Screeningtools postoperatieve pijn
Uitgangsvraag
Welke preoperatieve screeningtools zijn geschikt om de mate van postoperatieve pijn bij kinderen te voorspellen?
Aanbeveling
Gebruik geen screeningtool om postoperatieve pijn bij kinderen te voorspellen.
Overwegingen
Voor- en nadelen van de interventie en de kwaliteit van het bewijs
In de systematische literatuuranalyse werden twee artikelen gevonden die onderzoek deden naar ieder een andere screeningtool die preoperatief werd ingezet om postoperatieve (chronische) pijnscores te voorspellen bij adolescenten. Narayanasamy (2022) onderzocht in de Verenigde Staten de Pediatric Pain Screening Tool (PPST). Wildemeersch et al. (2018) onderzocht in België een screeningtool bestaande uit vragenlijsten die psychologische constructen meten, die mogelijke risicofactoren zijn voor postoperatieve pijn (i.e. depressieve klachten, angstklachten, angstige aanleg, eigenwaarde). De gerapporteerde resultaten wijzen op een mogelijk acceptabele test-hertest betrouwbaarheid van de PPST en een mogelijk lage voorspellende (criterium) validiteit van de PPST en de psychologische screeningtool. Echter, de gevonden/gerapporteerde resultaten leveren onvoldoende bewijs om conclusies te kunnen trekken m.b.t. de validiteit en betrouwbaarheid van deze screeningtools in het voorspellen van (chronische) postoperatieve pijn bij kinderen.
Waarden en voorkeuren van patiënten (en evt. hun verzorgers)
Voor kinderen en hun ouders/verzorgers is het van belang dat postoperatieve pijn met een screeningstool zou kunnen worden opgespoord, zodat dit zou kunnen resulteren in snellere en betere pijnbestrijding. Ook zou dit mogelijkheden kunnen bieden voor het voorkomen van postoperatieve pijn.
Kosten (middelenbeslag)
Kosten zijn hier niet van toepassing.
Aanvaardbaarheid, haalbaarheid en implementatie
De aanvaardbaarheid, haalbaarheid en implementatie is hier niet van toepassing, omdat geen aanbevelingen worden gedaan een bepaald screeningstool is implementeren.
Rationale van de aanbeveling: weging van argumenten voor en tegen de interventies
Er is geen evidence voor het gebruik van een screeningtool voor het voorspellen van postoperatieve pijn bij kinderen. Mogelijk kan de Pediatric Pain Screening Tool (PPST) als meetinstrument voor pijn worden toegepast. Dit komt terug in latere modules van deze richtlijn.
Onderbouwing
Achtergrond
Inadequate postoperatieve pijnbestrijding is geassocieerd met hogere pijnscores en vergroot de kans op het ontwikkelen van chronische postoperatieve pijn. Anderzijds is het doel om met de laagst mogelijke hoeveelheid van opioïden de postoperatieve pijnbestrijding uit te voeren om het risico op bijwerkingen van de opioïden zo laag mogelijk te houden en de kans op chronische postoperatieve opioïde behandeling te verminderen.
Acute matige tot ernstige postoperatieve pijn na heelkundige en urologische ingrepen bij kinderen (0-18 jaar) heeft een incidentie van 25-70% op de dag van chirurgie (Wilson, 2017). Na een tonsillectomie of een appendectomie gaf 22% van de kinderen (4-18 jaar) aan meer pijnstilling te willen in de eerste 24 uur na chirurgie (Stamer, 2021). De prevalentie van chronische postoperatieve pijn bij kinderen (6-18 jaar) 12 maanden na chirurgie wordt geschat op 20% (range 14.5 – 38 %) (Rabbitts, 2017).
Het doel van een preoperatieve screeningtool is om te voorspellen welke patiënten at risk zijn voor hogere postoperatieve pijnscores.
Conclusies / Summary of Findings
|
No GRADE |
Insufficient evidence was found regarding the validity and reliability of both the Pediatric Pain Screening Tool (PPST) and a preoperative psychological screening tool (consisting of the RSES, HADS and STAI) for predicting (chronic) postoperative pain in children. |
Samenvatting literatuur
Two studies were included in the analysis of the literature. Important study characteristics and results are summarized in the evidence tables.
Description of studies
Narayanasamy et al. (2022) evaluated the Pediatric Pain Screening Tool (PPST) as a preoperative screening tool to identify those at higher risk for chronic postsurgical pain (CPSP). The PPST is a nine item questionnaire divided in two domains, a physical and a psychosocial domain. Total score ranges from 0 to 9. The physical subscale is focused on assessing the presence of comorbid pain, functional ability and quality of life measures such as attending school, walking and sleep quality. The psychosocial subscale is focused on assessing pain related fear, anxiety, catastrophizing, depression and pain inconvenience.
Adolescents with a diagnosis of idiopathic scoliosis undergoing posterior spine fusion or with a diagnosis of pectus excavatum undergoing Nuss procedure were recruited in this prospective, observational cohort study performed in 3 pediatric institutions in the USA. A total of 164 participants were enrolled, 109 to 144 participants were included in analyses.
Preoperatively, participants needed to complete the PPST, PainDETECT, Child Anxiety Sensitivity Index (CASI), Patient Related Outcome Measures Information System – depression scale (PROMIS-DS), PROMIS pain interference scale (PROMIS-PIS), Functional Disability Inventory (FDI), Pediatric Quality of Life (PedsQL), the Insomnia Severity Index (ISI), and a Pain Intensity Numerical Rating Scale (NRS) for average pain over last 4 weeks (0−10). PPST, FDI, PedsQL and Pain intensity NRS were assessed again at 6 and 12 months postoperatively. Chronic Postsurgical Pain (CPSP) was considered present if a participant reported a pain score on a Pain Intensity Numerical Rating Scale (NRS) of NRS ≥ 4 (indicating moderate to severe pain) over the previous month or during the time of pain assessment at 6 months post-surgery or 12 months after surgery.
Analyses that were done and relevant for our research question: Construct validity was analysed by doing confirmatory factor analysis (structural validity) and by determining scale variability (i.e. measurement invariance between subgroups of age, race, sex, surgery type), convergent validity (i.e. correlations with tests measuring constructs known to be risk factors of CPSP) and discriminative validity (i.e. ability to differentiate between CPSP and non-CPSP groups; could also be considered as predictive criterion validity). Furthermore, using PPST (sub)scale scores at 6 and 12 months, differences between CPSP and no-CPSP groups were examined, and concurrent associations with 6-12 months FDI and PedsQL scores (concurrent criterion validity). Test-retest reliability for PPST was assessed in the non-CPSP group using the preoperative and 6-month measurements.
Wildemeersch et al. (2018) evaluated a preoperative psychological screening tool to predict postoperative pain intensity and interference, and coping. Adolescents scheduled for elective pectus surgery were recruited in this prospective single-center observational cohort study performed in the anesthesiology department of the Antwerp University Hospital in Belgium. In total 21 participants were enrolled. The preoperative screening tool was performed online and consisted of various questionnaires of which good psychometric properties have been established previously, but only regarding the psychological constructs they were designed to measure: the Rosenberg self-esteem scale (RSES), Hospital Anxiety and Depression Scale (HADs) and State-Trait Anxiety Inventory (STAI). Pain was assessed with a numeric pain rating scale assessment (NRS) postoperatively in-hospital (+/- 6 day stay) and until 7 days after hospital discharge. Pain intensity and interference was assessed using the Multidisciplinary Pain Inventory (MPI) and coping with the Coping Pain Questionnaire (CPQ), both at 7 days after hospital discharge.
Analyses that were done and relevant for our research question: One could consider that a form of construct validity was analysed by determining correlations between the psychological constructs that were measured by the preoperative screening tool. Criterion validity (“predictive”) was analysed by determining correlations between the preoperative psychological screening tool and postoperative pain measurements.
Results
Validity (critical outcome)
The domain validity refers to the degree to which an outcome measure measures the construct it purports to measure and contains the measurement properties content validity (including face validity), construct validity (including structural validity, hypotheses testing, and cross-cultural validity/measurement invariance) and criterion validity.
Pediatric Pain Screening Tool (PPST)
Structural validity
Narayanasamy et al. (2022) performed a confirmatory factor analysis (CFA) to test whether measures of the construct were consistent with construct domain. The standardized regression weights for item loading on PPST sub-score factors ranged from 0.610 to 0.782. Goodness of fit for CFA was confirmed using chi-square statistic (p=.249), Root Mean Square Error of Approximation (Estimate 0.037, 95% CI 0.00−0.086) and Comparative Fit Index (0.990). Standardized model correlation between factor 1 (PPST physical, items 1-4) and factor 2 (PPST psychosocial, items 5-8) was 0.975 (2-tailed P value <.001 for all CFA factor loadings).
Measurement invariance
Measurement invariance was determined by evaluating possible differences in median PPST total scores across subgroups using Wilcoxon rank-sum tests or Kruskal-Wallis Test. Comparisons were made between three age groups (<12; ≥12 and <18; ≥18) sex (male; female), race (Caucasian; African-American), surgery type (pectus; spine) and the three surgical sites. No statistically significant PPST scale variability was found in the cohort (p≥.423).
Convergent validity
Convergent validity was assessed by determining correlations between preoperative PPST scores and scores on questionnaires that assess the same constructs, but more broadly than the PPST; the PPST specifically assesses these constructs in their relation to pain. It was found that preoperative PPST total scores positively correlated with preoperative pain scores (Spearman Correlation Coefficient [SCC] 0.672; p<.001; strong association), CASI anxiety (SCC 0.357; p< .001; weak association), preoperative PROMIS measures for depressive symptoms (SCC0.569; p< .001; moderate association), PROMIS pain interference (SCC 0.501; p< .001; moderate association) and ISI insomnia measurement (SCC 0.567; p<.001; moderate association) and negatively with PedsQL quality of life measures (SCC -0.460 to -0.614; p< .001; moderate-strong association). Similarly, preoperative PPST subscale scores were also all significantly correlated with the constructs in the same direction as PPST total score: all moderate-strong associations, except for the association with CASI anxiety which was weak.
Discriminative validity/predictive criterion validity
Narayanasamy et al. (2022) defined risk groups for chronic postsurgical pain (CPSP), functional disability and quality of life by postsurgical NRS ≥ 4 scores, FDI upper tertile and PedsQL lower tertile scores respectively. Logistic regression models were fitted using PPST scores to predict CPSP, PPST physical subscale score to predict FDI and PPST psychosocial subscale score to predict PedsQL psychosocial score. Receiver operant Characteristic (ROC) curves and the area under the curve (AUC) were calculated. Optimal cut-off on predicted probability and PPST total and subscale scores were determined by maximizing Youden's index (Youden Index = sensitivity + specificity -1 [or weighted Youden Index considering the cost of false positive and false negative]). Based on these PPST (sub)scores, Risk for CPSP was stratified as low (<10%), medium (10−30%) and high (>30%).
With an AUC of 0.63, the discriminative validity of the PPST total score in distinguishing CPSP and non-CPSP was poor. A 95% confidence interval was not reported for the AUC. Using the as optimal determined PPST total score cut-off of 2, sensitivity was 63.9% and specificity was 64.7%. The PPST < 2 group (N = 57) still had a medium risk for CPSP (22.81%). PPST ≥ 2 group (N = 47) had high risk for CPSP (48.94%).
With regard to CPSP related constructs / outcomes assumed part of the pain experience, i.e. functional disability and quality of life, these are the results:
The discriminative validity of the PPST physical subscale score in distinguishing groups on functional disability seemed slightly better (acceptable) with an AUC of 0.70, but a 95% confidence interval was not reported and with an optimal cut-off of 2, sensitivity was low at 51.7%. Specificity was better with 91.2%.
The discriminative validity of the PPST psychosocial subscale score in distinguishing groups on quality of life was acceptable based on an AUC of 0.76, but a 95% confidence interval was again not reported and with an optimal cut-off of 2, sensitivity was only 61.1% and specificity 82.3%.
Concurrent criterion validity
Associations between preoperative, and 6 and 12 months postoperative PPST - total and subscale - and CPSP/non-CPSP groups were evaluated using Wilcoxon rank-sum tests.
It was shown that CPSP and non-CPSP groups median PPST total scores differed only nominally both preoperatively and postoperatively; their interquartile ranges (IQR) largely overlapped, and no statistical differences (significance at p<.025) were found: non-CPSP preop median 2 (IQR 0.5, 4) vs. CPSP preop median 1 (IQR 0, 3), p=.026; non-CPSP postop 6 and 12 months median 1 (IQR 0, 3) vs. CPSP postop 6 and 12 months median 2 (IQR 1, 4), p=.04.
