Intrathoracaal borstwandblokken
Uitgangsvraag
Wat is de rol van borstwandblokken (paravertebraal block) ten opzichte van epidurale pijnstilling bij patiënten die een intrathoracale chirurgische procedure ondergaan?
Aanbeveling
Geef een lichte voorkeur aan een continue paravertebrale blok boven een epiduraal voor thoracotomieën en VATS.
Overwegingen
Voor- en nadelen van de interventie en de kwaliteit van het bewijs
Er is een systematische literatuuranalyse uitgevoerd naar effecten van een continue paravertebraal blok en epidurale pijnstilling op postoperatieve pijn, postoperatieve opioïdengebruik en adverse events. Er werd literatuur gevonden voor de vergelijking bij twee typen chirurgie: thoracotomie en VATS. Postoperatieve pijn op 0, 6, 12, 24 en 48 uur na de ingreep was de cruciale uitkomstmaat, en chronische pijn, gebruik van opioïden en complicaties waren belangrijke uitkomstmaten voor klinische besluitvorming. Alle studies hadden methodologische beperkingen, waardoor er mogelijk risico is op vertekening van de studieresultaten (risk of bias) bij de subjectieve uitkomstmaten.
1. Thoracotomie
Voor postoperatieve pijn werd geen klinisch relevant verschil gevonden voor geen van de tijdstippen (lage GRADE).
Voor pijn gemeten op 6 uur na de ingreep komt postoperatieve pijn mogelijk in dezelfde mate voor, en voor pijn gemeten op 24 uur na de ingreep was de evidentie van te lage bewijskracht (zeer lage GRADE) om een conclusie te trekken.
Het bewijs op het gebied van chronische pijn suggereert een vergelijkbaar effect op 3 en 12 maanden.
Wat betreft de uitkomstmaat gebruik van opioïden was de evidentie van te lage bewijskracht (zeer lage GRADE) om een conclusie te trekken.
Het bewijs voor incidentie van adverse event hypotensie was redelijk en suggereert een klinisch relevant verschil van een lagere incidentie in het voordeel van paravertebraal blok.
De overall bewijskracht, namelijk de laagste bewijskracht van de cruciale uitkomstmaat, komt uit op zeer laag.
2. VATS
Voor postoperatieve pijn op 0-1 uur tijdens rust, werd een mogelijk klinisch relevant verschil gevonden in het voordeel van epidurale pijnstelling (lage GRADE). Voor de andere tijdstippen werd geen klinisch relevant verschil gevonden (lage GRADE) of was de evidentie van de bewijskracht te laag om een conclusie te kunnen trekken (zeer lage GRADE).
Op het gebied van chronische pijn was er voor deze vergelijking geen onderzoek. Wat betreft de uitkomstmaat gebruik van opioïden was de evidentie van te lage bewijskracht (zeer lage GRADE) om een conclusie te trekken.
Het bewijs voor de complicatie hypotensie was laag, er werd mogelijk een klinisch relevant verschil gevonden van een lagere incidentie in het voordeel van paravertebraal blok.
De overall bewijskracht, namelijk de laagste bewijskracht van de cruciale uitkomstmaat, komt uit op zeer laag.
Samenvattend is er een minimaal beter analgetisch effect van een epiduraal vergeleken met een continue paravertebraal blok gemeten middels postoperatieve pijnscores en opioïdengebruik, maar met meer kans op hypotensie.
Over een eventueel verschil in effect op chronische pijn van continue paravertebrale en epidurale analgesie bij VATS en thoracotomie kan geen uitspraak worden gedaan, dit is een kennishiaat.
Er is een zwakke bewijskracht qua analgetisch effect (ten voordeel van epiduraal) vergeleken met de iets sterkere bewijskracht qua complicaties (hypotensie: ten voordeel van continue paravertebraal).
Waarden en voorkeuren van patiënten (en evt. hun verzorgers)
Als een continue paravertebraal blok ook intra-operatief bv. door de thoraxchirurg zelf zou ingebracht kunnen worden, dan wordt dit door patiënten als minder belastend ervaren. Bij de afweging tussen wellicht een iets beter analgetisch effect van een epiduraal blok en minder complicaties bij een continue paravertebraal blok zouden naast eventuele comorbiditeit van de patiënt, diens waarden en voorkeuren meegenomen kunnen worden.
Kosten (middelenbeslag)
Vaak wordt dezelfde locoregionale set gebruikt voor een continue paravertebraal blok als voor een epiduraal blok; en worden beiden postoperatief vervolgd door een APS-team. Daardoor zou er geen verschil in kosten zijn tussen beide technieken
Aanvaardbaarheid, haalbaarheid en implementatie
Aanvaardbaarheid, haalbaarheid en implementatie is niet expliciet onderzocht. Maar epiduraal en continue paravertebraal blok kunnen allebei als een standaard locoregionale techniek in de anesthesiologie beschouwd worden. Er is echter wel meer ervaring (exposure) onder anesthesiologen met epiduraal dan een continue paravertebraal. Er is geen vergelijkende studie naar de leercurve tussen een epiduraal en paravertebraal, maar van het paravertebraal blok is bekend dat deze technisch moeilijker uitvoerbaar is (Coveney, 1998). Specifieke complicaties voor epidurale analgesie zijn het optreden van hypotensie (redelijk frequent) en ernstige neurologische schade(zelden). Continue paravertebraal heeft als belangrijkste complicatie een pneumothorax. Een paravertebraal valt onder intermediair bloedingen-risico versus epiduraal hoog-risico.
In het merendeel van de beschreven studies werd het continue paravertebraal blok ingebracht door de anesthesioloog (12 van de 20 studies). Met name in de meest recente studies gepubliceerd in de laatste 10 jaar werd dit echogeleid uitgevoerd. Mogelijk kan dit leiden tot effectievere pijnstilling (Patnaik 2018), hoewel dit niet expliciet is geëvalueerd in deze module.
Vaak kan een continue paravertebraal blok ook intra-operatief en zelf door de thoraxchirurg ingebracht worden. In 8 van de 20 beschreven studies werd het continue paravertebraal blok intra-operatief ingebracht door de operateur. Dit zou qua tijdsinvestering voor inbrengen van het blok en belasting voor de patiënt (slapend versus wakker) een duidelijk voordeel van een continue paravertebraal blok zijn.
Rationale van de aanbeveling: weging van argumenten voor en tegen de interventies
De overall bewijskracht, namelijk de laagste bewijskracht op pijnscores en opioïdengebruik, komt uit op zeer laag. Voor thoracotomieën en VATS geeft een continue paravertebraal blok dezelfde mate van pijnstilling in vergelijking met een epiduraal blok. Gezien er minder hypotensie (en kans op ernstige neurologische complicaties) van een continue paravertebraal te verwachten zijn, bestaat er een lichte voorkeur voor een continue paravertebraal blok boven een epiduraal. Ondanks dat beide technieken tot het standaard arsenaal van de anesthesioloog behoren, is er meer ervaring met epidurale pijnbestrijding. Aandacht voor scholing is nodig.
Onderbouwing
Achtergrond
Van oudsher werd epidurale pijnstilling gebruikt bij patiënten die een intrathoracale chirurgische procedure ondergaan. Recenter worden meer loco-regionale borstwandblokken gebruikt, zoals erector spinae (ESP) blok, intercostaal (IN) blok, en met name paravertebraal blok (PVB). Deze laatste staat ook als lichte voorkeur in vorige richtlijn boven epiduraal en wordt in Nederland samen met de epiduraal waarschijnlijk het vaakst gebruikt in deze categorie operaties. Vandaar dat voor deze module de focus ligt op de vergelijking tussen een epiduraal en een continue paravertebraal blok.
De vraag die de werkgroep heeft voor deze module is als volgt: Heeft een continue paravertebraal blok de voorkeur boven epiduraal bij intrathoracale chirurgie?
Conclusies / Summary of Findings
Conclusions
- Postoperative pain
|
Low GRADE |
Use of continuous PVB may result in little to no difference in postoperative pain at 2-6, 24, and 48 hours at rest and coughing/ physiotherapy when compared with the use of a TEB in adults undergoing thoracotomy.
Source: Yeung, 2016; Li, 2021 |
- Chronic postoperative pain
|
Very low GRADE |
The evidence is very uncertain about the effect of the use of continuous PVB compared with the use of TEB for chronic postoperative pain at 6 months in adults undergoing thoracotomy.
Source: Khoronenko, 2018; Li, 2021 |
|
Low GRADE |
Use of continuous PVB may result in little to no difference in chronic postoperative pain at 3 and 12 months when compared with the use of a TEB in adults undergoing thoracotomy.
Sources: Li, 2021 |
- Postoperative opioid consumption
|
Very low GRADE |
The evidence is very uncertain about the effect of the use of continuous PVB compared with the use of TEB for postoperative opioid consumption in adults undergoing thoracotomy.
Source: Huang, 2020 |
- Adverse events
Bleeding
|
No GRADE |
No evidence was found.
Source: - |
Hypotension
|
Moderate GRADE |
Use of continuous PVB likely results in a lower incidence of hypotension in adults undergoing thoracotomy when compared with the use of TEB.
Source: Yeung, 2016 |
Surgery type 2: Video-Assisted Thoractomy (VAT)
1. Postoperative pain
Okajima (2015) and Yeap (2021) provided absolute data for postoperative pain comparing PVB with TEB. Results are presented in table 4. Yeap (2021) made a distinction between PVB catheter (PVB-A) and single-injection PVB (PVB-B). in the study of Okajima (2015) four blocks of PVB and one catheter insertion were performed (results presented under PVB-B).
1.1. Postoperative pain at 0-1h
1.1.1. At rest
Okajima (2015) reported a median difference in pain scores at rest between PVB (n=36) and TEB (n=33) of 2.5 in favour of PVB. This difference was considered clinically relevant.
Yeap (2021) reported a median differences in pain scores at rest between PVB catheter (n=40) and TEB (n=40) of 1 in favour of PVB and no difference in pain scores at rest between single-injection PVB (n=40) and TEB (n=40). The difference between PVB catheter and TEB was considered clinically relevant.
1.1.2. At mobilization
Yeap (2021) reported a median differences in pain scores at mobilization between PVB catheter (n=40) and TEB (n=40) of 0.5 in favour of PVB and no differences in pain scores at mobilization between single-injection PVB (n=40) and TEB (n=40). The differences were not considered clinically relevant.
1.2. Postoperative pain at 6h
1.2.1. At rest
Okajima (2015) reported no difference in pain scores at rest between PVB (n=36) and TEB (n=33).
1.3. Postoperative pain at 12h
1.3.1. At rest
Okajima (2015) reported no difference in pain scores at rest between PVB (n=36) and TEB (n=33).
1.4. Postoperative pain at 24h
1.4.1. At rest
Okajima (2015) reported no difference in pain scores at rest between PVB (n=36) and TEB (n=33).
Yeap (2021) reported a median difference of pain scores at rest between PVB catheter (n=40) and TEB (n=40) of 1 in favour of TEB and a median differences of pain scores at rest between single-injection PVB (n=40) and TEB (n=40) of 0.5 in favour of TEB. The difference between PVB catheter and TEB was considered clinically relevant.
1.4.2. At mobilization
Yeap (2021) reported a median difference of pain scores at mobilization between PVB catheter (n=40) and TEB (n=40) of 0.5 in favour of TEB and a median differences of pain scores at rest between single-injection PVB (n=40) and TEB (n=40) of 1 in favour of TEB. The difference between single-injection PVB and TEB was considered clinically relevant.
1.5. Postoperative pain at 48h
1.5.1. At rest
Okajima (2015) reported no difference in pain scores at rest between PVB (n=36) and TEB (n=33).
Yeap (2021) reported a median difference of pain scores at rest between PVB catheter (n=40) and TEB (n=40) of 1 in favour of TEB and a median difference of pain scores at rest between single-injection PVB (n=40) and TEB (n=40) of 2 in favour of TEB. These differences were considered clinically relevant.
1.5.2. At mobilization
Yeap (2021) reported a median differences of pain scores at mobilization between PVB catheter (n=40) and TEB (n=40) of 1 in favour of TEB and a median differences of pain scores at rest between single-injection PVB (n=40) and TEB (n=40) of 2 in favour of TEB. These differences were considered clinically relevant.
Table 4. Postoperative pain scores in median (IQR).
|
Study |
Pain |
PVB-A |
n |
PVB-B
|
n |
TEB |
n |
Median difference |
|
|
PVB-A vs. TEB |
PVB-B vs. TEB |
||||||||
|
0-1h |
|||||||||
|
Okajima (2015) |
VRS (0-10) at rest |
- |
- |
1,5 (0-6) |
36 |
4 (0-7) |
33 |
- |
-2,5 in favour of PVB, clinically relevant |
|
Yeap (2021) |
VAS (0-10) at rest |
5.0 (3.0-8.0) |
40 |
6.0 (3.5-8.0) |
40 |
6.0 (3.5-8.0) |
40 |
-1 in favour of PVB, clinically relevant |
0, none, no |
|
|
VAS (0-10) at mobilization |
6.5 (4.0-9.0) |
40 |
7.0 (5.0-9.0) |
40 |
7.0 (5.0-9.0) |
40 |
-0,5 in favour of PVB, not clinically relevant |
0, none, no |
|
6h |
|||||||||
|
Okajima (2015) |
VRS (0-10) at rest |
- |
- |
1 (0-3) |
36 |
1 (0-3,25) |
33 |
- |
0, none, no |
|
12h |
|||||||||
|
Okajima (2015) |
VRS (0-10) at rest |
- |
- |
1 (0-3) |
36 |
1 (0-1,25) |
33 |
- |
0, none, no |
|
24h |
|||||||||
|
Okajima (2015) |
VRS (0-10) at rest |
- |
- |
1 ( 0-3) |
36 |
1 (0-2) |
33 |
- |
0, none, no |
|
Yeap (2021) |
VAS (0-10) at rest |
4.0 (3.0-5.5) |
40 |
4.5 (2.5-6.0) |
40 |
3.0 (1.0-5.0) |
40 |
1 in favour of TEB, clinically relevant |
0,5 In favour of TEB, not clinically relevant |
|
|
VAS (0-10) at mobilization |
6.5 (5.0-8.0) |
40 |
7.0 (5.0-8.0) |
40 |
6.0 (2.0-7.0) |
40 |
0,5 in favour of TEB, not clinically relevant |
1 In favour of TEB, clinically relevant |
|
POD2/48h |
|||||||||
|
Okajima (2015) |
VRS (0-10) at rest |
- |
- |
1 (0-3) |
36 |
1 (0-3,5) |
33 |
- |
0, none, no |
|
Yeap (2021) |
VAS (0-10) at rest |
3.0 (1.0-5.0) |
40 |
4.0 (2.0-6.0) |
40 |
2.0 (1.0-4.0) |
40 |
1 in favour of TEB, clinically relevant |
2 In favour of TEB, clinically relevant |
|
|
VAS (0-10) at mobilization |
5.0 (3.0-7.0) |
40 |
6.0 (3.0-8.0) |
40 |
4.0 (2.0-6.0) |
40 |
1 in favour of TEB, clinically relevant |
2 In favour of TEB, clinically relevant |
PVB-A = paravertebral block catheter; PVB-B = single-injection paravertebral block TEB = thoracic epidural blockade.
Unfortunately, the other RCTs reported postoperative pain scores at the different timepoints in graphs, from which the absolute data could not be extracted. Ding (2018) measured postoperative pain using a verbal rating score (VRS; scale of 0–10, with 0 indicating no pain and 10 indicating the worst pain imaginable) assessed at 2, 6, 12, 24, and 48 h after surgery at rest and after coughing. The authors report no significant difference in VRS during coughing among the two groups at 2 hours after surgery. At 6 and 12 hours after surgery, patients in the TEB group had a lower VRS during coughing as compared to the PVB group. There were no significant differences in VRS during coughing among the three groups at 24 and 48 hours after surgery.
Kosiński (2016) measured postoperative pain according to VAS at 1, 24 and 48 hours after the procedures. Both static (at rest) and dynamic assessments (on cough) were performed. The authors report significant differences in the measurements at 24 hours after surgery — both at rest and on coughing (P = 0.01 and P = 0.023, respectively); and in static pain at 48 hours after surgery (P = 0.026), in favour PVB.
Lai (2021) measured VAS at rest and on coughing at PACU, 6, 24 and 48 hours postoperatively. The authors report significant differences in pain at 24 hours at rest and on coughing (P = 0.001 and P < 0.001, respectively), in favour of TEB, and no significant differences between the two groups at other time points.
Because only the statistical significance of the results was presented and no absolute values were reported, no interpretation can be given for the clinical relevance of these results.
2. Chronic postoperative pain
Not reported.
3. Postoperative opioid consumption
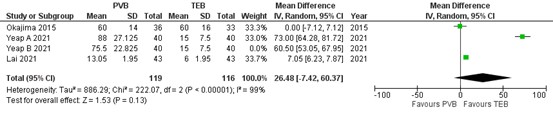
Three RCTs reported on postoperative opioid consumption. Results of the meta-analysis is presented in figure 5. As mentioned earlier, Yeap (2021) made a distinction between PVB catheter (A) and single-injection PVB (B). Because Okajima (2015) and Lai (2021) reported on PVB catheter, only the group A of Yeap (2021) was included in the meta-analysis. The mean difference (MD) of opioid consumption between PVB (n=119) and TEB (n=116) was 26.48 (95% CI -7.42 to 60.37) in favour of TEB. This difference was clinically relevant.
Figure 5. Postoperative opioid consumption.
Opioids were converted into equianalgesic doses of i.v. morphine for analysis (i.v. morphine 10 mg =i.v. fentanyl 100 μg = i.v. sufentanil 10 μg). Random effects model; df: degrees of freedom; I2: statistical heterogeneity; CI: confidence interval; Z: p-value of pooled effect. PVB = paravertebral block; TEB = epidural anesthesia
Ding (2018) and Kosiński (2016) reported postoperative opioid consumption in graphs from which the absolute date could not be extracted. Ding (2018) compared the total dose of sufentanil used during the 48-h postoperative period. Compared to the PVB group (n= 36), patients in the TEA group (n=32) received lower doses of sufentanil in the 48 h after surgery (p=0.005). Kosiński (2016) reported the emergency PCA-administered morphine doses (mg h-1) per day in the follow-up time of 72 hours. The comparative analysis of both groups (PVB, n=26; TEB, n=25) did not reveal any significant differences. The mean dose was 0.4 mg h-1 on day 0, 0.37 mg h-1 on day 1, 0.21 on day 2 and 0.14 mg h-1 on day 3. Because only the statistical significance and combined mean values of the comparative analyses were presented and no absolute values were reported, no interpretation can be given for the clinical relevance of these results.
4. Adverse events
4.1. Bleeding
Not reported.
4.2. Hypotension
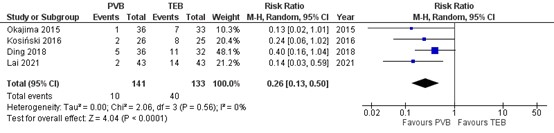
Four RCTs reported on the incidence of hypotension. Results are presented in figure 6. The number of patients with hypotension was higher in patients treated with TEB (40 of 133, 30%) compared to patients treated with PVB (10 of 141, 7.1%) (RR=0.26 95% CI 0.13 to 0.50). This difference is considered clinically relevant.
Figure 6. Incidence of hypotension .
Random effects model; df: degrees of freedom; I2: statistical heterogeneity; CI: confidence interval; Z: p-value of pooled effect. PVB = paravertebral block; TEB = epidural anesthesia
Level of evidence of the literature
The level of evidence for all outcomes was based on randomized studies and therefore starts at high.
The level of evidence regarding the outcome measure postoperative pain at 0-1 at rest and mobilization was downgraded by 2 levels to low because of study limitations (risk of bias, -1) and number of included patients (imprecision, -1).
The level of evidence regarding the outcome measure postoperative pain at 6 and 12 hours at rest was downgraded by 2 levels to low because of study limitations (risk of bias, -1) and number of included patients (imprecision, -1).
The level of evidence regarding the outcome measure postoperative pain 24 hours at rest was downgraded by 3 levels to very low because of study limitations (risk of bias, -1); conflicting results (inconsistency, -1); and number of included patients (imprecision, -1).
The level of evidence regarding the outcome measure postoperative pain 24 hours at mobilization was downgraded by 3 levels to very low because of study limitations (risk of bias, -1); and number of included patients (imprecision, -2).
The level of evidence regarding the outcome measure postoperative pain at 48 hours at rest was downgraded by 3 levels to very low because of study limitations (risk of bias, -1); conflicting results (inconsistency, -1); and number of included patients (imprecision, -1).
