procedure. |
48 hours |
CI-AKI, dialysis, adverse events |
Source of funding not reported |
Low** |
|
Klima, 2012 |
N at baseline Intervention: 87 Control: 89
Age (median, IQR) Intervention: 78 (70 to 82) years Control: 75 (70 to 82) years
Sex (male) Intervention: 57 (66%) Control: 55 (62%)
eGFR ± SD I: 43 ± 11 C: 43 ± 12
|
Intervention: The initial intravenous bolus was 3 mL/kg/h of 166 mEq/L sodium bicarbonate for 1 h immediately before radiocontrast injection. Following this, patients received the same fluid at a rate of 1 mL/kg/h during the contrast exposure and for 6 h after the procedure. Control: The infusion of 0.9% sodium chloride was administered at a continuous rate of 1 mL/kg/h, beginning from 8 p.m. on the day before the procedure and for at least 12h after the procedure.
|
48 hours |
CIN, dialysis, adverse events |
Commercial and non-commercial funding |
Low** |
|
Lee, 2011 |
N at baseline Intervention: 193 Control: 189
Age (median, IQR) Intervention: 69 (63 to 73) years Control: 68 (67 to 72) years
Sex (male) Intervention: 135 (70%) Control: 134 (71%)
eGFR: C: 46 (37-53) |
Intervention: Sodium bicarbonate infusion (154 mEq/L in dextrose and water) was begun 1 hour before the start of contrast injection, starting at 3 ml/kg/hour and decreasing to 1 ml/ kg/hour during the procedure and for 6 hours after completion of the procedure. All patients received NAC 1,200 mg 2 times/day for 2 days starting the day before the index procedure. Control: 0.9% sodium chloride 1 ml/kg/hour for 12 hours before and after the procedure. All patients received NAC 1,200 mg 2 times/day for 2 days starting the day before the index procedure.
|
48 hours for laboratory parameters 6 months for clinical parameters
|
CIN, dialysis, adverse events |
Source of funding not reported |
Low** |
|
Maioli, 2008 |
N at baseline Intervention: 250 Control: 250
Age (median, IQR) Intervention: 74 (67 to 79) years Control: 74 (70 to 79) years
Sex (male) Intervention: 142 (57%) Control: 152 (61%)
eGFR ± SD: I: 43 ± 11 C: 42 ± 10 |
Intervention: Sodium bicarbonate (154 mEq/l in dextrose and water) received 3 ml/kg for 1 h before contrast medium, followed by an infusion of 1 ml/kg/h for 6 h after the procedure. All patients received 600 mg oral NAC twice a day from the day before to the day after the procedure. Control: 1 ml/kg/h 0.9% sodium chloride for 12 h before and after the procedure.
|
5 days |
CIN, dialysis |
Source of funding not reported |
Low** |
|
Nieto-Rios, 2014 |
N at baseline Intervention: 107 Control: 113
Age (mean, SD) Intervention: 61 years (SD=17) Control: 60 years (SD=17)
Sex (male) Intervention: 61 (57%) Control: 65 (58%)
Baseline sCr (mg/dL): I: 1.3 ± 0.3 C: 1.3 ± 0.3 |
Intervention: 3 ml/kg of sodium bicarbonate solution (150 mEq/L) one hour prior to procedure and then drip rate was decreased to 1 ml/ kg/hour until 6 hours post procedure Control: 1 ml/ kg/hour of normal sodium chloride solution, starting 12 hours before and continuing 12 hours after iohexol contrast.
|
5 days |
CIN, adverse events |
Source of funding not reported |
Low** |
|
Shavit, 2009 |
N at baseline Intervention: 51 Control: 36
Age (mean, SD) Intervention: 72 years (SD=10) Control: 71 years (SD=9)
Sex (male) Intervention: 43 (84%) Control: 25 (70%)
eGFR (ml/min/1.73m2) ± SD: Intervention: 43 ± 11 Control: 40 ± 10
|
Intervention: 154 mEq/L sodium bicarbonate in 5% dextrose in water mixed by adding 154 mL of 1,000 mEq/L sodium bicarbonate to 846 mL of 5% dextrose in water. The initial IV bolus was 3 mL/kg for 1 hour before cardiac catheterization. Following this bolus, patients received the same fluid at a rate of 1 mL/kg per hour during the contrast exposure and for 6 hours after the procedure. For patients weighing more than 110 kg, the initial fluid bolus and drip were limited to those doses administered to patients weighing 110 kg. Control: 12-hour infusion of 154 mEq/L (0.9%) sodium chloride at a rate of 1 mL/kg per hour before cardiac catheterization and NAC 600 mg × 2/d orally the day before and the day of the procedure. |
2 days |
CI-AKI, dialysis |
Source of funding not reported |
Unclear** |
|
||||||
|
Chong, 2015
|
N at baseline Intervention: 157 Control: 156
Age (mean, SD) Intervention: 69.0 years (SD=10.1) Control: 67.0 years (SD=10.2)
Sex (male) Intervention: 113 (72%) Control: 121 (77.6%)
|
Intervention: sustained IV infusion of 154 mEq/L sodium chloride (0.9% sodium chloride) at a rate of 1mL/kg/h from12 h before cardiac catheterisation or PCI. The infusion was continued for 6 h after the procedure. 1.2 g oral NAC (2 tablets of 600 mg NAC dissolved in approximately 250 mL of water) twice a day for 3 consecutive days, starting from the day before cardiac catheterisation (to a total of 6 doses). For patients weighing more than 110 kg, the infusion was limited to a 110 kg patient dose. Control: 1.2 g oral NAC and an abbreviated IV infusion of 154 mEq/L sodium bicarbonate at a rate of 3 mL/kg/h for 1 h before cardiac catheterisation or PCI, and 1 mL/kg/h during and until 6 h after the procedure. The patients were not infused with 0.9% sodium chloride. The sodium bicarbonate solution had the same osmolality as the sodium chloride solution, and was infused for 12 h prior to the procedure to avoid over-alkalinisation. |
48 hours |
CIN, dialysis |
Source of funding not reported |
Unclear** |
|
Garcia, 2018 (subgroup analysis of Weisbord 2018) |
N at baseline Intervention: 593 Control: 568
Age (mean, SD) Intervention: 69.5 years (SD=8.4) Control: 69.1 years (SD=8.4)
|
Intervention: 0.9% sodium chloride Control: 1.26% sodium bicarbonate. |
Up to 90 days |
CA-AKI |
Funded by The VACooperative Studies Program and the National Health and Medical Research Council of Australia. Some authors received grants /fees from the pharmacy. |
Low (all outcomes) |
|
Motohiro, 2011 |
N at baseline Intervention: 77 Control: 78
Age (mean, SD) Intervention: 74 years (SD=7) Control: 71 years (SD=9)
Sex (male) Intervention: 49 (64%) Control: 66 (59%) |
Intervention: 0.9% sodium chloride for 12 hours before and after the procedure. Control: 0.9% sodium chloride for 12 hours before and after the procedure. |
1 month |
CIN, dialysis, adverse events |
Source of funding not reported |
Low** |
|
Tamura, 2009 |
N at baseline Intervention: 72 Control: 72
Age (mean, SD) Intervention: 73 years (SD=8) Control: 72 years (SD=10)
Sex (male) Intervention: 60 (83%) Control: 66 (92%) |
Intervention: Standard hydration with sodium chloride plus single-bolus intravenous administration of sodium bicarbonate (20 ml /20 mEq; Meyron 84, Otsuka Pharmaceutical, Inc., Tokyo, Japan) 5 minutes before contrast exposure Control: Standard hydration with sodium chloride alone.
|
3 days |
CIN, dialysis, adverse events |
Source of funding not reported |
Low** |
|
Ueda, 2011 |
N at baseline Intervention: 30 Control: 29
Age (mean, SD) Intervention: 77 years (SD=9) Control: 75 years (SD=10)
Sex (male) Intervention: 24 (79%) Control: 22 (77%)
|
Intervention: Intravenous bolus injection of 154 mEq/L of sodium bicarbonate at a dose of 0.5 ml/kg, as soon as possible after they were admitted, before the administration of the contrast medium. Intravenous infusion of 154 mEq/L sodium bicarbonate at 1 ml/kg/hour during and for 6 hours after the coronary procedure. Control: Intravenous bolus injection of 154 mEq/L of sodium chloride at a dose of 0.5 ml/kg, as soon as possible after they were admitted, before the administration of the contrast medium. Intravenous infusion of 154 mEq/L sodium bicarbonate at 1 ml/kg/hour during and for 6 hours after the coronary procedure. |
2 days |
CIN, dialysis, adverse events |
Source of funding not reported. |
Low** |
|
Weisbord, 2018 |
N at baseline Intervention: 2482 Control: 2511
Age (mean, SD) Intervention: 69.7 years (SD=8.3) Control: 69.9 years (SD=8.1)
Sex (male) Intervention: 2320 (93.5%) Control: 2351 (93.6%) |
Intervention: 0.9% sodium chloride Control: 1.26% sodium bicarbonate. |
Up to 90 days |
CA-AKI, dialysis, adverse events |
Funded by the U.S. Department of Veterans Affairs Office of Research and Development and the National Health and Medical Research Council of Australia. Some authors received grant support/fees from the pharmacy. |
Low (all outcomes) |
|
||||||
|
Kooiman, 2014 |
N at baseline Intervention: 267 Control: 281
Age (mean, SD) Intervention: 72.0 years (SD=10) Control: 73 years (SD=10)
Sex (male) Intervention: 160 (60%) Control: 171 (61%) |
Intervention: 250 mL intravenous 1.4% sodium bicarbonate 1 hour prior to CE-CT without hydration post-CE-CT Control: 2000 mL of intravenous 0.9% sodium chloride, 1000 mL prior to and 1000 mL post-CE-CT |
96 hours |
CI-AKI, dialysis, adverse events |
Non-commercial source of funding |
Low** |
* For further details, see risk of bias table in the appendix
** The risk of bias was based on the risk of bias assessment performed in the guideline of 2017
Results
1. Post-contrast acute kidney injury (PC-AKI) (critical)
Depending on the design, the RCTs comparing sodium to bicarbonate hydration were categorized into several groups:
- Short schedule NaHCO3 (3ml/kg/h 1 hour pre and 1ml/kg/h 6 hours post CM administration) vs. short schedule NaCl in patients with impaired kidney function undergoing CAG and/or PCI. A total of 11 RCTs with 2,192 patients and 237 PC-AKI events were identified (Adolph, 2008; Boccalandro, 2020; Boucek, 2013; Brar, 2008; Gomes, 2012; Manari, 2014; Masuda, 2007; Ozcan, 2007; Ratcliffe, 2009; Recio-Mayoral, 2007; Solomon 2015). No significant difference was found between patients that underwent bicarbonate versus sodium chloride hydration: Risk Ratio (RR): 0.89 (95% CI: 0.57 – 1.39), p=0.63, I2=56%, as shown in Figure 1.
- Short schedule NaHCO3 (3ml/kg/h 1 hour pre and 1ml/kg/h 6 hours post CM administration) vs. long schedule NaCl (1ml/kg/h 12 hours before and after CM administration) in patients with impaired kidney function undergoing CAG and/or PCI. A total of 9 RCTs (Briguori, 2007; Castini, 2010; Hafiz, 2012; Klima, 2012; Koc 2013; Lee, 2011; Maioli, 2008; Nieto Rios, 2014; Shavit, 2009) with 2,994 patients and 272 PC-AKI events were identified that compared bicarbonate hydration to sodium chloride pre- and posthydration (1ml/kg, 12hour pre- and post) for coronary angiography. No significant difference was found between patients that underwent bicarbonate versus sodium chloride hydration: Risk Ratio (RR): 1.23 (95% CI: 0.81 – 1.87), p=0.33, I2=47% as shown in Figure 2.
- All other hydration schedules comparing bicarbonate plus sodium chloride or bicarbonate only to either sodium chloride or bicarbonate only. A total of 6 RCTs (Chong, 2015; Garcia, 2018; Motohiro, 2011; Tamura, 2009; Ueda, 2011; Weisbord, 2018) with 5,661 patients and 503 PC-AKI cases, were considered suitable for this literature summary. The studies were considered too heterogenous in terms of hydration fluid content and hydration schemes in control group and treatment group to be considered for pooling. Chong, 2015 reported that PC-AKI incidences were 10/153 (6.5%) in the group receiving NaCl plus NAC, and 16/151 (10.6%) in the group bicarbonate plus NAC. The difference in PC-AKI incidence between groups was not significant. Motohiro, 2011 reported that 2/78 patients in the bicarbonate plus sodium chloride group versus 10/77 in the standard hydration group (RR: 0.20, 95% CI: 0.04 to 0.87) developed PC-AKI, thus the incidence of PC-AKI was lower in the combination group. Tamura, 2009 also reported lower rates of PC-AKI in the bolus group: 1/72 versus 9/72 (RR: 0,11; 95% CI: 0.01 to 0.85. The results of Ueda, 2011 were similar, although the difference in incidence of PC-AKI was not statistically significant: 2/30 versus 8/29 PC-AKI cases; RR: 0.24 (95% CI: 0.06 to 1.04). Weisbord (2018) reported that 206 of the 2482 patients (8.3%) who received NaCl had CA-AKI as compared to 239 of the 2511 patients (9.5%) who received NaHCO3 (RR=0.87, 95%CI 0.73 to 1.04). In addition, Garcia (2018) reported that 71 of the 593 patients (12.0%) who underwent coronary angiography with PCI and received NaCl had CA-AKI as compared to 64 of the 568 patients (11.3%) who received NaHCO3 (RR=1.06, 95%CI 0.77 to 1.46).
- Kooiman, 2014 reported a PC-AKI incidence of 4.1% in CT patients receiving 250ml NaHCO3 (ultrashort schedule) precontrast versus 5.1% (p=0.23) receiving pre- and post-CM hydration with NaCl 0,9%..
Figure 1. Pooled analysis of PC-AKI risk in patients receiving short schedules of hydration with either bicarbonate or sodium chloride for CAG/PCI
Figure 2. Pooled analysis of PC-AKI risk in patients receiving short schedules for bicarbonate versus long schedule for sodium chloride for CAG/PCI
2. Start renal replacement therapy (RRT) (critical)
Depending on the design, the RCTs comparing sodium to bicarbonate hydration were categorized into several groups:
- Short schedule NaHCO3 (3ml/kg/h 1 hour pre and 1ml/kg/h 6 hours post CM administration) vs. short schedule NaCl in patients with impaired kidney function undergoing CAG and/or PCI. A total of nine RCT’s (Adolph, 2008; Boccalandro, 2020; Boucek, 2013; Brar, 2008; Merten, 2004; Ozcan, 2007; Ratcliffe, 2009; Recio-Mayoral, 2007; Solomon, 2015) reported the requirement of dialysis.
- Short schedule NaHCO3 (3ml/kg/h 1 hour pre and 1ml/kg/h 6 hours post CM administration) vs. long schedule NaCl (1ml/kg/h 12 hours before and after CM administration) in patients with impaired kidney function undergoing CAG and/or PCI. A total of seven studies (Briguori, 2007; Castini, 2010; Hafiz, 2012; Klima, 2011; Lee, 2011; Maioli, 2008; Nieto-Rios, 2014; Shavit, 2009) reported the requirement of dialysis.
- All other hydration schedules comparing bicarbonate plus sodium chloride or bicarbonate only to either sodium chloride or bicarbonate only. A total of five studies (Chong, 2015; Motohiro, 2011; Tamuro, 2009; Ueda, 2011; Weisbord, 2018) reported the requirement of dialysis.
- The requirement for dialysis in patients receiving ultrashort schedule bicarbonate versus pre- and post-CM hydration with NaCl was reported by the study of Kooiman 2014.
In table 5, the dialysis requirement is shown for all the sodium chloride versus sodium bicarbonate hydration comparisons. Because of the diversity of the included studies and the low number of events, these studies were not pooled. The studies found in the update of 2025 had a greater sample size and more events occurred and were described in more detail below.
Boccalandro (2020) reported that 6 of the 190 patients (3.2%) who received NaCl had procedure related renal replacement therapy as compared to 8 of the 192 patients (4.2%) who received NaHCO3 (RR=0.76, 95%CI 0.27 to 2.14).
Weisbord (2018) reported that 29 of the 2482 patients (1.2%) who received NaCl needed dialysis by 90 days as compared to 32 of the 2511 patients (1.3%) who received NaHCO3 (RR=0.92, 95% CI 0.56 to 1.51).
