Borstwandblokken bij mammachirurgie
Uitgangsvraag
Welk borstwandblok heeft de voorkeur bij patiënten die mammachirurgie ondergaan?
Aanbeveling
Overweeg PECS II (pectoserratus plane block) en SAP als borstwandblok van voorkeur bij mammachirurgie.
Overwegingen
Voor- en nadelen van de interventie en de kwaliteit van het bewijs
Er is een systematische literatuur analyse uitgevoerd naar de gunstige en ongunstige effecten van verschillende soorten borstwandblokken bij mammachirurgie. Er werd literatuur gevonden voor zes vergelijkingen:
- SAP-blok versus PV-blok;
- ESP-blok versus PV-blok;
- ESP-blok versus SAP-blok;
- PECS II-blok versus PV-blok;
- PECS II-blok versus SAP-blok;
- PECS II-blok versus ESP-blok.
Postoperatieve pijn op 0, 6, 12 en 24 uur na de ingreep was de cruciale uitkomstmaat, en chronische pijn, gebruik van opioïden en complicaties waren belangrijke uitkomstmaten voor klinische besluitvorming.
Voor postoperatieve pijn, de cruciale uitkomstmaat, werd voor slechts enkele van de vergelijkingen een klinisch relevant verschil gevonden: op de PACU was er voordeel voor het PV-blok en op 12 uur een voordeel van het SAP-blok. PECS II geeft in vergelijking met een PV en een SAP-blok minder pijn op de PACU. Voor de overige vergelijkingen op het gebied van postoperatieve pijn werd geen klinisch relevant verschil gevonden in de eerste 24u.
Hieronder staan per vergelijking de belangrijkste conclusies beschreven.
Alle studies hadden methodologische beperkingen, waardoor er mogelijk risico is op vertekening van de studieresultaten (risk of bias) bij de subjectieve uitkomstmaten.
Vergelijking 1: SAP-blok versus PV-blok
Voor postoperatieve pijn werd een klinisch relevant verschil gevonden voor pijn gemeten in PACU in het voordeel van PV-blok en op 12 uur na de ingreep in het voordeel van SAP-blok.
Voor pijn gemeten op 6 uur na de ingreep komt postoperatieve pijn mogelijk in dezelfde mate voor, en voor pijn gemeten op 24 uur na de ingreep was de evidentie van te lage bewijskracht (zeer lage GRADE) om een conclusie te trekken.
Op het gebied van chronische pijn was er voor deze vergelijking geen evidentie.
Het bewijs voor het gebruik van opioïden suggereert een vergelijkbaar gebruik bij toepassing van beide blokken.
Wat betreft de uitkomstmaat complicaties was de evidentie van te lage bewijskracht (zeer lage GRADE) om een conclusie te trekken.
De overall bewijskracht, namelijk de laagste bewijskracht van de cruciale uitkomstmaat, komt uit op zeer laag. De resultaten kunnen daarom geen richting geven aan de besluitvorming over SAP-blok versus PV-blok voor mammachirurgie.
Vergelijking 2: ESP-blok versus PV-blok
Voor postoperatieve pijn 0-1 uur na de ingreep en 24 uur na de ingreep was de literatuur onzeker over het effect van het type blok (zeer lage GRADE). Voor postoperatieve pijn gemeten tussen 4-6 uur en 8-12 uur na de ingreep suggereert de literatuur geen (klinisch relevant) verschil. De overall bewijskracht, namelijk de laagste bewijskracht van de cruciale uitkomstmaten, komt uit op zeer laag en kan geen richting geven aan de besluitvorming over de keuze tussen ESP-blok versus PV-blok voor mammachirurgie.
Op het gebied van chronische pijn en complicaties was er geen evidentie. Voor het gebruik van opioïden suggereert de literatuur geen (klinisch relevant) verschil.
Vergelijking 3: ESP-block versus SAP-blok
Voor de uitkomstmaten postoperatieve pijn (gemeten op 0, 6 , 12 en 24 uur na de ingreep) en gebruik van opioïden was de evidentie van te lage bewijskracht (zeer lage GRADE) om een conclusie te trekken. Op het gebied van belangrijke uitkomstmaat chronische pijn en complicaties was er geen evidentie. De overall bewijskracht, namelijk de laagste bewijskracht van de cruciale uitkomstmaat, komt uit op zeer laag en kan geen richting geven aan de besluitvorming over de keuze tussen ESP-blok versus SAP-blok voor mammachirurgie.
Vergelijking 4: PECS II-blok versus PV-blok
Er werd een klinisch relevant verschil gevonden voor postoperatieve pijn gemeten direct na de ingreep in het voordeel van PECS II. Voor pijn gemeten op 6, 12, en 24 uur na de ingreep suggereert het bewijs geen (klinisch relevant) verschil.
Voor het gebruik van opioïden was de evidentie van te lage bewijskracht (zeer lage GRADE) om een conclusie te trekken. Voor chronische pijn en complicaties werd er geen literatuur gevonden. Deze resultaten kunnen daarom geen richting geven aan de besluitvorming over PECS II- versus PV-blok voor mammachirurgie.
Vergelijking 5: PECS II-blok versus SAP-blok
In een studie werd er een klinisch relevant verschil gevonden voor postoperatieve pijn gemeten direct na de ingreep in het voordeel van PECS II. In de andere studie werd geen klinisch relevant verschil gevonden. Voor pijn gemeten op de overige tijdstippen was de evidentie van te lage bewijskracht (zeer lage GRADE) om een conclusie te trekken. Ook voor chronische pijn, gebruik van opioïden en complicaties was de evidentie van te lage bewijskracht (zeer lage GRADE) om een conclusie te trekken.
Vergelijking 6: PECS II-blok versus ESP-blok
Voor postoperatieve pijn direct na de ingreep was de evidentie van te lage bewijskracht (zeer lage GRADE) om een conclusie te trekken. Het bewijs voor postoperatieve pijn op 6, 12 en 24 uur gemeten na de ingreep suggereert geen verschil in het gebruik van beide blokken. De overall bewijskracht, namelijk de laagste bewijskracht van de cruciale uitkomstmaten, komt uit op zeer laag en kan geen richting geven aan de besluitvorming over de keuze tussen PECS I/ II-blokken versus ESP-blok voor mammachirurgie.
Het bewijs voor het gebruik van opioïden suggereert een vergelijkbaar gebruik bij toepassing van beide blokken. Er is geen klinisch relevant verschil volgens de grenzen bepaald door deze werkgroep voor deze richtlijn. Echter, gezien de lage pijnscores en bekend beperkt morfinegebruik kan een verschil in bijna 5 mg morfine mogelijk toch wijzen op een klinisch relevant verschil tussen de blokken.
Voor complicaties was de evidentie van te lage bewijskracht (zeer lage GRADE) om een conclusie te trekken. Op het gebied van chronische pijn was er geen evidentie.
Samenvattend was de bewijskracht van alle beschreven uitkomstmaten voor alle vergelijkingen laag tot zeer laag (GRADE). Hier ligt een kennislacune. De literatuur kan onvoldoende richting geven aan de besluitvorming. De aanbeveling in deze module is daarom gebaseerd op aanvullende argumenten, waaronder expert opinie, en waar mogelijk aangevuld met literatuur.
Postoperatieve pijn
Er zijn over het algemeen lage pijnscores (gemiddelde/mediaan NRS 2 – 4), waardoor het vinden van een MCID van 1 op een 10-punts schaal of 10 mm op een 100 mm schaal relatief moeilijker. Hoewel niet kan worden voldaan aan de criteria voor een MCID, lijkt er soms enig voordeel te zijn voor een blok. Zo is bijvoorbeeld PECSII in het voordeel ten opzichte PV (6, 12, en 24uur) en ESP (PACU, 6, 12 en 24u). PV is in vergelijking met ESP op PACU, 6 en 24u in het voordeel (12u geen verschil); SAP is enkel op 6 uur mogelijk wat meer in het voordeel ten opzichte van een PV-blok; maar in vergelijking met een ESP-blok, heeft een ESP meer voordeel op 12 uur.
Postoperatief opioïdengebruik
Op het gebied van postoperatief opioïdengebruik werd geen klinisch relevant verschil gevonden voor alle vergelijkingen. Ook hier is het relevant om te vermelden dat het gemiddelde opioïdengebruik relatief laag is (maximaal 19,6 ± 2,7 mg/24uur in 1 studie, veelal <10 mg/24 uur).
Chronische pijn
De RCT van Fuiji is de enige van de 26 geïncludeerde studies die het effect van het blok op chronische pijn heeft geëvalueerd, zij vonden minder patiënten met matige tot ernstige chronische pijn in de groep die een PECS II blok (4/40) hebben gehad in vergelijking met een SAP blok (13/40). Gezien het zeer beperkte aantal uitkomsten over het verschil in chronische pijn tussen de verschillende blokken kunnen hier geen conclusies aan verbonden worden, en ligt hier een kennislacune.
Adverse events
Op het gebied van de adverse events werden geen klinisch relevante verschillen gevonden ofwel kon er geen conclusie aan worden verbonden vanwege te lage bewijskracht.
Blanco beschreef het PECS I in 2011, het PECS II in 2012 en het SAP-blok ESP in 2013 (Blanco, 2011; 2012; 2013). Forero heeft het ESP beschreven in 2016 (Forero, 2016). Hierdoor is er tot op heden nog beperkte literatuur betreffende de vergelijking tussen de verschillende blokken.
Het zijn allen fasciale plane blokken, waarbij een hoog volume moet worden toegediend om een analgetisch effect te krijgen.
- PECS II is een PECS I (n. pectoralis medialis en lateralis, tussen m. pectoralis major en minor) met daarbij een lateralere injectie tussen m. pectoralis minor en m. serratus anterior (laterale en anterieure tak van n. Intercostalis Th2-4). In deze analyse is veelal PECS II onderzocht in vergelijking met andere blokken, bij één vergelijking is PECS I verricht.
- Bij een SAP (tussen de m. latissimus dorsi en m. serratus anterior muscles of onder de m. serratus anterior) zouden de n. intercostobrachialis, de n. cutaneus lateralis vd n. intercostalis (Th 3-9), de n. thoracis longus en de n. thoracodorsalis verdoofd kunnen worden.
- Bij ESP wordt in het plane onder de m. erector spinae lokaal anestheticum toegediend, waarbij sommige studies aantonen dat er spreiding is naar de paravertebrale ruimte en andere naar de dorsale rami van de spinale zenuw.
De spreiding van het lokaal anestheticum is variabel, wat wil zeggen dat bij injectie met eenzelfde volume en op eenzelfde locatie, het verdoofde gebied kan variëren (Yang 2018, Dautzenberg 2019). Dit is tevens aangetoond voor PV-blokkades (Karmaker 2000).
Daarnaast is er bij de geïncludeerde studies verschillende doseringen lokaal anestheticum gegeven, wat het vergelijken onderling bemoeilijkt.
Generaliseerbaarheid
Ondanks dat enkel studies werden geïncludeerd met oncologische mammachirurgie, vallen hier ook verschillende type chirurgie (borstsparende mammachirurgie, mastectomie, met of zonder okselklierdissectie) onder, welke gepaard gaande met variërende ernst postoperatieve pijn. Of resultaten naar niet-oncologische mammachirurgie of andere extra-thoracale ingrepen ge-extrapoleert kunnen worden, wordt niet beantwoord in deze module. Desalniettemin zouden de gevonden resultaten in overweging kunnen worden genomen bij deze ingrepen.
Waarden en voorkeuren van patiënten (en evt. hun verzorgers)
Voor patiënten is het belangrijk om een adequate postoperatieve pijnstilling te hebben, met zo min mogelijk complicaties. De voorkeur gaat ernaar uit om indien mogelijk het gebruik van opioïden te beperken. Hierdoor zal het risico op de bijwerkingen door opioïden afnemen, zoals obstipatie, sufheid en postoperatieve misselijkheid en braken.
Wat betreft postoperatieve pijn, chronische pijn, opioïdengebruik en bijwerkingen zijn er geen klinische relevante verschillen gevonden wat voor een doorslaggevende keuze kan geven voor een bepaald blok.
Bijwerkingen komen niet vaak voor, maar er zijn hierin wel potentiële verschillen te bemerken. Een PV heeft als belangrijkste complicatie een pneumothorax, voor de overige blokken kan dit lokaal anesthetica toxiciteit zijn.
Het PECS- en SAP-blok zijn wellicht wat comfortabeler bij de uitvoer, gezien patiënten tijdens de procedure kunnen blijven liggen; bij een ESP- en PV-blok moet de patiënt ofwel zitten, op de zij of buik draaien.
Belangrijk is om de mogelijkheden te bespreken met de patiënt, gezien er geen duidelijke voorkeur is op basis van de literatuur ten aanzien van het effect op pijn of opioïden, maar er op basis van eventuele bijwerkingen of manier van uitvoeren van de procedure wel een voorkeur kan zijn.
Kosten (middelenbeslag)
De kosten van het plaatsen van een borstwandblok zullen voor ieder borstwandblok gelijk zijn gezien voor iedere blok dezelfde materialen en medicatie nodig is. Ook het scholen van het personeel en de tijdsinvestering zullen voor de verschillende blokken gelijk zijn.
Aanvaardbaarheid, haalbaarheid en implementatie
De borstwandblokken verschillen in het gemak van uitvoer. Er is geen vergelijkende studie naar de leercurve tussen de verschillende blokken, maar van het paravertebraal blok is bekend dat deze technisch moeilijker uitvoerbaar is (Coveney, 1998).
Voor de procedure zijn dezelfde materialen nodig (naald – medicatie – echo). Voor de uitvoer van een PV/ESP-blok moet de patiënt op de buik/zijligging/zitten. Een PECS II/SAP-blok kan liggend worden uitgevoerd, wat comfortabeler kan zijn. Er zijn tot op heden geen evaluaties gedaan naar de verschillende interventies met betrekking tot de implementatie, danwel de haalbaarheid.
Voor het implementeren van een borstwandblok zal er behoudens scholing en het beschikbaar zijn van materialen een korte tijdsinvestering vragen van de operateur, OK-team en anesthesioloog. Indien de anesthesioloog ervaren is in het plaatsen van een borstwandblok zal dit bij de gemiddelde patiënt weinig tijd innemen (< 5 minuten). De aanvaardbaarheid van een borstwandblok zal niet beperkt zijn gezien het de postoperatieve zorg voor de patiënt optimaliseert door het verminderen van de behoefte aan opioïden en mogelijke bijwerkingen hiervan ten opzichte van geen gebruik van een borstwandblok.
Rationale van de aanbeveling: weging van argumenten voor en tegen de interventies
Er werd voor postoperatieve pijn, slechts bij enkele van de vergelijkingen een klinisch relevant verschil gevonden: in de vergelijking tussen PV-blok en SAP-blok, was er op de PACU voordeel voor het PV-blok en op 12 uur een voordeel van het SAP blok. PECS II geeft in vergelijking met een PV-blok en een SAP-blok minder pijn op de PACU.
Er werd geen klinisch relevant verschil aangetoond in postoperatief opioïdgebruik en bijwerkingen tussen de borstwandblokken. Chronische pijn is weinig onderzocht en er is te veel onzekerheid om hier een conclusies aan te verbinden.
Gezien de beperkte beschikbare literatuur is er weinig bewijs om één blok boven het andere blok te stellen. De keuze van het borstwandblok zou gebaseerd moeten zijn op het type chirurgie, de praktische uitvoerbaarheid en tevens de ervaring van de anesthesioloog.
Onderbouwing
Achtergrond
Na oncologische mammachirurgie kan zelfs na een kleinere ingreep matige-ernstige postoperatieve pijn voorkomen, daarnaast is er een hoge prevalentie van chronische pijn variërend van 25-50% (Cregg 2013, Juhl 2016). Een meta-analyse in 2010 (Schnabel 2010) toonde tot 48 uur postoperatief lagere pijnscores en opioïdengebruik aan bij patiënten die mammachirurgie ondergingen en een paravertebraal (PV) blokkade kregen in vergelijking met geen PV-blok. Karmaker toonde in 2014 eenzelfde incidentie maar minder ernstige chronische pijn en minder effect op de kwaliteit van leven bij patiënten die een PV-blokkade hadden gehad. Het toepassen van een locoregionale techniek (borstwandblok) naast algehele anesthesie heeft dus gunstige effecten. Na bovenstaande studies zijn relatief nieuw fasciale-plane technieken onderzocht, zoals het Erector Spinae Plane (ESP), Serratus Anterior Plane (SAP) en PECS I en II blokken (pectoserratus plane blokken). Verschillende reviews die ESP (Huang 2020), SAP (Xie 2021) of PECS (Meißner 2021) onderzochten geven aan dat elke techniek an sich een gunstig effect heeft op postoperatieve pijn, maar het is momenteel onduidelijk welk blok de voorkeur heeft.
Conclusies / Summary of Findings
- Postoperative pain
|
Very low GRADE |
The evidence is very uncertain about the effect of the use of ESP block compared with the use of a PV block for postoperative pain at PACU arrival and 24 hours in adults undergoing breast surgeries.
Source: Gurkan 2020; Swisher 2020; Ghamry 2019 |
|
Low GRADE |
Use of ESP block may result in little to no difference in postoperative pain at 4-6 and 8-12 hours when compared with the use of a PV block in adults undergoing breast surgeries.
Source: Gurkan 2020; Ghamry 2019 |
- Chronic postoperative pain
|
No GRADE |
No evidence was found.
Source: - |
- Opioid consumption
|
Low GRADE |
Use of ESP block may result in little to no difference in postoperative opioid consumption when compared with the use of a PV block in adults undergoing breast surgeries.
Source: Gurkan 2020; Moustafa, 2020; Swisher 2020; Ghamry 2019 |
- Adverse events
|
No GRADE |
No evidence was found.
Source: - |
Comparison 3: Erector spinae plane (ESP) block versus serratus anterior plane (SAP) block
Results
Two studies were included for this comparison (Jiang, 2021; Shrivastava, 2021).
1. Postoperative pain
Jiang (2021) only provided p-values. They reported that postoperative pain scores (NRS, 0-10) in the ESP block group at 0.5 (P < .001), 6 (P = .002), 12 (P = .003) and 24 (P = .026) hours after surgery when patients were active was significantly lower than that in the SAP group. Because no absolute values were reported, no interpretation can be given for the clinical relevance of these results.
1.1. Postoperative pain at PACU arrival
Srivastava (2021) reported no difference in pain scores (VAS, 0-100 mm) between ESP block (n=25) and SAP block (n=25) and therefore this result was not clinically relevant.
1.2. Postoperative pain at 6 hours
Srivastava (2021) reported no difference in pain scores (VAS, 0-100 mm) between ESP block (n=25) and SAP block (n=25) and therefore this result was not clinically relevant.
1.3. Postoperative pain at 12 hours
Srivastava (2021) reported a mean difference of -10 in pain scores (VAS, 0-100 mm) between ESP block (n=25) and SAP block (n=25), in favour of patients who received ESP block. This difference was considered clinically relevant.
1.4. Postoperative pain at 24 hours
Srivastava (2021) reported no difference in pain scores between ESP block (n=25) and SAP block (n=25) and therefore this result was not clinically relevant.
2. Chronic postoperative pain
Not reported.
3. Postoperative opioid consumption
Both Jiang (2021) and Shrivastava (2021) reported postoperative tramadol consumption in 24 hours and were conversed to equianalgesic doses of i.v. morphine for analysis (conversion factor 0.1). In Jiang (2021), the mean difference (MD) of opioid consumption between ESP block (n=30) and SAP block (n=30) was 4.07 (95% CI 2.58 to 5.55) in favour of SAP block (i.e., more opioids required in ESPB group). The difference was considered not clinically relevant.
In Shrivastava (2021), the MD of opioid consumption between ESP block (n=25) and SAP block (n=25) was -0.58 (95% CI -0.84 to -0.32) in favour of ESP block (i.e., more opioids required in SAP group). The difference was considered not clinically relevant.
4. Adverse events
Not reported.
Level of evidence of the literature
The level of evidence for all outcomes under this comparison was based on randomized studies and therefore started at high.
For the outcome postoperative pain at 0, 6, 12 and 24 hours, the level of evidence was downgraded by 3 levels to very low because of study limitations (some concerns/ high risk of bias, -1), and only one small study was included on which a conclusion can be based (imprecision -2).
For the outcome chronic postoperative pain no GRADE could be given, because none of the studies reported on this outcome.
For the outcome postoperative opioid consumption, the level of evidence was downgraded by 3 levels to very low because of study limitations (some concerns risk of bias, -1), variance across studies (inconsistency, -1) and the small sample size (imprecision, -1).
For the outcome adverse events no GRADE could be given, because none of the studies reported on this outcome.
Conclusion
- Postoperative pain
|
Very low GRADE |
The evidence is very uncertain about the effect of the use of ESP block compared with the use of a SAP block for postoperative pain at 0, 6, 12, and 24 hours in adults undergoing breast surgeries.
Source: Shrivastava, 2021 |
- Chronic postoperative pain
|
No GRADE |
No evidence was found.
Source: - |
- Postoperative opioid consumption
|
Very low GRADE |
The evidence is very uncertain about the effect of the use of ESP block compared with the use of a SAP block for postoperative opioid consumption in adults undergoing mastectomies or breast surgeries.
Source: Jiang, 2021; Shrivastava, 2021 |
- Adverse events
|
No GRADE |
No evidence was found.
Source: - |
Comparison 4: PECS II block (pectoserratus plane block) versus paravertebral (PV) block
Results
One systematic review (SR) including seven RCTs (Elshanbary, 2021, including Abo-Sabaa, 2019; Siddeshwara, 2019; Tripathy 2019; Annamalai 2017; Syal & Chandel 2017; Kulhari 2016; Wahba & Kamal 2014) and one additional RCT (Jain, 2020) were included for this comparison.
1. Postoperative pain
1.1. Postoperative pain at PACU arrival
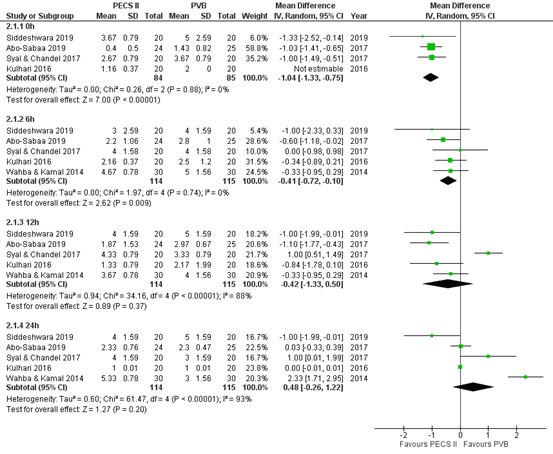
Pain scores at PACU arrival were reported by four RCTs (Abo-Sabaa, 2019; Siddeshwara, 2019; Syal & Chandel 2017; Kulhari 2016). The results are presented in figure 2. The mean difference (MD) of pain scores between PECS II block (n=84) and PV block (n=85) was -1.04 (95% CI -1.33 to -0.75), which is a clinically relevant difference in favour of PECS II block.
