Negatieve druktherapie
Uitgangsvraag
Wat is het effect van profylactisch gesloten incisionele negatieve druktherapie bij patiënten die chirurgische ingrepen ondergaan op het risico van een postoperatieve wondinfectie (POWI)?
Aanbeveling
Gebruik postoperatief negatieve druktherapie op de gesloten incisie ter preventie van postoperatieve wondinfecties.
Houd bij het prioriteren van chirurgische wonden voor negatieve druktherapie rekening met de a priori kans op POWI.
Overwegingen
Voor- en nadelen van de interventie en de kwaliteit van het bewijs
De analyses uitgevoerd door Groenen (eClinicalMedicine, 2023) laten het effect zien van iNDT op het percentage POWI’s vergeleken met standaard wondverzorging met reguliere wondverbanden bij chirurgische patiënten. De resultaten toonden een significante, klinisch relevante vermindering van POWI’s bij gebruik van iNDT in vergelijking met standaard wondverzorging, met een hoog niveau van bewijs (7,9% vs. 11,6% (RR 0.67; 95% CI: 0.59–0.76).
Het effect van iNDT verschilde niet van standaard wondverzorging voor secundaire uitkomstmaten zoals wonddehiscentie, seroma, hematoom, mortaliteit, necrose, aantal heropnames of aantal heroperaties. Voor huidblaarvorming werd een vijfvoudige toename gevonden bij patiënten met iNDT vergeleken met standaard wondverbanden. De heterogeniteit was hoog (I2 = 74%, en de incidentie van huidblaarvorming varieerde sterk, tussen 3% en 28%. Huidblaarvorming werd gemeld als een minimaal behandelbare bijwerking, maar moet in overweging worden genomen en worden besproken met patiënten voordat iNDT wordt gestart. De zekerheid van de effecten voor de secundaire uitkomstmaten varieerde van GRADE matig tot laag.
Er is geen biologische reden waarom men een verschil in effect tussen verschillende soorten chirurgie zou verwachten. Willekeurige opsplitsing van de beschikbare gegevens over chirurgische subspecialismen zonder bewijs voor effectmodificatie ondermijnt de statistische kracht en brengt het risico van schijnresultaten met zich mee. Desondanks willen zorgverleners resultaten opgesplitst per subspecialisme, en daarom worden subgroep analyses op basis van het type operatie gevraagd.
Het type operatie verschilde tussen de onderzoeken. Uit een subgroep analyse bleek dat iNDT het risico op POWI vermindert bij patiënten die een buikoperatie, orthopedische of traumachirurgie, vaatchirurgie of hartchirurgie ondergaan, terwijl er geen effect van iNDT was op POWI bij gynaecologische of verloskundige chirurgie, plastische chirurgie, algemene chirurgie en borstchirurgie.
De opgenomen studies gebruikten verschillende niveaus van subatmosferische druk. De subgroep analyse toonde aan dat zowel een subatmosferische druk van 75-80 mmHg als een subatmosferische druk van 120-125 mmHg beide effectief waren bij het verminderen van het percentage POWI. Slechts één studie gebruikte een zelfregulerende subatmosferische druk variërend van 50-200 mmHg, wat niet effectief was bij het verminderen van het POWI-percentage.
Aanvullende sensitiviteitsanalyses werden uitgevoerd om te evalueren of de betrokkenheid van de industrie of de studiekwaliteit onze bevindingen beïnvloedde. De aanvullende analyses toonden aan dat iNDT effectief was bij het verminderen van het POWI-percentage, ongeacht het niveau van betrokkenheid van de industrie (zie Subgroup analysis on the effect of iNPWT versus standard wound care on SSI across different levels of industry involvement. I2: statistical heterogeneity; CI: confidence interval). Soortgelijke resultaten werden gevonden voor de studiekwaliteit. Studies werden gecategoriseerd op basis van het algehele risico op bias met behulp van de ROB-2 tool. De analyses toonden aan dat iNDT effectief was bij het verminderen van het POWI-percentage, ongeacht de studiekwaliteit (zie Subgroup analysis on the effect of iNPWT versus standard wound care on SSI across different levels of risk of bias (low versus some concerns versus high risk of bias). ROB: risk of bias; I2: statistical heterogeneity; CI: confidence interval.).
Over het algemeen vermindert iNDT het POWI-percentage in vergelijking met standaard wondverzorging bij patiënten die chirurgische ingrepen ondergaan, vooral bij degenen die abdominale, orthopedische of traumachirurgie, vaatchirurgie of hartchirurgie ondergaan, met subatmosferische drukniveaus van zowel 75-80 mmHg als 120-125 mmHg.
Internationale richtlijnen
De CDC-richtlijn geeft geen aanbeveling voor iNDT, terwijl de WHO-richtlijn het gebruik van iNDT aanbeveelt bij volwassen patiënten met primair gesloten chirurgische incisies in hoog risico wonden, met als doel het voorkomen van POWI. De bevindingen van Groenen (2023) ondersteunen de aanbeveling van de WHO om iNDT te gebruiken bij patiënten die chirurgische ingrepen ondergaan.
Waarden en voorkeuren van patiënten (en evt. hun verzorgers)
De analyse van de effectiviteit houdt geen rekening met het ongemak van iNDT noch met de praktische overwegingen. iNDT kan de noodzaak voor dagelijkse verbandwisselingen wegnemen en lijkt daarom gunstig voor de patiënt. Echter, het apparaat kan niet losgekoppeld worden voor het douchen en in sommige gevallen beperkt de plaatsing van het apparaat de mobiliteit van de patiënt. Mobiliteitsbeperkingen zijn sterk afhankelijk van de locatie van de wond. Bovendien werd een significante toename van huidblaarvorming gevonden bij patiënten met iNDT. Huidblaarvorming werd gemeld als een minimaal en behandelbare bijwerking. Desalniettemin moet hiermee rekening worden gehouden en worden besproken met patiënten voordat iNDT wordt gestart.
Over het algemeen moeten de nadelige effecten en de voordelen zorgvuldig tegen elkaar worden afgewogen in samenspraak met de patiënt, idealiter met behulp van de principes van gedeelde besluitvorming.
Kosten (middelenbeslag)
De directe kosten van POWI kunnen onder andere een langer ziekenhuisverblijf, heropname, poliklinische en spoedbezoeken, verdere chirurgie en langdurige antibioticabehandeling omvatten. Indirecte kosten zijn moeilijker te kwantificeren, maar kunnen verloren productiviteit van de patiënt en het gezin omvatten, evenals een tijdelijke of permanente afname van functionele of mentale capaciteit. De geschatte kosten voor het beheersen van POWI verschillen sterk, van minder dan 400 dollar per geval voor oppervlakkige POWI tot meer dan 30.000 dollar per geval voor ernstige infecties van organen of ruimtes.
De studie van Eckmann (2022) onderzocht de prevalentie en de klinische en economische impact van chirurgische site-infecties (SSI) in Duitse ziekenhuizen. Patiënten met SSI hadden significant hogere mortaliteit (9,3% versus 4,5%) en een langere opnameduur (28 versus 12 dagen). De kosten per geval waren ook significant hoger voor de SSI-groep (19.008 versus 9.040 euro). Daarom moet de preventie van POWI na de operatie een hoge prioriteit krijgen.
De kosten voor iNDT-apparaten variëren van €120 tot €185 (130 dollar tot 200 dollar) per apparaat en daarom lijkt het gebruik van iNDT zeer kostenefficiënt te zijn.
Aanvaardbaarheid, haalbaarheid en implementatie
iNDT-apparaten zijn wijdverspreid verkrijgbaar en makkelijk in gebruik. Het is een simpel aan te brengen apparaat na geringe training van personeel. Een gespecialiseerde wondverpleegkundige is niet vereist. Er lijken geen majeure barrières te zijn voor de implementatie van iNDT.
Rationale van de aanbeveling: weging van argumenten voor en tegen de interventies
Aangezien iNDT effectief is gebleken in het verminderen van POWI, wijdverspreid verkrijgbaar en makkelijk in gebruik, beveelt de werkgroep het gebruik van iNDT aan bij patiënten die chirurgische ingrepen ondergaan met als doel POWI te verminderen. Hoewel de literatuuranalyse enkele studies m.b.t. volwassen patiënten heeft geïncludeerd is het niet aannemelijk dat het werkingsmechanisme bij kinderen anders is.
De kans op POWI kan a priori variëren afhankelijk van factoren zoals de mate van contaminatie, risicoprofiel van de patiënt en het specifieke type chirurgische ingreep. Echter wordt er een algemene aanbeveling gedaan omdat er geen biologische rationale is voor een relatief verschil in effect van iNPWT op POWI tussen de verschillende specialismen.
Subgroep analyse per type chirurgie resulteert in ondermijning van de statistische kracht door o.a. een kleinere sample size per subgroep waardoor resultaten onnauwkeuriger worden met een breed betrouwbaarheidsinterval. Dit geldt ook voor opsplitsing van data in hoog risico versus laagrisicogroepen. Het relatieve effect is vergelijkbaar over diverse subgroepen; Het absolute effect is naar verwachting groter bij patiëntpopulatie met een hoog risico op POWI.
De geplande duur van de behandeling lijkt geen belangrijke voorspeller van het effect op SSI (p = 0,69) – zie bijlage. Studies waarin een langere geplande behandelduur met NPWT werd gebruikt, hadden geen verschil in SSI. Tussen 5-7 dagen lijkt een optimale behandelduur.
Onderbouwing
Achtergrond
Postoperatieve wondcomplicaties (PWC’s) vormen een last voor patiënten, chirurgen en beleidsmakers, omdat PWC’s het risico op morbiditeit, mortaliteit en kosten verhogen. Met het toepassen van incisionele negatieve drukwondtherapie (iNDT) wordt verondersteld dat bacteriële contaminatie van chirurgische wonden voor her-epitheliasatie wordt voorkomen, de bloedstroom wordt verbetert en lymfedrainage wordt bevorderd, terwijl oedeem, hematoom of seroma-ophoping beperkt wordt. Deze werkmechanismen suggereren dat iNDT niet alleen kan helpen bij het voorkomen van POWI’s, maar ook wonddehiscentie, huidnecrose, seroma en mogelijk hematoom voorkomt.
Zwanenburg et al. (2019) voerde een systematische review (SR) uit en vond dat iNDT het risico op een POWI vermindert met een hoog niveau van bewijs, en mogelijk ook het risico op wonddehiscentie, huidnecrose en seroma, zij het met een laag tot zeer laag niveau van bewijs. Vanwege het beperkte aantal studies dat destijds beschikbaar was, includeerde Zwanenburg et al. (2019) niet-gerandomiseerde studies. Sindsdien zijn er tal van RCT’s gepubliceerd, en is een systematische update van de literatuur nodig. In deze module is een SR, meta-analyse en een GRADE-beoordeling uitgevoerd van gerandomiseerde studies om het effect van iNDT als interventie te evalueren, met het gebruik van standaard wondverbanden als controle op de preventie van PWC’s.
Conclusies / Summary of Findings
iNPWT versus standard wound care
|
High GRADE |
iNPWT reduces surgical site infections compared to standard wound care in patients undergoing surgical procedures.
Source: Groenen, 2023 |
|
Moderate GRADE |
iNPWT may reduce wound dehiscence compared to standard wound care in patients undergoing surgical procedures.
Source: Groenen, 2023 |
|
Low GRADE |
iNPWT may have little or no effect on the number of reoperations compared to standard wound care in patients undergoing surgical procedures.
Source: Groenen, 2023 |
|
Moderate GRADE |
iNPWT may reduce seroma compared to standard wound care in patients undergoing surgical procedures.
Source: Groenen, 2023 |
|
Low GRADE |
iNPWT may reduce hematoma compared to standard wound care in patients undergoing surgical procedures.
Source: Groenen, 2023 |
|
Low GRADE |
iNPWT may have little or no effect on the mortality compared to standard wound care in patients undergoing surgical procedures.
Source: Groenen, 2023 |
|
Low GRADE |
iNPWT may have little or no effect on the number of readmissions compared to standard wound care in patients undergoing surgical procedures.
Source: Groenen, 2023 |
|
Moderate GRADE |
iNPWT may increase skin blistering compared to standard wound care in patients undergoing surgical procedures.
Source: Groenen, 2023 |
|
Low GRADE |
iNPWT may reduce necrosis compared to standard wound care in patients undergoing surgical procedures.
Source: Groenen, 2023 |
Samenvatting literatuur
Description of studies
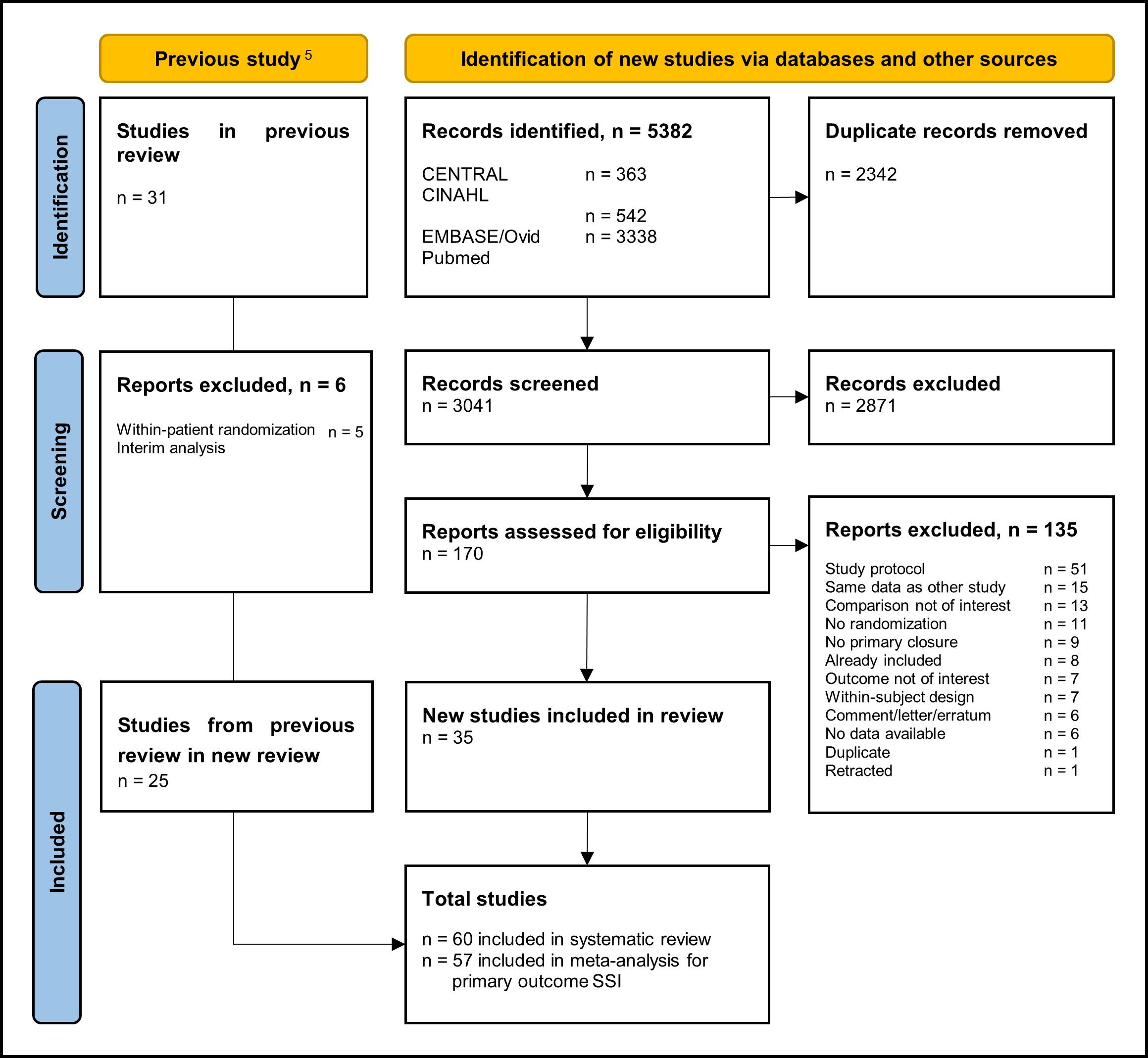
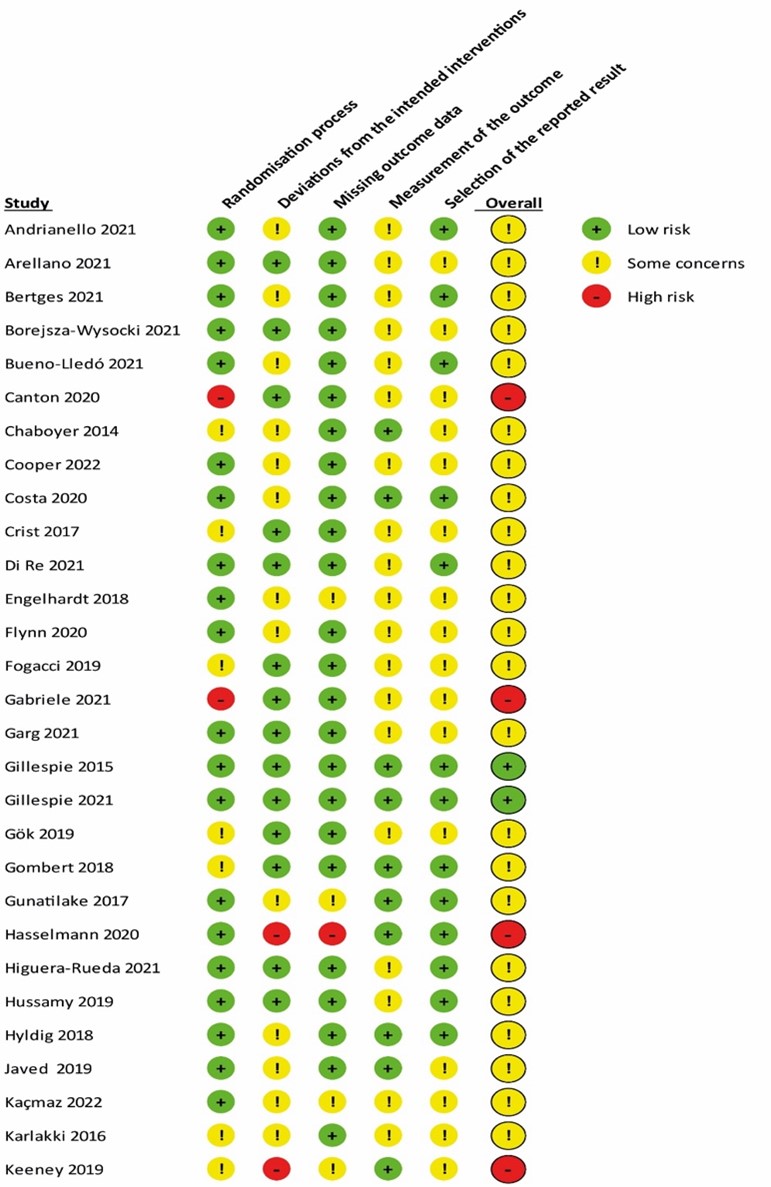
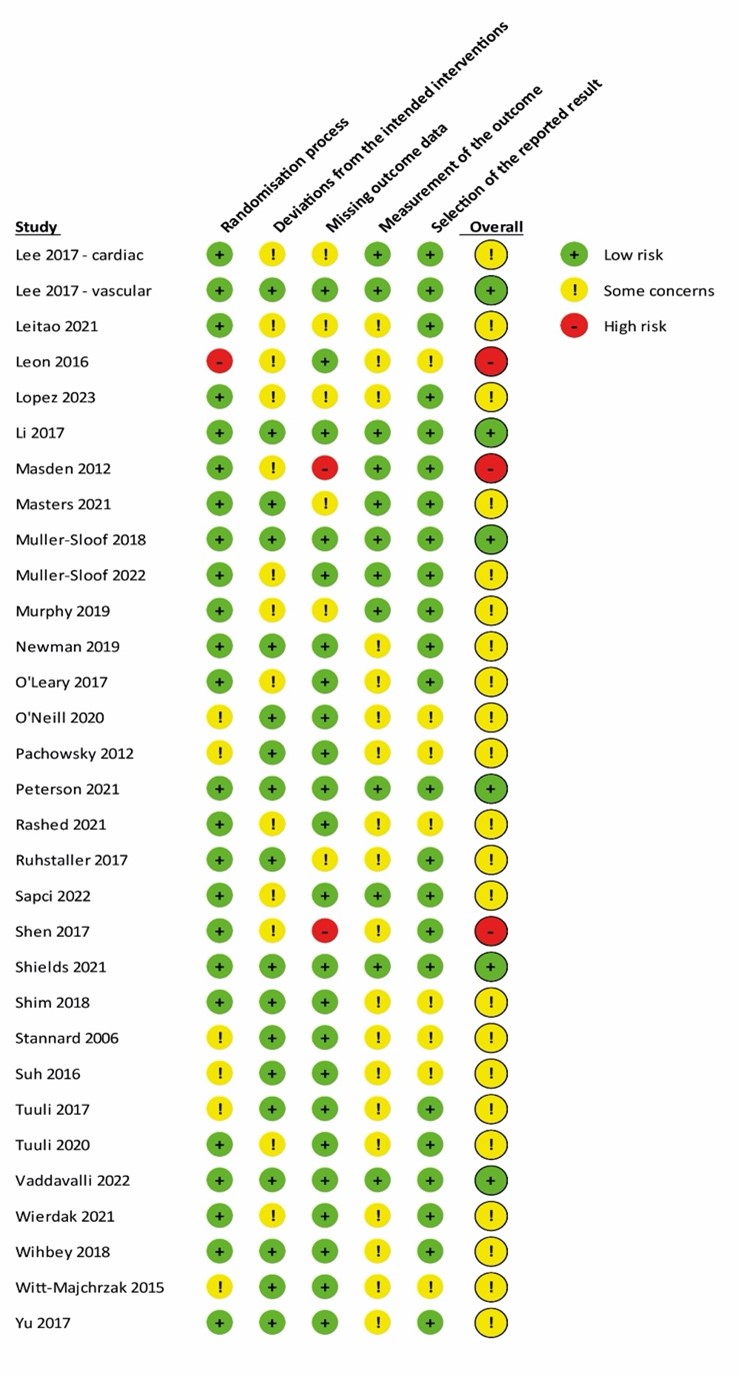
Sixty studies were included in the systematic review. A detailed PRISMA flow diagram for updating systematic reviews is presented in Figure 1. Zwanenburg et. al included 31 RCTs from which five were excluded due to methodological issues (within patient randomization), and the other study is an interim analysis of another included trial. Together with the 35 studies included from this update, we finally included 60 studies in the systematic review. Study characteristics of the included RCTs are summarized in table 1 in Study characteristics of included randomized controlled trials. The risk of bias assessment is summarized in the risk of bias tables in Risk of Bias Assessment ROB2.
Figure 1. PRISMA 2020 flow diagram for updated systematic reviews which included searches of databases and registers only.
Type of surgery
Type of surgery differed across the included studies included in the systematic review. Eighteen studies (n= 2,327) evaluated prophylactic closed incisional negative pressure therapy in patients after abdominal surgery, thirteen studies (n= 3,593) in patients with trauma or orthopedic surgery, eleven studies (n= 6,260) in patients after obstetrics or gynecology surgery, seven studies (n= 987) in patients after vascular surgery, four studies (n=316) in patients after cardiothoracic surgery, 3 studies (n= 182) in patients after plastic surgery, three studies (n= 138) in patients with general surgery, and one study (n= 100) in patients with breast surgery.
Industry involvement
An overview of all statements on industry involvement of studies is presented in Statements Industry involvement. Studies were categorized into four main categories to determine the influence of industry involvement: category 1= no industry funding or involvement, category 2= industry funding, without involvement in trial design, category 3= industry involvement in trial design, and category 4 = no information. Sixteen studies (n= 3,412) report no industry involvement, in 19 studies (n= 7,237) the industry (partially) funded the trial without involvement in the design, in 13 studies (n= 2,285) there was industry involvement in the trial design, and in twelve studies (n = 969) no information was provided on industry involvement.