1-year RRT
Boccalandro (2020) reported that after 1 year, 1 of the 190 patients (0.5%) who received NaCl required permanent renal replacement therapy as compared to 4 of the 192 patients (2.1%) who received NaHCO3 (RR=0.25, 95%CI 0.03 to 2.24).
5-year RRT
Boccalandro (2020) reported that after 5-years, 22 of the 190 patients (11.6%) who received NaCl required renal replacement therapy as compared to 19 of the 192 patients (9.9%) who received NaHCO3 (RR=1.17, 95%CI 0.66 to 2.09).
3. Acute renal failure (important)
Not reported.
4. Irreversible loss of kidney function (important)
Weisbord (2018) reported that 28 of the 2511 patients (1.1%) who received NaCl had persistent kidney impairment by 90 days as compared to 25 of the 2482 patients (1.0%) who received NaHCO3 (RR=1.11, 95%CI 0.65 to 1.89).
5. Adverse events (important)
Depending on the design, the RCTs comparing sodium to bicarbonate hydration were categorized into several groups:
- Short schedule NaHCO3 (3ml/kg/h 1 hour pre and 1ml/kg/h 6 hours post CM administration) vs. short schedule NaCl in patients with impaired kidney function undergoing CAG and/or PCI. A total of five studies (Boccalandro, 2020; Merten, 2004; Ozcan, 2007; Ratcliffe, 2009; Recio-Mayoral, 2007) reported the adverse event heart failure.
- Short schedule NaHCO3 (3ml/kg/h 1 hour pre and 1ml/kg/h 6 hours post CM administration) vs. long schedule NaCl (1ml/kg/h 12 hours before and after CM administration) in patients with impaired kidney function undergoing CAG and/or PCI. A total of four studies (Hafiz, 2012; Klima, 2011; Lee, 2011; Nieto-Rios, 2014) reported the adverse event heart failure.
- All other hydration schedules comparing bicarbonate plus sodium chloride or bicarbonate only to either sodium chloride or bicarbonate only. Four studies (Motohiro, 2011; Tamuro, 2009; Ueda, 2011; Weisbord, 2018) reported the adverse event heart failure.
- Kooiman 2014 reported the adverse event heart failure in patients receiving ultrashort schedule bicarbonate versus pre- and post-CM hydration with NaCl.
In table 5, the adverse events (heart failure) are shown for all the sodium chloride versus sodium bicarbonate hydration comparisons. Because of the diversity of the included studies and the low number of events, these studies were not pooled. The studies found in the update of 2025 had a greater sample size and more events occurred and were described in more detail below.
Boccalandro (2020) reported that 26 of the 190 patients (13.7%) who received NaCl had heart failure as compared to 24 of the 192 patients (12.5%) who received NaHCO3 (RR=1.09, 95%CI 0.65 to 1.84).
Weisbord (2018) reported that 166 of the 2482 patients (6.7%) who received NaCl had heart failure as compared to 201 of the 2511 patients (8.0%) who received NaHCO3 (RR=0.84, 95%CI 0.69 to 1.02). In the study, on average 344 (274-445) ml of fluids were given before contrast, 114 (74-170) ml during contrast and 570 (471-669) ml after contrast, which is substantially less than in the original long schedule of 1ml/kg/h for 12h pre- and for 12h post contrast administration. Of note, only patients with decompensated heart failure requiring intensive therapy such as dobutamine or isolated ultrafiltration were excluded from this trial. Decisions on discontinuation of RAS blockers and diuretics were left to the treating physicians. There were no other relevant differences in adverse events between the two groups.
Table 5. Dialysis requirement and adverse events in bicarbonate versus sodium chloride infusion
|
Author and date |
Dialysis |
Heart failure or edema |
||
|
|
Bicarbonate |
Sodium chloride |
Bicarbonate |
Sodium chloride |
|
Patients receiving short schedules of hydration with either bicarbonate or sodium chloride for CAG/PCI |
||||
|
Adolph, 2008 |
0/71 |
0/74 |
NR |
NR |
|
Boccalandro, 2020 |
8/192 |
6/190 |
24/192 |
26/190 |
|
Boucek, 2013 |
1/51 |
2/49 |
NR |
NR |
|
Brar, 2008 |
2/175 |
4/178 |
NR |
NR |
|
Gomes, 2012 |
NR |
NR |
NR |
NR |
|
Masuda, 2004 |
NR |
NR |
NR |
NR |
|
Merten, 2004 |
1/30 |
3/29 |
11/30 |
11/29 |
|
Ozcan, 2007 |
1/88 |
1/88 |
0/88 |
0/88 |
|
Ratcliffe, 2009 |
0/42 |
0/36 |
0/42 |
0/36 |
|
Recio-Mayoral, 2007 |
1/56 |
3/55 |
1/56 |
2/55 |
|
Solomon, 2015 |
8/195 |
6/196 |
NR |
NR |
|
Total |
22/900 |
25/895 |
36/408 |
39/398 |
|
Patients receiving short schedules for bicarbonate versus long schedule for sodium chloride for CAG/PCI |
||||
|
Briguori, 2007 |
1/108 |
1/111 |
NR |
NR |
|
Castini, 2010 |
0/52 |
0/51 |
NR |
NR |
|
Hafiz, 2012 |
0/159 |
0/151 |
0/159 |
0/151 |
|
Klima, 2011 |
0/169 |
0/89 |
0/169 |
0/89 |
|
Koc, 2013 |
NR |
NR |
NR |
NR |
|
Lee, 2011 |
10/193 |
3/189 |
0/193 |
0/189 |
|
Maioli, 2008 |
1/250 |
1/252 |
NR |
NR |
|
Nieto-Rios, 2014 |
NR |
NR |
8/103 |
7/113 |
|
Shavit, 2009 |
0/51 |
0/36 |
NR |
NR |
|
Total |
12/982 |
5/879 |
8/624 |
7/542 |
|
Patients receiving bicarbonate or sodium chloride hydration in “other” hydration schemes for coronary angiography |
||||
|
Chong, 2015 |
0/157 |
1/153 |
NR |
NR |
|
Motohiro, 2011 |
0/78 |
0/77 |
0/78 |
0/77 |
|
Tamuro, 2009 |
0/72 |
1/72 |
0/72 |
0/72 |
|
Ueda, 2011 |
0/30 |
0/29 |
0/30 |
0/29 |
|
Weisbord, 2018 |
32/2511 |
29/2482 |
201/2511 |
166/2482 |
|
Total |
32/2848 |
31/2813 |
201/2691 |
166/2660 |
|
Patients receiving ultrashort schedule bicarbonate versus pre- and post-CM hydration with NaCl |
||||
|
Kooiman, 2014 |
0/264 |
0/274 |
0/267 |
6/281 |
NR: not reported
Q3: Prehydration versus pre- and posthydration
Description of studies
A total of three studies were included in the analysis of the literature. One RCT was found in the search of 2017 and two studies were added in 2025. Important study characteristics and results are summarized in table 7. The assessment of the risk of bias is summarized in the risk of bias tables (under the tab ‘Evidence tabellen’).
Kooiman (2014) performed a randomized controlled, non-inferiority trial to compare a 1-hour schedule of 250ml NaHCO3 1.4% versus 1000 ml NaCl 0.9% in 4-12h pre- and 4-12h post-CM administration in 548 CT-patients.
Kooiman (2018) performed a multicenter randomized non-inferiority trial to determine whether pre-hydration with 1-hour sodium bicarbonate is non-inferior to pre- and post-hydration with sodium chloride in CKD patients undergoing elective cardiovascular diagnostic or other interventional contrast procedures requiring intra-arterial contrast administration. Patients 18 years or older with an eGFR <45 or an eGFR 45-60 ml/min in combination with diabetes mellitus or at least two other risk factors for the development of PC-AKI (i.e., peripheral arterial disease, congestive heart failure, age > 75 years, anemia, use of diuretics or non-steroidal anti-inflammatory drugs) were included. In total, 168 patients received 1-hour pre-procedural intravenous hydration with 250 ml 1.4% sodium bicarbonate and 165 patients received periprocedural intravenous hydration with 0.9% sodium chloride, 1000 ml in 4-12 hours prior to and 1000 ml in 4-12 hours following contrast administration (total volume 2000 ml). Groups were comparable at baseline except for an imbalance in type of contrast procedure. In the bicarbonate group 19.6% received contrast for CAG and 3% for PCI (18.2 and 1.8%, respectively in the sodium chloride group). Outcomes of interest were incidence of CI-AKI and need for dialysis.
Van Mourik (2018) performed an open-label single-center, unblinded, randomised non-inferiority trial to determine whether a short hydration protocol of 1 h sodium bicarbonate is non-inferior to conventional hydration with 24 h sodium chloride in consecutive patients with symptomatic aortic valve stenosis and impaired renal function who underwent pre-transcatheter aortic valve implantation (TAVI) computed tomography angiography (CTA). Patients with CKD classification 3a or above (eGFR <60ml/min/1.73m2 based on the modification of diet in renal disease formula), age >18 years and planned for CTA prior to TAVI were included. 80% of the patients in the short hydration group were using diuretics and 63% in the conventional group and a small percentage was using other nephrotoxic drugs (5% and 11%, respectively). In the short hydration group in 91% of the patients the nephrotoxic medication was interrupted on the day of the CTA and in 75% in the conventional group. In total, 39 patients received 1h sodium bicarbonate prior to CTA (1.4% 3ml/kg/h) and 35 patients received 24h sodium chloride (0.9% 1ml/kg/h) 8h prior to CTA and 16h post-CTA. Groups were comparable at baseline. The outcome of interest was the occurrence of PC-AKI.
Table 7. Characteristics of included studies
|
Study |
Participants |
Comparison |
Follow-up |
Outcome measures |
Comments |
Risk of bias (per outcome measure)* |
|
Individual studies |
||||||
|
Kooiman, 2014 |
N at baseline Intervention: 267 Control: 281
Age (mean, SD) Intervention: 72.0 years (SD=10) Control: 73 years (SD=10)
Sex (male) Intervention: 160 (60%) Control: 171 (61%)
|
Intervention: 250 mL intravenous 1.4% sodium bicarbonate 1 hour prior to CE-CT without hydration post-CE-CT Control: 2000 mL of intravenous 0.9% sodium chloride, 1000 mL prior to and 1000 mL post-CE-CT |
96 hours |
CI-AKI, dialysis, adverse events |
Non-commercial source of funding |
Low (all outcomes) |
|
Kooiman, 2018 |
N at baseline Intervention: 168 Control: 165
Age (mean, SD) Intervention: 73.0 years (SD=9.2) Control: 72.5 years (SD=8.8)
Sex (male) Intervention: 105 (62.5%) Control: 110 (66.7%)
|
Intervention: 1-hour pre-procedural intravenous hydration using 250 ml 1.4% sodium bicarbonate Control: periprocedural intravenous hydration with 0.9% sodium chloride, 1000 ml in 4±12 hours prior to and 1000 ml in 4±12 hours following contrast administration (total volume 2000 ml) |
Up to 2 months |
Incidence of PC-AKI, need for dialysis |
The study was funded by the Bronovo Hospital Research Foundation, a charity foundation. The sponsor did not have any influence on the design of the trial, data collection, analyses, interpretation or the writing of this manuscript. The authors have declared that no competing interests exist. |
Low (all outcomes) |
|
Van Mourik, 2021 |
N at baseline Intervention: 39 Control: 35
Age (median, IQR) Intervention: 81.2 (77.7–84.9) years Control: 83 (80.7–86.4) years
Sex (male) Intervention: 20 (51%) Control: 13 (37%)
|
Intervention: 1h sodium bicarbonate prior to CTA, 1.4% 3ml/kg/h Control: 24h sodium chloride, 0.9% 1ml/kg/h, 8h prior to CTA, 16h post-CTA |
Up to 5 days |
Occurrence of PC-AKI, adverse events |
Source of funding not reported. The authors declared that no competing interests exist. |
Low (all outcomes) |
* For further details, see risk of bias table in the appendix
Results
1. Post-contrast acute kidney injury (PC-AKI) (critical)
Three studies reported the incidence of PC-AKI (Kooiman, 2014; Kooiman, 2018; Van Mourik, 2018) (figure 1). In total, 19 of the 466 patients (4.1%) who received prehydration with NaHCO3 had PC-AKI as compared to 26 of the 469 patients (5.5%) who received pre- and posthydration (RR=0.74, 95%CI 0.42 to 1.32). This difference is clinically relevant favoring prehydration.
Figure 1. Incidence of PC-AKI for comparison between prehydration and pre- and posthydration
2. Start renal replacement therapy (RRT) (critical)
Three studies reported the start of renal replacement therapy for the comparison between prehydration and pre- and posthydration (Kooiman, 2014; Kooiman, 2018; Van Mourik, 2018). Since no events occurred, the results were not pooled.
Kooiman (2014) reported that no patients who either received prehydration with NaHCO3 (n=264) or pre- and posthydration with NaCl (n=274) developed a need for dialysis.
Kooiman (2018) reported that no patient who either received prehydration with NaHCO3 (n=163) or pre- and posthydration with NaCl (n=160) required dialysis.
Van Mourik (2018) reported that none of the patients who either received prehydration with NaHCO3 (n=39) or pre- and posthydration with NaCl (n=35)
needed dialysis.
3. Acute renal failure (important)
Not reported.
4. Irreversible loss of kidney function (important)
Not reported.
5. Adverse events
Kooiman (2014) reported that acute heart failure due to volume expansion (based on the treating physician’s clinical judgement) occurred in none of the patients who received prehydration with NaHCO3 (n=267) versus 6 of the 281 patients (2.1%) who received pre- and posthydration with NaCl (RR=0.08, 95%CI 0.00 to 1.43).
Van Mourik (2018) reported that only 1 of the 35 patients (2.9%) who received pre- and posthydration with NaCl developed acute heart failure, while this did not occur in patients who received prehydration with NaHCO3 (n=39) (RR=0.30, 95%CI 0.01 to 7.13).
Question 4 and 5, not part of the update in 2024.
Q4 Oral versus intravenous hydration
Description of studies
One RCT (Cho, 2010) was considered suitable for inclusion in the literature summary. Cho, 2010 the RCT using both pre- and post-hydration consisted of 91 patients with sCr >97,2 µmol/l or eGFR <60 ml/min/1.73m2 undergoing elective CAG. They were randomly assigned into 4 groups: A, NaCl 154mEq (0.9%)/l 3ml/kg/h 1 hour pre and 1ml/kg/h 6 hours post CM. B. NaHCO3 154mEq/l, same schedule as NaCl. C. 500ml of water, 4-2 hours pre-CM administration, followed by 600ml of water post contrast administration. D, C + 3.9g oral NaHCO3 pre-CM and 1.95g oral NaHCO3 post CM.
Results
Cho, 2010 also found no significant difference in the incidence of PC-AKI in all 4 groups: A 22.2%, B 9.5%, C 4.5% and D 4.8% (p>0.05).
Quality of evidence
For the comparison of oral versus intravenous hydration in all patients the level of evidence was graded as low due to imprecision and heterogeneity of included studies.
Q5 Hydration with controlled diuresis
Description of studies
Five Italian studies, all RCTs, describe the same technique, consisting of an extracorporeal circuit for continuous fluid infusion, combined with a Foley catheter for measuring urinary production (Barbanti, 2015; Briguori, 2011; Marenzi, 2012; Usmiani, 2016; Visconti, 2016) in respectively 112, 292, 170, 123, and 48 patients. This system is capable of delivering sterile replacement solution in an amount matched to the volume of urine produced, thereby avoiding hypovolemia and fluid overload. It displays urine and replacement volume and alerts to replace the fluid bag or drain the urine bag. After an initial bolus of 250ml NaCl 0.9% infused over 30 minutes, patients receive furosemide, 0.25mg/kg, to achieve a urinary flow of at least 300ml/h. Once this is achieved, the procedure is performed. The system keeps urinary flow >300ml/h for the next 4 hours, balancing between more NaCl and low dose furosemide.
Two of these three papers describe patients undergoing CAG and/or PCI (Marenzi, 2012; Usmiani, 2016), two papers describe patients undergoing Transcatheter Aortic Valve Implantation (TAVI) (Barbanti, 2015; Visconti, 2016) and one describes a mixed group of CAG and peripheral angiography (Briguori, 2011). All patients had eGFR <60 ml/min/1.73m2, in one paper <30 ml/min/1.73m2 (Briguori, 2011). The control group of each study had a different hydration schedule (sodium chloride versus bicarbonate versus a combination of both). Therefore, pooling of the studies was not possible due to heterogeneity.
