Gabapentine
Uitgangsvraag
Wat is de plaats van de toevoeging van gabapentine aan de zorg voor kinderen die een chirurgische ingreep ondergaan?
Aanbeveling
Dien geen gabapentine toe bij kinderen in het kader van multimodale pijnstilling perioperatief.
Overweeg de meerdaagse toediening van gabapentine in geselecteerde patiëntengroepen; bij (oncologische) amputatie chirurgie of (een hoog risico op) neuropathische pijn.
Overwegingen
Voor- en nadelen van de interventie en de kwaliteit van het bewijs
Er is literatuuronderzoek verricht naar de gunstige en ongunstige effecten van het toevoegen van gabapentine aan de standaardzorg bij kinderen die een chirurgische ingreep ondergaan. In totaal zijn er 12 randomized controlled trials geïncludeerd, waarvan het aantal patiënten per arm varieerde van 17 tot 44. De gemiddelde leeftijd van de kinderen varieerde van 62,8 maanden tot 15,8 jaar. Er is in de studies gekeken naar verschillende soorten operaties (adenotonsillectomie, orthopedische chirurgie, thoraxchirurgie, wervelkolomchirurgie) en verschillende soorten pijnstilling (meerdere soorten opioïden en paracetamol).
Postoperatieve pijn was gedefinieerd als cruciale uitkomstmaat. Voor postoperatieve pijn was de bewijskracht zeer laag tot laag. Er is afgewaardeerd voor beperkingen in de studieopzet (risico op bias), tegenstrijdige resultaten, betrouwbaarheidsintervallen die de grens van klinische besluitvorming overschreden en het aantal geïncludeerde patiënten. Hiermee komt de totale bewijskracht uit op zeer laag. Op basis van de literatuur over de cruciale uitkomstmaat kan geen richting worden gegeven aan de aanbevelingen.
Angst, opioïdconsumptie, postoperatieve mobilisatiesnelheid, complicaties en verblijfsduur in het ziekenhuis waren gedefinieerd als belangrijke uitkomstmaten. Voor deze belangrijke uitkomstmaten was de bewijskracht zeer laag tot laag. Bij deze uitkomstmaten is afgewaardeerd voor tegenstrijdige resultaten, onduidelijke betrouwbaarheidsintervallen, een zeer klein aantal events en het aantal geïncludeerde patiënten. Op basis van de literatuur over de belangrijke uitkomstmaten kan dus ook geen richting worden gegeven aan de aanbevelingen.
De literatuur kan in dit geval dus onvoldoende richting geven aan de besluitvorming. De aanbeveling is daarom gebaseerd op aanvullende argumenten waaronder expert opinie, waar mogelijk aangevuld met (indirecte) literatuur.
Ook op basis van expert opinie wordt het routinematig toedienen van gabapentine binnen een multimodaal behandelplan bij kinderen perioperatief niet geadviseerd.
Hierbij wordt wel de aanvulling gedaan dat vanuit de volwassenliteratuur er een zwak bewijs is dat gabapentine mogelijk wel de kans op chronische postoperatieve pijn verminderen. Toegespitst op kinderen is op dit moment voor deze uitkomstmaat onvoldoende wetenschappelijke literatuur beschikbaar (Doleman, 2023).
Er is een zwak bewijs in de afgeleide literatuur (Wang, 2018) dat bij oncologische amputaties in kinderen de kans op postoperatieve fantoompijn verkleind zou kunnen worden door het routinematig gebruik van gabapentine perioperatief. Voor deze groep zou het starten van gabapentine overwogen kunnen worden; zeker in het geval van risicofactoren (hoge pijnscores preoperatief, neuropathische pijn preoperatief, oudere leeftijd). Hierbij wordt meegewogen dat het complicatie risico danwel risico op bijwerkingen van gabapentine laag is.
De gevonden wetenschappelijke literatuur laat zien dat wanneer het geven van gabapentine overwogen wordt dit ten minste dient te gebeuren in het kader van een regime van meerdaagse toediening. Eenmalige toediening lijkt niet zinvol.
Waarden en voorkeuren van patiënten (en evt. hun verzorgers)
Gabapentine is een medicament met relatief weinig bijwerkingen en complicaties bij toediening. Derhalve is bij toediening van de medicatie over het algemeen sprake van een lage belasting voor de patiënt. In de praktijk worden bijwerkingen (duizeligheid, sufheid, gedragsveranderingen (zoals ongeremder of agressiever gedrag)) soms wel gezien, met name bij de start van de medicatie, waardoor een goede kosten-baten analyse van belang is bij het voorschrijven van de medicatie. Gezien het feit dat vanuit de literatuur (behoudens eventueel in geselecteerde groepen, zie boven) geen meerwaarde van de medicatie gezien wordt lijkt het niet zinvol om deze interventie routinematig aan ouders en patiënten aan te bieden danwel deze met hen te bespreken als optie voor pijnstilling. Zoals eerder beschreven kan overwogen worden om in geselecteerde gevallen (amputaties, hoog risico op chronische postoperatieve pijn of neuropathische pijn) deze medicatie wel als optie met patiënt en ouders te bespreken.
Kosten (middelenbeslag)
De kosten van gabapentine zijn zeer laag en derhalve geen relevante factor die meegewogen dient te worden in het wel/niet voorschrijven van deze medicatie
Aanvaardbaarheid, haalbaarheid en implementatie
Implementatie van het routinematig toedienen van gabapentine als perioperatieve pijnstilling is in principe makkelijk te realiseren. Medicatie is goedkoop en wordt volledig vergoed. Orale toediening is in meerdere vormen (zoals drank, capsules) beschikbaar, waardoor voor alle leeftijdsgroepen te gebruiken. Medicatie is geregistreerd in het kinderformularium als verantwoord in het gebruik bij kinderen. Doseeradviezen worden ook gegeven in het kinderformularium (Gabapentine, Kinderformularium). Gezien het feit dat er echter geen aantoonbaar effect wordt gezien van het routinematig toedienen van gabapentine perioperatief bij kinderen wordt implementatie in een multimodaal pijnbeleid van de deze medicatie niet geadviseerd.
Rationale van aanbeveling -1: weging van argumenten voor en tegen de interventies
Er is sprake van een lage kwaliteit en kwantiteit van wetenschappelijk bewijsmateriaal als het gaat om het routinematig gebruik van gabapentine in het kader van een multimodaal pijnbeleid bij kinderen. Tevens is er maar bij een beperkt aantal ingrepen (ATE, wervelkolomchirurgie en onderste ledemaat chirurgie) gekeken naar het effect van deze medicatie bij kinderen perioperatief. In de beperkte beschikbare literatuur wordt geen aanwijzing gezien voor een betere pijnstilling, minder opioïdengebruik of een betere perioperatieve angstbestrijding bij het gebruik van gabapentine. Hierbij valt op te merken dat de vergeleken doseringen, het doseringsregime en het type medicatie erg uiteenlopen.
Op basis van de op dit moment beschikbare literatuur ziet de werkgroep echter geen toegevoegde waarde van het routinematig toedienen van gabapentine bij kinderen als perioperatieve pijnstiller.
Rationale van aanbeveling -2: weging van argumenten voor en tegen de interventies
Op basis van expert opinion en aanpalende literatuur zijn er aanwijzingen dat gabapentine mogelijk de kans op chronische pijn en fantoompijn in geselecteerde groepen zouden kunnen verminderen. Vanuit de wetenschappelijk literatuur is het echter niet mogelijk om een harde uitspraak te doen over het nut van het toedienen van gabapentine voor deze indicatie. Gezien de relatief beperkte bijwerkingen, lage kosten en het gemak van toediening van de medicatie kan in geselecteerde groepen (amputaties, grote kans op neuropathische pijn) gabapentine perioperatief in overleg met patiënt en ouders overwogen worden. Een eenmalige preoperatieve dosis wordt hierin niet zinvol geacht, meerdaagse toediening mogelijk wel.
Onderbouwing
Achtergrond
Gabapentine maakt nog geen deel uit van de standaard multimodale perioperatieve therapie voor kinderen in veel ziekenhuizen. Voor volwassenen wordt deze medicatie veel meer toegepast in een multimodaal pijnbeleid perioperatief. Kosten voor deze medicatie zijn laag, en implementatie kost weinig moeite. De vraag is of er voldoende bewijs is voor deze therapie bij kinderen, onder welke omstandigheden danwel bij welke ingrepen deze medicatie zinvol zou kunnen zijn, wat de mogelijke bijwerkingen zijn en of implementatie van deze medicatie in het kader van multimodale perioperatieve pijnstilling bij kinderen zinvol zou kunnen zijn.
Conclusies / Summary of Findings
|
Very low GRADE |
The evidence is very uncertain about the effect of gabapentinoids on postoperative pain at 0 hours compared with standard care in children undergoing a surgical procedure.
Source: Haddadi, 2020; Rusy, 2010 |
|
Low GRADE |
Gabapentinoids may have no effect on postoperative pain at 6 hours compared with standard care in children undergoing a surgical procedure.
Source: Amin, 2011; Haddadi, 2020; Helenius, 2020; Mayell, 2014 |
|
Low GRADE |
Gabapentinoids may have no effect on postoperative pain at 24 hours compared with standard care in children undergoing a surgical procedure.
Source: Gettis, 2022; Haddadi, 2020; Helenius, 2020; Mayell, 2014 |
|
Very low GRADE |
The evidence is very uncertain about the effect of gabapentinoids on anxiety compared with standard care in children undergoing a surgical procedure.
Source: Tomaszek, 2020 |
|
Low GRADE |
Gabapentinoids may have no effect on postoperative opioid consumption at 24 hours compared with standard care in children undergoing a surgical procedure.
Source: Mayell, 2014; Helenius, 2020; |
|
Low GRADE |
Gabapentinoids may have little to no effect on total postoperative opioid consumption compared with standard care in children undergoing a surgical procedure.
Source: Amin, 2011; Anderson, 2020; Helenius, 2020; Tomaszek, 2020 |
|
No GRADE |
No evidence was found for the effect of gabapentinoids on time to postoperative mobilization compared with standard care in children undergoing a surgical procedure.
Source: - |
|
Low GRADE |
Gabapentinoids may have no effect on nausea compared with standard care in children undergoing a surgical procedure.
Source: Amin, 2011; Anderson, 2020; Fenikowski, 2022; Gettis, 2022; Haddadi, 2020; Helenius, 2020; Mayell, 2014; Rusy, 2010; Tomaszek, 2020 |
|
No GRADE |
No evidence was found for the effect of gabapentinoids on drowsiness compared with standard care in children undergoing a surgical procedure.
Source: - |
|
Low GRADE |
Gabapentinoids may reduce agitation compared with standard care in children undergoing a surgical procedure.
Source: Filho, 2019; Marouf, 2018; Salman, 2013 |
|
Very low GRADE |
The evidence is very uncertain about the effect of gabapentinoids on dizziness compared with standard care in children undergoing a surgical procedure.
Source: Fenikowski, 2022; Gettis, 2020; Marouf, 2018; Mayell, 2014; Salman, 2013; Tomaszek, 2020 |
|
Low GRADE |
Gabapentinoids may have no effect on length of stay when compared with standard care in children undergoing a surgical procedure.
