Profylactische maatregelen om hypersensitiviteitsreacties na CM te voorkomen
Uitgangsvraag
Welke profylactische maatregelen zouden moeten worden genomen bij patiënten met een verhoogd risico op hypersensitiviteitsreacties na contrastmiddel(CM)-toediening?
Deze vraag bevat de volgende categorieën:
- Patiënten met voorgaande (acute) hypersensitiviteitsreacties na jodiumhoudend CM of gadoliniumhoudend CM
- Patiënten met voorgaande doorbraakreactie na CM
- Patiënten met een voorgaande hypersensitiviteitsreactie na meerdere CM
- Patiënten met een voorgaande niet-acute (vertraagde) hypersensitiviteitsreactie na jodiumhoudend CM of gadoliniumhoudend CM
- Kruisreactiviteit tussen CM
- Documentatie van hypersensitiviteitsreacties
Aanbeveling
Bij alle patiënten met een (gedocumenteerde) geschiedenis van een hypersensitiviteitsreactie op een jodiumhoudend contrastmiddel of een gadoliniumhoudend contrastmiddel, overweeg een alternatieve beeldvormingstechniek. Wanneer dit niet mogelijk is, overweeg om onderzoek zonder contrastmiddel uit te voeren, maar alleen als dit een acceptabele reductie in diagnostische kwaliteit oplevert.
1. Patiënten met voorgaande (acute) hypersensitiviteitsreacties na jodiumhoudend contrastmiddel of gadoliniumhoudend contrastmiddel
Bij patiënten met een (gedocumenteerde) geschiedenis van een milde acute hypersensitiviteitsreactie door jodiumhoudend contrastmiddel of gadoliniumhoudende contrastmiddel:
- Behandel deze patiënten als elke andere patiënt, aangezien er geen risico is op het ontwikkelen van een matige of ernstige overgevoeligheidsreactie.
Bij patiënten met een (gedocumenteerde) geschiedenis van een matige tot ernstige acute overgevoeligheidsreactie door jodiumhoudend contrastmiddel of gadoliniumhoudende contrastmiddel:
- Stel het onderzoek uit en verwijs naar een allergoloog.
Als er geen tijd is om de patiënt naar een allergoloog te verwijzen:
- Kies een ander jodiumhoudend contrastmiddel of gadoliniumhoudend contrastmiddel als het contrastmiddel dat de reactie veroorzaakte bekend is*
- Overweeg om een test te doen door eerst 10% van het contrastmiddel te geven en de patiënt
>15 minuten te observeren: vooral bij ernstige reacties en wanneer het contrastmiddel dat de reactie veroorzaakte onbekend is
- Observeer de patiënt ≥ 30 min met behoud van intraveneuze toegang
- Wees alert op een nieuwe overgevoeligheidsreactie
*Zie ook flow charts
2. Patiënten met voorgaande doorbraakreactie na contrastmiddelen
Verwijs patiënten met een doorbraak overgevoeligheidsreactie op jodiumhoudend contrastmiddel of gadoliniumhoudend contrastmiddel altijd naar een allergoloog voor huidtesten met verschillende jodiumhoudende contrastmiddelen en gadoliniumhoudende contrastmiddelen.
*Zie ook flow charts
3. Patiënten met een voorgaande hypersensitiviteitsreactie na meerdere contrastmiddelen
Verwijs patiënten met een overgevoeligheidsreactie na meerdere jodiumhoudende contrastmiddelen of gadoliniumhoudende contrastmiddelen (ofwel 2 of meer jodiumhoudende contrastmiddelen, ofwel 2 of meer gadoliniumhoudende contrastmiddelen, ofwel een jodiumhoudend contrastmiddel én een gadoliniumhoudend contrastmiddel) altijd naar een allergoloog. Pas daarnaast dezelfde principes als hierboven omschreven toe.
*Zie ook flow charts
4. Patiënten met een voorgaande niet-acute (vertraagde) hypersensitiviteitsreactie na jodiumhoudend contrastmiddel of gadoliniumhoudend contrastmiddel
Bij patiënten met (verdenking op) een eerdere ernstige niet-acute cutane overgevoeligheidsreactie waarbij alarmsymptomen** aanwezig waren:
- Geef geen jodiumhoudend contrastmiddel of gadoliniumhoudend contrastmiddel
- Verwijs de patiënt direct naar een allergoloog.
Bij patiënten met een geschiedenis van een milde-matige niet-acute cutane overgevoeligheidsreactie waarbij alarmsymptomen** ontbraken:
- Kies een ander jodiumhoudend contrastmiddel of gadoliniumhoudend contrastmiddel als het contrastmiddel dat de reactie veroorzaakte bekend is*
- Geef instructies aan de patiënt als de reactie opnieuw optreedt om foto’s van de huidlaesies te maken en naar de radiologie-afdeling te sturen voor beoordeling
*Zie ook flow charts
5. Kruisreactiviteit tussen contrastmiddelen
Kruisreactiviteit is het meest relevant bij allergische hypersensitiviteitsreacties. Er is een hogere kans op kruisreactiviteit bij:
- Jodiumhoudend contrastmiddel met een N-(2,3 hydroxypropyl)-carbamoyl zijketen
- Macrocyclisch gadolinium-houdend contrastmiddel
De allergoloog bepaalt door middel van huidtesten met verschillende jodiumhoudende contrastmiddelen en gadoliniumhoudende contrastmiddelen:
- De oorzaak van de allergische reactie
- Kruisreactiviteit tussen verschillende contrastmiddelen
- Suggesties voor veilige alternatieve contrastmiddelen
6. Documentatie van hypersensitiviteitsreacties
De arts die verantwoordelijk is voor de toediening van het contrastmiddel is ook verantwoordelijk voor accurate documentatie van de hypersensitiviteitsreactie in het verslag van de beeldvorming.
De arts die verantwoordelijk is voor de toediening van het contrastmiddel of de allergoloog is ook verantwoordelijk voor accurate documentatie van de hypersensitiviteitsreactie in het elektronisch patiëntendossier.
Documenteer altijd op naam van het specifieke contrastmiddel en dit moet alleen gedaan worden door artsen of allergologen met ervaring op het gebied van contrastmiddelen.
Registreer het volgende na elke overgevoeligheidsreactie op contrastmiddelen:
- De plaats, datum en tijd van de contrast toediening - in het verslag van de beeldvorming en in het elektronisch patiëntendossier.
- De naam en dosis (volume, concentratie) van het specifieke contrastmiddel - in het verslag van de beeldvorming en in het elektronisch patiëntendossier.
- Het type overgevoeligheidsreactie, acuut of laat - in het verslag van de beeldvorming en in het elektronisch patiëntendossier.
- Alle symptomen en vitale parameters (bloeddruk, pols, ademsnelheid, zuurstof saturatie) van de patiënt - in het verslag van de beeldvorming en in het elektronisch patiëntendossier.
- De behandeling die werd gegeven en de respons van de patiënt daarop - in het verslag van de beeldvorming en in het elektronisch patiëntendossier.
- Gegevens van klinische follow-up en adviezen voor toekomsten premedicatie - in het verslag van de beeldvorming en in het elektronisch patiëntendossier.
- Gegevens over consultatie van een allergoloog over toekomstige contrastmiddeltoediening - in het elektronisch patiëntendossier.
Wanneer het om een ernstige of ongebruikelijke hypersensitiviteitsreactie gaat is de arts die verantwoordelijk is voor toediening van het contrastmiddel ook verantwoordelijk voor accurate rapportering naar de nationale farmacologie-autoriteit LAREB.
Overwegingen
Primarily, in patients with a (documented) history of a hypersensitivity reaction to a contrast medium, an alternative imaging modality should be considered. The more severe the reaction, the stronger omitting a contrast medium should be considered. For mild reactions in which alternative imaging modalities are of substantially inferior quality, the risk – benefit ratio may shift. In many cases, CT with iodine-based contrast media can be replaced by ultrasound, with or without contrast agents, or MRI, with or without contrast agents. When this is not possible, consider performing the examination without a contrast medium, but only if this has an acceptable degree of diagnostic quality. For this, close communication with the referring specialist is mandatory.
Use of premedication
In premedication, two types of drugs are used: H1-antihistamines and corticosteroids. Often, they are used concomitantly, making their individual effect difficult to assess, particularly since there are many variations in premedication schedules. H1-antihistamine monotherapy is not common practice in Europe and the US, but has been used successfully in milder HSRs, particularly by Korean research groups.
H1-antihistamines block histamine receptors on various effector cells, blocking the effect of one of the pivotal players in direct mast cell responses. However, mast cells and basophils secrete various other substances that are not blocked by these drugs. The main side effect of the older H1-antihistamines that are available for intravenous administration is drowsiness/sedation. For the newer nonsedating antihistamines this effect is usually mild, but these are mainly available for oral administration.
Corticosteroids have various effect on the immune system, including mast cells, and therefore can block both mast cell degranulation by upregulating inhibitory signalling receptors, and inhibit cytokine production through suppression of gene transcription. (Andrade, 2004; Park, 2009) These membrane stabilizing effects require that administration is started >6h before contrast media administration. Unfortunately, this comes with a less favourable side effect profile, particularly with higher doses and repeated exposure.
The old protocols for premedication shown below (Greenberger, 1981; Greenberger, 1986; Lasser, 1994) are still in widespread use. The Greenberger protocol is popular in the USA, while the Lasser protocol is more frequently used in Europe. There is no literature to establish an optimal indication or protocol. Recently, the Greenberger protocol has been modified into shorter options with intravenous administration for inpatients (Mervak, 2017).
Greenberger protocol (elective examinations 1981, 1984):
- Prednisolone 50 mg IV - 13h, 7h and 1h before the procedure.
- Diphenhydramine 50 mg IV - 1h before the procedure.
Greenberger protocol (emergency examinations 1986):
- Hydrocortisone 200 mg IV - immediately and every 4h until procedure is finished.
- Diphenhydramine 50 mg IV - 1h before the procedure Lasser protocol (elective examinations 1994).
- Methylprednisolone 32 mg IV - 12h and 2h before the procedure.
The evidence regarding the effectivity of corticosteroids and antihistamines for pharmacological prevention is very heterogeneous and of low quality; moreover, it stems from the time of use of high osmolar, ionic ICM (Delaney 2006; Tramer, 2006; Davenport, 2017). It seems that prophylactic premedication can prevent the number of hypersensitivity reactions after contrast administration, but premedication mainly reduces the number of mild reactions and therefore the total number of reactions (Lasser, 1994), and not the number of severe reactions (Jung, 2016). It has been shown that premedication can cause brief hyperglycaemia (Davenport, 2010), but may also be associated with longer hospital stay, increased costs, and worse clinical outcomes (Davenport, 2016).
