Subacromiale decompressie chirurgie bij SAPS
Uitgangsvraag
Do adults with atraumatic shoulder pain for more than 3 months diagnosed as subacromial pain syndrome (SAPS), also labelled as rotator cuff disease, benefit from subacromial decompression surgery?
Uitgangsvraag (NL)
Hebben volwassenen gediagnosticeerd met SAPS of Rotator Cuff disease (RCD) voordeel van subacromiale decompressie chirurgie in vergelijking met een niet-operatieve behandeling?
Aanbeveling
We issue a strong recommendation against subacromial decompression surgery for patients with SAPS because we believe that the undesirable consequences (i.e. serious adverse events and practical issues) clearly outweigh the desirable consequences. Further, the quality of the evidence is high for key outcomes - pain, function, and quality of life. Results - excluding important benefits - are consistent in the two low risk of trials of placebo- surgery. Even the high risk of bias trials did not suggest important benefits to patients. We expect very little variability in patient values and preferences.
The panel concluded that almost all informed patients would choose to avoid surgery because there is no benefit but there are harms and it is burdensome. Subacromial decompression surgery should not be offered to patients with SAPS. However, there is substantial uncertainty in what alternative treatment is best.
Aanbeveling (NL)
We adviseren tegen het uitvoeren van subacromiale decompressie chirurgie
Overwegingen
Understanding the recommendation
The panel concluded that almost all well informed patients would decline surgery and therefore made a strong recommendation against subacromial decompression surgery. The panel was confident that surgery provides no important benefit on pain, function, quality of life, and global perceived effect informed by moderate to high certainty evidence in a one year timeframe. Surgery also comes with burdens and the risk of harm (see main infographic). Clinicians should not offer patients subacromial decompression surgery unprompted, and clinicians, public healthcare providers, and others should make efforts to educate the public regarding the ineffectiveness of surgery. Although we did not take costs and resources into account beyond direct costs to patients (such as out-of pocket costs), surgery cannot be cost effective given the lack of important benefit, potential for harm, and associated costs.
The subacromial pain syndrome (SAPS) is an umbrella term for syndromes that are caused by multiple disorders in and around the subacromial space. The following conditions can be distinguished: partial or complete cuff ruptures, tendinitis calcarea, acromioclavicular arthrosis, biceps tenosynovitis, biceps tendinitis and a-traumatic instability. At this point the umbrella term SAPS does not provide enough information to tell anything about which syndrome is actually described or which treatment should be used.
To prevent inclusion bias in future research there should be differentiated in the earlier described diagnoses/conditions.
Uncertainty
Clinicians and patients might question what other therapies could be offered to patients diagnosed with SAPS or rotator cuff disease and whether any therapy is effective. Here we recognise the limitation of the BMJ Rapid Recommendations, made to provide guidance on new evidence that might change practice. For guidance on treatment alternatives beyond surgery, we point readers to a clinically focused overview article (Whittle, 2015) and to guidelines with a broader scope. The whole area of best management of SAPS is uncertain, as reflected in the following brief summary on available treatment options:
- Glucocorticoid injections and NSAIDs may provide moderate to small short term benefits on shoulder pain compared with placebo (Whittle, 2015; Arroll, 2005)
- Exercise, manual therapy, and electrotherapies are of uncertain benefit to patients compared with watchful waiting, and guidelines vary in their recommendations.25 26
- A holistic approach to care, with appropriate communication including reassurance and
- education, is likely to benefit patients but is poorly studied.27
- Key research questions to inform decision makers and future guidelines include:
- What are the best strategies to de-implement inefficient and potentially harmful subacromial decompression surgery for SAPS?
- How can we educate patients and clinicians to understand and adopt evidence, particularly when it goes against accepted beliefs?
We have high certainty in the estimates of effect for pain, function and quality of life, based on two low risk of bias trials with surgery. We have moderate certainty for the estimate of global perceived effect and risk for serious adverse events (due to indirectness of population in observational studies) and low certainty for the outcome return to work and frozen shoulder (imprecise estimate from trial data). The evidence is based on two linked systematic reviews; one on minimally important differences in pain, shoulder and quality of life in adults with shoulder pain and another review of 7 trials on benefits and harms of subacromial decompression surgery in adults with SAPS, which also included 54 observational studies investigating harms after such surgery.
Preference and values
The panel believes that all or almost all patients would place a high value on avoiding even minimal risk of complications and burden from surgery, if it is not helpful.
Resources and other considerations
With our international focus our recommendations take a patient-centred perspective rather than a health care systems perspective where cost-effectiveness becomes more important. That said, without any demonstrated benefit on patient-important outcomes surgery is unlikely to be cost-effective, neither from a healthcare payer nor from a societal perspective.
Management options
Clinicians and patients might question what other therapies could be offered to patients diagnosed with SAPS or RCD, and whether any therapy is effective. Here we recognise the limitations of our BMJ Rapid Recommendations, made to provide guidance on new evidence that might change practice. For guidance on treatment alternatives beyond surgery we point readers to a clinically focused overview article and to guidelines with a broader scope (Table 1 in the BMJ publication). The whole area of best management of SAPS is uncertain, as reflected in the following brief summary on available treatment options:
- Glucocorticoid injections and NSAIDs may provide moderate to small short-term benefits on shoulder pain when compared to placebo
- Exercise, manual therapy and electrotherapies are also of uncertain benefit to patients compared to watchful waiting, and guidelines vary in their recommendations
- A holistic approach to care, appropriate communication, including reassurance and education is likely to benefit patients, but is poorly studied.