Source: Anderson, 2020; Helenius, 2020 |
Samenvatting literatuur
Description of studies
An overview of the relevant study characteristics is presented in Table 1. More detailed information can be found in the evidence tables. As shown in Table 1, studies were conducted in children undergoing various surgeries. Patients per arm varied from 17 to 44. The mean age of patients varied from 62.8 months to 15.8 years.
Table 1. Study characteristics
|
Author, year |
Population (I/C), mean age; sex M/F |
Surgical procedure |
Intervention |
Control |
Pain scale |
Type of opioid used |
|
Amin, 2011 |
35/35, 5.3 and 4.8 years; 19/16 and 20/15 |
Adenotonsillectomy |
Oral gabapentin (syrup) 10mg/kg, single dose preoperative, 2 hours before induction of anesthesia |
Oral acetaminophen, 20mg/kg, 2 hours before induction of anesthesia |
VAS graded 0 to 10 |
Pethidine |
|
Anderson, 2020 |
24/26, 14.8 and 14.2 years, 5/19, 7/19 |
Posterior spinal fusion |
Oral gabapentin, preoperative 15 mg/kg and postoperative: 10 mg/kg every 8h |
Preoperative oral placebo 15mg/kg, postoperative 10 mg/kg every 8h |
VAS 10-cm line |
Morphine equivalent (intravenous hydromorphone, morphine, fentanyl, and oral oxycodone) |
|
Fenikowski, 2022 |
28/28, median 14 and 15 years, 27/1 and 23/5 |
Modified Ravitch procedure |
Oral gabapentin, preoperative 15mg/kg and postoperative: twice (6am, 6pm) 7.5 mg/kg for 3 days |
Placebo capsules instead of gabapentin |
NRS (0-10) |
Morphine |
|
Filho, 2019 |
40/44, 62.8 and 84.8 months, 22/18 and 31/13
|
Unilateral lower limb surgery |
Oral gabapentin solution, single dose, 10 mg/kg to 600 mg, 1 to 2 hours before surgery |
Placebo syrup, 1 to 2 hours before surgery |
CRIES (3mo-1yr), CHIPPS (1-5yrs), WBFS modified to 10 points (6-16yrs) |
Morphine |
|
Gettis, 2022 |
25/23, 6.8 and 6.6 years, 20/5 and 15/8 |
Tonsillectomy/ Adenoidectomy |
Oral gabapentin solution (10mg/ml), single dose, 15mg/kg (max 600mg), 30-60 min prior to transfer to operating room |
Oral placebo suspension, similar timing |
WBFS or VAS |
Oxycodone, hydrocodone, fentanyl, morphine, hydromorphone |
|
Haddadi, 2020 |
30/30, 10.40 and 8.37 years, not reported. |
Adenotonsillectomy |
Oral gabapentin suspension, single dose 10mg/kg, 2h before anesthesia + placebo (suppository) after intubation and maintenance of anesthesia |
Placebo gabapentin suspension 2h before anesthesia, acetaminophen suppository 40 mg/kg following intubation and maintenance of anesthesia |
WBFS (0-10) |
Morphine |
|
Helenius, 2020 |
32/31, 15.8 and 15.5 years, 11/21 and 10/21 |
Pedicle screw instrumentation for adolescent idiopathic scoliosis (AIS), spondylolisthesis, or Scheuermann kyphosis |
Oral pregabalin, preoperative (two doses): 2 mg/kg rounded up to next 25 mg on preoperative evening 12 h and approximately 2h before the induction of anesthesia. Maximum 150 mg twice a day. Postoperative: twice a day for 5 days |
Placebo: similar-looking capsules with similar timing |
Verbal NRS from 0 to 10 |
Oxycodone |
|
Marouf, 2018 |
30/30, 6.9 and 6.7 years, 16/14 and 17/13 |
Adenotonsillectomy |
Pregabalin syrup, single dose, 30 min before commencement anesthesia, concentration 100mg/ml, 1.5 mg/kg |
Placebo syrup 30 min before commencement anesthesia |
Faces Pain Scale-Revised (0-10) |
None (only acetaminophen used) |
|
Mayell, 2014 |
18/17, 14.7 and 15.0 years, 2/16 and 4/13 |
Idiopathic scoliosis surgery |
Gabapentin capsules, 600mg dose, 1h before surgery |
Placebo capsules 1h before surgery |
NRS score 0-10 |
Morphine or morphine equivalent |
|
Rusy, 2010 |
29/30, 14.8 and 14.2 years, 7/22 and 7/23 |
Pediatric spinal fusion |
Gabapentin, 15 mg/kg, capsule or liquid, 25-30 min before transportation to operating room. Postoperative: 5mg/kg 3 times per day for 5 days. |
Placebo capsule or liquid, similar timing |
Verbal NRS (0-10) |
Morphine (hydromorphone doses were multiplied by 5 to obtain morphine equivalents in 1 patient who received hydromorphone) |
|
Salman, 2013 |
23/23, median 5.6 and 5.3 years, 8/15 and 11/12 |
Tonsillectomy and adenoidectomy |
Gabapentin, 15 mg.kg-1 dissolved in 10 ml of saline orally, 30 min. before the induction of anesthesia |
Placebo: 10 ml of saline 30 min before the induction of anesthesia |
OPS
|
None (only acetaminophen used) |
|
Tomaszek, 2020 |
20/20, median 13 and 15 years, 16/4 and 18/2
|
Ravitch modified method |
Gabapentin, preoperative: 15mg/kg 1h before surgery. Postoperative: 7.5 mg/kg twice a day (6am, 6pm) for 3 days |
Placebo, similar timing |
NRS; range 0–10 |
Fentanyl |
CRIES = Children's Revised Impact of Event Scale, CHIPPS = Children's and Infants' Postoperative Pain Scale, NRS = numeric rating scale, OPS = Objective pain scale, VAS = visual analog scale, WBFS = Wong baker faces pain rating scale
Results
1. Postoperative pain
1.1 Postoperative pain at 0 hours post-surgery
Two studies reported postoperative pain at 0 hours post-surgery.
Haddadi (2020) used the Wong-Baker Faces Pain Rating Scale (WBFS) to report postoperative pain. The mean pain score was 3.20 (SD 1.94) in the intervention group and 2.73 (SD 1.57) in the control group. The mean difference (MD) of 0.47 (95%CI -0.42 to 1.36) is not considered clinically relevant.
Rusy (2010) used a verbal numerical rating scale (NRS, 0-10) to report postoperative pain in the post anesthesia recovery unit (PARU). The mean pain score was 2.5 (SD 2.8) in the intervention group and 6.0 (SD 2.4) in the control group. The MD of -3.50 (95%CI -4.83 to -2.17) is considered clinically relevant and is in favor of the intervention group.
1.1 Postoperative pain at 6 hours post-surgery
Two studies reported postoperative pain at 6 hours post-surgery and two studies reported postoperative pain at 8 hours post-surgery.
Amin (2011) used a visual analog scale (VAS) graded from 0 to 10 to report postoperative pain. At 6 hours after surgery, the mean pain score was 4.05 (SD 1.61) in the intervention group and 5.20 (SD 0.89) in the control group. The MD of -1.15 (95% CI -1.76 to -0.54) is considered clinically relevant and is in favor of the intervention group.
Haddadi (2020) used the WBFS to report postoperative pain. In the 6th hour after surgery the mean pain score was 1.33 (SD 0.84) in the intervention group and 1.53 (SD 1.07) in the control group. The MD of -0.20 (95% CI -0.69 to 0.29) is not considered clinically relevant.
Helenius (2020) used a verbal NRS from 0 to 10 to report postoperative pain after 8 hours. Postoperative pain was only reported graphically for the entire study population. For a subgroup of adolescent idiopathic scoliosis (AIS) patients (N=25/26) mean scores and SDs were reported. At 8 hours after surgery, the mean pain score was 3.1 (SD 2.3) in the intervention group and 3.3 (SD 2.2) in the control group. The MD of -0.20 (95% CI -1.44 to 1.04) is not considered clinically relevant.
Mayell (2014) assessed pain scores with a NRS (0-10) at 8 hours postoperatively. The difference between the groups was described as not significant, but median scores were only reported graphically in box and whisker plots. As a result, the clinical relevance cannot be determined.
1.1 Postoperative pain at 24 hours post-surgery
Four studies reported the outcome postoperative pain at 24 hours post-surgery.
Gettis (2022) reported median pain scores of 4 on a scale of 10 (WBFS and VAS) for both groups at 24 hours postoperatively. However, no other information was given and therefore the clinical relevance cannot be determined.
Haddadi (2020) used the WBFS (0-10) to report postoperative pain. In the 24th hour after surgery the mean pain score was 0.93 (SD 1.26) in the intervention group and 0.60 (SD 0.62) in the control group. The MD of 0.33 (95% CI -0.17 to 0.83) is not considered clinically relevant.
Helenius (2020) used a verbal NRS from 0 to 10 to report postoperative pain. Postoperative pain was reported in a graph for the entire study population without absolute numbers. Mean scores and SDs were however reported for a subgroup of adolescent idiopathic scoliosis (AIS) patients (N=25/26). The mean pain score was 3.3 (SD 2.6) in the intervention group and 3.5 (SD 2.2) in the control group. The MD of -0.20 (95% CI -1.52 to 1.12) is not considered clinically relevant.
Mayell (2014) assessed pain scores with the NRS (0-10) at 24 hours postoperatively. The difference between the groups was described as not significant, but median scores were only reported graphically in box and whisker plots. As a result, the clinical relevance cannot be determined.
2. Anxiety
Two studies reported the outcome anxiety.
Fenikowski (2022) reported postoperative state and trait anxiety for the intervention and control group. State and trait anxiety were assessed with the State-Trait Anxiety Inventory for children between 9 and 14 years (STAI-C, range: 20-60 points) and adolescents (STAI, range: 20-28 points). The results were expressed as sten scores with a range of 1–10 (5–6 sten = a moderate level of anxiety, >7 sten = a high level of anxiety). The difference was described as statistically non-significant, but without absolute data this cannot be verified, and the clinical relevancy cannot be interpreted.
Tomaszek (2020) reported postoperative state anxiety levels (range 1–10 sten) in a figure. The median level of state anxiety was reported to be lower in the intervention group on postoperative day 3 compared to before surgery (6 vs 7), while the level of postoperative anxiety remained the same as the preoperative level for the control group (6 vs 6). This difference is clinically relevant in favor of the intervention group.
3. Postoperative opioid consumption
3.1 Opioid consumption at 24 hours
Two studies reported opioid consumption at 24 hours.
Mayell (2014) reported cumulative postoperative opioid consumption provided as morphine equivalents in mg/kg. After 24 hours the mean total reported consumption was 1.29 (SD 0.44) in the intervention group and 1.46 (SD 0.68) in the control group. The MD of -0.17 (95% CI -0.55 to 0.21) is not considered clinically relevant.
Helenius (2020) reported cumulative oxycodone consumption in mg/kg at 24 hours presented as median values (95%CI). The median cumulative consumption was 0.70 mg/kg (0.63-0.81) in the experimental group and 0.72 mg/kg (0.66-0.87) in the control group. The difference of 0.02mg/kg (2.8%) is not considered clinically relevant.
3.2 Total opioid consumption
Four studies reported total opioid consumption.
