Cell saver
Uitgangsvraag
Wat zijn de (on)gunstige effecten van het gebruik van cell saver bij patiënten die cardiochirurgie ondergaan?
Aanbeveling
Pas routinematig gebruik van cell saver bij hartchirurgie met cardiopulmonaire bypass toe gedurende de gehele operatie, omdat hiermee de kans op een bloedtransfusie wordt verminderd.
Houd rekening met:
-
Het potentiële verlies aan plasma en bloedplaatjes door het cell saver proces leidt niet tot een toegenomen transfusiebehoefte van deze bloedproducten, maar grote volumina van geretransfundeerd cell saver bloed (15-20% van het circulerend bloedvolume) kunnen de hemostase beïnvloeden.
-
Het gebruik van de cell saver zou geassocieerd kunnen zijn met een verhoogde kans op een infectie (sternum- en beenwond, long- en urineweginfectie). Dit moet worden afgewogen tegen het infectierisico dat met bloedtransfusie gepaard gaat.
-
De kosten van het inzetten van een cell saver moeten worden afgewogen tegen de kosten van bloedtransfusie en/of bloedproducten.
Overwegingen
Voor- en nadelen van de interventie en de kwaliteit van het bewijs
Er is een literatuuronderzoek verricht naar de vergelijking tussen het gebruik van de cell saver versus geen cell saver bij volwassen patiënten die cardiochirurgie ondergaan. Er is één systematische review en er zijn 14 RCTs geïncludeerd die deze vergelijking hebben onderzocht. Als cruciale uitkomstmaat werd gevonden dat het aantal patiënten dat een bloedtransfusie kreeg lager is bij het gebruik van een cell saver (Risk Ratio 0.73, 95% CI 0.73-0.91). De vraag of de cell saver invloed had op het totaal aantal bloedtransfusies kon niet worden beantwoord. Het infectierisico (sternum- en beenwond, long- en urineweginfectie) was verhoogd in de cell saver groep (Risk Ratio 1.29, 95% CI 1.00-1.67). De overige uitkomstmaten lieten conflicterende resultaten zien. Er was geen klinisch relevant verschil (zoals gedefinieerd door de werkgroep) in blootstelling aan plasma of bloedplaatjes transfusie, postoperatief hemoglobinegehalte, postoperatief bloedverlies, postoperatieve stroke of neurologische stoornissen, cardiovasculaire complicaties (waaronder myocardinfarct en boezemfibrilleren), nierfalen, intensive care of ziekenhuis verblijfsduur, en mortaliteit. De bewijskracht werd als laag tot zeer laag beoordeeld.
De geïncludeerde meta-analyse van Wang (2009) laat zien dat het gebruik van een cell saver de blootstelling aan zowel bloedtransfusie als bloedcomponenten (plasma, bloedplaatjes) vermindert. Subanalyses suggereren dat de cell saver voordeel heeft wanneer gebruikt voor verwerken van bloed uit het operatiegebied (shed blood) en/of resterende bloed uit de hartlong machine gedurende de gehele operatie. Het cell saver proces waarbij de cell saver alleen gebruikt wordt tijdens cardiopulmonale bypass heeft geen significant effect op bloed conservatie en verhoogt de toediening van plasma. Als deze meta-analyse wordt gecombineerd met de beschikbare additionele RCT’s, die niet eenduidig het tijdsvenster van bloedcollectie rapporteren, dan resulteert het gebruik van een cell saver alleen in een lager aantal patiënten dat een bloedtransfusie krijgt.
De heterogeniteit van de geïncludeerde RCT’s is voor een deel verklaarbaar door de niet uniforme vraagstelling van de studies. Deze liepen uiteen van bloed conservatie tot beïnvloeding van de systemische inflammatoire respons geassocieerd met de hartlong machine. Daarnaast lopen de studies uiteen in de tijdsperioden dat de cell saver werd ingezet (alleen tijdens CPB of gedurende de gehele hartoperatie), en de bron van het bloed dat het cell saver proces heeft doorlopen (cardiotomy suction bloed, bloed uit het operatiegebied, en resterende bloed uit de hartlong machine). De vraag of alleen het opvangen van cardiotomy suction bloed van invloed is op de bloedtransfusie behoefte, kon niet worden beantwoord.
In 8 van de 14 studies werd de hoeveelheid geprocessed bloed door de cell saver vermeld. Grotere volumina aan cell saver bloed kunnen leiden tot stollingsstoornissen ten gevolge van verdunning van stollingsfactoren, activatie van fibrinolyse, en een resterend heparine effect.
Campbell (2011) toont aan dat reïnfusie van cell saver bloed leidt tot veranderingen in thromboelastometrie (ROTEM) parameters overeenkomend met een verdunnings coagulopathie. Dit wordt bevestigd in twee andere studies bij hartchirurgie die niet in de analyse zijn opgenomen. In een pilotstudie toont Adam (2020; niet opgenomen in analyse) aan dat de mediane postoperatieve fibrinogeen concentratie met 50% verminderde vergeleken met de preoperatieve concentratie. Deze bevinding wordt bevestigd door Son (2020; niet opgenomen in analyse) die aantoont dat een grotere geretransfundeerde hoeveelheid cell saver bloed (15-20% van het circulerend bloedvolume) tot een afname leidt van de FIBTEM maximal clot firmness <8 mm. Deze parameter wordt beschouwd als een risicofactor voor verdunnings coagulopathie waarvoor aanvulling met fibrinogeen is geïndiceerd. Daarnaast is uit deze studie gebleken dat er nog steeds heparine aanwezig kan zijn in het geretransfundeerde cell saver bloed, hetgeen zeker bij grotere hoeveelheden tot een coagulopathie kan leiden.
Er zijn een aantal factoren die de interpretatie van het effect van een cell saver verder beperken. De transfusie trigger voor bloedtransfusie werd in 9 van de 14 studies vermeld. Dit bemoeilijkte een conclusie van het effect op het postoperatieve hemoglobinegehalte door de cell saver. Daarnaast werd in minder dan de helft van de studies de indicatie (transfusietrigger) tot het toedienen van bloedproducten vermeld. Hoewel het gebruik van point-of-care stollingstesten (thromboelastografie of -metrie) in de hartchirurgie wordt geadviseerd in de internationale richtlijnen, werd hier in drie studies gebruik van gemaakt. In drie andere studies werden stollingsparameters in het lab bepaald. Uit onze analyse blijkt dat er meer plasma suppletie nodig is bij toepassing van de cell saver, echter de hoeveelheden bereiken geen klinisch relevant verschil. De uitkomsten voor bloedplaatjes suppletie zijn divers en laten ook geen klinisch relevant verschil zien. Ook hier geldt dat grotere volumina van cell saver bloed kunnen leiden tot een relatief verlies aan plasma en bloedplaatjes wat kan resulteren in stollingsstoornissen (Al-Khabori, 2015; niet opgenomen in analyse).
Het verhoogde infectierisico bij gebruik van de cell saver vergt nadere aandacht. In de studie van van Klarenbosch (2020) wordt statistisch onderbouwd dat het effect van de cell saver op het optreden van een infectie bijna net zo groot is als een transfusie van 1-2 eenheden RBC. Als het gebruik van een cell saver tot deze hoeveelheid bloedtransfusie besparing leidt, dan vallen de voor- en nadelen van de cell saver wat betreft het infectierisico tegen elkaar weg. Vermeijden (2015) rapporteert over het potentiële effect van leucocyten reductie met een filter en laat zien dat het gebruik van de cell saver met of zonder filter leidt tot een lager percentage patiënten die een bloedtransfusie krijgt. Het heeft geen effect op blootstelling aan plasma of bloedplaatjestransfusie. Er wordt niet gerapporteerd over het risico op infecties met een leucocyten filter.
De 2017 EACTS/EACTA ‘Guidelines on patient blood management for adult cardiac surgery’ concludeert dat het routinematig gebruik van de cell saver moet worden overwogen om bloedtransfusies te vermijden, maar retransfusie van grote volumina cell saver bloed (>1000ml) zou de hemostase kunnen beïnvloeden (Class IIa, Level B).
De 2021 STS/SCA/AmSECT/SABM ‘Update to the Clinical Practice Guidelines on Patient Blood Management’ concludeert dat het routinematig gebruik van de cell saver bijdraagt aan bloed conservatie tijdens hartoperaties met CPB (Class I, Level A).
De 2023 ESAIC update 2022 ‘Guideline severe perioperative bleeding’ adviseert het gebruik van de cell saver aangezien het bijdraagt aan het bloed conservatie tijdens grote hartchirurgische ingrepen (Class 1, Level B).
De bewijskracht voor het routinematig gebruik van de cell saver in bovenstaande internationale richtlijnen is discrepant met onze richtlijn waaruit een lage tot zeer lage bewijskracht wordt geconcludeerd. Daarvoor zijn meerdere redenen aan te wijzen waaronder de PICO criteria, de inclusiedatum van de studies (beperkt tot de recentere literatuur vanaf het jaar 2000), en het ontbreken van informatie betreffende transfusie van bloed of bloedproducten. Ook zijn studies die niet alleen gefocussed waren op hartchirurgie en studies die meerdere cell saver systemen hebben vergeleken niet opgenomen in de analyse. Daarnaast erkennen de internationale richtlijnen de zwakke methodologische kwaliteit van de meeste studies waardoor de bevindingen gebiased kunnen zijn ten gunste van de cell saver. Bij afwezigheid van gepubliceerd bewijs werd in de internationale richtlijnen overgegaan tot een expert consensus verklaring om tot een classificatie te komen die essentieel is voor de dagelijkse praktijk.
Waarden en voorkeuren van patiënten (en evt. hun verzorgers)
Patiënten en hun naasten vinden het belangrijk om pre-operatief uitgebreid geïnformeerd over wat hen bij een hartoperatie te wachten staat, inclusief de potentiële complicaties. Hierbij komen bloedverlies en eventuele bloedtransfusies of toediening van bloedproducten aan de orde. De kans op een bloedtransfusie neemt toe bij een preoperatieve anemie, re-operatie of complexe hartchirurgie. In het algemeen volstaat deze informatie aangezien patiënten geen directe invloed op het perioperatieve proces kunnen uitoefenen, zoals het gebruik van een cell saver. Bij Jehova getuigen zal het hartcentrum moeten bespreken of zij het risico op het onthouden van transfusies wil aanvaarden of de patiënt doorverwijzen naar een ander hartcentrum.
Kosten (middelenbeslag)
Vanuit value-based health care oogpunt is kosteneffectiviteit mede van belang om tot een verantwoorde keuze voor wel of geen cell saver te komen. Slechts één studie (Xie, 2015) rapporteert een kostenreductie bij gebruik van een cell saver. Echter, deze studie is niet goed te vergelijken met de situatie in Nederland. De kosten van het inzetten van een cell saver moeten worden afgewogen tegen de kosten van bloedtransfusie en/of bloedproducten waarbij een minimale, en wellicht ook maximale hoeveelheid cell saver bloed (>1000ml) dat wordt geretransfundeerd in overweging moeten worden genomen.
Aanvaardbaarheid, haalbaarheid en implementatie
Het implementeren van deze richtlijn is een voorwaarde om de zorg voor hartchirurgische patiënten te optimaliseren. Het toepassen van de cell saver als onderdeel van de interventies die aan een beter PBM bijdragen, vindt al op grote schaal plaats in Nederland. Het alleen toepassen van de cell saver ter vermindering van het aantal patiënten dat een bloedtransfusie krijgt is geen doel op zich, maar is als onderdeel van een PBM-programma van toegevoegde waarde.
Rationale van de aanbeveling: weging van argumenten voor en tegen de interventies
In de geïncludeerde studies werd de cell saver voornamelijk gebruikt voor bloedconservatie en niet om redenen van orgaanprotectie of beïnvloeding van de inflammatoire respons. De heterogeniteit van de geïncludeerde RCT’s en meta-analyse kon grotendeels verklaard worden door een niet uniforme vraagstelling betreffende de verschillende perioden van cell saver gebruik (pre- en/of post-CPB, tijdens CPB, verwerking resterend bloed uit HLM, en postoperatief). Daarnaast werden de volumina van zowel het opgevangen bloed als het verwerkte geretransfundeerde bloed niet altijd vermeld. Dit bemoeilijkte de interpretatie van de uitkomstmaten. Geconcludeerd kon worden dat het toepassen van de cell saver gedurende de gehele operatie de kans op een allogene bloedtransfusie vermindert.
Het verwerken van het opgevangen bloed leidt tot een verlies aan plasma en bloedplaatjes met mogelijke gevolgen voor de hemostase. Uit de studies die dit rapporteerden bleek de cell saver tot meer plasma transfusie te leiden, echter dit bereikte geen klinisch relevant verschil. De resultaten voor bloedplaatjes transfusie waren divers en leiden niet tot een klinisch relevant verschil. Wel zijn er aanwijzingen uit andere studies dat een groter volume van geretransfundeerd cell saver bloed (15-20% van het circulerend bloedvolume) aanleiding kan geven tot stollingsstoornissen.
De verhoogde kans op een infectie na het gebruik van de cell saver is een opvallende bevinding waar tevens een nuancering bij aangebracht moet worden. Dit risico moet namelijk afgewogen worden tegen het infectierisico dat bloedtransfusie met zich meebrengt. Dat zou statistisch gezien overeenkomen met 1-2 eenheden RBC transfusie. Als laatste speelt, zeker in de huidige tijd, het kostenaspect van het gebruik van medische middelen. Per ziekenhuis zal moeten worden afgewogen of de kosten van een cell saver opwegen tegen de kosten van bloedtransfusie en/of bloedproducten. Het inzetten van alleen het opvangreservoir alvorens het gehele cell saver systeem op te bouwen is al een gebruikelijke stap om tot kostenreductie te komen.
Onderbouwing
Achtergrond
Cell savers worden tijdens hartoperaties gebruikt om het hemoglobinegehalte te verbeteren en bloedtransfusies te verminderen. Meer dan 80% van de Nederlandse hartcentra gebruikt standaard de cell saver als onderdeel van Patient Blood Management (PBM). Desondanks bestaat er een grote variatie in het gebruik van bloed- en/of bloedproducten. Het gebruik van cell saver wordt in het algemeen als kosteneffectief beschouwd in relatie tot de kosten die bloedtransfusie met zich meebrengt. Echter, de reductie in bloedtransfusie kan leiden tot een toegenomen transfusie van stollingsfactoren en bloedplaatjes. Daarnaast is het onvoldoende duidelijk of cardiotomy suction bloed onderdeel moet zijn van het cell saving principe wegens activatie van stolling, fibrinolyse en inflammatie.
Conclusies / Summary of Findings
Number of patients transfused
|
Low GRADE |
Treatment with cell saver may reduce the number of patients transfused with red blood cells when compared with treatment without cell saver in adult patients undergoing cardiac surgery. Source: Aghdaii, 2012; Damgaard, 2010; Gu, 2008; Scrascia, 2012; Vermeijden, 2015; Wang, 2009 |
Blood product consumption
|
Very low GRADE |
The evidence is very uncertain about the effect of treatment with cell saver on plasma transfusion when compared with treatment without cell saver in adult patients undergoing cardiac surgery. Source: Reyes, 2011; Scrascia, 2012; Tachias, 2022; Van Klarenbosch, 2020; Vermeijden, 2015
The evidence is very uncertain about the effect of treatment with cell saver on platelet transfusion when compared with treatment without cell saver in adult patients undergoing cardiac surgery. Source: Reyes, 2011; Scrascia, 2012; Shen, 2016; Tachias, 2022; van Klarenbosch, 2020; Vermeijden, 2015; Xie, 2015 |
Postoperative blood loss
|
Very low GRADE |
The evidence is very uncertain about the effect of treatment with cell saver on 6h and 24h postoperative blood loss when compared with treatment without cell saver in adult patients undergoing cardiac surgery. Source: Aghdaii, 2012; Bauer, 2017; Gorki, 2020; Prieto, 2013; Reyes, 2011 |
|
Low GRADE |
Treatment with cell saver may result in little to no difference in 12h postoperative blood loss when compared with treatment without cell saver in adult patients undergoing cardiac surgery. Source: Gorki, 2020; Van Klarenbosch, 2020; Vermeijden, 2015 |
Hemoglobin concentration
|
Low GRADE |
Treatment with cell saver may result in little to no difference in postoperative hemoglobin concentration when compared with treatment without cell saver in adult patients undergoing cardiac surgery. Source: Bauer, 2017; Campbell, 2011; Damgaard, 2010; Gu, 2008; Reyes, 2011; Scrascia, 2012; Tachias, 2022; Van Klarenbosch, 2020; Vermeijden, 2015 |
Adverse events
|
Very low GRADE |
The evidence is very uncertain about the effect of treatment with cell saver on stroke when compared with treatment without cell saver in adult patients undergoing cardiac surgery. Source: Damgaard, 2010; Vermeijden, 2015
The evidence is very uncertain about the effect of treatment with cell saver on renal failure, neurological complications, atrial fibrillation, and myocardial infarction when compared with treatment without cell saver in adult patients undergoing cardiac surgery. Source: Bauer, 2017; Damgaard, 2010; Gorki, 2020; Prieto, 2013; Scrascia, 2012; Shen, 2016; Vermeijden, 2015 |
|
Low GRADE |
Treatment with cell saver may increase infection when compared with treatment without cell saver in adult patients undergoing cardiac surgery. Source: Bauer, 2017; Shen, 2016; Van Klarenbosch, 2020 |
Length of stay
|
Very low GRADE |
The evidence is very uncertain about the effect of treatment with cell saver on length of hospital and ICU stay when compared with treatment without cell saver in adult patients undergoing cardiac surgery. Source: Bauer, 2017; Damgaard, 2010; Gu, 2008; Prieto, 2013; Reyes, 2011; Scrascia, 2012; Shen, 2016; Van Klarenbosch, 2020; Vermeijden, 2015; Xie, 2015 |
Mortality
|
Very low GRADE |
The evidence is very uncertain about the effect of treatment with cell saver on mortality when compared with treatment without cell saver in adult patients undergoing cardiac surgery. Source: Aghdaii, 2012; Bauer, 2017; Gorki, 2020; Prieto, 2013; Reyes, 2011; Scrascia, 2012; Shen, 2016 |
Samenvatting literatuur
Description of studies
The systematic review by Wang (2009) investigated the safety and efficacy of the use of a cell saver in patients undergoing cardiac surgery. Randomized controlled trials (RCTs) studying adult patients undergoing cardiac surgery, being allocated randomly to a cell saver group versus a no cell saver group, and reporting at least one relevant clinical or economical outcome, were eligible for inclusion. If a cell saver was only used postoperatively or if cell saver blood was reinfused without washing, the study was excluded. Electronic searches were performed in MEDLINE, Cochrane CENTRAL, EMBASE, Current Contents, Database of abstracts of reviews of effects, NHS economic evaluation database, and international network of agencies for health technology assessment databases from the date of their inception to November 2008. In this literature analysis, only the studies performed after 2000 were included. Data of 9 published randomized controlled trials, including a total of 1341 patients were included. Meta-analyses were performed in the systematic review. The study reported the following relevant outcome measures: number of patients transfused (red blood cells), adverse events, hemoglobin concentration, length of stay, and mortality.
Gu (2008) performed a randomized study to explore whether mechanical cell salvage use in patients undergoing cardiac surgery with cardiopulmonary bypass (CPB) effects postoperative outcomes. Patients for elective coronary artery bypass grafting, single valve replacement, or a combined procedure were eligible for trial participation. In total, 40 patients were eligible and were randomized into two groups. The intervention group received cell saver use and the control group did not receive cell saver use. For the intervention group (n=20), conventional cardiotomy suction was not used, but the wound blood was collected in a cell saver reservoir. The salvaged blood was processed with a continuous auto transfusion system, and the residual blood in the heart-lung machine was transferred to the cell saver reservoir and processed by the cell saver. For the control group (n=20), conventional cardiotomy suction was used. The residual blood in the heart-lung machine was retransfused through a standard blood transfusion system. The duration of the follow-up was until the postoperative morning. The study reported the following relevant outcome measures: number of patients transfused (red blood cells), hemoglobin concentration, and length of stay.
Damgaard (2010) performed a randomized study to investigate whether intraoperative use of a cell saver for coronary operations using CPB reduces systemic inflammatory responses. Patients older than 18 years with a need for coronary artery bypass grafting (CABG) who gave informed consent were eligible for trial participation. In total, 29 patients were eligible and were randomized into two groups. The intervention group received cell saver use and the control group did not receive cell saver use. For the intervention group (n=15), cell saving of pericardial suction blood and residual blood in the CPB circuit after perfusion was performed. For the control group (n=14), the suction blood and the CPB circuit blood was retransfused directly. The duration of the follow-up was until the postoperative morning. The study reported the following relevant outcome measures: number of patients transfused (red blood cells), adverse events, postoperative blood loss, and length of stay.
Campbell (2011) performed a pilot study to investigate the relationship between the use of intraoperative cell salvage and the viscoelastic properties of clot formation in patients undergoing coronary bypass surgery. Patients scheduled for elective first-time coronary bypass surgery were eligible for trial participation. In total, 20 patients were eligible and were randomized into two groups. The intervention group received cell saver use and the control group did not receive cell saver use. For the intervention group (n=10), blood was salvaged before and after CPB and the residual CPB volume was processed using a continuous auto transfusion system. For the control group (n=10), no cell salvage system was used. The residual CPB volume was transfused unprocessed after CPB. In both groups, cardiotomy suction blood was returned to the venous reservoir. The duration of the follow-up was not reported but was at least until 4 hours post-surgery. The study reported the following relevant outcome measures: postoperative blood loss, and hemoglobin concentration.
Reyes (2011) performed a prospective randomized clinical trial to explore whether the use of a cell saver system reduces the need for blood transfusion in low-risk patients undergoing cardiac surgery. Patients undergoing cardiac surgery with the use of CPB were eligible for trial participation. In total, 63 patients were eligible and were randomized into two groups. The intervention group received cell saver use and the control group did not receive cell saver use. For the intervention group (n=34), a cell saver device was used during the entire procedure, recovering, and concentrating the blood in the circuits. Cardiotomy suction was used, and this blood was transfused to patients. For the control group (n=29), no cell saver was used. All the blood in the surgical field was aspired only using the cardiotomy suction. The duration of follow-up was 30 days after surgery. The study reported the following relevant outcome measures: blood product consumption (plasma, postoperative blood loss, hemoglobin concentration, length of stay, and mortality.
Scrascia (2012) performed a prospective randomized controlled trial to investigate the effect of blood salvage through a cell saver on hemoglobin levels and on coagulation and fibrinolysis activation. Patients undergoing first-time, elective, isolated CABG were eligible for trial participation. In total, 34 patients were eligible and were randomized into two groups. The intervention group received cell saver use and the control group did not receive cell saver use. For the intervention group (n=17), a cell saving system was used to collect, salvage, and wash the residual blood and transfuse it back to the patient. The CPB blood was suctioned by cardiotomy suckers and returned to the venous reservoir. For the control group (n=17), no cell salvage system was used. CPB blood was not transfused back to the patients. The duration of the follow-up was five days after surgery. The study reported the following relevant outcome measures: number of patients transfused (red blood cells), blood product consumption (platelets, plasma), adverse events, postoperative blood loss, hemoglobin concentration, length of stay, and mortality.