1.2. Postoperative pain at 6 hours
Pain scores at 6 hours post-surgery were reported by five RCTs (Abo-Sabaa, 2019; Siddeshwara, 2019; Syal & Chandel 2017; Kulhari 2016; Kulhari 2016). The results are presented in figure 2. The MD of pain scores between PECS II block (n=114) and PV block (n=115) was -0.41 (95% CI -0.72 to -0.10) in favour of PECS II block. This difference is not clinically relevant.
1.3. Postoperative pain at 12 hours
Pain scores at 12 hours post-surgery were reported by five RCTs (Abo-Sabaa, 2019; Siddeshwara, 2019; Syal & Chandel 2017; Kulhari 2016; Kulhari 2016). The results are presented in figure 2. The MD of pain scores between PECS II block (n=114) and PV block (n=115) was -0.42 (95% CI -1.33 to 0.50) in favour of PECS II block. This difference is not clinically relevant.
1.4. Postoperative pain at 24 hours
Pain scores at 24 hours post-surgery were reported by 5 RCTs (Abo-Sabaa, 2019; Siddeshwara, 2019; Syal & Chandel 2017; Kulhari 2016; Kulhari 2016). The results are presented in figure 2. The MD of pain scores between PECS II block (n=114) and PV block (n=115) was 0.48 (95% CI -0.26 to 1.22) in favour of PV block. This difference is not clinically relevant.
The additional study by Jain (2020) only reported very limited information, that postoperative pain scores (VAS, 0-10 cm) were not significantly different among the groups (PECS II versus PVB) in the postoperative 24 hour period. Because only the statistical significance of the results was presented and no absolute values were reported, no interpretation can be given for the clinical relevance of these results.
Figure 2. Postoperative pain at 0h, 6h, 12h and 24h.
Pain in the first 24 hours postoperatively assessed by a 10-point VAS or NRS scale; random effects model; df: degrees of freedom; I2: statistical heterogeneity; CI: confidence interval; Z: p-value of pooled effect. PECS II = pectoralis nerve II block; PVB = paravertebral block
2. Chronic postoperative pain
Not reported.
3. Postoperative opioid consumption
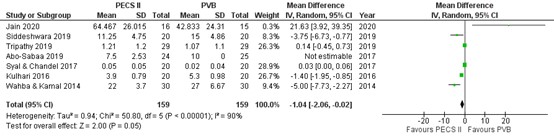
Seven studies reported postoperative use of morphine or fentanyl on which we performed a meta-analysis (Figure 3; Jain, 2020; Siddeshware, 2019; Tripathy, 2019; Abo-Sabaa, 2019; Syal & Chandel, 2017; Kulhari, 2016; Wahba & Kamal, 2014). Opioids were converted into equianalgesic doses of i.v. morphine for analysis (i.v. morphine 10 mg = i.v. fentanyl 100 μg = i.v. sufentanil 10 μg).
The mean difference (MD) of opioid consumption between PECS II block (n=159) and PV block (n=159) was -1.04 (95% CI -2.06 to -0.02) in favour of PECS II block. The difference was considered not clinically relevant.
Figure 3. Total postoperative morphine consumption in the first 24 h after surgery (mg)
Opioids were converted into equianalgesic doses of i.v. morphine for analysis (i.v. morphine 10 mg = i.v. fentanyl 100 μg = i.v. sufentanil 10 μg); random effects model; df: degrees of freedom; I2: statistical heterogeneity; CI: confidence interval; Z: p-value of pooled effect. PECS II = pectoralis nerve II block; PVB = paravertebral block.
- Adverse events
Jain (2020) reported that no complications such as inadvertent vascular puncture, pneumothorax, or hypotension occurred in any group (n PECS II = 15; n PVB = 15). This risk difference of 0 is considered not clinically relevant.
Level of evidence of the literature
The level of evidence for all outcomes under this comparison was based on randomized studies and therefore started at high.
For the outcome postoperative pain at PACU arrival, the level of evidence was downgraded by 2 levels to low because of study limitations (some concerns / high risk of bias, -1), and the sample size was small (imprecision, -1).
For the outcome postoperative pain at 6 hours, the level of evidence was
downgraded by 2 levels to low because of study limitations (some concerns / high risk of bias, -1), and the confidence interval crossing the clinical decision threshold (imprecision, -1).
For the outcome postoperative pain at 12 and 24 hours, the level of evidence was downgraded by 3 levels to very low because of study limitations (some concerns / high risk of bias, -1), variance across studies indicated by the variance of point estimates in different directions of the effect across studies (inconsistency, -1), and the confidence intervals crossing the clinical decision threshold (imprecision, -1).
For the outcome chronic pain, no GRADE could be given, because none of the studies reported on this outcome.
For the outcome postoperative opioid consumption, the level of evidence was downgraded by 3 levels to very low because of study limitations (some concerns / high risk of bias, -1), wide variance of point estimates across studies (inconsistency, -1), and the confidence intervals crossing the clinical decision threshold (imprecision, -1).
For the outcome adverse events, the level of evidence was downgraded by 3 levels to very low because it was a study with high risk of bias (-1), and only one study was included in which no events occurred (imprecision -2).
Conclusion
- Postoperative pain
|
Low GRADE |
Use of PECS II block (pectoserratus plane block) may result in less postoperative pain at PACU arrival when compared with the use of a PV block in adults undergoing mastectomy.
Use of PECS II block (pectoserratus plane block) may result in no to little difference in postoperative pain at 6, 12 and 24 hours when compared with the use of a PV block in adults undergoing mastectomy.
Source: Abo-Sabaa, 2019; Siddeshwara, 2019; Syal & Chandel 2017; Kulhari, 2016; Wahba & Kamal, 2014 |
- Chronic postoperative pain
|
No GRADE |
No evidence was found.
Source: - |
- Postoperative opioid consumption
|
Very low GRADE |
The evidence is very uncertain about the effect of the use of PECS II block (pectoserratus plane block) compared with the use of a PV block for postoperative opioid consumption in adults undergoing mastectomies.
Source: Jain, 2020; Abo-Sabaa, 2019; Siddeshwara, 2019; Tripathy, 2019; Syal & Chandel, 2017; Kulhari, 2016; Wahba & Kamal, 2014 |
- Adverse events
|
Very low GRADE |
The evidence is very uncertain about the effect of the use of PECS II block (pectoserratus plane block) compared with the use of a PV block for adverse events in adults undergoing mastectomies.
Source: Jain, 2020 |
Comparison 5: PECS II block (pectoserratus plane block) versus serratus anterior plane (SAP) block
Results
Three RCTs were included (Bakeer, 2020; Jain, 2020; Fujii, 2019).
1. Postoperative pain
Jain (2020) reported very limited information, that postoperative pain scores (VAS, 0-10cm) were not significantly different among the groups (PECS II block versus SAP block) in the postoperative 24-hour period. Because only the statistical significance of the results was presented, and no absolute values were reported, no interpretation can be given for the clinical relevance of these results.
1.1. Postoperative pain at PACU arrival
Pain scores at PACU arrival were reported by two RCTs (Bakeer, 2020; Fujii, 2019, Table 6). Bakeer (2020) reported no difference in pain scores in rest between PECS II block (n=60) and SAP block (n=60) and therefore this result was not clinically relevant. The study reported a median difference of -1 in pain scores during movement between PECS II block (n=60) and SAP block (n=60), in favour of PECS II block. This difference was considered clinically relevant.
Fujii (2019) reported a median difference of -5 in pain scores between PECS II block (n=40) and SAP block (n=40), in favour of PECS II block. This difference was considered not clinically relevant.
Table 6. Post operative pain scores at PACU arrival.
|
Study |
Pain |
PECS II block |
n |
SAP block |
n |
Median difference |
In favour of |
Clinically relevant? |
|
Bakeer (2020) |
VAS (0-10 cm) during rest, in median (range) |
0 (0–3) |
60 |
0 (0–2) |
60 |
0 |
None |
n.a. |
|
|
VAS (0-10 cm) during movement, in median (range) |
0 (0–3) |
60 |
1 (0–3) |
60 |
-1 |
PECS II block |
Yes |
|
Fujii (2019) |
VAS (0-100mm) in median (IQR) |
18 (11–27) |
40 |
23 (11–35) |
40 |
-5 |
None |
No |
IQR=interquartile range
1.2. Postoperative pain at 6 hours
Not reported.
1.3. Postoperative pain at 12 hours
Bakeer (2020) reported no difference in pain scores (VAS, 0-10 cm) in rest or during movement between PECS II block (n=60) and SAP block (n=60) and therefore this result was not clinically relevant.
1.4. Postoperative pain at 24 hours
Bakeer (2020) reported no difference in pain scores (VAS, 0-10 cm) in rest or during movement between PECS II block (n=60) and SAP block (n=60) and therefore this result was not clinically relevant.
2. Chronic pain
One RCT reported on chronic pain. Fuiji (2019) reported chronic pain scores, scored on a 11-point numerical rating scale (NRS), defined as: 1–3 mild pain; 4–6 moderate pain; and 7–10 severe pain. The number of patients with moderate or severe chronic pain was lower in the PECS II group (4 of 40, 10%) compared to patients with moderate or severe chronic pain was lower in the SPB group (13 of 40, 33%) (RR=0.31 95% CI 0.11 to 0.86). This difference was considered clinically relevant.
3. Postoperative opioid consumption
Two RCTs reported on opioid consumption (Bakeer, 2020; Jain, 2020). Bakeer (2020) reported that those patients who requested morphine in the PECS and SAP groups consumed a single dose of 2 mg. Therefore, there is no clinically relevant difference in mean opioid consumption between the groups (risk difference = 0).
For the study of Jain (2020) opioids were converted into equianalgesic doses of i.v. morphine for analysis (i.v. morphine 10 mg = i.v. fentanyl 100 μg = i.v. sufentanil 10 μg). The mean difference of opioid consumption between PECS II block (n=16) and SAP block (n=15) was 22.97 (95% CI 7.23 to 38.71) in favour of SAP block. The difference was considered clinically relevant.
4. Adverse events
Two RCTs reported on adverse events (Bakeer, 2020; Jain, 2020). Bakeer (2020) reported that no cases of pneumothorax, local anesthetic toxicity, or opioid side effects as respiratory depression, pruritus, or urinary retention were recorded in any group (n PECSS II = 60; n SAP = 60). This risk difference of 0 is considered not clinically relevant.
Jain (2020) reported that no complications like inadvertent vascular puncture, pneumothorax, or hypotension occurred in any group (n PECSS II = 15; n SAP = 15). This risk difference of 0 is considered not clinically relevant.
Level of evidence of the literature
The level of evidence for all outcomes under this comparison was based on randomized studies and therefore starts at high.
For the outcome postoperative pain at PACU, the level of evidence was downgraded by 2 levels to low because of study limitations (some concerns risk of bias, -1), and small studies (imprecision -1).
For the outcome postoperative pain at 12h and 24h, the level of evidence was downgraded by 3 levels to very low because of study limitations (some concerns/ high risk of bias, -1), and only one study was included on which a conclusion can be based (imprecision -2).
For the outcome chronic postoperative pain, the level of evidence was downgraded by 3 levels to very low because of study limitations (some concerns risk of bias, -1), and only one small study was included on which a conclusion can be based (imprecision -2).
For the outcome postoperative opioid consumption, the level of evidence was downgraded by 3 levels to very low because of study limitations (high risk of bias, -1), variance across studies (inconsistency, -1) and the small sample size (imprecision, -1).
For the outcome adverse events, the level of evidence was downgraded by 3 levels to very low because it was a study with high risk of bias (-1), and only two small studies were included in which no events occurred (imprecision -2).
Conclusion
- Postoperative pain
|
Low GRADE |
The literature is inconclusive about the effect of PECS II block (pectoserratus plane block) versus SAP block on postoperative pain at PACU in adults undergoing mastectomies.
Source: Bakeer, 2020; Fuiji, 2019 |
|
Very low GRADE |
The evidence is very uncertain about the effect of the use of PECS II block (pectoserratus plane block) compared with the use of a SAP block for postoperative pain at 0-1 and 24 hours in adults undergoing mastectomies.
Source: Bakeer, 2020 |
- Chronic postoperative pain
|
Very low GRADE |
The evidence is very uncertain about the effect of the use of PECS II block (pectoserratus plane block) compared with the use of a SAP block for chronic postoperative pain in adults undergoing mastectomies.
Source: Fuiji, 2019 |
- Postoperative opioid consumption
|
Very low GRADE |
The evidence is very uncertain about the effect of the use of PECS II block (pectoserratus plane block) compared with the use of a SAP block for postoperative opioid consumption in adults undergoing mastectomies.
Source: Bakeer, 2020; Jain, 2020 |
- Adverse events
|
Very low GRADE |
The evidence is very uncertain about the effect of the use of PECS II block (pectoserratus plane block) compared with the use of a SAP block for adverse events in adults undergoing mastectomies.
Source: Bakeer, 2020; Jain, 2020 |
Comparison 6: PECS II block (pectoserratus plane block) versus erector spinae plane (ESP) block
Results
One systematic review (SR) with three RCTs (Elshanbary 2021, including Altiparmak, 2018; Gad, 2019; Sinha, 2019) was included.
1. Postoperative pain
1.1. Postoperative pain at PACU arrival
Not reported.
1.2. Postoperative pain at 6 hours
Gad (2019) reported a mean difference (MD) of pain scores between PECS II block (n=23) and ESP block (n=24) of -0.25 (95% CI -0.61 to 0.11) in favour of PECS II block.
Sinha (2019) reported a mean difference (MD) of pain scores between PECS II block (n=30) and ESP block (n=30) of -0.57 (95% CI -0.97 to -0.17) in favour of PECS II block. These differences were considered not clinically relevant.
1.3. Postoperative pain at 12 hours
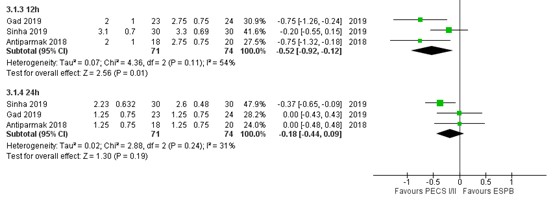
Pain scores at 12 hours post-surgery were reported by three RCTs (Gad, 2019; Sinha, 2019; Altiparmak, 2018), as outlined in figure 4. The MD of pain scores between PECS II block (n=71) and ESP block (n=74) was -0.52 (95% CI -0.92 to -0.12) in favour of PECS II block. This difference was considered not clinically relevant.
1.4. Postoperative pain at 24 hours
Pain scores at 24 hours post-surgery were reported by three RCTs (Gad, 2019; Sinha, 2019; Altiparmak, 2018), as outlined in figure 4. The MD of pain scores between PECS II block (n=71) and ESP block (n=74) was -0.18 (95% CI -0.44 to 0.09) in favour of PECS II block. This difference was considered not clinically relevant.
Figure 4. Postoperative pain at 12h and 24h.
Pain in the first 24 hours postoperatively assessed by VAS or NRS scale, transformed to a 10-point scale for comparison; Random effects model; df: degrees of freedom; I2: statistical heterogeneity; CI: confidence interval; Z: p-value of pooled effect. PECS = pectoralis nerve block; ESPN = erector spinae plane block.
2. Chronic postoperative pain
Not reported.
3. Postoperative opioid consumption
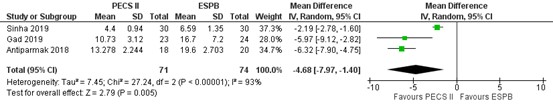
All three studies of the SR reported postoperative opioid use of morphine or tramadol in the first 24 hours after surgery, as outlined in figure 5 (Sinha, 2019; Gad, 2018; Antiparmak, 2018). Opioids were conversed to equianalgesic doses of i.v. morphine for analysis (conversion factor 0.1). The mean difference of opioid consumption between PECS II block (n=71) and ESP block (n=74) was -4.68 (95% CI -7.90 to -1.40) in favour of PECS II block (i.e., more opioids required in ESPB group). The difference was considered not clinically relevant.
Figure 5. Postoperative morphine consumption (mg).
Opioids were converted to into equianalgesic doses of i.v. morphine for comparison (conversion factor 0.1); random effects model; df: degrees of freedom; I2: statistical heterogeneity; CI: confidence interval; Z: p-value of pooled effect. PECS = pectoralis nerve block; ESPN = erector spinae plane block.
4. Adverse events
Not reported.
Level of evidence of the literature
The level of evidence for all outcomes under this comparison was based on randomized studies and therefore starts at high.
For the outcome postoperative pain at PACU arrival no GRADE could be given, because none of the studies reported on this outcome.
For the outcome postoperative pain at 6, 12 and 24 hours, the level of evidence was downgraded by 2 levels to low because of study limitations (some concerns risk of bias, -1) and the confidence intervals crossing the clinical decision threshold (imprecision, -1).
For the outcome chronic postoperative pain, no GRADE could be given, because none of the studies reported on this outcome.
For the outcome postoperative opioid consumption, the level of evidence was downgraded by 2 levels to low because of study limitations (some concerns risk of bias, -1), and the sample size was small (imprecision, -1).
For the outcome adverse events no GRADE could be given, because none of the studies reported on this outcome.
Conclusion
- Postoperative pain
|
No GRADE |
No evidence was found for postoperative pain at PACU arrival.
Source: - |
|
Low GRADE |
Use of PECS II block (pectoserratus plane block) may result in little to no difference in postoperative pain at 6, 12 and 24 hours when compared with the use of a ESP block in adults undergoing mastectomy.
Source: Altiparmak, 2018; Gad, 2019; Sinha, 2019 |
- Chronic postoperative pain
|
No GRADE |
No evidence was found.
Source: - |
- Postoperative opioid consumption
|
Low GRADE |
Use of PECS II block (pectoserratus plane block) may result in little to no difference in postoperative opioid consumption when compared with the use of a ESP block in adults undergoing mastectomy.
Source: Altiparmak, 2018; Gad, 2019; Sinha, 2019 |
- Adverse events
|
No GRADE |
No evidence was found.
Source: - |
Samenvatting literatuur
Description of studies
The main study characteristics of all included randomized controlled trials (RCTs) are outlined in table 2. As shown in the table, studies included various surgical procedures, different thoracic wall block solutions and follow-up time.
The systematic review and meta-analysis by Elshanbary (2021) compared PECS II block methods with other blocks in patients undergoing breast cancer surgeries. The review included 47 studies, with the search last updated in July 2020. Seven RCTs that were in line with PICO 2 (i.e., comparison of PECS II block and PV block) and were included in the current analysis (Abo-Sabaa 2019; Siddeshwara 2019; Tripathy 2019; Annamalai 2017; Syal & Chandel 2017; Kulhari 2016; Wahba & Kamal 2014). The seven RCTs were rated with a moderate to low risk of bias by the authors of the systematic review. Three other RCTs were in line with the PICO 3 (i.e., comparison of PECS II block (pectoserratus plane block) and ESP block) and were included in the current analysis (Antiparkmak, 2018; Gad, 2019; Sinha, 2019). The three RCTs were rated with a moderate to low risk of bias by the authors of the systematic review.
The systematic review and meta-analysis by Xiong (2021) assessed the postoperative analgesic effects of ESP block and PV block for thoracic and breast surgery (i.e., PICO 4). The systematic review included 10 RCTs, resulting from a literature search up to April 5, 2021. Four RCTs were included in the current analysis (Gurkan, 2020; Moustafa, 2020; Swisher, 2020; Ghamry, 2019). The included studies were all rated with a high risk of bias by the authors of the systematic review.