Performance measurement
As per GRADE guidance, our strong recommendation against subacromial decompression surgery can be used as a performance or quality of care measure and it is reasonable to tie the use of surgery to funding decisions or penalties. The non-use of surgery in patients with SAPS as a performance measure may be especially relevant given that such surgery continues to be performed frequently, despite accumulating evidence of no net benefit.
Implementation
The previous guideline SAPS was published in 2012. Since then a remarkable decline in subacromial decompression surgery (Veen, 2019) was seen in the Netherlands, up to 60% in 2018. These figures show that de previous guideline was implemented well in the Netherlands and that orthopaedic surgeons adhered to the recommendations.
Onderbouwing
Achtergrond
Up to a quarter of adults have experienced shoulder pain over the past year, and it represents the third most common musculoskeletal problem (Bot, 2005; Luime, 2004). About half of those affected will recover completely within six months (Bruls, 2015). Pain beyond three months is associated with poorer recovery, disability, and reduced ability to work. Subacromial pain is the most common form (up to 70%) of shoulder pain, and it can impair the ability to work or do household tasks (Mitchell, 2005; Harkness, 2003; van der Windt, 2000). Most patients presenting with subacromial pain, without a history of trauma, receive a diagnosis of subacromial pain syndrome (SAPS), shoulder impingement, or rotator cuff disease. Each of these labels describe similar clinical presentations, but there is inconsistency about how they are defined and overlap between these diagnoses. Here, we use the term SAPS (see box 1 for details of its presentation). This recommendation addresses the role of surgery for adults with symptoms lasting more than three months, who approach health professionals for treatment.
|
Common symptoms—Pain at the upper outer arm when lifting the arm (classically a painful arc through shoulder abduction), difficulty moving the arm (especially with forward flexion, external rotation, and abduction), reduced strength in the arm, and sleep problems due to Pain (Hermans, 2013; Whittle, 2015) Key differential diagnoses—Adhesive capsulitis (“frozen shoulder”) and glenohumeral osteoarthritis (Whittle, 2015; Brox, 2003) Imaging—Patients with SAPS can have degeneration and partial thickness rotator cuff tears or abnormalities in the subacromial bursa on imaging. These imaging findings are also common in people without symptoms (Gill, 2014) Pathophysiology—Remains poorly understood. Cadaver studies suggested that pain might occur from rotator cuff tendons being caught (“impinging”) between the acromion or coracoacromial ligament and the humerus (Neer, 1972). These studies provided the initial rationale for subacromial decompression surgery Abnormal MRI findings—Were highly prevalent in both symptomatic as well as asymptomatic shoulders. Only the frequencies of full-thickness tears in the supraspinatus tendon and glenohumeral osteoarthritis were higher (approximately 10%) in the symptomatic shoulder according to the investigators findings (Barreto, 2019). |
|
Box 1: Details of subacromial pain syndrome (SAPS) |
Current Practice
