Niet-medicamenteuze interventies
Uitgangsvraag
Wat is de plaats van niet-medicamenteuze interventies als vervanging van of in aanvulling op medicamenteuze sedatie?
Aanbeveling
Overweeg het toevoegen van niet-medicamenteuze interventies, die afleiden van pijn en angst, aan medicamenteuze PSA om hiermee het comfort vergroten.
Overweeg hierbij met name de minimaal belastende interventies, zoals muziek, video of virtual reality beleving.
Bespreek met de patiënt welke niet-medicamenteuze mogelijkheden er zijn en beslis samen welke niet-medicamenteuze behandeling het beste bij de patiënt en de situatie past.
Overweeg om het effect bij het gebruik van de niet-medicamenteuze interventies te meten en documenteren door middel van objectieve scores voor patiënttevredenheid, pijnbeleving en duur van de procedure.
Overwegingen
Voor- en nadelen van de interventie en de kwaliteit van het bewijs
In de literatuur wordt geen overtuigend bewijs gevonden voor het toevoegen van afleiding aan PSA. De bewijskracht is zeer laag. Dit komt voornamelijk omdat de studies niet geblindeerd zijn, er veel verschillende patiëntenpopulaties worden onderzocht en de studieresultaten niet eenduidig dezelfde kant op wijzen. Er ligt hier een kennislacune.
Daarnaast wordt er in de literatuur ook geen overtuigend bewijs gevonden voor toevoegen van hypnose aan PSA. De studies suggereren een kortere duur van de procedure. De studieresultaten voor de uitkomstmaat pijn wijzen niet eenduidig dezelfde kant op. De bewijskracht is laag tot zeer laag. Dit komt voornamelijk omdat de studies niet geblindeerd zijn en er veel verschillende patiëntpopulaties worden onderzocht. Voor de uitkomstmaat patiënttevredenheid ontbreekt het überhaupt aan wetenschappelijke onderbouwing. Er ligt hier een kennislacune. Van de andere kant impliceert “absence of evidence” niet altijd ook “evidence of absence”. Een multimodale benadering van pijn en angst kan soms meer effect hebben dan de som der delen. Betreffende de mogelijke uitkomsten van niet-medicamenteuze interventies bij het toedienen van PSA, is het belangrijk te realiseren dat een (verschil in) dosering van een sedativum weliswaar goed te meten is, maar daarmee niet vanzelfsprekend klinisch relevant hoeft te zijn.
Waarden en voorkeuren van patiënten (en evt. hun verzorgers)
Vanuit de pragmatische invalshoek, klinkt het logisch dat comfort verhogende omstandigheden gepaard zouden kunnen gaan met meer patiënttevredenheid en daaraan gekoppeld angstreductie. Deze uitspraak is gebaseerd op ervaring in de praktijk en niet onderbouwd in de literatuur. Goede informatie vooraf over een procedure en sedatie kan angst verminderen.
Daarnaast is voor het ervaren van pijn essentieel dat er een koppeling is tussen een nociceptieve prikkel en de bewuste beleving hiervan (corticaal proces).
Indien een niet-medicamenteuze interventie kan leiden tot verlaagde aandacht/awareness/bewustzijn (afleiding, verleggen van focus) van een nociceptieve prikkel, is het aannemelijk dat deze als minder/niet pijnlijk kan worden ervaren door een patiënt, ook als er nog reflexmatige reacties (bijvoorbeeld bewegen, terugtrekken, verhoogde hartslag, ademhaling of bloeddruk) zichtbaar zijn. Bespreek met de patiënt welke niet-medicamenteuze mogelijkheden er zijn en beslis samen welke niet-medicamenteuze behandeling het beste bij de patiënt en de situatie past.
Kosten (middelenbeslag)
Het zou wenselijk zijn als aannames en veronderstellingen onderbouwd worden door sterke wetenschappelijke data en kosten/baten analyse. Dit is niet altijd haalbaar en gangbare werkwijze is dat, zolang niet-medicamenteuze interventies gepaard gaan met beperkte kosten en minimale belasting voor een patiënt en procedure, empirische data verkregen wordt uit de praktijk. Belangrijk kader m.b.t. kostenbeheersing, is het voorkomen van inzet van extra personeel en/of dure materialen voor het bewerkstelligen van een niet-medicamenteuze interventie. Als er hoge aanschafkosten van materialen (bijv. VR-bril vs. koptelefoon) zijn en/of aanzienlijke tijdsinvesteringen nodig (bijv. EMDR) rondom een niet-medicamenteuze interventie, terwijl er geen verschil in uitkomst is, dan kunnen deze lasten meegenomen worden in de afweging om de niet-medicamenteuze interventie te implementeren.
Aanvaardbaarheid, haalbaarheid en implementatie
Pragmatische en empirische afwegingen zullen leidend moeten zijn om een goede balans in de voor- en nadelen van een implementatie te kunnen onderbouwen. Door praktijkvariatie van zowel interventies als PSA (middelen), zijn de lokale PSA-teams de meest aangewezen betrokkenen, om de haalbaarheid en kosten-baten balans op te maken en af te stemmen met de lokale sedatie commissies.
Rationale van aanbeveling 1: weging van argumenten voor en tegen de interventies
De werkgroep ziet potentiële meerwaarde in het gebruik van niet-medicamenteuze interventies, mits deze op lokaal niveau wenselijk en haalbaar worden geacht en voldoende afgewogen worden onderbouwd.
Gezien de beperkte level of evidence voor de verschillende methoden, dienen deze interventies als aanvullend te worden beschouwd, niet ter vervanging van PSA.
Minimaal invasieve technieken (bijv. muziek, video of VR) kunnen geïntegreerd worden met medicamenteuze PSA.
Rationale van aanbeveling 2: weging van argumenten voor en tegen de interventies
Indien niet-medicamenteuze interventies worden toegepast, is het belangrijk om hierbij te overwegen de relevante uitkomst criteria te formuleren en deze te registreren.
Door praktijkvariatie van zowel interventies als PSA (middelen), zijn de lokale PSA-teams de meest aangewezen betrokkenen, om deze te formuleren en te integreren in het patiëntendossier. Objectieve scores voor patiënttevredenheid, pijnbeleving, duur van de procedures en medicatie doseringen, kunnen indicatief zijn voor de uitkomst van een interventie. Hoewel deze scores niet altijd even klinisch relevant of significant zijn, kan er wel een indicatieve interpretatie aan gegeven worden en kunnen ze gebruikt worden voor follow up.
Onderbouwing
Achtergrond
Bij procedurele sedatie en analgesie (PSA) wordt primair gedacht aan medicamenteuze behandeling, terwijl anxiolyse en pijnervaring ook gedempt kunnen worden door niet-medicamenteuze interventies. Zo bestaan er naast het toedienen van hypnotische en/of analgetische middelen voor PSA ook veel niet-medicamenteuze interventies, zoals virtual reality, video’s, neuro linguïstisch programmeren, hypnose, cognitieve therapie, EMDR, muziek, ademhalingsoefeningen of een prikkelarme omgeving. Deze kunnen in plaats van sedatiemiddelen of als adjuvans aan medicamenteuze sedatie worden ingezet.
Op dit moment is er veel onduidelijk over welke niet-medicamenteuze technieken bewezen van meerwaarde zijn bij de PSA van volwassenen. Er zijn wel lokale initiatieven die nieuwe technieken proberen, maar er bestaat nog geen landelijke richtlijn over. Daarnaast bestaat er grote praktijkvariatie op zowel het gebied van de soorten technieken als hoe deze technieken worden ingezet.
Conclusies / Summary of Findings
Conclusions (1. Distraction)
Patient satisfaction (crucial)
|
low GRADE |
Distraction may increase patient satisfaction when compared with standard care in adult patients undergoing procedural sedation and analgesia.
Sources: Bechtold, 2006; Moon, 2018; Wang, 2014 |
Pain (crucial)
|
Very low GRADE |
The evidence is very uncertain about the effect of distraction on pain when compared with standard care in adult patients undergoing procedural sedation and analgesia.
Sources: Bechtold, 2006; De Silva, 2016; Ebrahimi, 2020; Kang, 2006; Sjölander, 2019; Wang, 2014 |
Procedure duration (important)
|
low GRADE |
Distraction may decrease the procedure duration when compared with standard care in adult patients undergoing procedural sedation and analgesia.
Sources: Akdoğan, 2021; Bechtold, 2006; De Silva, 2016; Fang, 2016; Kang, 2006; Kukreja, 2020; Kulkarni, 2012; Moon, 2018; Wang, 2014 |
Conclusions (2. Breathing exercises, relaxation techniques)
Patient satisfaction (crucial)
|
no GRADE |
No evidence was found regarding the effect of hypnosis in addition to standard care on patient satisfaction when compared with standard care in adult patients undergoing procedural sedation and analgesia. Sources: - |
Pain (crucial)
|
very low GRADE |
The evidence is very uncertain about the effect of hypnosis in addition to standard care on pain when compared with standard care alone in adult patients undergoing procedural sedation and analgesia. Sources: Izanloo, 2015; Mackey, 2018; Noergaard, 2019 |
Procedure duration (important)
|
low GRADE |
Hypnosis in addition to standard care may result in little to no difference in procedure duration when compared with standard care in adult patients undergoing procedural sedation and analgesia. Sources: Noergaard, 2019 |
Samenvatting literatuur
Description of studies
1. Distraction
Wang (2014) performed a systematic review and meta-analysis to determine effects of music during several types of endoscopies. Only randomized controlled trials (RCTs) were included that executed these procedures with and without music. Several databases were searched for RCTs published up to July 2013, including the Cochrane Library, PubMed, and Embase. Twenty-one RCTs comparing patients undergoing various endoscopic examinations with and without music were included, with a total sample size of 2,134. In all trials, standard care was used as the control (no music) condition. The music was self-selected in ten of the trials, and selected by the researchers in eleven trials. In two studies, patients aged younger than 18 years old were included, and in two studies age was not reported. The Cochrane Risk of Bias tool for RCTs was used assess quality of the studies. Overall, the authors report low risk of bias in the included studies. The reported outcome measures in the study were: patient satisfaction, pain, sedative dose, and procedure duration.
Akdoğan (2021) performed an RCT to compare music therapy with a non-music condition in patients undergoing elective non-oncological orthopedic surgery under spinal anesthesia. No information about the time and setting of the study was reported. Exclusion criteria were hearing loss, professional music practitioners, patients using medication that could affect the hypothalamo-hypophyseal and sympathetic system, patients who did not like the type of music. Note that new patients were recruited in place of patients who were excluded for any reason to reach the planned sample size. Fifty patients were included in the study, of which 25 to the music group (mean age: 41.96, SD: 11.41 y) and 25 to the no music group (mean age: 43.00, SD: 11.26 y). Propofol infusion was started at 1 mg/kg/hour in patients who underwent spinal anesthesia in the operation room. The reported outcome measure in the study was: procedure duration. The study has a high risk of bias as the study was not blinded.
Bashiri (2018) performed a randomized controlled double-blinded study to assess effects of music therapy in addition to sedation administered during endoscopy and colonoscopy in adult patients. The study was conducted between June and October 2017 in a hospital gastroenterology unit in Ankara, Turkey. Patients with endoscopic ultrasound and endoscopic retrograde cholangiopancreatography were excluded from the study. Patients were randomized to one of four groups:
- conscious sedation with music (n=33, median age: 44, age range 28-65);
- conscious sedation without music (n=25, median age: 46, age range 28-69);
- deep sedation with music (n=41, median age: 42, age range 24-65);
- deep sedation without music (n=55, median age: 44, range 28-60).
Patients were sedated either by gastroenterologists (conscious sedation) or by anesthesiologists (deep sedation). In the conscious sedation groups, 2 mg of midazolam was administered. In the deep sedation groups, patients were administered 1-2 mg of midazolam, 0.1-0.3 mg/kg of ketamine, and 1-3 mg/kg of propofol. Incremental 20 mg of propofol was administered when the patient moved or felt pain. Music was applied through headphones. In the control groups, no music was played through the headphones. Patients chose their favorite music. The reported outcome measure in the study was: sedation dose. The study has a risk of bias due to baseline differences between the groups.
Bechtold (2006) performed and RCT to compare effects of relaxing music versus no music during colonoscopy under low-dose conscious sedation in 116 patients. No information about the time and setting of the study was reported. Eighty-five patients were randomized the music group (mean age: 58.5 y, SD: not reported), and 81 to the no music group (mean age: 54.1 y, SD: not reported). Exclusion criteria were: patients with a history of prior colon resection, patients scheduled to undergo same-day esophagogastroduodenoscopy (EGD) and colonoscopy, and inability to give informed consent. In the music group, a CD player was playing relaxing music upon the entrance of the patient into the procedure room. Procedures were started after the patient received 50 mg of meperidine and 1 or 2 mg of midazolam. Additional medication was given at the discretion of the endoscopist. The reported outcome measures in the study were: patient satisfaction, pain, procedure duration and sedation dose. The study has a risk of bias as only the patients were blinded.
De Silva (2016) performed an RCT to compare the effects of audio (AD) and visual distraction (VD) compared to standard care in reducing discomfort and the need for sedation during colonoscopy. The study was carried out in the Professorial Endoscopy Unit, Colombo North Teaching Hospital, Ragama, Sri Lanka, from May 2014 to May 2015. Exclusion criteria were: visual or hearing impairment, allergies or hypersensitivity to premedication, patients who had had abdominal surgery or colectomy, personal history of anxiety or psychiatric disorder, and pregnancy. Two-hundred patients were randomized to one of three groups: 66 patients with a mean age of 54.4 (SD: 12.5) listened to music of their choice, 67 patients with a mean age of 51.5 (SD: 14.6) watched a movie of their choice, and 67 patients with a mean age of 56.5 (SD: 13.8) did not listen to music or watched a movie. Patient controlled analgesia and sedation were administered to all three groups. Sedation consisted of 25 mg of pethidine in 5-mg aliquots and 2.5mg of midazolam in 0.5-mg aliquots. The reported outcome measures in the study were: pain, procedure duration and sedation dose. The study has a risk of bias as the study was not double blinded.
Ebrahimi (2020) performed a prospective pilot study to assess the effect of music on the use of opiates and benzodiazepines and levels of pain and anxiety in adult patients undergoing invasive coronary angiography. The study was conducted between November 2017 and May 2018 in a medical and nursing department Los Angeles, USA. Exclusion criteria included acute myocardial infarction requiring emergency ICA, documented history of major hearing problems or deafness, and contraindication to standard care medications. Seventy-two patients were randomized and enrolled in the study, with 35 randomized to the music group (mean age: 69.57, SD: 8.92) and 37 to the standard care control group (mean age: 68.51, SD: 6.48). All patients received local anesthesia with two percent lidocaine subcutaneously. Midazolam (0.5-1.0 mg) and/or fentanyl (25-50 μg) were administered intravenously to all subjects who requested pharmacologic sedation. Thus, not all patients were sedated pre-emptively. In the music group, music was self-selected and played prior, during, and after the procedure. Music was delivered through a portable media player with single-use, disposable ear buds. Note that patients and caregivers were not blind to the intervention. The reported outcome measures in the study were: pain and sedation dose. The study has a risk of bias as the study was not double blinded.
Fang (2016) performed a study in adult patients (≥ 18 y) to investigate clinical efficacy, safety, and feasibility of wearing video glasses during elective interventional radiologic (IR) procedures. The study was conducted between August 2012 and August 2013 in New York, USA. Exclusion criteria were patients undergoing emergency procedures, requirement of general anesthesia, history of hearing difficulties, visual difficulties, or epileptic seizures. Eighty-three patients were randomized, of which 39 to the experimental group wearing video glasses, and 44 to the control group without video glasses. During the IR procedure, all patients in the experimental group were fitted with video glasses and earpieces. The patient chose a video from a preset list of videos, which included documentaries and general audience–rated movies. Total doses of sedation (intravenous midazolam) and analgesia (intravenous fentanyl) used during the procedure were recorded for both groups. Mean age in the experimental group was 53.1 (SD: 15.2), and 56.4 (SD: 13.5) in the control group. The reported outcome measure in the study was: procedure duration. The study has a risk of bias as the study was not blinded and the groups of patients were very heterogeneous.
Huang (2020) performed a single-center, randomized controlled trial on the effects of immersive virtual reality therapy (IVR) on intravenous patient-controlled sedation during elective total knee and total hip arthroplasty under regional anesthesia. The study was conducted at St Vincent’s Hospital in Melbourne, Australia between February and June 2016. Twenty-five patients were randomized to the experimental group (median age: 65, IQR: 57, 68), which received immersive virtual reality (a form of distraction therapy) and propofol patient-controlled sedation (PCS). Twenty-five control patients received propofol PCS alone (median age: 70, IQR: 64, 72). Both groups were able to control their intra-operative sedation using propofol, with instruction to use it whenever they felt too aware or anxious. Each press of the PCS supplied a propofol bolus of 400mcg/kg Ideal Body Weight with a 5-minute lockout period. The reported outcome measure in the study was: sedation dose. The study has a risk of bias as the study was not blinded.
Kang (2008) performed and RCT to determine whether playing music can reduce bispectral index (BIS) values during 1.2 μg/mL propofol sedation in patients undergoing total knee replacement under spinal-epidural anesthesia. The study was conducted in the operating room of a hospital in Seoul, South Korea. Exclusion criteria were: contraindication to regional anesthesia, patients taking herbal medicines or steroid drugs at least 6 months before surgery, or who had a history of chronic psychiatric drug use, poor auditory, renal, or liver function, and cardiopulmonary disease. Sixty-three patients were randomized to one of three groups: 20 to the noise group (standard operating room noise; mean age: 69.2, SD: 7.3 y), 19 to the silence group (ambient noise was blocked; mean age: 68.0, SD: 6.3 y), and 20 to the music group (self-selected music was played through headphones; mean age: 67.8, SD: 7.0 y). The reported outcome measures in the study were: pain and procedure duration. The study has a risk of bias as the study was not double blinded and only performed in the elderly.
Kukreja (2020) performed an RCT to assess effects of music therapy compared with a no music control group on sedation requirements, anxiety levels, and patient satisfaction for adult patients undergoing total knee arthroplasty under spinal anesthesia. The study was conducted between October 2018 and December 2019 in the UAB Highlands Hospital in Birmingham, USA. Exclusion criteria included hearing impairment secondary to age or disease, and any contraindications for spinal anesthesia. Fifty-seven patients were randomized and enrolled in the study, with 29 randomized to the music group (mean age: 65.14, SD: 1.82 y) and 28 to the standard care control group (mean age: 61.68, SD: 2.27 y). Intraoperatively, all patients were given a standardized dose of 1 mg midazolam and 50 mcg fentanyl, as well as started on a propofol infusion. The Bispectral Index Monitor was used to confirm moderate sedation (65-75), where a reading of 70 is labeled as a general representation of moderate sedation. Preoperatively, all patients in both groups received continuous adductor canal block for postoperative analgesia. Music was self-selected and delivered through a pair of study-provided headphones. Note that patients in the control group did not wear headphones during the operation. The reported outcome measures in the study were: procedure duration and sedation dose. The study has a risk of bias as the study was not blinded and a high proportion of the controls did not complete the study.