Regarding the control group, Briguori, 2011 used 154 mEq/L of sodium bicarbonate in dextrose and water, mixed in the hospital pharmacy by adding 154mL of 1000 mEq/L sodium bicarbonate (i.e. sodium bicarbonate 8.4%) to 846 mL of 5% dextrose in water (D5W), slightly diluting the dextrose concentration to 4.23%. The initial intravenous bolus was 3 mL/kg per hour for at least 1 hour before contrast injection. Then, all patients received the same fluid at a rate of 1 mL/kg per hour during contrast exposure and for 6 hours after the procedure. All patients enrolled in this group received NAC orally at a dose of 1200 mg twice daily the day before and the day of administration of the contrast agent (for a total of 2 days). In this group, an additional NAC dose (1200 mg diluted in 100 mL sodium chloride) was administered intravenously during the procedure. The total NAC dose was 6g.
The control group of Marenzi, 2012 received a continuous intravenous infusion of isotonic sodium chloride at a rate of 1 ml/kg/h (0.5 ml/kg/h in case of left ventricular ejection fraction <40%) for at least 12 h before and 12 h after the procedure. The control group of Usmiani, 2016 received 1000 mL isotonic sodium chloride i.v. administration 12 h before procedure (rate-adjusted according to LVEF: 20– 40mL/h if LVEF<30%, 80–120 mL/h if LVEF 30–50%, 200 mL/h if LVEF >50%), plus 3 mL/kg/h sodium bicarbonate 1.4% solution by i.v. infusion for 1 h before procedure, plus 5000mg of Vitamin C and 1200mg NAC administered orally. After the procedure the patients received 1mL/kg/h sodium bicarbonate 1.4% solution IV for 6 hours, plus 5000mg of vitamin C and 1200mg NAC administered orally on the following day.
Barbanti, 2015 included 112 patients undergoing Transcatheter Aortic Valve Implantation (TAVI) who were randomly assigned to either the controlled diuresis group (n=56) or the control group (intravenous sodium chloride solution at a rate of 1 ml/kg/h 12 h before TAVI, during contrast exposure, and for 6 h after the procedure).
Viconti, 2016 describes also a group of patients undergoing TAVI (n=48) with either controlled diuresis or bicarbonate schedule (same schedule as Briguori, 2011). In total, 48 patients were assigned (non-randomly) to the RenalGuard therapy group (n=22) or the control group (n=26). Because the above-mentioned studies used different hydration schemes and methods, the studies could not be pooled.
Brar, 2014 described a slightly different approach: during CAG, a left ventricular catheter was placed in order to measure left ventricular end-diastolic pressure. This was done in 178 patients with eGFR <60 ml/min/1.73m2 and one or more additional risk factors, such as diabetes, congestive heart failure, hypertension and age >75 years. The control group consisted of 172 patients with the same characteristics, undergoing the same procedure. Both groups received a bolus infusion, NaCl 0.9%, 3ml/kg/h, 1 hour pre-CAG. The control group received the same fluid at the same rate for 4 hours post CAG. The rate of post contrast fluid in the research group was dependent on the left ventricular end-diastolic pressure: <13mmHg 5ml/kg/h, 13 to 18mmHg 3ml/kg/h and >18mmHg 1.5ml/kg/h.
Another approach, described by Qian, 2016, is invasively measuring central venous pressure (CVP) and CVP-guided fluid administration in 264 patients. CVP <6mmHg 3ml/kg/h, CVP 6-12mmHg 1.5ml/kg/h, CVP>12mmHg 1ml/kg/h NaCl 0.9% 6 hours pre and 12 hours post CM administration. The control group received NaCl 1ml/kg/h 6 hours pre and 12 hours post CM administration. All patients were scheduled for CAG and/or PCI, had an eGFR 15-60 ml/min/1.73m2 and LVEF <50% (Qian, 2016).
Results
Briguori, 2011, Marenzi, 2012 and Usmiani, 2015 all reported a significantly lower incidence of PC-AKI in patients who received controlled diuresis. Briguori, 2011 found an incidence of PC-AKI of 11% in the forced diuresis group versus 20.5% in the control group (p=0.025) in patients with an eGFR <30mL/min/1.73m2. After 1 month, mortality was similar in the intervention (6/146) and control (6/146) group, p=0.99; need for dialysis arose in 7/146 patients in the control group versus 1/146 in the intervention group, p=0.03.
Marenzi, 2012 found an incidence of PC-AKI of 4,6% in the forced diuresis group versus 18% in the control group (p=0.005). In-hospital mortality was similar in the intervention (1/87) and control (2/82) group, p=0.53. Need for dialysis arose in 1/87 patients in the intervention group versus 3/83 in the control group, p=0.29.
Usmiani, 2016 found an incidence of PC-AKI of 7% in the forced diuresis group versus 25% in the control group (p=0.01). One-year mortality was not significantly different in the intervention (4/59) and control (8/65) group, p=0.46. Need for dialysis arose in 0/59 patients in the intervention group versus 2/65 in the control group, (p-value not reported).
Barbanti reported that the incidence of CI-AKI was lower in the controlled diuresis group compared to the control group (intravenous), controlled diuresis: 4/56 (5.4%) vs control: 13/56 (13.3%) (p=0.014).
Visconti, 2016 reported that PC-AKI occurred in 10/26 (38.5%) patients in the control group and in 1/22 (4.5%) patients in the RenalGuard group (p=0.005, odds ratio [OR] 0.076, 95% confidence interval [CI]: 0.009-0.66).
Brar, 2014 described that PC-AKI occurred in 16.3% of the patients in the control group vs. 6.7% in the research group (p=0.005). After 6 months, mortality was lower in the intervention (1/196) compared to the control (8/200) group, p=0.037. Need for dialysis arose in 1/196 patients in the intervention group versus 4/200 in the control group, p=0.37.
Qian, 2016 reported that PC-AKI occurred in 15.9% in the CVP group vs. 29.5% in the standard hydration group (p=0.006). Need for dialysis arose in 4/134 patients in the intervention group versus 13/135 in the control group, p=0.019. Acute pulmonary edema occurred in 5/134 patients in the intervention group versus 4/135 in the control group, p=0.50. Mortality rates were not reported.
Quality of evidence
For the comparison of controlled diuresis versus IV hydration in all patients the level of evidence was graded as low due to imprecision and heterogeneity of included studies.
Zoeken en selecteren
An updated systematic review of the literature was performed to answer the following question(s):
Which hydration strategy has to be used for patients that receive intravascular iodine containing contrast media and have a high risk of PC-AKI?
Table 1. PICO
| Patients | Patients with impaired renal function (chronic kidney disease, chronic renal failure, CKD stage 3 or higher, eGFR <60 ml/min/1.73m2) undergoing radiological or cardiological examinations with iodine-containing contrast media |
|
Intervention - 1 Intervention - 2 Intervention – 3 Intervention – 4* Intervention – 5* |
Hydration Hydration with NaCl Pre-hydration Oral hydration Hydration with controlled diuresis |
|
Control - 1 Control - 2 Control – 3 Control – 4* Control – 5* |
No hydration Hydration with NaHCO3 Pre- and posthydration Intravenous hydration Standard hydration |
| Outcomes | Post-contrast acute kidney injury (PC-AKI), start renal replacement therapy, acute renal failure, irreversible loss in kidney function, adverse events |
| Other selection criteria | Study design: systematic reviews, randomized controlled trials or observational studies |
* In 2024 an updated literature search was performed for question 1-3 but not for question 4 and 5 (search performed in June 2015). Therefore the search and select and summary of literature sections distinguishes question 4 and 5 from 1-3
Relevant outcome measures
The guideline panel considered Post-Contrast AKI (PC-AKI), end stage renal failure and start of renal replacement therapy as a critical outcome measures for decision making; and acute renal failure, irreversible loss of kidney function and adverse events (especially heart failure related to hydration procedure) as important long-term outcome measures.
The guideline defined PC-AKI as described in the chapter 2.1 PC-AKI: Definities, terminologie en klinisch verloop in the Dutch guideline “Veilig gebruik van contrastmiddelen (NVvR, 2017)”.
A priori, the guideline panel did not define the other outcome measures listed above but used the definitions used in the studies.
The guideline panel defined the following as a minimal clinically (patient) important difference:
- Post-contrast acute kidney injury (PC-AKI): relative risk <0.91 or >1.10.
- Complications of PC-AKI (start renal replacement therapy, acute renal failure, irreversible loss in kidney function, adverse events): relative risk <0.91 or >1.10.
A difference of at least 10% in relative risk was defined as a minimal clinically (patient) important difference; by expert opinion of the working group (no literature was available to substantiate the decision). To illustrate, if PC-AKI occurs with an incidence of 10% in the patient population, a difference of 10% of relative risk would mean a difference of 1% in absolute risk. Thus, the number needed to treat would be 100, ergo: a doctor would need to treat 100 patients to prevent one case of PC-AKI. When the incidence of PC-AKI is 5%, a difference of 10% in relative risk would mean a difference of 0.5% in absolute risk, and a number needed to treat of 200.
Search and select (Methods)
For question 1-3 a follow-up systematic literature search was performed by a medical information specialist using the following bibliographic databases: Embase.com and Ovid/Medline. Both databases were searched from 2017 to 21 October 2024 for systematic reviews, RCTs and observational studies. Systematic searches were completed using a combination of controlled vocabulary/subject headings (e.g., Emtree-terms, MeSH) wherever they were available and natural language keywords. The overall search strategy was derived from three primary search concepts: (1) impaired renal function; (2) iodine-containing contrast media; (3) hydration. Duplicates were removed using EndNote software. After deduplication a total of 571 records were imported for title/abstract screening. Initially, 85 studies were selected based on title and abstract screening. After reading 35 studies in full text, 27 studies were excluded (see the exclusion table under the tab ‘Evidence tabellen’), and 8 studies were included.
For question 4 and 5: The databases Medline (OVID), Embase and the Cochrane Library were searched from January 2000 to 17th of June 2015 using relevant search terms for systematic reviews (SRs), randomized controlled trials (RCTs) and observational studies (OBS). The literature search procured 858 hits: 183 SRs, 572 RCTs and 103 OBS. An update of the search on April 14th 2017 retrieved an additional 138 studies. Based on title and abstract a total of 47 studies were initially selected, and a total of 17 studies based on the updated search (64 in total). After examination of full text, a total of 19 + 10 (29 in total) studies were excluded and 28 + 7 studies definitely included in the literature summary. Of these one RCT (Cho, 2010) was considered suitable for inclusion in the literature summary for question 4 about Oral versus intravenous hydration. For question 5 about hydration with controlled diuresis, seven studies were included: Barbanti, 2015; Briguori, 2011; Marenzi, 2012; Usmiani, 2016; Visconti, 2016, Brar, 2014 and Qian, 2016.The search and select and literature analysis of 2017 can be found in the appendix.
Referenties
- Boccalandro F, Shreyder K, Harmon L, Dhindsa M, Fahim T, Sheikh S. Five-Year Follow-Up of Patients With Radio-Contrast-Induced Acute Renal Injury: Can Intravenous Sodium Bicarbonate Improve Long-Term Outcomes? Cardiovasc Revasc Med. 2021 Oct;31:61-68. doi: 10.1016/j.carrev.2020.11.017. Epub 2020 Nov 18. PMID: 33250404.
- Barbanti M, Gulino S, Capranzano P, et al. Acute kidney injury with the renalguard system in patients undergoing transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2015 Oct;8(12):1595-604.
- Brar SS, Aharonian V, Mansukhani P, et al. Haemodynamic-guided fluid administration for the prevention of contrast-induced acute kidney injury: the POSEIDON randomised controlled trial. Lancet. 2014;383(9931):1814-23.
- Briguori C, Visconti G, Focaccio A, et al. Renal Insufficiency After Contrast Media Administration Trial II (REMEDIAL II) RenalGuard system in high-risk patients for contrast-induced acute kidney injury. Circulation. 2011;124(11):1260-9.
- Chen SL, Zhang J, Yei F, Zhu Z, Liu Z, Lin S, Chu J, Yan J, Zhang R, Kwan TW. Clinical outcomes of contrast-induced nephropathy in patients undergoing percutaneous coronary intervention: a prospective, multicenter, randomized study to analyze the effect of hydration and acetylcysteine. Int J Cardiol. 2008 Jun 6;126(3):407-13. doi: 10.1016/j.ijcard.2007.05.004. Epub 2007 Jul 24. PMID: 17651830.
- Cho R, Javed N, Traub D, et al. Oral Hydration and Alkalinization is Noninferior to Intravenous Therapy for Prevention of Contrast-Induced Nephropathy in Patients with Chronic Kidney Disease. J Intervent Cardiol. 2010;23(5):460-6.
- Cossette F, Trifan A, Prévost-Marcotte G, Doolub G, So DF, Beaubien-Souligny W, Abou-Saleh D, Tanguay JF, Potter BJ, Ly HQ, Menkovic I, Cieza T, Avram R, Bastiany A, Marquis-Gravel G. Tailored hydration for the prevention of contrast-induced acute kidney injury after coronary angiogram or PCI: A systematic review and meta-analysis. Am Heart J. 2025 Jan 4;282:93-102. doi: 10.1016/j.ahj.2025.01.002. Epub ahead of print. PMID: 39756561.
- Garcia S, Bhatt DL, Gallagher M, Jneid H, Kaufman J, Palevsky PM, Wu H, Weisbord SD; PRESERVE Trial Group. Strategies to Reduce Acute Kidney Injury and Improve Clinical Outcomes Following Percutaneous Coronary Intervention: A Subgroup Analysis of the PRESERVE Trial. JACC Cardiovasc Interv. 2018 Nov 26;11(22):2254-2261. doi: 10.1016/j.jcin.2018.07.044. PMID: 30466822.
- Kooiman J, Sijpkens YW, de Vries JP, et al. A randomized comparison of 1-h sodium bicarbonate hydration versus standard peri-procedural saline hydration in patients with chronic kidney disease undergoing intravenous contrast-enhanced computerized tomography. Nephrol Dial Transplant. 2014;29(5):1029-36.
- Kooiman J, Sijpkens YW, van Buren M, Groeneveld JH, Ramai SR, van der Molen AJ, Aarts NJ, van Rooden CJ, Cannegieter SC, Putter H, Rabelink TJ, Huisman MV. Randomised trial of no hydration vs. sodium bicarbonate hydration in patients with chronic kidney disease undergoing acute computed tomography-pulmonary angiography. J Thromb Haemost. 2014 Oct;12(10):1658-66. doi: 10.1111/jth.12701. Epub 2014 Sep 26. PMID: 25142085.
- Kooiman J, de Vries JPM, Van der Heyden J, Sijpkens YWJ, van Dijkman PRM, Wever JJ, van Overhagen H, Vahl AC, Aarts N, Verberk-Jonkers IJAM, Brulez HFH, Hamming JF, van der Molen AJ, Cannegieter SC, Putter H, van den Hout WB, Kilicsoy I, Rabelink TJ, Huisman MV. Randomized trial of one-hour sodium bicarbonate vs standard periprocedural saline hydration in chronic kidney disease patients undergoing cardiovascular contrast procedures. PLoS One. 2018 Feb 8;13(2):e0189372. doi: 10.1371/journal.pone.0189372. PMID: 29420536; PMCID: PMC5805164.
- Luo Y, Wang X, Ye Z, Lai Y, Yao Y, Li J, Liu X. Remedial hydration reduces the incidence of contrast-induced nephropathy and short-term adverse events in patients with ST-segment elevation myocardial infarction: a single-center, randomized trial. Intern Med. 2014;53(20):2265-72. doi: 10.2169/internalmedicine.53.1853. Epub 2014 Oct 15. PMID: 25318787.
- Maioli M, Toso A, Leoncini M, Micheletti C, Bellandi F. Effects of hydration in contrast-induced acute kidney injury after primary angioplasty: a randomized, controlled trial. Circ Cardiovasc Interv. 2011 Oct 1;4(5):456-62. doi: 10.1161/CIRCINTERVENTIONS.111.961391. Epub 2011 Oct 4. PMID: 21972403.
- Marenzi G, Ferrari C, Marana I, et al. Prevention of contrast nephropathy by furosemide with matched hydration: the MYTHOS (induced diuresis with matched hydration compared to standard hydration for contrast induced nephropathy prevention) trial. JACC Cardiovasc Intervent. 2012;5(1):90-7.
