Botulinetoxinezuur A bij aandrangincontinentie
Uitgangsvraag
Wat is de plaats van botuline toxinezuur A bij vrouwen en mannen met idiopathische, refractaire aandrangincontinentie?
Aanbeveling
Bied BoNT-A blaasinjecties aan bij patiënten met idiopathische, refractaire aandrangincontinentie bij wie conservatieve of medicamenteuze behandeling tot onvoldoende verbetering van klachten leidt.
Bespreek met patiënten met idiopathische, refractaire aandrangincontinentie:
- Het risico op urineweginfectie en urineretentie waarvoor zelfkatheterisatie bij BoNT-A injecties.
- De noodzaak voor herhaalde interventie door tijdelijke werkzaamheid van BoNT-A.
Overwegingen
Voor- en nadelen van de interventie en de kwaliteit van het bewijs
Botulinetoxine-A (BoNT-A) heeft een positief effect op de klachten van aandrang incontinentie vergeleken met placebo behandeling. Het aantal urinary urge incontinence (UUI) episodes is met een klinisch relevant verschil verlaagd na het toedienen van BoNT-A, namelijk met gemiddeld 2 incontinentie episodes per dag. Belangrijke nadelen van BoNT-A injecties zijn het voorkomen van blaasontstekingen en urineretentie. De kwaliteit van leven werd op veel verschillende manieren gemeten. Over het algemeen nam de kwaliteit van leven na interventie met BoNT-A meer toe dan na interventie met placebo.
Van de 13 geïncludeerde, gerandomiseerde studies werden 10 studies ondersteund door de farmaceutische leverancier van BoNT-A. De mogelijke beïnvloeding van de resultaten is gecorrigeerd door aanpassing van de GRADE bij positief uitvallende resultaten. Ten aanzien van de nadelen is deze correctie niet toegepast, aangezien de bewijslast van de nadelen niet afgezwakt hoeven te worden door mogelijke beïnvloeding door de industrie. Dit heeft geleid tot een daling in waardering voor de cruciale uitkomstmaat UUI-episodes naar een bewijskracht GRADE gemiddeld. Hierdoor is ook de overall bewijskracht gemiddeld. Voor de uitkomstmaat risico’s was de GRADE bewijskracht gemiddeld voor blaasontsteking, en laag tot zeer laag voor alle andere bijwerkingen. Voor de kwaliteit van leven is de GRADE bewijskracht laag, mede omdat de data niet gepoold kon worden.
Er is geen bewijs gevonden voor de effectiviteit en veiligheid van Botulinetoxine-B.
Hoewel de richtlijn met name bedoeld is voor vrouwen met UUI is ervoor gekozen de zoekvraag ruimer op te stellen en mannen erin te betrekken. Zodat er geen literatuur verloren zou gaan. Gezien het percentage vrouw in alle geïncludeerde studies >75% betrof, en er gerandomiseerd is, denken wij toch een betrouwbare aanbeveling te kunnen geven voor vrouwen met aandrang incontinentie.
Er worden verschillende doseringen gebruikt, 50, 100, 150, 200 en 300 IU. Hoewel 300 IU de meest effectieve dosering is, wordt dit in de praktijk niet aanbevolen door de toename op het risico zelfkatheterisatie (Denys, 2012; Dmochowski, 2010). Dosering 100 IU en 200 IU zijn even effectief, waarbij in 100 IU eveneens minder zelfkatheterisatie nodig is (Chapple, 2013).
De plaats waar zou moeten worden geïnjecteerd om het beste effect te krijgen is nog niet geheel duidelijk. Studies lijken elkaar tegen te spreken of de injecties juist wel of juist niet in het trigonum plaatst zouden moeten vinden (Mangera, 2011; Kuo, 2011).
Standaardisering van de uitkomstmaten is in 2017 gestart en in een ICHOM-richtlijn vastgelegd (ICHOM, 2017). Sinds deze richtlijn is er nog geen publicatie geweest met de uitkomsten van deze uitkomstmaten.
Over de werkzame duur van de injecties kunnen we geen goede uitspraken doen o.b.v. de PICO. Een studie van Nitti uit 2016 met 3.5 jaar follow-up laat een gemiddelde werkzaamheid van 7.6 maanden zien waarbij de werkzaamheid niet afneemt. Buiten de UWIs worden er geen extra complicaties gezien (Nitti, 2016).
De angst om te moeten zelf-katheteriseren is de voornaamste reden om te stoppen met de behandeling (Downson, 2012).
Voor de specifieke (kwetsbare) oudere patiëntenpopulatie kan er geen uitspraak gedaan worden o.b.v. de PICO. Een recente review bevestigt dit (Manns, 2022). In een studie naar voorspellers van effectiviteit wordt gezien dat BoNT-A bij oudere patiënten minder lang werkt en minder effectief is in verminderen van UI episodes (Hendrickson, 2022).
Op basis van de gehanteerde PICO kunnen wij geen conclusies trekken over de vergelijking met medicatie of sacrale neuromodulatie bij patiënten met UUI (studies die BoNT-A vergeleken met andere behandelingen en niet met placebo werden actief geëxcludeerd). In deze geïncludeerde studies, namen alle participanten deel na gefaalde conservatieve of medicamenteuze therapie. Daarbij wisselde de follow-up duur van 6 weken tot 6 maanden.
Een RCT die bij 249 vrouwen met UUI 100IU BoNT-A vergeleek met solifenacine 5-10mg liet vergelijkbare verbetering van de UUI-episodes zien na 6 maanden (van 5 naar 3.3 en 3.4 respectievelijk); echter 27% van de BoNT-A groep was genezen vs 13% van de solifenacine groep. Deze resultaten stonden tegenover een hoger risico op urineretentie (5% vs 0%) en UWI (33% vs 13%) en een lagere kans op een droge mond (31% vs 46%) (Visco 2012). Deze resultaten zijn onder andere samengevat in een systematic review/ netwerk meta-analyse waarbij 65 RCTs geïncludeerd werden die BoNT-A met medicatie vergeleek ter behandeling van OAB. Na 12 weken waren alle behandelingen waren beter dan placebo therapie. Wanneer BoNT-A vergeleken werd met medicatie, hadden de patiënten die BoNT-A kregen meer kans op >50% of >100% vermindering van UUI episodes/dag (Drake 2017). Een RCT die BoNT-A vergeleek met PTNS bij 60 patiënten met OAB liet zien dat na 9 maanden de BoNT-A op alle uitkomsten effectiever was dan PTNS ten koste van 2 patiënten die CIC ondergingen 2 patiënten behandeld werden voor een UWI (Sherif 2017).
Een systematische review van 2 geïncludeerde studies met in totaal 88 patiënten, indiceerde een positief effect van botox behandeling op het seksueel functioneren van patiënten met OAB (Shawer, 2022). Verbetering werd gezien op behoefte, opwinding, lubricatie, orgasme en tevredenheid. Er werd geen verbetering gezien in dyspareunie.
Kosten (middelenbeslag)
BoNT-A is kostbaar. De injectie kan meestal poliklinisch worden gegeven, eventueel onder lokale anesthesie. Afgewogen tegen de kosten van de behandeling zal het verminderde gebruik van incontinentiemateriaal en toegenomen QoL staan. Er is een Amerikaanse studie gepubliceerd waarbij verschillende behandelopties (100EH botox, SNM, PTNS, medicatie) vergeleken wordt met best supportive care in een Markov model (Murray, 2019). Deze studie laat zien dat botox de hoogste QALY geeft van deze behandelopties.
Aanvaardbaarheid, haalbaarheid en implementatie
De aanvaarbaarheid, haalbaarheid en implementatie zal geen probleem zijn, aangezien de behandeling al wordt toegepast in de huidige praktijk.
Rationale van de aanbeveling
BoNT-A leidt tot minder incontinentie episodes en minder hinder van mictieklachten in vergelijking met vrouwen die placebo behandeling kregen. Echter, de BoNT-A injectie gaat wel gepaard met significant meer bijwerkingen zoals urineweginfectie, urineretentie, dysurie en mogelijk bacteriurie. Laat vrouwen vóór de blaasinjectie oefenen met zelfkatheterisatie.
Onderbouwing
Achtergrond
In Nederland is Botuline A (Botox®) injecties in de blaaswand een van de vervolgbehandelingen bij vrouwen met aandrangsincontinentie nadat conservatieve en medicamenteuze behandelingen hebben gefaald. Er zijn verschillende typen Botulines; Botuline A, AbobotulinumtoxinA (Dysport®), OnabotulinumtoxinA (Botox®) en IncobotulinumtoxinA (Xeomin®) en Botuline B (Myobloc®). Enkel Botulinetoxinezuur A is beschikbaar in Nederland en wordt daarom opgenomen in deze richtlijn. Botulinetoxinezuur A heeft een tijdelijk paralyserend effect van de m. detrusor door passagere blokkade van de acetylcholinereceptor in de synapsspleet. De behandeling is kostbaar, moet vaak herhaaldelijk worden toegepast en kan gepaard gaan met bijwerkingen (urineretentie en urineweginfectie) en is daarom voorbehouden aan de tweede lijn. Met behulp van bovenstaande uitgangsvraag wordt onderzocht hoe effectief en veilig blaasinjecties met botulinetoxinezuur A (BoNT-A) is bij patiënten met aandrangincontinentie.
Conclusies / Summary of Findings
- BotulinumtoxinA
1. Urinary urge incontinence episodes
|
Moderate GRADE |
OnabotulinumtoxinA likely reduces urinary urge incontinence episodes when compared with placebo in patients with idiopathic urinary urge incontinence.
Sources: Chapple 2013; Dowson, 2011; Nitti, 2013; Sahai, 2007; Yokoyama, 2020 |
2 Adverse events
2.1 Urinary tract infection
|
Moderate GRADE |
OnabotulinumtoxinA likely increases incidence of urinary tract infections when compared to placebo in patients with idiopathic urinary urge incontinence.
Sources: Chapple, 2013; Denys, 2011; Dmochowski, 2010; Dowson, 2011; Flynn, 2008; Herschorn, 2017; Liao, 2022; McCammon, 2021; Nitti, 2013; Tincello, 2011; Yokoyama, 2020 |
2.2 Urinary retention
|
Low GRADE |
OnabotulinumtoxinA may increase incidence of urinary retention when compared to placebo in patients with idiopathic urinary urge incontinence.
Sources: Chapple, 2013; Denys, 2011; Dmochowski, 2010; Dowson, 2011; Herschorn, 2017; McCammon, 2021; Nitti, 2013; Yokoyama, 2020 |
2.3 Dysuria
|
Low GRADE |
OnabotulinumtoxinA may increase dysuria when compared to placebo in patients with urge incontinence.
Sources: Chapple, 2013; Herschorn, 2017; Liao, 2022; McCammon, 2021; Nitti, 2013; Yokoyama, 2020 |
2.4 Other adverse events
|
Very low GRADE |
The evidence is very uncertain about the effect of onabotulinumtoxinA on other adverse events when compared to placebo in patients with urge incontinence.
Sources: Chapple, 2013; Herschorn, 2017; Liao, 2022; McCammon, 2021; Nitti, 2013; Yokoyama, 2020 |
- Quality of life
|
Low GRADE |
OnabotulinumtoxinA may increase quality of life when compared to placebo in patients with urge incontinence.
Sources: Chapple, 2013; Denys, 2011; Dmochowski, 2010; Dowson, 2011; Flynn, 2008; Herschorn, 2017; Jabs, 2013; Liao, 2022; McCammon, 2021; Nitti, 2013; Sahai, 2007; Tincello, 2011; Yokoyama, 2020 |
Samenvatting literatuur
Description of studies
All included studies report on results of interventions with botulinum toxin A (OnabotulinumtoxinA, BoNT-A). One systematic review was selected (Ramos, 2017).
Ramos (2017) performed a systematic review and meta-analysis on the efficacy and safety of OnabotulinumtoxinA treatment versus placebo in patients with overactive bladder (OAB). MEDLINE, EMBASE, LILACS, Cochrane, GreyNet, and OpenGrey were searched in June 2012. Inclusion criteria were studies on humans, subjects 18 years or older, published in English or Spanish, randomized controlled trials, clinical trials, and multicenter studies. Furthermore, studies selected had to compare different doses of OnabotulinumtoxinA, the use of OnabotulinumtoxinA vs placebo, or the use of OnabotulinumtoxinA vs antimuscarinics. In total, 11 studies (n=2149) were included, of which 8 studies (n=1825) complied with our PICO (Chapple, 2013; Denys, 2011; Dmochowski, 2010; Dowson, 2011; Flynn, 2008; Nitti, 2013; Sahai, 2007; Tincello, 2011). A meta-analysis was performed to assess urinary urge incontinence episodes, urinary urge episodes, urinary frequency, adverse events, urinary retention, and urinary tract infection. Risk of bias was assessed using the Cochrane Handbook (version 5.0).