Few studies have focused on H1-antihistamine monotherapy, and these are biased to patients with mild reactions (Lee, 2016; Park, 2018). In a large Korean multicentre study logistic regression analysis showed that changing the ICM (odds ratio 0.51; 95% CI: 0.36, 0.73) and premedication with H1-antihistamines (odds ratio 0.53; 95% CI: 0.33, 0.86) were protective against recurrent reactions (Cha, 2019).
Many studies report a use of antihistamine and corticosteroid combination premedication; often these regimens are stratified according to the severity of the previous HSR (antihistamines only in mild HSR; antihistamines + corticosteroids in moderate to severe HSR) (Lee, 2016; Park 2017; Park, 2018) or adapted based to the clinicals preference. Corticosteroid monotherapy has rarely been used in older studies (from the high osmolar, ionic ICM era) and their findings cannot reasonably be extrapolated to the current low osmolar, nonionic contrast media (Lasser, 1994) To our knowledge, there are no studies available in which prescription of premedication has been randomized. The currently discussed studies show no additional beneficial effect of corticosteroid premedication in preventing a recurrent HSR. (Park, 2018; Cha, 2019)
Not surprisingly, the Joint Task Force on Practice Parameters of the American Academy of Allergy, Asthma, and Immunology and the American College of Allergy, Asthma, and Immunology concluded in 2020 that “Evidence is lacking to support the role of glucocorticoid routine premedication in patients receiving low-osmolar or iso-osmolar ICMs to prevent recurrent radiocontrast media anaphylaxis” (Shaker, 2020).
In a recent study by McDonald (2021), published after our literature search, 1,973 high-risk patients with a history of HSR were retrospectively studied. Prophylactic measures consisted of changing the ICM and/or steroid premedication, with or without antihistamines. Only patients with a complete steroid premedication protocol (i.e., 2 doses of 32mg of methylprednisolone at 12 and 2 hours before) CT were include in the steroid group; patients with an incomplete protocol were put in the ‘not-steroid-premedicated’ group. In 4,360 examinations, 280 HSR occurred in 224 patients (11%), of which 19 (7%) were more severe than the previous HSR. Patients who received a different ICM with or without steroid premedication had a significantly lower rate of recurrent HSR than those who received the same ICM with steroid premedication (same ICM and steroid premedication: 80 of 423 examinations [19%]; different ICM and no steroid premedication: 10 of 322 examinations [3%]; odds ratio [OR], 0.14 [95% CI: 0.06, 0.33]; P , .001; different ICM and steroid premedication: five of 166 patients [3%]; OR, 0.12 [95% CI: 0.04, 0.36]; P < .001). A sub analysis of the first CT scans only revealed that patients who received the same ICM had a similar risk of recurrent HSR, regardless of whether they received steroid premedication. (Steroid premedication: 44 of 172 patients [26%] vs. no premedication: 73 of 298 patients [25%]; OR, 1.00 [95% CI: 0.64, 1.57]; P = .99).
Although there is less data on the effectivity of premedication in GBCA, the few studies available show comparable results. Premedication with antihistamines and corticosteroid did not eliminate moderate or severe reactions to gadobenate dimeglumine (Bhatti, 2018). Both premedication protocols employed by Ryoo (2019) (antihistamine, systemic steroid plus antihistamine) did not show a recurrence-lowering effect, compared with the non- premedicated cases (antihistamine administration [OR, 1.180; 95% CI, 0.647–2.154; P = 0.589] and systemic steroid plus antihistamine [OR, 1.668; 95% CI, 0.609–4.565; P = 0.316]).
Finally, there is a paucity of data on the benefits of premedication for non-severe nonimmediate hypersensitivity reactions. Most of these reactions are self-limiting or can be treated symptomatically. In the very recent large Korean analysis, changing the type of GBCA and premedication were preventive, but premedication was only preventive in nonimmediate reactions (Ahn YH, 2022). Major international guidelines suggest performing allergologic skin testing, but do not recommend the use of premedication for non-severe nonimmediate reactions (ACR, 2022; ESUR, 2018; Torres, 2021).
Changing of a specific contrast medium
In recent years, changing the culprit ICM has become a frequently employed prophylactic strategy that is used as an alternative or a complementary measure to premedication, the latter particularly if the change has been made empirically without performing skin tests.
A large comparative study with 771 patients showed that changing the CM was more effective than premedication in the prevention of adverse reactions (Abe, 2016). Similar results were achieved in patient cohorts with mild or moderate-severe HSR where changing the contrast medium led to fewer recurrent HSR (Park, 2017; Park, 2018).
A large retrospective study on 1,963 patients showed that changing the culprit ICM only led to significantly lower rates of recurrent HSR, odds ratio of 0.14 [95% CI: 0.06, 0.33]. Additional, corticosteroid premedication did not offer additional protection, odds ratio of 0.12 [95% CI: 0.04, 0.36] (McDonald, 2021). In severe HSR, skin testing is useful to provide a safe alternative ICM (Ahn, 2022; Sohn, 2021).
In a very recent meta-analysis (Umakoshi, 2022), published after our literature search, six retrospective observational studies at moderate to severe risk of bias assessed 4,329 patients in the ICM-change-group and 2,826 in the no-change group. Changing ICM was associated with a reduced risk of recurrent hypersensitivity reaction by 61% (risk ratio = 0.39; 95% credible interval [CrI]: 0.24, 0.58). Adverse events associated with ICM-change were not reported. It was concluded that in observational evidence of limited quality, ICM– change was associated with a reduced risk of recurrent immediate hypersensitivity reaction in patients with a prior ICM-induced hypersensitivity reaction.
In MRI, the experience of changing the culprit GBCA is more limited. In patients with mild immediate HSR, changing the contrast agent could reduce the recurrence rate (Ryoo, 2019). In a small study with mild to moderate HSR to a variety of linear and macrocyclic GBCA, empiric switching to gadoterate reduced the rate of recurrent HSR, independent of premedication with either corticosteroids and H1-antihistamines or corticosteroids only (Walker, 2021).
These findings are in line with the pathogenetic concept that the allergic reactions are not directed against a ubiquitous part of all ICM or GBCA (i.e., not against iodide), but are directed against a specific allergen that is unique to one or more contrast media; switching to a contrast medium that does not contain this epitope will prevent a recurrent allergic reaction. Unfortunately, the exact allergens/epitopes have not been identified and since contrast media are structurally related, the allergen may be present in other contrast media as well, leading to cross-reactivity for those specific agents. As a result, empiric switching of contrast media does not fully prevent a recurrent HSR. For ICM, the presence of the N-(2,3 dihydroxypropyl)-carbamoyl side chain may play a role in the HSR; after a HSR to an ICM containing this side chain, it is advised to switch to an ICM lacking this side chain (iobitridol, iopamidol), preferably supported by a negative skin test (Lerondeau, 2016).
Evidence to decision
There is no evidence that premedication reduces the risk of life-threatening anaphylactic reactions. The evidence for its role in less severe (moderate to mild) HSR remains weak and conflicting. Therefore, the GDG has decided to not advice premedication in patients with an history of immediate HSR to CM.
Corticosteroids do not appear to prevent immediate HSR to GBCA. Contrary, corticosteroids have significant side effects, particularly with cumulative use and in susceptible patients.
Antihistamines reduce the recurrence risk in milder reactions, but it remains uncertain if they also reduce the risk or ameliorate symptoms in moderate to severe reactions, as they are usually given in combination with steroids. Also, antihistamines have side effects, especially sedating side effects can occur (e.g., preventing driving a car). Changing the culprit CM as sole or complementary prophylactic measure significantly lowered the HSR recurrence rate for both ICM and GBCA.
Preferably the CM change is based on negative skin tests; if these are not available, an empiric but educated change should be performed, in which the currently known risks for cross-reactivity are considered (Table 1. Cross-reactivity rates between pairs of ICM in skin positive patients with immediate hypersensitivity reactions to iodine-based contrast media and Table 2. Cross-reactivity rates between pairs of ICM in skin positive patients with immediate hypersensitivity reactions to iodine-based contrast media). In case of an unknown previous culprit CM a testing dose of 10% of the alternative CM can be considered, especially in case of a previous severe reaction.
Breakthrough hypersensitivity reactions to contrast media
It’s becoming increasingly clear that premedication is far from perfect. In premedicated patients so-called “breakthrough” hypersensitivity reactions can occur despite premedication. These are usually of similar severity as the original culprit reaction and are seldom severe (Davenport, 2017; Mervak 2015), but occasionally are of greater severity than the index reaction (Bhatti, 2018).
Iodine-based contrast media
A large study of antihistamine premedication in patients with mild HSR showed no benefit of premedication with a breakthrough reaction frequency of 11%, identical to using no premedication (Lee, 2016).
In a study using a stratified premedication protocol, the frequency of breakthrough reactions was 17%. Most of these reactions (89%) were mild and required no treatment. In severe HSR underdosage of premedication led to a significant increase in breakthrough reactions (Lee, 2017).
Kim (2018) studied the effect of the administration route on breakthrough reactions. Re- exposure to intravascular CM yielded a breakthrough frequency of 19,5%. The number of reactions after extravascular CM was negligible.
Gadolinium-based contrast agents
Walker (2019) showed a high rate (35%) of breakthrough reactions in patients with HSR to gadobutrol. Both culprit and breakthrough HSR were usually mild but may escalate in severity. This rate is very similar to the rate in a previous large prospective study on HSR after gadobutrol (Power, 2016).
In a meta-analysis of breakthrough reactions, a similar 39% rate of breakthrough HSR was found. The frequency was similar between macrocyclic and protein-binding linear GBCA (Walker, 2020).
Evidence to decision
The frequency of breakthrough reactions varies on the severity of the culprit reaction and the specific premedication protocol. Rates after ICM vary between 2-20%, but rates after GBCA administration are higher, in the order of 35-40%. Most of the reactions are of similar severity as the culprit reaction, but incidental escalation in severity may be found.