Preoperative and postoperative PPST physical subscale scores also differed only nominally between CPSP/non-CPSP groups, not statistically (significance at p<.025): non-CPSP preop median 1 (IQR 0, 2) vs. CPSP preop median 0 (IQR 0, 1.5), p=.029; non-CPSP postop 6 months median 0 (IQR 0, 2) vs. CPSP postop 6 months median 1 (IQR 0, 2), p=.03; non-CPSP postop 12 months median 1 (IQR 0, 3) vs. CPSP postop 12 months median 1 (IQR 0, 2), p=.20.
CPSP and non-CPSP groups did have statistically different PPST psychosocial subscale scores at 12 months postoperatively: non-CPSP 12 months median 0 (IQR 0, 1) vs. CPSP 12 months median 1 (IQR 0, 3), p=.02. On the other time-points, PPST psychosocial subscale scores differed only nominally, not statistically between CPSP/non-CPSP groups:
non-CPSP preop median 1 (IQR 0, 2) vs. CPSP preop median 0 (IQR 0, 1.5), p=.092; non-CPSP 6 months median 0 (IQR 0, 2) vs. CPSP 6 months median 1 (IQR 0, 2), p=.19.
The association between 6 and 12 months postoperative PPST - total and subscale - scores and postoperative functional disability and quality of life (related to CPSP / assumed part of the pain experience) was assessed with Spearman Correlations Coefficients. Results showed that the association between postoperative PPST and PedsQL scores was less strong postoperatively than preoperatively shown in the analyses regarding convergent validity; postoperative associations were weak to moderate in strength (SCC .025 to SCC -0.495) and not always statistically significant (p=.200 to p<.001). Associations between postoperative PPST - total and subscale - scores and postoperative FDI functional disability scores were weak (SCC 0.247 to SCC 0.351) and not always statistically significant (p=.266 to p<.001).
These results, which show an overall poor concurrent criterion validity of the PPST for the prediction of chronic postoperative pain and poor to acceptable criterion validity of PPST for the prediction of constructs related to chronic postoperative pain (i.e. disability and quality of life), are in line with the poor to acceptable discriminative/predictive criterion validity and overall low sensitivity scores that were found in this study in the prediction of chronic postoperative pain, disability and quality of life.
Preoperative psychological screening tool
Construct validity
Wildemeersch et al. (2018) determined Spearman’s correlation coefficients to assess associations between the different psychological questionnaires within the screening tool. Associations between the psychological constructs measured would have been expected. However, in this research population (n = 21) the associations between HADS depressive characteristics and HADS anxiety characteristics and between HADS depressive characteristics and RSES self-esteem were small and not statistically significant (R= 0.31, p=.21 and R= -0.15, p=.56 respectively). A small-moderate association was reported between HADS anxiety characteristics and RSES self -esteem (R= -0.49, p=.04). STAI trait anxiety was moderately-strongly associated with HADS depressive characteristics, HADS anxiety characteristics, and RSES self-esteem (R= 0.52, p=.03, R= 0.55, p=.02, and R= -0.72, p<.001 respectively).
Criterion validity (predictive)
To assess if the preoperative scores on psychological questionnaires included in the screening tool were associated with postoperative pain scores on the NRS, MPI pain severity and interference, and CPQ pain coping strategies, Spearman’s correlation coefficients were determined. Correlations between preoperative psychological scores and postoperative pain scores were overall small, not in the direction as would be expected (e.g. higher scores on preoperative HADS correlated with lower NRS pain scores after discharge) and not statistically significant (0.09 ≤ p ≤ 0.96). Only STAI trait anxiety showed a moderate positive association and RSES self-esteem a moderate negative association with MPI pain interference, R= 0.58, p=.02 and R= -0.62, p=.01 respectively.
Small and non-statistically significant associations were found between most preoperative psychological scores and postoperative pain coping strategies (i.e. CPQ passive coping, catastrophizing and self-control; .12 ≤ p ≤ .99), except for preoperative HADS anxiety characteristics which showed a moderate positive correlation with a passive postoperative coping style, R= 0.55, p=.03. However, postoperative pain and pain coping styles were only weakly and non-significantly associated (0.04 ≤ R ≤ 0.26, .32 ≤ p ≤ .87).
Overall, these results hint towards a poor criterion validity for this preoperative psychological screening tool for the prediction of postoperative pain but the population sample was too small and the analyses were too limited to reach any conclusions about validity of this screening tool.
Reliability (important outcome)
The domain reliability refers to the degree to which the measurement is free from measurement error, and it contains the measurement properties internal consistency, test-retest reliability, and measurement error.
For PPST, the test-retest reliability was reported in Narayanasamy (2022). For the preoperative psychological screening tool, reliability was not assessed for the combination of included questionnaires in predicting postoperative pain (NB: reliability of the individual psychological tests has been established in previous research).
Pediatric Pain Screening Tool (PPST)
Test-retest reliability
Test-retest reliability was assessed within the group of non-CPSP patients using intraclass correlation coefficient (ICC) based on a 2-way mixed-effects model using the preoperative and 6 month postoperative PPST scores: ICC = 0.68 (P< .001). This is an indication of moderate/acceptable test-retest reliability.
Level of evidence of the literature
Generally, the COSMIN method is used to assess the quality of evidence regarding research on measurement properties of tools/tests/questionnaires and the GRADE method is used to assess the quality of evidence regarding research comparing interventions and diagnostic tests. However, neither method could be applied here.
Included studies were not set up with the goal to evaluate the measurement properties of a screening tool designed to predict (chronic) postoperative pain. Furthermore, evaluated questionnaires were not designed to predict future pain; they were either designed to measure the concurrent pain experience or designed to measure psychosocial constructs. As a result, many properties weren’t assessed or were not assessed in the way that COSMIN requires. Both studies included were more prognostic in nature in that they examined the possible use of different questionnaires in predicting (chronic) postoperative pain, however, not using multivariate prognostic models. Therefore, the level of evidence regarding the validity and reliability of the PPST and a pre-operative psychological screening tool consisting of various psychological questionnaires for the prediction of (chronic) postoperative pain in children could not be assessed systematically via the COSMIN and/or GRADE method.
Although quality of the evidence could not be systematically assessed, it can be mentioned that both studies had flaws in their report of methods and results which raises concerns of bias. In Narayanasamy et al. (2022), key results mentioned in text did not always match results mentioned in tables or in their abstract, the number of participants per analysis was not always clear, the number of participants over 18 years of age was not mentioned, and they often failed to report confidence intervals. In Wildemeersch et al. (2018), the number of participants per analysis was not clear, nor the pain scores used in the correlational analyses. Both studies report on adolescents undergoing surgery; validity and reliability of both tools remain to be evaluated in a younger population (concerns of indirectness). Sample sizes, especially in Wildemeersch et al. (2018) were small (concerns of imprecision).
Zoeken en selecteren
A systematic review of the literature was performed to answer the following question: What is the validity and reliability of preoperative screening tools for predicting (chronic) postoperative pain in children?
P: Children 0-18 years old
I: Preoperative screening tools for predicting (chronic) postoperative pain*
C: Screening tools compared to each other (if relevant)
O: Validity, reliability
* Screening tools may include the following:
- Preoperative Pain Stimulation Methods, such as quantitative sensory testing mechanical/thermal/electric
- Preoperative Psychometric Evaluations, such as
- State Trait Anxiety Inventory (STAI)
- Hospital Anxiety Depression Scale (HADS)
- Pain Catastrophizing Scale (PCS)
- Pittsburgh Sleep Quality Index (PSQI)
- Anticipated pain, perceived analgesic needs, and psychological traits to predict pain
Relevant outcome measures
The guideline development group considered validity as a critical outcome measure for decision making; and reliability as an important outcome measure for decision making.
The measurement properties were defined following the taxonomy of the Consensus-based Standards for the selection of health Measurement INstruments (COSMIN) (Mokkink, 2010).
The working group defined the discriminative validity of the screening tools as follows: Area Under the Curve, AUC < 0.7: poor; 0.7 ≤ AUC < 0.8: acceptable; 0.8 ≤ AUC < 0.9: excellent; AUC ≥ 0.9: outstanding.
The working group defined the reliability of the screening tools as follows: Intraclass Correlation Coefficient, ICC < 0.5: poor; 0.5 ≤ ICC < 0.75: moderate; 0.75 ≥ ICC < 0.9: good; ICC≥ 0.9: excellent.
Search and select (Methods)
The databases Medline (via OVID) and Embase (via Embase.com) were searched with relevant search terms from 2007 until 16 November 2021. The detailed search strategy is depicted under the tab Methods. The systematic literature search resulted in 342 hits. Studies were selected based on the following criteria:
- Being a systematic review, randomized controlled trial (RCT) or observational study (cohort study).
- Reporting the validity and/or reliability of a screening tool for predicting (chronic) postoperative pain in children aged 0 to 18 years old.
Six studies were initially selected based on title and abstract screening. After reading the full text, four studies were excluded (see the table with reasons for exclusion under the tab Methods), and two studies were included.
Referenties
- 1 - Mokkink, L. B., Terwee, C. B., Patrick, D. L., Alonso, J., Stratford, P. W., Knol, D. L., ... & de Vet, H. C. (2010). The COSMIN study reached international consensus on taxonomy, terminology, and definitions of measurement properties for health-related patient-reported outcomes. Journal of clinical epidemiology, 63(7), 737-745.
- 2 - Mokkink, L. B., Prinsen, C., Patrick, D. L., Alonso, J., Bouter, L. M., de Vet, H. C., ... & Mokkink, L. (2018). COSMIN methodology for systematic reviews of patient-reported outcome measures (PROMs). User manual, 78(1).
- 3 - Narayanasamy, S., Yang, F., Ding, L., Geisler, K., Glynn, S., Ganesh, A., ... & Chidambaran, V. (2022). Pediatric Pain Screening Tool: A Simple 9-Item Questionnaire Predicts Functional and Chronic Postsurgical Pain Outcomes After Major Musculoskeletal Surgeries. The Journal of Pain, 23(1), 98-111.
- 4 - Rabbitts, J. A., Fisher, E., Rosenbloom, B. N., & Palermo, T. M. (2017). Prevalence and predictors of chronic postsurgical pain in children: a systematic review and meta-analysis. The journal of pain, 18(6), 605-614.
- 5 - Wildemeersch, D., Bernaerts, L., DHondt, M., & Hans, G. (2018). Preliminary evaluation of a web-based psychological screening tool in adolescents undergoing minimally invasive pectus surgery: single-center observational cohort study. JMIR Mental Health, 5(2), e9806.
- 6 - Wilson, C. A., Sommerfield, D., Drake?Brockman, T. F., Lagrange, C., Ramgolam, A., & von Ungern?Sternberg, B. S. (2017). A prospective audit of pain profiles following general and urological surgery in children. Pediatric Anesthesia, 27(11), 1155-1164.
Evidence tabellen
Evidence table for intervention studies
|
Study reference |
Study characteristics |
Patient characteristics 2 |
Intervention Screening Tool |
Outcomes predicted by the screening tool Comparison / control (C) 3
|
Follow-up |
Outcome measures and effect size 4 |
Comments |
|
Narayanasamy, 2022 |
Type of study: prospective, observational, longitudinal study / cohort study
Setting and country: 3 pediatric institutions, USA
Funding: Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1 TR001425 through pilot funding and R01AR075857 through National Institute of Arthritis and Musculoskeletal and Skin Diseases. (PI: Chidambaran). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflicts of interest: None of the authors have any conflicts of interest to disclose. |
Inclusion criteria: Children and adolescents with an American Society of Anesthesiologists physical status ≤2 (mild systemic disease), aged 8 years and above, regardless of sex or race, with a diagnosis of idiopathic scoliosis undergoing posterior spine fusion surgery and subjects with a diagnosis of pectus excavatum undergoing Nuss procedure.
Exclusion criteria: Patients with history of opioid use in the past 6 months, liver and renal disease, pregnant or breastfeeding women, developmental delay, cancer, and those not fluent in written and/or spoken English.
N total at baseline: 164 enrolled. 144 completed perioperative protocol and included in analysis for PPST convergent and discriminative validity. 109 completed CPSP at 6 and/or 12 monts and were included in PPST-CPSP association analysis.
