Off-label azathioprine - Leprosy reaction
Uitgangsvraag
What is the safety and efficacy of off-label treatment with azathioprine in patients with dermatological diseases?
- Leprosy type 1 reaction
Aanbeveling
|
Weak |
There is a weak recommendation for the use of azathioprine with concomitant oral steroids for the treatment of a severe leprosy type 1 reaction. Although there was no significant beneficial effect of azathioprine compared with conventional (steroid) treatment, a possible steroid sparing effect was suggested.
Extra attention should be given to safety aspects when prescribing azathioprine (uncertain off-label safety). |
Overwegingen
|
Important subjects to consider |
Remarks |
|
Uncertainty in the estimates of likely benefit, and likely risk, inconvenience, and costs*
* estimates for benefit (efficacy/effectiveness) and safety are ranked by the working group as very certain, certain, uncertain or very uncertain. |
-One randomized trial (low quality of evidence) has demonstrated no significant difference in therapeutic effect of AZA and prednisolone treatment compare with prednisolone treatment alone. There was a significant beneficial effect compared with baseline symptoms. Uncertain estimate. -There is an uncertain steroid sparing effect. -Uncertainty about the off-label safety of azathioprine. -Costs may vary with the number of follow-up visits and dosage of azathioprine. |
|
Importance of the outcome that treatment prevents |
-Diminishing the skin symptoms of leprosy. |
|
Magnitude of treatment effect*
* the magnitude of treatment effect is ranked by the working group as good, moderate, low, no effect or worsening |
-Skin symptom improvement was seen in 52% of the patients after 3 months treatment. Moderate effect. |
|
Precision of estimate of treatment effect*
* estimates are ranked by the working group as very certain, certain, uncertain or very uncertain. |
-Descriptive outcome. Uncertain. |
|
Risks associated with therapy |
-See section “general treatment considerations” and “safety” |
|
Burdens of Therapy |
-During the first weeks of therapy laboratory monitoring at weekly intervals is necessary, afterwards every one to three months. See also section “general treatment considerations”. |
|
Risk of target event |
-Skin symptoms are the target event, so the risk is 100%. |
|
Costs |
- Costs of AZA are between € 10,14 and 10,17 for 15 days 3dd 50 mg, not included are the costs for delivery, laboratory monitoring and visits to the clinic. (*www.medicijnkosten.nl). |
|
Varying Values between patients |
- |
|
Other |
Duration of treatment was considered to short by the working group to evaluate treatment effect. |
Onderbouwing
Achtergrond
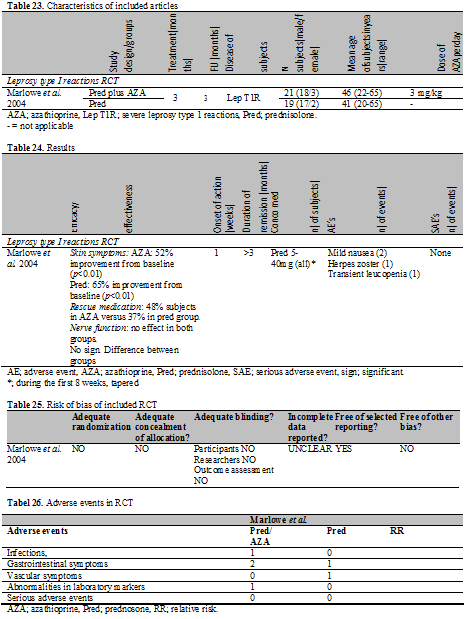
In total, 1 RCT published in 2004 was found in which patients with leprosy type 1 reaction were treated with AZA. To measure the efficacy in this trial, a special instrument was made, which was not previously validated. This outcome tool assessed three categories of symptoms. Category A includes skin symptoms and nerve pain, category B sensory testing and category C voluntary muscle testing. Furthermore the need for rescue medication was assessed as an aspect of efficacy.
Conclusies / Summary of Findings
|
Low |
The available RCT is of low quality because it has very serious limitations in study quality (Table 23). |
Samenvatting literatuur
RCT
Methodological quality
Marlowe et al. performed a non blinded randomized controlled trial. However, methods of randomization and concealment of allocation were not described. Nonetheless, the two treatment groups were described in great detail and were comparable at randomization. All predefined outcomes were addressed in the article, so selective outcome reporting was considered unlikely. An overview of the methodological quality can be seen in the risk of bias table (Table 25).