Cross-reactivity between specific contrast media (see also Introduction to chapter 3.5 Follow up strategieën na hypersensitiviteitsreacties na CM)
In most studies on contrast media hypersensitivity, the term cross-reactivity is used when patients have a HSR to two or more different contrast media, or if there are positive skin tests for two or more contrast media. In the latter case, it is not always entirely certain whether the skin test positivity is clinically relevant, as a drug provocation test is generally not performed. It has recently been suggested to discriminate polyvalent reactivity from cross-reactivity. Polyvalent reactivity comprises patients that have positive skin tests to multiple contrast media. It is argued that the term cross-reactivity should be reserved for polyvalent reactivity within a defined chemical group (e.g., with a N-(2,3 dihydroxypropyl)- carbamoyl side chain), and that multiple positive reactions against non-group CM should be defined as individual reactivity that is probably more prominent between contrast media (Schmid, 2021). However, this is a much stricter definition than has been used in most studies and for clarity we here stick to the broader definition of cross-reactivity.
Iodine -based contrast media
Schrijvers (2018) found most cross-reactivity between agents with a N-(2,3 dihydroxypropyl)-carbamoyl side chain. For immediate HSR, iomeprol and iopromide showed the highest test positivity (41%), while for nonimmediate HSR this was between ioversol and iomeprol (55%) (Table 1. Cross-reactivity rates between pairs of ICM in skin positive patients with immediate hypersensitivity reactions to iodine-based contrast media and Table 2. Cross-reactivity rates between pairs of ICM in skin positive patients with immediate hypersensitivity reactions to iodine-based contrast media).
Sohn (2021) showed in 250 patients with positive skin tests, polyvalent reactivity to at least 2 different ICM in 157 patients. The highest frequency was between iomeprol and iohexol (36%). The frequency was higher in pairs with common N-(2,3 dihydroxypropyl)-carbamoyl side chains than between CM with non-common side chains. This was significant for severe immediate HSR. In contrast, Gamboa (2021) found in IgE-mediated allergic reactions that cross-reactivity of iomeprol with iopamidol, iopromide, and iobitridol was low. In their study, iopamidol was a valid alternative in patients with IgE-mediated allergy to iomeprol and negative skin tests to iopamidol. The culprit ICM itself can be administered safely in patients having experienced nonallergic immediate hypersensitivity. In the CIRTACI study on immediate HSR it was also shown that cross-reactivity was predominantly present in allergic immediate reactions, but seldom in nonallergic immediate HSR (Clement, 2018).
In 43 patients with skin tests for nonimmediate HSR, Gaudin (2019) showed a high rate of cross-reactivity between ICM, that followed the Lerondeau classification (Lerondeau, 2016). Iobitridol was a well-tolerated alternative ICM in 77% of patients. Very similar findings have been found in a 19/142 patients with non-immediate HSR and positive intradermal tests (Gracia Bara, 2019).
In an older meta-analysis of 21 studies on skin testing, extensive data are presented on the frequency of cross-reactivity in immediate and nonimmediate reactions (Yoon, 2015). The percentage of cross-reactivity is in general lower than the percentages found in other studies (Schrijvers, 2018: Sohn, 2021). This may be related to the inclusion of older studies with a lower overall positive yield of the skin test.
Table 1. Cross-reactivity rates between pairs of ICM in skin positive patients with non-immediate hypersensitivity reactions to iodine-based contrast media
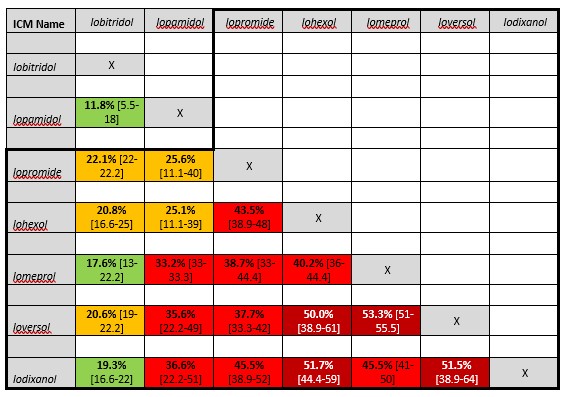
Average percentages and [range] of findings by Yoon 2015, Schrijvers 2018 and Sohn 2021. ICM containing the common N-(2,3-dihydroxypropyl) carbamoyl side chain is grouped within the black line. Risk of cross-reactivity is marked as very low (dark green, <10%), low (green, 10-20%), medium (orange 20-30%), high (red, 30-50%) and very high (dark red, >50%).
Table 2. Cross-reactivity rates between pairs of ICM in skin positive patients with immediate hypersensitivity reactions to iodine-based contrast media
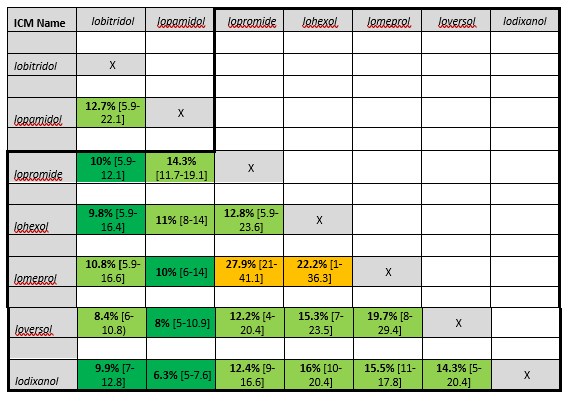
Average percentages [range] of findings by Yoon, 2015 and Schrijvers, 2018. ICM containing the common N-(2,3- dihydroxypropyl) carbamoyl side chain are grouped within the black lines. Risk of cross-reactivity is marked as very low (dark green, <10%), low (green, 10-20%), medium (orange 20-30%), high (red, 30-50%) and very high (dark red, >50%).
Gadolinium-based contrast agents
The CIRTACI study showed that a high percentage of Ring-Mesmer type 3-4 reactions after contrast media administration were allergic. Cross-reactivity among GBCA was only shown in these allergic immediate HSR. The overall number of cross-reactivity reactions was higher for GBCA than for ICM, but the number of patients was low for GBCA (Clement, 2018).
In a 7-year retrospective analysis of patients with hypersensitivity to GBCA, 13,6% (18/132) had positive skin tests and were deemed allergic. Cross-reactivity occurred in 38% and was more frequent among the macrocyclic GBCA. Cross-reactivity between macrocyclic and linear GBCA also occurred (Mankouri, 2021).
In a small retrospective study, Grüber (2021) showed cross-reactivity among macrocyclic GBCA and between macrocyclic and linear GBCA, but not among linear GBCA.
In a small case-series of 5 patients with immediate HSR to gadobutrol, only cross-reactivity with gadoterate was demonstrated (Gallardo-Higueras, 2021).
Evidence to decision
In ICM cross-reactivity is common in allergic immediate and even more in nonimmediate HSR. It occurs most frequently among ICM with a common N-(2,3 dihydroxypropyl)- carbamoyl side chain such as iopromide, iohexol, ioversol, iomeprol and iodixanol.
In GBCA cross-reactivity in allergic HSR is more common than with ICM and is especially prevalent among macrocyclic GBCA.
Serum tryptase evaluation and skin testing are key in diagnosing allergic vs. nonallergic HSR and skin tests can identify safe alternative contrast media for future diagnostic studies.
Unknown severity of previous hypersensitivity reaction to contrast media
Unfortunately, there is a lack of data about the recurrence rate and severity of HSR to CM of patients in which there is no data about the severity of the initial HSR. Although in our daily practice this is a substantial part of the population, in studies these patients are not included. Therefore, we want to stress the importance of proper documentation (see below).
A practical guideline to assess the severity of the initial reaction can be adapted from the Hartwig’s Severity Assessment Scale (Hartwig, 1992):
- Did the hypersensitivity reaction to contrast media caused permanent harm to the patient?
- Was the hypersensitivity reaction to contrast media reason for admission to the hospital or reason for increasing of hospital stay?
- Was the hypersensitivity reaction to contrast media treated with an adrenaline auto- injector (Epipen)?
The GDG advice to treat patients in line with a previous mild reaction if these questions are answered with ‘no’. In case one of these questions is answered with ‘yes’ patient should be treated as having a previous severe reaction.
Documentation of hypersensitivity reactions to contrast media
With an increasing use of changing between specific contrast media and the use of skin testing for identifying possible safe alternatives to culprit contrast media causing hypersensitivity reactions, proper documentation in the electronic patient record (EPR) has become very important.
However, the practice is quite different. Documentation in the EPR is not well standardized, is often done by physicians without any experience in the administration of contrast media, and is therefore often insufficient and incomplete (Ananthakrishnan, 2021; Deng, 2019). Recommendations for standardization have recently been published (Böhm, 2020). In selected institutions semi-structured tools for documentation of adverse events have only just been developed and implemented (Lang, 2022).
We would like to re-iterate the recommendations from Safe Use of Contrast Media, part 2: It is mandatory that the physician responsible for the administration of the CM or (EPR only) the drug allergy specialist accurately records the following:
- The place, date, and time of CM administration in the imaging report and in the electronic patient record.
- The specific contrast medium name and dose (volume, concentration) in the imaging report and in the electronic patient record.
- The type of hypersensitivity reaction, immediate or non-immediate, in the imaging report and in the electronic patient record.
- All patient symptoms and vital signs (blood pressure, pulse, respiration rate, oxygen saturation) in the imaging report and in the electronic patient record.
- The treatment given, and the response of the patient to the treatment in the imaging report and in the electronic patient record.
- Any clinical follow-up and advice on need for future premedication in the imaging report and in the electronic patient record.
- Any results of the consultation with a drug allergy specialist on future CM administration in the electronic patient record.
In addition:
- The presence of a documented allergic or nonallergic hypersensitivity reaction in the electronic patient record allergy registry (“allergie registratie”). It is essential that this reporting should be based on the name of the specific contrast medium and be done by radiologists/cardiologists or drug allergy specialists with experience in the use of contrast media.
- If the adverse reaction to a contrast medium is severe or unusual, the physician responsible for the administration of the CM or the drug allergy specialist should report all details of the reaction to the National Pharmacovigilance Authority (LAREB).
Recommendations and flowcharts
|
In all patients with a (documented) history of a hypersensitivity reaction to an iodine-based contrast medium or a gadolinium-based contrast agent, consider an alternative imaging modality. When this is not possible, consider performing an unenhanced exam, but only if the reduction in diagnostic quality is acceptable.