Important prognostic factors2: Age, median ± interquartile range: All (N=109): 14.67 (13.24, 15.95) No CPSP (N=71): 14.82 (13.5, 16.24) CPSP (N=38): 14.51 (13.53, 15.79) P value: .543
Sex, (Female/Male); Female %: All (N=109): 74/35; 64.81% No CPSP (N=71): 39/31; 56% CPSP (N=38): 34/4; 89% P value <.001
Race, (Caucasian/ African-American/ Other); Caucasian%: All (N=109): 88/13/8; 83.02% No CPSP (N=71): 63/3/4; 90% CPSP (N=38): 25/10/1; 69% P value <.001
Surgery type (N, %): All (N=109): Spine surgery (81, 74.31%) Pectus surgery (28, 25.69%) No CPSP (N=71): S: 49, 69.02% P: 22, 30.98% CPSP (N=38): S: 32, 84.21% P: 6, 15.79% P value .084
|
Pediatric Pain Screening Tool (PPST) Nine items in 2 domains- physical and psychosocial.
PPST total scores range from 0 to 9. Physical subscale scores range from 0 to 4. Psychosocial subscale scores range from 0 to 5.
Physical subscale is focused on assessing presence of comorbid pain, functional ability and quality of life measures such as attending school, walking and sleep quality.
Psychosocial subscale is focused on assessing pain related fear, anxiety, catastrophizing, depression and pain inconvenience.
Patients were instructed to consider the previous 2 weeks while answering the questions.
|
Predicted outcomes:
Primary Outcome: Chronic Postsurgical Pain (CPSP) was considered present if a participant reported a pain score on a Pain Intensity Numerical Rating Scale (NRS) of NRS ≥ 4 (indicating moderate to severe pain) over the previous month or during the time of pain assessment at 6 months post-surgery or 12 months after surgery.
Secondary Outcomes: Functional disability and quality of life at 3 to 6 months and 10 to 12 months as continuous variables. Measured by: Functional Disability Index (FDI): 5-point Likert scale with total score range 0 to 60. Pediatric Quality of Life measure (PedsQL): 23 items with total score range 0 to 92.
Also comparisons to: PainDETECT: Seven questions and visual chart to mark area of pain and radiation. Total score range 0 to 38; score >19 indicates likely neuropathic component.
Only baseline/ preoperative comparisons: Child Anxiety Sensitivity Index (CASI): 18 items with total score range 18 to 54.
NIH Patient-Reported Outcome Measurement Information System (PROMIS) Pediatric Short Form v2.0 Depressive Symptoms 8a: 8 items with total raw score range 8 to 40 which is converted into a T score based on a table. T score of 50 is average for US populations.
PROMIS Pediatric SF v2.0 Pain Interference 8a: 8 items utilizing a 7-d recall period. total score range 8 to 40.
Insomnia Severity Index (ISI): cutoff score of 10 had a 86.1% sensitivity and 87.7% specificity for detecting insomnia cases.
|
Length of follow-up: Patients were asked to complete the Pain Intensity (NRS), FDI, Pediatric Quality of Life measure (PedsQL), PPST and painDETECT questionnaires at 3 to 6 months and 10 to 12 months after surgery.
Incomplete outcome data: Of 164 patients, 109 patients had outcomes. Hence, approximately one-third were lost follow-up.
Reasons: Withdrawal (5) due to remaining intubated postop, delays, cancellations, complications, and incomplete questionnaires (15) => 144 participants completed perioperative protocols. Due to not responding to follow-up request: 39 lost => 105 participants with CPSP 6 months outcome; another 38 lost of those 105, but plus 4 participants who did not respond at 6 months => 71 participants with CPSP 12 month outcome.
Comparison of subjects with outcomes vs those lost to follow-up On comparing subjects who had outcomes to those who didn’t due to loss to follow up, the groups were comparable in all measures (age, sex, surgical type and race composition, preoperative PPST total and sub-scores and preoperative PedsQL) except FDI, with higher values (median (IQR) of 8 (3−14) in the loss to follow up group compared to 4 (0−9.5 in the group with outcomes (P= .016).
The incidence of CPSP was 27/105 (25.7%) at 6 months and 20/71 (28.2%) at 12 months, as subjects lost to follow up between 6 and 12 months mostly were those who did not have CPSP at 6 months (N = 29).
|
Construct validity: Structural validity (Confirmatory Factor Analysis (CFA); used to test whether measures of the construct were consistent with construct domain): The standardized regression weights for item loading on PPST sub-score factors ranged from 0.610 to 0.782. Goodness of fit for CFA was confirmed using chi-square statistic (P= .249), Root Mean Square Error of Approximation (Estimate 0.037 (95% CI 0.00−0.086)) and Comparative Fit Index (0.990). Standardized model correlation between factor 1 (ppst1-4) and factor 2 (ppst5-8) was 0.975 (2-tailed P value <.001 for all CFA factor loadings).
Construct validity: Measurement invariance (PPST Scale Variability; PPST scores were analyzed using Wilcoxon rank-sum tests or Kruskal-Wallis Test to evaluate differences by sex, race, surgery type and surgical site): Preoperative PPST scores ranged from 0 to 7 (2.1 ± 2.31). No statistically significant PPST scale variability was found in the cohort.
Convergent validity (Correlation of Preoperative PPST Score and Sub-Scores With Known Risk Factors of CPSP): Spearman Correlations Coefficients and P values are reported for PPST physical score; psychosocial score; total score:
*P value less than .05 was considered significant.
Discriminative validity (also predictive criterion validity) ROC and (maximum) Youden’s indices were determined (1) for PPST total scores in predicting CPSP (2) for PPST physical subscale scores in predicting FDI (3) for PPST psychosocial subscale score in predicting PedsQL
Optimal PPST (sub)score cut-offs are reported, the AUC for prediction and sensitivity, specificity (1) CPSP: cut-off = 2, AUC=0.63, sens 63.9%, spec 64.7% (2) FDI: cut-off = 2, AUC= 0.70, sens 51.7%, spec 91.2% (3)PedsQoL: cut-off = 2, AUC=0.76, sens 61.1%, spec 82.3%
PPST < 2 group (N = 57) still had a medium risk* for CPSP (22.81%). PPST ≥ 2 group (N = 47) had high risk* for CPSP (48.94%).
*risk for CPSP was stratified as low (<10%), medium (10−30%) and high (>30%)
Concurrent criterion validity Associations between PPST (total, 1−4, and 5−9) and CPSP at preop, 6 and 12 months were tested using Wilcoxon rank-sum tests (tests if CPSP and non-CPSP differ on PPST total, physical, and/or psychosocial scores).
Median (Interquartile range) and P values were reported for PPST physical score; psychosocial score; total score:
Preoperatively CPSP (No) 1 (0,2); 1 (0,2); 2 (0.5,4) CPSP (Yes): 0 (0 ,1.5); 0 (0 ,1.5); 1 (0,3) p= .029; p= .092; p= .026
At 6 months: CPSP (No): 0 (0,2); 0 (0,2); 1 (0,3) CPSP (Yes): 1 (0,2); 1 (0,2); 2 (1,4) p= .03; p= .19; p= .04 At 12 months: CPSP (No): 1 (0,3); 0 (0,1); 1 (0,3) CPSP (Yes): 1 (0,2); 1 (0,3); 2 (1,4) p= .20; p= .02*; p= .04
The relationship between PPST (total and subscales) with secondary outcomes (postoperative FDI and PedsQL total and subscales at 6 and 12 months after surgery): Spearman Correlations Coefficients and P values are reported for PPST physical score; psychosocial score; total score: At 6 months
At 12 months
*P value less than .025 was considered statistically significant.
Test-retest reliability for PPST (assessed using intraclass correlation coefficient (ICC) based on a 2-way mixed-effects model and preoperative and postoperative 6 month PPST within non-CPSP patients): ICC = 0.68 (P< .001) |
Text and tables do not always match in numbers and/or are unclear:
It is unclear if the “pain scores” mentioned in the tables refer to the NRS scores (but they probably do).
Specifically for table 2. Demographics, Preoperative Pain, Psychosocial and Functional Characteristics for CPSP and Non-CPSP Cohorts: Participants with CPSP are reported to have higher pain scores and more medications at preop/baseline, but in the table participants without CPSP seem to have higher pain scores and more medications. Also, the results for PPST, CASI, PROMIS and ISI seem to be placed into the wrong column with worse scores for the participants without CPSP while in the text, participants with CPSP are said to have the worse scores.
Not all participants were < 18. How many were older was not reported.
Associations between PPST (total, 1−4, and 5−9) and CPSP at 6 and 12 months were tested using Wilcoxon rank-sum tests, but results of the statistical tests are not reported (such as W, z, effect size r), only CPSP and non-CPSP group median and IQR are reported and a p-value.
The abstract mentions differences between CPSP and non-CPSP on PPST total and physical (sub)scale while in text and table groups only differed significantly on the psychosocial subscale.
Predictive or concurrent analyses? => It is unclear if preoperative PPST was used in the analyses concerning its prediction of CPSP (probably yes). Further analyses concerning correlations between PPST and CPSP or Functional disability and quality of life seem to use concurrent scores at 6 and 12 months.
95% CI for AUC were not reported.
For the Wilcoxon rank-sum tests, pre-op authors considered P<.05 significant and postop P<.025. |
|
Wildemeersch, 2018 |
Type of study: prospective single-center observational cohort study
Setting and country: Anesthesiology Department, Antwerp University Hospital (UZA), Belgium
Funding: Not reported.
Conflict of interest: None declared. |
Inclusion criteria: Patients scheduled for elective pectus surgery between June 2017 and August 2017.
Exclusion criteria: Patients with a history of a psychiatric disease, chronic opioid use (more than three months) or revision surgery were excluded.
N total at baseline: 21
Important prognostic factors2: Age, mean ± SD: 14.81 ± 1.33
Gender (male: female): 20:1 (95% men)
Type of deformity (N; PE: PC)*: 15:6
*PE: pectus excavatum; PC: pectus carinatum |
Preoperative psychological screening tool
An online preoperative psychological inventory was performed using the Rosenberg self-esteem scale (RSES), Hospital Anxiety and Depression Scale (HADs) and State-Trait Anxiety Inventory (STAI).
|
Predicted outcomes Postoperatively, pain intensity and interference was assessed using the Multidisciplinary Pain Inventory (MPI), Coping Pain Questionnaire (CPQ) and numeric pain rating scale assessment (NRS).
Comparisons: Correlations between the screening tool’s questionnaire RSES, HADS, and STAI scores
|
Length of follow-up: Post-op in-hospital registration +/- 6 days, NRS was assessed and + until 7 days after hospital discharge. MPI, CPQ, were assessed 7 days after hospital discharge.
Loss-to-follow-up, N (%) In a flow-chart they report that N=21 were analyzed for psychological variables, and only 1 patient refused to participate in the final interview (only loss to FU with regard to the eHealth feasibility), but in the text they report that only 18 (86%) adolescents completed the pre-op questionnaires, and only 16 (76%) fully completed the post-op questionnaires. No further details (are those 16 out of the initial 18?) nor reasons for this loss to FU are reported. |
Associations between questionnaire scores were determined with Spearman’s correlation coefficient. Statistical significance was considered when p < 0.05.
Construct validity Correlation between preoperative psychological questionnaire scores:
(1) Self-esteem (RSES) (2) Depressive characteristics (HADS) (3) Anxiety characteristics (HADS) (4) Anxiety trait (STAI)
(1)-(2): R= -0.15, p=0.56 (1)-(3): R= -0.49, p=0.04* (1)-(4): R= -0.72, p<0.001* (2)-(3): R= 0.31, p=0.21 (2)-(4): R= 0.52, p=0.03* (3)-(4): R= 0.55, p=0.02*
Criterion validity (predictive) Correlation between preoperative psychological screening and postoperative pain:
(A) Postoperative pain scores (NRS) (in-hospital) (B) Postoperative pain scores (NRS) (after discharge) (C) Pain Severity (MPI) (D) Pain Interference (MPI)
(1)-(A): R= -0.24, p=0.34 (1)-(B): R= 0.18, p=0.51 (1)-(C): R= 0.09, p=0.75 (1)-(D): R= -0.62, p=0.01*
(2)-(A):R= 0.01, p=0.96 (2)-(B): R= -0.44, p=0.87 (2)-(C): R= 0.11, p=0.70 (2)-(D): R= 0.46, p=0.09
(3)-(A): R= 0.26, p=0.30 (3)-(B): R= -0.32, p=0.22 (3)-(C): R= -0.2, p=0.47 (3)-(D): R= 0.46, p=0.09
(4)-(A): R= 0.22, p=0.38 (4)-(B): R= -0.13, p=0.64 (4)-(C): R= -0.08, p=0.78 (4)-(D): R= 0.58, p=0.02*
Correlations with pain coping strategies: Only Anxiety (HADS) was positively correlated with passive pain coping (R= 0.55, p=0.03*). No other correlations between preoperative psychological screening and coping strategies. No significant correlation between coping strategies and pain (NRS?) was found: passive coping index vs pain, R= 0.26, p=0.32; catastrophizing vs pain, R= 0.04, p=0.87 |
Only correlations reported. N in the analyses unclear.