Aghdaii (2012) performed a randomized clinical trial to explore the role of heparin in the retransfusion blood in the disturbance in coagulation and increase in blood loss. Patients undergoing primary, elective, on-pump CABG surgery, aged between 30 and 70 years, with a left ventricular ejection fraction ≥ 45%, a pump time of less than 2 hours, and an aortic clumping time of less than 45 minutes were eligible for trial participation. In total, 50 patients were eligible and were randomized into two groups. The intervention group received cell saver use and the control group did not receive cell saver use. For the intervention group (n=25), intraoperative cell salvage of shed blood was performed. The blood from the wound area and operative field, as well as the blood within the CPB circuit was collected in the cell saver and transfused to the patient. For the control group (n=25), no cell salvage system was used, patients only received homologous blood. The duration of the follow-up was 24 hours post-surgery. The study reported the following relevant outcome measures: postoperative blood loss, and mortality.
Pietro (2013) performed a randomized controlled trial to investigate the role of a cell saver device in the inflammatory response to cardiac surgery. Low-risk patients undergoing cardiac surgery with the use of CBP were eligible for trial participation. In total 57 patients were eligible and were randomized into two groups. The intervention group received cell saver use and the control group did not receive cell saver use. For the intervention group (n=29), a cell saver was used during cardiac surgery, all blood in the circuit was recovered, concentrated, and transfused to patients. Cardiotomy suction was applied, and this blood was reinfused during CPB. For the control group (n=28), no cell saver was used, only cardiotomy suction was used to aspirate and reinfuse all blood in the surgical field. The duration of the follow-up was 30 days after the procedure. The study reported the following relevant outcome measures: adverse events, postoperative blood loss, length of stay, and mortality.
Vermeijden (2015) performed a randomized multi-center trial to investigate filtration of salvaged blood in combination with the use of a cell saving device in patients undergoing cardiac surgery. Adult patients, scheduled in the morning for elective coronary bypass grafting, valve surgery or a combined procedure, were eligible for trial participation. In total, 738 patients were eligible and were randomized into four groups. In the three intervention groups cardiotomy suction blood, blood from the surgical field, and residual blood from the heart-lung machine were collected and processed as described. For intervention group I (n=192), collected blood was washed with a cell saver, and retransfused through a standard transfusion set. For intervention group II (n=180), collected blood was washed with a cell saver, and retransfused through a Leukocyte Depletion (LD) filter. For intervention group III (n=182), collected blood was only retransfused through a LD filter. For the control group (n=184), conventional cardiotomy suction was used, blood from the surgical field was discarded after reversal of heparin, and residual heart-lung machine blood was retransfused without cell saving or filter. From this study only intervention group I and the control group results were compared. The duration of the follow-up was length of stay, including 1-year mortality. The study reported the following relevant outcome measures: number of patients transfused (red blood cells), blood product consumption (platelets, plasma), adverse events, postoperative blood loss, hemoglobin concentration, length of stay, and mortality.
Xie (2015) performed a prospective randomized controlled clinical trial to evaluate efficacy, safety, and cost-effectiveness of the use of a cell saver in patients undergoing high-bleeding-risk cardiac surgery. Patients who provided written consent, were scheduled for cardiac surgery with CPB, surgery combined with aortic valve replacement and mitral valve replacement, Bentall, or reoperation, and who met at least two of the following criteria: age > 70 years, body surface area < 1.6m2, renal dysfunction, liver insufficiency, coagulation disorders, hemoglobin levels < 130 g/L in males or <120 g/L in females, platelet count <50x109/L, or intake of aspirin 3 days before surgery or clopidogrel 7 days before surgery, were eligible for trial participation. In total 150 patients were eligible and were randomized into two groups. The intervention group received cell saver and the control group did not receive cell saver. For the intervention group (n=73), shed blood and residual blood were filtrated, centrifugated, washed, concentrated, and retransfused to the patients, using a cell saver. For the control group (n=71), shed blood during the period of non-heparinization and residual blood were sucked into a suction apparatus and were discarded. The duration of the follow-up was not reported. The study reported the following relevant outcome measures: blood product consumption (platelets, plasma), postoperative blood loss, length of stay, and cost-effectivity.
Shen (2016) performed a prospective randomized controlled trial to evaluate the impact of the use of a cell saver on blood coagulation in patients undergoing high-bleeding-risk cardiac surgery with CPB. Patients scheduled for cardiac surgery with CPB, either multiple valves replacement or Bentall or reoperation, and who met at least two of the following criteria: age > 70 years, body surface area < 1.6m2, Cr >15 mg/L, liver Child Pugh B or C, R (TEG) >10 min, hemoglobin levels < 13 g/dL in males or <12 g/dL in females, platelet count <50x109/L, or drug withdrawal <3 days (aspirin) or <7 days (clopidogrel), were eligible for trial participation. In total, 110 patients were eligible and were randomized into two groups. The intervention group received cell saver use and the control group did not receive cell saver use. For the intervention group (n=54), shed blood and residual blood were filtrated, centrifugated, washed, concentrated, and retransfused to the patients, using a cell saver. For the control group (n=51), shed blood during the period of non-heparinization and residual blood were sucked into a suction apparatus and were discarded. The duration of the follow-up was not reported. The study reported the following relevant outcome measures: blood product consumption (platelets, plasma, adverse events, postoperative blood loss, length of stay, and mortality.
Bauer (2017) performed a prospective randomized controlled study to find out about the impact of the use of cell salvage on shed blood versus direct return of shed blood on biomarkers for systemic inflammation using minimal invasive extracorporeal circulation (MiECC). Patients aged between 18 and 95, with a body weigh between 55 and 150 kg, undergoing elective isolated CABG surgery were eligible for trial participation. In total, 76 patients were eligible and randomized into two groups. The intervention group received cell saver use and the control group did not receive cell saver use. For the intervention group (n=36), suction blood was separated, and cell salvage was performed before the blood was retransfused. For the control group (n=40), suction blood was separated and directly retransfused into the circuit without any treatment. The duration of the follow-up was not reported. The study reported the following relevant outcome measures: adverse events, postoperative blood loss, hemoglobin concentration, length of stay, and mortality.
Van Klarenbosch (2020) performed a study using data of a randomized controlled trial on cell salvage and leukocyte depletion filter use to explore postoperative infection data. Adult patients, scheduled for elective coronary artery bypass (CABG), valve surgery, or a combined procedure were eligible for trial participation. In total, 716 patients were eligible and were randomized into two groups. The intervention group received cell saver use and the control group did not receive cell saver use. For the intervention group (n=364), blood was collected from the surgical field, through cardiotomy suction, and from the heart-lung machine. This blood was washed using a cell saver and retransfused to the patients. For the control group (n=352), blood was either collected and filtered during CPB and retransfused, or conventional cardiotomy suction was used, blood from the surgical field was discarded before and after heparinisation, and residual heart-lung machine blood was retransfused without processing. The duration of the follow-up was not reported. The study reported the following relevant outcome measures: RBC transfusion, blood product consumption (plasma, platelets), adverse events, postoperative blood loss, and length of stay.
Gorki (2020) performed a study using data of a randomized controlled trial to investigate whether the rise of coagulation and inflammatory markers is caused by direct recirculation of pericardial fluids. Patients who underwent a first-time operation of isolated coronary disease with at least three target vessels, a left ventricular ejection fraction >40%, and age between 18 and 85 years were eligible for trial participation. In total, 48 patients were eligible and were randomized into two groups. The intervention group received cell saver use and the control group did not receive cell saver use. For the intervention group (n=24), shed pericardial fluids were collected separately and (if exceeding 500 mL) retransfused after the use of a cell saver. For the control group (n=24), pericardiotomy suction was used to retransfuse pericardial blood directly. The duration of the follow-up was not reported but was at least 30 days because mortality and adverse events were reported after 30 days. The study reported the following relevant outcome measures: adverse events, postoperative blood loss, and mortality.
Tachias (2022) performed a prospective randomized controlled study to investigate the effects of centrifuged end-product on bleeding and transfusion rates in adult patients undergoing cardiac surgery. Patients who gave written informed consent, aged 18 years or older who underwent cardiac surgery, either coronary bypass surgery, valve surgery, aortic replacement surgery, or mixed surgery, with CPB lasting for at least 90 minutes, were eligible for trial participation. In total, 209 patients were eligible and were randomized into two groups. The intervention group received cell saver use and the control group did not receive cell saver use. For the intervention group (n=99), the cell salvage reservoir collected lost blood from the moment of pericardiotomy to CPB, and after CPB to the end of surgery. The blood was retransfused to the patients. For the control group (n=110), allogeneic red cell transfusion according to the centre’s transfusion policy was performed, without the use of a cell saver. The duration of the follow-up was not reported. The study reported the following relevant outcome measures: blood product consumption (plasma, and hemoglobin concentration.
Results
Number of patients transfused (critical)
Number of patients transfused red blood cells (peri-operative)
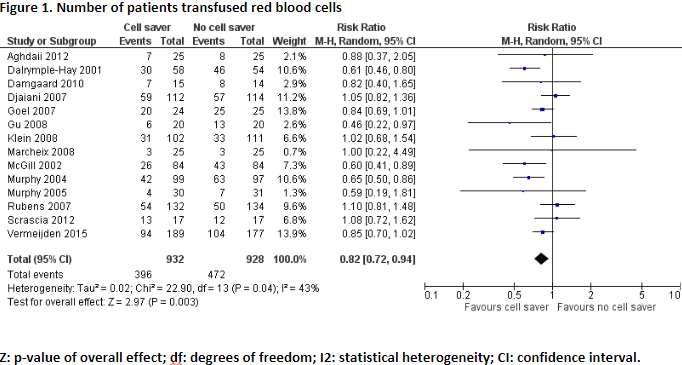
Six studies, including one meta-analysis, reported the outcome measure number of patients transfused red blood cells (Aghdaii, 2012; Damgaard, 2010; Gu, 2008; Scrascia, 2012; Vermeijden, 2015; Wang, 2009).
In total, 396 of the 932 patients (42%) who received treatment with cell saver received red blood cell transfusion, and 472 of the 928 patients (51%) who received treatment without cell saver received red blood cell transfusion. Pooled data from these studies showed a pooled risk ratio of 0.82 (95% CI 0.72 to 0.94), in favour of the patients who received treatment with cell saver (Figure 1). This difference is considered clinically relevant.
Blood product consumption (critical)
Plasma transfusion
Five studies reported the outcome measure plasma transfusion (Reyes, 2011; Scrascia, 2012; Tachias, 2022; Van Klarenbosch, 2020; Vermeijden, 2015). Due to heterogeneity in reporting the outcome measure, the results were not pooled.
Reyes (2011) reported total units plasma transfused. This was converted to mean number of plasma units transfused. The patients who received treatment with cell saver (n=34) received a mean of 0.24 units, and the patients who received treatment without cell saver (n=29) received a mean of 0.10 units. The mean difference between the groups was 0.14 units plasma, in favour of the patients who received treatment without cell saver. This difference is not considered clinically relevant.
Scrascia (2012) reported total units plasma transfused. This was converted to mean units of plasma transfused. The patients who received treatment with cell saver (n=17) received a mean of 0.59 units, and the patients who received treatment without cell saver (n=17) received no plasma transfusion. The mean difference between the groups was 0.59 units plasma, in favour of the patients who received treatment without cell saver. This difference is not considered clinically relevant.
Tachias (2022) reported mean units plasma transfused. The patients who received treatment with cell saver (n=99) received a mean of 1.0 units (SD ± 1.9 units), and the patients who received treatment without cell saver (n=110) received a mean of 0.9 units (SD ± 1.7 units). The mean difference between the two groups was 0.10 units plasma (95% CI -0.39 to 0.59), in favour of the patients who received treatment without cell saver. This difference is not considered clinically relevant.
Van Klarenbosch (2020) reported mean units plasma transfused. The patients who received treatment with cell saver (n=364) received a mean of 0.6 units (SD ± 1.5 units), and the patients who received treatment without cell saver (n=352) received a mean of 0.4 units (SD ± 1.1 units). The mean difference between the two groups was 0.20 units plasma (95% CI 0.01 to 0.39), in favour of the patients who received treatment without cell saver. This difference is not considered clinically relevant.
Vermeijden (2015) reported total units plasma transfused. This was converted to mean units of plasma transfused. The patients who received treatment with cell saver (n=189) received a mean of 0.51 units, and the patients who received treatment without cell saver (n=177) received a mean of 0.36 units. The mean difference between the groups was 0.15 units plasma, in favour of the patients who received treatment without cell saver. This difference is not considered clinically relevant.
Platelet transfusion
Seven studies reported the outcome measure platelet transfusion (Reyes, 2011; Scrascia, 2012; Shen, 2016; Tachias, 2022; van Klarenbosch, 2020; Vermeijden, 2015; Xie, 2015). Due to heterogeneity in reporting the outcome measure, the results were not pooled.
Reyes (2011) reported the number of platelet bags transfused. This was converted to mean units of platelets transfused per patient. The patients who received treatment with cell saver (n=34) received a mean of 0.09 units, and the patients who received treatment without cell saver (n=29) received no platelets. The mean difference between the groups was 0.09 units platelets, in favour of the patients who received treatment without cell saver. This difference is not considered clinically relevant.
Scrascia (2012) reported total units platelets transfusion. The patients who received treatment with cell saver (n=17) or without cell saver (n=17) received no platelets transfusion. This means there was no difference between the two groups.
Shen (2016) reported mean units of platelets transfused. The patients who received treatment with cell saver (n=53) received a mean of 1.81 units (SD ± 3.56 units), and the patients who received treatment without cell saver (n=50) received a mean of 1.92 units (SD ± 3.94 units). The mean difference between the groups was -0.11 units platelets (95% CI -1.56 to 1.34), in favour of the patients who received treatment with cell saver. This difference is not considered clinically relevant.
Tachias (2022) reported mean units of platelets transfused. The patients who received treatment with cell saver (n=99) received a mean of 2.7 units, and the patients who received treatment without cell saver (n=110) received a mean of 2.0 units. The mean difference between the two groups was 0.7 units platelets, in favour of the patients who received treatment without cell saver. This difference is not considered clinically relevant.
Van Klarenbosch (2020) reported mean units of platelets transfused. The patients who received treatment with cell saver (n=364) received a mean of 0.2 units (SD ± 0.6 units), and the patients who received treatment without cell saver (n=352) received a mean of 0.2 units (SD ± 0.5 units). This means there was no difference between the two groups.
Vermeijden (2015) reported total units of platelets transfused. This was converted to mean units of platelets transfused. Both the patients who received treatment with cell saver (n=189) and the patients who received treatment without cell saver (n=177) received a mean 0.17 units platelets. This means there was no difference between the two groups.
Xie (2015) reported units of platelets transfusion perioperatively. The patients who received treatment with cell saver (n=72) received a mean of 1.97 platelet units (SD ± 3.57 units), and the patients who received treatment without cell saver (n=69) received a mean of 1.91 units (SD ± 3.42 units). The mean difference between the two groups was 0.06 units platelets (95% CI -1.09 to 1.21), in favour of the patients who received treatment without cell saver. This difference is not considered clinically relevant.
Postoperative blood loss (important)
6h postoperative blood loss
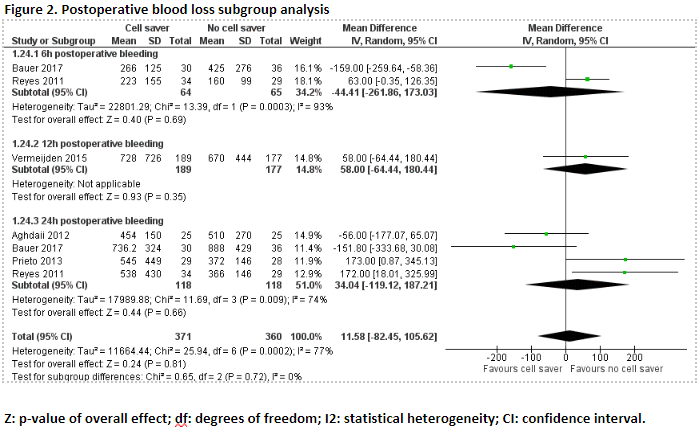
Two studies reported 6h postoperative blood loss (Bauer, 2017; Reyes, 2011). As only two studies were included, the results were not pooled.
Bauer (2017) reported 6 hours postoperative blood loss. The patients who received treatment with cell saver (n=30) had a mean blood loss of 266 mL (SD ± 125 mL), and the patients who received treatment without cell saver (n=36) had a mean blood loss of 425 mL (SD ± 276 mL). The mean difference between the groups was -159.00 mL (95% CI -259.64 to -58.36), in favour of the patients who received treatment with cell saver. This difference is not considered clinically relevant.
Reyes (2011) reported 6h postoperative blood loss. The patients who received treatment with cell saver (n=34) had a mean blood loss of 223 mL (SD ± 155 mL), and the patients who received treatment without cell saver (n=29) had a mean blood loss of 160 mL (SD ± 99 mL). The mean difference between the groups was 63.00 mL (95% CI -0.35 to 126.35), in favour of the patients who received treatment without cell saver. This difference is not considered clinically relevant.
12h postoperative blood loss
Three studies reported 12h postoperative blood loss (Gorki, 2020; Van Klarenbosch, 2020; Vermeijden, 2015). Due to study heterogeneity, the results were not pooled.
Gorki (2020) reported 12h postoperative blood loss. The patients who received treatment with cell saver (n=23) had a mean blood loss of 330 mL (range 260 to 415), and the patients who received treatment without cell saver (n=24) had a mean blood loss of 285 mL (range 250 to 415). The mean difference between the two groups was 45 mL, in favour of the patients who received treatment without cell saver. This difference is not considered clinically relevant.
Van Klarenbosch (2020) reported 12-h postoperative blood loss. The patients who received treatment with cell saver (n=364) had a mean blood loss of 688 mL (SD ± 623 mL), and the patients who received treatment without cell saver (n=352) had a mean blood loss of 721 mL (SD ± 528 mL). The mean difference between the two groups was -33.00 mL (95% CI -117.49 to 51.49), in favour of the patients who received treatment with cell saver. This difference is not considered clinically relevant.
Vermeijden (2015) reported 12-h postoperative blood loss. The patients who received treatment with cell saver (n=189) had a mean blood loss of 728 mL (SD ± 726 mL), and the patients who received treatment without cell saver (n=177) had a mean blood loss of 670 mL (SD ± 444 mL). The mean difference between the two groups was 58.00 mL (95% CI -64.44 to 180.44), in favour of the patients who received treatment without cell saver. This difference is not considered clinically relevant.
24h postoperative blood loss
Five studies reported 24h postoperative blood loss (Aghdaii, 2012; Bauer, 2017; Gorki, 2020; Prieto, 2013; Reyes, 2011).
In total, 24h postoperative blood loss was reported for 118 patients who received treatment with cell saver, and 118 who patients received treatment without cell saver. Pooled data from four studies showed a pooled mean difference of 34.04 mL (95% CI -119.12 to 187.21), in favour of the patients who received treatment without cell saver (Figure 2). This difference is not considered clinically relevant.
Gorki (2020) reported 24h postoperative blood loss. The patients who received treatment with cell saver (n=23) had a mean blood loss of 570 mL (range 415 to 840), and the patients who received treatment without cell saver had a mean blood loss of 605 mL (range 495 to 720). The mean difference between the two groups was -35 mL, in favour of the patients who received treatment with cell saver. This difference is not considered clinically relevant.
A subgroup analysis was performed of 6h, 12h and 24h postoperative blood loss. The results from van Klarenbosch (2020) regarding the 12h postoperative blood loss were excluded from the analysis because the data were obtained from the same patient population as the study from Vermeijden (2015). Pooled data of this subgroup analysis showed a pooled mean difference of 4.45 mL (95% CI -74.08 to 82.97), in favour of the patients who received treatment without cell saver (Figure 2). This difference is not considered clinically relevant.
Postoperative hemoglobin concentration (important)
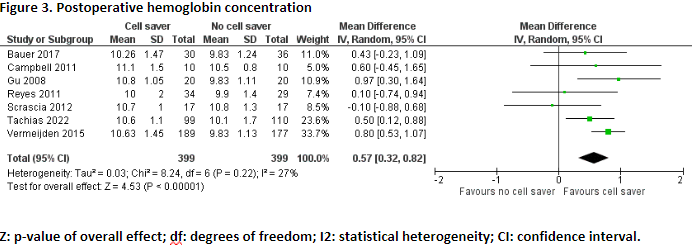
Eight studies reported the outcome measure postoperative hemoglobin concentration (Bauer, 2017; Campbell, 2011; Damgaard, 2010; Gu, 2008; Reyes, 2011; Scrascia, 2012; Tachias, 2022; Vermeijden, 2015).
In total, postoperative hemoglobin concentration was reported for 399 patients who received treatment with cell saver, and for 399 patients who received treatment without cell saver. Pooled data from these six studies showed a pooled mean difference of 0.57 g/dL (95% CI 0.32 to 0.82), in favour of the patients who received treatment with cell saver (Figure 3). This difference is not considered clinically relevant.
Damgaard (2010) reported postoperative (ICU) hemoglobin concentration. The patients who received treatment with cell saver (n=15) had a mean postoperative hemoglobin concentration of 6.2 mmol/L, and the patients who received treatment without cell saver (n=14) had a mean postoperative hemoglobin concentration of 5.5 mmol/L. The difference in mean postoperative hemoglobin concentration between the two groups was 0.7 mmol/L, in favour of the patients who received treatment with cell saver. This difference is considered clinically relevant.
Adverse events (important)
Stroke
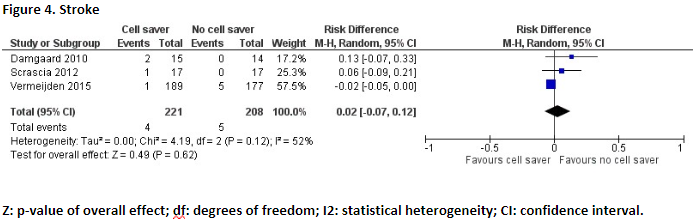
Three studies reported the outcome measure stroke (Damgaard, 2010; Scrascia, 2012; Vermeijden, 2015).
In total, 4 of the 221 (1,8%) patients who received treatment with cell saver suffered from stroke, and 5 of the 208 patients (2,4%) who received treatment without cell saver suffered from stroke. Pooled data of these three studies showed a pooled risk difference of 0.02 (95% CI -0.07 to 0.12) (Figure 4). This difference is not considered clinically relevant.
Neurological complications
Three studies reported the outcome measure neurological complications (Prieto, 2013; Shen, 2016; Bauer, 2017;). Due to study heterogeneity, the results were not pooled.