Amin (2011) reported the total postoperative pethidine consumption from the time of extubation. The mean consumption in mg was 8 (SD 10.05) in the intervention group and 16.25 (SD 11.57) in the control group. The MD of -8.25 mg (95% CI -13.33 to -3.17) is considered clinically relevant in favor of the intervention group.
Anderson (2020) reported opioid consumption in morphine equivalents. The total medication in mg/kg during postoperative days 0-6 is reported. The mean amount of medication was 3.38 mg/kg (SD 1.79) in the intervention group and 5.05 mg/kg (SD 3.16) in the control group. The MD of -1.67 mg/kg (95% CI -3.08 to -0.26) is considered clinically relevant in favor of the intervention group.
Helenius (2020) reported cumulative oxycodone consumption in mg/kg at discharge presented as median values (95%CI). The median cumulative consumption was 2.90 mg/kg (2.75-3.51) in the experimental group and 3.07 mg/kg (2.96-3.88) in the control group. The difference of 0.17 mg/kg (5.5%) is not considered clinically relevant.
Tomaszek (2020) reported total fentanyl consumption in µg for postoperative days 0-3. The total consumption was 1,279 µg (SD 384) in the intervention group and 1,266 µg (SD 396) in the control group. The MD of 13 µg (95% CI -228.75 to 254.75) is not considered clinically relevant.
4. Time to postoperative mobilization
No studies reported the outcome time to postoperative mobilization.
5. Complications
5.1 Nausea
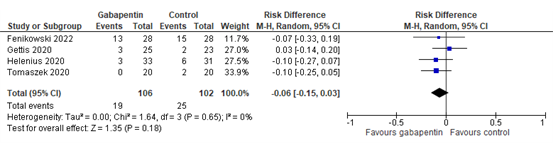
Nine studies reported the outcome nausea. Four studies reported nausea incidence or nausea and vomiting incidence (Fenikowski 2022, Gettis 2022, Helenius 2020, Tomaszek 2020). The results of these studies are pooled in Figure 1. The pooled risk difference of -0.06 (95%CI -0.15 to 0.03) is not considered clinically relevant.
Five studies also reported the outcome nausea and found no significant differences between the two groups (Amin 2011, Anderson 2020, Haddadi 2020, Mayell 2014, Rusy 2010). These studies did however not report nausea incidence per group and therefore the results of these studies could not be used to determine the clinical relevance.
Figure 1. Nausea incidence.
5.2 Drowsiness
No studies reported the outcome drowsiness.
5.3 Agitation
Three studies reported the outcome agitation.
Filho (2019) reported the frequency of postoperative agitation in the first operative hour. The postoperative effects were classified into 4 categories: dizziness, calmness, sedation and agitation. In the experimental group, 5 (12.5%) patients were agitated compared with 16 (36.3%) patients in the control group. The RR of 0.34 (95%CI 0.14, 0.85) is considered clinically relevant in favor of the experimental group.
Marouf (2018) measured emergence agitation on a 5-point scale (EAS; 1 = sleeping; 2 = awake, calm; 3 = irritable, crying; 4 = inconsolable crying; 5 = intense restlessness, disorientation). Mean and SD scores were reported at 10, 20 and 30 minutes after surgery:
- 10 min: intervention group mean score 2.66 (SD 1.18) vs control group mean score 3.4 (SD 1): MD -0.74 (95% CI -1.29, -0.19)
- 20 min: intervention group mean score 2.2 (SD 1.12) vs control group mean score 3.16 (SD 0.87): MD -0.96 (95% CI -1.47, -0.45)
- 30 min: intervention group mean score 2 (SD 1.01) vs control group mean score 3.06 (SD 0.78): MD -1.06 (95%CI -1.52, -0.60)
These mean differences are considered clinically relevant in favor of the experimental group.
Salman (2013) reported median emergence agitation (1: sleeping; 2: awake, calm; 3: irritable, crying; 4: inconsolable crying; 5: severe restlessness, disorientation) and range at 10, 20 and 30 minutes after surgery:
- 10 min: 4 (1-5) vs 5 (3-5)
- 20 min: 3 (1-5) vs 4 (2-5)
- 30 min: 2 (2-5) vs 4 (2-5)
The differences were described as significantly lower in the experimental group at 20 and 30 minutes, but without absolute data this cannot be checked and as a result the clinical relevance cannot be determined.
5.4 Dizziness
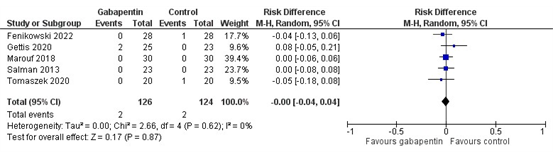
Six studies reported the outcome dizziness. Five studies reported dizziness incidence (Fenikowski 2022, Gettis 2022, Marouf 2018, Salman 2013, Tomaszek 2020). The results of these studies are pooled in Figure 2. The pooled risk difference of -0.00 (95% CI -0.04 to 0.04) is not considered clinically relevant.
Mayell (2014) only reported the total number of dizziness incidences per group instead of the number of patients who reported dizziness. No significant differences were found with regard to gabapentin-associated side effects, but without absolute data this cannot be checked. As a result, the clinical relevance cannot be determined.
Figure 2. Dizziness incidence.
6. Length of stay
Two studies reported length of hospital stay in days.
Anderson (2020) reported days hospitalized for both groups. The mean number of days was 4.5 (SD 0.7) in the intervention group and 4.5 (SD 0.9) in the control group. The MD of 0.00 (95% CI -0.45 to 0.45) is not considered clinically relevant.
Helenius (2020) reported mean length of stay for both groups but did not report the standard deviation. For the intervention group the mean length of stay was 6.5 days (range 4-10 days) and for the control group the mean length of stay was 6.8 days (range 5-9 days) The difference was described as statistically non-significant, but without absolute data this cannot be verified, and the clinical relevance cannot be interpreted.
Level of evidence of the literature
The level of evidence for all outcomes was based on randomized controlled trials and therefore started at high.
The level of evidence regarding the outcome measure postoperative pain at 0 hours was downgraded by three levels to very low because of study limitations (risk of bias, -1) conflicting results (inconsistency, -1) and the confidence interval crossing the threshold of clinical relevance (imprecision, -1).
The level of evidence regarding the outcome measure postoperative pain at 6 hours was downgraded by two levels to low because of conflicting results (inconsistency, -1) and a low number of included patients (imprecision, -1).
The level of evidence regarding the outcome measure postoperative pain at 24 hours was downgraded by two levels to low because of a low number of included patients (imprecision, -2).
The level of evidence regarding the outcome measure anxiety was downgraded by three levels to very low because of unclear confidence intervals and the number of included patients (imprecision, -3).
The level of evidence regarding the outcome measure opioid consumption at 24 hours was downgraded by two levels to low because of number of included patients (imprecision, -2).
The level of evidence regarding the outcome measure total opioid consumption was downgraded by two levels to low because of conflicting results (inconsistency, -1) and number of included patients (imprecision, -1).
The level of evidence regarding the outcome measure time to postoperative mobilization could not be graded since no studies reported this outcome.
The level of evidence regarding the outcome measure nausea was downgraded by two levels to low because of number of included patients (imprecision, -2).
The level of evidence regarding the outcome measure drowsiness could not be graded since no studies reported this outcome.
The level of evidence regarding the outcome measure agitation was downgraded by two levels to low because of the confidence interval crossing the thresholds of clinical relevance (imprecision, -2).
The level of evidence regarding the outcome measure dizziness was downgraded by three levels to very low because of a very low number of events (imprecision, -3).
The level of evidence regarding the outcome measure length of stay was downgraded by two levels to low because of unclear confidence intervals and number of included patients (imprecision, -2).
Zoeken en selecteren
A systematic review of the literature was performed to answer the following question:
What are the (un)favorable effects of adding antiepileptic drugs (gabapentinoids: pregabalin/gabapentin) to standard care in children undergoing a surgical procedure?
| P: | Children undergoing a surgical procedure |
| I: | Pregabalin/Gabapentin + standard care |
| C: | Standard care (placebo or acetaminophen) |
| O: |
Postoperative pain Anxiety Opioid consumption Time to postoperative mobilization Complications Length of stay |
Relevant outcome measures
The guideline development group considered postoperative pain as a critical outcome measure for decision making; and anxiety, opioid consumption, postoperative mobilization speed, complications and length of stay as an important outcome measure for decision making.
The working group defined the outcome measures as follows:
- Postoperative pain à PACU/ 0 hours, 6 and 24 hours (at rest; if nothing was reported about the condition in which pain was assessed (at rest or during mobilization) it was assumed pain was measured at rest)
- Opioid consumption à postoperative consumption in morphine equivalents; after 24 hours, total
- Complications à nausea, drowsiness, chronic pain (pain > 3 months (based on the international association study of pain (IASP)), agitation, dizziness
- Length of stay à length of stay in hospital in days
A priori, the working group did not define the outcome measures 'anxiety' and 'time to postoperative mobilization' but used the definitions used in the studies.
The working group defined the following clinically important differences:
- Postoperative pain: 1 point difference on a 10-point pain scale and 10 mm difference on a 100 mm pain scale.
- Anxiety: 10% difference between groups
- Opioid consumption: 20% difference between groups
- Time to postoperative mobilization: 1 day
- Complications: 10% difference, risk ratio (RR) ≤0.91 or ≥1.10; risk difference (RD) 0.10.
- Length of stay: 1 day.
Search and select (Methods)
The databases Medline (via OVID) and Embase (via Embase.com) were searched with relevant search terms until 10 December 2022. The detailed search strategy is depicted under the tab Methods. The systematic literature search resulted in 655 hits. Studies were selected based on the following criteria:
- RCT or systematic review
- Comparing standard care + Pregabalin/Gabapentin with standard care (placebo or acetaminophen)
- Study population: children undergoing a surgical procedure
- Reporting at least one outcome as defined in the PICO
- Published ≥ 2000.
Initially, 20 studies were selected based on title and abstract screening. After reading the full text, 8 studies were excluded (see the table with reasons for exclusion under the tab Methods), and 12 studies were included.
Results
In total, 12 studies were included in the analysis of the literature. Important study characteristics and results are summarized in the evidence tables. The assessment of the risk of bias is summarized in the risk of bias tables.
Referenties
- Amin SM, Amr YM. Comparison between preemptive gabapentin and paracetamol for pain control after adenotonsillectomy in children. Anesth Essays Res. 2011 Jul-Dec;5(2):167-70. doi: 10.4103/0259-1162.94758. PMID: 25885382; PMCID: PMC4173413.
- Anderson DE, Duletzke NT, Pedigo EB, Halsey MF. Multimodal pain control in adolescent posterior spinal fusion patients: a double-blind, randomized controlled trial to validate the effect of gabapentin on postoperative pain control, opioid use, and patient satisfaction. Spine Deform. 2020 Apr;8(2):177-185. doi: 10.1007/s43390-020-00038-z. Epub 2020 Feb 5. PMID: 32026435.
- Doleman B, Mathiesen O, Sutton AJ, Cooper NJ, Lund JN, Williams JP. Non-opioid analgesics for the prevention of chronic postsurgical pain: a systematic review and network meta-analysis. Br J Anaesth. 2023 Jun;130(6):719-728. doi: 10.1016/j.bja.2023.02.041. Epub 2023 Apr 12. PMID: 37059625; PMCID: PMC10251124.
