Prolongatie van antimicrobiële profylaxe
Uitgangsvraag
Wat is het effect van postoperatieve profylactische toediening van antibiotica bij patiënten die een operatie ondergaan op de preventie van postoperatieve wondinfecties?
Aanbeveling
Ter preventie van postoperatieve wondinfecties is het niet noodzakelijk of wenselijk de perioperatieve intraveneuze antibiotische profylaxe te continueren na de operatie.
De toediening van perioperatieve intraveneuze antibiotische profylaxe (ter voorkoming van postoperatieve wondinfecties) dient te voldoen aan de volgende voorwaarden:
- Eerste dosering <60 min voor incisie of aanleggen bloedleegte gegeven
- Direct postoperatief staken
Voor herdosering verwijst de werkgroep naar de SWAB-richtlijn Peri-operatieve profylaxe (2019). (paragraaf Organisatie, timing en duur van de profylaxe)
Overwegingen
Voor- en nadelen van de interventie en de kwaliteit van het bewijs
Als matig beoordeeld bewijs uit een meta-analyse van 58 gerandomiseerde gecontroleerde onderzoeken (RCT's) met 20.918 deelnemers toonde geen doorslaggevend bewijs voor het voordeel van het voortzetten van antibiotische profylaxe na de operatie voor het verminderen van de incidentie van postoperatieve wondinfecties (POWI’s) in vergelijking met onmiddellijke stopzetting. Vergelijkingen van postoperatieve regimes van verschillende duur toonde eveneens geen doorslaggevend bewijs in het voordeel van langdurige regimes.
Subgroep analyse toonde aan dat de effectiviteit van het stopzetten van antibiotische profylaxe na de operatie afhankelijk is of de chirurgische antibiotische profylaxe in de praktijk juist is toegepast. Wanneer de profylaxe juist is toegepast (d.w.z. tijdige toediening van de eerste dosis en herhaalde toediening indien aangewezen volgens de duur van de ingreep), toonde de studies met matige bewijskracht aan dat er geen voordeel is in het voortzetten van antibiotische profylaxe na de operatie voor het verminderen van POWI in vergelijking met het stopzetten ervan. Studies met matige bewijskracht toonden aan dat het voortzetten van antibiotische profylaxe na de operatie alleen effectief was wanneer de chirurgische antibiotische profylaxe niet juist was toegepast.
Enig bewijs uit verkennende analyse geeft aan dat het voortzetten van antibiotische profylaxe na de operatie het risico op POWI geassocieerd met maxillofaciale en cardiale chirurgie zou kunnen verminderen; echter, er waren heel weinig studies beschikbaar voor de subgroep maxillofaciale chirurgie en hartchirurgie.
Wanneer kosten en bijwerkingen werden gerapporteerd, leek het voortzetten na de operatie de kosten te verhogen en leidde het tot meer bijwerkingen. Antibioticagebruik is geassocieerd met belangrijke bijwerkingen op een tijdsafhankelijke manier (Branch-Elliman 2019, Hartbarth 2000, Stevens 2011). Op hun beurt zijn deze bijwerkingen geassocieerd met een aanzienlijke economische last die bijdraagt aan aanvullende verwervings- en administratiekosten in verband met het voortzetten van antibiotische profylaxe na de operatie (Cunha 2018, Dubbekerke 2012, OECD 2018).
Internationale richtlijnen
Deze bevindingen zijn in lijn met de aanbevelingen van de CDC (Berrios-Torres 2017) en de WHO (WHO 2018) over dit onderwerp. Beide richtlijnen raden niet aan om extra antimicrobiële profylaxe na de operatie toe te dienen ter voorkoming van POWI. De NICE-richtlijn heeft geen aanbeveling gedaan.
Waarden en voorkeuren van patiënten (en evt. hun verzorgers)
Voor de individuele patiënt kan een kortere duur van antibiotica leiden tot een korter ziekenhuisverblijf en minder gebruik van antibiotica, met de bijbehorende bijwerkingen. Bijwerkingen van de gebruikte antibiotica zijn vaak gastro-intestinale klachten zoals misselijkheid, braken, diarree en buikpijn. Minder antibiotica zullen waarschijnlijk leiden tot minder bijwerkingen. Bovendien is er meer onderzoek gedaan naar de negatieve impact van antibiotica op de darmmicrobiota met zowel korte- als langetermijngevolgen voor de gezondheid (Patangia, 2022; Ramirez 2020). Bovendien kan minder antibiotica leiden tot minder antimicrobiële resistentie tegen bepaalde antibiotica.
Het is belangrijk om patiënten te informeren over het nut en de noodzaak van de behandeling. Voor de rol van de patiënt bij het herkennen van een infectie verwijst de werkgroep naar de module Patiëntbetrokkenheid van deze richtlijn.
Kosten (middelenbeslag)
Kosten werden matig gerapporteerd, zo niet volledig, en er konden geen zinvolle meta-analyses worden uitgevoerd om deze resultaten te beoordelen. Wanneer kosten werden gerapporteerd, leek het voortzetten van profylaxe na de operatie de kosten te verhogen.
Aanvaardbaarheid, haalbaarheid en implementatie
Er zijn geen problemen te verwachten met betrekking tot de haalbaarheid van de verlenging van antimicrobiële profylaxe voor implementatie in de klinische praktijk.
Rationale van de aanbeveling: weging van argumenten voor en tegen de interventies
We hebben geen bewijs gevonden voor het voordeel van het voortzetten van antibiotische profylaxe na de operatie in vergelijking met het stopzetten op het verminderen van de incidentie van POWI. Opmerkelijk genoeg bood het voortzetten na de operatie geen extra voordeel bij het voorkomen van POWI, vooral wanneer chirurgische antibiotische profylaxe juist werd toegepast. Onze bevindingen ondersteunen de aanbevelingen van de WHO tegen het voortzetten van chirurgische antibiotische profylaxe na de operatie. Gezien de bijbehorende nadelige effecten, met name antimicrobiële resistentie, heeft deze veelvoorkomende toepassing in de praktijk geen basis. Toegenomen bewustzijn en educatie zijn vereist onder zowel zorgprofessionals als patiënten, vooral door inspanningen voor stewardship te prioriteren onder chirurgen en anesthesisten en te blijven aandringen op andere preventiemaatregelen naast chirurgische antibiotische profylaxe. Toekomstig onderzoek om het voordeel van het voortzetten van antibiotische profylaxe na de operatie te verduidelijken, indien aanwezig, zou vooraf de monitoring van bijwerkingen moeten specificeren, gedetailleerde gegevens verstrekken over kosten en het tijdstip van toediening voorafgaand aan de operatie en herhaalde toediening van antibiotica tijdens de operatie moeten standaardiseren.
Onderbouwing
Achtergrond
Het gebruik van antibiotica staat momenteel onder toezicht vanwege zorgen over de opkomst van antimicrobiële resistentie en andere schadelijke bijwerkingen. Wereldwijd is ongeveer één op de zes voorschriften voor antibiotica in het ziekenhuis bedoeld voor chirurgische antibiotische profylaxe, die vaak gedurende meerdere dagen na de operatie wordt voortgezet. Hoewel de effectiviteit van een juiste antibiotische profylaxe bij het voorkomen van postoperatieve wondinfecties (POWIs) bij aangewezen procedures algemeen erkend is, suggereren toenemende bewijzen dat een enkele preoperatieve dosis antibiotica, met extra intra-operatieve toediening indien nodig, mogelijk even effectief is als een langdurig postoperatief regime bij verschillende procedures.
Conclusies / Summary of Findings
|
Moderate GRADE |
Postoperative continuation of surgical prophylactic intravenous antibiotics is unlikely to reduce the number of postoperative surgical site infections, if the prophylactic intravenous antibiotics have been correctly administered (first dose less than 60 minutes before incision, perioperative re-dosing four hours after the previous dose and in case of excessive peroperative bleeding).
Bronnen: De Jonge + update (Aberg, 1993; Abro, 2014; Abubakar, 2001; Adaji, 2020; Ahmed, 2019; Balbo, 1991; Baquin, 2004; Bates, 1992; Becker, 2008; Becker, 1991; Bentley, 1999; Berry, 2019; Bidkar, 2014; ; Bozorgzadeh, 1999; Buckley, 1990; Campos, 2015; Cartana, 1994; Carroll, 2003; Chang, 2005; Chauhan, 2018; Cioca, 2002; Crist, 2018; Danda, 2010; Davis, 2016; Eshghpour, 2014; Fujita, 2015; Fujita, 2007; Fridrich, 1994; Garcia, 2020; Gargotta, 1991; Gupta, 2010; Haga, 2012; Hall, 1998; Hanif, 2015; Hellbusch, 2008; Hussain, 2012; Imamura, 2012; Irato, 1997; Ishibashi, 2014; Ishibashi, 2009; Jansisyanont, 2008; Jiang, 2004; Kang, 2009; Karren, 1993; Kim, 2017; Kow, 1995; Lau, 1990; Liberman, 1995; Lin, 2011; Lindeboom, 2003; Liu, 2008; Loozen, 2017; Lyimo, 2013; Madadi, 2019; Maier, 1992; Mann, 1990; McArdle, 1995; Meijer, 1993; Mohri, 2007; Mui, 2005; Niederhauser, 1997; Nooyen, 1994; Nusrath, 2020; Olak, 1991; Orjuela, 2020; Orlando, 2015; Otani, 2004; Rajabi, 2012; Rajan, 2005; Regimbeau, 2014; Righi, 1996; Sadraei-Moosavi, 2017; Salih, 2018; Santibañes, 2018; Sawyer, 1990; Scher, 1997; Sgroi, 1990; Shaheen, 2014; Su, 2005; Sugawara, 2016; Suzuki, 2011; Takayama, 2019; Takemoto, 2015; Tamayo, 2008; Tan, 2020; Togo, 2007; Tsang, 1992; Turano, 1992; Unemura, 2000; Urquhart, 2019; Wahab, 2013; Westen, 2015; Yamamoto, 2018; Yang, 2001). |
Samenvatting literatuur
Description of studies
Ninety-eight studies were included in the analysis of the literature, in which 10,227 patients were involved.
Fifty-eight RCTs compared postoperative continuation of antibiotic prophylaxis of any duration with direct postoperative discontinuation (Aberg, 1993; Abro, 2014; Balbo, 1991; Bates, 1992; Becker, 2008; Berry, 2019; Buckley, 1990; Campos, 2015; Cartana, 1994; Chauhan, 2018; Cioca, 2002; Crist, 2018; Danda, 2010; Fujita, 2007; Gargotta, 1991; Haga, 2012; Hall, 1998; Hellbusch, 2008; Hussain, 2012; Imamura, 2012; Irato, 1997; Jiang, 2004; Kang, 2009; Kim, 2017; Kow, 1995; Liberman, 1995; Lindeboom, 2003; Loozen, 2017; Lyimo, 2013; Madadi, 2019; Maier, 1992; Mann, 1990; Meijer, 1993; Mohri, 2007; Mui, 2005; Nooyen, 1994; Nusrath, 2020; Olak, 1991; Orjuela, 2020; Orlando, 2015; Rajabi, 2012; Rajan, 2005; Regimbeau, 2014; Sadraei-Moosavi, 2017; Salih, 2018; Santibañes, 2018; Scher, 1997; Sgroi, 1990; Shaheen, 2014; Su, 2005; Suzuki, 2011; Tamayo, 2008; Tan, 2020; Tsang, 1992; Turano, 1992; Unemura, 2000; Wahab, 2013; Westen, 2015).
One RCT compared postoperative continuation of antibiotic prophylaxis (multiple doses) for less than 24 hours with a single postoperative dose (Karren, 1993).
Thirty-one RCTs compared postoperative continuation of antibiotic prophylaxis for more than 24 hours with postoperative continuation equal or less than 24 hours (Abubakar, 2001; Ahmed, 2019; Baquin, 2004; Becker, 1991; Bentley, 1999; Bidkar, 2014; Bozorgzadeh, 1999; Carroll, 2003; Chang, 2005; Eshghpour, 2014; Fujita, 2015; Fridrich, 1994; Garcia, 2020; Hanif, 2015; Ishibashi, 2014; Ishibashi, 2009; Jansisyanont, 2008; Lau, 1990; Lin, 2011; Liu, 2008; Madadi, 2019; McArdle, 1995; Mui, 2005; Niederhauser, 1997; Rajabi, 2012; Righi, 1996; Takayama, 2019; Takemoto, 2015; Urquhart, 2019; Yamamoto, 2018; Yang, 2001).
Seven RCTs compared postoperative continuation of antibiotic prophylaxis for more than 48 hours with postoperative continuation equal or less than 48 hours (Adaji, 2020; Davis, 2016; Gupta, 2010; Otani, 2004; Sawyer, 1990; Sugawara, 2016; Togo, 2007).
One RCT compared postoperative continuation of antibiotic prophylaxis for more than 72 hours with postoperative continuation equal or less than 72 hours (Park, 2010).
Results
1. Surgical site infections
A. Postoperative continuation versus immediate discontinuation
SSI for postoperative continuation versus immediate discontinuation were reported in 58 studies (Aberg, 1993; Abro, 2014; Balbo, 1991; Bates, 1992; Becker, 2008; Berry, 2019; Buckley, 1990; Campos, 2015; Cartana, 1994; Chauhan, 2018; Cioca, 2002; Crist, 2018; Danda, 2010; Fujita, 2007; Gargotta, 1991; Haga, 2012; Hall, 1998; Hellbusch, 2008; Hussain, 2012; Imamura, 2012; Irato, 1997; Jiang, 2004; Kang, 2009; Kim, 2017; Kow, 1995; Liberman, 1995; Lindeboom, 2003; Loozen, 2017; Lyimo, 2013; Madadi, 2019; Maier, 1992; Mann, 1990; Meijer, 1993; Mohri, 2007; Mui, 2005; Nooyen, 1994; Nusrath, 2020; Olak, 1991; Orjuela, 2020; Orlando, 2015; Rajabi, 2012; Rajan, 2005; Regimbeau, 2014; Sadraei-Moosavi, 2017; Salih, 2018; Santibañes, 2018; Scher, 1997; Sgroi, 1990; Shaheen, 2014; Su, 2005; Suzuki, 2011; Tamayo, 2008; Tan, 2020; Tsang, 1992; Turano, 1992; Unemura, 2000; Wahab, 2013; Westen, 2015).
The results were pooled in a meta-analysis. The pooled number of SSI in the postoperative continuation group was 543/10691 (5.1%), compared to 588/10227 (5.7%) in the immediate discontinuation group. This resulted in a pooled relative risk ratio (RR) of 0.91 (95% CI 0.81 to 1.02), in favour of the postoperative continuation group (figure 1). This was not considered as a clinically relevant difference.
The studies were divided in three different categories:
(I) studies that adhered to the best standard of practice versus studies that did not adhere to the best standard of practice;
(II) studies that timed the first dose within 60 minutes before surgery versus studies that did not time the first dose within 60 minutes before surgery; and
(III) studies that specified intraoperative repeated administration when indicated versus studies that did not specify intraoperative repeated administration when indicated.
|
Analysis |
N |
SSI in longer regimen |
SSI shorter regimen |
Relative risk (95%CI) |
|
Overall analysis |
58 |
543 of 10.691 |
588 of 10.227 |
0.91 (0.81 - 1.02) |
|
Adherence to current best practice standards of SAP (repeat dose + timing correct) |
||||
|
Yes |
29 |
234 of 5.564 |
215 of 5.184 |
1.06 (0.88 - 1.28) |
|
No |
29 |
543 of 10.691 |
588 of 10.227 |
0.81 (0.68 - 0.96) |
|
Timing of first dose specified and within 60 min before surgery |
||||
|
Yes |
39 |
354 of 7.214 |
352 of 6.831 |
0.99 (0.86 - 1.15) |
|
No |
19 |
189 of 3.477 |
236 of 3.396 |
0.77 (0.61 - 0.96) |
|
Intraoperative repeat administration specified when indicated |
||||
|
Yes |
39 |
303 of 7.042 |
317 of 6.576 |
0.92 (0.79 - 1.08) |
|
No |
19 |
240 of 3.649 |
271 of 3.651 |
0.88 (0.72 - 1.08) |
Table 1. Meta-analysis and subgroup analyses of incidence of SSI associated with postoperative continuation versus postoperative discontinuation of antibiotic prophylaxis.
I. Adherent to best standards of practice versus not adherent to best standards of practice
Current best practice standards for surgical antibiotic prophylaxis are described in the American Society of Health-System Pharmacists clinical practice guidelines on antimicrobial prophylaxis in surgery (Bratzler 2013):
1) timing of the first preoperative dose within 60 min before incision;
2) repeat administration when the procedure duration exceeded two times the half-life of the antibiotic used.
The subgroup analyses indicates that compliance with best practice standards for surgical antibiotic prophylaxis significantly modified the association between postoperative continuation of antibiotic prophylaxis and the incidence of SSI (Figure 1).
In the subgroup analysis of 29 trials that were not compliant with abovementioned best practice standards of surgical antibiotic prophylaxis, continuation of antibiotic prophylaxis after surgery resulted in significant less SSI, compared with its immediate discontinuation (RR 0.81 [0.68-0.96]; corresponding heterogeneity was moderate (I² = 13%).
When the analysis was restricted to 29 trials that were compliant with abovementioned best practice standards of surgical antibiotic prophylaxis, there was no benefit of postoperative continuation of antibiotic prophylaxis (1.06 [0.89-1.28]). The corresponding heterogeneity in effect size was low (I² < 0.1%).
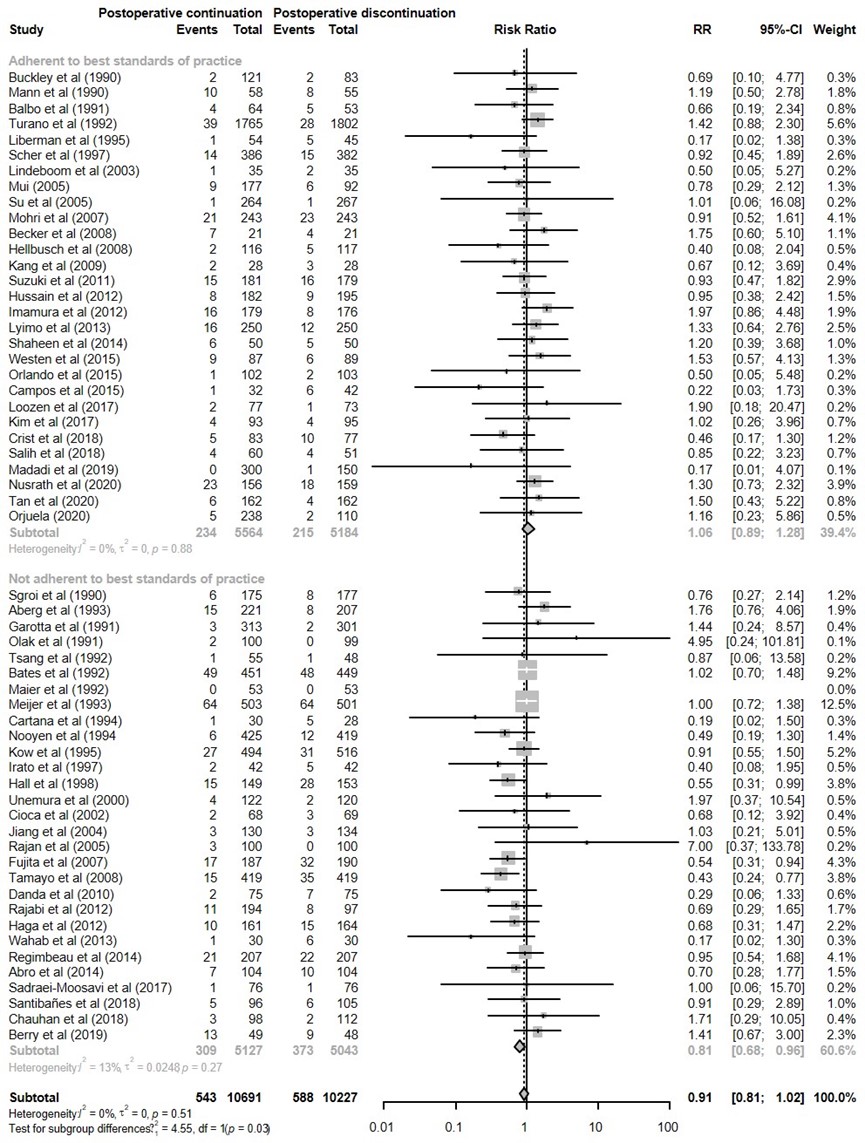
Figure 1. Forest plot showing the comparison between postoperative continuation of antibiotic prophylaxis to immediate discontinuation of antibiotic prophylaxis for surgical site infections (SSI) - Adherent to best standards of practice versus not adherent to best standards of practice. Pooled relative risk ratio (RR), random effects model. Z: p-value of overall effect; df: degrees of freedom; I2; SD: standard deviation; statistical heterogeneity; CI: confidence interval.
II. Timing of first dose within 60 minutes before surgery versus timing of first dose not within 60 minutes before surgery
Adequate timing alone did affect the effect estimate (Figure 2). In 19 trials where the first preoperative dose given >60 min before incision, continuation of antibiotic prophylaxis after surgery prevented SSI compared with its immediate discontinuation, RR 0.77 (95% CI 0.61 – 0.96). In the 39 studies with adequate timing of the first dose of antibiotic prophylaxis (<60 min prior to incision) there was no benefit found for continuation of antibiotic prophylaxis after surgery, RR 0.99 (0.86 - 1.15).
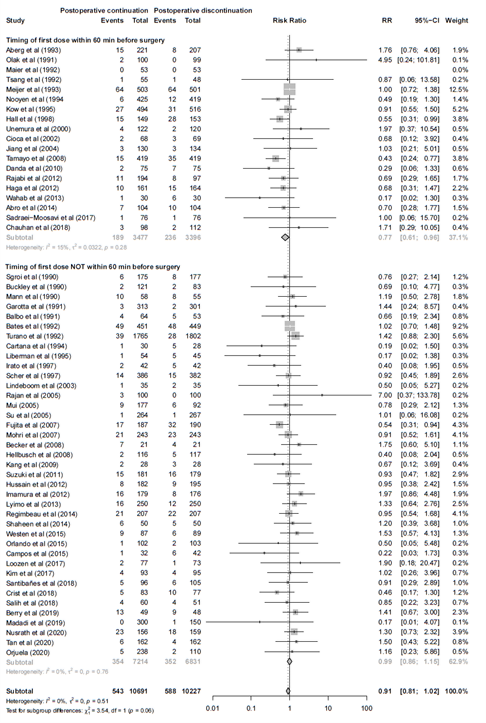
Figure 2. Forest plot showing the comparison between postoperative continuation of antibiotic prophylaxis to immediate discontinuation of antibiotic prophylaxis for surgical site infections (SSI) - Timing of first dose within 60 minutes before surgery versus timing of first dose not within 60 minutes before surgery. Pooled relative risk ratio (RR), random effects model. Z: p-value of overall effect; df: degrees of freedom; I2; SD: standard deviation; statistical heterogeneity; CI: confidence interval.
