Introductie hypersensitiviteitsreacties
Introduction to Hypersensitivity Reactions to Contrast Media
|
Disclaimer: This narrative review has been written by members of the Guideline Development Group so that non-specialized readers can follow the Modules about Hypersensitivity more easily. It was not part of the actual guideline process with structured literature analyses. |
The increased use of contrast media (CM) may give rise to an increased absolute number of total hypersensitivity reactions (HSR). The relative number of immediate HSR has decreased since the introduction of nonionic, low-osmolar ICM, while the number of non-immediate HSR is on the rise, due to an increased use of iso-osmolar ICM (Rosado Ingelmo, 2016).
Terminology and Definitions (see also 'Definitions of Adverse Drug Reactions')
The following definitions and terminology are based on the standard terminology recommended by the World Allergy Organisation (Cordona, 2020; Demoly, 2014; Johansson, 2004). When dealing with CM, the term allergy should be avoided as much as possible.
Hypersensitivity: Objectively reproducible symptoms or signs, initiated by exposure to a defined stimulus that is tolerated by normal subjects.
Drug Hypersensitivity Reaction (DHR): adverse effects of drugs that clinically resemble allergic reactions (‘pseudo-allergic’). These include adverse reactions that are immune or nonimmune mediated.
Drug Allergy: Hypersensitivity reactions that are associated with an immune mechanism for which evidence can be shown in the form of drug-specific antibodies or activated T lymphocytes.
Immediate (acute, early) hypersensitivity reaction to contrast media: an adverse reaction that occurs within 1 hour of contrast agent injection. Acute reactions can either be allergy-like (IgE-mediated or not) hypersensitivity reactions or chemotoxic responses.
Non-immediate (delayed, late) hypersensitivity reaction to contrast media: an adverse reaction that occurs between 1 hour and 1 week after contrast agent injection.
Figure 1 Schematic of adverse drug reaction types
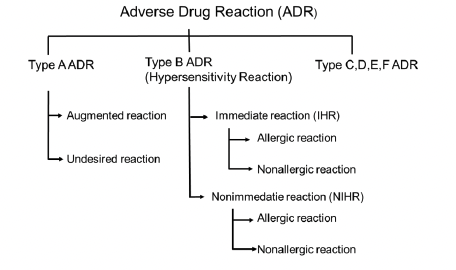
Adverse drug reaction (ADR): a response to a medicine which is noxious and unintended, and which occurs at doses normally used in man (WHO definition) (See Figure 1 Schematic of adverse drug reaction types).
ADR can be classified in multiple types, and for contrast media types A, B and D are most relevant. Type A (augmented) reactions result from an exaggeration of a drug’s normal pharmacological actions when given at the usual therapeutic dose and are normally dose dependent. These include all physiologic reactions. Type B (bizarre) reactions are novel responses that are not expected from the known pharmacological actions of the drug. These are less common, and so may only be discovered for the first time after a drug has already been made available for general use. These include allergic or non-allergic hypersensitivity reactions. Type D, or ‘delayed’ reactions, become apparent sometime after the use of a medicine. The timing of these may make them more difficult to detect. These include Nephrogenic Systemic Fibrosis (NSF) or iodine-induced hyperthyroidism (Edwards, 2000).
Anaphylaxis: Anaphylaxis is a severe, life-threatening systemic hypersensitivity reaction characterized by being rapid in onset with potentially life-threatening airway, breathing, or circulatory problems and is usually, although not always, associated with skin and mucosal changes (Cordona, 2020; WHO ICD-11 definition).
Anaphylaxis is highly likely when any one of the following 2 criteria are fulfilled (Cordona, 2020):
1. Acute onset of an illness (minutes to several hours) with simultaneous involvement of the skin, mucosal tissue, or both (e.g., generalized hives, pruritus or flushing, swollen lips-tongue-uvula)
And at least one of the following:
- Respiratory compromise (e.g., dyspnoea, wheezing/bronchospasm, stridor, reduced PEF, hypoxemia)
- Reduced blood pressure or associated symptoms of end-organ dysfunction (e.g., hypotonia [collapse], syncope, incontinence)
- Severe gastrointestinal symptoms (e.g., severe crampy abdominal pain, repetitive vomiting), especially after exposure to non-food allergens
2. Acute onset of hypotensiona or bronchospasmb or laryngeal involvementc after exposure to a known or highly probable allergend for that patient (minutes to several hours), even in the absence of typical skin involvement.
Note: a hypotension defined as a decrease in systolic BP greater than 30% from that person's baseline, or a systolic BP less than <90 mmHg. b. Excluding lower respiratory symptoms triggered by common inhalant allergens or food allergens perceived to cause “inhalational” reactions in the absence of ingestion. c. Laryngeal symptoms include stridor, vocal changes, odynophagia. d. An allergen is a substance (usually a protein) capable of triggering an immune response that can result in an allergic reaction. Most allergens act through an IgE-mediated pathway, but some non-allergen triggers can act independent of IgE (for example, via direct activation of mast cells).
Immediate hypersensitivity reactions to contrast media
Pathophysiology
Hypersensitivity reactions to CM are poorly understood. Recent research suggests that hypersensitivity reactions to nonionic CM are a heterogeneous disease. It can develop from multiple mechanisms such as IgE-dependent, complement dependent, direct membrane effects of CM, and possibly other mechanisms that have not been identified yet (Zhai, 2017). When an allergic drug reaction is suspected, DHR is the preferred term, because true drug allergy and nonallergic DHR may be difficult to differentiate based on the clinical presentation alone, especially in cases of acute severe DHR (Demoly, 2014).
Allergy-like hypersensitivity reactions may or may not be truly IgE-mediated. In general, allergy can be either antibody- or cell-mediated. Cell-mediated reactions usually occur after one or several days, while antibody-mediated reactions tend to be more immediate. A well-known reason for immediate reactions is the presence of antigen specific IgE antibodies attached to the surface of mast cells and basophil granulocytes. After cross-linking of IgE antibodies on the surface of these cells, a degranulation process follows, resulting in production of histamine and many other mediator substances. Other stimuli can also cause degranulation such as the degree of ionization, osmolality, and temperature of the injected solution. Some drugs such as fluoroquinolones are known to cause histamine release without the presence of specific IgE, via non-IgE-dependent activation routes of the mast cell (McNeil, 2015).
Compared to reactions to iodine-based CM, reactions to gadolinium-based CA are more frequently IgE-mediated, and thus true allergic reactions (Clement, 2018).
