Bèta-3 receptor agonist
Uitgangsvraag
Wat is de waarde van een bèta-3-receptor agonist (sympathicomimeticum) bij UI in de tweede- en derdelijnszorg in vergelijking met geen behandeling of antimuscarinicum dan wel een combinatie?
Aanbeveling
Gebruik van beta3 sympathicomimetica kan overwogen worden als alternatief voor antimuscarinica, indien antimuscarinica onvoldoende effect genereren of de bijwerkingen intolerabel zijn. Staak de behandeling indien er na 6 weken geen verbetering van klachten optreedt.
Het toepassen van combinatietherapie met een beta3 sympathicomimeticum, in plaats van ophogen van antimuscarinica kan overwogen worden. Let hierbij op tolereerbaarheid bijwerkingen en staak de behandeling indien er na 6 weken geen verbetering van klachten optreedt.
Overweeg de bloeddruk te evalueren bij gelijktijdig gebruik van beta3 sympathicomimetica en antihypertensiva.
Overwegingen
Voor- en nadelen van de interventie en de kwaliteit van het bewijs
Er is een literatuuronderzoek verricht naar de effectiviteit en veiligheid van beta-3 receptor agonisten in de behandeling van urine-incontinentie (UI). In totaal zijn er 10 RCT’s gevonden die beta-3 receptor agonisten vergeleken met placebo, een antimuscarinicum of een combinatie van een beta-3 receptor agonist en een antimuscarinicum.
1. Beta-3 receptor agonist versus placebo
Voor de vergelijking tussen mirabegron(/vibegron) en placebo werd de bewijskracht voor de uitkomstmaten volume voided/micturition en urinary incontinence episodes/24h beoordeeld als gemiddeld vanwege risico op bias door de rol van de studie sponsors bij de opzet en uitvoering van de studie. Het gebruik van mirabegron bij volwassenen met UI leidt mogelijk niet tot een klinisch relevant verschil in uitgescheiden volume per mictie of tot minder urine-incontinentie episodes per 24 uur (er zijn minder urine-incontinentie episodes/24u in de Beta-3 agonist groep vergeleken met placebo -0.26 (95%CI -0.39 to -0.14). Voor de overige uitkomstmaten komt de bewijskracht uit op laag of zeer laag. De overall bewijskracht is hierdoor laag, wat betekent dat nieuwe studies kunnen leiden tot andere inzichten. Er kunnen op basis van alleen de literatuur geen sterke aanbevelingen geformuleerd worden over de waarde van mirabegron bij volwassenen met UI in de tweede- en derdelijnszorg.
2. Beta-3 receptor agonist versus antimuscarinica
Voor de vergelijking tussen mirabegron/vibegron en antimuscarinica werd de bewijskracht voor alle uitkomstmaten beoordeeld als laag of zeer laag. Redenen hiervoor zijn onder andere risico op bias, brede betrouwbaarheidsintervallen, verscheidenheid in de definities van uitkomstmaten, conflicterende resultaten en kleine onderzoekspopulaties. Dit leidt tot een zeer lage overall bewijskracht. Dit betekent dat nieuwe studies kunnen leiden tot andere inzichten. Er kunnen op basis van alleen de huidige literatuur geen eenduidige aanbevelingen geformuleerd worden over de waarde van mirabegron/vibegron vergeleken met antimuscarinica bij volwassenen met UI in de tweede- en derdelijnszorg.
3. Beta-3 receptor agonist versus combinatie (beta-3 receptor agonist + antimuscarinica)
Voor de vergelijking tussen mirabegron en een combinatie van mirabegron met solifenacin werd de bewijskracht voor alle uitkomstmaten beoordeeld als laag of zeer laag. Redenen hiervoor zijn risico op bias, brede betrouwbaarheidsintervallen, conflicterende resultaten en kleine onderzoekspopulaties. Dit leidt tot een zeer lage overall bewijskracht, wat betekent dat andere studies kunnen leiden tot nieuwe inzichten. Herschorn (2017) vermeldt bijvoorbeeld een afname van incontinentie episodes per 24u van -1.76 in de mirabegron groep (n = 406), en -1.98 in de combinatie therapie groep (n = 816), maar omdat er geen spreiding wordt gerapporteerd is de grade systematiek niet toe te passen. Er kunnen dus op basis van alleen de literatuur geen sterke aanbevelingen geformuleerd worden over de waarde van mirabegron vergeleken met een combinatie van mirabegron met solifenacin bij volwassenen met UI in de tweede- en derdelijnszorg.
Naast de hierboven genoemde literatuur analyse is ook gekeken naar aanbevelingen vanuit andere (Europese en landelijke) richtlijnen aangaande de behandeling van aandrang urine-incontinentie met beta-3 agonisten:
In de richtlijn van de European Association of Urology (EAU, 2020) over non-neurogenic female LUTS wordt het volgende beschreven over het gebruik van beta-3 agonisten bij vrouwen:
Mirabegron en vibegron zijn beter dan placebo voor de verbetering van OAB/UUI symptomen. Beta-3 agonists zijn net zo effectief als antimuscarinica in de behandeling van OAB, maar met minder klachten van droge mond. De meest genoemde bijwerkingen in de beta-3 agonisten groep waren hypertensie (7.3%), nasopharyngitis (3.4%) en urineweginfecties (3%), maar deze waren vergelijkbaar met de placebo groep. Het is onduidelijk of dit bijwerkingen betreffen of toevalsbevindingen zijn. Aanbeveling vanuit de EAU richtlijn is dan ook om beta-3 agonisten te gebruiken als alternatief voor antimuscarinica als conservatieve behandeling faalt. De tweede aanbeveling luidt dat patiënten die inadequaat behandeld zijn met solifenacin 5 mg meer baat kunnen hebben bij de toevoeging van mirabegron, dan bij het ophogen van de dosis solifenacine.
Tevens wordt in dezelfde EAU-richtlijn gesteld dat Mirabegron effectief en veilig is bij ouderen. In deze richtlijn wordt ook aandacht besteed aan medicatie gebruik bij kwetsbare ouderen. Literatuur uit de EAU-richtlijn komt op veel vlakken niet overeen met de gevonden literatuur in de samenvatting van deze module. Omdat een overzicht van selectiecriteria en redenen voor excluderen van studies niet gepresenteerd is binnen deze EAU richtlijn, kunnen oorzaken voor verschillen met onze uitkomsten niet worden achterhaald.
Raadpleeg voor medicatie bij ouderen de betreffende module.
In de NVOG richtlijn urine incontinentie bij vrouwen (verwacht 2023) wordt het volgende geschreven over beta 3 sympathicomimetica:
Er zijn geen studies geïncludeerd die beta3-agonisten (bijv., mirabregon) bestuderen in een populatie van uitsluitend vrouwen, dit is genoteerd als kennislacune. Er wordt in deze richtlijn wel melding gemaakt van studies die beta-3 sympathicomimetica bij gemengde populaties hebben onderzocht:
De EMPOWUR-trial bestudeert het nog niet in Nederland beschikbare vibegron (Staskin, 2020). In deze internationale dubbel geblindeerde RCT werden 1518 patiënten (85% vrouw) geïncludeerd die 12 weken vibegron 75mg, tolterodine 4mg of placebo kregen. In de vibegron groep had 52.4% een ≥75% reductie van urge incontinentie episodes vs. 47.6% van de patiënten die tolterodine kregen en 36.38% in de placebogroep (p<0.05). De resultaten van deze studie zijn terug te vinden in de literatuursamenvatting van deze module.
Een ander artikel beschrijft de veiligheid en effectiviteit van alle door de producent gesponsorde fase 2-4 studies met mirabegron met antimuscarinica (solifenacine en tolterodine) en placebo in mannen en vrouwen (65-76% is vrouw in deze studies) met OAB wereldwijd (Chapple, 2020). Medicatie-gerelateerde bijwerkingen komen meer voor bij antimuscarinica, dan bij mirabegron en de placebogroep. Het vóórkomen van hypertensie was gelijk in de antimuscarinica, mirabegron en in de placebogroep. Mirabegron zou een gunstiger bijwerkingenprofiel hebben bij ouderen en patiënten met bekende obstipatie. Hierbij moet opgemerkt worden dat dit onderzoek gefinancierd is door de producent. Deze studie is echter niet opgenomen in de huidige literatuursamenvatting omdat dit een gepoolde analyse van meerdere studies betreft, zonder systematische selectiecriteria.
In de SYNERGY II dubbel geblindeerde multicenter RCT werden 1829 patiënten (80% vrouw) geïncludeerd met urge urine-incontinentie. Zij kregen solifenacine 5mg, mirabegron 50mg of een combinatie van beiden gedurende 1 jaar (Mueller, 2019). Combinatietherapie gaf minder incontinentie episodes vergeleken met mirabegron of solifenacine monotherapie (versus mirabegron: adjusted mean difference (AMD) −0.5, 95%CI −0.7 tot −0.2, p < 0.001; versus solifenacine: AMD −0.1, 95% CI −0.4 tot 0.1, p = 0.002). Daarnaast werd een verlaagde mictiefrequentie geregistreerd (versus mirabegron: AMD −0.5, 95% CI −0.8 tot −0.2, p < 0.001; versus solifenacine: AMD −0.4, 95% CI −0.7 tot −0.1, p = 0.004). De publicatie van Mueller (2019) is echter niet meegenomen in de literatuursamenvatting van dit hoofdstuk, gezien dit een subgroep analyse betreft van de SYNERGY-trial door Gratzke (2018), en dus een analyse op observationele data is. Een andere publicatie van de SYNERGY-trial laat de PROs (patient reported outcomes) zien waarbij de combinatietherapie verbetering geeft op HRQOL-parameters zoals de OAB-q symptom bother score (Robinson, 2018). Deze publicatie is echter niet meegenomen in de literatuursamenvatting, omdat de gerapporteerde uitkomsten niet overeenkwamen met de vooraf gedefinieerde PICO.
Op basis hiervan wordt geconcludeerd dat mirabegron mogelijk effectiever is dan placebo en even effectief als antimuscarinica voor de verbetering van OAB/urge incontinentie symptomen. Hierbij wordt genoteerd dat dit niet naar voren is gekomen uit de samenvatting van de literatuur van die richtlijn (omdat de PICO-vraag zich heeft gelimiteerd tot literatuur met uitsluitend vrouwen), maar dus wel wordt beschreven in de EAU richtlijn urine-incontinentie. Het uiteindelijke advies van de NVOG richtlijn urine-incontinentie bij vrouwen is dan ook om mirabegron aan te bieden als alternatief voor antimuscarinica bij gebrek aan effect of hinderlijke bijwerkingen.
De RCT van Kosilov (2015) staat beschreven in de module ‘Medicamenteuze behandeling ouderen’ van deze richtlijn. Hierin werd ten opzichte van placebo een gemiddelde afname van 2,1 episodes van urine-incontinentie gezien voor mirabegron. Echter, kon gezien het ontbreken van spreidingsmaten deze uitkomst in de betreffende module niet met de Grade systematiek beoordeeld worden op kwaliteit.
Op basis van de huidige PICO en literatuurselectie, met analyse en toepassing van de Grade systematiek, is er in de huidige richtlijn echter onvoldoende bewijs dat beta3 receptor sympathicomimetica effectief zijn in de behandeling van OAB/urge incontinentie, vergeleken met placebo, of met antimuscarinica. Combinatietherapie (antimuscarinica met een beta3 sympathicomimeticum) geeft op basis van alleen de literatuur ook geen sterke verbetering bij volwassenen met UI in de tweede- en derdelijnszorg.
Deze bevindingen conflicteren echter met de aanbevelingen van de recent herziene EAU richtlijn non-neurogenic female LUTS en de NVOG richtlijn urine-incontinentie bij vrouwen zoals hierboven beschreven. De EAU baseert zich bij haar aanbevelingen mede op studies met een observationeel design. De selectiecriteria voor studies en de weging die de EAU hieraan geeft, zijn niet gepubliceerd.
Door de strenge literatuurselectie ontbreken observationele cohort studies in de analyse van deze module van de NVU, wat het verschil in uitkomst met de EAU mogelijk voor een deel zou kunnen verklaren. Dit is echter niet te achterhalen. Probleem is verder dat er ten eerste geen duidelijk antwoord gegeven kan worden op de vraag wat het minimal clinically important difference is waarop de literatuur succes of falen van therapie beoordeelt. Een klein verschil in klinische relevantie wordt nu beschouwd als niet relevant, terwijl de huidige analyse juist allemaal hele kleine verschillen ten faveure van beta3 sympathicomimetica toont. Ten tweede zijn de studieresultaten bijna allemaal gebaseerd op een ‘mean’, terwijl de uitkomsten van deze studies vaak niet normaal verdeeld zijn, waardoor het beter zou zijn om een ‘median’ te bepalen. Verder is er geen verschil in effectiviteit tussen gebruik van beta3 sympathicomimetica en antimuscarinica aangetoond. Daarnaast laten alle studies een groot placebo-effect zien. Er zijn ook geen studies gedaan naar patiënttevredenheid.
Dit maakt het lastig om de huidige resultaten te interpreteren en om een concreet behandeladvies te geven. Na ampel beraad wordt geadviseerd dat gebruik van beta3 sympathicomimetica niet de behandeling van voorkeur is, maar wel overwogen kan worden als antimuscarinica falen of de bijwerkingen intolerabel zijn. Na 6 weken moet geëvalueerd worden of de behandeling aanslaat. Indien er geen verbetering optreedt, dient het beta3 sympathicomimeticum te worden gestaakt.
Waarden en voorkeuren van patiënten (en evt. hun verzorgers)
De tolerantie (balans tussen effectiviteit en bijwerkingen) van antimuscarinica en mirabegron is onderzocht in o.a. de PREFER-studie (Staskin, 2018), waarbij patiënten (73% vrouw) met OAB mirabegron met tolterodine in een cross-over design als monotherapie gedurende 3 maanden gebruikten. De ‘medication tolerability score’ en klinische verbetering was meer uitgesproken in de mirabegron groep dan bij tolterodine groep, en meer uitgesproken bij vrouwen, patiënten ouder ≥65 jaar en patiënten zonder incontinentie bij start van de studie. Een alternatief voor de behandeling van aandrang urine-incontinentie in plaats van conservatieve therapie (middels bekkenfysiotherapie/blaastraining en medicatie) is PTNS, danwel botox of neuromodulatie. Deze behandelingen zijn echter veel tijdrovender (PTNS) en invasiever (PTNS, botox, neuromodulatie) voor de patiënt.
Kosten (middelenbeslag)
Ten aanzien van de kosten varieert de prijs van antimuscarinica per dag tussen de € 0.24 (solifenacine 10mg/oxybutinine 5mg) en de € 0.90 (fesoterodine 4mg/mirabegron 50mg) (Farmacotherapeutisch Kompas, solfenacine, oxybutinine, fesoterodine, mirabegron 2023). Op jaarbasis kan dit een behoorlijke impact geven op het eigen risico (zie onderstaande tabel). Voor deze geneesmiddelen hoeft naast het eigen risico niet te worden bijbetaald.
Aanvaardbaarheid, haalbaarheid en implementatie
Bij mirabegron is de tijd tot maximaal effect langer dan bij antimuscarinica, namelijk rond de 6 weken. Raadzaam is het recept voor maximaal 6 weken uit te schrijven met het oog op duurzaamheid en na 6 weken telefonisch de behandeling te evalueren t.a.v. effect en bijwerkingen. Patiënten kunnen geïnstrueerd worden bij hinderlijke bijwerkingen de medicatie te staken en eerder met de behandelaar contact op te nemen. Frequent gerapporteerde bijwerkingen van mirabegron zijn tachycardie (1-10%) en palpitaties (0,1-1%). Daarnaast adviseert de FDA (U.S. Food and Drug Administration 2015) periodieke bloeddrukcontrole bij patiënten die mirabegron gebruiken, maar additionele studies zijn vereist om een volledige beoordeling van cardiale effecten ten tijde van gebruik van mirabegron te kunnen verrichten.
Rationale van de aanbeveling: weging van argumenten voor en tegen de interventies
De werkgroep concludeert na ampel beraad dat gebruik van beta3 sympathicomimetica niet de behandeling van voorkeur is bij aandrang urine-incontinentie, maar wel overwogen kan worden als antimuscarinica falen of de bijwerkingen intolerabel zijn. Na 6 weken moet geëvalueerd worden of de behandeling aanslaat. Indien er geen verbetering optreedt, dient het beta3 sympathicomimeticum te worden gestaakt.
Daarnaast kan mirabegron de bloeddruk verhogen. De FDA adviseert dan ook om een bloeddrukcontrole bij patiënten die mirabegron gebruiken, maar additionele studies zijn vereist om een volledige beoordeling van cardiale effecten ten tijde van gebruik van mirabegron te kunnen verrichten. Wees bovendien alert op relevante interacties op CYP-enzymniveau indien er sprake is van polyfarmacie.
Onderbouwing
Achtergrond
Antimuscarinica (ook vaak anticholinergica genoemd) vormen de hoeksteen van de medicamenteuze behandeling van urge urine-incontinentie (UUI). De werkzaamheid berust op blokkade van de muscarine-receptoren in de blaaswand. Dit vermindert de detrusorcontractiliteit en verandert het blaasgevoel. Ieder antimuscarinicum heeft een eigen farmacologisch profiel (bijvoorbeeld met betrekking tot affiniteit voor muscarine-receptoren of interacties), farmacokinetiek (bijvoorbeeld vetoplosbaarheid en halfwaardetijd) en toedieningsvorm (orale directe/vertraagde afgiftepreparaten, transdermaal, intravesicaal).
De meest voorkomende bijwerking van antimuscarinica is een droge mond, maar ook obstipatie, wazig zien, moeheid en cognitieve disfunctie kunnen vóórkomen. Mensen met een droge mond zullen geneigd zijn meer te gaan drinken; het is niet duidelijk of deze toegenomen vochtinname ook leidt tot het tenietdoen van het behandeleffect.
Mirabegron is een bèta-3 sympathicomimeticum dat sinds 1 april 2014 in Nederland beschikbaar is. In de setting van de verrichte trials (en dus de specifieke patiëntengroepen die daarin zijn onderzocht) lijken de adrenerg gemedieerde bijwerkingen van mirabegron mild en klinisch amper relevant te zijn. Ten tijde van het schrijven van deze update, is nog onbekend wanneer vibegron beschikbaar zal zijn op de Nederlandse markt.
Deze module gaat in op de waarde van medicatie. Raadpleeg voor conservatieve behandelingen zoals blaastraining de betreffende modules in deze richtlijn.
Conclusies / Summary of Findings
1. Beta-3 receptor agonist versus placebo/no treatment
1. Urinary incontinence episodes/24h
|
Moderate GRADE |
Beta-3 receptor agonist treatment likely results in little to no difference in urinary incontinence episodes/24h when compared with placebo treatment in adults with UI.
Source: Abrams, 2015; Herschorn, 2017; Khullar, 2013; Kuo, 2015; Nitti, 2013; Yamaguchi, 2014; Yamaguchi, 2015. |
2. Urgency episodes/24h
|
Low GRADE |
Beta-3 receptor agonist treatment may result in little to no difference in urgency episodes/24h when compared with placebo treatment in adults with UI.
Source: Abrams, 2015; Khullar, 2013; Kuo, 2015; Yamaguchi, 2014; Yamaguchi, 2015. |
3. Volume voided/micturition
|
Moderate GRADE |
Beta-3 receptor agonist treatment likely results in little to no difference in volume voided/micturition when compared with placebo treatment in adults with UI.
Source: Abrams, 2015; Herschorn, 2017; Khullar, 2013; Kuo, 2015; Nitti, 2013; Yamaguchi, 2014; Yamaguchi, 2015. |
4. Micturitions/24h
|
Low GRADE |
Beta-3 receptor agonist treatment may result in little to no difference in micturitions/24h when compared with placebo treatment in adults with UI.
Source: Abrams, 2015; Herschorn, 2017; Khullar, 2013; Kuo, 2015; Nitti, 2013; Shin, 2018; Yamaguchi, 2014; Yamaguchi, 2015. |
5. Total urine volume
|
no GRADE |
No evidence was found regarding the effect of beta-3 receptor agonist treatment on total urine volume compared with placebo treatment in adults with UI.
Source: - |
6. Adverse events
|
Low GRADE |
Beta-3 receptor agonist treatment may result in little to no difference in adverse events when compared with placebo treatment in adults with UI.
Source: Abrams, 2015; Herschorn, 2017; Khullar, 2013; Kuo, 2015; Shin, 2018; Yamaguchi, 2014; Yamaguchi, 2015. |
6a. Blood pressure
|
Very low GRADE |
The evidence is very uncertain about the effect of beta-3 receptor agonist treatment on blood pressure when compared with placebo treatment in adults with UI.
Source: Abrams, 2015; Kuo, 2015; Shin, 2018; Yamaguchi, 2014. |
6b. Hypertension
|
Low GRADE |
Beta-3 receptor agonist treatment may result in little to no difference in hypertension when compared with placebo treatment in adults with UI.
Source: Abrams, 2015; Khullar, 2013; Kuo, 2015; Nitti, 2013; Yamaguchi, 2014; Yamaguchi, 2015. |
6c. Pulse rate
|
Low GRADE |
Beta-3 receptor agonist treatment may result in little to no difference in pulse rate when compared with placebo treatment in adults with UI.
Source: Abrams, 2015; Shin, 2018; Yamaguchi, 2014; Yamaguchi, 2015. |
6d. Tachycardia
|
Very low GRADE |
The evidence is very uncertain about the effect of beta-3 receptor agonist treatment on tachycardia when compared with placebo treatment in adults with UI.
Source: Abrams, 2015; Nitti, 2013; Yamaguchi, 2014; Yamaguchi, 2015. |
6e. Palpitations
|
Very Low GRADE |
The evidence is very uncertain about the effect of beta-3 receptor agonist treatment on palpitations when compared with placebo treatment in adults with UI.
Source: Yamaguchi, 2015. |
2. Beta-3 receptor agonist versus antimuscarinic
1. Urinary incontinence episodes/24h
|
Very low GRADE |
The evidence is very uncertain about the effect of beta-3 receptor agonist treatment on urinary incontinence episodes/24h when compared with antimuscarinic treatment in adults with UI.
Source: Herschorn, 2017; Staskin, 2021; Suzuki, 2021. |
2. Urgency episodes/24h
|
Very low GRADE |
The evidence is very uncertain about the effect of beta-3 receptor agonist treatment on urgency episodes/24h when compared with antimuscarinic treatment in adults with UI.
Source: Staskin, 2021; Suzuki, 2021. |
3. Volume voided/micturition
|
Low GRADE |
Beta-3 receptor agonist treatment may result in little to no difference in volume voided/micturition when compared with antimuscarinic treatment in adults with UI.
Source: Abrams, 2015; Herschorn, 2017; Suzuki, 2021. |
4. Micturitions/24h
|
Low GRADE |
Beta-3 receptor agonist treatment may result in little to no difference in micturitions/24h when compared with antimuscarinic treatment in adults with UI.