III. Intraoperative repeat administration specified when indicated versus intraoperative repeat administration not specified when indicated
Adequate repeat administration alone did not affect the effect estimate (Figure 3). In the 39 studies with adequate repeat administration antibiotic prophylaxis the pooled relative risk was 0.92 (95% CI 0.79 – 1.08), versus no adequate timing RR 0.88 (95% CI 0.72 – 1.08) in 19 studies.
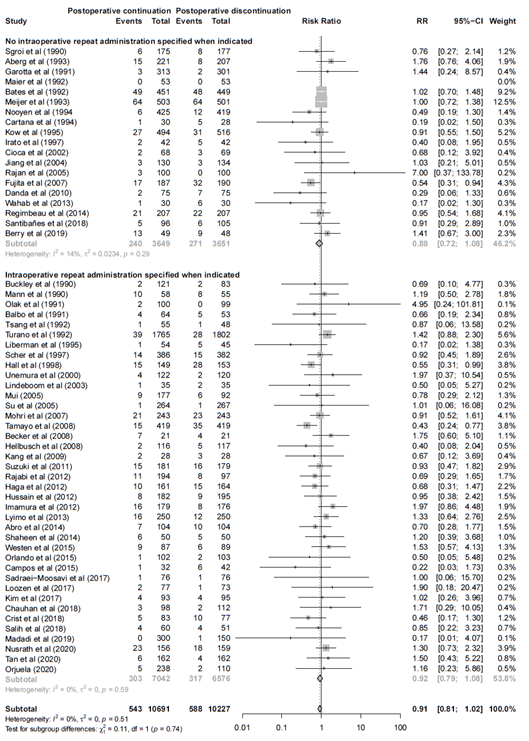
Figure 3. Forest plot showing the comparison between postoperative continuation of antibiotic prophylaxis to immediate discontinuation of antibiotic prophylaxis for surgical site infections (SSI) - Intraoperative repeat administration specified when indicated versus intraoperative repeat administration not specified when indicated. Pooled relative risk ratio (RR), random effects model. Z: p-value of overall effect; df: degrees of freedom; I2; SD: standard deviation; statistical heterogeneity; CI: confidence interval.
B. Postoperative postoperative continuations of antibiotic prophylaxis (multiple doses) for <24h versus a single postoperative dose
One study (Karran, 1993) compared postoperative continuation (multiple doses) for <24h versus a single dose after surgery. The number of SSI was 44/113 (38.9%) in the group with longer antibiotic prophylaxis, versus 39/114 (34.2%) in the group with a single postoperative dose. This resulted in a RR of 0.82 (0·57–1·40). This was not considered as a clinically relevant difference.
C. Postoperative continuation of surgical antibiotic prophylaxis for more than 24 hours versus postoperative continuation of surgical antibiotic prophylaxis equal or less than 24 hours
SSI for postoperative continuation for more than 24 hours versus postoperative continuation of surgical antibiotic prophylaxis equal or less than 24 hours were reported in 31 studies (Abubakar, 2001; Ahmed, 2019; Baquin, 2004; Becker, 1991; Bentley, 1999; Bidkar, 2014; Bozorgzadeh, 1999; Carroll, 2003; Chang, 2005; Eshghpour, 2014; Fujita, 2015; Fridrich, 1994; Garcia, 2020; Hanif, 2015; Ishibashi, 2014; Ishibashi, 2009; Jansisyanont, 2008; Lau, 1990; Lin, 2011; Liu, 2008; Madadi, 2019; McArdle, 1995; Mui, 2005; Niederhauser, 1997; Rajabi, 2012; Righi, 1996; Takayama, 2019; Takemoto, 2015; Urquhart, 2019; Yamamoto, 2018; Yang, 2001). The results were pooled in a meta-analysis. The pooled number of SSI in the postoperative continuation for more than 24 hours group was 244/2920 (8.4%), compared to 275/2944 (9.3%) in the postoperative continuation equal or less than 24 hours group. This resulted in a pooled relative risk ratio (RR) of 0.88 (95% CI 0.66 to 1.17), in favour of the postoperative continuation for more than 24 hours group (figure 4). This was not considered as a clinically relevant difference.
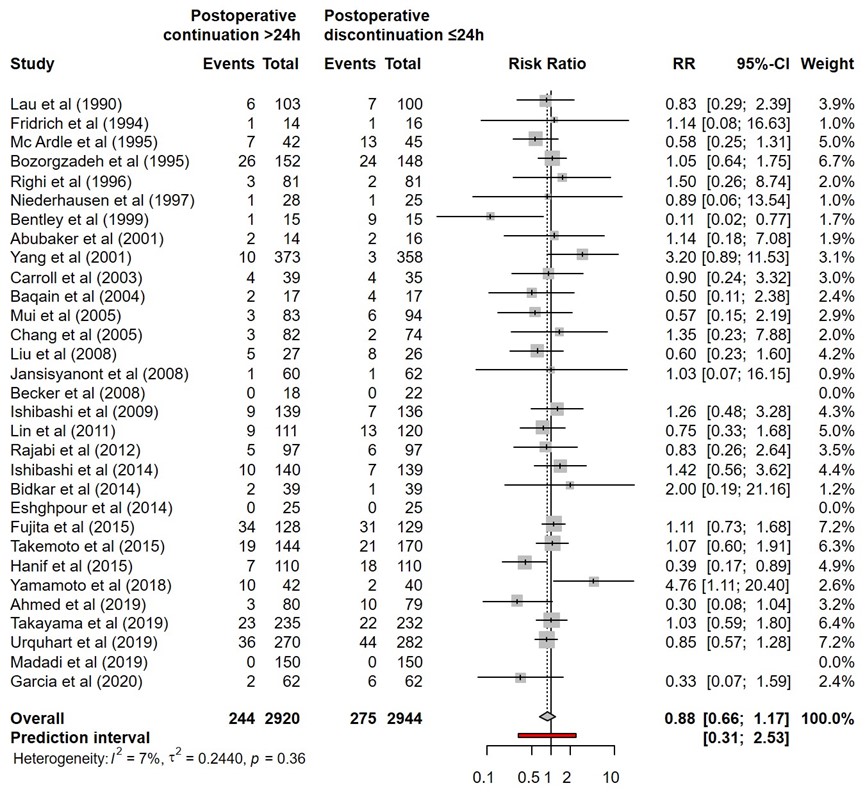
Figure 4. Forest plot showing the comparison between postoperative continuation of antibiotic prophylaxis for more than 24 hours to postoperative continuation of antibiotic prophylaxis equal or less than 24 hours for surgical site infections (SSI). Pooled relative risk ratio (RR), random effects model. Z: p-value of overall effect; df: degrees of freedom; I2; SD: standard deviation; statistical heterogeneity; CI: confidence interval.
D. Postoperative continuation of surgical antibiotic prophylaxis for more than 48 hours versus postoperative continuation of surgical antibiotic prophylaxis equal or less than 48 hours
SSI for postoperative continuation for more than 48 hours versus postoperative continuation of surgical antibiotic prophylaxis equal or less than 48 hours were reported in seven studies (Adaji, 2020; Davis, 2016; Gupta, 2010; Otani, 2004; Sawyer, 1990; Sugawara, 2016; Togo, 2007). The results were pooled in a meta-analysis. The pooled number of SSI in the postoperative continuation for more than 48 hours group was 56/493 (11.4%), compared to 38/504 (7.5%) in the postoperative continuation equal or less than 48 hours group. This resulted in a pooled relative risk (RR) of 1.44 (95% CI 0.90 to 2.31), in favor of the postoperative continuation equal or less than 48 hours group (Figure 5). This was considered as a clinically relevant difference.
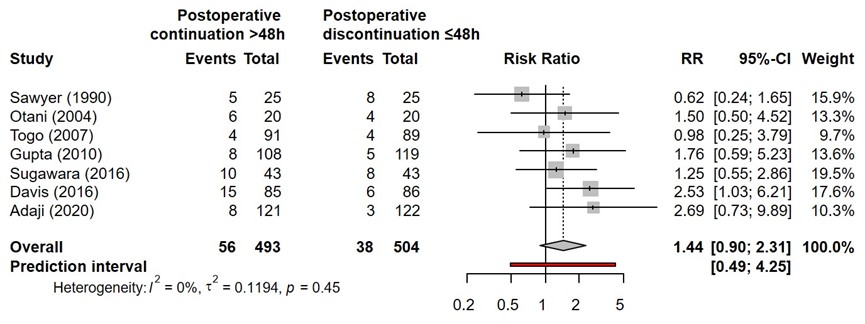
Figure 5. Forest plot showing the comparison between postoperative continuation of antibiotic prophylaxis for more than 48 hours to postoperative continuation of antibiotic prophylaxis equal or less than 48 hours for surgical site infections (SSI). Pooled relative risk ratio (RR), random effects model. Z: p-value of overall effect; df: degrees of freedom; I2; SD: standard deviation; statistical heterogeneity; CI: confidence interval.
E. Postoperative continuation of surgical antibiotic prophylaxis for more than 72 hours versus postoperative continuation of surgical antibiotic prophylaxis equal or less than 72 hours
SSI for postoperative continuation for more than 72 hours versus postoperative continuation of surgical antibiotic prophylaxis equal or less than 72 hours was reported in one study (Park, 2020).
The number of SSI for more than 72 hours antibiotics prophylaxis was 3/125 (2.4%), versus 4/130 (3.1%) in the postoperative continuation equal or less than 72 hours. This resulted in a RR of 0.61 (0.14 – 2.63). This was not considered as a clinically relevant difference.
2. Exploratory subgroup analysis
To investigate potential procedure-specific effects, we also did post-hoc exploratory subgroup analyses by procedure type.
|
Analysis |
SG |
N |
SSI in longer regimen |
SSI shorter regimen |
Relative risk (95%CI) |
|
Overall analysis |
|
58 |
543 of 10.691 |
588 of 10.227 |
0.91 (0.81 - 1.02) |
|
Subgroup analyses |
|||||
|
Maxillofacial surgery |
A |
6 |
9 of 268 |
27 of 279 |
0.38 (0.18 - 0.80) |
|
B |
3 |
4 of 95 |
11 of 105 |
0.44 (0.14 - 1.39) |
|
|
Cardiac surgery |
A |
3 |
21 of 1.144 |
48 of 988 |
0.43 (0.26 - 0.71) |
|
B |
1 |
0 of 300 |
1 of 150 |
0.17 (0.01- 4.08) |
|
|
Vascular Surgery |
A |
1 |
15 of 149 |
28 of 153 |
0.55 (0.31 - 0.99) |
|
B |
0 |
NA |
NA |
NA |
|
|
Appendectomy |
A |
7 |
35 of 798 |
34 of 604 |
0.75 (0.47 - 1.20) |
|
B |
4 |
22 of 473 |
24 of 383 |
0.76 (0.43 - 1.37) |
|
|
Colorectal surgery |
A |
2 |
32 of 368 |
48 of 269 |
0.68 (0.40 - 1.15) |
|
B |
1 |
15 of 181 |
16 of 179 |
0.93 (0.47 - 1.82) |
|
|
Upper GI surgery |
A |
4 |
51 of 647 |
51 of 636 |
0.98 (0.62 - 1.54) |
|
B |
3 |
41 of 486 |
36 of 472 |
1.11 (0.63 - 1.97) |
|
|
Cholecystectomy |
A |
6 |
39 of 693 |
37 of 712 |
1.06 (0.69 – 1.64) |
|
B |
2 |
6 of 170 |
5 of 168 |
1.19 (0.37 - 3.86) |
|
|
Hepatobiliary Surgery |
A |
1 |
64 of 503 |
64 of 501 |
1.00 (0.72 - 1.38) |
|
B |
0 |
NA |
NA |
NA |
|
|
Mixed general surgery |
A |
9 |
187 of 3.773 |
170 of 3.817 |
1.11 (0.91 - 1.35) |
|
B |
4 |
83 of 2.328 |
65 of 2.364 |
1.30 (0.95 - 1.78) |
|
|
Caesarean section |
A |
4 |
37 of 549 |
27 of 551 |
1.38 (0.85 - 2.22) |
|
B |
4 |
37 of 549 |
27 of 551 |
1.38 (0.85 - 2.22) |
|
|
Gynaecological surgery |
A |
3 |
4 of 336 |
11 of 337 |
0.37 (0.12 - 1.17) |
|
B |
1 |
1 of 264 |
1 of 267 |
1.01 (0.06 -16.08) |
|
|
Ortho/Trauma surgery |
A |
4 |
12 of 633 |
19 of 578 |
0.57 (0.28 - 1.18) |
|
B |
3 |
9 of 320 |
17 of 277 |
0.48 (0.22 - 1.06) |
|
|
Thoracic surgery |
A |
2 |
5 of 230 |
3 of 233 |
1.44 (0.36 - 5.87) |
|
B |
0 |
NA |
NA |
NA |
|
|
Head and neck surgery |
A |
2* |
13 of 159 |
8 of 156 |
1.60 (0.43 - 5.95) |
|
B |
1 |
10 of 58 |
8 of 55 |
1.19 (0.50 - 2.78) |
|
|
Transplantation surgery |
A |
2 |
14 of 151 |
11 of 151 |
1.29 (0.63 - 2.64) |
|
B |
1 |
1 of 102 |
2 of 203 |
0.50 (0.05 - 5.48) |
|
|
SG: Subgroup, A: Overall analysis, B: Adherence to current standards of practice subgroup, N: Number of studies, SSI: Surgical site infection, 95%CI: 95% confidence interval, NA: Not available, tau2: Tau-squared, MR: Meta-regression, MA: Meta-analysis, % of heterogeneity variance explained: * One study excluded from analysis because of no events in both arms |
|||||
Table 2. Results of the subgroup analysis by surgical subspecialty
3. Adverse events
24 studies (17 included in the primary analysis) described possible harmful effects or adverse events related to surgical antibiotic prophylaxis (Table 2). Of these, 18 studies could not attribute adverse events to antibiotic use in both the intervention and control groups (Becker 2008, Carrol 2003, Cartana 1994, Danda 2010, Eshghpour 2014, Fujita 2015, Imamura 2012, Kang 2009, Lindeboom 2003, Liu 2008, Loozen 2017, Maier 1992, Mohri 2007, Rajabi 2012, Regimbeau 2007, Righi 1996, Sawyer 1990, Suzuki 2011). The remaining six studies reported increased adverse events in the groups with prolonged regimens (Bidkar 2014, Karran 1993, Mui 2005, Rajan 2005, de Santibañes 2018, Turano 1992). Of these, one study reported increased cases of C difficile infection in the prolonged postoperative continuation group (Mui 2005). The other studies reported an increased frequency of rash and pruritus, erythema, phlebitis, hypotension, gastrointestinal disturbance (including nausea and diarrhoea), and unspecified local and systemic side-effects with postoperative continuation of antibiotic prophylaxis. No study reported on antimicrobial resistance. Owing to heterogeneity between studies in the comparisons made and the outcomes measured, no meta-analysis could be done of adverse effects.
|
Study |
Adverse event definition |
Longer postoperative regimens |
Shorter postoperative regimens |
|
Mui 2005¶ |
Clostridium difficile confirmed by fecal clostridium toxin |
5 of 177 |
0 of 92 |
|
Karran 1993 † |
Hypotension, phlebitis, rash, erythema |
5 of 114 |
1 of 113 |
|
Turano 1992 * |
Thrombophlebitis, allergic reaction and gastrointestinal disturbances |
40 of 1517 |
10 of 1700 |
|
Bidkar 2014 ‡ |
Gastrointestinal disturbances |
19 of 39 |
1 of 39 |
|
Rajan 2005 * |
Nausea, diarrhea, skin rash, pruritus |
29 of 100 |
2 of 100 |
|
de Santibañes 2018 * |
Unspecified |
4 of 96 |
3 of 105 |
|
Liu 2008 ‡ |
No adverse events attributable to antibiotic use in both the intervention and control group. |
||
|
Carrol 2003 ‡ |
No adverse events attributable to antibiotic use in both the intervention and control group. |
||
|
Righi 1996 ‡ |
No adverse events attributable to antibiotic use in both the intervention and control group. |
||
|
Maier 1992 * |
No adverse events attributable to antibiotic use in both the intervention and control group. |
||
|
Sawyer 1990 § |
No adverse events attributable to antibiotic use in both the intervention and control group. |
||
|
Kang 2009 * |
No adverse events attributable to antibiotic use in both the intervention and control group. |
||
|
Lindeboom 2003 * |
No adverse events attributable to antibiotic use in both the intervention and control group. |
||
|
Suzuki 2011 * |
No adverse events attributable to antibiotic use in both the intervention and control group. |
||
|
Fujita 2015 * |
No adverse events attributable to antibiotic use in both the intervention and control group. |
||
|
Imamura 2012 * |
No adverse events attributable to antibiotic use in both the intervention and control group. |
||
|
Mohri 2007 * |
No adverse events attributable to antibiotic use in both the intervention and control group. |
||
|
Regimbeau 2007 * |
No adverse events attributable to antibiotic use in both the intervention and control group. |
||
|
Becker 2008 * |
No adverse events attributable to antibiotic use in both the intervention and control group. |
||
|
Cartana 1994 * |
No adverse events attributable to antibiotic use in both the intervention and control group. |
||
|
Eshghpour 2014 ‡ |
No adverse events attributable to antibiotic use in both the intervention and control group. |
||
|
Loozen 2017 * |
No adverse events attributable to antibiotic use in both the intervention and control group. |
||
|
Rajabi 2012 ¶ |
No adverse events attributable to antibiotic use in both the intervention and control group. |
||
|
Danda 2010 * |
No adverse events attributable to antibiotic use in both the intervention and control group. |
||
|
* Postoperative continuation vs immediate discontinuation of SAP; † Postoperative continuation for 24h vs a single dose after surgery; ‡ Postoperative continuation for >24h vs ≤ 24h; § Postoperative continuation for >48h vs ≤ 48h; ¶ Postoperative continuation vs immediate discontinuation of SAP and Postoperative continuation for >24h vs ≤ 24h |
|||
Table 3. Studies reporting adverse events related to SAP
4. Studies reporting costs of SAP continuation
Five studies (Chang 2005, Liberman 1995, Orlando 2015, Rajan 2005, Su 2005) addressed cost-effectiveness and reported a cost increase associated with longer antibiotic prophylaxis regimens, in some cases as a result of treatment for side-effects and hospitalisation time in addition to prophylaxis treatment, which varied from US$36.90 to $78.95 (Table 4). None of these studies calculated costs associated with the emergence of antimicrobial resistance. All five studies were done in high-income countries (Australia, Italy, Taiwan, and the USA).
|
Study |
Cost included |
Cost postoperative continuation |
Cost postoperative discontinuation |
Absolute difference |
Relative difference |
|
Liberman 1995* |
Antibiotics |
$ 54.80 |
$ 17.90 |
+ $ 36.90 |
3.06 |
|
Chang 2005† |
Total costs |
$ 1,768.00 |
$ 1,728.00 |
+ $ 40.00 |
1.02 |
|
Rajan 2005* |
Total costs |
$ 93.45 |
$ 14.50 |
+ $ 78.95 |
6.44 |
|
Su 2005* |
Antibiotics |
$ 48.00 |
$ 3.50 |
+ $ 44.50 |
13.71 |
|
Orlando 2015 |
Antibiotics |
$ 38.80 |
$ 3.88 |
+ $ 34.92 |
10.00 |
|
* Postoperative continuation vs immediate discontinuation of SAP; † Postoperative continuation for>24 h vs ≤ 24 h |
|||||
Table 4: Studies reporting costs of SAP continuation
Level of evidence of the literature
Surgical site infections
The level of evidence regarding the outcome SSI was derived from randomized controlled trials and therefore started high. The level of evidence was downgraded by one level because of risk of bias. The level of evidence was considered as moderate.
Zoeken en selecteren
A systematic review of the literature was performed to answer the following question: What is the effect of postoperative continuation of antibiotic prophylaxis on the incidence of SSI compared with its postoperative discontinuation in adult patients undergoing surgical procedures?
P: Adult patients undergoing any surgical procedure.
I: Postoperative continuation of antibiotic prophylaxis.
C: Postoperative discontinuation of antibiotic prophylaxis.
O: Surgical site infections (SSI).
Relevant outcome measures
The guideline development group considered surgical site infections as a critical outcome for decision making.
The working group defined a threshold of 10% for continuous outcomes and a relative risk (RR) for dichotomous outcomes of <0.80 and >1.25 as a minimal clinically (patient) important difference.
Search and select (Methods)
The databases Medline (via OVID) and Embase (via Embase.com) were searched with relevant search terms, based on the literature search of de Jonge (2020) from the 24th of July 2018 up to the 28th of January 2021. The detailed search strategy is available on request via https://richtlijnendatabase.nl/. The systematic literature search resulted in 992 hits. Studies were selected based on the following criteria: systematic reviews and randomized controlled trials on the postoperative administration of antibiotics to prevent postoperative wound infections. One hundred eighty-eight studies were initially selected based on title and abstract screening. After reading the full text, 90 studies were excluded (see the table with reasons for exclusion in the table of excluded studies under the tab 'Evidence tabellen'), and 98 studies were included.
Results
Ninety-eight studies were included in the analysis of the literature under the tab 'Samenvatting literatuur'. Important study characteristics and results and quality assessments are summarized in the evidence tables and risk of bias tables under the 'evidence tabellen' tab.
Referenties
- Aberg, C., and M. Thore. "Single Versus Triple Dose Antimicrobial Prophylaxis in Elective Abdominal Surgery and the Impact on Bacterial Ecology." Journal of hospital infection 18.2 (1991): 149-54.
- Abro, A. H., et al. "Single Dose Versus 24 - Hours Antibiotic Prophylaxis against Surgical Site Infections." Journal of the Liaquat University of Medical and Health Sciences 13.1 (2014): 27-31.
- Abubaker, A. O., and M. K. Rollert. "Postoperative Antibiotic Prophylaxis in Mandibular Fractures: A Preliminary Randomized, Double-Blind, and Placebo-Controlled Clinical Study." Journal of Oral and Maxillofacial Surgery 59.12 (2001): 1415-19.
- Adaji, J. A., et al. "Short Versus Long-Term Antibiotic Prophylaxis in Cesarean Section: A Randomized Clinical Trial." Niger Med J 61.4 (2020): 173-79.
