Gebruik van LAMA
Uitgangsvraag
Wat is het effect van toevoegen van een LAMA aan de behandeling van ICS/LABA bij patiënten met niet-ernstig astma?
Aanbeveling
Overweeg een proefbehandeling LAMA bij patiënten met astma die aanhoudend ziektelast ervaren ondanks ICS/LABA (medium/high dosis).
Overwegingen
Voor- en nadelen van de interventie en de kwaliteit van het bewijs
Er werden vijf studies gevonden die het effect van het toevoegen van een LAMA aan behandeling met ICS en LABA bij patiënten met niet-ernstig astma onderzochten. Er zijn meerdere studies geïncludeerd die één device (ICS/LABA/LAMA) versus ICS/LABA hebben geïncludeerd en één studie heeft ICS/LABA + LAMA versus ICS/LABA geïncludeerd. Over het algemeen kan geconcludeerd worden dat het toevoegen van een LAMA naast een ICS/LABA combinatie de longfunctie minimaal wat verbetert (83 ml). Desondanks geeft het geen verbetering van de astma klachten (ACQ) danwel een reductie in het aantal astma exacerbaties. Er is geen duidelijke subgroep te definiëren die een grotere of juist geen respons laat zien van aanvullende behandeling met tiotropiumbromide (Kerstjens, 2016).
De bewijslast is matig tot laag van de studies die zijn geïncludeerd.
Waarden en voorkeuren van patiënten (en evt. hun verzorgers)
Voor de patiënt is het belangrijk dat zijn/haar astma zo stabiel mogelijk is met zo min mogelijk medicatie. Echter bij aanhoudende ziektelast zal een extra inhalatie geen grote belasting zijn voor patiënten die reeds bekend zijn met het gebruik van inhalatie medicatie
Kosten (middelenbeslag)
Het toevoegen van inhalatie medicatie zal kosten met zich meebrengen. Een LAMA generiek kost €0.87-1.13 per dag.
Aanvaardbaarheid, haalbaarheid en implementatie
Het toevoegen van een extra inhalatie is haalbaar en zal niet een belemmering zijn bij patiënten die reeds bekend zijn met het gebruik van inhalatiemedicatie. Vanwege het ontbreken van sterke bewijslast is de werkgroep van mening dat in sommige gevallen toch een proefbehandeling met een LAMA kan worden overwogen. Voorkeur heeft een proefbehandeling van maximaal 3 maanden met een LAMA, welke toegevoegd wordt aan de reeds bestaande ICS/LABA. Monitoring kan plaatsvinden middels ACQ, spirometrie en anamnestisch welbevinden van de patiënt. Wanneer een positief effect van de toevoeging van de LAMA wordt gevonden, heeft het de voorkeur om de inhalatie devices los van elkaar te blijven voorschrijven. Dit om MART toe te kunnen blijven passen. De mogelijkheid van het MART toepassen van een triple-combinatie is niet onderzocht.
Rationale van de aanbeveling: weging van argumenten voor en tegen de interventies
Bij patiënten met ernstig astma geeft het toevoegen van een LAMA naast ICS/LABA een exacerbatie reductie (Horita 2023). In de uitgangsvraag richten wij ons specifiek op niet-ernstig astma. De bewijsvoering laat geen voordeel zien van het toevoegen van LAMA op exacerbaties en de ACQ. Er is een verbetering van FEV1, maar de werkgroep acht deze niet klinisch relevant. Vanwege het ontbreken van sterke bewijslast is de werkgroep van mening dat in sommige gevallen toch een proefbehandeling met een LAMA kan worden overwogen.
Of een specifiek fenotype astma duidelijk meer baat heeft bij een toevoeging van een LAMA is in de gebruikte studies niet onderzocht. De werkgroep acht meer onderzoek noodzakelijk, momenteel kan niet aan iedere patiënt met niet-ernstig astma geadviseerd worden een LAMA toe te voegen.
Onderbouwing
Achtergrond
The combination of inhaled corticosteroids and long-acting β2 sympathomimetics (ICS/LABA), so-called 'dual therapy' is the basis of the treatment of asthma. This can be applied in increasing doses. If symptoms persist, adding a long-acting anticholinergic (LAMA) ('triple therapy') may be considered. Currently, it is unknown whether adding LAMA to ICS/LABA is beneficial. And if so, whether there is a difference when administering triple therapy in one device versus multiple devices.
Conclusies / Summary of Findings
|
Moderate GRADE |
In adults with non-severe asthma, triple therapy (ICS+LABA+LAMA) probably results in little to no difference in number of patients with ≥1 exacerbation during the study period as compared to dual therapy (ICS+LABA).
Source: Kerstjens, 2020 |
|
Low GRADE |
In adults with non-severe asthma, triple therapy (ICS+LABA+LAMA) may result in little to no difference in number of exacerbations per 52 weeks as compared to dual therapy (ICS+LABA).
Sources: Lee, 2021; both studies of Virchow, 2019 |
|
Moderate GRADE |
In adults with non-severe asthma, triple therapy (ICS+LABA+LAMA) probably results in little to no difference in number of visits to the first aid department as compared to dual therapy (ICS+LABA).
Sources: Lee, 2021; both studies of Virchow, 2019 |
|
Moderate GRADE |
In adults with non-severe asthma, triple therapy (ICS+LABA+LAMA) probably results in little to no difference in number of severe exacerbations (needing hospitalization or need of oral corticosteroids) as compared to dual therapy (ICS+LABA).
Sources: Lee, 2021; both studies of Virchow, 2019 |
|
Moderate GRADE |
In adults with non-severe asthma, triple therapy (ICS+LABA+LAMA) probably results in little to no difference in Asthma Control Questionnaire (ACQ) score as compared to dual therapy (ICS+LABA).
Sources: Kerstjens, 2020; Lee, 2021; both studies of Virchow, 2019; Zhang, 2018 |
|
Low GRADE |
In adults with non-severe asthma, triple therapy (ICS+LABA+LAMA) may result in an increased forced expiratory volume in one second as compared to dual therapy (ICS+LABA).
Sources: Kerstjens, 2020; Lee, 2021; both studies of Virchow, 2019; Zhang, 2018 |
Samenvatting literatuur
Description of studies
Five studies with a total of 8280 participants were included. Four of the five studies were multinational blinded randomized controlled trials with multiple treatment arms comparing ICS+LABA versus ICS+LABA+LAMA in different dosages (IRIDIUM by Kerstjens 2020, CAPTAIN by Lee 2021, TRIMARAN by Virchow 2019, TRIGGER by Virchow 2019). Those four studies started with a two- or three-week run-in period in which all participants were administered the same kind and dosage of treatment (ICS+LABA) after which they were randomized. All participants were aged 18 years or older, had a FEV1 of <80% or <85% of predicted normal, a FEV1 reversibility of ≥12% and 200mL after salbutamol/albuterol, and an Asthma Control Questionnaire-score of ≥1.5 points. Also, they were all using ICS+LABA before study entry and had at least one asthma exacerbation that required medical treatment with systemic corticosteroids in the year before study entry. The other study (Zhang 2018) was a randomized trial with 160 participants that were aged 18 years or older, had a FEV1 of 60 to 80% of predicted normal and a variability in FEV1 of <30%. Details regarding pre-study medication use were not provided. 80 out of 160 participants of the study of Zhang 2018 received the intervention/comparison of our interest.
The investigated LAMA was tiotropium in two studies and was dosed as 2.5µg two inhalations once daily (TRIGGER) or as 18µg once per day (Zhang, 2018). Three studies investigated glycopyrronium in dosages of 10µg two inhalations twice daily (TRIMARAN and TRIGGER) or once daily 50µg (IRIDIUM). One study investigated umeclidinium in dosages of 31.25µg or 62.5µg (CAPTAIN).
In the IRIDIUM, CAPTAIN, TRIMARAN and TRIGGER studies, the triple therapy was administered as fixed dose combination (e.g., in one device). In Zhang (2018), two devices were used: ICS+LABA in one device and LAMA in another device.
All studies performed before and after treatment measurements and reported data as change from baseline. Follow-up ranged from 8 to 52 weeks.
Results
1. Asthma control (number of patients with ≥1 exacerbation during the study period; number of exacerbations per 52 weeks; number of visits to the first aid department; number of severe exacerbations needing hospitalization; number of severe exacerbations in need of oral corticosteroids)
Data regarding exacerbations were reported in four studies (IRIDIUM, CAPTAIN, TRIMARAN, TRIGGER). The studies used various definitions for the severeness of an exacerbation as stated in the table below.
Table 1. Definitions of an asthma exacerbation
|
Study |
Moderate asthma exacerbation |
Severe asthma exacerbation |
|
IRIDIUM |
≥2 of the following: - progressive increase of ≥1 asthma symptom - increased use of rescue medication - deterioration in lung function lasting for ≥2 days but no need of systemic corticosteroids for ≥2 days or hospitalization.
A mild asthma exacerbation was diagnosed when 1 of those criteria was met, an investigator confirmed that the exacerbation was clinically significant and went beyond day-to-day variation in asthma control. |
An aggravation of asthma symptoms (e.g., shortness of breath, cough, wheezing, chest tightness) requiring systemic corticosteroids for ≥3 consecutive days or an emergency room visit, hospitalization owing to asthma, or death due to asthma. |
|
CAPTAIN |
Deterioration in either asthma symptoms or lung function, or increased rescue bronchodilator use, that required a physician-directed temporary change in maintenance treatment. |
Exacerbation requiring admission to a hospital or a visit to an emergency department due to the need for systemic corticosteroids, or asthma deterioration requiring systemic corticosteroid use (or doubling of the current maintenance systemic corticosteroid dose) for ≥3 days. |
|
TRIMARAN and TRIGGER |
≥1 of the following: - nocturnal awakenings due to asthma requiring a SABA for 2 consecutive nights or ≥0.75 increase from baseline in daily symptom score on 2 consecutive days - increase from baseline in use of SABA on 2 consecutive days (minimum increase 4 puffs per day) - ≥20% decrease in PEF from baseline on ≥2 consecutive mornings or evenings, or ≥20% decrease in FEV1 from baseline - a visit to an emergency department or a study site for asthma treatment not requiring systemic corticosteroids |
Worsening of asthma that required treatment with systemic corticosteroids for ≥3 days (with any associated emergency department visit or admission to hospital). |
1.1 Number of patients with ≥1 exacerbation during the study period
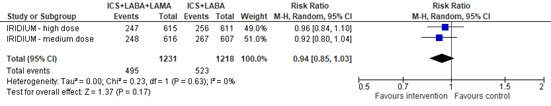
The total number of participants with any exacerbation (mild, moderate and/or severe asthma exacerbations) over 52 weeks was reported in the IRIDIUM study. The pooled risk ratio for an asthma exacerbation with ICS+LABA+LAMA versus ICS+LABA was 0.94 (95% CI 0.85 to 1.03), in favor of ICS+LABA+LAMA (figure 1).
Figure 1. Forest plot of the number of participants with an asthma exacerbation in the group of participants using ICS+LABA+LAMA versus participants using ICS+LABA
1.2 Number of exacerbations per 52 weeks
Three studies reported this outcome (CAPTAIN, TRIMARAN, TRIGGER).
Regarding moderate and/or severe exacerbations, a total of 3170 exacerbations occurred among 3056 participants in the ICS+LABA+LAMA group. This reflects an exacerbation rate per participant of 1.04 (95% CI 1.00 to 1.07). In the ICS+LABA group, a total of 2685 exacerbations occurred among 1958 participants: exacerbation rate per participant 1.37 (95% CI 1.32 to 1.42).