Kulkarni (2012) performed an RCT on the effects of playing patient-selected music during interventional radiological (IR) procedures on the doses of sedation and analgesia and anxiety levels of adult patients. The study was conducted at the Department of Radiology, Gartnavel General Hospital, Glasgow, UK. Patients were not included in the study in case of emergency procedures, procedures under general anesthesia, inability to consent, or when they had difficulty hearing. Note that 17 patients declined participation in the study because they insisted on listening to music during the procedure. During the IR procedure, headphones were applied to all patients. The 50 patients in the experimental group (mean age: 57, SD: 16) had self-selected music played to them and the 50 control patients (mean age: 59, SD: 15) had no music played during the procedure. Patient sedation and analgesia were administered as required following the departmental protocol. Note that sedation and/or analgesia were not administered pre-emptively in all procedures. The primary outcomes were reductions in the doses of drugs for sedation (midazolam) and analgesia (fentanyl). The reported outcome measures in the study were: procedure duration and sedation dose. The study has a risk of bias as the study was not double blinded.
Moon (2018) performed a prospective parallel non-crossover single-blind RCT to investigate effects of virtual reality (VR) distraction during endoscopic urologic surgery under spinal anesthesia. The study was conducted at the Seoul National University Hospital in Korea between March and November 2017. Inclusion criteria were scheduled endoscopic urologic surgery, including holmium laser enucleation of the prostate (HOLLEP) or transurethral resection of bladder tumor), and an ASA physical status classification of I–III. Patients with a history of chronic use of sedatives or narcotics (>6 months), alcohol or drug abuse, baseline pulse oximetry saturation of less than 90%, and baseline hemodynamic or respiratory instability were excluded. Thirty-seven patients were randomized, of which 18 to the VR group without sedative and 19 to the pharmacologic sedation group. The patients assigned to the VR group were subjected to a 30-min VR program. Patients watched an underwater view of the ocean while listening to narrations designed to induce relaxation and meditation. Sedation in the control group consisted of repeat doses of midazolam 1–2 mg every 30 min during the procedure. Median age in the VR group was: 69 (IQR: 65-70), and 69 (IQR: 63-72) in the control group. The reported outcome measures in the study were: patient satisfaction and procedure time. The study has a risk of bias as the study was not completely blinded.
Sjolander (2019) performed a single-blind RCT to examine whether patients’ experiences could be improved during colonoscopy by designing the examination room to include a digital screen showing calm nature films. The study was conducted at an endoscopy unit in Sweden between 2015 and 2017. Patients were randomized to the intervention group (i.e., the room showing films) or the control group (standard room). Mean age and SD of the 68 patients in the intervention group was 60 y (SD: 2.0), and 61 y (SD: 1.8) of the 69 control patients. In the intervention group, a loudspeaker playing nature sounds such as birdsong or flowing water was placed under the pillow on the examination bed. Five different films of calm nature scenes were randomly shown on the screen. All the colonoscopies were performed according to clinical routine. Each patient was offered an intravenous injection of a sedative drug (midazolam) and an analgesic drug (alfentanil). The reported outcome measure in the study was: pain. The study has a risk of bias as the study was not completely blinded.
Results (distraction)
Patient satisfaction (crucial)
This outcome measure was reported in three studies (Bechtold, 2006; Moon, 2018; Wang, 2014).
Five studies included in the SR from Wang (2014) reported patient satisfaction during endoscopy procedures. The satisfaction score was measured by different methods, therefore the standardized mean difference (SMD) was used for the meta-analysis. Individual study data, as well as the total number of patients included in this meta-analysis was not reported. The level of satisfaction was improved in the music group (SMD = 1.83, 95% CI 0.76 to 2.91). This means that the addition of music results in a higher patient satisfaction compared to the standard procedure. This is considered clinically relevant.
In the RCT performed by Bechtold (2006), 85 patients listened to relaxing music during a colonoscopy, 81 patients did not hear music during their procedure. Patient satisfaction was measured using three different scales: Experience I scale (1 = pleasant, 2 = tolerable, 3 = difficult, 4 = unacceptable); Experience II scale (1 = much better than I expected, 2 = somewhat better than I expected, 3 = about what I expected, 4 = somewhat worse than I expected, 5 = much worse than I expected); Experience III scale (100 mm scale where 0 represents pleasant and 100 represents worst experience I ever have). Patients in both the intervention and control group reported a tolerable patient satisfaction, using the Experience I scale. This means that the patient satisfaction was similar in both groups. When patient satisfaction is measured using the Experience II scale, the intervention group reported a median score of 1 (much better than I expected), and the control group reported a median score of 2 (somewhat better than I expected). This means that patients in the intervention group have a higher patient satisfaction compared to the control group. This difference is clinically relevant. When patient satisfaction is measured using the Experience III scale, the intervention group reported a mean satisfaction of 22.5, compared to 28.1 in the control group. This means that patients in the intervention group have a higher patient satisfaction (mean difference 5.6), however this difference is not considered clinically relevant.
In the RCT performed by Moon (2018), 18 patients received virtual reality distraction during endoscopic prostatectomy under spinal anesthesia, and 19 patients received pharmacological sedation. Patient satisfaction was measured on a 5-point Likert-like verbal rating scale according to a prespecified score-defining table (1 = extremely dissatisfied, 2 = dissatisfied, 3 = undecided, 4 = satisfied, 5 = extremely satisfied). Both groups reported a median patient satisfaction of 5, indicating that patients were extremely satisfied in both groups.
Pain (crucial)
The outcome measure was reported in six studies (Bechtold, 2006; De Silva, 2016; Ebrahimi, 2020; Kang, 2006; Sjölander, 2019; Wang, 2014). Due to heterogeneity in study populations and reporting of outcome measures, the study results were not pooled.
In the SR from Wang (2014) patient’s perception of pain during the procedures was measured on a linear analog scale from 0 (no pain) to 10 (maximal pain) or a VAS scale ranging from 0 (maxima pain) to 100 (no pain). Individual study data, as well as the total number of patients included in this meta-analysis was not reported. The overall effect of the meta-analysis based on the LAS favored music for pain reduction (weighted mean difference (WMD) -1.53, 95% CI -2.53 to -0.53). This means that the addition of music results in more pain reduction compared to the standard procedure, however the effect was small.
In the RCT performed by Bechtold (2006), 85 patients listened to relaxing music during a colonoscopy, and 81 patients did not hear music during their procedure. Pain was reported on a 100 mm scale where 0 represents not painful at all and 100 represents unbearable. It is unclear whether the patients were rating postoperative pain or pain experienced during the procedure. Standard deviations were not reported in this study. Patients who listened to music reported a mean pain intensity of 25.3 mm, while patients in the control group reported a mean pain intensity of 28.1 mm. The mean difference was 2.8 mm, in favour of the intervention group. However, this difference was not considered clinically relevant.
In the RCT performed by De Silva (2016), 66 patients listened to music during a colonoscopy in addition to standard treatment, and 67 patients received standard treatment only. Pain perceived by the patient during the procedure was reported on a 10-point VAS scale. Patients who listened to music reported a median pain intensity of 3 (IQR 2-4), while patients in the control group reported a pain intensity of 5 (IQR 3-8). This means that patients who listened to music had a lower pain score. This difference was clinically relevant.
In the RCT performed by Ebrahimi (2020), 35 patients listened to music during invasive coronary angiography without preplanned standard conscious sedation, and 37 patients received standard conscious sedation. Patients reported the periprocedural pain levels according to the Wong-Baker Faces analog pain rating scale. Patients who listened to music reported a mean pain of 0.96 (SD 1.40), whilst patients in the standard sedation group reported a mean pain of 0.92 (SD 2.18). The mean difference was 0.04 (95% CI -0.59 to 0.17). This difference was not considered clinically relevant.
In the RCT performed by Kang (2006), 20 patients listened to music during a total knee replacement, and 20 patients received standard care. Patients reported their postprocedure pain levels based on a 100-point VAS scale. Patients who listened to music reported a median pain level of 10 (IQR 0 to 66), patients in the control group reported a median pain level of 23 (IQR 0 to 70). This means that patients in the intervention group had a lower median pain score compared to the control group. This difference was considered clinically relevant.
In the RCT performed by Sjölander (2019), 68 patients received video distraction during colonoscopy, and 69 patients did not receive video distraction. The pain score was reported on a 10-point VAS scale, 15 minutes after the procedure. Patients who received distraction reported a mean pain intensity of 0.87 (SEM 0.17) and patients who did not receive distraction reported a mean pain intensity of 0.82 (SEM 0.17). This means that patients in the distraction group had on average a higher pain score (mean difference 0.05, 95% CI -0.42 to 0.52). However, this difference was not considered clinically relevant.
In the RCT performed by De Silva (2016), 67 patients watched a movie during a colonoscopy in addition to standard treatment, and 67 patients received standard treatment only. Pain was reported on a 10-point VAS scale. Patients who watched a movie reported a pain intensity of 4 (IQR 2-6), while patients in the control group reported a pain intensity of 5 (IQR 3-8). This means that patients who watched a movie have a lower pain score. This difference was clinically relevant.
Procedure duration (important)
This outcome measure was reported in eight studies (Akdoğan, 2021; Bechtold, 2006; De Silva, 2016; Fang, 2016; Kang, 2006; Kukreja, 2020; Kulkarni, 2012; Moon, 2018; Wang, 2014).
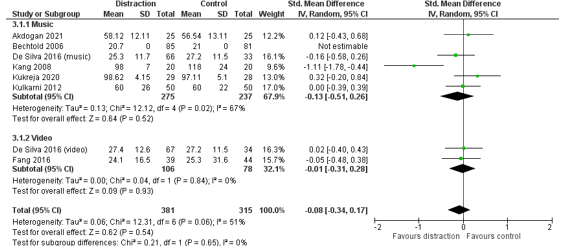
In total, 381 patients had distraction during their procedure, and 315 patients received standard care (control group) (Figure 1). The pooled effect estimate was SMD -0.08 (95% CI -0.34 to 0.17) in favour of distraction. This effect is somewhat larger in the subgroup of music distraction (SMD -0.13, 95% CI -0.51 to 0.26). This means that distraction, and specifically music distraction, results in a slightly shorter procedure duration, but the effect is small.
Figure 1. Outcome measure procedure duration for het comparison distraction versus standard care
Z: p-value pooled effect; df: degrees of freedom; I2: statistic heterogeneity; CI: confidence interval.
Wang (2014) did not report individual study data or the number of patients included in their meta-analysis. They included nine studies in their meta-analysis and report a shorter procedure duration in the intervention group, compared to the control group (WMD -0.88, 95% CI -2.52 to 0.75), however the effect is small.
Moon (2018) did not report the mean procedure duration with standard deviation, and was therefore not included in this meta-analysis. In the RCT performed by Moon (2018), 18 patients received virtual reality distraction during endoscopic prostatectomy under spinal anesthesia, and 19 patients received pharmacological sedation. The procedure took 40 minutes in the VR group (IQR 35 to 75) and 45 minutes in the control group (IQR 30 to 60). This means that the procedure took 5 minutes shorter when patients received VR distraction. This difference is not considered clinically relevant.
Level of evidence of the literature (distraction)
Patient satisfaction (crucial)
The level of evidence regarding this outcome measure comes from randomized clinical trials and therefore starts high. The level of evidence was downgraded by two levels because the studies were not blinded and lack of reporting individual study data and / or confidence intervals (risk of bias, 1 level); heterogeneity in study results (inconsistency, 1 level). The level of evidence is low.
Pain (crucial)
The level of evidence regarding this outcome measure comes from randomized clinical trials and therefore starts high. The level of evidence was downgraded by three levels because the studies were not blinded (risk of bias, 1 level); heterogeneity in study results (inconsistency, 1 level); and because the confidence intervals of the control and intervention group show a great amount of overlap (imprecision, 1 level). The level of evidence is very low.
Procedure duration (important)
The level of evidence regarding this outcome measure comes from randomized clinical trials and therefore starts high. The level of evidence was downgraded by two levels because of limitations in study design (risk of bias, 1 level) and heterogeneity in study results (inconsistency, 1 level).
2. Breathing exercises, relaxation techniques
None of the studies investigated effects of breathing exercises or relaxation techniques during/preceding PSA in patients that (will) undergo PSA.
3. Hypnosis, neuro-linguistic programming
Noergaard (2019) performed a systematic review and meta-analysis to determine effects of hypnotic analgesia in management of pain, anxiety, analgesic consumption, procedure length and adverse events in adults (18 years or older) undergoing minimally invasive procedures. Clinically controlled trials in which hypnosis was used together with pharmacological analgesia compared to pharmacological analgesia alone during invasive procedures were included. Several databases were searched for published and unpublished studies in July 2018, including MEDLINE via PubMed, CINAHL, the Cochrane Library, JBI Library, Scopus, Swemed+ and PsycINFO. Ten studies were included with a total sample size of 1,365 participants. Nine RCTs (Hizli, 2015; Lang, 2000, 2006, 2008; Lang, Joyce, Spiegel, Hamilton, & Lee, 1996; Marc, 2008, 2007; Shenefelt, 2013; Slack, 2009) and one quasi‐experimental study were included (Norgaard, 2013). The studies were published between 1996–2015 from four different countries: Six from the USA, two from Canada, one from Turkey and one from Denmark. The procedures in the studies included first‐trimester pregnancy termination, needle myography, biopsies, tumor treatments, angiographies, ablations, and skin lesion excisions. In all studies, patients were conscious during the procedure. Hypnotic analgesia was compared to standard care in all studies. In five studies, an extra arm in the randomization process was used, which was not included in the review: empathetic attention behavior, recorded hypnosis, or hypnosis without pain suggestion, respectively. Duration of the intervention differed between the studies but the content of the intervention was comparable, since all studies used an induction, guided imagery with analgesia suggestions and progressive muscle relaxation. In five studies, the intervention was provided periprocedural, in three studies the intervention started up to 20 min before the procedure and lasted throughout the procedure, and in two studies the intervention was provided before the procedure for 10–20 min and not during the procedure. In one study, participants listened to a 20‐min audio program, using a CD player with headphones. The intervention was provided to patients by a research assistant or an extra physician in all but one study. In one study, the intervention was provided by one of the procedure nurses in the procedure room. Risk of bias was assessed by the authors using the Cochrane Collaboration tool. Overall risk of bias across studies was moderate to high, due to performance and detection bias, which occurred in most of the studies. The reported outcome measures in the study were: pain, sedative dose, and procedure duration.
Izanloo (2015) performed a quasi-experimental trial (open-label, simple randomization) on the effects of conversational hypnosis compared with a control group receiving standard care on anxiety, endoscopy-related complications, and patient satisfaction during upper GI endoscopy. The study was conducted at the endoscopy unit of Razavi hospital in Mashhad, Iran between October and January 2013. In both groups, patients were sedated with 30-50 mg propofol until the desired level of sedation was achieved. In addition, continuous infusions of 100 - 300 mg/h were administered. The conversational hypnosis group consisted of 93 patients with a mean age of 50.46 (SD: 13.12) years. The control group consisted of 47 patients with a mean age of 54.89 (SD: 14.26) years. Outcome measures were: systolic blood pressure, diastolic blood pressure, mean blood pressure, pulse oximetry, heart rate, questionnaires of vital signs, degree of pain, satisfaction from the procedure (10-point scale), nausea, vomiting, and hiccups. All measures were collected before and after the procedure. The study has a high risk of performance bias. The reported outcome measures in the study were: patient satisfaction and pain.
Mackey (2018) performed a RCTs on the effects of hypnosis as an adjunct to intravenous sedation in patients between 18 and 25 years old undergoing third molar extraction in an outpatient setting (dental office) in Pennsylvania, USA. In total, 143 patients were randomly assigned to the treatment or control group. The treatment group consisted of 71 patients who listened to a rapid conversational induction and therapeutic suggestions via headphones throughout the entire surgical procedure along with a standard sedation dose of intravenous anesthetic. Standard sedation consisted of 50 mcg Fentanyl, 3 mg Midazolam, and 100 mg Propofol. This was given intravenously along with 8 mg Decadron. The control group consisted of 72 patients. The control patients also received intravenous anesthesia but listened to music without any hypnotic intervention. Mean age was not reported per group. Twenty-four patients were excluded for having a body weight greater than 100 kg (n=8), for having previous hypnotic experience (n=6), or because they were younger than 18 or older than 25 years old (n=10). The study has a high risk of performance bias. The reported outcome measures in the study were: pain and sedation dose.
Results (hypnosis, NLP etc)
Patient satisfaction (crucial)
None of the included studies reported the outcome measure patient satisfaction.
Pain (crucial)
The outcome measure pain was reported in three studies (Izanloo, 2015; Mackey, 2018; Noergaard, 2019).
In the RCT performed by Izanloo (2015), 93 patients received hypnosis in addition to standard treatment and 47 patients received standard treatment alone. Pain was collected based on a 10-point scale (0-10). Patients who received hypnosis reported a mean pain score of 0.28 (SD 0.89), patients who received standard treatment reported a mean pain score of 0.49 (SD 1.17). The mean difference is 0.21 (95% CI -0.17 to 0.59), in favour of standard treatment with hypnosis. This difference is not considered clinically relevant.
In the RCT performed by Mackey (2018) 71 patients received hypnosis in addition to standard treatment and 72 patients received standard treatment only. Patients that underwent hypnosis had a mean pain score of 2.69 (SD 1.56) during the procedure, while patients who only received standard care received had a mean pain score of 4.49 (SD 1.46). The mean difference was 1.80 (95% CI 1.30 to 2.30), in favour of the intervention group. This is considered clinically relevant.
Ten studies included in the SR from Noergaard (2019) reported pain. Even though nine out of ten studies used the same validated instrument to assess pain intensity (VAS scale 0-10 or 0-100), the results were calculated and reported differently, precluding a meta-analysis. Results of patient-related pain intensity were difficult to summarize because the pain was measured at different times. In eight individual studies, no statistically significant differences in patient-related pain intensity between control and intervention group in general were found. The results of the individual studies are shown in the Evidence Table. The studies indicate less pain intensity in the groups of patients that received hypnosis, however the differences were small. This is not considered clinically relevant.
Procedure duration (important)
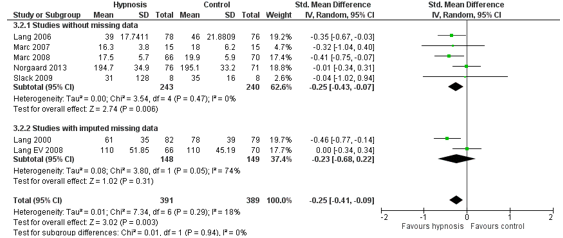
The outcome measure procedure duration was reported in one SR (Noergaard, 2019). This SR included seven RCT’s in the meta-analysis. In total, 391 patients received hypnotic analgesia in addition to standard care (experimental group), and 389 patients received standard care only (control group). The heterogeneity between these studies was 18% (Figure 2). The pooled effect estimate was SMD -0.25 (95% CI -0.41 to – 0.09) in favour of the addition of hypnotic analgesia. This means that the addition of hypnotic analgesia results in a shorter procedure duration, but the effect is small.