Source: Herschorn, 2017; Staskin, 2021. |
5. Total urine volume
|
Very low GRADE |
The evidence is very uncertain about the effect of beta-3 receptor agonist treatment on total urine volume when compared with antimuscarinic treatment in adults with UI.
Source: Suzuki, 2021. |
6. Adverse events
|
Very low GRADE |
The evidence is very uncertain about the effect of beta-3 receptor agonist treatment on adverse events when compared with antimuscarinic treatment in adults with UI.
Source: Abrams, 2015; Herschorn, 2017; Staskin, 2021; Suzuki, 2021. |
6a. Blood pressure
|
Low GRADE |
Beta-3 receptor agonist treatment may result in little to no difference in blood pressure when compared with antimuscarinic treatment in adults with UI.
Source: Abrams, 2015. |
6b. Hypertension
|
Low GRADE |
Beta-3 receptor agonist treatment may result in little to no difference in hypertension when compared with antimuscarinic treatment in adults with UI.
Source: Abrams, 2015; Staskin, 2021. |
6c. Pulse rate
|
Low GRADE |
Beta-3 receptor agonist treatment may result in little to no difference in pulse rate when compared with antimuscarinic treatment in adults with UI.
Source: Abrams, 2015. |
6d. Tachycardia
|
Very low GRADE |
The evidence is very uncertain about the effect of beta-3 receptor agonist treatment on tachycardia when compared with antimuscarinic treatment in adults with UI.
Source: Abrams, 2015. |
6e. Palpitations
|
no GRADE |
No evidence was found regarding the effect of beta-3 receptor agonist treatment on palpitations compared with antimuscarinic treatment in adults with UI.
Source: - |
3. Beta-3 receptor agonist versus combination (beta-3 receptor agonist + antimuscarinic)
1. Urinary incontinence episodes/24h
|
no GRADE |
No GRADE-assessment could be performed.
Source: Herschorn, 2017. |
2. Volume voided/micturition
|
Low GRADE |
Beta-3 receptor agonist treatment may result in little to no difference in volume voided/micturition when compared with combination treatment in adults with UI.
Source: Abrams, 2015; Herschorn, 2017. |
3. Micturitions/24h
|
no GRADE |
No GRADE-assessment could be performed.
Source: Herschorn, 2017. |
4. Urgency episodes/24h, 5. Total urine volume
|
no GRADE |
No evidence was found regarding the effect of beta-3 receptor agonist treatment on urgency episodes/24h and total urine volume compared with combination treatment in adults with UI.
Source: - |
6. Adverse events
|
Very low GRADE |
The evidence is very uncertain about the effect of beta-3 receptor agonist treatment on adverse events when compared with combination treatment in adults with UI.
Source: Abrams, 2015; Herschorn. |
6a. Blood pressure
|
Low GRADE |
Beta-3 receptor agonist treatment may result in little to no difference in blood pressure when compared with combination treatment in adults with UI.
Source: Abrams, 2015. |
6b. Hypertension
|
Low GRADE |
Beta-3 receptor agonist treatment may result in little to no difference in hypertension when compared with combination treatment in adults with UI.
Source: Abrams, 2015. |
6c. Pulse rate
|
Low GRADE |
Beta-3 receptor agonist treatment may result in little to no difference in pulse rate when compared with combination treatment in adults with UI.
Source: Abrams, 2015. |
6d. Tachycardia
|
Low GRADE |
Beta-3 receptor agonist treatment may result in little to no difference in tachycardia when compared with combination treatment in adults with UI.
Source: Abrams, 2015. |
6e. Palpitations
|
no GRADE |
No evidence was found regarding the effect of beta-3 receptor agonist treatment on palpitations compared with combination treatment in adults with UI.
Source: - |
Samenvatting literatuur
1. Beta-3 receptor agonist versus placebo/no treatment
Description of studies
For the first comparison, a total of eight RCTs were found (Abrams, 2015; Herschorn, 2017; Khullar, 2013; Kuo, 2015; Nitti, 2013; Shin, 2018; Yamaguchi, 2014; Yamaguchi, 2015). Study characteristics, including the outcome measures reported, are shown in Table 1. Results for studies in the elderly population are described in the ‘chapter drug treatment for elderly’ in this guideline.
Table 1: study characteristics of included studies for comparison 1
|
Study |
Patients |
Intervention |
Comparison |
Outcomes of interest reported |
N |
Follow-up |
||
|
Characteristics |
Type/dose |
Characteristics |
Type |
|||||
|
Abrams, 2015 |
Adults with OAB > 3 months |
n = 78 Mean age (SD): 53.4 (14) Female (%): 66.7 Mean BMI (SD): 26.6 (3.6) |
Mirabegron (50 mg) tablet, and two placebo tablets once daily orally |
n = 81 Mean age (SD): 54.6 (13.4) Female (%): 66.7 Mean BMI (SD): 27.1 (13.6) |
Three placebo tablets once daily orally |
Volume voided/micturition Micturitions/24h Incontinence episodes/24h Urgency episodes/24h Adverse events Blood pressure Hypertension Pulse rate Tachycardia |
159 |
14 weeks |
|
Herschorn, 2017 |
Adults with wet OAB > 3 months |
n = 422 Mean age (SD): 56.7 (13.3) Female (%): 76.5 Mean BMI (SD): 28.3 (6.0) |
Mirabegron (50 mg) tablet once daily orally |
n = 429 Mean age (SD): 57.9 (13) Female (%): 76.2 Mean BMI (SD): 28.7 (6.1) |
Placebo tablet once daily orally |
Volume voided/micturition Micturitions/24h Incontinence episodes/24h Adverse events |
851 |
14 weeks |
|
Khullar, 2013 |
Adults with OAB > 3 months |
n = 493 Mean age (SD): 59.1 (12.4) Female (%): 72.4 Mean BMI (SD): 27.5 (4.9) |
Mirabegron (50 mg) orally once daily |
n = 494 Mean age (SD): 59.2 (12.3) Female (%): 72.1 Mean BMI (SD): 27.8 (5.0) |
Placebo orally once daily |
Volume voided/micturition Micturitions/24h Incontinence episodes/24h Urgency episodes/24h Adverse events Hypertension |
987 |
12 weeks + 30 days |
|
Kuo, 2015 |
Adults with OAB > 3 months |
n = 338 Mean age (SD): 54.3 (14.2) Female (%): 67.5 Mean BMI (SD): N.R. |
Mirabegron (50 mg) orally once daily |
n = 323 Mean age (SD): 55.3 (13.6) Female (%): 69.7 Mean BMI (SD): N.R. |
Placebo orally once daily |
Volume voided/micturition Micturitions/24h Incontinence episodes/24h Urgency episodes/24h Adverse events Blood pressure Hypertension |
661 |
14 weeks |
|
Nitti, 2013 |
Adults with OAB > 3 months |
n = 442 Mean age (SD): 59.2 (13.5) Female (%): 72.9 Mean BMI (SD): 30.0 (6.6) |
Mirabegron (50 mg) |
n = 453 Mean age (SD): 60.1 (13.8) Female (%): 76.2 Mean BMI (SD): 30.4 (7.4) |
Placebo |
Volume voided/micturition Micturitions/24h Incontinence episodes/24h Hypertension Tachycardia |
895 |
12 weeks + 30 days |
|
Shin, 2018 |
Adult males with OAB > 12 weeks |
n = 310 Mean age (SD): 66.4 (9.5) Mean BMI (SD): 24.2 (2.8) |
Mirabegron (50 mg) orally once daily |
n = 154 Mean age (SD): 65.2 (10) Mean BMI (SD): 23.9 (3.7)
|
Placebo orally once daily |
Micturitions/24h Adverse events Blood pressure Pulse rate |
464 |
12 weeks + 14 weeks extended treatment period |
|
Yamaguchi, 2014 |
Adults with OAB > 24 weeks |
n = 369 Mean age (SD): 58.3 (13.9) Female (%): 84.3 Mean BMI (SD): N.R. |
Mirabegron (50 mg) orally once daily |
n = 368 Mean age (SD): 58.2 (14.2) Female (%): 84.2 Mean BMI (SD): N.R. |
Placebo orally once daily |
Volume voided/micturition Micturitions/24h Incontinence episodes/24h Urgency episodes/24h Adverse events Blood pressure Hypertension Pulse rate Tachycardia |
737 |
14 weeks |
|
Yamaguchi, 2015 |
Adults with OAB > 24 weeks |
n = 208 Mean age (SD): 56.2 (13.6) Female (%): 85.1 Mean BMI (SD): N.R. |
Mirabegron (50 mg) orally once daily |
n = 211 Mean age (SD): 55.7 (12.9) Female (%): 80.1 Mean BMI (SD): N.R. |
Placebo orally once daily |
Volume voided/micturition Micturitions/24h Incontinence episodes/24h Urgency episodes/24h Adverse events Hypertension Pulse rate Tachycardia Palpitations |
419 |
12 weeks |
|
Abbreviations: OAB = overactive bladder; BMI = body mass index; SD = standard deviation. |
||||||||
Results
1. Urinary incontinence episodes/24h
Seven studies reported on the number of urinary incontinence episodes per 24h (Table 1). Abrams (2015), Herschorn (2017), Khullar (2013) and Kuo (2015) reported on the number of urinary incontinence episodes per 24h, defined as the adjusted mean change from baseline to end of treatment. Nitti (2013) reported the adjusted mean change from baseline to final visit. Yamaguchi (2014) reported the mean change from baseline to final assessment, and Yamaguchi (2015) the mean change from baseline to end of study.
Data of Abrams (2015), Herschorn (2017) and Kuo (2015) could not be pooled because no absolute change or SE/SD values were reported. Abrams (2015) reported that a reduction in the number of urinary incontinence episodes/24h was observed at end of treatment in the mirabegron group (n = 78) as well as the placebo group (n = 81). Herschorn (2017) reported a mean change in urinary incontinence episodes/24h of -1.76 in the mirabegron group (n = 406), and -1.34 in the placebo group (n = 412). Kuo (2015) reported a baseline number of incontinence episodes of 2.4 (SD 2.5) in the mirabegron group (n = 338), and 2.4 (SD 2.7) in the placebo group (n = 323). It was mentioned that mirabegron was associated with improvement in urinary incontinence episodes/24h over time.
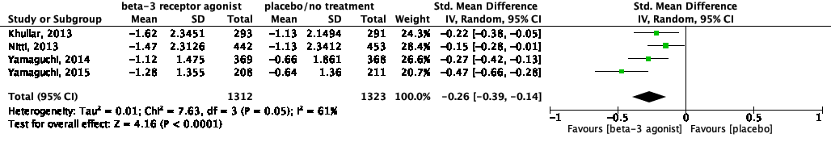
The pooled data show a standardized mean difference of -0.26 (95%CI -0.39 to -0.14), favoring beta-3 receptor agonists (Figure 1). This difference was not considered clinically relevant.
Figure 1: The effect of a beta-3 agonist on urinary incontinence episodes/24h.
Z: p-value of the pooled effect; df: degrees of freedom; I2: statistic heterogeneity; CI: confidence interval.
2. Urgency episodes/24h
Five studies reported on the number of urgency episodes per 24h (Table 1). Abrams (2015), Khullar (2013) and Kuo (2015) reported on the number of urinary incontinence episodes per 24h, defined as the adjusted mean change from baseline to end of treatment. Yamaguchi (2014) reported the mean change from baseline to final assessment, and Yamaguchi (2015) the mean change from baseline to end of study.
Data of Abrams (2015) and Kuo (2015) could not be pooled because no absolute (SE/SD) values were reported. Abrams (2015) reported no absolute values, but a graph shows that there is a larger (non-significant) increase in the mean number of urgency episodes/24h from baseline to end of treatment in the mirabegron group (n = 78) compared to the placebo group (n = 81). Kuo (2015) reported a baseline number of urgency episodes/24h of 5.2 (SD 4.6) in the mirabegron group (n = 338), and 5.6 (SD 5.3) in the placebo group (n = 323).
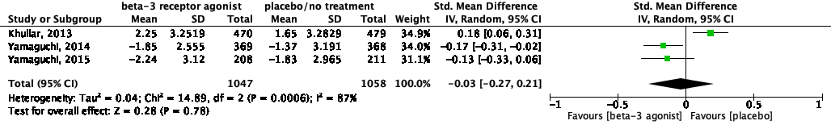
The pooled data show a standardized mean difference of -0.03 (95%CI -0.27 to 0.21), favoring beta-3 receptor agonists (Figure 2). This difference was not considered clinically relevant.
Figure 2: The effect of a beta-3 agonist on urgency episodes/24h.
Z: p-value of the pooled effect; df: degrees of freedom; I2: statistic heterogeneity; CI: confidence interval.
3. Volume voided/micturition
Seven studies reported on the volume voided per micturition (Table 1). Abrams (2015), Herschorn (2017), Khullar (2013) and Kuo (2015) reported on volume voided per micturition, defined as the adjusted mean change from baseline to end of treatment. Nitti (2013) reported the adjusted mean change from baseline to final visit. Yamaguchi (2014) reported the mean change from baseline to final assessment, and Yamaguchi (2015) the mean change from baseline to end of study.
Data of Herschorn (2017) and Kuo (2015) could not be pooled because no absolute change or SE/SD values were reported. Herschorn (2017) reported a mean change in volume voided/micturition of 21.99 in the mirabegron group (n = 408), and 8.44 in the placebo group (n = 413). Kuo (2015) reported a baseline volume voided/micturition of 147.8 (SD 52.7) for the mirabegron group (n = 338), and 152.6 (SD 55.0) for the placebo group (n = 323). This study mentioned that the magnitude of the increase in mean volume voided/micturition was numerally larger in the mirabegron group compared to the placebo group at all timepoints.
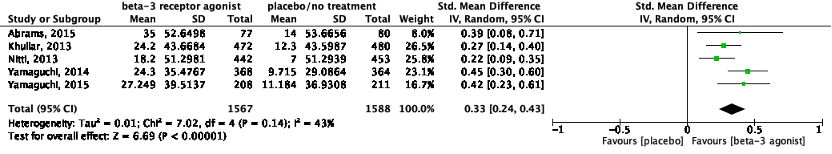
The pooled data show a standardized mean difference of 0.33 (95%CI 0.24 to 0.43) ml, favoring beta-3 receptor agonists (Figure 3). This difference was not considered clinically relevant.
Figure 3: The effect of a beta-3 agonist on volume voided/micturition.
Z: p-value of the pooled effect; df: degrees of freedom; I2: statistic heterogeneity; CI: confidence interval.
4. Micturitions/24h
Eight studies reported on the number of micturitions per 24 hour (Table 1). Abrams (2015), Herschorn (2017), Khullar (2013) and Kuo (2015) reported on micturitions per 24h, defined as the adjusted mean change from baseline to end of treatment. Nitti (2013) reported the adjusted mean change from baseline to final visit. Yamaguchi (2014) reported the mean change from baseline to final assessment, and Shin (2018) and Yamaguchi (2015) the mean change from baseline to end of study.
Data of Abrams (2015), Herschorn (2017) and Kuo (2015) could not be pooled because no absolute change or SE/SD values were reported. Abrams (2015) reported no absolute values, but a graph shows that there is a larger (non-significant) decrease from baseline to end of treatment in the mean number of micturitions/24h in the mirabegron group compared (n = 78) to the placebo group (n = 81). Herschorn (2017) reported a mean change in micturitions/24h of -2.03 in the mirabegron group (n = 406), and -1.64 in the placebo group (n = 412). Kuo (2015) reported a baseline number of micturitions/24h of 12.1 (SD 4.1) in the mirabegron group (n = 338), and 12.6 (SD 4.9) in the placebo group (n = 323). They reported that the mean number of micturitions/24h decreased over time in both groups.
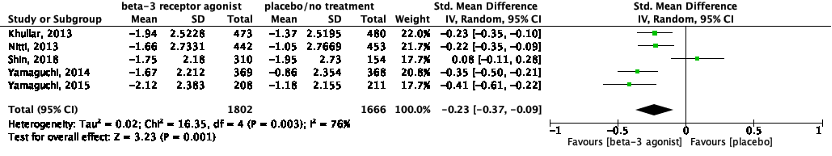
The pooled data show a standardized mean difference of -0.23 (95%CI -0.37 to -0.09), favoring beta-3 receptor agonists (Figure 4). This difference was not considered clinically relevant.
Figure 4: The effect of a beta-3 agonist on micturitions/24h.
Z: p-value of the pooled effect; df: degrees of freedom; I2: statistic heterogeneity; CI: confidence interval.
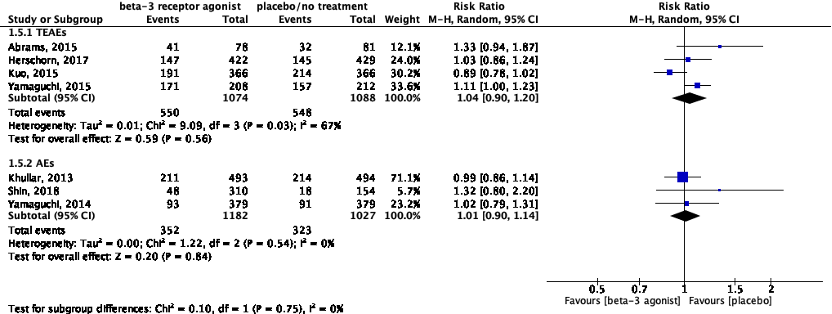
5. Adverse events
Seven studies reported on adverse events (Table 1). Abrams (2015), Herschorn (2017), Kuo (2015) and Yamaguchi (2015) reported on the frequency of treatment-emergent adverse events (TEAEs), whereas Khullar (2013), Shin (2018) and Yamaguchi (2014) reported on the total of adverse events (AEs).
Data of these studies was pooled with two subgroups because of the diversity in definitions of adverse events (Figure 5). For subgroup 1, the relative risk was 1.04 (95%CI 0.90 to 1.20), favoring placebo. This difference was not considered clinically relevant. For subgroup 2, the relative risk was 1.01 (95%CI 0.90 to 1.14), favoring placebo. This difference was not considered clinically relevant.
Figure 5: The effect of a beta-3 agonist on adverse events.
Z: p-value of the pooled effect; df: degrees of freedom; I2: statistic heterogeneity; CI: confidence interval.
a. Blood pressure (continuous outcome): mean difference between groups
Two studies reported on blood pressure (Table 1) as a continuous outcome. Data could not be pooled due to the diversity in reporting of the outcome measure blood pressure.
Abrams (2015) reported on blood pressure, defined as the adjusted mean change from baseline to end of treatment. The mean change in systolic blood pressure was 0.7 (SD 9.8) for the mirabegron group (n = 78) and -2.6 (SD 9.81) for the placebo group (n = 81). The standardized mean difference was 0.33 (95%CI 0.02 to 0.65) in favor of placebo treatment, which was not considered clinically different. For diastolic blood pressure, the mean change was 0.3 (SD 6.7) for the mirabegron group, and -1.2 (SD 6.7) for the placebo group. The standardized mean difference was 0.22 (95%CI -0.09 to 0.53), favoring placebo treatment, which was not considered clinically different.
Shin (2018) reported on blood pressure, defined as the mean change from baseline to final visit. The mean change in systolic blood pressure was -0.21 (SD 12.0) for the mirabegron group (n = 310), and 0.76 (SD 12.5) for the placebo group (n = 154). Standardized mean difference was -0.08 (95%CI -0.27 to 0.11) in favor of mirabegron treatment, which was not considered clinically different. For diastolic blood pressure, the mean change was 0.13 (SD 9.1) for the mirabegron group, and 0.7 (SD 8.4) for the placebo group. The standardized mean difference was -0.06 (95%CI -0.26 to 0.13), favoring mirabegron treatment, which was not considered clinically different.
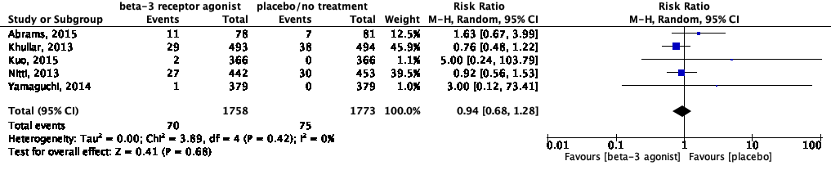
b. Blood pressure (dichotomous outcome): Hypertension/increased blood pressure
Eight studies reported on hypertension (Table 1). All studies reported on the incidence of hypertension. Data of Yamaguchi (2015) could not be pooled because nothing was reported about the incidence of hypertension in the placebo group. Yamaguchi (2015) reported hypertension in 1 out of 208 (0.5%) patients in the mirabegron group.
Kuo (2015) reported on the proportion of patients with an increased blood pressure. Blood pressure was increased in 1 out of 366 (0.3%) patients in the placebo group, and in 0 out of 366 patients in the mirabegron group. The risk ratio was 0.33 (95%CI 0.01 to 8.16) in favor of mirabegron treatment, which was considered clinically relevant.
Yamaguchi (2014) reported on the proportion of patients with an increased blood pressure. Blood pressure was increased in 1 out of 379 (0.3%) patients in the placebo group, and in 0 out of 379 patients in the mirabegron group. The risk ratio was 0.33 (95%CI 0.01 to 8.16) in favor of mirabegron treatment, which was considered clinically relevant.
The pooled data for hypertension show a risk ratio of 0.94 (95%CI 0.68 to 1.28), favoring beta-3 receptor agonists (Figure 6). This difference was not considered clinically relevant.
Figure 6: The effect of a beta-3 agonist on hypertension.
Z: p-value of the pooled effect; df: degrees of freedom; I2: statistic heterogeneity; CI: confidence interval.
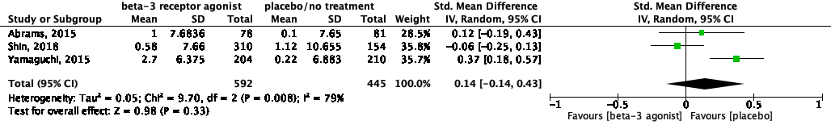
c. Pulse rate
Four studies reported on pulse rate (Table 1). Abrams (2015) reported on pulse rate, defined as the adjusted mean change from baseline to end of treatment. Shin (2018) reported on pulse rate, defined as the mean change from baseline to final visit. Yamaguchi (2015) reported on pulse rate, defined as the change from baseline to end of study.
The pooled data show a standardized mean difference of 0.14 (95%CI -0.14 to 0.43), favoring placebo treatment (Figure 7). This difference was not considered clinically relevant.
Figure 7: The effect of a beta-3 agonist on pulse rate.
Z: p-value of the pooled effect; df: degrees of freedom; I2: statistic heterogeneity; CI: confidence interval.
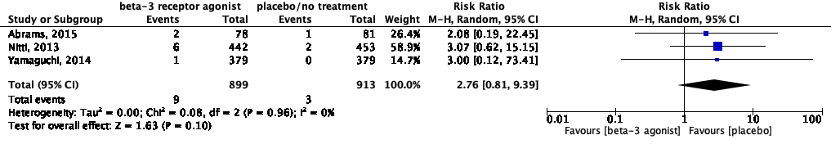
d. Tachycardia/increased heart rate
Four studies reported on tachycardia (Table 1). All studies reported on the incidence of tachycardia. Data of Yamaguchi (2015) could not be pooled because nothing was reported about the incidence of tachycardia in the placebo group. Yamaguchi (2015) reported tachycardia in 1 out of 208 (0.5%) patients in the mirabegron group.
