Femoro-popliteaal: Gecoate ballonnen en stents
Uitgangsvraag
Wat is de optimale endovasculaire behandeling van het femoro-popliteale traject?
De uitgangsvraag bevat de volgende deelvragen:
- Wat is het optimale type ballon (gecoat/ongecoat) bij endovasculaire behandeling van perifeer arterieel vaaatlijden in het femoro-popliteale traject?
- Wat is het optimale type stent (gecoat/ongecoat) bij endovasculaire behandeling van perifeer arterieel vaaatlijden in het femoro-popliteale traject?
Aanbeveling
Gebruik gecoate ballonnen voor de primaire endovasculaire behandeling van het femoro-popliteale traject.
Overweeg gecoate stents indien er een indicatie is voor stentplaatsing in het femoro-popliteale traject.
Overwegingen
Voor- en nadelen van de interventie en de kwaliteit van het bewijs
Er is literatuuronderzoek gedaan naar de voor- en nadelen van ballonnen en stents gecoat met een geneesmiddel vergeleken met niet-gecoate ballonnen of stents bij endovasculaire behandeling van patiënten met claudicatio intermittens en CLTI in het femoro-popliteale traject. Als cruciale uitkomstmaten werden patency/re-stenose en klinisch gedreven re-interventies gedefinieerd. Kwaliteit van leven, wondgenezing, amputatie en mortaliteit werden beschouwd als belangrijke uitkomstmaten voor de besluitvorming.
Er werd één systematische review en zeven gerandomiseerde trials gevonden. Angioplastiek met een gecoate ballon leek verbeteringen te geven in patency en het aantal re-interventies. Amputatie, mortaliteit en kwaliteit van leven konden geen richting geven aan de besluitvorming. De uitkomstmaat wondgenezing werd niet gerapporteerd in de studies. De beschreven studies hebben met name patiënten geïncludeerd met claudicatio intermittens klachten. In deze groep patiënten zien we dan ook nauwelijks wonden, amputaties en mortaliteit. Endovasculaire behandelingen met gecoate stents leek verbeteringen te geven in patency en het aantal re-interventies na langer dan 2 jaar en in mortaliteit tot 2 jaar. Er werd geen verbetering in kwaliteit van leven gevonden na behandeling met gecoate stents. De uitkomstmaat wondgenezing werd niet gerapporteerd in de studies. Op basis van de literatuur zou de voorkeur dus uitgaan naar gebruik van een gecoate ballon en een gecoate stent, ten opzichte van ongecoate ballon of stent.
Bij de behandeling van claudicatio intermittens zou men misschien wel moeten kiezen voor kwaliteit van leven als belangrijkste uitkomstmaat. Deze uitkomst is echter niet verschillend in de beschreven studies. De reden hiervoor is waarschijnlijk, dat patiënten met een symptomatische re-stenose voor het einde van de follow-up een re-interventie ondergaan. Dit is dan ook precies wat uit de analyse naar voren komt: de patiënten in beide groepen (gecoate ballonnen en stents vs. niet-gecoate ballonnen en stents) hebben aan het einde van de studie dezelfde kwaliteit van leven. Echter, om dit te bereiken ondergaan de patiënten die initieel behandeld zijn met niet-gecoate ballonnen en stents meer re-interventies vanwege een slechtere patency.
In 2018 publiceerde Katsanos een meta-analyse van alle op dat moment beschikbare randomised controlled trials over de behandeling met Paclitaxel gecoate ballonnen en stents. (Katsanos, 2018). De conclusie was dat Paclitaxel gecoate ballonnen en stents op de lange termijn leidde tot meer mortaliteit. Ondanks uitgebreide analyses kon geen goede verklaring voor deze uitkomst gegeven worden. Deze publicatie leidde tot een advies van de Food and Drug Administration (FDA) om de Paclitaxel gecoate ballonnen en stents niet meer te gebruiken. Mocht een behandelaar deze devices toch willen gebruiken, dan moesten de patiënten goed geïnformeerd worden over het verhoogde risico op mortaliteit op de lange termijn. Recenter onderzoek ontkracht echter deze verhoogde kans op mortaliteit op de lange termijn. Er is extra data beschikbaar gekomen, waaronder extra follow-up van de bestaande studies, extra informatie over de vitale status van patiënten in de bestaande studies, maar ook data van nieuwe studies. De FDA heeft naar aanleiding van deze nieuwe literatuur en hernieuwde analyses besloten, dat er geen aanwijzingen (meer) zijn voor een verhoogd risico op mortaliteit na behandeling met gecoate ballonnen en stents. Dit statement werd op 11 juli 2023 gepubliceerd en overgenomen door de Europese verenigingen voor interventieradiologie (CIRSE) en vaatchirurgie (ESVS). (https://www.fda.gov/medical-devices/letters-health-care-providers/update-paclitaxel-coated-devices-treat-peripheral-arterial-disease-unlikely-increase-risk-mortality).
Zowel gecoate ballonnen als stents resulteren na behandeling van het femoro-popliteale traject in een betere patency en minder re-interventies. Echter, of gecoate ballonnen dan wel stents betere resultaten geven na behandeling van het femoro-popliteale traject is nog onduidelijk. De BASIL-3 trial, de SWEDEPAD 1 en 2 trials en de FOREST-trial gaan hier meer duidelijkheid over geven.
Waarden en voorkeuren van patiënten (en evt. hun verzorgers)
Voor patiënten is het van belang zo min mogelijk re-interventies te ondergaan. Zij zijn derhalve gebaat bij een goede patency en minder re-interventies. Derhalve heeft een primaire endovasculaire behandeling van het femoro-popliteale traject middels een gecoate ballon of stent de voorkeur.
Kosten (middelenbeslag)
Gecoate ballonnen en stents zijn duur (400-1000 euro). Echter, het gebruik van deze producten leidt tot een betere patency en dientengevolge minder re-interventies. De kosten worden op die manier (deels) weer terugverdiend en derhalve lijkt het gebruik van deze dure producten goed te verdedigen.
Aanvaardbaarheid, haalbaarheid en implementatie
Gecoate ballonnen en stents worden in de meeste ziekenhuizen al jaren gebruikt als standaardbehandeling voor het femoro-popliteale traject. Het is wel van belang te benoemen dat bij gebruik van gecoate ballonnen en stents voldoende kennis van het behandelprotocol moet zijn. Zaken als predilatatie, geographic miss en atherectomie van sommige trajecten dragen bij aan de beschreven resultaten.
Rationale van de aanbeveling: weging van argumenten voor en tegen de interventies
Gecoate ballonnen en stents zijn bij de endovasculaire behandeling van het femoro-popliteale traject geassocieerd met een betere patency en aantal re-interventies dan niet-gecoate ballonnen en stents. De literatuur is redelijk eenduidig ten aanzien van deze conclusie.
Onderbouwing
Achtergrond
Endovascular treatment of patients with peripheral arterial disease has increased dramatically in recent decades due to improved techniques and increased expertise. The major advantage of endovascular treatment is its minimally invasive nature, however, the disadvantage is its limited patency compared to surgical revascularisation. To improve the patency of endovascular treatment, several technical developments have become available, such as the use of stents and coating balloons and stents with antiproliferative drugs. Balloons and stents are coated with, for example, Paclitaxel or Sirolimus to reduce the risk of intima hyperplasia and restenosis. Especially in the femoro-popliteal and infragenicular pathways, there is a lot of experience with coated endovascular balloons and stents.
Treatment of stenotic and occluding vascular disease is indicated in patients presenting with critical ischemia (Chronic Limb Treating Ischemia; CLTI). Patients with intermittent claudication (CI) who do not respond adequately to treatment by supervised gait training are also eligible for endovascular revascularization.
While treatment of patients with CI is aimed at improving walking distance, patients with CLTI often have one or more wounds on the foot. The goal of treatment is therefore to heal these wounds.
Conclusies / Summary of Findings
Drug-coated balloon angioplasty vs. conventional balloon angioplasty
Primary patency (critical)
One year
|
Low GRADE |
Drug-coated balloon angioplasty may increase primary patency when compared with conventional balloon angioplasty in patients with symptomatic peripheral arterial disease in the femoro-popliteal region.
Source: Koeckerling, 2023; Liao, 2022; Ni, 2022 |
Two years
|
Low GRADE |
Drug-coated balloon angioplasty may increase primary patency when compared with conventional balloon angioplasty in patients with symptomatic peripheral arterial disease in the femoro-popliteal region.
Source: Koeckerling, 2023 |
More than two years
|
Low GRADE |
Drug-coated balloon angioplasty may increase primary patency when compared with conventional balloon angioplasty in patients with symptomatic peripheral arterial disease in the femoro-popliteal region.
Source: Hausegger, 2024; Koeckerling, 2023; Lyden, 2022; Teichgräber, 2022 |
Clinically driven target lesion revascularization (critical)
One year
|
Moderate GRADE |
Drug-coated balloon angioplasty likely reduces clinically driven target lesion revascularization when compared with conventional balloon angioplasty in patients with symptomatic peripheral arterial disease in the femoro-popliteal region.
Source: Koeckerling, 2023; Liao, 2022 |
Two years
|
Moderate GRADE |
Drug-coated balloon angioplasty likely reduces clinically driven target lesion revascularization when compared with conventional balloon angioplasty in patients with symptomatic peripheral arterial disease in the femoro-popliteal region.
Source: Koeckerling, 2023 |
More than two years
|
Moderate GRADE |
Drug-coated balloon angioplasty likely reduces clinically driven target lesion revascularization at more than two years when compared with conventional balloon angioplasty in patients with symptomatic peripheral arterial disease in the femoro-popliteal region.
Source: Hausegger, 2024; Koeckerling, 2023; Lyden, 2022; Teichgräber, 2022 |
Amputation (important)
One year
|
Very low GRADE |
The evidence is very uncertain about the effect of drug-coated balloon angioplasty on amputation at one year when compared with conventional balloon angioplasty in patients with symptomatic peripheral arterial disease in the femoro-popliteal region.
Source: Koeckerling, 2023; Liao, 2022; Ni, 2022 |
Two years
|
Very low GRADE |
The evidence is very uncertain about the effect of drug-coated balloon angioplasty on amputation at two years when compared with conventional balloon angioplasty in patients with symptomatic peripheral arterial disease in the femoro-popliteal region.
Source: Koeckerling, 2023 |
More than two years
|
Very low GRADE |
The evidence is very uncertain about the effect of drug-coated balloon angioplasty on amputation at more than two years when compared with conventional balloon angioplasty in patients with symptomatic peripheral arterial disease in the femoro-popliteal region.
Source: Hausegger, 2024; Koeckerling, 2023; Lyden, 2022; Teichgräber, 2022 |
Mortality (important)
One year
|
Very low GRADE |
The evidence is very uncertain about the effect of drug-coated balloon angioplasty on mortality at one year when compared with conventional balloon angioplasty in patients with symptomatic peripheral arterial disease in the femoro-popliteal region.
Source: Koeckerling, 2023; Liao, 2022; Ni, 2022 |
Two years
|
Very low GRADE |
The evidence is very uncertain about the effect of drug-coated balloon angioplasty on mortality at two years when compared with conventional balloon angioplasty in patients with symptomatic peripheral arterial disease in the femoro-popliteal region.
Source: Koeckerling, 2023 |
More than two years
|
Very low GRADE |
The evidence is very uncertain about the effect of drug-coated balloon angioplasty on mortality at more than two years when compared with conventional balloon angioplasty in patients with symptomatic peripheral arterial disease in the femoro-popliteal region.
Source: Hausegger, 2024; Koeckerling, 2023; Lyden, 2022; Teichgräber, 2022 |
Wound healing (important)
|
No GRADE |
No evidence was found regarding the effect of drug-coated balloon angioplasty on wound healing when compared with conventional balloon angioplasty in patients with symptomatic peripheral arterial disease in the femoro-popliteal region.
Source: - |
Quality of life (important)
One year
|
Low GRADE |
Drug-coated balloon angioplasty may result in little to no difference in quality of life when compared conventional balloon angioplasty in patients with symptomatic peripheral arterial disease in the femoro-popliteal region.
Source: Liao, 2022 |
Drug-eluting stents vs. bare-metal stents
Primary patency (critical)
One year
|
Moderate GRADE |
Drug-eluting stents likely result in little to no difference in primary patency at one year when compared with bare-metal stents in patients with symptomatic peripheral arterial disease in the femoro-popliteal region.
Source: Fransson, 2023; Gouëffic, 2022; Koeckerling, 2023 |
Two years
|
Moderate GRADE |
Drug-eluting stents likely result in little to no difference in primary patency at two years when compared with bare-metal stents in patients with symptomatic peripheral arterial disease in the femoro-popliteal region.
Source: Fransson, 2023; Koeckerling, 2023 |
More than two years
|
Low GRADE |
Drug-eluting stents may increase primary patency at more than two years when compared with bare-metal stents in patients with symptomatic peripheral arterial disease in the femoro-popliteal region.
Source: Koeckerling, 2023 |
Clinically driven target lesion revascularization (critical)
One year
|
Very low GRADE |
The evidence is very uncertain about the effect of drug-eluting stents on clinically driven target lesion revascularization at one year when compared with bare-metal stents in patients with symptomatic peripheral arterial disease in the femoro-popliteal region.
Source: Fransson, 2023; Gouëffic, 2022; Koeckerling, 2023 |
Two years
|
Low GRADE |
Drug-eluting stents may result in little to no difference in clinically driven target lesion revascularization at two years when compared with bare-metal stents in patients with symptomatic peripheral arterial disease in the femoro-popliteal region.
Source: Fransson, 2023; Koeckerling, 2023 |
More than two years
|
Low GRADE |
Drug-eluting stents may reduce clinically driven target lesion revascularization at more than two years when compared with bare-metal stents in patients with symptomatic peripheral arterial disease in the femoro-popliteal region.
Source: Koeckerling, 2023 |
Amputation (important)
One year
|
Very low GRADE |
The evidence is very uncertain about the effect of drug-eluting stents on amputation at one year when compared with bare-metal stents in patients with symptomatic peripheral arterial disease in the femoro-popliteal region.
Source: Gouëffic, 2022; Koeckerling, 2023 |
Two years
|
Very low GRADE |
The evidence is very uncertain about the effect of drug-eluting stents on amputation at two years when compared with bare-metal stents in patients with symptomatic peripheral arterial disease in the femoro-popliteal region.
Source: Fransson, 2023; Koeckerling, 2023 |
Mortality (important)
One year
|
Very low GRADE |
The evidence is very uncertain about the effect of drug-eluting stents on mortality at one year when compared with bare-metal stents in patients with symptomatic peripheral arterial disease in the femoro-popliteal region.
Source: Gouëffic, 2022; Koeckerling, 2023 |
Two years, more than two years
|
Very low GRADE |
The evidence is very uncertain about the effect of drug-eluting stents on mortality at two years and more than two years when compared with bare-metal stents in patients with symptomatic peripheral arterial disease in the femoro-popliteal region.
Source: Fransson, 2023; Koeckerling, 2023 |
Wound healing (important)
|
No GRADE |
No evidence was found regarding the effect of drug-coated balloon angioplasty on wound healing when compared with conventional balloon angioplasty in patients with symptomatic peripheral arterial disease in the femoro-popliteal region.
Source: - |
Quality of life (important)
One year
|
Moderate GRADE |
Drug-eluting stents likely result in little to no difference in quality of life when compared with bare-metal stents in patients with symptomatic peripheral arterial disease in the femoro-popliteal region.
Source: Gouëffic, 2022 |
Samenvatting literatuur
Description of studies
Table 5 outlines the main study characteristics. Not all studies made a distinction between CI and CLTI in their populations.
Koeckerling (2023) performed a systematic review and meta-analysis to compare efficacy and safety outcomes for balloon angioplasty, bare-metal stents, drug-coated balloons, drug-eluting stents, covered stents, and atherectomy. Inclusion criteria were: -Randomized trials with active controls, comparing efficacy or safety endpoints between two or more of the following device-based interventions: BA, DCB, DES, BMS, covered stents with atherectomy, reporting exact porportions of participants with aortoiliac and femoropopliteal disease, primarily investigated de novo atherosclerotic lesions, proportion of study participants with IC exceeded 70% or estimates for IC patients were provided separately. Trials examining isolated infrapopliteal artery disease were excluded. The following databases were searched from inception through November 2021: EMBASE, MEDLINE, Web of Science Core Collection, Cochrane Central Register of Controlled Trials, and Google Scholar. In total, 51 randomized controlled trials were included in the systematic review, but only 20 randomized controlled trials were included in this analysis. The follow-up duration differed between the included RCTs. Studies were categorized into short-term follow-up (up to 1 year), mid-term follow-up (1-2 years), and long-term follow-up (more than 2 years). The following relevant outcome measures were reported by the systematic review: primary patency, target-lesion revascularization, amputation, and mortality.
Fransson (2023) performed a prospective, single centre, randomized controlled trial to compare drug eluting stent implantation with bare metal stent implantation in a subgroup of chronic limb threatening ischemia patients with lesions in the superficial femoral artery and the P1-P2 portion of the popliteal artery. Patients with CLTI and lesions in the SFA and first part of PA, with a target vessel diameter of 4-8 mm and at least one crucial artery patent to the foot were eligible for trial participation. Exclusion criteria were age below 18 years, ongoing or planned pregnancy, and patient unwillingness to participate. In total, 49 patients were eligible and were randomized to receive intervention or control. The intervention group (n=27) received Zilver Flex drug eluting stents. The control group (n=22) received Zilver Flex bare metal stents. The duration of the follow-up was 24 months (mortality records were monitored up to 5 years). The study reported the following relevant outcome measures: patency/restenosis, re-intervention based on symptomatic restenosis, amputation, and mortality.