Prieto (2013) reported neurological complications. In total, 0 of the 29 patients (0%) who received treatment with cell saver suffered from neurological complications, and 2 of the 28 patients (7.1%) who received treatment without cell saver suffered from neurological complications. The risk ratio was 0.19 (95% CI 0.01 to 3.86), in favour of the patients who received treatment with cell saver. This difference is considered clinically relevant.
Shen (2016) reported cognitive decline. In total, 0 of the 53 patients (0%) who received treatment with cell saver suffered from cognitive decline, and 3 of the 50 patients (6%) who received treatment without cell saver suffered from cognitive decline. The risk ratio was 0.13 (95% CI 0.01 to 2.55), in favour of the patients who received treatment with cell saver. This difference is considered clinically relevant.
Bauer (2017) reported neuropsychological deficits. In total, 3 of the 30 patients (10%) who received treatment with cell saver suffered from neuropsychological deficits, and 3 of the 36 patients (8%) who received treatment without cell saver suffered from neuropsychological deficits. The risk ratio was 1.20 (95% CI 0.26 to 5.52), in favour of the patients who received treatment without cell saver. This difference is not considered clinically relevant.
Cardiovascular complications
Seven studies reported the outcome measure cardiovascular complications (Bauer, 2017; Damgaard, 2010; Gorki, 2020; Prieto, 2013; Scrascia, 2012; Shen, 2016; Vermeijden, 2015). Due to study heterogeneity, the results were not pooled.
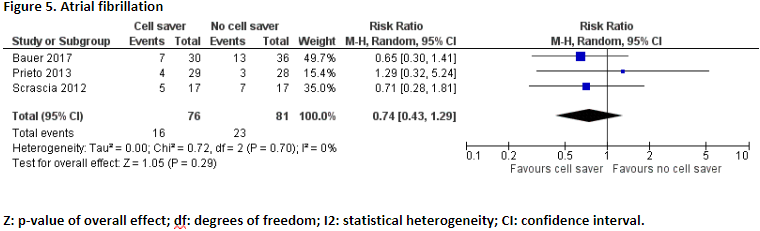
Scrascia (2012), Prieto (2013), and Bauer (2017) reported the number of patients who suffered from atrial fibrillation. In total, 16 of the 76 patients (21%) who received treatment with cell saver suffered from atrial fibrillation and 23 of the 81 patients (28%) who received treatment without cell saver suffered from atrial fibrillation. Pooled data of these three studies showed a pooled risk ratio of 0.74 (95% CI 0.43 to 1.29), in favour of the patients who received treatment with cell saver (Figure 5). This difference is considered clinically relevant.
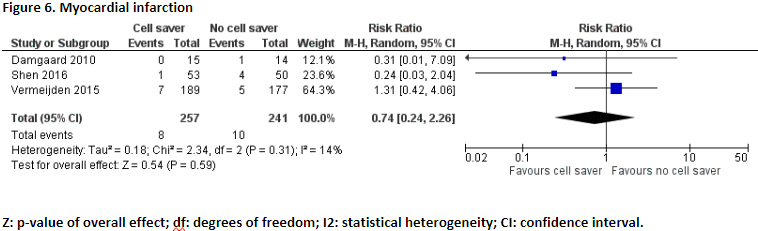
Damgaard (2010), Vermeijden (2015), and Shen (2016) reported the number of patients who suffered from myocardial infarction. In total, 8 of the 257 patients (3,1%) who received treatment with cell saver suffered from myocardial infarction and 10 of the 241 patients (4,1%) who received treatment without cell saver suffered from myocardial infarction. Pooled data of these three studies showed a pooled risk ratio of 0.74 (95% CI 0.24 to 2.26), in favour of the patients who received treatment with cell saver (Figure 6). This difference is considered clinically relevant.
Shen (2016) reported the number of patients who suffered from cardiovascular failure. In total, 6 of 53 patients (11.3%) who received treatment with cell saver suffered from cardiovascular failure, and 7 of the 50 patients (14.0%) who received treatment without cell saver suffered from cardiovascular failure. The risk ratio was 0.81 (95% CI 0.29 to 2.24), in favour of the patients who received treatment with cell saver. This difference is not considered clinically relevant.
Gorki (2020) reported the number of patients who experienced major adverse cardiac and cerebrovascular events. There were no major adverse cardiac and cerebrovascular events in patients who received treatment with cell saver (n=24) and without cell saver (n=24).
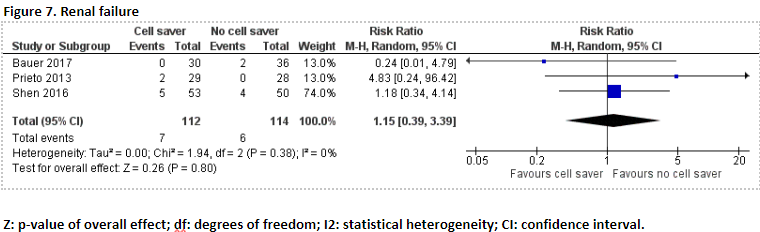
Renal failure
Three studies reported the outcome measure renal failure (Bauer, 2017; Prieto, 2013; Shen, 2016).
In total, 7 of the 112 patients (6.2%) who received treatment with cell saver suffered from renal failure, and 6 of the 114 patients (5.2%) who received treatment without cell saver suffered from renal failure. Pooled data of these three studies showed a pooled risk ratio of 1.15 (95% CI 0.39 to 3.39), in favour of the patients who received treatment without cell saver (Figure 7). This difference is not considered clinically relevant.
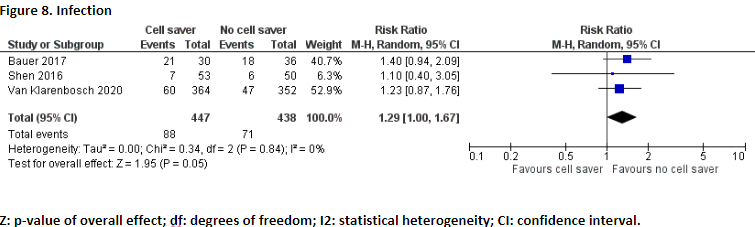
Infection
Three studies reported the outcome measure infection (Bauer, 2017; Shen, 2016; Van Klarenbosch, 2020). Bauer (2017) reported superficial and deep sternal infection. Shen (2016) reported wound and other infections, Van Klarenbosch (2020) reported lung infection, surgical site infection (superficial and deep), and urinary infection.
In total, 88 of the 447 patients (19.7%) who received treatment with cell saver suffered from infection, and 71 of the 438 patients (16.2%) who received treatment without cell saver suffered from infection. Pooled data of these three studies showed a pooled risk ratio of 1.29 (95% CI 1.00 to 1.67), in favour of the patients who received treatment without cell saver (Figure 8). This difference is considered clinically relevant.
Length of stay (important)
Length of hospital stay (days)
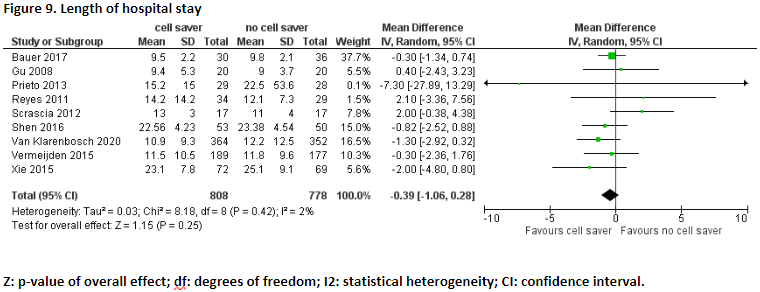
Ten studies reported the outcome measure length of hospital stay (Bauer, 2017; Damgaard, 2010; Gu, 2008; Prieto, 2013; Reyes, 2011; Scrascia, 2012; Shen, 2016; Van Klarenbosch, 2020; Vermeijden, 2015; Xie, 2015).
In total, length of hospital stay was reported for 808 patients who received treatment with cell saver, and 778 patients who received treatment without cell saver. Pooled data from these nine studies showed a pooled mean difference of -0.39 (95% CI -1.06 to 0.28), in favour of patients who received treatment with cell saver (Figure 9). This difference is not considered clinically relevant.
Damgaard (2010) reported postoperative admission days. The patients who were treated with cell saver had a mean admission of 6 days (range 4-14). The patients who were treated without cell saver had a mean admission of 6 days (range 5-12). This means there was no difference in mean admission days between the patients who received treatment with cell saver and the patients who received treatment without cell saver.
Length of ICU stay (days)
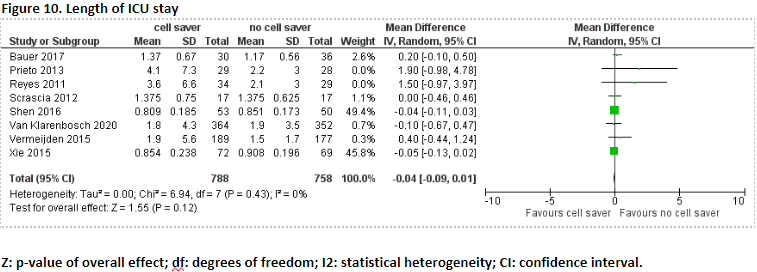
Eight studies reported the outcome measure length of ICU stay (Bauer, 2017; Prieto, 2013; Reyes, 2011; Scrascia, 2012; Shen, 2016; Van Klarenbosch, 2020; Vermeijden, 2015; Xie, 2015).
In total, 788 patients received treatment with cell saver, and 758 patients received treatment without cell saver. Pooled data of these eight studies showed a pooled mean difference of -0.04 (95% CI -0.09 to 0.01), in favour of patients who received treatment with cell saver (Figure 10). This difference is not considered clinically relevant.
Mortality (important)
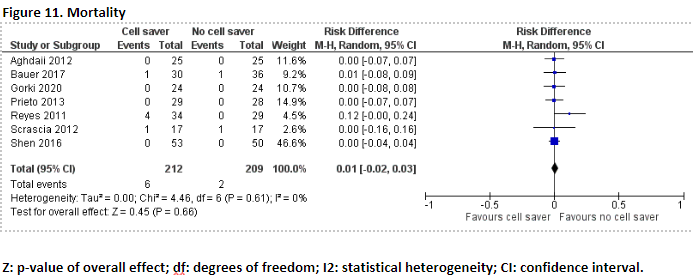
Seven studies, including one meta-analysis, reported the outcome measure mortality (Aghdaii, 2012; Bauer, 2017; Gorki, 2020; Prieto, 2013; Reyes, 2011; Scrascia, 2012; Shen, 2016). Mortality was not universally defined in these studies, ranging from hospital to 30-day mortality.
In total, 6 of the 212 patients (2.8%) who received treatment with cell saver died, and 2 of the 209 patients (1.0%) who received treatment without cell saver died. Pooled data of these seven studies showed pooled risk difference of 0.01 (95% CI -0.02 to 0.03), in favour of the patients who received treatment without cell saver (Figure 11). This difference is not considered clinically relevant.
Level of evidence of the literature
Number of patients transfused
The level of evidence regarding the outcome measure number of patients transfused red blood cells was based on RCTs and therefore starts high. The level of evidence was downgraded by 2 levels because of study limitations (risk of bias, -1), and because the confidence interval exceeds the levels for clinical relevance (imprecision, -1). The level of evidence is therefore low.
Blood product consumption
The level of evidence regarding the outcome measure platelet transfusion was based on RCTs and therefore starts high. The level of evidence was downgraded by 3 levels because of study limitations (risk of bias, -1), and because of a very small number of included patients (imprecision, -2). The level of evidence is therefore very low.
The level of evidence regarding the outcome measure plasma transfusion was based on RCTs and therefore starts high. The level of evidence was downgraded by 3 levels because of study limitations (risk of bias, -1), because of a small study population (imprecision, -1), and because of conflicting results (inconsistency, -1). The level of evidence is therefore very low.
Postoperative blood loss
The level of evidence regarding the outcome measure 6h and 24h postoperative blood loss was based on RCTs and therefore starts high. The level of evidence was downgraded by 3 levels because of study limitations (risk of bias, -1), because the confidence interval exceeds the levels for clinical relevance (6h postoperative blood loss) or a small number of patients (24h postoperative blood loss) (imprecision, -1), and because of conflicting results (inconsistency, -1). The level of evidence is therefore very low.
The level of evidence regarding the outcome measure 12h postoperative blood loss was based on RCTs and therefore starts high. The level of evidence was downgraded by 2 levels because of study limitations (risk of bias, -1), and because of conflicting results (inconsistency, -1). The level of evidence is therefore low.
Hemoglobin concentration
The level of evidence regarding the outcome measure hemoglobin concentration was based on RCTs and therefore starts high. The level of evidence was downgraded by 2 levels because of study limitations (risk of bias, -1), and because of a small number of included patients (imprecision, -1). The level of evidence is therefore low.
Adverse events
The level of evidence regarding the outcome measure stroke was based on RCTs and therefore starts high. The level of evidence was downgraded by 3 levels because of study limitations (risk of bias, -1), because of a small number of included patients (imprecision, -1), and because of conflicting results (inconsistency, -1). The level of evidence is therefore very low.
The level of evidence regarding the outcome measures renal failure, neurological complications, atrial fibrillation, and myocardial infarction was based on RCTs and therefore starts high. The level of evidence was downgraded by 4 levels because of study limitations (risk of bias, -1), because the confidence interval exceeds the levels for clinical relevance (imprecision, -2), and because of conflicting results (inconsistency, -1). The level of evidence is therefore very low.
The level of evidence regarding the outcome measure infection was based on RCTs and therefore starts high. The level of evidence was downgraded by 2 levels because of study limitations (risk of bias, -1), and because the confidence interval exceeds the levels for clinical relevance (imprecision, -1). The level of evidence is therefore low.
Length of stay
The level of evidence regarding the outcome measure length of hospital and ICU stay was based on RCTs and therefore starts high. The level of evidence was downgraded by 3 levels because of study limitations (risk of bias, -1), because the confidence interval exceeds the levels for clinical relevance (length of hospital stay) or because of the small number of included patients (length of ICU stay) (imprecision, -1), and because of conflicting results (inconsistency, -1). The level of evidence is therefore very low.
Mortality
The level of evidence regarding the outcome measure mortality was based on RCTs and therefore starts high. The level of evidence was downgraded by 3 levels because of study limitations (risk of bias, -1), and because the confidence interval exceeds the levels for clinical relevance (imprecision, -2). The level of evidence is therefore very low.
Zoeken en selecteren
A systematic review of the literature was performed to answer the following question:
What are the (un)favorable effects of using cell saver versus not using cell saver in adult patients undergoing cardiac surgery?
| P: | adult patients undergoing cardiac surgery |
| I: | use of cell saver |
| C: | no use of cell saver |
| O: | number of patients transfused, blood product consumption, adverse events, postoperative blood loss, hemoglobin concentration, length of stay, mortality, cost-effectivity |
Relevant outcome measures
The guideline development group considered number of patients transfused and blood product consumption as critical outcome measures for decision making; and adverse events, postoperative blood loss, hemoglobin concentration, length of stay, mortality, and cost-effectivity as important outcome measures for decision making.
The working group defined the outcome measures as follows:
-
Number of patients transfused: red blood cells
-
Blood product consumption: platelets and plasma (usually described as fresh frozen plasma (FFP)
A priori, the working group did not define the other outcome measures listed above but used the definitions used in the studies.
The working group defined the following differences as a minimal clinically (patient) important differences:
-
Number of patients transfused: 10% (RR < 0.9 and RR > 1.1)
-
Blood product consumption: 1 unit
-
Adverse events: 25% (RR < 0.8 and RR > 1.25; RD > 0.25)
-
Postoperative blood loss: 250 mL
-
Hemoglobin concentration: 0.625 mmol/liter, 1.0 g/dl
-
Length of stay: 1 day
-
Mortality: 10% (RR < 0.9 and RR > 1.1)
The databases Medline (via OVID) and Embase (via Embase.com) were searched with relevant search terms from 01-01-2000 until 06-10-2022. The detailed search strategy is depicted under the tab Methods. The systematic literature search resulted in 205 hits. Studies were selected based on the following criteria: systematic reviews or RCTs comparing the use of cell saver versus no use of cell saver in adult patients undergoing cardiac surgery. In total, 38 studies were initially selected based on title and abstract screening. After reading the full text, 23 studies were excluded (see the table with reasons for exclusion under the tab Methods), and 15 studies were included.
Results
One systematic review and fourteen randomized controlled trials were included in the analysis of the literature. Important study characteristics and results are summarized in the evidence tables. The assessment of the risk of bias is summarized in the risk of bias tables.
Referenties
- Adam EH, Funke M, Zacharowski K, et al. Impact of intraoperative cell salvage on blood coagulation factor concentrations in patients undergoing cardiac surgery. Anesth Analg 2020;130:1389-1395.
- Aghdaii, N., Kabiri, M., Yazdanian, F., & Ghaffarinejad, M. H. (2012). Effect of retransfusion of heparin remaining in the salvaged blood on postoperative blood loss in coronary artery bypass grafting: Comparison with homologous blood transfusion (running title: Postoperative blood loss in CABG).?Iranian Heart Journal,?13(2), 24-34.
- Al-Khabori M, Al-Riyami AZ, Baskaran B, Siddiqi M, Al-Sabti H. Discriminatory power of the intraoperative cell salvage use in the prediction of platelet and plasma transfusion in patients undergoing cardiac surgery. Transfus Apher Sci. 2015 Oct;53(2):208-12. doi: 10.1016/j.transci.2015.03.019. Epub 2015 Mar 28. PMID: 25863410.
- Bauer A, Hausmann H, Schaarschmidt J, Scharpenberg M, Troitzsch D, Johansen P, Nygaard H, Eberle T, Hasenkam JM. Shed-blood-separation and cell-saver: an integral Part of MiECC? Shed-blood-separation and its influence on the perioperative inflammatory response during coronary revascularization with minimal invasive extracorporeal circulation systems - a randomized controlled trial. Perfusion. 2018 Mar;33(2):136- 147. doi: 10.1177/0267659117728195. Epub 2017 Sep 22. PMID: 28937313.
- Campbell J, Holland C, Richens D, Skinner H. Impact of cell salvage during cardiac surgery on the thrombelastomeric coagulation profile: a pilot study. Perfusion. 2012 May;27(3):221-4. doi: 10.1177/0267659111432567. Epub 2011 Dec 20. PMID: 22185951.
- Damgaard S, Nielsen CH, Andersen LW, Bendtzen K, Tvede M, Steinbrüchel DA. Cell saver for on-pump coronary operations reduces systemic inflammatory markers: a randomized trial. Ann Thorac Surg. 2010 May;89(5):1511-7. doi: 10.1016/j.athoracsur.2010.02.003. PMID: 20417770.
- Gorki H, Nakamura J, Kunert A, Hoenicka M, Liebold A. Pericardial fluids or Cardiopulmonary Bypass-Is There a Major Culprit for Changes in Coagulation and Inflammation? Thorac Cardiovasc Surg. 2020 Apr;68(3):219-222. doi: 10.1055/s-0039-1677836. Epub 2019 Feb 6. PMID: 30727012.
- Gu YJ, Vermeijden WJ, de Vries AJ, Hagenaars JA, Graaff R, van Oeveren W. Influence of mechanical cell salvage on red blood cell aggregation, deformability, and 2,3- diphosphoglycerate in patients undergoing cardiac surgery with cardiopulmonary bypass. Ann Thorac Surg. 2008 Nov;86(5):1570-5. doi: 10.1016/j.athoracsur.2008.07.052. PMID: 19049752.
- Kietaibl S, Ahmed A, Afshari A, Albaladejo P, Aldecoa C, Barauskas G, De Robertis E, Faraoni D, Filipescu DC, Fries D, Godier A, Haas T, Jacob M, Lancé MD, Llau JV, Meier J, Molnar Z, Mora L, Rahe-Meyer N, Samama CM, Scarlatescu E, Schlimp C, Wikkelsø AJ, Zacharowski K. Management of severe peri-operative bleeding: Guidelines from the European Society of Anaesthesiology and Intensive Care: Second update 2022. Eur J Anaesthesiol. 2023 Apr 1;40(4):226-304. doi: 10.1097/EJA.0000000000001803. PMID: 36855941.
- Prieto MA, Guash S, Mendez JC, Munoz C, Planas A, Reyes G. Does use of cell saver decrease the inflammatory response in cardiac surgery? Asian Cardiovasc Thorac Ann. 2013 Feb;21(1):37-42. doi: 10.1177/0218492312446838. PMID: 23430418.
- Reyes G, Prieto M, Alvarez P, Orts M, Bustamante J, Santos G, Sarraj A, Planas A. Cell saving systems do not reduce the need of transfusion in low-risk patients undergoing cardiac surgery. Interact Cardiovasc Thorac Surg. 2011 Feb;12(2):189-93. doi: 10.1510/icvts.2010.251538. Epub 2010 Nov 30. PMID: 21118833.
- Scrascia G, Rotunno C, Nanna D, Rociola R, Guida P, Rubino G, de Luca Tupputi Schinosa L, Paparella D. Pump blood processing, salvage and re-transfusion improves hemoglobin levels after coronary artery bypass grafting, but affects coagulative and fibrinolytic systems. Perfusion. 2012 Jul;27(4):270-7. doi: 10.1177/0267659112442236. Epub 2012 Mar 22. PMID: 22440640.
- Shen S, Zhang J, Wang W, Zheng J, Xie Y. Impact of intra-operative cell salvage on blood coagulation in high-bleeding-risk patients undergoing cardiac surgery with cardiopulmonary bypass: a prospective randomized and controlled trial. J Transl Med. 2016 Jul 29;14(1):228. doi: 10.1186/s12967-016-0986-6. PMID: 27473326; PMCID: PMC4966771.
- Son K, Yamada T, Tarao K, et al. Effects of cardiac surgery and salvaged blood transfusion on thromboelastometry variables. J Cardiothorac Vasc Anesth 2020;34:2375-82. doi: 10.1053/j.jvca.2020.02.009
- Tachias F, Samara E, Petrou A, Karakosta A, Siminelakis S, Apostolakis E, Tzimas P. The Effect of Cell Salvage on Bleeding and Transfusion Needs in Cardiac Surgery. Anesthesiol Res Pract. 2022 Sep 1;2022:3993452. doi: 10.1155/2022/3993452. PMID: 36092853; PMCID: PMC9458370.
- Task Force on Patient Blood Management for Adult Cardiac Surgery of the European Association for Cardio-Thoracic Surgery (EACTS) and the European Association of Cardiothoracic Anaesthesiology (EACTA); Boer C, Meesters MI, Milojevic M, Benedetto U, Bolliger D, von Heymann C, Jeppsson A, Koster A, Osnabrugge RL, Ranucci M, Ravn HB, Vonk ABA, Wahba A, Pagano D. 2017 EACTS/EACTA Guidelines on patient blood management for adult cardiac surgery. J Cardiothorac Vasc Anesth. 2018 Feb;32(1):88-120. doi: 10.1053/j.jvca.2017.06.026. Epub 2017 Sep 30. PMID: 29029990.