- Nijssen EC, Rennenberg RJ, Nelemans PJ, Essers BA, Janssen MM, Vermeeren MA, Ommen VV, Wildberger JE. Prophylactic hydration to protect renal function from intravascular iodinated contrast material in patients at high risk of contrast-induced nephropathy (AMACING): a prospective, randomised, phase 3, controlled, open-label, non-inferiority trial. Lancet. 2017 Apr 1;389(10076):1312-1322. doi: 10.1016/S0140-6736(17)30057-0. Epub 2017 Feb 21. PMID: 28233565.
- Nijssen EC, Nelemans PJ, Rennenberg RJ, van Ommen V, Wildberger JE. Prophylactic Intravenous Hydration to Protect Renal Function From Intravascular Iodinated Contrast Material (AMACING): Long-term Results of a Prospective, Randomised, Controlled Trial. EClinicalMedicine. 2018 Nov 9;4-5:109-116. doi: 10.1016/j.eclinm.2018.10.007. PMID: 31193613; PMCID: PMC6537536.
- Qian G, Fu Z, Guo J, et al. Prevention of contrast-induced nephropathy by central venous pressure guided fluid administration in chronic kidney disease and congestive heart failure patients. JACC Cardiovasc Intervent. 2016;9(1):89-96.
- Timal RJ, Kooiman J, Sijpkens YW, de Vries JPP, Verberk-Jonkers IJ, Brulez HF, van Buren M, van der Molen AJ, Cannegieter SC, Putter H, van den Hout WB, Jukema JW, Rabelink TJ, Huisman MV. Effect of no prehydration vs sodium bicarbonate prehydration prior to contrast-enhanced computed tomography in the prevention of postcontrast acute kidney injury in adults with chronic kidney disease: the Kompas randomized clinical trial. JAMA Internal Medicine. 2020;180(4):533-541.
- Usmiani T, Andreis A, Budano C, et al. AKIGUARD (Acute Kidney Injury GUARding Device) trial: in-hospital and one-year outcomes. medicine Cardiovasc Med (Hagerstown, Md.). 2016;17(7):530-7.
- van Mourik MS, van Kesteren F, Planken RN, Stoker J, Wiegerinck EMA, Piek JJ, Tijssen JG, Koopman MG, Henriques JPS, Baan J Jr, Vis MM. Short versus conventional hydration for prevention of kidney injury during pre-TAVI computed tomography angiography. Neth Heart J. 2018 Sep;26(9):425-432. doi: 10.1007/s12471-018-1133-1. PMID: 30039383; PMCID: PMC6115307.
- Visconti G, Focaccio A, Donahue M, et al. RenalGuard System for the prevention of acute kidney injury in patients undergoing transcatheter aortic valve implantation. EuroIntervention. 2016 Apr 8;11(14):e1658-61.
- Wang Z, Song Y, A G, Li Y. Role of Hydration in Contrast-Induced Nephropathy in Patients Who Underwent Primary Percutaneous Coronary Intervention. Int Heart J. 2019 Sep 27;60(5):1077-1082. doi: 10.1536/ihj.18-725. Epub 2019 Aug 23. PMID: 31447466.
- Weisbord SD, Gallagher M, Jneid H, Garcia S, Cass A, Thwin SS, Conner TA, Chertow GM, Bhatt DL, Shunk K, Parikh CR, McFalls EO, Brophy M, Ferguson R, Wu H, Androsenko M, Myles J, Kaufman J, Palevsky PM; PRESERVE Trial Group. Outcomes after Angiography with Sodium Bicarbonate and Acetylcysteine. N Engl J Med. 2018 Feb 15;378(7):603-614. doi: 10.1056/NEJMoa1710933. Epub 2017 Nov 12. PMID: 29130810.
Evidence tabellen
Risk of bias table for intervention studies (randomized controlled trials; based on Cochrane risk of bias tool and suggestions by the CLARITY Group at McMaster University)
Question 1: hydration versus no hydration
|
Study reference
(first author, publication year) |
Was the allocation sequence adequately generated?
Definitely yes Probably yes Probably no Definitely no |
Was the allocation adequately concealed?
Definitely yes Probably yes Probably no Definitely no |
Blinding: Was knowledge of the allocated interventions adequately prevented?
Were patients blinded?
Were healthcare providers blinded?
Were data collectors blinded?
Were outcome assessors blinded?
Were data analysts blinded?
Definitely yes Probably yes Probably no Definitely no |
Was loss to follow-up (missing outcome data) infrequent?
Definitely yes Probably yes Probably no Definitely no |
Are reports of the study free of selective outcome reporting?
Definitely yes Probably yes Probably no Definitely no |
Was the study apparently free of other problems that could put it at a risk of bias?
Definitely yes Probably yes Probably no Definitely no |
Overall risk of bias If applicable/necessary, per outcome measure
LOW Some concerns HIGH
|
|
Chen, 2008 |
Probably no;
Reason: Only reported that patients were randomly divided. |
No information
|
No information |
Probably yes;
Reason: No loss to follow-up or missing data. |
Probably yes;
Reason: All relevant outcomes were reported. |
Probably yes;
Reason: No other problems noted. |
HIGH (all outcomes) |
|
Nijssen, 2017 |
Definitely yes;
Reason: Randomisation was computer generated using the ALEA screening and enrolment application software. |
Probably yes;
Reason: Laboratory personnel processing samples for serum creatinine values were masked to treatment allocation, with samples being labelled with coded stickers only. Minimisation ensured that allocated treatment was unpredictable. |
Probably no;
Reason: Open label design because blinding patients or nursing and research staff was not feasible due to the obvious difference in treatment of hydrated and non-hydrated patients.
|
Probably no;
Reason: Loss to follow-up was frequent but similar for intervention and control group. |
Definitely yes
Reason: All relevant outcomes were reported and matched with protocol. |
Definitely yes;
Reason: No other problems noted. |
LOW (dialysis, adverse events)
Some concerns (PC-AKI, end-stage renal failure, residual decline in kidney function) |
|
Nijssen, 2018 |
Definitely yes;
Reason: Randomisation was computer generated using the ALEA screening and enrolment application software. |
Probably yes;
Reason: Laboratory personnel processing samples for serum creatinine values were masked to treatment allocation, with samples being labelled with coded stickers only. Minimisation ensured that allocated treatment was unpredictable. |
Probably no;
Reason: Open label design because blinding patients or nursing and research staff was not feasible due to the obvious difference in treatment of hydrated and non-hydrated patients.
|
Probably no;
Reason: No loss to follow-up for 1-year dialysis outcome. For long-term renal events, follow-up was frequent but almost similar for both groups. |
Probably yes;
Reason: All relevant outcomes were reported. |
Definitely yes;
Reason: No other problems noted. |
LOW (dialysis)
Some concerns (end-stage renal failure, decline in kidney function)
|
|
Timal, 2020 |
Definitely yes;
Reason: Randomization was performed using Project Manager Internet Server (PROMISE) web-based software |
No information |
Probably no;
Reason: No blinding was performed but not feasible in this setting and probably no influence on outcomes. |
Probably yes;
Reason: Loss to follow-up was infrequent and missing data was similar in both groups except for the outcome persisting decline of renal function. |
Probably yes;
Reason: All relevant outcomes were reported and matched with protocol. |
Definitely yes;
Reason: No other problems noted. |
LOW (incidence of PC-AKI, end-stage renal failure, dialysis, adverse events)
Some concerns (decrease in residual kidney function) |
Question 2: NaCl versus NaHCO3
|
Study reference
(first author, publication year) |
Was the allocation sequence adequately generated?
Definitely yes Probably yes Probably no Definitely no |
Was the allocation adequately concealed?
Definitely yes Probably yes Probably no Definitely no |
Blinding: Was knowledge of the allocated interventions adequately prevented?
Were patients blinded?
Were healthcare providers blinded?
Were data collectors blinded?
Were outcome assessors blinded?
Were data analysts blinded?
Definitely yes Probably yes Probably no Definitely no |
Was loss to follow-up (missing outcome data) infrequent?
Definitely yes Probably yes Probably no Definitely no |
Are reports of the study free of selective outcome reporting?
Definitely yes Probably yes Probably no Definitely no |
Was the study apparently free of other problems that could put it at a risk of bias?
Definitely yes Probably yes Probably no Definitely no |
Overall risk of bias If applicable/necessary, per outcome measure
LOW Some concerns HIGH
|
|
Boccalandro, 2021 |
Probably yes;
Reason: Simple randomization based on an automated Excel database (Microsoft Corp.) was performed. |
Probably yes;
Reason: An allocation concealment technique was used. |
Definitely yes;
Reason: Double-blinded study (patients and investigators were blinded to the hydration regimen) |
Definitely yes;
Reason: Loss to follow-up was infrequent and similar for both groups. |
Probably yes;
Reason: All relevant outcomes were reported. |
Probably yes;
Reason: No other problems noted. |
LOW (all outcomes) |
|
Weisbord, 2018 |
Definitely yes;
Reason: A computer-generated permuted-block plan was used.
|
Definitely yes;
Reason: Central randomization was used. |
Probably yes;
Reason: Patients and trial investigators were unaware of trial-group assignments. |
Probably yes;
Reason: No loss to follow-up or missing data. |
Probably yes;
Reason: All relevant outcomes were reported and matched with protocol. |
Probably yes;
Reason: No other problems noted. |
LOW (all outcomes) |
Question 3: Prehydration versus pre- and posthydration
|
Study reference
(first author, publication year) |
Was the allocation sequence adequately generated?
Definitely yes Probably yes Probably no Definitely no |
Was the allocation adequately concealed?
Definitely yes Probably yes Probably no Definitely no |
Blinding: Was knowledge of the allocated interventions adequately prevented?
Were patients blinded?
Were healthcare providers blinded?
Were data collectors blinded?
Were outcome assessors blinded?
Were data analysts blinded?
Definitely yes Probably yes Probably no Definitely no |
Was loss to follow-up (missing outcome data) infrequent?
Definitely yes Probably yes Probably no Definitely no |
Are reports of the study free of selective outcome reporting?
Definitely yes Probably yes Probably no Definitely no |
Was the study apparently free of other problems that could put it at a risk of bias?
Definitely yes Probably yes Probably no Definitely no |
Overall risk of bias If applicable/necessary, per outcome measure
LOW Some concerns HIGH
|
|
Kooiman, 2018 |
Definitely yes;
Reason: Randomization was performed in a 1: 1 ratio using a computer generated allocation sequence using block randomization by a certified online program. |
Definitely no;
Reason: Open-label design. |
Definitely no;
Reason: Open-label design. |
Probably yes;
Reason: Loss to follow-up was infrequent and similar in both groups. Same for missing data. |
Probably yes;
Reason: All relevant outcomes were reported. |
Probably yes;
Reason: No other problems noted. |
LOW (all outcomes) |
|
Van Mourik, 2018 |
Definitely yes;
Reason: Randomisation was performed electronically, and block randomization was used. |
Definitely no;
Reason: Open-label trial.
|
Definitely no;
Reason: Unblinded open-label trial. |
Probably yes;
Reason: Loss to follow-up was infrequent and similar in both groups. |
Probably yes;
Reason: All relevant outcomes were reported. |
Probably yes;
Reason: No other problems noted. |
LOW (all outcomes)
|
Risk of bias table for interventions studies (cohort studies based on risk of bias tool by the CLARITY Group at McMaster University)
Question 2: NaCl versus NaHCO3
|
Author, year |
Selection of participants
Was selection of exposed and non-exposed cohorts drawn from the same population?
|
Exposure
Can we be confident in the assessment of exposure?
|
Outcome of interest
Can we be confident that the outcome of interest was not present at start of study?
|
Confounding-assessment
Can we be confident in the assessment of confounding factors? |
Confounding-analysis
Did the study match exposed and unexposed for all variables that are associated with the outcome of interest or did the statistical analysis adjust for these confounding variables? |
Assessment of outcome
Can we be confident in the assessment of outcome? |
Follow up
Was the follow up of cohorts adequate? In particular, was outcome data complete or imputed?
|
Co-interventions
Were co-interventions similar between groups?
|
Overall Risk of bias
|
|
Definitely yes, probably yes, probably no, definitely no |
Definitely yes, probably yes, probably no, definitely no |
Definitely yes, probably yes, probably no, definitely no |
Definitely yes, probably yes, probably no, definitely no |
Definitely yes, probably yes, probably no, definitely no |
Definitely yes, probably yes, probably no, definitely no |
Definitely yes, probably yes, probably no, definitely no |
Definitely yes, probably yes, probably no, definitely no |
Low, Some concerns, High |
|
|
Garcia, 2018 |
Definitely yes;
Reason: Participants were selected from the same study. |
Definitely yes;
Reason: Derived from previous study. |
Definitely yes;
Reason: Outcome related to intervention. |
Probably yes;
Reason: Derived from previous study.
