Adenotomie bij otitis media (rOMA, pOME, ACMOM)
Uitgangsvraag
Wat is de plaats van adenotomie bij de behandeling van otitis media (rOMA, pOME, ACMOM)?
Aanbeveling
Verricht niet standaard een adenotomie naast het plaatsen van trommelvliesbuisjes in de behandeling van kinderen met recidiverende otitis media acuta (rOMA), persisterende otitis media met effusie (pOME) en ACMOM, gezien het geringe positieve effect.
Bespreek met ouders de beperkte effectiviteit van adenotomie (al dan niet naast behandeling met trommelvliesbuisjes) en de bijbehorende risico’s.
Overwegingen
Voor- en nadelen van de interventie en de kwaliteit van het bewijs
Er is een literatuuronderzoek gedaan naar de effecten van een adenotomie vergeleken met geen adenotomie als behandeling voor verschillende vormen van otitis media (recidiverende otitis media acuta; rOMA, persisterende otitis media met effusie; pOME en actieve chronische mucosale otitis media; ACMOM).
De geïncludeerde studies laten geen sterk bewijs zien voor een betere resolutie van otitis media na adenotomie vergeleken met geen adenotomie (cruciale uitkomstmaat). Er lijkt geen verschil te zijn in het uitblijven van episodes van otitis media. Daarbij suggereert het bewijs dat er geen verschil is in de gemiddelde hoeveelheid episoden van otitis media (cruciale uitkomstmaat) en de aanwezigheid van een loopoor (belangrijke uitkomstmaat). De bewijskracht voor deze gevonden effecten is (zeer) laag, mede door beperkingen in de studieopzet (waaronder gebrek aan blindering) en conflicterende resultaten. Voor de belangrijke uitkomstmaat gehoorverlies werd ook slechts bewijs met een zeer lage bewijskracht gevonden, waardoor er nog veel onzekerheid bestaat over het daadwerkelijke effect van een adenotomie op gehoorverlies bij otitis media. De uitkomstmaat bijwerkingen werd in slechts 1 studie gerapporteerd. In zowel de adenotomie groep als in de controlegroep werden geen bijwerkingen gerapporteerd. Een deel van de geïncludeerde studies in het review van Van den Aardweg (2010) rapporteert de volgende postoperatieve complicaties na een adenotomie: Gates (1987) rapporteerde 1 kind (1%) met een terugkerende nabloeding. Paradise (1999) rapporteerde complicaties bij 4 kinderen (4%) waaronder maligne hyperthermie, pneumonie en postoperatieve velofaryngeale insufficiëntie. De MRC-studygroup (2012) rapporteerde dat er bij 1 van de 165 kinderen (0.6%) die een adenotomie heeft ondergaan een nabloeding is opgetreden. Hoewel er dus een risico bestaat op complicaties bij een adenotomie, treden deze slechts op in een zeer klein deel van de kinderen dat de interventie ondergaat. Omdat er geen data gerapporteerd is over de hoeveelheid complicaties in de controlegroepen, zijn deze resultaten niet in de literatuursamenvatting beschreven.
De uitkomstmaten pijn, kwaliteit van leven en taal-spraak ontwikkeling werden niet gerapporteerd in de geïncludeerde studies. Het is dan ook onduidelijk wat het effect van adenotomie is op deze parameters. Er was één studie waarin kwaliteit van leven werd onderzocht (Kujala 2014). Vanwege de visuele weergave van de resultaten kon deze niet geïncludeerd worden in de literatuursamenvatting. In deze studie wordt geconcludeerd dat ondanks dat het aantal episoden van rOMA afneemt, behandeling met buisjes en adenotomie niet leidt tot een verbetering van de kwaliteit van leven van het kind, vergeleken met het enkel plaatsen van buisjes.
In de meerderheid van de geïncludeerde studies werd adenotomie uitgevoerd in combinatie met het plaatsen van trommelvliesbuisjes. Dit komt overeen met de Nederlandse praktijk, waarin het uitvoeren van enkel een adenotomie voor de behandeling van otitis media over het algemeen niet aan de orde is.
Een inidividual patient data (IPD) analyse van Boonacker (2014) includeerde een groot deel van de studies die ook geïncludeerd zijn in de literatuursamenvatting van deze richtlijn module. Op basis van de individuele patiënten data concluderen zij dat een adenotomie leidt tot een lager risico op falen van behandeling in vergelijking met een afwachtend beleid (RR 0.67 95% CI: 0.45 tot 0.99). Gecombineerd met buisjes, is het additionele effect van adenotomie, vergeleken met buisjes alleen, verwaarloosbaar (RR: 1.01 95% BI: 0.81 tot 0.24). In deze IPD-analyse worden verschillende subgroepen onderscheiden, waaronder kinderen met rOMA < 2 jaar en kinderen met pOME ≥ 4 jaar. Voor deze twee groepen lijkt het voordeel van een adenotomie het grootst te zijn (adjusted RR: 0.63, 95% BI 0.47 tot 0.85 en adjusted RR: 0.77, 95% BI 0.68 tot 0.86 respectievelijk). Mogelijk hebben deze subgroepen dus wel baat bij behandeling met een adenotomie. Over de daadwerkelijke effectiviteit van adenotomie bij kinderen met rOMA > 2 jaar en kinderen met pOME > 4 jaar bestaat een kennishiaat. Voor andere subgroepen werd geen, of een minder groot effect van adenotomie gevonden.
Bij kinderen met pOME, rOMA, ACMOM in combinatie met klachten van nasale obstructie of pediatrische OSA, kan een adenotomie wel overwogen worden ter behandeling van nasale (obstructie)klachten (zie ZATT-richtlijn en de richtlijn Obstructief slaapapneu (OSA) bij kinderen)
Waarden en voorkeuren van patiënten (en evt. hun verzorgers)
Ouders van kinderen met recidiverende of persisterende klachten willen graag van de klachten af omdat zowel het kind, alsook de ouders vaak lijden onder de oorontstekingen met onrustige nachten als gevolg en verzuim van kinderdagverblijf of school. Op basis van het gevonden bewijs is het echter onduidelijk of adenotomie, met name in combinatie met buisjes, ook daadwerkelijk leidt tot minder oorontstekingen en/of klachten bij kinderen.
Kosten (middelenbeslag)
Een ingreep in de vorm van adenotomie is kostbaarder dan een afwachtend beleid. Vanuit kosten-perspectief zal een afwachtend beleid dus de voorkeur hebben.
Aanvaardbaarheid, haalbaarheid en implementatie
Adenotomie wordt veel uitgevoerd. Er is echter veel praktijkvariatie. Voor de behandeling van otitis media wordt adenotomie meestal uitgevoerd in combinatie met het plaatsen van trommelvliesbuisjes. Adenotomie wordt vaak gedaan wegens obstructieve neusklachten en soms in combinatie met otitiden. Tevens wordt een adenotomie vaak uitgevoerd in combinatie met een tonsillectomie. Het is niet bekend voor welke klachten de adenotomie met name wordt geïndiceerd.
Rationale van de aanbeveling: weging van argumenten voor en tegen de interventies
Op basis van het gevonden bewijs lijken er geen sterke argumenten te zijn voor de behandeling van rOMA, pOME en ACMOM met een adenotomie. Zowel adenotomie alleen, als adenotomie in combinatie met trommelvliesbuisjes laat slechts geringe effecten zien op het verloop van rOMA en pOME bij kinderen. Patiënten en ouders zijn gebaat bij een effectieve behandeling van de klachten, echter is het onduidelijk of een adenotomie ook daadwerkelijk leidt tot minder oorontstekingen en/of klachten.
Onderbouwing
Achtergrond
Recidiverende otitis media acuta (rOMA), persisterende otitis media met effusie (pOME) en actieve chronische mucosale otitis media (ACMOM) worden vaak geassocieerd met tubadysfunctie, bovenste luchtweginfecties en aandoeningen van de nasofarynx. Bij kinderen speelt mogelijk het adenoïd een bijdragende rol. Er zijn echter verschillende inzichten over het nut van een adenotomie in de behandeling van otitis media, leidend tot variatie in beleid. De werkgroep heeft zich de vraag gesteld of en in welk mate het uitvoeren van een adenotomie bijdraagt aan het verminderen van klachten van rOMA, pOME en ACMOM bij kinderen tot 18 jaar.
Conclusies / Summary of Findings
Recurrence of otitis media – resolution of otitis media
|
Very low GRADE |
The evidence is very uncertain about the effect of adenoidectomy compared with no adenoidectomy on the outcome resolution of otitis media in children with otitis media (rAOM and pOME)
Source: Van den Aardweg 2010 (Nguyen, 2004; Koivunen, 2004; Black, 1990; Dempster, 1993; Maw, 1986; Roydhouse, 1980); Kujala (2012); Hao (2019) |
Recurrence of otitis media – Episodes of otitis media
|
Low GRADE |
Adenoidectomy may result in little to no difference in episodes of otitis media when compared with no adenoidectomy in children with otitis media (rAOM and pOME)
Source: Van den Aardweg 2010 (Paradise, 1990; Paradise, 1999; Hammaren-Malmi, 2005; Matilla, 2003; Casselbrant, 2009) |
Presence of otorrhea
|
Very low GRADE |
The evidence is very uncertain about the effect of adenoidectomy compared with no adenoidectomy on the outcome presence of otorrhea in children with otitis media (rAOM and pOME)
Source: Van den Aardweg 2010 (Paradise 1990) |
Hearing loss
|
Very low GRADE |
The evidence is very uncertain about the effect of adenoidectomy compared with no adenoidectomy on the outcome hearing loss in children with otitis media (pOME)
Source: Van den Aardweg 2010 (Dempster, 1999; Maw, 1986), MRC study group (2012), Hao (2019) |
Complications
|
Low GRADE |
Adenoidectomy may result in little to no difference in complications when compared with no adenoidectomy in children with otitis media (pOME)
Source: Kujala (2012) |
Pain, Quality of life, Language, and speech development
|
No GRADE |
No evidence was found regarding the effect of adenoidectomy compared to no adenoidectomy on pain, quality of life, and language and speech development.