It is mentioned: The NRS mean score after the first five postoperative days was calculated. Patients continued the pain intensity registration through the platform until completion of the postoperative questionnaires, 7 days after hospital discharge. => it is not mentioned if again an after-discharge mean score was calculated and used in analyses.
Not examined in this article, but mentioned with regard to the validity and reliability of the individual psychological questionnaires for measuring psychological dimensions, not pain:
consistency (>0.85) and 1 month test/retest reliability (r>0.70) in α adolescents, healthy adults, and military samples.
|
Notes:
- Prognostic balance between treatment groups is usually guaranteed in randomized studies, but non-randomized (observational) studies require matching of patients between treatment groups (case-control studies) or multivariate adjustment for prognostic factors (confounders) (cohort studies); the evidence table should contain sufficient details on these procedures
- Provide data per treatment group on the most important prognostic factors [(potential) confounders]
- For case-control studies, provide sufficient detail on the procedure used to match cases and controls
- For cohort studies, provide sufficient detail on the (multivariate) analyses used to adjust for (potential) confounders
Table of excluded studies
|
Author and year |
Title |
Reason for exclusion |
|
Agarwal, 2006 |
Neonatal pain in surgical neonate |
Full text not found. No abstract. Title does not suggest relevance. |
|
Bringuier, 2009 |
The perioperative validity of the visual analog anxiety scale in children: A discriminant and useful instrument in routine clinical practice to optimize postoperative pain management |
Wrong study design/wrong I/O: Predicting post-op pain wasn't the main goal / outcome, and therefore hardly any information was reported. They only report: Postoperative pain did not differ significantly in the preoperative anxiety groups (POD1: P=0.47; DD: P=0.61). Furthermore, they report significant correlation p-values for simultaneous assessment of anxiety and pain postop, which is not relevant for this UV. |
|
Choo, 2010 |
Skin conductance fluctuations correlate poorly with postoperative self-report pain measures in school-aged children |
Wrong study design/wrong I/O: They examined if fluctuations in postop NFSC measurements can assess / predict /correlate with concurrent postop pain fluctuations and analgesic needs. |
|
Pedersen, 2020 |
Pressure pain thresholds in children before and after surgery: a prospective study |
Wrong study design: prediction of postop pain by preop algometry was not assessed. |
Verantwoording
Beoordelingsdatum en geldigheid
Publicatiedatum : 09-06-2023
Beoordeeld op geldigheid : 10-05-2023
Algemene gegevens
De ontwikkeling/herziening van deze richtlijnmodule werd ondersteund door het Kennisinstituut van de Federatie Medisch Specialisten (www.demedischspecialist.nl/kennisinstituut) en werd gefinancierd uit de Kwaliteitsgelden Medisch Specialisten (SKMS).
De financier heeft geen enkele invloed gehad op de inhoud van de richtlijnmodule.
Samenstelling werkgroep
Voor het ontwikkelen van de richtlijnmodule is in 2021 een multidisciplinaire werkgroep ingesteld, bestaande uit vertegenwoordigers van alle relevante specialismen (zie hiervoor de Samenstelling van de werkgroep) die betrokken zijn bij de zorg voor kinderen met pijn.
Kernwerkgroep
- Drs. M.A. (Maarten) Mensink, kinderanesthesioloog en pijnarts, werkzaam in het Prinses Máxima Centrum voor Kinderoncologie te Utrecht, NVA, voorzitter
- Drs. J.F. (Joanne) Goorhuis, algemeen kinderarts, werkzaam in het Medisch Spectrum Twente, NVK
- Dr. F (Felix) Kreier, algemeen kinderarts, werkzaam in het OLVG te Amsterdam, NVK
- Drs. M.S. (Sukru) Genco, algemeen kinderarts, werkzaam in het OLVG te Amsterdam, NVK
- Dr. S.H. (Steven) Renes, anesthesioloog-pijnspecialist, werkzaam in het Radboud UMC te Nijmegen, NVA
- Dr. P. (Petra) Honig-Mazer, psychotherapeut, werkzaam in het Erasmus MC Sophia te Rotterdam, PAZ/LVMP
- Drs. M. (Marjorie) de Neef, kinder-IC verpleegkundige, werkzaam in het Amsterdam UMC, V&VN
- R. (Rowy) Uitzinger, junior projectmanager en beleidsmedewerker, Stichting Kind en Ziekenhuis, tot 1 juni 2022
- E.C. (Esen) Doganer, junior projectmanager en beleidsmedewerker, Stichting Kind en Ziekenhuis, vanaf 1 juni 2022
Werkgroep
- Drs. L.A.M. (Lonneke) Aarts, algemeen kinderarts, werkzaam in het RadboudUMC Amalia kinderziekenhuis te Nijmegen, NVK
- Prof. dr. W.J.E. (Wim) Tissing, kinderoncoloog, werkzaam in het UMCG te Groningen en Prinses Máxima Centrum te Utrecht, NVK
- Prof. dr. S.N. (Saskia) de Wildt, kinderintensivist, werkzaam in het RadboudUMC te Nijmegen, NVK
- Prof. dr. N.M. (Nico) Wulffraat, kinderreumatoloog, werkzaam in het UMC Utrecht te Utrecht, NVK
- Drs. A.M. (Arine) Vlieger, algemeen kinderarts, werkzaam in het St. Antonius Ziekenhuis te Utrecht, NVK
- Dr. S.H.P. (Sinno) Simons, kinderarts-neonatoloog, werkzaam in het Erasmus MC Sophia te Rotterdam, NVK
- Drs. K. (Karina) Elangovan, kinderanesthesioloog, werkzaam in het Erasmus MC Sophia te Rotterdam, NVA
- Dr. C.M.G. (Claudia) Keyzer – Dekker, kinderchirurg, werkzaam in het Erasmus MC Sophia te Rotterdam, NVvH
- T. (Thirza) Schuerhoff, pedagogisch medewerker, werkzaam in het Prinses Máxima Centrum voor Kinderoncologie te Utrecht, Vakgroep Medische Pedagogische zorg
- A.P. (Annette) van der Kaa, kinderfysiotherapeut, werkzaam in het Erasumc MC Sophia te Rotterdam, KNGF en NVFK
Klankbordgroep
- Drs. J. (Judig) Blaauw, kinderrevalidatiearts, VRA
- Dr. H. (Hanneke) Bruijnzeel, AIOS, werkzaam in het UMC Utrecht te Utrecht, NVKNO
- Dr. A.M.J.W. (Anne-Marie) Scheepers, ziekenhuisapotheker, werkzaam in het MUMC te Maastricht, NVZA
- Dr. S.A. (Sylvia) Obermann-Borst, ervaringsdeskundige ouder & huisarts-epidemioloog, Care4Neo (voorheen Vereniging van Ouders van Couveusekinderen - VOC)
- Dr. I.H. (Ilse) Zaal-Schuller, arts voor verstandelijk gehandicapten/kaderarts palliatieve zorg i.o., werkzaam bij Prinsenstichting Purmerend/ AmsterdamUMC locatie AMC, NVAVG
- C. (Charlotte) Langemeijer, CliniClowns Nederland
- M. (Miep) van der Doelen, CliniClowns Nederland
- Dr. E. (Eva) Schaffrath, anesthesioloog, werkzaam in het Maastricht UMC te Maastricht, PROSA Kenniscentrum
- M. (Mirjam) Jansen op de Haar, HME-MO Vereniging Nederland
Met ondersteuning van
- Dr. J. (Janneke) Hoogervorst – Schilp, adviseur, Kennisinstituut van de Federatie Medisch Specialisten
- Dr. C. (Cécile) Overman, adviseur, Kennisinstituut van de Federatie Medisch Specialisten
Belangenverklaringen
De Code ter voorkoming van oneigenlijke beïnvloeding door belangenverstrengeling is gevolgd. Alle werkgroepleden hebben schriftelijk verklaard of zij in de laatste drie jaar directe financiële belangen (betrekking bij een commercieel bedrijf, persoonlijke financiële belangen, onderzoeksfinanciering) of indirecte belangen (persoonlijke relaties, reputatiemanagement) hebben gehad. Gedurende de ontwikkeling of herziening van een module worden wijzigingen in belangen aan de voorzitter doorgegeven. De belangenverklaring wordt opnieuw bevestigd tijdens de commentaarfase.
Een overzicht van de belangen van (kern)werkgroepleden en klankbordgroepleden en het oordeel over het omgaan met eventuele belangen vindt u in onderstaande tabel. De ondertekende belangenverklaringen zijn op te vragen bij het secretariaat van het Kennisinstituut van de Federatie Medisch Specialisten.
|
Betrokkenen |
Functie |
Nevenfuncties |
Gemelde belangen |
Ondernomen actie |
|
Werkgroep |
||||
|
Aarts |
Algemeen kinderarts in het Amalia kinderziekenhuissinds november 2017 |
Interne functies onbetaald: 1. voorzitter Pijn werkgroep Amalia kinderziekenhuis. 2. Verbonden aan werkgroep procedurele sedatie bij kinderen. 3. Implementatie VR in Amalia. |
Onderzoek naar effect comfort talk technieken; maar eenmalige subsidie gekregen voor uitvoer. Geen extern belang qua uitkomst. |
Geen actie |
|
Blaauw |
Kinderrevalidatiearts |
Geen |
Geen |
Geen actie |
|
Bruijn, de (interim) |
Kinderrevalidatiearts bij Revalidatie Friesland |
Lid kwaliteitscommissie VRA (deels betaald, vacatiegeld) |
Geen |
Geen actie |
|
Doelen |
CliniClown bij stichting CliniClowns 28 uur per week |
Bestuurslid theaterproducties Natuurtheater de Kersouwe in Heeswijk Dinther (onbetaald) |
Hoofddoel van mijn bijdrage aan de werkgroep is de kennis en ervaring van CliniClowns in te zetten bij het geven van feedback op de gemaakte stukken. |
Geen actie |
|
Elangovan |
Universitair medisch specialist Anesthesioloog-pijnspecialist; ErasmusMC |
Geen |
Geen |
Geen actie |
|
Genco |
Kinderarts, OLVG, Amsterdam |
- Eigenaar Genco Med. Beheer B.V. |
Directe belangen bij eigen B.V. maar geen relatie met de bezigheden van de werkgroep. Bijvangst van het project kan zijn: nieuwe kennis en ervaring om binnen onze organisatie te delen. |
Geen actie |
|
Goorhuis |
Algemeen kinderarts - acute kindergeneeskunde |
Geen |
Geen |
Geen actie |
|
Haar, van der |
Freelance consultant Moonshot |
Bestuurslid HME-MO Vereniging Nederland |
Geen |
Geen actie |
|
Kaa, van der |
Kinderfysiotherapeut |
-Docent Master Kinderfysiotherapie bij Breederode Hogeschool - Universitair docent -Kinderfysiotherapeut 1e lijn (Fysio van der Linden) |
Geen |
Geen actie |
|
Keyzer-Dekker |
Kinderchirurg Sophia Kinderziekenhuis ErasmusMC te Rotterdam |
APLS instructeur SSHK Riel, dagvergoeding |
Geen |
Geen actie |
|
Kreier |
Kinderarts OLVG Amsterdam |
Medeauteur en -oprichter “De hamster in je brein” |
Geen |
Geen actie |
|
Mensink* |
kinderanesthesioloog - pijnarts - Prinses Máxima Centrum voor kinderoncologie |
Bestuurslid sectie Pijn&palliatieve geneeskunde NVA - onbetaalde functie |
Geen |
Geen actie |
|
Neef, de |
Verpleegkundig onderzoeker, Kinder IC, Amsterdam UMC |
Geen |
Geen |
Geen actie |
|
Petra Honig-Mazer |
Erasmus MC - Sophia Kinderziekenhuis Afdeling Kinder- en Jeugdpsychiatrie/psychologie Unit Psychosociale Zorg Psychotherapeut BIG |
Kleine eigen praktijk: Praktijk voor Psychotherapie Honig-Mazer, betaald |
Geen |
Geen actie |
|
Renes |
Anesthesioloog-pijnspecialist Radboudumc |
Kwaliteitsvisitaties Nederlandse Vereniging Anesthesiologie, vacatiegeld |
Geen |
Geen actie |
|
Schuerhoff |
Medisch Pedagogisch Zorgverlener |
Geen |
Geen |
Geen actie |
|
Simons |
Kinderarts - neonatoloog - klinisch farmacoloog (Universitair Medische Specialist) |
Lid geneesmiddelencommissie Erasmus MC (onbetaald) |
Geen |
Geen actie |
|
Tissing |
Kinderoncoloog, Hoogleraar supportive care in de kinderoncologie. 0.6 fte Prinses Maxima Centrum, 0,4 fte UMCG |
PI van onderzoek naar app over invloed van laagdrempelig contact op pijn bij patiënten met kanker. |
Geen |
Geen actie |
|
Uitzinger |
Junior Project manager en beleidsmedewerker Stichting kind en ziekenhuis |
Geen |
Geen |
Geen actie |
|
Vlieger |
1. Kinderarts St Antonius ziekenhuis Nieuwegein |
1. Les geven via Cure en Care en via het Prinses MAxima Centrum op het gebiedvan hypnose bij kinderen. Dit is betaald. |