- Fenikowski D, Tomaszek L, Mazurek H, Gawron D, Maciejewski P. The Effects of Gabapentin on Post-Operative Pain and Anxiety, Morphine Consumption and Patient Satisfaction in Paediatric Patients Following the Ravitch Procedure-A Randomised, Double-Blind, Placebo-Controlled, Phase 4 Trial. J Clin Med. 2022 Aug 11;11(16):4695. doi: 10.3390/jcm11164695. PMID: 36012932; PMCID: PMC9409887.
- Gettis M, Brown AM, Fujimoto A, Wetzel M, Thomsen J. Gabapentin Premedication to Reduce Postoperative Pain for Pediatric Tonsillectomy/Adenoidectomy: A Pilot Study. J Perianesth Nurs. 2022 Oct;37(5):626-631. doi: 10.1016/j.jopan.2021.11.011. Epub 2022 Mar 5. PMID: 35256248.
- Haddadi S, Marzban S, Parvizi A, Nemati S, Chohdari A, Atrkar Roshan Z, Ramezani H. Effects of Gabapentin Suspension and Rectal Acetaminophen on Postoperative Pain of Adenotonsillectomy in Children. Iran J Otorhinolaryngol. 2020 Jul;32(111):197-205. doi: 10.22038/ijorl.2020.38811.2283. PMID: 32850507; PMCID: PMC7423084.
- Helenius LL, Oksanen H, Lastikka M, Pajulo O, Löyttyniemi E, Manner T, Helenius IJ. Preemptive Pregabalin in Children and Adolescents Undergoing Posterior Instrumented Spinal Fusion: A Double-Blinded, Placebo-Controlled, Randomized Clinical Trial. J Bone Joint Surg Am. 2020 Feb 5;102(3):205-212. doi: 10.2106/JBJS.19.00650. PMID: 31770296.
- Mayell A, Srinivasan I, Campbell F, Peliowski A. Analgesic effects of gabapentin after scoliosis surgery in children: a randomized controlled trial. Paediatr Anaesth. 2014 Dec;24(12):1239-44. doi: 10.1111/pan.12524. Epub 2014 Sep 17. PMID: 25230144.
- Marouf HM. Effect of Pregabalin Premedication on Emergence Agitation in Children after Sevoflurane Anesthesia: A Randomized Controlled Study. Anesth Essays Res. 2018 Jan-Mar;12(1):31-35. doi: 10.4103/aer.AER_223_17. PMID: 29628550; PMCID: PMC5872889.
- Pinto Filho WA, Silveira LHJ, Vale ML, Fernandes CR, Alves Gomes J. The Effect of Gabapentin on Postoperative Pain of Orthopedic Surgery of Lower Limb by Sciatic and Femoral Blockage in Children: A Clinical Trial. Anesth Pain Med. 2019 Aug 6;9(4):e91207. doi: 10.5812/aapm.91207. PMID: 31754608; PMCID: PMC6825328.
- Rusy LM, Hainsworth KR, Nelson TJ, Czarnecki ML, Tassone JC, Thometz JG, Lyon RM, Berens RJ, Weisman SJ. Gabapentin use in pediatric spinal fusion patients: a randomized, double-blind, controlled trial. Anesth Analg. 2010 May 1;110(5):1393-8. doi: 10.1213/ANE.0b013e3181d41dc2. PMID: 20418301.
- Salman AE, Camk?ran A, O?uz S, Dönmez A. Gabapentin premedication for postoperative analgesia and emergence agitation after sevoflurane anesthesia in pediatric patients. Agri. 2013;25(4):163-8. doi: 10.5505/agri.2013.98852. PMID: 24264551.
- Tomaszek L, Fenikowski D, Maciejewski P, Komotajtys H, Gawron D. Perioperative Gabapentin in Pediatric Thoracic Surgery Patients-Randomized, Placebo-Controlled, Phase 4 Trial. Pain Med. 2020 Aug 1;21(8):1562-1571. doi: 10.1093/pm/pnz207. PMID: 31596461.
- Wang X, Yi Y, Tang D, Chen Y, Jiang Y, Peng J, Xiao J. Gabapentin as an Adjuvant Therapy for Prevention of Acute Phantom-Limb Pain in Pediatric Patients Undergoing Amputation for Malignant Bone Tumors: A Prospective Double-Blind Randomized Controlled Trial. J Pain Symptom Manage. 2018 Mar;55(3):721-727. doi: 10.1016/j.jpainsymman.2017.11.029. Epub 2017 Dec 6. PMID: 29221844.
Evidence tabellen
Evidence table for intervention studies
Risk of bias table for intervention studies (randomized controlled trials; based on Cochrane risk of bias tool and suggestions by the CLARITY Group at McMaster University)
|
Study reference
(first author, publication year) |
Was the allocation sequence adequately generated?
Definitely yes Probably yes Probably no Definitely no |
Was the allocation adequately concealed?
Definitely yes Probably yes Probably no Definitely no |
Blinding: Was knowledge of the allocated interventions adequately prevented?
Were patients blinded?
Were healthcare providers blinded?
Were data collectors blinded?
Were outcome assessors blinded?
Were data analysts blinded? Definitely yes Probably yes Probably no Definitely no |
Was loss to follow-up (missing outcome data) infrequent?
Definitely yes Probably yes Probably no Definitely no |
Are reports of the study free of selective outcome reporting?
Definitely yes Probably yes Probably no Definitely no |
Was the study apparently free of other problems that could put it at a risk of bias?
Definitely yes Probably yes Probably no Definitely no |
Overall risk of bias If applicable/necessary, per outcome measure
LOW Some concerns HIGH
|
|
Amin, 2011 |
Probably yes
Reason: Patients were randomly allocated equally (35 patients) in each group |
Definitely yes
Reason: The randomization was performed using sealed numbered envelopes indicating the group of each patient. |
Probably yes
Reason: A blind nurse who did not participate in patients’ follow up read the number and made group assignments.
A blinded chief nurse who did not participate in data collection confirmed that each patient ingested the medications as was scheduled.
The pain intensity was assisted by a person who was blind to study.
No information on outcome assessors and data-analysts. |
Probably yes
Reason: No loss to follow-up reported |
Probably yes
Reason: No research protocol available. Outcomes mentioned in methods section are reported. |
No information. |
LOW (all outcomes) |
|
Anderson, 2020 |
Probably yes
Reason: Subjects were randomized into either the experimental or control group by the OHSU research pharmacy via block randomization upon patient enrollment to result in equal-sized groups at study completion. |
No information |
Probably yes
Reason: Patients, caretakers, and providers remained blinded to group assignments.
In protocol: Masking: Quadruple (Participant, Care Provider, Investigator, Outcomes Assessor)
(blinding of data analysts not reported) |
Definitely yes
Reason: Loss to follow-up was infrequent and similar in both groups |
Definitely yes
Reason: All relevant outcomes from protocol/ methods section are reported. |
No information.
|
LOW (all outcomes) |
|
Fenikowski, 2022 |
Probably yes
Reason: Randomized, (allocation ratio 1:1) |
No information
|
Probably yes
Reason:
From protocol: Masking: Quadruple (Participant, Care Provider, Investigator, Outcomes Assessor)
The children and their parents, nursing staff, surgeons, anaesthesiologists, investigators and data analysts were blinded to group assignments. It should be noted that the principal investigator performed both the duties of the study director and was responsible for data analysis. |
Definitely yes
Reason: Lost to follow-up n=0 reported for both groups |
Probably/definitely yes
Reason: All relevant outcomes from protocol/ methods section are reported. |
No information.
|
LOW (all outcomes) |
|
Filho, 2019 |
Probably yes
Reason: Our team randomized patients with a drawing software. |
No information |
Probably yes
Reason: The pharmacologist was blinded to solutions. For operative and postoperative period, the professionals didn’t know the group they had evaluated.
(blinding of data analysts not reported) |
Probably yes
Reason: Loss to follow-up was infrequent in intervention and control group.
|
Probably yes
Reason: All relevant outcomes from protocol/ methods section are reported. |
No information. |
LOW (all outcomes) |
|
Gettis, 2022 |
Definitely yes
Reason: Patients were randomized at a 1:1 ratio. Randomization was performed using random permuted blocks of size 2 and 4. Only the IDS pharmacist had access to the table. |
No information |
Probably yes
Reason: All ambulatory surgery staff, patients, and families as well as the research team were blinded to group assignments. […] the IDS pharmacist assigned a patient identification number sequentially and dispensed either gabapentin or placebo according to the randomization table.
(blinding of data analysts not reported) |
Probably yes
Reason: Loss to follow-up was infrequent in intervention and control group. |
Probably yes
Reason: All relevant outcomes from protocol/ methods section are reported. |
No information. |
LOW (all outcomes) |
|
Hadaddi , 2020 |
Definitely yes
Reason: The children were divided into two groups (..) based on quadruple random blocks. At the initiation of the study, the equences of the blocks were selected by simple random sampling, (..) The subjects were assigned into two groups based on the gradual referral of the patients who met the inclusion criteria. |
Definitely yes
Reason: Random sampling, sealed in the envelopes, and stored at Anesthesiology Research Center. |
Probably yes
Reason: The children, anesthesiologist, and ear, nose, throat surgeon were blinded to the study groups. The anesthesiologist performing the study was unaware of the constituents of the drugs and allocation of the groups. An anesthetist who was responsible for the questionnaires with different parameters was also unaware of group assignment; accordingly, blinding was satisfactorily maintained throughout the study course.
(blinding of data analysts not reported) |
Probably yes
Reason: No loss to follow-up reported.
|
Probably yes:
Reason: All relevant outcomes from methods section/ trial registration are reported. |
Funding and conflicts of interest not reported.
Groups not comparable at baseline: the differences in the weight and age of the children were statistically significant between the two groups and the subjects in the gabapentin group were approximately 3 kg heavier and 2 years older than those in the acetaminophen group. |
Some concerns (all outcomes) |
|
Helenius , 2020 |
Probably yes
Reason: Randomization to the study groups (1:1) was performed by the Department of Pharmacy on the basis of a predetermined list with blocks of 20 patients. |
No information |
Probably yes
Reason: The investigators, patients, parents, nursing staff, and surgeons were blinded to group assignment.
(blinding of data collectors and analysts not reported) |
Probably yes
Reason: Loss to follow-up was infrequent in intervention and control group. |
Probably yes
Reason: All relevant outcomes from methods section/ trial registration are reported. |
No information.
|
LOW (all outcomes) |
|
Marouf, 2018 |
Probably yes
Reason: Randomization was performed using computer program to create a list of numbers.
|
Definitely yes
Reason: Each number (which referred to one group) was sealed in an opaque envelope. Each parent was asked to choose one envelope and give it to a person who compared the number with the computer-created list and then allocated the child to one group. |
Probably yes
Reason: The individual who assigned the patient to the group was different from the one who formulated the drugs. The anesthesiologist who gave anesthesia and collected data and the child and his parents were unaware of the child's group.