Regarding severe exacerbations, a total of 670 exacerbations occurred among 2769 participants in the ICS+LABA+LAMA group: exacerbation rate per participant 0.24 (95% CI 0.22 to 0.26) versus 568 exacerbations among 1958 participants in the ICS+LABA group: exacerbation rate per participant 0.29 (95% CI 0.27 to 0.32). This corresponds with a risk ratio of 0.83 (95% CI 0.76 to 0.92) for a severe exacerbation in the ICS+LABA+LAMA group versus the ICS+LABA group, favoring ICS+LABA+LAMA.
The total number of participants with a moderate and/or severe exacerbation in the ICS+LABA+LAMA group differs from the total number of participants with a severe exacerbation in the ICS+LABA+LAMA group, because data regarding severe exacerbations in the group using tiotropium in the TRIGGER study were not available.
1.3 Number of visits to the first aid department
The number of visits to the first aid department was not specifically reported, though emergency department visit was stated in the definitions of moderate or severe exacerbations (see table 1).
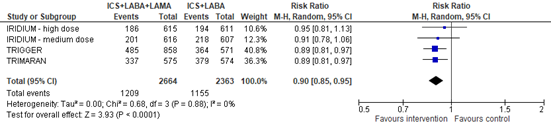
The number of patients with a moderate or severe exacerbation was reported in three studies (IRIDIUM, TRIMARAN, TRIGGER). The pooled risk ratio for a moderate or severe exacerbation with ICS+LABA+LAMA compared with ICS+LABA was 0.90 (95% CI 0.85 to 0.95), in favor of ICS+LABA+LAMA (figure 2).
Figure 2. Forest plot of the number of participants with a moderate or severe asthma exacerbation in the group of participants using ICS+LABA+LAMA versus participants using ICS+LABA
1.4 Number of severe exacerbations needing hospitalization; number of severe exacerbations in need of oral corticosteroids
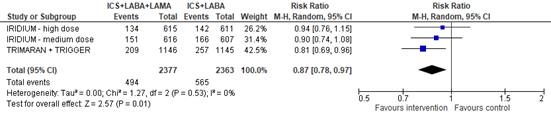
The number of patients with a severe exacerbation, thus requiring systemic corticosteroids and/or hospitalization, was reported in three studies (IRIDIUM, TRIMARAN, TRIGGER). Data for hospitalization and need for oral corticosteroids were not reported separately. The pooled risk ratio for a severe exacerbation was 0.87 (95% CI 0.78 to 0.97), favoring ICS+LABA+LAMA (figure 3). Note that data regarding severe exacerbations were not available for the group using tiotropium in the TRIGGER study.
Figure 3. Forest plot of the number of participants with a severe asthma exacerbation in the group of participants using ICS+LABA+LAMA versus participants using ICS+LABA
2. Asthma Control Questionnaire (ACQ) score
ACQ-7 scores were reported in four studies (IRIDIUM, CAPTAIN, TRIMARAN, TRIGGER). A lower score reflects less impairment and better controlled asthma. Each study reported the data in a different way, disallowing pooling of data.
The IRIDIUM study reported changes from baseline measured at week 26 and treatment differences. The groups using medium-dose and high-dose ICS+LABA+LAMA had a ACQ-7 change from baseline score of -0.974 and -0.975, respectively, whereas the corresponding ICS+LABA groups had ACQ-7 change from baseline score of -0.903 and -0.989, respectively. No confidence intervals or standard deviations were reported. The treatment difference between the medium-dose ICS+LABA+LAMA versus medium-dose ICS+LABA group was -0.071 (95% CI -0.151 to 0.010), favoring ICS+LABA+LAMA. For the high-dose groups, the treatment difference was 0.014 (95% CI -0.066 to 0.094), favoring ICS+LABA.
The CAPTAIN study reported changes from baseline measured at week 24 and treatment differences. The mean change from baseline for the medium-dose ICS+LABA+LAMA group was -0.73 (95% CI -0.78 to -0.69), for the high-dose ICS+LABA+LAMA group -0.77 (95% CI -0.81 to -0.72) and for the ICS+LABA group -0.68 (95% CI -0.73 to -0.63). This resulted in a treatment difference for the medium-dose ICS+LABA+LAMA versus ICS+LABA group of -0.06 (95% CI -0.12 to 0.01), favoring ICS+LABA+LAMA. For the high-dose ICS+LABA+LAMA versus ICS+LABA group this difference was -0.09 (95% CI -0.16 to -0.02), in favor of ICS+LABA+LAMA.
The TRIMARAN and TRIGGER studies reported adjusted mean differences at week 26 and 52. In TRIMARAN, at week 26, the adjusted mean difference was -0.043. No confidence intervals were reported, though this was reported to be not statistically significantly different. In TRIGGER (glycoperronium only), at week 26, the adjusted mean difference was -0.083 which was reported to be statistically significantly different with a p-value of <0.05.
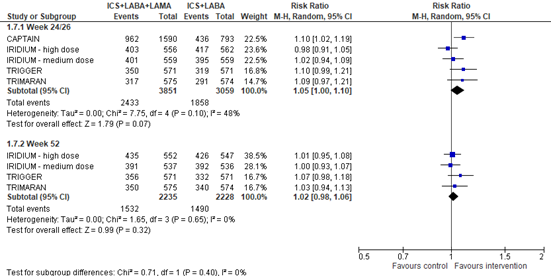
All these four studies also reported the number of participants with ≥0.5 points improvement (decrease) in ACQ-7 score compared to baseline, so-called ACQ-7 score responders. The pooled risk ratio for ≥0.5 points improvement at week 24 (CAPTAIN) or 26 (IRIDIUM, TRIMARAN, TRIGGER) was 1.05 (95% CI 1.00 to 1.10), favoring ICS+LABA+LAMA. At week 52, the pooled risk ratio was 1.02 (95% CI 0.98 to 1.06), in favor of ICS+LABA+LAMA (figure 4).
Figure 4. Forest plot of the number of participants with ≥0.5 points improvement in ACQ-7 score at week 24 or 26 (upper figure) or week 52 (lower figure) among participants using ICS+LABA+LAMA versus participants using ICS+LABA
Please note that for the TRIGGER study data regarding glycopyrronium were included
3. Spirometry (forced expiratory volume in one second (FEV1))
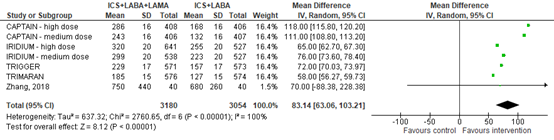
All five studies assessed FEV1 and reported it as change from baseline in mL. The mean difference of FEV1 change from baseline between ICS+LABA+LAMA and ICS+LABA was 83.14 mL (95% CI 63.06 to 103.21) (figure 5).
Figure 5. Forest plot of change from baseline FEV1 among participants using ICS+LABA+LAMA versus participants using ICS+LABA
Please note:
- For the CAPTAIN study, only the high dose arms of umeclidinium and controls were included (medium dose in the plot refers to the dose of fluticasone furoate.
- For the TRIGGER study, the glycopyrronium and control arms were included, thus not the tiotropium arm.
- Standard deviations of the IRIDIUM study were imputed
Level of evidence of the literature
According to GRADE, randomized controlled trials (RCTs) start at a high level of evidence.
Asthma-exacerbations
The level of evidence regarding the outcome measure ‘number of patients with ≥1 exacerbation during the study period’ was downgraded to moderate GRADE. Downgraded by one level for imprecision (due to reporting in only one study).
The level of evidence regarding the outcome measure ‘number of exacerbations per 52 weeks’ was downgraded to low GRADE. Downgraded by one level for indirectness (due to data availability for moderate/severe and severe exacerbations only) and one level for imprecision (due to overlap of the lower limit of the 95% confidence interval with the minimal clinically important difference).
The level of evidence regarding the outcome measure ‘number of visits to the first aid department’ was downgraded to moderate GRADE. Downgraded by one level for indirectness (because visits to the first aid department was expressed as the number of moderate and/or severe exacerbations).
The level of evidence regarding the outcome measure ‘number of severe exacerbations (needing hospitalization or need of oral corticosteroids)’ was downgraded to moderate GRADE. Downgraded by one level for imprecision (due to overlap of the lower limit of the 95% confidence interval with the minimal clinically important difference).
Asthma Control Questionnaire (ACQ) score
The level of evidence regarding the outcome measure ‘ACQ-score’ was downgraded to moderate GRADE. Downgraded by one level for indirectness (due to assessment of ACQ-responders because the impossibility to pool data regarding actual scores).
Spirometry
The level of evidence regarding the outcome measure ‘forced expiratory volume in one second’ was downgraded to low GRADE. Downgraded by one level for risk of bias (due to limitations in study design) and one level for inconsistency (due to statistical heterogeneity).
Zoeken en selecteren
A systematic review of the literature was performed to answer the following question:
‘What is the effectiveness of adding LAMA to ICS/LABA compared to ICS/LABA alone in patients with non-severe asthma?’
| P: | Patients with non-severe asthma (≥ 18 years of age) |
| I: |
Addition of LAMA (tiotropium, glycopyrronium, umeclidinium) on top of ICS/LABA (triple therapy) |
| C: | ICS/LABA treatment (dual therapy) |
| O: |
Asthma exacerbations, ACQ score, FEV1 |
- asthma exacerbations (number of patients with ≥1 exacerbation during the study period; number of exacerbations per 52 weeks; number of visits to the first aid department; number of severe exacerbations needing hospitalization; number of severe exacerbations in need of oral corticosteroids);
- asthma Control Questionnaire (ACQ) score;
- spirometry (forced expiratory volume in one second (FEV1).
Relevant outcome measures
The guideline development group considered ACQ score as a critical outcome measure for decision making; and asthma exacerbations and FEV1 as an important outcome measure for decision making.
The working group defined a 25% difference for dichotomous outcomes (RR< 0.8 or >1.25) and 0.5 standard deviation for continuous outcomes as minimal clinically (patient) important difference, in accordance with the GRADE default boundaries.
Search and select (Methods)
The databases Medline (via OVID) and Embase (via Embase.com) were searched with relevant search terms from January 1, 2000 until May 10, 2024. The detailed search strategy is depicted under the tab Methods. The systematic literature search resulted in 541 hits. Studies were selected based on the PICO criteria and study design (systematic reviews of RCTs and RCTs). 60 studies were initially selected based on title and abstract screening. After reading the full text, 55 studies were excluded (see the table with reasons for exclusion under the tab Methods), and five studies were included. One of those five studies was a corrigendum to another study and one report described two studies. Thus, the analysis of the literature is based on five studies from four reports.
Results
Five studies were included in the analysis of the literature. Important study characteristics and results are summarized in the evidence tables. The assessment of the risk of bias is summarized in the risk of bias tables.
Referenties
- Horita N, Goto A, Shibata Y, Ota E, Nakashima K, Nagai K, Kaneko T. Long-acting muscarinic antagonist (LAMA) plus long-acting beta-agonist (LABA) versus LABA plus inhaled corticosteroid (ICS) for stable chronic obstructive pulmonary disease (COPD). Cochrane Database Syst Rev. 2017 Feb 10;2(2):CD012066. doi: 10.1002/14651858.CD012066.pub2. Update in: Cochrane Database Syst Rev. 2023 Jun 5;6:CD012066. doi: 10.1002/14651858.CD012066.pub3. PMID: 28185242; PMCID: PMC6464543.
- Kerstjens HAM, Maspero J, Chapman KR, van Zyl-Smit RN, Hosoe M, Tanase AM, Lavecchia C, Pethe A, Shu X, D'Andrea P; IRIDIUM trial investigators. Once-daily, single-inhaler mometasone-indacaterol-glycopyrronium versus mometasone-indacaterol or twice-daily fluticasone-salmeterol in patients with inadequately controlled asthma (IRIDIUM): a randomised, double-blind, controlled phase 3 study. Lancet Respir Med. 2020 Oct;8(10):1000-1012. doi: 10.1016/S2213-2600(20)30190-9. Epub 2020 Jul 9. PMID: 32653074.