- Tibi P, McClure RS, Huang J, Baker RA, Fitzgerald D, Mazer CD, Stone M, Chu D, Stammers AH, Dickinson T, Shore-Lesserson L, Ferraris V, Firestone S, Kissoon K, Moffatt-Bruce S. STS/SCA/AmSECT/SABM Update to the Clinical Practice Guidelines on Patient Blood Management. Ann Thorac Surg. 2021 Sep;112(3):981-1004. doi: 10.1016/j.athoracsur.2021.03.033. Epub 2021 Jun 30. PMID: 34217505.
- van Klarenbosch J, van den Heuvel ER, van Oeveren W, de Vries AJ. Does Intraoperative Cell Salvage Reduce Postoperative Infection Rates in Cardiac Surgery? J Cardiothorac Vasc Anesth. 2020 Jun;34(6):1457-1463. doi: 10.1053/j.jvca.2020.01.023. Epub 2020 Jan 22. PMID: 32144053.
- Vermeijden WJ, van Klarenbosch J, Gu YJ, Mariani MA, Buhre WF, Scheeren TW, Hagenaars JA, Tan ME, Haenen JS, Bras L, van Oeveren W, van den Heuvel ER, de Vries AJ. Effects of cell-saving devices and filters on transfusion in cardiac surgery: a multicenter randomized study. Ann Thorac Surg. 2015 Jan;99(1):26-32. doi: 10.1016/j.athoracsur.2014.08.027. Epub 2014 Nov 12. PMID: 25440265.
- Wang G, Bainbridge D, Martin J, Cheng D. The efficacy of an intraoperative cell saver during cardiac surgery: a meta-analysis of randomized trials. Anesth Analg. 2009 Aug;109(2):320-30. doi: 10.1213/ane.0b013e3181aa084c. PMID: 19608798.
- Xie Y, Shen S, Zhang J, Wang W, Zheng J. The efficacy, safety and cost-effectiveness of intra- operative cell salvage in high-bleeding-risk cardiac surgery with cardiopulmonary bypass: a prospective randomized and controlled trial. Int J Med Sci. 2015 Apr 1;12(4):322-8. doi: 10.7150/ijms.11227. PMID: 25897293; PMCID: PMC4402435.
Evidence tabellen
Evidence table SR:
|
Study reference |
Study characteristics |
Patient characteristics |
Intervention (I) |
Comparison / control (C)
|
Follow-up |
Outcome measures and effect size |
Comments |
|
Wang, 2009
(Individual study characteristisc deduced from Wang, 2009) |
SR and meta-analysis of 31 RCTs
Literature search up to November 2008
A: Allen, 2007 B: Anderson, 2000 C: Borowiec, 1997 D: Carrier, 2006 E: Daane, 2003 F: Dalrymple-Hay, 2001 G: Damgaard, 2006 H: Dietrich, 1989 I: Djaiani, 2007 J: Eichert, 2001 K: Goel, 2007 L: Jewell, 2003 M: Klein, 2008 N: Laub, 1993 O: Marcheix, 2008 P: McGill, 2002 Q: Merville, 1991 R: Murphy, 2005 S: Murphy, 2004 T: Niranjan, 2006 U: Nuttall, 2006 V: Perttila, 1994 W: Rubens, 2007 X: Song, 2006 Y: Svenmarker, 2003 Z: Svenmarker, 2004 α: Tempe, 2001 β: Walpoth, 1999 γ: Westerberg, 2006 δ: Wiefferink, 2007 ε: Winton, 1982
Study design: Systematic Review of 31 Randomized Controlled Trials
Setting and country: A: UK B: Sweden C: Sweden D: Canada E: Netherlands F: UK G: Denmark H: Germany I: Canada J: Germany K: India L: UK M: UK N: USA O: Canada P: USA Q: French R: UK S: UK T: UK U: USA V: Finland W: Canada X: China Y: Sweden Z: Sweden α: India β: Switzerland γ: Sweden δ: Netherlands ε: Canada
Source of funding: Not reported.
Conflicts of interest: Not reported. |
Inclusion criteria SR: Adult patients undergoing cardiac surgery, randomized allocation to a cell saver (CS) group versus no cell saver group, reporting at least one relevant clinical or economical outcome, studies blinded and unblinded, in any language.
Exclusion criteria SR: If a CS was used only in the postoperative period and in trials in which the CS blood was reinfused without washing.
Important patient characteristics at baseline: N: A: 37 B: 20 C: 16 D: 40 E: 40 F: 112 G: 59 H: 50 I: 226 J: 40 K: 49 L: 20 M: 213 N: 50 O: 50 P: 168 Q: 120 R: 61 S: 196 T: 80 U: 59 V: 30 W: 266 X: 32 Y: 33 Z: 60 α: 40 β: 20 γ: 25 δ: 30 ε: 40
Surgery type: A: OPCAB B: CCAB C: CCAB D: CCAB E: CCAB, valve F: CCAB, valve G: OPCAB H: CCAB I: CCAB J: CCAB K: OPCAB L: CCAB M: CCAB, valve N: CCAB O: CCAB P: CCAB Q: CCAB R: OPCAB S: CCAB T: CCAB, OPCAB U: CCAB V: CCAB, valve W: CCAB, valve X: Valve Y: CCAB Z: CCAB α: Valve β: CCAB γ: CCAB δ: CCAB ε: CCAB, valve
Age and gender in the CS and control group were comparable at baseline. |
CS use: the use of an extracorporeal cell saving device, either used during CPB only, pre-CPB and post-CPB only, or throughout the entire operation.
Method of cell saver: A: Shed blood B: Shed blood C: Cardiotomy suction blood during CPB D: Cardiotomy suction blood during CPB E: Residual CPB volume F: Residual CPB volume G: Shed blood H: Residual CPB volume I:Cardiotomy suction blood during CPB J: Residual CPB volume K: Shed blood L: Cardiotomy suction blood during CPB M: Shed and residual CPB blood N: Shed and residual CPB blood O: Cardiotomy suction blood during CPB P: Shed and residual CPB blood Q: Shed and residual CPB blood R: Shed blood S: Shed blood T: Shed blood U: Cardiotomy suction blood during CPB V: Cardiotomy suction blood during CPB W: Cardiotomy suction blood during CPB X: Shed and residual CPB blood Y: Cardiotomy suction blood during CPB Z: Cardiotomy suction blood during CPB α: Shed blood β: Residual CPB volume γ: Cardiotomy suction blood δ: Shed and residual CPB blood ε: Shed blood
|
No CS use.
|
End point of follow-up: Not reported.
For how many participants were no complete outcome data available? Not reported.
|
Patients transfused any blood product OR [95% CI] G: 0.50 [0.17, 1.48] H: 0.18 [0.01, 4.04] I: 1.39 [0.82, 2.35] M: 1.00 [0.56, 1.77] N: 0.11 [0.02, 0.51] P: 0.48 [0.26, 0.90] Q: 0.44 [0.21, 0.92] R: 0.36 [0.11, 1.22] S: 0.43 [0.24, 0.77] W: 1.28 [0.78, 2.10] Y: 2.15 [0.34, 13.80] α: 0.04 [0.00, 0.68] δ: 0.57 [0.13, 2.50] ε: 1.29 [0.32, 5.17]
Pooled effect (random effects model / fixed effects model): - Intervention: 43.8% - Control: 51.2% OR 0.63 [95% CI 0.43 to 0.94] favoring the use of cell saver. Heterogeneity (I2): 61
Patients transfused red blood cells (RBCs) OR [95% CI] F: 0.19 [0.07, 0.46] I: 1.11 [0.66, 1.88] K: 0.09 [0.00, 1.76] M: 1.03 [0.57, 1.85] N: 0.16 [0.04, 0.67] O: 1.00 [0.18, 5.51] P: 0.43 [0.23, 0.80] Q: 1.00 [0.14, 7.43] R: 0.53 [0.14, 2.03] S: 0.40 [0.22, 0.71] W: 1.16 [0.71, 1.90] β: 1.50 [0.26, 8.82]
Pooled effect (random effects model / fixed effects model): - Intervention: 37.4% - Control: 45.4% OR 0.60 [95% CI 0.39, 0.92] favoring the use of cell saver. Heterogeneity (I2): 63
Patients transfused fresh frozen plasma OR [95% CI] I: 2.38 [1.18, 4.81] K: 1.47 [0.29, 7.37] M: 1.49 [0.50, 4.45] N: 1.00 [0.21, 4.76] O: 0.48 [0.04, 5.65] P: 1.09 [0.48, 2.49] Q: 0.45 [0.15, 1.42] R: 0.33 [0.01, 8.51] S: 0.70 [0.31, 1.57] W: 1.47 [0.63, 3.44]
Pooled effect (random effects model / fixed effects model): - Intervention: 13.1% - Control: 11.3% OR 1.16 [95% CI 0.82, 1.66] favoring no use of cell saver. Heterogeneity (I2): 8
Patients transfused platelets OR [95% CI] E: 2.43 [0.51, 11.51] I: 0.94 [0.42, 2.10] K: 0.76 [0.24, 2.39] M: 1.33 [0.39, 4.48] N: 0.13 [0.02, 0.73] O: 2.09 [0.18, 24.61] P: 0.69 [0.30, 1.61] Q: 1.00 [0.06, 16.37] R: 0.30 [0.05, 1.61] S: 0.98 [0.44, 2.17] W: 1.43 [0.56, 3.68]
Pooled effect (random effects model / fixed effects model): - Intervention: 10.9% - Control: 12.1% OR 0.90 [95% CI 0.63, 1.28] favoring the use of cell saver. Heterogeneity (I2): 3
Total allogenic blood product transfused (mL) Pooled effect (random effects model / fixed effects model): - Intervention, mean: 525 - Control, mean: 781 WMD -256 mL [95% CI -416, -95] favoring the use of cell saver. Heterogeneity (I2): 63
Red blood cells transfused, unit Pooled effect (random effects model / fixed effects model): - Intervention, mean: 1.16 - Control, mean: 1.65 WMD -0.43 u [95% CI -0.87, 0.01] favoring the use of cell saver. Heterogeneity (I2): 86
Fresh frozen plasma transfused, unit Pooled effect (random effects model / fixed effects model): - Intervention, mean: 0.81 - Control, mean: 0.89 WMD -0.08 u [95% CI -0.20, 0.04] favoring the use of cell saver. Heterogeneity (I2): 0
Platelets transfused, unit Pooled effect (random effects model / fixed effects model): - Intervention, mean: 0.28 - Control, mean: 0.63 WMD -0.36 u [95% CI -0.98, 0.27] favoring the use of cell saver. Heterogeneity (I2): 73
All-cause mortality Pooled effect (random effects model / fixed effects model): - Intervention: 1.2% - Control: 2.1% OR 0.65 [95% CI 0.25, 1.68] favoring the use of cell saver. Heterogeneity (I2): 0
Stroke or TIA Pooled effect (random effects model / fixed effects model): - Intervention: 1.0% - Control: 1.7% OR 0.59 [95% CI 0.20, 1.76] favoring the use of cell saver. Heterogeneity (I2): 0
Acute myocardial infarction Pooled effect (random effects model / fixed effects model): - Intervention: 4.5% - Control: 5.2% OR 0.84 [95% CI 0.38, 1.83] favoring the use of cell saver. Heterogeneity (I2): 0
Atrial fibrillation Pooled effect (random effects model / fixed effects model): - Intervention: 28.0% - Control: 29.5% OR 0.92 [95% CI 0.69, 1.23] favoring the use of cell saver. Heterogeneity (I2): 0
Renal dysfunction Pooled effect (random effects model / fixed effects model): - Intervention: 3.6% - Control: 4.0% OR 0.86 [95% CI 0.41, 1.80] favoring the use of cell saver. Heterogeneity (I2): 0
Infection Pooled effect (random effects model / fixed effects model): - Intervention: 7.6% - Control: 6.2% OR 1.25 [95% CI 0.75, 2.10] favoring no use of cell saver. Heterogeneity (I2): 0
Reexploration for bleeding Pooled effect (random effects model / fixed effects model): - Intervention: 2.6% - Control: 4.4% OR 0.61 [95% CI 0.31, 1.20] favoring the use of cell saver. Heterogeneity (I2): 0
Postoperative chest tube drainage (mL) Pooled effect (random effects model / fixed effects model): - Intervention, mean: 792 - Control, mean: 807 WMD -14.9 mL [95% CI -61.8, 31.9] favoring the use of cell saver. Heterogeneity (I2): 23
24-h postoperative hemoglobin concentration (mg/dL) Pooled effect (random effects model / fixed effects model): - Intervention, mean: 10.6 - Control, mean: 10.4 WMD 0.20 mg/dL [95% CI -0.26, 0.65] favoring the use of cell saver. Heterogeneity (I2): 91
Intensive care unit length of stay (days) Pooled effect (random effects model / fixed effects model): - Intervention, mean: 1.2 - Control, mean: 1.2 WMD 0.00 days [95% CI -0.13, 0.13] Heterogeneity (I2): 0
Hospital length of stay (days) Pooled effect (random effects model / fixed effects model): - Intervention, mean: 7.7 - Control, mean: 7.9 WMD -0.20 days [95% CI -0.54, 0.15] favoring the use of cell saver. Heterogeneity (I2): 12 |
Intraoperative use of a cell saver during cardiac surgery decreased the odds of exposure to any allogenic blood transfusion by 37% and red blood cell transfusion by 40%. Postoperative complications did not differ significantly between the two groups.
No GRADE performed.
Sensitivity analysis of results by high versus low Jadad scores: significant relationship between study quality and effect size. Higher quality studies reported more conservative effects on reducing odds of patient exposure to red blood cells.
Subgroup analysis was performed for subgroups of patients undergoing off-pump versus on-pump surgery. This subgroup analysis showed that a cell saver has similar effects on all outcomes, regardless of undergoing off-pump surgery or on-pump surgery, except for 24h postoperative hemoglobin concentration and total blood volume transfused. - 24h postoperative hemoglobin concentration: more improvement with a cell saver in patients undergoing off-pump surgery compared to on-pump surgery (WMD 1.0 vs 0.04 mg/dL for off-pump vs on-pump). - Total blood volume transfused: more improvement with a cell saver in patients undergoing on-pump surgery compared to off-pump surgery (WMD -89 mL vs -318 mL for off-pump vs on-pump).
Subgroup analysis was also performed for the different time periods of cell saver use. CS1 includes studies using a CS during CPB for cardiotomy suction blood only and CS2 includes studies using CS for shed and/or residual blood pre-CPB or post-CPB. There was less cell saver benefit in CS1 studies than in CS2 studies with respect to transfusion of any allogeneic blood product, allogeneic RBC transfusion, and allogeneic plasma transfusion. In addition, there was less cell saver benefit in CS1 studies with respect to units of RBCs transfused and 24h postoperative Hb.
|
Evidence table RCTs:
|
Study reference |
Study characteristics |
Patient characteristics 2 |
Intervention (I) |
Comparison / control (C) 3
|
Follow-up |
Outcome measures and effect size 4 |
Comments |
|
Gu, 2008 |
Randomized controlled trial
Setting and country: University Medical Centre Groningen, the Netherlands
Source of funding: Not reported.
Conflicts of interest: Not reported. |
Inclusion criteria: Patients undergoing cardiopulmonary bypass (CPB) for elective coronary artery bypass grafting, single valve replacement, or a combined procedure.
Exclusion criteria: Patients less than 18 years or over 80 years old, patients presenting for emergency operation.
N total at baseline: Intervention: 20 Control: 20
Important prognostic factors: Age ± SD: Intervention: 68 ± 9 Control: 66 ± 11
Gender (M/F): Intervention: 13/7 Control: 13/7
Length ± SD: Intervention: 174 ± 10 Control: 172 ± 9
Weight ± SD: Intervention: 82 ± 13 Control: 78 ± 13
Surgery (CABG/VR/CABG+VR): Intervention: 18/1/1 Control: 16/3/1
X-clamp time ± SD: Intervention: 53 ± 16 Control: 62 ± 19
CPB time ± SD: Intervention: 92 ± 24 Control: 96 ± 34
Groups were comparable at baseline. |
Intervention: The wound blood from both the pericardium and the pleural space was collected in a cell saver reservoir. Conventional cardiotomy suction was not used. The reservoir was primed with 100 mL of normal saline with 30.000 IU/L of heparin. The salvaged blood was processed with a Continuous AutoTransfusion System cell saver. The residual blood in the heart-lung machine after CPB was collected in a transfusion bag, transferred to the cell saver reservoir and processed also by the cell saver. |
Control: Conventional cardiotomy suction was used for the salvage of wound blood that was returned without cell saver processing. The residual blood in the heart-lung machine after CPB was collected in a transfusion bag and retransfused through a standard blood transfusion system. |
Length of follow-up: Postoperative morning.
Loss-to-follow-up: Not reported.
Incomplete outcome data: Not reported. |
Hemoglobin concentration (mmol/L): Intervention: 6.7 ± 0.65 Control: 6.1 ± 0.69 P=0.012
Chest drainage (mL/24h): Intervention: 460 ± 347 Control: 400 ± 222 P=0.533
RBC transfusion (mL): Intervention: 270 ± 455 Control: 375 ± 400 P=0.443
Patients receiving RBC transfusion: Intervention: 6/20 Control: 13/20 P=0.056
Hospital stay (days): Intervention: 9.4 ± 5.3 Control: 9.0 ± 3.7 P=0.811
|
|
|
Damgaard, 2010 |
Randomized controlled study
Setting and country: Department of Cardiothoracic Surgery Rigshospitalet, Copenhagen, Denmark
Source of funding: Grants from the Danish Heart Foundation and the former Copenhagen Hospital Corporation.
Conflicts of interest: Not reported. |
Inclusion criteria: Patients older than 18 years, need for CABG, informed consent.
Exclusion criteria: Off-pump, redo or valve operations, current infection or antibiotic treatment, s-creatinine concentration exceeding 200 µmol/L, liver disease, immune disease, and anti-inflammatory or immune-modulating treatment (except for nonsteroidal anti-inflammatory drugs and aspirin).
N total at baseline: Intervention: 15 Control: 15
Important prognostic factors: Age median (IQR): Intervention: 66 (53-72) Control: 68 (65-74)
Female sex (%): Intervention: 3 (20) Control: 3 (21)
Hemoglobin, mmol/L median (IQR): Intervention: 8.9 (7.9-9.2) Control: 8.5 (7.8-9.0)
Previous myocardial infarction (MI): Intervention: 6 (40) Control: 9 (64)
MI within one week: Intervention: 1 (7) Control: 0
Groups were comparable at baseline. |
Intervention: Cell saving of pericardial suction blood and residual blood in the CPB circuit after perfusion. |
Control: Direct retransfusion of the suction blood and the CPB circuit blood. |
Length of follow-up: Postoperative morning.
Loss-to-follow-up: Intervention: 0
Control: 1 Reason: anaphylactic reaction.
Incomplete outcome data: Not reported. |
Median (IQR) / No. (%) Net blood loss (mL): Intervention: 250 (200-280) Control: 475 (250-660)
Admission (days): Intervention: 6 (4-14) Control: 6 (5-12) P=0.649
Drain blood (mL): Intervention: 600 (530-1160) Control: 605 (420-800) P=0.470
RBC transfusion during entire admission (U): Intervention: 0 (0-4) Control: 1 (0-3) P=0.816
Patients received RBC transfusion: Intervention: 7 (47) Control: 8 (57) P=0.537
Myocardial infarction: Intervention: 0 Control: 1 (7) P=0.483
Reoperation: Intervention: 1 (7) Control: 2 (14) P=0.598
Stroke: Intervention: 2 (13) Control: 0 P=0.483 |
|
|
Campbell, 2011 |
Pilot study
Setting and country: Nottingham University Hospitals, UK
Source of funding: No specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Conflicts of interest: None declared. |
Inclusion criteria: Patients scheduled for elective first-time coronary bypass surgery.
Exclusion criteria: Patients older than 70 years, with a known clotting deficiency, taking warfarin or antiplatelet rugs within 5 days of surgery, or who had a pre-operative platelet count <150 x 109 L-1.
N total at baseline: Intervention: 10 Control: 10
Important prognostic factors2: Age (SD): Intervention: 62 (10) Control: 64 (10)
Sex male/female: Intervention: 9/1 Control: 9/1
Hemoglobin concentration (g/dL): Intervention: 14.3 (1.4) Control: 14.3 (1.0)
Euro SCORE: Intervention: 2.1 (1.3) Control: 2.9 (2.1)
Groups were comparable at baseline. |
Intervention: Blood was salvaged prior to the administration of heparin and after reversal with protamine and the residual CPB volume was processed before transfusion after bypass using a continuous auto transfusion system. Cardiotomy blood was returned to the venous reservoir. |
Control: No cell saver was used. The residual CPB volume was transfused unprocessed after protamine administration. Cardiotomy blood was returned to the venous reservoir. |
Length of follow-up: Not reported.
Loss-to-follow-up: Not reported.
Incomplete outcome data: Not reported.
|
Hemoglobin concentration (g/dL): Intervention: 11.1 (1.5) Control: 10.5 (0.8) P=0.2
Blood volume in chest drains 4 hours after surgery (mL): Intervention: 382 (211) Control: 348 (117) P=0.3
Platelet transfusion (n patients): Intervention: 2 Control: 1 |
|
|
Reyes, 2011 |
Prospective randomized clinical trial
Setting and country: Hospital Universitario de La Princesa, Madrid, Spain
Source of funding: Not reported.
Conflicts of interest: Not reported. |
Inclusion criteria: Patients undergoing cardiac surgery with the use of CPB.
Exclusion criteria: Patients over 80 years old, redo or emergency surgery, with aorta, endocarditis or pericardio disease, with combined procedures or requiring triple-valve surgery, patients that do not accept the use of blood products, with a logistic EuroSCORE >10%, and with a high risk of bleeding if the patient had two or more of the following conditions: - Preoperative creatinine >2.2 mg/ml - Liver insufficiency - Severe lung disease - Body surface area <1.6m2 - Preoperative hemoglobin levels <13 g/dL in males and <12 g/dL in females - Platelet count below 50.000/mL or any platelet dysfunction - Patients with coagulation disorders - Intake of aspirin three days prior to surgery or clopidogrel seven days prior to surgery.
N total at baseline: Intervention: 34 Control: 29
Important prognostic factors2: Age ± SD: Intervention: 65.5 ± 12.1 Control: 63.7 ± 12.7
Sex (female): Intervention: 10 (29.4%) Control: 11 (37.9%)
Stroke: Intervention: 1 (2.9%) Control: 5 (17.2%)
Logistic EuroSCORE: Intervention: 4.4 ± 2.7 Control: 4.3 ± 3
Groups were comparable at baseline. |
Intervention: A cell saver device was used during the entire procedure. All remaining blood inside the circuits was recovered and concentrated by the cell saver, cardiotomy suction was used and this blood was transfused to the patients using a 200 micron filter. |
Control: No cell saver was used. All blood in the surgical field was aspirated only using the cardiotomy suction. |
Length of follow-up: 30 days after surgery
Loss-to-follow-up: Not reported. No loss-to-follow-up.