First line treatment options for SAPS include simple analgesia such as paracetamol, non-steroidal anti-inflammatory drugs (NSAIDs), glucocorticoid injections, and exercise therapy (Whittle, 2015). Subacromial acromial decompression surgery is a second line treatment option for patients with more longstanding symptoms. Current guidelines provide inconsistent recommendations. Such surgery includes removal of the subacromial bursa (bursectomy and removal of bone from the under surface of the acromion (acromioplasty). Surgeons initially performed subacromial decompression surgery as an open procedure. It evolved to less invasive keyhole surgery: arthroscopy. Despite trials dating back to 1993 (Brox, 1993) and systematic reviews failing to demonstrate benefit from surgery, (Coghlan, 2008) the number of arthroscopies performed has risen dramatically, although there is substantial geographical variation (Vitale, 2010; Judge, 2014). There were 21 000 procedures performed in NHS hospitals in 2010, which cost approximately £50 million (Judge, 2014).
Disclaimer
The information in this guideline module is adapted from Rapid Recommendation published in the BMJ (Vandvik, 2019). For more information see:
Vandvik PO, Lähdeoja T, Ardern C, et al. Subacromial decompression surgery for adults with shoulder pain: a clinical practice guideline. BMJ 2019;364:l294
Or visit the website of MAGICapp: https://app.magicapp.org/public/guideline/nBMa0L
Conclusies
PICO 1
PICO 2
Samenvatting literatuur
Description of studies and results
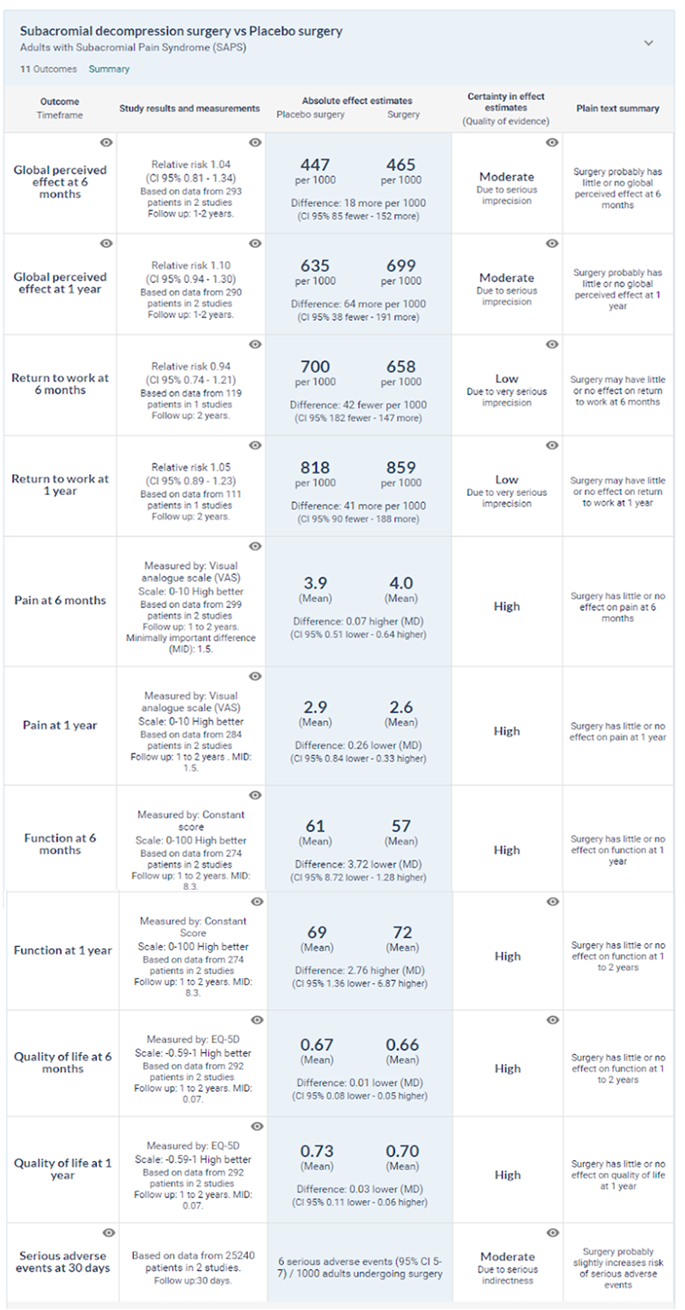
What are the benefits and harms of subacromial decompression surgery? The linked systematic review and meta-analysis pooled data from seven randomised controlled trials with 1014 participants diagnosed with SAPS (Lahdoaja, 2019). In general, the patients included in the trials are representative of patients with SAPS presenting to primary care centres and outpatient clinics. Participants were around 49 years (median) and had had symptoms for around two years (median). Planned evaluation of trials at lower risk of bias The panel planned to focus on evidence at lower risk of bias. Two trials included placebo surgery and were at low risk of bias (Paavola, 2018; Beard, 2018). At one year after treatment, they showed that surgery did not have meaningful benefit over placebo surgery:
- High certainty evidence for little or no effect on
- Pain (mean difference −0.26 (95% confidence interval −0.84 to 0.33), MID 1.5)
- Function (mean difference 2.8 (−1.4 to 6.9), MID 8.3)
- Health related quality of life (mean difference −0.03 points (−0.11 to 0.06), MID 0.07)
- Moderate certainty evidence for little or no global perceived effect (risk ratio 1.10 (0.94 to 1.30))
- Low certainty evidence for little or no effect on return to work (risk ratio 1.05 (0.89 to 1.23)).
Similar results were seen at six months, two years, and at five year follow-up, with the latter supported by low certainty evidence due to imprecise estimates from unblinded trials (Lahdoaja, 2019)
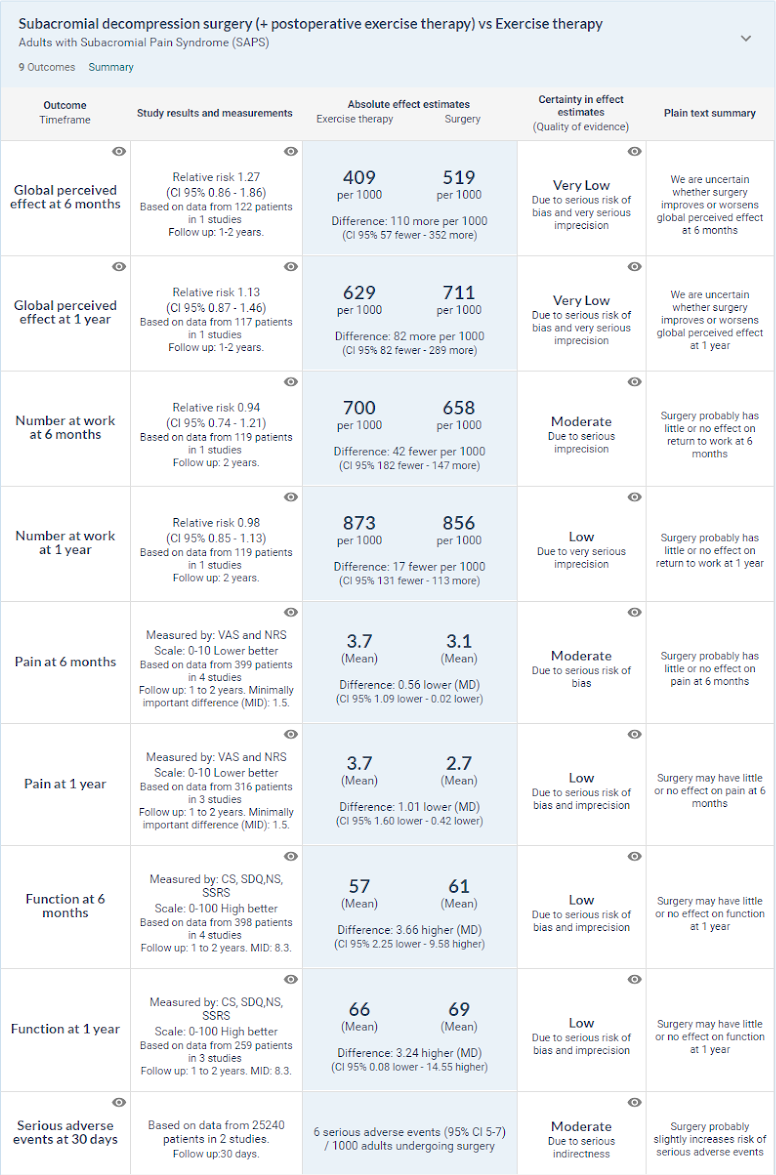
Planned evaluation of surgery compared with exercise therapy
This analysis compared subacromial decompression surgery (including postoperative exercise therapy) with exercise therapy alone. Six trials reported such comparisons, and all were at high risk of bias due to lack of blinding. Some had imprecise estimates of effect. Compared with exercise therapy, there was no important benefit of surgery on pain, function, quality of life, global perceived effect, and return to work (Lahdoaja, 2019). About a third (32%) of all participants included in the trials continued to have more than minor symptoms (such as mild to moderate pain) at one year, irrespective of treatment. The average pain scores in the trials at two years were 1.6 to 3.0 units (0-10 scale), reflecting mild to moderate pain.