Aangezien ik les geef op het gebied van non-farmacologische methoden om pijn en angst te voorkomen cq te behandelen kan ik daar theoretisch voordeel van ondervinden als nog meer afdelingen hun personeel geschoold wilt hebben in non-farmacologische technieken. in de richtlijn komen uiteraard geen namen van bedrijven te staan, dus ik verwacht geen evident voordeel. Mijn mede eigenaars van het skills4comfort bedrijf. Overigens loopt dit al heel goed. Iedereen is gelukkig al overtuigd van het belang van het aanleren van positief taalgebruik, afleiden, echt contact maken en een vertrouwensband opbouwen. |
Uitsluiten van besluitvorming voor modules over non-farmacologische pijnbestrijding. Mag wel meelezen. |
|
Wildt, de |
Kinderarts-intensivist, hoogleraar klinische farmacologie, Radboudumc |
Directeur stichting Nederlands Kenniscentrum Farmacotherapie Kinderen (detachering |
Patent: Gebruik van PENK voor nierfunctiebepaling bij kinderen (aangevraagd). Bill and Melinda Gates Foundation: model-informed dosing for pediatric dosing |
Geen actie |
|
Wulffraat |
Hoogleraar kinderimmunologie/reumatologie. UMCU |
Voorzitter (coordinator) ERN-RITA (european reference network (onbetaald). |
Onze vakgroep heeft zeer veel extern gefinancierd onderzoek. Er is geen direct belang van de financier bij deze richtlijn. Onderzoekslijn chronische pijn bij jeugdreuma is puur academisch. Mensink (aangesteld in PMC) is hier de promovendus. Ik ben de promotor. |
Geen actie |
|
Klankbordgroep |
||||
|
Bruijnzeel |
Arts-assistent Keel-, Neus- en Oorheelkunde, UMC Utrecht |
Kerngroep Pediatrie (KNO vereniging) - onbetaald |
Geen |
Geen actie |
|
Scheepers |
ziekenhuisapotheker, Maastricht UMC+ |
Geen |
Geen |
Geen actie |
|
Obermann-Borst |
Coördinator Wetenschap bij Care4Neo 10 u per week |
Coördinator Wetenschap bij Care4Neo 75% betaald/25% vrijwillig verzorgen van bijdrage vanuit patientenperspectief aan wetenschap, richtlijnen en kwaliteit van zorg namens de patientenvereniging voor ouders van en voor kinderen die te vroeg, te klein en/of ziek geboren zijn. |
Geen |
Geen actie |
|
Zaal Schuller |
Arts voor verstandelijk gehandicapten |
Arts voor verstandelijk gehandicapten, betaald. |
Geen |
Geen actie |
|
Schaffrath |
Kinderanesthesioloog MUMC |
Faculty member PROSA (tegen dagvergoeding) |
Geen |
Geen actie |
* Voorzitter
Inbreng patiëntenperspectief
Er werd aandacht besteed aan het patiëntenperspectief door afvaardiging van Stichting Kind en Ziekenhuis in de kernwerkgroep en Care4Neo, CliniClowns Nederland en HME-MO Vereniging Nederland in de klankbordgroep. Op verschillende momenten is input gevraagd tijdens een invitational conference en bij het opstellen van het raamwerk. Het verslag van de invitational conference [zie aanverwante producten] is besproken in de werkgroep. De verkregen input is meegenomen bij het opstellen van de uitgangsvragen, de keuze voor de uitkomstmaten en bij het opstellen van de overwegingen. De conceptrichtlijn is tevens voor commentaar voorgelegd aan de patiëntenorganisaties en de eventueel aangeleverde commentaren zijn bekeken en verwerkt.
Werkwijze
AGREE
Deze richtlijnmodule is opgesteld conform de eisen vermeld in het rapport Medisch Specialistische Richtlijnen 2.0 van de adviescommissie Richtlijnen van de Raad Kwaliteit. Dit rapport is gebaseerd op het AGREE II instrument (Appraisal of Guidelines for Research & Evaluation II; Brouwers, 2010).
Knelpuntenanalyse en uitgangsvragen
Voorafgaand aan de voorbereidende fase is een invitational conference georganiseerd over herkenning en behandeling van pijn binnen de kindzorg. Een verslag hiervan is opgenomen onder aanverwante producten. Daarnaast werd tijdens de voorbereidende fase van de richtlijn een schriftelijke knelpunteninventarisatie gehouden. Op basis van de uitkomsten van de knelpuntenanalyse zijn door de werkgroep concept-uitgangsvragen opgesteld en definitief vastgesteld.
Uitkomstmaten
Na het opstellen van de zoekvraag behorende bij de uitgangsvraag inventariseerde de werkgroep welke uitkomstmaten voor de patiënt relevant zijn, waarbij zowel naar gewenste als ongewenste effecten werd gekeken. Hierbij werd een maximum van acht uitkomstmaten gehanteerd. De werkgroep waardeerde deze uitkomstmaten volgens hun relatieve belang bij de besluitvorming rondom aanbevelingen, als cruciaal (kritiek voor de besluitvorming), belangrijk (maar niet cruciaal) en onbelangrijk. Tevens definieerde de werkgroep tenminste voor de cruciale uitkomstmaten welke verschillen zij klinisch (patiënt) relevant vonden.
Methode literatuursamenvatting
Een uitgebreide beschrijving van de strategie voor zoeken en selecteren van literatuur en de beoordeling van de risk-of-bias van de individuele studies is te vinden onder ‘Zoeken en selecteren’ onder Onderbouwing. De beoordeling van de kracht van het wetenschappelijke bewijs wordt hieronder toegelicht.
Beoordelen van de kracht van het wetenschappelijke bewijs
De kracht van het wetenschappelijke bewijs werd bepaald volgens de GRADE-methode. GRADE staat voor ‘Grading Recommendations Assessment, Development and Evaluation’ (zie https://www.gradeworkinggroup.org/). De basisprincipes van de GRADE-methodiek zijn: het benoemen en prioriteren van de klinisch (patiënt) relevante uitkomstmaten, een systematische review per uitkomstmaat, en een beoordeling van de bewijskracht per uitkomstmaat op basis van de acht GRADE-domeinen (domeinen voor downgraden: risk of bias, inconsistentie, indirectheid, imprecisie, en publicatiebias; domeinen voor upgraden: dosis-effect relatie, groot effect, en residuele plausibele confounding).
GRADE onderscheidt vier gradaties voor de kwaliteit van het wetenschappelijk bewijs: hoog, redelijk, laag en zeer laag. Deze gradaties verwijzen naar de mate van zekerheid die er bestaat over de literatuurconclusie, in het bijzonder de mate van zekerheid dat de literatuurconclusie de aanbeveling adequaat ondersteunt (Schünemann, 2013; Hultcrantz, 2017).
|
Definitie |
|
|
Hoog |
|
|
Redelijk |
|
|
Laag |
|
|
Zeer laag |
|
Bij het beoordelen (graderen) van de kracht van het wetenschappelijk bewijs in richtlijnen volgens de GRADE-methodiek spelen grenzen voor klinische besluitvorming een belangrijke rol (Hultcrantz, 2017). Dit zijn de grenzen die bij overschrijding aanleiding zouden geven tot een aanpassing van de aanbeveling. Om de grenzen voor klinische besluitvorming te bepalen moeten alle relevante uitkomstmaten en overwegingen worden meegewogen. De grenzen voor klinische besluitvorming zijn daarmee niet één op één vergelijkbaar met het minimaal klinisch relevant verschil (Minimal Clinically Important Difference, MCID). Met name in situaties waarin een interventie geen belangrijke nadelen heeft en de kosten relatief laag zijn, kan de grens voor klinische besluitvorming met betrekking tot de effectiviteit van de interventie bij een lagere waarde (dichter bij het nuleffect) liggen dan de MCID (Hultcrantz, 2017).
COSMIN
The COSMIN Risk of Bias tool was used to assess the quality of single studies for each measurement property. Thereby, the worst-score-counts method was used to determine the risk of bias, this means that the lowest rating given in a box determines the final rating, i.e. the quality of the study. The result of each study on a measurement property (figure 1) were rated against the updated criteria for good measurement properties (Table 1). Each result was rated as either sufficient (+), insufficient (–), or indeterminate (?).
Table 1. Criteria for good measurement properties (Mokkink, 2018)
|
Measurement property |
Rating[1] |
Criteria |
|
Structural validity |
+ |
CTT: CFA: CFI or TLI or comparable measure >0.95 OR RMSEA <0.06 OR SRMR <0.08[2] IRT/Rasch: No violation of unidimensionality[3]: CFI or TLI or comparable measure >0.95 OR RMSEA <0.06 OR SRMR <0.08 AND no violation of local independence: residual correlations among the items after controlling for the dominant factor < 0.20 OR Q3's < 0.37 AND no violation of monotonicity: adequate looking graphs OR item scalability >0.30 AND adequate model fit: IRT: χ2 >0.01 Rasch: infit and outfit mean squares ≥ 0.5 and ≤ 1.5 OR Z-standardized values > ‐2 and <2 |
|
? |
CTT: Not all information for ‘+’ reported IRT/Rasch: Model fit not reported |
|
|
- |
Criteria for ‘+’ not met |
|
|
Internal consistency |
+ |
At least low evidence[4] for sufficient structural validity[5] AND Cronbach's alpha(s) ≥ 0.70 for each unidimensional scale or Subscale.[6] |
|
? |
Criteria for “At least low evidence for sufficient structural validity” not met |
|
|
- |
At least low evidence4 for sufficient structural validity5 AND Cronbach’s alpha(s) < 0.70 for each unidimensional scale or Subscale.6 |
|
|
Reliability |
+ |
ICC or weighted Kappa ≥ 0.70 |
|
? |
ICC or weighted Kappa not reported |
|
|
- |
ICC or weighted Kappa < 0.70 |
|
|
Measurement error |
+ |
SDC or LoA < MIC |
|
? |
MIC not defined |
|
|
- |
SDC or LoA > MIC |
|
|
Hypotheses testing for construct validity |
+ |
The result is in accordance with the hypothesis[7] |
|
? |
No hypothesis defined (by the review team) |
|
|
- |
The result is not in accordance with the hypothesis |
|
|
Cross‐cultural validity\measurement invariance |
+ |
No important differences found between group factors (such as age, gender, language) in multiple group factor analysis OR no important DIF for group factors (McFadden's R2 < 0.02) |
|
? |
No multiple group factor analysis OR DIF analysis performed |
|
|
- |
Important differences between group factors OR DIF was found |
|
|
Criterion validity |
+ |
Correlation with gold standard ≥ 0.70 OR AUC ≥ 0.70 |
|
? |
Not all information for ‘+’ reported |
|
|
- |
Correlation with gold standard < 0.70 OR AUC < 0.70 |
|
|
Responsiveness |
+ |
The result is in accordance with the hypothesis OR AUC ≥ 0.70 |
|
? |
No hypothesis defined (by the review team) |
|
|
- |
The result is not in accordance with the hypothesis OR AUC < 0.70 |
|
|
AUC: area under the curve, CFA: confirmatory factor analysis, CFI: comparative fit index, CTT: classical test theory, DIF: differential item functioning, ICC: intraclass correlation coefficient, IRT: item response theory, LoA: limits of agreement, MIC: minimal important change, RMSEA: Root Mean Square Error of Approximation, SEM: Standard Error of Measurement, SDC: smallest detectable change, SRMR: Standardized Root Mean Residuals, TLI = Tucker‐Lewis Index |
||
[1] “+” = sufficient, ” –“ = insufficient, “?” = indeterminate
[2] To rate the quality of the summary score, the factor structures should be equal across studies
[3] unidimensionality refers to a factor analysis per subscale, while structural validity refers to a factor analysis of a (multidimensional) patient‐reported outcome measure
[4] As defined by grading the evidence according to the GRADE approach
[5] This evidence may come from different studies
[6] The criteria ‘Cronbach alpha < 0.95’ was deleted, as this is relevant in the development phase of a PROM and not when evaluating an existing PROM.