Negative pressure
Different negative pressure values were used for iNPWT. In thirty studies (n= 6,318) the negative pressure in the iNPWT group was set to 120-125 mmHg, twenty-five studies (n= 7,293) used a negative pressure of 75-80 mmHg, one study (n= 44) used an adjustable device with a negative pressure between 50 and 200 mmHg, and one study (n= 100) used a system with oscillating cyclic negative pressure between 50 and 125 mmHg.
iNPWT devices
Different iNPWT devices were used. Twenty-three studies (n= 5,538) used the Prevena with a negative pressure of 125 mmHg, 23 studies (n= 7,129) used the PICO with a negative pressure set to 80 mmHg, five studies (n= 258) used the VAC, of which two with a negative pressure of 125 mmHg, one with an negative pressure between 50-200 mmHg, one set to 75 mmHg and one did not mention the pressure. Two studies (n= 151) used the CuraVac with a negative pressure of 75 mmHg or a cyclic negative pressure regulation ranging from 50 to 125, one study (n= 104) used the VivanoTec with a negative pressure of 125 mmHg, one study (n=71) used the VSD with a negative pressure of 125 mmHg, one study (n=72) used a custom made device with a negative pressure of 120 mmHg, one study (n= 75) used the NANOVA with a negative pressure of 125 mmHg, and another study (n= 311) did not report the type of de device but reported a negative pressure of 125 mmHg.
Study quality
Risk of bias was assessed using the ROB2-tool. In total ten studies (n= 2,746) had low risk of bias, 43 studies (n= 10,015) had some concerns and 7 studies (n= 1,142) had high risk of bias.
Results
Primary outcome
Surgical Site infections
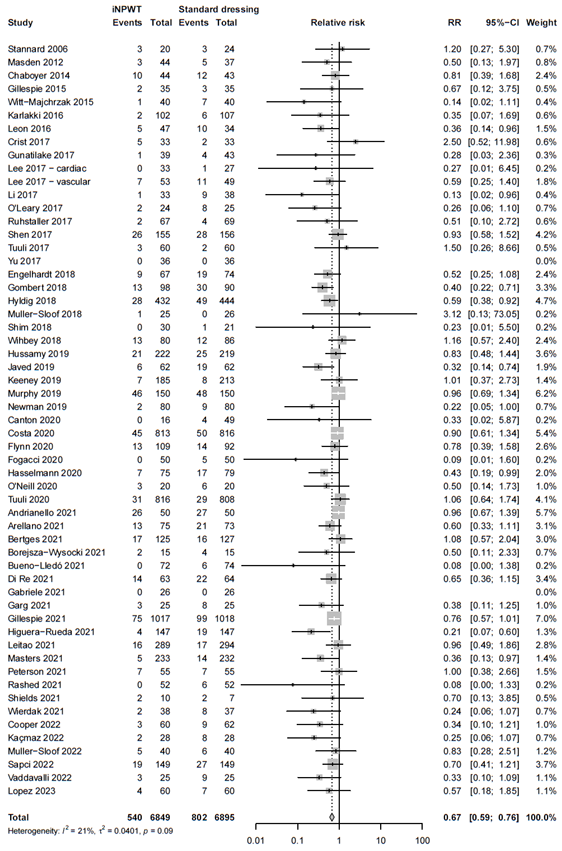
In total 13,744 patients were randomly assigned to receive iNPWT (n= 6,849) or standard wound care (n= 6,895). In total 1,342 SSI were reported, corresponding with an overall SSI rate of 9.8%. The meta-analysis showed that 540 of 6,849 patients had SSI in de iNPWT group (7.9%), and 802 of 6,895 patients had SSI in the control group (11.6%), resulting in an overall relative risk (RR) of 0.67 (95%CI 0.59 - 0.76), a statistically significant difference favoring the iNPWT group. Heterogeneity between studies was moderate (I2= 21%, τ2 = 0.0401, p = 0.09). The forest plot for SSI is presented in Figure 2.
Figure 2. Risk Ratio for the effect of iNPWT versus standard wound care on SSI, I2: statistical heterogeneity; CI: confidence interval.
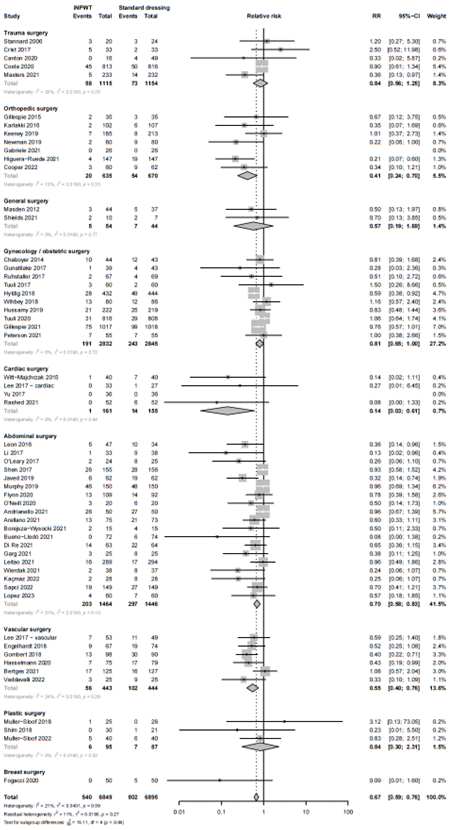
Subgroup analysis: type of surgery
We performed a subgroup analysis to evaluate the efficacy of iNPWT on SSI across different types of surgery. The subgroup analysis with forest plot is presented in Figure 3. A statistically significant difference favoring iNPWT on SSI was found in abdominal surgery (RR 0.66; 95%CI 0.54 - 0.81), orthopedic or trauma surgery (RR 0.64; 95%CI 0.46 - 0.89), vascular surgery (RR 0.55; 95%CI 0.39 to 0.77) and cardiac surgery (RR 0.14; 95%CI 0.03 to 0.62). No statistically significant difference between groups for SSI was found in obstetric surgery (RR 0.82; 95%CI 0.66 - 1.03), plastic surgery (RR 0.84; 95%CI 0.30 – 2.33), general surgery (RR 0.57; 95%CI 0.19 - 1.72) and breast surgery (RR 0.09; 95%CI 0.01 - 1.60).
Figure 3A. Subgroup analysis on the effect of iNPWT versus standard wound care on SSI across different types of surgery (clustering orthopedic and trauma surgery), I2: statistical heterogeneity; CI: confidence interval.
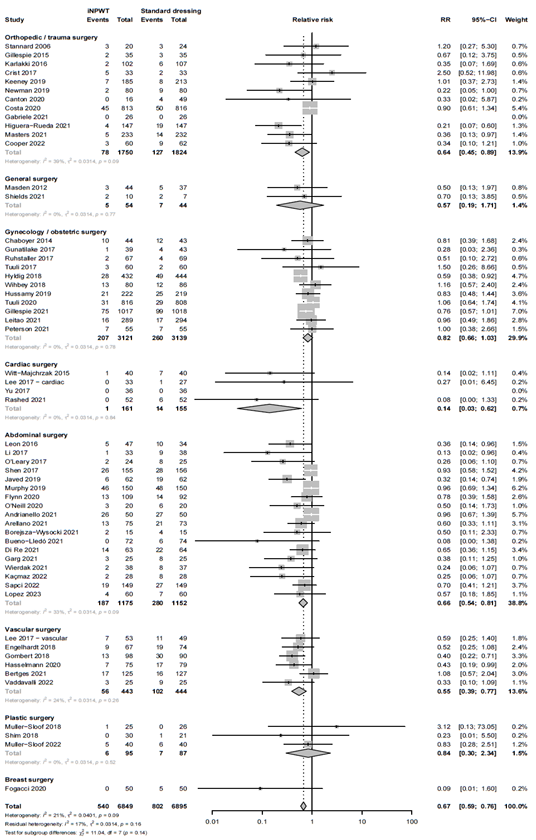

Figure 3B. Subgroup analysis on the effect of iNPWT versus standard wound care on SSI across different types of surgery (splitting orthopedic and trauma surgery), I: statistical heterogeneity; CI: confidence interval.
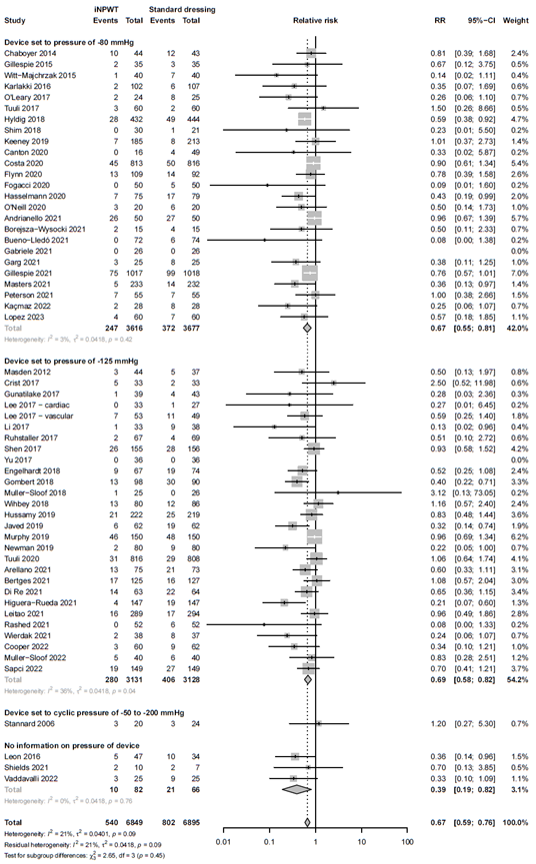
Subgroup analysis: negative pressure
The negative pressure differed across the studies. To evaluate whether the height of the negative pressure influences the efficacy of iNPWT on SSI we performed a subgroup analysis. The forest plot of the subgroup analysis is presented in Figure 4. Both, a negative pressure of 75-80 mmHg and a negative pressure of 120-125 mmHg were effective for the prevention of SSI, RR 0.67 (95%CI 0.55 - 0.81) and RR 0.69 (95%CI 0.58 - 0.82), respectively. There was no significant difference in SSI seen in a device with negative pressure ranging from 50-200 mmHg versus standard wound care (RR 1.20; 95%CI 0.27 - 5.30).
Figure 4. Subgroup analysis on the effect of iNPWT versus standard wound care on SSI across different subatmospheric pressures, I2: statistical heterogeneity; CI: confidence interval.
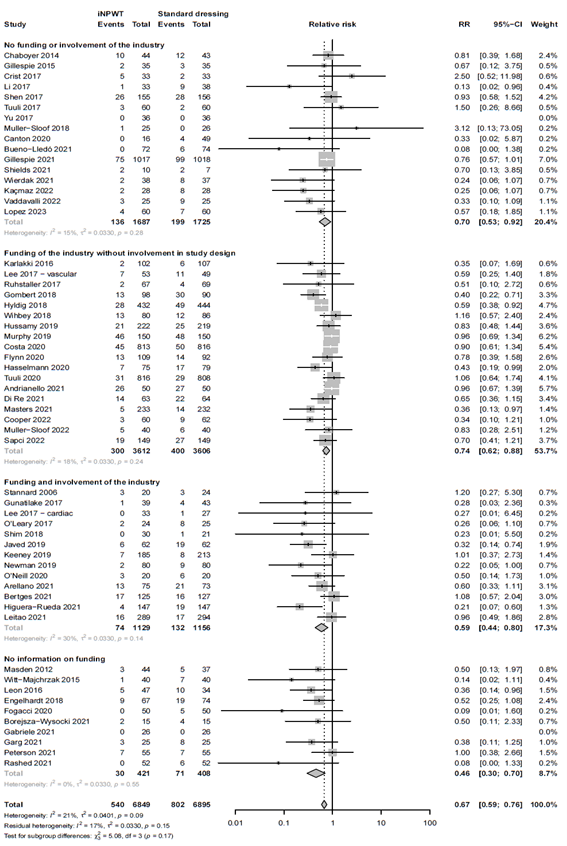
Subgroup analysis: industry involvement
To evaluate whether industry involvement influences the efficacy of iNPWT on SSI we performed a subgroup analysis. Subgroup analysis revealed that the efficacy of iNPWT on SSI was significantly across the different categories; category 1; no industry funding or involvement (RR 0.70; 95%CI 0.53 - 0.92), category 2; industry funding, without involvement in trial design (RR 0.74; 95%CI 0.62 - 0.88), category 3; industry involvement in trial design (RR 0.59; 95%CI 0.44 - 0.80), and category 4; no information (RR 0.46; 95%CI 0.30 - 0.70). The forest plot is presented in Figure 5.
Figure 5. Subgroup analysis on the effect of iNPWT versus standard wound care on SSI across different levels of industry involvement. I2: statistical heterogeneity; CI: confidence interval.
Subgroup analysis: study quality
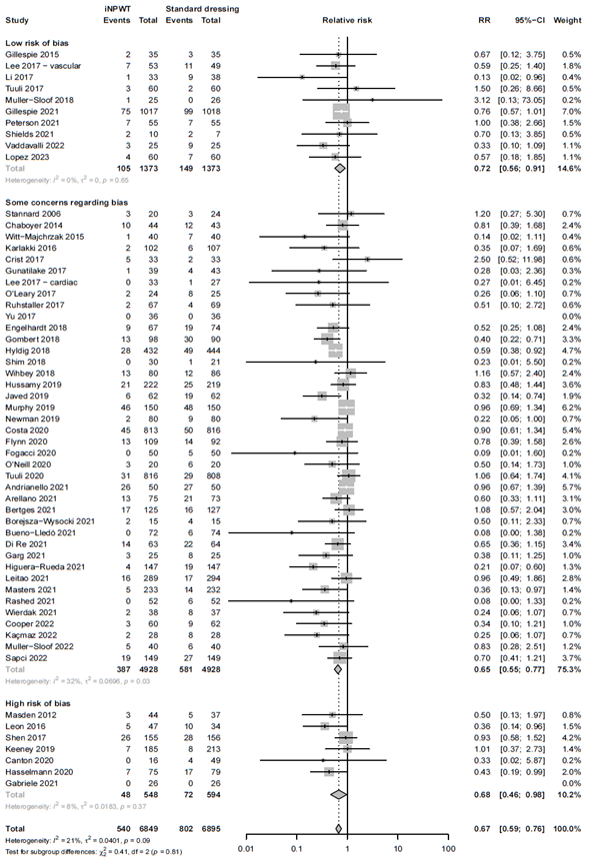
We evaluated if the quality of the included studies influences the efficacy of iNPWT by performing a sensitivity analysis. The results of the sensitivity analysis excluding studies at high risk of bias showed comparable results to the overall analysis. The efficacy of iNPWT on SSI in studies with low risk of bias or those with some concerns was RR 0.72 (95%CI 0.56 - 0.90), and RR 0.65 (95%CI 0.55 - 0.77). This was similar in studies with high risk of bias RR 0.68 (95%CI 0.46 - 0.98). The sensitivity analysis, separating studies with high risk of bias from those with low or risk of bias is presented in Figure 6.
Figure 6A. Subgroup analysis on the effect of iNPWT versus standard wound care on SSI across different levels of risk of bias (low versus some concerns versus high risk of bias). ROB: risk of bias; I2: statistical heterogeneity; CI: confidence interval.
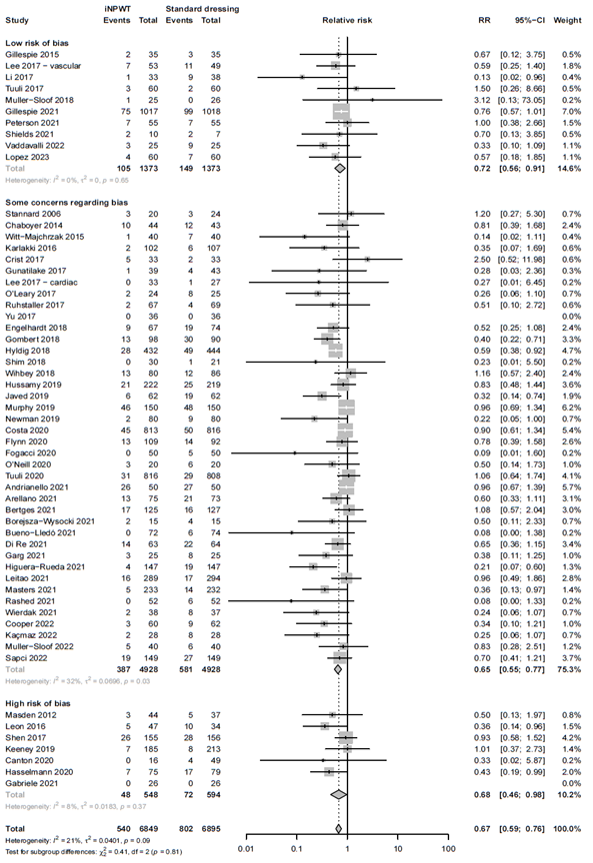
Figure 6B. Subgroup analysis on the effect of iNPWT versus standard wound care on SSI across different levels of risk of bias (low/some concerns versus high risk of bias). ROB: risk of bias; I2: statistical heterogeneity; CI: confidence interval.
Secondary outcomes
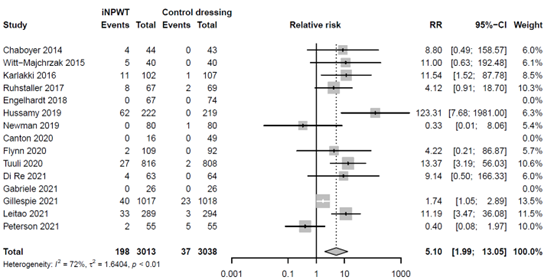
There was no significant difference between the effect of iNPWT versus standard wound care on wound dehiscence (RR 0.85; 95%CI 0.71 - 1.02), reoperation (RR 0.91; 95%CI 0.69 - 1.20), seroma (RR 0.83; 95% 0.65 - 1.06), hematoma (RR 0.77; 95%CI 0.48 - 1.23), mortality (RR 0.94; 95%CI 0.58 - 1.52), readmission (RR 0.96; 95%CI 0.69 - 1.35) and necrosis (RR 0.46; 95%CI 0.14 - 1.46). Skin blistering increased significantly with the use of iNPWT (RR 5.10; 95%CI 1·99 - 13·05) with high between study heterogeneity (I2 = 72%, τ2 = 1·6404, p < 0·01). All results are presented in the evidence table in Evidence table, all RR with corresponding 95% CI.
Wound dehiscence
The effect of iNPWT on wound dehiscence was evaluated in 8,867 patients. In total 719 patients had wound dehiscence, corresponding with an overall wound dehiscence rate of 8.1%. There was no significant difference in wound dehiscence in patients treated with iNPWT compared to standard wound dressings (RR 0.85; 95%CI 0.71 -1.02). The forest plot of the meta-analysis is presented in Figure 7.
Figure 7. Risk Ratio for the effect of iNPWT versus standard wound care on wound dehiscence, I2: statistical heterogeneity; CI: confidence interval.

Seroma
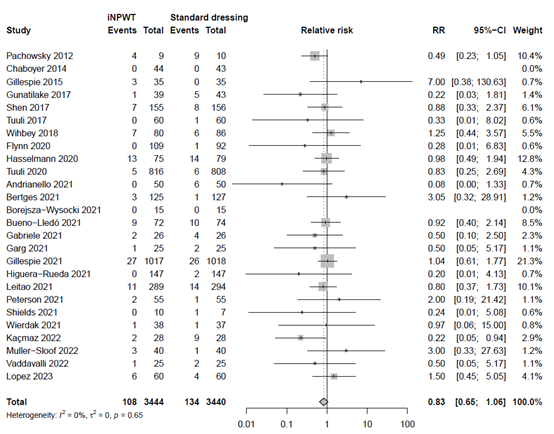
The effect of iNPWT on seroma was evaluated in 6,884 patients. In total 242 patients had seroma after surgery, corresponding with an overall seroma rate of 3.5%. There was no significant difference in seroma between patients treated with iNPWT compared to standard wound care (RR 0.83; 95%CI; 0.65 to 1.06). The forest plot of the meta-analysis is presented in Figure 8.
Figure 8. Risk Ratio for the effect of iNPWT versus standard wound care on seroma, I2: statistical heterogeneity; CI: confidence interval.
Hematoma
The effect of iNPWT on necrosis was evaluated in 6,827 patients. In total 79 patients had had hematoma after surgery, corresponding with an overall hematoma rate of 1.2%. There was no significant difference in hematoma between patients treated with iNPWT compared to standard wound care (RR 0.77; 95%CI 0.48 to 1.23). The forest plot with meta-analysis is presented in Figure 9.
Figure 9. Risk Ratio for the effect of iNPWT versus standard wound care on hematoma, I2: statistical heterogeneity; CI: confidence interval.
Necrosis
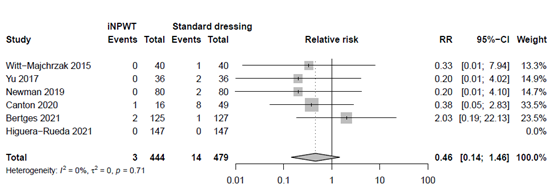
The effect of iNPWT on hematoma was evaluated in 923 patients. In total 17 patients had had hematoma after surgery, corresponding with an overall necrosis rate of 1.8%. There was no significant difference in necrosis between patients treated with iNPWT compared to standard wound care (RR 0.46; 95%CI 0.14 to 1.46). The forest plot with meta-analysis is presented in Figure 10.
Figure 10. Risk Ratio for the effect of iNPWT versus standard wound care on necrosis, I2: statistical heterogeneity; CI: confidence interval.
Readmission
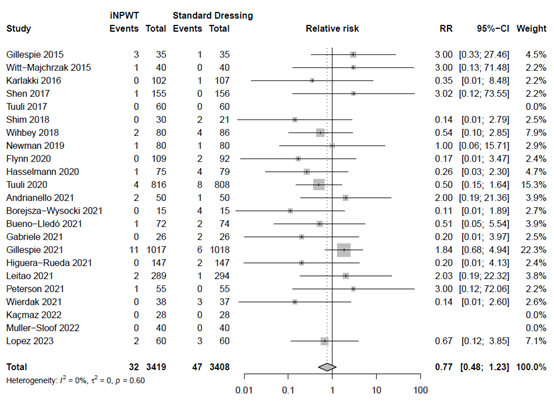
The effect of iNPWT on readmission was evaluated in 6,487 patients. In total 227 readmissions were reported, corresponding with an overall readmission rate of 3.5%. There was no significant difference in number of readmissions between patients treated with iNPWT compared to standard wound care (RR 0.96; 95%CI 0.69 to 1.35). The forest plot with meta-analysis is presented in Figure 11.
Figure 11. Risk Ratio for the effect of iNPWT versus standard wound care on readmission, I2: statistical heterogeneity; CI: confidence interval.
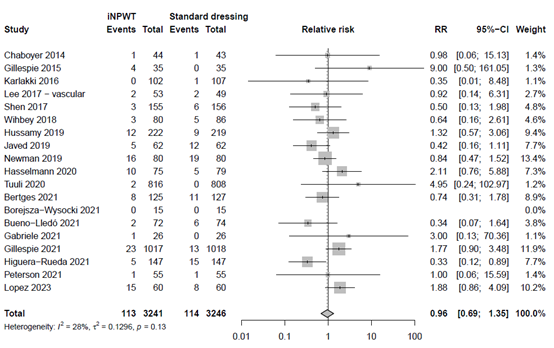
Reoperation
The effect of iNPWT on reoperation was evaluated in 9,320 patients. In total 197 reoperations were reported, corresponding with an overall reoperation rate of 2.1%. There was no significant difference in number of reoperations between patients treated with iNPWT compared to standard wound care (RR 0.91; 95%CI 0.69 to 1.20). The forest plot with meta-analysis is presented in Figure 12.