Harms
Potential harms from surgery were incompletely reported in the trials. The trials were also underpowered to detect rare events. There were around 12 more frozen shoulders per 1000 patients undergoing subacromial decompression surgery, based on the two placebo controlled trials (low certainty evidence). Because harms data from randomised trials were anticipated to be so limited, the guideline panel requested the systematic review to include observational studies designed to evaluate harms after subacromial decompression surgery.(Lahdoaja, 2019). The systematic review assessed 140 publications in full text, of which four reported results from a large prospective cohort study from the United States considered to represent best current evidence on serious harms (Gill, 2014; Neer, 1972; Paavola, 2018; Beard, 2018; Hao, 2019; Lahdeoja, 2019; Karjalainen, 2019; Diercks, 2014; Brox, 1993; Coghlan, 2008; Vitale, 2010; Judge, 2014; Shields, 2015). This registry study investigated 30-day complications resulting in readmission to hospitals after mixed arthroscopic procedures including subacromial decompression surgery from 2006 to 2013 (Brox, 2003).
The risk of serious harms after mixed shoulder arthroscopic procedures was 0.5% (95% confidence interval 0.4% to 0.7%) during years 2006-11 and 0.6% (0.5% to 0.7%) during 2011-13. Reported harms included events such as major bleeding, deep infections, serious anaesthetic complications, venous thromboembolism, and peripheral nerve injury. The indirectness caused by inclusion of mixed arthroscopic shoulder procedures in the registry study results in moderate certainty evidence for estimated harms.
Level of evidence of the literature
Use this link to see all conclusions and GRADE levels: https://app.magicapp.org/goto/guideline/nBMa0L/rec/42100
Zoeken en selecteren
The article of Vandvik (2019) published in the BMJ is used to answer this clinical questions. is published in the BMJ (Vandvik, 2019).
Two PICOs were investigated:
PICO 1:
P: Adults with Subacromial Pain Syndrome (SAPS)
I: Subacromial decompression surgery
C: Placebo surgery
PICO 2:
P: Adults with Subacromial Pain Syndrome (SAPS)
I: Subacromial decompression surgery (+postoperatieve exercise therapy)
C: Exercise therapy
Outcome measures and minimally important differences
The international panel included patients with lived experience of shoulder pain and surgery, orthopaedic surgeons, physiotherapists, rheumatologists, general internists, general practitioners, epidemiologists, and methodologists. The panel initially decided on the scope of the recommendation and the outcomes that are most important to patients. The panel identified the following important outcomes: pain, patient global perceived effect, physical function, participation in work and recreation activities, health related quality of life, development of full-thickness rotator cuff tears, and potential harms from surgery (such as adhesive capsulitis [iatrogenic frozen shoulder], death, infection, venous thromboembolism, and anaesthesia related events). This selection was also informed by the Outcome Measures in Rheumatology (OMERACT) preliminary shoulder trial core domain outcome set (Buchbinder, 2017). To inform the recommendation the panel members requested two systematic reviews addressing the following questions:
- What is the smallest change in pain, function and quality of life that patients with shoulder conditions such as SAPS consider important—the minimally important difference—to make surgery worthwhile? Such patient-reported outcomes measures (PROMs) were measured with a variety of instruments in the trials and are challenging to interpret.
- What are the benefits and harms of subacromial decompression surgery in patients with SAPS, as compared to placebo and nonoperative management strategies?
Parallel teams conducted these systematic reviews (Hao, 2019; Lahdeoja, 2019) Another team updated a Cochrane systematic review synchronised with this BMJ Rapid Recommendation (Karjalainen, 2019). The panel asked the review team to explore potential subgroup effects for risk of bias in trials and different types of comparisons to surgery, such as exercise therapy. The panel used this evidence and followed BMJ Rapid Recommendations procedures for creating a trustworthy recommendation. This includes the GRADE approach.
What is the minimum difference in symptoms and function important to patients? The systematic review of minimally important differences (MIDs) identified 22 original studies of 5562 patients. They reported results for 74 MID estimates judged to be of variable and mostly low credibility (Hao, 2019). The most credible MID estimates were used to help interpret the results of the systematic review, as shown in the infographic. The panel were, due to credible estimates, confident that patients valued
- A difference in pain of at least 1.5 units as important (visual analogue scale 0-10)
- A difference in function of at least 8.3 units as important (constant score 0-100)
The panel were less confident in the difference in health related quality of life reported by patients to be important (EQ 5-D, MID 0.07 units, low credibility median estimate). The panel met by videoconference to discuss the evidence and formulate a recommendation. The panel considered the balance of benefits, harms, and burdens of surgery versus placebo surgery and nonoperative treatments, the certainty of the evidence for each outcome, typical and expected variations in patient values and preferences, as well as feasibility and acceptability (practical issues) (Alonso-Coello, 2016). Recommendations using GRADE can be strong or weak, for or against a course of action (Guyatt, 2008). The panel made the recommendation from an individual patient’s perspective assuming that all options were available and affordable to the patient. It does not take a public health, societal, or health payer perspective. Healthcare systems can adapt these recommendations by including costs and other key issues of relevance, contextualised to national and local circumstances.