(blinding of outcome assessors and data analysts not reported) |
Definitely yes
Reason: No child was excluded from the study. |
Probably yes
Reason: All relevant outcomes from methods section are reported. |
No information.
|
LOW (all outcomes) |
|
Mayell, 2014 |
Probably yes
Reason: Patients were randomized using a standardized randomization by our Department of Pharmacy. |
No information |
Probably yes
Reason: randomized double-blinded placebo-controlled study, All outcome measurements were taken by blinded observers.
|
Probably yes
Reason: Loss to follow-up was infrequent in intervention and control group. |
Probably yes
Reason: All relevant outcomes from methods section are reported. |
No information.
|
LOW (all outcomes) |
|
Rusy, 2010 |
Probably yes
Reason: A hospital pharmacy clinical coordinator randomized participants to each study group (gabapentin or placebo), with stratification by surgeon before premedication for surgery. |
No information |
Definitely yes
Reason: The patients, parents, nursing staff, surgeons, acute pain staff managing the patients, and study principal investigator collecting postoperative data were blinded to group assignment.
(blinding of data analysts not reported) |
Probably yes
Reason: Loss to follow-up was infrequent in intervention and control group. |
Probably yes
Reason: All relevant outcomes from methods section/ trial registration are reported. |
No information on conflicts of interest.
|
LOW (all outcomes) |
|
Salman, 2013 |
Probably yes
Reason: The patients were randomly assigned (…) using a randomization list. |
No information |
Probably yes
Reason: Drugs were prepared by an investigator who was not involved in the group assignment. The anesthesiologists and data collectors and parents and observers in the recovery room were blinded to treatment group.
Anesthesia duration, and time to eye opening and extubation times were recorded by an observer blinded to the group assignment.
(blinding of data analysts not reported) |
Probably yes
Reason: No loss to follow-up reported. |
Probably yes
Reason: All relevant outcomes from methods section are reported. |
Funding not reported.
|
LOW (all outcomes) |
|
Tomaszek, 2020 |
Definitely yes
Reason: Patients were randomly assigned to one of two parallel groups (gabapentin vs placebo) in a 1:1 ratio.
The hospital pharmacy was responsible for assigning patients to individual groups (gabapentin and placebo) based on a computer-generated list that was prepared before recruitment by the study director. |
Definitely yes
Reason: Next, the capsules, packed in an envelope marked with the personal identity number code of the patient, were delivered to the ward. The nurses administered the medicine given in the individual order card as “gabapentin.” In a second sealed envelope, which was stored in a designated place in the operating suite, the pharmacist placed the information about whether the patient received gabapentin or placebo; the envelope could be opened only in the case of life-threatening complications. After the treatment was finished, the sealed envelope was returned to the pharmacist. |
Definitely yes
Reason: With the exception of the hospital pharmacy, participants, care providers (physicians, nurses, psychologist), investigators, and data analysts were blinded. One of the principal investigators had several roles to fulfill in the study as study director and data analyst. |
Probably yes
Reason: Loss to follow-up was infrequent in intervention and control group. |
Probably yes
Reason: All relevant outcomes from methods section/ trial registration are reported. |
No information.
|
LOW (all outcomes) |
Table of excluded studies
|
Reference |
Reason for exclusion |
|
Salah Abdelgalil A, Shoukry AA, Kamel MA, Heikal AMY, Ahmed NA. Analgesic Potentials of Preoperative Oral Pregabalin, Intravenous Magnesium Sulfate, and their Combination in Acute Postthoracotomy Pain. Clin J Pain. 2019 Mar;35(3):247-251. doi: 10.1097/AJP.0000000000000673. PMID: 30730476. |
Wrong target population (data for children not reported separately) |
|
Amin SM. Evaluation of gabapentin and dexamethasone alone or in combination for pain control after adenotonsillectomy in children. Saudi J Anaesth. 2014 Jul;8(3):317-22. doi: 10.4103/1658-354X.136417. Retraction in: Saudi J Anaesth. 2018 Oct-Dec;12(4):662. PMID: 25191179; PMCID: PMC4141377. |
Article was retracted |
|
Egunsola O, Wylie CE, Chitty KM, Buckley NA. Systematic Review of the Efficacy and Safety of Gabapentin and Pregabalin for Pain in Children and Adolescents. Anesth Analg. 2019 Apr;128(4):811-819. doi: 10.1213/ANE.0000000000003936. PMID: 30451725. |
No meta-analysis, review also includes studies that do not fit the target population (no surgery) |
|
Helenius L, Yrjälä T, Oksanen H, Pajulo O, Löyttyniemi E, Taittonen M, Helenius I. Pregabalin and Persistent Postoperative Pain Following Posterior Spinal Fusion in Children and Adolescents: A Randomized Clinical Trial. J Bone Joint Surg Am. 2021 Aug 23. doi: 10.2106/JBJS.21.00153. Epub ahead of print. PMID: 34424869. |
No useful outcomes reported |
|
Koh WS, Leslie K. Postoperative analgesia for complex spinal surgery. Curr Opin Anaesthesiol. 2022 Oct 1;35(5):543-548. doi: 10.1097/ACO.0000000000001168. Epub 2022 Jul 27. PMID: 35900754. |
No systematic review, data for children not reported separately |
|
Talaat SM, El-Gendy HA. Effect of pregabalin versus midazolam premedication on the anesthetic and analgesic requirements in pediatric day-case surgery: A randomized controlled trial. Egyptian Journal of Anaesthesia. 2021 Jan 1;37(1):50-6. |
Wrong comparison (control group was midozalam) |
|
Zhu A, Benzon HA, Anderson TA. Evidence for the Efficacy of Systemic Opioid-Sparing Analgesics in Pediatric Surgical Populations: A Systematic Review. Anesth Analg. 2017 Nov;125(5):1569-1587. doi: 10.1213/ANE.0000000000002434. PMID: 29049110. |
Broad review, included only 2 relevant RCTs (those 2 are included separately) |
|
Wang X, Yi Y, Tang D, Chen Y, Jiang Y, Peng J, Xiao J. Gabapentin as an Adjuvant Therapy for Prevention of Acute Phantom-Limb Pain in Pediatric Patients Undergoing Amputation for Malignant Bone Tumors: A Prospective Double-Blind Randomized Controlled Trial. J Pain Symptom Manage. 2018 Mar;55(3):721-727. doi: 10.1016/j.jpainsymman.2017.11.029. Epub 2017 Dec 6. PMID: 29221844. |
No useful outcomes reported |
Verantwoording
Beoordelingsdatum en geldigheid
Publicatiedatum : 18-12-2024
Beoordeeld op geldigheid : 17-12-2024
Algemene gegevens
De ontwikkeling/herziening van deze richtlijn werd ondersteund door het Kennisinstituut van de Federatie Medisch Specialisten (www.demedischspecialist.nl/kennisinstituut) en werd gefinancierd uit de Kwaliteitsgelden Medisch Specialisten (SKMS). De financier heeft geen enkele invloed gehad op de inhoud van de richtlijnmodule.
Samenstelling werkgroep
Voor het ontwikkelen van de richtlijn is in 2022 een multidisciplinaire werkgroep ingesteld, bestaande uit vertegenwoordigers van alle relevante specialismen (zie hiervoor de Samenstelling van de werkgroep) die betrokken zijn bij de zorg voor kinderen met postoperatieve pijn.
Werkgroep
Dr. L.M.E. (Lonneke) Staals, anesthesioloog, voorzitter, NVA
Dr. C.M.A. (Caroline) van den Bosch, anesthesioloog-pijnspecialist, NVA
Drs. A.W. (Alinde) Hindriks-Keegstra, anesthesioloog, NVA
Drs. G.A.J. (Geranne) Hopman, anesthesioloog, NVA
Drs. L.J.H. (Lea) van Wersch, anesthesioloog, NVA
Dr. C.M.G. (Claudia) Keyzer-Dekker, kinderchirurg, NVvH
Drs. F.L. (Femke) van Erp Taalman Kip, orthopedisch kinderchirurg, NOV
Dr. L.M.A. (Laurent) Favié, ziekenhuisapotheker, NVZA
J. (Jantine) Boerrigter-van Ginkel, verpleegkundig specialist kinderpijn, V&VN
S. (Sharine) van Rees-Florentina, recovery verpleegkundige, BRV
E.C. (Esen) Doganer en M. (Marjolein) Jager, beleidsmedewerker, Kind & Ziekenhuis
Klankbordgroep
Dr. L.M. (Léon) Putman, cardiothoracaal chirurg, NVT
R. (Remko) ter Riet, MSc, anesthesiemedewerker/physician assistant, NVAM
Drs. L.I.M. (Laura) Meltzer, KNO-arts, NVKNO
Met ondersteuning van
Dr. L.M.P. Wesselman, adviseur, Kennisinstituut van de Federatie Medisch Specialisten
I. van Dijk, junior adviseur, Kennisinstituut van de Federatie Medisch Specialisten
Belangenverklaringen
De Code ter voorkoming van oneigenlijke beïnvloeding door belangenverstrengeling is gevolgd. Alle werkgroepleden hebben schriftelijk verklaard of zij in de laatste drie jaar directe financiële belangen (betrekking bij een commercieel bedrijf, persoonlijke financiële belangen, onderzoeksfinanciering) of indirecte belangen (persoonlijke relaties, reputatiemanagement) hebben gehad. Gedurende de ontwikkeling of herziening van een module worden wijzigingen in belangen aan de voorzitter doorgegeven. De belangenverklaring wordt opnieuw bevestigd tijdens de commentaarfase.
Een overzicht van de belangen van werkgroepleden en het oordeel over het omgaan met eventuele belangen vindt u in onderstaande tabel. De ondertekende belangenverklaringen zijn op te vragen bij het secretariaat van het Kennisinstituut van de Federatie Medisch Specialisten.