Remember: Not all symptoms experienced by patients in the hour after contrast agent injections are adverse reactions to the contrast agent. Patient anxiety may cause symptoms after contrast agent administration, known as the Lalli effect (Lalli, 1974).
Clinical features and risk factors
The same acute adverse reactions are seen after intravascular administration of iodine-based contrast media and after gadolinium-based contrast agents or ultrasound contrast agents.
The term adverse drug reaction (ADR) is wider than hypersensitivity reactions, and includes several chemotoxic effects of CM injection (ADR type A), such as a feeling of warmth, dry mouth, or mild pain during injection, etc. Therefore, incidence figures between studies on hypersensitivity reactions and studies on ADR (for example post-marketing surveillance studies) can vary.
In Radiology, hypersensitivity reactions are usually discriminated into mild, moderate, or severe reactions as outlined below. It must be realized that in Allergology other classifications are used, discriminating reactions as allergic, non-allergic, or type A adverse reactions (see Figure 1 Schematic of adverse drug reaction types and Torres, 2021).
The chance that a reaction can be classified as allergic is lower when the reaction is mild or moderate. It is important to note that re-exposure to CM after an initial mild reaction never causes a moderate or severe reaction (Lee, 2017; Davenport, 2009).
Mild reactions include allergy-like hypersensitivity reactions such as scattered urticaria/pruritus, limited cutaneous oedema, itchy/scratchy throat, nasal congestion, and sneezing/conjunctivitis/ rhinorrhoea. This category also includes physiologic reactions such as limited nausea/vomiting, transient flushing/warmth/chills, headache/dizziness/anxiety, altered taste, mild hypertension or spontaneously resolving vasovagal reactions (ACR, 2022; ESUR, 2018; Wang, 2008).
Moderate reactions include allergy-like reactions such as diffuse urticaria/pruritus, diffuse erythema with stable vital signs, facial oedema without dyspnoea, throat tightness/hoarseness without dyspnoea, and mild wheezing/bronchospasm. Physiologic reactions include protracted nausea/vomitus, hypertensive urgency, isolated chest pain, and vasovagal reactions responsive to treatment (ACR, 2022; ESUR, 2018; Wang, 2008).
Severe reactions include allergy-like reactions such as diffuse erythema with hypotension, diffuse/facial oedema with dyspnoea, laryngeal oedema with stridor, and severe wheezing/ bronchospasm with hypoxia, and generalized anaphylactic reaction/shock. Severe physiologic reactions include treatment-resistant vasovagal reactions, arrhythmia, hypertensive emergencies, and convulsions/seizures. Also, to this category belong pulmonary oedema and cardiopulmonary arrest (ACR, 2022; ESUR, 2018; Wang, 2008).
Risk factors
Risk factor analysis is often done by retrospective observational studies without control groups (see also chapter chapter 3.5.3 Risk Factors for Hypersensitivity Reactions to Contrast Media). Risk factors for hypersensitivity are not fully established. Additional risk factors for immediate HSR that are common to allergic drug reactions include poorly controlled bronchial asthma, concomitant medications (e.g., ACE inhibitors, ß-blockers, and proton pump inhibitors), rapid administration of the drug, mastocytosis, autoimmune diseases, and viral infections (Rosado Ingelmo, 2016).
In Radiology literature, the most consistently reported risk factors for hypersensitivity reactions to CM are (ACR, 2022):
1. A prior hypersensitivity reaction to contrast media.
2. A history of allergy, particularly multiple severe allergies (atopy).
3. A history of asthma requiring treatment.
Female gender could not be substantiated as an independent risk factor for hypersensitivity reactions, but age may be relevant (Endrikat, 2022).
Incidence of acute hypersensitivity reactions
Incidence after iodine-based contrast media
The incidence is highest after iodine-based contrast media and lowest after ultrasound contrast agents. The incidence of acute adverse reactions has declined considerably after the introduction of low-osmolar and iso-osmolar iodine-based contrast media (ACR, 2022; ESUR, 2018).
In the early days of low-osmolar media, the classic Japanese study (Katayama, 1990) reported relatively high adverse drug reaction rates after nonionic CM of up to 3,1%, with severe and very severe reactions occurring in 0,44%. In contrast, more recent studies with large patient cohorts focusing more specifically on hypersensitivity (allergic-like) reactions have shown considerably lower incidence rates of 0,15 to 0,69% with severe reactions occurring in 0,005 to 0,013% (Hunt, 2009; Mortele, 2005; Wang, 2008).
Hypersensitivity reactions after non-vascular CM administration (either oral, rectal, intraductal, intravesical or intra-articular) are rare (see also the overview in Safe Use of Contrast Media, part 2). Such reactions occur slower, and the incidence is much lower than after intravascular administration and will be influenced by the integrity and condition of the wall of the cavity into which the contrast agent is administered (for example inflamed mucosa may lead to leakage into the intravascular compartment). Nevertheless, severe reactions can occur, even with non-vascular CM administration (Davis, 2015).
Incidence using specific iodinated contrast media
Large post-marketing surveillance studies of iobitridol and iodixanol have shown acute adverse events of 0,58-0,59% with severe events in 0,004 to 0,010% (Maurer, 2011; Zhang, 2014). A third study using iopromide is more difficult to compare due to different definitions, and had higher rates of 2,49% and 0,034%, respectively (Palkowitsch, 2014). It must be noted that physiologic reactions (feeling of warmth, metallic taste) make up a considerable part of these events.
More recently, the hypersensitivity reaction rate after iopromide was 0,74% in adults and 0,38% in elderly (Endrikat, 2022). In the same study population, the hypersensitivity reaction rate was 0.7% after intravenous administration vs. 0.2% after intra-arterial administration (Endrikat, 2020).
In addition, several retrospective observational studies have looked at differences in acute hypersensitivity rates among iodine-based CM. Although imperfect, these studies indicate a somewhat higher rate for iopromide and iomeprol compared to other CM (An, 2019; Gomi, 2010; Kim, 2017; Seong, 2014). It remains controversial whether iobitridol has a lower percentage, as indicated in one study (Kim, 2017).
Incidence after gadolinium-based contrast agents
Recent studies in large adult patient cohorts focusing on hypersensitivity (allergic-like) reactions have shown low incidence rates of 0,06-0,17% with severe reactions occurring in 0,003-0,006% (Aran, 2015; Behzadi, 2018; Dillman, 2007; Prince, 2011). More recent studies showed overall rates of 0,15-0,40%. For severe reactions rates were 0,002-0,004% in general populations and 0,033% in a population undergoing cardiac MRI (Ahn, 2022; McDonald, 2019; Uhlig, 2019).