Search and select (Methods)
The evidence is based on two linked systematic reviews; one on minimally important differences in pain, shoulder and quality of life in adults with shoulder pain (Hao, 2019) and another review of 7 trials on benefits and harms of subacromial decompression surgery in adults with SAPS (Lahdeoja, 2019), which also included 54 observational studies investigating harms after such surgery
Results
Two systematic reviews were included in the analysis of the literature. Important study characteristics and results are summarized in the evidence tables.
Referenties
- Alonso-Coello P, Oxman AD, Moberg J, et al. GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 2: Clinical practice guidelines. BMJ 2016;353:i2089. 10.1136/bmj. i2089 pmid:27365494.
- Arroll B, Goodyear-Smith F. Corticosteroid injections for painful shoulder: a meta-analysis. Br J Gen Pract 2005;55:224-8.pmid:15808040.
- Barreto RPG, Braman JP, Ludewig PM, Ribeiro LP, Camargo PR. Bilateral magnetic resonance imaging findings in individuals with unilateral shoulder pain. J Shoulder Elbow Surg. 2019 Sep;28(9):1699-1706. doi: 10.1016/j.jse.2019.04.001. Epub 2019 Jul 3. PubMed PMID: 31279721.
- Beard DJ, Rees JL, Cook JA, et al. CSAW Study Group. Arthroscopic subacromial decompression for subacromial shoulder pain (CSAW): a multicentre, pragmatic, parallel group, placebo-controlled, three-group, randomised surgical trial. Lancet 2018;391:329-38. 10.1016/S0140- 6736(17)32457-1 pmid:29169668.
- Bot SD, van der Waal JM, Terwee CB, et al. Incidence and prevalence of complaints of the neck and upper extremity in general practice. Ann Rheum Dis 2005;64:118-23. 10.1136/ard.2003.01934910.1136/ ard.2003.019349 pmid:15608309.
- Brox JI, Staff PH, Ljunggren AE, Brevik JI. Arthroscopic surgery compared with supervised exercises in patients with rotator cuff disease (stage II impingement syndrome). BMJ 1993;307:899-903. 10.1136/ bmj.307.6909.899 pmid:8241852.
- Brox JI. Regional musculoskeletal conditions: shoulder pain. Best Pract Res Clin Rheumatol 2003;17:33-56. 10.1016/S1521-6942(02)00101- 8 pmid:12659820.
- Bruls VE, Bastiaenen CH, de Bie RA. Prognostic factors of complaints of arm, neck, and/or shoulder: a systematic review of prospective cohort studies. Pain 2015;156:765-88. 10.1097/j. pain.0000000000000117 pmid:25659066.
- Buchbinder R, Page MJ, Huang H, et al. Shoulder Core Outcome Set Special Interest Group. A preliminary core domain set for clinical trials of shoulder disorders: a report from the OMERACT 2016 Shoulder Core Outcome Set Special Interest Group. J Rheumatol 2017;44:1880-3. 10.3899/jrheum.161123 pmid:28089972.
- Coghlan JA, Buchbinder R, Green S, Johnston RV, Bell SN. Surgery for rotator cuff disease. Cochrane Database Syst Rev 2008;(1):CD005619. pmid:18254085.
- Diercks R, Bron C, Dorrestijn O, et al. Dutch Orthopaedic Association. Guideline for diagnosis and treatment of subacromial pain syndrome: a multidisciplinary review by the Dutch Orthopaedic Association. Acta Orthop 2014;85:314-22. 10.3109/17453674.2014.920991 pmid:24847788.
- Gill TK, Shanahan EM, Allison D, Alcorn D, Hill CL. Prevalence of abnormalities on shoulder MRI in symptomatic and asymptomatic older adults. Int J Rheum Dis 2014;17:863-71. 10.1111/1756-185X.12476 pmid:25294682.
- Guyatt GH, Oxman AD, Vist GE, et al. GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924-6. 10.1136/ bmj.39489.470347.AD pmid:18436948.
- Hao Q, Devji T, Zeraatkar D, et al. Minimal important differences for improvement in shoulder condition patient-reported outcomes: a systematic review to inform a BMJ Rapid Recommendation. BMJ Open 2019; doi:10.1136/bmjopen-2018-028777.
- Harkness EF, Macfarlane GJ, Nahit ES, Silman AJ, McBeth J. Mechanical and psychosocial factors predict new onset shoulder pain: a prospective cohort study of newly employed workers. Occup Environ Med 2003;60:850-7. 10.1136/oem.60.11.850 pmid:14573715.
- Hermans J, Luime JJ, Meuffels DE, Reijman M, Simel DL, Bierma- Zeinstra SM. Does this patient with shoulder pain have rotator cuff disease?: The Rational Clinical Examination systematic review. JAMA 2013;310:837-47. 10.1001/jama.2013.276187 pmid:23982370.