- Lee LA, Bailes Z, Barnes N, Boulet LP, Edwards D, Fowler A, Hanania NA, Kerstjens HAM, Kerwin E, Nathan R, Oppenheimer J, Papi A, Pascoe S, Brusselle G, Peachey G, Sule N, Tabberer M, Pavord ID. Efficacy and safety of once-daily single-inhaler triple therapy (FF/UMEC/VI) versus FF/VI in patients with inadequately controlled asthma (CAPTAIN): a double-blind, randomised, phase 3A trial. Lancet Respir Med. 2021 Jan;9(1):69-84. doi: 10.1016/S2213-2600(20)30389-1. Epub 2020 Sep 9. Erratum in: Lancet Respir Med. 2021 Feb;9(2):e18. doi: 10.1016/S2213-2600(20)30602-0. PMID: 32918892. Correction: Correction to Lancet Respir Med 2021; 9: 69-84. Lancet Respir Med. 2021 Feb;9(2):e18. doi: 10.1016/S2213-2600(20)30602-0. Epub 2021 Jan 4. Erratum for: Lancet Respir Med. 2021 Jan;9(1):69-84. doi: 10.1016/S2213-2600(20)30389-1. PMID: 33412106.
- Virchow JC, Kuna P, Paggiaro P, Papi A, Singh D, Corre S, Zuccaro F, Vele A, Kots M, Georges G, Petruzzelli S, Canonica GW. Single inhaler extrafine triple therapy in uncontrolled asthma (TRIMARAN and TRIGGER): two double-blind, parallel-group, randomised, controlled phase 3 trials. Lancet. 2019 Nov 9;394(10210):1737-1749. doi: 10.1016/S0140-6736(19)32215-9. Epub 2019 Sep 30. PMID: 31582314.
- Zhang L, Huang G, Jin L, Han S. Therapeutic Effects of a Long-Acting Cholinergic Receptor Blocker, Tiotropium Bromide, on Asthma. Med Sci Monit. 2018 Feb 15;24:944-950. doi: 10.12659/msm.907950. PMID: 29446377; PMCID: PMC5822933.
- Zinnige Zorg - Verbetersignalement Astma. Zorginstituut Nederland. 2021. Accessed Online 15-11-2024 from:
Evidence tabellen
Evidence table for intervention studies
|
Study reference |
Study characteristics |
Patient characteristics |
Intervention (I) |
Comparison / control (C)
|
Follow-up |
Outcome measures and effect size |
Comments |
|
Kerstjens, 2020 |
Type of study: Phase III, five-armed, multicenter, randomized, double-blind, double-dummy, active-controlled, parallel-group.
Setting and country: Private clinics, universities and hospital sites in 41 countries.
Funding and conflicts of interest: Funded by Novartis Pharmaceuticals, East Hanover. |
Inclusion criteria: - men and women aged 18 - 75 y - asthma diagnosis ≥1y before screening - FEV1 <80% of predicted normal - increase in FEV1 ≥12% and 200mL after salbutamol/ albuterol - symptomatic at run-in - ACQ-7 score ≥1.5 - ≥1 asthma exacerbation requiring medical care and systemic corticosteroids in the 12 months before screening - receiving medium or high dose ICS+LABA ≥3 months and at stable dose ≥1 month before screening
Exclusion criteria: - smoking tobacco products ≤6 months before screening - smoking history >10 pack-years - chronic lung disease other than asthma - ≥1 asthma exacerbation requiring hospitalization or emergency room visit and systemic corticosteroids in the 6 weeks before screening - respiratory tract infection or asthma worsening ≤4 weeks before screening or during the run-in period - clinically significant comorbidities
N at baseline: Total: 3092 Intervention 1 / 2: 620 / 619 Control 1 / 2 / 3: 617 / 618 / 618
Other characteristics mean (SD): - Age: 52.2 (12.7) years - Duration of asthma: 18.1 ( 15.3) years - Baseline ACQ-7 score: 2.5 ( 0.6) - Pre-bronchodilator FEV1: 1.6L (0.6) - Predicted pre-bronchodilator FEV1: 54.8 (13.7) - FEV1 reversibility increase: 27.7% (20.2) |
All participants received fluticasone 250µg + salmeterol 50µg twice daily in the two weeks before randomization.
Intervention arms: 1. Medium-dose MF-IND-GLY: mometasone 80µg + indacaterol 150µg + glycopyrronium 50 µg in the evening via Breezhaler + placebo twice daily via Diskus 2. High-dose MF-IND-GLY: mometasone 160µg + indacaterol 150µg + glycopyrronium 50 µg in the evening via Breezhaler + placebo twice daily via Diskus
|
All participants received fluticasone 250µg + salmeterol 50µg twice daily in the two weeks before randomization.
Control arms: 1. Medium-dose MF-IND: mometasone 160µg + indacaterol 150µg in the evening via Breezhaler + placebo twice daily via Diskus 2. High-dose MF-IND: mometasone 320µg + indacaterol 150µg in the evening via Breezhaler + placebo twice daily via Diskus 3. High-dose FLU-SAL: fluticasone 500µg + salmeterol 50µg twice daily via Diskus + placebo in the evening via Breezhaler
|
Length of follow-up: 52 weeks
Loss-to-follow-up: Intervention 1: 0 Intervention 2: 1 Control 1: 0 Control 2: 2 Control 3: 1
Incomplete outcome data: Intervention 1: 74 Intervention 2: 59 Control 1: 65 Control 2: 61 Control 3: 62 Reasons: mainly due to subject or guardian decision.
|
FEV1 change from baseline at week 26: Intervention 1: + 299mL Intervention 2: +320mL Control 1: +223mL Control 2: +255mL Control 3: +201mL
- Int 1 vs control 1: Δ76mL (95% CI 41-111), p<0.001 - Int 2 vs control 2: Δ65mL (95% CI 31-99), p<0.001 - Int 1 vs control 3: Δ99mL (95% CI 64-133), p<0.001 - Int 2 vs control 3: Δ119mL (95% CI 85-154), p<0.001
ACQ-7 score change from baseline at week 26: Intervention 1: -0.974 Intervention 2: -0.975 Control 1: -0.903 Control 2: -0.989 Control 3: -0.889
- Int 1 vs control 1: Δ-0.071 (95% CI -0.151-0.010), p=0.085 - Int 2 vs control 2: Δ0.014 (95% CI -0.066-0.094), p=0.73 - Int 1 vs control 3: Δ-0.084 (95% CI -0.164—0.0015), p=0.038 - Int 2 vs control 3: Δ-0.086 (95% CI -0.165—0.006), p=0.0034
Annualized rate of moderate/severe exacerbations over 52 weeks: - Int 1 vs control 1: rate ratio 0.87 (95% CI 0.71-1.06), p=0.17 - Int 2 vs control 2: rate ratio 0.85 (95% CI 0.68-1.04), p=0.12
Mean daily number of puffs of rescue medication, change from baseline over 52 weeks: Intervention 1: -0.81 (0.058) Intervention 2: -0.88 (0.058) Control 1: -0.72 (0.058) Control 2: -0.83 (0.058) Control 3: -0.76 (0.058)
- Int 1 vs control 1: Δ-0.10 (95% CI -0.25-0.05), p=0.211 - Int 2 vs control 2: Δ-0.04 (95% CI -0.19-0.10), p=0.563 - Int 1 vs control 3: Δ-0.06 (95% CI -0.20—0.03), p=0.468 - Int 2 vs control 3: Δ-0.12 (95% CI -0.27—0.03), p=0.117 |
Study acronym: IRIDIUM |
|
Lee, 2020 |
Type of study: Phase IIIA, multicenter, randomized, double-blind, active-controlled, parallel-group.
Setting and country: Hospitals and primary care centers across 15 countries.
Funding and conflicts of interest: Funded by GSK.
|
Inclusion criteria: - adults aged ≥18 years - FEV1 30-85% of predicted normal - increase in FEV1 ≥12% and 200mL after salbutamol/ albuterol - ACQ-6 score ≥1.5 - healthcare contact or temporary change in asthma therapy for acute asthma symptoms in the year before screening - receiving daily ICS+LABA ≥12 and at stable dose ≥6 weeks before pre-screening
Exclusion criteria: - COPD diagnosis or concurrent respiratory disorder - current smokers - former smokers with smoking history ≥10 pack-years N at baseline: Total: 2436 Intervention 1/2/3/4: 405/406/404/408 Control 1/2: 407/406
Other characteristics mean (SD): - Age: 53.2 (13.1) years - Disease duration: 21.2 (15.3) years - Baseline ACQ-7 score: 2.12 (0.70) - Pre-bronchodilator FEV1: 2023mL (678) - Predicted pre-bronchodilator FEV1: 68.18% (14.76) - FEV1 reversibility increase: 29.92% (18.12) |
All participants received fluticasone propionate 250µg + salmeterol 50µg twice daily via Diskus for three weeks and fluticasone furoate 100µg + vilanterol 25µg in the subsequent two weeks before randomization.
Intervention arms: 1. FF/UMEC/VI 100/31.25/25µg: fluticasone furoate 100µg + umeclidinium 31.25µg + vilanterol 25µg 2. FF/UMEC/VI 100/62.5/25µg: fluticasone furoate 100µg + umeclidinium 62.5µg + vilanterol 25µg 3. FF/UMEC/VI 200/31.25/25µg: fluticasone furoate 200µg + umeclidinium 31.25µg + vilanterol 25µg 4. FF/UMEC/VI 200/62.5/25µg: fluticasone furoate 200µg + umeclidinium 62.5µg + vilanterol 25µg |
All participants received fluticasone propionate 250µg + salmeterol 50µg twice daily via Diskus for three weeks and fluticasone furoate 100µg + vilanterol 25µg in the subsequent two weeks before randomization.
Control arms: 1. FF/VI 100/25µg: fluticasone furoate 100µg + vilanterol 25µg 2. FF/VI 200/25µg: fluticasone furoate 200µg + vilanterol 25µg
|
Length of follow-up: Maximal 52 weeks
Loss-to-follow-up: 18 in total
Incomplete outcome data: 162 in total Reasons: mainly due to patient withdrawal.
|
FEV1 change from baseline at week 24: Intervention 1: + 120mL Intervention 2: +134mL Intervention 3: + 157mL Intervention 4: +168mL Control 1: +24mL Control 2: +76mL
- Int 1 vs control 1: Δ96mL (95% CI 52-139), p<0.001 - Int 2 vs control 1: Δ110mL (95% CI 66-153), p<0.001 - Int 3 vs control 2: Δ82mL (95% CI 39-125), p=0.002 - Int 4 vs control 2: Δ92mL (95% CI 49-135), p<0.001
ACQ-7 score change from baseline at week 24: Intervention 1+3 pooled: -0.73 Intervention 2+4 pooled: -0.77 Control pooled: -0.68
- Int 1+3 vs control: Δ-0.06 (95% CI -0.12-0.01), p=0.094 - Int 2+4 vs control: Δ-0.09 (95% CI -0.16--0.02), p=0.0084
Mean annualized rate of moderate/severe exacerbations over 52 weeks: Intervention 1+3 pooled: 0.68 Intervention 2+4 pooled: 0.61 Control pooled: 0.70
- Int 1+3 vs control: rate ratio 0.97 (95% CI 0.81-1.17), p=0.78 - Int 2+4 vs control: rate ratio 0.87 (95% CI 0.72-1.05), p=0.15 |
Study acronym: CAPTAIN |
|
Virchow, 2019 |
Type of studies: Two phase III, multicenter, randomized, double-blind, active-controlled, parallel-group.
Setting and country: Secondary and tertiary centers across 17 countries.