|
Werkgroeplid |
Functie |
Nevenfuncties |
Gemelde belangen |
Ondernomen actie |
|
L.M.E. Staals (voorzitter) |
Anesthesioloog Sectorhoofd Kinder- en Obstetrische anesthesiologie Universitair Docent Erasmus MC Sophia Kinderziekenhuis, Rotterdam |
Lid wetenschapcommissie Sectie Kinderanesthesiologie (NVA) (onbetaald) Lid scientific forum ESAIC/Devices abd Technology (onbetaald) Lid werkgroep Landelijke Kwaliteitsregistratie Amandeloperaties (NVKNO/NVA) (onbetaald) |
MSD/ Merck: i.v.m. een clinical trial naar sugammadex bij kinderen: Consultant of Global Clinical Trial Operations in the Netherlands. Betaald (inkomsten gaan op onderzoekskostenplaats van de afdeling Anesthesiologie Erasmus MC Sophia). Dit onderzoek gaat over sugammadex (antagonist voor spierverslapping). Klinisch onderzoek gedaan naar postoperatieve pijnstilling bij kinderen na buikchirurgie, d.m.v. wondcatheter met lokaal anestheticum (nog niet gepubliceerd, daarom niet meegenomen in search van de richtlijn). Er is geen belang bij het advies van de richtlijn. |
Geen restricties |
|
C.M.A. van den Bosch |
Anesthesioloog - pijnspecialist Prinses Maxima Centrum |
Geen |
Geen |
Geen restricties |
|
A.W. Hindriks-Keegstra |
Anesthesioloog UMC Utrecht
|
Geen |
VR ter behandeling van postoperatieve pijn en angst bij kinderen. |
Geen restricties. Extern gefinancierd onderzoek valt buiten bestek van de richtlijn |
|
G.A.J. Hopman |
Anesthesioloog, Radboud UMC, Nijmegen |
Geen |
Geen |
Geen restricties |
|
L.J.H. van Wersch |
Anesthesioloog, Maasziekenhuis Pantein |
Geen |
Geen |
Geen restricties |
|
C.M.G. Keyzer-Dekker |
Kinderchirurg, Erasmus MC Sophia. |
Geen |
Geen |
Geen restricties |
|
F.L. van Erp Taalman Kip |
Orthopedisch kinderchirurg, Erasmus Medisch Centrum Rotterdam |
-Docent Fontys Hogeschool Eindhoven, curriculum kinder- podotherapie -Docent TNO Leiden, onderwijs Jeugdartsen, - Trainer stichting Skills4Comfort |
Geen |
Geen restricties |
|
L.M.A. Favié |
Ziekenhuisapotheker Erasmus MC |
Geen |
Geen |
Geen restricties |
|
J.Boerrigter-van Ginkel |
Verpleegkundig Specialist Kinderpijn, Wilhelmina Ziekenhuis Utrecht. |
Geen |
Geen |
Geen restricties |
|
S. van Rees-Florentina |
Recovery verpleegkundige Flevoziekenhuis Almere |
Bestuurslid BRV BRN Nederland
|
Geen |
Geen restricties |
|
E.C. Doganer |
Stichting Kind&Ziekenhuis Junior Projectmanager/beleidsmedewerker |
Geen |
Geen |
Geen restricties |
|
M. Jager |
Stichting Kind&Ziekenhuis Junior Projectmanager/beleidsmedewerker |
Begeleider C bij Sherpa, betaald |
Geen |
Geen restricties |
|
Klankbordgroeplid |
Functie |
Nevenfuncties |
Gemelde belangen |
Ondernomen actie |
|
L.M. Putman |
Congenitaal cardio-thoracaal chirurg, Leids Universitair Medisch Centrum & Amsterdam UMC, voltijd functie |
Geen |
Geen |
Geen restricties |
|
R. ter Riet |
Anesthesiemedewerker/Physician Assistant Anesthesiologie/Pijngeneeskunde |
Voorzitter NVAM, Voorzitter commissie (acute) pijn NVAM/V&VN |
Geen |
Geen restricties |
|
L.I.M. Meltzer |
Beatrix ziekenhuis Gorinchem, Rivas zorggroep |
Geen |
Geen |
Geen restricties |
Inbreng patiëntenperspectief
Er werd aandacht besteed aan het patiëntenperspectief door zitting van een afgevaardigde van de patiëntenvereniging (Stichting Kind & Ziekenhuis) in de werkgroep. De conceptrichtlijn is tevens voor commentaar voorgelegd aan de Patiëntenfederatie Nederland en Stichting Kind & Ziekenhuis en de eventueel aangeleverde commentaren zijn bekeken en verwerkt.
Wkkgz & Kwalitatieve raming van mogelijke substantiële financiële gevolgen
Kwalitatieve raming van mogelijke financiële gevolgen in het kader van de Wkkgz
Bij de richtlijn is conform de Wet kwaliteit, klachten en geschillen zorg (Wkkgz) een kwalitatieve raming uitgevoerd of de aanbevelingen mogelijk leiden tot substantiële financiële gevolgen. Bij het uitvoeren van deze beoordeling zijn richtlijnmodules op verschillende domeinen getoetst (zie het stroomschema op de Richtlijnendatabase).
Uit de kwalitatieve raming blijkt dat er waarschijnlijk geen substantiële financiële gevolgen zijn, zie onderstaande tabel.
|
Module |
Uitkomst raming |
Toelichting |
|
Module Gabapentine |
Geen substantiële financiële gevolgen |
Hoewel uit de toetsing volgt dat de aanbeveling(en) breed toepasbaar zijn (>40.000 patiënten), volgt ook uit de toetsing dat het overgrote deel (±90%) van de zorgaanbieders en zorgverleners al aan de norm voldoet en/of het geen nieuwe manier van zorgverlening of andere organisatie van zorgverlening betreft, het geen toename in het aantal in te zetten voltijdsequivalenten aan zorgverleners betreft en het geen wijziging in het opleidingsniveau van zorgpersoneel betreft. Er worden daarom geen substantiële financiële gevolgen verwacht. |
Werkwijze
AGREE
Deze richtlijnmodule is opgesteld conform de eisen vermeld in het rapport Medisch Specialistische Richtlijnen 2.0 van de adviescommissie Richtlijnen van de Raad Kwaliteit. Dit rapport is gebaseerd op het AGREE II instrument (Appraisal of Guidelines for Research & Evaluation II; Brouwers, 2010).
Knelpuntenanalyse en uitgangsvragen
Tijdens de voorbereidende fase inventariseerde de werkgroep de knelpunten in de zorg voor kinderen met postoperatieve pijn. De werkgroep beoordeelde de aanbevelingen uit de eerdere richtlijn Postoperatieve pijn (NVA, 2013) op noodzaak tot revisie. Het raamwerk van de richtlijn voor volwassenen is ook kritisch bekeken als uitgangspunt. Op basis van de uitkomsten van de knelpuntenanalyse zijn door de werkgroep concept-uitgangsvragen opgesteld en definitief vastgesteld.
Uitkomstmaten
Na het opstellen van de zoekvraag behorende bij de uitgangsvraag inventariseerde de werkgroep welke uitkomstmaten voor de patiënt relevant zijn, waarbij zowel naar gewenste als ongewenste effecten werd gekeken. Hierbij werd een maximum van acht uitkomstmaten gehanteerd. De werkgroep waardeerde deze uitkomstmaten volgens hun relatieve belang bij de besluitvorming rondom aanbevelingen, als cruciaal (kritiek voor de besluitvorming), belangrijk (maar niet cruciaal) en onbelangrijk. Tevens definieerde de werkgroep tenminste voor de cruciale uitkomstmaten welke verschillen zij klinisch (patiënt) relevant vonden.
Methode literatuursamenvatting
Een uitgebreide beschrijving van de strategie voor zoeken en selecteren van literatuur is te vinden onder ‘Zoeken en selecteren’ onder Onderbouwing. Indien mogelijk werd de data uit verschillende studies gepoold in een random-effects model. Review Manager 5.4 werd gebruikt voor de statistische analyses. De beoordeling van de kracht van het wetenschappelijke bewijs wordt hieronder toegelicht.
Beoordelen van de kracht van het wetenschappelijke bewijs
De kracht van het wetenschappelijke bewijs werd bepaald volgens de GRADE-methode. GRADE staat voor ‘Grading Recommendations Assessment, Development and Evaluation’ (zie http://www.gradeworkinggroup.org/). De basisprincipes van de GRADE-methodiek zijn: het benoemen en prioriteren van de klinisch (patiënt) relevante uitkomstmaten, een systematische review per uitkomstmaat, en een beoordeling van de bewijskracht per uitkomstmaat op basis van de acht GRADE-domeinen (domeinen voor downgraden: risk of bias, inconsistentie, indirectheid, imprecisie, en publicatiebias; domeinen voor upgraden: dosis-effect relatie, groot effect, en residuele plausibele confounding).
GRADE onderscheidt vier gradaties voor de kwaliteit van het wetenschappelijk bewijs: hoog, redelijk, laag en zeer laag. Deze gradaties verwijzen naar de mate van zekerheid die er bestaat over de literatuurconclusie, in het bijzonder de mate van zekerheid dat de literatuurconclusie de aanbeveling adequaat ondersteunt (Schünemann, 2013; Hultcrantz, 2017).
|
GRADE |
Definitie |
|
Hoog |
|
|
Redelijk |
|
|
Laag |
|
|
Zeer laag |
|
Bij het beoordelen (graderen) van de kracht van het wetenschappelijk bewijs in richtlijnen volgens de GRADE-methodiek spelen grenzen voor klinische besluitvorming een belangrijke rol (Hultcrantz, 2017). Dit zijn de grenzen die bij overschrijding aanleiding zouden geven tot een aanpassing van de aanbeveling. Om de grenzen voor klinische besluitvorming te bepalen moeten alle relevante uitkomstmaten en overwegingen worden meegewogen. De grenzen voor klinische besluitvorming zijn daarmee niet één op één vergelijkbaar met het minimaal klinisch relevant verschil (Minimal Clinically Important Difference, MCID). Met name in situaties waarin een interventie geen belangrijke nadelen heeft en de kosten relatief laag zijn, kan de grens voor klinische besluitvorming met betrekking tot de effectiviteit van de interventie bij een lagere waarde (dichter bij het nuleffect) liggen dan de MCID (Hultcrantz, 2017).
De beoordelingen van de literatuur en de conclusies zijn gedaan op basis van de GRADE systematiek. De werkgroep vindt het belangrijk om relevante beperkingen hiervan aan te geven.
De klinische vragen in deze richtlijn gaan veelal over een reductie van postoperatieve pijn en opioïdenconsumptie bij een individuele patiënt. Onderzoeken beschrijven de verschillen op groepsniveau, over studies met verschillende patiëntpopulaties en operaties heen. Opioïdenconsumptie is sterk afhankelijk van tijdstip, ingreep en ernst van de pijn. Door het werken met een absolute drempelwaarde in mg (i.p.v. een relatieve drempelwaarde in %) bereiken resultaten gemeten op vroege postoperatieve tijdstippen en in studies met ingrepen met relatief lage opioïdenconsumptie vaak niet de MCID. Daarbij komt ook dat de doelgroep van de huidige richtlijn enorm varieert in lengte en gewicht (van prematuur tot adolescent). Lengte en gewicht heeft grote invloed op het analgetische effect van een specifieke dosering, waardoor alleen kijken naar milligrammen niet volstaat. Waar mogelijk is ook de relatieve reductie in procenten beschreven.
De keuze van de MCID (absoluut verschil in pijnscore of opioïdenconsumptie) heeft een bepaalde mate van willekeurigheid en is niet absoluut te zien. Ook zijn de conclusies zo geformuleerd (en geven alleen beperkt antwoord op het effect op een individuele patiënt voor een specifieke ingreep). In de literatuur worden de eindpunten pijnscores en opioïdenconsumptie separaat van elkaar weer gegeven, suggererend dat deze onafhankelijk van elkaar zijn. Echter kunnen deze twee eindpunten niet onafhankelijk van elkaar beoordeeld worden; in ieder protocol is opgenomen dat pijn behandeld moet worden. Deze separate beoordeling geeft niet altijd een adequaat antwoord op de klinische vraag naar het analgetische effect van een interventie.
Daarnaast worden multimodale componenten als aparte interventies beoordeeld, echter de klinische vraag is naar de effectiviteit als bouwsteen van een multimodale werkwijze.
Voor doseringsadviezen wordt er verwezen naar betrouwbare bronnen, zoals het farmacotherapeutisch kompas of het kinderformularium.
