Clonidine als additivum aan lokaal anestheticum
Uitgangsvraag
Wat is de plaats van alfa-2-agonist clonidine als additivum aan lokaal anestheticum aan het locoregionaal of neuraxiaal blok bij kinderen die een chirurgische ingreep ondergaan?
Voor clonidine intraveneus/oraal, zie module Clonidine.
Aanbeveling
Overweeg clonidine toe te voegen als adjuvans aan een lokaal anestheticum voor een perifere of neuraxiale zenuwblokkade, wanneer een verlengend analgetisch effect gewenst is.
Overwegingen
Voor- en nadelen van de interventie en de kwaliteit van het bewijs
Er is literatuuronderzoek verricht naar de gunstige en ongunstige effecten van de toevoeging van de alfa-2-agonist clonidine aan het locoregionaal blok bij kinderen die een chirurgische ingreep ondergaan. Er zijn 28 RCT’s geïncludeerd in de literatuursamenvatting. De operaties die de kinderen ondergingen waren divers. In de meeste studies werd clonidine als additivum aan een lokaal anestheticum bij een caudaal blok onderzocht, in enkele studies betrof het een perifere zenuwblokkade, zoals dorsaal penis blok of buikwandblok.
De studies waren doorgaans klein en hadden methodologische beperkingen. Hierdoor was er risico op vertekening van resultaten (risk of bias) en werd de optimale populatiegrootte (OIS) niet behaald door kleine aantallen. Daarom is de bewijskracht voor alle uitkomstmaten in meer of mindere mate afgewaardeerd. Bovendien werden in de geïncludeerde studies verschillende meet- en rapportage methoden gebruikt (bijvoorbeeld verschillende pijn meetinstrumenten zoals Children’s Hospital of Eastern Ontario Pain Scale (CHEOPS) en de
Children and Infants Postoperative Pain Scale (CHIPPS); en verschillen in rapportages zoals mediaan of gemiddelden) of waren de resultaten gelimiteerd beschreven in de studies, waardoor het bij de meeste uitkomstmaten niet mogelijk was om de resultaten te poolen. Derhalve zijn de resultaten vaak per studie beschreven.
Postoperatieve pijn op 0, 6 en 24 uur was gedefinieerd als cruciale uitkomstmaat. Voor postoperatieve pijn was de bewijskracht laag. De totale bewijskracht komt daarmee uit op laag. Alleen op 6 uur na de operatie werd een mogelijk pijn verlagend effect gevonden in het voordeel van de toevoeging van clonidine; op 0 uur en 24 uur na was een geen verschil in effect.
Angst, postoperatief gebruik van opioïden, en adverse events (apneu, ademhalingsdepressie, hypotensie, sedatie, en delirium) waren gedefinieerd als belangrijke uitkomstmaten.
Voor adverse events apneu en delier werd geen bewijs gevonden. Voor de uitkomstmaten hypotensie en sedatie is de bewijskracht literatuur laag en toont het bewijs dat er mogelijk geen verschil is in effect tussen de onderzochte groepen.
Samenvattend kan de literatuur onvoldoende richting geven aan de besluitvorming. De aanbeveling is daarom gebaseerd op aanvullende argumenten waaronder expert opinie, waar mogelijk aangevuld met (indirecte) literatuur.
Clonidine heeft mogelijk een positief effect op postoperatieve pijn na 6 uur wanneer dit wordt toegevoegd aan lokaal anesthetica. Er is zijn beperkte resultaten op latere tijdspunten, dus over de duur van het mogelijke positieve effect is geen uitspraak te doen. Er zijn geen nadelige effecten van het toedienen van clonidine als adjuvans naar voren gekomen in deze literatuurstudie. Het toevoegen van clonidine kan dan ook veilig gebruikt worden als adjuvans en is te overwegen.
Waarden en voorkeuren van patiënten (en evt. hun verzorgers)
Voor de ouders en de patiënt is goede pijnbestrijding met zo min mogelijke bijwerkingen belangrijk. Van de patiënt of ouders van de patiënt kan men niet verwachten dat deze zoveel kennis van medicatie heeft om de keuze voor toevoeging van clonidine aan het locoregionaal blok kan maken te bespreken. Deze keuze dient te worden gemaakt door de behandelend zorgverlener, op basis van zijn/haar kennis en ervaring. De patiënt en ouders mogen verwachten van de behandelend arts dat deze de kennis heeft van de medicatie om een keuze van medicament te maken.
Kosten (middelenbeslag)
Clonidine is een goedkoop middel en zal bij implementatie niet tot substantieel hogere kosten leiden. Ten opzichte van andere alfa-2-agonisten, zoals dexmedetomidine, is het een goedkoper medicament.
Aanvaardbaarheid, haalbaarheid en implementatie
De werkgroep is van mening dat implementatie er geen bezwaren zijn op het gebied van aanvaardbaarheid of haalbaarheid. Clonidine behoort tot de standaard anesthesiemiddelen en is daardoor breed beschikbaar. Bij de keuze voor dit middel dienen uiteraard de contra-indicaties van clonidine in acht genomen te worden.
Rationale van de aanbeveling: weging van argumenten voor en tegen de interventies
Er zijn aanwijzingen dat toevoegen van clonidine mogelijk een positief additief effect heeft op de analgetisch effect van lokaal anesthetica bij perifere en neuraxiale zenuwblokkades. Het toevoegen van clonidine aan een lokaal anestheticum lijkt niet te leiden tot een toename van adverse events. De medicatie brengt minimale kosten met zich mee en minimale bezwaren ten aanzien van implementatie. Dit alles in acht nemend is het toevoegen van clonidine aan lokaal anesthetica te overwegen.
Het Kinderformularium heeft een doseringsadvies beschreven voor clonidine als adjuvans bij andere analgetica (Clonidine, Kinderformularium).
Onderbouwing
Achtergrond
Regelmatig worden er zowel neuraxiale blokken (caudaal of epiduraal) geplaatst bij kinderen als perifere zenuwblokkades. Bij beide groepen kan het gaan om een singleshot techniek of met een katheter voor continue pijnstilling. Er wordt dan gebruik gemaakt van een lokaal anestheticum waarbij soms een additivum gebruikt wordt. Clonidine, een alfa-2-agonist kan toegevoegd worden als additivum. Het doel van een additivum is de duur van het blok verlengen en het gebruik van opioïden te verlagen en mogelijk angst te verminderen.
Conclusies / Summary of Findings
Postoperative pain
|
Low GRADE |
Clonidine as adjuvant to a local anesthetic may result in little to no difference in postoperative pain at PACU arrival/ 0 hours when compared with a local anesthetic alone in children undergoing a surgical procedure.
Source: Anouar 2016; Bhati 2022; El-Hennway 2009; Joshi 2004; Koul 2009; Mostafa 2021; Potti 2017; Tripi 2005; Visoiu 2021 |
|
Low GRADE |
Clonidine as adjuvant to a local anesthetic may result in reduced postoperative pain at 6 hours when compared with a local anesthetic alone in children undergoing a surgical procedure.
Source: Anouar 2016; Bhati 2022; Jindal 2011; Narasimhamurthy 2016; Parameswari 2010; Potti 2017; Visoiu 2021 |
|
Low GRADE |
Clonidine as adjuvant to a local anesthetic may result in little to no difference in postoperative pain at 24 hours when compared with a local anesthetic alone in children undergoing a surgical procedure.
Source: Anouar 2016; Bhati 2022; El-Hennway 2009; Joshi 2004; Mostafa 2021; Narasimhamurthy 2016; Potti 2017; Visoiu 2021 |
Anxiety
|
Very low GRADE |
The evidence is very uncertain about the effect of clonidine as adjuvant to a local anesthetic on anxiety when compared with local anesthetic alone in children undergoing a surgical procedure.
Source: Visoiu, 2021 |
Postoperative opioid consumption
|
Very low GRADE |
The evidence is very uncertain about the effect of clonidine as adjuvant to a local anesthetic on postoperative opioid consumption in PACU when compared with local anesthetic alone in children undergoing a surgical procedure.
Source: Anouar 2016; Joshi 2004; Tripi 2005 |
|
Very low GRADE |
The evidence is very uncertain about the effect of clonidine as adjuvant to a local anesthetic on postoperative opioid consumption in 24 hours when compared with local anesthetic alone in children undergoing a surgical procedure.
Source: Joshi 2004; Tripi 2005 |
|
Very low GRADE |
The evidence is very uncertain about the effect of clonidine as adjuvant to a local anesthetic on total postoperative opioid consumption when compared with local anesthetic alone in children undergoing a surgical procedure.
Source: Akin 2010; De Negri 2001; Ivani 2000; Kalachi 2007; Tripi 2005 |
Adverse events
|
No GRADE |
No evidence was found regarding the effect of clonidine as adjuvant to a local anesthetic compared with local anesthetic alone on adverse events apnea and postoperative emergence delirium in children undergoing abdominal surgery.
Source: - |
|
Low GRADE |
Clonidine as adjuvant to a local anesthetic may result in little to no difference in adverse events respiratory depression and hypotension when compared with a local anesthetic alone in children undergoing a surgical procedure.
Sources respiratory depression: Akbas 2005; Akin 2010; Bajwa 2010; Bhati 2022; El-Hennway 2009; Koul 2009; Narasimhamurthy 2016; Parameswari 2010; Potti 2017; Sanwatsarka 2017; Shaikh 2015; Singh 2012; Tripi 2005
Sources hypotension: Akbas 2005; Akin 2010; El-Hennway 2009; Parameswari 2010; Potti 2017; Sanwatsarka 2017; Shaikh 2015; Singh 2012; Tripi 2005 |
|
Low GRADE |
Clonidine as adjuvant to a local anesthetic may result in little to no difference in sedation when compared with a local anesthetic alone in children undergoing a surgical procedure.
Source: Bawja 2010; Ivani 2000; Jindal 2011; Koul 2009; Potti 2017; Mostafa 2021; Narasimhamurthy 2016; Sanwatsarka 2017; Tripi 2005; Visoiu 2021 |
Samenvatting literatuur
Description of studies
A description with study characteristics of each RCT is presented in Table 1. As shown in the table, studies were conducted in patients undergoing various surgeries. Number of patients per arm varied from 15 to 50. The mean age of children in the studies varies from 3 years to almost 14 years. The percentage of males was often not specified, but for the studies that did report the sex of the patients, the male percentage was high.
Table 1: Descriptives of included studies.