Data of Yamaguchi (2014) could not be pooled because of the diversity in reporting of the outcome measure pulse rate. Yamaguchi (2014) reported the proportion of patients who had an increased heart rate. Heart rate was increased in 0 out of 379 patients in the placebo group, and in 1 out of 379 (0.3%) patients in the mirabegron group.
The pooled data show a risk ratio of 2.76 (95%CI 0.81 to 9.39), favoring placebo treatment (Figure 8). This difference was considered clinically relevant.
Figure 8: The effect of a beta-3 agonist on tachycardia.
Z: p-value of the pooled effect; df: degrees of freedom; I2: statistic heterogeneity; CI: confidence interval.
e. Palpitations
One study reported on palpitations (Table 1). Yamaguchi (2015) reported treatment-related palpitations in 1 out of 212 (0.5%) patients in the placebo group and 4 out of 208 (1.9%) patients in the mirabegron group. The risk ratio was 4.08 (95%CI 0.46 to 36.17) in favor of placebo treatment, which was considered clinically relevant.
6. Total urine volume
None of the studies reported on the outcome measure total urine volume.
Level of evidence of the literature
The level of evidence of the literature was assessed per outcome measure, using the GRADE-methodology.
1. Urinary incontinence episodes/24h
The level of evidence regarding urinary incontinence episodes/24h started as high because it was based on RCTs and was downgraded by one level to moderate because the sponsors of most studies had a role in the design and conduct of the study (Abrams, 2015; Khullar, 2013; Kuo, 2015; Nitti, 2013; Yamaguchi, 2014; Yamaguchi, 2015) (risk of bias: -1).
2. Urgency episodes/24h
The level of evidence regarding urgency episodes/24h started as high because it was based on RCTs and was downgraded by two levels to low because the sponsors of all studies had a role in the design and conduct of the study (Abrams, 2015; Khullar, 2013; Kuo, 2015; Yamaguchi, 2014; Yamaguchi, 2015) (risk of bias: -1) and because of conflicting results (inconsistency: -1).
3. Volume voided/micturition
The level of evidence regarding volume voided/micturition started as high because it was based on RCTs and was downgraded by one level to moderate because the sponsors of most studies had a role in the design and conduct of the study (Abrams, 2015; Khullar, 2013; Kuo, 2015; Nitti, 2013; Yamaguchi, 2014; Yamaguchi, 2015) (risk of bias: -1).
4. Micturitions/24h
The level of evidence regarding micturitions/24h started as high because it was based on RCTs and was downgraded by two levels to low because the sponsors of most studies had a role in the design and conduct of the study (Abrams, 2015; Khullar, 2013; Kuo, 2015; Nitti, 2013; Yamaguchi, 2014; Yamaguchi, 2015) (risk of bias: -1) and because of conflicting results (inconsistency: -1).
5. Adverse events
The level of evidence regarding adverse events started as high because it was based on RCTs and was downgraded by two levels to low because the sponsors of most studies had a role in the design and conduct of the study (Abrams, 2015; Khullar, 2013; Kuo, 2015; Yamaguchi, 2014; Yamaguchi, 2015) (risk of bias: -1), and because of the diversity in definitions of adverse events (indirectness: -1).
a. Blood pressure
The level of evidence regarding blood pressure started as high because it was based on RCTs and was downgraded by three levels to very low because the sponsors of most studies had a role in the design and conduct of the study (Abrams, 2015; Kuo, 2015; Yamaguchi, 2014) (risk of bias: -1), because of conflicting results (inconsistency: -1), because the confidence interval crosses the border of clinical relevance (imprecision: -1).
b. Hypertension
The level of evidence regarding hypertension started as high because it was based on RCTs and was downgraded by two levels to low because the sponsors of all studies had a role in the design and conduct of the study (Abrams, 2015; Khullar, 2013; Kuo, 2015; Nitti, 2013; Yamaguchi, 2014; Yamaguchi, 2015) (risk of bias: -1), and because the confidence interval crosses the border of clinical relevance (imprecision: -1).
c. Pulse rate
The level of evidence regarding pulse rate started as high because it was based on RCTs and was downgraded by two levels to low because the sponsors of most studies had a role in the design and conduct of the study (Abrams 2015; Shin, 2018; Yamaguchi, 2014; Yamaguchi, 2015) (risk of bias: -1), and because of conflicting results (inconsistency: -1).
d. Tachycardia
The level of evidence regarding tachycardia started as high because it was based on RCTs and was downgraded by three levels to very low because the sponsors of all studies had a role in the design and conduct of the study (Abrams 2015; Nitti, 2013; Yamaguchi, 2014; Yamaguchi, 2015) (risk of bias: -1), and because the confidence interval crosses the border of clinical relevance and the low number of events (imprecision: -2).
e. Palpitations
The level of evidence regarding palpitations started as high because it was based on RCTs and was downgraded by three levels to very low because the sponsor of the study had a role in the design and conduct of the study (risk of bias: -1), and because the confidence interval crosses the borders of clinical relevance and the low number of events (imprecision: -2).
6. Total urine volume
None of the studies reported on this outcome measure and could therefore not be graded.
2. Beta-3 receptor agonist versus antimuscarinic
Description of studies
For the second comparison, a total of four RCTs were found (Abrams, 2015; Herschorn, 2017; Staskin, 2021; Suzuki, 2021). Study characteristics, including the outcome measures reported, are shown in Table 2.
Table 2: study characteristics of included studies for comparison 2
|
Study |
Patients |
Intervention |
Comparison |
Outcomes of interest reported |
N |
Follow-up |
||
|
Characteristics |
Type/dose |
Characteristics |
Type |
|||||
|
Abrams, 2015 (SYMPHONY) |
Adults with OAB > 3 months |
n = 78 Mean age (SD): 53.4 (14) Female (%): 66.7 Mean BMI (SD): 26.6 (3.6) |
Mirabegron (50 mg) tablet, and two placebo tablets once daily orally |
n = 79 Mean age (SD): 56.1 (11.7) Female (%): 64.6 Mean BMI (SD): 27.3 (4.8) |
Solifenacin (2.5 mg) tablet, and two placebo tablets once daily orally |
Volume voided/micturition Adverse events Blood pressure Hypertension Pulse rate Tachycardia |
391 |
14 weeks |
|
n = 156 Mean age (SD): 54.2 (15.5) Female (%): 66 Mean BMI (SD): 26.3 (3.9) |
Solifenacin (5 mg) tablet, and two placebo tablets once daily orally |
|||||||
|
n = 78 Mean age (SD): 55 (12.8) Female (%): 67.9 Mean BMI (SD): 27.2 (3.7) |
Solifenacin (10 mg) tablet, and two placebo tablets once daily orally |
|||||||
|
Herschorn, 2017 (SYNERGY) |
Adults with wet OAB > 3 months |
n = 422 Mean age (SD): 56.7 (13.3) Female (%): 76.5 Mean BMI (SD): 28.3 (6.0) |
Mirabegron (50 mg) tablet once daily orally |
n = 423 Mean age (SD): 58.2 (12.8) Female (%): 78.3 Mean BMI (SD): 28.5 (5.9) |
Solifenacin (5 mg) tablet once daily orally |
Volume voided/micturition Micturitions/24h Incontinence episodes/24h Adverse events |
845 |
14 weeks |
|
Staskin, 2021 (EMPOWUR) |
Adults with OAB |
n = 92 Mean age (SD): 58.8 (13.7) Female (%): 79.3 Mean BMI (SD): N.R. |
Vibegron (75 mg) orally once daily |
n = 91 Mean age (SD): 62.1 (12.1) Female (%): 76.9 Mean BMI (SD): N.R. |
Tolterodine extended release (4 mg) orally once daily |
Micturitions/24h Incontinence episodes/24h Urgency episodes/24h Adverse events Hypertension |
183 |
40 weeks of extension (52 weeks of total treatment) |
|
Suzuki, 2021 |
Adult females with OAB > 8 weeks |
n = 49 Mean age (SD): 72.7 (11.3) Mean BMI (SD): 23.3 (3.4) |
Mirabegron (50 mg) orally once daily |
n = 51 Mean age (SD): 68.2 (11.3) Mean BMI (SD): 24.1 (3.9) |
Oxybutynin patch (73.5 mg) placed on the lower abdomen or thighs once daily |
Volume voided/micturition Incontinence episodes/24h Urgency episodes/24h Total urine volume Adverse events |
100 |
8 weeks |
Results
1. Urinary incontinence episodes/24h
Three studies reported on the number of urinary incontinence episodes per 24 hour (Table 2). Herschorn (2017) reported on urinary incontinence episodes/24h, defined as the adjusted mean change from baseline to end of treatment. Staskin (2021) reported on the LS mean change from baseline to end of treatment. Suzuki (2021) reported on urgency incontinence episodes/24h, defined as the mean change from baseline to end of treatment. Data could not be pooled because no absolute SE/SD values were reported in Herschorn (2017) resulting in limited data.
Herschorn (2017) reported a mean change in urinary incontinence episodes/24h of -1.76 in the mirabegron group (n = 406), and -1.79 in the antimuscarinic group (n = 413).
Staskin (2021) reported a mean change in urinary incontinence episodes/24h of -2.5 (SD 2.0) in the vibegron group (n = 176), and -1.9 (SD 2.4) in the antimuscarinic group (n = 136). The standardized mean difference was -0.28 (95%CI -0.50 to -0.05) in favor of vibegron treatment, which was not considered clinically different.
Suzuki (2021) reported a mean change in urgency incontinence episodes/24h of -0.6 (SD 1.0) in the mirabegron group (n = 49), and -1.1 (SD 2.0) in the antimuscarinic group (n = 51). The standardized mean difference was 0.31 (95%CI -0.08 to 0.71) in favor of antimuscarinic treatment (oxybutynin), which was not considered clinically different.
2. Urgency episodes/24h
Two studies reported on the number of urgency episodes per 24 hour (Table 2). Staskin (2021) reported on the LS mean change from baseline to end of treatment. Suzuki (2021) reported on urinary urgency/24h, defined as the mean change from baseline to end of treatment. Data could not be pooled because of the limited availability of data.
Staskin (2021) reported a mean change SD in urgency episodes/24h of -3.4 (SD 4.7) in the vibegron group (n = 176), and -3.2 (SD 4.1) in the antimuscarinic group (n = 136). The standardized mean difference was -0.04 (95%CI -0.27 to 0.18) in favor of vibegron treatment, which was not considered clinically different.
Suzuki (2021) reported a mean change in urinary urgency/24h of -1.3 (SD 1.6) in the mirabegron group (n = 49), and -1.7 (SD 2.7) in the antimuscarinic group (n = 51). The standardized mean difference was 0.18 (95%CI -0.21 to 0.57) in favor of antimuscarinic treatment (oxybutynin), which was not considered clinically different.
3. Volume voided/micturition (5 mg)
Three studies reported on the volume voided per micturition (Table 2). Abrams (2015) and Herschorn (2017) reported on volume voided per micturition, defined as the adjusted mean change from baseline to end of treatment. Suzuki (2021) reported on the mean change from baseline to end of treatment. Data could not be pooled because no absolute SE/SD values were reported in Herschorn (2017).
Abrams (2015) reported a mean change in volume voided per micturition of 35 (SD 53) in the mirabegron group (n = 78), and 36 (SD 53) in the antimuscarinic group (n = 150). The standardized mean difference was -0.02 (95%CI -0.29 to 0.26) in favor of antimuscarinic treatment, which was not considered clinically different.
Herschorn (2017) reported a mean change in volume voided per micturition of 22 in the mirabegron group (n = 408), and 31 in the antimuscarinic group (n = 411).
Suzuki (2021) reported a mean change in volume voided per micturition of 27.8 (SD 36.1) in the mirabegron group (n = 49), and 36.8 (SD 48.4) in the antimuscarinic group (n = 51). The standardized mean difference was -0.21 (95%CI -0.60 to 0.18) in favor of antimuscarinic treatment, which was not considered clinically different.
4. Micturitions/24h
Two studies reported on the number of micturitions per 24 hour (Table 2). Herschorn (2017) reported on micturitions/24h, defined as the adjusted mean change from baseline to end of treatment. Staskin (2021) reported on the LS mean change from baseline to end of treatment. Data could not be pooled because no absolute SE/SD values were reported in Herschorn (2017) resulting in limited data.
Herschorn (2017) reported a mean change in micturitions/24h of -2.03 in the mirabegron group (n = 406), and -2.20 in the antimuscarinic group (n = 413).
Staskin (2021) reported a mean change in micturitions/24h of -2.4 (SD 2.7) in the vibegron group (n = 176), and -2.0 (SD 2.9) in the antimuscarinic group (n = 136). The mean difference was -0.40 (95%CI -1.04 to 0.24) in favor of vibegron treatment, which was not considered clinically different.
5. Adverse events
Four studies reported on adverse events (Table 2). Abrams (2015) and Herschorn (2017) reported on the frequency of treatment-emergent adverse events (TEAEs). Staskin (2021) reported on the proportion of patients with at least one TEAE. Suzuki (2021) reported on the frequency of any adverse event. Data could not be pooled because of the diversity in reporting of the outcome measure adverse events.
Abrams (2015) reported TEAEs in 41 out of 78 (52.6%) patients in the mirabegron group, and in 70 out of 156 (44.9%) patients in the antimuscarinic group. The risk ratio was 1.17 (95%CI 0.89 to 1.54) in favor of antimuscarinic treatment (solifenacin), which was not considered clinically different.
Herschorn (2017) reported TEAEs in 147 out of 422 (34.8%) patients in the mirabegron group, and 149 out of 423 (35.2%) patients in the antimuscarinic group. The risk ratio was 0.99 (95%CI 0.82 to 1.19) in favor of mirabegron treatment, which was not considered clinically different.
Staskin (2021) reported at least one TEAE in 171 out of 273 (62.6%) patients in the vibegron group, and 126 out of 232 (54.3%) patients in the antimuscarinic group. The risk ratio was 1.15 (95%CI 0.99 to 1.34) in favor of vibegron treatment, which was not considered clinically different.
Suzuki (2021) reported adverse events in 1 out of 49 (2%) patients in the mirabegron group, and in 26 out of 51 (51%) patients in de antimuscarinic group. The risk ratio was 0.04 (95%CI 0.01 to 0.28) in favor of mirabegron treatment, which was clinically different. Among these adverse events, in the Oxybutynin patch group application site dermatitis was seen 20 times, dry mouth 9 times, and other adverse events once. In the Mirabegron group, the adverse event was labeled as constipation.
a. Blood pressure
One study reported on blood pressure (Table 2). Abrams (2015) reported on blood pressure, defined as the adjusted mean change from baseline to end of treatment. The mean change in systolic blood pressure was 0.7 (SD 9.8) for the mirabegron group (n = 78) and -1.7 (SD 9.9) for the antimuscarinic group (n = 156). The mean difference was 2.4 (95%CI -0.27 to 5.07) in favor of antimuscarinic treatment (solifenacin), which was not considered clinically different. For diastolic blood pressure, the mean change was 0.3 (SD 6.7) for the mirabegron group, and -0.6 (SD 6.7) for the antimuscarinic group. The mean difference was 0.90 (95%CI -0.93 to 2.73), favoring antimuscarinic treatment, which was not considered clinically different.
b. Hypertension
Two studies reported on hypertension (Table 2), defined as the incidence of hypertension. Abrams (2015) reported hypertension in 11 out of 78 (14.1%) patients in the mirabegron group, and in 18 out of 156 (11.5%) patients in the antimuscarinic group. The risk ratio was 1.22 (95%CI 0.61 to 2.46) in favor of antimuscarinic treatment (solifenacin), which was not considered clinically different.
Staskin (2021) reported hypertension in 24 out of 273 (8.8%) patients in the vibegron group, and 20 out of 232 (9.6%) patients in the antimuscarinic group. The risk ratio was 1.02 (95%CI 0.58 to 1.80) in favor of antimuscarinic treatment (tolterodine), which was not considered clinically different.
c. Pulse rate
One study reported on pulse rate (Table 2), defined as the adjusted mean change from baseline to end of treatment. Abrams (2015) reported a mean change in pulse rate of 1.0 (SD 7.7) in the mirabegron group (n = 78), and 0.1 (SD 7.7) in the antimuscarinic group (n = 156). The mean difference was 0.90 (95%CI -1.19 to 2.99) in favor of antimuscarinic treatment (solifenacin), which was not considered clinically different.
d. Tachycardia
One study reported on tachycardia (Table 2), defined as the incidence of tachycardia. Abrams (2015) reported tachycardia in 2 out of 78 (2.6%) patients in the mirabegron group, and 6 out of 156 (3.8%) patients in the antimuscarinic group. The risk ratio was 0.67 (95%CI 0.14 to 3.23) in favor of mirabegron treatment, which was clinically different.
e. Palpitations
None of the studies reported on the outcome measure palpitations.
Level of evidence of the literature
The level of evidence of the literature was assessed per outcome measure, using the GRADE-methodology.
1. Urinary incontinence episodes/24h
The level of evidence regarding urinary incontinence episodes/24h started as high because it was based on RCTs and was downgraded by three levels to very low because of conflicting results (inconsistency: -1), diversity in the definitions of urinary incontinence episodes/24h (indirectness: -1) and because the confidence interval crosses the border of clinical relevance (imprecision: -1).
2. Urgency episodes/24h
The level of evidence regarding urgency episodes/24h started as high because it was based on RCTs and was downgraded by three levels to very low because of conflicting results (inconsistency: -1), diversity in the definitions of urinary incontinence episodes/24h (indirectness: -1) and because the confidence interval crosses the border of clinical relevance (imprecision: -1).
3. Volume voided/micturition
The level of evidence regarding volume voided/micturition started as high because it was based on RCTs and was downgraded by two levels to low because the sponsor of one study had a role in the design and conduct of the study (Abrams, 2015) (risk of bias: -1), and because the confidence interval crosses the border of clinical relevance (imprecision: -1).
4. Micturitions/24h
The level of evidence regarding micturitions/24h started as high because it was based on RCTs and was downgraded by two levels to low because of conflicting results (inconsistency: -1), and because the confidence interval crosses the border of clinical relevance (imprecision: -1).
5. Total urine volume
The level of evidence regarding total urine volume started as high because it was based on RCTs and was downgraded by three levels to very low because of the low number of included patients and the width of the confidence interval (imprecision: -3).
6. Adverse events
The level of evidence regarding adverse events started as high because it was based on RCTs and was downgraded by three levels to very low because the sponsor of one study had a role in the design and conduct of the study (Abrams, 2015) (risk of bias: -1), the diversity in definitions of adverse events (indirectness: -1), and because the confidence interval crosses the border of clinical relevance (imprecision: -1).
a. Blood pressure
The level of evidence regarding blood pressure started as high because it was based on RCTs and was downgraded by two levels to low because the sponsor of this study had a role in the design and conduct of the study (risk of bias: -1), and because of the low number of patients (imprecision: -1).
b. Hypertension
The level of evidence regarding hypertension started as high because it was based on RCTs and was downgraded by two levels to low because the sponsor of one study had a role in the design and conduct of the study (Abrams, 2015) (risk of bias: -1), and because the confidence interval crosses the border of clinical relevance (imprecision: -1).
c. Pulse rate
The level of evidence regarding pulse rate started as high because it was based on RCTs and was downgraded by two levels to low because the sponsor of this study had a role in the design and conduct of the study (risk of bias: -1), and because of the low number of patients (imprecision: -1).
d. Tachycardia
The level of evidence regarding tachycardia started as high because it was based on RCTs and was downgraded by three levels to very low because the sponsor of this study had a role in the design and conduct of the study (risk of bias: -1), and because the confidence interval crosses the border of clinical relevance and the low number of events (imprecision: -2).
e. Palpitations
None of the studies reported on this outcome measure and could therefore not be graded.
3. Beta-3 receptor agonist versus combination (beta-3 receptor agonist + antimuscarinic)
Description of studies
For the third comparison, a total of two RCTs were found (Abrams, 2015; Herschorn, 2017). Study characteristics, including the outcome measures reported, are shown in Table 3.
Table 3: study characteristics of included studies for comparison 3
|
Study |
Patients |
Intervention |
Comparison |
Outcomes of interest reported |
N |
Follow-up |
||
|
Characteristics |
Type/dose |
Characteristics |
Type |
|||||
|
Abrams, 2015 (SYMPHONY) |
Adults with OAB > 3 months |
n = 78 Mean age (SD): 53.4 (14) Female (%): 66.7 Mean BMI (SD): 26.6 (3.6) |
Mirabegron (50 mg) tablet, and two placebo tablets once daily orally |
n = 149 Mean age (SD): 53.7 (14.6) Female (%): 67.1 Mean BMI (SD): 26.5 (4.0) |
Mirabegron 50 mg + solifenacin 2.5 mg tablet, and two placebo tablets once daily orally |
Volume voided/micturition Adverse events Blood pressure Hypertension Pulse rate Tachycardia |
461 |
14 weeks |
|
n = 153 Mean age (SD): 54.1 (14.1) Female (%): 66.0 Mean BMI (SD): 26.5 (3.6) |
Mirabegron 50 mg + solifenacin 5 mg tablet, and two placebo tablets once daily orally |
|||||||
|
n = 81 Mean age (SD): 55.5 (13.8) Female (%): 66.7 Mean BMI (SD): 26.3 (3.3) |
Mirabegron 50 mg + solifenacin 10 mg tablet, and two placebo tablets once daily orally |
|||||||
|
Herschorn, 2017 (SYNERGY) |
Adults with wet OAB > 3 months |
n = 422 Mean age (SD): 56.7 (13.3) Female (%): 76.5 Mean BMI (SD): 28.3 (6.0) |
Mirabegron (50 mg) tablet once daily orally |
n = 848 Mean age (SD): 57.6 (13.4) Female (%): 76.8 Mean BMI (SD): 28.6 (5.9) |
Mirabegron 50 mg + solifenacin 5 mg tablet once daily orally |
Volume voided/micturition Micturitions/24h Incontinence episodes/24h Adverse events |
1270 |
14 weeks |
Results
1. Urinary incontinence episodes/24h
One study reported on the number of urinary incontinence episodes per 24 hour (Table 3), defined as the adjusted mean change from baseline to end of treatment. Herschorn (2017) reported a mean change in urinary incontinence episodes/24h of -1.76 in the mirabegron group (n = 406), and -1.98 in the combination treatment group (n = 816). Since no measures of dispersion were reported, this result was not evaluated using grade.
2. Volume voided/micturition
Two studies reported on volume voided per micturition (Table 3). Abrams (2015) and Herschorn (2017) reported on volume voided per micturition, defined as the adjusted mean change from baseline to end of treatment.
Abrams (2015) reported a mean change in volume voided per micturition of 35 (SD 53) in the mirabegron group (n = 78), and 54 (SD 53) in the combination treatment group (n = 150). The mean difference was -19.2 (95%CI -33.67 to -4.73) in favor of combination treatment, which was not considered clinically different.