Gouëffic (2022) performed a prospective, multicenter, randomized controlled trial to evaluate the patency of the Eluvia drug eluting stent, a polymer-coated paclitaxel-eluting stent, compared with bare metal stents for the treatment of femoropopliteal artery lesions. Patients with presentation with Rutherford class 2, 3 or 4 symptomatology, lesions in the native SFA or proximal popliteal artery with stenosis ≥70% by visual angiographic assessment, vessel diameter of 4-6 mm, and total lesion length of 30-120 mm were eligible for inclusion. The exclusion criteria were dialysis treatment; target lesion or vessel previously treated with a drug-coated balloon within the prior 12 months or previously stented; prior SFA or proximal popliteal artery surgery in target limb; heavy calcification; and intraprocedural use of atherectomy, laser, or other debulking devices. In total, 775 patients were eligible and were randomized to receive intervention or control. The intervention group (n=508) received drug eluting stents. The control group (n=267) received bare metal stents. The duration of the follow-up was 12 months. The study reported the following relevant outcome measures: patency/restenosis, re-intervention based on symptomatic restenosis, quality of life, amputation, and mortality.
Hausegger (2024) performed a follow-up study of a prospective randomized controlled trial to evaluate long-term patient safety and treatment efficacy. Previous patients from the Freeway stent study with stenosis or occlusion in the superficial femoral artery and proximal popliteal artery segment were eligible for inclusion. In total, 150 patients were included. The intervention group (n=76) received a nitinol stent plus freeway drug-eluting balloon. The control group (n=74) received a nitinol stent plus non-paclitaxel PTA. The follow-up duration was 5 years. The study reported the following relevant outcome measures: patency/restenosis, re-intervention based on symptomatic restenosis, amputation, and mortality.
Liao (2022) performed a prospective, single center randomized controlled trial to assess the efficacy of the Orchid drug-coated balloon for the treatment of femoropopliteal artery disease versus percutaneous transluminal angioplasty. Patients 18 to 85 years with de novo stenosis of at least 70% or occlusion lesions between 40 and 200mm long in the femoropopliteal artery, artery diameter at 4 to 8 mm, Rutherford category 2 to 5 in the target limb, and at least 1 non occluded vessel runoff to the foot were eligible for trial participation. Exclusion criteria were acute or subacute thrombus or aneurysm in the target vessel; the guidewire failed to cross the target lesion; severe flow-limiting dissections grade D) or residual stenosis >70% are generated after predilation; serum creatinine >2.5 mg/dL; allergy to aspirin, heparin, clopidogrel, paclitaxel, or contrast agent; patients with bilateral lower limb lesions need to be treated at the same time; prior bypass surgery or stent implantation of the target vessel; planned amputation of the target limb; and life expectancy <1 years. In total, 60 patients were eligible and were randomized to receive intervention or control. The intervention group (n=30) received Orchid drug-coated balloon. The control group (n=30) received PTA. The duration of follow-up was 12 months. The study reported the following relevant outcome measures: patency/restenosis, re-intervention based on symptomatic restenosis, quality of life, amputation, and mortality.
Lyden (2022) performed a multicenter randomized controlled trial to examine safety and efficacy of the Stellarex drug-coated angioplasty balloon for the treatment of femoropopliteal disease. Inclusion and exclusion criteria were reported in the ILLUMENATE Pivotal trial. In total, 300 patients were included. The intervention group (n=200) received Stellarex drug-coated angioplasty balloon. The control group (n=100) received POBA. The follow-up duration was 4 years. The study reported the following relevant outcome measures: patency/restenosis, re-intervention based on symptomatic restenosis, amputation, and mortality.
Ni (2022) performed a prospective, multicenter randomized controlled trial to evaluate the safety and efficacy of a novel ZENFlow carrier-free drug-coated balloon in the treatment of femoropopliteal artery occlusive disease. Patients 18–85 years of age, who had severe intermittent claudication or ischemic rest pain or minor tissue loss (Rutherford Clinical Category 3–5); with stenosis of 70–99% with lesion lengths ≤30 cm, or a complete occlusion with lengths of ≤10 cm involving the superficial femoral or popliteal arteries (or both) were eligible for trial participation. Exclusion criteria wereacute thrombus in the target vessels
severe renal or hepatic dysfunction; known contraindication or allergy to aspirin, clopidogrel, heparin, or paclitaxel; life expectancy <1 year; vessel stenosis or occlusion due to Buerger’s disease or autoimmune arteritis; pregnancy; and immunosuppressive agent therapy. In total, 192 patients were eligible and were randomized to receive intervention or control. The intervention group (n=93) received a drug-coated balloon with paclitaxel. The control group (n=99) received an uncoated balloon. The duration of the follow-up was 12 months. The study reported the following relevant outcome measures: patency/restenosis, re-intervention based on symptomatic restenosis, amputation, and mortality.
Teichgräber (2022) performed a prospective, multicenter randomized controlled trial to compare drug-coated balloon angioplasty with POBA in patients with femoropopliteal artery disease of Rutherford category 2-4. Patients of Rutherford category 2-4 and single femoropopliteal target lesions ≤15 cm were eligible for trial participation. In total, 171 patients were eligible and were randomized to receive intervention or control. The intervention group (n=85) received drug-coated balloon catheters, coated with paclitaxel. The control group (n=86) received uncoated balloon catheters. The duration of the follow-up was 5 years. The study reported the following relevant outcome measures: patency/restenosis, re-intervention based on symptomatic restenosis, amputation, and mortality.
Table 5. Study characteristics.
|
Study |
Disease severity |
Comparison |
N (I/C) |
Follow-up |
|
Liao, 2022 |
Rutherford class 2-5 |
Orchid DCB vs. PTA |
N=60 (30/30) |
12 months |
|
Lyden, 2022 |
NR |
Stellarex drug-coated angioplasty balloon vs. POBA |
N=300 (200/100) |
4 years |
|
Ni, 2022 |
Rutherford class 3-5 |
ZENFlow paclitaxel-coated balloon vs. uncoated balloon |
N=192 (93/99) |
12 months |
|
Gouëffic, 2022 |
Rutherford class 2-5 |
Eluvia DES coated stent vs. BMS bare nitinol stent |
N=775 (508/267) |
12 months |
|
Teichgräber, 2022 |
Rutherford class 2-5 |
Luminor 35 DCB catheter vs. uncoated balloon catheter |
N=171 (85/86) |
5 years |
|
Fransson, 2023 |
Rutherford class median 5 (IQR 4-5) |
Zilver Flex DES vs. Zilver Flex BMS |
N=49 (27/22) |
24 months (mortality 5 years) |
|
Hausegger, 2024 |
NR |
Nitinolstent plus FREEWAY drug-eluting balloon postdilatation vs. nitinolstent plus non-patclitaxel PTA |
N=150 (76/74) |
5 years |
|
Koeckerling, 2023 |
NR |
Drug-coated balloon angioplasty vs. conventional balloon angioplasty OR drug eluting stents vs. bare metal stents |
20 studies with in total n=3769 patients |
Different follow-up time (at least 1 year to up to more than 2 years). |
Results
Drug-coated balloon angioplasty vs. conventional balloon angioplasty
One systematic review and five studies reported the comparison drug-coated balloon angioplasty versus conventional balloon angioplasty (Hausegger, 2024; Koeckerling, 2023; Liao, 2022; Lyden, 2022; Ni, 2022; Teichgräber, 2022).
Primary patency (critical)
One year
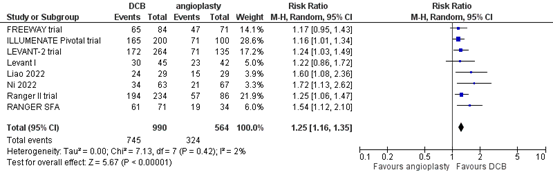
Eight studies reported the outcome measure primary patency at one year (FREEWAY trial; ILLUMENATE Pivotal trial; Levant I; LEVANT-2 trial; Liao, 2022; Ni, 2022; RANGER SFA; Ranger II trial). In total, 745 of the 990 patients who received drug-coated balloon angioplasty had primary patency at 1 year, compared with 324 of the 564 patients who received conventional balloon angioplasty (Figure 1). The risk ratio was 1.25 (95% CI 1.16 to 1.35), in favour of the patients who received drug-coated balloon angioplasty. This difference is considered clinically relevant.
Figure 1. Primary patency at one year (DCB)
Z: p-value of overall effect; df: degrees of freedom; I2: statistical heterogeneity; CI: confidence interval.
Two years
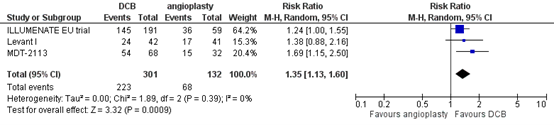
Three studies reported the outcome measure primary patency at two years (ILLUMENATE EU trial; Levant I; MDT-2113). In total, 223 of the 301 patients who received drug-coated balloon angioplasty had primary patency at two years, compared with 68 of the 132 patients who received conventional balloon angioplasty (Figure 2). The risk ratio was 1.35 (95% CI 1.13 to 1.60), in favour of the patients who received drug-coated balloon angioplasty. This difference is considered clinically relevant.
Figure 2. Primary patency at two years (DCB)
Z: p-value of overall effect; df: degrees of freedom; I2: statistical heterogeneity; CI: confidence interval.
More than two years
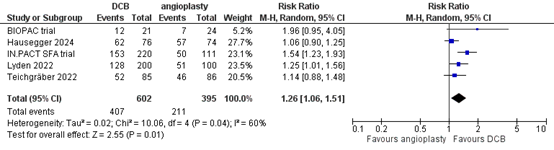
Five studies reported the outcome measure primary patency at more than two years (BIOPAC trial; Hausegger, 2024; IN.PACT SFA trial; Lyden, 2022; Teichgräber, 2022). In total, 407 of the 602 patients who received drug-coated balloon angioplasty had primary patency at more than two years, compared with 211 of the 395 patients who received conventional balloon angioplasty (Figure 3). The risk ratio was 1.26 (95% CI 1.06 to 1.51), in favour of the patients who received drug-coated balloon angioplasty. This difference is considered clinically relevant.
Figure 3. Primary patency at more than two years (DCB)
Z: p-value of overall effect; df: degrees of freedom; I2: statistical heterogeneity; CI: confidence interval.
Clinically driven target lesion revascularization (critical)
One year
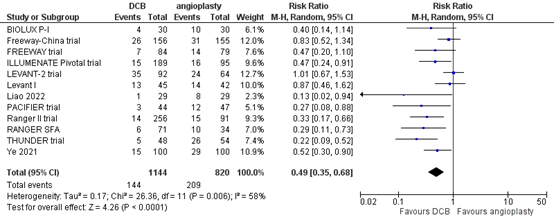
Twelve studies reported the outcome measure clinically driven target lesion revascularization at one year (BIOLUX P-I; FREEWAY trial; Freeway-China trial; ILLUMENATE Pivotal trial; Levant I; LEVANT-2 trial; Liao, 2022; PACIFIER trial; RANGER SFA; Ranger II trial; THUNDER trial; Ye, 2021). In total, 144 of the 1144 patients who received drug-coated balloon angioplasty underwent clinically driven target lesion revascularization at one year, compared with 209 of the 820 patients who received conventional balloon angioplasty (Figure 4). The risk ratio was 0.49 (95% CI 0.35 to 0.68), in favour of the patients who received drug-coated balloon angioplasty. This difference is considered clinically relevant.
Figure 4. Clinically driven target lesion revascularization at one year (DCB)
Z: p-value of overall effect; df: degrees of freedom; I2: statistical heterogeneity; CI: confidence interval.
Two years
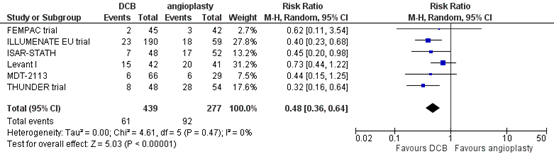
Six studies reported the outcome measure clinically driven target lesion revascularization at two years (FEMPAC trial; ILLUMENATE EU trial; ISAR-STATH; Levant I; MDT-2113; THUNDER trial). In total, 61 of the 439 patients who received drug-coated balloon angioplasty underwent clinically driven target lesion revascularization at two years, compared with 92 of the 277 patients who received conventional balloon angioplasty (Figure 5). The risk ratio was 0.48 (95% CI 0.36 to 0.64), in favour of the patients who received drug-coated balloon angioplasty. This difference is considered clinically relevant.
Figure 5. Clinically driven target lesion revascularization at two years (DCB)
Z: p-value of overall effect; df: degrees of freedom; I2: statistical heterogeneity; CI: confidence interval.
More than two years
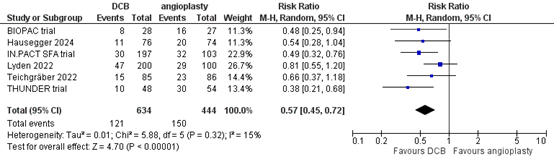
Six studies reported the outcome measure clinically driven target lesion revascularization at more than two years (BIOPAC trial; Hausegger, 2024; IN.PACT SFA trial; Lyden, 2022; Teichgräber, 2022; THUNDER trial). In total, 121 of the 634 patients who received drug-coated balloon angioplasty underwent clinically driven target lesion revascularization at more than two years, compared with 150 of the 444 patients who received conventional balloon angioplasty (Figure 6). The risk ratio was 0.57 (95% CI 0.45 to 0.72), in favour of the patients who received drug-coated balloon angioplasty. This difference is considered clinically relevant.
Figure 6. Clinically driven target lesion revascularization at more than two years (DCB)
Z: p-value of overall effect; df: degrees of freedom; I2: statistical heterogeneity; CI: confidence interval.
Amputation (important)
One year
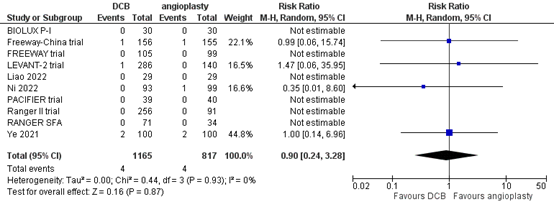
Ten studies reported the outcome measure amputation at one year (BIOLUX P-I; FREEWAY trial; Freeway-China trial; LEVANT-2 trial; Liao, 2022; Ni, 2022; PACIFIER trial; RANGER SFA; Ranger II trial; Ye, 2021). In total, 4 of the 1165 patients who received drug-coated balloon angioplasty underwent amputation at one year, compared with 4 of the 817 patients who received conventional balloon angioplasty (Figure 7). The risk ratio was 0.90 (95% CI 0.24 to 3.28), and the risk difference was 0.00 (95% CI -0.01 to 0.01) in favour of the patients who received drug-coated balloon angioplasty. This difference is not considered clinically relevant.
Figure 7. Amputation at one year (DCB)
Z: p-value of overall effect; df: degrees of freedom; I2: statistical heterogeneity; CI: confidence interval.
Two years
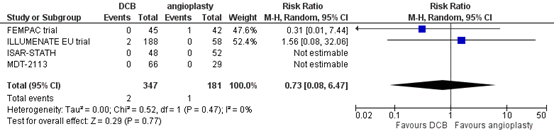
Four studies reported the outcome measure amputation at two years (FEMPAC trial; ILLUMENATE EU trial; ISAR-STATH; MDT-2113). In total, 2 of the 347 patients who received drug-coated balloon angioplasty underwent amputation at two years, compared with 1 of the 181 patients who received conventional balloon angioplasty (Figure 8). The risk ratio was 0.73 (95% CI 0.08 to 6.47) and the risk difference was 0.00 (95% CI -0.02 to 0.02), in favour of the patients who received drug-coated balloon angioplasty. This difference is not considered clinically relevant.
Figure 8. Amputation at two years (DCB)
Z: p-value of overall effect; df: degrees of freedom; I2: statistical heterogeneity; CI: confidence interval.
More than two years
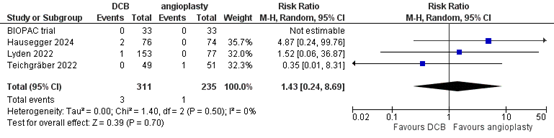
Four studies reported the outcome measure amputation at more than two years (BIOPAC trial; Hausegger, 2024; Lyden, 2022; Teichgräber, 2022). In total, 3 of the 311 patients who received drug-coated balloon angioplasty underwent amputation at more than two years, compared with 1 of the 235 patients who received conventional balloon angioplasty (Figure 9). The risk ratio was 1.43 (95% CI 0.24 to 8.69) and the risk difference was 0.01 (95% CI -0.01 to 0.02), in favour of the patients who received conventional balloon angioplasty. This difference is not considered clinically relevant.
Figure 9. Amputation at more than two years (DCB)
Z: p-value of overall effect; df: degrees of freedom; I2: statistical heterogeneity; CI: confidence interval.