Demography
There are two treatment groups; group I received AZA (3.0 mg/kg/day) and prednisolone (from 5 mg/day to 40mg/day) during the first 8 weeks in a reduction schedule and group II received prednisolone for 12 weeks in a reduction schedule (from 5 mg/day to 40 mg/day). The 3 months period of active treatment was followed by a 3 months follow-up period. In total, 40 subjects were enrolled and completed the study. The diagnosis of severe leprosy type 1 reaction was established by the presence of erythematous and raised skin lesions with or without evidence of neuritis in patients with borderline tuberculoid, borderline lepromatous or lepromatous leprosy. TMPT activity was not measured prior to initiation of AZA (Table 23).
Efficacy
The skin symptoms improved in 11/21 patients (52%) compared to baseline in the prednisolone plus AZA group versus improvement in 12/19 patients 65% in the prednisolone group at week 12; the improvement was significant in both groups. This effect was sustained in the follow up period. Need for rescue medication in the first 12 weeks was found in 48% of the subjects in AZA group versus 37% in prednisolone group. At 12 weeks and 24 weeks, the nerve function (sensory and voluntary muscle testing) showed no improvement in both groups. Nerve pain and tenderness did improve in both groups. There was no statistically significant difference between the groups concerning all the above mentioned outcomes (Table 24).
Referenties
- 1 - Marlowe SNS. Clinical outcomes in a randomized controlled study comparing azathioprine and prednisolone versus prednisolone alone in the treatment of severe leprosy type 1 reactions in Nepal. Transactions of the Royal Society of Tropical Medicine and Hygiene 2004; 98: 602-9.
Evidence tabellen
Verantwoording
Beoordelingsdatum en geldigheid
Publicatiedatum : 30-08-2014
Beoordeeld op geldigheid : 22-06-2010
A guideline can only be leading, if it is maintained on a continuous base, with systematic monitoring of medical scientific literature as well as regular contributions from clinical practice. In case of important developments, it can be decided that the complete working group shall meet to propose amendments, which will be distributed among the various professional groups. A revision will be planned at least every five years.
Doel en doelgroep
Objective
A guideline is a document with recommendations to support patient care in daily practice. The guideline is based on results of scientific research and subsequent formation of opinion, aimed at deciding on the appropriate medical intervention. A guideline and the documents derived from it, give recommendations for the treatment of patients, including psychosocial care and patient information.
Intended users
The guideline is intended for members of the medical and paramedical professional group, including: dermatologists, general practitioners, pharmacists and dermatology nurses, nurse practitioners and physician assistants. A text derived from the guideline is available for patients.
Samenstelling werkgroep
A working group was appointed for the development of the guideline. This group consisted of dermatologists, pharmacists and a general practitioner from Lareb (the Dutch pharmacovigilance centre). During the formation of the group, the geographical distribution of its members was taken into account as well as a balanced representation of academic and non-academic employment . The members of the working group have acted independently and not a single member received any favour aimed at influencing the guidelines.
|
J.G. (Jan Gerrit) van der Schroeff, MD, PhD |
Dermatologist, Chairman working group |
|
J.J.E. (Jannes) van Everdingen, MD, PhD |
Director NVDV |
|
M. E. (Mandy) Schram, MD |
PhD candidate and resident Dermatology |
|
P. (Pieter) van der Valk, MD,PhD |
Dermatologist |
|
W.R. (William) Faber MD. PhD, FRCP |
Dermatologist |
|
A.Y. (Amber) Goedkoop, MD, PhD |
Dermatologist |
|
R.J. (Rinke) Borgonjen, MD |
PhD candidate |
|
A. (Annemieke) Horikx, PharmD |
Pharmacist KNMP |
|
E.P. (Eugène) van Puijenbroek, MD |
General practitioner Lareb |
|
R.I.F. (Rutger) van der Waal, MD, PhD |
Dermatologist |
|
A. (Annemieke) Floor, PharmD |
Pharmacist-researcher |
|
W. (Wouter) Goldtschmidt, MD, PhD |
Dermatologist |
|
E.L. (Noortje) Swart PhD |
Clinical pharmacist |
|
Ph. I. (Phyllis) Spuls, MD, PhD |
Dermatologist |
Belangenverklaringen
List of conflicts of interest
None reported
Methode ontwikkeling
Evidence based
Implementatie
During the various phases of developing the draft guideline, the implementation of the guideline and the actual workability of the recommendations are taken into account as much as is possible. The guideline is distributed to all relevant professional groups and hospitals through the internet and in various medical journals attention will be given to the guideline.