Herschorn (2017) reported a mean change in volume voided per micturition of 22 in the mirabegron group (n = 408), and 40 in the combination treatment group (n = 821).
3. Micturitions/24h
One study reported on the number of micturitions per 24 hour (Table 3), defined as the adjusted mean change from baseline to end of treatment. Herschorn (2017) reported a mean change in micturitions/24h of -2.03 in the mirabegron group (n = 406), and -2.59 in the combination treatment group (n = 816). Since no measures of dispersion were reported, this result was not evaluated using grade.
4. Adverse events
Two studies reported on adverse events (Table 3). Both studies reported on the frequency of treatment-emergent adverse events (TEAEs).
Abrams (2015) reported TEAEs in 41 out of 78 (52.6%) patients in the mirabegron group, and in 67 out of 153 (43.8%) patients in the combination treatment group. The risk ratio was 1.20 (95%CI 0.91 to 1.58) in favor of combination treatment (mirabegron + solifenacin), which was not considered clinically different. Herschorn (2017) reported TEAEs in 147 out of 422 (34.8%) patients in the mirabegron group, and in 314 out of 848 (37%) patients in the combination treatment group. The risk ratio was 0.94 (95%CI 0.80 to 1.10) in favor of mirabegron treatment, which was not considered clinically different.
a. Blood pressure
One study reported on blood pressure (Table 3). Abrams (2015) reported on blood pressure, defined as the adjusted mean change from baseline to end of treatment. The mean change in systolic blood pressure was 0.7 (SD 9.8) for the mirabegron group (n = 78) and -2.1 (SD 9.9) for the combination treatment group (n = 153). The mean difference was 2.8 (95%CI 0.12 to 5.48) in favor of combination treatment (mirabegron + solifenacin), which was not considered clinically different. For diastolic blood pressure, the mean change was 0.3 (SD 6.7) for the mirabegron group, and -0.8 (SD 6.8) for the combination treatment group. The mean difference was 1.1 (95%CI -0.74 to 2.94), favoring combination treatment, which was not considered clinically different.
b. Hypertension
One study reported on hypertension (Table 3), defined as the incidence of hypertension. Abrams (2015) reported hypertension in 11 out of 78 (14.1%) patients in the mirabegron group, and in 9 out of 153 (5.9%) patients in the combination treatment group. The risk ratio was 2.40 (95%CI 1.04 to 5.54) in favor of combination treatment (mirabegron + solifenacin), which was considered clinically different.
c. Pulse rate
One study reported on pulse rate (Table 3), defined as the adjusted mean change from baseline to end of treatment. Abrams (2015) reported a mean change in pulse rate of 1.0 (SD 7.7) in the mirabegron group (n = 78), and 0.6 (SD 7.7) in the combination treatment group (n = 153). The mean difference was 0.40 (95%CI -1.69 to 2.49) in favor of combination treatment (mirabegron + solifenacin), which was not considered clinically different.
d. Tachycardia
One study reported on tachycardia (Table 3), defined as the incidence of tachycardia. Abrams (2015) reported tachycardia in 2 out of 78 (2.6%) patients in the mirabegron group, and 3 out of 153 (2.0%) patients in the combination treatment group. The risk ratio was 1.31 (95%CI 0.22 to 7.66) in favor of combination treatment (mirabegron + solifenacin), which was considered clinically different.
e. Urgency episodes/24h, f. Palpitations, g. Total urine volume
None of the studies reported on the outcome measures urgency episodes/24h, palpitations and total urine volume.
Level of evidence of the literature
The level of evidence of the literature was assessed per outcome measure, using the GRADE-methodology.
1. Urinary incontinence episodes/24h
The outcome measure urinary incontinence episodes/24h could not be graded, because no standard deviation or standard error values were reported.
2. Volume voided/micturition
The level of evidence regarding volume voided/micturition started as high because it was based on RCTs and was downgraded by two levels to low because the sponsors of one study had a role in the design and conduct of the study (Abrams, 2015) (risk of bias: -1), and because the confidence interval crosses the border of clinical relevance (imprecision: -1).
3. Micturitions/24h
The outcome measure micturitions/24h could not be graded, because no standard deviation or standard error values were reported.
4. Adverse events
The level of evidence regarding adverse events started as high because it was based on RCTs and was downgraded by three levels to very low because the sponsors of one study had a role in the design and conduct of the study (Abrams, 2015) (risk of bias: -1), because of conflicting results (inconsistency: -1), and because the confidence interval is crossing the border of clinical relevance (imprecision: -1).
a. Blood pressure
The level of evidence regarding blood pressure started as high because it was based on RCTs and was downgraded by two levels to low because the sponsor of this study had a role in the design and conduct of the study (risk of bias: -1), and because the confidence interval is crossing the border of clinical relevance (imprecision: -1).
b. Hypertension
The level of evidence regarding hypertension started as high because it was based on RCTs and was downgraded by two levels to low because the sponsor of this study had a role in the design and conduct of the study (risk of bias: -1), and because the confidence interval is crossing the border of clinical relevance (imprecision: -1).
c. Pulse rate
The level of evidence regarding pulse rate started as high because it was based on RCTs and was downgraded by two levels to low because the sponsor of this study had a role in the design and conduct of the study (risk of bias: -1), and because of the low number of included patients (imprecision: -1).
d. Tachycardia
The level of evidence regarding tachycardia started as high because it was based on RCTs and was downgraded by two levels to low because the sponsor of this study had a role in the design and conduct of the study (risk of bias: -1), and because the confidence interval is crossing the border of clinical relevance (imprecision: -1).
e. Urgency episodes/24h, f. Palpitations, g. Total urine volume
None of the studies reported on the outcome measures urgency episodes/24h, palpitations and total urine volume and could therefore not be graded.
Zoeken en selecteren
A systematic review of the literature was performed to answer the following question: What is the efficacy of a beta-3 receptor agonist (sympathomimetic) in adults with UI compared to placebo/no treatment, antimuscarinic or a combination?
P: Adults with urine incontinence (UI)
I: Beta-3 receptor agonist (sympathomimetic: mirabegron [50 mg] or vibegron [75 mg])
C1: Placebo/no treatment
C2: Antimuscarinic (e.g., oxybutynin, solifenacin, tolterodine, darifenacin, fesoterodine)
C3: Combination of an antimuscarinic and beta-3 receptor agonist
O: Volume voided per micturition, number of micturitions per 24h, number of urinary incontinence episodes per 24h, number of urgency episodes per 24h, adverse events, blood pressure, hypertension, pulse rate, tachycardia, palpitations
Relevant outcome measures
The guideline development group considered number of urinary incontinence episodes per 24h and number of urgency episodes per 24h as critical outcome measures for decision making; and volume voided per micturition, number of micturitions per 24h, adverse events, blood pressure, hypertension, pulse rate, tachycardia and palpitations as important outcome measures for decision making.
A priori, the working group did not define the outcome measures listed above but used the definitions used in the studies.
The working group defined the following minimal clinically (patient) important differences:
- Improvement of overactive bladder complaints:
- Urinary Distress Inventory (UDI-6): ≥ 8 points difference between groups (Barber, 2009)
In all other cases, the working group defined a 25% difference for dichotomous outcomes (0.8 > RR > 1.25), and 0.5 SD or -0.5 > SMD > 0.5 for continuous outcomes as a minimal clinically (patient) important difference.
Search and select (Methods)
The databases Medline (via OVID) and Embase (via Embase.com) were searched with relevant search terms from 2010 until 18 September 2022. The detailed search strategy is depicted under the tab Methods. The systematic literature search resulted in 479 hits. Studies were selected based on the following criteria:
- Systematic reviews (searched in at least two databases, and detailed search strategy, risk of bias assessment and results of individual studies available) or randomized controlled trials;
- Patients aged ≥ 18 years
- Studies including >20 (ten in each study arm) patients;
- Full-text English language publication; and
- Studies according to the PICO.
Initially, 89 studies were selected based on title and abstract screening. After reading the full text, 86 studies were excluded (see the table with reasons for exclusion under the tab Methods), and 3 studies were included. One of these studies (Mostafaei, 2022) is a systematic review and network meta-analysis, also investigating other treatment options for adults with UI that were not conform our PICO. Studies that were conform our PICO were extracted from the systematic review (Abrams, 2015; Herschorn, 2017; Khullar, 2013; Kuo, 2015; Nitti, 2013; Shin, 2018; Yamaguchi, 2014; Yamaguchi, 2015). These studies were all found in our own search as well. Two additional RCTs (Staskin, 2021; Suzuki, 2021) published after the search date of the systematic review, were also included.
Results
Ten studies were included in the analysis of the literature. Important study characteristics and results are summarized in the evidence tables. The assessment of the risk of bias is summarized in the risk of bias tables.
Because the PICO consisted of three comparators, this module is divided into three submodules:
1. Beta-3 receptor agonist versus placebo/no treatment
2. Beta-3 receptor agonist versus antimuscarinic
3. Beta-3 receptor agonist versus combination (antimuscarinic + beta-3 receptor agonist)
All submodules include a summary of the literature including a description of the included studies, results, grading of the level of evidence and conclusions.
Referenties
- 1 - Abrams P, Kelleher C, Staskin D, Rechberger T, Kay R, Martina R, Newgreen D, Paireddy A, van Maanen R, Ridder A. Combination treatment with mirabegron and solifenacin in patients with overactive bladder: efficacy and safety results from a randomised, double-blind, dose-ranging, phase 2 study (Symphony). Eur Urol. 2015 Mar;67(3):577-88. doi: 10.1016/j.eururo.2014.02.012. Epub 2014 Feb 19. PMID: 24612659.
- 2 - EAU Guidelines. Edn. presented at the EAU Annual Congress Milan March 2023. ISBN 978-94-92671-19-6.
- 3 - Herschorn S, Chapple CR, Abrams P, Arlandis S, Mitcheson D, Lee KS, Ridder A, Stoelzel M, Paireddy A, van Maanen R, Robinson D. Efficacy and safety of combinations of mirabegron and solifenacin compared with monotherapy and placebo in patients with overactive bladder (SYNERGY study). BJU Int. 2017 Oct;120(4):562-575. doi: 10.1111/bju.13882. Epub 2017 Jun 8. PMID: 28418102.
- 4 - Khullar V, Amarenco G, Angulo JC, Cambronero J, H¿ye K, Milsom I, Radziszewski P, Rechberger T, Boerrigter P, Drogendijk T, Wooning M, Chapple C. Efficacy and tolerability of mirabegron, a ?(3)-adrenoceptor agonist, in patients with overactive bladder: results from a randomised European-Australian phase 3 trial. Eur Urol. 2013 Feb;63(2):283-95. doi: 10.1016/j.eururo.2012.10.016. Epub 2012 Nov 6. PMID: 23182126.
- 5 - Kuo HC, Lee KS, Na Y, Sood R, Nakaji S, Kubota Y, Kuroishi K. Results of a randomized, double-blind, parallel-group, placebo- and active-controlled, multicenter study of mirabegron, a ?3-adrenoceptor agonist, in patients with overactive bladder in Asia. Neurourol Urodyn. 2015 Sep;34(7):685-92. doi: 10.1002/nau.22645. Epub 2014 Aug 17. PMID: 25130281.
- 6 - Michel MC, Cardozo L, Chermansky CJ, Cruz F, Igawa Y, Lee KS, Sahai A, Wein AJ, Andersson KE. Current and Emerging Pharmacological Targets and Treatments of Urinary Incontinence and Related Disorders. Pharmacol Rev. 2023 Jul;75(4):554-674. doi: 10.1124/pharmrev.121.000523. Epub 2023 Mar 14. PMID: 36918261.
- 7 - Mostafaei H, Salehi-Pourmehr H, Jilch S, Carlin GL, Mori K, Quhal F, Pradere B, Grossmann NC, Laukhtina E, Schuettfort VM, Aydh A, Sari Motlagh R, Knig F, Roehrborn CG, Katayama S, Rajwa P, Hajebrahimi S, Shariat SF. Choosing the Most Efficacious and Safe Oral Treatment for Idiopathic Overactive Bladder: A Systematic Review and Network Meta-analysis. Eur Urol Focus. 2022 Jul;8(4):1072-1089. doi: 10.1016/j.euf.2021.08.011. Epub 2021 Sep 22. PMID: 34563481.
- 8 - Nitti VW, Auerbach S, Martin N, Calhoun A, Lee M, Herschorn S. Results of a randomized phase III trial of mirabegron in patients with overactive bladder. J Urol. 2013 Apr;189(4):1388-95. doi: 10.1016/j.juro.2012.10.017. Epub 2012 Oct 16. PMID: 23079373.
- 9 - Shin DG, Kim HW, Yoon SJ, Song SH, Kim YH, Lee YG, Joo KJ, Bae JH, Kang TW, Jeong SJ, Woo SH, Yoo ES, Son H, Koo KC, Kim SW. Mirabegron as a treatment for overactive bladder symptoms in men (MIRACLE study): Efficacy and safety results from a multicenter, randomized, double-blind, placebo-controlled, parallel comparison phase IV study. Neurourol Urodyn. 2019 Jan;38(1):295-304. doi: 10.1002/nau.23852. Epub 2018 Oct 12. PMID: 30311691.
- 10 - Yamaguchi O, Marui E, Kakizaki H, Homma Y, Igawa Y, Takeda M, Nishizawa O, Gotoh M, Yoshida M, Yokoyama O, Seki N, Ikeda Y, Ohkawa S. Phase III, randomised, double-blind, placebo-controlled study of the ?3-adrenoceptor agonist mirabegron, 50?mg once daily, in Japanese patients with overactive bladder. BJU Int. 2014 Jun;113(6):951-60. doi: 10.1111/bju.12649. Epub 2014 Mar 17. PMID: 24471907.
- 11 - Yamaguchi O, Marui E, Igawa Y, Takeda M, Nishizawa O, Ikeda Y, Ohkawa S. Efficacy and Safety of the Selective ?3 -Adrenoceptor Agonist Mirabegron in Japanese Patients with Overactive Bladder: A Randomized, Double-Blind, Placebo-Controlled, Dose-Finding Study. Low Urin Tract Symptoms. 2015 May;7(2):84-92. doi: 10.1111/luts.12053. Epub 2014 Mar 11. PMID: 26663687.
Evidence tabellen
Evidence tables
|
Study reference |
Study characteristics |
Patient characteristics |
Intervention (I) |
Comparison / control (C)
|
Follow-up |
Outcome measures and effect size |
Comments |
|
Abrams, 2015 |
Type of study: Multicenter RCT.
Setting and country: 141 sites in 20 European countries.
Funding: The study was funded by Astellas.
Conflicts of interest: Potentially; several researchers received consultancy fees, grants, speaker fees, and patents and royalties from pharmaceutical companies. And some researchers involved in this study are employees of a pharmaceutical company.
|
Inclusion criteria: - 18 years or older. - Symptoms of OAB (urgency, urinary frequency, and/or urgency incontinence) for >3 months. - Eight or more micturitions per 24h and one urgency episode or more per 24h (with or without incontinence)
Exclusion criteria: - Average total daily urine volume >3000 ml. - Severe hypertension
N total at baseline: Placebo: 81 Mira (50 mg): 78 Soli (2.5 mg): 79 Soli (5 mg): 156 Soli (10 mg): 78 Soli (2.5 mg) + Mira (50 mg): 149 Soli (5 mg) + Mira (50 mg): 153 Soli (10 mg) + Mira (50 mg): 81
Important prognostic factors2: Age ± SD Placebo: 54.6 ± 13.4 Mira (50 mg): 53.4 ± 14.0 Soli (2.5 mg): 56.1 ± 11.7 Soli (5 mg): 54.2 ± 15.5 Soli (10 mg): 55.0 ± 12.8 Soli (2.5 mg) + Mira (50 mg): 53.7 ± 14.6 Soli (5 mg) + Mira (50 mg): 54.1 ± 14.1 Soli (10 mg) + Mira (50 mg): 55.5 ± 13.8
Sex (%female) Placebo: 66.7 Mira (50 mg): 66.7 Soli (2.5 mg): 64.6 Soli (5 mg): 66.0 Soli (10 mg): 67.9 Soli (2.5 mg) + Mira (50 mg): 67.1 Soli (5 mg) + Mira (50 mg): 66.0 Soli (10 mg) + Mira (50 mg): 66.7
Groups comparable at baseline? Yes, baseline characteristics were comparable, but incontinence, urgency and frequency were less severe in the placebo group.
|
Combinations of solifenacin (2.5/5/10 mg) and mirabegron (50 mg)
|
Solifenacin (2.5/5/10 mg) monotherapy Mirabegron (50 mg) monotherapy Placebo
|
Length of follow-up: 14 weeks
Loss-to-follow-up: N (total) = 67
Reasons: Withdrawal by patient (n = 27) Adverse event (n = 18) Protocol violation (n = 14) Lack of efficacy (n = 3) Lost to follow-up (n = 3) Other (n = 2)
Incomplete outcome data: Not reported, except loss of follow-up as above.
|
Voided volume per micturition Adjusted mean change (SE) from baseline to end of treatment Placebo: 14 (6) Mira (50 mg): 35 (6) Soli (2.5 mg): 36 (6) Soli (5 mg): 36 (4) Soli (10 mg): 36 (6) Soli (2.5 mg) + Mira (50 mg): 42 (4) Soli (5 mg) + Mira (50 mg): 54 (4) Soli (10 mg) + Mira (50 mg): 62 (6)
Frequency of TEAEs no, (%) Placebo: 32 (40) Mira (50 mg): 41 (53) Soli (2.5 mg): 32 (41) Soli (5 mg): 70 (45) Soli (10 mg): 47 (60) Soli (2.5 mg) + Mira (50 mg): 61 (41) Soli (5 mg) + Mira (50 mg): 67 (44) Soli (10 mg) + Mira (50 mg): 48 (59)
Blood pressure Adjusted mean change (SE) from baseline to end of treatment Systolic Placebo: -2.6 (1.1) Mira (50 mg): 0.7 (1.1) Soli (2.5 mg): -2 (1.1) Soli (5 mg): -1.7 (0.8) Soli (10 mg): -2.7 (1.1) Soli (2.5 mg) + Mira (50 mg): -0.6 (0.8) Soli (5 mg) + Mira (50 mg): -2.1 (0.8) Soli (10 mg) + Mira (50 mg): -0.4 (1.1)
Diastolic Placebo: -1.2 (0.7) Mira (50 mg): 0.3 (0.8) Soli (2.5 mg): -1.2 (0.8) Soli (5 mg): -0.6 (0.5) Soli (10 mg): 0 (0.8) Soli (2.5 mg) + Mira (50 mg): 0.2 (0.6) Soli (5 mg) + Mira (50 mg): -0.8 (0.6) Soli (10 mg) + Mira (50 mg): -0.2 (0.7)
Hypertension no, (%) Placebo: 7 (9) Mira (50 mg): 11 (14) Soli (2.5 mg): 8 (10) Soli (5 mg): 18 (12) Soli (10 mg): 5 (6) Soli (2.5 mg) + Mira (50 mg): 11 (7) Soli (5 mg) + Mira (50 mg): 7 (9) Soli (10 mg) + Mira (50 mg): 11 (14)
Pulse rate Adjusted mean change (SE) from baseline to end of treatment Placebo: 0.1 (0.85) Mira (50 mg): 1.0 (0.87) Soli (2.5 mg): 0.1 (0.87) Soli (5 mg): 0.1 (0.62) Soli (10 mg): 0.9 (0.87) Soli (2.5 mg) + Mira (50 mg): 1.1 (0.63) Soli (5 mg) + Mira (50 mg): 0.6 (0.62) Soli (10 mg) + Mira (50 mg): 1.3 (0.85)
Tachycardia no, (%) Placebo: 1 (1) Mira (50 mg): 2 (3) Soli (2.5 mg): 2 (3) Soli (5 mg): 6 (4) Soli (10 mg): 2 (3) Soli (2.5 mg) + Mira (50 mg): 7 (5) Soli (5 mg) + Mira (50 mg): 3 (2) Soli (10 mg) + Mira (50 mg): 3 (4) |
Authors conclusion: a combination of mirabegron and solifenacin demonstrated significant improvements in mean voided volume, micturition frequency, and urgency over solifenacin (5 mg) monotherapy, without increasing adverse effects associated with AM therapy compared with mirabegron or solifenacin monotherapy.
Limitations: 1) low proportion of incontinent individuals in the study population, 2) lack of power to detect a meaningful effect in secondary efficacy parameters (due to small sample sizes). |
|
Herschorn, 2017 |
Type of study: Multicenter RCT.
Setting and country: 435 sites in 42 countries.
Funding: This study was funded by Astellas Pharma Europe B.V.
Conflicts of interest: Potentially; several researchers received consultancy fees, grants, speaker fees, personal fees from pharmaceutical companies. And some researchers involved in this study are full-time employees of a pharmaceutical company.
|
Inclusion criteria: - 18 years or older - Symptoms of wet OAB for >3 months - In patients with mixed stress UI/UUI, UUI had to be the predominant factor - On average >8 micturitions/24h, >1 urgency episodes/24h, and >3 UI episodes over the 7-day micturition diary
Exclusion criteria: - Evidence of a urinary tract infection (urine culture containing >100.000 cfu/ml). - Average total daily urine volume >3000 ml. - Serum creatinine > 150 μmol/l, AST and/or ALT >2 x ULN, or GGT >3 x ULN, or total bilirubin >2 ULN.
N total at baseline: Placebo: 429 Mira 50 mg: 422 Soli 5 mg: 423 Soli 5 mg + Mira 50 mg: 848
Important prognostic factors2: Age ± SD Placebo: 57.9 ± 13.0 Mira 50 mg: 56.7 ± 13.3 Soli 5 mg: 58.2 ± 12.8 Soli 5 mg + Mira 50 mg: 57.6 ± 13.4
Sex (%male) Placebo: 23.3 Mira 50 mg: 23.5 Soli 5 mg: 21.7 Soli 5 mg + Mira 50 mg: 23.2
Groups comparable at baseline? Yes, no significant differences between the groups regarding baseline. |
Combinations of solifenacin (5 mg) and mirabegron (50 mg) daily
|
Daily: Solifenacin (5 mg) monotherapy Mirabegron (50 mg) monotherapy Placebo
|
Length of follow-up: 14 weeks
Loss-to-follow-up: Placebo: 43 Reasons: no study group (n = 2), AE (n = 13), lack of efficacy (n = 1), lost to follow-up (n = 4), protocol violation (n = 2), withdrawal by patient (n = 21).
Mira 50 mg: 50 Reasons: no study group (n = 4), AE (n = 12), lost to follow-up (n = 4), protocol violation (n = 3), withdrawal by patient (n = 23), other reasons (n = 4).
Soli 5 mg: 37 Reasons: no study group (n = 2), AE (n = 9), lack of efficacy (n = 2), lost to follow-up (n = 2), protocol violation (n = 5), withdrawal by patient (n = 16), other reasons (n = 1).