- Ahmed, M., et al. "Perioperative Antibiotic Use for Surgical Site Infection in Penetrating Hollow Viscus Injury - a Placebo-Controlled Study." Pakistan Journal of Medical & Health Sciences 13.4 (2019): 851-54.
- Balbo, G., et al. "Antibiotic Prophylaxis with Mezlocillin in Gastric Surgery. Comparison between Two Regimen. [Italian]." Chirurgia 4.7-8 (1991): 412-16.
- Baqain, Z. H., et al. "Antibiotic Prophylaxis for Orthognathic Surgery: A Prospective, Randomised Clinical Trial." Br J Oral Maxillofac Surg 42.6 (2004): 506-10.
- Bates, T., et al. "A Randomized Trial of One Versus Three Doses of Augmentin as Wound Prophylaxis in at-Risk Abdominal Surgery." Postgraduate Medical Journal 68.804 (1992): 811-16.
- Becker, A., L. Koltun, and J. Sayfan. "Impact of Antimicrobial Prophylaxis Duration on Wound Infection in Mesh Repair of Incisional Hernia - Preliminary Results of a Prospective Randomized Trial." European Surgery - Acta Chirurgica Austriaca 40.1 (2008): 37-40.
- Bentley, K.C., T.W. Head, and G.A. Aiello. "Antibiotic Prophylaxis in Orthognathic Surgery: A 1-Day Versus 5-Day Regimen." J.Oral Maxillofac.Surg 57.3 (1999): 226-30.
- Berrios-Torres, S. I., et al. "Centers for Disease Control and Prevention Guideline for the Prevention of Surgical Site Infection, 2017." JAMA Surg 152.8 (2017): 784-91.
- Berry, P. S., et al. "Intraoperative Versus Extended Antibiotic Prophylaxis in Liver Transplant Surgery: A Randomized Controlled Pilot Trial." Liver Transplantation 25.7 (2019): 1043-53.
- Bidkar, V.G., et al. "Perioperative Only Versus Extended Antimicrobial Usage in Tympanomastoid Surgery: A Randomized Trial." Laryngoscope 124.6 (2014): 1459-63.
- Bozorgzadeh, A., et al. "The Duration of Antibiotic Administration in Penetrating Abdominal Trauma." Am.J.Surg 177.2 (1999): 125-31.
- Branch-Elliman, W., et al. "Association of Duration and Type of Surgical Prophylaxis with Antimicrobial-Associated Adverse Events." JAMA Surg 154.7 (2019): 590-98.
- Bratzler, D. W., et al. "Clinical Practice Guidelines for Antimicrobial Prophylaxis in Surgery." Am J Health Syst Pharm 70.3 (2013): 195-283.
- Buckley, R., et al. "Perioperative Cefazolin Prophylaxis in Hip Fracture Surgery." Canadian Journal of Surgery 33.2 (1990): 122-25.
- Campos, G. B. P., et al. "Efficacy Assessment of Two Antibiotic Prophylaxis Regimens in Oral and Maxillofacial Trauma Surgery: Preliminary Results." International Journal of Clinical and Experimental Medicine 8.2 (2015): 2846-52.
- Carroll, W. R., et al. "Three-Dose Vs Extended-Course Clindamycin Prophylaxis for Free-Flap Reconstruction of the Head and Neck." Archives of otolaryngology--head & neck surgery 129.7 (2003): 771-4.
- Cartana, J., et al. "Antibiotic Prophylaxis in Wertheim-Meigs Surgery. A Single Dose Vs Three Doses." European Journal of Gynaecological Oncology 15.1 (1994): 14-18.
- Chang, W. C., et al. "Short Course of Prophylactic Antibiotics in Laparoscopically Assisted Vaginal Hysterectomy." Journal of Reproductive Medicine for the Obstetrician and Gynecologist 50.7 (2005): 524-28.
- Chauhan, V. S., et al. "Can Post-Operative Antibiotic Prophylaxis Following Elective Laparoscopic Cholecystectomy Be Completely Done Away with in the Indian Setting? A Prospective Randomised Study." Journal of Minimal Access Surgery 14.3 (2018): 192-96.
- Cioaca, R. E., et al. "Comparative Study of Clinical Effectiveness of Antibiotic Prophylaxis in Aseptic Mouth-Jaw- and Facial Surgery. [German]." Mund-, Kiefer- und Gesichtschirurgie : MKG 6.5 (2002): 356-59.
- Crist, B. D., et al. "Evaluating the Duration of Prophylactic Post-Operative Antibiotic Agents after Open Reduction Internal Fixation for Closed Fractures." Surg Infect (Larchmt) 19.5 (2018): 535-40.
- Cunha, C. B. "The Pharmacoeconomic Aspects of Antibiotic Stewardship Programs." Med Clin North Am 102.5 (2018): 937-46.
- Danda, A. K., et al. "Single-Dose Versus Single-Day Antibiotic Prophylaxis for Orthognathic Surgery: A Prospective, Randomized, Double-Blind Clinical Study." Journal of Oral and Maxillofacial Surgery 68.2 (2010): 344-46.
- Davis, Cm, et al. "Prevalence of Surgical Site Infections Following Orthognathic Surgery: A Double-Blind, Randomized Controlled Trial on a 3-Day Versus 1-Day Postoperative Antibiotic Regimen." Journal of oral and maxillofacial surgery 75.4 (2017): 796-804.
- de Santibañes, M., et al. "Extended Antibiotic Therapy Versus Placebo after Laparoscopic Cholecystectomy for Mild and Moderate Acute Calculous Cholecystitis: A Randomized Double-Blind Clinical Trial." Surgery (2018).
- Dubberke, E. R., and M. A. Olsen. "Burden of Clostridium Difficile on the Healthcare System." Clin Infect Dis 55 Suppl 2.Suppl 2 (2012): S88-92.
- Eshghpour, M., et al. "Value of Prophylactic Postoperative Antibiotic Therapy after Bimaxillary Orthognathic Surgery: A Clinical Trial." Iranian Journal of Otorhinolaryngology 26.77 (2014): 207-10.
- Fridrich, K. L., B. E. Partnoy, and D. L. Zeitler. "Prospective Analysis of Antibiotic Prophylaxis for Orthognathic Surgery." Int J Adult Orthodon Orthognath Surg 9.2 (1994): 129-31.
- Fujita, S., et al. "Randomized, Multicenter Trial of Antibiotic Prophylaxis in Elective Colorectal Surgery: Single Dose Vs 3 Doses of a Second-Generation Cephalosporin without Metronidazole and Oral Antibiotics." Archives of Surgery 142.7 (2007): 657-61.
- Fujita, T., and H. Daiko. "Optimal Duration of Prophylactic Antimicrobial Administration and Risk of Postoperative Infectious Events in Thoracic Esophagectomy with Three-Field Lymph Node Dissection: Short-Course Versus Prolonged Antimicrobial Administration." Esophagus 12.1 (2015): 38-43.
- Garcia, E. S., et al. "Postoperative Antibiotic Prophylaxis in Reduction Mammaplasty: A Randomized Controlled Trial." Plastic and reconstructive surgery 145.6 (2020): 1022e‐28e.
- Garotta, F., and F. Pamparana. "Antimicrobial Prophylaxis with Ceftizoxime Versus Cefuroxime in Orthopedic Surgery. Ceftizoxime Orthopedic Surgery Italian Study Group." J Chemother 3 Suppl 2 (1991): 34-5.
- Gupta, A., et al. "Comparison of 48 H and 72 H of Prophylactic Antibiotic Therapy in Adult Cardiac Surgery: A Randomized Double Blind Controlled Trial." J.Antimicrob.Chemother. 65.5 (2010): 1036-41.
- Haga, N., et al. "A Prospective Randomized Study to Assess the Optimal Duration of Intravenous Antimicrobial Prophylaxis in Elective Gastric Cancer Surgery." International Surgery 97.2 (2012): 169-76.
- Hall, J. C., et al. "Duration of Antimicrobial Prophylaxis in Vascular Surgery." American Journal of Surgery 175.2 (1998): 87-90.
- Hanif, A; Gillani, M; Alia, I; Farooq Dar, U; Mirza, A. "Comparison of Surgical Site Infection Rate in Case of Penetrating Hollow Viscus Injury after Perioperative Antibiotics Use for 24 Hours Versus 5 Days." P J M H S 9.4 (2015): 1396-98.
- Harbarth, S., et al. "Prolonged Antibiotic Prophylaxis after Cardiovascular Surgery and Its Effect on Surgical Site Infections and Antimicrobial Resistance." Circulation 101.25 (2000): 2916-21.
- Hellbusch, L. C., et al. "Single-Dose Vs Multiple-Dose Antibiotic Prophylaxis in Instrumented Lumbar Fusion--a Prospective Study." Surgical neurology 70.6 (2008): 622-7; discussion 27.
- Hussain, M.I., et al. "Role of Postoperative Antibiotics after Appendectomy in Non-Perforated Appendicitis." J.Coll.Physicians Surg Pak. 22.12 (2012): 756-59.
- Imamura, H., et al. "Intraoperative Versus Extended Antimicrobial Prophylaxis after Gastric Cancer Surgery: A Phase 3, Open-Label, Randomised Controlled, Non-Inferiority Trial." Lancet Infect Dis 12.5 (2012): 381-7.
- Irato, S., et al. "Prophylaxis with Single Administration of Cefotetan in Patients Undergoing Abdominal Hysterectomy. [Italian]." Giornale Italiano di Ostetricia e Ginecologia 19.4 (1997): 235-36.
- Ishibashi, K., et al. "Short-Term Intravenous Antimicrobial Prophylaxis for Elective Rectal Cancer Surgery: Results of a Prospective Randomized Non-Inferiority Trial." Surgery Today 44.4 (2014): 716-22.
- Ishibashi, K., et al. "Short-Term Intravenous Antimicrobial Prophylaxis in Combination with Preoperative Oral Antibiotics on Surgical Site Infection and Methicillin-Resistant Staphylococcus Aureus Infection in Elective Colon Cancer Surgery: Results of a Prospective Randomized Trial." Surgery Today 39.12 (2009): 1032-39.
- Jansisyanont, P., et al. "Antibiotic Prophylaxis for Orthognathic Surgery: A Prospective, Comparative, Randomized Study between Amoxicillin-Clavulanic Acid and Penicillin." J Med Assoc Thai 91.11 (2008): 1726-31.
- Jiang, L., et al. "Prophylactic Cefuroxime in General Thoracic Surgery. [Chinese]." Chinese Journal of Antibiotics 29.7 (2004): 412-14.
- Kang, S. H., J. H. Yoo, and C. K. Yi. "The Efficacy of Postoperative Prophylactic Antibiotics in Orthognathic Surgery: A Prospective Study in Le Fort I Osteotomy and Bilateral Intraoral Vertical Ramus Osteotomy." Yonsei Medical Journal 50.1 (2009): 55-59.
- Kim, E. Y., et al. "Is There a Real Role of Postoperative Antibiotic Administration for Mildmoderate Acute Cholecystitis? A Prospective Randomized Controlled Trial." Journal of Hepato-Biliary-Pancreatic Sciences 24.10 (2017): 550-58.
- Kow, L., et al. "Comparison of Cefotaxime Plus Metronidazole Versus Cefoxitin for Prevention of Wound Infection after Abdominal Surgery." World Journal of Surgery 19.5 (1995): 680-86.
- Lau, W. Y., et al. "Systemic Antibiotic Regimens for Acute Cholecystitis Treated by Early Cholecystectomy." Aust N Z J Surg 60.7 (1990): 539-43.
- Liberman, M.A., et al. "Single-Dose Cefotetan or Cefoxitin Versus Multiple-Dose Cefoxitin as Prophylaxis in Patients Undergoing Appendectomy for Acute Nonperforated Appendicitis." J Am Coll Surg 180 (1995): 77-80.
- Lin, M. H., et al. "Prospective Randomized Study of Efficacy of 1-Day Versus 3-Day Antibiotic Prophylaxis for Preventing Surgical Site Infection after Coronary Artery Bypass Graft." Journal of the Formosan Medical Association 110.10 (2011): 619-26.
- Lindeboom, J. A., E. M. Baas, and F. H. Kroon. "Prophylactic Single-Dose Administration of 600 Mg Clindamycin Versus 4-Time Administration of 600 Mg Clindamycin in Orthognathic Surgery: A Prospective Randomized Study in Bilateral Mandibular Sagittal Ramus Osteotomies." Oral surgery, oral medicine, oral pathology, oral radiology, and endodontics 95.2 (2003): 145-9.
- Liu, S. A., et al. "Preliminary Report of Associated Factors in Wound Infection after Major Head and Neck Neoplasm Operations--Does the Duration of Prophylactic Antibiotic Matter?" Journal of laryngology and otology 122.4 (2008): 403-8.
- Loozen, C. S., et al. "Randomized Clinical Trial of Extended Versus Single-Dose Perioperative Antibiotic Prophylaxis for Acute Calculous Cholecystitis." Br J Surg 104.2 (2017): e151-e57.
- Lyimo, F. M., et al. "Single Dose of Gentamicin in Combination with Metronidazole Versus Multiple Doses for Prevention of Post-Caesarean Infection at Bugando Medical Centre in Mwanza, Tanzania: A Randomized, Equivalence, Controlled Trial." BMC Pregnancy and Childbirth 13.123 (2013).
- Madadi, S., et al. "Postoperative Antibiotic Prophylaxis in the Prevention of Cardiac Implantable Electronic Device Infection." Pacing and clinical electrophysiology : PACE 42.2 (2019): 161‐65.
- Maier, W., and J. Strutz. "Perioperative Single-Dose Prophylaxis with Cephalosporins in Ent Surgery. A Prospective Randomized Study. [German]." Laryngo- Rhino- Otologie 71.7 (1992): 365-69.
- Mann, W., and J. Maurer. "[Perioperative Short-Term Preventive Antibiotics in Head-Neck Surgery]." Laryngo- rhino- otologie 69.3 (1990): 158-60.
- McArdle, C. S., et al. "Value of Oral Antibiotic Prophylaxis in Colorectal Surgery." British journal of surgery 82.8 (1995): 1046-8.
- Meijer, W. S., and P. I. Schmitz. "Prophylactic Use of Cefuroxime in Biliary Tract Surgery: Randomized Controlled Trial of Single Versus Multiple Dose in High-Risk Patients. Galant Trial Study Group." British journal of surgery 80.7 (1993): 917-21.
- Mohri, Y., et al. "Randomized Clinical Trial of Single- Versus Multiple-Dose Antimicrobial Prophylaxis in Gastric Cancer Surgery." British Journal of Surgery 94.6 (2007): 683-88.
- Mui, L. M., et al. "Optimum Duration of Prophylactic Antibiotics in Acute Non-Perforated Appendicitis." ANZ Journal of Surgery 75.6 (2005): 425-28.
- Niederhauser, U., et al. "Cardiac Surgery in a High-Risk Group of Patients: Is Prolonged Postoperative Antibiotic Prophylaxis Effective?" J Thorac Cardiovasc Surg 114.2 (1997): 162-8.
- Nooyen, S. M., et al. "Prospective Randomised Comparison of Single-Dose Versus Multiple-Dose Cefuroxime for Prophylaxis in Coronary Artery Bypass Grafting." European journal of clinical microbiology & infectious diseases 13.12 (1994): 1033-7.
- Nusrath, S., et al. "Single-Dose Prophylactic Antibiotic Versus Extended Usage for Four Days in Clean-Contaminated Oncological Surgeries: A Randomized Clinical Trial." Indian Journal of Surgical Oncology 11.3 (2020): 378-86.
- OECD. Organisation for Economic Co-Operation and Development. Stemming the Superbug Tide: Just a Few Dollars More. Nov 7, 2018. Https://Www.Oecd.Org/Health/Stemming-the-Superbug-Tide- 9789264307599-En.Htm (Accessed April 28, 2020).
- Olak, J., et al. "Randomized Trial of One-Dose Versus Six-Dose Cefazolin Prophylaxis in Elective General Thoracic Surgery." Annals of Thoracic Surgery 51.6 (1991): 956-58.
- Orjuela, A., and L. P. Cardozo. "Comparison of Two Prophylactic Antibiotic Protocols in Implantable Cardiac Stimulation Devices: “Comprofilaxia”." Revista colombiana de cardiologia 27.4 (2020): 330‐36.
- Orlando, G., et al. "One-Shot Versus Multidose Perioperative Antibiotic Prophylaxis after Kidney Transplantation: A Randomized, Controlled Clinical Trial." Surgery (United States) 157.1 (2015): 104-10.
- Otani, S., et al. "Feasibility of Short-Term Antibiotic Prophylaxis after Pulmonary Resection. [Japanese]." Kyobu geka The Japanese journal of thoracic surgery. 57.13 (2004): 1171-74; discussion 75-76.
- Patangia, D., et al. “Impact of antibiotics on the human microbiome and consequences for host health.” Microbiology Open. 11.1 (2022): e1260.
- Rajabi-Mashhadi, M. T., et al. "Optimum Duration of Perioperative Antibiotic Therapy in Patients with Acute Non-Perforated Appendicitis: A Prospective Randomized Trial." Asian Biomedicine 6.6 (2012): 891-94.
- Rajan, G. P., et al. "Antibiotic Prophylaxis in Septorhinoplasty? A Prospective, Randomized Study." Plastic and reconstructive surgery 116.7 (2005): 1995-98.
- Ramirez, J. et al. “Antibiotics as Major Disruptors of Gut Microbiota.” Frontiers in Cellular and Infection Microbiology 10 (2020): 572912.
- Regimbeau, J. M., et al. "Effect of Postoperative Antibiotic Administration on Postoperative Infection Following Cholecystectomy for Acute Calculous Cholecystitis: A Randomized Clinical Trial." JAMA - Journal of the American Medical Association 312.2 (2014): 145-54.
- Righi, M., et al. "Short-Term Versus Long-Term Antimicrobial Prophylaxis in Oncologic Head and Neck Surgery." Head Neck 18.5 (1996): 399-404.
- Sadraei-Moosavi, S-M, N Nikhbakhsh, and A-A Darzi. "Postoperative Antibiotic Therapy after Appendectomy in Patients with Non-Perforated Appendicitis." Caspian journal of internal medicine 8.2 (2017): 104-07.
- Salih, E. K., et al. "Comparative Study of Single Dose Per-Operative Metronidazole Versus Multiple Doses Postoperative Metronidazole in Acute Non-Complicated Appendicitis: A View on Postoperative Complications." Journal of Krishna Institute of Medical Sciences University 7.4 (2018): 78-84.
- Sawyer, R., et al. "Metronidazole in Head and Neck Surgery--the Effect of Lengthened Prophylaxis." Otolaryngol.Head Neck Surg 103.6 (1990): 1009-11.
- Scher, K. S. "Studies on the Duration of Antibiotic Administration for Surgical Prophylaxis." American surgeon 63.1 (1997): 59-62.
- Sgroi, G., et al. "Prophylactic Antibiotics in Abdominal Surgery: A Single Peroperative Dose Versus Ultra Short-Term Prophylaxis." Chirurgia 3.12 (1990): 652-6.
- Shaheen, S., and S. Akhtar. "Comparison of Single Dose Versus Multiple Doses of Anitibiotic Prophylaxis in Elective Caesarian Section." Journal of Postgraduate Medical Institute 28.1 (2014): 83-86.
- Stevens, V., et al. "Cumulative Antibiotic Exposures over Time and the Risk of Clostridium Difficile Infection." Clin Infect Dis 53.1 (2011): 42-8.
- Su, H. Y., et al. "Prospective Randomized Comparison of Single-Dose Versus 1-Day Cefazolin for Prophylaxis in Gynecologic Surgery." Acta Obstetricia et Gynecologica Scandinavica 84.4 (2005): 384-89.
- Sugawara, G., et al. "Duration of Antimicrobial Prophylaxis in Patients Undergoing Major Hepatectomy with Extrahepatic Bile Duct Resection: A Randomized Controlled Trial." Ann Surg 267.1 (2018): 142-48.
- Suzuki, T., et al. "Optimal Duration of Prophylactic Antibiotic Administration for Elective Colon Cancer Surgery: A Randomized, Clinical Trial." Surgery 149.2 (2011): 171-78.
- Takayama, T., et al. "Antimicrobial Prophylaxis for 1 Day Versus 3 Days in Liver Cancer Surgery: A Randomized Controlled Non-Inferiority Trial." Surgery Today 49.10 (2019): 859-69.
- Takemoto, R. C., et al. "Appropriateness of Twenty-Four-Hour Antibiotic Prophylaxis after Spinal Surgery in Which a Drain Is Utilized: A Prospective Randomized Study." The Journal of bone and joint surgery American volume. 97.12 (2015): 979-86.
- Tamayo, E., et al. "Comparative Study of Single-Dose and 24-Hour Multiple-Dose Antibiotic Prophylaxis for Cardiac Surgery." Journal of Thoracic and Cardiovascular Surgery 136.6 (2008): 1522-27.
- Tan, X., et al. "Effects of Antibiotics on Prevention of Infection, White Blood Cell Counts, and C-Reactive Protein Levels at Different Times in the Perioperative Period of Cesarean Section " International journal of clinical pharmacology and therapeutics 58.6 (2020): 310‐15.
- Togo, S., et al. "Duration of Antimicrobial Prophylaxis in Patients Undergoing Hepatectomy: A Prospective Randomized Controlled Trial Using Flomoxef." Journal of Antimicrobial Chemotherapy 59.5 (2007): 964-70.
- Tsang, T. M., P. K. Tam, and H. Saing. "Antibiotic Prophylaxis in Acute Non-Perforated Appendicitis in Children: Single Dose of Metronidazole and Gentamicin." Journal of the Royal College of Surgeons of Edinburgh 37.2 (1992): 110-2.
- Turano, A. "New Clinical Data on the Prophylaxis of Infections in Abdominal, Gynecologic, and Urologic Surgery. Multicenter Study Group." American journal of surgery 164.4 A Suppl (1992): 16S-20S.
- Unemura, Y., et al. "Prevention of Postoperative Infection Following Laparoscopic Cholecystectomy - Comparison between Single Dose and 2-Day Dose Administration of Antibiotic Prophylaxis. [Japanese]." Japanese Journal of Gastroenterological Surgery 33.12 (2000): 1880-84.
- Urquhart, J. C., et al. "The Effect of Prolonged Postoperative Antibiotic Administration on the Rate of Infection in Patients Undergoing Posterior Spinal Surgery Requiring a Closed-Suction Drain: A Randomized Controlled Trial." Journal of Bone and Joint Surgery - American Volume 101.19 (2019): 1732-40.
- Wahab, P. U. A., et al. "Antibiotic Prophylaxis for Bilateral Sagittal Split Osteotomies: A Randomized, Double-Blind Clinical Study." International Journal of Oral and Maxillofacial Surgery 42.3 (2013): 352-55.