Figure 2. Outcome measure procedure duration hypnotic analgesia in addition to standard care versus standard care alone.
Z: p-value pooled effect; df: degrees of freedom; I2: statistic heterogeneity; CI: confidence interval.
Level of evidence of the literature (hypnosis, NLP etc)
Patient satisfaction (crucial)
The level of evidence could not be graded as this outcome measure was not reported in the included studies.
Pain(crucial)
The level of evidence regarding this outcome measure comes from randomized clinical trials and therefore starts high. The level of evidence was downgraded by three levels because of lack of blinding (risk of bias, 1 level); differences in included patients and reporting of outcome measures (inconsistency, 1 level); and because the confidence interval exceeds the values of clinical relevance (imprecision, 1 level). The level of evidence is very low.
Procedure duration (important)
The level of evidence regarding this outcome measure comes from randomized clinical trials and therefore starts high. The level of evidence was downgraded by two levels because of lack of blinding (risk of bias, 1 level); and because the effect size exceeds the boundaries of clinical relevance (imprecision, 1 level). The level of evidence is low.
Zoeken en selecteren
A systematic review of the literature was performed to answer the following question:
What is the effect of non-pharmacological interventions during/preceding PSA in patients that (will) undergo PSA on effectiveness of PSA and patient satisfaction?
| P (Patients): | adult patients undergoing procedural sedation and analgesia (PSA) (>18 years old) |
| I (Intervention): |
non-pharmacological intervention (instead of or in addition to pharmacological intervention), namely:
|
| C (Comparison): | pharmacological intervention only (no non-pharmacological intervention), standard care, or placebo intervention (potentially in addition to pharmacological intervention) |
| O (Outcomes): | patient satisfaction, pain, procedure duration |
Relevant outcome measures
The guideline development group considered patient satisfaction and pain as critical outcome measures for decision making. Procedure duration and sedation dose were considered as important outcome measures for decision making.
A priori, the working group did not further define the outcome measures listed above but used the definitions used in the studies.
The working group defined the following differences as minimal clinically important differences:
- Patient satisfaction: 10% for continuous outcomes, 1 point on 7-point Likert, 25% difference in relative risk (RR) for dichotomous outcomes.
- Pain: 10% for continuous outcomes (VAS 1/10 or 10/100)
- Procedure duration: to decide by the health care professional, based on type of procedure; for the current summary, 25% of the procedure time was used
Search and select (Methods)
The databases Medline (via OVID) and Embase (via Embase.com) were searched with relevant search terms until April 2021. The detailed search strategy is depicted under the tab Methods. Studies published before 2005 were excluded. The systematic literature search resulted in 817 hits.
Studies were selected based on the following criteria: systematic reviews and randomized controlled trials on non-pharmacological interventions and sedation in adults. In total, 44 studies were initially selected based on title and abstract screening. After reading the full text, 28 studies were excluded (see table with reasons for exclusion under the tab Methods), and 16 studies were included.
Results
Sixteen studies were included in the analysis of the literature: two systematic reviews and seventeen randomized clinical trials. Important study characteristics and results are summarized in the evidence tables. The assessment of the risk of bias is summarized in the risk of bias tables.
Referenties
- Akdogan A, Arslan M, Erceyes N. The effect of sufi music on sedation in patients under spinal anesthesia during orthopedic surgery. Ann Clin Anal Med 2021;12(2):204-207
- Bashiri M, Akçal? D, Co?kun D, Cindoruk M, Dikmen A, Çifdalöz BU. Evaluation of pain and patient satisfaction by music therapy in patients with endoscopy/colonoscopy. Turk J Gastroenterol. 2018 Sep;29(5):574-579. doi: 10.5152/tjg.2018.18200. PMID: 30260780; PMCID: PMC6284616.
- Bechtold ML, Perez RA, Puli SR, Marshall JB. Effect of music on patients undergoing outpatient colonoscopy. World J Gastroenterol. 2006 Dec 7;12(45):7309-12. doi: 10.3748/wjg.v12.i45.7309. PMID: 17143946; PMCID: PMC4087488.
- De Silva AP, Niriella MA, Nandamuni Y, Nanayakkara SD, Perera KR, Kodisinghe SK, Subasinghe KC, Pathmeswaran A, de Silva HJ. Effect of audio and visual distraction on patients undergoing colonoscopy: a randomized controlled study. Endosc Int Open. 2016 Nov;4(11):E1211-E1214. doi: 10.1055/s-0042-117630. Epub 2016 Oct 20. PMID: 27853748; PMCID: PMC5110335.
- Ebrahimi R, Shroyer AL, Dennis P, Currier J, Lendvai Wischik D. Music Can Reduce the Need for Pharmacologic Conscious Sedation During Invasive Coronary Angiography. J Invasive Cardiol. 2020 Nov;32(11):440-444. Epub 2020 Oct 22. PMID: 33087584.
- Fang AS, Movva L, Ahmed S, Waldman D, Xue J. Clinical Efficacy, Safety, and Feasibility of Using Video Glasses during Interventional Radiologic Procedures: A Randomized Trial. J Vasc Interv Radiol. 2016 Feb;27(2):260-7. doi: 10.1016/j.jvir.2015.09.023. Epub 2015 Nov 25. PMID: 26626861.
- Huang MY, Scharf S, Chan PY. Effects of immersive virtual reality therapy on intravenouspatient-controlled sedation during orthopaedic surgery under regional anesthesia: A randomized controlled trial. PLoS One. 2020 Feb 24;15(2):e0229320. doi: 10.1371/journal.pone.0229320. PMID: 32092098; PMCID: PMC7039521.
- Izanloo A, Fathi M, Izanloo S, Vosooghinia H, Hashemian A, Sadrzadeh SM, Ghaffarzadehgan K. Efficacy of Conversational Hypnosis and Propofol in Reducing Adverse Effects of Endoscopy. Anesth Pain Med. 2015 Oct 24;5(5):e27695. doi: 10.5812/aapm.27695. PMID: 26587402; PMCID: PMC4644316.
- Kang JG, Lee JJ, Kim DM, Kim JA, Kim CS, Hahm TS, Lee BD. Blocking noise but not music lowers bispectral index scores during sedation in noisy operating rooms. J Clin Anesth. 2008 Feb;20(1):12-6. doi: 10.1016/j.jclinane.2007.06.005. PMID: 18346603.
- Kukreja P, Talbott K, MacBeth L, Ghanem E, Sturdivant AB, Woods A, Potter WA, Kalagara H. Effects of Music Therapy During Total Knee Arthroplasty Under Spinal Anesthesia: A Prospective Randomized Controlled Study. Cureus. 2020 Mar 24;12(3):e7396. doi: 10.7759/cureus.7396. PMID: 32337122; PMCID: PMC7179990.
- Kulkarni S, Johnson PC, Kettles S, Kasthuri RS. Music during interventional radiological procedures, effect on sedation, pain and anxiety: a randomised controlled trial. Br J Radiol. 2012 Aug;85(1016):1059-63. doi: 10.1259/bjr/71897605. Epub 2012 Mar 14. PMID: 22422386; PMCID: PMC3587064.
- Mackey EF. An Extension Study Using Hypnotic Suggestion as an Adjunct to Intravenous Sedation. Am J Clin Hypn. 2018 Apr;60(4):378-385. doi: 10.1080/00029157.2017.1416279. Erratum in: Am J Clin Hypn. 2020 Apr;62(4):427-428. PMID: 29485375.
- Moon JY, Shin J, Chung J, Ji SH, Ro S, Kim WH. Virtual Reality Distraction during Endoscopic Urologic Surgery under Spinal Anesthesia: A Randomized Controlled Trial. J Clin Med. 2018 Dec 20;8(1):2. doi: 10.3390/jcm8010002. PMID: 30577461; PMCID: PMC6352098.
- Noergaard MW, Håkonsen SJ, Bjerrum M, Pedersen PU. The effectiveness of hypnotic analgesia in the management of procedural pain in minimally invasive procedures: A systematic review and meta-analysis. J Clin Nurs. 2019 Dec;28(23-24):4207-4224. doi: 10.1111/jocn.15025. Epub 2019 Sep 3. PMID: 31410922.
- Sjölander A, Jakobsson Ung E, Theorell T, Nilsson Å, Ung KA. Hospital Design with Nature Films Reduces Stress-Related Variables in Patients Undergoing Colonoscopy. HERD. 2019 Oct;12(4):186-196. doi: 10.1177/1937586719837754. Epub 2019 Mar 26. PMID: 30913926.
- Wang MC, Zhang LY, Zhang YL, Zhang YW, Xu XD, Zhang YC. Effect of music in endoscopy procedures: systematic review and meta-analysis of randomized controlled trials. Pain Med. 2014 Oct;15(10):1786-94. doi: 10.1111/pme.12514. Epub 2014 Aug 19. PMID: 25139786.
Evidence tabellen
|
Study reference |
Study characteristics |
Patient characteristics |
Intervention (I) |
Comparison / control (C)
|
Follow-up |
Outcome measures and effect size |
Comments |
|
Noergaard, 2019
Study characteristics and results are extracted from the SR (unless stated otherwise) |
SR and meta-analysis of RCTs and nonrandomized controlled trials were included in this review.
Literature search up to July 2018.
A: Lang, 2000 B: Lang, 2008 C: Lang, 1996 D: Lang, 2006 E: Marc, 2008 F: Marc, 2007 G: Slack, 2009 H: Shenefelt, 2013 I: Hizli, 2015 J: Norgaard, 2013
Study design: RCT
Setting and Country: A: outpatients, USA B: outpatients, USA C: inpatients, USA D: outpatients, USA E: outpatients, Canada F: outpatients, Canada G: outpatients, USA H: outpatients, USA I: outpatients, Turkey J: inpatients, Denmark
Source of funding and conflicts of interest: None identified.
|
Inclusion criteria SR: - Quantitative randomised or nonrandomised controlled trials in English, Danish, Swedish and Norwegian. - Studies with adult patients (18 years and older) - interventions: studies that evaluated hypnotic analgesia provided together with usual pain medication used during minimally invasive procedures. - hypnosis could be provided either face to face or as a pre-recorded version without limits on the length of the intervention. - Comparators in the included studies were usual analgesics or usual care typical for the institution. - studies
Exclusion criteria SR: Studies were excluded if hypnotic analgesia had been used during open surgery, during dental procedures, labour, during burn treatment and other noninvasive procedures. In addition, studies where hypnotic analgesia was used for children and adolescents were excluded.
10 studies included.
Important patient characteristics at baseline: Number of patients A: 241 B: 201 C: 30 D: 236 E: 350 F: 30 G: 26 H: 39 I: 64 J: 147
Age (years) A: median 56 B: median 50 C: mean 66.5 D: median 49 E: mean 26.3/24.2 F: mean 27.0/25.6 G: mean 51 H: 59.2/66.1/55.9 I: 63.5 J: 59.9 / 59.5
Sex (% male): A: 47% B: 36.8% C: 100% D: 0.5% E: 0% F: 0% G: 65.4% H: 59% I: 100% J: 71% / 66%
Groups comparable at baseline? In all studies, the intervention and control group were similar and treated identically except for the exposure of the intervention. |
Describe intervention:
A: self-hypnotic relaxation together with empathic attentive behaviour in addition to control, access to intravenous analgesia with Fentanyl and Midazolam
B: self-hypnotic relaxation together with empathic attentive behaviour in addition to control, access to intravenous analgesia with Fentanyl and Midazolam
C: hypnosis with combined elements of relaxation training and guided imagery for induction of self-hypnotic process, access to intravenous analgesia with Fentanyl and Midazolam
D: self-hypnotic relaxation together with empathic attentive behaviour
E: hypnotic relaxation, session 20 minutes before the procedure guided by 1 or 2 hypnotherapists, access to intravenous analgesia with Fentanyl and Midazolam
F: hypnotic relaxation session 20 minutes before the procedure and throughout the procedure guided by hypnotist practitioner, access to pain medication (not described), access to N2O.
G: listened to an audio programme for 20 minutes in duration with hypnotic induction with analgesic suggestion just before the procedure, no access to pain medication.
H: hypnotic induction followed by self-guided imagery from the start and throughout the procedure, access to intravenous analgesia with Fentanyl and Midazolam
I: hypnosis sessions were standardised to last 10 minutes before the procedure guided by a physician.
J: structured attentive behaviour together with standardized guidance to self-hypnotic relaxation, access to intravenous analgesia with Fentanyl and Midazolam
|
Describe control:
A: usual care, access to intravenous analgesia with Fentanyl and Midazolam
B: usual care, access to intravenous analgesia with Fentanyl and Midazolam
C: usual care, access to intravenous analgesia with Fentanyl and Midazolam
D: usual care, no pain medication used but local anaesthetic
E: usual care, access to intravenous analgesia with Fentanyl and Midazolam
F: usual care, access to pain medication (not described), access to N2O
G: usual care, no access to pain medication
H: usual care, access to intravenous analgesia with Fentanyl and Midazolam
I: usual care
J: usual care, access to intravenous analgesia with Fentanyl and Midazolam (patient push button to require pain medication)
|
End-point of follow-up: Not reported.
For how many participants were no complete outcome data available? Not reported.
|
Seven studies were included in the meta-analysis: Lang, 2000; Lang, 2006; Lang, 2008; Marc, 2008; Marc 2007; Norgaard, 2013; Slack, 2019.
Outcome measure-1: patient satisfaction Not reported.
Outcome measure-2: procedure duration Pooled effect (random effects model): -0.25 (CI 95%: -0.41, -0.09). favoring the intervention. Heterogeneity (I2): 18%
Outcome measure-3: pain/discomfort Results were calculated and reported differently even though nine out of ten studies used the same validated instrument to assess pain intensity; a VAS scale 0–10 or 0–100.
A: Lang (2000): Pain intensity: NRS -=10 every 15 minutes and calculation of slopes: slope 0.03 versus slope 0.09
B: Lang (2008) Pain intensity, NRS every 0-10 every 15 minutes and calculation in slopes; median (IQR): 15-30 minutes: 0 (0-2) versus 1 (0-3); and 30-45 minutes: 0 (0-2) versus 2 (0-4)
C: Lang (1996): pain intensity NRS 0-10 every 20 minutes; median; time average rating: intervention 1.2 (1.0-8.3) versus control 2.5 (1.0-6.3); max average rating intervention: 2.0 (0-10) versus 5.0 (2-9)
D: Lang (2006): pain intensity NRS every 10 minutes and calculation of slopes: slope 0.34 versus 0.53
E: Marc (2008): Pain intensity NRS 0-10 Pain I: 11.8 (17.4) vs. 12.9 (17.4) Pain II: 9.9 (17.0) vs. 13.6 (19.0) Pain III: 39.7 (25.4) vs. 42.1 (27.9) pain IV: 14.4 (19.5) vs. 16.2 (20.3)
F: Marc (2006) Not significant (no data)
G: Slack (2009): VAS 0-100, mean (SD) worst pain: 49 (30) vs 67 (25) average pain 25 (22) vs 35 (26)
H: Shenefelt (2013) Not significant (no data)
I: Hizli (2015) pain intensity (VAS 0-10) mean (range) postintervention, preprocedure 1 (0-8) versus 3 (0-9).
J: Norgaard (2013) number of spontaneous expressed pain: intervention mean 1.4 (SD 1.2) versus control mean 2.8 (SD 1.8)
Outcome measure-4: sedation dose In five out of ten studies, the amount of Fentanyl and Midazolam (pain medication) used periprocedural was measured as an outcome and was calculated to be significantly less in the intervention group compared to the control group (Lang, 2000, 2008, 1996; Marc, 2008; Norgaard, 2013). In all but one study, the results were reported without SD, precluding a meta-analysis.
Fentanyl µg/Midazolam mg A: mean 22.50/0.45 intervention versus 47.50/0.95 control. B: mean 50(25-100)/1(0.50-2) intervention versus 75 (37.50-125)/1.5(0.75-2.50) control. C: mean 7(0-75)/0.14(0-1.50) intervention versus mean 50.39(0-125)/1.05(0-2.50) control. E: mean 39.39/1.08 intervention versus mean 49.71/1.62 F: 36% (CI 95% 16–61) of patients in the hypnosis group chose N20 sedation periprocedural compared to 87% (CI 95% 61–97) in the control group (p < .01). J: mean 220.70 (93) /0 intervention versus 292 (107) / 0 control
The consumption of pain medication was reduced between 21%–86% in these five individual studies in the intervention group. |
Facultative:
The majority of the studies were evaluated weak. The overall risk of bias across studies evaluated moderate to high. The two most common risk of bias among the studies were performance and detection bias, which occurred in most of the studies. The nature of the intervention prohibited blinding of the treatment to the participants and the majority of the studies had weaknesses due to the criteria about blinding of those assessing outcomes.
|
|
Wang, 2014
Study characteristics and results are extracted from the SR (unless stated otherwise) |
SR and meta-analysis of RCT’s.
Literature search up to July 2013.
A: Bampton, 1997 B: Björkman, 2013 C: Chan, 2003 D: Chlan, 2000 E: Colt, 1999 F: Costa, 2020 G: Danhauer, 2007 H: Dubois, 1995 I: El-Hassan, 2009 J: Harikumar, 2006 K: Hayes, 2003 L: Kotwal, 1998 M: Lee, 2002 N: Lopez, 2004 O: Ovayolu, 2006 P: Palakanis, 1994 Q: Schiemann, 2002 R: Smolen, 2002 S: Triller, 2006 T: Uedo, 2004 U: Yeo, 2013
Study design: RCT
Setting and Country: A: Australia B: Sweden C: China D: USA E: USA F: Italy G: USA H: USA I: UK J: Kerala K: USA L: India M: China N: Spain O: Turkey P: USA Q: Germany R: USA S: Slovenia T: Japan U: Korea
Source of funding and conflicts of interest: There is no any potential conflict of interest in the article. The study is not supported by any financial grants. |
Inclusion criteria SR: Patients undergoing various endoscopic examinations. Intervention and control as described in the next columns.
Exclusion criteria SR: Studies were excluded if the raw data could not be extracted.
21 studies included
Important patient characteristics at baseline:
N intervention /control A: 28/31 B: 60/60 C: 112/108 D: 30/34 E: 30/30 F: 56/53 G: 56/58 H: 21/28 I: 92/88 J: 38/40 K: 100/98 L: 54/50 M: 55/55 N: 63/55 O: 30/30 P: 25/25 Q: 59/60 R: 16/16 S: 93/107 T: 15/14 U: 35/35
Age: A: >18 B: 18-80 C: 18-65 D: >18 E: >18 F: 18-75 G: 18-60 H: NR I: ≥18 J: 15-60 K: ≥18 L: NR M: 16-75 N: 18-75 O: 18-75 P: 20-76 Q: 18-80 R: >18 S: >18 T: 40-69 U: >20
Groups comparable at baseline? Yes |
Describe intervention:
All subjects in the intervention group listened to music before and/or during the procedure, played on a compact disc (CD) player or other modalities with or without earphones and the style of music was not
A: relaxation music, before and during procedure B: Sedative music, during procedure C: slow-rhythm music, during procedure D: self-selected music, during procedure E: self-selected music, during procedure F: self-selected music, during procedure G: relaxation music, during procedure H: new wave music, during procedure I: self-selected music, 15 minutes before the procedure J: self-selected music, during procedure K: self-selected music, 15 minutes before the procedure L: Indian classical music, before and during procedure M: self-selected music, during procedure N: classical tracks, during procedure O: Turkish classical music, before and during procedure P: sedative music, during procedure Q: self-selected music, during procedure R: self-selected music, during procedure S: self-selected music, during procedure T: easy listening style, during procedure U: classical music, before and during procedure
|
Describe control:
The usual care group underwent the procedure as typically conducted without music
|
End-point of follow-up: Not reported.