Five RCTs were selected (Herschorn, 2017; Jabs, 2013; Liao, 2022; McCammon, 2021; Yokoyama, 2020) that complied with our PICO and were published after the aforementioned systematic review of Ramos et al.
Herschorn (2017) performed a multicenter, double-blind, randomized, placebo-controlled study from March 2013 to March 2015, at 68 sites in North America and Europe. The aim of the study was to compare the efficacy and safety of onabotulinumtoxinA or solifenacin versus placebo in overactive bladder patients with urinary incontinence. Men and women were included if they had OAB for >6 months, 2 UUI per day and >7 voids per day, no UUI-free day and no predominant stress incontinence. The intervention group (n=145, 61.4 ± 12.8 years, 84.8% female) consisted of intradetrusor 100U BoNT-A injections. The control group (n=60, 61.2 ± 12.2 years, 85% female) intervention consisted of placebo injections. Length of follow up was 12 weeks blinded, and 24 weeks unblinded. Outcomes of interest include QoL and adverse events.
Jabs (2013) performed a randomized, double-blind controlled trial from February 2008 to September 2009 in Canada. The aim of the study was to study the efficacy of intradetrusor injections of botulinum toxin A for non-neurogenic urinary urge incontinence. Women >18 years old were included in this study. The intervention group (n=11, 63 ± 9.4 years) received intradetrusor 100U BoNT-A injections. The control group (n=10, 63.8 ± 11.2 years) received placebo injections. Length of follow up was 6 months. Outcomes of interest include QoL.
Liao (2022) performed a multicenter, randomized, double-blind, placebo-controlled trial from April 2016 to December 2018, in 17 sites across China. The aim of the study was to evaluate the efficacy and safety of Hengli® Chinese botulinum toxin type A in patients with OAB. Women and men 18-75 years old with >8 micturitions per day were included in this study. The intervention group (n=144, 47.75 ± 14.20 years, 82.39% female) received intradetrusor 100U BoNT-A injections. The control group intervention (n=72, 46.39 ± 15.55 years, 85.92% female) consisted of placebo injections. Length of follow up was 24 weeks. Outcomes of interest were QoL score (International Prostate Symptom Score-QoL Subscore) and adverse events.
McCammon (2021) performed a randomized, multicenter, placebo-controlled, phase IV study from November 2013 to January 2017, in 44 sites in the United States. The aim of the study was to assess the efficacy and tolerability of onabotulinumtoxinA in patients with OAB. Men and women were included if they had OAB for 6 months or longer, were inadequately managed by an anticholinergic, had 3 or more recorded episodes of urgency UI (UUI), 1 or less UUI-free day, and 24 or greater micturitions on a 3-day paper bladder diary during screening. The intervention group (n=129, 60.8 ± 12.7 years, 88.4% female) received intradetrusor 100U BoNT-A injections. The control group intervention (n=125, 60.9 ± 12.1 years, 89.6% female) consisted of placebo injections. Length of follow up was 12 weeks. Outcomes of interest were QoL (KHQ score) and adverse events.
Yokoyama (2020) performed a phase III, randomized, double-blind, placebo-controlled trial from August 2016 to November 2018, at 53 sites in Japan. The aim of the study was to evaluate the efficacy and safety of onabotulinumtoxinA in patients with overactive bladder and urinary incontinence. Men and women were included if they were ≥20 years old with OAB, had 3 or more episodes of UUI and had a mean of 8 or more micturitions per day in a 3-day diary. The intervention group (n=124, 65.6 ± 12.43 years, 76% female) received intradetrusor 100U BoNT-A injections. The control group intervention (n=124, 66.2 ± 12.19 years, 74% female) consisted of placebo injections. Length of follow up was 48 weeks. Outcomes of interest were UUI, QoL (KHQ score) and adverse events.
Results
Thirteen studies were included in our analysis (Chapple, 2013; Denys, 2011; Dmochowski, 2010; Dowson, 2011; Flynn, 2008; Nitti, 2013; Sahai, 2007; Tincello, 2011; Herschorn, 2017; Jabs, 2013; Liao, 2022; McCammon, 2021; Yokoyama, 2020). All studies report on the effects of onabotulinumtoxinA versus placebo.
- Urinary urge incontinence episodes
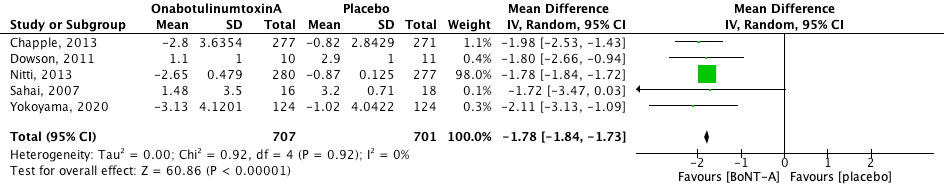
Urinary urge incontinence (UUI) episodes were measured per day. The pooled effect was mean difference -1.78 [95% CI -1.84 to -1.73] favouring OnabotulinumtoxinA (heterogeneity (I2): 0%). The result is statistically significant and clinically relevant. Denys (2012) reported a UUI decline of -1.5 for placebo, 50U BoNT-A and 150U BoNT-A, and a decline of -2.7 for 100U BoNT-A. Dmochowksi reported a mean change from baseline of –17.4 for placebo, and –20.7, –18.4, –23.0, –19.6 and –19.4 for BoNT-A dose groups of 50U, 100U, 150U, 200U and 300U. These results could not be pooled because not enough information was given.
Figure 1. Change in urinary urge incontinence episodes.
- Adverse events
2.1 Urinary tract infection
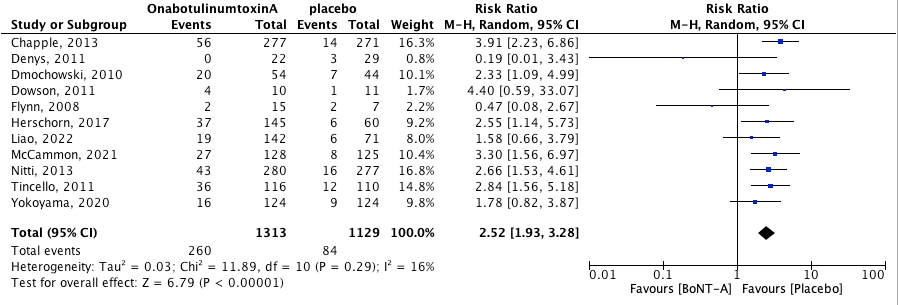
Urinary tract infection as most often determined by positive urine culture and/or leukocyturia. The pooled effect was RR 2.52 [95% CI 1.93 to 3.38] favoring placebo (heterogeneity (I2): 61%). This means that the risk of urinary tract infections is 152% higher in the BoNT-A group when compared to the placebo group. This result is both statistically significant and clinically relevant.
Figure 2. Risk of urinary tract infection.
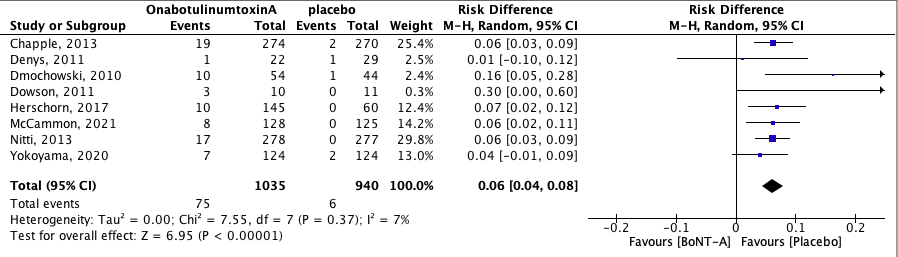
2.2 Urinary retention
Urinary retention was defined in the studies as post-void residue of ≥200 mL or <350 mL with associated symptoms or as the need for clean-intermittent catheterization due to retention. The pooled effect was RD 0.06 [95% CI 0.04 to 0.08] favoring placebo (heterogeneity (I2): 7%). This means that the absolute risk of urinary retention was 6% higher in the BoNT-A group when compared to the placebo group. Number needed to treat for an additional harmful outcome (NNTH) is 15.1 (absolute risk reduction (ARR) = (6/940)-(75/1035); NNTH = 1/ARR). This result is statistically significant and clinically relevant (NNTH for clinically relevant RR of 0.8 is 500) (Buckingham, 2008).
Figure 3. Risk of urinary retention.
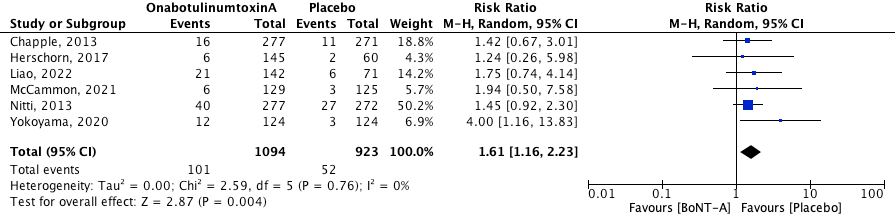
2.3 Dysuria
Dysuria was defined as self-reported pain and/or discomfort during urination. The pooled effect was RR 1.61 [95% CI 1.16 to 2.23] favoring placebo (heterogeneity (I2): 0%). This result is statistically significant and clinically relevant. The NNTH is 27.8 (1/ARR = 1/((52/923)-(101/1094)) = 27.8).
Figure 4. Risk of dysuria.
2.4 Other adverse events
Bladder adverse events
Chapple (2013) reported bacteriuria in 17 patients (6.2%) receiving OnabotulinumtoxinA versus 9 patients (3.3%) receiving placebo. Nitti (2013) reported bacteriuria in 23 patients (8.3%) receiving OnabotulinumtoxinA versus 10 patients (3.7%) receiving placebo. Herschorn (2017) reported bacteriuria in 11 patients (7.6%) receiving OnabotulinumtoxinA, versus 3 patients (5.0%) receiving placebo.
Chapple (2013) reported haematuria in 10 patients (3.6%) receiving OnabotulinumtoxinA versus 2 patients (0.7%) receiving placebo. Liao (2022) reported haematuria was reported in 3 patients (2.11%) receiving OnabotulinumtoxinA, versus 1 patient (1.41%) receiving placebo.
Chapple (2013) reported residual urine volume in 9 patients (3.3%) receiving OnabotulinumtoxinA versus 2 patients (0.7%) receiving placebo.
Liao (2022) reported ALT (Alanine transaminase) elevation in 3 patients (2.11%) receiving OnabotulinumtoxinA versus 0 patients receiving placebo.
Airway adverse events
Herschorn (2017) reported nasopharyngitis was reported in 2 patients (1.4%) receiving OnabotulinumtoxinA, versus 3 patients (5.0%) receiving placebo. Yokoyama (2020) reported nasopharyngitis in 15 patients (12%) receiving OnabotulinumtoxinA versus 11 patients (9%) receiving placebo.
Herschorn (2017) reported dry mouth in 4 patients (2.8%) receiving OnabotulinumtoxinA, versus 0 patients receiving placebo.
McCammon (2021) reported bronchitis in 1 patient (0.8%) receiving OnabotulinumtoxinA and in 4 patients (3.2%) receiving placebo. Cough was reported in 0 patients receiving OnabotulinumtoxinA and in 4 patients (3.2%) receiving placebo.
Tincello (2011) and Jabs (2013) did not report on adverse events.
Pooling was not possible because of the heterogeneity in outcomes reported as adverse events in the included studies. Overall, slightly more adverse were reported after intervention with onabotulinumtoxinA compared to placebo.
- Quality of life
3.1 UDI-6
Urogenital Distress Inventory (UDI-6) is a questionnaire that was used to determine urogenital symptoms and its burden on a patient. The higher the score, the more of a burden the patient experiences. Four studies (Jabs, 2013; Dowson, 2011; Flynn, 2008; Sahai, 2007) reported UDI-6 as measurement for QoL (table 1). Overall, burden of symptoms improved more for patients receiving OnabotulinumtoxinA compared to placebo, however, this improvement is not statistically significant.