*See also flow charts |
1. Patients with previous immediate (acute) hypersensitivity reactions to iodine-based contrast media or gadolinium-based contrast agents
|
In patients with a (documented) history of a mild immediate hypersensitivity reaction to an iodine-based contrast medium or a gadolinium-based contrast agent:
*See also flow charts |
|
In patients with a (documented) history of a moderate or severe hypersensitivity reaction to iodine-based contrast media or gadolinium-based contrast agents
If there is no time to refer the patient to a drug allergy specialist:
*See also flow charts |
2. Patients with a previous breakthrough reaction to contrast media
|
In patients with a breakthrough hypersensitivity reaction to iodine-based contrast media or gadolinium-based contrast agents, always refer to a drug allergy specialist for skin testing with a panel of different iodine-based contrast media or gadolinium-based contrast agents.
*See also flow charts |
3. Patients with previous hypersensitivity reactions to multiple contrast media
|
In patients with hypersensitivity reactions to multiple iodine-based or gadolinium-based contrast media (either two or more different iodine-based contrast media or gadolinium- based contrast agents or to an iodine-based contrast medium and a gadolinium-based contrast agent) apply the same as above, but always refer the patient to a drug allergy specialist.
*See also flow charts |
4. Patients with previous nonimmediate (delayed) hypersensitivity reactions to iodine- based contrast media or gadolinium-based contrast agents
|
|
In patients with a history of a mild-moderate nonimmediate skin eruption without danger signs¹:
*See also flow charts |
¹ Danger signs: erosive and/or haemorrhagic lesions, blistering and skin disruption, mucosal involvement, extracutaneous organ involvement (high fever, abnormal liver / kidney values, lymphadenopathy)
² Consider cross-reactivity of contrast media (see Table 1. Cross-reactivity rates between pairs of ICM in skin positive patients with immediate hypersensitivity reactions to iodine-based contrast media and Table 2. Cross-reactivity rates between pairs of ICM in skin positive patients with immediate hypersensitivity reactions to iodine-based contrast media) and an increased risk for NIHR with use of iso-osmolar ICM.
|
Assessment of severity of previous hypersensitivity reaction when information in patient file is lacking can be performed by asking patient the following questions:
The GDG advice to treat patients in line with a previous mild reaction if these questions are answered with ‘no’. In case one of these questions is answered with ‘yes’ patient should be treated as having a previous severe reaction.
*See also flow charts |
5. Cross-reactivity between contrast media
|
Cross-reactivity is most relevant in allergic hypersensitivity reactions. It occurs with a higher frequency among:
|
|
The drug allergy specialist determines through skin testing with a panel of different iodine-based contrast media and gadolinium-based contrast agents:
|
6. Documentation of hypersensitivity reactions
|
The physician responsible for the administration of the contrast medium should accurately document the hypersensitivity reaction in the imaging report. |
|
The physician responsible for the administration of the contrast medium or the drug allergy specialist should accurately document the hypersensitivity reaction in the electronic patient dossier. |
|
It is essential that reporting should be based on the name of the specific contrast medium and be done by physicians or drug allergy specialists with experience in the use of contrast media.
After all hypersensitivity reactions to contrast media, the following should be registered:
|
|
The physician responsible for the administration of the contrast medium or the drug allergy specialist should accurately document severe or unusual hypersensitivity reactions to the National Pharmacovigilance Authority LAREB. |
*See also Introduction to chapter 3.5 Follow up strategieën na hypersensitiviteitsreacties na CM
Onderbouwing
Achtergrond
Patients reporting a previous hypersensitivity reaction (HSR) to contrast media are at increased risk of developing a recurrent hypersensitivity reaction upon re-exposure (see Module 3.5.3 Risk Factors for Hypersensitivity Reactions to Contrast Media). It is unclear what the best strategy is to prevent such a recurrent hypersensitivity reaction.
Options include complete avoidance of contrast media and performing alternative imaging techniques, which may lead to inferior quality of the diagnostic modality or higher costs, depending on the modality used. Alternatively, contrast media can be alternated to a different agent, and/or so-called premedication may be employed. Premedication consists of antihistamines with or without corticosteroids, with the aim to prevent a hypersensitivity reaction. Different protocols for premedication (Greenberger, 1981; Greenberger, 1984; Greenberger, 1986; Lasser, 1994) are still in widespread use, often slightly modified, but there is no literature to establish an optimal indication or protocol. The older protocols have been challenged by newer, shorter options for inpatients (Mervak, 2017). Moreover, the use of premedication is a current topic of debate, as the literature on the effectiveness of premedication prior to CM administration remains unclear and particularly corticosteroids have relevant adverse effects.
All types of contrast media can give hypersensitivity reactions. See chapter 3.5 Follow-up Strategies after Hypersensitivity Reactions to Contrast Media.
All types of contrast media will be evaluated: iodine-based, gadolinium-based, microbubble, CM. Also, all types of administration routes will be covered, intravascular (intravenous or intra-arterial), oral and rectal, intracavitary (joints or bladder), and intraductal (bile or pancreatic ducts). See separate chapter for nonvascular CM administration.
Conclusies / Summary of Findings
|
Very low GRADE |
The evidence is very uncertain about the effect of premedication on hypersensitivity reactions to contrast media when compared with no premedication or a different premedication strategy in patients undergoing examinations with iodine-based contrast media.
Cha, 2019; Mervak, 2017; Park, 2017; Park, 2018; Specjalski, 2020; Tramer, 2006 |
|
Very low GRADE |
The evidence is very uncertain about the effect of premedication on hypersensitivity reactions to contrast when compared with no premedication or a different premedication strategy in patients undergoing examinations with gadolinium-based contrast agents.
Bhatti, 2018; Ryoo, 2019; Walker, 2021 |
Samenvatting literatuur
Description of studies – Iodine-based contrast media
Cha (2019) described a multicentre registry study aiming to identify the prevalence, patterns, risk factors, and preventive measures for ICM-related HSRs. Between March 2017 and October 2017, a total of 196 081 patients who underwent contrast-enhanced CT examinations using ICM were enrolled from seven participating institutions. Regimens for premedication were as follows: for patients who reported a mild index reaction, 4 mg of intravenous chlorpheniramine 30 minutes before ICM administration; for patients who reported a moderate index reaction, 40 mg of intravenous methylprednisolone and 4 mg of intravenous chlorpheniramine 1 hour before ICM administration; and for patients who reported a severe index reaction, 40 mg of intravenous methylprednisolone 4 hours and 1 hour before ICM administration and 4 mg of intravenous chlorpheniramine 1 hour before ICM administration via the intravenous cannula inserted for ICM injection.
Mervak (2017) described a retrospective cohort study aiming to determine if the allergic-like breakthrough reaction rate of intravenous corticosteroid prophylaxis administered 5 hours before contrast material–enhanced CT is noninferior to that of a traditional 13-hour oral regimen. All subjects were premedicated for a prior allergic like or unknown-type reaction to iodine-based contrast material. A noninferiority margin of 4.0% was selected to allow for no more than a clinically negligible 6.0% breakthrough reaction rate in the cohort that received 5-hour intravenous corticosteroid prophylaxis. The breakthrough reaction rate for a cohort of 202 patients who received accelerated 5-hour IV corticosteroid prophylaxis before contrast material–enhanced CT for a prior allergic-like or unknown-type reaction to iodine- based contrast media was compared with a previously published breakthrough reaction rate from the same institution for a similar group of subjects who received a 13-hour oral premedication regimen for the same indication (2.1%; 13 of 626). Only allergic-like breakthrough reactions were considered for this study; physiologic reactions were ignored, because they are not considered relevant to corticosteroid prophylaxis.
Park (2017) described a retrospective cohort study aiming to evaluate the outcomes of re- exposure to low osmolar iodine-based contrast medium (LOCM) in patients with a history of moderate-to-severe hypersensitivity reaction (HSR) who underwent contrast-enhanced computed tomography after the initial HSR. Premedication was defined as antihistamines or systemic steroids prescribed with the aim of preventing recurrence of HSR. The premedication regimens used at the time of re-exposure were determined according to the decision of the physicians in charge. Steroids and antihistamines were administered 0.5–1 hour before re-exposure to LOCM.
Park (2018) described a retrospective cohort aiming to evaluate premedication protocols involving administration of antihistamines and multidose corticosteroids that have
been widely used in prevention of recurrent HSRs to ICM. The outcomes of patients with mild HSR who subsequently underwent contrast material–enhanced CT between January 2012 and December 2015 were analysed. For premedication, 4 mg of chlorpheniramine was intravenously administered 30 minutes prior to re-exposure to ICM For patients with a mild index reaction. The initial HSR event was defined as the first occurrence of an immediate HSR to ICM. Recurrent HSR events were defined as an immediate HSR at repeated exposure to ICM after the initial event.
Specjalski (2020) described a prospective observational study aiming to determine efficacy of premedication before medical procedures with the use of iodine-based contrast media in patients with a history suggesting a hypersensitivity reaction after their past use. Out of 152 patients consulted due to adverse reactions after ICM (85 women and 67 men, aged 43–90), 101 were selected with a history suggesting a mild hypersensitivity reaction (urticaria, itching, skin redness, malaise etc.). All patients had an indication for ICM administration in the near future. Premedication was given with cetirizine (10 mg) and prednisone (20 mg or 50 mg, randomly assigned) 13, 7 and 1 h before the ICM administration. Patients with a history of a severe drug hypersensitivity reaction, including anaphylaxis, unstable asthma, renal insufficiency, or unstable heart insufficiency were excluded from the study. They also excluded patients with isolated subjective vasomotor symptoms (nausea, sweating, feeling of warmth etc.). Patients were randomly assigned to one of the premedication arms: 10 mg cetirizine + 20 mg prednisone or 10 mg cetirizine + 50 mg prednisone. The premedication was given orally 13, 7 and 1 h before the ICM administration. Subjects were observed 24 h after the ICM administration.
One systematic review (Tramer, 2006) included 9 RCTs in this analysis. The goal of this review was to review the efficacy of pharmacological prevention of serious reactions to iodine-based contrast media. A systematic search was performed up to October 2005. The pre-specified inclusion criteria were random allocation of patients, use of premedication alone or in combination, presence of a placebo or a no treatment control group, and reporting of presence or absence of allergic reactions. A total of 9 trials with 10,011 adult patients were included in the review analysis. No RCTs that answered the search questions were found that were published after this systematic review.