- Westen, E. H. M. N., et al. "Single-Dose Compared with Multiple Day Antibiotic Prophylaxis for Cesarean Section in Low-Resource Settings, a Randomized Controlled, Noninferiority Trial." Acta Obstetricia et Gynecologica Scandinavica 94.1 (2015): 43-49.
- World Health Organization (WHO). Global Guidelines for the Prevention of Surgical Site Infection, Second Edition. Geneva: World Health Organization; 2018. Licence: Cc by-Nc-Sa 3.0 Igo.
- Yamamoto, T., et al. "Dual-Center Randomized Clinical Trial Exploring the Optimal Duration of Antimicrobial Prophylaxis in Patients Undergoing Pancreaticoduodenectomy Following Biliary Drainage." Annals of Gastroenterological Surgery 2.6 (2018): 442-50.
- Yang, Z. "[Short-Term Versus Long-Term Antimicrobial Prophylaxis in Abdominal Surgery: A Multicenter Open Randomized Comparative Trial]." Zhonghua Wai Ke Za Zhi 39.10 (2001): 770-2.
Evidence tabellen
|
|
Author, Year |
Scope, participants |
Type of surgery |
Wound class. |
CDC SSI definition, Follow-up |
Intervention |
Control |
|
|
|
Postoperative continuation of surgical antibiotic prophylaxis vs. postoperative discontinuation of surgical antibiotic prophylaxis |
|||||||||
|
1 |
Sadraei-Moosavi 2018 |
Single centre 152* |
Appendectomy (open, uncomplicated) |
II-III |
No |
1g Ceftriaxone & 0.5g Metronidazole IV preoperatively + 24h postoperatively |
1g Ceftriaxone & 0.5g Metronidazole IV preoperatively |
No |
Yes |
|
2 |
Hussain 2012 |
Single centre 377 |
Appendectomy (open, uncomplicated) |
II-III |
Noa, 30 days |
Cefuroxime & Metronidazole IV preoperatively + 1x postoperatively |
Cefuroxime & Metronidazole IV preoperatively |
Yes |
Yes |
|
3 |
Liberman 1995 |
Single centre 99* |
Appendectomy (open uncomplicated) |
II-III |
Noa, 3 weeks |
2g Cefoxitin IV preoperatively + 3x q 6h postoperatively |
2g Cefoxitin IV preoperatively |
Yes |
Yes |
|
4 |
Tsang 1992 |
Single centre 103** |
Appendectomy (open, uncomplicated) |
II-III |
Noa, 4 weeks |
1.5 mg/kg Gentamicin IV & 7.5 mg/kg Metronidazole IV preoperatively +2x q 8h postoperatively |
1.5 mg/kg Gentamicin IV & 7.5 mg/kg Metronidazole IV preoperatively |
No |
Yes |
|
5 |
Salih 2018 |
Single centre 111* |
Appendectomy (open, uncomplicated) |
II-III |
CDC, 10 days |
0.5 mg metronidazole IV preoperatively + 9x q 8h postoperatively |
0.5 mg metronidazole IV preoperatively |
Yes |
Yes |
|
6 |
Suzuki 2011 |
Single centre 370 |
Colorectal surgery |
II-III |
Nof, 30 days |
1g Flomoxef IV preoperatively + 4x q 12h |
1g Flomoxef IV preoperatively |
Yes |
Yes |
|
7 |
Fujita 2007 |
Multi centre 377 |
Colorectal surgery |
II-III |
Nod, NR |
1g Cefmetazole IV preoperatively + 2x q 8h |
1g Cefmetazole IV preoperatively |
Yes |
No |
|
8 |
Imamura 2012 |
Multi centre 355 |
Upper GI surgery |
II |
CDC, 30 days |
1g of Cefazolin IV preoperatively +1 x direct postoperative & 4x q 12h postoperative |
1g of Cefazolin IV preoperatively |
No |
Yes |
|
9 |
Haga 2012 |
Single centre 325 |
Upper GI surgery |
II |
CDC, 30 days |
1g of Cefazolin IV preoperatively + 5x q 12h postoperatively |
1g of Cefazolin IV preoperatively |
No |
Yes |
|
10 |
Balbo 1991 |
Multi centre 117 |
Upper GI surgery |
II-III |
Nov, 30 days |
2g Mezlocillin IV preoperatively + 2x q 6h postoperatively |
2g Mezlocillin iv preoperatively |
Yes |
Yes |
|
11 |
Mohri 2007 |
Multi centre 486 |
Upper GI surgery |
II |
CDC, 6 weeks |
1g Cefazolin IV or 1.5 g Ampicillin sulbactam IV preoperatively + 7x q 12h postoperatively |
1g Cefazolin IV or 1.5 g Ampicillin sulbactam IV preoperatively |
Yes |
Yes |
|
12 |
Chauhan 2018 |
Single centre 210* |
Laparoscopic Cholecystectomy |
II-III |
Nod, 30 days |
1g Ceftriaxone IV preoperatively + 4x q 12h postoperatively |
1g Ceftriaxone IV preoperatively |
No |
No |
|
13 |
Santibañes 2018 |
Single centre 201 |
Laparoscopic Cholecystectomy |
II-III |
Nod, 30 days |
Ampicillin sulbactam IV q 6h preoperatively (admission – surgery, < 5 days) + 1g Amoxicillin/Clavulanic acid PO 15x q 8h |
Ampicillin sulbactam IV q 6h preoperatively (admission until surgery, < 5 days) + 1g Placebo PO 15x q 8h |
No |
No |
|
14 |
Kim 2017 |
Multi centre 188 |
Laparoscopic Cholecystectomy |
II-III |
Yes, 30 days |
1g Cefoxitin IV preoperatively + q 8h IV or PO if tolerated until POD 3 |
1g Cefoxitin IV preoperatively + placebo q 8h IV or PO if tolerated until POD 3 |
Yes |
Yes |
|
15 |
Loozen 2017 |
Single centre 150 |
Laparoscopic Cholecystectomy |
II-III |
Nou |
2g Cefazolin IV preoperatively + 0.75g Cefazoline IV & 0.5g Metronidazole IV 9x q 8h |
2g Cefazolin IV preoperatively |
Yes |
Yes |
|
16 |
Regimbeau 2014 |
Multi centre 414 |
Open or laparoscopic Cholecystectomy |
II-III |
CDC, 30 days |
2g Amoxiclav IV 3dd before surgery & preoperatively + 15x q 8h IV or PO if tolerated |
2g Amoxiclav IV 3dd before surgery & preoperatively |
Yes |
No |
|
17 |
Unemura 2000 |
Multi centre 242 |
Laparoscopic cholecystectomy |
II-III |
Noa, NR |
2g Cephalosporin IV preoperatively + 4x q 12h postoperatively |
2g Cephalosporin IV preoperatively |
No |
Yes |
|
18 |
Meijer 1993 |
Multi centre 1004 |
Hepatobiliary surgery |
II |
Noi, 4-6 weeks |
1.5g Cefuroxime IV preoperatively + 0.75g Cefuroxime IV 2x q 8h postoperatively |
1.5g Cefuroxime IV preoperatively |
No |
No |
|
19 |
Abro 2014 |
Single centre 208 |
Mixed general surgery |
I-III |
Noj, 35 days |
2g Ceftriaxone IV preoperatively + 1g Ceftriaxone IV 2x q 8h postoperatively (& 0.25g Gentamicin & 0.5g Metronidazole when indicated) |
2g Ceftriaxone IV preoperatively (& 0.25g Gentamicin & 0.5g Metronidazole when indicated) |
No |
Yes |
|
20 |
Becker 2008 |
Single centre 44 |
Mixed general surgery |
I |
CDC, 30 days |
1g Cefazoline IV preoperatively + 3dd postoperatively until drains were removed |
1g Cefazoline IV preoperatively |
Yes |
Yes |
|
21 |
Scher 1997 |
Single centre 768 |
Mixed general surgery |
II |
Nod, NR |
1g of Cefazolin IV preoperatively + 1g Cefazolin IV 3x q 8h postoperatively |
1g of Cefazolin IV preoperatively |
Yes |
Yes |
|
22 |
Kow 1995 |
Single centre 1010* |
Mixed general surgery |
II-III |
Nob, 4-6 weeks |
2g Cefoxitin IV & 0.5 Metronidazole IV preoperatively + 2x q 6h postoperatively |
2g Cefoxitin IV & 0.5g Metronidazole IV preoperatively |
No |
No |
|
1g Cefotaxime IV & 0.5g metronidazole IV preoperatively + 2x q 6h postoperatively |
1g Cefotaxime IV & 0.5g metronidazole IV preoperatively |
||||||||
|
23 |
Nusrath 2020 |
Single centre 312 |
Mixed oncological surgery |
II |
CDC, 30 days |
1.5g Cefuroxime IV preoperatively + 12x q 8h 1.5g Cefuroxime IV postoperatively |
1.5g Cefuroxime IV preoperatively |
Yes |
No |
|
24 |
Turano 1992 |
Single centre 3567* |
Abdominal, Gynaecological and Urological surgery |
II-III |
Noa, 7 days |
1g Cefotaxime IV preoperatively + 2x q 6h after the first dose |
1g Cefotaxime IV preoperatively |
Yes |
Yes |
|
25 |
Bates 1992 |
Multi centre 900* |
Mixed general surgery |
II-IV |
Nob, 30 days |
0.25g/0.125g Amoxicillin/clavulanic acid IV preoperatively + 2x q 8h postoperatively |
0.25g/0.125g Amoxicillin/clavulanic acid IV preoperatively |
Yes |
No |
|
26 |
Aberg 1991 |
Single centre 428* |
Mixed general surgery |
II-III |
Noa, 30 days |
1.5g Cefuroxime IV preoperatively + 2x q 8h (& 0.5g metronidazole when indicated) |
1.5g Cefuroxime IV preoperatively (& 0.5g metronidazole when indicated) |
No |
No |
|
27 |
Sgroi 1990 |
Single centre 352 |
Mixed general |
II-III |
Noa, NR |
1 x Cephalosporin preoperatively + 2x q 8h postoperatively |
1 x Cephalosporin preoperatively |
Yes |
No |
|
28 |
Westen 2015 |
Multi centre 176 |
C-section |
II |
Nok, 30 days |
1g Ampicillin IV & 0.5g Metronidazole IV preoperatively + 0.5 Amoxicillin & 0.5g Metronidazole IV 2x q 8h postoperatively followed by 0.5g Amoxicillin PO and 0.4g metronidazole PO 9x q 8h |
1g Ampicillin IV & 0.5g Metronidazole IV preoperatively |
Yes |
Yes |
|
29 |
Shaheen 2014 |
Single centre 100 |
C-section |
II |
Nol, 6 weeks |
1g Cefotaxime IV preoperatively + 2 x q 12h postoperatively followed by 0.4g Cefuroxime PO for 5 days |
1g Cefotaxime IV preoperatively |
Yes |
Yes |
|
30 |
Lyimo 2013 |
Single centre 500 |
C-section |
II |
CDC, 30 days |
3 mg/kg Gentamicin IV & 0.5g Metronidazole I + preoperatively Metronidazole 0.5g 3x q 8h postoperatively |
3 mg/kg Gentamicin IV & 0.5g Metronidazole IV preoperatively |
Yes |
Yes |
|
31 |
Tan 2020 |
Single centre 486 |
C-section |
II |
Noae, NI |
2 g Cefuroxime IV preoperatively + 3 days postoperatively |
2 g Cefuroxime IV preoperatively |
Yes |
Yes |
|
32 |
Su 2005 |
Single centre 532 |
Gynaecological surgery |
II |
Nom, 90 days |
1g Cefazolin preoperatively + 3x q 6h postoperatively |
1g Cefazolin IV preoperatively |
Yes |
Yes |
|
33 |
Irato 1997 |
Single centre 84 |
Gynaecological surgery |
II-III |
Now, NR |
2g cefotetan IV preoperatively + 10x q 12h |
2g cefotetan IV preoperatively |
Yes |
No |
|
34 |
Cartaña 1994 |
Single centre 58 |
Gynaecological surgery |
II |
Nod, 4 days |
4g Piperacillin preoperatively + 2x q 6h postoperatively |
4g Piperacillin IV preoperatively |
Yes |
No |
|
35 |
Buckley 1990 |
Single centre 204 |
Orthopaedic / trauma surgery |
I |
Noa, 6 weeks |
2g Cefazolin IV preoperatively + 1g Cefazolin 3x q 6h postoperatively |
2g Cefazolin IV preoperatively |
Yes |
Yes |
|
36 |
Garotta 1991 |
Multi centre 614 |
Orthopaedic / trauma surgery |
I |
Noc, 1 year |
2g Ceftizoxime IV preoperatively + 1x q 12h postoperatively |
2g Ceftizoxime IV preoperatively |
Yes |
No |
|
37 |
Hellbusch 2008 |
Multi centre 233 |
Orthopaedic / trauma surgery |
I |
Noo, >21 days |
1g<100kg<2g Cefazolin IV preoperatively + 9x q 8h postoperatively followed by 0.5g Cephalexin PO 28x q 6h |
1g<100kg<2g Cefazolin IV preoperatively |
Yes |
Yes |
|
38 |
Crist 2018 |
Single centre 227 |
Orthopaedic / trauma surgery |
I |
Nox |
1g<100kg<2g Cefazolin IV preoperatively + 2x q 8h postoperatively |
1g<100kg<2g Cefazolin IV preoperatively + 2x q 8h Saline |
Yes |
Yes |
|
39 |
Nooyen 1994 |
Single centre 844 |
Cardiothoracic surgery |
I |
Noc, NR |
20mg/kg Cefuroxime IV preoperatively + 0.75g Cefuroxime IV 9x q 8h postoperatively |
20mg/kg Cefuroxime IV preoperatively |
Yes |
No |
|
40 |
Tamayo 2008 |
Single centre 838 |
Cardiothoracic surgery |
I |
CDC, 12 months |
2g Cefazolin IV preoperativel + 1g Cefazolin IV 2x q 8h postoperatively |
2g Cefazolin IV preoperatively |
No |
Yes |
|
41 |
Olak 1991 |
Single centre 199 |
Cardiothoracic surgery |
II |
Noa, 6 weeks |
2g Cefazolin IV preoperatively + 1g Cefazolin IV 5x q 8h postoperatively |
2g Cefazolin IV preoperatively |
No |
Yes |
|
42 |
Orjuela 2020 |
Single centre 360 |
Cardiac surgery |
I |
Noaf, 2 years |
1g Cefazolin IV preoperatively + 1g Cefazolin IV 3x q 8h postoperatively |
1g Cefazolin IV preoperatively |
Yes |
Yes |
|
43 |
Madadi 2019 |
Single centre 300 |
Cardiothoracic surgery |
I |
Noaa, 30 days |
1-2 g Cephazolin IV preoperatively + Cephazolin IV 3x q 8h |
1-2 g Cephazolin IV preoperatively |
Yes |
Yes |
|
1-2 g Cephazolin IV preoperatively + Cephazolin IV 3x q 8h & Ciprofloxacin PO 14x q 12h |
1-2 g Cephazolin IV preoperatively |
Yes |
Yes |
||||||
|
44 |
Jiang 2004 |
Multi centre 264 |
Thoracic surgery |
II-III |
CDC, 30 days |
1.5g cefuroxime IV preoperatively + 15x 0.75g q 8h postoperatively |
1.5g cefuroxime IV preoperatively |
No |
No |
|
45 |
Hall 1998 |
Single centre 302 |
Vascular surgery |
I |
Noc, 42 days |
3.0g/0.1g Ticarcillin Clavulanic acid IV preoperatively + q 6h postoperatively until lines were removed |
3.0g/0.1g Ticarcillin Clavulanic acid IV preoperatively |
No |
Yes |
|
46 |
Orlando 2015 |
Multi centre 205 |
Transplant surgery |
I |
CDC, 30 days |
2g Cefazolin IV or 1g Cefotaxime IV preoperatively + q 12h postoperatively until removal of Foley catheter |
2g Cefazolin IV or 1g Cefotaxime IV preoperatively |
Yes |
Yes |
|
47 |
Berry 2019 |
Single centre 97
|
Liver transplant surgery |
II |
CDC, 30 days |
3.375 g Piperacillin/Tazobactam IV OR 2g Cefepime IV & 0.5 mg Metronidazole IV OR 1g Vancomycin IV & 0.4 mg Ciprofloxacin IV preoperatively + 8x q 8h 3.375 g Piperacillin/Tazobactam IV OR 2g Cefepime IV & 0.5 mg Metronidazole IV OR 1g Vancomycin IV & 0.4 mg Ciprofloxacin IV postoperatively |
3.375 g Piperacillin/Tazobactam IV OR 2g Cefepime IV & 0.5 mg Metronidazole IV OR 1g Vancomycin IV & 0.4 mg Ciprofloxacin IV preoperatively
|
Yes |
No |
|
48 |
Maier 1992 |
Single centre 106 |
Head and neck surgery |
I-II |
Nod, NR |
1.5 g Cefuroxime IV preoperatively + 2x q 8h postoperatively |
1.5 g Cefuroxime IV preoperatively |
Yes |
No |
|
49 |
Mann 1990 |
Single centre 113 |
Head and neck surgery |
II |
Noa, NR |
2g Cefotiam IV & 0.5g Metronidazole IV preoperatively + 2x q 8h postoperatively |
2g Cefotiam IV & 0.5g Metronidazole IV preoperatively |
Yes |
Yes |
|
50 |
Rajan 2005 |
Single centre 200 |
Head and neck surgery |
II |
Nod, 30 days |
2.2g Amoxicillin / clavulanic acid IV preoperatively + 1g Amoxicillin/ clavulanic acid PO 14x q 12h postoperatively |
2.2g Amoxicillin / clavulanic acid IV preoperatively |
Yes |
No |
|
51 |
Campos 2015 |
Single centre 74 |
Maxillofacial surgery |
I-II |
Noe, 6 weeks |
2g Cefazolin IV preoperatively + 1g Cefazolin IV 4x q 6h postoperatively |
2g Cefazolin IV preoperatively |
Yes |
Yes |
|
52 |
Lindeboom 2003 |
Single centre 70 |
Maxillofacial surgery |
II |
Nos, 3 months |
0.4g Clindamycin IV preoperatively + Clindamycin IV 4x q 6h postoperatively |
0.4g Clindamycin IV preoperatively |
Yes |
Yes |
|
53 |
Cioaca 2002 |
Single centre 140* |
Maxillofacial surgery |
II |
Noa, 14 days |
2.4 mg Amoxicillin/ Clavulanic acid IV preoperatively + 15x q 8h postoperatively |
2.4 mg Amoxicillin/ Clavulanic acid IV preoperatively |
No |
No |
|
2g Cefazolin IV preoperatively + 15x q 8h postoperatively |
2g Cefazolin IV preoperatively |
||||||||
|
54 |
Wahab 2013 |
Single centre 60* |
Maxillofacial surgery |
II |
CDC, 2 months |
1g Amoxicillin IV preoperatively + 0.5g Amoxicillin IV 2x q 4h postoperatively |
1g Amoxicillin IV preoperatively |
No |
No |
|
55 |
Danda 2010 |
Single centre 150* |
Maxillofacial surgery |
II |
Nob, 4 weeks |
1g Ampicillin IV preoperatively + Ampicillin 0.5g IV 4x q 6h postoperatively |
1g Ampicillin IV preoperatively |
No |
No |
|
56 |
Kang 2009 |
Single centre 56 |
Maxillofacial surgery |
II |
CDC, 2 weeks |
1g Cefpiramide IV preoperatively + 6x q 12h postoperatively |
1g Cefpiramide IV preoperatively |
Yes |
Yes |
|
57 |
Rajabi 2012 |
Single centre 291* |
Appendectomy (open, uncomplicated) |
II-III |
Noa, 10 days after discharge |
1g Ceftriaxone IV & 0.5g Metronidazole IV preoperatively + 1g Ceftriaxone IV q 12h & 0.5g Metronidazole IV q 8h For 1 OR 3 days postoperatively |
1g Ceftriaxone IV & 0.5g Metronidazole IV preoperatively |
No |
Yes |
|
58 |
Mui 2005 |
Single centre 269* |
Appendectomy (open, uncomplicated) |
II-III |
Noa, 30 days |
1.5g Cefuroxime IV & 0.5 g Metronidazole IV preoperatively + 2x postoperatively OR a 5-day course IV until PO was tolerated (Cefuroxime 0.25g 2dd + metronidazole 0.4g 3dd) |
1.5g Cefuroxime IV & 0.5 g Metronidazole IV preoperatively |
Yes |
Yes |
|
Postoperative continuation of surgical antibiotic prophylaxis for multiple postoperative doses <24h vs. postoperative continuation of surgical antibiotic prophylaxis for one postoperative dose |
|||||||||
|
59 |
Karran 1993 |
Single centre 227 |
Colorectal surgery |
II-III |
Nog, 6-8 weeks |
1g Imipenem IV preoperatively + 1x 3h postoperatively followed by 0.5 Imipenem IV 2x q 8 h |
1g Imipenem IV preoperatively + 1x 3h postoperatively |
No |
No |
|
Postoperative continuation of surgical antibiotic prophylaxis > 24h vs postoperative continuation of surgical antibiotic prophylaxis <= 24h |
|||||||||
|
60 |
Rajabi 2012 |
Single centre 194* |
Appendectomy (open, uncomplicated) |
II-III |
Noa, 10 days after discharge |
1.5g Cefuroxime IV & 0.5 g Metronidazole IV preoperatively + 1g Ceftriaxone 6x q 12h & 0.5g Metronidazole IV q 9x q 8h postoperatively |
1.5g Cefuroxime IV & 0.5 g Metronidazole IV preoperatively + 1g Ceftriaxone 2x q 12h & 0.5g Metronidazole IV 3x q 8h postoperatively |
No |
Yes |
|
61 |
Mui 2005 |
Single centre177* |
Appendectomy (open, uncomplicated) |
II-III |
Noa, 30 days |
1.5g Cefuroxime IV & 0.5 g Metronidazole IV preoperatively + 5-day course IV until PO was tolerated (Cefuroxime 250mg 2dd + metronidazole 400mg 3dd) |
1.5g Cefuroxime IV & 0.5 g Metronidazole IV preoperatively + 2x for 1 day postoperatively |
Yes |
Yes |
|
62 |
Ishibashi 2014 |
Single centre 297 |
Colorectal surgery |
II-III |
CDC, 30 days |
1g Flomoxef IV + 1x 1h postoperatively followed by 4x q 12h |
1g Flomoxef IV + 1x 1h postoperatively |
No |
Yes |
|
63 |
Ishibashi 2009 |
Single centre 275 |
Colorectal surgery |
II-III |
CDC, 30 days |
1g Cefotiam IV or Cefmetazole IV + 1x 1h postoperatively followed by 4 x q 12h |