Table 2. Characteristics of included studies.
|
Author, year
|
N (I/C) |
Surgical procedure |
Intervention |
Control |
Follow-up |
|
Comparison 1: SAP block versus PV block |
SAP block |
PV block |
|
||
|
Arora, 2021 |
20/20 |
Total mastectomy |
3-5 ml lignocaine 2%; 0.4 mL.kg-1 ropivacaine 0.5% |
3-5 ml lignocaine 2%; 0.4 mL.kg-1 ropivacaine 0.5% |
24h |
|
Gabriel, 2021 |
49/51 |
Non-mastectomy (unilateral or bilateral) |
20 ml (unilateral) or 16 ml (bilateral) epinephrine 1:400 000 |
20 ml (unilateral) or 16 ml (bilateral) epinephrine 1:400 000 |
POD 1 (morning) |
|
Jain, 2020 |
15/15 |
Unilateral mastectomy |
ropivacaine 0.375% |
ropivacaine 0.375% |
24h |
|
Amin, 2018 |
30/30 |
Unilateral mastectomy |
0.4 ml/kg of bupivacaine 0.25% with 5 μg/ml epinephrine |
15–20 ml of 0.25% bupivacaine with 5 μg/ml epinephrine |
3 days |
|
Gupta, 2017 |
25/25 |
Modified radical mastectomy with axillary dissection |
20 ml bupivacaine 0.5% |
20 ml bupivacaine 0.5% |
72h |
|
Comparison 2: ESP block versus PV block |
ESP block |
PV block |
|
||
|
Xiong, 2021: |
|
|
|
|
|
|
Gurkan, 2020 |
25/25 |
Breast surgery |
20 ml bupivacaine 0.25% |
20 ml bupivacaine 0.25% |
24h |
|
Moustafa, 2020 |
45/45 |
Breast surgery |
20 ml bupivacaine 0.25% |
20 ml bupivacaine 0.25% |
24h |
|
Swisher, 2020 |
50/50 |
Breast surgery |
25 ml ropivacaine unilateral 0.5% |
25 ml ropivacaine unilateral 0.5% |
24h |
|
Ghamry, 2019 |
35/35 |
Breast surgery |
20 ml bupivacaine 0.25% |
20 ml bupivacaine 0.25% |
24h |
|
Comparison 3: ESP block versus SAP block |
ESP block |
SAP block |
|
||
|
Jiang, 2021 |
30/30 |
Modified radical mastectomy |
20 ml ropivacaine 0.5% |
20 ml ropivacaine 0.5% |
24h |
|
Shrivastava, 2021 |
25/25 |
Breast surgery |
25 ml bupivacaine 0.25% |
25 ml bupivacaine 0.25% |
24h |
|
Comparison 4: PECS II block versus PV block |
PECS II block |
PV block |
|
||
|
Jain, 2020 |
16/15 |
Elective unilateral mastectomies |
0.375% ropivacaine |
0.375% ropivacaine |
24h |
|
Elshanbary, 2021: |
|
|
|
|
|
|
Abo-Sabaa, 2019 |
24/25 |
Modified radical mastectomy, lumpectomy, simple mastectomy, axillary lymph node dissection |
20 ml bupivacaine 0.25%; 10 ml bupivacaine 0.25% |
20 ml bupivacaine 0.25% |
24h |
|
Siddeshwara, 2019 |
20/20 |
Modified radical mastectomy |
15 ml mixture of levobupivacaine 0.25% and dexamethasone; 10 ml mixture of levobupivacaine 0.25% and dexamethasone |
25 ml levobupivacaine 0.25% and dexamethasone |
24h |
|
Tripathy, 2019 |
29/29 |
Modified radical mastectomy with axillary lymph node dissection |
30 ml lignocaine 0.1%; bupivacaine 0.25%; 1 μg/kg dexmedetomidine
|
30 ml lignocaine 0.1%; bupivacaine 0.25%; 1 μg/kg dexmedetomidine
|
24h |
|
Annamalai, 2017 |
30/30 |
Modified radical mastectomy |
- |
- |
24h |
|
Syal & Chandel 2017 |
20/20 |
Modified radical mastectomy |
10 ml bupivacaine (50 mg) 0.5% with 0.5 ml adrenaline 1:10000 (50 μg); 10 ml bupivacaine 0.5% (50 mg) with 0.5 ml adrenaline 1:10000 (50 μg) |
20 ml bupivacaine 0.5%; 1 ml adrenaline 1:10000 [100 μg]
|
24h |
|
Kulhari, 2016 |
20/20 |
Modified radical mastectomy |
25 ml ropivacaine 0.5%
|
25 ml ropivacaine 0.5% |
24h |
|
Wahba & Kamal 2014 |
30/30 |
Modified radical mastectomy |
10 ml levobupivacaine 0.25%; 20 ml levobubivacaine 0.25% |
15–20 ml levobupivacaine 0.25% |
24h |
|
Comparison 5: PECS II block versus SAP block |
PECS II block |
SAP block |
|
||
|
Bakeer, 2020 |
60/60
|
Unilateral modified radical mastectomy |
30 ml bupivacaine 0.25% |
30 ml bupivacaine 0.25%
|
24h |
|
Jain, 2020 |
16/15 |
Elective unilateral mastectomies with or without axillary dissection |
30 ml ropivacaine 0.375% |
30 ml ropivacaine 0.375% |
24h |
|
Fujii, 2019 |
40/40 |
mastectomy |
10 ml ropivacaine 0.5%; 20 ml ropivacaine 0.5% |
30 ml ropivacaine 0.5% |
6 months (Chronic pain) |
|
Comparison 6: PECS II block versus ESP block |
PECS II block |
ESP block |
|
||
|
Elshanbary, 2021: |
|
|
|
|
|
|
Altiparmak, 2018 |
18/20 |
Breast cancer |
10 ml bupivacaine 0.25%; 20 ml bupivacaine 0.25% |
2-3 ml isotonic saline solution; 20 ml bupivacaine |
24h |
|
Gad, 2019 |
23/24 |
Breast cancer |
10 ml +20 ml levobupivacaine 0.25% + 0.5 μ/kg dexmedetomidine |
20 ml levobupivacaine 0.25% + 0.5 μ/kg dexmedetomidine |
24h |
|
Sinha, 2019 |
30/30 |
Breast cancer |
2 ml lignocaine 2%; 15 ml ropivacaine 0.2%; 10 ml ropivacaine 0.2% |
2 ml lignocaine 2%; 20 ml ropivacaine 0.2% |
24h |
ESP = erector spinae plane; PV = paravertebral; PECS = pectoralis nerve; SAP = serratus anterior plane; USG = ultrasound guided; h = hours
Comparison 1: Serratus Anterior Plane (SAP) block versus paravertebral (PV) block
Results
Five RCTs were included for this comparison (Arora, 2022; Gabriel, 2021; Jain, 2020; Amin, 2018; Gupta, 2017).
- Postoperative pain
All five studies reported postoperative pain up to 24 hours, assessed by VAS or NRS scales. Jain (2020) reported very limited information, that postoperative pain scores (VAS, 0-10cm) were not significantly different among the groups (SAP block versus PV block) in the postoperative 24 hour period. Because only the statistical significance of the results was presented and no absolute values were reported, no interpretation can be given for the clinical relevance of these results.
1.1 Postoperative pain at PACU arrival
Pain scores at PACU arrival were reported by three RCTs (Arora, 2022; Gabriel, 2021; Amin, 2018). Arora (2022) reported that postoperative pain scores (NRS, 0-10) were significantly lower in the SAP block group as compared to the PV block group during (p=0.007). Because only statistical p-values were given and no absolute values were reported, no interpretation can be given for the clinical relevance of these results. The median differences reported by Gabriel (2021) and Amin (2018) are presented in Table 2 and were considered clinically relevant in favour of PV block in both studies.
Table 2. Postoperative pain scores at PACU arrival, median in cm.
|
study |
Pain |
SAP block |
n |
PV block |
n |
95%CI |
Median difference |
In favour of |
Clinically relevant? |
|
Gabriel (2021) |
NRS-scores (0-10) in median (IQR) |
4.0 (0-5.5) |
49 |
0 (0-3.0) |
51 |
−3.00 to −0.00 |
4.0 |
PV block |
Yes |
|
Amin (2018) |
VAS (0-10 cm) during rest, in median (range) |
1 (0-4) |
30 |
0 (0-4) |
30 |
- |
1 |
PV block |
Yes |
|
VAS (0-10 cm) during movement, in median (range) |
1 (0-4) |
30 |
0 (0-4) |
30 |
- |
1 |
PV block |
Yes |
IQR=interquartile range
1.2 Postoperative pain at 6 hours
Pain scores at 6 hours post-surgery were reported by two RCT (Arora, 2022; Gupta, 2017). Arora (2022) reported that postoperative pain scores (NRS, 0-10) were significantly lower in the SAP block group as compared to the PV block group (p=0.040). Because only statistical p-values were given and no absolute values were reported, no interpretation can be given for the clinical relevance of these results. The mean difference reported in the study by Gupta (2017) (-0.16 in favour of SAP block) was not considered clinically relevant (see table 3).
Table 3. Postoperative pain scores at 6 hours, mean in cm (SD not reported).
|
Study |
Pain |
SAP block |
n |
PV block |
n |
Mean difference |
In favour of |
Clinically relevant? |
|
Gupta (2017) |
VAS (0-100mm) |
3.2 |
25 |
3.36 |
25 |
-0.16 |
SAP block |
No |
1.3 Postoperative pain at 12 hours
Pain scores at 12 hours post-surgery were reported by one RCT (Amin, 2018). The difference in pain scores in rest and movement were clinically relevant in favour of SAP block.
Table 4. Postoperative pain scores at 12 hours, median (range) in cm (SD not reported).
|
Study |
Pain |
SAP block |
n |
PV block |
n |
Median difference |
In favour of |
Clinically relevant? |
|
Amin (2018) |
VAS (0-10 cm) during rest |
2 (0-4) |
30 |
3 (0-5) |
30 |
-1 |
SAP block |
Yes |
|
VAS (0-10 cm) during movement |
2 (0-4) |
30 |
3 (1-6) |
30 |
-1 |
SAP block |
Yes |
1.4 Postoperative pain at 24 hours
Pain scores at 24 hours post-surgery were reported by four RCTs (Arora, 2022; Gabriel, 2021; Amin, 2018; Gupta, 2017). Arora (2022) reported that postoperative pain scores (NRS, 0-10) were significantly lower in the SAP block group as compared to the PV block group (p=0.032). Because only statistical p-values were given and no absolute values were reported, no interpretation can be given for the clinical relevance of these results.
The median differences of 1.0 between SAP block and PV block in the study by Gabriel (2021) was considered clinically relevant in favour of PV block. The mean difference of -1 between SAP and PV block during movement in the study by Amin (2018) was considered clinically relevant in favour of SAP block. The pain scores reported by the studies Gupta (2017) (median difference -0.56) and Amin (2018) for pain in rest (mean difference 0), were considered not clinically relevant.
Table 5. Postoperative pain scores at 24 hours, in median and means.
|
Study |
Pain |
SAP block |
n |
PV block |
n |
Median difference/ mean difference |
In favour of |
Clinically relevant? |
|
Gabriel (2021) |
NRS-scores (0-10) in median (IQR) |
3.0 (2.0-4.0) |
49 |
2.0 (0.5-3.0) |
51 |
1.0 |
PV block |
Yes |
|
Amin (2018) |
VAS (0-10 cm) during rest, in median (range) |
3 (0-5) |
30 |
3 (0-5) |
30 |
0 |
None |
n.a. |
|
VAS (0-10 cm) during movement, in median (range) |
3 (0-6) |
30 |
4 (0-6) |
30 |
-1 |
SAP block |
Yes |
|
|
Gupta (2017) |
VAS (0-100mm) |
2.48 |
25 |
3.04 |
25 |
-0.56 |
SAP block |
No |
2. Chronic postoperative pain
Not reported.
3. Postoperative opioid consumption
Four studies reported postoperative use of morphine or fentanyl on which we performed a meta-analysis (figure 1; Gabriel, 2021; Jain, 2020; Amin, 2018; Gupta, 2017). Opioids were converted into equianalgesic doses of i.v. morphine for analysis (i.v. morphine 10 mg = i.v. fentanyl 100 μg = i.v. sufentanil 10 μg). For the study of Gabriel (2021), means and standard deviation (SD) were estimated from the medians and IQRs using the method by Hozo (2005).
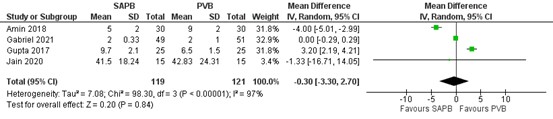
The mean difference in morphine i.v. between SAP block (n=119) and PV block (n=121) was -0.30 mg in favour of SAP block (95% CI -3.30 to 2.70). This difference was not considered clinically relevant.
Figure 1. Postoperative morphine consumption (mg)
Random effects model; df: degrees of freedom; I2: statistical heterogeneity; CI: confidence interval; Z: p-value of pooled effect. SAPB = serratus anterior plane block; PVB = paravertebral block
4. Adverse events
One RCT reported on adverse events (Jain, 2020). Jain (2020) reported that no complications like inadvertent vascular puncture, pneumothorax, or hypotension occurred in any group (n SAP block = 15; n PV block = 15).
Level of evidence of the literature
The level of evidence for all outcomes under this comparison was based on randomized studies and therefore started at high.
For the outcome postoperative pain at PACU arrival, the level of evidence was downgraded by two levels to low because of study limitations (some concerns / high risk of bias, -1), and the confidence intervals crossing the clinical decision threshold (imprecision, -1).
For the outcome postoperative pain at 6 hours and 12 hours, the level of evidence was downgraded by two levels to low because of study limitations (some concerns / high risk of bias, -1) and only one study with a small sample was included per time point (imprecision, -1).
For the outcome postoperative pain at 24 hours, the level of evidence was downgraded by three levels to very low because of study limitations (some concerns / high risk of bias, -1), variance in direction of effect across the studies (inconsistency, -1) and only two studies with small samples were included (imprecision, -1).
For the outcome chronic postoperative pain no GRADE could be given, because none of the studies reported on this outcome.
For the outcome postoperative opioid consumption, the level of evidence was downgraded by two levels to low because of study limitations (some concerns / high risk of bias, -1) and inconsistency of results indicated by wide variance of point estimates across studies with minimal overlap (inconsistency, -1).
For the outcome adverse events, the level of evidence was downgraded by three levels to very low because it was a study with high risk of bias (-1), and only one study was included in which no events occurred and therefore the outcome of interest was not met (imprecision -2).
Zoeken en selecteren
A systematic review of the literature was performed to answer the following question:
What are the favourable and unfavourable effects of various thoracic wall blocks in adult patients undergoing mamma surgery?
PICO’s
|
Patients |
patients with mamma surgery |
|
|
Comparison |
Intervention |
Control |
|
1 |
SAP |
PV |
|
2 |
ESP |
PV |
|
3 |
ESP |
SAP |
|
4 |
PECS II (pectoserratus plane block) |
PV |
|
5 |
PECS II (pectoserratus plane block) |
SAP |
|
6 |
PECS II (pectoserratus plane block) |
ESP |
|
Outcome measures |
|
|
ESP= erector spinae plane block; PECS II=pectoralis nerve blocks II; PV= paravertebral (PV) block; SAP=serratus anterior plane block
Relevant outcome measures
The guideline development group considered postoperative pain as a critical outcome measure for decision making; and chronic pain and postoperative opioid consumption and adverse events, as important outcome measures for decision making.
The working group defined the outcome measures as follows:
Postoperative pain (at rest and during mobilization/cough): Validated pain scale (Visual Analogue Scale (VAS) or Numeric Rating Scale (NRS) at post-anesthesia care unit (PACU) arrival, 6, 12, 24. Postoperative opioid consumption: defined as the total consumption in the first 24 hours after surgery (in Morphine Milligram Equivalent; MME). Chronic postoperative pain: pain > 3 months, in line with the international association study of pain (IASP). Adverse events: block failure or insufficient block effect, local anesthetic toxicity, and additionally also pneumothorax for paravertebral block.
The working group defined one point as a minimal clinically (patient) important difference on a 10-point pain scale and 10 mm on a 100 mm pain scale. Regarding postoperative opioid consumption, a difference of 10 mg was considered clinically relevant. For dichotomous variables, a difference of 10% was considered clinically relevant (RR ≤0.91 or ≥1.10; RD 0.10). For standardized mean differences (SMD), 0.5 was considered clinically relevant.
Search and select (Methods)
The databases Medline (via OVID) and Embase (via Embase.com) were searched with relevant search terms until 5-5-2022. The detailed search strategy is depicted under the tab Methods. The systematic literature search resulted in 440 hits. Studies were selected based on the following criteria:
Inclusion criteria:
- Systematic review of RCTs or RCT
- Mamma surgery
- Published ≥ 2005
- Patients ≥18 years
- Conform PICO
Exclusion criteria:
- compared with no block
- comparison of two variants of the same block
- comparisons with other blocks: thoracic spinal (blockade, parasternal/rhomboid) intercostal block, transversus thoracic muscle plane, retrolaminar block, interpleural block, serratus-intercostal fascial plane
- no original research
- n<20 per arm
A total of 59 studies (including 7 systematic reviews and 52 additional RCTs) were initially selected based on title and abstract screening. After reading the full text, 44 studies (including 4 systematic reviews and 40 additional RCTs) were excluded (see the table with reasons for exclusion under the tab Methods), and 15 studies were included.
Results
Two systematic reviews (reporting on 14 RCTs) and 13 additional single RCTs were included in the analysis of the literature.
Six comparisons between various thoracic wall blocks could be made from the included literature:
- serratus anterior plan (SAP) block versus paravertebral (PV) block;
- erector spinae plan (ESP) block versus paravertebral (PV) block;
- erector spinae plane (ESP) block versus serratus anterior plane (SAP) block;
- pectoralis nerve block (PECS) II (pectoserratus plane block) versus paravertebral (PV) block;
- pectoralis nerve block (PECS) II block (pectoserratus plane block) versus serratus anterior plane (SAP) block.
- pectoral nerve block (PECS) II block (pectoserratus plane block) versus erector spinae plane (ESP) block;
Table 1 Overview of studies per block-comparison.
|
I/C |
SAP |
PV |
ESP |
|
SAP |
|
Arora (2022) Gabriel (2021) Jain (2020) Amin (2018) Gupta (2017) |
|
|
ESP |
Jiang (2021) Shrivastava (2021) |
Xiong (2021) |
|
|
PECS II (pectoserratus plane block) |
Bakeer (2020) Jain (2020) Fuiji (2021) |
Elshanbary (2021) Jain (2020) |
Elshanbary (2021) |
The summary of the literature and conclusions are divided into these comparisons. Important study characteristics and results are summarized in the evidence tables. The assessment of the risk of bias is summarized in the risk of bias tables.
Referenties
- Amin SRM, Abdelrahman EA, El Shahat Afify E, Elsayed EM. Ultrasound-guided serratus anterior plane block versus thoracic paravertebral block for postmastectomy analgesia. Benha Med J 2018; 35:429436.
- Arora S, Ovung R, Bharti N, Yaddanapudi S, Singh G. Efficacy of serratus anterior plane block versus thoracic paravertebral block for postoperative analgesia after breast cancer surgery - a randomized trial. Braz J Anesthesiol. 2022 Sep-Oct;72(5):587-592. doi: 10.1016/j.bjane.2021.09.017. Epub 2021 Oct 7. PMID: 34627832; PMCID: PMC9515677.
- Bakeer AH, Kamel KM, Abdelgalil AS, Ghoneim AA, Abouel Soud AH, Hassan ME. Modified Pectoral Nerve Block versus Serratus Block for Analgesia Following Modified Radical Mastectomy: A Randomized Controlled Trial. J Pain Res. 2020 Jul 14;13:1769-1775.
- Blanco R. The 'pecs block': a novel technique for providing analgesia after breast surgery. Anaesthesia. 2011 Sep;66(9):847-8. doi: 10.1111/j.1365-2044.2011.06838.x. PMID: 21831090.
- Blanco R, Fajardo M, Parras Maldonado T. Ultrasound description of Pecs II (modified Pecs I): a novel approach to breast surgery. Rev Esp Anestesiol Reanim. 2012 Nov;59(9):470-5. doi: 10.1016/j.redar.2012.07.003. Epub 2012 Aug 29. PMID: 22939099.
- Blanco R, Parras Maldonado T. Reply to the article entitled "Ultrasound description of Pecs II (modified Pecs I): a novel approach to breast surgery". Reply of the authors. Rev Esp Anestesiol Reanim. 2013 May;60(5):296-7. doi: 10.1016/j.redar.2013.01.002. Epub 2013 Feb 27. PMID: 23453236.
- Coveney E, Weltz CR, Greengrass R, Iglehart JD, Leight GS, Steele SM, Lyerly HK Use of paravertebral block anesthesia in the surgical management of breast cancer: experience in 156 cases Ann Surg. 1998 Apr;227(4):496-501.
- Cregg R, Anwar S, Farquhar-Smith P. Persistent postsurgical pain. Curr Opin Support Palliat Care. 2013;7(2):144-52.
- Dautzenberg KHW, Zegers MJ, Bleeker CP, Tan ETH, Vissers KCP, van Geffen GJ, van der Wal SEI. Unpredictable Injectate Spread of the Erector Spinae Plane Block in Human Cadavers Anesth Analg. 2019 Nov;129(5):e163-e166.
- Elshanbary AA, Zaazouee MS, Darwish YB, Omran MJ, Elkilany AY, Abdo MS, Saadeldin AM, Elkady S, Nourelden AZ, Ragab KM. Efficacy and Safety of Pectoral Nerve Block (Pecs) Compared With Control, Paravertebral Block, Erector Spinae Plane Block, and Local Anesthesia in Patients Undergoing Breast Cancer Surgeries: A Systematic Review and Meta-analysis. Clin J Pain. 2021 Dec 1;37(12):925-939.
- Forero M, Adhikary SD, Lopez H, Tsui C, Chin KJ. The Erector Spinae Plane Block: A Novel Analgesic Technique in Thoracic Neuropathic Pain. Reg Anesth Pain Med. 2016 Sep-Oct;41(5):621-7. doi: 10.1097/AAP.0000000000000451. PMID: 27501016.
- Fujii T, Shibata Y, Akane A, Aoki W, Sekiguchi A, Takahashi K, Matsui S, Nishiwaki K. A randomised controlled trial of pectoral nerve-2 (PECS 2) block vs. serratus plane block for chronic pain after mastectomy. Anaesthesia. 2019 Dec;74(12):1558-1562. doi: 10.1111/anae.14856. Epub 2019 Sep 19. PMID: 31535722.
- Gabriel RA, Swisher MW, Sztain JF, Curran BP, Said ET, Abramson WB, Khatibi B, Alexander BS, Finneran JJ, Wallace AM, Armani A, Blair S, Dobke M, Suliman A, Reid C, Donohue MC, Ilfeld BM. Serratus anterior plane versus paravertebral nerve blocks for postoperative analgesia after non-mastectomy breast surgery: a randomized controlled non-inferiority trial. Reg Anesth Pain Med. 2021 Sep;46(9):773-778. doi: 10.1136/rapm-2021-102785. Epub 2021 Jun 22. PMID: 34158376; PMCID: PMC8380889.
- Gupta K, Srikanth K, Girdhar KK, Chan V. Analgesic efficacy of ultrasound-guided paravertebral block versus serratus plane block for modified radical mastectomy: A randomised, controlled trial. Indian J Anaesth. 2017 May;61(5):381-386. doi: 10.4103/ija.IJA_62_17. PMID: 28584346; PMCID: PMC5444215.
- Huang W, Wang W, Xie W, Chen Z, Liu Y. Erector spinae plane block for postoperative analgesia in breast and thoracic surgery: A systematic review and meta-analysis. J Clin Anesth. 2020 Nov;66:109900. doi: 10.1016/j.jclinane.2020.109900. Epub 2020 Jun 2. PMID: 32502778.
- Jain D, Mohan VK, Bhoi D, Batra RK, Kashyap L, Shende D, Hussain SY, Srivastava A, Seenu V. Analgesic efficacy and spread of local anesthetic in ultrasound-guided paravertebral, pectoralis II, and serratus anterior plane block for breast surgeries: A randomized controlled trial. Saudi J Anaesth. 2020 Oct-Dec;14(4):464-472. doi: 10.4103/sja.SJA_822_19. Epub 2020 Sep 24. PMID: 33447188; PMCID: PMC7796746.
- Jiang CW, Liu F, Zhou Q, Deng W. Comparison of rhomboid intercostal nerve block, erector spinae plane block and serratus plane block on analgesia for modified radical mastectomy: A prospective randomised controlled trial. Int J Clin Pract. 2021 Oct;75(10):e14539. doi: 10.1111/ijcp.14539. Epub 2021 Jul 2. PMID: 34133831.
- Juhl AA, Christiansen P, Damsgaard TE. Persistent Pain after Breast Cancer Treatment: A Questionnaire-Based Study on the Prevalence, Associated Treatment Variables, and Pain Type. J Breast Cancer. 2016 Dec;19(4):447-454. doi: 10.4048/jbc.2016.19.4.447. Epub 2016 Dec 23. PMID: 28053634; PMCID: PMC5204052.
- Karmakar MK, Samy W, Li JW, Lee A, Chan WC, Chen PP, Ho AMH Thoracic paravertebral block and its effects on chronic pain and health-related quality of life after modified radical mastectomy. Reg Anesth Pain Med. 2014 Jul-Aug;39(4):289-98.