|
Author, year
|
Population (I/C), mean age; sex (M/F) |
Surgical procedure |
Intervention |
Control |
|
Akbas, 2005
Turkey |
N: 25/ 25 Age (years) ± SD: 6.08 ± 2.87/ 5.64 ± 3.13 M:F: not reported |
Inguinal hernia repair and circumcision |
ropivacaine 0.2% 0.75 ml/kg) plus clonidine 1 µg/kg) (group RC)* caudal block |
received ropivacaine 0.2%, 0.75 ml/kg) (group R)*
*Drugs were diluted in 0.9% saline (0.75 ml/kg ) |
|
Akin, 2010
Turkey |
N (I1/I2/C): 20/ 20/ 20 Age (years) ± SD: 4.1 ± 2/ 4.1 ± 2/ 4.1 ± 2 M:F: not reported |
Inguinal hernia repair or orchidopexy surgery |
I1: Levobupivacaine 0.25% 0.75 ml/kg) and 2 µg/kg) clonidine caudally and 5 ml normal saline i.v. (Group L-Ccau)
I2: Levobupivacaine 0.25%, 0.75 ml/kg) and 2 µg/kg) clonidine in 5 ml normal saline i.v. (Group L-Civ) |
Levobupivacaine 0.25% 0.75 ml/kg) and 5 ml normal saline i.v (group L) |
|
Anouar, 2016
Tunisia |
N: 20/ 20 Age (months) ± SD: 30 ±3.12/ 25.2 ± 5 M:F: 20:0/20:0 |
Day-case male circumcision |
ml/Kg of bupivacaine 0.5% with 1 µg/kg of clonidine in each side dorsal penile nerve block |
0.1 ml/kg of bupivacaine 0.5% with placebo in each side |
|
Bajwa, 2010
India |
N: 22/ 22 Age (years) ± SD: 3.4 ± 1.42 / 3.1 ± 1.68 M:F: not reported |
Elective lower abdominal surgery (hernia surgery)
|
0.25% ropivacaine, 0.5 ml/kg, with an addition of 2 µg/kg clonidine via the caudal route* |
0.25% ropivacaine, 0.5 ml/kg*
*with a total volume being constant at 0.5 ml/kg in both the study groups |
|
Bhati 2022
India |
N: 20/ 20 Age (years) ± SD: 3.85 ± 1.39/ 3.40 ± 1.31 M:F: 20:0/20:0 |
Infraumbilical elective surgery |
caudal injection of 0.25% levobupivacaine at a dose of 1 mL/kg body weight with clonidine 0.5 µg/kg |
caudal injection of 0.25% levobupivacaine at a dose of 1 mL/kg body weight |
|
De Mey, 2000
Belgium |
N: 30/ 30 Age (months) ± SD: 39.1 ± 29.4/ 38.3 ± 32.2 M:F: 30:0/30:0 |
Hypospadias repair |
0.5 mL kg−1 bupivacaine 0.25% caudally + 1 μg kg−1 clonidine (group II) |
0.5 mL kg−1 bupivacaine 0.25% caudally (group I) |
|
De Negri, 2001
Italy |
N (I1/I2/I3/C): 15/ 15/ 15/ 15 Age (months) ± SD: 31 ±10/ 28±14/ 32 ±9/ 28 ± 12 M:F: 15:0/ 15:0/ 15:0/ 15:0 |
Hypospadias repair
|
I1: ropivacaine 0.08% 0.16 mg/kg/h plus clonidine 0.04 mg/kg/h (Group RC1)
I2: ropivacaine 0.08% 0.16 mg/kg/h plus clonidine 0.08 mg/kg/h (Group RC2)
I3: ropivacaine 0.08% 0.16 mg/kg/h plus clonidine 0.12 mg/kg/h (Group RC3) Epidural |
plain ropivacaine 0.1% 0.2 mg/kg/h (Group R) |
|
El-Hennway, 2009
Egypt |
N: 30/ 30 Age (months) (range): 45 (6–69)/ 43 (7–66) M:F: not reported |
Lower abdominal surgeries |
bupivacaine 0.25% (1 ml/ kg) with clonidine 2 mg/kg in normal saline 1 ml caudal block |
bupivacaine 0.25% (1 ml/kg) with normal saline 1 ml |
|
Ivani, 2000
Italy |
N: 20/ 20 Median age (years) (range): 3 (1–7)/ 3 (1–7) M:F: 16:4/ 18:2 |
Elective sub-umbilical surgery |
caudal block with 1 ml/kg of ropivacaine 0.1% with the addition of clonidine 2 mg/kg
|
caudal block with 1 ml/kg of ropivacaine 0.2%
|
|
Jindal, 2011
India |
N: 25/ 25 Age (months) ± SD: 8.48 ± 7.11/ 7.92 ± 6.48 M:F: 17:8/19:6 |
Elective cleft lip repair |
clonidine from a 150 μg/ml ampoule was diluted and 1 μg/kg was drawn with a tuberculin syringe and added to 0.5 ml of 0.5% bupivacaine; the resulting mixture was reconstituted with saline to a volume of 1 ml to maintain a bupivacaine concentration of 0.25% |
solution of 1 ml 0.25% bupivacaine solution was prepared by adding 0.5 ml saline to 0.5 ml of 0.5% bupivacaine |
|
Joshi, 2004
USA |
N: 18/ 17 Age (months) ± SD: 28 ± 22 / 44 ± 31 M:F: 3:15/ 2:15 |
Unilateral hernia, hydrocelectomy, or orchidopexy |
2 µg/kg) of clonidine (100 µg/ml) in addition to the bupivacaine. Caudal block |
equal volume of preservative-free normal saline |
|
Koul, 2009
India |
N: 20/ 20 Age (years) ± SD: 3.45 ± 2.06/ 3.28 ± 1.65 M:F: not reported |
Inguinalherniotomy |
Caudal epidural injection of 0.75ml/kg of 0.25% bupivacaine with 2 μg/kg of clonidine
|
Caudal epidural injection of 0.75 ml/kg of 0.25% bupivacaine |
|
Laha, 2012
India |
N: 15/ 15 Age (years) ± SD: 4.8 (±2.6)/ 4.7 (±2.6) M:F: 11:4/11:4 |
Lower abdominal, perineal, and lower limb surgeries
|
caudal injection of mixture of ropivacaine 0.2% (1 ml/kg) with clonidine 2 μg/kg |
caudal injection of plain ropivacaine 0.2% (1 ml/kg) |
|
Mostafa, 2021
Egypt |
N: 30/ 30 Age (years) ± SD: 5.68 ± 1.7/ 5.37 ± 1.6 M:F: not reported |
Laparoscopic orchiopexy |
levobupivacaine plus clonidine transversus abdominis plane block |
levobupivacaine plus normal saline
|
|
Narasimhamurthy, 2016
India |
N: 30/ 30 Age (years) ± SD: 4.8 + 1.7/ 4.7 + 1.7 M:F: 25:5/ 26:4 |
Infraumbilical surgeries (circumcision, herniotomy and orchidopexy) |
mixture of 0.2% Ropivacaine and preservative free Clonidine 1 μg/kg. caudal block |
mixture of 0.2% Ropivacaine and normal saline |
|
Parameswari, 2010
India |
N: 50/ 50 Age (months): 19/ 21 M:F: 47:3/ 47:3
|
Subumbilical surgeries under general anesthesia (circumcision; orchidopexy; herniotomy) |
Bupivacaine with 1 μg/kg clonidine caudal block |
Plain bupivacaine |
|
Potti, 2017
India |
N (I1/I2/C): 25/ 25/ 25 Age (years) ± SD: 4.7 ± 2/ 4.6 ± 1/ 4.32 ± 2 M:F: not reported |
Elective infraumbilicial surgeries |
I1: Group B (n = 25) received levobupivacaine 0.25% 1 mL/kg with 1 µg/kg clonidine caudally and 5 mL of normal saline i.v
I2: Group C (n = 25) received levobupivacaine 0.25% 1 mL/kg caudally and 1 µg/kg clonidine in 5 mL normal saline i.v |
Group A (n = 25) received levobupivacaine 0.25% 1 mL/kg caudally and 5 mL of normal saline i.v
|
|
Priolkar, 2016
India |
N: 30/ 30 Age (years) ± SD: 4.43+1.52/ 4.63+1.73 M:F: not reported
|
Infraumbilical operations |
Group: BC: Mixture of 1 ml/kg of 0.125% bupivacaine with preservative free clonidine 1 μg/kg. Caudal block |
Group: B: 1 ml/kg of 0.125% bupivacaine solution |
|
Rawat, 2019
India |
N: 32/ 32 Age (years) ± SD: 4.14 ± 1.05/ 4.16 ± 1.87 M:F: 15:7/14:8 |
Elective perineal surgeries |
Group III – 0.25% levobupivacaine (1 mL/kg) with clonidine 1 µg/kg Caudal block |
Group I - 0.25% levobupivacaine (1 mL/kg) |
|
Sanwatsarkar, 2017
India
|
N: 25/ 25 Age (years) ± SD: 6.28 ± 1.21/ 6.64 ± 1.29 M:F: 23:2/22:3 |
Umbilical surgeries under general anesthesia |
Group BC received 1 ml/kg 0.25% bupivacaine + 1 μg/kg clonidine in normal saline Caudal block |
Group B received 1 ml/ kg 0.25% bupivacaine in normal saline |
|
Shaikh, 2015
India |
N: 30/ 30 Age (months) ± SD: 58.30 ± 27.92/ 74.40 ± 37.34 M:F: 27:3/ 28:2
|
Elective sub-umbilical, perineal and lower limb surgeries |
Group B received caudal 0.25% bupivacaine 1 ml/Kg with clonidine 1 μg/Kg as an adjuvant made to 0.5 ml with normal saline |
Group A received caudal 0.25% bupivacaine plain with 0.5 ml Normal saline |
|
Singh, 2012
Nepal |
N: 20/ 20 Age (years) ± SD: 5.45±2.5 / 6.10 ± 2.19 M:F: not reported |
Below umbilical surgeries |
Group BC received 0.75 ml/kg of 0.25% bupivacaine with 1 μg/ kg of clonidine in normal saline Caudal block |
Group B received 0.75 ml/kg of 0.25% bupivacaine in normal saline |
|
Tripi, 2005
India |
N: 18/ 17 Age (months) ± SD: 59.0 ± 30.4 / 67.6 ± 30.5 M:F: 2:16/ 5:12 |
Ureteroneocystostomy |
1 ml/kg 0.125% bupivacaine with 1 µg/kg clonidine Caudal block |
preincision caudal block consisting of either 1 ml/kg 0.125% bupivacaine |
|
Visoiu, 2021
USA |
N: 26/ 24 Median age (years) (Q1,Q3): 13.0 (11.0, 15.0)/ 13.0 (11.5, 15.5) %F: 46/ 54
|
Laparoscopic appendectomy |
The R+C group received two 20 ml syringes with 10 ml of ropivacaine 0.5 % and 1 µg/kg of clonidine (100 µg =1 ml) for a total volume of 11 ml. Each patient in the R+C group received a total of 2 µg/kg clonidine. Rectus sheath nerve block |
The RO group received two 20 ml syringes, each with 10 ml of ropivacaine 0.5% and a 1 ml of normal saline |
Results
- Postoperative pain
In the included studies different pain scales were used to assess postoperative pain. Table 2 includes the descriptions of these different scales.
Table 2. Different pain scales used to assess postoperative pain.
|
Name |
Abbreviation |
Scoring |
Score range |
Meaning |
|
Children’s Hospital of Eastern Ontario Pain Scale (CHEOPS) |
CHEOPS |
6 categories of pain behavior: Cry, facial, verbal, torso, touch, and legs |
0 to 2 or 1 to 3 is assigned to each activity, total score range 4-13 |
higher scores indicating greater level of pain |
|
Children and Infants Postoperative Pain Scale (CHIPPS) |
CHIPPS |
4 items: crying, facial expression, trunk's posture, legs' posture and motor restlessness |
0 to 2 points per item, total score range 0-8 |
higher scores indicating greater level of pain |
|
FACES scale |
- |
five categories: crying, facial expression, position of torso, position of legs and motor restlessness |
Complication score 1, none; 2, moderate; 3, severe |
higher scores indicating greater level of pain |
|
FACES Pain Rating Scale (Wong-Baker) |
- |
6 faces; the first face represents a pain score of 0, and indicates "no hurt". The second face represents a pain score of 2, and indicates "hurts a little bit". The third face represents a pain score of 4, and indicates "hurts a little more". The fourth face represents a pain score of 6, and indicates "hurts even more" |
0-6 |
higher scores indicating greater level of pain |
|
Face, Legs, Activity, Cry, Consolability (FLACC) Behavioral Pain Scale |
FLACC |
5 categories: face, legs, activity, cry, and consolability |
0 to 2 points per category, total score range 0-10 |
higher scores indicating greater level of pain |
|
Numeric pain rating scale |
NRS |
0 being no pain and 10 the worst imaginable pain |
0-10 |
higher scores indicating greater level of pain |
|
Objective Pain Scale |
OPS |
4 pain behaviors: crying, movement, agitation, and verbalization |
0 to 2 points per category, total score range 0-10
|
higher scores indicating greater level of pain |
|
Visual analog scale |
VAS |
A continuous scale ranging from no pain to worst pain |
0 to 10 |
higher scores indicating greater level of pain |
1.1. Postoperative pain at PACU arrival/ 0 hours
Nine studies reported on postoperative pain at PACU arrival or pain assessed at 0 hours. All studies used different measurement and reporting methods. Therefore, the results are described per study.
Anoar (2016) assessed pain using the CHEOPS and reported mean pain scores (± standard deviation, SD). The mean pain scores were 5.8 (± 0.9) and 6.4 (± 0.8) in the clonidine (n=20) and control group (n=20), respectively (MD -0.60, 95% CI -1.13 to -0.07). This difference is not clinically relevant.
Bhati (2022) used the OPS and reported only mean pain scores. The mean pain score was 0 for both the intervention (n=20) and control group (n=20), respectively (MD 0).
El-Hennway (2009) assessed pain with the FLACC and reported participant’s pain intensity in percentages per score. For the intervention group the distribution of the scores was as follows, score 0= 45%, score 1= 40%, score 2= 15%, and for the control group scores were 0 = 25%, score 1= 45%, score 2= 30%.
Joshi (2004) assessed with the FACES scale reported by a nurse and reported mean pain scores (±SD). A pain score of 6.7 (±1.5) in the intervention group (n=18) and a pain score of 6.8 (±1.7) the control group (n=17) was found (mean difference (MD) -0.10; 95 % CI -1.16 to 0.96). This difference is not clinically relevant.
Koul (2009) assessed pain with the OPS at 30 minutes, 1 hour and 2 hours and reported mean OPS scores (±SD) over these time-intervals. The mean pain scores were 4.65 (± 0.25) and 4.55 (± 0.25) in the intervention (n=20) and control group (n=20), respectively (MD - 0.10 95% CI -0.05 to 0.25). This difference is not clinically relevant.
Mostafa (2021) assessed pain using the CHEOPS and reported mean pain scores (±SD). The mean pain scores were 4.00 (± 0.1) and 4.10 (± 0.3) in the intervention (n=30) and control group (n=30), respectively (MD - 0.10 95% CI -0.47 to 0.27). This difference is not clinically relevant.
Potti (2017) assessed pain using the CHIPPS and authors reported the number of patients experiencing each score (Table 3).
Table 3. CHIPPS pain scores at 1 hour (Potti 2017).
|
CHIPPS score |
0 |
1 |
2 |
3 |
≥4 |
|
I1: (n = 25) levobupivacaine 0.25% 1 mL/kg with 1 mug/kg clonidine caudally and 5 mL of normal saline i.v |
25 (100%) |
|
|
|
|
|
I2: (n = 25) levobupivacaine 0.25% 1 mL/kg caudally and 1 mug/kg clonidine in 5 mL normal saline i.v |
25 (100%) |
|
|
|
|
|
C: (n = 25) levobupivacaine 0.25% 1 mL/kg caudally and 5 mL of normal saline i.v |
20 (80%) |
3 (12%) |
2 (8%) |
|
|
Tripi (2005) assessed pain using the FACES Pain Rating Scale for children 1 to 3 years old, and
the FLACCS for children 4 years or older. Only mean values were reported; the mean pain scores were 0.90 and 1.18 in the intervention (n=18) and control group (n=17), respectively (MD -0.28). This difference is not clinically relevant.
Visoiu (2021) assessed pain using a NRS and reported mean pain scores (±SD). The mean pain scores were 1.20 (± 2.55) and 1.79 (± 2.78) in the intervention (n=25) and control group (n=19), respectively (MD -0.59, 95% CI -2.19 to 1.01). This difference is not clinically relevant.
1.2. Postoperative pain at 6 hours
Seven studies reported on postoperative pain at 6 hours. All studies used different measurement and reporting methods. Therefore, the results are described per study.