Source: - |
Samenvatting literatuur
Description of studies
Systematic reviews
Van den Aardweg (2010) performed a systematic review to assess the effectiveness of adenoidectomy versus non-surgical management or ventilation tubes in children with otitis media. The databases the Cochrane Ear, Nose and Throat Disorders Group Trials Register; the Cochrane Central Register of Controlled Trials (CENTRAL); PubMed; EMBASE; CINAHL; Web of Science; BIOSIS Previews; Cambridge Scientific Abstracts; mRCT and additional sources were searched for relevant articles published until March 2009. Randomized controlled trials on adenoidectomy for otitis media compared with non-surgical treatment, or ventilation tubes alone were considered relevant for inclusion. Additional inclusion criteria were: minimum follow-up of at least six months and children up to 18 years diagnosed with otitis media. Studies on any type of otitis media (recurrent acute otitis media; rAOM and persistent otitis media with effusion; pOME) were included. In total, 14 trials were included in the systematic review, of which 10 were considered relevant for the purpose of this guideline (Black 1990; Casselbrant 2009; Dempster 1993; Hammarén-Malmi 2005; Koivunen 2004; Maw 1986; Nguyen 2004; Paradise 1990; Paradise 1999; Roydhouse 1980). Reason for excluding the other trials was that the outcome measures that were reported did not meet the predefined PICO. In the review, the studies were subdivided into three different comparisons: adenoidectomy versus watchful waiting, adenoidectomy + unilateral tube (in one ear) versus unilateral ear tube only (in the other ear) and adenoidectomy + bilateral tubes versus bilateral tubes only. Baseline characteristics of the included are presented in Table 1. Outcomes included resolution of otitis media, referring the number of children not experiencing rAOM of pOME during the follow-up period; intervention failure, defined as the child experiencing 2 AOM episodes in 2 months, or 3 AOM episodes in 6 months, or having middle ear effusion for at least 2 months; number of episodes per year (rAOM and/or pOME) and hearing loss. Adverse events were also reported as an outcome, however these were reported as post-operative complications. Since not all children in the control group underwent surgery, a comparison of adverse events between the two groups can’t be made.
Table 1: baseline characteristics of the relevant studies included in the systematic review by Van den Aardweg (2010)
OME = otitis media with effusion, rAOM = recurrent acute otitis media, mo. = months,
|
Author |
Population |
OM type |
Intervention |
Comparator |
|
Black 1990 |
n = 149 aged 4 – 9 years |
Bilateral OME |
adenoidectomy + (myringotomy) + unilateral tube |
(myringotomy) + unilateral tubes |
|
Casselbrant 2009 |
n = 98 aged 24 months – 47 mo. |
OME |
adenoidectomy + myringotomy (+ tubes) |
myringotomy + tubes |
|
Dempster 1993 |
n = 78 aged 3 – 12 |
bilateral titis media |
adenoidectomy + unilateral tube |
unilateral tube |
|
Hammarén-Malmi 2005 |
n = 217 aged 1 – 4 years |
rAOM or pOME |
adenoidectomy + tubes |
tubes |
|
Koivunen 2004 |
n = 180, aged 10 mo. – 2 years |
≥ 3 episodes of acute otitis media |
adenoidectomy |
chemoprophylaxis, placebo |
|
Maw 1986 |
n = 150, aged 2- 9 years |
bilateral OME |
adenoidectomy + tubes |
adenotonsillectomy OR neither + tubes |
|
Nguyen 2004 |
n = 72 aged 18 months – 18 years |
rAOM or OME |
adenoidectomy + tubes |
tubes |
|
Paradise 1990 |
n = 99, aged 1-15 years |
rAOM (after tubes) |
adenoidectomy |
control |
|
Paradise 1999 |
n = 304 aged 3-15 years |
rAOM, pOME |
adenoidectomy |
control |
|
Roydhouse 1980 |
n = 169 aged 2- 24 years |
OME |
adenoidectomy + tubes |
tubes |
Randomized controlled trials
MRC study group (2012) performed a randomized controlled trial to determine the adjuvant effects of adenoidectomy with short-stay ventilation tubes to hearing over 3.5 years in children with pOME. Children were recruited from 11 different ENT-departments across the United Kingdom between April 1994 and January 1998. Children aged 3.25 years – 6.75 years on a first visit, with no previous ear surgery were eligible to participate. Additional eligibility criteria were: a biliterate B + B or B + C2 tympanogram combination and better ear hearing loss (HL) > 20 dB HL averaged across 0.5, 1, 2, and 4 kHZ and air-bone gap > 10 dB. Children with a HL > 40 dB were not excluded from randomization. The children were randomized to one of the three treatment arms: bilateral Shepard ventilation tubes with adjuvant adenoidectomy, only bilateral Shepard ventilation tubes or further watchful waiting. The results of the children randomized to the adenoidectomy + ventilation tubes group (n = 128) were compared to the results of the randomized to the tubes only group (n = 126). Follow-up was at 3 months, 6 months, 12 months, 18 months, and 24 months. Some patients were lost-to-follow-up however, the loss-to-follow-up rates were similar across groups and missing data was imputed. Also, some patients switched over to another treatment arm, this did not introduce bias since an intention-to-treat analysis was used. The results at different follow-up durations were summarized into two periods: 3 plus 6 months, and 12, 18 plus 24 months. Outcomes included air conduction hearing thresholds and adverse events. Since the adverse event were not specified for the different treatment arms, a comparison could not be made.
Hao (2019) performed a randomized controlled trial to evaluate the effect of ventilation tubes combined with adenoidectomy and only tube insertion as treatment for otitis media with effusion in children. Children presenting with OME at a Chinese hospital between September 2013 and January 2015 were eligible to participate. Children aged between 3 and 6 years old, with surgical indications after conservative treatment failure were included in the trial. Exclusion criteria were: children with mixed deafness, children with craniofacial deformities and children with incomplete clinical follow-up data. Children were randomized to treatment with adenoidectomy and ventilation tubes (n = 98) or ventilation tubes alone (n = 86). Outcomes were assessed at 3 months and 6 months follow-up. The outcomes included recovery, defined as complete disappearance of clinical symptoms and middle ear effusion) and hearing thresholds.
Kujala (2012) performed a randomized controlled trial to evaluate the efficacy of insertion of ventilation tubes with and without adenoidectomy for preventing rAOM in children. Children were recruited from an otorhinolaryngology department of a hospital in Finland. Inclusion criteria were: aged between 10 months and 2 years, at least 3 AOM episodes during the past 6 months. Exclusion criteria were: chronic otitis media with effusion, a priori adenoidectomy or ventilation tubes, cranial anomalies, documented immunological disorders or ongoing antimicrobial prophylaxis for a disease other than AOM. Children were randomly allocated to receive adenoidectomy and ventilation tubes, ventilation tubes alone or neither (control condition). Outcomes of the children receiving adenoidectomy and ventilation tubes (n = 100) were compared to the outcomes of the children receiving tubes alone (n = 100). Follow-up duration was one year, with follow-up visits at least every 4 months. Outcomes included intervention failure, defined as the child experiencing 2 AOM episodes in 2 months, or 3 AOM episodes in 6 months, or having middle ear effusion for at least 2 months and adverse events.
Results
Otitis media recurrence
Since the outcome measure otitis media recurrence was reported in various ways, this outcome measure is subdivided into two different measures.
- Resolution of otitis media: referring to the number of children not experiencing rAOM or pOME during the follow-up period. Some of the included studies only reported treatment failure. For these studies, the number of children with resolution of otitis media was calculated by subtracting the number of children with treatment failure from the study population.
- Episodes of otitis media: referring to the mean number of episodes children experienced during the follow-up period.
a. Resolution of otitis media
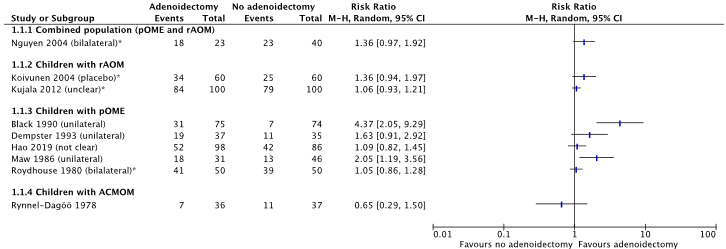
Nine studies reported the outcome resolution of otitis media at 12 months follow-up (from van den Aardweg, 2010: Nguyen, 2004; Koivunen, 2004; Black, 1990; Dempster, 1993; Maw, 1986; Roydhouse, 1980; Rynnel-Dagöö, 1978; Kujala, 2012; Hao 2019). All studies reported a Relative Risk Ratio (RR) in favor of treatment with adenoidectomy, see Figure 1. Due to large heterogeneity between studies (in study protocols and co-interventions) the studies were not pooled in a meta-analysis (I2 = 74%).
