Eerstelijnsbehandeling CRPC
Uitgangsvraag
Wat is de aanbevolen eerstelijnsbehandeling bij patiënten met een hormoonresistent prostaatcarcinoom (CRPC)?
De uitgangsvraag omvat de volgende deelvragen:
- Wat is de plaats van ‘androgen receptor signaling inhibitors’ (ARSI) als aanvullende behandeloptie bij ADT gebruik?
- Bij patiënten met niet-gemetastaseerde CRPC
- Bij patiënten met gemetastaseerde CRPC
- Wat is de plaats van chemotherapie als aanvullende behandeloptie bij ADT gebruik?
- Wat is de plaats van radioligand therapie als aanvullende behandeloptie bij ADT gebruik?
Aanbeveling
Zet de testosterononderdrukking voort bij patiënten die voldoen aan de criteria voor nmCRPC met een oplopend PSA. Geef geen aanvullende behandeling.
Overweeg om een PSMA PET scan te maken om metastasen aan te tonen.
Indien er hiermee beperkte metastasen worden aangetoond, overweeg een verder expectatief beleid.
Start een vervolgbehandeling wanneer metastasen aangetoond zijn middels CT- en skeletscan.
Geef bij een progressief mCRPC bij een patiënt die ‘chemo-fit’ is, docetaxel 75 mg/m2 plus prednison 5 mg bid iedere 3 weken maximaal 10 kuren.
Overweeg als behandeloptie voor behandeling van asymptomatisch of licht symptomatisch mCRPC - abiraterone 1000 mg plus prednison 5 mg bid of enzalutamide 160 mg.
Geef bij patiënten met een progressief mCRPC die niet ‘chemo-fit’ zijn of om andere redenen niet aan chemotherapie toekomen: abiraterone 1000 mg plus prednison 5 mg bid
of enzalutamide 160 mg. Overweeg radium-223 (6 injecties gegeven met intervallen van 4 weken) bij beperkte lymfekliermetastasering en afwezigheid van viscerale metastasen en contra-indicaties voor ARSI en chemotherapie.
Bespreek de patiënt in een MDO.
Bespreek de keuze van de therapie en de beste timing van het starten van de therapie met de patiënt.
Overwegingen
Indien er sprake is van een biochemische of radiologische progressie onder testosteronverlagende therapie, dan dient aangetoond te worden of er sprake is van CRPC naar de geldende criteria. Een belangrijk discussiepunt is de beste timing van de start van een volgende palliatieve behandeling en de keuze van de meest aangewezen therapie. De beschikbaarheid van gerandomiseerde studies die een keuze voor een volgende behandeling onderbouwen zijn zeer beperkt. Bespreking binnen het multidisciplinaire team is nu een vereiste. Een aanvullende behandeling met een klassieke androgeen receptor blokker zoals bicalutamide wordt als obsoleet beschouwd.
nmCRPC is een nieuwe entiteit en wordt gedefinieerd als een oplopend serum PSA bij een patiënt met een adequaat onderdrukt serum testosteron, terwijl er middels een CT van thorax en abdomen en skeletscan, geen metastasen aantoonbaar zijn. Er zijn drie redenen waarom deze nieuwe indicatie niet of nauwelijks van belang is voor de Nederlandse praktijk. Ten eerste zijn Nederlandse urologen en radiotherapeuten behoudend met het starten van testosteron-deprivatie en zetten deze veelal pas in op het moment dat er metastasen zijn aangetoond. Ten tweede is in Nederland de PSMA-PET scan breed beschikbaar. Een studie heeft aangetoond dat meer dan 90% van de patiënten die voldoen aan de criteria voor nmCRPC, op PSMA PET scan wel aantoonbare metastasen hebben en 55% afstand metastasen (M1) (Fendler, 2019). Hierdoor komt de entiteit nmCRPC nauwelijks voor in Nederland. Een derde punt van reserve is dat de studies metastasen-vrije overleving als primair eindpunt hadden. Hierdoor konden deze middelen niet volgens PASKWIL criteria worden beoordeeld.
Bij patiënten met een mCRPC kan er feitelijk voor drie verschillende klassen behandelingen gekozen worden, namelijk een behandeling met chemotherapie (docetaxel, cabazitaxel), een behandeling met hormonale middelen (abiraterone of enzalutamide), of een behandeling met radionucliden (radium-223). Al deze behandelingen worden vergoed, zijn beschikbaar en er is een wisselende hoeveelheid ervaring mee.
Abiraterone en enzalutamide zijn geregistreerd voor behandeling van patiënten met een asymptomatisch of gering symptomatisch mCRPC. Er zijn geen gegevens wat de optimale keuze is tussen deze twee middelen. Ook radium-223 kan gegeven worden in eerstelijns behandeling, aangezien latere behandeling met chemotherapie nog steeds mogelijk is, omdat invloed op beenmergfunctie minimaal is. Echter, in 2018 heeft PRAC, de geneesmiddelen veiligheidscommissie van EMA, geadviseerd dat radium-223 gebruik beperkt moet worden tot patiënten die tenminste twee eerdere behandelingen hebben gehad en patiënten voor wie geen andere behandelopties meer zijn. Dit naar aanleiding van de ERA223 studie waarin een hoger percentage fracturen en sterfte werd gevonden in de met radium-223 en abiraterone behandelde studiearm.
Verschillen in bijwerkingen, geneesmiddeleninteracties en co morbiditeit kunnen een rol spelen bij de keuze tussen de beschikbare middelen. Het lijkt voor de hand te liggen om bij een fitte patiënt en een uitgebreide ziekte met een agressief beloop, primair te kiezen voor chemotherapie. Daarbij moet meegewogen worden dat het niet zeker is of in een latere fase van het ziekte beloop patiënten nog fit genoeg zijn voor chemotherapie.
De voorkeur van de patiënt moet bij het vaststellen van het behandelbeleid zeker gewicht in de schaal leggen. Het is vaak behulpzaam om met een patiënt niet alleen de verschillende behandelingsmethoden te bespreken, maar ook uit te leggen dat de beste resultaten bereikt worden als een patiënt uiteindelijk met middelen uit alle drie deze methoden behandeld wordt. Een eerste keuze voor een bepaalde behandeling sluit latere behandeling met andere middelen niet uit en er zijn ook geen harde aanwijzingen dat een eerste keuze automatisch latere keuzen onmogelijk maakt.
Patiënten met bij diagnose gemetastaseerde ziekte worden veelal in het vroege, hormoon gevoelige stadium met chemotherapie of een ARSI behandeld, direct na het starten van een primaire hormonale behandeling (androgeen deprivatie therapie). Er zijn geen gegevens over de optimale behandelkeuze indien er na deze vroege behandeling progressie optreedt (er mCRPC wordt vastgesteld) en er een indicatie bestaat voor een vervolgbehandeling. Het lijkt het beste deze vragen bij een langdurig therapievrij interval te benaderen alsof er een eerstelijns keuze gemaakt moet worden, terwijl er bij progressie onder therapie of een heel kort therapievrij interval een “tweedelijns keuze” gemaakt moet worden. In het bepalen van de sequentie van de behandelingen is te adviseren de verschillende klassen middelen te alterneren. Dit geldt in het bijzonder voor de ARSI’s abiraterone en enzalutamide. Deze middelen kennen een belangrijke kruisresistentie en de kans op een repons op een tweede ARSI, direct na progressie op een eerste ARSI is minimaal (Khalaf, 2019).
Voor- en nadelen van de interventie en de kwaliteit van het bewijs
Op het moment van schrijven van de richtlijn, zijn er vier eerste lijn behandelopties voor patiënten met progressief mCRPC en een indicatie en mogelijkheid voor een behandeling (docetaxel, abiraterone, enzalutamide en radium-223). Alle vier behandelmogelijkheden hebben een vergelijkbaar overlevingsvoordeel laten zien als eerste lijn behandeling. Daarnaast hebben meerdere secundaire eindpunten in de studies, waaronder kwaliteit van leven, een gunstig effect laten zien van deze middelen. De bewijskracht van de studies naar een meerwaarde van de middelen is sterk. Er zitten echter belangrijke verschillen in de kwaliteit en bewijskracht van de studies wat betreft secondaire eindpunten. Daarmee zijn docetaxel en de twee ARSI’s te overwegen als eerste lijn mCRPC behandeling. Ook voor radium-223 is aangetoond dat deze een waarde heeft als eerste lijn behandeling, echter dit op basis van een subgroep analyse. Bovendien heeft de EMA geadviseerd deze behandeling niet als eerste lijn mCRPC therapie toe te passen. Een algemeen voorbehoud bij de conclusies van de studies is dat deze inmiddels ouder zijn en patiënten in cross-over niet met een van de nieuwere therapieën zijn behandeld. Daarnaast zijn er weinig gegevens waarop de voorkeur voor een eerste lijn behandeloptie, boven een ander, gebaseerd kan zijn.
Waarden en voorkeuren van patiënten
De behandeling van mCRPC is palliatief. De genoemde middelen hebben alle bijwerkingen en de overlevingswinst die met de middelen mediaan wordt bereikt, is beperkt. Daarmee is kwaliteit van leven van de patiënt belangrijk om in de beslissingen mee te nemen. Een belangrijk aspect hiervan is dat de voorkeur van de patiënt, na verkrijgen van complete informatie, zwaar weegt in de beslissing. Deze dient te worden geïncorporeerd in de medische overwegingen wat betreft of, en met welk middel, patiënt als eerste lijn mCRPC therapie zal worden behandeld. Aangezien de overlevingswinsten, maar ook de voordelen op secundaire eindpunten van de verschillende middelen elkaar niet veel ontlopen, tellen intensiviteit van de behandeling en te verwachten bijwerkingen zwaar in de keuze voor een eerste lijn behandeling. Zo kan een relatief jonge patiënt, met weinig comorbiditeit, docetaxel als eerste lijn behandeling verkiezen en daarmee alle vervolgopties openhouden, terwijl een oudere patiënt met veel comorbiditeit, afziet van chemotherapie en een ARSI prefereert.
Kosten (middelenbeslag)
Er zijn grote verschillen in de prijs tussen de eerste lijn mCRPC behandelopties. Op het moment van schrijven van deze richtlijn, zijn docetaxel en abiraterone behandeling veruit het goedkoopste. Het is een maatschappelijke discussie of deze verschillen in kosten de veelal beperkte overlevingswinst rechtvaardigen. Het is ook een plicht voor de behandelaar, om deze middelen alleen voor te schrijven binnen de indicatie en kritisch te blijven op de werkzaamheid en de behandeling niet onnodig lang voort te zetten. Per september 2022 is het patent op abiraterone vervallen en zijn er meerdere aanbieders van een generiek product. Daarmee is abiraterone op dit moment de goedkoopste behandeloptie. Daarom, is het vanuit het perspectief van maatschappelijke verantwoordelijkheid wenselijk om te kiezen voor abiraterone wanneer een ARSI geïndiceerd is, als er medisch-inhoudelijk geen argumenten zijn om voor enzalutamide te kiezen.
Aanvaardbaarheid, haalbaarheid en implementatie
De genoemde middelen vallen onder vergoede zorg en zijn voorbehouden aan behandelaars die geschoold zijn om te werken met deze middelen en patiënten categorie. Er zijn geen bijzondere omstandigheden nodig voor behandeling met docetaxel en ARSI’s en worden door alle oncologische centra aangeboden in Nederland. Daarmee zijn deze behandelingen breed beschikbaar. Uitzondering hierop is radium-223, welke alleen kan worden toegediend door centra met een afdeling nucleaire geneeskunde met de vereiste vergunningen.
Rationale van de aanbeveling: weging van argumenten voor en tegen de diagnostische procedure
- Wat is de plaats van ‘androgen receptor signaling inhibitors’ (ARSI) als aanvullende behandeloptie bij ADT?
- Bij patiënten met niet-gemetastaseerde CRPC
nmCRPC is een in Nederland weinig voorkomende entiteit. Daarnaast hadden de studies niet overall survival als primair eindpunt. Daarmee is het advies om patiënten te vervolgen tot aantoonbare metastasen (CT, skelet scan) en dan vervolgbehandeling te overwegen.
-
- Bij patiënten met gemetastaseerde CRPC
Voor patiënten met gemetastaseerd CRPC en een indicatie voor een vervolgbehandeling is een ARSI een van de behandelopties. De aangetoonde overlevingswinst van deze middelen is vergelijkbaar met die van docetaxel. De keuze zal veelal op een ARSI vallen, wanneer een patiënt in het hormoongevoelige stadium met docetaxel is behandeld. Abiraterone en enzalutamide hebben een vergelijkbare overlevingswinst laten zien. Keuze voor abiraterone of enzalutamide kan worden gebaseerd op het bijwerkingen profiel of de combinatie van abiraterone met prednison wenselijk is. Daarnaast zijn de lage kosten van abiraterone ook een belangrijk argument om voor dit middel te kiezen.
- Wat is de plaats van chemotherapie als aanvullende behandeloptie bij ADT?
Docetaxel chemotherapie heeft als eerste lijn behandeling een gelijke overlevingswinst laten zien als abiraterone en enzalutamide. Docetaxel is een intensievere behandeling dan ARSI, wat betreft ziekenhuisbezoeken en bijwerkingen. Toch zal de keuze op docetaxel vallen, wanneer een patiënt eerder met een ARSI is behandeld in het hormoongevoelige stadium. Daarnaast wordt gekozen voor docetaxel wanneer de ziekte een snel en agressief beloop heeft. Er is echter geen bewijs dat docetaxel in deze patiënten effectiever is of sneller werkt dan de ARSI’s.
- Wat is de plaats van radioligand therapie als aanvullende behandeloptie bij ADT?
Effectiviteit van radium-223 als eerste lijn behandeling van mCRPC is aangetoond in een subgroep analyse. Hiermee is de bewijslast van effectiviteit als eerste lijn behandeling minder dan van de andere opties. Daarnaast zijn er door de geneesmiddelenveiligheidscommissie van de EMA, adviezen uitgegaan voor plaatsbepaling van dit middel binnen de behandelopties voor mCRPC. Radium-223 wordt geadviseerd in te zetten als derde lijn behandeling en in de eerste lijn alleen voor patiënten voor wie geen verdere behandelopties meer zijn.
Onderbouwing
Achtergrond
Hormoonresistent prostaatkanker is het stadium van de ziekte waarin testosteronsynthese onderdrukking in de testikels, geen remming van ziekte meer bewerkstelligt. Sedert de introductie van docetaxel in 2004, zijn er meerdere behandelmogelijkheden voor CRPC bijgekomen waarvan een overlevingswinst is aangetoond. De overlevingswinst van deze middelen is echter beperkt tot 3-4 maanden. Een belangrijke vraag blijft de optimale sequentie van deze behandelingen. Gerandomiseerde studies beantwoorden deze vraag veelal niet. Deze module behandelt de keuze voor een eerste lijn behandeling.
Conclusies / Summary of Findings
Sub question 1a ARSI nmCRPC
1. Survival
|
High GRADE |
ARSI, combined with ADT, versus ADT alone or placebo results in an increased survival (metastasis free survival, progression free survival, overall survival, and long term survival) in patients with non metastatic castration resistant prostate cancer (nmCRPC).
Sources: Fizazi, 2019; Hussain, 2018; Small, 2019; Smith 2018; Smith, 2019 and Sternberg, 2020 |
2. Toxicity
|
High GRADE |
ARSI, combined with ADT, versus ADT alone or placebo results in comparable adverse events any grade in patients with nmCRPC. ARSI, combined with ADT, versus ADT alone or placebo results in higher adverse events ≥ grade 3 in patients with nmCRPC.