Soli 5 mg + Mira 50 mg: 85 Reasons: no study group (n = 13), AE (n = 26), lack of efficacy (n = 1), lost to follow-up (n = 3), protocol violation (n = 4), withdrawal by patient (n = 34), other reasons (n = 4).
Incomplete outcome data: Not reported, except loss of follow-up as above.
|
Mean number of urinary incontinence episodes per 24h Adjusted change from baseline to end of treatment Placebo: -1.3 Mira 50 mg: -1.8 Soli 5 mg: -1.8 Soli 5 mg + Mira 50 mg: -2.0
Mean number of micturitions per 24h Adjusted change from baseline to end of treatment Placebo: -1.6 Mira 50 mg: -2.0 Soli 5 mg: -2.2 Soli 5 mg + Mira 50 mg: -2.6
Mean volume voided per micturition Adjusted change from baseline to end of treatment Placebo: 8.4 Mira 50 mg: 22.0 Soli 5 mg: 31.0 Soli 5 mg + Mira 50 mg: 39.7
Frequency of TEAEs no, (%) Placebo: 145 (34) Mira 50 mg: 147 (35) Soli 5 mg: 149 (35) Soli 5 mg + Mira 50 mg: 314 (37)
|
Authors conclusion: combination therapy of mirabegron and solifenacin improved the most relevant OAB symptoms (urgency and UI episodes) compared with monotherapy.
Limitations: study population was very comparable with populations of previous mirabegron monotherapy studies. |
|
Khullar, 2013 |
Type of study: Multicenter RCT.
Setting and country: 189 sites in 27 countries in Europe and Australia.
Funding: Astellas Pharma Global Development sponsored this study.
Conflicts of interest: Potentially; several researchers are member of the advisory board of pharmaceutical companies. Researchers also received consultancy fees, grants and payment for lectures from pharmaceutical companies, and some researchers involved are employees of a pharmaceutical company. |
Inclusion criteria: - 18 years or older - Symptoms of OAB for >3 months - Average micturition frequency of eight or more times per 24h period and at least three episodes of urgency, with or without incontinence.
Exclusion criteria: - Stress incontinence or stress-predominant mixed incontinence - Average total daily urine volume >3000 ml
N total at baseline: Placebo: 494 Mira 50 mg: 493 Mira 100 mg: 496 Tol 4 mg: 495
Important prognostic factors2: Age ± SD Placebo: 59.2 ± 12.3 Mira 50 mg: 59.1 ± 12.4 Tol 4 mg: 59.1 ± 12.9
Sex (%male) Placebo: 27.9 Mira 50 mg: 27.6 Tol 4 mg: 27.1
Groups comparable at baseline? Yes. |
Mirabegron (50 mg) orally once daily |
Placebo or tolterodine ER (4 mg) orally once daily |
Length of follow-up: 12 weeks + 30 days
Loss-to-follow-up: Placebo: 44 Reasons: eligibility criterion not met (n = 5), adverse event (n = 13), lack of efficacy (n = 5), withdrew consent (n = 11), lost to follow-up (n = 4), protocol violation (n = 2), randomised but did not receive study drug (n = 2), other (n = 2).
Mira 50 mg: 57 Reasons: eligibility criterion not met (n = 8), adverse event (n = 25), lack of efficacy (n = 6), withdrew consent (n = 9), lost to follow-up (n = 3), protocol violation (n = 3), randomised but did not receive study drug (n = 1), other (n = 2).
Tol 4 mg: 50 Reasons: eligibility criterion not met (n = 4), adverse event (n = 24), lack of efficacy (n = 3), withdrew consent (n = 9), lost to follow-up (n = 5), protocol violation (n = 3), other (n = 2).
Incomplete outcome data: Not reported, except loss of follow-up as above.
|
Mean number of incontinence episodes per 24h Mean change (SE) from baseline to final visit Placebo: -1.1 (0.13) Mira 50 mg: -1.6 (0.14) Tol 4 mg: -1.2 (0.14)
Mean number of micturitions per 24h Mean change (SE) from baseline to final visit Placebo: -1.4 (0.12) Mira 50 mg: -1.9 (0.12) Tol 4 mg: -1.6 (0.12)
Volume voided per micturition Adjusted mean change (SE) from baseline to final visit Placebo: 12 (2) Mira 50 mg: 24 (2) Tol 4 mg: 25 (2)
Adjusted mean change (SE) from baseline to final visit Placebo: -1.7 (0.2) Mira 50 mg: -2.3 (0.2) Tol 4 mg: -2.1 (0.2)
Frequency of TEAEs no, (%) Placebo: 214 (43) Mira 50 mg: 211 (43) Tol 4 mg: 231 (47)
Hypertension no, (%) Placebo: 38 (8) Mira 50 mg: 29 (6) Tol 4 mg: 40 (8)
|
Authors conclusion: mirabegron has a proven efficacy and good tolerability, and therefore represents a new class of treatment for OAB.
Limitations: 1) long-term safety, efficacy and persistence of mirabegron cannot be extrapolated from this study, 2) head-to-head comparison of mirabegron and tolterodine was not allowed due to the study design, 3) a high placebo response diminished the treatment effect in this study, 4) this study included a significant proportion of patients who had previously discontinued antimuscarinic treatment, which may have influenced the tolterodine treatment effect. |
|
Kuo, 2015 |
Type of study: Multicenter RCT.
Setting and country: 67 sites in Taiwan, Korea, China and India.
Funding: This study was funded by Astellas Inc.
Conflicts of interest: Potentially; several researchers received consultancy fees, speaker fees and grants from, and conducted clinical trials for pharmaceutical companies. Also, some researchers are employees of the study sponsor. |
Inclusion criteria: - Symptoms of OAB for at least 12 weeks. - >8 micturitions per 24h on average - >1 episodes of urgency or urgency incontinence per 24h on average
Exclusion criteria: - Stress urinary incontinence as a predominant symptom - Urinary tract infection, urinary stone, interstitial cystitis or a history of recurrent urinary tract infection - Postvoid residual volume of >100 ml or a clinically significant lower urinary tract obstructive disease - Average total daily urine volume >3000 ml - Uncontrolled hypertension sitting systolic blood pressure (>180 mmHg or diastolic blood pressure >110 mmHg) - Pulse rate >110 beats per minute - Indwelling catheter or practices intermittent self-catheterization
N total at baseline: Placebo: 323 Mira: 338 Tol: 333
Important prognostic factors2: Age ± SD Placebo: 55.3 ± 13.6 Mira: 54.3 ± 14.2 Tol: 53.9 ± 14.5
Sex (%male) Placebo: 30.3 Mira: 32.5 Tol: 36.0
Groups comparable at baseline? Yes. |
Mirabegron (50 mg) orally, once daily after breakfast |
Placebo or tolterodine ER (4 mg) orally, once daily after breakfast |
Length of follow-up: 14 weeks
Placebo: 77 Reasons: eligibility criteria not met (n = 23), adverse event (n = 14), lack of efficacy (n = 7), withdrawal of consent (n = 21), lost to follow-up (n = 6), protocol deviation (n = 2), other (n = 4).
Mira: 61 Reasons: eligibility criteria not met (n = 18), adverse event (n = 9), lack of efficacy (n = 4), withdrawal of consent (n = 21), lost to follow-up (n = 3), protocol deviation (n = 4), other (n = 2).
Tol: 67 Reasons: eligibility criteria not met (n = 17), adverse event (n = 15), lack of efficacy (n = 2), withdrawal of consent (n = 24), lost to follow-up (n = 7), other (n = 2).
Incomplete outcome data: Not reported, except loss of follow-up as above. |
Frequency of TEAEs no, (%) Placebo: 214 (59%) Mira: 191 (52%) Tol: 260 (70%)
Hypertension no, (%) Placebo: 0 (0) Mira: 2 (0.5) Tol: 3 (0.8)
Blood pressure increased no, (%) Placebo: 1 (0.3) Mira: 0 (0) Tol: 1 (0.3) |
Authors conclusion: mirabegron is superior to placebo in reducing the frequency of micturitions in patients with symptoms of OAB.
Limitations: 1) low amount of severely affected patients included. |
|
Nitti, 2013 |
Type of study: Multicenter RCT.
Setting and country: 132 sites in the United States and Canada.
Funding: Medical writing assistance was supported by Astellas.
Conflicts of interest: None reported. |
Inclusion criteria: - 18 years or older - OAB symptoms for 3 or more months
Exclusion criteria: - Clinically relevant stress incontinence or mixed stress/urgency incontinence with stress as the predominant factor - An indwelling catheter - Evidence of a symptomatic urinary tract infection - Chronic inflammation - Bladder stones - Previous pelvic radiation therapy - Previous or current malignant disease of the pelvic organs - Severe hypertension (sitting average SBP 180 mmHg or greater and/or average sitting DBP 110 mmHg or greater) - Use of OAB medications which could not be stopped safely at screening.
N total at baseline: Placebo: 453 Mira 50 mg: 442
Important prognostic factors2: Age ± SD Placebo: 60.1 ± 13.8 Mira 50 mg: 59.2 ± 13.5
Sex (%male) Placebo: 23.8 Mira 50 mg: 27.1
Groups comparable at baseline? Yes |
Once daily mirabegron (50 mg) |
Once daily placebo |
Length of follow-up: 12 weeks + 30 days
Loss-to-follow-up: Placebo: 15.2% Reason: discontinuations due to adverse events (3.7%)
Mira 50 mg: 13.3% Reason: discontinuations due to adverse events (4.1%)
Incomplete outcome data: Not reported, except loss of follow-up as above. |
Number of incontinence episodes per 24h Change (SE) from baseline to final visit Placebo: -1.1 (0.11) Mira 50 mg: -1.5 (0.11)
Number of micturitions per 24h Change (SE) from baseline to final visit Placebo: -1.1 (0.13) Mira 50 mg: -1.7 (0.13)
Volume voided per micturition Change (SE) from baseline to final visit Placebo: 7 (2.4) Mira 50 mg: 18 (2.4)
Number of urgency episodes per 24h Change (SE) from baseline to final visit Placebo: -0.8 (0.16) Mira 50 mg: -1.6 (0.16)
Hypertension no, (%) Placebo: 30 (7) Mira 50 mg: 27 (6)
Tachycardia no, (%) Placebo: 2 (0.4) Mira 50 mg: 6 (1.4)
|
Authors conclusion: mirabegron was shown to be efficacious across objective and subjective endpoints in OAB patients. Mirabegron could provide an alternative therapeutic option for OAB, particularly in patients whose OAB is inadequately treated with current antimuscarinic therapy.
|
|
Shin, 2018 |
Type of study: Multicenter RCT
Setting and country: Korea.
Funding: This study was funded by Astellas Pharma Korea, Inc.
Conflicts of interest: All authors completed the ICMJE form for the disclosure of potential conflicts of interest. |
Inclusion criteria: - Males aged >20 years - Symptoms of OAB persistent for at least 12 weeks - Average of 8 or more micturition episodes per 24h - Score of 2 (one urgency episode or more per week) or greater in the urgency score section of the OAB symptoms scale
Exclusion criteria: - Risk or history of acute urinary retention - PSA levels >10 ng/ml or suspected prostate cancer - Voiding volume over 3000 ml per day - Suspicion of stress urinary incontinence - Postvoid residual volume >200 ml, voided volume <125 ml and Qmax <5 ml/sec - Subjects that received non-drug treatment, including bladder training or pelvic floor muscle exercise within the 4 weeks prior to screening
N total at baseline: Placebo: 154 Mira: 310
Important prognostic factors2: Age ± SD Placebo: 65.2 ± 10.0 Mira: 66.4 ± 9.5
Groups comparable at baseline? Yes, but the mirabegron group had larger prostate volume and fewer 24h micturition episodes. |
Once daily mirabegron (50 mg) |
Once daily placebo |
Length of follow-up: 12 weeks + 14 weeks extended treatment period
Loss-to-follow-up: Placebo: 45 Reasons: lack of efficacy (n = 1), adverse event (n =3), withdrawal by patient (n = 10), lost to follow-up (n = 5), compliance <70%, >130% (n = 2), protocol violation (n = 21), other (n = 3).
Mira 50 mg: 85 Reasons: lack of efficacy (n = 3), adverse event (n = 10), withdrawal by patient (n = 24), lost to follow-up (n = 6), compliance <70%, >130% (n = 3), protocol violation (n = 29), other (n = 10).
Incomplete outcome data: Not reported, except loss of follow-up as above. |
Number of micturition episodes per 24h Mean change (SD) from baseline to final visit Mirabegron: -1.8 (2.2) Placebo: -2.0 (2.7)
Frequency of AEs no, (%) Mirabegron: 48 (15.5) Placebo: 18 (11.7)
Blood pressure Mean change (SD) from baseline to final visit Systolic Mirabegron: -0.21 Placebo: 0.76
Diastolic Mirabegron: 0.13 Placebo: 0.7
Pulse rate Mean change (SD) from baseline to final visit Mirabegron: 0.58 Placebo: 1.12
|
Authors conclusion: a daily dose of 50 mg mirabegron for 12 weeks can be an effective treatment that alleviates urgency and storage symptoms in male patients with OAB.
Limitations: 1) small study population, 2) short follow-up, 3) there was no wash-out period of combined drugs to treat lower urinary tract symptoms (might have confounded the results found), 4) large variation of the primary efficacy variables within each group. |
|
Staskin, 2021 |
Type of study: RCT extension study.
Setting and country: 109 sites in the United States.
Funding: Editorial support was funded by Urovant Sciences.
Conflicts of interest: None declared. |
Inclusion criteria: - 18 years or older - History of OAB diagnosed by a physician 3 or more months before screening - Meet the diary-based criteria for either wet or dry OAB (i.e., urinary urgency with or without urge incontinence) - Completion of the EMPOWER study - Demonstration of >80% compliance with self-administration of the study treatment in EMPOWUR and to have completed >4 diary days for EMPOWUR week 12
Exclusion criteria: - Urine volume output of greater than 3000 ml - Inability to complete EMPOWUR for any reason - Change in history or current evidence of any clinically significant condition that could confound study results - Uncontrolled hyperglycaemia - Uncontrolled hypertension - Resting heart rate >100 beats per minute
N total at baseline: 40 weeks Vibe: 92 Tol: 91
52 weeks Vibe: 181 Tol: 141
Important prognostic factors2: Age ± SD 40 weeks Vibe: 58.8 ± 13.7 Tol: 62.1 ± 12.1
52 weeks Vibe: 62.1 ± 12.4 Tol: 60.6 ± 13.0
Sex (%female) 40 weeks Vibe: 79.3 Tol: 76.9
52 weeks Vibe: 77.3 Tol: 79.4
Groups comparable at baseline? Yes |
Once daily vibegron (75 mg) |
Once daily tolterodine ER (4 mg) |
Length of follow-up: 40 weeks of extension (52 total weeks of treatment)
Loss-to-follow-up: 40 weeks Vibe: 13 (14.1%) Reasons: withdrew consent (6.5%), lost to follow-up (4.3%), adverse event (1.1%), death (1.1%), other (1.1%).
Tol: 19 (20.9%) Reasons: withdrew consent (7.7%), adverse event (4.4%), lost to follow-up (3.3%), withdrawal by investigator (1.1%), other (4.4%).
52 weeks Vibe: 26 (14.3%) Reasons: withdrew consent (6.0%), adverse event (1.6%), lost to follow-up (3.3%), withdrawal by investigator (0.5%), lack of efficacy (0.5%), protocol deviation (0.5%), other (1.6%).
Tol: 18 (12.8%) Reasons: withdrew consent (5.7%), adverse event (2.8%), lost to follow-up (1.4%), withdrawal by investigator (0.7%), withdrawal by sponsor (0.7%), lack of efficacy (0.7%), other (0.7%).
Incomplete outcome data: Not reported, except loss of follow-up as above. |
Daily number of micturitions LS mean change (95%CI) from baseline to final visit Vibe: -2.4 (-2.9 to -2.0) Tol: -2.0 (-2.5 to -1.5)
Daily number of urgency episodes LS mean change (95%CI) from baseline to final visit Vibe: -3.4 (-4.0 to -2.7) Tol: -3.2 (-4.0 to -2.5)
Daily number of urinary incontinence episodes LS mean change (95%CI) from baseline to final visit Vibe: -2.5 (-2.8 to -2.2) Tol: -1.9 (-2.3 to -1.6)
Frequency of TEAEs no, (%) Vibe: 171 (63) Tol: 126 (54)
Hypertension no, (%) Vibe: 24 (9) Tol: 20 (9)
|
Authors conclusion: vibegron treatment showed favorable long-term efficacy, safety and tolerability in OAB patients. Vibegron represents an interesting long-term treatment option for patients with OAB.
Limitation: potential for selection bias for patients who elected to enter the EMPOWUR extension |
|
Suzuki, 2021 |
Type of study: Multicenter RCT.
Setting and country: 11 urology clinics and centers in Japan.
Funding: This study was sponsored by Hisamitsu Pharmaceutical.
Conflicts of interest: None declared. |
Inclusion criteria: - Female patients aged >20 years - OAB symptoms for >8 weeks - Eight or more micturitions per 24h - One or more urinary urgency episodes per 24h - Two or more nocturnal micturitions per night
Exclusion criteria: - >100 ml postvoid residual urine - Difficulty in walking by themselves - Urethral stricture - Bladder stone - Bladder tumor - Urinary tract infection - Pregnancy - Pelvic organ prolapse Neuropsychiatric disorder associated with neurogenic bladder, including cerebrovascular disorder - Use of medicines for urinary tract dysfunction within 2 weeks before enrolment
N total at baseline: Mira: 49 Oxy: 51
Important prognostic factors2: Age ± SD Mira: 72.7 ± 11.3 Oxy: 68.2 ± 11.3
Groups comparable at baseline? Yes |
Mirabegron (50 mg) orally once daily |
Oxybutynin (73.5 mg) patch placed on the lower abdomen or thighs once daily |
Length of follow-up: 8 weeks
Loss-to-follow-up: Mira: 5 Reasons: withdrew consent (n = 3), ineligible after enrolment (n = 1), adverse event (n = 1).
Oxy: 10 Reasons: withdrew consent (n = 4), ineligible after enrolment (n = 1), adverse event (n = 5).
Incomplete outcome data: Not reported, except loss of follow-up as above. |
24h urinary urgency Mean changes from baseline to end of treatment Mira: -1.3 ± 1.6 Oxy: -1.7 ± 2.7
24h urgency incontinence Mean changes from baseline to end of treatment Mira: -0.6 ± 1 Oxy: -1.1 ± 2
Total urine volume Mean changes from baseline to end of treatment Mira: -57 ± 402 Oxy: -58 ± 491
Mean voided urine volume Mean changes from baseline to end of treatment Mira: 28 ± 36 Oxy: 37 ± 48
Frequency of AEs no, (%) Mira: 1 (2) Oxy: 26 (51) |
Authors conclusion: the oxybutynin patch shows promising results for the treatment of OAB.
Limitations: 1) not enough power to evaluate differences among groups but only to compare before and after administration of each drug, 2) only objective evaluation of bladder function was carried out, 3) only female OAB patients were included in this study, 4) statistical differences in age between the two groups were present, 5) statistical improvement of N-QOL could not be detected, so it remains unclear if the oxybutynin patch has therapeutic effects on nocturia in the long term.
|
|
Yamaguchi, 2014 |
Type of study: Multicenter RCT.
Setting and country: Japan.
Funding: This study was sponsored by Astellas Pharma Inc.
Conflicts of interest: Potentially; several researchers received grants, consultancy fees, speaker fees and payments for lectures from pharmaceutical companies. Also, one researcher is a member of the advisory boards of a pharmaceutical company. |
Inclusion criteria: - 20 years or older - OAB symptoms for >24 weeks - On average >8 micturitions per 24h and >1 urgency episode per 24h and/or >1 urgency incontinence episode per 24h
Exclusion criteria: - Diagnosis of genuine stress incontinence - Average total daily urine volume >3000 ml - Postvoid residual urine volume of at least 100 ml
N total at baseline: Placebo: 368 Mira: 369 Tol: 368
Important prognostic factors2: Age ± SD Placebo: 58.2 ± 14.2 Mira: 58.3 ± 13.9 Tol: 58.3 ± 13.7
Sex (%male) Placebo: 15.8 Mira: 15.7 Tol: 17.4
Groups comparable at baseline? Yes |
Active mirabegron group: mirabegron (50 mg) tablet and tolterodine placebo capsule once daily |
Active tolterodine control: tolterodine (4 mg) capsule and mirabegron placebo tablet once daily
Placebo group: mirabegron placebo tablet and tolterodine placebo capsule once daily |
Length of follow-up: 14 weeks
Loss-to-follow-up: Placebo: 31 Reasons: adverse events (n = 9), inadequate efficacy (n = 3), withdrew consent (n = 12), protocol deviations (n = 5), other (n = 2).
Mira: 31 Reasons: adverse events (n = 15), inadequate efficacy (n = 4), withdrew consent (n = 8), protocol deviations (n = 3), other (n = 1).
Tol: 23 Reasons: adverse events (n = 13), inadequate efficacy (n = 2), withdrew consent (n = 1), protocol deviations (n = 2), other (n = 5).
Incomplete outcome data: Not reported, except loss of follow-up as above. |
Volume voided per micturition Mean change (SD) from baseline to final assessment Placebo: 10 ± 29 Mira: 24 ± 35 Tol: 29 ± 35
Micturitions per 24h Mean change (SD) from baseline to final assessment Placebo: -0.9 ± 2.3 Mira: -1.7 ± 2.2 Tol: -1.4 ± 2.2
Urgency episodes per 24h Mean change (SD) from baseline to final assessment Placebo: -1.4 ± 3.2 Mira: -1.9 ± 2.5 Tol: -1.7 ± 2.6
Incontinence episodes per 24h Mean change (SD) from baseline to final assessment Placebo: -0.7 ± 1.9 Mira: -1.1 ± 1.5 Tol: -1 ± 1.6 Frequency of AEs no, (%) Placebo: 91 (24) Mira: 93 (25) Tol: 131 (35)
Hypertension no, (%) Placebo: 0 (0) Mira: 0 (0) Tol: 3 (0.8)
Tachycardia no, (%) Placebo: 0 (0) Mira: 1 (0.3) Tol: 0 (0)
Blood pressure increased no, (%) Placebo: 1 (0.3) Mira: 0 (0) Tol: 2 (0.5)
Pulse rate increased no, (%) Placebo: 0 (0) Mira: 1 (0.3) Tol: 1 (0.3) |
Authors conclusion: this study supports mirabegron as an attractive treatment alternative in the management of OAB compared to antimuscarinic therapy.
Limitation: low mean number of nocturia episodes at baseline. |
|
Yamaguchi, 2015 |
Type of study: Multicenter RCT.
Setting and country: Japan.
Funding: This study and editorial support were funded by Astellas Pharma Inc.