Table 1. Included results of studies reporting UDI-6.
|
Study |
BoNT-A |
|
Placebo |
|
Mean change between groups at FU (95% CI) |
|
|
Baseline |
Follow-up |
Baseline |
Follow-up |
|
|
Dowson (2011) |
10.3 |
8.2 (12 weeks) |
8.3 |
8.6 (12 weeks) |
-1.2 (-6.1 to 3.7) |
|
Flynn (2008) |
49.6 |
31.0 (6 weeks) |
42.9 |
46.0 (6 weeks) |
Not reported |
|
Jabs (2013) |
57.6 (13.7) |
29.1 (16.2) (6 months) |
62.2 (17.3) |
51.1 (26.9) (6 months) |
17.4 (−1.9 to 36.6) |
|
Sahai (2007) |
10.75 |
5.13 (12 weeks) |
9.50 |
10.00 (12 weeks) |
-4.87 (-7.83 to -2.96) |
3.2 King’s Health Questionnaire (KHQ)
The KHQ consists of seven multi-item domains (Role Limitations, Social Limitations, Physical Limitations, Personal Relationships, Emotions, Sleep/Energy, and Severity/Coping Measures) and two single-item domains (General Health Perception and Incontinence Impact). A higher score on the scale means a heavier burden on the patient. The bigger the reduction (change from baseline) the bigger the improvement from baseline.
Six studies (Chapple, 2013; Dmochowski, 2010; Herschorn, 2017; McCammon, 2021; Nitti, 2013; Yokoyama, 2020) reported on KHQ Role limitations domain, and six reported on social limitations domain (see table 2). Overall, QoL improved more for patients receiving OnabotulinumtoxinA compared to placebo.
Table 2. Included results of studies reporting KHQ score.
|
Study |
Role Limitations (change from baseline) |
|
Social Limitations (change from baseline) |
|
Symptoms component (change from baseline) |
|
|
|
BoNT-A |
Placebo |
BoNT-A |
Placebo |
BoNT-A |
Placebo |
|
Chapple (2013) |
-26.5 |
-5.0 |
-16.2 |
-1.3 |
|
|
|
Dmochowski (2010) |
|
|
|
|
50U: -10 100U: -11 150U: -16 200U: -16 300U: -18 |
-9 |
|
Herschorn (2017) |
-31.2 (-36.2, -26.2) |
-15.1 (-22.8, -7.4) |
-13.7 (-16.9, -10.4) |
-6.1 (-11.2, -1.1) |
|
|
|
McCammon (2021) |
-37.8 |
-16.7 |
-20.4 |
-6.0 |
|
|
|
Nitti (2013) |
-24.3 |
-2.4 |
-17.3 |
-3.8 |
|
|
|
Yokoyama (2020) |
-21.09 ± 2.997 |
-6.48 ± 2.976 |
-13.36 ± 2.983 |
-4.95 ± 2.945 |
|
|
3.3 I-QoL
Five studies (Chapple, 2013; Denys, 2011; Nitti, 2013; Tincello, 2011; McCammon, 2021) reported QoL with the Incontinence Quality of Life (I-QoL) questionnaire (Table 3). For this questionnaire, a minimal important difference of 10 points is standardized. The higher the score (change from baseline), the bigger the improvement from baseline. Overall, QoL improved more for patients receiving OnabotulinumtoxinA compared to placebo.
Table 3. Included results of studies reporting I-QoL.
|
Study |
BoNT-A Change from baseline Total summary score: |
Placebo Change from baseline Total summary score: |
|
Chapple (2013) |
23.1 |
6.3 |
|
Denys (2011) |
50U: 8 100U: 35 150U: 30 |
8 |
|
McCammon (2021) |
27.1 |
9.8 |
|
Nitti (2013) |
21.9 |
6.8 |
|
Tincello (2011) |
55.11 (IQR 23.30–78.41) |
27.27 (IQR 18.18–46.59) |
Pooling of data regarding the outcome QoL was not possible because of the heterogeneity in outcomes reported in the included studies. Overall, QoL increased more after intervention with onabotulinumtoxinA compared to placebo.
Level of evidence of the literature
- Urinary urge incontinence episodes
The level of evidence regarding the outcome measure urinary urge incontinence episodes started at High and was downgraded with one level to a moderate GRADE due to influence of pharmaceutical industry and results in favor of the pharmaceutical industry (publication bias).
- Adverse events
2.1 Urinary tract infection
The level of evidence regarding the outcome measure urinary tract infections started at High and was downgraded with one level to a moderate GRADE because of bias due to non-blinding of patients and researchers (-1, risk of bias). Although influence of pharmaceutical industry could be present, downgrading for publication bias (risk of bias) is not necessary in this case since results are not in favor of the pharmaceutical industry.
2.2 Urinary retention
The level of evidence regarding the outcome measure urinary retention started at High and was downgraded by two levels to a low GRADE because of small number of events (-1, imprecision), and bias due to non-blinding of patients and researchers (-1, risk of bias). Although influence of pharmaceutical industry could be present, downgrading for publication bias (risk of bias) is not necessary in this case since results are not in favor of the pharmaceutical industry.
2.3 Dysuria
The level of evidence regarding the outcome measure dysuria started at High and was downgraded by two levels to a low GRADE because of conflicting results (-1, inconsistency) and bias due to non-blinding of patients and researchers (-1, risk of bias). Although influence of pharmaceutical industry could be present, downgrading for publication bias (risk of bias) is not necessary in this case since results are not in favor of the pharmaceutical industry.
2.4 Other adverse events
The level of evidence regarding other adverse events started at High and was downgraded by three levels to very low GRADE because of conflicting results and methodological heterogeneity (-2, inconsistency), and bias due to non-blinding of patients and researchers (-1, risk of bias). Although influence of pharmaceutical industry could be present, downgrading for publication bias (risk of bias) is not necessary in this case since results are not in favor of the pharmaceutical industry.
- Quality of life
The level of evidence regarding other adverse events started at High and was downgraded by two levels to a very low GRADE because of methodological heterogeneity (-2, inconsistency), and due to the influence of pharmaceutical industry and results in favor of the pharmaceutical industry (-1, publication bias).
Zoeken en selecteren
A systematic review of the literature was performed to answer the following question: What is the effectiveness and safety of intravesical botulin toxin A or B in women and men with urinary urge incontinence who had failed conservative and pharmacological therapy?
P: Women and men with idiopathic urinary urge incontinence who had failed conservative and pharmacological therapy.
I: Intravesical botulin A.
C: Placebo or no treatment.
O: Effect on incontinence (urinary urge incontinence episodes), adverse events (e.g., urinary tract infections (UTIs), urinary retention), quality of life (QoL).
Relevant outcome measures
The guideline development group considered effect on incontinence as a critical outcome measure for decision making; and adverse events and quality of life as important outcome measures for decision making.
The working group defined the outcome measures as follows:
- Effect on incontinence: urge incontinence episodes (UUI)
- Quality of life (QoL): patient reported outcome measure (PROM).
- Adverse events: all adverse events were included.
If the burden of urinary urge incontinence symptoms was measured with the Urinary Distress Inventory (UDI-6), the working group defined a difference of 8 points as a minimal clinically (patient) important difference (Barber, 2009). In all other cases, the working group defined the GRADE-standard limit of 25% difference for dichotomous outcomes (RR < 0.8 or > 1.25), and 10% for continuous outcomes as a minimal clinically (patient) important difference. For the King’s Health Questionnaire (KHQ) score a change of 5.0 was determined to be the minimal important difference (MID). A higher score on the scale means a heavier burden on the patient. The bigger the reduction (change from baseline) the bigger the improvement from baseline.
Search and select (Methods)
The databases Medline (via OVID) and Embase (via Embase.com) were searched with relevant search terms until February 26th, 2021. The detailed search strategy is depicted under the tab Methods. The systematic literature search resulted in 692 hits. Systematic reviews were selected. Fifty-five studies were initially selected based on title and abstract screening. After reading the full text, 53 studies were excluded (see Table of excluded studies). The initial search was aimed at BoNT-A and BoNT-B. Because no literature was found on BoNT-B and because it is not available in the Netherlands, we removed BoNT-B from our PICO. One systematic review was selected. Included RCTs were then analyzed for inclusion criteria and quality. Since the systematic review included was performed in 2016, an additional search was done on 21-03-2022 from 2016 onward for RCTs, and from 26-02-2021 onwards for systematic reviews. This new search resulted in 186 hits. Of those, 50 were selected based on title and abstract screening. After reading full text, 45 articles were excluded and 5 RCTs were included.
Results
One systematic review was selected, of which eight studies were included in the analysis of the literature. Five additional RCTs were selected. Important study characteristics and results are summarized in the evidence table. The assessment of the risk of bias is summarized in the risk of bias table.
Referenties
- Barber MD, Spino C, Janz NK, Brubaker L, Nygaard I, Nager CW, Wheeler TL; Pelvic Floor Disorders Network. The minimum important differences for the urinary scales of the Pelvic Floor Distress Inventory and Pelvic Floor Impact Questionnaire. Am J Obstet Gynecol. 2009 May;200(5):580.e1-7.
- Buckingham J, Fisher B, Saunders D. Evidence Bases Medicine Toolkit Ð Intervention calculations.
- Chapple C, Sievert KD, MacDiarmid S, Khullar V, Radziszewski P, Nardo C, Thompson C, Zhou J, Haag-Molkenteller C. OnabotulinumtoxinA 100 U significantly improves all idiopathic overactive bladder symptoms and quality of life in patients with overactive bladder and urinary incontinence: a randomised, double-blind, placebo-controlled trial. Eur Urol. 2013 Aug;64(2):249-56.
- Denys P, Le Normand L, Ghout I, Costa P, Chartier-Kastler E, Grise P, Hermieu JF, Amarenco G, Karsenty G, Saussine C, Barbot F; VESITOX study group in France. Efficacy and safety of low doses of onabotulinumtoxinA for the treatment of refractory idiopathic overactive bladder: a multicentre, double-blind, randomised, placebo-controlled dose-ranging study. Eur Urol. 2012 Mar;61(3):520-9.
- Dmochowski R, Chapple C, Nitti VW, Chancellor M, Everaert K, Thompson C, Daniell G, Zhou J, Haag-Molkenteller C. Efficacy and safety of onabotulinumtoxinA for idiopathic overactive bladder: a double-blind, placebo controlled, randomized, dose ranging trial. J Urol. 2010 Dec;184(6):2416-22.
- Dowson C, Sahai A, Watkins J, Dasgupta P, Khan MS. The safety and efficacy of botulinum toxin-A in the management of bladder oversensitivity: a randomised double-blind placebo-controlled trial. Int J Clin Pract. 2011 Jun;65(6):698-704.
- Dowson C, Watkins J, Khan MS, Dasgupta P, Sahai A. Repeated botulinum toxin type A injections for refractory overactive bladder: medium-term outcomes, safety profile, and discontinuation rates. Eur Urol. 2012 Apr;61(4):834-9.
- Flynn MK, Amundsen CL, Perevich M, Liu F, Webster GD. Outcome of a randomized, double-blind, placebo controlled trial of botulinum A toxin for refractory overactive bladder. J Urol. 2009 Jun;181(6):2608-15.
- Hendrickson WK, Xie G, Rahn DD, Amundsen CL, Hokanson JA, Bradley M, Smith AL, Sung VW, Visco AG, Luo S, Jelovsek JE. Predicting outcomes after intradetrusor onabotulinumtoxina for non-neurogenic urgency incontinence in women. Neurourol Urodyn. 2022 Jan;41(1):432-447.
- Herschorn S, Kohan A, Aliotta P, McCammon K, Sriram R, Abrams S, Lam W, Everaert K. The Efficacy and Safety of OnabotulinumtoxinA or Solifenacin Compared with Placebo in Solifenacin Nave Patients with Refractory Overactive Bladder: Results from a Multicenter, Randomized, Double-Blind Phase 3b Trial. J Urol. 2017 Jul;198(1):167-175.
- ICHOM Ð OVERACTIVE OVERACTIVE BLADDER DATA COLLECTION REFERENCE GUIDE; Version 1.2.2 Revised: April 10th, 2017
- Jabs C, Carleton E. Efficacy of botulinum toxin a intradetrusor injections for non-neurogenic urinary urge incontinence: a randomized double-blind controlled trial. J Obstet Gynaecol Can. 2013 Jan;35(1):53-60. doi: 10.1016/s1701-2163(15)31049-5. PMID: 23343798.
- Kuo HC. Bladder base/trigone injection is safe and as effective as bladder body injection of onabotulinumtoxinA for idiopathic detrusor overactivity refractory to antimuscarinics. Neurourol Urodyn. 2011 Sep;30(7):1242-8.