For how many participants were no complete outcome data available? Not reported
|
Outcome measure-1: patient satisfaction Satisfaction score was measured by different methods, and the SMD was used in this meta-analysis. Overall, the level of satisfaction was improved in the music group (SMD = 1.83, 95% CI [0.76, 2.91]).
Outcome measure-2: pain The overall effect of the meta-analysis based on the LAS favored music for pain reduction (WMD = - 1.53, 95% CI [- 2.53, - 0.53]).
utcome measure-3: procedure duration There was no significant difference in duration of procedure between groups (WMD = -0.08, 95% CI [- 2.52, 0.75]).
Outcome measure-3: sedation dose The meta-analysis showed that music did not reduce the dose used of sedative medications during examinations (WMD -0.53 (-1.39 to 0.33).
|
Facultative:
Our meta-analysis suggested that music may offer benefit for patients undergoing endoscopy.
Individual study results and forest plots are not reported in the study. |
Table of quality assessment for systematic reviews of RCTs and observational studies
Based on AMSTAR checklist (Shea et al.; 2007, BMC Methodol 7: 10; doi:10.1186/1471-2288-7-10) and PRISMA checklist (Moher et al 2009, PLoS Med 6: e1000097; doi:10.1371/journal.pmed1000097)
|
Study
First author, year |
Appropriate and clearly focused question?1
Yes/no/unclear |
Comprehensive and systematic literature search?2
Yes/no/unclear |
Description of included and excluded studies?3
Yes/no/unclear |
Description of relevant characteristics of included studies?4
Yes/no/unclear |
Appropriate adjustment for potential confounders in observational studies?5
Yes/no/unclear/notapplicable |
Assessment of scientific quality of included studies?6
Yes/no/unclear |
Enough similarities between studies to make combining them reasonable?7
Yes/no/unclear |
Potential risk of publication bias taken into account?8
Yes/no/unclear |
Potential conflicts of interest reported?9
Yes/no/unclear |
|
Noergaard, 2019 |
Yes |
Yes |
Yes, all ten studies that met the criteria for this SR were included. |
Yes |
Not applicable |
Yes, JBI-MAStARI |
Yes
|
Yes
To minimise publication bias, we did a comprehensive search for unpublished or grey literature (The Cochrane Collaboration, 2011) without finding any. |
No, only for the SR, not for the individual studies. |
|
Wang, 2014 |
Yes |
Yes |
No, potentially relevant studies that are excluded at final selection were not referenced with reasons. |
Yes |
Not applicable |
Yes, risk of bias tabel included for all studies. |
Unclear, they report that they use a random model when I² is > 50%.
The procedures and type of intervention have some differences. Subgroup analyses may have been more appropriate. |
Yes
Funnel plot was included. |
No, only for the SR, not for the individual studies. |
- Research question (PICO) and inclusion criteria should be appropriate and predefined
- Search period and strategy should be described; at least Medline searched; for pharmacological questions at least Medline + EMBASE searched
- Potentially relevant studies that are excluded at final selection (after reading the full text) should be referenced with reasons
- Characteristics of individual studies relevant to research question (PICO), including potential confounders, should be reported
- Results should be adequately controlled for potential confounders by multivariate analysis (not applicable for RCTs)
- Quality of individual studies should be assessed using a quality scoring tool or checklist (Jadad score, Newcastle-Ottawa scale, risk of bias table etc.)
- Clinical and statistical heterogeneity should be assessed; clinical: enough similarities in patient characteristics, intervention and definition of outcome measure to allow pooling? For pooled data: assessment of statistical heterogeneity using appropriate statistical tests (e.g. Chi-square, I2)?
- An assessment of publication bias should include a combination of graphical aids (e.g., funnel plot, other available tests) and/or statistical tests (e.g., Egger regression test, Hedges-Olken). Note: If no test values or funnel plot included, score “no”. Score “yes” if mentions that publication bias could not be assessed because there were fewer than 10 included studies.
- Sources of support (including commercial co-authorship) should be reported in both the systematic review and the included studies. Note: To get a “yes,” source of funding or support must be indicated for the systematic review AND for each of the included studies.
This table is also suitable for diagnostic studies (screening studies) that compare the effectiveness of two or more tests. This only applies if the test is included as part of a test-and-treat strategy – otherwise the evidence table for studies of diagnostic test accuracy should be used.
|
Study reference |
Study characteristics |
Patient characteristics 2 |
Intervention (I) |
Comparison / control (C) 3
|
Follow-up |
Outcome measures and effect size 4 |
Comments |
|
Akdoğan, 2021 |
Type of study: RCT
Setting and country: Turkey
Funding and conflicts of interest: None of the authors received any type of financial support that could be considered potential conflict of interest regarding the manuscript or its submission. |
Inclusion criteria: Patients in the ASA I-II risk group, aged 18-60, who will undergo elective non oncologic orthopedic surgery, with the approval of the local ethics committee and informed consent from the patients.
Exclusion criteria: Those who have hearing loss, are professional music practitioners, are using medications that can affect the hypothalamo-hypophyseal and sympathetic system. In addition, patients who did not like this type of music and stated that they did not want to listen were not included in the study.
N total at baseline: Intervention: 25 Control: 25
Important prognostic factors2:
Age (years): I: 41.96 (+/- 11.41) C: 43.00 (+/- 11.26)
Sex: F/M I: 11/14 C: 13/12
Groups comparable at baseline? Yes
|
Describe intervention (treatment/procedure/test):
The patients were made listen to works from Turkish Sufi Music Hüseyni Mode with an mp3 player (SONY NVZ-B172), using headsets. The patients were told to adjust the volume of the music according to their preferences.
|
Describe control (treatment/procedure/test):
No music. |
Length of follow-up: 30 minutes postoperative.
Loss-to-follow-up: Not reported.
Incomplete outcome data: None.
|
Outcome measures and effect size (include 95%CI and p-value if available):
Procedure duration: I: 58.12 (+/- 12.11) min. C: 56.54 (+/- 13.11) min. |
|
|
Bashiri, 2018 |
Type of study: RCT
Setting and country: Turkey
Funding and conflicts of interest: The authors have no conflicts of interest to declare.
The authors declared that this study has received no financial support. |
Inclusion criteria: Patients who were 18-70 years old, American Anesthesiologist Association (ASA) status I-III, and scheduled For endoscopy/ colonoscopy were included in the study after verbal and written approval was obtained.
Exclusion criteria: Patients scheduled for endoscopic ultrasound or endoscopic retrograde colangiopancreaticography and having difficulty in communication were excluded from the study.
N total at baseline: Group 1: 25 Group 2: 33 Group 3: 55 Group 4: 41
Important prognostic factors2: Age median (min-max) Group 1: 46 (28-69) Group 2: 44 (28-65) Group 3: 44 (28-60) Group 4: 42 (24-65)
Male/female Group 1: 4/21 Group 2: 13/21 Group 3: 26/29 Group 4: 15/26
Groups comparable at baseline? There were more women than men in all groups.
|
Describe intervention (treatment/procedure/test):
In the music group, patients listened to a 30-minute recording of their favorite music.
Group 2) conscious sedation Group 4) deep sedation
|
Describe control (treatment/procedure/test):
In the control group, the headphone was on without any music.
Group 1) conscious sedation Group 3) deep sedation
|
Length of follow-up: Until after the intervention.
Loss-to-follow-up: Not reported.
Incomplete outcome data: Not reported.
|
Outcome measures and effect size (include 95%CI and p-value if available):
Patient satisfaction: Satisfaction was assessed by Likert scale (1, very satisfied; 2, satisfied; 3, undetermined; and 4, not satisfied). Data was not provided.
In the sedation and music groups, patients were satisfied with their procedure and declared that they would prefer the same method for their next endoscopy. No significant difference was found in patient satisfaction between the groups.
Sedation dose (propofol (mg)): Group 3: 204.54 (SD 42.24) Group 4: 146.34 (SD 32.38)
Sedation dose (midazolam (mg)): Group 3: 2 (SD 0) Group 4: 1.3 (SD 0.48)
Sedation dose (ketamine (mg)): Group 3: 19.63 (SD 2.69) Group 4: 16.12 (SD 4.45)
Data was not provided for Group 1 and 2.
|
The study included conscious and deep sedation. |
|
Bechtold, 2006 |
Type of study: RCT
Setting and country: USA
Funding and conflicts of interest: |
Inclusion criteria: Patients presenting for elective outpatient colonoscopy.
Exclusion criteria: history of prior colon resection, patients scheduled to undergo same-day esophagogastroduodenoscopy (EGD) and colonoscopy, and inability to give informed consent.
N total at baseline: Intervention: 85 Control: 81
Important prognostic factors2: Age I: 58.5 C: 54.1
Sex: I: 51.8 % F C: 48.1 % F
Groups comparable at baseline? They were similar in terms of gender (51.8% females for the music group vs 48.1% for the non-music group, P = 0.64) and history of prior colonoscopy (36.5% vs 30.9%, P = 0.45). The music group was slightly older (58.5 years vs 54.1 years, P = 0.036), and reported less pre-procedural anxiety (36.3-mm vs 45.1-mm, borderline significance at P = 0.053). |
Describe intervention (treatment/procedure/test):
In the music group, a CD player was playing relaxing music upon the entrance of the patient into the procedure room. We played the same music for all patients in the music group: “Watermark” by Enya (Reprise Records, a Time Warner Company, 1988), which contains 12 tracks (ranging from 1:59 to 4:25 in length). The CD was set on repeat.
|
Describe control (treatment/procedure/test):
No music. |
Length of follow-up: Post procedure.
Loss-to-follow-up: Intervention: N (%) Reasons (describe)
Control: N (%) Reasons (describe)
Incomplete outcome data: Intervention: N (%) Reasons (describe)
Control: N (%) Reasons (describe)
|
Outcome measures and effect size (include 95%CI and p-value if available):
Experience I scale (1-4): I: 2 C: 2
Experience 2 scale (1-5) I: 1 C: 2
Experience 3 scale (100 mm scale) I: 22.5 C: 28.1
Pain: (100 mm VAS scale) I: 25.3 mm C: 25.4 mm
Procedure time: I: 20.7 minutes C: 21 minutes
Sedation dose (Meperidine) I: 57 mg C: 54.6 mg
Sedation dose (Midazolam) I: 1.92 mg C: 1.85 mg
|
|
|
De Silva, 2016 |
Type of study: RCT
Setting and country: teaching hospital, Sri Lanka.
Funding and conflicts of interest: none. |
Inclusion criteria: Consecutive patients with an indication to undergo elective day-case colonoscopy for medical indications were enrolled in the study in the absence of exclusion criteria.
Exclusion criteria: The exclusion criteria included visual or hearing impairment, allergies or hypersensitivity to premedication, patients who had had abdominal surgery or colectomy, personal history of anxiety or psychiatric disorder, and pregnancy.
N total at baseline: Intervention AD: 66 Intervention VD: 67 Control: 67
Important prognostic factors2: Age (years) I - AD: 54.4 (SD 12.5) I – VD: 51.5 (SD 14.6) C: 56.5 (SD 13.8)
Sex: I - AD: 49% M I – VD: 64% M C: 52% M
Groups comparable at baseline? Yes
|
Describe intervention (treatment/procedure/test):
All patients were administered midazolam and pethidine for sedation and analgesia, respectively, irrespective of the study group to which they were allocated.
Audio distraction (AD): The AD group was allowed to listen to the music of their choice during colonoscopy. The AD group listened to their choice of music through the SONY head mounted set.
Visual distraction: The VD group was allowed to watch a film of their choice with sound using a SONY head mounted display.
The duration of the movie or video clip and the play list were more than 20 minutes to cover the maximum predicted duration for the colonoscopy. |
Describe control (treatment/procedure/test):
All patients were administered midazolam and pethidine for sedation and analgesia, respectively, irrespective of the study group to which they were allocated.
The control group only received standard sedation during the colonoscopy. The control group had the SONY head mounted set on but with nothing playing for the duration of the procedure. |
Length of follow-up: Not reported.
Loss-to-follow-up: No loss to follow-up.
Incomplete outcome data: Data was complete.
|
Outcome measures and effect size (include 95%CI and p-value if available):
Pain (10-point VAS scale): Median + IQR AD: 3 (2-4) VD: 4 (2-6) C:5 (3-8)
Duration of the procedure: Total procedure time (SD) in minutes (from setting up of intervention and premedication to recovery from premedication) was similar among the three groups: 25.3 (11.7) for AD, 27.4 (12.6) for VD, 27.2 (11.5) for group C, respectively (P=0.593).
Sedation dose (pethidine): AD: 10 (5-15) VD: 10 (10-15) C: 15 (5-20)
Sedation dose (midazolam): AD: 1.5 (1.0 – 2.0) VD: 1.5 (1.5 – 2.0) C: 2 (1.0 – 2.5)
|
|
|
Ebrahimi, 2020 |
Type of study: RCT (pilot)
Setting and country: USA
Funding and conflicts of interest: The authors report no conflicts of interest regarding the content herein. |
Inclusion criteria: (1) age >18 years old; (2) non-emergent ICA; and (3) ability and willingness to participate in the study and sign the informed consent form
Exclusion criteria: (1) acute myocardial infarction requiring emergency ICA; (2) documented history of major hearing problems or deafness; (3) contraindication to SCS medications; and (4) unwillingness to comply with study procedures and requirements.
N total at baseline: Intervention: 35 Control: 37
Important prognostic factors2: Age, years (SD) I: 69.57 (8.92) C: 68.51 (6.48)
Sex: I: 2.9% F C: 8.1% F
Groups comparable at baseline? Yes.
|
Describe intervention (treatment/procedure/test):
For those randomized to the Music group, music was started approximately 20 minutes prior to arrival in the cardiac catheterization laboratory (CCL), and continued throughout the procedure and during the recovery period up to 1 hour. Choice of music was based on each subject’s stated preferences (specific genre, artists, or songs were searched in one of the mainstream digital music libraries). Music was delivered through a portable media player with single-use, disposable ear buds. The appropriate volume was adjusted by CCL nursing staff per patient request. Ear buds were used in only 1 ear of each subject to ensure adequate communication between the subject and the attending provider and CCL staff.
After “timeout” (the process of identifying the patient and the intended procedure) and prior to administration of local anesthesia with 2% lidocaine subcutaneously (defined as the start time for the procedure), all subjects were assessed for their levels of pain and anxiety by the nursing staff, and were asked whether they wanted to receive pharmacologic conscious sedation. The administered dose of these medications depended on age, weight, renal function, and other clinical criteria, and was chosen at the discretion of the operator.
|
Describe control (treatment/procedure/test):
Planned standard conscious sedation with pre-ICA midazolam and/or fentanyl without any background music.
SCS was not administered to those in the SCS arm if they requested to not receive SCS. |
Length of follow-up: Not reported.
Loss-to-follow-up: No loss to follow-up
Incomplete outcome data: Data was complete.
|
Outcome measures and effect size (include 95%CI and p-value if available):
Pain: I: 0.96 (SD 1.40) C: 0.92 (SD 2.18)
Sedation dose (Midazolam): I: 0.37 mg C: 0.68 mg SD was not reported.
Sedation dose (Fentanyl): I: 15.71 ug C: 20.95 ug SD was not reported. |
|
|
Fang, 2016 |
Type of study: RCT
Setting and country: prospective single-institution study, USA
Funding and conflicts of interest: None of the authors have identified a conflict of interest.
The study received pilot funding via a University of Rochester Medical Center Department of Imaging Science Fischer Fund Grant. |
Inclusion criteria: Patients 18 years of age or older, of either sex and any ethnicity, were recruited provided they spoke English, were undergoing an outpatient interventional radiologic procedure, and were able to provide informed consent.
Exclusion criteria: Patients undergoing emergency procedures; required general anaesthesia; had a history of hearing difficulties; visual difficulties; or epileptic seizures; were unable or unwilling to understand or provide informed consent or were unable to complete study procedures or unable to tolerate study constraints.
N total at baseline: Intervention: 39 Control:44
Important prognostic factors2: Age (mean, SD) I: 53.1 (15.2) C: 56.4 (13.5)
Sex: I: 56% M C: 70% M
Groups comparable at baseline? Patient demographics were similar between groups.
|
Describe intervention (treatment/procedure/test):
Patients were fitted with WideView XL Edition video glasses (iTVGoggles) and accompanying earpieces. The patient had the option of choosing from a preset list of videos, which included documentaries and general audience-rated movies.
In addition to standard treatment.
|
Describe control (treatment/procedure/test):
No video glasses. |
Length of follow-up: Postprocedure.
Loss-to-follow-up: None.
Incomplete outcome data: Intervention: 8 Control: 5
Patients decided to take of the video glasses during the procedure (N=5), the study was interrupted during the procedure (N=5), the patient was too sedated or in too much pain to complete the study protocol. |
Outcome measures and effect size (include 95%CI and p-value if available):
Patient satisfaction Patient satisfaction was not compared between the intervention and control group.
Pain There were no significant differences in pain score between the two groups. *Scores are not reported.
Procedure duration I: 24.1 minutes (16.5) C: 25.3 minutes (31.6)
Sedation dose Sedation dose is not reported.
|
|
|
Huang, 2020 |
Type of study: RCT
Setting and country: Single center, Australia
Funding and conflicts of interest: The author(s) received no specific funding for this work.
The authors have declared that no competing interests exist.
|
All patients undergoing elective knee or hip joint replacement surgery under regional anesthesia were eligible for enrolment.
Inclusion criteria: Inclusion criteria were English-speaking patients, 18 years of age and over, with no significant cardiovascular or respiratory disease.
Exclusion criteria: patients receiving general anesthesia, cognitive impairment preventing the use of subjective outcome surveys, visual or hearing impairment and non-English speaking patients.
N total at baseline: Intervention: 25 Control: 25
Important prognostic factors2: Age: I: 65 (IQR 57 – 68) C:70 (IQR 64 – 72)
Sex: I: 48% M C: 52% M
Groups comparable at baseline? The median age in the IVR group was younger by five years, other baseline characteristics seem to be well balanced between 2 groups.
|
Describe intervention (treatment/procedure/test):
The IVR group received IVR using one of two available HMD setups depending on availability, with both including noise-cancelling headphones.
|
Describe control (treatment/procedure/test):
The control group received no distraction and did not wear any HMD or headphones.
Standard care: Both groups were able to control their intra-operative sedation using propofol via patient-controlled sedation (PCS), consisting of an Alaris pump and PCA module with instruction to use it whenever they felt too aware or anxious. Each press of the PCS supplied a propofol bolus of 400mcg/kg Ideal Body Weight with a 5-minute lockout period. |
Length of follow-up: Postprocedure.