Anoar (2016) used the CHEOPS and reported mean pain scores (±SD). The mean pain scores were 4.5 (±0.5) and 6.2 (±1.1) in the intervention (n=20) and control group (n=20), respectively (MD -1.70, 95% CI -2.23 to -1.17). This difference is clinically relevant, in favor of clonidine.
Bhati (2022) used the OPS and reported only mean pain scores. The mean pain score was 0.75 versus 5 for the intervention (n=20) and control group (n=20), respectively (MD -4.25). This difference is clinically relevant, in favor of clonidine.
Jindal (2011) assessed pain with the FLACC and reported participant’s pain intensity in percentages per predefined score-ranges. For the intervention group (n=25) the distribution of the scores was as follows, 0-3 (no pain to mild pain) 24 (96%) 4-6 (moderate pain) 1 (4%); and for the control group (n=25) the scores were 0-3 (no pain to mild pain) 21 (84%) 4-6 (moderate pain) 3 (12%).
Narasimhamurthy (2016) assessed pain with the FLACC and reported number of patients with a score ≥ 4. Zero of 30 patients (0%) in the clonidine group; and 18 of 30 patients (60%) in the control group had a pain score ≥ 4. This difference in percentages is in favor of clonidine.
Parameswari (2010) assessed pain with the FLACC scale and reported results into categorized scores of four groups of pain severity (0 = No pain; 1 - 3 = Mild pain; 4 - 7 = Moderate pain; 8 - 10 = Severe pain). In the clonidine group, 33 of 50 patients (66%) had no to mild pain compared to 12 of 50 patients (24%) in the control group; and 17 patients (34%) in the clonidine group experienced moderate to severe pain compared to 38 patients (76%) in the control group. This difference in percentages in in favor of clonidine.
Potti (2017) assessed pain using the CHIPPS and reported the number of patients experiencing each score (Table 4). The difference between both clonidine groups and the control group is in favor of the clonidine groups.
Table 4. CHIPPS pain scores at 6 hours (Potti 2017).
|
CHIPPS score |
0 |
1 |
2 |
3 |
≥4 |
|
I1: (n = 25) levobupivacaine 0.25% 1 mL/kg with 1 mug/kg clonidine caudally and 5 mL of normal saline i.v |
6 (24%) |
18 (72%) |
1 (4%) |
|
|
|
I2: (n = 25) levobupivacaine 0.25% 1 mL/kg caudally and 1 mug/kg clonidine in 5 mL normal saline i.v |
1 (4%) |
5 (20%) |
12 (48%) |
4 (16%) |
3 (12%) |
|
C: (n = 25) levobupivacaine 0.25% 1 mL/kg caudally and 5 mL of normal saline i.v |
|
|
1 (4%) |
|
24 (96%) |
Visoiu (2021) assessed pain using a NRS and reported mean pain scores (±SD). The mean pain scores were 2.28 (± 2.49) and 2.43 (± 2.95) in the intervention (n=18) and control group (n=14), respectively (MD -0.15, 95% CI -2.08 to 1.78). This difference is not clinically relevant.
1.3. Postoperative pain at 24 hours
Seven studies reported on postoperative pain at 24 hours. All studies used different measurement and reporting methods. Therefore, the results are described per study.
Anoar (2016) used the CHEOPS and reported mean pain scores (±SD). The mean pain scores were 5.8 (± 0.9) and 7.7 (± 0.4) in the intervention (n=20) and control group (n=20), respectively (MD -1.90, 95% CI -2.33 to -1.47). This difference is clinically relevant.
Bajwa (2010) used the OPS and reported mean pain scores (±SD). The mean pain scores were 3.58 (± 0.40) and 3.72 (± 0.42) in the intervention (n=22) and control group (n=22), respectively (MD -0.14; 95% CI -0.38 to 0.10). This difference is not clinically relevant.
El-Hennway (2009) assessed pain with the FLACC and reported participant’s pain intensity in percentages per score. At 24 hours postoperatively, for the intervention group (n=30) the distribution of the scores was as follows, score 1 (30%), score 2 (40%), score 3 (30%), and for the control group (n=30) score 1 (40%), score 2 (40%), score 2 (20%).
Joshi (2004) assessed pain at 24 hours postoperatively based on parent reported visual analog scale (VAS) scores (0 representing no pain, and 10 representing worst imaginable pain) at rest and during movement. At rest, a pain score of 1.9 (±2.7) in the intervention group (n=18) and a pain score of 1.8 (±1.9) in the control group (n=17) was found (MD 0.10; 95 % CI -1.44 to 1.64).
This difference is not clinically relevant. During movement, a pain score of 3.6 (±3.3) in the intervention group (n=18) and a pain score of 3.2 (±2.3) the control group (n=17) was found (MD 0.40; 95 % CI -1.48 to 2.28). This difference is not clinically relevant.
Mostafa (2021) assessed pain using CHEOPS and reported mean pain scores (±SD). The mean pain scores were 4.13 (± 0.3) and 4.13 (± 0.3) in the intervention (n=30) and control group (n=30), respectively (MD 0.00, 95% CI -0.45 to 0.15). This difference is not clinically relevant.
Narasimhamurthy (2016) assessed pain with the FLACC scale and reported number of patients with a score ≥ 4. Three of 30 patients in the clonidine group (10%); and 2 of 30 patients in the control group (6.6%) had a pain score ≥ 4.
Potti (2017) assessed pain using the CHIPPS and reported the number of patients experiencing each score (Table 5).
Table 5. CHIPPS pain scores at 24 hours (Potti 2017)
|
CHIPPS score |
0 |
1 |
2 |
3 |
≥4 |
|
I1: (n = 25) levobupivacaine 0.25% 1 mL/kg with 1 mug/kg clonidine caudally and 5 mL of normal saline i.v |
|
1 (4%) |
3 (12%) |
1 (4%) |
20 (80%) |
|
I2: (n = 25) levobupivacaine 0.25% 1 mL/kg caudally and 1 mug/kg clonidine in 5 mL normal saline i.v |
|
|
1 (4%) |
|
24 (96%) |
|
C: (n = 25) levobupivacaine 0.25% 1 mL/kg caudally and 5 mL of normal saline i.v |
|
|
|
|
25 (100%) |
1.4. Reporting of postoperative pain with incomplete information
Ten other included studies did measure postoperative pain but only reported limited or unreadable data in the results section:
Akbas (2005) used the observational Oucher Pain Scale and only reported that there were no statistically significant differences among the groups with respect to the pain scoring.
Akin (2010) used the CHIPPS and presented results in a graph from which the data are not readable. The graph shows that the control group had higher pain scores during the first postoperative hour but were similar with the intervention group at 6h and 24h postoperatively.
De Mey (2000) used a VAS in children older than 5 years and CHEOPS in children less than 5 years of age, but no absolute pain scores are reported in the results section. The authors only report that the pain scores at 2, 6 and 12h postoperatively were not significantly different among the groups.
De Negri (2001) used the CHEOPS once in the recovery room and every 4h if the patient was awake, but only reported that pain scores were lower in the group receiving ropivacaine plus clonidine 0.12 mg compared to ropivacaine plus lower doses of clonidine (0.04 and 0.08 mg) and plain ropivacaine.
Ivani (2000) used the OPS and registered the mean of the maximum scores for each individual patient who did not require supplemental analgesia during the 24h study period. They report that OPS scores were similar between the control (mean: 1, range: 0–4; n=20) and the intervention (mean: 1, range: 0–4; n=20) groups (MD: 0).
Laha (2012) used the CHEOPS at PACU, 4h, 8h and 24h, and presented results in a graph from which the data are not readable. The graph shows that pain scores were (slightly) higher for the clonidine group compared to the control group - with pain scores increasing over time.
Rawat (2019) used the CHIPSS and presented results in a graph from which the data are not readable. The graph shows that pain scores are lower in the clonidine compared to the control group at 0, 6, and 12h after surgery.
Sanwatsarka (2017) used the FLACC scale and presented results in a graph from which the data are not readable. The authors report that: FLACC pain score never reached ≥4 during the first 3h after surgery in both groups; the number of patients with FLACC pain score ≥4 were significantly more in the control group at the end of 4th (46%), 8th (56%) and 12th (72%) hour postoperatively compared to the clonidine group; more children in the control group had moderate to severe pain at 4h, 8h and 12h postoperatively, compared to children in the clonidine group.
Shaikh (2015) used the FLACC scale and presented data in a graph from which the data are not readable. The graph shows at that the FLACC score begins at a score of 0 at 0h postoperatively for both groups; that the clonidine group has a much lower score compared to the control group at 4-8hr; and that at 24h postoperatively the clonidine group still has lower pain scores, but there is less difference with the control group.
Singh (2012) used the FLACC scale and presented results in a graph from which the data are not readable. In this study, efficacy of ketamine, fentanyl and clonidine were compared with a control group and the authors report that the FLACC pain score in the clonidine group was statistically significant lower compared to the other groups.
- Anxiety
Only one study (Visoiu, 2021) reported on the outcome anxiety. In children undergoing bilateral rectus sheath blocks for laparoscopic appendectomy surgery, they used the State-Trait Anxiety Inventory for Children (scores can range from 20 to 80, with higher scores correlating with greater anxiety) six hours after rectus sheath injections to score postoperative anxiety. The mean STAIC anxiety scores were 29.0 (26.0, 32.0) and 30.0 (27.0, 30.0) in the clonidine (n=23) and control group (n=18), respectively (MD -1 in favor of the clonidine group).
3. Postoperative opioid consumption
3.1. Postoperative opioid consumption in PACU
Three studies reported on postoperative opioid consumption in PACU. All three studies used different reporting methods, which meant that the results could not be pooled, and the results are described per study. In the study by Anouar (2016) supplemental analgesia of intravenous nalbuphine increments of 0.2 µg/kg was provided if the CHEOPS pain score was >7. The authors reported that no patient in the intervention group (n=20) nor in the control group (n=20) needed nalbuphine [RD: 0; 95% CI -0.09 to 0.09]. This difference is not clinically relevant.
In the study by Joshi (2004) fentanyl was given if the child had observable moderate or severe pain based on the faces scale (compilation score ≥2; see postoperative pain section, Table 2). The dose of fentanyl was 0.6 (±0.4) µg/kg in the intervention group compared to 0.8 (± 0.5) µg/kg in the control group (MD -0.20, 95% CI -0.50 to 0.10). This difference is not clinically relevant.
In the study by Tripi (2005) only mean intravenous morphine requirements for rescue therapy were reported (no SD). The consumption of morphine in PACU was 0.02 mg/kg in the intervention group compared to 0.05 mg/kg in the control group (MD 0.03). This difference is clinically relevant in favor of the control group.
3.2. Postoperative opioid consumption in 24 hours
Two studies reported on postoperative opioid consumption in 24h. In the study by Joshi (2004) doses of tylenol/codeine were given by the parents if their child appeared to be in pain. The mean number of doses of tylenol/codeine at 24h was 2.6 (±1.4) in the intervention group compared to 2.8 (± 2.5) in the control group (MD 0.20, 95% CI -1.13 to 1.53). This difference is not clinically relevant.
Tripi (2005) only reported mean intravenous morphine requirements for rescue therapy at 24h were reported (no SD). The consumption of morphine at 24h was 0.1 mg/kg in the intervention group compared to 0.2 mg/kg in the control group (MD 0.1). This difference is clinically relevant in favor of the intervention group.
3.3. Postoperative opioid consumption in total postoperative period
Five studies reported on postoperative opioid consumption in total postoperative period. All studies used different reporting methods, which meant that the results could not be pooled, and the results are described per study.
In the study by Akin (2010) patients with a CHIPPS score ≥4 were given tramadol oral drops (2 mg/kg). The authors reported on the number of patients requiring tramadol in the first 24h postoperatively: 19 of 20 (95%) patients who received clonidine caudally (I1); 13 of 20 (65%) patients who received clonidine in normal saline (I2) and 17 of 20 (85%) patients who only received levobupivacaine (C) required the rescue analgesic (RR I1 vs C: 1.12; 95% CI 0.91 to 1.38; RR I2 vs C: 0.76; 95% CI 0.53 to 1.11).
In the study by De Negri (2001) each child was prescribed acetaminophen/codeine suppositories to be given by the nurse if patients had CHEOPS scores >9 on two consecutive assessments 5min apart during the 48h study period. No absolute values were presented in the results section, but the authors report that the median number of postoperative analgesic doses was 4.0 in the control group, 4.0 in the group receiving clonidine 0.04 mg, 1.0 in in the group receiving clonidine 0.08 mg, and 1.0 in the group receiving clonidine 0.04 mg.
In the study by Ivani (2000) a fixed combination paracetamol (350 mg)-codeine (15 mg) suppository was administered if the patient’s OPS score was >5. Fewer patients needed supplemental paracetamol-codeine suppositories during the 24-h study period in the intervention group (2 of 20; 10%) compared to the control group (9 of 20; 45%) (RR: 0.22; 95% CI 0.05 to 0.90).
In the study by Kalachi (2007) tramadol 1–2 mg/kg IV was used for rescue analgesia if the pain score was ≥30 mm on a 0-100 mm VAS. Need for rescue analgesic during the first 24h after surgery was reported as yes/no. In the clonidine group, 29 of 42 (69%) needed tramadol compared to 26 of 63 patients in the control group (RR 1.67, 95% CI 1.17 to 2.39).
In the study by Tripi (2005) total number of patients requiring mean intravenous morphine requirements for rescue therapy during the 24h study period was reported. Five of 18 patients (27.7%) in the clonidine group received no postoperative morphine, compared to 1 of 15 (6.7%) in the control group (RR 4.17, 95% CI 0.54 to 31.88).
4. Adverse events
4.1. Apnea
Not reported.