- Liao L, Liu Q, Cong H, Xu Z, Li E, Weng Z, Jiang H, Liu B, Huang X, Xia S, Wen W, Wu J, Shi G, Wang Y, Li P, Yu Y, Fang Z, Zheng J, Tian Y, Shang D, Li H, Huang Z, Zhou L, Xiao Y, Zhang Y, Wang J, Zhang X, Zhang P, Wang D, Zhang X, Xie K, Wang B, Ma L, Tian X, Chen L, Dong J. Hengli¨ÊChinese Botulinum Toxin Type A for Treatment of Patients With Overactive Bladder: A Multicenter, Prospective, Randomized, Double-Blind, Placebo-Controlled Trial. Front Pharmacol. 2022 Feb 18;13:840695.
- Mangera A, Andersson KE, Apostolidis A, Chapple C, Dasgupta P, Giannantoni A, Gravas S, Madersbacher S. Contemporary management of lower urinary tract disease with botulinum toxin A: a systematic review of botox (onabotulinumtoxinA) and dysport (abobotulinumtoxinA). Eur Urol. 2011 Oct;60(4):784-95.
- Manns K, Khan A, Carlson KV, Wagg A, Baverstock RJ, Trafford Crump R. The use of onabotulinumtoxinA to treat idiopathic overactive bladder in elderly patients is in need of study. Neurourol Urodyn. 2022 Jan;41(1):42-47.
- McCammon K, Gousse A, Kohan A, Glazier D, Gruenenfelder J, Bai Z, Patel A, Hale D. Early and Consistent Improvements in Urinary Symptoms and Quality of Life With OnabotulinumtoxinA in Patients With Overactive Bladder and Urinary Incontinence: Results From a Randomized, Placebo-controlled, Phase IV Clinical Trial. Female Pelvic Med Reconstr Surg. 2021 Jul 1;27(7):450-456.
- Murray B, Hessami SH, Gultyaev D, Lister J, Dmochowski R, Gillard KK, Stanisic S, Tung A, Boer R, Kaplan S. Cost-effectiveness of overactive bladder treatments: from the US payer perspective. J Comp Eff Res. 2019 Jan;8(1):61-71.
- Nitti VW, Dmochowski R, Herschorn S, Sand P, Thompson C, Nardo C, Yan X, Haag-Molkenteller C; EMBARK Study Group. OnabotulinumtoxinA for the treatment of patients with overactive bladder and urinary incontinence: results of a phase 3, randomized, placebo controlled trial. J Urol. 2013 Jun;189(6):2186-93.
- Ramos H, Torres Castellanos L, Ponce Esparza I, Jaramillo A, Rodrguez A, Moreno Bencardino C. Management of Overactive Bladder With OnabotulinumtoxinA: Systematic Review and Meta-analysis. Urology. 2017 Feb;100:53-58. .
- Sahai A, Khan MS, Dasgupta P. Efficacy of botulinum toxin-A for treating idiopathic detrusor overactivity: results from a single center, randomized, double-blind, placebo controlled trial. J Urol. 2007 Jun;177(6):2231-6.
- Tincello DG, Kenyon S, Abrams KR, Mayne C, Toozs-Hobson P, Taylor D, Slack M. Botulinum toxin a versus placebo for refractory detrusor overactivity in women: a randomised blinded placebo-controlled trial of 240 women (the RELAX study). Eur Urol. 2012 Sep;62(3):507-14. doi: 10.1016/j.eururo.2011.12.056. Epub 2012 Jan 5. PMID: 22236796.
- Shawer S, Khunda A, Waring GJ, Ballard P. Impact of intravesical onabotulinumtoxinA (Botox) on sexual function in patients with overactive bladder syndrome: a systematic review and meta-analysis. Int Urogynecol J. 2022 Feb;33(2):235-243.
- Yokoyama O, Honda M, Yamanishi T, Sekiguchi Y, Fujii K, Nakayama T, Mogi T. OnabotulinumtoxinA (botulinum toxin typeÊA) for the treatment of Japanese patients with overactive bladder and urinary incontinence: Results of single-dose treatment from a phaseÊIII, randomized, double-blind, placebo-controlled trial (interim analysis). Int J Urol. 2020 Mar;27(3):227-234.
Evidence tabellen
Systematic review
|
Study reference |
Study characteristics |
Patient characteristics |
Intervention (I) |
Comparison / control (C)
|
Follow-up |
Outcome measures and effect size |
Comments |
|
Ramos, 2017
N.B., study characteristics and results are extracted from the SR (unless stated otherwise) |
Study design: SR and meta-analysis of RCTs
Literature search up to June 2012; 8/11 studies included in this analysis
A: Dowson, 2011 B: Flynn, 2009 C: Sahai, 2007 D: Dmochowski, 2010 E: Denys, 2011 F: Tincello, 2011 G: Chapple, 2013 H: Nitti, 2013
Study design: Multicenter, randomized, double-blind: D, E, G, H Single-center, randomized, double-blind: A, B, C, F
Setting and Country: A: UK B: USA C: UK D: USA E: France F: UK G: Europe and USA H: USA and Canada
Source of funding and conflicts of interest: A: Funded by National Institute of Health Research, to Guy’s & St Thomas’ NHS Foundation Trust in partnership with King’s College London and King’s College Hospital NHS Foundation Trust Authors have financial interest and/or other relationship with the industry. B: National Institutes of Health National Institute on Aging Grant #R21 AG25490-01. Authors have financial interest and/or other relationship with the industry. C: Supported by a grant from the British Urological Foundation. Authors have financial interest and/or other relationship with the industry D: Authors have financial interest and/or other relationship with the industry E: Sponsored by the ‘‘Assistance Publique – Hopitaux de Paris’’ and was funded by a grant of the French Ministry of Health (AOM03111). These organisations assisted in the collection, analysis, and interpretation of data as well as in manuscript preparation. Authors have financial interest and/or other relationship with the industry F: Sponsored by grants from The Moulton Charitable Trust and Wellbeing of Women. The sponsor remained independent of the design, conduct, and analysis of the study. Authors have financial interest and/or other relationship with the industry G: Allergan, Inc. helped design and conduct the study; collect, manage, analyse, and interpret the data; and prepare, review, and approve the manuscript. Authors have financial interest and/or other relationship with the industry H: Authors have financial interest and/or other relationship with the industry I: Authors have financial interest and/or other relationship with the industry |
Inclusion criteria SR: RCT OnabotulinumtoxinA injection in the detrusor muscle the control group: placebo, anticholinergic medications, or comparisons of different doses of OnabotulinumtoxinA
Exclusion criteria SR: none mentioned
Important patient characteristics at baseline: N, mean age A: I: n=10, 49.6±19 yrs C: n=11, 46.7±17 yrs B: I: n=15 / C: n=7 66 (range: 41 to 85) yrs C: I: n=16, 49.8 yrs C: n=18, 50.8 yrs D: I: n=268 / C: n=43 58.8 yrs E: I: n=70 / C: n=29 61.6 ± 14.0 yrs F: I: n=122, 60.7 (range 50.8–67.8) yrs C: n=118, 58.2 (range 51.5–69.2) yrs G: I: n=277, 59.5±15.5 yrs C: n=271, 59.2±14.1 yrs H: I: n=278, 61.7±12.7 yrs C: n=272, 61.0±13.1 yrs
Sex: A: I: 80% F / C: 64% F B: unknown C: I: 56% F / C: 55% F D: 92% F E: 87.9% F F: 100% F G: I: 88.1% F / C: 84.5% F H: I: 90% F / C: 88.4% F |
A: 100U BoNT-A, 10 injections at baseline B: 200U, 300U BoNT-A, 10-12 injections at baseline C: 200U BoNT-A, 20 injections at baseline D: 50U, 100U, 150U, 200U, 300U BoNT-A, 20 injections at baseline E: 50U, 100U, 150U BoNT-A, 15 injections at baseline F: 200U BoNT-A, 20 injections at baseline G: 100U BoNT-A, 20 injections at baseline H: 100U BoNT-A, 20 injections at baseline |
A: placebo, 10 injections at baseline B: placebo, 10-12 injections at baseline C: placebo, 20 injections at baseline D: placebo, 20 injections at baseline E: placebo, 15 injections at baseline F: placebo, 20 injections at baseline G: placebo, 20 injections at baseline H: placebo, 20 injections at baseline |
End-point of follow-up: A: 24 weeks B: 6 weeks C: 6 months D: 36 weeks E: 6 months F: 6 months G: 12 weeks H: 24 weeks
For how many participants were no complete outcome data available? A: I: n=2 (15%) / C: n=0 B: I: n=0 / C: n=0 C: <2% D: I: n=34 (13%) / C: n=7 (16%) E: I: n=6 (8%) / C: n=2 (6%) F: I: n=22 (18%) / C: n=19 (16%) G: I: n=27 (10%) / C: n=32 (12%) H: I: n=29 (10%) / C: n=29 (11%)
|
Outcome measure-1 Defined as change in urinary urge incontinence episodes Effect measure: mean difference [95% CI]: A: -1,80 (-2.66, -0.94) C: -1.72 (-3.47, 0.03) G: -1.98 (1.69, -2.27) H: -1.78 (-1.84, -1.72)
Outcome measure-2 Adverse events Urinary tract infections (UTI): Effect measure: risk ratio [95% CI]: A: 4.40 (0.59, 33.07) B:0.47 (0.08, 2.67) D: 2.33 (1.09, 4.99) E: 0.19 (0.01, 3.43) G: 3.91 (1.53, 4.61) H: 2.66 (1.53, 4.61)
Urinary retention defined as post-void residue of 200 mL: Effect measure: risk ratio [95% CI]: A: 7.65 (0.44, 131.75) D:8.15 (1.08, 61.22) E: 1.32 (0.09, 19.93) G: 9.36 (2.20, 39.80) H: 48.48 (2.96, 793.24)
Outcome measure-3 Quality of life Not reported in SR. |
Facultative: Brief description of author’s conclusion: “Intravesical injections of OnabotulinumtoxinA compared with placebo showed a statistically significant improvement in the treatment of overactive bladder. Adverse events were more frequent among patients treated with OnabotulinumtoxinA.”
Personal remarks on study quality, conclusions, and other issues (potentially) relevant to the research question: all studies were sponsored by pharmaceutical companies. Most of the authors have declared conflicts of interest. |
Intervention studies
|
Study reference |
Study characteristics |
Patient characteristics |
Intervention (I) |
Comparison / control (C)
|
Follow-up |
Outcome measures and effect size |
Comments |
|
Herschorn, 2017 |
Type of study: multicenter, double-blind, randomized, placebo-controlled study
Setting and country: North America and Europe
Funding and conflicts of interest: “This study was sponsored by Allergan plc, Dublin, Ireland. The authors would like to acknowledge Dr. Charles Pignataro, MD, formerly of Allergan plc, for his contributions to this study. Assistance with the writing and development of the manuscript was provided by Jennifer L. Giel, PhD, on behalf of Evidence Scientific Solutions, Philadelphia, Pennsylvania, and was funded by Allergan plc.” |
Inclusion criteria: - adults with OAB for >6 months - 2 UUI per day and >7 voids per day - no UUI-free day - no predominant SUI
Exclusion criteria: - OAB due to neurological reason - predominance of stress UI - previous/current therapy with solifenacin - previous/current botulinum toxin therapy for any urological condition - use of any treatments for OAB within 7 days of start of screening
N total at baseline: Intervention: 145 Control: 60
Important prognostic factors2: Age ± SD: I: 61.4 ± 12.8 C: 61.2 ± 12.2
Sex: I: 123 (84.8%) F C: 51 (85%) F
Groups comparable at baseline? Yes |
Describe intervention (treatment/procedure/test):
100U BoNT-A, detrusor injections, plus oral placebo
After 6 weeks, patient could ask for increase of oral medication (placebo in this case).