* The number of children with resolution of otitis media was calculated by subtracting the number with failure from the total population (Nguyen 2004; Koivunen 2004; Kujala 2012; Roydhouse 1980)
Figure 1. Forest plot showing the comparison adenoidectomy versus no adenoidectomy for the outcome resolution of otitis media. Pooled relative risk ratio, random effects model. Z: p-value of overall effect; df: degrees of freedom; SD: standard deviation; I2; statistical heterogeneity; CI: confidence interval.
b. Episodes of otitis media (OME or AOM)
Five studies reported the (mean) number of episodes of otitis media at 12 months follow-up (from Van den Aardweg, 2010; Paradise, 1990; Paradise, 1999; Hammaren-Malmi, 2005; Matilla, 2003; Casselbrant, 2009). Three studies reported a lower mean number of episodes in the first 12 months after adenoidectomy, compared to no adenoidectomy (Paradise, 1999; Paradise, 1990; Matilla, 2003). Two studies reported a higher mean number of episodes in the first 12 months after adenoidectomy, compared to no adenoidectomy (Hammaren-Malmi, 2005; Casselbrant, 2009). However, differences are small and appear to be within a similar range, see Table 2.
Due to the lack of standard deviations, and large heterogeneity between studies, the results could not be pooled in a meta-analysis.
Table 2. Overview of the studies reporting the outcome ‘episodes of OM’. rAOM: recurrent acute otitis media; pOME: persistent otitis media with effusion; SD: standard deviation
|
|
Population |
Intervention |
Control |
Difference |
|
Paradise 1999
|
Combined population rAOM + pOME n = 201, 100/101 |
Mean rate of AOM episodes (< 10 days) in first year 1.8 per subject
|
Mean rate of AOM episodes (< 10 days) in first year 2.1 |
Can’t be calculated because SDs are not reported |
|
Paradise 1990
|
Combined population rAOM + pOME n = 99, 52/47 |
Mean number of episodes OME in first year 1.06 |
Mean number of episodes OME in first year 1.45 |
Can’t be calculated because SDs are not reported |
|
Hammaren-Malmi 2005
|
Combined population rAOM + pOME |
Mean number of episodes OM 1.73 ± 1.8 |
Mean number of episodes OM 1.44 ± 1.5 |
Mean difference: 0.28 (95% CI: -0.16, 0.72)
|
|
Matilla 2003
|
rAOM (with and without effusion) n = 137, 63/74 |
2.05 episodes per person-year |
2.40 episodes per person year |
Can’t be calculated because SDs are not reported |
|
Casselbrant 2009
|
OME N = 98, no information |
Number of episodes of AOM after 18 months 7 |
Number of episodes of AOM after 18 years 6 |
Can’t be calculated because SDs are not reported |
Presence of otorrhea
One study reported the outcome presence of otorrhea (from van den Aardweg 2010; Paradise 1990). The number of secondary otorrhea episodes per subject was 0.13 in the adenoidectomy group (n = 52) and 0.13 in the control group (n = 47) at 12 months follow-up. At 24- and 36-months follow-up, the number of otorrhea episodes per subjects were 0.09 and 0.05 in the adenoidectomy and 0.04 and 0.07 in the control group, respectively. Due to the lack of standard deviations, the mean difference could not be calculated.
Pain
None of the included studies reported the outcome measure ‘pain’ for the comparison adenoidectomy versus no adenoidectomy as treatment of otitis media in children.
Hearing loss
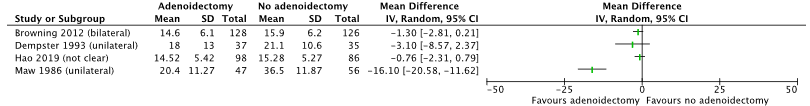
Five studies reported the outcome hearing loss (from Van den Aardweg 2010; Dempster, 1993; Black, 1990; Maw, 1986; MRC study group 2012; Hao 2019). Hearing loss was measured in dB as air conduction hearing loss at 6 months FU. In all studies the study population only included children with pOME. The studies reported lower levels of hearing loss in patients undergoing adenoidectomy, compared to no adenoidectomy, see Figure 2.
Due to large heterogeneity between studies (in study protocols and co-interventions) the studies were not pooled in a meta-analysis (I2 = 74%).
Figure 2. Forest plot showing the comparison adenoidectomy versus no adenoidectomy for the outcome hearing loss. Pooled relative risk ratio, random effects model. Z: p-value of overall effect; df: degrees of freedom; SD: standard deviation; I2; statistical heterogeneity; CI: confidence interval.
Black (1990; from Van den Aardweg) only reported the difference in dB between the treatment groups. At 6 months follow-up a difference of 4.3 dB (95% CI: -1.4 to 9.9) was found. It is not clear whether this result was in favor of treatment with adenoidectomy, or without.
Complications
Kujala (2012) reported no serious complications (hemorrhage or anesthetic complications) in both treatment arms meaning 0/100 children undergoing adenoidectomy with tubes experienced serious complications, and 0/100 children undergoing receiving tubes only experienced serious complications.
Quality of life
None of the included studies reported the outcome measure ‘quality of life’ for the comparison adenoidectomy versus no adenoidectomy as treatment of otitis media in children.
Language and speech development:
None of the included studies reported the outcome measure ‘language and speech development’ for the comparison adenoidectomy versus no adenoidectomy as treatment of otitis media in children.
Level of evidence of the literature
Otitis Media Recurrence – resolution of otitis media
The level of evidence regarding the outcome measure resolution of otitis media was derived from randomized controlled trials and therefore started high. The level of evidence was downgraded by three levels because of study limitations including lack of blinding (-1 risk of bias); conflicting results (-1 inconsistency) and the 95% confidence intervals crossing the boundaries of clinical decision making (-1 imprecision). The final level of evidence was graded ‘very low’.
Otitis Media Recurrence – episodes of otitis media
The level of evidence regarding the outcome measure episodes of otitis media was derived from randomized controlled trials and therefore started high. The level of evidence was downgraded by two levels because of study limitations including lack of blinding (-1 risk of bias); and conflicting results (-1 inconsistency). The final level of evidence was graded ‘low’.
Presence of otorrhea
The level of evidence regarding the outcome measure presence of otorrhea was derived from randomized controlled trials and therefore started high. The level of evidence was downgraded by three levels because of study limitations including lack of blinding (-1 risk of bias); conflicting results (-1 inconsistency); and low number of included patients (- 1 imprecision). The final level of evidence was graded ‘very low’.
Hearing loss
The level of evidence regarding the outcome measure hearing loss was derived from randomized controlled trials and therefore started high. The level of evidence was downgraded by three levels because of study limitations including lack of blinding (-1 risk of bias); conflicting results (-1 inconsistency); and wide 95% confidence intervals (-1 imprecision). The final level of evidence was graded ‘very low’.
Complications
The level of evidence regarding the outcome measure complications was derived from randomized controlled trials and therefore started high. The level of evidence was downgraded by two levels because of study limitations including lack of blinding (-1 risk of bias) and low number of included patients (-1 imprecision). The final level of evidence was graded ‘low’.
The level of evidence for the outcome measures pain, quality of life and language and speech development could not be graded as it was not reported in the included studies.
Zoeken en selecteren
A systematic review of the literature was performed to answer the following question:
What are the favorable and unfavorable effects of adenoidectomy compared with no adenoidectomy for children with recurrence acute otitis media (rAOM), persistent otitis media with effusion (pOME) or chronic suppurative otitis media (CSOM)?
P: patients with rAOM, pOME, CSOM (0-18 years)
I: adenoidectomy
C: no adenoidectomy
O: pain, otitis media recurrence, presence of otorrhea, hearing loss, complications, quality of life, language, and speech development
Relevant outcome measures
The guideline development group considered recurrence of otitis media as a critical outcome measure for decision making; and pain, otorrhea, hearing loss, complications, quality of life and language and speech development as an important outcome measure for decision making.
A priori, the guideline development group did not define the outcome measures listed above but used the definitions used in the studies.
The guideline development group defined the following thresholds as a minimal clinically (patient) important difference; for dichotomous outcomes: a RR < 0.80 and RR < 1.25, for continuous outcomes a 10% difference. For the outcome measure hearing level, a minimal clinically (patient) important difference of 10 dB was considered clinically relevant.
Search and select (Methods)
The databases Medline (via OVID) and Embase (via Embase.com) were searched with relevant search terms until the 3rd of May in 2023. The detailed search strategy is depicted under the tab Methods. The systematic literature search resulted in 417 hits. Studies were selected based on the following criteria: systematic reviews and RCTS comparing adenoidectomy with no adenoidectomy as treatment for otitis media in children (0-18 years).
No adenoidectomy referred to any watchful waiting and/or myringotomy and/or treatment with tubes (but only if both the intervention and control group received tubes). Co-interventions had to be similar across the intervention and control condition, studies comparing adenoidectomy with tubes were excluded.
21 studies were initially selected based on title and abstract screening. After reading the full text, 17 studies were excluded (see the table with reasons for exclusion under the tab Methods), and four studies were included.