Sources: Sopeña Sutil, 2021; Smith, 2021; Sternberg, 2021 |
3. Costs
|
No GRADE |
The outcome costs was not assessed by the included studies. |
4. Quality of life
|
High GRADE |
ARSI, combined with ADT, versus ADT alone or placebo results in a comparable quality of life at follow up in patients with nmCRPC.
Sources: Fizazi, 2019; Hussain, 2018 and Smith, 2018 |
Sub question 1b ARSI mCRPC
1. Survival
|
High GRADE |
ARSI versus placebo results in an increased survival (overall survival, progression free survival and long term survival) in patients with metastatic castration resistant prostate cancer (mCRPC).
Sources: Beer, 2014; Morris, 2015 and Ryan, 2015 |
2. Toxicity
|
High GRADE |
At the end of the study period (ca. 2 year follow up), ARSI versus placebo results in comparable adverse events any grade and adverse events ≥ grade 3 in patients with mCRPC.
At long term (ca. 4 year follow up), ARSI results in comparable adverse events any grade but higher adverse events ≥ grade 3 in patients with mCRPC.
Sources: Beer, 2014; Ryan, 2013, Ryan, 2015 |
3. Costs
|
No GRADE |
The outcome costs was not assessed by the included studies in patients with mCRPC. |
4. Quality of life
|
High GRADE |
ARSI versus placebo results in an improved quality of life in patients with mCRPC.
Sources: Beer, 2014 and Ryan, 2013 |
Sub question 3. Radioligand therapy
1. Survival
|
Moderate GRADE |
Radioligand therapy versus placebo probably results in an increased survival (overall survival, progression free survival and death related to prostate cancer) in patients with metastatic castration resistant prostate cancer (mCRPC).
Sources: Parker, 2013 and Parker, 2018 |
2. Toxicity
|
High GRADE |
Radioligand therapy versus placebo results in comparable adverse events of any grade in patients with mCRPC.
Sources: Parker, 2013 and Parker, 2018 |
3. Costs
|
No GRADE |
The outcome costs was not assessed by the included studies. |
4. Quality of life
|
Moderate GRADE |
Radioligand therapy versus placebo probably results in a not clinically relevant change in quality of life at follow up compared to baseline in patients with mCRPC.
Sources: Parker, 2013 and Parker, 2018 |
Samenvatting literatuur
1. ARSI
Sub question 1a ARSI nmCRPC
Description of studies
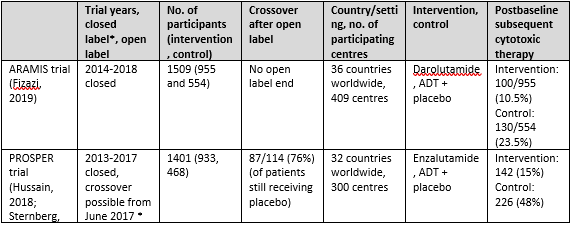
Three trials were included for sub question 1a: the ARAMIS trial with one publication (Fizazi, 2019), the PROSPER trial with 2 publications (Hussain, 2018 and Sternberg, 2020) and the SPARTAN trial with 3 publications (Small, 2019; Smith, 2018 and Smith, 2021). An overview of study characteristics can be found in Table 1.
Table 1. Overview study characteristics ARAMIS, PROSPER and SPARTAN
The ARAMIS trial
The ARAMIS trial (Fizazi, 2019) assessed the effect of darolutamide versus placebo in patients with non-metastatic castration-resistant prostate cancer (nmCRPC). The
trial was conducted in 409 centers in 36 countries worldwide. The primary endpoint was metastasis free survival, whereas secondary outcomes were overall survival, time to pain progression, time to chemotherapy and time to a symptomatic skeletal event. The median follow up time was 18 months. Patients were randomly assigned in a 2:1 ratio to receive the intervention (twice daily 600mg darolutamide) or matched placebo and ADT. Patients discontinued therapy because of protocol-defined progression, adverse events or withdrawal of consent. Patients that took a prohibited therapy (described in protocol) had to leave the trial but were followed for survival. A total of 1509 patients (955 darolutamide and 554 placebo) were assessed for the intention-to-treat analysis. Groups were comparable at baseline.
The PROSPER trial
The PROSPER trial (Hussain, 2018 and Sternberg, 2020) investigated the effect of enzalutamide versus placebo in patients with nmCRPC. The trial was conducted in more than 300 sites in 32 countries worldwide. The primary endpoint was metastasis free survival, whereas secondary outcomes were overall survival, time to PSA progression, PSA response rate, time to first use of a subsequent antineoplastic therapy, time to first use of chemotherapy, time to pain progression, health-related quality of life and adverse events. Median follow-up time was 23 months for the primary analysis and 48 months for the long term (third interim) analysis. Patients were randomly assigned in a 2:1 ratio to receive the intervention (160 mg enzalutamide once daily) or placebo once daily, next to continuation of ADT (either with a gonadotropin-releasing hormone agonist or antagonist or with previous bilateral orchiectomy). After the primary analysis, patients were unblinded and given the opportunity to switch to the enzalutamide group for the open-label extension. A total of 87/114 (76%) decided to switch. At the long term follow up (ca 4 years) 31% of enzalutamide treated patients had discontinued treatment because of disease progression, adverse events or other reasons. 1401 patients (933 enzalutamide and 468 placebo) were randomized and evaluated in an intention to treat analysis. Groups were comparable at baseline.
The SPARTAN trial
The SPARTAN trial (Small, 2019; Smith, 2018 and Smith, 2021) examined the effect of apalutamide versus placebo in patients with nmCRPC. The trial was conducted in 332 centers in 26 countries in North America, Europe, and the Asia–Pacific region. The primary endpoint was metastasis free survival, whereas secondary outcomes were time to metastasis, progression-free survival, time to symptomatic progression, overall survival and time to initiation of chemotherapy. The median follow up time was 20 months for the first interim analysis (Smith, 2018) and 52 months for the long term analysis (Smith, 2021). Patients were randomly assigned in a 2:1 ratio to receive apalutamide (240 mg per day) or matched placebo and ADT. After the primary analysis, patients in the placebo group were unblinded and given the opportunity to cross over to the open label apalutamide group. After unblinding, 19% patients of the placebo group without disease progression decided to switch to apalutamide. At the long term follow up, 70% of the intervention group and 100% of placebo group had discontinued treatment because of progressive disease, adverse events or other reasons. A total of 1207 patients (806 intervention and 401 control) were assessed in an intention-to-treat analysis. Groups were comparable at baseline.
Results
1. Survival
An overview of study characteristics and corresponding hazard ratios for all survival outcomes can be found in Table 2.
Table 2 Overview survival outcomes reported by ARAMIS, PROSPER and SPARTAN
|
|
Interim analysis |
Long term analysis |
|||
|
|
1.1 Survival overall (HR, 95%CI) |
1.2 Metastasis free survival (HR, 95% CI) |
1.3 Progression free survival (HR, 95% CI) |
1.4 Death related to prostate cancer (HR, 95% CI) |
1.5 Long term survival (HR, 95% CI) |
|
ARAMIS trial (Fizazi, 2019) |
0.71 (0.51-0.99) |
0.41 (0.34-0.49) |
0.13 (0.11-0.16) |
NR |
NR |
|
PROSPER trial (Hussain, 2018; Sternberg, 2020) |
0.80 (0.58-1.09) |
0.29 (0.24-0.35) |
0.07 (0.05-0.08) |
0.81 (0.72-0.91) |
0.73 (0.61-0.89) |
|
SPARTAN trial (Small, 2019; Smith 2018 and 2019) |
0.70 (0.47-1.04) |
0.27 (0.22-0.34) |
0.29 (0.24-0.36) |
NR |
0.78 (0.64-0.96) |
|
Results pooled |
0.74 (0.60-0.90) |
0.32 (0.29-0.36) |
0.14 (0.13-0.16) |
0.81 (0.72-0.91) |
0.76 (0.66-0.87) |
Sources: Fizazi, 2019; Hussain, 2018; Small, 2019; Smith 2018; Smith, 2019 and Sternberg, 2020
Interim analysis
1.1 Overall survival
Overall survival at first interim follow up was reported by three trials (ARAMIS, PROSPER and SPARTAN). The outcome was defined as the number of patients still alive at the end of the trial period. The outcome was assessed at the first interim follow up at 18, 23 and 20 months follow up respectively.
In the ARAMIS trial, overall survival was reported in 877/955 (91.8%) in the treatment group versus 496/554 (89.5%) in the placebo group (HR (95%CI): 0.71 (0.50-0.99)) (Fizazi, 2019).
In the PROSPER trial, overall survival was reported in 830/933 (89%) in the treatment group versus 402/468 (87%) in the placebo group (HR (95%CI): 0.80 (0.58-1.09)) (Hussain, 2018).
In the SPARTAN trial, overall survival was reported with HR (95%CI): 0.70 (0.47-1.04) at the first interim analysis at 20 month-follow up. For this analysis, absolute survival numbers were not given (Smith, 2018).
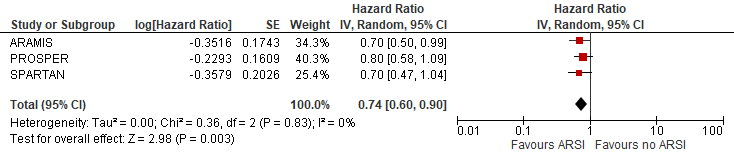
The pooled overall survival (using the first interim analysis numbers of SPARTAN) was estimated at HR (95%): 0.74 (0.60-0.90) favouring ARSI (Figure 1). The risk reduction in overall death was estimated at 26% due to ARSI.
Figure 1 Overall survival
N.B. Follow up at median 18, 20 and 23 months for ARAMIS, PROSPER and SPARTAN respectively.
Sources: Fizazi, 2019; Hussain, 2018 and Smith, 2018
1.2 Metastases free survival
Metastases free survival at first interim follow up was reported by three trials (ARAMIS, PROSPER and SPARTAN). The outcome was defined as the median number of months before metastases were diagnosed. The outcome was assessed at the first interim follow up at 18, 23 and 20 months follow up respectively.
In the ARAMIS trial, median metastases free survival was reported as 40.4 months in the treatment group versus 18.4 months in the placebo group (HR (95%CI): 0.41 (0.34-0.50) favouring treatment) (Fizazi, 2019).
In the PROSPER trial, median metastases free survival was reported as 36.6 months in the treatment group versus 14.7 months in the placebo group (HR (95%CI): 0.29 (0.24-0.35) favouring treatment) (Hussain, 2018).
In the SPARTAN trial, median metastases free survival was reported as 40.5 months in the treatment group versus 16.2 months in the placebo group (HR (95%CI): 0.27 (0.22-0.34) favouring treatment) (Smith, 2018).
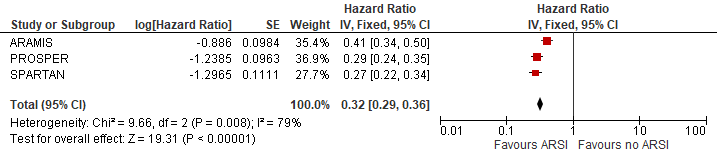
The pooled metastases free survival was estimated at HR (95%CI): 0.32 (0.29-0.36) favouring ARSI (Figure 2). The risk reduction in metastases was estimated at 68% due to ARSI.
Figure 2 Metastases free survival
N.B. Follow up at median 18, 20 and 23 months for ARAMIS, PROSPER and SPARTAN respectively.
Sources: Fizazi, 2019; Hussain, 2018 and Smith, 2018
1.3 Overall survival at the long term follow up
Two trials (PROSPER and SPARTAN) reported an extended follow-up of 48 and 52 months, respectively, and established overall survival of the intervention and placebo arms. The outcome was defined as the number of patients still alive at the long term follow up and the median number of months still alive. The outcome was measured at the long term open label extension at a median follow up of 48 and 52 months respectively. (Rodriguez-Vida, 2022; Smith, 2021; Tombal, 2022; Wenzel, 2022)
In the PROSPER trial, 645/933 patients (69%) were still alive in the treatment group compared to 290/468 (62%) in the placebo group. Median survival was reported as 67 months in the treatment group compared to 56 months in the placebo group (HR (95%CI): 0.73 (0.61-0.89) favouring treatment).
In the SPARTAN trial, at the long term follow up, 532/806 patients (66%) were still alive in the treatment group compared to 247/401 patients (62%) in the placebo group. Median survival was reported as 73.9 months in the treatment group versus 59.9 months in the placebo group (HR (95%): 0.78 (0.64-0.96) favouring treatment).
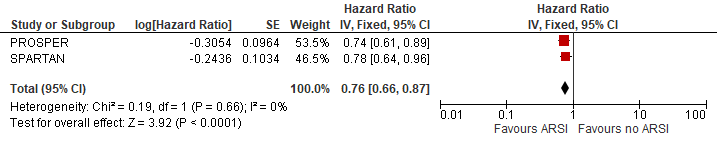
The pooled overall survival was estimated at HR (95%CI): 0.76 (0.66-0.87) (figure 5). The reduction in death was estimated at 24% due to ARSI treatment.
Figure 5 Long term survival
N.B. Follow up median follow up at 48 and 52 months for PROSPER and SPARTAN
Sources: Smith, 2021 and Sternberg, 2020
2. Toxicity
Data on toxicity was reported by three trials (ARAMIS, PROSPER and SPARTAN). Data was separately assessed for any event, grade ≥3 events and events leading to death. An overview can be found in table 3. In apalutamide treated patients, grade 3 and higher rash and hypertension, in enzalutamide treated patients grade 3 and higher fatigue and hypertension and in darolutamide treated patients fatigue was more common than in placebo treated patients
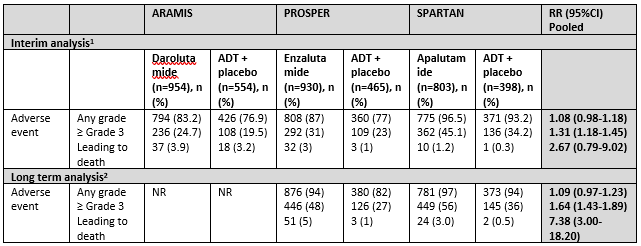
Table 3 Toxicity overview reported by ARAMIS, PROSPER and SPARTAN
1At the end of the trial period, after planned inclusion of app. N=1500 (ARAMIS), N=1440 (PROSPER) and N=1200 (SPARTAN) and before reveal of treatment and crossover allowance, median follow up 18 (ARAMIS), 23 (PROSPER) and 20 months (SPARTAN).
2At the long term follow up, after crossover allowance, intention-to-treat analysis, median follow up 48 (PROSPER) and 52 months (SPARTAN)
Sources: Sopeña Sutil (2021) for interim analysis and Smith, 2021 and Sternberg, 2021 for long term analysis
Interim analysis
Adverse events
Adverse events of any grade were common among treatment groups (varying 87% to 96%) and not significantly higher in the treatment groups compared to placebo (3 trials, n=4104, RR (95%CI) 1.08 (0.98-1.18)). However ≥ Grade 3 adverse events were more common in the treatment groups (3 trials, n=4104, RR (95%CI) 1.31 (1.18-1.45)). Any adverse event leading to death was not significantly higher in the treatment groups (3 trials, n=4104, RR (95%CI) 2.67 (0.79-9.02)) (Sopeña Sutil, 2021).