In a large meta-analysis, the overall rate was 92 per 100,000 gadolinium-based contrast agent (GBCA) injections (0,09%) with severe reactions occurring in 5,2 per 100,000 injections (0,005%). It was shown that the type of GBCA is of influence on the number of reactions. Linear nonionic GBCA had an incidence of 15 per 100,000 and linear ionic GBCA of 52 per 100,000. However, these GBCA are no longer available in Europe. The macrocyclic GBCA had slightly higher rates, macrocyclic ionic GBCA 90 per 100,000 and macrocyclic nonionic GBCA 160 per 100,000. The highest rate was for linear ionic GBCA with protein-binding, 170 per 100,000 injections (Behzadi, 2018).
Comparing specific GBCA, in one study more hypersensitivity reactions occurred after gadobenate and gadobutrol compared with gadodiamide or gadoterate injection (McDonald, 2019), while in another study most acute reactions occurred with gadoteridol and most delayed reactions with gadoterate (Ahn, 2022).
Breakthrough, protracted and biphasic hypersensitivity reactions
So-called “breakthrough” hypersensitivity reactions are recurring reactions despite premedication with corticosteroids and H1-antihistamines. The occurrence in published series is variable, 2 to 17%. These reactions are most often of similar severity as the original (culprit) reaction for which premedication was prescribed. Breakthrough reactions can be severe in incidental cases. Unfortunately, no data on the number of IgE-mediated reactions are available (Davenport, 2009; Mervak, 2015).
While most hypersensitivity reactions to CM are uniphasic, other patterns may also occur. A protracted reaction is defined as a reaction lasting > 5h in which symptoms incompletely resolve. This pattern is rare following CM, occurring in only 4% of anaphylactic (severe) reactions and may be predicted by a low responsiveness to initial adrenaline therapy (Kim, 2018).
A biphasic reaction is defined as a reaction recurring 0 to 72h after an initial hypersensitivity reaction. The median time for start of the second reaction is 8 to 12h after the first reaction. This pattern is also rare, occurring in 10% of anaphylactic (severe) reactions (Rohacek, 2014). Usually, the second reaction is of similar severity or milder than the initial reaction. Predictors for biphasic anaphylaxis are severe initial symptoms requiring adrenaline redosing or a long (> 40 min) duration of the initial reaction. An observation time of 6-12h after the initial anaphylactic reaction has resolved is practical (Lee, 2016; Kim, 2018 and 2019). The use of corticosteroids in this setting is controversial and is not recommended (Gabrielli 2019; Lee, 2016; Simons, 2015).
For ultrasound contrast agents the risk is low, but no large series have been published to date. Most adverse reactions are cardiovascular, and the incidence of hypersensitivity reactions is 0,009% with severe reactions occurring in 0,004% (Khawaja, 2010).
Classification
Historically, hypersensitivity reactions to CM have been graded as mild, moderate, or severe. This radiological classification shows overlap with other used classifications, such as the World Allergy Organisation (WAO) classification (Johansson, 2004) and modifications of the Ring - Messmer classification of allergic reactions (Ring, 1977; Table 1 Severity grading of anaphylactic reactions (modified Ring and Messmer)).
Table 1 Severity grading of anaphylactic reactions (modified Ring and Messmer)
|
Grade |
Skin |
Abdomen |
Airways |
Cardiovascular |
|
I |
Itch Flush Urticaria Angioedema |
- |
- |
- |
|
II |
Itch Flush Urticaria Angioedema |
Nausea Cramps |
Rhinorrhoea Hoarseness Dyspnoea |
Tachycardia (> 20 bpm) Hypertension (>20 mm Hg) Arrhythmia |
|
III |
Itch Flush Urticaria Angioedema |
Vomiting Defecation |
Laryngeal oedema Bronchospasm Cyanosis |
Shock |
|
IV |
Itch Flush Urticaria Angioedema |
Vomiting Defecation |
Respiratory arrest |
Cardiac arrest |
Classification according to the most severe symptom, no symptom is mandatory
A practical summary classification of acute hypersensitivity reactions to contrast media for radiological practices may be (free after ACR, 2022; ESUR, 2018):
Mild: Itching, sneezing, flushing, conjunctivitis, rhinorrhoea, epiphora, nausea, short-duration, or incidental vomiting, altered taste, limited (localized) scattered urticaria.
Moderate: Generalized or extensive urticaria, diffuse erythema without hypotension, facial or angioedema without dyspnoea, mild wheezing/bronchospasm, protracted vomiting, mild isolated hypotension.
Severe: Severe wheezing/bronchospasm, profound hypotension, pulmonary oedema, generalized anaphylactic reaction, seizures/convulsions, respiratory arrest, and cardiac arrest.
It is important to note that re-exposure to CM after an initial mild reaction never causes a moderate or severe reaction (Lee, 2017; Davenport, 2009). In addition to this, the risk of an IgE-mediated allergic reaction (and thus the risk of severe reactions in case of re-exposure) is low in moderate reactions without cutaneous symptoms. Therefore, in the classification most used in allergology only reactions with cutaneous symptoms (urticaria or angioedema) are classified as allergic-like (Torres, 2021).
Nonimmediate (late, delayed) hypersensitivity reactions to Contrast Media
Clinical features
A nonimmediate hypersensitivity reaction (NIHR) is a delayed hypersensitivity reaction > 1h after contrast administration (usually > 24h). NIHR usually presents as a maculopapular exanthema (MPE): skin rash consisting of patches (maculae) and nodules (papulae) spread over body and extremities. It normally heals within days to weeks, and if treatment is required, topical or oral steroids can be applied.
Many patients show a variety of nonspecific symptoms, which include headache, nausea, dizziness, gastro-intestinal upset, mild fever, and arm pain (Bellin, 2011; Christiansen, 2000). When compared to control populations (Loh, 2010), skin rashes with erythema and swelling are the most frequent true nonimmediate hypersensitivity reactions. Most patients present with cutaneous symptoms like other drug-induced skin eruptions, usually in the form of a macular or maculopapular exanthema. The exanthema usually occurs 2 to 10 days after first exposure to ICM and 1 to 2 days after re-exposure to the same ICM. Most reactions are mild to moderate in severity, are usually self-limiting and resolve within 1 week (Bellin, 2011).