Results
One systematic review, and three randomized controlled trials were included in the analysis of the literature. Important study characteristics and results are summarized in the evidence tables. The assessment of the risk of bias is summarized in the risk of bias tables.
Referenties
- Hao J, Chen M, Liu B, Yang Y, Liu W, Ma N, Han Y, Liu Q, Ni X, Zhang J. Compare two surgical interventions for otitis media with effusion in young children. Eur Arch Otorhinolaryngol. 2019 Aug;276(8):2125-2131. Doi: 10.1007/s00405-019-05421-9. Epub 2019 May 24. Erratum in: Eur Arch Otorhinolaryngol. 2019 Jun 18;: PMID: 31127413.
- Kujala T, Alho OP, Luotonen J, Kristo A, Uhari M, Renko M, Kontiokari T, Pokka T, Koivunen P. Tympanostomy with and without adenoidectomy for the prevention of recurrences of acute otitis media: a randomized controlled trial. Pediatr Infect Dis J. 2012 Jun;31(6):565-9. Doi: 10.1097/INF.0b013e318255ddde. PMID: 22466327.
- MRC Multicentre Otitis Media Study Group. Adjuvant adenoidectomy in persistent bilateral otitis media with effusion: hearing and revision surgery outcomes through 2 years in the TARGET 132esign132ies trial. Clin Otolaryngol. 2012 Apr;37(2):107-16. Doi: 10.1111/j.1749-4486.2012.02469.x. PMID: 22443163.
- Van den Aardweg MT, Schilder AG, Herkert E, Boonacker CW, Rovers MM. Adenoidectomy for otitis media in children. Cochrane Database Syst Rev. 2010 Jan 20;(1):CD007810. Doi: 10.1002/14651858.CD007810.pub2. PMID: 20091650.
Evidence tabellen
Evidence table for systematic review of RCTs and observational studies (intervention studies)
|
Study reference |
Study characteristics |
Patient characteristics |
Intervention (I) |
Comparison / control I
|
Follow-up |
Outcome measures and effect size |
Comments |
|
Van den Aardweg, 2010
Study characteristics and results are extracted from the SR (unless stated otherwise) |
SR and meta-analysis of RCTs
Literature search up to march 2009]
A: Black 1990, B: Casselbrant 2009, C: Dempster 1993, D: Hammarén-Malmi 2005, E: Koivunen 2004, F: Maw 1986, G: Nguyen 2004, H: Paradise 1990, I: Paradise 1999, J: Roydhouse 1980,
Study design: all RCT
Setting and Country: Not reported in SR
Source of funding and conflicts of interest: Not reported in SR
|
Inclusion criteria SR:
Exclusion criteria SR:
14 studies were included, of which 10 were considered relevant for this guideline
Important patient characteristics at baseline:
N, mean age A: n = 149; 4 – 9 years B: n = 98; 24 – 47 months C: n = 78; 3- 12 years D: n = 217; 1-4 years E: n = 180; 10 months – 2 years F: n = 150; 2 – 9 years G: n = 72; 18 months – 18 years H: n = 99; 1 – 15 years I: n = 304; 3- 15 years J: n = 169; 2 – 24 years
*information on sex was not presented in SR
Type of OM: A: Bilateral OME B: Uni- or bilateral middle ear effusion after extrusion of ventilation tubes C: Bilateral OME D: Combined population (rAOM and pOME) E: rAOM F: Bilateral pOME G: recurrent AOM or OME H: rAOM or pOME after tympanostomy tube extrusion I: rAOM or pOME J: pOME
Groups comparable at baseline? Probably yes |
Describe intervention:
A: adenoidectomy + (myringotomy) + unilateral tube B: adenoidectomy + myringotomy (+ tubes) C: adenoidectomy + unilateral tube D: adenoidectomy + tubes E: adenoidectomy F: adenoidectomy + tubes G: adenoidectomy + tubes H: adenoidectomy I: adenoidectomy J: adenoidectomy + tubes K: adenoidectomy |
Describe control:
A: (myringotomy) + unilateral tubes B: myringotomy + tubes C: unilateral tube D: tubes E: chemoprophylaxis, placebo F: adenotonsillectomy OR neither + tubes G: tubes H: control I: adenotonsillectomy, control group J: tubes K: adenoidectomy |
End-point of follow-up:
A: 7 weeks, 6 months, 12 months and 24 months, loss to follow-up 15% after 12 months and 39% after 24 months;
B: 18 months, 36 months Loss to follow-up: no information
C: 6 months, 12 months Loss to follow-up 8% after 12 months follow up;
D: 12 months Loss to follow-up: 9% after 12 months follow up;
E: 12 months, 24 months
Loss to follow-up: 28% at in non-adenoidectomy group, 3%in the adenoidectomy group after 24 months follow up
F: 6 months, 12 months Loss to follow-up: 10% after 24 months follow up
G: 12 months Loss to follow-up: 12 after 12 months follow up
H: 12 months, 24 months, 36 months Loss to follow-up: 13% after 12 months, 27% after 24 months and 47% after 36 months
I: 12 months, 24 months, 36 months
Loss to follow-up: I: 23% after 12 months, 34% after 24 months and 47% after 36 months follow up
J: 12 months, 24 months, 36 months Loss to follow-up: 3% after 12 months, 22 children (13%) after 24 months and 38 (22%) after 36 months follow up;
|
Outcome measure-1: resolution of OM RR of patients resolving from OM.
A: 4.37 (95% CI: 2.05, 9.29) C: 1.63 (95% CI: 0.91, 2.92] E: 1.36 (95% CI: 0.94, 1.97)* F: 2.05 (95% CI: 1.19, 3.56]. G: 1.36 (95% CI: 0.97, 1.92)* J: 1.05 (95% CI: 0.86, 1.28)*
*proportion of patients with resolution calculated by substracting the patients with treatment failure from the total study population
Outcome measure: episodes of OM B (OME): I: 7 / C: 6 D: I: 1.73 ± 1.8 /C: 1.44 ± 1.5 H (OME): I: 1.06 / C: 1.45 ( I (AOM) 1.8 / C: 2.1
Outcome measure: hearing loss A: MD: 4.3 (-1.4 to 9.9) C: MD: -3.10 (-8.57 to 2.37) F: MD: -16.10 (-20.58 to -11.62)
Outcome measure: otorrhea
H: 0.13. vs 0.13
|
Facultative:
Brief description of author’s conclusion: “Our review shows a significant benefit of adenoidectomy as far as the resolution of middle ear eGusion in children with OME is concerned. However, the benefit to hearing is small and the eGects on changes in the tympanic membrane are unknown. The risks of operating should be weighed against these potential benefits”
Included trials not considered relevant (+ reason) - Fillau-Nikolajsen 1980 (no relevant outcome measures reported) - Gates 1987 (no relevant outcome measures reported) - Matilla 2003 (no relevant outcome measures reported) - Rynnel-Dagoo 1978 (part of the children had additional insertion of tubes; not equal between intervention and control)
|
Evidence table for intervention studies (randomized controlled trials and non-randomized observational studies [cohort studies, case-control studies, case series])1
|
Study reference |
Study characteristics |
Patient characteristics 2 |
Intervention (I) |
Comparison / control I 3
|
Follow-up |
Outcome measures and effect size 4 |
Comments |
|
Browning 2012 |
Type of study: RCT
Setting and country: Study at 11 ENT departments in the UK (1994 – 1998)
Funding and conflicts of interest: None |
Inclusion criteria: - children aged 3.25 to 6.75 years on first visit - no previous ear surgery - bilateral B + B or B + C2 tympanogram combination - Better ear HL >20 dB HL average across 0.5, 1, 2 and 4 kHz and airbone gap > 10 dB - pOME
Exclusion criteria: - children with binaural HL > 40 dB, were not randomised
N total at baseline: Intervention: 128 Control: 126
Important prognostic factors2: For example age ± SD: I: 64.5 ± 10.3 mo. C: 62.5 ± 10.2 mo.