Loss-to-follow-up: None.
Incomplete outcome data: No incomplete outcome data.
|
Outcome measures and effect size (include 95%CI and p-value if available):
Patient satisfaction Additional questions regarding patient satisfaction found that all patients in both treatment groups were satisfied with their experience. Data was not provided, group comparison is not possible.
Pain Only the change scores are reported, group comparison is not possible.
Sedation dose The total median use in the control and IVR groups was 80 (25, 180) mg and 112 (0, 150) mg respectively (p = 0.88).
The median fentanyl use in the IVR group and the control group over the course of the entire procedure was 37.5 (0, 50) mcg and 0 (0, 50) mcg respectively (p = 0.22), while the median mida zolam use over the procedure was 2.3 (2, 3) mg and 1.5 (1, 2) mg respectively (p = 0.10). |
It should also be noted that almost half of the patients approached for this study declined, preferring to have analgesia and sedation managed by the anesthesiologist without IVR. |
|
Kang, 2008 |
Type of study: RCT Setting and country: operating room, South Korea
Funding and conflicts of interest: Not reported. |
Inclusion criteria: In the sedation study, 63 ASA physical status I and II patients (aged 55-78 years), who were scheduled to undergo total knee replacement surgery for degenerative arthritis of the knee, were enrolled in this randomized investigation.
Exclusion criteria: Patients who had any contraindication to regional anesthesia, took herbal medicines or steroid drugs at least 6 months before surgery, or who had a history of chronic psychiatric drug use were excluded from the study. Preoperative hearing acuity was checked, and we excluded from the study those patients with poor auditory function. Patients with poor renal function, poor liver function, or cardiopulmonary disease were also excluded from the study.
N total at baseline: Intervention: 20 Control: 20
Important prognostic factors2: Age I: 67.8 (SD 7.0) C: 69.2 (SD 7.3)
Sex: (MF) I: 2/18 C: 2/18
Groups comparable at baseline? Age, gender, level of sensory block, and operation time were similar in the groups.
|
Describe intervention (treatment/procedure/test):
A headset only was applied to patients in the music group
|
Describe control (treatment/procedure/test):
The noise group patients were exposed to the ambient OR noise. |
Length of follow-up: Postprocedure.
Loss-to-follow-up: None
Incomplete outcome data: Intervention: 1 One patient was excluded because he was given an increased target concentration of propofol for irritability during surgery.
Control: 1 One patient was excluded because Midazolam was injected during the spinal procedure to control anxiety.
|
Outcome measures and effect size (include 95%CI and p-value if available):
Pain: I: 10 (IQR 0-66) C: 23 (IQR 0-70)
Procedure duration: I: 98 (SD 7) C: 118 (SD 24)
|
The study contained a third arm: silence group in which ambient noise was blocked.
A power calculation was performed, 20 patients per group. |
|
Kukreja, 2020 |
Type of study: RCT
Setting and country: USA
Funding and conflicts of interest: The authors declare no conflicts of interest. |
Inclusion criteria: Patients greater than 18 years of age and of American Society of Anesthesiologists (ASA) Class I, II, or III undergoing total knee arthroplasty under spinal anesthesia were approached for participation.
Exclusion criteria: Patients with hearing impairment secondary to age or disease and any contraindications for spinal anesthesia were excluded from enrollment.
N total at baseline: Intervention: 38 Control: 42
Important prognostic factors2: Age: I: 65.14 (SD 1.82) C: 61.68 (SD 2.27)
Sex: I: 31% M C: 36% M
Groups comparable at baseline? Statistical analysis of these values indicates that the groups were similar.
|
Describe intervention (treatment/procedure/test):
The patients in the music group chose a genre of music (blues, classical, country, gospel, jazz, classical, rhythm and blues, rock, soundtrack) and self-selected a volume level.
|
Describe control (treatment/procedure/test):
No music. |
Length of follow-up:
Loss-to-follow-up: Intervention: 7 Reasons (describe)
Control: 4 Reasons (describe)
Incomplete outcome data: Intervention: 2 Conversion to general anaesthesia.
Control: 10 Conversion to general anaesthesia.
|
Outcome measures and effect size (include 95%CI and p-value if available):
Procedure time: I: 98.62 minutes (SD 4.15) C: 97.11 minutes (SD 5.10)
Patient satisfaction: Data was only presented for the intervention group, comparison between the groups was not possible.
Sedation dose (propofol dose per surgical minute per kg (mg/min/kg) I: 0.05 (SD 0.004) C:0.06 (SD 0.004)
|
All patients received 1 mg of midazolam and 50 mcg of fentanyl as sedatives for performing the nerve block.
Another interesting observation from this study (CONSORT flowchart) revealed that 23% of allocated “control” group patients were converted to general anesthesia as compared to only 5% in the “music” group.
Music therapy in this study did not decrease propofol requirements for sedation during total knee Arthroplasty. |
|
Kulkarni, 2012 |
Type of study: RCT
Setting and country: UK
Funding and conflicts of interest: Not reported. |
Inclusion criteria: All adult patients undergoing an IR procedure in our centre were considered for the study.
Exclusion criteria: Patients undergoing emergency procedures, procedures under general anaesthesia, unable to consent, hearing difficulty.
If the patient’s selected music was unavailable, the patient was excluded.
N total at baseline: Intervention: 50 Control: 50
Important prognostic factors2: Age: I: 57 (SD 16) C: 59 (SD 15)
Sex: I: 62% M C: 54% M
Groups comparable at baseline? Patients randomised to the music group had a significantly higher pulse rate at baseline than patients in the control group (77 vs 71 beats per min). No other Baseline characteristic differed significantly between the study groups. |
Describe intervention (treatment/procedure/test):
Self-selected music, via headphones at a comfortable volume.
|
Describe control (treatment/procedure/test):
Headphones without music. |
Length of follow-up: Postprocedure.
Loss-to-follow-up: None.
Incomplete outcome data: None.
|
Outcome measures and effect size (include 95%CI and p-value if available):
Procedure duration (min): I: 60 (SD 26) C: 60 (SD 22)
Sedation dose: Significantly lower doses of midazolam sedation were recorded in the music group, with a mean dose [standard deviation (SD)] of 1.3mg (2.2 mg) compared with 2.1mg (2.3 mg) in the control group (p50.027).
|
17 patients wanted music to be played during the procedure and refused consent for randomisation. |
|
Moon, 2018 |
Type of study: RCT
Setting and country: Korea
Funding and conflicts of interest: This study was not supported by any external fund.
The authors declared that they have no conflicts of interest. |
Inclusion criteria: scheduled endoscopic urologic surgery, including holmium laser enucleation of the prostate (HOLLEP) or transurethral resection of bladder tumor), and an American Society of Anesthesiologists (ASA) physical status classification of I–III.
Exclusion criteria: history of chronic use of sedatives or narcotics (>6 months), alcohol or drug abuse, baseline pulse oximetry saturation of less than 90%, and baseline hemodynamic or respiratory instability (initial systolic arterial pressure <80 mmHg, respiratory rate >25 or <10 breaths/min).
N total at baseline: Intervention: 18 Control: 19
Important prognostic factors2: Age: I: 69 (IQR 65-70) C: 69 (IQR 63-72)
All men: (endoscopic prostatectomy)
Groups comparable at baseline? The patient demographics and comorbidity were comparable.
|
Describe intervention (treatment/procedure/test):
30-min VR program via a head-mounted display and earphone connected to an android cell phone. The program was designed for relaxation and distraction from anxiety or pain of patients.
The patients watched a VR underwater view of the ocean while listening to narrations designed to induce relaxation and meditation. If the surgery was not completed within 30 min, the patient watched the VR program again.
|
Describe control (treatment/procedure/test):
The sedation group underwent pharmacological sedation with midazolam using an initial bolus dose of 1–2 mg and a maintenance dose of 1–2 mg every 10–30 min. Oxygen was supplied at 5 L/min via a facemask in the sedation group but not in the VR group. However, to blind the surgeon to group assignment, the VR headset and earphone were also used in the sedation group and a facemask was also used but without an oxygen supply in the VR group. |
Length of follow-up: 30 minutes postoperatively.
Loss-to-follow-up: No missing data.
Incomplete outcome data: No missing data.
|
Outcome measures and effect size (include 95%CI and p-value if available):
Patient satisfaction I: 5 (5-5) C: 4 (4-5)
Procedure duration I: 40 (IQR 35-75) C: 45 (IQR 30-60)
|
|
|
Sjölander, 2019 |
Type of study: RCT
Setting and country: Sweden.
Funding and conflicts of interest: |
Inclusion criteria: Patients who were referred for a routine colonoscopy on an outpatient basis were asked to participate in the study.
The study included patients undergoing colonoscopy for any reason such as diarrhea, inflammation, bleeding, polyp, evaluation of treatment, tumor, pain, ulcerative colitis, colitis, diverticulosis, anemia, constipation, or altered stools.
Exclusion criteria: Not reported.
N total at baseline: Intervention: 68 Control: 69
Important prognostic factors2: Age: I: 60.4 (SEM 2.0) C: 60.9 (SEM 1.8)
Sex: (M/F) I: 35/33 C: 28/41
Groups comparable at baseline? Yes |
Describe intervention (treatment/procedure/test):
A large digital screen with surrounding frame (85.8 52.4 in.) was mounted on the wall directly in front of the patient when lying on their left side. The frame and the wall that surrounded the screen were painted in a soft light green color (NCS: S 2020-B90G). A loudspeaker playing nature sounds such as birdsong or flowing water was placed under the pillow on the examination bed. The patients could choose to have the sound on or off.
Five different films of calm nature scenes were randomly shown on the screen. |
Describe control (treatment/procedure/test):
Patients in the control group were investigated in one of the traditionally equipped rooms. |
Length of follow-up: 15 minutes after the procedure.
Loss-to-follow-up: Not reported.
Incomplete outcome data: Not reported.
|
Outcome measures and effect size (include 95%CI and p-value if available):
Pain (VAS 10) – 15 minutes after the procedure I: 0.87 (SD 0.17) C:0.82 (SD0.17)
|
The patients were informed orally and in writing that the study was about the impact of design but were told nothing about the nature of the intervention.
Each patient was offered an intravenous injection of a sedative drug (midazolam) and an analgesic drug (alfentanil).
Power calculation: 64 per group.
The patients in the study had the opportunity to listen to natural sounds while watching the digital screen and the patients could choose to have the sound on or off. Thus, the intervention includes both audio and visual stimuli. The study does not take into account whether it is the audio or the visual stimuli that contributes to the effect or a combination of these. |
Evidence table for intervention studies (randomized controlled trials and non-randomized observational studies [cohort studies, case-control studies, case series])1
This table is also suitable for diagnostic studies (screening studies) that compare the effectiveness of two or more tests. This only applies if the test is included as part of a test-and-treat strategy – otherwise the evidence table for studies of diagnostic test accuracy should be used.
|
Study reference |
Study characteristics |
Patient characteristics 2 |
Intervention (I) |
Comparison / control (C) 3
|
Follow-up |
Outcome measures and effect size 4 |
Comments |
|
Izanloo, 2015 |
Type of study: quasi-experimental trial
Setting and country: endoscopy unit, Iran
Funding and conflicts of interest: This study was supported by education and research department of Razavi hospital. |
Inclusion criteria: The inclusion criteria were having at least middle school education, the minimum age of 18 and the maximum age of 85, lacking any history of psychological problem, and submitting a consent form for participating in the study.
Exclusion criteria: The exclusion criterion was lack of willingness to participate in the study and using opioid and benzodiazepine agents.
N total at baseline: Intervention: 93 Control: 47
Important prognostic factors2: For example age ± SD: I:50.46 (13.12) C: 54.89 (14.26)
Sex: I: 55% F C: 64% F
Groups comparable at baseline? There was no significant difference between the control and experiment groups in terms of demographic variables and clinical data. However, in the experiment group, the onset time of sedation after receiving anesthetic (mean = 28.39) was significantly lower than that of the control group (mean = 33.39) (P = 0.02). |
Describe intervention (treatment/procedure/test):
The experimental group was sedated by propofol according to standard protocol (see control) and conversational hypnosis. Conversational hypnosis includes indirect suggestions, which are designed to mislead, confuse, or force a patient to think about the meaning of these indirect suggestions, the different possibilities, and how they are applied to them personally. We talked to the patients for about 5 to 10 minutes.
|
Describe control (treatment/procedure/test):
Sedation was initiated by an anesthesiologist, using 30 - 50 mg of propofol until the desired level of sedation was achieved. Continuous infusions of 100 - 300 mg/h were also used. |
Length of follow-up: Not reported.
Loss-to-follow-up: Not reported.
Incomplete outcome data: Not reported.
|
Outcome measures and effect size (include 95%CI and p-value if available):
Patient satisfaction The degree of satisfaction in the experiment group was higher than that of the control group. However, the difference was insignificant (P = 0.368) (data not shown).
Pain: In the experiment group, the pain score with a mean of 0.28 (± 0.89) was lower than that of the control group (0.49 ± 1.17), but this difference was not significant (P = 0.25).
|
|
|
Mackey, 2018 |
Type of study: RCT
Setting and country: dental practice (suburbs and rural community), USA
Funding and conflicts of interest: Not reported. |
Inclusion criteria: Only clients who reported English as their first language were included to avoid any interpretive issues.
Exclusion criteria: Subjects that had previous hypnotic experiences were excluded from the study. Any individual who reported a major mental illness as defined in the Diagnostic and Statistical Manual of Mental Disorders (4th ed. [DSM-IV-TR]; American Psychiatric Association, 2000) was excluded from the study. Subjects reporting the following disorders (Axis I): schizophrenia and psychotic disorders and (Axis II): paranoid personality disorder, schizoid personality disorder, and schizotypal personality disorder, were excluded.
Individuals who were taking prescription medications that affect heart rate and/or blood pressure (i.e., beta blockers). Patients reporting a history of illicit drug use or admitting to the use of drugs that influence the normal physiologic or psychologic processes (narcotics, cocaine, inhalants, etc.) were excluded from the study. Patients who reported an allergy to sulfites were eliminated from participation in the study.
N total at baseline: Intervention: 71 Control: 72
Important prognostic factors2: Not reported.
Groups comparable at baseline? Unclear |
Describe intervention (treatment/procedure/test):
The treatment group received standard IV sedation with relaxing background music played through headphones along with a pre-recorded rapid induction and therapeutic suggestion throughout the entire surgical procedure.
|
Describe control (treatment/procedure/test):
The control group received standard IV sedation with the addition of relaxing music played through headphones that the subject wore during the entire surgical procedure. |
Length of follow-up: The patients were contacted by phone on the first post-operative day.
Loss-to-follow-up: It was necessary to exclude 24 people from this study. Eight individuals were excluded for having a body weight greater than 100 kg, 6 individuals were excluded for having previous hypnotic experience, and 10 individuals were excluded due to age less than 18 or older than 25.
Unknown whether this was in the control or treatment group.
Incomplete outcome data: Not reported.
|
Outcome measures and effect size (include 95%CI and p-value if available):
Pain I: 2.69 (1.56) C: 4.49 (1.46) MD: -1.80 (-2.30; -1.30). SMD -1.19 (-1.54; -0.83).
Sedation dose I: 32.39 (69.74) C: 155.76 (62.58) MD: -123.37 (-145.27; -101.47) SMD -1.85 (-2.25; -1.46).
|
Extension study of Mackey, 2009.
Data on the outcome measures is extracted from Pulling, 2020 (erratum of Mackey, 2018). |
Notes:
- Prognostic balance between treatment groups is usually guaranteed in randomized studies, but non-randomized (observational) studies require matching of patients between treatment groups (case-control studies) or multivariate adjustment for prognostic factors (confounders) (cohort studies); the evidence table should contain sufficient details on these procedures
- Provide data per treatment group on the most important prognostic factors [(potential) confounders]
- For case-control studies, provide sufficient detail on the procedure used to match cases and controls
- For cohort studies, provide sufficient detail on the (multivariate) analyses used to adjust for (potential) confounders
Risk of bias table for intervention studies (randomized controlled trials; based on Cochrane risk of bias tool and suggestions by the CLARITY Group at McMaster University)
|
Study reference
(first author, publication year) |
Was the allocation sequence adequately generated? a
Definitely yes Probably yes Probably no Definitely no |
Was the allocation adequately concealed?b
Definitely yes Probably yes Probably no Definitely no |
Blinding: Was knowledge of the allocated interventions adequately prevented?c
Were patients blinded? Were healthcare providers blinded? Were data collectors blinded? Were outcome assessors blinded? Were data analysts blinded?
Definitely yes Probably yes Probably no Definitely no |
Was loss to follow-up (missing outcome data) infrequent?d
Definitely yes Probably yes Probably no Definitely no |
Are reports of the study free of selective outcome reporting?e
Definitely yes Probably yes Probably no Definitely no |
Was the study apparently free of other problems that could put it at a risk of bias?f
Definitely yes Probably yes Probably no Definitely no |
Overall risk of bias If applicable/necessary, per outcome measureg
LOW Some concerns HIGH
|
|
Akdoğan, 2021 |
Definitely yes;
Reason: The software on the “www.randomizer.org” website was used for randomization. |
Probably yes;
Reason: The software on the “www.randomizer.org” website was used for randomization. |
No information;
Reason: nothing is reported on blinding. |
No information;
Reason: no loss to follow-up reported. |
Probably yes;
Reason: All outcomes mentioned in the methods section are reported in the results section. |
Probably no;
Reason: small sample size, power calculation is not provided. |
HIGH
Reason: It is unclear whether the study is blinded. This may have influenced the results. |
|
Bashiri, 2018 |
Definitely yes;
Reason: Patients were randomized to music treatment or control groups by drawing of lots. |
Probably yes;
Reason: Patients were randomized to music treatment or control groups by drawing of lots. |
Definitely yes;
Reason: An endoscopy personnel applied music administered through headphones so that the investigator and the endoscopist were blinded.
Patients were blinded. |
No information;
Reason: no loss to follow-up reported. |
Probably yes.