Long term analysis
Adverse events at long term assessment
Adverse events at the long term analysis was reported by two studies: PROSPER (Sternberg, 2020) and SPARTAN (Smith, 2021). The outcome was assessed at a median follow up of 48 months (PROSPER) and 52 months (SPARTAN).
Adverse events at any grade were not significantly higher at the long term follow up (2 trials, n=2596, RR (95%CI) 1.09 (0.97-1.23)). The number of patients with ≥ Grade 3 adverse events was significantly higher in the treatment group (RR (95%CI) 1.64 (1.43-1.89) and adverse events leading to death was higher in the treatment group 7.38 (3.00-18.20).
In the PROSPER, <1% of patients in the control group and 2% of patients in the enzalutamide treated group died of a cardiovascular event. According to the PROSPER research group itself, none of the cardiovascular events in the treatment group could have been related to the treatment medication (Sternberg, 2020).
4. Quality of life
Interim analysis
All three trials reported Health related Quality of Life (HRQoL) as FACT-P, EORTC-5D-3L and EORTC-QLQ-PR25 questionnaires scores, however all three trials used different ways of reporting. One trial reported mean FACT-P scores and various EORTC questionnaires scores at 16 weeks follow up compared to baseline (ARAMIS, Fizazi, 2019), one trial reported mean time in months to a decrease of the FACT-P score of at least 10 points (PROSPER, Hussain, 2018) and one trial reported the change in total FACT-P score from baseline to 29 months (SPARTAN, Smith, 2018). Variation in health related Quality of life reporting strategies, makes comparison of the trials challenging.
ARAMIS reported similar mean FACT-P and various EORTC-scores at 16w follow up in the intervention and control groups, suggesting no difference in HRQoL. PROSPER reported mean time to degradation in HRQoL questionnaires scores of 11.1 months in both groups, also suggesting no difference in HRQoL. SPARTAN reported a reduction in FACT-P score from baseline of 0.99 and 3.29 at the 29m follow up in the intervention and control arms of the study, respectively. With a threshold of 10 points for a clinical significant change, also HRQoL assessment in SPARTAN suggests no difference in HRQoL between intervention and control ars. With that, all three trials reported comparable quality of life scores at follow up (Fizazi, 2019; Hussain, 2018 and Smith, 2018).
Level of evidence of the literature
The level of evidence (GRADE method) is determined per comparison and outcome measure and is based on results from RCTs and therefore starts at level ‘high’.
The level of evidence regarding the outcome measure survival was not further downgraded.
The level of evidence regarding the outcome measure toxicity was not further downgraded.
The outcome costs was not assessed by the included studies.
The level of evidence regarding the outcome measure quality of life was not further downgraded.
Conclusions
1. Survival
|
High GRADE |
ARSI, combined with ADT, versus ADT alone or placebo results in an increased survival (metastasis free survival, progression free survival, overall survival, and long term survival) in patients with non metastatic castration resistant prostate cancer (nmCRPC).
Sources: Fizazi, 2019; Hussain, 2018; Small, 2019; Smith 2018; Smith, 2019 and Sternberg, 2020 |
2. Toxicity
|
High GRADE |
ARSI, combined with ADT, versus ADT alone or placebo results in comparable adverse events any grade in patients with nmCRPC. ARSI, combined with ADT, versus ADT alone or placebo results in higher adverse events ≥ grade 3 in patients with nmCRPC.
Sources: Sopeña Sutil, 2021; Smith, 2021; Sternberg, 2021 |
3. Costs
|
No GRADE |
The outcome costs was not assessed by the included studies. |
4. Quality of life
|
High GRADE |
ARSI, combined with ADT, versus ADT alone or placebo results in a comparable quality of life at follow up in patients with nmCRPC.
Sources: Fizazi, 2019; Hussain, 2018 and Smith, 2018 |
Sub question 1b ARSI mCRPC
Description of studies
Two trials were included for sub question 1b: the COU-AA-302-study with five publications (Basch, 2013; Morris, 2015; Rathkopf, 2014; Ryan, 2013 and Ryan, 2015) and the PREVAIL trial with two publications (Beer, 2014 and Loriot, 2015). Since publication of the previous version of this module (2016), no new publications appeared.
Table 5 Overview study characteristics COU-AA-302 and PREVAIL
|
|
Trial years |
No. of participants (intervention, control) |
Country/setting, no. of participating centres |
Intervention, control |
Postbaseline subsequent cytotoxic therapy |
|
The COU-AA-302 study |
2010-2012 |
1088 (546, 542) |
151, 12 countries |
Abiraterone-prednisone, prednisone alone |
Intervention: 252/546 (46%) Control: 339/542 (63%)* |
|
The PREVAIL study |
2010-2012 |
1717 (872, 845) |
Ca 180 centres worldwide, not exactly reported |
Enzalutamide, placebo |
Intervention: 382/872 (44%) Control: 642/845 (76%) |
*Docetaxel and Cabazitaxel added
The COU-AA-302 study
The COU-AA-302 study assessed the effect of abiraterone plus prednisone versus prednisone alone in chemotherapy-naïve patients with metastatic castration-resistant prostate cancer (mCRPC). The study is a phase-3 RCT conducted in 151 centers in 12 countries (in collaboration with US and European agencies). The primary end point was radiographic progression of prostate cancer at a bone scan or computed tomography (CT) or magnetic resonance imaging (MRI) or death by any cause. Three interim analyses for overall survival (OS) were planned at approximately 15%, 40% and 55% of expected deaths. At the first interim analysis (December 2010) median follow up time was 8.3 months, at the second interim analysis (December 2011) median follow up time was 27.2 months and median follow up time at the third interim analysis (May 2012) was not reported. Patients were randomly assigned in a 1:1 ratio to receive abiraterone acetate 1,000 mg daily + prednisone 5 mg or placebo + prednisone. Patients discontinued therapy because of radiographic or clinical progression (protocol-defined), adverse events or withdrawal of consent. A total of 1088 patients (546 Abiraterone-prednisone and 542 placebo-prednisone) were successfully randomized (Morris, 2015).
The PREVAIL study
The PREVAIL study investigated the effect of enzalutamide versus placebo in chemotherapy-naïve patients with metastatic castration-resistant prostate cancer (mCRPC). The phase-3 RCT was conducted in approximately 180 centers worldwide (exact locations not mentioned). The primary end points were radiographic progression-free survival and overall survival. At the planned interim analysis (May 2012) for survival after 516 deaths of planned enrollment of 1680 patients, median follow up was approximately 22 months. Patients were randomly assigned in a 1:1 ratio to receive either enzalutamide (160mg daily) or placebo capsules. Treatment discontinuation was the result of death, withdrawal of consent, disease progression (predefined), adverse events or protocol violation. A total of 1717 participants were successfully randomized over 872 enzalutamide and 845 placebo group.
Results
1. Survival
An overview of study characteristics and corresponding hazard ratios for all survival outcomes can be found in table 6.
Table 6 Overview survival outcomes reported by COU-AA-302 and PREVAIL
|
|
Interim analysis |
Long term analysis |
||
|
|
1.1 Survival overall (HR, 95%CI) |
1.2 Progression free survival (HR, 95% CI) |
1.3 Death related to prostate cancer (RR, 95% CI) |
1.4 Long term survival (HR, 95% CI) |
|
The COU-AA-302 study |
0.75 (0.61 to 0.93) |
0.53 (0.45 to 0.62) |
NR |
0.81 (0.70 to 0.93) |
|
The PREVAIL study |
0.71 (0.60 to 0.84) |
0.19 (0.15 to 0.23) |
No long term follow up performed. |
No long term follow up performed. |
|
Results pooled |
0.73 (0.64 to 0.83) |
Not pooled, different definitions and follow up time |
NR |
0.81 (0.70 to 0.93) |
Sources: Beer, 2014; Morris, 2015 and Ryan, 2015
Interim analysis
1.1 Overall survival
Overall survival at first interim follow up was reported by two trials (COU-AA-302 and PREVAIL). The outcome was defined as the number of deaths (out of total number of patients) at the end of the trial period. The outcome was assessed at the first interim follow up at 27 months (COU-AA-302) and 22 months (PREVAIL).
The COU-AA-302 reported 147 deaths in the abiraterone (total number still alive not reported) + prednisone group versus 186 deaths in the prednisolone alone group (HR (95%CI): 0.75 (0.61-0.93) (Morris, 2015). The PREVAIL study reported 241/872 (28%) of patients died in the enzalutamide group versus 299/845 (35%) of patients died in the placebo group (HR (95%CI): 0.71 (0.60-0.84). The pooled overall survival was estimated at HR (95%CI): 0.73 (0.64-0.83) favouring ARSI. The risk reduction in death was estimated at 27% due to ARSI.
Figure 6 Overall survival at first interim analysis at 27 and 22 months for COU-AA-302 and PREVAIL respectively
1.2 Progression free survival
Progression free survival at first interim follow up was reported by two trials (COU-AA-302 and PREVAIL). COU-AA-302 defined the outcome as “time from random assignment to the first occurrence of either progression by bone scan, progression by computed tomography (CT) or magnetic resonance imaging as defined by modified RECIST (version 1.0), or death resulting from any cause (COU-AA-302)”. PREVAIL defined the outcome as “the rate of radiographic progression-free survival at the 12months follow up”. Because of different study definitions it was decided not to pool data.
The COU-AA-302 estimated the median time to disease progression at the first interim follow up at 16.5 months abiraterone plus prednisone group versus 8.3 months in the placebo group (HR (95%CI): 0.53 (0.45-0.62) (follow up 27 months). The PREVAIL study reported the rate of radiographic progression-free survival at the 12 months follow up, which was 65% in the enzalutamide group versus 14% in the placebo group (HR (95%CI): 0.19 (0.15-0.23).
Table 7 Progression-free survival reported by COU-AA-302 and PREVAIL
|
|
Progression free survival treatment group |
Progression free survival control group |
Hazard ratio (95%CI) |
|
COU-AA-302 |
16.5 months1 |
8.3 months1 |
0.53 (0.45-0.62) |
|
PREVAIL |
65%2 |
14%2 |
0.19 (0.15-0.23) |
1Median follow up time 27 months. 2Median follow up time 12 months.
Sources: Beer, 2014 (PREVAIL) and Morris, 2015 (COU-AA-203)
Long term analysis
1.3 Death related to prostate cancer at long term follow up
The two included studies did not report this outcome measure.
1.4 Long term survival
Long term survival was reported by one study (COU-AA-302). COU-AA-302 defined the outcome as the risk of death in the treatment group compared to the placebo group at a follow up of a median of 49.2 months.
In the COU-AA-302 trial, 352/546 (65%) deaths occurred in the abiraterone acetate group versus 387/542(71%) in the placebo group (HR (95%CI): 0.81 (0.70–0.93). Median overall survival was 34.7 months in the treatment group versus 30.3 months in the placebo group. The reduction in death due to ARSI was estimated at 19%.
Table 8 Long term survival at long term follow up reported by COU-AA-302
|
|
Overall death treatment group |
Overall death control group |
Hazard ratio (95%CI) |
|
COU-AA-302 |
352/546 (65%)1 |
387/542 (71%)1 |
0.81 (0.70–0.93) |
2 Median follow up time 49 months.
Source: Ryan, 2015 (COU-AA-302)
2 Toxicity
Both trials (COU-AA-302 and PREVAIL) reported on toxicity. COU-AA-302 reported adverse events at long term follow up at a median follow up time of 49 months. An overview can be found in table 9. In abiraterone treated patients, grade 3 and higher cardiac disorders and increased liver enzymes were more common than in control patients, in enzalutamide treated patients, grade 3 and higher hypertension was more common than in control patients.
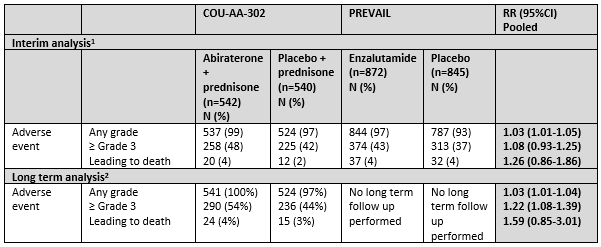
Table 9 Toxicity overview reported in COU-AA-302 and PREVAIL
1At the end of the trial period, after planned enrolment of n=1000 and median follow up time 22 months for COU-AA-302 and after planned enrolment of n=1680 and median follow up time 22 months for PREVAIL
2At the long term follow up, after crossover allowance, intention-to-treat analysis, median follow up 49 months for COU-AA-302
Source: Beer, 2014; Ryan, 2013, Ryan, 2015
Interim analysis
Adverse events
Adverse events at any grade were common and occurred in 99% and 97% in the treatment groups (abiraterone and enzalutamide respectively) versus 97% and 93% in the placebo groups (RR (95%CI): 1.03 (1.01 to 1.05). Adverse events ≥grade 3 occurred in 48% and 43% in the abiraterone and enzalutamide group respectively versus 43% and 37% in the placebo groups ((RR (95%CI): 1.08 (0.93 to 1.25). Adverse events leading to death occurred in 4% and 4% in the abiraterone and enzalutamide group respectively versus 2% and 4% in the placebo groups (HR (95%CI): 1.26 (0.86 to 1.86) (Beer, 2014 and Ryan, 2013). The COU-AA-302 study reported the most common adverse events leading to death were general disorders, including disease progression, a decline in physical health, and infections including
pneumonia and respiratory tract infection.
Long term analysis
Adverse events at long term analysis
The COU-AA-302 study reported on adverse events in the long term analysis at a median follow up of 49.2 months. Adverse events at any grade occurred in 100% of treated patients and 97% of placebo patients. Adverse events ≥ grade 3 occurred in 54% of treatment group patients versus 44% of placebo group patients (RR (95%CI): 1.22 (1.08-1.39). Adverse events leading to death occurred in 4% of treatment group patients versus 2% of placebo group patients (RR (95%CI): 1.59 (0.85 to 3.01). The COU-AA-302 study reported three patients (1%) in the treatment group had general physical health deterioration as a sign of clinical progression resulting in death versus three cases (1%) in the placebo group (Ryan, 2015)
3. Costs
Prices (November 2022) for Enzalutamide and Abiraterone are reported at 113,62 and 113,57 euro per day, respectively, while prices for abiraterone have decreased significantly since the patent has expired in September 2022 (source: Zorginstituut Nederland)
4. Quality of life
Quality of life was measured with the FACT-P score by both studies (COU-AA-302 and PREVAIL). The outcome measure was defined as the number of months until a decline of 10 points or more occurs of the FACT-P total score in both studies (Beer, 2014 (supplementary data) and Ryan, 2013). An overview can be found in table 10.
Table 10 Quality of life reported in COU-AA-203 and PREVAIL
|
|
COU-AA-302 |
PREVAIL |
Pooled results |
||
|
|
Abiraterone + prednisone (n=546) N (%) |
Placebo + prednisone (n=542) N (%) |
Enzalutamide (n=872) N (%) |
Placebo (n=845) N (%) |
HR (95%CI) |
|
Quality of life deterioration, measured with FACT-P |
12.7 mo. |
8.3 mo. |
11.3 mo. |
5.6 mo. |
|
Sources: Beer, 2014 and Ryan, 2013
The COU-AA-302 reported a median time of 12.7 months until quality of life decline occurred in the treatment group versus 8.3 months in the placebo group. The PREVAIL reported a median time of 11.3 months versus 5.6 months until quality of life decline occurred. The pooled results were HR (95%CI): 0.69 (0.56-0.86) favouring treatment.