Figure 12. Risk Ratio for the effect of iNPWT versus standard wound care on reoperation, I2: statistical heterogeneity; CI: confidence interval.
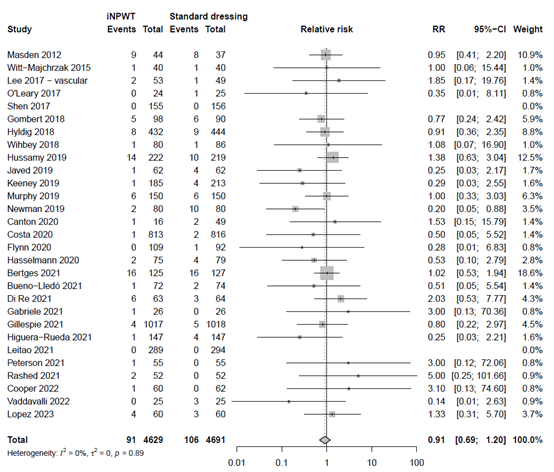
Mortality
The effect of iNPWT on mortality was evaluated in 8,105 patients. In total 66 mortalities were reported, corresponding with an overall reoperation rate of 0.8%. There was no significant difference in number of deaths between patients treated with iNPWT compared to standard wound care (RR 0.94; 95%CI 0.58 to 1.52). The forest plot with meta-analysis is presented in Figure 13.
Risk Ratio for the effect of iNPWT versus standard wound care on mortality, I2: statistical heterogeneity; CI: confidence interval.
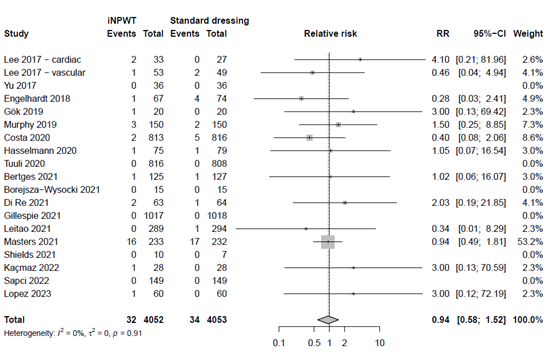
Skin blistering
The effect of iNPWT on skin blistering was evaluated in 6,051 patients. In total 235 patients experience skin blistering, corresponding with an overall reoperation rate of 3.9%. There was a large significant difference in number of skin blistering between patients treated with iNPWT compared to standard wound care (RR 5.10; 95%CI 1.99 to 13.05). The forest plot with meta-analysis is presented in Figure 14.
Figure 14. Risk Ratio for the effect of iNPWT versus standard wound care on skin blistering, I2: statistical heterogeneity; CI: confidence interval.
Adverse events
Adverse events of the skin related to the study intervention, including skin blistering and pain, were mentioned in 32 RCTs and are listed in Adverse events. Five studies reported no adverse events in both study arms.
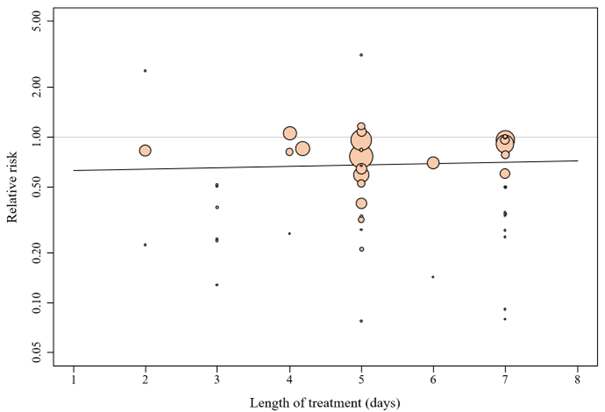
Duration of treatment
Meta-regression analysis showed that intended duration of treatment is not a significant effect size predictor (p = 0.69). Studies with longer intended iNPWT treatment duration were not associated with a difference in SSI, with a regression coefficient of 0.020. The bubble plot is presented in Figure 15.
Figure 15. Bubble plot of intended duration of treatment
Meta-regression showed that intended duration of treatment is not a significant effect size predictor (p = 0.69). Studies with longer intended duration of iNPWT treatment were not associated with a larger reduction in SSI, with a regression coefficient of 0.020. This means that for every additional intended day, the effect size (relative risk) is expected to rise by 0.020.
Level of evidence of the literature
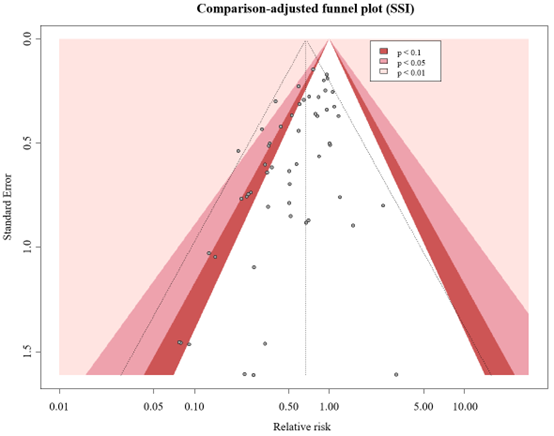
The GRADE approach for rating the certainty of estimates of treatment effects was used. Since all included studies are randomized controlled trials, the rating for the GRADE starts high for all outcomes. Each outcome can be downgraded due to one of the following reasons: risk of bias: the quality assessment of the individual studies is presented in the risk of bias tables in Risk of Bias Assessment ROB2; inconsistency: similarity of point estimates, extent of overlap of confidence intervals, and statistical criteria including tests of heterogeneity and I2; imprecision: For point estimates with 95%CIs that crosses the null-effect threshold and boundaries for clinical decision making we downgraded with one or two dimensions. If the boundaries are not crossed, we did not downgrade. Publication bias: The comparison-adjusted funnel plot showed no sign of small-study effects (see funnel plot diagrams). The certainty of the evidence, and absolute effects per outcome are presented in the GRADE-table in GRADE-table. A visual presentation of the absolute differences between iNPWT and standard wound care with GRADE certainty levels is presented in Figure 16.
Figure 16. Visual presentation showing the absolute differences between iNPWT and standard wound care with GRADE certainty levels per outcome measure.
|
|
Certainty assessment |
№ of patients |
Effect |
Certainty |
||||||||
|
|
№ of studies |
Study design |
Risk of bias |
Inconsistency |
Indirectness |
Imprecision |
Other considerations |
iNPWT |
Control dressings |
Relative |
Absolute |
|
|
Primary outcome |
||||||||||||
|
SSI |
57 |
RCTs |
Not serious |
Not serious (I2 = 21%) |
Not serious |
Not serious |
None |
540 / 6849 (7·9%) |
802 / 6895 (11·6%) |
RR 0·67 (0·59 - 0·76) |
38 fewer per 1.000 |
⨁⨁⨁⨁ High |
|
Secondary outcomes |
||||||||||||
|
Wound dehiscence |
35 |
RCTs |
Not serious |
Not serious (I2 = 13%) |
Not serious |
Serious† (-1) |
None |
332 / 4417 (7·5%) |
387 / 4450 (8·7%) |
RR 0·85 (0·71 - 1·02) |
13 fewer per 1.000 |
⨁⨁⨁◯ Moderate |
|
Reoperation |
29 |
RCTs |
Not serious |
Not serious (I2 = 0%) |
Not serious |
Serious‡ (-2) |
None |
91 / 4629 (2·0%) |
106 / 4691 (2·3%) |
RR 0·91 (0·69 - 1·20) |
2 fewer per 1.000 |
⨁⨁◯◯ |
|
Seroma |
26 |
RCTs |
Not serious |
Not serious (I2 = 0%) |
Not serious |
Serious† (-1) |
None |
108 / 3444 (3·1%) |
134 / 3440 (3·9%) |
RR 0·83 (0·65 - 1·06) |
7 fewer per 1.000 |
⨁⨁⨁◯ Moderate |
|
Hematoma |
23 |
RCTs |
Not serious |
Not serious (I2 = 0%) |
Not serious |
Serious‡ (-2) |
None |
32 / 3419 (0·9%) |
47 / 3408 (1·4%) |
RR 0·77 (0·48 - 1·23) |
3 fewer per 1.000 |
⨁⨁◯◯ |
|
Mortality |
19 |
RCTs |
Not serious |
Not serious (I2 = 0%) |
Not serious |
Serious‡ (-2)
|
None |
32 / 4052 (0·8%) |
34 / 4053 (0·8%) |
RR 0·94 (0·58 - 1·52) |
1 fewer per 1.000 |
⨁⨁◯◯ Low |
|
Readmission |
19 |
RCTs |
Not serious |
Not serious (I2 = 28%) |
Not serious |
Serious‡ (-2) |
None |
113 / 3241 (3·5%) |
114 / 3246 (3·5%) |
RR 0·96 (0·69 - 1·23) |
1 fewer per 1.000 |
⨁⨁◯◯ |
|
Skin blistering |
11 |
RCTs |
Not serious |
Serious (-1) (I2 = 72%) |
Not serious |
Not serious
|
None |
198 / 3013 (6·6%) |
37 / 3808 (1·2%) |
RR 5·10 (1·99 - 13·05) |
50 more per 1.000 |
⨁⨁⨁◯ Moderate |
|
Necrosis |
6 |
RCTs |
Not serious |
Not serious (I2 = 0%) |
Not serious |
Serious‡ (-2) |
None |
3 / 444 (0·7%) |
14 / 479 (2·9%) |
RR 0·46 (0·14 - 1·46) |
16 fewer per 1.000 |
⨁⨁◯◯ Low |
|
NS = not serious; *Risk of bias (Appendix 10); † 95% CI overlaps no effect; ‡ 95% CI overlaps no effect but fails to exclude considerable benefit or harm (default relative risk reduction >0·20); § 95%CI excludes no effect but fails to exclude considerable benefit or harm (default relative risk reduction >0·20). |
||||||||||||
Figure 17. Comparison-adjust funnel plot
Zoeken en selecteren
A systematic review of the literature was performed to answer the following question:
‘What is the effect of closed incisional negative pressure wound therapy (iNPWT) vs. conventional dressings on SSI in adult patients undergoing surgical procedures?’
P: Adult patients undergoing surgical procedures
I: Closed incisional negative pressure therapy (iNPWT)
C: No prophylactic iNPWT (conventional dressing)
O: Surgical site infections, wound dehiscence, reoperation, seroma, hematoma, mortality, readmission, skin blistering, skin necrosis
Relevant outcome measures
The guideline development group considered SSI as a critical outcome measure for decision making and wound dehiscence, seroma, hematoma, skin necrosis, readmission, and reoperations as important outcomes for clinical decision making.
The working group defined a threshold of 10% for continuous outcomes and a relative risk (RR) for dichotomous outcomes of <0.80 and >1.25 as a minimal clinically (patient) important difference.
Search and select (Methods)
This systematic review is reported according to the Preferred Reporting Items for updating Systematic Reviews (PRISMA) statement). We update the review by Zwanenburg et al. who searched the literature up to December 18, 2018. In this update we searched the databases Pubmed, Medline (via OVID) and Embase (via Embase.com) up to 24-10-2022. Search terms included: surgical site infection, post-operative wound infection, post-operative care, prophylactic closed incisional NPWT, vacuum assisted closure, wound care, dressing. The detailed search strategy is available on request via https://richtlijnendatabase.nl/.
We included RCTs that compared closed incisional NPWT (iNPWT) with standard wound care compromising the use of conventional dressings (no iNPWT). Non-randomized studies, cross-over studies, opinion papers, proceedings, editorials, studies in paediatric patients, animal studies and studies not focusing on primary wound closure were excluded. Two reviewers (HJ and HG) independently executed title and abstract screening and full text review of potential eligible studies. Discrepancies between the two reviewers were resolved through discussion and, if necessary, the senior author (MAB) was consulted. Additional articles were identified by backward and forward citation tracking of previously published studies.
The update of the search resulted in 5383 hits. Five studies were additionally included based on citation tracking. One hundred sixty-nine records were initially selected based on title and abstract screening. After reading the full-text, 135 studies were excluded, and 34 additional studies were included (see the 'Samenvatting literatuur' tab). Reasons for exclusion after full text review are reported in the exclusion table in the table of excluded studies under the 'Evidence tabellen' tab.
Referenties
- Anderson V, Chaboyer W, Gillespie BM, Fenwick J. The use of negative pressure wound therapy dressing in obese women undergoing caesarean section:: a pilot study. Evidence Based Midwifery. 2014;12(1):23.
- Andrianello S, Landoni L, Bortolato C, et al. Negative pressure wound therapy for prevention of surgical site infection in patients at high risk after clean-contaminated major pancreatic resections: A single-center, phase 3, randomized clinical trial. Surgery. May 2021;169(5):1069-1075. doi:10.1016/j.surg.2020.10.029
- Arellano M, Barragan Serrano C, Guedea M, et al. Surgical Wound Complications after Colorectal Surgery with Single-Use Negative-Pressure Wound Therapy Versus Surgical Dressing over Closed Incisions: A Randomized Controlled Trial. Adv Skin Wound Care. Dec 1 2021;34(12):657-661. doi:10.1097/01.ASW.0000756512.87211.13
- Astagneau P, Rioux C, Golliot F, Brucker G, Group INS. Morbidity and mortality associated with surgical site infections: results from the 1997-1999 INCISO surveillance. J Hosp Infect. Aug 2001;48(4):267-74. doi:10.1053/jhin.2001.1003
- Atkins BZ, Tetterton JK, Petersen RP, Hurley K, Wolfe WG. Laser Doppler flowmetry assessment of peristernal perfusion after cardiac surgery: beneficial effect of negative pressure therapy. Int Wound J. Feb 2011;8(1):56-62. doi:10.1111/j.1742-481X.2010.00743.x
- Beecher SM, O'Leary DP, McLaughlin R, Kerin MJ. The Impact of Surgical Complications on Cancer Recurrence Rates: A Literature Review. Oncol Res Treat. 2018;41(7-8):478-482. doi:10.1159/000487510
- Bertges DJ, Smith L, Scully RE, et al. A multicenter, prospective randomized trial of negative pressure wound therapy for infrainguinal revascularization with a groin incision. J Vasc Surg. Jul 2021;74(1):257-267 e1. doi:10.1016/j.jvs.2020.12.100
- Borejsza-Wysocki M, Bobkiewicz A, Francuzik W, et al. Effect of closed incision negative pressure wound therapy on incidence rate of surgical site infection after stoma reversal: a pilot study. Wideochir Inne Tech Maloinwazyjne. Dec 2021;16(4):686-696. doi:10.5114/wiitm.2021.106426
- Bueno-Lledo J, Franco-Bernal A, Garcia-Voz-Mediano MT, Torregrosa-Gallud A, Bonafe S. Prophylactic Single-use Negative Pressure Dressing in Closed Surgical Wounds After Incisional Hernia Repair: A Randomized, Controlled Trial. Ann Surg. Jun 1 2021;273(6):1081-1086. doi:10.1097/SLA.0000000000004310
- Canton G, Fattori R, Pinzani E, Monticelli L, Ratti C, Murena L. Prevention of postoperative surgical wound complications in ankle and distal tibia fractures: results of Incisional Negative Pressure Wound Therapy. Acta Biomed. Dec 30 2020;91(14-S):e2020006. doi:10.23750/abm.v91i14-S.10784
- Chaboyer W, Anderson V, Webster J, Sneddon A, Thalib L, Gillespie BM. Negative Pressure Wound Therapy on Surgical Site Infections in Women Undergoing Elective Caesarean Sections: A Pilot RCT. Healthcare (Basel). Sep 30 2014;2(4):417-28. doi:10.3390/healthcare2040417
- Cooper HJ, Santos WM, Neuwirth AL, et al. Randomized Controlled Trial of Incisional Negative Pressure Following High-Risk Direct Anterior Total Hip Arthroplasty. Journal of Arthroplasty. Aug 2022;37(8):S931-S936. doi:10.1016/j.arth.2022.03.039
- Costa ML, Achten J, Knight R, et al. Effect of Incisional Negative Pressure Wound Therapy vs Standard Wound Dressing on Deep Surgical Site Infection After Surgery for Lower Limb Fractures Associated With Major Trauma: The WHIST Randomized Clinical Trial. JAMA. Feb 11 2020;323(6):519-526. doi:10.1001/jama.2020.0059
- Crist BD, Oladeji LO, Khazzam M, Della Rocca GJ, Murtha YM, Stannard JP. Role of acute negative pressure wound therapy over primarily closed surgical incisions in acetabular fracture ORIF: A prospective randomized trial. Injury. Jul 2017;48(7):1518-1521. doi:10.1016/j.injury.2017.04.055
- Di Re AM, Wright D, Toh JWT, et al. Surgical wound infection prevention using topical negative pressure therapy on closed abdominal incisions - the 'SWIPE IT' randomized clinical trial. J Hosp Infect. Apr 2021;110:76-83. doi:10.1016/j.jhin.2021.01.013
- Eckmann C, Kramer A, Assadian O, et al. Clinical and economic burden of surgical site infections in inpatient care in Germany: A retrospective, cross-sectional analysis from 79 hospitals. PLoS One. 2022;17(12):e0275970. doi:10.1371/journal.pone.0275970
- Eckmann, C., Kramer, A., Assadian, O., Flessa, S., Huebner, C., Michnacs, K., Muehlendyck, C., Podolski, K. M., Wilke, M., Heinlein, W., & Leaper, D. J. (2022). Clinical and economic burden of surgical site infections in inpatient care in Germany: A retrospective, cross-sectional analysis from 79 hospitals. PloS one, 17(12), e0275970. https://doi.org/10.1371/journal.pone.0275970
- Engelhardt M, Rashad NA, Willy C, et al. Closed-incision negative pressure therapy to reduce groin wound infections in vascular surgery: a randomised controlled trial. Int Wound J. Jun 2018;15(3):327-332. doi:10.1111/iwj.12848
- Flynn J, Choy A, Leavy K, et al. Negative Pressure Dressings (PICO(TM)) on Laparotomy Wounds Do Not Reduce Risk of Surgical Site Infection. Surg Infect (Larchmt). Apr 2020;21(3):231-238. doi:10.1089/sur.2019.078
- Fogacci T, Cattin F, Semprini G, Frisoni G, Fabiocchi L, Samorani D. The negative pressure therapy with PICO as a prevention of surgical site infection in high-risk patients undergoing breast surgery. Breast J. May 2020;26(5):1071-1073. doi:10.1111/tbj.13659
- Gabriele L, Gariffo G, Grossi S, Ipponi E, Capanna R, Andrean L. Closed Incisional Negative Pressure Wound Therapy (ciNPWT) in Oncological Orthopedic Surgery: Preliminary Report. Surg Technol Int. May 20 2021;38:451-454. doi:10.52198/21.STI.38.OS1429
- Galiano RD, Hudson D, Shin J, et al. Incisional Negative Pressure Wound Therapy for Prevention of Wound Healing Complications Following Reduction Mammaplasty. Plast Reconstr Surg Glob Open. Jan 2018;6(1):e1560. doi:10.1097/GOX.0000000000001560
- Garg A, Jayant S, Gupta AK, Bansal LK, Wani A, Chaudhary P. Comparison of closed incision negative pressure wound therapy with conventional dressing in reducing wound complications in emergency laparotomy. Pol J Surg. 2021;93(5)doi:10.5604/01.3001.0014.9759
- Gillespie BM, Rickard CM, Thalib L, et al. Use of Negative-Pressure Wound Dressings to Prevent Surgical Site Complications After Primary Hip Arthroplasty: A Pilot RCT. Surg Innov. Oct 2015;22(5):488-95. doi:10.1177/1553350615573583
- Gillespie BM, Webster J, Ellwood D, et al. Closed incision negative pressure wound therapy versus standard dressings in obese women undergoing caesarean section: multicentre parallel group randomised controlled trial. BMJ. May 5 2021;373:n893. doi:10.1136/bmj.n893
- Gok MA, Kafadar MT, Yegen SF. Comparison of negative-pressure incision management system in wound dehiscence: A prospective, randomized, observational study. J Med Life. Jul-Sep 2019;12(3):276-283. doi:10.25122/jml-2019-0033
- Gombert A, Babilon M, Barbati ME, et al. Closed Incision Negative Pressure Therapy Reduces Surgical Site Infections in Vascular Surgery: A Prospective Randomised Trial (AIMS Trial). Eur J Vasc Endovasc Surg. Sep 2018;56(3):442-448. doi:10.1016/j.ejvs.2018.05.018
- Groenen H, Jalalzadeh H, Buis DR, Dreissen YEM, Goosen JHM, Griekspoor M, Harmsen WJ, IJpma FFA, van der Laan MJ, Schaad RR, Segers P, van der Zwet WC, de Jonge SW, Orsini RG, Eskes AM, Wolfhagen N, Boermeester MA. Incisional negative pressure wound therapy for the prevention of surgical site infection: an up-to-date meta-analysis and trial sequential analysis. EClinicalMedicine. 2023 Jul 24;62:102105. doi: 10.1016/j.eclinm.2023.102105. PMID: 37538540; PMCID: PMC10393772.
- Gunatilake RP, Swamy GK, Brancazio LR, et al. Closed-Incision Negative-Pressure Therapy in Obese Patients Undergoing Cesarean Delivery: A Randomized Controlled Trial. AJP Rep. Jul 2017;7(3):e151-e157. doi:10.1055/s-0037-1603956
- Hasselmann J, Bjork J, Svensson-Bjork R, Acosta S. Inguinal Vascular Surgical Wound Protection by Incisional Negative Pressure Wound Therapy: A Randomized Controlled Trial-INVIPS Trial. Ann Surg. Jan 2020;271(1):48-53. doi:10.1097/SLA.0000000000003364
- Higuera-Rueda CA, Emara AK, Nieves-Malloure Y, et al. The Effectiveness of Closed-Incision Negative-Pressure Therapy Versus Silver-Impregnated Dressings in Mitigating Surgical Site Complications in High-Risk Patients After Revision Knee Arthroplasty: The PROMISES Randomized Controlled Trial. J Arthroplasty. Jul 2021;36(7S):S295-S302 e14. doi:10.1016/j.arth.2021.02.076
- Hinrichs WL, Lommen EJ, Wildevuur CR, Feijen J. Fabrication and characterization of an asymmetric polyurethane membrane for use as a wound dressing. J Appl Biomater. Winter 1992;3(4):287-303. doi:10.1002/jab.770030408
- Horasan ES, Dag A, Ersoz G, Kaya A. Surgical site infections and mortality in elderly patients. Med Mal Infect. Oct 2013;43(10):417-22. doi:10.1016/j.medmal.2013.07.009
- Howell RD, Hadley S, Strauss E, Pelham FR. Blister formation with negative pressure dressings after total knee arthroplasty. Current Orthopaedic Practice. 2011;22(2):176-179.