- Meißner M, Austenfeld E, Kranke P, Zahn PK, Pogatzki-Zahn EM, Meyer-Frießem CH, Weibel S, Schnabel A. Pectoral nerve blocks for breast surgery: A meta-analysis. Eur J Anaesthesiol. 2021 Apr 1;38(4):383-393. doi: 10.1097/EJA.0000000000001403. PMID: 33259450.
- Schnabel A, Reichl SU, Kranke P, Pogatzki-Zahn EM, Zahn PK. Efficacy and safety of paravertebral blocks in breast surgery: a meta-analysis of randomized controlled trials. Br J Anaesth. 2010 Dec;105(6):842-52. doi: 10.1093/bja/aeq265. Epub 2010 Oct 14. Erratum in: Br J Anaesth. 2013 Sep;111(3):522. PMID: 20947592.
- Shrivastava, A. and Gour, V. and Chandrakant and Hingwe, S. To compare the effectiveness of erector spinae plane block with serratus anterior plane block for breast surgery -A comparative study. Journal of Cardiovascular Disease Research. 2021; 12: 6.
- Xie C, Ran G, Chen D, Lu Y. A narrative review of ultrasound-guided serratus anterior plane block. Ann Palliat Med. 2021 Jan;10(1):700-706. doi: 10.21037/apm-20-1542. Epub 2020 Dec 31. PMID: 33440981.
- Xiong C, Han C, Zhao D, Peng W, Xu D, Lan Z. Postoperative analgesic effects of paravertebral block versus erector spinae plane block for thoracic and breast surgery: A meta-analysis. PLoS One. 2021 Aug 25;16(8):e0256611. doi: 10.1371/journal.pone.0256611. PMID: 34432822; PMCID: PMC8386864.
- Yang HM, Choi YJ, Kwon HJ, O J, Cho TH, Kim SH. Comparison of injectate spread and nerve involvement between retrolaminar and erector spinae plane blocks in the thoracic region: a cadaveric study. Anaesthesia. 2018;73:12441250.
Evidence tabellen
Evidence tables
Risk of bias table for intervention studies (randomized controlled trials; based on Cochrane risk of bias tool and suggestions by the CLARITY Group at McMaster University)
|
Study reference
(first author, publication year) |
Was the allocation sequence adequately generated?
Definitely yes Probably yes Probably no Definitely no |
Was the allocation adequately concealed?
Definitely yes Probably yes Probably no Definitely no |
Blinding: Was knowledge of the allocated interventions adequately prevented?
Were patients blinded?
Were healthcare providers blinded?
Were data collectors blinded?
Were outcome assessors blinded?
Were data analysts blinded?
Definitely yes Probably yes Probably no Definitely no |
Was loss to follow-up (missing outcome data) infrequent?
Definitely yes Probably yes Probably no Definitely no |
Are reports of the study free of selective outcome reporting?
Definitely yes Probably yes Probably no Definitely no |
Was the study apparently free of other problems that could put it at a risk of bias?
Definitely yes Probably yes Probably no Definitely no |
Overall risk of bias If applicable/necessary, per outcome measure
LOW Some concerns HIGH
|
|
Comparison 1: SAP versus PV |
|||||||
|
Arora, 2022 |
Probably yes;
Reason: Randomization with computer generated random numbers, but method not further specified.
|
Probably yes;
Reason: Opaque, sealed envelopes were used. However, it is not described how/ by whom they were opened.
|
Probably no;
Reason: Patients and health care providers (anesthesiologist) not blinded. Outcome assessors and data collectors blinded. Blinding of analysts not reported. |
Probably no;
Reason: loss to follow-up was not described, but it was reported that one patient in the SAPB group had no sensory deficit and was considered as block failure. |
Definitely yes
Reason: All relevant outcomes were reported. |
Probably no;
Reason: it is not clear how patients were recruited and how many of eligible patients were excluded based on the exclusion criteria. |
Some concerns
Allocation, blinding and unclear recruitment and selection of patients. |
|
Gabriel, 2021 |
Probably yes;
Reason: Randomization using a computer-generated list, but method not further specified..
|
Probably yes;
Reason: Opaque, sealed envelopes were used. However, it is not described how/ by whom they were opened.
|
Probably no;
Reason: Patients, health care providers (anesthesia team) and outcome assessors (nurses) blinded. Blinding of data collectors and analysts not reported. |
Definitely no;
Reason: is was reported that there was no loss to follow-up, and that no patients were excluded from the analysis. |
Definitely yes;
Reason: All relevant outcomes were reported. |
Definitely yes;
Reason: No other problems noted. |
Some concerns |
|
Jain, 2020 |
Probably no;
Reason: only reported that there was random allocation but not the method.
|
Probably yes;
Reason: Opaque, sealed envelopes were used. It is not described how/by whom they were opened. |
Definitely no;
Reason: non-blinded RCT. |
Definitely no;
Reason: is was reported that there was no loss to follow-up, and that no patients were excluded from analysis.
|
Definitely no;
Reason: data on postoperative pain were only limited presented in the text. |
Definitely yes;
Reason: No other problems noted
|
HIGH
Unclear allocation, non-blinded RCT, data on postoperative pain limited presented. |
|
Amin, 2018 |
Probably yes;
Reason: Randomization with computer generated random numbers, but method not further specified. |
Probably yes;
Reason: sealed envelopes were used, but it is not described how/ by whom they were opened. |
Probably no;
Reason: single-blinded. Patients were blinded and health care providers were not blinded (different procedures for the groups were described). Blinding of data collectors, outcome assessors and analysts not reported. |
Probably no;
Reason: loss to follow-up was not described, but results on outcome measures are available for the included samples (n=60; I=30, C=30) |
Definitely yes;
Reason: All relevant outcomes were reported |
Probably no;
Reason: it is not clear how patients were recruited and how many of eligible patients were excluded based on the exclusion criteria. |
Some concerns
Single-blinded (patients) RCT, unclear patient recruitment. |
|
Gupta, 2017 |
Probably yes;
Reason: Randomization with computer generated random numbers, but method not further specified.
|
Probably yes;
Reason: sealed envelopes were used, but it is not described how/by whom they were opened.
|
Probably yes;
Reason: double-blinded, patients and health care providers were blinded. Blinding of data collectors, outcome assessors and analysts not reported. |
Definitely no;
Reason: is was reported that there was no loss to follow-up, and that no patients were excluded from analysis. |
Probably no;
Reason: Results on post-operative pain (mean visual analogue scales) only presented in a figure, without SD/ CI in numbers. |
Definitely yes;
Reason: No other problems noted
|
Some concerns
Outcome postoperative pain |
|
Comparison 3: ESP versus SAP |
|||||||
|
Jiang, 2020 |
Definitely yes;
Reason: Randomization with computer generated random numbers, created by an independent researcher.
|
Probably no;
Reason: not described
|
Probably yes;
Reason: health care providers and data collectors blinded (blinding of patients, outcome assessors and analysts not reported) |
Definitely yes;
Reason: There was no loss to follow-up in intervention and control group. |
Probably no;
Reason: Results provided in figures, no absolute values available for postoperative pain |
Definitely yes;
Reason: No other problems noted
|
Some concerns
Method of allocation unclear, limited reporting of outcome postoperative pain
|
|
Shrivastava, 2021 |
Probably yes;
Reason: Randomization with computer generated random numbers, but method not further specified. |
Probably no;
Reason: not described
|
Probably no;
Reason: not described
|
Probably yes;
Reason: no loss to follow-up in intervention and control group, but N not described in tables |
Probably no;
Reason: Results postoperative pain provided without standard deviations |
Definitely yes;
Reason: No other problems noted
|
HIGH
Method of allocation unclear, blinding unclear, limited reporting of outcome postoperative pain |
Comparison 4: PECS II versus PV |
|||||||
|
Jain, 2020 |
Probably no;
Reason: only reported that there was random allocation but not the method.
|
Probably yes;
Reason: Opaque, sealed envelopes were used. It is not described how/ by whom they were opened.
“Allocation concealment was done by sequentially numbered, opaque, sealed envelopes.” |
Definitely no;
Reason: non-blinded RCT |
Definitely no;
Reason: is was reported that there was no loss to follow-up, and that no patients were excluded from analysis.
|
Definitely no;
Reason: data on postoperative pain were only limited presented in the text. |
Definitely yes;
Reason: No other problems noted
|
HIGH
Unclear allocation, non-blinded RC, data on postoperative pain limited presented |
|
Comparison 5: PECS II versus SAP |
|||||||
|
Bakeer, 2020 |
Probably yes;
Reason: Central randomization with computer generated random numbers, but method not further specified.
|
Probably yes;
Reason: Opaque, sealed envelopes were used. It is not described how/ by whom they were opened.
|
Probably yes;
Reason: Patients, health care providers and data collectors blinded (blinding of Patients, outcome assessors and analysts not reported). |
Probably yes;
Reason: Loss to follow-up was infrequent in intervention and control group. |
Definitely yes;
Reason: All relevant outcomes were reported. |
Definitely yes;
Reason: No other problems noted.
|
Some concerns |
|
Jain, 2020 |
Probably no;
Reason: only reported that there was random allocation but not the method
|
Probably yes;
Reason: Opaque, sealed envelopes were used. It is not described how they were opened.
“Allocation concealment was done by sequentially numbered, opaque, sealed envelopes.” |
Definitely no;
Reason: non-blinded RCT |
Definitely no;
Reason: is was reported that there was no loss to follow-up, and that no patients were excluded from analysis
|
Definitely no;
Reason: data on postoperative pain were only limited presented in the text |
Definitely yes;
Reason: No other problems noted
|
HIGH
Unclear allocation, non-blinded RCT Data on postoperative pain limited presented |
|
Fujii, 2019 |
Definitely yes;
Reason: Randomization with computer generated random numbers, but method not further specified.
|
Probably no;
Reason: not described
|
Probably yes;
Reason: Patients, health care providers and data collectors blinded (blinding of Patients, outcome assessors and analysts not reported). |
Definately yes;
Reason: Loss to follow-up was infrequent in intervention and control group. |
Definitely yes
Reason: All relevant outcomes were reported; |
Definitely yes;
Reason: No other problems noted
|
Some concerns
Method of allocation unclear |
Randomization: generation of allocation sequences have to be unpredictable, for example computer generated random-numbers or drawing lots or envelopes. Examples of inadequate procedures are generation of allocation sequences by alternation, according to case record number, date of birth or date of admission.
Allocation concealment: refers to the protection (blinding) of the randomization process. Concealment of allocation sequences is adequate if patients and enrolling investigators cannot foresee assignment, for example central randomization (performed at a site remote from trial location). Inadequate procedures are all procedures based on inadequate randomization procedures or open allocation schedules..
Blinding: neither the patient nor the care provider (attending physician) knows which patient is getting the special treatment. Blinding is sometimes impossible, for example when comparing surgical with non-surgical treatments, but this should not affect the risk of bias judgement. Blinding of those assessing and collecting outcomes prevents that the knowledge of patient assignment influences the process of outcome assessment or data collection (detection or information bias). If a study has hard (objective) outcome measures, like death, blinding of outcome assessment is usually not necessary. If a study has “soft” (subjective) outcome measures, like the assessment of an X-ray, blinding of outcome assessment is necessary. Finally, data analysts should be blinded to patient assignment to prevents that knowledge of patient assignment influences data analysis.
Lost to follow-up: If the percentage of patients lost to follow-up or the percentage of missing outcome data is large, or differs between treatment groups, or the reasons for loss to follow-up or missing outcome data differ between treatment groups, bias is likely unless the proportion of missing outcomes compared with observed event risk is not enough to have an important impact on the intervention effect estimate or appropriate imputation methods have been used.
Selective outcome reporting: Results of all predefined outcome measures should be reported; if the protocol is available (in publication or trial registry), then outcomes in the protocol and published report can be compared; if not, outcomes listed in the methods section of an article can be compared with those whose results are reported.
Other biases: Problems may include: a potential source of bias related to the specific study design used (e.g. lead-time bias or survivor bias); trial stopped early due to some data-dependent process (including formal stopping rules); relevant baseline imbalance between intervention groups; claims of fraudulent behavior; deviations from intention-to-treat (ITT) analysis; (the role of the) funding body (see also downgrading due to industry funding https://kennisinstituut.viadesk.com/do/document?id=1607796-646f63756d656e74). Note: The principles of an ITT analysis implies that (a) participants are kept in the intervention groups to which they were randomized, regardless of the intervention they actually received, (b) outcome data are measured on all participants, and (c) all randomized participants are included in the analysis.
Overall judgement of risk of bias per study and per outcome measure, including predicted direction of bias (e.g. favors experimental, or favors comparator). Note: the decision to downgrade the certainty of the evidence for a particular outcome measure is taken based on the body of evidence, i.e. considering potential bias and its impact on the certainty of the evidence in all included studies reporting on the outcome.
Table of quality assessment for systematic reviews of RCTs and observational studies
Based on AMSTAR checklist (Shea et al.; 2007, BMC Methodol 7: 10; doi:10.1186/1471-2288-7-10) and PRISMA checklist (Moher et al 2009, PLoS Med 6: e1000097; doi:10.1371/journal.pmed1000097)
|
Study
First author, year |
Appropriate and clearly focused question?1
Yes/no/unclear |
Comprehensive and systematic literature search?2
Yes/no/unclear |
Description of included and excluded studies?3
Yes/no/unclear |
Description of relevant characteristics of included studies?4
Yes/no/unclear |
Appropriate adjustment for potential confounders in observational studies?5
Yes/no/unclear/notapplicable |
Assessment of scientific quality of included studies?6
Yes/no/unclear |
Enough similarities between studies to make combining them reasonable?7
Yes/no/unclear |
Potential risk of publication bias taken into account?8
Yes/no/unclear |
Potential conflicts of interest reported?9
Yes/no/unclear |
|
Comparison 2: ESP versus PV |
|||||||||
|
Xiong, 2021 |
Yes |
Yes |
Yes |
Yes |
N/A |
Yes, all included studies had high risk of bias |
Yes |
Yes, not detected |
Unclear Not specified for included studies |
|
Comparison 3: PECS II versus ESP |
|||||||||
|
Elshanbary, 2021 |
Yes |
Yes |
Yes |
No, information on measurement method of outcomes is lacking |
N/A |
Yes, included studies had moderate to low risk of bias |
Yes |
Yes |
Unclear Not specified for included studies |
|
Comparison 4: PECS II versus PV |
|||||||||
|
Elshanbary, 2021 |
Yes |
Yes |
Yes |
No, information on measurement method of outcomes is lacking |
N/A |
Yes, included studies had moderate to low risk of bias |
Yes |
Yes |
Unclear Not specified for included studies |
Evidence table for intervention studies
|
Study reference |
Study characteristics |
Patient characteristics 2 |
Intervention (I) |
Comparison / control (C) 3
|
Follow-up |
Outcome measures and effect size 4 |
Comments |
|
Comparison 1: SAP versus PV |
|||||||
|
Arora (2022) |
Study design: RCT
Setting and country:
Funding and conflicts of interest: Funding: not reported Conflict: reported that the authors declare no conflicts of interest. |
Inclusion criteria: (1)ASA (American Society of Anesthesiologists) physical status I---II female patients (2)age group of 18-65 years, (3) scheduled to undergo total mastectomy (4) with axillary clearance (5) under general anesthesia
Exclusion criteria: The patients who had (1) local infection at the block site, (2) coagulopathy, (3) morbid obesity (BMI > 40 kg.m-2), (4) allergy to local anesthetics, (5) decreased pulmonary reserve, (6) uncontrolled hypertension or ischemic heart disease, (7) renal dysfunction, and (8) pre-existing neurological deficits and psychiatric illness were excluded.
N total at baseline: I: 20 C: 20
Important prognostic factors2: age (years) mean ± SD I: 50.8 ± 9.5 C: 48.2 ± 9.8
ASA I:II (n) I: 15:5 C: 16:4
Groups comparable at baseline? The groups were comparable regarding the patients’ age, weight, height, and ASA physical status. Although more patients in the SAPB group had sensory spread at the level of T1, T2, and T6 as compared to the TPVB group, it was not statistically significant. |
SAP
3-5 ml lignocaine 2%; 0.4 mL.kg-1 ropivacaine 0.5% |
PV
3-5 ml lignocaine 2%; 0.4 mL.kg-1 ropivacaine 0.5% |
Length of follow-up: 24 hours after surgery in the postanesthesia care room
Loss-to-follow-up: No loss to follow up
Incomplete outcome data: 1 patient in the SAP group had no sensory deficit and considered as block failure. |
Postoperative pain* NRS
“Post-operative pain scores were significantly lower in the SAPB group as compared to the TPVB group (p < 0.05) during the first 2 hours and then at 6, 8, and 24 hours after surgery”
p-values: ½ h < 0.001 1 h = 0.007 6 h = 0.040 12 h = 0.080 24 h = 0.032
Adverse events No block- related adverse effects were reported in any group of patients. |
Study aim: to compare the efficacy of SAPB with the thoracic paravertebral block (TPVB) for postoperative analgesia after breast cancer surgery.
*results presented in figure without mean-values
**Patients with NRS more than 3 or those demanding analgesics received diclofenac sodium 75 mg intravenously. If NRS was remained high 30 minutes after administration of diclofenac, then injection tramadol 1 mg.kg-1 was given.
Author conclusion: The SAPB group patients also showed lower postoperative pain scores and demanded less rescue analgesia in comparison to the TPVB group. The postoperative tramadol consumption was low in both groups.
In conclusion, we found SAPB superior to TPVB in terms of prolonged duration of postoperative analgesia and reduced rescue analgesic requirement after radical mastectomy in breast cancer patients. Therefore, SAPB may be a viable alternative to the TPVB, which is technically more challenging and have a higher potential for adverse effects. Further studies are required to compare the efficacy and safety of SAPB with other chest wall blocks. As SAPB usually provides 4-6 hours of postoperative analgesia in these patients, the role of additives can also be assessed. |
|
Gabriel (2021) |
Study design: RCT (randomized, subject-masked, parallel-arm, active-controlled study)
Setting and country: University of California San Diego, La Jolla, California, USA
Funding and conflicts of interest: Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Conflict: The University of California has received funding and product for other research projects from Epimed International (Farmers Branch, Texas, USA); Infutronics (Natick, Massachusetts, USA); and SPR Therapeutics (Cleveland, Ohio, USA) for the following authors: RG, MWS, JFS, ETS, BK, JJF, AMW and BMI. RG is a consultant for Avanos (Alpharetta, Georgia, USA).
|
Inclusion criteria: (1) Adults (≥ 18 years) (2) unilateral or bilateral breast surgery (3) moderate postoperative pain anticipated (4) a planned single-injection regional analgesic (including lumpectomy with axillary node biopsy/resection, breast reconstruction, breast reduction, mastopexy, and implant expander removal/placem)
Exclusion criteria: (1) simple lumpectomy (with no other procedure), (2) planned regional analgesic with perineural catheter placement, (3) morbid obesity as defined as a body mass index >40 kg/m2 , (4) renal insufficiency (preoperative creatinine >1.5 mg/ dL), (5) current chronic opioid use (daily equivalent of >30 mg of morphine within the 2 weeks prior to surgery and duration of use >4 weeks), (5) history of opioid abuse, (6) any comorbidity that results in moderate or severe functional limitation, (7) inability to communicate with the investigators or hospital staff, (8) pregnancy, (9) incarceration and allergy to study medications (ropivacaine) (10) Patients undergoing mastectomy were excluded since they are offered a continuous PVB at the institution as standard care.
N total at baseline: I: 49 C: 51
Important prognostic factors2:
age (years) median (IQR) I: 55.0 (44.0–64.0) C: 53.0 (41.5–61.5)
Female sex n (%) I: 49 (100%) C: 48 (94.1%)
Bilateral procedure n (%) I: 17 (34.7%) C: 19 (38.0%)
Groups comparable at baseline? Yes, no imbalances in baseline characteristics
|
SAP
Local anesthetic (0.5% ropivacaine) with 1:400 000 of epinephrine was deposited deep to the serratus anterior muscle (20 mL for unilateral surgery, 16 mL on each side for bilateral surgery) |
PV
Local anesthetic (0.5% ropivacaine) with 1:400 000 of epinephrine was injected at the appropriate level(s) after negative aspiration (20 mL for unilateral surgery, 16 mL on each side for bilateral surgery) |
Length of follow-up: 1 day postoperatively (follow-up call)
Loss-to-follow-up: No loss to follow-up in either group (POD1)
Incomplete outcome data: No incomplete outcome data/ exclusion from analyses in either group
|
Postoperative pain 11-point NRS (0–10, 0=no pain, 10=worst pain imaginable)
Postoperative pain PACU Median (IQR)
I: 4.0 (0–5.5) C: 0 (0–3.0)
Postoperative pain morning POD1 (average) (+/-24h) Median (IQR)
I: 3.0 (2.0–4.0) C: 2.0 (0.5–3.0)
Opioid consumption*** intravenous morphine equivalents (MEQ) in mg = MME.
Opioid consumption morning POD1 (+/-24h) Median (IQR)
I: 2.0 (2.0–4.0) C: 2.0 (0–4.0) p= 0.24
in mean ± SD:
I: 2 ± 0.33 C: 2 ± 1
Adverse events There were no protocol deviations or adverse events related to the block procedures. |
Study aim: to perform a randomized, subject-masked, parallel-arm, active-controlled study comparing PVB to serratus block for non-mastectomy breast surgery patients.
! Patients undergoing mastectomy were excluded since they are offered a continuous PVB at our institution as standard care.
* authors: The lower limit of the 95% CI was less than −1.25, which is lower than the prespecified framework for non-inferiority, and therefore, non-inferiority was not demonstrated. Note that the upper limit is less than 0–5 decimal places (−2.66×10−5), demonstrating inferiority.
** authors: In this case, the upper limit of the 95% CI was on the positive side of 0–6 decimal places (2.49×10−6). Since the 95% CI lower limit of −4.5 was less than our prespecified margin of −2.0, we failed to conclude that the serratus block is non-inferior to the PVB.
*** Opioid consumption was analyzed as intravenous morphine equivalents (MEQ). Intraoperative and postoperative intravenous fentanyl, intravenous hydromorphone, and oral oxycodone were converted to intravenous MEQ
Author discussion/conclusion: serratus blocks provided inferior analgesia compared with PVBs; and, regarding opioid requirements, we were unable to demonstrate at least non-inferiority of serratus blocks. Furthermore, there were several differences in our secondary outcomes favoring PVB, including need of antiemetics in the PACU, opioid consumption in the PACU, and worst/least NRS pain scores in the PACU and up to POD 1. Of note, all blocks were performed by fellowship-trained attendings or current fellows overseen by the attendings who all have a great deal of experience with both blocks. Thus, providers with less experience—especially with PVBs—may still elect to perform the less efficacious fascial plane block due to risk/benefit ratio concerns; while practitioners skilled at PVB have reason not to change their current practice for breast surgery.