The level of evidence regarding the outcome measure postoperative pain at 48 hours at mobilization was downgraded by 3 levels to very low because of study limitations (risk of bias, -1); and number of included patients (imprecision, -2).
The level of evidence regarding the outcome measure postoperative opioid consumption was downgraded by 3 levels to very low because of study limitations (risk of bias, -1); conflicting results (inconsistency, -1); and limited numbers of included patients (imprecision, -1).
The level of evidence regarding the outcome measure hypotension was downgraded by 2 levels to low because of study limitations (risk of bias, -1); and limited number of included patients (imprecision, -1).
Conclusions
- Postoperative pain
|
Low GRADE |
Use of continuous PVB may result in less postoperative pain at 0-1 hours at rest, when compared with the use of a TEB in adults undergoing VATS.
Use of continuous PVB may result little to no difference in postoperative pain at 0-1 hours at mobilization, when compared with the use of a TEB in adults undergoing VATS.
Use of continuous PVB may result little to no difference in postoperative pain 6 and 12 hours at rest, when compared with the use of a TEB in adults undergoing VATS.
Source: Okajima, 2015; Yeap, 2021 |
|
Very Low GRADE |
The evidence is very uncertain about the effect of the use of continuous PVB compared with the use of TEB for postoperative pain at 24 and 48 hours at rest and at mobilization in adults undergoing VATS.
Source: Okajima, 2015; Yeap, 2021 |
- Chronic postoperative pain
|
No GRADE |
No evidence was found.
Source: - |
- Postoperative opioid consumption
|
Very low GRADE |
The evidence is very uncertain about the effect of the use of continuous PVB compared with the use of TEB for postoperative opioid consumption in adults undergoing VATS.
Source: Okajima, 2015; Yeap, 2021; Lai, 2021 |
- Adverse events
Bleeding
|
No GRADE |
No evidence was found.
Source: - |
Hypotension
|
Low GRADE |
Use of continuous PVB may result in a lower incidence of hypotension in adults undergoing VATS when compared with the use of TEB.
Source: Okajima, 2015; Kosiński, 2016; Ding, 2018; Lai, 2021 |
Samenvatting literatuur
Description of studies
Main study characteristics of all included randomized controlled trials (RCTs) are outlined in table 1. As shown in the table, studies included various surgical procedures, different thoracic wall block solutions and follow-up time.
Yeung (2016) conducted a systematic review and meta-analysis of RCTs to compare the two regional techniques of TEB and PVB in adults undergoing elective thoracotomy. The systematic review included 14 studies involving 798 participants. The review reports that the included studies demonstrated high heterogeneity in insertion and use of both regional techniques. In addition, the included studies had a moderate to high potential for bias, lacking details of randomisation, group allocation concealment or arrangements to blind participants or outcome assessors.
Table 1. Characteristics of included studies.
|
Author, year |
N (I/C) |
Intervention |
Control |
Follow-up |
||
|
Method of insertion |
Method of use |
Method of insertion |
Method of use |
|||
|
|
Continuous PVB |
|
TEB |
|
|
|
From Yeung, 2016: |
||||||
|
Bimston, 1999
|
30/20 |
Inserted under direct vision by surgeon. |
18 ml 0.5% bupivacaine Bolus followed by infusion of 0.1% bupivacaine with 10 μg/ml fentanyl, 10 - 15 ml/ hr. |
Percutaneously by landmark technique before induction of GA. |
Uncertain whether catheter was used during operation.
|
48h |
|
Casati, 2006
|
21/21 |
Percutaneously by landmark techniquebefore induction of GA. |
Percutaneously by landmark technique before induction of GA. |
Percutaneously by landmark technique before induction of GA. |
5 ml bolus of 0.75% ropivacaine. |
48h |
|
De Cosmo, 2002
|
25/25 |
Inserted under direct vision by surgeon. |
Used at the end of operation only.
|
Percutaneously by landmark technique before induction of GA. |
5 ml bolus of 0.2% ropivacaine and sufentanil 10 Zg given as bolus. Catheter used during operation if required. |
48h |
|
Grider, 2012
|
50/25 |
Inserted under direct vision by surgeon. |
Used at the end of operation only. |
Percutaneously by landmark technique before induction of GA. |
Used at the end of operation only. |
48h |
|
Gulbahar, 2020 |
25/19 |
Inserted under direct vision by surgeon. |
Used at the end of operation only. |
Percutaneously by landmark technique before induction of GA. |
Used at the end of operation only. |
48h |
|
Ibrahim, 2009
|
25/25 |
Percutaneously by landmark technique before induction of GA. |
15 - 20 ml 0.5% ropivacaine bolus followed by 0.375% ropivacaine 0.1 ml/kg/hr infusion. |
Percutaneously by landmark technique before induction of GA. |
15 - 20 ml 0.5% ropivacaine bolus followed by 0.375% ropivacaine 0.1 ml/kg/hr infusion. |
48h |
|
Kobayashi, 2013 |
35/35 |
Inserted under direct vision by surgeon. |
Used at the end of operation only. |
Percutaneously by landmark technique before induction of GA. |
Used at the end of operation only. |
48h |
|
Matthews, 1989 |
10/10 |
Percutaneously by landmark at the end of procedure. |
Used at the end of operation only. |
Percutaneously by landmark technique before induction of GA. |
Used at the end of operation only. |
48h |
|
Messina, 2009 |
12/12 |
Percutaneously by landmark technique before induction of GA. |
Used at the end of operation only. |
Percutaneously by landmark technique before induction of GA. |
Used at the end of operation only. |
48h |
|
Pintaric, 2011 |
16/16 |
Percutaneously by landmark technique before induction of GA. |
0.5% levopubivacaine with 30 μg/kg morphine. |
Percutaneously by landmark technique before induction of GA. |
0.25% levopubivacaine with 30 μg/kg morphine. |
48h |
|
Richardson, 1999 |
46/54 |
Inserted under direct vision by surgeon. |
20 ml bolus of 0.5% bupivacaine, followed by infusion at 0.1 ml/kg/hr. |
Percutaneously by landmark technique before induction of GA. |
10 - 15 ml bolus of 0.25% bupivacaine.
|
48h |
|
Huang, 2020
|
45 (IA)/32 (IB)/39 (C) |
Percutaneously, ultrasound-guided, before induction of GA. |
Group A: interplanar approach in an oblique axial transverse section.
A dose of 1 mg kg-1 of 0.33% of ropivacaine + a dose of 0.7 mg kg-1 of 0.33% ropivacaine added every 4 h during the operation.
Group B: interplanar approach in sagittal parasagittal section.
A dose of 1 mg kg-1 of 0.33% of ropivacaine + a dose of 0.7 mg kg-1 of 0.33% ropivacaine added every 4 h during the operation. |
Percutaneously, ultrasound-guided, before induction of GA. |
Injection of 0.25% of ropivacaine (3 ml) via the catheter. Another 3–5 ml was added every 5 min during the operation + 0.25% of ropivacaine 3–5 ml every 2 h during the operation. |
48h |
|
Khoronenko, 2018
|
100/100
|
Percutaneously, ultrasound-guided, before induction of GA. |
sevoflurane (0.8–1 MAC), fentanyl (0.05–0.1 mg i.v. every 15–30 minutes when the SBP increased by more than 15% from the baseline value or was >140 mmHg). |
Percutaneously by landmark technique before induction of GA. |
sevoflurane (0.8–1 MAC), fentanyl (0.05–0.1 mg i.v. every 15–30 minutes when the SBP increased by more than 15% from the baseline value or was >140 mmHg). |
6 months |
|
Li, 2021 |
42/41 |
Percutaneously, ultrasound-guided, before induction of GA. |
single-shot 20 mL of 0.25% ropivacaine combined with 1 µg/kg dexmedetomidine. |
Percutaneously by landmark technique before induction of GA. |
continuous infusion of sufentanil (0.2 μg/mL) and ropivacaine 0.06% at 5–10 mL/h during the operation. |
12 months |
|
Tamura, 2017 |
36/36
|
Inserted under direct vision by surgeon. |
20 mL of 0.375% ropivacaine as a bolus, followed by a 300-mL of continuous infusion of 0.2% ropivacaine at 5 mL/h. |
Percutaneously by landmark technique before induction of GA. |
5 mL of 0.375% ropivacaine as a bolus, followed by a 300-mL continuous infusion of 0.2% ropivacaine at 5 mL/h. |
42h |
|
2) VATS |
|
|
Continuous PVB |
|
TEB |
|
|
Ding, 2018 |
36/32
|
Percutaneously, ultrasound-guided, before induction of GA. |
single-dose 0.5% ropivacaine before the operation |
Percutaneously by landmark technique before induction of GA. |
0.5% ropivacaine and a single dose of epidural morphine (0.03 mg/kg) after extubating |
48hr |
|
Kosiński, 2016 |
26/25
|
Percutaneously by landmark technique before induction of GA. |
continuous infusion of 0.25% bupivacaine |
Percutaneously by landmark technique before induction of GA. |
continuous infusion of 0.25% bupivacaine |
72h |
|
Lai, 2021 |
43/43
|
Inserted under direct vision by surgeon (upon completion of surgery) |
initial bolus of 0.5% ropivacaine 0.1 mL/kg and then 0.1 mL/kg/h infusion for 48 h postoperatively. |
Percutaneously by landmark technique before induction of GA. |
ropivacaine 0.15% with 6 mcg/mL of hydromorphone and was administered via a pump starting at 2 mL/h for 48 h postoperatively. |
72h |
|
Okajima, 2015 |
36/33 |
Percutaneously, ultrasound-guided, before induction of GA. |
three single blocks with 5 ml of 0.5 % ropivacaine + 15 ml of 0.5 % ropivacaine for catheter insertion + continuous postoperative infusion (0.1 % ropivacaine plus fentanyl at 0.4 mg/day) for 36 h. |
Percutaneously by landmark technique before induction of GA. |
bolus dose (5–7 ml) of 0.25–0.375 % ropivacaine + continuous postoperative infusion (0.1 % ropivacaine plus fentanyl at 0.4 mg/day) for 36 h. |
POD2 |
|
Yeap, 2021 |
40 (IA)/40 (IB)/40 (C) |
Percutaneously, ultrasound-guided, before induction of GA. |
Group A: Ultrasound-guided PVB catheter.
Ropivacaine, 0.2%, was delivered postoperatively at a rate of 10 mL/h by infusion pump.
Group B: ultrasound-guided single-injection PVB
30 mL of 0.5% ropivacaine. |
Percutaneously, ultrasound-guided, before induction of GA. |
epidural mixture of 0.125% bupivacaine and 0.05 mg/mL of hydromorphone (starting at the end of the surgery). |
6 months |
PVB = paravertebral block; TEB = thoracic epidural blockade; h = hours.
Surgery type 1: Thoracotomy
Results
1. Postoperative pain
1.1. Postoperative pain at 2 to 6 hours
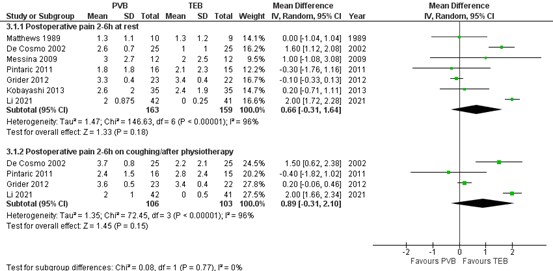
The systematic review by Yeung (2016) reported pain scores at 2 to 6 hours at rest and on coughing/ after physiotherapy for six RCTs. The additional RCT (Li, 2021) reported on postoperative pain scores at 6 hours which was added to the results of Yeung (2016) and on which a meta-analysis was performed. The results are presented in figure 1.
1.1.1. At rest
The mean difference (MD) of pain scores at rest between PVB (n=163) and TEB (n=159) was 0.66 (95% CI -0.31 to 1.64) in favour of TEB. This difference is not clinically relevant.
1.1.2. On coughing/ after physiotherapy
The mean difference (MD) of pain scores at rest between PVB (n=106) and TEB (n=103) was 0.89 (95% CI -0.31 to 2.10) in favour of TEB. This difference is not clinically relevant.
Figure 1. Postoperative pain at 2-6h at rest and on coughing/after physiotherapy.
Pain 2-6 hours postoperatively assessed by a 10-point VAS scale; random effects model; df: degrees of freedom; I2: statistical heterogeneity; CI: confidence interval; Z: p-value of pooled effect. PVB = paravertebral block; TEB = thoracic epidural blockade
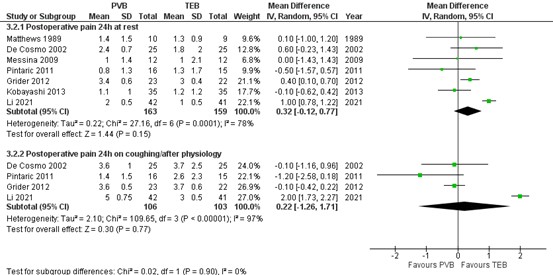
1.2. Postoperative pain at 24 hours
Pain scores at 24 hours at rest and on coughing/ after physiotherapy were reported by Yeung (2016; including 6 RCTs) and Li (2021) on which a meta-analysis could be performed. The results are presented in figure 2.
1.1.1. At rest
The mean difference (MD) of pain scores at rest between PVB (n=163) and TEB (n=159) was 0.32 (95% CI -0.12 to 0.77) in favour of TEB. This difference is not clinically relevant.
1.1.2. On coughing/ after physiotherapy
The mean difference (MD) of pain scores at rest between PVB (n=106) and TEB (n=103) was 0.22 (95% CI -1.26 to 1.71) in favour of TEB. This difference is not clinically relevant.
Figure 2. Postoperative pain at 24 at rest and on coughing/after physiotherapy
Pain hours postoperatively assessed by a 10-point VAS scale; random effects model; df: degrees of freedom; I2: statistical heterogeneity; CI: confidence interval; Z: p-value of pooled effect. PVB = paravertebral block; TEB = thoracic epidural blockade
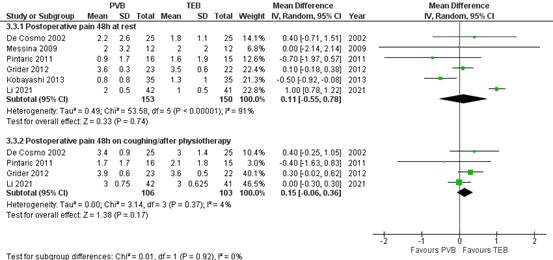
1.3. Postoperative pain at 48 hours
Pain scores at 48 hours at rest and on coughing/ after physiotherapy were reported by Yeung (2016; including 5 RCTs) and Li (2021) on which a meta-analysis could be performed. The results are presented in figure 3.
1.3.1. At rest
The mean difference (MD) of pain scores at rest between PVB (n=153) and TEB (n=150) was 0.11 (95% CI -0.55 to 0.78) in favour of TEB. This difference is not clinically relevant.
1.3.2. On coughing/ after physiotherapy
The mean difference (MD) of pain scores at rest between PVB (n=106) and TEB (n=103) was 0.15 (95% CI -0.06 to 0.36) in favour of TEB. This difference is not clinically relevant.
Figure 3. Postoperative pain at 48h at rest and on coughing/after physiotherapy
Pain 48 hours postoperatively assessed by a 10-point VAS scale; random effects model; df: degrees of freedom; I2: statistical heterogeneity; CI: confidence interval; Z: p-value of pooled effect. PVB = paravertebral block; TEB = thoracic epidural blockade
Two other additional single RCTs reported postoperative pain scores at the different timepoints in graphs from which the absolute data could not be extracted. In Huang (2020) the numerical rating scale (NRS) scores at rest and during coughing at 2, 4, 8, 12, 24, 36, and 48 hours after the surgery were recorded. The graphs with results on postoperative pain show that the NRS scores of the PVB-A and PBV-B groups are higher than TEB during both modalities and for all measured time-points. In addition, the authors reported that the rate of moderate pain at rest was similar, but the rate of moderate pain during coughing was the lowest in the TEB group. Furthermore, they reported that the TEB group had the least moderate pain during resting and coughing from 24 to 48 hours. Because no absolute values were reported, no interpretation can be given for the clinical relevance of these results.
Tamura (2017) recorded postoperative pain using a visual analog scale (VAS) while coughing, moving, and at rest 1, 2, 6, 12, 18, and 42 hours after a bolus injection of ropivacaine. The graphs with results on postoperative pain show that scores of the PVB group are higher than the TEB group for all modalities and each time-point. For all three modalities the pain for both PVB and TEB decrease over time. Also, for all three modalities, the difference between PVB and TEB for the earlier time-points is greater (about 80 mm for PVB versus 40/50 mm for TEB at 1 hour) than the difference at the later time-points (about 40/50 mm for PVB versus 20/30 for TEB at 42 hours). The authors additionally reported that all mean VAS scores in the TEB group were significantly lower than those in the PVB group. Because only the statistical significance of the results were presented and no absolute values were reported, no interpretation can be given for the clinical relevance of these results.
2. Chronic postoperative pain
Two RCTs reported on chronic pain. Khoronenko (2018) reported on the intensity of chronic pain on a VAS scale (0 for no pain and 100 mm for worst possible pain). Static and dynamic pain components were assessed 6 months after surgery. Li (2021) reported chronic pain scores at rest scored on a verbal rating scale (VRS; 0 for no pain, and 10 for the worst pain). Results of both studies are presented in table 2. Only for chronic pain at 3 months, Li (2021) reported a clinically different difference in favour of TEB.
Table 2. Chronic postoperative pain scores.
|
Study |
Pain |
PVB |
n |
TEB |
n |
Mean difference, 95% CI / median difference |
In favour of |
Clinically relevant? |
|
3 months |
||||||||
|
Li (2021) |
VRS (0-10) during rest, in median (range) |
1 (0-2) |
42 |
0 (0-1) |
41 |
1 |
TEB |
Yes |
|
6 months |
||||||||
|
Khoronenko (2018) |
VAS (0-100 mm) during rest in mean ± SD |
0.16 ± 0.39 |
100 |
0.10 ± 0.30 |
100 |
0.06, -0.04 to 0.16 |
TEB |
No |
|
|
VAS (0-100 mm) dynamic in mean ± SD |
0.59 ± 0.98 |
100 |
0.38 ± 0.79 |
100 |
0.21, -0.04 to 0.46
|
TEB |
No |
|
Li (2021) |
VRS (0-10) during rest, in median (range) |
0 (0-2) |
42 |
0 (0-1) |
41 |
0 |
None |
No |
|
12 months |
||||||||
|
Li (2021) |
VRS (0-10) during rest, in median (range) |
0 (0-1) |
42 |
C: 0 (0-0) |
41 |
0 |
None |
No |
PVB = paravertebral block; TEB = epidural anesthesia
3. Postoperative opioid consumption
The systematic review by Yeung (2016) did not report on postoperative opioid consumption. Of the additional included RCTs, only Huang (2020) reported on postoperative opioid consumption (in morphine milligram equivalents; MME). In the PVB group, they made a distinction between A) the interplanar approach in an oblique axial transverse section and B) the interplanar approach in sagittal parasagittal section. Results are presented in table 3. The mean difference in MME between PBV-A (n=45) and TEB (n=39) was 6.51 mg in favour of TEB (95% CI 3.77 to 9.25). The mean difference in MME between PBV-B (n=32) and TEB (n=39) was 6.74 mg in favour of TEB (95% CI 3.88 to 9.60). These differences were not clinically relevant.
Table 3. Postoperative opioid consumption in morphine milligram equivalents (MME) reported by Huang (2020).
|
PVB-A mean±SD |
n |
PVB-B mean±SD |
N |
TEB mean±SD |
n |
Mean difference [95% CI] |
|
|
PVB-A vs. TEB |
PVB-B vs. TEB |
||||||
|
8.75 ± 7.93 |
45 |
8.98 ± 7.09 |
32 |
2.24 ± 4.64 |
39 |
6.51 [3.77, 9.25]
|
6.74 [3.88, 9.60] |
PVB = paravertebral block; TEB = epidural anesthesia
4. Adverse events
4.1. Bleeding
Not reported.
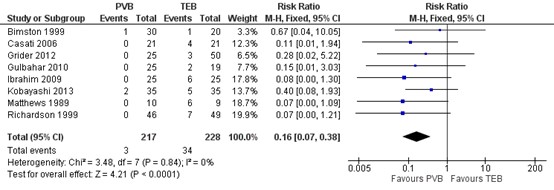
4.2. Hypotension
Eight RCTs included in Yeung (2016) reported on the incidence of hypertension. Results are presented in figure 4. The number of patients with hypotension was higher in patients treated with TEB (34 of 228, 14.9%) compared to patients treated with PVB (3 of 217, 0.9%) (RR=0.16 95% CI 0.07 to 0.38). This difference is considered clinically relevant.