- Hussamy DJ, Wortman AC, McIntire DD, Leveno KJ, Casey BM, Roberts SW. Closed Incision Negative Pressure Therapy in Morbidly Obese Women Undergoing Cesarean Delivery: A Randomized Controlled Trial. Obstet Gynecol. Oct 2019;134(4):781-789. doi:10.1097/AOG.0000000000003465
- Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. Jun 2 2015;162(11):777-84. doi:10.7326/M14-2385
- Hyldig N, Vinter CA, Kruse M, et al. Prophylactic incisional negative pressure wound therapy reduces the risk of surgical site infection after caesarean section in obese women: a pragmatic randomised clinical trial. BJOG. Apr 2019;126(5):628-635. doi:10.1111/1471-0528.15413
- Javed AA, Teinor J, Wright M, et al. Negative Pressure Wound Therapy for Surgical-site Infections: A Randomized Trial. Ann Surg. Jun 2019;269(6):1034-1040. doi:10.1097/SLA.0000000000003056
- Kacmaz HY, Baser M, Sozuer EM. Effect of Prophylactic Negative-Pressure Wound Therapy for High-Risk Wounds in Colorectal Cancer Surgery: A Randomized Controlled Trial. Advances in Skin & Wound Care. Nov 2022;35(11):597-603. doi:10.1097/01.Asw.0000874168.60793.10
- Karlakki SL, Hamad AK, Whittall C, Graham NM, Banerjee RD, Kuiper JH. Incisional negative pressure wound therapy dressings (iNPWTd) in routine primary hip and knee arthroplasties: A randomised controlled trial. Bone Joint Res. Aug 2016;5(8):328-37. doi:10.1302/2046-3758.58.BJR-2016-0022.R1
- Keeney JA, Cook JL, Clawson SW, Aggarwal A, Stannard JP. Incisional Negative Pressure Wound Therapy Devices Improve Short-Term Wound Complications, but Not Long-Term Infection Rate Following Hip and Knee Arthroplasty. J Arthroplasty. Apr 2019;34(4):723-728. doi:10.1016/j.arth.2018.12.008
- Kilpadi DV, Cunningham MR. Evaluation of closed incision management with negative pressure wound therapy (CIM): hematoma/seroma and involvement of the lymphatic system. Wound Repair Regen. Sep-Oct 2011;19(5):588-96. doi:10.1111/j.1524-475X.2011.00714.x
- Koek MBG, van der Kooi TII, Stigter FCA, et al. Burden of surgical site infections in the Netherlands: cost analyses and disability-adjusted life years. J Hosp Infect. Nov 2019;103(3):293-302. doi:10.1016/j.jhin.2019.07.010
- Kwon J, Staley C, McCullough M, et al. A randomized clinical trial evaluating negative pressure therapy to decrease vascular groin incision complications. J Vasc Surg. Dec 2018;68(6):1744-1752. doi:10.1016/j.jvs.2018.05.224
- Lee AJ, Sheppard CE, Kent WD, Mewhort H, Sikdar KC, Fedak PW. Safety and efficacy of prophylactic negative pressure wound therapy following open saphenous vein harvest in cardiac surgery: a feasibility study. Interact Cardiovasc Thorac Surg. Mar 1 2017;24(3):324-328. doi:10.1093/icvts/ivw400
- Lee K, Murphy PB, Ingves MV, et al. Randomized clinical trial of negative pressure wound therapy for high-risk groin wounds in lower extremity revascularization. J Vasc Surg. Dec 2017;66(6):1814-1819. doi:10.1016/j.jvs.2017.06.084
- Leitao MM, Jr., Zhou QC, Schiavone MB, et al. Prophylactic Negative Pressure Wound Therapy After Laparotomy for Gynecologic Surgery: A Randomized Controlled Trial. Obstet Gynecol. Feb 1 2021;137(2):334-341. doi:10.1097/AOG.0000000000004243
- Leon MB, C.; Garcia Perez, J.C.; Guedea, M.; Sanz, G.; Gonzalez, C.; Arrayo, A.; Rubio, I.; Gonzalez, C,; Gazo, J.; Cantero-Cid, R. Negative pressure therapy to reduce SSI in open colorectal surgery: prospective, randomised and multicenter study. Colorectal Disease. 2016;18:6-12. doi:10.1111/codi.13441
- Li PY, Yang D, Liu D, Sun SJ, Zhang LY. Reducing Surgical Site Infection with Negative-Pressure Wound Therapy After Open Abdominal Surgery: A Prospective Randomized Controlled Study. Scand J Surg. Sep 2017;106(3):189-195. doi:10.1177/1457496916668681
- Lopez-Lopez V, Hiciano-Guillermo A, Martinez-Alarcon L, et al. Postoperative negative-pressure incision therapy after liver transplant (PONILITRANS study): A randomized controlled trial. Surgery. Apr 2023;173(4):1072-1078. doi:10.1016/j.surg.2022.11.011
- Mahmoud NN, Turpin RS, Yang G, Saunders WB. Impact of surgical site infections on length of stay and costs in selected colorectal procedures. Surg Infect (Larchmt). Dec 2009;10(6):539-44. doi:10.1089/sur.2009.006
- Masden D, Goldstein J, Endara M, Xu K, Steinberg J, Attinger C. Negative pressure wound therapy for at-risk surgical closures in patients with multiple comorbidities: a prospective randomized controlled study. Ann Surg. Jun 2012;255(6):1043-7. doi:10.1097/SLA.0b013e3182501bae
- Masters J, Cook J, Achten J, Costa ML, Group WS. A feasibility study of standard dressings versus negative-pressure wound therapy in the treatment of adult patients having surgical incisions for hip fractures: the WHISH randomized controlled trial. Bone Joint J. Apr 2021;103-B(4):755-761. doi:10.1302/0301-620X.103B4.BJJ-2020-1603.R1
- Meeker J, Weinhold P, Dahners L. Negative pressure therapy on primarily closed wounds improves wound healing parameters at 3 days in a porcine model. J Orthop Trauma. Dec 2011;25(12):756-61. doi:10.1097/BOT.0b013e318211363a
- Muller-Sloof E, de Laat E, Kenc O, et al. Closed-Incision Negative-Pressure Therapy Reduces Donor-Site Surgical Wound Dehiscence in DIEP Flap Breast Reconstructions: A Randomized Clinical Trial. Plastic and Reconstructive Surgery. Oct 2022;150:38s-47s. doi:10.1097/Prs.0000000000009541
- Muller-Sloof E, de Laat HEW, Hummelink SLM, Peters JWB, Ulrich DJO. The effect of postoperative closed incision negative pressure therapy on the incidence of donor site wound dehiscence in breast reconstruction patients: DEhiscence PREvention Study (DEPRES), pilot randomized controlled trial. J Tissue Viability. Nov 2018;27(4):262-266. doi:10.1016/j.jtv.2018.08.005
- Murphy PB, Knowles S, Chadi SA, et al. Negative Pressure Wound Therapy Use to Decrease Surgical Nosocomial Events in Colorectal Resections (NEPTUNE): A Randomized Controlled Trial. Ann Surg. Jul 2019;270(1):38-42. doi:10.1097/SLA.0000000000003111
- Newman JM, Siqueira MBP, Klika AK, Molloy RM, Barsoum WK, Higuera CA. Use of Closed Incisional Negative Pressure Wound Therapy After Revision Total Hip and Knee Arthroplasty in Patients at High Risk for Infection: A Prospective, Randomized Clinical Trial. J Arthroplasty. Mar 2019;34(3):554-559 e1. doi:10.1016/j.arth.2018.11.017
- Nordmeyer M, Pauser J, Biber R, et al. Negative pressure wound therapy for seroma prevention and surgical incision treatment in spinal fracture care. Int Wound J. Dec 2016;13(6):1176-1179. doi:10.1111/iwj.12436
- O'Leary DP, Peirce C, Anglim B, et al. Prophylactic Negative Pressure Dressing Use in Closed Laparotomy Wounds Following Abdominal Operations: A Randomized, Controlled, Open-label Trial: The P.I.C.O. Trial. Ann Surg. Jun 2017;265(6):1082-1086. doi:10.1097/SLA.0000000000002098
- O'Neill CH, Martin RCG, 2nd. Negative-pressure wound therapy does not reduce superficial SSI in pancreatectomy and hepatectomy procedures. J Surg Oncol. Sep 2020;122(3):480-486. doi:10.1002/jso.25980
- Pachowsky M, Gusinde J, Klein A, et al. Negative pressure wound therapy to prevent seromas and treat surgical incisions after total hip arthroplasty. Int Orthop. Apr 2012;36(4):719-22. doi:10.1007/s00264-011-1321-8
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. Mar 29 2021;372:n71. doi:10.1136/bmj.n71
- Pauser J, Nordmeyer M, Biber R, et al. Incisional negative pressure wound therapy after hemiarthroplasty for femoral neck fractures - reduction of wound complications. Int Wound J. Oct 2016;13(5):663-7. doi:10.1111/iwj.12344
- Perry KL, Rutherford L, Sajik DM, Bruce M. A preliminary study of the effect of closed incision management with negative pressure wound therapy over high-risk incisions. BMC Vet Res. Nov 9 2015;11:279. doi:10.1186/s12917-015-0593-4
- Peterson AT, Bakaysa SL, Driscoll JM, Kalyanaraman R, House MD. Randomized controlled trial of single-use negative-pressure wound therapy dressings in morbidly obese patients undergoing cesarean delivery. Am J Obstet Gynecol MFM. Sep 2021;3(5):100410. doi:10.1016/j.ajogmf.2021.100410
- Pleger SP, Nink N, Elzien M, Kunold A, Koshty A, Boning A. Reduction of groin wound complications in vascular surgery patients using closed incision negative pressure therapy (ciNPT): a prospective, randomised, single-institution study. Int Wound J. Feb 2018;15(1):75-83. doi:10.1111/iwj.12836
- PREVENA F. Incision Management System 510(k) Premarket Notification. Available at: https://www.accessdata.fda.gov/cdrh_docs/pdf10/. Accessed August 14, 2018.
- Rashed A, Csiszar M, Beledi A, Gombocz K. The impact of incisional negative pressure wound therapy on the wound healing process after midline sternotomy. Int Wound J. Feb 2021;18(1):95-102. doi:10.1111/iwj.13497
- Ruhstaller K, Downes KL, Chandrasekaran S, Srinivas S, Durnwald C. Prophylactic Wound Vacuum Therapy after Cesarean Section to Prevent Wound Complications in the Obese Population: A Randomized Controlled Trial (the ProVac Study). Am J Perinatol. Sep 2017;34(11):1125-1130. doi:10.1055/s-0037-1604161
- Sapci I, Hull T, Ashburn JH, et al. Effect of Incisional Negative Pressure Wound Therapy on Surgical Site Infections in High Risk Re-Operative Colorectal Surgery: A Randomized Controlled Trial. Dis Colon Rectum. May 2021;64(5)
- Schweizer ML, Cullen JJ, Perencevich EN, Vaughan Sarrazin MS. Costs Associated With Surgical Site Infections in Veterans Affairs Hospitals. JAMA Surg. Jun 2014;149(6):575-81. doi:10.1001/jamasurg.2013.4663
- Sears ED, Wu L, Waljee JF, Momoh AO, Zhong L, Chung KC. The Impact of Deep Sternal Wound Infection on Mortality and Resource Utilization: A Population-based Study. World J Surg. Nov 2016;40(11):2673-2680. doi:10.1007/s00268-016-3598-7
- Shen P, Blackham AU, Lewis S, et al. Phase II Randomized Trial of Negative-Pressure Wound Therapy to Decrease Surgical Site Infection in Patients Undergoing Laparotomy for Gastrointestinal, Pancreatic, and Peritoneal Surface Malignancies. J Am Coll Surg. Apr 2017;224(4):726-737. doi:10.1016/j.jamcollsurg.2016.12.028
- Shields DW, Razii N, Doonan J, Mahendra A, Gupta S. Closed incision negative pressure wound therapy versus conventional dressings following soft-tissue sarcoma excision: a prospective, randomized controlled trial. Bone Joint Open. Dec 2021;2(12):1049-1056. doi:10.1302/2633-1462.212.Bjo-2021-0103.R1
- Shim HS, Choi JS, Kim SW. A Role for Postoperative Negative Pressure Wound Therapy in Multitissue Hand Injuries. Biomed Res Int. 2018;2018:3629643. doi:10.1155/2018/3629643
- Stannard JP, Robinson JT, Anderson ER, McGwin G, Jr., Volgas DA, Alonso JE. Negative pressure wound therapy to treat hematomas and surgical incisions following high-energy trauma. J Trauma. Jun 2006;60(6):1301-6. doi:10.1097/01.ta.0000195996.73186.2e
- Stannard JP, Volgas DA, McGwin G, 3rd, et al. Incisional negative pressure wound therapy after high-risk lower extremity fractures. J Orthop Trauma. Jan 2012;26(1):37-42. doi:10.1097/BOT.0b013e318216b1e5
- Sterne JAC, Savovic J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. Aug 28 2019;366:l4898. doi:10.1136/bmj.l4898
- Suh H, Lee AY, Park EJ, Hong JP. Negative Pressure Wound Therapy on Closed Surgical Wounds With Dead Space: Animal Study Using a Swine Model. Ann Plast Surg. Jun 2016;76(6):717-22. doi:10.1097/SAP.0000000000000231
- Suh HS, Hong JP. Effects of Incisional Negative-Pressure Wound Therapy on Primary Closed Defects after Superficial Circumflex Iliac Artery Perforator Flap Harvest: Randomized Controlled Study. Plast Reconstr Surg. Dec 2016;138(6):1333-1340. doi:10.1097/PRS.0000000000002765
- Tuuli MG, Liu J, Tita ATN, et al. Effect of Prophylactic Negative Pressure Wound Therapy vs Standard Wound Dressing on Surgical-Site Infection in Obese Women After Cesarean Delivery: A Randomized Clinical Trial. JAMA. Sep 22 2020;324(12):1180-1189. doi:10.1001/jama.2020.13361
- Tuuli MG, Martin S, Stout MJ, et al. 412: Pilot randomized trial of prophylactic negative pressure wound therapy in obese women after cesarean delivery. American Journal of Obstetrics and Gynecology. 2017;216(1)doi:10.1016/j.ajog.2016.11.670
- Urban J. A. (2006). Cost analysis of surgical site infections. Surgical infections, 7 Suppl 1, S19–S22. https://doi.org/10.1089/sur.2006.7.s1-19
- Vaddavalli VV, Savlania A, Behera A, Kaman L, Rastogi A, Abuji K. Prophylactic Incisional Negative Pressure Wound Therapy Versus Standard Dressing After Major Lower Extremity Amputation: A Randomized Controlled Trial. Journal of Vascular Surgery. Apr 2022;75(4):14s-14s.
- Wierdak M, Pisarska-Adamczyk M, Wysocki M, et al. Prophylactic negative-pressure wound therapy after ileostomy reversal for the prevention of wound healing complications in colorectal cancer patients: a randomized controlled trial. Tech Coloproctol. Feb 2021;25(2):185-193. doi:10.1007/s10151-020-02372-w
- Wihbey KA, Joyce EM, Spalding ZT, et al. Prophylactic Negative Pressure Wound Therapy and Wound Complication After Cesarean Delivery in Women With Class II or III Obesity: A Randomized Controlled Trial. Obstet Gynecol. Aug 2018;132(2):377-384. doi:10.1097/AOG.0000000000002744
- Witt-Majchrzak A, Zelazny P, Snarska J. Preliminary outcome of treatment of postoperative primarily closed sternotomy wounds treated using negative pressure wound therapy. Pol Przegl Chir. Feb 3 2015;86(10):456-65. doi:10.2478/pjs-2014-0082
- Yu Y, Song Z, Xu Z, et al. Bilayered negative-pressure wound therapy preventing leg incision morbidity in coronary artery bypass graft patients: A randomized controlled trial. Medicine (Baltimore). Jan 2017;96(3):e5925. doi:10.1097/MD.0000000000005925
- Zwanenburg PR, Tol BT, de Vries FEE, Boermeester MA. Incisional Negative Pressure Wound Therapy for Surgical Site Infection Prophylaxis in the Post-Antibiotic Era. Surg Infect (Larchmt). Nov/Dec 2018;19(8):821-830. doi:10.1089/sur.2018.212
- Zwanenburg PR, Tol BT, Obdeijn MC, Lapid O, Gans SL, Boermeester MA. Meta-analysis, Meta-regression, and GRADE Assessment of Randomized and Nonrandomized Studies of Incisional Negative Pressure Wound Therapy Versus Control Dressings for the Prevention of Postoperative Wound Complications. Ann Surg. Jul 2020;272(1):81-91. doi:10.1097/SLA.0000000000003644
Evidence tabellen
Risk of Bias Assessment ROB2
Evidence table, all RR with corresponding 95% CI
|
|
No. studies |
SSIs / participants iNPWT |
SSIs / participants standard wound care |
RR (95% CI)* |
GRADE |
|
Primary outcome |
|||||
|
SSI overall |
57 |
540 / 6849 (7·9%) |
802 / 6895 (11·6%) |
0·67 (0·59 - 0·76) |
High |
|
Type of Surgery |
p value for subgroup differences = 0·14 |
||||
|
Abdominal |
18 |
187 / 1175 (15·9%) |
280 / 1152 (24·3%) |
0·66 (0·54 - 0·81) |
|
|
Breast |
1 |
0 / 50 (0%) |
5 / 50 (10·0%) |
0·09 (0·01 - 1·60) |
|
|
Cardiac |
4 |
1 / 161 (0·6%) |
14 / 155 (9·0%) |
0·14 (0·03 - 0·62) |
|
|
General |
2 |
5 / 54 (9·3%) |
7 / 44 (15·9%) |
0·57 (0·19 - 1·72) |
|
|
Obstetric |
11 |
207 / 3121 (6·6%) |
260 / 3139 (8·3%) |
0·82 (0·66 - 1·03) |
|
|
Orthopedic / trauma |
12 |
78 / 1750 (4·5%) |
127 / 1824 (7·0%) |
0·64 (0·46 - 0·89) |
|
|
Plastic |
3 |
6 / 95 (6·3%) |
7 / 87 (8·0%) |
0·84 (0·30 - 2·34) |
|
|
Vascular |
6 |
56 /443 (12·6%) |
102 / 444 (23·0%) |
0·55 (0·39 - 0·77) |
|
|
Industry involvement |
p value for subgroup differences = 0·17 |
||||
|
No funding or involvement |
16 |
136 / 1687 (7·1%) |
199 / 1752 (11·4%) |
0·70 (0·53 - 0·92) |
|
|
Funding, no involvement |
18 |
300 / 3612 (8·3%) |
400 / 3606 (11·1%) |
0·74 (0·62 - 0·88) |
|
|
Funding + involvement |
13 |
74 / 1129 (6·6%) |
132 / 1156 (11·4%) |
0·59 (0·44 - 0·80) |
|
|
No information |
10 |
30 / 421 (7·1%) |
71 / 408 (17·4%) |
0·46 (0·30 - 0·70) |
|
|
Risk of Bias |
p value for subgroup differences = 0·81 |
||||
|
Low risk of bias |
10 |
105 / 1373 (7·6%) |
149 / 1373 (10·9%) |
0·72 (0·56 - 0·91) |
|
|
Some concerns |
40 |
387 / 4928 (7·9%) |
581 / 4928 (11·8%) |
0·65 (0·55 - 0·77) |
|
|
Low + Some concerns |
50 |
492 / 6301 (7·8%) |
730 / 6301 (11·6%) |
0·67 (0·58 - 0·77) |
|
|
High risk of bias |
7 |
48 / 548 (8·8%) |
72 / 594 (12·1%) |
0·68 (0·46 - 0·98) |
|
|
Pressure of the device |
p value for subgroup differences = 0·45 |
||||
|
-125 mmHg |
28 |
280 / 3131 (8·9%) |
406 / 3128 (13·0%) |
0·69 (0·58 - 0·82) |
|
|
-80 mmHg |
25 |
247 / 3616 (6·8%) |
372 / 3677 (10·1%) |
0·67 (0·55 - 0·81) |
|
|
Cyclic pressure |
1 |
3 / 20 (15·0%) |
3 / 24 (12·5%) |
1·20 (0·27 - 5·30) |
|
|
No information |
3 |
10 / 82 (12·2%) |
21 / 66 (31·8%) |
0·39 (0·19 - 0·82) |
|
|
Secondary outcomes |
|||||
|
Wound dehiscence |
35 |
332 / 4417 (7·5%) |
387 / 4450 (8·7%) |
0·85 (0·71 - 1·02) |
Moderate |
|
Reoperation |
29 |
91 / 4629 (2·0%) |
106 / 4691 (2·3%) |
0·91 (0·69 - 1·20) |
Low |
|
Seroma |
26 |
108 / 3444 (3·1%) |
134 / 3440 (3·9%) |
0·83 (0·65- 1·06) |
Moderate |
|
Hematoma |
23 |
32 / 3419 (0·9%) |
47 / 3408 (1·4%) |
0·77 (0·48 - 1·23) |
Low |
|
Mortality |
19 |
32 / 4052 (0·8%) |
34 / 4053 (0·8%) |
0·94 (0·58 - 1·52) |
Low |
|
Readmission |
19 |
113 / 3241 (3·5%) |
114 / 3246 (3·5%) |
0·96 (0·69 - 1·35) |
Low |
|
Skin blistering |
15 |
198 / 3013 (6·6%) |
37 / 3038 (1.2%) |
5·10 (1·99 - 13·05) |
Moderate |
|
Necrosis |
6 |
3 / 444 (0·7%) |
14 / 479 (2·9%) |
0·46 (0·14 - 1·46) |
Low |
|
* Studies with no events in both arms were excluded from quantitative analysis |
|||||
GRADE-table
Main outcome presenting the overall RR (95%CI), absolute effect estimates, GRADE certainty levels of the evidence and a plain language summary.