Reason: All outcomes mentioned in the methods section are reported in the results section. |
Probably no;
Reason: The groups were not comparable at baseline, more women than men in all groups + baseline pain scores were different between the groups. |
Some concerns
Reason: Baseline differences between the groups. |
|
Bechtold, 2006 |
Definitely yes;
Reason: Randomization was carried out by the use of opaque envelopes, half of which containing a piece of paper that said “music,” and the other half containing a piece of paper that said “no music.” |
Probably yes;
Reason: Randomization was carried out by the use of opaque envelopes, half of which containing a piece of paper that said “music,” and the other half containing a piece of paper that said “no music.” |
Probably no;
Reason: Only the patients were blinded to the intervention. |
No information
Reason: no loss to follow-up reported. |
Probably yes;
Reason: All outcomes mentioned in the methods section are reported in the results section. |
Probably no;
Reason: Some differences at baseline (less anxiety). |
HIGH
Reason: only patients were blinded to the intervention. |
|
De Silva, 2016 |
Definitely yes;
Reason: Using computer generated numbers, patients were randomly as signed to three groups. |
Probably yes;
Reason: Using computer generated numbers, patients were randomly as signed to three groups. |
Probably yes;
Reason: The endoscopist was blinded. The randomization procedure and the display mounting were performed by doctors who were not endoscopists. |
Definitely yes;
Reason: The study reported no loss to follow-up. |
Probably yes;
Reason: All outcomes mentioned in the methods section are reported in the results section. |
Probably no;
Reason: The study was terminated during an interim analysis as these results show unequivocal benefit of AD over VD or sedation alone. |
Some concerns
Reason: Early termination and the study was not completely blinded. |
|
Ebrahimi, 2020 |
No information
Reason: The randomization procedure was not described. |
No information
Reason: The randomization procedure was not described. |
Definitely no;
Reason: Blinding was not performed and is difficult to accomplish because of the nature of the intervention. |
Definitely yes;
Reason: the study reported no low to follow-up. |
Probably yes;
Reason: All outcomes mentioned in the methods section are reported in the results section. |
Probably no;
Reason: small sample size, power calculation is not provided. |
HIGH
Reason: small sample size and blinding was not performed. |
|
Fang, 2016 |
Probably no;
Reason: Patients were randomized to an experimental group (video glasses) or control group (no video glasses) based On block randomization (block size of 4). The small block size may predispose the study to selection bias. |
Definitely yes;
Reason: The randomization process was concealed with the use of sealed and consecutively numbered envelopes. |
Definitely no;
Reason: The patients and staff members were aware of who was in the experimental and control groups, which could lead to potential bias. |
Probably no;
Reason: The study reported no loss to follow-up but incomplete outcome data was high (>10% in both groups). |
Probably no;
Reason: The before and after measurements are not shown, only the differences; which makes it hard to interpretate. |
Probably no;
Reason: The groups included in the analysis were heterogeneous; patients underwent various types of outpatient procedures. |
HIGH
Reason: no blinding, risk of selection bias, heterogeneous groups of patients. |
|
Huang, 2020 |
Probably yes;
Reason: randomized to the IVR or control group by computer-generated randomization in Microsoft Excel 2016 with the process blocked every 10 patients to ensure 5 in each arm |
No information
Reason: nothing is reported about randomization concealment. |
Definitely no;
Reason: The study was not blinded. |
Probably yes;
Reason: The study reported no loss to follow-up. |
Probably no;
Reason: The before and after measurements are not shown, only the differences; which makes it hard to interpretate and compare. |
Probably yes;
Reason: Some patients got bored during the intervention, more engaging content was needed. |
HIGH
Reason: no blinding, |
|
Kang, 2008 |
Probably yes;
Reason: Patients were randomized using an sealed envelope assignment system. |
Probably yes;
Reason: Patients were randomized using an sealed envelope assignment system. : |
Probably no;
Reason: The design of the study mentions a single-blinded study. The authors do not mention who was blinded. |
Definitely yes;
Reason: Only two patients were excluded during the study. |
Probably yes;
Reason: All outcomes mentioned in the methods section are reported in the results section. |
Probably no;
Reason: The study was only performed in elderly people. Therefore probably not generalizable to all adults. |
HIGH
Reason: The study was not double blinded and only performed in the elderly. |
|
Kukreja, 2020 |
Probably yes;
Reason: Participants were randomized using computer-generated random numbers. |
Probably yes;
Reason: Participants were randomized using computer-generated random numbers. |
No information;
Reason: The study did not report any details on blinding. |
Definitely no;
Reason: loss to follow-up and incomplete outcome data were frequent. |
Probably yes;
Reason: All outcomes mentioned in the methods section are reported in the results section. |
Probably no;
Reason: Another interesting observation from this study (CONSORT flowchart) revealed that 23% of allocated “control” group patients were converted to general anesthesia as compared to only 5% in the “music” group. |
HIGH
Reason: The study was not blinded, and 23% of the control group converted to general anesthesia, compared to only 5% in the music group which may have affected the results. |
|
Kulkarni, 2012 |
Definitely yes;
Reason: Patients were randomised in blocks to either the study (music) group or the control (no music) group. Block size was randomly chosen to be four or six, with equal probability. This was a computer-generated randomisation process, with the sequencing concealed by sealed and consecutively numbered envelopes. |
Probably yes;
Reason: Patients were randomised in blocks to either the study (music) group or the control (no music) group. Block size was randomly chosen to be four or six, with equal probability. This was a computer-generated randomisation process, with the sequencing concealed by sealed and consecutively numbered envelopes. |
Probably no;
Reason: No details were reported on blinding. As all patients had headphones on, the healthcare providers were probably blinded. |
Definitely yes;
Reason: no missing data was reported. |
Probably yes;
Reason: All outcomes mentioned in the methods section are reported in the results section. |
Probably no;
Reason: It was a small study. |
HIGH
Reason: the study is not completely blinded. |
|
Moon, 2018 |
Definitely yes;
Reason: Randomization was conducted by a number table generated from an internet-based computer program (www.randomization.com). |
Definitely yes;
Reason: A staff anesthesiologist who is independent of our study made a random allocation sequence, enrolled participants and assigned participants to interventions. The study participants were allocated using sequentially numbered, opaque, sealed envelopes containing random allocation numbers. The sealed envelope was opened after induction of anesthesia by the attending anesthesiologist, who was not one of the trial investigators. |
Definitely no;
Reason: The patients and attending anesthesiologists could not be blinded to group assignment |
Definitely yes;
Reason: no missing data was reported. |
Probably yes;
Reason: All outcomes mentioned in the methods section are reported in the results section. |
Probably no;
Reason: the study was a small single-center trial, so its external validity may be limited. |
HIGH
Reason: patients were not blinded. This may certainly have affected the outcome measure patient satisfaction. |
|
Sjölander, 2019 |
Definitely no;
Reason: In order to randomly place the patients in either intervention or control group, they were chronologically assigned from the inclusion list. |
No information;
Reason: the randomization procedure is not reported. |
Probably no;
Reason: The study was single blind, and only the investigator was aware of the intervention design. |
Definitely yes;
Reason: no missing data was reported. |
Probably yes;
Reason: All outcomes mentioned in the methods section are reported in the results section. |
Probably no;
Reason: single center study, may provide guidance for other endoscopic units, but are not fully translatable to other hospital units. |
Some concerns
Reason: patients were not blinded but were unaware of the intervention design. Single center may hamper external validity. |
Risk of bias table for intervention studies (randomized controlled trials; based on Cochrane risk of bias tool and suggestions by the CLARITY Group at McMaster University)
Research question:
|
Study reference
(first author, publication year) |
Was the allocation sequence adequately generated? a
Definitely yes Probably yes Probably no Definitely no |
Was the allocation adequately concealed?b
Definitely yes Probably yes Probably no Definitely no |
Blinding: Was knowledge of the allocated interventions adequately prevented?c
Were patients blinded?
Were healthcare providers blinded?
Were data collectors blinded?
Were outcome assessors blinded?
Were data analysts blinded?
Definitely yes Probably yes Probably no Definitely no |
Was loss to follow-up (missing outcome data) infrequent?d
Definitely yes Probably yes Probably no Definitely no |
Are reports of the study free of selective outcome reporting?e
Definitely yes Probably yes Probably no Definitely no |
Was the study apparently free of other problems that could put it at a risk of bias?f
Definitely yes Probably yes Probably no Definitely no |
Overall risk of bias If applicable/necessary, per outcome measureg
LOW Some concerns HIGH
|
|
Izanloo, 2021 |
No information;
Reason: Based on the mean age and level of education (diploma, bachelor of science, master, or higher degrees), the patients were randomly assigned into experimental and control groups |
No information
Reason: Based on the mean age and level of education (diploma, bachelor of science, master, or higher degrees), the patients were randomly assigned into experimental and control groups |
No information
Reason: Blinding is not reported. Due to the nature of the intervention, patients were not blinded. |
No information
Reason: Nothing was reported on loss to follow-up. |
Probably yes;
Reason: All outcomes mentioned in the abstract of the study were reported. |
Probably no;
Reason: relevant baseline imbalance between the groups (the onset time of sedation after receiving anesthetics) |
HIGH (all outcomes)
The study has a quasi-experimental study design. The method of randomization is not clearly described and blinding was not reported. As the outcome measures are subjective, this could have resulted in bias. |
|
Mackey, 2018 |
Definitely no;
Reason: Random assignment of the participants was accomplished by having the oral surgery assistant assign them to the treatment or control group in alternating fashion. |
Probably no;
Reason: Random assignment of the participants was accomplished by having the oral surgery assistant assign them to the treatment or control group in alternating fashion. |
No information;
Reason: The researcher was blind to which subjects belonged to the treatment or experimental groups.
Furthermore, nothing is reported on blinding. |
Definitely no;
Reason: It was necessary to exclude 24 people from this study. Eight individuals were excluded for having a body weight greater than 100 kg, 6 individuals were excluded for having previous hypnotic experience, and 10 individuals were excluded due to age less than 18 or older than 25. Unclear whether this was in the experimental or control group. |
Probably yes;
Reason: All outcomes mentioned in the abstract of the study were reported. |
Probably no;
Reason: Nothing is reported about the demographics of the two groups. It is therefore unknown whether the groups are comparable. Furthermore, the study size is small. |
HIGH (all outcomes)
The method of randomization is not clearly described and the blinding procedure is not fully described. As the outcome measures are subjective, this could have resulted in bias. |
- Randomization: generation of allocation sequences have to be unpredictable, for example computer generated random-numbers or drawing lots or envelopes. Examples of inadequate procedures are generation of allocation sequences by alternation, according to case record number, date of birth or date of admission.
- Allocation concealment: refers to the protection (blinding) of the randomization process. Concealment of allocation sequences is adequate if patients and enrolling investigators cannot foresee assignment, for example central randomization (performed at a site remote from trial location). Inadequate procedures are all procedures based on inadequate randomization procedures or open allocation schedules..
- Blinding: neither the patient nor the care provider (attending physician) knows which patient is getting the special treatment. Blinding is sometimes impossible, for example when comparing surgical with non-surgical treatments, but this should not affect the risk of bias judgement. Blinding of those assessing and collecting outcomes prevents that the knowledge of patient assignment influences the process of outcome assessment or data collection (detection or information bias). If a study has hard (objective) outcome measures, like death, blinding of outcome assessment is usually not necessary. If a study has “soft” (subjective) outcome measures, like the assessment of an X-ray, blinding of outcome assessment is necessary. Finally, data analysts should be blinded to patient assignment to prevents that knowledge of patient assignment influences data analysis.
- If the percentage of patients lost to follow-up or the percentage of missing outcome data is large, or differs between treatment groups, or the reasons for loss to follow-up or missing outcome data differ between treatment groups, bias is likely unless the proportion of missing outcomes compared with observed event risk is not enough to have an important impact on the intervention effect estimate or appropriate imputation methods have been used.
- Results of all predefined outcome measures should be reported; if the protocol is available (in publication or trial registry), then outcomes in the protocol and published report can be compared; if not, outcomes listed in the methods section of an article can be compared with those whose results are reported.
- Problems may include: a potential source of bias related to the specific study design used (e.g. lead-time bias or survivor bias); trial stopped early due to some data-dependent process (including formal stopping rules); relevant baseline imbalance between intervention groups; claims of fraudulent behavior; deviations from intention-to-treat (ITT) analysis; (the role of the) funding body. Note: The principles of an ITT analysis implies that (a) participants are kept in the intervention groups to which they were randomized, regardless of the intervention they actually received, (b) outcome data are measured on all participants, and (c) all randomized participants are included in the analysis.
- Overall judgement of risk of bias per study and per outcome measure, including predicted direction of bias (e.g. favors experimental, or favors comparator). Note: the decision to downgrade the certainty of the evidence for a particular outcome measure is taken based on the body of evidence, i.e. considering potential bias and its impact on the certainty of the evidence in all included studies reporting on the outcome.
Table of excluded studies
|
Author and year |
Reason for exclusion |
|
Argstatter, 2006 |
The relevant outcome measures were not reported in this study. |
|
Åžen, 2009 |
Full text not available. |
|
Bansal, 2010 |
Full text not available. |
|
Bechtold, 2009 |
The SR of Wang (2014) is more recent. |
|
Björkman, 2013 |
Included in Wang (2014). |
|
Bruno, 2020 |
Full text not available. |
|
Bytzer, 2007 |
Does not match PICO: wrong intervention. |
|
Chang, 2011 |
Unclear whether the study contains PSA, and the relevant outcome measures were not reported in this study. |
|
Costa, 2010 |
Included in Wang (2014). |
|
Di Nasso, 2016 |
Doet not match PICO: only local anesthesia. |
|
Dogan, 2016 |
The relevant outcome measures were not reported in this study. |
|
Hamidi, 2017 |
Doet not match PICO: only local anesthesia. |
|
Harikumar, 2006 |
Included in Wang (2014). |
|
Mackey, 2009 |
The included study from Mackey (2018) is an update of this study. |
|
Maeyama, 2009 |
Full text not available. |
|
Marc, 2007 |
Included in Noergaard (2019). |
|
Meier, 2021 |
Full text not available. |
|
Munn, 2012 |
The SR does not match PICO: research question too broad. |
|
Munn, 2013 |
Does not match PICO: children. |
|
Ovayolu, 2006 |
Included in Wang (2014). |
|
Pongraweewan, 2005 |
Does not match PICO: wrong comparison |
|
Rudin, 2007 |
The SR of Wang (2014) is more recent. |
|
Spagnuolo, 2020 |
This study is not a RCT |
|
Tam, 2008 |
The SR of Wang (2014) is more recent. |
|
Tran, 2020 |
The comparison (noise cancelling) does not match PICO. |
|
Yeo, 2013 |
Included in Wang (2014). |
|
Zhang, 2005 |
Does not match PICO: surgical patients. |
|
Zhang, 2012 |
The study is not about PSA. |
Verantwoording
Beoordelingsdatum en geldigheid
Publicatiedatum : 23-05-2024
Beoordeeld op geldigheid : 23-05-2024
Algemene gegevens
De ontwikkeling/herziening van deze richtlijnmodule werd ondersteund door het Kennisinstituut van de Federatie Medisch Specialisten (www.demedischspecialist.nl/kennisinstituut) en werd gefinancierd uit de Kwaliteitsgelden Medisch Specialisten (SKMS). De financier heeft geen enkele invloed gehad op de inhoud van de richtlijnmodule.
Samenstelling werkgroep
Voor het ontwikkelen van de richtlijnmodule is in 2020 een multidisciplinaire werkgroep ingesteld, bestaande uit vertegenwoordigers van alle relevante specialismen (zie hiervoor de Samenstelling van de werkgroep) die betrokken zijn bij sedatie en/of analgesie bij volwassen patiënten.
Werkgroep
- Prof. dr. B. Preckel (voorzitter), anesthesioloog, Amsterdam UMC locatie AMC, NVA
- dr. C.R.M. Barends, anesthesioloog, UMCG, NVA
- L.R.M. Braam, BSc. Sedatie Praktijk Specialist, Catharina Ziekenhuis, NVAM
- drs. R. Brethouwer, abortusarts, Beahuis & Bloemenhovekliniek Heemstede, NGvA
- dr. J.M. van Dantzig, cardioloog, Catharina Ziekenhuis, NVVC
- drs. V.A.A. Heldens, anesthesioloog, Maxima MC, NVA
-
dr. C. Heringhaus, SEH-arts/anesthesioloog, LUMC (t/m 12-2022), Medisch manager Hyperbare Zuurstoftherapie Goes, MCHZ (vanaf 01-2023), NVSHA
- T. Jonkergouw, MA. Adviseur Patiëntbelang, Patiëntenfederatie Nederland (tot april 2023)
- Broere, M. Junior beleidsadviseur, Patiëntenfederatie Nederland (vanaf april 2023)
- dr. M. Klemt-Kropp, MDL-arts, Noordwest Ziekenhuisgroep, NVMDL
- drs. B.M.F. van der Leeuw, anesthesioloog, Albert Schweitzer ziekenhuis, NVA
- S. Reumkens, MSc. Physician Assistant, Diakonessenhuis, NAPA
Klankbordgroep
- drs. T.E.A. Geeraedts, radioloog, Erasmus MC, NVvR
- drs. J. Friederich, gynaecoloog, Noordwest Ziekenhuisgroep, NVOG
- dr. E.H.F.M. van der Heijden, longarts, Radboud UMC, NVALT
- drs. J. de Hoog, oogarts, Amsterdam UMC locatie AMC, NOG
- drs. A. Kanninga, arts voor verstandelijk gehandicapten, Cordaan, NVAVG
- drs. H.W.N. van der Pas, tandarts, UMC Utrecht, VMBZ
- dr. ir. C. van Pul, klinisch fysicus, Maxima MC, NVKF
- dr. R.J. Robijn, MDL-arts, Rijnstate, NVMDL
- drs. W.S. Segers, klinisch Geriater, Catharina Ziekenhuis, NVKG
- Prof. dr. A. Visser, hoogleraar geriatrische tandheelkunde, UMCG en Radboud UMC, KNMT
Met ondersteuning van:
- dr. L. Wesselman, adviseur, Kennisinstituut van Medisch Specialisten
- dr. S.N. Hofstede, senior adviseur, Kennisinstituut van Medisch Specialisten
- drs. I. van Dusseldorp, senior literatuurspecialist, Kennisinstituut van Medisch Specialisten
Belangenverklaringen
De Code ter voorkoming van oneigenlijke beïnvloeding door belangenverstrengeling is gevolgd. Alle werkgroepleden hebben schriftelijk verklaard of zij in de laatste drie jaar directe financiële belangen (betrekking bij een commercieel bedrijf, persoonlijke financiële belangen, onderzoeksfinanciering) of indirecte belangen (persoonlijke relaties, reputatiemanagement) hebben gehad. Gedurende de ontwikkeling of herziening van een module worden wijzigingen in belangen aan de voorzitter doorgegeven. De belangenverklaring wordt opnieuw bevestigd tijdens de commentaarfase.