Mortality (important)
One year
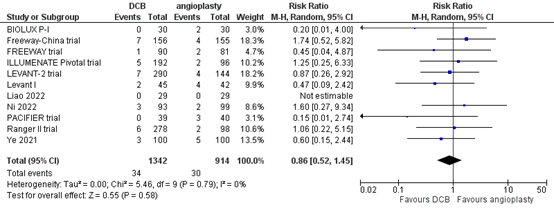
Eleven studies reported the outcome measure mortality at one year (BIOLUX P-I; FREEWAY trial; Freeway-China trial; ILLUMENATE Pivotal trial; Levant I; LEVANT-2 trial; Liao, 2022; Ni, 2022; PACIFIER trial; Ranger II trial; Ye, 2021). In total, 34 of the 1342 patients who received drug-coated balloon angioplasty had died at one year, compared with 30 of the 914 patients who received conventional balloon angioplasty (Figure 10). The risk ratio was 0.86 (95% CI 0.52 to 1.45), in favour of the patients who received drug-coated balloon angioplasty. This difference is not considered clinically relevant.
Figure 10. Mortality at one year (DCB)
Z: p-value of overall effect; df: degrees of freedom; I2: statistical heterogeneity; CI: confidence interval.
Two years
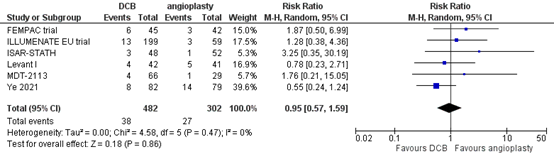
Six studies reported the outcome measure mortality at two years (FEMPAC trial; ILLUMENATE EU trial; ISAR-STATH; Levant I; MDT-2113; Ye, 2021). In total, 38 of the 482 patients who received drug-coated balloon angioplasty had died at two years, compared with 27 of the 302 patients who received conventional balloon angioplasty (Figure 11). The risk ratio was 0.95 (95% CI 0.57 to 1.59), in favour of the patients who received drug-coated balloon angioplasty. This difference is not considered clinically relevant.
Figure 11. Mortality at two years (DCB)
Z: p-value of overall effect; df: degrees of freedom; I2: statistical heterogeneity; CI: confidence interval.
More than two years
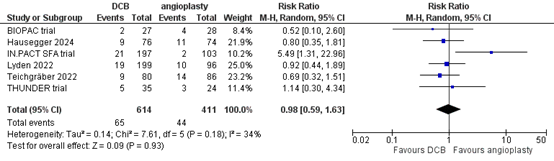
Six studies reported the outcome measure mortality at more than two years (BIOPAC trial; Hausegger, 2024; IN.PACT SFA trial; Lyden, 2022; Teichgräber, 2022; THUNDER trial). In total, 65 of the 614 patients who received drug-coated balloon angioplasty had died at more than two years, compared with 44 of the 411 patients who received conventional balloon angioplasty (Figure 12). The risk ratio was 0.98 (95% CI 0.59 to 1.63), in favour of the patients who received drug-coated balloon angioplasty. This difference is not considered clinically relevant.
Figure 12. Mortality at more than two years (DCB)
Z: p-value of overall effect; df: degrees of freedom; I2: statistical heterogeneity; CI: confidence interval.
Wound healing (important)
None of the included studies reported the outcome measure wound healing.
Quality of life (important)
One year
One study reported the outcome measure quality of life at one year (Liao, 2022). Quality of life was defined as change from baseline by EQ-5D index. The patients who received drug-coated balloon angioplasty (n=30) had a mean change of 0.092 (SD ± 0.142), compared with a mean change of 0.085 (SD ± 0.147) for the patients who received conventional balloon angioplasty (n=30). The mean difference was 0.007 (95% CI -0.07 to 0.08), in favour of the patients who received drug-coated balloon angioplasty. This difference is not considered clinically relevant.
Drug-eluting stents vs. bare-metal stents
One systematic review and two studies reported the comparison drug-eluting stents versus bare-metal stents at different follow-up times (Fransson, 2023; Gouëffic, 2022; Koeckerling, 2023).
Primary patency (critical)
One year
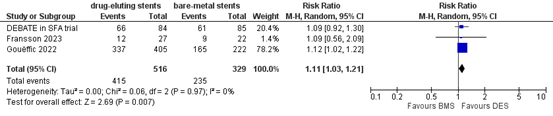
Three studies reported the outcome measure primary patency at 1 year (DEBATE in SFA trial; Fransson, 2023; Gouëffic, 2022). In total, 415 of the 516 patients who received drug-eluting stents had primary patency at one year, compared with 235 of the 329 patients who received bare-metal stents (Figure 13). The risk ratio was 1.11 (95% CI 1.03 to 1.21), in favour of the patients who received drug-eluting stents. This difference is not considered clinically relevant.
Figure 13. Primary patency at one year (DES)
Z: p-value of overall effect; df: degrees of freedom; I2: statistical heterogeneity; CI: confidence interval.
Two years
Two studies reported the outcome measure primary patency at two years (BATTLE trial; Fransson, 2023). Since only two studies reported the outcome measure, the results were not pooled.
BATTLE trial reported primary patency at two years. In total, 63 of the 85 patients (74.6%) who received drug-eluting stents had primary patency, compared with 68 of the 86 patients (78.8%) who received bare-metal stents. The risk ratio was 0.94 (95% CI 0.79 to 1.11), in favour of the patients who received bare-metal stents. This difference is not considered clinically relevant.
Fransson (2023) reported primary patency at 24 months. In total, 9 of the 27 patients (33%) who received drug-eluting stents had primary patency at two years, compared with 9 of the 22 patients (41%) who received bare-metal stents. The risk ratio was 0.81 (95% CI 0.39 to 1.70), in favour of the patients who received bare-metal stents. This difference is not considered clinically relevant.
More than two years
One study reported the outcome measure primary patency at more than two years (Zilver PTX trial). Since only one study reported the outcome measure, the results could not be pooled. In total, 38 of the 59 patients who received drug-eluting stents had primary patency at more than two years, compared with 12 of the 61 patients who received bare-metal stents. The risk ratio was 3.27 (95% CI 1.91 to 5.63), in favour of the patients who received drug-eluting stents. This difference is considered clinically relevant.
Clinically driven target lesion revascularization (critical)
One year
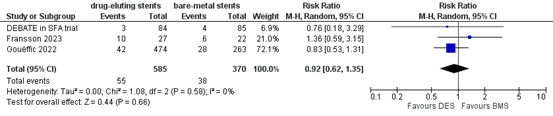
Three studies reported the outcome measure clinically driven target lesion revascularization at one year (DEBATE in SFA trial; Fransson, 2023; Gouëffic, 2022). In total, 55 of the 585 patients who received drug-eluting stents underwent clinically driven target lesion revascularization at one year, compared with 38 of the 370 patients who received bare-metal stent (Figure 14). The risk ratio was 0.92 (95% CI 0.62 to 1.35), in favour of the patients who received drug-eluting stents. This difference is not considered clinically relevant.
Figure 14. Clinically driven target lesion revascularization at one year (DES)
Z: p-value of overall effect; df: degrees of freedom; I2: statistical heterogeneity; CI: confidence interval.
Two years
Two studies reported the outcome measure clinically driven target lesion revascularization at two years (BATTLE trial; Fransson, 2023). Since only two studies reported the outcome measure, the results were not pooled.
BATTLE trial reported clinically driven target lesion revascularization at two years. In total, 12 of the 85 patients (14.4%) who received drug-eluting stents underwent clinically driven target lesion revascularization, compared with 11 of the 86 patients (12.4%) who received bare-metal stents. The risk ratio was 1.10 (95% CI 0.52 to 2.36), in favour of the patients who received bare-metal stents. This difference is not considered clinically relevant.
Fransson (2023) reported clinically driven target lesion revascularization at 24 months. In total, 11 of the 27 patients (41%) who received drug-eluting stents underwent clinically driven target lesion revascularization at two years, compared with 8 of the 22 patients (36%) who received bare-metal stents. The risk ratio was 1.12 (95% CI 0.55 to 2.29), in favour of the patients who received bare-metal stents. This difference is not considered clinically relevant.
More than two years
One study reported the outcome measure clinically driven target lesion revascularization at more than two years (Zilver PTX trial). Since only one study reported the outcome measure, the results could not be pooled. In total, 9 of the 59 patients (16.1%) who received drug-eluting stents underwent clinically driven target lesion revascularization, compared with 17 of the 61 patients (28.0%) who received bare-metal stents. The risk ratio was 0.55 (95% CI 0.27 to 1.13), in favour of the patients who received drug-eluting stents. This difference is considered clinically relevant.
Amputation
One year
Two studies reported the outcome measure amputation at one year (DEBATE in SFA trial; Gouëffic, 2022). Since only two studies reported the outcome measure, the results were not pooled.
DEBATE in SFA trial reported amputation at one year. In total, 0 of the 84 patients (0%) who received drug-eluting stents underwent amputation, compared with 0 of the 85 patients (0%) who received bare-metal stents. There was no difference between the two groups.
Gouëffic (2022) reported amputation at one year. In total, 1 of the 474 patients (0.2%) who received drug-eluting stents underwent amputation, compared with 0 of the 263 patients (0%) who received bare-metal stents. The risk ratio was 1.67 (95% CI 0.07 to 40.79), and the risk difference was 0.00 (95% CI -0.01 to 0.01), in favour of the patients who received bare-metal stents. This difference is not considered clinically relevant.
Two years
Two studies reported the outcome measure amputation at two years (BATTLE trial; Fransson, 2023). Since only two studies reported the outcome measure, the results were not pooled.
BATTLE trial reported amputation at two years. In total, 0 of the 84 patients (0%) who received drug-eluting stents underwent amputation, compared with 0 of the 85 patients (0%) who received bare-metal stents. There was no difference between the two groups.
Fransson (2023) reported amputation at 24 months. In total, 4 of the 27 patients (15%) who received drug-eluting stents underwent amputation, compared with 0 of the 22 patients (0%) who received bare-metal stents. The risk ratio was 7.39 (95% CI 0.42 to 130.28), in favour of the patients who received bare-metal stents. This difference is considered clinically relevant.
Mortality
One year
Two studies reported the outcome measure mortality at one year (DEBATE in SFA trial; Gouëffic, 2022). Since only two studies reported the outcome measure, the results were not pooled.
DEBATE in SFA trial reported mortality at one year. In total, 5 of the 84 patients (6.0%) who received drug-eluting stents had died at one year, compared with 1 of the 85 patients (1.2%) who received bare-metal stents. The risk ratio was 5.06 (95% CI 0.60 to 42.39), in favour of the patients who received bare-metal stents. This difference is considered clinically relevant.
Gouëffic (2022) reported all-cause mortality at one year. In total, 13 of the 474 patients (2.7%) who received drug-eluting stents had died at one year, compared with 3 of the 263 (1.1%) who received bare-metal stents. The risk ratio was 2.40 (95% CI 0.69 to 8.36), in favour of the patients who received bare-metal stents. This difference is considered clinically relevant.
Two years
Two studies reported the outcome measure mortality at two years (BATTLE trial; Fransson, 2023). Since only two studies reported the outcome measure, the results were not pooled.
BATTLE trial reported mortality at two years. In total, 5 of the 85 patients (6.4%) who received drug-eluting stents had died at two years, compared with 1 of the 86 patients (1.2%) who received bare-metal stents. The risk ratio was 5.06 (95% CI 0.60 to 42.40), in favour of the patients who received bare-metal stents. This difference is considered clinically relevant.
Fransson (2023) reported at two years. In total, 3 of the 27 patients (11%) who received drug-eluting stents had died at two years, compared with 4 of the 22 patients (18%) who received bare-metal stents. The risk ratio was 0.61 (95% CI 0.15 to 2.45), in favour of the patients who received drug-eluting stents. This difference is considered clinically relevant.
More than two years
Two studies reported the outcome measure mortality at more than two years (Fransson, 2023; Zilver PTX trial). Since only two studies reported the outcome measure, the results were not pooled.
Fransson (2023) reported mortality at five years. In total, 6 of the 27 patients (22%) who received drug-eluting stents had died at five years, compared with 5 of the 22 patients (23%) who received bare-metal stents. The risk ratio was 0.98 (95% CI 0.34 to 2.78), in favour of the patients who received drug-eluting stents. This difference is not considered clinically relevant.
Zilver PTX trial reported mortality at five years. In total, 6 of the 59 patients (10.2%) who received drug-eluting stents had died at five years, compared with 10 of the 61 patients (16.9%) who received bare-metal stents. The risk ratio was 0.62 (95% CI 0.24 to 1.60), in favour of the patients who received drug-eluting stents. This difference is considered clinically relevant.
Wound healing
None of the studies reported the outcome measure wound healing.
Quality of life
One year
One study reported the outcome measure health-related quality of life (Gouëffic, 2022). Since only one study reported the outcome measure, the results could not be pooled. The patients who received drug-eluting stents (n=444) had a quality of life score of 0.9 (SD ± 0.2), compared with a quality of life score of 0.9 (SD ± 0.1) for the patients who received bare-metal stents (n=246). The mean difference was 0.0 (95% CI -0.02 to 0.02). There was no difference in quality of life between the groups.
Level of evidence
The level of evidence for all outcomes was based on randomized controlled trials and therefore started high.
Drug-coated balloon vs. balloon angioplasty
Primary patency
One year, two years, more than two years
The level of evidence regarding the outcome measure primary patency was downgraded by two levels to LOW due to study limitations (risk of bias because of unclear randomization process and missing outcome data, -1), and because the confidence interval exceeds the upper level for clinical relevance, indicating uncertainty of the reported effect size (imprecision, -1).
Clinically driven target lesion revascularization
One year, two years, more than two years
The level of evidence regarding the outcome measure clinically driven target lesion revascularization at two years and at more than two years was downgraded by one level to MODERATE due to study limitations (risk of bias because of unclear randomization process, missing outcome data, and selection of reported results, -1).
Amputation
One year
The level of evidence regarding the outcome measure amputation at one year was downgraded by three levels to VERY LOW due to the wide confidence intervals and the limited number of events reported, indicating large uncertainty of the reported effect size (imprecision, -3).
Two years, more than two years
The level of evidence regarding the outcome measure amputation at two or more than two years was downgraded by four levels to VERY LOW due to study limitations (risk of bias because of unclear randomization process and missing outcome data, -1), and due to the wide confidence intervals and the limited number of events reported, indicating large uncertainty of the reported effect size (imprecision, -3).
Mortality
One year, two years, more than two years
The level of evidence regarding the outcome measure mortality was downgraded by three levels to VERY LOW due to study limitations (risk of bias, because of missing outcome data, selection of reported results, and unclear randomization process, -1), and because the confidence interval exceeds both levels for clinical relevance, indicating large uncertainty of the reported effect size (imprecision, -2).
Wound healing
The level of evidence regarding the outcome measures wound healing could not be determined due to the absence of data.
Quality of life
One year
The level of evidence regarding the outcome measure quality of life at one year was downgraded by two levels to LOW due to a very small sample size (imprecision, -2).
Drug-eluting stents vs. bare metal stents
Primary patency
One year
The level of evidence regarding the outcome measure primary patency at one year was downgraded by one level to MODERATE due to study limitations (risk of bias, because of missing outcome data and unclear randomization process, -1).
Two years
The level of evidence regarding the outcome measure primary patency at two years was downgraded by one level to MODERATE due to a small sample size (imprecision, -1).
More than two years
The level of evidence regarding the outcome measure primary patency at more than two years was downgraded by two levels to LOW due to study limitations (risk of bias, because of deviations from intended interventions and missing outcome data, -1), and due to a small sample size (imprecision, -1).
Clinically driven target lesion revascularization
One year
The level of evidence regarding the outcome measure clinically driven target lesion revascularization at one year was downgraded by three levels to VERY LOW due to study limitations (risk of bias, because of missing outcome data and unclear randomization process, -1), and because the confidence interval exceeds both levels for clinical relevance, indicating large uncertainty of the reported effect size (imprecision, -2).
Two years
The level of evidence regarding the outcome measure clinically driven target lesion revascularization at two years was downgraded by two levels to LOW because the confidence interval exceeds both levels for clinical relevance, indicating large uncertainty of the reported effect size (imprecision, -2).
More than two years
The level of evidence regarding the outcome measure clinically driven target lesion revascularization at more than two years was downgraded by two levels to LOW due to study limitations (risk of bias, because of deviations from intended interventions and missing outcome data, -1), and because the confidence interval exceeds the lower level for clinical relevance, indicating uncertainty of the reported effect size (imprecision, -1).
Amputation
One year
The level of evidence regarding the outcome measure amputation at one year was downgraded by three levels to VERY LOW due to the wide confidence intervals and the limited number of events reported, indicating large uncertainty of the reported effect size (imprecision, -3).
Two years
The level of evidence regarding the outcome measure amputation at two years was downgraded by three levels to VERY LOW due to the wide confidence intervals and the limited number of events reported, indicating large uncertainty of the reported effect size (imprecision, -3).
Mortality
One year
The level of evidence regarding the outcome measure mortality at one year was downgraded by three levels to VERY LOW due to the wide confidence intervals and the limited number of events reported, indicating large uncertainty of the reported effect size (imprecision, -3).
Two years
The level of evidence regarding the outcome measure mortality at two years was downgraded by three levels to VERY LOW because the confidence interval exceeds both levels for clinical relevance, indicating large uncertainty of the reported effect size (imprecision, -2), and due to conflicting results (inconsistency, -1).