At weeks 12, 18, or 24, patients could request and receive open-label onabotulinumtoxinA |
Describe control (treatment/procedure/test):
Placebo injections, plus oral placebo
After 6 weeks, patient could ask for increase of oral medication (placebo in this case)
At weeks 12, 18, or 24, patients could request and receive open-label onabotulinumtoxinA |
Length of follow-up: 12 weeks blinded, 24 weeks unblinded
Loss-to-follow-up/incomplete outcome data: Intervention: N=14 (9.7%) Reasons: Lack of efficacy: n=1 Lost to follow up: n=3 Adverse Events: n=5 Personal reasons: n=1 Other: n=4
Control: N=5 (8.3%) Reasons: Lost to follow up: n=2 Adverse Events: n=1 Other: n=2
|
Outcome measures and effect size (include 95%CI and p-value if available):
Adverse events: UTI: I: 37 (25.5%) C: 6 (10%)
Urinary retention: I: 10 (6.9%) C: 0%
Dry mouth: I: 2.8% C: 0%
Dysuria: I: 6 (4.1%) C: 2 (3.3%)
Bacteriuria: I: 11 (7.6%) C: 3 (5.0%)
Nasopharyngitis: I: 2 (1.4%) C: 3 (5.0%)
QoL: KHQ Role Limitations (change from baseline) I: -31.2 (-36.2, -26.2) C: -15.1 (-22.8, -7.4) P<0.001
Social Limitations domains (change from baseline) I: -13.7 (-16.9, -10.4) C: -6.1 (-11.2, -1.1) P=0.014 |
Oral placebo was administered to every patient because a 2nd intervention arm was present with oral medication, namely solifenacin (5/10mg) (plus placebo injection). |
|
Jabs, 2013 |
Type of study: single-institution, randomized, double-blind controlled trial
Setting and country: tertiary care hospital, Canada, February 2008 and September 2009
Funding and conflicts of interest: “Allergan Inc. (Markham, Ontario) provided an honorarium for a urologist experienced in the use of botulinum toxin to be the first author’s preceptor. Allergan Inc. also provided eight vials of botulinum toxin for the day of training. There was no financial support for this study.” |
Inclusion criteria: - women >18 years old - Clinical diagnosis of urinary urge incontinence with resistance to or intolerance of anticholinergic medication - Willingness and ability to use self-catheterization if necessary
Exclusion criteria: - Urinary urge incontinence secondary to neurologic disease - Known allergy or sensitivity to any of the components in the study medication - Pregnant and/or breast-feeding - The medical conditions of myasthenia gravis, Eaton-Lambert syndrome, or amytrophic lateral sclerosis - Symptomatic urinary retention or post-void residual of > 200 mL - Anticoagulation therapy - Familial bleeding disorder - Previous bladder pathology - Participation in another drug study - Previous botulinum toxin treatment for urological condition
N total at baseline: Intervention: 11 Control: 10
Important prognostic factors2: age ± SD: I: 63 ± 9.4 C: 63.8 ± 11.2
Sex: 100% F
Groups comparable at baseline? Yes |
Describe intervention (treatment/procedure/test):
100U BoNT-A intradetrusor, in 10 mL
|
Describe control (treatment/procedure/test):
Placebo (saline) injection |
Length of follow-up: 6 months
Loss-to-follow-up: none
Incomplete outcome data: none
|
Outcome measures and effect size (include 95%CI and p-value if available):
QoL: UDI-6 score (change from baseline) I: -28.5 C: -11.1 Mean change between groups: 17.4 (−1.9 to 36.6) |
‘’Five patients in the treatment group and seven in the control group continued to use anticholinergic medications throughout the study.’’ |
|
Liao, 2022 |
Type of study: multicenter, randomized, double-blind, placebo-controlled trial
Setting and country: April 2016 to December 2018, 17 sites across China
Funding and conflicts of interest: “This study was funded by the National Natural Science Foundation of China, Grant/Award Number: No. 81870523.” |
Inclusion criteria: - 18–75 years old with a primary diagnosis of idiopathic OAB - >8 micturitions per day
Exclusion criteria: - dysuria - post-void residual (PVR) - urine volume >50 ml with spontaneous micturition - longterm indwelling catheterization or CIC, - previous botulinum toxin treatment for any urologic condition within 6 months before screening - bladder or prostate cancer - any urologic abnormalities or diseases, such as urinary tract infection (UTI) and urolithiasis
N total at baseline: Intervention: 144 Control: 72
Important prognostic factors2: For example age ± SD: I: 47.75 ± 14.20 C: 46.39 ± 15.55
Sex: I: 117 (82.39%) F C: 61 (85.92%) F
Groups comparable at baseline? Yes |
Describe intervention (treatment/procedure/test):
100U (Hengli) BoNT-A
|
Describe control (treatment/procedure/test):
Placebo |
Length of follow-up: 24 weeks
Incomplete outcome data: Intervention: n=19 (13.2%) Reasons (describe): Adverse events: n=1 Lack of efficacy: n=1 Lost to follow up: n=3 Personal reasons: n=12 Other: n=2
Control: n=8 (11.1%) Reasons (describe): Adverse events: n=1 Lost to follow up: n=2 Personal reasons: n=4 Other: n=1
|
Outcome measures and effect size (include 95%CI and p-value if available):
QoL score (mean change from baseline) I: −1.00 (0.00, −2.00) C: −0.50 (0.00, −2.00)
Adverse events: UTI I: 19 (13.38) C: 6 (8.45)
Dysuria: I: 21 (14.79) C: 6 (8.45)
Haematuria I: 3 (2.11) C: 1 (1.41)
ALT (Alanine transaminase) elevation I: 3 (2.11) C: 0 (0) |
|
|
McCammon, 2021 |
Type of study: randomized, multicenter, placebo-controlled, phase IV study
Setting and country: 44 sites in the United States between November 2013 and January 2017
Funding and conflicts of interest: “This study and its analysis were sponsored by Allergan plc, Dublin, Ireland. Medical writing and editorial assistance was provided by Jennifer L. Giel, PhD, CMPP, and Karen Pemberton, PhD, CMPP, on behalf of Evidence Scientific Solutions, Inc., Philadelphia, PA, and was funded by Allergan plc, Dublin, Ireland. All authors met the ICMJE authorship criteria. Neither honoraria nor payments were made for authorship. Kurt McCammon has served as a consultant/ advisor and meeting participant/lecturer for AMS and Solace; as study investigator for AMS, Allergan plc, Astellas, Solace, and Uroplasty; and is a board member/officer/trustee for IVU med. Angelo Gousse has served as a consultant/advisor and investigator for Allergan plc and Precision Medical Devices. Alfred Kohan has been an investigator for Allergan plc, Ipsen, Myovant, and Watson; and a speaker for Allergan plc and Watson. David Glazier has been a study investigator for Allergan plc. Jennifer Gruenenfelder has served as a consultant and study investigator for Allergan plc. Zhanying Bai and Anand Patel are employees of Allergan plc. Douglass Hale has served as a consultant/advisor and meeting participant/ lecturer and has received research funding from Allergan plc.” |
Inclusion criteria: - Adults who had OAB for 6 months or longer - were inadequately managed by an anticholinergic - had recorded 3 or more episodes of urgency UI (UUI), 1 or less UUI-free day, and 24 or greater micturitions on a 3-day paper bladder diary during screening.
Exclusion criteria: - OAB caused by any neurologic condition; - predominance of stress UI, surgery, - disease other than OAB that might affect bladder function - PVR greater than 100 mL - and a 24-hour total urine void volume greater than 3000 mL during screening
N total at baseline: Intervention: 129 Control: 125
Important prognostic factors2: age ± SD: I: 60.8 ± 12.7 C: 60.9 ± 12.1
Sex: I: n=114 (88.4%) F C: n=112 (89.6%) F
Groups comparable at baseline? Yes |
Describe intervention (treatment/procedure/test):
100U BoNT-A, intradetrusor, 20 injections (0.5 mL each)
|
Describe control (treatment/procedure/test):
Placebo |
Length of follow-up: 12 weeks
Incomplete outcome data: Intervention: n=13 (10.1%) Reasons (describe): Adverse events: n=2 Lost to follow up: n=4 Personal reasons: n=4 Other: n=3
Control: n=4 (3.2%) Reasons (describe): Personal reasons: n=2 Protocol violation: n=1 Other: n=1
|
Outcome measures and effect size (include 95%CI and p-value if available):
QoL KHQ Incontinence impact domain (change from baseline) I: -34.4 C: -13.5
KHQ Role Limitations (change from baseline) I: -37.8 C: -16.7
KHQ Social Limitations (change from baseline) I: -20.4 C: -6.0 MID is -5.0
Adverse events: UTI I: 27 (21.1%) C: 8 (6.4%)
Urinary retention I: 8 (6.3%) C: 0
Dysuria: I: 6 (4.7%) C: 3 (2.4%)
Bronchitis I: 1 (0.8%) C: 4 (3.2%)
Cough I: 0 C: 4 (3.2%) |
|
|
Yokoyama, 2020 |
Type of study: phase III, randomized, double-blind, placebo-controlled trial
Setting and country: August 2016 to November 2018 at 53 sites in Japan
Funding and conflicts of interest: GlaxoSmithKline sponsored and provided funding for the study. |
Inclusion criteria: - ≥20 years with OAB - three or more episodes of UUI - a mean of eight or more micturitions per day in a 3-day diary
Exclusion criteria: - previous botulinum toxin treatment for any urological condition - stress-dominant mixed UI - any diseases, functional abnormalities or bladder surgery that might affect bladder function - a PVR urine volume >100 mL
N total at baseline: Intervention: 124 Control: 124
Important prognostic factors2: age ± SD: I: 65.6 ± 12.43 C: 66.2 ± 12.19
Sex: I: 94 (76%) F C: 92 (74%) F
Groups comparable at baseline? Yes |
Describe intervention (treatment/procedure/test):
100U BoNT-A, intradetrusor, 20 injections (0.5 mL each)
|
Describe control (treatment/procedure/test):
Placebo |
Length of follow-up: 48 weeks
Incomplete outcome data: Intervention: n=9 (7.3%) Reasons (describe): Protocol deviation: n=2 Withdrew consent: n=3 Adverse event: n=2 Reached protocol defined stopping criteria n=2
Control: n=5 (4.0%) Reasons (describe) Adverse event: n=1 Withdrew consent: n=3 Reached protocol defined stopping criteria n=1 |
Outcome measures and effect size (include 95%CI and p-value if available):
UUI episodes (adjusted mean change from baseline ± SE) I: -3.13 ± 0.370 C: -1.02 ± 0.363 Difference: -2.12 (-3.07 to -1.17)
QoL KHQ Role Limitations (mean change from baseline ± SE) I: -21.09 ± 2.997 C: -6.48 ± 2.976
KHQ Social Limitations (mean change from baseline ± SE) I: -12.36 ± 2.983 C: -4.95 ± 2.945
Adverse events: UTI I: 16 (13%) C: 9 (7%)
Nasopharyngitis I: 15 (12%) C: 11 (9%)
Dysuria: I: 12 (10%) C: 3 (2%)
Urinary retention: I: 7 (6%) C: 2 (2%) |
|
Risk of bias tables
Systematic review
|
Study
First author, year |
Appropriate and clearly focused question?
Yes/no/unclear |
Comprehensive and systematic literature search?
Yes/no/unclear |
Description of included and excluded studies?
Yes/no/unclear |
Description of relevant characteristics of included studies?
Yes/no/unclear |
Appropriate adjustment for potential confounders in observational studies?
Yes/no/unclear/not applicable |
Assessment of scientific quality of included studies?
Yes/no/unclear |
Enough similarities between studies to make combining them reasonable?
Yes/no/unclear |
Potential risk of publication bias taken into account?
Yes/no/unclear |
Potential conflicts of interest reported?
Yes/no/unclear |
|
Ramos, 2016 |
Yes |
Yes |
Yes |
Yes |
Not applicable |
Yes |
Yes |
No |
Yes |
Intervention studies
|
Study reference
(first author, publication year) |
Was the allocation sequence adequately generated?
Definitely yes Probably yes Probably no Definitely no |
Was the allocation adequately concealed?
Definitely yes Probably yes Probably no Definitely no |
Blinding: Was knowledge of the allocated interventions adequately prevented?
Were patients blinded?
Were healthcare providers blinded?
Were data collectors blinded?
Were outcome assessors blinded?
Were data analysts blinded?
Definitely yes Probably yes Probably no Definitely no |
Was loss to follow-up (missing outcome data) infrequent?
Definitely yes Probably yes Probably no Definitely no |
Are reports of the study free of selective outcome reporting?
Definitely yes Probably yes Probably no Definitely no |
Was the study apparently free of other problems that could put it at a risk of bias?