- Judge A, Murphy RJ, Maxwell R, Arden NK, Carr AJ. Temporal trends and geographical variation in the use of subacromial decompression and rotator cuff repair of the shoulder in England. Bone Joint J 2014;96-B:70- 4. 10.1302/0301-620X.96B1.32556 pmid:24395314
- Karjalainen TV, Jain NB, Page CM, et alSubacromial decompression surgery for rotator cuff disease. Cochrane Database Syst Rev 2019;(1):CD005619. 10.1002/14651858.CD005619.pub3.
- Lähdeoja T, Karjalainen T, Jokihaara J, et alSubacromial decompression surgery versus conservative management in patients with shoulder pain: a systematic review with meta-analysis. Br J Sports Med 2019; 10.1136/ bjsports-2018-100486 .
- Luime JJ, Koes BW, Hendriksen IJ, et al. Prevalence and incidence of shoulder pain in the general population; a systematic review. Scand J Rheumatol 2004;33:73-81.
- Mitchell C, Adebajo A, Hay E, Carr A. Shoulder pain: diagnosis and management in primary care. BMJ 2005;331:1124-8. 10.1136/ bmj.331.7525.1124 pmid:16282408.
- Neer CS 2nd. Anterior acromioplasty for the chronic impingement syndrome in the shoulder: a preliminary report. J Bone Joint Surg Am 1972;54:41-50. 10.2106/00004623-197254010- 00003 pmid:5054450
- Paavola M, Malmivaara A, Taimela S, et al. Finnish Subacromial Impingement Arthroscopy Controlled Trial (FIMPACT) Investigators. Subacromial decompression versus diagnostic arthroscopy for shoulder impingement: randomised, placebo surgery controlled clinical trial. BMJ 2018;362:k2860. 10.1136/bmj.k2860 pmid:30026230.
- Veen EJD, Stevens M, Koorevaar CT, Diercks RL. Appropriate care for orthopedic patients: effect of implementation of the Clinical Practice Guideline for Diagnosis and Treatment of Subacromial Pain Syndrome in the Netherlands. Acta Orthop. 2019 Jun;90(3):191-195. doi: 10.1080/17453674.2019.1593641. Epub 2019 Apr 1. PubMed PMID: 30931669; PubMed Central PMCID: PMC6534224.
- Vitale MA, Arons RR, Hurwitz S, Ahmad CS, Levine WN. The rising incidence of acromioplasty. J Bone Joint Surg Am 2010;92:1842-50. 10.2106/ JBJS.I.01003 pmid:20686058.
- Whittle S, Buchbinder R. In the clinic. Rotator cuff disease. Ann Intern Med 2015;162:ITC1-15. 10.7326/AITC201501060 pmid:25560729.
- van der Windt DA, Thomas E, Pope DP, et al. Occupational risk factors for shoulder pain: a systematic review. Occup Environ Med 2000;57:433-42. 10.1136/oem.57.7.433 pmid:10854494.
Evidence tabellen
Verantwoording
Autorisatiedatum en geldigheid
Laatst beoordeeld : 05-03-2021
Laatst geautoriseerd : 05-03-2021
Geplande herbeoordeling : 01-01-2018
Uiterlijk in 2017 bepaalt het bestuur van de Nederlandse Orthopaedische Vereniging of deze richtlijn nog actueel is. Zo nodig wordt een nieuwe werkgroep geïnstalleerd om de richtlijn te herzien. De geldigheid van de richtlijn komt eerder te vervallen indien nieuwe ontwikkelingen aanleiding zijn een herzieningstraject te starten.
De Nederlandse Orthopaedische Vereniging is als houder van deze richtlijn de eerstverantwoordelijke voor de actualiteit van deze richtlijn. De andere aan deze richtlijn deelnemende wetenschappelijk verenigingen of gebruikers van de richtlijn delen de verantwoordelijkheid en informeren de eerstverantwoordelijke over relevante ontwikkelingen binnen hun vakgebied.
|
Geldigheid en Onderhoud
Module[1] |
Regiehouder(s)[2] |
Jaar van autorisatie |
Eerstvolgende beoordeling actualiteit richtlijn[3] |
Frequentie van beoordeling op actualiteit[4] |
Wie houdt er toezicht op actualiteit[5] |
Relevante factoren voor wijzigingen in aanbeveling[6] |
|
Subacromial decompression surgery |
NOV |
2021 |
2026 |
1x per 5 jaar |
NOV |
|
[1] Naam van de module
[2] Regiehouder van de module (deze kan verschillen per module en kan ook verdeeld zijn over meerdere regiehouders)
[3] Maximaal na vijf jaar
[4] (half)Jaarlijks, eens in twee jaar, eens in vijf jaar
[5] regievoerende vereniging, gedeelde regievoerende verenigingen, of (multidisciplinaire) werkgroep die in stand blijft
[6] Lopend onderzoek, wijzigingen in vergoeding/organisatie, beschikbaarheid nieuwe middelen
Algemene gegevens
Met ondersteuning van de Orde van Medisch Specialisten. De richtlijnontwikkeling werd gefinancierd uit de Kwaliteitsgelden Medisch Specialisten (SKMS)
Doel en doelgroep
De breed samengestelde werkgroep heeft zich ten doel gesteld om de werkvelden van de eerstelijns, tweedelijns en sociaal geneeskundige sector een onderbouwd overzicht te geven van diagnostiek, interventies en meetinstrumenten, bruikbaar voor patiënten met schouderklachten die gekenmerkt worden door pijnklachten in het abductietraject.
Deze richtlijn is geschreven voor alle leden van de beroepsgroepen die betrokken zijn bij de zorg voor patiënten met SAPS.