More than two years
The level of evidence regarding the outcome measure mortality at more than two years was downgraded by three levels to VERY LOW due to the wide confidence intervals and the limited number of events reported, indicating large uncertainty of the reported effect size (imprecision, -3).
Wound healing
The level of evidence regarding the outcome measures wound healing could not be determined due to the absence of data.
Quality of life
One year
The level of evidence regarding the outcome measure quality of life at one year was downgraded by one level to MODERATE due to study limitations (risk of bias, because of missing outcome data, -1).
Zoeken en selecteren
A systematic review of the literature was performed to answer the following question:
What are the benefits and harms of treatment with a drug-coated balloon or stent compared with treatment with an uncoated balloon or stent, in patients with peripheral
Table 1. PICO 1
|
Patients |
patients with peripheral arterial disease in the femoro-popliteal region |
|
Intervention |
drug-coated balloon |
|
Control |
uncoated balloon |
|
Outcomes |
patency/restenosis, re-intervention based on symptomatic restenosis, quality of life, wound healing, amputation, mortality |
|
Other selection criteria |
Study design: systematic reviews and randomized controlled trials |
Table 2. PICO 2
|
Patients |
patients with with peripheral arterial disease in the femoro-popliteal region |
|
Intervention |
drug-coated stent |
|
Control |
uncoated stent |
|
Outcomes |
patency/restenosis, re-intervention based on symptomatic restenosis, quality of life, wound healing, amputation, mortality |
|
Other selection criteria |
Study design: systematic reviews and randomized controlled trials |
Relevant outcome measures
The guideline development group considered patency/restenosis and re-intervention based on symptomatic restenosis as critical outcome measures for decision making; and quality of life, amputation, wound healing and mortality as important outcome measures for decision making.
The working group did not define the outcome measures listed above a priori, but used the definitions used in the studies.
The working group defined 25% as a minimal clinically (patient) important difference for dichotomous outcomes (relative risk ≤0.80 or ≥1.25), and 10% of the maximum score for quality of life scores.
Search and select (Methods)
On the 10th of August, relevant search terms were used to search for systematic reviews, RCT and observational studies about the optimal treatment for patients with infragenicular/infrapopliteal arterial occlusive disease in the databases Embase.com and Ovid/Medline. The search resulted in 475 unique hits. Studies were selected based on the following criteria: (1) randomized controlled trials in (2) patients with peripheral arterial disease in either the femoro-popliteal or the crural region, in which (3) drug-coated balloons or stents were compared with uncoated balloons or stents. Twenty-six publications were initially selected based on title and abstract screening. After reading the full text, eighteen studies were excluded (see the table with reasons for exclusion under the tab Methods), and eight studies were included.
Results
One systematic review and seven randomized trials were included in the analysis of the literature. Important study characteristics and results are summarized in the evidence tables. The assessment of the risk of bias is summarized in the risk of bias tables.
Referenties
- Fransson T, Gottsäter A, Abdulrasak M, Malina M, Resch T. Randomized clinical Trial Comparing drug Eluting Stent Zilver PTX® Versus Bare Metal Stent Zilver Flex® for Treatment of Lesions in Femoral and Popliteal Arteries in Chronic Limb Threatening Ischemia. Vasc endovascular Surg. 2023 Oct;57(7):706-716. doi: 10.1177/15385744231171746. Epub 2023 Apr 21. PMID: 37085152.
- Gouëffic Y, Torsello G, Zeller T, Esposito G, Vermassen F, Hausegger KA, Tepe G, Thieme M, Gschwandtner M, Kahlberg A, Schindewolf M, Sapoval M, Diaz-Cartelle J, Stavroulakis K; EMINENT Investigators. Efficacy of a Drug-Eluting Stent Versus Bare Metal Stents for Symptomatic Femoropopliteal Peripheral Artery Disease: Primary Results of the EMINENT Randomized Trial. Circulation. 2022 Nov 22;146(21):1564-1576. doi: 10.1161/CIRCULATIONAHA.122.059606. Epub 2022 Oct 18. PMID: 36254728.
- Hausegger K, Kurre W, Schröder H, Dambach J, Stahnke S, Loewe C, Schürmann K, Fischbach R, Textor J, Schäfer S, Müller-Hülsbeck S. Long-Term Follow-up and Mortality Rate of Patients of the Randomized Freeway Stent Study. Cardiovasc Intervent Radiol. 2024 Feb;47(2):186-193. doi: 10.1007/s00270-023-03646-0. Epub 2024 Jan 25. PMID: 38273128; PMCID: PMC10844456.
- Katsanos K, Spiliopoulos S, Kitrou P, Krokidis M, Karnabatidis D. Risk of Death Following application of Paclitaxel-Coated Balloons and Stents in the Femoropopliteal Artery of the Leg: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J Am Heart Assoc. 2018 Dec 18;7(24):e011245. doi: 10.1161/JAHA.118.011245. PMID: 30561254; PMCID: PMC6405619.
- Koeckerling D, Raguindin PF, Kastrati L, Bernhard S, Barker J, Quiroga Centeno AC, Raeisi-Dehkordi H, Khatami F, Niehot C, Lejay A, Szeberin Z, Behrendt CA, Nordanstig J, Muka T, Baumgartner I. Endovascular revascularization strategies for aortoiliac and femoropopliteal artery disease: a meta-analysis. Eur Heart J. 2023 Mar 14;44(11):935-950. doi: 10.1093/eurheartj/ehac722. PMID: 36721954; PMCID: PMC10011342.
- Liao CJ, Song SH, Li T, Zhang YZAW. Orchid drug-coated balloon versus standard percutaneous transluminal angioplasty for the treatment of femoropopliteal artery disease: 12-month result of the randomized controlled trial. Vascular. 2022 Jun;30(3):448-454. doi: 10.1177/17085381211013968. Epub 2021 May 22. PMID: 34024196.
- Lyden SP, Faries PL, Niazi KAK, Sachar R, Jain A, Brodmann M, Werner M, Sood A, Krishnan P. No Mortality Signal With Stellarex Low-Dose Paclitaxel DCB: ILLUMENATE Pivotal 4-Year Outcomes. J Endovasc Ther. 2022 Dec;29(6):929-936. doi: 10.1177/15266028211068769. Epub 2022 Jan 8. PMID: 35000470.
- Ni L, Ye W, Zhang L, Jin X, Shu C, Jiang JS, Yang M, Wu DM, Li M, Yu GF, Yang J, Huang JH, Wang XB, Li XQ, Jiang WL, Wu ZQ, Liu CW. A Multicenter Randomized Trial Assessing ZENFlow Carrier-Free Drug-Coated Balloon for the Treatment of Femoropopliteal Artery Lesions. Front Cardiovasc Med. 2022 Mar 15;9:821672. doi: 10.3389/fcvm.2022.821672. PMID: 35391838; PMCID: PMC8982076.
- Teichgräber U, Lehmann T, Ingwersen M, Aschenbach R, Zeller T, Brechtel K, Blessing E, Lichtenberg M, von Flotow P, Heilmeier B, Sixt S, Brucks S, Erbel C, Beschorner U, Werk M, Riambau V, Wienke A, Klumb C, Thieme M, Scheinert D. Long-Term Effectiveness and Safety of Femoropopliteal Drug-Coated Balloon Angioplasty : 5-Year Results of the Randomized Controlled EffPac Trial. Cardiovasc Intervent Radiol. 2022 Dec;45(12):1774-1783. doi: 10.1007/s00270-022-03265-1. Epub 2022 Sep 11. PMID: 36088609; PMCID: PMC9705448.
Evidence tabellen
|
Study reference |
Study characteristics |
Patient characteristics |
Intervention (I) |
Comparison / control (C)
|
Follow-up |
Outcome measures and effect size |
Comments |
|
Koeckerling, 2023
Individual study characteristics and results are extracted from the individual studies. |
SR and meta-analysis of RCTs
Literature search up to 10th of December 2020
Drug-coated balloon vs. balloon angioplasty A: ILLUMENATE Pivotal trial B: IN.PACT SFA trial C: ILLUMENATE EU trial D: MDT-2113 E: RANGER SFA F: BIOPAC trial G: LEVANT-2 trial H: Ranger II trial I: Levant 1 J: FREEWAY trial K: THUNDER trial L: BIOLUX P-I M: FEMPAC trial N: PACIFIER trial O: Ye, 2021 P: Freeway-China trial Q: ISAR-STATH R: BATTLE trial S: DEBATE in SFA trial T: Zilver PTX trial
Study design: RCT
Setting and Country: Single centre and multicentre studies in Asia, Europe, America and Australia.
Source of funding and conflicts of interest: [commercial / non-commercial / industrial co-authorship]
|
Inclusion criteria SR: -Randomized trials with active controls -comparing efficacy or safety endpoints between two or more of the following device-based interventions: BA, DCB, DES, BMS, covered stents with atherectomy -Reporting exact porportions of participants with aortoiliac and femoropopliteal disease -Primarily investigated de novo atherosclerotic lesions -Proportion of study participants with IC exceeded 70% or estimates for IC patients were provided separately
Exclusion criteria SR: Trials examining isolated infrapopliteal artery disease.
51 studies included in the SR 20 studies included in this analysis
Important patient characteristics at baseline: Age (median, IQR) 68 (66-70) years
Sex median % male (IQR): 68% (63.5-72)
Diabetes median (IQR): 37.5% (31.3-47.3)
Groups comparable at baseline? Not reported. |
Intervention: A-Q: Drug-coated balloon R-T: Drug eluting stents
|
Control: A-Q: balloon angioplasty R-T: bare metal stents
|
End-point of follow-up: Maximum follow-up duration, n (%): -Short-term (<1y): 0 -Mid-term (1-2y): 20 (39%) -Long-term (>2y): 31 (61%)
For how many participants were no complete outcome data available? Not reported.
|
Primary patency See Results.
Target-lesion revascularization See Results.
Major amputations See Results.
All-cause mortality See Results.
|
Risk of bias (high, some concerns or low): Tool used by authors: revised Cochrane Risk-of-Bias 2 tool.
A: Some concerns (randomization process, measurement of outcomes, overall bias) B: Low C: Low D: Low E: Some concerns (deviations from intended interventions, overall bias) F: Some concerns (deviations from intended interventions, measurement of outcomes, overall bias) G: Low H: Some concerns (randomization process, overall bias) I: High (missing outcome data, overall bias), some concerns (measurement of outcomes) J: Some concerns (randomization process, deviations from intended interventions, missing outcome data, overall bias) K: High (missing outcome data, selection of reported results, overall bias), some concerns (measurement of outcomes) L: Some concerns (deviations from intended interventions, measurement of outcomes, overall bias) M: Some concerns (missing outcome data, measurement of outcomes, selection of reported results, overall bias) N: Some concerns (randomization process, overall bias) O: Low P: Some concerns (measurement of outcomes, overall bias) Q: High (missing outcome data, overall bias) R: Some concerns (deviations from intended interventions, missing outcome data, overall bias) S: Some concerns (missing outcome data, overall bias) T: High (deviations from intended interventions, missing outcome data, overall bias), some concerns (measurement of outcomes, selection of reported results)
Author’s conclusion: DCB angioplasty was associated with significantly higher primary patency and reduced TLR risk compared with BA in low-complexity, femoropopliteal lesions across all time points, primary BMS implantation was associated with statistically significant efficacy benefits over provisional stenting in non-complex femoropopliteal lesions at short- and mid-term follow-up, but not in long-term. No statistically significant differences in mid-term efficacy were observed for DES over BMS in femoropopliteal arteries, and there was no randomized evidence supporting stand-alone or adjunctive atherectomy over alternative endovascular strategies. |
|
Study reference |
Study characteristics |
Patient characteristics 2 |
Intervention (I) |
Comparison / control (C) 3
|
Follow-up |
Outcome measures and effect size 4 |
Comments |
|
Liao, 2022
Orchid China
ChiCTR 1900023619 |
Type of study: RCT
Setting and country: Single center, China
Funding and conflicts of interest: The author(s) received no financial support for the research, authorship, and/or publication of this article. The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. |
Inclusion criteria: 18 to 85 years patients with de novo stenosis of at least 70% or occlusion lesions between 40 and 200mm long in the femoropopliteal artery, artery diameter at 4 to 8 mm, Rutherford category 2 to 5 in the target limb, and at least 1 non occluded vessel runoff to the foot.
Exclusion criteria: (1) acute or subacute thrombus or aneurysm in the target vessel; (2) the guidewire failed to cross the target lesion; (3) severe flow-limiting dissections grade D) or residual stenosis >70% are generated after predilation; (4) serum creatinine >2.5 mg/dL; (5) allergy to aspirin, heparin, clopidogrel, paclitaxel, or contrast agent; (6) patients with bilateral lower limb lesions need to be treated at the same time; (7) prior bypass surgery or stent implantation of the target vessel; (8) planned amputation of the target limb; and (9) life expectancy <1 years.
N total at baseline: Intervention: 30 Control: 30
Important prognostic factors2: Age, mean ± SD: I: 69.2±9.0 C: 68.3±8.6
Sex: I: 60% M C: 67% M
Diabetes I: 46.7% C: 53.3%
Rutherford class 2 I: 20.0% C: 26.7%
Rutherford class 3 I: 40.0% C: 33.3%
Rutherford class 4 I: 33.3% C: 36.7%
Rutherford class 5 I: 6.7% C: 3.3%
Groups were comparable at baseline |
Orchid DCB
(Acotec Scientific, Beijing, China)
coated with paclitaxel at a dose of 3.0 lg/mm2 in a urea excipient
|
PTA
(Admiral Xtreme uncoated balloon) |
Length of follow-up: 12 months
Loss-to-follow-up: I: 1/30 (missed visit) C:1/30 (missed visit)
Incomplete outcome data: none
|
Patency/restenosis Defined as primary patency at 12 months I: 24/29 (82.8%) C: 15/29 (48.3%)
Re-intervention based on symptomatic restenosis Defined as clinically driven target lesion revascularization I: 1/29, 3.5% C: 8/29, 27.6%
Quality of life Defined as change from baseline by EQ-5D Index I: 0.092 ± 0.142 C: 0.085 ± 0.147
Wound healing Not reported
Amputation Defined as target limb major amputation I: 0/29 C: 0/29
Mortality Defined as limb related death I: 0/29 C: 0/29 |
|
|
Lyden, 2022
ILLUMENATE Pivotal
NCT 01858428 |
Type of study: RCT
Setting and country: multicenter, US and EU
Funding and conflicts of interest: The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This trial was originally funded by Covidien who divested of the product to Spectranetics when acquired by Medtronic. Spectranetics was then acquired by Covidien, then Philips. The company and the National Co-PIs were responsible for design and conduct of the study, in the collection, analysis, interpretation of the data, and in the preparation, review and approval of the manuscript. The author(s) declared numerous potential conflicts of interest with respect to the research, authorship, and/or publication of this article (list too long to include in table). |
Inclusion criteria: Specified in previous publication.
Exclusion criteria: Specified in previous publication.
N total at baseline: Intervention: 200 Control: 100
Important prognostic factors2: Age, mean: I: 68.3 C: 69.8
Sex: I: 56.0% M C: 64.0% M
Diabetes I: 49.5% C: 52.0%
Groups were comparable at baseline. |
Stellarex (Spectranetics LLC, Colorado Springs, CO, an affiliate of Philips North America LLC, ambridge, MA) drug-coated angioplasty balloon |
POBA |
Length of follow-up: 4 years
Loss-to-follow-up: Not specified
Incomplete outcome data: Not specified
|
Patency/restenosis Defined as primary patency at 36 months I: 64.2% (Kaplan-Meier) C: 51.0% at 36 months, 24.5% of DCB subjects and 13.0% of PTA subjects had missing data.
Re-intervention based on symptomatic restenosis Defined as freedom from CD-TLR I: 76.7%, 95% CI = 69.8%–82.3% C: 71.0%, 95% CI = 60.5%–79.2%
Quality of life Not reported
Wound healing Not reported
Amputation Defined as major amputation I: 1/153 C: 0/77
All-cause mortality 36 months I: 19/199, 9.5% C: 10/96, 10.4%
48 months I: 30/192, 15.6% C: 14/92, 15.2%
|
|
|
Hausegger, 2024
Freeway Stent Study |
Type of study: RCT
Setting and country: Multicenter, Germany and Austria
Funding and conflicts of interest: This study was funded by Eurocor GmbH. S. Stahnke and J. Dambach were employees of Eurocor Tech GmbH, the other authors declare that they have no conflict of interest.
|
Inclusion criteria: Specified in previous publication.
Exclusion criteria: Specified in previous publication.
N total at baseline: Original RCT Intervention: 105 Control: 99 Present study I: 76 C: 74
Important prognostic factors2: Age, mean ± SD: I: 65.5±9.5 C: 64.8±9.3
Sex: I: 76.0% M C: 78.1% M
Diabetes I: 26.7% C: 27.4%
Rutherford class: Not reported.
Groups were comparable at baseline. |
Nitinolstent plus FREEWAY drug-eluting balloon postdilatation.