4.2. Respiratory depression
Thirteen studies reported on the incidence of respiratory depression (a decrease in oxygen saturation <93%, requiring oxygen by face mask). Figure 1 shows that there is no difference in risk between the pooled clonidine group (n=335) and pooled control group (n=334) (RD 0.00, 95% CI -0.02 TO 0.02).
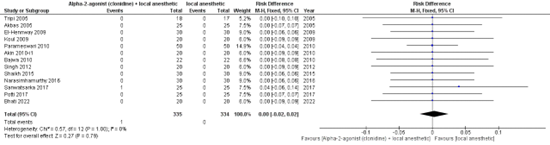
Figure 1: respiratory depression.
4.3. Hypotension
Ten studies reported incidence of hypotension (systolic blood pressure (SBP) <70 mm Hg). Figure 2 shows that there is no difference in risk between the pooled clonidine group (n=304) and pooled control group (n=324) (RD 0.03, 95% CI -0.00 to 0.07).

Figure 2: Hypotension.
4.4. Sedation
In total 20 studies reported on sedation levels. Eleven studies reported data that could be interpreted for (clinical) relevance (Table 6). The other studies (n=9) only reported limited information or unreadable data in the results section and are described below the table. All studies used different measurement and reporting methods. Therefore, the results are described per study.
Table 6. Sedation scores per study.
|
Study |
Sedation scale |
Time |
Reporting |
Clonidine group |
N |
Control group |
N |
Outcome (MD, RD) |
Clinically relevant? |
|
Bawja (2010) |
three-point scale (opening of eyes: 3 = spontaneously, 2 = to verbal command, 1 = to physical shaking, 0 =not arousable) |
24 h time interval (10-min interval after extubation and thereafter at intervals of 1, 2, 4, 6, 8, 12, 18 and 24 h) |
Mean (±SD) |
2.86 (± 0.52) |
22 |
2.68 (± 0.56) |
22 |
MD 0.18, 95% CI -0.14 to 0.50 |
No |
|
Ivani (2000) |
a four-point scale [0 (alert)–3 (not arousable)] |
Until the patients were once again alert as indicated by spontaneous eye opening |
Mean (range) |
mean: 1, range: 0–2 |
20 |
mean: 1, range: 0–1 |
20 |
MD 0 |
No |
|
Jindal (2011) |
University of Michigan sedation scale (UMSS) (0-3 score range) |
At extubation (PACU) |
% per sedation score [0:1:2:3] |
24%, 48%, 24%, 4% |
25 |
16%, 40%, 32%, 16% |
25 |
Similar distribution |
No |
|
Koul (2009) |
four-point sedation score (1: asleep, not arousable by verbal contact; 2: asleep, arousable by verbal contact; 3: drowsy not sleeping; 4: alert/aware) |
30minutes, 1 hour and 4 hours after the operation |
mean sedation scores over these time-intervals |
2.8 (± 0.45) |
20 |
2.83 (± 0.47) |
20 |
MD -0.03 95% CI -0.32 to 0.26 |
No |
|
Potti (2017) |
Ramsay Sedation Score |
1h, 6h, 12h |
Mean (±SD) |
PACU (1h) I_B: 2.52 ± 0.51 I_C: 2.40 ± 0.50
6 hours I_B: 2.00 ± 0.00 I_C: 2.00 ± 0.00
12 hours I I_B: 2.00 ± 0.00 I_C: 2.00 ± 0.00
|
25 |
PACU (1h) 2.40 ± 0.50
6 hours 2.00 ± 0.00
24 hours not reported
|
25 |
PACU (1h) 2.40 ± 0.50
6 hours 2.00 ± 0.00
|
|
|
Mostafa (2021) |
five-point sedation score (1. awake and alert, 2. sleeping but easily arouses to voice or light touch, 3. Arouses to loud voice or shaking, 4. arouses with painful stimuli and 5. cannot be aroused) |
After the block |
Mean (±SD) |
1.33 (± 0.1) |
30 |
1.17 (± 0.1) |
30 |
MD 0.16, 95% CI 0.11 to 0.21 |
No |
|
Narasimhamurthy (2016) |
three-point sedation score (0- awake, 1- arousable by voice, 2- arousable to pain, 3- unarousable) |
First 2 hours |
Number of patients per score |
Score = 0 22 of 30
Score = 1 8 of 30 |
30 |
Score = 0 22 of 30
Score =1 8 of 30 |
30 |
Score = 0 RD 0.00 95% CI -0.22 to 0.22
Score =1 RD 0.00 95% CI -0.22 to 0.22 |
No |
|
Sanwatsarka (2017) |
a four-point sedation score (1 Asleep, not arousable by verbal contact; 2 Asleep, arousable by verbal contact; 3 Drowsy not sleeping; 4 Alert/awake) |
0,1,2,4,8,12h |
Mean (±SD) |
0h 1.88±0.2112
4h 3.08±0.2208
12h 3.72±0.4032 |
25 |
0h 2.84±0.2688
4h 3.8±0.32
12h 4±0.0 |
25 |
0h MD -0.96 95% CI -1.09 to -0.83
4h MD -0.72 95% CI -0.87 to -0.57
12h MD -0.28 |
No |
|
Tripi (2005) |
5-point sedation score ranging from 1 (asleep, no response to painful stimulus) to 5 (crying, agitated or restless). |
15-minute intervals in the PACU |
Mean |
Initial score 3.2
All scores 3.4 |
18 |
Initial score 3.0
All scores 3.4 |
17 |
Initial score MD 0.2
All scores MD 0 |
No |
|
Visoiu (2021) |
Sedation Level (0, 1, 2)
|
PACU |
% of patients per score [0, 1, 2] |
18%, 77%, 5% |
26 |
19%, 76%, 5% |
24 |
Similar distribution |
No |
MD= mean difference; PACU=post anesthesia care unit; RD=risk difference; SD=standard deviation
Akbas (2005) used a three-point score (1, awake; 2, asleep but arousable by verbal contact; 3, asleep and not arousable by verbal contact) and only reported that sedation scores were higher in the clonidine group for the first 1-h period after the operation compared to the control group.
Akin (2010) used the Ramsay Sedation Score (1, awake: agitated or restless or both; 2, awake: cooperative, oriented, and tranquil; 3, awake but responds to commands only; 4, asleep: brisk response to light glabellar tap or loud auditory stimulus), but the data were presented in a graph from which the data was not readable. The authors reported that the mean postoperative sedation score was higher in the group that received levobupivacaine + clonidine caudally at 30-, 60- and 240-min post procedure compared to the group that received only levobupivacaine (control) and the group that received levobupivacaine + clonidine i.v. but not at extubation, 15 and 120 min and 6, 12 and 24 h postoperatively.
Bhati (2022) used the Ramsay Sedation Score at PACU at arrival and then every hour, but only reported in the results section that the score was 3 at 60 minutes post-block and that all children were awake and alert 120 minutes post-block.
De Negri (2001) assessed the degree of sedation with a three-point sedation scale based on eye opening (0 5 eyes open spontaneously; 1 5 eyes open to speech; 2 5 eyes open in response to physical stimulation) in the recovery room and every 4 h if the patient was awake for 48 h study period and presented data in a figure that was not readable. It was only reported that no statistical difference was found among the different study groups.
Joshi (2004) sedation level was scored with 0 corresponding to ‘asleep’ and 10 corresponding to ‘awake’. In the results section no absolute values are reported, but author report “there was no difference between the groups with respect to sedation the night of surgery or the following morning.”
Kaaibachi (2005) using a three-point scale (0, alert; 1, asleep but easy arousable; 2, asleep but not easily arousable) to measure sedation. In the results section they only report that there was no significant difference in the sedation scores between both groups either in recovery room (sedation score 2, 5% in the clonidine group versus 48% in the control group) or during the first 6 h.
Rawat (2019) used the Ramsay Sedation Score and presented data in a (bar) graph from which the values are not readable. The graph shows that sedation scores are higher in the clonidine group compared to the control group over all time points (0, 2, 4, 6, 8, 10, 12 h post-surgery).
Shaikh (2015) used a sedation score 0-3 according to child’s alertness and arousability. Data were presented in a figure and not readable. Sedation scores up to 8 hours postoperatively were lower in the control group as compared to the clonidine group. At 8 hours, both groups had a sedation score of 0.
Singh (2012) assessed sedation by using a four-point sedation score (Patient sedation score was defined as 1: Asleep, not arousable by verbal contact 2: Asleep, arousable by verbal contact, 3: Drowsy, not sleeping, 4: Alert/ awake) at every hour for the first eight hours. Data presented in figure not readable and further not reported by the authors. From the figure it can be observed that the intervention group had higher sedation scores throughout all timepoints as compared to the control group.
4.5. Postoperative emergence delirium
Not reported.
Level of evidence of the literature
The level of evidence for all outcome measures started as high, since the included studies were RCTs.
The level of evidence regarding the outcome measure postoperative pain at PACU arrival/ 0 hours was downgraded by two levels to low because of study limitations (risk of bias, -1); and number of included patients (imprecision, -1).
The level of evidence regarding the outcome measure postoperative pain at 6 hours was downgraded by two levels to low because of study limitations (risk of bias, -1); and number of included patients (imprecision, -1).
The level of evidence regarding the outcome measure postoperative pain at 24 hours was downgraded by two levels to low because of study limitations (risk of bias); and number of included patients (imprecision, -1).
The level of evidence regarding the outcome measure anxiety was downgraded by three levels to very low because only one study with a limited number of included patients was included (imprecision, -3).
The level of evidence regarding the outcome measure postoperative opioid consumption in PACU was downgraded by three levels to very low because of study limitations (risk of bias, -1); and number of included patients (imprecision, -2).
The level of evidence regarding the outcome measure postoperative opioid consumption in 24 hours was downgraded by three levels to very low because of study limitations (risk of bias, -1); and number of included patients (imprecision, -2).
The level of evidence regarding the outcome measure postoperative opioid consumption in the total postoperative period was downgraded by three levels to very low because of study limitations (risk of bias, -1); and number of included patients (imprecision, -2).
The level of evidence regarding the adverse events apnea and postoperative emergence delirium could not be assessed as no evidence was available.
The level of evidence regarding the adverse event respiratory depression was downgraded by one level to moderate because of study limitations (risk of bias, -1).
The level of evidence regarding the adverse event hypotension was downgraded by two levels to low because of study limitations (risk of bias, -1); and conflicting results (inconsistency, -1).
The level of evidence regarding the adverse event sedation was downgraded by two levels to low because of study limitations (risk of bias, -1); and number of included patients (imprecision, -1).
Zoeken en selecteren
A systematic review of the literature was performed to answer the following question: What is the effect of adding an alpha-2-agonist (clonidine) to the locoregional block compared with not adding alpha-2-agonist to the locoregional block in children undergoing a surgical procedure?
| P: | Children undergoing a surgical procedure |
| I: | Alpha-2-agonist (clonidine) + local anesthetic (ropivacaine or L-bupivacaine) |
| C: | Local anesthetic (ropivacaine or L-bupivacaine) |
| O: |
Postoperative pain Anxiety Postoperative opioid consumption Adverse events |
Relevant outcome measures
The guideline development group considered postoperative pain as a critical outcome measure for decision making; and anxiety, postoperative opioid consumption, complications and postoperative emergence delirium as important outcome measures for decision making.
The working group defined the outcome measures as follows:
- Postoperative pain à PACU / 0 hours, 6 and 24 hours (at rest; if nothing was reported about the condition in which pain was assessed (at rest or during mobilization) it was assumed pain was measured at rest)
- A priori, the working group did not define the outcome measure 'anxiety' but used the definitions used in the studies.
- Postoperative opioid consumption à morphine equivalent in PACU, at 24 hours and in total
- Adverse events à apnea, respiratory depression, hypotension, sedation, postoperative emergence delirium
The working group defined one point as a minimal clinically (patient) important difference on a 10-point pain scale and 10 mm on a 100 mm pain scale. Regarding postoperative opioid consumption, a difference of 20% was considered clinically relevant. For dichotomous variables, a difference of 10% was considered clinically relevant (RR ≤0.91 or ≥1.10; RD 0.10).
Search and select (Methods)
The databases Medline (via OVID) and Embase (via Embase.com) were searched with relevant search terms until 12-2-2023 + 18-2-2023. The detailed search strategy is depicted under the tab Methods. The systematic literature search resulted in 419 unique hits. Studies were selected based on the following criteria;
Inclusion criteria:
- Systematic review (SR) of randomized controlled trails (RCTs) or RCT
- Published ≥ 2000
- Children
- Conform PICO
Exclusion criteria:
- No original research
- N≤10 per arm
A total of 86 studies (16 SRs, 70 RCTs) were initially selected based on title and abstract screening. After reading the full text, 61 studies were excluded (see the table with reasons for exclusion under the tab Methods), and 24 studies (all RCTs) were included.
Results
A total of 24 RCTs were included in the analysis of the literature. Important study characteristics and results are summarized in the evidence tables. The assessment of the risk of bias is summarized in the risk of bias tables.
Referenties
- Akbas M, Akbas H, Yegin A, Sahin N, Titiz TA. Comparison of the effects of clonidine and ketamine added to ropivacaine on stress hormone levels and the duration of caudal analgesia. Paediatr Anaesth. 2005 Jul;15(7):580-5. doi: 10.1111/j.1460-9592.2005.01506.x. PMID: 15960642.
- Akin A, Ocalan S, Esmaoglu A, Boyaci A. The effects of caudal or intravenous clonidine on postoperative analgesia produced by caudal levobupivacaine in children. Paediatr Anaesth. 2010 Apr;20(4):350-5. doi: 10.1111/j.1460-9592.2010.03259.x. Epub 2010 Feb 11. PMID: 20158620.