Funding and conflicts of interest: Funded by Chiesi Farmaceutici. |
Inclusion criteria: - adults aged 18-75 years - asthma diagnosis ≥1 year - asthma diagnosis before age 40 - FEV1 <80% of predicted normal - increase in FEV1 ≥12% and 200mL after salbutamol - ACQ-7 score ≥1.5 - ≥1 asthma exacerbation requiring systemic corticosteroids or emergency department visit in the previous 12 months - receiving a stable dose of ICS+LABA ≥4 weeks before study entry (TRIMARAN: medium dose, TRIGGER: high dose)
Exclusion criteria: - history of near fatal asthma or previous ICU admission for asthma - severe exacerbation in the 4 weeks before study entry or run-in period - other substantial lung disease - current smokers - former smokers with smoking history ≥10 pack-years - current treatment with monoclonal antibodies or biological drugs - clinically significant cardiovascular conditions or laboratory abnormalities - unstable concurrent disease
N at baseline: - TRIMARAN Total: 1155 Intervention: 579 Control: 576 - TRIGGER Total: 1437 Intervention 1 / 2: 573 / 288 Control: 576
Other characteristics approximately, no total group numbers presented: - Age: 52 years - Disease duration: 24 years - Baseline ACQ-7 score: 2.4 - Pre-bronchodilator FEV1: 1.6L - Predicted pre-bronchodilator FEV1: 53% - FEV1 reversibility increase: 33% |
All participants received beclometasone dipropionate 100µg + formoterol fumarate 6µg two inhalations twice daily in the two weeks before randomization.
TRIMARAN: Intervention arm: 1. BDP/FF/G group: beclometasone dipropionate 100µg + formoterol fumarate 6µg + glycopyrronium 10µg two inhalations twice daily via pressurized metered-dose inhaler.
TRIGGER: Intervention arms: 1. BDP/FF/G group: beclometasone dipropionate 200µg + formoterol fumarate 6µg + glycopyrronium 10µg two inhalations twice daily via pressurized metered-dose inhaler. 2. BDP/FF/tiotropium group: beclometasone dipropionate 200µg + formoterol fumarate 6µg two inhalations twice daily + tiotropium 2.5µg two inhalations once daily |
All participants received beclometasone dipropionate 100µg + formoterol fumarate 6µg two inhalations twice daily in the two weeks before randomization.
TRIMARAN: Control arm: 1. BDP/FF group: beclometasone dipropionate 100µg + formoterol fumarate 6µg + two inhalations twice daily via pressurized metered-dose inhaler.
TRIGGER: Control arm: 1. BDP/FF group: beclometasone dipropionate 100µg + formoterol fumarate 6µg + two inhalations twice daily via pressurized metered-dose inhaler.
|
Length of follow-up: 52 weeks
Loss-to-follow-up: TRIMARAN: Intervention: 2 Control: 2
TRIGGER: Intervention 1: 0 Intervention 2: 1 Control: 1
Incomplete outcome data: TRIMARAN: Intervention: 34 Control: 35
TRIGGER: Intervention 1: 37 Intervention 2: 25 Control: 40
|
Predose FEV1 change from baseline at week 26: TRIMARAN intervention: +185mL TRIMARAN control: +127mL TRIGGER intervention 1: +229mL TRIGGER intervention 2: +274mL TRIGGER control: +157mL
- TRIMARAN: MD 57mL (95% CI 15-99, p=0.0080 - TRIGGER int 1 vs control: MD 73mL (95% CI 26-120, p=0.0025 - TRIGGER int 1 vs int 2: MD -45mL (95% CI -103-13, p=0.13
Annualized rate of moderate/severe exacerbations: - TRIMARAN: rate ratio 0.85 (95% CI 0.73-0.99, p=0.033 - TRIGGER int 1 vs control: rate ratio 0.88 (95% CI 0.75-1.03, p=0.11 - TRIGGER int 1 vs int 2: rate ratio 1.07 (95% CI 0.88-1.30, p=0.50
|
Study acronym: TRIMARAN (medium dose study) and TRIGGER (high dose study) |
|
Zhang, 2018 |
Type of study: Randomized trial
Setting and country: One hospital in China
Funding and conflicts of interest: None reported |
Inclusion criteria: In accordance with GINA 2009: - adults aged ≥18 years - FEV1 60-80% of predicted normal or FEV1 60-80% of personal best - variability in FEV1 or PEF <30% - correct and timely use of inhalers and inhalants
Exclusion criteria: - use of systemic glucocorticoids within the last month - use of inhalation or oral ICS, LABA, SABA, anticholinergic agents, antihistamines, leukotriene antagonists or theophyllines ≤48 hours before study entry - critical diseases besides asthma - severe respiratory, heart, renal or hepatic failure - myocardial infarction or severe arrhythmia ≤6 months - pregnant or lactating women - use of receptor antagonist - lung disease in addition to asthma - pulmonary lobectomy - contraindications to salmeterol or tiotropium bromide
N at baseline: Total: 160 Intervention 1 / 2: 40 / 40 Control 1 / 2: 40 / 40
Other characteristics approximately, no total group numbers presented: - Age: 36 years - Disease duration: 20 years - FEV1: 2L |
Intervention arms: 1. Spiriva-Seretide: tiotropium 18µg once per day + salmeterol 50µg + fluticasone propionate 250µg 1 inhalation twice per day (C) 2. Spiriva-Flixotide: tiotropium 18µg once per day + fluticasone propionate 250µg twice per day |
Control arms: 1. Flixotide: fluticasone propionate 250µg twice per day 2. Seretide: salmeterol 50µg + fluticasone propionate 250µg 1 inhalation twice per day (B) |
Length of follow-up: 8 weeks
Loss-to-follow-up: 0
Incomplete outcome data: 0
|
FEV1 change from baseline: Intervention 1: +0.75L (SD 0.44) Control 2: +0.68L (SD 0.26)
Number of times used salbutamol to alleviate symptoms: Intervention 1: 1.2 (SD 1.0) Control 2: 2.5 (SD 0.85) |
Only the comparison of intervention 1 vs control 2 is relevant to our PICO. |
Risk of bias table for intervention studies
|
Study reference (first author, year) |
Was the allocation sequence adequately generated?
|
Was the allocation adequately concealed?
|
Blinding:
|
Was loss to follow-up (missing outcome data) infrequent?
|
Are reports of the study free of selective outcome reporting?
|
Was the study apparently free of other problems that could put it at a risk of bias? |
Overall risk of bias If applicable/necessary, per outcome measure
|
|
Kerstjens, 2020 |
Definitely yes
Reason: Authors used interactive response technology. |
Definitely yes
Reason: Randomization number was used to link the patient to the package of investigational drug. |
Definitely yes
Reason: Blinding of patients, investigator staff, persons performing the assessments and data analysts. |
Definitely no
Reason: 4 out of 3092 were loss to follow-up. |
Definitely yes
Reason: Protocol checked. |
Probably no
Reason: Farmacy sponsored trial. The funder of the study had a role in the study design, data collection and data analysis, oversaw study conduct and was responsible for study report preparation. |
Low risk of bias
Reason: Large trial with correct randomizations and blinding. |
|
Lee, 2020 |
Definitely yes
Reason: Authors used a validated computerized system and interactive response technology. |
Definitely yes
Reason: Authors used centra based randomization. |
Definitely yes
Reason: Blinding of patients, investigators and the funder. |
Definitely no
Reason: 18 out of 2436 were loss to follow-up. |
Definitely yes
Reason: Protocol checked. |
Probably no
Reason: Farmacy sponsored trial. The funder of the study had a role in study design, data collection, data analysis, data interpretation and writing of the report. |
Low risk of bias
Reason: Large trial with correct randomizations and blinding. |
|
Virchow, 2019 |
Definitely yes
Reason: Authors used interactive response technology. |
Probably no
Reason: No information provided. |
Definitely yes / definitely no
Reason: - TRIMARAN: Blinding of patients, investigators, site staff, and sponsor personnel. - TRIGGER: Intervention group 2 was open-label. |
Definitely no
Reason: 4 out of 1155 and 2 out 1437 were loss to follow-up. |
Definitely yes
Reason: Protocol checked. |
Probably no
Reason: Farmacy sponsored trial. The funder of the study had a role in the study design and data analysis, oversaw study conduct, and was responsible for study report preparation. |
TRIMARAN: Low risk of bias
Reason: Large trial with correct randomizations and blinding.
TRIGGER: Some concerns
Reason: Large trial with correct randomizations but partly open-label. |
|
Zhang, 2018 |
Probably no
Reason: No information provided. |
Probably no
Reason: No information provided. |
Probably no
Reason: No information provided. |
Definitely no
Reason: No loss to follow-up. |
Probably yes
Reason: Outcome measures stated in the Methods section are reported. |
Probably no
Reason: no information about statistics, population is unclear. |
High risk of bias
Reason: Probably inadequate randomization and blinding. |
Table of excluded studies
|
Reference |
Reason for exclusion |
|
Tiotropium - What role in asthma?. Drug and Therapeutics Bulletin. 2015; 53 (9) :102-104 |
Wrong publication type |
|
Adams, K. S. and Lowe, D. K. Tiotropium for adults with inadequately controlled persistent asthma. Annals of Pharmacotherapy. 2013; 47 (1) :117-123 |
Wrong publication type |
|
Agusti, A. and Fabbri, L. and Lahousse, L. and Singh, D. and Papi, A. Single inhaler triple therapy (SITT) in asthma: Systematic review and practice implications. Allergy: European Journal of Allergy and Clinical Immunology. 2022; 77 (4) :1105-1113 |
Wrong publication type |
|
Befekadu, E. and Onofrei, C. and Colice, G. L. Tiotropium in asthma: A systematic review. Journal of Asthma and Allergy. 2014; (7) :11-21 |
Wrong publication type and wrong population |
|
Bollmeier, S. G. and Lee, S. Y. The emerging role of tiotropium for patients with asthma. Annals of Pharmacotherapy. 2013; 47 (5) :704-713 |
Wrong publication type |
|
Bollmeier, Suzanne G. and Prosser, Theresa R. Patient perspectives on fluticasone-vilanterol versus other corticosteroid combination products for the treatment of asthma. Patient preference and adherence. 2016; 10 :825-36 |