Conflicts of interest: Potentially; several researchers received grants, consultancy fees, speaker fees and payments for lectures from pharmaceutical companies. Also, one researcher is a member of the advisory boards of a pharmaceutical company. |
Inclusion criteria: - Outpatients aged >20 years - OAB symptoms for >24 weeks - An average of >8 micturitions per 24h and >1 urgency episode and/or >1 urgency incontinence episode per 24h
Exclusion criteria: - Polyuria exceeding 3000 ml in mean daily micturition volume - Clear diagnosis of stress incontinence
N total at baseline: Placebo: 211 Mira 50 mg: 208
Important prognostic factors2: Age ± SD Placebo: 55.7 ± 12.9 Mira 50 mg: 56.2 ± 13.6
Sex (%male) Placebo: 19.9 Mira 50 mg: 14.9
Groups comparable at baseline? Yes |
Oral once daily mirabegron (50 mg) tablet |
Oral once daily placebo tablet |
Length of follow-up: 12 weeks
Loss-to-follow-up: Placebo: 16 Reasons: adverse events (n = 6), insufficient therapeutic effect (n = 4), consent withdrawal (n = 1), protocol deviation (n = 5).
Mira 50 mg: 13 Reasons: adverse events (n = 8), consent withdrawal (n = 2), protocol deviation (n = 2), other (n = 1).
Incomplete outcome data: Not reported, except loss of follow-up as above. |
Micturitions per 24h Mean change (SD) from baseline to end of study Placebo: -1.2 ± 2.2 Mirabegron: -2.1 ± 2.4
Urgency episodes per 24h Mean change (SD) from baseline to end of study Placebo: -1.8 ± 3 Mirabegron: -2.2 ± 3.1
Incontinence episodes per 24h Mean change (SD) from baseline to end of study Placebo: -0.6 ± 1.4 Mirabegron: -1.2 ± 1.5
Volume voided per micturition Mean change from baseline to end of study Placebo: 11.2 ± 36.9 Mirabegron: 27.3 ± 39.5
Frequency of TEAEs no, (%) Placebo: 157 (74) Mirabegron: 171 (82)
Pulse rate Mean change (SD) from baseline to end of study Placebo: 0.22 ± 6.9 Mirabegron: 2.7 ± 6.4
Hypertension no, (%) Placebo: 0 (0) Mirabegron: 1 (0.5)
Tachycardia no, (%) Placebo: 0 (0) Mirabegron: 1 (0.5)
Palpitations no, (%) Placebo: 1 (0.5) Mirabegron: 4 (1.9) |
Authors conclusion: this study confirms the efficacy and safety of mirabegron, and support the recommended dose of 50 mg.
|
Risk of bias tables
|
Study reference
|
Was the allocation sequence adequately generated?
|
Was the allocation adequately concealed? |
Blinding: Was knowledge of the allocated interventions adequately prevented? Were patients/healthcare providers/data collectors/outcome assessors/data analysts blinded? |
Was loss to follow-up (missing outcome data) infrequent?
|
Are reports of the study free of selective outcome reporting?
|
Was the study apparently free of other problems that could put it at a risk of bias?
|
Overall risk of bias If applicable/necessary, per outcome measure
|
|
Abrams, 2015 |
Probably yes
Reason: assigned in a 2:1 ratio for primary compared with secondary treatment groups. |
Probably yes
Reason: not specifically reported but no reason to doubt that the allocation was adequately concealed. |
Definitely yes
Reason: double-blind trial using a double-blind, double-dummy technique. Run-in period was single-blinded. |
Definitely yes
Reason: primary reasons for discontinuation were withdrawal of consent, adverse events and protocol violation. Missing outcome data is balanced in numbers across intervention groups, with similar reasons. |
Probably yes
Reason: registered at ClinicalTrials.gov (NCT01340027). Primary and (most) secondary outcomes reported as prespecified in register. No reason to doubt that study is free of selective outcome reporting. |
Definitely yes
Reason: no other problems reported. |
LOW (some information is missing but it seems uncertain that it affected the outcomes) |
|
Herschorn, 2017 |
Probably yes
Reason: assigned to treatment in a 2:1 ratio between the combined therapy, monotherapy and placebo treatment arms. |
Probably yes
Reason: not specifically reported but no reason to doubt that the allocation was adequately concealed. |
Definitely yes
Reason: single-blind run-in period, double-blind treatment period, and single-blind run-out period. |
Definitely yes
Reason: primary reasons for discontinuation were adverse events or withdrawal by the patient. Missing outcome data is balanced in numbers across intervention groups, with similar reasons. |
Definitely yes
Reason: registered at ClinicalTrials.gov (NCT01972841). Primary and secondary outcomes reported as prespecified in register. |
Probably yes
Reason: primary objective of the study was not met. |
Some concerns (some information is missing, and the primary objective of the study was not met) |
|
Khullar, 2013 |
Definitely yes
Reason: computer-generated randomisation scheme with stratification by country was used. |
Definitely yes
Reason: allocation was accomplished via an interactive response system. |
Definitely yes
Reason: single-blind run-in period, followed by a double-blind treatment period. |
Definitely yes
Reason: primary reasons for discontinuation were adverse events and withdrawal of consent. Missing outcome data is balanced in numbers across intervention groups, with similar reasons. |
Probably yes
Reason: registered at ClinicalTrials.gov (NCT01972841). Primary and (most) secondary outcomes reported as prespecified in register. No reason to doubt that study is free of selective outcome reporting. |
Definitely yes
Reason: no other problems reported. |
LOW (no reasons to suspect bias) |
|
Kuo, 2015 |
Definitely yes
Reason: computer-generated randomisation scheme with stratification by site was used. |
Probably yes
Reason: not specifically reported but no reason to doubt that the allocation was adequately concealed. |
Definitely yes
Reason: single-blind run-in period, followed by a double-blind treatment period. |
Definitely yes
Reason: primary reasons for discontinuation were not meeting the eligibility criteria and withdrawal of consent. Missing outcome data is balanced in numbers across intervention groups, with similar reasons. |
Definitely yes
Reason: registered at ClinicalTrials.gov (NCT01043666). Primary and secondary outcomes reported as prespecified in register. |
Definitely yes
Reason: no other problems reported. |
LOW (no reasons to suspect bias) |
|
Nitti, 2013 |
Definitely yes
Reason: assigned to mirabegron (50/100 mg) or matching placebo in a 1:1:1 ratio using a computer-generated randomization scheme. |
Probably yes
Reason: not specifically reported but no reason to doubt that the allocation was adequately concealed. |
Definitely yes
Reason: single-blind run-in period, followed by a double-blind treatment period. |
Definitely yes
Reason: primary reason for discontinuation was adverse events. Missing outcome data is balanced in numbers across intervention groups, with similar reasons. |
Probably yes
Reason: registered at ClinicalTrials.gov (NCT00662909). Primary and (most) secondary outcomes reported as prespecified in register. No reason to doubt that study is free of selective outcome reporting. |
Definitely yes
Reason: no other problems reported. |
LOW (no reasons to suspect bias) |
|
Shin, 2018 |
Probably yes
Reason: randomization in a 2:1 ratio into the mirabegron or placebo group. |
Probably yes
Reason: not specifically reported but no reason to doubt that the allocation was adequately concealed. |
Definitely yes
Reason: double-blinded trial |
Definitely yes
Reason: primary reasons for discontinuation were withdrawal by patient and protocol violation. Missing outcome data is balanced in numbers across intervention groups, with similar reasons. |
Probably yes
Reason: no registration in register of clinical trials known. Study protocol has been published but is not available.
|
Probably no
Reason: large baseline characteristic differences, and no appropriate statistical analysis was used. |
Some concerns (some problems reported that could have affected the outcomes) |
|
Staskin, 2021 |
Definitely yes
Reason: central web-based randomization in a 1:1 ratio into the vibegron or tolterodine group |
Definitely yes
Reason: central web-based interactive response system. |
Definitely yes
Reason: double-blinded trial |
Definitely yes
Reason: the study completion rate was 85% overall, with similar completion rates for both treatment groups. Primary reason for discontinuation in both groups was withdrawal of consent, followed by adverse events. |
Definitely yes
Reason: registered at ClinicalTrials.gov (NCT03583372). Primary and secondary outcomes reported as prespecified in register. |
Probably no
Reason: potential selection bias for patients who elected to enter the EMPOWUR extension study, no sample size calculation. |
Some concerns (some problems reported that could have affected the outcomes) |
|
Suzuki, 2021 |
Definitely yes
Reason: central web-based randomization in a 1:1 ratio into the oxybutynin or mirabegron group |
Definitely yes
Reason: alliance clinical research support system was used |
Unknown |
Probably yes
Reason: six participants (9.8%) discontinued the study due to adverse events. Five patients in the oxybutynin group and one patient in the mirabegron group. |
Probably yes
Reason: no registration in register of clinical trials known. Study protocol has been published but is not available. |
Probably no
Reason: no sample size calculation was performed, small differences in baseline characteristics, objective evaluation for the effects of drugs. |
Some concerns (crucial information about blinding is missing, and problems are reported that could have affected the outcomes) |
|
Yamaguchi, 2014 |
Definitely yes
Reason: randomization in a 1:1:1 ratio using a block size of six |
Probably yes
Reason: not specifically reported but no reason to doubt that the allocation was adequately concealed. |
Definitely yes
Reason: single-blind run-in period, followed by a double-blind treatment period. |
Definitely yes
Reason: primary reasons for discontinuation were adverse events and withdrawal of consent. Missing outcome data is balanced in numbers across intervention groups, with similar reasons. |
Definitely yes
Reason: registered at ClinicalTrials.gov (NCT00966004). Primary and secondary outcomes reported as prespecified in register. |
Definitely yes
Reason: no other problems reported. |
LOW (no reasons to suspect bias) |
|
Yamaguchi, 2015 |
Probably yes
Reason: not specifically reported but no reason to doubt that the sequence was adequately generated. |
Probably yes
Reason: not specifically reported but no reason to doubt that the allocation was adequately concealed. |
Definitely yes
Reason: single-blind run-in period, followed by a double-blind treatment period. |
Definitely yes
Reason: primary reasons for discontinuation were adverse events, protocol deviation and withdrawal of consent. Missing outcome data is balanced in numbers across intervention groups, with similar reasons. |
Definitely yes
Reason: registered at ClinicalTrials.gov (NCT00527033). Primary and secondary outcomes reported as prespecified in register. |
Definitely yes
Reason: no other problems reported. |
LOW (no reasons to suspect bias) |
Table of excluded studies
|
Reference |
Reason for exclusion |
|
Abrams P, Kelleher C, Staskin D, Kay R, Martan A, Mincik I, Newgreen D, Ridder A, Paireddy A, van Maanen R. Combination treatment with mirabegron and solifenacin in patients with overactive bladder: exploratory responder analyses of efficacy and evaluation of patient-reported outcomes from a randomized, double-blind, factorial, dose-ranging, Phase II study (SYMPHONY). World J Urol. 2017 May;35(5):827-838. doi: 10.1007/s00345-016-1908-1. Epub 2016 Aug 11. PMID: 27514371. |
Wrong publication type (exploratory responder analyses) |
|
Alcántara Montero A. Long-term safety and efficacy of mirabegron and solifenacin in combination compared with monotherapy in patients with overactive bladder: SYNERGY II study. Actas Urol Esp (Engl Ed). 2019 Jan-Feb;43(1):51-52. English, Spanish. doi: 10.1016/j.acuro.2018.06.002. Epub 2018 Jul 17. PMID: 30025617. |
Foreign language |
|
Alcántara-Montero A. Combined treatment of solifenacin and mirabegron, an alternative in patients with overactive bladder (BESIDE study). Actas Urol Esp. 2016 Nov;40(9):593-594. English, Spanish. doi: 10.1016/j.acuro.2016.03.005. Epub 2016 Apr 25. PMID: 27126125. |
Foreign language |
|
Batista JE, Kölbl H, Herschorn S, Rechberger T, Cambronero J, Halaska M, Coppell A, Kaper M, Huang M, Siddiqui E; BEYOND study group. The efficacy and safety of mirabegron compared with solifenacin in overactive bladder patients dissatisfied with previous antimuscarinic treatment due to lack of efficacy: results of a noninferiority, randomized, phase IIIb trial. Ther Adv Urol. 2015 Aug;7(4):167-79. doi: 10.1177/1756287215589250. PMID: 26445596; PMCID: PMC4580095. |
Less recent and complete compared to Mostafaei (2022) |
|
Chapple CR, Amarenco G, López Aramburu MA, Everaert K, Liehne J, Lucas M, Vik V, Ridder A, Snijder R, Yamaguchi O; BLOSSOM Investigator Group. A proof-of-concept study: mirabegron, a new therapy for overactive bladder. Neurourol Urodyn. 2013 Nov;32(8):1116-22. doi: 10.1002/nau.22373. Epub 2013 Feb 19. PMID: 23424164. |
Published prior to February 2021 (search period Mostafaei, 2022) |
|
Chapple CR, Cardozo L, Nitti VW, Siddiqui E, Michel MC. Mirabegron in overactive bladder: a review of efficacy, safety, and tolerability. Neurourol Urodyn. 2014 Jan;33(1):17-30. doi: 10.1002/nau.22505. Epub 2013 Oct 11. PMID: 24127366. |
Wrong publication type (review) |
|
Chapple CR, Dvorak V, Radziszewski P, Van Kerrebroeck P, Wyndaele JJ, Bosman B, Boerrigter P, Drogendijk T, Ridder A, Van Der Putten-Slob I, Yamaguchi O; Dragon Investigator Group. A phase II dose-ranging study of mirabegron in patients with overactive bladder. Int Urogynecol J. 2013 Sep;24(9):1447-58. doi: 10.1007/s00192-013-2042-x. Epub 2013 Mar 8. PMID: 23471546; PMCID: PMC3745617. |
Published prior to February 2021 (search period Mostafaei, 2022) |
|
Chapple CR, Kaplan SA, Mitcheson D, Klecka J, Cummings J, Drogendijk T, Dorrepaal C, Martin N. Randomized double-blind, active-controlled phase 3 study to assess 12-month safety and efficacy of mirabegron, a β(3)-adrenoceptor agonist, in overactive bladder. Eur Urol. 2013 Feb;63(2):296-305. doi: 10.1016/j.eururo.2012.10.048. Epub 2012 Nov 6. PMID: 23195283. |
Published prior to February 2021 (search period Mostafaei, 2022) |
|
Frankel J, Staskin D, Varano S, Kennelly M, Newman DK, Rosenberg MT, Jankowich RA, Shortino D, Mudd PN Jr, Girman CJ. Interpretation of the Meaningfulness of Symptom Reduction with Vibegron in Patients with Overactive Bladder: Analyses from EMPOWUR. Adv Ther. 2022 Feb;39(2):959-970. doi: 10.1007/s12325-021-01972-8. Epub 2021 Dec 18. PMID: 34921665; PMCID: PMC8866263. |
Wrong outcome (patient perception of improvement) |
|
Frankel J, Varano S, Staskin D, Shortino D, Jankowich R, Mudd PN Jr. Vibegron improves quality-of-life measures in patients with overactive bladder: Patient-reported outcomes from the EMPOWUR study. Int J Clin Pract. 2021 May;75(5):e13937. doi: 10.1111/ijcp.13937. Epub 2021 Jan 22. PMID: 33332699; PMCID: PMC8244055. |
Wrong outcome (patient-reported QoL outcomes) |
|
Gratzke C, van Maanen R, Chapple C, Abrams P, Herschorn S, Robinson D, Ridder A, Stoelzel M, Paireddy A, Yoon SJ, Al-Shukri S, Rechberger T, Mueller ER. Long-term Safety and Efficacy of Mirabegron and Solifenacin in Combination Compared with Monotherapy in Patients with Overactive Bladder: A Randomised, Multicentre Phase 3 Study (SYNERGY II). Eur Urol. 2018 Oct;74(4):501-509. doi: 10.1016/j.eururo.2018.05.005. Epub 2018 Jun 1. PMID: 29866467. |
Published prior to February 2021 (search period Mostafaei, 2022) |
|
Griebling TL, Campbell NL, Mangel J, Staskin D, Herschorn S, Elsouda D, Schermer CR. Effect of mirabegron on cognitive function in elderly patients with overactive bladder: MoCA results from a phase 4 randomized, placebo-controlled study (PILLAR). BMC Geriatr. 2020 Mar 18;20(1):109. doi: 10.1186/s12877-020-1474-7. PMID: 32183741; PMCID: PMC7079371. |
Wrong outcome (cognitive function) |
|
Herschorn S, Barkin J, Castro-Diaz D, Frankel JM, Espuna-Pons M, Gousse AE, Stölzel M, Martin N, Gunther A, Van Kerrebroeck P. A phase III, randomized, double-blind, parallel-group, placebo-controlled, multicentre study to assess the efficacy and safety of the β₃ adrenoceptor agonist, mirabegron, in patients with symptoms of overactive bladder. Urology. 2013 Aug;82(2):313-20. doi: 10.1016/j.urology.2013.02.077. Epub 2013 Jun 13. Erratum in: Urology. 2013 Dec;82(6):1457. PMID: 23769122. |
Published prior to February 2021 (search period Mostafaei, 2022) |
|
Herschorn S, Staskin D, Schermer CR, Kristy RM, Wagg A. Safety and Tolerability Results from the PILLAR Study: A Phase IV, Double-Blind, Randomized, Placebo-Controlled Study of Mirabegron in Patients ≥ 65 years with Overactive Bladder-Wet. Drugs Aging. 2020 Sep;37(9):665-676. doi: 10.1007/s40266-020-00783-w. PMID: 32725584; PMCID: PMC7473960. |
Wrong population (elderly) |
|
Herschorn S, Staskin D, Tu LM, Fialkov J, Walsh T, Gooch K, Schermer CR. Patient-reported outcomes in patients with overactive bladder treated with mirabegron and tolterodine in a prospective, double-blind, randomized, two-period crossover, multicenter study (PREFER). Health Qual Life Outcomes. 2018 Apr 19;16(1):69. doi: 10.1186/s12955-018-0892-0. PMID: 29673355; PMCID: PMC5909214. |
Wrong outcome (patient-reported outcomes) |
|
Hsiao SM, Chang TC, Chen CH, Wu WY, Lin HH. Comparisons of the Clinical Outcomes and Urodynamic Effects of Mirabegron versus Tolterodine Treatment for Female Overactive Bladder Syndrome: A Subgroup Analysis of a Controlled, Randomised, Prospective Study. Low Urin Tract Symptoms. 2018 Sep;10(3):215-220. doi: 10.1111/luts.12167. Epub 2017 Apr 23. PMID: 28436145. |
Published prior to February 2021 (search period Mostafaei, 2022) |
|
Inoue M, Yokoyama T. Comparison of Two Different Drugs for Overactive Bladder, Solifenacin and Mirabegron: A Prospective Randomized Crossover Study. Acta Med Okayama. 2019 Oct;73(5):387-392. doi: 10.18926/AMO/57368. PMID: 31649364. |
Published prior to February 2021 (search period Mostafaei, 2022) |
|
Khullar V, Amarenco G, Angulo JC, Blauwet MB, Nazir J, Odeyemi IA, Hakimi Z. Patient-reported outcomes with the β3 -adrenoceptor agonist mirabegron in a phase III trial in patients with overactive bladder. Neurourol Urodyn. 2016 Nov;35(8):987-994. doi: 10.1002/nau.22844. Epub 2015 Aug 19. PMID: 26288118. |
Wrong outcome (patient-reported outcomes) |
|
Khullar V, Cambronero J, Angulo JC, Wooning M, Blauwet MB, Dorrepaal C, Martin NE. Efficacy of mirabegron in patients with and without prior antimuscarinic therapy for overactive bladder: a post hoc analysis of a randomized European-Australian Phase 3 trial. BMC Urol. 2013 Sep 18;13:45. doi: 10.1186/1471-2490-13-45. PMID: 24047126; PMCID: PMC3849064. |
Wrong publication type (post-hoc analysis irrelevant subgroups) |
|
Kinjo M, Sekiguchi Y, Yoshimura Y, Nutahara K. Long-term Persistence with Mirabegron versus Solifenacin in Women with Overactive Bladder: Prospective, Randomized Trial. Low Urin Tract Symptoms. 2018 May;10(2):148-152. doi: 10.1111/luts.12151. Epub 2016 Dec 2. PMID: 27911988. |
Wrong population (elderly) |
|
Kosilov K, Loparev S, Ivanovskaya M, Kosilova L. A randomized, controlled trial of effectiveness and safety of management of OAB symptoms in elderly men and women with standard-dosed combination of solifenacin and mirabegron. Arch Gerontol Geriatr. 2015 Sep-Oct;61(2):212-6. doi: 10.1016/j.archger.2015.06.006. Epub 2015 Jun 25. PMID: 26169181. |
Wrong population (elderly) |
|
Andersson KE, Martin N, Nitti V. Selective β₃-adrenoceptor agonists for the treatment of overactive bladder. J Urol. 2013 Oct;190(4):1173-80. doi: 10.1016/j.juro.2013.02.104. Epub 2013 Feb 28. PMID: 23458471. |
Wrong publication type (overview of available literature) |
|
Mirabegron Extended-Release Tablets (Myrbetriq): Treatment of Overactive Bladder (OAB) with Symptoms of Urgency, Urgency Incontinence and Urinary Frequency [Internet]. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2015 Jul. PMID: 26962607. |
Less recent and complete compared to Mostafaei (2022) |
|
Athanasiou S, Pitsouni E, Grigoriadis T, Zacharakis D, Salvatore S, Serati M. Mirabegron in female patients with overactive bladder syndrome: What's new? A systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2020 Aug;251:73-82. doi: 10.1016/j.ejogrb.2020.05.018. Epub 2020 May 15. PMID: 32480182. |
Less recent and complete compared to Mostafaei (2022) |
|