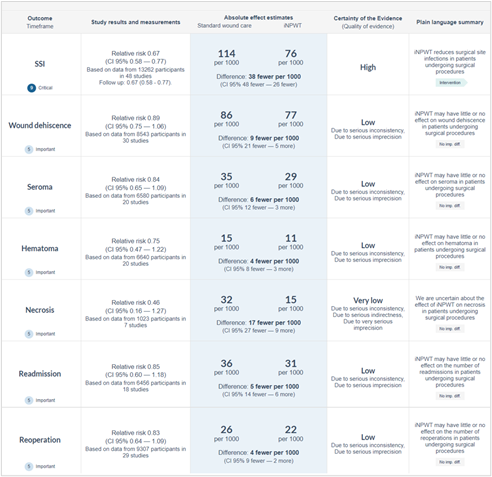
Table of excluded studies
|
|
Study |
Reason for exclusion |
|
1 |
Abesamis 20191 |
No randomization |
|
2 |
Achten 20182 |
Protocol |
|
3 |
ACTRN12619000785101, 20193 |
Protocol |
|
4 |
Anderson4 |
Interim analysis of included study (Chaboyer 20145) |
|
5 |
Biao 20196 |
Comparison not of interest |
|
6 |
Brennfleck 20207 |
Protocol |
|
7 |
Brown 20208 |
Protocol |
|
8 |
Campolier 20199 |
Conference abstract of included study (Costa 202010) |
|
9 |
Carrano 202111 |
No primary closure |
|
10 |
Chaboyer 202112 |
Secondary analysis of included study (Gillespie 202113) |
|
11 |
Chang 201814 |
Comment |
|
12 |
Chen 201915 |
Comparison not of interest |
|
13 |
Chetter 202116 |
Protocol |
|
14 |
ChiCTR1900022165, 201917 |
Protocol |
|
15 |
ChiCTR2000034266, 2020 18 |
Protocol |
|
16 |
Chu 201819 |
Comparison not of interest |
|
17 |
Clark 201920 |
Outcome not of interest |
|
18 |
Cocjin 201921 |
Comparison not of interest |
|
19 |
Cook 201922 |
No primary closure |
|
20 |
Costa 201823 (Health Technol Assess.) |
No primary closure |
|
21 |
Costa 201824 (JAMA) |
No primary closure |
|
22 |
Costa 202025 (Health Technol Assess.) |
Same data as included study (Costa 202010,JAMA) |
|
23 |
CTRI/2019/05/019225, 201926 |
Protocol |
|
24 |
CTRI/2019/08/020895, 201927 |
Protocol |
|
25 |
CTRI/2019/09/021388, 201928 |
Protocol |
|
26 |
Dadras 202229 |
Comparison not of interest |
|
27 |
Darwisch 202030 |
Conference abstract: no data available |
|
28 |
Davis 202031 |
Comparison not of interest |
|
29 |
Di Re 202032 |
Protocol |
|
30 |
Dondossola 202033 |
Letter to the editor |
|
31 |
Donlon 201934 |
Protocol |
|
32 |
DRKS00015136, 201935 |
Protocol |
|
33 |
DRKS00021494, 202036 |
Protocol |
|
34 |
Engelhardt 201837 |
Already included |
|
35 |
Fang 202038 |
No randomization |
|
36 |
Fernandes 202139 |
Conference abstract: no randomisation |
|
37 |
Ferrando 202140 |
Conference abstract: trial protocol |
|
38 |
Fogacci 201941 |
Conference abstract of included study (Fogacci 201942) |
|
39 |
Galiano 201843 |
Within-subject experimental design |
|
40 |
Gombert 201844 |
Already included |
|
41 |
Gombert 201945 |
No randomization |
|
42 |
Gombert 202046 |
Erratum |
|
43 |
Gonzalez 202047 |
Conference abstract: no data available |
|
44 |
Haddad 202148 |
Conference abstract of ongoing trial (NCT03773575) |
|
45 |
Halama 201949 |
Outcome not of interest |
|
46 |
Hasselmann 201950 |
Conference abstract of Hasselmann 202052 (Ann Surg.) |
|
47 |
Hasselmann 202051 (Surg Infect. [Larchmt]) |
Same data as Hasselmann 202052 (Ann Surg.) |
|
48 |
Howell 201153 |
Within-subject experimental design |
|
49 |
Hyldig 201954 |
Already included |
|
50 |
Hyldig 201955 |
Outcome not of interest |
|
51 |
Jaimes 202056 |
Conference abstract: outcome not of interest |
|
52 |
Javed 201957 |
Already included |
|
53 |
Jenkins 202258 |
Outcome not of interest |
|
54 |
Jørgensen 201859 |
Protocol |
|
55 |
BJOG. 2019 Apr;126(5):636 (No author listed) 60 |
Comment |
|
56 |
KCT0004063, 201961 |
Protocol |
|
57 |
Kim 202062 |
Protocol |
|
58 |
Knight 201963 |
Protocol |
|
59 |
Kojima 202164 |
No primary closure |
|
60 |
Kuncewitch 201965 |
Same data as Shen 201766 |
|
61 |
Kwon 201867 |
Within-subject experimental design |
|
62 |
Lee 201768 |
Already included |
|
63 |
Leitao 202069 |
Conference abstract of included study (Leitao 202170) |
|
64 |
Lopez 202271 |
Conference abstract of included study (Lopez-Lopez 202372) |
|
65 |
Low 202273 |
Protocol |
|
66 |
Lozano-Balderas 201774 |
No primary closure |
|
67 |
Lychagin 202075 |
Comparison not of interest |
|
68 |
Martin 201976 |
Conference abstract of included study (O’neill 202077) |
|
69 |
Masters 201878 |
Same data as Masters 202179 |
|
70 |
Molina 202180 |
Conference abstract: no data available |
|
71 |
Mondal 202281 |
Comparison not of interest |
|
72 |
Mujahid 202082 |
Outcome not of interest |
|
73 |
Muller-Sloof 201883 |
Already included |
|
74 |
Murphy 201984 |
Already included |
|
75 |
Myllykangas 202185 |
No randomization |
|
76 |
NCT03815370, 201986 |
Protocol |
|
77 |
NCT03816293, 201987 |
Protocol |
|
78 |
NCT03820219, 201988 |
Protocol |
|
79 |
NCT03871023, 201989 |
Protocol |
|
80 |
NCT03886818, 201990 |
Protocol |
|
81 |
NCT03900078, 201991 |
Protocol |
|
82 |
NCT03905213, 201992 |
Protocol |
|
83 |
NCT03935659, 201993 |
Protocol |
|
84 |
NCT03948412, 201994 |
Protocol |
|
85 |
NCT04003038, 201995 |
Protocol |
|
86 |
NCT04039659, 201996 |
Protocol |
|
87 |
NCT04063111, 201997 |
Protocol |
|
88 |
NCT04088162, 201998 |
Protocol |
|
89 |
NCT04110353, 201999 |
Protocol |
|
90 |
NCT04174183, 2019100 |
Protocol |
|
91 |
NCT04265612, 2020101 |
Protocol |
|
92 |
NCT04434820, 2020102 |
Protocol |
|
93 |
NCT04453319, 2020103 |
Protocol |
|
94 |
NCT04455724, 2020104 |
Protocol |
|
95 |
NCT04496180, 2020105 |
Protocol |
|
96 |
NCT04520841, 2020106 |
Protocol |
|
97 |
NCT04539015, 2020107 |
Protocol |
|
98 |
NCT04584957, 2020108 |
Protocol |
|
99 |
Newman 2019, 2020109 |
Already included |
|
100 |
Ni 2020110 |
No primary closure |
|
101 |
Nip 2020111 |
Conference abstract: no randomisation |
|
102 |
Nordmeyer112 |
No data available |
|
103 |
Ozkan 2020113 |
No randomisation |
|
104 |
Paim / RBR-5c8y6v, 2019114 |
Protocol |
|
105 |
Pape 2021115 |
Conference abstract: secondary analysis of included study (Tuuli 2020116) |
|
106 |
Park 2019117 |
No randomization |
|
107 |
Pauser 2016118 |
Comparison not of interest (drain in wound) |
|
108 |
Png 2020119 |
Same as Costa 202010 |
|
109 |
Pleger 2018120 |
Within-subject experimental design |
|
110 |
Rajabaleyan 2019121 |
Conference abstract: no primary closure |
|
111 |
Rezk 2019122 |
Protocol |
|
112 |
Sandy-Hodgetts 2017123 |
Protocol |
|
113 |
Sandy-Hodgetts 2020124 |
Protocol |
|
114 |
Sapci 2021125 |
Conference abstract of included study (Sapci 2022126) |
|
115 |
Schmid 2020127 |
Within-subject experimental design |
|
116 |
Schwartzmann 2021128 |
No randomization |
|
117 |
Seidel 2020129 |
Comparison not of interest |
|
118 |
Seidel 2020130 |
No primary closure |
|
119 |
Serra 2019131 |
No randomization |
|
120 |
Shim 2018132 |
Duplicate |
|
121 |
Stannard 2012133 |
Within-subject experimental design |
|
122 |
Sun 2019134 |
Comparison not of interest |
|
123 |
Svensson-Björk 2021135 |
Outcome not of interest |
|
124 |
Szmeja 2020136 |
Conference abstract: no data available |
|
125 |
Tanaydin 2018137 |
Within-subject experimental design |
|
126 |
Tanaydin 2018138 |
Erratum |
|
127 |
Venkatadass 2013139 |
Retracted |
|
128 |
Wang 2019140 |
Comparison not of interest |
|
129 |
Wilkin 2021141 |
Protocol |
|
130 |
Wilkin 2021141 |
Protocol, duplicate |
|
131 |
Wilkin 2022142 |
Conference abstract: protocol |
|
132 |
Yang 2020143 |
Comparison not of interest |
|
133 |
Yilmaz 2022144 |
Protocol |
|
134 |
Zhao 2020145 |
No randomization |
|
135 |
Zwanenburg 2020146 |
Comment |
|
1. Abesamis GM, Chopra S, Vickery K, Deva AK. A Comparative Trial of Incisional Negative-Pressure Wound Therapy in Abdominoplasty. Plast Reconstr Surg Glob Open. May 2019;7(5):e2141. doi:10.1097/GOX.0000000000002141. 2. Achten J, Vadher K, Bruce J, et al. Standard wound management versus negative-pressure wound therapy in the treatment of adult patients having surgical incisions for major trauma to the lower limb - a two-arm parallel group superiority randomised controlled trial: protocol for Wound Healing in Surgery for Trauma (WHIST). Bmj Open. Jun 2018;8(6)doi:ARTN e022115; 10.1136/bmjopen-2018-022115. 3. ACTRN12619000785101. Negative Pressure Wound Therapy to Reduce Incisional Wound Infections - A Randomised Control Trial. https://trialsearchwhoint/Trial2aspx?TrialID=ACTRN12619000785101. 2019. 4. Anderson V, Chaboyer W, Gillespie BM, Fenwick J. The use of negative pressure wound therapy dressing in obese women undergoing caesarean section:: a pilot study. Evidence Based Midwifery. 2014;12(1):23. 5. Chaboyer W, Anderson V, Webster J, Sneddon A, Thalib L, Gillespie BM. Negative Pressure Wound Therapy on Surgical Site Infections in Women Undergoing Elective Caesarean Sections: A Pilot RCT. Healthcare (Basel). Sep 30 2014;2(4):417-28. doi:10.3390/healthcare2040417. 6. Biao Y, Shan W, Yan Z, L C. Treatment of chronic refractory wounds with negative pressure wound therapy and platelet-rich plasma: accelerating the re-epithelialization of wounds and increasing. Chinese Journal of Tissue Engineering Research. 23(26):4181. 7. Brennfleck FW, Linsenmeier L, Junger HHG, et al. Negative pressure wound therapy (NPWT) on closed incisions to prevent surgical site infection in high-risk patients in hepatopancreatobiliary surgery: study protocol for a randomized controlled trial—the NP-SSI trial. Trials. 2020 2020;21(1). 8. Brown S, Nixon J, Ransom M, et al. Multiple Interventions for Diabetic Foot Ulcer Treatment Trial (MIDFUT): study protocol for a randomised controlled trial. BMJ open. 2020 2020;10(4). 9. Campolier M, Knight R, Spoors L, Achten J, Costa M. Assessing the quality of data collection in clinic; lessons from the wound healing in surgical trauma (WHiST) RCT. Trials. 2019 2019;20. 10. Costa ML, Achten J, Knight R, et al. Effect of Incisional Negative Pressure Wound Therapy vs Standard Wound Dressing on Deep Surgical Site Infection After Surgery for Lower Limb Fractures Associated With Major Trauma: The WHIST Randomized Clinical Trial. Jama. 2020 2020;323(6):519-526. 11. Carrano FM, Maroli A, Carvello M, et al. Negative-pressure wound therapy after stoma reversal in colorectal surgery: a randomized controlled trial. Bjs Open. Nov 9 2021;5(6)doi:ARTN zrab116; 10.1093/bjsopen/zrab116 12. Chaboyer W, Ellwood D, Thalib L, et al. Incidence and predictors of surgical site infection in women who are obese and give birth by elective caesarean section: A secondary analysis. Aust N Z J Obstet Gynaecol. Apr 2022;62(2):234-240. doi:10.1111/ajo.13428 13. Gillespie BM, Webster J, Ellwood D, et al. Closed incision negative pressure wound therapy versus standard dressings in obese women undergoing caesarean section: multicentre parallel group randomised controlled trial. BMJ. May 5 2021;373:n893. doi:10.1136/bmj.n893 14. Chang EI. Discussion: Comparison between Negative-Pressure Fixation and Film Dressing in Wound Management after Tissue Expansion: A Randomized Controlled Trial. Plastic and reconstructive surgery. 2018 2018;142(1):42-43. 15. Chen SQ, Liu WC, Zhang ZZ, et al. [Application of closed negative pressure irrigation and suction device in the treatment of high perianal abscess]. Zhonghua Wei Chang Wai Ke Za Zhi. 2019 2019;22(4):364-369. 16. Chetter I, Arundel C, Martin BC, et al. Negative pressure wound therapy versus usual care for surgical wounds healing by secondary intention (SWHSI-2 trial): study protocol for a pragmatic, multicentre, cross surgical specialty, randomised controlled trial. Trials. Oct 25 2021;22(1):739. doi:10.1186/s13063-021-05662-2 17. ChiCTR1900022165. Topical continuous delivery of non-pressurised oxygen combined with negative pressure wound therapy for chronic wounds: A Randomized controlled trial. https://trialsearchwhoint/Trial2aspx?TrialID=ChiCTR1900022165. 2019; 18. ChiCTR2000034266. The effect and mechanism of negative pressure wound therapy on the survival rate and scar formation of modified Meek skin graft: a prospective randomized controlled study. https://trialsearchwhoint/Trial2aspx?TrialID=ChiCTR2000034266. 2020 2020; 19. Chu W, Liu S, Wang Y, Li J, Liu H. Compressed fixation combined with vacuum-assisted closure for treating acute injury of the heel fat pad. Medical Science Monitor. 2018 2018;24:9466-9472. 20. Clark JM, Rychlik S, Harris J, Seikaly H, Biron VL, O'Connell DA. Donor site morbidity following radial forearm free flap reconstruction with split thickness skin grafts using negative pressure wound therapy. Le Journal d'oto-rhino-laryngologie et de chirurgie cervico-faciale [Journal of otolaryngology - head & neck surgery]. 2019 2019;48(1):21. 21. Cocjin HGB, Jingco JKP, Tumaneng FDC, Coruña JMR. Wound-Healing Following Negative-Pressure Wound Therapy with Use of a Locally Developed AquaVac System as Compared with the Vacuum-Assisted Closure (VAC) System. Journal of bone and joint surgery American volume. 2019 2019;101(22):1990‐1998. 22. Cook R, Thomas V, Martin R. Negative pressure dressings are no better than standard dressings for open fractures. BMJ (Online). 2019 2019;364 23. Costa ML, Achten J, Bruce J, et al. Negative-pressure wound therapy versus standard dressings for adults with an open lower limb fracture: The WOLLF RCT. Health Technology Assessment. 2018 2018;22(73):v-162. 24. Costa ML, Achten J, Bruce J, et al. Effect of negative pressure wound therapy vs standard wound management on 12-month disability among adults with severe open fracture of the lower limb the wollf randomized clinical trial. JAMA - Journal of the American Medical Association. 2018 2018;319(22):2280-2288. 25. Costa ML, Achten J, Knight R, et al. Negative-pressure wound therapy compared with standard dressings following surgical treatment of major trauma to the lower limb: the WHiST RCT. Health Technol Assess. 2020 2020;24(38):1-86. 26. CTRI/2019/05/019225. Clinical effectiveness of indigenous NPWT system Vacon for treatment of non healing wounds as compared to conventional wound therapy. 2019. 27. CTRI/2019/08/020895. To evaluate negative pressure dressings in decreasing surgical site infections after emergency laparotomy: a randomized controlled study. https://trialsearchwhoint/Trial2aspx?TrialID=CTRI/2019/08/020895. 2019. 28. CTRI/2019/09/021388. To compare number of infection between normal wounds dressing and wounds with machine dressing in open wounds after emergency operations of abdomen with pus: a comparison study. https://trialsearchwhoint/Trial2aspx?TrialID=CTRI/2019/09/021388. 2019 2019; 29. Dadras M, Ufton D, Sogorski A, et al. Closed-Incision Negative-Pressure Wound Therapy after Resection of Soft-Tissue Tumors Reduces Wound Complications: Results of a Randomized Trial. Plastic and Reconstructive Surgery. May 2022;149(5)doi:10.1097/Prs.0000000000009023 30. Darwisch A, Fajfrova Z. Negative pressure therapy after median sternotomy on closed incision: A randomized controlled study. Thoracic and Cardiovascular Surgeon. 2020 2020;68 31. Davis KE, La Fontaine J, Farrar D, et al. Randomized clinical study to compare negative pressure wound therapy with simultaneous saline irrigation and traditional negative pressure wound therapy for complex foot infections. Wound Repair Regen. 2020 2020;28(1):97-104. 32. Di Re AM, Wright D, Toh JWT, et al. Surgical wound infection prevention using topical negative pressure therapy on closed abdominal incisions – the ‘SWIPE IT’ randomized clinical trial. Journal of Hospital Infection. 2021 2021;110:76-83. 33. Dondossola D, Antonelli B, Rossi G. Vacuum-assisted wound closure and liver transplantation: new perspective and challenges. Updates Surg. Mar 2020;72(1):223-224. doi:10.1007/s13304-019-00693-6 34. Donlon NE, Boland PA, Kelly ME, et al. Prophylactic negative wound therapy in laparotomy wounds (PROPEL trial): randomized controlled trial. Int J Colorectal Dis. 2019 2019;34(11):2003-2010. 35. DRKS00015136. Negative pressure wound therapy (NPWT) on closed incisions to prevent surgical site infection in HPB-surgery. https://trialsearchwhoint/Trial2aspx?TrialID=DRKS00015136. 2019. 36. DRKS00021494. Single use negative pressure wound therapy system (Prevena ™) compared to standard wound care after spinal surgery. https://trialsearchwhoint/Trial2aspx?TrialID=DRKS00021494. 2020. 37. Engelhardt M, Rashad NA, Willy C, et al. Closed-incision negative pressure therapy to reduce groin wound infections in vascular surgery: a randomised controlled trial. International Wound Journal. 2018 2018;15(3):327-332. 38. Fang CL, Changchien CH, Chen MS, Hsu CH, Tsai CB. Closed incision negative pressure therapy following abdominoplasty after breast reconstruction with deep inferior epigastric perforator flaps. Int Wound J. 2020 2020;17(2):326-331. 39. Fernandes U, Marçal A, Pereira R, et al. The Impact of Closed Incision Negative Pressure Therapy on Postoperative Oncologic Breast Surgery Outcomes. European Journal of Surgical Oncology. 2021 2021;47(2):e45. 40. Ferrando P, Castellano I, Folli S, et al. A national multicenter randomized controlled trial to evaluate Closed Incision Negative Pressure Therapy in oncological breast surgery. European Journal of Surgical Oncology. 2021 2021;47(2):e16-e17. 41. Fogacci T, Cattin F, Samorani D. The negative pressure therapy with PICO as a prevention of surgical site infection in high risk patients undergoing breast surgery. Annals of Oncology. 2019 2019;30:iii42. 42. Fogacci T, Cattin F, Semprini G, Frisoni G, Fabiocchi L, Samorani D. The negative pressure therapy with PICO as a prevention of surgical site infection in high-risk patients undergoing breast surgery. Breast J. May 2020;26(5):1071-1073. doi:10.1111/tbj.13659 43. Galiano RD, Hudson D, Shin J, et al. Incisional Negative Pressure Wound Therapy for Prevention of Wound Healing Complications Following Reduction Mammaplasty. Plast Reconstr Surg Glob Open. Jan 2018;6(1):e1560. doi:10.1097/GOX.0000000000001560 44. Gombert A, Babilon M, Barbati ME, et al. Closed Incision Negative Pressure Therapy Reduces Surgical Site Infections in Vascular Surgery: A Prospective Randomised Trial (AIMS Trial). Eur J Vasc Endovasc Surg. Sep 2018;56(3):442-448. doi:10.1016/j.ejvs.2018.05.018 45. Gombert A, Babilon M, Barbati M, et al. Closed-incision Negative-pressure Therapy Reduces Surgical Site Infections in Vascular Surgery: A Prospective Randomised Controlled Trial (Aims Trial). European Journal of Vascular and Endovascular Surgery. 2019 2019;58(6):e359. 46. Gombert A, Babilon M, Barbati M, et al. Correction: Closed incisional negative pressure therapy may reduce surgical site infection rate following endophlebectomy with complementary polytetrafluoroethylene arteriovenous fistula of the common femoral vein (Journal of Vascular Surgery: Venous and Lymphatic Disorders (2020) 8(1) (89–94), (S2213333X1930438X), (10.1016/j.jvsv.2019.08.010)). Journal of Vascular Surgery: Venous and Lymphatic Disorders. 2020 2020;8(2):339. 47. Gonzalez MG, Elisa Barske M, Kjellsson KB, Saboda K, Hill MG. Topical negative pressure wound therapy to prevent wound complications following cesarean delivery in high risk obstetric patients. Reproductive Sciences. 2020 2020;27(1):136A-137A. 48. Haddad T, Bocchese S, Staley C, et al. Preliminary Analysis of Negative Pressure to Prevent Lower Extremity Amputation Wound Complications: Pilot Data From a Randomized Clinical Trial. Journal of Vascular Surgery. Sep 2021;74(3):E153-E153. 49. Halama D, Dreilich R, Lethaus B, Bartella A, Pausch NC. Donor-site morbidity after harvesting of radial forearm free flaps-comparison of vacuum-assisted closure with conventional wound care: A randomized controlled trial. J Craniomaxillofac Surg. 2019 2019;47(12):1980-1985. 50. Hasselmann J, Björk J, Svensson-Björk R, Acosta S. Inguinal Vascular Surgical Wound Protection by Incisional Negative Pressure Wound Therapy – A Randomized Controlled Trial – INVIPS Trial. European Journal of Vascular and Endovascular Surgery. 2019 2019;58(6):e726-e727. 51. Hasselmann J, Björk J, Svensson-Björk R, Butt T, Acosta S. Proposed Classification of Incision Complications: Analysis of a Prospective Study on Elective Open Lower-Limb Revascularization. Surg Infect (Larchmt). 2020 2020;21(4):384-390. 52. Hasselmann J, Bjork J, Svensson-Bjork R, Acosta S. Inguinal Vascular Surgical Wound Protection by Incisional Negative Pressure Wound Therapy: A Randomized Controlled Trial-INVIPS Trial. Ann Surg. Jan 2020;271(1):48-53. doi:10.1097/SLA.0000000000003364. 53. Howell RD, Hadley S, Strauss E, Pelham FR. Blister formation with negative pressure dressings after total knee arthroplasty. Current Orthopaedic Practice. 2011;22(2):176-179. 54. Hyldig N, Vinter CA, Kruse M, et al. Prophylactic incisional negative pressure wound therapy reduces the risk of surgical site infection after caesarean section in obese women: a pragmatic randomised clinical trial. BJOG. Apr 2019;126(5):628-635. doi:10.1111/1471-0528.15413. 55. Hyldig N, Joergensen JS, Wu C, et al. Cost-effectiveness of incisional negative pressure wound therapy compared with standard care after caesarean section in obese women: a trial-based economic evaluation. BJOG. 2019 2019;126(5):619-627. 56. Jaimes HG, B. Performance, safety, and efficacy of a single-use negative pressure wound therapy system for surgically closed incision sites and skin grafts: A prospective multi-center follow-up study. 2020. 57. Javed AA, Teinor J, Wright M, et al. Negative Pressure Wound Therapy for Surgical-site Infections: A Randomized Trial. Ann Surg. 2019 2019;269(6):1034-1040. 58. Jenkins S, Komber M, Mattam K, Briffa N. Negative pressure wound therapy in patients with diabetes undergoing left internal thoracic artery harvest: A randomized control trial. J Thorac Cardiovasc Surg. Apr 9 2022;doi:10.1016/j.jtcvs.2022.01.060 59. Jørgensen MG, Toyserkani NM, Hyldig N, et al. Prevention of seroma following inguinal lymph node dissection with prophylactic, incisional, negative-pressure wound therapy (SEROMA trial): study protocol for a randomized controlled trial [published correction appears in Trials. 