Samenstelling werkgroep
- Dr. C. Bron, fysiotherapeut, Koninklijk Nederlands Genootschap voor Fysiotherapie
- Prof. dr. R.L. Diercks, orthopedisch chirurg, Nederlandse Orthopaedische Vereniging, voorzitter
- Dr. O. Dorrestijn, orthopedisch chirurg, Nederlandse Orthopaedische Vereniging
- Dr. C.G.M. Meskers, revalidatiearts, Nederlandse Vereniging van Revalidatieartsen
- Drs. R.J. Naber, bedrijfsarts, Nederlandse Vereniging voor Arbeids- en Bedrijfsgeneeskunde
- Drs. T.J.W. de Ruiter, revalidatiearts, Nederlandse Vereniging van Revalidatieartsen
- Dr. W.J. Willems, orthopedisch chirurg, Nederlandse Orthopaedische Vereniging
- Dr. J.C. Winters, huisarts, Nederlands Huisartsen Genootschap
- Dr. H.J. van der Woude, radioloog, Nederlandse Vereniging voor Radiologie
Met ondersteuning van:
- Mw. drs. S.B. Muller-Ploeger, junior adviseur afdeling Ondersteuning Professionele Kwaliteit, Orde van Medisch Specialisten
- Mw. dr. N.H.J. van Veen, adviseur afdeling Ondersteuning Professionele Kwaliteit, Orde van Medisch Specialisten
- Mw. Ir. I.W. Loman, junior adviseur afdeling Ondersteuning Professionele Kwaliteit, Orde van Medisch Specialisten (indicatoren)
Voor het ontwikkelen van de richtlijn is in 2010 een multidisciplinaire werkgroep ingesteld, bestaande uit vertegenwoordigers van alle relevante specialismen die betrokken zijn bij de zorg voor patiënten met SAPS (zie hiervoor de samenstelling van de werkgroep).
De werkgroepleden zijn door hun beroepsverenigingen gemandateerd voor deelname. De werkgroep werkte gedurende twee jaar aan de totstandkoming van de richtlijn.
Belangenverklaringen
De werkgroepleden hebben schriftelijk verklaard of ze in de laatste vijf jaar een (financieel ondersteunde) betrekking onderhielden met commerciële bedrijven, organisaties of instellingen die in verband staan met het onderwerp van de richtlijn.
Geen van de werkgroepleden heeft aangegeven belangen te hebben die mogelijk kunnen interfereren met de besluitvorming in de werkgroep ten aanzien van de interpretatie van het wetenschappelijk bewijs en het opstellen van aanbevelingen.
Inbreng patiëntenperspectief
Het patiëntenperspectief wordt gezien als een waardevolle aanvulling bij het tot stand komen van deze richtlijn. Er is literatuuronderzoek gedaan naar de patiënttevredenheid na behandeling van SAPS. Ook is er een onderzoek onder patiënten met SAPS verricht waarbij onder meer werd gevraagd wat de relatie was tussen hun werkzaamheden, hun sportieve activiteiten en de schouderklachten en of ze na afsluiting van de specialistische behandeling aanvullende behandeling hadden gezocht. De resultaten staan beschreven in Hoofdstuk 10.
Methode ontwikkeling
Evidence based
Implementatie
In de verschillende fasen van de richtlijnontwikkeling is rekening gehouden met de implementatie van de richtlijn en de praktische uitvoerbaarheid van de aanbevelingen. Daarbij is uitdrukkelijk gelet op factoren die de invoering van de richtlijn in de praktijk kunnen bevorderen of belemmeren.
Na autorisatie zal de richtlijn digitaal worden verspreid onder alle relevante beroepsgroepen. Ook zal de richtlijn te downloaden zijn vanaf de website van de Kwaliteitskoepel: www.kwaliteitskoepel.nl.
Uit de richtlijn zullen toetsitems ten behoeve van een Richtlijntoets ontwikkeld worden. De Richtlijntoets bestaat uit web-based meerkeuze vragen die direct gekoppeld zijn aan de conclusies en aanbevelingen van een richtlijn en wordt geaccrediteerd.
Werkwijze
AGREE
Deze richtlijn is opgesteld conform de eisen in het rapport ‘Richtlijnen 2.0’ van de adviescommissie Richtlijnen van de Orde van Medisch Specialisten. Dit rapport is gebaseerd op het AGREE II instrument (Appraisal of Guidelines for Research & Evaluation II) (http://www.agreetrust.org). Dit is een internationaal breed geaccepteerd instrument voor de beoordeling van de kwaliteit van richtlijnen.
Knelpuntenanalyse, uitgangsvragen en uitkomstmaten
Op basis van de uitkomsten van de knelpuntenanalyse zijn door de voorzitter en de adviseur concept uitgangsvragen opgesteld. Deze zijn met de werkgroep besproken, waarna de werkgroep de definitieve uitgangsvragen heeft vastgesteld. Vervolgens inventariseerde de werkgroep welke uitkomstmaten voor de patiënt relevant zijn, namelijk vermindering van pijn en verbetering van schouderfunctie.
Strategie voor zoeken en selecteren van literatuur
De databases waarin is gezocht, de zoekactie of gebruikte trefwoorden van de zoekactie en de gehanteerde selectiecriteria zijn te vinden onder 'zoekverantwoording'.
Beoordelen en samenvatten van de literatuur
Individuele studies werden systematisch beoordeeld, op basis van op voorhand opgestelde methodologische kwaliteitscriteria (zie Tabel 1.1 en 1.2). De belangrijkste bevindingen uit de literatuur zijn beschreven in de samenvatting van de literatuur en in evidence-tabellen (zie Bijlage 3). Het wetenschappelijke bewijs werd samengevat in één of meerdere conclusies, waarbij het niveau van het meest relevante bewijs is weergegeven (zie Tabel 1.3).