- Anouar J, Mohamed S, Sofiene A, Jawhar Z, Sahar E, Kamel K. The analgesic effect of clonidine as an adjuvant in dorsal penile nerve block. Pan Afr Med J. 2016 Apr 21;23:213. doi: 10.11604/pamj.2016.23.213.5767. PMID: 27347302; PMCID: PMC4907766.
- Bajwa SJ, Kaur J, Bajwa SK, Bakshi G, Singh K, Panda A. Caudal ropivacaine-clonidine: A better post-operative analgesic approach. Indian J Anaesth. 2010 May;54(3):226-30. doi: 10.4103/0019-5049.65368. PMID: 20885869; PMCID: PMC2933481.
- Bhati K, Saini N, Aeron N, Dhawan S. A Comparative Study to Evaluate the Efficacy of Dexmedetomidine and Clonidine to Accentuate the Perioperative Analgesia of Caudal 0.25% Isobaric Levobupivacaine in Pediatric Infraumbilical Surgeries. Cureus. 2022 Aug 9;14(8):e27825. doi: 10.7759/cureus.27825. PMID: 36106237; PMCID: PMC9455914.
- de Mey JC, Strobbet J, Poelaert J, Hoebeke P, Mortier E. The influence of sufentanil and/or clonidine on the duration of analgesia after a caudal block for hypospadias repair surgery in children. Eur J Anaesthesiol. 2000 Jun;17(6):379-82. doi: 10.1046/j.1365-2346.2000.00690.x. PMID: 10928438.
- De Negri P, Ivani G, Visconti C, De Vivo P, Lonnqvist PA. The dose-response relationship for clonidine added to a postoperative continuous epidural infusion of ropivacaine in children. Anesth Analg. 2001 Jul;93(1):71-6. doi: 10.1097/00000539-200107000-00016. PMID: 11429342.
- El-Hennawy AM, Abd-Elwahab AM, Abd-Elmaksoud AM, El-Ozairy HS, Boulis SR. Addition of clonidine or dexmedetomidine to bupivacaine prolongs caudal analgesia in children. Br J Anaesth. 2009 Aug;103(2):268-74. doi: 10.1093/bja/aep159. Epub 2009 Jun 18. PMID: 19541679.
- Ivani G, De Negri P, Conio A, Amati M, Roero S, Giannone S, Lönnqvist PA. Ropivacaine-clonidine combination for caudal blockade in children. Acta Anaesthesiol Scand. 2000 Apr;44(4):446-9. doi: 10.1034/j.1399-6576.2000.440415.x. PMID: 10757579.
- Jindal P, Khurana G, Dvivedi S, Sharma JP. Intra and postoperative outcome of adding clonidine to bupivacaine in infraorbital nerve block for young children undergoing cleft lip surgery. Saudi J Anaesth. 2011 Jul;5(3):289-94. doi: 10.4103/1658-354X.84104. PMID: 21957409; PMCID: PMC3168347.
- Joshi W, Connelly NR, Freeman K, Reuben SS. Analgesic effect of clonidine added to bupivacaine 0.125% in paediatric caudal blockade. Paediatr Anaesth. 2004 Jun;14(6):483-6. doi: 10.1111/j.1460-9592.2004.01229.x. PMID: 15153211.
- Koul A, Pant D, Sood J. Caudal clonidine in day-care paediatric surgery. Indian J Anaesth. 2009 Aug;53(4):450-4. PMID: 20640207; PMCID: PMC2894500.
- Mostafa MF, Hamed E, Amin AH, Herdan R. Dexmedetomidine versus clonidine adjuvants to levobupivacaine for ultrasound-guided transversus abdominis plane block in paediatric laparoscopic orchiopexy: Randomized, double-blind study. Eur J Pain. 2021 Feb;25(2):497-507. doi: 10.1002/ejp.1689. Epub 2020 Nov 17. PMID: 33128801.
- Narasimhamurthy GC, Patel MD, Menezes Y, Gurushanth KN. Optimum Concentration of Caudal Ropivacaine & Clonidine - A Satisfactory Analgesic Solution for Paediatric Infraumbilical Surgery Pain. J Clin Diagn Res. 2016 Apr;10(4):UC14-7. doi: 10.7860/JCDR/2016/18946.7665. Epub 2016 Apr 1. PMID: 27190923; PMCID: PMC4866221.
- Parameswari A, Dhev AM, Vakamudi M. Efficacy of clonidine as an adjuvant to bupivacaine for caudal analgesia in children undergoing sub-umbilical surgery. Indian J Anaesth. 2010 Sep;54(5):458-63. doi: 10.4103/0019-5049.71047. PMID: 21189886; PMCID: PMC2991658.
- Potti LR, Bevinaguddaiah Y, Archana S, Pujari VS, Abloodu CM. Caudal Levobupivacaine Supplemented with Caudal or Intravenous Clonidine in Children Undergoing Infraumbilical Surgery: A Randomized, Prospective Double-blind Study. Anesth Essays Res. 2017 Jan-Mar;11(1):211-215. doi: 10.4103/0259-1162.200233. PMID: 28298787; PMCID: PMC5341679.
- Priolkar S, D'Souza SA. Efficacy and Safety of Clonidine as an Adjuvant to Bupivacaine for Caudal Analgesia in Paediatric Infra-Umbilical Surgeries. J Clin Diagn Res. 2016 Sep;10(9):UC13-UC16. doi: 10.7860/JCDR/2016/19404.8491. Epub 2016 Sep 1. PMID: 27790555; PMCID: PMC5072055.
- Rawat J, Shyam R, Kaushal D. A Comparative Study of Tramadol and Clonidine as an Additive to Levobupivacaine in Caudal Block in Pediatric Patients Undergoing Perineal Surgeries. Anesth Essays Res. 2019 Oct-Dec;13(4):620-624. doi: 10.4103/aer.AER_127_19. Epub 2019 Dec 16. PMID: 32009705; PMCID: PMC6937898.
- Sanwatsarkar S, Kapur S, Saxena D, Yadav G, Khan NN. Comparative study of caudal clonidine and midazolam added to bupivacaine during infra-umbilical surgeries in children. J Anaesthesiol Clin Pharmacol. 2017 Apr-Jun;33(2):241-247. doi: 10.4103/0970-9185.209739. PMID: 28781453; PMCID: PMC5520600.
- Shaikh SI, Atlapure BB. Clonidine as an adjuvant for bupivacaine in caudal analgesia for sub-umbilical surgery: A prospective randomized double blind study. Anaesthesia, Pain & Intensive Care. 2019 Jan 27:240-6.
- Singh J, Shah RS, Vaidya N, Mahato PK, Shrestha S, Shrestha BL. Comparison of ketamine, fentanyl and clonidine as an adjuvant during bupivacaine caudal anaesthesia in paediatric patients. Kathmandu Univ Med J (KUMJ). 2012 Jul-Sep;10(39):25-9. doi: 10.3126/kumj.v10i3.8013. PMID: 23434957.
- Tripi PA, Palmer JS, Thomas S, Elder JS. Clonidine increases duration of bupivacaine caudal analgesia for ureteroneocystostomy: a double-blind prospective trial. J Urol. 2005 Sep;174(3):1081-3. doi: 10.1097/01.ju.0000169138.90628.b9. PMID: 16094063.
- Visoiu M, Scholz S, Malek MM, Carullo PC. The addition of clonidine to ropivacaine in rectus sheath nerve blocks for pediatric patients undergoing laparoscopic appendectomy: A double blinded randomized prospective study. J Clin Anesth. 2021 Aug;71:110254. doi: 10.1016/j.jclinane.2021.110254. Epub 2021 Mar 19. PMID: 33752119.
Evidence tabellen
Evidence table for intervention studies (randomized controlled trials and non-randomized observational studies [cohort studies, case-control studies, case series])
|
Study reference (first author, publication year) |
Was the allocation sequence adequately generated?
Definitely yes Probably yes Probably no Definitely no |
Was the allocation adequately concealed?
Definitely yes Probably yes Probably no Definitely no |
Blinding: Was knowledge of the allocated interventions adequately prevented? Were patients blinded? Were healthcare providers blinded? Were data collectors blinded? Were outcome assessors blinded? Were data analysts blinded? |
Was loss to follow-up (missing outcome data) infrequent?
Definitely yes Probably yes Probably no Definitely no |
Are reports of the study free of selective outcome reporting?
Definitely yes Probably yes Probably no Definitely no |
Was the study apparently free of other problems that could put it at a risk of bias?
Definitely yes Probably yes Probably no Definitely no |
Overall risk of bias If applicable/necessary, per outcome measure
LOW Some concerns HIGH |
|
Akbas 2005 |
Probably no
Reason: not reported |
Probably no
Reason: not reported |
Probably yes
Reason: Drugs were diluted in 0.9% saline (0.75 mlÆkg) and prepared by a staff anesthesiologist not otherwise involved in the study. |
Probably yes
Reason: not reported, complete outcome data for 25 included patients per arm. |
Probably yes
Reason: All relevant outcomes were reported, but no absolute data were reported for all outcomes. |
Probably yes
Reason: funding and conflicts not reported. |
High
Reason: allocation, randomization and blinding |
|
Akin 2010 |
Probably no
Reason: not reported |
Probably yes
Reason: Children were randomized, using a sealed envelope technique, to one of the three treatment groups before caudal block was performed |
Probably yes
Reason: The study drug was prepared by an investigator unaware of the group assignment. |
Probably yes
Reason: not reported, complete outcome data for 25 included patients per arm. |
Probably yes
Reason: All relevant outcomes were reported, but no absolute data were reported for all outcomes. |
Probably yes
Reason: funding and conflicts not reported. |
High
Reason: allocation, blinding |
|
Anouar 2016 |
Probably no
Reason: not reported |
Probably yes
Reason: Each patient was randomly assigned to one of the two groups by drawing from a sealed envelope |
Probably yes
Reason: double-blind study. After surgery, patients were observed in PACU (Post Anesthesia Care Unit) for 6 hours by a nurse blinded to the study. |
Probably yes
Reason: not reported, complete outcome data for 25 included patients per arm. |
Probably yes
Reason: All relevant outcomes were reported, but no absolute data were reported for all outcomes. |
Probably yes
Reason: funding and conflicts not reported. |
Some concerns
Reason: allocation |
|
Bajwa 2010 |
Definitely yes
Reason: patients were randomly allocated according to a computer generated randomization |
Probably no
Reason: not reported |
Probably yes
Reason: double-blind study. The syringes for the study solutions were prepared by a senior resident of the anesthesiology department who was given written protocols for drug preparation and was unaware of the patients and operation theatre team. |
Probably yes
Reason: not reported, complete outcome data for 25 included patients per arm. |
Probably yes
Reason: All relevant outcomes were reported, but no absolute data were reported for all outcomes. |
Definitely yes
Reason: No other problems noted |
Some concerns
Reason: allocation |
|
Bhati 2022 |
Definitely yes
Reason: Children enlisted for the study were randomly allocated into three groups using a computer-generated randomization chart |
Probably no
Reason: not reported |
Definitely yes
Reason: double-blind study. The volume of each local anesthetic solution was prepared by one anesthetist in a coded transparent 10 mL syringe and labeled with the patient’s study number. All healthcare personnel, parents and guardians, and the anesthetist who performed the block were blinded to the caudal medications administered. |
Definitely no
Reason: consort diagram (figure 1) shows there were no dropouts, all patients were followed up till 24 hours. |
Probably yes
Reason: all relevant outcomes were reported, but mainly descriptive |
Definitely yes
Reason: No other problems noted |
Some concerns
Reason: Allocation, reporting of results |
|
De Negri 2001 |
Probably no
Reason: it is only stated that patients were randomly allocated into 2 equal groups |
Probably no
Reason: not reported |
Definitely no
Reason: Randomized, observer-blinded study. Assessments of the variables studied were recorded by nurse observers unaware of the mixture used for epidural infusions. |
Probably no
Reason: not reported |
Probably yes
Reason: All relevant outcomes were reported, but no absolute data were reported for all outcomes. |
Definitely yes
Reason: No other problems noted |
High
Reason: blinding, allocation and reporting of results |
|
El-Hennway 2009 |
Definitely yes
Reason: Using a computer-generated list, the subjects were randomly and evenly assigned into three groups: A, B, and C.