Discrimination should be made between mild-to-moderate NIHR and rare severe NIHR with danger signs, the so-called severe cutaneous adverse drug reactions (SCAR), such as drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, toxic epidermal necrolysis (TEN), acute generalized exanthemic pustulosis (AGEP), and Stevens-Johnson syndrome (SJS) (Brockow, 2019; Soria, 2021).
Pathophysiology
There is evidence that drug-specific T-cells play an important role in nonimmediate hypersensitivity reactions. In skin reactions an infiltrate in the dermis consisting of activated CD4+ or CD8+ T-cells and eosinophils is usually found (Christiansen, 2000 and 2003; Schönmann, 2020).
In vitro studies have shown two different pathways of CM recognition which both require major histocompatibility complex (MHC) molecules for stimulation: a) direct binding of CM to the T-cell receptor or MHC molecule (p-i concept), and b) after uptake and processing by antigen-presenting cells and presented to T-cells via MHC-II molecules ((pro)hapten concept) (Keller, 2009).
The hapten-independent pathway could explain results of cross-reactivity analyses that revealed that CM-specific activated T-cell clones reacted to CM with shared structural elements.
It has been postulated that CM do not induce a primary immune response, but instead interact with receptors on activated memory T-cells raised against other foreign substances (non-allergic NIHR). Patients with nonimmediate hypersensitivity should not be at risk for an immediate hypersensitivity reaction (mediated by IgE or other mechanisms) upon re-exposure to CM.
Risk factors
Established risk factors for nonimmediate hypersensitivity reactions to iodine-based CM include a previous hypersensitivity reaction and IL-2 immunotherapy. Most CM-associated nonallergic NIHR are associated with iso-osmolar CM (ACR, 2022; Bellin, 2011; ESUR, 2018).
Patients with a history of nonimmediate hypersensitivity reactions to ICM are not at increased risk for immediate HSR to ICM as these reactions are mechanistically unrelated (Christiansen, 2003; Mazori, 2018).
Incidence of nonimmediate hypersensitivity reactions
The frequency of nonimmediate hypersensitivity reactions to CM varies greatly between studies and is believed to be between 1-3% of patients after iodine-based CM administration and only very rarely after gadolinium-based CA administration (Bellin, 2011; Christiansen, 2000).
Incidence using specific iodine-based CM
Nonimmediate skin reactions tend to be more common after iodixanol (Benin, 2011; Sutton, 2003). The incidence of nonimmediate hypersensitivity reactions is not significantly different for the other iodine-based low-osmolar CM (Bellin, 2011).
Cross-reactivity between contrast media
Cross-reactivity between iodine-based CM
Most of the current cross-reactivity data come from skin testing. Cross-reactivity in late hypersensitivity reactions is probably caused by the presence of CM-specific T-cells, some of which may show a broad cross-reactivity pattern. There may be a link between the chemical structure of iodine-based CM and the pattern of cross-reactivity, but results are inconsistent.
Several studies have shown considerable cross-reactivity between different iodine-based CM, but specific data on immediate versus nonimmediate hypersensitivity reactions are lacking until now. In the larger studies, most cross-reactivity has been seen between the nonionic dimer iodixanol and its monomer iohexol, with relatively fewer positive skin reactions with iobitridol (Clement, 2018; Hasdenteufel, 2011; Lerondeau, 2016; Yoon, 2015).
Based on cross-reactivity patterns iodine-based CM may be divided in three groups, with relatively high intra-group cross-reactivity but less intergroup cross-reactivity (Lerondeau, 2016). Based on additional data, it seems reasonable to add iopromide to group A as well and possibly remove ioxithalamate and iopamidol (Schrijvers, 2018).
Table 2 Cross-reactivity grouping of iodine-based CM (Lerondeau, 2016) may be helpful for selecting an alternative agent for imaging studies.
Table 2 Cross-reactivity grouping of iodine-based CM (Lerondeau, 2016)
|
Group A |
Group B |
Group C |
|
Ioxithalamate (Telebrix) |
Iobitridol (Xenetix) |
Amidotrizoate (Gastrografin) |
|
Iopamidol (Iopamiro) |
Ioxaglate (Hexabrix) |
|
|
Iodixanol (Visipaque) |
||
|
Iohexol (Omnipaque) |
||
|
Ioversol (Optiray) |
||
|
Iomeprol (Iomeron) |
||
|
Iopromide (Ultravist) |
Note: Iopamidol and Ioxaglate are no longer available on the market in The Netherlands
Cross-reactivity between gadolinium-based CM
Information on cross-reactivity between GBCA is limited to case reports. Skin testing and provocation tests in such cases have shown that cross-reactivity among macrocyclic GBCA may be more extensive than among linear GBCA (Gallardo Higueras, 2021; Grüber, 2021).
Cross-reactivity between iodine-based and gadolinium-based CM
A recent study examined the risk of reactions to both iodine-based CM and gadolinium-based CA in the same patient in a large patient cohort. The incidence of primary hypersensitivity reactions was 0,047% and the incidence of secondary reactions 0,024%. Nearly all reactions were mild, requiring no treatment. Therefore, cross-reactivity between iodine-based and gadolinium-based CM is an extremely rare event (Sodagari, 2018).
Literature
Ahn YH, Kang DY, Park SB, Kim HH, Kim HJ, Park GY, et al. Allergic-like hypersensitivity reactions to Gadolinium-Based Contrast Agents: an 8-year cohort study of 154 539 patients. Radiology 2022; 303(2): 329-336.
American College of Radiology. ACR Manual on contrast media, v2022. Available at: [URL]. Accessed: 22. May 2022.
Guideline Safe Use of Contrast Media part 3 Guideline for Authorization Phase November 2022 116
An J, Jung H, Kwon OY, Kang Y, Lee JH, Won HK, et al. Differences in adverse reactions among iodinated contrast media: analysis of the KAERS database. J Allergy Clin Immunol Pract 2019; 7: 2205-2211.
Aran S, Shaqdan KW, Abujudeh HH. Adverse allergic reactions to linear ionic gadolinium-based contrast agents: experience with 194,400 injections. Clin Radiol 2015; 70; 466-475.
Behzadi AH, Zhao Y, Farooq Z, Prince MR. Immediate allergic reactions to gadolinium-based contrast agents: systematic review and meta-analysis. Radiology 2018; 286: 471-482.
Bellin MF, Stacul F, Webb JAW, Thomsen HS, Morcos SK, Almén T, et al. Late adverse reactions to iodine-based contrast media: an update. Eur Radiol 2011; 21: 2305-2310.