Level of evidence of the literature
The level of evidence (GRADE method) is determined per comparison and outcome measure and is based on results from RCTs and therefore starts at level ‘high’.
The level of evidence regarding the outcome measure survival was not further downgraded.
The level of evidence regarding the outcome measure toxicity was not further downgraded.
The outcome costs was not assessed by the included studies.
The level of evidence regarding the outcome measure quality of life was not further downgraded.
Conclusions
1. Survival
|
High GRADE |
ARSI versus placebo results in an increased survival (overall survival, progression free survival and long term survival) in patients with metastatic castration resistant prostate cancer (mCRPC).
Sources: Beer, 2014; Morris, 2015 and Ryan, 2015 |
2. Toxicity
|
High GRADE |
At the end of the study period (ca. 2 year follow up), ARSI versus placebo results in comparable adverse events any grade and adverse events ≥ grade 3 in patients with mCRPC.
At long term (ca. 4 year follow up), ARSI results in comparable adverse events any grade but higher adverse events ≥ grade 3 in patients with mCRPC.
Sources: Beer, 2014; Ryan, 2013, Ryan, 2015 |
3. Costs
|
No GRADE |
The outcome costs was not assessed by the included studies in patients with mCRPC. |
4. Quality of life
|
High GRADE |
ARSI versus placebo results in an improved quality of life in patients with mCRPC.
Sources: Beer, 2014 and Ryan, 2013 |
Sub question 2. Chemotherapy
For this sub question, no studies were found. All studies that were found lacked a control group without chemotherapy treatment or ADT alone. More information can be found in the Table of excluded studies. Therefore, no systematic literature analysis to this question was performed.
In short: Docetaxel was the first therapeutic intervention that showed an overall survival benefit over a drug that has not shown to improve overall survival (mitoxantrone) (Tannock et al NEJM, 2004). Six years later, the TROPIC study was published which showed that cabazitaxel treated patients, previously treated with docetaxel, had a survival benefit over treatment with mitoxantrone (de Bono, Lancet, 2010). The 2019 CARD study showed that patients previously treated with docetaxel and an ARSI, derived a longer overall survival when treated with cabazitaxel than patients treated with an ARSI after progressing on another ARSI (de Wit, NEJM, 2019).
Sub question 3. Radioligand therapy
Description of studies
One trial was included for sub question 3 radioligand therapy: the ALSYMPCA trial with six publications (Hoskin, 2014; Nome, 2015; Parker, 2013 and Parker, 2016 & Sartor, 2014 and Sartor, 2017). An overview of the study can be found in table 11.
Table 11 Overview study characteristics ALSYMPCA
|
|
Trial years |
No. of participants (intervention, control) |
Country/setting, no. of participating centres |
Intervention, control |
Postbaseline subsequent cytotoxic therapy |
|
The ALSYMPCA trial |
2008-2011 |
921 (614, 307) |
136 centres, 19 countries |
Ra-223, placebo |
NR |
The ALSYMPCA trial
The ALSYMPCA trial assessed the effect of radium-223 (ra-223) on bone metastases in patients with mCRPC. The trial was conducted in 136 study centers in 19 countries. Patients with skeletal metastases were included, but no visceral involvement (Nome, 2015). A single enlarged lymph node was allowed. The primary end point was overall survival, whereas time to the first skeletal event and various biochemical end points were also assessed (Parker, 2013). The first interim analysis occurred when approximately 50% of deaths had occurred (i.e. 320 deaths, n=901) (Parker, 2013). The second interim analysis took place at the end of the trial period when the intended 900 participants were included. At this moment 528 deaths had occurred (n=921), before planned early discontinuation and allowance for crossover for ethical reasons on the basis of the first interim analysis (Parker, 2013). Afterwards several post-hoc assessments at n=921 were performed (Hoskin, 2014, Parker, 2013 and Sartor, 2017).
Patients were stratified according to previous use or nonuse of docetaxel, baseline alkaline phosphatase level and current use or nonuse of bisphosphonate. Subsequently, they were randomized in a 2:1 ratio to receive up to six intravenous injections of ra-223 or placebo. At the time of the first analysis, approximately 58% had received all six injections in the ra-223 group and 47% in the placebo group. The dose was 50 kBq per kilogram of body weight. One injection was administered every four weeks. Besides this, patients received routine care provided at the treatment center such as radiation therapy, glucocorticoids, antiandrogens, ketoconazole, or estrogens. Chemotherapy and hemibody external radiotherapy was not allowed during the trial period. Planned follow up was 3 years. Patients discontinued treatment because of adverse events, clinical progression, crossover or withdrawal. Groups were comparable at baseline.
1. Survival
An overview of study characteristics and corresponding hazard ratios for all survival outcomes can be found in table 12.
Table 12 Overview survival outcomes ALSYMPCA
|
|
Interim analysis |
Long term analysis |
||
|
|
1.1 Survival overall (HR, 95%CI) |
1.2 Progression free survival (HR, 95% CI) |
1.3 Death related to prostate cancer (RR, 95% CI) |
1.4 Long term survival (HR, 95% CI) |
|
ALSYMPCA |
0.70 (0.58-0.83) |
0.66 (0.52-0.83) |
NR |
NR |
Sources: Parker, 2013 and Parker, 2018
Interim analysis
1.1 Overall survival at first interim follow up
Overall survival at first interim follow up was defined as the time from randomization until the date of death, regardless of the cause (Parker, 2013). The outcome was assessed at the end of the trial period when n=921 patients were included. At this moment, the median number of injections was six in the ra-223 group and five in the placebo group. The median follow up time in months was not reported.
Table 12 Overall survival at interim analysis
|
|
Overall survival treatment group |
Overall survival control group |
Hazard ratio (95%CI) |
|
ALSYMPCA |
14.9 months |
11.3 months |
0.70 (0.58-0.83) |
Sources: Parker, 2013
The study reported an overall survival of median 14.9 months in the treatment group versus 11.2 months in the control group (HR (95%CI): 0.70 (0.58-0.83)). It is estimated ra-223 prolonged survival with app. 4 months.
1.2 Progression free survival
Parker (2013) defined the outcome as time since randomization until first skeletal event occurred. The outcome was assessed at the end of the trial period (see above).
Table 13 Progression free survival at first interim follow up when app. 50% of deaths had occurred n=809
|
|
PFS toch? treatment group |
PFS control group |
Hazard ratio (95%CI) |
|
ALSYMPCA |
15.6 months |
9.8 months |
0.66 (0.52-0.83) |
Sources: Parker, 2013
The study reported time until first symptomatic event of median 15.6 months in the intervention group versus 9.8 months in the placebo group (HR (95%CI): 0.66 (0.52-0.83)). It is estimated ra-223 prolonged time until symptomatic skeletal event with app. 6 months.
Long term analysis
Not reported.
1.4 Long term survival
Not reported.
2 Toxicity
ALSYMPCA reported on toxicity. The authors reported on adverse events (any grade, grade 3-4 and serious adverse events). They did not report on adverse events leading to death. Median follow up time at interim analysis is not reported. Median follow up at long term analysis was 13 months (0-36) in the ra-223 group and 9 months (0-36) in the placebo group. An overview can be found in table 14. Grade 3 and higher adverse events were infrequent in the trial and no significant differences between the intervention and control arm were observed.
Table 14 Toxicity overview reported in ALSYMPCA
|
ALSYMPCA |
||||
|
|
|
Radium-223 (n=600) N (%) |
Placebo (n=301) N (%) |
RR (95% CI) |
|
Interim analysis1 |
||||
|
Adverse event |
Any grade ≥ Grade 3 Leading to death |
558 (93) 339 (56) NR |
290 (96) 188 (62) NR |
0.97 (0.93-1.00) 0.90 (0.81-1.01) NR |
|
Long term analysis2 – at the long term follow up, after crossover allowance |
||||
|
Adverse event |
Any grade ≥ Grade 3 Leading to death |
564 (94) 350 (58) 98 (16) |
292 (97) 194 (65) 68 (23) |
0.96 (0.94-1.00) 0.91 (0.81-1.01) 0.72 (0.54-0.95) |
1At the end of the trial period, after planned inclusion of 900 participants and before reveal of treatment and crossover allowance, median follow up in months not reported, n=901
2At the long term follow up, after crossover allowance, intention-to-treat analysis, median follow up 13 months in ra-223 and 9 months in placebo group, n=901
Sources: Parker, 2013 and Parker, 2018
Interim analysis
Adverse events
Adverse events were defined as any adverse event for which medical treatment was required, and determined at the interim analysis when the intended study population was admitted (n=901) (Parker, 2013).
Adverse events at any grade were common among both groups and not significantly different among groups (RR (95%CI): 0.97 (0.93-1.00). ≥ Grade 3 events were also not significantly different among groups ( RR (95%CI): 0.90 (0.81-1.01)). The authors did not report adverse events leading to death, however they did report one grade 5 hematologic adverse event was possibly related to the study drug: thrombocytopenia in a
patient in the radium-223 group, who died from pneumonia with hypoxemia without bleeding.
Long term analysis
Adverse events at long term analysis
Parker (2018) assessed the toxicity after extended follow up (median 13 and 9 months in intervention and control group respectively). They performed intention-to-treat (all patients were analyzed to the assigned groups), although data of the radium-223 + crossover group can be found in the article. A total of 901 (of 921) participants entered the safety data analysis, of which 195 (33%) in the intervention group and 134 (45%) in the placebo group did not proceed for 3-year follow up. Of patients that did proceed in the 3-year follow up, 355 (88%) in the ra-223 group and 153 (92%) in the placebo group discontinued during the three-year follow up because of death (70% in treatment group), patient request (7% in treatment group), disease progression (3% in treatment group), lost to follow up (<2%) or adverse events (<1%). These numbers were comparable in the placebo group. In total, 48 (12%) of ra-223 patients completed the three-year follow up versus 12 (7%) in the placebo group.
Adverse events at any grade were non-significantly different among groups (RR (95%CI): 0.96 (0.94-1.00) and ≥ grade 3 events were also non-significantly different (RR (95%CI): 0.91 (0.81-1.01)). The number of adverse events leading to death, however, was significantly higher in the placebo group (RR (95%CI): 0.72 (0.54-0.95). According to Parker (2018) two deaths were possibly related to treatment: one patient who received two injections died of myocardial infarction or bowel ischemia at 8w after the first injection, and one patient who received one injection died of general health deterioration and multiple organ failure at 4w after the injection.
3. Costs
Not reported.
4. Quality of life
ALSYMPCA assessed quality of life with the FACT-P scale (0-156 with higher scores indicating better quality of life).
The study reported a mean reduction at 16 weeks follow up compared to baseline of 2.6 points in the treatment group and 6.8 points in the placebo group, which are both not clinically significant. An increase of ≥10 points was regarded as a meaningful change. In the intervention group, 25% had a meaningful change versus 16% of the placebo group. This result was statistically significant in favour of the intervention but not clinically different (≥10 points).
Table 15 Change in Quality of life at 16 weeks as reported in ALSYMPCA
|
|
ALSYMPCA |
Pooled results |
|
|
|
Radium-223 (n=614) |
Placebo (n=307) |
HR (95%CI) |
|
Quality of life measured with FACT-P |
-2.7 25% ≥10 points change |
-6.8 16% ≥10 points change |
Not reported, p=0.006 |
Sources: Parker, 2013
Level of evidence of the literature
The level of evidence (GRADE method) is determined per comparison and outcome measure and is based on results from RCTs and therefore starts at level ‘high’.
The level of evidence regarding the outcome measure survival was downgraded by one level because of risk of bias (no clear reporting of the median follow up time at the first and second interim analysis and no clear reporting of long term survival outcome measures).
The level of evidence regarding the outcome measure toxicity was not further downgraded.
The outcome costs was not assessed by the included studies.
The level of evidence regarding the outcome measure quality of life was downgraded by one level because of unclear outcome measure reporting (effect measures are missing).
Conclusions
1. Survival
|
Moderate GRADE |
Radioligand therapy versus placebo probably results in an increased survival (overall survival, progression free survival and death related to prostate cancer) in patients with metastatic castration resistant prostate cancer (mCRPC).
Sources: Parker, 2013 and Parker, 2018 |
2. Toxicity
|
High GRADE |
Radioligand therapy versus placebo results in comparable adverse events of any grade in patients with mCRPC.
Sources: Parker, 2013 and Parker, 2018 |
3. Costs
|
No GRADE |
The outcome costs was not assessed by the included studies. |
4. Quality of life
|
Moderate GRADE |
Radioligand therapy versus placebo probably results in a not clinically relevant change in quality of life at follow up compared to baseline in patients with mCRPC.
Sources: Parker, 2013 and Parker, 2018 |
Zoeken en selecteren
A systematic review of the literature was performed to answer the following question: What is the effect of ARSI, in chemotherapy-naïve patients with non-metastatic and metastatic castration-resistant prostate cancer (nmCRPC and mCRPC)?
1. ARSI
1a ARSI nmCRPC
Research question: What is the effect of ARSI + ADT versus ADT alone in patients with non-metastatic castration-resistant prostate cancer (nmCRPC) as a first line treatment?
P: Chemotherapy-naïve patients with nmCRPC
I: ARSI (abiraterone, enzalutamide, apalutamide, darolutamide) combined with ADT
C: ADT alone
O: Survival, toxicity, costs, quality of life
1b ARSI mCRPC
Research question: What is the effect of ARSI + ADT versus ADT alone in patients with metastatic castration-resistant prostate cancer (mCRPC) as a first line treatment?
P: Chemotherapy-naïve patients with mCRPC
I: ARSI (abiraterone, enzalutamide, apalutamide) combined with ADT
C: ADT alone
O: Survival, toxicity, costs, quality of life
2. Chemotherapy
Research question: What is the effect of chemotherapy + ADT versus ADT alone in patients with metastatic castration-resistant prostate cancer (mCRPC) as a first line treatment?
P: Chemotherapy-naïve patients with mCRPC
I: Chemotherapy combined with ADT
C: ADT alone
O: Survival, toxicity, costs, quality of life
3. Radioligand therapy
Research question: What is the effect of radioligand therapy + ADT versus ADT alone in patients with metastatic castration-resistant prostate cancer (mCRPC) as a first line treatment?
P: Chemotherapy-naïve patients with mCRPC
I: Radioligand combined with ADT
C: ADT alone
O: Survival, toxicity, costs, quality of life
Relevant outcome measures
The guideline development group considered survival (progression free survival, metastases free survival and overall survival) as a critical outcome measure for decision making; and toxicity, costs and quality of life as important outcome measures for decision making.
The working group defined the outcome measures as follows:
- Survival was defined as overall survival (regardless of cause), metastasis-free survival (in non-metastatic patients) and progression-free survival.
- Toxicity was defined as adverse events that were more frequent in the treatment arm than in the control arm of the registration trials and could potentially be related to treatment.
- Costs was defined as reported by ‘Zorginstituut Nederland’ (if reported).
- Quality of life was defined as measured with a validated questionnaire such as FACT-P or EQ-5D and reported in the registration trials.