Werkwijze
During a period of year ( meetings) the working group worked on a draft guideline. An expert group made a bottleneck analysis during the preparatory phase. The expert group compiled a list of drugs which are frequently subscribed for off-label use in dermatology. The listed drugs were prioritized according to frequency of use and occurrence of potential serious adverse events. The members of the working group had the opportunity to propose alterations in the list of selected drugs The members of the working group agreed on composing a guideline about the off-label use of the following six selected drugs:
- Azathioprine
- Cyclosporine
- Methotrexate
- sulfasalazine dapsone
- Hydroxychloroquine
The working group agreed that the outcomes efficacy/effectiveness and safety are crucial for decision making. . The working group started by making a draft guideline for azathioprine and decided that the applied methods would serve as a blueprint for the other five drugs. Useful literature was found by systematic searches and by checking of references (see “Methodology of literature search”). The members of the working group assessed the relevant literature with regard to content and quality. Subsequently, conclusions were drawn and recommendations were made for off-label use of the selected drugs by the members of the working group. The final version of the guideline was approved by all scientific societies involved on.
Methodology of literature search
Research question
For each selected drug a research question according to PICO was made.
PICO stands for:
- Participants/population: population of patients with a dermatological disease who are treated with a drug that is not registered for the use in this particular disease.
- Intervention: the selected drug.
- Comparison: any other treatment (e.g. other systemic therapy, placebo, quality of life intervention), in case of lack of a control group; no other treatment.
- Outcome: safety and/or efficacy.
Search strategy
For each selected drug a standardized search was performed in the Medline (by PubMed) (1950-2009), EMBASE (1980-2009) and CENTRAL databases. This search strategy was designed by a literature specialist of the department ‘Professionele Kwaliteit van de Orde van Medisch Specialisten’. Also references of included articles were screened for eligibility.
Pre-selection with keywords
After the searches were uploaded in Reference Manager, articles labeled with possible keywords for exclusion were selected. A sample was taken of these selected articles to check if there were any relevant articles in that selection. The sample size was either 20 or 50 articles, depending on the number of articles labeled with a specific keyword. If the sample didn’t contain any relevant articles, all the articles labeled with a specific keyword were excluded.
In the searches of cyclosporine, methotrexate, dapsone, hydroxychloroquine and sulfasalazine articles with the keywords ‘case report’ were excluded after a sample of 50 articles didn’t reveal any relevant articles for inclusion.
In addition, articles with the following keywords were excluded after a sample of 20 articles didn’t show any relevant articles:
|
Cyclosporine |
Dapsone |
||
|
- |
Transplantation |
- |
Leprosy |
|
- |
Transplantation immunology |
- |
Mycobarterium leprae |
|
- |
Transplantation immunology [Physiology] |
- |
Pneumocystis carinii |
|
- |
Acute graft rejection [Complication] |
- |
Toxoplasmosis |
|
- |
Acute graft rejection [Diagnosis] |
- |
Spider |
|
- |
Acute graft rejection [Drug therapie] |
Methotrexate* |
|
|
- Acute graft versus host disease |
- |
Psoriasis |
|
|
- |
Bone Marrow Transplantation |
- |
Reumathoid arthritis |
|
- |
Breast cancer |
- |
Leukemia |
|
- |
Graft Survival |
- |
Osteosarcoom |
|
- |
Graft Recipient |
- |
Lymphoma |
|
- |
Kidney Graft |
- |
Bladder |
|
- |
Kidney Transplantation |
- |
Breast Cancer |
|
- |
Liver Transplantation |
- |
Mycosis |
|
- |
Proteinuria |
- |
Multiple sclerosis |
|
- |
Nephritis |
- |
Colitis |
|
- |
Irradiation |
- |
Asthma |
|
- |
Heart transplantation |
- Cancer + skin + cutaneous |
|
|
- |
Vitamin |
Sulfasalazine |
|
|
- |
Psoriasis |
- |
Rheumatoid arthritis |
|
Hydroxychloroquine: |
- |
Arthritis |
|
|
- |
Rheumatic disease |
- |
Crohn |
|
- |
Systemic lupus erythematodes |
- |
Ulcerative colitis |
|
- |
Discoid lupus erythematodes |
|
|
|
- |
Lupus erythematosus |
|
|
* In the methotrexate search articles with the note ‘review’ were excluded after a sample of 20 articles didn’tcontain any relevant articles.