Brockow K, Ardern-Jones MR, Mockenhaupt M, Aberer W, Barbaud A, Caubet JC, et al. EAACI position paper on how to classify cutaneous manifestations of drug hypersensitivity. Allergy 2019; 74: 14-27.
Cardona V, Ansotegui IJ, Ebisawa M, El-Gamal Y, Fernandez Rivas M, Fineman S, et al. World allergy organization anaphylaxis guidance 2020. World Allergy Organ J. 2020; 13(10): 100472.
Christiansen C, Pichler WJ, Skotland T. Delayed allergy-like reactions to X-ray contrast media: mechanistic considerations. Eur Radiol 2000; 10: 1965-1975.
Christiansen C. Current knowledge regarding the pathophysiology of the late adverse reactions to X-ray contrast media. GE Healthcare, 2003.
Clement O, Dewachter P, Mouton-Faivre C, Nevoret C, Guilloux L, Bloch Morot E, et al. Immediate hypersensitivity to contrast agents: The French 5-year CIRTACI study. EClinicalMedicine. 2018; 1: 51-61.
European Society of Urogenital Radiology Contrast Media Safety Committee. ESUR Guidelines on contrast safety, v10, 2018. Available at: [URL]. Accessed: 22. May 2022.
Davenport MS, Cohan RH, Caoili EM, Ellis JH. Repeat contrast medium reactions in premedicated patients: frequency and severity. Radiology 2009; 253: 372-379.
Davis L. Anaphylactoid reactions to the nonvascular administration of water-soluble iodinated contrast media. AJR Am J Roentgenol 2015; 2014: 1140-1145.
Demoly P, Adkinson NF, Brockow K, Castells M, Chiriac AM, Greenberger PA, et al. International Consensus on drug allergy. Allergy 2014; 69: 420-437.
Dillman JR, Ellis JH, Cohan RH, Strouse PJ Jan SC. Frequency and severity of acute allergic-like reactions to gadolinium-containing i.v. contrast media in children and adults. AJR Am J Roentgenol 2007; 189(6): 1533-1538.
Edwards IR, Aronson JK. Adverse drug reactions: definitions, diagnosis, and management. Lancet 2000; 356(9237): 1255-1259.
Endrikat J, Michel A, Kölbach R, Lengsfeld P, Vogtländer K. Risk of hypersensitivity reactions to iopromide after intra-arterial versus intravenous administration: a nested case-control analysis of 133,331 patients. Invest Radiol 2020; 55: 38-44.
Endrikat J, Chernova J, Gerlinger C, Pracz M, Lengsfeld P, Bhatti A, Michel A. Risk of hypersensitivity reactions to iopromide in children and elderly: an analysis of 132,850 patients from 4 observational studies and pharmacovigilance covering >288 million administrations. Invest Radiol 2022; 57(5): 318-326.
Gabrielli S, Clarke A, Morris J, Eisman H, Gravel J, Enarson P, et al. Evaluation of prehospital management in a Canadian emergency department anaphylaxis cohort. J Allergy Clin Immunol Pract 2019; 7: 2232-2238.e3.
Gallardo-Higueras A, Moreno EM, Muñoz-Bellido FJ, Laffond E, Gracia-Bara MT, Macias EM, et al. Patterns of cross-reactivity in patients with immediate hypersensitivity reactions to gadobutrol. J Investig Allergol Clin Immunol. 2021; 31: 504-506.
Gomi T, Nagamoto M, Hasegawa M, Katoh A, Sugiyama M, Murata N, et al. Are there any differences in acute adverse reactions among five nonionic iodinated contrast media? Eur Radiol 2010; 20: 1631-1635.
Grüber HP, Helbling A, Jörg L. Skin test results and cross-reactivity patterns in IgE- and T-cell-mediated allergy to gadolinium-based contrast agents. Allergy Asthma Immunol Res 2021; 13: 933-938.
Hasdenteufel F, Waton J, Cordebar V, Studer M, Collignon O, Luyasu S, et al. Delayed hypersensitivity reactions caused by iodixanol: an assessment of cross-reactivity in 22 patients (letter). J Allergy Clin Immunol 2011; 128: 1356-1357.
Hunt CH, Kallmes DF, Thielen KR. Frequency and severity of adverse effects of iodinated and gadolinium contrast materials: retrospective review of 456,930 doses. AJR Am J Roentgenol 2009; 193: 1124-1127.
Johansson SG, Bieber T, Dahl R, Friedmann PS, Lanier BQ, Lockey RF, et al. Revised nomenclature for allergy for global use: Report of the Nomenclature Review Committee of the World Allergy Organisation, October 2003. J Allergy Clin Immunol 2004; 113: 832-836.
Katayama H, Yamguchi K, Kozuka T, Takashima T, Seez P, Matsuura K. Adverse reactions to ionic and nonionic contrast media. A report from the Japanese committee on the safety of contrast media. Radiology 1990; 175: 621-628.
Keller M, Lerch M, Britschi M, Tache V, Gerber BO, Luthi M, et al. Processing-dependent and -independent pathways for recognition of iodinated contrast media by specific human T-cells. Clin Exp Allergy 2009; 40: 257-268.
Khawaja OA, Sheikh KA, Al-Mallah MH. Meta-analysis of adverse cardiovascular events associated with echocardiographic contrast agents. Am J Cardiol 2010; 106: 742-747.
Kim SR, Lee JH, Park KH, Park HJ, Park JW. Varied incidence of immediate adverse reactions to low-osmolar non-ionic iodide radiocontrast media used in computed tomography. Clin Exp Allergy 2017; 47: 106-112.
Kim TH, Yoon SH, Lee SY, Choi YH, Park CM, Kang HR, Cho SH. Biphasic and protracted anaphylaxis to iodinated contrast media. Eur Radiol 2018; 28: 1242-1252.
Kim TH, Yoon SH, Hong H, Kang HR, Cho SH, Lee SY. Duration of observation for detecting a biphasic reaction in anaphylaxis: a meta-analysis. Int Arch Allergy Immunol 2019; 179: 31-36.
Lalli AF. Urographic contrast media reactions and anxiety. Radiology 1974; 112: 267-271.
Lee S, Sadosty AT, Campbell RL. Update on biphasic anaphylaxis. Curr Opin Allergy Clin Immunol 2016; 16: 346-351.