This randomized active controlled study suggests that PVB provides improvements in short-term postoperative analgesia compared with serratus blocks following non-mastectomy breast surgery. |
|
Jain (2020) |
Study design: RCT; prospective, randomized interventional nonblinded study
Setting and country: India Institute of Medical Sciences, New Delhi, India
Funding and conflicts of interest Funding: Nil. Conflict: reported that there are no conflicts of interest |
Inclusion criteria: (1) ASA grades I and II (2) female patients (3) aged 18–65 years (4) undergoing elective unilateral mastectomies with or without axillary dissection
Exclusion criteria: (1) Patients with infection at the site of proposed block, (2) chest wall deformity, (3) coagulopathy, or (4) receiving any anticoagulants, (5) body mass index ≥35 kg/m2 , (6) mental retardation were excluded from the study
N total at baseline: I: 15 C: 15
Important prognostic factors2:
age (years) mean ± SD I: 60.87±11.9 C: 44.47±11.62
ASA (I/II) n (%) I: 11 (73.33)/4 (26.67) C: 12 (80)/3 (20)
Groups comparable at baseline? Yes, p-values demographic variables non-significant
|
USG SAP
0.375% ropivacaine |
USG single injection PV
0.375% ropivacaine |
Length of follow-up: Not specifically reported, but it is stated that patient was kept in PACU for 24 h postoperative period and the outcomes measurements were performed up to 24h
Loss-to-follow-up: Reported there was no loss to follow up
Incomplete outcome data: I: SAP group: all received allocated intervention C: TVPB group: all received allocated intervention
|
Postoperative pain (VAS 0‑10) at rest and ipsilateral abduction of arm* with 0 corresponding to no pain and 10 being the worst imaginable pain.
“Pain scores were not significantly different among the group in the postoperative 24 h period. Only three patients gave a response at immediate postoperative and therefore VAS scores were not assessed at this point of time. At 6 h, VAS scores were reaching significant difference among PECS and SAP block.”
Opioid consumption: Postoperative fentanyl requirement in 24h in µg (mean ± SD)[95%CI]
I: 415 ± 182.44 [95% CI 323–507] C: 428.33 ± 243.1 [95% CI 308.33–548.33]
(IV morphine 10 mg = IV fentanyl 100 μg = IV sufentanil 10 μg)
In MME: I: 41.5 ± 18.244 [95% CI 32.3–50.7] C: 42.833 ± 24.31 [95% CI 30.833–54.833]
Adverse events No complication like inadverdent vascular puncture, pneumothorax, hypotension was seen in any group.
|
Study aim: to compare the postoperative 24‑h fentanyl consumption in each of the three blocks. Secondary objectives were to compare spread of local anesthetic, sensory blockade produced, onset of the sensory blockade, intraoperative opioids requirement, pain scores for 24 h postoperative period, time to first postoperative analgesia requirement and incidence of postoperative nausea and vomiting (PONV).
* Pain scores (VAS 1‑10) were measured at rest and ipsilateral abduction of arm at 1, 2, 3, 6, 12, and 24‑hour postoperative. à very limited results
Author conclusion: TPVB and SAP group provide equivalent analgesia in patients undergoing mastectomies. PECS II block is inferior to TPVB and SAP block in providing analgesia for breast surgeries. Single‑shot TPVB and SAP block also result in greater spread of the drug along with sensory block compared to PECS II block. SAP block is easier to perform than TPVB with lesser chances of complications and results in faster onset. Thus, we recommend SAP block for patients undergoing mastectomies for effective analgesia |
|
Amin (2018) |
Study design: RCT (Prospective, single-blinded randomized study)
Setting and country: Benha University Hospital, Egypt
Funding and conflicts of interest: Funding: Nil. Conflict: no conflicts or interest |
Inclusion criteria: (1) Female patients (2) age of 30 and 60 years, (3) with cancer breast, (4) American Society of Anesthesia (ASA) I and II, (5) undergoing unilateral mastectomy operation
Exclusion criteria: (1) Refusal to participate, (2) morbid obesity (BMI >40 kg/m2 ), (3) renal insufficiency (creatinine >1.5 mg/dl), (4) current chronic analgesic therapy (daily use >4 weeks), (5) diabetes mellitus with polyneuritis, (6) generalized or local infection, (7) chronic pain in the anterolateral region of the chest or axilla, (8) inability to communicate with the medical staff and (9) ASA physical status III and IV
N total at baseline: I: 30 C: 30
Important prognostic factors2: age (years) mean ± SD I: 45±7 C: 42±8
ASA median (range) I: 1 (1-2) C: 1 (1-2)
Groups comparable at baseline? Yes, no significant statistical difference as regards to age, weight, height, and ASA status, between both groups with a P value greater than 0.05.
|
US-guided SAP
0.4 ml/kg of bupivacaine 0.25% with 5 μg/ml epinephrine
|
US-guided PV
15–20 ml of 0.25% bupivacaine with 5 μg/ml epinephrine |
Length of follow-up: Not reported, but hospital stay length up to 3 days
Loss-to-follow-up: Not reported
Incomplete outcome data: Not reported
|
Postoperative pain at rest VAS (0-10cm)
Median (range)
Postoperative pain at PACU I: 1 (0-4) C: 0 (0-4) P=0.301
Postoperative pain 12h I: 2 (0-4) C: 3 (0-5) p<0.001**
Postoperative pain 24h I: 3 (0-5) C: 3 (0-5) P=0.087
Postoperative pain at movement VAS (0-10cm)
Median (range)
Postoperative pain at PACU I: 1 (0–4) C: 0 (0-4) P= 0.384
Postoperative pain 12h I: 2 (0-4) C: 3 (1–6) P=0.044*
Postoperative pain 24h I: 3 (0–6) C: 4 (0–6) P=0.416
Opioid consumption: Pain rescue analgesia consumption in the first 24h* Morphine (5mg IV)
mean ± SD
I: 5±2 C: 9±2 p=0.01
Adverse events It was reported there were no complications related to the blocks.
|
Study aim: to evaluate the analgesic efficacy and safety of SAPB in comparison with TPVB for postmastectomy pain.
Author conclusion: The US- guided SAPB and TPVB provide good analgesia postmastectomy, but SAPB has a superior analgesic profile, with a longer duration of analgesia, with delayed requirement for the first rescue analgesia & 24 hours reduced analgesic consumption.
* Postoperative analgesia regimen was standard in all groups. When the VAS score was greater than 4, the patients were given morphine (5 mg IV).
|
|
Gupta (2017) |
Type of study: RCT (double‑blind, randomised, comparative study)
Setting and country: Vardhman Mahavir Medical College and Safdarjung Hospital, New Delh,, India.
Funding and conflicts of interest: Funding: Nil. Conflict: no conflict of interest |
Inclusion criteria: (1)patients undergoing MRM with axillary dissection, (2) age 18–65 years, weight 40–85 kg and American Society of Anesthesiologists Physical Status I or II.
Exclusion criteria: contraindications to nerve block, for example, coagulopathy and local infection at the site of block, local anaesthetic allergy and significant neurological, cardiac, renal, hepatic or respiratory disease and patients planned for breast conservative surgery/simple mastectomy with axillary clearance
N total at baseline: I: 25 C: 25
Important prognostic factors2: age ± SD: I: 48.9±4.0 C: 50±10.4
ASA (I/II): I: 23/2 C: 23/2
Groups comparable at baseline? Yes, it is reported that the demographic data of the two groups were comparable and that the intra‑.and post‑operative haemodynamic parameters were also comparable
|
ultrasound‑guided SAP
20 ml of 0.5% bupivacaine
|
ultrasound‑guided PV
20 ml of 0.5% bupivacaine
|
Length of follow-up: Not reported, but outcome measures reported up to 72h after surgery
Loss-to-follow-up: I: 0 C: 0
Incomplete outcome data: I: 0 C: 0
|
Postoperative pain VAS (0–100 mm) Mean
Postoperative pain 6h I: 3.2 C: 3.36 p=0.46
Postoperative pain 24h I: 2.48 C: 3.04 p=0.09
Opioid consumption: Postoperative patient-controlled analgesia morphine consumption within 24h mean ± SD I: 9.7±2.1 C: 6.5±1.5 P<0.001
Adverse events None of the patients in either group had any other complication. |
Study aim: to compare the analgesic efficacy of ultrasound‑guided PVB and SPB in patients undergoing MRM with axillary dissection.
The primary objective of this study was to determine the difference in the duration of analgesia of the two blocks. The secondary objectives were to compare the morphine consumption in 24, 48 and 72 h after surgery, visual analogue scale (VAS) pain scores and morphine‑related side effects (nausea/vomiting).
Author conclusion: The ultrasound‑guided SPB and PVB provide good analgesia post‑MRM, but PVB has a superior analgesic profile, with a longer duration of analgesia
the authors report on a VAS scale of 0-100mm, yet report centimeters. |
|
Comparison 3: ESP versus SAP |
|||||||
|
Jiang (2021) |
Study design: RCT; prospective
Setting and country: Affiliated Hospital of Jiaxing University, Jiaxing, China
Funding and conflicts of interest Funding: This study was supported by The Key Discipline Established with Zhejiang Province and Jiaxing City Jointly (2019-ss-ttyx) and Key Discipline of Anesthesiology of Jiaxing City (2019-zc-06). Conflict: The author reports no conflicts of interest in this work.
|
Inclusion criteria: (1) the patients needed to be aged between 18 and 80 years, (2) the American Society of Anesthesiologists (ASA) grade required was 1-2, (3) and patients needed to be prepared to undergo MRM in our hospital.
Exclusion criteria: (1) patients with shock or coma, (2) abnormal blood coagulation, (3) infection in the planned block area, (4) severe nerve injury on the side of the limb, (5) patients with a history of chronic pain and taking an analgesic, (6) patients with psychiatric diseases, (7) patents on radiotherapy and/or chemotherapy, (8) patients allergic to LAs (eg lidocaine and ropivacaine) and general anaesthetics, (9) patients and/or their families that refused surgical anaesthesia, (10) previous history of breast surgery or other chest surgery and (11) a body mass index (BMI) >34 kg/m2.
N total at baseline: I: 30 C: 30
age (years) mean ± SD I: 52.10 ± 11.50 C: 54.73 ± 13.60
ASA class I/II I: 12/18 C: 13/17
Duration of surgery (min) I: 100.96 ± 29.30 C: 109.36 ± 21.91
Groups comparable at baseline? Yes, no significant differences in basic characteristics
|
SAP
0.5% ropivacaine (20 mL) |
ESP
20 mL of 0.5% ropivacaine |
Length of follow-up: 24h
Loss-to-follow-up/ Incomplete outcome data: Lost to follow up = 0 Analysed = 30 per group
|
Postoperative pain* 11-point Numerical Rating Scale (NRS), which ranges from “0” (meaning “no pain”) to “10” (meaning “worst pain imaginable”)
Results presented in figures, no absolute values available.
“the postoperative NRS score in the ESP block group at 0.5 (P < .001), 1 (P < .001), 3 (P = .037), 6 (P = .002), 12 (P = .003), 18 (P < .001) and 24 (P = .026) h after surgery when patients were active was significantly lower than that in the SAB group”
Opioid consumption: Postoperative tramadol consumption in 24 h* Mean ± SD
I: 314.33 ± 18.88 mg C: 273.67 ± 36.90 mg
Adverse events block-related complications such as pneumothorax, bleeding, allergy and LA toxicity
Nothing reported. |
Study aim: to analyse the postoperative analgesic effects of US-guided RIB, ESP and SAB after MRM
*Within 24 hours after the operation, the patients received an intravenous injection of tramadol 1-2 mg/kg in the surgical ward for pain relief until the NRS pain score was ≤3 in accordance with hospital policy. [..] If the postoperative NRS was greater than 3 points, tramadol 1-2 mg/kg was administered intravenously, and then the pain was evaluated after 30 minutes
Author conclusion: S-RIB and the ESP block can effectively reduce the dosage of tramadol within 24 hours after MRM, and they can effectively relieve pain within 24 hours after MRM when compared with SAB. |
|
Shrivastava (2021) |
Study design: RCT; prospective
Setting and country: ABV Government Medical College, Vidisha, Madhya Pradesh, India
Funding and conflicts of interest Funding: - Conflict: -
|
Inclusion criteria: Patients with American Society of Anesthesiologists physical status Classes I and II
Exclusion criteria: Any patient with known allergy to the drugs used in the study, coagulation abnormalities, infection at the local site
N total at baseline: I: 25
age (years) mean ± SD I: 44.40 ±10.45
Duration of surgery (min) I: 55±12
Groups comparable at baseline? Yes, no significant differences in basic characteristics
|
SAP
25 mL of 0.25% bupivacaine |
ESP
25 mL of 0.25% bupivacaine |
Length of follow-up: 24h
Loss-to-follow-up/ Incomplete outcome data:
No loss to follow up/ incomplete outcome data |
Postoperative pain * visual analog scale (VAS) using a ruler graded from 0 to 100 mm, where 0 = no pain and 100 = the worst imaginable pain
0h I: 20
6h I: 20
12h I: 10
24h I: 10
Opioid consumption: postoperative tramadol consumption in 24 h
I: 9.6±2.25 |
Study aim: to compare the erector spinae plane (ESP) block and the serratus anterior plane (SAP) block, stating which is better for postoperative pain control
Author conclusion: The Serratus anterior plane block reduced postoperative tramadol and other analgesic requirements and reduced pain scores more effectively than Erector spinae plane block in the first 24 h in breast surgeries. However these blocks and the effectiveness in case of intraoperative pain relief can be tried in optimal concentrations of local anesthetics so as to reduce the complications by General anaesthesia, hereby optimising one of the limitations of this study
* When VAS score was ≥30, intravenous diclofenac 75 mg was given. If VAS was still ≥30, tramadol 50 mg was given till VAS |
|
Comparison 4: PECS II versus PV |
|||||||
|
Jain (2020) |
Study design: RCT; prospective, randomized interventional nonblinded study
Setting and country: India Institute of Medical Sciences, New Delhi, India
Funding and conflicts of interest Funding: Nil. Conflict: reported that there are no conflicts of interest |
Inclusion criteria: (1) ASA grades I and II (2) female patients (3) aged 18–65 years (4) undergoing elective unilateral mastectomies with or without axillary dissection
Exclusion criteria: (1) Patients with infection at the site of proposed block, (2) chest wall deformity, (3) coagulopathy, or (4) receiving any anticoagulants, (5) body mass index ≥35 kg/m2 , (6) mental retardation were excluded from the study
N total at baseline: I: 16 C: 15
Important prognostic factors2:
age (years) mean ± SD I: 45.47±8.98 C: 44.47±11.62
ASA (I/II) n (%) I: 12 (80)/3 (20) C: 12 (80)/3 (20)
Groups comparable at baseline? Yes, p-values demographic variables non-significant
|
USG modified PECS (PECS II) block
0.375% ropivacaine |
USG single injection PV
0.375% ropivacaine |
Length of follow-up: Not specifically reported, but it is stated that patient was kept in PACU for 24 h postoperative period and the outcomes measurements were performed up to 24h
Loss-to-follow-up: Reported there was no loss to follow up
Incomplete outcome data: I: 1 did not receive allocated intervention (anatomy distorted due to previous surgery) C: all received allocated intervention
|
Postoperative pain (VAS 0‑10) at rest and ipsilateral abduction of arm* with 0 corresponding to no pain and 10 being the worst imaginable pain.
“Pain scores were not significantly different among the group in the postoperative 24 h period. Only three patients gave a response at immediate postoperative and therefore VAS scores were not assessed at this point of time. At 6 h, VAS scores were reaching significant difference among PECS and SAP block.”
Opioid consumption: Postoperative fentanyl requirement in 24h in µg
(mean ± SD)[95%CI]
I: 644.67 ± 260.15 [95% CI 514.67–774.67] C: 428.33 ± 243.1 [95% CI 308.33–548.33]
(IV morphine 10 mg = IV fentanyl 100 μg = IV sufentanil 10 μg)
In MME: I: 64.467 ± 26.015 C: 42.833 ± 24.31
Adverse events No complication like inadverdent vascular puncture, pneumothorax, hypotension was seen in any group.
|
Study aim: to compare the postoperative 24‑h fentanyl consumption in each of the three blocks. Secondary objectives were to compare spread of local anesthetic, sensory blockade produced, onset of the sensory blockade, intraoperative opioids requirement, pain scores for 24 h postoperative period, time to first postoperative analgesia requirement and incidence of postoperative nausea and vomiting (PONV).
* Pain scores (VAS 1‑10) were measured at rest and ipsilateral abduction of arm at 1, 2, 3, 6, 12, and 24‑hour postoperative. à very limited results
Author conclusion: TPVB and SAP group provide equivalent analgesia in patients undergoing mastectomies. PECS II block is inferior to TPVB and SAP block in providing analgesia for breast surgeries. Single‑shot TPVB and SAP block also result in greater spread of the drug along with sensory block compared to PECS II block. SAP block is easier to perform than TPVB with lesser chances of complications and results in faster onset. Thus, we recommend SAP block for patients undergoing mastectomies for effective analgesia |
|
Comparison 5: PECS II versus SAP |
|||||||
|
Bakeer (2020) |
Study design: RCT; prospective
Setting and country: National Cancer Institute, Egypt
Funding and conflicts of interest Funding: not reported Conflict: the authors report no conflicts of interest
|
Inclusion criteria: aged 18–60 years, with an American Society of Anesthesiologists (ASA) class II scheduled for unilateral modified radical mastectomy (MRM).
Exclusion criteria: Patients with a history of bleeding diathesis, relevant drug allergy, opioid dependence, local sepsis, psychiatric illnesses
N total at baseline: I1: 60 I2: 60
age (years) mean ± SD I1: 50.8±8.9 12: 50.9±6.8
Duration of surgery (min) I1: 155±53 12: 158±34
Groups comparable at baseline? There was no significant difference in the patients’ demographic data and duration of surgery between all groups |
I1: PECSII block with 30 mL of bupivacaine 0.25%
|
I2: SAP using the same volume of bupivacaine 0.25% |
Length of follow-up: 24h
Loss-to-follow-up/ Incomplete outcome data:
two patients in the control group refused to continue the study, three patients in the PECS group, and two patients in the serratus group were excluded due to failed block
sample for analyses: I1: 57 I2: 58 |
Postoperative pain visual analogue scale (VAS) where 0 =no pain and 10 = the worst imaginable pain
median (range)
At rest immediate postop I1: 0 (0–3) I2: 0 (0–2)
at 12h I1: 0 (0–3) I2: 0 (0–2)
at 24h I1: 0 (0–2) I2: 0 (0–2)
At shoulder movement immediate postop I1: 0 (0–3) I2: 1 (0–3)
at 12h I1: 1 (0–3) I2: 1 (0–3)
at 24h I1: 1 (0–3) I2: 1 (0–3)
Opioid consumption: postoperative morphine consumption in 24 h* I1: single dose of 2 mg I2: single dose of 2 mg
No. (%) of patients requiring postoperative morphine I1: 11 (19.3%) I2: 14 (24.1%)
Adverse events No cases of pneumothorax, local anesthetic toxicity, or opioid side effects as respiratory depression, pruritus, or urinary retention were recorded |
Study aim: We assumed that PECSII could safely provide a better analgesic profile with an opioid-sparing effect than SAPB. We performed this study to evaluate this assumption.
Primary and secondary outcome measures: The total amount of morphine consumed in the first 24 postoperative hours was the primary outcome measure.
Secondary outcomes were the intraoperative fentanyl requirements, time to first rescue analgesia and VAS scores at rest and during shoulder movement immediately postoperative (defined as 15 min post-extubation) and at 1 hour, 4 hours, 8 hours, 12 hours and 24 hours postoperatively. Ramsey sedation score (RSS) was assessed at the same time points. Heart rate and MAP were recorded intraoperatively (1-minute pre-incision, 1-minute postincision) and postoperatively at 1, 4, 8, 12, 24 hours. Postoperative nausea and vomiting (PONV) were rated on a four-point verbal scale (none =no nausea, mild = nausea but no vomiting, moderate = vomiting one attack, severe = vomiting > one attack). PONV was treated by 10mg metoclopramide slowly IV. All other complications such as pneumothorax, local anesthetic systemic toxicity, respiratory depression defined as a respiratory rate
* Fewer patients in the PECS and SAPB groups needed morphine injections during the first 24 hours compared to the control group (p < 0.001). Those who requested morphine in the PECS and SAPB groups consumed a single dose of 2 mg, while the median consumed dose in the control group was 7 mg (range: 2–12) (p < 0.001) |
|
Jain (2020) |
Study design: RCT; prospective, randomized interventional nonblinded study
Setting and country: India Institute of Medical Sciences, New Delhi, India
Funding and conflicts of interest Funding: Nil. Conflict: reported that there are no conflicts of interest |
Inclusion criteria: (1) ASA grades I and II (2) female patients (3) aged 18–65 years (4) undergoing elective unilateral mastectomies with or without axillary dissection
Exclusion criteria: Patients with infection at the site of proposed block, chest wall deformity, coagulopathy, or receiving any anticoagulants, body mass index ≥35 kg/m2 , mental retardation were excluded from the study
N total at baseline: I: 16 C: 15
Important prognostic factors2:
age (years) mean ± SD I: 45.47±8.98 C: 60.87±11.9
ASA (I/II) n I: 12/3 C: 11/4
Surgical duration in min I: 109±27.14 C: 100.67±28.53
Groups comparable at baseline? Yes, p-values demographic variables non-significant |
USG modified PECS (PECS II) block
30ml 0.375% ropivacaine |
USG SAP
30ml 0.375% ropivacaine |
Length of follow-up: Not specifically reported, but it is stated that patient was kept in PACU for 24 h postoperative period and the outcomes measurements were performed up to 24h
Loss-to-follow-up: Reported there was no loss to follow up
Incomplete outcome data: I: PECS group: did not receive allocated intervention (anatomy distorted due to previous surgery)
C: SAP group: all received allocated intervention |
Postoperative pain (VAS 0‑10) at rest and ipsilateral abduction of arm* with 0 corresponding to no pain and 10 being the worst imaginable pain.
“Pain scores were not significantly different among the group in the postoperative 24 h period. Only three patients gave a response at immediate postoperative and therefore VAS scores were not assessed at this point of time. At 6 h, VAS scores were reaching significant difference among PECS and SAP block.”
Opioid consumption: Postoperative fentanyl requirement in 24h in µg (mean ± SD)[95%CI] I: PECS: 644.67 ± 260.15 [95% CI 514.67–774.67] C: SAP: 415 ± 182.44 [95% CI 323–507]
Adverse events No complication like inadverdent vascular puncture, pneumothorax, hypotension was seen in any group.
|
Study aim: to compare the postoperative 24‑h fentanyl consumption in each of the three blocks. Secondary objectives were to compare spread of local anesthetic, sensory blockade produced, onset of the sensory blockade, intraoperative opioids requirement, pain scores for 24 h postoperative period, time to first postoperative analgesia requirement and incidence of postoperative nausea and vomiting (PONV).