Figure 4. Incidence of hypotension.
Random effects model; df: degrees of freedom; I2: statistical heterogeneity; CI: confidence interval; Z: p-value of pooled effect. PVB = paravertebral block; TEB = epidural anesthesia
Level of evidence of the literature
The level of evidence for all outcomes was based on randomized studies and therefore started at high.
The level of evidence regarding the outcome measure postoperative pain at 2-6 hours, 24 hours and 48 hours at rest and coughing/ physiotherapy was downgraded by 2 levels to low because of study limitations (risk of bias, -1) and conflicting results (inconsistency, -1).
The level of evidence regarding the outcome measure chronic postoperative pain at 3 and 12 months was downgraded by 3 levels to very low because of study limitations (risk of bias, -1); and number of included patients (imprecision, -2). The level of evidence regarding the outcome measure chronic postoperative pain at 6 months was downgraded by 2 levels to low because of study limitations (risk of bias, -1); and number of included patients (imprecision, -1).
The level of evidence regarding the outcome measure postoperative opioid consumption was downgraded by 3 levels to very low because of study limitations (risk of bias, -1); and because only one study with a limited number of included patients was available (imprecision, -2).
The level of evidence regarding the outcome measure hypotension was downgraded by 1 level to moderate because of study limitations (risk of bias, -1).
Zoeken en selecteren
A systematic review of the literature was performed to answer the following question:
What is the effect of thoracic wall blocks on postoperative pain, rescue medication (incl. postoperative opioid usage) and adverse events in adult patients undergoing an intrathoracic surgical procedure?
P (patients) Patients undergoing intrathoracic surgery
I (intervention) continuous paravertebral block (PVB)
C (control) thoracic epidural blockade (TEB)
O (outcome measure) postoperative pain
postoperative opioid consumption
chronic postoperative pain
adverse events
Relevant outcome measures
The guideline development group considered postoperative pain as a critical outcome measure for decision making; and chronic pain and postoperative opioid consumption and adverse events, as important outcome measures for decision making.
The working group defined the outcome measures as follows:
Postoperative pain (at rest and during mobilization/cough): Validated pain scale (Visual Analogue Scale (VAS) or Numeric Rating Scale (NRS) at post-anesthesia care unit (PACU) arrival, 6, 12, 24 and 48 hours post-surgery. Postoperative opioid consumption: defined as the total consumption in the first 24 hours after surgery (in Morphine Milligram Equivalent; MME). Chronic postoperative pain: pain > 3 months, in line with the international association study of pain (IASP). Adverse events: bleeding or hypotension.
The working group defined one point as a minimal clinically (patient) important difference on a 10-point pain scale and 10 mm on a 100 mm pain scale. Regarding postoperative opioid consumption, a difference of 10 mg was considered clinically relevant. For dichotomous variables, a difference of 10% was considered clinically relevant (RR ≤0.91 or ≥1.10; RD 0.10).
Search and select (Methods)
The databases Medline (via OVID) and Embase (via Embase.com) were searched with relevant search terms until 4-5-2022. The detailed search strategy is depicted under the tab Methods. The systematic literature search resulted in 435 hits. Studies were selected based on the following criteria:
Inclusion criteria:
- Systematic review of RCTs or RCT
- Thoracotomy or VATS
- Patients ≥18 years
- Conform PICO
Exclusion criteria:
- comparison of two variants of the same block
- comparisons with other blocks
- no original research
- n<20 per arm
A total of 68 studies (including 17 systematic reviews and 51 additional RCTs) were initially selected based on title and abstract screening. After reading the full text, 58 studies were excluded (see the table with reasons for exclusion under the tab Methods), and 10 studies were included.
Results
One Cochrane systematic review (reporting on 11 RCTs) and nine additional single RCTs were included in the analysis of the literature. Literature was found for comparisons between paravertebral block (PVB) versus thoracic epidural blockade (TEB) for two surgery types: 1) thoracotomy (15 studies) and 2) video-assisted thoracoscopic surgery (VATS) (5 studies). The summary of the literature and conclusions are divided into these surgery types. Important study characteristics and results are summarized in the evidence tables. The assessment of the risk of bias is summarized in the risk of bias tables.
Referenties
- Coveney E, Weltz CR, Greengrass R, Iglehart JD, Leight GS, Steele SM, Lyerly HK Use of paravertebral block anesthesia in the surgical management of breast cancer: experience in 156 cases Ann Surg. 1998 Apr;227(4):496-501.
- Ding W, Chen Y, Li D, Wang L, Liu H, Wang H, Zeng X. Investigation of single-dose thoracic paravertebral analgesia for postoperative pain control after thoracoscopic lobectomy - A randomized controlled trial. Int J Surg. 2018 Sep;57:8-14. doi: 10.1016/j.ijsu.2018.07.006. Epub 2018 Jul 26. PMID: 30056127.
- Huang QW, Li JB, Huang Y, Zhang WQ, Lu ZW. A Comparison of Analgesia After a Thoracoscopic Lung Cancer Operation with a Sustained Epidural Block and a Sustained Paravertebral Block: A Randomized Controlled Study. Adv Ther. 2020 Sep;37(9):4000-4014.
- Khoronenko V, Baskakov D, Leone M, Malanova A, Ryabov A, Pikin O, Golovashchenko M. Influence of Regional Anesthesia on the Rate of Chronic Postthoracotomy Pain Syndrome in Lung Cancer Patients. Ann Thorac Cardiovasc Surg. 2018 Aug 20;24(4):180-186.
- Kosi?ski S, Fry?lewicz E, Wi?koj? M, ?miel A, Zieli?ski M. Comparison of continuous epidural block and continuous paravertebral block in postoperative analgaesia after video-assisted thoracoscopic surgery lobectomy: a randomised, non-inferiority trial. Anaesthesiol Intensive Ther. 2016;48(5):280-287. doi: 10.5603/AIT.2016.0059. PMID: 28000203.
- Lai J, Situ D, Xie M, Yu P, Wang J, Long H, Lai R. Continuous Paravertebral Analgesia versus Continuous Epidural Analgesia after Video-Assisted Thoracoscopic Lobectomy for Lung Cancer: A Randomized Controlled Trial. Ann Thorac Cardiovasc Surg. 2021 Oct 20;27(5):297-303. doi: 10.5761/atcs.oa.20-00283. Epub 2021 Feb 16. PMID: 33597333; PMCID: PMC8560537.
- Li XL, Zhang J, Wan L, Wang J. Efficacy of Single-shot Thoracic Paravertebral Block Combined with Intravenous Analgesia Versus Continuous Thoracic Epidural Analgesia for Chronic Pain After Thoracotomy. Pain Physician. 2021 Sep;24(6):E753-E759. PMID: 34554693.
- Okajima H, Tanaka O, Ushio M, Higuchi Y, Nagai Y, Iijima K, Horikawa Y, Ijichi K. Ultrasound-guided continuous thoracic paravertebral block provides comparable analgesia and fewer episodes of hypotension than continuous epidural block after lung surgery. J Anesth. 2015 Jun;29(3):373-378. doi: 10.1007/s00540-014-1947-y. Epub 2014 Nov 15. PMID: 25398399.
- Patnaik R, Chhabra A, Subramaniam R, Arora MK, Goswami D, Srivastava A, Seenu V, Dhar A. Comparison of Paravertebral Block by Anatomic Landmark Technique to Ultrasound-Guided Paravertebral Block for Breast Surgery Anesthesia: A Randomized Controlled Trial. Reg Anesth Pain Med. 2018 May;43(4):385-390.
- Tamura T, Mori S, Mori A, Ando M, Yokota S, Shibata Y, Nishiwaki K. A randomized controlled trial comparing paravertebral block via the surgical field with thoracic epidural block using ropivacaine for post-thoracotomy pain relief. J Anesth. 2017 Apr;31(2):263-270.
- Yeap YL, Wolfe JW, Backfish-White KM, Young JV, Stewart J, Ceppa DP, Moser EAS, Birdas TJ. Randomized Prospective Study Evaluating Single-Injection Paravertebral Block, Paravertebral Catheter, and Thoracic Epidural Catheter for Postoperative Regional Analgesia After Video-Assisted Thoracoscopic Surgery. J Cardiothorac Vasc Anesth. 2020 Jul;34(7):1870-1876.
- Yeung JH, Gates S, Naidu BV, Wilson MJ, Gao Smith F. Paravertebral block versus thoracic epidural for patients undergoing thoracotomy. Cochrane Database Syst Rev. 2016 Feb 21;2(2):CD009121.
Evidence tabellen
Evidence tables
Evidence table for systematic review of RCTs and observational studies (intervention studies)
Research question: Wat is de rol van borstwandblokken (paravertebral block, erector spinae plane block, Intercostal nerve block) ten opzichte van epidurale pijnstilling bij patiënten die een intrathoracale chirurgische procedure ondergaan?
|
Study reference |
Study characteristics |
Patient characteristics |
Intervention (I) = PVB |
Comparison / control (C) = TEB
|
Follow-up |
Outcome measures and effect size |
Comments |
||
|
Method of insertion |
Method of use |
Method of insertion |
Method of use |
||||||
|
Yeung 2016
PS., study characteristics and results are extracted from the SR (unless stated otherwise) |
SR and meta-analysis of randomized controlled trials (RCTs) comparing PVB with TEB in thoracotomy, including upper gastrointestinal surgery
Literature search up to [month/year]
A: Bimston 1999 B: Cesati 2006 C: De Cosmo, 2002 D: Grider, 2012 E: Gulbahar, 2020 F: Ibrahim, 2009 H: Kobayashi, 2013 I: Matthews, 1989 J: Messina 2009 M: Pintaric, 2011 N: Richardson, 1999
Study design: RCT
Setting and Country: A: 2 hospitals, USA B: single centre, Italy C: single centre, Italy D: single centre, USA E: -, Turkey F: single centre, Egypt H: single centre, Japan I: -, UK J: single centre, Italy M: single centre, Slovenia N: single centre, UK
Source of funding and conflicts of interest: |
Inclusion/exclusion criteria SR: 1) Types of studies: We included only randomized controlled trials (RCTs). We have excluded quasi-randomized trials, for example where allocation was determined by days of the week or hospital number. 2) Types of participants: We included all adults undergoing elective thoracotomy including for upper gastrointestinal surgery. 3) Types of interventions: We included continuous thoracic epidural infusions using local anaesthetics, opioids, and any adjuvant therapies. The comparator was continuous paravertebral blockade using local anaesthetics and adjuvant therapies.
11 studies included
Important patient characteristics at baseline: Number of patients; characteristics important to the research question and/or for statistical adjustment (confounding in cohort studies); for example, age, sex, bmi, ...
N (I/C) A: 30/20 B: 21/21 C: 25/25 D: 50/25 E: 25/19 F: 25/25 H: 35/35 I: 10/10 J: 12/12 M: 16/16 N: 46/54
Groups comparable at baseline? |
A: Inserted under direct vision by surgeon B: Percutaneously by landmark technique before induction of GA C: Inserted under direct vision by surgeons D: Inserted under direct vision by surgeon E: Inserted under direct vision by surgeon F: Percutaneously by landmark technique before induction of GA H: Inserted under direct vision by surgeon I: Percutaneously by landmark at the end of procedure J: Percutaneously by landmark technique before induction of GA M: Percutaneously by landmark technique before induction of GA N: Inserted by surgeon under direct vision
|
A: 18 ml 0.5% bupivacaine bolus followed by infusion of 0.1% bupivacaine with 10 `g/ml fentanyl, 10 - 15 ml/ hr B: Pre-op Injections by landmark technique before induction of GA 15 ml of 0.75% ropivacaine C: Used at the end of operation only D: Used at the end of the operation only E: Used at the end of operation only F: 15 - 20 ml 0.5% ropivacaine bolus followed by 0.375% ropivacaine 0.1 ml/kg/ hr infusion H: Used at the end of operation only I: Used at the end of operation only J: Used at the end of operation only M: 0.5% levopubivacaine with 30 `g/kg morphine, dose depends on height N: Pre-op Injections by landmark technique before induction of GA 20 ml of 0.5% bupivacaine
|
A: Percutaneously by landmark technique before induction of GA B: Percutaneously by landmark technique before induction of GA C: Percutaneously by landmark technique before induction of GA D: Percutaneously by landmark technique before induction of GA E: Percutaneously by landmark technique before induction of GA F: Percutaneously by landmark technique before induction of GA H: Percutaneously by landmark technique before induction of GA I: Percutaneously by landmark technique at the end of procedure J: Percutaneously by landmark technique before induction of GA M: Percutaneously by landmark technique before induction of GA N: Percutaneously by landmark technique before induction of GA
|
A: Uncertain whether catheter was used during operation B: 5 ml bolus of 0.75% ropivacaine C: 5 ml bolus of 0.2% ropivacaine and sufentanil 10 Zg given as bolus. Catheter used during operation if required D: Used at the end of operation E: Used at the end of operation F: 5 - 8 ml of 0.5% ropivacaine bolus followed by 0.375% ropivacaine 0.1 ml/kg.hr infusion H: Used at the end of operation I: Used at the end of operation J: Used at the end of operation M: 0.25% levopubivacaine with 30 `g/kg morphine, dose depends on height N: 10 - 15 ml bolus of 0.25% bupivacaine
|
End-point of follow-up: 48 hours
For how many participants were no complete outcome data available? (intervention/control) There was no study reporting high levels of missing data (less than 15% in all cases). However, we rated two studies at high risk of bias: Gulbahar 2010 excluded 6/50 participants (12%), but all from the epidural arm; Perttunen 1995 excluded 6/51 randomized participants (12%).
|
Postoperative pain Visual analogue scale scores (VAS) were used in all of the studies but the scales were different; a majority of studies used the 0 to 10 scale, but Kaiser 1998 used VAS in 0 to 4 categories (reported as mean and standard deviation (SD)), and two studies (Ibrahim 2009; Perttunen 1995) used VAS 0 to 5.
2-6h at rest SMD [95% CI]:
C: 1.82 [1.16, 2.49] D: -0.25 [-0.83, 0.34] H: 0.10 [-0.37, 0.57] I: 0.00 [-0.90, 0.90] J: 0.37 [-0.44, 1.18] M: -0.14 [-0.85, 0.56]
Pooled effect (random effects model): SMD 0.32, 95% CI -0.30 to 0.94
2-6h during coughing/ on movement SMD [95% CI]:
C: 0.93 [0.34, 1.52] D: 0.43 [-0.16, 1.02] M: -0.20 [-0.90, 0.51]
Pooled effect (random effects model): SMD 0.41, 95% CI -0.20 to 1.03
24h at rest SMD [95% CI]:
C: 0.39 [-0.17, 0.95] D: 0.77 [0.16, 1.37] H: -0.09 [-0.56, 0.38] I: 0.08 [-0.82, 0.98] J: 0.00 [-0.80, 0.80] M: -0.32 [-1.03, 0.39]
Pooled effect (random effects model): SMD 0.16, 95% CI -0.17 to 0.48
24h during coughing/ on movement SMD [95% CI]:
C: -0.05 [-0.61, 0.50] D: -0.18 [-0.76, 0.41] M: -0.61 [-1.33, 0.12]
Pooled effect (random effects model): SMD -0.23, 95% CI -0.58 to 0.12
Adverse events; hypotension RR [95% CI] A: 0.67 [0.04, 10.05] B: 0.11 [0.01, 1.94] D: 0.28 [0.02, 5.22] E: 0.15 [0.01, 3.03] F: 0.08 [0.00, 1.30] H: 0.40 [0.08, 1.93] I: 0.07 [0.00, 1.09] N: 0.07 [0.00, 1.21]
Pooled effect (fixed effects model): RR 0.16, 95% CI 0.07 to 0.38
|
Aim: To compare the two regional techniques of TEB and PVB in adults undergoing elective thoracotomy with respect to: 1. analgesic efficacy; 2. the incidence of major complications (including mortality); 3. the incidence of minor complications; 4. length of hospital stay; 5. cost effectiveness.
|
Evidence table for intervention studies
|
Study reference |
Study characteristics |
Patient characteristics 2 |
Intervention (I) |
Comparison / control (C) 3
|
Follow-up |
Outcome measures and effect size 4 |
Comments |
|
PVB versus Epidural anesthesia - thoractomy |
|||||||
|
Huang, 2020 |
Type of study: RCT
Setting and country: single centre, China
Funding and conflicts of interest: funding: This research was supported by Fujian Natural Science Foundation, China (No. 2018J01206). The journal’s Rapid Service Fee was funded by the authors. CI: Qiao-Wen Huang, Jia-Bin Li, Ye Huang, Wen-Qing Zhang and Zhi-Wei Lu have nothing to disclose |
Inclusion criteria: elective unilateral thoracoscopic surgery for lung cancer (ASA II–III, aged less than 80 years old) from February 1, 2019 to October 31, 2019 in the Zhangzhou Affiliated Hospital of Fujian Medical University.
Exclusion criteria: all patients that had a history of thoracic spine surgery; coagulopathy; a BMI of 28 kg m-2 or higher; a history of drug allergies; ASA C IV; and patients that refused to participate in the trial
Surgery: thoracoscopic surgery for lung cancer
N total at baseline: Intervention - T: 45 Intervention – P: 32 Control -E: 39
Important prognostic factors2: For example age ± SD: I -T (n=50): 56.86 ± 10.31 I -P (n=50): 58.50 ± 9.61 C (n=48): 58.58 ± 9.68
Sex (M/F): I I -T (n=50): 31/19 I -P (n=50): 23/27 C (n=48): 30/18
Groups comparable at baseline? There was no significant difference in the general characteristics of the three groups of patients
|
ultrasound-guided continuous thoracic paravertebral block
2 subgroups: the interplanar approach in an oblique axial transverse section—T group;
0.33% of ropivacaine (AstraZeneca, imported drug registration no. H20140763, Lot NAZB) was injected at a dose of 1 mg kg-1
A dose of 0.7 mg kg-1 of 0.33% ropivacaine was added every 4 h during the operation
the interplanar approach in sagittal parasagittal section—P group
0.33% of ropivacaine (AstraZeneca, imported drug registration no. H20140763, Lot NAZB) was injected at a dose of 1 mg kg-1
A dose of 0.7 mg kg-1 of 0.33% ropivacaine was added every 4 h during the operation
|
continuous epidural analgesia (E-group)
injection of 0.25% of ropivacaine (3 ml) via the catheter. Another 3–5 ml was added every 5 min and the blocked segments by perceived less or lost sensation in response to cold and pinprick were tested until the blocked segments covered the surgical incision. The physician also recorded the plane stabilization time and the range of the blocking plane, and added 0.25% of ropivacaine 3–5 ml every 2 h during the operation. |
Length of follow-up: 48h
After randomization (n=150, 50 patients in each group)
Loss-to-follow-up: Intervention - T: 5 Reasons: haemorrhage (n=1); conversion of surgery approach (n=1); benign nodules (n=2); failure puncture (n=1)
Intervention – P: 18 Reasons: conversion of surgery approach (n=2); benign nodules (n=1); failure puncture (n=2); failure catheter placement (n=13)
Control -E: 11 Reasons: refuse (n=2); benign nodules (n=1); failure puncture (n=7);
Incomplete outcome data: n.a.
|
Postoperative pain numerical rating scale (NRS) scores at rest and during coughing at 2 h, 4 h, 8 h, 12 h, 24 h, 36 h, and 48 h after the surgery were recorded
Data per time-point reported in figure, not readable.
NRS ≥ 4 during 0–24 h post operation (n) I -T: 38 I -P: 26 C : 27
NRS ≥ 4 during 24–48 h post operation (n) I -T: 29 I -P: 23 C : 9
Opioid consumption
0–24 h opioids consumption (MME) I -T: 8.75 ± 7.93 I -P: 8.98 ± 7.09 C : 2.24 ± 4.64
24–48 h opioids consumption (MME) I -T: 10.28 ± 6.94 I -P: 9.38 ± 7.78 C : 1.60 ± 4.47
|
Study objective: to compare the challenge of puncture and catheterization and the effect of postoperative analgesia of ultrasound-guided continuous thoracic paravertebral block and the continuous epidural analgesia in patients receiving thoracoscopic surgery for lung cancer
Study conclusion: The continuous analgesia technique of paravertebral space catheterization cannot replace the continuous epidural analgesia in thoracoscopic lung cancer surgery as the latter technique is still considered to be the gold standard |
|
Khoronenko, 2018
|
Type of study: RCT*
Setting and country: single centre, France
Funding and conflicts of interest: funding: The study was only supported by departmental funds. CI: All authors have no conflict of interest. |
Inclusion criteria: adult patients and American Society of Anesthesiologists (ASA) physical status from I to III
Exclusion criteria: general anesthesia within 7 days before study inclusion, administration of an experimental drug within 30 days before surgery, preoperative pain syndrome, or use of analgesics, acute unstable angina, acute myocardial infarction documented by laboratory findings within the past 6 weeks, heart rate (HR) <50 beats per minute (bpm), systolic blood pressure (SBP) < 100 mmHg, heart block, and preoperative vasopressor administration.