Lee SY, Yang MS, Choi YH, Park CM, Park HW, Cho SH, Kang HR. Stratified premedication strategy for the prevention of contrast media hypersensitivity in high-risk patients. Ann Allergy Asthma Immunol. 2017; 118(3): 339-344.e1.
Lerondeau B, Trechot P, Waton J, Poreaux C, Luc A, Schmutz JL, et al. Analysis of cross-reactivity among radiocontrast media in 97 hypersensitivity reactions (letter). J Allergy Clin Immunol 2016; 137: 633-635.
Loh S, Bagheri S, Katzberg RW, Fung MA, Li CS. Delayed adverse reaction to contrast-enhanced CT: a prospective single-center study comparison to control group without enhancement. Radiology 2010; 255:764–771.
Maurer M, Heine O, Wolf M, Freyhardt P, Schnapauff D, Hamm B. Safety and tolerability of iobitridol in general and in patients with risk factors: results in more than 160,000 patients. Eur J Radiol 2011; 80: 357-362.
Mazori DR, Nagler AR, Pomerantz MK. Delayed cutaneous reactions to iodinated contrast. Cutis 2018; 101: 433-435.
McDonald JS, Hunt CH, Kolbe AB, Schmitz JJ, Hartman RP, et al. Acute adverse events following Gadolinium-Based Contrast Agent administration: a single-center retrospective study of 281,945 injections. Radiology 2019; 292: 620-627.
McNeil BD, Pundir P, Meeker S, Han L, Undem BJ, Kulka M, Dong X. Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions. Nature. 2015; 519(7542): 237-241.
Mervak BM, Davenport MS, Ellis JH, et al. Rates of breakthrough reactions in inpatients at high risk receiving premedication before contrast-enhanced CT. AJR Am J Roentgenol. 2015; 205: 77-84.
Mortele KJ, Oliva MR, Ondategui S, Ros PR, Silverman SG. Universal use of nonionic iodinated contrast medium for CT: evaluation of safety in a large urban teaching hospital. AJR Am J Roentgenol 2005; 184:31-34.
Palkowitsch PK, Bostelmann S, Lengsfeld P. Safety and tolerability of iopromide intravascular use: a pooled analysis of three non-interventional studies in 132,012 patients. Acta Radiol 2014, 55: 707-714.
Prince MR, Zhang H, Zou Z, Staron RB and Brill PW. Incidence of immediate gadolinium contrast media reactions. AJR Am J Roentgenol 2011; 196: W138-W143.
Ring J, Messmer K. Incidence and severity of anaphylactoid reactions to colloid volume substitutes. Lancet 1977; 1(8009): 466-469.
Rohacek M, Edenhofer H, Bircher A, Bingisser R. Biphasic anaphylactic reactions: occurrence and mortality. Allergy 2014; 69: 791–797.
Rosado Ingelmo A, Doña Diaz I, Cabañas Moreno R, et al. Clinical Practice Guidelines for Diagnosis and Management of Hypersensitivity Reactions to Contrast Media. J Investig Allergol Clin Immunol 2016; 26(3): 144-155.
Schönmann C, Brockow K. Adverse reactions during procedures: Hypersensitivity to contrast agents and dyes. Ann Allergy Asthma Immunol 2020; 124: 156-164.
Schrijvers R, Breynaert C, Ahmedali Y, Bourrain JL, Demoly P, Chiriac AM. Skin testing for suspected iodinated contrast media hypersensitivity. J Allergy Clin Immunol Pract 2018; 6: 1246-1254.
Seong JM, Choi NK, Lee J, Chang Y, Kim YJ, Yang BR, et al. Comparison of the safety of seven iodinated contrast media. J Korean Med Sci 2013; 28: 1703-1710.
Simons FE, Ebisawa M, Sanchez-Borges M, Thong BY, Worm M, Tanno LK, et al. 2015 update of the evidence base: World Allergy Organisation anaphylaxis guidelines. World Allergy Organ J 2015; 8: 32.
Sodagari F, Mozaffary A, Wood III CG, Schmitz B, Miller FH, Yaghmai V. Reactions to both nonionic iodinated and gadolinium-based contrast media: incidence and clinical characteristics. AJR Am J Roentgenol 2018; 210: 1-5.
Soria A, Amsler E, Bernier C, Milpied B, Tétart F, Morice C, et al.; FISARD group. DRESS and AGEP Reactions to Iodinated Contrast Media: A French Case Series. J Allergy Clin Immunol Pract 2021; 9: 3041-3050.
Sutton AGC, Finn P, Campbell PG, et al. Early and late reactions following the use of iopamidol 340, iomeprol 350 and iodixanol 320 in cardiac catheterization. J Invasive Cardiol 2003; 15: 133-138.
Torres MJ, Trautmann A, Böhm I, Scherer K, Barbaud A, Bavbek S, et al. Practice parameters for diagnosing and managing iodinated contrast media hypersensitivity. Allergy 2021; 76: 1325-1339.
Uhlig J, Lücke C, Vliegenthart R, Loewe C, Grothoff M, Schuster A, et al.; ESCR MRCT Registry contributors. Acute adverse events in cardiac MR imaging with gadolinium-based contrast agents: results from the European Society of Cardiovascular Radiology (ESCR) MRCT Registry in 72,839 patients. Eur Radiol. 2019; 29(7): 3686-3695.
Wang CL, Cohan RL, Ellis JH, Caoli EM, Wang G, Francis IR. Frequency, outcome, and appropriateness of treatment of nonionic iodinated contrast media reactions. AJR Am J Roentgenol 2008; 191: 409-415.
Yoon SH, Lee SY, Kang HR, Kim JY, Hahn S, Park CM, et al. Skin tests in patients with hypersensitivity reaction to iodinated contrast media: a meta-analysis. Allergy. 2015; 70(6): 625-637.
Zhai L, Guo X, Zhang H, Jin Q, Zeng Q, Tang X and Gao C. Nonionic iodinated contrast media related immediate reactions; A mechanism study of 27 patients. Leg Med 2017; 24: 56-62.
Zhang BC, Hou L, Lv B, Xu YW. Post-marketing surveillance study with iodixanol in 20,185 Chinese patients from routine clinical practices. Br J Radiol 2014; 87: 20130325.