|
Probably yes;
Reason: Exploratory analyses were performed. |
Probably yes;
Reason: Derived from previous study and pre-defined. |
No information;
Reason: Only lost to follow-up reported for total group, not for subgroup |
Probably yes;
Reason: Co-interventions were similar. |
Low (all outcomes |
Table of excluded studies
|
Reference |
Reason for exclusion |
|
Ahmed K, McVeigh T, Cerneviciute R, Mohamed S, Tubassam M, Karim M, Walsh S. Effectiveness of contrast-associated acute kidney injury prevention methods; a systematic review and network meta-analysis. BMC Nephrol. 2018 Nov 13;19(1):323. doi: 10.1186/s12882-018-1113-0. PMID: 30424723; PMCID: PMC6234687. |
Wrong population: no impaired renal function |
|
Aktürk E, Aşkın L, Taşolar H, Kurtoğlu E, Türkmen S, Tanrıverdi O, Uzel KE. Evaluation of contrast nephropathy in percutaneous treatment of chronic total occlusions. Interv Med Appl Sci. 2019 Jun;11(2):95-100. doi: 10.1556/1646.11.2019.15. PMID: 32148912; PMCID: PMC7044539. |
Wrong population: excluded eGFR <60 |
|
Ali-Hasan-Al-Saegh S, Mirhosseini SJ, Ghodratipour Z, Sarrafan-Chaharsoughi Z, Rahimizadeh E, Karimi-Bondarabadi AA, Haddad F, Shahidzadeh A, Mahdavi P, Dehghan AM, Tahernejad M, Shahidzadeh A, Dehghan H, Ghanei A, Lotfaliani M, Weymann A, Zeriouh M, Popov AF, Sabashnikov A. Strategies Preventing Contrast-Induced Nephropathy After Coronary Angiography: A Comprehensive Meta-Analysis and Systematic Review of 125 Randomized Controlled Trials. Angiology. 2017 May;68(5):389-413. doi: 10.1177/0003319716661445. Epub 2016 Aug 1. PMID: 27485363. |
Wrong population: no impaired renal function |
|
Alonso P, Sanz J, García-Orts A, Reina S, Jiménez S, Osca J, Cano O, Andrés A, Sancho-Tello MJ, Martínez L. Usefulness of Sodium Bicarbonate for the Prevention of Contrast-Induced Nephropathy in Patients Undergoing Cardiac Resynchronization Therapy. Am J Cardiol. 2017 Nov 1;120(9):1584-1588. doi: 10.1016/j.amjcard.2017.07.058. Epub 2017 Aug 1. PMID: 28844518. |
Wrong population: no impaired renal function |
|
Birmpili P, Pearson T, Zywicka EM, Jackson J, Chandrasekar R. Effect of contrast administration on the renal function of predialysis patients undergoing fistuloplasty. J Vasc Surg. 2022 Oct;76(4):1066-1071. doi: 10.1016/j.jvs.2022.06.003. Epub 2022 Jun 13. PMID: 35709861. |
Observational study |
|
Bottinor W, Chawla R, Danyi P, Patel K, Turlington J, Sangal K, Hong W, Perera RA, Jovin IS. Intravenous Fluid Therapy Is Associated with a Reduced Incidence of Contrast-Induced Nephropathy but not with a Reduced Long-Term Incidence of Renal Dysfunction After Cardiac Catheterization. Cardiovasc Revasc Med. 2020 Jan;21(1):20-23. doi: 10.1016/j.carrev.2019.07.020. Epub 2019 Jul 24. PMID: 31378387. |
Wrong population: no impaired renal function |
|
Cai Q, Jing R, Zhang W, Tang Y, Li X, Liu T. Hydration Strategies for Preventing Contrast-Induced Acute Kidney Injury: A Systematic Review and Bayesian Network Meta-Analysis. J Interv Cardiol. 2020 Feb 11;2020:7292675. doi: 10.1155/2020/7292675. PMID: 32116474; PMCID: PMC7036123. |
No studies after search date (2017) |
|
Chen F, Lu J, Yang X, Liu D, Wang Q, Geng X, Xiao B, Zhang J, Liu F, Gu G, Cui W. Different hydration methods for the prevention of contrast-induced nephropathy in patients with elective percutaneous coronary intervention: a retrospective study. BMC Cardiovasc Disord. 2023 Jun 24;23(1):323. doi: 10.1186/s12872-023-03358-w. PMID: 37355592; PMCID: PMC10290803. |
Wrong population: no impaired renal function |
|
Giacoppo D, Gargiulo G, Buccheri S, Aruta P, Byrne RA, Cassese S, Dangas G, Kastrati A, Mehran R, Tamburino C, Capodanno D. Preventive Strategies for Contrast-Induced Acute Kidney Injury in Patients Undergoing Percutaneous Coronary Procedures: Evidence From a Hierarchical Bayesian Network Meta-Analysis of 124 Trials and 28 240 Patients. Circ Cardiovasc Interv. 2017 May;10(5):e004383. doi: 10.1161/CIRCINTERVENTIONS.116.004383. PMID: 28487354. |
No additional studies after search date previous guideline (2017) |
|
Hagikura A, Goto K, Takebayashi H, Kikuta Y, Kobayashi K, Sato K, Taniguchi M, Hiramatsu S, Kawai Y, Kohno H, Kusuyama T, Haruta S. The Role of Saline and Sodium Bicarbonate Preprocedural Hydration to Prevent Mid-term Renal Insufficiency in Patients with Chronic Kidney Disease Undergoing Percutaneous Coronary Intervention. Intern Med. 2019 Apr 15;58(8):1057-1065. doi: 10.2169/internalmedicine.1442-18. Epub 2018 Dec 18. PMID: 30568126; PMCID: PMC6522405. |
Retrospective study |
|
Jiang Y, Chen M, Zhang Y, Zhang N, Yang H, Yao J, Zhou Y. Meta-analysis of prophylactic hydration versus no hydration on contrast-induced acute kidney injury. Coron Artery Dis. 2017 Dec;28(8):649-657. doi: 10.1097/MCA.0000000000000514. PMID: 28692484. |
No additional studies after search date previous guideline (2017) |
|
Khan MI, Naseem M, Ullah S, Ahmed S, Khattak IQ, Khan MR. IMPACT OF CONTRAST-INDUCED NEPHROPATHY PREVENTION STRATEGIES ON PCI OUTCOMES IN HIGH-RISK PAKISTANI PATIENTS. Journal of Population Therapeutics and Clinical Pharmacology. 2024;31(6):3066-3071 |
No comparison between hydration and no hydration for CKD group |
|
Khan SU, Khan MU, Rahman H, Khan MS, Riaz H, Novak M, Opoku-Asare I, Kaluski E. A Bayesian network meta-analysis of preventive strategies for contrast-induced nephropathy after cardiac catheterization. Cardiovasc Revasc Med. 2019 Jan;20(1):29-37. doi: 10.1016/j.carrev.2018.06.005. Epub 2018 Jun 12. PMID: 30757995. |
Wrong population: no impaired renal function |
|
Liu Y, Hong D, Wang AY, Guo R, Smyth B, Liu J, Sun G, Chen S, Tan N, Jardine M, Brieger D, Shaman A, Islam S, Chen J, Gallagher M. Effects of intravenous hydration on risk of contrast induced nephropathy and in-hospital mortality in STEMI patients undergoing primary percutaneous coronary intervention: a systematic review and meta-analysis of randomized controlled trials. BMC Cardiovasc Disord. 2019 Apr 8;19(1):87. doi: 10.1186/s12872-019-1054-y. PMID: 30961544; PMCID: PMC6454772. |
Wrong population: no impaired renal function |
|
Locham S, Rodriguez A, Balceniuk MD, Mix D, Newhall K, Doyle A, Glocker R, Ellis J, Stoner M. Contrast-Associated Acute Kidney Injury in High-Risk Patients Undergoing Peripheral Vascular Interventions. Vasc Endovascular Surg. 2023 Aug;57(6):583-591. doi: 10.1177/15385744231162941. Epub 2023 Mar 7. PMID: 36880982. |
Observational study |
|
Miao S, Xue ZK, Zhang YR, Zhang H, Che JJ, Liu T, Tao HY, Li G, Chen KY. Comparison of Different Hydration Strategies in Patients with Very Low-Risk Profiles of Contrast-Induced Nephropathy. Med Sci Monit. 2021 Apr 30;27:e929115. doi: 10.12659/MSM.929115. PMID: 33927176; PMCID: PMC8095087. |
Wrong population: eGFR >60 mL/min/1.73 m2 |
|
Michel P, Amione-Guerra J, Sheikh O, Jameson LC, Bansal S, Prasad A. Meta-analysis of intravascular volume expansion strategies to prevent contrast-associated acute kidney injury following invasive angiography. Catheter Cardiovasc Interv. 2021 Nov 15;98(6):1120-1132. doi: 10.1002/ccd.29387. Epub 2020 Nov 13. PMID: 33185335. |
Kooiman 2018 and Weisbord 2018 included in search; no other studies after search date |
|
Nijssen EC, Nelemans PJ, Rennenberg RJ, Theunissen RA, van Ommen V, Wildberger JE. Prophylaxis in High-Risk Patients With eGFR < 30 mL/min/1.73 m2: Get the Balance Right. Invest Radiol. 2019 Sep;54(9):580-588. doi: 10.1097/RLI.0000000000000570. PMID: 31033672. |
Observational study |
|
Pakfetrat M, Malekmakan L, Salmanpour Z, Nikoo MH, Izadpanah P. Comparison of Normal Saline, Ringer's Lactate, and Sodium Bicarbonate for Prevention of Contrast-induced Nephropathy in Patients with Coronary Angiography: A Randomized Double-blind Clinical Trial. Indian J Nephrol. 2019 Jan-Feb;29(1):22-27. doi: 10.4103/ijn.IJN_48_17. PMID: 30814789; PMCID: PMC6375023. |
Wrong population: no impaired renal function |
|
Park S, Kim DK, Jung HY, Kim CD, Cho JH, Cha RH, Jeong JC, Kim S, Kim HJ, Ban TH, Chung BH, Lee JP, Park JT, Han SH, Yoo TH, Ryu DR, Moon SJ, Lee JE, Huh W, Kang EW, Chang TI, Joo KW. Efficacy and Safety of a Balanced Salt Solution Versus a 0.9% Saline Infusion for the Prevention of Contrast-Induced Acute Kidney Injury After Contrast-Enhanced Computed Tomography. Kidney Med. 2020 Feb 21;2(2):189-195. doi: 10.1016/j.xkme.2019.12.003. PMID: 32734238; PMCID: PMC7380376. |
Wrong comparison |
|
Shilbayeh SAR. Efficacy of sodium bicarbonate versus normal saline in the prevention of contrast-induced nephropathy among cardiac patients: a cohort study in Saudi Arabia. MMSL. 2022;91(1):18-28. |
No subgroup for CKD |
|
Su X, Xie X, Liu L, Lv J, Song F, Perkovic V, Zhang H. Comparative Effectiveness of 12 Treatment Strategies for Preventing Contrast-Induced Acute Kidney Injury: A Systematic Review and Bayesian Network Meta-analysis. Am J Kidney Dis. 2017 Jan;69(1):69-77. doi: 10.1053/j.ajkd.2016.07.033. Epub 2016 Oct 1. PMID: 27707552. |
Wrong population: no impaired renal function |
|
Valette X, Desmeulles I, Savary B, Masson R, Seguin A, Sauneuf B, Brunet J, Verrier P, Pottier V, Orabona M, Samba D, Viquesnel G, Lermuzeaux M, Hazera P, Dutheil JJ, Hanouz JL, Parienti JJ, du Cheyron D. Sodium Bicarbonate Versus Sodium Chloride for Preventing Contrast-Associated Acute Kidney Injury in Critically Ill Patients: A Randomized Controlled Trial. Crit Care Med. 2017 Apr;45(4):637-644. doi: 10.1097/CCM.0000000000002267. PMID: 28181941. |
Wrong population: no subgroup about chronic kidney disease; only stable renal function (unstable renal function defined as a serum creatinine increase greater than or equal to 0.3 mg/dL within the previous 48 hours or anuria within the previous 12 hours) |
|
Walker H, Guthrie GD, Lambourg E, Traill P, Zealley I, Plumb A, Bell S. Systematic review and meta-analysis of prophylaxis use with intravenous contrast exposure to prevent contrast-induced nephropathy. Eur J Radiol. 2022 Aug;153:110368. doi: 10.1016/j.ejrad.2022.110368. Epub 2022 May 23. PMID: 35636024. |
No additional studies after search date previous guideline (2017) |
|
Wang Z, Song Y, A G, Li Y. Role of Hydration in Contrast-Induced Nephropathy in Patients Who Underwent Primary Percutaneous Coronary Intervention. Int Heart J. 2019 Sep 27;60(5):1077-1082. doi: 10.1536/ihj.18-725. Epub 2019 Aug 23. PMID: 31447466. |
Wrong population |
|
Yan P, Duan SB, Luo XQ, Zhang NY, Deng YH. Effects of intravenous hydration in preventing post-contrast acute kidney injury in patients with eGFR < 30 mL/min/1.73 m2. Eur Radiol. 2023 Dec;33(12):9434-9443. doi: 10.1007/s00330-023-09858-9. Epub 2023 Jun 27. PMID: 37368109. |
Observational study |
|
Zaki HA, Bashir K, Iftikhar H, Alhatemi M, Elmoheen A. Evaluating the Effectiveness of Pretreatment With Intravenous Fluid in Reducing the Risk of Developing Contrast-Induced Nephropathy: A Systematic Review and Meta-Analysis. Cureus. 2022 May 8;14(5):e24825. doi: 10.7759/cureus.24825. PMID: 35693368; PMCID: PMC9172963. |
No additional studies after search date previous guideline (2017) |
Verantwoording
Beoordelingsdatum en geldigheid
Publicatiedatum : 09-12-2025
Beoordeeld op geldigheid : 09-12-2025
Samenstelling werkgroep
Voor het ontwikkelen van de richtlijnmodule is in 2023 een multidisciplinair cluster ingesteld. Het cluster Beeldvormende diagnostiek bestaat uit meerdere richtlijnen, zie hier voor de actuele clusterindeling. De stuurgroep bewaakt het proces van modulair onderhoud binnen het cluster. De expertisegroepsleden geven hun expertise in, indien nodig. De volgende personen uit het cluster zijn betrokken geweest bij de herziening van deze module:
Clusterstuurgroep
- Drs. N. (Nanko) de Graaf, voorzitter, radioloog, NVvR (tot 17-02-2025)
- Dr. S. (Stef) Levolger, voorzitter, (interventie)radioloog, NVvR (voorzitter vanaf 10-03-2025)
- Dr. P.M. (Marc) van der Zee, cardioloog, NVvC
- Dr. J.W. (Jan Willem) Hinnen, vaatchirurg, NVvH
- Dr. Ir. M. (Marcel) van Straten, klinisch fysicus, NVKF
Clusterexpertisegroep
- Dr. H.W. (Henk) van Hamersvelt, internist-nefroloog, NIV
- Drs. M. (Marije) Koning, AIOS interne geneeskunde – differentiatie nefrologie, NIV
- Drs. A.J. (Aart) van der Molen, radioloog, NVvR
- Drs. M.J.P. (Mariska) Rossius, radioloog, NVvR
- Dr. N. (Neeltje) Coolen, patiëntvertegenwoordiger, Nierpatiënten Vereniging Nederland
Met ondersteuning van
- Drs. D.A.M. (Danique) Middelhuis, adviseur, Kennisinstituut van de Federatie Medisch Specialisten
- Drs. A.L.J. (Andrea) Kortlever - van der Spek, adviseur, Kennisinstituut van de Federatie Medisch Specialisten (tot 01-07-2024)
- Dr. L. (Lotte) Houtepen, adviseur, Kennisinstituut van de Federatie Medisch Specialisten (vanaf 01-07-2024)
Belangenverklaringen
Een overzicht van de belangen van de clusterleden en het oordeel over het omgaan met eventuele belangen vindt u in onderstaande tabel. De ondertekende belangenverklaringen zijn op te vragen bij het secretariaat van het Kennisinstituut van de Federatie Medisch Specialisten via secretariaat@kennisinstituut.nl.
Clusterstuurgroepleden
|
Naam |
Hoofdfunctie |
Nevenwerkzaamheden |
Persoonlijke financiële belangen |
Persoonlijke relaties |
Extern gefinancierd onderzoek |
Intellectuele belangen en reputatie |
Overige belangen |
Datum |
Restrictie |
|
Nanko de Graaf* |
Academisch Medisch Specialist / Radioloog (betaald) Erasmus MC Rotterdam |
Bestuurslid sectie Techniek, NVvR (onbetaald) Bestuurslid NCS (Ned. Comm. Stralingsdosimetrie (onbetaald) Voorzitter richtlijn Veilig gebruik van intraveneuze contrastmiddelen bij kinderen (vacatiegelden) Adviescommissie Pocus (point of care ultrasound van de NVvR bij de NVK (onbetaald) |
Geen |
Geen |
Geen |
Geen
|
Geen |
23-1-2023 |
Geen restrictie |
|
Stef Levolger* |
(Interventie)radioloog. Vrijgevestigd medisch specialist. Lid vakgroep Radiologie Maasstad Ziekenhuis/ MSB Maasstad Ziekenhuis. |
Arts-commissielid MEC-U (lokale METC). Vacatiegelden. |
Geen persoonlijke financiële belangen. |
Geen financieel baat bij de partner. Geen financieel baat in verdere directe omgeving voor zover bekend. |
-Medeaanvrager Zorginstuut Nederland (ZiN) Veelbelovende zorg subsidieregeling: ‘ThrOmbectomy in high-Risk Pulmonary Embolism – Device versus thrOmbolysis NetherLands’ (TORPEDO-NL) - Lokaal PI Short MRI Surveillance (SMS) studie. KWF gefinancierd. - ZonMw DoelmatigheidsOnderzoek Voorbereidende studies: ‘What are the direct and indirect costs for PRG and PEG in The Netherlands’ * ZiN/ZonMw - Trombectomie versus IV-trombolyse. Hoog risico longembolie patiënten - Geen projectleider * ZonMw - Pilotstudie. Microcosting. PRG versus PEG gastrostomie - Geen projectleider * KWF - Leverkankerscreening. Echografie versus MRI. Surveillance - Geen projectleider |
Geen |
Geen |
27-8-2024 |
Geen restrictie |
|
Marc van der Zee |
Cardioloog St Jansdal |
Coördinator onderzoek binnen St Jansdal ziekenhuis incl. aansturing onderzoeksverpleegkundigen |
Geen |
Geen |
Geen |
Geen |
Geen |
22-11-2022 |
Geen restrictie |
|
Jan-willem Hinnen |
Vaatchirurg Jeroen Bosch Ziekenhuis, ‘s Hertogenbosch |
Bestuur Nederlandse Vereniging voor Vaatchirurige Betrokken bij onderzoeken welke niet gerelateerd zijn aan het cluster |
Geen |
Geen |
Geen |
Geen |
Geen |
19-02-2023 |
Geen restrictie |
|
Marcel van Straten |
Klinisch Fysicus Erasmus MC |
Lid Commissie Straling NVKF |
n.v.t. |
n.v.t. |
Marcel is principal investigator van de lijn Physics in CT Technology. In samenwerking met Siemens wordt de nieuwe acquisitie- (het maken van scans) en postprocessingtechnieken (het verwerken van informatie uit scans) op met name de photon-counting scanner geëvalueerd. Verschillende onderwepen uit het cluster zijn gerelateerd aan het onderzoek. Echter, worden er binnen het cluster geen aanbevelingen opgesteld die herleidbaar zijn tot fabrikanten, zoals Siemans. Tevens begeleid hij PhD-studenten die een thesis schrijven over dit onderwerp. |
n.v.t. |
n.v.t. |
9-1-2024 |
Geen restrictie |
Betrokken clusterexpertisegroepleden
|
Naam |
Hoofdfunctie |
Nevenwerkzaamheden |
Persoonlijke financiële belangen |
Persoonlijke relaties |
Extern gefinancierd onderzoek |
Intellectuele belangen en reputatie |
Overige belangen |
Datum |
Restrictie |
|
Henk van Hamersvelt |
Gepensioneerd internist-nefroloog met nul-uren aanstelling bij afdeling Nierziekten van Radboudumc te Nijmegen |
Onbezoldigd voorzitter richtlijnencommissie van de Nederlandse Federatie voor Nefrologie |
Geen |
Geen |
Geen |
Geen |
Geen |
22-11-2022 |
Geen restrictie |
|
Marije Koning |
AIOS interne geneeskunde - differentiatie nefrologie LUMC. |
Geen |
Geen |
Geen |
Ja. Onderzoek naar vascularisatie van vanuit stamcellen gegenereerde nierorganoiden. RegMedXB: Vascularisatie van nierorganoiden. Nefrosearch: Metabolomics van nierorganoiden. The Novo Nordisk Foundation Center for Stem Cell Medicine (reNEW). Vascularisatie van nierorganoiden |
Geen |
Geen |
28-11-2024 |
Geen restrictie |
|
Aart van der Molen |
Radioloog LUMC Leiden 100% |
Lid Expert Group Gadopiclenol Nederland - Guerbet Nederland - Consultancy (alleen vergaderingen betaald) |
Geen |
Geen |
NVT |
Geen |
Geen |
5-6-2023 |
Geen restrictie |
|
Mariska Rossius |
Radioloog Erasmus MC Rotterdam |
Secretaris sectiebestuur thoraxradiologie medisch coördinator planning LDOnderwijs coördinator thorax |
Geen |
Geen |
Geen |
Geen |
Geen |
2-8-2024 |
Geen restrictie |
|
Neeltje Coolen |
Coördinator Patiëntenparticipatie Kwaliteit van Zorg, Nierpatiënten Vereniging Nederland |
Geen |
Geen |
Geen |
AstraZeneca: Psychosocial impact of COVID-19 on immunocompromised individuals - a cross sectional survey in the Netherlands
Characteristics of immunocompromised individuals admitted to the ICU due to the Omicron corona variant - a descriptive study in the Netherlands |
Geen |
Geen |
30-11-2023 |
Geen restrictie |
Inbreng patiëntenperspectief
Kwalitatieve raming van mogelijke financiële gevolgen in het kader van de Wkkgz
Bij de richtlijnmodule voerden de clusterleden conform de Wet kwaliteit, klachten en geschillen zorg (Wkkgz) een kwalitatieve raming uit om te beoordelen of de aanbevelingen mogelijk leiden tot substantiële financiële gevolgen. Bij het uitvoeren van deze beoordeling is de richtlijnmodule op verschillende domeinen getoetst (zie het stroomschema bij Werkwijze).