[7] The results of all studies should be taken together and it should then be decided if 75% of the results are in accordance with the hypotheses
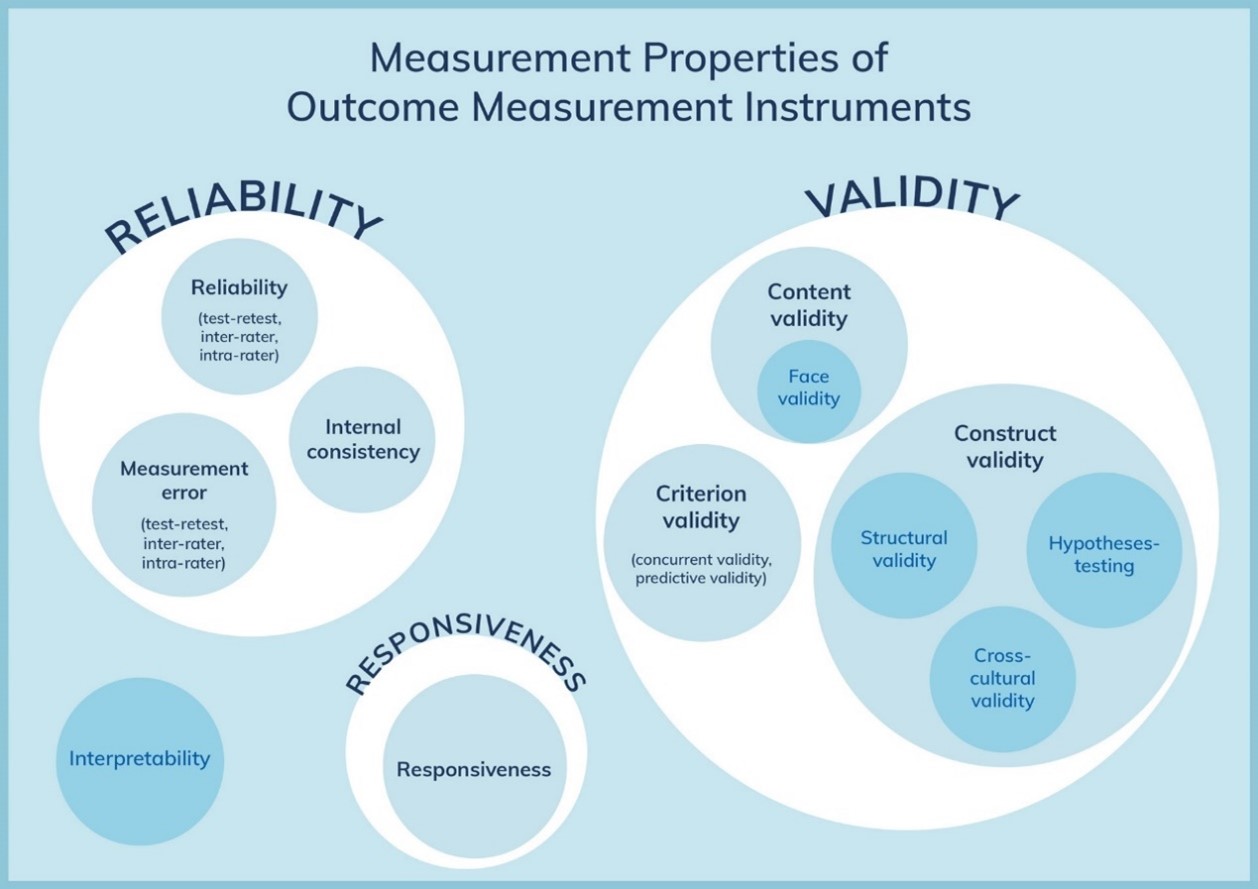
Figure 1. The COSMIN taxonomy of measurement properties (www.cosmin.nl)
The level of evidence of the literature was evaluated as described in the COSMIN user manual for systematic reviews of patient-reported outcome measures (Mokkink, 2018). The following four factors were taken into account: (1) risk of bias (i.e. the methodological quality of the studies), (2) inconsistency (i.e. unexplained inconsistency of results across studies), (3) imprecision (i.e. total sample size of the available studies), and (4) indirectness (i.e. evidence from different populations than the population of interest in the review). The quality of evidence could be downgraded with one level (e.g. from high to moderate evidence) if there is serious risk of bias, with two levels (e.g. from high to low) if there is very serious risk of bias, or with three levels (i.e. from high to very low) if there is extremely risk of bias. The quality of the evidence could be downgraded with one or two levels for inconsistency, imprecision (-1 if total N=50-100; -2 if total N<50) and indirectness.
Overwegingen (van bewijs naar aanbeveling)
Om te komen tot een aanbeveling zijn naast (de kwaliteit van) het wetenschappelijke bewijs ook andere aspecten belangrijk en worden meegewogen, zoals aanvullende argumenten uit bijvoorbeeld de biomechanica of fysiologie, waarden en voorkeuren van patiënten, kosten (middelenbeslag), aanvaardbaarheid, haalbaarheid en implementatie. Deze aspecten zijn systematisch vermeld en beoordeeld (gewogen) onder het kopje ‘Overwegingen’ en kunnen (mede) gebaseerd zijn op expert opinion. Hierbij is gebruik gemaakt van een gestructureerd format gebaseerd op het evidence-to-decision framework van de internationale GRADE Working Group (Alonso-Coello, 2016a; Alonso-Coello 2016b). Dit evidence-to-decision framework is een integraal onderdeel van de GRADE-methodiek.
Formuleren van aanbevelingen
De aanbevelingen geven antwoord op de uitgangsvraag en zijn gebaseerd op het beschikbare wetenschappelijke bewijs en de belangrijkste overwegingen, en een weging van de gunstige en ongunstige effecten van de relevante interventies. De kracht van het wetenschappelijk bewijs en het gewicht dat door de werkgroep wordt toegekend aan de overwegingen, bepalen samen de sterkte van de aanbeveling. Conform de GRADE-methodiek sluit een lage bewijskracht van conclusies in de systematische literatuuranalyse een sterke aanbeveling niet a priori uit, en zijn bij een hoge bewijskracht ook zwakke aanbevelingen mogelijk (Agoritsas, 2017; Neumann, 2016). De sterkte van de aanbeveling wordt altijd bepaald door weging van alle relevante argumenten tezamen. De werkgroep heeft bij elke aanbeveling opgenomen hoe zij tot de richting en sterkte van de aanbeveling zijn gekomen.
In de GRADE-methodiek wordt onderscheid gemaakt tussen sterke en zwakke (of conditionele) aanbevelingen. De sterkte van een aanbeveling verwijst naar de mate van zekerheid dat de voordelen van de interventie opwegen tegen de nadelen (of vice versa), gezien over het hele spectrum van patiënten waarvoor de aanbeveling is bedoeld. De sterkte van een aanbeveling heeft duidelijke implicaties voor patiënten, behandelaars en beleidsmakers (zie onderstaande tabel). Een aanbeveling is geen dictaat, zelfs een sterke aanbeveling gebaseerd op bewijs van hoge kwaliteit (GRADE gradering HOOG) zal niet altijd van toepassing zijn, onder alle mogelijke omstandigheden en voor elke individuele patiënt.
|
Implicaties van sterke en zwakke aanbevelingen voor verschillende richtlijngebruikers |
||
|
|
Sterke aanbeveling |
Zwakke (conditionele) aanbeveling |
|
Voor patiënten |
De meeste patiënten zouden de aanbevolen interventie of aanpak kiezen en slechts een klein aantal niet. |
Een aanzienlijk deel van de patiënten zouden de aanbevolen interventie of aanpak kiezen, maar veel patiënten ook niet. |
|
Voor behandelaars |
De meeste patiënten zouden de aanbevolen interventie of aanpak moeten ontvangen. |
Er zijn meerdere geschikte interventies of aanpakken. De patiënt moet worden ondersteund bij de keuze voor de interventie of aanpak die het beste aansluit bij zijn of haar waarden en voorkeuren. |
|
Voor beleidsmakers |
De aanbevolen interventie of aanpak kan worden gezien als standaardbeleid. |
Beleidsbepaling vereist uitvoerige discussie met betrokkenheid van veel stakeholders. Er is een grotere kans op lokale beleidsverschillen. |
Organisatie van zorg
In de knelpuntenanalyse en bij de ontwikkeling van de richtlijnmodule is expliciet aandacht geweest voor de organisatie van zorg: alle aspecten die randvoorwaardelijk zijn voor het verlenen van zorg (zoals coördinatie, communicatie, (financiële) middelen, mankracht en infrastructuur). Randvoorwaarden die relevant zijn voor het beantwoorden van deze specifieke uitgangsvraag zijn genoemd bij de overwegingen. Meer algemene, overkoepelende, of bijkomende aspecten van de organisatie van zorg worden behandeld in de module Organisatie van zorg.
Commentaar- en autorisatiefase
De conceptrichtlijnmodule werd aan de betrokken (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd ter commentaar. De commentaren werden verzameld en besproken met de werkgroep. Naar aanleiding van de commentaren werd de conceptrichtlijnmodule aangepast en definitief vastgesteld door de werkgroep. De definitieve richtlijnmodule werd aan de deelnemende (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd voor autorisatie en door hen geautoriseerd dan wel geaccordeerd.
Literatuur
Agoritsas T, Merglen A, Heen AF, Kristiansen A, Neumann I, Brito JP, Brignardello-Petersen R, Alexander PE, Rind DM, Vandvik PO, Guyatt GH. UpToDate adherence to GRADE criteria for strong recommendations: an analytical survey. BMJ Open. 2017 Nov 16;7(11):e018593. doi: 10.1136/bmjopen-2017-018593. PubMed PMID: 29150475; PubMed Central PMCID: PMC5701989.
Alonso-Coello P, Schünemann HJ, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Rada G, Rosenbaum S, Morelli A, Guyatt GH, Oxman AD; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ. 2016 Jun 28;353:i2016. doi: 10.1136/bmj.i2016. PubMed PMID: 27353417.
Alonso-Coello P, Oxman AD, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Vandvik PO, Meerpohl J, Guyatt GH, Schünemann HJ; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 2: Clinical practice guidelines. BMJ. 2016 Jun 30;353:i2089. doi: 10.1136/bmj.i2089. PubMed PMID: 27365494.
Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, Fervers B, Graham ID, Grimshaw J, Hanna SE, Littlejohns P, Makarski J, Zitzelsberger L; AGREE Next Steps Consortium. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010 Dec 14;182(18):E839-42. doi: 10.1503/cmaj.090449. Epub 2010 Jul 5. Review. PubMed PMID: 20603348; PubMed Central PMCID: PMC3001530.
Hultcrantz M, Rind D, Akl EA, Treweek S, Mustafa RA, Iorio A, Alper BS, Meerpohl JJ, Murad MH, Ansari MT, Katikireddi SV, Östlund P, Tranæus S, Christensen R, Gartlehner G, Brozek J, Izcovich A, Schünemann H, Guyatt G. The GRADE Working Group clarifies the construct of certainty of evidence. J Clin Epidemiol. 2017 Jul;87:4-13. doi: 10.1016/j.jclinepi.2017.05.006. Epub 2017 May 18. PubMed PMID: 28529184; PubMed Central PMCID: PMC6542664.
Medisch Specialistische Richtlijnen 2.0 (2012). Adviescommissie Richtlijnen van de Raad Kwalitieit. http://richtlijnendatabase.nl/over_deze_site/over_richtlijnontwikkeling.html
Neumann I, Santesso N, Akl EA, Rind DM, Vandvik PO, Alonso-Coello P, Agoritsas T, Mustafa RA, Alexander PE, Schünemann H, Guyatt GH. A guide for health professionals to interpret and use recommendations in guidelines developed with the GRADE approach. J Clin Epidemiol. 2016 Apr;72:45-55. doi: 10.1016/j.jclinepi.2015.11.017. Epub 2016 Jan 6. Review. PubMed PMID: 26772609.
Schünemann H, Brożek J, Guyatt G, et al. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group, 2013. Available from http://gdt.guidelinedevelopment.org/central_prod/_design/client/handbook/handbook.html.