* Pain scores (VAS 1‑10) were measured at rest and ipsilateral abduction of arm at 1, 2, 3, 6, 12, and 24‑hour postoperative. à very limited results
Conclusion: TPVB and SAP group provide equivalent analgesia in patients undergoing mastectomies. PECS II block is inferior to TPVB and SAP block in providing analgesia for breast surgeries. Single‑shot TPVB and SAP block also result in greater spread of the drug along with sensory block compared to PECS II block. SAP block is easier to perform than TPVB with lesser chances of complications and results in faster onset. Thus, we recommend SAP block for patients undergoing mastectomies for effective analgesia |
|
Fuiji 2019 |
Study design: RCT
Setting and country: Nagoya University Hospital, Nagoya, Japan
Funding and conflicts of interest No external funding or competing interests declared |
Inclusion criteria: women scheduled for breast cancer resection by mastectomy at the Nagoya University Hospital, Nagoya, Japan, between August 2015 and August 2018. Participants were ≥ 20 years old and ASA physical status 1 or 2.
Exclusion criteria: We did not study women: < 20 years old or ASA physical status > 2; with estimated glomerular filtration rate < 30 ml.min1 .1.73 m2 or Child–Pugh liver score ≥ B; allergic to ropivacaine; who had mastectomy that was radical, partial or followed by immediate reconstruction or within six months of previous mastectomy.
N total at baseline: I: 40 C: 40
Important prognostic factors2:
age (years) mean ± SD I: 58.4 ± 12.7 C: 57.9 ± 13.4
ASA (I/II) n (%) I: 26/14 C: 30/10
Operation time (min) I: 111 ± 36 C: 122 ± 49
Groups comparable at baseline? Yes, no sig. p-values on baseline characteristics
|
PECS II block
10 ml ropivacaine 0.5% and 20 ml ropivacaine 0.5% |
SAP
30 ml ropivacaine 0.5% |
Length of follow-up: 6 postoperative months
Loss-to-follow-up: no loss to follow up
Incomplete outcome data: Not reported, but clear that there was none |
Postoperative pain 24h a 0–100 mm visual analogue scale (VAS)
median (IQR [range])
I: 18 (11–27 [0–61]) mm C: 23 (11–35 [0–70]) mm
Opioid consumption: Morphine consumption in first 24 h
median (IQR [range])
I: 4 (2–7 [0–37]) mg C: 6 (3–9 [1–25]) mg
Chronic pain after 6 months 11-point numerical rating scale (NRS), defined as: 1–3, mild pain; 4–6, moderate pain; and 7–10, severe pain
moderate or severe chronic pain n (%)
I: 4/40 (10%) C: 13/40 (33%) adjusted OR (95%CI) 0.23 (0.07–0.80), p = 0.02
|
Study aim: to test the effects of the PECS 2 block compared with the serratus plane block for pain after mastectomy.
Primary and secondary outcomes We defined the primary outcome as the rate of pain worse than mild at six postoperative months.
Secondary outcomes at six postoperative months were the rate of participants without pain and the health-related quality of life. Acute secondary outcomes were morphine consumption within 24 postoperative hours and the pain score.
Authors conclusion: In conclusion, the PECS 2 block reduced chronic pain six months after mastectomy compared with serratus plane block |
Notes:
- Prognostic balance between treatment groups is usually guaranteed in randomized studies, but non-randomized (observational) studies require matching of patients between treatment groups (case-control studies) or multivariate adjustment for prognostic factors (confounders) (cohort studies); the evidence table should contain sufficient details on these procedures
- Provide data per treatment group on the most important prognostic factors [(potential) confounders]
- For case-control studies, provide sufficient detail on the procedure used to match cases and controls
- For cohort studies, provide sufficient detail on the (multivariate) analyses used to adjust for (potential) confounders
Evidence table for systematic review of RCTs and observational studies (intervention studies)
Research question: What are the favorable and unfavorable effects of various thoracic wall blocks in adult patients undergoing mamma surgery?
|
Study reference |
Study characteristics |
Patient characteristics |
Intervention (I) |
Comparison / control (C)
|
Follow-up |
Outcome measures and effect size |
Comments |
|
Comparison 2: ESP versus PV |
|||||||
|
Xiong, 2021
[individual study characteristics deduced from Xiong, 2021]
PS., study characteristics and results are extracted from the SR (unless stated otherwise) |
SR and meta-analysis of
Literature search up to April 2021
10 RCTs, 4 included in current analysis
A: Gurkan, 2020 B: Moustafa, 2020 C: Swisher, 2020 D: Ghamry, 2019
Study design: RCT
Setting and Country: Single-center, China
Source of funding and conflicts of interest: Funding: This study was supported by the Science and Technology Research Project of Jinhua (http:// kjj.jinhua.gov.cn/art/2021/4/2/art_1229317787_ 3832377.html), grant number (2021-4-004). Xiong Chang was responsible for this. The sponsors decided to publish this study Conflict: The authors have declared that no competing interests exist.
|
Inclusion criteria SR: (1) RCTs; (2) Adult patients (over 18 years old) who underwent thoracic or breast surgery; (3) Interventional use of PVB for postoperative analgesia (PVB group); (4) Use of ESPB for postoperative analgesia (ESPB group) in the control group.
Primary outcomes RS: Pain scores at rest and movement at 0–1, 4–6, 8–12, and 24 hours after surgery
Secondary outcomes SR: Opioid (morphine equivalent) consumption at 24 hours after surgery; Incidence of additional analgesia in 24 hours post-operation; Time required for completing block procedure; Incidence of postoperative nausea and vomiting (PONV); publication bias
Exclusion criteria SR: (1) Incomplete studies; (2) Unreported relevant outcomes.
10 studies included (4 in current analysis)
Important patient characteristics at baseline:
N, mean age (I/C) A: 25/25, 49.08/49.4 B: 45/45, - C: 50/50, 55.4/55.4 D: 35/35, 37.7/41
Duration of surgery (min.) A: 89.4/82.2 B: - C: 77.9/75.7 D: 170/ 173.2
Country A: Turkey B: Egypt C: USA D: Egypt
Groups comparable at baseline? Yes |
ESP
A: 20 ml 0.25% bupivacaine B: 20 ml 0.25% bupivacaine C: 25 ml 0.5% ropivacaine unilateral D: 20 ml of 0.25% bupivacaine
|
PV
A: 20 ml 0.25% bupivacaine B: 20 ml 0.25% bupivacaine C: 25 ml 0.5% ropivacaine unilateral D: 20 ml of 0.25% bupivacaine
|
End-point of follow-up: Not reported, but results up to 24h post-surgery
For how many participants were no complete outcome data available? (intervention/control)
Not reported
|
Postoperative pain (MDs were reported)
Mean ± SD
At rest 0-1h A: I: 2 ± 2.22 C: I: 3 ± 1.48 D: I: 1.33 ± 0.74
N=100 (MD =-0.39, 95% CI [-1.12, 0.35]. heterogeneity (P=0.06, I2=65%). [but comparison different way around]
At rest 4-6h A: I: 1 ±1.48 D: I: 1.62 ± 0.5
N=60 (MD =-0.12, 95% CI [-0.34, 0.10]. heterogeneity (P=1.00, I2=0%). [but comparison different way around]
At rest 8-12h A: I: 0.66 ± 1.48 D: I: 3 ± 1.48
N=60 (MD =0.30, 95% CI [-0.29, 0.88]. heterogeneity (P=0.88, I2=0%). [but comparison different way around]
At rest 24h A: I: 0.33 ± 0.74 C: I: 3 ± 1.48 D: I: 3.5 ± 2.2
N=60 (MD =-0.03, 95% CI [-0.53, 0.47]. heterogeneity (P=0.31, I2=16%). [but comparison different way around]
Postoperative opioid consumption at 24h
Mean ± SD
A: I: 5.6 ± 3.43 B: I: 6.17 ± 2.08 C: I: 5 ± 7.63 D: I: 26.7 ± 2.1
N=155 (MD =0.31, 95% CI [-0.34, 0.97]. heterogeneity (P=0.39, I2=1%). [but comparison different way around]
|
SR aim: to assess the postoperative analgesic effects of PVB and ESPB for thoracic and breast surgery
SR conclusion: The postoperative analgesic effects of PVB versus ESPB are distinguished by the surgical site. For thoracic surgery, the postoperative analgesic effect of PVB is better than that of ESPB; for breast surgery, the postoperative analgesic effects of PVB and ESPB are similar. At the same time, there is no significant statistical difference between the two postoperative analgesic techniques in PONV, but the block procedure time required for ESPB is significantly shorter than that for PVB. Therefore, when choosing postoperative analgesia techniques, the results of this meta-analysis recommend PVB for thoracic surgery and ESPB for breast surgery.
Quality assessment: The quality of included RCTs was assessed by two independent researchers (Chang Xiong and Chengpeng Han) using the Cochrane Collaboration Risk of Bias tool and the Jadad Score.
Six studies [18,20–24] displayed a high risk of bias arising from detection, attrition and reporting. The remaining four articles [19,25–27] had unclear risks of bias. The outcomes of risk assessment are presented in Fig 2. All trials were identified as high quality, according to the Jadad Score. The Jadad Score is shown in Table 1.
A, B, C, D = high risk of bias
No GRADE
Publication bias No substantial asymmetry was detected throughout visual examination of the funnel plot (S1 Fig). Also, the Egger’s test showed that the P-value for each outcome was greater than 0.1, indicating no publication bias existed in this meta-analysis (S1 Table).
|
|
Comparison 4: PECS II versus PV |
|||||||
|
Elshanbary, 2021
[individual study characteristics deduced from Elshanbary, 2021]
PS., study characteristics and results are extracted from the SR (unless stated otherwise) |
SR and meta-analysis of
Literature search up to July 2020
A: Abo-Sabaa 2019 B: Siddeshwara 2019 C: Tripathy 2019 D: Annamalai 2017 E: Syal &Chandel 2017 F: Kulhari 2016 G: Wahba & Kamal 2014
Study design: RCT [parallel]
Setting and Country: Multi-center, Egypt and Palestine
Source of funding and conflicts of interest: Funding: not reported Conflict: the authors declare no conflict of interest
|
Inclusion criteria SR: (1) female adult patients undergoing breast cancer surgeries (2) studies that compared Pecs I or Pecs II to other methods of analgesia.
Exclusion criteria SR: (1) observational, retrospective, and animal studies, theses, reviews, abstracts, nonrandomized clinical trials, and studies including patients undergoing breast augmentation surgery
47 studies included (7 in current analysis)
Important patient characteristics at baseline:
N, mean age (I/C) A: 24/25, 48.37/47.8
ASA Status I:II (I/C) A: 14:16/15:15
Type of surgery A: MRM, lumpectomy, simple mastectomy, ALND
|
PECS II
*A: 20 ml of 0.25% bupivacaine+ 10 ml of 0.25% bupivacaine *B: 15 ml mixture of levobupivacaine 0.25% and dexamethasone + 10 ml mixture of levobupivacaine 0.25% and dexamethasone *C: 30 ml of 0.1% lignocaine + 0.25% bupivacaine + 1 μg/kg dexmedetomidine *D: - *E: 10 ml 0.5% bupivacaine (50 mg) with 0.5 ml of adrenaline 1:10000 (50 μg)+ 10 ml of bupivacaine 0.5% (50 mg) with 0.5 ml of adrenaline 1:10000 (50 μg) *F: ropivacaine 0.5%, 25 ml *G: 10 ml of levobupivacaine 0.25% + 20 ml levobubivacaine 0.25%
|
PV
*A: 20 ml of 0.25% bupivacaine *B: 25 ml levobupivacaine 0.25% and dexamethasone C: 30 ml of 0.1% lignocaine + 0.25% bupivacaine + 1 μg/kg dexmedetomidine *D: - *E: 20 ml of 0.5% bupivacaine to which 1 ml of 1:10000 dilution adrenaline [100 μg] *F: ropivacaine 0.5%, 25 ml *G: 15–20 ml of levobupivacaine 0.25% |
End-point of follow-up:
For how many participants were no complete outcome data available? (intervention/control) Not reported
|
Postoperative pain Not reported how measured.
Mean ± SD
At 0h A: I: 0.4 ± 0.5 C: 1.43 ± 0.82 B: I: 3.67 ± 0.79 C: 5 ± 2.59 C: - D: - E: I: 2.67 ± 0.79 C: 3.67 ± 0.79 F: I: 1.16 ± 0.37 C: 2 ± 0 G: -
N=91 (SMD =-1.15, 95% CI [-1.63, -0.67], P= 0.17). Heterogeneity (P<0.00001, I2=43%)
At 6h A: I: 2.2 ± 1.06 C: 2.8 ± 1 B: I: 3 ± 2.59 C: 4 ± 1.59 C: - D: - E: I: 4 ± 1.58 C: 4 ± 1.58 F: I: 2.16 ± 0.37 C: 2.5 ± 1.2 G: I: 4.67 ± 0.73.90.8 C: 5 ± 1.56
N=121 (SMD =-0.34, 95% CI [-0.60, -0.09]. heterogeneity (P=0.69, I2=0%)
At 12h A: I: 1.87 ± 1.53 C: 2.97 ± 0.67 B: I: 4 ± 1.59 C: 5 ± 1.59 C: - D: - E: I: 4.33 ± 0.79 C: 3.33 ± 0.79 F: I: 1.33 ± 0.79 C: 2.17 ± 1.99 G: I: 3.67 ± 0.78 C: 4 ± 1.56
N=120 (SMD =-0.24, 95% CI [-0.92, 0.44]. heterogeneity (P<0.0001, I2=85%)
At 24h A: I: 2.33 ± 0.76 C: 2.3 ± 0.47 B: I: 4 ± 1.59 C: 5 ± 1.59 C: - D: - E: I: 4 ± 1.59 C: 3 ± 1.59 F: I: 1 ± 0.01 C: 1 ± 0.01 G: I: 5.33 ± 0.78 C: 3 ± 1.56
N=120 (SMD =0.38, 95% CI [-0.42, 1.18]. heterogeneity (P<0.0001, I2=89%)
Postoperative opioid consumption Not reported how measured or which opioid
Mean ± SD
A: I: 7.5 ± 2.53 C: 10 ± 0 B: I: 11.25 ± 4.75 C: 15 ± 4.86 C: I: 1.21 ± 1.2 C: 1.07 ± 1.1 D: - E: I: 0.5 ± 0.5 C: 0.2 ± 0.4 F: I: 3.9 ± 0.79 C: 5.3 ± 0.98 G: I: 22 ± 3.7 C: 27 ± 6.67
N= 287 (SMD =-0.48, 95% CI [-1.20, 0.24], P= 0.19) (Fig. 4). Pooled results were heterogeneous (P<0.00001, I2=86%) and the heterogeneity could not be solved by leave 1 out.
|
*extracted from individual studies
Aim: to compare Pecs block methods with control and other techniques, including PVB, ESPB, and LA methods, and to measure the effect of the addition of dexmedetomidine to Pecs II block.
*Methods: A: Pain: VAS 0-10cm Opioid: total morphine rescue B: Pain: NRS Opioid: morphine C: Pain: VAS 0-10cm Opioid: - D: E: Pain: VAS 0-10cm Opioid: fentanyl F: Pain: VAS 0-10 Opioid: patient-controlled morphine G: Pain: NRS 0-10 Opioid: morphine
Brief description of conclusion individual studies (table 2) A: Simple mastectomy, with a lack of contraindications and side effects complications producing any side effects, compared with PVB
Author conclusion: In conclusion, the Pecs block increases the analgesia quality in BC surgeries; reduces intraoperative and postoperative opioid consumption compared with control and ESPB. Furthermore, Pecs II block reduces the number of patients required analgesia and increases the duration of analgesia compared with control. Compared with PVB, Pecs II is better regarding pain relief. The combination of Pecs II with dexmedetomidine showed lower postoperative opioid consumption with increased duration of analgesia and better pain relief. We recommend Pecs II block to be a first-line option for analgesia in BC surgery.
Quality assessment: The quality of the included RCTs was moderate to high. We analyzed all outcomes related to BC surgeries. We used the random-effect model for analysis and did a sensitivity analysis, and assessed the publication bias.
GRADE not included |
|
Comparison 6: PECS II versus ESP |
|||||||
|
Elshanbary, 2021
[individual study characteristics deduced from Elshanbary, 2021]
PS., study characteristics and results are extracted from the SR (unless stated otherwise) |
SR and meta-analysis of
Literature search up to July 2020
A: Altıparmak, 2018 B: Gad, 2019 C: Sinha, 2019
Study design: RCT [parallel]
Setting and Country: Multi-center, Egypt and Palestine
Source of funding and conflicts of interest: Funding: not reported Conflict: the authors declare no conflict of interest
|
Inclusion criteria SR: (1) female adult patients undergoing breast cancer surgeries (2) studies that compared Pecs I or Pecs II to other methods of analgesia.
Exclusion criteria SR: (1) observational, retrospective, and anim23al studies, theses, reviews, abstracts, nonrandomized clinical trials, and studies including patients undergoing breast augmentation surgery
47 studies included (3 in current analysis)
Important patient characteristics at baseline:
N, mean age (I/C) A: 18/20; 53.56/57.55 B: 23/24; 53.80/53.63 C: 30/30; 49.6/48.61
ASA Status I:II (I/C) A: 8:10/7:13 B: - C: 20:10/22:8
Type of surgery A: MRM+ALND C: MRM
*Country A: Turkey C: India
Groups comparable at baseline? |
PECS II
*A: 10 ml of 0.25% bupivacaine + 20 ml of bupivacaine 0.25% *B: 10ML+20ML - 0.25% levobupivacaine plus 0.5 μ/kg dexmedetomidine *C: 2 ml of 2% lignocaine; 15 ml of 0.2% ropivacaine; 10 ml of 0.2% ropivacaine |
ESP
*A: 2-3ml of isotonic saline solution + 20 ml of bupivacaine *B: 20ML - 0.25% levobupivacaine plus 0.5 μ/kg dexmedetomidine *C: 2 ml of 2% lignocaine; 20 ml of 0.2% ropivacaine was injected
|
*End-point of follow-up: A: 24h B: 24h C: 24h
*For how many participants were no complete outcome data available? A: I: 2/ C: 0 B: I: 2/ C: 1 (lost to follow-up) C: none
|
Postoperative pain Not reported how measured.
Mean ± SD
At 6h A: I: - C: - B: I: 20 ± 5 C: 22.5 ± 7.5 C: I: 2.73 ± 0.78 C: 3.3 ± 0.82
At 12h A: I: 2 ± 1 C: 2.75 ± 0.75 B: I: 20 ± 10 C: 27.5 ± n7.5 C: 3.1 ± 0.7 C: 3.3 ± 0.69
At 24h A: I: 1.25 ± 0.75 C: 1.25 ± 0.75 B: I: 12.5 ± 7.5 C: 12.5 ± 7.5 C: I: 2.23 ± 0.632 C: 2.6 ± 0.48
Postoperative opioid consumption Not reported how measured or which opioid
Mean ± SD
A: I: 132.78 ± 22.44 C: 196 ± 27.03 B: I: 10.73 ± 3.12 C: 16.7 ± 7.2 C: I: 4.4 ± 0.94 C: 6.59 ± 1.35
|
*extracted from individual studies
Aim: to compare Pecs block methods with control and other techniques, including PVB, ESPB, and LA methods, and to measure the effect of the addition of dexmedetomidine to Pecs II block.
*Method: A: Pain: NRS 0-10 Opioid: tramadol Opioid: morphine Opioid: morphine (mg)
Brief description of conclusion individual studies (table 2) A: Pecs II block decreased postsurgical opioid usage and pain scores compared with ESPB
Author conclusion: In conclusion, the Pecs block increases the analgesia quality in BC surgeries; reduces intraoperative and postoperative opioid consumption compared with control and ESPB. Furthermore, Pecs II block reduces the number of patients required analgesia and increases the duration of analgesia compared with control. Compared with PVB, Pecs II is better regarding pain relief. The combination of Pecs II with dexmedetomidine showed lower postoperative opioid consumption with increased duration of analgesia and better pain relief. We recommend Pecs II block to be a first-line option for analgesia in BC surgery.
Quality assessment/ publication bias: The quality of the included RCTs was moderate to high. We analyzed all outcomes related to BC surgeries. We used the random-effect model for analysis and did a sensitivity analysis, and assessed the publication bias.
GRADE not included |
Table of excluded studies
|
Reference |
Reason for exclusion |
|
Singh, 2022 |
wrong study design |
|
Genc, 2022 |
PICO; wrong block |
|
Abu Elyazed, 2021 |
PICO; wrong block |
|
Kaur, 2020 |
PICO: serratus-intercostal fascial plane vs PECS II |
|
Elsabeeny, 2020 |
PICO: 1. intravenous morphine vs 2. SAPB and ESPB |
|
Kundra, 2013 |
PICO: Comparison of paravertebral and interpleural block |
|
Karmakar, 2014 |
PICO: continuous vs single shot TPB |
|
Eldeen, 2016 |
PICO: PECS II vs thoracic spinal blockade |
|
Murouchi, 2016 |
PICO: RLB or paravertebral block (PVB) |
|
Ueshima, 2017 |
Article is retracted |
|
Song, 2020 |
PICO: PECS I + SIPB (PS) or PSI block + PECSI + SIPB (PSP) groups. |
|
Song, 2020 |
PICO: PECS I + SIPB (PS) or PSI |
|
Sotome, 2021 |
PICO: Erector spinae plane block versus retrolaminar block |
|
Rokhtabnak, 2021 |
PICO: Pectoral Nerve Block II Versus Conventional Intercostal Nerve Block |
|
Ciftci, 2021 |
PICO: Ultrasound-Guided Type-II Pectoral Nerve Block and Rhomboid Intercostal Block |
|
Huang, 2020 |
Primarily thoracic studies, almost no mamma surgeries, not a complete and usable SR |
|
Fanelli, 2021 |
Other SR's are more recent and/or complete; only opioid consumption as outcome measure |
|
Leong, 2021 |
Other SR's are more recent and/or complete |
|
Hong, 2021 |
Other SR's are more recent and/or complete |
|
Singh, 2018 |
Other SR's are more recent and/or complete |
|
Versyck, 2019 |
Other SR's are more recent and/or complete |
|
Grape, 2020 |
Other SR's are more recent and/or complete |
|
Singh, 2020 |
Commentary: geen volledige SR |
|
Wahba, 2014 |
In included SR |
|
Kulhari, 2016 |
In included SR |
|
Hetta, 2016 |
In included SR |
|
Gupta, 2017 |
In included SR |
|
Syal, 2017 |
In included SR |
|
Altıparmak, 2019 |
In included SR |
|
El Ghamry, 2019 |
In included SR |
|
Gad, 2019 |
In included SR |
|
Joshi, 2019 |
In included SR |
|
Siddeshwara, 2019 |
In included SR |
|
Sinha, 2019 |
In included SR |
|
Tripathy, 2019 |
In included SR |
|
Moustafa, 2020 |
In included SR |
|
Swisher, 2020 |
In included SR |
|
Gürkan, 2020 |
In included SR |
|
Chin, 2021 |
No original research (descriptive review) |
|
Guan, 2021 |
Not found |
|
Parikh, 2018 |
Comparsion with ERAS, observational study design |
|
Huan, 2020 |
PICO: comparsion intercostal nerve block |
|
Meissner, 2021 |
PICO: vs no or sham block (vergelijking met RA is onduidelijk beschreven, SR niet bruikbaar) |
|
Asserson, 2021 |
PICO: nerve block vs no block (bovendien alleen in body contouring breast surgery) |
|
Li, 2021 |
PICO: ESPB vs no block |
|
Jacobs, 2020 |
Guideline, not usable as SR and not conform PICO (broader) |
|
Abi-Rafeh, 2022 |
Excluded |
|
Wu, 2021 |
In Chinese language |
|
Martsiniv, 2020 |
Not english language |
|
Wang. 2019 |
In Chinese language |
Verantwoording
Beoordelingsdatum en geldigheid
Publicatiedatum : 26-09-2023
Beoordeeld op geldigheid : 01-09-2023
Algemene gegevens
De ontwikkeling/herziening van deze richtlijnmodule werd ondersteund door het Kennisinstituut van de Federatie Medisch Specialisten (www.demedischspecialist.nl/kennisinstituut) en werd gefinancierd uit de Kwaliteitsgelden Medisch Specialisten (SKMS).