|
Module |
Uitkomst raming |
Toelichting |
|
Hydratie en complicaties |
Geen financiële gevolgen |
Hoewel uit de toetsing volgt dat de aanbeveling(en) breed toepasbaar zijn (>40.000 patiënten), volgt uit de toetsing dat het overgrote deel (±90%) van de zorgaanbieders en zorgverleners al aan de norm voldoet of het geen nieuwe manier van zorgverlening of andere organisatie van zorgverlening betreft, het geen toename in het aantal in te zetten voltijdsequivalenten aan zorgverleners betreft en het geen wijziging in het opleidingsniveau van zorgpersoneel betreft. Er worden daarom geen financiële gevolgen verwacht. |
Werkwijze
Voor meer details over de gebruikte richtlijnmethodologie verwijzen wij u naar de Werkwijze. Relevante informatie voor de ontwikkeling/herziening van deze richtlijnmodule is hieronder weergegeven.
Zoekverantwoording
Algemene informatie
|
Cluster/richtlijn: Cluster Beeldvormende diagnostiek - UV1 Hydratie en complicaties |
|
|
Uitgangsvraag/modules: Welke hydratiestrategie dient te worden toegepast bij patiënten die intravasculair jodiumhoudend contrastmiddel (CM)-toediening ondergaan en een hoog PC-AKI risico hebben? |
|
|
Database(s): Embase.com, Ovid/Medline |
Datum: 21 oktober 2024 |
|
Periode: vanaf 2017 |
Talen: geen restrictie |
|
Literatuurspecialist: Esther van der Bijl |
Rayyan review: https://new.rayyan.ai/reviews/1198413/overview |
|
BMI-zoekblokken: voor verschillende opdrachten wordt (deels) gebruik gemaakt van de zoekblokken van BMI-Online https://blocks.bmi-online.nl/ Deduplication: voor het ontdubbelen is gebruik gemaakt van http://dedupendnote.nl/ |
|
|
Toelichting: Voor deze vraag is gezocht op de elementen impaired renal function EN iodine-containing contrast media EN hydration.
De sleutelartikelen worden gevonden met deze search. |
|
|
Te gebruiken voor richtlijntekst: In de databases Embase.com en Ovid/Medline is op 21 oktober 2024 systematisch gezocht naar systematische reviews, RCTs en observationele studies over hydratie bij patiënten die jodiumhoudend contrastmiddel toegediend krijgen en een hoog PC-AKI risico hebben. De literatuurzoekactie leverde 571 unieke treffers op. |
|
Zoekopbrengst
21-10-2024
|
|
EMBASE |
OVID/MEDLINE |
Ontdubbeld |
|
SR |
90 |
62 |
94 |
|
RCT |
231 |
144 |
261 |
|
Observationele studies |
207 |
90 |
216 |
|
Totaal |
528 |
296 |
571* |
*in Rayyan
Zoekstrategie
Embase.com - 21 oktober 2024
|
No. |
Query |
Results |
|
#1 |
'kidney failure'/exp OR 'chronic kidney failure'/exp OR 'estimated glomerular filtration rate'/exp OR 'kidney disease'/de OR 'kidney function'/de OR (((kidney* OR renal*) NEAR/3 (disease* OR disorder* OR failure* OR insufficienc* OR injur* OR function*)):ti,ab,kw) OR 'nephropath*':ti,ab,kw OR egfr:ti,ab,kw OR gfr:ti,ab,kw OR 'estimated gfr':ti,ab,kw OR 'glomerular filtration rat*':ti,ab,kw OR 'glomerulofiltration rat*':ti,ab,kw OR 'glomerulus filtration rat*':ti,ab,kw OR ckd:ti,ab,kw OR nephrotoxicit*:ti,ab,kw OR 'aki':ti,ab,kw |
1229889 |
|
#2 |
'contrast medium'/exp OR 'iodinated contrast medium'/exp OR 'percutaneous coronary intervention'/exp OR 'angiography'/exp OR 'arteriography'/exp OR (((contrast OR radiocontrast) NEAR/3 (agent* OR material* OR media OR medium OR iodinated OR iodine OR iodized OR administrat* OR dose OR doses OR dosage OR enhanced OR exposure)):ti,ab,kw) OR 'percutaneous coronary intervention':ti,ab,kw OR 'contrast-induc*':ti,ab,kw OR 'radiocontrast-induc*':ti,ab,kw OR 'angiograph*':ti,ab,kw OR 'arteriogram*':ti,ab,kw OR 'arteriograph*':ti,ab,kw OR aortogram*:ti,ab,kw OR aortograph*:ti,ab,kw OR iodinated*:ti,ab,kw OR iodine*:ti,ab,kw OR 'iodixanol'/exp OR 'cardiascan':ti,ab,kw OR 'du 6807':ti,ab,kw OR 'du6807':ti,ab,kw OR 'iodixanol':ti,ab,kw OR 'iodixlu':ti,ab,kw OR 'iodixonal':ti,ab,kw OR 'optiprep':ti,ab,kw OR 'visipaque':ti,ab,kw OR 'xanoimage':ti,ab,kw OR 'meglumine ioxaglate plus sodium ioxaglate'/exp OR 'ag 62 27':ti,ab,kw OR 'ag 6227':ti,ab,kw OR 'ag6227':ti,ab,kw OR 'hexabrix':ti,ab,kw OR 'iomeprol'/exp OR 'b 16880':ti,ab,kw OR 'b16880':ti,ab,kw OR 'imeron':ti,ab,kw OR 'iomeprol':ti,ab,kw OR 'iomeron':ti,ab,kw OR 'iopamidol'/exp OR 'angiotron':ti,ab,kw OR 'b 15000':ti,ab,kw OR 'b15000':ti,ab,kw OR 'gastromiro':ti,ab,kw OR 'iopamidol':ti,ab,kw OR 'iopamidole':ti,ab,kw OR 'iopamigita':ti,ab,kw OR 'iopamilu':ti,ab,kw OR 'iopamiro':ti,ab,kw OR 'iopamiron':ti,ab,kw OR 'iopasen':ti,ab,kw OR 'iopasentis':ti,ab,kw OR 'iopathek':ti,ab,kw OR 'ipobraz':ti,ab,kw OR 'isovue':ti,ab,kw OR 'jopamiro':ti,ab,kw OR 'jopamirol':ti,ab,kw OR 'jopamiron':ti,ab,kw OR 'livealthimage':ti,ab,kw OR 'lopamidol':ti,ab,kw OR 'niopam':ti,ab,kw OR 'pamimage':ti,ab,kw OR 'scanlux':ti,ab,kw OR 'solutrast':ti,ab,kw OR 'sq 13396':ti,ab,kw OR 'sq13396':ti,ab,kw OR 'iohexol'/exp OR 'accupaque':ti,ab,kw OR 'exypaque':ti,ab,kw OR 'hexoimage':ti,ab,kw OR 'hexopaque':ti,ab,kw OR 'iohexagita':ti,ab,kw OR 'iohexlu':ti,ab,kw OR 'iohexol':ti,ab,kw OR 'iovision':ti,ab,kw OR 'kopaq':ti,ab,kw OR 'myelo-kit':ti,ab,kw OR 'nioscan':ti,ab,kw OR 'nycodenz':ti,ab,kw OR 'omnipaque':ti,ab,kw OR 'omnitrast':ti,ab,kw OR 'oraltag':ti,ab,kw OR 'win 39424':ti,ab,kw OR 'win39424':ti,ab,kw OR 'ioversol'/exp OR 'ioversol':ti,ab,kw OR 'mp 328':ti,ab,kw OR 'mp328':ti,ab,kw OR 'optiimage':ti,ab,kw OR 'optiject':ti,ab,kw OR 'optiray':ti,ab,kw OR 'iopromide'/exp OR 'iopromide':ti,ab,kw OR 'mypromide':ti,ab,kw OR 'ultraimage':ti,ab,kw OR 'ultravist':ti,ab,kw OR 'zk 35760':ti,ab,kw OR 'zk35760':ti,ab,kw OR 'iobitridol'/exp OR 'iobitridol':ti,ab,kw OR 'xenetix':ti,ab,kw OR 'ioxaglic acid'/exp OR 'er 60':ti,ab,kw OR 'er 61':ti,ab,kw OR 'er60':ti,ab,kw OR 'er61':ti,ab,kw OR 'ioxaglamate sodium':ti,ab,kw OR 'ioxaglate':ti,ab,kw OR 'ioxaglate sodium':ti,ab,kw OR 'ioxaglic acid':ti,ab,kw OR 'ioxaglinic acid':ti,ab,kw OR 'p 286':ti,ab,kw OR 'p286':ti,ab,kw OR 'sodium ioxaglate':ti,ab,kw OR 'iosimenol'/exp OR 'iosimenol':ti,ab,kw OR 'iosmin':ti,ab,kw |
1089925 |
|
#3 |
#1 AND #2 |
66977 |
|
#4 |
'contrast induced nephropathy'/exp OR ((('rc-induc*' OR 'contrast induc*' OR 'radiocontrast induc*') NEAR/4 (nephropath* OR nephrotoxicit* OR renal OR kidney*)):ti,ab,kw) OR 'ci-aki':ti,ab,kw OR 'pc-aki':ti,ab,kw |
8800 |
|
#5 |
#3 OR #4 |
67543 |
|
#6 |
'sodium chloride'/exp OR 'alcathion':ti,ab,kw OR 'dendritis':ti,ab,kw OR 'flexivial':ti,ab,kw OR 'gingivyl':ti,ab,kw OR 'halite':ti,ab,kw OR 'hypersal':ti,ab,kw OR 'hyposaline*':ti,ab,kw OR 'natrium chloride':ti,ab,kw OR 'physiological solution':ti,ab,kw OR 'purex':ti,ab,kw OR 'saline*':ti,ab,kw OR 'salt':ti,ab,kw OR 'sodium chloride':ti,ab,kw OR 'sodiumchloride':ti,ab,kw OR 'isotonic saline'/exp OR 'bicarbonate'/exp OR 'baros':ti,ab,kw OR 'bicarbonate*':ti,ab,kw OR 'colevac':ti,ab,kw OR 'dicarbonate*':ti,ab,kw OR 'hydrocarbonate*':ti,ab,kw OR 'meylon':ti,ab,kw OR 'neut':ti,ab,kw OR 'thamicarb':ti,ab,kw OR 'hydration'/exp OR 'hydrat*':ti,ab,kw OR 'prehydrat*':ti,ab,kw OR 'posthydrat*':ti,ab,kw |
752534 |
|
#7 |
#5 AND #6 |
4063 |
|
#8 |
#7 AND [2017-2025]/py NOT ('conference abstract'/it OR 'editorial'/it OR 'letter'/it OR 'note'/it) NOT (('animal'/exp OR 'animal experiment'/exp OR 'animal model'/exp OR 'nonhuman'/exp) NOT 'human'/exp) |
878 |
|
#9 |
'meta analysis'/exp OR 'meta analysis (topic)'/exp OR metaanaly*:ti,ab OR 'meta analy*':ti,ab OR metanaly*:ti,ab OR 'systematic review'/de OR 'cochrane database of systematic reviews'/jt OR prisma:ti,ab OR prospero:ti,ab OR (((systemati* OR scoping OR umbrella OR 'structured literature') NEAR/3 (review* OR overview*)):ti,ab) OR ((systemic* NEAR/1 review*):ti,ab) OR (((systemati* OR literature OR database* OR 'data base*') NEAR/10 search*):ti,ab) OR (((structured OR comprehensive* OR systemic*) NEAR/3 search*):ti,ab) OR (((literature NEAR/3 review*):ti,ab) AND (search*:ti,ab OR database*:ti,ab OR 'data base*':ti,ab)) OR (('data extraction':ti,ab OR 'data source*':ti,ab) AND 'study selection':ti,ab) OR ('search strategy':ti,ab AND 'selection criteria':ti,ab) OR ('data source*':ti,ab AND 'data synthesis':ti,ab) OR medline:ab OR pubmed:ab OR embase:ab OR cochrane:ab OR (((critical OR rapid) NEAR/2 (review* OR overview* OR synthes*)):ti) OR ((((critical* OR rapid*) NEAR/3 (review* OR overview* OR synthes*)):ab) AND (search*:ab OR database*:ab OR 'data base*':ab)) OR metasynthes*:ti,ab OR 'meta synthes*':ti,ab |
1071220 |
|
#10 |
'clinical trial'/exp OR 'randomization'/exp OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp OR 'placebo'/exp OR 'prospective study'/exp OR rct:ab,ti OR random*:ab,ti OR 'single blind':ab,ti OR 'randomised controlled trial':ab,ti OR 'randomized controlled trial'/exp OR placebo*:ab,ti |
4127462 |
|
#11 |
'major clinical study'/de OR 'clinical study'/de OR 'case control study'/de OR 'family study'/de OR 'longitudinal study'/de OR 'retrospective study'/de OR 'prospective study'/de OR 'comparative study'/de OR 'cohort analysis'/de OR ((cohort NEAR/1 (study OR studies)):ab,ti) OR (('case control' NEAR/1 (study OR studies)):ab,ti) OR (('follow up' NEAR/1 (study OR studies)):ab,ti) OR (observational NEAR/1 (study OR studies)) OR ((epidemiologic NEAR/1 (study OR studies)):ab,ti) OR (('cross sectional' NEAR/1 (study OR studies)):ab,ti) |
8454461 |
|
#12 |
'case control study'/de OR 'comparative study'/exp OR 'control group'/de OR 'controlled study'/de OR 'controlled clinical trial'/de OR 'crossover procedure'/de OR 'double blind procedure'/de OR 'phase 2 clinical trial'/de OR 'phase 3 clinical trial'/de OR 'phase 4 clinical trial'/de OR 'pretest posttest design'/de OR 'pretest posttest control group design'/de OR 'quasi experimental study'/de OR 'single blind procedure'/de OR 'triple blind procedure'/de OR (((control OR controlled) NEAR/6 trial):ti,ab,kw) OR (((control OR controlled) NEAR/6 (study OR studies)):ti,ab,kw) OR (((control OR controlled) NEAR/1 active):ti,ab,kw) OR 'open label*':ti,ab,kw OR (((double OR two OR three OR multi OR trial) NEAR/1 (arm OR arms)):ti,ab,kw) OR ((allocat* NEAR/10 (arm OR arms)):ti,ab,kw) OR placebo*:ti,ab,kw OR 'sham-control*':ti,ab,kw OR (((single OR double OR triple OR assessor) NEAR/1 (blind* OR masked)):ti,ab,kw) OR nonrandom*:ti,ab,kw OR 'non-random*':ti,ab,kw OR 'quasi-experiment*':ti,ab,kw OR crossover:ti,ab,kw OR 'cross over':ti,ab,kw OR 'parallel group*':ti,ab,kw OR 'factorial trial':ti,ab,kw OR ((phase NEAR/5 (study OR trial)):ti,ab,kw) OR ((case* NEAR/6 (matched OR control*)):ti,ab,kw) OR ((match* NEAR/6 (pair OR pairs OR cohort* OR control* OR group* OR healthy OR age OR sex OR gender OR patient* OR subject* OR participant*)):ti,ab,kw) OR ((propensity NEAR/6 (scor* OR match*)):ti,ab,kw) OR versus:ti OR vs:ti OR compar*:ti OR ((compar* NEAR/1 study):ti,ab,kw) OR (('major clinical study'/de OR 'clinical study'/de OR 'cohort analysis'/de OR 'observational study'/de OR 'cross-sectional study'/de OR 'multicenter study'/de OR 'correlational study'/de OR 'follow up'/de OR cohort*:ti,ab,kw OR 'follow up':ti,ab,kw OR followup:ti,ab,kw OR longitudinal*:ti,ab,kw OR prospective*:ti,ab,kw OR retrospective*:ti,ab,kw OR observational*:ti,ab,kw OR 'cross sectional*':ti,ab,kw OR cross?ectional*:ti,ab,kw OR multicent*:ti,ab,kw OR 'multi-cent*':ti,ab,kw OR consecutive*:ti,ab,kw) AND (group:ti,ab,kw OR groups:ti,ab,kw OR subgroup*:ti,ab,kw OR versus:ti,ab,kw OR vs:ti,ab,kw OR compar*:ti,ab,kw OR 'odds ratio*':ab OR 'relative odds':ab OR 'risk ratio*':ab OR 'relative risk*':ab OR 'rate ratio':ab OR aor:ab OR arr:ab OR rrr:ab OR ((('or' OR 'rr') NEAR/6 ci):ab))) |
15474998 |
|
#13 |
#8 AND #9 - SR |
90 |
|
#14 |
#8 AND #10 NOT #13 - RCT |
231 |
|
#15 |
#8 AND (#11 OR #12) NOT (#13 OR #14) - Observationeel |
207 |
|
#16 |
#13 OR #14 OR #15 - Totaal |
528 |