Description of studies – Gadolinium-based contrast agents
Bhatti (2018) described a retrospective cohort study aiming to determine the severity of breakthrough reactions to gadobenate dimeglumine in patients premedicated with a 13- hour premedication regimen. The final study population consisted of 19 breakthrough reactions to gadobenate dimeglumine in 19 subjects (18 female, 1 male) with a mean age of 51 years (range, 28-90 years) and a mean administered volume of gadobenate dimeglumine of 17 mL (range, 9-30 mL). Hypersensitivity reactions to gadobenate that were not preceded by premedication (n = 97) were explored as a comparator group. All premedication regimens were 13 hours in length, consisting of 150 mg oral prednisone (50mg 13, 7, and 1 hour before contrast material) and 50 mg oral diphenhydramine (1 hour before contrast material).
Ryoo (2019) described a retrospective cohort study aiming to evaluate the effectiveness of changing the contrast agent and single-dose premedication for HSR recurrence prevention in patients with a history of mild immediate HSR to GBCA who subsequently underwent enhanced magnetic resonance imaging. Intravenous chlorpheniramine 4 mg, 30 minutes before the GBCA administration, or intravenous methylprednisolone sodium succinate 40 mg plus chlorpheniramine 4 mg, 1 hour before the GBCA administration, was administrated as premedication regimen. Recurrence rates of immediate HSR were compared according to prevention strategies. The GBCA that was used at the initial HSR event was defined as the culprit agent. An immediate HSR event at re-exposure to a GBCA after the initial HSR was defined as recurrent HSR.
Walker (2021) described a prospective observational efficacy trial aiming to evaluate HSR rate to GBCA among patients with history of HSR to GBCA, empirically given an alternative GBCA prior to repeat administration. Patients with prior HSR to GBCA received 13-hour oral corticosteroid and diphenhydramine premedication prescription with switching of GBCA to gadoterate.
Results – Iodine-based contrast media
Cha (2019) studied 196081 patients (mean age 59.1 ±16.0 years; 53% men) who underwent ICM administration. The overall prevalence of HSRs was 0.73% (1433 of 196081), and severe reactions occurred in 0.01% (17 of 196081). Among the 196081 patients, 570 patients reported experiencing an HSR to ICM in the past, and 94.9% (541 of 570) patients underwent preventive measures before ICM administration.
Premedication only was conducted in 213 patients (37.4%, 213 of 570; 187 patients received antihistamine only and 26 patients received antihistamine with corticosteroids) and change of ICM only was performed in 52 patients (9.1%, 52 of 570). In 276 patients (48.4%, 276 of 570), both premedication and change of ICM were performed (203 received antihistamine with change of ICM and 73 received antihistamine and corticosteroids with change of ICM).
Among 570 patients who had experienced an HSR to ICM in the past, 195 patients experienced recurrent HSR, whereas 375 patients did not show any symptoms of recurrence. A total of 176 of 541 patients (32.5%) experienced recurrent HSR despite premedication and/or change of ICM. Of those 176 patients, 158 patients received pretreatment (n= 131 antihistamines only, n= 27 antihistamines plus corticosteroids) and their reactions were thus considered breakthrough reactions. In addition, recurrent events occurred in 92 of 328 (28.1%) patients for whom culprit agents were changed. Logistic regression analysis showed that use of premedication with antihistamine (OR, 0.5; P = .01) and change in the generic profile of ICM (OR, 0.5; P < 0.001) were preventive against recurrent HSR.
Mervak (2017) showed that significantly more subjects receiving a 13-hour oral regimen had a prior reaction to iodine-based contrast material of unknown type (38% vs 15%, P= .0001), and significantly more subjects who received an accelerated IV regimen had a prior mild reaction to iodine-based contrast material (51% vs 34%, P=.0001). The breakthrough reaction rate for 5-hour intravenous prophylaxis was 2.5% (five of 202 patients; 95% CI: 0.8%, 5.7%), which was noninferior to the 2.1% (13 of 626 patients; 95% CI: 1.1%, 3.5%) rate for the 13-hour regimen (P =.018). The upper limits of the confidence interval for the difference between the two rates was 3.7% (0.4%; 95% CI: 21.6%, 3.7%), which was within the 4.0% noninferiority margin. All breakthrough reactions were of equal or lesser severity to those of the index reactions (two severe, one moderate, and one mild reaction).
Park (2017) included 150 patients from the 11 included centres. The proportion of males was 49.3% and the mean age was 61.7 ± 11.5 years. Among a total of 328 cases of re- exposure, the ICM was changed in 59.1% and systemic steroids were administered as premedication in 37.2% of cases at the time of re-exposure. Among 180 re-exposures without steroid premedication following moderate initial HSR, changing the ICM significantly reduced the recurrence rate of HSR (22.5% vs. 11.0%; P = 0.037). Among 92 re-exposures premedicated with systemic steroids following moderate initial HSR, the recurrence rate of HSR did not significantly differ (30.6% vs. 16.1% with the same vs. different ICM; P = 0.100). Among 23 re-exposures without steroid premedication following severe initial HSR, the recurrence rate was similar irrespective of whether the same ICM was used or not (33.3% vs. 23.5%; P = 0.632). On the other hand, among 26 cases premedicated with systemic steroids following a severe initial HSR, the recurrence rate was only 9.5% (2/21) when a different ICM was used, whereas four out of five cases (80.0%) using the same ICM experienced recurrence (P = 0.005). Steroid premedication did not result in improvement of the overall outcomes at the subsequent re-exposure (16.5% vs. 23.0%, P = 0.250). Next, the subjects premedicated with systemic steroids into two groups were divided according to the dose of steroids. The recurrence rate of HSR was not statistically different between subjects premedicated with a steroid equivalent to < 40 mg (19.7%; 13/66) or ≥40 mg of prednisolone (26.8%; 15/56) (P = 0.353) The risk of recurrent HSR was 67.1% lower in cases where the implicated ICM was changed to another one (OR: 0.329; P = 0.001). However, steroid premedication did not show protective effects against recurrent HSR.
Park (2018), report a total of 1178 patients (men 47.5%, 55.8 ±11.2 years) with mild immediate HSR were re-exposed to ICM 3533 times. Among these patients, 1056 patients (89.6%) experienced allergy-like reactions and 122 patients (10.4%) developed gastrointestinal reactions. Premedication with an antihistamine had a significant recurrence- lowering effect; the recurrence rate was 16.6% in non-premedicated patients, but decreased to 10.7% when antihistamine premedication was administered (OR, 0.569; 95% CI: 0.443, 0.731; P=.001) Regardless of whether contrast media was replaced or not, administration of antihistamine premedication lowered the recurrence rate significantly (with the same contrast media: OR, 0.627; 95% CI: 0.430, 0.912; P = .015; with different contrast media: OR, 0.584; 95% CI: 0.4240, 0.776; P=.001) With re-exposure to the culprit agent without premedication, the recurrence rate was 31.1% (85 of 273 examinations). The recurrence rate decreased to 12% (105 of 872 examinations; P=.001) by only changing the culprit agent and to 7.6% (148 of 1947 examinations; P=.001) by using the combination of changing the ICM and antihistamine premedication. Changing the ICM plus antihistamine premedication was also helpful in reducing the recurrence of gastrointestinal symptoms from 16.1% to 1.8% (P=.020). However, despite changing of the ICM, some combinations of ICM did not show a prophylactic effect.
In Specjalski (2020), 76 patients underwent the radiologic procedure with premedication with antihistamine and a lower (40 patients; 3x 20mg) or higher dose (36 patients; 3x 50mg) of prednisone. Four of them (5%) reported a cutaneous hypersensitivity reaction (urticaria, itching, redness) and one dyspnoea. There was no statistically significant difference in relation to the premedication protocol (p = 0.1306).
Tramer (2006) reported 9 trials (including 10,011 adults) tested H1 antihistamines, corticosteroids, and an H1 +H2 blocker combination. No trial included exclusively patients with a history of allergic reactions. Many outcomes were not allergy related, and only a few were potentially life threatening. No reports on death, cardiopulmonary resuscitation, irreversible neurological deficit, or prolonged hospital stays were found. In two trials, 3/778 (0.4%) patients who received oral methylprednisolone 2×32 mg or intravenous prednisolone 250 mg had laryngeal oedema compared with 11/769 (1.4%) controls (odds ratio 0.31, 95% confidence interval 0.11 to 0.88). In two trials, 7/3093 (0.2%) patients who received oral methylprednisolone 2×32 mg had a composite outcome (including shock, bronchospasm, and laryngospasm) compared with 20/2178 (0.9%) controls (odds ratio 0.28, 0.13 to 0.60). In one trial, 1/196 (0.5%) patient who received intravenous clemastine 0.03 mg/kg and cimetidine 2 to 5 mg/kg had angio-oedema compared with 8/194 (4.1%) controls (odds ratio 0.20, 0.05 to 0.76).
Results – Gadolinium-based contrast agents
Bhatti (2018) showed that premedication was most commonly given (63% [12/19]) for a previous hypersensitivity reaction to gadolinium-based contrast media (GBCM); in 37%(7/19), it was given for a different risk factor. In those premedicated for a previous allergic-like reaction to GBCM of known severity (n = 9), the breakthrough reaction severity was the same as index reaction severity in 56% (5/9), less severe in 11% (1/9), and of greater severity in 33% (3/9). Two severe breakthrough reactions occurred; both were in subjects premedicated for risk factors other than a previous GBCM reaction. No subjects died. Five subjects were re-exposed to GBCM a total of 9 times; no repeat breakthrough reactions occurred.
Ryoo (2019) studied a total of 185 patients with a history of mild immediate HSR to GBCA who were re-exposed to GBCA 397 times during the study period. The overall recurrence rate was 19.6% (78/397). Changing the culprit GBCA significantly reduced the recurrence rate, compared with reusing the culprit GBCA (6.9%, 9/130 and 25.8%, 69/267; P < 0.001). The recurrence rate was lowest when the GBCA was changed to a different molecular structure class from the culprit agent, followed by changing to CM with the same molecular structure and reusing the culprit GBCA (6.2%, 7/113 vs 11.8%, 2/17 vs 25.8%, 69/267; P < 0.001). Single-dose premedication demonstrated no significant prophylactic effect on recurrence (20.4%, 17/98 vs 17.3%, 61/299 with and without premedication, respectively; P= 0.509). The recurrence rate of cases with antihistamine administration was 19.9%, and the recurrence rate of cases with systemic steroid plus antihistamine administration was 25.9%. Both premedication protocols did not show a recurrence-lowering effect, compared with the non-premedicated cases (antihistamine administration [OR, 1.180; 95% CI, 0.647–2.154; P = 0.589] and systemic steroid plus antihistamine [OR, 1.668; 95% CI, 0.609–4.565; P = 0.316]). Premedication in addition to changing CM also showed no additional prophylactic effect (7.2%, 7/97 and 6.1%, 2/33, respectively; P = 0.821).