Sex: I: 48% Male C: 48% Male
Acute OM episodes, >6 per year I: 127 N: 126
Groups comparable at baseline? Probably yes
|
Describe intervention:
Bilateral Shepards Ventilation tubes with adjuvant adenoidectomy
|
Describe control
Bilateral Shepards Ventilation tubes
|
Length of follow-up: 3, 6, 12, 18 and 24 months post-randomization
Loss-to-follow-up: at 6 months:
n attending the FU visit
Intervention: 106 Control: 112
Incomplete outcome data: All models used imputation for missing data
Some patients transferred to the other intervention, however, all analyses were performed as-randomised (intention to treat),
|
Outcome measures and effect size (include 95%CI and p-value if available):
Average HL at 3 to 6 months FU I: 14.6 ± 6.1 C: 15.9 ± 6.2 MD: -1.30 [-2.81, 0.21]
|
The author’s concluded that: “Adjuvant adenoidectomy doubles benefit from short-stay ventilation tubes by extending better hearing through the second year in children aged 3.25– 6.75 years with persistent otitis media with effusion with at least a 20 dB HL in both ears” |
|
Hao 2019 |
Type of study: RCT
Setting and country: Recruitment at the Otolargyngology department at Hospital in China (Single centre) from September 2013 to January 2015
Funding and conflicts of interest: None |
Inclusion criteria: - children with OME - aged 3 – 6 years - having surgical indications after conservative treatment failure
Exclusion criteria: Children with: - mixed deafness - craniofacial deformities - incomplete clinical follow-up data - tube indwelling time < 6 months
N total at baseline: N = 184 Intervention: 98 Control: 86
Important prognostic factors2: For example age ± SD: I: 5.03 ± 0.95 C: 5.21 ± 1.02
Sex: I: 64% male C: 64% male
Groups comparable at baseline? Probably yes (no statistically significant difference between groups)
|
Describe intervention (treatment/procedure/test):
Adenoidectomy + tympanostomy tube insertion
Group A
|
Describe control (treatment/procedure/test):
Tympanostomy tube insertion
Group B |
Length of follow-up: 3 months and 6 months post-operatively
Loss-to-follow-up: Intervention: N (%) Reasons (describe)
Control: N (%) Reasons (describe)
Incomplete outcome data: Children with incomplete follow-up data were excluded
|
Outcome measures and effect size (include 95%CI and p-value if available):
Clinical curative effect (= disappearance of clinical symptoms and middle ear effusion, hearing recovery) I: 52/98 (53.06%) C: 42/86 (48.84%)
Air conduction hearing threshold (6 months FU) I: 14.52 ± 5.42 C: 15.28 ± 5.27 |
The author’s concluded that: “Tube insertion combined with adenoidectomy is more effective than ventilation tubes in the treatment of young children with OME, and the same results were found for children under four years of age.” |
|
Kujala 2012 |
Type of study: RCT
Setting and country: Recruitment at Otorhinolargyngology department at a hospital (single centre) in Finland March 2002 – June 2004
Funding and conflicts of interest: None |
Inclusion criteria: - rAOM, at least 3 episodes during past 6 months - aged 10 months - 2 years
Exclusion criteria: - OME - prior adenoidectomy or tubes - cranial anomalies - immunological disorders - ongoing antimicrobial prophylaxis
N total at baseline: Intervention: 100 Control: 100
Important prognostic factors2: For example age ± SD: I: 17.7 ± 4.3 months C: 16.1 ± 4.0 months
Sex: I: 58% male C: 57% male
Groups comparable at baseline? Probably yes
|
Describe intervention (treatment/procedure/test):
Adenoidectomy with ventilation tubes
Donaldson silicone tubes |
Describe control (treatment/procedure/test):
Ventilation tubes
Donaldson silicone tubes
|
Length of follow-up: At least every 4 months for 1 year
Loss-to-follow-up: Intervention: n = 6 Reasons (describe)
Control: n = 18 Reasons (describe)
Analysed according to intention to treat principle.
Analysis until failure or drop-out
|
Outcome measures and effect size (include 95%CI and p-value if available):
Failure of intervention (2 AOM episodes in 3 months or 3 episodes in 6 months). I: 16/100 C: 21/00
|
The author’s concluded that: “Insertion of ventilation tubes alone or with adenoidectomy was effective in preventing recurrent AOM episodes in children younger than 2 years of age” |
Table of quality assessment for systematic reviews of RCTs and observational studies
Based on AMSTAR checklist (Shea et al.; 2007, BMC Methodol 7: 10; doi:10.1186/1471-2288-7-10) and PRISMA checklist (Moher et al 2009, PloS Med 6: e1000097; doi:10.1371/journal.pmed1000097)
|
Study
First author, year |
Appropriate and clearly focused question?1
Yes/no/unclear |
Comprehensive and systematic literature search?2
Yes/no/unclear |
Description of included and excluded studies?3
Yes/no/unclear |
Description of relevant characteristics of included studies?4
Yes/no/unclear |
Appropriate adjustment for potential confounders in observational studies?5
Yes/no/unclear/not applicable |
Assessment of scientific quality of included studies?6
Yes/no/unclear |
Enough similarities between studies to make combining them reasonable?7
Yes/no/unclear |
Potential risk of publication bias taken into account?8
Yes/no/unclear |
Potential conflicts of interest reported?9
Yes/no/unclear |
|
Van den Aardweg, 2010 |
Yes
Reason: PICO and research question are defined |
Yes:
Reason: Search period and strategy are described, MEDLINE was searched |
Yes
Reason: exclusion table included |
Yes
Reason: overview of individual study characteristics reported |
Not applicable |
Yes
Reason: Cochrane RoB tool was used |
Yes
Reason I2 was calculated. No pooling if heterogeneity was considered large |
Yes
Reason: it was stated that: due to large variety in the outcome measures it was not possible to make a funnel plot to explore publication bias |
Unclear:
Reason: ‘other biases’ were identified but no clear overview of conflicts of interest/funding per study |
1. Research question (PICO) and inclusion criteria should be appropriate and predefined
2. Search period and strategy should be described; at least Medline searched; for pharmacological questions at least Medline + EMBASE searched
3. Potentially relevant studies that are excluded at final selection (after reading the full text) should be referenced with reasons
4. Characteristics of individual studies relevant to research question (PICO), including potential confounders, should be reported
5. Results should be adequately controlled for potential confounders by multivariate analysis (not applicable for RCTs)
6. Quality of individual studies should be assessed using a quality scoring tool or checklist (Jadad score, Newcastle-Ottawa scale, risk of bias table etc.)
7. Clinical and statistical heterogeneity should be assessed; clinical: enough similarities in patient characteristics, intervention, and definition of outcome measure to allow pooling? For pooled data: assessment of statistical heterogeneity using appropriate statistical tests (e.g., Chi-square, I2)?
8. An assessment of publication bias should include a combination of graphical aids (e.g., funnel plot, other available tests) and/or statistical tests (e.g., Egger regression test, Hedges-Olken). Note: If no test values or funnel plot included, score “no”. Score “yes” if mentions that publication bias could not be assessed because there were fewer than 10 included studies.
9. Sources of support (including commercial co-authorship) should be reported in both the systematic review and the included studies. Note: To get a “yes,” source of funding or support must be indicated for the systematic review AND for each of the included studies.
Risk of bias table for intervention studies (randomized controlled trials; based on Cochrane risk of bias tool and suggestions by the CLARITY Group at McMaster University)
|
Study reference
(first author, publication year) |
Was the allocation sequence adequately generated?
Definitely yes Probably yes Probably no Definitely no |
Was the allocation adequately concealed?
Definitely yes Probably yes Probably no Definitely no |
Blinding: Was knowledge of the allocated interventions adequately prevented?
Were patients blinded?
Were healthcare providers blinded?
Were data collectors blinded?
Were outcome assessors blinded?
Were data analysts blinded?
Definitely yes Probably yes Probably no Definitely no |
Was loss to follow-up (missing outcome data) infrequent?
Definitely yes Probably yes Probably no Definitely no |
Are reports of the study free of selective outcome reporting?
Definitely yes Probably yes Probably no Definitely no |
Was the study apparently free of other problems that could put it at a risk of bias?
Definitely yes Probably yes Probably no Definitely no |
Overall risk of bias If applicable/necessary, per outcome measure
LOW Some concerns HIGH
|
|
Browning 2012 |
Definitely yes;
Reason: randomization according to a computer-generated random number sequence |
Definitely yes;
Reason: telephone call from the nurse/research assistant immediately communicated to the parents |
Definitely no
Reason: outcome assessors and data analysts were blinded; however, patients and health care providers je were not (due to nature of the intervention) |
Probably yes;
Reason: Loss to follow-up was infrequent in intervention and control group (94%) It was stated that imputation was used, but not which type of imputation. |
Definitely yes;
Reason: all outcomes stated in the method section are reported, outcomes of different FU periods were combined. |
Probably yes;
Reason: No other sources of bias could be identified |
All outcome measures: some concerns, due to impossibility to blind patients and health care providers |
|
Hao 2019 |
Probably no
Reason: not clear how allocation sequence was generated
It was stated that: all patients were randomly divided to Group A or Group B according to different surgical methods |
Probably no
Reason: no information
|
Definitely no
Reason: no information on blinding procedures was reported; nature of the intervention makes blinding unlikely |
Unclear
Reason: it was stated that children with incomplete data were excluded |
Probably yes
Reason: outcomes stated in method section are reported |
Probably yes;
Reason: No other sources of bias could be identified |
All outcome measures: high risk of bias due to unclear randomization and allocation procedures and lack of blinding |
|
Kujala 2012 |
Probably yes
Reason: children were randomly allocated using permuted blocks with a size of 3
|
Definitely yes
Reason: consecutively numbered sealed opaque enveloped were used |
Definitely no
Reason: it was stated that ‘investigators and parents cannot be blinded’ (due to nature of the intervention) |
Probably yes
Reason: loss to follow-up was infrequent in intervention and control group, intention to treat analysis was performed |
Probably yes;
Reason: outcomes stated in method section are reported |
Probably yes;
Reason: No other sources of bias could be identified Reason: |
All outcome measures: some concerns, due to impossibility to blind patients and health care providers |
Randomization: generation of allocation sequences have to be unpredictable, for example computer generated random-numbers or drawing lots or envelopes. Examples of inadequate procedures are generation of allocation sequences by alternation, according to case record number, date of birth or date of admission.
Allocation concealment: refers to the protection (blinding) of the randomization process. Concealment of allocation sequences is adequate if patients and enrolling investigators cannot foresee assignment, for example central randomization (performed at a site remote from trial location). Inadequate procedures are all procedures based on inadequate randomization procedures or open allocation schedules.