|
Probably no
Reason: not reported |
Definitely yes
Reason: All health-care personnel providing direct patient care, the subjects, and their parents or guardians were blinded to the caudal medications administered. All medications were prepared by pharmacy staff not participating in the study except for preparing the drugs. They received and kept the computer-generated table of random numbers according to which random group assignment was performed. After obtaining subjects weight, and according to the randomizing table, the volume to be injected in the caudal block was prepared in syringes with labels indicating only the serial number of the patient. |
Definitely no
Reason: 60 patients were enrolled into the study and none of the 60 attempted caudal blocks was perceived as being a failed attempt |
Probably yes
Reason: all relevant outcomes were reported, but absolute data not readable |
Definitely yes
Reason: No other problems noted |
Some concerns
Reason: allocation |
|
Ivani 2001 |
Definitely yes
Reason: Computer generated randomization was used to divide the patients |
Probably no
Reason: not reported |
Definitely no
Reason: observer-blinded study. Observers unaware of which drug had been administered evaluated the onset of the block and duration of postoperative analgesia. |
Probably no
Reason: 40 patients were enrolled and loss-to follow up is not reported, but results show no missing data |
Probably yes
Reason: all relevant outcomes were reported, but absolute data not readable |
Definitely yes
Reason: No other problems noted |
Some concerns
Reason: Allocation and lack of blinding |
|
Jindal 2011 |
Definitely yes
Reason: For randomization, computer generated numbers equal to the number of patients who were scheduled for the study was put into serially labelled opaque sealed envelopes. Before surgery, an envelope containing the random number was drawn for each patient. |
Definitely yes
Reason: See text in left column |
Probably no
Reason: It is only reported that the patient, anesthesiologist who performed the block, and surgeon were blinded to which anesthetic agent would be used. |
Probably no
Reason: 50 patients were enrolled and loss-to follow up is not reported, but results show no missing data |
Probably yes
Reason: all relevant outcomes were reported, but absolute data not reported or not readable |
Definitely yes
Reason: No other problems noted |
Some concerns
Reason: lack of blinding |
|
Joshi 2004 |
Probably no
Reason: not reported |
Probably no
Reason: not reported |
Probably no
Reason: It is only reported that a nurse blinded to group assignment treated postoperative pain. |
Probably no
Reason: 36 patients were enrolled and loss-to follow up is not reported, but results show no missing data |
Probably yes
Reason: all relevant outcomes were reported, but absolute data not reported or not readable |
Reason: No other problems noted |
High
Reason: allocation, blinding, outcome reporting |
|
Koul 2009 |
Probably no
Reason: not reported |
Probably no
Reason: not reported |
Probably no
Reason: it is reported this was a double-blind study, but no further information is provided on allocation and blinding. |
Probably no
Reason: it is not reported how many patients were enrolled and whether there was loss to follow-up. |
Probably yes
Reason: all relevant outcomes were reported, but absolute data not reported or not readable |
Reason: No other problems noted |
High
Reason: allocation, blinding, follow-up |
|
Laha, 2012 |
Probably no
Reason: not reported |
Probably no
Reason: not reported |
Probably no
Reason: it is reported this was a double-blind study, but no further information is provided on allocation and blinding. |
Probably no
Reason: it is not reported how many patients were enrolled and whether there was loss to follow-up. |
Probably yes
Reason: all relevant outcomes were reported, but absolute data not reported or not readable |
Reason: No other problems noted |
High
Reason: allocation, blinding, follow-up |
|
Mostafa, 2021 |
Probably yes
Reason: randomization computer- generated table using Microsoft Excel by a statistician who did not participate in the patients’ management |
Probably yes
Reason: not reported |
Probably yes
Reason: double blind study; the trial was planned that neither the investigators (surgeons and anesthesiologists) nor the patients’ guardians were aware of the group allocation and the drug combination received. An independent investigator who was not involved in performing the TAP block, or engaged with patient monitoring and data collection was entrusted with preparing and administering the study drugs. |
Definitely no
Reason: CONSORT flow diagram |
Definitely yes
Reason: all (mean) data of relevant outcome measures provided |
Reason: No other problems noted |
Low |
|
Narasimhamurthy 2016 |
Probably yes
Reason: randomization method is not reported
|
Probably yes
Reason: the patients were randomly allocated into two groups. |
Probably yes
Reason: The attending anesthesiologist administered the appropriate drug according to the code in the envelope. Name, age, hospital number, diagnosis and procedure were written in the same envelope, sealed and handed over to the investigator at the end of the procedure. A second observer did the patient assessment and data collection. The anesthesiologist who was collecting the data was blinded to the contents of the study drug. After all the cases had been completed at the end of the study, the code was broken, study drug contents revealed, and data compiled. |
Probably no
Reason: not reported |
Probably yes
Reason: all (mean) data of relevant outcome measures provided |
Reason: No other problems noted |
Some concerns
Reason: randomization method |
|
Parameswari 2010 |
Probably no
Reason: Randomization was done by picking random lots from a sealed bag |
Probably no
Reason: not reported |
Probably no
Reason: The drug was loaded by an anesthesiologist who did not participate in the study. Post-operative assessment was done by another anesthesiologist in the PACU who was not aware of the drug administered and by a nurse in the ward who was also blinded. |
Probably no
Reason: not reported |
Probably yes
Reason: all relevant outcomes were reported, but absolute data not reported or not readable |
Reason: No other problems noted |
Some concerns
Reason: allocation and blinding |
|
Potti 2017 |
Probably yes
Reason: patients were randomly allocated to one of the three groups by a computer-generated list and delivered in opaque, sealed numbered envelopes. |
Probably yes
Reason: see text in left column |
Probably no
Reason: double blind study, anesthesiologist who performed blocks was blinded to study drug, no further information stated
|
Probably no
Reason: not reported |
Probably yes
Reason: all relevant outcomes were reported, but absolute data not reported or not readable |
Reason: No other problems noted |
Some concerns
Reason: blinding, patients |
|
Priolkar 2016 |
Probably no
Reason: not reported |
Probably no
Reason: not reported |
Probably no
Reason: no information reported other than it was a double-blind study |
Probably no
Reason: not reported |
Probably yes
Reason: all relevant outcomes were reported, but absolute data not reported or not readable |
Reason: No other problems noted |
High
Reason: allocation, blinding, follow-up |
|
Rawat, 2019 |
Probably yes
Reason: use of a computer-generated list |
Probably no
Reason: not reported |
Probably no
Reason: no information reported other than it was a double-blind study |
Probably no
Reason: not reported |
Probably yes
Reason: all relevant outcomes were reported, but absolute data not reported or not readable |
Reason: No other problems noted |
Some concerns
Reason: allocation, blinding |
|
Sanwatsarkar, 2017 |
Probably yes
Reason: Randomization was done by picking random lots from a sealed bag |
Probably no
Reason: not reported |
Probably no
Reason: only stated that the drug was loaded by an anesthesiologist who did not participate in the study. |
Probably yes
Reason: not reported |
Probably yes
Reason: all relevant outcomes were reported, but absolute data not reported or not readable |
Reason: No other problems noted |
Some concerns
Reason: allocation, blinding |
|
Shaikh, 2015
|
Probably no
Reason: simple lottery method |
Probably no
Reason: not reported |
Probably no
Reason: all healthcare personnel, the patients and parents/guardians were blinded |
Probably no
Reason: not reported |
Probably yes
Reason: all relevant outcomes were reported, but absolute data not reported or not readable |
Reason: No other problems noted |
Some concerns
Reason: allocation |
|
Singh, 2012
|
Probably no
Reason: not reported |
Probably no
Reason: not reported |
Probably no
Reason: it is only reported that the caudal anesthesia was given by anesthesia technicians, who were blinded about the drugs being used |
Probably no
Reason: not reported |
Probably yes
Reason: all relevant outcomes were reported, but absolute data not reported or not readable |
Reason: No other problems noted |
High
Reason: allocation, blinding, follow-up |
|
Tripi, 2005 |
Probably no
Reason: randomization by random number assignment |
Probably no
Reason: not reported |
Probably yes
Reason: The anesthesiologist, surgeon and nurses caring for each patient were blinded to the contents of the administered solution, which was prepared by an anesthesia provider not caring for the patient. |
Probably no
Reason: not reported |
Probably no
Reason: results only in text, without absolute values |
Reason: No other problems noted |
High
Reason: allocation, reporting of results |
|
Visoiu 2021 |
Definitely yes
Reason: computer-generated random number table. |
Probably yes
Reason: The study medications were prepared and labeled as study drug by the pharmacy and released in identical syringes with a volume of 11 ml each. |
Probably yes
Reason: Patients as well as all members of the care team, except the pharmacist, were blinded to group allocation. |
Definitely no
Reason: loss to follow-up described in consort flow diagram |
Probably yes
Reason: all (mean) data of relevant outcome measures provided
|
Reason: No other problems noted |
Low |
Table of excluded studies
|
Reference |
Reason for exclusion |
|
Ansermino, M. and Basu, R. and Vandebeek, C. and Montgomery, C. |
more recent SR available |
|
Engelman, E. and Marsala, C. |
no quality assessment |
|
Lundblad, M. and Trifa, M. and Kaabachi, O. and Ben Khalifa, S. and Fekih Hassen, A. and Engelhardt, T. and Eksborg, S. and Lönnqvist, P. A. |
more recent SR available |
|
Schnabel, A. and Poepping, D. M. and Pogatzki-Zahn, E. M. and Zahn, P. K. |
no quality assessment |
|
Shah, Ushma J. and Karuppiah, Niveditha and Karapetyan, Hovhannes and Martin, Janet and Sehmbi, Herman |
more recent SR available |
|
Wang, Y. and Guo, Q. and An, Q. and Zhao, L. and Wu, M. and Guo, Z. and Zhang, C. |
no quality assessment |
|
Xiong, C. and Han, C. and Lv, H. and Xu, D. and Peng, W. and Zhao, D. and Lan, Z. |
more recent SR available |
|
Ahuja, S. and Aggarwal, M. and Joshi, N. and Chaudhry, S. and Madhu, S. V. |
Not conform PICO (I/C): wrong comparison (fentanyl vs. clonidine) |
|
Bhowmick, D. K. and Akhtaruzzaman, K. M. and Ahmed, N. and Islam, M. S. and Hossain, M. M. and Islam, M. M. |
Article not retrectable |
|
Bonisson, A. C. M. and Fernandes, M. L. and Araújo, G. F. and Vieira, F. E. and Noronha, L. M. and Gomez, R. S. |
Wrong language: Portuguese |
|
Chandrakant, Prasad and Vinod Kumar, Verma and Arvind, Kumar and Neeraj, Kumar and Gunjan, Kumar |
Not conform PICO (I/C): wrong comparison (different doses of clonidine were compared) |
|
Gogoi, Saurov and Saikia, Diganta and Dey, Sandeep |
Not conform PICO (I/C): wrong comparison (clonidine vs. dexmedetomidine) |
|
Kaabachi, O. and Rajeb, A. B. and Mebazaa, M. and Safi, H. and Jelel, C. and Ghachem, M. B. and Ammar, M. S. B. |
Not conform PICO (O): none of the crucial outcomes are reported |
|
Khakurel, S. and Sapkota, S. and Karki, A. J. |
Not conform PICO (O): none of the crucial outcomes are reported |
|
Klamt, J. G. and Garcia, L. V. and Stocche, R. M. and Meinberg, A. C. |
Not conform PICO (I/C): wrong comparison (different doses of clonidine were compared) |
|
Kumar, S. and Kumar, N. |
Not conform PICO (O): other primary outcomes and for outcomes of interest (pain) the article only reports statistically significant values among all the analysed one |
|
Lak, M. and Araghizadeh, H. and Shayeghi, S. and Khatibi, B. |
Not conform PICO (O): none of the crucial outcomes are reported |
|
Manickam, A. and Vakamudi, M. and Parameswari, A. and Chetan, C. |
Not conform PICO (O): none of the crucuial outcomes are reported |
|
Neogi, M. and Bhattacharjee, D. P. and Dawn, S. and Chatterjee, N. |
Not conform PICO (O): none of the crucial outcomes are reported |
|
Oner, S. O. and Tercan, E. and Boyaci, A. and Velibasoglu, H. and Ersoy, O. and Esmaoglu, A. |
Wrong language: Turkish |
|
Paul, S. and Bhattacharjee, D. P. and Nayek, S. and Chatterjee, N. and Sinha, N. |