Schünemann HJ, Oxman AD, Brozek J, Glasziou P, Jaeschke R, Vist GE, Williams JW Jr, Kunz R, Craig J, Montori VM, Bossuyt P, Guyatt GH; GRADE Working Group. Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. BMJ. 2008 May 17;336(7653):1106-10. doi: 10.1136/bmj.39500.677199.AE. Erratum in: BMJ. 2008 May 24;336(7654). doi: 10.1136/bmj.a139.
Schünemann, A Holger J [corrected to Schünemann, Holger J]. PubMed PMID: 18483053; PubMed Central PMCID: PMC2386626.
Wessels M, Hielkema L, van der Weijden T. How to identify existing literature on patients' knowledge, views, and values: the development of a validated search filter. J Med Libr Assoc. 2016 Oct;104(4):320-324. PubMed PMID: 27822157; PubMed Central PMCID: PMC5079497.
Zoekverantwoording
Embase
|
No. |
Query |
Results |
|
#18 |
#16 NOT #15 NOT #13 OBS |
123 |
|
#17 |
#15 NOT #13 RCT |
51 |
|
#16 |
#12 AND #14 |
183 |
|
#15 |
#8 AND #14 |
58 |
|
#14 |
#6 AND #11 |
210 |
|
#13 |
#6 AND #7 SR |
33 |
|
#12 |
#9 OR #10 |
14362882 |
|
#11 |
'intermethod comparison'/exp OR 'data collection method'/exp OR 'validation study'/exp OR 'feasibility study'/exp OR 'pilot study'/exp OR 'psychometry'/exp OR 'reproducibility'/exp OR reproducib*:ab,ti OR 'audit':ab,ti OR psychometr*:ab,ti OR clinimetr*:ab,ti OR clinometr*:ab,ti OR 'observer variation'/exp OR 'observer variation':ab,ti OR 'discriminant analysis'/exp OR 'validity'/exp OR reliab*:ab,ti OR valid*:ab,ti OR 'coefficient':ab,ti OR 'internal consistency':ab,ti OR (cronbach*:ab,ti AND ('alpha':ab,ti OR 'alphas':ab,ti)) OR 'item correlation':ab,ti OR 'item correlations':ab,ti OR 'item selection':ab,ti OR 'item selections':ab,ti OR 'item reduction':ab,ti OR 'item reductions':ab,ti OR 'agreement':ab,ti OR 'precision':ab,ti OR 'imprecision':ab,ti OR 'precise values':ab,ti OR 'test-retest':ab,ti OR ('test':ab,ti AND 'retest':ab,ti) OR (reliab*:ab,ti AND ('test':ab,ti OR 'retest':ab,ti)) OR 'stability':ab,ti OR 'interrater':ab,ti OR 'inter-rater':ab,ti OR 'intrarater':ab,ti OR 'intra-rater':ab,ti OR 'intertester':ab,ti OR 'inter-tester':ab,ti OR 'intratester':ab,ti OR 'interobeserver':ab,ti OR 'inter-observer':ab,ti OR 'intraobserver':ab,ti OR 'intertechnician':ab,ti OR 'inter-technician':ab,ti OR 'intratechnician':ab,ti OR 'interexaminer':ab,ti OR 'inter-examiner':ab,ti OR 'intraexaminer':ab,ti OR 'interassay':ab,ti OR 'inter-assay':ab,ti OR 'intraassay':ab,ti OR 'intra-assay':ab,ti OR 'interindividual':ab,ti OR 'inter-individual':ab,ti OR 'intraindividual':ab,ti OR 'intra-individual':ab,ti OR 'interparticipant':ab,ti OR 'inter-participant':ab,ti OR 'intraparticipant':ab,ti OR 'kappa':ab,ti OR 'kappas':ab,ti OR 'coefficient of variation':ab,ti OR repeatab*:ab,ti OR ((replicab*:ab,ti OR 'repeated':ab,ti) AND ('measure':ab,ti OR 'measures':ab,ti OR 'findings':ab,ti OR 'result':ab,ti OR 'results':ab,ti OR 'test':ab,ti OR 'tests':ab,ti)) OR generaliza*:ab,ti OR generalisa*:ab,ti OR 'concordance':ab,ti OR ('intraclass':ab,ti AND correlation*:ab,ti) OR 'discriminative':ab,ti OR 'known group':ab,ti OR 'factor analysis':ab,ti OR 'factor analyses':ab,ti OR 'factor structure':ab,ti OR 'factor structures':ab,ti OR 'dimensionality':ab,ti OR subscale*:ab,ti OR 'multitrait scaling analysis':ab,ti OR 'multitrait scaling analyses':ab,ti OR 'item discriminant':ab,ti OR 'interscale correlation':ab,ti OR 'interscale correlations':ab,ti OR (('error':ab,ti OR 'errors':ab,ti) AND (measure*:ab,ti OR correlat*:ab,ti OR evaluat*:ab,ti OR 'accuracy':ab,ti OR 'accurate':ab,ti OR 'precision':ab,ti OR 'mean':ab,ti)) OR 'individual variability':ab,ti OR 'interval variability':ab,ti OR 'rate variability':ab,ti OR 'variability analysis':ab,ti OR ('uncertainty':ab,ti AND ('measurement':ab,ti OR 'measuring':ab,ti)) OR 'standard error of measurement':ab,ti OR sensitiv*:ab,ti OR responsive*:ab,ti OR ('limit':ab,ti AND 'detection':ab,ti) OR 'minimal detectable concentration':ab,ti OR interpretab*:ab,ti OR (small*:ab,ti AND ('real':ab,ti OR 'detectable':ab,ti) AND ('change':ab,ti OR 'difference':ab,ti)) OR 'meaningful change':ab,ti OR 'minimal important change':ab,ti OR 'minimal important difference':ab,ti OR 'minimally important change':ab,ti OR 'minimally important difference':ab,ti OR 'minimal detectable change':ab,ti OR 'minimal detectable difference':ab,ti OR 'minimally detectable change':ab,ti OR 'minimally detectable difference':ab,ti OR 'minimal real change':ab,ti OR 'minimal real difference':ab,ti OR 'minimally real change':ab,ti OR 'minimally real difference':ab,ti OR 'ceiling effect':ab,ti OR 'floor effect':ab,ti OR 'item response model':ab,ti OR 'irt':ab,ti OR 'rasch':ab,ti OR 'differential item functioning':ab,ti OR 'dif':ab,ti OR 'computer adaptive testing':ab,ti OR 'item bank':ab,ti OR 'cross-cultural equivalence':ab,ti |
6920436 |
|
#10 |
'case control study'/de OR 'comparative study'/exp OR 'control group'/de OR 'controlled study'/de OR 'controlled clinical trial'/de OR 'crossover procedure'/de OR 'double blind procedure'/de OR 'phase 2 clinical trial'/de OR 'phase 3 clinical trial'/de OR 'phase 4 clinical trial'/de OR 'pretest posttest design'/de OR 'pretest posttest control group design'/de OR 'quasi experimental study'/de OR 'single blind procedure'/de OR 'triple blind procedure'/de OR (((control OR controlled) NEAR/6 trial):ti,ab,kw) OR (((control OR controlled) NEAR/6 (study OR studies)):ti,ab,kw) OR (((control OR controlled) NEAR/1 active):ti,ab,kw) OR 'open label*':ti,ab,kw OR (((double OR two OR three OR multi OR trial) NEAR/1 (arm OR arms)):ti,ab,kw) OR ((allocat* NEAR/10 (arm OR arms)):ti,ab,kw) OR placebo*:ti,ab,kw OR 'sham-control*':ti,ab,kw OR (((single OR double OR triple OR assessor) NEAR/1 (blind* OR masked)):ti,ab,kw) OR nonrandom*:ti,ab,kw OR 'non-random*':ti,ab,kw OR 'quasi-experiment*':ti,ab,kw OR crossover:ti,ab,kw OR 'cross over':ti,ab,kw OR 'parallel group*':ti,ab,kw OR 'factorial trial':ti,ab,kw OR ((phase NEAR/5 (study OR trial)):ti,ab,kw) OR ((case* NEAR/6 (matched OR control*)):ti,ab,kw) OR ((match* NEAR/6 (pair OR pairs OR cohort* OR control* OR group* OR healthy OR age OR sex OR gender OR patient* OR subject* OR participant*)):ti,ab,kw) OR ((propensity NEAR/6 (scor* OR match*)):ti,ab,kw) OR versus:ti OR vs:ti OR compar*:ti OR ((compar* NEAR/1 study):ti,ab,kw) OR (('major clinical study'/de OR 'clinical study'/de OR 'cohort analysis'/de OR 'observational study'/de OR 'cross-sectional study'/de OR 'multicenter study'/de OR 'correlational study'/de OR 'follow up'/de OR cohort*:ti,ab,kw OR 'follow up':ti,ab,kw OR followup:ti,ab,kw OR longitudinal*:ti,ab,kw OR prospective*:ti,ab,kw OR retrospective*:ti,ab,kw OR observational*:ti,ab,kw OR 'cross sectional*':ti,ab,kw OR cross?ectional*:ti,ab,kw OR multicent*:ti,ab,kw OR 'multi-cent*':ti,ab,kw OR consecutive*:ti,ab,kw) AND (group:ti,ab,kw OR groups:ti,ab,kw OR subgroup*:ti,ab,kw OR versus:ti,ab,kw OR vs:ti,ab,kw OR compar*:ti,ab,kw OR 'odds ratio*':ab OR 'relative odds':ab OR 'risk ratio*':ab OR 'relative risk*':ab OR 'rate ratio':ab OR aor:ab OR arr:ab OR rrr:ab OR ((('or' OR 'rr') NEAR/6 ci):ab))) |
12676523 |
|
#9 |
'major clinical study'/de OR 'clinical study'/de OR 'case control study'/de OR 'family study'/de OR 'longitudinal study'/de OR 'retrospective study'/de OR 'prospective study'/de OR 'comparative study'/de OR 'cohort analysis'/de OR ((cohort NEAR/1 (study OR studies)):ab,ti) OR (('case control' NEAR/1 (study OR studies)):ab,ti) OR (('follow up' NEAR/1 (study OR studies)):ab,ti) OR (observational NEAR/1 (study OR studies)) OR ((epidemiologic NEAR/1 (study OR studies)):ab,ti) OR (('cross sectional' NEAR/1 (study OR studies)):ab,ti) |
6767914 |
|
#8 |
'randomized controlled trial'/exp OR random*:ti,ab OR (((pragmatic OR practical) NEAR/1 'clinical trial*'):ti,ab) OR ((('non inferiority' OR noninferiority OR superiority OR equivalence) NEAR/3 trial*):ti,ab) OR rct:ti,ab,kw |
1839814 |
|
#7 |
'meta analysis'/exp OR 'meta analysis (topic)'/exp OR metaanaly*:ti,ab OR 'meta analy*':ti,ab OR metanaly*:ti,ab OR 'systematic review'/de OR 'cochrane database of systematic reviews'/jt OR prisma:ti,ab OR prospero:ti,ab OR (((systemati* OR scoping OR umbrella OR 'structured literature') NEAR/3 (review* OR overview*)):ti,ab) OR ((systemic* NEAR/1 review*):ti,ab) OR (((systemati* OR literature OR database* OR 'data base*') NEAR/10 search*):ti,ab) OR (((structured OR comprehensive* OR systemic*) NEAR/3 search*):ti,ab) OR (((literature NEAR/3 review*):ti,ab) AND (search*:ti,ab OR database*:ti,ab OR 'data base*':ti,ab)) OR (('data extraction':ti,ab OR 'data source*':ti,ab) AND 'study selection':ti,ab) OR ('search strategy':ti,ab AND 'selection criteria':ti,ab) OR ('data source*':ti,ab AND 'data synthesis':ti,ab) OR medline:ab OR pubmed:ab OR embase:ab OR cochrane:ab OR (((critical OR rapid) NEAR/2 (review* OR overview* OR synthes*)):ti) OR ((((critical* OR rapid*) NEAR/3 (review* OR overview* OR synthes*)):ab) AND (search*:ab OR database*:ab OR 'data base*':ab)) OR metasynthes*:ti,ab OR 'meta synthes*':ti,ab |
733409 |
|
#6 |
#5 AND [1-1-2007]/sd NOT ('conference abstract'/it OR 'editorial'/it OR 'letter'/it OR 'note'/it) NOT (('animal experiment'/exp OR 'animal model'/exp OR 'nonhuman'/exp) NOT 'human'/exp) |
716 |
|
#5 |
#1 AND #2 AND #3 AND #4 |
1149 |
|
#4 |
'preoperative period'/exp OR 'pre operati*':ti,ab,kw OR preoperati*:ti,ab,kw OR 'before surger*':ti,ab,kw OR presurg*:ti,ab,kw OR 'pre surg*':ti,ab,kw |
726647 |
|
#3 |