Reopening of original study Reasons for loss to follow-up compared to original study unclear |
Nitinolstent plus non-paclitaxel PTA postdilatation. |
Length of follow-up: 5-year follow-up
Loss-to-follow-up: Not applicable
Incomplete outcome data: Intervention: N=1 (1.3%) Reasons: missing vital data
Control: N=1 (1.4%) Reasons: missing vital data
|
Patency/restenosis Defined as rate of combined stent stenosis and very late stent thrombosis at 5 years I: 18.0% C: 22.9%
Re-intervention based on symptomatic restenosis Defined as freedom from clinically driven target lesion revascularization at 5 years I: 85.3% C:72.7% HR 0.48 (95% CI 0.25 to 0.93)
Quality of life Not reported.
Wound healing Not reported.
Amputation Defined as freedom from major or minor amputation I: 97.1% C: 100.0%
Mortality Defined as all-cause mortality at 5 years I: 12.0% C: 15.0% RR 0.81 (95% CI 0.35 to 1.90) |
Author’s conclusion: The present study did not find a mortality signal as seen in the 2018 meta-analysis data. To date, no plausible biological mechanism ahs been identified to explain the mortality, and no cause of death was found to be associated with the use of paclitaxel at doses administered with drug eluting devices. The efficacy results clearly demonstrate the clinical benefit of drug-eluting balloon treatment over a 5 year period. |
|
Ni, 2022
NCT 03844724 |
Type of study: RCT
Setting and country: multicenter, China
Funding and conflicts of interest: No funding specified. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. |
Inclusion criteria: 18–85 years of age had severe intermittent claudication or ischemic rest pain or minor tissue loss (Rutherford Clinical Category 3–5); stenosis of 70–99% with lesion lengths ≤30 cm, or a complete occlusion with lengths of ≤10 cm involving the superficial femoral or popliteal arteries (or both).
Exclusion criteria: (1) acute thrombus in the target vessels; (2) severe renal or hepatic dysfunction; (3) known contraindication or allergy to aspirin, clopidogrel, heparin, or paclitaxel; (4) life expectancy <1 year; (5) vessel stenosis or occlusion due to Buerger’s disease or autoimmune arteritis; (6) pregnancy; and (7) immunosuppressive agent therapy.
N total at baseline: Intervention: 93 Control: 99
Important prognostic factors2: Age, mean ± SD: I: 68.8 ± 8.3 C: 68.1 ± 10.5
Sex: I: 72.0% M C: 71.7% M
Diabetes I: 49.5% C: 46.5%
Rutherford class 3 I: 67.7% C: 67.7%
Rutherford class 4 I: 18.3% C: 22.2%
Rutherford class 5 I: 14.0% C: 10.1%
Groups were comparable at baseline. |
ZENFlow paclitaxel-coated balloon (PCB) catheter (Zylox Medical Device Inc., Zhejiang, China), a carrier-free DCB coated with paclitaxel (3 μg/mm2 ± 1 μg/mm2) |
uncoated balloon |
Length of follow-up: 12 months
Loss-to-follow-up: I: 3/93 C: 6/99
Incomplete outcome data: Not specified
|
Patency/restenosis Defined as primary patency at 12 months I: 54.0 (34/63) C: 31.3 (21/67)
Re-intervention based on symptomatic restenosis Defined as CD-TLR at 6 months I: 5.4 (5/93) C: 19.2 (19/99)
Quality of life Not reported
Wound healing Not reported
Amputation Defined as target limb major amputation I: 0 (0) C: 1 (1.0)
All-cause mortality at 12 months I: 3 (3.2) C: 2 (2.0) |
Authors’ conclusions: In conclusion, in this prospective, multicenter, randomized trial, the novel ZENFlow PCB was superior to standard PTA and had a favorable safety profile in patients with symptomatic femoropopliteal artery disease. |
|
Gouëffic, 2022
EMINENT study |
Type of study: RCT
Setting and country: 10 European countries (Austria, Belgium, France, Germany, Ireland, Italy, Spain, Switzerland, The Netherlands, the United Kingdom)
Funding and conflicts of interest: The study was funded and sponsored by Boston Scientific Corp, Marlborough MA.
Dr. Gouëffic has received research funding as well as personal fees and grants. Dr. Torsello, Dr. Zeller, Dr. Vermassen, Dr. Tepe, Dr. Kahlberg, Dr. Schindewolf, Dr. Sapoval, Dr. Diaz-Cartelle, and Dr. Stavroulakis received grants, travel grants, honoraria, or funding too. |
Inclusion criteria: All the following inclusion criteria were required to be met: presentation with Rutherford category 2, 3 or 4 symptomatology; lesions in the native SFA or proximal popliteal artery with stenosis ≥70% by visual angiographic assessment; vessel diameter of 4 to 6 mm; and total lesion length of 30 to 210 mm.
Exclusion criteria: Presence of any of the following exclusion criteria justified exclusion: dialysis treatment; target lesion or vessel previously treated with a drug-coated balloon within the prior 12 months or previously stented; prior SFA or proximal popliteal artery surgery in target limb; heavy calcification; and intraprocedural use of atherectomy, laser, or other debulking devices.
N total at baseline: Intervention: 508 Control: 267
Important prognostic factors2: Age, mean ± SD: I: 68.9±8.7 C: 68.9±9.1
Sex: I: 71.5% M C:67.4 % M
Diabetes (medically-treated) I: 31.9% C: 32.6%
Rutherford class 2 I: 29.6% C: 35.2%
Rutherford class 3 I: 66.3% C: 62.2%
Rutherford class 4 I: 3.6% C: 2.6%
Rutherford class 5 I: 0.4% C: 0.0%
Groups were comparable at baseline. |
Eluvia DES, a self-expanding nitinol stent coated with a fluorinated polymer and paclitaxel at a dose density of 0.167 µg/mm2 stent surface area. (stent length 150mm until November 2017, from November 2017 stent length 120 mm). |
BMS, self-expanding bare nitinol stents. |
Length of follow-up: 12 months
Loss-to-follow-up: Intervention: N=32 (6.3%) Reasons: withdrew (n=20), died (n=12)
Control: N=12 (4.5%) Reasons: withdrew (n=8), died (n=3)
Incomplete outcome data: Intervention: N=23 (4.5%) Reasons: missed visit/no data/out of window
Control: N=7 (2.6%) Reasons: missed visit/no data/out of window
|
Patency/restenosis Defined as peak systolic velocity ratio ≤2.4 at the 12 month visit in the absence of clinically driven target lesion revascularization or bypass of the target lesion I: 83.2% (337/405) C: 74.3% (165/222) Difference 8.9% (95% CI 2.1 to 15.7)
Re-intervention based on symptomatic restenosis Defined as target lesion revascularization I: 8.9% (42/474) C: 10.6% (28/263) Difference -1.8% (-6.3 to 2.7)
Quality of life Defined as health related quality of life (EuroQol 5-dimension 5-level questionnaire). Percentages of patients with improved scores at 12 months. Mobility I: 66.4% (295/444) C: 64.2% (158/246) Self-care I: 8.8% (39/445) Usual activities I: 38.0% (169/445) C: 37.0% (91/246) Pain/discomfort I: 53.6% (238/444) C: 58.1% (143/246) Anxiety/depression I: 22.5% (100/444) C: 20.0% (49/245)
Wound healing Not reported.
Amputation Defined as target limb major amputation I: 0.2% (1/474) C: 0.0% (0/263) Difference 0.2% (-0.2 to 0.6)
All-cause mortality I: 2.7% (13/474) C: 1.1% (3/263) Difference 1.6% (-0.3 to 3.6) |
Authors’ conclusion: Eluvia is the first DES to demonstrate superior 1-year primary patency compared with any globally marketed BMS in an adequately designed randomized trial. The trial supports the benefit of a polymer-based paclitaxel-eluting stent for treating SFA or proximal popliteal artery lesions of intermediate length. |
|
Teichgräber, 2022
EffPac trial |
Type of study: RCT
Setting and country: 11 German sites.
Funding and conflicts of interest: Open Access funding enabled and organized by Projekt DEAL. The study was supported by iVascular S.L.U., Barcelona, Spain and Endoscout, Freiburg, Germany. UT is a consultant for iVascular. TZ is coprincipal investigator of the ILICO study, a study sponsored by iVascular. Other authors declare no conflict of interest.
|
Inclusion criteria:
Exclusion criteria:
-post-menopausal (12 months natural amenorrhea or 6 months amenorrhea with serum FSH > 40mlU/ml) -sterilization after bilateral ovariectomy with or without hysterectomy -using an effective method of birth control for the duration of the trial: implants, injectables, combined oral contraceptives, intrauterine device (in place for a period of at least 2 months prior to screening) and with negative serum pregnancy test -sexual abstinence -vasectomy partner
N total at baseline: Intervention: 85 Control: 86
Important prognostic factors2: Age, mean ± SD: I: 68 ± 8 C: 68 ± 9
Sex: I: 60% M C: 70% M
Diabetes I: 36% C: 41%
Rutherford class 2 I: 15% C: 21%
Rutherford class 3 I: 81% C: 78%
Rutherford class 4 I: 2% C: 1%
Rutherford class 5 I: 1% C: 0%
Groups were comparable at baseline |
Luminor 35 DCB catheter (iVascular). Coated with paclitaxel at a surface concentration of 3 µg/mm2 and an organic ester excipient. |
Uncoated balloon catheters. |
Length of follow-up: 5 years
Loss-to-follow-up: Until 6 months Intervention: N=9 (10.6%) Reasons: withdrawal
Control: N=10 (11.6%) Reasons: withdrawal (n=8), death (n=2)
6 months – 1 year Intervention: N=1 (1.2%) Reasons: death
Control: N=0 (0%)
1 year – 2 years Intervention: N=15 (17.6%) Reasons: withdrawal
Control: N=20 (23.3%) Reasons: missed visits (n=2), withdrawal (n=18)
2 years – 42 months Intervention: N=8 (9.4%) Reasons: missed visit (n=1), withdrawal (n=5), death (n=2)
Control: N=7 (8.1%) Reasons: missed visit (n=2), death (n=5)
42 months – 5 years Intervention: N=6 (7.1%) Reasons: death
Control: N=7 (8.1%) Reasons: death
Incomplete outcome data: Not reported.
|
Patency/restenosis Defined as absence of >50% diameter restenosis of the target lesion by angiography or a peak systolic velocity ratio at <2.4 by duplex ultrasonography without the need for TLR. I: 61.4% C: 53.5%
Re-intervention based on symptomatic restenosis Defined as: freedom from clinically driven target lesion revascularization (CD-TLR) at five years I: 82.1% C: 73.7%
Quality of life Not reported.
Wound healing Not reported.
Amputation Defined as major target limb amputation I: 0/49 C: 2% (1/51)
All-cause death: I: 11% (9/80) C: 16% (14/86) |
Authors’ conclusion: Long-term follow-up of the EffPac trial showed superiority in terms of primary patency after femoropopliteal Luminor 35 DCB angioplasty compared to POBA over a period of 5 years. This finding was reflected by freedom from TLR, however, no longer with a significant difference. Femoropopliteal Luminor 35 DCB angioplasty is a sustainably efficacious and safe treatment approach. |
|
Fransson, 2023
|
Type of study: RCT
Setting and country: Sweden
Funding and conflicts of interest: supported by the Cook Medical, with limited unrestricted support. The authors and corresponding institution are solely responsible for data collection and interpretation. The authors declared no potential conflicts of interest. |
Inclusion criteria: CLTI and lesions in the SFA and first part of PA (P1-P2), target vessel diameter 4-8 mm and at least one crural artery patent to the foot.
Exclusion criteria: Age <18 years, ongoing or planned pregnancy, and patient unwillingness to participate.
N total at baseline: Intervention: 27 Control: 22
Important prognostic factors2: Age, median (IQR): I: 74 (68-79) C: 76 (70-80)
Sex: I: 56% M C: 50% M
Diabetes I: 59% C: 41%
Rutherford Class, median (IQR): I: 5 (4-5) C: 5 (4-5)
Groups were comparable at baseline. |
Zilver Flex DES (Drug eluting stents) |
Zilver Flex BMS (Bare metal stents) |
Length of follow-up: 24 months (national mortality records were monitored up to 5 years)
Loss-to-follow-up: Intervention: N=3 (11.1%) Reasons: death
Control: N=4 (18.2%) Reasons: death
Incomplete outcome data: Not reported.
|
Patency/restenosis Defined as primary patency 12 months I: 44% (12/27) C: 41% (9/22)
Defined as primary patency 24 months I: 33% (9/27) C: 41% (9/22)
Re-intervention based on symptomatic restenosis Defined as target lesion revascularization 12 months I: 37% (10/27) C: 27% (6/22)
Defined as target lesion revascularization 24 months I: 41% (11/27) C: 36% (8/22)
Quality of life Not reported.
Wound healing Not reported.
Amputation Defined as amputation rate 24 months I: 15% (4/27) C: 0%
Mortality: Defined as mortality 2 years I: 11% (3/27) C: 18% (4/22)
Defined as mortality 5 years I: 22% (6/27) C: 23% (5/22)
|
Authors’ conclusion: This study could not demonstrate superiority of DES compared to BMS in the treatment of long FP lesions in patients with CLTI, but was limited by insufficient patient inclusion. |
Risk of bias tabel
|
Study reference
(first author, publication year) |
Was the allocation sequence adequately generated?
Definitely yes Probably yes Probably no Definitely no |
Was the allocation adequately concealed?
Definitely yes Probably yes Probably no Definitely no |
Blinding: Was knowledge of the allocated interventions adequately prevented?
Were patients blinded?
Were healthcare providers blinded?
Were data collectors blinded?
Were outcome assessors blinded?
Were data analysts blinded?
Definitely yes Probably yes Probably no Definitely no |
Was loss to follow-up (missing outcome data) infrequent?
Definitely yes Probably yes Probably no Definitely no |
Are reports of the study free of selective outcome reporting?
Definitely yes Probably yes Probably no Definitely no |
Was the study apparently free of other problems that could put it at a risk of bias?
Definitely yes Probably yes Probably no Definitely no |
Overall risk of bias If applicable/necessary, per outcome measure
LOW Some concerns HIGH
|
|
Liao, 2022 |
Definitely yes
Reason: The randomization sequence was computer-generated. |
Probably yes
Reason: The randomization sequence was only opened after angiographic confirmation that the patient met all inclusion criteria and none of the exclusion criteria. |
Definitely no
Reason: The treating physicians were aware of the treatment choice because the DCBs looked different from uncoated balloons. Patients, follow-up investigators, laboratory personnel and evaluators were unaware of treatment choices. |
Definitely yes
Reason: Loss to follow-up was infrequent in intervention and control group. |
Definitely yes
Reason: All relevant outcomes were reported. |
Probably no
Reason: Limited number of patients enrolled, restricted to Chinese patients. Longer-term follow-up is needed. |
LOW (all outcomes)
|
|
Lyden, 2022 |
No information
|
No information |
Definitely no
Reason: Imaging core laboratories and clinical events committee were blinded, the operator could not be blinded to the treatment of the patient. |
No information |
Definitely yes
Reason: All relevant outcome measures were reported.