Walker (2021) evaluated 26 patients with mild (92.3% [24/26]) or moderate (7.7% [2/26]) HRS to gadobutrol (53.8% [14/26]), gadoxetate (3.8% [1/26]), and gadopentetate (3.8% [1/26]). In 38.5% (10/26), inciting GBCA was unknown but was likely gadobutrol or gadopentetate based on availability. Most patients were female (84.6% [22/26]). The mean patient age was 52.1 ± 15.8 years. From 27 gadoterate administrations, 59.3% (16/27) patients received corticosteroid and diphenhydramine premedication, 11.1% (3/27) received only diphenhydramine, and 29.6% (8/27) with no premedication. Among the 26 included patients, 2 patients, both female, with a history of immediate HR to gadobutrol had a breakthrough HR to gadobutrol despite adequately dosed corticosteroid premedication. Hypersensitivity reaction rate after empiric switching to gadoterate was 3.7% (1 mild reaction; 95% CI, 0.09%–18.9%) overall with no difference in patients with (6.3% [1/16]; 95% CI, 0.15%–28.7%) or without (0%; [0/11] upper bound 95% CI, 25.0%) corticosteroid premedication.
Summary of study’s conclusions – Iodine-based contrast media
Use of premedication with antihistamine (OR, 0.5; P = .01) was preventive against recurrent HSR (Cha, 2019).
A change in the culprit ICM and premedication with antihistamine are useful for reducing the recurrence of HSRs (Cha, 2019).
Accelerated intravenous premedication with corticosteroids beginning 5 hours before contrast-enhanced CT has a breakthrough reaction rate noninferior to that of a 13-hour oral premedication regimen (Mervak, 2017).
In patients with moderate-to-severe HSR, steroid premedication only shows limited effectiveness. Steroid premedication did not result in improvement of the overall outcomes at the subsequent re-exposure (16.5% vs. 23.0%, P = 0.250). Steroid premedication did not show protective effects against recurrent HSR (Park, 2017).
Premedication with an antihistamine had a significant recurrence-lowering effect (OR, 0.569; 95% CI: 0.443, 0.731; P=.001) in mild HSR (Park, 2018).
Premedication with cetirizine and prednisone before radiologic procedures, regardless of dosage of the corticosteroid, proved to be efficient in patients with a history suggesting hypersensitivity to iodine-based contrast media (Specjalski, 2020).
Summary of study’s conclusions – Gadolinium-based contrast agents
Premedication with antihistamine and corticosteroid does not eliminate moderate or severe reactions to gadobenate dimeglumine and recurrent reactions can be of greater severity than index reactions (Bhatti, 2018).
Both premedication protocols (antihistamine, systemic steroid plus antihistamine) did not show a recurrence-lowering effect, compared with the non-premedicated cases (antihistamine administration [OR, 1.180; 95% CI, 0.647–2.154; P = 0.589] and systemic steroid plus antihistamine [OR, 1.668; 95% CI, 0.609–4.565; P = 0.316]) (Ryoo, 2019).
Empirically switching GBCAs, with or without the use of corticosteroid premedication, can substantially reduce the rate of hypersensitivity breakthrough reactions (Walker, 2021).
Level of evidence of the literature
The quality of certainty of evidence for the outcome allergic / hypersensitivity reaction was downgraded from low to very low due to risk of bias (as described below), heterogeneity of included studies, indirectness, and imprecision of outcome measures (low numbers of events).
The risk of bias of the included studies was deemed high due to high risk of bias in selection of participants, selection of the outcome of interest and Confounding analysis.
Zoeken en selecteren
A systematic review of the literature was performed to answer the following question: What are the effects of prophylactic measures to prevent hypersensitivity reactions after contrast media administration?
P (Patients): Patients undergoing radiological examinations with contrast media.
I (Intervention): Prophylactic measure to prevent hypersensitivity reactions after contrast administration.
C (Comparison): No prophylactic measure or a different prophylactic measure to prevent hypersensitivity reactions after contrast administration.
O (Outcome): Allergic reactions to contrast media, hypersensitivity reaction, type I/type IV, severe allergic reaction.
Relevant outcome measures
The working group considered allergic / hypersensitivity reactions to contrast as critical outcome measures for the decision-making process.
Search and select (Methods)
The databases Medline (via OVID) and Embase (via Embase.com) were searched with relevant search terms until April 22nd, 2021. The detailed search strategy is depicted under the tab Methods. The systematic literature search resulted in 400 hits. Studies were selected based on the following criteria
- Adult patients undergoing radiological examinations with contrast media.
- Evaluation of effectiveness of prophylactic measures to prevent hypersensitivity reactions after contrast administration.
- Reports predefined outcome measure: hypersensitivity reactions.
- No reports of case series or exploratory findings (n ≥ 10).
Based on title and abstract a total of twenty-three studies were selected. After examination of full text, a total of fifteen studies were excluded and eight studies were included in the literature summary. Reason for exclusion is reported in Table of excluded studies in the Appendices to modules.
The most important study characteristics and results were included in the evidence tables. The evidence tables and assessment of individual study quality are included.
Referenties
- Abe S, Fukuda H, Tobe K, Ibukuro K. Protective effect against repeat adverse reactions to iodinated contrast medium: Premedication vs. changing the contrast medium. Eur Radiol. 2016; 26(7): 2148-2154.
- Ahn KM, Ahn YH, Cho MK, Kang DY, Lee SY, Kang HR. Validation of practical pathway in patients with anaphylaxis to low osmolar contrast media: a retrospective cohort study. J Allergy Clin Immunol Pract. 2022; 10(10): 2685-2692.e2.
- Ahn YH, Kang DY, Park SB, Kim HH, Kim HJ, Park GY, et al. Allergic-like hypersensitivity reactions to gadolinium-based contrast agents: an 8-year cohort study of 154,539 patients. Radiology. 2022; 303(2): 329-336.
- American College of Radiology. ACR Manual on contrast media, v2022. Available at: [URL].
Accessed: 1 April 2022. - Ananthakrishnan L, Parrott DT, Mielke N, Xi Y, Davenport MS. Fidelity of electronic documentation for reactions prompting premedication to iodine-based contrast media. J Am Coll Radiol. 2021; 18(7): 982-989.
- Andrade MV, Hiragun T, Beaven MA. Dexamethasone suppresses antigen-induced activation of phosphatidylinositol 3-kinase and downstream responses in mast cells.
J Immunol. 2004; 172(12): 7254. - Bhatti ZS, Mervak BM, Dillman JR, Davenport MS. Breakthrough reactions to gadobenate dimeglumine. Invest Radiol. 2018; 53: 551-554.
- Böhm IB, van der Molen AJ. Recommendations for standardized documentation of contrast medium-induced hypersensitivity. J Am Coll Radiol. 2020; 17(8): 1027-1028.
- Cha MJ, Kang DY, Lee W, Yoon SH, Choi YH, Byun JS, et al. Hypersensitivity reactions to iodinated contrast media: a multicenter study of 196 081 patients. Radiology. 2019; 293: 117-124.
- Clement O, Dewachter P, Mouton-Faivre C, Nevoret C, Guilloux L, Bloch Morot et al.
Immediate Hypersensitivity to Contrast Agents: The French 5-year CIRTACI Study. EClinicalMedicine. 2018; 1: 51-61. - Davenport MS, Cohan RH, Caoili EM, Ellis JH. Hyperglycemic consequences of
corticosteroid premedication in an outpatient population. AJR Am J Roentgenol. 2010; 194(6): W483-W488. - Davenport MS, Mervak BM, Ellis JH, Dillman JR, Dunnick NR, Cohan RH. Indirect cost and harm attributable to oral 13-hour inpatient corticosteroid prophylaxis before contrast- enhanced CT. Radiology. 2016; 279(2): 492-501.
- Davenport MS, Cohan RH. The evidence for and against corticosteroid prophylaxis in at-risk patients. Radiol Clin North Am. 2017; 55(2): 413-421.
- Delaney A, Carter A, Fisher M. The prevention of anaphylactoid reactions to iodinated radiological contrast media: a systematic review. BMC Med Imaging. 2006; 6: 2.
- Deng F, Li MD, Wong A, Kowalski LT, Lai KH, Digumarthy SR, Zhou L. Quality of documentation of contrast agent allergies in electronic health records. J Am Coll Radiol 2019; 16: 1027-1035.
- European Society of Urogenital Radiology Contrast Media Safety Committee. ESUR Guidelines on contrast safety, v10, 2018. Available at: [URL] Accessed: 1 April 2022.
- Gallardo-Higueras A, Moreno EM, Muñoz-Bellido FJ, Laffond E, Gracia-Bara MT, Macias EM, et al. Patterns of cross-reactivity in patients with immediate hypersensitivity reactions to gadobutrol. J Investig Allergol Clin Immunol. 2021; 31(6): 504-506.
- Gamboa P, Sánchez de Vicente J, Galán C, Jáuregui I, Segurola A, García-Lirio E, et al.
Tolerance to iopamidol in patients with confirmed allergic immediate hypersensitivity to iomeprol. J Allergy Clin Immunol Pract. 2021; 9(5): 2101-2103.e1. - Gaudin O, Deschamps O, Duong TA, Gener G, Paul M, Luciani A, et al. Cutaneous tests, and interest of iobitridol in non-immediate hypersensitivity to contrast media: A case series of 43 patients. J Eur Acad Dermatol Venereol. 2020; 34(4): e178-e180.
- Gracia-Bara MT, Moreno E, Laffond E, Muñoz-Bellido F, Lázaro M, Macías E, et al. Tolerability of iobitridol in patients with non-immediate hypersensitivity reactions to iodine-based contrast media. Allergy. 2019; 74(1): 195-197.
- Greenberger PA, Patterson R, Simon R, Lieberman P, Wallace W. Pretreatment of high-risk patients requiring radiographic contrast media studies. J Allergy Clin Immunol. 1981; 67(3): 185-187.
- Greenberger PA, Patterson R, Radin RC. Two pretreatment regimens for high-risk patients receiving radiographic contrast media. J Allergy Clin Immunol. 1984; 74(4 Pt 1): 540-
543. - Greenberger PA, Halwig JM, Patterson R, Wallemark CB. Emergency administration of radiocontrast media in high-risk patients. J Allergy Clin Immunol. 1986; 77(4) :630-634.