Wrong comparison |
|
Bonini M, Scichilone N. Tiotropium in asthma: back to the future of anticholinergic treatment. Clin Mol Allergy. 2017 Dec 4;15:20. doi: 10.1186/s12948-017-0076-1. PMID: 29213218; PMCID: PMC5713051. |
Wrong publication type |
|
Cazzola, M. and Calzetta, L. and Rinaldi, B. and De Novellis, V. and Rogliani, P. and Matera, M. G. Clinical characteristics, treatment patterns and adherence in patients with asthma on multiple inhaler triple therapy: a review of findings. Expert Review of Respiratory Medicine. 2022; 16 (11) :1205-1212 |
Wrong publication type |
|
Cazzola, M. and Ora, J. and Rogliani, P. and Matera, M. G. Role of muscarinic antagonists in asthma therapy. Expert Review of Respiratory Medicine. 2017; 11 (3) :239-253 |
Wrong publication type |
|
Cazzola, M. and Puxeddu, E. and Matera, M. G. and Rogliani, P. A potential role of triple therapy for asthma patients. Expert Review of Respiratory Medicine. 2019; 13 (11) :1079-1085 |
Wrong publication type |
|
Clinical Review Report: Indacaterol Acetate-Glycopyrronium Bromide-Mometasone Furoate (Enerzair Breezhaler): (Novartis Pharmaceuticals Canada Inc.): Indication: Asthma maintenance, adults [Internet]. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2021 Jan. PMID: 33877750. |
Wrong publication type |
|
Dahl, R. and Engel, M. and Dusser, D. and Halpin, D. and Kerstjens, H. A. M. and Zaremba-Pechmann, L. and Moroni-Zentgraf, P. and Busse, W. W. and Bateman, E. D. Safety and tolerability of once-daily tiotropium Respimat® as add-on to at least inhaled corticosteroids in adult patients with symptomatic asthma: A pooled safety analysis. Respiratory Medicine. 2016; 118 :102-111 |
Wrong comparison |
|
de Llano, L. P. and Naval, E. and Mejía, N. and Domínguez-Ortega, J. Inhaled indacaterol/glycopyrronium/mometasone furoate fixed-dose combination in moderate-to-severe asthma. Expert Review of Respiratory Medicine. 2022; 16 (1) :1-15 |
Wrong publication type |
|
Evans DJ, Kew KM, Anderson DE, Boyter AC. Long-acting muscarinic antagonists (LAMA) added to inhaled corticosteroids (ICS) versus higher dose ICS for adults with asthma. Cochrane Database Syst Rev. 2015 Jul 21;2015(7):CD011437. doi: 10.1002/14651858.CD011437.pub2. PMID: 26196545; PMCID: PMC8666143. |
Wrong comparison |
|
- Gessner C, Kornmann O, Maspero J, van Zyl-Smit R, Krüll M, Salina A, Gupta P, Bostel S, Fucile S, Conde LG, Pfister P. Fixed-dose combination of indacaterol/glycopyrronium/mometasone furoate once-daily versus salmeterol/fluticasone twice-daily plus tiotropium once-daily in patients with uncontrolled asthma: A randomised, Phase IIIb, non-inferiority study (ARGON). Respir Med. 2020 Aug-Sep;170:106021. doi: 10.1016/j.rmed.2020.106021. Epub 2020 May 27. Erratum in: Respir Med. 2020 Dec;175:106186. doi: 10.1016/j.rmed.2020.106186. PMID: 32843164. - Correction: Gessner C, Kornmann O, Maspero J, van Zyl-Smit R, Krüll M, Salina A, Gupta P, Bostel S, Fucile S, Conde LG, Pfister P. Corrigendum to "Fixed-dose combination of indacaterol/glycopyrronium/mometasone furoate once-daily versus salmeterol/fluticasone twice-daily plus tiotropium once-daily in patients with uncontrolled asthma: A randomised, Phase IIIb, non-inferiority study (ARGON)" [Respir. Med. 170 (Aug-Sep 2020) 106021]. Respir Med. 2020 Dec;175:106186. doi: 10.1016/j.rmed.2020.106186. Epub 2020 Oct 18. Erratum for: Respir Med. 2020 Aug - Sep;170:106021. doi: 10.1016/j.rmed.2020.106021. PMID: 33082033. |
Wrong comparison |
|
Halpin, D. M. and Kaplan, A. G. and Russell, R. K. Why choose tiotropium for my patient? A comprehensive review of actions and outcomes versus other bronchodilators. Respiratory Medicine. 2017; 128 :28-41 |
Wrong publication type |
|
Holguin, F. and Cardet, J. C. and Chung, K. F. and Diver, S. and Ferreira, D. S. and Fitzpatrick, A. and Gaga, M. and Kellermeyer, L. and Khurana, S. and Knight, S. and McDonald, V. M. and Morgan, R. L. and Ortega, V. E. and Rigau, D. and Subbarao, P. and Tonia, T. and Adcock, I. M. and Bleecker, E. R. and Brightling, C. and Boulet, L. P. and Cabana, M. and Castro, M. and Chanez, P. and Custovic, A. and Djukanovic, R. and Frey, U. and Frankemölle, B. and Gibson, P. and Hamerlijnck, D. and Jarjour, N. and Konno, S. and Shen, H. and Vitary, C. and Bush, A. Management of severe asthma: A European Respiratory Society/American Thoracic Society guideline. Pulmonologiya. 2021; 31 (3) :272-295 |
Wrong publication type |
|
Jonas DE, Wines RCM, DelMonte M, Amick HR, Wilkins TM, Einerson BD, Schuler CL, Wynia BA, Shilliday BB. Drug Class Review: Controller Medications for Asthma: Final Update 1 Report [Internet]. Portland (OR): Oregon Health & Science University; 2011 Apr. PMID: 22132427. |
Wrong intervention/ comparison |
|
Kew KM, Dahri K. Long-acting muscarinic antagonists (LAMA) added to combination long-acting beta2-agonists and inhaled corticosteroids (LABA/ICS) versus LABA/ICS for adults with asthma. Cochrane Database Syst Rev. 2016 Jan 21;2016(1):CD011721. doi: 10.1002/14651858.CD011721.pub2. PMID: 26798035; PMCID: PMC9440477. |
PICO matches, but 2 out of 3 studies regard severe asthma patients (wrong population) and 1 study has a wrong comparison |
|
Lee, S. W. and Kim, H. J. and Yoo, K. H. and Park, Y. B. and Park, J. Y. and Jung, J. Y. and Moon, J. Y. and Byun, M. K. and Kim, S. W. and Kim, Y. H. Long-acting anticholinergic agents in patients with uncontrolled asthma: A systematic review and meta-analysis. International Journal of Tuberculosis and Lung Disease. 2014; 18 (12) :1421-1430 |
Wrong comparison |
|
Lou, L. L. and Gong, H. H. and Zhang, M. Q. and Gao, J. M. Efficacy and safety of tiotropium in the treatment of severe persistent asthma: Meta-analysis. Acta Academiae Medicinae Sinicae. 2016; 38 (1) :62-68 |
Wrong population |
|
Luz, M. I. and Aguiar, R. and Morais-Almeida, M. The reality of LAMAs for adult asthmatic patients. Expert Review of Respiratory Medicine. 2020; :1087-1094 |
Wrong publication type |
|
Mansfield, L. and Bernstein, J. A. Tiotropium in asthma: From bench to bedside. Respiratory Medicine. 2019; 154 :47-55 |
Wrong publication type |
|
Mansfield, L. and Duong-Quy, S. and Craig, T. Burden of Asthma and Role of 2.5 µg Tiotropium Respimat® as an Add-On Therapy: A Systematic Review of Phase 2/3 Trials. Advances in Therapy. 2019; 36 (10) :2587-2599 |
Wrong population, wrong comparison |
|
Matera, M. G. and Belardo, C. and Rinaldi, M. and Rinaldi, B. and Cazzola, M. New perspectives on the role of muscarinic antagonists in asthma therapy. Expert Review of Respiratory Medicine. 2020; 14 (8) :817-824 |
Wrong publication type |
|
Meltzer, E. O. and Berger, W. E. A review of the efficacy and safety of once-daily tiotropium Respimat 2.5 micrograms in adults and adolescents with asthma. Allergy and Asthma Proceedings. 2018; 39 (1) :14-26 |
Wrong publication type |
|
Molimard, M. and Till, D. and Stenglein, S. and Singh, D. and Krummen, M. Inhalation devices for long-acting β2-agonists: Efficiency and ease of use of dry powder formoterol inhalers for use by patients with asthma and COPD. Current Medical Research and Opinion. 2007; 23 (10) :2405-2413 |
Wrong publication type |
|
Muiser, S. and Gosens, R. and van den Berge, M. and Kerstjens, H. A. M. Understanding the role of long-acting muscarinic antagonists in asthma treatment. Annals of Allergy, Asthma and Immunology. 2022; 128 (4) :352-360 |
Wrong publication type |
|
Oba Y, Anwer S, Patel T, Maduke T, Dias S. Addition of long-acting beta2 agonists or long-acting muscarinic antagonists versus doubling the dose of inhaled corticosteroids (ICS) in adolescents and adults with uncontrolled asthma with medium dose ICS: a systematic review and network meta-analysis. Cochrane Database Syst Rev. 2023 Aug 21;8(8):CD013797. doi: 10.1002/14651858.CD013797.pub2. PMID: 37602534; PMCID: PMC10441001. |
Wrong comparison |
|
Ora, J. and Calzetta, L. and Ritondo, B. L. and Matera, M. G. and Rogliani, P. Current long-acting muscarinic antagonists for the treatment of asthma. Expert Opinion on Pharmacotherapy. 2021; 22 (17) :2343-2357 |
Wrong publication type, wrong population |
|
Pelaia, C. and Crimi, C. and Crimi, N. and Ricciardi, L. and Scichilone, N. and Valenti, G. and Bonavita, O. and Andaloro, S. and Morini, P. and Rizzi, A. and Pelaia, G. Indacaterol/glycopyrronium/mometasone fixed dose combination for uncontrolled asthma. Expert Review of Respiratory Medicine. 2022; 16 (2) :183-195 |
Wrong publication type |
|
Pizzichini, M. M. M. and Kerstjens, H. A. M. and Pizzichini, E. Current role of anticholinergic drugs in the treatment of asthma: Key messages for clinical practice. Polskie Archiwum Medycyny Wewnetrznej. 2015; 125 (11) :859-866 |
Wrong publication type |
|
Quirce, S. and Domínguez-Ortega, J. and Barranco, P. Anticholinergics for treatment of Asthma. Journal of Investigational Allergology and Clinical Immunology. 2015; 25 (2) :84-93 |
Wrong publication type |
|
Rashid, Q. and Klein, R. Tiotropium in the treatment of patients with asthma. Southern Medical Journal. 2014; 107 (5) :330-337 |
Wrong publication type |
|
Rodrigo, G. J. and Castro-Rodríguez, J. A. What is the role of tiotropium in asthma?: A systematic review with meta-analysis. Chest. 2015; 147 (2) :388-396 |
Either wrong comparison or wrong population |
|
Rogliani P, Ritondo BL, Calzetta L. Triple therapy in uncontrolled asthma: a network meta-analysis of phase III studies. Eur Respir J. 2021 Sep 2;58(3):2004233. doi: 10.1183/13993003.04233-2020. PMID: 33509960. |