Overwegingen (van bewijs naar aanbeveling)
Om te komen tot een aanbeveling zijn naast (de kwaliteit van) het wetenschappelijke bewijs ook andere aspecten belangrijk en worden meegewogen, zoals aanvullende argumenten uit bijvoorbeeld de biomechanica of fysiologie, waarden en voorkeuren van patiënten, kosten (middelenbeslag), aanvaardbaarheid, haalbaarheid en implementatie. Deze aspecten zijn systematisch vermeld en beoordeeld (gewogen) onder het kopje ‘Overwegingen’ en kunnen (mede) gebaseerd zijn op expert opinion. Hierbij is gebruik gemaakt van een gestructureerd format gebaseerd op het evidence-to-decision framework van de internationale GRADE Working Group (Alonso-Coello, 2016a; Alonso-Coello 2016b). Dit evidence-to-decision framework is een integraal onderdeel van de GRADE methodiek.
Formuleren van aanbevelingen
De aanbevelingen geven antwoord op de uitgangsvraag en zijn gebaseerd op het beschikbare wetenschappelijke bewijs en de belangrijkste overwegingen, en een weging van de gunstige en ongunstige effecten van de relevante interventies. De kracht van het wetenschappelijk bewijs en het gewicht dat door de werkgroep wordt toegekend aan de overwegingen, bepalen samen de sterkte van de aanbeveling. Conform de GRADE-methodiek sluit een lage bewijskracht van conclusies in de systematische literatuuranalyse een sterke aanbeveling niet a priori uit, en zijn bij een hoge bewijskracht ook zwakke aanbevelingen mogelijk (Agoritsas, 2017; Neumann, 2016). De sterkte van de aanbeveling wordt altijd bepaald door weging van alle relevante argumenten tezamen. De werkgroep heeft bij elke aanbeveling opgenomen hoe zij tot de richting en sterkte van de aanbeveling zijn gekomen.
In de GRADE-methodiek wordt onderscheid gemaakt tussen sterke en zwakke (of conditionele) aanbevelingen. De sterkte van een aanbeveling verwijst naar de mate van zekerheid dat de voordelen van de interventie opwegen tegen de nadelen (of vice versa), gezien over het hele spectrum van patiënten waarvoor de aanbeveling is bedoeld. De sterkte van een aanbeveling heeft duidelijke implicaties voor patiënten, behandelaars en beleidsmakers (zie onderstaande tabel). Een aanbeveling is geen dictaat, zelfs een sterke aanbeveling gebaseerd op bewijs van hoge kwaliteit (GRADE gradering HOOG) zal niet altijd van toepassing zijn, onder alle mogelijke omstandigheden en voor elke individuele patiënt.
|
Implicaties van sterke en zwakke aanbevelingen voor verschillende richtlijngebruikers |
||
|
|
||
|
|
Sterke aanbeveling |
Zwakke (conditionele) aanbeveling |
|
Voor patiënten |
De meeste patiënten zouden de aanbevolen interventie of aanpak kiezen en slechts een klein aantal niet. |
Een aanzienlijk deel van de patiënten zouden de aanbevolen interventie of aanpak kiezen, maar veel patiënten ook niet. |
|
Voor behandelaars |
De meeste patiënten zouden de aanbevolen interventie of aanpak moeten ontvangen. |
Er zijn meerdere geschikte interventies of aanpakken. De patiënt moet worden ondersteund bij de keuze voor de interventie of aanpak die het beste aansluit bij zijn of haar waarden en voorkeuren. |
|
Voor beleidsmakers |
De aanbevolen interventie of aanpak kan worden gezien als standaardbeleid. |
Beleidsbepaling vereist uitvoerige discussie met betrokkenheid van veel stakeholders. Er is een grotere kans op lokale beleidsverschillen. |
Organisatie van zorg
In de knelpuntenanalyse en bij de ontwikkeling van de richtlijn is expliciet aandacht geweest voor de organisatie van zorg: alle aspecten die randvoorwaardelijk zijn voor het verlenen van zorg (zoals coördinatie, communicatie, (financiële) middelen, mankracht en infrastructuur). Randvoorwaarden die relevant zijn voor het beantwoorden van deze specifieke uitgangsvraag zijn genoemd bij de overwegingen. Meer algemene, overkoepelende, of bijkomende aspecten van de organisatie van zorg worden behandeld in de module Organisatie van zorg.
Commentaar- en autorisatiefase
De conceptrichtlijn werd aan de betrokken (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd ter commentaar. De commentaren werden verzameld en besproken met de werkgroep. Naar aanleiding van de commentaren werd de conceptrichtlijn aangepast en definitief vastgesteld door de werkgroep. De definitieve richtlijn werd aan de deelnemende (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd voor autorisatie en door hen geautoriseerd dan wel geaccordeerd.
Literatuur
Agoritsas T, Merglen A, Heen AF, Kristiansen A, Neumann I, Brito JP, Brignardello-Petersen R, Alexander PE, Rind DM, Vandvik PO, Guyatt GH. UpToDate adherence to GRADE criteria for strong recommendations: an analytical survey. BMJ Open. 2017 Nov 16;7(11):e018593. doi: 10.1136/bmjopen-2017-018593. PubMed PMID: 29150475; PubMed Central PMCID: PMC5701989.
Alonso-Coello P, Schünemann HJ, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Rada G, Rosenbaum S, Morelli A, Guyatt GH, Oxman AD; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ. 2016 Jun 28;353:i2016. doi: 10.1136/bmj.i2016. PubMed PMID: 27353417.
Alonso-Coello P, Oxman AD, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Vandvik PO, Meerpohl J, Guyatt GH, Schünemann HJ; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 2: Clinical practice guidelines. BMJ. 2016 Jun 30;353:i2089. doi: 10.1136/bmj.i2089. PubMed PMID: 27365494.
Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, Fervers B, Graham ID, Grimshaw J, Hanna SE, Littlejohns P, Makarski J, Zitzelsberger L; AGREE Next Steps Consortium. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010 Dec 14;182(18):E839-42. doi: 10.1503/cmaj.090449. Epub 2010 Jul 5. Review. PubMed PMID: 20603348; PubMed Central PMCID: PMC3001530.
Hultcrantz M, Rind D, Akl EA, Treweek S, Mustafa RA, Iorio A, Alper BS, Meerpohl JJ, Murad MH, Ansari MT, Katikireddi SV, Östlund P, Tranæus S, Christensen R, Gartlehner G, Brozek J, Izcovich A, Schünemann H, Guyatt G. The GRADE Working Group clarifies the construct of certainty of evidence. J Clin Epidemiol. 2017 Jul;87:4-13. doi: 10.1016/j.jclinepi.2017.05.006. Epub 2017 May 18. PubMed PMID: 28529184; PubMed Central PMCID: PMC6542664.
Medisch Specialistische Richtlijnen 2.0 (2012). Adviescommissie Richtlijnen van de Raad Kwalitieit. http://richtlijnendatabase.nl/over_deze_site/over_richtlijnontwikkeling.html
Neumann I, Santesso N, Akl EA, Rind DM, Vandvik PO, Alonso-Coello P, Agoritsas T, Mustafa RA, Alexander PE, Schünemann H, Guyatt GH. A guide for health professionals to interpret and use recommendations in guidelines developed with the GRADE approach. J Clin Epidemiol. 2016 Apr;72:45-55. doi: 10.1016/j.jclinepi.2015.11.017. Epub 2016 Jan 6. Review. PubMed PMID: 26772609.
Schünemann H, Brożek J, Guyatt G, et al. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group, 2013. Available from http://gdt.guidelinedevelopment.org/central_prod/_design/client/handbook/handbook.html.
Zoekverantwoording
Literature search strategy
|
Database(s): Embase, Medline (Ovid) |
Datum: 10-12-2022 |
|
Periode: 2000- heden |
Talen: nvt |
Zoekopbrengst
|
|
EMBASE |
OVID/Medline |
Ontdubbeld |
|
SRs |
113 |
16 |
120 |
|
RCTs |
141 |
48 |
113 |
|
OBS |
552 |
50 |
422 |
|
Totaal |
806 |
114 |
655 |
Zoekstrategie
Embase
|
No. |
Query |
Results |
|
#13 |
#5 AND #10 |
552 |
|
#12 |
#5 AND #7 |
141 |
|
#11 |
#5 AND #6 |
113 |
|
#10 |
#8 OR #9 |
15531480 |
|
#9 |
'case control study'/de OR 'comparative study'/exp OR 'control group'/de OR 'controlled study'/de OR 'controlled clinical trial'/de OR 'crossover procedure'/de OR 'double blind procedure'/de OR 'phase 2 clinical trial'/de OR 'phase 3 clinical trial'/de OR 'phase 4 clinical trial'/de OR 'pretest posttest design'/de OR 'pretest posttest control group design'/de OR 'quasi experimental study'/de OR 'single blind procedure'/de OR 'triple blind procedure'/de OR (((control OR controlled) NEAR/6 trial):ti,ab,kw) OR (((control OR controlled) NEAR/6 (study OR studies)):ti,ab,kw) OR (((control OR controlled) NEAR/1 active):ti,ab,kw) OR 'open label*':ti,ab,kw OR (((double OR two OR three OR multi OR trial) NEAR/1 (arm OR arms)):ti,ab,kw) OR ((allocat* NEAR/10 (arm OR arms)):ti,ab,kw) OR placebo*:ti,ab,kw OR 'sham-control*':ti,ab,kw OR (((single OR double OR triple OR assessor) NEAR/1 (blind* OR masked)):ti,ab,kw) OR nonrandom*:ti,ab,kw OR 'non-random*':ti,ab,kw OR 'quasi-experiment*':ti,ab,kw OR crossover:ti,ab,kw OR 'cross over':ti,ab,kw OR 'parallel group*':ti,ab,kw OR 'factorial trial':ti,ab,kw OR ((phase NEAR/5 (study OR trial)):ti,ab,kw) OR ((case* NEAR/6 (matched OR control*)):ti,ab,kw) OR ((match* NEAR/6 (pair OR pairs OR cohort* OR control* OR group* OR healthy OR age OR sex OR gender OR patient* OR subject* OR participant*)):ti,ab,kw) OR ((propensity NEAR/6 (scor* OR match*)):ti,ab,kw) OR versus:ti OR vs:ti OR compar*:ti OR ((compar* NEAR/1 study):ti,ab,kw) OR (('major clinical study'/de OR 'clinical study'/de OR 'cohort analysis'/de OR 'observational study'/de OR 'cross-sectional study'/de OR 'multicenter study'/de OR 'correlational study'/de OR 'follow up'/de OR cohort*:ti,ab,kw OR 'follow up':ti,ab,kw OR followup:ti,ab,kw OR longitudinal*:ti,ab,kw OR prospective*:ti,ab,kw OR retrospective*:ti,ab,kw OR observational*:ti,ab,kw OR 'cross sectional*':ti,ab,kw OR cross?ectional*:ti,ab,kw OR multicent*:ti,ab,kw OR 'multi-cent*':ti,ab,kw OR consecutive*:ti,ab,kw) AND (group:ti,ab,kw OR groups:ti,ab,kw OR subgroup*:ti,ab,kw OR versus:ti,ab,kw OR vs:ti,ab,kw OR compar*:ti,ab,kw OR 'odds ratio*':ab OR 'relative odds':ab OR 'risk ratio*':ab OR 'relative risk*':ab OR 'rate ratio':ab OR aor:ab OR arr:ab OR rrr:ab OR ((('or' OR 'rr') NEAR/6 ci):ab))) |