Ovid/Medline - 21 oktober 2024
|
# |
Searches |
Results |
|
1 |
exp Renal Insufficiency/ or exp Kidney Failure, Chronic/ or exp Glomerular Filtration Rate/ or *Kidney Diseases/ or ((kidney* or renal*) adj3 (disease* or disorder* or failure* or insufficienc* or injur* or function*)).ti,ab,kf. or nephropath*.ti,ab,kf. or egfr.ti,ab,kf. or gfr.ti,ab,kf. or estimated gfr.ti,ab,kf. or glomerular filtration rat*.ti,ab,kf. or glomerulofiltration rat*.ti,ab,kf. or glomerulus filtration rat*.ti,ab,kf. or ckd.ti,ab,kf. or nephrotoxicit*.ti,ab,kf. or aki.ti,ab,kf. |
680591 |
|
2 |
exp Contrast Media/ or exp Percutaneous Coronary Intervention/ or exp Angiography/ or ((contrast or radiocontrast) adj3 (agent* or material* or media or medium or iodinated or iodine or iodized or administrat* or dose or doses or dosage or enhanced or exposure)).ti,ab,kf. or percutaneous coronary intervention.ti,ab,kf. or contrast-induc*.ti,ab,kf. or radiocontrast-induc*.ti,ab,kf. or angiograph*.ti,ab,kf. or arteriogram*.ti,ab,kf. or arteriograph*.ti,ab,kf. or aortogram*.ti,ab,kf. or aortograph*.ti,ab,kf. or iodinated*.ti,ab,kf. or iodine*.ti,ab,kf. or cardiascan.ti,ab,kf. or du 6807.ti,ab,kf. or du6807.ti,ab,kf. or iodixanol.ti,ab,kf. or iodixlu.ti,ab,kf. or iodixonal.ti,ab,kf. or optiprep.ti,ab,kf. or visipaque.ti,ab,kf. or xanoimage.ti,ab,kf. or exp Ioxaglic Acid/ or ag 62 27.ti,ab,kf. or ag 6227.ti,ab,kf. or ag6227.ti,ab,kf. or hexabrix.ti,ab,kf. or b 16880.ti,ab,kf. or b16880.ti,ab,kf. or imeron.ti,ab,kf. or iomeprol.ti,ab,kf. or iomeron.ti,ab,kf. or exp Iopamidol/ or angiotron.ti,ab,kf. or b 15000.ti,ab,kf. or b15000.ti,ab,kf. or gastromiro.ti,ab,kf. or iopamidol.ti,ab,kf. or iopamidole.ti,ab,kf. or iopamigita.ti,ab,kf. or iopamilu.ti,ab,kf. or iopamiro.ti,ab,kf. or iopamiron.ti,ab,kf. or iopasen.ti,ab,kf. or iopasentis.ti,ab,kf. or iopathek.ti,ab,kf. or ipobraz.ti,ab,kf. or isovue.ti,ab,kf. or jopamiro.ti,ab,kf. or jopamirol.ti,ab,kf. or jopamiron.ti,ab,kf. or livealthimage.ti,ab,kf. or lopamidol.ti,ab,kf. or niopam.ti,ab,kf. or pamimage.ti,ab,kf. or scanlux.ti,ab,kf. or solutrast.ti,ab,kf. or sq 13396.ti,ab,kf. or sq13396.ti,ab,kf. or exp Iohexol/ or accupaque.ti,ab,kf. or exypaque.ti,ab,kf. or hexoimage.ti,ab,kf. or hexopaque.ti,ab,kf. or iohexagita.ti,ab,kf. or iohexlu.ti,ab,kf. or iohexol.ti,ab,kf. or iovision.ti,ab,kf. or kopaq.ti,ab,kf. or myelo-kit.ti,ab,kf. or nioscan.ti,ab,kf. or nycodenz.ti,ab,kf. or omnipaque.ti,ab,kf. or omnitrast.ti,ab,kf. or oraltag.ti,ab,kf. or win 39424.ti,ab,kf. or win39424.ti,ab,kf. or ioversol.ti,ab,kf. or mp 328.ti,ab,kf. or mp328.ti,ab,kf. or optiimage.ti,ab,kf. or optiject.ti,ab,kf. or optiray.ti,ab,kf. or iopromide.ti,ab,kf. or mypromide.ti,ab,kf. or ultraimage.ti,ab,kf. or ultravist.ti,ab,kf. or zk 35760.ti,ab,kf. or zk35760.ti,ab,kf. or iobitridol.ti,ab,kf. or xenetix.ti,ab,kf. or exp Ioxaglic Acid/ or er 60.ti,ab,kf. or er 61.ti,ab,kf. or er60.ti,ab,kf. or er61.ti,ab,kf. or ioxaglamate sodium.ti,ab,kf. or ioxaglate.ti,ab,kf. or ioxaglate sodium.ti,ab,kf. or ioxaglic acid.ti,ab,kf. or ioxaglinic acid.ti,ab,kf. or p 286.ti,ab,kf. or p286.ti,ab,kf. or sodium ioxaglate.ti,ab,kf. or iosimenol.ti,ab,kf. or iosmin.ti,ab,kf. |
700016 |
|
3 |
1 and 2 |
27564 |
|
4 |
(((rc-induc* or contrast induc* or radiocontrast induc*) adj4 (nephropath* or nephrotoxicit* or renal or kidney*)) or ci-aki or pc-aki).ti,ab,kf. |
3948 |
|
5 |
3 or 4 |
27592 |
|
6 |
exp Sodium Chloride/ or exp Bicarbonates/ or alcathion.ti,ab,kf. or dendritis.ti,ab,kf. or flexivial.ti,ab,kf. or gingivyl.ti,ab,kf. or halite.ti,ab,kf. or hypersal.ti,ab,kf. or hyposaline*.ti,ab,kf. or natrium chloride.ti,ab,kf. or physiological solution.ti,ab,kf. or purex.ti,ab,kf. or saline*.ti,ab,kf. or salt.ti,ab,kf. or sodium chloride.ti,ab,kf. or sodiumchloride.ti,ab,kf. or baros.ti,ab,kf. or bicarbonate*.ti,ab,kf. or colevac.ti,ab,kf. or dicarbonate*.ti,ab,kf. or hydrocarbonate*.ti,ab,kf. or meylon.ti,ab,kf. or neut.ti,ab,kf. or thamicarb.ti,ab,kf. or hydrat*.ti,ab,kf. or prehydrat*.ti,ab,kf. or posthydrat*.ti,ab,kf. |
545558 |
|
7 |
5 and 6 |
1583 |
|
8 |
limit 7 to yr="2017 -Current" |
485 |
|
9 |
8 not (comment/ or editorial/ or letter/) not ((exp animals/ or exp models, animal/) not humans/) |
411 |
|
10 |
meta-analysis/ or meta-analysis as topic/ or (metaanaly* or meta-analy* or metanaly*).ti,ab,kf. or systematic review/ or cochrane.jw. or (prisma or prospero).ti,ab,kf. or ((systemati* or scoping or umbrella or "structured literature") adj3 (review* or overview*)).ti,ab,kf. or (systemic* adj1 review*).ti,ab,kf. or ((systemati* or literature or database* or data-base*) adj10 search*).ti,ab,kf. or ((structured or comprehensive* or systemic*) adj3 search*).ti,ab,kf. or ((literature adj3 review*) and (search* or database* or data-base*)).ti,ab,kf. or (("data extraction" or "data source*") and "study selection").ti,ab,kf. or ("search strategy" and "selection criteria").ti,ab,kf. or ("data source*" and "data synthesis").ti,ab,kf. or (medline or pubmed or embase or cochrane).ab. or ((critical or rapid) adj2 (review* or overview* or synthes*)).ti. or (((critical* or rapid*) adj3 (review* or overview* or synthes*)) and (search* or database* or data-base*)).ab. or (metasynthes* or meta-synthes*).ti,ab,kf. |
783100 |
|
11 |
exp clinical trial/ or randomized controlled trial/ or exp clinical trials as topic/ or randomized controlled trials as topic/ or Random Allocation/ or Double-Blind Method/ or Single-Blind Method/ or (clinical trial, phase i or clinical trial, phase ii or clinical trial, phase iii or clinical trial, phase iv or controlled clinical trial or randomized controlled trial or multicenter study or clinical trial).pt. or random*.ti,ab. or (clinic* adj trial*).tw. or ((singl* or doubl* or treb* or tripl*) adj (blind$3 or mask$3)).tw. or Placebos/ or placebo*.tw. |
2792904 |
|
12 |
Epidemiologic studies/ or case control studies/ or exp cohort studies/ or Controlled Before-After Studies/ or Case control.tw. or cohort.tw. or Cohort analy$.tw. or (Follow up adj (study or studies)).tw. or (observational adj (study or studies)).tw. or Longitudinal.tw. or Retrospective*.tw. or prospective*.tw. or consecutive*.tw. or Cross sectional.tw. or Cross-sectional studies/ or historically controlled study/ or interrupted time series analysis/ [Onder exp cohort studies vallen ook longitudinale, prospectieve en retrospectieve studies] |
4858135 |
|
13 |
Case-control Studies/ or clinical trial, phase ii/ or clinical trial, phase iii/ or clinical trial, phase iv/ or comparative study/ or control groups/ or controlled before-after studies/ or controlled clinical trial/ or double-blind method/ or historically controlled study/ or matched-pair analysis/ or single-blind method/ or (((control or controlled) adj6 (study or studies or trial)) or (compar* adj (study or studies)) or ((control or controlled) adj1 active) or "open label*" or ((double or two or three or multi or trial) adj (arm or arms)) or (allocat* adj10 (arm or arms)) or placebo* or "sham-control*" or ((single or double or triple or assessor) adj1 (blind* or masked)) or nonrandom* or "non-random*" or "quasi-experiment*" or "parallel group*" or "factorial trial" or "pretest posttest" or (phase adj5 (study or trial)) or (case* adj6 (matched or control*)) or (match* adj6 (pair or pairs or cohort* or control* or group* or healthy or age or sex or gender or patient* or subject* or participant*)) or (propensity adj6 (scor* or match*))).ti,ab,kf. or (confounding adj6 adjust*).ti,ab. or (versus or vs or compar*).ti. or ((exp cohort studies/ or epidemiologic studies/ or multicenter study/ or observational study/ or seroepidemiologic studies/ or (cohort* or 'follow up' or followup or longitudinal* or prospective* or retrospective* or observational* or multicent* or 'multi-cent*' or consecutive*).ti,ab,kf.) and ((group or groups or subgroup* or versus or vs or compar*).ti,ab,kf. or ('odds ratio*' or 'relative odds' or 'risk ratio*' or 'relative risk*' or aor or arr or rrr).ab. or (("OR" or "RR") adj6 CI).ab.)) |
5813056 |
|
14 |
9 and 10 - SR |
62 |
|
15 |
(9 and 11) not 14 - RCT |
144 |
|
16 |
(9 and (12 or 13)) not (14 or 15) - Observationeel |
90 |
|
17 |
14 or 15 or 16 - Totaal |
296 |
Search van 2017 (behorend bij subvragen 4 & 5)
Systematic reviews
|
Database |
Search terms |
Total |
|
Medline (OVID)
2000-heden Engels, |
1 exp Contrast Media/ or ((contrast adj3 iodine) or (contrast adj3 medi*)).ti,ab. (108416) 2 Sodium Chloride/ or exp Cardiac Catheterization/ or exp Bicarbonates/ or Rehydration Solutions/ or exp Fluid Therapy/ or (hydrat* or prehydrat* or posthydrat* or rehydrat* or 'volume expansion' or (pre adj1 hydrat*) or (post adj1 hydrat*) or ((oral or iv or intravenous) adj1 (hydrat* or fluid)) or (sodium adj2 (chloride* or bicarbonate*)) or nacl or ((heart or cardiac) adj2 catheterization*)).ti,ab. (262412) 3 exp Kidney Diseases/ or (((kidney or renal) adj2 (disease* or injur* or failure*)) or nephropath* or (renal adj2 (insufficienc* or function* or disease* or failure*))).ti,ab. (525125) 4 1 and 2 and 3 (911) 5 (((contrast* or ci) adj2 (nephropath* or 'kidney injury' or aki or nephrotoxicity)) or cin or ciaki).ti,ab. (8859) 6 Sodium Chloride/ or exp Cardiac Catheterization/ or exp Bicarbonates/ or Rehydration Solutions/ or exp Fluid Therapy/ or (hydrat* or prehydrat* or posthydrat* or rehydrat* or 'volume expansion' or (pre adj1 hydrat*) or (post adj1 hydrat*) or ((oral or iv or intravenous) adj1 (hydrat* or fluid)) or (sodium adj2 (chloride* or bicarbonate*)) or nacl or ((heart or cardiac) adj2 catheterization*)).ti,ab. (262412) 7 5 and 6 (644) 8 4 or 7 (1049) 9 limit 8 to (yr="2000 -Current" and (dutch or english)) (775) 10 (meta-analysis/ or meta-analysis as topic/ or (meta adj analy$).tw. or ((systematic* or literature) adj2 review$1).tw. or (systematic adj overview$1).tw. or exp "Review Literature as Topic"/ or cochrane.ab. or cochrane.jw. or embase.ab. or medline.ab. or (psychlit or psyclit).ab. or (cinahl or cinhal).ab. or cancerlit.ab. or ((selection criteria or data extraction).ab. and "review"/)) not (Comment/ or Editorial/ or Letter/ or (animals/ not humans/)) (236842) 11 9 and 10 (69) – 66 uniek 12 (exp clinical trial/ or randomized controlled trial/ or exp clinical trials as topic/ or randomized controlled trials as topic/ or Random Allocation/ or Double-Blind Method/ or Single-Blind Method/ or (clinical trial, phase i or clinical trial, phase ii or clinical trial, phase iii or clinical trial, phase iv or controlled clinical trial or randomized controlled trial or multicenter study or clinical trial).pt. or random*.ti,ab. or (clinic* adj trial*).tw. or ((singl* or doubl* or treb* or tripl*) adj (blind$3 or mask$3)).tw. or Placebos/ or placebo*.tw.) not (animals/ not humans/) (1459903) 13 9 and 12 (333) 14 13 not 11 (278) |
177 |
|
Embase (Elsevier) |
'contrast medium'/exp OR (contrast NEAR/3 iodine):ab,ti OR (contrast NEAR/3 medi*):ab,ti AND (hydrat*:ab,ti OR prehydrat*:ab,ti OR posthydrat*:ab,ti OR rehydrat*:ab,ti OR 'volume expansion':ab,ti OR (pre NEAR/1 hydrat*):ab,ti OR (post NEAR/1 hydrat*):ab,ti OR ((oral OR iv OR intravenous) NEAR/1 (hydrat* OR fluid)):ab,ti OR (sodium NEAR/2 (chloride* OR bicarbonate)):ab,ti OR nacl:ab,ti OR ((heart OR cardiac) NEAR/2 catheterization):ab,ti OR 'sodium chloride'/exp OR 'heart catheterization'/exp OR 'bicarbonate'/exp OR 'oral rehydration solution'/exp OR 'hydration'/exp) AND ('kidney disease'/exp OR 'kidney function'/exp OR ((kidney or renal) NEAR/2 (disease* OR injur* OR failure*)):ab,ti OR nephropath*:ab,ti OR (renal NEAR/2 (insufficienc* OR function* OR disease* OR failure*)):ab,ti)