Verantwoording
Beoordelingsdatum en geldigheid
Publicatiedatum : 28-11-2022
Beoordeeld op geldigheid : 28-11-2022
Validity
The Radiological Society of the Netherlands (NVvR) will determine around 2027 if this guideline (per module) is still valid and applicable. If necessary, the scientific societies will form a new guideline group to revise the guideline. The validity of a guideline can be shorter than 5 years, if new scientific or healthcare structure developments arise, that could be a reason to commence revisions. The Radiological Society of the Netherlands is the owner of this guideline and thus primarily responsible for the actuality of the guideline. Other scientific societies that have participated in the guideline development share the responsibility to inform the primarily responsible scientific society about relevant developments in their field.
Algemene gegevens
General Information
The Kennisinstituut van de Federatie Medisch Specialisten (www.kennisinstituut.nl) assisted the guideline development group. The guideline was financed by Stichting Kwaliteitsgelden Medisch Specialisten (SKMS) which is a quality fund for medical specialists in The Netherlands.
Samenstelling werkgroep
Guideline development group (GDG)
A multidisciplinary guideline development group (GDG) was formed for the development of the guideline in 2020. The GDG consisted of representatives from all relevant medical specialization fields which were using intravascular contrast administration in their field.
All GDG members have been officially delegated for participation in the GDG by their scientific societies. The GDG has developed a guideline in the period from June 2020 until November 2022. The GDG is responsible for the complete text of this guideline.
Guideline development group
- Dekkers I.A. (Ilona), clinical epidemiologist and radiologist, Leiden University Medical Center, Leiden
- Geenen R.W.F. (Remy), radiologist, Noordwest Ziekenhuisgroep, Alkmaar
- Kerstens M.N. (Michiel), internist-endocrinologist, University Medical Centre Groningen
- Krabbe J.G. (Hans), clinical chemist-endocrinologist, Medisch Spectrum Twente, Enschede
- Rossius M.J.P. (Mariska), radiologist, Erasmus Medical Centre, Rotterdam
- Uyttenboogaart M. (Maarten), neurologist and neuro-interventionalist, University Medical Centre Groningen
- van de Luijtgaarden K.M. (Koen), vascular surgeon, Maasstad Ziekenhuis, Rotterdam
- van der Molen A.J. (Aart), chair guideline development group, radiologist, Leiden University Medical Center, Leiden
- van der Wolk S.L. (Sabine), gynaecologist-obstetrician, Haga Ziekenhuis, Den Haag
- van de Ven A.A.J.M. (Annick), internist-allergologist-immunologist, University Medical Centre Groningen (until 1.7.2022)
- van der Houwen, T.B. (Tim), internist-allergologist-immunologist, Amsterdam University Medical Center (from 1.7.2022)
Invited experts
- van Maaren M.S. (Maurits), internist-allergologist-immunologist, Erasmus MC, Rotterdam
Belangenverklaringen
Conflicts of interest
The GDG members have provided written statements about (financially supported) relations with commercial companies, organisations or institutions that were related to the subject matter of the guideline. Furthermore, inquiries have been made regarding personal financial interests, interests due to personal relationships, interests related to reputation management, interest related to externally financed research and interests related to knowledge valorisation. The statements on conflict of interest can be requested from the administrative office of Kennisinstituut van de Federatie Medisch Specialisten (secretariaat@kennisinstituut.nl) and were summarised below.
|
Last name |
Function |
Other positions |
Personal financial interests |
Personal relations |
Reputation management |
Externally financed research |
Knowledge valorisation |
Other interests |
Signed |
Actions |
|
Dekkers IA |
Radiologist, LUMC |
Clinical Epidemiologist
Member of contrast media safety committee, European Society of Urogenital Radiology (no payment)
Member, Gadolinium Research and Education Committee, European Society of Magnetic Resonance in Medicine, and Biology (no payment) |
No |
No |
No |
No |
No |
Received consultancy fees from Guerbet, 2019- 2022 |
July 24th, 2020, Reaffirmed October 12th, 2022 |
No restrictions: received in part 3 of the guideline speaker fees, but this guideline does not mention specific medication, not of working mechanism, nor of side effects. |
|
Geenen RWF |
Radiologist, Noordwest ziekenhuisgroep /Medisch specialisten Noordwest |
Member of contrast media safety committee, European Society of Urogenital Radiology (no payment) |
No |
No |
No |
No |
No |
No |
April 11th, 2020, Reaffirmed October 12th, 2022 |
No restrictions |
|
Houwen T, van der |
Internist - Immunologist - Allergologist, Amsterdam UMC, also seconded allergologist in Huid Medisch Centrum |
None |
None |
None |
None |
None |
None |
None |
July 11th, 2022 Reaffirmed October 12th, 2022 |
No restrictions |
|
Kerstens MN |
Internist- endocrinologist, UMCG |
Chairman Bijniernet (no payment) |
No |
No |
No |
No |
No |
No |
July 1st, 2020, reaffirmed October 25th, 2022 |
No restrictions |
|
Krabbe JG |
Clinical chemist, Medisch Spectrum Twente |
No |
No |
No |
No |
No |
No |
No |
September 1st, 2020, Reaffirmed October 13th, 2022 |
No restrictions |
|
Luijtgaarden KM, van de |
Vascular surgeon, Maasland Ziekenhuis |
No |
No |
No |
No |
No |
No |
No |
August 1st, 2020, reaffirmed October 26th, 2022 |
No restrictions |
|
Molen AJ, van der |
Radiologist LUMC |
Member of contrast media safety committee, European Society of Urogenital Radiology (no payment)
Member, Gadolinium Research and Education Committee, European Society of Magnetic Resonance in Medicine, and Biology (no payment) |
No |
No |
No |
No |
No |
Received consultancy fees from Guerbet, 2019- 2022 |
July, 24th, 2020 Reaffirmed October 12th, 2022 |
No restrictions: received in part 3 of the guideline speaker fees, but this guideline does not mention Specific medication, not of working mechanism, nor of side effects. |
|
Rossius MJP |
Radiologist Erasmus Medical Centre |
Medical coordinator (no payment) |
No |
No |
No |
No |
No |
No |
April 7th, 2020, Reaffirmed October 13th, 2022 |
No restrictions |
|
Uyttenboogaart M |
Neurologist and neuro- interventionalist UMCG |
Advisor International Federation of Orthopaedic Manipulative Physical Therapist / Nederlandse Vereniging Manuele Therapie |
No |
No |
Subsidy Hart Stichting for CONTRAST (Consortium of New Treatments in Acute Stroke): WP8 Stroke logistics and Epidemiology: financing of 2 PhD students by the Hart Stichting / PPS Allowance |
Work package leader CONTRAST (Consortium of New Treatments in Acute Stroke): WP8 Stroke logistics and Epidemiology |
No |
No |
June 30th, 2020, reaffirmed October 26th, 2022 |
No restrictions: the CONTRAST consortium wp8 is only about organisation and treatment of stroke. Stroke is not in this guideline. |
|
Ven AAJM, van de |
Internist- allergologist- immunologist, UMCG |
Education and research related to work as internist- allergist |
No |
No |
No |
No |
No |
No |
April 7th, 2020, Reaffirmed October 19th, 2022 |
No restrictions |
|
Wolk S, van der |
Gynaecologist- obstetrician, Haga Ziekenhuis |
No |
No |
No |
No |
No |
No |
No |
June 30th, 2021, reaffirmed October 25th, 2022 |
No restrictions |
Inbreng patiëntenperspectief
Input of patient’s perspective
The guideline does not address a specific adult patient group, but a diverse set of diagnoses. Therefore, it was decided to invite a broad spectrum of patient organisations for the stakeholder consultation. The stakeholder consultation was performed at the beginning of the process for feedbacking on the framework of subjects and clinical questions addressed in the guideline, and during the commentary phase to provide feedback on the concept guideline. The list of organisations which were invited for the stakeholder consultation can be requested from the Kennisinstituut van de Federatie Medisch Specialisten (secretariaat@kennisinstituut.nl). In addition, patient information on safe use of contrast media in pregnancy and lactation was developed for Thuisarts.nl, a platform to inform patients about health and disease.