An overall validation of this method was provided by the double search strategy on azathioprine. An initial/broad search (thus without using keywords) was compared with the search that used specific keywords for exclusion. Articles with the keywords ‘case report’, ‘polymyositis’ and ‘idiopatic thrombocytopenic purpera’ were excluded after a sample showed no relevant articles.
We found that all studies that were included in initial/broad search were present in the search using keywords for exclusion. This validates the method of excluding articles by using keywords.
Selection of articles
All articles with title and abstract referring to off-label treatment with the predefined drug in patients with dermatological diseases were selected. To determine eligibility, the full text of the selected articles was screened according to the predefined in-and exclusion criteria. Data on methodological quality, study characteristics, efficacy and safety were extracted by using a data extraction form. All stages of literature selection and data extraction were performed by two independent reviewers. Disagreements about study selection and data extraction were solved by discussion.
In- and exclusion criteria
Selection of the articles was performed by using the following pre-defined in- and exclusion criteria. Inclusion criteria:
- The article concerns the selected drug and
- The selected drug is used in the treatment of a dermatological disease for which that particular drug is not registered.
Exclusion criteria:
- Case reports with less than 5 subjects*
- Lack of data on safety and efficacy
- Articles concerning treatment other than systemic treatment with the selected drug
- Animal studies
- In vitro studies
- Double publications
- Articles concerning diseases that are primarily treated by other specialists
- Language other than English, French, German and Dutch
No restrictions were imposed regarding age, gender, skin type and number of subjects in a study and date of publication.
* A random sample of the excluded articles was taken to check if any relevant adverse effects were missed.
Data-extraction
Of all the included articles, data were extracted by two independent reviewers. This was done by using a standardized data extraction form. Disagreements on data extraction were solved by discussion.
Data- extraction was performed on:
- Methodological quality
- Demographics
- Efficacy
- Safety
Methodological quality
Randomized controlled trials (RCT’s) were assessed following the criterion grading system described in the Cochrane Handbook for systematic reviews of interventions 5.0.0 (updated February 2008). To assess the risk of bias withinincluded RCT’s, the following parameters for methodological quality were used; sequence generation, concealment of allocation, blinding (of participants, researchers and outcome assessment), reporting of incomplete data, presence of selective outcome reporting and other potential threats to validity.
The methodological quality of cohort studies was assessed by using the checklists for cohort studies described by the Dutch Cochrane Centre.
Demographics
Data of demographics were extracted concerning:
- Study design: randomized? controlled? prospective, retrospective?
- Treatment arms
- Disease of the subjects: severity, stage, subtype, duration
- Previous medications
- Diagnostics: what was the method of diagnosis? Clinical, histopathological, other diagnostic criteria?
- Subjects: number, male/female, age, subgroups
- Duration of treatment
- Duration of follow up
- Concomitant medication
- Dosing schedule of the selected drug
Efficacy/effectiveness
- Used outcome parameters: clinical assessment, global assessment, quality of life measurement, laboratory markers, onset of effect, duration of remission, relapse rate, etc)
- Severity outcomes: the result of the used outcome parameters. Differences between baseline and end of the study and between treatment groups.
Safety
Safety is an important issue in off-label use of medication. The working group scored all adverse events, including a special focus on serious adverse effects. Within the included studies, every study that reported (serious) adverse events was taken into account. Adverse events reported in RCT’s or cohorts will be compared with the adverse events that occurred in the control group. If possible a relative risk will be calculated.
Extracted safety data:
- Adverse events: which? how many? at what time during treatment or after treatment?
- Serious adverse events: which? how many? at what time during treatment or after treatment?
- Withdrawals due to adverse events?
An Adverse Event (AE) was defined as an unfavorable and unintended sign, including an abnormal laboratory finding, symptom or disease associated with the use of a medical treatment or procedure, regardless whether it is considered related to the medical treatment or procedure, that occurs during the course of the study.