Surgery: thoracotomy for non-small-cell lung cancer
N total at baseline: I1: 100
Important prognostic factors2: For example age ± SD: I1: 58 ± 9
Sex (m/f): I1: 77/23
Groups comparable at baseline? The groups were comparable regarding demographic features, blood loss, and type and duration of surgery
|
I1: PVB
Propofol (2 mg/kg), fentanyl (0.002 mg/kg), ketamine (25 mg), and rocuronium (0.6 mg/kg) were administered for induction. After endotracheal intubation, anesthesia was maintained with sevoflurane (0.8–1 MAC), fentanyl (0.05–0.1 mg IV every 15–30 minutes when the SBP increased by more than 15% from the baseline value or was >140 mmHg).
|
TEA
Propofol (2 mg/kg), fentanyl (0.002 mg/kg), ketamine (25 mg), and rocuronium (0.6 mg/kg) were administered for induction. After endotracheal intubation, anesthesia was maintained with sevoflurane (0.8–1 MAC), fentanyl (0.05–0.1 mg IV every 15–30 minutes when the SBP increased by more than 15% from the baseline value or was >140 mmHg).
|
Length of follow-up: 6 months
Loss-to-follow-up (total)*: Screened: 347 of whom 47 met exclusion criteria
Reasons: 17 patients suffered from an atrioventricular block I–II, 23 patients had a HR less than 50 bpm, and seven patients had preoperative hypotension with SBP less than 100 mmH.
Incomplete outcome data: Not reported
|
Chronic pain Horizontal VAS: 0 = no pain and 100 mm = worst possible pain. Static and dynamic pain components were assessed 7 days, 1 month, and 6 months after surgery
6 months – static (means ± standard deviation) I1: 0.16 ± 0.39 C: 0.10 ± 0.30
6 months – dynamic (means ± standard deviation) I1: 0.59 ± 0.98 C: 0.38 ± 0.79
|
*note that this is a study with 3 comparisons: PVB, INB and TEA
Stjudy objective: to assess whether the type of regional anesthesia influenced the incidence of chronic postthoracotomy pain syndrome (CPTPS).
Study conclusion: Patients who were administered TEA had less incidence and reduced intensity of CPTPS when compared with INB. Six months after surgery, the incidences of CPTPS were 23%, 34%, and 40% in the TEA, PVB, and INB groups, respectively. We did not find differences in CPTPS frequency and intensity between PVB and other groups. A pain syndrome that persists more than 1 month after surgery should be considered a predictor of pain syndrome chronicity.
|
|
Li, 2021 |
Type of study: RCT
Setting and country: single-centre, China
Funding and conflicts of interest: funding: There was no external funding in the preparation of this manuscript. CI: h author certifies that he or she, or a member of his or her immediate family, has no commercial association (i.e., consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted manuscript. |
Inclusion criteria: patients scheduled for elective thoracotomy
Exclusion criteria: contraindication to the use of TEA or TPVB, pregnancy, a history of cardiovascular and gastroesophageal surgery, preexisting pain syndrome, psychological disorders, coagulopathy, and severe hepatic, cardiovascular, or renal disorders. Standard posterolateral thoracotomy was chosen for all patients.
Surgery: elective thoracotomy
N total at baseline: Intervention: 42 Control: 41
Important prognostic factors2: For example age ± SD: I: 60.6 ± 9.0 C: 63.0 ± 9.6
Sex (ratio F/M) I: 16/26 C: 9/32
Groups comparable at baseline? Yes, the 2 groups of patients were comparable in surgical and demographic data
|
single-shot TPVB (ultrasound guided) combined with intravenous analgesia (P)
20 mL of 0.25% ropivacaine (Naropin, AstraZeneca, Gothenburg, Sweden) combined with 1 µg/kg dexmedetomidine (Ai Bei Ning, Jiangsu Hengrui Medicine, Lianyungang, China) was injected after a gentle aspiration test for blood or air (13,14).
In these patients, the intravenous PCA (sufentanil, basal rate of 0.03 µg/ kg/h, bolus dose of 0.01 µg/kg, lockout time 15 min) was used for 48 hours postoperatively.
|
continuous TEA (E)
epidural infusion of sufentanil (0.2 μg/mL) and ropivacaine 0.06% was started at 5–10 mL/h before the skin incision and maintained during the operation. Within 48 hours after the operation, the epidural patient-controlled analgesia (PCA) (0.06% ropivacaine + 0.2μg/mL sufentanil, basal rate was 5 mL/h, 3 mL PCA at a lock-out time of 30 minutes) was used. |
Length of follow-up: 12 months post operation
Loss-to-follow-up: Before allocation I: 3 (of 48) Reason did not receive allocated intervention due to inoperability (2) and reoperation (1)
C: 3 (of 48) Reason did not receive allocated intervention due to inoperability
After allocation Intervention:3 Reasons 3 death
Control: 4 Reasons 4 death
Incomplete outcome data: Intervention:0 Control: 0
|
Postoperative pain Verbal Rating Scale (VRS: 0 for no pain, and 10 for the worst pain) at rest and during coughing at 6, 24 and 48 h
Outcome data presented in box plots
6h at rest (median, IQR) I: 2 (0-3.5) In mean±SD I: 2 ± 0.875
24h at rest (median, IQR) I: 2 (2-4)
In mean±SD I: 2 ± 0.5
48h at rest (median, IQR) I: 2 (0,5-2,5)
In mean±SD I: 2 ± 0.5
><
6h at coughing (median, IQR) I: 2 (1-5)
In mean±SD I: 2 ± 1
24h at coughing (median, IQR) I: 5 (3-6)
In mean±SD I: 5 ± 0.75
48h at coughing (median, IQR) I: 3 (2-5)
In mean±SD I: 3 ± 0.75
Chronic pain Verbal Rating Scale (VRS: 0 for no pain, and 10 for the worst pain) at rest at 3, 6 and 12 months
Outcome data presented in box plots
3m at rest (median, IQR) I: 1 (0-2) 6m at rest (median, IQR) I: 0 (0-2)
12m at rest (median, IQR) I: 0 (0-1)
|
Study objective: to evaluate the impact of single-shot TPVB combined with intravenous analgesia versus continuous thoracic epidural analgesia (TEA) on chronic pain incidence after thoracotomy
Study conclusion: In conclusion, our results show that patients who received continuous TEA had less acute pain intensity within 24 hours after operation and lower chronic pain incidence at rest at 3 and 12 months postoperation when compared with single-shot TPVB combined with intravenous analgesia. In addition, acute pain intensity within 24 hours postoperation appears to be a predictor of chronic pain. Therefore, these results suggest the importance of aggressive management of acute pain postoperation, not only for the immediate benefit but possibly also to prevent the transition to chronicity. |
|
Tamura, 2017 |
Type of study: RCT
Setting and country: single centre, Japan
Funding and conflicts of interest: not reported |
Inclusion criteria: lung cancer patients scheduled for elective single-side lung lobectomy with antero- or verticalaxillary incision were recruited during an 18-month period. The patients were aged 20–80 years and had an American Society of Anesthesiologists physical status of I–II.
Exclusion criteria: The exclusion criteria were the same as those in our previous study [10] with the following additional criteria applied: combined resection of parietal pleura, coagulation disorder, thrombocytopenia, anti-coagulation therapy, and heart failure
Surgery: single-side lung lobectomy
N total at baseline: (analyses) Intervention: 36 Control: 36
Important prognostic factors2: For example age ± SD: I: 67.1 ± 8.7 C: 66.9 ± 9.7
Sex (male:female): I: 29:7 C: 28:8
Groups comparable at baseline? Yes, demographic data and surgical characteristics, including duration of anesthesia, operation, and hospitalization, were comparable in both groups
|
PVB catheter (P)
20 mL of 0.375% ropivacaine was administered as a bolus, followed by a 300-mL of continuous infusion of 0.2% ropivacaine at 5 mL/h
|
Epidural catheter (E)
5 mL of 0.375% ropivacaine (Anapeine injection; AstraZeneca K.K.) was administered as a bolus, followed by a 300-mL continuous infusion of 0.2% ropivacaine using an infusion pump (Multirate Infusor LV; Baxter Healthcare Co. Inc., Deerfield, IL) at 5 mL/h
|
Length of follow-up: 42h
Loss-to-follow-up: Intervention: 4 of 40 Reason: withdrawal
Control: 4 of 40 Reason: withdrawal
Incomplete outcome data: Intervention: 0 Control: 0
|
Postoperative pain visual analog scale (VAS) while coughing, moving, and at rest 2 h after the bolus injection of ropivacaine; these evaluations were also performed at 1, 6, 12, 18, and 42 h after the injection. The VAS consisted of a 100-mm line (0 mm, no pain; 100 mm, worst pain imaginable).
Outcome data presented in line charts that are not readable.
All mean VAS scores in group E were significantly lower than those in group P throughout the observation period.
|
Study objective: to evaluate analgesic efficacy between paravertebral block via the surgical field (PVB-sf), in which the catheter was inserted into the ventral side of the sympathetic trunk in the paravertebral space by a thoracic surgeon under thoracoscopic visualization, and epidural block (Epi) using ropivacaine for post-thoracotomy pain relief
Study conclusion: In conclusion, Epi was superior to PVB-sf, in which the catheter was inserted into the ventral side of the sympathetic trunk in the paravertebral space, for post-thoracotomy pain management, as indicated by differences in the effective sensory block range of local anesthetics. |
|
PVB versus Epidural anesthesia - VATS |
|||||||
|
Ding, 2018 |
Type of study: RCT
Setting and country: single centre, China
Funding and conflicts of interest: funding: First Excellent Young People Project of the Second Hospital of Harbin Medical University IC: The authors declare that they have no competing interests. |
Inclusion criteria: patients classified as American Society of Anesthesiology Physical Status I/III undergoing elective video-assisted thoracoscopic lobectomy. Patients included in the study were between the ages of 18 and 70 years; had no neurologic or psychiatric illnesses, renal or hepatic insufficiency, pregnancy, diabetes, allergy to local anesthetics, or coagulation abnormalities; and were not undergoing anticoagulant therapy.
Exclusion criteria: Not reported
Surgery: elective video-assisted thoracoscopic lobectomy
N total at baseline: (analyses) Intervention: 36 Control: 32
Important prognostic factors2: For example age ± SD: I: 56.7 ± 9.3 C: 53.1 ± 10.9
Sex (% male): I: 63.9 C: 46.9
Groups comparable at baseline? There were no differences between groups in terms of patient, surgery, and PVB characteristics and intraoperative data
|
single-dose thoracic paravertebral analgesia
single-dose 0.5% ropivacaine PVB before the operation combined with the PCIA (0.01 μg/kg demand dose and 15 min lockout period with 0.03 μg/kg/h background infusion) after extubation during the 48-h postoperative period |
Thoracic epidural analgesia
intraoperative thoracic epidural anesthesia with 0.5% ropivacaine and a single dose of epidural morphine (0.03 mg/kg) after extubation, combined with the same PCIA scheme. |
Length of follow-up: 48h
Loss-to-follow-up: I: 2 Reasons: 1 thoractomy, 1 sensory block level did not reach the required level
C: 3 Reasons: 2 thoractomy, 1 sensory block level did not reach the required level
Incomplete outcome data: Intervention:0 Control: 0
|
Postoperative pain Verbal rating score (VRS; scale of 0–10, with 0 indicating no pain and 10 indicating the worst pain imaginable) was assessed at 2, 6, 12, 24, 36, and 48 h after surgery at rest and after coughing.
Data in figure, not readable
Postoperative opioid consumption total dose of sufentanil used during the 48-h postoperative period
Data in figure, not readable
Adverse events Hypotension n (%) I: 5, 13.9 C: 11, 34.4
|
Study objective: to investigate the postoperative analgesic effect of TEA, PVB, and PVB with dexmedetomidine in patients undergoing thoracoscopic lobectomy |
|
Kosiński, 2016 |
Type of study: RCT
Setting and country: single centre, Poland
Funding and conflicts of interest: not reported |
Inclusion criteria: patients qualified for VATS lobectomy due to cancer, aged 18-85 years, of both genders, ASA I-III, an understanding of the principles of VAS pain assessment and no chronic pain
Exclusion criteria: technical failures to insert an epidural or paravertebral catheter, abandonment of resection (e.g., in cases of neoplastic dissemination), conversion of VATS to thoracotomy, anatomical obstacles to drug distribution found intraoperatively, cases in which the VAS assessment of pain severity was infeasible (e.g., postoperative delirium), use of other drugs affecting pain sensations, artificial lung ventilation, discontinuation of local anaesthesia for technical reasons (e.g., catheter slipping out or damage), and the use of drugs or doses that were not included in the study protocol.
Surgery: Video-assisted (VATS) lung lobectomy
N total at baseline: (for analysis) Intervention: 26 Control: 25
Important prognostic factors2: Age, mean (range): I: 64.7 (44–73) C: 59.9 (28–78)
Sex (female): I: 12 (46%) C: 10 (40%)
Groups comparable at baseline? yes
|
PVB
continuous infusion of 0.25% bupivacaine with epinephrine, intravenous ketoprofen and paracetamol |
TEA
continuous infusion of 0.25% bupivacaine with epinephrine, intravenous ketoprofen and paracetamol
|
Length of follow-up: 72h
Loss-to-follow-up: Intervention: 11 Reasons: 1 failure of installation; 7 lesser extent of resection; 1 non-standard technique; 1 postoperative artificial ventilation; 1 technical failure.
Control: 16 Reasons: 7 failure of installation; 1 resection abandonment; 1 lesser extent of resection; 3 conversions to thoracotomy; 1 postoperative artificial ventilation; 2 violations of protocol; 1 technical failure.
Incomplete outcome data: n.a.
|
Postoperative pain VAS was evaluated 1, 4, 8, 24, 36, 48 and 72 h after the procedures using a standard ruler. In each case, static (at rest) and dynamic assessments (on cough).
data provided in graphs, not readable.
Significant differences were found in the measurements at 24 h — both at rest and on coughing (P = 0.01 and P = 0.023, respectively) — and in static pain at 36 h and 48 h (P = 0.025 and P = 0.026, respectively).
Postoperative opioid consumption Postoperative morphine dosage (Emergency PCA-administered morphine doses (mg h-1))
data provided in graphs, not readable.
The comparative analysis of both groups did not reveal any significant differences in the postoperative morphine dosage. The mean dose was 0.4 mg h-1 on day 0, 0.37 mg h-1 on day 1, 0.21 on day 2 and 0.14 mg h-1 on day 3
Adverse events I: 2 C: 8
|
epidural blocks were performed using the classic paramedian approach technique, whereas paravertebral blocks were performed according to the Eason and Wyatt method.
Study objective: to compare the analgaesic efficacy of continuous thoracic epidural block (TEA) and percutaneous continuous paravertebral block (PVB) in patients undergoing video-assisted lung lobectomy.
Study conclusion: 1. Pain following VATS lobectomy is severe and requires the use of complex techniques of postoperative analgaesia, including the methods of regional anaesthesia. 2. Continuous paravertebral block using the classical landmark puncture technique is as equally effective as epidural block for multimodal analgaesia. 3. Continuous paravertebral block has a better profile of safety than epidural block, which is particularly visible in the lower incidences of hypotension and urinary retention |
|
Lai, 2021 |
Type of study: RCT
Setting and country: single centre, China
Funding and conflicts of interest: funding: not reported, CI: All authors have no conflict of interests to declare. |
Inclusion criteria: patients aged 18–80 years old who were undergoing VATS lobectomy, American Society of Anesthesiologists (ASA) physical status class I–III, an understanding of the principle of visual rating scale (VAS) pain assessment and no chronic pain.
Exclusion criteria: patients who were on anticoagulation, patients taking opioids for greater than three weeks prior to surgery, patients with a contraindication to regional anesthesia such as infection close to the site of puncture, allergy to local anesthetics, and those with a history of chronic pain, severe cardiovascular disease, liver or renal insufficiency, a change in surgery type, conversion of VATS to thoracotomy, accidental catheter slipping, and patient refusal.
Surgery: VATS lobectomy
N total at baseline: Intervention: 43 Control: 43
Important prognostic factors2: For example age ± SD: I: 59 ± 9 C: 57 ± 9
Sex (% female): I: 16 (37.2) C: 20 (46.5)
Groups comparable at baseline? There were no significant differences between the two groups in terms of demographics
|
continuous paravertebral analgesia (P)*
Patients in the paravertebral group received an initial bolus of 0.5% ropivacaine 0.1 mL/kg and then 0.1 mL/kg/h infusion for 48 h postoperatively.
|
continuous epidural analgesia (E)*
The epidural infusion consisted of ropivacaine 0.15% with 6 mcg/mL of hydromorphone and was administered via a pump (Opon, Jiangsu aipeng Medical Technology Co., Ltd, Nantong, China) starting at 2 mL/h for 48 h postoperatively. |
Length of follow-up: 72h
Loss-to-follow-up:
Randomization: n = 46 Intervention: 3 Reasons: 1 converted to open procedure; 2 catheter slipping out
Randomization: n = 45 Control: 2 Reasons: 1 converted to open procedure; 1 catheter slipping out
Incomplete outcome data: Intervention: 0 (of 43) Control: 0 (of 43)
|
Postoperative pain at rest and on coughing using an 11-point VAS scale (0 = no pain, 10 = worst imaginable pain).
Data is provided in figures (line chart) that is not readable.
The comparative analysis of pain using GLM demonstrated slight intergroup differences. The U test showed significant differences in pain at 24 h at rest and on coughing (P = 0.001 and P < 0.001, respectively). There were no differences between the two groups at other time points.
Postoperative opioid consumption Cumulative oxycodone consumption 24h (mg) (mean ± standard deviation) I: 8.7 ± 1.3 C: 4.0 ± 1.3
To MME (conversion factor 1.5): I: 13.05 ± 1.95 C: 6 ± 1.95
Adverse events Hypotension (number of patients (%)) I: 2 (4.7) |
Study objective: to compare the analgesia efficacy and side effects of continuous paravertebral analgesia and continuous epidural analgesia for postoperative pain after VATS lobectomy
* All patients also benefited from IV patient controlled analgesia (PCA) of oxycodone 50 mg and palonosetron 0.075 mg mixed with normal saline to a total volume of 100 mL. The disposable PCA device was set to deliver no background infusion and 2 mL on-demand bolus with a lockout time of 5 min.
Study conclusion: Though TEA has more adverse events than PVB, it is more analgesic effective than PVB in patients undergoing VATS lobectomy. TEA may be superior to PVB in patients undergoing thoracoscopic lobectomy. |
|
Okajima, 2015 |
Type of study: RCT
Setting and country: single centre, Japan
Funding and conflicts of interest: not reported |
Inclusion criteria: consecutive patients (age: 18–75 years) with ASA physical status I–III scheduled for VATS with an axillary skin incision of 6–8 cm at Nishi-Kobe Medical Center in Kobe, Japan, between August 2008 and October 2009.