Studies will be included separately (no exclusion table) |
|
Salpeter, S. R. An update on the safety of long-acting β-agonists in asthma patients using inhaled corticosteroids. Expert Opinion on Drug Safety. 2010; 9 (3) :407-419 |
Wrong publication type |
|
Sharma, Ashish and Kerstjens, Huib A. M. and Aalbers, Rene and Moroni-Zentgraf, Petra and Weber, Benjamin and Dahl, Ronald Pharmacokinetics of tiotropium administered by Respimat R in asthma patients: Analysis of pooled data from Phase II and III clinical trials. Pulmonary pharmacology & therapeutics. 2017; 42 :25-32 |
Wrong outcome |
|
Sobieraj DM, Baker WL, Weeda ER, Nguyen E, Coleman CI, White CM, Lazarus SC, Blake KV, Lang JE. Intermittent Inhaled Corticosteroids and Long-Acting Muscarinic Antagonists for Asthma [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2018 Mar. Report No.: 17(18)-EHC027-EF. PMID: 29741837. |
Wrong population |
|
Wang, L. and Zhou, R. and Xie, X. Tiotropium added to low- to medium-dose inhaled corticosteroids (ICS) versus low- to medium-dose ICS alone for adults with mild to moderate uncontrolled persistent asthma: A systematic review and meta-analysis. Journal of Asthma. 2019; 56 (1) :69-78 |
Wrong comparison |
|
Wechsler, M. E. and Oppenheimer, J. J. Open-inhaler versus single-inhaler triple therapy (long-acting muscarinic antagonist, inhaled corticosteroid, and long-acting β2-agonist) in asthma patients: a narrative review. Journal of Asthma. 2023; 60 (9) :1633-1645 |
Wrong publication type |
|
Westby M, Benson M, Gibson P. Anticholinergic agents for chronic asthma in adults. Cochrane Database Syst Rev. 2004;2004(3):CD003269. doi: 10.1002/14651858.CD003269.pub2. PMID: 15266477; PMCID: PMC6483359. |
Wrong comparison |
|
Zhou, H. and Liu, C. Efficacy and safety of vilanterol and fluticasone furoate/vilanterol in the treatment of asthma: A systematic review and meta-analysis. International Journal of Clinical and Experimental Medicine. 2016; 9 (6) :8898-8911 |
Wrong comparison |
|
Beeh KM, Moroni-Zentgraf P, Ablinger O, Hollaenderova Z, Unseld A, Engel M, Korn S. Tiotropium Respimat® in asthma: a double-blind, randomised, dose-ranging study in adult patients with moderate asthma. Respir Res. 2014 Jun 3;15(1):61. doi: 10.1186/1465-9921-15-61. PMID: 24890738; PMCID: PMC4066691. |
Wrong comparison |
|
Casale TB, Aalbers R, Bleecker ER, Meltzer EO, Zaremba-Pechmann L, de la Hoz A, Kerstjens HAM. Tiotropium Respimat® add-on therapy to inhaled corticosteroids in patients with symptomatic asthma improves clinical outcomes regardless of baseline characteristics. Respir Med. 2019 Oct-Nov;158:97-109. doi: 10.1016/j.rmed.2019.09.014. Epub 2019 Sep 30. PMID: 31654891. |
Wrong comparison |
|
FitzGerald JM, Sadatsafavi M. Triple therapy in a single inhaler: a new option for uncontrolled asthma. Lancet. 2019 Nov 9;394(10210):1690-1692. doi: 10.1016/S0140-6736(19)32216-0. Epub 2019 Sep 30. PMID: 31582315. |
Wrong publication type |
|
Hoshino M, Ohtawa J, Akitsu K. Effects of the addition of tiotropium on airway dimensions in symptomatic asthma. Allergy Asthma Proc. 2016 Nov;37(6):147-153. doi: 10.2500/aap.2016.37.3991. PMID: 27931291. |
Wrong population |
|
Kerstjens HA, Engel M, Dahl R, Paggiaro P, Beck E, Vandewalker M, Sigmund R, Seibold W, Moroni-Zentgraf P, Bateman ED. Tiotropium in asthma poorly controlled with standard combination therapy. N Engl J Med. 2012 Sep 27;367(13):1198-207. doi: 10.1056/NEJMoa1208606. Epub 2012 Sep 2. PMID: 22938706. |
Wrong population |
|
Kerwin E, Dorinsky P, Patel M, Rossman K, Reisner C, Maes A, Darken P, Griffis D, Fjällbrant H. A randomized controlled trial of glycopyrrolate administered by metered dose inhaler in patients with uncontrolled asthma despite ICS/LABA treatment. J Asthma. 2022 Jul;59(7):1420-1432. doi: 10.1080/02770903.2021.1938603. Epub 2021 Aug 1. PMID: 34338132. |
Wrong population |
|
Nakamura Y, Hozawa S, Sagara H, Ohbayashi H, Lee LA, Crawford J, Tamaoki J, Nishi T, Fowler A. Efficacy and safety of once-daily, single-inhaler fluticasone furoate/umeclidinium/vilanterol versus fluticasone furoate/vilanterol in Japanese patients with inadequately controlled asthma: the CAPTAIN study. Curr Med Res Opin. 2021 Sep;37(9):1657-1665. doi: 10.1080/03007995.2021.1944849. Epub 2021 Jul 14. PMID: 34162298. |
Subgroup analysis of a larger study |
|
Ohta K, Ichinose M, Tohda Y, Engel M, Moroni-Zentgraf P, Kunimitsu S, Sakamoto W, Adachi M. Long-Term Once-Daily Tiotropium Respimat® Is Well Tolerated and Maintains Efficacy over 52 Weeks in Patients with Symptomatic Asthma in Japan: A Randomised, Placebo-Controlled Study. PLoS One. 2015 Apr 20;10(4):e0124109. doi: 10.1371/journal.pone.0124109. PMID: 25894430; PMCID: PMC4404354. |
Wrong comparison |
|
Park SY, Kim S, Kim JH, Kim SH, Lee T, Yoon SY, Kim MH, Moon JY, Yang MS, Jung JW, Kim JH, Choi JH, Park CS, Kim S, Lee J, Kwon JW, Hur GY, Kim SH, Kim HK, Shin YS, Kim SH, Nam YH, Jang AS, Park SY, Kim TB; Cohort for Reality and Evolution of Adult Asthma in Korea (COREA) Study Group. A Randomized, Noninferiority Trial Comparing ICS + LABA with ICS + LABA + LAMA in Asthma-COPD Overlap (ACO) Treatment: The ACO Treatment with Optimal Medications (ATOMIC) Study. J Allergy Clin Immunol Pract. 2021 Mar;9(3):1304-1311.e2. doi: 10.1016/j.jaip.2020.09.066. Epub 2020 Nov 9. PMID: 33184024. |
Wrong population |
|
Price D, Kaplan A, Jones R, Freeman D, Burden A, Gould S, von Ziegenweidt J, Ali M, King C, Thomas M. Long-acting muscarinic antagonist use in adults with asthma: real-life prescribing and outcomes of add-on therapy with tiotropium bromide. J Asthma Allergy. 2015 Jan 14;8:1-13. doi: 10.2147/JAA.S76639. PMID: 25609985; PMCID: PMC4298307. |
Wrong study design |
|
Sagara H, D'Andrea P, Tanase AM, Pethe A, Tanaka Y, Matsuo K, Hosoe M, Nakamura Y. Long-term safety of once-daily indacaterol acetate/glycopyrronium bromide/mometasone furoate high-dose, and indacaterol acetate/mometasone furoate high-dose, in Japanese patients with inadequately controlled asthma: Results from two open-label, 52-week studies. J Asthma. 2023 Feb;60(2):403-411. doi: 10.1080/02770903.2022.2056048. Epub 2022 May 30. PMID: 35348408. |
Wrong comparison / wrong population |
Verantwoording
Beoordelingsdatum en geldigheid
Publicatiedatum : 04-11-2025
Beoordeeld op geldigheid : 12-09-2025
Algemene gegevens
De ontwikkeling/herziening van deze richtlijnmodule werd ondersteund door het Kennisinstituut van de Federatie Medisch Specialisten (www.demedischspecialist.nl/kennisinstituut) en werd gefinancierd uit de Stichting Kwaliteitsgelden Medisch Specialisten (SKMS). De financier heeft geen enkele invloed gehad op de inhoud van de richtlijnmodule.
Samenstelling werkgroep
Voor het ontwikkelen van de richtlijnmodule is in 2022 een multidisciplinaire werkgroep ingesteld, bestaande uit vertegenwoordigers van alle relevante specialismen (zie hiervoor de samenstelling van de werkgroep) die betrokken zijn bij de zorg voor patiënten met niet-acute astma in de tweedelijn.
Werkgroep
- Dr. B. (Bas) Langeveld, longarts, werkzaam in het Deventer ziekenhuis te Deventer, NVALT (voorzitter)
- Dr. A. (Astrid) Aardenburg – van Huisstede, longarts, werkzaam in Noordwest Ziekenhuisgroep
- Dr. M (Marijke) Amelink, longarts, werkzaam in het Spaarne Gasthuis te Haarlem, NVALT
- Drs. L.M. (Leonie) Imming, longarts, werkzaam in Medisch Spectrum Twente te Enschede, NVALT
- Dr. B. (Bart) Hilvering, longarts, werkzaam in het Amsterdam UMC te Amsterdam, NVALT
- Dr. J.N.G. (Hanneke) Oude Elberink, allergoloog, werkzaam in het Universitair Medisch Centrum Groningen te Groningen, NIV/NVvAKI
- Dr. M.E. (Marjolein) Cornet, KNO-arts, werkzaam in het Alrijne ziekenhuis te Leiden, NVKNO
- Prof. dr. J.W.M. (Jean) Muris, huisarts en hoogleraar Huisartsgeneeskunde, NHG
- Drs. M.H.A. (Mariëtte) Scholma-Bronsema, verpleegkundige specialist Astma/COPD/OSA, werkzaam in het Wilhelmina Ziekenhuis te Assen, V&VN
- Drs. Y. (Yvonne) Kappe, senior beleidsadviseur, Longfonds & VND
- Drs. E.M. (Esther) van der Roest, ervaringsdeskundige, VND
- S.T. (Saskia) van Dorst M, ervaringsdeskundige, Longfonds
Klankbordgroep
- Wendy Bokxem, Verpleegkundig specialist longgeneeskunde i.o, werkzaam in Ziekenhuisgroep Twente te Hengelo, V&VN
Met ondersteuning van
- M. (Mark) van Eck, junior adviseur, Kennisinstituut van de Federatie Medisch Specialisten
- Dr. J. (Janneke) Hoogervorst-Schilp, senior adviseur, Kennisinstituut van de Federatie Medisch Specialisten
- Dr. A.N. (Nynke) Kampstra, adviseur, Kennisinstituut van de Federatie Medisch Specialisten
Belangenverklaringen
De Code ter voorkoming van oneigenlijke beïnvloeding door belangenverstrengeling is gevolgd. Alle werkgroepleden hebben schriftelijk verklaard of zij in de laatste drie jaar directe financiële belangen (betrekking bij een commercieel bedrijf, persoonlijke financiële belangen, onderzoeksfinanciering) of indirecte belangen (persoonlijke relaties, reputatiemanagement) hebben gehad. Gedurende de ontwikkeling of herziening van een module worden wijzigingen in belangen aan de voorzitter doorgegeven. De belangenverklaring wordt opnieuw bevestigd tijdens de commentaarfase.
Een overzicht van de belangen van werkgroepleden en het oordeel over het omgaan met eventuele belangen vindt u in onderstaande tabel. De ondertekende belangenverklaringen zijn op te vragen bij het secretariaat van het Kennisinstituut van de Federatie Medisch Specialisten.