2018 Oct 19;19(1):570]. Trials. 2018;19(1):441. Published 2018 Aug 15. doi:10.1186/s13063-018-2757-6 60. Should negative pressure wound therapy be used at the time of caesarean in obese women? BJOG: An International Journal of Obstetrics and Gynaecology. 2019 2019;126(5):636. 61. KCT0004063. The effectiveness of negative pressure wound dressing for the wound healing after stoma closure: An prospective, open-label, randomized control study. https://trialsearchwhoint/Trial2aspx?TrialID=KCT0004063. 2019. 62. Kim S, Kang SI. The effectiveness of negative-pressure wound therapy for wound healing after stoma reversal: a randomised control study (SR-PICO study). Trials. 2020 2020;21(1):24. 63. Knight R, Spoors LM, Costa ML, Dutton SJ. Wound Healing In Surgery for Trauma (WHIST): statistical analysis plan for a randomised controlled trial comparing standard wound management with negative pressure wound therapy. Trials. 2019 2019;20(1):186. 64. Kojima K, Goto M, Nagashima Y, et al. Effectiveness of negative pressure wound therapy for the wound of ileostomy closure: a multicenter, phase II randomized controlled trial. Bmc Surgery. Dec 28 2021;21(1)doi:ARTN 442 10.1186/s12893-021-01446-2 65. Kuncewitch MP, Blackham AU, Clark CJ, et al. Effect of Negative Pressure Wound Therapy on Wound Complications Post-Pancreatectomy. Am Surg. 2019 2019;85(1):1-7. 66. Shen P, Blackham AU, Lewis S, et al. Phase II Randomized Trial of Negative-Pressure Wound Therapy to Decrease Surgical Site Infection in Patients Undergoing Laparotomy for Gastrointestinal, Pancreatic, and Peritoneal Surface Malignancies. J Am Coll Surg. Apr 2017;224(4):726-737. doi:10.1016/j.jamcollsurg.2016.12.028 67. Kwon J, Staley C, McCullough M, et al. A randomized clinical trial evaluating negative pressure therapy to decrease vascular groin incision complications. J Vasc Surg. Dec 2018;68(6):1744-1752. doi:10.1016/j.jvs.2018.05.224 68. Lee K, Murphy PB, Ingves MV, et al. Randomized clinical trial of negative pressure wound therapy for high-risk groin wounds in lower extremity revascularization. Journal of Vascular Surgery. 2017 2017;66(6):1814-1819. 69. Leitao MM, Zhou Q, Schiavone MB, et al. A phase 3 randomized controlled trial of preventive negative pressure wound therapy in postoperative incision management. Gynecologic Oncology. 2020 2020;159:53. 70. Leitao MM, Jr., Zhou QC, Schiavone MB, et al. Prophylactic Negative Pressure Wound Therapy After Laparotomy for Gynecologic Surgery: A Randomized Controlled Trial. Obstet Gynecol. Feb 1 2021;137(2):334-341. doi:10.1097/AOG.0000000000004243 71. Lopez VL, Martinez-Alarcon L, Hiciano-Guillermo A, et al. Postoperative negative-pressure incision therapy following liver transplant (ponilitrans study): a randomized controlled trial. Transplantation. Aug 2022;106(8s):101-101. 72. Lopez-Lopez V, Hiciano-Guillermo A, Martinez-Alarcon L, et al. Postoperative negative-pressure incision therapy after liver transplant (PONILITRANS study): A randomized controlled trial. Surgery. Apr 2023;173(4):1072-1078. doi:10.1016/j.surg.2022.11.011 73. Low EZ, Nugent TS, O'Sullivan NJ, et al. Application of PREVENA (Surgical Incision Protection System) in reducing surgical site infections following reversal of ileostomy or colostomy: the PRIC study protocol. International Journal of Colorectal Disease. May 2022;37(5):1215-1221. doi:10.1007/s00384-022-04153-3 74. Lozano-Balderas G, Ruiz-Velasco-Santacruz A, Diaz-Elizondo JA, Gomez-Navarro JA, Flores-Villalba E. Surgical Site Infection Rate Drops to 0% Using a Vacuum-Assisted Closure in Contaminated/Dirty Infected Laparotomy Wounds. Am Surg. May 1 2017;83(5):512-514. 75. Lychagin AV, Rosenberg N, Gritsyuk AA. Evaluation of the potential complications of surgical wound drainage in primary total hip arthroplasty: a prospective controlled double-blind study. Hip Int. 2020 2020:1120700020941749. 76. Martin RCG, O'Neill CH. Negative-pressure therapy for hepatectomy and pancreatectomy: A randomized trial for surgical site infection prevention. HPB. 2019 2019;21:S26-S27. 77. O'Neill CH, Martin RCG, 2nd. Negative-pressure wound therapy does not reduce superficial SSI in pancreatectomy and hepatectomy procedures. J Surg Oncol. Sep 2020;122(3):480-486. doi:10.1002/jso.25980 78. Masters JPM, Achten J, Cook J, Dritsaki M, Sansom L, Costa ML. Randomised controlled feasibility trial of standard wound management versus negative-pressure wound therapy in the treatment of adult patients having surgical incisions for hip fractures. BMJ Open. 2018 2018;8(4) 79. Masters J, Cook J, Achten J, Costa ML, Group WS. A feasibility study of standard dressings versus negative-pressure wound therapy in the treatment of adult patients having surgical incisions for hip fractures: the WHISH randomized controlled trial. Bone Joint J. Apr 2021;103-B(4):755-761. doi:10.1302/0301-620X.103B4.BJJ-2020-1603.R1 80. Molina AC, Pla MJ. Negative Pressure Therapy in the Prevention of Surgical Wound Complications in Breast Oncoplastic Surgery. A Prospective Randomized Study. International Journal of Gynecological Cancer. Mar 2021;31:A372-A372. doi:10.1136/ijgc-2021-ESGO.657 81. Mondal A, Ali MS, Galidevara I, Arumugam M. Effect of Incisional Negative Pressure Wound Therapy Following Incisional Hernia Repair-A Randomised Controlled Trial. JCDR Feb 2022;16(2):1-4. doi: 10.7860/JCDR/2022/51153.15955. 82. Mujahid AM, Khalid FA, Ali N, Sajjad Y, Khan H, Tarar MN. Vacuum-assisted Closure in Integration of Skin Graft Over Scalp Wounds: A Randomised Control Trial. J Coll Physicians Surg Pak. 2020 2020;30(2):163-167. 83. Muller-Sloof E, de Laat HEW, Hummelink SLM, Peters JWB, Ulrich DJO. The effect of postoperative closed incision negative pressure therapy on the incidence of donor site wound dehiscence in breast reconstruction patients: DEhiscence PREvention Study (DEPRES), pilot randomized controlled trial. Journal of Tissue Viability. 2018 2018;27(4):262-266. 84. Murphy PB, Knowles S, Chadi SA, et al. Negative Pressure Wound Therapy Use to Decrease Surgical Nosocomial Events in Colorectal Resections (NEPTUNE): A Randomized Controlled Trial. Ann Surg. 2019 2019;270(1):38-42. 85. Myllykangas HM, Halonen J, Husso A, Vaananen H, Berg LT. Does Incisional Negative Pressure Wound Therapy Prevent Sternal Wound Infections? Thorac Cardiovasc Surg. Jan 2022;70(1):65-71. doi:10.1055/s-0041-1731767 86. NCT03815370. A Non-Traumatic Binder for Temporary Abdominal Wall Closure. https://clinicaltrialsgov/show/NCT03815370. 2019. 87. NCT03816293. SUpPress SSI - Single Use Negative Pressure Wound Therapy (NPWT) to Reduce Surgical Site Infections. https://clinicaltrialsgov/show/NCT03816293. 2019 2019. 88. NCT03820219. Incisional Negative Pressure Wound Therapy in Patients Undergoing Spine Surgery. https://clinicaltrialsgov/show/NCT03820219. 2019. 89. NCT03871023. Prophylactic Negative Wound Therapy in Laparotomy Wounds. https://clinicaltrialsgov/show/NCT03871023. 2019. 90. NCT03886818. Efficacy of Negative Pressure Wound Therapy After Total Ankle Arthroplasty. https://clinicaltrialsgov/show/NCT03886818. 2019. 91. NCT03900078. Incisional Negative Pressure Wound Therapy for Resection of Soft Tissue Tumors. https://clinicaltrialsgov/show/NCT03900078. 2019. 92. NCT03905213. Prevention of Surgical Wound Infection. https://clinicaltrialsgov/show/NCT03905213. 2019. 93. NCT03935659. Negative Pressure Wound Therapy for Surgical Site Infection Prevention in Common Femoral Artery Exposure. https://clinicaltrialsgov/show/NCT03935659. 2019. 94. NCT03948412. Negative Pressure Wound Therapy (PREVENA) Versus Standard Dressings for Incision Management After Renal Transplant. https://clinicaltrialsgov/show/NCT03948412. 2019. 95. NCT04003038. Negative Pressure Wound Therapy in Healing Abdominal Incision in Obese Patients Undergoing Breast Reconstruction Surgery. https://clinicaltrialsgov/show/NCT04003038. 2019. 96. NCT04039659. POstoperative Negative-pressure Incision Therapy Following LIver TRANSplant: a Randomized Controlled Trial. https://clinicaltrialsgov/show/NCT04039659. 2019. 97. NCT04063111. Role of Vacuum in Open Fracture Tibia Grade III Type B. https://clinicaltrialsgov/show/NCT04063111. 2019. 98. NCT04088162. The Use of Post-operative NPWT Dressing in the Prevention of Infectious Complications After Ostomy Reversal Surgery. https://clinicaltrialsgov/show/NCT04088162. 2019. 99. NCT04110353. Prophylactic Closed Incision Negative Pressure Wound Therapy on Abdominal Wounds - Clinical and Economic Perspectives. https://clinicaltrialsgov/show/NCT04110353. 2019. 100. NCT04174183. Evaluation of the Effectiveness of a Closed-incision Negative-pressure Therapy (Prevena®) on Bilateral Groin Incision. https://clinicaltrialsgov/show/NCT04174183. 2019. 101. NCT04265612. Effect of the Negative Pressure Therapy Dressing Compared With Hydrogel Dressing. https://clinicaltrialsgov/show/NCT04265612. 2020. 102. NCT04434820. External Negative Pressure Dressing System vs. Traditional Wound Dressing for Cesarean Section Incision in Obese Women. https://clinicaltrialsgov/show/NCT04434820. 2020. 103. NCT04453319. Efficacy of Negative Pressure Wound Closure Therapy by PICO System in Prevention of Complications of Femoral Artery Exposure. https://clinicaltrialsgov/show/NCT04453319. 2020. 104. NCT04455724. Negative Pressure Incisional Wound Therapy for High-risk Ventral Hernia Repair: a Randomized Controlled Trial. https://clinicaltrialsgov/show/NCT04455724. 2020. 105. NCT04496180. Prevena to Prevent Surgical Site Infection After Emergency Abdominal Laparotomy. https://clinicaltrialsgov/show/NCT04496180. 2020. 106. NCT04520841. Clinical Trial Comparing Negative Pressure Wound Therapy and Standard Dry Dressings. https://clinicaltrialsgov/show/NCT04520841. 2020. 107. NCT04539015. Assess the Efficacy of Prevena Plus vs SOC to Closed Incision in Pts Undergoing CAWR and Other Laparotomy Procedures. https://clinicaltrialsgov/show/NCT04539015. 2020. 108. NCT04584957. Prophylactic Negative Pressure Wound Therapy (VAC) in Gynecologic Oncology (G.O.). https://clinicaltrialsgov/show/NCT04584957. 2020. 109. Newman JM, Siqueira MBP, Klika AK, Molloy RM, Barsoum WK, Higuera CA. Use of Closed Incisional Negative Pressure Wound Therapy After Revision Total Hip and Knee Arthroplasty in Patients at High Risk for Infection: A Prospective, Randomized Clinical Trial. J Arthroplasty. 2019 2019;34(3):554-559.e1. 110. Ni Z, Sun J, Qi S. Therapeutic Effect of Topical Negative Pressure Therapy/Vacuum-Associated Closure Therapy on Cephalic Facial Skin Abscess. Surg Infect (Larchmt). 2020 2020;21(8):722-725. 111. Nip L, Fatayer H, Rusius V, Bramley M. Surgical Site Infection (SSI) and seroma rates in oncoplastic breast patients using negative pressure wound dressings. European Journal of Surgical Oncology. 2020 2020;46(6):e34-e35. 112. Nordmeyer M, Pauser J, Biber R, et al. Negative pressure wound therapy for seroma prevention and surgical incision treatment in spinal fracture care. Int Wound J. 2016;13(6):1176-1179. doi:10.1111/iwj.12436. 113. Ozkan B, Markal Ertas N, Bali U, Uysal CA. Clinical Experiences with Closed Incisional Negative Pressure Wound Treatment on Various Anatomic Locations. Cureus. 2020 2020;12(6):e8849. 114. RBR-5c8y6v. The Wound action effects of a Simple Suction Dressing. http://wwwwhoint/trialsearch/Trial2aspx?TrialID=RBR-5c8y6v. 2019 2019; 115. Pape K, Tuuli MG, Neal CM, et al. Predictors of surgical-site infection after cesarean delivery in obese women receiving evidence-based preventive measures. American Journal of Obstetrics and Gynecology. 2021 2021;224(2):S652-S653. 116. Tuuli MG, Liu J, Tita ATN, et al. Effect of Prophylactic Negative Pressure Wound Therapy vs Standard Wound Dressing on Surgical-Site Infection in Obese Women After Cesarean Delivery: A Randomized Clinical Trial. JAMA. Sep 22 2020;324(12):1180-1189. doi:10.1001/jama.2020.13361. 117. Park KU, Clemens MW, Lange CE, Bridges CA, Checka CM. Novel Use of Incisional Negative Pressure Wound Therapy for Management of High-risk Breast Incisions. Ann Surg. 2019 2019;270(6):e73-e74. 118. Pauser J, Nordmeyer M, Biber R, et al. Incisional negative pressure wound therapy after hemiarthroplasty for femoral neck fractures - reduction of wound complications. Int Wound J. 2016;13(5):663-667. doi:10.1111/iwj.12344. 119. Png ME, Madan JJ, Dritsaki M, et al. Cost-utility analysis of standard dressing compared with incisional negative-pressure wound therapy among patients with closed surgical wounds following major trauma to the lower limb. Bone Joint J. 2020 2020;102(8):1072-1081. 120. Pleger SP, Nink N, Elzien M, Kunold A, Koshty A, Boning A. Reduction of groin wound complications in vascular surgery patients using closed incision negative pressure therapy (ciNPT): a prospective, randomised, single-institution study. Int Wound J. Feb 2018;15(1):75-83. doi:10.1111/iwj.12836 121. Rajabaleyan P. Vacuum assisted closure versus on-demand re-laparotomy in patients with faecal or diffuse peritonitis: a multicenter randomized controlled trial (VACOR). Colorectal disease. 2019 2019;21:17‐. 122. Rezk F, Åstrand H, Acosta S. Incisional negative pressure wound therapy for the prevention of surgical site infection after open lower limb revascularization – Rationale and design of a multi-center randomized controlled trial. Contemporary Clinical Trials Communications. 2019 2019;16 123. Sandy-Hodgetts K, Leslie GD, Parsons R, Zeps N, Carville K. Prevention of postsurgical wound dehiscence after abdominal surgery with NPWT: a multicentre randomised controlled trial protocol. Journal of wound care. 2017 2017;26:S23-S26. 124. Sandy-Hodgetts K, Parsons R, Norman R, Fear MW, Wood FM, White SW. Effectiveness of negative pressure wound therapy in the prevention of surgical wound complications in the cesarean section at-risk population: a parallel group randomised multicentre trial-the CYGNUS protocol. BMJ Open. 2020 2020;10(10):e035727. 125. Sapci I, Hull T, Ashburn JH, et al. The American Society of Colon and Rectal Surgeons 2021 Annual Scientific Meeting Abstracts. Diseases of the Colon & Rectum. 2021;64(5):e109-e364. doi:10.1097/dcr.0000000000002029 126. Sapci I, Hull T, Ashburn JH, et al. Effect of Incisional Negative Pressure Wound Therapy on Surgical Site Infections in High Risk Re-Operative Colorectal Surgery: A Randomized Controlled Trial. Dis Colon Rectum. May 2021;64(5) 127. Schmid SC, Seitz AK, Haller B, et al. Final results of the PräVAC trial: prevention of wound complications following inguinal lymph node dissection in patients with penile cancer using epidermal vacuum-assisted wound closure. World J Urol. 2021 2021;39(2):613-620. 128. Schwartzmann E, Sy M, Sharma M, Mankowski B, Jemielity M, Perek B. Negative pressure wound therapy for surgical site infection after sternotomy and its role in preparing the wound for reconstruction. Kardiochir Torakochirurgia Pol. Sep 2021;18(3):190-191. doi:10.5114/kitp.2021.109414 129. Seidel D, Storck M, Lawall H, et al. Negative pressure wound therapy compared with standard moist wound care on diabetic foot ulcers in real-life clinical practice: results of the German DiaFu-RCT. BMJ open. 2020 2020;10(3):e026345. 130. Seidel D, Diedrich S, Herrle F, et al. Negative Pressure Wound Therapy vs Conventional Wound Treatment in Subcutaneous Abdominal Wound Healing Impairment: The SAWHI Randomized Clinical Trial. JAMA Surg. 2020 2020;155(6):469-478. 131. Serra F, Sergi W, Spatafora F, et al. Negative pressure wound therapy (NPWT) after cytoreductive surgery (CRS) and intraperitoneal chemotherapy (HIPEC) for peritoneal surface malignancies: preliminary report. G Chir. 2019 2019;40(6):578-582. 132. Shim HS, Choi JS, Kim SW. A Role for Postoperative Negative Pressure Wound Therapy in Multitissue Hand Injuries. Biomed Res Int. 2018;2018:3629643. doi:10.1155/2018/3629643 133. Stannard JP, Volgas DA, McGwin G, 3rd, et al. Incisional negative pressure wound therapy after high-risk lower extremity fractures. J Orthop Trauma. Jan 2012;26(1):37-42. doi:10.1097/BOT.0b013e318216b1e5 134. Sun W, Gao JH, Zhu LG, et al. Compression therapy following posterior lumbar interbody fusion: a prospective, randomized, clinical study. BMC Surg. 2019 2019;19(1):161. 135. Svensson-Björk R, Saha S, Acosta S, et al. Cost-effectiveness analysis of negative pressure wound therapy dressings after open inguinal vascular surgery – The randomised INVIPS-Trial. Journal of Tissue Viability. 2021 2021;30(1):95-101. 136. Szmeja J, Borejsza-Wysocki M, Bobkiewicz A, Krokowicz L, Banasiewicz T, Szmyt K. The Comparison of Quality of Life in Patients with Pilonidal Sinus Disease. Negative Pressure Wound Therapy Versus Standard Wound Dressings - A Randomized Pilot Study. Gastroenterology. 2020 2020;158(6):S‐1601‐. 137. Tanaydin V, Beugels J, Andriessen A, Sawor JH, van der Hulst RRWJ. Randomized Controlled Study Comparing Disposable Negative-Pressure Wound Therapy with Standard Care in Bilateral Breast Reduction Mammoplasty Evaluating Surgical Site Complications and Scar Quality. Aesthetic plastic surgery. 2018 2018;42(4):927-935. 138. Tanaydin V, Beugels J, Andriessen A, Sawor JH, van der Hulst RRWJ. Erratum: Correction to: Randomized Controlled Study Comparing Disposable Negative-Pressure Wound Therapy with Standard Care in Bilateral Breast Reduction Mammoplasty Evaluating Surgical Site Complications and Scar Quality (Aesthetic plastic surgery (2018) 42 4 (927-935)). Aesthetic plastic surgery. 2018 2018;42(4):1176. 139. Venkatadass K, Bittersohl B, Fornari ED, Bomar JD, Hosalkar H. Erratum: Does incisional wound VAC after major Hip surgery in obese pediatric patients reduce wound infection and scar formation? A pilot study pediatrics (Clinical Orthopaedics and Related Research). Clinical Orthopaedics and Related Research. 2013 2013;471(8):2730. 140. Wang T, Li X, Fan L, et al. Negative pressure wound therapy promoted wound healing by suppressing inflammation via down-regulating MAPK-JNK signaling pathway in diabetic foot patients. Diabetes Res Clin Pract. 2019 2019;150:81-89. 141. Collaborative SUNRRISE Study Group on behalf of the Northwest Research Collaborative and the West Midlands Research Collaborative. An international pragmatic randomised controlled trial to compare a single use negative pressure dressing versus a surgeon's preference of dressing to reduce the incidence of surgical site infection following emergency laparotomy: the SUNRRISE Trial Protocol. Colorectal Dis. Dec 4 2020;doi:10.1111/codi.15474 142. Wilkin R. The SUNRRISE Trial – Single Use Negative pressure dressing for Reduction In Surgical site infection following Emergency laparotomy. 2022; 143. Yang ML, Zhou XJ, Zhu YG, et al. [Clinical efficacy and influencing factors of different modes of continuous negative pressure wound therapy on venous ulcer wounds of lower limbs]. Zhonghua Shao Shang Za Zhi. 2020 2020;36(12):1149-1158. 144. Yilmaz M, Thorn A, Sorensen MS, Jensen CL, Petersen MM. Effect of negative pressure wound therapy after surgical removal of deep-seated high-malignant soft tissue sarcomas of the extremities and trunk wall-study protocol for a randomized controlled trial. Trials.Jun 18 2022;23(1)doi:ARTN 507; 10.1186/s13063-022-06468-6 145. Zhao N, Liu Y, Yue J, et al. Negative pressure drainage-assisted irrigation for maxillofacial space infection. Oral Dis. 2020 2020;26(7):1586-1591. 146. Zwanenburg PR, Timmer AS, Boermeester MA. Incisional Negative Pressure Wound Therapy After Surgery for Major Trauma-Related Fractures. Jama. 2020 2020;323(22):2343-2344. |
||
Study characteristics of included randomized controlled trials
|
Study |
N total |
Procedure |
Wound class |
SAP |
iNPWT device |
mmHg |
Min. days |
Control dressings |
Industry involvement in design |
ROB |
|
Andrianello 2021 |
100 |
Pancreatectomy |
II |
Yes |
PICO |
-80 * |
7 |
GBDs |
No |
Some concerns |
|
Arellano 2021 |
148 |
Colorectal surgery |
II-III |
Yes |
Prevena |
-125 * |
7 |
GBDs |
Yes |
Some concerns |
|
Bertges 2021 |
252 |
Open femoral vascular surgery |
I / IV |
Yes |
Prevena |
-125 * |
5 |
GBDs |
Yes |
Some concerns |
|
Borejsza-Wysocki 2021 |
30 |
Stoma reversal surgery |
III |
Yes |
PICO |
-80 |
7 |
GBDs |
Not reported |
Some concerns |
|
Bueno-Lledó 2021 |
146 |
Hernia repair |
I |
Yes |
PICOn |
-80 |
7 |
GBDs |
No |
Some concerns |
|
Canton 2020 |
65 |
Lower extremity fracture surgery |
I |
Yes |
PICO |
-80 |
7 |
GBDs |
No |
High |
|
Chaboyer 2014 |
87 |
Caesarean section |
II |
Yes |
PICO |
-80 * |
4 |
HBDs |
No |
Low |
|
Cooper 2022 |
120 |
Total hip arthroplasty |
I |
NR |
Prevena |
-125 * |
7 |
HBDs |
No |
Some concerns |
|
Costa 2020 |
1629 |
Lower extremity fracture surgery |
I |
NR |
PICO |
-80 * |
7 |
GBDs |
No |
Some concerns |
|
Crist 2017 |
66 |
Acetabular fracture surgery |
I |
Yes |
VAC |
-125 |
2 |
GBDs |
No |
Some concerns |
|
Di Re 2021 |
127 |
Open abdominal surgery |
I-IV |
Yes |
Prevena |
-125 |
5 |
GBDs or HBDs |
No |
Some concerns |
|
Engelhardt 2018 |
132 |
Open femoral vascular surgery |