Tabel 1.1 GRADE bewijsniveaus van interventiestudies
|
Bewijsniveau |
Interventie onderzoek (voorbeelden) |
|
Hoog |
RCTs zonder ernstige beperkingen Observationele studies met zeer grote effecten en zonder ernstige beperkingen |
|
Matig |
RCTs met ernstige beperkingen Observationele studies met grote effecten en zonder ernstige beperkingen |
|
Laag |
RCTs met zeer ernstige beperkingen Observationele studies zonder ernstige beperkingen |
|
Zeer laag |
RCTs met zeer ernstige beperkingen en inconsistente resultaten Observationele studies met ernstige beperkingen Niet-systematische klinische observaties (vb. case series of case reports) |
Tabel 1.2 EBRO bewijsniveaus van diagnostisch accuratesse onderzoek of onderzoek naar etiologie en prognose
|
Bewijs niveau |
Diagnostisch accuratesse onderzoek |
Etiologie, prognose |
|
A1 |
Meta-analyse van min. 2 onafhankelijk van elkaar uitgevoerde onderzoeken van A2-niveau |
|
|
A2 |
Onderzoek t.o.v. een referentietest (‘gouden standaard’) met tevoren gedefinieerde afkapwaarden en onafhankelijke beoordeling van resultaten, met voldoende grote serie van opeenvolgende patiënten die allen de index- en referentietest hebben gehad |
Prospectief cohort onderzoek van voldoende omvang en follow-up, waarbij adequaat gecontroleerd is voor ‘confounding’ en selectieve follow-up voldoende is uitgesloten. |
|
B |
Onderzoek t.o.v. een referentietest, maar niet met alle kenmerken die onder A2 zijn genoemd |
Prospectief cohort onderzoek, maar niet met alle kenmerken als genoemd onder A2 of retrospectief cohort onderzoek of patiëntcontrole onderzoek |
|
C |
Niet-vergelijkend onderzoek |
|
Tabel 1.3 Niveau van bewijskracht van de conclusie op basis van de aan de conclusie ten grondslag liggende literatuur
|
Niveau |
Conclusie gebaseerd op |
|
1 |
Voor therapeutische interventiestudies: studies van hoge kwaliteit Voor diagnostisch accuratesse onderzoek of prognose, etiologie of bijwerkingen: onderzoek van niveau A1 of tenminste 2 onafhankelijk van elkaar uitgevoerde onderzoeken van niveau A2 |
|
2 |
Voor therapeutische interventiestudies: studies van matige kwaliteit Voor diagnostisch accuratesse onderzoek of prognose, etiologie of bijwerkingen: 1 onderzoek van niveau A2 of tenminste 2 onafhankelijk van elkaar uitgevoerde onderzoeken van niveau B |
|
3 |
Voor therapeutische interventiestudies: studies van lage kwaliteit Voor diagnostisch accuratesse onderzoek of prognose, etiologie of bijwerkingen: 1 onderzoek van niveau B of tenminste 2 onafhankelijk van elkaar uitgevoerde onderzoeken van niveau C |
|
4 |
Voor therapeutische interventiestudies: studies van zeer lage kwaliteit Voor diagnostisch accuratesse onderzoek of prognose, etiologie of bijwerkingen: 1 onderzoek van niveau C |
Overwegingen
Voor een aanbeveling zijn, naast het wetenschappelijke bewijs, ook andere aspecten belangrijk, zoals de expertise van de werkgroepleden, patiëntenvoorkeuren, kosten, beschikbaarheid van voorzieningen of organisatorische zaken. Deze aspecten zijn, voor zover niet wetenschappelijk onderzocht, vermeld onder het kopje ‘Overwegingen’.
Formuleren van aanbevelingen
De aanbevelingen geven een antwoord op de uitgangsvraag en zijn gebaseerd op het beschikbare wetenschappelijke bewijs en de belangrijkste overwegingen.
Indicatorontwikkeling
Gelijktijdig met het ontwikkelen van de conceptrichtlijn werden er interne kwaliteitsindicatoren ontwikkeld om het toepassen van de richtlijn in de praktijk te volgen en te versterken. Meer informatie over de methode van indicatorontwikkeling is op te vragen bij de afdeling Ondersteuning Professionele Kwaliteit (OPK) van de Orde van Medisch Specialisten. In Hoofdstuk 11 zijn een drietal indicatoren opgenomen.
Kennislacunes
Tijdens de ontwikkeling van deze richtlijn is systematisch gezocht naar onderzoek waarvan de resultaten bijdragen aan een antwoord op de uitgangsvragen. Bij elke uitgangsvraag is door de werkgroep nagegaan of er (aanvullend) wetenschappelijk onderzoek gewenst is. Een overzicht van aanbevelingen voor nader (vervolg)onderzoek staat in Hoofdstuk 12.
Commentaar- en autorisatiefase
De conceptrichtlijn is aan de betrokken (wetenschappelijke) verenigingen voorgelegd voor commentaar. De commentaren zullen worden verzameld en besproken met de werkgroep. Naar aanleiding van de commentaren zal de conceptrichtlijn aangepast en definitief vastgesteld worden door de werkgroep. De definitieve richtlijn zal aan de betrokken (wetenschappelijke) verenigingen voorgelegd worden voor autorisatie.
Zoekverantwoording
Zoekacties zijn opvraagbaar. Neem hiervoor contact op met de Richtlijnendatabase.