The working group defined the limits of clinical relevance for the crucial outcome measure ‘overall survival’; an overall survival benefit >12 weeks OR a hazard ratio ≤0.7 due to treatment, which is in line with the current PASKWIL criteria used by the ‘NVMO commissie ter Beoordeling van Oncologische Middelen (cieBOM) to evaluate palliative treatments. For the other outcomes (survival, toxicity, quality of life), the GRADE default limits ≤0.8HR/RR≥1.25 were used, unless different limits for clinical relevance were given by the included studies.
Search and select (Methods)
On April 16th, 2021 the Medline (via OVID) and Embase (via Embase.com) databases were searched using relevant search terms for systematic reviews (SRs) and randomized controlled trials (RCTs) published from 2014 onwards. The new literature (2014-2021) is added to the literature from the original module published in 2016. The detailed search strategies are depicted under the tab Methods.The literature search yielded 1172 unique hits. Studies were selected based on the following criteria:
- Systematic reviews (SRs), (network) meta analyses, or Randomized Controlled Trials (RCTs).
- Involving chemotherapy-naïve patients with non-metastatic and metastatic castration resistant prostate cancer.
- Comparing ARSI, chemotherapy or targeted therapy with ADT versus ADT alone or ADT + placebo.
- Assessing survival, toxicity, costs or quality of life in the selected studies.
- In case of multiple publications, the most recent publication was selected, or the publication which addressed an study outcome of specific interest.
For sub question 1 ARSI, 22 studies were initially selected, 17 were excluded and 5 studies were included. For sub question 1a ARSI nmCRPC 3 studies were included and sub question 1b ARSI mCRPC 2 studies were included.
For sub question 2 chemotherapy, 7 studies were initially selected and none were included.
For sub question 3 radioligand, 11 studies were initially selected and one was included.
See ‘Table of excluded studies’ for reasons of exclusion under tab Methods.
Results
6 studies were included in the analysis of the literature. Important study characteristics and results are summarized in the evidence tables. The assessment of the risk of bias is summarized in the risk of bias tables.
Referenties
- 1 - Basch E, Autio K, Ryan CJ, Mulders P, Shore N, Kheoh T, Fizazi K, Logothetis CJ, Rathkopf D, Smith MR, Mainwaring PN, Hao Y, Griffin T, Li S, Meyers ML, Molina A, Cleeland C. Abiraterone acetate plus prednisone versus prednisone alone in chemotherapy-naive men with metastatic castration-resistant prostate cancer: patient-reported outcome results of a randomised phase 3 trial. Lancet Oncol. 2013 Nov;14(12):1193-9.
- 2 - Beer TM, Armstrong AJ, Rathkopf DE, Loriot Y, Sternberg CN, Higano CS, Iversen P, Bhattacharya S, Carles J, Chowdhury S, Davis ID, de Bono JS, Evans CP, Fizazi K, Joshua AM, Kim CS, Kimura G, Mainwaring P, Mansbach H, Miller K, Noonberg SB, Perabo F, Phung D, Saad F, Scher HI, Taplin ME, Venner PM, Tombal B; PREVAIL Investigators. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014 Jul 31;371(5):424-33
- 3 - Di Nunno V, Mollica V, Santoni M, Gatto L, Schiavina R, Fiorentino M, Brunocilla E, Ardizzoni A, Massari F. New Hormonal Agents in Patients With Nonmetastatic Castration-Resistant Prostate Cancer: Meta-Analysis of Efficacy and Safety Outcomes. Clin Genitourin Cancer. 2019 Oct;17(5):e871-e877
- 4 - Fendler, Wolfgang P., et al. "Prostate-Specific Membrane Antigen Ligand Positron Emission Tomography in Men with Nonmetastatic Castration-Resistant Prostate CancerDisease Burden by PSMA-PET in nmCRPC." Clinical Cancer Research 25.24 (2019): 7448-7454.
- 5 - Fizazi K, Shore N, Tammela TL, Ulys A, Vjaters E, Polyakov S, Jievaltas M, Luz M, Alekseev B, Kuss I, Kappeler C, Snapir A, Sarapohja T, Smith MR; ARAMIS Investigators. Darolutamide in Nonmetastatic, Castration-Resistant Prostate Cancer. N Engl J Med. 2019 Mar 28;380(13):1235-1246.
- 6 - Hird AE, Magee DE, Bhindi B, Ye XY, Chandrasekar T, Goldberg H, Klotz L, Fleshner N, Satkunasivam R, Klaassen Z, Wallis CJD. A Systematic Review and Network Meta-analysis of Novel Androgen Receptor Inhibitors in Non-metastatic Castration-resistant Prostate Cancer. Clin Genitourin Cancer. 2020 Oct;18(5):343-350.
- 7 - Hoskin P, Sartor O, O'Sullivan JM, Johannessen DC, Helle SI, Logue J, Bottomley D, Nilsson S, Vogelzang NJ, Fang F, Wahba M, Aksnes AK, Parker C. Efficacy and safety of radium-223 dichloride in patients with castration-resistant prostate cancer and symptomatic bone metastases, with or without previous docetaxel use: a prespecified subgroup analysis from the randomised, double-blind, phase 3 ALSYMPCA trial. Lancet Oncol. 2014 Nov;15(12):1397-406.
- 8 - Hussain M, Fizazi K, Saad F, Rathenborg P, Shore N, Ferreira U, Ivashchenko P, Demirhan E, Modelska K, Phung D, Krivoshik A, Sternberg CN. Enzalutamide in Men with Nonmetastatic, Castration-Resistant Prostate Cancer. N Engl J Med. 2018 Jun 28;378(26):2465-2474.
- 9 - Khalaf DJ, Annala M, Taavitsainen S, Finch DL, Oja C, Vergidis J, Zulfiqar M, Sunderland K, Azad AA, Kollmannsberger CK, Eigl BJ, Noonan K, Wadhwa D, Attwell A, Keith B, Ellard SL, Le L, Gleave ME, Wyatt AW, Chi KN. Optimal sequencing of enzalutamide and abiraterone acetate plus prednisone in metastatic castration-resistant prostate cancer: a multicentre, randomised, open-label, phase 2, crossover trial. Lancet Oncol. 2019 Dec;20(12):1730-1739.
- 10 - Loriot Y, Miller K, Sternberg CN, Fizazi K, De Bono JS, Chowdhury S, Higano CS, Noonberg S, Holmstrom S, Mansbach H, Perabo FG, Phung D, Ivanescu C, Skaltsa K, Beer TM, Tombal B. Effect of enzalutamide on health-related quality of life, pain, and skeletal-related events in asymptomatic and minimally symptomatic, chemotherapy-naive patients with metastatic castration-resistant prostate cancer (PREVAIL): results from a randomised, phase 3 trial. Lancet Oncol. 2015 May;16(5):509-21.
- 11 - Morris MJ, Molina A, Small EJ, de Bono JS, Logothetis CJ, Fizazi K, de Souza P, Kantoff PW, Higano CS, Li J, Kheoh T, Larson SM, Matheny SL, Naini V, Burzykowski T, Griffin TW, Scher HI, Ryan CJ. Radiographic progression-free survival as a response biomarker in metastatic castration-resistant prostate cancer: COU-AA-302 results. J Clin Oncol. 2015 Apr 20;33(12):1356-63.
- 12 - Nome R, Hernes E, Bogsrud TV, Bjøro T, Fosså SD. Changes in prostate-specific antigen, markers of bone metabolism and bone scans after treatment with radium-223. Scand J Urol. 2015 Jun;49(3):211-7.
- 13 - Parker C, Nilsson S, Heinrich D, Helle SI, O'Sullivan JM, Fosså SD, Chodacki A, Wiechno P, Logue J, Seke M, Widmark A, Johannessen DC, Hoskin P, Bottomley D, James ND, Solberg A, Syndikus I, Kliment J, Wedel S, Boehmer S, Dall'Oglio M, Franzén L, Coleman R, Vogelzang NJ, O'Bryan-Tear CG, Staudacher K, Garcia-Vargas J, Shan M, Bruland ØS, Sartor O; ALSYMPCA Investigators. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013 Jul 18;369(3):213-23.
- 14 - Parker CC, Coleman RE, Sartor O, Vogelzang NJ, Bottomley D, Heinrich D, Helle SI, O'Sullivan JM, Fosså SD, Chodacki A, Wiechno P, Logue J, Seke M, Widmark A, Johannessen DC, Hoskin P, James ND, Solberg A, Syndikus I, Kliment J, Wedel S, Boehmer S, Dall'Oglio M, Franzén L, Bruland ØS, Petrenciuc O, Staudacher K, Li R, Nilsson S. Three-year Safety of Radium-223 Dichloride in Patients with Castration-resistant Prostate Cancer and Symptomatic Bone Metastases from Phase 3 Randomized Alpharadin in Symptomatic Prostate Cancer Trial. Eur Urol. 2018 Mar;73(3):427-435.
- 15 - Rathkopf DE, Smith MR, de Bono JS, Logothetis CJ, Shore ND, de Souza P, Fizazi K, Mulders PF, Mainwaring P, Hainsworth JD, Beer TM, North S, Fradet Y, Van Poppel H, Carles J, Flaig TW, Efstathiou E, Yu EY, Higano CS, Taplin ME, Griffin TW, Todd MB, Yu MK, Park YC, Kheoh T, Small EJ, Scher HI, Molina A, Ryan CJ, Saad F. Updated interim efficacy analysis and long-term safety of abiraterone acetate in metastatic castration-resistant prostate cancer patients without prior chemotherapy (COU-AA-302). Eur Urol. 2014 Nov;66(5):815-25.
- 16 - Rodriguez-Vida A, Rodríguez-Alonso A, Useros-Rodríguez E, et al. Impact of New Systemic Therapies in Overall Survival in Non-Metastatic Castration Resistant Prostate Cancer: Systematic Review and Meta-Analysis. Clin Genitourin Cancer. 2022;20(2):197.e1-197.e10.
- 17 - Ryan CJ, Smith MR, de Bono JS, Molina A, Logothetis CJ, de Souza P, Fizazi K, Mainwaring P, Piulats JM, Ng S, Carles J, Mulders PF, Basch E, Small EJ, Saad F, Schrijvers D, Van Poppel H, Mukherjee SD, Suttmann H, Gerritsen WR, Flaig TW, George DJ, Yu EY, Efstathiou E, Pantuck A, Winquist E, Higano CS, Taplin ME, Park Y, Kheoh T, Griffin T, Scher HI, Rathkopf DE; COU-AA-302 Investigators. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013 Jan 10;368(2):138-48
- 18 - Ryan CJ, Smith MR, Fizazi K, Saad F, Mulders PF, Sternberg CN, Miller K, Logothetis CJ, Shore ND, Small EJ, Carles J, Flaig TW, Taplin ME, Higano CS, de Souza P, de Bono JS, Griffin TW, De Porre P, Yu MK, Park YC, Li J, Kheoh T, Naini V, Molina A, Rathkopf DE; COU-AA-302 Investigators. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2015 Feb;16(2):152-60.
- 19 - Sartor O, Coleman R, Nilsson S, Heinrich D, Helle SI, O'Sullivan JM, Fosså SD, Chodacki A, Wiechno P, Logue J, Widmark A, Johannessen DC, Hoskin P, James ND, Solberg A, Syndikus I, Vogelzang NJ, O'Bryan-Tear CG, Shan M, Bruland ØS, Parker C. Effect of radium-223 dichloride on symptomatic skeletal events in patients with castration-resistant prostate cancer and bone metastases: results from a phase 3, double-blind, randomised trial. Lancet Oncol. 2014 Jun;15(7):738-46.
- 20 - Sartor O, Coleman RE, Nilsson S, Heinrich D, Helle SI, O'Sullivan JM, Vogelzang NJ, Bruland Ø, Kobina S, Wilhelm S, Xu L, Shan M, Kattan MW, Parker C. An exploratory analysis of alkaline phosphatase, lactate dehydrogenase, and prostate-specific antigen dynamics in the phase 3 ALSYMPCA trial with radium-223. Ann Oncol. 2017 May 1;28(5):1090-1097
- 21 - Small EJ, Saad F, Chowdhury S, Oudard S, Hadaschik BA, Graff JN, Olmos D, Mainwaring PN, Lee JY, Uemura H, De Porre P, Smith AA, Zhang K, Lopez-Gitlitz A, Smith MR. Apalutamide and overall survival in non-metastatic castration-resistant prostate cancer. Ann Oncol. 2019 Nov 1;30(11):1813-1820.
- 22 - Smith MR, Saad F, Chowdhury S, Oudard S, Hadaschik BA, Graff JN, Olmos D, Mainwaring PN, Lee JY, Uemura H, Lopez-Gitlitz A, Trudel GC, Espina BM, Shu Y, Park YC, Rackoff WR, Yu MK, Small EJ; SPARTAN Investigators. Apalutamide Treatment and Metastasis-free Survival in Prostate Cancer. N Engl J Med. 2018 Apr 12;378(15):1408-1418.
- 23 - Smith MR, Saad F, Chowdhury S, Oudard S, Hadaschik BA, Graff JN, Olmos D, Mainwaring PN, Lee JY, Uemura H, De Porre P, Smith AA, Brookman-May SD, Li S, Zhang K, Rooney B, Lopez-Gitlitz A, Small EJ. Apalutamide and Overall Survival in Prostate Cancer. Eur Urol. 2021 Jan;79(1):150-158.
- 24 - Sopeña Sutil R, Silva Ruiz J, Garcia Gomez B, Romero-Otero J, Garcia-Gonzalez L, Duarte Ojeda JM, de Velasco G, Castellano Gauna D, Rodriguez Antolin A. Seizures and Neuropsychiatric Toxicity in Patients with Non-Metastatic CRPC Treated with New Antiandrogens: Systematic Review and Meta-Analysis. Oncol Res Treat. 2021;44(4):154-163.
- 25 - Sternberg CN, Fizazi K, Saad F, Shore ND, De Giorgi U, Penson DF, Ferreira U, Efstathiou E, Madziarska K, Kolinsky MP, Cubero DIG, Noerby B, Zohren F, Lin X, Modelska K, Sugg J, Steinberg J, Hussain M; PROSPER Investigators. Enzalutamide and Survival in Nonmetastatic, Castration-Resistant Prostate Cancer. N Engl J Med. 2020 Jun 4;382(23):2197-2206.
- 26 - Tombal B, Sternberg CN, Hussain M, et al. Matching-adjusted indirect treatment comparison of the efficacy of enzalutamide versus apalutamide for the treatment of nonmetastatic castration-resistant prostate cancer. ESMO Open. 2022;7(3):100510.
- 27 - Wenzel M, Nocera L, Collà Ruvolo C, et al. Overall survival and adverse events after treatment with darolutamide vs. apalutamide vs. enzalutamide for high-risk non-metastatic castration-resistant prostate cancer: a systematic review and network meta-analysis [published correction appears in Prostate Cancer Prostatic Dis. 2023 Mar 10;:]. Prostate Cancer Prostatic Dis. 2022;25(2):139-148.
Evidence tabellen
1a ARSI nmCRPC
Research question: What is the effect of ARSI + ADT versus ADT alone in patients with non-metastatic castration-resistant prostate cancer (nmCRPC)?