Definitely yes Probably yes Probably no Definitely no |
Overall risk of bias If applicable/necessary, per outcome measure
Low Some concerns High
|
|
Herschorn, 2017 |
Probably yes
Reason: “Randomization was stratified by center and number of UUI episodes (≤ 9 or >9 as recorded in a 3-day diary) at baseline” |
Probably yes
Reason: no information in text. |
Probably no
Reason: Blinding was ended at 12 weeks. No information on blinding of data analysts |
Probably yes
Reason: more missing outcomes due to adverse events in intervention group, although overall numbers are equal. |
Definitely yes
Reason: similar outcomes as described in the protocol. |
Probably no
Reason: study and publication was sponsored by pharmaceutical company Allergan |
Some concerns
Reason: Blinding was ended after 12 weeks (follow-up until 24 weeks). Results might be biased. |
|
Jabs, 2013 |
Definitely yes
Reason: a 1:1 allocation scheme was used. |
Definitely yes
Reason: a concealed envelope was used. |
Definitely yes
Reason: patient and surgeon were blinded until six months (end of trial). Only the nurse (preparing the injection) was aware of the intervention. |
Definitely yes
Reason: no loss to follow up. |
Probably yes
Reason: no information in text. |
Probably yes
Reason: although Allergan has provided for BoNT-A, no financial support was granted. No further influence of the industry.
|
Low
Reason: no concerns |
|
Liao, 2022 |
Definitely yes
Reason: an Interactive Web Response System (IWRS) was used for randomization. |
Probably yes
Reason: no information in text. |
Definitely yes
Reason: patients and researchers were blinded. |
Probably yes
Reason: more missing outcomes due to personal reasons in intervention group, although overall numbers are equal. |
Probably yes
Reason: no information in text. |
No information |
Low
Reason: no concerns |
|
McCammon, 2021 |
Definitely yes
Reason: an interactive voice response/ interactive web response system was used. |
Probably yes
Reason: no information in text. |
Definitely no
Reason: no evidence of blinding |
Probably no
Reason: numbers are not close (more in intervention group) |
Probably yes
Reason: no information in text. |
Definitely no
Reason: study and publication was sponsored by pharmaceutical company Allergan |
High
Reason: no blinding was performed. |
|
Yokoyama, 2020 |
Probably yes
Reason: no information in text. |
Probably yes
Reason: no information in text. |
Probably yes
Reason: double-blind RCT. Although not specifically mentioned blinding of analysts. |
Probably yes
Reason: some differences in incomplete outcomes. |
Definitely yes
Reason: similar outcomes as described in the protocol. |
No information |
Low
Reason: no concerns
|
Table of excluded studies
|
Reason for exclusion |
|
|
Abdelwahab, 2015 |
Wrong study design (prospective cohort) |
|
Abrar, 2021 |
Wrong study design (no comparison with placebo, predictors) |
|
Al-Azzawi, 2020 |
Wrong study design (no comparison with placebo) |
|
Allahdin, 2012 |
Exclusion, broad focus treatment options |
|
Amundsen, 2018 |
Wrong study design (no comparison with placebo); wrong outcome (economics) |
|
Anger, 2010 |
Exclusion, very old, 23 articles however only 3 RCTs |
|
Apostolidis, 2014 |
Wrong study design (no comparison with placebo) |
|
Arruda, 2018 |
Exclusion, better systematic review available |
|
Balzarro, 2019 |
Exclusion, impact OAB on female sexual function |
|
Bijnens, 2021 |
Wrong publication type (conference abstract) |
|
Broome, 2016 |
Wrong study design (no comparison with placebo) |
|
Chai, 2017 |
Wrong study design (reviewing assessment methods) |
|
Chancellor, 2013 |
Exclusion, broad focus patient group |
|
Chapple, 2013 |
Included in systematic review |
|
Chibelean, 2015 |
Exclusion, no meta-analysis, focus on inclusion RCTs and SRs |
|
Chua, 2015 |
Exclusion, not conform PICO different focus beta-3 adrenergic receptor agonists |
|
Chuang, 2014 |
Wrong comparison (no onabotulinumtoxin A) |
|
Cooley, 2019 |
Exclusion, wrong study design, prospective studies |
|
Cox, 2014 |
Wrong study design (no comparison with placebo) |
|
Cruz, 2014 |
Wrong population (neurogenic OAB) |
|
Cui, 2021 |
Wrong study design (no comparison with placebo) |
|
Cui, 2013 |
Exclusion, better systematic review available |
|
Cui, 2015 |
Exclusion, better systematic review available |
|
Davis, 2015 |
Exclusion, not conform PICO, different focus site of injection |
|
De, 2022 |
Wrong study design (prospective cohort) |
|
De, 2019 |
Wrong study design (no comparison with placebo) |
|
Dequirez, 2021 |
Wrong study design (review on other interventions) |
|
Doherty, 2019 |
Exclusion, specific P detrusor overactivity recalcitrant to initial injections |
|
Drake, 2017 |
Exclusion, better systematic review available |
|
Duthie, 2011 |
Exclusion, Duthie 2011 is an update |
|
Duthie, 2011 |
Exclusion, review Duthie was already in NVU guideline 2014 |
|
Eldred-Evans, 2017 |
Exclusion, wrong study design, no systematic review |
|
El-Hefnawy, 2021 |
Wrong study design (no comparison with placebo) |
|
Everaert, 2015 |
Wrong study design (no comparison with placebo) |
|
Freemantle, 2016 |
Wrong study design (no comparison with placebo); wrong outcome (economics); better systematic review available |
|
Geoffrion, 2018 |
Exclusion, pharmacotherapy |
|
Gerjevic, 2021 |
Wrong study design (no comparison with placebo); wrong outcome (economics) |
|
Ginsberg, 2017 |
Wrong study design (no comparison with placebo) |
|
Gong, 2020 |
Exclusion, also patients with neurogenic detrusor overactivity. Only reported Episodes of Urinary Incontinence (UI) per Week for NDO and not for IOAB. |
|
Gonzalez, 2021 |
Wrong study design (prospective cohort) |
|
Gu, 2017 |
Exclusion, outcome UI episodes per week, only reported NDO (neurogenic detrusor overactivity) |
|
Hamid, 2021 |
Exclusion, wrong study design, prospective study |
|
Harvie, 2020 |
wrong study design (no comparison with placebo); wrong outcome (economics) |
|
He, 2021 |
Exclusion, comparison Botox A and SNM |
|
Hendrickson, 2022 |
Wrong study design (prognostic prediction model) |
|
Hsieh, 2016 |
Exclusion, wrong study design, no systematic review |
|
Jambusaria, 2014 |
Exclusion, wrong study design, no systematic review |
|
Jo, 2018 |
Exclusion, not conform PICO, other focus site of injection |
|
Johnston, 2019 |
Maybe, quality of life in patients with overactive bladder |
|
Kim, 2016 |
Wrong study design (no comparison with placebo) |
|
Komesu, 2018 |
Wrong study design (no comparison with placebo) |
|
Kopcsay, 2022 |
Wrong study design (no comparison with placebo) |
|
Krhut, 2016 |
Wrong study design (no onabotulinumtoxin A) |
|
Ksibi, 2009 |
Exclusion, old, only 3 RCTs (low quality) |
|
Kuo, 2014 |
Wrong study design (not onabotulinumtoxin A) |
|
Kuo, 2020 |
Exclusion, wrong study design, editorial |
|
Lee, 2019 |
Exclusion, specific about minimally invasive intravesical injection and also patients with Interstitial Cystitis/Bladder Pain Syndrome included. |
|
Liao, 2016 |
Wrong study design (no comparison with placebo) |
|
Lo, 2020 |
Exclusion, better systematic review available |
|
Lozano-Ortega, 2019 |
Exclusion, no comparison with placebo |
|
MacDonald, 2007 |
Exclusion, old with 2 trials about Botox A |
|
Mangera, 2011 |
Exclusion, not conform PICO, Botox versus Dysport |
|
Mangera, 2014 |
Exclusion, P is too broad, review of a systematic review |
|
Manns, 2021 |
Wrong publication type (study protocol) |
|
Miotla, 2017 |
Wrong study design (no comparison with placebo) |
|
Moga, 2015 |
Wrong study design (no comparison with placebo) |
|
Murray, 2019 |
Wrong study design (no comparison with placebo); wrong outcome (economics) |
|
Ni, 2018 |
Exclusion, value of repeat injections |
|
Nitti, 2017 |
Included in systematic review |
|
Nitti, 2016 |
Wrong study design (prospective cohort) |
|
Nitti, 2021 |
Exclusion, broad focus interventions, Botox A in abstract |
|
Niu, 2018 |
Exclusion, comparison Botox A and SNM |
|
Nobrega, 2018 |
Wrong study design (no comparison with placebo) |
|
Olivera, 2016 |
Maybe, focus on Non antimuscarinic treatment (broad focus, no meta-analysis) |
|
Orasanu, 2013 |
Exclusion, wrong study design, no systematic review |
|
Owen, 2017 |
Wrong outcome (prediction model) |
|
Rachaneni, 2017 |
Exclusion, not conform PICO, other focus. OAB with versus without detrusor overactivity |
|
Seth, 2013 |
Maybe, broad focus on patients with lower urinary tract dysfunction (LUTD) |
|
Shawer, 2022 |
Wrong study design (no comparison with placebo) |
|
Sherif, 2017 |
Wrong study design (no comparison with placebo) |
|
Sievert, 2014 |
Included in systematic review |
|
Stamm, 2018 |
Exclusion, inconsistency definitions |
|
Sun, 2015 |
Exclusion, search period unclear 'last 5 years' |
|
Suskind, 2019 |
Wrong study design (prospective cohort) |
|
Tubaro, 2015 |
Maybe, a comparison with placebo? Systematic review? |
|
Tyagi, 2017 |
Exclusion, not conform PICO, other focus. Including injection-free onabotulinumtoxin A chemodenervation |
|
Van, 2015 |
Exclusion, comparison several types of Botox A in broad patient group |
|
Visco, 2016 |
Wrong study design (no comparison with placebo) |
|
Visco, 2016 |
Wrong study design (no comparison with placebo); wrong outcome (economics) |
|
Werneburg, 2022 |
Wrong study design (no comparison with placebo) |
|
Wu, 2019 |
Exclusion, neurogenic detrusor overactivity |
|
Yuan, 2017 |
Exclusion, neurogenic detrusor overactivity |
Verantwoording
Beoordelingsdatum en geldigheid
Publicatiedatum : 13-11-2023
Beoordeeld op geldigheid : 07-11-2023
Algemene gegevens
De ontwikkeling/herziening van deze richtlijnmodule werd ondersteund door het Kennisinstituut van de Federatie Medisch Specialisten (www.demedischspecialist.nl/kennisinstituut) en werd gefinancierd uit de Kwaliteitsgelden Medisch Specialisten (SKMS). De financier heeft geen enkele invloed gehad op de inhoud van de richtlijnmodules.
Samenstelling werkgroep
Voor het ontwikkelen van de richtlijnmodules zijn in 2020 per module schrijvers en meelezers benoemd. Deze personen werden aangewezen als vertegenwoordigers door de relevante beroepsgroepen die betrokken zijn bij de in de module beschreven zorg (zie hiervoor de Samenstelling van de werkgroep). Alle schrijvers van modules vallend onder één richtlijn vormden samen een schrijfgroep. Alle meelezers van modules vallend onder één richtlijn vormden samen een clusterwerkgroep. In totaal resulteerde dit dus in zes werkgroep en zes clusterwerkgroepen.