Exclusion criteria: allergy to non-steroidal anti-inflammatory drugs (NSAIDs) or LA, renal or hepatic failure, body mass index over 30 kg/m2 , infection at injection site, coagulative abnormality, and inability to comprehend pain scoring
Surgery: video-assisted thoracic surgery (VATS) (i.e., lung surgery including lobectomy, segmentectomy, wedge resection)
N total at baseline: (complete data) Intervention: 36 Control: 33
Important prognostic factors2: For example age ± SD: I: 61.0 ± 15.9 C: 63.0 ± 11.1
Sex (m/f): I: 20/16 C: 24/9
Type of surgery Lobectomy I: 11 C: 15
Segmentectomy I: 2 C: 2
Wedge resection 1: 23 C: 16
Groups comparable at baseline? patients’ characteristics did not differ between the two groups
|
ultrasound-guided PVB
three single blocks with 5 ml of 0.5 % ropivacaine
|
TEB with the addition of fentanyl to ropivacaine
An epidural bolus dose (5–7 ml) of 0.25–0.375 % ropivacaine was administered before skin incision
|
Length of follow-up: POD2
Loss-to-follow-up: Intervention: 9 Reason : Extended resection(n=4) Failed blocks(n=4) Cancelled operation(n=1)
Control: 9 Reason: Extended resection(n=9) Re-operations(n=1 ) Failed blocks(n=1) Postoperative renal failure(n=1)
Incomplete outcome data: Intervention: N (%) Reasons (describe)
Control: N (%) Reasons (describe)
|
Postoperative pain* verbal rating scale for pain at rest (VRS; 0 = none, 10 = maximum pain)
Outcome data presented in box plots
0h (median, IQR) I: 1,5 (0-6) C: 4 (0-7)
6h (median, IQR) I: 1 (0-3) C: 1 (0-3,25)
12h (median, IQR) I: 1 (0-3) C: 1 (0-1,25)
24h (median, IQR) I: 1 ( 0-3) C: 1 (0-2)
POD2 (median, IQR) I: 1 (0-3) C: 1 (0-3,5)
Postoperative opioid consumption Postoperative consumptions of fentanyl I: 0.6 ± 0.14 mg C: 0.6 ± 0.16 mg
Adverse events Hypotension I: 1 of 36 C: 7 of 33
|
Study objective: to compare postoperative analgesia, side effects, and complications between ultrasound-guided PVB (USG-PVB) and TEB with the addition of fentanyl to ropivacaine after lung surgery
* When the patients suffered from severe pain despite this protocol for analgesia, intravenous fentanyl was administered and then those patients were withdrawn from this study
Study conclusion: In conclusion, USG-PVB provided similar postoperative analgesia to TEB for patients undergoing VATS. PVB had better advantages in terms of the maintenance of hemodynamics and the prevention of hypotension. On the other hand, PVB combined with fentanyl caused a high incidence of PONV, similarly to TEB, so further trials are required to search for an adequate dosage. We conclude that USG-PVB and TEB are similarly acceptable for postoperative analgesia after VATS. |
|
Yeap, 2021 |
Type of study: RCT
Setting and country: single centre, USA
Funding and conflicts of interest: Funding: Indiany University; IC: The authors declare no conflicts of interest |
Inclusion criteria: All VATS cases scheduled by thoracic surgeons at Indiana University Health-University Hospital , American Society of Anesthesiologists (ASA) classes 1 to 4, ≥18 years of age, and patient desiring regional anesthesia for adjunct postoperative pain control.
Exclusion criteria: contraindication to TEA or PVB, history of substance abuse in the prior 6 months, opioid tolerance, necessitation for intubation after surgery, and known allergy or contraindication to any study medications (oxycodone/acetaminophen, bupivacaine, or ropivacaine)
Surgery: VAT
N total at baseline: I1: 40 I2: 40 C: 40
Important prognostic factors2: For example age ± SD: I1: 63.5 (48.5-70.0) I2: 62.0 (45.5-69.5) C: 61.0 (52.0-70.0)
Sex (female): I1: 16 (40.0%) I2: 19 (47.5%) C: 25 (62.5%)
Groups comparable at baseline? All characteristics were similar among groups except for over-representation (p = 0.026) by Caucasian race in the TEA group (97.5% v 80.0% and 82.5% for single-injection PVB or PVB catheter, respectively). Also, the median time to place the PVB catheter (12.0 min) was longer than the time required for the singleinjection PVB (6.0 min) or TEA (5.0 min; p < 0.001).
|
I1: Ultrasound-guided PVB catheter
Ropivacaine, 0.2%, was delivered postoperatively at a rate of 10 mL/h by infusion pump (OnQ Pain Relief System, Kimberly-Clark, Roswell, GA)
I2: ultrasound-guided single-injection PVB
30 mL of 0.5% ropivacaine
|
TEA
epidural mixture of 0.125% bupivacaine and 0.05 mg/mL of hydromorphone (starting at the end of the surgery) |
Length of follow-up: 6 months
Loss-to-follow-up: 53 in total for 6 month measurement
I1: 17 I2: 18 C: 18
Reason: not described.
Incomplete outcome data: Not reported
|
Postoperative pain visual analog scale pain scores (at rest and with knee flexion)
1h – rest in median (IQR) I1: 5.0 (3.0-8.0) I2: 6.0 (3.5-8.0) C: 6.0 (3.5-8.0)
24h - rest in median (IQR) I1: 4.0 (3.0-5.5) I2: 4.5 (2.5-6.0) C: 3.0 (1.0-5.0)
48h - rest in median (IQR) I1: 3.0 (1.0-5.0) I2: 4.0 (2.0-6.0) C: 2.0 (1.0-4.0)
1h – knee flexion in median (IQR) I1: 6.5 (4.0-9.0) I2: 7.0 (5.0-9.0) C: 7.0 (5.0-9.0)
24h - knee flexion in median (IQR) I1: 6.5 (5.0-8.0) I2: 7.0 (5.0-8.0) C: 6.0 (2.0-7.0)
48h - knee flexion in median (IQR) I1: 5.0 (3.0-7.0) I2: 6.0 (3.0-8.0) C: 4.0 (2.0-6.0)
Postoperative opioid consumption morphine milligram equivalents [MME]
24h in median (IQR) I1: 88.0 (34.5-143.0) I2: 75.5 (30.5-121.8) C: 15.0 (0-30.0)
In mean±SD I2: 75.5± 22,825 C: 15.0 ±7,5
Chronic pain at 6 months (n=67) (single-injection PVB, n = 23; PVB catheter, n = 22; TEA, n = 22)
Chronic postsurgical pain indicators (based on 13 survey questions) were similar for all 3 groups. |
Study objective: to compare adjunct analgesic modalities for VATS, including paravertebral nerve blockade (PVB) and thoracic epidural anesthesia (TEA).
Study conclusion: In conclusion, pain scores and opioid consumption after VATS procedures were lower with TEA than either PVB technique. Although these results support TEA as the superior technique, the small difference in pain scores may not be clinically significant, and the risk of rare adverse events with TEA might lead one to consider single-injection PVB. In the authors’ institution, single-injection PVB is performed for most VATS cases owing to the shorter placement time and lower risk for serious adverse events. Clearly, use of PVB catheters adds no benefit and cannot be justified. |
Notes:
- Prognostic balance between treatment groups is usually guaranteed in randomized studies, but non-randomized (observational) studies require matching of patients between treatment groups (case-control studies) or multivariate adjustment for prognostic factors (confounders) (cohort studies); the evidence table should contain sufficient details on these procedures
- Provide data per treatment group on the most important prognostic factors [(potential) confounders]
- For case-control studies, provide sufficient detail on the procedure used to match cases and controls
- For cohort studies, provide sufficient detail on the (multivariate) analyses used to adjust for (potential) confounders
Table of quality assessment for systematic reviews of RCTs and observational studies
Based on AMSTAR checklist (Shea et al.; 2007, BMC Methodol 7: 10; doi:10.1186/1471-2288-7-10) and PRISMA checklist (Moher et al 2009, PLoS Med 6: e1000097; doi:10.1371/journal.pmed1000097)
|
Study
First author, year |
Appropriate and clearly focused question?1
Yes/no/unclear |
Comprehensive and systematic literature search?2
Yes/no/unclear |
Description of included and excluded studies?3
Yes/no/unclear |
Description of relevant characteristics of included studies?4
Yes/no/unclear |
Appropriate adjustment for potential confounders in observational studies?5
Yes/no/unclear/notapplicable |
Assessment of scientific quality of included studies?6
Yes/no/unclear |
Enough similarities between studies to make combining them reasonable?7
Yes/no/unclear |
Potential risk of publication bias taken into account?8
Yes/no/unclear |
Potential conflicts of interest reported?9
Yes/no/unclear |
|
Yeung, 2016 |
Yes |
Yes |
Yes |
Yes, but data on sex is missing in the SR |
Not applicable |
Yes
|
Yes |
Yes |
No |
Risk of bias table for intervention studies (randomized controlled trials; based on Cochrane risk of bias tool and suggestions by the CLARITY Group at McMaster University)
|
Study reference
(first author, publication year) |
Was the allocation sequence adequately generated?
Definitely yes Probably yes Probably no Definitely no |
Was the allocation adequately concealed?
Definitely yes Probably yes Probably no Definitely no |
Blinding: Was knowledge of the allocated interventions adequately prevented?
Were patients blinded?
Were healthcare providers blinded?
Were data collectors blinded?
Were outcome assessors blinded?
Were data analysts blinded?
Definitely yes Probably yes Probably no Definitely no |
Was loss to follow-up (missing outcome data) infrequent?
Definitely yes Probably yes Probably no Definitely no |
Are reports of the study free of selective outcome reporting?
Definitely yes Probably yes Probably no Definitely no |
Was the study apparently free of other problems that could put it at a risk of bias?
Definitely yes Probably yes Probably no Definitely no |
Overall risk of bias If applicable/necessary, per outcome measure
LOW Some concerns HIGH
|
|
PVB versus Epidural anesthesia – thoracotomy |
|||||||
|
Casati, 2006 |
Probably yes
Reason: computer generated sequence |
Probably no
Reason: sealed envelopes, but not described how or by whom they were opened |
Probably no
Reason: blinded observer study |
Probably no
Reason: not reported, but data provided for all included patients |
Definitely yes
Reason: all relevant outcomes were reported |
Probably yes
Reason: outcome data provided bar charts or summary measures in the text |
Some concerns
Reason: allocation and reporting |
|
Khoronenko, 2018 |
Probably no
Reason: not reported/ described |
Probably no
Reason: not reported/ described |
Probably no
Reason: not reported/ described |
Probably no
Reason: not reported, but data provided for all included patients
|
Definitely yes
Reason: all relevant outcomes were reported |
Definitely yes;
Reason: No other problems noted |
Some concerns
Reason: allocation and blinding |
|
Li, 2021 |
Probably yes
Reason: computer-generated random numbers |
Probably no
Reason: not reported/ described |
Definitely not
Reason: reported as a limitation |
Probably yes
Reason: flowchart of inclusion and exclusion from eligibility to analyses |
Definitely yes
Reason: all relevant outcomes were reported |
Definitely yes;
Reason: No other problems noted |
Some concerns
Reason: allocation and blinding |
|
Tamura, 2017 |
Probably yes
Reason: computer-generated randomization program |
Probably no
Reason: not reported/ described |
Probably no
Reason: An anesthesiologist blinded to the analgesic method carried out the evaluations of pain (VAS) and sensory deprivation (cold sign, pin prick tests) |
Probably yes
Reason: flowchart of inclusion and exclusion from eligibility to analyses |
Definitely yes
Reason: all relevant outcomes were reported |
Definitely yes;
Reason: No other problems noted |
Some concerns
Reason: allocation and blinding |
|
PVB versus Epidural anesthesia – VATS |
|||||||
|
Ding, 2018 |
Probably no
Reason: not reported/ described |
Probably no
Reason: not reported/ described |
Probably no
Reason: not reported/ described |
Probably yes
Reason: 2 cases in intervention group and 3 cases in comparison group with similar reasons, but no test was performed |
Definitely yes
Reason: all relevant outcomes were reported |
Probably yes
Reason: outcome data provided bar charts or summary measures in the text |
Some concerns
Reason: allocation, blinding and reporting |
|
Kosiński, 2016 |
Probably no
Reason: not reported/ described |
Probably no
Reason: not reported/ described |
Probably no
Reason: not reported/ described |
Probably yes
Reason: flowchart of inclusion and exclusion from eligibility to analyses |
Definitely yes
Reason: all relevant outcomes were reported |
Probably yes;
Reason: outcome data postoperative pain in chart that is not readable
|
Some concerns
Reason: allocation and blinding |
|
Lai, 2021 |
Probably yes
Reason: randomization was performed using a computer-generated randomization sequence by an investigator not involved in patient care or perioperative assessment |
Probably no
Reason: not reported/ described |
Probably no
Reason: blinding was not performed except for investigator who was blinded to the group allocation and who collected all outcome and perioperative data |
Probably yes
Reason: 3 cases in intervention group and 2 cases in comparison group with similar reasons, but no test was performed |
Definitely yes
Reason: all relevant outcomes were reported |
Probably yes;
Reason: outcome data postoperative pain in chart that is not readable
|
Some concerns
Reason: allocation and blinding |
|
Okaijma, 2015 |
Probably no
Reason: only reported that patients were randomly assigned using the sealed envelope technique
|
Probably no
Reason: only reported that patients were randomly assigned using the sealed envelope technique
|
Probably no
Reason: not reported/ described |
Probably yes
Reason: flowchart of inclusion and exclusion from eligibility to analyses |
Definitely yes
Reason: all relevant outcomes were reported |
Definitely yes;
Reason: No other problems noted |
Some concerns
Reason: allocation and blinding |
|
Yeap, 2021 |
Probably yes
Reason: performed using Research Randomizer (https://www.randomizer.org).
|
Probably no
Reason: not reported/ described |
Probably no
Reason: only described that there was a blinded investigator for outcome assessment |
Probably no
Reason: not reported/ described |
Definitely yes
Reason: all relevant outcomes were reported |
Definitely yes;
Reason: No other problems noted |
Some concerns
Reason: allocation and blinding |
Table of excluded studies
|
Reference |
Reason for exclusion |
|
D'Ercole F, Arora H, Kumar PA. Paravertebral Block for Thoracic Surgery. J Cardiothorac Vasc Anesth. 2018 Apr;32(2):915-927. doi: 10.1053/j.jvca.2017.10.003. Epub 2017 Oct 4. PMID: 29169795. |
Not suitable as basis SR |
|
Harky A, Clarke CG, Kar A, Bashir M. Epidural analgesia versus paravertebral block in video-assisted thoracoscopic surgery. Interact Cardiovasc Thorac Surg. 2019 Mar 1;28(3):404-406. doi: 10.1093/icvts/ivy265. PMID: 30169855. |
Not suitable as basis SR |
|
Liang XL, An R, Chen Q, Liu HL. The Analgesic Effects of Thoracic Paravertebral Block versus Thoracic Epidural Anesthesia After Thoracoscopic Surgery: A Meta-Analysis. J Pain Res. 2021 Mar 26;14:815-825. doi: 10.2147/JPR.S299595. PMID: 33814927; PMCID: PMC8009548. |
Not suitable as basis SR |
|
Norum HM, Breivik H. A systematic review of comparative studies indicates that paravertebral block is neither superior nor safer than epidural analgesia for pain after thoracotomy. Scand J Pain. 2010 Jan 1;1(1):12-23. doi: 10.1016/j.sjpain.2009.10.003. PMID: 29913936. |
Not suitable as basis SR |
|
Ren P, Du Y, He G, Jiang D. Efficacy and safety of general anesthesia combined with paravertebral blockade on postoperative recovery in patients undergoing pulmonary surgery: a systematic review and meta-analysis. J Thorac Dis. 2022 Feb;14(2):431-442. doi: 10.21037/jtd-22-103. PMID: 35280484; PMCID: PMC8902126. |
Not suitable as basis SR |
|
Scarfe AJ, Schuhmann-Hingel S, Duncan JK, Ma N, Atukorale YN, Cameron AL. Continuous paravertebral block for post-cardiothoracic surgery analgesia: a systematic review and meta-analysis. Eur J Cardiothorac Surg. 2016 Dec;50(6):1010-1018. doi: 10.1093/ejcts/ezw168. Epub 2016 May 30. PMID: 27242357. |
Cardio |
|
Xu M, Hu J, Yan J, Yan H, Zhang C. Paravertebral Block versus Thoracic Epidural Analgesia for Postthoracotomy Pain Relief: A Meta-Analysis of Randomized Trials. Thorac Cardiovasc Surg. 2022 Aug;70(5):413-421. doi: 10.1055/s-0040-1722314. Epub 2021 Jan 21. PMID: 33477177. |
Not suitable as basis SR |
|
Balzani E, Rosboch GL, Ceraolo E, Lyberis P, Filippini C, Piccioni F, Guerrera F, Ruffini E, Pedoto A, Brazzi L. The effect of peripheral regional analgesia in thoracic surgery: a systematic review and a meta-analysis of randomized-controlled trials. Tumori. 2023 Feb;109(1):6-18. doi: 10.1177/03008916221081891. Epub 2022 Mar 31. PMID: 35361015. |
Not suitable as basis SR |
|
Shi, Z. and Li, J. C. and Jiang, X. J. Paravertebral block versus epidural block for post-thoracotomy analgesia: A systematic review. Chinese Journal of Evidence-Based Medicine, 2014. |
Too old; more recent reviews available |
|
Júnior Ade P, Erdmann TR, Santos TV, Brunharo GM, Filho CT, Losso MJ, Filho GR. Comparison between continuous thoracic epidural and paravertebral blocks for postoperative analgesia in patients undergoing thoracotomy: Systematic review. Braz J Anesthesiol. 2013 Sep-Oct;63(5):433-42. doi: 10.1016/j.bjane.2013.10.002. Epub 2013 Nov 19. PMID: 24565302. |
Too old; more recent reviews available |
|
Davies RG, Myles PS, Graham JM. A comparison of the analgesic efficacy and side-effects of paravertebral vs epidural blockade for thoracotomy--a systematic review and meta-analysis of randomized trials. Br J Anaesth. 2006 Apr;96(4):418-26. doi: 10.1093/bja/ael020. Epub 2006 Feb 13. Erratum in: Br J Anaesth. 2007 Nov;99(5):768. PMID: 16476698. |
Too old; more recent reviews available |
|
Detterbeck FC. Efficacy of methods of intercostal nerve blockade for pain relief after thoracotomy. Ann Thorac Surg. 2005 Oct;80(4):1550-9. doi: 10.1016/j.athoracsur.2004.11.051. PMID: 16181921. |
Too old; more recent reviews available |
|
Ding X, Jin S, Niu X, Ren H, Fu S, Li Q. A comparison of the analgesia efficacy and side effects of paravertebral compared with epidural blockade for thoracotomy: an updated meta-analysis. PLoS One. 2014 May 5;9(5):e96233. doi: 10.1371/journal.pone.0096233. PMID: 24797238; PMCID: PMC4010440. |
Too old; more recent reviews available |
|
Baidya DK, Khanna P, Maitra S. Analgesic efficacy and safety of thoracic paravertebral and epidural analgesia for thoracic surgery: a systematic review and meta-analysis. Interact Cardiovasc Thorac Surg. 2014 May;18(5):626-35. doi: 10.1093/icvts/ivt551. Epub 2014 Jan 31. PMID: 24488821. |
Too old; more recent reviews available |
|
Joshi GP, Bonnet F, Shah R, Wilkinson RC, Camu F, Fischer B, Neugebauer EA, Rawal N, Schug SA, Simanski C, Kehlet H. A systematic review of randomized trials evaluating regional techniques for postthoracotomy analgesia. Anesth Analg. 2008 Sep;107(3):1026-40. doi: 10.1213/01.ane.0000333274.63501.ff. PMID: 18713924. |
Too old; more recent reviews available |
|
Steinthorsdottir KJ, Wildgaard L, Hansen HJ, Petersen RH, Wildgaard K. Regional analgesia for video-assisted thoracic surgery: a systematic review. Eur J Cardiothorac Surg. 2014 Jun;45(6):959-66. doi: 10.1093/ejcts/ezt525. Epub 2013 Nov 27. PMID: 24288340. |
Too old; more recent reviews available |
|
Aly, H. M. and Mousa, E. A. and Mohammad, S. A. and Nishiwaki, K. A randomized comparison of ultrasound guided thoracic para vertebral block and epidural analgesia for post-thoracotomy pain relief and respiratory function. Egyptian Journal of Anaesthesia. 2010; 26 (3) 189-198 |
Not available |
|
Biswas S, Verma R, Bhatia VK, Chaudhary AK, Chandra G, Prakash R. Comparison between Thoracic Epidural Block and Thoracic Paravertebral Block for Post Thoracotomy Pain Relief. J Clin Diagn Res. 2016 Sep;10(9):UC08-UC12. doi: 10.7860/JCDR/2016/19159.8489. Epub 2016 Sep 1. Retraction in: J Clin Diagn Res. 2020 Jul;14(7):ZZ01. PMID: 27790554; PMCID: PMC5072054. |
retracted article: The article had to be retracted as the data has errors which could mislead the readers. This error was brought to the editorial notice, post-publication by one of the reader. |
|
Casati A, Alessandrini P, Nuzzi M, Tosi M, Iotti E, Ampollini L, Bobbio A, Rossini E, Fanelli G. A prospective, randomized, blinded comparison between continuous thoracic paravertebral and epidural infusion of 0.2% ropivacaine after lung resection surgery. Eur J Anaesthesiol. 2006 Dec;23(12):999-1004. doi: 10.1017/S0265021506001104. Epub 2006 Jul 7. PMID: 16824243. |
Included in SR Yeung (2016), which is included in the literature review. |
|
Çetin, Y. and Atalan, N. and Ugur, I. and Kudsioǧlu, T. and Yapici, N. and Aykaç, Z. Comparison of epidural and single-shot paravertebral block analgesia after thoracotomy. Gogus-Kalp-Damar Anestezi ve Yogun Bakim Dernegi Dergisi. 2017; 23(2) 43-47 |
Language: Turkish |
|
Dango S, Harris S, Offner K, Hennings E, Priebe HJ, Buerkle H, Passlick B, Loop T. Combined paravertebral and intrathecal vs thoracic epidural analgesia for post-thoracotomy pain relief. Br J Anaesth. 2013 Mar;110(3):443-9. doi: 10.1093/bja/aes394. Epub 2012 Nov 14. PMID: 23151421. |
PICO: combi with intrathecal opioid (ITO)
|
|
De Cosmo G, Aceto P, Campanale E, Congedo E, Clemente A, Mascia A, et al. Comparison between epidural and paravertebral intercostal nerve block with ropivacaine aIer thoracotomy: eGects on pain relief, pulmonary function and patient satisfaction. Acta Medica Romana 2002;40(4):340-7. |
Included in SR Yeung (2016), which is included in the literature review. |
|
Debreceni G, Molnár Z, Szélig L, Molnár TF. Continuous epidural or intercostal analgesia following thoracotomy: a prospective randomized double-blind clinical trial. Acta Anaesthesiol Scand. 2003 Oct;47(9):1091-5. doi: 10.1034/j.1399-6576.2003.00208.x. PMID: 12969101. |
PICO: outcomes |
|
Dhole S, Mehta Y, Saxena H, Juneja R, Trehan N. Comparison of continuous thoracic epidural and paravertebral blocks for postoperative analgesia after minimally invasive direct coronary artery bypass surgery. J Cardiothorac Vasc Anesth. 2001 Jun;15(3):288-92. doi: 10.1053/jcan.2001.23271. PMID: 11426357. |
PICO: coronary artery bypass surgery
|
|
El Shora HA, El Beleehy AA, Abdelwahab AA, Ali GA, Omran TE, Hassan EA, Arafat AA. Bilateral Paravertebral Block versus Thoracic Epidural Analgesia for Pain Control Post-Cardiac Surgery: A Randomized Controlled Trial. Thorac Cardiovasc Surg. 2020 Aug;68(5):410-416. doi: 10.1055/s-0038-1668496. Epub 2018 Aug 16. PMID: 30114712. |
PICO: cardiac surgery |
|
Kashiwagi Y, Iida T, Kunisawa T, Iwasaki H. [Efficacy of Ultrasound-guided Thoracic Paravertebral Block Compared with the Epidural Analgesia in Patients Undergoing Video-assisted Thoracoscopic Surgery]. Masui. 2015 Oct;64(10):1010-4. Japanese. PMID: 26742399. |
Language: Japanese |
|
Kobayashi R, Mori S, Wakai K, Fukumoto K, Saito T, Katayama T, et al. Paravertebral block via the surgical field versus epidural block for patients undergoing thoracotomy: a randomised clinical trial. Surgery Today 2013;43(9):963-9. [PUBMED: 23702705] |
Included in SR Yeung (2016), which is included in the literature review. |
|
Li, Z. G. and Ma, W. Q. and Huang, H. B. Comparison of intravenous fentanyl, thoracic epidural bupivacaine/fentanyl, or intercostal nerve blockade plus intravenous fentanyl for treatment of post-thoracotomy analgesia ventilation function. Chinese Journal of Clinical Rehabilitation. 2003; 7 (23): 3236-3237. |
Language: Chinese |
|
Mehta Y, Arora D, Sharma KK, Mishra Y, Wasir H, Trehan N. Comparison of continuous thoracic epidural and paravertebral block for postoperative analgesia after robotic-assisted coronary artery bypass surgery. Ann Card Anaesth. 2008 Jul-Dec;11(2):91-6. doi: 10.4103/0971-9784.41576. PMID: 18603748.