Een overzicht van de belangen van werkgroepleden en het oordeel over het omgaan met eventuele belangen vindt u in onderstaande tabel. De ondertekende belangenverklaringen zijn op te vragen bij het secretariaat van het Kennisinstituut van de Federatie Medisch Specialisten.
|
Werkgroeplid |
Functie |
Nevenfuncties |
Gemelde belangen |
Ondernomen actie |
|
Preckel |
Anesthesioloog, hoogleraar anesthesiologie (in het bijzonder veiligheid in het perioperatieve proces) Amsterdam Universitair Medische Centra locatie AMC |
Onbetaalde nevenwerkzaamheden – commissie-werkzaamheden:
Lid Patient Safety and Quality Committee of the European Society of Anesthesiologists;
Lid Patient Safety Committee van de World Federation of Societies of Anesthesiologists;
Lid Raad Wetenschap en Innovatie van de Federatie Medisch Specialisten FMS;
Lid Commissie Wetenschap & Innovatie van de Nederlandse Vereniging voor Anesthesiologie NVA
Representative Council European Association of Cardiothoracic Anesthesiology and Intensive Care (EACTAIC)
Hoger leidinggevend personeel (penningmeester) van de “Stichting ter bevordering van de wetenschap en opleiding in de anesthesiologie”;
|
Research grants: European Society of Anesthesiology and Intensive Care ESAIC ZonMw NovoNordisk Netherland
Advisory board Sensium Healthcare United Kingdom
Geen van de gemelde belangen heeft relatie met het onderwerp van het advies/de richtlijn
|
Geen actie vereist |
|
Barends |
Anesthesioloog in het Universitair Medisch Centrum Groningen |
Geen |
Geen |
Geen actie vereist |
|
Braam |
Sedatiepraktijkspecialist Catharina Ziekenhuis Eindhoven |
Lid sedatie commissie NVAM (onbetaald) |
Geen |
Geen actie vereist |
|
Brethouwer |
Abortusarts te Beahuis & Bloemenhovekliniek (0,56 fte) en SAA (0,22 fte), Medisch coördinator Beahuis&Bloemenhovekliniek (0,33 fte) |
Penningmeester van het Nederlands Genootschap van Abortusartsen (onbetaald) Voorzitter landelijke werkgroep PSA van het NGvA (onbetaald) Bestuurslid van FIAPAC, een Europese abortus organisatie (onbetaald) |
Geen |
Geen actie vereist |
|
Broere |
Junior beleidsadviseur Patiëntenbelang - fulltime |
geen |
geen |
Geen actie vereist |
|
van Dantzig |
Cardioloog vrij gevestigd, Catharina Ziekenhuis 100% |
Lid Plenaire Visitatie Commissie NVVC (onbetaald) |
Op onze afdeling wordt extern gefinancierd onderzoek uitgevoerd maar niet op het gebied van de werkgroep. |
Geen actie vereist |
|
Heldens |
Anesthesioloog Maxima MC |
Geen |
Geen |
Geen actie vereist |
|
Heringhaus |
Vanaf 01-2023 Medisch manager Hyperbare Zuurstoftherapie Goes, MCHZ
t/m 12-2022 SEH-arts KNMG |
Trainingen voor verschillende onderwerpen gerelateerd aan acute zorg, hyperbare geneeskunde, PSA |
Geen |
Geen actie vereist |
|
Jonkergouw |
Junior beleidsadviseur - Patiëntenfederatie Nederland - 32 tot 36 uur per week |
Vrijwilliger activiteiten - Diabetes Vereniging Nederland - Zeer incidenteel |
Geen |
Geen actie vereist |
|
Klemt-Kropp |
MDL-arts, Noordwest Ziekenhuisgroep Alkmaar - Schagen - Den Helder (0.9 fte) |
Secretaris Concilium Gastroenterologicum, NVMDL tot 11 april 2022 (niet betaald) Voorzitter PSA commissie NVMDL (niet betaald) Docent Teach the Teacher AUMC en Noordwest Ziekenhuisgroep cursussen voor aios en medisch specialisten (betaalde functie, ongeveer 40 Std. per jaar)
Voorzitter Stichting MDL Holland-Noord (KvK 56261225) vanaf okt. 2012 t/m 31-12-2019. De stichting heeft in de laatste 3 jaar grants ontvangen van de farmaceutische industrie en van de farmaceutische industrie gesponsorde onderzoeken gefaciliteerd:
1. Ondersteuning optimalisering van zorg voor IBD-patiënten. Therapeutic drugmonitoring en PROMs bij patiënten met IBD. Zorgverbetertraject. PhD student, looptijd van 2015 tot op heden. Tot 2018 grant van Dr. Falk Pharma. vanaf 2018 grant van Janssen Cilag Geen relatie met sedatie 2.Retrieval of patients chronically infected with Hepatitis B or Hepatitis C in Northern Holland. Afgesloten 2018. Project gefinancierd met grant van Gilead. Geen relatie met sedatie 3. SIPI. Screening op Infectieuze aandoeningen in Penitentiaire Inrichtingen. Project gefinancierd met grants van AbbVie, MSD en Gilead. Project begin 2019 afgesloten. Geen relatie met sedatie 4. 3DUTCH trial. Een observationeel onderzoek naar de effectiviteit van een behandeling van chronische hepatitis C met een combinatie van de antivirale middelen ombitasvir - paritaprevir /ritonavir, ± dasabuvir, ± ribavirine. Sponsor AbbVie. Studie afgesloten Jan. 2018 Geen relatie met sedatie 5. Remsima switch IFX9501 - An open-label, multicenter, non- inferiority monitoring program to investigate the quality of life, efficacy and safety in subjects with Crohn’s Disease (CD), Ulcerative Colitis (UC) in stable remission after switching from Remicade® (infliximab) to Remsima® (infliximab biosimilar) L016-048. Sponsor: Munipharma. Afgelsoten augustus 2018. Geen relatie met sedatie 6. NASH - NN9931-4296 Investigation of efficacy and safety of three dose levels of subcutaneous semaglutide once daily versus placebo in subjects with non-alcoholic steatohepatitis L016-061. Sponsor NovoNordisk. Studie afgesloten Feb. 2020. Geen relatie met sedatie 7. Randomized, Double-blind, Placebo-controlled, Parallel-group Efficacy and Safety Study of SHP647 as Induction Therapy in Subjects with Moderate to Severe Crohn's Disease (CARMEN CD 305). SHD-647-305. Sponsor Shire. Studie loopt sinds 2019. Geen relatie met sedatie 8. Randomized, Double-blind, Placebo-controlled, Parallel-group Efficacy and Safety Study of SHP647 as Maintenance Therapy in Subjects with Moderate to Severe Crohn's Disease (CARMEN CD 307). SHD-647-307. Sponsor Shire. Studie loopt sinds 2019. Geen relatie met sedatie 9. Estimating the prevalence of advanced liver fibrosis in a population cohort in care in Northern-Holland with the use of the non-invasive FIB-4 index. Grant van Gilead. Onderzoek afgesloten sept. 2019. Geen relatie met sedatie |
Incidenteel deelname aan advisory boards van de farmaceutische industrie (Gilead, Janssen Cilag, AbbVie: (hepatologische onderwerpen, vooral hepatitis C) Incidenteel voordrachten tijdens symposia gesponsord van de farmaceutische industrie (Gilead, AbbVie)
|
Geen actie vereist; meeste studies afgerond; nr. 1,7,8 lopen. Sponsoren (Dr. Falk Pharma & Shire) hebben geen relatie met sedatie. |
|
Reumkens |
Physician Assistant Anesthesiologie Radboud UMC |
Voorzitter vakgroep PA Anesthesiologie NAPA |
Geen |
Geen actie vereist |
|
Van der Leeuw |
Anesthesioloog |
Geen |
Geen |
Geen actie vereist |
|
Klankbordgroeplid |
Functie |
Nevenfuncties |
Gemelde belangen |
Ondernomen actie |
|
Robijn |
Mdl arts rijnstate ziekenhuis Arnhem |
Geen |
Geen |
Geen actie vereist |
|
Van der Heijden |
Longarts |
Voormalig Secretaris Sectie Interventie longziekten NVALT (onbezoldigd)
Lid Board of National Delegates European Association of Bronchology and International Pulmonology (namens NL, onbezoldigd)
|
Buiten het veld van deze richtlijn heeft mijn afdeling de afgelopen 3 jaar vergoedingen ontvangen voor de volgende activiteiten: - unrestricted research grants: Pentax Medical Europe, Philips, Astra Zeneca, Johnson&Johnson. - adviseur / consultant: Pentax Medical, Philips IGT, Johnson&Johnson. - spreker: Pentax Medical. |
Geen actie vereist |
|
Van der Pas |
Tandarts, UMC Utrecht |
Commissielid Horace Wells van de KNMT, stimulatie van de intercollegiale samenwerking bij tandheelkundige behandeling van bijzondere zorggroepen met farmacologische ondersteuning. Onbetaald. Voormalig commissielid Bijzondere Zorggroepen van de KNMT, toegankelijkheid van mondzorg voor kwetsbare zorggroep. Betaald via vacatiegelden. Gastdocent opleiding mondzorgkunde HU. Lezing mondzorg aan mensen met een verstandelijke beperking. Betaald. Gastdocent opleiding verpleegkundig-specialist GGZ. Lezing mondzorg in de geestelijke gezondheidszorg Betaald. Cursusleider lichte sedatie in de mondzorg, BT Academy. Meerdaagse cursus voor tandartsen en mondhygienisten om zich te bekwamen in lichte sedatie, m.n. training in de inhalatiesedatie met lachgas-zuurstof mengsel middels titratietechniek. Betaald. |
Geen |
Geen actie vereist |
|
De Hoog |
Oogarts in Amsterdam UMC (0,2 fte.) en Retina Operatie Centrum Amstelveen (0,4 fte.). Medisch manager Retina Operatie Centrum (0,2 fte.) |
Voorzitter Werkgroep Vitreoretinale Chirurgie Nederland (onbetaald) Lid redactieraad vaktijdschrift 'De Oogarts', uitgave van BPM-medica (onbezoldigd) Medeorganisator Eilanddagen (bijscholing uveïtis voor oogartsen, onbetaald) |
Geen |
Geen actie vereist |
|
Geeraedts |
Interventieradioloog Afdeling Radiologie en Nucleaire geneeskunde Erasmus Medisch Centrum, Rotterdam |
Geen |
Geen |
Geen actie vereist |
|
Van Pul |
Klinisch fysicus in Maxima Medisch Centrum |
Universitair Hoofd Docent aan de Technische Universiteit van Eindhoven (0,2 fte). Daar supervisor van PhD studenten bij HTSM (NWO-TTW gesubsidieerd) project waaraan ook een industriële partner deelneemt (https://www.nwo.nl/projecten/15345-0).
|
Geen |
Geen actie vereist |
|
Friederich |
Gynaecoloog NWZ Den Helder, Algemeen gynaecoloog met als aandachtsgebieden benigne gynaecologie, minimaal invasieve chirurgie en bekkenbodemproblematiek |
Vicevoorzitter calamiteitencommissie NVZ lid klachtencommissie NWZ Den Helder |
Geen |
Geen actie vereist |
|
Segers |
Klinisch geriater, St. Jans Gasthuis, Weert
|
Klinisch farmacoloog in opleiding, Catharina ziekenhuis, Eindhoven Onbetaald |
Geen |
Geen actie vereist |
|
Kanninga |
Arts Verstandelijk Gehandicapten (arts VG) bij Cordaan Amsterdam Anesthesioloog niet praktiserend |
Geen |
Geen |
Geen actie vereist |
|
Visser |
Hoogleraar geriatrische tandheelkunde fulltime (1 fte) - Afdeling Gerodontologie, Centrum voor Tandheelkunde en Mondzorgkunde, Universitair Medisch Centrum Groningen, Rijksuniversiteit Groningen, Nederland - Afdeling Gerodontologie, Faculteit Tandheelkunde, Radboud UMC, Radboud Universiteit Nijmegen, Nederland |
Geen |
Geen |
Geen actie vereist |
Inbreng patiëntenperspectief
Inbreng patiëntenperspectief
Er werd aandacht besteed aan het patiëntenperspectief door het uitnodigen van de Patiëntenfederatie Nederland voor de invitational conference. Het verslag hiervan (zie aanverwante producten) is besproken in de werkgroep. Aanvullend heeft een afgevaardigde van de Patiëntenfederatie Nederland deelgenomen in de werkgroep. De verkregen input is meegenomen bij het opstellen van de uitgangsvragen, de keuze voor de uitkomstmaten en bij het opstellen van de richtlijn. De conceptrichtlijn is tevens voor commentaar voorgelegd aan de Patiëntenfederatie Nederland en de aangeleverde commentaren zijn bekeken en verwerkt.
Kwalitatieve raming van mogelijke financiële gevolgen in het kader van de Wkkgz
Bij de richtlijn is conform de Wet kwaliteit, klachten en geschillen zorg (Wkkgz) een kwalitatieve raming gedaan of de aanbevelingen mogelijk leiden tot substantiële financiële gevolgen. Bij het uitvoeren van deze beoordeling zijn richtlijnmodules op verschillende domeinen getoetst (zie ook het hiervoor gebruikte stroomschema dat als uitgangspunt voor de beoordeling is gebruikt).
Uit de kwalitatieve raming blijkt dat er waarschijnlijk geen substantiële financiële gevolgen zijn. Een overzicht van uitkomsten van de kwalitatieve raming met bijbehorende toelichting vindt u in onderstaande tabel.
|
Module |
Uitkomst raming |
Toelichting |
|
Module Niet-medicamenteuze interventies |
geen substantiële financiële gevolgen |
Hoewel uit de toetsing volgt dat de aanbeveling(en) breed toepasbaar zijn (>40.000 patiënten), volgt ook uit de toetsing dat het overgrote deel (±90%) van de zorgaanbieders en zorgverleners al aan de norm voldoet en het geen nieuwe manier van zorgverlening of andere organisatie van zorgverlening betreft, het geen toename in het aantal in te zetten voltijdsequivalenten aan zorgverleners betreft en het geen wijziging in het opleidingsniveau van zorgpersoneel betreft. |
Werkwijze
AGREE
Deze richtlijnmodule is opgesteld conform de eisen vermeld in het rapport Medisch Specialistische Richtlijnen 2.0 van de adviescommissie Richtlijnen van de Raad Kwaliteit. Dit rapport is gebaseerd op het AGREE II instrument (Appraisal of Guidelines for Research & Evaluation II; Brouwers, 2010).
Knelpuntenanalyse en uitgangsvragen
Tijdens de voorbereidende fase inventariseerde de werkgroep de knelpunten in de zorg voor patiënten die procedurele sedatie en/of analgesie ondergaan. Tevens zijn er knelpunten aangedragen door de Nederlandse Vereniging voor Anesthesiologie, de Nederlandse Vereniging voor Heelkunde, de Nederlandse Vereniging voor Obstetrie en Gynaecologie, Nederlandse Vereniging voor Cardiologie, de Nederlandse Vereniging van Maag-Darm-Leverartsen, de Vereniging voor Keel-Neus-Oorheelkunde en Heelkunde van het Hoofd-Halsgebied, de Nederlandse Vereniging voor Intensive Care, de Nederlandse Internisten Vereniging, de Nederlandse Vereniging van Spoedeisende Hulp Artsen, het Nederlands Genootschap van Abortusartsen, de Nederlandse Vereniging van Anesthesiemedewerkers, de Verpleegkundigen & Verzorgenden Nederland, de Nederlandse Vereniging voor Mondziekten, Kaak- en Aangezichtschirurgie, de Vereniging Mondzorg voor Bijzondere Zorggroepen, Stichting Kind & Ziekenhuis, Inspectie Gezondheidszorg en Jeugd en de Vereniging van Artsen voor Verstandelijk Gehandicapten via een invitational conference. Een verslag hiervan is opgenomen onder aanverwante producten. Op basis van de uitkomsten van de knelpuntenanalyse zijn door de werkgroep concept-uitgangsvragen opgesteld en definitief vastgesteld.
Uitkomstmaten
Na het opstellen van de zoekvraag behorende bij de uitgangsvraag inventariseerde de werkgroep welke uitkomstmaten voor de patiënt relevant zijn, waarbij zowel naar gewenste als ongewenste effecten werd gekeken. Hierbij werd een maximum van acht uitkomstmaten gehanteerd. De werkgroep waardeerde deze uitkomstmaten volgens hun relatieve belang bij de besluitvorming rondom aanbevelingen, als cruciaal (kritiek voor de besluitvorming), belangrijk (maar niet cruciaal) en onbelangrijk. Tevens definieerde de werkgroep tenminste voor de cruciale uitkomstmaten welke verschillen zij klinisch (patiënt) relevant vonden.
Methode literatuursamenvatting
Een uitgebreide beschrijving van de strategie voor zoeken en selecteren van literatuur is te vinden onder ‘Zoeken en selecteren’ onder Onderbouwing. Indien mogelijk werd de data uit verschillende studies gepoold in een random-effects model. Review Manager 5.4 werd gebruikt voor de statistische analyses. De beoordeling van de kracht van het wetenschappelijke bewijs wordt hieronder toegelicht.
Beoordelen van de kracht van het wetenschappelijke bewijs
De kracht van het wetenschappelijke bewijs werd bepaald volgens de GRADE-methode. GRADE staat voor ‘Grading Recommendations Assessment, Development and Evaluation’ (zie http://www.gradeworkinggroup.org/). De basisprincipes van de GRADE-methodiek zijn: het benoemen en prioriteren van de klinisch (patiënt) relevante uitkomstmaten, een systematische review per uitkomstmaat, en een beoordeling van de bewijskracht per uitkomstmaat op basis van de acht GRADE-domeinen (domeinen voor downgraden: risk of bias, inconsistentie, indirectheid, imprecisie, en publicatiebias; domeinen voor upgraden: dosis-effect relatie, groot effect, en residuele plausibele confounding).
GRADE onderscheidt vier gradaties voor de kwaliteit van het wetenschappelijk bewijs: hoog, redelijk, laag en zeer laag. Deze gradaties verwijzen naar de mate van zekerheid die er bestaat over de literatuurconclusie, in het bijzonder de mate van zekerheid dat de literatuurconclusie de aanbeveling adequaat ondersteunt (Schünemann, 2013; Hultcrantz, 2017).
|
GRADE |
Definitie |
|
Hoog |
|
|
Redelijk |
|
|
Laag |
|
|
Zeer laag |
|
Bij het beoordelen (graderen) van de kracht van het wetenschappelijk bewijs in richtlijnen volgens de GRADE-methodiek spelen grenzen voor klinische besluitvorming een belangrijke rol (Hultcrantz, 2017). Dit zijn de grenzen die bij overschrijding aanleiding zouden geven tot een aanpassing van de aanbeveling. Om de grenzen voor klinische besluitvorming te bepalen moeten alle relevante uitkomstmaten en overwegingen worden meegewogen. De grenzen voor klinische besluitvorming zijn daarmee niet één op één vergelijkbaar met het minimaal klinisch relevant verschil (Minimal Clinically Important Difference, MCID). Met name in situaties waarin een interventie geen belangrijke nadelen heeft en de kosten relatief laag zijn, kan de grens voor klinische besluitvorming met betrekking tot de effectiviteit van de interventie bij een lagere waarde (dichter bij het nul effect) liggen dan de MCID (Hultcrantz, 2017).
Overwegingen (van bewijs naar aanbeveling)
Om te komen tot een aanbeveling zijn naast (de kwaliteit van) het wetenschappelijke bewijs ook andere aspecten belangrijk en worden meegewogen, zoals aanvullende argumenten uit bijvoorbeeld de biomechanica of fysiologie, waarden en voorkeuren van patiënten, kosten (middelenbeslag), aanvaardbaarheid, haalbaarheid en implementatie. Deze aspecten zijn systematisch vermeld en beoordeeld (gewogen) onder het kopje ‘Overwegingen’ en kunnen (mede) gebaseerd zijn op expert opinion. Hierbij is gebruik gemaakt van een gestructureerd format gebaseerd op het evidence-to-decision framework van de internationale GRADE Working Group (Alonso-Coello, 2016a; Alonso-Coello 2016b). Dit evidence-to-decision framework is een integraal onderdeel van de GRADE methodiek.