Voorzitter project (technisch voorzitter)
Timmermans A. (Anne), gynaecoloog, AmsterdamUMC, NVOG
Werkgroep Urine-incontinentie bij vrouwen
Engberts M.K. (Marian), urogynaecoloog, Isala Ziekenhuis te Zwolle, NVOG
Klerkx W.M. (Wenche), urogynaecoloog, St. Antonius Ziekenhuis te Utrecht, NVOG
Koldewijn E.L. (Evert), uroloog, Catharina Ziekenhuis te Eindhoven, NVU
Labrie J. (Julien), gynaecoloog, Spaarne Gasthuis te Haarlem, NVOG
Martens F. (Frank), uroloog, Radboudumc te Nijmegen, NVU
Steures P. (Pieternel), urogynaecoloog, Jeroen Bosch Ziekenhuis te Den Bosch, NVOG
Clusterwerkgroep Urine-incontinentie bij vrouwen
Adamse C. (Corine), geregistreerd bekkenfysiotherapeut en klinisch epidemioloog, Antonius Ziekenhuis Sneek, docent Master opleiding Bekkenfysiotherapie, SOMT Amersfoort, NVFB/KNGF
Bosch M. (Marlies), patiëntvertegenwoordiger, Bekkenbodem4all
Dos Santos A. (Ana), bekkenfysiotherapeut MSc, PelviCentrum te Leiden, NVFB/KNGF
Lagro-Janssen A.L.M. (Toine), prof, huisarts n.p., RadboudUMC Nijmegen, NHG
Teunissen T.A.M. (Doreth), huisarts, RadboudUMC te Nijmegen, NHG
Ondersteuning project
Abdollahi M. (Mohammadreza), adviseur Kennisinstituut van de Federatie van Medisch Specialisten
Labeur Y.J. (Yvonne), adviseur, Kennisinstituut van de Federatie Medisch Specialisten
Sussenbach A.E. (Annelotte), junior adviseur Kennisinstituut van de Federatie van Medisch Specialisten
Verhoeven M. (Maxime), adviseur, Kennisinstituut van de Federatie van Medisch Specialisten
Projectleiding
Augustus 2022- nu Mostovaya I.M. (Irina) (projectleider), senior adviseur, Kennisinstituut van de Federatie Medisch Specialisten
April 2020 tot augustus 2021: Bijlsma-Rutte A. (Anne), adviseur, Kennisinstituut van de Federatie van Medisch Specialisten
September 2021 tot januari 2022: Venhorst K. (Kristie), adviseur, Kennisinstituut van de Federatie van Medisch Specialisten
Februari 2022 tot juni 2022: Göthlin M. (Mattias), adviseur, Kennisinstituut van de Federatie van Medisch Specialisten
Belangenverklaringen
De Code ter voorkoming van oneigenlijke beïnvloeding door belangenverstrengeling is gevolgd. Alle werkgroepleden hebben schriftelijk verklaard of zij in de laatste drie jaar directe financiële belangen (betrekking bij een commercieel bedrijf, persoonlijke financiële belangen, onderzoek financiering) of indirecte belangen (persoonlijke relaties, reputatiemanagement) hebben gehad. Gedurende de ontwikkeling of herziening van een module worden wijzigingen in belangen doorgegeven. De belangenverklaring wordt opnieuw bevestigd tijdens de commentaarfase.
Een overzicht van de belangen van werkgroepleden en het oordeel over het omgaan met eventuele belangen vindt u in onderstaande tabel. De ondertekende belangenverklaringen zijn op te vragen bij het secretariaat van het Kennisinstituut van de Federatie Medisch Specialisten.
|
Werkgroeplid |
Functie |
Nevenfuncties |
Gemelde belangen |
Ondernomen actie |
|
Timmermans (technisch voorzitter van het project) |
Gynaecoloog, Amsterdam UMC (0.5 fte) |
Commissie kwaliteitsdocumenten NVOG (onbetaald); projectgroep Gynae Goes Green NVOG (onbetaald) |
Geen |
Geen actie |
|
Urine-incontinentie bij vrouwen - werkgroep |
||||
|
Engberts |
Urogynaecoloog ISALA |
Trainer Altis ® Sling voor Coloplast, betaald |
Geen |
Niet betrokken bij de besluitvorming rondom (fasci)slings. |
|
Klerkx |
Urogynaecoloog, St. Antonius Ziekenhuis |
Geen |
Geen |
Geen actie |
|
Koldewijn |
Uroloog 100% Catharina ziekenhuis Eindhoven |
Voorzitter Stichting Opleidingen Medici (SOM). Stichting acquireert gelden voor promotieonderzoek: onbetaald |
Geen |
Geen actie |
|
Labrie |
Gynaecoloog Spaarne Gasthuis |
Medisch Manager vakgroep gynaecologie gevaceerd |
Geen |
Geen actie |
|
Martens |
Uroloog, radboudumc |
Geen |
OASIS trial, implantaat PTNS, BlueWind, multicenter, PI Nijmegen. |
Geen trekker van module over PTNS/TENS. |
|
Steures |
Urogynaecoloog Jeroen Bosch Ziekenhuis, Den Bosch |
Geen |
Geen |
Geen actie |
|
Urine-incontinentie bij vrouwen - clusterwerkgroep |
||||
|
Adamse |
Geregistreerd bekkenfysiotherapeut en klinisch epidemioloog, Antonius Ziekenhuis Sneek. |
Commissielid Wetenschapscommissie NVFB Commissielid Richtlijn chronische bekkenpijn FMS Docent Master opleiding Bekkenfysiotherapie, SOMT Amersfoort |
Geen |
Geen actie |
|
Bosch |
Zie boven |
Zie boven |
Zie boven |
Zie boven |
|
Dos Santos |
Geregistreerd bekkenfysiotherapeut MSc bij PelviCentrum - Centrum voor Bekkenfysiotherapie Leiden |
Lid van NVFB Wetenschappelijke Commissie. Vergoeding van de reiskosten en bijwonen van vergaderingen. |
Deelname aan het ontwikkelen van de richtlijn kan ervoor zorgen dat collega vaker gaan verwijzen naar mijn praktijk vanwege meer bekendheid. Mogelijk belangen bij bescherming positie bekkenfysiotherapie. |
Geen actie |
|
Lagro-Janssen |
Geen werkgever |
Adviseur centrum Seksueel Geweld Gelderland-Zuid en Midden (onbetaald) |
Geen |
Geen actie |
|
Teunissen |
Huisarts, zelfstandig 0,6 fte Docent, senior onderzoeker Radboudumc afdeling eerstelijnsgeneeskunde 0,4 fte |
Huisarts -> huisartswerkzaamheden (betaald) Radboudumc -> docent senior onderzoeker (betaald) |
Geen |
Geen actie |
Inbreng patiëntenperspectief
Er werd aandacht besteed aan het patiëntenperspectief door het uitnodigen van Patiëntenfederatie Nederland en Stichting Bekkenbodem4All voor de schriftelijke knelpunteninventarisatie en voor deelname aan de clusterwerkgroepen. De verkregen input is meegenomen bij het opstellen van de uitgangsvragen, de keuze voor de uitkomstmaten en bij het opstellen van de overwegingen. De richtlijn is voor commentaar voorgelegd aan Patiëntenfederatie Nederland en Stichting Bekkenbodem4All en de eventueel aangeleverde commentaren worden bekeken en verwerkt.
Werkwijze
AGREE
Deze richtlijnmodule is opgesteld volgens de eisen vermeld in het rapport Medisch Specialistische Richtlijnen 2.0 van de adviescommissie Richtlijnen van de Raad Kwaliteit. Dit rapport is gebaseerd op het AGREE II instrument (Appraisal of Guidelines for Research & Evaluation II; Brouwers, 2010).
Knelpuntenanalyse en uitgangsvragen
Tijdens de voorbereidende fase inventariseerden de werkgroep de knelpunten in de zorg en de actualiteit van de aanbevelingen beschreven in de te reviseren modules. Tevens zijn er knelpunten aangedragen door de Nederlandse Vereniging voor Obstetrie en Gynaecologie (NVOG), de Nederlandse Vereniging van Maag-Darm-Leverartsen (NVMDL), Vereniging Klinische Genetica Nederland (VKGN), Inspectie Gezondheidszorg en Jeugd (IGJ), Koninklijke Nederlandse Organisatie van Verloskundigen (KNOV), Nederlands Huisartsen Genootschap (NHG), Nederlandse Vereniging voor Bekkenfysiotherapie (NVFB) / Koninklijk Nederlands Genootschap voor Fysiotherapie (KNGF), Nederlandse Vereniging van Ziekenhuizen (NVZ), Patiëntenfederatie Nederland (PFN), Zorginstituut Nederland (ZiNL), Zelfstandige Klinieken Nederland (ZKN) en Zorgverzekeraars Nederland (ZN) via een schriftelijke knelpunteninventarisatie.
Op basis van de uitkomsten van de knelpuntenanalyse zijn door de werkgroep concept-uitgangsvragen opgesteld en definitief vastgesteld.
Uitkomstmaten
Na het opstellen van de zoekvraag behorende bij de uitgangsvraag inventariseerde de werkgroep welke uitkomstmaten voor de patiënt relevant zijn, waarbij zowel naar gewenste als ongewenste effecten werd gekeken. Hierbij werd een maximum van acht uitkomstmaten gehanteerd. De werkgroep waardeerde deze uitkomstmaten volgens hun relatieve belang bij de besluitvorming rondom aanbevelingen, als cruciaal (kritiek voor de besluitvorming), belangrijk (maar niet cruciaal) en onbelangrijk. Ook definieerde de werkgroep tenminste voor de cruciale uitkomstmaten welke verschillen zij klinisch (patiënt) relevant vonden.
Methode literatuursamenvatting
Een uitgebreide beschrijving van de strategie voor zoeken en selecteren van literatuur en de beoordeling van de risk-of-bias van de individuele studies is te vinden onder ‘Zoeken en selecteren’ onder Onderbouwing. De beoordeling van de kracht van het wetenschappelijke bewijs wordt hieronder toegelicht.
Beoordelen van de kracht van het wetenschappelijke bewijs
De kracht van het wetenschappelijke bewijs werd bepaald volgens de GRADE-methode. De basisprincipes van de GRADE-methodiek zijn: het benoemen en prioriteren van de klinisch (patiënt) relevante uitkomstmaten, een systematische review per uitkomstmaat, en een beoordeling van de bewijskracht per uitkomstmaat op basis van de acht GRADE-domeinen (domeinen voor downgraden: risk of bias, inconsistentie, indirectheid, imprecisie, en publicatiebias; domeinen voor upgraden: dosis-effect relatie, groot effect, en residuele plausibele confounding).
GRADE onderscheidt vier gradaties voor de kwaliteit van het wetenschappelijk bewijs: hoog, redelijk, laag en zeer laag. Deze gradaties verwijzen naar de mate van zekerheid die er bestaat over de literatuurconclusie, in het bijzonder de mate van zekerheid dat de literatuurconclusie de aanbeveling adequaat ondersteunt (Hultcrantz, 2017; Schünemann, 2013).
|
GRADE |
Definitie |
|
Hoog |
|
|
Redelijk |
|
|
Laag |
|
|
Zeer laag |
|
Bij het beoordelen (graderen) van de kracht van het wetenschappelijk bewijs in richtlijnen volgens de GRADE-methodiek spelen grenzen voor klinische besluitvorming een belangrijke rol (Hultcrantz, 2017). Dit zijn de grenzen die bij overschrijding aanleiding zouden geven tot een aanpassing van de aanbeveling. Om de grenzen voor klinische besluitvorming te bepalen moeten alle relevante uitkomstmaten en overwegingen worden meegewogen. De grenzen voor klinische besluitvorming zijn daarmee niet één op één vergelijkbaar met het minimaal klinisch relevant verschil (Minimal Clinically Important Difference, MCID). Met name in situaties waarin een interventie geen belangrijke nadelen heeft en de kosten relatief laag zijn, kan de grens voor klinische besluitvorming met betrekking tot de effectiviteit van de interventie bij een lagere waarde (dichter bij het nul effect) liggen dan de MCID (Hultcrantz, 2017).
Overwegingen (van bewijs naar aanbeveling)
Om te komen tot een aanbeveling zijn naast (de kwaliteit van) het wetenschappelijke bewijs ook andere aspecten belangrijk en worden meegewogen, zoals aanvullende argumenten uit bijvoorbeeld de biomechanica of fysiologie, waarden en voorkeuren van patiënten, kosten (middelenbeslag), aanvaardbaarheid, haalbaarheid en implementatie. Deze aspecten zijn systematisch vermeld en beoordeeld (gewogen) onder het kopje ‘Overwegingen’ en kunnen (mede) gebaseerd zijn op expert opinion. Hierbij is gebruik gemaakt van een gestructureerd format gebaseerd op het evidence-to-decision framework van de internationale GRADE Working Group (Alonso-Coello, 2016a; Alonso-Coello 2016b). Dit evidence-to-decision framework is een integraal onderdeel van de GRADE-methodiek.
Formuleren van aanbevelingen
De aanbevelingen geven antwoord op de uitgangsvraag en zijn gebaseerd op het beschikbare wetenschappelijke bewijs en de belangrijkste overwegingen, en een weging van de gunstige en ongunstige effecten van de relevante interventies. De kracht van het wetenschappelijk bewijs en het gewicht dat door de werkgroep wordt toegekend aan de overwegingen, bepalen samen de sterkte van de aanbeveling. Volgens de GRADE-methodiek sluit een lage bewijskracht van conclusies in de systematische literatuuranalyse een sterke aanbeveling niet a priori uit, en zijn bij een hoge bewijskracht ook zwakke aanbevelingen mogelijk (Agoritsas, 2017; Neumann, 2016). De sterkte van de aanbeveling wordt altijd bepaald door weging van alle relevante argumenten tezamen. De werkgroep heeft bij elke aanbeveling opgenomen hoe zij tot de richting en sterkte van de aanbeveling zijn gekomen.