- Grüber HP, Helbling A, Jörg L. Skin test results and cross-reactivity patterns in IgE- and T-cell- mediated allergy to gadolinium-based contrast agents. Allergy Asthma Immunol Res. 2021; 13(6): 933-938.
- Hartwig SC, Siegel J, Schneider PJ. Preventability and severity assessment in reporting adverse drug reactions. Am J Hosp Pharm. 1992; 49: 2229–2232.
- Jung JW, Choi YH, Park CM, Park HW, Cho SH, Kang HR. Outcomes of corticosteroid prophylaxis for hypersensitivity reactions to low osmolar contrast media in high-risk patients. Ann Allergy Asthma Immunol. 2016; 117(3): 304-309.e1.
- Kim YS, Choi YH, Cho YJ, Lee S, Yoon SH, Park CM, Kang HR. Incidence of breakthrough reaction in patients with prior acute allergic-like reactions to iodinated contrast media according to the administration route. Korean J Radiol. 2018; 19(2): 352-357.
- Lang M, Deng F, Singh R, DeFuria CL, Saini S, Alkasab TK. Implementation of a semistructured clinical event documentation tool for acute adverse contrast reactions. J Am Coll Radiol. 2022; 19(5): 655-662.
- Lasser EC, Berry CC, Mishkin MM, Williamson B, Zheutlin N, Silverman JM. Pretreatment with corticosteroids to prevent adverse reactions to nonionic contrast media. AJR Am J Roentgenol. 1994; 162(3): 523-526.
- Lee SH, Park HW, Cho SH, Kim SS. The efficacy of single premedication with antihistamines for radiocontrast media hypersensitivity. Asia Pac Allergy. 2016; 6(3): 164-167.
- Lee SY, Yang MS, Choi YH, Park CM, Park HW, Cho SH, Kang HR. Stratified premedication strategy for the prevention of contrast media hypersensitivity in high-risk patients. Ann Allergy Asthma Immunol. 2017; 118(3): 339-344.e1.
- Lerondeau B, Trechot P, Waton J, Poreaux C, Luc A, Schmutz JL, Paris C, Barbaud A. Analysis of cross-reactivity among radiocontrast media in 97 hypersensitivity reactions. J Allergy Clin Immunol. 2016; 137(2): 633-635.e4.
- Mankouri F, Gauthier A, Srisuwatchari W, Moutou A, Borushko A, Popa L, et al.
Hypersensitivity to gadolinium-based contrast agents: A single-center retrospective analysis over 7 years. J Allergy Clin Immunol Pract. 2021; 9(4): 1746-1749.e2. - McDonald JS, Larson NB, Kolbe AB, Hunt CH, Schmitz JJ, Maddox DE, et al. Prevention of allergic-like reactions at repeat CT: steroid pretreatment versus contrast material substitution. Radiology. 2021; 301(1): 133-140.
- Mervak BM, Davenport MS, Ellis JH, Cohan RH. Rates of breakthrough reactions in inpatients at high risk receiving premedication before contrast-enhanced CT. AJR Am J Roentgenol. 2015; 205(1): 77-84.
- Mervak BM, Cohan RH, Ellis JH, Khalatbari S, Davenport MS. Intravenous corticosteroid premedication administered 5 hours before CT compared with a traditional 13-hour oral regimen. Radiology. 2017; 285:425-433.
- Park HJ, Park JW, Yang MS, Kim MY, Kim SH, Jang GC, Nam YH, Kim GW, Kim S, Park HK, Jung JW, Park JS, Kang HR. Re-exposure to low osmolar iodine-based contrast media in patients with prior moderate-to-severe hypersensitivity reactions: A multicentre retrospective cohort study. Eur Radiol. 2017; 27:2886-2893.
- Park SJ, Kang DY, Sohn KH, Yoon SH, Lee W, Choi YH, Cho SH, Kang HR. Immediate mild reactions to CT with iodine-based contrast media: strategy of contrast media re- administration without corticosteroids. Radiology. 2018; 288:710-716.
- Park SK, Beaven MA. Mechanism of upregulation of the inhibitory regulator, src-like adaptor protein (SLAP), by glucocorticoids in mast cells. Mol Immunol. 2009;46(3):492. Epub 2008 Nov 25.Power S, Talbot N, Kucharczyk W, Mandell DM. Allergic-like reactions to the MR Imaging contrast agent gadobutrol: A prospective study of 32 991 consecutive injections. Radiology. 2016; 281(1): 72-77.
- Ryoo CH, Choi YH, Cheon JE, Yoon SH, Kang HR, Park SJ, Lee W. Preventive effect of changing contrast media in patients with a prior mild immediate hypersensitivity reaction to gadolinium-based contrast agent. Invest Radiol. 2019; 54: 633-637.
- Schmid AA, Böhm IB. Cross-Reactivity and Polyvalent Reactivity in Patients with Iodine-based Contrast Medium Allergy: How to Use the Terms Correctly. Int Arch Allergy Immunol. 2021; 182(8): 725-727.
- Schrijvers R, Breynaert C, Ahmedali Y, Bourrain JL, Demoly P, Chiriac AM. Skin testing for suspected iodine-based contrast media hypersensitivity. J Allergy Clin Immunol Pract. 2018; 6(4): 1246-1254.
- Sohn KH, Seo JH, Kang DY, Lee SY, Kang HR. Finding the optimal alternative for immediate hypersensitivity to low-osmolar iodine-based contrast. Invest Radiol. 2021; 56(8): 480- 485.
- Shaker MS, Wallace DV, Golden DBK, et al. Anaphylaxis: a 2020 practice parameter update, systematic review, and Grading of Recommendations, Assessment, Development and
Evaluation (GRADE) analysis. J Allergy Clin Immunol 2020; 145(4): 1082–1123. - Specjalski K, Górska L, Wajda B, Chełmiñska M, Jassem E. Oral premedication in patients with a history suggesting hypersensitivity to iodinated contrast media. Adv Dermatol Allergol. 2020; 37(4): 520-523.
- Tramer MR, von Elm E, Loubeyre P, Hauser C. Pharmacological prevention of serious anaphylactic reactions due to iodinated contrast media: systematic review. BMJ 2006; 333(7570): 675-682.
- Umakoshi H, Nihashi T, Takada A, Hirasawa N, Ishihara S, Takehara Y, et al. Iodinated contrast media substitution to prevent recurrent hypersensitivity reactions: A systematic review and meta-analysis. Radiology. 2022; 305(2): 341-349.
- Walker DT, Chakraborty S, Schieda N. Single-center retrospective analysis of breakthrough allergic-like reactions to gadobutrol. Invest Radiol. 2019; 54(7): 448-451.
- Walker DT, Davenport MS, McGrath TA, McInnes MDF, Shankar T, Schieda N. Breakthrough hypersensitivity reactions to gadolinium-based contrast agents and strategies to decrease subsequent reaction rates: a systematic review and meta-analysis.
Radiology. 2020; 296(2): 312-321. - Walker DT, McGrath TA, Glikstein R, Chakraborty S, Blanchette C, Schieda N. Empiric switching of gadolinium-based contrast agents in patients with history of previous immediate hypersensitivity reaction to GBCA: A prospective single-center, single-arm efficacy trial. Invest Radiol. 2021; 56:369-373.
- Yoon SH, Lee SY, Kang HR, Kim JY, Hahn S, Park CM, et al. Skin tests in patients with hypersensitivity reaction to iodinated contrast media: a meta-analysis. Allergy. 2015; 70(6): 625-637.
Verantwoording
Beoordelingsdatum en geldigheid
Laatst beoordeeld : 28-11-2022
Validity
The Radiological Society of the Netherlands (NVvR) will determine around 2027 if this guideline (per module) is still valid and applicable. If necessary, the scientific societies will form a new guideline group to revise the guideline. The validity of a guideline can be shorter than 5 years, if new scientific or healthcare structure developments arise, that could be a reason to commence revisions. The Radiological Society of the Netherlands is the owner of this guideline and thus primarily responsible for the actuality of the guideline. Other scientific societies that have participated in the guideline development share the responsibility to inform the primarily responsible scientific society about relevant developments in their field.
Algemene gegevens
General Information
The Kennisinstituut van de Federatie Medisch Specialisten (www.kennisinstituut.nl) assisted the guideline development group. The guideline was financed by Stichting Kwaliteitsgelden Medisch Specialisten (SKMS) which is a quality fund for medical specialists in The Netherlands.
Samenstelling werkgroep
Guideline development group (GDG)
A multidisciplinary guideline development group (GDG) was formed for the development of the guideline in 2020. The GDG consisted of representatives from all relevant medical specialization fields which were using intravascular contrast administration in their field.
All GDG members have been officially delegated for participation in the GDG by their scientific societies. The GDG has developed a guideline in the period from June 2020 until November 2022. The GDG is responsible for the complete text of this guideline.