Blinding: neither the patient nor the care provider (attending physician) knows which patient is getting the special treatment. Blinding is sometimes impossible, for example when comparing surgical with non-surgical treatments, but this should not affect the risk of bias judgement. Blinding of those assessing and collecting outcomes prevents that the knowledge of patient assignment influences the process of outcome assessment or data collection (detection or information bias). If a study has hard (objective) outcome measures, like death, blinding of outcome assessment is usually not necessary. If a study has “soft” (subjective) outcome measures, like the assessment of an X-ray, blinding of outcome assessment is necessary. Finally, data analysts should be blinded to patient assignment to prevents that knowledge of patient assignment influences data analysis.
Lost to follow-up: If the percentage of patients lost to follow-up or the percentage of missing outcome data is large, or differs between treatment groups, or the reasons for loss to follow-up or missing outcome data differ between treatment groups, bias is likely unless the proportion of missing outcomes compared with observed event risk is not enough to have an important impact on the intervention effect estimate or appropriate imputation methods have been used.
Selective outcome reporting: Results of all predefined outcome measures should be reported; if the protocol is available (in publication or trial registry), then outcomes in the protocol and published report can be compared; if not, outcomes listed in the methods section of an article can be compared with those whose results are reported.
Other biases: Problems may include: a potential source of bias related to the specific study design used (e.g., lead-time bias or survivor bias); trial stopped early due to some data-dependent process (including formal stopping rules); relevant baseline imbalance between intervention groups; claims of fraudulent behavior; deviations from intention-to-treat (ITT) analysis; (the role of the) funding body. Note: The principles of an ITT analysis imply that (a) participants are kept in the intervention groups to which they were randomized, regardless of the intervention they actually received, (b) outcome data are measured on all participants, and (c) all randomized participants are included in the analysis.
Overall judgement of risk of bias per study and per outcome measure, including predicted direction of bias (e.g., favors experimental, or favors comparator). Note: the decision to downgrade the certainty of the evidence for a particular outcome measure is taken based on the body of evidence, i.e., considering potential bias and its impact on the certainty of the evidence in all included studies reporting on the outcome.
Table of excluded studies
|
Reference |
Reason for exclusion |
|
Kujala T, Alho OP, Kristo A, Uhari M, Renko M, Pokka T, Koivunen P. Quality of life after surgery for recurrent otitis media in a randomized controlled trial. Pediatr Infect Dis J. 2014 Jul;33(7):715-9. Doi: 10.1097/INF.0000000000000265. PMID: 24445832. |
Data only presented in graphs |
|
To, K., L. Harrison, and M. Daniel. “Management of otitis media with effusion and recurrent acute otitis media.” The Otorhinolaryngologist (2013). |
Overview of all treatment options for otitis media |
|
Jabeen, Farhat, et al. “Comparison of rate of recurrence of otitis media with effusion in children treated by myringotomy and ventilating tube insertion with patients treated by additional adenoidectomy.” Rawal Medical Journal 44.3 (2019): 513-520. |
Outcomes (recurrence) only at 3-month follow-up |
|
Boonacker CW, Rovers MM, Browning GG, Hoes AW, Schilder AG, Burton MJ. Adenoidectomy with or without grommets for children with otitis media: an individual patient data meta-analysis. Health Technol Assess. 2014 Jan;18(5):1-118. Doi: 10.3310/hta18050. PMID: 24438691; PMCID: PMC4780935. |
Systematic review with individual patient data analysis. |
|
Mikals SJ, Brigger MT. Adenoidectomy as an adjuvant to primary tympanostomy tube placement: a systematic review and meta-analysis. JAMA Otolaryngol Head Neck Surg. 2014 Feb;140(2):95-101. Doi: 10.1001/jamaoto.2013.5842. PMID: 24287958. |
Low quality SR |
|
Cheong KH, Hussain SS. Management of recurrent acute otitis media in children: systematic review of the effect of different interventions on otitis media recurrence, recurrence frequency and total recurrence time. J Laryngol Otol. 2012 Sep;126(9):874-85. Doi: 10.1017/S0022215112001338. Epub 2012 Jul 5. PMID: 22874133. |
Observational studies and studies on drug treatment included in analysis |
|
Berkman ND, Wallace IF, Steiner MJ, Harrison M, Greenblatt AM, Lohr KN, Kimple A, Yuen A. Otitis Media With Effusion: Comparative Effectiveness of Treatments [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2013 May. Report No.: 13-EHC091-EF. PMID: 23762917. |
Studies on other management (e.g drug treatment) included |
|
Wallace IF, Berkman ND, Lohr KN, Harrison MF, Kimple AJ, Steiner MJ. Surgical treatments for otitis media with effusion: a systematic review. Pediatrics. 2014 Feb;133(2):296-311. Doi: 10.1542/peds.2013-3228. Epub 2014 Jan 6. PMID: 24394689. |
Non-RCTS and observational studies included |
|
Tian X, Liu Y, Wang M, Liu H. [A systematic review of adenoidectomy in the treatment of otitis media with effusion in children]. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2014 Apr;29(8):723-5. Chinese. PMID: 26248446. |
Article in Chinese |
|
Tian, Xiao-Yan, Mei-Qun Wang, and Yue-Hui Liu. “A systematic review of adenoidectomy in the treatment of otitis media with effusion in children.” Int J Clin Exp Med 11.10 (2018): 10639-10645. |
Low quality SR (no table with baseline characteristics or excluded studies) |
|
Xu WM, Ye YH. [Effect of tympanostomy tube insertion with adenoidectomy for children with recurrent otitis media with effusion]. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2016 Dec;30(23):1873-1875. Chinese. Doi: 10.13201/j.issn.1001-1781.2016.23.009. PMID: 29798016. |
Article in Chinese |
|
Williamson I. Otitis media with effusion in children. BMJ Clin Evid. 2011 Jan 12;2011:0502. PMID: 21477396; PMCID: PMC3275303. |
Studies on other management esignies (e.g drug treatment) |
|
Schilder AG, Marom T, Bhutta MF, Casselbrant ML, Coates H, Gisselsson-Solén M, Hall AJ, Marchisio P, Ruohola A, Venekamp RP, Mandel EM. Panel 7: Otitis Media: Treatment and Complications. Otolaryngol Head Neck Surg. 2017 Apr;156(4_suppl):S88-S105. Doi: 10.1177/0194599816633697. PMID: 28372534. |
Search strategy of revie w not clear, no table of excluded studies |
|
Popova D, Varbanova S, Popov TM. Comparison between myringotomy and tympanostomy tubes in combination with adenoidectomy in 3-7-year-old children with otitis media with effusion. Int J Pediatr Otorhinolaryngol. 2010 Jul;74(7):777-80. Doi: 10.1016/j.ijporl.2010.03.054. PMID: 20399511. |
Wrong comparator: both groups received adenoidectomy |
|
van den Aardweg, M. T., Schilder, A. G., Herkert, E., Boonacker, C. W., & Rovers, M. (2009). Adenoidectomy for recurrent or chronic nasal symptoms and middle ear disease in children up to 18 years of age. Cochrane Database of Systematic Reviews, (2). |
Wrong study esign: study protocol |
|
Marchisio P, Chonmaitree T, Leibovitz E, Lieberthal A, Lous J, Mandel E, McCormick D, Morris P, Ruohola A. Panel 7: Treatment and comparative effectiveness research. Otolaryngol Head Neck Surg. 2013 Apr;148(4 Suppl):E102-21. Doi: 10.1177/0194599812465397. PMID: 23536528. |
More recent panel paper available (Schilder, 2017) |
|
Casselbrant ML, Mandel EM, Rockette HE, Kurs-Lasky M, Fall PA, Bluestone CD. Adenoidectomy for otitis media with effusion in 2-3-year-old children. Int J Pediatr Otorhinolaryngol. 2009 Dec;73(12):1718-24. Doi: 10.1016/j.ijporl.2009.09.007. Epub 2009 Oct 12. PMID: 19819563; PMCID: PMC2787742. |
Included in SR van den Aardweg 2010 |
Verantwoording
Beoordelingsdatum en geldigheid
Laatst beoordeeld : 29-04-2024
Algemene gegevens
De ontwikkeling/herziening van deze richtlijnmodule werd ondersteund door het Kennisinstituut van de Federatie Medisch Specialisten (www.demedischspecialist.nl/kennisinstituut) en werd gefinancierd uit de Kwaliteitsgelden Medisch Specialisten (SKMS). De financier heeft geen enkele invloed gehad op de inhoud van de richtlijnmodule.
Samenstelling werkgroep
Voor het ontwikkelen van de richtlijnmodule is in 2021 een multidisciplinaire werkgroep ingesteld, bestaande uit vertegenwoordigers van alle relevante specialismen (zie hiervoor de Samenstelling van de werkgroep) die betrokken zijn bij de zorg voor patiënten met Otitis Media (in de tweede lijn).