|
Probably no
Reason: The trial was underpowered to detect differences on any individual endpoint, and hence exposed to type II error. The results from the study are not directly generalizable to other DCBs. |
HIGH (all outcomes) |
|
Hausegger, 2024 |
No information |
Probably no
Reason: randomization with envelopes, but the study was open. |
Definitely no
Reason: Open, randomized study. |
Definitely yes
Reason: Loss-to-follow-up was infrequent (approximately 1%) in both the intervention and control group. |
Definitely yes
Reason: All relevant outcome measures were reported. |
Definitely no
Reason: The study was a reopening of a former RCT, recontacting patients by telephone or during a routine check-up. The original study was not powered for statistical analysis of 5-year mortality or TLR between the two treatment arms. |
HGH (all outcomes) |
|
Ni, 2022 |
Definitely yes
Reason: Randomization was computer-generated. |
Definitely yes
Reason: The generated numbers were sealed in envelopes, which were only opened after the evaluation of the target lesions. |
Definitely no
Reason: Due to visual differences the treating physician and catheterization laboratory staff were not blinded to the treatment assignment. The patients and radiologist/sonographer were blinded. |
Definitely yes
Reason: Loss-to-follow-up was infrequent in intervention and control group. |
Definitely yes
Reason: All relevant outcome measures were reported. |
Probably no
Reason: number of patients who agreed to angiography and duplex ultrasound was less than optimal, higher prevalence of longer lesion length in the DCB group, short follow-up duration. |
LOW (all outcomes) |
|
Gouëffic, 2022 |
Probably yes
Reason: Randomization was stratified by study site and lesion length. For each site, a computer-generated list of random treatment allocations using random permuted blocks of various sizes within each stratum was used. |
Probably no
Reason: No information. |
Definitely no
Reason: study staff were not blinded. Site personnel conducting clinical follow-up assessments were blinded whenever possible, patients were blinded, and core laboratory personnel and the CEC were blinded. |
Definitely no
Reason: Loss-to-follow-up was 10.8% in the intervention group, and 7.1% in the control group. |
Definitely yes
Reason: All relevant outcome measures were reported. |
Probably no
Reason: poor presentation of women and populations that are not White. The study reflects common practice in a limited geographic region. A pooled comparator group design was used. Follow-up occurred during COVID-19, which affected clinical trial conduct. |
HIGH (all outcomes) |
|
Teichgräber, 2022 |
No information
|
No information |
Probably no
Reason: participants and core laboratory staff members were blinded. No information about blinding of treating physicians. |
Definitely no
Reason: Loss-to-follow-up was above 10% in both the intervention and control group within 1 year. |
Definitely yes
Reason: All relevant outcome measures were reported. |
Probably no
Reason: The study was not powered to assess a difference in long-term all-cause mortality. The results cannot be transferred automatically to other DCB types. |
HIGH (all outcomes) |
|
Fransson, 2023 |
No information
Reason: the staff performed randomization. |
Definitely yes
Reason: Randomization was performed with blinded envelopes. |
Definitely no
Reason: The treating physician and staff were not blinded, patients were blinded. |
Definitely no
Reason: Loss to follow-up was 11.1% in the intervention group and 18.2% in the control group. |
Definitely yes
Reason: All relevant outcome measures were reported. |
Definitely no
Reason: Limited number of patients (underpowered), relative high rate of technical failure, non-consecutive randomization because patients were treated without being included in the study. |
Some concerns (all outcomes) |
Exclusie tabel
|
Reference |
Reason for exclusion |
|
Briody H, Kearns CA, Lee MJ. Mortality, Safety, and Effectiveness of Paclitaxel-Containing Balloons and Stents in the Femoropopliteal Artery: Systematic Review and Meta-Analysis of Randomized Controlled Trials since 2018. J Vasc Interv Radiol. 2024 Feb 28:S1051-0443(24)00198-2. doi: 10.1016/j.jvir.2023.12.574. Epub ahead of print. PMID: 38428483. |
More complete SR used |
|
Cao S, He T, Xie J, Feng H, Liu K, Qu B, Wu X. Drug-coated balloon angioplasty versus balloon angioplasty for treating patients with in-stent restenosis in the femoropopliteal artery: A meta-analysis. Medicine (Baltimore). 2021 Apr 23;100(16):e25599. doi: 10.1097/MD.0000000000025599. PMID: 33879723; PMCID: PMC8078449. |
Wrong P: in-stent restenosis |
|
Diehm N, Schneider H. Cost-effectiveness analysis of paclitaxel-coated balloons for endovascular therapy of femoropopliteal arterial obstructions. J Endovasc Ther. 2013 Dec;20(6):819-25. doi: 10.1583/13-4416R.1. PMID: 24325699. |
More complete SR used |
|
Dinh K, Limmer AM, Chen AZL, Thomas SD, Holden A, Schneider PA, Varcoe RL. Mortality Rates After Paclitaxel-Coated Device Use in Patients With Occlusive Femoropopliteal Disease: An Updated Systematic Review and Meta-Analysis of Randomized Controlled Trials. J Endovasc Ther. 2021 Oct;28(5):755-777. doi: 10.1177/15266028211023505. Epub 2021 Jun 9. PMID: 34106028. |
More complete SR used |
|
D'Oria M, Mastrorilli D, Secemsky E, Behrendt CA, Veraldi G, DeMartino R, Mani K, Budtz-Lilly J, Scali S, Saab F, Calvagna C, Mezzetto L, Ruaro B, Lepidi S. Robustness of Longitudinal Safety and Efficacy After Paclitaxel-Based Endovascular Therapy for Treatment of Femoro-Popliteal Artery Occlusive Disease: An Updated Systematic Review and Meta-Analysis of Randomized Controlled Trials. Ann Vasc Surg. 2024 Apr;101:164-178. doi: 10.1016/j.avsg.2023.11.024. Epub 2023 Dec 26. PMID: 38154491. |
More complete SR used |
|
Duda SH, Pusich B, Richter G, Landwehr P, Oliva VL, Tielbeek A, Wiesinger B, Hak JB, Tielemans H, Ziemer G, Cristea E, Lansky A, Bérégi JP. Sirolimus-eluting stents for the treatment of obstructive superficial femoral artery disease: six-month results. J Invasive Cardiol. 2004 Jan;16 Suppl A:15A-19A. PMID: 23573600. |
Wrong intervention |
|
He Z, Wang H, Lin F, Ding W, Chen K, Zhang Z. The safety and efficacy of different endovascular treatments for in-stent restenosis of the femoropopliteal artery: A network meta-analysis. Vasc Med. 2022 Jun;27(3):239-250. doi: 10.1177/1358863X211070327. Epub 2022 Feb 15. PMID: 35164613. |
Wrong P: in-stent restenosis |
|
Katsanos K, Spiliopoulos S, Teichgräber U, Kitrou P, Del Giudice C, Björkman P, Bisdas T, de Boer S, Krokidis M, Karnabatidis D. Editor's Choice - Risk of Major Amputation Following Application of Paclitaxel Coated Balloons in the Lower Limb Arteries: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Eur J Vasc Endovasc Surg. 2022 Jan;63(1):60-71. doi: 10.1016/j.ejvs.2021.05.027. Epub 2021 Jul 27. PMID: 34326002. |
More complete SR used |
|
Katsogridakis E, Ballance L, Cawley O, Antoniou GA. Drug-eluting stents for the treatment of complex femoro-popliteal disease: a systematic review and meta-analysis. J Cardiovasc Surg (Torino). 2022 Jun;63(3):299-307. doi: 10.23736/S0021-9509.18.10614-8. Epub 2018 Aug 29. PMID: 30168308. |
More complete SR used |
|
Li M, Tu H, Yan Y, Guo Z, Zhu H, Niu J, Yin M. Meta-analysis of outcomes from drug-eluting stent implantation in femoropopliteal arteries. PLoS One. 2023 Sep 21;18(9):e0291466. doi: 10.1371/journal.pone.0291466. PMID: 37733656; PMCID: PMC10513203. |
More complete SR used |
|
Mao J, Sedrakyan A, Goodney PP, Malone M, Cavanaugh KJ, Marinac-Dabic D, Eldrup-Jorgensen J, Bertges DJ; Society for Vascular Surgery Vascular Quality Initiative and the Vascular Implant Surveillance and Interventional Outcomes Network. Editor's Choice - Real World Study of Mortality After the Use of Paclitaxel Coated Devices in Peripheral Vascular Intervention. Eur J Vasc Endovasc Surg. 2023 Jan;65(1):131-140. doi: 10.1016/j.ejvs.2022.08.014. Epub 2022 Aug 23. PMID: 36007713; PMCID: PMC9839562. |
More complete SR used |
|
Nowakowski P, Uchto W, Hrycek E, Kachel M, Ludyga T, Polczyk F, Żurakowski A, Kaźmierczak P, Granada JF, Nowakowska I, Kiesz RS, Milewski KP, Buszman PE, Buszman PP. Microcrystalline paclitaxel-coated balloon for revascularization of femoropopliteal artery disease: Three-year outcomes of the randomized BIOPAC trial. Vasc Med. 2021 Aug;26(4):401-408. doi: 10.1177/1358863X20988360. Epub 2021 Mar 9. PMID: 33686879. |
In SR |
|
Park JI, Ko YG, Lee YJ, Lee SJ, Hong SJ, Ahn CM, Kim JS, Kim BK, Hong MK, Yu CW, Rha SW, Park JK, Min PK, Yoon CH, Lee SR, Park SH, Choi DH. Long coverage with drug-eluting stents is superior to spot coverage for long femoropopliteal artery disease: PARADE II study. Front Cardiovasc Med. 2022 Oct 19;9:1022071. doi: 10.3389/fcvm.2022.1022071. PMID: 36337904; PMCID: PMC9626975. |
Wrong comparison |
|
Pecoraro F, Dinoto E, Pakeliani D, Mirabella D, Ferlito F, Bajardi G. Efficacy and one-year outcomes of Luminor® paclitaxel-coated drug-eluting balloon in the treatment of popliteal artery atherosclerosis lesions. Ann Vasc Surg. 2021 Oct;76:370-377. doi: 10.1016/j.avsg.2021.04.015. Epub 2021 May 2. PMID: 33951533. |
? |
|
Sachar R, Soga Y, Ansari MM, Kozuki A, Lopez L, Brodmann M, Schroë H, Ramanath VS, Diaz-Cartelle J, Zeller T; RANGER II SFA Investigators. 1-Year Results From the RANGER II SFA Randomized Trial of the Ranger Drug-Coated Balloon. JACC Cardiovasc Interv. 2021 May 24;14(10):1123-1133. doi: 10.1016/j.jcin.2021.03.021. PMID: 34016410. |
In SR |
|
Shishehbor MH, Scheinert D, Jain A, Brodmann M, Tepe G, Ando K, Krishnan P, Iida O, Laird JR, Schneider PA, Rocha-Singh KJ, Zeller T. Comparison of Drug-Coated Balloons vs Bare-Metal Stents in Patients With Femoropopliteal Arterial Disease. J Am Coll Cardiol. 2023 Jan 24;81(3):237-249. doi: 10.1016/j.jacc.2022.10.016. Epub 2022 Nov 1. PMID: 36332764. |
Wrong comparison |
|
Sun G, Liu J, Jia S, Zhang J, Zhuang B, Jia X, Fu W, Wu D, Wang F, Zhao Y, Guo P, Bi W, Wang S, Guo W; AcoArt I Trial Investigators. Comparison of drug-coated balloon angioplasty versus uncoated balloon angioplasty in treatment of total occlusions with severe femoropopliteal lesions: An additional analysis from the AcoArt I study. Vascular. 2021 Jun;29(3):340-349. doi: 10.1177/1708538120953663. Epub 2020 Sep 9. PMID: 32903168. |
Wrong study design |
|
Taneva GT, Pitoulias GA, Abu Bakr N, Kazemtash M, Muñoz Castellanos J, Donas KP. Assessment of Sirolimus- vs. paCLitaxEl-coated balloon angioPlasty In atherosclerotic femoropopliteal lesiOnS (ASCLEPIOS Study): preliminary results. J Cardiovasc Surg (Torino). 2022 Feb;63(1):8-12. doi: 10.23736/S0021-9509.21.12169-X. PMID: 35179337. |
Preliminary results |
|
Tepe G, Schnorr B, Albrecht T, Brechtel K, Claussen CD, Scheller B, Speck U, Zeller T. Angioplasty of femoral-popliteal arteries with drug-coated balloons: 5-year follow-up of the THUNDER trial. JACC Cardiovasc Interv. 2015 Jan;8(1 Pt A):102-8. doi: 10.1016/j.jcin.2014.07.023. PMID: 25616822. |
In SR |
|
Ullah W, Zghouzi M, Sattar Z, Ahmad B, Zahid S, Suleiman AM, Sattar Y, Khan MZ, Paul T, Bagur R, Qureshi MI, Fischman DL, Banerjee S, Prasad A, Alraies MC. Safety and efficacy of drug-coated balloon for peripheral artery revascularization-A systematic review and meta-analysis. Catheter Cardiovasc Interv. 2022 Mar;99(4):1319-1326. doi: 10.1002/ccd.30074. Epub 2022 Jan 18. PMID: 35043555. |
More complete SR used |
|
Xu Y, Liu J, Zhang J, Zhuang B, Jia X, Fu W, Wu D, Wang F, Zhao Y, Guo P, Bi W, Wang S, Guo W. Long-term safety and efficacy of angioplasty of femoropopliteal artery disease with drug-coated balloons from the AcoArt I trial. J Vasc Surg. 2021 Sep;74(3):756-762.e3. doi: 10.1016/j.jvs.2021.01.041. Epub 2021 Feb 15. PMID: 33600928. |
In SR |
|
Ye W, Zhang X, Dai X, Huang X, Liu Z, Jiang M, Liu C. Reewarm™ PTX drug-coated balloon in the treatment of femoropopliteal artery disease: A multi-center, randomized controlled trial in China. Int J Cardiol. 2021 Mar 1;326:164-169. doi: 10.1016/j.ijcard.2020.10.060. Epub 2020 Oct 28. PMID: 33127414. |
In SR |
|
Zhang B, Yang M, He T, Li X, Gu J, Zhang X, Dai X, Li X, Lu X, Lang D, Hu H, Chen X, Yang B, Gu H, Zhang X, Zou Y. Twelve-Month Results From the First-in-China Prospective, Multi-Center, Randomized, Controlled Study of the FREEWAY Paclitaxel-Coated Balloon for Femoropopliteal Treatment. Front Cardiovasc Med. 2021 Sep 10;8:686267. doi: 10.3389/fcvm.2021.686267. PMID: 34568443; PMCID: PMC8460758. |
In SR |
|
Zhang C, Yin G. Safety of paclitaxel-coated devices in the femoropopliteal arteries: A systematic review and meta-analysis. PLoS One. 2022 Oct 13;17(10):e0275888. doi: 10.1371/journal.pone.0275888. PMID: 36227807; PMCID: PMC9560511. |
More complete SR used |
|
Zhao S, Li L, Cui K. Network Analysis of Endovascular Treatment Strategies for Femoropopliteal Arterial Occlusive Disease. J Endovasc Ther. 2023 Aug;30(4):487-498. doi: 10.1177/15266028221090434. Epub 2022 Apr 8. PMID: 35392691. |
Wrong study design: NMA |
|
Zhou Y, Wang J, He H, Li Q, Li M, Li X, Shu C. Comparative effectiveness of endovascular treatment modalities for de novo femoropopliteal lesions in intermittent claudication: A network meta-analysis of randomized controlled trials. Int J Cardiol. 2021 Nov 15;343:122-130. doi: 10.1016/j.ijcard.2021.08.038. Epub 2021 Aug 27. PMID: 34461162. |
Wrong study design: NMA |
Verantwoording
Beoordelingsdatum en geldigheid
Publicatiedatum : 02-04-2025
Beoordeeld op geldigheid : 01-04-2025
Algemene gegevens
De ontwikkeling/herziening van deze richtlijnmodule werd ondersteund door het Kennisinstituut van de Federatie Medisch Specialisten (www.demedischspecialist.nl/kennisinstituut) en werd gefinancierd uit de Kwaliteitsgelden Medisch Specialisten (SKMS).
De financier heeft geen enkele invloed gehad op de inhoud van de richtlijnmodule.
Samenstelling werkgroep
Voor het ontwikkelen van de richtlijnmodule is in 2021 een multidisciplinaire werkgroep ingesteld, bestaande uit vertegenwoordigers van alle relevante specialismen (zie hiervoor de Samenstelling van de werkgroep) die betrokken zijn bij de zorg rondom patiënten met (chronisch) perifeer arterieel vaatlijden van de onderste extremiteit(en).
Werkgroep
- Dr. B. (Bram) Fioole (voorzitter), Nederlandse Vereniging voor Heelkunde
- Dr. C. (Çagdas) Ünlü, Nederlandse Vereniging voor Heelkunde
- Drs. J.L. (Jorg) de Bruin, Nederlandse Vereniging voor Heelkunde
- Prof. dr. B.M.E. (Barend) Mees, Nederlandse Vereniging voor Heelkunde
- Drs. D.A.F. (Daniel) van den Heuvel, Nederlandse Vereniging voor Radiologie
- Dr. S.W. (Sanne) de Boer, Nederlandse Vereniging voor Radiologie
- Dr. R. (Rinske) Loeffen), Nederlandse Internisten Vereniging
- Dr. M.E.L. (Marie-Louise) Bartelink, Nederlands Huisartsen Genootschap
- E. (Emilien) Wegerif, Nederlandse Vereniging voor Heelkunde
- J. (Jenny) Zwiggelaar, Koninklijk Nederlands Genootschap voor Fysiotherapie / Vereniging van Oefentherapeuten Cesar en Mensendieck
- G. (Gilaine) Kleian, Harteraad
- P.A.H. (Patricia) van Mierlo – van den Broek, MANP, Verpleegkundigen & Verzorgenden Nederland / VS Vaatchirurgie Netwerk
Met ondersteuning van:
- Dr. W. (Wouter) Harmsen, senior adviseur, Kennisinstituut van de Federatie Medisch Specialisten (tot 2023)
- Dr. R. (Romy) Zwarts - van de Putte, adviseur, Kennisinstituut van de Federatie Medisch Specialisten (tot 2023)
- Dr. M.S. (Matthijs) Ruiter, senior adviseur, Kennisinstituut van de Federatie Medisch Specialisten (vanaf 2023)
- M. (Mitchel) Griekspoor, MSc, adviseur, Kennisinstituut van de Federatie Medisch Specialisten (vanaf 2023)
Belangenverklaringen
De Code ter voorkoming van oneigenlijke beïnvloeding door belangenverstrengeling is gevolgd. Alle werkgroepleden hebben schriftelijk verklaard of zij in de laatste drie jaar directe financiële belangen (betrekking bij een commercieel bedrijf, persoonlijke financiële belangen, onderzoeksfinanciering) of indirecte belangen (persoonlijke relaties, reputatiemanagement) hebben gehad. Gedurende de ontwikkeling of herziening van een module worden wijzigingen in belangen aan de voorzitter doorgegeven. De belangenverklaring wordt opnieuw bevestigd tijdens de commentaarfase.