Bhide AA, Digesu GA, Fernando R, Khullar V. Use of mirabegron in treating overactive bladder. Int Urogynecol J. 2012 Oct;23(10):1345-8. doi: 10.1007/s00192-012-1724-0. Epub 2012 Mar 13. PMID: 22411211. |
Wrong publication type (review) |
|
Bragg R, Hebel D, Vouri SM, Pitlick JM. Mirabegron: a Beta-3 agonist for overactive bladder. Consult Pharm. 2014 Dec;29(12):823-37. doi: 10.4140/TCP.n.2014.823. PMID: 25521658; PMCID: PMC4605389. |
Wrong publication type (review) |
|
Bridgeman MB, Friia NJ, Taft C, Shah M. Mirabegron: β3-adrenergic receptor agonist for the treatment of overactive bladder. Ann Pharmacother. 2013 Jul-Aug;47(7-8):1029-38. doi: 10.1345/aph.1S054. Epub 2013 Jun 11. PMID: 23757386. |
Wrong publication type (review) |
|
Caremel R, Loutochin O, Corcos J. What do we know and not know about mirabegron, a novel β3 agonist, in the treatment of overactive bladder? Int Urogynecol J. 2014 Feb;25(2):165-70. doi: 10.1007/s00192-013-2161-4. Epub 2013 Aug 7. PMID: 23922008. |
Wrong publication type (review) |
|
Chen HL, Chen TC, Chang HM, Juan YS, Huang WH, Pan HF, Chang YC, Wu CM, Wang YL, Lee HY. Mirabegron is alternative to antimuscarinic agents for overactive bladder without higher risk in hypertension: a systematic review and meta-analysis. World J Urol. 2018 Aug;36(8):1285-1297. doi: 10.1007/s00345-018-2268-9. Epub 2018 Mar 19. PMID: 29556972. |
Less recent and complete compared to Mostafaei (2022) |
|
Cui Y, Zong H, Yang C, Yan H, Zhang Y. The efficacy and safety of mirabegron in treating OAB: a systematic review and meta-analysis of phase III trials. Int Urol Nephrol. 2014 Jan;46(1):275-84. doi: 10.1007/s11255-013-0509-9. Epub 2013 Jul 30. PMID: 23896942. |
Less recent and complete compared to Mostafaei (2022) |
|
De Nunzio C, Brucker B, Bschleipfer T, Cornu JN, Drake MJ, Fusco F, Gravas S, Oelke M, Peyronnet B, Tutolo M, van Koeveringe G, Madersbacher S. Beyond Antimuscarinics: A Review of Pharmacological and Interventional Options for Overactive Bladder Management in Men. Eur Urol. 2021 Apr;79(4):492-504. doi: 10.1016/j.eururo.2020.12.032. Epub 2021 Jan 2. PMID: 33402296. |
Less recent and complete compared to Mostafaei (2022) |
|
De Nunzio C, Presicce F, Pirozzi L, Castellan P, Schips L, Cindolo L, Lombardo R, Tubaro A. The Current Indications and the Benefits of Combining a β3-Agonist with an Anticholinergic for the Treatment of OAB. Curr Drug Targets. 2015;16(11):1198-206. doi: 10.2174/1389450116666150806124345. PMID: 26245475. |
Wrong publication type (review) |
|
Duong V, Iwamoto A, Pennycuff J, Kudish B, Iglesia C. A systematic review of neurocognitive dysfunction with overactive bladder medications. Int Urogynecol J. 2021 Oct;32(10):2693-2702. doi: 10.1007/s00192-021-04909-5. Epub 2021 Jul 2. PMID: 34213600. |
Wrong outcome (cognitive dysfunction) |
|
Fest J, Pfalzgraf D, Weiss C, Hetjens S. Evaluating the efficacy and tolerability of mirabegron, a β3-adrenoceptor agonist, for the treatment of overactive bladder: Systematic review and network meta-analysis. Journal of Clinical Urology. 2017;10(6):557-567. doi:10.1177/2051415817706045 |
Less recent and complete compared to Mostafaei (2022) |
|
Gratzke C, Chapple C, Mueller ER, Robinson D, Rolland C, Staskin D, Stoelzel M, Maanen RV, Siddiqui E. Efficacy and Safety of Combination Pharmacotherapy for Patients with Overactive Bladder: A Rapid Evidence Assessment. Eur Urol. 2019 Dec;76(6):767-779. doi: 10.1016/j.eururo.2019.07.010. Epub 2019 Aug 13. PMID: 31416636. |
Wrong publication type (rapid evidence assessment) |
|
Kuo, H. C., Lin, H. H., Yu, H. J., Cheng, C. L., Hung, M. J. and Lin, A. T. L. Results of a randomized, double-blind, placebo-controlled study of mirabegron in a Taiwanese population with overactive bladder and comparison with other clinical trials. Urol. Sc. 2015, 26(1):41-48. doi: 10.1016/j.urols.2014.12.010 |
Corrigendum of this study is already included (Kuo, 2015) |
|
Hou J, Xu F, Du H, Li N. Adverse events associated with mirabegron 50mg versus placebo: A systematic review and meta-analysis. Prog Urol. 2021 Sep;31(11):627-633. doi: 10.1016/j.purol.2021.05.005. Epub 2021 Jul 24. PMID: 34312078. |
Less recent and complete compared to Mostafaei (2022) |
|
Hsu FC, Weeks CE, Selph SS, Blazina I, Holmes RS, McDonagh MS. Updating the evidence on drugs to treat overactive bladder: a systematic review. Int Urogynecol J. 2019 Oct;30(10):1603-1617. doi: 10.1007/s00192-019-04022-8. Epub 2019 Jul 25. PMID: 31346670; PMCID: PMC6795617. |
Less recent and complete compared to Mostafaei (2022) |
|
Iino S, Kaneko M, Narukawa M. Factors influencing efficacy endpoints in clinical trials for new oral medicinal treatments for overactive bladder: a systematic literature review and meta-analysis. Int Urol Nephrol. 2018 Jun;50(6):1021-1030. doi: 10.1007/s11255-018-1869-y. Epub 2018 Apr 12. PMID: 29651695. |
Wrong outcome (factors influencing endpoints) |
|
Jayarajan J, Radomski SB. Pharmacotherapy of overactive bladder in adults: a review of efficacy, tolerability, and quality of life. Res Rep Urol. 2013 Dec 6;6:1-16. doi: 10.2147/RRU.S40034. PMID: 24400248; PMCID: PMC3862648. |
Wrong publication type (review) |
|
Jian Z, Yuan C, Li H, Zhang W, Wang K. Vibegron 50 mg is the optimal algorithm in the pharmacologic management of overactive bladder: outcomes from a systematic review and meta-analysis. Int Urol Nephrol. 2020 Dec;52(12):2215-2221. doi: 10.1007/s11255-020-02536-5. Epub 2020 Jun 9. PMID: 32519241. |
Wrong intervention (vibegron 50 mg) |
|
Kelleher C, Hakimi Z, Zur R, Siddiqui E, Maman K, Aballéa S, Nazir J, Chapple C. Efficacy and Tolerability of Mirabegron Compared with Antimuscarinic Monotherapy or Combination Therapies for Overactive Bladder: A Systematic Review and Network Meta-analysis. Eur Urol. 2018 Sep;74(3):324-333. doi: 10.1016/j.eururo.2018.03.020. Epub 2018 Apr 23. PMID: 29699858. |
Included studies not reported |
|
Kennelly MJ, Rhodes T, Girman CJ, Thomas E, Shortino D, Mudd PN Jr. Efficacy of Vibegron and Mirabegron for Overactive Bladder: A Systematic Literature Review and Indirect Treatment Comparison. Adv Ther. 2021 Nov;38(11):5452-5464. doi: 10.1007/s12325-021-01902-8. Epub 2021 Sep 18. PMID: 34537953; PMCID: PMC8520873. |
Less recent and complete compared to Mostafaei (2022) |
|
Kistler KD, Xu Y, Zou KH, Ntanios F, Chapman DS, Luo X. Systematic literature review of clinical trials evaluating pharmacotherapy for overactive bladder in elderly patients: An assessment of trial quality. Neurourol Urodyn. 2018 Jan;37(1):54-66. doi: 10.1002/nau.23309. Epub 2017 Aug 1. PMID: 28763112. |
Wrong population (elderly) |
|
Lozano-Ortega G, Walker DR, Johnston K, Mickle A, Harrigan S, Rogula B, Kristy RM, Hairston JC, Schermer CR. Comparative Safety and Efficacy of Treatments for Overactive Bladder Among Older Adults: A Network Meta-analysis. Drugs Aging. 2020 Nov;37(11):801-816. doi: 10.1007/s40266-020-00792-9. PMID: 32960422; PMCID: PMC7595992. |
Wrong population (elderly) |
|
Maman K, Aballea S, Nazir J, Desroziers K, Neine ME, Siddiqui E, Odeyemi I, Hakimi Z. Comparative efficacy and safety of medical treatments for the management of overactive bladder: a systematic literature review and mixed treatment comparison. Eur Urol. 2014 Apr;65(4):755-65. doi: 10.1016/j.eururo.2013.11.010. Epub 2013 Nov 18. PMID: 24275310. |
Less recent and complete compared to Mostafaei (2022) |
|
Obloza A, Kirby J, Yates D, Toozs-Hobson P. Indirect treatment comparison (ITC) of medical therapies for an overactive bladder. Neurourol Urodyn. 2017 Sep;36(7):1824-1831. doi: 10.1002/nau.23189. Epub 2017 Feb 21. PMID: 28220521. |
Wrong publication type (comparative study) |
|
Olivera CK, Meriwether K, El-Nashar S, Grimes CL, Chen CC, Orejuela F, Antosh D, Gleason J, Kim-Fine S, Wheeler T, McFadden B, Balk EM, Murphy M; Systematic Review Group for the Society of Gynecological Surgeons. Nonantimuscarinic treatment for overactive bladder: a systematic review. Am J Obstet Gynecol. 2016 Jul;215(1):34-57. doi: 10.1016/j.ajog.2016.01.156. Epub 2016 Feb 4. PMID: 26851599. |
Included only 3 relevant RCTs (mirabegron) |
|
Mitcheson HD, Samanta S, Muldowney K, Pinto CA, Rocha BA, Green S, Bennett N, Mudd PN Jr, Frenkl TL. Vibegron (RVT-901/MK-4618/KRP-114V) Administered Once Daily as Monotherapy or Concomitantly with Tolterodine in Patients with an Overactive Bladder: A Multicenter, Phase IIb, Randomized, Double-blind, Controlled Trial. Eur Urol. 2019 Feb;75(2):274-282. doi: 10.1016/j.eururo.2018.10.006. Epub 2018 Oct 25. PMID: 30661513. |
Wrong intervention (vibegron 3, 15, 50 and 100 mg) |
|
Mueller ER, van Maanen R, Chapple C, Abrams P, Herschorn S, Robinson D, Stoelzel M, Yoon SJ, Al-Shukri S, Rechberger T, Gratzke C. Long-term treatment of older patients with overactive bladder using a combination of mirabegron and solifenacin: a prespecified analysis from the randomized, phase III SYNERGY II study. Neurourol Urodyn. 2019 Feb;38(2):779-792. doi: 10.1002/nau.23919. Epub 2019 Jan 15. PMID: 30644570; PMCID: PMC6850571. |
Wrong population (elderly) |
|
Ohlstein EH, von Keitz A, Michel MC. A multicenter, double-blind, randomized, placebo-controlled trial of the β3-adrenoceptor agonist solabegron for overactive bladder. Eur Urol. 2012 Nov;62(5):834-40. doi: 10.1016/j.eururo.2012.05.053. Epub 2012 Jun 5. PMID: 22695239. |
Wrong intervention (solabegron) |
|
Otsuka A, Kageyama S, Suzuki T, Matsumoto R, Nagae H, Kitagawa M, Furuse H, Ozono S. Comparison of mirabegron and imidafenacin for efficacy and safety in Japanese female patients with overactive bladder: A randomized controlled trial (COMFORT study). Int J Urol. 2016 Dec;23(12):1016-1023. doi: 10.1111/iju.13231. Epub 2016 Sep 29. PMID: 27686226. |
Published prior to February 2021 (search period Mostafaei, 2022) |
|
Özkidik M, Coşkun A, Asutay MK, Bahçeci T, Hamidi N. Efficacy and tolerability of mirabegron in female patients with overactive bladder symptoms after surgical treatment for stress urinary incontinence. Int Braz J Urol. 2019 Jul-Aug;45(4):782-789. doi: 10.1590/S1677-5538.IBJU.2018.0518. PMID: 31136113; PMCID: PMC6837616. |
Published prior to February 2021 (search period Mostafaei, 2022) |
|
Robinson D, Kelleher C, Staskin D, Mueller ER, Falconer C, Wang J, Ridder A, Stoelzel M, Paireddy A, van Maanen R, Hakimi Z, Herschorn S. Patient-reported outcomes from SYNERGY, a randomized, double-blind, multicenter study evaluating combinations of mirabegron and solifenacin compared with monotherapy and placebo in OAB patients. Neurourol Urodyn. 2018 Jan;37(1):394-406. doi: 10.1002/nau.23315. Epub 2017 Jul 13. PMID: 28704584. |
Wrong outcome (patient-reported outcomes) |
|
Bragg R, Hebel D, Vouri SM, Pitlick JM. Mirabegron: a Beta-3 agonist for overactive bladder. Consult Pharm. 2014 Dec;29(12):823-37. doi: 10.4140/TCP.n.2014.823. PMID: 25521658; PMCID: PMC4605389. |
Wrong publication type (review) |
|
Rossanese M, Novara G, Challacombe B, Iannetti A, Dasgupta P, Ficarra V. Critical analysis of phase II and III randomised control trials (RCTs) evaluating efficacy and tolerability of a β₃-adrenoceptor agonist (Mirabegron) for overactive bladder (OAB). BJU Int. 2015 Jan;115(1):32-40. doi: 10.1111/bju.12730. Epub 2014 Jul 27. PMID: 24602031. |
Wrong publication type (review) |
|
Sebastianelli A, Russo GI, Kaplan SA, McVary KT, Moncada I, Gravas S, Chapple C, Morgia G, Serni S, Gacci M. Systematic review and meta-analysis on the efficacy and tolerability of mirabegron for the treatment of storage lower urinary tract symptoms/overactive bladder: Comparison with placebo and tolterodine. Int J Urol. 2018 Mar;25(3):196-205. doi: 10.1111/iju.13498. Epub 2017 Dec 3. PMID: 29205506. |
Less recent and complete compared to Mostafaei (2022) |
|
Shi H, Chen H, Zhang Y, Cui Y. The efficacy and safety of Vibegron in treating overactive bladder: A systematic review and pooled analysis of randomized controlled trials. Neurourol Urodyn. 2020 Jun;39(5):1255-1263. doi: 10.1002/nau.24387. Epub 2020 May 18. PMID: 32421908. |
Less recent and complete compared to Mostafaei (2022) |
|
Su S, Liang L, Lin J, Liu L, Chen Z, Gao Y. Systematic review and meta-analysis of the efficacy and safety of vibegron vs antimuscarinic monotherapy for overactive bladder. Medicine (Baltimore). 2021 Feb 5;100(5):e23171. doi: 10.1097/MD.0000000000023171. PMID: 33592817; PMCID: PMC7870164. |
Wrong intervention (wrong doses of vibegron) |
|
Wang J, Zhou Z, Cui Y, Li Y, Yuan H, Gao Z, Zhu Z, Wu J. Meta-analysis of the efficacy and safety of mirabegron and solifenacin monotherapy for overactive bladder. Neurourol Urodyn. 2019 Jan;38(1):22-30. doi: 10.1002/nau.23863. Epub 2018 Oct 23. PMID: 30350884. |
Less recent and complete compared to Mostafaei (2022) |
|
Wu T, Duan X, Cao CX, Peng CD, Bu SY, Wang KJ. The role of mirabegron in overactive bladder: a systematic review and meta-analysis. Urol Int. 2014;93(3):326-37. doi: 10.1159/000361079. Epub 2014 Aug 7. PMID: 25115445. |
Less recent and complete compared to Mostafaei (2022) |
|
Yi W, Yang Y, Yang J. Monotherapy with mirabegron had a better tolerance than the anticholinergic agents on overactive bladder: A systematic review and meta-analysis. Medicine (Baltimore). 2021 Oct 15;100(41):e27469. doi: 10.1097/MD.0000000000027469. PMID: 34731124; PMCID: PMC8519252. |
Less recent and complete compared to Mostafaei (2022) |
|
Staskin D, Frankel J, Varano S, Shortino D, Jankowich R, Mudd PN Jr. International Phase III, Randomized, Double-Blind, Placebo and Active Controlled Study to Evaluate the Safety and Efficacy of Vibegron in Patients with Symptoms of Overactive Bladder: EMPOWUR. J Urol. 2020 Aug;204(2):316-324. doi: 10.1097/JU.0000000000000807. Epub 2020 Feb 18. PMID: 32068484. |
Extension study included (Staskin, 2021) |
|
Staskin D, Herschorn S, Fialkov J, Tu LM, Walsh T, Schermer CR. A prospective, double-blind, randomized, two-period crossover, multicenter study to evaluate tolerability and patient preference between mirabegron and tolterodine in patients with overactive bladder (PREFER study). Int Urogynecol J. 2018 Feb;29(2):273-283. doi: 10.1007/s00192-017-3377-5. Epub 2017 Jun 15. PMID: 28620791; PMCID: PMC5780540. |
Published prior to February 2021 (search period Mostafaei, 2022) |
|
Torimoto K, Matsushita C, Yamada A, Goto D, Matsumoto Y, Hosokawa Y, Miyake M, Aoki K, Hirayama A, Tanaka N, Fujimoto K. Clinical efficacy and safety of mirabegron and imidafenacin in women with overactive bladder: A randomized crossover study (the MICRO study). Neurourol Urodyn. 2017 Apr;36(4):1097-1103. doi: 10.1002/nau.23050. Epub 2016 Jun 6. PMID: 27265880. |
Published prior to February 2021 (search period Mostafaei, 2022) |
|
Varano S, Staskin D, Frankel J, Shortino D, Jankowich R, Mudd PN Jr. Efficacy and Safety of Once-Daily Vibegron for Treatment of Overactive Bladder in Patients Aged ≥65 and ≥75 Years: Subpopulation Analysis from the EMPOWUR Randomized, International, Phase III Study. Drugs Aging. 2021 Feb;38(2):137-146. doi: 10.1007/s40266-020-00829-z. Epub 2021 Jan 20. PMID: 33469832; PMCID: PMC7882560. |
Wrong population (elderly) |
|
Vasudeva P, Kumar A, Yadav S, Kumar N, Chaudhry N, Prasad V, Nagendra Rao S, Yadav P, Patel S. Neurological safety and efficacy of darifenacin and mirabegron for the treatment of overactive bladder in patients with history of cerebrovascular accident: A prospective study. Neurourol Urodyn. 2021 Nov;40(8):2041-2047. doi: 10.1002/nau.24793. Epub 2021 Sep 13. PMID: 34516666. |
Wrong intervention (mirabegron 25 mg) |
|
Vecchioli Scaldazza C, Morosetti C. Comparison of Therapeutic Efficacy and Urodynamic Findings of Solifenacin Succinate versus Mirabegron in Women with Overactive Bladder Syndrome: Results of a Randomized Controlled Study. Urol Int. 2016;97(3):325-329. doi: 10.1159/000445808. Epub 2016 Apr 20. PMID: 27092789. |
Published prior to February 2021 (search period Mostafaei, 2022) |
|
Wagg A, Staskin D, Engel E, Herschorn S, Kristy RM, Schermer CR. Efficacy, safety, and tolerability of mirabegron in patients aged ≥65yr with overactive bladder wet: a phase IV, double-blind, randomised, placebo-controlled study (PILLAR). Eur Urol. 2020 Feb;77(2):211-220. doi: 10.1016/j.eururo.2019.10.002. Epub 2019 Nov 13. PMID: 31733990. |
Wrong population (elderly) |
|
Weber MA, Chapple CR, Gratzke C, Herschorn S, Robinson D, Frankel JM, Ridder AM, Stoelzel M, Paireddy A, van Maanen R, White WB. A strategy utilizing ambulatory monitoring and home and clinic blood pressure measurements to optimize the safety evaluation of noncardiovascular drugs with potential for hemodynamic effects: a report from the SYNERGY trial. Blood Press Monit. 2018 Jun;23(3):153-163. doi: 10.1097/MBP.0000000000000320. PMID: 29578880; PMCID: PMC5959217. |
Wrong outcome (evaluating blood pressure measurements) |
|
Weber MA, Haag-Molkenteller C, King J, Walker A, Mudd PN Jr, White WB. Effects of vibegron on ambulatory blood pressure in patients with overactive bladder: results from a double-blind, placebo-controlled trial. Blood Press Monit. 2022 Apr 1;27(2):128-134. doi: 10.1097/MBP.0000000000000572. PMID: 34699409; PMCID: PMC8893125. |
Wrong population (elderly) |
|
White, W.B., Chapple, C., Gratzke, C., Herschorn, S., Robinson, D., Frankel, J., Ridder, A., Stoelzel, M., Paireddy, A., van Maanen, R. and Weber, M.A. (2018), Cardiovascular Safety of the β3-Adrenoceptor Agonist Mirabegron and the Antimuscarinic Agent Solifenacin in the SYNERGY Trial. The Journal of Clinical Pharmacology, 58: 1084-1091. https://doi.org/10.1002/jcph.1107 |
Published prior to February 2021 (search period Mostafaei, 2022) |
|
Yamaguchi O, Kakizaki H, Homma Y, Igawa Y, Takeda M, Nishizawa O, Gotoh M, Yoshida M, Yokoyama O, Seki N, Okitsu A, Hamada T, Kobayashi A, Kuroishi K. Long-term safety and efficacy of antimuscarinic add-on therapy in patients with overactive bladder who had a suboptimal response to mirabegron monotherapy: A multicenter, randomized study in Japan (MILAI II study). Int J Urol. 2019 Mar;26(3):342-352. doi: 10.1111/iju.13868. Epub 2018 Dec 13. PMID: 30548692; PMCID: PMC7379522. |
Published prior to February 2021 (search period Mostafaei, 2022) |
|
Yoshida M, Takeda M, Gotoh M, Nagai S, Kurose T. Vibegron, a Novel Potent and Selective β3-Adrenoreceptor Agonist, for the Treatment of Patients with Overactive Bladder: A Randomized, Double-blind, Placebo-controlled Phase 3 Study. Eur Urol. 2018 May;73(5):783-790. doi: 10.1016/j.eururo.2017.12.022. Epub 2018 Feb 1. PMID: 29366513. |
Wrong intervention (vibegron 50, 100 mg) |
|
Yoshida M, Takeda M, Gotoh M, Yokoyama O, Kakizaki H, Takahashi S, Masumori N, Nagai S, Hashimoto K, Minemura K. Efficacy of novel β3 -adrenoreceptor agonist vibegron on nocturia in patients with overactive bladder: A post-hoc analysis of a randomized, double-blind, placebo-controlled phase 3 study. Int J Urol. 2019 Mar;26(3):369-375. doi: 10.1111/iju.13877. Epub 2018 Dec 17. PMID: 30557916; PMCID: PMC6912249. |
Wrong intervention (vibegron 50, 100 mg) |
|
Yoshida M, Takeda M, Gotoh M, Yokoyama O, Kakizaki H, Takahashi S, Masumori N, Nagai S, Minemura K. Efficacy of vibegron, a novel β3-adrenoreceptor agonist, on severe urgency urinary incontinence related to overactive bladder: post hoc analysis of a randomized, placebo-controlled, double-blind, comparative phase 3 study. BJU Int. 2020 May;125(5):709-717. doi: 10.1111/bju.15020. Epub 2020 Feb 23. PMID: 31991511; PMCID: PMC7318146. |
Wrong intervention (vibegron 50, 100 mg) |
|
Yoshida M, Takeda M, Gotoh M, Yokoyama O, Kakizaki H, Takahashi S, Masumori N, Nagai S, Minemura K. Cardiovascular safety of vibegron, a new β3-adrenoceptor agonist, in older patients with overactive bladder: Post-hoc analysis of a randomized, placebo-controlled, double-blind comparative phase 3 study. Neurourol Urodyn. 2021 Aug;40(6):1651-1660. doi: 10.1002/nau.24732. Epub 2021 Jun 17. PMID: 34139038; PMCID: PMC8362047. |
Wrong intervention (vibegron 50, 100 mg |
|
Zubiaur Líbano C, Poza-Barrasús JL, Valero Fernández EM. Quality of life and treatment persistence evaluation in Spanish patients treated with mirabegron. Results of the BELIEVE study. Actas Urol Esp (Engl Ed). 2020 May;44(4):224-232. English, Spanish. doi: 10.1016/j.acuro.2020.01.003. Epub 2020 Mar 4. PMID: 32145942. |
Wrong outcome (QoL and treatment persistence) |
Verantwoording
Beoordelingsdatum en geldigheid
Laatst beoordeeld : 11-04-2024
Algemene gegevens
De ontwikkeling/herziening van deze richtlijnmodule werd ondersteund door het Kennisinstituut van de Federatie Medisch Specialisten (www.demedischspecialist.nl/kennisinstituut) en werd gefinancierd uit de Kwaliteitsgelden Medisch Specialisten (SKMS). De financier heeft geen enkele invloed gehad op de inhoud van de richtlijnmodule.