Werkgroep
- Dhr. dr. H.J. (Jeroen) Rosingh (voorzitter), KNO-arts, Isala Zwolle; NVKNO
- Mevr. dr. E.H. (Jet) van den Akker, KNO-arts, Meander MC Amersfoort; NVKNO
- Dhr. dr. M.P. (Marc) van der Schroeff, KNO-arts, Erasmus MC Rotterdam; NVKNO
- Mevr. dr. S.A.C. (Sophie) Kraaijenga, KNO-arts, Rijnstate Arnhem; NVKNO
- Mevr. dr. J.E.C. (Esther) Wiersinga-Post, Klinisch Fysicus - audioloog, UMC Groningen; NVKF
- Mevr. H.F. (Francien) Miedema MSc, logopedist/klinisch gezondheidswetenschapper, Jeroen Bosch Ziekenhuis, ’s-Hertogenbosch; NVLF
- Dhr. dr. R.P. (Roderick) Venekamp, huisarts, UMC Utrecht; NHG
- Mevr. H. (Hester) Rippen, patiëntvertegenwoordiger; Stichting Kind en Ziekenhuis
Met ondersteuning van
- Mevr. dr. A. (Anja) van der Hout, adviseur, Kennisinstituut van de Federatie Medisch Specialisten (vanaf september 2022)
- Mevr. D.G. (Dian) Ossendrijver, MSc, junior adviseur, Kennisinstituut van de Federatie Medisch Specialisten
- Dhr. M. (Mitchel) Griekspoor, MSc, adviseur Kennisinstituut van de Federatie Medisch Specialisten
- Mevr. dr. I. (Iris) Duif, adviseur Kennisinstituut van de Federatie Medisch Specialisten (tot september 2022)
Belangenverklaringen
De Code ter voorkoming van oneigenlijke beïnvloeding door belangenverstrengeling is gevolgd. Alle werkgroepleden hebben schriftelijk verklaard of zij in de laatste drie jaar directe financiële belangen (betrekking bij een commercieel bedrijf, persoonlijke financiële belangen, onderzoeksfinanciering) of indirecte belangen (persoonlijke relaties, reputatiemanagement) hebben gehad. Gedurende de ontwikkeling of herziening van een module worden wijzigingen in belangen aan de voorzitter doorgegeven. De belangenverklaring wordt opnieuw bevestigd tijdens de commentaarfase.
Een overzicht van de belangen van werkgroepleden en het oordeel over het omgaan met eventuele belangen vindt u in onderstaande tabel. De ondertekende belangenverklaringen zijn op te vragen bij het secretariaat van het Kennisinstituut van de Federatie Medisch Specialisten.
|
Werkgroeplid |
Functie |
Nevenfuncties |
Gemelde belangen |
Ondernomen actie |
|
Rosingh |
- KNO-arts in lsala, Zwolle (werkend in MSB verband, dus niet in dienst van het ziekenhuis) - Plaatsvervangend opleider - KNO-arts in Ziekenhuis in Suriname, MMC Nickerie (september 2022 en november 2023) |
- Voorzitter Commissie Richtlijnen NVKNO - Opleider KNO en lid van consilium - Voorzitter ad interim commissie Kwaliteit & Veiligheid van (jan. 2023 – sep 2023) |
Geen |
Geen restricties |
|
Van der Schroeff |
KNO-arts, ErasmusMC |
Geen |
Geen |
Geen restricties |
|
Rippen |
- Directeur Stichting Kind en Ziekenhuis - Eigenaar Fiduz management (strategie, advies en projectmanagement) |
- Lid Raad van Toezicht MEEr-groep - Lid Adviesraad Medgezel - Coördinator European Association for Children in Hospital (EACH) - Bestuurslid College Perinatale zorg (CPZ) - AQUA De methodologische Advies- en expertgroep Leidraad voor Kwaliteitsstandaarden (AQUA) - Penningmeester Ervaringskenniscentrum Schouders - Voorzitter Landelijke Borstvoedingsraad - Voorzitter MKS Landelijke coördinatieteam Integrale Kindzorg - Voorzitter Expertiseraad Kenniscentrum kinderpalliatieve zorg - Lid Algemene Ledenvergadering VZVZ - Lid beoordelingscommissie KIDZ |
Geen |
Geen restricties |
|
Kraaijenga |
KNO-arts, Rijnstate ziekenhuis te Arnhem. |
Geen |
Geen |
Geen restricties |
|
Wiersinga-Post |
Klinisch fysicus – audioloog, UMC Groningen |
Geen |
Geen |
Geen restricties |
|
Miedema |
Logopedist, klinisch gezondheidswetenschapper Jeroen Bosch ziekenhuis, ‘s-Hertogenbosch |
Geen |
Geen |
Geen restricties |
|
Van den Akker |
KNO-arts, Meander Medisch Centrum Amersfoort |
-Lid Kerngroep kinder kno van de Nederlandse KNO vereniging, (betaald) - Voorzitter peersupport team meander medische centrum (onbetaald) |
Geen |
Geen restricties |
|
Venekamp |
- Praktiserend huisarts, Huisartsenpraktijk Verwielstraat te Waalwijk - Associate professor, Julius Centrum, UMC Utrecht |
NHG Autorisatiecommissie (vacatiegelden) |
Onze onderzoeksgroep van de afdeling Huisartsgeneeskunde van het Julius Centrum, UMC Utrecht, verricht onderzoek naar alledaagse infectieziekten dat wordt gefinancierd door (semi)overheid, met name ZonMw, en fondsen. |
Geen restricties |
Inbreng patiëntenperspectief
Er werd aandacht besteed aan het patiëntenperspectief door afvaardiging van Kind en Ziekenhuis in de richtlijnwerkgroep. De verkregen input is meegenomen bij het opstellen van de uitgangsvragen, de keuze voor de uitkomstmaten en bij het opstellen van de overwegingen. De conceptrichtlijn is tevens voor commentaar voorgelegd aan Kind en Ziekenhuis en de eventueel aangeleverde commentaren zijn bekeken en verwerkt.
Kwalitatieve raming van mogelijke financiële gevolgen in het kader van de Wkkgz
Bij de richtlijn is conform de Wet kwaliteit, klachten en geschillen zorg (Wkkgz) een kwalitatieve raming uitgevoerd of de aanbevelingen mogelijk leiden tot substantiële financiële gevolgen. Bij het uitvoeren van deze beoordeling zijn richtlijnmodules op verschillende domeinen getoetst (zie het stroomschema op de Richtlijnendatabase).
Uit de kwalitatieve raming blijkt dat er waarschijnlijk geen substantiële financiële gevolgen zijn, zie onderstaande tabel.
|
Module |
Uitkomst raming |
Toelichting |
|
Adenotomie bij otitis media (rOMA, pOME, ACMOM) |
geen financiële gevolgen |
Hoewel uit de toetsing volgt dat de aanbeveling(en) breed toepasbaar zijn (5.000-40.000 patiënten), volgt ook uit de toetsing dat het overgrote deel (±90%) van de zorgaanbieders en zorgverleners al aan de norm voldoet. Er worden daarom geen substantiële financiële gevolgen verwacht. |
Werkwijze
AGREE
Deze richtlijnmodule is opgesteld conform de eisen vermeld in het rapport Medisch Specialistische Richtlijnen 2.0 van de adviescommissie Richtlijnen van de Raad Kwaliteit. Dit rapport is gebaseerd op het AGREE II instrument (Appraisal of Guidelines for Research & Evaluation II; Brouwers, 2010).
Need-for-update en uitgangsvragen
Tijdens de voorbereidende fase inventariseerde de werkgroep de geldigheid van de modules binnen de richtlijn Otitis media bij kinderen in de tweede lijn (need-for-update). Naast de betrokken wetenschappelijke verenigingen en patiëntenorganisaties zijn hier ook andere stakeholders voor benaderd in 2020. Ook was er de mogelijkheid om nieuwe onderwerpen voor modules aan te dragen die aansluiten bij de richtlijn.
Op basis van de uitkomsten van deze inventarisatie zijn door de werkgroep geprioriteerd en daarbij zijn de uitgangsvragen opgesteld en definitief vastgesteld tijdens een vergadering.
Uitkomstmaten
Na het opstellen van de zoekvraag behorende bij de uitgangsvraag inventariseerde de werkgroep welke uitkomstmaten voor de patiënt relevant zijn, waarbij zowel naar gewenste als ongewenste effecten werd gekeken. Hierbij werd een maximum van acht uitkomstmaten gehanteerd. De werkgroep waardeerde deze uitkomstmaten volgens hun relatieve belang bij de besluitvorming rondom aanbevelingen, als cruciaal (kritiek voor de besluitvorming), belangrijk (maar niet cruciaal) en onbelangrijk. Tevens definieerde de werkgroep tenminste voor de cruciale uitkomstmaten welke verschillen zij klinisch (patiënt) relevant vonden.
Methode literatuursamenvatting
Een uitgebreide beschrijving van de strategie voor zoeken en selecteren van literatuur is te vinden onder ‘Zoeken en selecteren’ onder Onderbouwing. Indien mogelijk werd de data uit verschillende studies gepoold in een random-effects model. Review Manager 5.4 werd gebruikt voor de statistische analyses. De beoordeling van de kracht van het wetenschappelijke bewijs wordt hieronder toegelicht.
Beoordelen van de kracht van het wetenschappelijke bewijs
De kracht van het wetenschappelijke bewijs werd bepaald volgens de GRADE-methode. GRADE staat voor ‘Grading Recommendations Assessment, Development and Evaluation’ (zie http://www.gradeworkinggroup.org/). De basisprincipes van de GRADE-methodiek zijn: GRADE-methodiek zijn: het benoemen en prioriteren van de klinisch (patiënt) relevante uitkomstmaten, een systematische review per uitkomstmaat, en een beoordeling van de bewijskracht per uitkomstmaat op basis van de acht GRADE-domeinen (domeinen voor downgraden: risk of bias, inconsistentie, indirectheid, imprecisie, en publicatiebias; domeinen voor upgraden: dosis-effect relatie, groot effect, en residuele plausibele confounding).