Een overzicht van de belangen van werkgroepleden en het oordeel over het omgaan met eventuele belangen vindt u in onderstaande tabel. De ondertekende belangenverklaringen zijn op te vragen bij het secretariaat van het Kennisinstituut van de Federatie Medisch Specialisten.
|
Werkgroeplid |
Hoofdfunctie |
Project Tekstveld |
Nevenwerkzaamheden |
Persoonlijke Financiële belangen |
Persoonlijke relaties |
Extern gefinancierd onderzoek |
Intell. Belangen en reputatie |
Overige belangen |
Ondernomen actie |
|
Bram Fioole (vz.) |
Vaatchirurg |
Richtlijn Perifeer arterieel vaatlijden |
Opleider vaatchirurgie en algemene heelkunde Maasstadziekenhuis, onbetaald
Secretaris Stichting DEAll (stichting ter bevordering van wetenschappelijk onderzoek op het gebied van de vaatchirurgie en interventieradiologie), onbetaald.
|
Geen. |
Geen. |
Stichting DEAll/FOREST trial/projectleider-> ja Getinge/DISCOVER trial/projectleider-> ja Cook/Zephyr registry/ projectleider -> ja |
Geen. |
Geen. |
Geen restricties voor deelname aan de richtlijn. |
|
Marie-Louise Bartelink |
* 80% huisartsdocent en -onderzoeker, associate professor * 20% huisartsdocent
|
Richtlijn Perifeer arterieel vaatlijden |
Geen. |
Geen. |
Geen. |
ZonMw - EBM-leren in de huisartspraktijk, kwalittatieve studies - Projectleider |
Geen. |
Geen. |
Geen restricties voor deelname aan de richtlijn. |
|
Patricia van Mierlo – van den Broek |
Verpleegkundig Specialist in het specialisme Algemene Gezondheidszorg werkzaam bij de afdeling Vaatchirurgie.
|
Richtlijn Perifeer arterieel vaatlijden |
Als vrijwilliger werkzaam (onbetaald) als collectant voor een aantal goede doelen (KWF en dierenbescherming) |
Geen. |
Geen. |
Betrokken bij de "FOREST"-trial (randomized comparison of FemORal drug-Eluting balloons and Stents), een gerandomiseerd prospectief multicenter onderzoek waarbij de langere termijn resultaten van drug-eluting ballonnen en stents in het AFS traject onderzocht wordt (NL57055.101.16). Deze trial wordt gesponsord door de stichting DEAII (Dutch Endovascular Alliance).
|
Geen. |
Geen. |
Geen restricties voor deelname aan de richtlijn. |
|
Emilien Wegerif |
Arts onderzoeker |
Richtlijn Perifeer arterieel vaatlijden
|
Eigenaresse Caro-Jo Amsterdam, eenmanszaak betaald Caro-Jo Amsterdam is een Chaar sierraden merk
|
Ik heb geen financiële belangen bij de uitkomsten van de publicaties |
Geen. |
Geen. |
Geen. |
Geen. |
Geen restricties voor deelname aan de richtlijn. |
|
Jenny Zwiggelaar |
*Fysiotherapeut, hart-vaat-long gespecialiseerd
*Projectmedewerker Chronisch ZorgNet
Beide functies in loondienst |
Richtlijn Perifeer arterieel vaatlijden
|
Geen. |
Geen, enkel de bekende werkrelaties. |
Geen. |
Geen. |
Geen persoonlijk belangen of reputatiegewin. Ik treed in deze werkgroep als afgevaardigde van de beroepsgroep KNGF en zal enkel de kennis vanuit ChronischZorgnet (voorheen ClaudicatioNet) meebrengen.
|
Chronisch ZorgNet is op de hoogte en ondersteund de inbreng in deze werkgroep. Vanuit KNGF is de duidelijke opdracht om alle fysiotherapeuten met de specialisatie PAV te vertegenwoordigen. Dit zal naar verwachting ook beter zichtbaar worden bij het invoeren van het aantekenregister Vaat (als onderdeel van deelregistratie hart-vaat-long-fysiotherapeut). Er is een open en prettige communicatie onderling en geen belangenverstrengeling.
|
Geen restricties voor deelname aan de richtlijn. |
|
Rinske Loeffen |
Internist vasculair geneeskundige |
Richtlijn Perifeer arterieel vaatlijden
|
* Redactielid Focus Vasculair (onbetaald) * Werkgroep Tabaksontmoediging NVALT (onbetaald) * Werkgroep SKMS project Stop met Roken Zorg (onbetaald) * Werkgroep Acute boekje vasculaire geneeskunde (onbetaald).
|
Geen. |
Geen. |
Geen. |
Geen. |
Geen. |
Geen restricties voor deelname aan de richtlijn. |
|
Sanne de Boer |
Interventieradioloog |
Richtlijn Perifeer arterieel vaatlijden |
* Verschillende wetenschappelijke en beleidscommissies binnen de NVIR (onbetaald) * Institutioneel contract met Brainlab voor consultant werkzaamheden * Institutioneel contract met MUMC/CTCM * Incidenteel consultant werkzaamheden voor Philips, ook via institutioneel contract.
|
Zie nevenwerkzaamheden. |
Geen. |
|
Geen. |
Geen. |
Geen restricties. Onderwerp van advieswerk valt buiten de afbakening van de richtlijn. |
|
Çagdas Ünlü |
Vaatchirurg |
Richtlijn Perifeer arterieel vaatlijden
|
Projectleider CLEARPATH, studie na antistolling na endovasculaire behandeling ZonMw subsidie |
Geen. |
Geen. |
ZonMw (Clearpath) |
Geen. |
Geen. |
Geen restricties voor deelname aan de richtlijn. |
|
Daniel van den Heuvel |
Interventie Radioloog |
Richtlijn Perifeer arterieel vaatlijden
|
Proctor voor bedrijf LimFlow SA, Parijs. Betaald. Adjudicator voor Bioreact studie, Biotronic. Betaald. |
Zie nevenwerkzaamheden. Verder geen persoonlijke financiële belangen. |
Geen. |
Philips/Illumenate BTK PMS. Effectiviteit en veiligheid van de Stellarex .014 Ballon bij patiënten met kritieke ischemie ->ja. Micromedical/Heal study, PMS. Effectiviteit en veiligheid van de Microstent bij patiënten met kritieke ischemie -> ja. Philips/REPEAT study. Reproduceerbaarheid van 2D perfusie angiografie-> ja. |
Geen. |
Geen. |
Geen restricties voor deelname aan de richtlijn. |
|
Jorg de Bruin |
Vaatchirurg |
Richtlijn Perifeer arterieel vaatlijden
|
EMC; Klinisch en wetenschappelijk werk vaatchirurgie in academisch ziekenhuis |
Geen conflict of specifiek belang. |
Geen. |
Geen. |
Geen. |
Geen. |
Geen restricties voor deelname aan de richtlijn. |
|
Ilse Verstraaten |
Beleidsadviseur Harteraard (betaald) |
Richtlijn Perifeer arterieel vaatlijden
|
Geen. |
Geen. |
Geen. |
Geen. |
Geen. |
Geen. |
Geen restricties voor deelname aan de richtlijn.
|
|
Gilaine Kleian |
Junior projectmedewerker, Harteraad |
Richtlijn Perifeer arterieel vaatlijden
|
Geen. |
Geen. |
Geen. |
Geen. |
Geen. |
Geen. |
Geen restricties voor deelname aan de richtlijn.
|
|
Barend Mees |
Vaatchirurg |
Richtlijn Perifeer arterieel vaatlijden
|
* Secretaris bestuur Nederlandse Vereniging voor Vaatchirurgie * Lid Commissie Richtlijnen NVvH * Council Member European Society for Vascular Surgery * Member ESVS Steering Guideline Committee * Director European Vascular Course. * Bestuur Stichting Drie Lichten * Lid Onderzoekspijler Dutch Cardiovascular Alliance * Lid adviesraad Marfan patienten * Consultancy werkzaamheden voor Philips, Bentley Innomed. |
Geen. |
Geen. |
* Regmed XB: Cardiovascular Moonshot (Moonshot leader) * EU-Horizon 2020: PAPA-ARTIS (local PI) * InScIte: XS-graft (onderzoeksleider) * Philips: SAVER-registry (National PI) * ID3: Supersurg trial (local PI) * Cook: Zephyr registry (local PI) * Philips: FORS registry (local co-PI) * ZonMw: GENPAD study (local PI) |
*Ontwikkelaar van de mazeBox, een training tool voor endovasculaire technieken. |
Geen. |
Geen restricties voor deelname aan richtlijn. Genoemde onderzoek valt buiten de afbakening van de richtlijn. |
Inbreng patiëntenperspectief
Er werd aandacht besteed aan het patiëntenperspectief door het uitnodigen van Harteraad voor de schriftelijke knelpuntenanalyse. Het verslag hiervan is besproken in de werkgroep. De verkregen input is meegenomen bij het opstellen van de uitgangsvragen, de keuze voor de uitkomstmaten en bij het opstellen van de overwegingen. De conceptrichtlijn is tevens voor commentaar voorgelegd aan de deelnemers van de schriftelijke knelpuntenanalyse en de eventueel aangeleverde commentaren zijn bekeken en verwerkt.
Kwalitatieve raming van mogelijke financiële gevolgen in het kader van de Wkkgz
Bij de richtlijnmodule is conform de Wet kwaliteit, klachten en geschillen zorg (Wkkgz) een kwalitatieve raming uitgevoerd om te beoordelen of de aanbevelingen mogelijk leiden tot substantiële financiële gevolgen. Bij het uitvoeren van deze beoordeling is de richtlijnmodule op verschillende domeinen getoetst.
|
Module |
Uitkomst raming |
Toelichting |
|
Module 7: Femoro-popliteaal: Gecoate ballonnen en stents |
Geen substantiële financiële gevolgen |
Uit de toetsing volgt dat de aanbevelingen een toename in directe kosten kunnen hebben, maar met een kostenbesparend effect op de langere termijn. |
Werkwijze
AGREE
Deze richtlijnmodule is opgesteld conform de eisen vermeld in het rapport Medisch Specialistische Richtlijnen 2.0 van de adviescommissie Richtlijnen van de Raad Kwaliteit. Dit rapport is gebaseerd op het AGREE II instrument (Appraisal of Guidelines for Research & Evaluation II; Brouwers, 2010).
Knelpuntenanalyse en uitgangsvragen
Tijdens de voorbereidende fase inventariseerde de werkgroep de knelpunten in de zorg voor patiënten met (chronisch) perifeer arterieel vaatlijden van de onderste extremiteit(en). Tevens zijn er knelpunten aangedragen via een schriftelijke knelpuntenanalyse. Een verslag hiervan is opgenomen onder aanverwante producten.
Op basis van de uitkomsten van de knelpuntenanalyse zijn door de werkgroep concept-uitgangsvragen opgesteld en definitief vastgesteld.
Uitkomstmaten
Na het opstellen van de zoekvraag behorende bij de uitgangsvraag inventariseerde de werkgroep welke uitkomstmaten voor de patiënt relevant zijn, waarbij zowel naar gewenste als ongewenste effecten werd gekeken. Hierbij werd een maximum van acht uitkomstmaten gehanteerd. De werkgroep waardeerde deze uitkomstmaten volgens hun relatieve belang bij de besluitvorming rondom aanbevelingen, als cruciaal (kritiek voor de besluitvorming), belangrijk (maar niet cruciaal) en onbelangrijk. Tevens definieerde de werkgroep tenminste voor de cruciale uitkomstmaten welke verschillen zij klinisch (patiënt) relevant vonden.
Methode literatuursamenvatting
Een uitgebreide beschrijving van de strategie voor zoeken en selecteren van literatuur is te vinden onder ‘Zoeken en selecteren’ onder Onderbouwing. Indien mogelijk werd de data uit verschillende studies gepoold in een random-effects model. [Review Manager 5.4] werd gebruikt voor de statistische analyses. De beoordeling van de kracht van het wetenschappelijke bewijs wordt hieronder toegelicht.
Beoordelen van de kracht van het wetenschappelijke bewijs
De kracht van het wetenschappelijke bewijs werd bepaald volgens de GRADE-methode. GRADE staat voor ‘Grading Recommendations Assessment, Development and Evaluation’ (zie http://www.gradeworkinggroup.org/). De basisprincipes van de GRADE-methodiek zijn: het benoemen en prioriteren van de klinisch (patiënt) relevante uitkomstmaten, een systematische review per uitkomstmaat, en een beoordeling van de bewijskracht per uitkomstmaat op basis van de acht GRADE-domeinen (domeinen voor downgraden: risk of bias, inconsistentie, indirectheid, imprecisie, en publicatiebias; domeinen voor upgraden: dosis-effect relatie, groot effect, en residuele plausibele confounding).
GRADE onderscheidt vier gradaties voor de kwaliteit van het wetenschappelijk bewijs: hoog, redelijk, laag en zeer laag. Deze gradaties verwijzen naar de mate van zekerheid die er bestaat over de literatuurconclusie, in het bijzonder de mate van zekerheid dat de literatuurconclusie de aanbeveling adequaat ondersteunt (Schünemann, 2013; Hultcrantz, 2017).
|
GRADE |
Definitie |
|
Hoog |
|
|
Redelijk |
|
|
Laag |
|
|
Zeer laag |
|
Bij het beoordelen (graderen) van de kracht van het wetenschappelijk bewijs in richtlijnen volgens de GRADE-methodiek spelen grenzen voor klinische besluitvorming een belangrijke rol (Hultcrantz, 2017). Dit zijn de grenzen die bij overschrijding aanleiding zouden geven tot een aanpassing van de aanbeveling. Om de grenzen voor klinische besluitvorming te bepalen moeten alle relevante uitkomstmaten en overwegingen worden meegewogen. De grenzen voor klinische besluitvorming zijn daarmee niet één op één vergelijkbaar met het minimaal klinisch relevant verschil (Minimal Clinically Important Difference, MCID). Met name in situaties waarin een interventie geen belangrijke nadelen heeft en de kosten relatief laag zijn, kan de grens voor klinische besluitvorming met betrekking tot de effectiviteit van de interventie bij een lagere waarde (dichter bij het nuleffect) liggen dan de MCID (Hultcrantz, 2017).
Overwegingen (van bewijs naar aanbeveling)
Om te komen tot een aanbeveling zijn naast (de kwaliteit van) het wetenschappelijke bewijs ook andere aspecten belangrijk en worden meegewogen, zoals aanvullende argumenten uit bijvoorbeeld de biomechanica of fysiologie, waarden en voorkeuren van patiënten, kosten (middelenbeslag), aanvaardbaarheid, haalbaarheid en implementatie. Deze aspecten zijn systematisch vermeld en beoordeeld (gewogen) onder het kopje ‘Overwegingen’ en kunnen (mede) gebaseerd zijn op expert opinion. Hierbij is gebruik gemaakt van een gestructureerd format gebaseerd op het evidence-to-decision framework van de internationale GRADE Working Group (Alonso-Coello, 2016a; Alonso-Coello 2016b). Dit evidence-to-decision framework is een integraal onderdeel van de GRADE methodiek.
Formuleren van aanbevelingen
De aanbevelingen geven antwoord op de uitgangsvraag en zijn gebaseerd op het beschikbare wetenschappelijke bewijs en de belangrijkste overwegingen, en een weging van de gunstige en ongunstige effecten van de relevante interventies. De kracht van het wetenschappelijk bewijs en het gewicht dat door de werkgroep wordt toegekend aan de overwegingen, bepalen samen de sterkte van de aanbeveling. Conform de GRADE-methodiek sluit een lage bewijskracht van conclusies in de systematische literatuuranalyse een sterke aanbeveling niet a priori uit, en zijn bij een hoge bewijskracht ook zwakke aanbevelingen mogelijk (Agoritsas, 2017; Neumann, 2016). De sterkte van de aanbeveling wordt altijd bepaald door weging van alle relevante argumenten tezamen. De werkgroep heeft bij elke aanbeveling opgenomen hoe zij tot de richting en sterkte van de aanbeveling zijn gekomen.
In de GRADE-methodiek wordt onderscheid gemaakt tussen sterke en zwakke (of conditionele) aanbevelingen. De sterkte van een aanbeveling verwijst naar de mate van zekerheid dat de voordelen van de interventie opwegen tegen de nadelen (of vice versa), gezien over het hele spectrum van patiënten waarvoor de aanbeveling is bedoeld. De sterkte van een aanbeveling heeft duidelijke implicaties voor patiënten, behandelaars en beleidsmakers (zie onderstaande tabel). Een aanbeveling is geen dictaat, zelfs een sterke aanbeveling gebaseerd op bewijs van hoge kwaliteit (GRADE gradering HOOG) zal niet altijd van toepassing zijn, onder alle mogelijke omstandigheden en voor elke individuele patiënt.
|
Implicaties van sterke en zwakke aanbevelingen voor verschillende richtlijngebruikers |
||
|
|
||
|
|
Sterke aanbeveling |
Zwakke (conditionele) aanbeveling |
|
Voor patiënten |
De meeste patiënten zouden de aanbevolen interventie of aanpak kiezen en slechts een klein aantal niet. |
Een aanzienlijk deel van de patiënten zouden de aanbevolen interventie of aanpak kiezen, maar veel patiënten ook niet. |
|
Voor behandelaars |
De meeste patiënten zouden de aanbevolen interventie of aanpak moeten ontvangen. |
Er zijn meerdere geschikte interventies of aanpakken. De patiënt moet worden ondersteund bij de keuze voor de interventie of aanpak die het beste aansluit bij zijn of haar waarden en voorkeuren. |
|
Voor beleidsmakers |
De aanbevolen interventie of aanpak kan worden gezien als standaardbeleid. |
Beleidsbepaling vereist uitvoerige discussie met betrokkenheid van veel stakeholders. Er is een grotere kans op lokale beleidsverschillen. |
Organisatie van zorg
In de knelpuntenanalyse en bij de ontwikkeling van de richtlijnmodule is expliciet aandacht geweest voor de organisatie van zorg: alle aspecten die randvoorwaardelijk zijn voor het verlenen van zorg (zoals coördinatie, communicatie, (financiële) middelen, mankracht en infrastructuur). Randvoorwaarden die relevant zijn voor het beantwoorden van deze specifieke uitgangsvraag zijn genoemd bij de overwegingen. Meer algemene, overkoepelende, of bijkomende aspecten van de organisatie van zorg worden behandeld in module 14 en 15.