De financier heeft geen enkele invloed gehad op de inhoud van de richtlijnmodule.
Samenstelling werkgroep
Voor het ontwikkelen van de richtlijnmodule is in 2021 een multidisciplinaire werkgroep ingesteld, bestaande uit vertegenwoordigers van alle relevante specialismen (zie hiervoor de Samenstelling van de werkgroep) die betrokken zijn bij de zorg voor patiënten met postoperatieve pijn.
Samenstelling van de werkgroep
Werkgroep
Prof. dr. J. (Jörgen) Bruhn, anesthesioloog, (voorzitter) NVA
Prof. dr. dr. M.W. (Markus) Hollmann, anesthesioloog, NVA
Dr. M.F. (Markus) Stevens, anesthesioloog, NVA
Drs. L.J.H. (Lea) van Wersch, anesthesioloog, NVA
Dr. M.H.J. (Margot) Roozekrans, anesthesioloog, NVA
Dr. S.A.S. (Sandra) van den Heuvel, anesthesioloog/pijnspecialist, NVA
Drs. S.J. (Stijn) Westerbos, orthopeed, NOV
Drs. W.L. (Wilson) Li, cardiothoracaal chirurg, NVT
S.F. (Cedric) Lau MSc, ziekenhuisapotheker, NVZA
Dr. R.L.M. (Rianne) van Boekel, verpleegkundig pijnconsulent, V&VN
Drs. I.L. (Ilona) Thomassen-Hilgersom, patiëntvertegenwoordiger, Samenwerkingverband Pijnpatiënten naar één stem
Klankbordgroep
Drs. N.C. (Niels) Gritters van den Oever, intensivist, NVIC
J.P. (Patrick) Rensink, anesthesiemedewerker/pijnconsulent, NVAM
Dr. G. (Gijs) Helmerhorst, orthopeed, NOV
Dr. C.D. (Cor) de Kroon, gynaecoloog-oncoloog, NVOG
Dr. W.J. (Wietse) Eshuis, chirurg, NVvH
Dr. D. (Daphne) Roos, chirurg, NVvH
Met ondersteuning van
Dr. F. Willeboordse, senior adviseur, Kennisinstituut van de Federatie Medisch Specialisten
Dr. L.M.P. Wesselman, adviseur, Kennisinstituut van de Federatie Medisch Specialisten
Dr. L.M. van Leeuwen, adviseur, Kennisinstituut van de Federatie Medisch Specialisten
I. van Dijk, junior adviseur, Kennisinstituut van de Federatie Medisch Specialisten
Belangenverklaringen
De Code ter voorkoming van oneigenlijke beïnvloeding door belangenverstrengeling is gevolgd. Alle werkgroepleden hebben schriftelijk verklaard of zij in de laatste drie jaar directe financiële belangen (betrekking bij een commercieel bedrijf, persoonlijke financiële belangen, onderzoeksfinanciering) of indirecte belangen (persoonlijke relaties, reputatiemanagement) hebben gehad. Gedurende de ontwikkeling of herziening van een module worden wijzigingen in belangen aan de voorzitter doorgegeven. De belangenverklaring wordt opnieuw bevestigd tijdens de commentaarfase.
Een overzicht van de belangen van werkgroepleden en het oordeel over het omgaan met eventuele belangen vindt u in onderstaande tabel. De ondertekende belangenverklaringen zijn op te vragen bij het secretariaat van het Kennisinstituut van de Federatie Medisch Specialisten.
|
Werkgroeplid |
Functie |
Nevenfuncties |
Gemelde belangen |
Ondernomen actie |
|
Bruhn, voorzitter |
Professor & Afdelingshoofd afdeling Anesthesiologie, Radboud UMC |
Editorial Board Journal of Clinical Monitoring and Computing, onbetaald |
Geen |
Geen actie |
|
Hollmann |
Professor & Afdelingshoofd afdeling Anesthesiologie, Amsterdam UMC, locatie AMC |
|
Geen |
Restricties t.a.v. modules over ketamine en lidocaïne. |
|
Lau |
|
Nationale Werkgroep morbide obesitas en bariatrische chirurgie (KNMP), vergoeding voor bestede uren |
Geen belangenverstrengeling, promotie-onderzoek is op een ander vlak dan waar beoogde werkgroep zich over buigt
|
Geen actie |
|
Boekel, van |
|
|
Geen |
Geen actie |
|
Thomassen-Hilgersom |
Voorzitter Samenwerkingsverband Pijnpatiënten naar één stem vrijwilliger en geen werkgever |
|
Geen |
Geen actie |
|
Li |
Cardiothoracaal chirurg (Radboudumc, Nijmegen) |
Bestuurslid NVT (Nederlandse Vereniging voor Thoraxchirurgie) |
Radboudumc zal in 2021 meedoen aan een RCT naar de optimale vorm van pijnstilling ten tijde van longchirugie (epiduraal versus paravertebraal) OPtriAL - met ZonMw subsidie, geïniteerd vanuit het MMC |
Geen actie |
|
Roozekrans |
Anesthesioloog - Pijnspecialist - Noordwest Ziekenhuisgroep |
Geen |
Geen |
Geen actie |
|
Stevens |
Chef de Clinique kinderanesthesie AUMC locatie AMC |
|
Geen |
Geen actie |
|
Heuvel, van den |
Anesthesioloog-pijnarts, Radboud UMC |
Geen |
Geen |
Geen actie |
|
Wersch, van |
Anesthesioloog, Maasziekenhuis Pantein |
Geen |
Geen |
Geen actie |
|
Westerbos |
Orthopeed, Alrijne ziekenhuis |
Geen |
Geen |
Geen actie |
|
Gritters van den Oever |
Anesthesioloog-intensivist Treant Zorggroep |
|
Geen |
Geen actie |
|
Rensink |
|
|
Geen |
Geen actie |
|
Kroon, de |
Gynaecoloog-oncoloog Leids Univesitair Medisch Centrum (1.0 fte) |
|
Geen |
Geen actie |
|
Roos |
Chirurg |
Geen |
Geen |
Geen actie |
|
Eshuis |
Chirurg, Amsterdam UMC |
Geen |
Geen |
Geen actie |
|
Willeboordse |
Senior adviseur Kennisinstituut van de Federatie Medisch Specialisten |
Geen |
Partner werkzaam bij Janssen Vaccines, onderdeel van Johnsson &Johnsson, via partner ook financiële belangen (aandelen J&J) |
Geen actie |
|
Wesselman |
Adviseur Kennisinstituut van de Federatie Medisch Specialisten |
|
|
Geen actie |
|
Leeuwen, van |
Adviseur Kennisinstituut van de Federatie Medisch Specialisten |
|
|
Geen actie |
|
Dijk, van |
Junior adviseur Kennisinstituut van de Federatie Medisch Specialisten |
Geen |
Geen |
Geen actie |
Inbreng patiëntenperspectief
Er werd aandacht besteed aan het patiëntenperspectief door zitting van een afgevaardigde van de patiëntenvereniging (Pijnpatiënten naar één stem) in de werkgroep. De Patiëntenfederatie Nederland en Pijnpatiënten naar één stem werden uitgenodigd voor de invitational conference. Het verslag hiervan [zie aanverwante producten] is besproken in de werkgroep. De verkregen input is meegenomen bij het opstellen van de uitgangsvragen, de keuze voor de uitkomstmaten en bij het opstellen van de overwegingen. De conceptrichtlijn is tevens voor commentaar voorgelegd aan de Patiëntenfederatie Nederland en Pijnpatiënten naar één stem en de eventueel aangeleverde commentaren zijn bekeken en verwerkt.
Wkkgz & Kwalitatieve raming van mogelijke substantiële financiële gevolgen
Kwalitatieve raming van mogelijke financiële gevolgen in het kader van de Wkkgz
Bij de richtlijn is conform de Wet kwaliteit, klachten en geschillen zorg (Wkkgz) een kwalitatieve raming uitgevoerd of de aanbevelingen mogelijk leiden tot substantiële financiële gevolgen. Bij het uitvoeren van deze beoordeling zijn richtlijnmodules op verschillende domeinen getoetst (zie het stroomschema op de Richtlijnendatabase).
Uit de kwalitatieve raming blijkt dat er waarschijnlijk geen substantiële financiële gevolgen zijn, zie onderstaande tabel.
|
Module |
Uitkomst raming |
Toelichting |
|
Module Organisatie van Zorg |
Geen substantiële financiële gevolgen |
Hoewel uit de toetsing volgt dat de aanbeveling(en) breed toepasbaar zijn (>40.000 patiënten), volgt ook uit de toetsing dat het overgrote deel (±90%) van de zorgaanbieders en zorgverleners al aan de norm voldoet en/of het geen nieuwe manier van zorgverlening of andere organisatie van zorgverlening betreft, het geen toename in het aantal in te zetten voltijdsequivalenten aan zorgverleners betreft en het geen wijziging in het opleidingsniveau van zorgpersoneel betreft. Er worden daarom geen substantiële financiële gevolgen verwacht. |
|
Module Transitionele Pijn Service (TPS) |
Geen substantiële financiële gevolgen |
Uit de toetsing volgt dat de aanbevelingen breed toepasbaar zijn (>40.000 patiënten). Hoewel de aanbeveling aangeeft dat een andere of nieuwe manier van zorgverlening gewenst is (i.e. andere manier van samenwerking/afstemming tussen zorgverleners) waarbij een TPS-model met TPS-team geïnitieerd wordt, laten eerste kosten-effectiviteitsstudies kostenbesparingen zien. De verwachting is dat TPS leidt tot betere zorg-op-maat, waarbij chronische postoperatieve pijn zorg doelmatiger wordt behandeld. Per ziekenhuis zal de vorm, intensiteit en organisatie van het TPS-model variëren. Zo kunnen ziekenhuizen ook kiezen voor een minder uitgebreid TPS. De aanbeveling geeft relatief veel ruimte voor de precieze invulling. Alle overwegingen tezamen, worden er geen substantiële financiële gevolgen verwacht. |
|
Module Pijnmeting |
Geen substantiële financiële gevolgen |
Hoewel uit de toetsing volgt dat de aanbeveling(en) breed toepasbaar zijn (>40.000 patiënten), volgt ook uit de toetsing dat het overgrote deel (±90%) van de zorgaanbieders en zorgverleners al aan de norm voldoet en/of het geen nieuwe manier van zorgverlening of andere organisatie van zorgverlening betreft, het geen toename in het aantal in te zetten voltijdsequivalenten aan zorgverleners betreft en het geen wijziging in het opleidingsniveau van zorgpersoneel betreft. Er worden daarom geen substantiële financiële gevolgen verwacht. |
|
Module Niet-medicamenteuze interventies |
Geen substantiële financiële gevolgen |
Hoewel uit de toetsing volgt dat de aanbeveling(en) breed toepasbaar zijn (>40.000 patiënten), volgt ook uit de toetsing dat het geen nieuwe manier van zorgverlening of andere organisatie van zorgverlening betreft, het geen toename in het aantal in te zetten voltijdsequivalenten aan zorgverleners betreft en het geen wijziging in het opleidingsniveau van zorgpersoneel betreft. Er worden daarom geen substantiële financiële gevolgen verwacht. |
|
Module Buikwandblokken |
Geen substantiële financiële gevolgen |
Hoewel uit de toetsing volgt dat de aanbeveling(en) breed toepasbaar zijn (>40.000 patiënten), volgt ook uit de toetsing dat het overgrote deel (±90%) van de zorgaanbieders en zorgverleners al aan de norm voldoet en/of het geen nieuwe manier van zorgverlening of andere organisatie van zorgverlening betreft, het geen toename in het aantal in te zetten voltijdsequivalenten aan zorgverleners betreft en het geen wijziging in het opleidingsniveau van zorgpersoneel betreft. Er worden daarom geen substantiële financiële gevolgen verwacht. |
|
Module Borstwandblokken bij mammachirurgie |
Geen substantiële financiële gevolgen |
Hoewel uit de toetsing volgt dat de aanbeveling(en) breed toepasbaar zijn (>40.000 patiënten), volgt ook uit de toetsing dat het overgrote deel (±90%) van de zorgaanbieders en zorgverleners al aan de norm voldoet en/of het geen nieuwe manier van zorgverlening of andere organisatie van zorgverlening betreft, het geen toename in het aantal in te zetten voltijdsequivalenten aan zorgverleners betreft en het geen wijziging in het opleidingsniveau van zorgpersoneel betreft. Er worden daarom geen substantiële financiële gevolgen verwacht. |
|
Module Borstwandblokken intrathoracaal |
Geen substantiële financiële gevolgen |
Hoewel uit de toetsing volgt dat de aanbeveling(en) breed toepasbaar zijn (>40.000 patiënten), volgt ook uit de toetsing dat het overgrote deel (±90%) van de zorgaanbieders en zorgverleners al aan de norm voldoet en/of het geen nieuwe manier van zorgverlening of andere organisatie van zorgverlening betreft, het geen toename in het aantal in te zetten voltijdsequivalenten aan zorgverleners betreft en het geen wijziging in het opleidingsniveau van zorgpersoneel betreft. Er worden daarom geen substantiële financiële gevolgen verwacht. |
|
Module Continue Wond infusie |
Geen substantiële financiële gevolgen |
Hoewel uit de toetsing volgt dat de aanbeveling(en) breed toepasbaar zijn (>40.000 patiënten), volgt ook uit de toetsing dat het geen nieuwe manier van zorgverlening of andere organisatie van zorgverlening betreft, het geen toename in het aantal in te zetten voltijdsequivalenten aan zorgverleners betreft en het geen wijziging in het opleidingsniveau van zorgpersoneel betreft. Er worden daarom geen substantiële financiële gevolgen verwacht. |
|
Module Cryoanalgesie |
Geen substantiële financiële gevolgen |
Uit de toetsing volgt dat de aanbeveling(en) niet breed toepasbaar zijn (<5.000 patiënten) en zal daarom naar verwachting geen substantiële financiële gevolgen hebben voor de collectieve uitgaven. |
|
Module Dexmethason |
Geen substantiële financiële gevolgen |
Hoewel uit de toetsing volgt dat de aanbeveling(en) breed toepasbaar zijn (>40.000 patiënten), volgt ook uit de toetsing dat het overgrote deel (±90%) van de zorgaanbieders en zorgverleners al aan de norm voldoet en/of het geen nieuwe manier van zorgverlening of andere organisatie van zorgverlening betreft, het geen toename in het aantal in te zetten voltijdsequivalenten aan zorgverleners betreft en het geen wijziging in het opleidingsniveau van zorgpersoneel betreft. Er worden daarom geen substantiële financiële gevolgen verwacht. |
|
Module Gabapentinoïden |
Geen substantiële financiële gevolgen |
Hoewel uit de toetsing volgt dat de aanbeveling(en) breed toepasbaar zijn (>40.000 patiënten), volgt ook uit de toetsing dat het overgrote deel (±90%) van de zorgaanbieders en zorgverleners al aan de norm voldoet en/of het geen nieuwe manier van zorgverlening of andere organisatie van zorgverlening betreft, het geen toename in het aantal in te zetten voltijdsequivalenten aan zorgverleners betreft en het geen wijziging in het opleidingsniveau van zorgpersoneel betreft. Er worden daarom geen substantiële financiële gevolgen verwacht. |
|
Module Ketamine |
Geen substantiële financiële gevolgen |
Hoewel uit de toetsing volgt dat de aanbeveling(en) breed toepasbaar zijn (>40.000 patiënten), volgt ook uit de toetsing dat het overgrote deel (±90%) van de zorgaanbieders en zorgverleners al aan de norm voldoet en/of het geen nieuwe manier van zorgverlening of andere organisatie van zorgverlening betreft, het geen toename in het aantal in te zetten voltijdsequivalenten aan zorgverleners betreft en het geen wijziging in het opleidingsniveau van zorgpersoneel betreft. Er worden daarom geen substantiële financiële gevolgen verwacht. |
|
Module Magnesium |
Geen substantiële financiële gevolgen |
Hoewel uit de toetsing volgt dat de aanbeveling(en) breed toepasbaar zijn (>40.000 patiënten), volgt ook uit de toetsing dat het overgrote deel (±90%) van de zorgaanbieders en zorgverleners al aan de norm voldoet en/of het geen nieuwe manier van zorgverlening of andere organisatie van zorgverlening betreft, het geen toename in het aantal in te zetten voltijdsequivalenten aan zorgverleners betreft en het geen wijziging in het opleidingsniveau van zorgpersoneel betreft. Er worden daarom geen substantiële financiële gevolgen verwacht. |
|
Module Methadon |
Geen substantiële financiële gevolgen |
Hoewel uit de toetsing volgt dat de aanbeveling(en) breed toepasbaar zijn (>40.000 patiënten), volgt ook uit de toetsing dat het overgrote deel (±90%) van de zorgaanbieders en zorgverleners al aan de norm voldoet en/of het geen nieuwe manier van zorgverlening of andere organisatie van zorgverlening betreft, het geen toename in het aantal in te zetten voltijdsequivalenten aan zorgverleners betreft en het geen wijziging in het opleidingsniveau van zorgpersoneel betreft. Er worden daarom geen substantiële financiële gevolgen verwacht. |
|
Module Beta blokkers -Esmolol |
Geen substantiële financiële gevolgen |
Hoewel uit de toetsing volgt dat de aanbeveling(en) breed toepasbaar zijn (>40.000 patiënten), volgt ook uit de toetsing dat het overgrote deel (±90%) van de zorgaanbieders en zorgverleners al aan de norm voldoet en/of het geen nieuwe manier van zorgverlening of andere organisatie van zorgverlening betreft, het geen toename in het aantal in te zetten voltijdsequivalenten aan zorgverleners betreft en het geen wijziging in het opleidingsniveau van zorgpersoneel betreft. Er worden daarom geen substantiële financiële gevolgen verwacht. |
|
Module Alpha 2 agonist - Clonidine |
Geen substantiële financiële gevolgen |
Hoewel uit de toetsing volgt dat de aanbeveling(en) breed toepasbaar zijn (>40.000 patiënten), volgt ook uit de toetsing dat het overgrote deel (±90%) van de zorgaanbieders en zorgverleners al aan de norm voldoet en/of het geen nieuwe manier van zorgverlening of andere organisatie van zorgverlening betreft, het geen toename in het aantal in te zetten voltijdsequivalenten aan zorgverleners betreft en het geen wijziging in het opleidingsniveau van zorgpersoneel betreft. Er worden daarom geen substantiële financiële gevolgen verwacht. |
|
Module Alpha 2 agonist – Dexmedetomidine |
Geen substantiële financiële gevolgen |
Hoewel uit de toetsing volgt dat de aanbeveling(en) breed toepasbaar zijn (>40.000 patiënten), volgt ook uit de toetsing dat het overgrote deel (±90%) van de zorgaanbieders en zorgverleners al aan de norm voldoet en/of het geen nieuwe manier van zorgverlening of andere organisatie van zorgverlening betreft, het geen toename in het aantal in te zetten voltijdsequivalenten aan zorgverleners betreft en het geen wijziging in het opleidingsniveau van zorgpersoneel betreft. Er worden daarom geen substantiële financiële gevolgen verwacht. |
|
Module Lidocaïne |
Geen substantiële financiële gevolgen |
Hoewel uit de toetsing volgt dat de aanbeveling(en) breed toepasbaar zijn (>40.000 patiënten), volgt ook uit de toetsing dat het overgrote deel (±90%) van de zorgaanbieders en zorgverleners al aan de norm voldoet en/of het geen nieuwe manier van zorgverlening of andere organisatie van zorgverlening betreft, het geen toename in het aantal in te zetten voltijdsequivalenten aan zorgverleners betreft en het geen wijziging in het opleidingsniveau van zorgpersoneel betreft. Er worden daarom geen substantiële financiële gevolgen verwacht. |
|
Module Multimodale pijnbestrijding |
Geen substantiële financiële gevolgen |
Hoewel uit de toetsing volgt dat de aanbeveling(en) breed toepasbaar zijn (>40.000 patiënten), volgt ook uit de toetsing dat het overgrote deel (±90%) van de zorgaanbieders en zorgverleners al aan de norm voldoet en/of het geen nieuwe manier van zorgverlening of andere organisatie van zorgverlening betreft, het geen toename in het aantal in te zetten voltijdsequivalenten aan zorgverleners betreft en het geen wijziging in het opleidingsniveau van zorgpersoneel betreft. Er worden daarom geen substantiële financiële gevolgen verwacht. |
Werkwijze
AGREE
Deze richtlijnmodule is opgesteld conform de eisen vermeld in het rapport Medisch Specialistische Richtlijnen 2.0 van de adviescommissie Richtlijnen van de Raad Kwaliteit. Dit rapport is gebaseerd op het AGREE II instrument (Appraisal of Guidelines for Research & Evaluation II; Brouwers, 2010).
Knelpuntenanalyse en uitgangsvragen
Tijdens de voorbereidende fase inventariseerde de werkgroep de knelpunten in de zorg voor patiënten met postoperatieve pijn.