In de GRADE-methodiek wordt onderscheid gemaakt tussen sterke en zwakke (of conditionele) aanbevelingen. De sterkte van een aanbeveling verwijst naar de mate van zekerheid dat de voordelen van de interventie opwegen tegen de nadelen (of vice versa), gezien over het hele spectrum van patiënten waarvoor de aanbeveling is bedoeld. De sterkte van een aanbeveling heeft duidelijke implicaties voor patiënten, behandelaars en beleidsmakers (zie onderstaande tabel). Een aanbeveling is geen dictaat, zelfs een sterke aanbeveling gebaseerd op bewijs van hoge kwaliteit (GRADE gradering HOOG) zal niet altijd van toepassing zijn, onder alle mogelijke omstandigheden en voor elke individuele patiënt.
|
Implicaties van sterke en zwakke aanbevelingen voor verschillende richtlijngebruikers |
||
|
|
Sterke aanbeveling |
Zwakke (conditionele) aanbeveling |
|
Voor patiënten |
De meeste patiënten zouden de aanbevolen interventie of aanpak kiezen en slechts een klein aantal niet. |
Een aanzienlijk deel van de patiënten zouden de aanbevolen interventie of aanpak kiezen, maar veel patiënten ook niet. |
|
Voor behandelaars |
De meeste patiënten zouden de aanbevolen interventie of aanpak moeten ontvangen. |
Er zijn meerdere geschikte interventies of aanpakken. De patiënt moet worden ondersteund bij de keuze voor de interventie of aanpak die het beste aansluit bij zijn of haar waarden en voorkeuren. |
|
Voor beleidsmakers |
De aanbevolen interventie of aanpak kan worden gezien als standaardbeleid. |
Beleidsbepaling vereist uitvoerige discussie met betrokkenheid van veel stakeholders. Er is een grotere kans op lokale beleidsverschillen. |
Organisatie van zorg
In de knelpuntenanalyse en bij de ontwikkeling van de richtlijnmodule is expliciet aandacht geweest voor de organisatie van zorg: alle aspecten voor het verlenen van zorg (zoals coördinatie, communicatie, (financiële) middelen, mankracht en infrastructuur). Randvoorwaarden die relevant zijn voor het beantwoorden van deze specifieke uitgangsvraag zijn genoemd bij de overwegingen.
Commentaar- en autorisatiefase
De conceptrichtlijnmodule werd aan de betrokken (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd ter commentaar. De commentaren worden verzameld en besproken met de werkgroep. Naar aanleiding van de commentaren wordt de conceptrichtlijnmodule aangepast en definitief vastgesteld door de werkgroep. De definitieve richtlijnmodule wordt aan de deelnemende (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd voor autorisatie en akkoord.
Literatuur
Agoritsas T, Merglen A, Heen AF, Kristiansen A, Neumann I, Brito JP, Brignardello-Petersen R, Alexander PE, Rind DM, Vandvik PO, Guyatt GH. UpToDate adherence to GRADE criteria for strong recommendations: an analytical survey. BMJ Open. 2017 Nov 16;7(11):e018593.
Alonso-Coello P, Schünemann HJ, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Rada G, Rosenbaum S, Morelli A, Guyatt GH, Oxman AD; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ. 2016 Jun 28;353:i2016.
Alonso-Coello P, Oxman AD, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Vandvik PO, Meerpohl J, Guyatt GH, Schünemann HJ; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 2: Clinical practice guidelines. BMJ. 2016 Jun 30;353:i2089.
Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, Fervers B, Graham ID, Grimshaw J, Hanna SE, Littlejohns P, Makarski J, Zitzelsberger L; AGREE Next Steps Consortium. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010 Dec 14;182(18):E839-42.
Hultcrantz M, Rind D, Akl EA, Treweek S, Mustafa RA, Iorio A, Alper BS, Meerpohl JJ, Murad MH, Ansari MT, Katikireddi SV, Östlund P, Tranæus S, Christensen R, Gartlehner G, Brozek J, Izcovich A, Schünemann H, Guyatt G. The GRADE Working Group clarifies the construct of certainty of evidence. J Clin Epidemiol. 2017 Jul;87:4-13.
Medisch Specialistische Richtlijnen 2.0 (2012). Adviescommissie Richtlijnen van de Raad Kwaliteit.
Neumann I, Santesso N, Akl EA, Rind DM, Vandvik PO, Alonso-Coello P, Agoritsas T, Mustafa RA, Alexander PE, Schünemann H, Guyatt GH. A guide for health professionals to interpret and use recommendations in guidelines developed with the GRADE approach. J Clin Epidemiol. 2016 Apr;72:45-55.
Schünemann H, Brożek J, Guyatt G, et al. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group, 2013.
Schünemann HJ, Oxman AD, Brozek J, Glasziou P, Jaeschke R, Vist GE, Williams JW Jr, Kunz R, Craig J, Montori VM, Bossuyt P, Guyatt GH; GRADE Working Group. Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. BMJ. 2008 May 17;336(7653):1106-10. Erratum in: BMJ. 2008 May 24;336(7654).
Wessels M, Hielkema L, van der Weijden T. How to identify existing literature on patients' knowledge, views, and values: the development of a validated search filter. J Med Libr Assoc. 2016 Oct;104(4):320-324.
Zoekverantwoording
Literatuur zoekstrategie
Algemene informatie
|
Richtlijn: Richtlijn Urine Incontinentie >> herziening 4 modules in het ’24 modules project’ |
|
|
Uitgangsvraag: Wat is de effectiviteit van botuline A en B bij vrouwen met aandrangincontinentie? |
|
|
Database(s): Medline (OVID), Embase |
Datum: 26-02-2021 |
|
Periode: >2006 |
Talen: Engels, Nederlands |
|
Literatuurspecialist: Laura Boerboom |
|
|
BMI zoekblokken: voor verschillende opdrachten wordt (deels) gebruik gemaakt van de zoekblokken van BMI-Online https://blocks.bmi-online.nl/ Bij gebruikmaking van een volledig zoekblok zal naar de betreffende link op de website worden verwezen. |
|
|
Toelichting en opmerkingen:
→ Voor deze vraag is gezocht op de elementen aandrangincontinentie (in het blauw) en Botuline toxine A en B (in het groen).
→ De werkgroep had twee sleutelpublicaties, en deze opgegeven artikelen van Duthie (2011) en Chapple (2013) komen uit onderstaande search.
|
|
|
Te gebruiken voor richtlijnen tekst: In de databases Embase (via embase.com) en Medline (via OVID) is op 26-02-2021 met relevante zoektermen gezocht naar systematische reviews, RCT’s en observationele studies over de effectiviteit van botuline A en B bij vrouwen met aandrangincontinentie. De literatuurzoekactie leverde 692 unieke treffers op. |
|
Zoekopbrengst
|
|
EMBASE |
OVID/MEDLINE |
Ontdubbeld |
|
SRs |
127 |
101 |
145 |
|
RCTs |
280 |
229 |
336 |
|
Observationele studies |
143 |
203 |
211 |
|
Totaal |
550 |
533 |
692 |
Updated search
|
Richtlijn: Richtlijn Urine Incontinentie >> herziening 4 modules in het ’24 modules project’ |
|
|
Uitgangsvraag: Wat is de effectiviteit van botuline A en B bij vrouwen met aandrangincontinentie? |
|
|
Database(s): Medline (OVID), Embase |
Datum: 21-03-2022 |
|
Periode: >2013 |
Talen: Engels, Nederlands |
|
Literatuurspecialist: Linda Niesink-Boerboom |
|
|
BMI zoekblokken: voor verschillende opdrachten wordt (deels) gebruik gemaakt van de zoekblokken van BMI-Online https://blocks.bmi-online.nl/ Bij gebruikmaking van een volledig zoekblok zal naar de betreffende link op de website worden verwezen. |
|
|
Toelichting en opmerkingen:
→ Voor deze vraag is gezocht op de elementen aandrangincontinentie (in het blauw) en Botuline toxine A en B (in het groen).
→ Dit betreft een update van een eerdere search op 26-02-2021. Nu zijn alle RCT’s >2013 meegenomen, en de nieuwe SR’s sinds de vorige search.
|
|
|
Te gebruiken voor richtlijnen tekst: In de databases Embase (via embase.com) en Medline (via OVID) is op 21-03-2022 met relevante zoektermen gezocht naar systematische reviews en RCT’s over de effectiviteit van botuline A en B bij vrouwen met aandrangincontinentie. De literatuurzoekactie leverde 172 unieke RCT’s op en nog 14 nieuwe SR’s. |
|
|
|
EMBASE |
OVID/MEDLINE |
Ontdubbeld |
|
SRs |
115 |
85 |
124 (waarvan 14 nieuw) |
|
RCTs |
172 |
157 |
172 |
|
Totaal |
|
|
|
Zoekstrategie (zelfde als bij oorspronkelijke search)
|
Database |
Zoektermen |
||||||||||||||||||||||||||||||||||||
|
Embase
|
|
||||||||||||||||||||||||||||||||||||
|
Medline (OVID)
|
1 exp Urinary Bladder, Overactive/ or exp Urinary Incontinence, Urge/ or 'bladder overactivity'.ti,ab,kf. or 'overactive bladder'.ti,ab,kf. or 'detrusor overactivity'.ti,ab,kf. or 'overactive detrusor'.ti,ab,kf. or 'overactive urinary bladder'.ti,ab,kf. or 'urge incontinence'.ti,ab,kf. or 'urinary incontinence'.ti,ab,kf. or 'urine incontinence'.ti,ab,kf. (34102) 2 exp Botulinum Toxins, Type A/ or onabotulinumtoxina.ti,ab,kf. or onabotulinumtoxinb.ti,ab,kf. or abobotulinumtoxina.ti,ab,kf. or abobotulinumtoxinb.ti,ab,kf. or ((botox or 'botulin toxin*' or 'botulinum toxin*' or onabotulinumtoxin) adj3 (a or b)).ti,ab,kf. (12442) 3 1 and 2 (1063) 4 limit 3 to ((english or dutch) and yr="2006 -Current") (914) 5 (meta-analysis/ or meta-analysis as topic/ or (metaanaly* or meta-analy* or metanaly*).ti,ab,kf. or systematic review/ or cochrane.jw. or (prisma or prospero).ti,ab,kf. or ((systemati* or scoping or umbrella or "structured literature") adj3 (review* or overview*)).ti,ab,kf. or (systemic* adj1 review*).ti,ab,kf. or ((systemati* or literature or database* or data-base*) adj10 search*).ti,ab,kf. or ((structured or comprehensive* or systemic*) adj3 search*).ti,ab,kf. or ((literature adj3 review*) and (search* or database* or data-base*)).ti,ab,kf. or (("data extraction" or "data source*") and "study selection").ti,ab,kf. or ("search strategy" and "selection criteria").ti,ab,kf. or ("data source*" and "data synthesis").ti,ab,kf. or (medline or pubmed or embase or cochrane).ab. or ((critical or rapid) adj2 (review* or overview* or synthes*)).ti. or (((critical* or rapid*) adj3 (review* or overview* or synthes*)) and (search* or database* or data-base*)).ab. or (metasynthes* or meta-synthes*).ti,ab,kf.) not (comment/ or editorial/ or letter/ or ((exp animals/ or exp models, animal/) not humans/)) (480847) 6 (exp clinical trial/ or randomized controlled trial/ or exp clinical trials as topic/ or randomized controlled trials as topic/ or Random Allocation/ or Double-Blind Method/ or Single-Blind Method/ or (clinical trial, phase i or clinical trial, phase ii or clinical trial, phase iii or clinical trial, phase iv or controlled clinical trial or randomized controlled trial or multicenter study or clinical trial).pt. or random*.ti,ab. or (clinic* adj trial*).tw. or ((singl* or doubl* or treb* or tripl*) adj (blind$3 or mask$3)).tw. or Placebos/ or placebo*.tw.) not (animals/ not humans/) (2086600) 7 Epidemiologic studies/ or case control studies/ or exp cohort studies/ or Controlled Before-After Studies/ or Case control.tw. or (cohort adj (study or studies)).tw. or Cohort analy$.tw. or (Follow up adj (study or studies)).tw. or (observational adj (study or studies)).tw. or Longitudinal.tw. or Retrospective*.tw. or prospective*.tw. or consecutive*.tw. or Cross sectional.tw. or Cross-sectional studies/ or historically controlled study/ or interrupted time series analysis/ [Onder exp cohort studies vallen ook longitudinale, prospectieve en retrospectieve studies] (3649108) 8 4 and 5 (101) – SR’s 9 (4 and 6) not 8 (229) – RCT’s 10 (4 and 7) not 8 not 9 (203) – Observationele studies 11 8 or 9 or 10 (533) |