13679059 |
|
#8 |
'major clinical study'/de OR 'clinical study'/de OR 'case control study'/de OR 'family study'/de OR 'longitudinal study'/de OR 'retrospective study'/de OR 'prospective study'/de OR 'cohort analysis'/de OR cohort*:ab,ti OR (('case control' NEAR/1 (study OR studies)):ab,ti) OR (('follow up' NEAR/1 (study OR studies)):ab,ti) OR (observational NEAR/1 (study OR studies)) OR ((epidemiologic NEAR/1 (study OR studies)):ab,ti) OR (('cross sectional' NEAR/1 (study OR studies)):ab,ti) |
6908482 |
|
#7 |
'randomized controlled trial'/exp OR random*:ti,ab OR (((pragmatic OR practical) NEAR/1 'clinical trial*'):ti,ab) OR ((('non inferiority' OR noninferiority OR superiority OR equivalence) NEAR/3 trial*):ti,ab) OR rct:ti,ab,kw |
1992000 |
|
#6 |
'meta analysis'/exp OR 'meta analysis (topic)'/exp OR metaanaly*:ti,ab OR 'meta analy*':ti,ab OR metanaly*:ti,ab OR 'systematic review'/de OR 'cochrane database of systematic reviews'/jt OR prisma:ti,ab OR prospero:ti,ab OR (((systemati* OR scoping OR umbrella OR 'structured literature') NEAR/3 (review* OR overview*)):ti,ab) OR ((systemic* NEAR/1 review*):ti,ab) OR (((systemati* OR literature OR database* OR 'data base*') NEAR/10 search*):ti,ab) OR (((structured OR comprehensive* OR systemic*) NEAR/3 search*):ti,ab) OR (((literature NEAR/3 review*):ti,ab) AND (search*:ti,ab OR database*:ti,ab OR 'data base*':ti,ab)) OR (('data extraction':ti,ab OR 'data source*':ti,ab) AND 'study selection':ti,ab) OR ('search strategy':ti,ab AND 'selection criteria':ti,ab) OR ('data source*':ti,ab AND 'data synthesis':ti,ab) OR medline:ab OR pubmed:ab OR embase:ab OR cochrane:ab OR (((critical OR rapid) NEAR/2 (review* OR overview* OR synthes*)):ti) OR ((((critical* OR rapid*) NEAR/3 (review* OR overview* OR synthes*)):ab) AND (search*:ab OR database*:ab OR 'data base*':ab)) OR metasynthes*:ti,ab OR 'meta synthes*':ti,ab |
883358 |
|
#5 |
#4 AND [1-1-2000]/sd NOT ('conference abstract'/it OR 'editorial'/it OR 'letter'/it OR 'note'/it) NOT (('animal experiment'/exp OR 'animal model'/exp OR 'nonhuman'/exp) NOT 'human'/exp) |
1314 |
|
#4 |
#1 AND #2 AND #3 |
1597 |
|
#3 |
'adolescent'/exp OR 'baby'/exp OR 'boy'/exp OR 'child'/exp OR 'minors'/exp/mj OR 'pediatric patient'/exp OR 'pediatrics'/exp OR 'schoolchild'/exp OR infan*:ti,ab OR newborn*:ti,ab OR 'new born*':ti,ab OR perinat*:ti,ab OR neonat*:ti,ab OR baby*:ti,ab OR babies:ti,ab OR toddler*:ti,ab OR minors*:ti,ab OR boy:ti,ab OR boys:ti,ab OR boyfriend:ti,ab OR boyhood:ti,ab OR girl*:ti,ab OR kid:ti,ab OR kids:ti,ab OR child*:ti,ab OR children*:ti,ab OR schoolchild*:ti,ab OR adolescen*:ti,ab OR juvenil*:ti,ab OR youth*:ti,ab OR teen*:ti,ab OR pubescen*:ti,ab OR pediatric*:ti,ab OR paediatric*:ti,ab OR peadiatric*:ti,ab OR school:ti,ab OR school*:ti,ab OR prematur*:ti,ab OR preterm*:ti,ab |
5573175 |
|
#2 |
'surgery'/exp OR 'surgical patient'/exp OR 'surgical risk'/exp OR 'perioperative period'/exp OR surgic*:ti,ab,kw OR surger*:ti,ab,kw OR operation*:ti,ab,kw OR operative:ti,ab,kw OR presurg*:ti,ab,kw OR preoperati*:ti,ab,kw OR 'pre-surg*':ti,ab,kw OR 'pre-operati*':ti,ab,kw OR perisurg*:ti,ab,kw OR perioperati*:ti,ab,kw OR 'peri-surg*':ti,ab,kw OR 'peri-operati*':ti,ab,kw OR postsurg*:ti,ab,kw OR postoperati*:ti,ab,kw OR 'post-surg*':ti,ab,kw OR 'post-operati*':ti,ab,kw OR laparoscop*:ti,ab,kw |
7085934 |
|
#1 |
'gabapentin'/exp OR '1 (aminomethyl) cyclohexaneacetic acid':ti,ab,kw OR '2 [1 (aminomethyl) cyclohexyl] acetic acid':ti,ab,kw OR 'ci 945':ti,ab,kw OR 'ci945':ti,ab,kw OR 'dineurin':ti,ab,kw OR 'dm 1796':ti,ab,kw OR 'dm 5689':ti,ab,kw OR 'dm1796':ti,ab,kw OR 'dm5689':ti,ab,kw OR 'gabalept':ti,ab,kw OR 'gabaliquid geriasan':ti,ab,kw OR 'gabapen':ti,ab,kw OR 'gabapentin':ti,ab,kw OR 'gabatin':ti,ab,kw OR 'gantin':ti,ab,kw OR 'go 3450':ti,ab,kw OR 'go3450':ti,ab,kw OR 'goe 3450':ti,ab,kw OR 'goe3450':ti,ab,kw OR 'gralise':ti,ab,kw OR 'kaptin':ti,ab,kw OR 'keneil':ti,ab,kw OR 'neurontin':ti,ab,kw OR 'neurotonin':ti,ab,kw OR 'nupentin':ti,ab,kw OR 'sefelsa':ti,ab,kw OR 'serada':ti,ab,kw OR pregabalin:ti,ab,kw |
41928 |
Ovid/Medline
|
# |
Searches |
Results |
|
12 |
6 and 9 |
50 |
|
11 |
6 and 8 |
48 |
|
10 |
6 and 7 |
16 |
|
9 |
Epidemiologic studies/ or case control studies/ or exp cohort studies/ or Controlled Before-After Studies/ or Case control.tw. or cohort*.tw. or Cohort analy$.tw. or (Follow up adj (study or studies)).tw. or (observational adj (study or studies)).tw. or Longitudinal.tw. or Retrospective*.tw. or prospective*.tw. or consecutive*.tw. or Cross sectional.tw. or Cross-sectional studies/ or historically controlled study/ or interrupted time series analysis/ |
4349055 |
|
8 |
exp clinical trial/ or randomized controlled trial/ or exp clinical trials as topic/ or randomized controlled trials as topic/ or Random Allocation/ or Double-Blind Method/ or Single-Blind Method/ or (clinical trial, phase i or clinical trial, phase ii or clinical trial, phase iii or clinical trial, phase iv or controlled clinical trial or randomized controlled trial or multicenter study or clinical trial).pt. or random*.ti,ab. or (clinic* adj trial*).tw. or ((singl* or doubl* or treb* or tripl*) adj (blind$3 or mask$3)).tw. or Placebos/ or placebo*.tw. |
2528311 |
|
7 |
meta-analysis/ or meta-analysis as topic/ or (metaanaly* or meta-analy* or metanaly*).ti,ab,kf. or systematic review/ or cochrane.jw. or (prisma or prospero).ti,ab,kf. or ((systemati* or scoping or umbrella or "structured literature") adj3 (review* or overview*)).ti,ab,kf. or (systemic* adj1 review*).ti,ab,kf. or ((systemati* or literature or database* or data-base*) adj10 search*).ti,ab,kf. or ((structured or comprehensive* or systemic*) adj3 search*).ti,ab,kf. or ((literature adj3 review*) and (search* or database* or data-base*)).ti,ab,kf. or (("data extraction" or "data source*") and "study selection").ti,ab,kf. or ("search strategy" and "selection criteria").ti,ab,kf. or ("data source*" and "data synthesis").ti,ab,kf. or (medline or pubmed or embase or cochrane).ab. or ((critical or rapid) adj2 (review* or overview* or synthes*)).ti. or (((critical* or rapid*) adj3 (review* or overview* or synthes*)) and (search* or database* or data-base*)).ab. or (metasynthes* or meta-synthes*).ti,ab,kf. |
634619 |
|
6 |
limit 5 to yr="2000-Current" |
140 |
|
5 |
4 not ((exp animals/ or exp models, animal/) not humans/) not (letter/ or comment/ or editorial/) |
149 |
|
4 |
1 and 2 and 3 |
154 |
|
3 |
(child* or schoolchild* or infan* or adolescen* or pediatri* or paediatr* or neonat* or boy or boys or boyhood or girl or girls or girlhood or youth or youths or baby or babies or toddler* or childhood or teen or teens or teenager* or newborn* or postneonat* or postnat* or puberty or preschool* or suckling* or picu or nicu or juvenile?).tw. |
2828088 |
|
2 |
exp Surgical Procedures, Operative/ or exp Specialties, Surgical/ or exp Perioperative Period/ or surgic*.ti,ab,kf. or surger*.ti,ab,kf. or operation*.ti,ab,kf. or operative.ti,ab,kf. or presurg*.ti,ab,kf. or preoperati*.ti,ab,kf. or pre-surg*.ti,ab,kf. or pre-operati*.ti,ab,kf. or perisurg*.ti,ab,kf. or perioperati*.ti,ab,kf. or peri-surg*.ti,ab,kf. or peri-operati*.ti,ab,kf. or postsurg*.ti,ab,kf. or postoperati*.ti,ab,kf. or post-surg*.ti,ab,kf. or post-operati*.ti,ab,kf. or laparoscop*.ti,ab,kf. |
5118910 |
|
1 |
exp Gabapentin/ or gabapentin*.ti,ab,kf. or neurontin.ti,ab,kf. or neurotonin.ti,ab,kf. or nupentin.ti,ab,kf. or sefelsa.ti,ab,kf. or serada.ti,ab,kf. or pregabalin.ti,ab,kf. or dineurin.ti,ab,kf. or 'cyclohexaneacetic acid'.ti,ab,kf. or 'cyclohexyl acetic acid'.ti,ab,kf. or 'ci 945'.ti,ab,kf. or 'ci945'.ti,ab,kf. or 'dm 1796'.ti,ab,kf. or 'dm 5689'.ti,ab,kf. or 'dm1796'.ti,ab,kf. or 'dm5689'.ti,ab,kf. or 'gabalept'.ti,ab,kf. or 'gabaliquid geriasan'.ti,ab,kf. or 'gabapen'.ti,ab,kf. or 'gabapentin*'.ti,ab,kf. or 'gabatin*'.ti,ab,kf. or 'gantin'.ti,ab,kf. or 'go 3450'.ti,ab,kf. or 'go3450'.ti,ab,kf. or 'goe 3450'.ti,ab,kf. or 'goe3450'.ti,ab,kf. or 'gralise'.ti,ab,kf. or 'kaptin'.ti,ab,kf. or 'keneil'.ti,ab,kf. |
11412 |