|
PICO: robotic-assisted coronary artery bypass surgery
|
|
Messina M, Boroli F, Landoni G, Binami E, Dedola F, N'zepa Batonga J, et al. A comparison of epidural vs paravertebral blockade in thoracic surgery. Minerva Anestesiologica 2009;75(11):616-21. [PUBMED: 19881458] |
Included in SR Yeung (2016), which is included in the literature review. |
|
Murkerjee M, Goswami A, Gupta S, Sarbapalli D, Pal R, Kar S. Analgesia in post-thoracotomy patients: comparison between thoracic epidural and thoracic paravertebral blocks. Anesthesia Essays and Researches 2010;4(2):75-80. [DOI: 10.4103/0259-1162.73511] |
Included in SR Yeung (2016), which is included in the literature review. |
|
Nagaraja PS, Ragavendran S, Singh NG, Asai O, Bhavya G, Manjunath N, Rajesh K. Comparison of continuous thoracic epidural analgesia with bilateral erector spinae plane block for perioperative pain management in cardiac surgery. Ann Card Anaesth. 2018 Jul-Sep;21(3):323-327. doi: 10.4103/aca.ACA_16_18. PMID: 30052229; PMCID: PMC6078032. |
PICO: cardiac surgery |
|
Öztürk T, Topcu İ, Yaldız S, Özbakkaloğlu A, Aşık K, Yentürk A. Torakotomi sonrası postoperatif ağrı kontrolünde torakal epidural ve paravertebral bloğun karşılaştırılması [Comparison of thoracic epidural and paravertebral analgesia for postoperative pain control after thoracotomy]. Agri. 2016 Jan;28(1):32-8. Turkish. doi: 10.5505/agri.2015.22043. PMID: 27225610. |
Language: Turkish |
|
Szebla R, Machała W. Porównanie skuteczności ciagłego znieczulenia zewnatrzoponowego i przykregowego podczas operacji płucnych [Continuous epidural anaesthesia vs paravertebral block for lung surgery--a comparative study]. Anestezjol Intens Ter. 2008 Jul-Sep;40(3):152-5. Polish. PMID: 19469115. |
Language: Polish |
|
Yu S, Valencia MB, Roques V, Aljure OD. Regional analgesia for minimally invasive cardiac surgery. J Card Surg. 2019 Nov;34(11):1289-1296. doi: 10.1111/jocs.14177. Epub 2019 Aug 23. PMID: 31441548. |
PICO: cardiac surgery |
|
Zhang S, Han X, Zhou D, Sun M, Cang J, Miao C, Liang C. The effects of erector spinae plane block on perioperative opioid consumption and rehabilitation in video assisted thoracic surgery. BMC Anesthesiol. 2021 Dec 10;21(1):313. doi: 10.1186/s12871-021-01536-x. PMID: 34893026; PMCID: PMC8662884.
|
PICO: wrong comparison of 3 groups: group C (general anesthesia with patient-controlled intravenous analgesia [PCIA]), group T (general anesthesia with patient-controlled epidural analgesia [PCEA]), or group E (general anesthesia with continuous ESPB and PCIA) |
|
Concha M, Dagnino J, Cariaga M, Aguilera J, Aparicio R, Guerrero M. Analgesia after thoracotomy: epidural fentanyl/bupivacaine compared with intercostal nerve block plus intravenous morphine. J Cardiothorac Vasc Anesth. 2004 Jun;18(3):322-6. doi: 10.1053/j.jvca.2004.03.013. PMID: 15232813. |
N < 20 per arm
|
|
Kanazi GE, Ayoub CM, Aouad M, Abdallah F, Sfeir PM, Adham AB, El-Khatib MF. Subpleural block is less effective than thoracic epidural analgesia for post-thoracotomy pain: a randomised controlled study. Eur J Anaesthesiol. 2012 Apr;29(4):186-91. doi: 10.1097/EJA.0b013e32834fcef7. PMID: 22327109. |
PICO: wrong block comparison: subpleural analgesia with thoracic epidural analgesia in patients undergoing thoracotomy
|
|
Kingma BF, Eshuis WJ, de Groot EM, Feenstra ML, Ruurda JP, Gisbertz SS, Ten Hoope W, Marsman M, Hermanides J, Hollmann MW, Kalkman CJ, Luyer MDP, Nieuwenhuijzen GAP, Scholten HJ, Buise M, van Det MJ, Kouwenhoven EA, van der Meer F, Frederix GWJ, Cheong E, Al Naimi K, van Berge Henegouwen MI, van Hillegersberg R. Paravertebral catheter versus EPidural analgesia in Minimally invasive Esophageal resectioN: a randomized controlled multicenter trial (PEPMEN trial). BMC Cancer. 2020 Feb 22;20(1):142. doi: 10.1186/s12885-020-6585-1. PMID: 32087686; PMCID: PMC7036230. |
PICO: wrong outcomes: quality of postoperative recovery, as measured by the Quality of Recovery-40 (QoR-40) questionnaire on the morning of postoperative day, the integrated pain and systemic opioid score and patient satisfaction and pain experience according to the International Pain Outcomes (IPO) questionnaire, and cost-effectiveness. |
|
Olivier JF, Bracco D, Nguyen P, Le N, Noiseux N, Hemmerling T; Perioperative Cardiac Surgery Research Group (PeriCARG). A novel approach for pain management in cardiac surgery via median sternotomy: bilateral single-shot paravertebral blocks. Heart Surg Forum. 2007;10(5):E357-62. doi: 10.1532/HSF98.20071082. PMID: 17855198. |
PICO: cardiac surgery |
|
Scarci M, Joshi A, Attia R. In patients undergoing thoracic surgery is paravertebral block as effective as epidural analgesia for pain management? Interact Cardiovasc Thorac Surg. 2010 Jan;10(1):92-6. doi: 10.1510/icvts.2009.221127. Epub 2009 Oct 23. PMID: 19854794. |
wrong study design: narrative review
|
|
Takamori S, Yoshida S, Hayashi A, Matsuo T, Mitsuoka M, Shirouzu K. Intraoperative intercostal nerve blockade for postthoracotomy pain. Ann Thorac Surg. 2002 Aug;74(2):338-41. doi: 10.1016/s0003-4975(02)03710-4. PMID: 12173810. |
PICO: wrong comparison: epidural analgesia only (group A, n = 20) or epidural analgesia plus temporary, intraoperative intercostal nerve blockade
|
|
Wei W, Zheng X, Gu Y, Tang C, Yao Y. [Effects of different postoperative analgesic strategies on postoperative neurocognitive function and quality of recovery in elderly patients undergoing one lung ventilation]. Nan Fang Yi Ke Da Xue Xue Bao. 2020 Dec 30;40(12):1821-1825. Chinese. doi: 10.12122/j.issn.1673-4254.2020.12.19. PMID: 33380392; PMCID: PMC7835681. |
PICO: wrong outcomes: neurocognitive function and quality of recovery
|
|
Wong J, Cooper J, Thomas R, Langford R, Anwar S. Persistent Postsurgical Pain Following Thoracotomy: A Comparison of Thoracic Epidural and Paravertebral Blockade as Preventive Analgesia. Pain Med. 2019 Sep 1;20(9):1796-1802. doi: 10.1093/pm/pny293. PMID: 30789665.
|
wrong study design: observational study with data collected at 6 months
|
|
Yeung J, Middleton L, Tryposkiadis K, Kerr A, Daniels J, Naidu B, Melody T, Goebel A, Wilson M, Kumar S, Szentgyorgyi L, Flanagan S, Shah R, Worrall A, Gao F. Randomised controlled trial to investigate the effectiveness of thoracic epidural and paravertebral blockade in reducing chronic post-thoracotomy pain (TOPIC): a pilot study to assess feasibility of a large multicentre trial. BMJ Open. 2019 Jul 9;9(7):e023679. doi: 10.1136/bmjopen-2018-023679. Erratum in: BMJ Open. 2019 Aug 15;9(8):e023679corr1. PMID: 31292172; PMCID: PMC6624049. |
wrong study design: A pilot study to assess feasibility of a large multicentre trial
|
|
Yuge, O. and Totoki, T. and Namiki, A. and Hanaoka, K. A trial study to prevent the persistent pain following thoracotomy - The anesthetic management protocol. Anesthesia and Resuscitation. 2000; 36(4): 181-185.
|
wrong study design: trail study
|
|
Zhang W, Cong X, Zhang L, Sun M, Li B, Geng H, Gu J, Zhang J. Effects of thoracic nerve block on perioperative lung injury, immune function, and recovery after thoracic surgery. Clin Transl Med. 2020 Jul;10(3):e38. doi: 10.1002/ctm2.38. Epub 2020 Jul 8. PMID: 32639645; PMCID: PMC7418816. |
PICO: wrong comparison: general anesthesia group (GAL group), thoracic paravertebral nerve block (TPVB) combined with general anesthesia (TPL group), and TPVB (with paravertebral dexmedetomidine) combined with general anesthesia group (TDL group); general anesthesia group (GAE group), TPVB combined with general anesthesia group (TPE group), and thoracic epidural block combined with general anesthesia group (TEE group)
|
|
Misra S, Awal S. Does erector spinae plane block result in improved postoperative analgesia and enhanced recovery in adult patients after cardiac surgery? Interact Cardiovasc Thorac Surg. 2021 May 27;32(6):873-877. doi: 10.1093/icvts/ivab010. PMID: 33693671; PMCID: PMC8691566. |
PICO: cardiac surgery
|
|
Lin J, Liao Y, Gong C, Yu L, Gao F, Yu J, Chen J, Chen X, Zheng T, Zheng X. Regional Analgesia in Video-Assisted Thoracic Surgery: A Bayesian Network Meta-Analysis. Front Med (Lausanne). 2022 Apr 6;9:842332. doi: 10.3389/fmed.2022.842332. PMID: 35463038; PMCID: PMC9019113. |
Not suitable as basis SR |
|
Wedad, M. and Zaki, M. K. and Haleem, M. The effect of addition of wound infiltration with local anaesthetics to interpleural block on post-thoracotomy pain, pulmonary function and stress response in comparison to thoracic epidural and paravertebral block. Egyptian Journal of Anaesthesia. 2004; 20(1):67-72.
|
Too old |
|
Andreae MH, Andreae DA. Regional anaesthesia to prevent chronic pain after surgery: a Cochrane systematic review and meta-analysis. Br J Anaesth. 2013 Nov;111(5):711-20. doi: 10.1093/bja/aet213. Epub 2013 Jun 28. PMID: 23811426; PMCID: PMC3793661. |
Too old |
|
Chen R, Su S, Shu H. Efficacy and safety of rhomboid intercostal block for analgesia in breast surgery and thoracoscopic surgery: a meta-analysis. BMC Anesthesiol. 2022 Mar 16;22(1):71. doi: 10.1186/s12871-022-01599-4. Erratum in: BMC Anesthesiol. 2022 Apr 8;22(1):101. PMID: 35296252; PMCID: PMC8925179. |
PICO: wrong block: Rhomboid intercostal block and no comparison between blocks as an aim
|
|
Biçer C, Ünalan EN, Aksu R, Önal Ö, Güneş I. Adição de dexmedetomidina à bupivacaína em bloqueio paravertebral guiado por ultrassom potencializa o alívio da dor pós‐operatória em pacientes submetidos à toracotomia [Addition of dexmedetomidine to bupivacaine in ultrasonography-guided paravertebral blockade potentiates postoperative pain relief among patients undergoing thoracotomy]. Braz J Anesthesiol. 2019 Mar-Apr;69(2):144-151. doi: 10.1016/j.bjan.2018.11.002. Epub 2019 Jan 18. PMID: 30665671; PMCID: PMC9391857. |
Wrong language: Brazilian |
|
Huang QW, Lu ZW, Li JB, Zhang WQ, Jiang LW, Lin ZJ. A Comparison of Puncture and Continuous Pump Analgesia With Two Different Approaches to Thoracic Paravertebral Block for Thoracic Surgery. Front Surg. 2022 Feb 18;8:711205. doi: 10.3389/fsurg.2021.711205. PMID: 35252317; PMCID: PMC8894586. |
PICO: wrong outcomes |
Verantwoording
Beoordelingsdatum en geldigheid
Publicatiedatum : 26-09-2023
Beoordeeld op geldigheid : 01-09-2023
Algemene gegevens
De ontwikkeling/herziening van deze richtlijnmodule werd ondersteund door het Kennisinstituut van de Federatie Medisch Specialisten (www.demedischspecialist.nl/kennisinstituut) en werd gefinancierd uit de Kwaliteitsgelden Medisch Specialisten (SKMS).
De financier heeft geen enkele invloed gehad op de inhoud van de richtlijnmodule.
Samenstelling werkgroep
Voor het ontwikkelen van de richtlijnmodule is in 2021 een multidisciplinaire werkgroep ingesteld, bestaande uit vertegenwoordigers van alle relevante specialismen (zie hiervoor de Samenstelling van de werkgroep) die betrokken zijn bij de zorg voor patiënten met postoperatieve pijn.
Samenstelling van de werkgroep
Werkgroep
Prof. dr. J. (Jörgen) Bruhn, anesthesioloog, (voorzitter) NVA
Prof. dr. dr. M.W. (Markus) Hollmann, anesthesioloog, NVA
Dr. M.F. (Markus) Stevens, anesthesioloog, NVA
Drs. L.J.H. (Lea) van Wersch, anesthesioloog, NVA
Dr. M.H.J. (Margot) Roozekrans, anesthesioloog, NVA
Dr. S.A.S. (Sandra) van den Heuvel, anesthesioloog/pijnspecialist, NVA
Drs. S.J. (Stijn) Westerbos, orthopeed, NOV
Drs. W.L. (Wilson) Li, cardiothoracaal chirurg, NVT
S.F. (Cedric) Lau MSc, ziekenhuisapotheker, NVZA
Dr. R.L.M. (Rianne) van Boekel, verpleegkundig pijnconsulent, V&VN
Drs. I.L. (Ilona) Thomassen-Hilgersom, patiëntvertegenwoordiger, Samenwerkingverband Pijnpatiënten naar één stem
Klankbordgroep
Drs. N.C. (Niels) Gritters van den Oever, intensivist, NVIC
J.P. (Patrick) Rensink, anesthesiemedewerker/pijnconsulent, NVAM
Dr. G. (Gijs) Helmerhorst, orthopeed, NOV
Dr. C.D. (Cor) de Kroon, gynaecoloog-oncoloog, NVOG
Dr. W.J. (Wietse) Eshuis, chirurg, NVvH
Dr. D. (Daphne) Roos, chirurg, NVvH
Met ondersteuning van
Dr. F. Willeboordse, senior adviseur, Kennisinstituut van de Federatie Medisch Specialisten
Dr. L.M.P. Wesselman, adviseur, Kennisinstituut van de Federatie Medisch Specialisten
Dr. L.M. van Leeuwen, adviseur, Kennisinstituut van de Federatie Medisch Specialisten
I. van Dijk, junior adviseur, Kennisinstituut van de Federatie Medisch Specialisten
Belangenverklaringen
De Code ter voorkoming van oneigenlijke beïnvloeding door belangenverstrengeling is gevolgd. Alle werkgroepleden hebben schriftelijk verklaard of zij in de laatste drie jaar directe financiële belangen (betrekking bij een commercieel bedrijf, persoonlijke financiële belangen, onderzoeksfinanciering) of indirecte belangen (persoonlijke relaties, reputatiemanagement) hebben gehad. Gedurende de ontwikkeling of herziening van een module worden wijzigingen in belangen aan de voorzitter doorgegeven. De belangenverklaring wordt opnieuw bevestigd tijdens de commentaarfase.