Not conform PICO (O): none of the crucial outcomes are reported |
|
Ribeiro Jr, O. D. and de Abreu, L. C. and Valenti, V. E. and Cisternas, J. R. and Saletti, D. and Lima, C. J. B. and Silvestre, D. N. and Godoy, I. R. B. and Mello, L. G. M. and Nascimento, V. B. and Martins, L. C. |
Not conform PICO (O): crucial outcome measures not reported as data; only used as indication for purpose of evaluation of duration of postoperative analgesia |
|
Wheeler, M. and Patel, A. and Suresh, S. and Roth, A. G. and Birmingham, P. K. and Heffner, C. L. and Coté, C. J. |
Not conform PICO (C); bupivacaine and epinephrine |
|
Yildiz, T. S. and Korkmaz, F. and Solak, M. and Toker, K. |
Not conform PICO (O): crucial outcome measures not reported as data; only used as indication for purpose of evaluation of duration of postoperative analgesia |
|
Bock, M. and Kunz, P. and Schreckenberger, R. and Graf, B. M. and Martin, E. and Motsch, J. |
Not conform PICO (O): none of the crucial outcomes are reported |
|
Cucchiaro, G. and Dagher, C. and Baujard, C. and Dubousset, A. M. and Benhamou, D. |
Not conform PICO (I/C): wrong comparison (clonidine vs. morphine) |
|
Fernandes, M. L. and Tibúrcio, M. A. and Pires, K. C. C. and Gomez, R. S. |
Not conform PICO (C): bupivacaine 0.166% with epinephrine 1:600 |
|
Lundblad, Marit and Lonnqvist, Per-Arne |
Wrong study design |
|
Petroheilou, K. and Livanios, S. and Zavras, N. and Hager, J. and Fassoulaki, A. |
Not conform PICO (I/C): wrong comparison (clonidine vs. clonidine plus ropivacaine) and none of the crucial outcomes are reported |
|
Petrova, B. and Gavrillova, N. and Koceva, Sv and Boyadjieva, St and Yaneva, D. |
Not conform PICO (C): Chirocaine, Lidocaine |
|
Sardar, Arijit and Prasad, Ganga and Arora, Mahesh Kumar and Kashyap, Lokesh |
Not conform PICO (I/C): wrong comparison (midazolam and bupivacaine vs. midazolam with oral clonidine and bupivacaine) |
|
Archana, K. N. and Vyshnavi, S. and Ganesh, Vinutha |
Not conform PICO (O): none of the crucial outcomes are reported |
|
Barbero, G. E. and de Miguel, M. and Sierra, P. and Merritt, G. and Bora, P. and Borah, N. and Ciarallo, C. and Ing, R. and Bosenberg, A. and de Nadal, M. |
Not conform PICO (O): none of the crucial outcomes are reported |
|
Batra, Y. K. and Rakesh, S. V. and Panda, N. B. and Lokesh, V. C. and Subramanyam, R. |
Not conform PICO (O): none of the crucial outcomes are reported |
|
Chalkiadis, G. A. and Sommerfield, D. and Low, J. and Orsini, F. and Dowden, S. J. and Tay, M. and Penrose, S. and Pirpiris, M. and Graham, H. K. |
Not conform PICO (I/C): wrong comparison (clonidine vs. fentanyl) |
|
Cucchiaro, G. and Adzick, S. N. and Rose, J. B. and Maxwell, L. and Watcha, M. |
Not conform PICO (I/C): wrong comparisons (bupivacaine + fentanyl, bupivacaine + clonidine, bupivacaine + fentanyl + clonidine) |
|
Disma, N. and Frawley, G. and Mameli, L. and Pistorio, A. and Alberighi, O. D. C. and Montobbio, G. and Tuo, P. |
Not conform PICO (I/C): wrong comparison (Patients were allocated to one of three groups (msexcel |
|
Fonseca, N. M. and De Oliveira, C. A. |
Not conform PICO (P): wrong patient group (16 to 57 years of age) |
|
Giannoni, C. and White, S. and Enneking, F. K. and Morey, T. |
Not conform PICO (I/C): wrong comparison (tonsillar fossae of isotonic sodium chloride, ropivacaine, or ropivacaine plus clonidine prior to tonsil excision) |
|
Hansen, T. G. and Henneberg, S. W. and Walther-Larsen, S. and Lund, J. and Hansen, M. |
Not conform PICO (I/C): wrong comparison (caudal vs. i.v. clonidine) |
|
Ivani, G. and Conio, A. and De Negri, P. and Eksborg, S. and Lönnqvist, P. A. |
Not conform PICO (I/C): wrong comparison a caudal block (ropivacaine 0.2%, 1 ml·kg-1 + clonidine 2 μg·kg-1) vs. an ilioinguinal-iliohypogastric nerve block (ropivacaine 0.2%, 0.4 ml·kg-1 + clonidine 2 μg·kg-1) |
|
Jarraya, A. and Elleuch, S. and Zouari, J. and Smaoui, M. and Laabidi, S. and Kolsi, K. |
Not conform PICO (I/C): wrong comparison (bupivacaine with fentanyl and clonidine vs. bupivacaine with fentanyl) |
|
Khatavkar, S. S. and Lonkar, S. S. and Panchal, P. B. and Thatte, W. S. and Nagendra, S. and Tewari, D. |
Not conform PICO (I/C): wrong comparison (ropivacaine 0.25% plus fentanyl vs. ropivacaine 0.25% plus clonidine) |
|
Sharma, Rohan and Kamal, Geeta and Agarwal, Shilpa and Gupta, Anju and Gupta, Aikta and Kalra, Bhumika |
Not conform PICO (I/C): wrong comparison (different doses of clonidine were compared) |
|
Shukla, B. U. and Prabhakar, T. and Malhotra, K. |
Not conform PICO (I/C): wrong comparison (clonidine vs. fentanyl) |
|
Sinha, Chandni and Kumar, Bindey and Bhadani, Umesh Kumar and Kumar, Ajeet and Kumar, Amarjeet and Ranjan, Alok |
Not conform PICO (I/C): wrong comparison (dexamethasone vs. clonidine) |
|
Vetter, T. R. and Carvallo, D. and Johnson, J. L. and Mazurek, M. S. and Presson Jr, R. G. |
Not conform PICO (I/C): wrong comparison (clonidine vs. hydromorphone vs. morphine) |
|
Wheeler, Melissa and Patel, Arti and Suresh, Santhanam and Roth, Andrew G. and Birmingham, Patrick K. and Heffner, Corri L. and Cote, Charles J. |
Not conform PICO (I/C): wrong comparison (bupivacaine with fresh epinephrine and clonidine vs. bupivacaine with epinephrine) |
|
Kaabachi O, Zerelli Z, Methamem M, Abdelaziz AB, Moncer K, Toumi M. Clonidine administered as adjuvant for bupivacaine in ilioinguinal-iliohypogastric nerve block does not prolong postoperative analgesia. Paediatr Anaesth. 2005 Jul;15(7):586-90. doi: 10.1111/j.1460-9592.2005.01497.x. PMID: 15960643. |
Not conform PICO, wrong outcomes |
|
Cao JP, Miao XY, Liu J, Shi XY. An evaluation of intrathecal bupivacaine combined with intrathecal or intravenous clonidine in children undergoing orthopedic surgery: a randomized double-blinded study. Paediatr Anaesth. 2011 Apr;21(4):399-405. doi: 10.1111/j.1460-9592.2011.03543.x. PMID: 21371167. |
Not conform PICO, intrathecal administration of LA with or without clonidine iv |
Verantwoording
Beoordelingsdatum en geldigheid
Laatst beoordeeld : 17-12-2024
Algemene gegevens
De ontwikkeling/herziening van deze richtlijn werd ondersteund door het Kennisinstituut van de Federatie Medisch Specialisten (www.demedischspecialist.nl/kennisinstituut) en werd gefinancierd uit de Kwaliteitsgelden Medisch Specialisten (SKMS). De financier heeft geen enkele invloed gehad op de inhoud van de richtlijnmodule.
Samenstelling werkgroep
Voor het ontwikkelen van de richtlijn is in 2022 een multidisciplinaire werkgroep ingesteld, bestaande uit vertegenwoordigers van alle relevante specialismen (zie hiervoor de Samenstelling van de werkgroep) die betrokken zijn bij de zorg voor kinderen met postoperatieve pijn.
Werkgroep
Dr. L.M.E. (Lonneke) Staals, anesthesioloog, voorzitter, NVA
Dr. C.M.A. (Caroline) van den Bosch, anesthesioloog-pijnspecialist, NVA
Drs. A.W. (Alinde) Hindriks-Keegstra, anesthesioloog, NVA
Drs. G.A.J. (Geranne) Hopman, anesthesioloog, NVA
Drs. L.J.H. (Lea) van Wersch, anesthesioloog, NVA
Dr. C.M.G. (Claudia) Keyzer-Dekker, kinderchirurg, NVvH
Drs. F.L. (Femke) van Erp Taalman Kip, orthopedisch kinderchirurg, NOV
Dr. L.M.A. (Laurent) Favié, ziekenhuisapotheker, NVZA
J. (Jantine) Boerrigter-van Ginkel, verpleegkundig specialist kinderpijn, V&VN
S. (Sharine) van Rees-Florentina, recovery verpleegkundige, BRV
E.C. (Esen) Doganer en M. (Marjolein) Jager, beleidsmedewerker, Kind & Ziekenhuis
Klankbordgroep
Dr. L.M. (Léon) Putman, cardiothoracaal chirurg, NVT
R. (Remko) ter Riet, MSc, anesthesiemedewerker/physician assistant, NVAM
Drs. L.I.M. (Laura) Meltzer, KNO-arts, NVKNO
Met ondersteuning van
Dr. L.M.P. Wesselman, adviseur, Kennisinstituut van de Federatie Medisch Specialisten
I. van Dijk, junior adviseur, Kennisinstituut van de Federatie Medisch Specialisten
Belangenverklaringen
De Code ter voorkoming van oneigenlijke beïnvloeding door belangenverstrengeling is gevolgd. Alle werkgroepleden hebben schriftelijk verklaard of zij in de laatste drie jaar directe financiële belangen (betrekking bij een commercieel bedrijf, persoonlijke financiële belangen, onderzoeksfinanciering) of indirecte belangen (persoonlijke relaties, reputatiemanagement) hebben gehad. Gedurende de ontwikkeling of herziening van een module worden wijzigingen in belangen aan de voorzitter doorgegeven. De belangenverklaring wordt opnieuw bevestigd tijdens de commentaarfase.
Een overzicht van de belangen van werkgroepleden en het oordeel over het omgaan met eventuele belangen vindt u in onderstaande tabel. De ondertekende belangenverklaringen zijn op te vragen bij het secretariaat van het Kennisinstituut van de Federatie Medisch Specialisten.
|
Werkgroeplid |
Functie |
Nevenfuncties |
Gemelde belangen |
Ondernomen actie |
|
L.M.E. Staals (voorzitter) |
Anesthesioloog Sectorhoofd Kinder- en Obstetrische anesthesiologie Universitair Docent Erasmus MC Sophia Kinderziekenhuis, Rotterdam |
Lid wetenschapcommissie Sectie Kinderanesthesiologie (NVA) (onbetaald) Lid scientific forum ESAIC/Devices abd Technology (onbetaald) Lid werkgroep Landelijke Kwaliteitsregistratie Amandeloperaties (NVKNO/NVA) (onbetaald) |
MSD/ Merck: i.v.m. een clinical trial naar sugammadex bij kinderen: Consultant of Global Clinical Trial Operations in the Netherlands. Betaald (inkomsten gaan op onderzoekskostenplaats van de afdeling Anesthesiologie Erasmus MC Sophia). Dit onderzoek gaat over sugammadex (antagonist voor spierverslapping). Klinisch onderzoek gedaan naar postoperatieve pijnstilling bij kinderen na buikchirurgie, d.m.v. wondcatheter met lokaal anestheticum (nog niet gepubliceerd, daarom niet meegenomen in search van de richtlijn). Er is geen belang bij het advies van de richtlijn. |
Geen restricties |
|
C.M.A. van den Bosch |
Anesthesioloog - pijnspecialist Prinses Maxima Centrum |
Geen |
Geen |
Geen restricties |
|
A.W. Hindriks-Keegstra |
Anesthesioloog UMC Utrecht
|
Geen |
VR ter behandeling van postoperatieve pijn en angst bij kinderen. |
Geen restricties. Extern gefinancierd onderzoek valt buiten bestek van de richtlijn |
|
G.A.J. Hopman |
Anesthesioloog, Radboud UMC, Nijmegen |
Geen |
Geen |
Geen restricties |
|
L.J.H. van Wersch |
Anesthesioloog, Maasziekenhuis Pantein |
Geen |
Geen |
Geen restricties |
|
C.M.G. Keyzer-Dekker |
Kinderchirurg, Erasmus MC Sophia. |
Geen |
Geen |
Geen restricties |
|
F.L. van Erp Taalman Kip |
Orthopedisch kinderchirurg, Erasmus Medisch Centrum Rotterdam |
-Docent Fontys Hogeschool Eindhoven, curriculum kinder- podotherapie -Docent TNO Leiden, onderwijs Jeugdartsen, - Trainer stichting Skills4Comfort |
Geen |
Geen restricties |
|
L.M.A. Favié |
Ziekenhuisapotheker Erasmus MC |
Geen |
Geen |
Geen restricties |
|
J.Boerrigter-van Ginkel |
Verpleegkundig Specialist Kinderpijn, Wilhelmina Ziekenhuis Utrecht. |
Geen |
Geen |
Geen restricties |
|
S. van Rees-Florentina |
Recovery verpleegkundige Flevoziekenhuis Almere |
Bestuurslid BRV BRN Nederland
|
Geen |
Geen restricties |
|
E.C. Doganer |
Stichting Kind&Ziekenhuis Junior Projectmanager/beleidsmedewerker |
Geen |
Geen |
Geen restricties |
|
M. Jager |
Stichting Kind&Ziekenhuis Junior Projectmanager/beleidsmedewerker |
Begeleider C bij Sherpa, betaald |
Geen |
Geen restricties |
|
Klankbordgroeplid |
Functie |
Nevenfuncties |
Gemelde belangen |
Ondernomen actie |
|
L.M. Putman |
Congenitaal cardio-thoracaal chirurg, Leids Universitair Medisch Centrum & Amsterdam UMC, voltijd functie |
Geen |
Geen |
Geen restricties |
|
R. ter Riet |
Anesthesiemedewerker/Physician Assistant Anesthesiologie/Pijngeneeskunde |
Voorzitter NVAM, Voorzitter commissie (acute) pijn NVAM/V&VN |
Geen |
Geen restricties |
|
L.I.M. Meltzer |
Beatrix ziekenhuis Gorinchem, Rivas zorggroep |
Geen |
Geen |
Geen restricties |
Inbreng patiëntenperspectief
Er werd aandacht besteed aan het patiëntenperspectief door zitting van een afgevaardigde van de patiëntenvereniging (Stichting Kind & Ziekenhuis) in de werkgroep. De conceptrichtlijn is tevens voor commentaar voorgelegd aan de Patiëntenfederatie Nederland en Stichting Kind & Ziekenhuis en de eventueel aangeleverde commentaren zijn bekeken en verwerkt.
Wkkgz & Kwalitatieve raming van mogelijke substantiële financiële gevolgen
Kwalitatieve raming van mogelijke financiële gevolgen in het kader van de Wkkgz
Bij de richtlijn is conform de Wet kwaliteit, klachten en geschillen zorg (Wkkgz) een kwalitatieve raming uitgevoerd of de aanbevelingen mogelijk leiden tot substantiële financiële gevolgen. Bij het uitvoeren van deze beoordeling zijn richtlijnmodules op verschillende domeinen getoetst (zie het stroomschema op de Richtlijnendatabase).
Uit de kwalitatieve raming blijkt dat er waarschijnlijk geen substantiële financiële gevolgen zijn.
Werkwijze
AGREE
Deze richtlijnmodule is opgesteld conform de eisen vermeld in het rapport Medisch Specialistische Richtlijnen 2.0 van de adviescommissie Richtlijnen van de Raad Kwaliteit. Dit rapport is gebaseerd op het AGREE II instrument (Appraisal of Guidelines for Research & Evaluation II; Brouwers, 2010).