Incomplete outcome data: Not reported. No incomplete outcome data.
|
Number of patients that received blood transfusion: Intervention: 12 (35.3%) Control: 13 (41.2%) P=0.79
Total blood transfusions: Intervention: 31 Control: 28
Plasma bags transfused: Intervention: 8 Control: 3 P=0.77
Platelet bags transfused: Intervention: 3 Control: 0 P=0.08
6h postoperative bleeding (mL): Intervention: 223 ± 155 Control: 160 ± 99 P=0.06
24h postoperative bleeding (mL): Intervention: 538 ± 430 Control: 366 ± 146 P=0.05
Total length of stay (days): Intervention: 14.2 ± 14.2 Control: 12.1 ± 7.3 P=0.51
Length of stay in ICU (days): Intervention: 3.6 ± 6.6 Control: 2.1 ± 3 P=0.26
Postoperative fever: Intervention: 8 (23.5%) Control: 8 (27.6%) P=1
Hemoglobin at discharge (g/dL): Intervention: 10 ± 2 Control: 9.9 ± 1.4 P=0.80
Mortality: Intervention: 4 (11.8%) Control: 0 P=0.12
|
|
|
Scrascia, 2012 |
Prospective randomized controlled trial Policlinico University Hospital of Bari, Italy
Source of funding: No specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflicts of interest: None declared. |
Inclusion criteria: First-time, elective isolated CABG patients.
Exclusion criteria: Age > 80 years, pre-operative hemoglobin <12 g/dL, body surface area <1.7m2, redo or emergency surgery, valvular, thoracic aorta or combined procedures, liver insufficiency, platelet count below 50.000 or antiplatelet treatment taken within 5 days before surgery, pre-existing hemolytic or hemostatic disorders, anticoagulant treatment, inflammatory disorders or steroids treatment.
N total at baseline: Intervention: 17 Control: 17
Important prognostic factors2: Age ± SD: Intervention: 71 ± 8 Control: 66 ± 10
Sex (male (%)): Intervention: 8 (47) Control: 13 (76)
Previous myocardial infarction (%): Control: 4 (24)
Ejection Fraction (%): Intervention: 45 ± 12 Control: 50 ± 13
Groups were comparable at baseline.
|
Intervention: A cell saving system was used to collect residual blood remaining inside the CPB circuit at the end of surgery. Blood was salvaged using a double-lumen suction tube flushed with heparinized 0.9% normal saline, using the Hemonetics Cell Saver. The salvaged blood was washed and centrifuged and then transferred to the patient. During CPB, blood was suctioned by cardiotomy suckers and returned to the venous reservoir without processing. |
Control: No cell salvage was used. CPB blood was not transfused to the patients. |
Length of follow-up: Five days after surgery.
Loss-to-follow-up: Not reported. No loss-to-follow-up.
Incomplete outcome data: No incomplete outcome data. |
Intraoperative RBC (units): Intervention: 12 Control: 9 P=NS
Intraoperative Plt (units): Intervention: 0 Control: 0
Intraoperative plasma (units): Intervention: 1 Control: 0 P=NS
Patients receiving intra-op RBC: Intervention: 7 (41) Control: 7 (41) P=NS
Patients receiving intra-op plasma: Intervention: 1 (6) Control: 0 P=NS
Overall length of stay (days): Intervention: 13 ± 3 Control: 11 ± 4 P=NS
ICU length of stay (hours): Intervention: 33 ± 18 Control: 33 ± 15 P=NS
Total chest tube drainage (mL): Intervention: 749 ± 320 Control: 592 ± 264 P=NS
Post-op RBC transfusion (units): Intervention: 11 Control: 5 P= NS
Post-op Plt transfusion (units): Intervention: 0 Control: 0
Post-op plasma transfusion (units): Intervention: 9 Control: 0 P=NS
Patients receiving post-op RBC: Intervention: 6 (35) Control: 5 (29) P=NS
Patients receiving post-op plasma: Intervention: 5 (29) Control: 0 P=0.04
Atrial fibrillation: Intervention: 5 (29) Control: 7 (41) P=NS
Cerebrovascular accident: Intervention: 1 (6) Control: 0 P=NS Intervention: 1 (6) Control: 1 (6) P=NS
Hemoglobin concentration Tx - intraoperative (g/dL): Intervention: 8.0 ± 0.9 Control: 9.1 ± 1.5 P=0.020
Hemoglobin concentration T0 – 2 hours post CPB (g/dL): Intervention: 10.7 ± 1.3 Control: 10.2 ± 1.6 P=NS
Hemoglobin concentration T1 – 24h post CPB (g/dL): Intervention: 10.7 ± 1 Control: 10.8 ± 1.3 P=NS |
|
|
Aghdaii, 2012 |
Randomized clinical trial
Setting and country: Shaheed Rajaei Cardiovascular, Medical and Research Center, Tehran University of Medical Sciences, Tehran, Iran.
Source of funding: Not reported.
Conflicts of interest: Not reported. |
Inclusion criteria: Primary, elective, on-pump CABG surgery, age between 30 and 70 years, left ventricular ejection fraction ≥ 45%, pump time < 2 hours, and aortic clumping time <45 minutes.
Exclusion criteria: Known coagulation disorders, redo or emergency surgery, patients on Warfarin, heparin, or other systemic anticoagulant drugs and antiplatelet drugs such as Aspirin preoperatively, and co-existing diseases (renal and hepatic disease, diabetes mellitus, hypertension, and endocrine and hematology disorders).
N total at baseline: Intervention: 25 Control: 25
Important prognostic factors2: Age ± SD: Intervention: 55 ± 14 Control: 58 ± 5.4
Sex (male/female): Intervention: 17/8 Control: 16/9
LVEF (%): Intervention: 48 ± 4.5 Control: 48 ± 4.8
Groups were comparable at baseline. |
Intervention: Intraoperative cell salvage of shed blood. The blood aspired from the wound area and the operative field as well as the blood within the CPB circuit and the residual blood from the heart-lung machine was collected in the cell saver reservoir. It was washed and concentrated with a continuous-flow cell saver before transfusion. |
Control: No cell salvage system was used. Patients only receive homologous blood. |
Length of follow-up: 24 hours post-surgery.
Loss-to-follow-up: Not reported. No loss to follow-up.
Incomplete outcome data: Not reported. No incomplete outcome data.
|
Postoperative blood loss (mL); First 8 hours post-op: Intervention: 210 ± 103 Control: 242 ± 184 P=0.463
Second 8 hours post-op: Intervention: 112 ± 76 Control: 156 ± 127 P=0.144
Third 8 hours post-op: Intervention: 131 ± 79 Control: 105 ± 105 P=0.333
Total 24 hours post-op: Intervention: 454 ± 150 Control: 510 ± 270 P=0.362
Requirement to packed RBCs transfusion (mL): Intervention: 80 ± 160 Control: 460 ± 200 P=0.0001
Mean volume of blood transfusion (mL): Intervention: 504 ± 158 Control: 338 ± 123 P=0.0001
Packed cell units transfused: Intervention: 11 Control: 16
Patients that required packed RBCs postoperatively: Intervention: 7/25 Control: 8/25
Requirement for blood products during first 24 hours post-surgery (units): Intervention: 0.4 ± 0.8 (total 11) Control: 0.7 ± 1 (total 16)
Mortality (until discharge): Intervention: 0 Control: 0 |
|
|
Prieto, 2013 |
Randomized controlled trial
Setting and country: Hospital Universitario La Princesa, Madrid, Spain.
Source of funding: No specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Conflicts of interest: None declared. |
Inclusion criteria: Low risk, undergoing cardiac surgery with the use of CBP.
Exclusion criteria: Age over 80 years, redo or emergency surgery, aortic or pericardial disease, endocarditis, need for triple-valve surgery, refusal of blood products, logistic EuroSCORE >10%, and a high risk of bleeding if 2 or more of the following conditions were met: pre-operative creatinine >2.2 mg/mL, liver insufficiency, severe lung disease, body surface area <1.6m2, preoperative hemoglobin <13 g/dL in males or <12 g/dL in females, platelet count <50.000/mL or any platelet disorder, coagulation disorder, intake of aspirin 3 days before surgery or clopidogrel 7 days before surgery.
N total at baseline: Intervention: 29 Control: 28
Important prognostic factors2: Age ± SD: Intervention: 65.5 ± 12.1 Control: 63.3 ± 12.8
Sex (female (%)): Intervention: 8 (27.6) Control: 10 (35.7)
Stroke (%): Intervention: 1 (3.4)
Ejection fraction <50%: Intervention: 5 (17.9) Control: 2 (7.1)
Logistic EuroSCORE: Intervention: 4.5% ± 2.8% Control: 4.3% ± 3%
Groups were comparable at baseline. |
Intervention: The use of a cell saver during cardiac surgery. All remaining blood inside the circuit was recovered and concentrated and transfused to the patients. Cardiotomy suction was applied and this blood was reinfused during extracorporeal circulation. |
Control: No use of a cell saver. Only cardiotomy suction was used to aspirate all blood in the surgical field. |
Length of follow-up: 30 days after the procedure.
Loss-to-follow-up: Not reported. No loss to follow-up.
Incomplete outcome data: Not reported. No incomplete outcome data.
|
24h postoperative bleeding (mL): Intervention: 545 ± 449 Control: 372 ± 146 P=0.06
Intensive care unit stay (days): Intervention: 4.1 ± 7.3 Control: 2.2 ± 3 P=0.21
Hospital stay (days): Intervention: 15.2 ± 15 Control: 22.5 ± 53.6 P=0.49
Postoperative atrial fibrillation: Intervention: 4 (13.3%) Control: 3 (10.7%) P=1
Renal failure: Intervention: 2 (6.9%) Control: 0 P=0.49
Neurological complications: Intervention: 0 Control: 2 (7.1%) P=0.30
Mortality: Intervention: 0 Control: 0 |
|
|
Vermeijden, 2015 |
Randomized multi-center trial
Setting and country: Two academic and four non-academic centers, The Netherlands.
Source of funding:
Conflicts of interest: Not reported. |
Inclusion criteria: Adult patients, scheduled for elective coronary artery bypass grafting, valve surgery, or a combined procedure, scheduled in the morning.
Exclusion criteria: Scheduled for off-pump surgery, known coagulation disorders (except after the use of aspirin, clopidogrel, or low-molecular-weight heparin).
N total at baseline: Intervention I: 192 Intervention II: 180 Intervention III: 182 Control: 184
Important prognostic factors2: Age ± SD: Intervention I: 66 ± 9.5 Intervention II: 65 ± 9.7 Intervention III: 66 ± 10.5 Control: 66 ± 9.7
Sex (male, %): Intervention I: 134 (71) Intervention II: 140 (80) Intervention III: 132 (75) Control: 127 (71)
EuroSCORE: Intervention I: 4.2 ± 3.0 Intervention II: 4.3 ± 3.0 Intervention III: 4.7 ± 3.3 Control: 4.7 ± 3.4
Myocardial infarction (%): Intervention I: 23 Intervention II: 21 Intervention III: 28 Control: 27
Stroke (%): Intervention I: 4 Intervention II: 6 Intervention III: 7 Control: 6
Hemoglobin (mmol/L): Intervention I: 7.6 ± 0.9 Intervention II: 7.6 ± 0.9 Intervention III: 7.6 ± 0.9 Control: 7.5 ± 0.9
Surgery type (CABG/valve/CABG+valve): Intervention I: 116/54/19 Intervention II: 106/44/25 Intervention III: 110/33/32 Control: 115/37/25
It was not reported whether groups were comparable at baseline. |
Intervention I: Cardiotomy suction blood, blood from the surgical field, and residual heart lung machine blood was collected. This blood was washed with a cell saver, and retransfused through a standard transfusion set.
Intervention II: Cardiotomy suction blood, blood from the surgical field, and residual heart lung machine blood was collected. This blood was washed with a cell saver, and retransfused through an LD filter.
Intervention III: Cardiotomy suction blood, blood from the surgical field, and residual heart lung machine blood was collected. This blood was retransfused through an LD filter. |
Control: Cardiotomy suction blood and blood from the surgical field was discarded after reversal of heparin. Heart lung machine blood was retransfused through a standard transfusion set. |
Length of follow-up: Not reported.
Loss-to-follow-up: Intervention I: 3 Reason: Consent withdrawn, CPR, request surgeon.
Intervention II: 5 Reason: Consent withdrawn, off-pump surgery, tumour found, protocol violation, request surgeon.
Intervention III: 7 Reason: Consent withdrawn, off-pump surgery (2), protocol violation (3), exceptional blood loss.
Control: 7 Reason: Consent withdrawn, off-pump surgery (3), protocol violation, tumour found, aortic surgery.
Incomplete outcome data: Intervention III: 3 Reason: Transfusion data not available.
Control: 3 Reason: Transfusion data not available.
|
Blood collected (mL): Intervention I: 1310 ± 1186 Intervention II: 1537 ± 1541 Intervention III: 1463 ± 971 Control: NA
Total units RBC in first 24h: Intervention I: 205 Intervention II: 186 Intervention III: 255 Control: 244 RR 0.75 (95% CI 0.61 to 0.92) favoring cell saver. RR 1.02 (95% CI 0.83 to 1.25) favoring no filter.
Total units RBC during hospital admission: Intervention I: 358 Intervention II: 355 Intervention III: 429 Control: 357 RR 0.86 (95% CI 0.71 to 1.04) favoring cell saver. RR 1.13 (95% CI 0.93 to 1.38) favoring no filter.
Patients transfused RBC in first 24h (n, %): Intervention I: 76 (40) Intervention II: 61 (35) Intervention III: 90 (52) Control: 86 (49) RR 0.57 (95% CI 0.42 to 0.78) favoring cell saver. RR 0.95 (95% CI 0.70 to 1.29) favoring filter.
Patients transfused RBC during hospital admission (n, %): Intervention I: 94 (50) Intervention II: 79 (45) Intervention III: 103 (59) Control: 104 (59) RR 0.58 (95% CI 0.42 to 0.79) favoring cell saver. RR 0.92 (95% CI 0.67 to 1.25) favoring filter.
Total units plasma: Intervention I: 97 Intervention II: 109 Intervention III: 78 Control: 64 RR 1.39 (95% CI 1.04 to 1.85) favoring no cell saver. RR 1.22 (95% CI 0.91 to 1.62) favoring no filter.
Patients transfused with plasma (n, %): Intervention I: 30 (16) Intervention II: 30 (17) Intervention III: 24 (14) Control: 29 (16) RR 1.12 (95% CI 0.74 to 1.70) favoring no cell saver. RR 0.94 (95% CI 0.62 to 1.43) favoring filter.
Total units platelets: Intervention I: 32 Intervention II: 51 Intervention III: 32 Control: 30 RR 1.24 (95% CI 0.92 to 1.67) favoring no cell saver. RR 1.33 (95% CI 0.99 to 1.79) favoring no filter.
Patients transfused with platelets (n, %): Intervention I: 25 (13) Intervention II: 33 (19) Intervention III: 24 (14) Control: 22 (13) RR 1.25 (95% CI 0.82 to 1.91) favoring no cell saver. RR 1.30 (95% CI 0.85 to 1.99) favoring no filter.
Total units RBC, plasma, and platelets: Intervention I: 487 Intervention II: 515 Intervention III: 539 Control: 451 RR 0.96 (95% CI 0.78 to 1.17) favoring cell saver. RR 1.16 (95% CI 0.96 to 1.42) favoring no filter.
Patients transfused with any RBC, plasma, and platelets (n, %): Intervention I: 98 (52) Intervention II: 83 (47) Intervention III: 103 (59) Control: 108 (61) RR 0.67 (95% CI 0.49 to 0.91) favoring cell saver. RR 0.91 (95% CI 0.67 to 1.24) favoring filter.
12h blood loss chest tube (mL): Intervention I: 728 ± 726 Intervention II: 646 ± 487 Intervention III: 772 ± 597 Control: 670 ± 444 RR 0.90 (95% CI 0.82 to 0.98) favoring cell saver. RR 1.02 (95% CI 0.92 to 1.13) favoring no filter.
Hemoglobin day 1 (mmol/L): Intervention I: 6.6 ± 0.9 Intervention II: 6.6 ± 0.8 Intervention III: 6.3 ± 0.8 Control: 6.1 ± 0.7 RR 0.38 (95% CI 0.26 to 0.49) favoring cell saver. RR 0.05 (95% CI -0.06 to 0.17) favoring filter.
Reexploration, n (%): Intervention I: 15 (8) Intervention II: 14 (8) Intervention III: 17 (10) Control: 12 (7) RR 1.00 (95% CI 0.56 to 1.80) RR 1.17 (95% CI 0.65 to 2.11) favoring no filter.
Myocardial infarction, n (%): Intervention I: 7 (4) Intervention II: 1 (1) Intervention III: 5 (3) Control: 5 (3) RR 0.50 (95% CI 0.14 to 1.73) favoring cell saver. RR 0.38 (95% CI 0.11 to 1.32) favoring filter.
Stroke, n (%): Intervention I: 1 (1) Intervention II: 5 (3) Intervention III: 7 (4) Control: 5 (3) RR 0.36 (95% CI 0.10 to 1.22) favoring cell saver. RR 2.82 (95% CI 0.82 to 9.62) favoring no filter.
Length of stay intensive care unit (days): Intervention I: 1.9 ± 5.6 Intervention II: 1.7 ± 2.4 Intervention III: 2.4 ± 4.7 Control: 1.5 ± 1.7 RR 0.95 (95% CI 0.79 to 1.12) favoring cell saver. RR 1.18 (95% CI 0.99 to 1.39) favoring no filter.
Length of stay hospital (days): Intervention I: 11.5 ± 10.5 Intervention II: 10.3 ± 7.8 Intervention III: 12.7 ± 15.0 Control: 11.8 ± 9.6 RR 0.89 (95% CI 0.80 to 0.89) favoring cell saver. RR 0.98 (95% CI 0.87 to 1.08) favoring filter.
One-year mortality, n (%): Intervention I: 1 (1) Intervention II: 6 (3) Intervention III: 8 (5) Control: 5 (3) RR 0.36 (95% CI 0.11 to 1.23) favoring cell saver. RR 3.32 (95% CI 0.99 to 11.0) favoring no filter. |
|
|
Xie, 2015 |
Prospective, randomized, controlled clinical trial
Setting and country: Zhejiang Provincial People’s Hospital, Hangzhou, Zhejiang, China
Source of funding: The study was funded by the Natural Science Foundation of Zhejiang Province.
Conflicts of interest: No competing interests exist. |
Inclusion criteria: Provision of written consent, scheduled for cardiac surgery with CPB, surgery combined with aortic valve replacement and mitral valve replacement, or Bentall (aortic valve replacement and ascending aorta and aortic root replacement), or reoperation (single or multiple valve replacement), meet at least two of the following conditions: - Age > 70 years - Body surface area < 1.6m2 - Renal dysfunction - Liver insufficiency - Coagulation disorders - Hemoglobin levels < 130 g/L in males or <120 g/L in females - Platelet count <50x109/L - Intake of aspirin 3 days before surgery or clopidogrel 7 days before surgery
Exclusion criteria: Not stated.
N total at baseline: Intervention: 75 Control: 75
Important prognostic factors2: Age ± SD: Intervention: 51.7 ± 15.6 Control: 53.1 ± 15.1
Sex (male, %): Intervention: 35 (48.6) Control: 29 (42.0)
Surgery type (multiple valve/Bentall/reoperation): Intervention: 47/10/15 Control: 46/8/15
Groups were comparable at baseline. |
Intervention: Shed blood during the period of non-heparinization was sucked into the cell saver reservoir. Residual blood in the CPB circuit was sucked into the cell saver reservoir. After filtration, centrifugation, washing, and concentration the blood was retransfused to the patients. |
Control: Shed blood during the period of non-heparinization and residual blood were sucked into suction apparatus and were discarded. |
Length of follow-up: Not reported.
Loss-to-follow-up: Intervention: 3 Reason: Did not receive allocated intervention due to equipment failure (2), died within 24 hours post-surgery (1).
Control: 6 Reason: Received CS after surgical request due to heavy bleeding (4), died within 24 hours post-surgery (2).
|
ICU stay (hours): Intervention: 20.5 ± 5.7 Control: 21.8 ± 4.7 P=0.253
Hospital stay (days): Intervention: 23.1 ± 7.8 Control: 25.1 ± 9.1 P=0.263
Intraoperative blood loss (mL): Intervention: 1425.6 ± 162.4 Control: 1347.5 ± 179.8 P=0.105
Perioperative allogeneic blood transfusion RBC (units): Intervention: 2.01 ± 2.75 Control: 5.39 ± 3.28 P<0.0001
Perioperative allogeneic blood transfusion plasma (mL): Intervention: 113.9 ± 202.7 Control: 133.3 ± 220.6 P=0.725
Perioperative allogeneic blood transfusion PLT (units): Intervention: 1.97 ± 3.57 Control: 1.91 ± 3.42 P=0.879
Costs total blood transfusion (USD): Intervention: 360.5 ± 140.8 Control: 194.4 ± 152.4 P=0.001
Costs total hospital (USD): Intervention: 16725.3 ± 2271.7 Control: 16142.2 ± 2572.3 P=0.211
Costs allogeneic blood transfusion (USD): - RBC: Intervention: 45.7 ± 54.3 Control: 122.7 ± 65.6 P<0.001 - Plasma: Intervention: 14.8 ± 37.5 Control: 17.3 ± 32.4 P=0.825 - PLT: Intervention: 56.1 ± 51.5 Control: 54.4 ± 42.7 P=0.978 |
Outcome measure cost-effectivity is studied:
Cases: 72 Price of allogeneic RBC: 22.8 USD/unit. Quantity of autologous RBC transfusion: 4.09 units. Price of autologous blood transfusion: 243.9 USD Cost of reduced (USD): -150.6 Cost-effectiveness: No
|
|
Shen, 2016 |
Prospective randomized controlled trial
Setting and country: Zhejiang Provincial People’s Hospital, Hangzhou, Zhejiang, China.
Source of funding: The study was funded by the Natural Science Foundation of Zhejiang Province.
Conflicts of interest: No competing interests exist. |
Inclusion criteria: Scheduled for cardiac surgery with CPB, multiple valves replacement or Bentall, or reoperation, meet at least two of the following conditions: age >70 years, BSA <1.6m2, Cr >15 mg/L, liver Child-Pugh B or C, R (TEG) >10 min, HB <13 g/dL (male) or <12 g/dL (female), PLT <50x109/L, and drug withdrawal <3 days (aspirin) or <7 days (clopidogrel).
Exclusion criteria: Emergency cardiac surgery with CPB, first time single valve replacement.