Implementatie
Implementation
During different phases of guideline development, implementation and practical enforceability of the guideline were considered. The factors that could facilitate or hinder the introduction of the guideline in clinical practice have been explicitly considered. The implementation plan can be found in the ‘Appendices to modules’. Furthermore, quality indicators were developed to enhance the implementation of the guideline. The indicators can also be found in the ‘Appendices to modules’.
Werkwijze
Methodology
AGREE
This guideline has been developed conforming to the requirements of the report of Guidelines for Medical Specialists 2.0 by the advisory committee of the Quality Counsel (www.kwaliteitskoepel.nl). This report is based on the AGREE II instrument (Appraisal of Guidelines for Research & Evaluation II) (www.agreetrust.org), a broadly accepted instrument in the international community and based on the national quality standards for guidelines: “Guidelines for guidelines” (www.zorginstituutnederland.nl).
Identification of subject matter
During the initial phase of the guideline development, the GDG identified the relevant subject matter for the guideline. The framework is consisted of both new matters, which were not yet addressed in part 1 and 2 of the guideline, and an update of matters that were subject to modification (for example in case of new published literature). Furthermore, a stakeholder consultation was performed, where input on the framework was requested.
Clinical questions and outcomes
The outcome of the stakeholder consultation was discussed with the GDG, after which definitive clinical questions were formulated. Subsequently, the GDG formulated relevant outcome measures (both beneficial and harmful effects). The GDG rated the outcome measures as critical, important and of limited importance (GRADE method). Furthermore, where applicable, the GDG defined relevant clinical differences.
Search and select
For clinical questions, specific search strategies were formulated, and scientific articles published in several electronic databases were searched. First, the studies that potentially had the highest quality of research were reviewed. The GDG selected literature in pairs (independently of each other) based on the title and abstract. A second selection was performed by the methodological advisor based on full text. The databases used, selection criteria and number of included articles can be found in the modules, the search strategy in the appendix.
Quality assessment of individual studies
Individual studies were systematically assessed, based on methodological quality criteria that were determined prior to the search. For systematic reviews, a combination of the AMSTAR checklist and PRISMA checklist was used. For RCTs the Cochrane risk of bias tool and suggestions by the CLARITY Group at McMaster University were used, and for cohort studies/observational studies the risk of bias tool by the CLARITY Group at McMaster University was used. The risk of bias tables can be found in the separate document Appendices to modules.
Summary of literature
The relevant research findings of all selected articles were shown in evidence tables. The evidence tables can be found in the separate document Appendices to modules. The most important findings in literature were described in literature summaries. When there were enough similarities between studies, the study data were pooled.
Grading quality of evidence and strength of recommendations
The strength of the conclusions of the included studies was determined using the GRADE- method. GRADE stands for Grading Recommendations Assessment, Development and Evaluation (see http://www.gradeworkinggroup.org) (Atkins, 2004). GRADE defines four levels for the quality of scientific evidence: high, moderate, low, or very low. These levels provide information about the certainty level of the literature conclusions (http://www.guidelinedevelopment.org/handbook).
The evidence was summarized in the literature analysis, followed by one or more conclusions, drawn from the body of evidence. The level of evidence for the conclusions can be found above the conclusions. Aspects such as expertise of GDG members, local expertise, patient preferences, costs, availability of facilities and organisation of healthcare aspects are important to consider when formulating a recommendation. These aspects are discussed in the paragraph justifications. The recommendations provide an answer to the clinical question or help to increase awareness and were based on the available scientific evidence and the most relevant justifications.
Appendices
Internal (meant for use by scientific society or its members) quality indicators were developed with the guideline and can be found in the separate document Appendices to modules. In most cases, indicators were not applicable. For most questions, additional scientific research on the subject is warranted. Therefore, the GDG formulated knowledge gaps to aid in future research, which can be found in the separate document Appendices to modules.
Commentary and authorisation phase
The concept guideline was subjected to commentaries by the involved scientific societies. The list of parties that participated in the commentary phase can be requested from the Kennisinstituut van de Federatie Medisch Specialisten (secretariaat@kennisinstituut.nl). The commentaries were collected and discussed with the GDG. The feedback was used to improve the guideline; afterwards the GDG made the guideline definitive. The final version of the guideline was offered to the involved scientific societies for authorization and was authorized.
Literature
Brouwers MC, Kho ME, Browman GP, et al. AGREE Next Steps Consortium. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010; 182(18): E839-E842.
Medisch Specialistische Richtlijnen 2.0. Adviescommissie Richtlijnen van de Raad Kwaliteit, 2012. Available at: [URL].
Schünemann H, Brożek J, Guyatt G, et al. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group, 2013. Available at: [URL].
Schünemann HJ, Oxman AD, Brozek J, et al. Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. BMJ. 2008;336(7653):1106- 1110. Erratum published in: BMJ 2008;336(7654).
Ontwikkeling van Medisch Specialistische Richtlijnen: stappenplan. Kennisinstituut van Medisch Specialisten, 2020.