GRADE onderscheidt vier gradaties voor de kwaliteit van het wetenschappelijk bewijs: hoog, redelijk, laag en zeer laag. Deze gradaties verwijzen naar de mate van zekerheid die er bestaat over de literatuurconclusie, in het bijzonder de mate van zekerheid dat de literatuurconclusie de aanbeveling adequaat ondersteunt (Schünemann, 2013; Hultcrantz, 2017).
|
GRADE |
Definitie |
|
Hoog |
|
|
Redelijk |
|
|
Laag |
|
|
Zeer laag |
|
Bij het beoordelen (graderen) van de kracht van het wetenschappelijk bewijs in richtlijnen volgens de GRADE-methodiek spelen grenzen voor klinische besluitvorming een belangrijke rol (Hultcrantz, 2017). Dit zijn de grenzen die bij overschrijding aanleiding zouden geven tot een aanpassing van de aanbeveling. Om de grenzen voor klinische besluitvorming te bepalen moeten alle relevante uitkomstmaten en overwegingen worden meegewogen. De grenzen voor klinische besluitvorming zijn daarmee niet één op één vergelijkbaar met het minimaal klinisch relevant verschil (Minimal Clinically Important Difference, MCID). Met name in situaties waarin een interventie geen belangrijke nadelen heeft en de kosten relatief laag zijn, kan de grens voor klinische besluitvorming met betrekking tot de effectiviteit van de interventie bij een lagere waarde (dichter bij het nul effect) liggen dan de MCID (Hultcrantz, 2017).
Overwegingen (van bewijs naar aanbeveling)
Om te komen tot een aanbeveling zijn naast (de kwaliteit van) het wetenschappelijke bewijs ook andere aspecten belangrijk en worden meegewogen, zoals aanvullende argumenten uit bijvoorbeeld de biomechanica of fysiologie, waarden en voorkeuren van patiënten, kosten (middelenbeslag), aanvaardbaarheid, haalbaarheid en implementatie. Deze aspecten zijn systematisch vermeld en beoordeeld (gewogen) onder het kopje ‘Overwegingen’ en kunnen (mede) gebaseerd zijn op expert opinion. Hierbij is gebruik gemaakt van een gestructureerd format gebaseerd op het evidence-to-decision framework van de internationale GRADE Working Group (Alonso-Coello, 2016a; Alonso-Coello 2016b). Dit evidence-to-decision framework is een integraal onderdeel van de GRADE methodiek.
Formuleren van aanbevelingen
De aanbevelingen geven antwoord op de uitgangsvraag en zijn gebaseerd op het beschikbare wetenschappelijke bewijs en de belangrijkste overwegingen, en een weging van de gunstige en ongunstige effecten van de relevante interventies. De kracht van het wetenschappelijk bewijs en het gewicht dat door de werkgroep wordt toegekend aan de overwegingen, bepalen samen de sterkte van de aanbeveling. Conform de GRADE-methodiek sluit een lage bewijskracht van conclusies in de systematische literatuuranalyse een sterke aanbeveling niet a priori uit, en zijn bij een hoge bewijskracht ook zwakke aanbevelingen mogelijk (Agoritsas, 2017; Neumann, 2016). De sterkte van de aanbeveling wordt altijd bepaald door weging van alle relevante argumenten tezamen. De werkgroep heeft bij elke aanbeveling opgenomen hoe zij tot de richting en sterkte van de aanbeveling zijn gekomen.
In de GRADE-methodiek wordt onderscheid gemaakt tussen sterke en zwakke (of conditionele) aanbevelingen. De sterkte van een aanbeveling verwijst naar de mate van zekerheid dat de voordelen van de interventie opwegen tegen de nadelen (of vice versa), gezien over het hele spectrum van patiënten waarvoor de aanbeveling is bedoeld. De sterkte van een aanbeveling heeft duidelijke implicaties voor patiënten, behandelaars en beleidsmakers (zie onderstaande tabel). Een aanbeveling is geen dictaat, zelfs een sterke aanbeveling gebaseerd op bewijs van hoge kwaliteit (GRADE gradering HOOG) zal niet altijd van toepassing zijn, onder alle mogelijke omstandigheden en voor elke individuele patiënt.
|
Implicaties van sterke en zwakke aanbevelingen voor verschillende richtlijngebruikers |
||
|
|
Sterke aanbeveling |
Zwakke (conditionele) aanbeveling |
|
Voor patiënten |
De meeste patiënten zouden de aanbevolen interventie of aanpak kiezen en slechts een klein aantal niet. |
Een aanzienlijk deel van de patiënten zouden de aanbevolen interventie of aanpak kiezen, maar veel patiënten ook niet. |
|
Voor behandelaars |
De meeste patiënten zouden de aanbevolen interventie of aanpak moeten ontvangen. |
Er zijn meerdere geschikte interventies of aanpakken. De patiënt moet worden ondersteund bij de keuze voor de interventie of aanpak die het beste aansluit bij zijn of haar waarden en voorkeuren. |
|
Voor beleidsmakers |
De aanbevolen interventie of aanpak kan worden gezien als standaardbeleid. |
Beleidsbepaling vereist uitvoerige discussie met betrokkenheid van veel stakeholders. Er is een grotere kans op lokale beleidsverschillen. |
Organisatie van zorg
In de knelpuntenanalyse en bij de ontwikkeling van de richtlijnmodule is expliciet aandacht geweest voor de organisatie van zorg: alle aspecten die randvoorwaardelijk zijn voor het verlenen van zorg (zoals coördinatie, communicatie, (financiële) middelen, mankracht en infrastructuur). Randvoorwaarden die relevant zijn voor het beantwoorden van deze specifieke uitgangsvraag zijn genoemd bij de overwegingen van de verschillende modules.
Commentaar- en autorisatiefase
De conceptrichtlijnmodule werd aan de betrokken (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd ter commentaar. De commentaren werden verzameld en besproken met de werkgroep. Naar aanleiding van de commentaren werd de conceptrichtlijnmodule aangepast en definitief vastgesteld door de werkgroep. De definitieve richtlijnmodule werd aan de deelnemende (wetenschappelijke) verenigingen en (patiënt) organisaties voorgelegd voor autorisatie en door hen geautoriseerd dan wel geaccordeerd.
Literatuur
Agoritsas T, Merglen A, Heen AF, Kristiansen A, Neumann I, Brito JP, Brignardello-Petersen R, Alexander PE, Rind DM, Vandvik PO, Guyatt GH. UpToDate adherence to GRADE criteria for strong recommendations: an analytical survey. BMJ Open. 2017 Nov 16;7(11):e018593. Doi: 10.1136/bmjopen-2017-018593. PubMed PMID: 29150475; PubMed Central PMCID: PMC5701989.
Alonso-Coello P, Schünemann HJ, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Rada G, Rosenbaum S, Morelli A, Guyatt GH, Oxman AD; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ. 2016 Jun 28;353:i2016. Doi: 10.1136/bmj.i2016. PubMed PMID: 27353417.
Alonso-Coello P, Oxman AD, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Vandvik PO, Meerpohl J, Guyatt GH, Schünemann HJ; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 2: Clinical practice guidelines. BMJ. 2016 Jun 30;353:i2089. Doi: 10.1136/bmj.i2089. PubMed PMID: 27365494.
Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, Fervers B, Graham ID, Grimshaw J, Hanna SE, Littlejohns P, Makarski J, Zitzelsberger L; AGREE Next Steps Consortium. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010 Dec 14;182(18):E839-42. Doi: 10.1503/cmaj.090449. Epub 2010 Jul 5. Review. PubMed PMID: 20603348; PubMed Central PMCID: PMC3001530.
Hultcrantz M, Rind D, Akl EA, Treweek S, Mustafa RA, Iorio A, Alper BS, Meerpohl JJ, Murad MH, Ansari MT, Katikireddi SV, Östlund P, Tranæus S, Christensen R, Gartlehner G, Brozek J, Izcovich A, Schünemann H, Guyatt G. The GRADE Working Group clarifies the construct of certainty of evidence. J Clin Epidemiol. 2017 Jul;87:4-13. Doi: 10.1016/j.jclinepi.2017.05.006. Epub 2017 May 18. PubMed PMID: 28529184; PubMed Central PMCID: PMC6542664.
Medisch Specialistische Richtlijnen 2.0 (2012). Adviescommissie Richtlijnen van de Raad Kwalitieit. http://richtlijnendatabase.nl/over_deze_site/over_richtlijnontwikkeling.html
Neumann I, Santesso N, Akl EA, Rind DM, Vandvik PO, Alonso-Coello P, Agoritsas T, Mustafa RA, Alexander PE, Schünemann H, Guyatt GH. A guide for health professionals to interpret and use recommendations in guidelines developed with the GRADE approach. J Clin Epidemiol. 2016 Apr;72:45-55. Doi: 10.1016/j.jclinepi.2015.11.017. Epub 2016 Jan 6. Review. PubMed PMID: 26772609.
Schünemann H, Brożek J, Guyatt G, et al. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group, 2013. Available from http://gdt.guidelinedevelopment.org/central_prod/_design/client/handbook/handbook.html .
Zoekverantwoording
Zoekacties zijn opvraagbaar. Neem hiervoor contact op met de Richtlijnendatabase.