Een overzicht van de belangen van werkgroepleden en het oordeel over het omgaan met eventuele belangen vindt u in onderstaande tabel. De ondertekende belangenverklaringen zijn op te vragen bij het secretariaat van het Kennisinstituut van de Federatie Medisch Specialisten.
|
Werkgroeplid |
Functie |
Nevenfuncties |
Gemelde belangen |
Ondernomen actie |
|
Bruhn, voorzitter |
Professor & Afdelingshoofd afdeling Anesthesiologie, Radboud UMC |
Editorial Board Journal of Clinical Monitoring and Computing, onbetaald |
Geen |
Geen actie |
|
Hollmann |
Professor & Afdelingshoofd afdeling Anesthesiologie, Amsterdam UMC, locatie AMC |
|
Geen |
Restricties t.a.v. modules over ketamine en lidocaïne. |
|
Lau |
|
Nationale Werkgroep morbide obesitas en bariatrische chirurgie (KNMP), vergoeding voor bestede uren |
Geen belangenverstrengeling, promotie-onderzoek is op een ander vlak dan waar beoogde werkgroep zich over buigt
|
Geen actie |
|
Boekel, van |
|
|
Geen |
Geen actie |
|
Thomassen-Hilgersom |
Voorzitter Samenwerkingsverband Pijnpatiënten naar één stem vrijwilliger en geen werkgever |
|
Geen |
Geen actie |
|
Li |
Cardiothoracaal chirurg (Radboudumc, Nijmegen) |
Bestuurslid NVT (Nederlandse Vereniging voor Thoraxchirurgie) |
Radboudumc zal in 2021 meedoen aan een RCT naar de optimale vorm van pijnstilling ten tijde van longchirugie (epiduraal versus paravertebraal) OPtriAL - met ZonMw subsidie, geïniteerd vanuit het MMC |
Geen actie |
|
Roozekrans |
Anesthesioloog - Pijnspecialist - Noordwest Ziekenhuisgroep |
Geen |
Geen |
Geen actie |
|
Stevens |
Chef de Clinique kinderanesthesie AUMC locatie AMC |
|
Geen |
Geen actie |
|
Heuvel, van den |
Anesthesioloog-pijnarts, Radboud UMC |
Geen |
Geen |
Geen actie |
|
Wersch, van |
Anesthesioloog, Maasziekenhuis Pantein |
Geen |
Geen |
Geen actie |
|
Westerbos |
Orthopeed, Alrijne ziekenhuis |
Geen |
Geen |
Geen actie |
|
Gritters van den Oever |
Anesthesioloog-intensivist Treant Zorggroep |
|
Geen |
Geen actie |
|
Rensink |
|
|
Geen |
Geen actie |
|
Kroon, de |
Gynaecoloog-oncoloog Leids Univesitair Medisch Centrum (1.0 fte) |
|
Geen |
Geen actie |
|
Roos |
Chirurg |
Geen |
Geen |
Geen actie |
|
Eshuis |
Chirurg, Amsterdam UMC |
Geen |
Geen |
Geen actie |
|
Willeboordse |
Senior adviseur Kennisinstituut van de Federatie Medisch Specialisten |
Geen |
Partner werkzaam bij Janssen Vaccines, onderdeel van Johnsson &Johnsson, via partner ook financiële belangen (aandelen J&J) |
Geen actie |
|
Wesselman |
Adviseur Kennisinstituut van de Federatie Medisch Specialisten |
|
|
Geen actie |
|
Leeuwen, van |
Adviseur Kennisinstituut van de Federatie Medisch Specialisten |
|
|
Geen actie |
|
Dijk, van |
Junior adviseur Kennisinstituut van de Federatie Medisch Specialisten |
Geen |
Geen |
Geen actie |
Inbreng patiëntenperspectief
Er werd aandacht besteed aan het patiëntenperspectief door zitting van een afgevaardigde van de patiëntenvereniging (Pijnpatiënten naar één stem) in de werkgroep. De Patiëntenfederatie Nederland en Pijnpatiënten naar één stem werden uitgenodigd voor de invitational conference. Het verslag hiervan [zie aanverwante producten] is besproken in de werkgroep. De verkregen input is meegenomen bij het opstellen van de uitgangsvragen, de keuze voor de uitkomstmaten en bij het opstellen van de overwegingen. De conceptrichtlijn is tevens voor commentaar voorgelegd aan de Patiëntenfederatie Nederland en Pijnpatiënten naar één stem en de eventueel aangeleverde commentaren zijn bekeken en verwerkt.
Wkkgz & Kwalitatieve raming van mogelijke substantiële financiële gevolgen
Kwalitatieve raming van mogelijke financiële gevolgen in het kader van de Wkkgz
Bij de richtlijn is conform de Wet kwaliteit, klachten en geschillen zorg (Wkkgz) een kwalitatieve raming uitgevoerd of de aanbevelingen mogelijk leiden tot substantiële financiële gevolgen. Bij het uitvoeren van deze beoordeling zijn richtlijnmodules op verschillende domeinen getoetst (zie het stroomschema op de Richtlijnendatabase).
Uit de kwalitatieve raming blijkt dat er waarschijnlijk geen substantiële financiële gevolgen zijn, zie onderstaande tabel.
|
Module |
Uitkomst raming |
Toelichting |
|
Module Organisatie van Zorg |
Geen substantiële financiële gevolgen |
Hoewel uit de toetsing volgt dat de aanbeveling(en) breed toepasbaar zijn (>40.000 patiënten), volgt ook uit de toetsing dat het overgrote deel (±90%) van de zorgaanbieders en zorgverleners al aan de norm voldoet en/of het geen nieuwe manier van zorgverlening of andere organisatie van zorgverlening betreft, het geen toename in het aantal in te zetten voltijdsequivalenten aan zorgverleners betreft en het geen wijziging in het opleidingsniveau van zorgpersoneel betreft. Er worden daarom geen substantiële financiële gevolgen verwacht. |
|
Module Transitionele Pijn Service (TPS) |
Geen substantiële financiële gevolgen |
Uit de toetsing volgt dat de aanbevelingen breed toepasbaar zijn (>40.000 patiënten). Hoewel de aanbeveling aangeeft dat een andere of nieuwe manier van zorgverlening gewenst is (i.e. andere manier van samenwerking/afstemming tussen zorgverleners) waarbij een TPS-model met TPS-team geïnitieerd wordt, laten eerste kosten-effectiviteitsstudies kostenbesparingen zien. De verwachting is dat TPS leidt tot betere zorg-op-maat, waarbij chronische postoperatieve pijn zorg doelmatiger wordt behandeld. Per ziekenhuis zal de vorm, intensiteit en organisatie van het TPS-model variëren. Zo kunnen ziekenhuizen ook kiezen voor een minder uitgebreid TPS. De aanbeveling geeft relatief veel ruimte voor de precieze invulling. Alle overwegingen tezamen, worden er geen substantiële financiële gevolgen verwacht. |
|
Module Pijnmeting |
Geen substantiële financiële gevolgen |
Hoewel uit de toetsing volgt dat de aanbeveling(en) breed toepasbaar zijn (>40.000 patiënten), volgt ook uit de toetsing dat het overgrote deel (±90%) van de zorgaanbieders en zorgverleners al aan de norm voldoet en/of het geen nieuwe manier van zorgverlening of andere organisatie van zorgverlening betreft, het geen toename in het aantal in te zetten voltijdsequivalenten aan zorgverleners betreft en het geen wijziging in het opleidingsniveau van zorgpersoneel betreft. Er worden daarom geen substantiële financiële gevolgen verwacht. |
|
Module Niet-medicamenteuze interventies |
Geen substantiële financiële gevolgen |
Hoewel uit de toetsing volgt dat de aanbeveling(en) breed toepasbaar zijn (>40.000 patiënten), volgt ook uit de toetsing dat het geen nieuwe manier van zorgverlening of andere organisatie van zorgverlening betreft, het geen toename in het aantal in te zetten voltijdsequivalenten aan zorgverleners betreft en het geen wijziging in het opleidingsniveau van zorgpersoneel betreft. Er worden daarom geen substantiële financiële gevolgen verwacht. |
|
Module Buikwandblokken |
Geen substantiële financiële gevolgen |
Hoewel uit de toetsing volgt dat de aanbeveling(en) breed toepasbaar zijn (>40.000 patiënten), volgt ook uit de toetsing dat het overgrote deel (±90%) van de zorgaanbieders en zorgverleners al aan de norm voldoet en/of het geen nieuwe manier van zorgverlening of andere organisatie van zorgverlening betreft, het geen toename in het aantal in te zetten voltijdsequivalenten aan zorgverleners betreft en het geen wijziging in het opleidingsniveau van zorgpersoneel betreft. Er worden daarom geen substantiële financiële gevolgen verwacht. |
|
Module Borstwandblokken bij mammachirurgie |
Geen substantiële financiële gevolgen |
Hoewel uit de toetsing volgt dat de aanbeveling(en) breed toepasbaar zijn (>40.000 patiënten), volgt ook uit de toetsing dat het overgrote deel (±90%) van de zorgaanbieders en zorgverleners al aan de norm voldoet en/of het geen nieuwe manier van zorgverlening of andere organisatie van zorgverlening betreft, het geen toename in het aantal in te zetten voltijdsequivalenten aan zorgverleners betreft en het geen wijziging in het opleidingsniveau van zorgpersoneel betreft. Er worden daarom geen substantiële financiële gevolgen verwacht. |
|
Module Borstwandblokken intrathoracaal |
Geen substantiële financiële gevolgen |
Hoewel uit de toetsing volgt dat de aanbeveling(en) breed toepasbaar zijn (>40.000 patiënten), volgt ook uit de toetsing dat het overgrote deel (±90%) van de zorgaanbieders en zorgverleners al aan de norm voldoet en/of het geen nieuwe manier van zorgverlening of andere organisatie van zorgverlening betreft, het geen toename in het aantal in te zetten voltijdsequivalenten aan zorgverleners betreft en het geen wijziging in het opleidingsniveau van zorgpersoneel betreft. Er worden daarom geen substantiële financiële gevolgen verwacht. |
|
Module Continue Wond infusie |
Geen substantiële financiële gevolgen |
Hoewel uit de toetsing volgt dat de aanbeveling(en) breed toepasbaar zijn (>40.000 patiënten), volgt ook uit de toetsing dat het geen nieuwe manier van zorgverlening of andere organisatie van zorgverlening betreft, het geen toename in het aantal in te zetten voltijdsequivalenten aan zorgverleners betreft en het geen wijziging in het opleidingsniveau van zorgpersoneel betreft. Er worden daarom geen substantiële financiële gevolgen verwacht. |
|
Module Cryoanalgesie |
Geen substantiële financiële gevolgen |
Uit de toetsing volgt dat de aanbeveling(en) niet breed toepasbaar zijn (<5.000 patiënten) en zal daarom naar verwachting geen substantiële financiële gevolgen hebben voor de collectieve uitgaven. |
|
Module Dexmethason |
Geen substantiële financiële gevolgen |
Hoewel uit de toetsing volgt dat de aanbeveling(en) breed toepasbaar zijn (>40.000 patiënten), volgt ook uit de toetsing dat het overgrote deel (±90%) van de zorgaanbieders en zorgverleners al aan de norm voldoet en/of het geen nieuwe manier van zorgverlening of andere organisatie van zorgverlening betreft, het geen toename in het aantal in te zetten voltijdsequivalenten aan zorgverleners betreft en het geen wijziging in het opleidingsniveau van zorgpersoneel betreft. Er worden daarom geen substantiële financiële gevolgen verwacht. |
|
Module Gabapentinoïden |
Geen substantiële financiële gevolgen |
Hoewel uit de toetsing volgt dat de aanbeveling(en) breed toepasbaar zijn (>40.000 patiënten), volgt ook uit de toetsing dat het overgrote deel (±90%) van de zorgaanbieders en zorgverleners al aan de norm voldoet en/of het geen nieuwe manier van zorgverlening of andere organisatie van zorgverlening betreft, het geen toename in het aantal in te zetten voltijdsequivalenten aan zorgverleners betreft en het geen wijziging in het opleidingsniveau van zorgpersoneel betreft. Er worden daarom geen substantiële financiële gevolgen verwacht. |
|
Module Ketamine |
Geen substantiële financiële gevolgen |
Hoewel uit de toetsing volgt dat de aanbeveling(en) breed toepasbaar zijn (>40.000 patiënten), volgt ook uit de toetsing dat het overgrote deel (±90%) van de zorgaanbieders en zorgverleners al aan de norm voldoet en/of het geen nieuwe manier van zorgverlening of andere organisatie van zorgverlening betreft, het geen toename in het aantal in te zetten voltijdsequivalenten aan zorgverleners betreft en het geen wijziging in het opleidingsniveau van zorgpersoneel betreft. Er worden daarom geen substantiële financiële gevolgen verwacht. |
|
Module Magnesium |
Geen substantiële financiële gevolgen |
Hoewel uit de toetsing volgt dat de aanbeveling(en) breed toepasbaar zijn (>40.000 patiënten), volgt ook uit de toetsing dat het overgrote deel (±90%) van de zorgaanbieders en zorgverleners al aan de norm voldoet en/of het geen nieuwe manier van zorgverlening of andere organisatie van zorgverlening betreft, het geen toename in het aantal in te zetten voltijdsequivalenten aan zorgverleners betreft en het geen wijziging in het opleidingsniveau van zorgpersoneel betreft. Er worden daarom geen substantiële financiële gevolgen verwacht. |
|
Module Methadon |
Geen substantiële financiële gevolgen |
Hoewel uit de toetsing volgt dat de aanbeveling(en) breed toepasbaar zijn (>40.000 patiënten), volgt ook uit de toetsing dat het overgrote deel (±90%) van de zorgaanbieders en zorgverleners al aan de norm voldoet en/of het geen nieuwe manier van zorgverlening of andere organisatie van zorgverlening betreft, het geen toename in het aantal in te zetten voltijdsequivalenten aan zorgverleners betreft en het geen wijziging in het opleidingsniveau van zorgpersoneel betreft. Er worden daarom geen substantiële financiële gevolgen verwacht. |
|
Module Beta blokkers -Esmolol |
Geen substantiële financiële gevolgen |
Hoewel uit de toetsing volgt dat de aanbeveling(en) breed toepasbaar zijn (>40.000 patiënten), volgt ook uit de toetsing dat het overgrote deel (±90%) van de zorgaanbieders en zorgverleners al aan de norm voldoet en/of het geen nieuwe manier van zorgverlening of andere organisatie van zorgverlening betreft, het geen toename in het aantal in te zetten voltijdsequivalenten aan zorgverleners betreft en het geen wijziging in het opleidingsniveau van zorgpersoneel betreft. Er worden daarom geen substantiële financiële gevolgen verwacht. |
|
Module Alpha 2 agonist - Clonidine |
Geen substantiële financiële gevolgen |
Hoewel uit de toetsing volgt dat de aanbeveling(en) breed toepasbaar zijn (>40.000 patiënten), volgt ook uit de toetsing dat het overgrote deel (±90%) van de zorgaanbieders en zorgverleners al aan de norm voldoet en/of het geen nieuwe manier van zorgverlening of andere organisatie van zorgverlening betreft, het geen toename in het aantal in te zetten voltijdsequivalenten aan zorgverleners betreft en het geen wijziging in het opleidingsniveau van zorgpersoneel betreft. Er worden daarom geen substantiële financiële gevolgen verwacht. |
|
Module Alpha 2 agonist – Dexmedetomidine |
Geen substantiële financiële gevolgen |
Hoewel uit de toetsing volgt dat de aanbeveling(en) breed toepasbaar zijn (>40.000 patiënten), volgt ook uit de toetsing dat het overgrote deel (±90%) van de zorgaanbieders en zorgverleners al aan de norm voldoet en/of het geen nieuwe manier van zorgverlening of andere organisatie van zorgverlening betreft, het geen toename in het aantal in te zetten voltijdsequivalenten aan zorgverleners betreft en het geen wijziging in het opleidingsniveau van zorgpersoneel betreft. Er worden daarom geen substantiële financiële gevolgen verwacht. |
|
Module Lidocaïne |
Geen substantiële financiële gevolgen |
Hoewel uit de toetsing volgt dat de aanbeveling(en) breed toepasbaar zijn (>40.000 patiënten), volgt ook uit de toetsing dat het overgrote deel (±90%) van de zorgaanbieders en zorgverleners al aan de norm voldoet en/of het geen nieuwe manier van zorgverlening of andere organisatie van zorgverlening betreft, het geen toename in het aantal in te zetten voltijdsequivalenten aan zorgverleners betreft en het geen wijziging in het opleidingsniveau van zorgpersoneel betreft. Er worden daarom geen substantiële financiële gevolgen verwacht. |
|
Module Multimodale pijnbestrijding |
Geen substantiële financiële gevolgen |
Hoewel uit de toetsing volgt dat de aanbeveling(en) breed toepasbaar zijn (>40.000 patiënten), volgt ook uit de toetsing dat het overgrote deel (±90%) van de zorgaanbieders en zorgverleners al aan de norm voldoet en/of het geen nieuwe manier van zorgverlening of andere organisatie van zorgverlening betreft, het geen toename in het aantal in te zetten voltijdsequivalenten aan zorgverleners betreft en het geen wijziging in het opleidingsniveau van zorgpersoneel betreft. Er worden daarom geen substantiële financiële gevolgen verwacht. |
Werkwijze
AGREE
Deze richtlijnmodule is opgesteld conform de eisen vermeld in het rapport Medisch Specialistische Richtlijnen 2.0 van de adviescommissie Richtlijnen van de Raad Kwaliteit. Dit rapport is gebaseerd op het AGREE II instrument (Appraisal of Guidelines for Research & Evaluation II; Brouwers, 2010).
Knelpuntenanalyse en uitgangsvragen
Tijdens de voorbereidende fase inventariseerde de werkgroep de knelpunten in de zorg voor patiënten met postoperatieve pijn.
De werkgroep beoordeelde de aanbevelingen uit de eerdere richtlijn Postoperatieve pijn (NVA, 2013) op noodzaak tot revisie. Tevens zijn er knelpunten aangedragen door relevante partijen middels een invitational conference.
Op basis van de uitkomsten van de knelpuntenanalyse zijn door de werkgroep concept-uitgangsvragen opgesteld en definitief vastgesteld.
Uitkomstmaten
Na het opstellen van de zoekvraag behorende bij de uitgangsvraag inventariseerde de werkgroep welke uitkomstmaten voor de patiënt relevant zijn, waarbij zowel naar gewenste als ongewenste effecten werd gekeken. Hierbij werd een maximum van acht uitkomstmaten gehanteerd. De werkgroep waardeerde deze uitkomstmaten volgens hun relatieve belang bij de besluitvorming rondom aanbevelingen, als cruciaal (kritiek voor de besluitvorming), belangrijk (maar niet cruciaal) en onbelangrijk. Tevens definieerde de werkgroep tenminste voor de cruciale uitkomstmaten welke verschillen zij klinisch (patiënt) relevant vonden.
Methode literatuursamenvatting
Een uitgebreide beschrijving van de strategie voor zoeken en selecteren van literatuur is te vinden onder ‘Zoeken en selecteren’ onder Onderbouwing. Indien mogelijk werd de data uit verschillende studies gepoold in een random-effects model. Review Manager 5.4 werd gebruikt voor de statistische analyses. De beoordeling van de kracht van het wetenschappelijke bewijs wordt hieronder toegelicht.
Beoordelen van de kracht van het wetenschappelijke bewijs
De kracht van het wetenschappelijke bewijs werd bepaald volgens de GRADE-methode. GRADE staat voor ‘Grading Recommendations Assessment, Development and Evaluation’ (zie http://www.gradeworkinggroup.org/). De basisprincipes van de GRADE-methodiek zijn: het benoemen en prioriteren van de klinisch (patiënt) relevante uitkomstmaten, een systematische review per uitkomstmaat, en een beoordeling van de bewijskracht per uitkomstmaat op basis van de acht GRADE-domeinen (domeinen voor downgraden: risk of bias, inconsistentie, indirectheid, imprecisie, en publicatiebias; domeinen voor upgraden: dosis-effect relatie, groot effect, en residuele plausibele confounding).
GRADE onderscheidt vier gradaties voor de kwaliteit van het wetenschappelijk bewijs: hoog, redelijk, laag en zeer laag. Deze gradaties verwijzen naar de mate van zekerheid die er bestaat over de literatuurconclusie, in het bijzonder de mate van zekerheid dat de literatuurconclusie de aanbeveling adequaat ondersteunt (Schünemann, 2013; Hultcrantz, 2017).
|
GRADE |
Definitie |
|
Hoog |
|
|
Redelijk |
|
|
Laag |
|
|
Zeer laag |
|
Bij het beoordelen (graderen) van de kracht van het wetenschappelijk bewijs in richtlijnen volgens de GRADE-methodiek spelen grenzen voor klinische besluitvorming een belangrijke rol (Hultcrantz, 2017). Dit zijn de grenzen die bij overschrijding aanleiding zouden geven tot een aanpassing van de aanbeveling. Om de grenzen voor klinische besluitvorming te bepalen moeten alle relevante uitkomstmaten en overwegingen worden meegewogen. De grenzen voor klinische besluitvorming zijn daarmee niet één op één vergelijkbaar met het minimaal klinisch relevant verschil (Minimal Clinically Important Difference, MCID). Met name in situaties waarin een interventie geen belangrijke nadelen heeft en de kosten relatief laag zijn, kan de grens voor klinische besluitvorming met betrekking tot de effectiviteit van de interventie bij een lagere waarde (dichter bij het nuleffect) liggen dan de MCID (Hultcrantz, 2017).