N total at baseline: Intervention: 55 Control: 55
Important prognostic factors2: Age ± SD: Intervention: 50.42 ± 15.43 Control: 52.53 ± 15.65
Sex (male %): Intervention: 27 (50.94) Control: 24 (48.00)
Surgery type (multiple valve/Bentall/reoperation): Intervention: 36/8/9 Control: 33/7/10
HB levels lower than 13 mg/dL: Intervention: 21 (39.62) Control: 23 (46.00)
HB levels lower than 12 mg/dL: Intervention: 17 (32.08) Control: 18 (36.00)
Groups were comparable at baseline. |
Intervention: Shed blood during the period of non-heparinization was sucked into the cell saver. Residual blood in the CPB circuit was sucked into the cell saver reservoir. After filtration, centrifugation, washing, and concentration the blood was retransfused to the patients. |
Control: Shed blood and residual blood were sucked into suction apparatus and were discarded. |
Length of follow-up: Not reported.
Loss-to-follow-up: Intervention: 2 Reason: Did not receive allocated intervention due to equipment failure (1), died within 24 hours post-surgery (1).
Control: 5 Reason: Received CS after surgical request due to heavy bleeding (4), died within 24 hours post-surgery (1).
|
Length of ICU stay (hours): Intervention: 19.42 ± 4.43 Control: 20.42 ± 4.16 P=0.099
Length of hospital stay (days): Intervention: 22.56 ± 4.23 Control: 23.38 ± 4.54 P=0.206
Intraoperative blood loss (mL): Intervention: 1640.96 ± 87.73 Control: 1609.03 ± 137.91 P=0.072
Perioperative allogeneic blood transfusion RBC (units): Intervention: 2.11 ± 2.66 Control: 5.40 ± 3.48 P<0.0001
Perioperative allogeneic blood transfusion plasma (mL): Intervention: 118.76 ± 253.82 Control: 129.00 ± 284.92 P=0.953
Perioperative allogeneic blood transfusion PLT (units): Intervention: 1.81 ± 3.56 Control: 1.92 ± 3.94 P=0.916
Excessive bleeding Intervention: 17 (32.08) Control: 8 (16.00) RR 4.58 (95% CI 1.02 to 18.34), favoring no cell saver.
Myocardial infarction Intervention: 1 (1.89) Control: 4 (8.00) RR 0.15 (95% CI 0.01 to 1.99), favoring cell saver.
Cognitive decline Intervention: 0 Control: 3 (6.00) RR <0.001 (95% CI <0.001 to 999.99), favoring cell saver.
Cardiovascular failure Intervention: 6 (11.32) Control: 7 (14.00) P=0.682
Infection Intervention: 7 (13.21) Control: 6 (12.00) P=0.948
Renal failure Intervention: 5 (9.43) Control: 4 (8.00) P=1.000
Mortality Intervention: 0 Control: 0 |
|
|
Bauer, 2017 |
Prospective, randomized, controlled study
Setting and country: MediClin Heart Center Coswig, Coswig, Saxony-Anhalt, Germany.
Source of funding: Financed by third-party fund MECC-2016, granted both by the MediClin Heart Center Coswig and Maquet Cardiopulmonary AG.
Conflicts of interest: No potential conflicts of interest with regard to the research, authorship, and publication. |
Inclusion criteria: Age between 18 and 95 years, body weight between 55 and 150 kg, and undergoing elective isolated CABG surgery.
Exclusion criteria: Redo procedures, preoperative signs of inflammation, fever and elevated values of c-reactive protein, procalcitonin or leukocytes counts, intraoperative change in planned surgical treatment (valve and aortic surgery), intraoperative use of hemofiltration, switch to open ECC system, and intraoperative use of any anti-inflammatory drugs.
N total at baseline: Intervention: 36 Control: 40
Important prognostic factors2: Age ± SD: Intervention: 67.5 ± 9.9 Control: 66.7 ± 8.8
Sex (male): Intervention: 23 Control: 29
Previous acute myocardial infarction: Intervention: 7 (23.6%) Control: 5 (13.89%)
Hemoglobin concentration pre-operative (g/dL): Intervention: 8.78 ± 0.83 Control: 8.73 ± 1.02
Groups were comparable at baseline, apart from diabetes. |
Intervention: Shed blood separation and cell saver use before retransfusion. The suction blood was retransfused or washed between cross-clamping. Suction blood was separated and cell salvage was performed before the blood was retransfused. |
Control: The suction blood was separated and directly retransfused into the circuit without any treatment. |
Length of follow-up: Not reported.
Loss-to-follow-up: Intervention: 6 (7.9%)
Reason: Did not receive allocated intervention (change of indication) (n=3), abstained from written informed consent (n=1), intraoperative change to valve surgery (n=1), hemofiltration (n=1).
Control: 4 (5.3%)
Reason: Did not receive allocated intervention (change of indication) (n=2), intraoperative change to valve surgery (n=2). |
Re-transfused suction blood intraoperative (mL) + aRBC: Intervention: 488.9 ± 215.9 Control: 941.1 ± 493.9 Difference: -452.2 95% CI -637.4 to -267.1 P<0.00
RBC intraoperative: Intervention: 0 Control: 0.23 ± 0.7 Difference: -0.23 95% CI -0.51 to -0.053 P=0.088
RBC perioperative: Intervention: 0.97 ± 1.8 Control: 1 ± 1.5 Difference: 0.3 95% CI 0.31 to 1.63 P=0.92
Hemoglobin concentration (g/dL): - Admission ICU: Intervention: 6.17 ± 1.07 Control: 5.71 ± 0.96 95% CI -0.39 to 0.96 P=0.07 - 6 hours post ECC: Intervention: 7.27 ± 0.96 Control: 6.66 ± 0.75 95% CI 0.18 to 1.03 P=0.007 -24 hours post ECC: Intervention: 6.37 ± 0.91 Control: 6.1 ± 0.77 95% CI -0.14 to 0.69 P=0.19 - Discharge: Intervention: 6.97 ± 0.80 Control: 6.61 ± 0.83 95% CI -0.04 to 0.78 P=0.079
Bleeding (mL): - 6 hours post-op: Intervention: 266 ± 125 Control: 425 ± 276 95% CI -270.2 to -48.2 P=0.003 -24 hours post-op: Intervention: 763.2 ± 324 Control: 888 ± 429 95% CI -292 to -66.7 P=0.12
Massive bleeding (>1% body weight): Intervention: 1 (3) Control: 3 (8) P=0.62
ICU stay time (days): Intervention: 1.37 ± 0.67 Control: 1.17 ± 0.56 95% CI -0.102 to 72.7 P=0.50
Hospital stay time (days): Intervention: 9.5 ± 2.2 Control: 9.8 ± 2.1 95% CI -1.4 to 0.73 P=0.53
Superficial sternal infection: Intervention: 11 (37) Control: 9 (25) P=0.42
Deep sternal infection: Intervention: 1 (3) Control: 3 (8) P=0.62
Any other infections (arms/legs): Intervention: 9 (30) Control: 6 (17) P=0.25
New atrial fibrillation: Intervention: 7 (23) Control: 13 (36) P=0.29
Pericardial effusion: Intervention: 2 (7) Control: 1 (3) P=0.59
Renal insufficiency: Intervention: 0 Control: 2 (6) P=0.50
Abdominal complications: Intervention: 1 (3) Control: 0 P=0.45
Neuropsychological deficits (delirium): Intervention: 3 (10) Control: 3 (8) P=1.0
Pulmonary complications (atelectasis, pneumonia): Intervention: 1 (3) Control: 1 (3) P=1.0
Mortality: Intervention: 1 (3) Control: 1 (3) P=1.0 |
|
|
Van Klarenbosch, 2020 |
Data of a randomized controlled trial
Setting and country: Six cardiac surgery centers in the Netherlands.
Source of funding: The study was funded by The Netherlands Organization for Health Research and Development (ZonMw).
Conflicts of interest: None declared. |
Inclusion criteria: Adult patients, scheduled for elective coronary artery bypass grafting (CABG), valve surgery, or a combined procedure.
Exclusion criteria: Scheduled for off-pump surgery, known coagulation disorders (except after the use of aspirin, clopidogrel, or low-molecular-weight heparin).
N total at baseline: Intervention: 364 Control: 352
Important prognostic factors2: Age ± SD: Intervention: 65 ± 9.6 Control: 66 ± 10
Sex (male %): Intervention: 276 (76) Control: 256 (73)
EuroSCORE: Intervention: 4.3 ± 3.0 Control: 4.7 ± 3.4
Previous myocardial infarction (%): Intervention: 76 (21) Control: 95 (27)
Hemoglobin (g/dL): Intervention: 12.3 ± 1.5 Control: 12.3 ± 1.5
Surgery type (CABG/valve/CABG+valve): Intervention: 222/98/44 Control: 225/70/57
Groups were comparable at baseline, except for surgery type. |
Intervention: Blood was collected from the surgical field, through cardiotomy suction, and from the heart lung machine. The blood was washed using a cell saver and retransfused to the patients. |
Control: Blood was either collected and filtered during CPB and then retransfused, or blood from the surgical field was discarded before and after heparinization (using cardiotomy suction), and heart-lung machine blood was retransfused without processing. |
Length of follow-up: Not reported.
Loss-to-follow-up: Not applicable.
Incomplete outcome data: Not applicable.
|
Postoperative infections -Lung: Intervention: 30 (8.3) Control: 23 (6.6) -Saphenous vein wound: Intervention: 8 (2.2) Control: 8 (2.3) -Urinary: Intervention: 13 (3.6) Control: 9 (2.6) -Lung and wound: Intervention: 4 (1.1) Control: 4 (1.1) OR 2.291 (95% CI 1.177 to 4.460), favoring no cell saver.
Residual CPB blood (mL): Intervention: 795 ± 575 P=0.028
12-h blood loss (mL): Intervention: 688 ± 623 Control: 721 ± 528 P=0.451
RBC (units): Control: 2.3 ± 3.0 P=0.246 OR 0.275 (95% CI 0.176 to 0.432), favoring cell saver.
Plasma (units): Intervention: 0.6 ± 1.5 P=0.110
Platelets (units): Intervention: 0.2 ± 0.6 Control: 0.2 ± 0.5 P=0.243
Intensive care unit stay (days): Intervention: 1.8 ± 4.3 Control: 1.9 ± 3.5 P=0.664
Hospital stay (days): Intervention: 10.9 ± 9.3 Control: 12.2 ± 12.5 P=0.121 |
The study uses data of a previously performed randomized controlled trial. |
|
Gorki, 2020 |
Data from a prospective randomized trial
Setting and country: Universitätsklinik, Ulm, Germany.
Source of funding: Not reported.
Conflicts of interest: None declared. |
Inclusion criteria: First-time operation of isolated coronary disease with at least three target vessels, left ventricular ejection fraction >40%, age between 18 and 85 years.
Exclusion criteria: Emergency and coagulation disorders, increased bleeding risk or findings precluding one of the three investigated surgical techniques (porcelain aorta, diffuse calcified target vessels).
N total at baseline: Intervention: 24 Control: 24
Important prognostic factors2: Age (95% CI): Intervention: 68.8 (62.8 to 72.9) Control:
Gender (male): Intervention: 20 Control: 22
EuroSCORE II: Intervention: 1.28 (0.89 to 1.68) Control: 1.37 (0.92 to 1.78)
Groups were comparable at baseline, apart from one patient, who was operated by different surgeon. |
Intervention: Separate collection of shed pericardial fluids and (if exceeding 500 mL) retransfusion after the use of a cell saver. |
Control: The use of pericardiotomy suction to recirculate pericardial blood. |
Length of follow-up: Not reported, but at least 30 days.
Loss-to-follow-up: Not reported.
Incomplete outcome data: Not reported. |
Major adverse cardiac and cerebrovascular events: Intervention: 0 Control: 0 P=1
Mortality: Intervention: 0 Control: 0 P=1
Drainage loss 12 h (mL): Intervention: 330 (95% CI 260 to 415) Control: 285 (95% CI 250 to 415) P=0.437
Drainage loss 24 h (mL): Intervention: 570 (95% CI 415 to 840) Control: 605 (95% CI 495 to 720) P=0.6395 |
The study uses data of a previously performed randomized controlled trial.
All outcome measures: per protocol analysis. |
|
Tachias, 2022 |
Prospective randomized controlled study
Setting and country: University Hospital of Ioannina, Ioannina, Greece.
Source of funding: Not reported.
Conflicts of interest: None declared. |
Inclusion criteria: Written informed consent, age > 18 years, and cardiac surgery (coronary bypass surgery, valve surgery, aortic replacement surgery, or mixed surgery) with extracorporeal circulation lasting > 90 minutes.
Exclusion criteria: Failure of in-time anticoagulant medication discontinuation, emergency cases, and surgery without at least 90 minutes of ECC minutes (on- or off- pump surgery, pericardial effusion drainage, pacemaker manipulations).
N total at baseline: Intervention: 104 Control: 105
Important prognostic factors2: Age ± SD: Intervention: 66.28 ± 10 Control: 67.06 ± 10
Sex (male %): Intervention: 74 (76) Control: 87 (79)
EuroSCORE II: Intervention: 2.45 ± 2.3 Control: 2.12 ± 1.6
Surgery type (CABG/non-CABG/two operations/three operations/thoracic aorta operation (%)): Intervention: 61.62/25.26/10.00/1.01/6.06 Control: 63.63/29.09/7.27/0.0/2.73
Groups were comparable at baseline. |
Intervention: Intraoperative cell salvaging. The cell salvage reservoir collected lost blood from the moment of pericardiotomy to the ECC, and after ECC to the end of surgery. Blood from the surgical field was returned into the ECC reservoir during the surgery. The blood was retransfused to the patients. |
Control: Allogeneic red cell transfusions according to the center’s transfusion policy. |
Length of follow-up: Not reported.
Loss-to-follow-up: Dropout before randomization: 17 (7.5%) Reasons: actual duration of ECC was less than 90 minutes (intervention 5, control 5), last-minute decision to perform off-pump surgery (intervention 3, control 2), failure of sufficient postoperative data collection (intervention 1, control 1).
Five patients of the intervention group did not receive the collected blood due to delays in preparing the centrifuged product. These 5 patients were transferred to the control group.
|
RBC units transfused: Intervention: 2.6 ± 2.3 P>0.05
Plasma units transfused: Intervention: 1.0 ± 1.9 Control: 0.9 ± 1.7 P>0.05
PLT units transfused: Intervention: 2.7 ± 3.9 Control: 2.0 ± 2.9 P>0.05 Hemoglobin concentration at 24 hours (g/dL): Intervention: 10.6 ± 1.1 Control: 10.1 ± 1.7 P<0.05
|
|
Risk of Bias (SR):
|
Study
First author, year |
Appropriate and clearly focused question?1
Yes/no/unclear |
Comprehensive and systematic literature search?2
Yes/no/unclear |
Description of included and excluded studies?3
Yes/no/unclear |
Description of relevant characteristics of included studies?4
Yes/no/unclear |
Appropriate adjustment for potential confounders in observational studies?5
Yes/no/unclear/notapplicable |
Assessment of scientific quality of included studies?6
Yes/no/unclear |
Enough similarities between studies to make combining them reasonable?7
Yes/no/unclear |
Potential risk of publication bias taken into account?8
Yes/no/unclear |
Potential conflicts of interest reported?9
Yes/no/unclear |
|
Wang, 2009 |
Yes
Reason: The research question of the review and the research question of the guideline module are similar. |
Yes
Reason: Different data sources were researched (see evidence table). |
No
Reason: References and reasons for study exclusion at final selection were not reported |
Yes
Reason: Table with characteristics of included studies was included in SR. |
Not applicable
Reason: SR of 31 RCTs. |
Yes
Reason: Quality of individual studies was assessed using Jadad scores. |
Yes
Reason: Age and gender was comparable at baseline. Pooling of data possible for most outcome measures. Heterogeneity was tested using I2.
Subgroup analyses were performed. |
Yes
Reason: Funnel plots were visually inspected but not included in the SR. Tests for detecting publication bias were generally underpowered, especially for secondary outcomes. |
No
Reason: Sources of support and potential conflicts of interest were not reported for both the SR and the included studies. |
Risk of Bias (RCTs):
|
Study reference
(first author, publication year) |
Was the allocation sequence adequately generated?
Definitely yes Probably yes Probably no Definitely no |
Was the allocation adequately concealed?
Definitely yes Probably yes Probably no Definitely no |
Blinding: Was knowledge of the allocated interventions adequately prevented?
Were patients blinded?
Were healthcare providers blinded?
Were data collectors blinded?
Were outcome assessors blinded?
Were data analysts blinded? Definitely yes Probably yes Probably no Definitely no |
Was loss to follow-up (missing outcome data) infrequent?
Definitely yes Probably yes Probably no Definitely no |
Are reports of the study free of selective outcome reporting?
Definitely yes Probably yes Probably no Definitely no |
Was the study apparently free of other problems that could put it at a risk of bias?
Definitely yes Probably yes Probably no Definitely no |
Overall risk of bias If applicable/necessary, per outcome measure
LOW Some concerns HIGH
|
|
Gu, 2008 |
Definitely yes
Reason: Randomized according to a computer generated table. |
No information
Reason: Not reported. |
No information
Reason: Not reported. |
Probably yes
Reason: Not reported, but allocation of 40 patients and analysis performed for 40 patients. |
Probably yes
Reason: All outcomes in abstract reported in results. |
Definitely no
Reason: The study was underpowered. |
HIGH
Due to missing information about allocation concealment and blinding and the study being underpowered. |
|
Damgaard, 2010 |
No information
Reason: Not reported. |
No information
Reason: Not reported. |
No information
Reason: Not reported. |
Probably yes
Reason: Exclusion of one patient before start of study. Allocation of 29 patients and analysis performed for 29 patients. |
Probably yes
Reason: All outcomes in abstract reported in results. |
Definitely no
Reason: The study was underpowered with respect to assessment of complications. The trial was based on a few patients, therefore there is a risk of type II errors and larger variance. |
HIGH
Due to missing information about allocation concealment and blinding and the study’s small sample size. |
|
Campbell, 2011 |
No information
Reason: Not reported. |
Definitely yes
Reason: Patients were randomized by sealed envelope. |
Probably yes
Reason: Blinding of healthcare providers. |
Probably yes
Reason: Not reported, but allocation of 20 patients and analysis performed for 20 patients. |
Probably yes
Reason: All outcomes in abstract reported in results. |
Definitely no
Reason: The study was a pilot study of only 10 patients per arm. |
HIGH
Due to missing information about generation of allocation sequence and the study’s small sample size. |
|
Reyes, 2011 |
No information
Reason: Not reported. |
No information
Reason: Not reported. |
No information
Reason: Not reported. |
Probably yes
Reason: Not reported, but allocation of 63 patients and analysis performed for 20 patients. |
Probably yes
Reason: All outcomes in abstract reported in results. |
Probably no
Reason: Sample size too small to evaluate clinical effect of CS. |
HIGH
Due to missing information about allocation concealment, blinding and the study’s small sample size. |
|
Scrascia, 2012 |
Definitely yes
Reason: Randomization was computer generated. |
No information
Reason: Not reported. |
Probably yes
Reason: Double-blind trial, not specified who was blinded. |
Probably yes
Reason: Not reported, but allocation of 34 patients and analysis performed for 34 patients. |
Probably yes
Reason: All outcomes in abstract reported in results. |
Probably no
Reason: Small sample size, single-centre, no clinical statements could be made. |
Some concerns
Due to missing information about allocation concealment and the study’s small sample size. |
|
Aghdaii, 2012 |
No information
Reason: Not reported. |
No information
Reason: Not reported. |
Definitely yes
Reason: all study investigators, patients, surgeons, anesthetist, and persons involved in care were blinded. |
Probably yes
Reason: Not reported, but allocation of 50 patients and analysis performed for 50 patients. |
Probably yes
Reason: All outcomes in abstract reported in results. |
Probably no:
Reason: Small sample size. |
Some concerns
Due to missing information about allocation concealment and possible additional risk of bias. |
|
Prieto, 2013 |
Probably yes
Reason: Patients were randomized using a statistics programme. |
No information
Reason: Not reported. |
No information
Reason: Not reported. |
Probably yes
Reason: Not reported, but allocation of 57 patients and analysis performed for 57 patients. |
Probably yes
Reason: All outcomes in abstract reported in results. |
Probably no
Reason: Lack of statistical power for clinical endpoints and patient sample is not representative.