1b ARSI mCRPC
Research question: What is the effect of ARSI + ADT versus ADT alone in patients with metastatic castration-resistant prostate cancer (mCRPC)?
|
Study reference |
Study characteristics |
Patient characteristics |
Intervention (I) |
Comparison / control (C)
|
Follow-up |
Outcome measures and effect size |
Comments |
|
COU-AA-302
Publications: Basch, 2013; Morris, 2015; Rathkopf, 2014; Ryan, 2013 and Ryan, 2015 |
Type of study: RCT
Setting and country: 151 centers in 12 countries
Funding and conflicts of interest: data assessors were independent. Source of funding was Ortho Biotech Oncology Research & Development (unit of Cougar Biotechnology). |
Inclusion criteria: - age >18yo, - metastatic, histologically or cytologically confirmed adenocarcinoma of the prostate - previous ADT therapy - furthermore see article Ryan (2013).
Exclusion criteria: - visceral metastases - previous therapy with ketoconazole lasting more than 7 days
N total at baseline: Intervention: 546 Control: 542
Important prognostic factors: Groups were comparable at baseline. |
Describe intervention (treatment/procedure/test): Abiraterone at a dose of 1 g (administered as four 250-mg tablets) + prednisone 5 mg orally twice daily
|
Describe control (treatment/procedure/test): four placebo tablets once daily at least 1 hour before and 2 hours after a meal + prednisone 5 mg orally twice daily
|
Length of follow-up: bone scanning were performed every 8 weeks during the first 24 weeks and every 12 weeks thereafter. Patient-reported outcomes were assessed at baseline and at every visit with the use of the BPISF. FACT-P questionnaires were completed every third visit. The median follow-up duration for all patients was 22.2 months (Ryan, 2013). Long term median follow up of 49.2 months (Ryan, 2015).
Loss-to-follow-up: Intervention, N (%): 4 Reasons (describe): did not receive study drug.
Control, N (%): 2 Reasons (describe): did not receive study drug. These were not included in toxicity analysis, but were included in the survival analysis.
Incomplete outcome data: Intervention, N (%): 0
Control, N (%): 0
|
Outcome measures and effect size (include 95%CI and p-value if available):
1. Survival
Interim analysis
Number of deaths regardless of cause during trial period. I: 147 deaths C: 186 deaths HR (95%CI): 0.75 (0.61-0.93) (Morris, 2015)
1.2 Progression free survival Time from random assignment until first occurrence of progression by bone scan at median follow up of 27 months. I: 16.5 months C: 8.3 months HR (95%CI): 0.53 (0.45-0.62) (Beer, 2014 and Morris, 2015)
Long term analysis
1.3 Death related to prostate cancer Not reported.
1.4 Long term survival Overall death over long term follow up of median 49 months. I: 352/546 (65%) C: 387/542 (71%) HR (95%CI): 0.81 (0.70–0.93)
2. Toxicity
Interim analysis
Adverse event, grade 3-4 I: 258 (48) C: 225 (42) RR (95%CI): NR
Long term analysis Adverse event, grade 3-4 I: 290 (54%) C: 236 (44%) RR (95%CI): NR
3. Costs Not reported
4. Quality of life Number of months until a decline of >10 points occurred in FACT-P score I: 12.7 mo. C: 8.3 mo. HR (95%CI): 0.78 (0.66–0.92) |
Authors’ conclusion: “In summary, the results show benefit from the use of abiraterone in patients with asymptomatic or mildly symptomatic metastatic castration-resistant prostate cancer who have not received previous chemotherapy. These findings include increased rates of radiographic progression-free survival and overall survival, as well as clinically meaningful secondary end points, such as delays in the use of opiates for pain and chemotherapy and patient-reported outcomes related to health-related quality of life.”
|
|
PREVAIL
Publications: Beer, 2014 and Loriot, 2015 |
Type of study: RCT
Setting and country: 180 centers worldwide (exact locations not mentioned)
Funding and conflicts of interest: Funded by Medivation and Astellas Pharma. Disclosures found in the Appendix. “Dr. Armstrong reports grant support from Medivation/Astellas, Janssen, Sanofi Aventis, Dendreon, and Bayer during the conduct of the study, and personal fees from Medivation/Astellas, Janssen, Dendreon, Sanofi Aventis, and Bayer outside the submitted work.”
|
Inclusion criteria: - histologically confirmed adenocarcinoma of the prostate - documented metastases and PSA progression - despite receiving LHRH therapy or orchiectomy with serum level testosterone (<1.73 nmol/L) or less. - Concomitant ADT. - Additional inclusion criteria in article of Beer (2014).
Exclusion criteria: - cytotoxic chemotherapy before - visceral metastases - heart failure N total at baseline: Intervention: 832 Control: 801
Important prognostic factors: Groups were comparable at baseline. |
Describe intervention (treatment/procedure/test): oral enzalutamide (at a dose of 160 mg) once daily
|
Describe control (treatment/procedure/test): placebo once daily with or without food
|
Length of follow-up: Imaging was performed at the time of screening, at weeks 9, 17, and 25, and every 12 weeks thereafter. Total follow up was median 12 months for radiographic progression and median 22 months for overall survival outcomes.
Loss-to-follow-up: Intervention, N (%): 1 Reasons (describe): lost to follow up.
Control, N (%): 0
Incomplete outcome data: Intervention, N (%): 0 Reasons (describe): all participants except for one lost to follow in the intervention group were included in the safety (toxicity) analysis.
Control, N (%): 0 Reasons (describe): all participants were included in safety analysis.
|
Outcome measures and effect size (include 95%CI and p-value if available):
1. Survival
Interim analysis
Number of deaths regardless of cause during trial period. I: 241/872 (28%) C: 299/845 (35%) HR (95%CI): 0.71 (0.60-0.84)
1.2 Progression free survival Rate of radiographic progression at 12 months follow up. I: 65% C: 14% HR (95%CI): 0.19 (0.15-0.23)
Long term analysis No long term follow up performed.
2. Toxicity
Interim analysis
Adverse event, grade 3-4 I: 374 (43) C: 313 (37) RR (95%CI) 1.08 (0.93-1.25)
Long term analysis No long term follow up performed.
3. Costs Not reported.
4. Quality of life Number of months until decline of 10 points or more occurred on FACT-P total score. I: 11.3 mo. C: 5.6 mo. HR (95%CI): 0.63, confidence interval not reported. P<0.001 |
Authors’ conclusion: “In conclusion, in men with minimally symptomatic or asymptomatic metastatic prostate cancer who had not received chemotherapy, enzalutamide, an oral therapy with an excellent side-effect profile, significantly delayed radiographic disease progression or death, the need for cytotoxic chemotherapy, and the deterioration in quality of life and significantly improved overall survival.”
|
2 Chemotherapy
No evidence tables.
3 Radioligand therapy
Research question: What is the effect of radioligand therapy + ADT versus ADT alone in patients with metastatic castration-resistant prostate cancer (mCRPC)?
|
Study reference |
Study characteristics |
Patient characteristics |
Intervention (I) |
Comparison / control (C)
|
Follow-up |
Outcome measures and effect size |
Comments |
|
ALSYMPCA
Publications: Hoskin, 2014; Nome, 2015; Parker, 2013 and Parker, 2016 & Sartor, 2014 and Sartor, 2017. |
Type of study: RCT
Setting and country: 136 study centers in 19 countries
Funding and conflicts of interest: Funded by Algeta and Bayer HealthCare Pharmaceuticals. Conflicts of interest and disclosure forms are attached to online version of the article (Parker, 2013). No potential conflicts of interest relevant to this article. |
Inclusion criteria: - histologically confirmed, progressive castration-resistant prostate cancer - with two or more bone metastases detected on skeletal scintigraphy - no known visceral metastases - receiving the best standard of care - had received docetaxel - more see article Parker (2013).
Exclusion criteria: - received chemotherapy within the previous 4 weeks - had not recovered from adverse events due to chemotherapy. - more see Parker (2013)
N total at baseline: Intervention: 614 Control: 307
Important prognostic factors: Groups comparable at baseline (see Parker, 2013)
|
Describe intervention (treatment/procedure/test): six intravenous injections of radium-223 (at a dose of 50 kBq per kilogram of body weight), administered every four weeks. Randomized 2:1 ratio.
|
Describe control (treatment/procedure/test): A matching placebo injection, six injections administered every four weeks.
|
Length of follow-up: 3 years
Loss-to-follow-up: Intervention, N (%): 13 (2) Reasons (describe): withdrawal before first injection, not included in safety (toxicity) analysis
Control, N (%): 5 (2) Reasons (describe): withdrawal before first injection, not included in safety (toxicity) analysis
Incomplete outcome data: Intervention, N (%): 2 (0.2) Reasons (describe): “Two patients received no treatment and had missing dates of withdrawal.”
Control, N (%): 0
|
Outcome measures and effect size (include 95%CI and p-value if available):
1. Survival
Interim analysis
The time from randomization until the date of death, regardless of the cause. I: 14.9 mo. C: 11.3 mo. HR (95%CI): 0.70 (0.58-0.83)
1.2 Progression free survival Time since randomization until first skeletal event. I: 15.6 mo. C: 9.8 mo. HR (95%CI): 0.66 (0.52-0.83)
Long term analysis
1.3 Death related to prostate cancer Not reported.
1.4 Overall survival long term Not reported.
2. Toxicity
Interim analysis
Adverse event, grade 3-4 I: 339 (56) C: 188 (62) RR (95%CI): 0.90 (0.81-1.01)
Long term analysis
Adverse event, grade 3-4 I: 350 (58) C: 194 (65) RR (95%CI): 0.91 (0.81-1.01)
3. Costs Not reported.
4. Quality of life Mean reduction on FACT-P score (0-156 with higher scores indicating better QoL) from baseline to 16 week follow up. I: -2.7 C: -6.8 HR (95%CI): not reported, p<0.001 |
Authors’ conclusion: “This updated final long-term safety follow-up ALSYMPCA analysis shows that radium-223 is well tolerated in CRPC patients with symptomatic bone metastases, with minimal nonhematologic AEs, a low incidence of myelosuppression with long-term preservation of hematopoietic function, and no new safety signals.” (Parker, 2018) |
Risk of bias tables
1a ARSI nmCRPC
Research question: What is the effect of ARSI + ADT versus ADT alone in patients with non-metastatic castration-resistant prostate cancer (nmCRPC)?
|
Study reference
(first author, publication year) |
Was the allocation sequence adequately generated?
|
Was the allocation adequately concealed?
|
Blinding: Was knowledge of the allocated interventions adequately prevented?
Were patients blinded?
Were healthcare providers blinded?
Were data collectors blinded?
Were outcome assessors blinded?
Were data analysts blinded? |
Was loss to follow-up (missing outcome data) infrequent?
|
Are reports of the study free of selective outcome reporting?
|
Was the study apparently free of other problems that could put it at a risk of bias?
|
Overall risk of bias
|
|
ARAMIS Publication: Fizazi, 2019 |
Low risk
|
Low risk
|
Low risk
|
Low risk
|
Low risk
|
Low risk
|
LOW
|
|
SPARTAN Publications: Smith 2018, Small 2019 and Smith 2021 |
Low risk
|
Low risk
|
Low risk
|
Low risk
|
Low risk
|
Low risk
|
LOW
|
|
PROSPER Publications: Hussain, 2018 and Sternberg 2020 |
Low risk
|
Unclear risk |
Low risk
|
Low risk
|
Low risk
|
Low risk
|
LOW
|
Data derives from systematic review and meta-analysis by Sopeña Sutil e.a. (2021)
1b ARSI mCRPC
Research question: What is the effect of ARSI + ADT versus ADT alone in patients with metastatic castration-resistant prostate cancer (mCRPC)?
|
Study reference
(first author, publication year) |
Was the allocation sequence adequately generated?
|
Was the allocation adequately concealed?
|
Blinding: Was knowledge of the allocated interventions adequately prevented?
Were patients blinded?
Were healthcare providers blinded?
Were data collectors blinded?
Were outcome assessors blinded?
Were data analysts blinded? |
Was loss to follow-up (missing outcome data) infrequent?
|
Are reports of the study free of selective outcome reporting?
|
Was the study apparently free of other problems that could put it at a risk of bias?
|
Overall risk of bias
|
|
COU-AA-302 Publications: Basch, 2013; Morris, 2015; Rathkopf, 2014; Ryan, 2013 and Ryan, 2015 |
Low risk
|
Low risk
|
Low risk
|
Low risk
|
Low risk
|
Low risk
|
LOW
|
|
PREVAIL Publications: Beer, 2014 and Loriot, 2015 |
Low risk
|
Low risk
|
Low risk
|
Low risk
|
Low risk
|
Low risk
|
LOW
|
N.B. Also based on previous module’s assessment in 2016
2 Chemotherapy
No risk of bias tables.
3 Radioligand therapy
Research question: What is the effect of radioligand therapy + ADT versus ADT alone in patients with metastatic castration-resistant prostate cancer (mCRPC)?
|
Study reference
(first author, publication year) |
Was the allocation sequence adequately generated?
|
Was the allocation adequately concealed?
|
Blinding: Was knowledge of the allocated interventions adequately prevented?
Were patients blinded?
Were healthcare providers blinded?
Were data collectors blinded?
Were outcome assessors blinded?
Were data analysts blinded? |
Was loss to follow-up (missing outcome data) infrequent?
|
Are reports of the study free of selective outcome reporting?
|
Was the study apparently free of other problems that could put it at a risk of bias?