1g Cefotiam IV or 1g Cefmetazole IV + 1x 1h postoperatively |
No |
Yes |
|
64 |
McArdle 1995 |
Single centre 169 |
Colorectal surgery |
II-III |
Noa, 4 weeks after discharge |
0.5g Metronidazole IV & 0.12g Gentamicin IV + 0.5g Metronidazole IV & 0.08g Gentamicin 9x q 8h |
0.5g Metronidazole IV & 0.12g Gentamicin IV+ 0.5g Metronidazole IV & 0.08g gentamicin IV 2x q 8h |
Yes |
Yes |
|
65 |
Becker 1991 |
Single centre 40 |
Colorectal surgery |
II-III |
Nob, 56 days |
2g Cefoxitin IV preoperatively + 2x q 6h after the initial dose followed by 1g Cefoxitin IV 20x q 6h postoperatively |
2g Cefoxitin IV preoperatively + 2x q 6h after the initial dose |
Yes |
No |
|
66 |
Fujita 2015 |
Single centre 257 |
Upper GI surgery |
II |
CDC, 30d |
1g Cefmetazole IV 4x q 3h starting preoperatively + 4x q 12h postoperatively |
1g Cefmetazole IV 4x q 3h starting preoperatively |
Yes |
Yes |
|
67 |
Lau 1990 |
Single centre 203 |
Open cholecystectomy |
II-III |
Noh, 1 year |
2g Cefamandole IV preoperatively + 0.5g Cefamandole IV 30x q 6h after the initial dose |
2g Cefamandole IV preoperatively + 0.5g Cefamandole IV 2x q 6h after the initial dose |
Yes |
No |
|
68 |
Yang 2001 |
Multi centre 731 |
Mixed general |
II-III |
Nod, NR |
0.3g Nefibromycin IV & 0.5g metronidazole IV when needed + 9x q 8h postoperatively |
0.3g Nefibromycin IV & 0.5g metronidazole IV when needed + 2x q 8h postoperatively |
Yes |
No |
|
69 |
Bozorgzadeh 1999 |
Single centre 300* |
Mixed general surgery |
II-III |
CDC, 30 days |
1g Cefoxitin IV for 24 with the first dose given in the emergency department after determination of the requirement for laparotomy + 20x q 6h |
1g Cefoxitin IV for 24 with the first dose given in the emergency department after determination of the requirement for laparotomy + 4x q 6h |
No |
No |
|
70 |
Hanif 2015 |
Single centre 220* |
Mixed general surgery |
II-III |
Nod, NR |
1g Sulbactam IV & 0.5 g Cephaperazone IV preoperatively + 14x q 8h |
1g Sulbactam IV & 0.5 g Cephaperazone IV preoperatively + 2x q 8h |
Yes |
No |
|
71 |
Chang 2005 |
Single centre 156 |
Gynaecological surgery |
II |
Noo, 7 days after discharge |
2g Cephalothin IV & 0.08g Gentamicin IV preoperatively + 1g Cephalothin IV 5-10x q 6h & 0.06-0.08g Gentamicin IV 4-8x q 8h postoperatively |
2g Cephalothin IV & 0.08g Gentamicin IV preoperatively + 1g Cephalothin IV 4x q 6h & 0.06-0.08g Gentamicin IV 3x q 8h postoperatively |
Yes |
No |
|
72 |
Takemoto 2015 |
Single centre 314 |
Orthopaedic / trauma surgery |
I |
CDC, 1 year |
Cefazolin for drain duration starting preoperatively (average of 3.2 days) |
Cefazolin for 24h starting preoperatively |
Yes |
Yes |
|
73 |
Lin 2011 |
Single centre 231 |
Cardiothoracic surgery |
I |
CDC, 30 days |
1 gr Cefazolin preoperatively + 9x q 8h postoperatively |
1 gr Cefazolin preoperatively + 3x q 8h postoperatively |
No |
Yes |
|
74 |
Niederhauser 1997 |
Single centre 53 |
Cardiothoracic surgery |
I |
CDC, 3-540 days |
1g of cefazolin preoperatively + 2x q 8h postoperatively followed by Ticarcillin/clavunate 5.2g 6x q 8h & 0.5g Vancomycin q 12h until removal of IABP |
1g of cefazolin preoperatively + 2x q 8h postoperatively |
Yes |
Yes |
|
75 |
Liu 2008 |
Single centre 53 |
Head and neck surgery |
II |
CDC, 30 days |
0.3g Clindamycin IV preoperatively +12x q 6h postoperatively |
0.3g Clindamycin IV preoperatively + 4x q 6h postoperatively |
Yes |
Yes |
|
76 |
Carroll 2003 |
Single centre 74 |
Head and neck surgery |
II |
Nop, 7 days |
0.9g Clindamycin IV preoperatively +15x q 8h after the initial dose |
0.9g Clindamycin IV preoperatively + 3x q 8h after the initial dose |
Yes |
Yes |
|
77 |
Righi 1996 |
Single centre 162 |
Head and neck surgery |
II |
Nos, 20 days |
0.6g Clindamycin IV & Cefonicid 1g IV preoperatively + 0.6g Clindamycin IV 9x q 8h & Cefonicid 1g 3x q 12h postoperatively |
0.6g Clindamycin IV & Cefonicid 1g IV preoperatively + 0.6g Clindamycin IV 3x q 8h & Cefonicid 1g 1x q 12h postoperatively |
Yes |
No |
|
78 |
Bidkar 2014 |
Single centre 78* |
Head and neck surgery |
I-III |
Nod, 3 weeks |
1.5g Cefuroxime preoperatively + 0.75g Cefuroxime 2x q 12h postoperatively followed by 0.2g Cefixime PO 16x q 12h |
1.5g Cefuroxime Preoperatively + 0.75g Cefuroxime 2x q 12h postoperatively |
Yes |
Yes |
|
79
|
Abubaker 2001 |
Single centre 30 |
Maxillofacial surgery |
II |
Noe, 6 weeks |
2m U aqueous Penicillin-G IV q 4h from admission trough the preoperative and intraoperative phase and for 12h postoperatively followed by 0.5g penicillin PO 20x q 6h |
2m U aqueous Penicillin-G IV q 4h from admission trough the preoperative and intraoperative phase and for 12h postoperatively |
Yes |
Yes |
|
80 |
Eshghpour 2014 |
Single centre 50* |
Maxillofacial surgery |
II |
Nod, 6 weeks |
1g Cefazolin IV preoperatively + 1x q 4h after the initial dose followed by 0.5g Amoxicillin PO 21x q 8h |
1g Cefazolin IV preoperatively + 1x q 4h after the initial dose |
Yes |
Yes |
|
81 |
Jansisyanont 2008 |
Multi centre 122* |
Maxillofacial surgery |
II |
CDC, 6 weeks |
1.2g Amoxicillin / Clavulanic acid + 0.625g Amoxicillin / clavulanic acid PO 15x q 8h postoperatively |
1.2g Amoxicillin / Clavulanic acid preoperatively + 1x q 8h postoperatively |
Yes |
Yes |
|
2 million units of aqueous Penicillin IV + 0.5g Amoxicillin PO 15x q 8h postoperatively |
2m U of aqueous Penicillin IV preoperatively + 1x q 4h postoperatively |
||||||||
|
82 |
Baqain 2004 |
Single centre 34 |
Maxillofacial surgery |
II |
Not, 6 weeks |
1g Amoxicillin IV + 0.5g Amoxicillin IV 1x q 3h postoperatively followed by 0.5g Amoxicillin 15x q 8h |
1g Amoxicillin IV + 0.5g Amoxicillin IV 1x q 3h postoperatively |
No |
No |
|
83 |
Bentley 1999 |
Single centre 30 |
Maxillofacial surgery |
II |
CDC, 30 days |
2m U aqueous Penicillin-G IV preoperatively + 1x q 3h postoperatively after the last intraoperative dose followed by 1m U Penicillin-G IV 8x q 6h followed by 0.3g penicillin-V PO 8x q 6h |
2m U aqueous Penicillin-G IV preoperatively + 1x q 3h postoperatively after the last intraoperative dose |
No |
Yes |
|
84 |
Fridrich 1994 |
Single centre 30* |
Maxillofacial surgery |
II |
Nod, 8 weeks |
2m U Penicillin IV preoperatively + q 4h until IV discontinuation on postoperative day 1 followed by 0.5g Penicillin VK 28x q 6h |
2m U Penicillin IV preoperatively + a 2h until participants reached the recovery room, where the final dose was given |
Yes |
Yes |
|
85 |
Garcia 2020 |
Single centre 124 |
Mammaplasty |
I |
CDC, 30 days |
1g cephalothin IV preoperatively + 3x postoperatively q 6h followed by 500mg oral cephalexin 28x q 6h |
1g cephalothin IV preoperatively + 3x postoperatively q 6h followed by oral placebo 28x q 6h |
Yes |
No |
|
86 |
Ahmed 2019 |
Single centre 159 |
Hollow viscus injury |
III |
CDC, unknown |
1g sulbactam + cefoperazone and 0.5g metronidazole IV preoperatively + 16x postoperatively q 8h |
1g sulbactam + cefoperazone and 0.5g metronidazole IV preoperatively + 3x q 8h postoperatively |
Yes |
No |
|
87 |
Urquhart 2019 |
Single centre 282 |
Spinal surgery |
I |
CDC, 1 year |
2g cefazolin IV preoperatively + 12x q 8h postoperatively OR 1g vancomycin IV preoperatively + 6x q 12h |
2g cefazolin IV preoperatively + 3x q 8h postoperatively OR 1g vancomycin IV preoperatively + 2x q 12h |
Yes |
Yes |
|
88 |
Takayama 2019 |
Multi centre 480 |
Hepatobiliary surgery |
II |
CDC, 30 days |
1g flomoxef IV preoperatively + q 3h intra-operatively + 6x q 12h post-operatively |
1g flomoxef IV preoperatively + q 3h intra-operatively + 1x q 6h post-operatively |
Yes |
Yes |
|
89 |
Madadi 2019 |
Single centre 300 |
Cardiac surgery |
I |
Noag, 2 years |
1-2g cephazolin IV preoperatively + 3x q 8h postoperatively |
1-2g cephazolin IV preoperatively |
Yes |
Yes |
|
1-2g cephazolin IV preoperatively + 3x q 8h postoperatively followed by 250-500mg ciprofloxacin14x q12h oral |
|||||||||
|
90 |
Yamamoto 2018 |
Single centre 82 |
Hepatobiliary surgery |
II |
Noad, unknown |
1g cefozopran IV preoperatively + 9x q 12h postoperatively |
1g cefozopran IV preoperatively + 1x postoperatively |
Yes |
Yes |
|
Postoperative continuation of surgical antibiotic prophylaxis > 48h vs postoperative continuation of surgical antibiotic prophylaxis <= 48h |
|||||||||
|
91 |
Togo 2007 |
Single centre 180 |
Hepatobiliary surgery |
II |
CDC, 30 days |
1g Flomoxef IV preoperatively + 1x postoperatively followed by 2g Flomoxef IV 10x q 12h |
1g Flomoxef IV preoperatively + 1x postoperatively followed by 2g Flomoxef IV 4x q 12h |
Yes |
Yes |
|
92 |
Sugawara 2018 |
Single centre 86 |
Hepatobiliary surgery |
II-III |
CDC, 30 days |
Cefazoline IV (or in case of a positive culture, as culture indicated) preoperatively + 12x q8h |
Cefazoline IV (or in case of a positive culture, as culture indicated) preoperatively + 6x q8h |
Yes |
Yes |
|
93 |
Gupta 2010 |
Single centre 227 |
Cardiothoracic surgery |
I |
CDC, 30 days |
Ceftazidime Pentahydrate IV & Amikacin IV preoperatively + for 72h postoperatively |
Ceftazidime Pentahydrate IV & Amikacin IV preoperatively + for 48h postoperatively |
Yes |
Yes |
|
94 |
Otani 2004 |
Single centre 40 |
Thoracic surgery |
II-III |
Nod, 14 days |
1g Cefmetazole IV preoperatively + 1x directly postoperatively followed by 12 x q 12h |
1g Cefmetazole IV preoperatively + 1x directly followed by 2 x q 12h |
No |
No |
|
95 |
Sawyer 1990 |
Multi centre 50 |
Head and neck surgery |
II |
Nos, NR |
1g Cefazolin IV & 0.5g Metronidazole IV preoperatively + 1g Cefazolin IV 21x q 8h & 0.5g Metronidazole IV 28x q 6 h postoperatively |
1g Cefazolin IV & 0.5g Metronidazole IV preoperatively + 1g Cefazolin IV 6x q 8h & 0.5g Metronidazole IV 8x q 6 h postoperatively |
No |
Yes |
|
96 |
Davis 2017 |
Single centre 171* |
Maxillofacial surgery |
II |
CDC, 30 days / 1 year |
2g Cefazolin IV preoperatively + 3x q 8h postoperatively followed by 0.5g Cephalexin PO & 0.3g Clindamycin PO 8x q 6h |
2g Cefazolin IV preoperatively + 3x q 8h postoperatively |
Yes |
Yes |
|
97 |
Adaji 2020 |
Single centre 243 |
C-section |
III |
Noab, 14 days / 6 weeks |
0.75g Cefuroxime IV & 0.4g Metronidazole IV preoperatively + 0.75g cefuroxime IV 4x q 12h & 6x q 8h Metronidazole IV postoperatively followed by 0.5g Cefuroxime PO 10x q 12h & 0.4g Metronidazole PO 5x q 24h |
0.75g Cefuroxime IV & 0.4g Metronidazole IV preoperatively + 0.75g cefuroxime IV 4x q 12h & 6x q 8h Metronidazole IV postoperatively |
Yes |
No |
|
Postoperative continuation of surgical antibiotic prophylaxis > 72h vs postoperative continuation of surgical antibiotic prophylaxis <= 72h |
|||||||||
|
98 |
Park 2010 |
Multi centre 255 |
Colorectal surgery |
II-III |
CDC, 21 days |
1g Cefotetan IV preoperatively + 15x q 8h postoperatively |
1g Cefotetan IV preoperatively + 9x q 8h postoperatively |
Yes |
Yes |
|
CDC: Center for Disease Control and Prevention; SSI: Surgical Site infection; Wound class.: CDC Wound Classification;
a. Purulent discharge with or without culture b. Purulent discharge, or serous with a positive culture c. Discharge with a positive culture d. Wound infection, not otherwise specified e. Pus drainage at the fracture site or in the vicinity of the surgical intervention site; b) increased swelling 7 days after the operation; c) presence of a fistula in the area of the surgical intervention or at the site of the fracture, with active drainage; d) other clinical features observed by the evaluator, including typical signs of infection such as fever, oedema and localized redness. f. Purulent discharge or abscess g. Purulent discharge, positive bacteriological culture, abscess, peritonitis, septicaemia h. Purulent discharge, serous discharge + positive bacteriological cultures, serous discharge after the patient had returned home. Intra-peritoneal abscess was diagnosed by ultrasonic evidence of an abscess and by laparotomy i. 0: No sign of infection., 1: Minor infection (erythema, stitch abscess or skin edge necrosis)., 2: Major infection (purulent discharge or wound dehiscence). j. Pain at the operative site, persistent fever >38°C wound erythema, tenderness, wound discharge and dehiscence. k. Presence of erythema, purulent discharge, cellulitis or wound abscess, peritonitis, pelvic abscess or wound dehiscence. l. Superficial or deep infection, pus discharge, abscess formation, wound dehiscence, and hematoma formation m. Abdominal wound infection or trocar wound infection (including wound discharge or abscess). Pelvic abscess or tuba-ovarian abscess. Vaginal cuff abscess. Postoperative septicaemia. n. Pelvic cellulitis, vaginal cuff abscess, pelvic abscess, wound infection o. If the wound appeared red or oedematous or if there was drainage. p. A wound was considered infected if the colour became red or the wound was swollen. A pink wound that developed purulent discharge was also considered infected. q. Purulent drainage (either spontaneously or by incision) or muco-cutaneous fistula interpreted as wound infection. r. Major wound infection was defined as wound breakdown and undermining of tissues sufficient to allow packing of the wound. Lesser complications, such as cellulitis or a tiny fistula, allowing only entry of a cotton-tipped applicator were considered as minor. s. Presence of purulent drainage (either spontaneously or by incision), accompanied by pain or tenderness, localized swelling, redness, and heat or fever (>38.5° C) or an increase in localized swelling after an initial postoperative decrease of oedema, together with pain, discomfort, induration, and an increase in body temperature (>38.5° C). t. The need for additional antibiotics u. Wound infection Erythema of incision(s), pus and/or turbid fluid. Intra-abdominal abscess v. Purulent discharge, endoperitoneal abscess or diffuse peritonitis but not secondary to anastomotic leakage w. Infiltrate, dehiscence or Purulent secretion of the wound. x. Purulent drainage at the operative site with the presence of one or more of the classic signs and symptoms of inflammation (rubor, calor, tumor, dolor) z. Pus discharge from the wound, redness, tenderness and oedema. Intra-abdominal collection was defined as fluid collection inside the peritoneal cavity confirmed by ultrasound or CT aa. Pocket infection with localized inflammation without evidence of systemic infection. Pocket infection with positive blood culture but without evidence of infective endocarditis. Device-related endo- carditis according to the Duke modified criteria. ab. Partial or total dehiscence with the presence of purulent or serous wound discharge. ac. Postoperative body temperature of> 38 °C; with incision bleeding, redness and pain, inflammatory exudate, or possible dehiscence of incision. ad. Postoperative infectious complications were defined as: clinically relevant postoperative pancreatic fistula (CR-POPF) which was defined according to International Study Group (ISGPF) criteria, intra-abdominal abscess, postoperative cholangitis and wound infection. ae. Diagnosis of abdominal incision infection: Postoperative maternal body temperature of > 38 °C; with incision bleeding, redness and pain, inflammatory exudate, or possible dehiscence of incision. af. Infectious complications were defined as infection of the wound, presence of pus from the surgical wound requiring open drainage, bacteremia, or endocarditis. ag. Infections were classified in one of the following categories: (1) pocket infection with localized inflammation without evidence of systemic infection; (2) pocket infection with positive blood culture but without evidence of infective endocarditis; (3) device-related endocarditis according to the Duke modified criteria.11 |
|||||||||
Criteria for risk of bias assessment
|
Risk of bias domain |
Criteria for judgment |
|
Selection bias |
Low risk of bias: A random component was used in the sequence generation process and allocation was concealed High risk of bias: A non-random component was used or allocation was inadequately concealed. Unclear: Sequence generation or allocation concealment was insufficiently described for judgement. |
|
Performance bias |
Low risk of bias: Blinding of patients and investigators was described (e.g. with a placebo control group)
Hight risk of bias: There was no blinding of patients and investigators.
Unclear: Blinding of participants and investigators was insufficiently described for judgement |
|
Detection bias |
Low risk of bias: Outcome assessor blinding was ensured High risk of bias: Outcome assessors were not blinded Unclear: Blinding of outcome assessors was insufficiently described. |
|
Attrition bias |
Low risk of bias: An intention to treat analysis was conducted or attrition was low or balanced and unlikely to have affected the outcome
High risk of bias: Attrition was unbalanced or high relative to the event incidence and could have affected the outcome.