Werkgroep
|
Werkgroeplid |
Functie |
Nevenfuncties |
Gemelde belangen |
Ondernomen actie |
|
Dr. B. (Bas) Langeveld, (voorzitter) |
Longarts, Deventer ziekenhuis |
Incidenteel deelname adviesraad industrie (AstraZeneca, Novartis, GSK) over biologicals (betreft ernstig astma, dit valt buiten scope van de richtlijn) |
Deelname Studies: AstraZeneca: ANDHI, tot 2019 GSK: NIMBLE, start 2e kwartaal 2022 (PI) Studies betreffen middelen voor de behandeling van ernstig astma, dit valt buiten de inhoud van deze richtlijn. Opbrengst komt ten goede van de onderzoeksafdeling longziekten van het Deventer ziekenhuis |
Geen restricties (ernstig astma valt buiten deze richtlijn). Deelname adviesraad wordt neergelegd gedurende richltijnontwikkeling. |
|
Dr. A. (Astrid) Aardenburg – van Huisstede |
Longarts, Noordwest Ziekenhuisgroep |
Bijscholing huisartsen CASPIR (betaald) Principal Investigator diverse onderzoeken opgezet door farmacie (betaald) |
Voor alle genoemde studies lokale projectleider: * UMCG - High-flow therapie bij COPD * Astra Zeneca - Oberon studie (COPD) * Franciscus Gasthuis - Tricolon studie (COPD) * Rapsodie database ernstig astma - Geen projectleider |
Geen restricties |
|
Dr. M (Marijke) Amelink |
Longarts, Spaarne ziekenhuis |
Research waarbij unrestricted grant van teva (onbetaald voor mij) mbt ernstig astma. - spreker caspir cursus (betaald via caspir) - verschillende keren een adviesraad gedaan voor pharmacie (eenmalige vergoeding voor astra Z, GSK, Sanofi) - mede voorzitter noord NL symposium met Els Weersink (sponsor chiesi, onbetaald). Staat los van astma. Ik heb geen aandelen, opties of financiële belangen bij een bedrijf. Geen lopend betaald adviseurschap. |
1 unrestricted grand mbt ernstig astma |
Geen restricties |
|
Drs. L.M. (Leonie) Imming |
Longarts, MST |
Geen |
GSK: NIMBLE, start 2e kwartaal 2022. Studie betreft middelen voor de behandeling van ernstig astma, dit valt buiten de inhoud van deze richtlijn. |
Geen restricties |
|
Dr. B. (Bart) Hilvering |
Longarts, Amsterdam UMC |
Incidenteel advisory boards en voordrachten over ernstig astma door GSK, AstraZeneca, Sanofi, dit valt buiten de scope van de richtlijn |
Lokale PI voor de AIRLEAF en CLAIRLEAF studie, geïnitieerd door Boehringer Ingelheim, medicatie voor bronchiectasieen, dit valt buiten de scope van de richtlijn |
Geen restricties |
|
Dr. J.N.G. (Hanneke) Oude Elberink |
Internist-allergoloog, UMCG |
Geen |
Geen |
Geen restricties |
|
Dr. M.E. (Marjolein) Cornet |
KNO-arts, Alrijne ziekenhuis |
Geen |
Geen |
Geen restricties |
|
Prof. dr. J.W.M. (Jean) Muris |
Geen |
Geen |
Geen |
Geen restricties |
|
M.H.A. (Mariëtte) Scholma-Bronsema |
Verpleegkundig specialist longziekten Wilhelmina Ziekenhuis Assen |
Vrijwilliger longfonds voorzitter kwaliteitsteam Assen van de Huisartsen Zorg Drenthe. Lid van transmurale zorg aanpak astma-COPD (landelijke commissie). |
Geen |
Geen restricties |
|
Drs. Y. (Yvonne) Kappe |
Projectleider Longfonds en astmaVereniging Nederland en Davos
|
Geen |
Geen |
Geen restricties |
|
Drs. E.M. (Esther) van der Roest |
ErvaringsdeskundigeastmaVereniging Nederland en Davos |
Geen |
Geen |
Geen restricties |
|
S.T.M. (Saskia) van Dorst |
Ervaringsdeskundige Longfonds |
Geen |
Geen |
Geen restricties |
Klankbordgroep
|
Klankbordgroep lid |
Functie |
Nevenfuncties |
Gemelde belangen |
Ondernomen actie |
|
Drs. W. (Wendy) Bokxem |
Verpleegkundig specialist longgeneeskunde, ZGT Hengelo |
Geen |
Geen |
Geen restricties |
Inbreng patiëntenperspectief
Er werd aandacht besteed aan het patiëntenperspectief door afvaardiging van astmaVereniging Nederland en Davos en Longfonds in de werkgroep. De verkregen input is meegenomen bij het opstellen van de uitgangsvragen, de keuze voor de uitkomstmaten en bij het opstellen van de overwegingen (zie kopje waarden en voorkeuren van patiënten). De conceptrichtlijn is tevens voor commentaar voorgelegd aan de Patientenfederatie Nederland en de aangeleverde commentaren zijn bekeken en verwerkt.
Wkkgz & Kwalitatieve raming van mogelijke substantiële financiële gevolgen
Kwalitatieve raming van mogelijke financiële gevolgen in het kader van de Wkkgz. Bij de richtlijn is conform de Wet kwaliteit, klachten en geschillen zorg (Wkkgz) een kwalitatieve raming uitgevoerd of de aanbevelingen mogelijk leiden tot substantiële financiële gevolgen. Bij het uitvoeren van deze beoordeling zijn richtlijnmodules op verschillende domeinen getoetst (zie het stroomschema op de Richtlijnendatabase).
Uit de kwalitatieve raming blijkt dat er waarschijnlijk geen substantiële financiële gevolgen zijn, zie onderstaande tabel.
| Module |
Uitkomst raming |
Toelichting |
|
Gebruik van LAMA |
Geen financiële gevolgen |
Hoewel uit de toetsing volgt dat de aanbeveling(en) breed toepasbaar zijn (>40.000 patiënten), volgt uit de toetsing dat het overgrote deel (±90%) van de zorgaanbieders en zorgverleners al aan de norm voldoet. Er worden daarom geen financiële gevolgen verwacht. |
Werkwijze
AGREE
Deze richtlijnmodule is opgesteld conform de eisen vermeld in het rapport Medisch Specialistische Richtlijnen 2.0 van de adviescommissie Richtlijnen van de Raad Kwaliteit. Dit rapport is gebaseerd op het AGREE II instrument (Appraisal of Guidelines for Research & Evaluation II; Brouwers, 2010).
Knelpuntenanalyse en uitgangsvragen
Tijdens de voorbereidende fase inventariseerde de werkgroep de knelpunten in de zorg voor patiënten met niet-acute astma. Tevens zijn er (aanvullende) knelpunten aangedragen door de Nederlandse Associatie Physician Assistants, Longfonds en Astma Vereniging Nederland en Davos, NVALT-sectie astma & allergie, COPD & Astma Huisartsen Advies Groep en de Nederlandse Vereniging van Ziekenhuizen via een schriftelijke knelpuntenanalyse. Een verslag hiervan is opgenomen onder aanverwante producten. Op basis van de uitkomsten van de knelpuntenanalyse zijn door de werkgroep concept-uitgangsvragen opgesteld en definitief vastgesteld.
Uitkomstmaten
Na het opstellen van de zoekvraag behorende bij de uitgangsvraag inventariseerde de werkgroep welke uitkomstmaten voor de patiënt relevant zijn, waarbij zowel naar gewenste als ongewenste effecten werd gekeken. Hierbij werd een maximum van acht uitkomstmaten gehanteerd. De werkgroep waardeerde deze uitkomstmaten volgens hun relatieve belang bij de besluitvorming rondom aanbevelingen, als cruciaal (kritiek voor de besluitvorming), belangrijk (maar niet cruciaal) en onbelangrijk. Tevens definieerde de werkgroep tenminste voor de cruciale uitkomstmaten welke verschillen zij klinisch (patiënt) relevant vonden.
Methode literatuursamenvatting
Een uitgebreide beschrijving van de strategie voor zoeken en selecteren van literatuur is te vinden onder ‘Zoeken en selecteren’ onder Onderbouwing. Indien mogelijk werd de data uit verschillende studies gepoold in een random-effects model. Review Manager 5.4 werd gebruikt voor de statistische analyses. De beoordeling van de kracht van het wetenschappelijke bewijs wordt hieronder toegelicht.
Beoordelen van de kracht van het wetenschappelijke bewijs
De kracht van het wetenschappelijke bewijs werd bepaald volgens de GRADE-methode. GRADE staat voor ‘Grading Recommendations Assessment, Development and Evaluation’ (zie http://www.gradeworkinggroup.org/). De basisprincipes van de GRADE-methodiek zijn: het benoemen en prioriteren van de klinisch (patiënt) relevante uitkomstmaten, een systematische review per uitkomstmaat, en een beoordeling van de bewijskracht per uitkomstmaat op basis van de acht GRADE-domeinen (domeinen voor downgraden: risk of bias, inconsistentie, indirectheid, imprecisie, en publicatiebias; domeinen voor upgraden: dosis-effect relatie, groot effect, en residuele plausibele confounding).
GRADE onderscheidt vier gradaties voor de kwaliteit van het wetenschappelijk bewijs: hoog, redelijk, laag en zeer laag. Deze gradaties verwijzen naar de mate van zekerheid die er bestaat over de literatuurconclusie, in het bijzonder de mate van zekerheid dat de literatuurconclusie de aanbeveling adequaat ondersteunt (Schünemann, 2013; Hultcrantz, 2017).
|
GRADE |
Definitie |
|
Hoog |
|
|
Redelijk |
|
|
Laag |
|
|
Zeer laag |
|
Bij het beoordelen (graderen) van de kracht van het wetenschappelijk bewijs in richtlijnen volgens de GRADE-methodiek spelen grenzen voor klinische besluitvorming een belangrijke rol (Hultcrantz, 2017). Dit zijn de grenzen die bij overschrijding aanleiding zouden geven tot een aanpassing van de aanbeveling. Om de grenzen voor klinische besluitvorming te bepalen moeten alle relevante uitkomstmaten en overwegingen worden meegewogen. De grenzen voor klinische besluitvorming zijn daarmee niet één op één vergelijkbaar met het minimaal klinisch relevant verschil (Minimal Clinically Important Difference, MCID). Met name in situaties waarin een interventie geen belangrijke nadelen heeft en de kosten relatief laag zijn, kan de grens voor klinische besluitvorming met betrekking tot de effectiviteit van de interventie bij een lagere waarde (dichter bij het nuleffect) liggen dan de MCID (Hultcrantz, 2017).
Overwegingen (van bewijs naar aanbeveling)
Om te komen tot een aanbeveling zijn naast (de kwaliteit van) het wetenschappelijke bewijs ook andere aspecten belangrijk en worden meegewogen, zoals aanvullende argumenten uit bijvoorbeeld de biomechanica of fysiologie, waarden en voorkeuren van patiënten, kosten (middelenbeslag), aanvaardbaarheid, haalbaarheid en implementatie. Deze aspecten zijn systematisch vermeld en beoordeeld (gewogen) onder het kopje ‘Overwegingen’ en kunnen (mede) gebaseerd zijn op expert opinion. Hierbij is gebruik gemaakt van een gestructureerd format gebaseerd op het evidence-to-decision framework van de internationale GRADE Working Group (Alonso-Coello, 2016a; Alonso-Coello 2016b). Dit evidence-to-decision framework is een integraal onderdeel van de GRADE methodiek.
Formuleren van aanbevelingen
De aanbevelingen geven antwoord op de uitgangsvraag en zijn gebaseerd op het beschikbare wetenschappelijke bewijs en de belangrijkste overwegingen, en een weging van de gunstige en ongunstige effecten van de relevante interventies. De kracht van het wetenschappelijk bewijs en het gewicht dat door de werkgroep wordt toegekend aan de overwegingen, bepalen samen de sterkte van de aanbeveling. Conform de GRADE-methodiek sluit een lage bewijskracht van conclusies in de systematische literatuuranalyse een sterke aanbeveling niet a priori uit, en zijn bij een hoge bewijskracht ook zwakke aanbevelingen mogelijk (Agoritsas, 2017; Neumann, 2016). De sterkte van de aanbeveling wordt altijd bepaald door weging van alle relevante argumenten tezamen. De werkgroep heeft bij elke aanbeveling opgenomen hoe zij tot de richting en sterkte van de aanbeveling zijn gekomen.
In de GRADE-methodiek wordt onderscheid gemaakt tussen sterke en zwakke (of conditionele) aanbevelingen. De sterkte van een aanbeveling verwijst naar de mate van zekerheid dat de voordelen van de interventie opwegen tegen de nadelen (of vice versa), gezien over het hele spectrum van patiënten waarvoor de aanbeveling is bedoeld. De sterkte van een aanbeveling heeft duidelijke implicaties voor patiënten, behandelaars en beleidsmakers (zie onderstaande tabel). Een aanbeveling is geen dictaat, zelfs een sterke aanbeveling gebaseerd op bewijs van hoge kwaliteit (GRADE gradering HOOG) zal niet altijd van toepassing zijn, onder alle mogelijke omstandigheden en voor elke individuele patiënt.
|
Implicaties van sterke en zwakke aanbevelingen voor verschillende richtlijngebruikers |
||
|
|
Sterke aanbeveling |
Zwakke (conditionele) aanbeveling |
|
Voor patiënten |
De meeste patiënten zouden de aanbevolen interventie of aanpak kiezen en slechts een klein aantal niet. |
Een aanzienlijk deel van de patiënten zouden de aanbevolen interventie of aanpak kiezen, maar veel patiënten ook niet. |
|
Voor behandelaars |
De meeste patiënten zouden de aanbevolen interventie of aanpak moeten ontvangen. |
Er zijn meerdere geschikte interventies of aanpakken. De patiënt moet worden ondersteund bij de keuze voor de interventie of aanpak die het beste aansluit bij zijn of haar waarden en voorkeuren. |
|
Voor beleidsmakers |
De aanbevolen interventie of aanpak kan worden gezien als standaardbeleid. |
Beleidsbepaling vereist uitvoerige discussie met betrokkenheid van veel stakeholders. Er is een grotere kans op lokale beleidsverschillen. |
Organisatie van zorg
In de knelpuntenanalyse en bij de ontwikkeling van de richtlijnmodule is expliciet aandacht geweest voor de organisatie van zorg: alle aspecten die randvoorwaardelijk zijn voor het verlenen van zorg (zoals coördinatie, communicatie, (financiële) middelen, mankracht en infrastructuur). Randvoorwaarden die relevant zijn voor het beantwoorden van deze specifieke uitgangsvraag zijn genoemd bij de overwegingen. Meer algemene, overkoepelende, of bijkomende aspecten van de organisatie van zorg worden behandeld in de module Organisatie van zorg.