Guideline development group
- Dekkers I.A. (Ilona), clinical epidemiologist and radiologist, Leiden University Medical Center, Leiden
- Geenen R.W.F. (Remy), radiologist, Noordwest Ziekenhuisgroep, Alkmaar
- Kerstens M.N. (Michiel), internist-endocrinologist, University Medical Centre Groningen
- Krabbe J.G. (Hans), clinical chemist-endocrinologist, Medisch Spectrum Twente, Enschede
- Rossius M.J.P. (Mariska), radiologist, Erasmus Medical Centre, Rotterdam
- Uyttenboogaart M. (Maarten), neurologist and neuro-interventionalist, University Medical Centre Groningen
- van de Luijtgaarden K.M. (Koen), vascular surgeon, Maasstad Ziekenhuis, Rotterdam
- van der Molen A.J. (Aart), chair guideline development group, radiologist, Leiden University Medical Center, Leiden
- van der Wolk S.L. (Sabine), gynaecologist-obstetrician, Haga Ziekenhuis, Den Haag
- van de Ven A.A.J.M. (Annick), internist-allergologist-immunologist, University Medical Centre Groningen (until 1.7.2022)
- van der Houwen, T.B. (Tim), internist-allergologist-immunologist, Amsterdam University Medical Center (from 1.7.2022)
Invited experts
- van Maaren M.S. (Maurits), internist-allergologist-immunologist, Erasmus MC, Rotterdam
Methodological support
- Abdollahi M. (Mohammadreza), advisor, Knowledge Institute of the Federation Medical Specialists
- Labeur Y.J. (Yvonne), junior advisor, Knowledge Institute of the Federation Medical Specialists
- Mostovaya I.M. (Irina), senior advisor, Knowledge Institute of the Federation Medical Specialists
Belangenverklaringen
Conflicts of interest
The GDG members have provided written statements about (financially supported) relations with commercial companies, organisations or institutions that were related to the subject matter of the guideline. Furthermore, inquiries have been made regarding personal financial interests, interests due to personal relationships, interests related to reputation management, interest related to externally financed research and interests related to knowledge valorisation. The statements on conflict of interest can be requested from the administrative office of Kennisinstituut van de Federatie Medisch Specialisten (secretariaat@kennisinstituut.nl) and were summarised below.
|
Last name |
Function |
Other positions |
Personal financial interests |
Personal relations |
Reputation management |
Externally financed research |
Knowledge valorisation |
Other interests |
Signed |
Actions |
|
Dekkers IA |
Radiologist, LUMC |
Clinical Epidemiologist
Member of contrast media safety committee, European Society of Urogenital Radiology (no payment)
Member, Gadolinium Research and Education Committee, European Society of Magnetic Resonance in Medicine, and Biology (no payment) |
No |
No |
No |
No |
No |
Received consultancy fees from Guerbet, 2019- 2022 |
July 24th, 2020, Reaffirmed October 12th, 2022 |
No restrictions: received in part 3 of the guideline speaker fees, but this guideline does not mention specific medication, not of working mechanism, nor of side effects. |
|
Geenen RWF |
Radiologist, Noordwest ziekenhuisgroep /Medisch specialisten Noordwest |
Member of contrast media safety committee, European Society of Urogenital Radiology (no payment) |
No |
No |
No |
No |
No |
No |
April 11th, 2020, Reaffirmed October 12th, 2022 |
No restrictions |
|
Houwen T, van der |
Internist - Immunologist - Allergologist, Amsterdam UMC, also seconded allergologist in Huid Medisch Centrum |
None |
None |
None |
None |
None |
None |
None |
July 11th, 2022 Reaffirmed October 12th, 2022 |
No restrictions |
|
Kerstens MN |
Internist- endocrinologist, UMCG |
Chairman Bijniernet (no payment) |
No |
No |
No |
No |
No |
No |
July 1st, 2020, reaffirmed October 25th, 2022 |
No restrictions |
|
Krabbe JG |
Clinical chemist, Medisch Spectrum Twente |
No |
No |
No |
No |
No |
No |
No |
September 1st, 2020, Reaffirmed October 13th, 2022 |
No restrictions |
|
Luijtgaarden KM, van de |
Vascular surgeon, Maasland Ziekenhuis |
No |
No |
No |
No |
No |
No |
No |
August 1st, 2020, reaffirmed October 26th, 2022 |
No restrictions |
|
Molen AJ, van der |
Radiologist LUMC |
Member of contrast media safety committee, European Society of Urogenital Radiology (no payment)
Member, Gadolinium Research and Education Committee, European Society of Magnetic Resonance in Medicine, and Biology (no payment) |
No |
No |
No |
No |
No |
Received consultancy fees from Guerbet, 2019- 2022 |
July, 24th, 2020 Reaffirmed October 12th, 2022 |
No restrictions: received in part 3 of the guideline speaker fees, but this guideline does not mention Specific medication, not of working mechanism, nor of side effects. |
|
Rossius MJP |
Radiologist Erasmus Medical Centre |
Medical coordinator (no payment) |
No |
No |
No |
No |
No |
No |
April 7th, 2020, Reaffirmed October 13th, 2022 |
No restrictions |
|
Uyttenboogaart M |
Neurologist and neuro- interventionalist UMCG |
Advisor International Federation of Orthopaedic Manipulative Physical Therapist / Nederlandse Vereniging Manuele Therapie |
No |
No |
Subsidy Hart Stichting for CONTRAST (Consortium of New Treatments in Acute Stroke): WP8 Stroke logistics and Epidemiology: financing of 2 PhD students by the Hart Stichting / PPS Allowance |
Work package leader CONTRAST (Consortium of New Treatments in Acute Stroke): WP8 Stroke logistics and Epidemiology |
No |
No |
June 30th, 2020, reaffirmed October 26th, 2022 |
No restrictions: the CONTRAST consortium wp8 is only about organisation and treatment of stroke. Stroke is not in this guideline. |
|
Ven AAJM, van de |
Internist- allergologist- immunologist, UMCG |
Education and research related to work as internist- allergist |
No |
No |
No |
No |
No |
No |
April 7th, 2020, Reaffirmed October 19th, 2022 |
No restrictions |
|
Wolk S, van der |
Gynaecologist- obstetrician, Haga Ziekenhuis |
No |
No |
No |
No |
No |
No |
No |
June 30th, 2021, reaffirmed October 25th, 2022 |
No restrictions |
Inbreng patiëntenperspectief
Input of patient’s perspective
The guideline does not address a specific adult patient group, but a diverse set of diagnoses. Therefore, it was decided to invite a broad spectrum of patient organisations for the stakeholder consultation. The stakeholder consultation was performed at the beginning of the process for feedbacking on the framework of subjects and clinical questions addressed in the guideline, and during the commentary phase to provide feedback on the concept guideline. The list of organisations which were invited for the stakeholder consultation can be requested from the Kennisinstituut van de Federatie Medisch Specialisten (secretariaat@kennisinstituut.nl). In addition, patient information on safe use of contrast media in pregnancy and lactation was developed for Thuisarts.nl, a platform to inform patients about health and disease.
Implementatie
Implementation
During different phases of guideline development, implementation and practical enforceability of the guideline were considered. The factors that could facilitate or hinder the introduction of the guideline in clinical practice have been explicitly considered. The implementation plan can be found in the ‘Appendices to modules’. Furthermore, quality indicators were developed to enhance the implementation of the guideline. The indicators can also be found in the ‘Appendices to modules’.
Werkwijze
Methodology
AGREE
This guideline has been developed conforming to the requirements of the report of Guidelines for Medical Specialists 2.0 by the advisory committee of the Quality Counsel (www.kwaliteitskoepel.nl). This report is based on the AGREE II instrument (Appraisal of Guidelines for Research & Evaluation II) (www.agreetrust.org), a broadly accepted instrument in the international community and based on the national quality standards for guidelines: “Guidelines for guidelines” (www.zorginstituutnederland.nl).
Identification of subject matter
During the initial phase of the guideline development, the GDG identified the relevant subject matter for the guideline. The framework is consisted of both new matters, which were not yet addressed in part 1 and 2 of the guideline, and an update of matters that were subject to modification (for example in case of new published literature). Furthermore, a stakeholder consultation was performed, where input on the framework was requested.
Clinical questions and outcomes
The outcome of the stakeholder consultation was discussed with the GDG, after which definitive clinical questions were formulated. Subsequently, the GDG formulated relevant outcome measures (both beneficial and harmful effects). The GDG rated the outcome measures as critical, important and of limited importance (GRADE method). Furthermore, where applicable, the GDG defined relevant clinical differences.
Search and select
For clinical questions, specific search strategies were formulated, and scientific articles published in several electronic databases were searched. First, the studies that potentially had the highest quality of research were reviewed. The GDG selected literature in pairs (independently of each other) based on the title and abstract. A second selection was performed by the methodological advisor based on full text. The databases used, selection criteria and number of included articles can be found in the modules, the search strategy in the appendix.
Quality assessment of individual studies
Individual studies were systematically assessed, based on methodological quality criteria that were determined prior to the search. For systematic reviews, a combination of the AMSTAR checklist and PRISMA checklist was used. For RCTs the Cochrane risk of bias tool and suggestions by the CLARITY Group at McMaster University were used, and for cohort studies/observational studies the risk of bias tool by the CLARITY Group at McMaster University was used. The risk of bias tables can be found in the separate document Appendices to modules.
Summary of literature
The relevant research findings of all selected articles were shown in evidence tables. The evidence tables can be found in the separate document Appendices to modules. The most important findings in literature were described in literature summaries. When there were enough similarities between studies, the study data were pooled.
Grading quality of evidence and strength of recommendations
The strength of the conclusions of the included studies was determined using the GRADE- method. GRADE stands for Grading Recommendations Assessment, Development and Evaluation (see http://www.gradeworkinggroup.org) (Atkins, 2004). GRADE defines four levels for the quality of scientific evidence: high, moderate, low, or very low. These levels provide information about the certainty level of the literature conclusions (http://www.guidelinedevelopment.org/handbook).
The evidence was summarized in the literature analysis, followed by one or more conclusions, drawn from the body of evidence. The level of evidence for the conclusions can be found above the conclusions. Aspects such as expertise of GDG members, local expertise, patient preferences, costs, availability of facilities and organisation of healthcare aspects are important to consider when formulating a recommendation. These aspects are discussed in the paragraph justifications. The recommendations provide an answer to the clinical question or help to increase awareness and were based on the available scientific evidence and the most relevant justifications.
Appendices
Internal (meant for use by scientific society or its members) quality indicators were developed with the guideline and can be found in the separate document Appendices to modules. In most cases, indicators were not applicable. For most questions, additional scientific research on the subject is warranted. Therefore, the GDG formulated knowledge gaps to aid in future research, which can be found in the separate document Appendices to modules.
Commentary and authorisation phase
The concept guideline was subjected to commentaries by the involved scientific societies. The list of parties that participated in the commentary phase can be requested from the Kennisinstituut van de Federatie Medisch Specialisten (secretariaat@kennisinstituut.nl). The commentaries were collected and discussed with the GDG. The feedback was used to improve the guideline; afterwards the GDG made the guideline definitive. The final version of the guideline was offered to the involved scientific societies for authorization and was authorized.
Literature
Brouwers MC, Kho ME, Browman GP, et al. AGREE Next Steps Consortium. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010; 182(18): E839-E842.
Medisch Specialistische Richtlijnen 2.0. Adviescommissie Richtlijnen van de Raad Kwaliteit, 2012. Available at: [URL].
Schünemann H, Brożek J, Guyatt G, et al. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group, 2013. Available at: [URL].
Schünemann HJ, Oxman AD, Brozek J, et al. Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. BMJ. 2008;336(7653):1106- 1110. Erratum published in: BMJ 2008;336(7654).
Ontwikkeling van Medisch Specialistische Richtlijnen: stappenplan. Kennisinstituut van Medisch Specialisten, 2020.