De werkgroep beoordeelde de aanbevelingen uit de eerdere richtlijn Postoperatieve pijn (NVA, 2013) op noodzaak tot revisie. Tevens zijn er knelpunten aangedragen door relevante partijen middels een invitational conference.
Op basis van de uitkomsten van de knelpuntenanalyse zijn door de werkgroep concept-uitgangsvragen opgesteld en definitief vastgesteld.
Uitkomstmaten
Na het opstellen van de zoekvraag behorende bij de uitgangsvraag inventariseerde de werkgroep welke uitkomstmaten voor de patiënt relevant zijn, waarbij zowel naar gewenste als ongewenste effecten werd gekeken. Hierbij werd een maximum van acht uitkomstmaten gehanteerd. De werkgroep waardeerde deze uitkomstmaten volgens hun relatieve belang bij de besluitvorming rondom aanbevelingen, als cruciaal (kritiek voor de besluitvorming), belangrijk (maar niet cruciaal) en onbelangrijk. Tevens definieerde de werkgroep tenminste voor de cruciale uitkomstmaten welke verschillen zij klinisch (patiënt) relevant vonden.
Methode literatuursamenvatting
Een uitgebreide beschrijving van de strategie voor zoeken en selecteren van literatuur is te vinden onder ‘Zoeken en selecteren’ onder Onderbouwing. Indien mogelijk werd de data uit verschillende studies gepoold in een random-effects model. Review Manager 5.4 werd gebruikt voor de statistische analyses. De beoordeling van de kracht van het wetenschappelijke bewijs wordt hieronder toegelicht.
Beoordelen van de kracht van het wetenschappelijke bewijs
De kracht van het wetenschappelijke bewijs werd bepaald volgens de GRADE-methode. GRADE staat voor ‘Grading Recommendations Assessment, Development and Evaluation’ (zie http://www.gradeworkinggroup.org/). De basisprincipes van de GRADE-methodiek zijn: het benoemen en prioriteren van de klinisch (patiënt) relevante uitkomstmaten, een systematische review per uitkomstmaat, en een beoordeling van de bewijskracht per uitkomstmaat op basis van de acht GRADE-domeinen (domeinen voor downgraden: risk of bias, inconsistentie, indirectheid, imprecisie, en publicatiebias; domeinen voor upgraden: dosis-effect relatie, groot effect, en residuele plausibele confounding).
GRADE onderscheidt vier gradaties voor de kwaliteit van het wetenschappelijk bewijs: hoog, redelijk, laag en zeer laag. Deze gradaties verwijzen naar de mate van zekerheid die er bestaat over de literatuurconclusie, in het bijzonder de mate van zekerheid dat de literatuurconclusie de aanbeveling adequaat ondersteunt (Schünemann, 2013; Hultcrantz, 2017).
|
GRADE |
Definitie |
|
Hoog |
|
|
Redelijk |
|
|
Laag |
|
|
Zeer laag |
|
Bij het beoordelen (graderen) van de kracht van het wetenschappelijk bewijs in richtlijnen volgens de GRADE-methodiek spelen grenzen voor klinische besluitvorming een belangrijke rol (Hultcrantz, 2017). Dit zijn de grenzen die bij overschrijding aanleiding zouden geven tot een aanpassing van de aanbeveling. Om de grenzen voor klinische besluitvorming te bepalen moeten alle relevante uitkomstmaten en overwegingen worden meegewogen. De grenzen voor klinische besluitvorming zijn daarmee niet één op één vergelijkbaar met het minimaal klinisch relevant verschil (Minimal Clinically Important Difference, MCID). Met name in situaties waarin een interventie geen belangrijke nadelen heeft en de kosten relatief laag zijn, kan de grens voor klinische besluitvorming met betrekking tot de effectiviteit van de interventie bij een lagere waarde (dichter bij het nuleffect) liggen dan de MCID (Hultcrantz, 2017).
De beoordelingen van de literatuur en de conclusies zijn gedaan op basis van de GRADE systematiek. De werkgroep vindt het belangrijk om relevante beperkingen hiervan aan te geven.
De klinische vraag gaat vaak over een reductie van ernstige postoperatieve pijn en opioïdenconsumptie in een aantal patiënten met resp. over een klinisch relevante reductie van ernstige postoperatieve pijn opioïdenconsumptie bij een individuele patiënt. Hetzelfde geldt voor opioïdenconsumptie; de keuze van een absolute drempelwaarde in mg (i.p.v. een relatieve drempelwaarde in %) maakt dit afhankelijk van tijdstip, ingreep en ernst van de pijn: vroege postoperatieve tijdstippen en (studies met) ingrepen met relatief lage opioïdconsumptie kunnen vaak de MCID niet bereiken.
De keuze van de MCID heeft een bepaalde mate van willekeurigheid en is niet absoluut te zien. Ook zijn de conclusies zo geformuleerd (en geven alleen beperkt antwoord op het effect op een individuele patiënt voor een specifieke ingreep).
In de literatuur worden de eindpunten pijnscores en opioïdenconsumptie separaat van elkaar weer gegeven, suggererend dat deze onafhankelijk van elkaar zijn. Echter kunnen deze twee eindpunten niet onafhankelijk van elkaar beoordeeld worden; in ieder protocol is opgenomen dat pijn behandeld moet worden. Deze separate beoordeling geeft niet altijd een adequaat antwoord op de klinische vraag naar het analgetische effect van een interventie.
Daarnaast worden multimodale componenten als aparte interventies beoordeeld, echter de klinische vraag is naar de effectiviteit als bouwsteen van een multimodale werkwijze.
Overwegingen (van bewijs naar aanbeveling)
Om te komen tot een aanbeveling zijn naast (de kwaliteit van) het wetenschappelijke bewijs ook andere aspecten belangrijk en worden meegewogen, zoals aanvullende argumenten uit bijvoorbeeld de biomechanica of fysiologie, waarden en voorkeuren van patiënten, kosten (middelenbeslag), aanvaardbaarheid, haalbaarheid en implementatie. Deze aspecten zijn systematisch vermeld en beoordeeld (gewogen) onder het kopje ‘Overwegingen’ en kunnen (mede) gebaseerd zijn op expert opinion. Hierbij is gebruik gemaakt van een gestructureerd format gebaseerd op het evidence-to-decision framework van de internationale GRADE Working Group (Alonso-Coello, 2016a; Alonso-Coello 2016b). Dit evidence-to-decision framework is een integraal onderdeel van de GRADE methodiek.
De aanbevelingen geven antwoord op de uitgangsvraag en zijn gebaseerd op het beschikbare wetenschappelijke bewijs en de belangrijkste overwegingen, en een weging van de gunstige en ongunstige effecten van de relevante interventies. De kracht van het wetenschappelijk bewijs en het gewicht dat door de werkgroep wordt toegekend aan de overwegingen, bepalen samen de sterkte van de aanbeveling. Conform de GRADE-methodiek sluit een lage bewijskracht van conclusies in de systematische literatuuranalyse een sterke aanbeveling niet a priori uit, en zijn bij een hoge bewijskracht ook zwakke aanbevelingen mogelijk (Agoritsas, 2017; Neumann, 2016). De sterkte van de aanbeveling wordt altijd bepaald door weging van alle relevante argumenten tezamen. De werkgroep heeft bij elke aanbeveling opgenomen hoe zij tot de richting en sterkte van de aanbeveling zijn gekomen.
In de GRADE-methodiek wordt onderscheid gemaakt tussen sterke en zwakke (of conditionele) aanbevelingen. De sterkte van een aanbeveling verwijst naar de mate van zekerheid dat de voordelen van de interventie opwegen tegen de nadelen (of vice versa), gezien over het hele spectrum van patiënten waarvoor de aanbeveling is bedoeld. De sterkte van een aanbeveling heeft duidelijke implicaties voor patiënten, behandelaars en beleidsmakers (zie onderstaande tabel). Een aanbeveling is geen dictaat, zelfs een sterke aanbeveling gebaseerd op bewijs van hoge kwaliteit (GRADE gradering HOOG) zal niet altijd van toepassing zijn, onder alle mogelijke omstandigheden en voor elke individuele patiënt.
|
Implicaties van sterke en zwakke aanbevelingen voor verschillende richtlijngebruikers |
||
|
|
||
|
|
Sterke aanbeveling |
Zwakke (conditionele) aanbeveling |
|
Voor patiënten |
De meeste patiënten zouden de aanbevolen interventie of aanpak kiezen en slechts een klein aantal niet. |
Een aanzienlijk deel van de patiënten zouden de aanbevolen interventie of aanpak kiezen, maar veel patiënten ook niet. |
|
Voor behandelaars |
De meeste patiënten zouden de aanbevolen interventie of aanpak moeten ontvangen. |
Er zijn meerdere geschikte interventies of aanpakken. De patiënt moet worden ondersteund bij de keuze voor de interventie of aanpak die het beste aansluit bij zijn of haar waarden en voorkeuren. |
|
Voor beleidsmakers |
De aanbevolen interventie of aanpak kan worden gezien als standaardbeleid. |
Beleidsbepaling vereist uitvoerige discussie met betrokkenheid van veel stakeholders. Er is een grotere kans op lokale beleidsverschillen. |
Organisatie van zorg
In de knelpuntenanalyse en bij de ontwikkeling van de richtlijnmodule is expliciet aandacht geweest voor de organisatie van zorg: alle aspecten die randvoorwaardelijk zijn voor het verlenen van zorg (zoals coördinatie, communicatie, (financiële) middelen, mankracht en infrastructuur). Randvoorwaarden die relevant zijn voor het beantwoorden van deze specifieke uitgangsvraag zijn genoemd bij de overwegingen. Meer algemene, overkoepelende, of bijkomende aspecten van de organisatie van zorg worden behandeld in de module Organisatie van zorg.
Commentaar- en autorisatiefase
De conceptrichtlijnmodule werd aan de betrokken (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd ter commentaar. De commentaren werden verzameld en besproken met de werkgroep. Naar aanleiding van de commentaren werd de conceptrichtlijnmodule aangepast en definitief vastgesteld door de werkgroep. De definitieve richtlijnmodule werd aan de deelnemende (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd voor autorisatie en door hen geautoriseerd dan wel geaccordeerd.
Literatuur
Agoritsas T, Merglen A, Heen AF, Kristiansen A, Neumann I, Brito JP, Brignardello-Petersen R, Alexander PE, Rind DM, Vandvik PO, Guyatt GH. UpToDate adherence to GRADE criteria for strong recommendations: an analytical survey. BMJ Open. 2017 Nov 16;7(11):e018593. doi: 10.1136/bmjopen-2017-018593. PubMed PMID: 29150475; PubMed Central PMCID: PMC5701989.
Alonso-Coello P, Schünemann HJ, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Rada G, Rosenbaum S, Morelli A, Guyatt GH, Oxman AD; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ. 2016 Jun 28;353:i2016. doi: 10.1136/bmj.i2016. PubMed PMID: 27353417.
Alonso-Coello P, Oxman AD, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Vandvik PO, Meerpohl J, Guyatt GH, Schünemann HJ; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 2: Clinical practice guidelines. BMJ. 2016 Jun 30;353:i2089. doi: 10.1136/bmj.i2089. PubMed PMID: 27365494.
Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, Fervers B, Graham ID, Grimshaw J, Hanna SE, Littlejohns P, Makarski J, Zitzelsberger L; AGREE Next Steps Consortium. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010 Dec 14;182(18):E839-42. doi: 10.1503/cmaj.090449. Epub 2010 Jul 5. Review. PubMed PMID: 20603348; PubMed Central PMCID: PMC3001530.
Hultcrantz M, Rind D, Akl EA, Treweek S, Mustafa RA, Iorio A, Alper BS, Meerpohl JJ, Murad MH, Ansari MT, Katikireddi SV, Östlund P, Tranæus S, Christensen R, Gartlehner G, Brozek J, Izcovich A, Schünemann H, Guyatt G. The GRADE Working Group clarifies the construct of certainty of evidence. J Clin Epidemiol. 2017 Jul;87:4-13. doi: 10.1016/j.jclinepi.2017.05.006. Epub 2017 May 18. PubMed PMID: 28529184; PubMed Central PMCID: PMC6542664.
Medisch Specialistische Richtlijnen 2.0 (2012). Adviescommissie Richtlijnen van de Raad Kwalitieit. http://richtlijnendatabase.nl/over_deze_site/over_richtlijnontwikkeling.html
Neumann I, Santesso N, Akl EA, Rind DM, Vandvik PO, Alonso-Coello P, Agoritsas T, Mustafa RA, Alexander PE, Schünemann H, Guyatt GH. A guide for health professionals to interpret and use recommendations in guidelines developed with the GRADE approach. J Clin Epidemiol. 2016 Apr;72:45-55. doi: 10.1016/j.jclinepi.2015.11.017. Epub 2016 Jan 6. Review. PubMed PMID: 26772609.
Schünemann H, Brożek J, Guyatt G, et al. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group, 2013. Available from http://gdt.guidelinedevelopment.org/central_prod/_design/client/handbook/handbook.html.
Zoekverantwoording
Zoekverantwoording
Embase 5-5-2022
|
No. |
Query |
Results |
|
#10 |
#7 AND #9 |
1 |
|
#9 |
efficacy AND safety AND of AND pectoral AND nerve AND block AND pecs AND compared AND with AND control, AND paravertebral AND erector AND spinae AND plane AND block, AND local AND anesthesia AND in AND patients AND undergoing AND breast AND cancer AND surgeries AND a AND systematic AND review AND 'meta analysis' |
1 |
|
#8 |
#4 AND #6 (RCT’s) |
377 |
|
#7 |
#4 AND #5 (SR’s) |
101 |
|
#6 |
'clinical trial'/exp OR 'randomization'/exp OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp OR 'placebo'/exp OR 'prospective study'/exp OR rct:ab,ti OR random*:ab,ti OR 'single blind':ab,ti OR 'randomised controlled trial':ab,ti OR 'randomized controlled trial'/exp OR placebo*:ab,ti |
3530572 |
|
#5 |
'meta analysis'/exp OR 'meta analysis (topic)'/exp OR metaanaly*:ti,ab OR 'meta analy*':ti,ab OR metanaly*:ti,ab OR 'systematic review'/de OR 'cochrane database of systematic reviews'/jt OR prisma:ti,ab OR prospero:ti,ab OR (((systemati* OR scoping OR umbrella OR 'structured literature') NEAR/3 (review* OR overview*)):ti,ab) OR ((systemic* NEAR/1 review*):ti,ab) OR (((systemati* OR literature OR database* OR 'data base*') NEAR/10 search*):ti,ab) OR (((structured OR comprehensive* OR systemic*) NEAR/3 search*):ti,ab) OR (((literature NEAR/3 review*):ti,ab) AND (search*:ti,ab OR database*:ti,ab OR 'data base*':ti,ab)) OR (('data extraction':ti,ab OR 'data source*':ti,ab) AND 'study selection':ti,ab) OR ('search strategy':ti,ab AND 'selection criteria':ti,ab) OR ('data source*':ti,ab AND 'data synthesis':ti,ab) OR medline:ab OR pubmed:ab OR embase:ab OR cochrane:ab OR (((critical OR rapid) NEAR/2 (review* OR overview* OR synthes*)):ti) OR ((((critical* OR rapid*) NEAR/3 (review* OR overview* OR synthes*)):ab) AND (search*:ab OR database*:ab OR 'data base*':ab)) OR metasynthes*:ti,ab OR 'meta synthes*':ti,ab |
821778 |
|
#4 |
#3 AND [1-1-2000]/sd NOT ('conference abstract'/it OR 'editorial'/it OR 'letter'/it OR 'note'/it) NOT (('animal experiment'/exp OR 'animal model'/exp OR 'nonhuman'/exp) NOT 'human'/exp) |
734 |
|
#3 |
#1 AND #2 |
1403 |
|
#2 |
'nerve block'/exp OR 'autonomic blocker':ti,ab,kw OR 'autonomic blocking':ti,ab,kw OR 'autonomic nerve block':ti,ab,kw OR 'autonomic nerve blocking':ti,ab,kw OR 'conduction block':ti,ab,kw OR 'maxillary nerve block':ti,ab,kw OR 'nerve block':ti,ab,kw OR 'nerve block anaesthesia':ti,ab,kw OR 'nerve block anesthesia':ti,ab,kw OR 'nerve blockade':ti,ab,kw OR 'nerve blocking':ti,ab,kw OR 'nerve conduction block':ti,ab,kw OR 'neurogenic blockade':ti,ab,kw OR 'sensory nerve block':ti,ab,kw OR ((pectoralis NEAR/3 nerve NEAR/3 block*):ti,ab,kw) OR pecs:ti,ab,kw OR ((serratus NEAR/3 anterior NEAR/3 plane NEAR/3 block*):ti,ab,kw) OR ((paravertebral NEAR/3 block*):ti,ab,kw) OR ((erector NEAR/3 spinae NEAR/3 plane NEAR/3 block*):ti,ab,kw) OR 'esp block*':ti,ab,kw OR espb:ti,ab,kw OR psbp:ti,ab,kw |
56297 |
|
#1 |
'breast surgery'/exp OR 'mastectomy'/exp OR 'breast reconstruction'/exp OR 'breast surgery':ti,ab,kw OR 'breast operation':ti,ab,kw OR mammaplasty:ti,ab,kw OR 'breast reduction':ti,ab,kw OR 'breast cancer surger*':ti,ab,kw OR 'breast plastic surger*':ti,ab,kw OR 'mamma surger*':ti,ab,kw OR 'breast amputation':ti,ab,kw OR 'breast resection':ti,ab,kw OR 'mammectomy':ti,ab,kw OR 'mastectomy':ti,ab,kw OR mammaplast*:ti,ab OR ((breast NEAR/3 reconstruct*):ti,ab,kw) OR mammoplast*:ti,ab,kw OR ((mamma NEAR/3 reconstruct*):ti,ab,kw) |
103915 |
Pubmed 05-05-2022
|
Search |
Query |
Results |
|
#10 |
Search: #7 AND #9 |
|
|
#9 |
Search: efficacy AND safety AND of AND pectoral AND nerve AND block AND pecs AND compared AND with AND control, AND paravertebral AND erector AND spinae AND plane AND block, AND local AND anesthesia AND in AND patients AND undergoing AND breast AND cancer AND surgeries AND a AND systematic AND review AND 'meta analysis' - Articles found by citation matching |
|
|
#8 |
Search: #4 AND #6 (RCT’s) |
|
|
#7 |
Search: #4 AND #5 (SR’s) |
|
|
#6 |
Search: ((random*[tiab] AND (controlled[tiab] OR control[tiab] OR placebo[tiab] OR versus[tiab] OR vs[tiab] OR group[tiab] OR groups[tiab] OR comparison[tiab] OR compared[tiab] OR arm[tiab] OR arms[tiab] OR crossover[tiab] OR cross-over[tiab]) AND (trial[tiab] OR study[tiab])) OR ((single[tiab] OR double[tiab] OR triple[tiab]) AND (masked[tiab] OR blind*[tiab]))) |
|
|
#5 |
Search: ("Meta-Analysis" [Publication Type] OR "Meta-Analysis as Topic"[Mesh] OR metaanaly*[tiab] OR meta-analy*[tiab] or metanaly*[tiab] OR "Systematic Review" [Publication Type] OR systematic[sb] OR "Cochrane Database Syst Rev"[Journal] or prisma[tiab] OR preferred reporting items[tiab] OR prospero[tiab] OR ((systemati*[ti] OR scoping[ti] OR umbrella[ti] OR structured literature[ti]) AND (review*[ti] OR overview*[ti])) OR systematic review*[tiab] OR scoping review*[tiab] OR umbrella review*[tiab] OR structured literature review*[tiab] OR systematic qualitative review*[tiab] OR systematic quantitative review*[tiab] OR systematic search and review[tiab] OR systematized review[tiab] OR systematised review[tiab] OR systemic review[tiab] OR systematic literature review*[tiab] OR systematic integrative literature review*[tiab] OR systematically review*[tiab] OR scoping literature review*[tiab] OR systematic critical review[tiab] OR systematic integrative review*[tiab] OR systematic evidence review[tiab] OR Systematic integrative literature review*[tiab] OR Systematic mixed studies review*[tiab] OR Systematized literature review*[tiab] OR Systematic overview*[tiab] OR Systematic narrative review*[tiab] OR ((systemati*[tiab] OR literature[tiab] OR database*[tiab] OR data-base*[tiab] OR structured[tiab] OR comprehensive*[tiab] OR systemic*[tiab]) AND search*[tiab]) OR (Literature[ti] AND review[ti] AND (database*[tiab] OR data-base*[tiab] OR search*[tiab])) OR ((data extraction[tiab] OR data source*[tiab]) AND study selection[tiab]) OR (search strategy[tiab] AND selection criteria[tiab]) OR (data source*[tiab] AND data synthesis[tiab]) OR medline[tiab] OR pubmed[tiab] OR embase[tiab] OR Cochrane[tiab] OR ((critical[ti] OR rapid[ti]) AND (review*[ti] OR overview*[ti] OR synthes*[ti])) OR (((critical*[tiab] OR rapid*[tiab]) AND (review*[tiab] OR overview*[tiab] OR synthes*[tiab]) AND (search*[tiab] OR database*[tiab] OR data-base*[tiab]))) OR metasynthes*[tiab] OR meta-synthes*[tiab]) NOT ("Comment" [Publication Type] OR "Letter" [Publication Type] OR "Editorial" [Publication Type] OR (("Animals"[Mesh] OR "Models, Animal"[Mesh]) NOT "Humans"[Mesh])) |
|
|
#4 |
Search: #3 AND ("2000/01"[Date - Entrez] : "3000"[Date - Entrez]) |
|
|
#3 |
Search: #1 AND #2 |
|
|
#2 |
Search: "Nerve Block"[Mesh] OR "nerve block*"[tiab] OR ("serratus anterior plane"[tiab] OR "pectoralis nerve"[tiab] OR "pectoserratus plane"[tiab] OR "erector spinae plane"[tiab]) AND block*[tiab] OR PECS[tiab] OR ESPB[tiab] OR PSPB[tiab] |
|
|
#1 |
Search: "Breast Neoplasms/surgery"[Mesh] OR "Breast/surgery"[Mesh] OR "Mastectomy"[Mesh] OR "Mammaplasty"[Mesh] OR mammaplast*[tiab] OR "breast reconstruct*"[tiab] OR mammoplast*[tiab] OR "breast sparing surger*"[tiab] OR "breast reduction*"[tiab] OR "mamma surger*"[tiab] OR "breast amputation*"[tiab] OR mammectom*[tiab] OR mastectom*[tiab] OR mammaplast*[tiab] OR mammoplast*[tiab] OR "breast reconstruction*"[tiab] |