Commentaar- en autorisatiefase
De conceptrichtlijnmodule werd aan de betrokken (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd ter commentaar. De commentaren werden verzameld en besproken met de werkgroep. Naar aanleiding van de commentaren werd de conceptrichtlijnmodule aangepast en definitief vastgesteld door de werkgroep. De definitieve richtlijnmodule werd aan de deelnemende (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd voor autorisatie en door hen geautoriseerd dan wel geaccordeerd.
Literatuur
Agoritsas T, Merglen A, Heen AF, Kristiansen A, Neumann I, Brito JP, Brignardello-Petersen R, Alexander PE, Rind DM, Vandvik PO, Guyatt GH. UpToDate adherence to GRADE criteria for strong recommendations: an analytical survey. BMJ Open. 2017 Nov 16;7(11):e018593. doi: 10.1136/bmjopen-2017-018593. PubMed PMID: 29150475; PubMed Central PMCID: PMC5701989.
Alonso-Coello P, Schünemann HJ, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Rada G, Rosenbaum S, Morelli A, Guyatt GH, Oxman AD; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ. 2016 Jun 28;353:i2016. doi: 10.1136/bmj.i2016. PubMed PMID: 27353417.
Alonso-Coello P, Oxman AD, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Vandvik PO, Meerpohl J, Guyatt GH, Schünemann HJ; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 2: Clinical practice guidelines. BMJ. 2016 Jun 30;353:i2089. doi: 10.1136/bmj.i2089. PubMed PMID: 27365494.
Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, Fervers B, Graham ID, Grimshaw J, Hanna SE, Littlejohns P, Makarski J, Zitzelsberger L; AGREE Next Steps Consortium. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010 Dec 14;182(18):E839-42. doi: 10.1503/cmaj.090449. Epub 2010 Jul 5. Review. PubMed PMID: 20603348; PubMed Central PMCID: PMC3001530.
Hultcrantz M, Rind D, Akl EA, Treweek S, Mustafa RA, Iorio A, Alper BS, Meerpohl JJ, Murad MH, Ansari MT, Katikireddi SV, Östlund P, Tranæus S, Christensen R, Gartlehner G, Brozek J, Izcovich A, Schünemann H, Guyatt G. The GRADE Working Group clarifies the construct of certainty of evidence. J Clin Epidemiol. 2017 Jul;87:4-13. doi: 10.1016/j.jclinepi.2017.05.006. Epub 2017 May 18. PubMed PMID: 28529184; PubMed Central PMCID: PMC6542664.
Medisch Specialistische Richtlijnen 2.0 (2012). Adviescommissie Richtlijnen van de Raad Kwalitieit. http://richtlijnendatabase.nl/over_deze_site/over_richtlijnontwikkeling.html
Neumann I, Santesso N, Akl EA, Rind DM, Vandvik PO, Alonso-Coello P, Agoritsas T, Mustafa RA, Alexander PE, Schünemann H, Guyatt GH. A guide for health professionals to interpret and use recommendations in guidelines developed with the GRADE approach. J Clin Epidemiol. 2016 Apr;72:45-55. doi: 10.1016/j.jclinepi.2015.11.017. Epub 2016 Jan 6. Review. PubMed PMID: 26772609.
Schünemann H, Brożek J, Guyatt G, et al. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group, 2013. Available from http://gdt.guidelinedevelopment.org/central_prod/_design/client/handbook/handbook.html.
Zoekverantwoording
Algemene informatie
|
Cluster/richtlijn: Perifeer Arterieel Vaatlijden (PAV) – Femoro-popliteaal: Gecoate ballonnen en stents |
|
|
Uitgangsvraag/modules: Wat is de optimale behandeling van het femoro-popliteale traject (drug-eluting balloon en stents) |
|
|
Database(s): Ovid/Medline, Embase.com |
Datum: 10 april 2024 (update van 5 juni 2022) |
|
Periode: geen restrictie |
Talen: Engels, Nederlands |
|
Literatuurspecialist: Esther van der Bijl |
|
|
BMI-zoekblokken: voor verschillende opdrachten wordt (deels) gebruik gemaakt van de zoekblokken van BMI-Online https://blocks.bmi-online.nl/ Bij gebruikmaking van een volledig zoekblok zal naar de betreffende link op de website worden verwezen. |
|
|
Toelichting: Voor deze vraag is gezocht op de elementen:
Het sleutelartikel wordt gevonden met de zoekopdracht.
De resultaten staan in Rayyan: https://rayyan.ai/reviews/465123
|
|
|
Te gebruiken voor richtlijnen tekst: Nederlands In de databases Embase.com en Ovid/Medline is op 10 april 2024 een update gedaan van de search uit 2022. Er is met relevante zoektermen gezocht naar systematische reviews en RCT’s over behandeling met drug-eluting balloons en stents bij femoro-popliteaal letsel. De literatuurzoekactie leverde 69 nieuwe unieke treffers op. |
|
Zoekopbrengst 10 april 2024
|
|
EMBASE |
OVID/MEDLINE |
Rayyan |
Ontdubbeld ten opzichte van Rayyan |
|
SRs |
122 |
158 |
144 |
16 |
|
RCT |
196 |
268 |
237 |
53 |
|
Observationele studies |
236 |
227 |
225 |
|
|
Totaal |
318 |
426 |
|
69* |
*in Rayyan
Zoekopbrengst 5 juni 2022
|
|
EMBASE |
OVID/MEDLINE |
Ontdubbeld |
|
SRs |
108 |
130 |
144 |
|
RCT |
159 |
222 |
237 |
|
Observationele studies |
162 |
159 |
225 |
|
Totaal |
429 |
511 |
606 |
Zoekstrategie Embase.com 10 april 2024
|
No. |
Query |
Results |
|
#1 |
'leg ischemia'/exp/mj OR 'claudication'/exp/mj OR 'popliteal artery'/exp/mj OR (((popliteal* OR femoropopliteal* OR 'femoro popliteal') NEAR/3 arter*):ti,ab,kw) OR claudicatio*:ti,kw OR 'angina cruris':ti,kw OR 'dysbasia':ti,kw OR ((isch?em* NEAR/3 ('lower limb*' OR leg*)):ti,kw) OR 'chronic limb-threatening isch?em*':ti,ab,kw OR clti:ti,ab,kw OR 'critical limb isch?em*':ti,ab,kw |
23908 |
|
#2 |
'drug eluting cardiovascular stent'/exp OR 'drug-eluting balloon catheter'/exp OR (((eluting OR coated OR paclitaxel OR sirolimus OR everolimus) NEAR/3 (stent* OR balloon*)):ti,ab,kw) OR 'paclitaxel'/exp/mj OR 'sirolimus'/exp/mj OR 'everolimus'/exp/mj |
81487 |
|
#3 |
#1 AND #2 AND ([english]/lim OR [dutch]/lim) NOT ('conference abstract'/it OR 'editorial'/it OR 'letter'/it OR 'note'/it) NOT (('animal experiment'/exp OR 'animal model'/exp OR 'nonhuman'/exp) NOT 'human'/exp) |
601 |
|
#4 |
'meta analysis'/exp OR 'meta analysis (topic)'/exp OR metaanaly*:ti,ab OR 'meta analy*':ti,ab OR metanaly*:ti,ab OR 'systematic review'/de OR 'cochrane database of systematic reviews'/jt OR prisma:ti,ab OR prospero:ti,ab OR (((systemati* OR scoping OR umbrella OR 'structured literature') NEAR/3 (review* OR overview*)):ti,ab) OR ((systemic* NEAR/1 review*):ti,ab) OR (((systemati* OR literature OR database* OR 'data base*') NEAR/10 search*):ti,ab) OR (((structured OR comprehensive* OR systemic*) NEAR/3 search*):ti,ab) OR (((literature NEAR/3 review*):ti,ab) AND (search*:ti,ab OR database*:ti,ab OR 'data base*':ti,ab)) OR (('data extraction':ti,ab OR 'data source*':ti,ab) AND 'study selection':ti,ab) OR ('search strategy':ti,ab AND 'selection criteria':ti,ab) OR ('data source*':ti,ab AND 'data synthesis':ti,ab) OR medline:ab OR pubmed:ab OR embase:ab OR cochrane:ab OR (((critical OR rapid) NEAR/2 (review* OR overview* OR synthes*)):ti) OR ((((critical* OR rapid*) NEAR/3 (review* OR overview* OR synthes*)):ab) AND (search*:ab OR database*:ab OR 'data base*':ab)) OR metasynthes*:ti,ab OR 'meta synthes*':ti,ab |
733409 |
|
#5 |
'randomized controlled trial'/exp OR random*:ti,ab OR (((pragmatic OR practical) NEAR/1 'clinical trial*'):ti,ab) OR ((('non inferiority' OR noninferiority OR superiority OR equivalence) NEAR/3 trial*):ti,ab) OR rct:ti,ab,kw |
1839814 |
|
#6 |
'case control study'/de OR 'comparative study'/exp OR 'control group'/de OR 'controlled study'/de OR 'controlled clinical trial'/de OR 'crossover procedure'/de OR 'double blind procedure'/de OR 'phase 2 clinical trial'/de OR 'phase 3 clinical trial'/de OR 'phase 4 clinical trial'/de OR 'pretest posttest design'/de OR 'pretest posttest control group design'/de OR 'quasi experimental study'/de OR 'single blind procedure'/de OR 'triple blind procedure'/de OR (((control OR controlled) NEAR/6 trial):ti,ab,kw) OR (((control OR controlled) NEAR/6 (study OR studies)):ti,ab,kw) OR (((control OR controlled) NEAR/1 active):ti,ab,kw) OR 'open label*':ti,ab,kw OR (((double OR two OR three OR multi OR trial) NEAR/1 (arm OR arms)):ti,ab,kw) OR ((allocat* NEAR/10 (arm OR arms)):ti,ab,kw) OR placebo*:ti,ab,kw OR 'sham-control*':ti,ab,kw OR (((single OR double OR triple OR assessor) NEAR/1 (blind* OR masked)):ti,ab,kw) OR nonrandom*:ti,ab,kw OR 'non-random*':ti,ab,kw OR 'quasi-experiment*':ti,ab,kw OR crossover:ti,ab,kw OR 'cross over':ti,ab,kw OR 'parallel group*':ti,ab,kw OR 'factorial trial':ti,ab,kw OR ((phase NEAR/5 (study OR trial)):ti,ab,kw) OR ((case* NEAR/6 (matched OR control*)):ti,ab,kw) OR ((match* NEAR/6 (pair OR pairs OR cohort* OR control* OR group* OR healthy OR age OR sex OR gender OR patient* OR subject* OR participant*)):ti,ab,kw) OR ((propensity NEAR/6 (scor* OR match*)):ti,ab,kw) OR versus:ti OR vs:ti OR compar*:ti OR ((compar* NEAR/1 study):ti,ab,kw) OR (('major clinical study'/de OR 'clinical study'/de OR 'cohort analysis'/de OR 'observational study'/de OR 'cross-sectional study'/de OR 'multicenter study'/de OR 'correlational study'/de OR 'follow up'/de OR cohort*:ti,ab,kw OR 'follow up':ti,ab,kw OR followup:ti,ab,kw OR longitudinal*:ti,ab,kw OR prospective*:ti,ab,kw OR retrospective*:ti,ab,kw OR observational*:ti,ab,kw OR 'cross sectional*':ti,ab,kw OR cross?ectional*:ti,ab,kw OR multicent*:ti,ab,kw OR 'multi-cent*':ti,ab,kw OR consecutive*:ti,ab,kw) AND (group:ti,ab,kw OR groups:ti,ab,kw OR subgroup*:ti,ab,kw OR versus:ti,ab,kw OR vs:ti,ab,kw OR compar*:ti,ab,kw OR 'odds ratio*':ab OR 'relative odds':ab OR 'risk ratio*':ab OR 'relative risk*':ab OR 'rate ratio':ab OR aor:ab OR arr:ab OR rrr:ab OR ((('or' OR 'rr') NEAR/6 ci):ab))) |
13242898 |
|
#7 |
#3 AND #4 – SR’s |
122 |
|
#8 |
#3 AND #5 NOT #7 – RCT’s |
196 |
|
#9 |
#3 AND #6 NOT (#7 OR #8) – Observationele studies |
236 |
|
#10 |
#7 OR #8 OR #9 |
554 |
Zoekstrategie Ovid/Medline 10 april 2024
|
# |
Searches |
Results |
|
1 |
(exp Ischemia/ and exp Leg/) or exp Intermittent Claudication/ or exp Popliteal Artery/ or ((popliteal* or femoropopliteal* or femoro popliteal) adj3 arter*).ti,ab,kf. or claudicatio*.ti,ab,kf. or angina cruris.ti,ab,kf. or dysbasia.ti,ab,kf. or (isch?em* adj3 (lower limb* or leg*)).ti,ab,kf. |
35234 |
|
2 |
(*Angioplasty, Balloon/ and (eluting or coated or paclitaxel or sirolimus or everolimus).ti,ab,kf.) or exp *Drug-Eluting Stents/ or ((eluting or coated or paclitaxel or sirolimus or everolimus) adj3 (stent* or balloon*)).ti,ab,kf. or exp *Paclitaxel/ or exp *Sirolimus/ or exp *Everolimus/ |
44191 |
|
3 |
1 and 2 |
1127 |
|
4 |
limit 3 to (english language or dutch) |
1085 |
|
5 |
4 not (comment/ or editorial/ or letter/ or ((exp animals/ or exp models, animal/) not humans/)) |
932 |
|
6 |
meta-analysis/ or meta-analysis as topic/ or (metaanaly* or meta-analy* or metanaly*).ti,ab,kf. or systematic review/ or cochrane.jw. or (prisma or prospero).ti,ab,kf. or ((systemati* or scoping or umbrella or "structured literature") adj3 (review* or overview*)).ti,ab,kf. or (systemic* adj1 review*).ti,ab,kf. or ((systemati* or literature or database* or data-base*) adj10 search*).ti,ab,kf. or ((structured or comprehensive* or systemic*) adj3 search*).ti,ab,kf. or ((literature adj3 review*) and (search* or database* or data-base*)).ti,ab,kf. or (("data extraction" or "data source*") and "study selection").ti,ab,kf. or ("search strategy" and "selection criteria").ti,ab,kf. or ("data source*" and "data synthesis").ti,ab,kf. or (medline or pubmed or embase or cochrane).ab. or ((critical or rapid) adj2 (review* or overview* or synthes*)).ti. or (((critical* or rapid*) adj3 (review* or overview* or synthes*)) and (search* or database* or data-base*)).ab. or (metasynthes* or meta-synthes*).ti,ab,kf. |
738657 |
|
7 |
exp randomized controlled trial/ or randomized controlled trials as topic/ or random*.ti,ab. or rct?.ti,ab. or ((pragmatic or practical) adj "clinical trial*").ti,ab,kf. or ((non-inferiority or noninferiority or superiority or equivalence) adj3 trial*).ti,ab,kf. |
1704596 |
|
8 |
Case-control Studies/ or clinical trial, phase ii/ or clinical trial, phase iii/ or clinical trial, phase iv/ or comparative study/ or control groups/ or controlled before-after studies/ or controlled clinical trial/ or double-blind method/ or historically controlled study/ or matched-pair analysis/ or single-blind method/ or (((control or controlled) adj6 (study or studies or trial)) or (compar* adj (study or studies)) or ((control or controlled) adj1 active) or "open label*" or ((double or two or three or multi or trial) adj (arm or arms)) or (allocat* adj10 (arm or arms)) or placebo* or "sham-control*" or ((single or double or triple or assessor) adj1 (blind* or masked)) or nonrandom* or "non-random*" or "quasi-experiment*" or "parallel group*" or "factorial trial" or "pretest posttest" or (phase adj5 (study or trial)) or (case* adj6 (matched or control*)) or (match* adj6 (pair or pairs or cohort* or control* or group* or healthy or age or sex or gender or patient* or subject* or participant*)) or (propensity adj6 (scor* or match*))).ti,ab,kf. or (confounding adj6 adjust*).ti,ab. or (versus or vs or compar*).ti. or ((exp cohort studies/ or epidemiologic studies/ or multicenter study/ or observational study/ or seroepidemiologic studies/ or (cohort* or 'follow up' or followup or longitudinal* or prospective* or retrospective* or observational* or multicent* or 'multi-cent*' or consecutive*).ti,ab,kf.) and ((group or groups or subgroup* or versus or vs or compar*).ti,ab,kf. or ('odds ratio*' or 'relative odds' or 'risk ratio*' or 'relative risk*' or aor or arr or rrr).ab. or (("OR" or "RR") adj6 CI).ab.)) |
5663934 |
|
9 |
5 and 6 – SR’s |
158 |
|
10 |
(5 and 7) not 9 – RCT’s |
268 |
|
11 |
(5 and 8) not (9 or 10) – Observationele studies |
227 |
|
12 |
9 or 10 or 11 |
653 |