Unclear: Attrition was insufficiently described |
|
Reporting bias |
Low risk of bias: No outcomes mentioned in the study registration or protocol where omitted or altered. High risk of bias: Outcomes mentioned in the study registration or protocol where omitted or altered. Unclear: No registration or protocol was available |
|
Other bias |
Low risk of bias, unless other concerns existed on the validity of the study |
Risk of bias evaluation of the included studies
|
Author, Year |
Random sequence generation (selection bias) |
Allocation concealment (selection bias) |
Blinding of participants and personnel (performance bias) |
Blinding of outcome assessment (detection bias) |
Incomplete outcome data (attrition bias) |
Selective reporting (reporting bias) |
Other bias |
|
Sadraei-Moosavi 2018 |
Unclear |
Unclear |
Unclear |
Unclear |
Unclear |
Low |
Low |
|
|
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
All predefined outcomes reported |
No concerns |
|
Hussain 2012 |
Unclear |
Unclear |
Unclear |
Unclear |
Unclear |
Unclear |
Low |
|
|
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
No protocol or registration |
No concerns |
|
Liberman 1995 |
Unclear |
Unclear |
Unclear |
Unclear |
Unclear |
Unclear |
Low |
|
|
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
No protocol or registration |
No concerns |
|
Tsang 1992 |
High |
High |
High |
Unclear |
Unclear |
Unclear |
Low |
|
|
Randomized according to hospital numbers (even –odd) |
Randomized according to hospital numbers (even –odd) |
No blinding described and no allocation concealment |
Not (sufficiently) described |
Not (sufficiently) described |
No protocol or registration |
No concerns |
|
Suzuki 2011 |
Low |
Unclear |
Unclear |
Unclear |
Low |
Unclear |
Low |
|
|
Random number table |
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
Missing data balanced in numbers across intervention groups for similar reasons |
No protocol or registration |
No concerns |
|
Fujita 2007 |
Low |
Low |
High |
High |
Low |
Unclear |
Low |
|
|
Central computer randomisation |
Central computer randomisation |
Not blinded |
Not blinded |
Low attrition, unlikely to influence outcome |
No protocol or registration |
No concerns |
|
Imamura 2012 |
Low |
Low |
High |
High |
Low |
Low |
Low |
|
|
Mersenne twister randomisation |
Central randomisation |
Not blinded |
Not blinded |
Intention to treat analysis |
All predefined outcomes reported |
No concerns |
|
Haga 2012 |
Unclear |
Unclear |
Unclear |
Unclear |
Unclear |
Unclear |
Low |
|
|
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
No protocol or registration |
No concerns |
|
Balbo 1991 |
Unclear |
High |
Unclear |
Unclear |
Unclear |
Unclear |
Low |
|
|
Not (sufficiently) described |
Based on a randomisation list
|
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
No protocol or registration |
No concerns |
|
Mohri 2007 |
Low |
Low |
Low |
Low |
Low |
Unclear |
Low |
|
|
Central computer randomisation |
Central computer randomisation |
Blinded investigators and patients |
Independent outcome assessor |
Balanced in reason and groups, unlikely to affect outcome |
No protocol or registration |
No concerns |
|
Chauhan 2018 |
Unclear |
Unclear |
Unclear |
Unclear |
Unclear |
Unclear |
Low |
|
|
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
No protocol or registration |
No concerns |
|
Santibañes 2018 |
Low |
Low |
Low |
Low |
Low |
Low |
Low |
|
|
Not (sufficiently) described |
Not (sufficiently) described |
Blinded investigators and patients |
Blinded investigators and patients |
Intention to treat analysis |
All predefined outcomes reported |
No concerns |
|
Kim 2017 |
Low |
Low |
Low |
Low |
Low |
Low |
Low |
|
|
Computer randomisation |
Central allocation by independent investigator |
Blinded investigators and patients |
Blinded investigators and patients |
Missing outcomes balanced in reason and groups |
All predefined outcomes reported |
No concerns |
|
Loozen 2017 |
Low |
Low |
High |
Unclear |
Low |
Low |
Low |
|
|
Central computer randomisation |
Central computer randomisation |
Not blinded |
Not (sufficiently) described |
Missing outcomes balanced in reason and groups |
All predefined outcomes reported |
No concerns |
|
Regimbeau 2014 |
Low |
Low |
Unclear |
Low |
Low |
Unclear |
Low |
|
|
Central computer randomisation |
Central computer randomisation based |
Not (sufficiently) described |
Blinded outcome assessor |
Intention to treat analysis |
No protocol or registration |
No concerns |
|
Unemura 2000 |
High |
High |
Unclear |
Unclear |
Unclear |
Unclear |
Low |
|
|
Randomized by alternately selecting treatment allocation |
Randomized by alternately selecting treatment allocation |
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
No protocol or registration |
No concerns |
|
Meijer 1993 |
Low |
Low |
Low |
Low |
Low |
Unclear |
Low |
|
|
Central computer randomisation |
Central computer randomisation |
Blinded investigators and patients |
Blinded outcome assessor |
Intention to treat analysis |
No protocol or registration |
No concerns |
|
Abro 2014 |
Unclear |
Unclear |
Unclear |
Unclear |
High |
Unclear |
Low |
|
|
Not (sufficiently) described) |
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
High attrition relative to events. Could have affected outcome |
No protocol or registration |
No concerns |
|
Becker 2008 |
Low |
Unclear |
Low |
Unclear |
Unclear |
Unclear |
Low |
|
|
Drawing of envelopes |
Not (sufficiently) described |
Randomisation after procedure |
Not (sufficiently) described |
Not (sufficiently) described |
No protocol or registration |
No concerns |
|
Scher 1997 |
Low |
Low |
Unclear |
Unclear |
Unclear |
Unclear |
Low |
|
|
Central randomisation by random number chart* |
Central randomisation by random number chart * |
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
No protocol or registration |
No concerns |
|
Kow 1995 |
Unclear |
Unclear |
High |
Unclear |
Unclear |
Unclear |
Low |
|
|
Not (sufficiently) described |
Not (sufficiently) described |
Not blinded
|
Not (sufficiently) described |
Not (sufficiently) described |
No protocol or registration |
No concerns |
|
Turano 1992 |
Unclear |
High |
Unclear |
Unclear |
Unclear |
Unclear |
Low |
|
|
Not (sufficiently) described |
Open randomisation |
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
No protocol or registration |
No concerns |
|
Bates 1992 |
Low |
Unclear |
Unclear |
Low |
Low |
Unclear |
Low |
|
|
Randomized by random number table |
Not (sufficiently) described |
Not (sufficiently) described |
Blinded outcome assessor |
Attrition low and balanced. Unlikely to affect outcome |
No protocol or registration |
No concerns |
|
Aberg 1991 |
Unclear |
Unclear |
Unclear |
Unclear |
Unclear |
Unclear |
Low |
|
|
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
No protocol or registration |
No concerns |
|
Sgroi 1990 |
Unclear |
Unclear |
Unclear |
Unclear |
Unclear |
Unclear |
Low |
|
|
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
No protocol or registration |
No concerns |
|
Westen 2015 |
Unclear |
Low |
Unclear |
Low |
Low |
Low |
Low |
|
|
Not (sufficiently) described |
Sequentially numbered, opaque, sealed envelopes |
Not (sufficiently) described |
Blinded outcome assessor |
Intention to treat analysis |
All predefined outcomes reported |
No concerns |
|
Shaheen 2014 |
Low |
Unclear |
Unclear |
Unclear |
Unclear |
Unclear |
Low |
|
|
Shuffled cards |
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
No protocol or registration |
No concerns |
|
Lyimo 2013 |
Low |
Unclear |
High |
High |
Low |
Unclear |
Low |
|
|
Drawing of envelopes |
Not (sufficiently) described |
Not blinded |
Not blinded |
Intention to treat analysis |
No protocol or registration |
No concerns |
|
Su 2005 |
Low |
Unclear |
Unclear |
Unclear |
High |
Unclear |
Low |
|
|
Computer randomisation |
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
Unbalanced attrition. Could have affected outcome |
No protocol or registration |
No concerns |
|
Irato 1997 |
Unclear |
Unclear |
Unclear |
Unclear |
Unclear |
Unclear |
Low |
|
|
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
No protocol or registration |
No concerns |
|
Cartaña 1994 |
Low |
Unclear |
Unclear |
Unclear |
Unclear |
Unclear |
Low |
|
|
Random number table |
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
No protocol or registration |
No concerns |
|
Buckley 1990 |
Unclear |
Unclear |
Low |
Low |
High |
Unclear |
Low |
|
|
Not (sufficiently) described |
Not (sufficiently) described |
Blinded investigators and patients |
Blinded outcome assessors |
Unbalanced attrition. Could have affected outcome |
No protocol or registration |
No concerns |
|
Garotta 1991 |
Low |
Low |
Unclear |
Unclear |
Unclear |
Unclear |
Low |
|
|
Central computer randomisation |
Central computer randomisation |
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
No protocol or registration |
No concerns |
|
Hellbusch 2008 |
Unclear |
Unclear |
Unclear |
Unclear |
High |
Unclear |
Low |
|
|
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
High attrition relative to events. Could have affected outcome |
No protocol or registration |
No concerns |
|
Crist 2018 |
Unclear |
Low |
Low |
Low |
High |
Unclear |
Low |
|
|
Not (sufficiently) described |
Pharmacy controlled randomisation |
Blinded investigators and patients |
Blinded investigators and patients |
High attrition relative to events. Could have affected outcome |
No protocol or registration |
No concerns |
|
Nooyen 1994 |
Low |
Low |
Unclear |
Unclear |
High |
Unclear |
Low |
|
|
Central computer randomisation |
Central computer randomisation |
Not (sufficiently) described |
Not (sufficiently) described |
Unbalanced attrition. Could have affected outcome |
No protocol or registration |
No concerns |
|
Tamayo 2008 |
Low |
Unclear |
Unclear |
Low |
High |
Unclear |
Low |
|
|
Computerized randomisation |
Not (sufficiently) described |
Not (sufficiently) described |
Blinded outcome assessor |
High attrition relative to events. Could have affected outcome |
No protocol or registration |
No concerns |
|
Olak 1991 |
Low |
Low |
Low |
Low |
Unclear |
Unclear |
Low |
|
|
Central random number generation |
Central random number generation |
Blinded investigators and patients |
Blinded outcome assessor |
Not (sufficiently) described |
No protocol or registration |
No concerns |
|
Jiang 2004 |
Low |
High |
Unclear |
Unclear |
Unclear |
Unclear |
Low |
|
|
Random number list |
Open list |
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
No protocol or registration |
No concerns |
|
Hall 1998 |
Low |
Low |
Unclear |
Unclear |
Unclear |
Unclear |
Low |
|
|
Computer randomisation |
Sequentially numbered, opaque, sealed envelopes |
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
No protocol or registration |
No concerns |
|
Orlando 2015 |
Low |
Low |
Unclear |
Unclear |
Low |
Low |
Low |
|
|
Central computer randomisation |
Central computer randomisation |
Not (sufficiently) described |
Not (sufficiently) described |
All patients complied with the study protocol |
All predefined outcomes reported |
No concerns |
|
Maier 1992 |
High |
High |
High |
Unclear |
Unclear |
Unclear |
Low |
|
|
Randomisation by even and uneven days |
Randomisation by even and uneven days |
No blinding described and no allocation concealment |
Not (sufficiently) described |
Not (sufficiently) described |
No protocol or registration |
No concerns |
|
Mann 1990 |
Unclear |
Unclear |
Unclear |
Unclear |
Unclear |
Unclear |
Low |
|
|
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
No protocol or registration |
No concerns |
|
Rajan 2005 |
Low |
Unclear |
Low |
Unclear |
Unclear |
Unclear |
Low |
|
|
Drawing of envelopes |
Not (sufficiently) described |
Blinded investigators |
Not (sufficiently) described |
Not (sufficiently) described |
No protocol or registration |
No concerns |
|
Campos 2015 |
Unclear |
Unclear |
Unclear |
Unclear |
Unclear |
Unclear |
Low |
|
|
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
No protocol or registration |
No concerns |
|
Lindeboom 2003 |
Low |
High |
Unclear |
Low |
Unclear |
Unclear |
Low |
|
|
Random number list |
Open list |
Not (sufficiently) described |
Blinded outcome assessor |
Not (sufficiently) described |
No protocol or registration |
No concerns |
|
Cioaca 2002 |
Unclear |
Unclear |
Unclear |
Low |
Low |
Unclear |
Low |
|
|
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
Blinded outcome assessor |
Attrition low and balanced. Unlikely to affect outcome |
No protocol or registration |
No concerns |
|
Wahab 2013 |
Unclear |
Unclear |
Unclear |
Unclear |
Unclear |
Unclear |
Low |
|
|
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
No protocol or registration |
No concerns |
|
Danda 2010 |
Unclear |
Unclear |
Low |
Low |
Unclear |
Unclear |
Low |
|
|
Not (sufficiently) described |
Not (sufficiently) described |
Blinded investigators and patients |
Blinded outcome assessor |
Not (sufficiently) described |
No protocol or registration |
No concerns |
|
Kang 2009 |
Low |
Unclear |
Unclear |
Unclear |
Unclear |
Unclear |
Low |
|
|
Computer randomisation |
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
No protocol or registration |
No concerns |
|
Rajabi 2012 |
Unclear |
Unclear |
Low |
Unclear |
Unclear |
Unclear |
Low |
|
|
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
No protocol or registration |
No concerns |
|
Mui 2005 |
Unclear |
Unclear |
Unclear |
Unclear |
Unclear |
Unclear |
Low |
|
|
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
No protocol or registration |
No concerns |
|
Karran 1993 |
Unclear |
Unclear |
Unclear |
Unclear |
High |
Unclear |
Low |
|
|
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
High attrition relative to events. Could have affected outcome |
No protocol or registration |
No concerns |
|
Ishibashi 2014 |
Unclear |
Unclear |
Low |
Unclear |
Low |
Unclear |
Low |
|
|
Not (sufficiently) described |
Not (sufficiently) described |
Randomisation after procedure |
Not (sufficiently) described |
Attrition low and balanced. Unlikely to affect outcome |
No protocol or registration |
No concerns |
|
Ishibashi 2009 |
Unclear |
Unclear |
Low |
Unclear |
Unclear |
Unclear |
Low |
|
|
Not (sufficiently) described |
Not (sufficiently) described |
Randomisation after procedure |
Not (sufficiently) described |
Not (sufficiently) described |
No protocol or registration |
No concerns |
|
McArdle 1995 |
Unclear |
Unclear |
Unclear |
Unclear |
Unclear |
Unclear |
Low |
|
|
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
No protocol or registration |
No concerns |
|
Becker 1991 |
Unclear |
Unclear |
Low |
Low |
Unclear |
Unclear |
Low |
|
|
Not (sufficiently) described |
Not (sufficiently) described |
Blinded investigators and patients |
Blinded outcome assessor |
Not (sufficiently) described |
No protocol or registration |
No concerns |
|
Fujita 2015 |
Unclear |
Unclear |
Unclear |
Unclear |
Unclear |
Unclear |
Low |
|
|
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
No protocol or registration |
No concerns |
|
Lau 1990 |
Unclear |
Unclear |
Low |
Low |
High |
Unclear |
Low |
|
|
Not (sufficiently) described |
Not (sufficiently) described |
Randomisation after procedure |
Blinded outcome assessor |
High attrition relative to events. Could have affected outcome |
No protocol or registration |
No concerns |
|
Yang 2001 |
Unclear |
Unclear |
Unclear |
Unclear |
Unclear |
Unclear |
Low |
|
|
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
No protocol or registration |
No concerns |
|
Bozorgzadeh 1999 |
Unclear |
Unclear |
Unclear |
Unclear |
Unclear |
Unclear |
Low |
|
|
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
No protocol or registration |
No concerns |
|
Hanif 2015 |
High |
High |
Unclear |
Unclear |
Unclear |
Unclear |
Low |
|
|
Randomisation by alternating assignment |
Randomisation by alternating assignment |
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
No protocol or registration |
No concerns |
|
Chang 2005 |
Low |
Low |
Unclear |
Unclear |
Unclear |
Unclear |
Low |
|
|
Central computer randomisation |
Central computer randomisation |
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
No concerns |
|
Takemoto 2015 |
Low |
Unclear |
Low |
Low |
High |
Low |
Low |
|
|
Computer randomisation |
Not (sufficiently) described |
Investigators blinded |
Outcome assessors blinded |
High attrition relative to events. Could have affected outcome |
All predefined outcomes reported |
No concerns |
|
Lin 2011 |
Low |
unclear |
Unclear |
Low |
low |
Unclear |
Low |
|
|
Computer randomisation |
Not (sufficiently) described |
Not (sufficiently) described |
Blinded outcome assessor |
Intent-to-treat analysis |
No protocol or registration |
No concerns |
|
Niederhauser 1997 |
Low |
Unclear |
Low |
Unclear |
Low |
Unclear |
Low |
|
|
Randomisation list |
Not (sufficiently) described |
Randomisation after procedure |
Not (sufficiently) described |
All participants were analysed |
No protocol or registration
|
No concerns |
|
Liu 2008 |
Low |
Unclear |
Unclear |
Unclear |
Unclear |
Unclear |
Low |
|
|
Computer randomisation |
Not (sufficiently) described |
Randomisation after procedure |
Not (sufficiently) described |
Not (sufficiently) described |
No protocol or registration |
No concerns |
|
Carroll 2003 |
unclear |
unclear |
Unclear |
Unclear |
Unclear |
Unclear |
Low |
|
|
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
No protocol or registration |
No concerns |
|
Righi 1996 |
unclear |
unclear |
Unclear |
Unclear |
High |
Unclear |
Low |
|
|
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
High attrition relative to events. Could have affected outcome |
No protocol or registration |
No concerns |
|
Bidkar 2014 |
Low |
Unclear |
Low |
Low |
Low |
Unclear |
Low |
|
|
Computer randomisation |
Not (sufficiently) described |
Blinded investigators |
Blinded outcome assessors |
All participants were analysed |
No protocol or registration |
No concerns |
|
Abubaker 2001 |
Unclear |
Low |
Low |
Low |
High |
Unclear |
Low |
|
|
Not (sufficiently) described |
Central randomisation |
Blinded investigators and participants |
Blinded outcome assessors |
High attrition relative to events. Could have affected outcome |
No protocol or registration |
No concerns |
|
Eshghpour 2014 |
Unclear |
Unclear |
Unclear |
Unclear |
Low |
Unclear |
Low |
|
|
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
All participants were analysed |
No protocol or registration |
No concerns |
|
Jansisyanont 2008 |
Unclear |
Unclear |
Unclear |
Unclear |
High |
Unclear |
Low |
|
|
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
High attrition relative to events. Could have affected outcome |
No protocol or registration |
No concerns |
|
Baqain 2004 |
Low |
Low |
Low |
low |
Low |
Unclear |
Low |
|
|
Central randomisation by random list |
Central randomisation by random list |
Blinded investigators and patients |
Blinded outcome assessors |
All participants were analysed |
No protocol or registration |
No concerns |
|
Bentley 1999 |
Unclear |
Unclear |
Low |
Low |
Unclear |
Unclear |
Low |
|
|
Not (sufficiently) described |
Not (sufficiently) described |
Blinded investigators and patients |
Blinded outcome assessors |
Not (sufficiently) described |
No protocol or registration |
No concerns |
|
Fridrich 1994 |
Unclear |
Unclear |
Unclear |
Unclear |
Low |
Unclear |
Low |
|
|
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
All participants were analysed |
No protocol or registration |
No concerns |
|
Togo 2007 |
Low |
Low |
Unclear |
Unclear |
low |
unclear |
Low |
|
|
Central computer randomisation |
Central computer randomisation |
Not (sufficiently) described |
Not (sufficiently) described |
All participants were analysed |
No protocol or registration |
No concerns |
|
Sugawara 2018 |
Low |
Low |
Low |
Unclear |
Low |
Low |
Low |
|
|
Central computer randomisation |
Central computer randomisation |
Randomisation after procedure |
Not (sufficiently) described outcome |
All participants were analysed |
All predefined outcomes reported |
No concerns |
|
Gupta 2010 |
Low |
Low |
Low |
Low |
Low |
Unclear |
Low |
|
|
Randomisation by random number table |
Allocation concealed throughout the study |
Blinded investigators and patients |
Blinded outcome assessors |
Attrition low. Unlikely to affect outcome. |
No protocol or registration |
No concerns |
|
Otani 2004 |
Unclear |
Unclear |
Unclear |
Unclear |
Unclear |
Unclear |
Low |
|
|
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
No protocol or registration |
No concerns |
|
Sawyer 1990 |
Unclear |
Unclear |
Unclear |
Unclear |
Unclear |
Unclear |
Low |
|
|
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
No protocol or registration |
No concerns |
|
Davis 2017 |
Low |
Unclear |
Low |
Low |
High |
Low |
Low |
|
|
Randomisation by drawing envelopes |
Not (sufficiently) described |
Blinded investigators and patients |
Blinded outcome assessors |
High attrition relative to events. Could have affected outcome |
All predefined outcomes reported |
No concerns |
|
Park 2010 |
Unclear |
Unclear |
Unclear |
Unclear |
Unclear |
Unclear |
Low |
|
|
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
Not (sufficiently) described |
No protocol or registration |
No concerns |
|
* Information obtained through correspondence with author |
|||||||
Studies excluded after full text review
|
Author and year |
Reason for exclusion |
|
Kumar 20131 |
Incomparable regimen |
|
Ahn 20132 |
Not an RCT |
|
Fonseca 20063 |
Incomparable regimen |
|
Sevin 20074 |
Not an RCT |
|
Han 20145 |
Not an RCT |
|
Farran 20086 |
Did not address study question |
|
Schardey 19977 |
Did not address study question |
|
Vu 20148 |
Not an RCT |
|
Basoli 20089 |
Did not address study question |
|
Safdar 199210 |
Incomparable regimen |
|
Gidiri 201411 |
Incomparable regimen |
|
Kato 200712 |
Incomparable regimen |
|
Dahl A 200613 |
Not an RCT |
|
Kakimaru 201014 |
Not an RCT |
|
Kato 200615 |
Not an RCT |
|
Pedrini 200516 |
Not an RCT |
|
Righi 199517 |
Duplicate of Righi 1996 |
|
Adde 201218 |
Incomparable regimen |
|
Luaces 201019 |
Incomparable regimen |
|
Lacasa 200720 |
Incomparable regimen |
|
Jensen 199021 |
Incomparable regimen |
|
Boffi 199222 |
Duplicate of Gazzaniga 1992 |
|
Gazzaniga 199223 |
Incomparable regimen |
|
Mathur 201324 |
Incomparable regimen |
|
Kaczmarzyk 200725 |
Did not address study question |
|
Vargas-Mena 201226 |
Not an RCT |
|
Wu 199827 |
Did not address study question |
|
Ahmadi 200528 |
Did not address study question |
|
Morimoto 199829 |
Did not address study question |
|
Morimoto 199330 |
Not retrievable |
|
Hashizume 200431 |
Incomparable regimen |
|
Bonzanini 199332 |
Did not address study question |
|
Fukushima 201433 |
Congress abstract |
|
Badia 201134 |
Congress abstract |
|
Hashimoto 201435 |
Congress abstract |
|
Ijarotimi 201336 |
Not retrievable |
|
Shakya 201037 |
Not retrievable |
|
Ko 201038 |
Not retrievable |
|
Rajshekhar 200939 |
Congress abstract |
|
Patacchiola 200040 |
Did not address study question |
|