Knelpuntenanalyse en uitgangsvragen
Tijdens de voorbereidende fase inventariseerde de werkgroep de knelpunten in de zorg voor kinderen met postoperatieve pijn. De werkgroep beoordeelde de aanbevelingen uit de eerdere richtlijn Postoperatieve pijn (NVA, 2013) op noodzaak tot revisie. Het raamwerk van de richtlijn voor volwassenen is ook kritisch bekeken als uitgangspunt. Op basis van de uitkomsten van de knelpuntenanalyse zijn door de werkgroep concept-uitgangsvragen opgesteld en definitief vastgesteld.
Uitkomstmaten
Na het opstellen van de zoekvraag behorende bij de uitgangsvraag inventariseerde de werkgroep welke uitkomstmaten voor de patiënt relevant zijn, waarbij zowel naar gewenste als ongewenste effecten werd gekeken. Hierbij werd een maximum van acht uitkomstmaten gehanteerd. De werkgroep waardeerde deze uitkomstmaten volgens hun relatieve belang bij de besluitvorming rondom aanbevelingen, als cruciaal (kritiek voor de besluitvorming), belangrijk (maar niet cruciaal) en onbelangrijk. Tevens definieerde de werkgroep tenminste voor de cruciale uitkomstmaten welke verschillen zij klinisch (patiënt) relevant vonden.
Methode literatuursamenvatting
Een uitgebreide beschrijving van de strategie voor zoeken en selecteren van literatuur is te vinden onder ‘Zoeken en selecteren’ onder Onderbouwing. Indien mogelijk werd de data uit verschillende studies gepoold in een random-effects model. Review Manager 5.4 werd gebruikt voor de statistische analyses. De beoordeling van de kracht van het wetenschappelijke bewijs wordt hieronder toegelicht.
Beoordelen van de kracht van het wetenschappelijke bewijs
De kracht van het wetenschappelijke bewijs werd bepaald volgens de GRADE-methode. GRADE staat voor ‘Grading Recommendations Assessment, Development and Evaluation’ (zie http://www.gradeworkinggroup.org/). De basisprincipes van de GRADE-methodiek zijn: het benoemen en prioriteren van de klinisch (patiënt) relevante uitkomstmaten, een systematische review per uitkomstmaat, en een beoordeling van de bewijskracht per uitkomstmaat op basis van de acht GRADE-domeinen (domeinen voor downgraden: risk of bias, inconsistentie, indirectheid, imprecisie, en publicatiebias; domeinen voor upgraden: dosis-effect relatie, groot effect, en residuele plausibele confounding).
GRADE onderscheidt vier gradaties voor de kwaliteit van het wetenschappelijk bewijs: hoog, redelijk, laag en zeer laag. Deze gradaties verwijzen naar de mate van zekerheid die er bestaat over de literatuurconclusie, in het bijzonder de mate van zekerheid dat de literatuurconclusie de aanbeveling adequaat ondersteunt (Schünemann, 2013; Hultcrantz, 2017).
|
GRADE |
Definitie |
|
Hoog |
|
|
Redelijk |
|
|
Laag |
|
|
Zeer laag |
|
Bij het beoordelen (graderen) van de kracht van het wetenschappelijk bewijs in richtlijnen volgens de GRADE-methodiek spelen grenzen voor klinische besluitvorming een belangrijke rol (Hultcrantz, 2017). Dit zijn de grenzen die bij overschrijding aanleiding zouden geven tot een aanpassing van de aanbeveling. Om de grenzen voor klinische besluitvorming te bepalen moeten alle relevante uitkomstmaten en overwegingen worden meegewogen. De grenzen voor klinische besluitvorming zijn daarmee niet één op één vergelijkbaar met het minimaal klinisch relevant verschil (Minimal Clinically Important Difference, MCID). Met name in situaties waarin een interventie geen belangrijke nadelen heeft en de kosten relatief laag zijn, kan de grens voor klinische besluitvorming met betrekking tot de effectiviteit van de interventie bij een lagere waarde (dichter bij het nuleffect) liggen dan de MCID (Hultcrantz, 2017).
De beoordelingen van de literatuur en de conclusies zijn gedaan op basis van de GRADE systematiek. De werkgroep vindt het belangrijk om relevante beperkingen hiervan aan te geven.
De klinische vragen in deze richtlijn gaan veelal over een reductie van postoperatieve pijn en opioïdenconsumptie bij een individuele patiënt. Onderzoeken beschrijven de verschillen op groepsniveau, over studies met verschillende patiëntpopulaties en operaties heen. Opioïdenconsumptie is sterk afhankelijk van tijdstip, ingreep en ernst van de pijn. Door het werken met een absolute drempelwaarde in mg (i.p.v. een relatieve drempelwaarde in %) bereiken resultaten gemeten op vroege postoperatieve tijdstippen en in studies met ingrepen met relatief lage opioïdenconsumptie vaak niet de MCID. Daarbij komt ook dat de doelgroep van de huidige richtlijn enorm varieert in lengte en gewicht (van prematuur tot adolescent). Lengte en gewicht heeft grote invloed op het analgetische effect van een specifieke dosering, waardoor alleen kijken naar milligrammen niet volstaat. Waar mogelijk is ook de relatieve reductie in procenten beschreven.
De keuze van de MCID (absoluut verschil in pijnscore of opioïdenconsumptie) heeft een bepaalde mate van willekeurigheid en is niet absoluut te zien. Ook zijn de conclusies zo geformuleerd (en geven alleen beperkt antwoord op het effect op een individuele patiënt voor een specifieke ingreep). In de literatuur worden de eindpunten pijnscores en opioïdenconsumptie separaat van elkaar weer gegeven, suggererend dat deze onafhankelijk van elkaar zijn. Echter kunnen deze twee eindpunten niet onafhankelijk van elkaar beoordeeld worden; in ieder protocol is opgenomen dat pijn behandeld moet worden. Deze separate beoordeling geeft niet altijd een adequaat antwoord op de klinische vraag naar het analgetische effect van een interventie.
Daarnaast worden multimodale componenten als aparte interventies beoordeeld, echter de klinische vraag is naar de effectiviteit als bouwsteen van een multimodale werkwijze.
Voor doseringsadviezen wordt er verwezen naar betrouwbare bronnen, zoals het farmacotherapeutisch kompas of het kinderformularium.
Overwegingen (van bewijs naar aanbeveling)
Om te komen tot een aanbeveling zijn naast (de kwaliteit van) het wetenschappelijke bewijs ook andere aspecten belangrijk en worden meegewogen, zoals aanvullende argumenten uit bijvoorbeeld de biomechanica of fysiologie, waarden en voorkeuren van patiënten, kosten (middelenbeslag), aanvaardbaarheid, haalbaarheid en implementatie. Deze aspecten zijn systematisch vermeld en beoordeeld (gewogen) onder het kopje ‘Overwegingen’ en kunnen (mede) gebaseerd zijn op expert opinion. Hierbij is gebruik gemaakt van een gestructureerd format gebaseerd op het evidence-to-decision framework van de internationale GRADE Working Group (Alonso-Coello, 2016a; Alonso-Coello 2016b). Dit evidence-to-decision framework is een integraal onderdeel van de GRADE methodiek.
Formuleren van aanbevelingen
De aanbevelingen geven antwoord op de uitgangsvraag en zijn gebaseerd op het beschikbare wetenschappelijke bewijs en de belangrijkste overwegingen, en een weging van de gunstige en ongunstige effecten van de relevante interventies. De kracht van het wetenschappelijk bewijs en het gewicht dat door de werkgroep wordt toegekend aan de overwegingen, bepalen samen de sterkte van de aanbeveling. Conform de GRADE-methodiek sluit een lage bewijskracht van conclusies in de systematische literatuuranalyse een sterke aanbeveling niet a priori uit, en zijn bij een hoge bewijskracht ook zwakke aanbevelingen mogelijk (Agoritsas, 2017; Neumann, 2016). De sterkte van de aanbeveling wordt altijd bepaald door weging van alle relevante argumenten tezamen. De werkgroep heeft bij elke aanbeveling opgenomen hoe zij tot de richting en sterkte van de aanbeveling zijn gekomen.
In de GRADE-methodiek wordt onderscheid gemaakt tussen sterke en zwakke (of conditionele) aanbevelingen. De sterkte van een aanbeveling verwijst naar de mate van zekerheid dat de voordelen van de interventie opwegen tegen de nadelen (of vice versa), gezien over het hele spectrum van patiënten waarvoor de aanbeveling is bedoeld. De sterkte van een aanbeveling heeft duidelijke implicaties voor patiënten, behandelaars en beleidsmakers (zie onderstaande tabel). Een aanbeveling is geen dictaat, zelfs een sterke aanbeveling gebaseerd op bewijs van hoge kwaliteit (GRADE gradering HOOG) zal niet altijd van toepassing zijn, onder alle mogelijke omstandigheden en voor elke individuele patiënt.
|
Implicaties van sterke en zwakke aanbevelingen voor verschillende richtlijngebruikers |
||
|
|
||
|
|
Sterke aanbeveling |
Zwakke (conditionele) aanbeveling |
|
Voor patiënten |
De meeste patiënten zouden de aanbevolen interventie of aanpak kiezen en slechts een klein aantal niet. |
Een aanzienlijk deel van de patiënten zouden de aanbevolen interventie of aanpak kiezen, maar veel patiënten ook niet. |
|
Voor behandelaars |
De meeste patiënten zouden de aanbevolen interventie of aanpak moeten ontvangen. |
Er zijn meerdere geschikte interventies of aanpakken. De patiënt moet worden ondersteund bij de keuze voor de interventie of aanpak die het beste aansluit bij zijn of haar waarden en voorkeuren. |
|
Voor beleidsmakers |
De aanbevolen interventie of aanpak kan worden gezien als standaardbeleid. |
Beleidsbepaling vereist uitvoerige discussie met betrokkenheid van veel stakeholders. Er is een grotere kans op lokale beleidsverschillen. |
Organisatie van zorg
In de knelpuntenanalyse en bij de ontwikkeling van de richtlijn is expliciet aandacht geweest voor de organisatie van zorg: alle aspecten die randvoorwaardelijk zijn voor het verlenen van zorg (zoals coördinatie, communicatie, (financiële) middelen, mankracht en infrastructuur). Randvoorwaarden die relevant zijn voor het beantwoorden van deze specifieke uitgangsvraag zijn genoemd bij de overwegingen. Meer algemene, overkoepelende, of bijkomende aspecten van de organisatie van zorg worden behandeld in de module Organisatie van zorg.
Commentaar- en autorisatiefase
De conceptrichtlijn werd aan de betrokken (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd ter commentaar. De commentaren werden verzameld en besproken met de werkgroep. Naar aanleiding van de commentaren werd de conceptrichtlijn aangepast en definitief vastgesteld door de werkgroep. De definitieve richtlijn werd aan de deelnemende (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd voor autorisatie en door hen geautoriseerd dan wel geaccordeerd.
Literatuur
Agoritsas T, Merglen A, Heen AF, Kristiansen A, Neumann I, Brito JP, Brignardello-Petersen R, Alexander PE, Rind DM, Vandvik PO, Guyatt GH. UpToDate adherence to GRADE criteria for strong recommendations: an analytical survey. BMJ Open. 2017 Nov 16;7(11):e018593. doi: 10.1136/bmjopen-2017-018593. PubMed PMID: 29150475; PubMed Central PMCID: PMC5701989.
Alonso-Coello P, Schünemann HJ, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Rada G, Rosenbaum S, Morelli A, Guyatt GH, Oxman AD; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ. 2016 Jun 28;353:i2016. doi: 10.1136/bmj.i2016. PubMed PMID: 27353417.
Alonso-Coello P, Oxman AD, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Vandvik PO, Meerpohl J, Guyatt GH, Schünemann HJ; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 2: Clinical practice guidelines. BMJ. 2016 Jun 30;353:i2089. doi: 10.1136/bmj.i2089. PubMed PMID: 27365494.
Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, Fervers B, Graham ID, Grimshaw J, Hanna SE, Littlejohns P, Makarski J, Zitzelsberger L; AGREE Next Steps Consortium. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010 Dec 14;182(18):E839-42. doi: 10.1503/cmaj.090449. Epub 2010 Jul 5. Review. PubMed PMID: 20603348; PubMed Central PMCID: PMC3001530.
Hultcrantz M, Rind D, Akl EA, Treweek S, Mustafa RA, Iorio A, Alper BS, Meerpohl JJ, Murad MH, Ansari MT, Katikireddi SV, Östlund P, Tranæus S, Christensen R, Gartlehner G, Brozek J, Izcovich A, Schünemann H, Guyatt G. The GRADE Working Group clarifies the construct of certainty of evidence. J Clin Epidemiol. 2017 Jul;87:4-13. doi: 10.1016/j.jclinepi.2017.05.006. Epub 2017 May 18. PubMed PMID: 28529184; PubMed Central PMCID: PMC6542664.
Medisch Specialistische Richtlijnen 2.0 (2012). Adviescommissie Richtlijnen van de Raad Kwalitieit. http://richtlijnendatabase.nl/over_deze_site/over_richtlijnontwikkeling.html
Neumann I, Santesso N, Akl EA, Rind DM, Vandvik PO, Alonso-Coello P, Agoritsas T, Mustafa RA, Alexander PE, Schünemann H, Guyatt GH. A guide for health professionals to interpret and use recommendations in guidelines developed with the GRADE approach. J Clin Epidemiol. 2016 Apr;72:45-55. doi: 10.1016/j.jclinepi.2015.11.017. Epub 2016 Jan 6. Review. PubMed PMID: 26772609.
Schünemann H, Brożek J, Guyatt G, et al. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group, 2013. Available from http://gdt.guidelinedevelopment.org/central_prod/_design/client/handbook/handbook.html.
Zoekverantwoording
Zoekacties zijn opvraagbaar. Neem hiervoor contact op met de Richtlijnendatabase.