Commentaar- en autorisatiefase
De conceptrichtlijnmodule werd aan de betrokken (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd ter commentaar. De commentaren werden verzameld en besproken met de werkgroep. Naar aanleiding van de commentaren werd de conceptrichtlijnmodule aangepast en definitief vastgesteld door de werkgroep. De definitieve richtlijnmodule werd aan de deelnemende (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd voor autorisatie en door hen geautoriseerd dan wel geaccordeerd.
Literatuur
Agoritsas T, Merglen A, Heen AF, Kristiansen A, Neumann I, Brito JP, Brignardello-Petersen R, Alexander PE, Rind DM, Vandvik PO, Guyatt GH. UpToDate adherence to GRADE criteria for strong recommendations: an analytical survey. BMJ Open. 2017 Nov 16;7(11):e018593. doi: 10.1136/bmjopen-2017-018593. PubMed PMID: 29150475; PubMed Central PMCID: PMC5701989.
Alonso-Coello P, Schünemann HJ, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Rada G, Rosenbaum S, Morelli A, Guyatt GH, Oxman AD; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ. 2016 Jun 28;353:i2016. doi: 10.1136/bmj.i2016. PubMed PMID: 27353417.
Alonso-Coello P, Oxman AD, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Vandvik PO, Meerpohl J, Guyatt GH, Schünemann HJ; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 2: Clinical practice guidelines. BMJ. 2016 Jun 30;353:i2089. doi: 10.1136/bmj.i2089. PubMed PMID: 27365494.
Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, Fervers B, Graham ID, Grimshaw J, Hanna SE, Littlejohns P, Makarski J, Zitzelsberger L; AGREE Next Steps Consortium. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010 Dec 14;182(18):E839-42. doi: 10.1503/cmaj.090449. Epub 2010 Jul 5. Review. PubMed PMID: 20603348; PubMed Central PMCID: PMC3001530.
Hultcrantz M, Rind D, Akl EA, Treweek S, Mustafa RA, Iorio A, Alper BS, Meerpohl JJ, Murad MH, Ansari MT, Katikireddi SV, Östlund P, Tranæus S, Christensen R, Gartlehner G, Brozek J, Izcovich A, Schünemann H, Guyatt G. The GRADE Working Group clarifies the construct of certainty of evidence. J Clin Epidemiol. 2017 Jul;87:4-13. doi: 10.1016/j.jclinepi.2017.05.006. Epub 2017 May 18. PubMed PMID: 28529184; PubMed Central PMCID: PMC6542664.
Medisch Specialistische Richtlijnen 2.0 (2012). Adviescommissie Richtlijnen van de Raad Kwalitieit. http://richtlijnendatabase.nl/over_deze_site/over_richtlijnontwikkeling.html.
Neumann I, Santesso N, Akl EA, Rind DM, Vandvik PO, Alonso-Coello P, Agoritsas T, Mustafa RA, Alexander PE, Schünemann H, Guyatt GH. A guide for health professionals to interpret and use recommendations in guidelines developed with the GRADE approach. J Clin Epidemiol. 2016 Apr;72:45-55. doi: 10.1016/j.jclinepi.2015.11.017. Epub 2016 Jan 6. Review. PubMed PMID: 26772609.
Schünemann H, Brożek J, Guyatt G, et al. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group, 2013. Available from http://gdt.guidelinedevelopment.org/central_prod/_design/client/handbook/handbook.html.
Zoekverantwoording
Zoekstrategie
Embase (May 9, 2024)
|
No. |
Query |
Results |
|
#14 |
#13 NOT #11 |
233 |
|
#13 |
#9 AND #12 |
288 |
|
#12 |
'randomized controlled trial'/exp OR random*:ti,ab OR (((pragmatic OR practical) NEAR/1 'clinical trial*'):ti,ab) OR ((('non inferiority' OR noninferiority OR superiority OR equivalence) NEAR/3 trial*):ti,ab) OR rct:ti,ab,kw |
2194524 |
|
#11 |
#9 AND #10 |
188 |
|
#10 |
'meta analysis'/exp OR 'meta analysis (topic)'/exp OR metaanaly*:ti,ab OR 'meta analy*':ti,ab OR metanaly*:ti,ab OR 'systematic review'/de OR 'cochrane database of systematic reviews'/jt OR prisma:ti,ab OR prospero:ti,ab OR (((systemati* OR scoping OR umbrella OR 'structured literature') NEAR/3 (review* OR overview*)):ti,ab) OR ((systemic* NEAR/1 review*):ti,ab) OR (((systemati* OR literature OR database* OR 'data base*') NEAR/10 search*):ti,ab) OR (((structured OR comprehensive* OR systemic*) NEAR/3 search*):ti,ab) OR (((literature NEAR/3 review*):ti,ab) AND (search*:ti,ab OR database*:ti,ab OR 'data base*':ti,ab)) OR (('data extraction':ti,ab OR 'data source*':ti,ab) AND 'study selection':ti,ab) OR ('search strategy':ti,ab AND 'selection criteria':ti,ab) OR ('data source*':ti,ab AND 'data synthesis':ti,ab) OR medline:ab OR pubmed:ab OR embase:ab OR cochrane:ab OR (((critical OR rapid) NEAR/2 (review* OR overview* OR synthes*)):ti) OR ((((critical* OR rapid*) NEAR/3 (review* OR overview* OR synthes*)):ab) AND (search*:ab OR database*:ab OR 'data base*':ab)) OR metasynthes*:ti,ab OR 'meta synthes*':ti,ab |
1026297 |
|
#9 |
#6 NOT #7 AND [2000-2024]/py |
2061 |
|
#8 |
#6 NOT #7 |
2123 |
|
#7 |
#6 AND ('Conference Abstract'/it OR 'Conference Paper'/it OR 'Conference Review'/it OR 'Editorial'/it OR 'Letter'/it OR 'Note'/it) |
1048 |
|
#6 |
#5 NOT (('adolescent'/exp OR 'child'/exp) NOT ('adult'/exp OR 'aged'/exp OR 'middle aged'/exp)) |
3171 |
|
#5 |
#3 AND #4 |
3344 |
|
#4 |
'tiotropium bromide'/exp OR 'glycopyrronium'/exp OR 'glycopyrronium bromide plus indacaterol plus mometasone furoate'/exp OR 'umeclidinium'/exp OR ((triple NEAR/5 (therap* OR treat*)):ti,ab,kw) OR acopair:ti,ab,kw OR airtiyo:ti,ab,kw OR braltus:ti,ab,kw OR favynd:ti,ab,kw OR gregal:ti,ab,kw OR lutio:ti,ab,kw OR orseba:ti,ab,kw OR spiriva:ti,ab,kw OR srivasso:ti,ab,kw OR tiogiva:ti,ab,kw OR tiotropium*:ti,ab,kw OR tiotrus:ti,ab,kw OR tiova:ti,ab,kw OR asecryl:ti,ab,kw OR cuvposa:ti,ab,kw OR dartisla:ti,ab,kw OR enurev:ti,ab,kw OR gastrodyn:ti,ab,kw OR glersa:ti,ab,kw OR glycopyrrolat*:ti,ab,kw OR glycopyrronium*:ti,ab,kw OR lonhala:ti,ab,kw OR mobinul:ti,ab,kw OR nodapton:ti,ab,kw OR qbrexza:ti,ab,kw OR robinal:ti,ab,kw OR robinol:ti,ab,kw OR robinul:ti,ab,kw OR seebri:ti,ab,kw OR sialanar:ti,ab,kw OR sroton:ti,ab,kw OR strodin:ti,ab,kw OR tarodyl:ti,ab,kw OR tarodyn:ti,ab,kw OR tovanor:ti,ab,kw OR enerzair:ti,ab,kw OR zimbus:ti,ab,kw OR ellipta:ti,ab,kw OR incruse:ti,ab,kw OR rolufta:ti,ab,kw OR umeclidinium*:ti,ab,kw |
45109 |
|
#3 |
'asthma'/exp OR asthma*:ti,ab,kw |
366868 |
Ovid/Medline (May 9, 2024)
|
1 |
"Asthma"/ or asthma*.ti,ab,kf. |
207137 |
|
2 |
exp "Tiotropium Bromide"/ or exp "Glycopyrrolate"/ or GSK573719.nm. or ((triple adj5 (therap* or treat*)) or acopair or airtiyo or braltus or favynd or gregal or lutio or orseba or spiriva or srivasso or tiogiva or tiotropium* or tiotrus or tiova or asecryl or cuvposa or dartisla or enurev or gastrodyn or glersa or glycopyrrolat* or glycopyrronium* or lonhala or mobinul or nodapton or qbrexza or robinal or robinol or robinul or seebri or sialanar or sroton or strodin or tarodyl or tarodyn or tovanor or enerzair or zimbus or ellipta or incruse or rolufta or umeclidinium*).ti,ab,kf. |
20701 |
|
3 |
1 and 2 |
790 |
|
4 |
3 not ((exp "Adolescent"/ or exp "Child"/ or exp "Infant"/) not exp "Adult"/) |
744 |
|
5 |
limit 4 to yr="2000 -Current" |
726 |
|
6 |
meta-analysis/ or meta-analysis as topic/ or (metaanaly* or meta-analy* or metanaly*).ti,ab,kf. or systematic review/ or cochrane.jw. or (prisma or prospero).ti,ab,kf. or ((systemati* or scoping or umbrella or "structured literature") adj3 (review* or overview*)).ti,ab,kf. or (systemic* adj1 review*).ti,ab,kf. or ((systemati* or literature or database* or data-base*) adj10 search*).ti,ab,kf. or ((structured or comprehensive* or systemic*) adj3 search*).ti,ab,kf. or ((literature adj3 review*) and (search* or database* or data-base*)).ti,ab,kf. or (("data extraction" or "data source*") and "study selection").ti,ab,kf. or ("search strategy" and "selection criteria").ti,ab,kf. or ("data source*" and "data synthesis").ti,ab,kf. or (medline or pubmed or embase or cochrane).ab. or ((critical or rapid) adj2 (review* or overview* or synthes*)).ti. or (((critical* or rapid*) adj3 (review* or overview* or synthes*)) and (search* or database* or data-base*)).ab. or (metasynthes* or meta-synthes*).ti,ab,kf. |
744780 |
|
7 |
5 and 6 |
59 |
|
8 |
exp clinical trial/ or randomized controlled trial/ or exp clinical trials as topic/ or randomized controlled trials as topic/ or Random Allocation/ or Double-Blind Method/ or Single-Blind Method/ or (clinical trial, phase i or clinical trial, phase ii or clinical trial, phase iii or clinical trial, phase iv or controlled clinical trial or randomized controlled trial or multicenter study or clinical trial).pt. or random*.ti,ab. or (clinic* adj trial*).tw. or ((singl* or doubl* or treb* or tripl*) adj (blind$3 or mask$3)).tw. or Placebos/ or placebo*.tw. |
2723256 |
|
9 |
5 and 8 |
294 |
|
10 |
9 not 7 |
250 |