Urbanetz 199441 |
Not retrievable |
|
Cartana 199042 |
Not retrievable |
|
Ali 200643 |
Congress abstract |
|
Ricart-Hoffiz 201144 |
Congress abstract |
|
Rolle 199045 |
Not retrievable |
|
Orlando 201046 |
Congress abstract |
|
Navarro 199547 |
Did not address study question |
|
Lee 201248 |
Not retrievable |
|
Cheshani 201549 |
Not retrievable |
|
Ali 201250 |
Not retrievable |
|
Seker 201151 |
Not retrievable |
|
Bencini 199452 |
Not retrievable |
|
Lindeboom 200553 |
Did not address study question |
|
Marcucci 199054 |
Not retrievable |
|
Shahid 200755 |
Did not address study question |
|
Cuthbertson 199156 |
Did not address study question |
|
Akgur 199257 |
Did not address study question |
|
Garcia 201758 |
Did not address study question |
|
Ghosh 201759 |
Congress abstract |
|
Habibi 201660 |
Congress abstract |
|
Phillips 201661 |
Congress abstract |
|
Samson 201762 |
Congress abstract |
|
Chen 201863 |
Not retrievable |
|
Yalagachin 201864 |
Did not address study question |
|
Kate 202065 |
Congress abstract |
|
Mohammed 202066 |
Incomparable regimen |
|
Sabbagh 202067 |
Study protocol |
|
Togo 202068 |
Did not address study question |
|
Guler 201969 |
Incomparable regimen |
|
Han-Gyu 201970 |
Incomparable regimen |
|
Kirby 201971 |
Incomparable regimen |
|
Knight72 |
No surgery |
|
Nagata 201973 |
Study protocol |
|
Nitrushwa 201974 |
Congress abstract |
|
Omar 201975 |
Did not address study question |
|
Posillico 201976 |
Not an RCT |
|
Shigemura 201977 |
Study protocol |
|
Speich 201978 |
Study protocol |
|
Shah 201679 |
Conference abstract |
|
Griffiths 201880 |
Study protocol |
|
Knight 201881 |
Study protocol |
|
Montravers 201882 |
Did not address study question |
|
Yang 201783 |
Not an RCT |
|
Chen 201884 |
Not retrievable |
|
Shkedy 201885 |
Did not address study question |
|
Sittirai86 |
Did not address study question |
|
Van Oostveen87 |
Study protocol |
|
Yates 201888 |
Not an RCT |
|
Backes 201789 |
Did not address study question |
|
Vathul 201790 |
Conference abstract |
|
References 1. Kumar A, Patodia M, Pandove PK, Sharda VK, Pahwa S. Role of antibiotic prophylaxis in laparoscopic cholecystectomy: A randomized prospective study. Journal International Medical Sciences Academy 2013; 26(4): 209-11. 2. Ahn BK, Lee KH. Single-dose antibiotic prophylaxis is effective enough in colorectal surgery. ANZ Journal of Surgery 2013; 83(9): 641-5. 3. Fonseca SN, Kunzle SR, Junqueira MJ, Nascimento RT, de Andrade JI, Levin AS. Implementing 1-dose antibiotic prophylaxis for prevention of surgical site infection. Arch Surg 2006; 141(11): 1109-13; discussion 14. 4. Sevin A, Senen D, Sevin K, Erdogan B, Orhan E. Antibiotic use in abdominoplasty: prospective analysis of 207 cases. Journal of Plastic, Reconstructive and Aesthetic Surgery 2007; 60(4): 379-82. 5. Han JH, Jeong O, Ryu SY, Jung MR, Park YK. Efficacy of single-dose antimicrobial prophylaxis for preventing surgical site infection in radical gastrectomy for gastric carcinoma. Journal of Gastric Cancer 2014; 14(3): 156-63. 6. Farran L, Llop J, Sans M, et al. Efficacy of enteral decontamination in the prevention of anastomotic dehiscence and pulmonary infection in esophagogastric surgery. Dis Esophagus 2008; 21(2): 159-64. 7. Schardey HM, Joosten U, Finke U, et al. The prevention of anastomotic leakage after total gastrectomy with local decontamination. A prospective, randomized, double-blind, placebo-controlled multicenter trial. Ann Surg 1997; 225(2): 172-80. 8. Vu LT, Vittinghoff E, Nobuhara KK, Farmer DL, Lee H. Surgical site infections in neonates and infants: Is antibiotic prophylaxis needed for longer than 24 h? Pediatric Surgery International 2014; 30(6): 587-92. 9. Basoli A, Chirletti P, Cirino E, et al. A prospective, double-blind, multicenter, randomized trial comparing ertapenem 3 Vs 5 days in community-acquired intraabdominal infection. Journal of Gastrointestinal Surgery 2008; 12(3): 592-600. 10. Safdar CA, Hashmi MA. Antibiotic prophylaxis in paediatric surgery. JPMA The Journal of the Pakistan Medical Association 1992; 42(12): 286-8. 11. Gidiri MF, Ziruma A. A randomized clinical trial evaluating prophylactic single-dose vs prolonged course of antibiotics for caesarean section in a high HIV-prevalence setting. Journal of Obstetrics and Gynaecology 2014; 34(2): 160-4. 12. Kato Y, Shime N, Hashimoto S, et al. Effects of controlled perioperative antimicrobial prophylaxis on infectious outcomes in pediatric cardiac surgery. Critical care medicine 2007; 35(7): 1763-8. 13. A WD, Toksvig-Larsen S. Infection prophylaxis: A prospective study in 106 patients operated on by tibial osteotomy using the hemicallotasis technique. Archives of Orthopaedic and Trauma Surgery 2006; 126(7): 441-7. 14. Kakimaru H, Kono M, Matsusaki M, Iwata A, Uchio Y. Postoperative antimicrobial prophylaxis following spinal decompression surgery: Is it necessary? Journal of Orthopaedic Science 2010; 15(3): 305-9. 15. Kato D, Maezawa K, Yonezawa I, et al. Randomized prospective study on prophylactic antibiotics in clean orthopedic surgery in one ward for 1 year. Journal of Orthopaedic Science 2006; 11(1): 20-7. 16. Pedrini L, Pisano E, Sensi L, et al. Prophylaxis of vascular graft infection: Long-term results of a prospective study. Italian Journal of Vascular and Endovascular Surgery 2005; 12(4): 117-27. 17. Righi M, Manfredi R, Farneti G, Pasquini E, Romei Bugliari D, Cenacchi V. Clindamycin/cefonicid in head and neck oncologic surgery: one-day prophylaxis is as effective as a three-day schedule. Journal of chemotherapy (Florence, Italy) 1995; 7(3): 216-20. 18. Adde CA, Soares MS, Romano MM, et al. Clinical and surgical evaluation of the indication of postoperative antibiotic prescription in third molar surgery. Oral Surgery, Oral Medicine, Oral Pathology and Oral Radiology 2012; 114(SUPPL. 5): S26-S31. 19. Luaces-Rey R, Arenaz-Bua J, Lopez-Cedrun-Cembranos JL, Martinez-Roca C, Pertega-Diaz S, Sironvalle-Soliva S. Efficacy and safety comparison of two amoxicillin administration schedules after third molar removal. A randomized, double-blind and controlled clinical trial. Medicina Oral, Patologia Oral y Cirugia Bucal 2010; 15(4): e633-e8. 20. Lacasa JM, Jimenez JA, Ferras V, et al. Prophylaxis versus pre-emptive treatment for infective and inflammatory complications of surgical third molar removal: a randomized, double-blind, placebo-controlled, clinical trial with sustained release amoxicillin/clavulanic acid (1000/62.5 mg). International Journal of Oral and Maxillofacial Surgery 2007; 36(4): 321-7. 21. Jensen LS, Andersen A, Fristrup SC, et al. Comparison of one dose versus three doses of prophylactic antibiotics, and the influence of blood transfusion, on infectious complications in acute and elective colorectal surgery. British Journal of Surgery 1990; 77(5): 513-8. 22. Boffi L, Panebianco R. [A comparative study of 2 schedules of antibiotic prophylaxis using ceftazidime in the prevention of infections in elective surgery of the biliary surgery. Preliminary results]. Clinica terapeutica 1992; 140(3): 265-71. 23. Gazzaniga M, Chiodo G, Boffi L, Panebianco R, Fostini R. Prevention of infections in elective biliary tract surgery. Current therapeutic research, clinical and experimental 1992; 52(6): 935-43. 24. Mathur P, Trikha V, Farooque K, et al. Implementation of a short course of prophylactic antibiotic treatment for prevention of postoperative infections in clean orthopaedic surgeries. Indian Journal of Medical Research 2013; 137(1): 111-6. 25. Kaczmarzyk T, Wichlinski J, Stypulkowska J, Zaleska M, Panas M, Woron J. Single-dose and multi-dose clindamycin therapy fails to demonstrate efficacy in preventing infectious and inflammatory complications in third molar surgery. International journal of oral and maxillofacial surgery 2007; 36(5): 417-22. 26. Vargas-Mena R, Arredondo-Gomez E, Pavia-Carrillo EF. [Effect of a short antimicrobial prophylaxis regimen on the prevalence of postoperative infection in elective orthopedics and traumatology surgery]. Acta ortopedica mexicana 2012; 26(6): 369-74. 27. Wu CC, Yeh DC, Lin MC, Liu TJ, P'Eng F K. Prospective randomized trial of systemic antibiotics in patients undergoing liver resection. The British journal of surgery 1998; 85(4): 489-93. 28. Ahmadi AH, Cohen BE, Shayani P. A prospective study of antibiotic efficacy in preventing infection in reduction mammaplasty. Plastic and reconstructive surgery 2005; 116(1): 126-31. 29. Morimoto K, Kinoshita H. Once-daily use of ofloxacin for prophylaxis in breast cancer surgery. Chemotherapy 1998; 44(2): 135-41. 30. Morimoto K, Nakatani S, Sasaki Y, Kinoshita H. [Prospective randomized study on effect of duration of antimicrobial prophylaxis for mastectomy]. JpnJAntibiot 1993; 46(5): 404-10. 31. Hashizume T, Nishizawa R, Aizawa S, et al. Clinical Study of Using Prophylactic Antibiotics and Chemical Preparation for Elective Operation of Colorectal Cancer. [Japanese]. Japanese Journal of Gastroenterological Surgery 2004; 37(4): 375-83. 32. Bonzanini C, Ubiali P, Invernizzi R. [The use of piperacillin in the preoperative prophylaxis of colorectal surgery]. Minerva Chir 1993; 48(23-24): 1437-43. 33. Fukushima R, Konishi T, Mohri Y, et al. A prospective randomized study to assess the optimal duration of antimicrobial prophylaxis in total gastrectomy. Surgical infections 2014; 15: S-11. 34. Badia-Perez JM, Jimeno J, Aldeano A, et al. Randomised trial of a short course of postoperative antibiotic therapy in low-risk acute cholecystitis. Surgical infections 2011; 12 (2): A2-A3. 35. Hashimoto M, Kobayashi T, Ohdan H, et al. A randomised clinical trial to determine the period of antimicrobial prophylaxis administration after hepatocellular carcinoma surgery. Surgical infections 2014; 15 (3): A8. 36. Ijarotimi AO, Badejoko OO, Ijarotimi O, Loto OM, Orji EO, Fasubaa OB. Comparison of short versus long term antibiotic prophylaxis in elective caesarean section at the Obafemi Awolowo University Teaching Hospitals Complex, Ile-Ife, Nigeria. The Nigerian postgraduate medical journal 2013; 20(4): 325-30. 37. Shakya A, Sharma J. Comparison of single versus multiple doses of antibiotic prophylaxis in reducing post-elective caesarean section infectious morbidity. Kathmandu University Medical Journal 2010; 8(30): 179-84. 38. Ko JK, Cho YK, Yang HJ, Park CW, Park JS, Jun JK. A prospective multicenter randomized study on prophylactic antibiotics use in cesarean section performed at tertiary center. Korean Journal of Obstetrics and Gynecology 2010; 53(3): 227-34. 39. Rajshekhar S, Shetty J, Kumar P. Evaluation of short term antibiotic prophylaxis for emergency caesarean delivery. International Journal of Gynecology and Obstetrics 2009; 107: S313-S4. 40. Patacchiola F, Paolantonio L, Palermo P, Stefano L, Mascaretti G, Moscarini M. [Antibiotic prophylaxis of infective complications after cesarean section. Our experience]. Minerva ginecologica 2000; 52(10): 385-9. 41. Urbanetz AA, Lobo David G, De Deus Bueno JA, De Oliveira Marques L, De Oliveira LJ. Antibiotics in infectious prophylaxis in abdominal hysterectomy. [Portuguese]. Jornal Brasileiro de Ginecologia 1994; 104(8): 263-7. 42. Cartañá J, Yárnoz MC, Ruiz de Gopegui RM, Mascaró M, Cortés J. [Antibiotic prophylaxis with piperacillin in vaginal hysterectomy. Study of 1 dose versus 3 doses]. Enfermedades infecciosas y microbiología clínica 1990; 8(4): 218-21. 43. Ali M, Raza A. Role of single dose antibiotic prophylaxis in clean orthopedic surgery. J Coll Physicians Surg Pak 2006; 16(1): 45-8. 44. Ricart-Hoffiz P, Takemoto R, Park J, et al. Prospective, randomized study of surgical site infections with the use of perioperative antibiotics for 24 hours versus the duration of a drain after spinal surgery. Spine Journal 2011; 1): 23S. 45. Rolle A, Thetter O, Hallfeldt K, Mandelkow H, Schweiberer L. [Perioperative preventive use of antibiotics in thoracic surgery--results of a controlled randomized study with optocillin]. Pneumologie (Stuttgart, Germany) 1990; 44 Suppl 1: 291-2. 46. Orlando G, Manzia TM, Sorge R, et al. One-shot versus multidose perioperative antibiotic prophilaxis after kidney transplantation: Preliminary results from a prospective randomized multicenter study. American Journal of Transplantation 2010; 10: 325. 47. Navarro M, Scola E, Scola B, Ortiz P, Martinez T, Vega MF. [Prophylactic antibiotic therapy with amoxicillin-clavulanic acid in oropharyngeal surgery]. Acta OtorrinolaringolEsp 1995; 46(1): 41-4. 48. Lee JW, Lee JY, Kim SM, Kim MJ, Lee JH. Prophylactic antibiotics in intra-oral bone grafting procedures: a prospective, randomized, double-blind clinical trial. Journal of the Korean Association of Oral and Maxillofacial Surgeons 2012; 38(2): 90-5. 49. Cheshani MI, Hosseini G, Mostafavi-Toroghi H, Hakemi A, Eghbali K. Comparison of Perioperative Prophylactic Antibiotic Protocols in Preventing the Infectious Complications after Open Prostatectomy. International Medical Journal 2015; 22(1): 33-5 3p. 50. Ali M, Nadeem M, Shah SZA, Khan MM, Ahmad M, Ullah MA. Prolonged versus short course of Antibiotic prophylaxis in clean general surgery. Journal of Medical Sciences (Peshawar) 2012; 20(3): 128-32. 51. Seker D, Ugurlu C, Ergul Z, Akinci M, Olcucuoglu E, Kulacoglu H. Single dose prophylactic antibiotics may not be sufficient in elective pilonidal sinus surgery: An early terminated study. Turkiye Klinikleri Journal of Medical Sciences 2011; 31(1): 186-90. 52. Bencini PL, Signorini M, Galimberti M, Cavicchini S, Caputo R. Preoperative antibiotic prophylaxis in flexural surgery of difficult contamination-prone areas of the skin: The utility of a single dose of antibiotic. Journal of Dermatological Treatment 1994; 5(1): 17-9. 53. Lindeboom JA, Frenken JW, Valkenburg P, van den Akker HP. The role of preoperative prophylactic antibiotic administration in periapical endodontic surgery: a randomized, prospective double-blind placebo-controlled study. IntEndodJ 2005; 38(12): 877-81. 54. Marcucci L, Vellucci A, Miani P, et al. Antibiotic prophylaxis in ear, nose and throat surgery: a comparison of a single preoperative dose with three peri-operative doses of ceftazidime. Journal of hospital infection 1990; 15 Suppl A: 81-5. 55. Shahid U, Arain MA, Dar MI, Khan AB, Aftab S, Manan AU. The role of long-term antibiotics in the prevention of infection in postoperative cardiac surgeries. Journal of College of Physicians and Surgeons Pakistan 2007; 17(7): 394-7. 56. Cuthbertson AM, McLeish AR, Penfold JCB, Ross H. A comparison between single and double dose intravenous timentin for the prophylaxis of wound infection in elective colorectal surgery. Diseases of the Colon and Rectum 1991; 34(2): 151-5. 57. Akgur FM, Tanyel FC, Buyukpamukcu N, Hicsonmez A. Prophylactic antibiotics for colostomy closure in children: Short versus long course. Pediatric Surgery International 1992; 7(4): 279-81. 58. Garcia EDS, Veiga DF, Veiga-Filho J, et al. Postoperative antibiotic use in breast reduction: Preliminary results of a randomized controlled trial. Journal of Women's Health 2017; 26 (4): A54. 59. Ghosh P, Agrawal A, Regmi M. Single dose versus multiple dose antibiotic regimens in elective gynaecologic surgery: Results of a clinical controlled trial. Journal of Obstetrics and Gynaecology Research 2017; 43: 90-1. 60. Habibi Z, Nejat F. Single dose versus multiple dose perioperative prophylactic antibiotics in post-operative wound infection in pediatric cranial procedures with implanted skull materials: A single center clinical trial. Child's Nervous System 2016; 32 (10): 2025. 61. Phillips B, Fourman M, Bishawi M, et al. Are prophylactic postoperative antibiotics necessary for immediate breast reconstruction? Results of a prospective randomized clinical trial. Journal of the American College of Surgeons, 2016. http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/547/CN-01158547/frame.html (accessed. 62. Samson P, Gaunay G, Derisavifard S, et al. Prospective randomized trial of antibiotic prophylaxis duration for percutaneous nephrolithotomy in low-risk patients: Preliminary results. Journal of Endourology 2017; 31 (Supplement 2): A76-A7. 63. Chen J, Huang LG, Hu XJ. [The study of the rational use of antibiotics after nasal surgery]. Lin chuang er bi yan hou tou jing wai ke za zhi = Journal of clinical otorhinolaryngology, head, and neck surgery 2018; 32(13): 998-1001. 64. Yalagachin GH, Raman P, Huchchannavar S. A randomized controlled study of single-dose antibiotic prophylaxis for clean surgeries. Infectious Diseases in Clinical Practice 2018; 26(1): 39-44. 65. Kate V. Comparison of short course antimicrobial therapy vs. conventional antimicrobial therapy in patients with complicated intra-abdominal infections- a randomized controlled trial. Gastroenterology 158.6 (2020): S-1497 66. Mohammed SO, A Shuaibu SD, Gaya SA, Rabiu A. The efficacy of two doses versus 7 days' course of prophylactic antibiotics following cesarean section: An experience from Aminu Kano Teaching Hospital. Ann Afr Med. 2020;19(2):103-112. 67. Sabbagh C, Siembida N, Dupont H, et al. The value of post-operative antibiotic therapy after laparoscopic appendectomy for complicated acute appendicitis: a prospective, randomized, double-blinded, placebo-controlled phase III study (ABAP study). Trials. 2020;21(1):451. 68. Togo Y, Fukui K, Ueda Y, et al. Comparison of single- and multiple-dose cefazolin as prophylaxis for transurethral enucleation of prostate: A multicenter, prospective, randomized controlled trial by the Japanese Research Group for Urinary Tract Infection. Int J Urol. 2020;27(3):244-248. 69. Guler Y, Karabulut Z, Sengul S, Calis H. The effect of antibiotic prophylaxis on wound infections after laparoscopic cholecystectomy: A randomised clinical trial. Int Wound J. 2019;16(5):1164-1170. 70. Cha HG, Kwon JG, Han HH, Eom JS, Kim EK. Appropriate Prophylactic Antibiotic Use in Clean Wound Surgery Under Local Anesthesia. J Korean Med Sci. 2019;34(17):e135. 71. Kirby A, Asín-Prieto E, Burns FA, et al. Colo-Pro: a pilot randomised controlled trial to compare standard bolus-dosed cefuroxime prophylaxis to bolus-continuous infusion-dosed cefuroxime prophylaxis for the prevention of infections after colorectal surgery. Eur J Clin Microbiol Infect Dis. 2019;38(2):357-363. 72. Knight M, Chiocchia V, Partlett C, et al. Prophylactic antibiotics in the prevention of infection after operative vaginal delivery (ANODE): a multicentre randomised controlled trial [published correction appears in Lancet. 2019 Jun 15;393(10189):2394]. Lancet. 2019;393(10189):2395-2403. 73. Nagata K, Yamada K, Shinozaki T, et al. Non-inferior comparative study comparing one or two day antimicrobial prophylaxis after clean orthopaedic surgery (NOCOTA study): a study protocol for a cluster pseudo-randomized controlled trial comparing duration of antibiotic prophylaxis. BMC Musculoskelet Disord. 2019;20(1):533. 74. Nitrushwa D. " Single vs. extended antibiotics for prevention of surgical infection in emergent cesarean delivery in Rwanda." Am J Obstet Gynecol. 220.1 (2019): S414-S415. 75. Omar M, Selim M, El Sherif E, et al. Ciprofloxacin infusion versus third generation cephalosporin as a surgical prophylaxis for percutaneous nephrolithotomy: a randomized study. Cent European J Urol. 2019;72(1):57-61. 76. Posillico SE, Young BT, Ladhani HA, Zosa BM, Claridge JA. Current Evaluation of Antibiotic Usage in Complicated Intra-Abdominal Infection after the STOP IT Trial: Did We STOP IT?. Surg Infect (Larchmt). 2019;20(3):184-191. 77. Shigemura K, Yamamichi F, Nishimoto K, Kitagawa K, Fujisawa M. Protocol for a comparison study of 1-day (single dose) versus 2-day prophylactic antibiotic administration in Holmium Laser enucleation of the prostate (HoLEP): a randomized controlled trial. F1000Res. 2019;8:161. 78. Speich B, Bausch K, Roth JA, et al. Single-dose versus 3-day cotrimoxazole prophylaxis in transurethral resection or greenlight laser vaporisation of the prostate: study protocol for a multicentre randomised placebo controlled non-inferiority trial (CITrUS trial). Trials. 2019;20(1):142. 79. Shah P, Rosenberger L, Guidry C, Sawyer R. Impact of extended versus short course peri-operative antibiotics on surgical site infection in liver transplant patients. Surg Infect (Larchmt). 2016;17 Suppl 1:S1-S42. 80. Griffiths L, Samson P, Derisavifard S, Gaunay G, Leavitt DA. Prospective randomized trial of antibiotic prophylaxis duration for percutaneous nephrolithotomy in low-risk patients. J Endourol. 32.S2 (2018): P1-A573. 81. Knight M, Mottram L, Gray S, Partlett C, Juszczak E; ANODE collaborative group. Prophylactic antibiotics for the prevention of infection following operative vaginal delivery (ANODE): study protocol for a randomised controlled trial. Trials. 2018;19(1):395. 82. Montravers P, Tubach F, Lescot T, et al. Short-course antibiotic therapy for critically ill patients treated for postoperative intra-abdominal infection: the DURAPOP randomised clinical trial. Intensive Care Med. 2018;44(3):300-310. 83. Yang S, Liu G, Tang D, Cai D. Evaluation Intravenous Drip Cephazolin Prophylaxis of Breast Cancer Surgery Site Infection. J Craniofac Surg. 2017;28(6):e527-e531. 84. Chen J, Huang LG, Hu XJ. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2018;32(13):998-1001. 85.Shkedy Y, Stern S, Nachalon Y, et al. Antibiotic prophylaxis in clean head and neck surgery: A prospective randomised controlled trial. Clin Otolaryngol. 2018;43(6):1508-1512. 86. Sittitrai P, Siriwittayakorn C. Perioperative antibiotic prophylaxis in open tracheostomy: A preliminary randomized controlled trial. Int J Surg. 2018;54(Pt A):170-175. 87. van Oostveen RB, Romero-Palacios A, Whitlock R, et al. Prevention of Infections in Cardiac Surgery study (PICS): study protocol for a pragmatic cluster-randomized factorial crossover pilot trial [published correction appears in Trials. 2019 Oct 16;20(1):595]. Trials. 2018;19(1):688. 88. Yates AJ Jr; American Association of Hip and Knee Surgeons Evidence-Based Medicine Committee. Postoperative prophylactic antibiotics in total joint arthroplasty. Arthroplast Today. 2018;4(1):130-131. 89. Backes M, Dingemans SA, Dijkgraaf MGW, et al. Effect of Antibiotic Prophylaxis on Surgical Site Infections Following Removal of Orthopedic Implants Used for Treatment of Foot, Ankle, and Lower Leg Fractures: A Randomized Clinical Trial [published correction appears in JAMA. 2018 Mar 13;319(10 ):1051]. JAMA. 2017;318(24):2438-2445. 90. Vathul B, Pari M. Is antibiotic prophylaxis for open thyroidectomies necessary? a randomized trial in south indian population. Clujul Med. 2017;90;S88.
|
|
Verantwoording
Beoordelingsdatum en geldigheid
Laatst beoordeeld : 01-12-2024
Algemene gegevens
De ontwikkeling/herziening van deze richtlijnmodule werd ondersteund door het Kennisinstituut van de Federatie Medisch Specialisten (www.demedischspecialist.nl/kennisinstituut) en werd gefinancierd uit de Kwaliteitsgelden Medisch Specialisten (SKMS). De financier heeft geen enkele invloed gehad op de inhoud van de richtlijnmodule.