Samenstelling werkgroep
Voor het ontwikkelen van de richtlijnmodule is in 2021 een multidisciplinaire werkgroep ingesteld, bestaande uit vertegenwoordigers van alle relevante specialismen (zie hiervoor de Samenstelling van de werkgroep) die betrokken zijn bij de zorg voor patiënten met urine-incontinentie in de 2e- en 3e-lijnszorg.
Werkgroep
- Dhr. dr. M.R. (Michael) van Balken, uroloog, NVU (voorzitter)
- Dhr. dr. L.P.W. (Bart) Witte, uroloog, NVU
- Dhr. drs. M.A.C. (Martijn) Smits, uroloog, NVU
- Mevr. dr. M.K. (Marian) Engberts, urogynaecoloog, NVOG
- Mevr. dr. P. (Pieternel) Steures, urogynaecoloog, NVOG
- Mevr. drs. A.C. (Rianne) van der Meer, klinisch geriater, NVKG
- Mevr. C.W.L. (Tine) van den Bos, Bekkenbodem4All
Meelezers:
- Mevr. dr. T.A.M. (Doreth) Teunissen, huisarts, NHG
- Dhr. H.J. (Henk Jan) Mulder, urologie verpleegkundige, V&VN
- Mevr. drs. A. (Ana) Dos Santos, bekkenfysiotherapeut, NVFB
- Mevr. drs. C. (Corine) Adamse, bekkenfysiotherapeut, NVFB
- Dhr. dr. R.S. (Ramon) Dekker, internist, NIV
Met ondersteuning van:
- Mevr. dr. I.M. (Irina) Mostovaya, senior adviseur, Kennisinstituut van de Federatie Medisch Specialisten, Utrecht
- Mevr. drs. B. (Beatrix) Vogelaar, adviseur, Kennisinstituut van de Federatie Medisch Specialisten, Utrecht
- Mevr. drs. D.A.M. (Danique) Middelhuis, junior adviseur, Kennisinstituut van de Federatie Medisch Specialisten, Utrecht
- Mevr. drs. L. (Laura) van Wijngaarden, junior adviseur, Kennisinstituut van de Federatie Medisch Specialisten, Utrecht
Belangenverklaringen
De Code ter voorkoming van oneigenlijke beïnvloeding door belangenverstrengeling is gevolgd. Alle werkgroepleden hebben schriftelijk verklaard of zij in de laatste drie jaar directe financiële belangen (betrekking bij een commercieel bedrijf, persoonlijke financiële belangen, onderzoeksfinanciering) of indirecte belangen (persoonlijke relaties, reputatiemanagement) hebben gehad. Gedurende de ontwikkeling of herziening van een module worden wijzigingen in belangen aan de voorzitter doorgegeven. De belangenverklaring wordt opnieuw bevestigd tijdens de commentaarfase.
Een overzicht van de belangen van werkgroepleden en het oordeel over het omgaan met eventuele belangen vindt u in onderstaande tabel. De ondertekende belangenverklaringen zijn op te vragen bij het secretariaat van het Kennisinstituut van de Federatie Medisch Specialisten.
|
Werkgroep |
||||
|
Achternaam werkgroeplid |
Hoofdfunctie |
Nevenwerkzaamheden |
Gemelde belangen |
Actie |
|
Van Balken (voorzitter) |
Uroloog, Rijnstate Ziekenhuis, Arnhem |
Bestuurslid NVU (vacatie)
Presentatie gehouden op de Post ICS IUGA congres in 2021 waarvoor vergoeding van Astellas |
Transparantieregister 2018:
Deelname als onderzoeker aan Renova-trial (OASIS). Bedrijf: Bluewind. Alleen tegemoetkoming onkosten te maken voor ziekenhuiskosten e.d.
|
geen dienstverlening bedrijven tijdens Richtlijn- ontwikkeling
Extra kritisch commentaar gevraagd van onafhankelijke reviewers tijdens de commentaarfase
|
|
Engberts |
Urogynaecoloog bij Isala |
Trainer Altis Sling Coloplast -> tegen vergoeding
Presentatie gehouden op de Post ICS IUGA congres in 2021 waarvoor vergoeding van Astellas |
Trainer bij Coloplast SIMS Altis tegen SUI om die reden bij herziening richtlijn UI NVOG met name medicatie bij OAB in combinatie/PTNS beoordeeld
Ik doe ook onderzoek naar Bulkamid & Altis sling maar hier is tot op heden nog geen vergoeding voor.
|
Extra kritisch commentaar gevraagd van onafhankelijke reviewers tijdens de commentaarfase |
|
Witte |
Uroloog, Isala Klinieken, Zwolle, Nederland |
Secretaris Werkgroep Functionele en Reconstructieve Urologie van de Nederlandse Vereniging van Urologie, onbetaald.
|
In 2018 heb ik een vergoeding gekregen als spreker in opdracht van Pierre Fabre.
In ons ziekenhuis lopen verschillende studies die extern gefinancierd worden. Van de OASIS studie is de inclusie net gesloten. Het gaat om een implanteerbaar device voor de behandeling van aandrangsincontinentie. Wij hebben 4 patiënten geïncludeerd. Follow up is 12 maanden. |
Geen actie |
|
Van den Bos |
Bekkenfysiotherapeut zzp bij paramedisch centrum AdFysio te De Lier 0.5 fte |
Voorzitter Bekkenbodem4All - vrijwilligers vergoeding
|
Geen |
Geen actie |
|
Van der Meer |
Klinische geriater, Groene Hart Ziekenhuis Gouda, 0,7 fte
|
Geen |
Geen |
Geen actie |
|
Smits |
Uroloog, Maastricht UMC+ |
Lid Werkgroep Functionele en Reconstructieve urologie NVU (-)
Presentatie gehouden op de Post ICS IUGA congres in 2021 waarvoor vergoeding van Astellas |
Diensteverlening honorarium Medtronic 2020 665euro = Deelname advisory board Medtronic
Deelname als onderzoeker aan:
Enkel support financiële support voor onkosten ziekenhuis
geen dienstverlening bedrijven tijdens Richtlijn- ontwikkeling |
Extra kritisch commentaar gevraagd van onafhankelijke reviewers tijdens de commentaarfase |
|
Steures |
Urogynaecoloog, Jeroen Bosch Ziekenhuis, ’s Hertogenbosch |
Lid van landerlijke werkgroep bekkenbodem
Lid commissie Actualisatie en revisie gynaecologische richtlijnmodules. Modules: urine-incontinentie |
Geen |
Geen actie |
|
Klankbordgroep |
||||
|
Achternaam klankbordgroeplid |
Hoofdfunctie |
Nevenwerkzaamheden |
Gemelde belangen |
Actie |
|
Teunissen |
Huisarts, zelfstandig 0,6 fte
|
Geen |
Geen |
Geen |
|
Adamse |
Bekkenfysiotherapeut en Klinisch Epidemioloog, Antonius Ziekenhuis Sneek
|
Commissielid Wetenschapscommissie NVFB
|
Mogelijk positie bekkenfysiotherapie
|
Geen |
|
Dekker |
Internist ouderengeneeskunde bij Ziekenhuis Rivierenland
|
2021 – heden Commissielid NIV kerngroep Ouderengeneeskunde – Kwaliteit & Richtlijnen - onbetaald
|
Geen |
Geen |
|
Dos Santos |
Geregistreerd bekkenfysiotherapeut MSc bij Pelvicentrum centrum voor bekkenfysiotherapie Leiden
|
Lid van NVFB wetenschappelijke commissie vergoeding voor reiskosten en bijwonen vergaderingen
|
Deelname aan het ontwikkelen kan ervoor zorgen dat collega gaan verwijzen naar mijn praktijk vanwege meer bekendheid.
|
Geen |
|
Mulder |
Verpleegkundig specialist Martini Ziekenhuis Groningen
|
Geen |
Geen |
Geen |
Inbreng patiëntenperspectief
Er werd aandacht besteed aan het patiëntenperspectief door afvaardiging van Stichting Bekkenbodem4All in de werkgroep. De verkregen input is meegenomen bij het opstellen van de uitgangsvragen, de keuze voor de uitkomstmaten en bij het opstellen van de overwegingen (zie per module ook “Waarden en voorkeuren van patiënten”). De conceptrichtlijn is tevens voor commentaar voorgelegd aan Stichting Bekkenbodem4All en de eventueel aangeleverde commentaren zijn bekeken en verwerkt.
Kwalitatieve raming van mogelijke financiële gevolgen in het kader van de Wkkgz
Bij de richtlijn is conform de Wet kwaliteit, klachten en geschillen zorg (Wkkgz) een kwalitatieve raming uitgevoerd of de aanbevelingen mogelijk leiden tot substantiële financiële gevolgen. Bij het uitvoeren van deze beoordeling zijn richtlijnmodules op verschillende domeinen getoetst (zie het stroomschema op de Richtlijnendatabase).
Uit de kwalitatieve raming blijkt dat er waarschijnlijk geen substantiële financiële gevolgen zijn, zie onderstaande tabel.
Module |
Uitkomst raming |
Toelichting |
|
Module beta 3 receptor agonist |
Geen financiële gevolgen |
Hoewel uit de toetsing volgt dat de aanbeveling(en) breed toepasbaar zijn (>40.000 patiënten), volgt uit de toetsing dat [het overgrote deel (±90%) van de zorgaanbieders en zorgverleners al aan de norm voldoet OF het geen nieuwe manier van zorgverlening of andere organisatie van zorgverlening betreft, het geen toename in het aantal in te zetten voltijdsequivalenten aan zorgverleners betreft en het geen wijziging in het opleidingsniveau van zorgpersoneel betreft]. Er worden daarom geen financiële gevolgen verwacht. |
Werkwijze
AGREE
Deze richtlijnmodule is opgesteld conform de eisen vermeld in het rapport Medisch Specialistische Richtlijnen 2.0 van de adviescommissie Richtlijnen van de Raad Kwaliteit. Dit rapport is gebaseerd op het AGREE II instrument (Appraisal of Guidelines for Research & Evaluation II; Brouwers, 2010).
Knelpuntenanalyse en uitgangsvragen
Tijdens de voorbereidende fase inventariseerde de werkgroep de knelpunten in de zorg voor patiënten met urine-incontinentie in de 2e en 3e lijnszorg. De werkgroep beoordeelde de aanbeveling(en) uit de eerdere richtlijnmodule (NVU, 2014) op noodzaak tot revisie. Tevens zijn er knelpunten aangedragen door de Nederlandse Vereniging voor Klinische Geriatrie (NVKG), Nederlandse Vereniging voor Obstetrie & Gynaecologie (NVOG), Nederlandse Vereniging voor Urologie (NVU), Bekkenbodem4All (B4A), Inspectie voor de Gezondheidszorg en Jeugd (IGJ), Nederlands Huisartsen Genootschap (NHG), Nederlandse Vereniging voor Bekkenfysiotherapie (NVFB (in afstemming met de Koninklijk Nederlands Genootschap voor Fysiotherapie (KNGF)), Nederlandse Vereniging van Ziekenhuizen (NVZ), Patiëntenfederatie, Verpleegkundigen & Verzorgenden Nederland (V&VN), Zorginstituut Nederland (ZiNL), Zelfstandige Klinieken Nederland (ZKN) en Zorgverzekeraars Nederland (ZN) via een schriftelijke knelpunteninventarisatie.
Uitkomstmaten
Na het opstellen van de zoekvraag behorende bij de uitgangsvraag inventariseerde de werkgroep welke uitkomstmaten voor de patiënt relevant zijn, waarbij zowel naar gewenste als ongewenste effecten werd gekeken. Hierbij werd een maximum van acht uitkomstmaten gehanteerd. De werkgroep waardeerde deze uitkomstmaten volgens hun relatieve belang bij de besluitvorming rondom aanbevelingen, als cruciaal (kritiek voor de besluitvorming), belangrijk (maar niet cruciaal) en onbelangrijk. Tevens definieerde de werkgroep tenminste voor de cruciale uitkomstmaten welke verschillen zij klinisch (patiënt) relevant vonden.
Methode literatuursamenvatting
Een uitgebreide beschrijving van de strategie voor zoeken en selecteren van literatuur is te vinden onder ‘Zoeken en selecteren’ onder Onderbouwing. Indien mogelijk werd de data uit verschillende studies gepoold in een random-effects model. Review Manager 5.4 werd gebruikt voor de statistische analyses. De beoordeling van de kracht van het wetenschappelijke bewijs wordt hieronder toegelicht.
Beoordelen van de kracht van het wetenschappelijke bewijs
De kracht van het wetenschappelijke bewijs werd bepaald volgens de GRADE-methode. GRADE staat voor ‘Grading Recommendations Assessment, Development and Evaluation’ (zie http://www.gradeworkinggroup.org). De basisprincipes van de GRADE-methodiek zijn: het benoemen en prioriteren van de klinisch (patiënt) relevante uitkomstmaten, een systematische review per uitkomstmaat, en een beoordeling van de bewijskracht per uitkomstmaat op basis van de acht GRADE-domeinen (domeinen voor downgraden: risk of bias, inconsistentie, indirectheid, imprecisie, en publicatiebias; domeinen voor upgraden: dosis-effect relatie, groot effect, en residuele plausibele confounding).
GRADE onderscheidt vier gradaties voor de kwaliteit van het wetenschappelijk bewijs: hoog, redelijk, laag en zeer laag. Deze gradaties verwijzen naar de mate van zekerheid die er bestaat over de literatuurconclusie, in het bijzonder de mate van zekerheid dat de literatuurconclusie de aanbeveling adequaat ondersteunt (Schünemann, 2013; Hultcrantz, 2017).
|
GRADE |
Definitie |
|
Hoog |
|
|
Redelijk |
|
|
Laag |
|
|
Zeer laag |
|
Bij het beoordelen (graderen) van de kracht van het wetenschappelijk bewijs in richtlijnen volgens de GRADE-methodiek spelen grenzen voor klinische besluitvorming een belangrijke rol (Hultcrantz, 2017). Dit zijn de grenzen die bij overschrijding aanleiding zouden geven tot een aanpassing van de aanbeveling. Om de grenzen voor klinische besluitvorming te bepalen moeten alle relevante uitkomstmaten en overwegingen worden meegewogen. De grenzen voor klinische besluitvorming zijn daarmee niet één op één vergelijkbaar met het minimaal klinisch relevant verschil (Minimal Clinically Important Difference, MCID). Met name in situaties waarin een interventie geen belangrijke nadelen heeft en de kosten relatief laag zijn, kan de grens voor klinische besluitvorming met betrekking tot de effectiviteit van de interventie bij een lagere waarde (dichter bij het nuleffect) liggen dan de MCID (Hultcrantz, 2017).
Overwegingen (van bewijs naar aanbeveling)
Om te komen tot een aanbeveling zijn naast (de kwaliteit van) het wetenschappelijke bewijs ook andere aspecten belangrijk en worden meegewogen, zoals aanvullende argumenten uit bijvoorbeeld de biomechanica of fysiologie, waarden en voorkeuren van patiënten, kosten (middelenbeslag), aanvaardbaarheid, haalbaarheid en implementatie. Deze aspecten zijn systematisch vermeld en beoordeeld (gewogen) onder het kopje ‘Overwegingen’ en kunnen (mede) gebaseerd zijn op expert opinion. Hierbij is gebruik gemaakt van een gestructureerd format gebaseerd op het evidence-to-decision framework van de internationale GRADE Working Group (Alonso-Coello, 2016a; Alonso-Coello 2016b). Dit evidence-to-decision framework is een integraal onderdeel van de GRADE methodiek.
Formuleren van aanbevelingen
De aanbevelingen geven antwoord op de uitgangsvraag en zijn gebaseerd op het beschikbare wetenschappelijke bewijs en de belangrijkste overwegingen, en een weging van de gunstige en ongunstige effecten van de relevante interventies. De kracht van het wetenschappelijk bewijs en het gewicht dat door de werkgroep wordt toegekend aan de overwegingen, bepalen samen de sterkte van de aanbeveling. Conform de GRADE-methodiek sluit een lage bewijskracht van conclusies in de systematische literatuuranalyse een sterke aanbeveling niet a priori uit, en zijn bij een hoge bewijskracht ook zwakke aanbevelingen mogelijk (Agoritsas, 2017; Neumann, 2016). De sterkte van de aanbeveling wordt altijd bepaald door weging van alle relevante argumenten tezamen. De werkgroep heeft bij elke aanbeveling opgenomen hoe zij tot de richting en sterkte van de aanbeveling zijn gekomen.
In de GRADE-methodiek wordt onderscheid gemaakt tussen sterke en zwakke (of conditionele) aanbevelingen. De sterkte van een aanbeveling verwijst naar de mate van zekerheid dat de voordelen van de interventie opwegen tegen de nadelen (of vice versa), gezien over het hele spectrum van patiënten waarvoor de aanbeveling is bedoeld. De sterkte van een aanbeveling heeft duidelijke implicaties voor patiënten, behandelaars en beleidsmakers (zie onderstaande tabel). Een aanbeveling is geen dictaat, zelfs een sterke aanbeveling gebaseerd op bewijs van hoge kwaliteit (GRADE gradering HOOG) zal niet altijd van toepassing zijn, onder alle mogelijke omstandigheden en voor elke individuele patiënt.
|
Implicaties van sterke en zwakke aanbevelingen voor verschillende richtlijngebruikers |
||
|
|
||
|
|
Sterke aanbeveling |
Zwakke (conditionele) aanbeveling |
|
Voor patiënten |
De meeste patiënten zouden de aanbevolen interventie of aanpak kiezen en slechts een klein aantal niet. |
Een aanzienlijk deel van de patiënten zouden de aanbevolen interventie of aanpak kiezen, maar veel patiënten ook niet. |
|
Voor behandelaars |
De meeste patiënten zouden de aanbevolen interventie of aanpak moeten ontvangen. |
Er zijn meerdere geschikte interventies of aanpakken. De patiënt moet worden ondersteund bij de keuze voor de interventie of aanpak die het beste aansluit bij zijn of haar waarden en voorkeuren. |
|
Voor beleidsmakers |
De aanbevolen interventie of aanpak kan worden gezien als standaardbeleid. |
Beleidsbepaling vereist uitvoerige discussie met betrokkenheid van veel stakeholders. Er is een grotere kans op lokale beleidsverschillen. |
Organisatie van zorg
In de knelpuntenanalyse en bij de ontwikkeling van de richtlijnmodule is expliciet aandacht geweest voor de organisatie van zorg: alle aspecten die randvoorwaardelijk zijn voor het verlenen van zorg (zoals coördinatie, communicatie, (financiële) middelen, mankracht en infrastructuur). Randvoorwaarden die relevant zijn voor het beantwoorden van deze specifieke uitgangsvraag zijn genoemd bij de overwegingen. Meer algemene, overkoepelende, of bijkomende aspecten van de organisatie van zorg worden behandeld in de module Organisatie van zorg.
Commentaar- en autorisatiefase
De conceptrichtlijnmodule werd aan de betrokken (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd ter commentaar. De commentaren werden verzameld en besproken met de werkgroep. Naar aanleiding van de commentaren werd de conceptrichtlijnmodule aangepast en definitief vastgesteld door de werkgroep. De definitieve richtlijnmodule werd aan de deelnemende (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd voor autorisatie en door hen geautoriseerd dan wel geaccordeerd.
Literatuur
Agoritsas T, Merglen A, Heen AF, Kristiansen A, Neumann I, Brito JP, Brignardello-Petersen R, Alexander PE, Rind DM, Vandvik PO, Guyatt GH. UpToDate adherence to GRADE criteria for strong recommendations: an analytical survey. BMJ Open. 2017 Nov 16;7(11):e018593. doi: 10.1136/bmjopen-2017-018593. PubMed PMID: 29150475; PubMed Central PMCID: PMC5701989.
Alonso-Coello P, Schünemann HJ, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Rada G, Rosenbaum S, Morelli A, Guyatt GH, Oxman AD; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ. 2016 Jun 28;353:i2016. doi: 10.1136/bmj.i2016. PubMed PMID: 27353417.
Alonso-Coello P, Oxman AD, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Vandvik PO, Meerpohl J, Guyatt GH, Schünemann HJ; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 2: Clinical practice guidelines. BMJ. 2016 Jun 30;353:i2089. doi: 10.1136/bmj.i2089. PubMed PMID: 27365494.
Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, Fervers B, Graham ID, Grimshaw J, Hanna SE, Littlejohns P, Makarski J, Zitzelsberger L; AGREE Next Steps Consortium. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010 Dec 14;182(18):E839-42. doi: 10.1503/cmaj.090449. Epub 2010 Jul 5. Review. PubMed PMID: 20603348; PubMed Central PMCID: PMC3001530.
Hultcrantz M, Rind D, Akl EA, Treweek S, Mustafa RA, Iorio A, Alper BS, Meerpohl JJ, Murad MH, Ansari MT, Katikireddi SV, Östlund P, Tranæus S, Christensen R, Gartlehner G, Brozek J, Izcovich A, Schünemann H, Guyatt G. The GRADE Working Group clarifies the construct of certainty of evidence. J Clin Epidemiol. 2017 Jul;87:4-13. doi: 10.1016/j.jclinepi.2017.05.006. Epub 2017 May 18. PubMed PMID: 28529184; PubMed Central PMCID: PMC6542664.
Medisch Specialistische Richtlijnen 2.0 (2012). Adviescommissie Richtlijnen van de Raad Kwalitieit. http://richtlijnendatabase.nl/over_deze_site/over_richtlijnontwikkeling.html
Neumann I, Santesso N, Akl EA, Rind DM, Vandvik PO, Alonso-Coello P, Agoritsas T, Mustafa RA, Alexander PE, Schünemann H, Guyatt GH. A guide for health professionals to interpret and use recommendations in guidelines developed with the GRADE approach. J Clin Epidemiol. 2016 Apr;72:45-55. doi: 10.1016/j.jclinepi.2015.11.017. Epub 2016 Jan 6. Review. PubMed PMID: 26772609.
Schünemann H, Brożek J, Guyatt G, et al. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group, 2013. Available from http://gdt.guidelinedevelopment.org/central_prod/_design/client/handbook/handbook.html.
Zoekverantwoording
Zoekacties zijn opvraagbaar. Neem hiervoor contact op met de Richtlijnendatabase.