|
HIGH
Due to missing information about allocation concealment and blinding, and patient sample being not representative. |
|
Vermeijden, 2015 |
Definitely yes
Reason: Use of computer-generated randomization tables. |
Definitely yes
Reason: Allocation was done with sealed, sequentially numbered envelopes. |
Probably yes
Reason: The intraoperative part could not be blinded but all other caregivers were blinded to the intervention. |
Definitely yes
Reason: 22 of 738 (3%) patients dropped out. No missing outcome data. |
Probably yes
Reason: All outcomes in abstract reported in results. |
Probably yes
Reason: Analysis based on intention-to-treat principle. |
LOW
Due to adequate allocation concealment, infrequent loss-to-follow-up, no selective outcome reporting, and no additional risk of bias. |
|
Xie, 2015 |
Definitely yes
Reason: Patients were randomly assigned into two groups according to a randomization list. |
No information |
No information
Reason: Not reported. |
Definitely yes
Reason: Loss-to-follow-up was 3 of 141 (2.1%). No missing outcome data. |
Probably yes
Reason: All outcomes in abstract reported in results. |
Probably no
Reason: Relatively small sample size, no unified evaluation standard of high-bleeding-risk cardiac surgery. |
Some concerns
Due to missing information about allocation concealment and blinding, and additional bias, such as small sample size. |
|
Shen, 2016 |
Definitely yes
Reason: Patients were randomly assigned into two groups according to a randomization list. |
No information
Reason: Not reported. |
Probably no
Reason: Study was not double blinded. |
Definitely yes
Reason: Loss-to-follow-up was 2 of 105 (1.9%). No missing outcome data. |
Probably yes
Reason: All outcomes in abstract reported in results. |
Definitely no
Reason: Not double blind, no unified evaluation standard of high-bleeding-risk cardiac surgery. |
Some concerns
Due to missing information about allocation concealment, the study not being double blind, and additional bias. |
|
Bauer, 2017 |
No information
Reason: Not reported. |
No information
Reason: Not reported. |
No information
Reason: Not reported. |
Definitely yes
Reason: Loss-to-follow-up was 10 of 76 (13%). |
Probably yes
Reason: All outcomes in abstract reported in results. |
Probably no
Reason: Study was underpowered. |
HIGH
Due to missing information about allocation concealment and blinding, frequent loss-to-follow-up, and the study being underpowered. |
|
Van Klarenbosch, 2020 |
Probably yes
Reason: Sequentially numbered envelopes were used to randomize the patients. |
Definitely yes
Reason: Sealed, envelopes were used to randomize the patients. |
No information
Reason: Not reported. |
Not applicable
Reason: Study uses data from another RCT. |
Probably yes
Reason: All outcomes in abstract reported in results. |
Probably no
Reason: Data from another RCT are used, groups were not comparable with regard to surgery type. |
Some concerns
Due to missing information about blinding and study design. |
|
Gorki, 2020 |
Definitely yes
Reason: The software system R was used to generate random allocation sequences. |
Definitely yes
Reason: Sealed and sequentially numbered envelopes were provided by the instituted and opened by the surgeon. |
No information
Reason: Not reported. |
Probably yes
Reason: Not reported, but allocation of 48 patients and analysis performed for 48 patients. |
Probably yes
Reason: All outcomes in abstract reported in results. |
Probably no
Reason: Data from another RCT are used. |
Some concerns
Due to missing information about blinding and the use of data from another RCT. |
|
Tachias, 2022 |
Probably yes
Reason: The nurse anesthetist blindly drew a closed, randomizing envelope. |
Definitely yes
Reason: The nurse anesthetist blindly drew a closed, randomizing envelope. |
Probably no
Reason: Only blinding of the nurse who drew the randomizing envelope is reported. |
Probably yes
Reason: Dropout before randomization was 7.5%. After randomization, loss-to-follow-up or missing outcome data. |
Probably yes
Reason: All outcomes in abstract reported in results. |
Probably no
Reason: Some limitations are reported, such as the presence of some differences between the groups, but not statistically significant. |
Some concerns
Due to missing information about blinding and some additional limitations. |
Table of excluded studies
|
Reference |
Reason for exclusion |
|
Al Khabori M, Al Riyami A, Siddiqi MS, Sarfaraz ZK, Ziadinov E, Al Sabti H. Impact of cell saver during cardiac surgery on blood transfusion requirements: a systematic review and meta-analysis. Vox Sang. 2019 Aug;114(6):553-565. doi: 10.1111/vox.12824. Epub 2019 Jul 5. PMID: 31273821. |
Outcome measures are limited, therefore individual studies after the search date of Wang 2009 are included. |
|
Weltert L, Nardella S, Rondinelli MB, Pierelli L, De Paulis R. Reduction of allogeneic red blood cell usage during cardiac surgery by an integrated intra- and postoperative blood salvage strategy: results of a randomized comparison. Transfusion. 2013 Apr;53(4):790-7. doi: 10.1111/j.1537-2995.2012.03836.x. Epub 2012 Aug 6. PMID: 22882381. |
Does not comply with PICO: comparison between two cell saver systems. |
|
Meybohm P, Choorapoikayil S, Wessels A, Herrmann E, Zacharowski K, Spahn DR. Washed cell salvage in surgical patients: A review and meta-analysis of prospective randomized trials under PRISMA. Medicine (Baltimore). 2016 Aug;95(31):e4490. doi: 10.1097/MD.0000000000004490. Erratum in: Medicine (Baltimore). 2018 Apr;97(17):e0640. PMID: 27495095; PMCID: PMC4979849. |
Does not comply with PICO: not only focused on patients undergoing cardiac surgery. |
|
Carless PA, Henry DA, Moxey AJ, O'Connell D, Brown T, Fergusson DA. Cell salvage for minimising perioperative allogeneic blood transfusion. Cochrane Database Syst Rev. 2010 Apr 14;2010(4):CD001888. doi: 10.1002/14651858.CD001888.pub4. PMID: 20393932; PMCID: PMC4163967. |
Does not comply with PICO: not only focused on patients undergoing cardiac surgery. |
|
Vertrees RA, Conti VR, Lick SD, Zwischenberger JB, McDaniel LB, Shulman G. Adverse effects of postoperative infusion of shed mediastinal blood. Ann Thorac Surg. 1996 Sep;62(3):717-23. doi: 10.1016/s0003-4975(96)00390-6. PMID: 8783998. |
Study performed before the year 2000. |
|
Parrot D, Lançon JP, Merle JP, Rerolle A, Bernard A, Obadia JF, Caillard B. Blood salvage in cardiac surgery. J Cardiothorac Vasc Anesth. 1991 Oct;5(5):454-6. doi: 10.1016/1053-0770(91)90119-e. PMID: 1932650. |
Study performed before the year 2000. |
|
Gäbel J, Westerberg M, Bengtsson A, Jeppsson A. Cell salvage of cardiotomy suction blood improves the balance between pro- and anti-inflammatory cytokines after cardiac surgery. Eur J Cardiothorac Surg. 2013 Sep;44(3):506-11. doi: 10.1093/ejcts/ezt019. Epub 2013 Feb 12. PMID: 23404689. |
Does not comply with PICO: no information about transfusion. |
Verantwoording
Beoordelingsdatum en geldigheid
Publicatiedatum : 23-07-2024
Beoordeeld op geldigheid : 05-07-2024
Algemene gegevens
De ontwikkeling/herziening van deze richtlijnmodule werd ondersteund door het Kennisinstituut van de Federatie Medisch Specialisten (www.demedischspecialist.nl/kennisinstituut) en werd gefinancierd uit de Kwaliteitsgelden Medisch Specialisten (SKMS). De financier heeft geen enkele invloed gehad op de inhoud van de richtlijnmodule.
Samenstelling werkgroep
Voor het ontwikkelen van de richtlijnmodule is in 2021 een multidisciplinaire werkgroep ingesteld, bestaande uit vertegenwoordigers van alle relevante specialismen (zie hiervoor de Samenstelling van de werkgroep) die betrokken zijn bij de zorg rondom perioperatief bloed- en (anti)stollingsmanagement voor patiënten die cardiochirurgie ondergaan.
Werkgroep
- Dr. M. (Maarten) ter Horst (voorzitter), NVA, anesthesioloog, Erasmus MC
- Drs. J.M.A.A. (Joost) van der Maaten, NVA, anesthesioloog, UMC Groningen
- Dr. A.B.A. (Alexander) Vonk, NVT, cardiothoracaal Chirurg, Amsterdam UMC
- Drs. E.J. (Edgar) Daeter, NVT, cardiothoracaal chirurg, St. Antonius Ziekenhuis
- Dr. R.I. (Rick) Meijer, NIV/NVIVG, internist, Radboud UMC
- Dr. J.L.H. (Jean-Louis) Kerkhoffs, NIV, internist, HagaZiekenhuis
- Dr. A.J.G. (Gerard) Jansen, NIV, internist, Erasmus MC
- Prof. dr. A.P.J. (Alexander) Vlaar, NVIC, intensivist, Amsterdam UMC
- Prof. dr. ir. Y.M.C. (Yvonne) Henskens, NVKC, laboratoriumspecialist klinische chemie, Maastricht UMC
- Dr. N.R. (Nick) Bijsterveld, NVVC, cardioloog, Amsterdam UMC
- Dr. N. (Nienke) van Rein, NVZA, ziekenhuisapotheker, Leiden UMC
- I.G.J. (Ilse) Verstraaten, MSc, beleidsmedewerker Harteraad
Klankbordgroep
- Drs. K. (Karin) Gorter, NeSECC, klinisch perfusionist, UMC Utrecht
- Drs. A.R. (Arnold) van Oostrum, NeSECC, klinisch perfusionist, OLVG
- Dr. A.J. (Sander) Spanjersberg, NVA, cardioanesthesioloog, Isala Zwolle
- Dr. P.A.W. (Peter) te Boekhorst, NIV, internist, Erasmus Medisch Centrum
Met ondersteuning van
- Dr. R. (Romy) Zwarts-van de Putte, adviseur Kennisinstituut
- Drs. E.R.L. (Evie) Verweg, junior adviseur Kennisinstituut
Belangenverklaringen
De Code ter voorkoming van oneigenlijke beïnvloeding door belangenverstrengeling is gevolgd. Alle werkgroepleden hebben schriftelijk verklaard of zij in de laatste drie jaar directe financiële belangen (betrekking bij een commercieel bedrijf, persoonlijke financiële belangen, onderzoeksfinanciering) of indirecte belangen (persoonlijke relaties, reputatiemanagement) hebben gehad. Gedurende de ontwikkeling of herziening van een module worden wijzigingen in belangen aan de voorzitter doorgegeven. De belangenverklaring wordt opnieuw bevestigd tijdens de commentaarfase.
Een overzicht van de belangen van werkgroepleden en het oordeel over het omgaan met eventuele belangen vindt u in onderstaande tabel. De ondertekende belangenverklaringen zijn op te vragen bij het secretariaat van het Kennisinstituut van de Federatie Medisch Specialisten.
|
Werkgroeplid |
Functie |
Nevenfuncties |
Gemelde belangen |
Ondernomen actie |
|
ter Horst |
Anesthesioloog (Erasmus MC) |
Bestuurslid sectie cardio-anesthesiologie niet betaald Lid van de Landelijke gebruikersraad van Sanquin namens de NVA niet betaald Lid Leidraad EHH, CCU, ICCU gecoördineerd door kennisinstituut niet betaald lid EACTAIC subcommissie TEE niet betaald |
- Presentatie over eigen onderzoek CLS-Behring over ROTEM (verkoop fibrinogeen) (betaling aan het ziekenhuis, betaling voor tijd), hebben geen invloed op wat er verteld wordt. - Verricht onderzoek naar vermindering van bloedproducten verbruik bij cardiochirurgie: recent artikel over dit onderwerp gepubliceerd. Als er intellectueel belang is dan is dit minimaal. |
Volgens de werkgroep geen restrictie nodig. De betaling van de presentatie is puur voor de tijd die erin wordt gestoken, de financier heeft geen invloed op de inhoud van de presentatie. |
|
van der Maaten |
Anesthesioloog (UMCG) |
Lid RvT Nederlandse Hart Registratie (NHR) |
Geen |
Geen restrictie |
|
Vonk |
Cardiothoracaal Chirurg (AUMC Amsterdam) |
Geen |
Geen |
Geen restrictie |
|
Daeter |
Cardiothoracaal Chirurg (St. Antonius ziekenhuis Nieuwegein) |
Medisch manager eenheid hart antonius ziekenhuis |
Geen |
Geen restrictie |
|
Meijer |
Internist (Radboudumc) |
Geen |
Geen |
Geen restrictie |
|
Kerkhoffs |
Internist-hematoloog (HagaZiekenhuis Den Haag) |
Transfusie Specialist/senior onderzoeker, Sanquin Amsterdam, Research en transfusie consulten FTE 0.2 (onderzoek over bloedplaatjes, verder geen directe link met richtlijn). |
Geen |
Geen restrictie |
|
Jansen |
Internist-hematoloog (Erasmus MC) |
Wetenschappelijk voorzitter NVB |
Research funding: Principia, Argenx, CSL Behring, Sobi, EHA Research Fellowship (PI): personal fellowship for research. - Consultancy: Novartis, Amgen. Paid |
Geen restrictie. De onderzoeken hebben geen overlap met de onderwerpen die in de richtlijn worden behandeld.
|
|
Vlaar |
Intensivist (Amsterdam- UMC) |
Lid Medische adviesraad Sanquin |
PI van PACER trial (ZonMw gefinancierd) Werfen in kind sponsoring CSL Behring studie grant en consultancy LSBR Fellowship op het gebied van TACO VIDI op gebied van TRALI |
Geen restrictie. De farma gesponsorde studies hebben geen betrekking op de modules uit de opgestelde richtlijn. |
|
Henskens |
Klinisch chemicus en waarnemend hoofd Centraal diagnostisch Laboratorium (CDL) Maastricht UMC+, Hoogleraar Klinische Chemie, in het bijzonder hemostase. 0.9 fte (CDL, MUMC+), 0.1 fte Universiteit Maastricht, FHML (faculty of Health, Medicine and Life Sciences). |
VHL Vereniging hematologische laboratoria, voorzitter (onbetaald); Landelijke en regionale gebruikersraad Sanquin (onbetaald), TVB Tijd voor verbinding antistollingszorg Expert team, namens NFU (vacatiegelden), Richtlijn antithrombotisch beleid, op afroep namens NVKC (vacatiegelden), voorzitter concilium NVKC (vacatiegelden), Lid Raad Opleidng FMS (onbetaald). |
Projectleider van de studie: Laboratory Predictors of hemostasis and thrombosis. Financier Siemens, Stago, Roche, Werfen, Nodia (korting of gratis reagentia of apparatuur te leen van bovenstaande bedrijven, zij financieren geen studies). |
Exclusie besluitvorming aanbevelingen module over POC testfacilieit aangezien het extern gefinancierde onderzoek mogelijk tot belangenverstrengeling leidt. Het werkgroeplid heeft geen band met één specifieke firma, alle testen worden onderzocht.
|
|
Bijsterveld |
Cardioloog (Amsterdam UMC) |
Voorzitter NVVC werkgroep Cardiologie en Sport (onbetaald) |
Geen |
Geen restrictie |
|
van Rein |
Ziekenhuisapotheker, Klinische Farmacie en Toxicologie, LUMC. 0.83 FTE. Inkopen en logistiek geneesmiddelen, directe patientenzorg, trials, onderwijs. Assistant progessor, Klinische Epidemiologie, LUMC, 0.17 FTE. Begeleiden promovendi, beurzen schrijven, onderwijs. |
Richtlijn antitrombotisch beleid |
Extern gefinancierd onderzoek (geen projectleider) – ZonMw GGG: Distinct onderzoek: optimaliseren tromboseprophylaxe na orthopedische ingrepen + L-TRRiP studie: optimaliseren trombosebehandeling na eerste veneuze trombose. Ook extern gefinancierd onderzoek vanuit de Trombosestichting: balans bloedingen en trombose optimaliseren dmv proteomics, wel projectleider.
Daarnaast ook verantwoordelijk voor alle geneesmiddelen studies die lopen in het LUMC en het CHDR, maar neem daar niet zelf actief aan deel. |
Geen restrictie, de trials hebben geen overlap met de onderwerpen die in de richtlijn worden behandeld. |
|
Verstraaten |
Beleidsadviseur Harteraad |
Geen |
Geen |
Geen restrictie |
|
Gorter |
Klinisch perfusionist, Heartbeat Dutch Perfusion Service, UMCU |
Geen |
Projectleider van onderzoek verschillende ACT targets tijdens CPB. Financier medtronic. |
Geen restrictie, Gorter is onderdeel van de klankbordgroep en niet actief betrokken bij het formuleren van de aanbevelingen met betrekking tot ACT targets. |
|
van Oostrum |
Klinisch Perfusionist MCL (betaald) |
Voorzitter NeSECC (onbetaald) |
Praktijkvoordeel omdat ik werkzaam ben als klinisch perfusionist, en we hier in de praktijk op de OK baat bij kunnen hebben. |
Geen restrictie |
|
Spanjersberg |
Isala, MSB |
Geen |
Voordracht fibrinogeen in hartchirurgie (sponsor CSL) |
Geen restrictie |
|
te Boekhorst |
Internist-Hematoloog / transfusiespecialist, Erasmus MC |
Voorzitter stichting TRIP |
Geen |
Geen restrictie |
Inbreng patiëntenperspectief
Er werd aandacht besteed aan het patiëntenperspectief door benoem gebruikte methode: uitnodigen van de patiëntenvereniging Harteraad voor de invitational conference en een afgevaardigde van de patiëntenvereniging Harteraad in de werkgroep. De verkregen input is meegenomen bij het opstellen van de uitgangsvragen, de keuze voor de uitkomstmaten en bij het opstellen van de overwegingen. De conceptrichtlijn is tevens voor commentaar voorgelegd aan de patiëntenvereniging Harteraad en de eventueel aangeleverde commentaren zijn bekeken en verwerkt.
Kwalitatieve raming van mogelijke financiële gevolgen in het kader van de Wkkgz
Bij de richtlijn is conform de Wet kwaliteit, klachten en geschillen zorg (Wkkgz) een kwalitatieve raming uitgevoerd of de aanbevelingen mogelijk leiden tot substantiële financiële gevolgen. Bij het uitvoeren van deze beoordeling zijn richtlijnmodules op verschillende domeinen getoetst (zie het stroomschema op de Richtlijnendatabase).
Uit de kwalitatieve raming blijkt dat er waarschijnlijk geen substantiële financiële gevolgen zijn, zie onderstaande tabel.
Module |
Uitkomst raming |
Toelichting |
|
Module Cell saver |
Geen financiële gevolgen |
Hoewel uit de toetsing volgt dat de aanbeveling(en) breed toepassbaar zijn (5.000-40.000 patiënten), volgt ook uit de toetsing dat het overgrote deel (±90%) van de zorgaanbieders en zorgverleners al aan de norm voldoet. Er worden daarom geen financiële gevolgen verwacht. |
Werkwijze
AGREE
Deze richtlijnmodule is opgesteld conform de eisen vermeld in het rapport Medisch Specialistische Richtlijnen 2.0 van de adviescommissie Richtlijnen van de Raad Kwaliteit. Dit rapport is gebaseerd op het AGREE II instrument (Appraisal of Guidelines for Research & Evaluation II; Brouwers, 2010).
Knelpuntenanalyse en uitgangsvragen
Tijdens de voorbereidende fase inventariseerde de werkgroep de knelpunten in de zorg rondom perioperatief bloed- en (anti)stollingsmanagement voor patiënten die cardiochirurgie ondergaan. Tevens zijn er knelpunten aangedragen door de Nederlandse Vereniging voor Anesthesiologie (NVA), Nederlandse Vereniging voor Cardiologie (NVvC), Nederlandse Vereniging voor Intensive Care (NVIC), Nederlandse Vereniging voor Klinische Chemie en Laboratoriumgeneeskund (NVKC), Harteraad, Inspectie Gezondheidszorg en Jeugd (IGJ), Vereniging Innovatieve Geneesmiddelen (VIG), en de Nederlandse Vereniging voor Anesthesiologiemedewerkers (NVAM) via een schriftelijke knelpuntenanalyse. Een verslag hiervan is opgenomen onder aanverwante producten.
Op basis van de uitkomsten van de knelpuntenanalyse zijn door de werkgroep concept-uitgangsvragen opgesteld en definitief vastgesteld.
Uitkomstmaten
Na het opstellen van de zoekvraag behorende bij de uitgangsvraag inventariseerde de werkgroep welke uitkomstmaten voor de patiënt relevant zijn, waarbij zowel naar gewenste als ongewenste effecten werd gekeken. Hierbij werd een maximum van acht uitkomstmaten gehanteerd. De werkgroep waardeerde deze uitkomstmaten volgens hun relatieve belang bij de besluitvorming rondom aanbevelingen, als cruciaal (kritiek voor de besluitvorming), belangrijk (maar niet cruciaal) en onbelangrijk. Tevens definieerde de werkgroep tenminste voor de cruciale uitkomstmaten welke verschillen zij klinisch (patiënt) relevant vonden.
Methode literatuursamenvatting
Een uitgebreide beschrijving van de strategie voor zoeken en selecteren van literatuur is te vinden onder ‘Zoeken en selecteren’ onder Onderbouwing. Indien mogelijk werd de data uit verschillende studies gepoold in een random-effects model. Review Manager 5.4 werd gebruikt voor de statistische analyses. De beoordeling van de kracht van het wetenschappelijke bewijs wordt hieronder toegelicht.
Beoordelen van de kracht van het wetenschappelijke bewijs
De kracht van het wetenschappelijke bewijs werd bepaald volgens de GRADE-methode. GRADE staat voor ‘Grading Recommendations Assessment, Development and Evaluation’ (zie http://www.gradeworkinggroup.org/). De basisprincipes van de GRADE-methodiek zijn: het benoemen en prioriteren van de klinisch (patiënt) relevante uitkomstmaten, een systematische review per uitkomstmaat, en een beoordeling van de bewijskracht per uitkomstmaat op basis van de acht GRADE-domeinen (domeinen voor downgraden: risk of bias, inconsistentie, indirectheid, imprecisie, en publicatiebias; domeinen voor upgraden: dosis-effect relatie, groot effect, en residuele plausibele confounding).
GRADE onderscheidt vier gradaties voor de kwaliteit van het wetenschappelijk bewijs: hoog, redelijk, laag en zeer laag. Deze gradaties verwijzen naar de mate van zekerheid die er bestaat over de literatuurconclusie, in het bijzonder de mate van zekerheid dat de literatuurconclusie de aanbeveling adequaat ondersteunt (Schünemann, 2013; Hultcrantz, 2017).
|
GRADE |
Definitie |
|
Hoog |
|
|
Redelijk |
|
|
Laag |
|
|
Zeer laag |
|
Bij het beoordelen (graderen) van de kracht van het wetenschappelijk bewijs in richtlijnen volgens de GRADE-methodiek spelen grenzen voor klinische besluitvorming een belangrijke rol (Hultcrantz, 2017). Dit zijn de grenzen die bij overschrijding aanleiding zouden geven tot een aanpassing van de aanbeveling. Om de grenzen voor klinische besluitvorming te bepalen moeten alle relevante uitkomstmaten en overwegingen worden meegewogen. De grenzen voor klinische besluitvorming zijn daarmee niet één op één vergelijkbaar met het minimaal klinisch relevant verschil (Minimal Clinically Important Difference, MCID). Met name in situaties waarin een interventie geen belangrijke nadelen heeft en de kosten relatief laag zijn, kan de grens voor klinische besluitvorming met betrekking tot de effectiviteit van de interventie bij een lagere waarde (dichter bij het nuleffect) liggen dan de MCID (Hultcrantz, 2017).
Overwegingen (van bewijs naar aanbeveling)
Om te komen tot een aanbeveling zijn naast (de kwaliteit van) het wetenschappelijke bewijs ook andere aspecten belangrijk en worden meegewogen, zoals aanvullende argumenten uit bijvoorbeeld de biomechanica of fysiologie, waarden en voorkeuren van patiënten, kosten (middelenbeslag), aanvaardbaarheid, haalbaarheid en implementatie. Deze aspecten zijn systematisch vermeld en beoordeeld (gewogen) onder het kopje ‘Overwegingen’ en kunnen (mede) gebaseerd zijn op expert opinion. Hierbij is gebruik gemaakt van een gestructureerd format gebaseerd op het evidence-to-decision framework van de internationale GRADE Working Group (Alonso-Coello, 2016a; Alonso-Coello 2016b). Dit evidence-to-decision framework is een integraal onderdeel van de GRADE methodiek.
Formuleren van aanbevelingen
De aanbevelingen geven antwoord op de uitgangsvraag en zijn gebaseerd op het beschikbare wetenschappelijke bewijs en de belangrijkste overwegingen, en een weging van de gunstige en ongunstige effecten van de relevante interventies. De kracht van het wetenschappelijk bewijs en het gewicht dat door de werkgroep wordt toegekend aan de overwegingen, bepalen samen de sterkte van de aanbeveling. Conform de GRADE-methodiek sluit een lage bewijskracht van conclusies in de systematische literatuuranalyse een sterke aanbeveling niet a priori uit, en zijn bij een hoge bewijskracht ook zwakke aanbevelingen mogelijk (Agoritsas, 2017; Neumann, 2016). De sterkte van de aanbeveling wordt altijd bepaald door weging van alle relevante argumenten tezamen. De werkgroep heeft bij elke aanbeveling opgenomen hoe zij tot de richting en sterkte van de aanbeveling zijn gekomen.