I |
Yes |
Prevena |
-125 |
5 |
GBDs |
Not reported |
High |
|
Flynn 2020 |
201 |
Open abdominal surgery |
II-III |
Yes |
PICO |
-80 * |
7 |
NR |
No |
Some concerns |
|
Fogacci 2019 |
100 |
Breast surgery |
I |
NR |
PICO |
-80 |
7 |
GBDs |
Not reported |
Some concerns |
|
Gabriele 2021 |
52 |
Oncological orthopedic surgery |
I |
Yes |
PICO |
-80 |
7 |
‘Standard’ |
Not reported |
High |
|
Garg 2021 |
50 |
Emergency abdominal laparotomy |
I-IV? |
NR |
NR |
-75 |
3 |
GBDs |
Not reported |
Some concerns |
|
Gillespie 2015 |
70 |
Primary hip arthroplasty |
I |
Yes |
PICO |
-80 |
5§ |
HBDs |
No |
Low |
|
Gillespie 2021 |
2035 |
Caesarean section |
II |
Yes |
PICO |
-80 |
5 |
GBDs or GBDs |
No |
Low |
|
Gök 2019 |
40 |
General surgery |
II-IV |
NR |
Prevena |
-125 * |
7 |
‘Standard’ |
Not reported |
High |
|
Gombert 2018 |
204 |
Open femoral vascular surgery |
I |
Yes |
Prevena |
-125 |
5 |
GBDs |
No |
Low |
|
Gunatilake 2017 |
82 |
Caesarean section |
I-II |
Yes |
Prevena |
-125 |
5 |
GBDs or HBDs |
Yes |
High |
|
Hasselmann 2020 |
154 |
Inguinal vascular surgery |
I |
Yes |
PICO |
-80 |
NR |
GBDs |
No |
High |
|
Higuera-Rueda 2021 |
294 |
Knee arthroplasty |
I / IV |
NR |
Prevena |
-125 |
5 |
HBDs with silver |
Yes |
Some concerns |
|
Hussamy 2019 |
241 |
Caesarean section |
II |
Yes |
Prevena |
-125 |
2 |
GBDs |
No |
Some concerns |
|
Hyldig 2018 |
876 |
Caesarean section |
II |
Yes |
PICO |
-80 * |
5 |
GBDs |
No |
Some concerns |
|
Javed 2019 |
123 |
Open pancreaticoduodenectomy |
II |
Yes |
Prevena |
-125 |
5 |
GBDs |
No |
Low |
|
Kacmaz 2022 |
56 |
Colorectal cancer surgery |
II-III |
Yes |
PICO |
-80 |
7 |
GBDs |
No |
Some concerns |
|
Karlakki 2016 |
209 |
Hip and knee arthroplasty |
I |
Yes |
PICO |
-80 * |
7 |
GBDs or HBDs |
Yes |
Some concerns |
|
Keeney 2019 |
398 |
Hip and knee arthroplasty |
I |
NR |
PICO |
-80 |
7 |
GBDs |
Yes |
High |
|
Lee 2017, cardiac |
64 |
Saphenous vein harvest for CABG |
I |
NR |
Prevena |
-125 |
7§ |
GBDs |
Yes |
Low |
|
Lee 2017, vascular |
102 |
Open femoral vascular surgery |
I |
NR |
Prevena |
-125 * |
8§ |
GBDs |
No |
Low |
|
Leitao 2021 |
584 |
Open gynecologic surgery |
I-IV |
Yes |
Prevena |
-125 |
7§ |
‘Standard’ |
Yes |
Some concerns |
|
Leon 2016 |
81 |
Open colorectal surgery |
II |
NR |
NR |
NR |
NR |
‘Standard’ |
Not reported |
High |
|
Li 2017 |
71 |
Open abdominal surgery |
I-II |
Yes |
VSD |
-125 |
3 |
GBDs |
No |
Low |
|
Lopez 2023 |
120 |
Liver transplant surgery |
II |
Yes |
PICO |
-80 |
5 |
GBDs |
No |
Some concerns |
|
Masden 2012 |
81 |
Lower extremity or abdominal wound closure |
I |
NR |
VAC |
-125 |
3 |
Silicone gauze + silver dressing |
Not reported |
High |
|
Masters 2021 |
465 |
Hip fracture surgery |
I |
Yes |
PICO |
-80 |
NR |
‘Standard’ |
No |
Some concerns |
|
Muller-Sloof 2018 |
51 |
DIEAP or PAP donor-site closure |
I |
Yes |
Prevena |
-125 |
5 |
GBDs |
No |
Some concerns |
|
Muller-Sloof 2022 |
80 |
DIEP flap breast reconstruction |
I |
Yes |
Prevena |
-125 |
5 |
NR |
No |
Some concerns |
|
Murphy 2019 |
300 |
Colorectal surgery |
II |
Yes |
Prevena |
-125 |
5 |
GBDs |
No |
Some concerns |
|
Newman 2019 |
160 |
Hip and knee arthroplasty |
I |
Yes |
Prevena |
-125 * |
2 |
GBDs with silver |
Yes |
Some concerns |
|
O'Leary 2017 |
49 |
Open abdominal general and gynecological surgery |
I-III |
Yes |
PICO |
-80 |
4 |
HBDs |
Yes |
High |
|
O’Neill 2020 |
40 |
Pancreatectomy and hepatectomy |
II |
Yes |
PICO |
-80 * |
7 |
GBDs |
Yes |
Some concerns |
|
Pachowsky 2012 |
19 |
Total hip arthroplasty |
I |
Yes |
Prevena |
-125 * |
5 |
GBDs |
Yes |
Some concerns |
|
Peterson 2021 |
110 |
Caesarean section |
II |
Yes |
PICO |
-80 * |
7 |
GBDs |
Not reported |
Low |
|
Rashed 2021 |
104 |
Sternotomy |
I |
Yes |
VivanoTec |
-125 |
5 |
GBDs |
Not reported |
Some concerns |
|
Ruhstaller 2017 |
119 |
Caesarean section |
II |
Yes |
Prevena |
-125 * |
3 |
GBDs |
No |
High |
|
Sapci 2022 |
298 |
Colorectal surgery |
II-IV |
Yes |
Prevena |
-125 |
6 |
GBDs |
No |
Some concerns |
|
Shen 2017 |
265 |
Abdominal oncological resections |
II |
NR |
NR |
-125 |
4 |
GBDs |
No |
High |
|
Shields 2021 |
17 |
Soft-tissue sarcoma surgery |
I |
NR |
VAC |
NR |
NR |
HBDs |
No |
Low |
|
Shim 2018 |
51 |
Hand surgery |
I |
NR |
CuraVAC |
-75 |
3 |
GBDs |
Yes |
Some concerns |
|
Stannard 2006 |
44 |
Calcaneus, pilon, or tibial plateau fracture surgery |
I-III |
NR |
VAC |
-50 to -200 |
NR |
GBDs |
Yes |
Some concerns |
|
Suh 2016 |
100 |
SCIP flap harvest |
I |
Yes |
CuraVac |
-50 to -125 (cyclic) |
5 |
GBDs |
Not reported |
Some concerns |
|
Tuuli 2017 ¶ |
120 |
Caesarean section |
II |
NR |
PICO |
-80* |
4 |
‘Standard’ |
No (registration) |
Some concerns |
|
Tuuli 2020 |
1624 |
Caesarean section |
II |
Yes |
Prevena |
-125 |
4 |
GBDs |
No |
Some concerns |
|
Vaddavalli 2022 |
50 |
Lower extremity amputation because of peripheral arterial disease |
I |
NR |
CCNPWT |
NR |
6 |
GBDs |
No |
Low |
|
Wierdak 2021 |
75 |
Open abdominal surgery |
II-III |
Yes |
NANOVA |
-125 * |
3 |
NR |
No |
Some concerns |
|
Wihbey 2018 |
166 |
Caesarean section |
II |
Yes |
Prevena |
-125 * |
5 |
GBDs |
No |
Some concerns |
|
Witt-Majchrzak 2015 |
80 |
CABG |
I |
Yes |
PICO |
-80 |
6 |
GBDs |
Yes |
Some concerns |
|
Yu 2017 |
72 |
Saphenous vein harvest for CABG |
I |
Yes |
Custom |
-120 |
5 |
GBDs |
No |
Some concerns |
|
CABG, coronary artery bypass grafting; DIEAP, deep inferior artery perforator; GBDs, gauze-based dressings; HBDs, hydrocolloid-based dressings; NR, not reported; PAP, profunda artery perforator; SC, some concerns; SCIP, superficial circumflex artery perforator;
¶ Conference abstract * Pressure (mmHg) not mentioned in study § Number of days or time of discharge, whichever came first |
||||||||||
Statements Industry involvement
|
Study |
Statements on involvement of industry
|
Score |
|
Andrianello 2021 |
Smith & Nephew Healthcare (Hull, UK) supplied the devices used for the study. The company was not involved in the analysis of the trial. |
2 |
|
Arellano 2021 |
This study was sponsored by PREVENA Incision Management System, KCI, San Antonio, Texas, who provided the closed-incision negative-pressure therapy devices. |
3 |
|
Bertges 2021 |
The present investigator-initiated study was funded by Acelity KCI (San Antonio, Texas). |
3 |
|
Borejsza-Wysocki 2021 |
NR |
4 |
|
Bueno-Lledó 2021 |
No commercial involvement |
1 |
|
Canton 2020 |
No commercial involvement |
1 |
|
Chaboyer 2014 |
No commercial involvement |
1 |
|
Cooper 2022 |
This research was an investigator-initiated study that received limited support from Kinetic Concepts, Inc (San Antonio, TX; now 3M, Minneapolis, MN), who provided 62 surgical dressings for the study group at no cost to the participating institutions. No additional funding or support was received for study costs or research personnel. |
2 |
|
Costa 2020 |
Smith and Nephew provided incisional negative pressure wound therapy dressings (PICO single use negative pressure wound therapy system) to recruiting centers. The sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. |
2 |
|
Crist 2017 |
No commercial involvement |
1 |
|
Di Re 2021 |
KCI (San Antonio, TX, USA) provided the NPWT dressings and devices for this study. No funding was received for this controlled trial. |
2 |
|
Engelhardt 2018 |
NR |
4 |
|
Flynn 2020 |
This trial was funded by Smith and Nephew, the company that produces the PICO dressing. Smith and Nephew received ongoing updates of all findings but did not have any active input or editorial power over the study protocol, day-to-day running of the trial or reporting of findings. |
2 |
|
Fogacci 2019 |
NR |
4 |
|
Gabriele 2021 |
NR |
4 |
|
Garg 2021 |
NR |
4 |
|
Gillespie 2015 |
No commercial involvement |
1 |
|
Gillespie 2021 |
The trial was funded by a competitive peer reviewed grant (APP1081026) from the Australian National Health and Medical Research Council. The funders had no role in considering the study design or in the collection, analysis, or interpretation of data, the writing of the report, or the decision to submit the article for publication. |
1 |
|
Gök 2019 |
NR |
4 |
|
Gombert 2018 |
This investigator-initiated trial was funded by Acelity, San Antonio, TX, USA. The funder of this investigator-initiated trial had no role in study design, data collection, data analysis, data interpretation or writing of the article. |
2 |
|
Gunatilake 2017 |
The study was sponsored by KCI, an Acelity Company, San Antonio, TX. |
3 |
|
Hasselmann 2020 |
The research group received an unrestricted unconditional research grant of 15,550 USD and a donation of 100 PICO dressing kits from Smith and Nephew in 2013. |
2 |
|
Higuera-Rueda 2021 |
The present investigation was funded by KCI-3M, San Antonio, TX. |
3 |
|
Hussamy 2019 |
Study devices were provided by Kinetic Concepts Incorporated (San Antonio, Texas). This company had no input on the study design, collection, analysis, and interpretation of data, writing of the report, or the decision to submit the report for publication. |
2 |
|
Hyldig 2018 |
Funding for this peer-reviewed, investigator-initiated clinical trial was provided by grants from the University of Southern Denmark, Odense University Hospital, the Region of Southern Denmark, Lundbeckfonden, and an unrestricted grant from the iNPWT device manufacturer Smith & Nephew (devices and operating funding). None of these sources of funds had an influence on study design, data collection, data analyses, interpretation of results or writing of the report. |
2 |
|
Javed 2019 |
Funding: KCI/Acelity |
3 |
|
Kacmaz 2022 |
The authors received specific funding for this work by the Erciyes University Scientific Research Projects Unit. |
1 |
|
Karlakki 2016 |
The study was funded through a grant from Smith & Nephew UK, to cover the cost of NPWT dressings and data collection costs. C. Whitall declares she has received payment from Smith & Nephew for other work unrelated to this paper. |
2 |
|
Keeney 2019 |
Our institution received research funding from Smith & Nephew Orthopaedics that was related to this study. |
3 |
|
Lee 2017, cardiac |
This work was supported by KCI USA Incorporated, an Acelity Company. |
3 |
|
Lee 2017, vascular |
Kinetic Concepts Inc (San Antonio, Tex) donated all NPWT devices but had no influence on study design, data collection, management or any input on publication. |
2 |
|
Leitao 2021 |
The protocol was supported in part by KCI/Acelity. The role of the sponsor in the design, execution, analysis, reporting, and funding is fully disclosed. The sponsor reviewed the manuscript and provided general funding for research purposes. |
3 |
|
Leon 2016 |
NR |
4 |
|
Li 2017 |
No commercial involvement |
1 |
|
Lopez 2023 |
This research did not receive any specific funding from any agencies in the public, commercial, or not-for-profit areas. |
1 |
|
Masden 2012 |
Drs Attinger and Steinberg are consultants for Kinetic Concepts Incorporated |
4 |
|
Masters 2021 |
J. Masters and J. Cook report institutional grants (paid to University of Oxford) from the Royal College of Surgeons of England/Dunhill Medical Trust research training fellowship, Smith & Nephew (device supply), and the NIHR Oxford Biomedical Research Centre (research infrastructure support), all related to this study. M. Costa reports institutional research grant funding (paid to University of Oxford) from the National Institute for Health Research (NIHR), the European Union (EU), the Royal College of Surgeons (RCS) England, and Smith & Nephew, not related to this study. |
2 |
|
Muller-Sloof 2018 |
No commercial involvement |
1 |
|
Muller-Sloof 2022 |
This study was funded in part by GD Medical (Houten, The Netherlands). Before commencing this study, both parties, the Department of Plastic Surgery at Radboud University Medical Center and GD Medical, stated in a written agreement (signed on March 31, 2017) that GD Medical would not have sight of the data or analysis of this study and would not have any influence on reporting and publication of the presented manuscript. |
2 |
|
Murphy 2019 |
The study was funded by an industry grant from Kinetic Concepts Inc. (San Antonio, TX) in the amount of $87 000. The devices were also provided free of charge. KCI did not have any input on study design, data acquisition, date analysis, interpretation, and drafting of final manuscript. |
2 |
|
Newman 2019 |
This study was funded by a research grant provided by KCI/Acelity Inc. (San Antonio, TX). |
3 |
|
O'Leary 2017 |
Support was received from Smith and Nephew in the form of 25 PICO dressings. The authors were responsible for trial design, data analysis, and manuscript writing. The decision to publish the results of the trial was made together with the trial sponsor and study authors. |
3 |
|
O’Neill 2020 |
The PICO incisional negative pressure wound therapy devices used in this study were provided Smith & Nephew, Hull, UK. |
3 |
|
Pachowsky 2012 |
The PREVENA wound treatment system was provided by KCI free of charge. Matthias H. Brem gave scientific presentations for KCI. |
2 |
|
Peterson 2021 |
NR |
4 |
|
Rashed 2021 |
NR |
4 |
|
Ruhstaller 2017 |
All study devices were provided by Acelity. The funding sources had no role in study design, data collection, or analysis. |
2 |
|
Sapci 2022 |
Acelity/KCI provided the PrevenaTM Incision Management System used in this trial free of charge, without any other funding provided. Acelity/KCI had no input on data acquisition, analysis, or interpretation. |
2 |
|
Shen 2017 |
No commercial involvement |
1 |
|
Shields 2021 |
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article. Dressings used in the study were funded through a charitable contribution from the local health board, NHS Greater Glasgow and Clyde Endowments (Registered Charity Number: SC005895). |
1 |
|
Shim 2018 |
NR |
3 |
|
Stannard 2006 |
It is hereby declared that first author, James P. Stannard, MD, is a Consultant for Kinetics Concepts, Inc. (KCI), the manufacturer of the Negative Pressure Wound Therapy device that is the subject of this manuscript. Additionally, KCI has provided financial assistance in the form of a grant for a clinical study relative to the VAC. |
3 |
|
Suh 2016 |
NR |
4 |
|
Tuuli 2017 |
NR in paper. On clinicaltrails.gov “Sponsor: Washington University School of Medicine” |
1 |
|
Tuuli 2020 |
Acelity donated negative pressure devices and provided supplemental funding. The NIH and Acelity had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. Specifically, the funders had no right to veto publication or to control the decision regarding to which journal the manuscript was submitted. |
2 |
|
Vaddavalli 2022 |
The authors received funding from Department of General Surgery, Post Graduate Institute of Medical Education and Research (PGIMER), Chandigarh, India. 160012. |
1 |
|
Wierdak 2021 |
No commercial involvement |
1 |
|
Wihbey 2018 |
The devices used in this study were provided by an unrestricted research grant from KCI Medical (San Antonio, Texas). KCI Medical had no role in the study design, data collection, or data analysis. Final results of the study were shared with the company before manuscript submission; however, the investigators were not bound to incorporate KCI’s comments. |
2 |
|
Witt-Majchrzak 2015 |
NR |
4 |
|
Yu 2017 |
No commercial involvement |
1 |
|
1: no industry funding or involvement 2: industry funding, without involvement in trial design 3: industry involvement in trial design 4: no information
NR: not reported |
||
Adverse events
|
Study |
|
Adverse events |
|
Bertges 2021 |
iNPWT |
15 adverse events (undefined, no significant adverse events were attributed to PREVENA) |
|
Control |
19 adverse events |
|
|
Borejsza-Wysocki 2021 |
iNPWT |
VAS-scores 1st day: 4.0 ±1.1; 3rd day: 2.5 ±.0.9; 5th day: 2.3 ±1.1 |
|
Control |
VAS-scores 1st day: 4.9 ±0.9; 3rd day: 3.5 ±.0.9; 5th day: 2.9 ±0.9 |
|
|
Bueno-Lledó 2021 |
iNPWT |
No events attributable to iNPWT dressing (undefined) |
|
Control |
NR |
|
|
Canton 2020 |
iNPWT |
No events of pain, discomfort, intolerance, rash, itching and blisters |
|
Control |
NR |
|
|
Chaboyer 2014 |
iNPWT |
4 skin blistering, 0 dermatitis, 1 bleeding |
|
Control |
0 skin blistering, 1 dermatitis, 1 bleeding |
|
|
Costa 2020 |
iNPWT |
173 pain or tender |
|
Control |
188 pain or tender |
|
|
Di Re 2021 |
iNPWT |
4 skin blistering |
|
Control |
0 skin blistering |
|
|
Engelhardt 2018 |
iNPWT |
No events (not defined) |
|
Control |
NR |
|
|
Flynn 2020 |
iNPWT |
2 skin blistering, 0 pain |
|
Control |
0 skin blistering, 1 pain |
|
|
Gabriele 2021 |
iNPWT |
1 intolerance to treatment without complication (redding or blistering) |
|
Control |
NR |
|
|
Garg 2021 |
iNPWT |
0 pain |
|
Control |
NR |
|
|
Gillespie 2015 |
iNPWT |
8 bleeding |
|
Control |
1 bleeding |
|
|
Gillespie 2021 |
iNPWT |
40 skin blistering, 10 dermatitis |
|
Control |
23 skin blistering, 3 dermatitis |
|
|
Gombert 2018 |
iNPWT |
No events of Szilagyi grade II-III SSIs, myocardial infarction, or hospital re-admission because of SSI) |
|
Control |
NR |
|
|
Gunatilake 2017 |
iNPWT |
No serious events (not defined) |
|
Control |
No serious events (not defined) |
|
|
Hasselmann 2020 |
iNPWT |
No events attributable to the iNPWT dressing (undefined) |
|
Control |
NR |
|
|
Higuera-Rueda 2021 |
iNPWT |
61 treatment-emergent adverse events (based on the Safety Analysis Set, type of event not further described) |
|
Control |
71 treatment-emergent adverse events (based on the Safety Analysis Set, type of event not further described) |
|
|
Hussamy 2019 |
iNPWT |
62 skin blistering |
|
Control |
0 skin blistering |
|
|
Javed 2018 |
iNPWT |
No adverse skin reactions (not defined) |
|
Control |
No adverse skin reactions (not defined) |
|
|
Karlakki 2016 |
iNPWT |
11 skin blistering, 0 pain (no other adverse events described as outcome) |
|
Control |
1 skin blistering, 1 pain |
|
|
Lee 2017, cardiac |
iNPWT |
1 rash or eczema, 2 itching, 2 pain (other adverse events outcomes were heaviness, weakness, stiffness, paraesthesia, numbness, burning, discolouration, oedema) |
|
Control |
1 rash or eczema, 1 itching, 2 pain |
|
|
Leitao 2021 |
iNPWT |
33 skin blistering, 6 dermatitis, 6 pain (no other adverse events as outcome) |
|
Control |
3 skin blistering, 4 dermatitis, 2 pain |
|
|
Muller-Sloof 2018 |
iNPWT |
0 dermatitis, 3 pain (no other adverse events as outcome) |
|
Control |
0 dermatitis, 3 pain |
|
|
Muller-Sloof 2022 |
iNPWT |
No serious adverse events occurred in relation to the study. |
|
Control |
|
|
|
Murphy 2019 |
iNPWT |
6 bleeding. There were no reported adverse events from the NPWT dressing. |
|
Control |
5 bleeding |
|
|
Newman 2019 |
iNPWT |
0 skin blistering (no other adverse events as outcome) |
|
Control |
1 skin blistering |
|
|
Peterson 2021 |
iNPWT |
2 skin blistering |
|
Control |
5 skin blistering |
|
|
Ruhstaller 2017 |
iNPWT |
8 skin blistering, 4 discomfort with device (1 removed), 5 malfunctions with device |
|
Control |
2 skin blistering |
|
|
Sapci 2022 |
iNPWT |
There were no adverse events associated with the iNPWT dressing. |
|
Control |
NR |
|
|
Suh 2016 |
iNPWT |
No events of pain |
|
Control |
No events of pain |
|
|
Tuuli 2017 |
iNPWT |
2 skin reaction |
|
Control |
0 skin reaction |
|
|
Tuuli 2020 |
iNPWT |
27 skin blistering, 10 dermatitis, 14 pain |
|
Control |
2 skin blistering, 3 dermatitis, 1 pain |
|
|
Witt-Majchrzak 2015 |
iNPWT |
5 skin blistering |
|
Control |
0 skin blistering |
Verantwoording
Beoordelingsdatum en geldigheid
Publicatiedatum : 17-12-2024
Beoordeeld op geldigheid : 01-12-2024
Algemene gegevens
De ontwikkeling/herziening van deze richtlijnmodule werd ondersteund door het Kennisinstituut van de Federatie Medisch Specialisten (www.demedischspecialist.nl/kennisinstituut) en werd gefinancierd uit de Kwaliteitsgelden Medisch Specialisten (SKMS). De financier heeft geen enkele invloed gehad op de inhoud van de richtlijnmodule.
Samenstelling werkgroep
Voor het ontwikkelen van de richtlijnmodules 2 tot 16 is in 2020 op initiatief van de NVvH een multidisciplinaire werkgroep ingesteld, bestaande uit vertegenwoordigers van alle relevante specialismen (zie hiervoor de Samenstelling van de werkgroep) die betrokken zijn bij de zorg voor preventie van postoperatieve wondinfecties. Daarnaast is in 2022 op initiatief van het Samenwerkingsverband Richtlijnen Infectiepreventie (SRI) een separate multidisciplinaire werkgroep samengesteld voor de herziening van de WIP-richtlijn over postoperatieve wondinfecties: module 17-22. De ontwikkelde modules van beide werkgroepen zijn in deze richtlijn samengevoegd.
Belangenverklaringen
De Code ter voorkoming van oneigenlijke beïnvloeding door belangenverstrengeling is gevolgd. Alle werkgroepleden hebben schriftelijk verklaard of zij in de laatste drie jaar directe financiële belangen (betrekking bij een commercieel bedrijf, persoonlijke financiële belangen, onderzoek financiering) of indirecte belangen (persoonlijke relaties, reputatiemanagement) hebben gehad. Gedurende de ontwikkeling of herziening van een module worden wijzigingen in belangen aan de voorzitter doorgegeven. De belangenverklaring wordt opnieuw bevestigd tijdens de commentaarfase.