De beoordelingen van de literatuur en de conclusies zijn gedaan op basis van de GRADE systematiek. De werkgroep vindt het belangrijk om relevante beperkingen hiervan aan te geven.
De klinische vraag gaat vaak over een reductie van ernstige postoperatieve pijn en opioïdenconsumptie in een aantal patiënten met resp. over een klinisch relevante reductie van ernstige postoperatieve pijn opioïdenconsumptie bij een individuele patiënt. Hetzelfde geldt voor opioïdenconsumptie; de keuze van een absolute drempelwaarde in mg (i.p.v. een relatieve drempelwaarde in %) maakt dit afhankelijk van tijdstip, ingreep en ernst van de pijn: vroege postoperatieve tijdstippen en (studies met) ingrepen met relatief lage opioïdconsumptie kunnen vaak de MCID niet bereiken.
De keuze van de MCID heeft een bepaalde mate van willekeurigheid en is niet absoluut te zien. Ook zijn de conclusies zo geformuleerd (en geven alleen beperkt antwoord op het effect op een individuele patiënt voor een specifieke ingreep).
In de literatuur worden de eindpunten pijnscores en opioïdenconsumptie separaat van elkaar weer gegeven, suggererend dat deze onafhankelijk van elkaar zijn. Echter kunnen deze twee eindpunten niet onafhankelijk van elkaar beoordeeld worden; in ieder protocol is opgenomen dat pijn behandeld moet worden. Deze separate beoordeling geeft niet altijd een adequaat antwoord op de klinische vraag naar het analgetische effect van een interventie.
Daarnaast worden multimodale componenten als aparte interventies beoordeeld, echter de klinische vraag is naar de effectiviteit als bouwsteen van een multimodale werkwijze.
Overwegingen (van bewijs naar aanbeveling)
Om te komen tot een aanbeveling zijn naast (de kwaliteit van) het wetenschappelijke bewijs ook andere aspecten belangrijk en worden meegewogen, zoals aanvullende argumenten uit bijvoorbeeld de biomechanica of fysiologie, waarden en voorkeuren van patiënten, kosten (middelenbeslag), aanvaardbaarheid, haalbaarheid en implementatie. Deze aspecten zijn systematisch vermeld en beoordeeld (gewogen) onder het kopje ‘Overwegingen’ en kunnen (mede) gebaseerd zijn op expert opinion. Hierbij is gebruik gemaakt van een gestructureerd format gebaseerd op het evidence-to-decision framework van de internationale GRADE Working Group (Alonso-Coello, 2016a; Alonso-Coello 2016b). Dit evidence-to-decision framework is een integraal onderdeel van de GRADE methodiek.
De aanbevelingen geven antwoord op de uitgangsvraag en zijn gebaseerd op het beschikbare wetenschappelijke bewijs en de belangrijkste overwegingen, en een weging van de gunstige en ongunstige effecten van de relevante interventies. De kracht van het wetenschappelijk bewijs en het gewicht dat door de werkgroep wordt toegekend aan de overwegingen, bepalen samen de sterkte van de aanbeveling. Conform de GRADE-methodiek sluit een lage bewijskracht van conclusies in de systematische literatuuranalyse een sterke aanbeveling niet a priori uit, en zijn bij een hoge bewijskracht ook zwakke aanbevelingen mogelijk (Agoritsas, 2017; Neumann, 2016). De sterkte van de aanbeveling wordt altijd bepaald door weging van alle relevante argumenten tezamen. De werkgroep heeft bij elke aanbeveling opgenomen hoe zij tot de richting en sterkte van de aanbeveling zijn gekomen.
In de GRADE-methodiek wordt onderscheid gemaakt tussen sterke en zwakke (of conditionele) aanbevelingen. De sterkte van een aanbeveling verwijst naar de mate van zekerheid dat de voordelen van de interventie opwegen tegen de nadelen (of vice versa), gezien over het hele spectrum van patiënten waarvoor de aanbeveling is bedoeld. De sterkte van een aanbeveling heeft duidelijke implicaties voor patiënten, behandelaars en beleidsmakers (zie onderstaande tabel). Een aanbeveling is geen dictaat, zelfs een sterke aanbeveling gebaseerd op bewijs van hoge kwaliteit (GRADE gradering HOOG) zal niet altijd van toepassing zijn, onder alle mogelijke omstandigheden en voor elke individuele patiënt.
|
Implicaties van sterke en zwakke aanbevelingen voor verschillende richtlijngebruikers |
||
|
|
||
|
|
Sterke aanbeveling |
Zwakke (conditionele) aanbeveling |
|
Voor patiënten |
De meeste patiënten zouden de aanbevolen interventie of aanpak kiezen en slechts een klein aantal niet. |
Een aanzienlijk deel van de patiënten zouden de aanbevolen interventie of aanpak kiezen, maar veel patiënten ook niet. |
|
Voor behandelaars |
De meeste patiënten zouden de aanbevolen interventie of aanpak moeten ontvangen. |
Er zijn meerdere geschikte interventies of aanpakken. De patiënt moet worden ondersteund bij de keuze voor de interventie of aanpak die het beste aansluit bij zijn of haar waarden en voorkeuren. |
|
Voor beleidsmakers |
De aanbevolen interventie of aanpak kan worden gezien als standaardbeleid. |
Beleidsbepaling vereist uitvoerige discussie met betrokkenheid van veel stakeholders. Er is een grotere kans op lokale beleidsverschillen. |
Organisatie van zorg
In de knelpuntenanalyse en bij de ontwikkeling van de richtlijnmodule is expliciet aandacht geweest voor de organisatie van zorg: alle aspecten die randvoorwaardelijk zijn voor het verlenen van zorg (zoals coördinatie, communicatie, (financiële) middelen, mankracht en infrastructuur). Randvoorwaarden die relevant zijn voor het beantwoorden van deze specifieke uitgangsvraag zijn genoemd bij de overwegingen. Meer algemene, overkoepelende, of bijkomende aspecten van de organisatie van zorg worden behandeld in de module Organisatie van zorg.
Commentaar- en autorisatiefase
De conceptrichtlijnmodule werd aan de betrokken (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd ter commentaar. De commentaren werden verzameld en besproken met de werkgroep. Naar aanleiding van de commentaren werd de conceptrichtlijnmodule aangepast en definitief vastgesteld door de werkgroep. De definitieve richtlijnmodule werd aan de deelnemende (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd voor autorisatie en door hen geautoriseerd dan wel geaccordeerd.
Literatuur
Agoritsas T, Merglen A, Heen AF, Kristiansen A, Neumann I, Brito JP, Brignardello-Petersen R, Alexander PE, Rind DM, Vandvik PO, Guyatt GH. UpToDate adherence to GRADE criteria for strong recommendations: an analytical survey. BMJ Open. 2017 Nov 16;7(11):e018593. doi: 10.1136/bmjopen-2017-018593. PubMed PMID: 29150475; PubMed Central PMCID: PMC5701989.
Alonso-Coello P, Schünemann HJ, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Rada G, Rosenbaum S, Morelli A, Guyatt GH, Oxman AD; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ. 2016 Jun 28;353:i2016. doi: 10.1136/bmj.i2016. PubMed PMID: 27353417.
Alonso-Coello P, Oxman AD, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Vandvik PO, Meerpohl J, Guyatt GH, Schünemann HJ; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 2: Clinical practice guidelines. BMJ. 2016 Jun 30;353:i2089. doi: 10.1136/bmj.i2089. PubMed PMID: 27365494.
Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, Fervers B, Graham ID, Grimshaw J, Hanna SE, Littlejohns P, Makarski J, Zitzelsberger L; AGREE Next Steps Consortium. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010 Dec 14;182(18):E839-42. doi: 10.1503/cmaj.090449. Epub 2010 Jul 5. Review. PubMed PMID: 20603348; PubMed Central PMCID: PMC3001530.
Hultcrantz M, Rind D, Akl EA, Treweek S, Mustafa RA, Iorio A, Alper BS, Meerpohl JJ, Murad MH, Ansari MT, Katikireddi SV, Östlund P, Tranæus S, Christensen R, Gartlehner G, Brozek J, Izcovich A, Schünemann H, Guyatt G. The GRADE Working Group clarifies the construct of certainty of evidence. J Clin Epidemiol. 2017 Jul;87:4-13. doi: 10.1016/j.jclinepi.2017.05.006. Epub 2017 May 18. PubMed PMID: 28529184; PubMed Central PMCID: PMC6542664.
Medisch Specialistische Richtlijnen 2.0 (2012). Adviescommissie Richtlijnen van de Raad Kwalitieit. http://richtlijnendatabase.nl/over_deze_site/over_richtlijnontwikkeling.html
Neumann I, Santesso N, Akl EA, Rind DM, Vandvik PO, Alonso-Coello P, Agoritsas T, Mustafa RA, Alexander PE, Schünemann H, Guyatt GH. A guide for health professionals to interpret and use recommendations in guidelines developed with the GRADE approach. J Clin Epidemiol. 2016 Apr;72:45-55. doi: 10.1016/j.jclinepi.2015.11.017. Epub 2016 Jan 6. Review. PubMed PMID: 26772609.
Schünemann H, Brożek J, Guyatt G, et al. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group, 2013. Available from http://gdt.guidelinedevelopment.org/central_prod/_design/client/handbook/handbook.html.
Zoekverantwoording
Zoekverantwoording
Embase 2-5-2022
|
No. |
Query |
Results |
|
#16 |
#4 AND #15 |
2 |
|
#15 |
#13 OR #14 |
2 |
|
#14 |
postoperative AND analgesic AND effects AND of AND paravertebral AND versus AND erector AND spinae AND plane AND block AND for AND thoracic AND breast AND surgery AND a AND 'meta analysis' AND xiong |
1 |
|
#13 |
paravertebral AND block AND versus AND thoracic AND epidural AND for AND patients AND undergoing AND thoracotomy AND yeung |
1 |
|
#12 |
#4 AND #11 |
189 |
|
#11 |
#10 AND [1-1-2000]/sd NOT ('conference abstract'/it OR 'editorial'/it OR 'letter'/it OR 'note'/it) NOT (('animal experiment'/exp OR 'animal model'/exp OR 'nonhuman'/exp) NOT 'human'/exp) |
189 |
|
#10 |
#3 AND #9 |
311 |
|
#9 |
'epidural anesthesia'/exp OR 'dural blocking' OR 'epidural anaesthesia' OR 'epidural anaesthetic agent' OR 'epidural anesthesia' OR 'epidural anesthetic agent' OR 'epidural block' OR 'epidural blockade' OR 'extradural anaesthesia' OR 'extradural anesthesia' OR 'extradural block' OR 'lumbar epidural anaesthesia' OR 'lumbar epidural anesthesia' OR 'lumbar peridural anaesthesia' OR 'lumbar peridural anesthesia' OR 'lumbar peridural block' OR 'peridural anaesthesia' OR 'peridural anesthesia' OR 'peridural block' OR 'peridural blocking' |
38117 |
|
#8 |
#4 AND #6 |
384 |
|
#7 |
#4 AND #5 |
69 |
|
#6 |
'clinical trial'/exp OR 'randomization'/exp OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp OR 'placebo'/exp OR 'prospective study'/exp OR rct:ab,ti OR random*:ab,ti OR 'single blind':ab,ti OR 'randomised controlled trial':ab,ti OR 'randomized controlled trial'/exp OR placebo*:ab,ti |
3527495 |
|
#5 |
'meta analysis'/exp OR 'meta analysis (topic)'/exp OR metaanaly*:ti,ab OR 'meta analy*':ti,ab OR metanaly*:ti,ab OR 'systematic review'/de OR 'cochrane database of systematic reviews'/jt OR prisma:ti,ab OR prospero:ti,ab OR (((systemati* OR scoping OR umbrella OR 'structured literature') NEAR/3 (review* OR overview*)):ti,ab) OR ((systemic* NEAR/1 review*):ti,ab) OR (((systemati* OR literature OR database* OR 'data base*') NEAR/10 search*):ti,ab) OR (((structured OR comprehensive* OR systemic*) NEAR/3 search*):ti,ab) OR (((literature NEAR/3 review*):ti,ab) AND (search*:ti,ab OR database*:ti,ab OR 'data base*':ti,ab)) OR (('data extraction':ti,ab OR 'data source*':ti,ab) AND 'study selection':ti,ab) OR ('search strategy':ti,ab AND 'selection criteria':ti,ab) OR ('data source*':ti,ab AND 'data synthesis':ti,ab) OR medline:ab OR pubmed:ab OR embase:ab OR cochrane:ab OR (((critical OR rapid) NEAR/2 (review* OR overview* OR synthes*)):ti) OR ((((critical* OR rapid*) NEAR/3 (review* OR overview* OR synthes*)):ab) AND (search*:ab OR database*:ab OR 'data base*':ab)) OR metasynthes*:ti,ab OR 'meta synthes*':ti,ab |
820576 |
|
#4 |
#3 AND [1-1-2000]/sd NOT ('conference abstract'/it OR 'editorial'/it OR 'letter'/it OR 'note'/it) NOT (('animal experiment'/exp OR 'animal model'/exp OR 'nonhuman'/exp) NOT 'human'/exp) |
790 |
|
#3 |
#1 AND #2 |
1433 |
|
#2 |
((paravertebral NEAR/3 block*):ti,ab,kw) OR ((erector NEAR/3 spinae NEAR/3 plane NEAR/3 block*):ti,ab,kw) OR 'esp block*':ti,ab,kw OR ((intercostal NEAR/3 block*):ti,ab,kw) |
4184 |
|
#1 |
'thoracotomy'/exp OR 'thorax surgery'/exp OR 'chest operation*':ti,ab,kw OR 'pleura incision*':ti,ab,kw OR 'pleuracotom*':ti,ab,kw OR 'pleural incision*':ti,ab,kw OR 'pleurotom*':ti,ab,kw OR 'rethoracotom*':ti,ab,kw OR 'thoracotom*':ti,ab,kw OR 'thoracoscopic':ti,ab,kw OR 'video assisted thoracoscopic surgery'/exp OR 'vat*':ti,ab,kw OR 'video assisted thoracic surgery':ti,ab,kw OR 'video assisted thoracoscopic surgery':ti,ab,kw OR 'video-assisted thoracic surgery':ti,ab,kw OR 'videothoracoscopic surgery':ti,ab,kw OR 'video-assisted thoracoscopic surger*':ti,ab,kw OR 'robot assisted thoracoscopic surger*':ti,ab,kw OR 'robot-assisted thoracoscopic surger*':ti,ab,kw OR 'rats':ti,ab,kw OR 'robot assisted thoracic surger*':ti,ab,kw OR 'robot-assisted thoracic surger*':ti,ab,kw OR 'robotthoracoscopic surger*':ti,ab,kw OR 'funnel chest'/exp OR 'chonechondrosternon':ti,ab,kw OR 'foveated chest':ti,ab,kw OR 'foveated thorax':ti,ab,kw OR 'funnel breast':ti,ab,kw OR 'funnel chest':ti,ab,kw OR 'funnel thorax':ti,ab,kw OR 'koilosternia':ti,ab,kw OR 'pectus excavatum':ti,ab,kw OR 'nuss procedure'/exp OR 'ravitch procedure'/exp OR ravitch:ti,ab,kw OR 'cardiothoracic surger*':ti,ab,kw OR 'chest surger*':ti,ab,kw OR 'thoracic operation*:ti,ab,kw' OR 'thoracic surger*':ti,ab,kw OR 'thoracic surgical procedure*':ti,ab,kw OR 'thorax surger*':ti,ab,kw |
1832890 |
Pubmed 04-05-2022
|
Search |
Query |
Results |
|
#8 |
Search: #4 AND #6 (RCT’s) |
|
|
#7 |
Search: #4 AND #5 (SR’s) |
|
|
#6 |
Search: ((random*[tiab] AND (controlled[tiab] OR control[tiab] OR placebo[tiab] OR versus[tiab] OR vs[tiab] OR group[tiab] OR groups[tiab] OR comparison[tiab] OR compared[tiab] OR arm[tiab] OR arms[tiab] OR crossover[tiab] OR cross-over[tiab]) AND (trial[tiab] OR study[tiab])) OR ((single[tiab] OR double[tiab] OR triple[tiab]) AND (masked[tiab] OR blind*[tiab]))) |
|
|
#5 |
Search: ("Meta-Analysis" [Publication Type] OR "Meta-Analysis as Topic"[Mesh] OR metaanaly*[tiab] OR meta-analy*[tiab] or metanaly*[tiab] OR "Systematic Review" [Publication Type] OR systematic[sb] OR "Cochrane Database Syst Rev"[Journal] or prisma[tiab] OR preferred reporting items[tiab] OR prospero[tiab] OR ((systemati*[ti] OR scoping[ti] OR umbrella[ti] OR structured literature[ti]) AND (review*[ti] OR overview*[ti])) OR systematic review*[tiab] OR scoping review*[tiab] OR umbrella review*[tiab] OR structured literature review*[tiab] OR systematic qualitative review*[tiab] OR systematic quantitative review*[tiab] OR systematic search and review[tiab] OR systematized review[tiab] OR systematised review[tiab] OR systemic review[tiab] OR systematic literature review*[tiab] OR systematic integrative literature review*[tiab] OR systematically review*[tiab] OR scoping literature review*[tiab] OR systematic critical review[tiab] OR systematic integrative review*[tiab] OR systematic evidence review[tiab] OR Systematic integrative literature review*[tiab] OR Systematic mixed studies review*[tiab] OR Systematized literature review*[tiab] OR Systematic overview*[tiab] OR Systematic narrative review*[tiab] OR ((systemati*[tiab] OR literature[tiab] OR database*[tiab] OR data-base*[tiab] OR structured[tiab] OR comprehensive*[tiab] OR systemic*[tiab]) AND search*[tiab]) OR (Literature[ti] AND review[ti] AND (database*[tiab] OR data-base*[tiab] OR search*[tiab])) OR ((data extraction[tiab] OR data source*[tiab]) AND study selection[tiab]) OR (search strategy[tiab] AND selection criteria[tiab]) OR (data source*[tiab] AND data synthesis[tiab]) OR medline[tiab] OR pubmed[tiab] OR embase[tiab] OR Cochrane[tiab] OR ((critical[ti] OR rapid[ti]) AND (review*[ti] OR overview*[ti] OR synthes*[ti])) OR (((critical*[tiab] OR rapid*[tiab]) AND (review*[tiab] OR overview*[tiab] OR synthes*[tiab]) AND (search*[tiab] OR database*[tiab] OR data-base*[tiab]))) OR metasynthes*[tiab] OR meta-synthes*[tiab]) NOT ("Comment" [Publication Type] OR "Letter" [Publication Type] OR "Editorial" [Publication Type] OR (("Animals"[Mesh] OR "Models, Animal"[Mesh]) NOT "Humans"[Mesh])) |
|
|
#4 |
Search: #3 AND ("2000/01"[Date - Entrez] : "3000"[Date - Entrez]) |
|
|
#3 |
Search: #1 AND #2 |
|
|
#2 |
Search: (paravertebral[tiab] OR intercostal[tiab] OR "erector spinae plane"[tiab] OR ESP[tiab]) AND block*[tiab] |
|
|
#1 |
Search: "Thoracotomy"[Mesh] OR "Thoracic Surgical Procedures"[Mesh] OR thoracotom*[tiab] OR "chest operation*"[tiab] OR "pleura incision*"[tiab] OR pleuracotom*[tiab] OR "pleural incision*"[tiab] OR pleurotom*[tiab] OR rethoracotom*[tiab] OR "Thoracic Surgery, Video-Assisted"[Mesh] OR "video-assisted thoracoscopic surger*"[tiab] OR "video assisted thoracoscopic surger*"[tiab] OR vat*[tiab] OR "robot assisted thoracoscopic surger*"[tiab] OR rats[tiab] OR "robot assisted thoracic surger*"[tiab] OR "Funnel Chest"[Mesh] OR "funnel chest*"[tiab] OR chonechondrosternon[tiab] OR "funnel breast*"[tiab] OR "funnel thorax"[tiab] OR koilosternia[tiab] OR "pectus excavatum"[tiab] OR "nuss procedure*"[tiab] OR "ravitch procedure*"[tiab] OR "cardiothoracic surger*"[tiab] OR "chest surger*"[tiab] OR "thoraric operation*"[tiab] OR "thoracic surger*"[tiab] OR "thorax surger*"[tiab] |