OR ('contrast induced nephropathy'/exp/dm_pc OR ((contrast* OR ci) NEAR/2 (nephropath* OR 'kidney injury' OR aki OR nephrotoxicity)):ab,ti OR cin:ab,ti OR ciaki:ab,ti AND (hydrat*:ab,ti OR prehydrat*:ab,ti OR posthydrat*:ab,ti OR rehydrat*:ab,ti OR 'volume expansion':ab,ti OR (pre NEAR/1 hydrat*):ab,ti OR (post NEAR/1 hydrat*):ab,ti OR ((oral OR iv OR intravenous) NEAR/1 (hydrat* OR fluid)):ab,ti OR (sodium NEAR/2 (chloride* OR bicarbonate)):ab,ti OR nacl:ab,ti OR ((heart OR cardiac) NEAR/2 catheterization):ab,ti OR 'sodium chloride'/exp OR 'heart catheterization'/exp OR 'bicarbonate'/exp OR 'oral rehydration solution'/exp OR 'hydration'/exp)) AND ([dutch]/lim OR [english]/lim) AND [embase]/lim AND [2000-2015]/py
AND ('clinical trial'/exp OR 'randomization'/exp OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp OR 'placebo'/exp OR 'prospective study'/exp OR rct:ab,ti OR random*:ab,ti OR 'single blind':ab,ti OR 'randomised controlled trial':ab,ti OR 'randomized controlled trial'/exp OR placebo*:ab,ti) NOT 'conference abstract':it (484)
AND 'meta analysis'/de OR cochrane:ab OR embase:ab OR psychlit:ab OR cinahl:ab OR (systematic NEAR/1 (review OR overview)):ab,ti OR (meta NEAR/1 analy*):ab,ti OR metaanalys*:ab,ti OR 'data extraction':ab OR cochrane:jt OR 'systematic review'/de NOT (animal* NOT human*)), (137) - 82 uniek |
|
|
Cochrane (Wiley) |
((contrast* OR ci) NEAR/2 (nephropath* OR 'kidney injury' OR aki OR nephrotoxicity)):ab,ti OR cin:ab,ti OR ciaki:ab,ti AND (hydrat*:ab,ti OR prehydrat*:ab,ti OR posthydrat*:ab,ti OR rehydrat*:ab,ti OR 'volume expansion':ab,ti OR (pre NEAR/1 hydrat*):ab,ti OR (post NEAR/1 hydrat*):ab,ti OR ((oral OR iv OR intravenous) NEAR/1 (hydrat* OR fluid)):ab,ti OR (sodium NEAR/2 (chloride* OR bicarbonate)):ab,ti OR nacl:ab,ti OR ((heart OR cardiac) NEAR/2 catheterization)) 15 CDR, 45 DARE
11 CR’s niet relevant (CIN-HPV) >4 uniek, DARE 25 uniek, 2 niet relevant |
RCTs
|
Database |
Search terms |
Total |
|
Medline (OVID)
Engels,
2000-juni 2015 |
1 exp Contrast Media/ or ((contrast adj3 iodine) or (contrast adj3 medi*)).ti,ab. (110323) 2 Sodium Chloride/ or exp Cardiac Catheterization/ or exp Bicarbonates/ or Rehydration Solutions/ or exp Fluid Therapy/ or (hydrat* or prehydrat* or posthydrat* or rehydrat* or 'volume expansion' or (pre adj1 hydrat*) or (post adj1 hydrat*) or ((oral or iv or intravenous) adj1 (hydrat* or fluid)) or (sodium adj2 (chloride* or bicarbonate*)) or nacl or ((heart or cardiac) adj2 catheterization*)).ti,ab. (263883) 3 exp Kidney Diseases/ or (((kidney or renal) adj2 (disease* or injur* or failure*)) or nephropath* or (renal adj2 (insufficienc* or function* or disease* or failure*))).ti,ab. (527891) 4 1 and 2 and 3 (918) 5 (((contrast* or ci) adj2 (nephropath* or 'kidney injury' or aki or nephrotoxicity)) or cin or ciaki).ti,ab. (8912) 6 Sodium Chloride/ or exp Cardiac Catheterization/ or exp Bicarbonates/ or Rehydration Solutions/ or exp Fluid Therapy/ or (hydrat* or prehydrat* or posthydrat* or rehydrat* or 'volume expansion' or (pre adj1 hydrat*) or (post adj1 hydrat*) or ((oral or iv or intravenous) adj1 (hydrat* or fluid*)) or (sodium adj2 (chloride* or bicarbonate*)) or nacl or ((heart or cardiac) adj2 catheterization*)).ti,ab. or Water/ or water.ti,ab. or D5w.ti,ab. or Isotonic Solutions/ or Hypotonic Solutions/ or (ringer* adj3 (lactate or solution*)).ti,ab. or ((hypotonic or isotonic) adj3 solution*).ti,ab. or Hydroxyethyl Starch Derivatives/ or (Hydroxyethy* adj3 starch*).ti,ab. (818303) 7 5 and 6 (733) 8 4 or 7 (1140) 9 limit 8 to (yr="2000 -Current" and (dutch or english)) (818) 10 (meta-analysis/ or meta-analysis as topic/ or (meta adj analy$).tw. or ((systematic* or literature) adj2 review$1).tw. or (systematic adj overview$1).tw. or exp "Review Literature as Topic"/ or cochrane.ab. or cochrane.jw. or embase.ab. or medline.ab. or (psychlit or psyclit).ab. or (cinahl or cinhal).ab. or cancerlit.ab. or ((selection criteria or data extraction).ab. and "review"/)) not (Comment/ or Editorial/ or Letter/ or (animals/ not humans/)) (240088) 11 9 and 10 (72) 12 (exp clinical trial/ or randomized controlled trial/ or exp clinical trials as topic/ or randomized controlled trials as topic/ or Random Allocation/ or Double-Blind Method/ or Single-Blind Method/ or (clinical trial, phase i or clinical trial, phase ii or clinical trial, phase iii or clinical trial, phase iv or controlled clinical trial or randomized controlled trial or multicenter study or clinical trial).pt. or random*.ti,ab. or (clinic* adj trial*).tw. or ((singl* or doubl* or treb* or tripl*) adj (blind$3 or mask$3)).tw. or Placebos/ or placebo*.tw.) not (animals/ not humans/) (1471469) 13 9 and 12 (341) 14 13 not 11 (283) – 265 uniek 17 Epidemiologic studies/ or case control studies/ or exp cohort studies/ or Controlled Before-After Studies/ or Case control.tw. or (cohort adj (study or studies)).tw. or Cohort analy$.tw. or (Follow up adj (study or studies)).tw. or (observational adj (study or studies)).tw. or Longitudinal.tw. or Retrospective.tw. or prospective.tw. or Cross sectional.tw. or Cross-sectional studies/ or historically controlled study/ or interrupted time series analysis/ [Onder exp cohort studies vallen ook longitudinale, prospectieve en retrospectieve studies] (2160769) 22 21 not 19 (134) – vanaf 2007: 105 – 103 uniek –in afzonderlijk document |
572 RCTS
6 SRs new (177 SRs in earlier search strategy) |
|
Embase (Elsevier) |
'contrast medium'/exp OR (contrast NEAR/3 iodine):ab,ti OR (contrast NEAR/3 medi*):ab,ti
AND (hydrat*:ab,ti OR prehydrat*:ab,ti OR posthydrat*:ab,ti OR rehydrat*:ab,ti OR 'volume expansion':ab,ti OR (pre NEAR/1 hydrat*):ab,ti OR (post NEAR/1 hydrat*):ab,ti OR ((oral OR iv OR intravenous) NEAR/1 (hydrat* OR fluid*)):ab,ti OR (sodium NEAR/2 (chloride* OR bicarbonate)):ab,ti OR nacl:ab,ti OR ((heart OR cardiac) NEAR/2 catheterization):ab,ti OR water:ab,ti OR d5w:ab,ti OR (ringer* NEAR/3 (lactate OR solution*)):ab,ti OR ((hypotonic OR isotonic) NEAR/3 solution*):ab,ti OR (hydroxyethy* NEAR/3 starch*):ab,ti OR 'sodium chloride'/exp OR 'heart catheterization'/exp OR 'bicarbonate'/exp OR 'oral rehydration solution'/exp OR 'hydration'/exp OR 'water'/exp OR 'isotonic solution'/exp OR 'ringer lactate solution'/exp OR 'hetastarch derivative'/exp OR 'fluid balance'/exp)
AND ('kidney disease'/exp OR 'kidney function'/exp OR (kidney NEAR/2 (disease* OR injur* OR failure*)):ab,ti OR nephropath*:ab,ti OR (renal NEAR/2 (insufficienc* OR function* OR disease* OR failure*)):ab,ti)
OR ('contrast induced nephropathy'/exp/dm_pc OR ((contrast* OR ci) NEAR/2 (nephropath* OR 'kidney injury' OR aki OR nephrotoxicity)):ab,ti OR cin:ab,ti OR ciaki:ab,ti
AND (hydrat*:ab,ti OR prehydrat*:ab,ti OR posthydrat*:ab,ti OR rehydrat*:ab,ti OR 'volume expansion':ab,ti OR (pre NEAR/1 hydrat*):ab,ti OR (post NEAR/1 hydrat*):ab,ti OR ((oral OR iv OR intravenous) NEAR/1 (hydrat* OR fluid*)):ab,ti OR (sodium NEAR/2 (chloride* OR bicarbonate)):ab,ti OR nacl:ab,ti OR ((heart OR cardiac) NEAR/2 catheterization):ab,ti OR water:ab,ti OR d5w:ab,ti OR (ringer* NEAR/3 (lactate OR solution*)):ab,ti OR ((hypotonic OR isotonic) NEAR/3 solution*):ab,ti OR (hydroxyethy* NEAR/3 starch*):ab,ti OR 'sodium chloride'/exp OR 'heart catheterization'/exp OR 'bicarbonate'/exp OR 'oral rehydration solution'/exp OR 'hydration'/exp OR 'water'/exp OR 'isotonic solution'/exp OR 'ringer lactate solution'/exp OR 'hetastarch derivative'/exp OR 'fluid balance'/exp))
AND ([dutch]/lim OR [english]/lim) AND [embase]/lim AND [2000-2015]/py
AND ('clinical trial'/exp OR 'randomization'/exp OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp OR 'placebo'/exp OR 'prospective study'/exp OR rct:ab,ti OR random*:ab,ti OR 'single blind':ab,ti OR 'randomised controlled trial':ab,ti OR 'randomized controlled trial'/exp OR placebo*:ab,ti) NOT 'conference abstract':it
NOT 'meta analysis'/de OR cochrane:ab OR embase:ab OR psychlit:ab OR cinahl:ab OR (systematic NEAR/1 (review OR overview)):ab,ti OR (meta NEAR/1 analy*):ab,ti OR metaanalys*:ab,ti OR 'data extraction':ab OR cochrane:jt OR 'systematic review'/de NOT (animal* NOT human*)) (517) – 307 uniek |
Observational studies
|
Database |
Search terms |
Total |
|
Medline (OVID)
Engels,
2007-juni 2015 |
1 exp Contrast Media/ or ((contrast adj3 iodine) or (contrast adj3 medi*)).ti,ab. (110323) 2 Sodium Chloride/ or exp Cardiac Catheterization/ or exp Bicarbonates/ or Rehydration Solutions/ or exp Fluid Therapy/ or (hydrat* or prehydrat* or posthydrat* or rehydrat* or 'volume expansion' or (pre adj1 hydrat*) or (post adj1 hydrat*) or ((oral or iv or intravenous) adj1 (hydrat* or fluid)) or (sodium adj2 (chloride* or bicarbonate*)) or nacl or ((heart or cardiac) adj2 catheterization*)).ti,ab. (263883) 3 exp Kidney Diseases/ or (((kidney or renal) adj2 (disease* or injur* or failure*)) or nephropath* or (renal adj2 (insufficienc* or function* or disease* or failure*))).ti,ab. (527891) 4 1 and 2 and 3 (918) 5 (((contrast* or ci) adj2 (nephropath* or 'kidney injury' or aki or nephrotoxicity)) or cin or ciaki).ti,ab. (8912) 6 Sodium Chloride/ or exp Cardiac Catheterization/ or exp Bicarbonates/ or Rehydration Solutions/ or exp Fluid Therapy/ or (hydrat* or prehydrat* or posthydrat* or rehydrat* or 'volume expansion' or (pre adj1 hydrat*) or (post adj1 hydrat*) or ((oral or iv or intravenous) adj1 (hydrat* or fluid*)) or (sodium adj2 (chloride* or bicarbonate*)) or nacl or ((heart or cardiac) adj2 catheterization*)).ti,ab. or Water/ or water.ti,ab. or D5w.ti,ab. or Isotonic Solutions/ or Hypotonic Solutions/ or (ringer* adj3 (lactate or solution*)).ti,ab. or ((hypotonic or isotonic) adj3 solution*).ti,ab. or Hydroxyethyl Starch Derivatives/ or (Hydroxyethy* adj3 starch*).ti,ab. (818303) 7 5 and 6 (733) 8 4 or 7 (1140) 9 limit 8 to (yr="2000 -Current" and (dutch or english)) (818) 10 (meta-analysis/ or meta-analysis as topic/ or (meta adj analy$).tw. or ((systematic* or literature) adj2 review$1).tw. or (systematic adj overview$1).tw. or exp "Review Literature as Topic"/ or cochrane.ab. or cochrane.jw. or embase.ab. or medline.ab. or (psychlit or psyclit).ab. or (cinahl or cinhal).ab. or cancerlit.ab. or ((selection criteria or data extraction).ab. and "review"/)) not (Comment/ or Editorial/ or Letter/ or (animals/ not humans/)) (240088) 11 9 and 10 (72) 12 (exp clinical trial/ or randomized controlled trial/ or exp clinical trials as topic/ or randomized controlled trials as topic/ or Random Allocation/ or Double-Blind Method/ or Single-Blind Method/ or (clinical trial, phase i or clinical trial, phase ii or clinical trial, phase iii or clinical trial, phase iv or controlled clinical trial or randomized controlled trial or multicenter study or clinical trial).pt. or random*.ti,ab. or (clinic* adj trial*).tw. or ((singl* or doubl* or treb* or tripl*) adj (blind$3 or mask$3)).tw. or Placebos/ or placebo*.tw.) not (animals/ not humans/) (1471469) 13 9 and 12 (341) 14 13 not 11 (283) – 265 uniek 17 Epidemiologic studies/ or case control studies/ or exp cohort studies/ or Controlled Before-After Studies/ or Case control.tw. or (cohort adj (study or studies)).tw. or Cohort analy$.tw. or (Follow up adj (study or studies)).tw. or (observational adj (study or studies)).tw. or Longitudinal.tw. or Retrospective.tw. or prospective.tw. or Cross sectional.tw. or Cross-sectional studies/ or historically controlled study/ or interrupted time series analysis/ [Onder exp cohort studies vallen ook longitudinale, prospectieve en retrospectieve studies] (2160769) 22 21 not 19 (134) – vanaf 2007: 105 – 103 uniek –in afzonderlijk document |
103 obs. |