A Serious Adverse Events (SAE) was defined as any untoward medical occurrence that results in death, is life threatening, requires inpatient hospitalization or prolongation of existing hospitalization, results in persistent or significant disability/incapacity, is a congenital anomaly/birth defect or is reported in the study as such.
Handling of the data
Extracted data will be presented in tables and with accompanying text per disease following standardized means.
Level of evidence
The description and assessment of the articles according to the data extraction (see above) are listed in separate sections under the headers “Safety data off -label azathioprine” or “Efficacy/effectiveness data off-label azathioprine” and in tables (see section Tables).
Not all data extracted from articles are equally valuable. Therefore every set of articles is summarised in a conclusion, in which the level of the evidence is indicated according to the GRADE system (see boxes below). Consequently the recommendations in this guideline are based on evidence generated by scientific research, with emphasis on the outcomes safety and effectiveness/efficacy. The search results that were used are up to date until at least 01-10-2009, unless stated otherwise.
GRADE system
|
Type of evidence |
Randomized trial = high Observational study = low Any other evidence = very low |
|
Decrease* grade if |
Important inconsistency • Some or major uncertainty about directness • Imprecise or sparse data • High probability of reporting bias • Serious or very serious limitation to study quality |
|
Increase grade if |
• Strong evidence of association—significant relative risk of > 2 ( < 0.5) based on consistent evidence from two or more observational studies, with no plausible confounders (+1) • Very strong evidence of association—significant relative risk of > 5 ( < 0.2) based on direct evidence with no major threats to validity (+2) • Evidence of a dose response gradient (+1) • All plausible confounders would have reduced the effect (+1) |
|
*Each quality criterion can reduce the quality by one or, if very serious, by two levels. |
|
|
Conclusion
|
Development of the recommendations
For the development of a recommendation, other aspects than scientific evidence are also of importance, such as: patient preferences, availability of special techniques or expertise, organisational aspects, social consequences or costs. Known adverse events are also taken into account, as far as they were not already distilled from scientific literature. These aspects are discussed after the conclusion(s). On the basis of literature, the conclusion is here placed in the context of daily practice, and the pros and cons of the various treatments are balanced against each other. The final formulated recommendation is the result of the available evidence in combination with these considerations and can be formulated as a weak or strong recommendation in favour of a certain therapy or as a weak or strong recommendation against a certain therapy (see box below). The aim of this procedure and the formulation of the guideline using this ‘format’ is to enhance the transparency of the guideline. It leaves room for an efficient discussion during the meetings of the working group and moreover, it improves clarity for the user of the guideline.
|
Recommendation
|
Zoekverantwoording
Literature search
Between September 2009 and October 2009, a literature search in Medline (1950-2009), EMBASE (1980-2009) and CENTRAL was performed. As main search strategy ‘azathioprine’ and synonyms (not the active metabolites) were used in combination with all skin diseases; for example the search strategy in Medline:
1 derm*.jn. (22039)
2 Azathioprine/ (11859)
3 (azathioprine* or imuran* or immuran* or imurel*).ab. (8887)
4 (azathioprine* or imuran* or immuran* or imurel*).ti. (2856)
5 (azathioprine* or imuran* or imurel* or immuran*).kw. (72)
6 4 or 2 or 5 or 3 (16781)
7 6 and 1 (72)
8 exp Skin Diseases/ (669358)
9 6 and 8 (1705)
10 7 or 9 (1711)
11 limit 10 to (humans and (dutch or english or french or german)) (1535)
There was no limit with respect to the date of the publication. Literature references of all relevant articles found were checked in order to find additional articles. In addition, data published in Micromedex concerning azathioprine was studied to retrieve further potential relevant references regarding safety in off-label use. None were found.
Study selection and data extraction
All articles with title and abstract referring to off-label treatment with azathioprine of patients with dermatological diseases were selected by two reviewers. Next, to determine eligibility, the full text of the selected articles was screened by two reviewers. Disagreements were solved by discussion. Predefined in- and exclusion criteria are described in detail in the introduction section. Data on methodological quality, demographics, efficacy and safety were extracted by two independent reviewers using a data extraction form. Disagreements about data extraction were solved by discussion.