Formuleren van aanbevelingen
De aanbevelingen geven antwoord op de uitgangsvraag en zijn gebaseerd op het beschikbare wetenschappelijke bewijs en de belangrijkste overwegingen, en een weging van de gunstige en ongunstige effecten van de relevante interventies. De kracht van het wetenschappelijk bewijs en het gewicht dat door de werkgroep wordt toegekend aan de overwegingen, bepalen samen de sterkte van de aanbeveling. Conform de GRADE-methodiek sluit een lage bewijskracht van conclusies in de systematische literatuuranalyse een sterke aanbeveling niet a priori uit, en zijn bij een hoge bewijskracht ook zwakke aanbevelingen mogelijk (Agoritsas, 2017; Neumann, 2016). De sterkte van de aanbeveling wordt altijd bepaald door weging van alle relevante argumenten tezamen. De werkgroep heeft bij elke aanbeveling opgenomen hoe zij tot de richting en sterkte van de aanbeveling zijn gekomen.
In de GRADE-methodiek wordt onderscheid gemaakt tussen sterke en zwakke (of conditionele) aanbevelingen. De sterkte van een aanbeveling verwijst naar de mate van zekerheid dat de voordelen van de interventie opwegen tegen de nadelen (of vice versa), gezien over het hele spectrum van patiënten waarvoor de aanbeveling is bedoeld. De sterkte van een aanbeveling heeft duidelijke implicaties voor patiënten, behandelaars en beleidsmakers (zie onderstaande tabel). Een aanbeveling is geen dictaat, zelfs een sterke aanbeveling gebaseerd op bewijs van hoge kwaliteit (GRADE gradering HOOG) zal niet altijd van toepassing zijn, onder alle mogelijke omstandigheden en voor elke individuele patiënt.
|
Implicaties van sterke en zwakke aanbevelingen voor verschillende richtlijngebruikers |
||
|
|
||
|
|
Sterke aanbeveling |
Zwakke (conditionele) aanbeveling |
|
Voor patiënten |
De meeste patiënten zouden de aanbevolen interventie of aanpak kiezen en slechts een klein aantal niet. |
Een aanzienlijk deel van de patiënten zouden de aanbevolen interventie of aanpak kiezen, maar veel patiënten ook niet. |
|
Voor behandelaars |
De meeste patiënten zouden de aanbevolen interventie of aanpak moeten ontvangen. |
Er zijn meerdere geschikte interventies of aanpakken. De patiënt moet worden ondersteund bij de keuze voor de interventie of aanpak die het beste aansluit bij zijn of haar waarden en voorkeuren. |
|
Voor beleidsmakers |
De aanbevolen interventie of aanpak kan worden gezien als standaardbeleid. |
Beleidsbepaling vereist uitvoerige discussie met betrokkenheid van veel stakeholders. Er is een grotere kans op lokale beleidsverschillen. |
Organisatie van zorg
In de knelpuntenanalyse en bij de ontwikkeling van de richtlijnmodule is expliciet aandacht geweest voor de organisatie van zorg: alle aspecten die randvoorwaardelijk zijn voor het verlenen van zorg (zoals coördinatie, communicatie, (financiële) middelen, mankracht en infrastructuur). Randvoorwaarden die relevant zijn voor het beantwoorden van deze specifieke uitgangsvraag zijn genoemd bij de overwegingen. Meer algemene, overkoepelende, of bijkomende aspecten van de organisatie van zorg worden behandeld in de module Organisatie van zorg.
Commentaar- en autorisatiefase
De conceptrichtlijnmodule werd aan de betrokken (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd ter commentaar. De commentaren werden verzameld en besproken met de werkgroep. Naar aanleiding van de commentaren werd de conceptrichtlijnmodule aangepast en definitief vastgesteld door de werkgroep. De definitieve richtlijnmodule werd aan de deelnemende (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd voor autorisatie en door hen geautoriseerd dan wel geaccordeerd.
Literatuur
Agoritsas T, Merglen A, Heen AF, Kristiansen A, Neumann I, Brito JP, Brignardello-Petersen R, Alexander PE, Rind DM, Vandvik PO, Guyatt GH. UpToDate adherence to GRADE criteria for strong recommendations: an analytical survey. BMJ Open. 2017 Nov 16;7(11):e018593. doi: 10.1136/bmjopen-2017-018593. PubMed PMID: 29150475; PubMed Central PMCID: PMC5701989.
Alonso-Coello P, Schünemann HJ, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Rada G, Rosenbaum S, Morelli A, Guyatt GH, Oxman AD; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ. 2016 Jun 28;353:i2016. doi: 10.1136/bmj.i2016. PubMed PMID: 27353417.
Alonso-Coello P, Oxman AD, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Vandvik PO, Meerpohl J, Guyatt GH, Schünemann HJ; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 2: Clinical practice guidelines. BMJ. 2016 Jun 30;353:i2089. doi: 10.1136/bmj.i2089. PubMed PMID: 27365494.
Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, Fervers B, Graham ID, Grimshaw J, Hanna SE, Littlejohns P, Makarski J, Zitzelsberger L; AGREE Next Steps Consortium. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010 Dec 14;182(18):E839-42. doi: 10.1503/cmaj.090449. Epub 2010 Jul 5. Review. PubMed PMID: 20603348; PubMed Central PMCID: PMC3001530.
Hultcrantz M, Rind D, Akl EA, Treweek S, Mustafa RA, Iorio A, Alper BS, Meerpohl JJ, Murad MH, Ansari MT, Katikireddi SV, Östlund P, Tranæus S, Christensen R, Gartlehner G, Brozek J, Izcovich A, Schünemann H, Guyatt G. The GRADE Working Group clarifies the construct of certainty of evidence. J Clin Epidemiol. 2017 Jul;87:4-13. doi: 10.1016/j.jclinepi.2017.05.006. Epub 2017 May 18. PubMed PMID: 28529184; PubMed Central PMCID: PMC6542664.
Medisch Specialistische Richtlijnen 2.0 (2012). Adviescommissie Richtlijnen van de Raad Kwalitieit. http://richtlijnendatabase.nl/over_deze_site/over_richtlijnontwikkeling.html
Neumann I, Santesso N, Akl EA, Rind DM, Vandvik PO, Alonso-Coello P, Agoritsas T, Mustafa RA, Alexander PE, Schünemann H, Guyatt GH. A guide for health professionals to interpret and use recommendations in guidelines developed with the GRADE approach. J Clin Epidemiol. 2016 Apr;72:45-55. doi: 10.1016/j.jclinepi.2015.11.017. Epub 2016 Jan 6. Review. PubMed PMID: 26772609.
Schünemann H, Brożek J, Guyatt G, et al. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group, 2013. Available from http://gdt.guidelinedevelopment.org/central_prod/_design/client/handbook/handbook.html.
Zoekverantwoording
Zoekverantwoording
Algemene informatie
|
Richtlijn: PSA volwassenen |
|
|
Uitgangsvraag: UV14 Wat is het effect van non-farmacologische interventies tijdens/voorafgaande aan PSA in patiënten die PSA (zullen) ondergaan op complicaties, effectiviteit PSA, kwaliteit PSA en patiënttevredenheid ? |
|
|
Database(s): Ovid/Medline, Embase |
Datum: 20-4-2021 |
|
Periode: |
Talen: nvt |
|
Literatuurspecialist: Ingeborg van Dusseldorp |
|
|
BMI zoekblokken: voor verschillende opdrachten wordt (deels) gebruik gemaakt van de zoekblokken van BMI-Online https://blocks.bmi-online.nl/ Bij gebruikmaking van een volledig zoekblok zal naar de betreffende link op de website worden verwezen. |
|
|
Toelichting:
Voor deze vraag is gezocht met de volgende elementen:
Sedatie EN niet famacologische interventies (zie strategie) En volwassenen
Vanwege de vele interventies wordt veel literatuur gevonden. Er wordt voor gekozen om in eerste instantie de SRs en RCTs aan te bieden.
De vier sleutelartikelen worden gevonden. |
|
|
Te gebruiken voor richtlijnen tekst: In de databases Embase en Ovid/Medline is op 2021 met relevante zoektermen gezocht naar systematische reviews en RCTs over niet farmacologische interventies bij PSA. De literatuurzoekactie leverde 817 unieke treffers op. |
|
Zoekopbrengst
|
|
EMBASE |
OVID/MEDLINE |
Ontdubbeld |
|
SRs |
60 |
190 |
155* |
|
RCTs |
235 |
490 |
662* |
|
Observationele studies |
127 |
276 |
300 |
|
Overig |
|
|
|
|
Totaal |
|
|
817* |
*in Rayyan
Zoekstrategie
Embase
|
No. |
Query |
Results |
|
#18 |
#16 NOT #15 NOT #14 |
127 |
|
#17 |
#15 NOT #14 |
325 |
|
#16 |
#3 AND #13 |
367 |
|
#15 |
#2 AND #13 |
358 |
|
#14 |
#1 AND #13 |
60 |
|
#13 |
#12 AND [1-1-2005]/sd NOT (('adolescent'/exp OR 'child'/exp OR adolescent*:ti,ab OR child*:ti,ab OR schoolchild*:ti,ab OR infant*:ti,ab OR girl*:ti,ab OR boy*:ti,ab OR teen:ti,ab OR teens:ti,ab OR teenager*:ti,ab OR youth*:ti,ab OR pediatr*:ti,ab OR paediatr*:ti,ab OR puber*:ti,ab) NOT ('adult'/exp OR 'aged'/exp OR 'middle aged'/exp OR adult*:ti,ab OR man:ti,ab OR men:ti,ab OR woman:ti,ab OR women:ti,ab)) NOT ('conference abstract'/it OR 'editorial'/it OR 'letter'/it OR 'note'/it) NOT (('animal experiment'/exp OR 'animal model'/exp OR 'nonhuman'/exp) NOT 'human'/exp) |
898 |
|
#12 |
#10 AND #11 |
2473 |
|
#11 |
'comfort'/exp OR 'massage'/exp OR 'cooling'/exp OR 'illumination'/exp OR 'music'/exp OR 'music therapy'/exp OR 'neurolinguistic programming'/exp OR 'hypnosis'/exp OR 'virtual reality'/exp OR 'breathing exercise'/exp OR distract*:ti,ab,kw OR nonpharmacolog*:ti,ab,kw OR 'non pharmacolog*':ti,ab,kw OR nlp:ti,ab,kw OR 'neurolinguistic program*':ti,ab,kw OR hypnosis:ti,ab,kw OR 'breathing exercise*':ti,ab,kw OR 'virtual reality':ti,ab,kw OR video:ti,ab,kw OR movie:ti,ab,kw OR 'low stimul*':ti,ab,kw OR lighting:ti,ab,kw OR cooling:ti,ab,kw OR decor:ti,ab,kw OR comfort:ti,ab,kw OR massage:ti,ab,kw OR 'stress reduct*':ti,ab,kw |
453878 |
|
#10 |
'procedural sedation and analgesia'/exp OR 'procedural sedation'/exp OR 'sedation'/exp/mj OR 'sedati*':ti,kw OR 'deep sedation'/de OR 'conscious sedation'/de |
36628 |
|
#9 |
#5 OR #6 OR #7 OR #8 |
4 |
|
#8 |
31541245 AND voss |
1 |
|
#7 |
32092098 AND huang |
1 |
|
#6 |
11756911 AND lee |
1 |
|
#5 |
9710387 AND koch |
1 |
|
#4 |
interventions AND to AND reduce AND anxiety, AND distress AND the AND need AND for AND sedation AND in AND adult AND patients AND undergoing AND magnetic AND resonance AND imaging AND a AND systematic AND review AND munn |
1 |
|
#3 |
'major clinical study'/de OR 'clinical study'/de OR 'case control study'/de OR 'family study'/de OR 'longitudinal study'/de OR 'retrospective study'/de OR 'prospective study'/de OR 'comparative study'/de OR 'cohort analysis'/de OR ((cohort NEAR/1 (study OR studies)):ab,ti) OR (('case control' NEAR/1 (study OR studies)):ab,ti) OR (('follow up' NEAR/1 (study OR studies)):ab,ti) OR (observational NEAR/1 (study OR studies)) OR ((epidemiologic NEAR/1 (study OR studies)):ab,ti) OR (('cross sectional' NEAR/1 (study OR studies)):ab,ti) |
6460706 |
|
#2 |
('clinical trial'/exp OR 'randomization'/exp OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp OR 'placebo'/exp OR 'prospective study'/exp OR rct:ab,ti OR random*:ab,ti OR 'single blind':ab,ti OR 'randomised controlled trial':ab,ti OR 'randomized controlled trial'/exp OR placebo*:ab,ti) NOT 'conference abstract':it |
2500470 |
|
#1 |
('meta analysis'/exp OR 'meta analysis (topic)'/exp OR metaanaly*:ti,ab OR 'meta analy*':ti,ab OR metanaly*:ti,ab OR 'systematic review'/de OR 'cochrane database of systematic reviews'/jt OR prisma:ti,ab OR prospero:ti,ab OR (((systemati* OR scoping OR umbrella OR 'structured literature') NEAR/3 (review* OR overview*)):ti,ab) OR ((systemic* NEAR/1 review*):ti,ab) OR (((systemati* OR literature OR database* OR 'data base*') NEAR/10 search*):ti,ab) OR (((structured OR comprehensive* OR systemic*) NEAR/3 search*):ti,ab) OR (((literature NEAR/3 review*):ti,ab) AND (search*:ti,ab OR database*:ti,ab OR 'data base*':ti,ab)) OR (('data extraction':ti,ab OR 'data source*':ti,ab) AND 'study selection':ti,ab) OR ('search strategy':ti,ab AND 'selection criteria':ti,ab) OR ('data source*':ti,ab AND 'data synthesis':ti,ab) OR medline:ab OR pubmed:ab OR embase:ab OR cochrane:ab OR (((critical OR rapid) NEAR/2 (review* OR overview* OR synthes*)):ti) OR ((((critical* OR rapid*) NEAR/3 (review* OR overview* OR synthes*)):ab) AND (search*:ab OR database*:ab OR 'data base*':ab)) OR metasynthes*:ti,ab OR 'meta synthes*':ti,ab) NOT (('animal'/exp OR 'animal experiment'/exp OR 'animal model'/exp OR 'nonhuman'/exp) NOT 'human'/exp) NOT ('conference abstract'/it OR 'conference review'/it OR 'editorial'/it OR 'letter'/it OR 'note'/it) |
533576 |
Ovid/Medline
|
# |
Searches |
Results |
|
14 |
12 not 11 not 10 |
276 |
|
13 |
11 not 10 |
490 |
|
12 |
6 and 9 |
503 |
|
11 |
6 and 8 |
561 |
|
10 |
6 and 7 |
130 |
|
9 |
Epidemiologic studies/ or case control studies/ or exp cohort studies/ or Controlled Before-After Studies/ or Case control.tw. or cohort.tw. or Cohort analy$.tw. or (Follow up adj (study or studies)).tw. or (observational adj (study or studies)).tw. or Longitudinal.tw. or Retrospective*.tw. or prospective*.tw. or consecutive*.tw. or Cross sectional.tw. or Cross-sectional studies/ or historically controlled study/ or interrupted time series analysis/ [Onder exp cohort studies vallen ook longitudinale, prospectieve en retrospectieve studies] |
3808351 |
|
8 |
(exp clinical trial/ or randomized controlled trial/ or exp clinical trials as topic/ or randomized controlled trials as topic/ or Random Allocation/ or Double-Blind Method/ or Single-Blind Method/ or (clinical trial, phase i or clinical trial, phase ii or clinical trial, phase iii or clinical trial, phase iv or controlled clinical trial or randomized controlled trial or multicenter study or clinical trial).pt. or random*.ti,ab. or (clinic* adj trial*).tw. or ((singl* or doubl* or treb* or tripl*) adj (blind$3 or mask$3)).tw. or Placebos/ or placebo*.tw.) not (animals/ not humans/) |
2107174 |
|
7 |
(meta-analysis/ or meta-analysis as topic/ or (metaanaly* or meta-analy* or metanaly*).ti,ab,kf. or systematic review/ or cochrane.jw. or (prisma or prospero).ti,ab,kf. or ((systemati* or scoping or umbrella or "structured literature") adj3 (review* or overview*)).ti,ab,kf. or (systemic* adj1 review*).ti,ab,kf. or ((systemati* or literature or database* or data-base*) adj10 search*).ti,ab,kf. or ((structured or comprehensive* or systemic*) adj3 search*).ti,ab,kf. or ((literature adj3 review*) and (search* or database* or data-base*)).ti,ab,kf. or (("data extraction" or "data source*") and "study selection").ti,ab,kf. or ("search strategy" and "selection criteria").ti,ab,kf. or ("data source*" and "data synthesis").ti,ab,kf. or (medline or pubmed or embase or cochrane).ab. or ((critical or rapid) adj2 (review* or overview* or synthes*)).ti. or (((critical* or rapid*) adj3 (review* or overview* or synthes*)) and (search* or database* or data-base*)).ab. or (metasynthes* or meta-synthes*).ti,ab,kf.) not (comment/ or editorial/ or letter/ or ((exp animals/ or exp models, animal/) not humans/)) |
490888 |
|
6 |
5 not ((Adolescent/ or Child/ or Infant/ or adolescen*.ti,ab,kf. or child*.ti,ab,kf. or schoolchild*.ti,ab,kf. or infant*.ti,ab,kf. or girl*.ti,ab,kf. or boy*.ti,ab,kf. or teen.ti,ab,kf. or teens.ti,ab,kf. or teenager*.ti,ab,kf. or youth*.ti,ab,kf. or pediatr*.ti,ab,kf. or paediatr*.ti,ab,kf. or puber*.ti,ab,kf.) not (Adult/ or adult*.ti,ab,kf. or man.ti,ab,kf. or men.ti,ab,kf. or woman.ti,ab,kf. or women.ti,ab,kf.)) |
1645 |
|
5 |
limit 4 to yr="2005 -Current" |
2076 |
|
4 |
3 not ((exp animals/ or exp models, animal/) not humans/) not (letter/ or comment/ or editorial/) |
2792 |
|
3 |
1 and 2 |
3075 |
|
2 |
Patient Comfort/ or massage/ or music/ or music therapy/ or exp Neurolinguistic Programming/ or exp Hypnosis/ or exp Hypnosis, Anesthetic/ or exp virtual reality/ or exp Breathing Exercises/ or distraction*.ti,ab,kf. or nonpharmacolog*.ti,ab,kf. or "non pharmacolog*".ti,ab,kf. or nlp.ti,ab,kf. or "neurolinguistic program*".ti,ab,kf. or hypnosis.ti,ab,kf. or "breathing exercise*".ti,ab,kf. or "virtual reality".ti,ab,kf. or video.ti,ab,kf. or movie.ti,ab,kf. or lighting.ti,ab,kf. or cooling.ti,ab,kf. or decor.ti,ab,kf. or comfort.ti,ab,kf. or massage.ti,ab,kf. or (stress adj2 (reduct* or alleviat*)).ti,ab,kf. |
293320 |
|
1 |
exp Deep Sedation/ or exp Conscious Sedation/ or sedati*.ti,ab,kf. |
61529 |