'psychometry'/exp OR 'anxiety assessment'/exp OR 'pain assessment'/exp OR 'hospital anxiety and depression scale'/exp OR 'pain catastrophizing scale'/exp OR 'pittsburgh sleep quality index'/exp OR 'quantitative sensory testing'/exp OR 'state trait anxiety inventory'/exp OR ((quantitative NEAR/2 (test* OR inventor*)):ti,ab,kw) OR ((had NEAR/2 (scale OR score)):ti,ab,kw) OR 'hads':ti,ab,kw OR (((pain OR anxiety) NEAR/3 (scale* OR score* OR tool* OR test* OR instrument* OR inventor*)):ti,ab,kw) OR 'pittsburgh sleep quality index':ti,ab,kw OR ((screening NEAR/3 (tool* OR instrument*)):ti,ab,kw) OR psqi:ti,ab,kw OR 'stai-ch':ti,ab,kw OR psychometr*:ti,ab,kw |
526919 |
|
#2 |
'postoperative pain'/exp OR (((postoperati* OR 'post operati*' OR surgical OR postsurgical) NEAR/3 pain):ti,ab,kw) |
98347 |
|
#1 |
infan*:ti,ab OR newborn*:ti,ab OR 'new born*':ti,ab OR perinat*:ti,ab OR neonat*:ti,ab OR 'baby'/exp OR baby*:ti,ab OR babies:ti,ab OR toddler*:ti,ab OR 'minors'/exp/mj OR minor*:ti,kw OR 'boy'/exp OR boy:ti,ab OR boys:ti,ab OR boyfriend:ti,ab OR boyhood:ti,ab OR girl*:ti,ab OR kid:ti,ab OR kids:ti,ab OR 'child'/exp OR child*:ti,ab OR children*:ti,ab OR schoolchild*:ti,ab OR 'schoolchild'/exp OR adolescen*:ti,ab OR juvenil*:ti,ab OR youth*:ti,ab OR teen*:ti,ab OR pubescen*:ti,ab OR pediatric*:ti,ab OR paediatric*:ti,ab OR peadiatric*:ti,ab OR school:ti,ab OR school*:ti,ab OR prematur*:ti,ab OR preterm*:ti,ab OR 'pediatrics'/exp OR 'pediatric patient'/exp |
4710293 |
|
# |
Searches |
Results |
|
19 |
17 not 16 not 14 OBS |
81 |
|
18 |
16 not 14 RCT |
96 |
|
17 |
10 and 15 |
178 |
|
16 |
7 and 15 |
101 |
|
15 |
5 and 13 |
211 |
|
14 |
6 and 13 SR |
14 |
|
13 |
12 not ((exp animals/ or exp models, animal/) not humans/) not (letter/ or comment/ or editorial/) |
293 |
|
12 |
limit 11 to yr="2007 -Current" |
295 |
|
11 |
1 and 2 and 3 and 4 |
400 |
|
10 |
8 or 9 |
6637670 |
|
9 |
Epidemiologic studies/ or case control studies/ or exp cohort studies/ or Controlled Before-After Studies/ or Case control.tw. or cohort.tw. or Cohort analy$.tw. or (Follow up adj (study or studies)).tw. or (observational adj (study or studies)).tw. or Longitudinal.tw. or Retrospective*.tw. or prospective*.tw. or consecutive*.tw. or Cross sectional.tw. or Cross-sectional studies/ or historically controlled study/ or interrupted time series analysis/ [Onder exp cohort studies vallen ook longitudinale, prospectieve en retrospectieve studies] |
3992768 |
|
8 |
Case-control Studies/ or clinical trial, phase ii/ or clinical trial, phase iii/ or clinical trial, phase iv/ or comparative study/ or control groups/ or controlled before-after studies/ or controlled clinical trial/ or double-blind method/ or historically controlled study/ or matched-pair analysis/ or single-blind method/ or (((control or controlled) adj6 (study or studies or trial)) or (compar* adj (study or studies)) or ((control or controlled) adj1 active) or "open label*" or ((double or two or three or multi or trial) adj (arm or arms)) or (allocat* adj10 (arm or arms)) or placebo* or "sham-control*" or ((single or double or triple or assessor) adj1 (blind* or masked)) or nonrandom* or "non-random*" or "quasi-experiment*" or "parallel group*" or "factorial trial" or "pretest posttest" or (phase adj5 (study or trial)) or (case* adj6 (matched or control*)) or (match* adj6 (pair or pairs or cohort* or control* or group* or healthy or age or sex or gender or patient* or subject* or participant*)) or (propensity adj6 (scor* or match*))).ti,ab,kf. or (confounding adj6 adjust*).ti,ab. or (versus or vs or compar*).ti. or ((exp cohort studies/ or epidemiologic studies/ or multicenter study/ or observational study/ or seroepidemiologic studies/ or (cohort* or 'follow up' or followup or longitudinal* or prospective* or retrospective* or observational* or multicent* or 'multi-cent*' or consecutive*).ti,ab,kf.) and ((group or groups or subgroup* or versus or vs or compar*).ti,ab,kf. or ('odds ratio*' or 'relative odds' or 'risk ratio*' or 'relative risk*' or aor or arr or rrr).ab. or (("OR" or "RR") adj6 CI).ab.)) |
5014547 |
|
7 |
exp randomized controlled trial/ or random*.ti,ab,kf. or ((pragmatic or practical) adj clinical trial*).ti,ab,kf. or ((non-inferiority or noninferiority or superiority or equivalence) adj3 trial*).ti,ab,kf. or rct.ti,ab,kf. |
1405398 |
|
6 |
(meta-analysis/ or meta-analysis as topic/ or (metaanaly* or meta-analy* or metanaly*).ti,ab,kf. or systematic review/ or cochrane.jw. or (prisma or prospero).ti,ab,kf. or ((systemati* or scoping or umbrella or "structured literature") adj3 (review* or overview*)).ti,ab,kf. or (systemic* adj1 review*).ti,ab,kf. or ((systemati* or literature or database* or data-base*) adj10 search*).ti,ab,kf. or ((structured or comprehensive* or systemic*) adj3 search*).ti,ab,kf. or ((literature adj3 review*) and (search* or database* or data-base*)).ti,ab,kf. or (("data extraction" or "data source*") and "study selection").ti,ab,kf. or ("search strategy" and "selection criteria").ti,ab,kf. or ("data source*" and "data synthesis").ti,ab,kf. or (medline or pubmed or embase or cochrane).ab. or ((critical or rapid) adj2 (review* or overview* or synthes*)).ti. or (((critical* or rapid*) adj3 (review* or overview* or synthes*)) and (search* or database* or data-base*)).ab. or (metasynthes* or meta-synthes*).ti,ab,kf.) not (comment/ or editorial/ or letter/ or ((exp animals/ or exp models, animal/) not humans/)) |
530323 |
|
5 |
(instrumentation or methods).fs. or Validation Study/ or Comparative Study/ or exp Psychometrics/ or psychometr*.ti,ab,kf. or clinimetr*.mp. or clinometr*.mp. or exp Outcome Assessment, Health Care/ or outcome assessment.ti,ab,kf. or outcome measure*.mp. or exp Observer Variation/ or observer variation.ti,ab,kf. or exp Health Status Indicators/ or exp "reproducibility of results"/ or reproducib*.ti,ab,kf. or exp Discriminant Analysis/ or reliab*.ti,ab,kf. or unreliab*.ti,ab,kf. or valid*.ti,ab,kf. or coefficient.ti,ab,kf. or homogeneity.ti,ab,kf. or homogeneous.ti,ab,kf. or internal consistency.ti,ab,kf. or (cronbach* and (alpha or alphas)).ti,ab,kf. or (item and (correlation* or selection* or reduction*)).ti,ab,kf. or agreement.mp. or precision.mp. or imprecision.mp. or precise values.mp. or test-retest.ti,ab,kf. or (test and retest).ti,ab,kf. or (reliab* and (test or retest)).ti,ab,kf. or stability.ti,ab,kf. or interrater.ti,ab,kf. or inter-rater.ti,ab,kf. or intrarater.ti,ab,kf. or intra-rater.ti,ab,kf. or intertester.ti,ab,kf. or inter-tester.ti,ab,kf. or intratester.ti,ab,kf. or intra-tester.ti,ab,kf. or interobserver.ti,ab,kf. or inter-observer.ti,ab,kf. or intraobserver.ti,ab,kf. or intra-observer.ti,ab,kf. or intertechnician.ti,ab,kf. or inter-technician.ti,ab,kf. or intratechnician.ti,ab,kf. or intra-technician.ti,ab,kf. or interexaminer.ti,ab,kf. or inter-examiner.ti,ab,kf. or intraexaminer.ti,ab,kf. or intra-examiner.ti,ab,kf. or interassay.ti,ab,kf. or inter-assay.ti,ab,kf. or intraassay.ti,ab,kf. or intra-assay.ti,ab,kf. or interindividual.ti,ab,kf. or inter-individual.ti,ab,kf. or intraindividual.ti,ab,kf. or intra-individual.ti,ab,kf. or interparticipant.ti,ab,kf. or inter-participant.ti,ab,kf. or intraparticipant.ti,ab,kf. or intra-participant.ti,ab,kf. or kappa.ti,ab,kf. or kappa's.ti,ab,kf. or kappas.ti,ab,kf. or repeatab*.mp. or ((replicab* or repeated) and (measure or measures or findings or result or results or test or tests)).mp. or generaliza*.ti,ab,kf. or generalisa*.ti,ab,kf. or concordance.ti,ab,kf. or (intraclass and correlation*).ti,ab,kf. or discriminative.ti,ab,kf. or known group.ti,ab,kf. or factor analysis.ti,ab,kf. or factor analyses.ti,ab,kf. or factor structure.ti,ab,kf. or factor structures.ti,ab,kf. or dimension*.ti,ab,kf. or subscale*.ti,ab,kf. or (multitrait and scaling and (analysis or analyses)).ti,ab,kf. or item discriminant.ti,ab,kf. or interscale correlation*.ti,ab,kf. or error.ti,ab,kf. or errors.ti,ab,kf. or individual variability.ti,ab,kf. or (variability and (analysis or values)).ti,ab,kf. or (uncertainty and (measurement or measuring)).ti,ab,kf. or standard error of measurement.ti,ab,kf. or sensitiv*.ti,ab,kf. or responsive*.ti,ab,kf. or (limit and detection).ti,ab,kf. or minimal detectable concentration.ti,ab,kf. or interpretab*.ti,ab,kf. or ((minimal or minimally or clinical or clinically) and (important or significant or detectable) and (change or difference)).ti,ab,kf. or (small* and (real or detectable) and (change or difference)).ti,ab,kf. or meaningful change.ti,ab,kf. or ceiling effect.ti,ab,kf. or floor effect.ti,ab,kf. or item response model.ti,ab,kf. or irt.ti,ab,kf. or rasch.ti,ab,kf. or differential item functioning.ti,ab,kf. or dif.ti,ab,kf. or computer adaptive testing.ti,ab,kf. or item bank.ti,ab,kf. or cross-cultural equivalence.ti,ab,kf. |
10451293 |
|
4 |
exp Preoperative Period/ or pre operati*.ti,ab,kf. or preoperati*.ti,ab,kf. or before surger*.ti,ab,kf. or presurg*.ti,ab,kf. or pre surg*.ti,ab,kf. |
394021 |
|
3 |
exp Psychometrics/ or ((exp Symptom Assessment/ or assessment.ti,ab,kf.) and (exp Anxiety/ or exp Pain/)) or exp Visual Analog Scale/ or (had adj2 (scale or score)).ti,ab,kf. or hads.ti,ab,kf. or psychometr*.ti,ab,kf. or ((pain or anxiety or screening or quantitative) adj3 (scale* or score* or tool* or test* or instrument* or inventor*)).ti,ab,kf. or pittsburgh sleep quality index.ti,ab,kf. or psqi.ti,ab,kf. or stai-ch.ti,ab,kf. or psychometr*.ti,ab,kf. |
354577 |
|
2 |
exp Pain, Postoperative/ or ((postoperati* or post operati* or surgical or postsurgical) adj3 pain).ti,ab,kf. |
66973 |
|
1 |
(child* or schoolchild* or infan* or adolescen* or pediatri* or paediatr* or neonat* or boy or boys or boyhood or girl or girls or girlhood or youth or youths or baby or babies or toddler* or childhood or teen or teens or teenager* or newborn* or postneonat* or postnat* or puberty or preschool* or suckling* or picu or nicu or juvenile?).tw. |
2677163 |