Een overzicht van de belangen van werkgroepleden en het oordeel over het omgaan met eventuele belangen vindt u in onderstaande tabel. De ondertekende belangenverklaringen zijn op te vragen bij het secretariaat van het Kennisinstituut van de Federatie Medisch Specialisten.
|
Werkgroeplid |
Functie |
Nevenfuncties |
Gemelde belangen |
Ondernomen actie |
|
Mevr. prof. dr. M.A. Boermeester |
Chirurg |
* Medisch Ethische Commissie, Amsterdam UMC, locatie AMC * Antibiotica Commissie, Amsterdam UMC |
Persoonlijke financiële belangen Hieronder staan de beroepsmatige relaties met bedrijfsleven vermeld waarbij eventuele financiële belangen via de AMC Research B.V. lopen, dus institutionele en geen persoonlijke gelden zijn: Skillslab instructeur en/of spreker (consultant) voor KCI/3M, Smith&Nephew, Johnson&Johnson, Gore, BD/Bard, TELABio, GDM, Medtronic, Molnlycke.
Persoonlijke relaties Geen.
Extern gefinancierd onderzoek Institutionele grants van KCI/3M, Johnson&Johnson en New Compliance.
Intellectuele belangen en reputatie Ik maak me sterk voor een 100% evidence-based benadering van maken van aanbevelingen, volledig transparant en reproduceerbaar. Dat is mijn enige belang in deze, geen persoonlijk gewin.
Overige belangen Geen.
|
Extra kritische commentaarronde. |
|
Dhr. dr. M.J. van der Laan |
Vaatchirurg |
Vice voorzitter Consortium Kwaliteit van Zorg NFU, onbetaald
|
Persoonlijke financiële belangen Geen.
Persoonlijke relaties Geen.
Extern gefinancierd onderzoek Geen.
Intellectuele belangen en reputatie Geen.
Overige belangen Geen.
|
Geen.
|
|
Dhr. dr. W.C. van der Zwet |
Arts-microbioloog |
Lid Regionaal Coördinatie Team, Limburgs infectiepreventie & ABR Zorgnetwerk (onbetaald) |
||
|
Dhr. dr. D.R. Buis |
Neurochirurg |
Lid Hoofdredactieraad Tijdschrift voor Neurologie & Neurochirurgie - onbetaald |
||
|
Dhr. dr. J.H.M. Goosen |
Orthopaedisch Chirurg |
Inhoudelijke presentaties voor Smith&Nephew en Zimmer Biomet. Deze worden vergoed per uur. |
||
|
Mw. drs. H. Jalalzadeh |
Arts-onderzoeker |
Geen. |
Persoonlijke financiële belangen Geen.
Persoonlijke relaties Geen.
Extern gefinancierd onderzoek Geen.
Intellectuele belangen en reputatie Geen.
Overige belangen Geen. |
Geen.
|
|
Dhr. dr. N. Wolfhagen |
AIOS chirurgie |
|||
|
Mw. drs. H. Groenen |
Arts-onderzoeker |
|||
|
Dhr. dr. F.F.A. Ijpma |
Traumachirurg |
|||
|
Dhr. dr. P. Segers |
Cardiothoracaal chirurg |
|||
|
Mw. Y.E.M. Dreissen |
AIOS neurochirurgie |
|||
|
Dhr. R.R. Schaad |
Anesthesioloog |
Inbreng patiëntenperspectief
Er werd aandacht besteed aan het patiëntenperspectief door uitnodigen van de Patiëntenfederatie Nederland voor de invitational conference. De verkregen input is meegenomen bij het opstellen van de uitgangsvragen, de keuze voor de uitkomstmaten en bij het opstellen van de overwegingen. De conceptmodules zijn tevens voor commentaar voorgelegd aan de Patiëntenfederatie Nederland en de eventueel aangeleverde commentaren zijn bekeken en verwerkt. Voor de modules 17-22 was de patiëntfederatie vertegenwoordigd in de werkgroep.
Wkkgz & Kwalitatieve raming van mogelijke substantiële financiële gevolgen
Bij de richtlijn is conform de Wet kwaliteit, klachten en geschillen zorg (Wkkgz) een kwalitatieve raming uitgevoerd of de aanbevelingen mogelijk leiden tot substantiële financiële gevolgen. Bij het uitvoeren van deze beoordeling zijn richtlijnmodules op verschillende domeinen getoetst (zie het stroomschema op de Richtlijnendatabase).
Uit de kwalitatieve raming blijkt dat er waarschijnlijk geen substantiële financiële gevolgen zijn.
Voor module 8 (Negatieve druktherapie) geldt dat uit de toetsing volgt dat de aanbeveling(en) breed toepasbaar zijn (5.000 - 40.000 patiënten). Tevens volgt uit de toetsing dat het geen nieuwe manier van zorgverlening of andere organisatie van zorgverlening betreft. Er worden daarom geen substantiële financiële gevolgen verwacht.
Voor de overige modules en aanbevelingen geldt dat uit de toetsing volgt dat de aanbeveling(en) breed toepasbaar zijn (>40.000 patiënten). Tevens volgt uit de toetsing dat het overgrote deel (±90%) van de zorgaanbieders en zorgverleners al aan de norm voldoet en het geen nieuwe manier van zorgverlening of andere organisatie van zorgverlening betreft. Ook wordt geen toename in het aantal in te zetten voltijdsequivalenten aan zorgverleners verwacht of een wijziging in het opleidingsniveau van zorgpersoneel. Er worden daarom geen substantiële financiële gevolgen verwacht.
Methode ontwikkeling
Evidence based
Implementatie
Zie voor de implementatie het implementatieplan in het tabblad 'Bijlagen'.
Werkwijze
AGREE
Deze richtlijnmodule is opgesteld conform de eisen vermeld in het rapport Medisch Specialistische Richtlijnen 2.0 van de adviescommissie Richtlijnen van de Raad Kwaliteit. Dit rapport is gebaseerd op het AGREE II instrument (Appraisal of Guidelines for Research & Evaluation II; Brouwers, 2010).
Knelpuntenanalyse en uitgangsvragen
Tijdens de voorbereidende fase inventariseerde de werkgroepen de knelpunten in de zorg voor patiënten die chirurgie ondergaan. Tevens zijn er knelpunten aangedragen door middel van een invitational conference. De verslagen hiervan zijn opgenomen onder aanverwante producten.
Op basis van de uitkomsten van de knelpuntenanalyse zijn door de werkgroep concept-uitgangsvragen opgesteld en definitief vastgesteld.
Uitkomstmaten
Na het opstellen van de zoekvraag behorende bij de uitgangsvraag inventariseerde de werkgroep welke uitkomstmaten voor de patiënt relevant zijn, waarbij zowel naar gewenste als ongewenste effecten werd gekeken. Hierbij werd een maximum van acht uitkomstmaten gehanteerd. De werkgroep waardeerde deze uitkomstmaten volgens hun relatieve belang bij de besluitvorming rondom aanbevelingen, als cruciaal (kritiek voor de besluitvorming), belangrijk (maar niet cruciaal) en onbelangrijk. Tevens definieerde de werkgroep tenminste voor de cruciale uitkomstmaten welke verschillen zij klinisch (patiënt) relevant vonden.
Methode literatuursamenvatting
Een uitgebreide beschrijving van de strategie voor zoeken en selecteren van literatuur is te vinden onder ‘Zoeken en selecteren’ onder Onderbouwing. Indien mogelijk werd de data uit verschillende studies gepoold in een random-effects model. De beoordeling van de kracht van het wetenschappelijke bewijs wordt hieronder toegelicht.
Beoordelen van de kracht van het wetenschappelijke bewijs
De kracht van het wetenschappelijke bewijs werd bepaald volgens de GRADE-methode. GRADE staat voor ‘Grading Recommendations Assessment, Development and Evaluation’ (zie http://www.gradeworkinggroup.org/). De basisprincipes van de GRADE-methodiek zijn: het benoemen en prioriteren van de klinisch (patiënt) relevante uitkomstmaten, een systematische review per uitkomstmaat, en een beoordeling van de bewijskracht per uitkomstmaat op basis van de acht GRADE-domeinen (domeinen voor downgraden: risk of bias, inconsistentie, indirectheid, imprecisie, en publicatiebias; domeinen voor upgraden: dosis-effect relatie, groot effect, en residuele plausibele confounding).
GRADE onderscheidt vier gradaties voor de kwaliteit van het wetenschappelijk bewijs: hoog, redelijk, laag en zeer laag. Deze gradaties verwijzen naar de mate van zekerheid die er bestaat over de literatuurconclusie, in het bijzonder de mate van zekerheid dat de literatuurconclusie de aanbeveling adequaat ondersteunt (Schünemann, 2013; Hultcrantz, 2017).
|
GRADE |
Definitie |
|
Hoog |
|
|
Redelijk |
|
|
Laag |
|
|
Zeer laag |
|
Bij het beoordelen (graderen) van de kracht van het wetenschappelijk bewijs in richtlijnen volgens de GRADE-methodiek spelen grenzen voor klinische besluitvorming een belangrijke rol (Hultcrantz, 2017). Dit zijn de grenzen die bij overschrijding aanleiding zouden geven tot een aanpassing van de aanbeveling. Om de grenzen voor klinische besluitvorming te bepalen moeten alle relevante uitkomstmaten en overwegingen worden meegewogen. De grenzen voor klinische besluitvorming zijn daarmee niet één op één vergelijkbaar met het minimaal klinisch relevant verschil (Minimal Clinically Important Difference, MCID). Met name in situaties waarin een interventie geen belangrijke nadelen heeft en de kosten relatief laag zijn, kan de grens voor klinische besluitvorming met betrekking tot de effectiviteit van de interventie bij een lagere waarde (dichter bij het nuleffect) liggen dan de MCID (Hultcrantz, 2017).
Overwegingen (van bewijs naar aanbeveling)
Om te komen tot een aanbeveling zijn naast (de kwaliteit van) het wetenschappelijke bewijs ook andere aspecten belangrijk en worden meegewogen, zoals aanvullende argumenten uit bijvoorbeeld de biomechanica of fysiologie, waarden en voorkeuren van patiënten, kosten (middelenbeslag), aanvaardbaarheid, haalbaarheid en implementatie. Deze aspecten zijn systematisch vermeld en beoordeeld (gewogen) onder het kopje ‘Overwegingen’ en kunnen (mede) gebaseerd zijn op expert opinion. Hierbij is gebruik gemaakt van een gestructureerd format gebaseerd op het evidence-to-decision framework van de internationale GRADE Working Group (Alonso-Coello, 2016a; Alonso-Coello 2016b). Dit evidence-to-decision framework is een integraal onderdeel van de GRADE methodiek.
Formuleren van aanbevelingen
De aanbevelingen geven antwoord op de uitgangsvraag en zijn gebaseerd op het beschikbare wetenschappelijke bewijs en de belangrijkste overwegingen, en een weging van de gunstige en ongunstige effecten van de relevante interventies. De kracht van het wetenschappelijk bewijs en het gewicht dat door de werkgroep wordt toegekend aan de overwegingen, bepalen samen de sterkte van de aanbeveling. Conform de GRADE-methodiek sluit een lage bewijskracht van conclusies in de systematische literatuuranalyse een sterke aanbeveling niet a priori uit, en zijn bij een hoge bewijskracht ook zwakke aanbevelingen mogelijk (Agoritsas, 2017; Neumann, 2016). De sterkte van de aanbeveling wordt altijd bepaald door weging van alle relevante argumenten tezamen. De werkgroep heeft bij elke aanbeveling opgenomen hoe zij tot de richting en sterkte van de aanbeveling zijn gekomen.
In de GRADE-methodiek wordt onderscheid gemaakt tussen sterke en zwakke (of conditionele) aanbevelingen. De sterkte van een aanbeveling verwijst naar de mate van zekerheid dat de voordelen van de interventie opwegen tegen de nadelen (of vice versa), gezien over het hele spectrum van patiënten waarvoor de aanbeveling is bedoeld. De sterkte van een aanbeveling heeft duidelijke implicaties voor patiënten, behandelaars en beleidsmakers (zie onderstaande tabel). Een aanbeveling is geen dictaat, zelfs een sterke aanbeveling gebaseerd op bewijs van hoge kwaliteit (GRADE gradering HOOG) zal niet altijd van toepassing zijn, onder alle mogelijke omstandigheden en voor elke individuele patiënt.
|
Implicaties van sterke en zwakke aanbevelingen voor verschillende richtlijngebruikers |
||
|
|
Sterke aanbeveling |
Zwakke (conditionele) aanbeveling |
|
Voor patiënten |
De meeste patiënten zouden de aanbevolen interventie of aanpak kiezen en slechts een klein aantal niet. |
Een aanzienlijk deel van de patiënten zouden de aanbevolen interventie of aanpak kiezen, maar veel patiënten ook niet. |
|
Voor behandelaars |
De meeste patiënten zouden de aanbevolen interventie of aanpak moeten ontvangen. |
Er zijn meerdere geschikte interventies of aanpakken. De patiënt moet worden ondersteund bij de keuze voor de interventie of aanpak die het beste aansluit bij zijn of haar waarden en voorkeuren. |
|
Voor beleidsmakers |
De aanbevolen interventie of aanpak kan worden gezien als standaardbeleid. |
Beleidsbepaling vereist uitvoerige discussie met betrokkenheid van veel stakeholders. Er is een grotere kans op lokale beleidsverschillen. |
Organisatie van zorg
In de knelpuntenanalyse en bij de ontwikkeling van de richtlijnmodule is expliciet aandacht geweest voor de organisatie van zorg: alle aspecten die randvoorwaardelijk zijn voor het verlenen van zorg (zoals coördinatie, communicatie, (financiële) middelen, mankracht en infrastructuur). Randvoorwaarden die relevant zijn voor het beantwoorden van deze specifieke uitgangsvraag zijn genoemd bij de overwegingen. Meer algemene, overkoepelende, of bijkomende aspecten van de organisatie van zorg worden behandeld in de module Organisatie van zorg.
Commentaar- en autorisatiefase
De conceptrichtlijnmodule werd aan de betrokken (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd ter commentaar. De commentaren werden verzameld en besproken met de werkgroep. Naar aanleiding van de commentaren werd de conceptrichtlijnmodule aangepast en definitief vastgesteld door de werkgroep. De definitieve richtlijnmodule werd aan de deelnemende (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd voor autorisatie en door hen geautoriseerd dan wel geaccordeerd.
Adaptatie
Een aantal modules van deze richtlijn betreft een adaptatie van modules van de World Health Organization (WHO)-richtlijn ‘Global guidelines for the prevention of surgical site infection’ (WHO, 2018), te weten:
- Module Normothermie
- Module Immunosuppressive middelen
- Module Glykemische controle
- Module Antimicrobiële afdichtingsmiddelen
- Module Wondbeschermers bij laparotomie
- Module Preoperatief douchen
- Module Preoperatief verwijderen van haar
- Module Chirurgische handschoenen: Vervangen en type handschoenen
- Module Afdekmaterialen en operatiejassen
Methode
- Uitgangsvragen zijn opgesteld in overeenstemming met de standaardprocedures van het Kennisinstituut van de Federatie Medisch Specialisten.
- De inleiding van iedere module betreft een korte uiteenzetting van het knelpunt, waarbij eventuele onduidelijkheid en praktijkvariatie voor de Nederlandse setting wordt beschreven.
- Het literatuuronderzoek is overgenomen uit de WHO-richtlijn. Afhankelijk van de beoordeling van de actualiteit van de richtlijn is een update van het literatuuronderzoek uitgevoerd.
- De samenvatting van de literatuur is overgenomen van de WHO-richtlijn, waarbij door de werkgroep onderscheid is gemaakt tussen ‘cruciale’ en ‘belangrijke’ uitkomsten. Daarnaast zijn door de werkgroep grenzen voor klinische besluitvorming gedefinieerd in overeenstemming met de standaardprocedures van het Kennisinstituut van de Federatie Medisch Specialisten, en is de interpretatie van de bevindingen primair gebaseerd op klinische relevantie van het gevonden effect, niet op statistische significantie. In de meta-analyses zijn naast odds-ratio’s ook relatief risico’s en risicoverschillen gerapporteerd.
- De beoordeling van de mate van bewijskracht is overgnomen van de WHO-richtlijn, waarbij de beoordeling is gecontroleerd op consistentie met de standaardprocedures van het Kennisinstituut van de Federatie Medisch Specialisten (GRADE-methode; http://www.gradeworkinggroup.org/). Eventueel door de WHO gerapporteerde bewijskracht voor observationele studies is niet overgenomen indien ook gerandomiseerde gecontroleerde studies beschikbaar waren.
- De conclusies van de literatuuranalyse zijn geformuleerd in overeenstemming met de standaardprocedures van het Kennisinstituut van de Federatie Medisch Specialisten.
- In de overwegingen heeft de werkgroep voor iedere aanbeveling het bewijs waarop de aanbeveling is gebaseerd en de aanvaardbaarheid en toepasbaarheid van de aanbeveling voor de Nederlandse klinische praktijk beoordeeld. Op basis van deze beoordeling is door de werkgroep besloten welke aanbevelingen ongewijzigd zijn overgenomen, welke aanbevelingen niet zijn overgenomen, en welke aanbevelingen (mits in overeenstemming met het bewijs) zijn aangepast naar de Nederlandse context. ‘De novo’ aanbevelingen zijn gedaan in situaties waarin de werkgroep van mening was dat een aanbeveling nodig was, maar deze niet als zodanig in de WHO-richtlijn was opgenomen. Voor elke aanbeveling is vermeld hoe deze tot stand is gekomen, te weten: ‘WHO’, ‘aangepast van WHO’ of ‘de novo’.
Voor een verdere toelichting op de procedure van adapteren wordt verwezen naar de Bijlage Adapteren.
Literatuur
Agoritsas T, Merglen A, Heen AF, Kristiansen A, Neumann I, Brito JP, Brignardello-Petersen R, Alexander PE, Rind DM, Vandvik PO, Guyatt GH. UpToDate adherence to GRADE criteria for strong recommendations: an analytical survey. BMJ Open. 2017 Nov 16;7(11):e018593. doi: 10.1136/bmjopen-2017-018593. PubMed PMID: 29150475; PubMed Central PMCID: PMC5701989.
Alonso-Coello P, Schünemann HJ, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Rada G, Rosenbaum S, Morelli A, Guyatt GH, Oxman AD; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ. 2016 Jun 28;353:i2016. doi: 10.1136/bmj.i2016. PubMed PMID: 27353417.
Alonso-Coello P, Oxman AD, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Vandvik PO, Meerpohl J, Guyatt GH, Schünemann HJ; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 2: Clinical practice guidelines. BMJ. 2016 Jun 30;353:i2089. doi: 10.1136/bmj.i2089. PubMed PMID: 27365494.
Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, Fervers B, Graham ID, Grimshaw J, Hanna SE, Littlejohns P, Makarski J, Zitzelsberger L; AGREE Next Steps Consortium. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010 Dec 14;182(18):E839-42. doi: 10.1503/cmaj.090449. Epub 2010 Jul 5. Review. PubMed PMID: 20603348; PubMed Central PMCID: PMC3001530.
Hultcrantz M, Rind D, Akl EA, Treweek S, Mustafa RA, Iorio A, Alper BS, Meerpohl JJ, Murad MH, Ansari MT, Katikireddi SV, Östlund P, Tranæus S, Christensen R, Gartlehner G, Brozek J, Izcovich A, Schünemann H, Guyatt G. The GRADE Working Group clarifies the construct of certainty of evidence. J Clin Epidemiol. 2017 Jul;87:4-13. doi: 10.1016/j.jclinepi.2017.05.006. Epub 2017 May 18. PubMed PMID: 28529184; PubMed Central PMCID: PMC6542664.
Medisch Specialistische Richtlijnen 2.0 (2012). Adviescommissie Richtlijnen van de Raad Kwalitieit. http://richtlijnendatabase.nl/over_deze_site/over_richtlijnontwikkeling.html
Neumann I, Santesso N, Akl EA, Rind DM, Vandvik PO, Alonso-Coello P, Agoritsas T, Mustafa RA, Alexander PE, Schünemann H, Guyatt GH. A guide for health professionals to interpret and use recommendations in guidelines developed with the GRADE approach. J Clin Epidemiol. 2016 Apr;72:45-55. doi: 10.1016/j.jclinepi.2015.11.017. Epub 2016 Jan 6. Review. PubMed PMID: 26772609.
Schünemann H, Brożek J, Guyatt G, et al. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group, 2013. Available from http://gdt.guidelinedevelopment.org/central_prod/_design/client/handbook/handbook.html.
World Health Organization. Global guidelines for the prevention of surgical site infection,
second edition. Geneva: World Health Organization; 2018. (https://www.who.int/publications/i/item/9789241550475, accessed 12 June 2023).
Zoekverantwoording
Agoritsas T, Merglen A, Heen AF, Kristiansen A, Neumann I, Brito JP, Brignardello-Petersen R, Alexander PE, Rind DM, Vandvik PO, Guyatt GH. UpToDate adherence to GRADE criteria for strong recommendations: an analytical survey. BMJ Open. 2017 Nov 16;7(11):e018593. doi: 10.1136/bmjopen-2017-018593. PubMed PMID: 29150475; PubMed Central PMCID: PMC5701989.
Alonso-Coello P, Schünemann HJ, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Rada G, Rosenbaum S, Morelli A, Guyatt GH, Oxman AD; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ. 2016 Jun 28;353:i2016. doi: 10.1136/bmj.i2016. PubMed PMID: 27353417.
Alonso-Coello P, Oxman AD, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Vandvik PO, Meerpohl J, Guyatt GH, Schünemann HJ; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 2: Clinical practice guidelines. BMJ. 2016 Jun 30;353:i2089. doi: 10.1136/bmj.i2089. PubMed PMID: 27365494.
Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, Fervers B, Graham ID, Grimshaw J, Hanna SE, Littlejohns P, Makarski J, Zitzelsberger L; AGREE Next Steps Consortium. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010 Dec 14;182(18):E839-42. doi: 10.1503/cmaj.090449. Epub 2010 Jul 5. Review. PubMed PMID: 20603348; PubMed Central PMCID: PMC3001530.
Hultcrantz M, Rind D, Akl EA, Treweek S, Mustafa RA, Iorio A, Alper BS, Meerpohl JJ, Murad MH, Ansari MT, Katikireddi SV, Östlund P, Tranæus S, Christensen R, Gartlehner G, Brozek J, Izcovich A, Schünemann H,
Guyatt G. The GRADE Working Group clarifies the construct of certainty of evidence. J Clin Epidemiol. 2017 Jul;87:4-13. doi: 10.1016/j.jclinepi.2017.05.006. Epub 2017 May 18. PubMed PMID: 28529184; PubMed Central PMCID: PMC6542664.
Medisch Specialistische Richtlijnen 2.0 (2012). Adviescommissie Richtlijnen van de Raad Kwalitieit. http://richtlijnendatabase.nl/over_deze_site/over_richtlijnontwikkeling.html
Neumann I, Santesso N, Akl EA, Rind DM, Vandvik PO, Alonso-Coello P, Agoritsas T, Mustafa RA, Alexander PE, Schünemann H, Guyatt GH. A guide for health professionals to interpret and use recommendations in guidelines developed with the GRADE approach. J Clin Epidemiol. 2016 Apr;72:45-55. doi: 10.1016/j.jclinepi.2015.11.017. Epub 2016 Jan 6. Review. PubMed PMID: 26772609.
Schünemann H, Brożek J, Guyatt G, et al. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group, 2013. Available from http://gdt.guidelinedevelopment.org/central_prod/_design/client/handbook/handbook.html.
World Health Organization. Global guidelines for the prevention of surgical site infection,
second edition. Geneva: World Health Organization; 2018. (https://www.who.int/publications/i/item/9789241550475, accessed 12 June 2023).