|
Overall risk of bias
|
|
ALSYMPCA Publications: Hoskin, 2014; Nome, 2015; Parker, 2013 and Parker, 2016 & Sartor, 2014 and Sartor, 2017 |
Low risk
|
Low risk
|
Low risk
|
Low risk
|
Low risk
|
Unclear reporting, results are not reported in table of supplementary table, only in text and the total number of included patients per group for this outcome measure was not reported. |
LOW
|
N.B. Also based on previous module’s assessment in 2016
Table of excluded studies
1. ARSI
|
Reference |
Reason for exclusion |
|
Chi, 2015 |
Wrong population, patients progressing after abiraterone or enzalutamide. |
|
Chowdhury, 2020 |
Wrong analysis, matching-adjusted indirect comparison (MAIC) by weighting patients from SPARTAN study to baseline characteristics of PROSPER. |
|
de Bono, 2011 (CIE-BOM literature) |
Wrong population, patients progressing after chemotherapy. N.B. COU-AA-301 trial. |
|
Fang, 2017 |
Wrong comparison, abiraterone and enzalutamide were compared. |
|
Gracia, 2016 |
Wrong comparison; comparison of hypertension, neurological and psychiatric adverse events on enzalutamide and abiraterone acetate plus prednisone. Only abstract available. |
|
Kang, 2017 |
Wrong comparison, no control group with ADT, but with placebo or prednisone? |
|
Kumar, 2020 |
A more recent meta-analysis and more extensive publication is available by Hird (2020). |
|
Meunier, 2021 |
Wrong population, black population. |
|
Mori, 2020 |
A more recent version of this meta-analysis is available by Wenzel (2021), however the study by Wenzel did not consider different follow up times. Therefore, the meta-analysis of Hird (2020) was chosen. |
|
Roviello, 2016 "Role of the novel…" |
More recent SRs are available. This study uses the trials: AFFIRM, COU-AA-301, COU-AA-302, ELM-PC 4, ELM-PC 5, PREVAIL. The study included both patients naive to chemotherapy and patients progressing after chemotherapy. Subgroup analyses are unclear. |
|
Roviello, 2016 "Incidence and relative.." |
Wrong comparison, CYP-17 inhibitors and prednisone versus placebo and prednisone |
|
Roviello, 2020 |
More recent studies and SRs available. Study uses the JADAD-5-item scale for assessment, while others use the Cochrane tool or GRADE assessment. The more extensive meta-analysis of Hird (2020) was chosen. |
|
Scher, 2012 (CIE-BOM literature) |
Wrong population, patients progressing after chemotherapy. N.B. AFFIRM trial. |
|
Ternov, 2021 |
Wrong comparison, enzalutamide versus AAP for mCRPC. |
|
Wang, 2021 |
Wrong population, men with mCRPC in real world practice, inclusion of cohort studies with patients with mCRPC. Comparison abiraterone versus enzalutamide |
|
Wenzel, 2021 |
Wrong analysis. The study is an extension of Mori (2020) and included the most recent publications of SPARTAN and PROSPER, however the study did not analyse for different follow up times between studies. The goal of the study was to compare the three medicines. |
|
Zhang, 2017 |
Wrong comparison, indirect comparison between abiraterone acetate and enzalutamide |
2. Chemotherapy
|
Reference |
Reason for exclusion |
|
Berthold, 2008 |
Wrong comparison: no control group without chemotherapy or ADT alone |
|
de Bono, 2010 |
Wrong comparison: no control group without chemotherapy, patients progressing after docetaxel treatment |
|
de Bono, 2020 |
Wrong comparison: no control group without chemotherapy, patients progressing after enzalutamide or abiraterone treatment |
|
Leal, 2019 |
Wrong comparison: only two studies from 1995/1996 with control arm endocrine therapy only |
|
Petrylak, 2004 |
Wrong comparison: no control group without chemotherapy or ADT alone |
|
Song, 2018 |
Wrong comparison: no control arm with ADT only |
|
Tannock, 2004 |
Wrong comparison: no control group without chemotherapy or ADT alone |
2. Radioligand therapy
|
Reference |
Reason for exclusion |
|
de Bono, 2016 (CIE-BOM) |
Wrong population; patients progressing after chemotherapy. N.B. VISION trial. |
|
Dizdarevic, 2020 |
20 articles about prostate cancer (of which many articles about 1 RCT (ALSYMPCA) and the others about phase I and II studies) |
|
McGann, 2015 |
|
|
Nilsson, 2007 |
Wrong population; patients progressing after chemotherapy. N.B. Included in module of 2016. |
|
Nilsson, 2013 |
Wrong population; patients progressing after chemotherapy. N.B. Included in module of 2016. |
|
Sadaghiani, 2021 |
Does not comply to PICO |
|
Satapathy, 2021 |
|
|
Sartor, 2021 (CIE-BOM) |
Wrong population; patients progressing after chemotherapy. N.B. VISION trial. |
|
Terrisse, 2020 |
Control group no ADT. Reports overall survival and symptomatic skeletal event-free survival for alpha-emitting and beta-emitting radio-isotopes. |
|
von Eyben, 2020 |
No control group with ADT? No RCTs? |
Verantwoording
Beoordelingsdatum en geldigheid
Publicatiedatum : 22-12-2023
Beoordeeld op geldigheid : 22-12-2023
Bij het opstellen van de module heeft de werkgroep een inschatting gemaakt over de maximale termijn waarop herbeoordeling moet plaatsvinden en eventuele aandachtspunten geformuleerd die van belang zijn bij een toekomstige herziening (update). De geldigheid van de richtlijnmodule komt eerder te vervallen indien nieuwe ontwikkelingen aanleiding zijn een herzieningstraject te starten.
Algemene gegevens
De richtlijnontwikkeling werd ondersteund door het Kennisinstituut van de Federatie Medisch Specialisten (www.kennisinstituut.nl) en werd gefinancierd uit de Stichting Kwaliteitsgelden Medisch Specialisten (SKMS). Patiënten participatie bij deze richtlijn werd medegefinancierd uit de Stichting Kwaliteitsgelden Patiënten Consumenten (SKPC) binnen het programma KIDZ. De financier heeft geen enkele invloed gehad op de inhoud van de richtlijn.
Samenstelling werkgroep
Werkgroep
- Prof. dr. R.J.A. van Moorselaar, uroloog, werkzaam in het Amsterdam Universitair Medische Centra, locatie VUmc, NVU, voorzitter
- Prof. dr. J.O. Barentsz, radioloog, werkzaam in het RadboudUMC, NVvR
- Drs. R.J. van Alphen, internist-oncoloog werkzaam in het ETZ
- Drs. R. van der Giessen, patiënten vertegenwoordiger, Prostaatkankerstichting
- Prof. dr. L. Incrocci, radiotherapeut-oncoloog, werkzaam in het Erasmus MC, NVRO
- Dr. M.J.R. Janssen, nucleair geneeskundige, werkzaam in het RadboudUMC, NVvN
- Dr. G.J.L.H. van Leenders, patholoog, werkzaam in het Erasmus Medisch Centrum, NVvP
- Dr. I.M. van Oort, uroloog, werkzaam bij het RadboudUMC, NVU
- Dr. I. Schoots, radioloog, werkzaam bij het Erasmus MC, NVvR
- Dr. D.M. Somford, uroloog, werkzaam bij CWZ Nijmegen, NVU
- Dr. Y. Reisman, uroloog, seksuoloog NVVS, Flare-Health, Amstelveen, NVVS
- Drs. I. Zantingh, gz-psycholoog – seksuoloog NVVS, werkzaam bij Antoni van Leeuwenhoek, NVVS
- C.N. Tillier, verpleegkundig specialist urologie, werkzaam bij Antoni van Leeuwenhoek, V&VN
- Drs. H.A.M. Vanhauten, werkzaam bij het UMCG, radiotherapeut-oncoloog. NVRO
- Dr. E. Vegt, nucleair geneeskundige, werkzaam in het Erasmus MC, NVNG
- Dr. M. Tascilar, internist-oncoloog, werkzaam in Isala, NIV
- Dr. A. Bergman, internist-oncoloog werkzaam bij Antoni van Leeuwenhoek, NIV
Met ondersteuning van:
- Dr. I. Mostovaya, senior adviseur, Kennisinstituut van de Federatie Medisch Specialisten
- Dr. J. Boschman, senior adviseur, Kennisinstituut van de Federatie Medisch Specialisten
- Dr. M. van Son, adviseur, Kennisinstituut van de Federatie Medisch Specialisten
Belangenverklaringen
De KNMG-code ter voorkoming van oneigenlijke beïnvloeding door belangenverstrengeling is gevolgd. Alle werkgroepleden hebben schriftelijk verklaard of zij in de laatste drie jaar directe financiële belangen (betrekking bij een commercieel bedrijf, persoonlijke financiële belangen, onderzoeksfinanciering) of indirecte belangen (persoonlijke relaties, reputatiemanagement, kennisvalorisatie) hebben gehad. Een overzicht van de belangen van werkgroepleden en het oordeel over het omgaan met eventuele belangen vindt u in onderstaande tabel. De ondertekende belangenverklaringen zijn op te vragen bij het secretariaat van het Kennisinstituut van de Federatie Medisch Specialisten.
|
Werkgroeplid |
Functie |
Nevenfuncties |
Gemelde belangen |
Ondernomen actie |
|
* Van Moorselaar |
Uroloog, Amsterdam UMC |
Advisory board: Astellas, AstraZeneca Bayer, Janssen, Sanofi-Genzyme: Betaald Incoming president European Board of Urology: onbetaald Board member European School of Urology: onbetaald |
geen |
Geen actie. In transparantieregister diverse keren honoraria genoemd voor Astellas, AstraZeneca Bayer, Janssen, Sanofi-Genzyme (2018-2020). Bij herziening RL 2017 zat Jeroen ook al in deze adviescommisies (alleen AstraZeneca is erbij gekomen) |
|
Reisman |
Directeur en Seksuoloog Flare-Health, Amstelveen (0.5 fte) Hoofdopleider Seksuologie, RINO Amsterdam (zzp contract) |
Past-President European Society for Sexual Medicine (ESSM) - deelnemen aan het bestuur
Bestuurlid en Past-President Multidisciplinary Joint Committee for Sexual Medicine of the European Union for Medical Specialists (UEMS)
Co-director ESSM School and Advanced Course of Sexual Medicine
Voorzitter network workinggroup HPV information and education van European Cancer Organisation (ECCO)
Honorary Professor of Urology, Federal State Institute of Urology, Moscow
Visiting Professor of Andrology, Shanghai Jiao Tong University, China
Allemaal vrijwillig |
Adviseur Besins Health Care Spreker: Lundbeck, Lilly, Coloplast en Berlin-Chemie
Expertise op gebied van Onco-Seksuologie Editor "Cancer, Intimacy and Sexuality" |
Geen actie. |
|
Tillier |
Verpleegkundig Specialist Urologie NKI-AVL, Amsterdam |
EAUN Chair Elect (onbetaald) Consultant IPSEN bij het schrijven van informiatie boekje voor patienten over hormonale behandeling bij prostaatkanker |
Geen. |
Geen actie. |
|
Schoots |
Radioloog, Radiologie & Nucleair Geneeskunde, Erasmus MC (0.8 fte betaald) |
Research appointment, Radiologie, AVL-NKI (0.2 fte betaald) |
Advies functie - Quantib NV: radiologische software als hulp voor radiologische beoordeling van prostaat MRI - (betaald - aan werkgever Erasmus MC) |
Geen actie. De UV's die we updaten hebben geen betrekking op de prostaat MRI. |
|
Van der Giessen |
Lid Kwaliteits Groep Prostaatkanker Stichting (vrijwilliger) Lid Overleggroep NWO (Erasmus- vrijwilliger)) |
Geen. |
Geen. |
Geen actie. |
|
Vegt |
Nucleair geneeskundige - Erasmus MC Rotterdam |
Geen. |
Deelname aan de studie "ROTOR registry'' naar uitkomsten van Ra-223-therapie bj prostaatcarnicoom, gefinancieerd door Bayer. |
ROTOR registry heeft mogelijk enige betrekking op UV1 hormoongevoelig prostaatcarcinoom. Werkgroeplid is niet als trekker/meelezer betrokken bij deze UV. |
|
Van Oort |
Oncologisch Uroloog, Radboudumc Nijmegen |
Geen. |
adviseurschap bij Astellas, Sanofi, Janssen, Roche, Bayer
gefinancieerd onderzoek door: Astellas, Janssen, Bayer
Radboudumc heeft de SelectMdx urinetest ontwikkeld, eigendom ligt nu extern |
Geen actie. In transparantieregister diverse keren honoraria genoemd voor Astellas, Bayer, Janssen, Sanofi-Genzyme (2018-2020). Bij herziening RL 2017 zat Inge ook in deze adviescommisies. Onderzoek gefinancierd door de industrie (was bij 2017 werkgroep ook zo). |
|
Incrocci |
Radiotherapeut-oncoloog, Erasmus MC, Rotterdam |
Geen. |
Geen. |
Geen actie. |
|
Barentsz |
Hoogleraar Radiologie Radboudumc |
Geen. |
onbetaald adviseur van SPL Medical en Soteria Medical |
Geen actie. |
|
Bergman |
Internist-Oncoloog, Nederlands Kanker Instituut, Antoni van Leeuwenhoek |
'Bezoldigde sprekers/organisatie vergoedingen van Bayer, Astellas, Sanofi |
Participatie Advisory boards: Astelas, Jansen, Bayer, Sanofi.
Financiering Investigator Initiated Studies door: Astellas, Sanofi, Bayer, Amgen Participatie industry sponsored Studies van: Merck, Astellas, Jansen, Bayer, Astra Zeneca.
Bestuurslid Dutch Uro Oncology Study Group. |
Geen actie. Vergoedingen industrie en onderzoek gefinancierd door industrie |
|
Vanhauten |
Radiotherapeut oncoloog UMCG |
Bestuurslid Prostaatcentrum Noord-Nederland, onbetaald. Enkele malen per jaar lezing/panellid Astellas, Jansen, Prevents waarvoor sprekersvergoeding. |
Geen. |
Geen actie. |
|
Tascilar |
Internist-Oncoloog |
Commissie COM (NVMO), aanvragen van off label indicaties voor oncologische middelen, vacatiegelden Lid cieBOM, vacatiegelden Bestuurslid DRCG, onbetaald lid Raad van Advies Prostaatkankerstichting, onbetaald DUOS werkgroep gemetastaseerd prostaatcarcinoom, onbetaald |
Geen. |
Geen actie. |
|
Mensink |
VrÍjwilliger, Belangenbehartiger Kwliteit van Zorg bij Prostaatkankerstichting |
Geen. |
Geen. |
Geen actie. |
|
Janssen |
nucleair geneeskundige Radboudumc |
Voorzitter onderwijscommissie NVNG (onbetaald) lid onderwijscommissie NVvR (onbetaald lid commissie wetenschappelijke ontmoetingen NVNG docent landelijk differentiantenonderwijs nucleaire radiologie (onkostenvergoeding) |
PROPER-ABX studie, vergelijkende studie waarin Axumin en PSMA-1007 woren vergeleken qua diagnostische waarbij bij patienten met biochemische recidief prostaatcarcinoom, gefinancieerd door ABX. |
Geen actie. De UV's die we updaten hebben geen betrekking op Axumin en PSMA-1007. |
|
Van Alphen |
internist oncoloog bij ETZ te Tilburg fulltime bestuurslid NVMO 3 uur per week (betaald aan het MSB) |
Bestuurslid NVMO vacatie gelden 3 uur per week aan MSB NIV platform kwaliteit 4 avonden per jaar vacatie -> MSB Richtlijnen commissie NVMO 2 uur per maand vacatie -> MSB Richtlijnen commissie NIV 1 uur per maand vacatie -> MSB Commissie kwaliteit NVMO: 1 uur per maand vacatie MSB Inval vanuit NIV voor module hormoonsensitief prostaat carcinoom. |
ja, Studies met Pfizer, Bayer, Astellas, eigenlijk alle firma's die met prostaatkanker werken. Ik ken geen oncoloog in een groter perifeer ziekenhuis of academie die hier nee kan invullen. Hooguit neutraal als je voor alle firma's studies hebt lopen. |
Geen actie??? |
|
Zantingh |
gz-psycholoog / seksuoloog NVVS Antoni van Leeuwenhoek Centrum kwaliteit van leven
docent cursus Seksuologie in de GZ opleiding RINO Amsterdam
eigenaar / praktijkhouder Seksuologie Praktijk Utrecht |
onbetaald lid mediacommissie NVVS
onbetaald voorzitter NVVS Special Interest Group onco-seksuologie |
Geen. |
Geen actie. |
|
Van Leenders |
Patholoog, Erasmus MC, Rotterdam |
Bestuurslid Nederlandse Expertgroep Urologische Pathologie (NEUP), European Network of Uropathology (ENUP), International Society of Urological Pathology (ISUP)
Wetenschappelijke raad ProstaatKankerStichting (PKS) en stichting Egidius
Lidmaatschap EAU guideline committee prostate cancer, en International Collaboration on Cancer Reporting (ICCR) prostate cancer |
Geen |
Geen actie. |
|
Somford |
Uroloog CWZ en Prosper (1.0 FTE)
Uroloog Radboudumc (detachering) |
Voorzitter Werkgroep Oncologische Urologie (WOU) van de NVU
Voorzitter Clinical Audit Board (CAB) Multidisciplinaire Kwaliteitstregistratie Prostaakanker (DICA)
Voorzitter Lokale Toetsingscommissie CWZ
Lid Wetenschappelijke Commissie (NVU)
Associate Editor Frontiers in Oncology |
Research Grants (CWZ): KWF, Besins Health Care
Contracted research (CWZ): Janssen, Eli Lilly, Astellas, Blue Earth Diagnostics, Bayer, SPL Medical, QED Therapeutics
Advisory Boards: Astellas, Janssen, MSD, Bayer
Consultancy: Patient+
Lectures: Bayer, Janssen
|
Geen actie. Research grants hebben gene betrekking op herziene uitgangsvragen. |
Inbreng patiëntenperspectief
Er werd aandacht besteed aan het patiëntenperspectief door een afgevaardigde van een patiëntenvereniging, de ProstaatKankerStichting, in de werkgroep te laten participeren.