Samenstelling werkgroep
Voor het ontwikkelen van de richtlijnmodules 2 tot 16 is in 2020 op initiatief van de NVvH een multidisciplinaire werkgroep ingesteld, bestaande uit vertegenwoordigers van alle relevante specialismen (zie hiervoor de Samenstelling van de werkgroep) die betrokken zijn bij de zorg voor preventie van postoperatieve wondinfecties. Daarnaast is in 2022 op initiatief van het Samenwerkingsverband Richtlijnen Infectiepreventie (SRI) een separate multidisciplinaire werkgroep samengesteld voor de herziening van de WIP-richtlijn over postoperatieve wondinfecties: module 17-22. De ontwikkelde modules van beide werkgroepen zijn in deze richtlijn samengevoegd.
Belangenverklaringen
De Code ter voorkoming van oneigenlijke beïnvloeding door belangenverstrengeling is gevolgd. Alle werkgroepleden hebben schriftelijk verklaard of zij in de laatste drie jaar directe financiële belangen (betrekking bij een commercieel bedrijf, persoonlijke financiële belangen, onderzoek financiering) of indirecte belangen (persoonlijke relaties, reputatiemanagement) hebben gehad. Gedurende de ontwikkeling of herziening van een module worden wijzigingen in belangen aan de voorzitter doorgegeven. De belangenverklaring wordt opnieuw bevestigd tijdens de commentaarfase.
Een overzicht van de belangen van werkgroepleden en het oordeel over het omgaan met eventuele belangen vindt u in onderstaande tabel. De ondertekende belangenverklaringen zijn op te vragen bij het secretariaat van het Kennisinstituut van de Federatie Medisch Specialisten.
|
Werkgroeplid |
Functie |
Nevenfuncties |
Gemelde belangen |
Ondernomen actie |
|
Mevr. prof. dr. M.A. Boermeester |
Chirurg |
* Medisch Ethische Commissie, Amsterdam UMC, locatie AMC * Antibiotica Commissie, Amsterdam UMC |
Persoonlijke financiële belangen Hieronder staan de beroepsmatige relaties met bedrijfsleven vermeld waarbij eventuele financiële belangen via de AMC Research B.V. lopen, dus institutionele en geen persoonlijke gelden zijn: Skillslab instructeur en/of spreker (consultant) voor KCI/3M, Smith&Nephew, Johnson&Johnson, Gore, BD/Bard, TELABio, GDM, Medtronic, Molnlycke.
Persoonlijke relaties Geen.
Extern gefinancierd onderzoek Institutionele grants van KCI/3M, Johnson&Johnson en New Compliance.
Intellectuele belangen en reputatie Ik maak me sterk voor een 100% evidence-based benadering van maken van aanbevelingen, volledig transparant en reproduceerbaar. Dat is mijn enige belang in deze, geen persoonlijk gewin.
Overige belangen Geen.
|
Extra kritische commentaarronde. |
|
Dhr. dr. M.J. van der Laan |
Vaatchirurg |
Vice voorzitter Consortium Kwaliteit van Zorg NFU, onbetaald
|
Persoonlijke financiële belangen Geen.
Persoonlijke relaties Geen.
Extern gefinancierd onderzoek Geen.
Intellectuele belangen en reputatie Geen.
Overige belangen Geen.
|
Geen.
|
|
Dhr. dr. W.C. van der Zwet |
Arts-microbioloog |
Lid Regionaal Coördinatie Team, Limburgs infectiepreventie & ABR Zorgnetwerk (onbetaald) |
||
|
Dhr. dr. D.R. Buis |
Neurochirurg |
Lid Hoofdredactieraad Tijdschrift voor Neurologie & Neurochirurgie - onbetaald |
||
|
Dhr. dr. J.H.M. Goosen |
Orthopaedisch Chirurg |
Inhoudelijke presentaties voor Smith&Nephew en Zimmer Biomet. Deze worden vergoed per uur. |
||
|
Mw. drs. H. Jalalzadeh |
Arts-onderzoeker |
Geen. |
Persoonlijke financiële belangen Geen.
Persoonlijke relaties Geen.
Extern gefinancierd onderzoek Geen.
Intellectuele belangen en reputatie Geen.
Overige belangen Geen. |
Geen.
|
|
Dhr. dr. N. Wolfhagen |
AIOS chirurgie |
|||
|
Mw. drs. H. Groenen |
Arts-onderzoeker |
|||
|
Dhr. dr. F.F.A. Ijpma |
Traumachirurg |
|||
|
Dhr. dr. P. Segers |
Cardiothoracaal chirurg |
|||
|
Mw. Y.E.M. Dreissen |
AIOS neurochirurgie |
|||
|
Dhr. R.R. Schaad |
Anesthesioloog |
Inbreng patiëntenperspectief
Er werd aandacht besteed aan het patiëntenperspectief door uitnodigen van de Patiëntenfederatie Nederland voor de invitational conference. De verkregen input is meegenomen bij het opstellen van de uitgangsvragen, de keuze voor de uitkomstmaten en bij het opstellen van de overwegingen. De conceptmodules zijn tevens voor commentaar voorgelegd aan de Patiëntenfederatie Nederland en de eventueel aangeleverde commentaren zijn bekeken en verwerkt. Voor de modules 17-22 was de patiëntfederatie vertegenwoordigd in de werkgroep.
Wkkgz & Kwalitatieve raming van mogelijke substantiële financiële gevolgen
Bij de richtlijn is conform de Wet kwaliteit, klachten en geschillen zorg (Wkkgz) een kwalitatieve raming uitgevoerd of de aanbevelingen mogelijk leiden tot substantiële financiële gevolgen. Bij het uitvoeren van deze beoordeling zijn richtlijnmodules op verschillende domeinen getoetst (zie het stroomschema op de Richtlijnendatabase).
Uit de kwalitatieve raming blijkt dat er waarschijnlijk geen substantiële financiële gevolgen zijn.
Voor module 8 (Negatieve druktherapie) geldt dat uit de toetsing volgt dat de aanbeveling(en) breed toepasbaar zijn (5.000 - 40.000 patiënten). Tevens volgt uit de toetsing dat het geen nieuwe manier van zorgverlening of andere organisatie van zorgverlening betreft. Er worden daarom geen substantiële financiële gevolgen verwacht.
Voor de overige modules en aanbevelingen geldt dat uit de toetsing volgt dat de aanbeveling(en) breed toepasbaar zijn (>40.000 patiënten). Tevens volgt uit de toetsing dat het overgrote deel (±90%) van de zorgaanbieders en zorgverleners al aan de norm voldoet en het geen nieuwe manier van zorgverlening of andere organisatie van zorgverlening betreft. Ook wordt geen toename in het aantal in te zetten voltijdsequivalenten aan zorgverleners verwacht of een wijziging in het opleidingsniveau van zorgpersoneel. Er worden daarom geen substantiële financiële gevolgen verwacht.
Methode ontwikkeling
Evidence based
Implementatie
Zie voor de implementatie het implementatieplan in het tabblad 'Bijlagen'.
Werkwijze
AGREE
Deze richtlijnmodule is opgesteld conform de eisen vermeld in het rapport Medisch Specialistische Richtlijnen 2.0 van de adviescommissie Richtlijnen van de Raad Kwaliteit. Dit rapport is gebaseerd op het AGREE II instrument (Appraisal of Guidelines for Research & Evaluation II; Brouwers, 2010).
Knelpuntenanalyse en uitgangsvragen
Tijdens de voorbereidende fase inventariseerde de werkgroepen de knelpunten in de zorg voor patiënten die chirurgie ondergaan. Tevens zijn er knelpunten aangedragen door middel van een invitational conference. De verslagen hiervan zijn opgenomen onder aanverwante producten.
Op basis van de uitkomsten van de knelpuntenanalyse zijn door de werkgroep concept-uitgangsvragen opgesteld en definitief vastgesteld.
Uitkomstmaten
Na het opstellen van de zoekvraag behorende bij de uitgangsvraag inventariseerde de werkgroep welke uitkomstmaten voor de patiënt relevant zijn, waarbij zowel naar gewenste als ongewenste effecten werd gekeken. Hierbij werd een maximum van acht uitkomstmaten gehanteerd. De werkgroep waardeerde deze uitkomstmaten volgens hun relatieve belang bij de besluitvorming rondom aanbevelingen, als cruciaal (kritiek voor de besluitvorming), belangrijk (maar niet cruciaal) en onbelangrijk. Tevens definieerde de werkgroep tenminste voor de cruciale uitkomstmaten welke verschillen zij klinisch (patiënt) relevant vonden.
Methode literatuursamenvatting
Een uitgebreide beschrijving van de strategie voor zoeken en selecteren van literatuur is te vinden onder ‘Zoeken en selecteren’ onder Onderbouwing. Indien mogelijk werd de data uit verschillende studies gepoold in een random-effects model. De beoordeling van de kracht van het wetenschappelijke bewijs wordt hieronder toegelicht.
Beoordelen van de kracht van het wetenschappelijke bewijs
De kracht van het wetenschappelijke bewijs werd bepaald volgens de GRADE-methode. GRADE staat voor ‘Grading Recommendations Assessment, Development and Evaluation’ (zie http://www.gradeworkinggroup.org/). De basisprincipes van de GRADE-methodiek zijn: het benoemen en prioriteren van de klinisch (patiënt) relevante uitkomstmaten, een systematische review per uitkomstmaat, en een beoordeling van de bewijskracht per uitkomstmaat op basis van de acht GRADE-domeinen (domeinen voor downgraden: risk of bias, inconsistentie, indirectheid, imprecisie, en publicatiebias; domeinen voor upgraden: dosis-effect relatie, groot effect, en residuele plausibele confounding).
GRADE onderscheidt vier gradaties voor de kwaliteit van het wetenschappelijk bewijs: hoog, redelijk, laag en zeer laag. Deze gradaties verwijzen naar de mate van zekerheid die er bestaat over de literatuurconclusie, in het bijzonder de mate van zekerheid dat de literatuurconclusie de aanbeveling adequaat ondersteunt (Schünemann, 2013; Hultcrantz, 2017).
|
GRADE |
Definitie |
|
Hoog |
|
|
Redelijk |
|
|
Laag |
|
|
Zeer laag |
|
Bij het beoordelen (graderen) van de kracht van het wetenschappelijk bewijs in richtlijnen volgens de GRADE-methodiek spelen grenzen voor klinische besluitvorming een belangrijke rol (Hultcrantz, 2017). Dit zijn de grenzen die bij overschrijding aanleiding zouden geven tot een aanpassing van de aanbeveling. Om de grenzen voor klinische besluitvorming te bepalen moeten alle relevante uitkomstmaten en overwegingen worden meegewogen. De grenzen voor klinische besluitvorming zijn daarmee niet één op één vergelijkbaar met het minimaal klinisch relevant verschil (Minimal Clinically Important Difference, MCID). Met name in situaties waarin een interventie geen belangrijke nadelen heeft en de kosten relatief laag zijn, kan de grens voor klinische besluitvorming met betrekking tot de effectiviteit van de interventie bij een lagere waarde (dichter bij het nuleffect) liggen dan de MCID (Hultcrantz, 2017).
Overwegingen (van bewijs naar aanbeveling)
Om te komen tot een aanbeveling zijn naast (de kwaliteit van) het wetenschappelijke bewijs ook andere aspecten belangrijk en worden meegewogen, zoals aanvullende argumenten uit bijvoorbeeld de biomechanica of fysiologie, waarden en voorkeuren van patiënten, kosten (middelenbeslag), aanvaardbaarheid, haalbaarheid en implementatie. Deze aspecten zijn systematisch vermeld en beoordeeld (gewogen) onder het kopje ‘Overwegingen’ en kunnen (mede) gebaseerd zijn op expert opinion. Hierbij is gebruik gemaakt van een gestructureerd format gebaseerd op het evidence-to-decision framework van de internationale GRADE Working Group (Alonso-Coello, 2016a; Alonso-Coello 2016b). Dit evidence-to-decision framework is een integraal onderdeel van de GRADE methodiek.
Formuleren van aanbevelingen
De aanbevelingen geven antwoord op de uitgangsvraag en zijn gebaseerd op het beschikbare wetenschappelijke bewijs en de belangrijkste overwegingen, en een weging van de gunstige en ongunstige effecten van de relevante interventies. De kracht van het wetenschappelijk bewijs en het gewicht dat door de werkgroep wordt toegekend aan de overwegingen, bepalen samen de sterkte van de aanbeveling. Conform de GRADE-methodiek sluit een lage bewijskracht van conclusies in de systematische literatuuranalyse een sterke aanbeveling niet a priori uit, en zijn bij een hoge bewijskracht ook zwakke aanbevelingen mogelijk (Agoritsas, 2017; Neumann, 2016). De sterkte van de aanbeveling wordt altijd bepaald door weging van alle relevante argumenten tezamen. De werkgroep heeft bij elke aanbeveling opgenomen hoe zij tot de richting en sterkte van de aanbeveling zijn gekomen.
In de GRADE-methodiek wordt onderscheid gemaakt tussen sterke en zwakke (of conditionele) aanbevelingen. De sterkte van een aanbeveling verwijst naar de mate van zekerheid dat de voordelen van de interventie opwegen tegen de nadelen (of vice versa), gezien over het hele spectrum van patiënten waarvoor de aanbeveling is bedoeld. De sterkte van een aanbeveling heeft duidelijke implicaties voor patiënten, behandelaars en beleidsmakers (zie onderstaande tabel). Een aanbeveling is geen dictaat, zelfs een sterke aanbeveling gebaseerd op bewijs van hoge kwaliteit (GRADE gradering HOOG) zal niet altijd van toepassing zijn, onder alle mogelijke omstandigheden en voor elke individuele patiënt.
|
Implicaties van sterke en zwakke aanbevelingen voor verschillende richtlijngebruikers |
||
|
|
Sterke aanbeveling |
Zwakke (conditionele) aanbeveling |
|
Voor patiënten |
De meeste patiënten zouden de aanbevolen interventie of aanpak kiezen en slechts een klein aantal niet. |
Een aanzienlijk deel van de patiënten zouden de aanbevolen interventie of aanpak kiezen, maar veel patiënten ook niet. |
|
Voor behandelaars |
De meeste patiënten zouden de aanbevolen interventie of aanpak moeten ontvangen. |
Er zijn meerdere geschikte interventies of aanpakken. De patiënt moet worden ondersteund bij de keuze voor de interventie of aanpak die het beste aansluit bij zijn of haar waarden en voorkeuren. |
|
Voor beleidsmakers |
De aanbevolen interventie of aanpak kan worden gezien als standaardbeleid. |
Beleidsbepaling vereist uitvoerige discussie met betrokkenheid van veel stakeholders. Er is een grotere kans op lokale beleidsverschillen. |
Organisatie van zorg
In de knelpuntenanalyse en bij de ontwikkeling van de richtlijnmodule is expliciet aandacht geweest voor de organisatie van zorg: alle aspecten die randvoorwaardelijk zijn voor het verlenen van zorg (zoals coördinatie, communicatie, (financiële) middelen, mankracht en infrastructuur). Randvoorwaarden die relevant zijn voor het beantwoorden van deze specifieke uitgangsvraag zijn genoemd bij de overwegingen. Meer algemene, overkoepelende, of bijkomende aspecten van de organisatie van zorg worden behandeld in de module Organisatie van zorg.
Commentaar- en autorisatiefase
De conceptrichtlijnmodule werd aan de betrokken (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd ter commentaar. De commentaren werden verzameld en besproken met de werkgroep. Naar aanleiding van de commentaren werd de conceptrichtlijnmodule aangepast en definitief vastgesteld door de werkgroep. De definitieve richtlijnmodule werd aan de deelnemende (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd voor autorisatie en door hen geautoriseerd dan wel geaccordeerd.
Adaptatie
Een aantal modules van deze richtlijn betreft een adaptatie van modules van de World Health Organization (WHO)-richtlijn ‘Global guidelines for the prevention of surgical site infection’ (WHO, 2018), te weten:
- Module Normothermie
- Module Immunosuppressive middelen
- Module Glykemische controle
- Module Antimicrobiële afdichtingsmiddelen
- Module Wondbeschermers bij laparotomie
- Module Preoperatief douchen
- Module Preoperatief verwijderen van haar
- Module Chirurgische handschoenen: Vervangen en type handschoenen
- Module Afdekmaterialen en operatiejassen
Methode
- Uitgangsvragen zijn opgesteld in overeenstemming met de standaardprocedures van het Kennisinstituut van de Federatie Medisch Specialisten.
- De inleiding van iedere module betreft een korte uiteenzetting van het knelpunt, waarbij eventuele onduidelijkheid en praktijkvariatie voor de Nederlandse setting wordt beschreven.
- Het literatuuronderzoek is overgenomen uit de WHO-richtlijn. Afhankelijk van de beoordeling van de actualiteit van de richtlijn is een update van het literatuuronderzoek uitgevoerd.
- De samenvatting van de literatuur is overgenomen van de WHO-richtlijn, waarbij door de werkgroep onderscheid is gemaakt tussen ‘cruciale’ en ‘belangrijke’ uitkomsten. Daarnaast zijn door de werkgroep grenzen voor klinische besluitvorming gedefinieerd in overeenstemming met de standaardprocedures van het Kennisinstituut van de Federatie Medisch Specialisten, en is de interpretatie van de bevindingen primair gebaseerd op klinische relevantie van het gevonden effect, niet op statistische significantie. In de meta-analyses zijn naast odds-ratio’s ook relatief risico’s en risicoverschillen gerapporteerd.
- De beoordeling van de mate van bewijskracht is overgnomen van de WHO-richtlijn, waarbij de beoordeling is gecontroleerd op consistentie met de standaardprocedures van het Kennisinstituut van de Federatie Medisch Specialisten (GRADE-methode; http://www.gradeworkinggroup.org/). Eventueel door de WHO gerapporteerde bewijskracht voor observationele studies is niet overgenomen indien ook gerandomiseerde gecontroleerde studies beschikbaar waren.
- De conclusies van de literatuuranalyse zijn geformuleerd in overeenstemming met de standaardprocedures van het Kennisinstituut van de Federatie Medisch Specialisten.
- In de overwegingen heeft de werkgroep voor iedere aanbeveling het bewijs waarop de aanbeveling is gebaseerd en de aanvaardbaarheid en toepasbaarheid van de aanbeveling voor de Nederlandse klinische praktijk beoordeeld. Op basis van deze beoordeling is door de werkgroep besloten welke aanbevelingen ongewijzigd zijn overgenomen, welke aanbevelingen niet zijn overgenomen, en welke aanbevelingen (mits in overeenstemming met het bewijs) zijn aangepast naar de Nederlandse context. ‘De novo’ aanbevelingen zijn gedaan in situaties waarin de werkgroep van mening was dat een aanbeveling nodig was, maar deze niet als zodanig in de WHO-richtlijn was opgenomen. Voor elke aanbeveling is vermeld hoe deze tot stand is gekomen, te weten: ‘WHO’, ‘aangepast van WHO’ of ‘de novo’.
Voor een verdere toelichting op de procedure van adapteren wordt verwezen naar de Bijlage Adapteren.
Literatuur
Agoritsas T, Merglen A, Heen AF, Kristiansen A, Neumann I, Brito JP, Brignardello-Petersen R, Alexander PE, Rind DM, Vandvik PO, Guyatt GH. UpToDate adherence to GRADE criteria for strong recommendations: an analytical survey. BMJ Open. 2017 Nov 16;7(11):e018593. doi: 10.1136/bmjopen-2017-018593. PubMed PMID: 29150475; PubMed Central PMCID: PMC5701989.
Alonso-Coello P, Schünemann HJ, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Rada G, Rosenbaum S, Morelli A, Guyatt GH, Oxman AD; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ. 2016 Jun 28;353:i2016. doi: 10.1136/bmj.i2016. PubMed PMID: 27353417.
Alonso-Coello P, Oxman AD, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Vandvik PO, Meerpohl J, Guyatt GH, Schünemann HJ; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 2: Clinical practice guidelines. BMJ. 2016 Jun 30;353:i2089. doi: 10.1136/bmj.i2089. PubMed PMID: 27365494.
Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, Fervers B, Graham ID, Grimshaw J, Hanna SE, Littlejohns P, Makarski J, Zitzelsberger L; AGREE Next Steps Consortium. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010 Dec 14;182(18):E839-42. doi: 10.1503/cmaj.090449. Epub 2010 Jul 5. Review. PubMed PMID: 20603348; PubMed Central PMCID: PMC3001530.
Hultcrantz M, Rind D, Akl EA, Treweek S, Mustafa RA, Iorio A, Alper BS, Meerpohl JJ, Murad MH, Ansari MT, Katikireddi SV, Östlund P, Tranæus S, Christensen R, Gartlehner G, Brozek J, Izcovich A, Schünemann H, Guyatt G. The GRADE Working Group clarifies the construct of certainty of evidence. J Clin Epidemiol. 2017 Jul;87:4-13. doi: 10.1016/j.jclinepi.2017.05.006. Epub 2017 May 18. PubMed PMID: 28529184; PubMed Central PMCID: PMC6542664.
Medisch Specialistische Richtlijnen 2.0 (2012). Adviescommissie Richtlijnen van de Raad Kwalitieit. http://richtlijnendatabase.nl/over_deze_site/over_richtlijnontwikkeling.html
Neumann I, Santesso N, Akl EA, Rind DM, Vandvik PO, Alonso-Coello P, Agoritsas T, Mustafa RA, Alexander PE, Schünemann H, Guyatt GH. A guide for health professionals to interpret and use recommendations in guidelines developed with the GRADE approach. J Clin Epidemiol. 2016 Apr;72:45-55. doi: 10.1016/j.jclinepi.2015.11.017. Epub 2016 Jan 6. Review. PubMed PMID: 26772609.
Schünemann H, Brożek J, Guyatt G, et al. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group, 2013. Available from http://gdt.guidelinedevelopment.org/central_prod/_design/client/handbook/handbook.html.
World Health Organization. Global guidelines for the prevention of surgical site infection,
second edition. Geneva: World Health Organization; 2018. (https://www.who.int/publications/i/item/9789241550475, accessed 12 June 2023).
Zoekverantwoording
Zoekacties zijn opvraagbaar. Neem hiervoor contact op met de Richtlijnendatabase.