In de GRADE-methodiek wordt onderscheid gemaakt tussen sterke en zwakke (of conditionele) aanbevelingen. De sterkte van een aanbeveling verwijst naar de mate van zekerheid dat de voordelen van de interventie opwegen tegen de nadelen (of vice versa), gezien over het hele spectrum van patiënten waarvoor de aanbeveling is bedoeld. De sterkte van een aanbeveling heeft duidelijke implicaties voor patiënten, behandelaars en beleidsmakers (zie onderstaande tabel). Een aanbeveling is geen dictaat, zelfs een sterke aanbeveling gebaseerd op bewijs van hoge kwaliteit (GRADE gradering HOOG) zal niet altijd van toepassing zijn, onder alle mogelijke omstandigheden en voor elke individuele patiënt.
|
Implicaties van sterke en zwakke aanbevelingen voor verschillende richtlijngebruikers |
||
|
|
||
|
|
Sterke aanbeveling |
Zwakke (conditionele) aanbeveling |
|
Voor patiënten |
De meeste patiënten zouden de aanbevolen interventie of aanpak kiezen en slechts een klein aantal niet. |
Een aanzienlijk deel van de patiënten zouden de aanbevolen interventie of aanpak kiezen, maar veel patiënten ook niet. |
|
Voor behandelaars |
De meeste patiënten zouden de aanbevolen interventie of aanpak moeten ontvangen. |
Er zijn meerdere geschikte interventies of aanpakken. De patiënt moet worden ondersteund bij de keuze voor de interventie of aanpak die het beste aansluit bij zijn of haar waarden en voorkeuren. |
|
Voor beleidsmakers |
De aanbevolen interventie of aanpak kan worden gezien als standaardbeleid. |
Beleidsbepaling vereist uitvoerige discussie met betrokkenheid van veel stakeholders. Er is een grotere kans op lokale beleidsverschillen. |
Kennisvragen
De kennislacunes per module staan onder ‘Bijlagen’ en dan ‘Onderzoek’.
Inleiding
Tijdens de ontwikkeling van de richtlijn peri-operatief bloed en (anti)stollingsmanagement is systematisch gezocht naar onderzoeksbevindingen die nuttig konden zijn voor het beantwoorden van de uitgangsvragen. Een deel (of een onderdeel) van de hiervoor opgestelde zoekvragen is met het resultaat van deze zoekacties te beantwoorden, een groot deel echter niet. Door gebruik te maken van de evidence-based richtlijn ontwikkeling (EBRO) is duidelijk geworden dat er nog kennisvragen bestaan. De werkgroep is van mening dat (vervolg)onderzoek wenselijk is om in de toekomst een duidelijker antwoord te kunnen geven op vragen uit de praktijk.
Om deze reden heeft de werkgroep per module aangegeven op welke vlakken nader onderzoek gewenst is.
Organisatie van zorg
In de knelpuntenanalyse en bij de ontwikkeling van de richtlijnmodule is expliciet aandacht geweest voor de organisatie van zorg: alle aspecten die randvoorwaardelijk zijn voor het verlenen van zorg (zoals coördinatie, communicatie, (financiële) middelen, mankracht en infrastructuur). Randvoorwaarden die relevant zijn voor het beantwoorden van deze specifieke uitgangsvraag zijn genoemd bij de overwegingen. Meer algemene, overkoepelende, of bijkomende aspecten van de organisatie van zorg worden behandeld in de module Organisatie van zorg.
Commentaar- en autorisatiefase
De conceptrichtlijnmodule werd aan de betrokken (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd ter commentaar. De commentaren werden verzameld en besproken met de werkgroep. Naar aanleiding van de commentaren werd de conceptrichtlijnmodule aangepast en definitief vastgesteld door de werkgroep. De definitieve richtlijnmodule werd aan de deelnemende (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd voor autorisatie en door hen geautoriseerd dan wel geaccordeerd.
Literatuur
Agoritsas T, Merglen A, Heen AF, Kristiansen A, Neumann I, Brito JP, Brignardello-Petersen R, Alexander PE, Rind DM, Vandvik PO, Guyatt GH. UpToDate adherence to GRADE criteria for strong recommendations: an analytical survey. BMJ Open. 2017 Nov 16;7(11):e018593. doi: 10.1136/bmjopen-2017-018593. PubMed PMID: 29150475; PubMed Central PMCID: PMC5701989.
Alonso-Coello P, Schünemann HJ, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Rada G, Rosenbaum S, Morelli A, Guyatt GH, Oxman AD; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ. 2016 Jun 28;353:i2016. doi: 10.1136/bmj.i2016. PubMed PMID: 27353417.
Alonso-Coello P, Oxman AD, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Vandvik PO, Meerpohl J, Guyatt GH, Schünemann HJ; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 2: Clinical practice guidelines. BMJ. 2016 Jun 30;353:i2089. doi: 10.1136/bmj.i2089. PubMed PMID: 27365494.
Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, Fervers B, Graham ID, Grimshaw J, Hanna SE, Littlejohns P, Makarski J, Zitzelsberger L; AGREE Next Steps Consortium. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010 Dec 14;182(18):E839-42. doi: 10.1503/cmaj.090449. Epub 2010 Jul 5. Review. PubMed PMID: 20603348; PubMed Central PMCID: PMC3001530.
Hultcrantz M, Rind D, Akl EA, Treweek S, Mustafa RA, Iorio A, Alper BS, Meerpohl JJ, Murad MH, Ansari MT, Katikireddi SV, Östlund P, Tranæus S, Christensen R, Gartlehner G, Brozek J, Izcovich A, Schünemann H, Guyatt G. The GRADE Working Group clarifies the construct of certainty of evidence. J Clin Epidemiol. 2017 Jul;87:4-13. doi: 10.1016/j.jclinepi.2017.05.006. Epub 2017 May 18. PubMed PMID: 28529184; PubMed Central PMCID: PMC6542664.
Medisch Specialistische Richtlijnen 2.0 (2012). Adviescommissie Richtlijnen van de Raad Kwalitieit. http://richtlijnendatabase.nl/over_deze_site/over_richtlijnontwikkeling.html
Neumann I, Santesso N, Akl EA, Rind DM, Vandvik PO, Alonso-Coello P, Agoritsas T, Mustafa RA, Alexander PE, Schünemann H, Guyatt GH. A guide for health professionals to interpret and use recommendations in guidelines developed with the GRADE approach. J Clin Epidemiol. 2016 Apr;72:45-55. doi: 10.1016/j.jclinepi.2015.11.017. Epub 2016 Jan 6. Review. PubMed PMID: 26772609.
Schünemann H, Brożek J, Guyatt G, et al. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group, 2013. Available from http://gdt.guidelinedevelopment.org/central_prod/_design/client/handbook/handbook.html.
Zoekverantwoording
Zoekverantwoording
Algemene informatie
|
Richtlijn: NVT Perioperatief bloed- en (anti)stollingsmanagement voor cardiochirurgie in Nederland |
|
|
Uitgangsvraag: Wat zijn de (on)gunstige effecten van het gebruik van cell saver bij volwassen patiënten die cardiochirurgie ondergaan? |
|
|
Database(s): Ovid/Medline, Embase |
Datum: 6-10-2022 |
|
Periode: nvt |
Talen: nvt |
|
Literatuurspecialist: Ingeborg van Dusseldorp |
|
|
BMI zoekblokken: voor verschillende opdrachten wordt (deels) gebruik gemaakt van de zoekblokken van BMI-Online https://blocks.bmi-online.nl/ Bij gebruikmaking van een volledig zoekblok zal naar de betreffende link op de website worden verwezen. |
|
|
Toelichting: Voor deze vraag is gezocht met de volgende concepten: Cardiochirurgie AND cell saving AND volwassenen Alle sleutelartikelen worden gevonden
|
|
|
Te gebruiken voor richtlijnen tekst: In de databases Embase en Ovid/Medline is op 6-10-2022 met relevante zoektermen gezocht naar systematische reviews en RCTs over de effecten van het gebruik van cellsaver bij volwassen patiënten die cardiochirurgie ondergaan. De literatuurzoekactie leverde 205 unieke treffers op. |
|
Zoekopbrengst
|
|
EMBASE |
OVID/MEDLINE |
Ontdubbeld |
|
SRs |
43 |
30 |
45* |
|
RCTs |
163 |
12 |
160* |
|
Observationele studies |
334 |
245 |
422 |
|
Overig |
|
|
|
|
Totaal |
|
|
205* |
*in Rayyan
Zoekstrategie
Embase
|
No. |
Query |
Results |
|
#24 |
#15 AND #23 sleutelartikelen gevonden |
7 |
|
#23 |
#16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 sleutelartikelen |
7 |
|
#22 |
cell AND salvage AND of AND cardiotomy AND suction AND blood AND improves AND the AND balance AND between AND pro- AND 'anti inflammatory' AND cytokines AND after AND cardiac AND surgery |
1 |
|
#21 |
intraoperative AND cell AND salvage AND is AND associated AND with AND reduced AND postoperative AND blood AND loss AND transfusion AND requirements AND in AND cardiac AND surgery |
1 |
|
#20 |
effects AND of AND 'cell saving' AND devices AND filters AND on AND transfusion AND in AND cardiac AND surgery AND a AND multicenter AND randomized AND study |
1 |
|
#19 |
comparative AND evaluation AND of AND blood AND salvage AND techniques AND in AND patients AND undergoing AND cardiac AND surgery AND with AND cardiopulmonary AND bypass |
1 |
|
#18 |
does AND intraoperative AND cell AND salvage AND reduce AND postoperative AND infection AND rates AND in AND cardiac AND surgery AND klarenbosch |
1 |
|
#17 |
'cell saver' AND an AND integral AND 'shed blood separation' AND its AND influence AND on AND the AND perioperative AND inflammatory AND response AND during AND coronary AND revascularization AND with AND minimal AND invasive AND extracorporeal AND circulation AND systems |
1 |
|
#16 |
impact AND of AND cell AND saver AND during AND cardiac AND surgery AND on AND blood AND transfusion AND requirements AND a AND systematic AND review AND 'meta analysis' |
1 |
|
#15 |
#10 OR #11 OR #12 |
560 |
|
#14 |
#12 NOT #11 NOT #10 OBS |
354 |
|
#13 |
#11 NOT #10 RCT |
163 |
|
#12 |
#5 AND (#8 OR #9) |
527 |
|
#11 |
#5 AND #7 |
186 |
|
#10 |
#5 AND #6 SR |
43 |
|
#9 |
'case control study'/de OR 'comparative study'/exp OR 'control group'/de OR 'controlled study'/de OR 'controlled clinical trial'/de OR 'crossover procedure'/de OR 'double blind procedure'/de OR 'phase 2 clinical trial'/de OR 'phase 3 clinical trial'/de OR 'phase 4 clinical trial'/de OR 'pretest posttest design'/de OR 'pretest posttest control group design'/de OR 'quasi experimental study'/de OR 'single blind procedure'/de OR 'triple blind procedure'/de OR (((control OR controlled) NEAR/6 trial):ti,ab,kw) OR (((control OR controlled) NEAR/6 (study OR studies)):ti,ab,kw) OR (((control OR controlled) NEAR/1 active):ti,ab,kw) OR 'open label*':ti,ab,kw OR (((double OR two OR three OR multi OR trial) NEAR/1 (arm OR arms)):ti,ab,kw) OR ((allocat* NEAR/10 (arm OR arms)):ti,ab,kw) OR placebo*:ti,ab,kw OR 'sham-control*':ti,ab,kw OR (((single OR double OR triple OR assessor) NEAR/1 (blind* OR masked)):ti,ab,kw) OR nonrandom*:ti,ab,kw OR 'non-random*':ti,ab,kw OR 'quasi-experiment*':ti,ab,kw OR crossover:ti,ab,kw OR 'cross over':ti,ab,kw OR 'parallel group*':ti,ab,kw OR 'factorial trial':ti,ab,kw OR ((phase NEAR/5 (study OR trial)):ti,ab,kw) OR ((case* NEAR/6 (matched OR control*)):ti,ab,kw) OR ((match* NEAR/6 (pair OR pairs OR cohort* OR control* OR group* OR healthy OR age OR sex OR gender OR patient* OR subject* OR participant*)):ti,ab,kw) OR ((propensity NEAR/6 (scor* OR match*)):ti,ab,kw) OR versus:ti OR vs:ti OR compar*:ti OR ((compar* NEAR/1 study):ti,ab,kw) OR (('major clinical study'/de OR 'clinical study'/de OR 'cohort analysis'/de OR 'observational study'/de OR 'cross-sectional study'/de OR 'multicenter study'/de OR 'correlational study'/de OR 'follow up'/de OR cohort*:ti,ab,kw OR 'follow up':ti,ab,kw OR followup:ti,ab,kw OR longitudinal*:ti,ab,kw OR prospective*:ti,ab,kw OR retrospective*:ti,ab,kw OR observational*:ti,ab,kw OR 'cross sectional*':ti,ab,kw OR cross?ectional*:ti,ab,kw OR multicent*:ti,ab,kw OR 'multi-cent*':ti,ab,kw OR consecutive*:ti,ab,kw) AND (group:ti,ab,kw OR groups:ti,ab,kw OR subgroup*:ti,ab,kw OR versus:ti,ab,kw OR vs:ti,ab,kw OR compar*:ti,ab,kw OR 'odds ratio*':ab OR 'relative odds':ab OR 'risk ratio*':ab OR 'relative risk*':ab OR 'rate ratio':ab OR aor:ab OR arr:ab OR rrr:ab OR ((('or' OR 'rr') NEAR/6 ci):ab))) |
13508920 |
|
#8 |
'major clinical study'/de OR 'clinical study'/de OR 'case control study'/de OR 'family study'/de OR 'longitudinal study'/de OR 'retrospective study'/de OR 'prospective study'/de OR 'comparative study'/de OR 'cohort analysis'/de OR ((cohort NEAR/1 (study OR studies)):ab,ti) OR (('case control' NEAR/1 (study OR studies)):ab,ti) OR (('follow up' NEAR/1 (study OR studies)):ab,ti) OR (observational NEAR/1 (study OR studies)) OR ((epidemiologic NEAR/1 (study OR studies)):ab,ti) OR (('cross sectional' NEAR/1 (study OR studies)):ab,ti) |
6767914 |
|
#7 |
'randomized controlled trial'/exp OR random*:ti,ab OR (((pragmatic OR practical) NEAR/1 'clinical trial*'):ti,ab) OR ((('non inferiority' OR noninferiority OR superiority OR equivalence) NEAR/3 trial*):ti,ab) OR rct:ti,ab,kw |
1839814 |
|
#6 |
'meta analysis'/exp OR 'meta analysis (topic)'/exp OR metaanaly*:ti,ab OR 'meta analy*':ti,ab OR metanaly*:ti,ab OR 'systematic review'/de OR 'cochrane database of systematic reviews'/jt OR prisma:ti,ab OR prospero:ti,ab OR (((systemati* OR scoping OR umbrella OR 'structured literature') NEAR/3 (review* OR overview*)):ti,ab) OR ((systemic* NEAR/1 review*):ti,ab) OR (((systemati* OR literature OR database* OR 'data base*') NEAR/10 search*):ti,ab) OR (((structured OR comprehensive* OR systemic*) NEAR/3 search*):ti,ab) OR (((literature NEAR/3 review*):ti,ab) AND (search*:ti,ab OR database*:ti,ab OR 'data base*':ti,ab)) OR (('data extraction':ti,ab OR 'data source*':ti,ab) AND 'study selection':ti,ab) OR ('search strategy':ti,ab AND 'selection criteria':ti,ab) OR ('data source*':ti,ab AND 'data synthesis':ti,ab) OR medline:ab OR pubmed:ab OR embase:ab OR cochrane:ab OR (((critical OR rapid) NEAR/2 (review* OR overview* OR synthes*)):ti) OR ((((critical* OR rapid*) NEAR/3 (review* OR overview* OR synthes*)):ab) AND (search*:ab OR database*:ab OR 'data base*':ab)) OR metasynthes*:ti,ab OR 'meta synthes*':ti,ab |
733409 |
|
#5 |
#4 NOT ('conference abstract'/it OR 'editorial'/it OR 'letter'/it OR 'note'/it) NOT (('animal'/exp OR 'animal experiment'/exp OR 'animal model'/exp OR 'nonhuman'/exp) NOT 'human'/exp) |
845 |
|
#4 |
#3 NOT (('adolescent'/exp OR 'child'/exp OR adolescent*:ti,ab,kw OR child*:ti,ab,kw OR schoolchild*:ti,ab,kw OR infant*:ti,ab,kw OR girl*:ti,ab,kw OR boy*:ti,ab,kw OR teen:ti,ab,kw OR teens:ti,ab,kw OR teenager*:ti,ab,kw OR youth*:ti,ab,kw OR pediatr*:ti,ab,kw OR paediatr*:ti,ab,kw OR puber*:ti,ab,kw) NOT ('adult'/exp OR 'aged'/exp OR 'middle aged'/exp OR adult*:ti,ab,kw OR man:ti,ab,kw OR men:ti,ab,kw OR woman:ti,ab,kw OR women:ti,ab,kw)) |
1177 |
|
#3 |
#1 AND #2 |
1279 |
|
#2 |
'intraoperative blood salvage device'/exp OR 'autologous blood recovery':ti,ab,kw OR 'cell salvage'/exp OR (((blood OR cell) NEAR/3 (salvage OR sav*)):ti,ab,kw) |
5261 |
|
#1 |
'thorax surgery'/exp OR 'cardiovascular surgery'/exp OR (((cardi* OR chest* OR thoracic OR thorax OR coronar* OR myocard* OR heart) NEAR/3 (operat* OR resect* OR surg*)):ti,ab,kw) OR 'coronary bypass*':ti,ab,kw |
|
Ovid/Medline
|
# |
Searches |
Results |
|
14 |
12 not 11 not 10 OBS |
245 |
|
13 |
10 not 11 RCT |
12 |
|
12 |
4 and (8 or 9) |
377 |
|
11 |
5 and 7 |
155 |
|
10 |
5 and 6 SR |
30 |
|
9 |
Case-control Studies/ or clinical trial, phase ii/ or clinical trial, phase iii/ or clinical trial, phase iv/ or comparative study/ or control groups/ or controlled before-after studies/ or controlled clinical trial/ or double-blind method/ or historically controlled study/ or matched-pair analysis/ or single-blind method/ or (((control or controlled) adj6 (study or studies or trial)) or (compar* adj (study or studies)) or ((control or controlled) adj1 active) or "open label*" or ((double or two or three or multi or trial) adj (arm or arms)) or (allocat* adj10 (arm or arms)) or placebo* or "sham-control*" or ((single or double or triple or assessor) adj1 (blind* or masked)) or nonrandom* or "non-random*" or "quasi-experiment*" or "parallel group*" or "factorial trial" or "pretest posttest" or (phase adj5 (study or trial)) or (case* adj6 (matched or control*)) or (match* adj6 (pair or pairs or cohort* or control* or group* or healthy or age or sex or gender or patient* or subject* or participant*)) or (propensity adj6 (scor* or match*))).ti,ab,kf. or (confounding adj6 adjust*).ti,ab. or (versus or vs or compar*).ti. or ((exp cohort studies/ or epidemiologic studies/ or multicenter study/ or observational study/ or seroepidemiologic studies/ or (cohort* or 'follow up' or followup or longitudinal* or prospective* or retrospective* or observational* or multicent* or 'multi-cent*' or consecutive*).ti,ab,kf.) and ((group or groups or subgroup* or versus or vs or compar*).ti,ab,kf. or ('odds ratio*' or 'relative odds' or 'risk ratio*' or 'relative risk*' or aor or arr or rrr).ab. or (("OR" or "RR") adj6 CI).ab.)) |
5259989 |
|
8 |
Epidemiologic studies/ or case control studies/ or exp cohort studies/ or Controlled Before-After Studies/ or Case control.tw. or cohort.tw. or Cohort analy$.tw. or (Follow up adj (study or studies)).tw. or (observational adj (study or studies)).tw. or Longitudinal.tw. or Retrospective*.tw. or prospective*.tw. or consecutive*.tw. or Cross sectional.tw. or Cross-sectional studies/ or historically controlled study/ or interrupted time series analysis/ [Onder exp cohort studies vallen ook longitudinale, prospectieve en retrospectieve studies] |
4260811 |
|
7 |
exp randomized controlled trial/ or randomized controlled trials as topic/ or random*.ti,ab. or rct?.ti,ab. or ((pragmatic or practical) adj "clinical trial*").ti,ab,kf. or ((non-inferiority or noninferiority or superiority or equivalence) adj3 trial*).ti,ab,kf. |
1550591 |
|
6 |
meta-analysis/ or meta-analysis as topic/ or (metaanaly* or meta-analy* or metanaly*).ti,ab,kf. or systematic review/ or cochrane.jw. or (prisma or prospero).ti,ab,kf. or ((systemati* or scoping or umbrella or "structured literature") adj3 (review* or overview*)).ti,ab,kf. or (systemic* adj1 review*).ti,ab,kf. or ((systemati* or literature or database* or data-base*) adj10 search*).ti,ab,kf. or ((structured or comprehensive* or systemic*) adj3 search*).ti,ab,kf. or ((literature adj3 review*) and (search* or database* or data-base*)).ti,ab,kf. or (("data extraction" or "data source*") and "study selection").ti,ab,kf. or ("search strategy" and "selection criteria").ti,ab,kf. or ("data source*" and "data synthesis").ti,ab,kf. or (medline or pubmed or embase or cochrane).ab. or ((critical or rapid) adj2 (review* or overview* or synthes*)).ti. or (((critical* or rapid*) adj3 (review* or overview* or synthes*)) and (search* or database* or data-base*)).ab. or (metasynthes* or meta-synthes*).ti,ab,kf. |
621474 |
|
5 |
4 not ((exp animals/ or exp models, animal/) not humans/) not (letter/ or comment/ or editorial/) |
608 |
|
4 |
3 not ((Adolescent/ or Child/ or Infant/ or adolescen*.ti,ab,kf. or child*.ti,ab,kf. or schoolchild*.ti,ab,kf. or infant*.ti,ab,kf. or girl*.ti,ab,kf. or boy*.ti,ab,kf. or teen.ti,ab,kf. or teens.ti,ab,kf. or teenager*.ti,ab,kf. or youth*.ti,ab,kf. or pediatr*.ti,ab,kf. or paediatr*.ti,ab,kf. or puber*.ti,ab,kf.) not (Adult/ or adult*.ti,ab,kf. or man.ti,ab,kf. or men.ti,ab,kf. or woman.ti,ab,kf. or women.ti,ab,kf.)) |
631 |
|
3 |
1 and 2 |
686 |
|
2 |
exp Operative Blood Salvage/ or autologous blood recovery.ti,ab,kf. or ((blood or cell) adj3 (salvage or sav*)).ti,ab,kf. |
3216 |
|
1 |
Thoracic Surgery/ or exp Cardiovascular Surgical Procedures/ or ((cardi* or chest* or thoracic or thorax or coronar* or myocard* or heart*) adj4 (operat* or surg* or resect*)).ti,ab,kf. or coronary bypass*.ti,ab,kf. |
535740 |