Implementatie
In de verschillende fasen van het ontwikkelproces is rekening gehouden met de implementatie van de richtlijnmodule en de praktische uitvoerbaarheid van de aanbevelingen. Daarbij is uitdrukkelijk gelet op factoren die de invoering van de module in de praktijk kunnen bevorderen of belemmeren. De implementatietabel is te vinden bij de aanverwante producten.
Werkwijze
AGREE
Deze module is opgesteld conform de eisen vermeld in het rapport Medisch Specialistische Richtlijnen 2.0 van de adviescommissie Richtlijnen van de Raad Kwaliteit. Dit rapport is gebaseerd op het AGREE II instrument (Appraisal of Guidelines for Research & Evaluation II; Brouwers, 2010), dat een internationaal breed geaccepteerd instrument is. Voor een stap-voor-stap beschrijving hoe een evidence-based module tot stand komt wordt verwezen naar het stappenplan Ontwikkeling van Medisch Specialistische Richtlijnen van het Kennisinstituut van de Federatie Medisch Specialisten.
Knelpuntenanalyse
Uit de inventarisatie van de knelpunten door werkgroep/commissie Prostaatcarcinoom van de NVU (2017) bleek dat er een noodzaak was voor (revisie) van deze richtlijnmodule.
Uitgangsvraag en uitkomstmaten
Op basis van de uitkomsten van de knelpuntenanalyse is door de werkgroepleden en de adviseur een uitgangsvraag opgesteld. Vervolgens inventariseerde de werkgroep welke uitkomstmaten voor de patiënt relevant zijn, waarbij zowel naar gewenste als ongewenste effecten werd gekeken. De werkgroep waardeerde deze uitkomstmaten volgens hun relatieve belang bij de besluitvorming rondom aanbevelingen, als kritiek, belangrijk (maar niet kritiek) en onbelangrijk. Tevens definieerde de werkgroep tenminste voor de kritieke uitkomstmaten welke verschillen zij klinisch (patiënt) relevant vonden.
Strategie voor zoeken en selecteren van literatuur
Aan de hand van specifieke zoektermen werd gezocht naar gepubliceerde wetenschappelijke studies in (verschillende) elektronische databases. Tevens werd aanvullend gezocht naar studies aan de hand van de literatuurlijsten van de geselecteerde artikelen. In eerste instantie werd gezocht naar studies met de hoogste mate van bewijs. De werkgroepleden selecteerden de via de zoekactie gevonden artikelen op basis van vooraf opgestelde selectiecriteria. De geselecteerde artikelen werden gebruikt om de uitgangsvraag te beantwoorden. De geselecteerde databases waarin is gezocht en de gehanteerde selectiecriteria zijn te vinden in de module met desbetreffende uitgangsvraag. De zoekstrategie is opvraagbaar bij de Richtlijnendatabase, zie het tabblad Zoekverantwoording voor verdere details.
Kwaliteitsbeoordeling individuele studies
Individuele studies werden systematisch beoordeeld, op basis van op voorhand opgestelde methodologische kwaliteitscriteria, om zo het risico op vertekende studieresultaten (risk of bias) te kunnen inschatten. Deze beoordelingen kunt u vinden in de Risk of Bias (RoB) tabellen. De gebruikte RoB instrumenten zijn gevalideerde instrumenten die worden aanbevolen door de Cochrane Collaboration:
• AMSTAR – voor systematische reviews.
Samenvatten van de literatuur
De relevante onderzoeksgegevens van alle geselecteerde artikelen werden overzichtelijk weergegeven in evidence-tabellen. De belangrijkste bevindingen uit de literatuur werden beschreven in de samenvatting van de literatuur. Indien van toepassing: Bij een voldoende aantal studies en overeenkomstigheid (homogeniteit) tussen de studies werden de gegevens ook kwantitatief samengevat (meta-analyse) met behulp van Review Manager 5.
Beoordelen van de kracht van het wetenschappelijke bewijs
De kracht van het wetenschappelijke bewijs werd bepaald volgens de GRADE-methode. GRADE staat voor: Grading Recommendations Assessment, Development and Evaluation (zie http://www.gradeworkinggroup.org/).
GRADE onderscheidt vier gradaties voor de kwaliteit van het wetenschappelijk bewijs: hoog, redelijk, laag en zeer laag. Deze gradaties verwijzen naar de mate van zekerheid die er bestaat over de literatuurconclusie (Schünemann, 2013).
|
GRADE |
Definitie |
|
Hoog |
|
|
Redelijk |
|
|
Laag |
|
|
Zeer laag |
|
Formuleren van de conclusies
Voor elke relevante uitkomstmaat werd het wetenschappelijk bewijs samengevat in een of meerdere literatuurconclusies waarbij het niveau van bewijs werd bepaald volgens de GRADE-methodiek. De werkgroepleden maakten de balans op van elke interventie (overall conclusie). Bij het opmaken van de balans werden de gunstige en ongunstige effecten voor de patiënt afgewogen. De overall bewijskracht wordt bepaald door de laagste bewijskracht gevonden bij een van de kritieke uitkomstmaten. Bij complexe besluitvorming waarin naast de conclusies uit de systematische literatuuranalyse vele aanvullende argumenten (overwegingen) een rol spelen, werd afgezien van een overall conclusie. In dat geval werden de gunstige en ongunstige effecten van de interventies samen met alle aanvullende argumenten gewogen onder het kopje Overwegingen.
Overwegingen (van bewijs naar aanbeveling)
Om te komen tot een aanbeveling zijn naast (de kwaliteit van) het wetenschappelijke bewijs ook andere aspecten belangrijk en worden meegewogen, zoals de expertise van de werkgroepleden, de waarden en voorkeuren van de patiënt, kosten, beschikbaarheid van voorzieningen en organisatorische zaken. Deze aspecten worden, voor zover geen onderdeel van de literatuursamenvatting, vermeld en beoordeeld (gewogen) onder het kopje Overwegingen.
Formuleren van aanbevelingen
De aanbevelingen geven antwoord op de uitgangsvraag en zijn gebaseerd op het beschikbare wetenschappelijke bewijs en de belangrijkste overwegingen, en een weging van de gunstige en ongunstige effecten van de relevante interventies. De kracht van het wetenschappelijk bewijs en het gewicht dat door de werkgroep wordt toegekend aan de overwegingen, bepalen samen de sterkte van de aanbeveling. Conform de GRADE-methodiek sluit een lage bewijskracht van conclusies in de systematische literatuuranalyse een sterke aanbeveling niet a priori uit, en zijn bij een hoge bewijskracht ook zwakke aanbevelingen mogelijk. De sterkte van de aanbeveling wordt altijd bepaald door weging van alle relevante argumenten tezamen.
Randvoorwaarden (Organisatie van zorg)
Bij de ontwikkeling van de module is expliciet rekening gehouden met de organisatie van zorg: alle aspecten die een randvoorwaarde zijn voor het verlenen van zorg (zoals coördinatie, communicatie, (financiële) middelen, menskracht en infrastructuur). Randvoorwaarden die relevant zijn voor het beantwoorden van een specifieke uitgangsvraag maken onderdeel uit van de overwegingen bij de bewuste uitgangsvraag, randvoorwaarden die van invloed zijn op de implementatie van de aanbeveling zijn opgenomen in de implementatietabel.
Kennislacunes
Tijdens de ontwikkeling van deze module is systematisch gezocht naar onderzoek waarvan de resultaten bijdragen aan een antwoord op de uitgangsvraag. Er is nagegaan of (aanvullend) wetenschappelijk onderzoek gewenst is om de uitgangsvraag te kunnen beantwoorden. Mocht dit bij deze module het geval zijn, dan is er een aanbeveling voor het doen van onderzoek opgenomen in de bijlage Kennislacunes. Deze bijlage is te vinden onder de aanverwante producten.
Commentaar- en autorisatiefase
De conceptmodule werd aan de betrokken (wetenschappelijke) verenigingen, instanties en (patiënt) organisaties voorgelegd ter commentaar. De commentaren werden verzameld en besproken met de werkgroep. Naar aanleiding van de commentaren werd de conceptmodule aangepast en definitief vastgesteld door de werkgroep. De definitieve module werd aan de deelnemende (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd voor autorisatie en door hen geautoriseerd dan wel geaccordeerd. De commentaartabel is op te vragen bij het Kennisinstituut via secretariaat@kennisinstituut.nl
Literatuur
Brouwers MC, Kho ME, Browman GP, et al. AGREE Next Steps Consortium. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010;182(18):E839-42. doi: 10.1503/cmaj.090449. Epub 2010 Jul 5. Review. PubMed PMID: 20603348.
Medisch Specialistische Richtlijnen 2.0. Adviescommissie Richtlijnen van de Raad Kwalitieit. http://richtlijnendatabase.nl/over_deze_site/over_richtlijnontwikkeling.html. 2012.
Schünemann H, Brożek J, Guyatt G, et al. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group. Available from http://gdt.guidelinedevelopment.org/central_prod/_design/client/handbook/handbook.html. 2013.
Schünemann HJ, Oxman AD, Brozek J, et al. Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. BMJ. 2008;336(7653):1106-10. doi: 10.1136/bmj.39500.677199.AE. Erratum in: BMJ. 2008;336(7654). doi: 10.1136/bmj.a139. PubMed PMID: 18483053.
Ontwikkeling van Medisch Specialistische Richtlijnen: stappenplan. Kennisinstituut van de Federatie Medisch Specialisten.
Zoekverantwoording
Literatuur zoekstrategie
Zoekverantwoording
Algemene informatie
|
Richtlijn: Prostaatcarcinoom |
|
|
Uitgangsvraag: Wat is de plaats van ADT gecombineerd met ARSI of chemotherapie of radioligandtherapie of targeted therapie in vergelijking met alleen ADT in de behandeling van patiënten met hormoon-resistent gemetastaseerd of niet-gemetastaseerd hormoon-resistent prostaatcarcinoom? |
|
|
Database(s): Medline (OVID), Embase |
Datum: 26-07-2021 |
|
Periode: >2015 |
Talen: Engels, Nederlands |
|
Literatuurspecialist: Linda Niesink |
|
|
BMI zoekblokken: voor verschillende opdrachten wordt (deels) gebruik gemaakt van de zoekblokken van BMI-Online https://blocks.bmi-online.nl/ Bij gebruikmaking van een volledig zoekblok zal naar de betreffende link op de website worden verwezen. |
|
|
Toelichting en opmerkingen:
→ Voor deze vraag is gezocht op de elementen hormoon resistent prostaatcarcinoom (in het blauw) en androgeen deprivatie therapie (ADT) of ARSI óf chemotherapie óf radioligandtherapie óf targeted therapy (in het oranje). → de SPARTAN (2018) en PROSPER (2018) studies worden gevonden met de zoekstrategie. → resultaten staan in Rayyan.
|
|
|
Te gebruiken voor richtlijnen tekst: In de databases Embase (via embase.com) en Medline (via OVID) is op 26-07-2021 met relevante zoektermen gezocht naar systematische reviews en RCT’s over androgeen deprivatie therapie (ADT) gecombineerd met ARSI of chemotherapie of radioligandtherapie of targeted therapie bij patiënten met een hormoonresistent (niet-)gemetastaseerd prostaatcarcinoom. De literatuurzoekactie leverde 1.172 unieke treffers op. |
|
Zoekopbrengst
|
|
EMBASE |
OVID/MEDLINE |
Ontdubbeld |
|
SRs |
156 |
143 |
190 |
|
RCTs |
690 |
724 |
982 |
|
Totaal |
846 |
867 |
1172 |
Zoekstrategie
|
Database |
Zoektermen |
|||||||||||||||||||||||||||
|
Embase
|
|
|||||||||||||||||||||||||||
|
Medline (OVID)
|
1 exp Prostatic Neoplasms, Castration-Resistant/ or (castration adj2 resist* adj2 prostat*).ti,ab,kw. or hormone-refractory.ti,ab,kw. or (crpc or mcrpc or nmcrpc).ti,kf. (11143) 2 exp *Receptors, Androgen/ or ('androgen receptor signaling inhibit*' or 'arsi' or antiandrogen therap*).ti,kf. or exp *Abiraterone Acetate/ or exp *Drug Therapy/ or exp *Docetaxel/ or ('androgen receptor signaling inhibit* or arsi or 'antiandrogen therap*' or abirateron* or zytiga or enzalutamid* or apalutamid* or xtandi or radioligand* or xofigo or olparib or lynparza or chemotherap* or 'targeted therap*' or docetaxel or cabazitaxel or jevtana).ti,kf. or (radium adj3 "223").ti,kf. (540677) 3 1 and 2 (4733) 4 limit 3 to ((english or dutch) and yr="2015 -Current") (2813) 5 4 not (comment/ or editorial/ or letter/ or ((exp animals/ or exp models, animal/) not humans/)) (2554) 6 (meta-analysis/ or meta-analysis as topic/ or (metaanaly* or meta-analy* or metanaly*).ti,ab,kf. or systematic review/ or cochrane.jw. or (prisma or prospero).ti,ab,kf. or ((systemati* or scoping or umbrella or "structured literature") adj3 (review* or overview*)).ti,ab,kf. or (systemic* adj1 review*).ti,ab,kf. or ((systemati* or literature or database* or data-base*) adj10 search*).ti,ab,kf. or ((structured or comprehensive* or systemic*) adj3 search*).ti,ab,kf. or ((literature adj3 review*) and (search* or database* or data-base*)).ti,ab,kf. or (("data extraction" or "data source*") and "study selection").ti,ab,kf. or ("search strategy" and "selection criteria").ti,ab,kf. or ("data source*" and "data synthesis").ti,ab,kf. or (medline or pubmed or embase or cochrane).ab. or ((critical or rapid) adj2 (review* or overview* or synthes*)).ti. or (((critical* or rapid*) adj3 (review* or overview* or synthes*)) and (search* or database* or data-base*)).ab. or (metasynthes* or meta-synthes*).ti,ab,kf.) not (comment/ or editorial/ or letter/ or ((exp animals/ or exp models, animal/) not humans/)) (490203) 7 (exp clinical trial/ or randomized controlled trial/ or exp clinical trials as topic/ or randomized controlled trials as topic/ or Random Allocation/ or Double-Blind Method/ or Single-Blind Method/ or (clinical trial, phase i or clinical trial, phase ii or clinical trial, phase iii or clinical trial, phase iv or controlled clinical trial or randomized controlled trial or multicenter study or clinical trial).pt. or random*.ti,ab. or (clinic* adj trial*).tw. or ((singl* or doubl* or treb* or tripl*) adj (blind$3 or mask$3)).tw. or Placebos/ or placebo*.tw.) not (animals/ not humans/) (2105819) 8 5 and 6 (143) – SRs 9 (5 and 7) not 8 (724) - RCTs 10 8 or 9 (867)
|